DISTRIBUTION AGREEMENT
EXHIBIT 10.29

This Distribution Agreement (the "Agreement') is entered into by and between United Orthopedic Corporation (UOC), with principle business address at Xx. 00, Xxxx Xxx. 0, Xxxxxxx Xxxx, Xxxxxxx, 000 Xxxxxx (hereafter '`UOC" or "Company") and CPM Medical Inc. (hereafter "Distributor") having a principle place of business at 0000 Xxxxxxxxxx Xxxxx XxXxxxxx, Xxxxx 00000, XXX. (UOC and Distributor sometimes shall be collectively referred to as the "Parties" and individually as a "Party").
IN CONSIDERATION of the mutual promises and covenants set forth herein and for other good and valuable consideration, Company and Distributor agree as follow.
1. DEFINITIONS
|
|
1.1 |
"Products" shall mean those products listed in Addendum A attached hereto. The content and sort of Products may be changed, abandoned or added by Company, at Company's sole discretion, provided that Company gives ninety (90) days' prior written notice to Distributor. Company shall be under no obligation to continue the production of any product, except as provide herein. |
2. APPOINTMENT AND AUTHORITY OF DISTRIBUTOR
|
|
2.1 |
Appointment. Subject to the terms and conditions set forth herein, Company hereby appoint as Company's non-exclusive distributor for the Products, and Distributor hereby accepts such appointment. |
|
|
2.2 |
Sub distributors. Distributors shall not appoint sub distributors of Products without Company's prior specific written consent. Any appointed sub distributor is subject to the same terms and conditions set forth herein as the Distributor and that the Distributor is responsible for any non-compliance under the agreement of any appointed sub distributor. |
|
|
2.3 |
Conflict of Interest. During the term of this Agreement, Distributor shall not, without Company's prior written consent, represent, promote or otherwise try to sell any products that, in Company's judgment, compete with the Products covered by this Agreement. |
PAGE 1 OF 20

|
|
considered employees of Company. Distributor is not authorized to enter into any commitment or contract of any kind on behalf of Company, without the prior express written consent of the President of Company. Distributor hereby agrees to ensure that all of its employees and other representatives are aware of these restrictive covenants and obligations (and all others under this Agreement) and shall adhere to such. Distributor acknowledges and agrees that Distributor is responsible for and agrees to comply with obligations under federal and state tax laws relating to Distributor's obligations hereunder, including, without limitation, for payment of income and employment taxes related to Distributor's employees and agents. |
3. TERMS OF PRUCHASE PRODUCTS BY DISTRIBUTOR
|
|
3.1 |
Terms and Conditions. All purchases of Products by Distributor from Company during the term of this Agreement shall be subject to the terms and conditions of this Agreement. |
|
|
3.2 |
Prices. All prices of Products are F.O.B. Company's facility or as provided by written notice to Distributor (the "Distributor Site"). The Price List for Distributor shall be set forth in Addendum B attached hereto. Company has the right at any time to revise the prices in Addendum B with sixty (60) days advance written notice to Distributor. Such revisions shall apply to all orders received after the effective date of revision. Price changes shall not affect unfulfilled purchase orders accepted by Company prior to the effective date of the price change. |
|
|
3.3 |
Taxes. Distributor's Purchase Price does not include any foreign, federal, state or local taxes that may be applicable to the Products. In the event that such taxes are applicable and Company has the legal obligation to collect such taxes, Company shall be entitled to add to Distributor's invoice with the amount of such taxes and Distributor shall pay such amount unless Distributor provide Company with a valid tax exemption certificate authorized by the appropriate taxing authority. |
|
|
3.4 |
Payment. Payment term is net 60 days from the date of invoice. Full payment of Distributor's Purchase Price for the Products (including any freight, taxes or other applicable costs initially paid by Company but to be borne by Distributor) shall be in United States of America Dollars. All exchange, interest, banking, collecting and other charges shall be Distributor's responsibility. |
PAGE 2 OF 20

