EXECUTION VERSION ROYALTY AGREEMENT dated as of May 31, 2017 by and between AZIYO MED, LLC and LIGAND PHARMACEUTICALS INCORPORATED

EXECUTION VERSION
ROYALTY AGREEMENT
dated as of
May 31, 2017
by and between
AZIYO MED, LLC
and
LIGAND PHARMACEUTICALS INCORPORATED

- i -
TABLE OF CONTENTS
Page
ARTICLE I - Definitions .................................................................................... 1
SECTION 1.01. Defined Terms. ................................................................. 1
ARTICLE II - Payments by the Company; ............................................................... 9
SECTION 2.01. Buydown Payment. ........................................................... 9
SECTION 2.02. Periodic Royalties. ............................................................. 9
SECTION 2.03. Milestone Payments. ......................................................... 10
SECTION 2.04. Consent to Sale Transaction; No Assumed Obligations. ............... 11
ARTICLE III - Representations and Warranties of the Company ................................... 11
SECTION 3.01. Organization. .................................................................. 11
SECTION 3.02. Authorization. ................................................................ 11
SECTION 3.03. Governmental Authorization. ............................................... 11
SECTION 3.04. Ownership. ..................................................................... 11
SECTION 3.05. Litigation. ...................................................................... 12
SECTION 3.06. Compliance with Laws. ...................................................... 12
SECTION 3.07. Conflicts. ....................................................................... 12
SECTION 3.08. Current Indebtedness.......................................................... 13
SECTION 3.09. Financial Statements. ......................................................... 13
ARTICLE IV - Representations and Warranties of Ligand .......................................... 13
SECTION 4.01. Organization. ................................................................... 13
SECTION 4.02. No Assignment; Authorization. ............................................. 13
SECTION 4.03. Conflicts. ....................................................................... 13
ARTICLE V - Covenants ................................................................................... 14
SECTION 5.01. Access; Information. .......................................................... 14
SECTION 5.02. Confidentiality; Press Release. .............................................. 15
SECTION 5.03. Efforts; Further Assurance. .................................................. 15
SECTION 5.04. Remedies Event. .............................................................. 15
SECTION 5.05. Indebtedness; Sale of Revenue Interests................................. 16
SECTION 5.06. Remittance of Funds to Accounts. .......................................... 16
ARTICLE VI - Term and Termination .................................................................... 16
SECTION 6.01. Term. ............................................................................ 16
SECTION 6.02. Extension of the Term. ....................................................... 16
SECTION 6.03. Effect of Termination. ........................................................ 17
ARTICLE VII ................................................................................................ 17
SECTION 7.01. Survival. ........................................................................ 17
SECTION 7.02. Notices. ......................................................................... 17

- ii -
SECTION 7.03. Successors and Assigns. .................................................... 18
SECTION 7.04. Indemnification. ............................................................ 18
SECTION 7.05. No Implied Representations and Warranties. ............................. 20
SECTION 7.06. Independent Nature of Relationship. ..................................... 20
SECTION 7.07. Entire Agreement. ........................................................... 20
SECTION 7.08. Amendments; No Waivers. ............................................... 21
SECTION 7.09. Interpretation. ................................................................ 21
SECTION 7.10. Headings and Captions. ...................................................... 21
SECTION 7.11. Counterparts; Effectiveness .................................................. 21
SECTION 7.12. Severability .................................................................... 21
SECTION 7.13. Expenses. ...................................................................... 21
SECTION 7.14. Governing Law; Jurisdiction. ............................................. 22
ARTICLE VIII - Intercreditor Matters and Guarantee ............................................... 22
SECTION 8.01. Ligand Interests; Recharacterization. ................................... 22
SECTION 8.02. Other Ligand Security. .................................................... 23
SECTION 8.03. Priority. ..................................................................... 24
SECTION 8.04. Other Intercreditor Matters. ............................................... 24
SECTION 8.05. Control Agreements. ....................................................... 24
SECTION 8.06. Termination or Release. ................................................... 24
ARTICLE IX - Remedies .................................................................................. 25
SECTION 9.01. Remedies. ................................................................... 25
SECTION 9.02. Acceleration. .................................................................. 25

ROYALTY AGREEMENT
This ROYALTY AGREEMENT (as amended, supplemented or otherwise modified
from time to time, this “Agreement”) is dated as of May 31, 2017, by and between AZIYO
MED, LLC, a Delaware limited liability company (the “Company”); and LIGAND
PHARMACEUTICALS INCORPORATED, a Delaware corporation (“Ligand”).
RECITALS
WHEREAS, the Company wishes (i) to acquire from CorMatrix Cardiovascular, Inc.
(the “Seller”) all of its assets related or applicable to, or used in connection with, its business of
developing, manufacturing and commercializing the Products described herein (the “Sale
Transaction”), and (ii) in connection with such Sale Transaction, to obtain the consent of
Ligand to the Sale Transaction and enter into an agreement with Ligand setting forth the
obligations of the Company to Ligand with respect to such acquired interests and the sale of the
Products; and
WHEREAS, as a condition precedent to Ligand’s entering into this Agreement, Aziyo
Biologics, Inc., a Delaware corporation and the parent of the Company (the “Guarantor”), has
agreed to enter into a Guaranty Agreement guarantying the obligations of the Company under
Section 2.01, in substantially the form attached hereto as Exhibit A;
NOW, THEREFORE, in consideration of the mutual covenants, agreements
and representations and warranties set forth herein, the parties hereto agree as follows:
ARTICLE I - Definitions
SECTION 1.01. Defined Terms. As used in this Agreement, the following terms
shall have the meanings specified below:
“Affiliate” shall mean any Person that controls, is controlled by, or is under
common control with another Person. For purposes of this definition, “control” shall mean
direct or indirect ownership of a majority of the stock or other equity interests having the right
to vote for the election of directors or other members of the governing body of the entity.
“Applicable Royalty Percentage” shall mean (i) prior to the first $5,000,000
payment of the Buydown Payment, twenty percent (20.0%) or (ii) following such payment,
five percent (5.0%), provided that if the second $5,000,000 installment of the Buydown
Payment is not made on or before December 15, 2017, then the Applicable Royalty Percentage
shall be twenty percent (20.0%) from December 15, 2017, until such second payment is made.
“Asset Purchase Agreement” shall mean that certain Asset Purchase Agreement,
dated as of May 31, 2017, by and among the Seller, the Company and the Guarantor setting
forth the terms and conditions of the Sale Transaction.
“Audit Costs” shall mean, with respect to any audit of the books and records of
the Company or its Subsidiaries with respect to amounts payable or paid under this Agreement,
the reasonable out-of-pocket cost of such audit, including all fees, costs and expenses incurred

- 2 -
in connection therewith.
“Bankruptcy Event”. shall mean the occurrence of any proceeding being
instituted by or against the Company seeking to adjudicate it bankrupt or insolvent, or
seeking liquidation, winding up, reorganization, arrangement, adjustment, protection, relief
or composition of it or its debts under any law relating to bankruptcy, insolvency or
reorganization or relief of debtors, or seeking the entry of an order for relief or the
appointment of a receiver, trustee or other similar official for it or any substantial part of its
property, or the Company taking any action to authorize any of the actions set forth above.
Notwithstanding the foregoing, if such proceeding is instituted against the Company, no
Bankruptcy Event shall have occurred unless such proceeding remains undismissed,
undischarged or unbonded for a period of sixty (60) days.
“Books” shall mean all of the books and records of a Person, including ledgers,
federal and state tax returns, records regarding the Person’s assets or liabilities, the General
Collateral, business operations or financial condition, and all computer programs or storage or
any equipment containing such information.
“Business Day” shall mean any day other than a Saturday, a Sunday, any day
which is a legal holiday under the laws of the State of New York, or any day on which banking
institutions located in the State of New York are required by law or other governmental action
to close.
“Buydown Payment” shall have the meaning set forth in Section 2.01(a).
“CanGaroo Product Change of Control” shall mean the first to occur of any
Product Change of Control in respect of the CanGaroo Products.
“Collateral” means the Royalty Related Collateral and the General Collateral.
“Company” shall have the meaning set forth in the preamble.
“Company Change of Control” shall mean, with respect to the Company, the
first to occur of any of the following transactions:
(a) the acquisition by any Person or group (within the meaning of Sections
13(d)(3) or 14(d)(2) of the Securities Exchange Act of 1934, as amended) of beneficial
ownership of any capital stock of the Company, if after such acquisition, such Person or group
would be the "beneficial owner" (as defined in Rule 13d-3 under the Securities Exchange Act of
1934, as amended, but assuming that any convertible securities owned by such Person or group
or any controlled affiliates thereof are immediately exercisable), directly or indirectly, of
securities of the Company representing a majority of the voting power of the Company;
(b) a merger or consolidation of the Company, with any other Person, other
than a merger or consolidation which would result in the Company's voting securities
outstanding immediately prior thereto continuing to represent (either by remaining outstanding
or by being converted into voting securities of the surviving entity) a majority of voting power
of the Company immediately after such merger or consolidation; or

- 3 -
(c) the bona fide sale, lease, transfer, exclusive license or other disposition, in
a single transaction or series of related transactions, by the Company or any of its Subsidiaries
of all or substantially all the assets of the Company and its Subsidiaries, taken as a whole.
“Confidential Information” shall mean, as it relates to the Company and its
Affiliates and any of the Products, the Intellectual Property related to any of the Products,
confidential business information, financial data and other like information (including
ideas, research and development, know-how, formulas, schematics, compositions,
technical data, specifications, customer and supplier lists, pricing and cost information, and
business and marketing plans and proposals), inventory, ideas, algorithms, processes,
computer software programs or applications (in both source code and object code form),
client lists and tangible or intangible proprietary information or material, or such other
information that either Party identifies to the other as confidential or the nature of which or
the circumstances of the disclosure of which would reasonably indicate that such information
is confidential or proprietary. Notwithstanding the foregoing definition, Confidential
Information shall not include information that (a) is already in the public domain at the time
the information is disclosed, (b) thereafter becomes lawfully obtainable from other sources
who, to the knowledge of the recipient, have no obligation of confidentiality, (c) can be
shown to have been independently developed by the recipient or its representatives without
reference to any Confidential Information of the other Party or (d) is required to be disclosed
under laws, rules and regulations of any Governmental Authority applicable to the Company
or its Affiliates or Ligand or its Affiliates, as the case may be, or pursuant to the rules and
regulations of any securities exchange or trading system or pursuant to any other laws, rules or
regulations of any Governmental Authority having jurisdiction over the Company and its
Affiliates or Ligand and its Affiliates.
“Depository Bank” shall mean Silicon Valley Bank.
“Effective Date” shall mean the date of the closing of the Sale Transaction.
“Excluded Assets” shall mean (i) any deposit accounts exclusively used by the
Company for payroll, payroll taxes and other employee wage and benefit payments to or for
the benefit of the Company’s employees, (ii) the Permitted CD Collateral Accounts (as defined
in the MidCap Credit Facility), (iii) any fee interest in owned or leased real property (including
fixtures related thereto), (iv) any “intent to use” trademark application for which a statement
of use has not been filed with the United States Patent and Trademark Office, (v) any
motor vehicles or other assets subject to certificates of title, (vi) any equity interests of
subsidiaries that are not wholly-owned subsidiaries to the extent a security interest on such
equity interests is prohibited by the organizational or joint venture documents relating to
such equity interests, (vii) any voting equity interests of foreign subsidiaries in excess of 65%
of the outstanding voting equity interests of such subsidiaries and (viii) any assets over which
the granting of a security interest in such assets would be prohibited by applicable law
or contract or that would require governmental consent, approval, license or authorization,
in each case after giving effect to Section 9-406, 9-407, 9-408 or 9-409 of the Uniform
Commercial Code in the applicable jurisdiction or any other applicable law or principle of
equity.

