LICENSE AND ASSIGNMENT AGREEMENT
EXHIBIT 10.28
LICENSE AND ASSIGNMENT AGREEMENT
dated
16 JANUARY 2018
by
AETERNA ZENTARIS GMBH
the Licensor
and
The
i
Table of contents
|
1. |
Definitions and interpretation |
1 |
|
2. |
Grant of rights / license |
13 |
|
3. |
Governance |
15 |
|
4. |
Technology transfer and technical / regulatory assistance |
18 |
|
5. |
Development |
19 |
|
6. |
Supply |
22 |
|
7. |
Commercialization. |
25 |
|
8. |
Financial Provisions |
29 |
|
9. |
Trademarks |
32 |
|
10. |
Intellectual Property (except Trademarks) |
33 |
|
11. |
Confidentiality |
36 |
|
12. |
Representations, warranties and covenants |
38 |
|
13. |
Indemnity and insurance cover |
42 |
|
14. |
Duration and termination of the agreement |
45 |
|
15. |
Assignment/succession |
49 |
|
16. |
Governing law and disputes |
50 |
|
17. |
Miscellaneous |
51 |
|
Schedule 1 |
|
CMO Contracts |
|
|
|
Schedule 2 |
|
Technology Transfer Critical Materials and Timing |
|
|
|
Schedule 3 |
|
FDA Letters |
|
|
|
Schedule 4 |
|
Licensor Materials to be Transferred to Licensee |
|
|
|
Schedule 5 |
|
Disclosure Schedule |
|
|
|
Schedule 6 |
|
Domain Names |
|
|
|
Schedule 7 |
|
Licensor Patent Rights |
|
|
|
Schedule 8 |
|
Supply Chain |
|
|
|
Schedule 9 |
|
Development Plan |
|
|
|
Schedule 10 |
|
PIP Budget |
ii
License and Assignment Agreement
This Agreement is dated 16 January 2018 (“Effective Date”)
Between
, (the “"),; and
Strongbridge Ireland Limited, a corporation incorporated under the laws of Ireland, (the “"),.
The Licensor and the Licensee sometimes being referred to in this Agreement as a “Party” or together the “Parties”.
A. The Licensor owns or has the exclusive right to certain technology, intellectual property rights, regulatory files and confidential and/or proprietary information relating to the Product (as defined below).
B. The Licensor is interested in assigning the ownership of the Product Registrations (as defined below) and granting an exclusive license under the intellectual property to the Licensee to carry out development, manufacturing, registration and commercialization of the Product in the Territory (as defined below).
C. The Licensee, together with its Affiliates (as defined below), are engaged in the development, manufacturing and commercialization of pharmaceutical products in the Territory and possess the resources, skills and experience there necessary to perform their obligations under this Agreement.
The Parties now agree as follows:
1. Definitions and interpretation
“Adult Indication” means assessing GHD in adults.
“Affiliates” means an entity that has a relationship directly or indirectly with another entity such that either entity is Controlled by, Controlling, has the power to Control, or under common Control with the other entity, or a third party Controls, or has the power to Control, both of the entities, where “Control” means possession, directly or indirectly, of the power to direct or cause the direction of the activities, management and policies of the relevant entity and in the case of a corporate entity shall include but not be limited to the holding of more than fifty percent (50%) of the share capital of the entity or the equivalent power or authority to elect more than fifty percent (50%) of the board of directors of such entity or the equivalent management body. The Parties acknowledge that in the case of certain entities organized under the Laws of certain countries outside of the EU, the maximum percentage ownership permitted by Law for a foreign investor may be less than fifty percent (50%), and that in such case such lower percentage shall be substituted in the preceding sentence, provided that such foreign investor has the power to direct the management and policies of such entity.
“Agreement” means this License and Assignment Agreement together with its attached Schedules.
“Anti-Bribery Law” means the US Foreign Corrupt Practices Act, UK Xxxxxxx Xxx 0000 and any other applicable Law, rule, regulation, or other legally binding measure of any jurisdiction that relates to bribery, corruption, or, for purposes of U.S. Law, transfers of value to licensed healthcare providers.
1
“API” means the active pharmaceutical ingredient macimorelin acetate, and any metabolite, salt, ester, hydrate, solvate, isomer, enantiomer, free acid form, free base form, crystalline form, co-crystalline form, amorphous form, pro-drug, racemate, polymorph, chelate, stereoisomer, tautomer, or optically active form of any of the foregoing.
“Bankruptcy Event” means, with respect to either Party:
(a) a notice having been issued to convene any meeting for the purpose of passing a resolution or seeking a petition to wind up or liquidate that Party, or to seek bankruptcy or official administration, or such a resolution having been passed or such a petition having been issued, including a petition under title 11 of the United States Code or any similar statute, Law or regulation (except in relation to a solvent reconstruction or reorganization of that Party);
(b) an involuntary petition in an insolvency proceeding or any proceeding seeking reorganization, arrangement composition, readjustment, liquidation or similar relief under any statute, Law or regulation, is filed against a Party and is not dismissed or stayed within ninety (90) days of the filing thereof or that Party admits or accepts;
(c) a trustee in bankruptcy, receiver, administrative receiver, receiver and manager, court appointed receiver, interim receiver, custodian, sequestrator or similar officer is appointed in respect of that Party or over any part of that Party's assets or any third party takes steps to appoint such an officer in respect of that Party and is not dismissed, vacated or stayed within ninety (90) days of the appointment thereof and if stayed, is not dismissed or vacated within ninety (90) days of the such stay;
(d) a Party takes any step, (including starting negotiations), with a view to readjustment, rescheduling or deferral of any part of that Party's indebtedness including a moratorium with creditors, or proposes or makes any general assignment, composition or arrangement with or for the benefit of all or some of that Party's creditors or makes or suspends or threatens to suspend making payments to all or some of that Party's creditors or the Party submits to any type of voluntary arrangement with creditors; or
(e) a Party takes any step, (including starting negotiations), to file a petition or answer seeking for that Party any reorganization, arrangement, composition, conservatorship, winding up, readjustment, liquidation or similar relief under any statute, Law or regulation.
“Business Day” means between 9.00 am and 5.00 pm local time on a day other than a Saturday, Sunday, bank or other public holiday in Germany or any State in the USA.
“Calendar Quarter” means each period of three months ending on 31 March, 30 June, 30 September or 31 December.
“Calendar Year” means each successive period of twelve calendar (12) months commencing on 1 January.
“Children” means persons 18 years of age or younger including neonates, infants and adolescents.
“Clinical Trial” means the administration of the Product to humans for purposes of generating data on characteristics of the Product including, without limitation, any study carried out in order to obtain or maintain a Regulatory Approval in any country of the Territory.
“CMO” means a Person that conducts contract manufacture on behalf of another Person.
2
“CMO Contracts” means those contracts set out at Schedule 1.
“Commercial Information” means all information which is Controlled and relates to the pricing, reimbursement, marketing, promotion and selling of the Product including but not limited to (i) commercialization plans, strategic and implementation; and (ii) pharmaco-economic studies justifying pricing; and (iii) analysis of competitive products and environment including market research reports; and (iv) product positioning strategies (including unique selling proposition and understanding of competitors' positioning strategies) and promotional strategies (including Promotional Materials); and (v) Pricing Approval submissions and the content of bids for tenders; and (vi) virtual product and clinical support approaches and techniques via web page; and (vii) medical education strategies; and (viii) strategies used for building relationships with health insurance and managed care entities; and (ix) analysis of market sales and prescription data; (x) terms of contractual arrangements with purchasers; and (xi) customer lists.
“Commercialize” or “Commercialization” means any and all activities directed to commercialization, including marketing, promoting, detailing, importing, distributing, warehousing, offering for sale, having sold and/or selling a pharmaceutical product, including market research, pre-launch marketing and educational activities, and sampling.
“Commercially Reasonable Efforts” means, in respect of a Party, efforts and resources commonly used by a pharmaceutical company of a similar size to that Party, based on market capitalization, to Commercialize (or where the phrase 'Commercially Reasonable Efforts' is used in this Agreement in a context other than relating to Commercialization, to perform such other act as the operative language of this Agreement states) a product owned by such a company or to which it has rights, which product is at a similar stage in its development or product life and is of similar market potential to the Product, in each case taking into account issues of safety and efficacy, product profile, the proprietary position, the then-current competitive environment for such product and the likely timing of such product’s entry into the market, the regulatory environment and status of such product, and other relevant scientific, technical and commercial factors, in each case as measured by the facts and circumstances at the time such efforts are due and in relation to the country or countries to which such efforts pertain.
“Confidential Information” means, subject to the relevant carve-outs set forth in Section 11:
(a) the terms and conditions of this Agreement, for which each Party will be considered a Disclosing Party and a Recipient Party;
(b) the Dossier, Know How and Commercial Information within the Licensor IPR Package for which the Licensor will be considered the Disclosing Party and the Licensee shall be the Recipient Party;
(c) information within the Licensee IPR Package in relation to which the Licensee will be considered the Disclosing Party and the Licensor the Recipient Party; and
(d) any other non-public information, whether or not patentable, disclosed or provided by one Party to the other Party in connection with this Agreement, including, without limitation, information regarding such Party's strategy, business plans, objectives, research, technology, products, business affairs or finances including any non-public data relating to Commercialization of any product and other information of the type that is customarily considered to be confidential information by parties engaged in activities that are substantially similar to the activities being engaged in by the Parties under this Agreement, for which the Party making such disclosure will be considered the Disclosing Party and the receiver will be the Recipient Party.
3
“Control” (including variations such as “Controlled” and except as used in the definition of 'Affiliates') means with respect to any intellectual property or other rights, possession of the right, whether directly or indirectly, and whether by ownership, license or otherwise, to assign, or grant a license, sub-license or other right to or under, such intellectual property or other rights without violating the terms of any agreement or other arrangement with any Third Party.
“Cost of Manufacture” means the amounts paid or payable by the relevant Party to the CMO in respect of relevant Products or Materials supplied.
“CTD Format” means the format of a common technical document recommended by ICH or adopted by any Governmental Authority in the Territory responsible for marketing authorization or monitoring the marketing of the Product that are formatting requirements or methods for the submission of other or supplemental information or data to such Government Authority and as may be updated from time to time.
“CTM” shall mean Product in a form suitable for administration to humans in a Clinical Trial manufactured in accordance with the specifications and GMP and as required by any IND appropriately packaged and labelled.
“Data Room” means the data room hosted and operated by Xxxxxxx for and on behalf of the Licensor, entitled 'Macrilen';
“Development (and the corresponding verb “to Develop”) means all development and regulatory activities regarding the Product and the API in the Territory, including:
(a) carrying out the PIP or any other pediatric study required to obtain Regulatory Approval for the Product for the Pediatric Indication in any country in the Territory; and
(b) preparing, submitting, reviewing or developing data or information necessary for the purpose of submission to a Regulatory Authority to obtain or maintain and/or expand Regulatory Approval of the Product in the Territory including data management, statistical designs and studies, document preparation, and other related administrative costs.
“Development Costs” means the direct and indirect costs of the PIP currently anticipated to be five million to six million dollars ($5,000,000 –$6,000,000) as set forth in the PIP Budget, subject to any increases of such budget agreed by the Parties.
“Development Plan” means the plan for carrying out the PIP attached in Schedule 9, as such plan may be updated in accordance with Section 5, either in relation to the PIP or otherwise.
“Disclosing Party” means the Party which discloses Confidential Information to the other Party.
“Disclosure Schedule” means the disclosure schedule, in the form of Schedule 5.
“Documents” means analyses, books, CD-ROM, charts, comments, computations, designs, discs, diskettes, files, graphs, ledgers, notebooks, paper, photographs, plans, records, recordings, reports, research notes, tapes, web pages and websites, and any other graphic or written data or other media on which Know How is made available, communicated, or use whether permanently stored or available temporarily through other means, and advertising and Promotional Materials of any nature whatsoever including preparatory materials for the same.
4
“Dossier” means the information and data for the Product in a country in CTD Format (or otherwise) and as registered with a Regulatory Authority for the Regulatory Approval containing the administrative, safety, efficacy, quality, non-clinical and clinical data, chemistry and manufacturing control data and communications with the Regulatory Authority for the Product as it may change from time to time.
“EMA” means the European Medicines Agency.
“Encumbrance” means a mortgage, charge, pledge, lien, option, restriction, right of first refusal, right of pre-emption, third party right or interest, other encumbrance or security interest of any kind, or another type of preferential arrangement (including, without limitation, a title transfer or retention arrangement) having similar effect but for the purposes of this Agreement always excluding a license.
“EU” means the countries of the European Union from time to time which are currently Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and includes the United Kingdom regardless of future treaty or common relationships with the other countries in the EU or Europe.
“Europe” means that group of countries comprised of the EU plus (if they are not Member States) Iceland, Liechtenstein, Norway, Switzerland and UK.
“Existing CMO Contracts” has the meaning given to it in Section 6.1.
“Existing Indications” means the Adult Indication and the Pediatric Indication.
“Exploitation Agreement” means the exploitation agreement between (i) Aeterna Zentaris GmbH (formerly Zentaris GmbH); (ii) The Centre National De La Recherche Scientifique; (iii) The University of Montpellier 1; and (iv) The University of Montpellier 2, entered into in 2005.
“FDA” means the U.S. Food and Drug Administration.
“Final Report” means the clinical study report including attachments, quality assurance, monitoring reports, and raw data that record the performance or information generated from a Clinical Trial, whether or not compliant with ICH E3 or conducted under GCP, and that includes such personal health information or personal identifying information as may be necessary to assure the quality and integrity of the report.
“First Commercial Sale Date” means the date of the first commercial sale in an arm's length transaction to a Third Party of the Product for an Existing Indication in each country in the Territory by or on behalf of the Licensee, an Affiliate, Sub-licensee after obtaining Regulatory Approval and any Pricing Approvals for the Product necessary or desirable in such country. For clarity “First Commercial Sale Date” shall not be any date on which sale of Product for an Existing Indication occurs in the Territory for use on a compassionate use or named patient basis.
“Formal Presentation” means a presentation of the results of a Clinical Trial based on the data and conclusions set out in an Interim Report.
“GCP” means the then-current standards, practices and procedures for good clinical practices, including but not limited to those promulgated or endorsed by:
(c) the European Commission Directive 20/2001/EC relating to good clinical practice in clinical trials on medicinal products for human use;
5
(d) the ICH Harmonised Tripartite Guidelines for Good Clinical Practice (E6) and any other guidelines for good clinical practices for trials on medicinal products in the European Union;
(e) the FDA as set forth in the guidelines entitled "Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance," including related regulatory requirements imposed by the FDA and the provisions of 21 C.F.R. Part 312; and
(f) the equivalent applicable Law in any relevant country.
“GHD” means growth hormone deficiency.
“GLP” means the then-current standards, practices and procedures for good laboratory practices, including but not limited to those promulgated or endorsed by:
(g) the European Commission Directives 2004/9/EC and 2004/10/EC relating to the application of the principles of good laboratory practices as well as "The rules governing medicinal products in the European Union," Volume 3, Scientific guidelines for medicinal products for human use (ex - OECD principles of GLP);
(h) the then-current standards, practices and procedures promulgated or endorsed by the FDA as defined in 21 C.F.R. Part 58; and
(i) the equivalent applicable Law in any relevant country.
“GMP” means the then-current standards, practices and procedures for good laboratory practices, including but not limited to those promulgated or endorsed by:
(j) the European Commission Directives 2004/9/EC and 2004/10/EC relating to the application of the principles of good laboratory practices as well as "The rules governing medicinal products in the European Union," Volume 3, Scientific guidelines for medicinal products for human use (ex - OECD principles of GLP);
(k) the then-current standards, practices and procedures promulgated or endorsed by the FDA as defined in 21 C.F.R. Part 58; and
(l) the equivalent applicable Law in any relevant country;
“Government Authority” shall have the same meaning as Regulatory Authority.
“ICH” means the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
“IND” means an Investigational New Drug Application exemption or Clinical Trial Application filed with, acknowledged, or accepted by, Governmental Authorities prior to beginning clinical trials in humans in any country, or any comparable application to and acceptance by the Regulatory Authority of a country or group of countries prior to beginning clinical trials in humans in that country or in that group of countries.
“Interim Report” shall mean a written report that contains an initial analysis of the results of a Clinical Trial once that Clinical Trial has completed and the data from that Clinical Trial has been collated and analyzed and, in particular reporting, on the extent to which the endpoints of the Clinical Study have been met but which is not the Final Report, has not yet been subject to quality assurance and precedes the Final Report.
“JSC” means the joint steering committee established under Section 3.1.
6
“Know How” means technical and other information which is not in the public domain, including information and data comprising or relating to (i) non-clinical data including pharmacological, toxicological and metabolic data and results of all non-clinical studies relevant to the product; and (ii) clinical safety and efficacy data including data analyses, study reports and information contained in protocols, filings or other submissions to and responses from ethical committees and Regulatory Authorities; and (iii) safety (pharmacovigilance) data; and (iv) production facilities and processes including any drug master file, specifications, techniques, manufacturing line procedures, chemistry and manufacturing control data, standard operating procedures quality assurance and quality control processes and techniques, and all other documentation retained to comply with GMP procedures; and (v) information relating to contract manufacturers and the manufacturing supply chain of the Product, including API, fill finish, primary and secondary packaging (items (iv) and (v) together being “Manufacturing Know How”). Know How includes (a) Documents containing Know How; and (b) includes and covers any legal rights including trade secrets, copyright, database or design rights protecting such Know How. The fact that an item is known to the public shall not be taken to preclude the possibility that a compilation including the item, and/or a development relating to the item, is not known to the public.
“Knowledge” means a Party's and its Affiliates' understanding in good faith as possessed in the case of Licensor, by Xxxxxxx Xxxx, Xxxxxxx Xxxxxxx, Xxxxx Xxxxxxxx, Xxxxxxx Xxxxxx or Xxxxxxxx Xxxxxxx and in the case of Licensee, by Xxxxxxx Xxxx, Xxxxxxx Xxxxx and A. Xxxxx Xxxxx, of the relevant facts and information after performing a reasonable investigation with respect to such facts and information.
“Launch” means the initial transfer of the Product billed or invoiced by Licensee (or an Affiliate or Sub-licensees) to a non-Sub-licensee Third Party in the Territory following the Effective Date.
“Law” or “Laws” means any federal, state, local, municipal, foreign, international, multinational, or other constitution, law, statute, treaty, rule, regulation, ordinance, code, binding case law or principle of common law, rule or regulation, promulgated or issued by an Governmental Authority, as well as any Judgments, decrees, injunctions or agreements of any entity in the course of dispute resolution that might have binding or precedential effect on the Parties, and also includes any industry standard, third party certification, any technical or scientific standard to which adherence is required by any Governmental Authority and any rules or policies of non-governmental accreditation, standards, certification, or oversight bodies and includes any obligations or responsibilities applicable to holders of Product Registrations including, without limitation, pharmacovigilance and required annual or expedited reporting needed to maintain such Product Registration.
“Legal Requirement” means any applicable Law of any Government Authority including any amendment, extension or replacement thereof which is from time to time in force.
“License” means the rights and license granted by Licensor to Licensee pursuant to the terms of Section 2.1.
“Licensee CMO Contracts” has the meaning given to it in Section 6.2.
“Licensee IPR Package” means the intellectual property and other rights at any time during the Term Controlled by the Licensee or its Affiliates relating to the Product pursuant to Section 10.3 including but not limited to:
(a) Licensee Patent Rights;
(b) Know How;
7
(c) Dossiers;
(d) Commercial Information; and
(e) the Licensee Trademark.
“Licensee Know-How” means the Know-How Controlled by the Licensee at the date of termination of this Agreement solely to the extent related to the Product.
“Licensee Materials” means the Materials Controlled by the Licensee at the date of termination of this Agreement that are necessary to Manufacture the Product.
“Licensee Patent Rights” means all Patent Rights generated or developed by or on behalf of the Licensee and/or its Affiliates at any time during the Term of this Agreement containing claims covering the Product or the Manufacturing of the Product.
“Licensee Trademarks” means all Trademarks Controlled by the Licensee relating solely to the Product, but excludes the Licensor Trademark.
“Licensor IPR Package” means the intellectual property and other rights Controlled by the Licensor or its Affiliates relating to the Product (with the exception of the Licensor Materials, Controlled at any time during the Term) including but not limited to:
(a) the Licensor Patent Rights;
(b) Know How;
(c) Dossiers;
(d) the Commercial Information in relation to the Territory; and
(e) the Licensor Trademark.
“Licensor Materials” means those Materials of the Licensor as set out in Schedule 4.
“Licensor Patent Rights” means the Patent Rights Controlled by the Licensor at any time during the Term of this Agreement in the countries of the Territory containing claims covering the Product and approved uses of the Product, which at the Effective Date are those set out in Schedule 7.
“Licensor Supply Period” has the meaning given to it in Section 6.4.
“Licensor Trademark” means the Macrilen trademark registered in the Territory and any accompanying logos, trade names, trade dress and/or other indicia relating to the Product Controlled by the Licensor.