|
|
shall deliver Products to the carrier selected by Distributor. All freight, insurance, and other shipping expenses, as well as any special packing expense, shall be paid by Distributor. Distributor shall also bear all applicable taxes, duties, and similar charges that may be assessed against the Products after delivery to the carrier at Company's Distribution Site. |
|
|
3.6 |
Shipping Discrepancies and Packaging Damage. Shipping discrepancies and packaging damages must be reported within thirty (30) days from the date of invoice for corrections to be considered by UOC. |
|
|
3.7 |
Goods Return. Catalog items cannot be returned to Company unless a returned goods authorization (RA) number is issued by Company. The information required to obtain the authorization number includes the packing list or invoice number, purchase order number, and the reason for return. This number must be referenced in the return shipment to Company. |
|
|
A |
Sterile merchandise, which has been opened, or the packaging damaged, will not be credited upon return. |
|
|
B |
Special order or custom items will not be credited upon return. |
|
|
C |
Obsolete merchandise not listed in the current Addendum A cannot be returned. |
|
|
D |
Returns more than one-hundred-and-fifty (150) days from the date of the invoice will not be accepted |
|
|
E |
Restocking charges for returned items that were accepted by Company will be at 25% of invoice price. |
|
|
3.8 |
Product Defects. Distributor shall inspect all products promptly upon receipt thereof and may not return any defected product that fails to perform in any material way to meet the specifications set forth in Company's current brochure or related literature for that product. To return a defected product, Distributor shall, notify Company in writing of its rejection along with a completed Customer Complaint Form. Within seven (7) days after rejection, Distributor shall return to Company the rejected product, freight prepaid. As promptly as possible but no later than thirty (30) working days after receipt of properly rejected products, Company shall, at its option and expense, replace the products. Company shall reimburse the shipping charges back to Distributor for properly rejected products; conversely, Distributor shall be responsible for all shipping costs for Products that were not accepted by Company for rejection. |
4. WARRANTY FOR PRODUCTS
PAGE 3 OF 20

|
|
4.2 |
Limitation of Liability and Warranty. Company's liability arising out of this agreement and/or sale of the products shall be limited as follows: in no event shall Company be liable for costs of procurement of substitute goods by anyone. In no event shall Company be liable to distributor or any other entity for any special, consequential, incidental, or indirect damages, however caused, on any theory of liability or breach of warranty, whether or not Company has been advised on the possibility. Except for the express limited warranty set forth in previous Subsection 4.1 Standard Limited Warranty above, Company grants no implied warranties for the products, either in fact or by operation of law, by statute, or otherwise. |
|
|
4.3 |
Product Complaints. |
|
|
4.3.1 |
4.3.1 Each Party shall cooperate fully with the other Party in dealing with customer complaints concerning the Product(s) and shall take reasonable action to resolve promptly and follow up with regard to such complaints. |
|
|
4.3.2 |
Without limiting the generality of the foregoing, the Distributor shall: |
|
|
A |
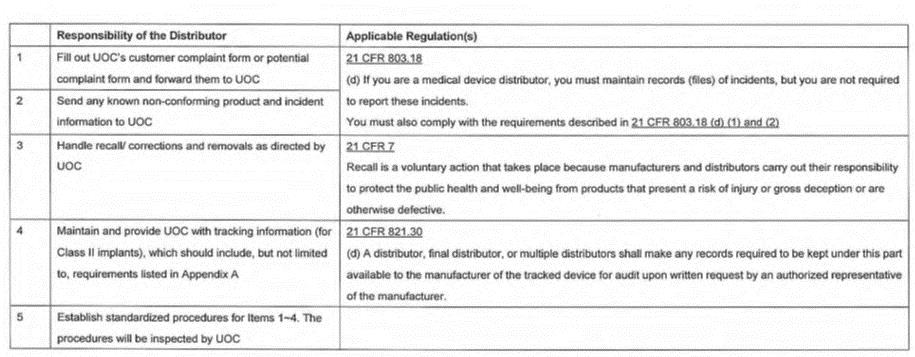
notify Company immediately of any adverse events or customer complaints, including, but not limited to, any untoward medical occurrence, death, life-threatening injury, or hospitalization conceivably associated with the use of ; |
|
|
B |
provide copies to Company within a commercially reasonable time of all customer complaints received by the Distributor relating to the Product(s); |
PAGE 4 OF 20

