AMENDED AND RESTATED EXCLUSIVITY AGREEMENT
Exhibit 10.8
***Text Omitted and Filed Separately
with the Securities and Exchange Commission.
Confidential Treatment Requested
Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the
Securities Act of 1933, as amended.
AMENDED AND RESTATED EXCLUSIVITY AGREEMENT
This Amended and Restated Exclusivity Agreement (Agreement) is entered into as of the Effective Date (defined below) by and between Silimed-Silicone e Instrumental Medico-Cirugico e Hospitalar LTDA, a company organized under the laws of Brazil on behalf of itself and any affiliated, controlled or otherwise related entity or person including, without limitation, any entity controlled or owned at least in part by the officers and partners (stockholders) of Silimed-Silicone e Instrumental Medico-Cirurgico e Hospitalar Ltda. (collectively, (Manufacturer) and Juliet Medical, Inc., a Delaware corporation (Company) as of the Effective Date.
Background
Manufacturer manufactures and sells, among other things, various silicone based medical devices, sterile or non-sterile, including but not limited to custom and/or non-custom implantable devices for physicians. Manufacturer and Silimed, Inc., a Texas corporation that is selling its assets, including certain contractual rights to Company pursuant to the Asset Purchase Agreement (as defined below) are parties to an Exclusive Agreement dated December 1, 1997 (the 1997 Agreement) granting Company the right to distribute and sell the Products (as defined below) in the United States. Pursuant to the 1997 Agreement, Company filed for regulatory approval of certain silicone and saline breast implant devices in the United States and is performing clinical trials for such silicone breast implants. Company is also selling tissue expanders, facial implants, testicular implants and other devices pursuant to 510k approvals. Manufacturer and Company desire to amend and restate the 1997 Agreement to reflect the change in circumstances as Company approaches commercial release of the silicone breast implant devices in the United States.
NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Manufacturer and Company agree to amend and restate the 1997 Agreement to read in full as follows:
1. DEFINITIONS
1.1 Definitions. Capitalized terms used in this Agreement and not otherwise defined herein shall have the meaning set forth below.
Affiliate means with respect to either party, any Person that, directly or indirectly, is controlled by, controls or is under common control with such party. For purposes of this definition, “control” means, with respect to any Person, the direct or indirect ownership of more than fifty percent (50%) of the voting interest in such Person or the possession otherwise, directly or indirectly, of the power to direct the management or policies of such Person.
Company IP means individually and collectively all IP Rights (a) owned by Company prior to December 1, 1997 or (b) acquired by Company from another Person at any time during the term of this Agreement or (c) conceived, authored, created or otherwise developed by or on behalf of Company on or after December 1, 1997 in each case to the extent that they relate to the Products or the Product Specifications (including processes and know-how for the manufacture thereof).
Confidential Information means all data, specifications, training and any other trade secrets or know-how related to the design, implementation, performance or manufacture of the Products, as well as all other information and data provided by either party to the other party pursuant to this Agreement in written or other tangible medium and marked as confidential except any portion thereof which: (i) is known to the receiving party, as evidenced by the receiving party’s written records, before receipt thereof under this Agreement; (ii) is disclosed to the receiving party by a third person who is under no obligation of confidentiality to the disclosing party hereunder with respect to such information and who otherwise has a right to make such disclosure; (iii) is or becomes generally known in the trade through no fault of the receiving party; or (iv) is independently developed by the receiving party, as evidenced by the receiving party’s written records, without access to such information.
Control means, with respect to any IP Rights, possession by a party or its Affiliates of the ability (whether by ownership, license, or otherwise) to grant access, a license, or a sublicense to such IP Rights without violating the
terms of any agreement or other arrangement with any third party as of the time such party would first be required hereunder to grant the other party such access, license, or sublicense.
Effective Date means the “Closing Date” under the certain Asset Purchase Agreement between Silimed, Inc., a Texas corporation, and Company (the Asset Purchase Agreement), which is the date on which Company will purchase from Silimed Inc. certain assets of Silimed, Inc. as described in the Asset Purchase Agreement. The Effective Date shall in no event be later than April 6, 2007.
Facility means Manufacturer’s manufacturing facility in Rio de Janeiro, Brazil.
FCA means “Free Carrier”, as that expression is defined in Incoterms 2000, ICC Publishing S.A.
Force Majeure means any event beyond the control of the parties, including, without limitation, fire, flood, riots, strikes, epidemics, acts of war or terrorism (declared or undeclared and including the continuance, expansion or new outbreak of any war, conflict or terrorism now in existence), embargoes and governmental actions or decrees.
Improvements means individually and collectively all discoveries, inventions, know-how, techniques, methodologies, modifications, improvements, works of authorship, designs and data (whether or not protectable under patent, copyright, trade secrecy or similar laws) relating to additions, developments, enhancements, updates and other changes in Products, including but not limited to any extensions of the label claims for any Product(s) and any new designs for the Product(s) that are conceived, created, discovered, developed, or reduced to practice or tangible medium of expression: (a) solely by one or more employees or consultants of Manufacturer at any time in the course of performing the transactions contemplated by this Agreement; or (b) jointly by one or more employees or consultants of Manufacturer and one or more employees or consultants of Company at any time in the course of performing the transactions contemplated by this Agreement; or (c) solely by one or more employees or consultants of Company at any time in the course of performing the transactions contemplated by this Agreement.
IP Rights means any and all of the following in any jurisdiction throughout the world: (a) inventions and patents and patent applications for same; (b) copyrights and registrations and applications for same; (c) trademarks, trade names, domain names and other indicia of source or origin; (d) software; (e) Confidential Information, including trade secrets and know-how; and (f) all other intellectual property rights or industrial property rights of any kind or nature (whether or not protectable under patent, copyright, trade secrecy or similar laws) that are conceived, discovered, developed, created or reduced to practice or tangible medium of expression by consultants or employees of a party to this Agreement (or by Silimed, Inc. in the case of Juliet Medical, Inc. as a party) without the use of IP Rights owned or Controlled by the other party to this Agreement (or by Silimed Inc. in the case of Juliet Medical, Inc. as a party) or are otherwise Controlled by such party (or by Silimed Inc. in the case of Juliet Medical, Inc. as a party).
Manufacturer IP means all (a) IP Rights owned by Manufacturer prior to December 1, 1997, (b) IP Rights acquired by Manufacturer from another Person, and (c) IP Rights conceived, authored, created or otherwise developed by or on behalf of Manufacturer at any time in each case to the extent that they relate to the Products or the Product Specifications (including processes and know-how for the manufacture thereof).
Person means any individual, corporation, association, partnership (general or limited), joint venture, trust, estate, limited liability company, limited liability partnership, unincorporated organization, government (or any agency or political subdivision thereof) or other legal entity or organization.
Products means all products for all medical applications which are now or which may hereafter become a part of the Manufacturer’s line of products falling within the scope of the Patents (to the extent Manufacturer has a Patent) all as further described in the applicable Product Specifications, including the silicone breast implants, saline breast implants, polyurethane breast implants, tissue expanders, testicular implants, body contouring implants, facial implants (including maxofacial implants and nostril retainers), urology products (including constrictors and vaginal stents), gastric balloon products and gastric band products identified in Attachment 1 together with any Improvements. Attachment 1 may be amended from time to time in accordance with Section 2.1(b) to add additional products or Improvements to Products as they become part of Manufacturer’s line of products.
Product Approvals means, for the United States, those regulatory approvals required for importation, promotion, pricing, marketing and sale of the Product in such country.
Product Specifications means the specifications set forth in Attachment 1 for the Products, as such specifications may be modified from time to time by the mutual agreement of the parties acting in accordance with Section 3.11 to reflect (i) the Product Approvals that issue with respect to the Products or (ii) Improvements and the addition of new products to Manufacturer’s product line.
Territory means the United States of America (including its territories).
Trademarks means (a) the trademarks, set forth on Attachment 3, and (b) any other trademarks, as may be agreed upon in writing from time to time by the parties for use by Company in connection with the promotion, marketing and sale of the Products in the Territory.
1.2 Other Defined Terms. Each of the following terms has the meanings ascribed to it in the section set forth opposite such term:
|
1997 Agreement |
|
Recitals |
|
AER |
|
Section 5.4 |
|
Agreement |
|
Recitals |
|
Applicable GMP |
|
Section 5.2 |
|
Company |
|
Recitals |
|
Excused Supply Failure |
|
Section 3.3 |
|
Firm Order Period |
|
Section 3.1 |
|
ICDR |
|
Section 9.4 |
|
Indemnified Party |
|
Section 7.2 |
|
Indemnifying Party |
|
Section 7.2 |
|
Losses |
|
Section 7.2 |
|
Manufacturer |
|
Recitals |
|
Regulatory Authority |
|
Section 5.2 |
|
Representative |
|
Section 9.1 |
|
RMA |
|
Section 3.10 |
|
SOPs |
|
Section 5.4 |
|
Supply Forecast |
|
Section 3.1. |
2. DISTRIBUTION RIGHTS; VALIDATION
2.1 Appointment. (a) Subject to terms and conditions of this Agreement: Manufacturer hereby appoints Company as Manufacturer’s exclusive distributor for the promotion, sale and delivery of the Products in the Territory. Company hereby accepts such appointment as exclusive distributor within the Territory. Company shall use its commercially reasonable efforts to promote and sell the Products in the Territory, subject to delays caused by Regulatory Authorities with respect to the issuance of Product Approvals and to adequate supply of the Products for resale in the Territory.
