ASSET PURCHASE AGREEMENT THIS ASSET PURCHASE AGREEMENT, dated as of February 21, 2023 (this “Agreement”), is made by and between Tricida, Inc., a Delaware corporation (“Seller”), and Renibus Therapeutics, Inc., a Delaware corporation (“Purchaser”)....

EXECUTION VERSION ASSET PURCHASE AGREEMENT BY AND BETWEEN TRICIDA, INC. AND RENIBUS THERAPEUTICS, INC. DATED AS OF FEBRUARY 21, 2023 Exhibit 10.1

i TABLE OF CONTENTS Page ARTICLE I THE ACQUISITION .................................................................................................1 Section 1.1. Acquired Assets ...........................................................................................1 Section 1.2. Excluded Assets ...........................................................................................2 Section 1.3. Assumed Liabilities .....................................................................................4 Section 1.4. Excluded Liabilities .....................................................................................4 Section 1.5. Assignment of Assigned Contracts ..............................................................6 Section 1.6. Assignment of Acquired Assets ...................................................................7 Section 1.7. Purchase Price; Deposit Funds.....................................................................8 Section 1.8. Withholding .................................................................................................9 Section 1.9. Purchase Price Allocation ............................................................................9 ARTICLE II THE CLOSING ......................................................................................................10 Section 2.1. Closing .......................................................................................................10 Section 2.2. Deliveries at the Closing ............................................................................10 Section 2.3. Contingent Payments .................................................................................11 ARTICLE III REPRESENTATIONS AND WARRANTIES OF SELLER ...............................12 Section 3.1. Qualification, Organization, Subsidiaries ..................................................12 Section 3.2. Authority of Seller .....................................................................................13 Section 3.3. Consents and Approvals ............................................................................13 Section 3.4. No Violations .............................................................................................13 Section 3.5. Books and Records ....................................................................................14 Section 3.6. Assets .........................................................................................................14 Section 3.7. Brokers or Finders......................................................................................14 Section 3.8. Litigation ....................................................................................................14 Section 3.9. Intellectual Property ...................................................................................14 Section 3.10. Material Contracts ......................................................................................15 Section 3.11. Compliance with Laws; Permits ................................................................16 Section 3.12. Environmental and Regulatory Matters .....................................................16 Section 3.13. Taxes ..........................................................................................................18 Section 3.14. Insurance ....................................................................................................18 Section 3.15. Warranties Exclusive .................................................................................18 ARTICLE IV REPRESENTATIONS AND WARRANTIES OF PURCHASER ......................19 Section 4.1. Qualification; Organization .......................................................................19 Section 4.2. Authority of Purchaser ...............................................................................19 Section 4.3. Consents and Approvals ............................................................................20

ii Section 4.4. No Violations .............................................................................................20 Section 4.5. Brokers or Finders......................................................................................20 Section 4.6. Legal Proceedings ......................................................................................20 Section 4.7. Financing....................................................................................................20 Section 4.8. Adequate Assurances Regarding Assigned Contracts ...............................20 Section 4.9. Disclaimer ..................................................................................................20 Section 4.10. Information ................................................................................................21 ARTICLE V COVENANTS ........................................................................................................21 Section 5.1. Conduct of Business Pending Closing .......................................................21 Section 5.2. Access and Information .............................................................................23 Section 5.3. Approvals and Consents; Cooperation; Notification .................................24 Section 5.4. Further Assurances.....................................................................................25 Section 5.5. Update of Seller Disclosure Schedules ......................................................26 Section 5.6. Other Actions .............................................................................................26 Section 5.7. The Sale Order ...........................................................................................26 Section 5.8. Cooperation with Respect to Bankruptcy Court Approvals ......................26 Section 5.9. Bankruptcy Court Filings ...........................................................................26 Section 5.10. Adequate Assurance and Performance ......................................................26 Section 5.11. Post-Closing Confidentiality ......................................................................27 Section 5.12. Transfer of Acquired Assets ......................................................................27 Section 5.13. Transition Services.....................................................................................27 Section 5.14. Permits .......................................................................................................27 Section 5.15. Post-Closing Diligence ..............................................................................28 Section 5.16. Additional Actions .....................................................................................28 Section 5.17. Net Sales Statements ..................................................................................28 Section 5.18. Audit Rights ...............................................................................................28 ARTICLE VI CONDITIONS PRECEDENT ..............................................................................29 Section 6.1. Condition(s) Precedent to Obligation of Seller and Purchaser ..................29 Section 6.2. Conditions Precedent to Obligation of Seller ............................................29 Section 6.3. Conditions Precedent to Obligation of Purchaser ......................................30 ARTICLE VII NO SURVIVAL ..................................................................................................31 Section 7.1. No Survival ................................................................................................31 ARTICLE VIII TERMINATION ................................................................................................31 Section 8.1. Termination ................................................................................................31 Section 8.2. Effect of Termination .................................................................................32

iii ARTICLE IX GENERAL PROVISIONS ....................................................................................33 Section 9.1. Tax Matters ................................................................................................33 Section 9.2. Bulk Sales ..................................................................................................34 Section 9.3. Public Announcements ..............................................................................34 Section 9.4. Notices .......................................................................................................34 Section 9.5. Descriptive Headings; Interpretative Provisions .......................................35 Section 9.6. No Strict Construction ...............................................................................36 Section 9.7. Entire Agreement; Assignment ..................................................................36 Section 9.8. Governing Law; Submission of Jurisdiction; Waiver of Jury Trial ...........36 Section 9.9. Expenses ....................................................................................................37 Section 9.10. Amendment ................................................................................................37 Section 9.11. Waiver ........................................................................................................37 Section 9.12. No Liability; Release .................................................................................37 Section 9.13. Counterparts; Effectiveness .......................................................................38 Section 9.14. Severability; Validity .................................................................................38 Section 9.15. Specific Performance .................................................................................38 Section 9.16. Remedies Cumulative ................................................................................38 Section 9.17. Conflicts; Deal Communications ...............................................................39 Section 9.18. Non-Waiver................................................................................................39 ARTICLE X DEFINITIONS........................................................................................................40 SCHEDULES Schedule 1.1(b) Assigned Contracts Schedule 1.1(h) Designated Parties Schedule 1.1(j) Acquired Assets & Rights Schedule 1.2(m) Excluded Assets & Rights Schedule 1.5(a) Cure Costs Schedule 10 Knowledge of Seller Schedule 11 Knowledge of Purchaser

ASSET PURCHASE AGREEMENT THIS ASSET PURCHASE AGREEMENT, dated as of February 21, 2023 (this “Agreement”), is made by and between Tricida, Inc., a Delaware corporation (“Seller”), and Renibus Therapeutics, Inc., a Delaware corporation (“Purchaser”). Seller and Purchaser are referred to individually herein as a “party” and collectively as the “parties.” Capitalized terms used herein and not otherwise defined shall have the respective meanings set forth in Article X. WHEREAS, Seller is engaged in the business of the development and commercialization of the Product (the “Business”); WHEREAS, on January 11, 2023 (the “Petition Date”), Seller filed a voluntary petition for relief commencing a case (the “Chapter 11 Case”) under Chapter 11 of Title 11 of the United States Code (the “Bankruptcy Code”) in the United States Bankruptcy Court for the District of Delaware (the “Bankruptcy Court”); WHEREAS, Purchaser desires to purchase and accept, and Seller desires to sell, convey, assign, transfer and deliver, or cause to be sold, conveyed, assigned, transferred and delivered, to Purchaser, all of the Acquired Assets, free and clear of all Encumbrances, and Purchaser is willing to assume, and Seller desires to assign and delegate to Purchaser, all of the Assumed Liabilities, all in the manner and subject to the terms and conditions set forth herein and in accordance with Sections 105, 363 and 365 of the Bankruptcy Code and the Sale Order (as defined herein) (such sale and purchase of the Acquired Assets and such assignment and assumption of the Assumed Liabilities, the “Acquisition”); and WHEREAS, the execution and delivery of this Agreement and Seller’s ability to consummate the Transactions are subject to, among other things, the entry of the Sale Order by the Bankruptcy Court under, inter alia, Sections 363 and 365 of the Bankruptcy Code. NOW, THEREFORE, in consideration of the foregoing and the respective representations, warranties, covenants and agreements set forth herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows. ARTICLE I THE ACQUISITION Section 1.1. Acquired Assets. On the terms and subject to the conditions set forth in this Agreement and subject to the entry of the Sale Order, at the Closing (or, with respect to the Assigned Contracts, on the Assignment Effective Date as set forth in Section 1.5), the Seller shall sell, assign, transfer, convey and deliver, or cause to be sold, assigned, transferred, conveyed and delivered, to Purchaser, and Purchaser shall purchase, acquire and accept from Seller, free and clear of all Encumbrances, other than Cure Costs, all right, title and interest of the Seller in, to or under the following assets, properties and rights (collectively, the “Acquired Assets”), in each case, wherever located and whether now existing or hereafter acquired:

2 (a) the Product, and all raw materials, active pharmaceutical ingredients, excipients, work-in-process, semi-finished and finished goods, supplies (including clinical drug supplies), samples, components, packaging materials, and other inventories owned by Seller that are primarily related to the Product (collectively, “Inventory”); (b) the Contracts listed, described or otherwise identified on Schedule 1.1(b), as such schedule may be amended from time to time pursuant to Section 1.5 (such Contracts, the “Assigned Contracts”); (c) the Seller Intellectual Property; (d) to the extent assignable, all rights under non-disclosure or confidentiality, invention and Intellectual Property assignment agreements executed for the benefit of Seller with current or former employees, consultants or contractors of Seller or with third parties to the extent related to the Acquired Assets; (e) all Books and Records to the extent relating to the Acquired Assets, other than Retained Books and Records (the “Acquired Books and Records”); (f) to the extent assignable or transferable, all Regulatory Authorizations and Regulatory Documentation associated with the Product or Seller Intellectual Property (the “Transferred Permits”); (g) all goodwill and other intangible assets primarily associated with the Acquired Assets, including all goodwill associated with the Seller Intellectual Property; (h) other than Avoidance Actions which are governed by clause (i) of this Section 1.1 and Tax related assets which are governed by Section 1.2(e), all rights, claims, rebates, refunds, causes of action, actions, suits or proceedings, hearings, audits, rights of recovery, rights of setoff, rights of recoupment, rights of reimbursement, rights of indemnity or contribution and other similar rights (known and unknown, matured and unmatured, accrued or contingent, regardless of whether such rights are currently exercisable) against the designated parties set forth on Schedule 1.1(h) (collectively, the “Designated Parties”), including all warranties, representations, guarantees, indemnities and other contractual claims (express, implied or otherwise) against the Designated Parties to the extent primarily related to the Acquired Assets or the Assumed Liabilities; (i) all avoidance claims or causes of action available to Seller under Chapter 5 of the Bankruptcy Code (including Sections 544, 545, 547, 548, 549, 550 and 553) or any similar actions under any other applicable Law (whether or not asserted as of the Closing Date) (collectively, “Avoidance Actions”) against the Designated Parties; and (j) the other assets and rights set forth in Schedule 1.1(j). Section 1.2. Excluded Assets. Notwithstanding anything contained in this Agreement to the contrary, Seller shall not be deemed to sell, transfer, assign or convey to

3 Purchaser any right, title and interest of Seller in, to or under the assets, properties and rights of Seller other than the Acquired Assets (such assets, properties and rights other than the Acquired Assets, the “Excluded Assets”). Without limiting the foregoing, Seller shall not be deemed to sell, transfer, assign or convey to Purchaser, and the Acquired Assets shall not include, any of the following assets, properties or rights of Seller: (a) all Cash including, for the avoidance of doubt, all prepaid deposits related to professional fee retainers and cash collateral security, if any; (b) the Equipment; (c) the Lease; (d) all insurance policies of Seller (including all current and prior director and officer or similar fiduciary or errors and omissions insurance policies and all rights thereunder and all proceeds thereof or other insurance policies as set forth on Schedule 3.14); (e) any Tax credits, Tax refunds, Tax deposits, Tax claims, Tax rebates, Tax attributes of Seller and prepaid Tax amounts of the Seller; (f) any shares of capital stock or other equity interests of Seller, any Affiliate thereof or any other Person or any securities convertible into, exchangeable for or exercisable for shares of capital stock or other equity interests of Seller, any Affiliate thereof or any other Person; (g) the Retained Books and Records; (h) professional retainers paid by the Seller to its advisors or representatives in connection with the Chapter 11 Case and the Transactions contemplated herein; (i) all rights, claims or causes of action of Seller arising under this Agreement or the Ancillary Documents, including (subject to Section 9.17) Deal Communications; (j) all rights, claims or causes of action by or in the right of Seller against (i) any current or former director, officer or service provider of any Seller or (ii) Patheon Austria GmbH & Co KG or Thermo Xxxxxx Scientific Inc. and any of their affiliates and subsidiaries (other than PPD Development, L.P.); (k) all Avoidance Actions, other than in respect of the Designated Parties as provided for in Section 1.1(i); (l) any other assets of the Seller that are not lawfully assignable or transferable; and (m) the other assets and rights set forth in Schedule 1.2(m).

4 Section 1.3. Assumed Liabilities. On the terms and subject to the conditions set forth in this Agreement and subject to the entry of the Sale Order, at the Closing (or, with respect to the Assigned Contracts, on the Assignment Effective Date as set forth in Section 1.5), in consideration for the sale, assignment, conveyance, transfer and delivery of the Acquired Assets to Purchaser, Purchaser (or Purchaser’s Designee) shall effective as of the Closing (or, with respect to the Assigned Contracts, effective on the Assignment Effective Date as set forth in Section 1.5) assume from Seller and agree to pay, perform and discharge, when due, in accordance with their respective terms and subject to the respective conditions thereof, only the following Liabilities of Seller (collectively, the “Assumed Liabilities”): (a) all Liabilities with respect to the Acquired Assets (other than the Assigned Contracts) arising out of or relating to Purchaser’s ownership or operation of the Acquired Assets (other than the Assigned Contracts) following the Closing; (b) all Liabilities arising under the Assigned Contracts (in each case, including all Cure Costs); provided, however, that, except for the payment of Cure Costs pursuant to Section 1.5, Purchaser shall not assume or agree to pay, discharge or perform any Liabilities of Seller under or with respect to any Assigned Contracts, including Liabilities arising out of any breach, misfeasance or under any other theory, to the extent relating to Seller’s conduct prior to the Assignment Effective Date; and (c) all Liabilities arising out of the Transferred Permits. Section 1.4. Excluded Liabilities. Notwithstanding anything contained in this Agreement to the contrary, Purchaser shall not assume, be obligated to assume, be deemed to have assumed, or be obliged to pay, perform or otherwise discharge, any Liabilities of Seller, whether existing on the Closing Date or arising thereafter, other than the Assumed Liabilities (such Liabilities other than Assumed Liabilities, the “Excluded Liabilities”). Without limiting the foregoing, Purchaser shall not be obligated to assume, and does not assume, and hereby disclaims all the Excluded Liabilities, including the following Liabilities of Seller or of any predecessor of Seller: (a) any Liability arising out of facts or circumstances in existence prior to the Closing Date and from or related to any breach, default under, failure to perform, torts related to the performance of, violations of Law, infringements or indemnities under, guaranties pursuant to and overcharges, underpayments or penalties on the part of Seller or any of its Affiliates under any Contract, agreement, arrangement or understanding to which Seller or any of its Affiliates is a party prior to the Closing Date; (b) except to the extent included in the Assumed Liabilities, any Liability arising from or related to the operation or condition of the Acquired Assets prior to the Closing or facts, actions, omissions, circumstances or conditions existing, occurring or accruing prior to the Closing; (c) all Taxes of Seller;

5 (d) all Liabilities of Seller relating to legal services, accounting services, financial advisory services, investment banking services or any other professional services (“Professional Services”) performed in connection with this Agreement and any of the Transactions or otherwise on behalf of Seller, and any pre-petition or post-petition claims for such Professional Services, including any brokerage fees, commissions, finders or similar fees incurred by Seller in connection with the Transactions; (e) all Liabilities or claims arising out of, relating to or with respect to the employment or performance of services for, or termination of employment or services for, or potential employment or engagement for the performance of services for, Seller (or any predecessor) of any individual Person or any Person acting as a professional employer organization, employee leasing company or providing similar services on or prior to the Closing (including as a result of the Transactions), including Liabilities or claims for wages, remuneration, compensation, stock options or other equity-based awards, vacation, paid time off, benefits, workers’ compensation, severance (including statutory severance), separation, termination, unfair labor practice, discrimination, classification, or notice pay or benefits (including under COBRA, except to the extent required by applicable Treasury Regulations issued under COBRA), claims under the WARN Act, or any other form of accrued or contingent compensation (including leave entitlements), irrespective of whether such Liabilities or claims are paid or made, as applicable, on, before or after Closing; (f) all Liabilities with respect to any current or former employee of Seller; (g) all Liabilities relating to Excluded Assets; (h) all Liabilities of Seller arising under or pursuant to Environmental Laws, including with respect to any real property owned, operated, leased or otherwise used by Seller, including any Liabilities for noncompliance with Environmental Laws or the release of hazardous materials by Seller on or prior to the Closing, whether known or unknown as of the Closing; (i) all Liabilities arising from or related to any claim, action, arbitration, audit, hearing, investigation, suit, litigation or other proceeding (whether civil, criminal, administrative, investigative, or informal and whether pending or threatened or having any other status) against Seller or any of its Affiliates, or related to the Acquired Assets or the Assumed Liabilities, pending or threatened or with respect to facts, actions, omissions, circumstances or conditions existing, occurring or accruing prior to the Closing Date; (j) all Liabilities of Seller in respect of Indebtedness; (k) all Liabilities arising in connection with any violation of any applicable Law or Order relating to the period prior to the Closing; (l) all Liabilities for fraud, breach of fiduciary duty, misfeasance or under any other theory relating to conduct, performance or non-performance of Seller or any of its current or former directors, officers, employees or service providers;

6 (m) all Liabilities to any equity holder of Seller; (n) all Liabilities arising from state or bankruptcy law theories of recovery, including fraudulent transfer; and (o) any other Liability of Seller that arises in relation to the period prior to the Closing and is not expressly included among the Assumed Liabilities. Section 1.5. Assignment of Assigned Contracts. (a) Schedule 1.5(a) sets forth, with respect to each Designated Contract, all amounts, costs and expenses required by the Bankruptcy Court to cure defaults, if any, under such Designated Contract so that it may be assumed and assigned to Purchaser pursuant to Sections 363 and 365 of the Bankruptcy Code (the “Cure Costs”). In accordance with the Bidding Procedures Order, Seller shall notify non-Seller counterparties to such Assigned Contracts listed on Schedule 1.1(b) as of the date hereof of the deadline to object to the Cure Costs and that, absent a timely objection, the Cure Costs with respect to any Assigned Contract shall be binding upon the non-Debtor parties to such Assigned Contract for all purposes in the Chapter 11 Case and will constitute a final determination of the Cure Costs required to be paid in connection with the assumption and assignment of such Assigned Contract. (b) Notwithstanding the foregoing, prior to the Confirmation Date, Purchaser may identify any Designated Contract that Purchaser desires to have included as, or excluded from being, an Assigned Contract in accordance with Section 1.5(d). Schedule 1.5(b) sets forth, with respect to each Designated Contract, (i) all Cure Costs relating to such Designated Contract and (ii) all out-of-pocket amounts, costs and expenses required by Seller to maintain such Designated Contract and timely pay all amounts owed thereunder when due payable through the Confirmation Date (the “Maintenance Costs”). Purchaser shall reimburse Seller for the approved Maintenance Costs set forth on Schedule 1.5(b) related to each Designated Contract through the earliest of (x) the Confirmation Date, (y) the Assignment Effective Date, and (z) the date on which Purchaser provides written notice to Seller that it has determined not to include such Designated Contract as an Assigned Contract (the earlier of such date, the “Maintenance Termination Date”). With respect to each Designated Contract, from the date hereof through the Maintenance Termination Date, Seller shall (A) timely pay all Maintenance Costs related to such Designated Contract, (B) use reasonable best efforts to comply in all material respects with its obligations thereunder and maintain such Designated Contract in full force and effect, and (C) not materially modify, amend, extend or terminate any Designated Contract or waive, release or assign any rights, obligations, or claims thereunder without the prior written consent of Purchaser. Notwithstanding the foregoing sentence, Seller has no obligation to pay any Cure Costs or Maintenance Costs that are not reimbursed by the Purchaser. (c) To the maximum extent permitted by the Bankruptcy Code and subject to the other provisions of this Section 1.5 and the Sale Order, on the Assignment Effective Date, (x) Seller shall assign the Assigned Contracts pursuant to Section 365 of the Bankruptcy Code and the Sale Order, subject to the provision of adequate assurance by Purchaser as may be required under

7 Section 365 of the Bankruptcy Code and payment by Purchaser of the Cure Costs, in respect of the Assigned Contracts and (y) Purchaser shall pay promptly all Cure Costs (if any) in connection with such assumption and assignment (as agreed to among Purchaser and Seller or as determined by the Bankruptcy Court) and assume and perform and discharge the Assumed Liabilities (if any) under the Assigned Contracts, pursuant to the Sale Order and the Bill of Sale & Assignment and Assumption Agreement. (d) Notwithstanding anything in this Agreement to the contrary, Purchaser may, following good faith consultation with Seller, amend or revise Schedule 1.1(b) setting forth the Assigned Contracts, in order to add any Designated Contract to, or eliminate any Designated Contract from, such schedule at any time during the period commencing from the date hereof and ending on the day prior to the Confirmation Date (the “Designation Deadline”). Automatically upon the addition of any Designated Contract to Schedule 1.1(b), it shall be an Assigned Contract for all purposes of this Agreement. If Purchaser adds one or more Designated Contracts to Schedule 1.1(b) after the date hereof and Seller has not previously notified the non-Seller counterparties to such Contracts pursuant to Section 1.5(a) (such Contracts, the “Additional Assigned Contracts”), Seller shall file any supplemental motion required to assume and assign such Additional Assigned Contracts and shall provide such supplemental notice as is required, and the hearing with respect to the assumption and assignment of such Additional Assigned Contracts may occur after the Sale Hearing. Automatically upon the removal of any Designated Contract from Schedule 1.1(b), such Designated Contract shall be an Excluded Asset (and for the avoidance of doubt shall cease to be an Assigned Contract) for all purposes of this Agreement, and no Liabilities arising thereunder shall be assumed or borne by Purchaser. (e) Following the Closing, for all purposes hereunder, the Acquired Assets shall be deemed to include the Assigned Contracts only after the Assigned Contracts (if any) are assigned to Purchaser on the Assignment Effective Date. Section 1.6. Assignment of Acquired Assets. Notwithstanding anything contained in this Agreement to the contrary, this Agreement shall not constitute an agreement to assign or transfer any Acquired Asset, if, notwithstanding the provisions of Sections 363 and 365 of the Bankruptcy Code, an attempt at assignment or transfer thereof, without the consent or approval required or necessary for such assignment or transfer, would constitute a violation of Law or a breach of an Assigned Contract or Transferred Permit. If, notwithstanding the provisions of Sections 363 and 365 of the Bankruptcy Code and the commercially reasonable efforts of Seller, such consent or approval is required but not obtained with respect to an Acquired Asset, neither Seller nor Purchaser shall be in breach of this Agreement nor shall the Purchase Price be adjusted nor (but subject to the termination rights set forth in Section 8.1) shall the Closing be delayed in respect of such Acquired Asset; provided, however, if the Closing occurs, then, with respect to any Acquired Asset for which consent or approval is required but not obtained, from and after the Closing for a period of no more than six (6) months, Seller shall reasonably cooperate, at Purchaser’s sole cost and expense, with Purchaser in any reasonable arrangement that Purchaser may request in its sole discretion to provide Purchaser with all of the benefits of the applicable Acquired Asset, including enforcement for the benefit of Purchaser of any and all rights of Seller against any party to the applicable Acquired Asset arising out of the breach or cancellation thereof

8 by such party; provided, however, to the extent that any such arrangement has been made at Purchaser’s direction to provide Purchaser with the benefits of the applicable Acquired Asset, from and after the Closing, Purchaser shall be responsible for, and shall promptly pay and perform all payment and other obligations in connection with such Acquired Asset (all of which shall constitute, and shall be deemed to be, Assumed Liabilities hereunder) to the same extent as if such Acquired Asset had been assigned or transferred at Closing. Any assignment to Purchaser of any Acquired Asset that shall, notwithstanding the provisions of Sections 363 and 365 of the Bankruptcy Code, require the consent or approval of any Person for such assignment as aforesaid shall be made subject to such consent or approval being obtained. Notwithstanding anything to the contrary contained herein, Purchaser shall reimburse, indemnify and hold harmless Seller and/or their Affiliates from any and all Liabilities incurred by Seller and/or their Affiliates in connection with any action taken by Seller at Purchaser’s or its Affiliates’ request pursuant to this Section 1.6. Section 1.7. Purchase Price; Deposit Funds. (a) The aggregate consideration for the Acquired Assets (the “Purchase Price”) shall be the sum of the following: (i) an amount in cash equal to $250,000 (“Base Cash Amount”), subject to Section 1.7(b); (ii) the assumption of the Assumed Liabilities including the assumption by Purchaser (or assumption by Purchaser’s Designee) of the obligation to pay the applicable counterparties of the applicable Assigned Contracts, the Cure Costs; and (iii) the right to receive the Contingent Payments as and to the extent they become due pursuant to Section 2.3 below. (b) Simultaneously with the execution of this Agreement, the parties shall execute the escrow agreement, in form and substance reasonably acceptable to Seller and Purchaser (the “Escrow Agreement”), with an escrow agent reasonably satisfactory to Seller and Purchaser (the “Escrow Agent”), and Purchaser shall deposit into escrow with the Escrow Agent an amount equal to $25,000 (such amount, together with all interest and other earnings accrued thereon, the “Deposit Funds”), by wire transfer of immediately available funds pursuant to the terms of the Escrow Agreement. The Deposit Funds shall be held as a trust fund and shall not be subject to any lien, attachment, trustee process or any other judicial process of any creditor of Seller or Purchaser. The Deposit Funds shall be released by the Escrow Agent and delivered to either (x) Purchaser or (y) Seller, as follows: (i) if the Closing shall occur, the Deposit Funds shall be applied towards the portion of the Base Cash Amount payable by Purchaser to Seller pursuant to Section 1.7(a)(i) and in such case, the Deposit Funds shall be released to Seller on the Closing Date (and Purchaser shall take all action necessary to cause such release to Seller by wire transfer of immediately available funds to such account of Seller set forth in the Escrow Agreement);

9 (ii) if this Agreement is terminated by Seller pursuant to Section 8.1(b)(iii) or Section 8.1(b)(iv), then the Seller shall have the right (in addition to any and all other rights which the Seller may have at law or in equity in connection with this Agreement) to retain the Deposit Funds, and the Deposit Funds shall be released to Seller within two (2) Business Days following such termination (and the parties shall take all action necessary to cause such release to Seller by wire transfer of immediately available funds to such account of Seller set forth in the Escrow Agreement); or (iii) if this Agreement is terminated other than in a manner provided by Section 1.7(b)(ii), the Deposit Funds shall be released to Purchaser within two (2) Business Days after such termination (and the parties shall take all action necessary to cause such release to Purchaser by wire transfer of immediately available funds to such account of Purchaser set forth in the Escrow Agreement). Section 1.8. Withholding. Notwithstanding anything to the contrary in this Agreement, Purchaser shall be entitled to deduct and withhold from any consideration payable hereunder such amounts as are required to be deducted and withheld with respect thereto under the Code or any other Tax Law. To the extent that amounts are so deducted and withheld, such amounts shall be treated for all purposes of this Agreement as having been paid to the Person in respect of which such deduction or withholding was made. Notwithstanding the foregoing, Purchaser shall: (i) consult with Seller in good faith prior to withholding any amounts payable to Seller, (ii) promptly, and in any event no later than five (5) Business Days prior to the date of the applicable payment, notify Seller in writing if Purchaser determines that any withholding or deduction is required under the Code or any applicable Tax Law with respect to any portion of payment to Seller (which written notice shall (x) include a copy of the calculation of the amount to be deducted and withheld and (y) identify any applicable provision of the Code or any applicable Tax Law pursuant to which such deduction or withholding is required), and (iii) provide Seller with reasonable opportunity to provide such forms or other evidence that would eliminate or reduce any such required deduction or withholding. Section 1.9. Purchase Price Allocation. The parties agree to allocate for Tax purposes (and, as applicable, to cause their respective Affiliates to allocate for Tax purposes) the Purchase Price and any other amounts treated as additional consideration for Tax purposes among the Acquired Assets in accordance with the following procedures and, to the extent applicable, in accordance with Section 1060 of the Code and the Treasury Regulations promulgated thereunder. Within one hundred twenty (120) days after the Closing Date, Purchaser shall deliver to Seller a proposed allocation of the Base Cash Amount, the Assumed Liabilities (to the extent properly taken into account for income Tax purposes) and any other amounts treated as additional consideration for income Tax purposes as of the Closing Date (the “Allocation”). If Seller notifies Purchaser in writing of any reasonable objections to one or more items reflected in the Allocation within thirty (30) days after Purchaser’s delivery thereof, Purchaser shall consider in good faith any such reasonable objections to the Allocation so provided by Seller. Each of the parties (a) shall (and shall cause its Affiliates to) prepare and file all Tax Returns (and Internal Revenue Service Forms 8594) in a manner consistent with the Allocation and (b) shall not (and shall cause its Affiliates not to) take any position on any Tax Return or in connection with any Tax proceeding