- 4 -
“Excluded Costs” shall mean the following items to the extent permitted by
generally accepted accounting principles: (i) value added or any other similar transaction taxes
accrued on sales invoices, (ii) sales discounts and all kinds of rebates, (iii) any orders or parts
thereof which are subsequently returned to the Company (or an Affiliate, agent or sublicensee
thereof, as applicable) and refunded to the customer or wholesaler, (iv) charges for late payment
collected from customers, registration charges and other service charges and (v) applicable
shipping charges.
“Existing Liens” shall mean (i) any liens or other security interests upon any
assets of the Company for the benefit of the lenders and other secured parties under the MidCap
Credit Facility, and (ii) the rights of the Seller and Xxxx Biotech Incorporation, an Indiana
corporation, under the respective cross license agreements entered into by the Company and
each of them in connection with the Sale Transaction, as they may be amended or modified
from time to time.
“Fiscal Quarter” shall mean each three (3) month period commencing
January 1, April l, July 1 or October 1, provided however that (a) the first Fiscal Quarter after
the Effective Date shall commence on the day after the Effective Date and continue to the end of
the first full Fiscal Quarter thereafter and (b) the last Fiscal Quarter of the Term shall end
upon the expiration or termination of this Agreement.
“Fiscal Year” shall mean the calendar year.
“General Collateral” shall have the meaning set forth in Section 8.02.
“Governmental Authority” shall mean any government, court, regulatory or
administrative agency or commission, or other governmental authority, agency or
instrumentality, whether foreign, federal, state or local (domestic or foreign).
“Guarantor” shall have the meaning set forth in the Recitals.
“Intellectual Property” shall mean all proprietary information; technical data;
laboratory notebooks; clinical data; priority rights; trade secrets; know-how;
confidential information; inventions (whether patentable or unpatentable and whether or not
reduced to practice or claimed in a pending patent application); Patents; registered or
unregistered trademarks, trade names, service marks, including all goodwill associated
therewith; registered and unregistered copyrights and all applications thereof; in each case that
are owned, controlled by, generated by, issued to, licensed to, licensed by or hereafter acquired
by or licensed by the Company or any of its Subsidiaries.
“Intercreditor Agreement” shall mean that certain Intercreditor Agreement,
by and among the MidCap Credit Facility Agent, Ligand and the Company, dated as of May 31,
2017, as it may be amended, supplemented or otherwise modified from time to time.
“Knowledge of the Company” shall mean the current actual knowledge,
information or belief held by Lode Debrabandere, Xxxxx Xxxxx and Xxxxxxxx XxXxxx Xxxxxxxx
after reasonable inquiry by such person into the relevant subject matter.

- 5 -
“Ligand” shall have the meaning set forth in the preamble.
“Ligand Account” shall mean the following account (or such other account as
Ligand may designate in writing (such designation to be made at least two (2) Business Days
prior to any payment owing to Ligand under this Agreement)):
Ligand Pharmaceuticals, Inc.
Bank of America Xxxxxxx Xxxxx
Account No. 1453127240
Routing No. 000000000
“Ligand Purchase Agreement” shall mean the Interest Purchase Agreement,
dated as of May 3, 2016, between the Seller and Ligand.
“Losses” shall mean collectively, any and all claims, damages, losses,
judgments, awards, penalties, liabilities, costs and expenses (including reasonable attorneys' fees
and expenses) incurred in connection with defending any action, suit or proceeding.
“Main Account” shall mean the deposit account maintained by the
Company at the Depository Bank with account number 3302165408.
“Material Adverse Change"” shall mean, with respect to the Company and its
Subsidiaries, any event, change, circumstance, occurrence, effect or state of facts that has caused
or is reasonably likely to cause a material adverse change in the business, operations, assets or
financial condition of the Company and its Subsidiaries, taken as a whole.
“Material Adverse Effect” shall mean (a) the effect of a Material Adverse
Change, (b) a material adverse effect on the validity or enforceability of this Agreement, (c)
the inability or failure of the Company to make the payments provided in this Agreement, (d) a
material adverse effect on the ability of the Company to perform any of its other material
obligations under this Agreement or (e) any material adverse effect on the Products or the ability
of the Company and its Subsidiaries to distribute, market and/or sell the Products.
“MidCap Credit Facility” shall mean that certain Credit and Security Agreement
(Revolving Loan) and that certain Credit and Security Agreement (Term Loan), as each may be
amended, amended and restated, supplemented or otherwise modified as of the date hereof, and
as each may be further amended, amended and restated. supplemented or otherwise modified
from time to time as permitted by the Intercreditor Agreement, by and among the Company
and the Guarantor, as Borrowers (as defined therein), MidCap Credit Facility Agent and the
Lenders (as defined therein) party thereto.
“MidCap Credit Facility Agent” shall mean MidCap Financial Trust, a Delaware
statutory trust, in its capacity as administrative agent under the MidCap Credit Facility or any
successor thereto in such capacity.
“Minimum Annual Royalty” shall mean with respect to (a) calendar year 2017,
zero, (b) calendar year 2018, $1,250,000, (c) calendar year 2019, $2,200,000, and (d) calendar
year 2020 and each calendar year thereafter during the Term, $2,750,000; provided, however,

- 6 -
that for the final Fiscal Year of the Term, the “Minimum Annual Royalty” shall mean the
applicable Minimum Annual Royalty multiplied by the fraction of such Fiscal Year that is
within the Term; provided, further, that if any Product, or any product enumerated in the
definition of any Product (or in any Schedule referenced in any such definition), is divested as a
result of a Product Change of Control or if any Product is withdrawn from the market for
regulatory or safety reasons, the Minimum Annual Royalty shall be reduced by an amount that is
the product of (a) the applicable Minimum Annual Royalty for the Fiscal Year in which such
Product Change of Control or withdrawal takes place and for each Fiscal Year thereafter times
(b) the fraction representing (x) the total sales of such Product or enumerated product subject
to such Product Change of Control or withdrawal in the twelve (12) calendar months
immediately preceding such Product Change of Control or withdrawal over (y) the aggregate
total sales of all Products in the twelve (12) calendar months immediately preceding such
Product Change of Control or withdrawal. For the avoidance of doubt, the Minimum Annual
Royalty shall be adjusted according to the foregoing proviso immediately as of any Product
Change of Control or withdrawal.
“Minimum Quarterly Payment” shall mean, with respect to any Fiscal Quarter
during the Term, an amount equal to the difference between (i) the applicable Minimum
Quarterly Royalty and (ii) the aggregate Monthly Royalties paid to Ligand with respect to such
Fiscal Quarter.
“Minimum Quarterly Royalty” shall mean the applicable Minimum
Annual Royalty divided by four; provided, however, that for the final Fiscal Quarter of the
Term, the “Minimum Quarterly Royalty” shall mean the applicable Minimum Annual Royalty
divided by four multiplied by the fraction of such Fiscal Quarter that is within the Term.
“Minimum Quarterly Royalty Overpayment” shall have the meaning set
forth in Section 2.02(b)(ii).
“Monthly Report” shall mean, with respect to the relevant Payment Month of
the Company, a report showing (a) the gross revenues of the Products for such Payment
Month, (b) the Net Sales Proceeds for such Payment Month, (c) the Excluded Costs for such
Payment Month, and (d) a reasonable calculation of the amount to which Ligand is entitled
for such Payment Month pursuant to Section 2.02(a) of this Agreement.
“Monthly Royalty” shall mean, with respect to each Payment Month, the
amount due to Ligand pursuant to Section 2.02(a) for such Payment Month.
“Net Sales Proceeds” shall mean the aggregate amount of sales proceeds
received by the Company (or an Affiliate, agent or sublicensee thereof, as applicable) and its
Subsidiaries for Products in any Payment Month during the Term, less Excluded Costs.
“Obligations” shall mean any and all payment obligations of the Company under
this Agreement.
“Pari Passu Collateral” shall have the meaning set forth in the Intercreditor
Agreement.

- 7 -
“Parties” shall mean Ligand, the Company and any other Person from time to
time made party to this Agreement, each a “Party.”
“Patent” shall mean all patents, patent rights, patent applications, patent
disclosures and invention disclosures issued or filed, together with all reissues, divisions,
continuations, revisions, term extensions, substitutes, supplementary protection certificates,
reexaminations, inter-partes reviews, post-grant oppositions or similar post-grant review
proceedings, including the inventions claimed in any of the foregoing and any priority rights
arising therefrom, that are issued or filed prior to the date hereof or during the remainder of the
Term, which are owned by the Company or any Subsidiary.
“Payment Month” shall mean each month-long period commencing on the first
day of each calendar month during the Term, provided however that (a) the first Payment
Month after the Effective Date shall commence on the day after the Effective Date and continue
until the end of the first full Payment Month thereafter and (b) the last Payment Month of the
Term shall end upon the expiration or termination of this Agreement.
“Permitted Liens” shall mean (i) the Existing Liens, (ii) the security interests
granted to Ligand pursuant to Article VIII, and (iii) any liens for taxes or other governmental
charges arising by operation of law in the ordinary course of business for sums which are not yet
due and payable.
“Permitted Transaction” shall mean any transaction (a) contemplated by the
MidCap Credit Facility, as in effect on the Effective Date, or any refinancing facility with
respect thereto and (b) during the Term whereby the Company incurs, creates, assumes or
permits to exist any indebtedness for borrowed money; provided that such transaction (x)
does not, except to the extent expressly contemplated by Section 8.06, result in any security
interest granted hereunder ceasing to be a valid and perfected security interest and (y) could
not reasonably be expected to impair the ability of the Company to comply with the requirements
to make the payments set forth in Section 2.01, Section 2.02 or Section 2.03.
“Person” shall mean an individual, corporation, partnership, limited
liability company, association, trust or other entity or organization, but not including a
government or political subdivision or any agency or instrumentality of such government or
political subdivision.
“Product Change of Control” shall mean, with respect to any Product, or any
product enumerated in the definition of any Product (or in any Schedule referenced in any such
definition), any sale or other transfer by the Company of substantially all of the assets primarily
used to commercialize such Product or such enumerated product or of the exclusive right to
commercialize such Product or such enumerated product.
“Products” shall mean (a) SIS tissue sheet products that were (i) marketed or
sold by the Seller under the trade name CorMatrix Carotid Repair, CorMatrix Vascular Repair,
CorMatrix Pericardial Repair & Reconstruction, Tyke or CorMatrix Cardiac Tissue Repair and
(ii) described in a Section 510(k) premarket notification cleared by the FDA on or prior to the
Closing Date, (b) SIS encasement structures for encapsulation of any cardiac implantable

- 8 -
electronic device (CIED) that were (i) marketed or sold by the Seller under the trade name
CanGaroo or CorMatrix CanGaroo and (ii) described in a Section 510(k) premarket notification
cleared by the FDA on or prior to the Closing Date (the “CanGaroo Products”), and (c) any
products substantially similar in design and application to the Products specified in clauses (a)
and (b) commercialized after the Closing Date during the Term, including in each case specified
in clauses (a) - (c), any modifications and improvements made to the tissue sheet structures or
the encasement structures of such Products that are to be commercialized for the applications in
the Aziyo Fields of Use, as defined in the Asset Purchase Agreement. For the purposes hereof,
the term “Products” shall also mean and include (x) CanGaroo Products for encasement of
neurologic devices and other subcutaneous implantable device applications, and (y) CanGaroo
Products composed of SIS plus antibiotics. Schedule A sets forth a complete list of all of the
Products at the Closing Date.
“Recharacterization” shall have the meaning set forth in the recitals to the
Intercreditor Agreement.
“Regulatory Agency” shall mean a Governmental Authority with
responsibility for the approval of the marketing and sale of surgical implants or other regulation
of surgical implants.
“Regulatory Approvals” shall mean all approvals (including, without
limitation, where applicable, pricing and reimbursement approval and schedule
classifications), product and/or establishment licenses, registrations or authorizations of any
Governmental Authority necessary for the manufacture, use, storage, import, export, transport,
offer for sale, or sale of any of the Products.
“Remedies Event” shall mean (x) a Bankruptcy Event, (y) a failure by the
Company to make a payment pursuant to Section 2,01, Section 2.02 or Section 2.03, provided
that no such failure shall constitute a Remedies Event unless such failure shall remain uncured
for thirty (30) days or (z) an Event of Default (as defined in the MidCap Credit Facility) under
the MidCap Credit Facility.
“Royalty Interests” shall mean the right to receive on a monthly basis cash in an
amount equal to the product of the Applicable Royalty Percentage multiplied by the Net Sales
Proceeds during the Term, pursuant to the terms and conditions of this Agreement. For the
avoidance of doubt, Royalty Interests shall not constitute accounts or payment intangibles (as
each term is defined in the UCC) giving rise to such cash amounts.
“Royalty Related Collateral” means (a) any accounts (as defined in Article 9 of
the UCC) with respect to the Products and the proceeds of such accounts, (b) the Special
Account, (c) any intellectual property acquired by the Company from the Seller and necessary
for the production, marketing or sale of the Products, including those set forth on Schedule B
hereto and (c) any Equipment and Inventory (as defined in Article 9 of the UCC) used in
connection with the production of any Product.
“Sale Transaction” shall have the meaning set forth in the Recitals.
“SEC” shall mean the U.S. Securities and Exchange Commission.

