ELAN PHARMA INTERNATIONAL LIMITED AND ENTREMED, INC. LICENSE AGREEMENT
INDEX
1.
|
Definitions and Interpretation | 1 | ||||
2.
|
The License | 7 | ||||
3.
|
Intellectual Property | 8 | ||||
4.
|
Non-Competition | 15 | ||||
5.
|
Registration, Marketing and the Promotion of the Product | 15 | ||||
6.
|
Financial Provisions | 15 | ||||
7.
|
Manufacture and Supply | 18 | ||||
8.
|
Payments, Reports and Audits | 19 | ||||
9.
|
Duration and Xxxxxxxxxxx | 00 | ||||
00.
|
Consequences of Termination | 21 | ||||
11.
|
Warranties, Indemnification and Liability | 22 | ||||
12.
|
Confidentiality | 25 | ||||
13.
|
Miscellaneous Provisions | 26 | ||||
Schedule 1
|
EPIL Patents | 31 | ||||
Schedule 2
|
EntreMed Patents | 39 | ||||
Schedule 3
|
Technological Competitors | 40 | ||||
Schedule 4
|
Commercial Unit Dose Cost of Goods Pricing | 41 | ||||
Schedule 5
|
Key Terms for Manufacturing and Supply Agreement | 42 | ||||
| i |
||||||
THIS LICENSE AGREEMENT is dated January 9, 2006
PARTIES:
| (1) | ELAN PHARMA INTERNATIONAL LIMITED, a company incorporated under the laws of Ireland, having its registered office at Monksland, Athlone, Co Westmeath, Ireland (“EPIL”); and | |
| (2) | ENTREMED, INC., a Delaware corporation, having its principal place of business at 0000 Xxxxxxx Xxxxxx Xxxxx, Xxxxxxxxx, XX 00000 (“EntreMed”). |
BACKGROUND:
| (A) | EPIL possesses certain proprietary technology as well as proprietary know-how and confidential information used or useful in the manufacture and use of pharmaceutical products containing nanoparticles. | |
| (B) | EntreMed possesses certain rights to intellectual property rights covering the Compound (as defined below). | |
| (C) | EntreMed wishes to obtain the right to utilize certain EPIL Intellectual Property to commercialize the Product in the Field in the Territory in accordance with the terms and conditions set out below. | |
| (D) | Simultaneously with or shortly after the execution of this Agreement, EntreMed intends to enter into a Services Agreement with EDDI, an EPIL Affiliate, that will contain a Development Plan for further research and development of the Product and that will xxxxx XXXX the exclusive right to supply the Product to EntreMed for preclinical, clinical and regulatory purposes. | |
| (E) | Following the execution of this Agreement and the Services Agreement, EPIL and EntreMed intend to enter into a Manufacturing and Supply Agreement that will establish the terms by which EPIL or an Affiliate will manufacture and supply to EntreMed its entire requirement of the Product for commercial supply in the Territory. |
TERMS:
The Parties agree as follows:
| 1. | DEFINITIONS AND INTERPRETATION | |
| 1.1. | Definitions. In this Agreement: | |
| “Affiliate” means any corporation or entity controlling, controlled or under common control with EPIL or EntreMed, as the case may be. For the purposes of this Agreement, “control” means the direct or indirect ownership of more than 50% of the issued voting shares or other voting rights of the subject entity to elect directors, or if not meeting the preceding criteria, any entity owned or controlled by or owning or controlling at the maximum control or ownership right permitted in the country where such entity exists. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 1
| “Agreement” means this license agreement (which expression shall be deemed to include its Recitals and Schedules). | ||
| “API” means active pharmaceutical ingredient. | ||
| “Business Days” means Monday to Friday inclusive, excluding any days on which the clearing banks are generally closed in Dublin and/or New York. | ||
| “cGMP” means the then current regulations set forth in 21 C.F.R. Parts 210-211, 820 or 21 C.F.R. Subchapter C (drugs) quality system regulations and the requirements thereunder imposed by the FDA and, as applicable, the equivalent regulations and requirements imposed by other RHAs in the Territory. | ||
| “Claims” means all and any claims (whether successful or otherwise), demands, settlement amounts, losses, liabilities, damages, costs and expenses, including reasonable attorneys’ fees and expenses and legal costs. | ||
| “Commercially Reasonable Efforts” means those efforts of a party which are consistent with those utilised by such party for its own internally developed or in-licensed pharmaceutical products, taking into account all factors that impact the manufacturing, development, marketing and sales of such products, as applicable. | ||
| “Compound” means EntreMed’s proprietary compound 2-methoxyestradiol (2ME2) having the following chemical formula: | ||
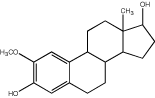 |
||
| For the avoidance of doubt, Compound does not include any analogs or derivatives of 2ME2 including, but not limited to, EntreMed compound ENMD-1198. | ||
| “Compound Data” means data relating to the Compound generated by EntreMed or EDDI pursuant to the Services Agreement and/or Development Plan. | ||
| “CSA” means the Clinical Supply Agreement dated July 12, 2004, as amended, entered into by EntreMed and EDDI including all of its appendices, schedules and exhibits thereto related to the Compound. | ||
| “DDP” (delivery duty paid) shall have the same meaning as in the ICC Incoterms 2000, International Rules for the Interpretation of Trade Terms, ICC Publications No. 560. | ||
| “Development Plan” shall have the meaning ascribed thereto in the Services Agreement. | ||
| “DMF” or “Drug Master File” means any confidential detailed information submitted by EPIL, EDDI or its Affiliates to any RHA in the Territory about any facilities, processes or articles that |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 1
| are used by EPIL, EDDI or its Affiliates in the manufacture, processing, packaging or storage of the Product. | ||
| “EDDI” means EPIL’s Affiliate, Elan Drug Delivery, Inc., a Delaware Corporation, having its principal place of business at 0000 Xxxxxxx Xxxxx, Xxxx xx Xxxxxxx, XX 00000. | ||
| “EEA” means the member states of the European Economic Area, as the same may change from time to time. | ||
| “Effective Date” means the date of this Agreement as set forth in the header on page 1 herein. | ||
| “EntreMed Improvements” means any and all improvements under the EntreMed Patents, the EntreMed Know-How and/or to the Compound that have been conceived, created, developed and/or otherwise invented by EntreMed or its Affiliates and/or EPIL or its Affiliates and such Parties’ Third Party contractors under the Services Agreement, or otherwise pursuant to this Agreement, the Manufacture and Supply Agreement, the CSA, the MEEA or the Letter Agreement. | ||
| “EntreMed Intellectual Property” means the EntreMed Know-How, the EntreMed Patents and the EntreMed Improvements and the Compound. | ||
| “EntreMed Know-How” means any and all rights owned, licensed or controlled by EntreMed or its Affiliates as of the Effective Date (otherwise than pursuant to the EPIL License) to any scientific, pharmaceutical or technical information, data, discovery, invention (whether patentable or not), know-how, substances, techniques, processes, systems, formulations and designs and expertise relating to the Compound which is not generally known to the public. | ||
| “EntreMed Patents” means any and all rights under any and all patent applications and/or patents, now existing, currently pending or hereafter filed, or acquired or licensed by EntreMed or its Affiliates relating to the Compound, including without limitation as set forth in Schedule 2, and any foreign counterparts thereof and all divisionals, continuations, continuations-in-part, any foreign counterparts thereof and all patents issuing on any of the foregoing and any foreign counterparts thereof, together with all registrations, reissues, re-examinations, supplemental protection certificates, or extensions thereof, and any foreign counterparts thereof. | ||
| “EntreMed Trademark” means EntreMed’s rights to use the trademark(s) Panzem® or such other trademarks as EntreMed may from time to time reasonably specify. | ||
| “EPIL Improvements” means * any and all improvements to the EPIL Patents, the EPIL Know-How, the EPIL Technology * that have been conceived, created, developed and/or otherwise invented by EPIL or its Affiliates and/or EntreMed or its Affiliates and such Parties’ Third Party contractors under the Services Agreement, or otherwise pursuant to this Agreement, the Manufacture and Supply Agreement, the CSA, the MEEA or the Letter Agreement. | ||
| “EPIL Intellectual Property” means the EPIL Know-How, the EPIL Patents and the EPIL Improvements. | ||
| “EPIL Know-How” means any and all rights owned, licensed or controlled by EPIL or its Affiliates as of the Effective Date to any scientific, pharmaceutical or technical information, data, discovery, invention (whether patentable or not), know-how, substances, techniques, processes, |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 2
| systems, formulations, designs and expertise relating to the EPIL Technology which is not generally known to the public. | ||
| “EPIL Patents” means any and all rights under any and all patent applications and/or patents, now existing, currently pending or hereafter filed by EPIL relating to the EPIL Technology, including without limitation as set forth in Schedule 1, and any foreign counterparts thereof and all divisionals, continuations, continuations-in-part, any foreign counterparts thereof and all patents issuing on any of the foregoing, and any foreign counterparts thereof, together with all registrations, reissues, re-examinations, supplemental protection certificates, or extensions thereof, and any foreign counterparts thereof. | ||
| “EPIL Technology” means EPIL’s proprietary technology directed to: | ||
| (a) nanoparticulate colloidal dispersions of compounds stabilized against agglomeration of the nanoparticles and EPIL’s proprietary methods, equipment and materials used for making such dispersions (such technology is commonly referred to by EPIL as NanoCrystal® Technology); and | ||
| (b) EPIL’s proprietary processes for converting said dispersions into intermediates and/or finished tablet or capsule formulations and the intermediates, tablets and capsules created using such proprietary processes including the NanoMill® System; and | ||
| as more fully described in the EPIL Patents and applied in the EPIL Know-How. | ||
| “EPIL Trademark” means “NanoCrystal®”, “NanoMill®” or such other trademarks as EPIL may from time to time reasonably specify. | ||
| “EU” means the member states of the European Union, as same may change from time to time in terms of member states. | ||
| “EXW” (ex works) has the same meaning as in the ICC Incoterms 2000, International Rules for the Interpretation of Trade Terms, ICC Publication No. 560. | ||
| “FDA” means the United States Food and Drug Administration or any other successor agency whose approval is necessary to market the Product in the US. | ||
| “Field” means oral prescription pharmaceutical products for use in humans. Diagnostics are specifically excluded. | ||
| “Force Majeure” means any cause or condition beyond the reasonable control of the Party obliged to perform, including acts of God, acts of government (in particular with respect to the refusal to issue necessary import or export licenses), fire, flood, earthquake, war, riots or embargoes or strikes affecting a Party. | ||
| “Generic” means each generic version of Product that is legally substitutable for the Product as follows: (i) in the US, is approved under 21 U.S.C. 505(j) (or any successor legislation) or which has an “AB” rating with respect to Product; (ii) in the EU, is authorised to be placed on the market in accordance with Article 10 of Directive 2001/83/EC as amended (when implemented by such member states) by Directive 2004/27 (or any successor legislation); (iii) in countries of the Territory other than the US and the EU where such pharmaceutical product: (a) contains the same active pharmaceutical ingredients as Product; (b) is approved by an abridged procedure that |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 3
| relies in whole or in part on safety and efficacy data generated for any approval of Product; and (c) has the same or substantially the same labelling as the Product. | ||
| “Governmental Authority” means all governmental and regulatory bodies, agencies, departments or entities, which regulate, direct or control commercial and other related activities in the Territory. | ||
| “Highly Confidential Information” shall have the meaning ascribed thereto in the Services Agreement. | ||
| “ICH” means the International Conference on Harmonization. | ||
| “ICH Qualifying Batch” means batches of Product intended for stability studies to support registration of the first commercial Product in the Territory. | ||
| “In Market” means the sale of the Product in the Territory by EntreMed, or where applicable, by a permitted sub-licensee, to an unaffiliated Third Party, such as a wholesaler, distributor, managed care organisation, hospital or pharmacy, and shall exclude the transfer pricing of the Product by one EntreMed Affiliate to another EntreMed Affiliate or a permitted sub-licensee. | ||
| “Letter Agreement” means the letter agreement entered into by EntreMed and EPIL dated May 9, 2005. | ||