(b) The Manufacturer shall promptly notify Company of any Improvements to Products and of any new products that become part of the Manufacturer’s line of products, and Manufacturer shall provide Company with such notice, the Products Specifications for such Improvements to Products or new products and the pricing for such new products (such pricing to be determined in accordance with Section 3.4). Such Improvements to Products and new products shall automatically become Products subject to this Agreement in accordance with Section 3.11.
2.2 Exclusive Dealing. (a) Manufacturer shall not provide Products to any third party if Manufacturer knows or has reason to believe that Products provided to such party have been or will be sold for use or used in the Territory. Company shall not provide Products to any third party if Company knows or has reason to believe that Products provided to such third party have been or will be sold for use, or used, outside of the Territory.
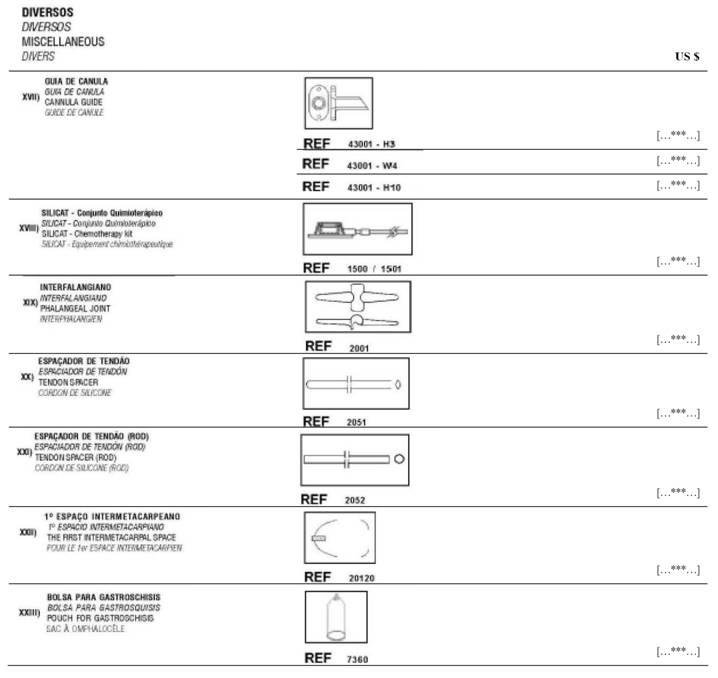
(b) Notwithstanding the provisions of Section 2.1 and Section 2.2(a), it is understood and agreed that Manufacturer is party to a supply agreement with […***…] for the distribution of Manufacturer’s […***…] product marketed as “Pouch of Gastroschisis” for pediatric applications. This Agreement shall in no way limit or otherwise affect such supply agreement with respect to such […***…] product.
(c) Except as otherwise provided in Section 2.2(b), Manufacturer shall notify Company if it receives any request to supply Products to any customer located in or for sale, use or distribution in the Territory. It is understood and agreed that it shall not be a breach of this Agreement for Manufacturer to have contact with such customers or customers located in the Territory; provided Manufacturer does not directly or indirectly sell, offer to sell, market, distribute, ship or deliver Products to customers located in the Territory.
(d) Company shall notify Manufacturer if it receives any request to supply Products to any customer for sale, use or distribution outside the Territory.
2.3 Competitive Products. During the term of this Agreement, Company shall not, and shall cause its Affiliates not to, market, sell or otherwise distribute in the Territory any product that is directly competitive with any Product that is then being commercially sold or clinically tested by Company in the Territory. It is understood and agreed that a product is “directly competitive with a Product” if it has an elastomer made of silicone and a filler made of either silicone or saline.
2.4 2.3A Production Line Validation. Manufacturer hereby represents and warrants to Company that Manufacturer has successfully validated all production line(s) at the Facility upon which Products are produced in accordance with all quality system regulations promulgated under the Applicable GMP.
2.5 Annual Meetings. (a) No later than ninety (90) days prior to each anniversary of the Effective Date, the parties shall meet, in person or by videoconference or teleconference, to confer regarding (i) the Company’s expectations with respect to Product development and commercialization in the next contract year so that the Manufacturer may consider any changes in the resource allocation required to address such expected demand for Products; and (ii) the Manufacturer’s expectations with respect to pricing for each Product for the next contract year so that the Company may consider its cost structure with respect to the next contract year. The purpose of such meetings is to serve as a venue for the parties to provide timely notice of their respective expectations for the next contract year as well as trends and developments they foresee in order to reduce the likelihood of surprises with respect to either Company’s demand for Products or Manufacturer’s pricing for Products. If meetings are held in person they shall be held at either the headquarters of Manufacturer or the headquarters of the Company on an alternating basis, unless the parties mutually agree to hold such meeting in an alternative venue. The party hosting the meeting shall be responsible for providing an agenda for each meeting at least seven (7) days in advance of such meeting and shall prepare written draft minutes of all meetings in reasonable detail and distribute such draft minutes to the other party for comment and review within seven (7) days after the relevant meeting. The party receiving such minutes shall have seven (7) days to provide comments. The party preparing the minutes shall incorporate timely received comments and distribute finalized minutes within thirty (30) days following the relevant meeting.
(b) The parties shall review and approve on a timely basis, but in any case, within thirty (30) days following receipt of finalized minutes, the expected forecasts for Products and the proposed pricing for Products for the next contract year. In the event that the Manufacturer does not approve the Company’s expected forecast for Products or the Company does not approve the Manufacturer’s proposed pricing for any Product(s) as presented at the meeting, then such party shall provide the other party with a detailed statement describing the basis of its concerns and the parties shall in good faith seek to resolve such matter prior to the commencement of the next contract year in accordance with the provisions of this Agreement, including Sections 3.1, 3.3 and 3.4.
3. PURCHASE OF PRODUCTS AND TERMS OF SALE.
3.1 Purchase Forecasts. During the term of this Agreement, Company shall provide to Manufacturer, on a quarterly basis, a non-binding rolling forecast of orders of each of the Products with respect to the next twelve (12) months (Supply Forecast). Notwithstanding the foregoing, the forecasts for […***…] of any 12 month period (Firm
***Confidential Treatment Requested
Order Period) covered by a Supply Forecast shall represent binding purchase obligations of Company to purchase all the Products set forth in the Supply Forecast for such Firm Order Period; provided, that (a) the forecast for […***…] of the Supply Forecast covering the period immediately following receipt of a Product Approval for the United States for a particular Product shall be considered part of the Firm Order Period for such Product and) shall represent a binding purchase obligation of Company to purchase all such Product for such Firm Order Period and (b) the forecast for all “custom” Products shall represent a binding purchase obligation of Company to purchase such “custom” Product.
3.2 Product Orders. (a) Orders for each Firm Order Period shall be placed by written purchase order and submitted by mail, electronic mail or facsimile, or by other means agreed upon by the parties. No order shall be binding upon Manufacturer until the same shall have been accepted in writing by Manufacturer (including acceptance by EDI). Manufacturer shall accept or reject all orders within five (5) days following receipt of same and shall deliver all orders that are accepted within ninety (90) days following the date such order is received. The parties covenant and agree that any standard printed terms of purchase/sale of Company and Manufacturer provided by either party to the other party shall be disregarded and that the provisions of this Agreement shall govern such purchase and sale.
(b) Company shall use […***…] efforts to maximize sales of Products during the term of this Agreement.
3.3 Obligation to Supply. (a) Manufacturer shall use […***…] efforts to accept and fill each order for Products submitted by Company; including orders that exceed the Supply Forecast for any month by up to […***…] percent ([…***…]%) of the amount in the Supply Forecast for such month delivered to Manufacturer ninety (90) days prior to such month. When allocating production with respect to filling orders for Products, Manufacturer shall ensure that Company orders are accepted and filled in a manner that does not differ in any material respect from the priority and fill rate Manufacturer applies to Products ordered by third parties or Manufacturer’s own sales organization; provided, however, the foregoing provision shall not apply to accepting or filling orders that exceed the Supply Forecast. Notwithstanding the foregoing, Manufacturer shall not be in breach of this Section 3.3 if Manufacturer’s failure to supply Products is due to a Force Majeure event or if Manufacturer’s failure is limited to quantities in excess of the quantities specified in this Section 3.3 (any such failure an Excused Supply Failure).
(b) If Manufacturer is unable to supply any Product(s) ordered by Company in accordance with the terms of this Agreement other than because of an Excused Supply Failure, then Manufacturer shall use […***…] efforts to remedy the problem, including by securing an alternative source of supply within a reasonable time at no cost to Company, and any such alternative source of supply shall be on terms substantially identical with the terms of this Agreement. If Manufacturer is unable to remedy such problem within […***…] after its initial failure to supply (measured from the date that delivery would have been due under Company’s binding Supply Forecast), then Manufacturer shall consult with Company and the parties shall work together to remedy the problem, which may include having Manufacturer reactivate its old manufacturing facility. If Manufacturer is unable to remedy the supply problem after an aggregate period of […***…] (or longer as agreed in writing by the parties), commencing with the date upon which such failure to supply began, then Company may at its option, and upon notice to Manufacturer, remedy the supply problem by manufacturing the applicable Product(s) itself or through an alternative source of supply (which shall not be deemed a violation by Company of Section 2.3) using the procedure specified in Section 3.3A or in the alternative, terminate this Agreement with respect to such Product(s) pursuant to Section 8.2 (last sentence).