“Losses” means any and all losses, damages, liabilities, costs and expenses (including, without limitation, reasonable attorneys' fees and expenses). In calculating “Losses”, the duty to mitigate on the part of the Party suffering the Losses shall be taken into account.
“Manufacture” or “Manufacturing” means to make, have made, produce, manufacture, process, fill, finish, package, label, perform quality control testing, perform quality assurance, release procedures, ship or store a compound or product or any component thereof. When used as a noun, “Manufacture” or “Manufacturing” means any and all activities involved in Manufacturing a compound or product or any component thereof.
“Material” means any quantities of raw materials (including, without limitation, active pharmaceutical ingredients, drug substance, precursors, intermediates, drug product and
8
excipients), labels, packaging materials, dedicated physical components, and dedicated equipment, reference standards or reagents, needed for the Manufacturing of the Product.
“NDA” means the new drug application filed with and approved by the FDA for the Product having NDA number 205598.
“NDA Product” means the Product that is the subject of the NDA.
“Net Sales” means:
(m) the gross amount invoiced by the Licensee, its Affiliates, Sub-licensees or Sub-distributors for sale of Products to third parties, less the following deductions relating to sales of the Products in each case as required by applicable GAAP:
(i) normal and customary trade, cash and quantity discounts accrued or actually given, credits, price adjustments or allowances for damaged products, returns or rejections of products;
(ii) chargeback payments and rebates (or the equivalent thereof) for the Product granted to group purchasing organizations, managed health care organizations or to federal, state/provincial, local and other governments, including their agencies, or to trade customers;
(iii) reasonable and customary freight, shipping insurance and other transportation expenses directly related to the sale of the Product (if actually borne by the Licensee, its Affiliates, or Sub-licensees without reimbursement from any Third Party);
(iv) required distribution commissions/fees payable to any Third Party providing distribution services to the Licensee; and
(n) sales, value-added, excise taxes, tariffs and duties, and other taxes and government charges directly related to the sale, to the extent that such items are included in the gross invoice price of the Product and actually borne by the Licensee, its Affiliates, or Sub-licensees without reimbursement from any Third Party (but not including taxes assessed against the income derived from such sale).
For these purposes sale of Products shall include the Product as Developed by the Licensee from time to time.
Nothing herein shall prevent Licensee or any of its Affiliates or Sub-licensees from making selling, distributing or invoicing Product at a discounted price for shipments to Third Parties in connection with clinical studies, sampling, compassionate sales, or an indigent program or similar bona fide arrangements in which such party agrees to forego a normal profit margin for good faith business reasons and notwithstanding anything herein to the contrary, to the extent such distribution is made without charge it shall not be deemed a sale for purposes of determining Net Sales. Sales or other commercial dispositions of Products (1) between Licensee and its Affiliates and/or its Sub-licensees (except where such Affiliates or Sub-licensees are an end user of the Product), or (2) provided to Third Parties without charge, in connection with research and development, Clinical Trials, compassionate use, humanitarian and charitable donations, or indigent programs or for use, in reasonable and customary quantities, as samples, shall in each case, be excluded from the computation of Net Sales, and no payments will be payable on such sales or such other commercial dispositions.
“New Indication” means an indication for the treatment of any disease or condition other than the Existing Indications.
9
“Outstanding CMO Contracts” has the meaning given to it in Section 6.1.
“Pediatric Indication” means assessing GHD in Children.
“Patent Rights” means:
(a) all national, regional and international patents and patent applications, including provisional patent applications; and
(b) all patent applications filed either from such patents, patent applications or provisional applications or from an application claiming priority from any of these, including divisionals, continuations, continuations-in-part, provisionals, converted provisionals, and continued prosecution applications; and
(c) any and all patents that have issued or in the future issue from the foregoing patent applications in paragraphs (a) and (b) above, including author certificates, inventor certificates, utility models, xxxxx patents and design patents and certificates of invention; and
(d) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, re-examinations and extensions (including any supplementary protection certificates and the like) of the foregoing patents or patent applications in paragraphs (a) to (c) above inclusive, and
(e) any similar rights, including so-called pipeline protection (where the subject matter previously disclosed was not previously patentable in a particular jurisdiction but subsequently becomes patentable subject matter in such jurisdiction), or any importation, revalidation, confirmation or introduction patent or registration patent or patent of additions to any such foregoing patent applications and patents.
“Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, incorporated association, joint venture or similar entity or organization, including a government or political subdivision, department or agency of a government.
"PIP" means the pediatric investigation plan, pediatric study plan, the pediatric study, pediatric drug development and/or any other clinical or pharmacological data collection as submitted to and agreed by EMA (April 11 2017) and which will support authorization of PGHD for the Product by EMA and as further requested by FDA in FDA’s Pediatric Written Request (PWR) under the Best Pharmaceuticals for Children Act (BPCA) which PWR stipulates that the PIP as submitted and agreed to by EMA will meet the requirements to obtain a period of pediatric exclusivity in the U.S..
“PIP Budget” means the budget attached hereto as Schedule 10, which budget relates to the PIP and sets out the direct and indirect costs of the PIP anticipated by the Parties as at the Effective Date as such budget may be updated from time to time in accordance with Section 5.5.
“Pricing Approval” means:
(a) such approval, agreement, determination or governmental decision establishing prices for the Product that can be charged and will be reimbursed by Government Authorities in countries in the Territory where Government Authorities or Government Authorities of such country approve or determine pricing for pharmaceutical products for reimbursement or otherwise; and
10
(b) a price established in a supply contract with a Government Authority following a tender process.
“Product” means any pharmaceutical product containing the API, including the product developed by the Licensor for the Existing Indications and approved by the FDA for marketing in the United States under the NDA.
“Product Registrations” means all applications (including any IND, and the NDA), orphan designations, new drug applications, abbreviated new drug applications, new drug submissions, and any comparable applications and submissions, together with any and all supplements or modifications or amendments thereto, whether existing, pending, withdrawn or in draft form, together with all correspondence to or from any Governmental or Regulatory Authority with respect thereto, prepared and submitted to any Governmental or Regulatory Authority in the Territory with respect to the Product including the NDA and any supplemental drug applications filed with and approved by the FDA or other Regulatory Authorities for the Product whether relating to the Pediatric Indication, a New Indication and/or other dosages of the Product.
“Promotional Materials” means all sales representative training materials and all written, printed, graphic, electronic, audio or video matter, including journal advertisements, sales visual aids, leave-behind items, reprints, direct mail, internet postings and sites and broadcast advertisements intended for use or used by or on behalf of Licensee or its Affiliates in connection with any promotion of a Product.
“Recipient Party” means the Party which receives Confidential Information from the other Party.
“Regulatory Approval” means any approval required from a Regulatory Authority to market and sell a pharmaceutical product in any country of the Territory including the NDA, but excluding any Pricing Approval required or commercially desirable.
“Regulatory Authority” means any supranational, national or local parliament, regional, state, county, city, town, village, municipal, district, commission, department or agency including FDA, EMA, or any competent authority in the EU, Europe or the United Kingdom or other country in which the Product holds marketing authorization, authority (including a listing authority in relation to a stock exchange), inspectorate, minister, ministry official, or other public or statutory Person (whether autonomous or not), multinational organization or any other body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory or taxing authority or power of any nature over the Parties.
“Regulatory Submissions” means any submission by a Person to a Regulatory Authority in connection with the Product.
“Royalty Term” means, Existing Indication by Existing Indication, and country by country of the Territory, the period that is from the First Commercial Sale Date until the later of either:
(a) the expiry of all Valid Claims of the Licensor Patent Rights in such country;
(b) the expiration of any regulatory marketing exclusivity period or other statutory designation that provides similar exclusivity for the Commercialization of the Product in such country; and
(c) ten (10) full Calendar Years following such First Commercial Sale Date.
Where “expiry” in relation to a Valid Claim for purposes of royalty calculations means the cessation of a claim to meet the definition of Valid Claim.
11
“Sub-licensee” means a Third Party to whom the Licensee or its Affiliates grants a sublicense in accordance with Section 2.
“Supply Chain” has the meaning given to it in Section 6.1.
“Taxes” means all taxes of any kind, and all charges, fees, customs, levies, duties, imposts, required deposits or other assessments, including all federal, state, local or foreign net income, capital gains, gross income, gross receipt, property, franchise, sales, use, excise, withholding, payroll, employment, social security, workers' compensation, unemployment, occupation, capital stock, ad valorem, value added, transfer, gains, windfall profits, net worth, asset, transaction, and other taxes, and any interest, penalties or additions to tax with respect thereto, imposed upon any individual or entity by any taxing authority or other governmental authority under the Laws of the applicable country in the Territory.
“Term” means the period commencing on the Effective Date and, unless earlier terminated in accordance with this Agreement, expiring country by country of the Territory at the end of the Royalty Term in such country.
“Term Loan Agreement” means the Term Loan Agreement, dated as of July 14, 2017, among Strongbridge U.S. Inc., a Delaware corporation, Strongbridge Biopharma Public Limited Company, a public limited company incorporated under the laws of Ireland, the Licensee, Cortendo Cayman Ltd., an exempted company incorporated in the Cayman Islands, Cortendo AB (Publ), a public limited liability company incorporated under the laws of Sweden, the subsidiary guarantors from time to time party thereto, the lenders from time to time party thereto and CRG Servicing LLC, a Delaware limited liability company, as amended, supplemented or otherwise modified from time to time.
“Territory” means the United States of America and Canada.
“Third Party” means a party other than either of the Parties or any of their respective Affiliates.
“Trademarks” means registered trademarks and applications thereof, unregistered trade or service marks, get up, logos, trade dress and company names in each case with any and all associated goodwill and all rights or forms of protection of a similar or analogous nature including rights which protect goodwill.
“Valid Claim” means either:
(a) a claim of an issued and unexpired patent included within Patent Rights, which has not been held revoked, unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction, unappealable or un-appealed within the time allowed for appeal, and which has not been admitted to be invalid or unenforceable through reissue or disclaimer or otherwise; or
(b) a claim of a pending patent application included within Patent Rights which claim was filed and is being prosecuted in good faith and has not been abandoned or finally disallowed without the possibility of appeal or refiling of the application, provided that no more than seven (7) years have passed since the earliest priority date for such application.
“Year” means each complete Calendar Year following the First Commercial Sale Date.
1.2 In this Agreement:
(a) the table of contents and headings are inserted for ease of reference only and shall not affect the interpretation of any provision of this Agreement;
12
(b) all references to a particular Section, paragraph or Schedule shall be a reference to that Section, paragraph or Schedule, in or to this Agreement as it may be amended from time to time pursuant to this Agreement;
(c) words in one gender include any other gender, words importing individuals import companies and vice versa, words in the singular include the plural and vice versa, and words importing the whole shall be treated as including a reference to any part thereof;
(d) references to a company shall include any company, corporation or other body corporate wherever and however incorporated or established;
(e) reference to the words “include” or “including” (or any similar term) are not to be construed as implying any limitation and general words introduced by the word “other” (or any similar term) shall not be given a restrictive meaning by reason of the fact that they are preceded by words indicating a particular class of acts, matters or things;
(f) references to “writing” or “written” includes any method of reproducing words or text in a legible and non-transitory form and, for the avoidance of doubt, shall include text transmitted by e-mail;
(g) references to “indemnify” and to “indemnifying” any person against any Losses by reference to any matter includes indemnifying and keeping that person indemnified against all Losses from time to time made, suffered or incurred as a direct or indirect consequence of or which would not have arisen but for that matter;
(h) when calculating the period of time before which, within which or following which any act is to be done or step taken pursuant to this Agreement, the date that is the reference date in calculating that period shall be excluded. If the last day of that period is not a Business Day, the period in question shall end on the next Business Day; and
(i) reference to any statute or regulation includes any modification, amendment or re-enactment that statute or regulation.
2. Grant of rights / license
2.1 The Licensor, subject to the terms of this Agreement, grants to the Licensee the exclusive, non-assignable (except in conjunction with an assignment of this Agreement under Section 15) right and license to use the Licensor IPR Package:
(a) to Commercialize the Product in the Territory;
(b) to Manufacture in any country the quantities of Product required for Commercialization in the Territory; and
(c) to Develop the Product for Commercialization in the Territory.
2.2 Subject to Section 15.2 and Section 2.3, the Licensee shall have the right to sublicense its rights under the License to an Affiliate or Third Party Sub-licensees without the consent of the Licensor, except in the case of a Third Party where in one transaction or a series of related transactions Licensee sublicenses substantially all of its rights under the License to such Third Party.
2.3 The Parties agree that:
13
(a) prior to the grant of a sub-license pursuant to Section 2.2, the Licensee shall serve written notice on the Licensor and such notice shall contain the following information:
(i) the identity of the proposed Sub-licensee; and
(ii) the reasons why the Licensee believes such Sub-licensee will be able to perform its obligation;
(b) where the proposed sub-license is to a Third Party where in one transaction or a series of related transactions Licensee seeks to sub-license substantially all of its rights under the License to such Third Party and the Licensor has a veto right under Section 2.2 above, then the notice to be provided to the Licensor pursuant to Section 2.3(a) must be provided at least six (6) weeks prior to entry into the proposed sub-license with such Third Party; and
(c) the Licensee (in its capacity as sub-licensor) shall remain responsible for all of its obligations hereunder and if the acts or omissions of any such Sub-licensee cause the Licensee to be in breach of this Agreement the Licensee shall be responsible therefor (with all the express consequences provided for under this Agreement and any implied consequences) regardless of any remedy which the Licensee may have against the Sub-licensee. In particular but without limitation, the Licensee shall perform its financial obligations under this Agreement regardless of a breach by any Sub-Licensee.
2.4 During such time as either Party owes Royalties to the other Party under this Agreement, each Party shall not, and shall procure that its Affiliates and Sub-licensees shall not, directly or indirectly, Develop, Manufacture or Commercialize in the Territory any product for assessing GHD in adults or Children other than the Product.
2.5 During the Term the Licensee, its Affiliates and Sub-Licensees shall refrain from:
(a) seeking or accepting any actual or potential orders for the Product that are known or are reasonably suspected to be for use outside the Territory and the Licensee shall refer to the Licensor all inquiries that the Licensee receives for the Product for sale or ultimate delivery outside the Territory; or
(b) establishing any branch or maintaining any distribution depot for the Product outside the Territory.
2.6 During the Term:
(a) the Licensor, its Affiliates and licensees (other than Licensee) shall refrain from seeking or accepting any actual or potential orders for the Product that are known or are reasonably suspected to be for use inside the Territory; and
(b) the Licensor shall refer to the Licensee all inquiries that the Licensor receives for the Product for sale or ultimate delivery inside the Territory.
2.7 For clarity, nothing in this Section 2 shall limit or restrict the Licensor, its Affiliates, or any Third Party licensee of the Licensor, from using the Licensor IPR Package in connection with the development or Commercialization of the Product outside of the Territory or in connection with the Manufacture of the Product outside of the Territory or inside the Territory for development or Commercialization outside the Territory.
14
3. Governance
3.1 With effect from the Effective Date the Parties shall establish and run a Joint Steering Committee (“JSC”).
3.2 The JSC shall be organized as follows:
(a) the JSC shall comprise four (4) persons (“JSC Members”) and the Licensor and the Licensee respectively shall be entitled to appoint two (2) JSC Members, including one person whose primary responsibility shall relate to Development, to remove any JSC Member appointed by it and to appoint any person to fill a vacancy arising from the removal or retirement of such JSC Member appointed by it. JSC Members must be appropriate for the primary function of the JSC in terms of their seniority, availability, function in their respective organization, training and experience. There will be a chairperson (“JSC Chairperson”) who will be one of the Licensee JSC Members;
(b) the Licensee and the Licensor respectively shall each notify the other of any change in the identities of their JSC Members. Both sides shall use reasonable efforts to keep an appropriate level of continuity in representation. JSC Members may be represented at any meeting by another person designated in writing by the absent JSC Member;
(c) JSC shall hold meetings in person as frequently as the members of the JSC may agree shall be necessary, and otherwise by teleconference or a video-conference, but no less frequently than four (4) times each Calendar Year in total. Dates of meetings shall be agreed by the Parties not less than thirty (30) days beforehand. Each Party shall submit the agenda items and associated materials that it wishes to be considered no later than seven (7) days prior to the meeting and it is agreed that Licensor JSC Members shall only be entitled to submit agenda items for inclusion which relate to matters that have or could potentially have a material impact on the Product, payments to be made pursuant to this Agreement or the Licensor more generally. The first meeting of the JSC will take place as soon as reasonably practicable after the Effective Date, but in no event later than ninety (90) days after the Effective Date, at such location as the members of the JSC may agree or, failing such agreement, Dublin, Ireland. Special meetings of the JSC may be called by any JSC Member upon reasonable written notice to the then current chairman of the JSC not more than twice in any Calendar Year absent a material matter requiring JSC attention that cannot reasonably be delayed until the next scheduled JSC meeting;
(d) if not held by teleconference or videoconference, the venue for meetings of the JSC shall be held at such location as the members of the JSC may agree or, failing such agreement, Dublin, Ireland. Each Party shall be responsible for its own expenses including travel and accommodation costs incurred in connection with JSC meetings; and
(e) the JSC Chairperson or its designee shall be responsible for preparing the minutes of any JSC meeting as soon as reasonably practicable thereafter, seeking unanimous approval of those minutes from the JSC Members, signing and dating the approved minutes and distributing a copy of the signed minutes to each Party.
3.3 The JSC shall have the following purposes:
(a) as regards Commercialization:
(i) to: (A) provide the Licensor with visibility into the Commercialization of the Product in the Territory by the Licensee via reports prepared in the ordinary course for the Licensee's internal purposes, provided that such internal reports
15
at a minimum provide the Licensor with the information set out in Section 7.2 at least quarterly, failing which the Licensee shall deliver such information in accordance with Section 7.2 irrespective of whether it is prepared in the ordinary course or not; (B) procure that the Licensor has reasonable access to Commercial Information of the Licensee relating solely to the Product; (C) ensure that the Licensor is informed regarding acceptance of the Product and Product quality complaints; and (D) provide a means for the Licensor to provide input for any activities relating to Commercialization; and
(ii) (to the extent materially relevant to the sale of the Product in the Territory, to provide the Licensee with visibility into the Commercialization of the Product outside the Territory by the Licensor, to include reasonable information in relation to overall sales volume, market messaging, branding, product positioning strategies, and the information to be provided to the Licensee as specified in Section 7,
and otherwise to act as a forum for general liaison and communication between the Parties in relation to Commercialization of the Product by the Licensee. It is agreed that neither Party shall be required to provide copies of term sheets or commercial agreements relating to grants to Third Parties of rights related to the Product, by Licensee in the Territory, or by the Licensor outside the Territory;
(b) provide each Party with visibility into the other Party's Development activities with respect to the Product other than in relation to the PIP, and to provide a forum for discussion of such Development activities;
(c) oversee and guide the Licensor's activities in carrying out the PIP, to consider reports from the Licensor in respect of the PIP, to provide a means for the Licensee to provide input and, where so provided hereunder, approval for any activities relating to the PIP and for general liaison and communication between the Parties in relation to the PIP;
(d) review and approve with respect to the PIP (including any amendments to any of the following):
(i) the investigational medicinal Product Dossier;
(ii) locations of Clinical Trial sites;
(iii) the identity of principal investigators for the Clinical Trial sites;
(iv) the investigator brochure;
(v) informed consent documentation;
(vi) proposed amendments by the Parties to the PIP Budget (it being noted that pursuant to Sections 3.5 and 5.5, the JSC may not increase the PIP Budget without the written consent of both Parties);
(vii) where a contract is to be entered into with a CRO in relation to the Product after the Effective Date, the identity of and the proposed contract with such CRO;
(viii) the identity and contracts with major vendors (e.g. site monitors); and
(ix) protocols prepared by the Licensor for the PIP;
(e) coordinate with respect to:
16
(i) the finalization of the each Party’s supply chain for the Product for its respective Territory;
(ii) the transition of responsibility for the Manufacture of the Product for the Territory from the Licensor to the Licensee; and
(iii) the general improvement of the supply chain of the Product, both inside and outside the Territory,
and for general liaison and communication between the Parties in relation to the Manufacture of the Product; and
(f) shall perform such other functions and responsibilities as are given to it under the express provisions of this Agreement but shall have no authority to amend any commercial terms of this Agreement or any matter that would cause any payments stated in this Agreement to be other than the amount of those terms as stated herein.
3.4 Each Party shall give reasonable consideration to input provided by the other Party to the JSC on all matters set forth in Section 3.3, provided however that the JSC shall have authority to make decisions solely with respect to the PIP. Such decisions shall be made by unanimous agreement of the JSC Members wherever possible with the Licensee JSC Members together having one vote on behalf of the Licensee and the Licensor JSC Members together having one vote on behalf of the Licensor. Both Parties will use their reasonable efforts to build consensus. All decisions of the JSC shall be minuted by or on behalf of the JSC Chairperson who shall seek unanimous approval of those minutes from the JSC Members, sign and date the approved minutes and promptly send a copy of the minutes of each JSC meeting to both Parties. The JSC shall exercise this authority in good faith and in accordance with the terms of this Agreement, and any decision by the JSC on such matters made in accordance with this Section 3.4 shall be binding upon the Parties. Subject to Section 3.5 below:
(a) in the event that agreement on a PIP-related decision cannot be reached within thirty (30) days, the matter shall be referred for resolution by negotiation between a representative appointed by Licensor, and for Licensee, the President and CEO of Strongbridge Biopharmaceuticals plc or his/her designee, and if the executives cannot reach agreement on the issues under consideration within ten (10) Business Days, then the matter shall be referred to a Third-Party pharmaceutical executive mutually and reasonably agreeable to the Parties and having no less than ten (10) years’ experience directing biopharmaceutical product development programs as set forth in Section 3.4(b);
(b) Expedited Expert Referral.