| “Manufacturing and Supply Agreement” means the agreement that will be entered into by EntreMed and EPIL or its Affiliate, for the manufacture and supply of Product in the Field in the Territory by EPIL or its Affiliate to EntreMed. | ||
| “MEEA” means the Master Experimental Evaluation Agreement dated May 18, 2004 entered into by EntreMed and EDDI including all of its appendices, schedules and exhibits thereto related to the Compound. | ||
| “* “ | ||
| “NanoMill® System” means the milling system designed and developed by or on behalf of EPIL and its Affiliates for preparing nanoparticulate dispersions for pharmaceutical formulations, including the milling equipment and appropriate milling media and stabilizing materials, and associated manuals, protocols and know-how. | ||
| “NCD” means NanoCrystal® colloidal dispersion. | ||
| “Net Sales” shall, subject to the provisions of Clause 6.7, mean in the case of Product sold by EntreMed, or by a sub-licensee, the aggregate gross In Market sales proceeds billed for the Product by EntreMed, or by a sub-licensee, as the case may be, in accordance with generally accepted accounting principles, less the following deductions relating to such sales, provided that all such deductions shall be commercially reasonable and consistent with standard industry practices and are actually paid and not recouped: |
| (i) | trade, cash or quantity discounts, allowances, adjustments and rejections, and any other adjustments, including those granted on account of price adjustments, billing errors, rebates, chargeback rebates, fees, reimbursements or similar payments granted or given to |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 4
| wholesalers or other distributors, buying groups, health care insurance carriers or other institutions; | |||
| (ii) | rebates, recalls (other than where the Product is replaced without charge), returns, rejected goods and damages goods not covered by insurance; | ||
| (iii) | price reductions or rebates imposed by Governmental Authorities and any payment in respect of sales to any Governmental Authority in respect of any government-subsidized program, including Medicare and Medicaid rebates or other payments under managed care agreements; | ||
| (iv) | sales, excise, turnover, inventory, value-added and similar taxes assessed on the royalty-bearing sale of such Product, but not including any taxes on income paid by or assessed against EntreMed or a sub-licensee; | ||
| (v) | transportation, importation, shipping, insurance and other handling expenses directly chargeable to the royalty-bearing sale of the Product, but only to the extent that such expenses are separately delineated in the applicable invoices; | ||
| (vi) | chargebacks granted to drug wholesalers or their customers in cases where there are not direct shipments to such customers by EntreMed or its sublicense. |
| For the avoidance of doubt, costs associated with changes to packaging or labelling shall not be deducted from Net Sales. | ||
| “Party” or “Parties” means EPIL and its Affiliates or EntreMed or its Affiliates, individually or collectively, as referred to herein. | ||
| “Pivotal Study” means a clinical study whose primary objective is to obtain a definitive evaluation of the therapeutic efficacy and safety of the Product in patients for the particular indication in question. | ||
| “Post-Phase 1b Clinical Study” in this Agreement means any clinical study initiated post phase I. | ||
| “Product” means liquid, tablet or capsule formulations for oral administration being developed pursuant to the Development Plan that incorporate the EPIL Technology and contain the Compound as the sole active ingredient. For the avoidance of doubt, injectable formulations are specifically excluded from this Agreement. | ||
| “Prosecute” means in relation to a class of intellectual property: |
| (a) | to secure the grant of any patent application within such class; | ||
| (b) | to file and prosecute patent applications on patentable inventions and discoveries relating to that class; | ||
| (c) | to defend all such applications against Third Party oppositions; and | ||
| (d) | to maintain in force any issued letters patent relating to the same |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 5
| and “Prosecution” has a corresponding meaning. | ||
| “Regulatory Application” means any regulatory application or any other application for marketing approval for the Product, which EntreMed will file with the FDA or with any other RHA in the Territory, including any supplements or amendments thereto which EntreMed may file. | ||
| “Regulatory Approval” means the final approval to market the Product in any country of the Territory, including pricing and reimbursement approval and any other approval which is required to launch the Product in the normal course of business. | ||
| “RHA” means any relevant government health authority (or any successor agency thereof) in any country of the Territory, including the FDA, whose approval is necessary to market and sell the Product in the relevant country of the Territory. | ||
| “Related Agreements” means the Services Agreement and the Manufacturing and Supply Agreement. | ||
| “Services Agreement” means the agreement, and all appendices, schedules, exhibits and amendments thereto, between EntreMed and EDDI that is signed simultaneously with this Agreement, or shortly thereafter, and that contains a Development Plan for EDDI to perform certain development activities for the formulation of the Product and such other services as may be agreed by the Parties. | ||
| “Technological Competitor” means a person or entity listed in Schedule 3, and divisions, subsidiaries and successors thereof * . For this definition “competitors” shall mean companies that have or use technology that is directed to and/or suitable for providing substantially similar or comparable enhancements to the solubility characteristics of active pharmaceutical ingredients as those provided by EPIL Technology. | ||
| “Term” means the term of this Agreement, as set out in Clause 9.3 herein. | ||
| “Territory” means all of the countries of the world. | ||
| “Third Party” means any individual or entity not a Party to this Agreement other than an Affiliate of a Party. | ||
| “US” or “USA” means the United States of America and its possessions and territories, including but not limited to Puerto Rico. | ||
| “Valid Claim” means any claim of an issued and unexpired patent included within the EPIL Intellectual Property which has not been held unenforceable, unpatentable or invalid by a decision of a court or government agency of competent jurisdiction, that is unappealable or unappealed within the time allowed for appeal, or which has not expressly been admitted by the holder of the patent or supplementary protection certification to any person to be invalid or unenforceable. | ||
| “VAT” or “Value Added Tax” means (i) any tax imposed in compliance with the Sixth Directive of the Council of the European Communities (77/388/EEC) and (ii) any other tax of a similar |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 6
| fiscal nature, whether imposed in a member state of the European Union or anywhere else in the Territory. | ||
| “$” and “US$” mean US Dollars. | ||
| 1.2. | Further Definitions. In addition, the following definitions have the meanings in the Clauses corresponding thereto, as set forth below: |
| Definition | Clause | |||
| “Compensation Fee” | 6.2 | |||
| “Confidential Information” | 12.1 | |||
| “Designated Manufacturer” | 7.3 | |||
| “Disclosing Party” | 12.14 | |||
| “Due Date” | 8.8 | |||
| “EntreMed Logo” | 3.6.1.2 | |||
| “EPIL License” | 2.1 | |||
| “Extended Term” | 9.2 | |||
| “Firm” | 3.5.5 | |||
| “Infringement Claim” | 3.4.1 | |||
| “Initial Term” | 9.1 | |||
| “License Milestone Payments” | 6.1 | |||
| “Manufacturing License” | 7.3 | |||
| “Notice” | 13.11.1 | |||
| “Statement” | 8.1 | |||
| “Quality Agreement” | Schedule 5, paragraph 4 |
| 1.3. | Interpretation. In this Agreement: |
| 1.3.1 | the singular includes the plural and vice versa, and unless the context or subject otherwise requires, references to words in one gender include references to the other genders; | ||
| 1.3.2 | unless the context otherwise requires, reference to a recital, article, paragraph, provision, clause or schedule is to a recital, article, paragraph, provision, clause or schedule of or to this Agreement; | ||
| 1.3.3 | the headings in this Agreement are inserted for convenience only and do not affect its construction; and | ||
| 1.3.4 | the expressions “include”, “includes”, “including”, “in particular” and similar expressions shall be construed without limitation. |
| 2. | THE LICENSE | |
| 2.1. | EPIL License to EntreMed. Subject to the terms of this Agreement, EPIL hereby grants to EntreMed an exclusive license (the “EPIL License”) to the EPIL Intellectual Property to import, export, use, conduct clinical evaluations in support of Regulatory Applications, offer for sale, market, distribute and sell the Product in the Field in the Territory, subject to * . For the avoidance of doubt, the EPIL License shall not include the right to perform any |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 7
| formulation and/or process development activities for the Product unless this has been agreed pursuant to the Services Agreement. | ||
| 2.2. | Sub-licensing. Subject to the prior written consent of EPIL which shall not be unreasonably withheld, delayed or conditioned, EPIL grants to EntreMed the right to grant sub-licenses in respect of the EPIL Intellectual Property to import, export, use, offer for sale, market, distribute and sell the Product in one or more countries of the Territory, subject to the following conditions: |
| 2.2.1 | Any sub-license granted shall be in the same terms as the terms of this Agreement insofar as they are applicable, mutatis mutandis, but excluding the right to grant a sub-license; | ||
| 2.2.2 | For the avoidance of doubt, EntreMed shall ensure that EPIL shall have the same rights of audit and inspection vis-à-vis a sub-licensee as EPIL has vis-à-vis EntreMed pursuant to this Agreement. | ||
| 2.2.3 | EntreMed shall be liable to EPIL and its Affiliates for all acts and omissions of any sub-licensee as though such acts and omissions were by EntreMed. | ||
| 2.2.4 | EntreMed shall not disclose any Confidential Information of EPIL or its Affiliates in its dealings with sublicensees without the prior written consent of EPIL, which consent shall not be unreasonably withheld or delayed. | ||
* |
|||
| 3. | INTELLECTUAL PROPERTY | |
| 3.1. | Ownership of Intellectual Property. |
| 3.1.1 | EPIL shall remain the sole owner of the EPIL Intellectual Property. | ||
| 3.1.2 | EntreMed shall remain the sole owner of the EntreMed Intellectual Property. |
| 3.2. | Patent Prosecution and Maintenance. |
| 3.2.1 | EPIL, at its sole discretion and expense, may Prosecute the EPIL Intellectual Property in the Territory. | ||
| 3.2.2 | EntreMed, at its sole discretion and expense, may Prosecute the EntreMed Intellectual Property in the Territory. | ||
| 3.2.3 | Each Party shall provide the other with reasonable support in the Prosecution of the EPIL Intellectual Property and the EntreMed Intellectual Property in respect of any inventions that were developed under this Agreement or Related Agreements and shall provide all information and/or data in its possession that is reasonably necessary or proper to support any relevant patent application in the Territory. Further, the Parties shall execute and deliver all documents and instruments that are reasonably necessary or proper to support the Prosecution of the EPIL Intellectual Property and the EntreMed Intellectual Property as the other Party may request. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 8
| 3.3. | Enforcement. |
| 3.3.1 | EPIL and EntreMed shall promptly inform each other in writing of any actual or alleged unauthorized use of the EPIL Intellectual Property or the EntreMed Intellectual Property by a Third Party of which it becomes aware and provide the other Party with any available evidence of such unauthorized use. | ||
| 3.3.2 | EPIL shall have the right to enforce for EPIL’s own benefit (including by agreement or by litigation) EPIL Intellectual Property at its own instigation. EntreMed shall reasonably cooperate with EPIL to enforce such rights. In the event such proceedings are related to the Product, EntreMed shall be kept advised at all times of such suit or proceedings brought by EPIL. | ||
| 3.3.3 | EntreMed shall have the right to enforce for EntreMed’s own benefit (including by agreement or through litigation) EntreMed Intellectual Property at its own instigation. EPIL shall reasonably cooperate with EntreMed to enforce such rights. In the event such proceedings are related to the Compound, EPIL shall be kept advised at all times of such suit or proceedings brought by EntreMed. |
| 3.4. | Infringement Claims. |
| 3.4.1 | Each of the Parties shall promptly notify the other Party in writing of any Claims made or brought against either of them alleging infringement or other unauthorised use of the proprietary rights of a Third Party arising from the manufacture, importation, use, offer for sale, sale or other commercialization of the Product in the Territory (“Infringement Claim”). | ||
