THIS AGREEMENT is made by and between:
EXHIBIT 10.55
THIS AGREEMENT is made by and between:
| (1) | AVECIA LIMITED, acting through its Avecia Biotechnology business, with offices at Hexagon Tower, Blackley, Manchester, M9 8ZS, England (“Avecia”); and |
| (2) | NUVELO, INC., a Delaware corporation with offices at 000 Xxxxxxx Xxxxxx, Xxxxxxxxx, XX 00000, XXX (“Nuvelo”). |
WHEREAS
| A | Avecia has experience and knowledge with regard to process development, fermentation and manufacture of recombinant proteins. |
| B | Nuvelo is carrying out research and development in relation to the Product (as defined below). |
| C | In anticipation of entering into a definitive agreement to carry out a range of activities in relation to the Product (as defined in this Agreement), Avecia and Nuvelo entered into an Interim Agreement on 21 January 2005 (the “Interim Agreement”) that set out the interim terms and conditions on which the following portions of the Project, consisting of (i) assessment and planning, (ii) transfer of process and assays, (iii) purchase of certain capital equipment, (iv) replicate 15L fermentation and purification runs and (v) GMP consultancy preparatory to GMP manufacture, would be carried out before execution of the definitive agreement. |
| D | Nuvelo now wishes Avecia to carry out activities in relation to the API (as defined below), including validation work in respect of the Process (as defined below). |
NOW IT IS HEREBY AGREED BY NUVELO AND AVECIA AS FOLLOWS:
| 1. | Definitions: |
| Affiliate | Any corporation, association or other business entity which directly or indirectly controls, is controlled by or is under common control with Avecia or Nuvelo and “control,” “controls” or “controlled” shall mean the legal power to direct or cause the direction of the general management and policies of such entity whether through the ownership of at least 50% of the voting securities or voting capital stock of such business entity, or any other comparable controlling equity or controlling ownership interest with respect to a business entity other than a corporation. | |
| API | The polypeptide referred to by Nuvelo as alfimeprase is the Active Pharmaceutical Ingredient. In accordance with Annexe 18 of the EU Guide to Good Manufacturing Practice 2002, titled “Good | |
1
| Manufacturing Practice for Active Pharmaceutical Ingredients,” an Active Pharmaceutical Ingredient is any substance or mixture of substances intended to be used in the manufacture of a drug (medicinal) product and that, when used in the production of a drug, becomes an active ingredient of the drug product. Such substances are intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure and function of the body. | ||
| API Specification | The specification for API attached to, and part of the Quality Agreement, which may be amended from time to time in accordance with the terms and conditions of this Agreement and the Quality Agreement. | |
| Applicable Laws | All applicable supranational, national or local laws, rules and regulations, including without limitation the United States Federal Food, Drug and Cosmetic Act, the regulations promulgated pursuant thereto, any applicable non-U.S. equivalents thereof, and any successor laws, rules or regulations thereto. | |
| Avecia Default | Means:
(a) A Failure by Avecia to use reasonable commercial endeavours to progress the Project;
(b) A Failure by Avecia to manufacture any Development Batch or any Validation Batch in accordance with cGMP, specifically including any failure to follow Avecia’s facilities’ Standard Operating Procedures that results in, or from which arises, a Defective Batch;
(c) Solely with respect to the centrifuge trials, a failure by Avecia to conduct the centrifuge trials in accordance with Avecia’s Standard Operating Procedures: (1) for the fermentation and harvest suite or suites which are applicable to the centrifuge trials in the reasonable determination of the Quality Team, a list of which shall be generated by the Quality Team; and (2) for, as reasonably modified from time to time as a result of the work carried out under the Project, any equipment to be used in the centrifuge trials;
(d) Solely with respect to the Development Batches:
(1) A failure by Avecia personnel—including without limitation lack of appropriate training, lack of attention to MBRs or Standard Operating | |
2
| Procedures, or lack of proper laboratory analysis—that results in, or from which arises, a Defective Batch;
(2) A failure of Avecia documentation—including without limitation inadequate Standard Operating Procedure or MBR documentation, improperly organized or lost documentation, or poor analysis of information incorporated into any such documentation—that results in, or from which arises, a Defective Batch;
(3) Adulteration—including without limitation adulteration from cleaning agents and/or other contaminants, or adulteration from using reagents other than in the order specified in the Work Programme or the MBRs for the Process—that results in, or from which arises, a Defective Batch;
(4) A failure of Avecia equipment—including without limitation poor maintenance, age of the equipment, computer malfunction, software malfunction or controller malfunction, equipment being out of specification or not properly calibrated, equipment not being validated for intended use, improper cleaning, improper cleaning cycles or failure to clean equipment—that results in, or from which arises, a Defective Batch;
(5) A Failure of an Avecia facility—including without limitation a cGMP violation in the facility, improper environmental controls, including HVAC system failure, poor construction or inadequate maintenance—that results in, or from which arises, a Defective Batch; or
(6) A failure of Avecia raw materials—including without limitation raw materials that are out of specification, quarantined, improperly tested, improperly processed or expired—that results in, or from which arises, a Defective Batch;
(e) For the avoidance of doubt, a failure of a centrifuge trial or the occurrence of a Defective Batch that is a Development Batch, that results from a factor—other than any of the factors listed in Clauses (a) through (d) above—which affects the Process or production of the API and was not known and could not reasonably have been known by Avecia at the commencement of the applicable stage of the Project shall not be considered to be an Avecia default; such factors include, without limitation:
(1) the Process does not perform as anticipated due to a change of scale; |
3
| (2) inability of the centrifuge to clarify the harvested broth to an acceptable level;
(3) failure during a centrifuge run of the pH control system to maintain a specified range; and
(4) failure during a centrifuge run of the feed strategy to generate the expected product yield or quality; or
(f) The occurrence of a Defective Batch during the Validation Campaign is, and is automatically deemed to be an Avecia Default, except upon the occurrence of a Defective Batch which results from a factor—other than any of the factors listed in Clauses (a) through (d) above—which affects the Process or production of the API and was not known and could not reasonably have been known by Avecia before the Defective Batch occurred, including, without limitation, a previously unknown factor not studied either as part of the Amgen programme transferred to Avecia or the Avecia laboratory work programme carried out pursuant to the Project (the preceding in this Clause (f) referred to herein as a “Validation Process Failure”). | ||
| Background Intellectual Property | Any Intellectual Property owned by or Controlled by a Party (where “Controlled” means the ability to grant a license or sublicense to the Intellectual Property):
(a) at the Commencement Date of this Agreement; or
(b) after the Commencement Date that is either: (1) acquired independently of the Project; or (2) developed independently of the Project by any employee of that Party without use of or reference to any of the Confidential Information disclosed by the other Party. | |
| Batch | A quantity of API produced using the Process that (a) is expected to have uniform character and quality within specified limits, and (b) is produced according to a single manufacturing run during the same cycle of the Process. | |
| Batch Dispute | The definition set forth in Clause 2.7(g). | |
| Cancellation Fee | A sum calculated in accordance with Schedule 4. The Cancellation Fee may include, as applicable as set forth in Schedule 4 and in accordance with the terms | |
4
| and conditions of this Agreement, the Development Batch Fee and/or the Validation Campaign Fee. | ||
| Certificate of Analysis | A document, the form of which is approved in advance by the Quality Team, that is prepared and issued by Avecia’s quality assurance department certifying analysis and cGMP manufacture of the API and its compliance with the API Specification. | |
| cGMP | Current Good Manufacturing Practices required by Regulatory Authorities with respect to the development, manufacture, storage and supply of any Batch, including current good manufacturing practices as incorporated in Annexe 18 of the EU Guide to Good Manufacturing Practice 2002, titled “Good Manufacturing Practice for Active Pharmaceutical Ingredients” (formerly ICH Q7A) and as interpreted in the USA Federal Register Vol 66 No. 186 (formerly ICH Q7A), and subject to any arrangements, additions or clarifications agreed in writing from time to time between the Parties in the Quality Agreement. | |
| Commencement Date | 21st January 2005. | |
| Completion | Completion of the Project as set out in Clause 4. | |
| Confidential Information | Shall have the meaning given in the Confidentiality Agreement, subject to Clause 7. New Intellectual Property shall be deemed to be Confidential Information disclosed by Nuvelo. | |
| Confidentiality Agreement | The confidentiality agreement entered into between the Parties and dated 21st September 2004, attached hereto as Schedule 7. | |
| Defective Batch | Either:
(a) A Batch that has not been produced in accordance with cGMP, specifically including all Standard Operating Procedures for Avecia’s facility and the Master Batch Records for the Process, and for which any non-conformances from the SOPs or the MBRs cannot be closed-out in accordance with the procedures for close-out of non-conformances set forth in the Quality Agreement; or
(b) A Batch, other than the first Development Batch or the Engineering Batch, which cannot be Released. | |
5
| Development Batch or cGMP Development Batch | A Batch manufactured during Development Batch Manufacture, specifically including the Engineering Batch. | |
| Development Batch Manufacture | The work to manufacture the development Batches and the Engineering Batch in accordance with Clause 2.3 and the Work Programme. | |
| Engineering Batch | A Batch manufactured immediately prior to the Validation Campaign for the purpose of re-testing the Process before commencement of the Validation Campaign. | |
| Equipment | Any equipment (such as, without limitation, columns, freezers, filtration skids, cassette holders, ammonia feed tanks) which is required to be purchased in order for Avecia to carry out the Project and which is set forth in Schedule 6 to this Agreement or set forth in the Work Programme or any Programme Amendment Order. | |
| Executive Committee or EC | The executive committee established pursuant to Clause 2.7. | |
| Expenditure | An amount paid or due and payable to a Third Party for the purchase of subcontractor services directly from the Third Party in accordance with Clause 13.2 or for the purchase of goods or materials directly from the Third Party. | |
| Force Majeure | Any cause beyond the reasonable control of the Party in question, which for the avoidance of doubt and without prejudice to the generality of the foregoing, may include governmental actions, war, riots, terrorism, civil commotion, fire, flood, epidemic, labour disputes (excluding labour disputes involving the work force or any part thereof of the Party in question), restraints or delays affecting shipping or carriers, inability or delay in obtaining supplies of adequate or suitable materials, and act of God, but shall not include failure of the Product in clinical trials or failure of the Product to gain regulatory approval. | |
| Handling Fee | A sum equivalent to 8% of the actual Expenditure for the Equipment or consumables purchased under Clauses 3.2(a)(1), 3.2(a)(3) and 3.3. | |
| Hold Time | Any period of time during Development Batch Manufacture or the Validation Campaign during which Batches are not being manufactured, whether by | |
6
| agreement so that modifications to procedures, processes and/or facilities or review thereof can be undertaken or as a result of a Nuvelo Delay or an Avecia Default. | ||
| Intellectual Property | Any Patent, trade secret, copyright or other industrial or intellectual property right. | |
| Invention | Any invention, innovation, improvement, development, discovery, computer program, device, method, know-how, process, technique or the like, whether or not written or otherwise fixed in any form or medium, regardless of the media on which contained and whether or not patentable or copyrightable. | |
| New Intellectual Property or New IP | Any Intellectual Property that claims or covers any New Invention. | |
| New Invention | Any Invention directly resulting from or directly arising out of Avecia’s performance of the Project. | |
| Non-Manufacturing Delay | A delay resulting from a decision by Nuvelo to delay the Project for reasons unrelated to: the Project; manufacture of the API under the Agreement; any Avecia Default; or any Process Failure. Non-Manufacturing Delay may include, without limitation, decisions made in response to the outcome of clinical trials of the API. | |
| Nuvelo Delay | Any delay in the Development Batch Manufacture or the Validation Campaign which is caused by Nuvelo including, without limitation, unreasonable refusal to agree to any matter requiring mutual agreement under this Agreement in a timely manner; but, each of the following is not, and shall not be deemed to be, a Nuvelo Delay: (i) any delay or refusal by Nuvelo to agree based upon or resulting from an Avecia Default; and (ii) any delay resulting from Nuvelo’s refusal to take a license under a Patented, Licensed Avecia Invention under Clause 5.2(c). | |
| Master Batch Record or MBR | A written description of the procedure to be followed for manufacturing a Batch of the API, including but not limited to the history of a Batch from the raw material stage up through and until completion of the Batch, a complete list of all active and inactive ingredients, components, weights and measures, descriptions of drug product containers, closures, packaging materials, and labelling and complete specifications for each, within the meaning of 21 Code of Federal Regulations 211.186, or its successor as in effect from | |
7
| time to time, and also in compliance with Annexe 18 of the EU Guide to Good Manufacturing Practice 2002, titled “Good Manufacturing Practice for Active Pharmaceutical Ingredients” (formerly ICH Q7A), or its successor as in effect from time to time. | ||
| Party or Parties | Avecia and Nuvelo are referred to individually herein as a “Party”, and collectively as the “Parties”. | |
| Patent | Any: (a) patent, including without limitation any inventor’s certificate or design patent, and any substitution, extension, registration, confirmation, reissue, re-examination or any like filing thereof related to a patent; and (b) any pending patent application, including without limitation any continuation, division or continuation-in-part thereof and any provisional application. | |
| Process | The process for the manufacture of API communicated to Avecia by, or on behalf of, Nuvelo and subsequently scaled-up by Avecia under and in accordance with this Agreement. | |
| Process Assumptions | The following: (a) requirements set forth in the Master Batch Records for the Process, (b) requirements set forth in Avecia’s Standard Operating Procedures, and (c) raw materials and consumables requirements and specifications. Any amounts of time necessary to conduct a particular part of the Process, also referred to as cycle times, are expressly excluded from Process Assumptions, irregardless of any statements to the contrary set forth in the Work Programme. | |
| Product | Any product that incorporates or contains the API. | |
| Programme Amendment Order | A document in the form set out in Schedule 3 detailing changes to the Project agreed upon and signed by both Parties. | |
| Project | The range of activities to be carried out under this Agreement in accordance with the Work Programme, the details of which are more fully set out in the Work Programme, and any additional or alternative work not set forth in the Work Programme that may be agreed in a Programme Amendment Order. | |
| Project Steering Committee or PSC | The Project steering committee established pursuant to Clause 2.7. | |
8
| Quality Agreement | The document, a copy of which is attached as Schedule 2, that sets forth, amongst other things:
(a) the mutually agreed quality standards applicable for the manufacture of the API under the Agreement in accordance with cGMP; and
(b) the roles and responsibilities of each Party’s personnel in relation to quality assurance matters under this Agreement; and
(c) the API Specifications. | |
| Quality Team | The Avecia Quality Unit together with the Nuvelo Quality Unit, as further set forth in the Quality Agreement. | |
| Regulatory Authority or Regulatory Authorities | The U.S. Food and Drug Administration, the European Agency for the Evaluation of Medicinal Products, and any equivalent governmental regulatory body in any territory in the world, and any successor entity or entities to any of the preceding. | |
| Regulatory Filing | Any and all correspondence or petitions to Regulatory Authorities for the purpose of registering the Product or the Process, or modifying or supplementing existing filings and subsequent amendments and supplements thereto, as required by Applicable Laws, in order to develop, manufacture, test, sell or distribute Product or the API under this Agreement. | |
| Release or Released | The process by which, in respect of each Batch:
(a) Avecia’s Quality Unit:
(1) reviews and approves completed Batch records;
(2) reviews and approves all campaign Batch records and buffer Batch records;
(3) closes out all non-conformances;
(4) closes out all change controls;
(5) issues a Certificate of Analysis;
(6) Issues a Certificate of Compliance; and
(b) Nuvelo’s Quality Unit:
(1) Reviews, and, if appropriate, accepts all Batch related manufacturing documentation; | |
9
| (2) Reviews, and, if appropriate, accepts QC Batch related analyses; and
(3) Reviews, and, if appropriate, accepts the Certificate of Analysis.