10 inconsistent with the Allocation, in each case, except to the extent otherwise required by applicable Law or by a “determination” within the meaning of Section 1313(a) of the Code (or any analogous provision of applicable state, local or non-U.S. Law). ARTICLE II THE CLOSING Section 2.1. Closing. Upon the terms and subject to the conditions hereof, the closing of the sale of the Acquired Assets and the assumption of the Assumed Liabilities contemplated hereby (the “Closing”) shall take place remotely by exchange of documents and signatures via email or other manner as may be mutually agreed upon by Xxxxxxxxx and Seller, at 10:00 a.m. Pacific Time as soon as possible (and in any event within two (2) Business Days) after the conditions set forth in ARTICLE VI have been satisfied or (if permissible) waived (except for such conditions that, by their nature, are to be satisfied at the Closing, but subject to the satisfaction or (if permissible) waiver thereof at the Closing), or at such time, date and place as the parties may mutually agree (the date of the Closing being herein referred to as the “Closing Date”). For purposes of this Agreement, upon the occurrence of the Closing, the Closing shall be deemed to have occurred at 12:01 a.m. Pacific Time, on the Closing Date. Section 2.2. Deliveries at the Closing. (a) At the Closing, Seller shall deliver to Purchaser: (i) a duly executed bill of sale and assignment and assumption agreement, in form and substance reasonably acceptable to Seller and Purchaser (the “Bill of Sale & Assignment and Assumption Agreement”), transferring the Acquired Assets and Assumed Liabilities to Purchaser; provided, that the assignment and assumption of the Assigned Contracts shall be effective upon the Confirmation Date rather than the Closing Date; (ii) duly executed assignments of Seller Intellectual Property, in form and substance reasonably acceptable to Seller and Purchaser (the “Intellectual Property Assignment Agreements”); (iii) such other instruments of assignment or conveyance duly executed by Seller as shall be reasonably requested or reasonably necessary to transfer the Acquired Assets to Purchaser in accordance with this Agreement; (iv) electronic copies of all Acquired Books and Records in Seller’s possession that relate to the development and manufacturing of the Products, including but not limited to all Regulatory Documentation and all clinical and primary source data; (v) a copy of all materials made available to the Purchaser in the electronic data room set up by the Seller in connection with this Agreement; (vi) a duly executed IRS Form W-9 from Seller; and

11 (vii) the certificates described in Section 6.3(c). (b) At the Closing, Purchaser shall deliver to Seller: (i) the Base Cash Amount (including causing any portion of the Base Cash Amount to be paid by release of the Deposit Funds to Seller to be released to Seller) by wire transfer of immediately available funds to an account or accounts designated by Seller; (ii) the Bill of Sale & Assignment and Assumption Agreement, duly executed by Purchaser; (iii) the Intellectual Property Assignment Agreements, duly executed by Xxxxxxxxx; (iv) the certificate(s) described in Section 6.2(c); and (v) evidence of the payment of all Cure Costs to the applicable Assigned Contract counterparties. Section 2.3. Contingent Payments. (a) Subject to adjustment pursuant to Section 2.3(e), Purchaser shall make or cause to be made a one-time milestone payment to Seller of $2,500,000 (the “FDA Milestone Payment”) within thirty (30) days after the first achievement, by Purchaser or any other Purchaser Party, of approval by the FDA of an NDA for a Product (the “FDA Milestone Event”), provided that the Product or any aspect of its composition of matter, method of use in the relevant Indication or its formulation is claimed, at the time of achievement, by any Valid Claim in the United States. The FDA Milestone Payment is payable one time only, regardless of the number of Products to achieve the FDA Milestone Event. Under no circumstances shall Purchaser be obligated to pay the Seller more than $2,500,000 pursuant to this Section 2.3(a). (b) Subject to Section 2.3(c), Purchaser shall make the following one-time milestone payments to Seller within thirty (30) days after the end of the Calendar Quarter in which the Aggregate Net Sales of the Product in the Territory first exceed the values indicated below. For clarity, the milestone payments in this Section 2.3(b) shall be additive, such that if more than one milestone specified below is achieved in the same Calendar Quarter, then the milestone payments for all such milestones shall be payable. Each milestone payment set forth below shall be payable only once, regardless of the number of times that the Aggregate Net Sales of the Product in the Territory exceed the indicated values. For clarity, the total milestone payments under this Section 2.3(b) shall in no event exceed $150,000,000.
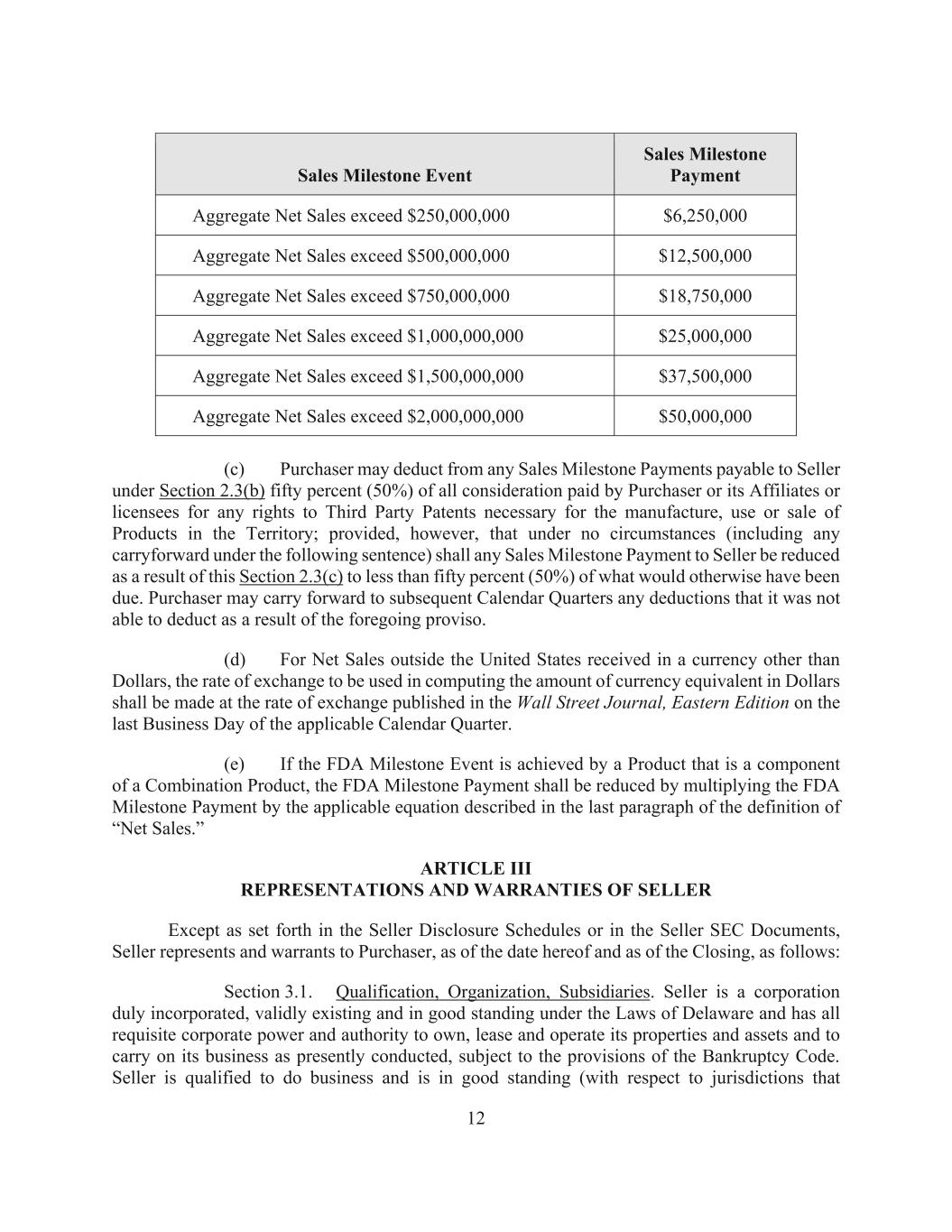
12 Sales Milestone Event Sales Milestone Payment Aggregate Net Sales exceed $250,000,000 $6,250,000 Aggregate Net Sales exceed $500,000,000 $12,500,000 Aggregate Net Sales exceed $750,000,000 $18,750,000 Aggregate Net Sales exceed $1,000,000,000 $25,000,000 Aggregate Net Sales exceed $1,500,000,000 $37,500,000 Aggregate Net Sales exceed $2,000,000,000 $50,000,000 (c) Purchaser may deduct from any Sales Milestone Payments payable to Seller under Section 2.3(b) fifty percent (50%) of all consideration paid by Purchaser or its Affiliates or licensees for any rights to Third Party Patents necessary for the manufacture, use or sale of Products in the Territory; provided, however, that under no circumstances (including any carryforward under the following sentence) shall any Sales Milestone Payment to Seller be reduced as a result of this Section 2.3(c) to less than fifty percent (50%) of what would otherwise have been due. Purchaser may carry forward to subsequent Calendar Quarters any deductions that it was not able to deduct as a result of the foregoing proviso. (d) For Net Sales outside the United States received in a currency other than Dollars, the rate of exchange to be used in computing the amount of currency equivalent in Dollars shall be made at the rate of exchange published in the Wall Street Journal, Eastern Edition on the last Business Day of the applicable Calendar Quarter. (e) If the FDA Milestone Event is achieved by a Product that is a component of a Combination Product, the FDA Milestone Payment shall be reduced by multiplying the FDA Milestone Payment by the applicable equation described in the last paragraph of the definition of “Net Sales.” ARTICLE III REPRESENTATIONS AND WARRANTIES OF SELLER Except as set forth in the Seller Disclosure Schedules or in the Seller SEC Documents, Seller represents and warrants to Purchaser, as of the date hereof and as of the Closing, as follows: Section 3.1. Qualification, Organization, Subsidiaries. Seller is a corporation duly incorporated, validly existing and in good standing under the Laws of Delaware and has all requisite corporate power and authority to own, lease and operate its properties and assets and to carry on its business as presently conducted, subject to the provisions of the Bankruptcy Code. Seller is qualified to do business and is in good standing (with respect to jurisdictions that

13 recognize such concept) as a foreign corporation or other entity in each jurisdiction where the ownership, leasing or operation of its assets or properties or conduct of its business requires such qualification, except where the failure to be so qualified or, where relevant, in good standing, would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. Section 3.2. Authority of Seller. Seller has all requisite corporate power and authority to execute and deliver and, subject to the entry and effectiveness of the Sale Order, to perform its obligations under this Agreement and each of the Ancillary Documents to which Seller is a party. The execution, delivery and performance of this Agreement and such Ancillary Documents by Seller and the consummation of the Transactions by the Seller have been duly and validly authorized and approved by all requisite corporate action of Seller, and subject to entry of the Sale Order no other corporate proceedings (pursuant to Seller’s organizational documents or otherwise) on the part of Seller is necessary to authorize the consummation of, and to consummate, the Transactions. Subject to the entry and effectiveness of the Sale Order, this Agreement and each such Ancillary Document have been, or at or prior to Closing (as the case may be) will be, duly and validly executed and delivered by Seller to the extent a party thereto, and, assuming the due authorization, execution and delivery of this Agreement and each such Ancillary Document by Purchaser, as applicable, constitute (except as such enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to creditors’ rights generally or general principles of equity or as contemplated by the Chapter 11 Case) a valid and binding agreement of Seller, enforceable against Seller in accordance with its terms. Section 3.3. Consents and Approvals. Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, and subject to the Sale Order, no consent, approval or authorization of, or declaration, filing or registration with, any Governmental Entity is necessary or required to be made or obtained by Seller or its Affiliates in connection with the execution, delivery and performance of this Agreement and the Ancillary Documents to which Seller is a party and the consummation of the Transactions, except for the filing of the Intellectual Property Assignment Agreements as applicable and the certain filings required to effectuate the transfer of the Transferred Permits or compliance with any applicable requirements of the Chapter 11 Case. Section 3.4. No Violations. Except as a result of the Chapter 11 Case or as described in Section 3.4 of the Seller Disclosure Schedule, neither the execution, delivery or performance of this Agreement and the Ancillary Documents by Seller nor the consummation by Seller of the Transactions will (a) conflict with or result in any violation or breach of any provisions of the certificate of incorporation, bylaws or other organizational documents of Seller, (b) conflict with or violate any Order or Law applicable to Seller or (c) result in the creation or imposition of any Encumbrance on any Acquired Asset, except in each case of the foregoing clauses (b) and (c), for breaches, violations, defaults or terminations that (x) would not reasonably be expected to have, a Material Adverse Effect or (y) are excused by or unenforceable as a result of the entry or effectiveness of the Sale Order.

14 Section 3.5. Books and Records. The Books and Records maintained with respect to the Business accurately and fairly reflect, in all material respects, the assets and Liabilities of Seller with respect to the Business. Section 3.6. Assets. Seller has good, valid, and marketable title to, or in the case of property leased by Seller, a valid leasehold interest in all of the Acquired Assets, free and clear of all Encumbrances other than Cure Costs. Section 3.7. Brokers or Finders. Other than as set forth on Section 3.7 of the Seller Disclosure Schedule, Seller has not employed or engaged any investment banker, broker or finder who is entitled to any fee or any commission in connection with this Agreement, the Ancillary Documents or the Transactions for which Purchaser is liable. Section 3.8. Litigation. Except as set forth in Section 3.8 of the Seller Disclosure Schedule and except for the Chapter 11 Case, there are no Actions pending or, to the Knowledge of Seller, threatened in writing against Seller relating to the Business or the Acquired Assets by or before, and there are no Orders outstanding with, any Governmental Entity that would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. Section 3.9. Intellectual Property. (a) Section 3.9(a) of the Seller Disclosure Schedule sets forth a true and complete list, as of the date hereof, of: (i) all Patents owned by Seller and included in the Seller Intellectual Property; (ii) all Trademarks owned by and included in the Seller Intellectual Property, and (iii) all Copyrights owned by and included in the Seller Intellectual Property, in each case including the jurisdiction in which each item has been registered or filed, application or serial number or similar identifier, the filing date, the applicable issuance, registration or grant date. (b) Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, all necessary filing, examination, registration, maintenance, annuity and renewal fees due or actions required (including proofs and working or use) in connection with the Seller Intellectual Property listed or required to be listed on Section 3.9(a) of the Seller Disclosure Schedule and having a due date on or before the date hereof have been paid or taken, as the case may be, and except as set forth in Section 3.9(b) of the Seller Disclosure Schedule, none of the Seller Intellectual Property is subject to any filing, examination, registration, maintenance, annuity and renewal fees falling due or actions required (including proofs of working or use) at the time of Closing. (c) Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, Seller has complied with all applicable Law in filing and prosecuting each item of the Seller Intellectual Property owned or licensed by Seller and all filings,

15 payments and other actions required to be made or taken to maintain each item thereof in full force and effect have been made by the applicable deadline. (d) Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, no interference, opposition, reissue, reexamination or other Action of any nature is or has been pending or, to the Knowledge of Seller, threatened, in which the scope, validity or enforceability of any Seller Intellectual Property is being, has been or could reasonably be expected to be contested or challenged, and no Actions are pending or, to the Knowledge of Seller, threatened which allege that Seller is infringing, misappropriating, diluting or otherwise violating the Intellectual Property of any Person. To the Knowledge of Seller, Xxxxxx has not received any written charge, complaint, claim, demand, notice or other written communication or notice from any Person claiming that the operation of the Business infringes, misappropriates, or violates any Intellectual Property of any Person. Section 3.10. Material Contracts. (a) Section 3.10(a) of the Seller Disclosure Schedule contains a true and complete list of each Contract described below in this Section 3.10(a) under which any of the Acquired Assets are bound or affected and to which Seller is a party (the “Material Contracts”): (i) each Contract relating to the Acquired Assets that (A) grants any option, right of first refusal, right of first offer or similar rights to any customer, vendor, supplier, distributor, contractor, collaborator, or other Person or (B) contains a covenant expressly limiting in any material respect the freedom of Seller (or that would limit in any material respect the freedom of Purchaser after the Closing) to engage in any business with any Person or in any geographic area or to compete with any Person; (ii) the contracts listed in Schedule 1.5(b); (iii) any Contract relating to the sale or disposition of Acquired Assets (other than a sale or disposition of Inventory in the Ordinary Course of Business); (iv) any Contract providing for the development of any material Seller Intellectual Property; and (v) any Contract that grants any ownership interest, any license, sublicense or other option or right to any Seller Intellectual Property or by which Seller is required to grant to any Person any right or license, any covenant not to assert/sue, release or other immunity from suit under or any other rights, to any Seller Intellectual Property. (b) True and complete copies of each Material Contract in effect as of the date hereof, including all amendments, modifications and waivers relating thereto, have been made available to Purchaser prior to the date hereof. To the Knowledge of Seller, no other party to any Material Contract is in breach of or default under the terms of any such Contract and no event exists which upon notice or the passage of time, or both, would reasonably be expected to (other than as a result of the Chapter 11 Case and any related bankruptcy filings) (i) give rise to any

16 material default, in the performance by Seller, or, to the Knowledge of Seller, by any other party under any of the Material Contracts or (ii) cause or permit the acceleration or other changes of any right or obligation or the loss of any benefit thereunder. Each of the Material Contracts is a legal, valid, binding and enforceable obligation of Seller and, to the Knowledge of Seller, of each other party thereto, and is in full force and effect. Seller has not received any written notice of any termination, default or event that with notice or lapse of time, or both, would constitute a default by Seller under any Material Contract, except for such defaults arising in connection with the Chapter 11 Case and any related bankruptcy filings or that otherwise, individually or in the aggregate, would not reasonably be expected to have a Material Adverse Effect. Section 3.11. Compliance with Laws; Permits. (a) Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, Seller, with respect to operation of the Business and the ownership and use of the Acquired Assets, is and during the past three (3) years has been in compliance with all Laws applicable to the Business and in possession of all Permits necessary for Seller to own, lease and use its properties and assets or to carry on its businesses at the relevant time. Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, all Permits currently held by Seller are in full force and effect, no default (with or without notice, lapse of time or both) has occurred under any such Permit, and Seller has not received any written notice from any Governmental Entity threatening to suspend, revoke, withdraw or modify any such Permit. (b) Neither Seller nor, to the Knowledge of Seller, any director, officer or employee acting on behalf of Seller, has during the past three (3) years (i) taken any action in violation of any applicable Anti-Corruption Law, (ii) offered, authorized, provided or given any payment or thing of value to any Person for the purpose of influencing any act or decision of such Person to unlawfully obtain or retain business or other advantage or (iii) taken any other action that would constitute an offer to pay, a promise to pay or a payment of money or anything else of value, or an authorization of such offer, promise or payment, directly or indirectly, to any representative of another company or entity in the course of their business dealings with Seller, in order to unlawfully induce such Person to act against the interest of his or her employer or principal. Section 3.12. Environmental and Regulatory Matters. (a) Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect: (i) Seller is, and has been during the past three (3) years, in compliance with all applicable Environmental Laws imposing obligations on or otherwise related to the Business, Product, Assumed Liabilities and Acquired Assets; (ii) Seller possesses all material permits and approvals issued pursuant to any applicable Law relating to the protection of the environment or, as such relates to exposure to Hazardous Substances, to health and safety that are required to conduct the Business, and are, and have been during the past three (3) years, in compliance with all such permits and approvals; (iii) no releases of Hazardous Substances have occurred at, on, from or under any real property currently leased by Seller in a manner that would

17 reasonably be expected to result in a Liability under any Environmental Laws; (iv) Seller has not received any written claim or notice from any Governmental Entity or other Person alleging that Seller is or may be in violation of or liable under, any Environmental Law; and (v) Seller has not entered into or agreed to any consent decree or order and is not subject to any judgment, decree or judicial order relating to compliance with Environmental Laws or the investigation, sampling, monitoring, treatment, remediation, removal or clean-up of Hazardous Substances. (b) Seller has made available to Purchaser true and complete copies of all material Regulatory Documentation and Regulatory Authorizations from or with the FDA and all other applicable Regulatory Authorities filed, submitted, exchanged, or held by Seller relating to the Product or necessary or useful to conduct the Business. Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, all such Regulatory Authorizations, if any, are (i) in full force and effect, (ii) validly registered and on file with applicable Regulatory Authorities, (iii) in compliance with all material filing and maintenance requirements, and (iv) in good standing, valid and enforceable. Seller has fulfilled and performed all of its material obligations with respect to such Regulatory Authorizations, and no event has occurred which allows, or after notice or lapse of time would allow, lapse, revocation, or termination thereof. (c) Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, (i) Seller is in compliance in all material respects with all applicable Health Laws that affect the Acquired Assets, the Assumed Liabilities or the Business, (ii) as of the date of this Agreement, Seller has not received any written or oral notice or other communication from any Regulatory Authority (A) withdrawing or placing any application or authorization applicable to the Product on “clinical hold” or requiring the termination or suspension or investigation of any pre-clinical studies or clinical trials of the Product or (B) alleging any violation of any Health Law and (iii) there are no investigations, suits, claims, Actions or proceedings pending, or to the Knowledge of Seller, threatened which allege any violation by Seller or any third party engaged by Seller with respect to the Product of any such Health Law. (d) All clinical trials conducted or being conducted with respect to the Products by or at the direction of Seller have been and are being conducted in compliance in all respects with the required experimental protocols, procedures and controls and in all material respects with all applicable Health Laws. Except as set forth at Section 3.12(d) of the Seller Disclosure Schedule, no clinical trial conducted by or, on behalf of, Seller has been terminated or suspended by any Regulatory Authority and Seller has not received any notification or other communication from any institutional review board, ethics committee or safety monitoring committee raising any issues that may result in a clinical hold or otherwise delay, materially restrict or otherwise limit or impair the use of any clinical studies proposed or currently conducted by, or on behalf of, Seller, or in which Seller has participated and, to the Knowledge of Seller, no such action has been threatened. (e) None of Seller or, to the Knowledge of Seller, any Person acting on Seller’s behalf has, with respect to the Product, (i) been subject to a Regulatory Authority shutdown or import or export prohibition or (ii) received any FDA Form 483, or other written Regulatory Authority notice of inspectional observations, “warning letters,” “untitled letters” or written

18 demand or written request to make any change to any Product or any processes or procedures, or any similar correspondence from any Regulatory Authority alleging or asserting non-compliance with any applicable Health Law or Regulatory Authorization and, to the Knowledge of Seller, no Regulatory Authority is considering such action. Section 3.13. Taxes. (a) (i) All income and other material Tax Returns required to be filed with respect to the Business, the Acquired Assets and Assumed Liabilities have been timely filed with the appropriate Taxing Authority in all jurisdictions in which such Tax Returns are required to be filed (after giving effect to any valid extensions of time in which to make such filings), and all such Tax Returns are true, complete and correct in all material respects; and (ii) all material Taxes payable with respect to the Business, the Acquired Assets and Assumed Liabilities, whether or not shown on any Tax Return, have been timely paid. (b) No audit or other proceeding with respect to any Taxes or Tax Returns with respect to the Business, the Acquired Assets or Assumed Liabilities is currently in progress, or has been proposed or threatened in writing. (c) Seller has not received written notice of any Tax deficiency outstanding, proposed or assessed, nor has Seller executed any waiver of any statute of limitations in respect of Taxes nor agreed to any extension of time with respect to a Tax assessment, collection or deficiency, in each case, with respect to the Business, the Acquired Assets or Assumed Liabilities. (d) There are no material liens for Taxes upon any of the Acquired Assets. (e) None of the Acquired Assets constitutes stock, partnership interests or any other equity interest in any Person for U.S. federal income Tax purposes. Section 3.14. Insurance. Section 3.14 of the Seller Disclosure Schedule sets forth with respect to the Business a complete and accurate list of the insurance policies of Seller as of the date hereof. Except as would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect and except as a result of the Chapter 11 Case, (a) all insurance policies set forth on Section 3.14 of the Seller Disclosure Schedule are in full force and effect and are valid and enforceable, (b) Seller is not in material breach of or default under any such insurance policies, (c) Seller has not taken any action or failed to take any action that (with or without notice or lapse of time, or both), would constitute such a breach or default or permit termination or modification of any of the insurance policies, and (d) all premiums due thereunder have been paid. There are no material claims under any of the insurance policies set forth on Section 3.14 of the Seller Disclosure Schedule for which coverage has been denied or disputed by the applicable insurance carrier (other than a customary reservation of rights notice). Section 3.15. Warranties Exclusive. EXCEPT AS EXPRESSLY SET FORTH IN THIS ARTICLE III (AS MODIFIED BY THE SELLER DISCLOSURE SCHEDULE) OR IN THE BILL OF SALE AND THE ASSUMPTION AGREEMENT, SELLER MAKES NO REPRESENTATION OR WARRANTY, STATUTORY, EXPRESS OR IMPLIED, WRITTEN

19 OR ORAL, AT LAW OR IN EQUITY, IN RESPECT OF ANY OF ITS ASSETS (INCLUDING THE ACQUIRED ASSETS), LIABILITIES (INCLUDING THE ASSUMED LIABILITIES) OR THE BUSINESS, INCLUDING, WITH RESPECT TO MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE, OR NON-INFRINGEMENT, AND ANY SUCH OTHER REPRESENTATIONS OR WARRANTIES ARE HEREBY EXPRESSLY DISCLAIMED AND NONE SHALL BE IMPLIED AT LAW OR IN EQUITY. NEITHER SELLER NOR ANY OTHER PERSON, DIRECTLY OR INDIRECTLY, HAS MADE OR IS MAKING, ANY REPRESENTATION OR WARRANTY, WHETHER WRITTEN OR ORAL, REGARDING ANY PRO-FORMA FINANCIAL INFORMATION, FINANCIAL PROJECTIONS OR OTHER FORWARD-LOOKING STATEMENTS OF SELLER. SELLER IS TRANSFERRING ALL ACQUIRED ASSETS ON AN “AS IS, WHERE IS, WITH ALL FAULTS” BASIS. ARTICLE IV REPRESENTATIONS AND WARRANTIES OF PURCHASER Purchaser hereby represents and warrants to Seller, as of the date hereof and as of the Closing, as follows: Section 4.1. Qualification; Organization. Purchaser is a corporation duly incorporated, validly existing and in good standing under the Laws of its jurisdiction of organization and has all requisite corporate or similar power and authority to own, lease and operate its properties and assets and to carry on its business as presently conducted. Purchaser is qualified to do business and is in good standing (with respect to jurisdictions that recognize such concept) as a foreign corporation or other entity in each jurisdiction where the ownership, leasing or operation of its assets or properties or conduct of its business requires such qualification, except where the failure to be so qualified or, where relevant, in good standing, would not, individually or in the aggregate, impair or delay its ability to perform its obligations under this Agreement. Section 4.2. Authority of Purchaser. Purchaser has all requisite corporate power and authority to execute and deliver and perform its obligations under this Agreement and each of the Ancillary Documents to which it is a party (subject to entry of the Sale Order). The execution, delivery and performance of this Agreement and such Ancillary Documents by Purchaser and the consummation of the Transactions have been duly and validly authorized and approved by all requisite corporate action of Purchaser, as applicable, and no other corporate proceedings (pursuant to any of Purchaser’s organizational documents or otherwise) on the part of Purchaser is necessary to authorize the consummation of, and to consummate the Transactions. This Agreement and each such Ancillary Document have been, or at or prior to Closing (as the case may be) will be, duly and validly executed and delivered by Purchaser to the extent a party thereto, and, assuming the due authorization, execution and delivery of this Agreement and each such Ancillary Document by Seller, as applicable, constitute (except as such enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to creditors’ rights generally or general principles of equity) a valid and binding agreement of Purchaser, as applicable, enforceable against Purchaser in accordance with its terms.

