MANUFACTURING & SUPPLY AGREEMENT
EXHIBIT 10.1
MANUFACTURING & SUPPLY AGREEMENT
This SUPPLY AGREEMENT (this “Agreement”), dated as of March 11, 2014 (the “Effective Date”), is by and between LNK International Inc., a New York Corporation (“Supplier”), and SCRIPSAMERICA INC., a Delaware company (“SCRIPSAMERICA”).
1. GENERAL TERMS OF PURCHASE AND SALE
1.1. Packages Manufactured, Assembled and Purchased. With respect to each of the products described on Schedule A hereto (the “Products”), Supplier shall provide the Product manufacturing, assembly, packaging, labeling, and packing services, and provide the Product components, as described on Schedule A hereto, and SCRIPSAMERICA shall purchase the Products from Supplier, pursuant to purchase orders submitted by SCRIPSAMERICA to Supplier from time to time in accordance with Section 3.4. The Products shall be manufactured, assembled, packaged, labeled, and packed for shipping in strict compliance with the procedures, standards, requirements, and other specifications set forth on Schedule B hereto (the “Specifications”). Schedule B will be amended to reflect any Specifications agreed upon in writing by SCRIPSAMERICA and Supplier after the execution of this Agreement. The Products will be manufactured, assembled, packaged, labeled, and packed at the facilities designated in Schedule B, and Supplier may not use any other facility for such work without SCRIPSAMERICA’s written consent. All facilities must comply with Section 5.4.
1.2. Pricing Fee and Payment Terms.
(a) Fees. SCRIPSAMERICA shall pay to Supplier the fees described on Schedule C hereto (the “Fees”). Such Fees constitute Supplier’s entire compensation for its performance under this Agreement and, except as otherwise specifically provided herein, SCRIPSAMERICA shall not be obligated to pay Supplier any other charges, costs (including regular inbound shipping costs), taxes or expenses. Subject to Supplier’s obligations under Section 3.5, SCRIPSAMERICA shall be obligated to pay all expedited inbound shipping charges that SCRIPSAMERICA initiates and shall arrange and pay all outbound shipping charges. The Fees are firm for the Term (as defined in Section 2.1), and may be adjusted during the Term only as provided on` Schedule C hereto. Representatives of SCRIPSAMERICA and Supplier will discuss in a transparent manner on the cost of raw material, direct labor costs and Fees prior to SCRIPSAMERICA issuing the Purchase Order.
(b) Invoices; Payment. Supplier shall issue invoices to SCRIPSAMERICA within Five (5) Business Days after the Products are shipped to SCRIPSAMERICA. SCRIPSAMERICA shall pay Supplier within (30) days from the date of receipt of each invoice that is properly supported by complete and correct bills of lading. All payments shall be made in U.S. dollars. As used herein, “Business Day” means any day other than a Saturday or Sunday or any other day on which banks in Pennsylvania are permitted or required by applicable law to be closed.
(c) Cost Reduction Initiatives. Supplier shall use all commercially reasonable efforts to establish and implement cost reduction initiatives including but not limited to production yield improvement, lower cost raw materials and other components. Supplier shall disclose to and discuss with SCRIPSAMERICA any cost reduction derived from the successful implementation of such initiatives and the parties shall negotiate an agreed upon allocation of such cost savings. Supplier and SCRIPSAMERICA shall meet every quarter to specifically discuss cost reduction initiative opportunities and the status of ongoing efforts. Best efforts shall be made by Supplier to propose cost reduction initiatives which reduce costs by a minimum of 5% for each product over the term of the agreement.
(d) Performance Management. Supplier and SCRIPSAMERICA shall jointly develop Key Performance Indicators (KPIs) that will be the basis for measuring the performance of the supplier. These KPIs will be reviewed at the end of each calendar quarter at a joint meeting of the Supplier and SCRIPSAMERICA.
1.3. Non - Exclusive Supply Arrangement. During the Term (as defined in Section 2.1 below) Supplier agrees that it or its Affiliates (as defined in Section 6.1(a)) will, continue to supply product, (a) manufacture, assemble, package, label, and/or pack for shipment on a non-exclusive basis. This product will not be sold in the United States of America.
| 1 |
2. TERM;
TERMINATION
2.1. Term. The term of this Agreement shall commence on the Effective Date and shall remain in full force and effect for one (1) year from the Effective Date (the “Term”), unless otherwise terminated earlier pursuant to Section 2.2. This Agreement shall renew automatically for renewal terms of one (1) year each unless either party submits a notice of termination no later than one hundred eighty (180) days prior to the expiration of the then current term.
2.2. Termination. This Agreement may be terminated in accordance with any of the following provisions:
(a) Default. If a party fails to perform or comply in any material respect with any of its obligations under this Agreement (except pursuant to a force majeure event set forth in Section 9.2 or a breach of Section 6.5), and such failure is not remedied within thirty (30) days after receipt of written notice of such failure, then the other party may terminate this Agreement effective upon expiration of such thirty (30) day cure period.
(b) Default Due to Force Majeure. If a party fails to perform or comply in any material respect with any of its obligations under this Agreement for a period of at least ninety (90) consecutive or cumulative days due to a force majeure event set forth in Section 9.2, then the other party may terminate this Agreement immediately upon written notice to the party suffering the force majeure event.
(c) Insolvency/Bankruptcy. If a party shall: (i) be unable to pay or admits in writing its inability to pay its debts as they mature; (ii) make a general assignment for the benefit of creditors; (iii) apply for or consent to the appointment of a receiver, trustee or liquidator of all or a substantial part of its assets; (iv) file a petition or be the subject of an involuntary petition in bankruptcy or for reorganization or for an arrangement pursuant to a bankruptcy act or insolvency which petition is not dismissed within ninety (90) days from such filing; or (v) be adjudicated as bankrupt or insolvent, then the other party may terminate this Agreement upon written notice to the first party.
(d) Breach of Confidentiality. If a party breaches its obligations under Section 6.5, then the other party may terminate this Agreement immediately upon written notice to the breaching party describing the breach.
(e) Suspension/Termination of Products. If SCRIPSAMERICA determines in its sole discretion that it will no longer market the Products then it may terminate this Agreement upon ninety (90) days prior notice to Supplier.
2.3. Notification. Supplier shall immediately notify SCRIPSAMERICA in writing if (a) there is anything that prohibits or restricts Supplier from doing business with or providing or licensing Technology (as defined in Section 6.1, to SCRIPSAMERICA, or (b) Supplier grants any competitor manufacturing a Competing Product preferential rights to any of Supplier’s Technology. In addition to any rights set forth in Section 2.2, SCRIPSAMERICA shall have the right to terminate this Agreement upon sixty (60) days written notice at any time after receipt of such written notice from Supplier.
2.4. Purchase of Inventories Upon Termination. Upon expiration of this Agreement, or termination by Supplier pursuant to Section 2.2(a), 2.2(b), 2.2(c), 2.2(d), or 2.3 SCRIPSAMERICA (a) shall purchase Supplier’s uncontaminated, usable packaging, work in process and finished goods inventories that (i) are unique to the Products, (ii) cannot otherwise be used by Supplier within six (6) months of termination, and (iii) are covered by firm SCRIPSAMERICA purchase orders or are long lead time items that SCRIPSAMERICA agreed in writing to purchase and (b) shall have the obligation to purchase Supplier’s uncontaminated, usable raw materials, at Supplier’s cost (including inbound shipping costs); provided, that, if SCRIPSAMERICA terminates this Agreement pursuant to Section 2.2(a), 2.2(b), 2.2(c), 2.2(d), or 2.3, then SCRIPSAMERICA shall have the right, but not the obligation, to purchase such inventories. SCRIPSAMERICA shall not be liable for any claim based upon any expenditure, investment or commitment made by or on behalf of Supplier or in connection with the establishment, development or maintenance of any business or goodwill of Supplier.