|
|
C |
keep and maintain records of all customer complaints received by Distributor relating to the Product(s); and |
|
|
D |
otherwise provide such assistance and information as Company reasonably requests to fulfill Company's complaint handling obligations for the Product(s), |
|
|
4.4 |
Adverse Event Reporting |
|
|
A |
Company shall be responsible for reporting adverse device events, malfunctions, incidents, near incidents and other reportable events related to the Product(s) pursuant to the Applicable Laws of any jurisdiction in which the Product(s) is marketed or sold, including, without limitation, FDA 21 C.F.R. Part 803, as may be amended from time to time ("MDR"). |
|
|
B |
The Distributor shall provide such assistance and information as Company reasonably requests to fulfill its adverse event reporting obligations for the Product(s). |
|
|
C |
The Company shall keep and maintain records of all customer complaints received by the Company relating to the Product(s) as required by 21 C.F.R. & 803.18 (or similar provisions of other Applicable Laws), as applicable; |
5. ADDITIONAL OBLIGATIONS OF DISTRIBUTOR
|
|
5.1 |
Health and Safety Laws and Regulations. Distributor shall comply fully, at its expense, with any and all applicable health and safety laws and regulations. |
|
|
5.2 |
Registration Licenses and Permits. Distributor agrees to use its best efforts to investigate, obtain any required government approval for, promote and distribute the Products, at its own expense as soon as feasible after the date of this Agreement, using generally the same channels and methods, exercising the same due diligence and adhering to the same standards which it employs with respect to other clinical and diagnostic products sold by Distributor, as well as distributors own products. Unless prohibited by local law, all such registrations and approvals obtained by Distributor shall be in the name of Company. |
|
|
5.3 |
Purchase Commitment. Distributor hereby agrees to purchase from the Company during the consecutive twenty-four-month period following the effective date of this Agreement the dollar amount of total purchases set forth on Addendum C (the "Annual Purchase Commitment"). The Annual Purchase Commitments for the following twelve-month period will be established before the end of the current twelve-month period. |
PAGE 5 OF 20

|
|
5.5 |
Customer and Sales Reporting. Distributor shall, at Distributor's own expense and consistent with the sales policies of Company: (I) place the Products in Distributor's literature as soon as possible; (ii) provide adequate contact with existing and potential customers on a regular basis, consistent with good business practice; (iii) assist Company in assessing customer requirements for the Products, including modifications and improvements thereto, in terms of quality, design, functional capability, and other features; and (iv) provide Company on a quarterly basis with a list of customers' feedback, a list of customers who have used Products and a list of institutions which have purchased Products, and a summary of the number of Products held by Distributor at the end of such quarter. |
|
|
5.6 |
Assignment of Products Rights. Upon termination of this Agreement for any reason whatsoever, Distributor agrees, to the full extent allowed by law in each jurisdiction, to assign, transfer, convey and set over unto Company, for no additional consideration, all registrations, license, permits, and other rights relating to sale, distribution and use of the Products. |
|
|
5.7 |
Limitation on Distributor's Rights to the Products. Distributor shall have no right to copy, modify or remanufacture any Product or part thereof. Distributor shall not make any changes, alterations, modifications or additions to the Products without prior written approval of Company. |
|
|
5.8 |
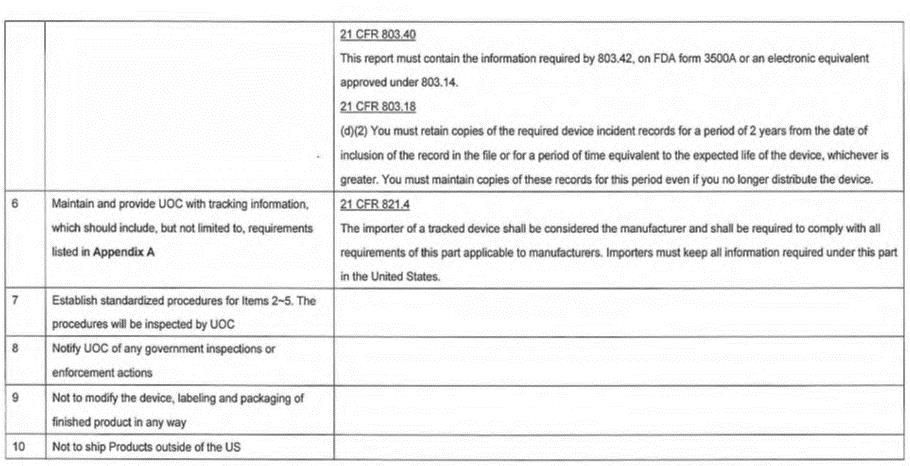
Compliance with U.S. Food and Drug Administration (FDA) Regulations. Distributor is required to fulfill the responsibilities of being an initial importer and a distributor governed by FDA, as outlined under the "Initial Importing and Distribution Regulatory Requirements", set forth in Addendum D attached hereto. Distributor acknowledges the receipt of the "Initial Importing and Distribution Regulatory Requirements" and represents that it has read and understood. Failure by Distributor to fulfill the responsibilities stated in Addendum D may result in, at Company's discretion, a termination of contract. |
PAGE 6 OF 20