(i) the Parties shall agree to the identify of such Third-Party pharmaceutical executive as promptly as practicable and in no event later than ten (10) days following the expiration of the ten (10) day period set forth in Section 3.4(a);
(ii) not later than ten (10) days following the expiration of the ten (10) day period set forth in Section 3.4(b)(i), each Party shall submit to the Third-Party pharmaceutical executive (with a copy to the other Party) a position paper setting forth the desired decision on the applicable matter and the rationale for such desired decision, including any supporting documentation provided to the JSC in relation to the matter under consideration thereto, such position paper not to exceed 5000 words (not including supporting documentation);
(iii) the Parties shall request the Third-Party pharmaceutical executive to render, not later than ten (10) days following the expiration of the ten (10) day period
17
set forth in Section 3.4(b)(ii) (or such other time to which the Parties and such executive may all agree), a decision in good faith and based solely on the arguments set forth in such position papers (and for the avoidance of doubt, neither Party shall be permitted to contact the Third-Party pharmaceutical executive to provide additional information or arguments), and such decision shall be restricted to the one of the desired outcomes set forth in the respective position papers of the Parties, and shall render such decision without providing more than the identity of the Party whose desired outcome constitutes such decision;
(iv) such decision by the Third-Party pharmaceutical executive shall be final and binding on the Parties; and
(v) the Party whose desired outcome is not adopted by the Third-Party pharmaceutical executive shall be responsible for payment of the fee owed to the Third-Party pharmaceutical executive .
3.5 The Parties agree that in no event shall the JSC or any dispute resolution process have the authority to increase the PIP Budget without the express written consent of both Parties in accordance with Section 5.5.
4. Technology transfer and technical / regulatory assistance1
The Licensor shall:
(a) maintain the Data Room until the first anniversary of the Effective Date (the “Closure Date”) and shall give nominated representatives of the Licensee access to such Data Room;
(b) as soon as reasonably practicable following the Closure Date, the Licensor shall provide to the Licensee two identical CD-ROM discs on which are contained copies of all Documents contained, on the Closure Date, within the Data Room;
(c) in accordance with the timing set forth in Schedule 2, to the extent not already in the Data Room, make available to the Licensee the Know How Controlled by it, the Dossier filed in respect of the NDA and the Commercial Information Controlled by it and details about the Licensor Trademarks and Licensor Patent Rights, including those items set forth on Schedule 2. Licensor shall provide those materials as soon as reasonably practicable after the Effective Date, and in no event later than any time specified on Schedule 2 for any particular item set out thereon; and
(d) immediately following execution of this Agreement, and before transfer of the Product Registrations (NDA, IND, ODD), provide the Licensee with a CD-ROM disc or discs containing all FDA correspondence related to these applications and the complete electronic copy of the NDA, IND and ODD in the exact format and active file links as submitted to the FDA in respect of the NDA and IND and as pdf files in respect of the ODD. This should include the FDA submission acknowledgements if filed electronically.
(e) provide not later than six months after the Effective Date the transfer of a copy of the electronic Trial Master File(s) for the NDA supporting studies.
1 Note to draft: Parties to discuss and determine how to handle the preservation of and access of Licensee to any clinical samples held by Licensor.
18
5. Development
5.1 In each Calendar Year that Development regarding the PIP is occurring, each Party shall at its own cost and expense (subject to Section 5.4) use Commercially Reasonable Efforts to carry out the activities specified in the Development Plan in relation to the PIP as the responsibility of such Party so as to meet the timelines set out in them.
5.2 During the period of Development regarding the PIP by the Licensor, it shall update the Development Plan on or before November 1st in each year and shall submit the same to the JSC for review and approval.
5.3 The Licensor shall:
(a) be responsible for the conduct of the PIP, subject to and in accordance with the timelines set out in the Development Plan and subject to the direction of the JSC;
(b) conduct all activities in relation to the PIP in compliance with all Legal Requirements, ethics committee, informed consent or similar approvals in relation thereto, and in compliance with GCP;
(c) provide periodic updates regarding the PIP to the JSC as reasonably requested by the JSC;
(d) permit Licensee (at its own cost) and upon reasonable notice to audit, during normal business hours, the trial master file and shall cooperate with any such audit; and
(e) promptly provide to the JSC the results of the PIP in the form of a copy of any Interim Report, a Formal Presentation and a copy of the Final Report as these occur, even if between JSC scheduled meetings.
5.4 The Licensee shall reimburse the Licensor for seventy per cent (70%) of the ongoing documented Development Costs reasonably incurred by the Licensor in accordance with the Development Plan and the PIP Budget. The Licensee shall reimburse its proportion of the Development Costs not later than sixty (60) days following receipt of the Licensor's invoices and supporting evidence of such Development Costs having been incurred in connection with the conduct of the PIP, which the Licensor shall submit as and when such Development Costs arise but no more frequently than on a quarterly basis, save where an invoice is for an amount equal to or greater than $100,000 whereupon the Licensor shall be permitted to invoice on a monthly basis. Payment shall be made by the Licensee within thirty (30) Business Days of the date of receipt of the Licensor's invoice, into such bank account as the Licensor shall specify from time to time. For the avoidance of doubt, the Licensor shall be responsible for the remaining thirty per cent (30%) of such Development Costs in connection with the conduct of the PIP.
5.5 During the period in which the PIP is ongoing, the Parties may, from time to time, together agree in writing to update the PIP Budget and where this occurs the proposed changes will be submitted to the JSC for review and discussion. Once agreed by the Parties in writing, the revised PIP Budget shall be the 'PIP Budget' for the purposes of this Agreement.
5.6 Upon a Bankruptcy Event of Licensor, Licensee shall have the right, but not the obligation, to assume full control of the PIP upon written notice to Licensor. Following any such notice, Licensor shall, to the extent permitted by Law, use its reasonable endeavors to promptly assign to Licensee or its designee all contracts associated with the PIP and shall cooperate
19
with Licensee to ensure a smooth transition of control of the PIP to Licensee, including by executing such documents requested by Licensee as are necessary to enable such transition. Licensee shall thereafter carry out the PIP as set forth in the Development Plan on behalf of itself with respect to the Territory and on behalf of Licensor outside the Territory. Licensee shall pay Licensor's thirty percent (30%) share of the cost and expenses of the PIP as set forth in the PIP Budget and shall be entitled to deduct all such costs and expenses from any amounts of royalties or milestones owed to Licensor hereunder prior to making any payments to Licensor.
Development other than the PIP
5.7 Except as expressly set forth above in relation to the PIP and subject to Section 5.7 below, each Party shall:
(a) be responsible for the conduct and all associated costs and expenses of the Development activities for the Product in its own territory and for the avoidance of doubt 'territory' shall mean the USA and Canada for the Licensee and the rest of the world for the Licensor;
(b) conduct all such Development activities in compliance with all Legal Requirements, ethics committee, informed consent or similar approvals in relation thereto, and in compliance with GCP;
(c) provide the JSC with a summary and update in reasonable detail of such Development activities at each meeting of the JSC; and
(d) provide the other Party with a copy of a near-final draft of each Clinical Trial protocol or update to a protocol for the Product at least forty-five (45) days prior to the date on which such protocols or updates are provided to any Regulatory Authority or Clinical Trial site, in order to permit such other Party to comment on such protocol or update, and shall reasonably consider in good faith any comments thereon provided by such other Party within thirty (30) days following such other Party's receipt of such copy of such protocol or update.
5.8 The Parties agree that where any Development activity of the Licensee is, in the Licensor's reasonable opinion, going to have a material adverse impact on the Development, Manufacture or Commercialization of the Product outside of the Territory for the Existing Indications, then the prior written consent of the of the Licensor will be required, such consent not to be unreasonably withheld, delayed or conditioned.
5.9 If Licensee wishes to Develop Product in the Territory for a New Indication, Licensee shall have the right to do so on the following conditions:
(a) the Licensee shall be solely responsible for the costs and expenses of the Development activities;
(b) the Licensee shall supply the Licensor with all material Know How, Dossiers and other Licensee IPR Package relating thereto, including a copy of each Final Report promptly following its preparation;
(c) subject to paragraph 5.9(d), the Licensee hereby grants to the Licensor a perpetual, irrevocable, exclusive, sub-licensable, right and license to use the Licensee IPR Package for the Product for such New Indication to Develop, manufacture and Commercialize the Product outside the Territory; and
(d) Licensor shall pay royalties to Licensee under such license on the same payment terms as, and at a rate equal to two percent (2%) less than the rate owed by, Licensee
20
to Licensor for sales of Products hereunder, with all applicable definitions and provisions having the Parties reversed, mutatis mutandis.
Regulatory Approvals
5.10 Assignment of the NDA and Domain Names.
(a) Subject to the terms and conditions set out in this Agreement, Licensor hereby sells, conveys, and transfers to Licensee, and Licensee hereby purchases and accepts the sale, conveyance and transfer of all of Licensor's right, title and interest in the Product Registrations (including the NDA and the IND and orphan designation with respect thereto, and all copyrights, data and information contained therein), and the domain names set forth on Schedule 6, in the Territory on the Effective Date, subject to Licensor’s express right to return of the Product Registrations and domain names upon certain terminations of the Agreement as set forth in Section 14.
(b) Promptly after the Effective Date, each Party shall file with the FDA letters and applications in compliance with 21 CFR § 314.72 in the forms set forth on Schedule 3 in order to record with the FDA the change in ownership of the NDA from Licensor to Licensee.
(c) Promptly following the first Marketing approval for a Product outside the Territory, to the extent permitted by applicable Law, Licensee shall ensure that any internet users from outside the Territory who access the domain names listed on Schedule 6 (or any variants of these that are now or in the future become owned by the Licensee or an Affiliate or agent of the Licensee), are redirected to Licensor’s website or another website designated by Licensor covering the Licensor Product outside the Territory, provided that Licensor shall provide a link on such website with reasonably prominent instructions so that visitors to its site from inside the Territory or otherwise with an interest in the Product in the Territory are directed to jump to Licensor’s website or another website designated by Licensor covering the Licensor Product inside the Territory.
5.11 The Licensee shall:
(a) subject to Section 5.11(b) below, at its sole cost and expense (subject to Section 5.3 with respect to costs of the PIP), use Commercially Reasonable Efforts to obtain any further Regulatory Approvals for the Product and any Pricing Approval required or commercially desirable in each country of the Territory, and such Regulatory Approvals shall, in all instances, be obtained by the Licensee in its own name and the Licensor shall provide its reasonable assistance to the Licensee to enable the Licensee to obtain the Regulatory Approvals in the Licensee's name; and
(b) use Commercially Reasonable Efforts following the Effective Date to obtain Regulatory Approval for the Product in Canada for the Adult Indication.
5.12 The Licensee shall:
(a) conduct all Development activities (other than the PIP which is the responsibility of the Licensor) in the Territory in compliance with all Legal Requirements;
(b) be responsible for the preparation of all submissions for the grant of a Regulatory Approval in the Territory; and
(c) as soon as reasonably practicable following its receipt of the final study report for the last necessary clinical trial for the PIP, prepare and make all submissions for the grant
21
of Regulatory Approval in each country in the Territory for the Product for the Pediatric Indication using Commercially Reasonable Efforts.
5.13 The Licensee shall be responsible for all filings and submissions in respect of Regulatory Approvals for the Product in the Territory as well as attending all meetings with Government Authorities and Regulatory Authorities in respect thereof and:
(a) shall provide, at the request of the Licensor, copies of all material proposed filings, in advance of the filing or submission thereof, for review and comment by the Licensor. The Licensee shall consider in good faith in its revision of the filing or submission, any comments made by Licensor; and
(b) shall inform the Licensor in advance of all meetings or conference calls with the Regulatory Authorities with respect to Products including on Pricing Approval issues and one Licensor representatives shall have the right to attend solely as a non-participating observer in the meeting, to the extent permitted by such Regulatory Authorities.
5.14 The Licensee shall, from the Effective Date, use Commercially Reasonable Efforts to maintain the orphan status of the NDA Product.
6. Supply
6.1 An illustration of the Licensor's proposed supply chain for the Product for the USA is set out in Schedule 8 of this Agreement (the “Supply Chain”). Certain of the CMO Contracts that comprise the Supply Chain have been entered into by the Licensor or its Affiliates as of the Effective Date (the “Existing CMO Contracts”) while others have not been concluded as at the Effective Date (the “Outstanding CMO Contracts”). The Licensee shall use Commercially Reasonable Efforts to procure a full supply chain of the Product for the USA to enable the launch of the Product as soon as reasonably practicable after the Effective Date and the Licensee shall provide such reasonable assistance as the Licensor may reasonably request, subject to the provisions of Section 6.3(f).
6.2 The Parties agree that the Licensor shall not be required to assign, transfer or novate any of the Existing CMO Contracts to the Licensee but the Parties shall have a joint option (meaning, for the avoidance of doubt, that both Parties must consent to the assignment, transfer or novation) to do so, which is jointly exercisable at any time within 90 days of the Effective Date. The Licensee shall:
(a) in respect of the Outstanding CMO Contracts, be entitled to enter into negotiations with the relevant CMOs in respect of such contracts, in place of the Licensor, and conclude such contracts in its own name or that of an Affiliate; and
(b) in respect of the Existing CMO Contracts, negotiate in good faith with the Licensor's CMOs or with alternative Third Party manufacturers with the intention of concluding its own direct terms with such CMOs or Third Party manufacturers,
and the contracts which the Licensee enters into pursuant to Sections 6.2(a) and (b) are together the “Licensee CMO Contracts”.
6.3 The Parties agree that:
(a) the Licensor shall provide to the Licensee such reasonable assistance as the Licensee shall reasonably request to help the Licensee to enter into the Licensee CMO Contracts;
22
(b) they shall work together in good faith following the Effective Date to ensure that, where they both use common CMOs in their respective supply chains, that they leverage volume discounts from such common CMOs when ordering Materials;
(c) each Party shall use reasonable efforts to ensure that they do not enter into any contract with a shared contracting party that disadvantages the other Party in such other Party’s dealing with such contracting party;
(d) each Party’s contracts with shared suppliers shall provide that in the event of a shortage or other restriction on the supply of a commonly purchased material from such suppliers, the supplier, as between the Parties, shall allocate available supply pro rata to the Parties based upon each Parties forecasts for such material in place prior to any knowledge of a potential or actual shortage thereof;
(e) if the Licensor and Licensee together choose to exercise the joint option (meaning, for the avoidance of doubt, that both Parties must consent to exercise of the option) referred to in Section 6.2 then the Parties shall, through the JSC, work together to assign, transfer or novate the Existing CMO Contracts to the Licensee whereupon such Existing CMO Contracts shall become Licensee CMO Contracts; and
(f) the Licensee shall reimburse the Licensor for all reasonable costs and expenses (including employee costs which shall be charged on an hourly basis) associated with assistance requested by Licensee from and provided by the Licensor (or its Affiliates or any of their respective employees or consultants) pursuant to this Section 6.
Interim Licensor Supply Arrangements
6.4 The Parties will co-operate and work in good faith during the transition period following the Effective Date, as regards supply arrangements. More particularly, and subject to Sections 6.5 and 6.6 below, insofar as the Licensee requires Materials for the Manufacture of the Product under any Existing CMO Contract then, for the period from the Effective Date until the date upon which the Licensee enters into the relevant Licensee CMO Contract (“Licensor Supply Period”):
(a) the Licensor shall procure that the relevant CMO supplies Materials to the Licensee or one of its Affiliates or CMOs, subject to the terms and conditions of such Existing CMO Contract, for the purposes of Manufacture by the Licensee or one of its Affiliates or CMOs of the Product for the Territory;
(b) the Licensor shall procure that the Licensor Materials set forth on Schedule 4 to be delivered to the Licensor, are instead delivered to such location as the Licensee notifies to the Licensor in writing prior to that date, and the Licensee shall within 10 Business Days of receipt of any such Licensor Material, pay to the Licensor an amount equal to the amount set forth on Schedule 4 for the corresponding Licensor Material so received by Licensee;
(c) the Licensor shall not, during the Licensor Supply Period, terminate the relevant Existing CMO Contract or amend it in a manner which would be adverse to the interests of the Licensee; and
(d) the Licensor shall, at Licensee’s cost and expense, take such actions in respect of such Existing CMO Contract as the Licensee may reasonably request, including where a CMO is in breach of the terms of an Existing CMO Contract, to enforce its rights against such CMO for and on behalf of the Licensee.
6.5 The Parties agree that:
23
(a) the liability of the Licensor to the Licensee pursuant to Section 6.4(a), shall be limited, in all instances other than a liability arising from the negligence or willful misconduct of the Licensor in connection with the performance of its obligations under Section 6.4(a), to the amount actually recovered by the Licensee from the relevant CMO;
(b) Licensor, promptly upon receipt thereof, shall send by electronic means copies of all invoices in respect of Materials supplied by the Licensor pursuant to Section 6.4(a) of this Agreement, which invoices Licensee shall pay on behalf of Licensor in accordance with the payment terms thereof, provided that if Licensee fails to make any such payment of a timely sent invoice copy for amounts not in dispute by Licensee (including invoices or portions of invoices previously but no longer in dispute), Licensor shall be entitled to pay such invoice (including any penalty or interest owed as a result of late payment thereof ) and to invoice Licensee for such payment, which Licensee shall pay to Licensor promptly. Licensee shall indemnify the Licensor and its Affiliates in respect of any claim made by a CMO against the Licensor as a result of the Licensee's failure to make any payments due to a CMO (including any disputed amounts that the Licensee incorrectly disputes); and
(c) the Licensor shall not be required to comply with its obligations pursuant to Section 6.4 to the extent that this would breach any applicable Laws and/or which would put it in breach of its obligations under any Existing CMO Contract.
6.6 The Parties agree that:
(a) the Licensee shall use Commercially Reasonable Efforts to take full responsibility for and management of supply of Products in the Territory as soon as reasonably practicable following the Effective Date; and
(b) to the extent that the Licensee does not take responsibility for and management of supply of Products by the date which is twelve (12) months from the Effective Date, then:
(i) the Parties shall enter into a supply agreement to govern such supply arrangements between the Parties (the "Supply Agreement"); and
(ii) subject to the terms of the Supply Agreement, the Licensor shall have no further obligations under Sections 6.4 - 6.9 of this Agreement (which relate to the interim supply arrangements); and
(c) with effect from the end of the Licensor Supply Period (or, if earlier, the date which is 9 months from the Effective Date), the Licensee shall either itself or through the Supply Agreement be responsible for supply, and shall manage the Manufacturing of all Materials and Product for sale in the Territory.
For the avoidance of doubt, the Licensor shall be responsible for and shall manage the Manufacturing of all Materials and Product for sale in countries outside of the Territories.
6.7 During the Licensor Supply Period, the Licensor will provide letters of access allowing access for the Licensee to the extent possible to the production sites of its CMOs, in each case where Materials have been manufactured for the Licensee pursuant to Section 6.4, in order to enable the Licensee to visit such sites and to review the Manufacturing and any documentation relating to the Materials or its Manufacturing maintained at such site to the extent necessary or required by applicable Law or the requirements of any Regulatory Authority or to prepare for any investigation or inspection that may be carried out by any Regulatory Authority.
24
6.8 The Parties shall cooperate and discuss in good faith with a view to agreeing a plan (including timeline and a detailed description of all documentation containing Manufacturing Know-How that will need to be provided to the Licensee) for the successful transfer of the Manufacturing Know-How Controlled by the Licensor to the Licensee during the Licensor Supply Period and on an ongoing basis thereafter in order to assist the Licensee in Manufacturing or having Manufactured the Product. Licensor agrees to make a technical transfer of any Manufacturing Know-How existing as of the Effective Date or created at any point during the Term which it Controls to the Licensee in accordance with the timelines agreed in any technical transfer plan (with respect to Manufacturing Know-How in existence as of the Effective Date and Controlled by Licensor) and within such time as is reasonably practicable after creation of such Manufacturing Know-How or upon the Licensor becoming aware of the existence of such Manufacturing Know-How (with respect to Manufacturing Know-How created or discovered during the Term). The Licensee agrees to make a technical transfer of any Licensee Manufacturing Know-How which it Controls created at any point during the Term to the Licensor within such time as is reasonably practicable after creation of such Licensee Manufacturing Know-How or upon the Licensee becoming aware of the existence of such Licensee Manufacturing Know-How. Except as set forth in Section 6.9, the costs of the transfer of Manufacturing Know-How shall be shared equally by the Parties.