For the purpose of this definition, “Accepts” means Avecia has received written notification from Nuvelo that Nuvelo accepts the documents referred to in Clause (b) above. In the absence of such written notification that Nuvelo does or does not accept such documents, upon the expiration of 30 calendar days from the date on which Nuvelo receives Avecia’s written notification of Avecia’s completion of the tasks referred to in Clause (a) above, Nuvelo is deemed to have accepted the documents referred to in Clause (b) above. A Batch that has been Released may be referred to as a “Released Batch”. A Released Batch, or set of Released Batches, may also be referred to as “Released API”. If Nuvelo rejects the documents referred to in Clause (b) above, the matter will be addressed in accordance with Clause 2.7(g). | ||
| Standard Operating Procedures or SOPs | Written procedures requiring uniform performance of specific functions, or uniform use of specific equipment or resources to ensure data, analysis and manufacturing quality and uniformity. | |
| Third Party | Any person or entity other than the Parties or their respective Affiliates. | |
| Unremedied Breach | A material breach of this Agreement which is not remedied within 30 calendar days after receipt of written notice from the non-breaching Party requiring rectification of the breach. | |
| Validation Batch | A Batch manufactured by Avecia as part of the Validation Campaign. | |
| Validation Campaign | The campaign carried out by Avecia to manufacture three (3) consecutive Validation Batches that can be Released out of up to five (5) anticipated Validation Batches, intended to demonstrate to Regulatory Authorities a high degree of assurance that the Process will consistently produce Batches meeting pre-determined acceptance criteria necessary for Release, as set out in more detail in Schedule 1 and the Quality Agreement. | |
| Validation Master Plan | A validation project plan developed by Avecia and reviewed, and if acceptable, approved by Nuvelo in writing which contains all of the validation activities for | |
10
| the Validation Campaign, including validation steps, deliverables, a time schedule and responsibilities. | ||
| Valid Claim | A claim of a Patent that: (a) in the case of a pending claim, is being prosecuted, has been pending for no more than 6 years and has not been abandoned or permitted to lapse, and (b) in the case of an issued claim, has not expired or been held invalid or unenforceable in a final binding court decision from which no appeal can be or is taken. | |
| Work Programme | The protocol for the performance of the Project by Avecia, agreed upon by the Parties and attached as Schedule 1 of this Agreement. The Work Programme sets forth the timing and requirements for the activities to be carried out under the Project, including the Work Programme Timeline, attached to the Work Programme as Appendix 2. | |
| 1.2 | Interpretation. References in this Agreement to “Schedules” refer to the Schedules incorporated into this Agreement, specifically including, without limitation, the Confidentiality Agreement, the Quality Agreement, and the Work Programme. To the extent that there is conflict between or ambiguity relating to, on the one hand, any or all of the Schedules and, on the other, the remainder of this Agreement, the wording of the remainder of this Agreement shall prevail. |
| 2. | Performance of Project |
| 2.1 | General. |
| (a) | Avecia shall carry out the Project in accordance with the terms and conditions of this Agreement and the Quality Agreement with reasonable skill and care and no less than the level of skill and care to be reasonably expected of a professional provider of such services. Avecia also shall perform the Project in compliance with all relevant professional standards, all Applicable Laws and cGMP. |
| (b) | The Parties acknowledge that, having regard to the fact that the work to be performed hereunder is by its nature developmental, Avecia does not guarantee to Nuvelo the achievement of a successful outcome for the Project, but will use reasonable commercial endeavours to ensure timely success. |
| 2.2 | Laboratory work and scale-up activities |
| (a) | Following the Commencement Date, Avecia shall carry out, and has carried out with respect to a portion thereof as set forth in the Interim Agreement, a range of activities, including |
11
| data acquisition and assessment, process transfer, replicate 15L runs, process characterisation, fermentation and downstream process, assay validation, process transfer and prep for large scale manufacture, laboratory based cleaning studies and centrifuge trials on two (2) 3000 litre Batches of the API, all as set out in more detail in the Work Programme. |
| (b) | Centrifuge Trials. |
| (1) | Obligations and Timing. Avecia shall carry out production of two (2) centrifuge trials on two (2) 3000 litre Batches of the API in accordance with its ABC5000 facilities’ Standard Operating Procedures, the Master Batch Records for the Process, all Applicable Laws and the terms and conditions of this Agreement. Avecia shall commence modifications of its ABC5000 facility for the centrifuge trials no later than week commencing July 4, 2005, Pacific Standard Time. If Avecia fails to commence the modifications of its ABC5000 facility necessary for the centrifuge trials before the expiration of the week commencing July 4, 2005, Pacific Standard Time, then Nuvelo is entitled to terminate this Agreement in accordance with Section 8.2 upon 10 calendar days written notice to Avecia, without payment of any Cancellation Fees if Avecia has not commenced the modifications before the expiration of the 10 calendar day period. |
| (2) | Process Failures; Additional Centrifuge Work. If neither of the two centrifuge trials is successful for any reason other than an Avecia Default, then the Parties shall meet to agree to an additional phase of process development work, to include the performance of further centrifuge trials (all such work referred to herein as “Additional Centrifuge Work”) and commercially reasonable terms for the performance of the Additional Centrifuge Work. The Additional Centrifuge Work shall be set forth in a Programme Amendment Order, such Programme Amendment Order to include revised timings for Development Batch Manufacture and Validation Campaign, as appropriate. If, other than as a result of an Avecia Default, the Additional Centrifuge Work does not result in at least one successful centrifuge trial and Development Batch Manufacture has not commenced before the expiration of December 31, 2005, Pacific Standard Time, then Nuvelo may either terminate the Agreement in accordance with Clause 8.2 upon a date that is 30 calendar days after December 31, 2005, Pacific Standard Time or call an Urgent Meeting of the PSC to discuss whether further Additional Centrifuge Work should be conducted. |
12
| (3) | Centrifuge Trials; Avecia Default. If neither of the two centrifuge trials is successful as a result of Avecia Default(s), Avecia shall promptly reprocess, rework if pre-approved in writing by an authorized representative of Nuvelo or re-perform, the centrifuge trials at Avecia’s cost and expense until either (i) one of the centrifuge trials is successful or; (ii) the centrifuge trials are unsuccessful other than as a result of an Avecia Default, at which time Clause 2.2(b)(2) will apply. |
| 2.3 | Development Batch Manufacture |
| (a) | Batch Production. Avecia shall carry out production of two (2) Development Batches (in accordance with the terms set forth below) in its ABC5000 facility in accordance with the terms and conditions of this Agreement, cGMP, Applicable Laws and the Work Programme, with the primary aim of testing and improving Batch records and operating procedures, clarifying any scale-up issues and ensuring operator familiarity with the Process. Avecia also shall carry out an Engineering Batch in accordance with Clause 2.3(e). Whilst Development Batch Manufacture is intended to produce Development Batches which can be Released, it is recognised that problems related to the Process that are not Avecia Defaults may preclude this. |
| (b) | Development Batch Failure. If the Quality Team is in agreement that neither of the two (2) Development Batches manufactured under Clause 2.3(a) can be Released, Avecia shall, at the direction and option of Nuvelo, either: |
| (1) | reprocess one of the two (2) Development Batches produced during Development Batch Manufacture in accordance with cGMP; |
| (2) | if pre-approved in writing by an authorized representative of Nuvelo, rework one of the two (2) Development Batches produced during Development Batch Manufacture in accordance with cGMP; |
| (3) | manufacture another Development Batch; or |
| (4) | immediately stop all work, as a result of a decision by Nuvelo to terminate the Agreement in accordance with Clause 8. |
| (c) | Next Actions Upon Development Batch Failure. |
| (1) | Avecia Default. In the event that the Quality Team is in agreement that the second Development Batch or both of the two (2) Development Batches manufactured under Clause 2.3(a) are Defective Batches as a result of an Avecia Default, Avecia shall rework, reprocess or |
13
| manufacture in accordance with Clause 2.3(b)—within 10 business days after the Quality Team notified the PSC of the Quality Team’s determination if the Avecia Default is of a nature that can be promptly corrected, otherwise, as soon as reasonably practicable thereafter—at Avecia’s cost and expense, until such time as Avecia generates a Development Batch that can be Released (or could have been Released but for an issue that is not an Avecia Default); but if, as a result of Avecia Default(s), Avecia fails to generate a Development Batch which can be Released within 3 months from commencement of such rework, reprocessing or further manufacture, then the PSC shall hold an Urgent Meeting to discuss whether or not yet another Development Batch should be generated. Nuvelo is entitled to terminate the Agreement, without payment of any Cancellation Fees, if Development Batches cannot be Released as a result of Avecia Default(s) before the expiration of the 3 month cure period provided in this Clause 2.3(c)(1). Nuvelo may terminate the Agreement during the 3 month cure period for Unremedied Breach if Avecia fails to generate a Development Batch that can be Released as a result of Avecia Default(s) and is not using commercially reasonable efforts to generate at least one Development Batch that can be Released per month during the three month cure period. |
| (2) | Process Failure. If the first 2 Development Batches cannot be Released for a reason other than Avecia Default, at the request of Nuvelo, Avecia shall rework, reprocess or manufacture Development Batches at a time and at a cost to Nuvelo to be agreed by the Parties in good faith and recorded in a Programme Amendment Order. If the rework, reprocessing or further manufacture results in another Development Batch which is a Defective Batch for a reason other than Avecia Default, then the PSC shall hold an Urgent Meeting to discuss whether or not yet another Development Batch should be generated. If and at such time as Avecia and Nuvelo agree that Process scale-up problems causing the Defective Batches in accordance with this Clause 2.3(c)(2) are or will be satisfactorily resolved, unless the Agreement is terminated by one the Parties, the Parties will agree to a revised timetable for manufacture of a number of Development Batches to be determined, upon commercially reasonable terms, in a Programme Amendment Order. |
| (d) | Successful Development Batch Release: Pre-Validation Campaign Review. If at least one (1) of the two (2) |
14
| Development Batches manufactured under Clause 2.3(a) can be Released or if rework, reprocessing or further manufacture carried out in accordance with Clause 2.3(b) results in a Development Batch which can be Released, the Project Steering Committee shall review the activities carried out to date, solely to evaluate operability of the Process and Avecia’s cGMP compliance in order to ensure that both Parties are confident of success in the Validation Campaign (“the Pre-Validation Campaign Review”). During the Pre-Validation Campaign Review, the PSC, with input from the Quality Team, shall discuss readiness to carry out the Validation Campaign, potential timing for its conduct and any other issues concerning the Process operability or cGMP compliance. This discussion will include a review and analysis of the actual performance of the Development Batches compared to the Process Assumptions, and an attempt by the PSC to reach agreement with respect to whether the Validation Campaign shall be delayed, not be delayed, or not be carried out at all. If during the Pre-Validation Campaign Review, the EC, after referral of the matter to it by the Project Steering Committee, is unable to reach a final decision in accordance with the terms of Clause 2.7(f) as to whether the Validation Campaign shall be delayed, not be delayed, or not be carried out at all—solely due to Process operability or Avecia cGMP compliance issues, or any other concerns which resulted in a Development Batch being a Defective Batch— then either Party may terminate this Agreement in accordance with and subject to the terms provided for termination set forth in this Agreement, including the possibility that the Parties may mutually agree to terminate the Agreement under Clause 8.4. |
| (e) | Proceeding to Validation Campaign. If at least one (1) Development Batch is Released, then following completion of, and subject to either agreement by the PSC or a determination made by the EC in the Pre-Validation Campaign Review, Avecia shall carry out an Engineering Batch and shall proceed to and shall carry out the Validation Campaign in accordance with the terms and conditions of this Agreement for the performance of the Project in accordance with Clause 2.1, the Work Programme and any revised timetable for performance of the Engineering Batch and the Validation Campaign resulting from Pre-Validation Campaign Review. Development Batch Manufacture shall be deemed to be complete when: (1) the Quality Team has completed Release of at least one Development Batch, and that Batch’s associated documentation has been delivered to Nuvelo; and (2) Avecia has completed manufacture of an Engineering Batch that is not a Defective Batch, and the Engineering Batch’s associated documentation has been delivered to Nuvelo. |
15
| (f) | Delivery of Defective Batches. In the event that during Development Batch Manufacture it is determined by the Quality Team that a Development Batch is a Defective Batch, the Defective Batch shall not be delivered to Nuvelo, unless Nuvelo requests it. If Nuvelo requests delivery of the Defective Batch, Avecia shall deliver such Defective Batch in accordance with Clause 4.2 and such Defective Batch may be used in research or development internally, labelled for non-human use or destroyed. |
| (g) | Quality Team Disagreement. If the Quality Team cannot reach agreement with respect to whether or not a Development Batch is a Defective Batch, or whether or not a Defective Batch resulted from an Avecia Default, the disagreement will be resolved in accordance with Clause 2.7(g). |
| 2.4 | Validation Campaign |
| (a) | cGMP Preparation |
| (1) | Avecia shall carry out cGMP preparation work following the decision that Avecia shall carry out the Validation Campaign under and in accordance with Clause 2.3(e) above. Such cGMP preparation work shall include the work identified in the Work Programme, the Quality Agreement and any additional work agreed under a Program Amendment Order pursuant to Clause 2.3(c)(2) or otherwise. |
| (2) | Avecia shall produce a Validation Master Plan for review, comment and approval by Nuvelo before the start of the Validation Campaign. |
| (3) | Avecia shall conduct the Validation Campaign in accordance with the terms and conditions of this Agreement and the Quality Agreement. The Quality Agreement sets forth how the Parties’ Quality Units will jointly review non-conformances, determine severity, assess their level of impact on the API and agree on actions which may include accepting a Validation Batch, failing a Validation Batch or declaring a Validation Batch to be a Defective Batch, disqualifying a Validation Batch from the series of three (3) consecutive Batches or passing or failing the entire Validation Campaign. |
| (b) | Manufacture of Validation Batches |
| (1) | Commencement of Validation Campaign. Avecia shall commence manufacture of Validation Batches following confirmation by the Quality Team that the |
16
| preparation work set forth in Clauses 2.4(a)(1) and 2.4(a)(2) has been completed and the Master Batch Records have been approved in accordance with the Quality Agreement. If the Quality Team is unable to reach agreement on such confirmation and approval, the matter shall be referred to the PSC for resolution. |
| (2) | Number of Validation Batches. Subject to Clause 2.4(b)(3) below, the Validation Campaign shall consist of up to five (5) Validation Batches with an expectation of producing three (3) Released consecutive successful production Batches and thus constitute a formal Validation Campaign. Avecia will ensure that sufficient time is scheduled in its ABC5000 facility to complete a Validation Campaign of up to five (5) Validation Batches. |
| (3) | 5th Validation Batch. In the event that the Quality Team determines that a fifth (5th) Validation Batch is required, other than as a result of an Avecia Default, in order to increase the chances of successful completion of the Validation Campaign, Avecia shall carry out manufacture of a fifth (5th) Validation Batch on the basis that Avecia will meet the costs of operating its ABC 5000 facility and Nuvelo shall pay to Avecia, as an additional technical consultancy fee, the actual Expenditures for any additional raw materials or consumables required to be purchased in order to manufacture such fifth (5th) Validation Batch, plus the Handling Fee, in accordance with Clause 3.2(b). |
| (4) | Documentation Review. During the Validation Campaign, the Parties shall carry out review of the Master Batch Records, campaign Batch records and other relevant documentation relating to each Validation Batch as it is manufactured. This review and assessment will involve the Nuvelo Quality Unit and such other of Nuvelo’s staff as Nuvelo directs, in Nuvelo’s discretion. |
| (5) | Completion of Validation Campaign. The Validation Campaign shall be deemed to be complete when the Quality Team determines that the Validation Campaign has completed three (3) Validation Batches that can be designated consecutive successful production Batches that have completed Release, and all associated documentation and a validation summary report have been delivered to Nuvelo in accordance with Clause 4.2. |
17
| (c) | Quality Unit Determination |
| (1) | Determination of Validation Success or Failure. The Quality Team shall determine in accordance with the Quality Agreement and by reference to cGMP whether the Validation Campaign has been successful. In the event that the Quality Team determines that the Validation Campaign has not been successful, then, as set forth in and in accordance with Clauses 2.4(c)(2) and 2.4(c)(3) below, Avecia shall repeat the Validation Campaign or Validation Batches (as appropriate) within 10 business days after the Quality Team notified the PSC of the Quality Team’s determination, if the cause of the failure of the Validation Campaign is of a nature that can be promptly corrected, otherwise, as soon as reasonably practicable thereafter. In the event of any dispute arising amongst the Quality Team, the matter will be resolved in accordance with Clause 2.7(g). |
| (2) | Avecia Default. If the Validation Campaign is determined not to be successful as a result of an Avecia Default, then the repeated Validation Campaign or Validation Batches (as appropriate) shall be promptly carried out by Avecia at Avecia’s cost and expense; but if the repeated Validation Campaign or Validation Batches (as appropriate) does not result in a successful Validation Campaign as a result of an Avecia Default within 3 months of commencement of such repeated Validation Campaign or Validation Batches (as appropriate), then the PSC shall hold an Urgent Meeting to discuss whether or not the Validation Campaign or Validation Batches (as appropriate) shall be repeated again. Nuvelo is entitled to terminate the Agreement, without payment of any Cancellation Fees, if, as a result of Avecia Default, the repeated Validation Campaign or Validation Batches (as appropriate) does not result in a successful Validation Campaign before the expiration of the 3 month cure period provided in this Clause 2.4(c)(2). Nuvelo may terminate the Agreement during the 3 month cure period for Unremedied Breach if Avecia fails to generate a successful Validation Campaign and is not using commercially reasonable efforts to generate at least 5 Validation Batches that could lead to a successful Validation Campaign during the three month cure period. |
| (3) | Validation Process Failure. If the Validation Campaign is determined not to be successful because of a Validation Process Failure, then, if requested in writing by Nuvelo, a repeated Validation Campaign or Validation Batches (as appropriate) shall be carried out by Avecia at a commercially reasonable cost to Nuvelo to be agreed in good faith by the Parties and set out in |
18
| a Programme Amendment Order. In the event that a repeated Validation Campaign carried out under this Clause is determined not to be successful, then the Parties shall meet to discuss what action should be taken. |
| (d) | Quality Team Disagreement. If the Quality Team cannot reach agreement with respect to whether or not a Validation Batch is a Defective Batch, or whether or not a Defective Batch resulted from an Avecia Default, the disagreement will be resolved in accordance with Clause 2.7(g). |
| 2.5 | Delays and Cancellations. Nuvelo shall have an option to, and otherwise may as a result of an Avecia Default, delay commencement of, or cancel Development Batch Manufacture or the Validation Campaign. The Parties also acknowledge that delays may occur as a result of Process Failures, as set forth below. In the event that Nuvelo decides to exercise such option or take such action, the following provisions shall apply: |
| (a) | Notice. Nuvelo shall give written notice to Avecia of its intention to delay or cancel Development Batch Manufacture or the Validation Campaign (as appropriate), and, in the case of delay, setting out the estimated length, or circumstances that would dictate the length, of the delay. Notice is deemed given at such time as either Party notifies the other Party in writing that the Validation Campaign will be delayed as a result of a Process Failure, and in such case, the PSC shall determine the estimated length of the delay. |
| (b) | Cancellation Fees; Non-Manufacturing Delays or Cancellation. With respect to any cancellation or Non-Manufacturing Delay, Nuvelo shall pay to Avecia the Cancellation Fees in consideration for technical consultancy into the effect of such Non-Manufacturing Delay or cancellation on the Project. |
| (c) | Process Failure Delay Fee. With respect to any delay of the Validation Campaign resulting from a Process Failure that results in a delay of the completion of the usage of Avecia’s manufacturing facilities for the Validation Campaign by more than two weeks after August 16, 2006, Nuvelo shall pay to Avecia, in consideration for technical consultancy into the effect of such delay on the Project, the sum of £212,470 pounds per week (the “Process Failure Delay Fee”) for each week beyond the expiration of two weeks after August 16, 2006, during which the completion continues to be delayed, until such time as Nuvelo has paid to Avecia an amount in Process Failure Delay Fees that is equal to the lesser of: £2,550,000 pounds; or the amount Nuvelo would have paid to Avecia in Cancellation Fees if notice had been given regarding a Non-Manufacturing Delay instead of a delay |
19
| resulting from a Process Failure. Avecia shall make commercially reasonable endeavours to raise revenue by utilising the production facility during the period during which the Validation Campaign was intended to take place but for the delay. Avecia shall refund to Nuvelo a sum equivalent to the revenue (net of raw materials and consumables Expenditure) raised as a result of such alternative use up to a maximum of 80% of the technical consultancy fee paid under this Clause 2.5(c). For the purposes of this Clause 2.5(c), “Process Failure” means the occurrence of a Defective Batch that results from a factor—other than any of the factors listed in paragraphs (a) through (d) of the definition of Avecia Default—which affects the Process or production of the API and was not known and could not reasonably have been known by Avecia prior to the time at which the factor became known, including, without limitation, a previously unknown factor not studied either as part of the Amgen programme transferred to Avecia or the Avecia laboratory work programme carried out pursuant to the Project. |
| (d) | Delay. The Parties shall meet to discuss availability of Avecia’s manufacturing facility for the delayed Development Batch Manufacture or the Validation Campaign (as appropriate) in accordance with Clause 2.3 and 2.4 and, except with respect to any delay resulting from or arising out of an Avecia Default, Nuvelo’s refusal to take a license under a Patented, Licensed Avecia Invention under Clause 5.2(c) or failure of the centrifuge trials, if the delay is in excess of six (6) months, a commercially reasonable amount payable by Nuvelo to Avecia in respect of such later commencement of Development Batch Manufacture or the Validation Campaign (as appropriate). |
| (e) | Effect of Delay on Project. Except with respect to a delay resulting from or arising out of an Avecia Default or Nuvelo’s refusal to take a license under a Patented, Licensed Avecia Invention under Clause 5.2(c), Avecia shall not be obliged to carry out the delayed Development Batch Manufacture or the Validation Campaign (as appropriate) until the Parties have reached agreement on the later commencement thereof, but will use reasonable commercial endeavours to reschedule the availability of its facility and appropriate personnel. In the case of a delay resulting from an Avecia default, if Nuvelo decides to have Avecia proceed with Development Batch Manufacture and/or the Validation Campaign, Avecia shall carry out the delayed Development Batch Manufacture or Validation Campaign in accordance with the terms and conditions set forth in Clauses 2.3 and 2.4. |
| (f) | Effect of Cancellation. Where Nuvelo elects to cancel Development Batch Manufacture, Avecia shall not be obliged to carry out the Validation Campaign, and except with respect |
20
| to any cancellation resulting from or arising out of an Avecia Default or failure of the centrifuge trials, the Validation Campaign shall also be deemed cancelled with effect from the date of notice of Nuvelo’s intention to cancel Development Batches Manufacture and the Validation Campaign. |
| 2.6 | Nuvelo Delay. |
| (a) | In the event of a Nuvelo Delay, Avecia and Nuvelo will use reasonable commercial endeavours to minimize such Nuvelo Delay or, subject to agreement by both Parties, to adjust the Development Batch Manufacture or the Validation Campaign schedule to accommodate such delay. Any Nuvelo Delay which cannot be avoided shall be considered to be Hold Time in respect of the affected Batch. |
| (b) | In the event that a Nuvelo Delay results in an inability for Avecia to carry out the Development Batch Manufacture or the Validation Campaign in accordance with the mutually agreed schedule therefor, then the Parties shall meet to discuss availability of Avecia’s manufacturing facility for the delayed Development Batch Manufacture or the Validation Campaign (as appropriate), and a commercially reasonable amount payable by Nuvelo to Avecia in respect of such later commencement of Development Batch Manufacture or the Validation Campaign (as appropriate). |
| 2.7 | Project Steering Committee, or PSC, Executive Committee, or EC, and Resolution of Batch Disputes. |
| (a) | Membership. |
| (1) | PSC Membership. The PSC shall have at least 6 and up to 8 members, within any case, an equal number of members appointed by each Party. Each Party’s initial membership on the PSC shall be as set forth in Schedule 5. Each Party may replace its PSC representatives at any time upon written notice to the other Party, provided that each Party shall appoint and maintain for the duration of the term of this Agreement representatives on the PSC of equivalent or higher position within that Party as the original representative(s) set forth on Schedule 5. The PSC shall keep minutes of its meetings and submit its meeting minutes to the EC members for EC and Party review. The host Party at each in-person meeting shall prepare the minutes for that meeting, otherwise, minute taking will alternate between the Parties for each meeting. |