2.5. Rights Upon Termination. Any termination of this Agreement shall be without prejudice to all other rights and remedies available to the parties under this Agreement or at law or in equity. If the Agreement is terminated by a party pursuant to Section 2.2(a) or (d), the defaulting party shall be responsible for all reasonable out of pocket expenses and losses incurred by such non-defaulting party resulting from the termination.
| 2 |
3. PRACTICES AND PROCEDURES
3.1. Supplier Responsibilities. As set forth in Schedule A, SCRIPSAMERICA or Supplier shall purchase and provide all raw materials, components, packaging, labeling and shipping materials, labor, utilities and equipment necessary to manufacture, assemble, package, and label the Products and pack the Products for shipping, all in strict compliance with the Specifications. Use of materials shall be on a first in, first out basis, unless otherwise agreed to in writing by SCRIPSAMERICA. Supplier shall prepare and deliver in a timely fashion all reports and information reasonably requested by SCRIPSAMERICA, including, product quality, and daily production and shipping reports. Upon the date hereof and each anniversary hereafter, Supplier shall provide SCRIPSAMERICA with a list of all assets that are located at any of Supplier’s facilities but are owned by SCRIPSAMERICA. If SCRIPSAMERICA purchases and provides any raw material and/or packaging/ancillary items, Supplier shall deduct those costs from the Purchase Order value and Invoice SCRIPSAMERICA for the balance amount.
3.2. Supplier Capacity. Supplier represents and warrants that it has sufficient capacity to supply the volumes of the Products. With the exception of any disruption in manufacturing caused by a Force Majeure Event, and subject to the maximum Product production volumes, if, in any calendar month during the Term, Supplier fails to deliver to SCRIPSAMERICA at least ninety-eight percent (98%) of the volume of Products ordered by SCRIPSAMERICA pursuant to its purchase order for such month (the “Minimum Production Volume”), then, for each Product for which Supplier failed to deliver the Minimum Production Volume, SCRIPSAMERICA shall receive a credit on its next purchase order (or purchase orders if the credit amount is larger than the price of the next single order) in an amount equal to (i) the number of Product units below the Minimum Production Volume that Supplier failed to deliver, multiplied by (ii) the per unit Product Fee then in effect for such Product; provided, that such credit will be applied to the total Product Fees contained in such purchase order and is not required to be used to offset Product Fees for the Product for which Supplier failed to meet the Minimum Production Volume; provided, further, that if this Agreement is terminated or expires before all of SCRIPSAMERICA’s credits are used, then SCRIPSAMERICA shall receive, within thirty (30) days of such termination or expiration date, a cash payment from Supplier for the entire value of any unused credits.
3.3. Inventories. Supplier shall be responsible for ordering, purchasing and maintaining all raw material and component inventories and for managing order quantities, lead times, and delivery dates for the Products. All unused materials for the Products shall be stored in Supplier's warehouse. Supplier shall be responsible for supplying an inventory report of all raw materials and components (either at Supplier’s facility or subject to issued purchase orders with Product raw material/component suppliers) within three (3) Business Days after the end of each calendar month for the Products. Supplier shall notify SCRIPSAMERICA immediately of any significant loss of materials and Supplier shall be responsible for all losses, shrinkage and scrap of materials associated with packaging and assembling the products, except where losses, shrinkage and scrap of materials are directly related to insufficient quality of materials delivered by the SCRIPSAMERICA-specified supplier of such components listed on Schedule A. Supplier shall perform an annual physical inventory relating to the Products owned by SCRIPSAMERICA at Supplier’s own expense and SCRIPSAMERICA shall bear the expense of any other physical inventories requested by SCRIPSAMERICA.
3.4. Shipment. Time of delivery of the Products by Supplier is of the essence. All sales of the Products under this Agreement shall be FOB (Incoterms 2000) Supplier’s facility located ▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇. Title to and risk of loss of such Products shall be transferred to SCRIPSAMERICA by Supplier upon delivery by Supplier to SCRIPSAMERICA’s designated carrier. If Supplier is more than seven (7) calendar days late in delivering, in whole or in part, any shipments of the Products to SCRIPSAMERICA due to the actions and/or omissions of Supplier, Supplier shall make all such late shipments to SCRIPSAMERICA as SCRIPSAMERICA directs, and Supplier shall pay all additional shipping costs and shipping expenses in connection with such late shipments. SCRIPSAMERICA shall ensure that the shipment of Products by its designated carriers complies with all applicable Federal, State and local laws, rules, regulations and ordinances (collectively, “Laws”), including, without limitation, the Toxic Substance Control Act.
3.5. Changes. SCRIPSAMERICA shall have the right to request changes from time to time to the Products, the Specifications or any other specifications or procedures. If Supplier believes that such changes would result in an increase or decrease in Supplier’s manufacturing, assembling, packaging, labeling and/or packing costs, Supplier shall promptly notify SCRIPSAMERICA of the amount of such increase or decrease in writing before Supplier agrees to make the change. SCRIPSAMERICA shall pay only those costs of such changes that SCRIPSAMERICA agrees to in writing, and all agreed upon changes shall be reflected in amendments to the appropriate Schedules hereto.
3.6. Special or Test Production. SCRIPSAMERICA shall have the right to request from time to time that Supplier manufacture the Products pursuant to an Experimental Order (“EO”) furnished by SCRIPSAMERICA. Prior to the issuance of an EO, Supplier will provide SCRIPSAMERICA with a written estimate of the feasibility, cost, and production forecast for such EO production. Supplier shall manufacture the Products in strict compliance with any EO. Supplier shall not manufacture any Products that do not strictly conform to the Specifications without a written EO signed by SCRIPSAMERICA. The written EO signed by SCRIPSAMERICA shall include Supplier’s terms for cost and production forecast. An EO production shall be conducted prior to the first purchase order required to be submitted to Supplier pursuant to Section 3.4. If SCRIPSAMERICA advises Supplier that the EO is confidential, Supplier shall restrict access to the EO and information concerning the EO to only those employees of Supplier who have a need to know and shall not permit any other third parties to view the EO, products made during the EO or other information concerning the EO without SCRIPSAMERICA’s prior written consent.
| 3 |
3.7. Destruction or Return of Materials. SCRIPSAMERICA shall have the right to require Supplier, at SCRIPSAMERICA’s option, to destroy or return obsolete, test or other materials, provided that SCRIPSAMERICA has paid for the materials to be destroyed or returned. SCRIPSAMERICA shall reimburse Supplier for any reasonable costs incurred in destroying or returning such materials. Supplier shall not be required to store at its facility any unused material or packaging component that has been in its possession for two (2) years, but has been inactive. Supplier shall notify SCRIPSAMERICA if any such unused materials or packaging components exist and SCRIPSAMERICA will respond promptly with instructions to return or destroy at SCRIPSAMERICA’s expense. Notwithstanding the foregoing sentence, SCRIPSAMERICA shall not be obligated to pay for any nonconforming products or materials that it requests Supplier to destroy nor shall SCRIPSAMERICA be required to reimburse Supplier for the costs incurred in destroying or returning such nonconforming materials. Upon SCRIPSAMERICA’s request, Supplier shall physically witness the destruction of such materials and shall provide written certification to SCRIPSAMERICA that such materials have been completely destroyed. At SCRIPSAMERICA’s option, SCRIPSAMERICA also may have a representative present to witness such destruction.
3.8. SCRIPSAMERICA Representative. Without compromising or disclosing any confidential trade secret (as that term is defined by the Uniform Trade Secrets Act) or proprietary information belonging to other customers of Supplier, SCRIPSAMERICA shall have the right to have a mutually agreed number of its representatives on-site at Supplier’s facilities to monitor Supplier’s performance under this Agreement, observe the manufacturing, assembling, packaging, labeling and packing processes, and coordinate shipments. The dates of such monitoring shall be mutually agreed upon by both SCRIPSAMERICA and Supplier within ten (10) calendar days prior to any such visit, and Supplier shall cooperate by supplying such office space, administrative assistance, and utilities (excluding long distance telephone services) to such SCRIPSAMERICA representatives. SCRIPSAMERICA shall be entitled to four (4) such monitoring visits for each twelve (12) month period during the Term.