- 9 -
“SIS” shall mean a solid sheet extracellular matrix composition prepared from
intestinal tissue.
“Special Account” shall mean the deposit account maintained by the
Company at the Depository Bank with account number 3302165412.
“Subsidiary” shall mean, with respect to the Company, a corporation,
partnership, joint venture, limited liability company or other business entity of which a majority
of the voting power (other than securities or interests having such power only by reason of the
happening of a contingency) are at the time owned beneficially or of record by the
Company.
“Sweep Event” shall have the meaning set forth in the Intercreditor Agreement.
“Term” shall have the meaning set forth in Section 6.01.
“Third Party” shall mean any Person other than Ligand and any Affiliate of
Ligand or the Company or any Subsidiary of the Company.
“Transfer” shall have the meaning set forth in Section 8.06(c).
“UCC” shall mean the Uniform Commercial Code as in effect in the State of
New York from time to time.
ARTICLE II - Payments by the Company;
SECTION 2.01. Buydown Payment. The Company shall make a payment to
Ligand in the amount of $10,000,000 (the “Buydown Payment”) in two (2) installments, the
first installment in the amount of $5,000,000 payable within thirty (30) days after the Effective
Date but no later than June 30, 2017, and the second installment in the amount of $5,000,000
payable on or before December 15, 2017, each of which shall be paid by wire transfer of
immediately available funds to the Ligand Account.
SECTION 2.02. Periodic Royalties.
(a) Monthly Royalties. The Company shall pay to Ligand, by wire transfer
of immediately available funds (i) within thirty (30) days after the end of each Payment Month,
an amount equal to the Applicable Royalty Percentage multiplied by the Net Sales Proceeds (if
any) received by the Company during such Payment Month, and (ii) on the Closing Date, the
monthly royalty amounts due and owing by Seller under the Ligand Purchase Agreement for the
Payment Months of March and April 2017 and when due the monthly royalty amount of Seller
thereunder for the Payment Month of May 2017.

- 10 -
(b) Minimum Quarterly Payments.
(i) Subject to clause (b)(ii), if, with respect to any Fiscal Quarter
that begins after December 31, 2017, the sum of (1) the aggregate Monthly Royalties
paid to Ligand during the Fiscal Year that includes such Fiscal Quarter plus (2) the
Minimum Quarterly Payments made to Ligand during such Fiscal Year is less than the
Minimum Quarterly Royalty multiplied by the number of completed Fiscal Quarters in
such Fiscal Year, then the Company shall pay to Ligand, by wire transfer of
immediately available funds within thirty (30) days after the end of such Fiscal
Quarter, an amount equal to the difference between (A) the Minimum Quarterly
Royalty multiplied by the number of completed Fiscal Quarters in such Fiscal Year
and (B) the sum of (1) the aggregate Monthly Royalties paid to Ligand with respect to
such Fiscal Year plus (2) the Minimum Quarterly Payments made to Ligand with
respect to such Fiscal Year.
(ii) Notwithstanding clause (b)(i), if, with respect to any Fiscal Year
that begins after December 31, 2017 in respect of which any Minimum Quarterly
Payment was made to Ligand, the aggregate Monthly Royalties paid in respect of such
Fiscal Year exceed the applicable Minimum Annual Royalty (the amount of any such
Minimum Quarterly Payments made in any such Fiscal Year, the “Minimum Royalty
Overpayment”), then any subsequent payment obligation owing by the Company
pursuant to this Agreement shall be automatically reduced and offset in the amount of
such Minimum Royalty Overpayment until such Minimum Royalty Overpayment is
extinguished.
(c) Payments to Ligand.
(i) Within thirty (30) days following the end of each Payment Month,
the Company shall disburse from the Special Account to the Ligand Account an
amount equal to the amount to which Ligand is entitled pursuant to Section 2.02(a)
of this Agreement (if any) for such Payment Month.
(ii) Within sixty (60) days following the end of each Fiscal
Quarter, the Company shall remit by wire transfer of immediately available funds to
the Ligand Account an amount equal to the amount to which Ligand is entitled pursuant
to Section 2.02(b) of this Agreement (if any) for such Fiscal Quarter.
(iii) If, after any Sweep Event occurs, the "notice of exclusive control"
giving rise to such Sweep Event is revoked prior to a Bankruptcy Event, to the
extent any Minimum Quarterly Royalty came due and was not paid pursuant to
[Section 2.1(b)] of the lntercreditor Agreement prior to such revocation, the Company
shall pay such Minimum Quarterly Royalty within thirty (30) days of such revocation.
SECTION 2.03. Milestone Payments.
(a) If and when the aggregate amount of Net Sales Proceeds received by the
Company during the Term equals $100,000,000, the Company shall pay $5,000,000 to Ligand,
which payment shall be made within forty-five (45) days thereof by wire transfer of immediately

- 11 -
available funds to the Ligand Account.
(b) If and when the aggregate amount of Net Sales Proceeds received by the
Company during the Term equals $300,000,000 or the occurrence of a CanGaroo Product
Change of Control during the Term, whichever is sooner, the Company shall pay an additional
$5,000,000 to Ligand, which payment shall be made within forty-five (45) days thereof by wire
transfer of immediately available funds to Ligand Account.
SECTION 2.04. Consent to Sale Transaction; No Assumed Obligations.
Ligand hereby consents to the Sale Transaction on the terms and conditions set forth in the Asset
Purchase Agreement. Notwithstanding any provision in this Agreement or any other writing to
the contrary, Ligand acknowledges and agrees that (i) Ligand does not have any right, title or
interest in or to any of the Products or any other assets acquired the Company in the Sales
Transaction, except for its interest in and to the Royalty Interests (and the security interests
granted to Ligand hereunder) during the Term, all as set forth herein, and (ii) neither the
Company nor any of its Affiliates have assumed or agreed to pay any liabilities or other
obligations of the Seller or any of its Affiliates to Ligand or its Affiliates of whatever nature,
whether presently in existence or arising or asserted hereafter, including without limitation, any
obligations or liabilities of the Seller or its Affiliates under the Ligand Purchase Agreement. All
such liabilities and obligations shall be retained by and remain obligations and liabilities of the
Seller and its Affiliates.
ARTICLE III - Representations and Warranties of the Company
SECTION 3.01. Organization. The Company is a limited liability company
duly organized, validly existing and in good standing under the laws of the State of Delaware
and has all limited liability company power and all licenses, authorizations, consents and
approvals required to carry on its business as proposed to be conducted in connection with this
Agreement. The Company has no Subsidiaries.
SECTION 3.02. Authorization. The Company has all necessary power and
authority to enter into, execute and deliver this Agreement and to perform all of the obligations
to be performed by it hereunder and to consummate the transactions contemplated
hereunder. This Agreement has been duly authorized, executed and delivered by the Company
and constitutes the valid and binding obligation of the Company, enforceable against
the Company in accordance with its terms, subject to applicable bankruptcy, insolvency,
reorganization, moratorium or similar laws affecting creditors' rights generally or general
equitable principles.
SECTION 3.03. Governmental Authorization. The execution and delivery by
the Company of this Agreement, and the performance by the Company of its obligations
hereunder, does not require any notice to, action or consent by, or in respect of, or filing with,
any Governmental Authority.
SECTION 3.04. Ownership.
(a) As of the date hereof, the Company owns or holds a valid license under
all of the Intellectual Property and the Regulatory Approvals which it currently purports to own

- 12 -
related to any of the Products, free and clear of all liens, except Permitted Liens. As of the date
hereof, the Company has not granted, nor does there exist, any lien on the Products except
Permitted Liens.
(b) There is no filed and served on the Company or, to the Knowledge of the
Company, threatened against the Company in writing any action, suit, proceeding, investigation
or claim by any Person to which the Company is a party that claims that the Intellectual
Property or the manufacture, use, marketing, sale, offer for sale, importation or distribution of
any Product infringes on any Person’s trade secrets or other intellectual property. The Company
has not received any written communication containing an offer to license to the Company, or a
request that the Company consider whether it wishes to obtain a license, under any intellectual
property owned by a third party, in each case, to make, use or sell a Product. To the Knowledge
of the Company, without any independent investigation or inquiry, there are no pending
unlicensed patent applications owned by any other Person that if a patent were to issue thereon
without modification or amendment, would limit or prohibit, in any material respect, the
manufacture, use or sale of any Product.
SECTION 3.05. Litigation. As of the date hereof, there is no (a) action, suit,
arbitration proceeding, claim, investigation or other proceeding pending or, to the Knowledge of
the Company, threatened against the Company or (b) any governmental inquiry pending or, to
the Knowledge of the Company, threatened against the Company, in each case with respect to
clause (a) or (b) above, which, if adversely determined, would question the validity of, or could
reasonably be expected to have a material adverse effect on the transactions contemplated by
this Agreement or could reasonably be expected to have a Material Adverse Effect. As of the
date hereof, there is no action, suit, arbitration proceeding, claim, investigation or other
proceeding pending or, to the Knowledge of the Company, threatened in writing against the
Company relating to any of the Products, the Intellectual Property related to any of the
Products or the Regulatory Approvals.
SECTION 3.06. Compliance with Laws. To the Knowledge of the Company,
the Company (a) is not in violation of, has not violated and is not under investigation with
respect to, and (b) has not been threatened to be charged with or been given written notice of
any violation of, any law, rule, ordinance or regulation of, or any judgment, order, writ,
decree, permit or license entered by any Governmental Authority applicable to the
Company which would reasonably be expected to have a Material Adverse Effect.
SECTION 3.07. Conflicts. Neither the execution and delivery of this Agreement
nor the performance or consummation of the transactions contemplated hereby by the Company
will: (a) contravene, conflict with, result in a breach or violation of, constitute a default
under, or accelerate the performance provided by, in any material respects any provision of (i)
any law, rule, ordinance or regulation of any Governmental Authority, or any judgment, order,
writ, decree, permit or license of any Governmental Authority, to which the Company or
any of its assets or properties are subject or bound or (ii) any contract, agreement,
commitment or instrument to which the Company is a party or by which the Company, or
any of its assets or property’s is bound or committed; (b) contravene, conflict with, result in
a breach or violation of, constitute a default under, or accelerate the performance provided
by, any provisions of the certificate of formation or limited liability company agreement (or

- 13 -
other organizational or constitutional documents) of the Company; (c) require any
notification to, filing with, or consent of, any Person or Governmental Authority, except
such consents that have been obtained at or prior to the date hereof; or (d) give rise to any
right of termination, cancellation or acceleration of any right or obligation of the
Company or to a loss of any right of the Company to distribute, market and/or sell any of the
Products, except, in the case of clause (a), (c) or (d) above, for any such breaches, defaults or
other occurrences that would not, individually or in the aggregate, have a Material Adverse
Effect.
SECTION 3.08. Current Indebtedness. Other than as set forth on Schedule 3.08,
there is no indebtedness (other than trade indebtedness in the ordinary course of business) for
borrowed money of the Company.
SECTION 3.09. Financial Statements. All financial statements for the
Company and Guarantor delivered to Ligand fairly present, in conformity with generally
accepted accounting principles, in all material respects, the consolidated financial condition and
consolidated results of operations of the Company and Guarantor.
ARTICLE IV - Representations and Warranties of Ligand
SECTION 4.01. Organization. Ligand is a corporation duly incorporated and
validly existing under the laws of the State of Delaware.
SECTION 4.02. No Assignment; Authorization. Ligand has not assigned,
transferred, pledged, granted a security interest in or otherwise disposed of any of its obligations
or rights under the Ligand Purchase Agreement, or any right, title or interest in or to the
Products, any Intellectual Property related to the Products or any revenues related to the
Products, except as provided herein, and has all necessary power and authority to enter into,
execute and deliver this Agreement and to perform all of the obligations to be performed by it
hereunder and to consummate the transactions contemplated hereunder. This Agreement has
been duly authorized, executed and delivered by Ligand and constitutes the valid and binding
obligation of Ligand, enforceable against Ligand in accordance with its respective terms,
subject, as to enforcement of remedies, to bankruptcy, insolvency, reorganization, moratorium
or similar laws affecting creditors' rights generally or general equitable principles.
SECTION 4.03. Conflicts. Neither the execution and delivery of this
Agreement nor the performance or consummation of the transactions contemplated hereby by
Ligand will: (a) contravene, conflict with, result in a breach or violation of, constitute a default
under, or accelerate the performance provided by, in any material respects any provision of (i)
any law, rule or regulation of any Governmental Authority, or any judgment, order, writ,
decree, permit or license of any Governmental Authority, to which Ligand or any of its assets or
properties may be subject or bound or (ii) any contract, agreement, commitment or instrument to
which Ligand is a party or by which Ligand or any of its assets or properties is bound or
committed; (b) contravene, conflict with, result in a breach or violation of, constitute a
default under, or accelerate the performance provided by, any provisions of the certificate of
incorporation or bylaws (or other organizational or constitutional documents) of Ligand; or (c)
require any notification to, filing with, or consent of, any Person or Governmental Authority,