(c) In the event that, for any material period of time (i.e., any period of time that may interrupt Manufacturer’s ability to supply then Products that are the subject of the then most recent Supply Forecast), the Manufacturer Facility or the Manufacturer production line becomes non-operational for any reason whatsoever, Manufacturer will promptly notify Company, and Manufacturer will ensure that the production line for the affected Products is restored and revalidated at the same rate and in a manner that does not differ in any material respect from the priority and restoration rate of any other production line(s) at the Facility.
3.3A Manufacturing Rights. (a) If Company notifies Manufacturer that Company will manufacture any Product(s) itself or through a third party as a result of a failure to supply by Manufacturer, Manufacturer shall (i) deliver to Company within thirty (30) days media embodying or disclosing all technology and proprietary or intellectual property rights necessary to enable Company or its designee to manufacture such Product(s) conforming with the applicable Product Specifications; and (ii) provide Company or its designee, upon request, with assistance
***Confidential Treatment Requested
in establishing a back-up manufacturing line. […***…] Company shall require any third party Company designates to manufacture Products pursuant to this Section 3.3A, to agree in writing to observe the terms of this Agreement relating to confidentiality and the manufacture of Products.
(b) It is understood and agreed that in no case shall […***…] pursuant to this Section 3.3A.
(c) Company may continue to exercise the manufacturing rights provided in this Section 3.3A until Manufacturer notifies Company that it is again able to supply Company’s needs for the applicable Product(s) and substantiates such claim to Company’s reasonable satisfaction. Upon such a showing, Company shall commence purchasing such Product(s) from Manufacturer and Company’s rights under this Section 3.3A shall terminate (with respect to such interruption in supply), provided that: (i) Company shall not be required to cancel any then outstanding purchase orders with any third party operating a back-up manufacturing line established pursuant to this Section 3.3A to the extent such orders have been accepted by such third party and are binding obligations of Company; and (ii) Manufacturer shall pay all cancellation costs incurred by Company in switching its purchases from such third party to Manufacturer. Company shall use commercially reasonable efforts to avoid significant cancellation fees in any third party contracts it enters pursuant to this Section 3.3A. Company shall not order Products from any third party operating a back-up manufacturing line established pursuant to this Section 3.3A for delivery more than […***…] months following the date of such order.
3.4 Product Prices. (a) The prices for each of the Products are set forth on Attachment 2.
(b) Except as otherwise agreed by the parties or as provided in this Section 3.4, Manufacturer will not increase prices for the Products. Not more than once annually, Manufacturer may increase the prices for the Products to reflect (i) increases in the cost of raw materials, or (ii) increases in the costs of production components utilized in the manufacture of such Products including utilities, packaging materials and labor utilized in such manufacturing plus a pro rata portion of factory overhead costs allocated to the Products purchased by Company pursuant to this Agreement in accordance with normal accounting practices for all products manufactured in the Facility. Price increases shall not include any costs attributable to idle plant capacity (that Manufacturer or any third party may have) or general corporate activities that are not primarily associated with the manufacture of Products, including, by way of example only, salaries and benefits of executive management, administrative support for such management, and all costs of the finance, purchasing, legal, business development and corporate development functions of Manufacturer or overhead (other than Facility overhead for the portions of the Facility used for manufacturing Products). Without limiting the foregoing, Manufacturer will use commercially reasonable efforts to maintain price increases to an amount that does not exceed […***…] percent ([…***…] %) per annum and shall include with any notice of a price increase documentation of such production cost increases. Company shall have the right, not more than once in any twelve (12) month period, upon reasonable notice to have an independent certified public accountant, selected by Company and reasonably acceptable to Manufacturer, inspect Manufacturer’s books and records for purposes of verifying Manufacturer’s actual production costs for the Products. Manufacturer shall make all applicable books and records available for such inspection during normal business hours at Manufacturer’s facility. Any such audit shall be at the expense of Company, unless such audit discloses that Manufacturer’s reported average production costs for the audited period are less than Manufacturer’s actual production costs for such period by more than […***…] percent ([…***…]%), in which case Manufacturer shall reimburse Company for such expenses. If any audit discloses that Manufacturer’s actual production cost for a Product is less than the reported production cost, Manufacturer shall promptly make payment to Company of an amount equal to the product of […***…].
(c) Company shall not be required to pay any royalty to Manufacturer for its sale of Product supplied by Manufacturer pursuant to this Agreement.
3.5 Intentionally Omitted.
***Confidential Treatment Requested
3.6 Resale Prices. Nothing contained herein shall be deemed to limit in any way the right of Company to determine the prices at which, or the terms on which, the Products purchased by Company may be resold by Company.
3.7 Payment. (a) Company shall pay for Products within thirty (30) days after the date of Manufacturer’s invoice. All payments shall be stated and paid in U.S. Dollars. Except for income taxes that may be assessed against Manufacturer, all taxes and charges that may be imposed by any government taxing authority on the amounts paid by Company to Manufacturer under this Agreement shall be paid by Company for Manufacturer’s account.
(b) In the event Company fails to make any payment hereunder in full when due that is not disputed in good faith, the amount due shall accrue interest beginning on the […***…] day following the final date on which such payment was due, calculated at the annual rate equal to […***…] ([…***…]%) above the prime interest rate reported in the Wall Street Journal for the due date, calculated from the due date until paid in full. Such payment when made shall be accompanied by all interest so accrued. Said interest and the payment and acceptance thereof shall not negate or waive the right of Manufacturer to any other remedy, legal or equitable, to which it may be entitled because of the delinquency of the payment. In the event Company disputes any payment it shall notify Company in accordance with Section 9.2 and shall pay all undisputed sums in accordance with this Agreement.
(c) Intentionally omitted.
(d) Nothing in this Section 3.7 shall waive any other rights and remedies Manufacturer may have under law or this Agreement, and all such rights and remedies set forth herein shall be considered cumulative with all other available rights and remedies.
3.8 Shipping. (a) Manufacturer shall arrange for shipment (according to Company directions) and invoicing to Company of the Products ordered by Company via common carrier (air freight), FCA Rio de Janeiro, Brazil to the carrier selected by Company. Company shall pay all customs, duties and other governmental charges, if any, related to importation and sale of the Product in the Territory following such FCA delivery, and shall have all responsibility for storing and clearing the Products through all United States customs and importation requirements. Manufacturer shall be responsible for clearing the products for export from Brazil.
(b) Manufacturer will supply Products to Company in finished and final packaged format for end user sale, (including all trade dress, labeling and warning and handling instructions) as documented in the applicable packaging specifications for each Product. The parties shall adopt such packaging specifications for each Product not later than six (6) months prior to the anticipated date of commercial launch for the applicable Product in the Territory and such packaging specifications shall be appended to this Agreement as Attachment 4.
3.9 Product Samples. (a) Manufacturer shall provide Company, promptly upon request, with a reasonable number samples of the Products of any current production run, not to exceed […***…] percent ([…***…]%) of such production run, for testing purposes. Such Product samples shall be shipped to Company in accordance with the provisions set forth in Section 3.8, and Company shall pay the price for such Products in the manner described in Section 3.7.
(b) Manufacturer shall also make available to Company, at Company’s expense and upon Company’s request, for regulatory approval purposes, sample Products in accordance with the applicable Product Specifications. In addition, Manufacturer shall supply Company, at no charge, with a mutually agreed number of units of each of the Products for demonstration and marketing purposes and will supply additional units at cost.
3.10 Acceptance. (a) Company shall notify Manufacturer within thirty (30) days of the receipt of a shipment of the Products (or for shipments delivered to Company’s customer within thirty (30) days following the receipt of the samples for such shipment requested by Company in accordance with Section 3.9(a)) of any apparent non-conformity of the Product to the applicable Product Specifications. If Company fails to so notify Manufacturer, it will be deemed to have accepted the Product; provided that the warranty provided in Section 6.2 and Manufacturer’s obligations under Section 7.2 shall survive acceptance of the Product by Company.
***Confidential Treatment Requested
(b) Manufacturer shall perform all in-process and finished product tests or checks required by the applicable Product Specifications and Applicable GMP. For purposes of this Agreement, such tests shall be considered routine and shall be performed at Manufacturer’s expense; provided that in the event that Company requests any tests in addition to the foregoing, Company shall compensate Manufacturer for the cost of such additional tests in accordance with a method to be mutually determined. All tests and test results shall be performed, documented and summarized by Manufacturer in accordance with the applicable Product Specifications and applicable laws, rules and regulations. Manufacturer shall immediately notify Company of any significant out-of-specification analytical results for either in process or finished Product test results.
(c) Company shall not be required to pay Manufacturer for any Products which have been properly rejected for failure to comply with Product Specifications. Manufacturer shall at its expense and at no further cost to Company replace any Products that do not conform to the Product Specifications. All defective units of the Product shall be returned to Manufacturer or its designee if requested by Manufacturer and at Manufacturer’s cost; upon Manufacturer’s request, Company will hold such Products at its premises for inspection by Manufacturer or its designee. Company shall notify Manufacturer in writing of its rejection of Product under this Section 3.10 within thirty (30) days of Company’s receipt thereof, shall request a Return Material Authorization (RMA) number and shall within thirty (30) days of receipt of such RMA number return a sample of such rejected Product to Manufacturer or its designee freight prepaid and properly insured, along with a reasonably detailed statement of the claimed defect and proof of date of purchase unless Manufacturer requests Company to hold such Products at its premises for inspection by Manufacturer or its designee. In the event Manufacturer determines that the returned (or held) Product is defective and properly rejected by Company, Manufacturer shall replace such defective Product (and shall provide Company with an RMA for the balance of the defective lot of Product which shall be returned to Manufacturer freight prepaid and properly insured unless Manufacturer requests that Company have such lot destroyed). Manufacturer shall deliver to Company, freight prepaid, all replacements for Products properly rejected, along with reimbursement of the shipment charges for return of the nonconforming Product (if any). In the event that any rejected Product is determined by Manufacturer to not be defective, Company shall reimburse Manufacturer for all costs and expenses related to the inspection and return of such Product to Company. If there is disagreement between the parties as to whether the Product meets Product Specifications, the parties shall have such Product tested by a mutually agreed upon third party and such third party’s determination as to whether such Product meets Product Specifications shall be binding on the parties. The expense for such testing shall be borne by Company unless it is determined that the Product does not meet the Product Specifications. Pending the outcome of such testing, Manufacturer shall have the right to suspend shipments of the applicable Products in respect of all existing and future orders of such Product.