20 Section 4.3. Consents and Approvals. No consent, approval, permit or authorization of, or declaration, filing or registration with, any Governmental Entity is necessary or required to be made or obtained by Purchaser or its Affiliates in connection with the execution, delivery and performance of this Agreement and the Ancillary Documents and the consummation of the Transactions. Section 4.4. No Violations. Neither the execution, delivery or performance of this Agreement and the Ancillary Documents by Purchaser nor the consummation by Purchaser of the Transactions will (a) conflict with or result in any violation or breach of any provisions of the certificate of incorporation, bylaws or other organizational documents of Purchaser, (b) conflict with or violate any Order or Law applicable to Purchaser or its properties, rights or assets, except in each case of the foregoing clauses, for breaches, violations, defaults or terminations that would not reasonably be expected to, individually or in the aggregate, impair or delay Purchaser’s ability to perform its obligations under this Agreement. Section 4.5. Brokers or Finders. Purchaser has not employed any investment banker, broker or finder in connection with the Transactions who might be entitled to any fee or any commission from Seller in connection with this Agreement or upon consummation of the Acquisition or any of the other Transactions. Section 4.6. Legal Proceedings. There is no Action or Order pending against, or to the Knowledge of Purchaser, threatened against or affecting, Purchaser before any arbitrator or any Governmental Entity which in any manner challenges or seeks to prevent, enjoin, alter or materially delay the Transactions or which would or would reasonably be expected to impair Purchaser’s ability to consummate the Transactions. Section 4.7. Financing. Purchaser has, or will at the time any payment is required, sufficient funds available to deliver the Purchase Price to Seller and consummate the Transactions, including the timely satisfaction of the Assumed Liabilities and payment of all applicable Cure Costs. Section 4.8. Adequate Assurances Regarding Assigned Contracts. To the Knowledge of Purchaser, there exist no facts or circumstances that would cause, or be reasonably expected to cause, Purchaser and/or its Affiliates not to qualify as “good faith” purchasers under Section 363(m) of the Bankruptcy Code. As of the Closing, Purchaser will be capable of satisfying the adequate assurance of future performance conditions contained in Sections 365(b)(1)(C) and 365(f) of the Bankruptcy Code with respect to the Assigned Contracts. Section 4.9. Disclaimer. Purchaser acknowledges that neither Seller nor any other Person is making, and Purchaser is not relying on, any representations or warranties whatsoever, statutory, expressed or implied, written or oral, at law or in equity, beyond those expressly made by Seller in Article IV hereof (as modified by the Seller Disclosure Schedules). Purchaser acknowledges that, except as expressly set forth in Article IV (as modified by the Seller Disclosure Schedules), neither Seller nor any other Person has, directly or indirectly, made any representation or warranty, statutory, expressed or implied, written or oral, at law or in equity, as

21 to the accuracy or completeness of any information that Seller furnished or made available to Purchaser and its Representatives in respect of the Business, and Seller’s operations, assets, stock, Liabilities, condition (financial or otherwise) or prospects. Purchaser acknowledges that neither Seller nor any other Person, directly or indirectly, has made, and Purchaser has not relied on, any representation or warranty, whether written or oral, regarding any pro-forma financial information, financial projections or other forward-looking statements of Seller, and Purchaser will make no claim with respect thereto. Purchaser acknowledges that the Acquired Assets are being transferred on an “AS IS, WHERE IS” basis. Section 4.10. Information. Purchaser has conducted such investigations of the Seller as it deems necessary and appropriate in connection with the execution and delivery of this Agreement and the Ancillary Documents to which Purchaser is a party and the consummation of the transactions contemplated hereby and thereby. Purchaser acknowledges that it and its Representatives have been permitted full and complete access to the books and records, facilities, equipment, Tax Returns, Contracts, insurance policies (or summaries thereof) and other properties and assets of Seller, that it and its Representatives have desired or requested to see or review, and that it and its Representatives have had a full opportunity to meet with the officers and employees of Seller to discuss the Business. Neither Seller nor any other Person (including any officer, director, member or partner of Seller or any of their Affiliates) shall have or be subject to any liability to Purchaser, or any other Person, resulting from Purchaser’s use of any information, documents or material made available to Purchaser in any “data rooms,” management presentations, due diligence or in any other form in expectation of the transactions contemplated hereby or by the Ancillary Documents. ARTICLE V COVENANTS Section 5.1. Conduct of Business Pending Closing. (a) Seller agrees that between the date hereof and the earlier of the Closing or the date, if any, on which this Agreement is validly terminated pursuant to ARTICLE VIII, except as set forth in Schedule 5.1(a) of the Seller Disclosure Schedule, and except (1) as expressly provided in this Agreement, (2) as consented to in writing by Purchaser (such consent not to be unreasonably withheld, conditioned or delayed), (3) for the consequences arising from the filing of the Chapter 11 Case and (4) as required by applicable Law, Seller shall: (i) use Commercially Reasonable Efforts to conduct the Business in all material respects in the Ordinary Course of Business; and (ii) use Commercially Reasonable Efforts to maintain and preserve the Acquired Assets. For the avoidance of doubt, in no event shall the taking of COVID-19 Measures by the Seller be deemed a breach of this Section 5.1(a).

22 (b) Seller agrees that between the date hereof and the earlier of the Closing or the date, if any, on which this Agreement is validly terminated pursuant to ARTICLE VIII, except as set forth in Schedule 5.1(b) of the Seller Disclosure Schedule, and except (1) as expressly provided in this Agreement, (2) as consented to in writing by Purchaser (such consent not to be unreasonably withheld, conditioned or delayed), (3) for the consequences arising from the filing of the Chapter 11 Case, and (4) as required by applicable Law, Seller shall not, with respect to the Business: (i) other than in the Ordinary Course of Business, sell, lease, license, assign, abandon, permit to lapse, transfer, exchange, swap or otherwise dispose of, or subject to any Encumbrance (other than Cure Costs) any of the Acquired Assets; (ii) fail to maintain, or allow to lapse, or abandon any Seller Intellectual Property; (iii) enter into or become bound by, terminate or materially amend or modify any material Contract relating to the acquisition or disposition or granting of any license with respect to any Seller Intellectual Property; (iv) materially modify, amend, extend or terminate any Assigned Contract or waive, release or assign any rights, obligations, or claims thereunder; (v) make, change or revoke any Tax election, adopt or change any method of Tax accounting, file any amended Tax Return, enter into any “closing agreement” within the meaning of Section 7121 of the Code (or any similar provision of state, local or non-U.S. Law), or surrender any right to claim a refund of Taxes, in each case, except to the extent such action would not be binding on Purchaser and would not reasonably be expected to increase the Taxes of Purchaser; (vi) cancel or fail to use commercially reasonable efforts to maintain in the Ordinary Course of Business Seller’s insurance policies included in, or covering any, Acquired Assets or to renew or replace existing insurance policies included in, or covering any, Acquired Assets following their termination; (vii) terminate or modify or waive in any material respect any right under any material Permit or otherwise fail to use best efforts to maintain all material Permits used in the operation of the Business or included in the Acquired Assets; (viii) file a motion, fail to timely contest a pleading seeking, or otherwise consent to a conversion of the Chapter 11 Case into a liquidation proceeding under Chapter 7 of the Bankruptcy Code; or (ix) agree or authorize, in writing or otherwise, to take any of the foregoing actions.

23 Without in any way limiting any party’s rights or obligations under this Agreement, the parties understand and agree that (i) nothing contained in this Agreement shall give Purchaser, directly or indirectly, the right to control or direct the operations of Seller, or the Business prior to the Closing and (ii) prior to the Closing, Seller shall exercise, consistent with, and subject to, the terms and conditions of this Agreement, complete control and supervision over the Business and its operations. Section 5.2. Access and Information. (a) From the date hereof and through the earlier of the Closing or the date, if any, on which this Agreement is validly terminated pursuant to ARTICLE VIII, Seller shall (at Purchaser’s sole cost and expense) afford Purchaser and its Representatives reasonable access during normal business hours upon reasonable advance notice to all of Seller’s properties, offices, employees and Books and Records to the extent related to the Acquired Assets and the Assumed Liabilities to make investigation of the Acquired Assets and the Assumed Liabilities as is reasonable. Any such investigation shall be conducted in a reasonable manner (and shall not unreasonably interfere with the operations of Seller or the Business), under reasonable circumstances and shall be subject to any applicable restrictions under applicable Law. Seller shall use commercially reasonable efforts to at Purchaser’s sole cost and expense to cause its Representatives to cooperate with Purchaser and Purchaser’s Representatives in connection with such investigation, and Purchaser and Purchaser’s Representatives shall cooperate with Seller and Seller’s Representatives to take all reasonable measures to minimize any disruption to the Business. Notwithstanding anything herein to the contrary, no such investigation shall be permitted to the extent that it would require Seller to disclose information subject to any privilege (including attorney-client privilege) or work product protection or breach any obligation of confidentiality or Contract. For the avoidance of doubt, information obtained pursuant to this Agreement including this Section 5.2(a) and the consummation of the Transactions shall be subject to the Confidential Disclosure Agreement. All requests for information or access requested by Purchaser from Seller pursuant to this Section 5.2(a) shall be only directed to the notice parties for Seller set forth in Section 9.4. (b) For a period of three (3) years after Closing (the “Preservation Period”), Purchaser will provide Seller, its successors and their Representatives with reasonable access (including the right to copy), during normal business hours upon reasonable advance notice, to the Acquired Books and Records, as well as, to employees, officers, advisors and accountants of Purchaser, in each case, for purposes relating to the Chapter 11 Case, the wind-down of the operations of Seller and its estate, actions to which Seller is a party (other than in connection with any litigation or dispute with Purchaser), insurance claims, Tax payments, returns or audits, the functions of any trusts established under a Chapter 11 plan of Seller or any other successors of Seller. Any such access shall be conducted in a reasonable manner (and shall not unreasonably interfere with the operations of Purchaser), under reasonable circumstances and shall be subject to any applicable restrictions under applicable Law. Notwithstanding anything herein to the contrary, no such access shall be permitted to the extent that it would require Purchaser to disclose information subject to any privilege (including attorney-client privilege) or work product protection or breach any obligation of confidentiality or Contract. For the avoidance of doubt,

24 information obtained pursuant to this Section 5.2(b) shall be subject to Section 5.11. All requests for information or access requested by Seller from Purchaser pursuant to this Section 5.2(b) shall be only directed to the notice parties for Purchaser set forth in Section 9.4. For purposes of this Section 5.2(b), references to “Seller” shall be construed, where applicable, to include any liquidating trust, plan administrator, or comparable Person or body bearing responsibility for the administration and wind-down of Seller’s operations, estates and Chapter 11 Case. In the event Purchaser wishes to destroy any Acquired Books and Records during the Preservation Period, Purchaser shall first provide twenty (20) Business Days’ prior written notice to Seller, and Seller shall have the right, at its option and expense, to take possession of such records within twenty (20) Business Days after notice thereof. (c) During the Preservation Period, Purchaser shall have reasonable access to the Books and Records in the possession of Seller to the extent that (i) such Books and Records relate to any period prior to the Closing Date and are not already in the possession of Purchaser and (ii) such access is reasonably required by Purchaser in connection with the Acquired Assets or the Assumed Liabilities. Any such access shall be conducted in a reasonable manner (and shall not unreasonably interfere with the operations of Seller), under reasonable circumstances and shall be subject to any applicable restrictions under applicable Law. Seller shall use commercially reasonable efforts to at Purchaser’s sole cost and expense to cause its Representatives to cooperate with Purchaser and Purchaser’s Representatives in connection with such access, and Purchaser and Purchaser’s Representatives shall cooperate with Seller and Seller’s Representatives to take all reasonable measures to minimize any disruption to the operations of Seller. Notwithstanding anything herein to the contrary, no such access shall be permitted to the extent that it would require Seller to disclose information subject to any privilege (including attorney-client privilege) or work product protection or breach any obligation of confidentiality or Contract. All requests for information or access requested by Purchaser from Seller pursuant to this Section 5.3(c) shall be only directed to the notice parties for Seller set forth in Section 9.4. Section 5.3. Approvals and Consents; Cooperation; Notification. (a) Subject to the terms and conditions of this Agreement (including Section 5.14), each party shall use commercially reasonable efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things necessary, proper or advisable under applicable Law to consummate the Transactions and to cause the conditions to the consummation of the Transactions set forth in Article VI to be satisfied as soon as practicable after the date hereof, including (i) preparing and filing or otherwise providing, in consultation with the other party and as promptly as practicable and advisable after the date hereof, all documentation to effect all necessary applications, notices, petitions, filings, and other documents and to obtain as promptly as practicable all waiting period expirations or terminations, consents, clearances, waivers, licenses, orders, registrations, approvals, permits, and authorizations necessary or advisable to be obtained from any third party or any Governmental Entity in order to consummate the Transactions, and (ii) taking all steps as may be necessary, subject to the limitations in this Section 5.3, to obtain all such waiting period expirations or terminations, consents, clearances, waivers, licenses, registrations, permits, authorizations, orders and approvals from any third party or any Governmental Entity. Purchaser shall be responsible for all filing fees payable in connection

25 with such filings; provided that all other costs and expenses incurred in connection with this Section 5.3 (including, for the avoidance of doubt, all costs and expenses payable by such party to any of its financial advisors, attorneys, accountants, advisors, consultants or other Representatives) shall be paid by the party incurring such costs and expenses. (b) Each party shall give prompt notice to the other party (i) of any notice or other communication from any Governmental Entity in connection with this Agreement or the Acquisition, or from any Person alleging that the consent of such Person is or may be required in connection with the Acquisition, (ii) of any legal proceeding commenced or, to the knowledge of such party, threatened against it or any of its Affiliates or otherwise relating to, involving or affecting such party or any of its Affiliates, in each case in connection with, arising from or otherwise relating to the Acquisition, and (iii) upon becoming aware of the occurrence or impending occurrence of any event or circumstance that would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect or which would reasonably be expected to prevent or materially delay or impede the consummation of the Acquisition; provided, however, that the delivery of any notice pursuant to this Section 5.3(b) shall not cure any breach of any representation or warranty requiring disclosure of such matter prior to the date hereof or otherwise limit or affect the remedies available hereunder to any party. (c) Notwithstanding the foregoing, the obligations of the parties to obtain any consent, approval or waiver from the Bankruptcy Court shall be governed exclusively by Section 5.7, Section 5.8, and Section 5.9. Section 5.4. Further Assurances. In addition to the provisions of this Agreement, from time to time after the Closing Date, Seller and Purchaser shall use commercially reasonable efforts to execute and deliver such other instruments of conveyance, transfer or assumption, as the case may be, and take such other actions as may be reasonably requested to implement more effectively the conveyance and transfer of the Acquired Assets to Purchaser and the assumption of the Assumed Liabilities by Purchaser and otherwise to effect the purposes of this Agreement and the Transactions. In furtherance and not in limitation of the foregoing if, following the Closing, (x) Seller receives or becomes aware that it holds any asset, property or right which constitutes an Acquired Asset, then Seller shall transfer such asset, property or right to Purchaser for no additional consideration, and pending such conveyance the parties shall reasonably cooperate with each other to provide Purchaser with all of the benefits of use of such asset, property or right and (y) Purchaser receives or becomes aware that it holds any asset, property or right which constitutes an Excluded Asset, then Purchaser shall transfer such asset, property or right to Seller as promptly as practicable for no additional consideration, and pending such conveyance the parties shall reasonably cooperate with each other to provide Seller with all of the benefits of use of such asset, property or right. Purchaser shall be responsible for all reasonable out-of-pocket transfer costs associated with the transfer of the Acquired Assets pursuant to this Section 5.4; provided that all other costs and expenses incurred in connection with this Section 5.4 (including, for the avoidance of doubt, all costs and expenses payable by such party to any of its financial advisors, attorneys, accountants, advisors, consultants or other Representatives) shall be paid by the party incurring such costs and expenses.

26 Section 5.5. Update of Seller Disclosure Schedules. From the date of this Agreement until the Closing Date, Seller shall as promptly as reasonably practicable deliver any new schedules or supplement or amend the Seller Disclosure Schedules with respect to any matter that, if existing, occurring or known at the date of this Agreement, would have been required to be set forth or described in the Seller Disclosure Schedules. Any such supplement or amendment shall be deemed to modify the Seller Disclosure Schedules for purposes of this Agreement except to the extent the matters set forth in such supplement or amendment are material to the Acquired Assets or the Business. Section 5.6. Other Actions. Purchaser covenants and agrees that, except (w) as expressly contemplated by this Agreement, (x) with the prior written consent of Seller (which consent shall not be unreasonably withheld, conditioned or delayed), (y) as otherwise required by Law or (z) to the extent not inconsistent with the Bankruptcy Code, the Federal Rules of Bankruptcy Procedure, any orders entered by the Bankruptcy Court in the Chapter 11 Case, after the date of this Agreement and prior to the Closing Date, Purchaser shall use commercially reasonable efforts not to take or agree to or commit to assist any other Person in taking any action (i) that would reasonably be expected to result in a failure of any of the conditions to the Closing or (ii) that would reasonably be expected to impair the ability of Purchaser or Seller to consummate the Closing in accordance with the terms hereof or to materially delay such consummation. Section 5.7. The Sale Order. Seller shall use its best efforts to obtain entry of the Sale Order (provided Purchaser is the Successful Bidder) in form and substance acceptable to Purchaser within ten (10) Business Days after Purchaser is determined to be the Successful Bidder. Section 5.8. Cooperation with Respect to Bankruptcy Court Approvals. Purchaser shall take such actions as are reasonably requested by Seller to assist in obtaining entry by the Bankruptcy Court of the Sale Order. Section 5.9. Bankruptcy Court Filings. Seller shall obtain Purchaser’s consent with respect to the proposed Sale Order prior to the presentation of such Sale Orders to the Bankruptcy Court. Seller shall consult with Purchaser with respect to any other pleadings or proposed Orders to be presented to the Bankruptcy Court relating to the Transactions, and the bankruptcy proceedings in connection therewith, and provide Purchaser with copies of applications, pleadings, notices, proposed Orders and other documents to be filed by Seller in the Chapter 11 Case that relate in any way to this Agreement, the Acquisition, the Bidding Procedures, the Bidding Procedures Order or the Sale Order prior to the making of any such filing with or submission to the Bankruptcy Court. Section 5.10. Adequate Assurance and Performance. Purchaser shall, and shall cause its Subsidiaries to, use commercially reasonable efforts to provide adequate assurance of the future performance by Purchaser of each Assigned Contract as required under Section 365 of the Bankruptcy Code. Purchaser and Seller agree that they will promptly take all actions reasonably required to assist in obtaining a Bankruptcy Court finding that there has been an adequate demonstration of adequate assurance of future performance under the Assigned Contracts pursuant to Section 365 of the Bankruptcy Code, such as furnishing timely requested and factually accurate

27 affidavits, non-confidential financial information and other documents or information for filing with the Bankruptcy Court and making Xxxxxxxxx’s and Seller’s Representatives available to testify before the Bankruptcy Court. Section 5.11. Post-Closing Confidentiality. (a) Seller and Purchaser hereby agree that the Confidential Disclosure Agreement shall terminate, and no party shall have any further obligations thereunder, effective concurrently with the Closing, other than Purchaser’s obligations with respect to the confidentiality and non-use of information exclusively related to the Excluded Assets and the Excluded Liabilities. (b) From and after the Closing and except as required by applicable Law, Seller shall, and shall cause its Affiliates and Representatives to, (i) treat and hold as confidential any information to the extent concerning the Acquired Assets or the Assumed Liabilities that is not, as of the Closing, generally available to the public (the “Confidential Information”) and (ii) refrain from using any of the Confidential Information for commercial purposes; provided, however, that “Confidential Information” shall not include (x) any information that becomes publicly available after the Closing Date through no fault of Seller or any of its Affiliates and Representatives, or (y) any information that after the Closing Date is legitimately received by Seller or any of its Affiliates or Representatives from a third Person (provided that such third Person is not bound by an obligation of secrecy with respect to such information). Seller agrees that in the event it (or any of its Affiliates or Representatives) is required by Law to use or disclose any Confidential Information, Seller shall inform Purchaser in advance of any such required disclosure, shall use commercially reasonable efforts to cooperate with Purchaser in obtaining a protective order or other protection in respect of such required disclosure, and shall limit such disclosure to the extent reasonably possible while still complying with such Law. Section 5.12. Transfer of Acquired Assets. Seller shall, at Purchaser’s sole cost and expense, make all necessary arrangements for Purchaser to take possession of Acquired Assets and to transfer the Acquired Assets to a location designated by Purchaser, to the extent requested by Purchaser, as promptly as practicable following the Closing. In furtherance of the foregoing, Seller shall, at Purchaser’s sole cost and expense, deliver or cause to be delivered to Purchaser, at the address set forth in Section 9.4, 95 kilograms of the Product in bulk and 5 kilograms of the Product in sachets on the Closing Date or as soon as reasonably practicable thereafter. Section 5.13. Transition Services. Following the Closing until the Confirmation, Purchaser shall be responsible for the approved Maintenance Costs set forth on Schedule 1.5(b). Section 5.14. Permits. As promptly as practicable after the Closing, Seller and Purchaser shall file with each applicable Governmental Entity the notices and information required pursuant to any applicable Law to transfer the Permits included in the Acquired Assets from Seller to Purchaser. The parties also agree to use all reasonable best efforts to take any and all other actions at Seller’s sole cost and expense required by any Governmental Entity to effect the transfer of the Transferred Permits from Seller to Purchaser. Purchaser shall be responsible for all filing

28 fees and reasonable out-of-pocket transfer costs payable in connection with this Section 5.14; provided that all other costs and expenses incurred in connection with this Section 5.14 (including, for the avoidance of doubt, all costs and expenses payable by such party to any of its financial advisors, attorneys, accountants, advisors, consultants or other Representatives) shall be paid by the party incurring such costs and expenses. Section 5.15. Post-Closing Diligence. Purchaser shall (directly or indirectly through any Purchaser Party and other Third Parties) use Commercially Reasonable Efforts to Develop (including seeking regulatory approval for) and Commercialize at least one Product. Upon the written request of Seller (such request to be provided within sixty (60) days after the end of the applicable calendar year), Purchaser shall prepare and provide Seller with a high level written report (which shall be maintained by Seller as Purchaser’s Confidential Information) summarizing its Development and Commercialization efforts with respect to the Products during such calendar year. Without limiting Purchaser’s obligations set forth in the first sentence of this Section 5.15, Xxxxxx agrees that any and all Development and/or Commercialization activities with respect to the Product, including any and all scientific, regulatory, clinical and business plans, strategies, designs and decisions shall be within the sole discretion of Purchaser. Section 5.16. Additional Actions. Neither Seller nor Purchaser will file any pleading or take any other action in the Bankruptcy Court or other court of competent jurisdiction with respect to this Agreement or the consummation of the Transactions that is inconsistent with performing and carrying out the provisions of this Agreement in accordance with the terms and subject to the conditions herein. Notwithstanding the foregoing, Purchaser hereby agrees and acknowledges that this Agreement and the Transactions are subject to Seller’s right and ability to solicit, negotiate, discuss, and consider higher or otherwise better competing bids with respect to the Acquired Assets, in each case in accordance with the Bidding Procedures Order. Section 5.17. Net Sales Statements. Within forty-five (45) days after the end of each calendar year, Purchaser shall have compiled a Net Sales Statement for such calendar year. Purchaser shall keep each such Net Sales Statement in its books and records. Section 5.18. Audit Rights. Upon reasonable advance written notice (and in no event less than fifteen (15) days’ advance written notice), Purchaser shall permit one (1) independent certified public accounting firm of nationally recognized standing selected by Seller and reasonably acceptable to Purchaser (the “Independent Accountant”) to have access at reasonable times during normal business hours to the books and records of Purchaser and its Affiliates as may be reasonably necessary to evaluate and verify Purchaser’s calculation of Net Sales during the prior three (3) year period, including the Net Sales Statements; provided that (a) such Independent Accountant shall enter into a customary confidentiality agreement reasonably satisfactory to Purchaser with respect to the confidential information of Purchaser or its Affiliates to be furnished pursuant to this Section 5.18, (b) such access does not unreasonably interfere with the conduct of the business of Purchaser or any of its Affiliates and (c) such Independent Accountant shall disclose to Seller only whether any milestone set forth in Section 2.3 has been achieved during the Calendar Quarter that is the subject of the inspection. The fees charged by such Independent Accountant shall be borne by Seller unless such Independent Accountant

29 concludes that any milestone payment should have been paid pursuant to Section 2.3 but was not paid when due, in which case, Purchaser shall bear the full cost of such audit. The audit rights set forth in this Section 5.18 may not be exercised more than once in any calendar year. If, in accordance with the procedures set forth in this Section 5.18, the Independent Accountant concludes that any milestone payment should have been paid but was not paid when due, Purchaser shall promptly, and in any event within thirty (30) days of the date the Independent Accountant delivers to Purchaser the Independent Accountant’s written report, pay Seller such milestone payment (to the extent not paid on a subsequent date), plus interest calculated at the rate of SOFR plus two percent (2%) per annum or the maximum rate allowed by applicable Law, whichever is lower, from when such milestone payment should have been paid, as applicable, to the date of actual payment. ARTICLE VI CONDITIONS PRECEDENT Section 6.1. Condition(s) Precedent to Obligation of Seller and Purchaser. The respective obligations of each party to consummate the Transactions shall be subject to the satisfaction or waiver by both Seller and Purchaser (to the extent permitted by applicable Law) at or prior to the Closing of each of the following conditions: (a) No Orders. No Governmental Entity shall have enacted, issued, promulgated, decreed or entered any Order from or after the date of this Agreement which remains in effect on the Closing Date and that has the effect of prohibiting (or delaying beyond the Outside Date) the consummation of the Transactions. (b) Sale Order. The Bankruptcy Court shall have entered the Sale Order in form and substance reasonably acceptable to Purchaser and such Sale Order shall be a Final Order (unless such Final Order requirement is waived by the Purchaser). Section 6.2. Conditions Precedent to Obligation of Seller. The obligations of Seller to effect the Transactions shall be subject to the satisfaction or waiver (to the extent permitted by applicable Law) by Seller at or prior to the Closing of the following conditions: (a) Representations and Warranties. The representations and warranties of Purchaser contained in Article IV of this Agreement shall be true and correct (disregarding any exception or qualification in such representations or warranties relating to “material” or “materiality”) as of the date hereof and as of the Closing Date (except to the extent such representations and warranties speak as of another date (other than the date of this Agreement), in which case such representations and warranties shall be true and correct as of such other date), except where the failure of such representations or warranties to be so true and correct (disregarding any exception or qualification in such representations or warranties relating to “material” or “materiality”) has not and would not reasonably be expected to, individually or in the aggregate, materially impair or materially delay Purchaser’s ability to perform its obligations under this Agreement or to consummate the Transactions;

30 (b) Covenants. The covenants and obligations of Purchaser to be performed or complied with at or prior to the Closing pursuant to this Agreement shall have been duly performed and complied with in all material respects; (c) Officer’s Certificates. Purchaser shall have delivered to Seller a certificate duly executed by an authorized officer of Purchaser certifying to the effect that the conditions set forth in Section 6.2(a) and Section 6.2(b) have been satisfied; and (d) Closing Documentation. Seller shall have received the other items to be delivered to it pursuant to Section 2.2(b). The foregoing conditions are for the benefit of Seller only and accordingly Seller will be entitled to waive compliance with any such conditions if it sees fit to do so, without prejudice to rights and remedies at Law and in equity and also without prejudice to any rights of termination or otherwise in the event of the failure to fulfill any other conditions in whole or in part. Section 6.3. Conditions Precedent to Obligation of Purchaser. The obligations of Purchaser to effect the Transactions shall be subject to the satisfaction or waiver (to the extent permitted by applicable Law) by Purchaser at or prior to the Closing of the following conditions: (a) Representations and Warranties. The representations and warranties of the Seller contained in Article III of this Agreement, (disregarding any exception or qualification in such representations and warranties relating to “material”, “materiality” or “Material Adverse Effect”), shall be true and correct as of the date hereof and as of the Closing Date (except to the extent such representations and warranties are expressly made as of a specific date, in which case such representations and warranties shall be so true and correct as of such specific date only), except for such failures to be true and correct as would not, individually or in the aggregate, have a Material Adverse Effect; (b) Covenants. The covenants and obligations of Seller to be performed or complied with at or prior to the Closing pursuant to this Agreement shall have been duly performed and complied with in all material respects; and (c) Officer’s Certificates. Seller shall have delivered to Purchaser a certificate duly executed by an executive officer of Seller certifying to the effect that the conditions set forth in Section 6.3(a) and Section 6.3(b) have been satisfied; and (d) Closing Documentation. Purchaser shall have received the other items to be delivered to it pursuant to Section 2.2(a). The foregoing conditions are for the benefit of Purchaser only and accordingly Purchaser will be entitled to waive compliance with any such conditions if it sees fit to do so, without prejudice to any rights and remedies at Law and in equity and also without prejudice to any of rights of termination or otherwise in the event of the failure to fulfill any other conditions in whole or in part.