- 14 -
except, in the case of the foregoing clause (a) or (c), for any such breaches, defaults or other
occurrences that would not, individually or in the aggregate, have a material adverse effect
on the ability of Ligand to perform any of its obligations under this Agreement.
ARTICLE V - Covenants
SECTION 5.01. Access; Information.
(a) Maintenance of Books and Records. During the Term, the
Company shall keep and maintain, or cause to be kept and maintained, at all times books of
account and records consistent with good business practices and customary industry standards
adequate to correctly reflect all payments paid and/or payable to Ligand with respect to the
Products.
(b) Inspection Rights. During the Term, Ligand shall have the right to
designate a Third Party independent public accounting firm (the “Ligand Representative”) to
visit the Company’s and its Subsidiaries’ offices and properties where the Company and its
Subsidiaries keep and maintain their books and records relating or pertaining to the Products for
the purpose of conducting an audit of such books and records with respect the payments due and
payable to Ligand under Section 2.02 or Section 2.03, and to inspect and audit such books and
records for such purpose, during normal business hours, and, upon at least ten (10) Business
Days’ written notice given by Ligand to the Company, the Company will provide such Ligand
Representative reasonable access to such books and records; provided, however, such inspection
and audit rights may only be exercised by Ligand once in each calendar year.
(c) Audit Costs. In the event any audit of the books and records of the
Company and its Subsidiaries relating to the the gross revenues of the Products or Net Sales
Proceeds conducted by Ligand and/or any of Ligand’s representatives reveals that the amounts
paid to Ligand hereunder for the period of such audit have been understated by more than ten
percent (10%) of the undisputed amounts due for the period subject to such audit, then the
Audit Costs in respect of such audit shall be borne by the Company; and in all other cases,
such Audit Costs shall be borne by Ligand.
(d) Monthly Reports. During the Term, the Company shall, promptly
after the end of each Payment Month of the Company (but in no event later than thirty (30) days
following the end of such Payment Month), produce and deliver to Ligand a Monthly
Report for such Payment Month.
(e) Periodic Reports. The Company shall deliver to Ligand the
following financial statements:
(i) Within forty-five (45) days after the end of each Fiscal Quarter
after the Effective Date, copies of the unaudited financial statements of the Company
for such Fiscal Quarter; and
(ii) Within one hundred twenty (120) days after the end of each Fiscal
Year after the Effective Date, copies of the audited consolidated financial statements of
the Guarantor and the Company for such Fiscal Year.

- 15 -
(f) Notice of Deposits Following a Sweep Event. Following a Sweep Event,
on the first Business Day of each week, the Company shall provide Ligand with notice of the
amount and the source of all deposits made during the preceding week into the Main Account.
SECTION 5.02. Confidentiality; Press Release.
(a) All Confidential Information furnished by the Company or Seller to
Ligand or by Ligand to the Company in connection with this Agreement and the transactions
contemplated hereby, as well as the terms, conditions and provisions of this Agreement, shall be
kept confidential by Ligand and the Company. Notwithstanding the foregoing, (i) the
Company and Ligand may disclose such Confidential Information to their partners, directors,
employees, managers, officers, investors, bankers, advisors, trustees and representatives, (ii) the
Company may disclose the terms, conditions and provisions of this Agreement to any
Third Party in connection with (and only in connection with) a transaction with such Third
Party that could reasonably be expected to result in (X) a Company Change of Control, (Y) a
Product Change of Control or (Z) a sale by the Company of a Subsidiary, division, product line,
or other significant portion of its business, and (iii) the Company and Ligand may disclose such
Confidential Information as may otherwise be required by applicable law, including filing this
Agreement with the SEC; provided, in the case of the foregoing clauses (i) and (ii) that such
Persons and such Third Parties shall be informed of the confidential nature of such information
and shall be obligated to keep such information confidential pursuant to the terms of this
Section 5.02(a); provided, further, that in the case of the foregoing clause (iii) Ligand shall
provide at least five (5) Business Days’ notice to the Company of any filing with the SEC and
consider in good faith a request for confidential treatment of any portion of this Agreement prior
to filing with the SEC.
(b) Notwithstanding the foregoing clause (a), Ligand may make a press
release or other announcement or public disclosure concerning this Agreement, provided that
such press release shall be (x) subject to prior review by the Company and (y) in form and
substance reasonably satisfactory to the Company taking into account any commercial
sensitivities of the Company.
SECTION 5.03. Efforts; Further Assurance. Subject to the terms and
conditions of this Agreement, each of Ligand and the Company will use its commercially
reasonable efforts to take, or cause to be taken, all actions and to do, or cause to be done, all
things necessary under applicable laws and regulations to consummate the transactions
contemplated by this Agreement. Ligand and the Company agree to execute and deliver such
other documents, certificates, agreements and other writings and to take such other actions as
may be reasonably necessary in order to consummate or implement expeditiously the
transactions contemplated by this Agreement.
SECTION 5.04. Remedies Event. During the Term, if a Remedies Event shall
have occurred and be continuing, subject to the Intercreditor Agreement, the Company shall
not, without the consent of Ligand, make a distribution to its member or members, or retire any
indebtedness for borrowed money, other than in connection with a Permitted Transaction, or
engage in any transaction that would result in a Company Change of Control.

- 16 -
SECTION 5.05. Indebtedness; Sale of Revenue Interests. Prior to the time
that the Buydown Payment is paid in full, unless Ligand shall otherwise consent in writing,
the Company shall not, other than in connection with any Permitted Transaction, (x) incur,
create, assume or permit to exist any indebtedness for borrowed money of the Company
other than indebtedness of the Company as of the Effective Date or (y) sell, assign, transfer
or convey any interests in the revenues generated by the Products to any Third Party other
than by the terms of this Agreement.
SECTION 5.06. Remittance of Funds to Accounts.
(a) Weekly Sweep to Special Account. The Company shall instruct the
Depository Bank to sweep any funds arising from the Royalty Interests contained in the Main
Account, no less frequently than once every week, into the Special Account, in accordance
with further instructions to be provided by the Company on a weekly basis, it being
understood that, in respect of the sweep contemplated under this Section 5.06(a), at the end
of each Payment Month, the Company may retain from disbursement from the Special
Account to Ligand any Excluded Costs deriving from any week covered by such Payment
Month so long as such Excluded Costs are reflected in the Monthly Report in respect of such
Payment Month.
(b) Special Account. The funds in the Special Account shall be held in
trust solely for the benefit of Ligand. The Company shall not take any action with respect
to the Special Account other than making (i) the instructions to the Depository Bank necessary
to effectuate the sweep contemplated in clause (a) of this Section 5.06, (y) any adjustment
(and corresponding withdrawal of excess funds) for Excluded Costs as necessary to
reconcile the balance of the Special Account with the amount to which Ligand is entitled
pursuant to Section 2.02(a), provided that such adjustment shall occur only in accordance with
and upon delivery of a Monthly Report calculating such Excluded Costs and (z) any
disbursement of funds from the Special Account to the Ligand Account in accordance with
Section 2.02(c)(i).
ARTICLE VI - Term and Termination
SECTION 6.01. Term. This Agreement shall commence on the Effective Date
and shall continue through and including the tenth anniversary of the Effective Date (the
“Term”).
SECTION 6.02. Extension of the Term. If any payments are accrued hereunder
on or prior to the end of the Term and are required to be made by one of the Parties
hereunder, this Agreement shall remain in full force and effect until any and all such payments
have been made in full. Upon expiration or termination of this Agreement in accordance
with its terms, all right, title and interest in and to the Royalty Interests shall automatically
revert to the Company (and the security interests granted to Ligand hereunder shall
automatically terminate), and Ligand will have no further rights in or with respect to the Royalty
Interests or other Collateral and all other rights and interests of Ligand hereunder shall terminate
(other than any contingent indemnification obligations with respect to which no claim has been
made).

- 17 -
SECTION 6.03. Effect of Termination. In the event of the termination of this
Agreement pursuant to Section 6.02, this Agreement shall forthwith become void, impose
no liability on the part of any Party hereto or its Affiliates, directors, officers, stockholders,
partners, managers or members and have no effect other than the provisions of this Section 6.03,
and Section 5.02, Section 6.02 and Article VII hereof, which shall survive any such termination.
ARTICLE VII Miscellaneous
SECTION 7.01. Survival. All representations and warranties made herein or in
any other writing delivered pursuant hereto shall survive the execution and delivery of this
Agreement and shall continue to survive until the expiration or termination of this Agreement
in accordance with Article VI.
SECTION 7.02. Notices. All notices, consents, waivers and communications
hereunder given by any Party to the other shall be in writing (including facsimile transmission)
and delivered personally, by telegraph, telecopy, telex or facsimile, by a recognized overnight
courier, or by dispatching the same by certified or registered mail, return receipt requested,
with postage prepaid, in each case addressed (with a copy by email):
If to Ligand to:
Ligand Pharmaceuticals Incorporated
00000 Xxxxx Xxxxxx Xxxxx Xxxx, Xxxxx 000
Xx Xxxxx, XX 00000
Attention: Xxxxxxx Xxxxxxx
Email: xxxxxxxx@xxxxxx.xxx
With a copy to:
Xxxxxx & Xxxxxxx LLP
00000 Xxxx Xxxxx Xxxxx
Xxx Xxxxx, XX 00000
Attention: Xxxxx Xxxxxxxxx
Email: xxxxxx.xxxxxxxxx@xx.xxx
If to the Company to:
Aziyo Med, LLC
00000 Xxxxxxxxxx Xxxxx, Xxxxx 000
Xxxxxx Xxxxxx, XX 00000
Attention: Xxxxxxx Xxxxx
Email: xxxxxx@xxxxx.xxx

- 18 -
With a copy to:
Xxxxxxx & Xxxxxxx LLP
Xxx Xxxxxxxxxxxx Xxxxx
Xxxxxxxx, XX 00000
Attention: Xxxx X. Xxxxxxxx, Xx.
Email: xxxxxxxxx@xxxxxxx.xxx
or to such other address or addresses as Ligand or the Company may from time to time
designate by notice as provided herein, except that notices of changes of address shall be
effective only upon receipt. All such notices, consents, waivers and communications shall: (a)
when posted by certified or registered mail, postage prepaid, return receipt requested, be
effective three (3) Business Days after dispatch, (b) when telegraphed, telecopied, telexed or
facsimiled, be effective upon receipt by the transmitting party of confirmation of complete
transmission, or (c) when delivered by a recognized overnight courier or in person, be effective
upon receipt when hand delivered.
SECTION 7.03. Successors and Assigns.
(a) The provisions of this Agreement shall be binding upon and inure to the
benefit of the parties hereto and their respective successors and assigns.
(b) Upon the consent of Ligand (which consent may not be
unreasonably withheld, delayed or conditioned for any proposed assignment to any
reasonably creditworthy proposed assignee), the Company may assign all or any applicable part
of its rights and obligations under this Agreement in respect of any Product Change of Control,
subject to the assumption by such proposed assignee of the obligations set forth in Section 2.01
and Section 2.02 with respect to such Product.
(c) Solely upon the consent of the Company (which consent may not be
unreasonably withheld, delayed or conditioned, other than in respect of any proposed
assignment to any direct competitor of the Company, in respect of which such consent may be
granted or withheld by the Company in its sole discretion), Ligand may assign any of its
obligations or rights under this Agreement without restriction; provided that,
notwithstanding the foregoing, Ligand may assign its rights and/or delegate its
obligations under this Agreement to an Affiliate, to any Person in a transaction in which Ligand
also assigns all of its right, title and interest in all or substantially all of its assets to the same
party contemporaneous with the assignment of this Agreement, or to a successor,
whether by way of merger, sale of stock or otherwise, without the Company's prior written
consent. In advance of any proposed assignment by Ligand to any proposed assignee,
Ligand shall provide to the Company any information concerning such proposed assignment
and such proposed assignee as may be reasonably requested by the Company.
SECTION 7.04. Indemnification.
(a) The Company hereby indemnifies and holds Ligand and its
Affiliates and any of their respective partners, directors, managers, members, officers,
employees and agents (each, a “Ligand Indemnified Party”) harmless from and against any