| (2) | EC membership. The EC shall be composed of 2 members, 1 representative of each Party. Each EC |
21
| member shall not be a member of the PSC, shall have obtained, and maintain, the level of vice president (or comparable title) or above, and shall be duly authorized by the Party it represents to resolve any and all disagreements of the PSC. The EC representatives are set forth on Schedule 5. Each Party may replace its EC representative with another qualifying individual at any time upon written notice to the other Party. The EC shall keep minutes of its meetings and prepare a report for Party review. Minute taking will alternate between the Parties for each meeting. |
| (b) | Power and Responsibilities. |
| (1) | PSC Powers and Responsibilities. The PSC shall have the following specific responsibilities and authority: |
| (i) | to review and approve the overall plan for process development, characterization, manufacturing and Release of Batches of Product; |
| (ii) | to review and approve resources and timelines for the Project, and any changes for the Project; |
| (iii) | to evaluate and manage any changes or other incidents that may occur during the course of the Project; |
| (iv) | to serve as a forum for the sharing of information between the Parties with respect to Project activities and Project progress; and |
| (v) | to evaluate Batch Disputes that arise at the Quality Team level in accordance with Clause 2.7(g). |
| (2) | XX Xxxxxx and Responsibilities. The EC shall support the PSC in decision-making and support the overall strategy for the Project. The EC shall have the following specific responsibilities and authority: |
| (i) | to resolve any disagreements of the PSC, but excluding Batch Disputes, in accordance with Clause 2.7(f); |
| (ii) | to review PSC meeting minutes and evaluate the effectiveness and composition of the PSC, providing any comments thereon back to the PSC for consideration; and |
| (iii) | to serve as a forum for information sharing between senior management of the Parties. |
22
| (c) | Limitations on PSC and EC. The PSC and the EC shall have no power to amend or waive compliance with this Agreement. Any amendments that alter the terms of this Agreement shall be implemented, if at all, pursuant to Clause 14 below. The PSC and EC shall have only the responsibility explicitly provided for them in Clause 2.7(b), and shall not have any other powers or responsibilities. |
| (d) | Regular Meetings. The Parties shall endeavour to schedule regular meetings of the PSC and EC at least 30 calendar days in advance. The PSC shall meet at least once a calendar quarter, and at least 2 regular meetings per year will be held in person. The EC shall meet twice a year, and shall decide whether or not it will meet in person, by teleconference or videoconference. Committee meetings held in person will alternate between sites designated by each Party and each Party shall be responsible for all of its own expenses of participating in PSC and EC meetings. With the consent of the representatives of each Party serving on the PSC, other representatives of each Party may attend meetings of the PSC. With the consent of each EC member, other representatives of each Party may attend meetings of the EC, or portions thereof. |
| (e) | Additional & Urgent Meetings. Upon mutual agreement of the PSC or EC, not to be unreasonably withheld, the Parties may schedule additional meetings of the PSC or EC as necessary to appropriately conduct the Project, and any such additional PSC or EC meetings will be held by teleconference or videoconference and will be held no later than 15 calendar days after reasonably requested by a Party. A meeting of the PSC may be requested on an urgent basis (“Urgent Meeting”) for the following reasons: Batch failure, excessive downtime in the facility being used to develop or manufacture Product, notice of a Batch Dispute, significant cGMP or Applicable Law violation or significant adulteration of Product or a Batch. An Urgent Meeting shall be held no later than 3 business days after requested by a Party. Urgent meetings may be held in-person, by teleconference or by videoconference. If the PSC cannot come to agreement on a matter before it at an Urgent Meeting, except with respect to a Batch Dispute, the matter shall be immediately referred to the EC for resolution. |
| (f) | Decision Making & Dispute Resolution. The PSC and EC will reasonably discuss all matters that come before them. Decisions of the PSC and EC will be made by unanimous agreement, with each Party having one vote on the PSC and one vote on the EC. If the PSC cannot come to agreement on an issue at the applicable PSC meeting, other than with |
23
| respect to a Batch Dispute, the PSC shall promptly refer the matter to the EC. The EC shall meet no later than 15 calendar days after referral of a matter to it by the PSC. The EC shall have 15 calendar days after meeting to resolve the matter. If the EC cannot mutually agree on a resolution of the matter before the expiration of the 15 day period, then Nuvelo is entitled to make the final decision for the EC on the matter, which Nuvelo decision shall not be unreasonable, with the exception of the following matters, which shall only be resolved by mutual agreement of the EC, Avecia’s agreement to such matters not be unreasonably withheld: (1) any matter which would require execution of a Programme Amendment Order; (2) any matter which would, other than as a result of an Avecia Default, adversely effect a manufacturing schedule— which schedule is not in conflict with the Project timeline set forth in the Work Program Timeline—established for a third party by Avecia, for use of Avecia’s facilities; or (3) materially change the Project timeline set forth in the Work Program Timeline. The decisions of the EC, whether determined by final decision of Nuvelo or mutual decision, in accordance with the preceding sentence, shall bind both of the Parties, except that: Avecia may refer a matter upon which Nuvelo made a final decision to dispute resolution in accordance with Clause 19.2 if in Avecia’s reasonable, good faith judgement, Nuvelo’s decision would (1) violate the express terms of this Agreement; or (2) result in a breach of Applicable Law. |
| (g) | Dispute Resolution for Batch Disputes. If a dispute arises amongst the Quality Team relating to whether or not a Batch of the API is a Defective Batch, or whether or not a Defective Batch results from or arises out of an Avecia Default (each of the preceding a “Batch Dispute”), such Batch Dispute shall be resolved as follows. |
| (1) | The Quality Team shall immediately notify the Project Steering Committee (PSC) in writing (the “Batch Dispute Notice”) that a Batch Dispute exists amongst the Quality Team. The Quality Team will discuss the Batch Dispute in good faith to attempt to reach agreement on: (i) whether a Batch is a Defective Batch and, if so, what course of action shall be taken to address it; and (ii) whether or not a Defective Batch resulted from or arose out of an Avecia Default. |
| (2) | In the event that the Quality Team fails to reach agreement on a Batch Dispute within 15 calendar days after sending the Batch Dispute Notice to the PSC, the Quality Team shall immediately refer the Batch Dispute to the PSC for discussion, by written notice to the PSC. Once the Batch Dispute has been referred to the PSC, the PSC has 5 business days from receipt of the referral notice to either resolve the Batch Dispute or |
24
| refer the Batch Dispute to an independent expert or laboratory, with the expertise necessary to reasonably resolve the Batch Dispute. |
| (3) | If the PSC cannot resolve the Batch Dispute or agree upon an independent expert or laboratory to resolve the Batch Dispute before the expiration of 5 business days after receiving the referral notice, the PSC shall immediately notify the EC of its failure in writing. Within no later than 2 business days after receiving notice of the failure from the PSC, each EC member shall nominate an independent expert who shall not: be a current or former employee, consultant or agent of a Party; have an immediate family member who is an employee, consultant or agent of a Party; or have any financial interest in a Party. Promptly thereafter, those two independent experts shall agree on a third independent expert who shall use any reasonable information, materials and data provided to him or her by the other two experts within 10 business days after his or her agreement to act as the third independent expert, to either promptly resolve the Batch Dispute or determine which independent Third Party laboratory will conduct the work necessary to promptly resolve the Batch Dispute. Such referral shall be solely for the purpose of establishing whether or not the applicable Batch is a Defective Batch and whether or not any Defective Batch results from or arises out of an Avecia Default. The decision of the independent expert, or independent laboratory, shall be made in writing and shall be binding upon the Parties. Whichever Party failed to accurately assess whether the Batch was or was not a Defective Batch, or whether or not a Defective Batch resulted from or arose out of an Avecia Default, shall bear the full cost and expense associated with the hiring and performance of the independent experts and/or laboratory. |
| 2.8 | Programme Amendment Orders. The Parties may agree to vary the Project and sums to be paid under Clause 3 as a result of a Project variation, so long as such variation is made in writing in a Programme Amendment Order. The Parties recognize that any of the following will require changes to the Work Programme that may cause a change in payments set out in Clause 3: |
| (a) | Nuvelo requires Avecia to carry out additional or different work to that specified in the Work Programme set forth in Schedule 1; or |
| (b) | the actual circumstances encountered in carrying out the Project differ from the then current Process Assumptions; or |
25
| (c) | in the event that there is a delay to the Project for any reason other than Avecia Default or Nuvelo’s refusal to take a license under a Patented, Licensed Avecia Invention, including a Nuvelo Delay; or |
| (d) | in the event that Additional Centrifuge Work is required as a result of a failure, other than one attributable to an Avecia Default, of the centrifuge trials pursuant to Clause 2.2(b); or |
| (e) | Hold Time, but excluding Hold Time resulting from or arising out of: (1) an Avecia Default or (2) Nuvelo’s refusal to take a license under a Patented, Licensed Avecia Invention. |
| 2.9 | Regulatory Matters and Regulatory Assistance. |
| (a) | During the Project and following Completion, Avecia will provide reasonable assistance to Nuvelo in respect of Nuvelo’s Regulatory Filing activities for the Product and the Process, including preparatory to and during Pre-Approval Inspection (PAI) and related quality unit support, as further set forth below. |
| (b) | At no additional cost to Nuvelo, Avecia shall: provide Nuvelo with any and all requested data created in connection with the development and manufacture of the API under this Agreement which is reasonably necessary to support submissions for regulatory approvals to Regulatory Authorities; and take all actions necessary to recreate or modify as reasonably necessary any documentation or materials provided by Avecia to Nuvelo for submission to Regulatory Authorities that were not provided to Nuvelo in a form or format reasonably acceptable to the applicable Regulatory Authority. Nuvelo shall advise Avecia on the form or format Nuvelo, or the Regulatory Authority, to the extent Nuvelo is aware of the Regulatory Authority’s requirements, may require prior to provision of such documentation or materials by Avecia. |
| (c) | Subject to payment by Nuvelo of a reasonable commercial rate for such assistance and Avecia’s reasonable expenses, Avecia shall develop any data requested by Nuvelo concerning the development, manufacture or quality assurance testing of the API which is necessary to support submissions for regulatory approvals to Regulatory Authorities, which data was not anticipated to be developed as part of the Project under the Work Programme or the Quality Agreement. |
| (d) | Nuvelo shall be responsible for preparing and filing all submissions for regulatory approvals of Product. Nuvelo will confirm Nuvelo interpretations of Avecia data in the Chemistry, Manufacturing and Controls (CMC) section of any |
26
| submissions to Regulatory Authorities with Avecia before submission to Regulatory Authorities. Nuvelo owns and shall own all Regulatory Filings related to the Product and the API. |
| (e) | At no additional cost to Nuvelo, to the extent Nuvelo needs to submit and make available any Master Batch Record in connection with obtaining any regulatory approvals from any Regulatory Authority, to the fullest extent permissible under Applicable Laws, Avecia shall provide Nuvelo with any and all information necessary for Nuvelo to make the Master Batch Record available to the applicable Regulatory Authority. |
| (f) | At no additional cost or expense to Nuvelo, Nuvelo shall have full access to and the right to use and reference, any correspondence, facility and engineering records and diagrams, validation documentation, Batch records, reports, analyses, regulatory requirements and any other data and documentation generated in connection with the Project, and Avecia shall provide Nuvelo with 1 full set of the foregoing documentation upon request. |
| (g) | Avecia will handle all waste resulting from or arising out of the Project in accordance with Applicable Laws. |
| (h) | The provision of data, information or copies of records from Avecia’s work is included in the fees set out in Clause 3. In the event that Avecia needs to generate additional information under paragraph (c) above or re-analyze data in an unanticipated way in response to a Regulatory Authority request, then that information and data shall be provided subject to agreement of a reasonable commercial rate based on the time/effort required. |
| 2.10 | Quality Audits by Nuvelo & SCAR Reporting. |
| (a) | Nuvelo reserves the right to conduct, twice-a-year, comprehensive quality audits of Avecia’s facilities, which may include a site tour, questioning of employees in work areas and review of quality system documentation to the appropriate quality standards. Avecia will be notified in advance of the intention to conduct an audit and the audit’s scope, and a mutually convenient date will be selected. During the audit, any non-conformances will be noted and documented in a report issued by Nuvelo within thirty (30) business days. Avecia will be requested to submit a written response and corrective action plan within thirty (30) business days of receipt of the report. Avecia will close all corrective actions that can be closed within 90 business days to the satisfaction of Nuvelo. Corrective actions that cannot be closed out within 90 business days will have a timeline for closure agreed with Nuvelo. |
27
| (b) | Supplier performance issues or non-conformances in the API will be indicated to Avecia, in addition to any notifications through the PSC or EC, in the form of a Nuvelo Supplier Corrective Action Report (SCAR)/Memo. Avecia will be requested to respond to the SCAR/Memo usually within thirty (30) business days post receipt. |
| 3. | Payments |
| 3.1 | Consideration. In consideration of Avecia carrying out the technical consultancy activities pursuant to the Project in accordance with the terms and conditions of this Agreement and in consideration for sale and delivery of API in accordance with the terms and conditions of this Agreement, Nuvelo shall pay to Avecia a sum of ten million pounds (£10,000,000), to be paid, as follows, if and only if the following terms and conditions set forth below are met: |
| Milestone Number |
Anticipated/Approximate Of Milestone |
Milestone/Invoice Trigger |
Amount Triggered by Milestone (£) | |||
| 1 |
Done and paid in full before the signature date of this Agreement | In consideration for technical consultancy in relation to assessment and planning, paid on the Commencement Date | 105,000 | |||
| 2 |
Done and paid in full before the signature date of this Agreement | In consideration for Avecia carrying out technical consultancy preparatory to GMP manufacture, paid on the Commencement Date | 50,000 | |||
| 3 |
Done and paid in full before the signature date of this Agreement | In consideration for technical consultancy pursuant to commencement of the Project, paid on 28th February 2005 | 300,000 | |||
| 4 |
Signature of this Agreement | In consideration for Avecia carrying out technical consultancy in relation to transfer of process and assays, payable on completion of transfer of process and analytical methods | 105,000 | |||
| 5 |
Signature of this Agreement | In consideration for technical consultancy pursuant to Process characterisation in the laboratory (Experimental set 1, based on RPN analysis), payable on commencement of the Process characterisation | 136,250 | |||
| 6 |
Signature of this Agreement | In consideration for technical consultancy pursuant to assay validation, payable on commencement of assay validation | 358,750 | |||
| 7 |
Signature of this Agreement | In consideration for technical consultancy pursuant to preparation of the Project plan, payable on agreement of the Project plan, including the manufacturing schedule by the PSC | 50,000 | |||
28
| 8 |
Signature of this Agreement | In consideration for technical consultancy pursuant to modification of ABC5000 facility modifications for centrifuge trials, payable on commencement of modifications | 350,000 | |||
| 9 |
18-Jul-05 | In consideration for technical consultancy pursuant to preparation of a cleaning study, payable on completion of the cleaning study and delivery of a report on such study | 10,000 | |||
| 10 |
08-Aug-05 | In consideration for technical consultancy pursuant to preparation of Batch records for centrifuge trials, payable on completion of such Batch records | 160,000 | |||
| 11 |
14-Aug-05 | In consideration for technical consultancy pursuant to preparation for Development Batch Manufacture, payable three (3) months prior to commencement of manufacture of the first Development Batch | 420,000 | |||
| 12 |
18-Aug-05 | In consideration for technical consultancy pursuant to the replicate 15L fermentation runs, payable on completion of the runs and delivery of the associated technical report | 54,500 | |||
| 13 |
22-Aug-05 | In consideration for technical consultancy in performance of the centrifuge runs, payable on commencement of the first centrifuge run | 200,000 | |||
| 14 |
01-Sep-05 | In consideration for technical consultancy pursuant to the centrifuge runs, payable on completion of the first centrifuge run | 250,000 | |||
| 15 |
07-Sep-05 | In consideration for technical consultancy pursuant to the replicate purification runs, payable on completion of the runs and delivery of the associated technical report | 54,500 | |||
| 16 |
26-Sep-05 | In consideration for technical consultancy pursuant to 100L labscale purification, payable on completion thereof | 44,000 | |||
| 17 |
26-Sep-05 | In consideration for technical consultancy pursuant to preparation of Batch records, payable on commencement of Batch record writing (approval of Process Specification for GMP Development Batches) | 88,500 | |||
29
| 18 |
28-Oct-05 | In consideration for technical consultancy pursuant to preparation for Development Batch Manufacture, payable on completion of the plant report for centrifuge runs | 200,000 | |||
| 19 |
14-Nov-05 | In consideration for technical consultancy pursuant to preparation for Development Batch Manufacture, payable on completion of the Batch records for Development Batch Manufacture | 88,500 | |||
| 20 |
14-Nov-05 | In consideration for technical consultancy pursuant to performance of Development Batch Manufacture, payable on commencement of Development Batch Manufacture | 751,250 | |||
| 21 |
18-Nov-05 | In consideration for technical consultancy pursuant to assay validation, payable upon completion of assay validation for the first 10 assays to be validated | 291,250 | |||
| 22 |
21-Nov-05 | In consideration for technical consultancy pursuant to characterisation of the Process in the laboratory (Experimental Set 1) payable on delivery of the fermentation technical report and purification technical report |
136,250 | |||
| 23 |
01-Dec-05 | As a first stage payment in consideration for sale and delivery of a Released Batch of API, payable on commencement of manufacture of the second Development Batch | 251,250 | |||
| 24 |
20-Jan-06 | In consideration for technical consultancy pursuant to assay validation, payable upon completion of assay validation for the remainder of the assays to be validated | 417,000 | |||
| 25 |
01-Mar-06 | As a final stage payment in consideration for sale and delivery of Released Development Batch(es), payable on Release of at least one Development Batch during Development Batch Manufacture | 510,000 | |||
| 26 |
12-Apr-06 | In consideration for technical consultancy during performance of Engineering Batch manufacture, payable on commencement of manufacture of the Engineering Batch | 424,940 | |||
30
| 27 |
12-Apr-06 | In consideration for technical consultancy in preparation of Batch records for the Validation Campaign, payable on completion of such Batch records | 120,600 | |||
| 28 |
26-Apr-06 | In consideration for technical consultancy during Engineering Batch manufacture, payable on completion of manufacture of the Engineering Batch, as evidenced by commencement of post-Batch cleaning of the facility | 424,940 | |||
| 29 |
26-Apr-06 | As a first stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on commencement of manufacture of the first Validation Batch | 424,940 | |||
| 30 |
26-Apr-06 | In consideration for technical consultancy pursuant to preparation of the Validation Master Plan under clause 2.4(a)(ii) of the Work Programme, payable on completion of Validation Master Plan | 50,000 | |||
| 31 |
26-Apr-06 | In consideration for technical consultancy pursuant to preparation of the Validation Master Plan, payable on execution of the Validation Master Plan | 99,000 | |||
| 32 |
12-May-06 | As a second stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on commencement of manufacture of the second Validation Batch | 424,940 | |||
| 33 |
29-May-06 | As a third stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on commencement of manufacture of the third Validation Batch | 424,940 | |||
| 34 |
12-Jun-06 | As a fourth stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on commencement of manufacture of the fourth Validation Batch | 424,940 | |||
31
| 35 |
26-Jun-06 |
As a fifth stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on commencement of manufacture of the fifth Validation Batch, if agreed under Clause 2.4(b)(3) | Payment in accordance with Clause 2.4(b)(3) | |||
| 36 |
16-Aug-06 |
As a sixth stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on Release of the first Validation Batch | 424,940 | |||
| 37 |
01-Sep-06 |
As a seventh stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on Release of the second Validation Batch | 424,940 | |||
| 38 |
18-Sep-06 |
As an eighth stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on Release of the third Validation Batch | 424,940 | |||
| 39 |
02-Oct-06 |
As a ninth stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on Release of the fourth Validation Batch, such stage payment to be the final stage payment for sale and delivery of API produced during the Validation Campaign, in the event that the PSC determines that a fifth Validation Batch is not required. | 424,940 | |||
| 40 |
18-Oct-06 |
As final stage payment in consideration for sale and delivery of Released API produced during the Validation Campaign, payable on Release of the fifth Validation Batch, in the event that the PSC determines that a fifth Validation Batch is not required | Payment in accordance with Clause 2.4(b)(3) | |||
| 41 |
01-Dec-06 |
In consideration for technical consultancy pursuant to preparation of validation reports, payable on delivery of such reports | 99,000 | |||
| 3.2 | Excluded Items. |
| (a) | The sums set out in Clause 3.1 above do not include: |
| (1) | Expenditure actually incurred by Avecia for major consumable items (including, without limitation, Expenditure associated with chromatography resins and filtration membranes) intended to be used and not reasonably usable for other purposes by Avecia, or |
32
| actually used for cGMP Batches (including centrifuge trials conducted under Clause 2.2, Development Batch Manufacture and the Validation Campaign); or |
| (2) | Expenditures actually incurred by Avecia for work conducted by subcontractors, subcontracted by Avecia in accordance with Clause 13.2, for the performance of the following assays, which assays are discussed in more detail in the Work Programme: (i) intact MS, (ii) DNA or equivalent, (iii) USP/EP Pyrogen, (iv) EPA Copper and Zinc, and (v) DNA sequence for cell bank; or |
| (3) | Expenditure actually incurred by Avecia in respect of other consumable items purchased for use in the Project (including, without limitation, disposable bags, tubing, hoses and chemical raw material costs), or actually used for cGMP Batches (including centrifuge trials conducted under Clause 2.2, Development Batch Manufacture and the Validation Campaign). |
| (b) | Avecia shall notify Nuvelo of the Expenditure incurred in respect of Clause 3.2(a)(3) and Avecia shall obtain Nuvelo’s approval in writing prior to incurring such Expenditure in Clause 3.2(a)(1) or 3.2(a)(2) and, subject to approval in respect of Expenditure under Clauses 3.2(a)(1) or 3.2(a)(2), such Expenditure shall thereupon form part of the consideration for the provision of the technical consultancy services (including qualification, quality assurance and quality control activities) by Avecia and Avecia shall invoice Nuvelo for: (1) such amounts; and (2) in the case of Expenditure incurred under Clauses 3.2(a)(1) and 3.2(a)(3), the Handling Fee. Upon any termination of this Agreement, all consumable items purchased by Avecia for the Project that are not consumed or otherwise worn beyond further use by their use in the Project shall, upon Nuvelo’s request, be sold to Nuvelo or Nuvelo’s designee for the sum total of £1 and delivered by Avecia, ready for shipment, into the custody of a shipper reasonably acceptable to Nuvelo or Nuvelo’s designee, the cost of shipment to be at Nuvelo’s cost and expense, for Nuvelo’s or its designee’s use and disposal, in its discretion. |
| 3.3 | Equipment. In order for Avecia to provide technical consultancy services work under this Agreement, it will be necessary for certain items of Equipment to be purchased by Avecia. Avecia will be authorised to proceed with the relevant services for which the Equipment is required on the following terms: |
| (a) | Avecia shall obtain, from an independent Third Party, at least 2 quotations for each item of Equipment and send a copy of the quotations by fax or email to Nuvelo and Nuvelo shall inform Avecia within 5 business days of receipt which |
33
| quotation Nuvelo approves and whether it authorises Avecia to proceed with the services for which the item of Equipment is required. In the absence of such approval, Avecia shall not proceed with the relevant services. |
| (b) | If Nuvelo gives its approval to the relevant quotation, Avecia shall be considered authorised to proceed with the relevant services set forth under the Work Programme or agreed upon Program Amendment Order and shall purchase the item of Equipment for such purpose. Any Equipment purchased by Avecia shall be dedicated to the Project, and shall not be used for any other use or purpose. Avecia shall keep the Equipment in good working order. The purchase price of the item of Equipment per the approved quotation shall thereupon form part of the consideration for the provision of the technical consultancy services (including qualification, quality assurance and quality control activities) by Avecia and Avecia shall invoice Nuvelo for: (i) such amount; and (ii) the Handling Fee. |
| (c) | Following completion or termination of the Project, whichever occurs first, at any time within 12 months following such completion or termination, Nuvelo shall be entitled to purchase all or any portion of the Equipment from Avecia for the sum total of £1.00 in its or their then current, good working condition, subject to payment of any outstanding and undisputed fees by Nuvelo to Avecia for work carried out under this Agreement in accordance with the terms and conditions of this Agreement and the Work Programme. |
| (d) | Nuvelo shall provide to Avecia reasonable prior written notice of its wish to purchase the Equipment so as to minimize any unplanned facility downtime and Nuvelo shall be responsible for all commercially reasonable removal costs and expenses. If the removal of any Equipment is performed by Nuvelo personnel, Nuvelo agrees to pay for the making good of any damage caused to Avecia’s facility as a result of such removal. If the removal is performed by Avecia personnel, Avecia agrees to pay for the making good of any damage caused to the Equipment being removed as a result of such removal. |