|
|
performing the surgery and the list of products implanted by unit catalog number and lot number. The name of the patient will be retained by Distributor and immediately reported to Company in the event of an actual recall of the products. Failure by Distributor to provide this information timely may be the cause for Company to cease shipment of products to Distributor until such information has been provided. Distributor is also required to follow the responsibilities of being an initial importer and a distributor, as outlined under the "Initial Importing and Distribution Regulatory Requirements", set forth in Addendum D attached hereto. Failure by Distributor to fulfill the responsibilities stated in Addendum D may result in, at Company's discretion, a termination of contract. |
|
|
5.10 |
Governmental Inspections and Inquiries. Distributor shall promptly, and in any event within three (3) business days after the date of receipt of notice, notify Company in writing of, and shall provide Company with copies of, any correspondence and other documentation received or prepared by the Distributor in connection with any of the following events to the extent necessary to meet the requirements of any Governmental or Regulatory Authority: |
|
|
(i) |
Receipt of a regulatory letter(such as a Warning Letter or untitled letter) , warning, recall notice, notice of inspection or similar communication from any Governmental or Regulatory Authority in connection with the storage, marketing, advertisement, sale and/or distribution of the Product(s); and |
|
|
(ii) |
Any Governmental or Regulatory Authority's comments relating to the Product(s) that may require a response or action by Company. |
|
|
(iii) |
Without limiting the generality of the foregoing, in the event that the Company or any Agent receives a letter or comments from any such Governmental or Regulatory Authority in connection with any of the Product(s) that requires a response or action by Company, the Distributor shall promptly provide to Company any data or information required in preparing such response that relates to storage, marketing, advertising, sale or distribution of the Product(s), and the Distributor will cooperate fully with Company in preparing such response. |
PAGE 7 OF 20

|
|
of any correspondence that relates to such inspection. Company may, at its election, send representatives to such facility and may participate in any portion of such inspection that relates to any of the Product(s). The Distributor shall furnish to Company copies of all material information supplied to, or supplied by, such Governmental or Regulatory Authority, including observations and responses within five (5) business days after the delivery of such information by the Governmental or Regulatory Authority. |
|
|
(v) |
The Company shall provide Distributor with a copy of any response related to such visit or inspection for Company's review and comment prior to submission of the response. The Distributor shall provide Company with a copy of the final response promptly after it is submitted to the Governmental or Regulatory Authority. |
|
|
(vi) |
In the event any Governmental Authority detains or seizes any of the Product(s) from the Distributor or any Agent, the Distributor shall promptly send retained samples of each applicable Product and duplicate reports relating to such seizure to Company, provided that such action does not violate any applicable laws, statutes, ordinances or regulations |
6. TERM AND TERMINATION
|
|
6.1 |
Term. This Agreement shall become effective on January 1", 2011 and shall continue in full force and effect for a fixed term of Two (2) years from such date and end on December 31st, 2012. |
|
|
6.2 |
Termination Without Cause. Either party may terminate this Agreement at any time and for any reason upon ninety (90) days prior written notice to the other party. |
|
|
6.3 |
Termination With Cause. |
|
|
6.3.1 |
Immediate Termination by Company. Notwithstanding the above, Company may immediately terminate this Agreement with Distributor upon any of the following events: |
|
|
(i) |
If Distributor becomes insolvent or files a voluntary petition in bankruptcy, or has filed for it such involuntary petition in bankruptcy (unless such involuntary petition is withdrawn or dismissed within thirty days after filing); |
PAGE 8 OF 20