4. INSPECTION AND AUDIT
4.1. Inspection. Without compromising Supplier's customers' confidential information, and on a mutually agreed upon date within one (1) week from written notice to Supplier, SCRIPSAMERICA shall have the right, during Supplier’s normal business hours, to inspect the Supplier’s facilities where the Products are being manufactured, assembled, packaged, labeled and packed and where materials used to manufacture, assemble, package, label and pack the Products are handled or stored, and to observe the manufacturing, assembling, packaging, labeling, storage, inspection, testing, packing, and shipping of the Products.
4.2. Audit. Supplier shall keep complete and accurate accounts, records, books, and data with respect to Supplier’s performance under this Agreement (the “Records”). SCRIPSAMERICA and its representatives shall have the right, at all reasonable times, to inspect, and audit the Records relating to Supplier’s performance under this Agreement and such other documents and records as may be reasonably necessary to verify Supplier’s performance of its obligations under this Agreement. Supplier shall retain all Records no longer than required by the US Food and Drug Administration requirements and will make the same available to SCRIPSAMERICA and its representatives within fifteen (15) Business Days after receipt of a written request for such Records from SCRIPSAMERICA.
5. QUALITY CONTROL & ASSURANCE; WARRANTIES & REPRESENTATIONS
5.1. Quality Control. Supplier shall conduct all quality control sampling and testing required by the Specifications. All such sampling and testing shall be conducted by qualified personnel. Supplier shall bear the cost of all equipment necessary to perform such sampling and testing as is required by SCRIPSAMERICA as of the date hereof. Written summaries of quality test results shall be available to SCRIPSAMERICA, at no cost, upon SCRIPSAMERICA’s request. Supplier shall retain records relating to its quality control testing for at least four (4) years after such testing is completed.
5.2. Supplier’s Warranties. Supplier warrants that (a) at the time of delivery of materials and packaging to SCRIPSAMERICA, it will have good and marketable title to all materials and packaging sold to SCRIPSAMERICA, and (b) all Products sold to SCRIPSAMERICA will strictly conform to the Specifications and SCRIPSAMERICA’s quality control standards, will be manufactured in accordance and comply with all applicable Laws and industry standards, will be manufactured using current Good Manufacturing Practices (“cGMP”), will be free from all defects in material and workmanship, and will be free and clear of all liens and encumbrances (together with all other warranties of Supplier set forth in this Agreement, the “Supplier Warranties”). THE SUPPLIER WARRANTIES ARE THE ONLY WARRANTIES OF SUPPLIER WITH RESPECT TO THIS AGREEMENT AND ARE IN LIEU OF ANY OTHER WARRANTIES, WHETHER EXPRESS, IMPLIED OR STATUATORY, INCLUDING BUT NOT LIMITED TO THOSE FOR MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR ANY OTHER WARRANTY ARISING OUT OF ANY SPECIFICATION. SCRIPSAMERICA HEREBY WAIVES ALL OTHER WARRANTIES OR GUARANTEES OF SUPPLIER WHETHER EXPRESS OR IMPLIED.
| 4 |
5.3. Certificates of Analysis and Manufacturing Compliance.
(a) Supplier shall test or cause to be tested each lot of Product purchased pursuant to this Agreement as per the Specifications. For each lot of Product tested, each test shall set forth the items tested, specifications, and test results in a certificate of analysis, which Supplier shall send or cause to be sent to SCRIPSAMERICA. SCRIPSAMERICA is entitled to rely on such certificates for all purposes of this Agreement
(b) Supplier shall provide or cause to be provided a certificate of manufacturing compliance or manufacturing lot record that will certify that the Products were manufactured in accordance with the Specifications and applicable cGMPs.
5.4. Facility Compliance. Supplier's facilities at which all of its work hereunder is to be performed (including all equipment and procedures used in such facilities) are, and at all times during the term of this Agreement will be, and all such work to be undertaken by Supplier hereunder will be, compliant with all applicable provisions of the Federal Food, Drug and Cosmetic Act and all other applicable Laws and government regulations. Supplier will promptly disclose to SCRIPSAMERICA any regulatory breaches upon notification by the Food and Drug Administration (“FDA”) or any other governmental authority.
5.5. Distribution Record. Supplier shall maintain distribution records that contain all of the appropriate information as specified in the cGMP regulations.
5.6. Regulatory Compliance. Supplier is responsible for cGMP compliance with all Laws as they apply to Supplier's facility. As long as the Products meet the Specifications, Supplier shall have no responsibility for compliance with Laws as they relate specifically to SCRIPSAMERICA’s use of ingredients, labeling or marketing. Supplier assumes responsibility for all contact with the FDA and other regulatory bodies as relates to the manufacture, assembly, packaging, labeling, and packing for shipment of the Products, even after the termination of this Agreement; provided, that Supplier shall (a) furnish SCRIPSAMERICA with copies of all reports and other correspondence received from any such regulatory bodies which relate to the Products, the facilities used to manufacture the Products, or the quality systems of the Supplier, and (b) provide SCRIPSAMERICA with draft copies of any related response to any regulatory body at least three (3) Business Days (as defined below) prior to submission of such response.
5.7. Regulatory Inspections.
(a) Supplier agrees to host inspections from any federal, state or provincial regulatory authority responsible for the supervision of Supplier’s operations, even after the termination of this Agreement.
(b) Supplier shall immediately inform SCRIPSAMERICA of any regulatory inspections which may involve the Products or related processes and shall make its best effort to prepare for such inspections. Supplier shall (i) furnish SCRIPSAMERICA with copies of all reports and analyses relating to such inspections and (ii) provide to SCRIPSAMERICA duplicate samples of the Products given to government agencies and duplicates of any photographs taken during the inspections (unless such pictures contain confidential or trade secret information). Supplier shall inform SCRIPSAMERICA of the findings of such an inspection and immediately provide a copy of the correspondence with the authorities, provided that the Products are concerned.
(c) In the above cases a copy of any regulatory report, FDA Form 483, or letter shall be provided to SCRIPSAMERICA within three (3) business days of receipt if it relates to the Products, the facilities used to manufacture the Products, or the quality systems of the Supplier.
(d) Supplier agrees to provide copies of any response to a regulatory report concerning the Products to any regulatory body.
5.8. Nonconforming Products. The total costs (including, without limitation, raw materials, packaging supplies, packing charges, proper disposal costs, product returns and recall costs) relating to the Products that do not comply with the Specifications, the Supplier Warranties or any other provision of this Agreement shall be the responsibility of Supplier. For purposes of clarification, if a Product is subject to a recall, the “total costs” of such recall would include, without limitation, all costs related to all Product units that are recalled (regardless of whether such units were conforming or non-conforming). If SCRIPSAMERICA believes that any Products do not comply with the Specifications, Supplier Warranties or any provisions of this Agreement, SCRIPSAMERICA shall notify Supplier of such nonconformance and, upon Supplier’s request, provide written details and deliver a sample of such nonconforming Products to Supplier. Supplier shall promptly notify SCRIPSAMERICA (and in any event within seven (7) calendar days) whether Supplier agrees that such Products are not in compliance. Supplier shall have the right to rework or dispose of nonconforming Products only with the written consent of SCRIPSAMERICA, which consent shall not be unreasonably withheld. Supplier shall replace any such nonconforming Products with conforming Products at Supplier’s expense within thirty (30) days after receipt of SCRIPSAMERICA's notice of nonconformity. Supplier shall be required to secure, deploy, and pay for all of the labor, materials and other resources (including, but not limited to, legal and regulatory advisors) necessary to address any Products that do not comply with the Specifications, the Supplier Warranties or any other provision of this Agreement, and SCRIPSAMERICA shall not be required to provide or make available to Supplier any labor, materials or other resources for any such purposes.
| 5 |
If SCRIPSAMERICA and Supplier are unable to agree as to whether certain Products comply with the Specifications or the Supplier Warranties, the parties shall cooperate to have the Products in dispute analyzed by an independent testing laboratory of recognized repute selected by SCRIPSAMERICA and approved by Supplier, which approval shall not be unreasonably withheld or delayed. The results of such laboratory testing shall be final and controlling. The fees and expenses of such laboratory testing shall be borne entirely by the party against whom such laboratory’s findings are made.