31 ARTICLE VII NO SURVIVAL Section 7.1. No Survival. The parties agree that the representations and warranties contained in this Agreement, any Ancillary Document or in any certificate or instrument delivered by or on behalf of Seller, any Affiliate of Seller or Purchaser or its Affiliates or Representatives pursuant to this Agreement, including the certificates delivered at Closing pursuant to Section 6.2(c) and Section 6.3(c), shall not survive the Closing hereunder, and none of the parties shall have any Liability to each other after the Closing for any breach thereof. The parties agree that the covenants contained in this Agreement or any Ancillary Document to be performed prior to or at the Closing shall terminate at the Closing and none of the parties shall have any Liability to each other after the Closing for any breach thereof. The covenants contained in this Agreement to be performed in any part after the Closing shall survive the Closing hereunder for the duration of the contemplated performance in respect of such covenant. ARTICLE VIII TERMINATION Section 8.1. Termination. (a) This Agreement may be terminated in writing by either Purchaser or Seller in the event that the Closing has not occurred on or before March 15, 2023, or such other date as may be mutually agreed in writing by the parties (the “Outside Date”); provided, however, that the right to terminate this Agreement pursuant to this Section 8.1(a) shall not be available to any party whose failure to perform any of its obligations under this Agreement required to be performed by it at or prior to the Closing results in the failure of the Closing to occur prior to the Outside Date. (b) This Agreement may also be terminated prior to the Closing: (i) by the mutual written agreement of Purchaser and Seller; (ii) by Purchaser by written notice to Seller, if (x) there shall have been a material breach of any of the covenants or agreements in this Agreement or any of the representations or warranties set forth in Article III of this Agreement on the part of Seller which breach, would result in, if occurring or continuing on the Closing Date, the failure of the conditions set forth in Section 6.3(a) or Section 6.3(b), as the case may be, or (y) Seller has breached the Bidding Procedures Order or the Sale Order, which such breach, in each case is not cured prior to the Outside Date (provided, that Purchaser is not then in material breach of any of the covenants, agreements, representations or warranties set forth in this Agreement); (iii) by Seller by written notice to Purchaser, if (x) there shall have been a material breach of any of the covenants or agreements in this Agreement or any of the representations or warranties set forth in Article IV of this Agreement on the part of

32 Purchaser which breach, would result in, if occurring or continuing on the Closing Date, the failure of the conditions set forth in Section 6.2(a) or Section 6.2(b), as the case may be, or (y) Purchaser has breached the Bidding Procedures Order or the Sale Order, which such breach, in each case is not cured prior to the Outside Date (provided, that Seller is not then in material breach of any of the covenants, agreements, representations or warranties set forth in this Agreement); (iv) by Seller by written notice to Purchaser, if the Sale Order with respect to the Transactions contemplated by this Agreement has been entered and (i) Seller has provided the Purchaser with written notice that Seller is prepared to consummate the Closing, (ii) the conditions to Closing in Section 6.1 and Section 6.3 have been satisfied (or waived by Seller, other than those conditions that by their nature can only be satisfied at Closing) and (iii) the Closing Date does not occur within three (3) Business Days of Seller providing Purchaser with such notice; (v) by Purchaser by written notice to Seller, if Seller files a motion requesting, consents to, fails to timely contest a pleading seeking, or the Bankruptcy Court, sua sponte, orders (A) a conversion of the Chapter 11 Case into a liquidation proceeding under Chapter 7 of the Bankruptcy Code, (B) the dismissal of the Chapter 11 Case, (C) the appointment of a Chapter 11 trustee or examiner or (D) the termination of the exclusivity period described in 11 U.S.C. § 1121(b); (vi) by either Seller or Purchaser by written notice to each other party (as applicable), if a Governmental Entity issues a final, non-appealable ruling or Order permanently prohibiting the Transactions; provided, however, that the right to terminate this Agreement pursuant to this Section 8.1(b)(vi) shall not be available to any party whose breach of any of its representations, warranties, covenants or agreements contained herein results in such ruling or Order; and (vii) by either Seller or Purchaser, if the Auction has occurred and a third party other than Purchaser is the Successful Bidder and Purchaser is not determined to be the Next-Highest Bidder. Section 8.2. Effect of Termination. (a) Except as otherwise provided in this Section 8.2, in the event of termination of this Agreement by either party in accordance with Section 8.1, all rights and obligations of the parties under this Agreement shall terminate without any Liability of any party to the other party, provided that the provisions of Section 1.7(b)(ii), Section 1.7(b)(iii), the Purchaser’s confidentiality obligations in Section 5.2(a), this Section 8.2 and ARTICLE IX (other than Section 9.1 and Section 9.2) shall expressly survive the termination of this Agreement, and provided further that the Confidential Disclosure Agreement shall survive any termination of this Agreement and nothing in this Section 8.2 shall relieve Purchaser or the Seller of their obligations under the Confidential Disclosure Agreement.

33 (b) The parties acknowledge that the agreements contained in this Section 8.2 are an integral part of the Transactions, that the damages resulting from termination of this Agreement under circumstances where Xxxxxx is entitled to the Deposit Funds are uncertain and incapable of accurate calculation and that the delivery of the Deposit Funds is not a penalty but rather shall constitute liquidated damages in a reasonable amount that will compensate Seller in the circumstances where Seller is entitled to the Deposit Funds for the efforts and resources expended and opportunities forgone while negotiating this Agreement and in reliance on this Agreement and on the expectation of the consummation of the Transactions, and that, without these agreements, Seller would not enter into this Agreement. If Purchaser fails to take any action necessary to cause the delivery of the Deposit Funds pursuant to the Escrow Agreement under circumstances where Seller is entitled to the Deposit Funds and, in order to obtain such Deposit Funds Seller commences a suit which results in a judgment in favor of Seller, Purchaser shall be responsible for, and shall pay to Seller an amount in cash equal to, the costs and expenses (including attorney’s fees) incurred by Seller in connection with such suit. ARTICLE IX GENERAL PROVISIONS Section 9.1. Tax Matters. (a) Purchaser shall be responsible for (and shall indemnify and hold harmless the Seller and its directors, officers, employees, Affiliates, agents, attorneys, successors and permitted assigns against) any sales, use, value added, duty, stamp, documentary stamp, filing, recording, registration, conveyance, transfer or similar fees or taxes or governmental charges (including any interest, fine, penalty, additions to Tax or additional amount thereon) payable in connection with the transactions contemplated by this Agreement, including any payments made in lieu of any such Taxes or governmental charges that become payable in connection with the transactions contemplated by this Agreement (“Transfer Taxes”). To the extent that any Transfer Taxes are required to be paid by the Seller (or such Transfer Taxes are assessed against the Seller), Purchaser shall promptly reimburse the Seller, as applicable, for such Transfer Taxes. The Seller shall take all actions reasonably requested by Purchaser to mitigate or otherwise reduce (and shall not take any actions which would increase or fail to mitigate or reduce) all such Transfer Taxes through securing exemptions therefrom or otherwise and shall continue to cooperate with Purchaser post-Closing to reduce the liability for, or to increase the amount or availability of any refund of, any such Transfer Taxes. (b) Seller shall be responsible for all Taxes of Seller. For the avoidance of doubt and except as provided in Section 9.1(a), Purchaser shall not assume any Liability hereunder for any Taxes of Seller, regardless of whether such Taxes are attributable to any period prior to or after the Closing Date. (c) Seller, on the one hand, or Purchaser, on the other hand, as the case may be (the “Reimbursing Party”), shall provide reimbursement for any Tax paid by the other (the “Paying Party”), all or a portion of which is the responsibility of the Reimbursing Party in accordance with the terms of this Agreement (including this Section 9.1). Within a reasonable time prior to the

34 payment of any such Tax, the Paying Party shall give notice to the Reimbursing Party of the Tax payable and the Paying Party’s and Reimbursing Party’s respective Liability therefor, although failure to do so shall not relieve the Reimbursing Party from its Liability hereunder except to the extent the Reimbursing Party is actually prejudiced thereby. (d) The parties shall provide each other with such assistance as reasonably may be requested by any of them in connection with (i) the preparation of any Tax Return, (ii) the determination of any Liability in respect of Taxes or the right to any refund, credit or prepayment in respect of Taxes (including pursuant to this Agreement) or (iii) any audit or other examination by any Taxing Authority, or any judicial or administrative proceeding with respect to any Taxes. Section 9.2. Bulk Sales. Purchaser hereby waives compliance with the requirements and provisions of any “bulk-transfer” or similar Laws of any jurisdiction that may otherwise be applicable with respect to the sale, conveyance, assignment or transfer of any or all of the Acquired Assets to Purchaser. Section 9.3. Public Announcements. Unless otherwise required by applicable Law or by obligations of Seller or Purchaser or their respective Affiliates pursuant to any listing agreement with or rules of any securities exchange or in order to enforce a party’s rights or remedies under this Agreement, and subject to the provisions of the Bankruptcy Code, the Sale Order, and Seller’s obligations in connection with the Chapter 11 Case, Seller and Purchaser shall consult with each other before issuing any press release or otherwise making any public statement with respect to this Agreement, the Ancillary Documents, the Transactions or the activities and operations of the other and shall not issue any such release or make any such statement without the prior written consent of the other. Section 9.4. Notices. Any notice pursuant to this Agreement must be in writing and will be deemed effectively given by the applicable party on the earliest of the date (a) three (3) Business Days after such notice is sent by registered U.S. mail, return receipt requested, (b) on which such notice is sent by email (to the extent that no “bounce back” or similar message indicating non-delivery is received with respect thereto), (c) one Business Day after such notice is deposited with an overnight courier service for next day delivery, or (d) on which such notice is delivered by hand (or, if delivery is refused, upon presentment); in each case to the appropriate address set forth below (or by such other address as the party may designate by notice to the other party from time to time): to Seller: Tricida, Inc. 0000 Xxxxxxxxx Xxxxx, Xxxxx 000 Xxxxx Xxx Xxxxxxxxx, XX 00000 Attention: Xxxxxx Xxxxxxxx, Xxxxx Xxxxxx and Xxx XxXxxxx Email: xxxxxxxxx@xxxxxxx.xxx; xxxxxxx@xxxxxxx.xxx and xxxxxxxx@xxxxxxx.xxx with a copy (which shall not constitute notice) to:

00 Xxxxxx Xxxxxx LLP 000 0xx Xxxxxx Xxx Xxxx, XX 00000 Attention: Xxxxxxxx Xxxxx and Xxxxx Xxxxx Email: xxxxxx@xxxxxx.xxx; xxxxxx@xxxxxx.xxx and xxxxxxxx@xxxxxx.xxx to Purchaser: Renibus Therapeutics, Inc. 000 Xxxxx Xxx, Xxxxx 000 Xxxxxxxxx, XX 00000 Attention: Xxxx Xxxxxx Email: xxxxxxx@xxxxxxx.xxx with a copy (which shall not constitute notice) to: Xxxxxx & Xxxxxxx LLP 00000 Xxxx Xxxxx Xxxxx Xxx Xxxxx, XX 00000 Attention: Xxxxxxx X. Xxxxxx; Xxx Xxxxxxx; Xxxxxx Xxxxxxx Email: Xxxxxxx.Xxxxxx@XX.xxx; Xxx.Xxxxxxx@xx.xxx; Xxxxxx.Xxxxxxx@xx.xxx Section 9.5. Descriptive Headings; Interpretative Provisions. The headings contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement. The words “hereof,” “herein” and “hereunder” and words of like import used in this Agreement shall refer to this Agreement as a whole and not to any particular provision of this Agreement. References to Articles, Sections and Schedules are to Articles, Sections and Schedules of this Agreement unless otherwise specified. All Schedules annexed hereto or referred to herein are hereby incorporated in and made a part of this Agreement as if set forth in full herein. Any capitalized terms used in any Schedule but not otherwise defined therein, shall have the meaning as defined in this Agreement. Any singular term in this Agreement shall be deemed to include the plural, and any plural term the singular. Where a word or phrase is defined herein, each of its other grammatical forms shall have a corresponding meaning. Whenever the words “include,” “includes” or “including” are used in this Agreement, they shall be deemed to be followed by the words “without limitation,” whether or not they are in fact followed by those words or words of like import. Except where the context otherwise requires, the word “or” is used in the inclusive sense (and/or). Whenever the last day for the exercise of any right or the discharge of any duty under this Agreement falls on other than a Business Day, the party having such right or duty shall have until the next Business Day to exercise such right or discharge such duty. Unless otherwise indicated, the word “day” shall be interpreted as a calendar day. References to “dollars” or “$” mean United States dollars, unless otherwise clearly indicated to the contrary. The word “notice” shall mean notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement. No summary of this Agreement prepared by or on behalf of any party shall affect the meaning or

36 interpretation of this Agreement. Time periods based on a number of days within or following which any payment is to be made or act is to be done shall be calculated by excluding the reference date in calculating such period and, if applicable, by extending the period to the next Business Day following if the last day of the period is not a Business Day. A reference to any law includes that law as amended and the regulations promulgated under such law. Section 9.6. No Strict Construction. Seller, on the one hand, and Purchaser, on the other hand, participated jointly in the negotiation and drafting of this Agreement, and, in the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as jointly drafted by Seller and Purchaser, and no presumption or burden of proof shall arise favoring or disfavoring any party by virtue of the authorship of any provision of this Agreement. Without limitation as to the foregoing, no rule of strict construction construing ambiguities against the draftsperson shall be applied against any Person with respect to this Agreement. Section 9.7. Entire Agreement; Assignment. This Agreement, the Ancillary Documents and the Confidential Disclosure Agreement constitute the entire agreement and supersede all other prior agreements and understandings, both written and oral, between the parties with respect to the subject matter hereof and thereof. Neither party to this Agreement may assign any of its rights or delegate any of its obligations under this Agreement without the prior written consent of the other party; provided, that Purchaser may assign any of its rights and obligations hereunder to any Affiliate of Purchaser (a “Purchaser Designee”) and, following the Closing, in whole or in part to any successor-in-interest to any Person acquiring all or any portion of the Acquired Assets; provided, further, that Seller may assign any of its rights to receive Contingent Payments pursuant to Section 2.3 to any administrator or trustee of Seller’s bankruptcy estate pursuant to Seller’s confirmed plan of liquidation in the Chapter 11 Case. Without limiting the foregoing, Purchaser acknowledges and agrees that if Purchaser assigns to any Person all or substantially all of Purchaser’s rights to the Compound, including the Program Patents, then Purchaser shall require such Person to assume Purchaser’s obligations under Section 2.3; provided that Purchaser shall remain liable for such obligations in the event that the market cap of such Person does not exceed $100 million. Any purported assignment in violation of this Section 9.7 shall be null and void. This Agreement is for the sole benefit of the parties (and their successors and permitted assigns) and nothing expressed or implied in this Agreement shall give or be construed to give any Person other than the parties (and their successors and permitted assigns) any legal or equitable right, remedy, or claim under or with respect to this Agreement or any provision of this Agreement. To the extent that Purchaser assigns any of its rights and obligations hereunder to a Purchaser Designee, upon the transfer of any Acquired Asset to, or the assumption of any Assumed Liability by, such Purchaser Designee, such Purchaser Designee shall be solely responsible for such Acquired Asset or Assumed Liability, as applicable; provided, however, assignment of this Agreement, or any of the rights, interests or obligations hereunder by Purchaser to any Person shall not relieve Purchaser of its obligations under Section 2.2(b)(i). Section 9.8. Governing Law; Submission of Jurisdiction; Waiver of Jury Trial. This Agreement shall be governed by and construed in accordance with the Laws of the State of Delaware. Any and all claims, controversies and causes of action arising out of or relating to this Agreement, whether sounding in contract, tort or statute, shall be governed by the Laws of the

37 State of Delaware, including its statutes of limitations, without giving effect to any conflict-of- laws or other rule that would result in the application of the Laws of a different jurisdiction. The parties agree that the exclusive jurisdiction and venue for any litigation arising out of this Agreement shall be in the Bankruptcy Court; provided, however, that if at the time of commencement of any such litigation, there is no longer a pending Chapter 11 Case or the Bankruptcy Court does not have jurisdiction or declines to exercise jurisdiction, the exclusive jurisdiction and venue for any litigation arising out of or relating to this Agreement, provided jurisdiction may be obtained under applicable Law, shall be in the state or federal courts in the State of Delaware, and each party hereby waives any objections they may have with respect thereto (including any objections based upon forum non conveniens). Each party hereby consents to service of process in the manner and at the address set forth in Section 9.4. EACH PARTY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY. Section 9.9. Expenses. Except as otherwise provided herein, whether or not the Transactions are consummated, all costs and expenses incurred in connection with this Agreement and the Transactions shall be paid by the party incurring such expenses. Section 9.10. Amendment. This Agreement may not be amended except by an instrument in writing signed by each party. Section 9.11. Waiver. At any time prior to the Closing, the parties may (a) extend the time for the performance of any of the obligations or other acts of the other party, (b) waive any inaccuracies in the representations and warranties contained herein or in any document delivered pursuant hereto and (c) waive compliance with any of the agreements or conditions contained herein. Any agreement on the part of a party to any such extension or waiver shall be valid only if set forth in an instrument in writing signed on behalf of such party. Section 9.12. No Liability; Release. (a) No past, present or future Representative of Seller or its Affiliates (collectively, the “Seller’s Group”), shall have any liability for any obligations or liabilities of Seller or Purchaser, as applicable, under this Agreement or any Ancillary Document or otherwise for any claim based upon, in respect of, relating to or by reason of, the transactions contemplated by this Agreement or and Ancillary Document. Any claim or Action based upon, arising out of, or related to this Agreement or any or any Ancillary Document may only be brought against Persons that are expressly named as parties hereto or thereto, and then only with respect to the specific obligations set forth herein or therein. Other than the parties, no other party shall have any liability or obligation for any of the representations, warranties, covenants, agreements, obligations or liabilities of any party under this Agreement or any Ancillary Document or of or for any Action based on, in respect of, or by reason of, the transactions contemplated hereby or thereby (including the breach, termination or failure to consummate such transactions), in each case whether based on contract, tort, fraud, strict liability, other legal requirements or otherwise and whether by piercing the corporate veil, by a claim by or on behalf of a party or another Person or otherwise.

38 (b) Effective upon the Closing Date, Purchaser acknowledges that it has no claim, counterclaim, setoff, recoupment, Action or cause of action of any kind or nature whatsoever against any of the Persons within the Seller’s Group, that directly or indirectly arises out of, is based upon, or is in any manner connected with any transaction, event, circumstances, action, failure to act or occurrence of any sort or type, including any approval or acceptance given or denied, whether known or unknown, which occurred, existed, was taken or begun prior to the consummation of the transactions contemplated hereunder and under the Ancillary Documents (collectively, the “Purchaser Released Claims”); and, should any Purchaser Released Claims nonetheless exist, Purchaser hereby (i) releases and discharges each member of the Seller’s Group from any liability whatsoever on such Purchaser Released Claims, and (ii) releases, remises, waives and discharges all such Purchaser Released Claims against the Seller’s Group. (c) Purchaser, to the fullest extent allowed under applicable Law, hereby waives and relinquishes all statutory and common law protections purporting to limit the scope or effect of a general release, whether due to lack of knowledge of any claim or otherwise, including, waiving and relinquishing the terms of any law which provides that a release may not apply to material unknown claims. (d) Notwithstanding anything herein to the contrary, Seller shall remain liable for complying with each covenant in this Agreement and the Ancillary Documents that survives the Closing by its nature or in accordance with its terms. Section 9.13. Counterparts; Effectiveness. This Agreement may be executed in multiple counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via electronic mail (including pdf or any electronic signature, e.g., xxx.xxxxxxxx.xxx) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes. Section 9.14. Severability; Validity. If any provision of this Agreement or the application thereof to any Person or circumstance is held invalid or unenforceable, the remainder of this Agreement, and the application of such provision to other Persons or circumstances, shall not be affected thereby, and to such end, the provisions of this Agreement are agreed to be severable. Section 9.15. Specific Performance. The parties agree that irreparable injury will occur in the event that any of the provisions of this Agreement is not performed in accordance with its specific terms or is otherwise breached. Each party shall be entitled to seek an injunction or injunctions to prevent or remedy any breaches or threatened breaches of this Agreement by any other party, to a decree or order of specific performance specifically enforcing the terms and provisions of this Agreement and to any further equitable relief. Section 9.16. Remedies Cumulative. Except as herein expressly set forth, no remedy conferred upon a party by this Agreement is intended to be exclusive of any other remedy

39 herein or by Law provided or permitted, but each shall be cumulative and shall be in addition to every other remedy given herein or now or hereafter existing at Law, in equity or by statute. Section 9.17. Conflicts; Deal Communications. (a) It is acknowledged by each of the parties that Seller has retained Sidley Austin LLP (“Sidley”) to act as its counsel in connection with the negotiation, documentation and consummation of this Agreement and the Transactions (the “Current Representation”), and that Purchaser does not have the status of a client of Sidley for conflict of interest arising from the Current Representation. Purchaser hereby agrees that after the Closing, Sidley may represent Seller or any of its Affiliates or any of their respective Representatives (any such Person, a “Permitted Seller Person”) in any matter involving or arising from the Current Representation, including any interpretation or application of this Agreement or any Ancillary Document, and including for the avoidance of doubt any Actions involving Purchaser or any of its Affiliates, and any Permitted Seller Person, even though the interests of such Permitted Seller Person may be directly adverse to Purchaser or any of its Affiliates, and even though Sidley may have represented Purchaser in a substantially related matter, or may be representing Purchaser in ongoing matters. Purchaser hereby waives and agrees not to assert (i) any claim that Sidley has a conflict of interest in any representation described in this Section 9.17(a) or (ii) any confidentiality obligation with respect to any communication between Sidley and any Permitted Seller Person occurring during the Current Representation. (b) Purchaser hereby agrees that all communications (whether before, at or after the Closing) between Sidley, any Permitted Seller Person, or any current or former director, officer or employee of Seller to the extent related to the Current Representation or any dispute arising under this Agreement (the “Deal Communications”), whether or not attorney-client privileged, and all rights to any other evidentiary privilege to the extent related thereto, and the protections afforded to information relating to representation of a client under applicable rules of professional conduct that may apply to such Deal Communications, shall be retained, owned, and controlled collectively by the Permitted Seller Persons and shall not pass to or be claimed by Purchaser or any of its Affiliates or their respective Representatives. To the extent that files or other materials maintained by Sidley constitute Deal Communications, only the Permitted Seller Persons shall hold property rights in such communications and Sidley shall have no duty to reveal or disclose any such files or other materials or any Deal Communications by reason of any attorney-client relationship between Sidley and any Permitted Seller Persons. (c) Notwithstanding the foregoing, if a dispute related to the Acquired Assets, the Assumed Liabilities or the transactions contemplated by this Agreement or any other Ancillary Document arises between Purchaser or its Affiliates, on the one hand, and a third party other than (and unaffiliated with) Seller and its Affiliates and Representatives, on the other hand, after the Closing, then Purchaser and its Affiliates may assert such attorney-client privilege to prevent disclosure to such third party of confidential communications by Sidley. Section 9.18. Non-Waiver. Neither the failure nor any delay by any party in exercising any right, power, or privilege under this Agreement or the documents referred to in this

40 Agreement shall operate as a waiver of such right, power or privilege, and no single or partial exercise of any such right, power, or privilege shall preclude any other or further exercise of such right, power, or privilege or the exercise of any other right, power, or privilege. To the maximum extent permitted by Law, (a) no waiver that may be given by a party shall be applicable except in the specific instance for which it is given, and (b) no notice to or demand on one party shall be deemed to be a waiver of any right of the party giving such notice or demand to take further action without notice or demand. ARTICLE X DEFINITIONS As used herein, the terms below shall have the following meanings: “Acquired Assets” is defined in Section 1.1. “Acquired Books and Records” is defined in Section 1.1(e). “Acquired Patents” means, collectively, all Patents included in the Acquired Assets. “Acquisition” is defined in the Recitals. “Action” means any claim, hearing, charge, action, suit, arbitration, litigation, mediation, grievance, audit, examination, inquiry, proceeding or investigation by or before any Governmental Entity or arbitrator. “Additional Assigned Contracts” is defined in Section 1.5(d). “Affiliate” of a specified Person means a Person who, directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, such specified Person, and the term “control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such Person, whether through ownership of voting securities, by contract or otherwise. “Aggregate Net Sales” means all Net Sales of the Products in the Territory on a cumulative basis subsequent to their First Commercial Sale. “Agreement” has the meaning set forth in the Preamble and shall include the Schedules annexed hereto or referred to herein. “Allocation” is defined in Section 1.9. “Ancillary Documents” means the Bill of Sale & Assignment and Assumption Agreement, Intellectual Property Assignment Agreements, Escrow Agreement, and each other agreement, document or instrument (other than this Agreement) executed and delivered by the parties in connection with the consummation of the Transactions.

41 “Anti-Corruption Law” means any Law related to combating bribery and corruption, including the OECD Convention on Combating Bribery of Foreign Officials in International Business Transactions, the UN Convention Against Corruption and any implementing legislation promulgated pursuant to such Conventions, the Foreign Corrupt Practices Act of 1977 and the U.K. Bribery Act 2010. “Assigned Contracts” is defined in Section 1.1(b). “Assignment Effective Date” means the date on which the assignment of an Assigned Contract (including any Designated Contract) is assigned to Purchaser in accordance with the terms hereof. “Assumed Liabilities” is defined in Section 1.3. “Auction” is defined in the Bidding Procedures. “Avoidance Actions” is defined in Section 1.1(i). “Bankruptcy Code” is defined in the Recitals. “Bankruptcy Court” is defined in the Recitals. “Base Cash Amount” is defined in Section 1.7(a)(i). “Bidding Procedures” means the bidding procedures approved by the Bankruptcy Court pursuant to the Bidding Procedures Order. “Bidding Procedures Order” means the Order (A) Approving Certain Bidding Procedures and the Form and Manner of Notice Thereof, (B) Scheduling an Auction and a Hearing on the Approval of the Sale of All or Substantially All of the Debtor’s Assets, (C) Establishing Certain Assumption and Assignment Procedures and Approving the Manner of Notice Thereof, and (D) Granting Related Relief entered by the Bankruptcy Court on January 26, 2023 in the Chapter 11 Case at docket number 100. “Bill of Sale & Assignment and Assumption Agreement” is defined in Section 2.2(a)(i). “Books and Records” means all documents of, or otherwise in the possession, custody or control of, or used by, Seller that primarily relate to the Acquired Assets or the Assumed Liabilities, including all files, instruments, papers, books, microfilms, photographs, letters, budgets, forecasts, ledgers, journals, title policies, lists of past, present or prospective customers, supplier lists, regulatory filings, technical documentation, and financial and tax records. “Business” is defined in the Recitals.