|
|
(ii) |
If Distributor fails to meet the Minimum Purchase Objectives in any year or for two consecutive quarters as set forth under the Terms set forth on Addendum C; |
|
|
(iii) |
If Distributor fails to cure any breach of any covenant, commitment or obligation under this Agreement within thirty days after receipt of written notice from Company of such breach; |
|
|
(iv) |
If Distributor commits a breach of a covenant, commitment or obligation under this Agreement which is of such a nature as not to be reasonable to cure, including but not limited to, a breach of Distributor's covenants respecting Confidential Information or of Distributor's covenants respecting conflicting activities; |
|
|
(v) |
If Distributor commits a crime or an act of fraud; |
|
|
(vi) |
If Distributor takes any action or asserts any claim against Company which in Company's judgment is detrimental to Company's business, reputation, or the relationship of the parties; |
|
|
(vii) |
If Distributor is not authorized by law; or |
|
|
(viii) |
If Distributor becomes the subsidiary of any other company, sold to another party or if control of Distributor (by ownership or by composition of the Board of Directors or otherwise) shall be substantially changed, or if this Agreement is assigned to any other person or entity without the acceptance of Company. |
|
|
6.3.2 |
Immediate Termination by Distributor. This Agreement may be terminated immediately by Distributor on written notice by Distributor to Company in any of the following events: |
|
|
(i) |
If Company becomes insolvent or files a voluntary petition in bankruptcy, or has filed for it an involuntary petition in bankruptcy (unless such involuntary petition is withdrawn or dismissed within thirty days after filing), or admits in writing an inability to pay its debts as they mature; or |
|
|
(ii) |
If Company fails to cure any breach of a covenant, commitment or obligation under this Agreement within thirty (30) days after receipt of written notice from Distributor to Company of such breach |
PAGE 9 OF 20

7. INTELLECTUAL PROPERTY RIGHTS AND CONFIDENTITALITY
|
|
7.1 |
Intellectual Property Rights. Distributor agrees that Company owns all right, title, and interest in the product lines that include the Products and in all of Company's patents, trademarks, trade names, inventions, copyrights, know-how, and trade secrets relating to the designs, manufacture, operation or service of the Products. The use by Distributor of any of these property rights is authorized only for the purposes herein set forth, and upon termination of this Agreement for any reason such authorization shall cease. Distributor shall not during the Term of this agreement or thereafter acquire or attempt to acquire in any manner whatsoever any right, title or interest in or to any of the trademarks, service marks, trade names, logos, or other signs or symbols identifying UOC or its related entities, or any xxxx, name, logo, sign or symbol confusingly similar thereto. |
|
|
7.2 |
Sale Conveys No Right to Manufacture or Copy. The Products are offered for sale and are sold by Company subject in every case to the condition that such sales does not convey any license, expressed or by implication, to manufacture, duplicate or otherwise copy or reproduce any of the Products. Distributor shall take appropriate steps with Distributor's customers, as Company may request, to inform them of and assure compliance with the restrictions contained in Section 8 Trademarks and Trade Name. |
PAGE 10 OF 20