6.9 For any change in a Manufacturing process or Material used for the Product (including the Product, itself) that are made as a result of a requirement of at least one Regulatory Authority in the Territory and at least one Regulatory Authority outside the Territory, the parties agree to split all costs and expenses related to developing and implementing such change (but not the cost of any Material used in the Product) with Licensee bearing seventy percent (70%) of such costs and expenses and Licensor bearing thirty percent (30%) of such costs and expenses
Licensee Supply of Materials
6.10 Where the Licensor seeks to obtain Product or Materials for the Manufacture of the Product for outside the Territories from the Licensee, then the Parties shall negotiate in good faith to enter into a supply agreement for such Product and/or Material on commercially reasonable terms.
PIP
6.11 Notwithstanding any other provision of this agreement, where the PIP is fully or partially conducted in the Territory, the Licensor shall be entitled to supply to the relevant Third Parties assisting with the PIP such amount of CTM as is necessary for the PIP.
7. Commercialization.
7.1 Licensee shall either itself or through its Affiliates, and Sub-licensees Commercialize the Product in the Territory using Commercially Reasonable Efforts and 'Commercially Reasonable Efforts' for these purposes shall include, but not be limited to the Licensee:
(a) as soon as reasonably practicable following the Effective Date hiring, at a minimum, 15 field specialists whose role shall solely relate to the Product and maintaining such minimum number of field specialists for at least the 12 month period following the first Launch; and
(b) ensuring, that notwithstanding the resources required by the Licensee for the preparation for and the launch of another product by the Licensee (or its Affiliates), it continues to provide an appropriate level of resources dedicated to the Commercialization of the Product.
25
7.2 The Licensee shall provide Licensor through the JSC with reports and other commercialization planning documents for the Product as are generated in the ordinary course for the Licensee's internal purposes. To the extent that such ordinary course reports and planning documents do not provide the Licensor with the following information on a quarterly basis, the Licensee shall be required to provide such information to the Licensor upon a quarterly basis: current sales forecasts and actual volume of sales of the Product in the Territory, the number of specialist sales staff dedicated wholly or partially to the Commercialization of the Product, any material decrease in either from time to time and the reasons therefor and the nature and scale of promotional activities in the Territory.
Pricing
7.3 Licensee will use Commercially Reasonable Efforts to secure required Pricing Approval for the Product in the Territory for each Existing Indication. The Licensee will have sole authority for determining and establishing the price and terms of sale (including any rebates or discounts) of Product in the Territory. Each Party shall provide the other Party with copies of any material Documents or other material pertaining to Pricing Approvals in such providing Party’s respective territory.
7.4 The Licensee or its Affiliates or Sub-licensees will be responsible for the creation, preparation, production and reproduction of all Promotional Materials and for filing, as required by Legal Requirements, all Promotional Materials with Regulatory Authorities in the Territory. Subject to Section 9, the Licensee shall use its own corporate name and/or logo or those of an Affiliate on Promotional Materials in connection with Commercialization of Product in the Territory, unless otherwise mutually agreed by the Parties.
Promotional claims/compliance
7.5 The Licensee shall not promote the Product for intended uses or indications other than those in the labelling as approved by the Regulatory Authority in the Territory from time to time nor shall the Licensee make any medical or promotional claims for any Product other than as permitted by Legal Requirements (which for the avoidance of doubt shall include the False Claims Act). When distributing information related to any Product or its use for the Existing Indications in the Territory (including information contained in scientific articles, reference publications and publicly available healthcare economic information), the Licensee must comply with all Legal Requirements (which for the avoidance of doubt shall include the False Claims Act) in the applicable country.
International Congresses / Symposia
7.6 For international congresses or symposia:
(a) where Licensee wishes to have a presence in a symposium or congress outside the Territory regarding the Product, Licensee shall notify Licensor in advance of Licensee's proposed presence, providing reasonable detail regarding such planned presence and seeking Licensor’s consent (such consent not to be unreasonably withheld, delated or conditioned) for such presence. Licensor, as soon as reasonably practicable, but in no event later than ten (10) days after receiving such notice, shall notify Licensee of Licensor either withholding consent (with the reasons therefor) or providing consent to such presence;
26
(b) where Licensor wishes to have a presence in a symposium or congress inside the Territory regarding the Product, Licensor shall notify Licensee in advance of Licensor's proposed presence, providing reasonable detail regarding such planned presence and seeking Licensee’s consent (such consent not to be unreasonably withheld, delated or conditioned) for such presence. Licensee, as soon as reasonably practicable, but in no event later than ten (10) days after receiving such notice, shall notify Licensor of Licensee either withholding consent (with the reasons therefor) or providing consent to such presence; and
(c) The Parties shall, to the extent reasonable and practicable, coordinate attendance at such conference or symposium (including displaying brand names, trademarks and logos for the Product, to the extent permitted by Law).
Product Complaints
7.7 The Licensee will be responsible for responding to product complaints regarding Product in the Territory and the Licensor for responding to product complaints regarding Product outside the Territory. If the Licensor receives complaints about Product for a country in the Territory, it will refer such complaints to the Licensee (and vice versa), and the Licensee will be responsible for responding thereto (and vice versa). Each Party shall, in all instances, keep the other fully appraised of matters in relation to such product complaints including by providing copies of all correspondence with the complainant.
Medical and consumer inquiries
7.8 The Licensee will be responsible for responding to medical questions or inquiries from members of the medical and paramedical professions and consumers regarding Product in the Territory in accordance with Laws. If the Licensor receives questions about Product for a country in the Territory, it will refer such questions to the Licensee, and the Licensee will be responsible for responding thereto. Each Party shall, in all instances, keep the other Party reasonably appraised of matters in relation to any such questions or inquiries that are material and/or frequently asked, including by providing copies of correspondence with the relevant members of the medical or paramedical professions or consumers.
Compliance with Laws
7.9 Each Party will perform its obligations under this Agreement in accordance with all Laws. Each Party shall obtain from the requisite Government Authorities any consents, licenses, permits, waivers, approvals, authorizations, clearances, registrations or orders required to be obtained or made by such Party in connection with the authorization, execution and delivery by such Party of this Agreement and its performance of its obligations under this Agreement including but not limited to the importation, exportation, sale and distribution of the Product.
Regulatory affairs
7.10 Post-Approval Regulatory Submissions.
(a) Subject to Section 7.10(b) below, as between the Parties, Licensee shall have sole responsibility, at its own expense, for preparing, filing and maintaining all Regulatory Submissions for Product in the Territory after the Effective Date, including Regulatory Submissions in connection with Development activities hereunder (collectively, “Post-Approval Regulatory Submissions”). Licensee shall use Commercially Reasonable Efforts to compile, submit and prosecute all Post-Approval Regulatory Submissions, in a format acceptable to the applicable Regulatory Authorities in the Territory. All Post-Approval Regulatory Submissions for Product in the Territory shall be filed in the name of Licensee. Licensee shall be responsible for
27
all communications and other dealings with the Regulatory Authorities relating to the Post-Approval Regulatory Submissions and Licensee shall be the legal and beneficial owner of all Post-Approval Regulatory Submissions.
(b) Prior to submitting a Post-Approval Regulatory Submission regarding a material matter (such as a supplemental change to the NDA), Licensee shall provide a draft (which may be wholly or partly in electronic form) to the Licensor for review and comment.
7.11 Adverse Event Reporting.
(a) Licensor shall be responsible for complying with all Legal Requirements governing adverse events both inside and outside the Territory that occur prior to the transfer of NDA to Licensee, and Licensor's responsibilities shall thereafter continue outside the Territory. Licensor shall submit copies of reports of adverse events to Licensee simultaneously with submission to the applicable Regulatory Authorities, and, following, transfer of the NDA to Licensee, Licensee shall submit copies of adverse events to Licensor simultaneously with submission to the applicable Regulatory Authorities in the Territory. Licensee shall be responsible for complying with all Legal Requirements governing adverse events in the Territory that occur after the transfer of the NDA to Licensee. Each Party shall notify the other in a timely manner and in any event within forty eight (48) hours of receiving any notice from a Regulatory Authority, independent review committee, data safety monitoring board or another similar Clinical Trial or post-marketing monitoring body alleging concern regarding a patient safety issue or other material information relevant to the safety or efficacy of Product.
(b) As will be more fully set forth in the Safety Agreement and agreed to pursuant to Section 7.11(d), Licensor shall be responsible at its own cost for establishing and maintaining a global safety database for the Product based on procedures and guidelines so agreed by the Parties for the operation of such database.
(c) If during the Product’s Development or Commercialization, the Product becomes subject to adverse effects or information of the type referred to in the last sentence of Section 7.11(a) is received, in each case which Licensee, in good faith, reasonably believes would seriously impact the long-term viability of Product in the Territory, Licensee shall determine whether or not there exists such serious impact on the long-term viability of such Product and, what if anything, the Parties should do to address the matter. If Licensee, upon consideration of the relevant facts and in its sole discretion, determines that the Parties are unable to successfully address and resolve the safety issue, Licensee shall provide written notice to Licensor of such determination, which notice shall set forth the reasons therefor, and Licensee may terminate its rights and obligations under this Agreement upon written notice.
(d) As promptly as practicable following the Effective Date, but in no event later than one hundred eighty (180) days thereafter, Licensee and Licensor will develop and agree upon safety data exchange procedures in a separate and detailed safety agreement (the “Safety Agreement”). Such agreement will describe the coordination of collection, investigation, reporting, and exchange of information concerning adverse events or any other safety problem of any significance, and product quality and product complaints involving adverse events, sufficient to permit each Party, its Affiliates, licensees or sublicensees to comply with its legal obligations. The safety data exchange procedures will be promptly updated if required by changes in Legal Requirements. In the event of any conflict or inconsistency between this Agreement
28
and the Safety Agreement with respect to: (i) safety-related matters, the Safety Agreement shall prevail; and (ii) any other matter, this Agreement shall prevail.
7.12 Regulatory Correspondence.
(a) Notification to Other Parties of Regulatory Correspondence. Each Party shall promptly (and in any event, within two (2) Business Days of the date of receipt of notice) notify the other Party in writing of, and shall provide the other Party with copies of, any correspondence and other documentation received or prepared by such Party in connection with any of the following events:
(i) receipt of a material regulatory letter, warning letter, Form 483, or similar item, from any Regulatory Authority directed to the Manufacture, packaging, and/or storage of Product, any Licensee facility or any contract Manufacturing facility associated with Licensee's supply of Product;
(ii) any recall or correction of any batch of Product; and
(iii) any regulatory comments relating to Product requiring a response or action by a Party.
(b) Regulatory Correspondence requiring a Licensor response. In the event that Licensor receives any material regulatory letter or comments from any Regulatory Authority directed to its development or Manufacture of Product requiring a response or action by Licensor, including, but not limited to, receipt of a Form 483 or a warning letter, Licensee (as applicable) will promptly provide Licensor with any data or information in Licensee's possession that is required by Licensor in preparing any response relating to Licensor's development of such Product, and will cooperate with Licensor's reasonable requests related to Licensor preparing such response.
(c) Regulatory Correspondence requiring a Licensee response. In the event that Licensee receives any material regulatory letter or comments from any Regulatory Authority relating to the development or Manufacture of Product, Licensor (as applicable) will promptly provide Licensee with any data or information required by Licensee in preparing any response relating to Licensor's development or Manufacture of Product, and will cooperate fully with Licensee in preparing such response. To the extent reasonably practicable (subject to the time a response is mandated), Licensee shall provide Licensor with a copy of each such response for Licensor's review and comment at least two (2) Business Days prior to Licensee's submission of the response.
7.13 Regulatory updates. During the Term, each Party will keep the other Party generally apprised of the status of any Regulatory Submissions related to Product inside and outside the Territory. Licensee shall immediately notify Licensor in writing upon receipt by Licensee of any Regulatory Approval to market Product in the Territory. Licensor shall immediately notify Licensee in writing upon receipt by Licensor of any Regulatory Approvals to market Product outside the Territory.
8. Financial Provisions
8.1 The Licensee will pay to the Licensor an upfront fee of twenty four million USD (US$24,000,000) within five (5) days of the Effective Date.
29
8.2 The Licensee will pay to the Licensor a milestone payment of five million USD (US$5,000,000) within thirty (30) days of the grant of Regulatory Approval in the USA for the Product for the Pediatric Indication.
8.3 The following one-time payments shall be paid by the Licensee to the Licensor in USD sixty (60) days following first achievement of the following Commercialization milestone events:
(a) cumulative Net Sales of Product in a Calendar Year reaching twenty five million dollars: Commercialization payment of four million dollars (US$4,000,000);
(b) cumulative Net Sales of Product in a Calendar Year reaching fifty million dollars: Commercialization payment of ten million dollars (US$10,000,000);
(c) cumulative Net Sales of Product in a Calendar Year reaching one hundred million dollars: Commercialization payment of twenty million dollars (US$20,000,000);
(d) cumulative Net Sales of Product in a Calendar Year reaching two hundred million dollars: Commercialization payment of forty million dollars (US$40,000,000); and
(e) cumulative Net Sales of Product in a Calendar Year reaching five hundred million dollars: Commercialization payment of one hundred million dollars (US$100,000,000).
8.4 Each of the milestone payments subject to Sections 8.2 and 8.3, shall only be payable by the Licensee upon the first occurrence of the applicable event whenever it occurs. Upon the occurrence of the applicable event the milestone payment shall be payable even if more than one occurs in a Calendar Year. Such milestone payments are non-refundable in any circumstances whatsoever and are not creditable against the royalties due under Section 8.6.
8.5 The Licensee shall report the occurrence of each milestone event under Sections 8.2 and 8.3 to the Licensor within forty-five (45) days of its occurrence and at the same time shall make the milestone payment to the Licensor for which Sections 8.2 and/or 8.3 provides.
Royalties; Taxes
8.6 The Licensee will pay to the Licensor royalties during the Royalty Term as set forth below:
Royalty = A + B where:
A equals fifteen per cent (15%) of that portion of Net Sales of Product in the Territory, which, during the Calendar Year in question, is less than or equal to seventy five million USD (US$75,000,000); and
B equals eighteen per cent (18%) of that portion of Net Sales of Product in the Territory, which, during the Calendar Year in question, is greater than seventy five million USD (US$75,000,000).
If, in the United States, if data exclusivity remains following expiry of the last Valid Claim in a United States patent or patent application, then the royalty rate calculated above shall be reduced to (A +B)/2 (and the remaining royalty payments shall be payable as a royalty on Know-How). In any country in the Territory where, during the Royalty Term, there are no Valid Claims of Licensor Patent Rights or data exclusivity protection for the Product for a particular Existing Indication, the royalty rate shall be reduced to five percent (5%) (and the
30
remaining royalty payments shall be payable as a royalty on Know-How) irrespective of the amount of Net Sales of Product.
8.7 To the extent that a New Indication is Developed and Commercialized by or on behalf of Licensee following the Effective Date then royalties will be payable upon the Net Sales of Product for such New Indication in accordance with Section 8.6. For these purposes:
(a) the time periods referred to in the definitions of 'Term' and 'Royalty Term' shall relate to the New Indication(s) and not the Existing Indication (and related definitions, including that of 'First Commercial Sale Date' shall be read accordingly);
(b) the provisions of Section 8.6 shall otherwise apply mutatis mutandis to Net Sales derived from such New Indication; and
(c) Net Sales of the Product for such New Indication shall be included in the calculation of the 'Commercialization Milestone Payments'.
8.8 For the avoidance of doubt, any royalties or other payments owed by Licensor under any agreement to which Licensor or any Affiliate of Licensor is a party, including the Exploitation Agreement, shall be the sole responsibility of Licensor.
8.9 All royalties due to the Licensor under this Agreement shall be calculated and payable on a Calendar Quarter basis, and shall be paid by the Licensee to the Licensor in USD within sixty (60) days following the end of each of 31 March, 30 June, 30 September and 31 December in each year (“60 day Period” and “Quarter End” respectively). Within sixty (60) days of each Quarter End the Licensee shall send to the Licensor a written report setting out (i) Product by Product and country by country the amount of Net Sales in such country during such quarter expressed in the local currency of that country and a breakdown of whether these relate to the Adult Indication, Pediatric Indication or New Indication; and (ii) the amount of the royalties due to the Licensor in relation to such Quarter. Any sales in a country other than the U.S., for purposes of determining the Net Sales amount shall be calculated using the average rate of exchange of the currency of such country for the applicable calendar month, as published in The Wall Street Journal, New York edition.
8.10 Taxes.
(a) Licensor shall be liable for all income and other Taxes (including interest) imposed upon any payments made by Licensee to Licensor under this Agreement (“Agreement Payments”). If applicable Laws, rules or regulations require the withholding of Taxes, Licensee shall make such withholding payments and shall subtract the amount thereof from the Agreement Payments. Licensee shall submit to Licensor appropriate proof of payment of the withheld Taxes as well as the official receipts within a reasonable period of time. Licensee shall provide Licensor reasonable assistance in order to allow Licensor to obtain the benefit of any present or future treaty against double taxation which may apply to the Agreement Payments.
(b) Licensee shall bear and be responsible for and pay all applicable Taxes related to:
(i) the transfer to Licensee of the Product Registrations and the license rights granted under this Agreement; and
(ii) the Development and Commercialization by Licensee of the Product in the Territory other than for the PIP.
8.11 Each Party and its Affiliates shall use all reasonable efforts to maintain complete and accurate records in sufficient detail to permit the other Party to confirm the accuracy of the amount to be reimbursed with respect to Development Costs, or other amounts to be paid,
31
reimbursed, credited, offset or shared hereunder incurred or generated (as applicable) by such Party's or Affiliate's achievement of sales milestones, royalty payments and other compensation or reimbursement payable under this Agreement. Upon reasonable prior notice, such records shall be open during regular business hours for a period of three (3) years from the creation of individual records for examination at the auditing Party's expense, and not more often than once each Calendar Year, by an independent certified public accountant selected by the auditing Party and reasonably acceptable to the audited Party or Affiliate for the sole purpose of verifying for the auditing Party the accuracy of the financial statements or reports or sales milestone notices furnished by the audited Party or Affiliate pursuant to this Agreement or of any payments made, or required to be made, by or to the audited Party or Affiliate to the other pursuant to this Agreement. Any such auditor shall not disclose the audited Party's or Affiliate's confidential information to the auditing Party, but shall, instead, report that there was or was not a discrepancy uncovered by the audit and if such a discrepancy was uncovered, the amount and direction of it. Any amounts shown to be owed but unpaid, or overpaid and in need of reimbursement, shall be paid or refunded (as the case may be) within thirty (30) days after the accountant's report, plus interest (as set forth in Section 8.13) from the original due date (unless challenged in good faith by the audited Party, in which case any undisputed portion shall be paid in accordance with the foregoing timetable, any dispute with respect to such challenge shall be resolved in accordance with Section 16, any remaining disputed portion shall be paid within thirty (30) days after resolution of the dispute, and interest shall accrue (as set forth in Section 8.13) with respect to the disputed portion from the original due date). The auditing Party shall bear the full cost of such audit unless such audit reveals an overpayment to, or an underpayment by, the audited Party or Affiliate that resulted from a discrepancy in a report that the audited Party or Affiliate provided to the other Party during the applicable audit period, which underpayment or overpayment was more than ten percent (10%) of the amount set forth in such report, in which case the audited Party or Affiliate shall bear the full cost of such audit. Each Party, at the request of the other Party, shall make available to the other Party the results of any audit performed by the non-requesting Party on such non-requesting Party's Sub-licensees hereunder.
8.12 All payments made to either Party under this Agreement shall be made by wire transfer to the account of such Party.
8.13 If a Party fails to make any payment due to the other Party hereunder on the due date for payment and the payment is not in dispute between the Parties, without prejudice to the other right or remedy available to the other Party, the Party owed such Payment shall be entitled to charge the Licensee interest (both before and after judgment) on the amount unpaid at the annual rate of LIBOR plus two per cent (2%) calculated on a daily basis until payment in full is made without prejudice to the Licensor's right to receive payment on the due date.
9. Trademarks
9.1 The Licensee shall have the option to use the Licensor Trademark in relation to the Commercialization of Product in the Territory. The use by the Licensee of the Licensor Trademark shall not constitute or imply any assignment or transfer of the Licensor Trademark or any goodwill associated with it. Any goodwill accrued in connection with the use of the Licensor Trademark shall accrue solely to the benefit of the Licensor. The Licensee shall ensure that each reference to and use of the Licensor Trade Xxxx by the Licensee in Promotional Materials is acceptable to the Licensor and is accompanied by an acknowledgement that the Licensor Trade Xxxx is owned by the Licensor and used by the Licensee under license.
32
9.2 The Licensee shall not challenge the validity of the Licensor Trademark and shall not aid or assist third parties to do so. Whatever use the Licensee makes of the Licensor Trademark shall inure to the sole and exclusive benefit of the Product in accordance with this Agreement.
9.3 Except as required by applicable Law, neither Party shall use the other Party's corporate name, or use any Trademarks of the other Party (other than the Licensor Trademark) in connection with any Promotional Materials or publication without the other Party's prior written consent which shall not be unreasonably withheld. The above restriction will not apply to representations that the Licensee is the exclusive licensee of the Licensor for the Product in the Territory.