(d) Company shall notify Manufacturer in writing of any shortage in quantity of any shipment of Product within thirty (30) days of receipt of such Product. In the event of such shortage, Manufacturer shall endeavor to make up and ship the shortage as promptly as possible, but with the substitute shipment occurring no later than thirty (30) days after notice, at no additional cost to Company.
3.11 Changes. (a) The Product Specifications may be modified or changed only by mutual agreement of the Company and Manufacturer. To the extent that such modification or change results in an increase or decrease in the cost of manufacturing any Product or requires additional capital investment or other material changes to the manufacturing process, the parties shall, contemporaneously with the modification to the Product Specifications if not earlier, jointly examine and mutually agree upon the consequences thereof and shall make an appropriate increase or decrease to the purchase price of such Product arising from such modification or change and amend Attachment 2 accordingly. At least six (6) weeks prior notice to the other party is required for any requested Product Specifications change; provided, however, that if any requested Product Specifications change requires additional Product Approval(s), the implementation of such requested change shall in no event be required until four (4) weeks after such approval(s) have been obtained.
(b) Manufacturer shall promptly contact Company in the event that Manufacturer anticipates making changes to any material used to manufacture the Products or in the event Manufacturer considers any such material used to manufacture the Products to be nonconforming or unacceptable and Manufacturer shall not be required to implement any change to the Product Specifications that it reasonably believes will prevent it from being able to perform in accordance with the terms of this Agreement unless such terms are modified. If Manufacturer notifies Company that it believes the preceding sentence is applicable the parties shall meet and attempt to resolve the matter
using the procedure specified in Section 9.2 if necessary; during any such period the Product will continue to be manufactured under the Product Specifications without such modification.
(c) Company shall promptly upon request supply Manufacturer with “flow charts” regarding FDA requirements for any changes made in the material, construction, processing or manufacturing for any Products and any future Products.
4. CONFIDENTIALITY; INTELLECTUAL PROPERTY RIGHTS
4.1 Confidentiality. (a) During the term of this Agreement and for […***…] years thereafter, Manufacturer and Company shall not use for any purpose other than this Agreement and shall not reveal or disclose to third parties the subject matter of this Agreement and any Confidential Information received from the other party as confidential in nature. Any Confidential Information disclosed by either party hereunder to the other party may be used only by employees of the other party or its affiliates who agree to be bound by such party’s obligations hereunder with respect to such Confidential Information and who have a genuine need to know such information for the purposes permitted by this Agreement. The parties shall take reasonable measures to assure that no unauthorized use or disclosure is made by others to whom access to such Confidential Information is granted. Nothing herein shall be construed as preventing a receiving party from using and disclosing any Confidential Information as necessary (i) in prosecuting or defending litigation in accordance with Section 4.3; (ii) in connection with the initiation and conduct of clinical trials; or (iii) in conducting research and development in accordance with this Agreement including with third party collaborators (if such collaborators are subject to written confidentiality agreements with such party).
(b) No public announcement or other disclosure to any third party concerning the existence of or terms of this Agreement shall be made, either directly or indirectly, by either party to this Agreement, except as may be legally required or as may be required for recording purposes, without first obtaining the written approval of the other party and agreement upon the nature and text of such announcement or disclosure. The party desiring to make any such public announcement or other disclosure (pursuant to legal requirement, for recording purposes or otherwise) shall provide the other party with a written copy of the proposed public statement, in reasonably sufficient time prior to public release in order to allow such other party to comment upon such announcement or disclosure.
4.2 Licenses. (a) Manufacturer hereby grants to Company an exclusive, royalty-free, non-transferable (except in accordance with section 10.4) license to use (i) the Trademarks in the Territory in connection with the promotion, marketing, advertising, and sale of Products in the Territory; and (ii) any other Manufacturer IP as necessary or useful to promote, sell and deliver the Products in the Territory. In addition, upon the occurrence of the events described in Section 3.3A and solely in accordance with Section 3.3A Company may practice such intellectual property as necessary or useful for purposes of manufacturing Product in accordance with Section 3.3A.
(b) If at any time Manufacturer believes that any Product(s) do not meet Manufacturer’s commercially reasonable quality expectations, Manufacturer will notify Company and Company will use commercially reasonable efforts to cure any such deficiency within a commercially reasonable period of time and unless such deficiency is resolved to Manufacturer’s reasonable satisfaction, not to be unreasonably withheld, Manufacturer may require Company to cease using the Trademarks in connection with the applicable Products. Upon Manufacturer’s request, Company will provide Manufacturer with samples of its usage of the Trademarks. Any use of the Trademarks will indicate that Manufacturer is the owner of the Trademarks. All uses of the Trademarks and all goodwill associated therewith will inure solely to the benefit of Manufacturer.
(c) Company shall use the Trademarks only in the manners expressly approved by Manufacturer in advance in writing. Company shall use the Trademarks to promote, market, advertise and sell the Products in the Territory. Company may elect to adopt other trademarks for use in the promotion, marketing and sale of the Products (in addition to the Trademarks), in which case Section 4.2(b)-(c) shall not apply to Company’s use of such other marks.
4.3 Infringement. (a) Each of Company and Manufacturer will notify the other party in writing of any infringement or unauthorized use or disclosure of the other party’s IP Rights in the Territory within thirty (30) days after it becomes aware of such infringement or unauthorized use or disclosure.
***Confidential Treatment Requested
(b) Subject to Sections 4.3(c) and 4.3(d), each party shall have the exclusive right (but not the obligation) at its own cost to take all legal action in the Territory it deems necessary or advisable to eliminate or minimize the consequences of any infringement or unauthorized use or disclosure of its IP Rights in the Territory.
(c) Without limiting the generality or applicability of Section 4.3(b), Manufacturer shall have the exclusive right (but not the obligation) at its own cost to take all legal action in the Territory it deems necessary or advisable to eliminate or minimize the consequences of any infringement or unauthorized use or disclosure of the Trademarks in the Territory. All proceeds realized upon any judgment or settlement regarding an action undertaken with respect to the Trademarks shall be retained by Manufacturer.
(d) Without limiting the generality or applicability of Section 4.3(b), Company shall have the exclusive right at its own cost to take all legal action in the Territory it deems necessary or advisable to eliminate or minimize the consequences of any infringement or unauthorized use or disclosure of the IP Rights other than Trademarks or Manufacturer Confidential Information as embodied in a Product. For the purpose of taking any such legal action, Company shall have the right, subject to Manufacturer’s prior written consent (which consent shall not be unreasonably withheld) to use the name of Manufacturer as plaintiff, either solely or jointly in accordance with the applicable rules of procedure. Manufacturer shall furnish Company with whatever written authority may be required in order to enable Company to use Manufacturer’s name in connection with any such legal action, and shall otherwise cooperate with Company (at Company’s expense) in connection with any such action. All proceeds realized upon any judgment or settlement regarding an action undertaken with respect to the IP Rights other than Trademarks or Manufacturer Confidential Information as embodied in a Product shall be used to reimburse Company for the costs of litigating or settling such matter and then shall be paid to Company and to Manufacturer in an amount that compensates Company for lost sales in respect of such infringement and Manufacturer in an amount that reflects the sums that would be due Manufacturer from Company based on the lost sales.
4.4 Ownership of IP Rights; Retained Rights. (a) This Agreement does not convey to Company any right, title or interest in or to any Manufacturer IP by implication, estoppel or otherwise except for the license rights expressly granted under Section 4.2. Title to the Manufacturer IP shall at all times remain vested in Manufacturer.
(b) This Agreement does not convey to Manufacturer any right, title or interest in or to any Company IP by implication, estoppel or otherwise except for the license rights expressly granted under Section 4.4(d). Title to the Company IP shall at all times remain vested in Company.
(c) Intentionally Omitted.
(d) Company hereby grants to Manufacturer a non-exclusive, royalty-free, fully paid-up license to use the Company IP to fulfill Manufacturer’s obligations to Company under this Agreement.
4.5 Improvements. If the parties jointly undertake any development, modification, enhancement or alteration of the Products after the Effective Date, then: (a) Company shall be the exclusive owner of all Improvements created solely by Company employees or consultants; (b) Manufacturer shall be the exclusive owner of all Improvements created solely by Manufacturer employees or consultants; and (c) the parties shall jointly own all Improvements created jointly by the parties; provided that Manufacturer hereby grants and agrees to grant to Company a perpetual, irrevocable, exclusive, transferable, fully paid-up, royalty-free right and license to use such Improvements in the Territory and Company hereby grants to Manufacturer a perpetual, irrevocable, exclusive, transferable, fully paid-up, royalty-free right and license to use such Improvements outside the Territory. If required, patent counsel mutually acceptable to the parties shall determine inventorship of all Improvements in accordance with U.S. patent law (and other U.S. intellectual property law, if applicable) and which party is entitled to ownership of such Improvements. Any disagreements will be resolved using the procedures described in Section 9.2 and Section 9.6.