|
|
but not limited to sales and financial data, customer information, manufacturing processes and specifications for the Products, shall be confidential information and Distributor shall not at any time, whether directly or indirectly, use (other than in the performance of its obligations hereunder) or disclose such information to any third party without the prior written consent of Company. All such information, know-how and trade secrets shall remain the sole property of Company. Distributor agrees to obtain from its employees, agents or sub distributors who have access to such information appropriate confidentiality or proprietary rights agreements. |
8. TRADEMARKS AND TRADE NAMES
|
|
8.1 |
Use. During the term of this Agreement, Distributor shall have the right to indicate to the public that Distributor is an authorized distributor of Company's Products and to advertise such Products under the trademarks, marks and trade names that Company may adopt from time to time (Company's Trademarks"). Distributor shall not alter or remove any Company's Trademark applied to the Products or packages at the factory or at Company's distribution facility. Except as set forth in this Section 9 PATENT, COPYRIGHT, AND TRADEMARK INDEMNITY, nothing contained in this Agreement shall grant to Distributor any rights, title or interest in Company's Trademarks. |
|
|
8.2 |
At no time during or after the term of this Agreement shall Distributor challenge or assist others to challenge Company's Trademarks or the registration thereof or attempt to register any trademarks, marks or trade name confusingly similar to those of Company. If Distributor violates, all sale and interests arising hereby of Distributor and whomever Distributor assists shall be deemed as an agreed compensation, which shall be paid by Distributor to Company. |
|
|
8.3 |
Approval of Representations. All representations of Company's Trademarks that Distributor intends to use shall first be submitted to Company for written approval, which shall not be unreasonably withheld, of design, color, and other details or shall be exact copies of those used by Company. If any of Company's Trademarks are to be used in conjunction with another trademark on or in relation to the Products, then Company's xxxx shall be presented equally legibly, equally prominently, and of equal or greater size that the other but nevertheless separated from the other so that each appears to be a xxxx in its own right, distinct from the other xxxx. |
9. INDEMNIFICATION
Company shall indemnify, defend and hold harmless Distributor, its officers, directors, shareholders and employees from and against all claims, causes of action, suits, costs and expenses (including reasonable legal fees), losses or liabilities resulting from any negligent
PAGE 11 OF 20

or wrongful act or omission on the part of Company, its employees, agents or representatives related to this Agreement and asserted by third parties and which relates to Company's performance hereunder including any of the Products violating any proprietary right of any third party. Company shall not indemnify Distributor for any claims, causes of action, suits, costs and expenses (including reasonable legal fees), losses or liabilities resulting from any negligent or wrongful act or omission on the part of Distributor, its employees, agents or representatives related to this Agreement,
10. OTHERS
The parties agree that the foregoing agreements and restrictions contained in Section 7, Section 8, and Section 9 shall survive after termination or expiration of this Agreement and, in the event of the Distributor's breach of any of the foregoing provisions, Company shall be entitled to equitable and injunctive relief against the Distributor in addition to other remedies available pursuant to this Agreement or applicable law.
PAGE 12 OF 20

The Addendums are the part of this Agreement and have the same effectiveness as the main text of this Agreement.
IN WITNESS WHEREOF, the undersigned are duly authorized to execute this Agreement on behalf of Company and Distributor, as applicable.
United Orthopedic Corporation
|
By |
|
By |
|
Print Name XXXXX XXX |
|
Print Name XXXX XXXXXX |
|
Title Chairman |
|
Title C.O.O |
|
Date |
|
Date |
PAGE 13 OF 20

Product List
Addendum A has 14 pages total attached hereto.
PAGE 14 OF 20


Page 1 of 13
Addendum A

Page 2 of 13
Addendum A

Page 3 of 13
Addendum A

Page 4 of 13
Addendum A

Page 5 of 13
Addendum A

Page 6 of 13
Addendum A

Page 7 of 13
Addendum A

Page 8 of 13
Addendum A

PAGE 15 OF 20

PAGE 16 OF 20

PAGE 17 OF 20

PAGE 18 OF 20

PAGE 19 OF 20

PAGE 20 OF 20

|
➢ |
A distributor, final distributor, or multiple distributor of any tracked device shall promptly provide the manufacturer tracking the device with the following information (21CFR 821): |
(1)The name and address of the distributor, final distributor or multiple distributors;
|
|
(2) |
The lot number, batch number, model number, or serial number of the device or other identifier used by the manufacturer to track the device; |
(3)The date the device was receive;
(4)The person from whom the device was received;
(5)The date the device was provided to the patient or for use in the patient;
(6)The name, mailing address, and telephone number of the prescribing physician;
|
|
(7) |
The date of the patient's death, or the date the device was returned to the manufacturer, permanently retired from use, or otherwise permanently disposed of. |
|
➢ |
A distributer shall provide such information to the manufacturer monthly. |
|
➢ |
Device tracking is required for the useful life of the device. |
|
➢ |
Information contained in such records that would identify patient or research subjects shall not be available for public disclosure. |
PAGE 21 OF 20