Supplementary Agreement on New Drug Methionine-Enkephalin Cooperation
Supplementary Agreement on New Drug Methionine-Enkephalin Cooperation
In October, 2012, Hubei Qianjiang Pharmaceutical Co., Ltd (a Chinese listed company) and TNI Biotech, Inc. (an American company) signed Cooperation Agreement on New Drug Methionine Enkephalin in Qianjiang, Hubei Province, China.
In August 5, 2014, Hubei Qianjiang Pharmaceutical Co., Ltd (hereinafter referred to as "Qianjiang Pharmaceutical ") and TNI Biotech, Inc. (hereinafter referred to as "TNI Biotech") signed Supplementary Cooperation Agreement on New Drug Methionine Enkephalin in America. TNI Biotech has established a great deal of studies since signed the agreements, including basic studies such as pharmaceutical studies, pharma toxicology studies, clinical trials and new-developed indications. Such trials work smoothly, and several results have got the approval of the Food and Drug Administration.
According to the reports of TNI Biotech, TNI Biotech has established phase I and II clinical trials as used for pancreatic cancer, and joined FDA meeting in August, 2013. In the meeting, FDA approved TNI Biotech in following aspects: developing XXXX as lyophilized power and with administration route by iv or subcutaneous approving phase I and II clinical results; holding meeting on End of Phase II and determination of Phase III in America after TNI Biotech complement pharmaceutical studies and pharma-toxicology studies on XXXX; evaluating the basic studies and trials by TNI Biotech on XXXX as used for treating liver cancer. FDA approved a phase IIB study but has required a PK and Dosing Trial be run in parallel with the Phase IIB Trials.
To date, Hubei Qianjiang Pharmaceutical Co., Ltd has established great of marketing research, and pre-trials on pharmaceutical and pharma-toxicology studies, which have obtained positive results. In order to accelerate clinical trials in both America and China and many other researches and developments, both parties agree:
| 1. | According to the previous agreements, Qianjiang Pharmaceutical has opened a co-administration account for the development of XXXX in China. |
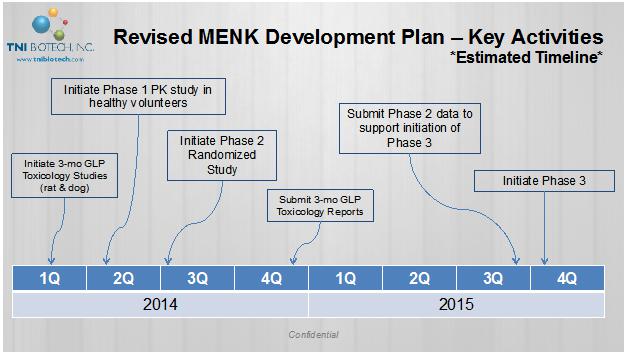
| 2. | The parties agree to immediately to initiate 3-mo GLP Toxicology Studies (rat & dog) within 30 days of the signing of this agreement. GLP Toxicology Studies Trials will be run in China in accordance with international standards and acceptable to the FDA. The studies will include |
Exploratory Toxicology (nGLP)
| • | Dose range finding studies |
| • | Different species and methods of administration |
| • | Multiple dosing regimens |
| • | Estimate the response vs. dose given |
Definitive Toxicology (GLP)
| • | Performed in collaboration with Xxxxxxx Laboratories (USA) and MPI/Medicillon (China) |
| • | General toxicology studies |
| • | Different species and methods of administration |
| • | Immunogenicity study with NHPs |
Special toxicology studies (planned)
| 3. | Xxxxxxxxx has raised the funds necessary to for clinical development and marketing of XXXX. Expenditure are subject to full budget approval by both Xxxxxxxxx and Cytocom and will be approved by Xx. Xx Xxxx, Qianjiang pharmaceutical, and, authorized by Xxxxxx Xxxxxxx, United States. |
| 4. | Cytocom and Qianjiang Pharmaceutical will meet immediately with the SFDA to determine that PK and Dosing Trials completed in the US will be acceptable to the SFDA. All developments and trial results run by Cytocom in America or EU will be used for requesting registration approval in China |
| 5. | Based on PK, Xxxxxx and Existing Trial results in US, Qianjiang pharmaceutical and Xx. Xxxxxx Xxxxxx and Xx. Xxxxxx Xxxxxxxx will meet with the SFDA to determine what additional data will be required to initiate corresponding trial in China |
This amendment will be going to effect after signature by two parties
| /s/ Ye Jige | August 6, 2014 | |||
| Xx. Xx Xxxx Chief Executive Officer | Date | |||
| Hubei Qianjiang Pharmaceutical Co., Ltd | ||||
| /s/ Xxxxxx Xxxxxxx | August 6, 2014 | |||
| Xxxxxx Xxxxxxx | Date | |||
| Chief Executive Officer | ||||
| TNI BioTech Inc | ||||
| /s/ Xxxxxx Xxxxxxx | August 6, 2014 | |||
| Cytocom Inc Chairman | Date | |||
| Xxxxxx Xxxxxxx |
EXHIBIT A
ESTIMATED DEVELOPMENT XXXX BUDGET AND TIMELINE
| XXXX Development | 6 Months | 12 Months | 18 Months | 24 Months | 30 Months | |||||||||||||||
| PK & Dosing Trial XXXX | $ | 1,200,000 | ||||||||||||||||||
| XXXX RE; Formulation | $ | 195,000 | ||||||||||||||||||
| XXXX CMC Package | $ | 600,000 | $ | 600,000 | ||||||||||||||||
| XXXX Animial Studies | $ | 850,000 | ||||||||||||||||||
| China Lab | $ | 60,000 | $ | 60,000 | $ | 60,000 | $ | 60,000 | $ | 60,000 | ||||||||||
| Pancaratic / Liver Cancer IIB/III | $ | 9,375,000 | $ | 9,375,000 | $ | 9,375,000 | ||||||||||||||
| TOTAL | $ | 2,905,000 | $ | 660,000 | $ | 9,435,000 | $ | 9,435,000 | $ | 9,435,000 | ||||||||||