| 3.4 | Invoices and Timing of Payments. Avecia shall promptly issue invoices for the sums set out in Clauses 3.1, 3.2 and 3.3 once such sums become payable in accordance with Clauses 3.1, 3.2 and 3.3. In respect of invoices in respect of sums set out in Clause 3.1, Nuvelo shall pay invoices received by Nuvelo on or before the 15th day of each month by the end of that month and shall pay invoices received by Nuvelo after the 15th day of each month by the end of the following month. In respect of invoices in respect of sums set out in Clause 3.2, Nuvelo shall pay such sums within 30 calendar days after Nuvelo’s receipt of the relevant invoice. In respect of |
34
| invoices in respect of sums set out in Clause 3.3, Nuvelo shall pay such invoices within ten (10) business days after receipt of the relevant invoice. Payments shall be made in Pounds Sterling. |
| 3.5 | Bank Account Details. All amounts payable to Avecia under this Agreement shall be paid by wire transfer, quoting invoice numbers of payment, to an account number specified in writing by Avecia. |
| 3.6 | Taxes. Any payment under this Agreement is stated exclusive of any Value Added Tax. It is the understanding of both Parties that the technical consultancy services being provided under this Agreement are outside the scope of UK VAT. However, in the event that UK VAT does become payable, the UK VAT shall be for the account of Nuvelo and Avecia shall use best efforts to assist Nuvelo in seeking recovery of such tax paid from the UK HM Customs and Excise. If Nuvelo is otherwise required to withhold tax applicable to any payment under this Agreement, Nuvelo shall promptly provide Avecia with the official receipts and such other evidence of payment as Avecia may reasonably request and Nuvelo shall render Avecia reasonable assistance in order to allow Avecia to obtain the benefit of any relevant present or future double taxation treaty. |
| 3.7 | Financial Tracking; Reporting. The PSC and Nuvelo’s financial team representatives will review and measure the growth in value of the Project by tracking resources used versus progress against the deliverables set out in the Work Programme. In respect of consumables, raw materials and any other items purchased by Avecia under Clause 3.2, Avecia will provide a breakdown by item and include copies of invoices for those items of an individual price of greater than £5,000, to the PSC and Nuvelo’s financial team representatives. Within 3 business days after the end of each month, Avecia will provide a financial ‘Earned Value Analysis’ report for that month to Nuvelo. Utilizing the Payment Schedule set forth in Clause 3.1, the report will summarize the approximate value of services provided to Nuvelo for each Project stage that Avecia actively performed during that month. Upon written request by Nuvelo, Avecia shall provide, within thirty days of receipt of Nuvelo’s written notice, documented proof of purchase for all Expenditures and Equipment, or for specified Expenditures and Equipment, passed through to Nuvelo in accordance with Sections 3.2, 3.3 and 3.4. Avecia will provide Nuvelo with a quarterly written report at each regular PSC meeting setting forth the activity status of its manufacturing suite(s) that are intended to be used or could be used for the Project, for the six months following the date of submission of the report to Nuvelo. The report will set forth the times when Avecia’s suites are available and when they are committed to other projects. |
35
| 4. | Completion of the Project |
| 4.1 | Completion. Completion of the Project will be deemed to have occurred when the Development Batch Manufacture, the Validation Campaign and any additional or alternative work agreed in a Programme Amendment Order have been completed. |
| 4.2 | Packaging, Shipping and Delivery. Deliveries of all material, including documentation, returned consumable items or Equipment under Clauses 3.2 or 3.3, and API will be made Ex Works, Avecia’s Billingham facility (Incoterms 2000), subject to the following. Avecia is responsible for packaging and labelling the API in anticipation of shipment. Avecia also shall employ a shipper, reasonably acceptable to Nuvelo, for the shipment of all Released API and any Defective Batch. Avecia will package the Released API or Defective Batch in validated containers under validated shipping conditions. Until the shipping conditions have been validated, Avecia will package the Released API or Defective Batch with temperature monitoring devices that are able to function properly during the shipping process, as further set forth in the Quality Agreement. Risk of loss for all Released API or Defective Batch shall transfer to Nuvelo after delivery of the Released API or Defective Batch to the Nuvelo-approved shipper by Avecia, except with respect to any loss resulting from a failure of Avecia to properly package and label the API for shipment. Nuvelo will purchase insurance to cover any loss to the Released API or Defective Batch once it is delivered to the shipper. Nuvelo agrees that it shall export the API sold to it promptly and in any case within 3 months of the final stage payment for the API and shall provide evidence of such export within 3 months thereof. |
| 4.3 | Payment on Completion. Nuvelo shall pay to Avecia within thirty (30) calendar days after Completion any sums required to be paid pursuant to Clause 3.2 above which may remain outstanding at Completion. Upon Completion, Nuvelo shall pay any sums for milestones triggered pursuant to Clause 3.1 in accordance with the payment terms set forth in Section 3.4. |
| 4.4 | Irrevocable Option for Future Manufacturing Period. |
| (a) | Avecia hereby grants Nuvelo an irrevocable option (the “Manufacturing Option”) to purchase, upon commercially reasonable terms to be agreed under Clause 4.4(c) below, 3 kilograms of Released API (“the Manufacturing Quantity”), to be manufactured in the stream in Avecia’s ABC5000 facility |
36
| in which the Process was validated under this Agreement, during the period commencing April 1, 2007 and expiring October 31, 2008 (“the Manufacturing Option Period”). |
| (b) | Following signature of this Agreement and whilst this Agreement remains in force, Nuvelo shall use reasonable commercial endeavours to provide Avecia with an estimate regarding the potential timing of the Manufacturing Quantity during the Manufacturing Option Period. The obligation to provide the estimate will commence on Release of the first Validation Batch and the forecast will be updated at each PSC meeting or on Nuvelo becoming aware of a change in its timeline if earlier. To exercise the Manufacturing Option, Nuvelo shall give Avecia written notice of its exercise not later than 1 calendar year before the date that Nuvelo expects Avecia to deliver the Manufacturing Quantity to Nuvelo. In the absence of providing Avecia with timely written election, the option in Clause 4.4(a) shall lapse. |
| (c) | No later than 30 calendar days after commencement of the Validation Campaign, Avecia and Nuvelo shall promptly commence negotiations in good faith for the supply of the API by Avecia to Nuvelo upon commercially reasonable terms (“Supply Agreement”). The Supply Agreement is anticipated to contain provisions related to anticipated volumes, pricing, forecasting mechanisms and quality control provisions, and such other commercially reasonable provisions. The Parties shall use reasonable commercial efforts to commence discussions regarding possible key principles of any Supply Agreement after the first successful Release of a Development Batch. Nuvelo shall have no obligation to enter into the Supply Agreement and may immediately halt negotiations at any time, as a result of an Avecia Default. |
| 5. | Intellectual Property & Confidential Information |
| 5.1 | Background Intellectual Property. Except with respect to the licenses granted in this Clause 5, nothing in this Agreement shall be deemed to imply any license to a Party to use the other Party’s Background Intellectual Property. Nothing in this Agreement shall affect the ownership by either Party of any of its Background Intellectual Property. |
| 5.2 | Non-exclusive Licenses under Background Intellectual Property and New Intellectual Property. |
| (a) | Project Use License. Subject to the terms and conditions of this Agreement, Nuvelo grants to Avecia: (1) a royalty free, non-exclusive licence, without the right to sublicense, under its Background Intellectual Property solely to perform and otherwise carry out the Project, and for no other use or |
37
| purpose; and (2) a royalty free, non-exclusive license, without the right to sublicense without Nuvelo’s prior written approval, under the New Intellectual Property solely to perform and otherwise carry out the Project. |
| (b) | Nuvelo Commercial License. Subject to the terms and conditions of this Agreement, specifically including Clause 5.2(c), Avecia hereby grants Nuvelo a royalty-free, irrevocable, non-exclusive, world-wide license, with power to sub-license through multiple levels of sublicensees, under any of Avecia’s Background Intellectual Property that is incorporated into the Process during the Project, to make, have made, use, sell, offer for sale and import the Product or the API or any form, including any filled or finished form, of the Product or API. |
| (c) | Royalty-Bearing License. If Avecia believes in good faith that the Process would be materially improved by the utilization in the Process of an Invention or Inventions covered by a Valid Claim or Valid Claims within Avecia’s Background IP, then if and only if Avecia has previously, and continues to only grant access to such Inventions to Third Parties under a royalty-bearing license (each such Invention a “Patented, Licensed Avecia Invention”), Avecia shall notify Nuvelo in writing regarding Avecia’s proposal to incorporate any Patented, Licensed Avecia Invention into the Process, and shall propose commercially reasonable terms for a license to the Patent or Patents containing the Valid Claim or Valid Claims covering the Patented, Licensed Avecia Invention(s). Nuvelo has no obligation whatsoever to agree to the utilization of any Patented, Licensed Avecia Invention in the Process, and Avecia shall not delay or discontinue the Project as a result of a decision by Nuvelo not to incorporate any Patented, Licensed Avecia Invention in the Process. If a duly authorized representative of Nuvelo agrees in writing to the utilization of a Patented, Licensed Avecia Invention in the Process, then Avecia shall grant Nuvelo a worldwide license under the Patent(s) containing the Valid Claim(s) that cover the Patented, Licensed Avecia Invention(s) to make, have made, use, sell, offer for sale and import the Product or the API or any form, including any filled or finished form, of the Product or API, subject to agreement upon commercially reasonable terms. |
| 5.3 | Ownership & Protection of New Intellectual Property. Any and all New Inventions, and any and all New Intellectual Property, shall belong to Nuvelo. Avecia shall, and shall ensure that its employees shall, at Nuvelo’s expense, perform all acts and execute all instruments necessary to assign to Nuvelo all right, title and interest in and to any and all New Invention and New Intellectual Property. Nuvelo is entitled to, but has no obligation, to file, prosecute, maintain, register, enforce or defend any and all New Intellectual |
38
| Property. All fees, costs and expenses connected with Nuvelo’s filing, prosecution, maintenance, enforcement or defense of New Intellectual Property shall be borne and paid by Nuvelo. |
| 5.4 | Non-exclusive license under New Intellectual Property. Subject to the terms and conditions of this Agreement, Nuvelo hereby grants to Avecia a royalty-free, irrevocable, non-exclusive, world-wide license, with power to sub-license, but without the ability to grant Avecia’s sublicensees the ability to grant sublicenses, under the New Intellectual Property for any use other than to make, have made, use, sell, offer for sale or import, keep or otherwise deal in Product or the API or any form, including any filled or finished form, of Product or API. |
| 5.5 | Provision of Technical Assistance and Process Transfer. If following Completion or following termination of this Agreement for any reason, Nuvelo requires Avecia’s technical assistance for the transfer of all or any portion of the Process to Nuvelo or a Third Party designee of Nuvelo, or other technical assistance, then Avecia shall promptly provide Nuvelo with all reasonable assistance necessary to transfer the Process to Nuvelo or Nuvelo’s Third Party designee, and Nuvelo will pay for Avecia’s reasonable, actual costs and expenses associated with transfer of the Process, except that if Nuvelo terminates for Avecia’s Unremedied Breach, Nuvelo shall only pay Avecia for one-third of Avecia’s actual costs and expenses associated with such assistance notwithstanding the provisions of Clause 9.1(b). |
| 5.6 | Limitation. Avecia shall not, and shall not use the Process to, make, have made, use, sell, offer for sale or import API or Product for any entity, other than Nuvelo, in any territory, without the prior written consent of a duly authorized representative of Nuvelo. |
| 6. | Warranties, Liability and Indemnity |
| 6.1 | Warranties. |
| (a) | Each Party warrants to the other that: |
| (1) | it has the necessary right and authority to enter into this Agreement; and |
| (2) | to its knowledge as of the signature date of this Agreement, the use of its Background Intellectual Property pursuant to this Agreement for the purposes set out in this Agreement will not infringe the Intellectual Property of a Third Party. |
| (b) | Avecia represents and warrants that it has not been debarred, nor does it intend to use for the Project any individual who has been debarred, under Section 306 of the Federal Food, Drug, |
39
| and Cosmetic Act, 21 U.S.C. §335a(a) or (b). In the event that Avecia becomes debarred, Avecia shall notify Nuvelo immediately. |
| 6.2 | Nuvelo Indemnity to Avecia. |
| (a) | Except as otherwise provided below in Clause 6.2(b), Nuvelo shall indemnify, defend and hold harmless Avecia, its Affiliates and their directors, officers, employees and agents (the “Avecia Indemnitees”) from and against any loss, claim, suit, liability, damage or expense, of whatsoever kind or nature (“Loss”), resulting from or arising out of: |
| (1) | actual or suspected infringement of the Intellectual Property of any Third Party arising from the Avecia Indemnitees’ application of Nuvelo’s Background IP or information provided to the Avecia Indemnitees by Nuvelo, in the performance of the Project; |
| (2) | Nuvelo or its Affiliates, or their directors, officers, employees or agent’s use of any results of the Project performed by the Avecia Indemnitees hereunder (including without limitation API or other materials manufactured); or |
| (3) | the negligence, wilful misconduct or breach of any Applicable Laws by Nuvelo or its Affiliates, or their directors, officers, employees or agents; |
| (b) | Nuvelo has no obligation to indemnify, defend or hold harmless the Avecia Indemnitees for any Loss to the extent resulting from or arising out of: |
| (1) | the actual or suspected infringement of the Intellectual Property of any Third Party arising from any of the Avecia Indemnitees’ application of Avecia’s Background IP or information not provided to the Avecia Indemnitees by Nuvelo or its Affiliates, or their officers, directors, employees or agents, in the performance of the Project; |
| (2) | the negligence or wilful misconduct of any of the Avecia Indemnitees; |
| (3) | any breach of this Agreement by any of the Avecia Indemnitees; |
| (4) | any failure by any of the Avecia Indemnitees to comply with Applicable Laws; or |
| (5) | in respect of the indemnity under paragraph (a)(2) of this Clause 6.2, Avecia’s continuing use of Nuvelo’s |
40
| Background IP, following Avecia receiving written notice, and supporting documentation, from Nuvelo that use of any of such Background IP results in the actual or suspected infringement of the intellectual property of any Third Party and instructing Avecia to cease use of that part of Nuvelo’s Background IP which results in such actual or suspected infringement. |
| 6.3 | Avecia Indemnity to Nuvelo. |
| (a) | Except as otherwise provided below in Clause 6.3(b), Avecia shall indemnify, defend and hold harmless Nuvelo and its Affiliates, and their officers, directors, employees and agents (“Nuvelo Indemnitees”) from and against any Loss resulting from or arising out of: |
| (1) | actual or suspected infringement of the Intellectual Property of any Third Party by any of the Avecia Indemnitees’ or Avecia subcontractor’s application of Avecia’s Background IP during the performance of the Project; |
| (2) | any of the Nuvelo Indemnitees’ use of any Avecia Background IP or any results of the Project generated by any of the Avecia Indemnitees, or any Avecia subcontractors, hereunder (including without limitation API or other materials manufactured) to the extent any of the Nuvelo Indemnitees’ use of the Avecia Background IP or any results of the Project results in the actual or suspected infringement of the Intellectual Property of any Third Party, but with respect to any results of the Project, solely to the extent such results were generated through any of the Avecia Indemnitees’ application of Avecia’s Background IP in the performance of the Project; or |
| (3) | the negligence, wilful misconduct or breach of any Applicable Laws by any of the Avecia Indemnitees; |
| (b) | Avecia has no obligation to indemnify, defend or hold harmless the Nuvelo Indemnitees for any Loss to the extent resulting from or arising out of: |
| (1) | the negligence or wilful misconduct of any of the Nuvelo Indemnitees; |
| (2) | any breach of this Agreement by any of the Nuvelo Indemnitees; |
| (3) | any failure by any of the Nuvelo Indemnitees to comply with Applicable Laws; or |
41
| (4) | in respect of the indemnity under paragraph (a)(2) of this Clause 6.3, Nuvelo’s continuing use of any results of the Project, following Nuvelo receiving written notice from Avecia, and supporting documentation, that use of such results of the Project results in the actual or suspected infringement of the intellectual property of any Third Party. |
| 6.4 | Indirect, Consequential and Special Damages Excluded. Except with respect to indemnity under Clauses 6.2 and 6.3 above or any breach of intellectual property or confidentiality provisions in accordance with Clauses 5 and 7, in no event shall either Party be liable for any special, incidental, consequential or indirect damages arising in any way out of or relating to this Agreement, however caused and on any theory of liability. This limitation will apply even if the other Party has been advised of the possibility of such damages. |
| 6.5 | Limitation. Neither Party’s liability under this Agreement will exceed the total amount that would be due to Avecia if the Project was fully completed, except with respect to any liability of Avecia arising under Clause 9.1(c). |
| 6.6 | Insurance. Each Party shall secure and maintain in full force and effect during the term of this Agreement policies of insurance providing coverage for (a) employer’s liability and (b) public and products liability having policy limits, deductibles and other terms appropriate to the conduct of that Party’s business. Evidence of such insurance in the form of a broker’s letter will be made available for examination upon request of the other Party. |
| 6.7 | General Conditions of Indemnification. Each Party’s agreement to indemnify, defend and hold the other harmless in accordance with Clauses 6.2 and 6.3 is conditioned on the indemnified Party: (a) providing prompt written notice to the indemnifying Party of any claim or lawsuit with potential for a Loss no more than ten (10) business days after the indemnified Party has knowledge of such claim or lawsuit, but the ten (10) day time period shall not preclude indemnity hereunder so long as the delay has not materially prejudiced defense of the claim or suit; (b) permitting the indemnifying Party to have full conduct and control to investigate, prepare for, defend against and settle any such claim or lawsuit; (c) providing the indemnifying Party, at the indemnifying Party’s reasonable expense, with the full cooperation and assistance in the investigation of, preparation for and defense of any such claim or lawsuit; and (d) not agreeing to any compromise or settlement materially adverse to the rights of the indemnified Party under this Agreement without the indemnifying Party’s prior written consent. This Clause 6.7 survives termination of this Agreement with respect to any Loss arising from the Parties’ performance during the term of this Agreement and with respect to any conditions and obligations that survive the termination or expiration of this Agreement. |
42
| 7. | Confidentiality |
| 7.1 | The provisions of the Confidentiality Agreement shall remain in full force and effect, except to the extent modified by the terms and conditions of this Agreement. |
| 7.2 | The obligations of confidentiality and non-disclosure set forth in the Confidentiality Agreement will continue for seven (7) years after the expiration or termination of this Agreement, and the Confidentiality Agreement shall not terminate or expire before the termination of this Agreement. |
| 7.3 | Avecia may use the Confidential Information solely for the purpose of the performance of the Project, except that Avecia may use New IP in accordance with the license set forth in Clause 5.4, subject to entry of any relevant Third Party customer or supplier into confidentiality obligations to protect the confidentiality of such New IP, which obligations are no less onerous than those contained in this Agreement. |
| 7.4 | The Confidentiality Agreement shall, with effect from the date hereof, be deemed to be extended to cover disclosures of information by either Party, together with any information acquired by, or otherwise exposed to, either Party as a result of site visits, in each case pursuant to and subject to this Agreement. |
| 8. | Duration and Termination |
| 8.1 | Commencement and Duration. This Agreement and the Project shall commence on the Commencement Date and shall continue (unless terminated in accordance with the provisions of Clauses 8.2, 8.3 or 8.4) until Completion. |
| 8.2 | Termination by Nuvelo. Subject to Clause 9, Nuvelo may terminate this Agreement at any time with or without cause, cause including Unremedied Breach, effective immediately upon written notice to Avecia. |
| 8.3 | Termination by Avecia. Avecia may terminate this Agreement immediately upon notice to Nuvelo in respect of an Unremedied Breach by Nuvelo, where material breach includes a failure by Nuvelo to pay an amount owed to Avecia under this Agreement within 10 business days of when it is due. |
| 8.4 | Termination by Agreement. Subject to Clause 9, this Agreement may be terminated by mutual agreement at any time prior to Completion. |
43
| 9. | Consequences of Termination |
| 9.1 | General. In the event of termination by either Party under Clause 8 above: |
| (a) | Termination other than for Unremedied Breach. Upon termination of this Agreement, other than for termination by Nuvelo in connection with an Unremedied Breach by Avecia, Nuvelo will pay Avecia: |
| (1) | all sums due and payable up to the date of termination but not yet paid, but excluding any payments (other than the Handling Fee) due in respect of subcontractors appointed under Clause 13.2 or consumable items or Equipment purchased under Clauses 3.2 and 3.3: (i) that can be returned; or (ii) the ordering of or contract for which can be cancelled; |
| (2) | all reasonable costs already incurred by Avecia up to the date of termination in accordance with the terms and conditions of this Agreement and uncancellable expenses incurred by Avecia after termination which could not reasonably be avoided after a reasonable attempt by Avecia to mitigate such costs, including reasonable cancellation charges payable by Avecia to a subcontractor appointed under Clause 13.2 or the seller of consumables or Equipment in respect of consumables or Equipment (i) that has been returned to the seller, or (ii) the order of or contract for which has been cancelled; and |
| (3) | the Cancellation Fee in consideration for technical consultancy services performed up to date of termination and in winding down and cancelling the Project, if and only if the Agreement is terminated by: (i) Avecia under Clause 8.3; or (ii) by Nuvelo in accordance with Clause 8.2, but excluding termination by Nuvelo (A) for Unremedied Breach; (B) due to Force Majeure affecting Avecia; or (C) in accordance with Clause 2.2(b). |
| (4) | Fifty percent (50%) of the Development Batch Fee that otherwise would have been due and payable, as calculated in accordance with Schedule 4, in consideration for technical consultancy services performed up to the date of termination and in winding down and cancelling the Project, if and only if, other than as a result of an Avecia Default, Nuvelo terminates the Agreement under Clause 8.2 as a result of a failure of the centrifuge trials and a failure by Avecia to commence Development Batch Manufacture before the expiration of December 31, 2005, Pacific Standard Time, in accordance with Section 2.2(b)(2). |
44
| (b) | Termination for Unremedied Breach; Avecia Default. If Nuvelo terminates for Avecia’s Unremedied Breach, no later than 5 days after the effective date of Nuvelo’s termination, Avecia shall pay to Nuvelo, as compensation for Nuvelo’s loss of monies paid to Avecia for work not performed in accordance with the terms and conditions of this Agreement, a sum equivalent to any and all monies paid to Avecia under this Agreement, less amounts paid for technical consultancy work completed by Avecia in accordance with the terms and conditions of this Agreement and not affected by the breach. In the event of an Avecia Default in the performance of any portion of the Project, Nuvelo is entitled to request Avecia to: (i) promptly repeat the applicable portion of the Project at Avecia’s own cost or (ii) reimburse Nuvelo for the price for that particular portion of the Project. Failure by Avecia to timely cure an Avecia Default results in an Unremedied Breach of the Agreement. |
| (c) | Currency Exchange Rate Losses. If Nuvelo terminates this Agreement for Unremedied Breach by Avecia, Avecia shall be liable for any currency exchange rate losses incurred by Nuvelo up through and until January 1, 2007, as a result of commitments made by Nuvelo to enable Nuvelo to pay Avecia the payments set forth in Clause 3.1 in British pounds sterling. Within 5 business days after the effective date of termination of the Agreement for Avecia’s Unremedied Breach, Nuvelo shall arrange to sell all British pounds sterling purchased by Nuvelo in anticipation of its obligation to make future payments under this Agreement, and Nuvelo shall also arrange to sell all contracts to purchase British pounds sterling purchased by Nuvelo in anticipation of its obligations to make future payments under this Agreement. Avecia shall pay to Nuvelo an amount equal to any currency exchange losses actually incurred by Nuvelo in connection with such sales, as reduced by any amounts owed by Nuvelo to Avecia as of the effective date of termination on account of services completed by Avecia in compliance with and prior to termination of this Agreement. Avecia will pay any undisputed amounts owed to Nuvelo under this Clause 9.1(c) within five (5) business days of its receipt of an invoice for such amounts from Nuvelo. All payments to Nuvelo under and in accordance with this Clause 9.1(c) will be made in United States dollars. |
| (d) | Subject to Section 6.5, the rights and remedies set out in this Clause 9.1 in respect of the consequences of termination do not limit any other remedies or damages to which a Party may be entitled in law or in equity. |
| 9.2 | Acquired Rights. Termination or expiry of this Agreement, for whatever reason, shall not prejudice the rights of either Party |
45
| acquired before such termination or expiry, including the right to payment for services actually performed under the Project in accordance with the terms and conditions of this Agreement, pursuant to Clause 3. |
| 9.3 | Survival. The provisions of Clauses 1.2, 2.9, 2.10(a), 3.3(c), 3.3(d), 3.5, 3.6, 3.7, 5, 6.2 through 6.5, 6.7 and 7 through 20 shall survive the termination or expiry of this Agreement for the term set forth in the applicable Clause, or if no period is specified, the earlier of perpetuity of the maximum amount of time permitted under Delaware law. |
| 10. | Independent Contractor |
Nothing in this Agreement shall create, or be deemed to create, a partnership or the relationship of principal and agent or employer and employee between the Parties. Each Party agrees to perform under this Agreement solely as an independent contractor.