| 3.4.2. | EntreMed shall indemnify and hold harmless Elan against all Infringement Claims related to the manufacture, importation, use, offer for sale, sale or other commercialization of the Product in the Territory resulting from: |
| 3.4.2.1 | a breach by EntreMed of its representations and warranties set forth in Clauses 11.2.3 to 11.2.6; or | ||
| 3.4.2.2 | intellectual property which is owned by, licensed to (which for clarity does not include the EPIL License which is governed pursuant to other terms and conditions contained in this Agreement) or controlled by EntreMed, any Affiliate of EntreMed or a permitted sub-licensee in the country in question, or which is/was generated pursuant to some agreement between EntreMed (or an Affiliate or permitted sub-licensee) on the one hand and a Third Party on the other. |
| 3.4.3 | Subject to Clauses 3.4.4, 3.4.5 and 3.4.6, EPIL shall indemnify and hold harmless EntreMed against all Infringement Claims resulting from a breach by EPIL of its representations and warranties set forth in Clauses * .. | ||
| For the avoidance of doubt, the parties agree that EntreMed shall indemnify and hold harmless EPIL against all claims (whether successful or otherwise), demands, settlement amounts, damages, losses, liabilities, costs and expenses |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 9
| (including reasonable attorneys’ fees) which may arise in connection with any Infringement Claim related to the manufacture, importation, use, offer for sale, sale or other commercialization of the Product in the Territory where such Infringement Claim does not result from a breach by EPIL of any of its representations or warranties set forth in Clauses * .. | |||
| 3.4.4 | Subject to Clause 3.4.5 and Clause 3.4.6, EPIL’s aggregate cumulative liability pursuant to Clause 3.4.3 (and/or under any other provision of this Agreement) in respect of those Infringement Claims for which EPIL is liable under Clause 3.4.3 shall not exceed certain limitations as follows: |
| 3.4.4.1 | * % ( * per cent)of any lump sum payment due to a Third Party as a result of a court order or settlement in respect of the Infringement Claim (including a claim for damages); and | ||
| 3.4.4.2 | * % ( * per cent) of any license fees due to a Third Party under any license entered into in accordance with Clause 3.5 (or as applicable, payments in the nature of a royalty on sales ordered to be paid to such Third Party by a court of competent jurisdiction in a final judgment under such an Infringement Claim). |
| 3.4.5 | EntreMed will be entitled to recover amounts due by EPIL to EntreMed under Clause 3.4.4 as a credit against the sums payable by EntreMed to EPIL under the provisions of Clause 6 during the Initial Term, provided however that the maximum credit which may be claimed by EntreMed will be * % ( * per cent) of the sums otherwise payable in that quarter. | ||
| Any deficit remaining in EntreMed’s recovery of amounts due by EPIL to EntreMed under Clauses 3.4.3 and 3.4.4 following recovery by EntreMed within the limitations set forth in this Clause 3.4.5 may be carried over to subsequent calendar quarters, subject always to the preceding paragraph. Any deficit at the end of the Initial Term shall be borne by EntreMed and EPIL shall have no liability to EntreMed in relation thereto. | |||
| For the avoidance of doubt, EntreMed shall indemnify and hold harmless EPIL against all Infringement Claims to the extent that they are in excess of the limits set forth in Clause 3.4.4 and this Clause 3.4.5. | |||
| 3.4.6 | The Parties hereby agree that EntreMed shall consult with EPIL prior to making references to EPIL Intellectual Property in any proceedings and obtain EPIL’s permission to make such references, except to the extent required by applicable law. Save as specifically provided otherwise in this Clause 3.4, the provisions of Clause 11.7 shall apply as regards the conduct of any Infringement Claim. | ||
| With reference to the provisions of Clause 11.7.4, EPIL and EntreMed shall consult as regards any actions EPIL or EntreMed proposes to take in order to mitigate any loss or liability in respect of any Infringement Claim, such as EntreMed ceasing to sell the Product, the Parties agreeing to modify the Product, or either or both of the Parties entering into a licensing or settlement negotiation with the Third Party. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 10
| In the event that the Parties are unable to agree on such action, (1) * and (2) EntreMed shall be entitled to take such action as it may reasonably consider expedient pursuant to the terms as set forth in Clause 3.5 herein. In the event that EntreMed fails to take such action as reasonably requested by EPIL, EntreMed shall indemnify and hold EPIL harmless against all Infringement Claims as they related to the manufacture, * of the Product in the Territory to the extent that they relate to the period after the date of the direction to take such action. |
| 3.5. | Third Party Licenses. |
| 3.5.1 | Notice. If EntreMed reasonably believes that the sale of Products would infringe the intellectual property rights of a Third Party unless a license is obtained from such Third Party, and that such infringement arises as a result of the incorporation of the EPIL Technology into the Product, EntreMed shall so inform EPIL by written notice, which shall include documents supporting EntreMed’s position and shall notify EPIL that it has thirty (30) days in which to respond. | ||
| 3.5.2 | Counter-Notice. EPIL shall have thirty (30) days to review the notice from EntreMed and to agree or disagree with EntreMed’s belief by written counter-notice. If EPIL disagrees with EntreMed’s belief, then EPIL shall provide EntreMed with documents supporting EPIL’s position. EntreMed shall have thirty (30) days from the date of receipt to review the documents from EPIL. Failure by EPIL to respond to EntreMed’s notice, or by EntreMed to respond to EPIL’s notice, shall be taken for the purposes of the decision as to whether to obtain a license under this Clause 3.5 (but for the avoidance of doubt, not for any other purpose whatsoever) as accession to the position of the other Party. The Parties agree that the time periods as set forth in this Clause 3.5.2 may be reasonably extended by the mutual written agreement of the Parties. | ||
| 3.5.3 | Use of Documents. All documents exchanged by the Parties shall be maintained in confidence and shall not be used for any other purpose than the resolution of the scope of a Third Party’s intellectual property rights as it pertains to the sale of a Product as set forth in this Agreement. | ||
| 3.5.4 | Resolution. If EPIL disagrees with EntreMed’s position pursuant to the terms as set forth in Clause 3.5.2 herein and if EntreMed maintains its original position after such review period, then the matter shall be referred first to the officers of EPIL and EntreMed having responsibility for the subject matter of the dispute, or their designees. Such officers, or their designees, as the case may be, shall negotiate in good faith to resolve such dispute in a mutually satisfactory manner. If such efforts do not result in a mutually satisfactory resolution of the dispute within thirty (30) days of such referral, the matter shall be referred to the chief executive officer of each Party, or their respective designees. | ||
| 3.5.5 | Final Resolution. If the Parties’ chief executive officers or their designees do not resolve the dispute within thirty (30) days of the matter being referred to them (or such longer time periods as may be mutually agreed in writing by the Parties), an independent mutually acceptable Third Party law firm with suitable expertise in the |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 11
| field of intellectual property in pharmaceuticals (the “Firm”) shall be appointed to determine whether, in its opinion, the sale of the Products would infringe such Third Party intellectual property. Once appointed, the Firm shall not be used by either Party for matters pertaining to the EPIL Intellectual Property or the EntreMed Intellectual Property, other than subsequent disputes under this Clause 3.5. The costs of the Firm shall be borne by the Party with whom the Firm disagrees. | |||
| 3.5.6 | Disputes Not To Be Reopened. The procedure in Clauses 3.5.1 to 3.5.5 shall not be used more than once in relation to any particular Third Party intellectual property allegedly infringed, absent new and relevant facts. | ||
| 3.5.7 | Negotiation. If the Parties or the Firm determine that a license should be obtained, EPIL shall have the initial right to negotiate such license. In the event that EPIL is unsuccessful in obtaining such a license within one hundred and twenty (120 days) of its first meeting with such Third Party, then EntreMed shall have the right to negotiate such license. | ||
| 3.5.8 | Terms. The Party attempting to negotiate the license shall: |
| 3.5.8.1 | use all Commercially Reasonable Efforts to achieve commercially reasonable terms; | ||
| 3.5.8.2 | keep the other Party reasonably informed of such negotiations; | ||
| 3.5.8.3 | submit to the other Party any draft terms before approval (insofar as they affect that other Party); | ||
| 3.5.8.4 | reasonably take into account any comments the other Party may have; and | ||
| 3.5.8.5 | without prejudice to the generality of the foregoing, use all Commercially Reasonable Efforts to ensure that the license is sub-licensable to EntreMed (in the case of EPIL) or freely transferable to EPIL (in the case of EntreMed). |
| 3.5.9 | Unrelated Licenses. Nothing in this Clause 3.5 shall be construed as affecting EntreMed’s rights to obtain licenses wholly unrelated to the incorporation of the EPIL Technology in the Product, at its own expense. |
| 3.6. | Trademarks. |
| 3.6.1 | EntreMed Trademark. |
| 3.6.1.1 | EntreMed shall market the Product in the Territory under the EntreMed Trademark. | ||
| 3.6.1.2 | EntreMed grants to EPIL and its Affiliates for the Term a royalty free, worldwide, non-exclusive license to the EntreMed Trademark and, if different, trademarks showing EntreMed’s corporate logo (the “EntreMed Logo”), for the purpose of EPIL’s promotion of its activities and of the EPIL Technology. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 12
| 3.6.1.3 | EPIL shall ensure that each reference to and use of the EntreMed Trademark and EntreMed Logo by EPIL is in a manner approved by EntreMed and accompanied by an acknowledgement, in a form approved by EntreMed, that the same is a trademark (or registered trademark) of EntreMed. | ||
| 3.6.1.4 | EPIL shall not use the EntreMed Trademark or EntreMed Logo in any way which might materially prejudice its distinctiveness or validity or the goodwill of EntreMed therein. | ||
| 3.6.1.5 | EPIL shall not use any trademarks or trade names so resembling the EntreMed Trademark or EntreMed Logo as to be likely to cause confusion or deception. | ||
| 3.6.1.6 | EntreMed will be entitled to conduct all enforcement proceedings relating to the EntreMed Trademark or EntreMed Logo and shall at its sole discretion decide what action, if any, to take in respect of any infringement or alleged infringement of the EntreMed Trademark or EntreMed Logo or passing-off or any other claim or counter-claim brought or threatened in respect of the use or registration of the EntreMed Trademark or EntreMed Logo. Any such proceedings shall be conducted at EntreMed’s expense and for its own benefit. |
| 3.6.2 | EPIL Trademark. |
| 3.6.2.1 | EntreMed shall prominently display the appropriate EPIL Trademark on the packaging of the Product and on all promotional materials in relation to the Product to acknowledge that EPIL Intellectual Property has been applied in developing and manufacturing the Product. | ||
| 3.6.2.2 | EPIL grants to EntreMed for the Term a paid-up, worldwide, non-exclusive license to the EPIL Trademark, solely for the purpose of fulfilling EntreMed’s obligations under this Clause 3.6.2.2 and exercising its rights under this Agreement. | ||
| 3.6.2.3 | EntreMed shall ensure that each reference to and use of the EPIL Trademark by EntreMed is in a manner approved by EPIL and accompanied by an acknowledgement, in a form approved by EPIL, that the same is a trademark (or registered trademark) of EPIL. | ||
| 3.6.2.4 | EntreMed shall not use the EPIL Trademark in any way which might materially prejudice its distinctiveness or validity or the goodwill of EPIL therein. | ||
| 3.6.2.5 | EntreMed shall not use any trademarks or trade names so resembling the EPIL Trademark as to be likely to cause confusion or deception. | ||
| 3.6.2.6 | EPIL will be entitled to conduct all enforcement proceedings relating to the EPIL Trademark and shall at its sole discretion decide what action, if any, to take in respect of any infringement or alleged infringement of the EPIL Trademark or passing-off or any other claim or counter-claim brought or |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 13
threatened in respect of the use or registration of the EPIL Trademark.