5.9. Representations.
(a) Each party represents and warrants to the other party that it has the full right and authority to enter into and perform this Agreement, that its performances hereunder will not conflict with or breach any other agreement to which it is a party, and that it is free of any obligations that would prevent or tend to impair the full performance of its obligations hereunder.
(b) Each party represents and warrants to the other party that any and all services performed by it hereunder shall be of a professional quality consistent with generally accepted industry standards for the performance of such types of services and will comply with all Laws.
(c) Except for the intellectual property of SCRIPSAMERICA referred to in Section 6 hereof, Supplier owns all right, title and interest in and to, or otherwise has lawful rights to use, the intellectual property used by Supplier in the manufacturing, assembly, packaging, labeling, and packing of the Products and Supplier has not received notice of any present or threatened claim, action or proceeding alleging that any part of its intellectual property infringes any third party's intellectual property rights, and SCRIPSAMERICA and its Affiliates may freely market and sell the Products without infringing any third party's intellectual property rights and without any royalty, fee or similar payment of any kind being or becoming due or payable by SCRIPSAMERICA or its Affiliates to any third party.
(d) Supplier represents and warrants to SCRIPSAMERICA (i) that the Products or any components or parts thereof purchased for the Products will not infringe upon the intellectual property of any third party, and (ii) that Supplier has obtained all necessary licenses, permits and permissions to use any third party intellectual property.
(e) SCRIPSAMERICA represents and warrants to that, to its actual knowledge as of the Effective Date, the Specifications for the Products will not infringe upon the patent, copyright or trademark rights of any third party. SCRIPSAMERICA further represents and warrants that it maintains all necessary governmental licenses, permits and approvals related to the sale and distribution of the Products.
6. INTELLECTUAL PROPERTY AND CONFIDENTIALITY6
6.1.
Definitions.
(a) “Affiliate” shall mean, with respect to any person, any other person who directly or indirectly controls or is controlled by, or is under common control with, such person; and “control” means, with respect to any person, the direct or indirect ability to direct or cause the direction of the management and policies of such person, whether through the ownership of voting securities, by contract or otherwise.
(b) “SCRIPSAMERICA Confidential Information” means any and all information or Technology that (i) concerns or relates to any aspect of the Products or the business of SCRIPSAMERICA and/or SCRIPSAMERICA, Inc. (“SCRIPSAMERICA”); (ii) is owned or used by SCRIPSAMERICA and/or SCRIPSAMERICA; or (iii) is, for any reason, identified or otherwise treated as confidential by SCRIPSAMERICA and/or SCRIPSAMERICA, in each instance, whether or not reduced to writing or other tangible medium of expression, and whether or not patented, patentable, capable of trade secret protection or protected as an unpublished or published work under the United States Copyright Act of 1976, as amended, except such information or Technology that Supplier can clearly show (A) was publicly known prior to the date of this Agreement; (B) subsequent to the date of this Agreement has become publicly known through no fault of Supplier; (C) was known to and documented by Supplier prior to the date of this Agreement and with respect to which Supplier was not and is not under any obligation of confidentiality; or (D) was disclosed to Supplier without restriction on disclosure or use by a third party who was not under any obligations of confidentiality (contractual or otherwise).
| 6 |
(c) “Supplier Confidential Information” means any and all manufacturing processes, technologies, procedures or any information regarding any of Supplier’s other manufacturing customers that relates to the manufacture of any products by Supplier, except such information or Technology which SCRIPSAMERICA and/or SCRIPSAMERICA can clearly show (A) was publicly known prior to the date of this Agreement; (B) subsequent to the date of this Agreement has become publicly known through no fault of SCRIPSAMERICA and/or SCRIPSAMERICA; (C) was known to and documented by SCRIPSAMERICA and/or SCRIPSAMERICA prior to the date of this Agreement and with respect to which SCRIPSAMERICA and SCRIPSAMERICA were not and are not under any obligation of confidentiality; or (D) was disclosed to SCRIPSAMERICA and/or SCRIPSAMERICA without restriction on disclosure or use by a third party who was not under any obligations of confidentiality (contractual or otherwise).
(d) “Patents” shall mean all United States and foreign patents and applications therefore (including continuations, divisionals, provisionals continuations-in-part, or reissues of patent applications and patents issuing thereon) owned by SCRIPSAMERICA or its Affiliates and relating to or concerning or on which any issued or pending claim reads on the Products, use of the Products, and/or manufacture of the Products.
(e) “Technology” means ideas, concepts, know-how, techniques, methods, models, processes, designs, data, software, apparatus, devices, molds, tooling, packaging or packaging materials, techniques, formulations, flow charts, block diagrams, reports, systems, sketches, compositions of matter, discoveries, developments, improvements, and inventions (whether or not patentable), patents, patent applications, works of authorship (whether or not copyrightable), information, algorithms, trade secrets, procedures, notes, summaries, results and conclusions.
6.2. Intellectual Property.
(a) LNK International (and its Affiliates) and ScripsAmerica Inc. agree that, as between them LNK and its Affiliates are the sole and exclusive owner of all rights, intellectual and otherwise, to (i) the Patents, (ii) all Technology relating to, concerning or incorporated in the Products, including, without limitation, (A) the formula for the Products, (B) processing techniques and operating procedures for manufacturing, assembling, packaging, labeling, and packing for shipment the Products (regardless of whether existing on the Effective Date or later developed by SCRIPSAMERICA, Supplier and/or any of their respective Affiliates), and (iii) the trademarks, trade names and trade dress used in connection with the packaging, marketing and sale of the Products. Supplier agrees that, as a result of performing under this Agreement, Supplier does not acquire any right, title or interest in any property, intellectual or otherwise, owned or controlled by SCRIPSAMERICA or its Affiliates.
(b) SCRIPSAMERICA and Supplier agree that, as between them, Supplier is the sole and exclusive owner of all rights, intellectual and otherwise, to (i) Supplier’s proprietary processing techniques and proprietary operating procedures for filling and packaging the Products, developed independently by Supplier, without the use of SCRIPSAMERICA Technology, and (ii) all of Suppliers intellectual property, trade secrets processes and applications in existence prior to the Effective Date (the items in clauses (i) and (ii) are collectively, the “Supplier IP”). SCRIPSAMERICA agrees that, as a result of performing under this Agreement, SCRIPSAMERICA does not acquire any right, title or interest in any property, intellectual or otherwise, owned or controlled by Supplier.
(c) During the Term of this Agreement, SCRIPSAMERICA shall sell the Products purchased from Supplier pursuant to this Agreement under its own trademarks and trade dress. The trademarks and trade dress will be clearly indicated in the Purchase Order given by SCRIPSAMERICA to the Supplier. Supplier acknowledges that such trademarks, trade dress, and any other designations of the Product labels and packages are the sole and exclusive property of SCRIPSAMERICA and its Affiliates, and that Supplier's labeling of the Product under SCRIPSAMERICA's trademarks and trade dress shall not be construed as granting any right in such trademarks or trade dress to Supplier.
(d) Each party covenants and agrees that it will not, nor will it cause or permit any of its Affiliates to, take or omit to take any action that is in any manner inconsistent with, or tends to diminish or impair, the other party’s or the other party’s Affiliate’s rights as set forth in this Section 6.2. Supplier agrees to assist in every proper and legal way to obtain, maintain and protect SCRIPSAMERICA’s rights in such property in the United States and all foreign countries.
| 7 |
6.3. Warranties Regarding Technology. Supplier hereby warrants that it has the right, as of the date of this Agreement, and hereafter will not impair such right, to make all transfers to SCRIPSAMERICA as set forth in this Agreement.
6.4. Left blank intentionally
6.5.
Confidentiality
(a) During the Term of this Agreement, and for the longer of either (i) five (5) years after termination of this Agreement or (ii) for so long as the SCRIPSAMERICA Confidential Information shall not be publicly known, Supplier shall not use any SCRIPSAMERICA Confidential Information, except to perform its obligations under this Agreement, or disclose any SCRIPSAMERICA Confidential Information to any third party, except, as authorized in writing by SCRIPSAMERICA or as required by applicable Laws. Upon termination of this Agreement or upon written request by SCRIPSAMERICA, Supplier shall deliver to SCRIPSAMERICA all SCRIPSAMERICA Confidential Information, as well as all documents, media, items and Technology comprising, embodying or relating to SCRIPSAMERICA Confidential Information, as well as any other documents or things belonging to SCRIPSAMERICA that may be in Supplier’s possession.