5. REGULATORY ACTIVITIES; APPROVALS
5.1 Regulatory Approvals. (a) Company (i) will have sole responsibility and authority, at its sole expense, for obtaining and maintaining Product Approvals for the Products from the FDA or other Regulatory Authority
necessary for the commercial import, export, distribution and sale of the Product in the Territory, and (ii) shall use its commercially reasonable efforts to obtain and maintain all such Product Approvals as soon as reasonably practicable after the Effective Date; this responsibility includes, without limitation, conducting and managing a clinical trial program in the United States, if necessary, as determined by Company. All regulatory’ filings with any Regulatory Authority in the Territory relating to the Product will be made in the name of Company or its designee. Manufacturer will use commercially reasonable efforts to cooperate with all of Company’s efforts to obtain and maintain Product Approvals for the Product, including by providing Company and the Regulatory Authority with such information and assistance as Company may reasonably request regarding the Product, including but not limited to, any and all surgical and clinical studies, documentation, certifications, bio-compatibility studies, and any other information which may be required by the Regulatory Authority or which may have a bearing or positive impact on the approval of any Products for which Company seeks Product Approvals; and Manufacturer will comply with all applicable regulatory requirements (including without limitation design controls, change controls, manufacturing and quality systems and Applicable GMP) reasonably necessary to obtain Regulatory Approval of the Product.
(b) Manufacturer shall, at its expense and its discretion, use commercially reasonable efforts to obtain and maintain any regulatory approvals required for the manufacture of the Product at the Manufacturer Facility. Such regulatory approvals for the manufacture of the product at the Manufacturer Facility shall be applied for and maintained in the name of Manufacturer. Company will use commercially reasonable efforts to cooperate with all of Manufacturer’s efforts to obtain and maintain regulatory approvals for the manufacture of the Product at the Facility, including, without limitation, by providing Manufacturer and the Regulatory Authority with such information and assistance as Manufacturer may reasonably request regarding the Product.
(c) Each party shall keep the other advised of regulatory interactions, activities and correspondence and the registration status of the Products and the Facility on a quarterly basis, except that matters requiring more immediate attention shall be communicated as soon as practicable. Each party shall promptly provide reasonable advice and assistance to the other party as may be necessary to obtain and maintain approvals for the Facility, Applicable GMPs for the manufacture of the Products and Product Approvals for the Products.
5.2 Quality Control. (a) Manufacturer shall manufacture the Products in accordance with the Product Specifications. Manufacturer will install and maintain effective quality control systems, conduct quality assurance testing and keep statistical process control records conforming to the then applicable good manufacturing practices regulations of the U.S. Food and Drug Administration under 21 CFR Part 820 or comparable regulations of any other any regulatory agency or authority that has authority to grant registrations, authorizations and approvals necessary for the commercial manufacture of the Products (each, a Regulatory Authority) (the Applicable GMP) and including specifically ISO 9001 certification and ISO 13485 certification.
(b) Manufacturer will comply with Applicable GMP requirements in its manufacturing of the Products. Prior to shipping any Product, Manufacturer will carry out the Product tests specified in the applicable Product Specifications on each Product. If any Product fails to meet the Product Specifications, the Product will be replaced by Manufacturer. No Product will be shipped to Company or its designee without passing all tests specified in the Product Specifications except with Company’s prior written approval.
(c) Manufacturer will maintain manufacturing quality documentation, including records of Manufacturer’s Product tests in accordance with the applicable regulations of the applicable Regulatory Authorities and Manufacturer will certify that Product was manufactured and tested in accordance with the applicable Product Specifications and regulatory requirements. Company may request copies of such manufacturing quality documentation and records and certifications as part of the inspections permitted under Section 5.3(a). Company will maintain quality control documentation, including records of Company’s Product Approval process and records related to Company’s promotion and marketing of the Product.
5.3 Inspections. (a) During regular business hours and upon reasonable advance notice, Manufacturer will permit Company and its agents to inspect the Facility and provide access to Manufacturer’s manufacturing quality control documentation and regulatory files related to the Products to the extent necessary for, and for the sole purpose of assessing Manufacturer’s compliance with the provisions of Sections 5.2 and 6.2 of this Agreement;
provided that such inspections shall be conducted in a manner that does not unreasonably disrupt operations of the Facility.
(b) Manufacturer will allow representatives of any Regulatory Authority with jurisdiction over the manufacture, marketing, distribution and sale of any of the Products in the Territory to inspect the Facility, and will cooperate with such representatives in every reasonable manner. Manufacturer will promptly provide Company with notice of any inspections of the Facility by inspectors of the FDA or any other Regulatory Authority reasonably related to its performance hereunder or the subject matter of this Agreement and will permit Company to attend such inspections. Manufacturer will provide Company with copies of any FDA Form 483 notices of adverse findings, regulatory letters or similar writings it receives from any Regulatory Authority setting forth adverse findings or non¬compliance with applicable laws, regulations or standards relating to the Products supplied by Manufacturer hereunder within two (2) days of its receipt thereof, and Manufacturer’s written response to such Regulatory Authority not later than the date of its submission thereof.
(c) If an inspection pursuant to Section 5.3(a) reveals that the Facility does not satisfy the requirements above in all material respects, then Company will promptly provide to Manufacturer written notice of such fact, which notice will contain in reasonable detail the deficiencies found in the manufacturing facilities and, if practicable, those steps Company believes Manufacturer should undertake in order to remedy such deficiencies. Manufacturer will remedy such deficiencies within a reasonable period of time after receipt of such written notice.
5.4 Vigilance; Recalls. (a) Attachment 5 contains the Company’s standard operating procedures (SOPs) as to Product recalls, which have been mutually agreed upon by the parties. If either party becomes aware of information about any Product indicating that it may not conform to the applicable Product Specifications, or that there are potential issues regarding safety or accuracy of results of Product, it will promptly so notify the other party. The parties will promptly confer to discuss such circumstances and to consider appropriate courses of action, which courses of action will be consistent with the SOPs.
(b) In the event that (i) Company determines that an event, incident, or circumstance may result in the need for a recall or other removal of the Product or any lot or lots thereof from the market; (ii) any Regulatory Authority in the Territory threatens to remove a Product from the market; or (iii) any Regulatory Authority in the Territory requires distribution of a “Dear Doctor” letter or its equivalent regarding the use of Product, Company shall promptly advise Manufacturer in writing, and shall provide Manufacturer with copies of all relevant correspondence, notices and the like. Notwithstanding anything the contrary herein, Company shall have final authority to make all decisions relating to any recall within the Territory and shall pay all costs associated with such recall of the Product for any reason, whether or not requested or ordered by any Regulatory Authority; provided that the foregoing does not limit Company’s right to proceed against Manufacturer pursuant to Section 6.2 to the extent such recall or withdrawal of the Product is the result of (i) any breach by Manufacturer of its duties under the Agreement or (ii) Manufacturer’s negligence or willful-misconduct. If Company initiates any recall of a Product, Manufacturer shall cooperate and provide reasonable assistance to Company in conducting such recall.
(c) Intentionally Omitted.
(d) With respect to any recall, withdrawal, or field correction of a Product, Company or its designee will make all contacts with the FDA and other Regulatory Authorities, and will be responsible for coordinating all of the necessary activities in connection with such recall, withdrawal, or field correction. Company and Manufacturer will coordinate any statements to customers and the media, including, but not limited to, press releases and interviews for publication or broadcast except as otherwise required by applicable law to assure patient safety, and neither party will issue any such statements without consulting with the other. The parties will reasonably cooperate with each other in the conduct of such activities and will perform any acts reasonably requested by the other party to facilitate the recall, withdrawal or field correction. Each party will keep the other party fully informed of progress and in relation to all material decisions or actions such party undertakes pursuant to this Section 5.4(d).
(e) Each party will notify the other party within three (3) business days (unless a shorter period is required under applicable laws or regulations) of any event or complaint that gives rise or could give rise to the need to file a adverse event report within the meaning of the Federal Food, Drug and Cosmetic Act of 1938, as amended or similar report under the laws or regulations administered by any Regulatory Authority (collectively, an AER),
with respect to any Product or the manufacture, distribution or use thereof in accordance with applicable regulations covering AER’s. Each such notice will be Confidential Information under this Agreement. If, as a result of any corrective action or any final, non-appealable or non-appealed governmental or court action, an AER is required to be issued for any Product sold hereunder, Company will bear the costs and expenses of and will be responsible for all corrective actions associated with such AER but the foregoing does not limit Company’s right to proceed against Manufacturer pursuant to Section 6.2 to the extent such AER is the direct result of any breach by Manufacturer of its duties under the Agreement.
5.5 Compliance with Laws. Company shall comply with all applicable laws and regulations pertaining to the importation, distribution, sales and marketing of the Products and performance by Company of its obligations under this Agreement. Manufacturer will comply with all applicable laws and regulations pertaining to the manufacture of the Products and in any other manner pertaining to performance by Manufacturer of its obligations under this Agreement.
5.6 FDA Approvals. Company shall use its commercially reasonable efforts to obtain promptly all Product Approvals, including, but not limited to, approvals from the FDA necessary for the commercial import, export, distribution and sale of the Product in the Territory.