Any such proceedings shall be conducted at EPIL’s expense and for its
own benefit.
| 4. | NON-COMPETITION |
In order to provide adequate protection of EPIL Know-How, EPIL Confidential Information and EPIL
Highly Confidential Information, EntreMed and its Affiliates shall not develop, market or sell in
combination with a Technological Competitor any * formulation containing the
Compound, other than the Product: (i) in the EEA for a period of * beginning on the date of
first In Market sale of the Product in the EEA, or (ii) elsewhere in the Territory * .
| 5. | REGISTRATION, MARKETING AND THE PROMOTION OF THE PRODUCT | |
| 5.1. | Regulatory Matters. Except as specified otherwise in this Agreement or in the Related Agreements, EntreMed shall own and shall be responsible for filing for and maintaining all necessary Regulatory Approvals, including for the manufacture of the Compound and any necessary export or import licenses in relation to the Compound and/or the Product. | |
| 5.2. | Diligent Efforts. EntreMed shall use Commercially Reasonable Efforts: |
| 5.2.1 | to obtain Regulatory Approvals for the Product; and | ||
| 5.2.2 | to market and promote the Product with a view to achieving the commercial potential of the Product throughout the Territory. |
| 5.3. | Required Markings. All trade packaging and marketing materials shall: |
| 5.3.1 | to the extent permitted by law, include due acknowledgement that the Product is developed and manufactured by EPIL or its Affiliate; and | ||
| 5.3.2 | have marked representative patent number(s) including that of the formulation patent in respect of the EPIL Patents on the Product, or otherwise reasonably communicate to the trade the existence of any EPIL Patents for the countries within the Territory in such a manner as to ensure compliance with, and enforceability under, applicable laws. |
| 5.4. | Launch. EntreMed shall use Commercially Reasonable Efforts to effect the * commercial launch of the Product: |
| 5.4.1 | in the USA * days of the Regulatory Approval in the USA, subject to the timely receipt of launch stocks under the Manufacturing and Supply Agreement and there not being any Infringement Claim pending; and | ||
| 5.4.2 | in the United Kingdom, Germany, France, Italy and Japan * days after the relevant Regulatory Approval, subject to the timely receipt of launch stocks under the Manufacturing and Supply Agreement and there not being any pending Infringement Claim. |
| 5.5. | Reporting. EntreMed shall promptly notify EPIL in writing of the date that the Product is administered in the first patient in a Post-Phase 1b Clinical Study and in the first patient in the |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 14
First Pivotal Study. EntreMed also shall promptly notify EPIL in writing of (i) the
submission date of all Regulatory Applications, (ii) the date that all such submissions are
accepted for filing by the relevant RHA, where applicable, and (iii) the date of all
Regulatory Approvals.
Following the first Regulatory Approval of the Product in a country in the Territory, the
Parties shall meet as often as reasonably requested by the other (not more than once per
calendar quarter). At such meetings, EntreMed shall report on the ongoing sales performance
of the Product in each country of the Territory, *
for the next quarterly
period. Such meetings may be held by telephone. If held in person, each Party shall be
responsible for its own costs in respect of travel and accommodation expenses in attending
such meetings.
| 6. | FINANCIAL PROVISIONS | |
| 6.1. | License Milestone Payments. In consideration of the grant of the EPIL License, EntreMed shall pay to EPIL the following non-refundable amounts: |
| 6.1.1 | US$ * upon the earlier of (i) EntreMed’s announcement of a Post-Phase1b Clinical Study or (ii) the administration of Product to the first patient in a Post-Phase1b Clinical Study; | ||
| 6.1.2 | Notwithstanding the milestone payment made under Clause 6.1.1(i) or 6.1.1(ii), US$ * upon the administration of Product to the first patient in a post-Phase1b clinical study; | ||
| 6.1.3 | US$ * upon the earlier of (i) EntreMed’s announcement of the first Pivotal Study or (ii) the administration of Product to the first patient in the first Pivotal Study in support of a Regulatory Application; | ||
| 6.1.4 | US$ * upon the administration of Product to the first patient in the first Pivotal Study in support of a Regulatory Application; | ||
| 6.1.5 | US$ * upon completion of production and packaging of the first ICH Qualifying Batch of Product; | ||
| 6.1.6 | US$ * upon the execution of all validation protocols and the completion of a validation report for the commercial manufacturing process; | ||
| 6.1.7 | US$ * upon the acceptance for filing of the first Regulatory Application in the US; | ||
| 6.1.8 | US$ * upon the acceptance for filing of the first Regulatory Application in the EEA * ; | ||
| 6.1.9 | US$ * upon the * acceptance for filing of the first Regulatory Application in Japan * ; | ||
| 6.1.10 | US$ * upon the (i) successful FDA pre-approval inspection (PAI) of a commercial manufacturing facility, as indicated by FDA * |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 15
* , (ii) notification by the FDA that the FDA waives the
PAI,
*
;
| 6.1.11 | US$ * upon the first In Market Sale in the US; | ||
| 6.1.12 | US$ * the first In Market sale in the EEA * ; and | ||
| 6.1.13 | US$ upon * the first In Market sale in Japan or * . | ||
| the payments described in this Clause 6.1 being “License Milestone Payments”. |
| 6.2. | Compensation Fee. In the event that EntreMed executes a Third Party agreement for the supply of Compound for * , EntreMed will pay to EPIL a compensation fee of * the “Compensation Fee”). For clarification purposes, the Compensation Fee will not be required to be paid in the event that EntreMed discontinues the development of the Product for any reason and * . | |
| 6.3. | Not Subject to Future Performance Obligations. The License Milestone Payments shall not be subject to future performance obligations of EPIL to EntreMed and shall not be applicable against future services provided by EPIL to EntreMed. | |
| The terms of Clause 6.1 relating to the License Milestone Payments are independent and distinct from the other terms of this Agreement. | ||
| 6.4 | Royalty on Sales for the Initial Term. In further consideration of the grant of the EPIL License, EntreMed shall pay to EPIL a non-refundable stepped royalty of * of aggregate Net Sales for the first * of annual aggregate Net Sales in the Territory increasing to * of aggregate Net Sales for all amounts of annual aggregate Net Sales greater than * in the Territory, for the Initial Term. | |
| 6.5 | Royalty on Sales for the Extended Term and any additional Extended Term. In further consideration of the continuing grant by EPIL to EntreMed of a license to use the EPIL Know-How during the Extended Term, EntreMed shall pay to EPIL a non-refundable royalty of * of aggregate Net Sales in the Territory provided this Agreement has not been terminated * pursuant to Clause 9. | |
| 6.6 | Generic Competition. If during any calendar quarter of the Extended Term, one or more Generics is marketed and all such Generics achieve in any country of the Territory an aggregate market share of not less than * percent ( * %) (calculated by reference to the total sales of the Product and all Generics, as demonstrated to the reasonable satisfaction of EPIL), the royalty payable to EPIL shall be reduced to * percent ( * %) in respect of that country in that calendar quarter. For the avoidance of doubt, no such reduction shall apply after such circumstances are eliminated, nor in any country where such circumstances do not apply. | |
| 6.7. | Bundling. During the Term, in the event that EntreMed, its Affiliates or a permitted Third Party sub-licensee shall sell to Third Party purchasers the Product together with other products, with pricing of the Product tied to pricing or volume of purchase of other products (by the method |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 16
commonly known in the pharmaceutical industry as “bundling”), then for the purposes of
determining the Net Sales of the Product, * . The Net Sales of such permitted
bundled products (meaning the combined price at which the Product and other product(s) are
sold) will be multiplied by a fraction, the numerator of which is standard price for the
Product multiplied by the number of units of Product sold in the bundled products and the
denominator of which is the numerator plus the sum of the standard prices of the other
products in the product bundle multiplied by the number of units of these products sold as
part of the product bundle.
For the avoidance of doubt, in the event that EntreMed, its Affiliates or a permitted Third
Party sub-licensee sells the Product with other products which (i) are not prescription
products and/or (ii) which are generic products, and the price attributable to the Product
is less than the average price of “arms length” sales to similar customers for the reporting
period in which sales occur (such sales to be excluded from the calculation of the average
price of “arms length” sales), Net Sales for any such sales shall be * .
| 6.8. | Method of calculation of fees. The Parties acknowledge and agree that the methods for calculating the milestones, royalties and fees under this Agreement are for the purposes of the convenience of the Parties, are freely chosen and not coerced. | |
| 7. | MANUFACTURE AND SUPPLY | |
| 7.1. | Manufacturing and Supply Agreement. The Parties agree that they, or their respective Affiliates, will negotiate in good faith a Manufacturing and Supply Agreement for the commercial supply of Product which they shall aim to execute on or before the first anniversary of entering into this Agreement. The Parties agree that the Manufacturing and Supply Agreement shall incorporate Clauses 7.2 and 7.3 and the key terms set out in Schedule 5. | |
| 7.2. | Supply. The Parties agree that EPIL or an Affiliate shall have the exclusive right to manufacture and supply to EntreMed, its Affiliates and permitted sub-licensees their entire requirement of the Product in the Territory for commercial purposes in accordance with the Manufacturing and Supply Agreement that will be negotiated in good faith between the Parties or between EntreMed and an Affiliate of EPIL. The cost of goods for Product supplied pursuant to the Manufacturing and Supply Agreement containing a * percent ( * %) API concentration shall be as set out in Schedule 4. The cost of goods for Product supplied under the Manufacturing and Supply Agreement * . The Parties further agree that the Manufacturing and Supply Agreement shall contain provisions relating to * | |
. |
||
| 7.3. | Right to Manufacturing License. Subject to the terms of this Agreement and the Manufacturing and Supply Agreement, the Parties further agree that in the event EPIL or any EPIL Affiliate is unable to manufacture the Product for commercial purposes for reasons of failure to supply (as such will be set out in more detail in the Manufacturing and Supply Agreement), EPIL shall grant to EntreMed a non-exclusive license under the EPIL Intellectual Property to make or have made the Product in the Field in the Territory (“the Manufacturing License”). Subject to the prior |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 17
written consent of EPIL which shall not be unreasonably withheld or delayed, EntreMed shall
have the right to grant a sub-license of the Manufacturing License *
.