| 11. | Entire Agreement |
This Agreement and with the Schedules attached hereto contain the entire agreement between the Parties with respect to the subject matter set forth therein and supersedes any previous agreements relating to the Project (including the Interim Agreement) and any understandings between the Parties with respect thereto.
| 12. | Announcements And Publicity |
Subject to the prior written approval of the other Party, a Party may make an official press release, announcement or other formal publicity relating to the transactions which are the subject of this Agreement. The Party wishing to make such release, announcement or publicity shall provide a copy of the text thereof to the other Party for its review, revision and approval, not to be unreasonably withheld or delayed. Nothing in this Clause 12 shall preclude disclosure of the terms of this Agreement as required by Applicable Laws, including without limitation the regulations of the United States Securities and Exchange Commission.
| 13. | Assignment and Subcontracting |
| 13.1 | This Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective legal successors but shall not otherwise be assignable by either Party, without the prior written consent of the other Party, which consent shall not be unreasonably withheld or delayed, except that either Party may assign this Agreement without consent to an Affiliate or a purchaser or acquirer of a Party, or of the part of a Party’s business to which this Agreement relates. |
46
| 13.2 | With Nuvelo’s prior written consent as to the ability to subcontract particular work and as to the identity of each subcontractor, Avecia may subcontract certain analytical work under the Project in accordance with the Quality Agreement, subject to inclusion in such subcontract of confidentiality and intellectual property provisions no less onerous than those contained herein, and commercially reasonable cancellation provisions. Except as provided otherwise in Section 3.2(a)(2) or in a Programme Amendment Order, Avecia is responsible for all costs, fees and Expenditures associated with any subcontracted work, and shall not pass any such costs, fees or Expenditures through to Nuvelo. Avecia shall be liable for any acts or omissions of any subcontractor as if such acts or omissions were Avecia’s own. |
| 14. | Variation |
No variation, modification or amendment of this Agreement shall bind either Party unless made in writing in the English language and agreed to in writing by duly authorized officers of both Parties.
| 15. | Illegality |
If any provision of this Agreement is agreed by the Parties to be illegal, void or unenforceable under any law that is applicable hereto or if any court of competent jurisdiction in a final decision so determines, this Agreement shall continue in force and effect except that the illegal, void or unenforceable provision shall be deemed to be excised herefrom with effect from the date of such agreement or judicial decision or such earlier date as the Parties may agree.
| 16. | Waiver |
A failure by either Party hereto to exercise or enforce any rights conferred upon it by this Agreement shall not be deemed to be a waiver of any such rights or operate so as to bar the exercise or enforcement thereof at any subsequent time or times.
| 17. | Notices |
| 17.1 | Formal Notices. Any formal notice required or permitted under this Agreement shall be in writing which may take the form of a letter or facsimile and shall be sent by prepaid post, facsimile, or hand delivery (including messenger service). The addresses for any such notice or other communication shall be those stated on the first page of this Agreement. |
47
| 17.2 | Other Communications. In addition to the methods set out in Clause 17.1, any communications other than formal notices between the Parties may be made by telephone or by email. |
| 17.3 | Change of Address. Any Party may, at any time by written notice to the other Party, change the address or the facsimile numbers to which notices or other communications shall be sent. All notices and other communications shall have been duly given or made (i) when delivered by hand (including by messenger service) upon delivery or (ii) when delivered by post upon delivery or (iii) when faxed upon receipt of a legible copy by recipient and production of a satisfactory transmission report by sender confirming transmission of the fax in full to the appropriate number by the fax machine which sent the fax. |
| 18. | Force Majeure |
Neither Party shall be liable to the other Party in any manner whatsoever for any failure or delay in performing its obligations under this Agreement if and to the extent, and for the duration, that such is due to Force Majeure. Without prejudice to Clause 8, any said failure or delay shall not give either Party the right to terminate this Agreement except, and to the extent that such Force Majeure continues for a period exceeding three (3) months. Termination as a result of Force Majeure shall take effect as if the Agreement had been terminated by mutual agreement under Clause 8.4.
| 19. | Law and Jurisdiction |
| 19.1 | Law. This Agreement is governed by and shall be construed and interpreted in accordance with the laws of the State of Delaware, United States of America. Any proceedings between the Parties shall be conducted in the English language. |
| 19.2 | Referral to Senior Managers. Prior to any dispute, difference or disagreement concerning this Agreement proceeding to litigation pursuant to Clauses 19.1 and 19.4, the Parties shall seek to resolve the matter within thirty (30) calendar days by referring it to the CFO of Avecia and the CEO of Nuvelo, each of which shall have the authority necessary to fully resolve the dispute. |
| 19.3 | Interim Steps. Neither of the Parties shall be deemed to be precluded from taking such interim formal steps as may be considered necessary to protect such Party’s position while the procedures referred to in Clause 19.2 are pursued. |
| 19.4 | Jurisdiction. Except as provided for in Clause 19.2, in relation to any legal action or proceedings to enforce this Agreement or arising out of or in connection with this Agreement each of the Parties irrevocably consents and submits to the exclusive jurisdiction and venue of the federal and state courts located in the State of Delaware. |
48
| 20. | General |
| 20.1 | Headings. The headings for each Clause or Paragraph or Section in this Agreement, and in the Schedules, have been inserted for convenience of reference only and are not intended to limit or expand on the meaning of the language contained in the particular clause, paragraph or section. |
| 20.2 | Ambiguities. Ambiguities, if any, in this Agreement shall not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision. |
| 20.3 | Counterparts. This Agreement may be executed in two or more counterparts, including facsimile counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. |
49
IN WITNESS WHEREOF, the authorized representatives of the Parties have executed this Agreement as of the last date set forth below, and upon its execution, this Agreement becomes effective as of the Commencement Date.
| For and on behalf of AVECIA LIMITED |
||||||||
| Signature | /s/ Xxxxx Xxx |
|||||||
| Name |
Xxxxx Xxx |
|||||||
| Position |
VP Biotechnology |
|||||||
| Date |
30 June 2005 |
|||||||
| For and on behalf of NUVELO INC. |
||||||||
| Signature | /s/ Xxx Xxxxxxxxx |
Signature | /s/ Xxxxxxx Xxxx | |||||
| Name |
Xxx Xxxxxxxxx |
Name |
Xxxxxxx Xxxx | |||||
| Position |
CFO |
Position |
SVP R&D | |||||
| Date |
30 June 2005 |
Date |
30 June 2005 | |||||
50
Schedule 1
The Work Programme
51
Schedule 2
Quality Agreement
52
Schedule 3
Programme Amendment Order
| (1) Project Title & Number |
(2) Date Project Started |
(3) P.A.O. Number | ||
| (4) Reason for P.A.O. | ||||
| (5) Amendment required and new milestones | ||||
| (6) Impact on price, time frame, resources | ||||
| (7) Amended payment schedule (if required) | ||||
53
| Authorisation |
||||
| for Avecia |
for customer | |||
54
Schedule 4
The Cancellation Fee
| A. | The Cancellation Fee shall be a percentage of: |
| (1) | in the case of Development Batch Manufacture, the total payment to be made in respect of Development Batch Manufacture for completion of milestones 11, 17, 19, 20, 23 and 25 under Clause 3.1, being £2,109,500 (“Development Batch Fee”) less any sums already received for milestones 11, 17, 19, 20, 23 and 25 under this Agreement in respect of Development Batch Manufacture at the date on which notice is given of termination, cancellation or Non-Manufacturing Delay. |
| (2) | in the case of the Validation Campaign, the total payment to be made in respect of the Validation Campaign for completion of milestones 26, 27, 28, 29, 30, 31, 32, 33, 34, 36, 37, 38, 39 and 41 under Clause 3.1, including manufacture of the Engineering Batch (or as otherwise agreed in writing), being £4,618,000 (“the Validation Campaign Fee”) less any sums already received for milestones 26, 27, 28, 29, 30, 31, 32, 33, 34, 36, 37, 38, 39 and 41 under this Agreement in respect of the Validation Campaign and the Engineering Batch under Clause 3.1 at the date on which notice is given of termination, cancellation or Non-Manufacturing Delay. |
| B. | The applicable percentage shall be dependent on the amount of notice of termination, cancellation or Non-Manufacturing Delay given by Nuvelo prior to the date for commencement of Development Batch Manufacture or the Validation Campaign (as appropriate). |
| C. | The Cancellation Fee shall be calculated as follows: |
| Notice Period |
Percentage of Validation Campaign Fee or Development Batch Fee (as appropriate) |
||
| Nine months or more |
0 | % | |
| Fewer than nine months but more than six months |
50 | % | |
| Six months or fewer but more than four months |
70 | % | |
| Four months or fewer but more than one month |
90 | % | |
| One month or less |
100 | % |
| D. | Avecia shall make commercially reasonable endeavours to raise revenue by utilising the production facility during the period during which |
55
| Development Batch Manufacture or the Validation Campaign (as appropriate) was intended to take place but for termination, cancellation on Non-Manufacturing Delay by Nuvelo. Avecia shall refund to Nuvelo a sum equivalent to the revenue (net of raw materials and consumables Expenditure) raised as a result of such alternative use up to a maximum of 80% of the Cancellation Fee paid. |
| E. | Where the Cancellation Fee is less than the amount already paid to Avecia by Nuvelo under this Agreement in consideration for Development Batch Manufacture or the Validation Campaign (as appropriate), Avecia shall pay to Nuvelo the difference between the applicable Cancellation Fee and the amount already paid in consideration for the Development Batch Manufacture or the Validation Campaign (as appropriate). |
56
Schedule 5
Committee Members
Project Steering Committee Members
| Nuvelo |
Avecia | |
| Xxxxx Xxxxx, VP Business Development Xxxxxx Xxxxxx, Director, R&D Finance Xx. Xxxx Xxxxxxx, Senior Director, PSM Xx. Xxxxx Xxxxxxx, Senior Director, Regulatory and QA |
Dr Xxxxxxx Xxxxxx, Business General Manager, Biologics Xxxxx Xxxxxxx, Head of Business Planning Dr Xxxx Xxxxxx, Head of R&D Xx Xxxxx Xxxxxxx, Head of Quality & Regulatory Affairs |
Executive Committee Members
| Nuvelo |
Avecia | |
| Dr. Xxxxxxx Xxxx Senior Vice President, Research and Development |
Dr Xxxxx Xxx, Senior Vice-President, Avecia Biotechnology |
57
Schedule 6
The Equipment
| Liquid Nitrogen Dewars (x2) and Data Logger |
| Depth Filtration System |
| 1000L jacketed bag holder |
| Delipid filter skid |
| 2x1000mm chromatography columns, hydraulic skid and spares |
| 2 x Freezers (-30°C & - 70°C) |
58
Schedule 7
The Confidentiality Agreement
See attached
59
Schedule 1 Work Programme
B2422 Work Programme
Scope Statement
Program to carry out technology transfer of the alfimeprase Process, Process characterization and validation of in-process and bulk API assays by Avecia and manufacture of two (2) cGMP Development Batches, one (1) Engineering Batch and up to five (5) Validation Batches of alfimeprase in Avecia’s ABC 5000 facility.
Contents
| Stage 1. | Data Acquisition and Assessment | |
| Stage 2. | Process Transfer & Fit | |
| Stage 3. | Capital Equipment Specification and Procurement | |
| Stage 4. | 15L Fermentation Runs | |
| Stage 5. | Laboratory Scale Purification Runs | |
| Stage 6. | Assay Transfer of Validated Methods | |
| Stage 7. | Assay Transfer | |
| Stage 8. | Assay Validation | |
| Stage 9. | Laboratory Process Characterisation (LPC) | |
| Stage 10. | Fermentation Scale-Up and Centrifuge Trials at 3000L scale | |
| Stage 11. | cGMP Preparation at 3000L scale | |
| Stage 12. | Two (2) cGMP Development Batches at 3000L scale | |
| Stage 13. | Validation Documentation | |
| Stage 14. | One (1) Engineering Batch at 3000L scale | |
| Stage 15. | Up to Five (5) Validation Batches at 3000L scale | |
| Appendix 1 | Process Assumptions | |
| Appendix 2 | B2422 Work Programme Timeline (see attachment) |
Detailed Work Programme
Stage 1. Data Acquisition and Assessment
| 1.1. | Objectives |
| 1.1.1. | To perform systematic review and gap analysis of Amgen technical information regarding the fermentation, harvest and purification steps for the alfimeprase Process. |
| 1.1.2. | To perform systematic review and gap analysis of Amgen technical information regarding the analytical testing for the alfimeprase Process. |
| 1.1.3. | To perform systematic review and gap analysis of Amgen technical information regarding the genetically modified organism (GMO; Pichia) for alfimeprase. |
| 1.2. | Activities |
| 1.2.1. | Review and evaluate all Amgen technical reports, laboratory notebooks, laboratory reports and previous process batch records to define the alfimeprase Process. |
Page 1 of 11
Schedule 1 Work Programme
| 1.2.2. | Review the analytical methods listed below. |
| Method number |
Assay |
Characteristic | ||
| A0819 |
Genotypic Verification (V) |
MCB and WCB Identity | ||
| A0662 |
Microbial Contaminants (V) |
Safety | ||
| A0359 |
Viable Cell Count (V) |
MCB and WCB Quality | ||
| A01382 |
AEX-Quant (V) |
Strength | ||
| A0848 |
Clot Lysis (validated) |
Potency | ||
| A0120 |
Absorbance at 280nm (validated) |
Strength | ||
| A0829 |
SEC (V) |
Purity | ||
| A0874 |
RP HPLC in-process (V) |
Purity | ||
| A0831 |
AEX-Qual (V) |
Purity | ||
| A0830 |
RP HPLC (V) |
Purity | ||
| A0815 |
SDS PAGE Coomassie (V) |
Purity, Identity | ||
| A0815 |
SDS PAGE Silver |
Purity | ||
| A0847 |
Peptide map (V) |
Identity | ||
| X0000 |
Xxxxxx XX OUTSOURCED (V) |
Identity | ||
| A0862 |
Anti Pichia EIA (V) |
Impurities | ||
| A01339 |
DNA or equivalent OUTSOURCED (V) |
Impurities | ||
| A0221 |
Appearance (V) |
Quality | ||
| USP/EP Pyrogen (rabbit) OUTSOURCED |
Safety | |||
| A0736 |
Bioburden (V) |
Safety | ||
| A0857 |
MES (V) |
Process clearance | ||
| A0837 |
Imidazole (V) |
Process clearance | ||
| A01102 |
EPA Copper and Zinc OUTSOURCED |
Buffer Monitoring | ||
| A0442 |
DNA sequence for cell bank OUTSOURCED (V) |
MCB and WCB Safety | ||
| A0719 |
Cell bank contamination (V) |
MCB and WCB Safety | ||
| A0404 |
Polysorbate 80 (V) |
Buffer monitoring | ||
| A0410 |
Sucrose and Mannitol (V) |
Buffer monitoring | ||
| A0225 |
Citrate (V) |
Buffer monitoring | ||
| A0302 |
Sulphate (V) |
Buffer monitoring | ||
| A0986 |
Tris (V) |
Buffer monitoring | ||
| EP Osmolality |
Quality | |||
| USP/EP pH |
Quality | |||
| USP/EP Conductivity |
Quality |
| (V) | denotes analytical methods that require some validation work. |
| 1.2.3. | Generate a GMO assessment to work with alfimeprase at Avecia. |
| 1.3. | Deliverables |
| 1.3.1. | Definition of a detailed work programme |
| 1.3.2. | Project timeline |
Page 2 of 11
Schedule 1 Work Programme
Stage 2. Process Transfer & Fit
| 2.1. | Objective |
| 2.1.1. | To perform a gap analysis to identify new facility equipment required to manufacture alfimeprase. |
| 2.2. | Activities |
| 2.2.1. | Based on the technical data review (Stage 1), Avecia to highlight the requirements for new equipment purchases to support manufacture of alfimeprase. |
| 2.3. | Deliverables |
| 2.3.1. | Scope of equipment requirements |
Stage 3. Capital Equipment Specification and Procurement
| 3.1. | Objectives |
| 3.1.1. | To purchase Equipment for use during Centrifuge Trials |
| 3.1.2. | To purchase Equipment for use during cGMP manufacture |
| 3.2. | Activities |
| 3.2.1. | Generate a user requirement specification (URS) for all new Equipment. |
| 3.2.2. | Obtain vendor quotes and generate bid comparisons (as appropriate) for all new Equipment for Nuvelo to review. |
| 3.2.3. | Upon receiving Nuvelo confirmation, place relevant order to purchase new Equipment and management of supplier regarding factory acceptance testing and delivery. |
| New Equipment for Centrifuge Trials |
Estimated cost £000s | |
| Liquid Nitrogen Dewars (x2) and Data Logger |
8.752 | |
| Total |
8.752 | |
| New Equipment for GMP manufacture |
Estimated cost £000s | |
| Depth Filtration System |
120 | |
| 1000L jacketed bag holder |
30 | |
| Delipid filter skid |
10 | |
| 2x1000mm chromatography columns, hydraulic skid and spares |
303 | |
| 2 x Freezers (-30°C & - 70°C) |
6 | |
| Total |
469 | |
Page 3 of 11
Schedule 1 Work Programme
| 3.2.4. | In addition Avecia would need to make certain plant modifications to ABC5000 stream 2 facility and provide items of a generic nature at Avecia cost. |
| Modification to fermenter control software |
| Trace heating on glucose line |
| Ammonia feed tank |
| Various tankage |
| 0.2 µm filtration skid |
| Small UF rig (Sartorius Beta) |
| 3.3. | Deliverables |
| 3.3.1. | URS for each new Equipment purchase. |
| 3.3.2. | Installed and qualified new Equipment fit for purpose. |
Stage 4. 15L Fermentation Runs
| 4.1. | Objectives |
| 4.1.1. | To demonstrate robustness and reproducibility of the fermentation Process at laboratory scale within Avecia (familiarisation). |
| 4.1.2. | To demonstrate the fermentation Process comparability after substitution of several raw materials (comparability). |