9.4 In the case of infringement or misuse of the Licensor Trademark, if the Licensor fails to initiate proceedings within three (3) months of the Licensee's notification thereof, the Licensee may give the Licensor notice requesting the Licensor to take such proceedings within thirty (30) days of the date of this second notice. If the Licensor fails to initiate such proceedings within such period, the Licensee shall be entitled to do so at its own cost and expense in which case it shall have sole conduct of any claim or proceedings. The Licensor shall, and shall procure that its Affiliates shall, reasonably assist and cooperate with the Licensee in any such claim, provided that the Licensee shall reimburse the Licensor for all reasonable out-of-pocket costs and expenses, if any, relating to such assistance and cooperation. Such reasonable assistance and cooperation of the Licensor and its Affiliates shall include but not be limited to the execution of such documents and the performance of such other acts, including being joined as a party to any proceedings, as may be reasonably required to facilitate such claim, including but not limited to such documents and acts that may, upon the Licensee's request, be required for the registration of the Licensee as exclusive licensee of the Licensor Trademark in the Territory at the trademark office in the relevant countries of the Territory. The Licensee shall have sole right to settle such proceedings provided such settlement does not directly or indirectly adversely affect the Licensor's rights and interests outside of the Territory, and shall be entitled to retain any financial payment awarded in such proceedings or agreed in any such settlement for its own account.
10. Intellectual Property (except Trademarks)
10.1 For the purposes of this Section 10, the “Licensor IPR Package” shall exclude the Licensor Trademark and the “Licensee IPR Package” shall exclude Trademarks.
10.2 Except as expressly set forth in this Agreement, nothing in this Agreement transfers ownership of the Licensor IPR Package to the Licensee.
10.3 The Licensee shall own all the Licensee IPR Package. The Licensee shall use Commercially Reasonable Efforts to procure that, under the terms of any appointment of a Sub-licensee, all Materials, Know How and Commercial Information generated or developed by or upon their behalf shall be Controlled by the Licensee.
10.4 The Licensee grants to the Licensor a perpetual, exclusive, sub-licensable, fully paid-up royalty free right and license to use the Licensee IPR Package outside the Territory for Development, Manufacture and Commercialization of the Product outside the Territory other than for a New Indication, which license rights are covered solely by the license set forth in Section 5.9.
10.5 The Licensor shall be solely responsible at its own cost and expense for the filing, prosecution, maintenance and/or defense of the Licensor Patent Rights in the Territory using reasonable efforts to prosecute all patent applications forming part of the Licensor Patent Rights to grant in the USA, including conducting any necessary or desirable claims or proceedings (including but not limited to any interference, reissue or re-examination or opposition or revocation proceedings). The Licensor shall keep the Licensee reasonably
33
informed of all filings made for the Licensor Patent Rights in the Territory including sending the Licensee a copy of any such filing and otherwise shall keep the Licensee informed of all material developments in relation to such Licensor Patent Rights and shall, upon the Licensee's request, provide the Licensee with copies of relevant Documents related to the filing, prosecution and maintenance of such Licensor Patent Rights. The Licensor shall give full consideration of, and absent a compelling reason to the contrary shall adopt any reasonable representation made by the Licensee in relation to the prosecution of the Licensor Patent Rights in the Territory when making any submission to a patent office (including the scope of foreign filings) and in the conduct of any proceedings in relation to such Licensor Patent Rights.
10.6 In the event that the Licensor declines to file or, having filed, declines to further prosecute, maintain and/or defend any pending Licensor Patent Rights in any country of the Territory, the Licensor shall provide the Licensee with written notice thereof. In the case where the Licensor has filed but is declining to further prosecute or maintain the Licensor Patent Rights, such notice shall be given at least thirty (30) days prior to the expiration of any official substantive deadline relating to such activities. In any of such circumstances the Licensee shall have the right to decide that the Licensee should file, continue to file, prosecute and maintain such Licensor Patent Rights and in such case the Licensee shall give written notice to the Licensor. The Licensor shall upon receipt of any such notice from the Licensee transfer to the Licensor all its files relating to the relevant Licensor Patent Rights and at the Licensee's cost and expense execute any documents to otherwise transfer control of such filing, prosecution and maintenance to the Licensor and thereafter the Licensee shall be responsible for the cost and expense of prosecuting and maintaining such Licensor Patent Rights. In addition the Licensor shall assign such Licensor Patent Rights to the Licensee for zero or nominal consideration, whereupon they shall not be part of any license rights granted to or through Licensor hereunder.
10.7 The Licensee shall be responsible at its own cost and expense and in its sole discretion for the filing, prosecution, maintenance and/or defense of Patent Rights in respect of inventions within Know How developed by the Licensee, its Affiliates or Sub-licensees relating to or potentially covering Product (the “Licensee Patents Rights”). The Licensee shall keep the Licensor informed of all material developments in relation to the Licensee Patent Rights and shall, upon the Licensor's request, provide the Licensor with copies of relevant documents related to the filing, prosecution and maintenance of the Licensee Patent Rights. The Licensee shall consider in good faith any reasonable representation made by the Licensor in relation to the prosecution of Patent Rights within the Licensee Patent Rights when making any submission to a patent office and in the conduct of any proceedings in relation to such Licensee Patent Rights in Europe.
10.8 In the event that the Licensee declines to file or, having filed, declines to further prosecute, maintain and/or defend any pending Licensee Patent Rights in any country of the Territory or Europe, the Licensee shall provide the Licensor with written notice thereof. In the case where the Licensee has filed but is declining to further prosecute or maintain such Licensee Patent Rights, such notice shall be given at least thirty (30) days prior to the expiration of any official substantive deadline relating to such activities. In any of such circumstances the Licensor shall have the right to decide that the Licensor should file, continue to file or prosecute such Licensee Patent Rights and in such case the Licensor shall give written notice to the Licensee. The Licensee shall upon receipt of any such notice from the Licensor transfer to the Licensor all its files relating to such Licensee Patent Rights and execute any documents necessary to transfer control of such filing, prosecution and maintenance to the Licensor. In addition the Licensee shall assign such Licensee Patent Rights to the Licensor for zero or nominal consideration, whereupon they shall not be part of any license rights granted to or through Licensee hereunder.
34
10.9 If either Party becomes aware of any actual, threatened or suspected infringement or misuse by a Third Party of any of the Licensor IPR Package relating to the Product in the Territory, it shall promptly inform the other Party in writing of all available evidence and details available to it concerning said infringement (the “Infringement Notice”). With respect to any actual, threatened or suspected infringement, the Licensee shall have the right, but not an obligation, at its own cost and expense to bring, defend or maintain any suit or action, in which case it shall have sole conduct of any claim or proceedings including any counterclaim for invalidity or unenforceability or any declaratory judgment action. The Licensor shall provide all necessary assistance to the Licensee in relation to such proceedings (including being joined as a party to any such proceedings) and the Licensee shall on demand by the Licensor indemnify the Licensor against reasonable costs of such activity that were approved by Licensee in advance in writing, provided that if the Licensor elects to be separately represented (which shall be at the Licensor's discretion), such separate representation shall be at the Licensor's sole cost and expense. The Licensee shall retain any award of costs and damages made or settlement sum paid, after recovery by the Licensor of its actual out-of-pocket costs and expenses. The Licensee will not (i) enter into any settlement of or make admission or stipulation in any such proceedings that admits any liability of Licensor or materially negatively impacts Licensor's rights hereunder or in the Licensor IPR Package without the Licensor's prior written consent, not to be unreasonably withheld or delayed.
10.10 If under Section 10.9, the Licensee fails to take any such proceedings within 90 days of any Infringement Notice, then the Licensor shall have the right, but not the obligation, upon 20 days prior notice to the Licensee at the Licensor's expense to bring, defend or maintain any suit or action or to control the conduct thereof against any actual, threatened or suspected infringement. The Licensee shall provide all necessary assistance to the Licensor in relation to such proceedings (including being joined as a party to any such proceedings). In any such action brought by the Licensor, the Licensor shall retain any award of costs and damages made or settlement sum paid, after recovery by the Licensee of its actual out-of-pocket costs and expenses. The Licensor will not enter into any settlement of or make admission or stipulation in any such proceedings that admits any liability of Licensee or materially negatively impacts Licensee's rights hereunder or in the Licensor IPR Package without the Licensee's prior written consent, not to be unreasonably withheld or delayed.
10.11 If either Party or its Affiliates receives formal notice from a Third Party that the Development, Manufacture or Commercialization of Product in the Territory under this Agreement infringes or otherwise violates the intellectual property rights of such Third Party in the Territory, then such Party must promptly notify the other Party in writing of this allegation. As soon as reasonably practicable after the receipt of such notice and at all times thereafter, the Parties will meet and consider the appropriate course of action with respect to such allegation of infringement. In any such instance, each Party will at its own cost, expense and liability, have the right to defend any action naming it; however, the Parties will at all times cooperate, share all material notices and filings in a timely manner, provide all reasonable assistance to each other and use good faith efforts mutually to agree upon an appropriate course of action, including, as appropriate, the preparation of material court filings and any discussions concerning a potential defense and/or settlement of any such claim. The rights and obligations in this Section 10.11 will apply even if only one Party defends any such claimed infringement action commenced by a Third Party. Neither Party will enter into any settlement of or make admission or stipulation in any such proceedings that admits any liability of the other Party or materially negatively impacts such other Party's rights hereunder or in the Licensor IPR Package without such other Party's prior written consent, not to be unreasonably withheld or delayed.
10.12 The Parties agree to cooperate in an effort to avoid loss of any of the Patent Rights forming part of the Licensor IPR Package or Licensee IPR Package including by executing any
35
documents as may be reasonably required. In particular, the Parties shall cooperate with each other in obtaining patent term extension or restoration or supplemental protection certificate (“Patent Term Extensions”) or their equivalents in any country of the Territory. Licensee shall have the sole right and shall use Commercially Reasonable Efforts to seek any available Patent Term Extensions in the Territory.
11.1 Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, each Party agrees that, during the Term and for seven (7) years thereafter, it shall, and shall cause its Affiliates, to keep confidential and not publish or otherwise disclose to any Third Party, and not use for any purpose other than as provided for in this Agreement, any Confidential Information of the other Party or any of its Affiliates, provided that each Party and its Affiliates may disclose the Confidential Information of the other Party or its Affiliates to the receiving Party's and its Affiliates' officers, directors, employees, agents and advisors who in each case are bound by commercially reasonable obligations of confidentiality with respect to the use and disclosure of such Confidential Information. Notwithstanding the foregoing, Confidential Information of a Party or its Affiliate shall exclude that portion of such information or materials that the receiving Party (or the receiving Party's Affiliate) can demonstrate by competent written proof:
(a) was generally available in the public domain at the time it was disclosed to the receiving party or subsequently came into the public domain through no fault of the receiving party; or
(b) was already known to the receiving Party or its Affiliate, other than under an obligation of confidentiality, at the time of disclosure by the other Party or is subsequently disclosed to the receiving Party or its Affiliate by a Third Party without obligations of confidentiality with respect thereto.
(c) is independently discovered or developed by the receiving Party or its Affiliate without the aid, application, or use of Confidential Information.
11.2 For clarity, specific aspects or details of Confidential Information shall not be deemed to be within the public domain or in the possession of the Recipient Party merely because the Confidential Information is embraced by more general information in the public domain or in the possession of the Recipient Party. Further, any combination of Confidential Information shall not be considered in the public domain or in the possession of the Recipient Party merely because individual elements of such Confidential Information are in the public domain or in the possession of the Recipient Party unless the combination is in the public domain or in the possession of the Recipient Party.
11.3 Notwithstanding the above obligations of confidentiality and non-use a Recipient Party may disclose Confidential Information:
(a) In responding to a valid order of a court of competent jurisdiction or other competent authority; provided that the receiving Party shall, to the extent reasonably practicable under the circumstances, first have given to the disclosing Party notice and a reasonable opportunity to quash the order or obtain a protective order requiring that the Confidential Information be held in confidence or used only for the purpose for which the order was issued; and provided further that if such order is not quashed or a protective order is not obtained, the Confidential Information disclosed shall be limited to the information that is legally required to be disclosed;
36
(b) to a Regulatory Authority as reasonably necessary to obtain Regulatory Approval in a particular jurisdiction to the extent consistent with the licenses granted under terms of this Agreement; and
(c) In complying with Applicable Law, provided that to the extent such disclosure is required to comply with a Legal Requirement, including to the extent such disclosure is required in publicly filed financial statements or other public statements under rules governing a stock exchange; provided, to the extent possible bearing in mind such Legal Requirements and subject to the next subsequent sentence of this Section (c), such Party shall provide the other Party with a copy of the proposed text of such statements or disclosure five (5) days in advance of the date on which the disclosure is to be made to enable the other Party to review and provide comments, unless a shorter review time is agreed. [If the compliance with a Legal Requirement requires filing of this Agreement, the filing Party shall to the extent possible seek confidential treatment of portions of this Agreement from the relevant Government Authority and shall provide the other Party with a copy of the proposed filings at least five (5) days prior to filing for the other Party to review any such proposed filing.]2 Each Party agrees that it will obtain its own legal advice with regard to its compliance with Legal Requirements and will not rely on any statements made by the other Party relating to such Legal Requirements;
(d) (i) to its actual or potential investment bankers; (ii) to existing and potential investors in connection with an offering or placement of securities for purposes of obtaining financing for its business and to actual and prospective lenders for the purpose of obtaining financing for its business; and (iii) to a bona fide potential acquirer or merger partner for the purposes of evaluating entering into a merger or acquisition, provided, however, any such persons must be obligated to substantially the same extent as set forth in Section 11 to hold in confidence and not make use of such Confidential Information for any purpose other than those permitted by this Agreement; and
(e) to its legal advisers for the purpose of seeking advice.
11.4 Nothing in this Section 11 restricts either Party from using or disclosing any of its own Confidential Information for any purpose whatsoever, subject always to the rights granted in this Agreement and provided always that the Licensee shall not under any circumstances publish the results of any clinical studies on Product without the prior written approval of the Licensor.
11.5 Following execution of this Agreement, each Party shall release a separate press release regarding this transaction and the wording of each press release shall be pre-agreed by the other Party. Other than such press releases, save as permitted in Section 11.3 neither Party shall make any public announcement or statement to the public containing Confidential Information without the prior written consent of the other. No such public announcements or statements shall be made without the prior review and consent of the appropriate individual designated for the purpose by the other Party
Publicity
11.6 Licensor, along with trial investigators, has submitted for publication a draft publication regarding the pivotal trial for the NDA Product. Promptly upon Licensor’s receipt thereof, Licensor agrees to provide a copy to Licensee a of all correspondence and editorial feedback regarding such publication for Licensee's review and comment, which comments Licensor
2 Note to draft: Parties to discuss whether or not the agreement will be afforded confidential treatment.
37
shall accept and incorporate into such publication prior to any resubmission or finalization thereof.
11.7 Subject to Section 7.6, other than with respect to the publication regarding the pivotal trial, the Parties shall each provide the other Party with copies of any publications or meeting abstracts relating to a Product for such other Party's review and comment at least thirty (30) days prior to submitting such publication to any journal or submitting or presenting it or an abstract thereof at any congress, conference or other public meeting, and each Party agrees to take reasonably into account any comments received from the other Party thereon.
11.8 Following final submission of the publication for the pivotal trial, (a) Licensor and its Affiliates and Sub-licensees shall refrain from engaging any study investigator, practitioners, opinion leaders, patient advocates and payers in the Territory regarding the Product for any reason, including scientific discourse, without the prior consent of Licensee (such consent not to be unreasonably withheld, delayed or conditioned); and (b) Licensee and its Affiliates and Sub-licensees shall refrain from engaging any study investigator, practitioners, opinion leaders, patient advocates and payers outside the Territory regarding the Product for any reason, including scientific discourse, without the prior consent of the Licensor (such consent not to be unreasonably withheld, delayed or conditioned).
12. Representations, warranties and covenants
12.1 Mutual Representations and Warranties. Each Party hereby represents, warrants, and covenants (as applicable) to the other Party as of the Effective Date as follows:
(a) Corporate Existence and Power. It is a company or corporation duly organized, validly existing, and in good standing under the Laws of the jurisdiction in which it is incorporated, and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as contemplated in this Agreement, including the right to grant or receive, as the case may be, the licenses granted and received hereunder.
(b) Authority and Binding Agreement. It has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder; it has taken all necessary corporate action on its part required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder; and this Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms.
(c) No Conflict. It is not a party to and will not enter into any agreement that would prevent it from granting the rights or exclusivity granted or intended to be granted to the other Party under this Agreement or performing its obligations under this Agreement.
(d) No Debarment. Such Party is not debarred, has not been convicted, and is not subject to debarment or conviction pursuant to Section 306 of the Federal Food, Drug and Cosmetics Act. To such Party's Knowledge, in the course of the Development of the API or Products, such Party has not used prior to the Effective Date and shall not use, during the Term, any employee, agent or independent contractor who has been debarred by any Regulatory Authority, or, to the best of such Party's Knowledge, is the subject of debarment proceedings by a Regulatory Authority or has been convicted pursuant to Section 306 of the FD&C Act.
(e) Anti-Corruption. Such Party, its Affiliates and their respective directors, officers, employees, agents or other persons or entities acting on its behalf (all the foregoing collectively “Representatives”) have conducted and will conduct their businesses as
38
they relate to this Agreement, in compliance with Anti-Bribery Laws. Without limiting the generality of the foregoing, such Party represents and warrants that it has and will have necessary procedures in place to prevent bribery and corrupt conduct by it and its Representatives in connection with the Development, Manufacture and Commercialization of the Product.
12.2 Representations, Warranties and Covenants by Licensor. Licensor hereby represents, warrants and covenants to Licensee, as of the Effective Date, as follows, except as set forth otherwise in the Disclosure Schedule:
(a) Licensor owns or has a valid right to grant the licenses hereunder to the Licensor IPR Package existing as of the Effective Date, including the NDA and Licensor Patent Rights, and Licensor has the right to make the assignments and grant the licenses to Licensee as purported to be granted pursuant to this Agreement. Neither Licensor nor any of its Affiliates has entered into any agreement granting any right, interest or claim in or to, any Licensor Patent Rights or Licensor Know-How to any Third Party (other than pursuant to the CMO Contracts included in the Data Room) that would conflict with the licenses to Licensee as purported to be granted pursuant to this Agreement.
(b) All Licensor Patent Rights are listed in Schedule 7. All Licensor Patent Rights are subsisting and are not invalid or unenforceable, in whole or in part, are being prosecuted in the patent offices indicated in Schedule 7 in accordance with Applicable Law, and all applicable fees have been paid on or before the Effective Date. The Licensor Patent Rights represent all Patent Rights within Licensor' or its Affiliates' ownership or Control that Licensor reasonably believes include claims covering the making, using, and composition of matter of the API or the Products, or the Development, Manufacture or Commercialization thereof, as of the Effective Date. Licensor has properly recorded in the relevant U.S. and Canadian patent offices the assignments, or other necessary documents, supporting its legal title to the Licensor Patent Rights. To the Licensor’ Knowledge, Licensor and its Affiliates have presented, or will present prior to the pertinent patent office deadlines, all relevant references, documents, or information of which it and the inventors are aware to the relevant patent examiner at the pertinent patent office, in connection with the prosecution of the pending patent applications included in the Licensor Patent Rights.
(c) There are no claims, judgments, or settlements against, or amounts with respect thereto, owed by Licensor or any of its Affiliates relating to the Product Registrations, the Licensor Patent Rights, or the Licensor Know-How. No claim or litigation has been brought or threatened in writing by any Person against Licensor alleging, and Licensor has no Knowledge of any reasonable basis for any such claim or allegation, whether or not asserted, that (i) the Licensor Patent Rights or the Licensor Know-How are invalid or unenforceable, or (ii) the Product Registrations, the Licensor Patent Rights, or the Licensor Know-How, or the disclosing, copying, making, assigning, or licensing of the Product Registrations, the Licensor Patent Rights, or the Licensor Know-How, or the Development, Manufacture, Commercialization or other exploitation of the API or Products as contemplated herein, does or will violate, infringe, misappropriate or otherwise conflict or interfere with, any Patent or other intellectual property or proprietary right of any Third Party.
(d) To Licensor's Knowledge, no Person is infringing or threatening to infringe or misappropriating or threatening to misappropriate the Licensor Patent Rights, the Licensor Know-How, or the Product Registrations. To Licensor’ Knowledge, there are no activities by Third Parties that would constitute infringement or misappropriation of the Licensor Technology (in the case of pending claims, evaluating them as if issued).
39
(e) Each Person who, to Licensor’s Knowledge, has or has had any rights in or to any Licensor Patent Rights or any Licensor Know-How, has assigned and has executed an agreement assigning its entire right, title, and interest in and to such Licensor Patent Rights and Licensor Know-How to Licensor. To Licensor's Knowledge, no current officer, employee, agent, or consultant of Licensor or any of its Affiliates is in violation of any term of any assignment or other agreement regarding the protection of Patents or other intellectual property or proprietary information of Licensor or such Affiliate or of any employment contract relating to the relationship of any such Person with Licensor.