- 19 -
and all Losses incurred or suffered by any Ligand Indemnified Party arising out of any breach of
any representation or warranty made by the Company in this Agreement.
(b) Ligand hereby indemnifies and holds the Company, its Affiliates and any
of their respective partners, directors, managers, officers, employees and agents (each, a
“Company Indemnified Party”) harmless from and against any and all Losses incurred or
suffered by a Company Indemnified Party arising out of any breach of any representation or
warranty made by Ligand in this Agreement.
(c) If any claim, demand, action or proceeding (including any
investigation by any Governmental Authority) shall be brought or alleged against an
indemnified party in respect of which indemnity is to be sought against an indemnifying
party pursuant to the preceding paragraphs, the indemnified party shall, promptly after
receipt of notice of the commencement of any such claim, demand, action or proceeding, notify
the indemnifying party in writing of the commencement of such claim, demand, action or
proceeding, enclosing a copy of all papers served, if any; provided, that the omission to so
notify such indemnifying party will not relieve the indemnifying party from any liability that it
may have to any indemnified party under the foregoing provisions of this Section 7.04
unless, and only to the extent that, such omission results in the forfeiture of, or has a material
adverse effect on the exercise or prosecution of, substantive rights or defenses by the
indemnifying party. In case any such action is brought by a third party against an
indemnified party and it notifies the indemnifying party of the commencement thereof, the
indemnifying party will be entitled to participate therein and, to the extent that it may wish,
jointly with any other indemnifying party similarly notified, to assume the defense thereof, with
counsel reasonably satisfactory to such indemnified party (who shall not, except with the
consent of the indemnified party, be counsel to the indemnifying party), and after notice
from the indemnifying party to such indemnified party of its election so to assume the defense
thereof, the indemnifying party will not be liable to such indemnified party under this
Section 7.04 for any legal or other expenses subsequently incurred by such indemnified party in
connection with the defense thereof other than reasonable costs of investigation. In any such
proceeding by a third party, an indemnified party shall have the right to retain its own counsel,
but the reasonable fees and expenses of such counsel shall be at the expense of such
indemnified party unless the indemnifying party and the indemnified party shall have
mutually agreed to the retention of such counsel. It is agreed that the indemnifying party
shall not, in connection with any proceeding or related proceedings in the same
jurisdiction, be liable for the reasonable fees and expenses of more than one separate law firm
(in addition to local counsel where necessary) for all such indemnified parties. The
indemnifying party shall not be liable for any settlement of any proceeding effected
without its written consent, but if settled with such consent or if there be a final judgment for
the plaintiff, the indemnifying party agrees to indemnify the indemnified party from and
against any loss or liability by reason of such settlement or judgment. No
indemnifying party shall, without the prior written consent of the indemnified party, effect any
settlement of any pending or threatened proceeding in respect of which any indemnified party is
or could have been a party and indemnity could have been sought hereunder by such
indemnified party, unless such settlement includes an unconditional release of such
indemnified party from all liability on claims that are the subject matter of such proceeding.

- 20 -
(d) Ligand's sole remedy shall be to recover any monetary damages
associated with a breach of a representation or warranty made by the Company in this
Agreement, subject to the other terms and provisions contained in this Agreement.
SECTION 7.05. No Implied Representations and Warranties. Each Party
acknowledges and agrees that, other than the representations and warranties specifically
contained in this Agreement, there are no representations or warranties of either Party or any
other Person either expressed or implied with respect to the Products or the Sale Transaction
or the other transactions contemplated hereby. Without limiting the foregoing,
Ligand acknowledges and agrees that (a) Ligand and its Affiliates, together with its and its
Affiliates’ representatives, have made their own investigation of the Products, the
Intellectual Property related to the Products and the Regulatory Approvals and are not
relying on any implied warranties or upon any other representation or warranty whatsoever,
including any representation or warranty as to the future amount or potential value of the
Products or Net Sales Proceeds, the amount of any payments by the Company hereunder or as to
the creditworthiness of the Company and (b) except as expressly set forth in any representation
or warranty in this Agreement, Ligand shall have no claim or right to indemnification by the
Company pursuant to Section 7.04 (or otherwise) with respect to any information, documents or
materials furnished by the Company or Seller or any of their respective representatives to
Ligand, any of its Affiliates, or any of its or its Affiliates’ representatives, including any
information, documents or material made available to Ligand, its Affiliates or any of its and its
Affiliates’ representatives in any data room, presentation, management presentation,
interview or any other form relating to the transactions contemplated hereby.
SECTION 7.06. Independent Nature of Relationship. (a) The relationship
between the Company and Ligand is solely that of obligor and obligee, and neither Ligand nor
the Company has any fiduciary or other special relationship with the other or any of their
respective Affiliates. Nothing contained herein shall be deemed to constitute the Company and
Ligand as a partnership, an association, a joint venture or other kind of entity or legal form for
any purposes, including any tax purposes.
(b) No officer or employee or agent of Ligand will be located at the premises
of the Company or any of its Affiliates, except in connection with an audit performed
pursuant to Section 5.01. No officer, manager or employee of Ligand shall engage in any
commercial activity with the Company or any of its Affiliates other than as contemplated herein
or as otherwise separately agreed in writing.
(c) Ligand and/or any of its Affiliates shall not at any time obligate the
Company, or impose on the Company any obligation, in any manner or respect to any Person
not a party hereto. The Company and/or any of its Affiliates shall not at any time obligate
Ligand, or impose on Ligand any obligation, in any manner or respect to any Person not a party
hereto.
SECTION 7.07. Entire Agreement. This Agreement, together with the
Exhibits and Schedules hereto (which are incorporated herein by reference), constitute the
entire agreement between the parties with respect to the subject matter hereof and
supersede all prior agreements (including any term sheet), understandings and

- 21 -
negotiations, both written and oral, between the Parties with respect to the subject matter of this
Agreement. Notwithstanding any other provision set forth herein, neither Seller nor
Guarantor is assuming any obligation or liability under the Ligand Purchase Agreement.
No representation, inducement, promise, understanding, condition or warranty not set forth
herein has been made or relied upon by either Party hereto. Neither this Agreement, nor any
provision hereof, is intended to confer upon any Person other than the parties hereto any rights
or remedies hereunder or in respect hereof.
SECTION 7.08. Amendments; No Waivers.
(a) Neither this Agreement nor any term or provision hereof may be
amended, changed or modified except with the written consent of all Parties. No waiver of
any right hereunder shall be effective unless such waiver is signed in writing by the Party
against whom such waiver is sought to be enforced.
(b) No failure or delay by either Party in exercising any right, power or
privilege hereunder shall operate as a waiver thereof nor shall any single or partial exercise
thereof preclude any other or further exercise thereof or the exercise of any other right,
power or privilege. The rights and remedies herein provided shall be cumulative and not
exclusive of any rights or remedies provided by law.
SECTION 7.09. Interpretation. When a reference is made in this
Agreement to an Article, Section, Schedule or Exhibit, such reference shall be to an
Article, Section, Schedule or Exhibit to this Agreement unless otherwise indicated. The
words “include”, “includes” and “including” when used herein shall be deemed in each case
to be followed by the words “without limitation.” Neither Party shall be or be deemed to be the
drafter of this Agreement for the purposes of construing this Agreement against one Party or the
other.
SECTION 7.10. Headings and Captions. The headings and captions in this
Agreement are for convenience and reference purposes only and shall not be considered a part
of or affect the construction or interpretation of any provision of this Agreement.
SECTION 7.11. Counterparts; Effectiveness.This Agreement may be executed
in two or more counterparts, each of which shall be an original, but all of which together shall
constitute one and the same instrument. Any counterpart may be executed by facsimile or pdf
signature and such facsimile or pdf signature shall be deemed an original.
SECTION 7.12. Severability. If any provision of this Agreement is held to be
invalid or unenforceable, the remaining provisions shall nevertheless be given full force and
effect.
SECTION 7.13. Expenses. Each of Ligand and the Company will pay all of
its own fees and expenses in connection with entering into and consummating the transactions
contemplated by this Agreement.

- 22 -
SECTION 7.14. Governing Law; Jurisdiction.
(a)This Agreement shall be governed by, and construed, interpreted and
enforced in accordance with, the laws of the State of New York, without giving effect to the
principles of conflicts of law thereof.
(b) Any legal action or proceeding with respect to this Agreement may be
brought in any state or federal court of competent jurisdiction in the State of New York,
County of New York. By execution and delivery of this Agreement, each Party hereby
irrevocably consents to and accepts, for itself and in respect of its property, generally and
unconditionally the exclusive jurisdiction of such courts. Each Party hereby further
irrevocably waives any objection, including any objection to the laying of venue or based on
the grounds of forum non conveniens, which it may now or hereafter have to the bringing of
any action or proceeding in such jurisdiction in respect of this Agreement.
(c) Each Party hereby irrevocably consents to the service of process out
of any of the courts referred to in clause (b) of this Section 7.14 in any such suit, action or
proceeding by the mailing of copies thereof by registered or certified mail, postage prepaid, to it
at its address set forth in this Agreement. Each Party hereby irrevocably waives any objection
to such service of process and further irrevocably waives and agrees not to plead or claim in
any suit, action or proceeding commenced hereunder that service of process was in any way
invalid or ineffective. Nothing herein shall affect the right of a party to serve process on the
other Party in any other manner permitted by law.
ARTICLE VIII - Intercreditor Matters and Guarantee
SECTION 8.01. Ligand Interests; Recharacterization.
(a) Notwithstanding anything herein to the contrary, it is the intention of the
Parties that the Royalty Interests are owned by Ligand, and such Royalty Interests shall be
treated as the property of Ligand for all purposes, other than federal and state income tax
purposes. The provisions of this Agreement shall be construed to further these intentions of the
Parties.
(b) The Royalty Interests and any amounts received by the Company in
respect of the Royalty Interests and, without limiting the foregoing, any cash deposited into
the Special Account in accordance with the terms hereof and any cash deposited into the
Main Account in respect of or consisting of the Royalty Interests (subject to any adjustments
for Excluded Costs) is not, and is not intended to be, the property of the Company (or, in the
event of a Bankruptcy Event, any estate created thereby by operation of applicable law or
otherwise) but is possessed by the Company in trust solely on behalf of Ligand pending
disbursement to Ligand or as otherwise provided in Section 5.06, in each case, as
contemplated hereby.
(c) If, notwithstanding subparagraph (b), the Royalty Interests are subject
to a Recharacterization, the Parties intend that the Company shall be deemed hereunder to
have granted, and the Company does hereby grant, to Ligand a first priority security interest
in favor of Ligand, to secure the obligations to make the payments under Section 2.01,

- 23 -
Section 2.02, and Section 2.03, including in respect of any acceleration thereof pursuant to
Section 9.02, in the Royalty Interests and all proceeds and products thereof, and the Special
Account and any cash or other funds, amounts or financial assets held therein or credited
thereto.
(d) No other liens or security interests (including any liens or security
interests in favor of the MidCap Credit Facility Agent) shall exist on the Special Account or
the cash or other funds, amounts or financial assets held therein or credited thereto other
than any customary liens of the Depository Bank.
(e) lf, notwithstanding clause (b), the conveyance of the Royalty Interests
is subject to a Recharacterization, the Parties intend that the Company shall be deemed
hereunder to have granted, and the Company does hereby grant (subject to the priorities
specified in the Intercreditor Agreement) a security interest in favor of Ligand, to secure the
obligations to make payments under Section 2.01, Section 2.02, and Section 2.03, including
for the avoidance of doubt any acceleration of any payments pursuant to Section 9.02, in
the Pari Passu Collateral.
SECTION 8.02. Other Ligand Security.
(a) In addition to the special rights and security interests provided in
Section 8.01, as security for the Company’s payment obligations in respect of any Minimum
Quarterly Royalties payable hereunder (including in respect of any acceleration thereof pursuant
to Section 9.02), the Company hereby grants (subject to the priorities specified in the
Intercreditor Agreement) a security interest in all of the following assets of the Company that
constitute “Collateral” under the MidCap Credit Facility (the “General Collateral”):
(i) All goods, Accounts (including health-care insurance receivables),
Equipment, Inventory, contract rights or rights to payment of money, leases, license
agreements, franchise agreements, General Intangibles, commercial tort claims,
documents, instruments (including any promissory notes), chattel paper (whether
tangible or electronic), cash, deposit accounts, investment accounts, commodity accounts
and other Collateral Accounts, all certificates of deposit, fixtures, letters of credit rights
(whether or not the letter of credit is evidenced by a writing), securities, and all other
investment property, supporting obligations, and financial assets, whether now owned or
hereafter acquired, wherever located (each capitalized term in this clause (i) not
otherwise defined in this Agreement or the MidCap Credit Facility, as defined in the
UCC); and
(ii) all the Company's Books relating to the foregoing, and any and all
claims, rights and interests in any of the above and all substitutions for, additions,
attachments, accessories, accessions and improvements to and replacements, products,
proceeds and insurance proceeds of any or all of the foregoing.
(b) Notwithstanding the foregoing, no security interest is or will be granted pursuant
to this Agreement in any right, title or interest of the Company under or in, and “Collateral” shall not
include, any Excluded Assets.