| 4.2. | Activities |
| 4.2.1. | Receive alfimeprase working cell bank (WCB) vials. |
| 4.2.2. | Generate and ensure technical approval of laboratory protocols with Nuvelo. |
| 4.2.3. | Perform four (4) fermentation runs at 15L using Avecia preferred supplier raw materials. Presentation of familiarisation data set to Nuvelo. |
| 4.2.4. | Receive the original alfimeprase raw material list and specifications from Nuvelo. |
| 4.2.5. | Review and technical approval of a comparability procedure drafted by Nuvelo. |
| 4.2.6. | Perform four (4) fermentation runs at 15L for comparability, two (2) using the original alfimeprase Process raw materials and two (2) using Avecia preferred supplier raw materials. Presentation of comparability data set to Nuvelo. |
| 4.2.7. | Generate familiarisation and comparability technical reports. |
| 4.3. | Deliverables |
| 4.3.1. | Interim fermentation familiarisation technical report. |
| 4.3.2. | Final fermentation comparability technical report. |
| 4.3.3. | QA review of limited data from familiarization and comparability technical reports for Nuvelo. |
Stage 5. Laboratory Scale Purification Runs
| 5.1. | Objectives |
| 5.1.1. | To demonstrate robustness and reproducibility of the purification Process at 15L laboratory scale within Avecia (familiarisation). |
| 5.1.2. | To demonstrate the purification Process comparability after substitution of several fermentation and purification raw materials (comparability). |
Page 4 of 11
Schedule 1 Work Programme
| 5.1.3. | To demonstrate cleanability of alfimeprase in relation to proteins used in cleaning validation studies. |
| 5.2. | Activities |
| 5.2.1. | Receive the alfimeprase fermentation diafiltered, concentrated, clarified broth (DCCB) from Nuvelo. |
| 5.2.2. | Perform two (2) purification runs through to final Product using Nuvelo DCCB. |
| 5.2.3. | Perform analytical testing of the purification Process using ‘Nuvelo DCCB’. |
| 5.2.4. | Receive the first 2 x 15L Avecia fermentation broth from Stage 4 familiarisation. Create DCCB and purify 2 x 15L to final Product. |
| 5.2.5. | Perform analytical testing of purification Process using Avecia DCCB. |
| 5.2.6. | Receive the second 2 x 15L Avecia fermentation broth from Stage 4 familiarisation. Create DCCB and purify 1 x 15L to final Product. |
| 5.2.7. | Perform analytical testing of Avecia DCCB purification Process. |
| 5.2.8. | Presentation of familiarisation data set to Nuvelo. |
| 5.2.9. | Generate interim familiarisation technical report. |
| 5.2.10. | Perform cleanability studies. |
| 5.2.11. | Review and technical approval of a comparability procedure drafted by Nuvelo. |
| 5.2.12. | Receive the next 4 x 15L Avecia fermentation broth from Stage 4 comparability. Create DCCB and purify 2 x 15L (one from each preferred raw material supplier) to final Product. |
| 5.2.13. | Perform analytical testing. |
| 5.2.14. | Generate comparability technical report. |
| 5.3. | Deliverables |
| 5.3.1. | Interim purification familiarisation technical report. |
| 5.3.2. | Final purification comparability technical report. |
| 5.3.3. | Cleanability technical report. |
| 5.3.4. | QA review of limited data from familiarization and comparability technical reports for Nuvelo. |
Stage 6. Assay Transfer of Validated Methods
| 6.1. | Objectives |
| 6.1.1. | To transfer the validated clot lysis and appearance analytical methods to Avecia QC. |
| 6.2. | Activities |
| 6.2.1. | Receive appropriate samples, historical data and reference standard from Nuvelo. |
| 6.2.2. | Jointly review and ensure QA approval of analytical transfer protocols with Nuvelo for the clot lysis and appearance assays. |
| 6.2.3. | Execute protocols and report data. |
| 6.2.4. | Generate validation reports. |
| 6.3. | Deliverables |
| 6.3.1. | Validated method transfer reports. |
Page 5 of 11
Schedule 1 Work Programme
Stage 7. Assay Transfer
| 7.1. | Objectives |
| 7.1.1. | To transfer the non-validated Amgen analytical methods to Avecia. |
| 7.1.2. | To transfer the non-validated Amgen analytical methods identified to be outsourced in Stage 1.2.2 to Avecia approved outsourced testing houses. |
| 7.2. | Activities |
| 7.2.1. | Receive appropriate samples, historical data and reference standard from Nuvelo. |
| 7.2.2. | Jointly review and ensure QA approval of analytical transfer protocols with Nuvelo for non-validated assays defined in stage 1.2.2. |
| 7.2.3. | Jointly review additional work required on assays to prepare for assay validation. |
| 7.2.4. | Execute transfer protocols and report data. |
| 7.2.5. | Generate transfer reports as determined by Nuvelo. |
7.3. Deliverables
| 7.3.1. | Method transfer data and reports (if required) for each assay. |
Stage 8. Assay Validation
| 8.1. | Objective |
| 8.1.1. | To ensure validation of all the analytical methods identified by (V) in analytical table from Stage 1.2.2 of the work programme, for API Release testing during the validation batches. |
| 8.2. | Activities |
| 8.2.1. | Generate validation sub-plan for analytical assays |
| 8.2.2. | Jointly review and ensure QA approval of analytical validation protocols with Nuvelo for non-validated assays identified by (V) in the analytical table from Stage 1.2.2 of the work programme. |
| 8.2.3. | Execute validation protocols in accordance with ICH Q2A and Q2B and report data. |
| 8.2.4. | Generate validation reports. |
| 8.3. | Deliverables |
| 8.3.1. | Method validation data and reports for each assay. |
Stage 9. Laboratory Process Characterisation (LPC)
| 9.1. | Objective |
| 9.1.1. | To evaluate key operational parameters and ranges for critical Process steps. |
| 9.2. | Activities for LPC experimental set #1 |
| 9.2.1. | Review and evaluate all Amgen technical reports and data from previous LPC studies. |
| 9.2.2. | Perform a RPN analysis. |
| 9.2.3. | Perform Fermentation Studies |
| 9.2.3.1. | Investigate degradation of seed fermenter media on sterilisation. |
| 9.2.3.2. | Investigate the addition of antifoam (heating to filter sterilise) for addition to fermenter. |
| 9.2.3.3. | Investigate seed fermenter inoculum volume. |
Page 6 of 11
Schedule 1 Work Programme
| 9.2.3.4. | Investigate the change over to time triggers at a specified feed flow rate. |
| 9.2.4. | Perform Purification Studies |
| 9.2.4.1. | Investigate the SPHP step to determine the optimum UV cut-off range. |
| 9.2.4.2. | Investigate the SPHP step and reduction in gradient range during elution. |
| 9.2.4.3. | Investigate the temperature of operation during the IMAC step. |
| 9.2.4.4. | Investigate the requirement for wash step #2 during the IMAC step. |
| 9.2.4.5. | Investigate the pH of the load and buffer material during the IMAC step. |
| 9.3. | Deliverables |
| 9.3.1. | RPN analysis report |
| 9.3.2. | LPC Experimental set 1 Fermentation technical report |
| 9.3.3. | LPC Experimental set 1 Purification technical report |
Stage 10. Fermentation Scale-Up and Centrifuge Trials at 3000L scale
| 10.1. | Objectives |
| 10.1.1. | Release of working cell bank (WCB) for cGMP use |
| 10.1.2. | To provide the first data on fermentation performance at 3000L scale |
| 10.1.3. | To provide early definition of centrifuge operating conditions which can only be determined empirically |
| 10.1.4. | To allow greater chance that the first cGMP Development batch would make useful Product and de-risk that stage of the programme. |
| 10.2. | Activities |
| 10.2.1. | Perform QC identity and purity testing of WCB and generate a Release Certificate of Analysis (CofA), approved by QA for the WCB. |
| 10.2.2. | Order all raw materials and consumables. |
| 10.2.3. | Generate draft manufacturing batch records. |
| 10.2.4. | Review and technical approval of a comparability procedure drafted by Nuvelo. |
| 10.2.5. | Carry out first 3000L fermentation and centrifuge trial. Analyse centrifuge performance data. |
| 10.2.6. | Carry out the second fermentation and centrifuge trial with parameters to mimic the operation of the first cGMP Development batch or with refined parameters based on failure of batch 1, if necessary. |
| 10.2.7. | Supply 4 x 50 L fermentation broth for lab-scale purification. Store 2 x 50L of fermentation broth for future purifications. |
| 10.2.8. | Perform 2 x 50L purification using broth from centrifuge trials to supply research grade material to Nuvelo. |
| 10.2.9. | Generate manufacturing trial summary report. |
| 10.3. | Deliverables |
| 10.3.1. | QC document for cell bank testing. |
| 10.3.2. | Release CofA for the WCB. |
| 10.3.3. | Draft manufacturing batch records. |
| 10.3.4. | Research grade material. |
Page 7 of 11
Schedule 1 Work Programme
| 10.3.5. | Manufacturing batch summary report. |
| 10.3.6. | Comparability report. |
Stage 11. cGMP Preparation at 3000L scale
| 11.1. | Objectives |
| 11.1.1. | To prepare for cGMP manufacture. |
| 11.2. | Activities |
| 11.2.1. | Generate a Process specification document. |
| 11.2.2. | Generate cGMP manufacturing batch records. |
| 11.2.3. | Generate QC document detailing the Release Product Specification (refer to Appendix 2). |
| 11.2.4. | Order all raw materials and consumables. |
| 11.2.5. | Review and technical approval of a comparability procedure drafted by Nuvelo. |
| 11.3. | Deliverables |
| 11.3.1. | Avecia QA approved Process specification document. |
| 11.3.2. | Avecia QA approved cGMP manufacturing batch records. |
| 11.3.3. | Avecia QA approved QC Document (Product Specification). |
Stage 12. Two (2) cGMP Development Batches at 3000L scale
| 12.1. | Objectives |
| 12.1.1. | To perform two (2) cGMP batches at 3000L scale |
| 12.1.2. | To supply cGMP Product from at least 1 x 3000L Batch for clinical use. |
| 12.2. | Activities |
| 12.2.1. | Run the first cGMP Development batch at 3000L scale. |
| 12.2.2. | Perform limited analytical testing according to Appendix 7 of the Quality Agreement. |
| 12.2.3. | Perform full analytical testing for API Release of the first batch (to be jointly agreed and conditional on all limited testing performed in stage 12.2.2 being completed successfully). |
| 12.2.4. | Perform QA batch disposition for API Release of first batch if all Release testing was performed. |
| 12.2.5. | Run the second cGMP Development batch at 3000L scale. |
| 12.2.6. | Perform limited analytical testing according to Appendix 7 of the Quality Agreement. |
| 12.2.7. | Perform full analytical testing for API Release of the second batch (to be jointly agreed and conditional on all limited testing performed in stage 12.2.6 being completed successfully). |
| 12.2.8. | Perform QA batch disposition for API Release of second batch. |
| 12.2.9. | Generate manufacturing batch summary reports |
| 12.2.10. | Review the Process assumptions and cycle times as part of pre-validation review. |
| 12.3. | Deliverables |
| 12.3.1. | Released cGMP API from at least 1 cGMP Development Batch to be supplied for clinical use. |
| 12.3.2. | Any material not Released for clinical use will be made available to Nuvelo for research purposes. |
| 12.3.3. | Manufacturing batch summary reports. |
Page 8 of 11
Schedule 1 Work Programme
Stage 13. Validation Documentation
| 13.1. | Objective |
| 13.1.1. | To generate and QA approval of validation documentation to support successful validation campaign. |
| 13.2. | Activities |
| 13.2.1. | Generate and approve the validation documentation shown below. |
| Document |
Purpose |
Accountable Function |
Approving Functions (Final formal Release by QA) | |||
| Validation Master Plan | Defines overview Validation strategy for Product | QA | QA,QC, M, R&D | |||
| Validation Sub Plans | Defines specific Validation plans for Project relating to Equipment Qualification Cleaning Validation Analytical Validation Process Validation Electronic systems Training |
Various | QA,QC, M, R&D | |||
| Process Validation Sub Plan | Defines specific activities to be undertaken in Process Validation programme And links these to relevant technical reports and the Development Report |
R&D | QA,QC, M, R&D | |||
| Process Validation Protocol | Document listing: Runs to be undertaken in the manufacturing campaign, the expected specification of material produced (and the acceptances ranges for this). Critical process control parameters and acceptance ranges. The results derived from the Process Validation Campaign shall be recorded in this document |
R&D | QA,QC, M, R&D | |||
| Process Validation Sub Plan Report | Report detailing and analysing the outcome and data derived from the Process Validation Campaign | R&D | QA,QC, M, R&D | |||
| Validation Summary Report | Summary document reviewing output from Validation Programme and formally closing it. | QA | QA,QC, M, R&D | |||
Page 9 of 11
Schedule 1 Work Programme
| 13.3. | Deliverables |
| 13.3.1. | Avecia QA approved validation documentation as listed above. |
Stage 14. One (1) Engineering Batch at 3000L scale
| 14.1. | Objectives |
| 14.1.1. | To re-familiarise with the Process after the extended hold period. |
| 14.1.2. | To de-risk the validation batches. |
| 14.2. | Activities |
| 14.2.1. | Order all raw materials and consumables. |
| 14.2.2. | Run one (1) engineering batch. |
| 14.2.3. | Perform limited analytical testing according to Appendix 4. |
| 14.3. | Deliverables |
| 14.3.1. | Manufacturing batch summary report. |
Stage 15. Up to Five (5) Validation Batches at 3000L scale
| 15.1. | Objective |
| 15.1.1. | To demonstrate consistent successful cGMP manufacture by performing up to five (5) consecutive batches with at least 3 consecutive batches conforming to approved validation documentation. |
| 15.1.2. | Perform Release Testing during validation batches. |
| 15.2. | Activities |
| 15.2.1. | Run up to five (5) consecutive cGMP manufacturing batches as per the approved validation documentation. |
| 15.2.2. | Justify both Process and Product quality conformance for three (3) consecutive batches as per the validation documentation. |
| 15.2.3. | Perform QA batch disposition for API Release of all acceptable Batches. |
| 15.3. | Deliverables |
| 15.3.1. | Released cGMP API, from 3 consecutive batches, to be supplied for clinical use. |
| 15.3.2. | Validation summary report. |
Page 10 of 11
Schedule 1 Work Programme
Appendix 1 Process Assumptions
Cycle Times
Fermentation & Harvest
The fermentation Process (from inoculation of the production vessel through to harvesting of the last of the culture) is estimated to be completed in no more than seven (7) days (168 hours). Occupation of any one (1) inoculum vessel is also required for no more than one (1) day (24 hours).
Purification
The Process is estimated to require no more than six (6) days (144 hours) occupation of the purification area for the process of each complete fermentation batch (including dispensing into final bulk containers).
The total cycle time for each Batch is estimated to be no more than fourteen (14) days in the ABC 5000 facility.
Occupancy times include time required for key in process control tests to be completed.
Appendix 2 B2422 Work Programme Timeline (see attachment)
Page 11 of 11
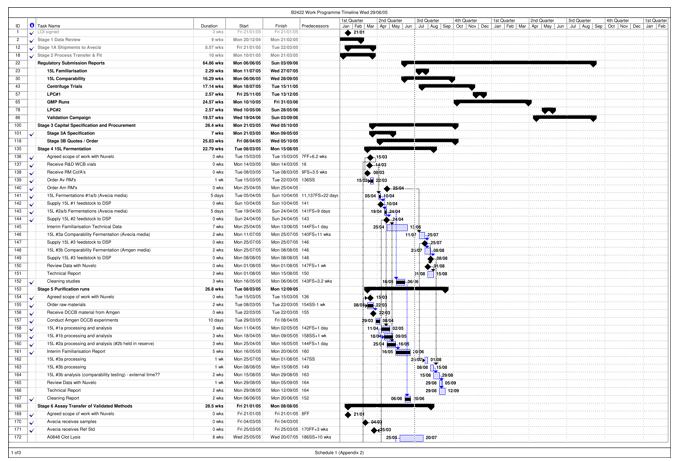
B2422 Work Programme Timeline Wed 29/06/05 ID Task Name Duration Start Finish Predecessors
1 LOI signed 0 wks Fri 21/01/05 Fri 21/01/05 2 Stage 1 Data Review 9 wks Mon 20/12/04 Mon 21/02/05
12 Stage 1A Shipments to Avecia 8.57 wks Fri 21/01/05 Tue 22/03/05 18 Stage 2 Process Transfer & Fit 10 wks Mon 10/01/05 Mon 21/03/05
22 Regulatory Submission Reports 64.86 wks Mon 06/06/05 Sun 03/09/06 23 15L Familiarisation 2.29 wks Mon 11/07/05 Wed 27/07/05
30 15L Comparability 16.29 wks Mon 06/06/05 Wed 28/09/05 43 Centrifuge Trials 17.14 wks Mon 18/07/05 Tue 15/11/05
57 LPC#1 2.57 wks Fri 25/11/05 Tue 13/12/05 65 GMP Runs 24.57 wks Mon 10/10/05 Fri 31/03/06
78 LPC#2 2.57 wks Wed 10/05/06 Sun 28/05/06 86 Validation Campaign 19.57 wks Wed 19/04/06 Sun 03/09/06
100 Stage 3 Capital Specification and Procurement 28.4 wks Mon 21/03/05 Wed 05/10/05 101 Stage 3A Specification 7 wks Mon 21/03/05 Mon 09/05/05
118 Stage 3B Quotes / Order 25.83 wks Fri 08/04/05 Wed 05/10/05 135 Stage 4 15L Fermentation 22.79 wks Tue 08/03/05 Mon 15/08/05
136 Agreed scope of work with Nuvelo 0 wks Tue 15/03/05 Tue 15/03/05 7FF+6.2 wks 137 Receive R&D WCB vials 0 wks Mon 14/03/05 Mon 14/03/05 16
138 Receive RM CofA’s 0 wks Tue 08/03/05 Tue 08/03/05 9FS+3.5 wks 139 Order Av RM’s 1 wk Tue 15/03/05 Tue 22/03/05 136SS
140 Order Am RM’s 0 wks Mon 25/04/05 Mon 25/04/05 141 15L Fermentations #1a/b (Avecia media) 5 days Tue 05/04/05 Sun 10/04/05 11,137FS+22 days
142 Supply 15L #1 feedstock to DSP 0 wks Sun 10/04/05 Sun 10/04/05 141 143 15L #2a/b Fermentations (Avecia media) 5 days Tue 19/04/05 Sun 24/04/05 141FS+9 days
144 Supply 15L #2 feedstock to DSP 0 wks Sun 24/04/05 Sun 24/04/05 143 145 Interim Familiarisation Technical Data 7 wks Mon 25/04/05 Mon 13/06/05 144FS+1 day
146 15L #3a Comparability Fermentation (Avecia media) 2 wks Mon 11/07/05 Mon 25/07/05 140FS+11 wks 147 Supply 15L #3 feedstock to DSP 0 wks Mon 25/07/05 Mon 25/07/05 146
148 15L #3b Comparability Fermentation (Amgen media) 2 wks Mon 25/07/05 Mon 08/08/05 146 149 Supply 15L #3 feedstock to DSP 0 wks Mon 08/08/05 Mon 08/08/05 148
150 Review Data with Nuvelo 0 wks Mon 01/08/05 Mon 01/08/05 147FS+1 wk 151 Technical Report 2 wks Mon 01/08/05 Mon 15/08/05 150
152 Cleaning studies 3 wks Mon 16/05/05 Mon 06/06/05 143FS+3.2 wks 153 Stage 5 Purification runs 26.8 wks Tue 08/03/05 Mon 12/09/05
154 Agreed scope of work with Nuvelo 0 wks Tue 15/03/05 Tue 15/03/05 136 155 Order raw materials 2 wks Tue 08/03/05 Tue 22/03/05 154SS-1 wk
156 Receive DCCB material from Amgen 0 wks Tue 22/03/05 Tue 22/03/05 155 157 Conduct Amgen DCCB experiments 10 days Tue 29/03/05 Fri 08/04/05
158 15L #1a processing and analysis 3 wks Mon 11/04/05 Mon 02/05/05 142FS+1 day 159 15L #1b processing and analysis 3 wks Mon 18/04/05 Mon 09/05/05 158SS+1 wk
160 15L #2a processing and analysis (#2b held in reserve) 3 wks Mon 25/04/05 Mon 16/05/05 144FS+1 day 161 Interim Familiarisation Report 5 wks Mon 16/05/05 Mon 20/06/05 160
162 15L #3a processing 1 wk Mon 25/07/05 Mon 01/08/05 147SS 163 15L #3b processing 1 wk Mon 08/08/05 Mon 15/08/05 149