6. REPRESENTATIONS AND WARRANTIES
6.1 Authorization; Enforceability. Each of Manufacturer and Company represents and warrants to the other that: (a) it is a corporation or limited liability company duly organized and validly existing under the laws of its incorporating jurisdiction; (b) it has all requisite corporate power and authority to enter into this Agreement; (c) it is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder and consummate the transactions contemplated hereby; (d) this Agreement is a valid and binding obligation of such party enforceable in accordance with its terms; and (e) its execution, delivery and performance of this Agreement will not conflict in any material fashion with the terms of any other agreement or instrument to which it is or becomes a party or by which it is or becomes bound, nor violate any law or regulation of any court, governmental body or administrative or other agency having authority over it.
6.2 Product Warranty. Manufacturer represents and warrants to Company, its successors, assigns, and customers and to users of the Products that each Product supplied to Company hereunder: (a) conforms to the applicable Product Specifications for the applicable period stated in Attachment 6, for each Product described therein; and (b) was manufactured, processed, labeled, packaged, stored and tested (in each case, while in the possession or control of Manufacturer) in accordance with the Product Specifications (including any applicable Product Approvals) and Applicable GMP. This warranty does not apply to any non-conformity of the Products resulting from (i) any alteration, misuse, mishandling or storage in an improper environment in each case by any party other than Manufacturer or its agents or (ii) any labeling approved by Company or any labeling required to be added to the Products by Company. THE FOREGOING WARRANTY IS THE SOLE AND EXCLUSIVE WARRANTY GIVEN BY MANUFACTURER WITH RESPECT TO THE PRODUCTS, AND MANUFACTURER DOES NOT GIVE OR MAKE, AND COMPANY IS NOT RELYING ON, ANY OTHER REPRESENTATIONS OR WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, TITLE OR NONINFRINGEMENT IN CONNECTION WITH THE PRODUCTS .
7. RISK ALLOCATION
7.1 LIMITATION OF LIABILITY. NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR LOST PROFITS OR FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, PUNITIVE OR EXEMPLARY DAMAGES IN CONNECTION WITH THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED BY THIS AGREEMENT, HOWEVER CAUSED, UNDER ANY THEORY OF LIABILITY INCLUDING CONTRACT, TORT (INCLUDING NEGLIGENCE) OR OTHERWISE, AND NOTWITHSTANDING THAT SUCH DAMAGES MAY HAVE BEEN IN THE REASONABLE CONTEMPLATION OF THE PARTIES. THE LIMITATIONS OF LIABILITY SET FORTH IN THIS SECTION 7.1 SHALL NOT APPLY TO COMPANY’S PAYMENT OBLIGATIONS SET FORTH IN THIS AGREEMENT, TO BREACH OF CONFIDENTIALITY OBLIGATIONS UNDER SECTION 4.1 OR VIOLATIONS OF
ANOTHER PARTY’S INTELLECTUAL PROPERTY RIGHTS AND SHALL NOT SERVE TO LIMIT A PARTY’S INDEMNIFICATION OBLIGATIONS PROVIDED IN SECTION 7.2 WITH RESPECT TO THIRD PARTY CLAIMS.
7.2 Indemnification. Subject to the provisions of Section 7.3, each of Manufacturer and Company (each, in such capacity, an Indemnifying Party) will defend, indemnify and hold harmless the other party, its subsidiaries, parent corporations, affiliates, officers, directors, employees, agents, or representatives and their respective successors and assigns (each, in such capacity, an Indemnified Party) from and against any claim, suit, demand, loss, damage, expense (including reasonable attorney’s fees of indemnitee(s) and those that may be asserted by a third party) or liability (collectively, Losses) imposed upon the Indemnified Party(s) by any third party to the extent arising from or related to: (a) any material breach of such Indemnifying Party’s representations and warranties under this Agreement; (b) any negligence or intentional misconduct by such Indemnifying Party (or its employees, agents or representatives) in performing its obligations under this Agreement; (c) in the case of Manufacturer as the Indemnifying Party an inherent defect in the Products or failure of the Product to comply with the Product Specifications; (d) in the case of Company as the Indemnifying Party (i) any labeling, package insert, warnings or other materials supplied or required by law or this Agreement to be supplied by or at the direction of Company; and (ii) any promotional claims with respect to any Product by Company or by an Affiliate, licensee, sublicensee, distributor or agent of Company; and (e) in the case of Manufacturer as the Indemnifying Party any claim that the Product infringes any patent or other intellectual property right of any third party other than cases in which such infringement arises from a modification proposed by Company to a Product Specification. The foregoing indemnification action shall not apply to the extent that such Losses arose as a result of any Indemnified Party’s negligence, intentional misconduct or breach of this Agreement.
7.3 Procedure. To receive the benefit of indemnification under Section 7.2, the Indemnified Party must (a) promptly notify the Indemnifying Party of a claim or suit; provided, that failure to give such notice shall not relieve Indemnifying Party of its indemnification obligations except to the extent that such failure prejudices the rights of Indemnifying Party; (b) provide reasonable cooperation to the Indemnifying Party (and its insurer), as reasonably requested, at Indemnifying Party’s cost and expense; and (c) tender to the Indemnifying Party (and its insurer) full authority to defend or settle the claim or suit; provided that no settlement requiring any admission by the Indemnified Party or that imposes any obligation on the Indemnified Party shall be made without the Indemnified Party’s consent, not to be unreasonably withheld. Neither party, as Indemnifying Party, has any obligation to indemnify the other party in connection with any settlement made without the Indemnifying Party’s written consent. The Indemnified Party has the right to participate at its own expense in the claim or suit and in selecting counsel therefor.
7.4 Insurance. Each party shall procure and maintain insurance or self-insurance, including product liability insurance, adequate to cover its obligations hereunder and which are consistent with normal business practices of prudent companies similarly situated at all times during which any Product is being clinically tested with human subjects or commercially distributed or sold by Company. It is understood that such insurance shall not be construed to create a limit of either party’s liability with respect to its indemnification obligations under Section 7.2. Each party shall provide the other with written evidence of such insurance (or financial information that describes the amounts available under any self-insurance facility) upon request. Each party shall provide the other with written notice at least fifteen (15) days prior to the cancellation, non-renewal or material change in such insurance or self-insurance which materially adversely affects the rights of the other party hereunder. If such party does not obtain replacement insurance or take other measures that allow it to provide comparable coverage within such 15-day period, the other party shall have the right to terminate this Agreement effective at the end of such fifteen (15) day period without notice or any additional waiting periods.
8. TERM AND TERMINATION
8.1 Term. This Agreement shall take effect as the Effective Date and shall remain in effect until the fifth anniversary of the Effective Date, unless sooner terminated in accordance with Section 8.2 of this Agreement (including the Sections referenced in Section 8.2) or extended in accordance with this Section 8.1. Manufacturer hereby grants Company, or its successors and assigns, an option to renew this Agreement, upon the same terms as herein set forth, for one (1) additional five (5) year period, which option to renew shall automatically take effect at the expiration of the then current term unless written notice of Company’s decision not to renew is delivered to
Manufacturer at least six (6) months prior to the expiration of the then current term. All applicable terms and conditions of this Agreement shall remain in effect during the renewal term, unless expressly amended by mutual agreement of the parties. During any notice period prior to the expiration of the initial term or any renewal term, each party will continue to perform and fulfill all of its respective obligations.
8.2 Termination. Subject to Sections 3.3(b) and 3.7 with respect to breaches addressed thereby, either party may terminate this Agreement at any time upon sixty (60) days notice to the other party in the event that the other party shall have breached any of its material obligations hereunder and shall not have cured such default prior to the expiration of the sixty (60) day period, provided that if the breach relates only to a single Product, then the terminating party shall be entitled to terminate this Agreement only with respect to the applicable Product. In addition, either party shall have the right to terminate this Agreement on a Product-by-Product basis upon thirty (30) days notice if a Force Majeure condition (excluding a Force Majeure condition that is the fault of the party seeking to terminate) has prevented performance by the other party for more than forty five (45) consecutive days or more than one hundred twenty (120) days in the aggregate in any 12-month period. The parties may also terminate this Agreement at any time on a Product-by-Product basis upon mutual written agreement of the parties. Company may also terminate this Agreement with respect to a Product upon notice to Manufacturer if Manufacturer fails to cure a breach with respect to such Product under Section 3.3(b) in accordance with Section 3.3(b).
8.3 Effect of Termination. (a) Termination of this Agreement shall not affect rights and obligations of either party that may have accrued prior to the effective date of termination or any obligation specifically stated to survive termination. The provisions of Sections 1, 4.1, 4.4-4.5, 5.2(c), 5.4(a)-(d), 6.1-6.2, 7.1-7.3, 8.3, 9, 10.1, 10.3 and 10.5-10.14 shall survive any expiration or termination of this Agreement in accordance with their respective terms. Upon termination of this Agreement for any reason and upon expiration of the applicable sell-out period specified in this Section 8.3, Company shall discontinue any and all use of the licenses granted under Section 4.2.
(b) Upon any termination (including expiration) of this Agreement, each party shall return to the other party or certify in writing to the other party that it has destroyed all documents and other tangible items it or its employees or agents have received or created pertaining, referring or relating to the Confidential Information of the other party; provided, however, that a party is permitted to retain one copy of such materials in its legal files to be used solely to verify compliance with its obligations hereunder.
(c) Upon any termination (including expiration) of this Agreement, Manufacturer and Company will cooperate to minimize disruption to customers for the Product in the Territory. Except in the event of termination arising from Company’s breach of this Agreement, and on a cash-in-advance basis only, Manufacturer will fill existing orders that Manufacturer has received and accepted and will accept additional orders from Company for additional Products that Company is, as of the date of termination notice, contractually obligated to furnish to its customers, to the extent Company does not have sufficient product in its inventory to fulfill such obligations; provided Company notifies Manufacturer of such transactions by the termination date. Any Products manufactured pursuant to the provisions of this Section 8.3(c) shall be subject to the provisions of Section 3 and Section 5 (except as expressly modified by this Section 8.3(c) with respect to payment terms).