42 “Business Day” means any day other than a Saturday, a Sunday or a day on which banks in San Francisco, California, Dallas, Texas or New York, New York are authorized or obligated by Law to close. “Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 or December 31. “Cash” means all cash and cash equivalents, restricted cash, including checks, commercial paper, treasury bills, certificates of deposit, investments, other deposits, instruments and marketable securities held in the name of or for the benefit of Seller and any bank accounts of Seller as of immediately prior to the Closing. “Chapter 11 Case” is defined in the Recitals. “Closing” is defined in Section 2.1. “Closing Date” is defined in Section 2.1. “COBRA” means the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended. “Code” means the Internal Revenue Code of 1986. “Commercialization” or “Commercialize” means any and all activities undertaken before and after Marketing Approval for any Product and that relate to the marketing, promoting, distributing, importing or exporting for sale, offering for sale and selling of the Product, and interacting with Regulatory Authorities regarding the foregoing. “Commercially Reasonable Efforts” means the application by the applicable party of diligent efforts and resources to fulfill the obligation at issue, consistent with the exercise of prudent scientific and business judgment and the level of efforts that a pharmaceutical company of comparable size and resources as those of such party and its Affiliates would normally devote to a product at a similar stage in its product life as the Products and having profit potential and strategic value comparable to that of the Products, taking into account, without limitation, commercial, legal and regulatory factors, target product profiles, product labeling, past performance, the regulatory environment and competitive market conditions in the therapeutic area, safety and efficacy of the Product, the strength of its proprietary position and such other factors as a similarly situated pharmaceutical company may reasonably consider, all based on conditions then prevailing. For clarity, Commercially Reasonable Efforts will not mean that a party guarantees that it will actually accomplish the applicable task or objective. “Compound” means the highly cross-linked, aliphatic amine polymer originally synthesized by Seller, also known as veverimer and formerly known as TRC101, which has the chemical name poly(allylamine-co- -diallyl-1,3-diaminopropane-co-1,2-diaminoethane) with the following structure:

43 a = residue of N,N’-diallyl-1,3-diaminopropane dihydrochloride (monomer and crosslinker); b = residue of allylamine (monomer); c = residue of 1,2-dichloroethane (ethylene crosslink between two amines); an ethylene linkage between two allylamine groups is shown as an example of one of many possible linkages between amines; and m = a larger number indicating an extended polymer network. “Confidential Disclosure Agreement” means the Confidential Disclosure Agreement, between Seller and Purchaser, dated January 19, 2023. “Confidential Information” is defined in Section 5.11(b). “Confirmation” mean entry of an Order by the Bankruptcy Court confirming the Seller’s plan of liquidation in the Chapter 11 Case. “Confirmation Date” means the date on which the Confirmation occurs. “Contingent Payment” means an FDA Milestone Payment or a Sales Milestone Payment. “Contract” means any agreement, contract, instrument, concession, franchise, note, option, bond, mortgage, indenture, trust document, loan or credit agreement, or other legally binding commitment or instrument. “COVID-19” means SARS-Co V-2 or COVID-19 and any evolutions, variants or mutations thereof or related or associated epidemics, pandemics or disease outbreaks. “COVID-19 Measures” means quarantine, “shelter in place,” “stay at home,” workforce reduction, social distancing, shut down, closure, sequester, safety or similar laws, directives, restrictions, guidelines, responses or recommendations of or promulgated by any Governmental

44 Entity, including the Centers for Disease Control and Prevention and the World Health Organization, or other reasonable actions taken in response to the foregoing or otherwise, in each case, in connection with or in response to COVID-19. “Cure Costs” is defined in Section 1.5(a). “Current Representation” is defined in Section 9.17(a). “Deal Communications” is defined in Section 9.17(b). “Deposit Funds” is defined in Section 1.7(b). “Designated Contracts” means the Contracts listed, described or otherwise identified on Schedule 1.5(b). “Designated Parties” is defined in Section 1.1(h). “Designation Deadline” is defined in Section 1.5(d). “Develop” or “Development” means, with respect to any Product, the performance of all pre-clinical and clinical development (including toxicology, pharmacology, test method development and stability testing, process development, formulation development, quality control development, statistical analysis, chemistry, manufacturing and controls (CMC) activities), clinical trials (excluding clinical trials conducted after regulatory approval of an NDA), manufacturing and regulatory activities that are required to obtain regulatory approval of Product. “Effect” means any change, effect, development, circumstance, condition, fact, state of facts, event or occurrence. “Encumbrance” means any lien (statutory or otherwise), pledge, hypothecation, mortgage, deed of trust, security interest, encumbrance, covenant, charge, claim, option, right of first refusal, easement, right of way, encroachment, occupancy right, preemptive right, charge, community property interest or restriction of any nature, including any restriction on use, voting, transfer, receipt of income or exercise of any other attribute of ownership, whether for value or no value and whether voluntary or involuntary (including by operation of Law or by judgment, levy, attachment, garnishment, bankruptcy or other legal or equitable proceedings). “Environmental Laws” means any Laws applicable to the Business which (a) regulate or relate to the protection or clean-up of the environment, the use, treatment, storage, transportation, presence, recycling, reclamation, reuse, generation, processing, production, remediation, handling, disposal or release of Hazardous Substances, the preservation or protection of waterways, groundwater, drinking water, air, wildlife, plants or other natural resources, or the health and safety of Persons or property or (b) impose Liability or responsibility with respect to any of the foregoing. The term “Environmental Laws” includes the following (including their implementing regulations and any state analogs): the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended by the Superfund Amendments and Reauthorization Act of 1986, 42

45 U.S.C. §§ 9601 et seq.; the Solid Waste Disposal Act, as amended by the Resource Conservation and Recovery Act of 1976, as amended by the Hazardous and Solid Waste Amendments of 1984, 42 U.S.C. §§ 6901 et seq.; the Federal Water Pollution Control Act of 1972, as amended by the Clean Water Act of 1977, 33 U.S.C. §§ 1251 et seq.; the Toxic Substances Control Act of 1976, as amended, 15 U.S.C. §§ 2601 et seq.; the Emergency Planning and Community Right-to-Know Act of 1986, 42 U.S.C. §§ 11001 et seq.; the Clean Air Act of 1966, as amended by the Clean Air Act Amendments of 1990, 42 U.S.C. §§ 7401 et seq.; and the Occupational Safety and Health Act of 1970, as amended, 29 U.S.C. §§ 651 et seq. “Equipment” means the tangible personal property owned, used or held for use by Seller, including, but not limited to, the physical assets and all leasehold improvements, furnishings, equipment, machinery, tools, supplies, spare parts, copiers, telecopy machines and other telecommunication equipment, cubicles and miscellaneous office materials owned by Seller. “ERISA” means the Employee Retirement Income Security Act of 1974, as amended. “Escrow Agent” is defined in Section 1.7(b). “Escrow Agreement” is defined in Section 1.7(b). “Excluded Liabilities” is defined in Section 1.4. “FDA” means the United States Food and Drug Administration. “FDA Milestone Event” is defined in Section 2.3(a). “FDA Milestone Payment” is defined in Section 2.3(a). “Final Order” means an order or judgment of the Bankruptcy Court (or any other court of competent jurisdiction) entered by the clerk of the Bankruptcy Court (or such other court) on the docket in the Chapter 11 Case (or the docket of such other court), which has not been modified, amended, reversed, vacated or stayed and as to which (i) the time to appeal, petition for certiorari, or move for a new trial, reargument or rehearing has expired and as to which no appeal, petition for certiorari or motion for new trial, reargument or rehearing shall then be pending or (ii) if an appeal, writ of certiorari new trial, reargument or rehearing thereof has been sought, such order or judgment of the Bankruptcy Court (or other court of competent jurisdiction) shall have been affirmed by the highest court to which such order was appealed, or certiorari shall have been denied, or a new trial, reargument or rehearing shall have been denied or resulted in no modification of such order, and the time to take any further appeal, petition for certiorari or move for a new trial, reargument or rehearing shall have expired, as a result of which such order shall have become final in accordance with Rule 8002 of the Federal Rules of Bankruptcy Procedure; provided, that the possibility that a motion under Rule 60 of the Federal Rules of Civil Procedure, or any analogous rule under the Bankruptcy Rules, may be filed relating to such order, shall not cause an order not to be a Final Order.

46 “First Commercial Sale” means the first sale to a Third Party of a Product in a given regulatory jurisdiction after Marketing Approval has been obtained in such jurisdiction. “GAAP” means United States generally accepted accounting principles. “Governmental Entity” means (a) any supranational, national, federal, state, county, municipal, local, or foreign government or any entity exercising executive, legislative, judicial, regulatory, taxing, or administrative functions of or pertaining to government, (b) any public international governmental organization or (c) any agency, division, bureau, department, commission, board, arbitral or other tribunal, branch or other political subdivision of any government, entity or organization described in the foregoing clause (a) or (b) of this definition. “Hazardous Substance” means any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive, corrosive, ignitable or flammable chemical, chemical compound, hazardous substance, material or waste that is subject to regulation or control under any Environmental Laws. “Health Laws” all Laws applicable to the Seller the purpose of which is to ensure the safety, efficacy and quality of medicines, biological products or pharmaceuticals by regulating the research, development, manufacturing and distribution of these products, including the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301), the Anti-Kickback Statute (42 U.S.C. Section 1320a-7b(b)), the Civil Monetary Penalties Law (42 U.S.C. § 1320a-7a), the Physician Payment Sunshine Act (42 U.S.C. § 1320a-7h), the Civil False Claims Act (31 U.S.C. Section 3729 et seq.), the criminal False Claims Law (42 U.S.C. § 1320a-7b(a)), all criminal laws relating to health care fraud and abuse, including but not limited to 18 U.S.C. Sections 286 and 287, and the health care fraud criminal provisions under HIPAA (42 U.S.C. Section 1320d et seq.), the exclusion laws (42 U.S.C. § 1320a-7), HIPAA and similar state and foreign privacy and data security laws such as the European Union General Data Protection Regulation, Medicare (Title XVIII of the Social Security Act), Medicaid (Title XIX of the Social Security Act), and any and all other comparable state, local, federal or foreign health care laws. “HIPAA” means the Health Insurance Portability and Accountability Act of 1996, as supplemented by the Health Information Technology for Clinical Health Act of the American Recovery and Reinvestment Act of 2009. “Indebtedness” means, with respect to any Person, without duplication: (a) the principal, interest and premium (if any) in respect of (i) indebtedness of such Person for money borrowed and (ii) indebtedness evidenced by notes, debentures, bonds or other similar instruments for the payment of which such Person is responsible or liable; (b) all obligations of such Person under leases required to be capitalized in accordance with GAAP; (c) all obligations of such Person for the reimbursement of any obligor on any letter of credit, banker’s acceptance or similar credit transaction; (d) all obligations of the type referred to in clauses (a) through (c) of any Persons for the payment of which such Person is responsible or liable, directly or indirectly, as obligor, guarantor, surety or otherwise, including guarantees of such obligations; (e) all obligations of the type referred to in clauses (a) through (d) of other Persons secured by any lien on any property or

47 asset of such Person (whether or not such obligation is assumed by such Person), and (f) all accrued interest, prepayment premiums or penalties, and fees and expenses related to any of the foregoing obligations. “Indication” means a generally acknowledged disease or condition. “Intellectual Property” means all intellectual property rights, including, all intellectual property rights arising from or in respect of the following: (i) all patents and applications therefor, including continuations, divisionals, continuations-in-part, or reissues of patent applications and patents issuing thereon (collectively, “Patents”), (ii) trademarks, service marks, trade names, service names, brand names, all trade dress rights, logos and corporate names and general intangibles of a like nature, together with the goodwill associated with any of the foregoing, and all applications, registrations and renewals thereof (collectively, “Trademarks”), (iii) copyrights and registrations and applications therefor (collectively, “Copyrights”), (iv) trade secrets and confidential inventions (including recipes, know-how, formulas, compositions and manufacturing and production processes and techniques), and (v) internet domain names, websites, web pages and social media accounts. “Intellectual Property Assignment Agreements” is defined in Section 2.2(a)(ii). “Inventory” is defined in Section 1.1(a). “IRS” means the United States Internal Revenue Service. “Knowledge of Purchaser” means the actual knowledge of the individuals listed on Schedule 11. “Knowledge of Seller” means the actual knowledge of the individuals listed on Schedule 10. “Law” means any law, statute, constitution, requirement, code, rule, regulation, order, ordinance, treaty, judgment or decree or other pronouncement of any Governmental Entity. “Lease” means the contract pursuant to which Seller leases the property at 0000 Xxxxxxxxx Xxxxx, Xxxxx 00, Xxxxx Xxx Xxxxxxxxx, Xxxxxxxxxx 00000, by and between Seller and Are-San Francisco No. 17, LLC dated April 4, 2014, as amended by that certain first amendment, dated August 2, 2017, that certain second amendment dated November 7, 2017, that certain third amendment dated August 14, 2019 and that certain fourth amendment dated December 14, 2020. “Liability” means any liability, debt, guarantee, claim, demand, commitment, Encumbrance or obligation (whether known or unknown, asserted or unasserted, direct or indirect, absolute or contingent, accrued or unaccrued, liquidated or unliquidated, or due or to become due) of every kind and description, including all costs and expenses related thereto. “Maintenance Costs” is defined in Section 1.5(b).

48 “Maintenance Termination Date” is defined in Section 1.5(b). “Marketing Approval” means, with respect to the Product in a particular country or regulatory jurisdiction, receipt of all approvals, including Pricing Approval, necessary for the commercial sale of the Product in such country or regulatory jurisdiction. “Material Adverse Effect” means (a) any Effect that, individually or in the aggregate, is materially adverse to the ability of Seller to consummate the Transactions or (b) any Effect that, individually or in the aggregate, results in a material adverse effect on the Acquired Assets or the Assumed Liabilities (taken as a whole); provided, however, that the fact that the Chapter 11 Case has been filed and that, accordingly, Seller has been conducting the Business in the Ordinary Course of Business as the same is being conducted as of the date of this Agreement in the Chapter 11 Case, any effect resulting from such filing or the prosecution of the Chapter 11 Case or any action taken by the Bankruptcy Court, shall not, in and of itself, be deemed to be a Material Adverse Effect for purposes of this definition; and provided, further, that no Effects resulting or arising from the following shall be deemed to constitute a Material Adverse Effect or shall be taken into account when determining whether a Material Adverse Effect exists or has occurred: (i) changes in general economic, financial, credit or securities markets or geopolitical conditions, (ii) general changes or developments in regulatory or macroeconomic conditions or the industries and markets in which the Business operates, (iii) the announcement of the Transactions or the identity of Purchaser, (iv) any action or omission by Purchaser in breach of this Agreement, (v) any action requested by Purchaser, (vi) changes in any applicable Laws or applicable accounting regulations or principles or the enforcement or interpretation thereof, (vii) any outbreak or escalation of hostilities or war or any act of terrorism or natural disaster or act of God, (viii) any failure to meet internal or published projections, forecasts, or revenue or earning predictions, (ix) any epidemics, pandemics or contagious disease outbreaks (including COVID-19) and any political or social conditions, including civil unrest, protests and public demonstrations; (x) any matter disclosed in the Seller SEC Documents, (xi) any action required or prohibited by the commencement of the Chapter 11 Case and (xii) any effect resulting from the public announcement or pendency of this Agreement, the identity of Purchaser, compliance with terms of this Agreement, the taking of any action permitted to be taken hereunder or in connection with the Transactions contemplated hereby, or the consummation of the Transactions, in each case, including on relationships, contractual or otherwise, with customers, suppliers, vendors or employees; provided, further, that the exceptions set forth in clauses (i), (ii), (vi), (vii) and (ix) above shall only apply to the extent that such Effect is materially and disproportionately adverse to the Business, taken as a whole, compared to other companies in the industry or market in which Seller operates (in which case, only the incremental disproportionate adverse effect may be taken into account in determining whether a Material Adverse Effect has occurred). “Material Contracts” is defined in Section 3.10(a). “NDA” means a New Drug Application, as defined in the U.S. Federal Food, Drug, and Cosmetic Act, as amended, and applicable regulations promulgated thereunder by the FDA.

49 “Net Sales” means the gross amount invoiced by Purchaser or any Purchaser Party for sales of a Product to Third Parties (“Gross Sales”) during the applicable Calendar Quarter, less the following deductions, in each case, (i) without duplication, (ii) where applicable with respect to Gross Sales, and (iii) as incurred in the Ordinary Course of Business in type and amount consistent with Purchaser’s standard practice, as determined in accordance with, and as recorded in revenues under, GAAP or IFRS: (a) trade discounts, including trade, cash and quantity discounts or rebates, credits or refunds (Including inventory management fees, discounts or credits); (b) allowances or credits actually granted upon claims, returns or rejections of products, including recalls, regardless of the party requesting such recall; (c) bad debts or provisions for bad debts; (d) charges included in the gross sales price for freight, insurance, transportation, postage, handling and any other charges relating to the sale, transportation, delivery or return of such Product; (e) customs duties, sales, excise and use taxes and any other governmental charges (including value added tax) actually paid in connection with the transportation, distribution, use or sale of such Product (but excluding what is commonly known as income taxes); (f) rebates and chargebacks or retroactive price reductions made to federal, state or local governments ( or their agencies or programs), or any Third Party payor, administrator or contractor, including managed health organizations; and (g) the portion of any deductions to gross invoice price imposed by Regulatory Authorities or other governmental entities, including the annual fee on branded prescription pharmaceutical manufacturers and importers under the US Affordable Care Act, that Purchaser allocates to sales of the Products. Sales between Purchaser and its Affiliates and licensees shall be disregarded for purposes of calculating Net Sales except if such purchaser is an end user; provided that any subsequent sales to any end user shall be included in Net Sales. Net Sales shall not be imputed to transfers of Products for use in any clinical trial, for bona fide charitable purposes, for compassionate use, for indigent patient programs or as free samples of Products. Notwithstanding the foregoing, in the event a Product is sold as a component of a Combination Product in any country, in any Calendar Quarter, Net Sales shall be calculated by multiplying the Net Sales of the Combination Product in such country during such Calendar Quarter (calculated by applying the formula set forth above as if it applied to sales of such Combination Product in such country) by the fraction A/(A+B), where A is the average Net Sales per unit sold of such Product(s) when sold separately in such country during such Calendar Quarter (calculated by determining the Net Sales of such Product(s) in such country during such Calendar Quarter in accordance with the formula set forth above and dividing such Net Sales by the number of units of such Product(s) sold in such country during such Calendar Quarter) and B is the average Net Sales per unit sold of the Other Products included in the Combination Product when sold separately in such country during such Calendar Quarter (calculated by determining the Net Sales of such Other Product(s) in such country during such Calendar Quarter by applying the formula set forth above as if it applied to sales of such Other Product(s) and dividing such Net Sales by the number of units of such Other Product(s) sold in such country during such Calendar Quarter). In the event that no separate sales of a Product or any Other Product(s) included in a Combination Product are made by Purchaser or its Affiliates or licensees during a Calendar Quarter in which such Combination Product is sold in a country, the average Net Sales per unit sold in the above described equation shall be replaced with Purchaser’s reasonable good faith estimate of the fair

50 market value of such Product(s) and each of the Other Product(s) included in such Combination Product. For purposes of this definition, “Combination Product” means a Royalty-Bearing Product sold in combination with or bundled with an Other Product for a single price. “Net Sales Statement” means a written statement of Purchaser, certified by the chief financial officer of Purchaser, setting forth in reasonable detail the calculation of Net Sales for each Calendar Quarter, each of which shall include (a) an itemized calculation of the gross amounts invoiced by the Purchaser Parties and applicable sublicensees for the Product sold to third parties, (b) an itemized calculation of the permitted deductions, and (c) to the extent that any of the amounts in clauses (a) or (b) are recorded in currencies other than United States Dollars, the exchange rates used for conversion of such foreign currency into United States Dollars. The Net Sales Statement shall be calculated in accordance with applicable accounting standards and shall be derived from the financial statements of Purchaser. “Next-Highest Bidder” is defined in the Bidding Procedures. “Order” means any order, injunction, judgment, decree, ruling, writ, assessment or award of a Governmental Entity. “Ordinary Course of Business” means the ordinary and usual course of normal day-to-day operations of Seller’s Business, as conducted by Seller consistent with past practice and except for the consequences relating to the filing of the Chapter 11 Case; provided, that in no event shall “Ordinary Course of Business” include any breach of Law or Contract, or violation of any Permit. “Other Product” means any active ingredient, component or product that is not itself a Product but is a regulated component required to effect treatment of an applicable Indication. “Outside Date” is defined in Section 8.1. “party” and “parties” are defined in the Preamble. “Paying Party” is defined in Section 9.1(c). “Permits” means all material consents, approvals, authorizations, clearances, certificates, notices, permits, registrations, or licenses issued by any Governmental Entity. “Permitted Seller Person” is defined in Section 9.17(a). “Person” means a natural person, partnership, corporation, limited liability company, business trust, joint stock company, trust, unincorporated association, joint venture, Governmental Entity or other entity or organization. “Petition Date” is defined in the Recitals. “Preservation Period” is defined in Section 5.2(b).

51 “Pricing Approval” means such governmental approval, agreement, determination or decision establishing prices for the Product that can be charged and/or reimbursed in regulatory jurisdictions where the applicable Governmental Entities approve or determine the price and/or reimbursement of pharmaceutical products and where such approval, agreement, determination or decision establishes prices for the Product that are acceptable to Purchaser in its sole discretion. “Product” means (a) the Compound and (b) any product or product candidate that is comprised of or contains the Compound. “Professional Services” is defined in Section 1.4(d). “Program Patents” means: (a) the Acquired Patents; (b) any and all provisionals, divisionals, continuations and continuations-in-part of the patents and patent applications referenced in the preceding subsection (a); (c) all foreign patent applications associated with the patent applications referenced in the preceding subsections (a) and (b); (d) all patents issued or issuing from the patent applications referenced in the preceding subsections (a) through (c); and (e) reissues, reexaminations, restorations (including supplemental protection certificates) and extensions of any patent or patent application referenced in the preceding subsections (a) through (d). “Purchase Price” is defined in Section 1.7(a). “Purchaser” is defined in the Recitals. “Purchaser Designee” is defined in Section 9.7. “Purchaser Parties” means Purchaser and its Affiliates, and with respect to any Products, any of their successors, assigns, or direct or indirect licensees or sublicensees (including at any tier of sublicense) of such Products. “Regulatory Authority” means any national or supranational Governmental Entity, including the FDA, with responsibility for granting any license, registrations or approvals with respect to the Products. “Regulatory Authorizations” means any approvals, clearances, authorizations, registrations, certifications, licenses and permits granted by any Regulatory Authority.

52 “Regulatory Documentation” means all (a) applications, submissions, registrations, or notifications submitted, or generated or prepared in preparation for, or anticipation of, submission, to a Regulatory Authority with a view to the filing, obtaining, updating or maintaining of any Regulatory Authorization, in each case including any investigational medicinal product dossier to the extent relating to the Product, (b) correspondence with or to Regulatory Authorities (including Regulatory Authorization letters, minutes and official contact reports relating to any communications with any Regulatory Authorities) with respect to the assets described in clause (a) above, (c) records contained in all pharmacovigilance and study databases, all adverse drug events, experience or reaction reports and associated documents, investigations of adverse drug event, experience or reaction reports, and any other information relevant to the assessment of safety or benefit-risk ratios, including, for the avoidance of doubt, all legacy data, in each case to the extent relating to the Product, and (d) non-clinical, clinical and other files, writings, drafts, notes, studies, reports, modules and other documents or data contained or referenced in or supporting any of the foregoing, in each case, that were acquired, developed, compiled, collected or generated by Seller or by any third party on behalf of Seller, in each case, to the extent used or related to the Product. “Reimbursing Party” is defined in Section 9.1(c). “Representatives” means, when used with respect to any Person, the directors, officers, employees, consultants, financial advisors, accountants, legal counsel, investment bankers and other agents, advisors and representatives of such Person and its Subsidiaries. “Retained Books and Records” means the company seal, minute books, stock certificates, stock or equity record books and ledgers, Tax Returns and other books, records and work papers related to Taxes paid or payable by Seller or its Affiliates, work papers and such other books and records as pertain to the organization, qualification to do business, existence or capitalization of Seller or any Affiliate thereof, books and records that Seller is required to retain under applicable Law, books and records that primarily relate to an Excluded Asset or Excluded Liability and all of Seller’s communications, documents, or materials primarily related to the Excluded Assets and the Excluded Liabilities or that are subject to any confidentiality, work product doctrine, common interest, joint defense privilege or other privilege, and electronic and tangible documents reflecting such communications and materials. “Royalty-Bearing Product” means any Product that, at the time of its First Commercial Sale, is covered by, or is developed in whole or in part by processes covered by, a Valid Claim, in the country where the Product is made, sold or used. “Sale Hearing” means the hearing conducted by the Bankruptcy Court to approve the Transactions. “Sale Order” means an Order of the Bankruptcy Court, in form and substance acceptable to the Purchaser, with any changes that Purchaser may approve in advance, which approval shall not be unreasonably withheld, conditioned, or delayed, that has not been stayed, vacated or stayed pending appeal, which, among other things, (a) approves, pursuant to Sections 105, 363 and 365

53 of the Bankruptcy Code, (i) the execution, delivery and performance by Seller of this Agreement, (ii) the sale of the Acquired Assets to Purchaser free and clear of all Encumbrances on the terms set forth herein, and (iii) the performance by Seller of its obligations under this Agreement; (b) authorizes the Seller to assume and assign to Purchaser the Assigned Contract; (c) finds that Purchaser is not a successor to the Seller, and (d) finds that Purchaser is a “good faith” buyer within the meaning of Section 363(m) of the Bankruptcy Code and grants Purchaser the full protection provided thereby. “Sales Milestone Event” means each Calendar Quarter with respect to which Sales Milestone Payments become due and payable in accordance with Section 2.3(b). “Sales Milestone Payment” means any payment that becomes due and payable pursuant to Section 2.3(b). “SEC” means the Securities and Exchange Commission. “Seller” is defined in the Recitals. “Seller Disclosure Schedule” means the disclosure schedules delivered by Seller to Purchaser immediately prior to the execution of this Agreement. “Seller Intellectual Property” means all Intellectual Property owned by Seller and used or held for use in the Business (other than Intellectual Property that is related to an Excluded Asset). “Seller SEC Documents” means all forms, statements, documents and reports filed or furnished by Seller with the SEC. “Seller’s Group” is defined in Section 9.12. “SOFR” means, with respect to any period, the daily Secured Overnight Financing Rate provided by the Federal Reserve Bank of New York as the administrator of the benchmark (or a successor administrator) on the Federal Reserve Bank of New York’s website, as of the date two calendar days prior to the first day of such period. “Subsidiary” means with respect to any Person, any corporation, limited liability company, partnership or other organization, whether incorporated or unincorporated, of which (a) at least a majority of the outstanding shares of capital stock, or other equity interests, having by their terms ordinary voting power to elect a majority of the board of directors or others performing similar functions with respect to such corporation, limited liability company, partnership or other organization is directly or indirectly owned or controlled by such Person or by any one or more of its Subsidiaries, or by such Person and one or more of its Subsidiaries, or (b) with respect to a partnership, such Person or any other Subsidiary of such Person is a general partner of such partnership. “Successful Bidder” is defined in the Bidding Procedures.

54 “Tax” or “Taxes” means any and all U.S. federal, state, local and non-U.S. taxes, assessments, levies, duties, tariffs, imposts and other similar charges and fees imposed by any Governmental Entity, including income, franchise, windfall or other profits, gross receipts, property, sales, use, net worth, capital stock, payroll, employment, social security, workers’ compensation, unemployment compensation, excise, withholding, ad valorem, stamp, transfer, value-added, occupation, environmental, disability, real property, personal property, registration, alternative or add-on minimum, or estimated tax, including any interest, penalty, additions to tax and any additional amounts imposed with respect thereto. “Tax Return” means any report, return, certificate, claim for refund, election, estimated Tax filing or declaration filed or required to be filed with any Governmental Entity with respect to Taxes, including any schedule or attachment thereto, and including any amendments thereof. “Taxing Authority” means any federal, state, local or foreign Governmental Entity or authority responsible for the imposition, collection or administration of any Tax. “Territory” means all of the world. “Third Party” means any Person other than Seller or Purchaser or an Affiliate of Seller or Purchaser. “Transactions” means the transactions contemplated by this Agreement and the Ancillary Documents. “Transfer Taxes” is defined in Section 9.1(a). “Transferred Permit” is defined in Section 1.1(f). “Valid Claim” means (a) any claim of an issued and unexpired patent in the Program Patents, (as may be extended through supplementary protection certificate or patent term extension), which claim (i) has not been revoked, held invalid or unenforceable by a patent office, court or other governmental agency of competent jurisdiction in a final and non-appealable judgment (or judgment from which no appeal was taken within the allowable time period) and (ii) has not been disclaimed, denied or admitted to be invalid or unenforceable through reissue, re- examination or disclaimer or otherwise or (b) any claim of a pending application in the Program Patents, which claim has not been pending for more than five (5) years from its earliest U.S. or foreign priority date. “WARN Act” means the federal Worker Adjustment and Retraining Notification Act, 29 U.S.C. § 2101 et seq. (1988) and any similar Laws, including Laws of any state, country or other locality that is applicable to a termination of employees, including the Cal-WARN Act, Cal. Labor Code §§ 1400-1408. [Signature Page Follows]

IN WITNESS WHEREOF, Seller has caused this Agreement to be executed on its behalf by its officers thereunto duly authorized, as of the date first above written. SELLER: TRICIDA, INC. By: Name: Xxxxxx Xxxxxxxx Title: Chief Executive Officer /s/ Xxxxxx Xxxxxxxx

[Signature Page to Asset Purchase Agreement] IN WITNESS WHEREOF, Purchaser has caused this Agreement to be executed on its behalf by its officers thereunto duly authorized, as of the date first above written. PURCHASER: RENIBUS THERAPEUTICS, INC. By: Name: Xxxxxx Xxxxxxx Xxxxxx Title: Co-Chief Executive Officer /s/ Xxxxxx Xxxxxxx Xxxxxx

Schedule 1.1(b) Assigned Contracts None, subject to modification pursuant to Section 1.5.