Additionally, in the event that EntreMed, or as the case may be, the sub-licensee of the
Manufacturing License entrusted with the manufacture of the Product (either, the “Designated
Manufacturer”) becomes
*
the Designated Manufacturer shall not be entitled to
exercise manufacturing rights hereunder (or under the sub-license of the Manufacturing
License, as applicable) or if manufacturing rights were previously exercised prior to the
event in question such manufacturing rights shall automatically revert to EPIL. In either
event, EPIL and EntreMed (or * ) shall co-operate in good faith to locate an
independent Third Party manufacturer who is not * and is capable of manufacturing the Product.
| 8. | PAYMENTS, REPORTS AND AUDITS | |
| 8.1. | Records. Commencing with the first In Market sale in the Territory, EntreMed shall keep true and accurate records of gross sales of the Product, the items deducted from the gross amount in calculating the Net Sales, the Net Sales and the royalties payable to EPIL under Clauses 6.4. and 6.5, as applicable EntreMed shall deliver to EPIL a written statement (the “Statement”) thereof within forty-five (45) days following the end of each calendar quarter, (or any part thereof in the first or last calendar quarter of this Agreement) for such calendar quarter. The Statement shall outline on a country-by-country basis, the calculation of the Net Sales from gross revenues during that calendar quarter, the applicable percentage rate, and a computation of the sums due to EPIL. The Parties’ financial officers shall agree upon the precise format of the Statement. | |
| 8.2. | Foreign Currency. Payments due on Net Sales of the Product based on sales amounts in a currency other than US$ shall first be calculated in the foreign currency and then converted to US$ on the basis of the exchange rate in effect for the purchase of US$ with such foreign currency quoted in the Wall Street Journal (or comparable publication if not quoted in the Wall Street Journal) over the five (5) business days prior to the end of the relevant calendar quarter in which such payment is due. | |
| 8.3. | VAT. All payments to EPIL are exclusive of any applicable value added or any other sales tax, for which EntreMed will be additionally liable if applicable. | |
| 8.4. | Taxes. If EntreMed is required by law to pay or withhold any income or other taxes on behalf of EPIL with respect to any monies payable to EPIL under this Agreement: |
| 8.4.1 | EntreMed shall deduct them from the amount of such monies due; | ||
| 8.4.2 | any such tax required to be paid or withheld shall be an expense of and borne solely by EPIL; | ||
| 8.4.3 | EntreMed shall promptly provide EPIL with a certificate or other documentary evidence to enable EPIL to support a claim for a refund or a foreign tax credit. |
| 8.5. | Double Tax Co-operation. EPIL and EntreMed agree to co-operate reasonably as may be necessary to take advantage of any double taxation agreements or similar agreements as may, from time to time, be available in order to enable EntreMed to make such payments to EPIL without any deduction or withholding. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 18
| 8.6. | Timing. Payments to EPIL shall be made as follows: |
| 8.6.1 | each of the License Milestone Payments shall be paid within fifteen (15) calendar days of the achievement of the relevant event to which they relate; and | ||
| 8.6.2 | payment of royalties shall be made upon provision of the Statement (45 days following the end of the calendar quarter). |
| 8.7. | Manner of Payment. All payments due hereunder shall be made in US$ to the designated bank account of EPIL in accordance with such timely written instructions as EPIL shall from time to time provide. | |
| 8.8. | Interest. Without prejudice to EPIL’s other remedies hereunder, EntreMed shall pay interest to EPIL on sums not paid to EPIL on the date on which payment should have been made pursuant to the applicable provisions of this Agreement (“Due Date”) over the period from the Due Date until the date of actual payment (both before and after judgement) at the Prime Rate publicly announced by Xxxxxx Guaranty Trust Company of New York at its principal office on the Due Date (or next to occur Business Day, if such date is not a Business Day) plus * %, such interest to payable on demand from time to time and compounded monthly. Interest shall be payable both before and after judgment. | |
| 8.9. | Audit. For the one hundred and eighty (180) day period following the close of each calendar year of the Agreement, EntreMed will, in the event that EPIL reasonably requests such access, provide EPIL’s independent certified accountants (reasonably acceptable to EntreMed) with access, during regular business hours, no more than once per calendar year unless a discrepancy is discovered, and subject to the confidentiality provisions as contained in this Agreement, to EntreMed’s books and records relating to the Product, solely for the purpose of verifying the accuracy and reasonable composition of the calculations under this Agreement for the calendar year then ended. | |
| 8.10. | Correction of Discrepancies. In the event of a discovery of a discrepancy, a correcting payment shall be made by EntreMed to EPIL together with interest at the rate specified in Clause 8.8. If the discrepancy exceeds * percent * %) of the amount due then additionally the cost of such accountants shall be borne by EntreMed. | |
| 9. | DURATION AND TERMINATION | |
| 9.1. | Initial Term. This Agreement shall be deemed to have come into force on the Effective Date and, subject to the rights of termination outlined in this Clause 9 and the provisions of applicable laws, will expire on a country-by-country basis: |
| 9.1.1 | on the* anniversary of the date of the first In Market sale of the Product in the country concerned; or | ||
| 9.1.2 | in any country upon the expiration of the life of the last to expire patent having a Valid Claim where such patent is included in the EPIL Intellectual Property in that country; | ||
| whichever date is* to occur (the “Initial Term”). |
| 9.2. | Continuation. At the end of the Initial Term in such country, this Agreement shall continue automatically for * year periods thereafter (collectively known as the “Extended Term”), |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 19
unless the Agreement has been terminated by * by serving one (1) year’s written notice
* immediately prior to the end of the Initial Term or any such additional Extended Term
| 9.3. | Term. The Initial Term and the Extended Term shall collectively be referred to as the “Term” in this Agreement. | |
| 9.4. | Breach / Insolvency. In addition to the rights of termination provided for elsewhere in this Agreement, either Party will be entitled forthwith to terminate this Agreement by written notice to the other Party if: |
| 9.4.1 | that other Party commits a material breach of any of the provisions of this Agreement, and fails to cure the same within sixty (60) days after receipt of a written notice from another Party hereto giving full particulars of the breach and requiring it to be remedied; provided, that if the breaching Party has proposed a course of action to cure the breach and is acting in good faith to cure same but has not cured the breach by the sixtieth (60th) day after receipt of a written notice, such period shall be extended by such period as is reasonably necessary to permit the breach to be cured, provided that such period shall not be extended by more than thirty (30) additional days, unless otherwise agreed in writing by the Parties; | ||
| 9.4.2 | that other Party goes into liquidation under the laws of any applicable jurisdiction (except for the purposes of amalgamation or reconstruction and in such manner that the company resulting therefrom effectively agrees to be bound by or assume the obligations imposed on that other Party under this Agreement); | ||
| 9.4.3 | a receiver, administrator, examiner, trustee or similar officer is appointed over all or substantially all of assets of that other Party under the laws of any applicable jurisdiction; or | ||
| 9.4.4 | any proceedings are filed or commenced by that other Party under bankruptcy, insolvency or debtor relief laws, or anything analogous to any of the foregoing under the laws of any applicable jurisdiction occurs in relation to that other Party. |
| 9.5. | Additional EntreMed Termination Rights. EntreMed shall be entitled to terminate this Agreement for any country or countries of the Territory by written notice to EPIL where: |
| 9.5.1 | the sale of the Product is prohibited by the RHA in such country or countries; | ||
| 9.5.2 | despite having used Commercially Reasonable Efforts, EntreMed is unable to obtain Regulatory Approval for the Product in such country or countries so as to permit a reasonable commercial return for EntreMed. |
| 9.6. | Additional EPIL Termination Rights. In further addition to the rights and termination provided for elsewhere in this Agreement, EPIL shall be entitled to terminate this Agreement for any country or countries of the Territory in the event that EntreMed fails to file a Regulatory Application in the United States within * of the date that Pivotal Studies are completed in relation to the first Product indication. However: |
| 9.6.1 | If, * , EntreMed has and is continuing to use Commercially Reasonable Efforts to complete the supporting clinical studies and file such US |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 20
Regulatory Application, EPIL will consider in good faith whether or not it deems it
appropriate to exercise such right of termination;
| 9.6.2 | In the event that the EntreMed’ failure to file such US Regulatory Application is due to EPIL’s or its Affiliate’s decision not to manufacture the Product, * ; | ||
| 9.6.3 | In the event that, during the Term, EntreMed, its sublicensee or their respective Affiliates * , EPIL, * , shall be entitled to terminate this Agreement and the Related Agreements in their entirety. |
| 9.7. | Cross-Termination. In the event this Agreement is terminated * , pursuant to the terms as set forth in this Clause 9, other than the surviving provisions as set forth in the Related Agreements, the MEEA and the CSA, as these agreements pertain to the Compound and/or the Product, if applicable, the Related Agreements, the MEEA and the CSA shall automatically terminate and be of no further legal force and effect as related to Compound and/or the Product. | |
| 10. | CONSEQUENCES OF TERMINATION | |
| 10.1. | General Consequence. Upon exercise of those rights of termination specified in Clause 9 herein or elsewhere in this Agreement, this Agreement shall, subject to Clauses 10.2, automatically terminate forthwith and be of no further legal force or effect. | |
| 10.2. | Specific Consequences. Upon termination of the Agreement by either Party, or upon termination by EPIL of the EPIL License for a particular country under Clauses 9.6 herein, the following shall be the consequences relating to the Territory or the particular country, as applicable: |
| 10.2.1 | any sums that were due from EntreMed to EPIL under the provisions of this Agreement prior to its termination or expiry, or become due by virtue of such termination or expiry, shall be paid in full within thirty (30) calendar days of termination of this Agreement or termination of the EPIL Licence in a particular country, as applicable, and EPIL shall not be liable to repay to EntreMed any amount of money paid or payable by EntreMed to EPIL up to the date of the termination of this Agreement; | ||
| 10.2.2 | where a License Milestone Payment is due by reference to some period after an event, and termination occurs after such event but before the due date for such License Milestone Payment, such License Milestone Payment shall become immediately due and payable and shall be paid in accordance with the provisions of Clause 10.2.1; and for the avoidance of doubt, EntreMed’ liability for any then accrued License Milestone Payments shall not otherwise be affected by virtue of termination of this Agreement; | ||
| 10.2.3 | all representations and warranties shall insofar are appropriate remain in full force and effect; | ||
| 10.2.4 | the provisions of this Agreement regarding with respect to confidentiality and non-use of materials or confidential information shall remain in effect for a further period of 10 (ten) years from such date of termination. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 21
| 10.2.5 | the rights of inspection and audit shall continue in force for the period referred to in the relevant provisions of this Agreement; | ||