(f) None of the intellectual property rights licensed hereunder by Licensor to Licensee and existing as of the Effective Date are owned or Controlled in whole or in part by any Third Party other than the rights under the CMO Contracts included in the Data Room.
(g) With respect to those portions of the Licensor Know-How the confidentiality of which is material to the Commercialization of Products, such portions of the Licensor Know-How have been kept confidential or have been disclosed to Third Parties only under terms of confidentiality. To Licensor' Knowledge, no breach of such confidentiality has been committed by any Third Party.
(h) There are no pending, and to Licensor's Knowledge there are no threatened, actions, claims, demands, suits, proceedings, arbitrations, grievances, citations, summonses, subpoenas, inquiries or investigations of any nature, civil, criminal, regulatory or otherwise, in law or in equity, against Licensor or any of its Affiliates or, to the Knowledge of Licensor, pending or threatened against any Third Party, in each case, relating to the transactions contemplated by this Agreement.
(i) Other than the Exploitation Agreement, Licensor has not entered into any agreement with a Third Party pursuant to which Licensor may be obligated to pay a royalty or other consideration with respect to sales of a Product, nor is there any agreement that the Licensor is a party to which conflicts with or restricts in any material manner, the rights assigned or licensed by Licensor to Licensee hereunder.
(j) Licensor is not, and to Licensor's Knowledge no other party to such agreement is, in breach of any obligations owed under the Exploitation Agreement.
(k) Licensor has provided or made available to Licensee, prior to the Effective Date, true, complete, and correct copies of all material adverse information with respect to the safety and efficacy of the API and Product which is known to Licensor.
(l) To Licensor's Knowledge, Licensor and its Affiliates and licensees have generated, prepared, maintained, and retained all Product Registrations that are required to be maintained or retained pursuant to and in accordance with applicable Law, and all such information is true, complete and correct and what it purports to be.
(m) Licensor and its Affiliates have conducted, and to Licensor's Knowledge, their respective contractors licensees and consultants have conducted, all Development of the API or the Products that they have conducted prior to the Effective Date in accordance with applicable Law. To Licensor’s Knowledge, Licensor has conducted, and has caused its licensees, contractors and consultants to conduct, any and all pre-clinical and clinical studies related to the API and Products in accordance with Applicable Law. To Licensor's Knowledge, Licensor and its Affiliates and licensees have employed (and, with respect to such tests and studies that Licensor will perform, will employ) Persons with appropriate education, knowledge and experience to conduct and to oversee the conduct of the PIP.
40
(n) To Licensor's Knowledge, neither Licensor nor any of its Affiliates or licensees, nor any of its or their respective officers, employees, or agents has made an untrue statement of material fact or fraudulent statement to the FDA or any other Regulatory Authority with respect to the Development of the API or the Products, failed to disclose a material fact required to be disclosed to the FDA or any other Regulatory Authority with respect to the Development of the API or the Products, or committed an act, made a statement, or failed to make a statement with respect to the Development of the API or the Products that could reasonably be expected to provide a basis for the FDA to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities”, set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto or any analogous Laws or policies in the Territory.
(o) The inventions claimed in the Licensor Patent Rights (i) were not conceived or made in connection with any research activities funded, in whole or in part, by the federal government of the United States or any agency thereof, (ii) are not a “subject invention” as that term is described in 35 U.S.C. Section 201(f), and (iii) are not otherwise subject to the provisions of the Xxxx-Xxxx Act.
12.3 Each Party undertakes to inform the other as soon as it becomes aware that any of the following events are reasonably likely to occur:
(a) cessation of conducting its business or trading;
(b) sale of all or any material portion of its assets or business;
(c) entry of a judgment against such Party in an amount that exceeds US$100,000 or the entry of any declaratory, injunctive or other equitable remedy or court order that would materially impair such Party's ability to continue to conduct its business or to perform its obligations under this Agreement;
(d) any attachment (including prejudgment attachment) of any material assets;
(e) failure to pay any wages to such Party's employees when due (except to the extent subject to a bona fide dispute);
(f) a financial institution which lends money to such Party declaring an event of default by the company under any bank loan or other debt instrument;
(g) entry by the Party into any restructuring or workout agreement, or similar agreement, relating to any material indebtedness (being debt exceeding US$100,000); or
(h) loss of any permits, licenses or governmental authorizations that are reasonably necessary in order for the Party to continue to engage in its current business.
12.4 Except as expressly set forth herein, each Party expressly disclaims and excludes any and all representations and warranties, express, implied, statutory or otherwise, including without limitation the warranties of merchantability and fitness for a particular purpose.
12.5 EXCEPT WITH RESPECT TO (1) AMOUNTS OWED TO A THIRD PARTY WHICH AMOUNTS FALL WITHIN THE INDEMNIFICATION OBLIGATIONS OF A PARTY UNDER SECTIONS 13.1 AND 13.2, (2) DAMAGES AVAILABLE FOR A PARTY'S BREACH OF ITS CONFIDENTIALITY OBLIGATIONS HEREUNDER, OR (3) DAMAGES AVAILABLE IN THE CASE OF A PARTY'S FRAUD, GROSS NEGLIGENCE OR INTENTIONAL MISCONDUCT, NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, PUNITIVE, OR INDIRECT DAMAGES ARISING FROM OR RELATING TO ANY
41
BREACH OF THIS AGREEMENT OR ANY TORT CLAIMS ARISING HEREUNDER, REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES.
12.6 Nothing in this Agreement shall be taken to exclude or limit either Party's liability to the extent that such liability cannot be excluded or limited in Law, including for death or personal injury, fraud or fraudulent misrepresentation.
12.7 The Parties agree that:
(a) Licensor shall use all reasonable efforts to negotiate and execute, as soon as reasonably practicable after the Effective Date, an amendment to the Exploitation Agreement which provides that upon the termination of the Exploitation Agreement The Centre National De La Recherche Scientifique will be obligated to enter into an agreement with the Licensee, in respect of the Territory, on substantially the same terms as those provided to the Licensor under the Exploitation Agreement including in respect of royalty payments; and
(b) the Licensee shall use all reasonable efforts to assist the Licensor in respect of its obligation at Section 12.7(a) above,
and it is agreed that the Licensor shall not be obligated pursuant to this Section 12.7 to agree to any increase in the royalties payable under the Exploitation Agreement.
13. Indemnity and insurance cover
13.1 Indemnification by Licensee. Subject to Sections 13.3 and 13.4, Licensee shall indemnify, defend and hold Licensor, its Affiliates, and their respective directors, officers, employees consultants, contractors, sub-licensees and agents (collectively, the “Licensor Indemnitees”) harmless from and against any and all claims, suits, proceedings or causes of action (“Claims”) brought against such Licensor Indemnitee, including any damages or other amounts payable with respect to such Claims, as well as any reasonable attorneys' fees and costs of litigation incurred as to any such Claim until the indemnifying Party has acknowledged that it will provide indemnification hereunder with respect to such Claim as provided below (collectively, “Damages”), in each case to the extent resulting from or based on:
(a) any development work done by Licensee for a Product, or any sale, use, importation, storage, handling, distribution or offer for sale or sale of Product by Licensee or any of its Affiliates or sub-licensees;
(b) Licensee's breach of this Agreement or any representation or warranty made by Licensee herein;
(c) the willful misconduct of, or violation of applicable Law by, Licensee, its Affiliates or Sub-licensees, or their respective employees, contractors or agents in the performance of this Agreement; and/or (d) breach of a contractual or fiduciary obligation owed by Licensee (including without limitation misappropriation of trade secrets).
(d) infringement or misappropriation with respect to the Development, Manufacture, or Commercialization of the Product in the Territory under the Licensee IPR Package or Licensee Trademarks.
and the foregoing indemnity obligation shall not apply to the extent such Claims or Damages result from any matter for which Licensor is required to indemnify Licensee.
42
consultants, contractors, sub-licensees and agents (collectively, the “Licensee Indemnitees”) harmless from and against any and all Third Party Claims brought against such Licensee Indemnitee, including any Damages resulting therefrom, in each case to the extent resulting from or based on:
(a) any development work done by Licensor for a Product, or any sale, use, importation, storage, handling, distribution or offer for sale or sale of Product by Licensor or any of its Affiliates or sub-licensees;
(b) Licensor's breach of this Agreement or any representation or warranty made by Licensor therein;
(c) the willful misconduct of, or violation of applicable Law by, Licensor, its Affiliates or sub-licensees, or their respective employees, contractors or agents in the performance of this Agreement;
(d) breach of a contractual or fiduciary obligation owed by Licensor (including without limitation misappropriation of trade secrets); or
(e) infringement or misappropriation with respect to the Development, Manufacture, or Commercialization of the NDA Product under the Licensor IPR Package or Licensor Trademarks.
and the foregoing indemnity obligation shall not apply to any Damages to the extent such Damages result from any matter for which Licensee is required to indemnify Licensor pursuant to Section 13.1(b), (c), or (d).
(a) Subject to Section 13.4, Licensor shall indemnify and hold the Licensee Indemnitees harmless from and against any and all Damages which a Licensee Indemnitee may incur or suffer arising out of any Third Party claim of property damage, bodily injury or death (including negligence, tort, strict liability or otherwise) to the extent caused by (i) any Licensor Design Defect or (ii) any sale, use, importation, storage, handling, distribution, offer for sale or sale of Product, or development of a Product conducted by Licensor. A “Licensor Design Defect” shall be a defect in the design or formulation of the NDA Product as such NDA Product is designed and formulated as set forth in the NDA on the Effective Date.
(b) Subject to Section 13.4 and except to the extent provided in subsection (a) above, Licensee shall defend, indemnify and hold the Licensor Indemnitees harmless from and against any and all Damages arising out of any Third Party claims of property damage, bodily injury or death (including negligence, tort, strict liability or otherwise) to the extent caused by (i) any sale, use, importation, storage, handling, distribution, offer for sale or sale of Product, or development of such Product conducted by, Licensee; or (ii) any Licensee Design Defect. A “Licensee Design Defect” shall be a defect in the design or formulation of the Product designed or formulated by Licensee that differs from the design or formulation of the NDA Product as of the Effective Date.
13.4 Indemnification Procedures. A Party seeking indemnification under Section 13.1, 13.2 or 13.3 hereof (the “Indemnitee”) shall promptly notify the other Party (the “Indemnitor”) in writing of any claim, lawsuit or other action in respect of which the Indemnitee, its Affiliates, or any of their respective directors, officers, employees and agents intend to claim such
43
indemnification. The Indemnitee shall permit, and shall cause its Affiliates and their respective directors, officers, employees and agents to permit the Indemnitor to have complete control of such defense (except as set forth below) so long as it promptly assumes the defense and prosecutes the defense with appropriate diligence and care. Notwithstanding the foregoing, such Indemnitor shall not have the right to defend or direct the defense of any such claim, lawsuit or other action that (x) is asserted directly by or on behalf of a Third Party that is a supplier or direct customer of the Indemnitee, or (y) seeks an injunction or other equitable relief against the Indemnitee. The Party controlling the defense hereunder (the “Defending Party”) shall have the authority, at its discretion, to settle any such claim, lawsuit or other action only with the prior written consent of the Party who is not controlling the defense (the “Non-Defending Party”), provided, however, that such consent shall not be unreasonably withheld or delayed so long as such settlement does not adversely affect the Non-Defending Party's rights hereunder or impose any obligations on the Non-Defending Party in addition to those set forth herein. The Defending Party and the Non-Defending Party, and their respective Affiliates, and their respective directors, officers, employees and agents shall cooperate fully with each other and their respective legal representatives in the investigation and defense of any claim, lawsuit or other action covered by this indemnification. The Defending Party shall keep the Non-Defending Party reasonably informed of the progress of the action and shall consider the comments and observations of the Non-Defending Party timely given in the course of the proceedings. If the Indemnitor is the Defending Party, the Indemnitee shall have the right, but not the obligation, to be represented by counsel of its own selection and expense. Notwithstanding the foregoing, the Indemnitee may be represented by separate counsel at the expense of the Indemnitor if a conflict of interest exists between the interests of the Indemnitor and Indemnitee so that a single counsel representing Indemnitor cannot adequately defend the rights of the Indemnitee.
13.6 Each Party shall maintain, at its own cost, the insurance coverages set forth in this Section 13.6; provided, however, the Licensee has the right, in its sole discretion, to self-insure in part or in whole for any such coverages:
(a) commencing as of the Effective Date, and thereafter for the period of time required under Section 13.7, each Party shall obtain and maintain on an ongoing basis, commercial general liability insurance, including contractual liability, in the minimum amount of ten million dollars ($10,000,000) per occurrence, combined single limit for bodily injury and property damage liability, and
(b) commencing as of the Effective Date, and thereafter for the period of time required under Section 13.7, each Party shall obtain and maintain on an ongoing basis, products liability insurance, including contractual liability, in the minimum amount of ten million dollars ($10,000,000) per occurrence, combined single limit for bodily injury and property damage liability.
13.7 Except to the extent that the Licensee self-insures as authorized under Section 13.6, the following provisions apply:
(a) All insurance coverages shall be primary insurance with respect to each Party's own participation under this Agreement, and shall be maintained with an insurance for the Licensee or companies having an A.M. Best's rating (or its equivalent) of A-XII or better.
(b) Each Party shall be provided additional insured status under the other party’s commercial general liability and products liability insurance policies.
44
(c) The insurance policies shall be under an occurrence form, but if only a claims-made form is available to a Party, then in such a case, such Party shall maintain the insurance coverage for at least five (5) years following such Party's completing performance of its obligations under this Agreement.
(d) Each Party's aggregate deductibles under its commercial general liability and products liability and other insurance policies shall be reasonably satisfactory to the other Party, taking into account the deductibles that are prudent and customary with respect to the activities in which it is engaged under this Agreement.
(e) Each Party's insurance coverage shall include an option to purchase tail coverage for claims arising during a period covered by such insurance (the policy period) but for which claims are not brought during the policy period, which tail period shall have a duration of at least five (5) years following the end of the policy period. The tail coverage will be purchased if the insurance coverage in place is cancelled or non-renewed.
(f) Each Party shall provide to the other Party its respective certificates of insurance evidencing the insurance coverages set forth in Section 13.6 Each Party shall provide to the other Party at least thirty (30) days prior written notice of any cancellation, non-renewal or material change in any of the insurance coverages. Each Party shall, upon receipt of written request from the other Party, provide renewal certificates to the other Party for as long as such Party is required to maintain insurance coverages hereunder.
14. Duration and termination of the agreement
14.1 The Agreement shall enter into force and effect on the Effective Date and shall remain in full force and effect country by country of the Territory for the duration of the Term, subject to earlier termination as provided in this Agreement. At the expiry of the Royalty Term in each country of the Territory the licenses granted by the Parties hereunder shall become perpetual, irrevocable, fully paid up and royalty free in such country.
14.2 The Licensee shall be entitled to terminate the Agreement:
(a) effective immediately upon prior written notice to the Licensor pursuant to Section 7.11(d), if the Licensee can demonstrate that there are reasonable good faith grounds to believe there is a safety concern related to the Product, or
(b) effective immediately upon prior written notice to the Licensor in case of withdrawal of the Regulatory Approval for the Product in the United States for whatever reason which the Licensee reasonably believes will be permanent;
(c) effective upon two hundred and seventy (270) days' prior written notice to the Licensor, for any reason or no reason; or
(d) pursuant to Section 14.4 below, which relates to a material breach of the Agreement by the Licensor.
14.3 The Licensor shall be entitled to terminate this Agreement pursuant to Section 14.4 below, which relates to a material breach of the Agreement by the Licensee.
14.4 Either Party (the non-breaching Party) shall have the right to terminate this Agreement upon giving ninety (90) days written notice to the other Party on the occurrence of a material breach by such other Party (the breaching Party) which material breach is incapable of remedy or which, in the case of a breach capable of remedy, shall not have been remedied
45
within such ninety (90) day notice period, provided that the non-breaching Party shall have identified in its notice the breach in reasonable detail. If the breach relates to:
(a) Canada then the Licensor shall only have the right to terminate this Agreement in relation Canada; or
(b) the USA then the Licensor shall have the right to terminate this Agreement in its entirety.
If the breaching Party in good faith disputes such material breach or disputes the failure to cure or remedy such material breach and provides written notice of that dispute to the non-breaching Party within the above notice period, then the matter will be addressed under the dispute resolution provisions in Section 16 and the non-breaching Party may not terminate this Agreement until it has been determined under Section 16 that the breaching Party is in material breach of this Agreement, and the breaching Party further fails to cure such breach within sixty (60) days after the conclusion of that dispute resolution procedure.
14.5 Rights in Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by either Party are, and shall otherwise be deemed to be, for purposes of section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that each Party, as licensee of certain rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code. The Parties further agree that, in the event of the occurrence of a Bankruptcy Event in respect of a Party (such Party, the “Debtor”) under the U.S. Bankruptcy Code, the other Party shall be entitled to a complete duplicate of (or complete access to, as appropriate) any intellectual property licensed to such other Party and all embodiments of such intellectual property, which, if not already in such other Party's possession, shall be promptly delivered to it:
(a) upon any such commencement of a bankruptcy proceeding upon such other Party's written request therefor, unless the Debtor elects to continue to perform all of its obligations under this Agreement; or
(b) if not delivered following the rejection of this Agreement by the Debtor upon written request therefor by the other Party.
14.6 Expiration of, or termination of this Agreement by Licensee pursuant to Section 14.4, shall result in the following:
(a) All rights granted by the Licensor to the Licensee in the Licensor IPR Package and Licensor Trademarks under the Agreement shall become fully-paid-up, royalty-free and perpetual.
(b) The rights and obligations of the Parties set forth in Section 10 shall continue until the date that is six (6) years after the last to expire Valid claim of a Patent Right Controlled by either Party shall have expired.
(c) The obligations with respect to monitoring and exchange of safety information set forth in Section 7.11 shall continue for as long as both Parties are Developing or Commercializing a Product anywhere in the World, with each Party bearing its own costs and expenses with respect thereto.
14.7 Termination of this Agreement by Licensee other than pursuant to Section 14.4, or by Licensor pursuant to Section 14.2(b), shall result in the following, which, if the termination is in relation to a country, shall only apply in such country:
46
(a) Termination of all rights granted by the Licensor to the Licensee under the Agreement and reversion to the Licensor of such rights. The Licensee shall immediately cease all use of the Licensor IPR Package and shall cease all Development, manufacturing and Commercialization activity. All Sub-licenses agreements and arrangements shall terminate simultaneously.
(b) The Licensee hereby grants to the Licensor a non-exclusive, perpetual, sub-licensable, fully paid-up royalty free right and license to use the Licensee IPR Package inside such Country for the Development and Commercialization of the Product in such country and for the Manufacture of Products in the Territory. Termination shall have no impact upon the Licensor's rights to use the Licensee IPR Package outside the Territory.
(c) The Licensee shall promptly transfer to the Licensor the Licensee Materials, Licensee Know How, Product Registrations and Commercial Information and Documents containing the same (including any of the same in the possession of a Sub-licensee).
(d) Commensurate with Legal Requirements, to the extent held by the Licensee, it shall as soon as reasonably practicable after termination transfer to the Licensor or its nominee all right, title and interest in all relevant Product Registrations held by the Licensee for the relevant Product(s), and the Licensee shall execute all necessary and appropriate letters to the FDA and other Regulatory Authorities in the Territory (if any) to ensure that ownership of the Product Registrations are transferred to the Licensor (i) for United States Product Registrations, within 15 days of termination, and for Canadian Product Registrations, within 70 days of termination. The date upon which a Product Registration is registered in the Licensor's name shall be known as the “Transfer Date”. In the event that such a transfer is not possible under Legal Requirements, the Licensee shall use reasonable efforts at Licensor's sole cost and expense to ensure that the Licensor has the benefit of the relevant Product Registrations and, to this end, consents to any Regulatory Authority in the Territory cross-referencing to the data and information on file with any Regulatory Authority as may be necessary to facilitate the granting of second Product Registrations to the Licensor in the terminated countries. In such circumstance as soon as the second Product Registrations are given to the Licensor, the Licensor will, so far as possible under Legal Requirements, cancel the corresponding first Product Registration. Further from the date of submission of the appropriate letters to the Regulatory Authorities in the Territory (if any) necessary to ensure that ownership of the Product Registrations is given to the Licensor until the Product Registrations are actually transferred to the Licensor or second Product Registrations are issued by such Regulatory Authorities, the Licensee shall, at Licensor's sole cost and expense:
(i) submit all necessary filings, correspondence and reports, attend all necessary meetings and otherwise conduct such other activities as are necessary to maintain the Product Registrations;
(ii) as soon as practicably possible submit to the Licensor copies of all filings and correspondence that are submitted to any Regulatory Authority to the extent they relate to all relevant Product(s) in the Territory; and
(iii) provide the Licensor with advance written notice of any meetings or telephone calls with any Regulatory Authority in the Territory relating to Product Registrations and allow the Licensor to participate in all such meetings or telephone calls.