164 15L #3b analysis (comparability testing)—external time?? 2 wks Mon 15/08/05 Mon 29/08/05 163 165 Review Data with Nuvelo 1 wk Mon 29/08/05 Mon 05/09/05 164
166 Technical Report 2 wks Mon 29/08/05 Mon 12/09/05 164 167 Cleaning Report 2 wks Mon 06/06/05 Mon 20/06/05 152
168 Stage 6 Assay Transfer of Validated Methods 28.5 wks Fri 21/01/05 Mon 08/08/05 169 Agreed scope of work with Nuvelo 0 wks Fri 21/01/05 Fri 21/01/05 8FF
170 Avecia receives samples 0 wks Fri 04/03/05 Fri 04/03/05 171 Avecia receives Ref Std 0 wks Fri 25/03/05 Fri 25/03/05 170FF+3 wks
172 A0848 Clot Lysis 8 wks Wed 25/05/05 Wed 20/07/05 186SS+10 wks
0xx Xxxxxxx 0xx Xxxxxxx 0xx Xxxxxxx 0xx Quarter 1st Quarter 2nd Quarter 3rd Quarter 4th Quarter 1st Quarter Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb
21/01
15/03 14/03 08/03 15/03 22/03
25/04 05/04 10/04 10/04 19/04 24/04 24/04
25/04 13/06
11/07 25/07 25/07 25/07 08/08 08/08 01/08 01/08 15/08 16/05 06/06
15/03 08/03 22/03 22/03 29/03 08/04 11/04 02/05 18/04 09/05 25/04 16/05 16/05 20/06
25/07 01/08 08/08 15/08 15/08 29/08 29/08 05/09 29/08 12/09 06/06 20/06
21/01
04/03 25/03
25/05 20/07
1 of 3 Schedule 1 (Appendix 2)
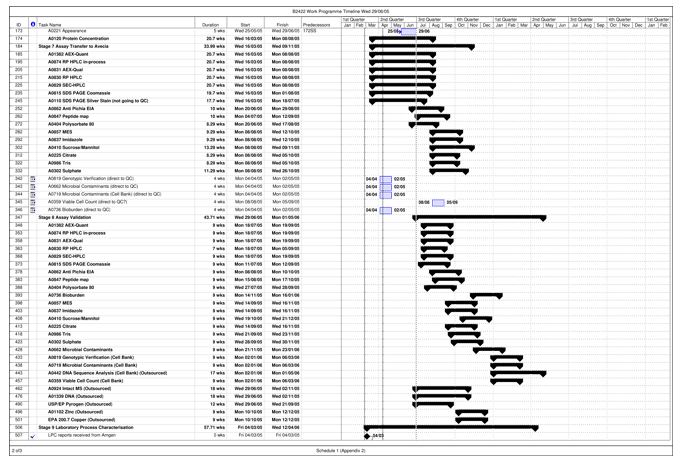
B2422 Work Programme Timeline Wed 29/06/05 ID Task Name Duration Start Finish
173 A0221 Appearance 5 wks Wed 25/05/05 Wed 29/06/05 174 A0120 Protein Concentration 20.7 wks Wed 16/03/05 Mon 08/08/05
184 Stage 7 Assay Transfer to Avecia 33.99 wks Wed 16/03/05 Wed 09/11/05 185 A01382 AEX-Quant 20.7 wks Wed 16/03/05 Mon 08/08/05
195 A0874 RP HPLC in-process 20.7 wks Wed 16/03/05 Mon 08/08/05 205 A0831 AEX-Qual 20.7 wks Wed 16/03/05 Mon 08/08/05
215 A0830 RP HPLC 20.7 wks Wed 16/03/05 Mon 08/08/05 225 A0829 SEC-HPLC 20.7 wks Wed 16/03/05 Mon 08/08/05
235 A0815 SDS PAGE Coomassie 19.7 wks Wed 16/03/05 Mon 01/08/05 245 A0110 SDS PAGE Silver Stain (not going to QC) 17.7 wks Wed 16/03/05 Mon 18/07/05
252 A0862 Anti Pichia EIA 10 wks Mon 20/06/05 Mon 29/08/05 262 A0847 Peptide map 10 wks Mon 04/07/05 Mon 12/09/05
272 A0404 Polysorbate 80 8.29 wks Mon 20/06/05 Wed 17/08/05 282 A0857 MES 9.29 wks Mon 08/08/05 Wed 12/10/05
292 A0837 Imidazole 9.29 wks Mon 08/08/05 Wed 12/10/05 302 A0410 Sucrose/Mannitol 13.29 wks Mon 08/08/05 Wed 09/11/05
312 A0225 Citrate 8.29 wks Mon 08/08/05 Wed 05/10/05 322 A0986 Tris 8.29 wks Mon 08/08/05 Wed 05/10/05
332 A0302 Sulphate 11.29 wks Mon 08/08/05 Wed 26/10/05 342 A0819 Genotypic Verification (direct to QC) 4 wks Mon 04/04/05 Mon 02/05/05
343 A0662 Microbial Contaminants (ditrect to QC) 4 wks Mon 04/04/05 Mon 02/05/05 344 A0719 Microbial Contaminants (Cell Bank) (ditrect to QC) 4 wks Mon 04/04/05 Mon 02/05/05
345 A0359 Viable Cell Count (direct to QC?) 4 wks Mon 08/08/05 Mon 05/09/05 346 A0736 Bioburden (direct to QC) 4 wks Mon 04/04/05 Mon 02/05/05
347 Stage 8 Assay Validation 43.71 wks Wed 29/06/05 Mon 01/05/06 348 A01382 AEX-Quant 9 wks Mon 18/07/05 Mon 19/09/05
353 A0874 RP HPLC in-process 9 wks Mon 18/07/05 Mon 19/09/05 358 A0831 AEX-Qual 9 wks Mon 18/07/05 Mon 19/09/05
363 A0830 RP HPLC 7 wks Mon 18/07/05 Mon 05/09/05
368 A0829 SEC-HPLC 9 wks Mon 18/07/05 Mon 19/09/05 373 A0815 SDS PAGE Coomassie 9 wks Mon 11/07/05 Mon 12/09/05
378 A0862 Anti Pichia EIA 9 wks Mon 08/08/05 Mon 10/10/05 383 A0847 Peptide map 9 wks Mon 15/08/05 Mon 17/10/05
388 A0404 Polysorbate 80 9 wks Wed 27/07/05 Wed 28/09/05 393 A0736 Bioburden 9 wks Mon 14/11/05 Mon 16/01/06
398 A0857 MES 9 wks Wed 14/09/05 Wed 16/11/05 403 A0837 Imidazole 9 wks Wed 14/09/05 Wed 16/11/05
408 A0410 Sucrose/Mannitol 9 wks Wed 19/10/05 Wed 21/12/05 413 A0225 Citrate 9 wks Wed 14/09/05 Wed 16/11/05
418 A0986 Tris 9 wks Wed 21/09/05 Wed 23/11/05 423 A0302 Sulphate 9 wks Wed 28/09/05 Wed 30/11/05
428 A0662 Microbial Contaminants 9 wks Mon 21/11/05 Mon 23/01/06 433 A0819 Genotypic Verification (Cell Bank) 9 wks Mon 02/01/06 Mon 06/03/06
438 A0719 Microbial Contaminants (Cell Bank) 9 wks Mon 02/01/06 Mon 06/03/06 443 A0442 DNA Sequence Analysis (Cell Bank) (Outsourced) 17 wks Mon 02/01/06 Mon 01/05/06
457 A0359 Viable Cell Count (Cell Bank) 9 wks Mon 02/01/06 Mon 06/03/06 462 A0924 Intact MS (Outsourced) 18 wks Wed 29/06/05 Wed 02/11/05
476 A01339 DNA (Outsourced) 18 wks Wed 29/06/05 Wed 02/11/05 490 USP/EP Pyrogen (Outsourced) 12 wks Wed 29/06/05 Wed 21/09/05
496 A01102 Zinc (Outsourced) 9 wks Mon 10/10/05 Mon 12/12/05 501 EPA 200.7 Copper (Outsourced) 9 wks Mon 10/10/05 Mon 12/12/05
506 Stage 9 Laboratory Process Characterisation 57.71 wks Fri 04/03/05 Wed 12/04/06 507 LPC reports received from Amgen 0 wks Fri 04/03/05 Fri 04/03/05
1st Quarter 2nd Quarter 3rd Quarter 4th Quarter 1st Quarter 2nd Quarter 3rd Quarter 4th Quarter 1st Quarter Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb
25/05 29/06
04/04 02/05 04/04 02/05 04/04 02/05
08/08 05/09 04/04 02/05
04/03
2 of 3 Schedule 1 (Appendix 2)
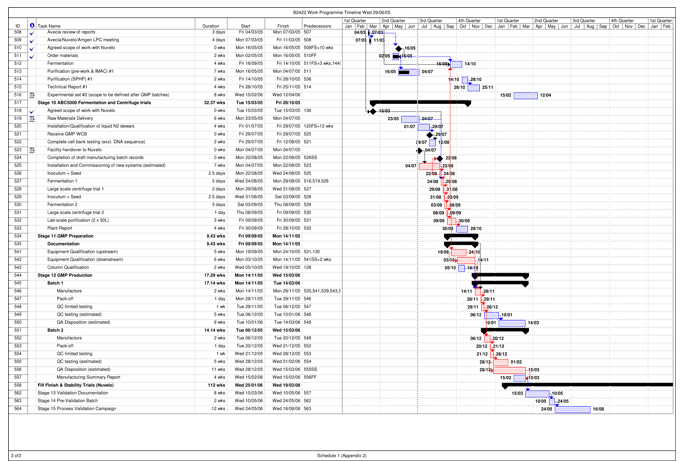
B2422 Work Programme Timeline Wed 29/06/05 ID Task Name Duration Start Finish Predecessors
508 Avecia review of reports 3 days Fri 04/03/05 Mon 07/03/05 507 509 Avecia/Nuvelo/Amgen LPC meeting 4 days Mon 07/03/05 Fri 11/03/05 508
510 Agreed scope of work with Nuvelo 0 wks Mon 16/05/05 Mon 16/05/05 508FS+10 wks 511 Order materials 2 wks Mon 02/05/05 Mon 16/05/05 510FF
512 Fermentation 4 wks Fri 16/09/05 Fri 14/10/05 511FS+3 wks,14416/004/10 513 Purification (pre-work & IMAC) #1 7 wks Mon 16/05/05 Mon 04/07/05 511
514 Purification (SPHP) #1 2 wks Fri 14/10/05 Fri 28/10/05 536 515 Technical Report #1 4 wks Fri 28/10/05 Fri 25/11/05 514
516 Experimental set #2 (scope to be defined after GMP batches) 8 wks Wed 15/02/06 Wed 12/04/06 517 Stage 10 ABC5000 Fermentation and Centrifuge trials 32.37 wks Tue 15/03/05 Fri 28/10/05
518 Agreed scope of work with Nuvelo 0 wks Tue 15/03/05 Tue 15/03/05 136 519 Raw Materials Delivery 6 wks Mon 23/05/05 Mon 04/07/05
520 Installation/Qualification of liquid N2 dewars 4 wks Fri 01/07/05 Fri 29/07/05 120FS+12 wks 521 Receive GMP WCB 0 wks Fri 29/07/05 Fri 29/07/05 520
522 Complete cell bank testing (excl. DNA sequence) 2 wks Fri 29/07/05 Fri 12/08/05 521 523 Facility handover to Nuvelo 0 wks Mon 04/07/05 Mon 04/07/05
524 Completion of draft manufacturing batch records 0 wks Mon 22/08/05 Mon 22/08/05 526SS 525 Installation and Commissioning of new systems (estimated) 7 wks Mon 04/07/05 Mon 22/08/05 523
526 Inoculum + Seed 2.5 days Mon 22/08/05 Wed 24/08/05 525 527 Fermentation 1 5 days Wed 24/08/05 Mon 29/08/05 518,519,526
528 Large scale centrifuge trial 1 2 days Mon 29/08/05 Wed 31/08/05 527 529 Inoculum + Seed 2.5 days Wed 31/08/05 Sat 03/09/05 528
530 Fermentation 2 5 days Sat 03/09/05 Thu 08/09/05 529 531 Large scale centrifuge trial 2 1 day Thu 08/09/05 Fri 09/09/05 530
532 Lab-scale purification (2 x 50L) 3 wks Fri 09/09/05 Fri 30/09/05 531 533 Plant Report 4 wks Fri 30/09/05 Fri 28/10/05 532
534 Stage 11 GMP Preparation 9.43 wks Fri 09/09/05 Mon 14/11/05 535 Documentation 9.43 wks Fri 09/09/05 Mon 14/11/05
541 Equipment Qualification (upstream) 5 wks Mon 19/09/05 Mon 24/10/05 531,130 542 Equipment Qualification (downstream) 6 wks Mon 03/10/05 Mon 14/11/05 541SS+2 wks
543 Column Qualification 2 wks Wed 05/10/05 Wed 19/10/05 128 544 Stage 12 GMP Production 17.29 wks Mon 14/11/05 Wed 15/03/06 545 Batch 1 17.14 wks Mon 14/11/05 Tue 14/03/06 546 Manufacture 2 wks Mon 14/11/05 Mon 28/11/05 535,541,539,543,5
547 Pack-off 1 day Mon 28/11/05 Tue 29/11/05 546 548 QC limited testing 1 wk Tue 29/11/05 Tue 06/12/05 547 549 QC testing (estimated) 5 wks Tue 06/12/05 Tue 10/01/06 548 550 QA Disposition (estimated) 9 wks Tue 10/01/06 Tue 14/03/06 549
551 Batch 2 14.14 wks Tue 06/12/05 Wed 15/03/06 552 Manufacture 2 wks Tue 06/12/05 Tue 20/12/05 548 553 Pack-off 1 day Tue 20/12/05 Wed 21/12/05 552 554 QC limited testing 1 wk Wed 21/12/05 Wed 28/12/05 553
555 QC testing (estimated) 5 wks Wed 28/12/05 Wed 01/02/06 554 556 QA Disposition (estimated) 11 wks Wed 28/12/05 Wed 15/03/06 555SS
557 Manufacturing Summary Report 4 wks Wed 15/02/06 Wed 15/03/06 556FF 558 Fill Finish & Stability Trials (Nuvelo) 112 wks Wed 25/01/06 Wed 19/03/08
562 Stage 13 Validation Documentation 8 wks Wed 15/03/06 Wed 10/05/06 557 563 Stage 14 Pre-Validation Batch 2 wks Wed 10/05/06 Wed 24/05/06 562 564 Stage 15 Process Validation Campaign 12 wks Wed 24/05/06 Wed 16/08/06 000
0xx Xxxxxxx 0xx Xxxxxxx 0xx Xxxxxxx 0xx Quarter 1st Quarter 2nd Quarter 3rd Quarter 4th Quarter 1st Quarter Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb
04/03 07/03 07/03 11/03 16/05 02/05 16/05 16/09 14/10 16/05 04/07 14/10 28/10 28/10 25/11 15/02 12/04 15/03
23/05 04/07 01/07 29/07 29/07 29/07 12/08 04/07 22/08 04/07 22/08 22/08 24/08 24/08 29/08 29/08 31/08 31/08 03/09 03/09 08/09 08/09 09/09 09/09 30/09 30/09 28/10
19/09 24/10 03/10 14/11 05/10 19/10 14/11 28/11 28/11 29/11 29/11 06/12 06/12 10/01 10/01 14/03
06/12 20/12 20/12 21/12 21/12 28/12 28/12 01/02 28/12 15/03 15/02 15/03 15/03 10/05 10/05 24/05 24/05 16/08
3 of 3 Schedule 1 (Appendix 2)
QUALITY AGREEMENT
Nuvelo, Inc.
000 Xxxxxxx Xxxxxx
Xxxxxxxxx, XX 00000
and
Avecia Biotechnology
Biologics Business
XX Xxx 0
Xxxxxxx Xxxxxx
Xxxxxxxxxx
Xxxxxxxxx
XX00 0XX
| Name | Name | |||||||
| Xxxxx X Xxxxxxx | Xxxxx X Xxxxxxx | |||||||
| Head of Quality and Regulatory Affairs | Sr. Director RA/QA | |||||||
| Date | Date | |||||||
| Date 30 Jun 05 | Modification 0 | Page 1 of 19 |
Index
| 1. | Quality Agreement | 4 | ||||
| 1.1 | Purpose |
4 | ||||
| 1.2 | Duration of Agreement |
4 | ||||
| 1.3 | API |
4 | ||||
| 1.4 | Regulatory Compliance |
4 | ||||
| 2. | Manufacturing cGMP Compliance | 5 | ||||
| 2.1 | Materials |
5 | ||||
| 2.2 | Documentation |
5 | ||||
| 2.3 | Change Control |
6 | ||||
| 2.4 | Process Control |
7 | ||||
| 2.5 | Training |
7 | ||||
| 2.6 | Calibration |
8 | ||||
| 3. | Quality Control | 8 | ||||
| 4. | Stability | 8 | ||||
| 5. | Quality Assurance | 9 | ||||
| 5.1 | Documentation |
9 | ||||
| 5.2 | Batch Disposition |
9 | ||||
| 5.3 | Internal Audits |
10 | ||||
| 5.4 | Audits by Nuvelo |
10 | ||||
| 5.5 | Regulatory Actions |
10 | ||||
| 6. | Dispute Resolution | 11 | ||||
| 7. | Qualification | 11 | ||||
| 7.1 | Equipment Computer Facility and Utility Qualification |
12 | ||||
| 7.2 | Laboratory Qualification |
12 | ||||
| 7.3 | Cleaning Verification and Validation |
12 | ||||
| Date 30 Jun 05 | Modification 0 | Page 2 of 19 |
| 8. |
Revisions |
12 | ||
| Appendix 1 |
Glossary of terms |
|||
| Appendix 2 |
Responsibilities |
|||
| Appendix 3 |
List of Contacts |
|||
| Appendix 4 |
Batch Disposition Roles and Responsibilities |
|||
| Appendix 5 |
Responsibilities for Shipment of the Manufactured API |
|||
| Appendix 6 |
Draft Release Testing Specification and Criteria for Acceptance for Bulk Alfimeprase API |
|||
| Appendix 7 |
Draft Limited Testing and Criteria for Acceptance to Demonstrate Acceptability of Process Performance for Bulk Alfimeprase API | |||
| Date 30 Jun 05 | Modification 0 | Page 3 of 19 |
| 1. | Quality Agreement |
| 1.1 | Purpose |
| 1.1.1 | This agreement defines the roles and responsibilities for both Avecia and Nuvelo with respect to quality assurance and regulatory compliance matters. |
| 1.1.2 | This Quality agreement also defines how Avecia Quality Unit and Nuvelo Quality Unit will interact with each other over Quality related issues. |
| 1.1.3 | This Quality agreement shall be incorporated with and constitute a part of the Commercial Agreement between Avecia and Nuvelo. |
| 1.2 | Duration of Agreement |
| 1.2.1 | The Quality agreement will be effective as of the Commencement Date of the Commercial Agreement and will expire with the termination of the Commercial Agreement. |
| 1.2.2 | Avecia QA will review, and up-date the Quality Agreement accordingly, in agreement with Nuvelo QA on completion of the centrifuge trial runs, GMP runs and prior to the Validation runs. |
| 1.3 | API |
| 1.3.1 | Avecia will manufacture all bulk Active Pharmaceutical Ingredient (API) in accordance with cGMP regulations. |
| 1.4 | Regulatory Compliance |
| 1.4.1 | Avecia will manufacture the API in compliance with all applicable EU regulations including the Rules and Guidance for Pharmaceutical Manufacturers and Distributors 2002 Annexe 18 GMP for API in the EU. |
| 1.4.2 | Avecia will manufacture the API in compliance with ICHQ7A, as incorporated in the Federal Register Vol 66 No186 (formerly ICHQ7A) and those sections applicable within the FDA regulations 210, 211, 600, 601 and 610. |
| 1.4.3 | Avecia will maintain all licenses and approvals necessary to manufacture the API and comply with all FDA regulations applicable to a manufacturer. |
| 1.4.4 | Avecia will test and release the API using validated analytical methods in compliance with FDA regulations and the most current versions of ICH guidance, including ICHQ2A, “Text on Validation of Analytical Procedures and ICHQ2B, “ Validation of Analytical Procedures: Methodology”. The analytical methods will be validated by Avecia prior to the execution of the validation lots. |
| 1.4.5 | Avecia will ensure that it is in compliance with all computer and software validation requirements in accordance to 21 CFR Part 11. |
| Date 30 Jun 05 | Modification 0 | Page 4 of 19 |
| 1.4.6 | Avecia will identify to Nuvelo in writing all raw materials in the product of direct or indirect animal origin. Avecia will source these materials from countries which are not listed in 9 CFR section 94.18 and further, the materials must have supporting Certificates of Compliance as required by the European Union Directive R 999/2001. |
| 2. | Manufacturing cGMP Compliance |
| 2.1 | Materials |
| 2.1.1 | Avecia will use raw materials, packaging and labelling components that are acceptable to Nuvelo and sampled, tested via methods approved by Avecia and stored in accordance with Avecia documentation and procedures. |
| 2.1.2 | Avecia is responsible for ensuring that all materials procured by Avecia for use in the API are in compliance with specifications for such raw materials. Avecia are also responsible for ensuring the raw materials are acceptable to Nuvelo for use in the manufacture of the API. Nuvelo shall confirm to Avecia the acceptability of the raw materials in writing. Raw materials are given an expiration date upon the satisfactory completion of all initial tests. Avecia will hold the relevant Certificates of Analysis for the materials. |
| 2.1.3 | Avecia will purchase raw materials from suppliers that are acceptable to Nuvelo and approved by Avecia. Nuvelo shall confirm to Avecia the acceptability of the suppliers in writing. |
| 2.1.4 | Avecia and Nuvelo will jointly agree to a list of key suppliers who will be audited by Avecia to ensure that they are in compliance with the FDA and EU regulations. |
| 2.2 | Documentation |
| 2.2.1 | Avecia will prepare the necessary documentation for each lot of API in its standard format as evidence that the API was manufactured in compliance with current GMP. |
| 2.2.2 | Avecia will retain batch documentation for at least one year after the expiry date of the batch, five years after the records of manufacture have been completed, or three years after the distribution of the batch whichever is the larger. Before disposing of batch documentation, Avecia will give advanced written warning of 3 months to Nuvelo. |
| 2.2.3 | Avecia will store the API under conditions approved by Nuvelo. Avecia will hold records of storage conditions according to Avecia’s Standard Operating Procedures. |
| 2.2.4 | Avecia and Nuvelo will agree on storage conditions, containers and delivery configuration. Avecia is responsible for packaging and labelling the API for shipment, and delivery of the API. Avecia will co-operate with Nuvelo for shipping arrangements. Avecia will deliver the API in validated shipping containers under validated shipping conditions. Until the shipping conditions have been validated, Avecia will deliver the API with temperature monitoring devices ready and capable of proper monitoring during shipment. Down loaded data from the temperature monitoring devices shall be sent to Nuvelo within 3 business days of the return of the Temp Tale.(see Appendix 5) |
| 2.2.5 | Delivery of API - Nuvelo will authorise Avecia to deliver upon submission of a Nuvelo delivery request form, to a location designated by Nuvelo. Delivery records, etc., will be documented and maintained by Avecia. |
| Date 30 Jun 05 | Modification 0 | Page 5 of 19 |
| 2.2.6 | Avecia will deliver samples upon the submission of a Nuvelo sample request form, and Avecia will co-operate with Nuvelo for transit arrangements. |
| 2.3 | Change Control |
These change activities will be documented in accordance with cGMP and shall be considered by Avecia to be records of the quality system, available for review and audit.
| 2.3.1 | Equipment |
Avecia shall provide Nuvelo written notice of any proposed changes to the processing equipment used to manufacture the API, including:
a) Design changes to process equipment.
b) Major repairs to process equipment, excluding routine preventative maintenance/repair activities. Replacement of equipment with identical equipment will be considered a major repair for the purposes of this agreement.
c) Scale up of lot size for manufacture of the component that does not involve use of alternative equipment.
| 2.3.2 | Components |
Avecia shall purchase components used to manufacture and package Nuvelo APIs in accordance with Nuvelo’s requirements. Avecia shall provide Nuvelo written notice of any proposed changes, including changes to the sources of, and processes for the manufacture for the raw materials, including packaging changes, to the specified raw materials or components used in the manufacture of the API. Avecia shall implement and maintain an appropriate quality agreement and purchasing controls with each raw material supplier, to ensure that Avecia is informed in advance of any such changes by its suppliers to raw material composition or manufacturing processes.
| 2.3.3 | Processes |
Avecia shall notify Nuvelo of planned process changes in writing, prior to implementation and subject to Nuvelo’s Quality Assurance (QA) and Process Sciences Manufacturing (PSM) groups’ approvals.