- 24 -
SECTION 8.03. Priority. (a) Pursuant and subject to the Intercreditor
Agreement, the security interest granted in Section 8.01(c) shall be for all purposes senior in
right to any other lien other than any customary liens of the Depository Bank.
(b) Pursuant and subject to the Intercreditor Agreement, the security interest
granted in Section 8.01(e) shall be pari passu with the Existing Liens.
(c) Pursuant and subject to the Intercreditor Agreement, the security interest
granted in Section 8.02 shall be for all purposes junior and subordinate (on a "silent second"
basis) to the Existing Liens.
SECTION 8.04. Other Intercreditor Matters. Ligand acknowledges that, the
MidCap Credit Facility Agent will not file any partial UCC-3 termination statement in respect
of the liens held by the MidCap Credit Facility Agent or otherwise release any of its Collateral
(as such term is defined under the MidCap Credit Facility) under the MidCap Credit Facility.
However, the Intercreditor Agreement shall contain an express acknowledgement by the
MidCap Credit Facility Agent that it has no security interest in or other rights in respect of the
Special Account or any cash held therein.
SECTION 8.05. Control Agreements.
(a) The Company agrees, with respect to the Special Account (upon
request of Ligand), to use commercially reasonable efforts to cause the Depository Bank to
agree to comply at any time with instructions from Ligand to the Depository Bank directing
the disposition of funds from time to time credited to the Special Account, without further
consent of the Company, pursuant to a customary deposit account control agreement in form
and substance satisfactory to Ligand. However, Ligand shall not give any such instructions
or withhold any withdrawal rights from the Company, unless a Remedies Event has occurred
and is continuing.
(b) Nothing herein is intended to affect or shall be construed as affecting
the rights of the MidCap Credit Facility Agent under any deposit account control agreement
in favor of it in respect of the Main Account.
SECTION 8.06. Termination or Release.
(a) Upon receipt by Ligand of an aggregate amount of $15,027,342 on or
after the Closing Date pursuant to this Agreement, all right, title and interest in and to the
Royalty Interest and the Collateral shall automatically revert to the Company, and Ligand will
have no further rights in or with respect to the Royalty Interest or the Collateral and all security
interests granted hereunder shall terminate and be released; provided, however, the other terms
and conditions of this Agreement shall remain in full force and effect, including without
limitation, the Company’s obligation to make the payments described in Article II during the
remainder of the Term.
(b) Upon the withdrawal of any Excluded Costs or other amounts from the
Special Account in accordance with the terms hereof, Ligand's security interest in such
amounts granted pursuant to Section 8.01(c) shall be automatically terminated and released.

- 25 -
(c) Subject to the Intercreditor Agreement, upon the sale, lease, transfer,
assignment or other disposition (a “Transfer”) of any assets of the Company (other than the
Royalty Interests and the Royalty Related Collateral) permitted under the MidCap Credit
Facility, all of Ligand's security interests in such assets shall be automatically terminated and
released.
(d) Subject to the Intercreditor Agreement, at the Company's request, Ligand
shall subordinate its liens and other rights with respect to any such assets or property or
terminate and release its liens with respect to any such assets or property (in each case other
than the Royalty Interests and the Royalty Related Collateral) in connection with any Permitted
Transaction.
(e) Upon the termination of the MidCap Credit Facility (or, if the MidCap
Credit Facility is refinanced by another debt facility secured by all Collateral (other than the
Royalty Interests and the Royalty Related Collateral), upon the termination of such refinancing
debt facility), Ligand's security interest in all Collateral (other than the Royalty Interests and the
Royalty Related Collateral) shall automatically be terminated and released.
(f) In connection with any termination or release pursuant to this
Section 8.06, Ligand shall execute and deliver to the Company all documents that the Company
shall reasonably request to evidence such termination or release. Ligand further agrees
that with respect to any deposit account (other than the Special Account) over which it has
control, it shall not give any instruction to the applicable bank until a Remedies Event has
occurred and is continuing.
ARTICLE IX - Remedies
SECTION 9.01. Remedies. If any Remedies Event shall occur and be
continuing, subject to the terms of the Intercreditor Agreement, Ligand may exercise all rights
and remedies of a secured party under the UCC or under any other applicable law and in
equity, provided that Ligand shall exercise any such remedy against the Special Account prior to
the exercise of any such remedy against any other Collateral.
SECTION 9.02. Acceleration. If any Remedies Event shall occur and be
continuing, subject to the terms of the Intercreditor Agreement, upon notice to the
Company, Ligand may declare all Minimum Quarterly Royalties required to be paid by the
Company from the date of such Remedies Event until the expiration of the Term to be due and
payable forthwith, whereupon the same shall immediately become due and payable.
[Remainder of this page intentionally left blank]



EXECUTION VERSION
GUARANTY AGREEMENT
THIS GUARANTY AGREEMENT (this “Agreement”), is dated as of May 31, 2017,
by AZIYO BIOLOGICS, INC., a Delaware corporation (the “Guarantor”), for the benefit of
LIGAND PHARMACEUTICALS INCORPORATED, a Delaware corporation (“Ligand”)
pursuant to a certain Royalty Agreement, dated as of the date hereof, with Aziyo Med, Inc., a
Delaware corporation (as such Agreement may be amended from time to time, the “Royalty
Agreement”). Unless otherwise defined herein or the context otherwise requires, capitalized
terms are used herein with the respective meanings set forth in the Royalty Agreement.
W I T N E S S E T H:
WHEREAS, Aziyo Med, LLC, a Delaware limited liability company (the “Company”)
is wholly subsidiary of the Guarantor, and the Guarantor acknowledges that it will benefit from
the Royalty Agreement and the transactions related thereto; and
WHEREAS, the Guarantor is willing to guaranty the payment by the Company of the
Buydown Payment under Section 2.01 of the Royalty Agreement as set forth below;
NOW, THEREFORE, in consideration of the premises and for other good and valuable
consideration (the receipt and sufficiency of which are hereby acknowledged), and intending to
be legally bound, the Guarantor hereby agrees as follows:
1. The Guarantor hereby absolutely and unconditionally guaranties to Ligand the
payment by the Company of the Buydown Payment under Section 2.01 of the Royalty
Agreement in accordance with the terms and conditions set forth therein, and the Guarantor
covenants and agrees that upon the failure by the Company to pay any installment of the
Buydown Payment on or prior to the due date, the Guarantor shall, upon demand by Ligand,
pay the same itself, or cause the same to be paid, as if the Guarantor were the obligor under
the Royalty Agreement. This Agreement shall terminate and be of no further force or effect
upon payment in full of the Buydown Payment.
2. The obligations of the Guarantor hereunder shall be absolute and unconditional,
and shall not be impaired, released, modified, stayed, limited, terminated or discharged in
whole in party, by any of the following and the Guarantor hereby freely and voluntarily waives
any defense based on any of the following: the validity, regularity or enforceability of the
Royalty Agreement or any provision thereof, or the absence of any action to enforce the same,
the recovery of any judgment against the Guarantor or any other party or any action to enforce
the same (other than to the extent that such recovery results in the full satisfaction and
performance of any obligations of the Guarantor hereunder), any failure or delay in the
enforcement of the obligations of the Company under the Royalty Agreement, all
presentments, demands for performance, notices of nonperformance, protests, notices of
protest, notices of dishonor, notices of acceptance of this Agreement or other notices to which
the Company may be entitled, the benefit or defense of any statute of limitations affecting the
liability of the Guarantor hereunder or the liability of the Company, any rights of subrogation,
reimbursement, exoneration, contribution and indemnity, and any rights or claims of any kind

2
or nature against the Company which arise out of or are caused by this Agreement, and any
rights to enforce any remedy which the Guarantor may have against the Company, any duty of
Ligand to advise the Guarantor of any information known to Ligand regarding the financial
condition of the Company or any setoff, counterclaim, recoupment, limitation or termination,
and irrespective of any other circumstances which might otherwise limit recourse against the
Guarantor or constitute a legal or equitable discharge or defense of a guarantor or surety,
provided that, notwithstanding the foregoing provisions of this Section 2, the Guarantor shall
not be required to perform or cause to be performed any obligation as to which the Company
would be discharged, released or otherwise excused under the provisions of the Royalty
Agreement. Notwithstanding anything set forth in this Agreement to the contrary, this
Agreement shall not be interpreted to impose upon the Guarantor any greater obligations than
the obligations of the Company under the Royalty Agreement as the terms thereof may be
modified, waived or released from time to time.
3. The obligations of the Guarantor hereunder shall not be subject to any
requirement that (i) any person first enforce any remedies it may have against the Company or
any other person, (ii) any person first seek to recover from the Company under the Royalty
Agreement before proceeding against the Guarantor hereunder, (iii) the existence of any matter
or circumstance concerning the ability (financial or otherwise) of the Company to perform its
obligations under the Royalty Agreement, or (iv) any other requirement, condition or matter,
whether or not similar to the foregoing, other than events, conditions or circumstances which,
under the express terms and provisions of the Royalty Agreement, discharge, release or
otherwise excuse performance by the Company of its obligations under the Royalty
Agreement. This is a guaranty of payment and performance and not merely of collection. The
obligations of the Guarantor with respect to its obligations hereunder shall not be subject to any
claim of the Guarantor against any other person (including, without limitation, Ligand) other
than a setoff, reduction, claim or defense expressly authorized under the Royalty Agreement.
4. The Guarantor shall maintain and do or cause to be done all things necessary to
preserve and keep in full force and effect its corporate existence. The Guarantor shall not seek
or permit the dissolution or liquidation of the Guarantor in whole or in part, merge into,
consolidate with, or in any way be acquired by any person, or transfer all or substantially all of
its assets to any person or persons, unless the surviving or resulting person (or, in the case of a
transfer of assets, the transferee) assumes all of the obligations of the Guarantor hereunder.
Neither the Guarantor’s obligation to make payment or render performance in accordance with
the terms of this Agreement nor any remedy for the enforcement thereof, shall be impaired,
modified, stayed, released, limited, terminated or discharged in any manner whatsoever by any
impairment, modification, change, release, limitation or stay of the liability of the Company or
its estate in bankruptcy or any remedy for the enforcement thereof resulting from the operation
of any present or future provision of Title 11 of the United States Code entitled “Bankruptcy,”
as now and hereafter in effect, or any successor statute or other statute or from the decision of
any court interpreting any of the same, and the Guarantor shall remain obligated under this
Agreement as if no such impairment, stay, modification, change, release or limitation had
occurred.