Schedule 1.1(h) Designated Parties 1. PRA (ICON) 2. AndersonBrecon dba PCI 3. PPD Development, L.P. (excluding Patheon Austria GmbH & Co KG and Thermo Xxxxxx Scientific Inc.) 4. Pharmoron UK 5. Medpace Research Labs 6. LifeScience Logistics

Schedule 1.1(j) Acquired Assets & Rights None.

Schedule 1.2(m) Excluded Assets & Rights 1. Tax refunds a. IRS employee retention credit; and b. German VAT. 2. Trade prepaids and credit balances a. Approximately $11,000 of credit balances with non-CRO vendors; and b. Approximately $16,000 of prepayments due to milestones with CRO COMAC. 3. Deposits and unused retainers a. Leases; and b. $100,000 retainer balance with Ernst & Xxxxx. 4. Unearned insurance premia. 5. Personal Property and equipment a. Lab equipment; and b. FF&E personal property.
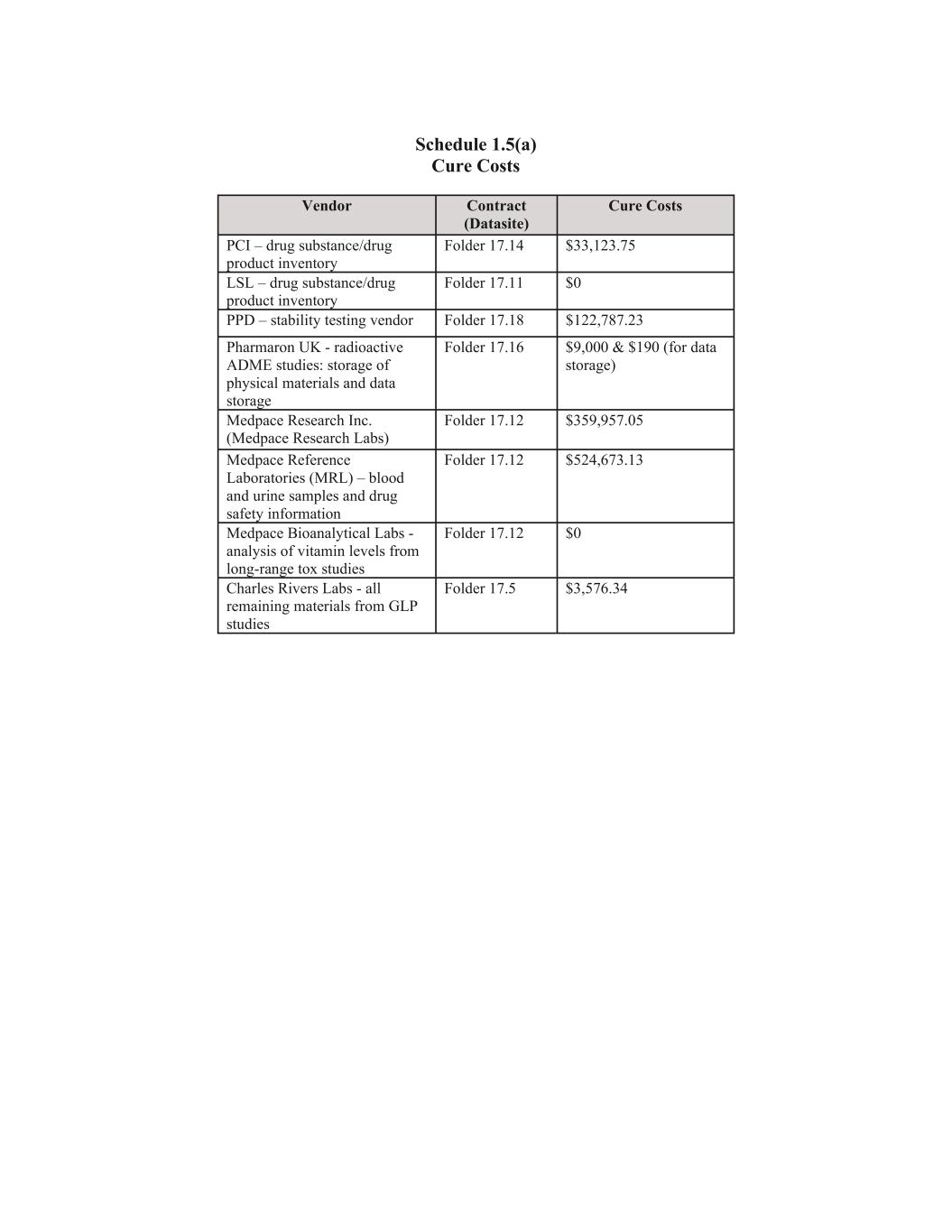
Schedule 1.5(a) Cure Costs Vendor Contract (Datasite) Cure Costs PCI – drug substance/drug product inventory Folder 17.14 $33,123.75 LSL – drug substance/drug product inventory Folder 17.11 $0 PPD – stability testing vendor Folder 17.18 $122,787.23 Pharmaron UK - radioactive ADME studies: storage of physical materials and data storage Folder 17.16 $9,000 & $190 (for data storage) Medpace Research Inc. (Medpace Research Labs) Folder 17.12 $359,957.05 Medpace Reference Laboratories (MRL) – blood and urine samples and drug safety information Folder 17.12 $524,673.13 Medpace Bioanalytical Labs - analysis of vitamin levels from long-range tox studies Folder 17.12 $0 Xxxxxxx Xxxxxx Labs - all remaining materials from GLP studies Folder 17.5 $3,576.34
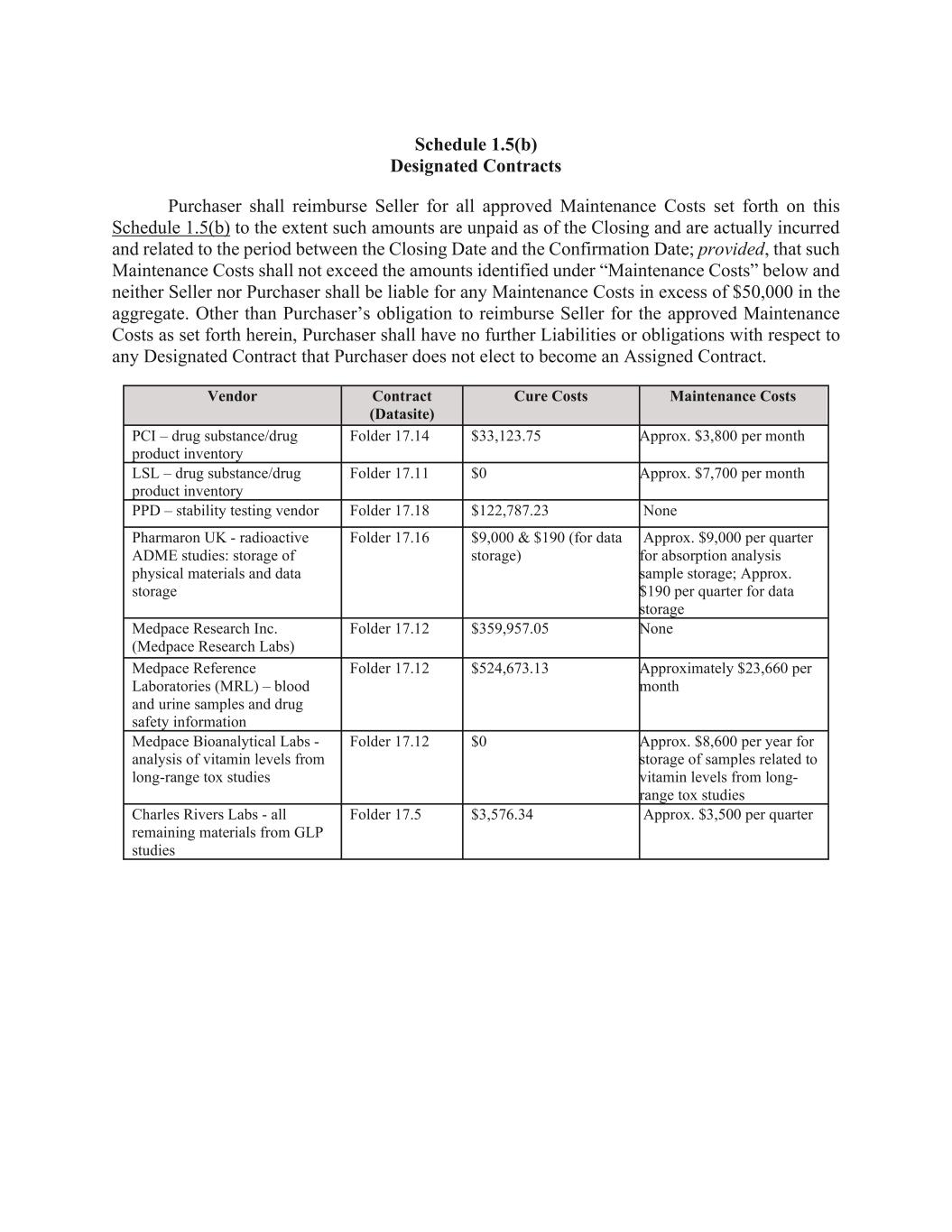
Schedule 1.5(b) Designated Contracts Purchaser shall reimburse Seller for all approved Maintenance Costs set forth on this Schedule 1.5(b) to the extent such amounts are unpaid as of the Closing and are actually incurred and related to the period between the Closing Date and the Confirmation Date; provided, that such Maintenance Costs shall not exceed the amounts identified under “Maintenance Costs” below and neither Seller nor Purchaser shall be liable for any Maintenance Costs in excess of $50,000 in the aggregate. Other than Purchaser’s obligation to reimburse Seller for the approved Maintenance Costs as set forth herein, Purchaser shall have no further Liabilities or obligations with respect to any Designated Contract that Purchaser does not elect to become an Assigned Contract. Vendor Contract (Datasite) Cure Costs Maintenance Costs PCI – drug substance/drug product inventory Folder 17.14 $33,123.75 Approx. $3,800 per month LSL – drug substance/drug product inventory Folder 17.11 $0 Approx. $7,700 per month PPD – stability testing vendor Folder 17.18 $122,787.23 None Pharmaron UK - radioactive ADME studies: storage of physical materials and data storage Folder 17.16 $9,000 & $190 (for data storage) Approx. $9,000 per quarter for absorption analysis sample storage; Approx. $190 per quarter for data storage Medpace Research Inc. (Medpace Research Labs) Folder 17.12 $359,957.05 None Medpace Reference Laboratories (MRL) – blood and urine samples and drug safety information Folder 17.12 $524,673.13 Approximately $23,660 per month Medpace Bioanalytical Labs - analysis of vitamin levels from long-range tox studies Folder 17.12 $0 Approx. $8,600 per year for storage of samples related to vitamin levels from long- range tox studies Xxxxxxx Xxxxxx Labs - all remaining materials from GLP studies Folder 17.5 $3,576.34 Approx. $3,500 per quarter

Schedule 10 Knowledge of Seller 1. Xxxxxxxx X. Xxxxxx 2. Xxxxxx XxXxxxx 3. Xxxxxx Xxxxxxxx

Schedule 11 Knowledge of Purchaser 1. Xxxxx Xxxxxxxxxx 2. Xxxx Xxxxxx 3. Xxxxx Xxxxxxx

SELLER DISCLOSURE SCHEDULES TO ASSET PURCHASE AGREEMENT BY AND BETWEEN TRICIDA, INC. AND RENIBUS THERAPEUTICS, INC. DATED AS OF FEBRUARY 21, 2023

These Seller Disclosure Schedules (the “Seller Disclosure Schedules”) are being furnished by Tricida, Inc., a Delaware corporation (“Seller”), in connection with the execution and delivery of that certain Asset Purchase Agreement, dated as of February 21, 2023 (the “Agreement”), by and between Seller and Renibus Therapeutics, Inc., a Delaware corporation. Unless the context otherwise requires, all capitalized terms used herein and not otherwise defined shall have the respective meanings assigned to them in the Agreement. Any summary of or reference to a document herein is qualified by the terms of such document, and the terms and provision of each such document are incorporated herein by reference. These Seller Disclosure Schedules are qualified in its entirety by reference to the Agreement, and shall not be construed as constituting representations and warranties, except as and to the extent provided in the Agreement. Nothing set forth in these Seller Disclosure Schedules shall be deemed to broaden the representations and warranties contained in the Agreement or to interpret the meaning of any of the representations or warranties in the Agreement. These Seller Disclosure Schedules have been arranged for purposes of convenience in separately numbered sections corresponding to the sections of the Agreement; provided, however, each section of these Seller Disclosure Schedules will be deemed to incorporate by reference all information disclosed in any other section of these Seller Disclosure Schedules, or in the Seller SEC Documents, and each disclosure will be deemed a disclosure against any representation or warranty set forth in the Agreement, in each case, to the extent the relevance of such disclosure to such other section of these Seller Disclosure Schedules or such other representation or warranty set forth in the Agreement is reasonably apparent on the face of such disclosure. The representations and warranties set forth in Article III of the Agreement are made and given subject to the disclosures contained in the Seller Disclosure Schedules and the Seller shall not be, or deemed to be, in breach of any such representations and warranties in respect of any matters so disclosed. Items disclosed in these Seller Disclosure Schedules, or in the Seller SEC Documents (but excluding any forward-looking disclosures set forth in any risk factor section, any disclosure in any section relating to forward-looking statements and any other disclosures included in such Seller SEC Documents to the extent they are predictive or forward-looking in nature), are not necessarily limited to the items that the Agreement requires to be reflected herein, and certain items disclosed in these Seller Disclosure Schedules are included solely for informational purposes or to avoid misunderstanding. The inclusion of any information in these Seller Disclosure Schedules shall not be deemed to be an admission or acknowledgement by Seller that such information (or any non-disclosed information) is material or would reasonably be expected to establish or define any standard of materiality for purposes of the Agreement. In disclosing the information in these Seller Disclosure Schedules, Seller expressly does not waive any attorney-client privilege associated with such information or any protection afforded by the work-product doctrine with respect to any of the matters disclosed or summarized herein. All references in the Seller Disclosure Schedules to the enforceability of agreements with third parties, the existence or non-existence of third-party rights, the absence of breaches or defaults by third parties or similar matters or statements, are intended only to set forth exceptions to or disclosure with respect to the representations, warranties, covenants and other agreements of Seller in the Agreement and are not intended to be admissible against any party to the Agreement by any Person who is not a party to the Agreement, or give rise to any claim or benefit to any Person who is not a party to the Agreement. In addition, the disclosure of any matter in the Seller Disclosure Schedules does not constitute an admission that such matter constitutes noncompliance with, or a violation of, any Law or contractual obligation. No Person, other than the parties to the Agreement, may rely for any purpose on the inclusion or exclusion of any items in the Seller Disclosure Schedules.

Schedule 3.4 No Violations None.

Schedule 3.7 Brokers or Finders 1. Xxxxxx Buckfire & Co. LLC, and its affiliate Xxxxxx, Xxxxxxxx & Co., Inc.

4876-1460-0529v.17 Schedule 3.8 Litigation 1. In Re Tricida Stockholder Derivative Litigation, Master File No. 1:21-cv-00205-RGA (D. Del. 2021) – Derivative Securities Case 2. MedPace Research Inc., et al. v. Tricida, Inc., Case No. A2204648 (X.X. Xxxxxxxx Cnty., Ohio 2022) – Contract Dispute 3. Xxxxx, et al. v. Tricida, Inc., et al., Case No. 21-cv-00076-HSG (N.D. Cal. 2021) – Securities Class Action 4. Xxxxx Xxxxx v. Tricida, Inc., WCAB No. ADJ17005589 (Cal.) – Workers’ Compensation Claim
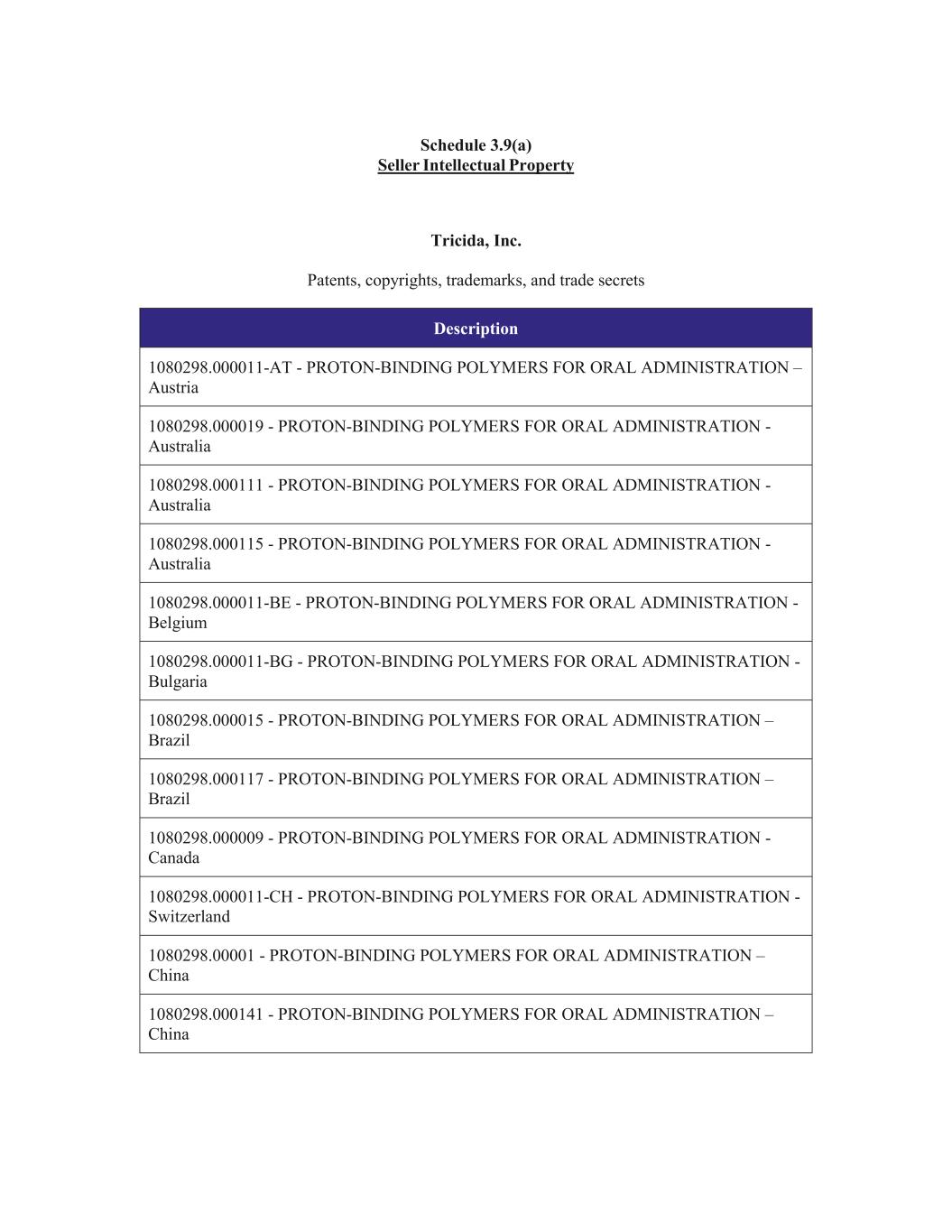
Schedule 3.9(a) Seller Intellectual Property Tricida, Inc. Patents, copyrights, trademarks, and trade secrets Description 1080298.000011-AT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Austria 1080298.000019 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Australia 1080298.000111 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Australia 1080298.000115 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Australia 1080298.000011-BE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Belgium 1080298.000011-BG - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Bulgaria 1080298.000015 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Brazil 1080298.000117 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Brazil 1080298.000009 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Canada 1080298.000011-CH - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Switzerland 1080298.00001 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – China 1080298.000141 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – China
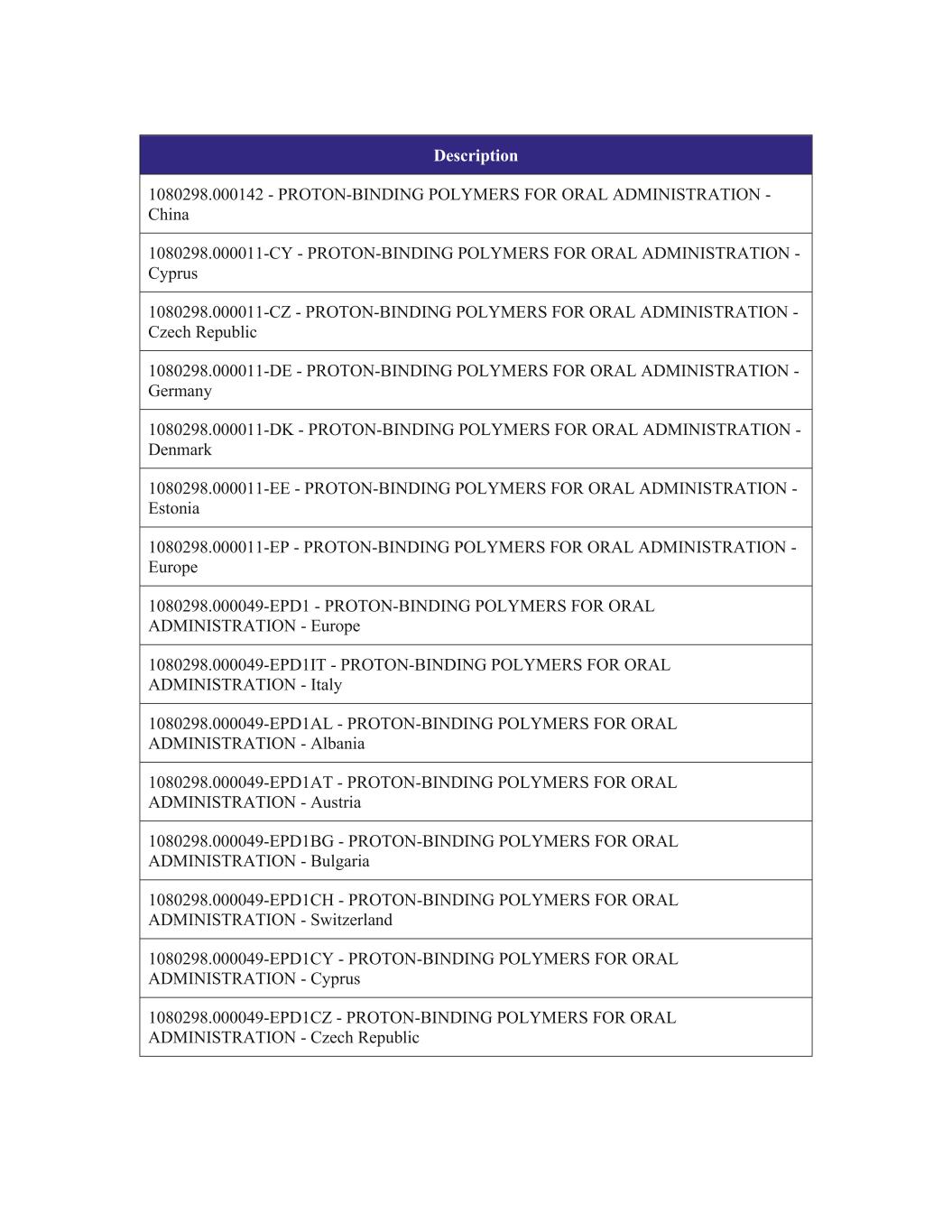
Description 1080298.000142 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - China 1080298.000011-CY - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Cyprus 1080298.000011-CZ - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Czech Republic 1080298.000011-DE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Germany 1080298.000011-DK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Denmark 1080298.000011-EE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Estonia 1080298.000011-EP - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Europe 1080298.000049-EPD1 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Europe 1080298.000049-EPD1IT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Italy 1080298.000049-EPD1AL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Albania 1080298.000049-EPD1AT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Austria 1080298.000049-EPD1BG - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Bulgaria 1080298.000049-EPD1CH - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Switzerland 1080298.000049-EPD1CY - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Cyprus 1080298.000049-EPD1CZ - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Czech Republic
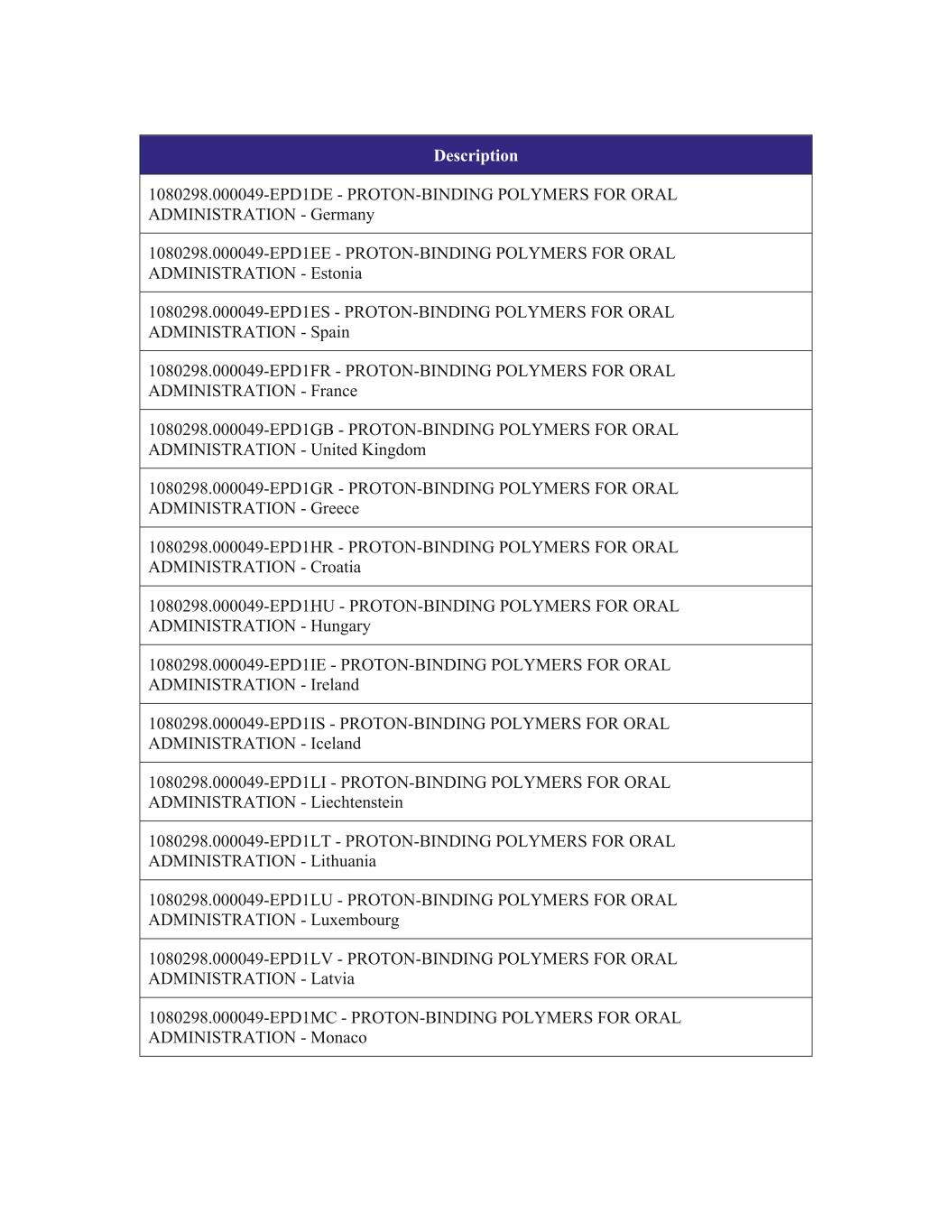
Description 1080298.000049-EPD1DE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Germany 1080298.000049-EPD1EE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Estonia 1080298.000049-EPD1ES - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Spain 1080298.000049-EPD1FR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - France 1080298.000049-EPD1GB - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United Kingdom 1080298.000049-EPD1GR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Greece 1080298.000049-EPD1HR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Croatia 1080298.000049-EPD1HU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hungary 1080298.000049-EPD1IE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Ireland 1080298.000049-EPD1IS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Iceland 1080298.000049-EPD1LI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Liechtenstein 1080298.000049-EPD1LT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Lithuania 1080298.000049-EPD1LU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Luxembourg 1080298.000049-EPD1LV - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Latvia 1080298.000049-EPD1MC - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Monaco