(b) During the Term of this Agreement, and for the longer of either (i) five (5) years after termination of this Agreement or (ii) for so long as the Supplier Confidential Information shall not be publicly known, SCRIPSAMERICA shall not use any Supplier Confidential Information, except to perform its obligations under this Agreement, or disclose any Supplier Confidential Information to any third party, except as authorized in writing by Supplier or as required by applicable Laws. Upon termination of this Agreement or upon written request of Supplier, SCRIPSAMERICA shall deliver to Supplier all Supplier Confidential Information, as well as all documents, media, items and Technology comprising, embodying or relating to the Supplier Confidential Information, as well as any other documents or things belonging to Supplier that may be in SCRIPSAMERICA’s possession.
(c) The provisions of this Section 6.5 shall supersede any other confidentiality agreements between the parties with respect to the subject matter hereof and such confidentiality agreements are hereby terminated as between SCRIPSAMERICA and Supplier. SCRIPSAMERICA and Supplier hereby confirm that all proprietary information previously disclosed by one to the other prior to the date of this Agreement shall be deemed SCRIPSAMERICA Confidential Information or Supplier Confidential Information, as applicable, as long as SCRIPSAMERICA or Supplier, respectively, have complied with the provisions of this Agreement to protect such SCRIPSAMERICA Confidential Information or Supplier Confidential Information.
(d) Supplier not to Replicate Product. Supplier shall not, under any circumstances, copy, replicate, imitate or reverse engineer any of SCRIPSAMERICA's products, including, but not limited to, the Product(s). Current Product is existing marketed LNK technology and not that of SCRIPSAMERICA.
(e) Injunctive Relief. Each party acknowledges and realizes that the other party's Confidential Information is special, unique and extraordinary and is vital to the other party. Accordingly, the parties acknowledge that the breach of this Section 6.5 by one of the parties will result in irreparable to the other party and that, therefore, in addition to any and all other remedies the other party may have pursuant to this Agreement, at law or in equity, it shall be entitled to institute and prosecute proceedings at law or in equity, in any court of competent jurisdiction, to obtain an injunction restraining the first party from violating or continuing to violate this Section 6.5. Each party agrees that the disclosing party's remedy at law would be inadequate and, therefore, agrees and consents that temporary and/or permanent injunctive relief may be sought in any proceeding which may be brought to enforce this Section 6.5 without the necessity or proof of actual damage.
(f) Agreement Confidential. The parties agree that the existence and contents of this Agreement (including any Schedules and attachments) is Confidential Information and shall not be disclosed to any third party without the prior written consent of the other party, except that in furtherance of this Agreement, and only to the extent reasonably necessary for this purpose, its existence or contents may be disclosed to the following who shall also be made subject to the restrictions on disclosure stated herein; (i) any Affiliate of the parties, (ii) governmental regulatory agencies, including, but not limited to, environmental protection authorities, (iii) contract laboratories, and (iv) suppliers of raw materials or components. This obligation of confidentiality shall not apply to disclosures required by law.
7. INDEMNIFICATION
7.1. Supplier’s Indemnification. Supplier shall indemnify, defend and hold harmless SCRIPSAMERICA and its Affiliates, shareholders, subsidiaries, directors, officers, employees, agents and representatives (each a “SCRIPSAMERICA Indemnitee”), from any and all liabilities, claims, losses, damages, judgments or awards, costs or expenses, including reasonable attorneys’ fees, of whatsoever nature and by whomsoever asserted, whether asserted by a third party or by a party to this Agreement, directly or indirectly, arising out of, resulting from or in any way connected with (a) any breach by Supplier of the terms of this Agreement; (b) non-compliance with the Specifications or the Supplier Warranties; (c) any non-compliance with any Laws applicable to Supplier’s obligations under this Agreement; (d) any governmental, regulatory or other proceedings to the extent any such proceedings result from Supplier’s failure to comply with the Specifications or the Supplier Warranties; (e) any recall or return of the Products initiated by Supplier or SCRIPSAMERICA, whether voluntarily or by order of any court or other duly empowered governmental or regulatory office, to the extent that Supplier’s failure to comply with the Specifications or the Supplier Warranties is responsible for such recall; or (f) any claim that the manufacture, use or sale of any of the Products infringes upon or violates any patent, trademark, copyright, trade secret or other proprietary rights of any third party so long as such claim is not based upon proprietary rights owned by SCRIPSAMERICA.
| 8 |
7.2. SCRIPSAMERICA’s Indemnification. SCRIPSAMERICA shall indemnify and hold harmless Supplier and its Affiliates, shareholders, subsidiaries, directors, officers, employees, agents and representatives (each a “Supplier Indemnitee”) from any and all liabilities, claims, losses, damages, judgments or awards, costs or expenses, including reasonable attorneys’ fees, of whatsoever nature and by whomsoever asserted, whether asserted by a third party or by a party to this Agreement, directly or indirectly, arising out of, resulting from or in any way connected with any breach by SCRIPSAMERICA of the terms of this Agreement.
7.3. Indemnification Procedures. Supplier or SCRIPSAMERICA, as applicable (in such capacity, the “Indemnitor”) shall promptly assume full and complete responsibility for the investigation, defense, compromise and settlement of any claim, suit or action arising out of or relating to the indemnified matters following written notice thereof from the SCRIPSAMERICA Indemnitee or Supplier Indemnitee, as applicable (the “Indemnitee”), which notice shall be given by the Indemnitee within ten (10) days of the Indemnitee’s knowledge of such claim, suit or action. Failure to provide such timely notice shall not eliminate the Indemnitor’s indemnification obligations to the Indemnitee unless, and only to the extent to which, such failure has substantially prejudiced the Indemnitor. Notwithstanding the foregoing, the Indemnitee shall have the right, in its sole discretion and at Indemnitee’s expense, to participate in or to defend or prosecute, through its own counsel, any claim suit or action for which it is entitled to indemnification by the Indemnitor; provided, however, that if the Indemnitee is advised in writing by its legal counsel that there is a conflict between the positions of the Indemnitor and the Indemnitee in conducting the defense of such action or that there are legal defenses available to the Indemnitee different from or in addition to those available to the Indemnitor, then counsel for the Indemnitee, at the Indemnitor’s expense, shall be entitled to conduct the defense to the extent necessary to protect the interests of the Indemnitee. The Indemnitor shall not enter into any compromise or settlement without the Indemnitee’s prior written consent, which consent shall not be unreasonably withheld, unless the settlement is limited to money paid by the Indemnitor, with no acknowledgment of wrongdoing by the Indemnitee and no other restriction on or liability to the Indemnitee. The absence of a complete and general release of all claims against Indemnitee shall be reasonable grounds for Indemnitee to refuse to provide written consent to a compromise or settlement. If the Indemnitor does not assume and diligently pursue the defense of such claim, suit or action, the Indemnitor shall reimburse the Indemnitee for the reasonable fees and expenses of any counsel retained by the Indemnitee to undertake or assist in such defense, and shall be bound by the results obtained by the Indemnitee.
7.4. Additional SCRIPSAMERICA Rights. In addition to the provisions of Sections 7.1, in the event the use or sale of any of the Products or any components or parts thereof is enjoined by a court of competent jurisdiction due to any claim of infringement or violation of any patent, trademark, copyright, trade secret, or other proprietary rights of any third party, Supplier, to the extent such claim is not based upon proprietary rights owned by SCRIPSAMERICA, shall promptly, at SCRIPSAMERICA’s option: (a) obtain for SCRIPSAMERICA, at no expense to SCRIPSAMERICA, the right to continue using the Products or components or parts thereof; (b) replace the infringing items at no expense to SCRIPSAMERICA, with a non-infringing item of equal performance and quality; or (c) modify, at no expense to SCRIPSAMERICA, the infringing items so that they become non-infringing.