3
5. No failure or delay in exercising any right, power or privilege hereunder or
under the Royalty Agreement shall operate as a waiver thereof nor shall any single or partial
exercise thereof preclude any other right, power or privilege. No waiver, amendment, release
or modification of this Agreement shall be established by conduct, custom or course of
dealing, but solely by an instrument in writing duly executed by the party against whom such
waiver, amendment, release or modification is sought to be enforced.
6. The Guarantor may, upon prior written notice to Ligand, assign all or any
portion of its rights and obligations under this Agreement or delegate any of its obligations
under this Agreement at any time so long as such assignee or delegee shall be creditworthy and
capable of performing the obligations of the Guarantor hereunder; and such assignment or
delegation shall relieve the Guarantor of any obligations or liabilities hereunder arising on or
after the date of the assignment or delegation so long as Ligand consents in advance to such
assignment or delegation, which consent shall not be unreasonably withheld, conditioned or
delayed. Any assignment in violation of this Section 6 shall be null and void and of no effect.
7. The invalidity or unenforceability of one or more provisions of this Agreement
shall not affect the validity or enforceability of the remaining portions of this Agreement.
8. Notwithstanding any performance by the Guarantor by reason of this
Agreement, the Guarantor agrees that it shall not be entitled to any claim whatsoever against
the Company in consequence of such performance. The Guarantor hereby irrevocably waives
any right to collect from the Company under any right or claim to subrogation (or right in the
nature of a right to subrogation) against the Company to which it would be entitled under
applicable law in consequence of such performance. If the Guarantor shall nonetheless receive
any amount or property in consequence of a right of subrogation or similar right, such amount
shall be held in trust by the Guarantor and paid over to, or upon the direction of, the Company
without further claim for its return. The foregoing limitation shall not preclude the Company
from making any payment to the Guarantor in respect of any performance by the Guarantor of
its obligations hereunder.
9. Notices and other communications to be given pursuant to this Agreement shall
be in writing and shall be delivered personally or sent by certified mail, return receipt
requested, or by facsimile (i) if to the Guarantor, at 00000 Xxxxxxxxxx Xxxxx, Xxxxx 000, Xxxxxx
Xxxxxx, Xxxxxxxx 00000, Attention: President, with a copy to Xxxxxxx & Xxxxxxx LLP, its
counsel, at Xxx Xxxxxxxxxxxx Xxxxx, Xxxxxxxx, Xxxxxxxxxxx 00000, Attention: Xxxx X.
Xxxxxxxx, Xx., and (ii) if to Ligand, at 00000 Xxxxx Xxxxxx Xxxxx Xxxx, Xxxxx 000, Xx Xxxxx,
Xxxxxxxxxx 00000, with a copy to Xxxxxx & Xxxxxxx LLP, its counsel, at 00000 Xxxx Xxxxx
Xxxxx, Xxx Xxxxx, Xxxxxxxxxx 00000, Attention: Xxxxx Xxxxxxxxx. Any notice given pursuant
to this Section 9 shall be effective upon receipt.
10. This Agreement shall be governed by the laws of the State of Delaware other
than its choice of law rules.
11. The Guarantor irrevocably (i) agrees that any suit, action or other legal

4
proceeding arising out of or relating to this Agreement may be brought in a court of record in
the State of Delaware or in the Courts of the United States of America, District of Delaware,
(ii) consents to the jurisdiction of each such court in any such suit, action or proceeding, and
(iii) waives any objection which the Guarantor may have to the laying of venue of any such
suit, action or proceeding in any of such courts and any claim that any such suit, action or
proceeding has been brought in an inconvenient forum.
[signature page follows]