| 10.2.6 | the license granted by EPIL to EntreMed of the EPIL Trademark under Clause 3.6.2.2 shall automatically terminate; | ||
| 10.2.7 | any other provision of this Agreement which, by its nature, is intended to continue after termination, shall survive termination; | ||
| 10.2.8 | any sub-license granted under Clause 2.2 shall automatically terminate;. |
| 11. | WARRANTIES, INDEMNIFICATION AND LIABILITY | |
| 11.1. | EPIL Warranties. EPIL represents and warrants to EntreMed as of the Effective Date, as follows: |
| 11.1.1 | EPIL has the right to enter into this Agreement and grant the EPIL License. | ||
| 11.1.2 | Other than the Merck Agreement, there are no agreements between EPIL and any Third Party that conflict with the EPIL License. | ||
| 11.1.3 | EPIL is the owner of the EPIL Patents. | ||
| 11.1.4 | EPIL or its Affiliates are not obligated to assign to any sublicensee or any Third Party any EntreMed Improvements made by EPIL or its Affiliates or made on EPIL’s behalf by its sublicensees or Third Party contract manufacturers. | ||
| 11.1.5 | Except for the oppositions in the European Patent Office of EP-B-499299 and EP-1185371 EPIL has not been notified or does not otherwise have knowledge of any infringement proceedings, actions, suits or complaints pending against nor any outstanding injunctions, judgments, orders, decrees, rulings or other charges against EPIL or any Affiliate of EPIL in connection with the EPIL Patents or the EPIL Know How in the Territory that may affect the making, using, or selling of the Product. | ||
| 11.1.6 | To the best knowledge of EPIL, with no special search, the use of the EPIL Technology in the making, using, selling or importation of the Product does not infringe any intellectual property rights of a Third Party patents in the Territory. |
| 11.2. | EntreMed Warranties. EntreMed represents and warrants to EPIL as of the Effective Date, as follows: |
| 11.2.1 | EntreMed has the right to enter into this Agreement and the Related Agreements. | ||
| 11.2.2 | There are no agreements between EntreMed and any Third Party that conflict with this Agreement. | ||
| 11.2.3 | EntreMed is the owner or exclusive licensee of the EntreMed Patents. | ||
| 11.2.4 | EntreMed or its Affiliates are not obligated to assign to any sublicensee or any Third Party any EPIL Improvements made by EntreMed or its Affiliates or made on EntreMed’s behalf by its sublicensees or Third Party contract manufacturers. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 22
| 11.2.5 | EntreMed has not been notified of any infringement proceedings, actions, suits or complaints pending against nor any outstanding injunctions, judgments, orders, decrees, rulings or other charges against EntreMed or any Affiliate of EntreMed in connection with the EntreMed Patents or the EntreMed Know-How in the Territory that may affect the making, using, or selling of the Product. | ||
| 11.2.6 | To the best knowledge of EntreMed, with no special search, the use of the EntreMed Intellectual Property in the making, using, selling or importation of the Product does not infringe any intellectual property rights of a Third Party in the Territory. |
| 11.3. | Mutual Indemnification. Each of the Parties shall indemnify and hold harmless the other Party against all Claims insofar as they arise out of or in connection with any breach by the first Party of any of its representations, obligations or warranties under this Agreement or from the first Party’s fraud or wilful misconduct. | |
| 11.4. | Infringement Claims. The Parties acknowledge that Clause 3.4 herein contains the Parties’ full agreement as regards liability for Infringement Claims, save to the extent that Clause 3.4 incorporates other provisions of this Agreement by specific cross-reference. | |
| 11.5. | Indemnification (Medical Claims). EntreMed shall indemnify EPIL against all Claims made or brought against EPIL or its Affiliates seeking damages for personal injury (including death) and/or for the cost of medical treatment, caused by or attributed to the Product. | |
| 11.6. | Sub-licensees. With reference to Clause 2.2.3 herein, EntreMed shall indemnify and hold harmless EPIL or its Affiliates to the extent that any Claims arise out of any such acts or omissions of any sub-licensee. | |
| 11.7. | Conduct of Claims. The Party seeking an indemnity shall: |
| 11.7.1 | fully and promptly notify the other Party of any Claim or proceedings, or threatened Claim or proceedings; | ||
| 11.7.2 | permit the indemnifying Party to take full control of such Claim or proceedings at its sole cost and expense, with counsel of the indemnifying Party’s choice, provided that the indemnifying Party shall reasonably and regularly consult with the indemnified Party in relation to the progress and status of such Claim or proceedings; | ||
| 11.7.3 | co-operate in the investigation and defence of such Claim or proceedings; and | ||
| 11.7.4 | take all reasonable steps to mitigate any loss or liability in respect of any such Claim or proceedings. |
The indemnifying Party may settle a Claim on terms which provide only for monetary relief
and do not include any admission of liability. Save as aforesaid, neither the indemnifying
Party nor the Party to be indemnified shall acknowledge the validity of, compromise or
otherwise settle any Claim without the prior written consent of the other, which shall not
be unreasonably withheld.
| 11.8. | Exclusion of Implied Warranties. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, ENTREMED ACKNOWLEDGES THAT THE EPIL LICENSE IS GRANTED ON AN “AS IS” BASIS, WITHOUT REPRESENTATION OR WARRANTY WHETHER EXPRESS OR IMPLIED INCLUDING WARRANTIES OF MERCHANTABILITY, FITNESS |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 23
FOR A PARTICULAR PURPOSE, OR INFRINGEMENT OF THIRD PARTY RIGHTS, AND ALL SUCH WARRANTIES ARE
EXPRESSLY DISCLAIMED TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAWS.
| 11.9. | Exclusion of Consequential Loss. WITHOUT PREJUDICE TO THE OBLIGATION OF EITHER PARTY TO INDEMNIFY THE OTHER IN RESPECT OF CLAIMS BY A THIRD PARTY, NOTWITHSTANDING ANYTHING TO THE CONTRARY IN THIS AGREEMENT, EPIL AND ENTREMED SHALL NOT BE LIABLE TO THE OTHER BY REASON OF ANY REPRESENTATION OR WARRANTY, CONDITION OR OTHER TERM OR ANY DUTY OF COMMON LAW, OR UNDER THE EXPRESS TERMS OF THIS AGREEMENT, FOR ANY INDIRECT, CONSEQUENTIAL, SPECIAL, INCIDENTAL OR PUNITIVE LOSS OR DAMAGE (WHETHER FOR LOSS OF CURRENT OR FUTURE PROFITS, LOSS OF ENTERPRISE VALUE OR OTHERWISE) AND WHETHER OCCASIONED BY THE NEGLIGENCE OF THE RESPECTIVE PARTIES, THEIR EMPLOYEES OR AGENTS OR OTHERWISE. | |
| 11.10. | Extension of Indemnification. Where this Agreement provides for the indemnification of a Party to this Agreement or for the limitation of a Party’s liability, such indemnification and/or limitation (as the case may be) shall also apply for the benefit of such Party’s Affiliates and the employees, officers, directors and agents of any of them, acting in such capacity. | |
| 11.11. | Indemnification of Affiliates. Where this Agreement provides for the indemnification of a Party, such indemnification shall also apply for the benefit of such Party’s Affiliates and the employees, officers, directors and agents of any of them, acting in such capacity. | |
| 12. | CONFIDENTIALITY | |
| 12.1. | Confidential Information: The Parties agree that it will be necessary, from time to time, to disclose to each other confidential and proprietary information, including without limitation, inventions, trade secrets, specifications, designs, data, know-how and other proprietary information relating to EPIL Intellectual Property, EntreMed Intellectual Property, the Product, processes, services and business of the disclosing Party. | |
| The foregoing shall be referred to collectively as “Confidential Information”. | ||
| 12.2. | Exclusion. Confidential Information shall be deemed not to include: |
| 12.2.1 | information which is in the public domain; | ||
| 12.2.2 | information which is made public through no breach of this Agreement; | ||
| 12.2.3 | information which is independently developed by a Party, as evidenced by such Party’s records; or | ||
| 12.2.4 | information that becomes available to a receiving Party on a non-confidential basis, whether directly or indirectly, from a source other than the other Party hereto, which source did not acquire this information on a confidential basis. |
| 12.3. | Use of Confidential Information. Any Confidential Information disclosed by the disclosing Party shall be used by the receiving Party exclusively for the purposes of fulfilling the receiving Party’s |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 24
obligations or exercising its rights under this Agreement or Related Agreements and for no
other purpose.
| 12.4. | Non-Disclosure. Except as otherwise specifically provided in this Agreement, each Party shall disclose Confidential Information of the other Party only to those employees, representatives and agents requiring knowledge thereof in connection with fulfilling the Party’s obligations under this Agreement, and not to any other Third Party. | |
| 12.5. | Obligation to Inform. Each Party further agrees to inform all such employees, representatives and agents of the terms and provisions of this Agreement relating to Confidential Information and to obtain their agreement hereto as a condition of receiving Confidential Information. | |
| 12.6. | Care. Each Party shall exercise the same standard of care as it would itself exercise in relation to its own confidential information (but in no event less than a reasonable standard of care) to protect and preserve the proprietary and confidential nature of the Confidential Information disclosed to it by the other Party. | |
| 12.7. | Return of Information. Upon termination or expiration of this Agreement, each Party shall promptly, upon the written request of the other Party, return all documents and any copies thereof containing Confidential Information belonging to, or disclosed by, such other Party, save that it may retain one copy of the same solely for the purposes of ensuring compliance with this Clause 12. | |
| 12.8. | Attribution. Any breach of this Clause 12 by any person informed by one of the Parties is considered a breach by the Party itself. | |
| 12.9. | Acknowledgment. The Parties agree that the obligations of this Clause 12 are necessary and reasonable in order to protect the Parties’ respective businesses. The Parties further agree that monetary damages may be inadequate to compensate a Party for any breach by the other Party of its covenants and agreements with respect to confidentiality, and that each Party shall be entitled to seek injunctive or other equitable relief against the threatened or continued breach of those provisions, in addition to with any other remedy which may be available. | |
| 12.10. | Compound Data. For the purpose of demonstrating to Third Parties the benefits of the EPIL Technology, EPIL shall be entitled, subject to the prior written consent of EntreMed which shall not be unreasonably withheld, conditioned or delayed, to disclose to Third Parties the numerical values underlying the Compound data provided that EPIL does not disclose EntreMed’s name or the name of the Compound and that the above consent requirement shall not be deemed to apply to any numerical values that have already been publicly disclosed by EntreMed. | |
| 12.11. | Announcements. No announcement or public statement concerning the existence, subject matter or any term of this Agreement, or its performance, shall be made by or on behalf of any Party without the prior written approval of the other, such approval not to be unreasonably withheld, conditioned or delayed. | |
| 12.12. | Joint Press Release. The Parties agree to discuss the issue of a joint press release announcing the execution of this Agreement. If the Parties decide not to issue a joint press release regarding this event, then each Party shall be entitled to issue its own press release, but the wording of each such release shall be agreed to by the other Party in writing before publication. Following the publication of said initial press release(s), each Party shall be free to disclose, without the other Parties’ prior written consent, the existence of this Agreement, the identity of the other Party and |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 25
the terms of the Agreement that have already been publicly disclosed in the initial press release(s) but in no circumstance may
either Party disclose any other information regarding the existence, subject matter, or any term of this Agreement (such as
confidential information or commercially sensitive information on financial terms) or its performance, without the prior
written consent of the other.