7.5 Limit on Types of Damages. Except as expressly provided herein, in no event will either party be responsible to the other party or any of its Affiliates or representatives (whether as an indemnifying party pursuant to this Section 7 or pursuant to any other provision in this Agreement), for any incidental, consequential, or punitive damages, even if the other party has been advised of the possibility of such damages.
8. INSURANCE.
8.1 Supplier Insurance. Supplier shall keep in force throughout the Term of this Agreement and for thirty-six (36) months following the termination of this Agreement commercial general liability insurance written on a claims made basis, including bodily injury, property damage, products liability and contractual liability coverages as respects this Agreement, with coverage of at least US$5,000,000 per occurrence and aggregate. Attached as Schedule F is a copy of a certificate of insurance that Supplier has provided to SCRIPSAMERICA from a financially responsible insurance company satisfactory to SCRIPSAMERICA, certifying such coverages, naming SCRIPSAMERICA as an additional insured, and requiring at least thirty (30) days prior written notice to SCRIPSAMERICA of any cancellation or material change thereof. Supplier shall also maintain worker’s compensation and other insurance in force in accordance with applicable Laws on all employees engaged by Supplier in any way on the work which is the subject of this Agreement. If Supplier fails to furnish such certificates, or, if at any time during the Term of this Agreement, SCRIPSAMERICA is notified of the cancellation or lapse of Supplier’s insurance as described above, and Supplier fails to rectify the same within ten (10) calendar days after notice from SCRIPSAMERICA, in addition to all other remedies available to SCRIPSAMERICA hereunder, SCRIPSAMERICA, at its option, may obtain such insurance and Supplier shall promptly reimburse SCRIPSAMERICA for the cost of the same. Failure of SCRIPSAMERICA to demand such certificate or other evidence of full compliance with these insurance requirements shall not be construed as a waiver of Supplier’s obligation to maintain such insurance. Any deductible and/or self insured retention, as applicable, are the sole responsibility of Supplier.
| 9 |
9. MISCELLANEOUS PROVISIONS
9.1.
Independent Contractor. Supplier is an independent contractor and not an agent, employee, partner, joint venture
partner, subsidiary or an affiliated entity of SCRIPSAMERICA. No party shall incur any debts or make any commitments on behalf
of the other, except to and only to the extent, if at all, specifically provided in this Agreement.
9.2. Force Majeure. Except as otherwise provided herein, neither party shall be liable to the other for any loss or failure to perform resulting from any act of God, fire, flood, explosion or other natural disaster, actions or impositions by Federal, state or local authorities, strike, labor dispute, vandalism, riot, commotion, act of public enemies, blockage or embargo or any other cause beyond the reasonable control of such party. Upon the occurrence of any such event that results in, or will result in, a delay or failure to perform, the party whose performance is delayed or prevented shall be relieved from fulfilling its obligations under this Agreement during the period of such force majeure event and shall immediately provide written notice to the other party of such occurrence and the anticipated effect of such occurrence. The party whose performance is affected shall use its best efforts to minimize disruptions in its performance and shall resume full performance of its obligations under this Agreement as soon as possible.
9.3. Notices. Any notice or other communication required or permitted to be given hereunder shall be in writing (including facsimile or similar transmission) and mailed (by certified mail, return receipt requested, postage prepaid), sent or delivered (including by way of overnight courier service) addressed as follows:
|
If to SCRIPSAMERICA:
SCRIPSAMERICA INC. ▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇ ▇▇▇▇▇ Att: ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇
|
If to Supplier:
LNK International Inc.
|
or to such other address as the parties may give notice to the others by like means. All such notices and communications, if mailed, shall be effective upon the earlier of (a) actual receipt by the addressee, or (b) the date shown on the return receipt of such mailing. All such notices and communications, if not mailed, shall be effective upon the earlier of (a) actual receipt by the addressee, (b) with respect to facsimile and similar electronic transmission, the earlier of (i) the time that electronic confirmation of a successful transmission is received or (ii) the date of transmission, if a confirming copy of the transmission also is sent by overnight courier service on the date of transmission, or (c) with respect to delivery by overnight courier service, one (1) day after deposit with such courier service, if delivery on such day by such courier is confirmed with the courier or the recipient. The parties further agree that delivery of a notice or other communication required or permitted to be given hereunder in writing may be given via email addressed to: (a) with respect to SCRIPSAMERICA, ▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇, and (b) with respect to Supplier, ▇▇▇▇ ▇▇▇▇▇▇. Such email notices and communications shall be effective on the date of transmission if a confirming copy of the transmission also is sent via overnight courier service on the date of transmission.
| 10 |
9.4. Successors and Assigns. This Agreement shall be binding on and shall inure to the benefit of the parties and their respective successors in interest and permitted assigns. Neither party shall assign this Agreement or any of its rights or obligations hereunder without the prior written consent of the other party; provided, however, that (a) Supplier may assign this Agreement and all, but not less than all, of its rights and obligations hereunder to any Affiliate, any successor by merger, or any purchaser of substantially all of the assets or stock of Supplier, if (and only if) such Affiliate, successor or purchaser satisfies SCRIPSAMERICA’s then current manufacturing requirements and capabilities for the Products as determined by SCRIPSAMERICA in its reasonable discretion; and (b) SCRIPSAMERICA may, without having to obtain Supplier’s consent, assign this Agreement and its rights and obligations hereunder to any Affiliate, any successor by merger, or any purchaser of substantially all of the assets or stock of SCRIPSAMERICA and/or SCRIPSAMERICA, Inc., and may collaterally assign its rights hereunder to any lender.
9.5. Severability. Any term or provision of this Agreement which is invalid or unenforceable in any jurisdiction shall, as to that jurisdiction, be ineffective to the extent of such invalidity or unenforceability without rendering invalid or unenforceable the remaining terms and provisions of this Agreement or affecting the validity or enforceability of any of the terms or provisions of this Agreement in any other jurisdiction. If any provision of this Agreement is so broad as to be unenforceable, the provision shall be interpreted to be only so broad as is enforceable.
9.6. Survival. Sections 2.4, 2.5, 3.8, 4, 5, 6, 7, 8, and 9 shall survive any termination or expiration of this Agreement.
9.7. Entire Agreement and Conflict. This Agreement (including the Schedules hereto), the Specifications and any other documents incorporated by reference, constitute the entire Agreement and supersede any previous agreement, whether written or oral, between the parties relating to the subject matter of this Agreement. In the event of any conflict, the terms and conditions of this Agreement shall prevail over the terms and conditions of any purchase order or other shipping, delivery, receiving, billing or other document used directly or indirectly by either party in performing this Agreement.
9.8. Amendment and Waiver. This Agreement may not be amended or modified in any respect, except by writing made and executed in the same manner as this Agreement. No provisions of this Agreement shall be waived by any act, omission or knowledge of the parties except by an instrument in writing expressly waiving such provisions and executed by the party against whom such waiver is claimed. No waiver of any default under or breach of this Agreement shall operate as a waiver of any other or subsequent default or breach.
9.9. Construction. This Agreement has been submitted to the scrutiny of, and has been negotiated by, all parties hereto and their counsel, and shall be given a fair and reasonable interpretation in accordance with the terms hereof, without consideration or weight being given to its having been drafted by any party hereto or its counsel.
9.10. Headings. The headings of this Agreement are for convenience only and shall be of no force or effect in construing or interpreting any of the provisions of this Agreement.
9.11. Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original, but all of which shall constitute one and the same instrument. Delivery of an executed counterpart of this Agreement by telefacsimile or PDF technology delivered via e-mail shall be equally as effective as delivery of a manually executed counterpart of this Agreement. Any Party delivering an executed counterpart of this Agreement by telefacsimile or PDF technology delivered via e-mail also shall deliver a manually executed counterpart of this Agreement, but the failure to deliver a manually executed counterpart shall not affect the validity, enforceability, or binding effect of this Agreement.