(d) If termination is based upon material breach by Company, Manufacturer shall have the right but not the obligation to repurchase some or all of the inventory of the Product held by Company or its Affiliates. The price for such inventory shall be the cost thereof actually paid by Company to Manufacturer. Upon issuance of an RMA to Company by Manufacturer, Company shall ship the Products to Manufacturer freight prepaid. If Manufacturer does not repurchase the inventory under this Section 8.3(d), Company shall have the right to sell out its inventory for a period of […***…] from the date of termination. Thereafter, Manufacturer shall have the right but not the obligation to repurchase any remaining inventory of the Product in accordance with the provisions of this Section 8.3(d).
9. DISPUTE RESOLUTION.
9.1 Designated Contacts. Each party will designate an individual (Representative) who will have the authority to represent such Party in all matters concerning the transactions contemplated by this Agreement. All communications should be addressed to the designated Representative. The initial Company Representative will be Xxxx Xxxxx. The initial Manufacturer Representative will be Xxxxxxx Xxxxxx.
***Confidential Treatment Requested
9.2 Issue Resolution. In the event that any dispute arises relating to this Agreement, the Representatives shall promptly meet and attempt to resolve same through good faith discussions. If the Representatives are unable to resolve any dispute to their mutual satisfaction within thirty (30) days after they commence discussions regarding same, and do not agree to extend the time for resolution of the issue at the end of their meeting, then either party may initiate alternative dispute resolution in accordance with Section 9.3. Pending resolution of any dispute, both parties will continue their performance under this Agreement including, without limitation, the payment of all amounts due to the other party that are not in dispute.
9.3 Arbitration. Any controversy or claim arising between the parties in connection with this Agreement that cannot be resolved using the procedure specified in sections 9.1-9.2, shall be resolved by binding arbitration in accordance with the terms and conditions of Sections 9.3-9.5; provided, that actions by either party seeking equitable or declaratory relief may be brought in court pursuant to Section 10.1 without resort to any of the provisions of this Section 9. This agreement to arbitrate shall continue in full force and effect despite the expiration, rescission or termination of this Agreement. All arbitration shall be undertaken in accordance with the federal policy in the United States favoring arbitration, as set forth in the Federal Arbitration Act, and the decision of the arbitrator(s) shall be enforceable in any court of competent jurisdiction. The parties knowingly and voluntarily waive their rights to have their dispute tried and adjudicated by a judge and jury except as expressly provided herein. The arbitrator(s) shall apply the law of New York and the arbitration shall be held in New York, New York, United States of America.
9.4 Arbitration Procedure. Any party may demand arbitration by sending written notice to the other party. The arbitration and the selection of the arbitrator(s) shall be conducted in accordance with such rules as may be agreed upon by the parties, or, failing agreement within thirty (30) days after arbitration is demanded, under the International Arbitration Rules of the American Arbitration Association’s International Centre for Dispute Resolutions (ICDR), as such rules may be modified by this Agreement. If the parties are unable to agree upon a single arbitrator within thirty (30) days following the date arbitration is demanded, then three (3) arbitrators shall be used, one selected by each party within ten (10) days after the conclusion of the thirty (30) day period and a third selected by the first two within ten (10) days thereafter. Unless the parties agree otherwise, they shall be limited in their discovery to directly relevant documents. Responses or objections to a document request shall be served twenty (20) days after receipt of the request. The arbitrator(s) shall resolve any discovery disputes.
9.5 Awards. The arbitrator(s) shall only have the authority to award actual money damages (with interest on unpaid amounts from the date due) and the arbitrator(s) shall not have the authority to award exemplary or punitive damages, and the parties expressly waive any claimed right to such damages. The arbitration shall be of each party’s individual claims only, and no claim of any other party shall be subject to arbitration in such proceeding. The costs and expenses of the arbitration, but not the costs and expenses of the parties, shall be shared equally by the parties; provided that if the arbitrator(s) determine(s) that one party prevailed in the proceeding, then the other party shall bear the entire cost and expense of the arbitration. If a party fails to proceed with arbitration, unsuccessfully challenges the arbitration award, or fails to comply with the arbitration award, the other party is entitled to costs, including reasonable attorneys’ fees, for having to compel arbitration or defend or enforce the award. Except as otherwise required by law, the parties and the arbitrator(s) shall maintain as confidential all information or documents obtained during the arbitration process, including the resolution of the dispute.
9.6 Intellectual Property. Notwithstanding the provisions of Sections 9.3-9.5, no claim or dispute relating to the ownership, use, enforceability or validity of any IP Rights shall be subject to arbitration; if any such claim or dispute arises and is not resolved by the parties in accordance with Section 9.2, then the parties shall resort to litigation to resolve such matter.
10. GENERAL PROVISIONS.
10.1 Governing Law; Venue. (a) This Agreement shall be governed and construed in accordance with the internal, substantive laws of New York, United States of America, to the exclusion of any choice or conflict of laws rule or provision that would result in the application of the substantive law of any other jurisdiction. The United Nations Convention on Contracts for the International Sale of Goods shall not apply to the transactions contemplated by this Agreement.
(b) Any action, suit or other proceeding pursuant to, arising under, or touching or concerning this Agreement or the transactions contemplated hereby shall be brought exclusively in any court of competent jurisdiction in New York, New York, United States of America. The parties agree to take any and all necessary or appropriate action to submit to the exclusive jurisdiction of any such court.
10.2 Waiver. No provision of or right under this Agreement shall be deemed to have been waived by any act or acquiescence on the part of either party, its agents or employees, but only by an instrument in writing signed by an authorized officer of each party. No waiver by either party of any breach of this Agreement by the other party shall be effective as to any other breach, whether of the same or any other term or condition and whether occurring before or after the date of such waiver.
10.3 Independent Contractors. Each party represents that it is acting on its own behalf as an independent contractor and is not acting as an agent for or on behalf of any third party. This Agreement and the relations hereby established by and between Company and Manufacturer do not constitute a partnership, joint venture, franchise, agency or contract of employment.
10.4 Assignment. Neither party may assign this Agreement or any of its rights and obligations under this Agreement without the prior written consent of the other party; provided, that either party may assign this Agreement without consent to: (a) any Person to which such party transfers all or substantially all of its assets or with which such party is consolidated or merged; or (b) any Affiliate; provided, further, that in each instance the assignee expressly assumes all obligations imposed on the assigning party by this Agreement in writing and the other party is promptly notified of such assignment. Any attempt to assign this Agreement or any rights or obligations hereunder other than as expressly permitted by this Section 10.4 shall be null and void.
10.5 Successors and Assigns. This Agreement shall bind and inure to the benefit of the parties hereto and their respective successors and permitted assigns.
10.6 Notices. Unless otherwise provided herein, any notice, report, payment or document to be given by one party to the other shall be in writing and shall be deemed given when delivered personally or mailed by certified or registered mail, postage prepaid (such mailed notice to be effective on the date which is three (3) business days after the date of mailing), or sent by internationally recognized overnight courier (such notice sent by courier to be effective one business day after it is deposited with such courier), or sent by telefax (such notice sent by telefax to be effective when sent, if confirmed by certified or registered mail or overnight courier as aforesaid) to the address set forth on the signature page to this Agreement or to such other place as any party may designate as to itself by written notice to the other party.
10.7 Severability. In the event any provision of this Agreement shall for any reason be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other term or provision hereof. The parties agree that they will negotiate in good faith or will permit a court to replace any provision hereof so held invalid, illegal or unenforceable with a valid provision which is as similar as possible in substance to the invalid, illegal or unenforceable provision.
10.8 Captions. Captions of the sections and subsections of this Agreement are for reference purposes only and do not constitute terms or conditions of this Agreement and shall not limit or affect the meaning or construction of the terms and conditions hereof.
10.9 Word Meanings. Words such as herein, hereinafter, hereof and hereunder refer to this Agreement as a whole and not merely to a section or paragraph in which such words appear, unless the context otherwise requires. The singular shall include the plural, and each masculine, feminine and neuter reference shall include and refer also to the others, unless the context otherwise requires.
10.10 Entire Agreement; Amendment. The terms and provisions contained in this Agreement (including the Attachments) constitute the entire understanding of the parties with respect to the transactions and matters contemplated hereby and supersede all prior or contemporaneous communications, representations, agreements and understandings relating to the subject matter hereof, including the 1997 Agreement. No representations,
inducements, promises or agreements, whether oral or otherwise, between the parties not contained in this Agreement shall be of any force or effect. No agreement or understanding amending or extending this Agreement including the Attachments or varying its terms (including any inconsistent terms in any purchase order, acknowledgment or similar form) shall be binding upon either party unless it is in a writing specifically referring to this Agreement and signed by’ a duly authorized representative of each of the parties.
10.11 Conflict or Inconsistency. In the event of any conflict or inconsistency between the terms and conditions of this Agreement and any terms or conditions set forth in any purchase order or other document relating to the transactions contemplated by this Agreement, the terms and conditions set forth in this Agreement shall prevail.
10.12 Rules of Construction. The parties agree that they have participated equally in the formation of this Agreement and that the language and terms of this Agreement shall not be construed against either party by reason of the extent to which such party or its professional advisors participated in the preparation of this Agreement.