| 12.13 | Other Disclosures. EntreMed shall be entitled to provide (i) a copy of this Agreement and Related Agreements in which all financial terms have been redacted to Third Parties that have expressed a genuine and legitimate interest in partnering or collaborating with EntreMed to commercialize the Product in the Territory or (ii) a full copy of this Agreement or Related Agreements to Third Parties that have expressed a genuine and legitimate interest in acquiring EntreMed or substantially all of EntreMed’s business assets. EPIL shall be entitled to provide a full copy of this Agreement and Related Agreements to Third Parties that have expressed an interest in acquiring EPIL Technology from EPIL as it relates to the manufacture or commercialization of Product. In each case, each such Third Party shall be bound by a confidentiality agreement with terms and conditions that are at least as restrictive as that set forth in this Article 12. The Parties further agree that EntreMed’s right to supply any such information to a Third Party that is a Technological Competitor shall require EPIL’s prior written consent, * . | |
| 12.14. | Required Disclosures. A Party (the “Disclosing Party”) will be entitled to make an announcement or public statement concerning the existence, subject matter or any term of this Agreement, or to disclose Confidential Information that the Disclosing Party is required to make or disclose pursuant to: | |
| 12.14.1 a valid order of a court or Governmental Authority; or | ||
| 12.14.2 any other requirement of law or any securities or stock exchange; | ||
| provided that if the Disclosing Party becomes legally required to make such announcement, public statement or disclosure hereunder, the Disclosing Party shall give the other Party prompt notice of such fact to enable the other Party to seek a protective order or other appropriate remedy concerning any such announcement, public statement or disclosure, including confidential treatment and/or appropriate redactions. | ||
| The Disclosing Party shall fully co-operate with the other Party in connection with that other Party’s efforts to obtain any such order or other remedy. If any such order or other remedy does not fully preclude announcement, public statement or disclosure, the Disclosing Party shall make such announcement, public statement or disclosure only to the extent that the same is legally required. | ||
| 13. | MISCELLANEOUS PROVISIONS | |
| 13.1. | Force Majeure. Neither Party shall be liable for failure or delay in the performance of any of its obligations under this Agreement if such failure or delay results from Force Majeure, but any such failure or delay shall be remedied by such Party as soon as practicable. | |
| 13.2. | Assignment. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 26
| 13.2.1 | EntreMed shall not be entitled to subcontract or delegate the whole or any part of its duties hereunder or to assign this Agreement to its Affiliate(s) where EntreMed becomes an Affiliate of a Technological Competitor unless the provisions of Clause 2.2 have been fully complied with. Subject to the foregoing, each Party be entitled without the consent of the other: |
| 13.2.1.1 | to subcontract or delegate the whole or any part of its duties hereunder to its Affiliate(s); and/or | ||
| 13.2.1.2 | to assign this Agreement to its Affiliate, provided that such assignment has no material adverse tax implications for the other Party. |
| 13.2.2 | Except as provided for in Clause 13.2.1, this Agreement may not be assigned by a Party without the prior written consent of the other, which shall not be unreasonably withheld or delayed. |
| 13.3. | Parties Bound. This Agreement shall be binding upon and run for the benefit of the Parties, their successors and permitted assigns. | |
| 13.4. | Relationship of the Parties. In this Agreement, nothing shall be deemed to constitute a partnership between the Parties or make either Party an agent for the other, for any purpose whatsoever. | |
| 13.5. | Entire Agreement. Without prejudice to Clause 13.13, this Agreement, together with the Related Agreements, constitutes the entire agreement and understanding between the Parties with respect to its subject matter, and except as expressly provided, supersedes all prior representations, writings, negotiations or understandings with respect to that subject matter. | |
| Nothing in this Clause 13.5 shall exclude any liability which any Party would otherwise have to the other Party or any right which either of them may have to rescind this Agreement in respect of any statements made fraudulently by the other prior to the execution of this Agreement or any rights which either of them may have in respect of fraudulent concealment by the other. | ||
| 13.6. | Severability. If any provision in this Agreement is deemed to be, or becomes invalid, illegal, void or unenforceable under applicable laws, such provision will be deemed amended to conform to applicable laws so as to be valid and enforceable, or if it cannot be so amended without materially altering the intention of the parties, it will be deleted, but the validity, legality and enforceability of the remaining provisions of this Agreement shall not be impaired or affected in any way. | |
| 13.7. | Further Assurance. Each Party shall do and execute, or arrange for the doing and executing of, each necessary act, document and thing reasonably within its power to implement this Agreement. | |
| 13.8. | Counterparts. This Agreement may be executed in any number of counterparts, each of which when so executed shall be deemed to be an original and all of which when taken together shall constitute this Agreement. | |
| 13.9. | Waivers. A failure to exercise or delay in exercising a right or remedy provided by this Agreement or by law does not constitute a waiver of the right or remedy or a waiver of other rights or remedies. No single or partial exercise of a right or remedy provided by this Agreement |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 27
or by law prevents further exercise of the right or remedy or the exercise of another right
or remedy.
| 13.10. | Variations. No variation of this Agreement shall be effective unless it is made in writing and signed by each of the Parties. | |
| 13.11. | Notices. |
| 13.11.1 | A notice under or in connection with this Agreement (a “Notice”): |
| 13.11.1.1 | shall be in writing; and | ||
| 13.11.1.2 | may be delivered personally or sent by first class post (and air mail if overseas) or by fax to the Party due to receive the Notice at its address set out below: |
| 13.11.2 | The address referred to in Clause 13.11.1.2 is: |
| (a) | in the case of EPIL: |
| Address: | Elan Pharma International Limited | |||||||||
| Monksland | ||||||||||
| Athlone | ||||||||||
| Co. Westmeath | ||||||||||
| Ireland | ||||||||||
| Fax: | x000 00 000 0000 | |||||||||
| Marked for the attention of : | Vice President and General Counsel | |||||||||
| Elan Drug Technologies | ||||||||||
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 28
| (b) | in the case of EntreMed: |
| Address: | EntreMed, Inc. | |||||
| 0000 Xxxxxxx Xxxxxx Xxxxx | ||||||
| Xxxxxxxxx, XX 00000 | ||||||
| Fax: | x0 000 000 0000 | |||||
| Marked for the attention of : | Vice President of Corporate Development | |||||
| 13.11.3 | Notice is deemed given: |
| 13.11.3.1 | if delivered personally, when the person delivering the notice obtains the signature of a person at the address referred to in Clause 13.11.1.2; | ||
| 13.11.3.2 | if sent by post, except air mail, two Business Days after posting it; | ||
| 13.11.3.3 | if sent by air mail, six Business Days after posting it; | ||
| 13.11.3.4 | if sent by fax, when confirmation of its transmission has been recorded by the sender’s fax machine. |
| 13.12 | Set-off. Each of the Parties will be entitled but not obliged to set-off against any amount of money payable to it by the other Party under this Agreement, any amount of money payable by it to the other Party under this Agreement. | |
| 13.13 | To the extent there is a conflict in the terms, conditions or procedures as set forth in this Agreement, the Related Agreements, the MEEA, and the CSA the terms, conditions or procedures of this Agreement shall govern. The Parties further agree that the Letter Agreement shall terminate in its entirety upon the Effective Date. | |
| 13.14 | Governing Law and Jurisdiction: This Agreement shall be governed by and construed in accordance with the laws of New York, without regard to its conflict of laws rules, and shall be subject to the exclusive jurisdiction of New York. |
***
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 29
SIGNED
/s/ Elan Pharma International Limited
Duly authorised for and on behalf of:
ELAN PHARMA INTERNATIONAL LIMITED
Duly authorised for and on behalf of:
ELAN PHARMA INTERNATIONAL LIMITED
SIGNED
/s/ EntreMed, Inc.
Duly authorised for and on behalf of:
ENTREMED, INC.
Duly authorised for and on behalf of:
ENTREMED, INC.
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 30
SCHEDULE 1 EPIL PATENTS
| Title of Invention | Country | Patent/Appln. No. | ||
Surface Modified Drug Nanoparticles |
U.S. | 5,145,684 | ||
| EU | EP 000000 | |||
| Xxxxxxxxx | AR 255241 | |||
| Xxxxxxxxx | 000000 | |||
| Xxxxxx | 2,059,432 | |||
| * | * | |||
| Columbia | 24635 | |||
| Finland | 108333 | |||
| Hungary | 221,586 | |||
| * | * | |||
| Israel | 100754 | |||
| Japan | 92/11226 | |||
| S. Korea | 200061 | |||
| Malaysia | MY-108134-A | |||
| Mexico | 176345 | |||
| New Zealand | 241362 | |||
| Norway | 303668 | |||
| Philippines | 29069 | |||
| Russia | 2066553 | |||
| Singapore | 55104 | |||
| Taiwan | NI-071312 | |||
Solid Dose Nanoparticulate Compositions Comprising a
Synergistic Combination of a Polymeric Surface Stabilizer
and Dioctyl Sodium Sulfosuccinate |
U.S. | 6,375,986 | ||
| * | * | |||
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 31
| Title of Invention | Country | Patent/Appln. No. | ||
| * | * | |||
| * | * | |||
| * | * | |||
Nanoparticulate dispersions comprising a synergistic
combination of a polymeric surface stabilizer and dioctyl
sodium sulfosuccinate |
U.S. | 6,592,903 | ||
Nanoparticulate Compositions of Angiogenesis Inhibitors |
U.S. | 10/392,403 | ||
| * | * | |||
| * | * | |||
| Japan | 2003-577857 | |||
| * | * | |||
Redispersible Nanoparticulate Film Matrices With
Protective Overcoats |
U.S. | 5,573,783 | ||
| Canada | 2,212,803 | |||
| Europe | EP 812187 | |||
| * | * | |||
Continuous Method of Grinding Pharmaceutical Substances |
U.S. | 5,718,388 | ||
| Argentina | Patent No. AR 253558 V1 | |||
| Canada | Patent. No. 2,190,134 | |||
| Taiwan | Patent No. NI-122518 | |||
| Europe | EP 804161 | |||
| Israel | Patent No. 113851 | |||
| Japan | Patent No. 3607294 | |||
| Malaysia | Patent No. MY-113,569-A | |||
| Philippines | Patent No. 31497 | |||
| Venezuela | Patent No. 0854/95 | |||
Method of Grinding Pharmaceutical Substances |
U.S. | 5,862,999 | ||
| Argentina | Patent No. AR 254497 V1 | |||
| * | * | |||
| Taiwan | Patent No. NI-112433 | |||
| Europe | EP 760653 | |||
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 32
| Title of Invention | Country | Patent/Appln. No. | ||
| Israel | Patent No. 113852 | |||
| * | * | |||
| Malaysia | Patent No. MY-112,458-A | |||
| Philippines | Patent No. 0-0000-00000 | |||
| Venezuela | Patent No. 0853-95 | |||
Methods for Preventing Crystal Growth and Particle
Aggregation in Nanoparticle Compositions |
U.S. | 6,267,989 | ||
| * | * | |||
| Canada | Patent No. 2,367,096 | |||
| Europe | EP 1161229 | |||
Process of Preparing Therapeutic Compositions Containing
Nanoparticles |
U.S. | 5,510,118 | ||
Use of Ionic Cloud Point Modifiers to Prevent Particle
Aggregation During Sterilization |
U.S. | 5,298,262 | ||
Use of Non-Ionic Cloud Point Modifiers to Minimize
Nanoparticulate Aggregation During Sterilization |
U.S. | 5,346,702 | ||
Use of Purified Surface Modifiers to Prevent Particle
Aggregation During Sterilization |
U.S. | 5,352,459 | ||
| Argentina | Patent No. AR 255267 V1 | |||
| Mexico | Patent No. 190632 | |||
Method of Preparing Stable Drug Nanoparticles |