Prior to any change being implemented, Nuvelo shall be given the opportunity to purchase components and API made by the existing process as assurance of continuity of supply.
| 2.3.4 | Other Activities |
Other activities, listed below, shall be documented in accordance with cGMP and shall be considered by Avecia to be records of the quality system, available for review and audit.
| Date 30 Jun 05 | Modification 0 | Page 6 of 19 |
a) Adjustments necessary to operate equipment efficiently and safely for the manufacture of the API. These may be recorded on quality documents; however, such documentation is only a requirement where it is specified on the Standard Operating Procedures used in that part of the processing.
b) Changes to lots of raw materials to make the API. These will be recorded as required by the quality system.
| 2.4 | Process Control |
| 2.4.1 | Inspections |
a) Raw materials, intermediates, components, containers, and container closures for the API manufactured will be inspected upon receipt per requirements of Avecia quality system. Documentation of those inspections will be retained in the batch record and appropriate samples will be maintained by Avecia.
b) Avecia and Nuvelo will work together to determine the required in-process inspections. In-process inspections may occur during the manufacture of the API. These inspection activities will be documented in lab notebooks, Batch Sheets and /or Test Sheets, whichever is appropriate. A copy will be retained in the batch record.
c) Cleanliness : The API will be manufactured in an environment that is clean and meets current Avecia facility, regulatory and safety standards, specifically including cGMP. The facility will be monitored on a regular basis during manufacturing according to Avecia approved procedures.
d) Identification and Traceability Criteria: All materials for making the API must be properly identified and traceable. Identification shall include part number and lot number as a minimum. The quality status of each of these must also be identified. The quality status may be identified electronically, marked on the container or referenced to a lab notebook.
e) Acceptance Criteria: Acceptance of the API is based on achievement of the acceptance criteria in the API Specification identified by Nuvelo requirements. A draft Specification is attached to this Quality Agreement as Appendix 6.
f) The specification will be jointly approved by Avecia and Nuvelo QA prior to use in manufacturing cGMP API lots and the approved specification will be held in a separate QC document. In the cGMP Development Batches and the Engineering Batch identified in the Work Programme, a limited set of release tests to demonstrate the suitability of the API for full release will be performed prior to performance of all required release testing. A draft of the proposed limited testing for these batches is attached to this Quality Agreement in Appendix 7.
| 2.5 | Training |
Personnel manufacturing and/or testing the API must be trained appropriately on the equipment and methods used. This training must be documented in a training record. The training records will be maintained by Avecia for inspection.
| Date 30 Jun 05 | Modification 0 | Page 7 of 19 |
| 2.6 | Calibration |
All equipment used to manufacture, test or store raw or intermediate materials or final APIs will be calibrated to meet the requirements of NIST standards.
| 3. | Quality Control |
| 3.1 | All analytical methods will be validated prior to the execution of the validation lots in accordance with FDA regulation and ICH guidelines. |
| 3.2 | The testing activities for the API that are to be performed by Avecia QC will be in accordance with the specifications agreed upon by Avecia and Nuvelo. Avecia will not sub contract any testing without prior written approval from Nuvelo. All sub contractor analytical laboratories will be audited by Avecia. Avecia warrants that all sub contractors used will be FDA and EU GMP compliant. |
| 3.3 | Avecia will notify Nuvelo of any critical or major investigations and Out of Specification (OOS), Out of Trend (OOT) results related to the In process testing and final testing of the API within 72 hours or next working day (Monday-Friday). Nuvelo approval of such documentation is required and will be shown by sign-off on the back-page of the copy of the original investigation/OOS/OOT form faxed by Avecia QA to Nuvelo QA. Notification will be provided for minor investigations, OOS and OOT results on a weekly basis and no approval by Nuvelo QA is required. |
| 3.4 | Avecia QC will retain sufficient samples of raw materials and the API from each batch to allow at least twice the quantity necessary to determine whether it meets the API Specifications, a draft of which is attached hereto as Appendix 6, excluding bioburden and endotoxin testing. |
| 3.5 | Avecia QC will retain samples for at least 1 year after the expiration date of the last lot of drug API containing the API. Avecia will inform Nuvelo of this date and give advanced written warning of expiration of 3 months to Nuvelo. |
| 3.6 | Avecia QC will produce a Certificate of Analysis for each batch of API confirming that the API has been tested via approved methods in accordance with the Specification in the jointly approved QC document. Avecia will perform release testing for the API within 30 calendar days after the last day of manufacture. Avecia will ship the API to Nuvelo/Nuvelo designated site within 90 calendar days of API release. |
| 3.7 | Avecia QA will target a bioburden level of £10 CFU/100mL. |
| 4. | Stability |
| 4.1 | Nuvelo is responsible, where appropriate, for determining that a routine stability monitoring program is implemented. |
| 4.2 | Avecia will provide Nuvelo or its designee with stability samples within two weeks of the last day of manufacture of each Batch and of each validation lot. |
| Date 30 Jun 05 | Modification 0 | Page 8 of 19 |
| 5. | Quality Assurance |
| 5.1 | Documentation |
| 5.1.1 | Avecia QA is responsible for ensuring creation of the Nuvelo API specific manufacturing master documentation. Avecia QA and Nuvelo QA will approve the batch documentation before commencement of manufacturing. Nuvelo QA are responsible for ensuring that the batch manufacturing records issued by Avecia QA for approval are done so within 2 business days of receipt of the final draft. |
| 5.1.2 | Avecia QA will approve Standard Operating Procedures required for the manufacture of the API. |
| 5.1.3 | Avecia QA will inform Nuvelo of any major and critical Non Conformance Reports generated during the manufacture of each batch of API within 2 business days. Nuvelo sign-off is required on each critical or major non-conformance reports before closure of investigation. Notification will be provided for all minor non-conformances on a weekly basis. |
| 5.1.4 | Avecia QA will inform Nuvelo of any changes to the master batch records, bills of material specification and equipment. |
| 5.1.5 | Avecia QA will notify on a weekly basis via e-mail or e-room all Change Controls raised. All major and critical change controls raised will require Nuvelo sign off prior to implementation. |
| 5.2 | Batch Disposition |
| 5.2.1 | For each batch of API, Avecia QA will provide to Nuvelo copies of the release, executed batch record, Certificate of Analysis, Certificate of Conformance, any deviation/change control reports. Nuvelo will review and approve, as appropriate, the batch record and then accordingly forward a signed-off copy of the API disposition form to Avecia as confirmation of Nuvelo’s acceptance of the batch. |
| 5.2.2 | Avecia QA is responsible for ensuring that the API has been manufactured according to the Specifications attached hereto as Appendix 6, Standard Operating Procedures, Master Batch Records and cGMP. |
| 5.2.3 | Reprocess/Rework: Avecia QA will not internally authorise any rework without written prior approval from Nuvelo. Reprocessing may be performed at Avecia with internal approval. The details of the reprocessing will be included in the batch record. Approval from Regulatory Affairs (RA), QA and PSM management will be required for any rework activities. |
| 5.2.4 | Nuvelo QA must respond within 2 business days to any Avecia QA issues reported during batch disposition. |
| 5.2.5 | The release of the API ready for shipping is the responsibility of Avecia QA. |
| 5.2.6 | Avecia QA will be responsible for ensuring that appropriate Batch Records are copied and dispatched to Nuvelo QA within 10 business days after final disposition. |
| Date 30 Jun 05 | Modification 0 | Page 9 of 19 |
| 5.2.7 | Responsibilities for shipment of the API are detailed in Appendix 5. |
| 5.2.8 | Batch Disposition roles and responsibilities are detailed in (Appendix 4). |
| 5.3 | Internal Audits |
| 5.3.1 | Avecia QA will carry out routine internal audits for GMP compliance according to approved schedules developed as part of the Avecia Quality System. |
| 5.4 | Audits by Nuvelo |
| 5.4.1 | Nuvelo reserves the right to conduct twice-a-year, comprehensive quality audits of Avecia’s facilities, which may include a site tour, questioning of employees in work areas and review of quality system documentation to the appropriate quality standards. Avecia will be notified in advance of the intention to conduct an audit and the audit’s scope, and a mutually convenient date will be selected. During the audit, any non-conformances will be noted and documented in a report issued by Nuvelo within thirty (30) business days. Avecia will be requested to submit an acceptable written response and corrective action plan within thirty (30) business days of receipt of the report. Avecia will close all corrective actions that can be closed within 90 business days to the satisfaction of Nuvelo. Corrective actions that cannot practically be closed out within 90 business days will have a timeline for closure agreed with Nuvelo. |
| 5.4.2 | Supplier performance issues or non-conformances in the API will be indicated to Avecia in the form of a Nuvelo Supplier Corrective Action Report (SCAR)/Memo. Avecia will be requested to respond to the SCAR/Memo usually within thirty (30) business days post receipt. |
| 5.5 | Regulatory Actions |
| 5.5.1 | Avecia QA will provide Nuvelo information and support for any Nuvelo regulatory submission (e.g., CMC) upon request. Nuvelo RA will supply Avecia with copies of the submitted information that relates to manufacture by Avecia. |
| 5.5.2 | Nuvelo will inform Avecia QA of any pertinent changes in the CMC section of its regulatory document. |
| 5.5.3 | Avecia QA will inform Nuvelo in writing of any change of any kind to the Process that may impact Nuvelo regulatory submissions. Avecia shall not make any change to the Process without Nuvelo’s written prior approval of the change. |
| 5.5.4 | Avecia QA will provide Nuvelo with advanced written notice of any FDA/MHRA regulatory inspections where possible. |
| 5.5.5 | Avecia will also allow Nuvelo representatives to be present at site during any FDA/regulatory audits relating to Nuvelo’s API. All responses to any FDA/MHRA findings must be reviewed and approved by Nuvelo prior to submission. |
| 5.5.6 | During other regulatory audits (for non-Nuvelo APIs), if any findings are identified, Avecia will inform Nuvelo where their API is potentially affected. |
| Date 30 Jun 05 | Modification 0 | Page 10 of 19 |
| 5.5.7 | Avecia will confirm Nuvelo interpretations of Avecia data in the CMC section before Nuvelo’s submission to Regulatory Agencies. |
| 6. | Dispute Resolution |
If a dispute arises amongst the Quality Team relating to whether or not a Batch of the API is a Defective Batch, or whether or not a Defective Batch results from or arises out of an Avecia Default (each of the preceding a “Batch Dispute”), such Batch Dispute shall be resolved as follows.
(1) The Quality Team shall immediately notify the Project Steering Committee (PSC) in writing (the “Batch Dispute Notice”) that a Batch Dispute exists amongst the Quality Team. The Quality Team will discuss the Batch Dispute in good faith to attempt to reach agreement on: (i) whether a Batch is a Defective Batch and, if so, what course of action shall be taken to address it; and (ii) whether or not a Defective Batch resulted from or arose out of an Avecia Default.
(2) In the event that the Quality Team fails to reach agreement on a Batch Dispute within 15 days after sending the Batch Dispute Notice to the PSC, the Quality Team shall immediately refer the Batch Dispute to the PSC for discussion, by written notice to the PSC. Once the Batch Dispute has been referred to the PSC, the PSC has 5 business days from receipt of the referral notice to either resolve the Batch Dispute or refer the Batch Dispute to an independent expert or laboratory, with the expertise necessary to reasonably resolve the Batch Dispute.
(3) If the PSC cannot resolve the Batch Dispute or agree upon an independent expert or laboratory to resolve the Batch Dispute before the expiration of 5 business days after receiving the referral notice, the PSC shall immediately notify the EC of its failure in writing. Within no later than 2 business days after receiving notice of the failure from the PSC, each EC member shall nominate an independent expert who shall not: be a current or former employee, consultant or agent of a Party; have an immediate family member who is an employee, consultant or agent of a Party; or have any financial interest in a Party. Promptly thereafter, those two independent experts shall agree on a third independent expert who shall use any reasonable information, materials and data provided to him or her by the other two experts within 10 business days after his or her agreement to act as the third independent expert, to either promptly resolve the Batch Dispute or determine which independent Third Party laboratory will conduct the work necessary to promptly resolve the Batch Dispute. Such referral shall be solely for the purpose of establishing whether or not the applicable Batch is a Defective Batch and whether or not any Defective Batch results from or arises out of an Avecia Default. The decision of the independent expert, or independent laboratory, shall be made in writing and shall be binding upon the Parties. Whichever Party failed to accurately assess whether the Batch was or was not a Defective Batch, or whether or not a Defective Batch resulted from or arose out of an Avecia Default, shall bear the full cost and expense associated with the hiring and performance of the independent experts and/or laboratory. .
| 7. | Qualification |
Avecia will generate the Validation Master Plan which contains the Validation Strategy. Avecia QA, Nuvelo QA and Nuvelo Process Sciences and Manufacturing will jointly approve the Validation Master Plan which contains appropriate validation sub plans for the project which will be approved by Avecia QA.
| Date 30 Jun 05 | Modification 0 | Page 11 of 19 |
| 7.1 | Equipment, Computer Facility and Utility Qualification |
| 7.1.1 | Avecia is responsible for ensuring that equipment, computer, facility, utility and support systems for the manufacture of Nuvelo API are qualified according to regulatory requirements including 21 CFR Part 11. Avecia will perform required Installation Qualification (IQ)/Operational Qualification (OQ)/Performance Qualification (PQ)/calibration activities. |
| 7.2 | Laboratory Qualification |
| 7.2.1 | Avecia is responsible for ensuring that all QC laboratories are in compliance with FDA cGMP regulations and are appropriately qualified in all of the methodology associated with Nuvelo’s APIs. |
| 7.2.2 | Validation methodology will be developed in compliance with ICH Q2A Text on Validation of Analytical Procedures and ICH Q2B Validation of Analytical Procedures; Methodology. The degree of validation of methods will be agreed with Nuvelo according to the status of the API. |
| 7.3 | Cleaning Validation and Verification |
| 7.3.1 | Avecia is responsible for ensuring that a cleaning verification is conducted, and documented in accordance with cGMP and that a cleaning verification of API contact parts used in the manufacture of Nuvelo API is carried out between batches of different APIs to prevent cross-contamination. |
| 7.3.2 | Nuvelo will provide information (solubility, toxicity, dose, etc.) to establish cleaning limits. |
| 8.0 | REVISIONS |
| Mod. No. |
DATE |
REVISION DETAILS |
BY | |||
| 0 |
30 Jun 05 | New Agreement | DH |
| Date 30 Jun 05 | Modification 0 | Page 12 of 19 |
Appendix 1
Glossary of Terms
API: Active Pharmaceutical Ingredient.
cGMP: Current Good Manufacturing Practice.
ICH: International Conference on Harmonization.
Certificate of Analysis: A compilation of the analytical test methods applied, specifications for each test and test results for a particular intermediate/finished API lot. The certificate should be reviewed and approved by QA management.
Certificate of Conformance: Certification confirming that an intermediate or finished API has been manufactured in compliance to the cGMP’s and that quality records associated have been reviewed and approved by QA management.
Commercial Agreement: the Agreement effective 21 January 2005 for the manufacture of the API by and between Avecia and Nuvelo.
Approved Suppliers: Suppliers approved within Avecia’s quality system and qualified using FDA/EU guidelines, and approved by Nuvelo.
Key Suppliers: Suppliers that supply key ingredients for the manufacture of the API. Change to these Suppliers would impact the API quality.
OOS: Out of Specification.
OOT: Out of Trend.
NIST: National Institute of Standards.
In-Process Control: Checks performed during production to monitor and if appropriate, to adjust the process and/or to ensure that the API conforms to the specifications attached to the QA Agreement as Appendix 6.
Critical Non-conformance: A non-conformance which would impact API quality.
Major Non-conformance: A non-conformance which has a potential to impact API quality and requires an investigation to assess the impact on API quality.
Minor Non-conformance: A non-conformance which has no impact on API quality
Reprocess: Reprocess is defined as repeat processing of a manufacturing step that does not affect the disposition of the API. For example: a 0.22 micron filtration when the filter integrity failed.
Rework: Upon prior written approval of Nuvelo, rework may occur on identification of a non-conformance associated with the finished API (this applies to intermediate stages of API also) that may affect the API quality. Rework is only performed upon the prior written approval of Nuvelo as a result of an investigation through the non-conformance reporting process or as a result of a change that occurred in the drug master file (ex: expiration date). For example: Relabeling of vials due to incorrect sample lot #. This would lead to an non-conformance and then the change would be approved by Nuvelo before commencement of the rework .
| Date 30 Jun 05 | Modification 0 | Page 13 of 19 |
Appendix 2
Responsibilities
| Responsibility |
Avecia |
Nuvelo | ||
| Formula |
ü | |||
| API Method |
ü | |||
| Scale-up Procedure |
ü | |||
| Qualification Procedures |
ü | ü | ||
| Stability Programme |
ü | |||
| Raw Material Selection |
ü | ü | ||
| Documentation for TSE risk |
ü | |||
| Specific API Information (safety, transport etc) |
ü | |||
| API ready for shipping |
ü | |||
| API Storage |
ü | |||
| API Specification |
ü | ü | ||
| Method of Analysis |
ü | |||
| Method of Analysis verification |
ü | ü | ||
| Analysis + Release |
ü | ü | ||
| Storage of API QC Samples |
ü | |||
| Manufacture of API |
ü | |||
| Batch Number Assignment |
ü | |||
| Expiry Date Assignment |
ü | ü | ||
| Manufacturing Documentation |
ü | |||
| In Process Controls |
ü | |||
| Audit of API Manufacturer |
ü | |||
| Deviation Management |
ü | ü | ||
| Change Management |
ü | ü | ||
| Retention of Records |
ü | |||
| Shipping Study |
ü | |||
| Date 30 Jun 05 | Modification 0 | Page 14 of 19 |
Appendix 3
List of Contacts
| Area of Responsibility | AVECIA Main Phone: x00 (0) 0000 000000 |
NUVELO Main Phone: 000-000-0000 | ||
| QA | Xxx Xxxxxxxx x00 (0) 0000 000000 xxx.xxxxxxxx@xxxxxx.xxx |
Xxxxx van der Linden Ph: 000-000-0000 xxxxxxxxxxxxx@xxxxxx.xxx | ||
| PSM | N/A | Xxxx Xxxxxxx Ph: 000-000-0000 xxxxxxxx@xxxxxx.xxx Xxxx Xxxxx Ph: 000-000-0000 xxxxxx@xxxxxx.xxx | ||
| RA | Xxxx Xxxxxxxxx x00 (0) 0000 000000 xxxx.xxxxxxxxx@xxxxxx.xxx |
Xxxxx X. Xxxxxxx Ph: 000-000-0000 xxxxxxxx@xxxxxx.xxx | ||
| QC | Xxxxx Xxxxxxx x00 (0) 0000 000000 xxxxx.xxxxxxx@xxxxxx.xxx |
Xxxxx Xxxx Ph: 000-000-0000 xxxxx@xxxxxx.xxx | ||
| Date 30 Jun 05 | Modification 0 | Page 15 of 19 |
Appendix 4
Batch Disposition Roles and Responsibilities.
| Responsibilities for API Batch Disposition |
Avecia QC |
Avecia QA |
Nuvelo | |||
| Final Review of all batch related manufacturing documentation |
Ö | |||||
| Review of QC batch related analyses |
Ö | |||||
| Final Review of QC batch related analyses |
Ö | |||||
| Generation of Certificate of Analysis (C of A) |
Ö | |||||
| Final Review of C of A |
Ö | |||||
| Final review of batch record and acceptance of the batch |
Ö | |||||
| Generation of Water and Environmental (WESRs) |
Ö | |||||
| Summary Reports for Manufacturing Period |
Ö | |||||
| Final Review of WESRs |
Ö | |||||
| BSE free statement for the batch |
Ö | |||||
| Review of completed Non Conformance Reports |
Ö | |||||
| Review of completed Change Control Reports |
Ö | |||||
| Review and Client Approval of Major/Critical NCRs, investigations, OOS and OOT results |
Ö | |||||
| Review and Client Approval of Major/Critical Change Control Reports |
Ö | |||||
| Final Approval of all Non Conformance Reports |
Ö | |||||
| Final Approval of all Change Control Reports |
Ö | |||||
| Final approval of Lab investigations |
Ö | |||||
| Notification of Minor, Changes, NCRs, investigations, OOS and OOT results to Nuvelo |
Ö | |||||
| Compliance check against Avecia batch disposition procedure |
Ö | |||||
| Batch Disposition |
Ö | |||||
| Dispatch Instruction |
Ö | |||||
| Copies of all batch documentation to Customer |
Ö |
| Date 30 Jun 05 | Modification 0 | Page 16 of 19 |
Appendix 5
Responsibilities for Delivery/Shipment of the Manufactured API
| Responsibilities |
Avecia |
Avecia QA |
Nuvelo | |||
| Provide Avecia with completed dispatch request form for shipment. Including, Batch number, quantity, reason for delivery, delivery contact and address. | ü | |||||
| Approve dispatch request form. | ü | |||||
| Agree dispatch time and date. | ü | ü | ü | |||
| Generate shipping documentation† | ü | ü | ||||
| Send documentation to courier for checks (if applicable). | ü | |||||
| Provide all documentation to Avecia QA prior to shipment. | ü | |||||
| Arrange transport, packaging conditions/configuration and temperature monitoring devices and downloaded data. | ü | ü | ||||
| Arrange insurance cover for shipment (if required) | ü | |||||
| Check dispatch batch records & shipping documentation. | ü | |||||
| Execute dispatch batch record | ü | |||||
| Inform recipient of shipment. | ü | ü | ||||
| Return of confirmation receipts from recipient to Avecia QA. | ü | |||||
| Complete Final Product Log (SP3703) | ü | |||||
| Arrange customer invoice if using Avecia’s courier account. | ü |
| † | Documentation includes: |
| a. | Cover letter (3 copies) |
| b. | MSDS (3 copies) |
| c. | CofA (3 copies) |
| d. | A signed statement of identity which details the quantity, container sizes, an accurate description of the material and the usage of the material. |
| e. | Customs invoice for shipments in EU. |
| f. | For deliveries to the United States. |
| i. | A signed US Customs declaration, indicating that the material is produced by recombinant microbial fermentation (including the strain) and stating the material does not contain any animal or cell culture derived products or additives such as albumin or serum. |
| ii. | For material that does contain any animal or cell culture derived products or additives such as albumin or serum, a USDA permit is required for US customs clearance. Obtaining a USDA permit is the responsibility of the customer. |
| iii. | A signed US Customs invoice stating the declared value of the shipment. |
| g. | Avecia finance department will confirm the type of commercial agreement, as either technical consultancy or sale of product. |
| i. | For ‘technical consultancy’ contracts, the declared value for all delivered material will be zero (0). |
| ii. | For ‘sale of product’ contracts the declared value for delivered material will be the cost of replacement. |
| Date 30 Jun 05 | Modification 0 | Page 17 of 19 |
Appendix 6
Draft Release Testing Specification and
Criteria for Acceptance for Bulk Alfimeprase API
| Parameter |
Method Type |
Acceptance Criterion | ||
| Appearance | Appearance | Clear, colorless liquid practically free of particulates | ||
| Purity | Bioburden | Report result | ||
| SDS-PAGE, Coomassie Stain | Report % main band; main band same position as standard | |||
| SE-HPLC | Main Peak ³ 95% | |||
| RP-HPLC | Main Peak ³ 88% | |||
| AE-HPLC | Main Peak ³ 93% | |||
| Impurities | Pyrogenicity | Absence of pyrogens (dose level of 10 mL/kg) | ||
| Nucleic Acid Content | £ 10 pg/1 mg alfimeprase | |||
| Yeast Contaminant Immunoassay | £0.01% (w/w) | |||
| Potency | Clot Lysis Activity | ³ 70% of standard activity | ||
| Identity | Molecular Weight | 22570 - 22583 Da | ||
| Peptide map | Conforms to standard | |||
| Quantity | Protein Concentration | 9.0 to 11.0 mg/mL | ||
| Excipients / Chemical Composition | PH | 7.9 ± 0.4 | ||
| Osmolality | 315 to 455 mOsm/kg | |||
| Zn Content | 0.43 to 0.65 mM | |||
| Polysorbate 80 | 0.005 to 0.015% (w/v) | |||
| Date 30 Jun 05 | Modification 0 | Page 18 of 19 |
Appendix 7
Draft Limited Testing and Criteria for Acceptance to Demonstrate Acceptability of Process Performance for Bulk Alfimeprase API
| Parameter |
Method Type |
Acceptance Criterion | ||
| Purity | Bioburden | Report result | ||
| RP-HPLC | Main Peak ³ 88% | |||
| AE-HPLC | Main Peak ³ 93% | |||
| Impurities | Yeast Contaminant Immunoassay |
£0.01% (w/w) | ||
| Potency | Clot Lysis Activity | ³ 70% of standard activity | ||
| Quantity | Protein Concentration | 9.0 to 11.0 mg/mL | ||
| Excipients / Chemical Composition | Osmolality | 315 to 455 mOsm/kg | ||
| Date 30 Jun 05 | Modification 0 | Page 19 of 19 |