Description 1080298.000049-EPD1MK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Macedonia 1080298.000049-EPD1MT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Malta 1080298.000049-EPD1NL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Netherlands 1080298.000049-EPD1NO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Norway 1080298.000049-EPD1PL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Poland 1080298.000049-EPD1PT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Portugal 1080298.000049-EPD1RO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Romania 1080298.000049-EPD1RS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Serbia 1080298.000049-EPD1SE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Sweden 1080298.000049-EPD1SI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovenia 1080298.000049-EPD1SK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovak Republic 1080298.000049-EPD1SM - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - San Marino 1080298.000049-EPD1TR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Turkey 1080298.00007 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Europe 1080298.000070-AL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Albania
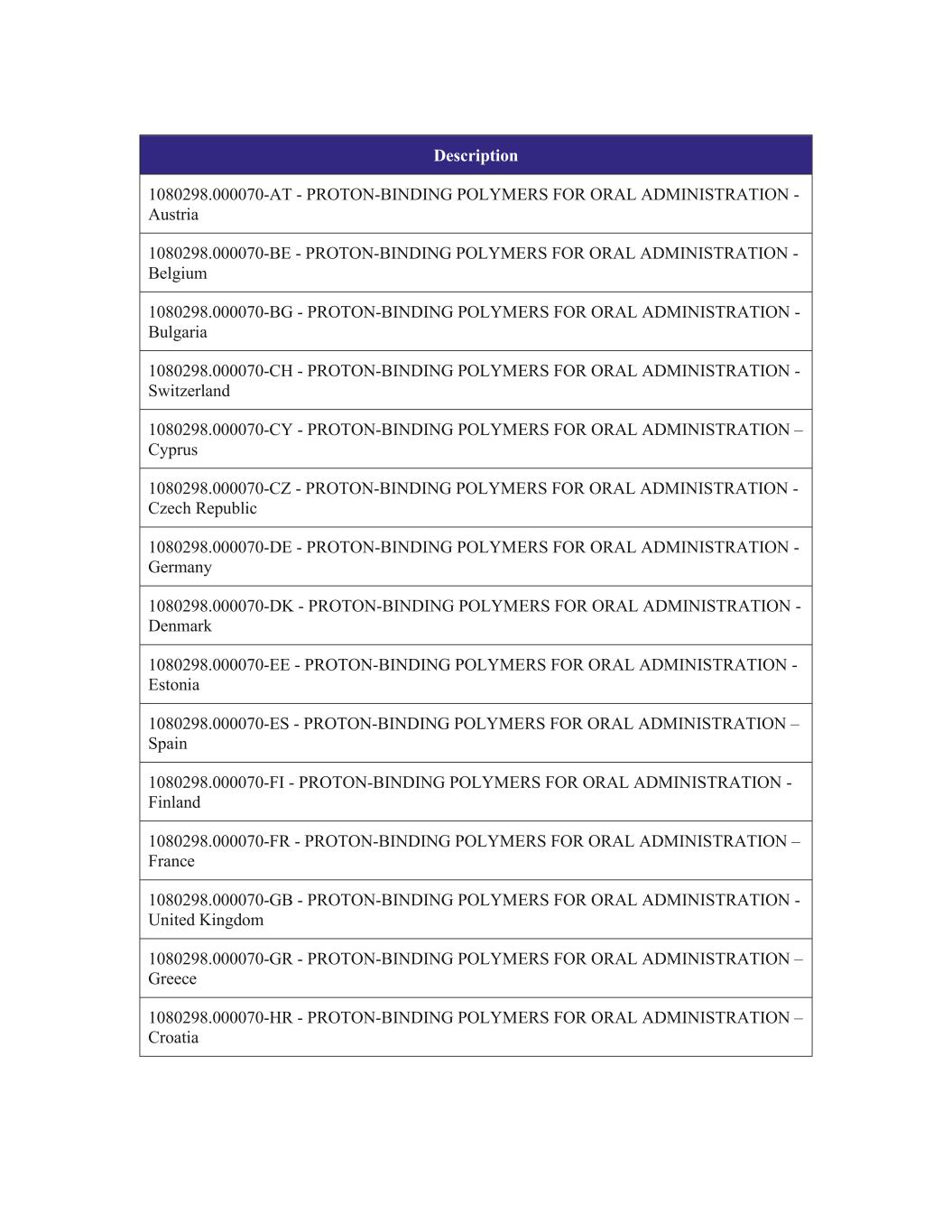
Description 1080298.000070-AT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Austria 1080298.000070-BE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Belgium 1080298.000070-BG - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Bulgaria 1080298.000070-CH - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Switzerland 1080298.000070-CY - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Cyprus 1080298.000070-CZ - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Czech Republic 1080298.000070-DE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Germany 1080298.000070-DK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Denmark 1080298.000070-EE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Estonia 1080298.000070-ES - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Spain 1080298.000070-FI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Finland 1080298.000070-FR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – France 1080298.000070-GB - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United Kingdom 1080298.000070-GR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Greece 1080298.000070-HR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Croatia
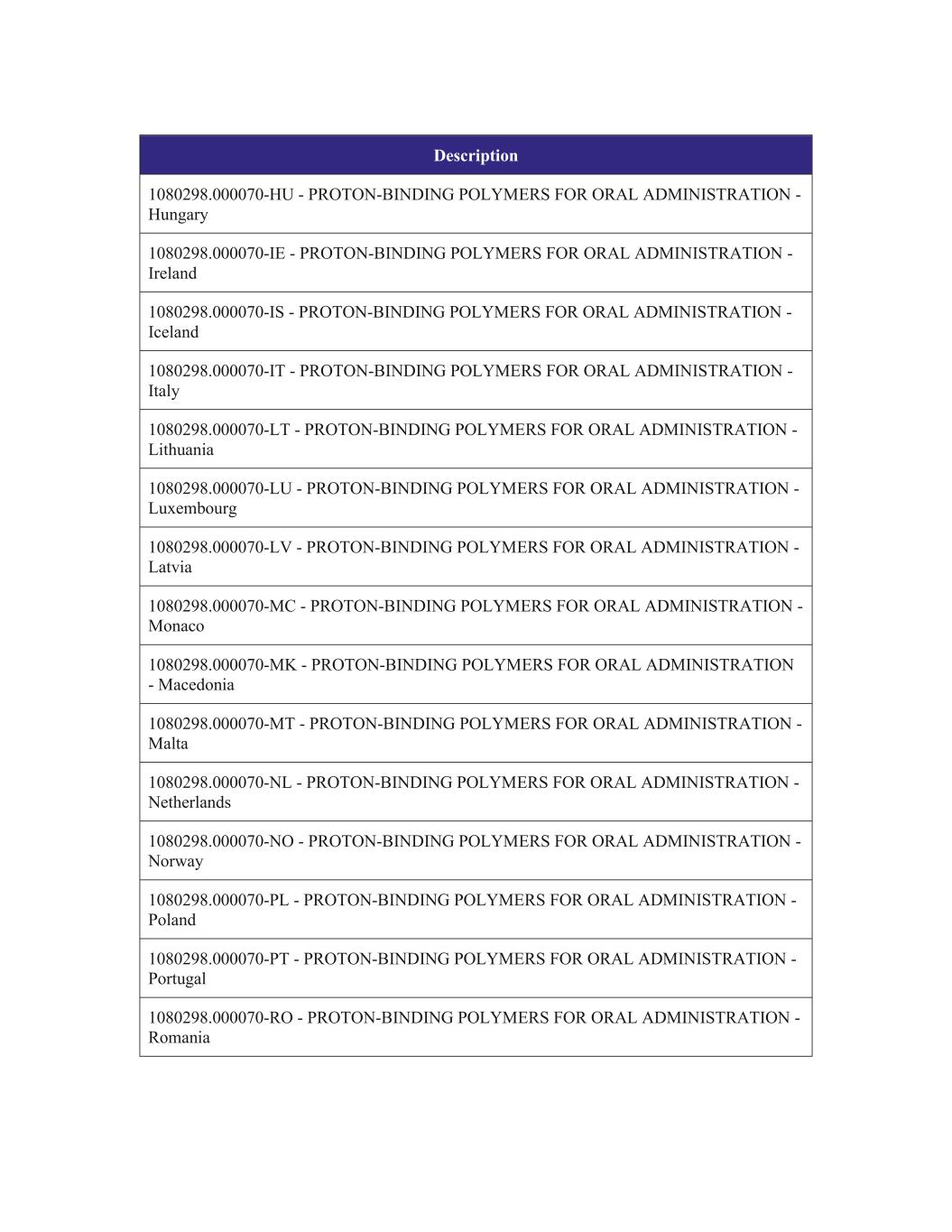
Description 1080298.000070-HU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hungary 1080298.000070-IE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Ireland 1080298.000070-IS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Iceland 1080298.000070-IT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Italy 1080298.000070-LT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Lithuania 1080298.000070-LU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Luxembourg 1080298.000070-LV - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Latvia 1080298.000070-MC - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Monaco 1080298.000070-MK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Macedonia 1080298.000070-MT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Malta 1080298.000070-NL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Netherlands 1080298.000070-NO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Norway 1080298.000070-PL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Poland 1080298.000070-PT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Portugal 1080298.000070-RO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Romania
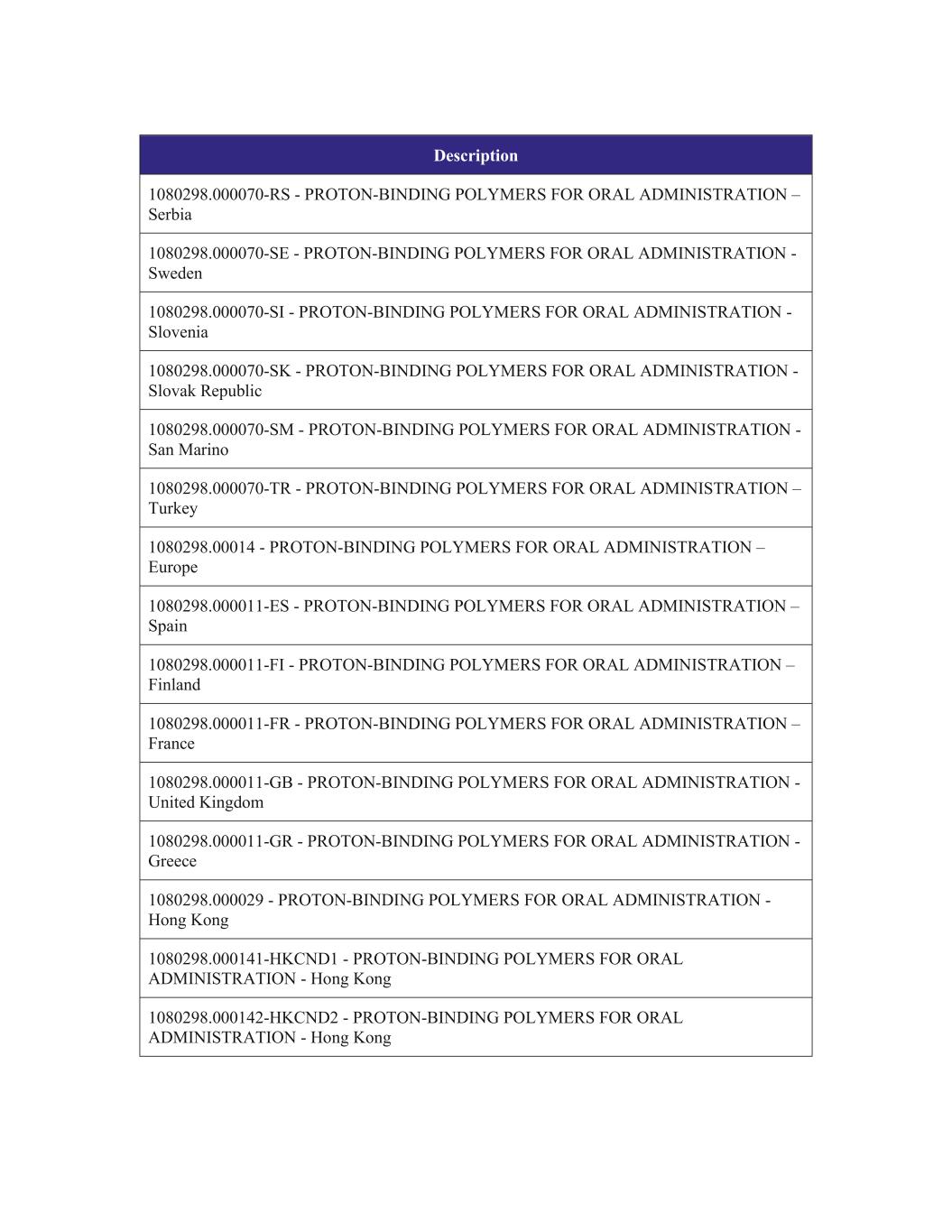
Description 1080298.000070-RS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Serbia 1080298.000070-SE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Sweden 1080298.000070-SI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovenia 1080298.000070-SK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovak Republic 1080298.000070-SM - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - San Marino 1080298.000070-TR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Turkey 1080298.00014 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Europe 1080298.000011-ES - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Spain 1080298.000011-FI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – Finland 1080298.000011-FR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION – France 1080298.000011-GB - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United Kingdom 1080298.000011-GR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Greece 1080298.000029 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000141-HKCND1 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000142-HKCND2 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong
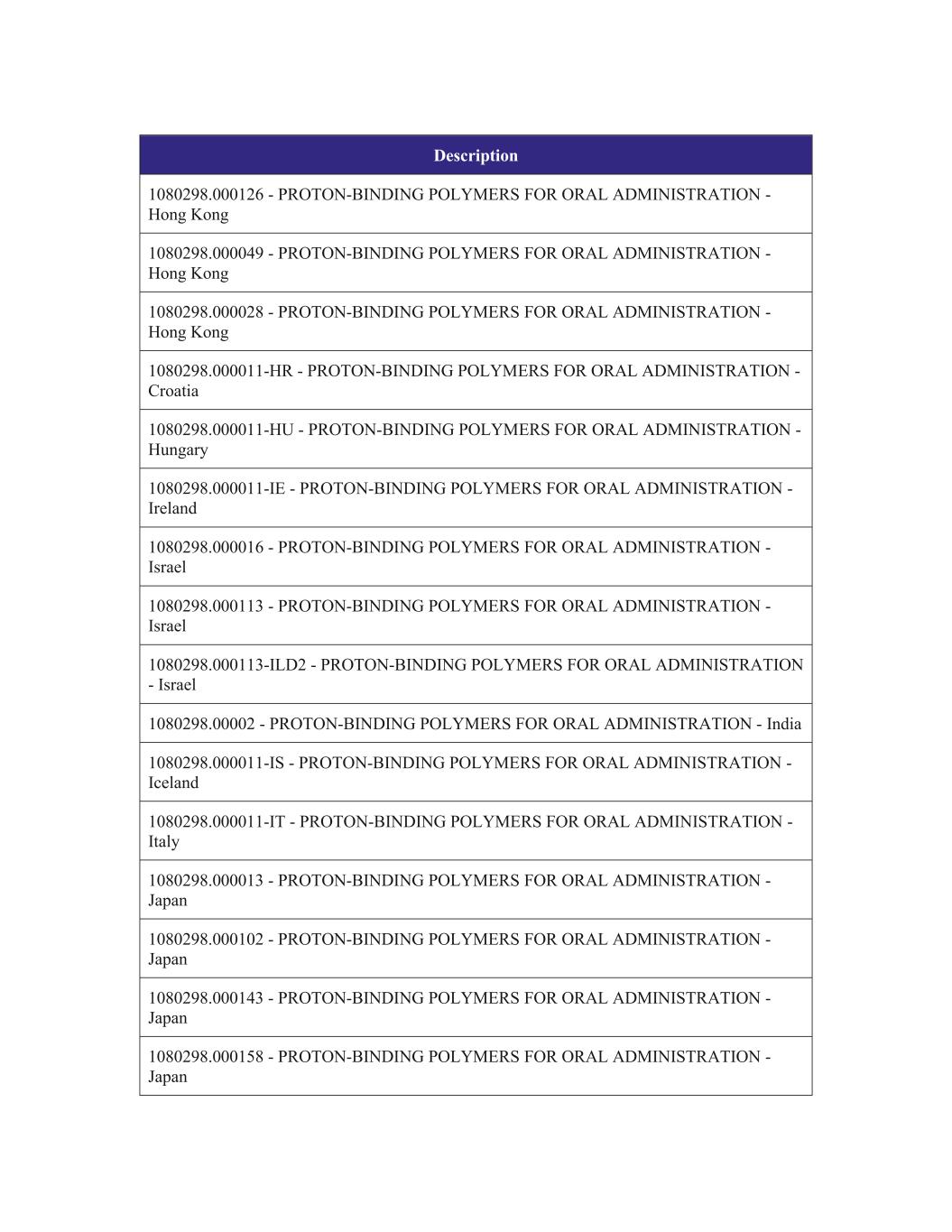
Description 1080298.000126 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000049 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000028 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000011-HR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Croatia 1080298.000011-HU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hungary 1080298.000011-IE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Ireland 1080298.000016 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Israel 1080298.000113 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Israel 1080298.000113-ILD2 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Israel 1080298.00002 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - India 1080298.000011-IS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Iceland 1080298.000011-IT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Italy 1080298.000013 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Japan 1080298.000102 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Japan 1080298.000143 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Japan 1080298.000158 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Japan
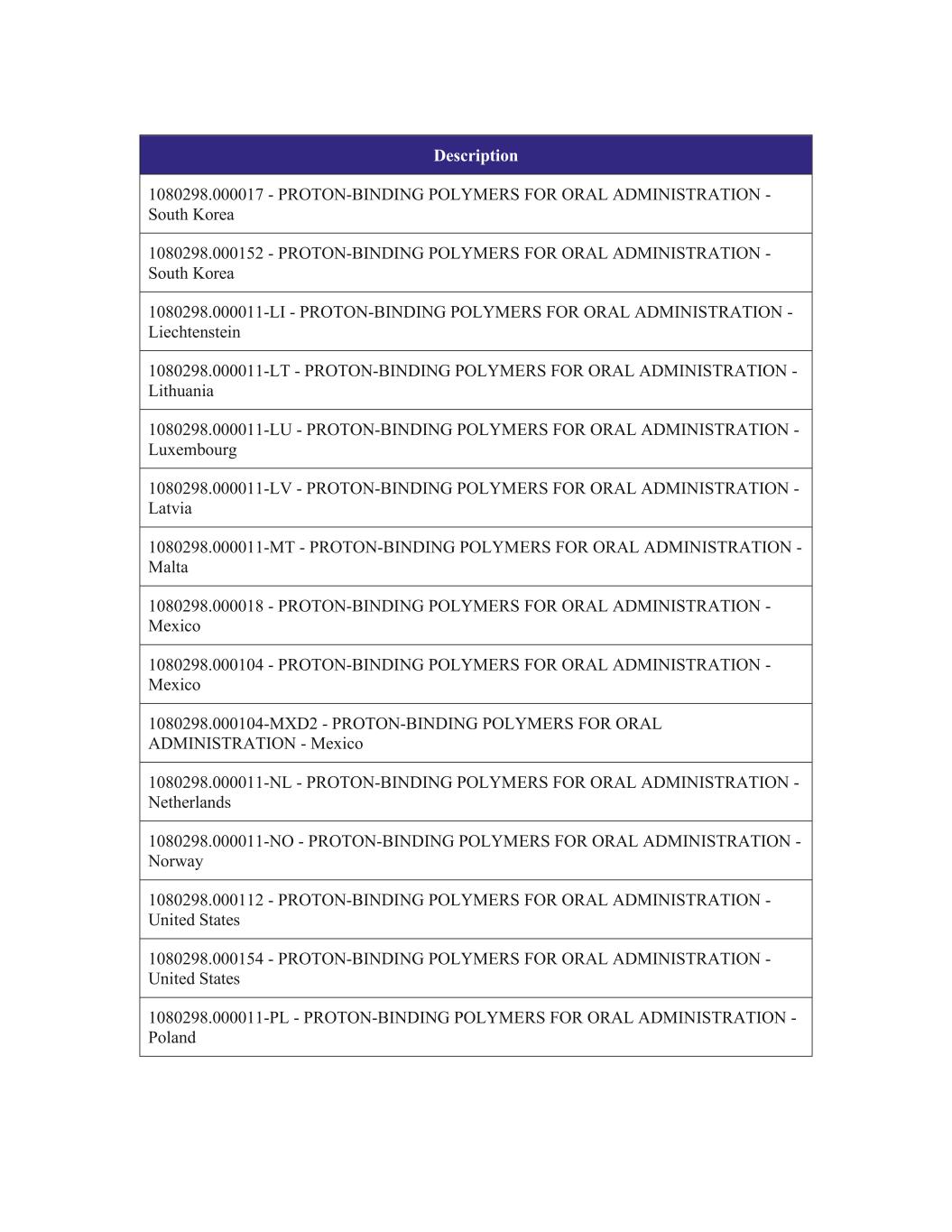
Description 1080298.000017 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - South Korea 1080298.000152 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - South Korea 1080298.000011-LI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Liechtenstein 1080298.000011-LT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Lithuania 1080298.000011-LU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Luxembourg 1080298.000011-LV - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Latvia 1080298.000011-MT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Malta 1080298.000018 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Mexico 1080298.000104 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Mexico 1080298.000104-MXD2 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Mexico 1080298.000011-NL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Netherlands 1080298.000011-NO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Norway 1080298.000112 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000154 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000011-PL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Poland
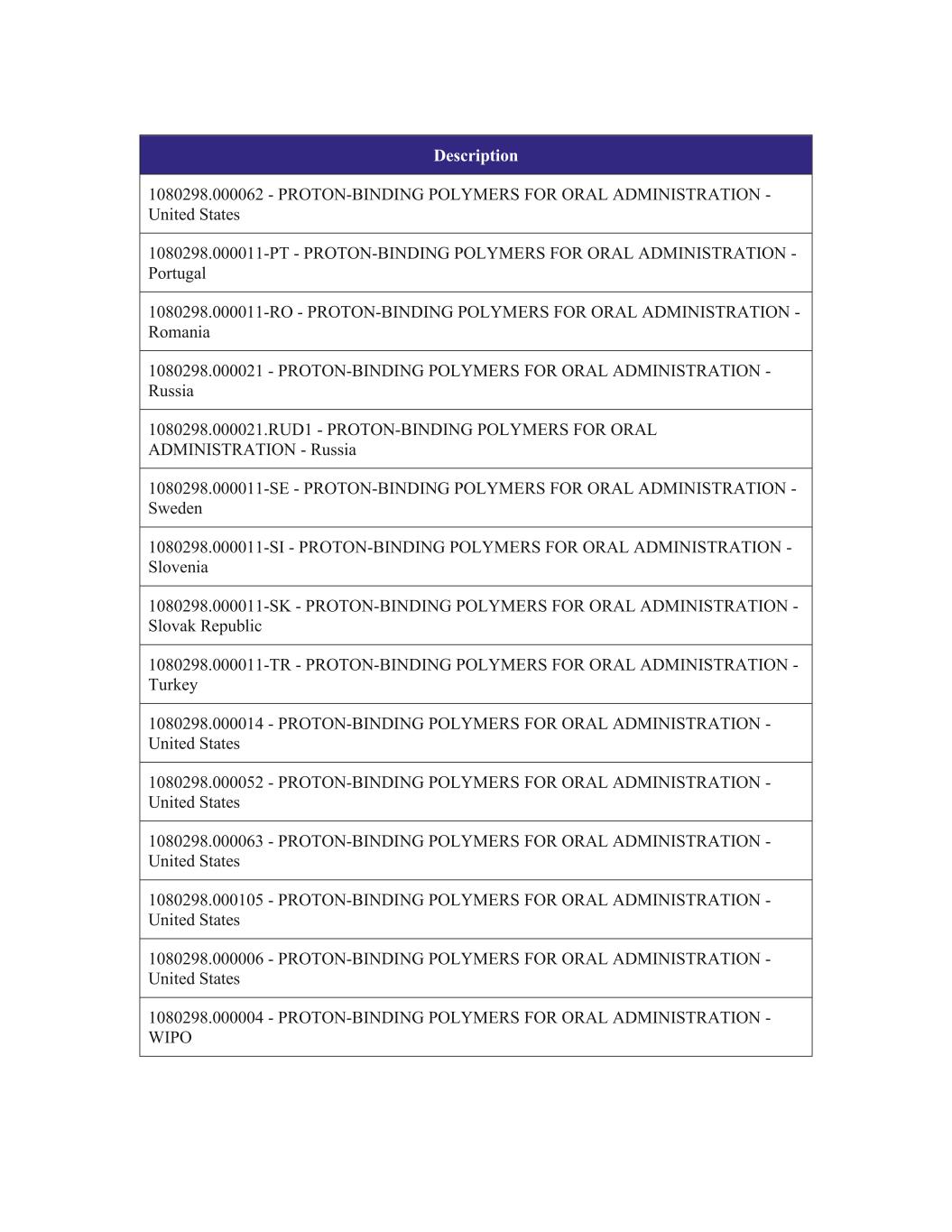
Description 1080298.000062 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000011-PT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Portugal 1080298.000011-RO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Romania 1080298.000021 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Russia 1080298.000021.RUD1 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Russia 1080298.000011-SE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Sweden 1080298.000011-SI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovenia 1080298.000011-SK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovak Republic 1080298.000011-TR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Turkey 1080298.000014 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000052 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000063 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000105 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000006 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000004 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - WIPO

Description 1080298.000123-WO - PROCESS FOR THE PREPARATION OF 1,3- BIS(ALLYLAMINO)PROPANE - WIPO 1080298.000049-EPD1BE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Belgium 1080298.000049-EPD1DK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Denmark 1080298.000049-EPD1FI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Finland 1080298.000045-AL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Albania 1080298.000045-AT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Austria 1080298.000037 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Australia 1080298.000037-AUD1 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Australia 1080298.000045-BA - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Bosnia 1080298.000045-BE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Belgium 1080298.000045-BG - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Bulgaria 1080298.000038 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Brazil 1080298.000038-BRD1 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Brazil 1080298.000054 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Canada 1080298.000162 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Canada