47
(e) The Licensee shall as soon as reasonably practicable notify the Licensor of the amount of stocks of Product owned by it (title not having passed to a customer) and the precise location of such stocks with corresponding stock numbers, and the amount of such stocks subject to customer orders but not yet delivered. The Licensor shall have the option (exercisable by written notice to the Licensee to be given not more than one (1) month termination), either (i) to inspect and purchase from the Licensee a price equal to Cost of Manufacture all of or any part of stocks of the Product held by the Licensee which are not subject to orders from customers and are in good and saleable condition or (ii) permit the Licensee to sell all or some of such stocks of Products for a period up to and no later than the Licensee holds its own Regulatory Approval for Product in the relevant country. All other stocks of Products shall be destroyed by the Licensee.
(f) The Licensee shall inform the Licensor of the details of all stocks including batch numbers sold by the Licensee, its Affiliates, or Sub-licensees in the previous six (6) months to distributors or wholesalers and the names and other details of such distributors or wholesalers.
(g) The Licensee shall be entitled to invoice and collect and retain payment for Products sold by Licensee prior to termination, and shall be responsible for any payments, rebates, administrative fees or chargebacks (“Commercial Payments”) due to customers (including those payments due under managed care agreements and agreements with health maintenance organization) for Products sold by the Licensee, its Affiliates and Sub-Licensees prior to the termination date and the Licensor shall be responsible for Commercial Payments to customers for Products sold or dispensed thereafter. In the event that, with respect to Products sold or dispensed during the Calendar Quarter in which the termination date occurs, the Parties cannot determine the date on which such Products were sold, then the Party that has made a Commercial Payment for such Product shall be entitled to obtain reimbursement from the other party on a pro rata basis for such Commercial Payment (with the Licensee's pro rata share constituting the proportion of days in the Calendar Quarter up to and including the termination date, and the Licensor's pro rata share constituting the proportion of days in such Quarter following the termination date). Either Party that has made a Commercial Payment, all or a portion of which is the responsibility of the other Party, shall submit a payment request to the other Party, with supporting documentation, and such other Party shall reimburse the requesting Party for the Commercial Payment (or portion thereof) for which it is responsible within thirty (30) days of receipt of such request.
(h) The Licensee shall be responsible for any rebates due to a governmental entity (“Government Rebates”) applicable to Product sold by the Licensee, its Affiliates, or Sub-licensees prior to the termination date. The Parties acknowledge that the request for such payments is submitted to the National Drug Code holder (or its equivalent outside the USA) of the Product and, as such, the Licensee will receive, process and pay Government Rebates on all Product bearing Licensee National Drug Code and shall within 30 days of payment, submit to the Licensor an invoice for the amounts of any Government Rebates made in relation to Product sold after the termination date. In the event that, with respect to Product sold or dispensed during the Calendar Quarter in which the termination date occurs, the Parties cannot determine the date on which such Product was sold or dispensed, then the Party that has made a Government Rebate for such Product shall be entitled to obtain reimbursement from the other Party on a pro rata basis for such Government Rebate (with the Licensee's pro rata share constituting the proportion of days in the Calendar Quarter up to and including the termination date, and the Licensor's pro rata share constituting the
48
proportion of days in such Calendar Quarter following the termination date). Either Party that has made a Government Rebate, all or a portion of which is the responsibility of the other Party, shall submit a payment request to the other Party, with supporting documentation, and such other Party shall reimburse the requesting Party for the Government Rebate (or portion thereof) for which it is responsible within thirty (30) days of receipt of such request.
(i) The Licensee shall be responsible for returns from any customer in respect of all Product sold by the Licensee, its Affiliates, or Sub-licensees prior to the termination date and the Licensor shall be responsible for returns in respect of all Product sold or dispensed after the termination date, regardless of when such return occurs. Neither Party shall seek to encourage Product returns or accept Product returns other than in the ordinary course of business save, in each case, with the written consent of the other Party. The Licensee shall process all Product returns received by it and shall destroy the returned Product. The Licensee shall reimburse the Licensor (within ten (10) days of request therefor) with respect to any returns of Product sold on or prior to the termination date for all costs and expenses relating to the return, including the actual cost of destruction and related administrative costs and expenses.
(j) The Licensee will transfer to the Licensor the domain name for the internet website established by the Licensee.
(k) To the extent permitted by applicable Law, for a period not to exceed ninety (90) days, Licensee will reasonably cooperate at Licensor’s sole cost and expense as reasonably requested by Licensor, in assisting with the smooth transition of the Commercialization of the Product in the Territory from Licensee to Licensor
14.8 Termination of the Agreement shall be without prejudice to any rights that shall have accrued to the benefit of either party before such termination, including the right of either party to receive or recover: (i) damages sustained by reason of the breach of the Agreement by the other party, or (ii) any payments for Products which may then be owing under the terms of the Agreement (including any invoice). In addition, the following provisions of this Agreement shall survive termination of this Agreement:
(a) Section 11 (Confidentiality);
(b) this Section 14 (Duration and termination of the agreement);
(c) Section 16 (Governing law and disputes); and
(d) any provision of this Agreement necessary for its interpretation or enforcement.
14.9 If:
(a) a Court pursuant to Section 16.2Error! Reference source not found. finds that Licensee is obligated to transfer the Product Registrations pursuant to Section 14.7(d) has not done so in a timely manner; or
(b) if Licensee agrees in any settlement or otherwise that it is obliged to transfer the Product Registrations pursuant to Section 14.7(d),
then the Licensor shall be entitled to equitable relief pursuant to Section 16.2 including specific performance of such obligation, without a need to post bond or other surety.
15.1 Subject to Section 15.2, this Agreement shall not be assignable nor shall the rights licensed hereunder or the ownership of the NDA (other than pursuant to Section 5.10) be transferable
49
in any way by either Party except by prior written consent of the other Party, not to be unreasonably withheld, conditioned or delayed; provided, however, that:
(a) other than with respect to assignment of the ownership of the NDA, which assignment shall require the consent of the other Party, not to be unreasonably withheld, conditioned or delayed, either Party may assign this Agreement in whole or in part to a corporate Affiliate on reasonable prior written notice to the other Party of such assignment on the condition that the assigning Party shall remain liable hereunder for the prompt payment and performance of all obligations of the assignee;
(b) this Agreement may be assigned by a Party to a Third Party in connection with a sale or transfer of all or substantially all of such Party's business or assets to which this Agreement relates or in connection with a merger or consolidation transaction involving such Third Party provided always that such Third Party gives a written deed of undertaking to the non-affected Party agreeing to abide by all the obligations under this Agreement of the assigning Party; and
(c) Licensee may pledge, grant a security interest, lien or charge in, or other encumbrance upon, any of Licensee’s rights or interests in this Agreement (and may assign this Agreement or the rights hereunder, in whole or in part in connection with any of the foregoing), including without limitation pursuant to the terms of the Term Loan Agreement (and any amendment, restatement, replacement or refinancing thereof) and any related documents.
15.2 If a Bankruptcy Event occurs with respect to the Licensee, then:
(a) the Licensee shall not be permitted to assign this Agreement or transfer its rights hereunder; and
(b) except as specified in Section 15.1(c), the Licensor shall not be deemed to have consented to: (i) any assignment by the Licensee of this Agreement; or (ii) the transfer of any of the Licensee rights contained in this Agreement.
15.3 This Agreement shall be binding upon, and shall inure to the benefit of, all permitted assigns.
16. Governing law and disputes
16.1 This Agreement shall be governed by and construed in accordance with the Laws of the State of New York, United States without regard to its conflicts of laws principles.
16.2 The Parties consent to the exclusive jurisdiction of the Federal courts and the State courts of the State of New York, in each case, located in the borough of Manhattan, City of New York (the "New York Courts") in any dispute, suit or action (each, a “Proceeding”) arising out of or relating to this Agreement or the transactions contemplated hereby, and hereby irrevocably and unconditionally (i) agrees not to commence any such Proceeding except in such New York Courts, (ii) agrees that any claim in respect of any such Proceeding may be heard and determined in the New York Courts, (iii) waives, to the fullest extent it may legally and effectively do so, any objection which it may now or hereafter have to the laying of venue of any such Proceeding in any such New York Court, and (iv) waives, to the fullest extent permitted by Law, the defense of an inconvenient forum to the maintenance of such Proceeding in any such New York Court. Each of the parties agrees that a final judgment in any such Proceeding shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by Law. THE PARTIES HEREBY IRREVOCABLY WAIVE, AND AGREE TO CAUSE THEIR RESPECTIVE AFFILIATES TO WAIVE, THE RIGHT TO TRIAL BY JURY IN SUCH PROCEEDINGS.
50
Neither Party shall be responsible for any delay or failure to perform its obligations under the Agreement or shall be liable to the other for loss or damages for any default or delay caused by conditions beyond its reasonable control, including but not limited to acts of God, governmental restrictions, declared or not declared wars or insurrections, strikes, terrorism, floods or work stoppages. If either of the Parties is so affected it shall give prompt written notice of such cause to the other Party. The Party giving such notice shall thereupon be excused from such of its obligations under the Agreement as it is thereby disabled from performing for so long as it is so disabled.
(a) Any notice (which term shall in this Section 17.2 include any other formal written communication) required to be given under this Agreement or in connection with the matters contemplated by it shall, except where otherwise specifically provided, be in writing in the English language.
(b) Any such notice shall be addressed as provided in Section 17.2(a) and may be:
(i) delivered by hand (which shall include by courier), in which case it shall be deemed to have been given upon delivery at the relevant address if it is delivered not later than 17.00 hours on a Business Day, or, if it is delivered later than 17.00 hours on a Business Day or at any time on a day which is not a Business Day, at 08.00 hours on the next Business Day; or
(ii) sent by electronic mail, in which case it shall be deemed to be given when the E-mail leaves the E-mail gateway of the sender where it leaves such gateway before 17.00 hours on any Business Day or in any other case at 08.00 hours on the next Business Day after it leaves such gateway and the onus shall be on the sender to prove the time that the E-mail left its gateway.
(c) The addresses and other details of the Parties for notices are, subject to Section 17.2(d):
If to the Licensor, addressed to:
Attention: Xxxxxxx Xxxx (President & Chief Executive Officer)
Address:
Æterna Zentaris, 000 Xxxxx Xxxxx, Xxxxxxxxxx, XX 00000
E-mail address: xxxxx@xxxxxxx.xxx
If to the Licensee, addressed to:
Attention: Chief Legal Officer
Address: 00 Xxxxxxxxx Xxxxxxx, Xxxxxx 0, X00 X000
E-mail address: x.xxxx@xxxxxxxxxxxxxxx.xxx
(d) Any Party to this Agreement may notify the other Party of any change to the address or any of the other details specified in Section 17.2(c), provided that such notification
51
shall only be effective on the date specified in such notice or two Business Days after the notice is given, whichever is later.
17.3 In proving service of any notice in accordance with Section 17.2, it shall be sufficient to prove that the envelope containing the notice was properly addressed and delivered by hand to the relevant address or that the e-mail was sent to the correct e-mail address, as the case may be.
Except as otherwise expressly provided in the Agreement, no other right, express or implied, is granted by the Agreement.
Each party agrees to execute, acknowledge and deliver such further instruments, and to do all such other reasonable acts, as may be necessary or appropriate in order to carry out the purposes and intent of the Agreement.
No amendment, modification or supplement of any provision of the Agreement shall be valid or effective unless made in writing and signed by a duly authorized officer or director of each party.
No provision of the Agreement shall be waived by any act, omission or knowledge of any party or its agents or employees except by an instrument in writing expressly waiving such provision and signed by a duly authorized officer or director of the waiving party. The waiver by either Party of a breach or a default of any provision of this Agreement by the other Party shall not be construed as a waiver of any succeeding breach of the same or any other provision, nor shall any delay or omission on the part of either Party to exercise or avail itself of any right, power or privilege that it has or may have hereunder operate as a waiver of any right, power or privilege by such Party.
The Agreement may be executed in any number of counterparts, each of which need not contain the signature of more than one party but all such counterparts taken together shall constitute one and the same agreement. A signed Agreement received by a party hereto via facsimile will be deemed an original, and binding upon the party who signed it. Facsimile signatures or signatures sent by email attachment or telecopy shall be valid and binding to the same extent as original signatures. This agreement shall not be effective until each party has executed at least one counterpart.
Whenever possible, each provision of the Agreement shall be interpreted in such manner as to be effective and valid under applicable Law, but if any provision of the Agreement is held to be prohibited by or invalid under applicable Law, such provision shall be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of the Agreement.
The relationship between the Licensor and the Licensee created by the Agreement is one of independent contractors and neither party shall have the power or authority to bind or obligate
52
the other. There is no employee-employer relationship or partnership relationship between the Licensor and the Licensee or any of its representatives.
Except as otherwise specifically provided herein, each party shall at their own expense in their respective countries, take such steps as may be required to satisfy any Laws or requirements with respect to declaring, filing, recording or otherwise rendering the Agreement valid.
Each party shall bear its own expenses and costs incurred in the negotiations leading up to and in preparation of the Agreement and of matters incidental to the Agreement.
17.13 Entire Agreement of the Parties
The Agreement (including the Schedules) shall constitute and contain the complete, final and exclusive understanding and agreement of the parties and cancels and supersedes any and all prior negotiations, correspondence, understanding and agreements, whether oral or written, between the Licensor and the Licensee respecting the subject matter thereof.
The Parties exclude the application of any international statutes on the sales of goods, including the United Nations Convention on International Contracts for the Sales of Goods.
The English version of the Agreement shall be deemed the official and governing instrument, notwithstanding any translations thereof.
[Signature Page Follows]
53
Intending to be legally bound, the parties have caused the Agreement to be executed by their duly authorized officers or directors as of the Effective Date.
Aeterna Zentaris GmbH
|
By: |
|||||
|
Name: |
Xxxxxxx Xxxx |
|
|
|
|
|
Title: |
|
|
|
|
|
|
Its: |
CEO |
|
|
|
|
Strongbridge Ireland Limited
|
By: |
|||||
|
Name: |
A. Xxxxx Xxxxx |
|
|
|
|
|
Title: |
|
|
|
|
|
|
Its: |
Director |
|
|
|
|
54
Schedule 1
CMO Contracts
|
Objective / Purpose |
CMO |
Address |
Contract |
Execution Date |
|
Manufacturing of Boc-AEZS-130, packaging, quality control, batch release, stability testing and distribution |
Flamma SpA |
Xxx Xxxxxxxx 00, 00000 Xxxxxxxx x’Xxxxx, Italy |
QA |
2017-09-12 |
|
SA |
n.a. |
|||
|
Manufacturing of AEZS-130 drug substance, packaging, labelling, quality control, batch release and stability testing |
PolyPeptide Laboratories France SAS |
0 xxx xx Xxxxxxxx, 00000 Xxxxxxxxxx, Xxxxxx |
QA |
2017-10-06* |
|
SA |
2013-08-21* |
|||
|
Manufacturing drug product, primary packaging, quality control, stability testing and batch release |
Allphamed Pharbil Arzneimittel GmbH |
Xxxxxxxxxxxxxxxxx 00, 00000 Xxxxxxxxxx, Xxxxxxx |
QA |
2017-09-27 |
|
SA |
2013-08-08* |
|||
|
Labeling, secondary packaging, batch release and distribution |
Corden Pharma GmbH |
Xxxx-Xxxx-Strasse, 68723 Plankstadt, Germany |
QA |
2017-11-21 |
|
SA |
In preparation, draft status |
|||
|
Storage of drug product stability samples |
Pharbil Pharma GmbH |
Xxxxxxxxxxxxx Xxxxxxx 00, 00000 Xxxxxxxxx, Xxxxxxx |
n.a. |
Internally regulated within NextPharma subsidiaries |
|
Batch certification for export from EU and final batch release to US market |
Aeterna Zentaris GmbH |
Xxxxxxxxxxxxxxxxxx 00, 00000 Xxxxxxxxx xx Xxxx, Xxxxxxx |
n.a. |
Internally regulated within Aeterna Zentaris |
|
Storage and distribution (3PL services) |
ICS AmerisourceBergen |
0000 Xxxxxxx Xxxxxxx, Xxxxxx, XX 00000, XXX |
Statements of Work |
In preparation, draft status |
SA: Supply Agreement, QA: Quality Agreement
* Update in progress
55
Schedule 2
Technology Transfer Critical Materials and Timing
To the extent not already in the Data Room:
|
Materials |
Timing for transfer |
|
Copy of a customer facing slide deck for MCO purposes. |
As soon as possible on or after the Effective Date |
|
|
|
|
Copies of any additional draft/final promotional documents. |
As soon as possible on or after the Effective Date |
|
Any medical affairs planning documents generated, including any physician lists, conference planning documents, speaker training materials, etc. |
As soon as possible on or after the Effective Date |
|
Drafts of any presentations or product-related manuscripts in preparation at the time of deal closing |
As soon as possible on or after the Effective Date |
|
|
|
|
Information pertaining to any requests in the last 12 months for supply of drug product or funding to support clinical research or expanded access to drug in the Territory |
As soon as possible on or after the Effective Date |
|
Copies of batch records and certificates of analysis for any Licensor Materials to be transferred to Licensee. This should include the batch records and certificates of analysis of the input materials used to make each batch of Licensor Materials (drug product, AEZS-130 drug substance, and Boc-AEZS-130 intermediate). |
Prior to or upon transfer of the applicable Licensor Material |
|
Copies of all pricing, access and reimbursement related material. Including requests for proposals, analysis and contracts. |
As soon as possible on or after the Effective Date |
56
Schedule 3
FDA Letters
FDA NDA TRANSFER LETTER
Month Date, Year
Xxxx-Xxxx Guettier, MD
Director
Food and Drug Administration
Center for Drug Evaluation and Research
Division of Metabolism and Endocrinology Products
0000-X Xxxxxxxxx Xxxx
Xxxxxxxxxx, XX 00000-0000
Re: NDA #205598 Macimorelin (AEZS-130, formally ARD-07) Granules for oral solution
GENERAL CORRESPONDENCE-Transfer of NDA Ownership
Dear Xx. Xxxxxxxx,
Reference is made to the NDA 205598 Macimorelin (AEZS-130, formally ARD-07) Granules for oral solution. Aeterna Zentaris Inc., hereby submits a General Correspondence-Transfer of NDA ownership to notify that we have transferred ownership of this application from Aeterna Zentaris Inc., to the new owner:
Strongbridge Ireland Limited,
00 Xxxxxxxxx Xxxxxxx, Xxxxxx 0, X00 X000
000 Xxxxxxxxxx Xxxxx, Xxxxx 000, Xxxxxxx, XX 00000
Attn: Xxxxx Xxxxxxxx, Vice President, Regulatory Affairs
e-mail: x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
Phone: 000-000-0000
Effective Month Date, Year, all rights and responsibilities regarding NDA 205598 are transferred to Strongbridge Ireland Limited (“Strongbridge”). Strongbridge will concurrently notify the Agency of its acceptance of ownership of the designation under separate cover. A complete copy of the NDA 205598, including IND #73,196 and Orphan Drug Designation #06-2255, has been provided to Strongbridge for their files.
If there are any questions, or if additional information is required, please contact me at XXX-XXX-XXXX.
Sincerely,
{Name}
{Title}
57
FDA NDA ACCEPTANCE LETTER
Month Date, Year
Xxxx-Xxxx Guettier, MD
Director
Food and Drug Administration
Center for Drug Evaluation and Research
Division of Metabolism and Endocrinology Products
0000-X Xxxxxxxxx Xxxx
Xxxxxxxxxx, XX 00000-0000
Re: NDA #205598 Macimorelin (AEZS-130, formally ARD-07) Granules for oral solution
GENERAL CORRESPONDENCE-Transfer of NDA Ownership
Dear Xx. Xxxxxxxx,
Pursuant to 21 CFR §314.72, Strongbridge Ireland Limited, hereby notifies the FDA of a change in ownership of NDA 205598 Macimorelin. As indicated in correspondence dated XXXX, from Aeterna Zentaris Inc. (copy enclosed), all rights to NDA 205598 Macimorelin have been transferred from Aeterna Zentaris Inc. to Strongbridge Ireland Limited, effective Month Date, Year with the following associated address:
Strongbridge Ireland Limited,
00 Xxxxxxxxx Xxxxxxx, Xxxxxx 0, X00 X000
Attn: Xxxxx Xxxxxxxx, Vice President, Regulatory Affairs
e-mail: x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
Phone: 000-000-0000
Fax: 000-000-0000
Strongbridge Ireland Limited, (“Strongbridge”) has made arrangements with Aeterna Zentaris Inc. to obtain a complete copy of the NDA 205598, including supplements, and records that are required to be kept under 21 CFR 314.81. In addition, Strongbridge reserves the right to request a copy of the application from FDA's files under the fee schedule in 20.45 of FDA's public information regulations. Strongbridge hereby commits to the agreements, promises, and conditions made by the former owner and contained in the application.
Provided herein is a new form FDA 356h signed by the Strongbridge agent of record.
If there are any questions, or if additional information is required, please contact me at 000-000-0000.