9.12. Language of Agreement and Notices. This Agreement is in the English language only, which shall be controlling in all respects, and all versions hereof in any other language shall be for accommodation only and shall not be binding on the parties. All notices and communications required or permitted to be given or made under this Agreement shall be in the English language.
| 11 |
9.13. Governing Law; Arbitration. This Agreement shall be governed by and construed in accordance with the internal laws of the State of Delaware U.S.A., without regard to conflict of law principles. All disputes, claims and other matters in controversy arising directly or indirectly out of or related to this Agreement, or the breach hereof, whether contractual or non-contractual, shall be determined by arbitration and shall be settled by a majority vote of three arbitrators, one of whom shall be appointed by SCRIPSAMERICA, one of whom shall be appointed by Supplier and the third of whom shall be appointed by the first two arbitrators. Persons eligible to be selected as arbitrators shall be limited to attorneys who have been in practice at least ten (10) years specializing in corporate matters, who have had both training and experience as arbitrators and who have had no prior relationship or business dealings with either SCRIPSAMERICA or Supplier or their respective directors and officers. If either SCRIPSAMERICA or Supplier fails to appoint an arbitrator within ten (10) days of a request in writing by the other party to do so or if the first two arbitrators cannot agree on the appointment of the third arbitrator, then the third arbitrator shall be appointed by the American Arbitration Association (the “AAA”), provided that such arbitrator also must meet the foregoing eligibility requirements. The arbitration shall be conducted in the English language in the City of Washington D.C in accordance with the commercial rules of the AAA then in effect, subject to any modifications agreed to in writing by the parties. The U.S. Federal Arbitration Act (the “FAA”) shall apply to the construction and interpretation of this Agreement to arbitrate. The arbitrators shall base their award on applicable law and judicial precedent and, unless both parties agree otherwise, shall include in such award the findings of fact and conclusions of law upon which the award is based and may include equitable relief. Judgment on the award rendered by the arbitrators may be entered in any court of competent jurisdiction. The arbitrators shall award recovery of reasonable attorneys’ fees and costs to the prevailing party. The arbitrators’ resolution of the dispute shall be final and binding, except that any party can appeal to the federal courts of the United States of America (located in the City of Washington D.C) or, if such federal courts do not have jurisdiction, to the courts of the State of New Jersey (located in the City of Washington D.C), to vacate and remand, or modify or correct the arbitration award for any of the grounds specified in the FAA or if the arbitrators committed prejudicial error in the application of substantive law to the established facts. The procedures specified in this Section 9.13 shall be the sole and exclusive procedures for resolution of disputes; provided, however, that nothing contained herein shall preclude any party from filing a judicial proceeding seeking equitable or injunctive relief.
9.14. Consent to Jurisdiction. With respect to each matter which is not subject to the mandatory arbitration provisions of Section 9.13, each of the parties hereby irrevocably and unconditionally consents to submit to the jurisdiction of the federal courts of the United States of America (located in the City of Washington D.C) or, if such federal courts do not have jurisdiction, to the courts of the State of New Jersey (located in the City of Newark) for any litigation arising out of or relating to this Agreement and the transactions contemplated hereby. Each of the parties hereto hereby irrevocably and unconditionally waives any objection to the laying of venue of any litigation arising out of this Agreement or the transactions contemplated hereby by the courts of the United States of America or the State of D.C, in each case, located in the City of Washington D.C, and hereby further irrevocably and unconditionally waives and agrees not to plead or claim in any such court that any such litigation brought in any such court has been brought in an inconvenient forum. Any judgment or other decision of any such court shall be enforceable, without further proceedings, against the named party anywhere in the world where such party is located, does business or has assets.
9.15. Carbon Taxes. Supplier shall be solely responsible for (a) any tax liabilities levied by any governmental body that relate in any way to carbon emissions, regardless of whether such carbon emission tax liabilities are levied against Supplier or SCRIPSAMERICA, and (b) purchasing, at Supplier’s cost, any carbon emissions credits that would in the future be required for Supplier to perform its obligations under this Agreement.
[Signature Page Follows]
| 12 |
SIGNATURE PAGE TO SUPPLY AGREEMENT
IN WITNESS WHEREOF, the parties have caused this Agreement to be duly authorized and executed as of the date first above written.
| SCRIPSAMERICA: | ||
| SCRIPSAMERICA | ||
| By: | /s/ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ | |
| Name: | ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ | |
| Title: | CEO | |
| SUPPLIER: | ||
| LNK International Inc. | ||
| By: | /s/ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ | |
| Name: | ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ | |
| Title: | EVP | |
| 13 |
SCHEDULE A
PRODUCTS AND SERVICES
PRODUCTS
| SCRIPSAMERICA Product | Product Name | Unit |
| ▇▇▇▇-▇▇▇-▇▇ BLBX | Acetaminophen 80 mg Rapid Melts Children's Meltaway Grape | [***] count |
| 1823-643-0l BLBX | Acetaminophen 80 mg Rapid Melts Children's Meltaway Cherry | [***] count |
| ▇▇▇▇-▇▇▇-▇▇ BLBX | Acetaminoph en 160 mg Rapid Melts Children's Meltaway Grape | [***] count |
| ▇▇▇▇-▇▇▇-▇▇ BLBX | Acetaminophen 160 mg Rapid Melts Children's Meltaway Cherry | [***] count |
SERVICES
Supplier will perform the following services with respect to the Products:
| · | Pack into unit carton boxes and insert product literature, as required |
| · | Shrink wrap 4 completed cartons together in front to back panel configuration |