[signature page to Ligand Guaranty Agreement]
IN WITNESS WHEREOF, the Guarantor has caused this Guaranty Agreement to be
executed on its behalf by one of its officers thereunto duly authorized on and as of the day and
year first above written.
AZIYO BIOLOGICS, INC.
By:
Name: Xxxxxxx X. Xxxxx
Title: Vice President, Finance and Treasurer
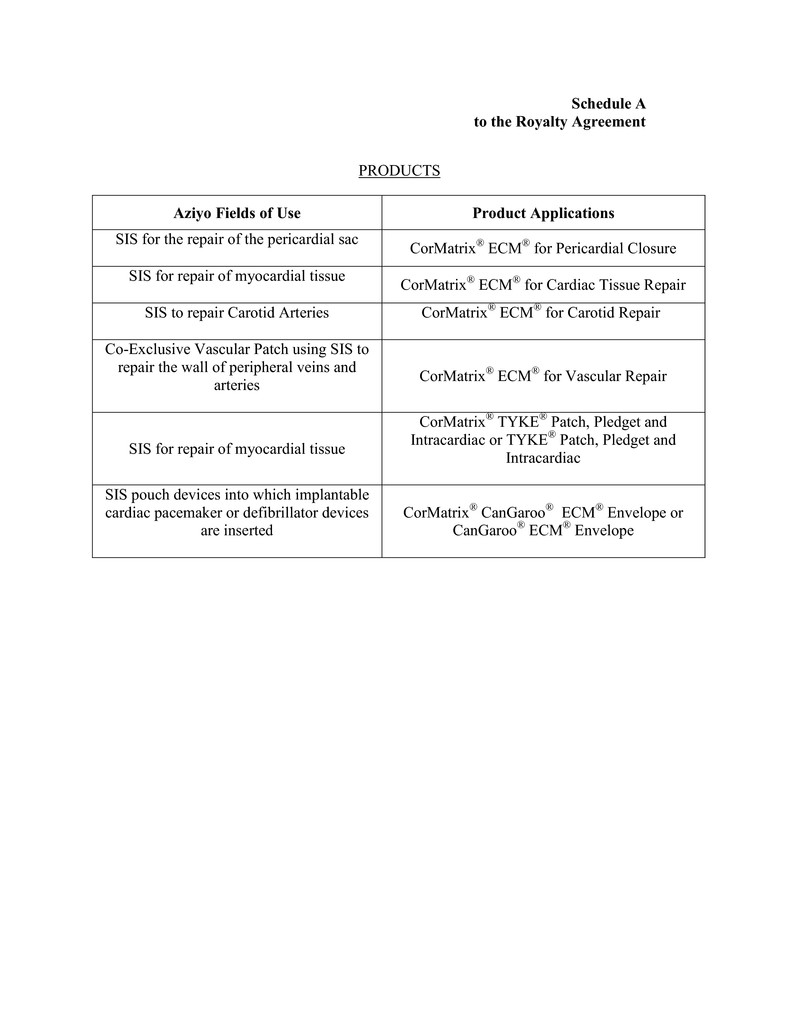
Schedule A
to the Royalty Agreement
PRODUCTS
Aziyo Fields of Use Product Applications
SIS for the repair of the pericardial sac
CorMatrix
® ECM® for Pericardial Closure
SIS for repair of myocardial tissue
CorMatrix
® ECM® for Cardiac Tissue Repair
SIS to repair Carotid Arteries
CorMatrix® ECM® for Carotid Repair
Co-Exclusive Vascular Patch using SIS to
repair the wall of peripheral veins and
arteries
CorMatrix® ECM® for Vascular Repair
SIS for repair of myocardial tissue
CorMatrix® TYKE® Patch, Pledget and
Intracardiac or TYKE® Patch, Pledget and
Intracardiac
SIS pouch devices into which implantable
cardiac pacemaker or defibrillator devices
are inserted
CorMatrix® CanGaroo® ECM® Envelope or
CanGaroo® ECM® Envelope
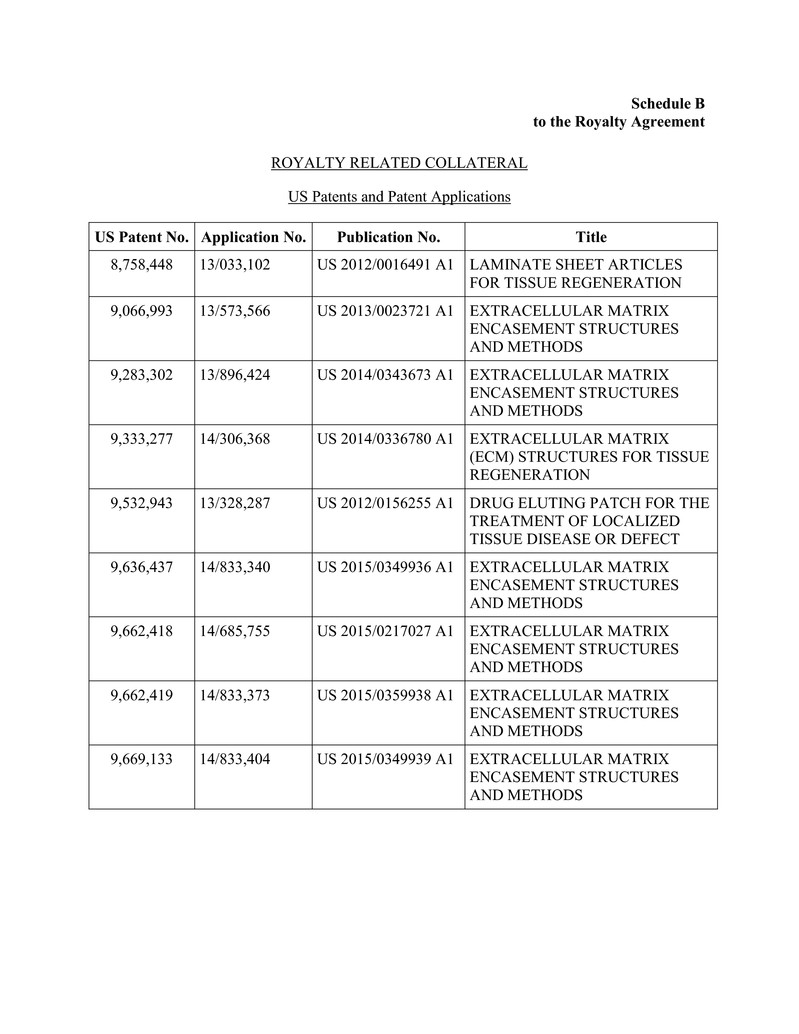
Schedule B
to the Royalty Agreement
ROYALTY RELATED COLLATERAL
US Patents and Patent Applications
US Patent No. Application No. Publication No. Title
8,758,448 13/033,102 US 2012/0016491 A1 LAMINATE SHEET ARTICLES
FOR TISSUE REGENERATION
9,066,993 13/573,566 US 2013/0023721 A1 EXTRACELLULAR MATRIX
ENCASEMENT STRUCTURES
AND METHODS
9,283,302 13/896,424 US 2014/0343673 A1 EXTRACELLULAR MATRIX
ENCASEMENT STRUCTURES
AND METHODS
9,333,277 14/306,368 US 2014/0336780 A1 EXTRACELLULAR MATRIX
(ECM) STRUCTURES FOR TISSUE
REGENERATION
9,532,943 13/328,287 US 2012/0156255 A1 DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED
TISSUE DISEASE OR DEFECT
9,636,437 14/833,340 US 2015/0349936 A1 EXTRACELLULAR MATRIX
ENCASEMENT STRUCTURES
AND METHODS
9,662,418
14/685,755 US 2015/0217027 A1 EXTRACELLULAR MATRIX
ENCASEMENT STRUCTURES
AND METHODS
9,662,419 14/833,373 US 2015/0359938 A1 EXTRACELLULAR MATRIX
ENCASEMENT STRUCTURES
AND METHODS
9,669,133
14/833,404 US 2015/0349939 A1 EXTRACELLULAR MATRIX
ENCASEMENT STRUCTURES
AND METHODS
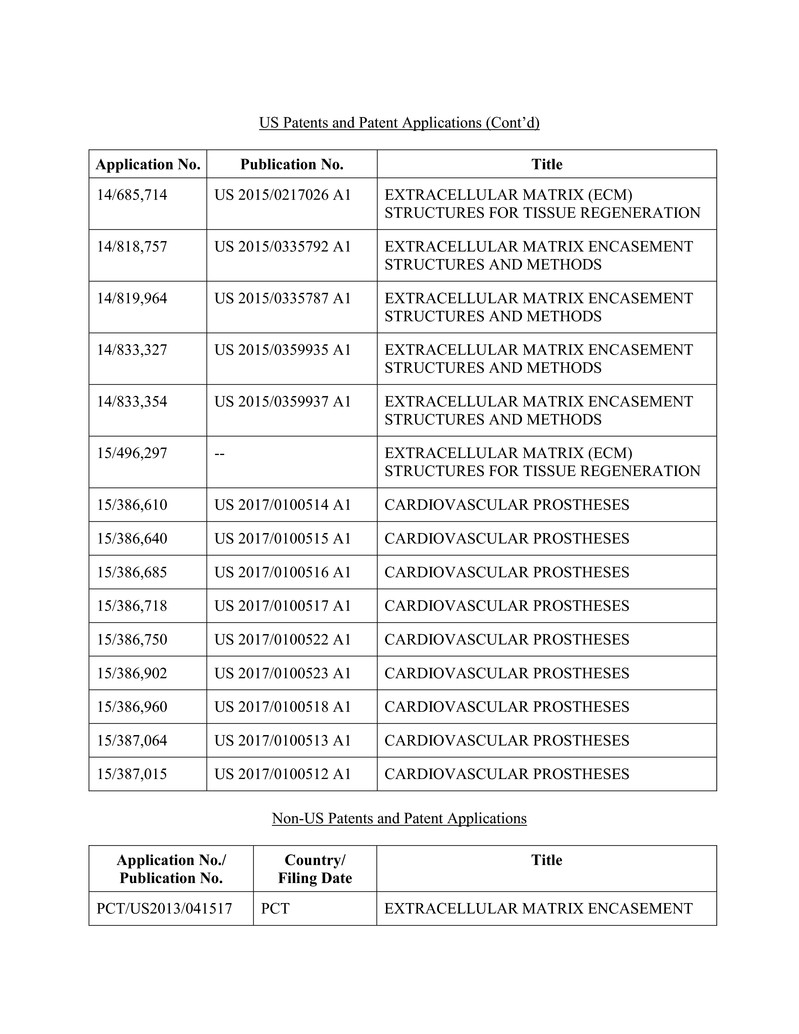
US Patents and Patent Applications (Cont’d)
Application No. Publication No. Title
14/685,714 US 2015/0217026 A1 EXTRACELLULAR MATRIX (ECM)
STRUCTURES FOR TISSUE REGENERATION
14/818,757 US 2015/0335792 A1 EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
14/819,964 US 2015/0335787 A1 EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
14/833,327 US 2015/0359935 A1 EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
14/833,354 US 2015/0359937 A1 EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
15/496,297 -- EXTRACELLULAR MATRIX (ECM)
STRUCTURES FOR TISSUE REGENERATION
15/386,610 US 2017/0100514 A1 CARDIOVASCULAR PROSTHESES
15/386,640 US 2017/0100515 A1 CARDIOVASCULAR PROSTHESES
15/386,685 US 2017/0100516 A1 CARDIOVASCULAR PROSTHESES
15/386,718 US 2017/0100517 A1 CARDIOVASCULAR PROSTHESES
15/386,750 US 2017/0100522 A1 CARDIOVASCULAR PROSTHESES
15/386,902 US 2017/0100523 A1 CARDIOVASCULAR PROSTHESES
15/386,960 US 2017/0100518 A1 CARDIOVASCULAR PROSTHESES
15/387,064 US 2017/0100513 A1 CARDIOVASCULAR PROSTHESES
15/387,015 US 2017/0100512 A1 CARDIOVASCULAR PROSTHESES
Non-US Patents and Patent Applications
Application No./
Publication No.
Country/
Filing Date
Title
PCT/US2013/041517 PCT EXTRACELLULAR MATRIX ENCASEMENT
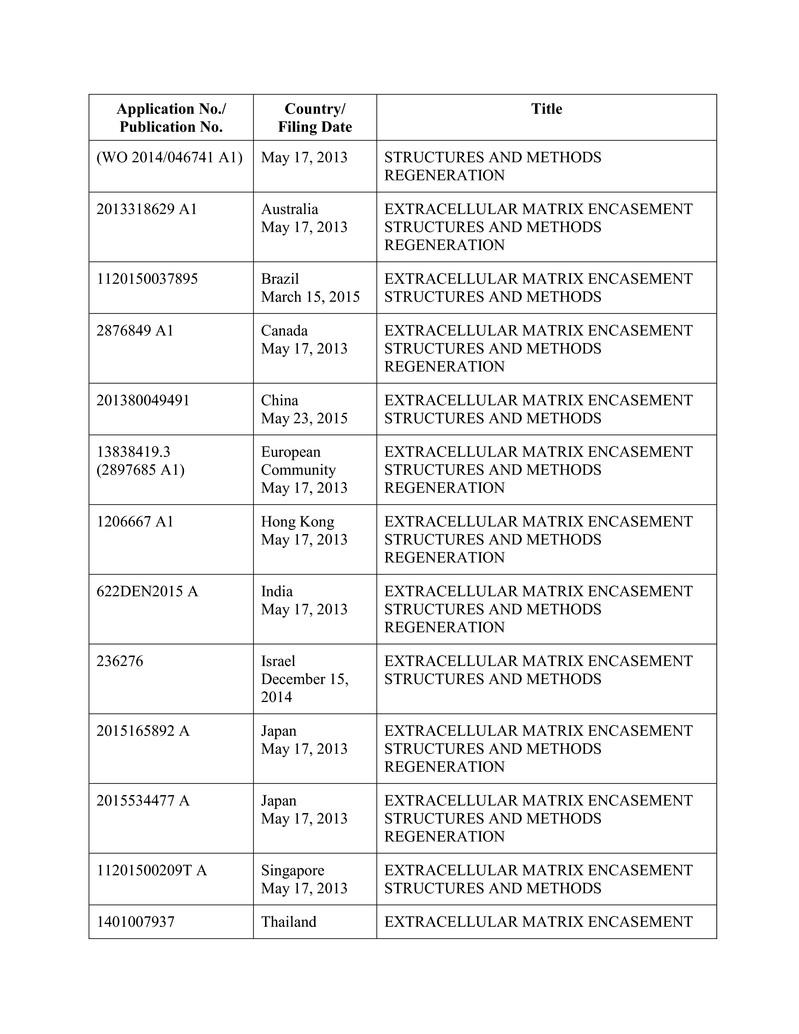
Application No./
Publication No.
Country/
Filing Date
Title
(WO 2014/046741 A1) May 17, 2013 STRUCTURES AND METHODS
REGENERATION
2013318629 X0 Xxxxxxxxx
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
1120150037895 Brazil
March 15, 2015
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
2876849 A1
Canada
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
201380049491 China
May 23, 2015
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
13838419.3
(2897685 A1)
European
Community
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
1206667 A1
Hong Kong
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
622DEN2015 A
India
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
000000 Xxxxxx
December 15,
2014
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
2015165892 A Japan
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
2015534477 A Japan
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
11201500209T A
Singapore
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
1401007937 Thailand EXTRACELLULAR MATRIX ENCASEMENT
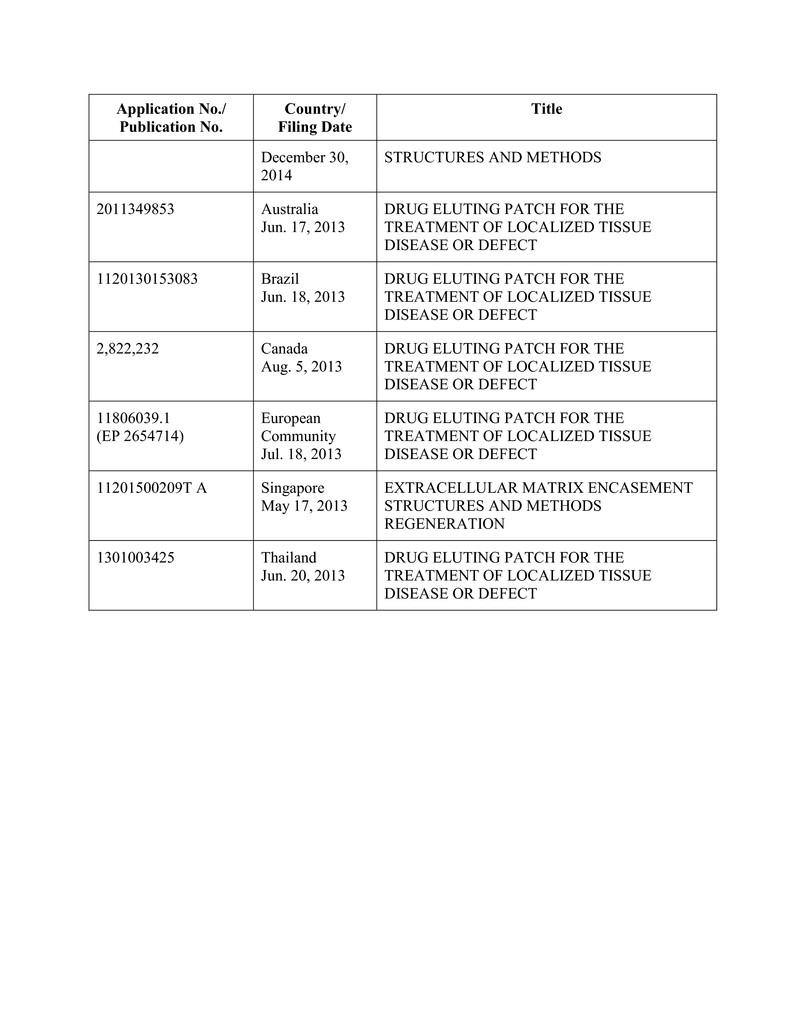
Application No./
Publication No.
Country/
Filing Date
Title
December 30,
2014
STRUCTURES AND METHODS
2011349853 Australia
Jun. 17, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
1120130153083 Brazil
Jun. 18, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
2,822,232 Canada
Aug. 5, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
11806039.1
(EP 2654714)
European
Community
Jul. 18, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
11201500209T A
Singapore
May 17, 2013
EXTRACELLULAR MATRIX ENCASEMENT
STRUCTURES AND METHODS
REGENERATION
1301003425 Thailand
Jun. 20, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
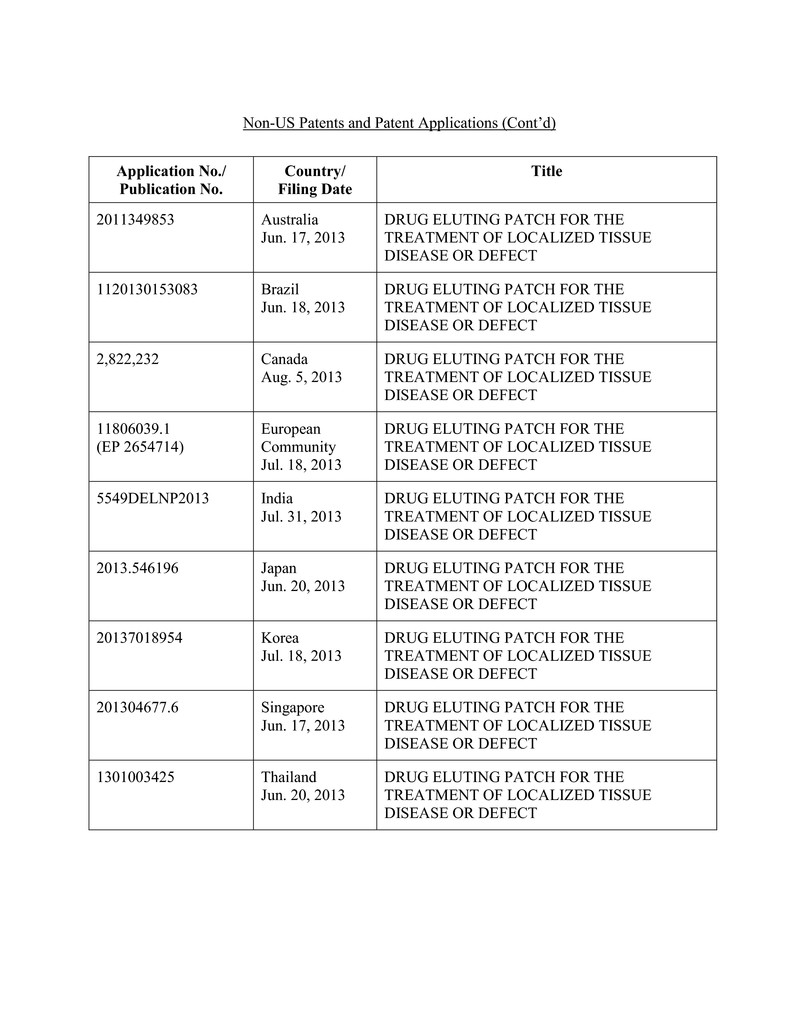
Non-US Patents and Patent Applications (Cont’d)
Application No./
Publication No.
Country/
Filing Date
Title
2011349853 Australia
Jun. 17, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
1120130153083 Brazil
Jun. 18, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
2,822,232 Canada
Aug. 5, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
11806039.1
(EP 2654714)
European
Community
Jul. 18, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
5549DELNP2013 India
Jul. 31, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
2013.546196 Japan
Jun. 20, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
20137018954 Korea
Jul. 18, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
201304677.6 Singapore
Jun. 17, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT
1301003425 Thailand
Jun. 20, 2013
DRUG ELUTING PATCH FOR THE
TREATMENT OF LOCALIZED TISSUE
DISEASE OR DEFECT

Schedule 3.08
to the Royalty Agreement
MidCap Credit Facility:
Credit and Security Agreement (Revolving Loan) providing for a revolving loan in the maximum principal
amount of $8,000,000.
Credit and Security Agreement (Term Loan) providing for a term loan in the maximum principal amount of
$12,000,000.