Sincerely,
Xxxxx Xxxxxxxx
Vice President, Regulatory Affairs
Strongbridge Biopharma (parent company of Strongbridge Ireland Limited)
000 Xxxxxxxxxx Xxxxx, Xxxxx 000
Xxxxxxx, XX 00000 XXX
Office: 000-000-0000
Mobile: 000-000-0000
x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
CC: Xxxxxx Xxxxxxx, Regulatory Project Manager
58
FDA IND TRANSFER LETTER
Month Date, Year
Xxxx-Xxxx Guettier, MD
Director
Food and Drug Administration
Center for Drug Evaluation and Research
Division of Metabolism and Endocrinology Products
0000-X Xxxxxxxxx Xxxx
Xxxxxxxxxx, XX 00000-0000
RE: IND # 73,196 Macimorelin (AEZS-130, Formerly ARD-07) Granules for Oral Solution
GENERAL CORRESPONDENCE-Transfer of IND Ownership
Dear Xx. Xxxxxxxx,
Reference is made to IND #73,196 originally submitted on February 7, 2007 for Macimorelin (AEZS-130, Formerly ARD-07) Granules for Oral Solution, as a diagnostic for growth hormone deficiency [GHD]. Aeterna Zentaris Inc., hereby submits a General Correspondence-Transfer of IND ownership to notify that we have transferred ownership of this application from Aeterna Zentaris Inc., to a new sponsor:
Strongbridge Ireland Limited,
00 Xxxxxxxxx Xxxxxxx, Xxxxxx 0, X00 X000
Attn: Xxxxx Xxxxxxxx, Vice President, Regulatory Affairs
e-mail: x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
Phone: 000-000-0000
Fax: 000-000-0000
Effective Month Date, Year, all rights and responsibilities regarding IND #73,196 are transferred to Strongbridge Ireland Limited (“Strongbridge”). Strongbridge will concurrently notify the Agency of its acceptance of ownership of the designation under separate cover. A complete copy of the IND #73,196 has been provided to Strongbridge for their files.
If there are any questions, or if additional information is required, please contact me at XXX-XXX-XXXX.
Sincerely,
{Name}
{Title}
59
FDA IND ACCEPTANCE LETTER
Month Date, Year
Xxxx-Xxxx Guettier, MD
Director
Food and Drug Administration
Center for Drug Evaluation and Research
Division of Metabolism and Endocrinology Products
0000-X Xxxxxxxxx Xxxx
Xxxxxxxxxx, XX 00000-0000
Re: IND # 73,196 Macimorelin (AEZS-130, Formerly ARD-07) Granules for Oral Solution
Transfer of IND Ownership
Dear Xx. Xxxxxxxx,
Reference is made to IND #73,196 originally submitted on February 7, 2007 for Macimorelin (AEZS-130, Formerly ARD-07) Granules for Oral Solution, as a diagnostic for growth hormone deficiency [GHD].
Pursuant to 00 XXX §000, Xxxxxxxxxxxx Xxxxxxx Limited, hereby notifies the FDA of a change in ownership of IND #73,196 for Macimorelin. As indicated in correspondence dated XXXX, from Aeterna Zentaris Inc., (copy enclosed), all rights to IND #73,196 for Macimorelin have been transferred from Aeterna Zentaris Inc. to Strongbridge Ireland Limited, effective Month Date, Year with the following associated address:
Strongbridge Ireland Limited,
00 Xxxxxxxxx Xxxxxxx, Xxxxxx 0, X00 X000
Attn: Xxxxx Xxxxxxxx, Vice President, Regulatory Affairs
e-mail: x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
Phone: 000-000-0000
Fax: 000-000-0000
Provided herein is a new form FDA 1571 signed by the Strongbridge U.S. Agent of record.
Strongbridge has made arrangements with Aeterna Zentaris Inc. to obtain a complete copy of the IND #73,196, including amendments, and records. Strongbridge hereby commits to the agreements, promises, and conditions made by the former owner and contained in the application.
If there are any questions, or if additional information is required, please contact me at 000-000-0000.
Sincerely,
Xxxxx Xxxxxxxx
Vice President, Regulatory Affairs
Strongbridge Biopharma (parent company of Strongbridge Ireland Limited)
000 Xxxxxxxxxx Xxxxx, Xxxxx 000
Xxxxxxx, XX 00000 XXX
Office: 000-000-0000
Mobile: 000-000-0000
x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
CC: Xxxxxx Xxxxxxx, Regulatory Project Manager
60
FDA ORPHAN DESIGNATION TRANSFER LETTER
Month Date, Year
Gayatri X. Xxx, MD., JD.
Director, Office of Orphan Products Development
Food and Drug Administration
WO32-5271
00000 Xxx Xxxxxxxxx Xxxxxx
Xxxxxx Xxxxxx, XX 000000-000
RE: Designation #06-2255, Macimorelin Acetate as a Diagnosis of Growth Hormone Deficiency
Transfer of Orphan Drug Designation Ownership
Dear Xx. Xxx,
Reference is made to Orphan Drug Designation (ODD) #06-2255, originally approved on May 14, 2007, for Macimorelin acetate as a Diagnosis of Growth Hormone. Aeterna Zentaris Inc., hereby submits a Transfer of Orphan Drug Designation ownership to notify that we have transferred ownership of this application from Aeterna Zentaris Inc., to a new sponsor:
Strongbridge Ireland Limited,
00 Xxxxxxxxx Xxxxxxx, Xxxxxx 0, X00 X000
Attn: Xxxxx Xxxxxxxx, Vice President, Regulatory Affairs
e-mail: x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
Phone: 000-000-0000
Fax: 000-000-0000
Effective Month Date, Year, all rights and responsibilities regarding ODD #06-2255 are transferred to Strongbridge Ireland Limited. Strongbridge Ireland Limited will concurrently notify the Agency of its acceptance of ownership of the designation under separate cover. A complete copy of the ODD #06-2255 has been provided to Strongbridge Ireland Limited for their files.
If there are any questions, or if additional information is required, please contact me at XXX-XXX-XXXX.
Sincerely,
{Name}
{Title}
61
FDA ORPHAN DESIGNATION ACCEPTANCE LETTER
Month Date, Year
Gayatri X. Xxx, MD., JD.
Director, Office of Orphan Products Development
Food and Drug Administration
WO32-5271
00000 Xxx Xxxxxxxxx Xxxxxx
Xxxxxx Xxxxxx, XX 000000-000
RE: Designation #06-2255, Macimorelin Acetate as a Diagnosis of Growth Hormone Deficiency
Transfer of Orphan Drug Designation Ownership
Dear Xx. Xxx,
Reference is made to Orphan Drug Designation (ODD) #06-2255 originally approved on May 14, 2007 for Macimorelin acetate as a Diagnosis of Growth Hormone.
Pursuant to 21 CFR §316.27, Strongbridge Ireland Limited hereby notifies the FDA of a change in ownership of IND #73,196 for Macimorelin. As indicated in correspondence dated XXXX, from Aeterna Zentaris Inc., (copy enclosed), all rights to IND #73,196 for Macimorelin have been transferred from Aeterna Zentaris Inc. to Strongbridge Ireland Limited (Strongbridge), effective Month Date, Year with the following associated address:
Strongbridge Ireland Limited,
00 Xxxxxxxxx Xxxxxxx, Xxxxxx 0, X00 X000
Attn: Xxxxx Xxxxxxxx, Vice President, Regulatory Affairs
e-mail: x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
Phone: 000-000-0000
Fax: 000-000-0000
Provided herein is a new form FDA 1571 signed by the Strongbridge agent of record.
Strongbridge has made arrangements with Aeterna Zentaris Inc. to obtain a complete copy of the ODD #06-2255, including amendments, and records. Strongbridge hereby commits to the agreements, promises, and conditions made by the former owner and contained in the application.
If there are any questions, or if additional information is required, please contact me at 000-000-0000.
Sincerely,
Xxxxx Xxxxxxxx
Vice President, Regulatory Affairs
Strongbridge Biopharma (parent company of Strongbridge Ireland Limited)
000 Xxxxxxxxxx Xxxxx, Xxxxx 000
Xxxxxxx, XX 00000 XXX
Office: 000-000-0000
Mobile: 000-000-0000
x.xxxxxxxx@xxxxxxxxxxxxxxx.xxx
CC: Xxxxxx Xxxxxxx, Regulatory Project Manager
62
Schedule 4
Licensor Materials to be Transferred to Licensee
API/ Drug substance
|
Batch |
Units |
QC-release ** till |
Shipment from |
Shipment to |
3PL*** |
Delivery date |
Costs |
Total Cost |
|
Batch T* |
937g |
05/2018 |
Polypetide SaS 0 xxx xx Xxxxxxxx 00000 Xxxxxxxxxx Xxxxxx |
Allpharmed PHARBIL GmbH (NextPharma) Xxxxxxxxxxxxx. 00 00000 Xxxxxxxxxx Xxxxxxx |
World Courier (AmerisourceBergen) |
Jun/ 2017 |
360,000 Euro |
360,000€ |
|
Batch U**** |
~1kg |
Planned QC-testing in Feb/2018 |
Polypetide SaS 0 xxx xx Xxxxxxxx 00000 Xxxxxxxxxx Xxxxxx |
Allpharmed PHARBIL GmbH (NextPharma) Xxxxxxxxxxxxx. 00 00000 Xxxxxxxxxx Xxxxxxx |
World Courier (AmerisourceBergen) |
Mar/ 0000 |
x000,000 Euro |
370,000€ |
*: relevant for the first DP-batch in scale (9,000-9,500 sachets)
**: QC-testing against the current Drug substance release specification (D-87575\RS_0830)
***: AeternaZentaris was responsible for the Shipment (Incoterms2010 EXW)
****: relevant for the second DP-batch in scale (9,000-9,500 sachets)
63
Drug product/ finished product
|
Batch |
Units |
Expiry date* |
Shipment from |
Shipment to |
3PL** |
Delivery date |
Costs |
Total Cost |
|
A1710002A |
1197 |
09/2021 |
CordenPharma GmbH Xxxx-Xxxx Xxxxxxx 00000 Xxxxxxxxxx, Xxxxxxx |
ICS Xxxxxx 000 Xxxxxxxxxxxxx Xxxx, Xxxxx 000 XX 00000 XX |
Schenker MHG (CordenPharma is responsible for the shipment; DDP acc. Incoterms2010) |
Feb 7th, 2018 |
44,5 Euro/unit + 6,500 Euro validation fee/batch |
59,766.50€ |
|
A1710003A |
1127 |
09/2021 |
CordenPharma GmbH Xxxx-Xxxx Xxxxxxx 00000 Xxxxxxxxxx, Xxxxxxx |
ICS Xxxxxx 000 Xxxxxxxxxxxxx Xxxx, Xxxxx 000 XX 00000 XX |
Schenker MHG (CordenPharma is responsible for the shipment; DDP acc. Incoterms2010) |
Feb 7th, 2018 |
44,5 Euro/unit + 6,500 Euro validation fee/batch |
56,651.50€ |
|
n/a |
9,000-9,500*** |
n/a |
CordenPharma GmbH Xxxx-Xxxx Xxxxxxx 00000 Xxxxxxxxxx, Xxxxxxx |
ICS Xxxxxx 000 Xxxxxxxxxxxxx Xxxx, Xxxxx 000 XX 00000 XX |
Schenker MHG (CordenPharma is responsible for the shipment; DDP acc. Incoterms2010) |
Apr/ 2018 |
8,9 Euro/unit + 6,500 Euro validation fee/batch |
86,600€-91,050€ |
|
n/a |
9,000-9,500**** |
n/a |
CordenPharma GmbH Xxxx-Xxxx Xxxxxxx 00000 Xxxxxxxxxx, Xxxxxxx |
ICS Xxxxxx 000 Xxxxxxxxxxxxx Xxxx, Xxxxx 000 XX 00000 XX |
Schenker MHG (CordenPharma is responsible for the shipment; DDP acc. Incoterms2010) |
Q3/ 2018 |
8,9 Euro/unit + 6,500 Euro validation fee/batch |
86,600€-91,050€ |
*: QC-testing against the current DrugProduct release specification (D-87575\RS_0974_02) and Finished Product specification (D-87575\PS_1072_01)
**: Document: “Process description for handling and flow of CordenPharma Airfreight shipments”
***: manufacturing of the first validation batch has been started on Jan 12th, 2018; API-batch T is applied
****: manufacturing of the second validation batch is planned in Q3/2018; API-batch U will be applied
64
Schedule 5
Disclosure Schedule
This Disclosure Schedule (this “Disclosure Schedule”) is furnished in connection with
that certain Licence and Assignment Agreement (the “Agreement”), dated on or around 16 January 2018, by and among Aeterna Zentaris GmbH, a corporation incorporated under the laws of Germany (the "Licensor”) and Strongbridge Ireland Limited, a corporation incorporated under the laws of Ireland (the "Licensee").
Capitalized terms used but not defined in this Disclosure Schedule have the meanings set
forth for them in the Agreement. The information in this Disclosure Schedule represents
exceptions to and qualifications of the representations and warranties set forth in the corresponding numbered sections of the Agreement, and is arranged to correspond to the numbered and lettered sections of the Agreement.
If there is any inconsistency between the statements in the body of the Agreement and
those in the Disclosure Schedule (other than specific exceptions set forth as such with respect to
representations or warranties stated in the Agreement), the statements in the body of the
Agreement will control. The mention of any matter or event in this Disclosure Schedule is not an
admission or acknowledgement, in and of itself, that such information is material either
individually or in combination with other matters or events disclosed herein, or has resulted in or
would result in a material adverse effect, or is outside the ordinary course of business. The
information contained in the Disclosure Schedule is disclosed solely for purposes of the
Agreement, and no information contained herein shall be deemed an admission to any third party
of any matter whatsoever, including violation of any law or breach of any agreement.
[Remainder of page intentionally left blank]
65
Schedule 12.2(b) and (c)
Licensor Patent Rights
The following papers identified by Licensee:
(1) EP1572: A novel peptide-mimetic GH secretagogue with potent and selective GH-releasing activity in man by Xxxxxxx et al

(2)
Targeting the Ghrelin Receptor
Orally Active GHS and Cortistatin Analogs
Xxxxxx Xxxxxxxxx et al, Endocrine, vol. 22, no. 1, 13–18, October 2003
(3) GH-releasing hormone and GH-releasing peptide-6 for diagnostic
testing in GH-deficient adults
by Xxxx Xxxxxxx et al; Lancet 2000; 356: 1137.42
66
Schedule 6
Domain Names
1. xxxxxxxx.xxx
2. xxxxxxxx.xxx
67
Schedule 7
Licensor Patent Rights
|
Title: |
"Growth Hormone Secretagogues" |
|||
|
Owner: |
Aeterna Zentaris GmbH |
|||
|
Internal No.: |
99/25 PH |
|
Internal No |
Country |
Application No |
Application Date |
Patent No |
Granted Date |
Expiry Date |
|
US 99/25 PH/2 |
USA |
09/880,498 |
13.06.2001 |
US 6,861,409 B2 |
01.03.2005 |
01.08.2022 |
|
US 99/25 PH/3 |
USA |
10/837,620 |
04.05.2004 |
US 7,297,681 B2 |
20.11.2007 |
01.08.2022 |
|
CA-PCT 99/25 PH |
Canada |
2,407,659 |
13.06.2001 |
2,407,659 |
09.11.2010 |
13.06.2021 |
|
Title: |
"Methods and kits to diagnose Growth Hormone Deficiency" |
|
Owner: |
Aeterna Zentaris GmbH |
|
Internal No.: |
06/05 Z |
|
Internal No |
Country |
Application No |
Application Date |
Patent No |
Granted Date |
Expiry Date |
|
US-PCT 06/05 Z |
USA |
12/279,805 |
19.02.2007 |
US 8,192,719 B2 |
05.06.2012 |
12.10.2027 |
|
Titel: |
"Method of Assessing Growth Hormone Deficiency Comprising Oral Administration Of a Macimorelin Containing Composition and Collecting One or Two Post-administration Samples" |
|
Owner: |
Aeterna Zentaris GmbH |
|
Internal No.: |
17/01 Z |
|
Prio-Anmeldung |
Country |
Application No |
Application Date |
Patent No |
Granted Date |
Expiry Date |
|
US-Prio 17/01 Z |
US |
62/609,059 |
21.12.2017 |
68
Schedule 8
Supply Chain
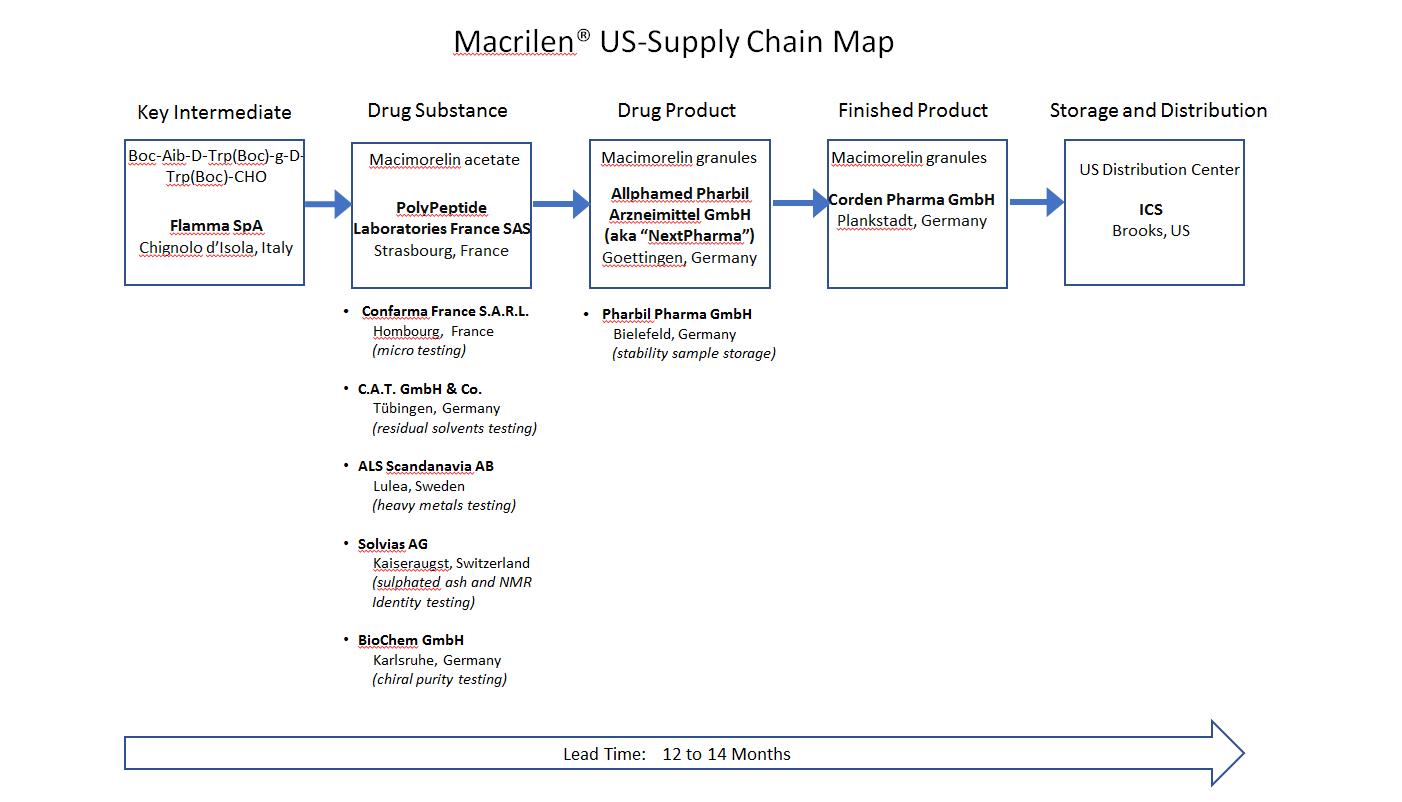
69
Schedule 9
Development Plan
1. Pediatric development plan,
based on the Pediatric Investigation Plan as agreed with the EMA:
o Pediatric development plan to be discussed with FDA
o Study 1: Ascending single oral doses of macimorelin in at least 24 stratified pediatric patients from 2 to less than 18 years of age with suspected GDH
o Study 2: Diagnostic efficacy and safety of macimorelin acetate in at least 50 stratified pediatric patients from 2 to less than 18 years of age with suspected GDH.
o Estimated approx. costs 3 – 3.5 Mio EUR
o Estimated approx. 4 years
2. Phase IV study:
o Collect data to support label change to two blood draws at 45 and 60 min to diagnose AGHD
o A US patent application directed to a Macrilen test with two blood draws was filed 21-Dec-17
o Estimated clinical duration approx. 6 months
o Estimated costs approx. 500 TEUR
70
Schedule 10
The proposal has been approved by EMA, but must be discussed with FDA and adapted if necessary.
Study AEZS-130-P01
Open label, single dose trial to investigate the pharmacokinetics, pharmacodynamics, safety and tolerability of macimorelin acetate after ascending single oral doses of macimorelin in paediatric patients from two to less than 18 years of age with suspected GHD.
Date of completion: by July 2020
Patient number: at least 24 subjects
Study AEZS-130-P02
Open label, single dose trial to determine the diagnostic efficacy and safety of macimorelin acetate in paediatric patients from two to less than 18 years of age with suspected GHD.
Date of completion: July 2022
Patient number: at least 50 subjects
Budget proposal total: US$5,000,000 – 6,000,000
71