| · | Place 6 shrink wrapped 4-packs into a shipper (side by side in a single layer) |
| · | Place 2 shipper labels around opposite diagonal corners of the shipper with the center tick ▇▇▇▇ on each label on the corner of the shipper |
| · | Print each completed shipper with a lot and expiration date on end panel (coding must not interfere with shipping label) |
| · | Palletize completed cases |
| · | Place corrugated pallet corner protectors on all 4 edges of the pallet |
| · | Stretch-wrap pallet |
PRODUCT COMPONENTS
Supplier will supply the following materials for the Products:
| · | Raw materials to formulate products |
| · | Stick Foil |
| · | Shippers |
| · | Shipper labels |
| · | Exterior shipping materials (pallets, stretch wrap, corner guards) |
| · | Cartons |
| [***] THE CONFIDENTIAL PORTION OF THIS AGREEMENT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE COMMISSION PURSUANT TO A REQUEST FOR CONFIDENTIALITY. |
| 14 |
SCHEDULE B
SPECIFICATIONS
PRODUCT SPECIFICATIONS
| · | [See attached specifications] |
APPROVED SUPPLIER FACILITY
| 15 |
FINISHED PRODUCT SPECIFICATION
44-449
LNK INTERNATIONAL, INC., ▇▇ ▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇. Y. 11788
| LNK Part No.: | 44-449 | Specification No.: | L-449 | Imprint: | 44-449 |
| Product: | ACETAMINOPHEN 160mg JUNIOR STRENGTH CHEWABLE GRAPE FLAVOR RAPIDTABS | ||||
| (Equivalent to Junior Strength Tylenol® Meltaways®) | |||||
| Written By: |  |
Date: | 02/08/2013 |
| Approved By: |  |
Date: | 02/12/2013 |
| Effective Date: | 02/14/2013 |

| SPECIFICATION | LIMITS |
| APPEARANCE | A ROUND PURPLE COLORED TABLET WITH GRAPE AROMA DEBOSSED 44-449 ON ONE SIDE AND WITH A DIMPLE, BISECT ON OTHER SIDE. |
| COLOR | PURPLE |
| IMPRINT | 44-449 |
| WEIGHT |
1300mg RANGE: 1235mg TO 1365mg |
| HARDNESS | 2.5 To 10.0 kp |
| THICKNESS | 0.228" TO 0.253" |
| DISSOLUTION | NOT LESS THAN 75% (Q) IS DISSOLVED IN 45 MINUTES. |
| ACTIVES | ACETAMINOPHEN 160mg |
| INACTIVES | CITRIC ACID, CROSPOVIDONE, DEXTRATES HYDRATED, D&C RED #▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇&▇ RED#27 ALUMINUM LAKE, ETHYLCELLULOSE, FD&C BLUE #1 ALUMINUM LAKE, FLAVORS, MAGNESIUM STEARATE, MANNITOL, POLYETHYLENE, STEARIC ACID, SUCRALOSE. |
| 16 |
FINISHED PRODUCT SPECIFICATION
44-452
LNK INTERNATIONAL, INC., ▇▇ ▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇. Y. 11788
| LNK Part No.: | 44-452 | |||
| Specification No.: | L-452 | |||
| Product: | ACETAMINOPHEN 80mg CHILDREN'S CHEWABLE GRAPE FLAVOR | |||
| RAPIDTABS | ||||
| Imprint: | 44-452 | |||
| Written By: |  |
Date: | 06/11/2012 |
| Approved By: |  |
Date: | 06/27/2012 |
| Effective Date: | 06/28/2012 |

| SPECIFICATION | LIMITS |
| APPEARANCE | A PURPLE, ROUND, CHEWABLE TABLET WITH GRAPE AROMA, DEBOSSED 44-452 ON ONE SIDE AND WITH A DIMPLE ON OTHER SIDE. |
| COLOR | PURPLE |
| IMPRINT | 44-452 |
| WEIGHT |
770mg RANGE: 732mg TO 808mg |
| HARDNESS | 2.5 To 13.0 kp |
| THICKNESS | 0.200" TO 0.230" |
| DISSOLUTION | NOT LESS THAN 75% (Q) IS DISSOLVED IN 45 MINUTES. |
| ACTIVES | ACETAMINOPHEN 80mg |
| INACTIVES | CITRIC ACID, CROSPOVIDONE, DEXTRATES, D&C RED #▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇&▇ RED#27 ALUMINUM LAKE, ETHYLCELLULOSE, FD&C BLUE #1 ALUMINUM LAKE, FLAVORS, MANNITOL, MAGNESIUM STEARATE, POLYETHYELENE, SUCRALOSE, STEARIC ACID.. |
| 17 |
FINISHED PRODUCT SPECIFICATION
44-643
LNK INTERNATIONAL, INC., ▇▇ ▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇. Y. 11788
| QAD Part # | 44-643 | Specification No.: | L-643 | Imprint: | 44-643 |
| Product: | Acetaminophen 80mg Children’s Cherry Flavor Chewable Rapid Tabs | ||||
| (Equivalent to Children’s Tylenol® Meltaways®) | |||||
| Written By: |  |
Date: | 05/22/2014 |
| Approved By: | Date: | 05/31/2014 | |
| Effective Date: | 06/21/2014 |

| SPECIFICATION | LIMITS |
| APPEARANCE | A Pink Round Colored Tablet Debossed 44-643 On One Side and With a Dimple on the Other Side. |
| COLOR | Pink |
| IMPRINT | 44-643 |
| WEIGHT |
770mg RANGE: 732mg TO 808mg |
| HARDNESS | 2.5 TO 13.0 KP |
| THICKNESS | 0.200" TO 0.230" |
| DISSOLUTION | Not Less Than 75% (Q) is dissolved in 45 minutes. |
| ACTIVES | Acetaminophen 80mg |
| INACTIVES |
Corn Starch, Citric acid, Crospovidone, Dextrates hydrated, D&C Red #▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇&▇ RED #30 Aluminium Lake, Ethyl cellulose, Flavor, Magnesium Stearate, Mannitol, Polyethylene, Stearic acid, Sucralose. |
| 18 |
FINISHED PRODUCT SPECIFICATION
44-644
LNK INTERNATIONAL, INC., ▇▇ ▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇. Y. 11788
| QAD Part # | 44-644 | Specification No.: | L-644 | Imprint: | 44-644 |
| Product: | Acetaminophen 160mg Junior Strength Cherry Flavor Chewable Rapid tabs | ||||
| (Equivalent to Junior Strength Tylenol® Meltaways®) | |||||
| Written By: |  |
Date: | 10/24/2013 |
|
Approved By: |
Date: |
11-11-13 | |
| Effective Date: | 11/12/13 |

| SPECIFICATION | LIMITS |
| APPEARANCE | A Pink Round Colored Tablet Debossed 44-644 On One Side and With a Dimple, Bisect on the Other Side. |
| COLOR | Pink |
| IMPRINT | 44-644 |
| WEIGHT |
1300mg RANGE: 1235mg TO 1365mg |
| HARDNESS | 2.5 TO 10.0 kp |
| THICKNESS | 0.228" TO 0.253" |
| DISSOLUTION | Not Less Than 75% (Q) is dissolved in 45 Minutes. |
| ACTIVES | Acetaminophen 160mg |
| INACTIVES | Corn Starch, Citric acid, Crospovidone, Dextrates hydrated, D&C Red #▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇&▇ RED #30 Aluminium Lake, Ethyl cellulose, Flavor, Magnesium Stearate, Mannitol, Polyethylene, Stearic acid, Sucralose. |
| 19 |
SCHEDULE C
PRICING
PRODUCT FEES
Term
The completed Products (i.e. Products manufactured, assembled, packaged, labeled, and packed for shipment) shall be priced as follows:
| Product Number | Product Name | Unit | Price per Unit |
|
▇▇▇▇-▇▇▇-▇▇ BLBX |
Acetaminophen 80 mg Rapid Melts Children's Meltaway Grape | [***] count | $[***] |
|
▇▇▇▇-▇▇▇-▇ l BLBX |
Acetaminophen 80 mg Rapid Melts Children's Meltaway Cherry | [***] count | $[***] |
|
▇▇▇▇-▇▇▇-▇▇ BLBX |
Acetaminoph en 160 mg Rapid Melts Children's Meltaway Grape | [***] count | $[***] |
|
▇▇▇▇-▇▇▇-▇▇ BLBX |
Acetaminophen 160 mg Rapid Melts Children's Meltaway Cherry | [***] count | $[***] |
PRODUCT FEE ADJUSTMENTS
[***]
| [***] THE CONFIDENTIAL PORTION OF THIS AGREEMENT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE COMMISSION PURSUANT TO A REQUEST FOR CONFIDENTIALITY. |
| 20 |
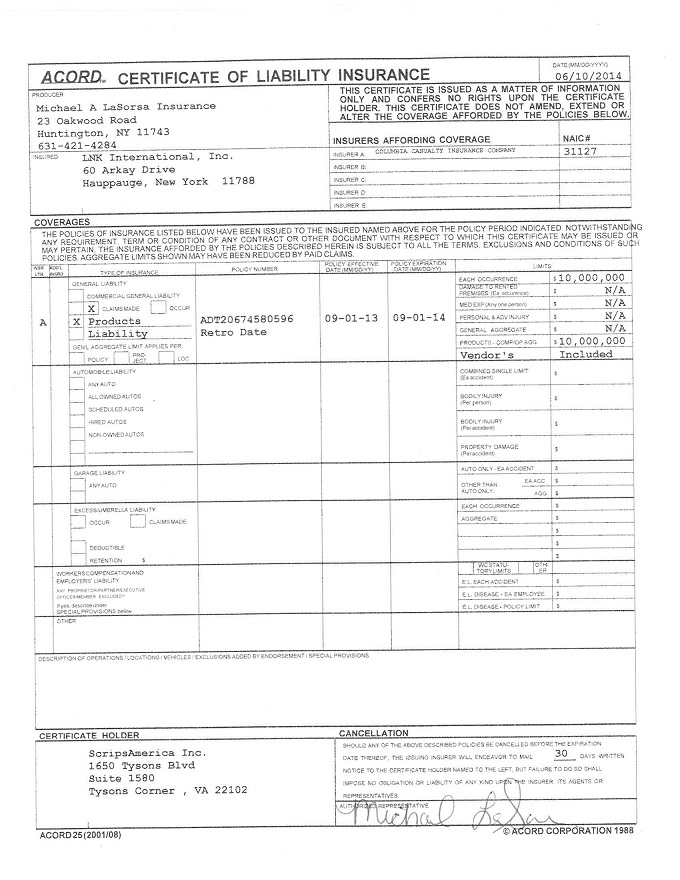
SCHEDULE F
CERTIFICATE OF INSURANCE
[See attached certificates]
| 21 |
| 22 |