U.S. | 5,534,270 | ||
Formulations of Compounds as Nanoparticulate Dispersions
in Digestible Oils or Fatty Acids |
U.S. | 5,571,536 | ||
| U.S. | 5,560,931 | |||
| Europe | EP 808154 | |||
Microprecipitation of Nanoparticulate Pharmaceutical Agents |
U. S. | 5,560,932 | ||
Pharmaceutical Compositions Containing Polyalkylene Block
Copolymers Which Gel at Physiological Temperature |
U.S. | 5,565,188 | ||
| U.S. | 5,705,194 | |||
| * | * | |||
| Europe | EP 810855 | |||
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 33
\
| Title of Invention | Country | Patent/Appln. No. | ||
| * | * | |||
Sulfated Non-Ionic Block Copolymer Surfactant as
Stabilizer Coatings for Nanoparticle Compositions |
U.S. | 5,569,448 | ||
Formulations of Oral Gastrointestinal Therapeutic Agents
in Combination with Pharmaceutically Acceptable Clays |
U. S. | 5,585,108 | ||
Butylene Oxide-Ethylene Oxide Block Copolymer Surfactants
as Stabilizer Coatings for Nanoparticle Compositions |
U.S. | 5,587,143 | ||
| * | * | |||
| Europe | EP 804162 | |||
| Israel | Patent No. 114354 | |||
| Japan | Patent No. 0000000 | |||
Sugar Based Surfactant for Nanocrystals |
U.S. | 5,622,938 | ||
Microprecipitation of Nanoparticulate Pharmaceutical
Agents Using Surface Active Material Derived From Similar
Pharmaceutical Agents |
U.S. | 5,716,642 | ||
Use of PEG-Derivatized Lipids as Surface Stabilizers for
Nanoparticle Compositions |
U.S. | 6,270,806 | ||
| * | * | |||
| Canada | Patent No. 2,362,508 | |||
| Europe | EP 1156788 | |||
Controlled Release of Nanoparticle Compositions |
* | * | ||
| * | * | |||
| * | * | |||
| * | * | |||
Bioadhesive Nanoparticle Compositions Having Cationic
Surface Stabilizers |
US | 6,428,814 | ||
| * | * | |||
| * | * | |||
| * | * | |||
| Europe | EP 1217993 | |||
Small Scale Mill and Method Thereof |
US | 6,431,478 | ||
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 34
| Title of Invention | Country | Patent/Appln. No. | ||
| US | 6,745,962 | |||
| * | * | |||
| * | * | |||
| * | * | |||
| Europe | EP 1185371 | |||
Rapidly Disintegrating Oral Dosage Form |
US | 6,316,029 | ||
| * | * | |||
| * | * | |||
| * | * | |||
| * | * | |||
Nanoparticulate Compositions Comprising Copolymers of
Vinyl Pyrrolidone and Vinyl Acetate as Surface Stabilizers |
* | * | ||
| * | * | |||
| * | * | |||
| * | * | |||
| * | * | |||
Stabilization of Chemical Compounds Using Nanoparticulate
Technology |
* | * | ||
| * | * | |||
| * | * | |||
| * | * | |||
Compositions Having a Combination of Controlled Release
and Immediate Release Characteristics |
U.S. | 6,908,626 | ||
| * | * | |||
| * | * | |||
| * | * | |||
Apparatus for Sanitary Wet Milling |
U.S. | 6,582,285 | ||
| * | * | |||
| * | * | |||
| * | * | |||
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
Page 35
| Title of Invention | Country | Patent/Appln. No. | ||
| * | * | |||
Fast Dissolving Dosage Forms Having Reduced Friability |
* | * | ||
| * | * | |||
| * | * | |||
| * | * | |||
| * | * | |||
Method of Grinding Pharmaceutical Substances |
U.S. | 5,518,187 | ||
| Argentina | Patent No. 251001 | |||
| Australia | Patent No. 660852 | |||
| * | * | |||
| Taiwan | Patent No. NI-69476 | |||
| Czech Republic | Patent No. 284802 | |||
| Europe | Patent No. EP 600528 | |||
| Finland | Patent No. 108399 | |||
| Hungary | Patent No. 210928 | |||
| * | * | |||
| South Korea | Patent No. 312798 | |||
| Malaysia | Patent No. MY-109,419-A | |||
| Mexico | Patent No. 189779 | |||
| New Zealand | Patent No. 248813 | |||
| * | * | |||
| Philippines | Patent No. 31118 | |||
| Slovak Republic | Patent No. 281078 | |||
| * | * | |||
| Venezuela | Patent No. 1484-93 | |||
Liquid Dosage Compositions of Stable Nanoparticulate
Active Agents |
* | * | ||
| * | * | |||
| * | * | |||
| * | * | |||
| * | * | |||
Low Viscosity Liquid Dosage Forms |
* | * | ||
| * | * | |||
| * | * | |||
| * | * | |||
| * | * | |||
System and Method for Milling Materials |
U.S. | U.S. Patent No. 6,742,734 | ||
* The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission.
Page 36
| Title of Invention | Country | Patent/Appln. No. | ||
| * | * | |||
| * | * | |||
| * | * | |||
| * | * | |||
| * | * | |||
Surface Modified Anticancer Nanoparticles |
U.S. | Patent No. 5,399,363 | ||
| Canada | Patent No. 2098242 | |||
| Europe | Patent No. EP 577215 | |||
| * | * | |||
| Mexico | Patent No. 205097 | |||
| Norway | Patent No. 308193 | |||
| Taiwan | Patent No. NI-079294 | |||
* The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission.
Page 37
SCHEDULE 2 ENTREMED PATENTS
| Serial No./ | Issue Date/ | |||||||
| Title | Country | Appl Type/Status | Filing Date | Patent No. | ||||
Estrogenic Compounds as |
US | Parent | 8/6/1993 | 4/2/1996 | ||||
Anti-Mitotic Agents |
05213-0190 | Issued | 08/102,767 | 5,504,074 | ||||
Estrogenic Compounds as |
Canada | Nat’l Phase-PCT | 2/5/1996 | |||||
Anti-Mitotic Agents |
05213-0190CA | Pending | 2,168,850 | |||||
Estrogenic Compounds as |
EPO | Nat’l Phase-PCT | 2/23/1996 | |||||
Anti-Mitotic Agents |
05213-0190EP | Issue Fee Paid | 94924120.2 | |||||
Estrogonic Compounds as |
Hong Kong | Nat’l Phase-PCT | 12/28/1998 | |||||
Anti-Mitotic Agents |
05213-0190HK | Pending | 98115897.5 | |||||
Estrogenic Compounds as |
Japan | Nat Phase PCT | 2/6/1996 | |||||
Anti-Mitotic Agents |
05213-0190JP | Pending | 506502/95 | |||||
Estrogenic Compounds as |
US | Continuation | 12/12/1995 | 8/26/1997 | ||||
Anti-Mitotic Agents |
05213-0191 | Issued | 08/571,265 | 5,661,143 | ||||
Estrogenic Compounds as |
EPO | Division | 8/1/2005 | |||||
Anti-Mitotic Agents |
05213-0191EP | Pending | 05016659.4 | |||||
Estrogenic Compounds as |
US | Division | 4/25/1997 | 4/6/4999 | ||||
Anti-Mitotic Agents |
05213-0192 | Issued | 08/838,699 | 5,892,069 | ||||
* The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission.
Page 38
SCHEDULE 3 TECHNOLOGICAL COMPETITORS
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
* The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission.
Page 39
SCHEDULE 4 COMMERCIAL UNIT DOSE COST OF GOODS PRICING
Commercial Product Cost of Goods per 1000mg unit dose
| * | ||||||||
| * r | * | * | * | * | ||||
*
|
* | * | * | * | ||||
*
|
* | * | * | * | ||||
*
|
* | * | * | * | ||||
*
|
* | * | * | * |
*
*
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
SCHEDULE 5
KEY TERMS FOR MANUFACTURING AND SUPPLY AGREEMENT
| 1. | EPIL to be the sole and exclusive supplier of Product to EntreMed in the Territory for commercial supplies._________________ | |
*
|
||
.
|
||
| 2. | EPIL to own and be responsible for (i) filing regulatory approvals in regard to EPIL Technology, (ii) DMFs that EPIL or its Affiliates may file in respect of EPIL Technology and the application of EPIL Technology as regards the Product and/or the manufacture of Product, and (iii) all necessary manufacturing approvals for the commercial manufacture of the Product. EntreMed to be responsible for filing for and maintaining all other necessary Regulatory Approvals, including for the manufacture of the Compound and any necessary export or import licenses in relation to the Compound and/or the Product. For the avoidance of doubt, EntreMed shall own and be responsible for the NDA and all other applications for regulatory approval for the Product. | |
| 3. | EPIL to supply Product that is manufactured in accordance with and conforms to agreed specifications and to all applicable laws and regulations for supply and manufacturing, including cGMP. Product to be provided * bulk packaging. Product packaging to conform to written standards that are to be agreed by the Parties. | |
| 4. | EntreMed to supply Compound on time, free of charge * EPIL’s facility and in sufficient quantities to enable EPIL to meet firm purchase orders. Compound to (i) conform to specifications, (ii) be accompanied by a certificate of analysis, and (iii) be supplied, packaged and labeled in accordance with EPIL requirements that are to be set out in a quality agreement (“Quality Agreement”) to be negotiated in good faith between the Parties in relation to the commercial manufacture and supply of Product. The Quality Agreement will also address issues related * . | |
| 5. | Detailed forecasting, ordering and delivery provisions to be negotiated in good faith between the Parties and to be fully set out in Manufacturing and Supply Agreement. | |
| 6. | The Parties to establish * | |
| 7. | Until the * anniversary of the date that the Product is provided for commercial
use in Territory, the price per unit of Product to be as per Schedule 4, * . To the extent that the characteristics of final Product vary from the Product as specified on Schedule 4, the price will be as per Schedule 4 adjusted as is reasonably and directly commensurate to such variance * . Price increases shall be * , although |
|
.
|
||
| 8. | EntreMed to have right to review and approve proposed changes in advance of their implementation specific to the Product manufacturing, testing, or controls documentation which require prior Regulatory Authority approval as well as any other changes that may be specified as requiring EntreMed approval in the Quality Agreement. | |
| 9. | EntreMed to have * consent for EPIL use of Third Party subcontractors. EntreMed to also have right to audit EPIL and subcontractors relevant to the Product manufacturing and testing no more than * per calendar year, unless for cause. | |
| 10. | EPIL to manufacture and supply commercial Product to EntreMed through the EPIL facility located in Athlone, Ireland. Within * after the first commercial sale of Product, * a second |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |
| manufacturing facility * that can manufacture and supply commercial Product in accordance with specifications, cGMPs and all relevant regulatory laws. * to be responsible for the costs of the process transfer to * manufacturing facility in anticipation of the commercial scale-up of the Product * and for the costs of process transfer, validation and maintenance * . Site transfers requested by * from and after the NDA filing other than those mentioned above shall be subject to reasonable prior consent and shall be at * cost. | ||
| 11. | EntreMed to order safety stock through agreed order and forecast procedures. * . | |
| 12. | EPIL to identify Third Parties that are reasonably acceptable to EntreMed for purposes of granting a Manufacturing License in the event of a failure to supply, an event that will be negotiated in good faith and defined by the Parties in the Manufacturing and Supply Agreement. The cost of such technology transfer shall be borne by * . | |
| 13. | Release and rejection provisions (e.g., defects and latent defects) reasonably acceptable to the Parties, with EPIL to have a specified time (e.g., * days) rectify the issue. EntreMed to be refunded where Product cannot be reworked or replaced within specified time, although Parties agree that EntreMed shall not be entitled to a refund where non-conformity is due to the supply of defective Compound or where the noncomformity is otherwise attributable to the negligent acts or omissions EntreMed. | |
| 14. | * to be responsible for coordinating any Product recall and ensuring that
recalls are conducted in a commercially reasonable manner. Costs of recall shall be borne by
EntreMed unless (i) the recall arises from EPIL’s failure to supply Product in accordance with
agreed specifications and cGMP or from the negligent acts or omissions of EPIL in
manufacturing the Product or from EPIL’s breaches of the Manufacturing and Supply Agreement
* .
|
|
| 15. | EPIL responsible for compliance to cGMP and applicable laws for supply and manufacture, adherence to specifications and indemnifications resulting from its breach thereof. EntreMed responsible for marketing and promotion, and for recalls and indemnification arising otherwise. Indemnification provisions will correspond to such responsibilities. | |
| 16. | Term of the manufacture and supply agreement will be the Term of the License Agreement. |
| * | The marked portions have been omitted pursuant to a request for confidential treatment and filed separately with the Securities Exchange Commission. |