Description 1080298.000045-CH - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Switzerland 1080298.000046 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - China 1080298.00015 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - China 1080298.000045-CY - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Cyprus 1080298.000045-CZ - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Czech Republic 1080298.000045-DE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Germany 1080298.000045-DK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Denmark 1080298.000045-EE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Estonia 1080298.000045 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Europe 1080298.000069 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Europe 1080298.000069-AL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Albania 1080298.000069-AT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Austria 1080298.000069-BA - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Bosnia 1080298.000069-BE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Belgium 1080298.000069-BG - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Bulgaria
Description 1080298.000069-CH - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Switzerland 1080298.000069-CY - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Cyprus 1080298.000069-CZ - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Czech Republic 1080298.000069-DE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Germany 1080298.000069-DK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Denmark 1080298.000069-EE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Estonia 1080298.000069-ES - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Spain 1080298.000069-FI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Finland 1080298.000069-FR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - France 1080298.000069-GB - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United Kingdom 1080298.000069-GR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Greece 1080298.000069-HR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Croatia 1080298.000069-HU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hungary 1080298.000069-IE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Ireland 1080298.000069-IS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Iceland
Description 1080298.000069-IT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Italy 1080298.000069-LT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Lithuania 1080298.000069-LU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Luxembourg 1080298.000069-LV - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Latvia 1080298.000069-MA - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Morocco 1080298.000069-MC - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Monaco 1080298.000069-MD - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Moldova 1080298.000069-ME - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Montenegro 1080298.000069-MK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Macedonia 1080298.000069-MT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Malta 1080298.000069-NL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Netherlands 1080298.000069-NO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Norway 1080298.000069-PL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Poland 1080298.000069-PT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Portugal 1080298.000069-RO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Romania
Description 1080298.000069-RS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Serbia 1080298.000069-SE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Sweden 1080298.000069-SI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovenia 1080298.000069-SK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovak Republic 1080298.000069-SM - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - San Marino 1080298.000069-TR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Turkey 1080298.000119 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Europe 1080298.000045-ES - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Spain 1080298.000045-FI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Finland 1080298.000045-FR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - France 1080298.000045-GB - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United Kingdom 1080298.000045-GR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Greece 1080298.000061 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000150-HKCND1 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000053 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong
Description 1080298.000069-HK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hong Kong 1080298.000045-HR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Croatia 1080298.000045-HU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Hungary 1080298.000045-IE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Ireland 1080298.000044 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Israel 1080298.000149 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Israel 1080298.000159 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Israel 1080298.000043 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - India 1080298.000045-IS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Iceland 1080298.000045-IT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Italy 1080298.000042 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Japan 1080298.000139 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Japan 1080298.000151 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Japan 1080298.000041 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - South Korea 1080298.000045-LI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Liechtenstein
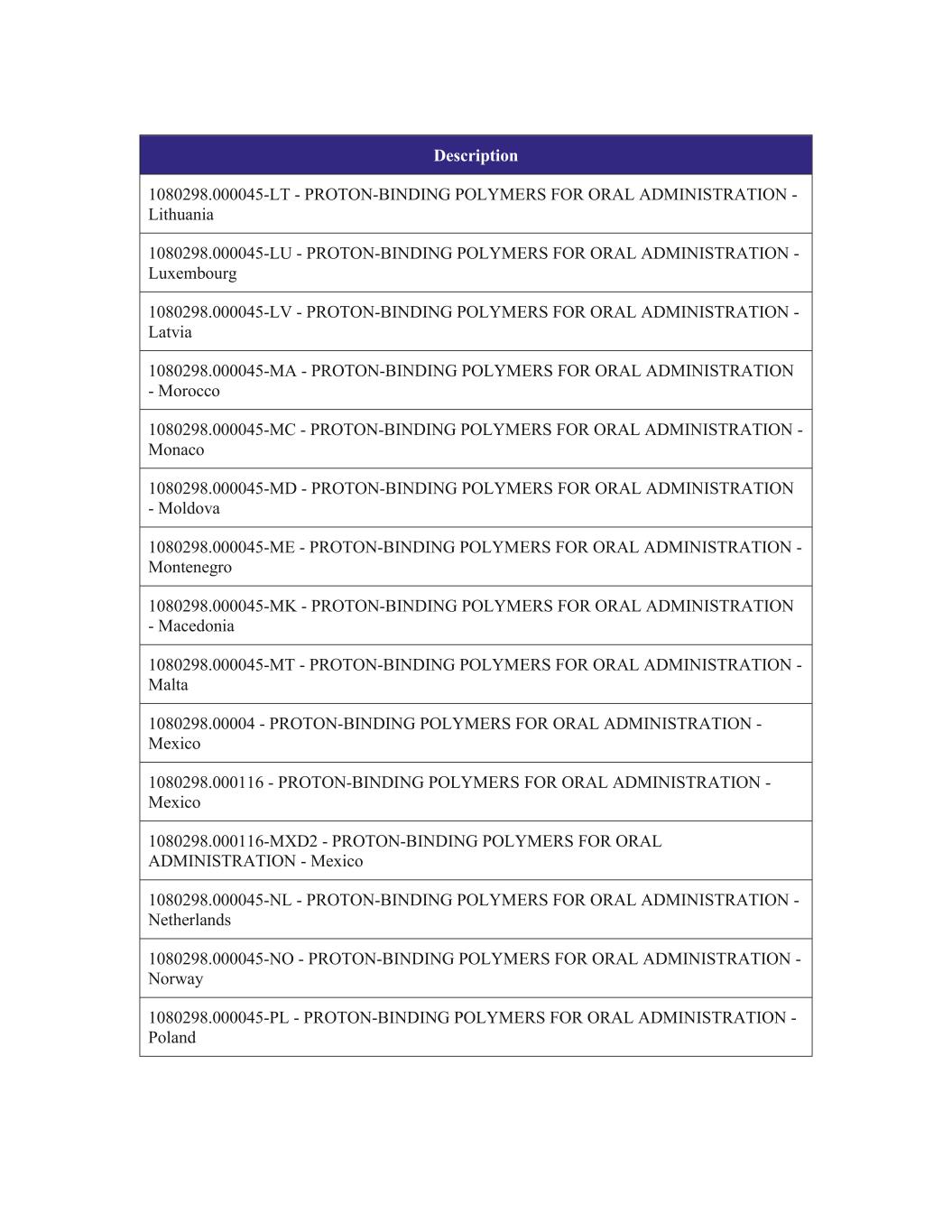
Description 1080298.000045-LT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Lithuania 1080298.000045-LU - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Luxembourg 1080298.000045-LV - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Latvia 1080298.000045-MA - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Morocco 1080298.000045-MC - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Monaco 1080298.000045-MD - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Moldova 1080298.000045-ME - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Montenegro 1080298.000045-MK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Macedonia 1080298.000045-MT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Malta 1080298.00004 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Mexico 1080298.000116 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Mexico 1080298.000116-MXD2 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Mexico 1080298.000045-NL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Netherlands 1080298.000045-NO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Norway 1080298.000045-PL - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Poland
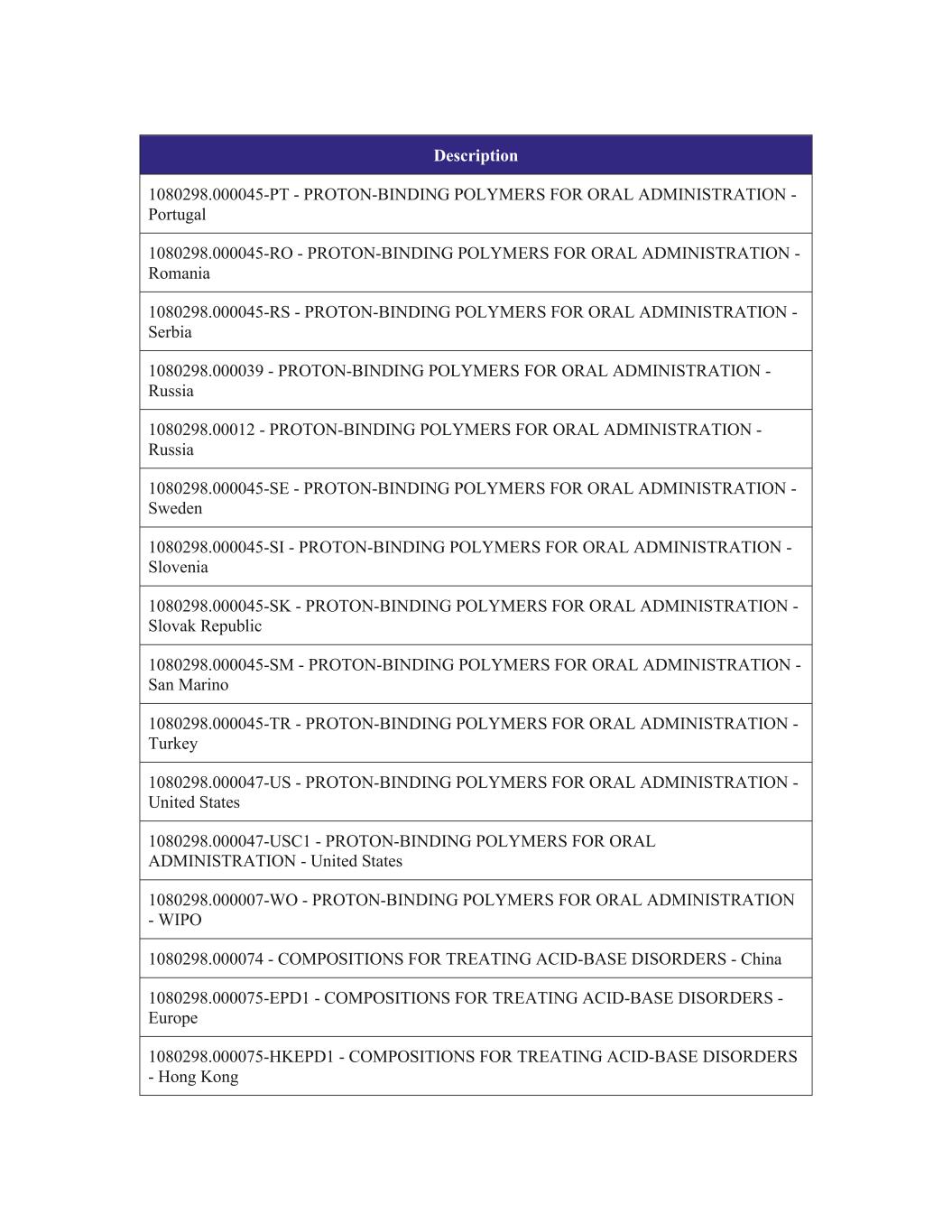
Description 1080298.000045-PT - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Portugal 1080298.000045-RO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Romania 1080298.000045-RS - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Serbia 1080298.000039 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Russia 1080298.00012 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Russia 1080298.000045-SE - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Sweden 1080298.000045-SI - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovenia 1080298.000045-SK - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Slovak Republic 1080298.000045-SM - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - San Marino 1080298.000045-TR - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - Turkey 1080298.000047-US - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000047-USC1 - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - United States 1080298.000007-WO - PROTON-BINDING POLYMERS FOR ORAL ADMINISTRATION - WIPO 1080298.000074 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - China 1080298.000075-EPD1 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - Europe 1080298.000075-HKEPD1 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - Hong Kong
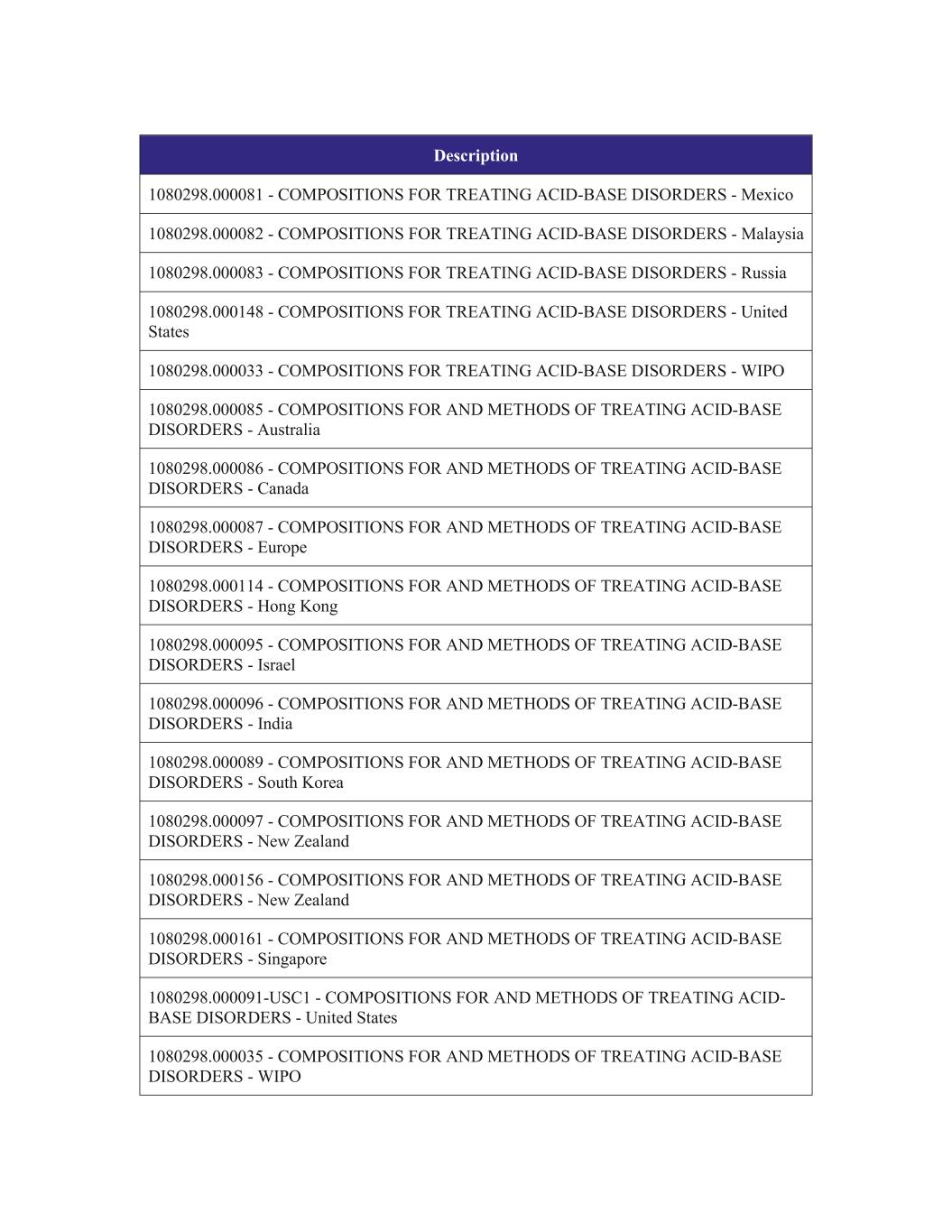
Description 1080298.000081 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - Mexico 1080298.000082 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - Malaysia 1080298.000083 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - Russia 1080298.000148 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - United States 1080298.000033 - COMPOSITIONS FOR TREATING ACID-BASE DISORDERS - WIPO 1080298.000085 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Australia 1080298.000086 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Canada 1080298.000087 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Europe 1080298.000114 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Hong Kong 1080298.000095 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Israel 1080298.000096 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - India 1080298.000089 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - South Korea 1080298.000097 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - New Zealand 1080298.000156 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - New Zealand 1080298.000161 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Singapore 1080298.000091-USC1 - COMPOSITIONS FOR AND METHODS OF TREATING ACID- BASE DISORDERS - United States 1080298.000035 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - WIPO
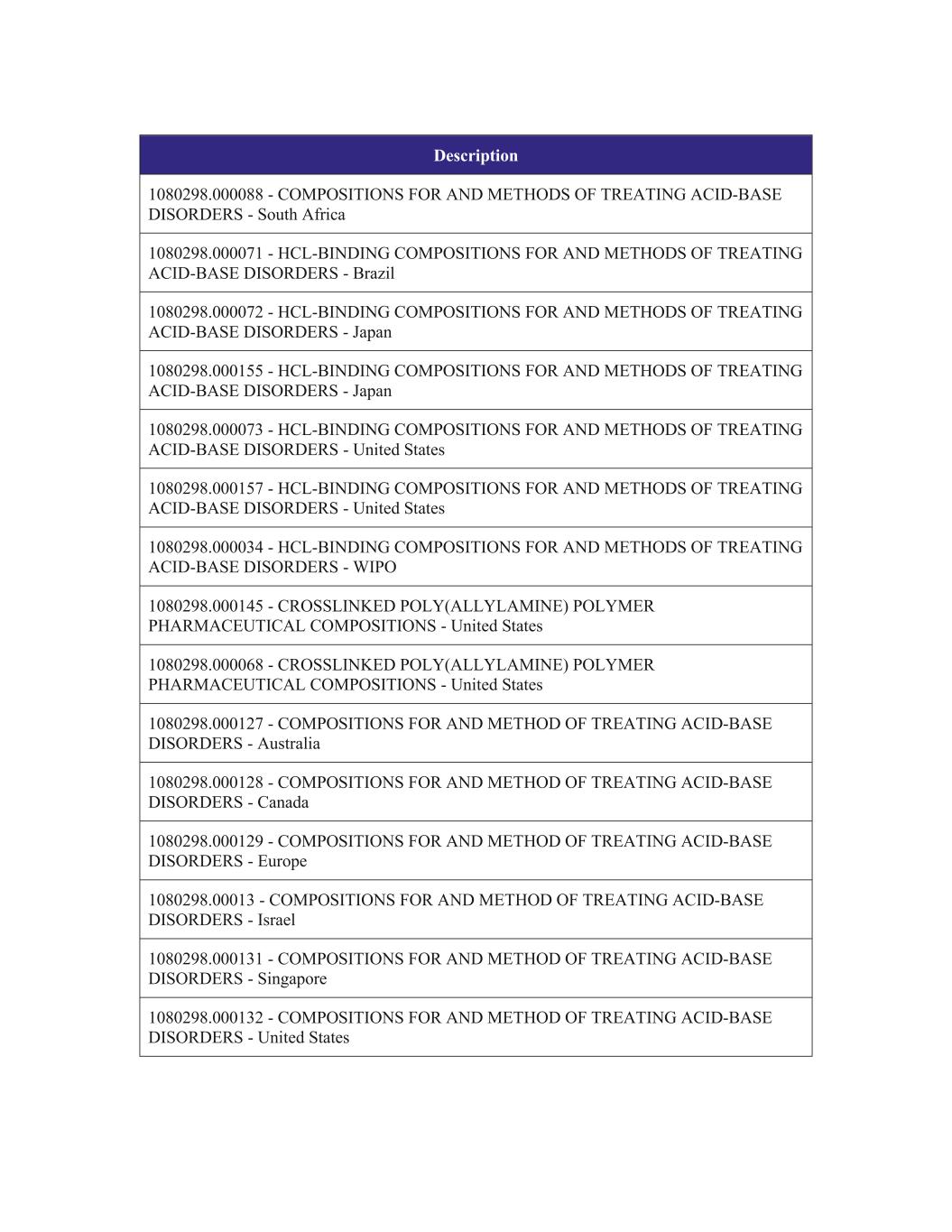
Description 1080298.000088 - COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - South Africa 1080298.000071 - HCL-BINDING COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Brazil 1080298.000072 - HCL-BINDING COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Japan 1080298.000155 - HCL-BINDING COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - Japan 1080298.000073 - HCL-BINDING COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - United States 1080298.000157 - HCL-BINDING COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - United States 1080298.000034 - HCL-BINDING COMPOSITIONS FOR AND METHODS OF TREATING ACID-BASE DISORDERS - WIPO 1080298.000145 - CROSSLINKED POLY(ALLYLAMINE) POLYMER PHARMACEUTICAL COMPOSITIONS - United States 1080298.000068 - CROSSLINKED POLY(ALLYLAMINE) POLYMER PHARMACEUTICAL COMPOSITIONS - United States 1080298.000127 - COMPOSITIONS FOR AND METHOD OF TREATING ACID-BASE DISORDERS - Australia 1080298.000128 - COMPOSITIONS FOR AND METHOD OF TREATING ACID-BASE DISORDERS - Canada 1080298.000129 - COMPOSITIONS FOR AND METHOD OF TREATING ACID-BASE DISORDERS - Europe 1080298.00013 - COMPOSITIONS FOR AND METHOD OF TREATING ACID-BASE DISORDERS - Israel 1080298.000131 - COMPOSITIONS FOR AND METHOD OF TREATING ACID-BASE DISORDERS - Singapore 1080298.000132 - COMPOSITIONS FOR AND METHOD OF TREATING ACID-BASE DISORDERS - United States
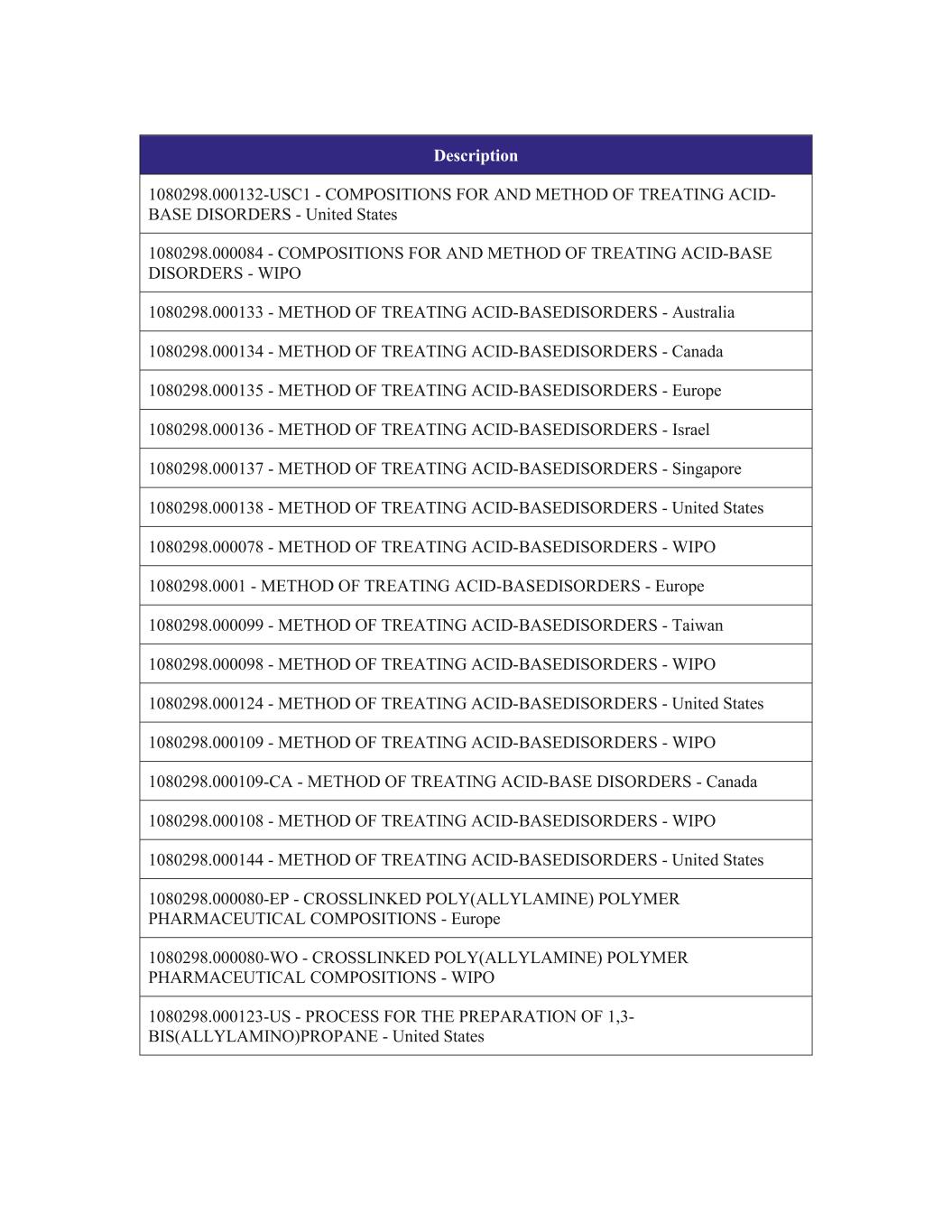
Description 1080298.000132-USC1 - COMPOSITIONS FOR AND METHOD OF TREATING ACID- BASE DISORDERS - United States 1080298.000084 - COMPOSITIONS FOR AND METHOD OF TREATING ACID-BASE DISORDERS - WIPO 1080298.000133 - METHOD OF TREATING ACID-BASEDISORDERS - Australia 1080298.000134 - METHOD OF TREATING ACID-BASEDISORDERS - Canada 1080298.000135 - METHOD OF TREATING ACID-BASEDISORDERS - Europe 1080298.000136 - METHOD OF TREATING ACID-BASEDISORDERS - Israel 1080298.000137 - METHOD OF TREATING ACID-BASEDISORDERS - Singapore 1080298.000138 - METHOD OF TREATING ACID-BASEDISORDERS - United States 1080298.000078 - METHOD OF TREATING ACID-BASEDISORDERS - WIPO 1080298.0001 - METHOD OF TREATING ACID-BASEDISORDERS - Europe 1080298.000099 - METHOD OF TREATING ACID-BASEDISORDERS - Taiwan 1080298.000098 - METHOD OF TREATING ACID-BASEDISORDERS - WIPO 1080298.000124 - METHOD OF TREATING ACID-BASEDISORDERS - United States 1080298.000109 - METHOD OF TREATING ACID-BASEDISORDERS - WIPO 1080298.000109-CA - METHOD OF TREATING ACID-BASE DISORDERS - Canada 1080298.000108 - METHOD OF TREATING ACID-BASEDISORDERS - WIPO 1080298.000144 - METHOD OF TREATING ACID-BASEDISORDERS - United States 1080298.000080-EP - CROSSLINKED POLY(ALLYLAMINE) POLYMER PHARMACEUTICAL COMPOSITIONS - Europe 1080298.000080-WO - CROSSLINKED POLY(ALLYLAMINE) POLYMER PHARMACEUTICAL COMPOSITIONS - WIPO 1080298.000123-US - PROCESS FOR THE PREPARATION OF 1,3- BIS(ALLYLAMINO)PROPANE - United States

Description 1080298.000123-WO - PROCESS FOR THE PREPARATION OF 1,3- BIS(ALLYLAMINO)PROPANE - WIPO 1080298.000163 - PHARMACEUTICAL COMPOSITIONS FOR THE TREATMENT OF LITHIASIS DISORDERS – WIPO 1080298.000153 - PHARMACEUTICAL COMPOSITIONS FOR THE TREATMENT OF LITHIASIS DISORDERS - United States

In re: Tricida, Inc. Internet domain names and websites xxxxxxxxxxxx.xxx xxxxxxxxxxxx.xxxx xxxxxxxxxxxx.xxx xxxxxxxxxxxx.xxx xxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxx.xxxx xxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxx.xxx xxxxxxxx.xxx xxxxxxxx.xxxx xxxxxxxx.xxx xxxxxxxx.xxx xxxxxxxx-xxx.xxx xxxxxxxx-xxx.xxxx xxxxxxxx-xxx.xxx xxxxxxxx-xxx.xxx xxxxxxxx.xxx xxxxxxxx.xxxx xxxxxxxx.xxx xxxxxxxx.xxx xxx.xxxxxxx.xxx xxxxxxx.xxx xxxxxxxx.xxx xxxxxxxx.xxxx xxxxxxxx.xxx xxxxxxxx.xxx xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxx.xxx xxxxxx.xxxx xxxxxx.xxx xxxxxx.xxx xx.xxxxxxx.xxx xxxxxxx.xxx

xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxxxxxxxxxxxx.xxxx xxxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxxxxxxxxxxx.xxxx xxxxxxxxxxxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxxxxxxxxxxx.xxx xxxx-xxxx.xxx xxxx-xxxx.xxxx xxxx-xxxx.xxx xxxx-xxxx.xxx xxxxxxxx.xxx xxxxxxxx.xxxx xxxxxxxx.xxx xxxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxxxx.xxx xxxxxxxxx.xxxx xxxxxxxxx.xxx xxxxxxxxx.xxx xxxxxxxx.xxx xxxxxxxx.xxxx xxxxxxxx.xxx xxxxxxxx.xxx XXXXXXX.XXX
xxxxxxx0xxxxxxx.xxx xxxxxxxxxxxxx.xxx xxxxxxxxxxxxx.xxx xxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxx.xxxx xxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxx.xxx xxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxxxxxxxxxx.xxx xxxxxxxxxxxxxxxxxxxxxxxx.xxx XXXXXXXX.XXX xxxxxxxx.xxx xxxxxxxx.xxxx xxxxxxxx.xxx xxxxxxxx.xxx xxxxxx-xxx.xxx xxxxxx-xxx.xxxx xxxxxx-xxx.xxx xxxxxx-xxx.xxx xxxxx-xxx.xxx xxxxxxxx.xxx xxxxxxxx.xxx xxxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxx.xxxx xxxxxxx.xxx xxxxxxx.xxx xxxxxxxxx.xxx
xxxxxxxxx.xxxx xxxxxxxxx.xxx xxxxxxxxx.xxx

Schedule 3.10(a) Material Contracts (i) None. (ii) None. (iii) None. (iv) None. (v) None.

Schedule 3.12(d) Regulatory Matters None.

Schedule 3.14 Insurance 1. Products / Clinical Trial Liability Insurance Policy, by and between Tricida, Inc., and Medmarc, expiring April 15, 2023. 2. Products / Clinical Trial Liability Insurance Policy, by and between Tricida, Inc., and Noetic Specialty, expiring April 15, 2023.

Schedule 5.1(a) Conduct of Business Pending Closing None.

Schedule 5.1(b) Seller Interim Operating Covenants None.