10.13 Counterparts. This Agreement may be executed in multiple counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
10.14 Force Majeure. Except as otherwise provided in this Agreement, a delay or failure of a party to comply with any obligation created by this Agreement that is caused by a Force Majeure condition shall not be a breach of this Agreement.
10.15 Further Assurances. Each party covenants and agrees that, subsequent to the execution and delivery of this Agreement and without any additional consideration, it will execute and deliver any further legal instruments and perform any acts which are or may become reasonably necessary to effectuate the purposes of this Agreement.
10.16 Canada. Manufacturer will, within thirty (30) days following the Effective Date seek to arrange a meeting among representatives of the Company and the party to which Manufacturer has granted distribution rights for products in the country of Canada (Anexxa Medical Technologies Inc.) for the purpose of determining whether the Company can persuade such distributor to give up its rights to Canada in exchange for a payment from Company. If Company and such distributor are able to reach a mutually satisfactory agreement under which such distributor will relinquish rights to distribute in Canada in exchange for a payment from Company then Manufacturer hereby covenants and agrees that: (a) it will permit such distributor to terminate such distribution agreement without payment of any termination or similar fee; and (b) effective upon the payment of such fee by Company, the country of Canada shall automatically be added to the definition of Territory under this Agreement and the provisions of the last sentence of Section 3.8(a) shall automatically be amended to include as Company’s responsibility clearing Products through all customs and import requirements for Canada.
[Remainder of this page intentionally left blank]
IN WITNESS WHEREOF the parties have caused this Agreement to be executed on their behalf by their duly
authorized representatives intending it to take effect as an instrument under seal as of the Effective Date.
|
Silimed-Silicone e Instrumental Medico-Cirugico e Hospitalar LTDA |
Juliet Medical, Inc. | |||
|
|
| |||
|
By: |
/s/ Xxxxxxx Xxxxxx, |
|
By: |
/s/Xxxx Xxxxx |
|
Name: Xxxxxxx Xxxxxx |
Name: Xxxx Xxxxx | |||
|
Title: Presidente |
Title: Chief Executive Officer | |||
|
|
| |||
|
Notice Address: |
Notice Address: | |||
|
Silimed-Silicone e Instrumental Medico-Cirugico |
Juliet Medical, Inc. | |||
|
E Hospitalar LTDA |
c/o Orbimed Advisors LLC | |||
|
R Xxxxxxxxxx Xxxxx, 374 |
New York, NY USA 00000 | |||
|
Xxx xx Xxxxxxx, Xxxxxx |
| |||
|
Fax: 00-000-000-000 |
Fax: 000.000.000.0000 | |||
|
Email: xxxxxxxxxxxxx@xxxxxxxx.xxx.xx |
Email: xxxx@xxxxxxxxxxxxx.xxx | |||
|
Attn: Xxxxxxx Xxxxxx, President |
Attn: Xxxx Xxxxx, Chief Executive Officer | |||
|
|
| |||
|
|
| |||
|
|
| |||
|
Attachment 1: Products; Product Specification |
| |||
|
Attachment 2: Pricing |
| |||
|
Attachment 3: Trademarks |
| |||
|
Attachment 4: Packaging Specifications |
| |||
|
Attachment 5: Standard Operating Procedures |
| |||
|
Attachment 6: Product Warranty Periods |
| |||
Attachment 1
Mammary Implants:
-Inflatable Mammary Implant (textured surface Posterior Valve)
-Inflatable Mammary Implant (textured surface Anterior Valve)
-Anatomical Inflatable Mammary Implant
-Anatomical Mammary Implant (silicone gel polyurethane foam coated)
-Anatomical Mammary Implant (silicone gel textured surface)
-Mammary Implant (silicone gel smooth surface)
-Mammary Implant (silicone gel polyurethane foam coated surface)
-Mammary Implant (silicone gel textured surface)
-Smooth Surface Mammary Implant (round shape)
-Textured Surface Mammary Implant (round shape)
-Nuance® Mammary Implant
-Enhance® Mammary Implant
-Quartzo® Mammary Implant
-Pitanguy/Xxxxxxx Mammary Implant
-Inferior Pole Anatomical System
Contour Implants:
-Calf Implant (filled)
-Calf Implant
-Gluteal Implant
-Round and Oval bases --4 Designs
-Round and Oval bases --5 Designs
-Quartzo
-Pectoral Implant
-Silicone Smooth Surface/Texture Surface
-Silicone Texture Only
Tissue Expanders:
-Tissue Expanders
-Anatomical Tissue Expanders (breast)
-Gingival Expander
Facial Implants:
-Eyelid Suspensor Implant
-Ear Implant
-Medgel Nasal Splint
-Nostril Retainer
-Anatomical Chin Implant
-Chin Implant
-Anatomical Malar Implant
-Malar Implant
-Implant for Nasal Dorsum
-Nasal Implant in “L” Shape
-Zygomatic Implant
Hand Surgery Products:
-Tendon Spacer
-Implant for the First Intermetacarpal Space
Urology Products:
-Inflatable Periurethral Constrictor
-Vesical Conformer
-Tube for Hypospadius
-Testicular Implant
-Silicone Gel
-Silicone Gel Xxxxx Surface
-Malleable Penile Implant
-Adjustable Penile Implant
-Vaginal Stent
Bariatric Products:
-Adjustable Gastric Band
-Gastric Balloon
Miscellaneous Products:
-SILICAT Chemotherapy Kit
-Guide for Canula
-Suspension Sheet for Mammoplasty
-Medgel
-Silicone Sheets and Blocks
Mammary Implants:
-Inflatable Mammary Implant (textured surface Posterior Valve)
-Inflatable Mammary Implant (textured surface Anterior Valve)
-Anatomical Inflatable Mammary Implant
-Anatomical Mammary Implant (silicone gel polyurethane foam coated)
-Anatomical Mammary Implant (silicone gel textured surface)
-Mammary Implant (silicone gel smooth surface)
-Mammary Implant (silicone gel polyurethane foam coated surface)
-Mammary Implant (silicone gel textured surface)
-Smooth Surface Mammary Implant (round shape)
-Textured Surface Mammary Implant (round shape)
-Nuance® Mammary Implant/Enhance® Mammary Implant
-Quartzo® Mammary Implant
-Pitanguy/Xxxxxxx Mammary Implant
-inferior Pole Anatomical System































Contour Implants:
-Calf Implant (filled)
-Calf Implant
-Gluteal Implant
-- Round and Oval bases --4 Designs
-- Round and Oval bases --5 Designs
-- Quartzo
-Pectoral Implant
-- Silicone Smooth Surface/Texture Surface
-- Silicone Texture Only














Tissue Expanders
-Tissue Expanders
- Anatomical Tissue Expanders (breast)
- Gingival Expander




























Urology Products:
-Inflatable Periurethral Constrictor
-Vesical Conformer
-Tube for Hypospadius
-Testicular Implant
-- Silicone Gel
-- Silicone Gel Xxxxx Surface
-Malleable Penile Implant
-Adjustable Penile Implant
-Vaginal Stent


















Bariatric Products
-Adjustable Gastric Bad
- Gastric Balloon




Miscellaneous Products:
-SILICAT Chemotherapy Kit
-Guide for Canula
-Suspension Sheet for Mammoplasty
-Medgel
-Silicone Sheets and Blocks
















SILIMED-Silicone e Instrumental Médico-Cirúrgico Hospitalar Ltda.
Xxx Xxxxxxxxxx Xxxxx, 000 – Xxx xx Xxxxxxx – BRASIL – 21240-660
· (5521) 3687-7140 / 3687-7141 - · (5521) 3687-7000
Attachment 3:
Trademarks
SILIMED
BIODESIGN
ENHANCE
NUANCE
MEME
REPLICON
Attachment 4
Packaging Specifications
SILIMED — 510(K) Products
General Information:
|
510(K) -PRODUCT |
UNITS/ |
STERILE (Y/N) |
PACKAGING |
|
K981851 - OVAL CARVING BLOCK |
1 |
Y |
Double Blister sealed with Tyvek in a cardboard box |
|
K974482 - GLUTEAL IMPLANT |
1 |
Y |
Double Blister sealed with Tyvek in a cardboard box |
|
K981852 - TISSUE EXPANDER |
1 |
Y |
Double Blister sealed with Tyvek in a cardboard box |
|
K042054 - PECTORAL IMPLANT |
1 |
Y |
Double Blister sealed with Tyvek in a cardboard box |
|
K974480 - CALF IMPLANT |
1 |
Y |
Double Blister sealed with Tyvek in a cardboard box |
|
K974479 - VAGINAL STENT |
1 |
Y |
Double Blister sealed with Tyvek in a cardboard box |
|
K981833 - NASAL IMPLANT |
1 |
Y |
Double plastic envelope in a pvc envelope |
|
K981850 - CHIN IMPLANT |
1 |
Y |
Double plastic envelope in a pvc envelope |
|
K980221 - NASAL RETAINER |
1 |
Y |
Double plastic envelope in a pvc envelope |
|
K981835 - MALAR IMPLANT
|
1
|
Y
|
Double plastic envelope in a pvc envelope
|
Unit for Shipping:
· SILIMED does not use pallets for shipping.
· The large unit for shipping has the cubical weight of 61.95Kg. The quantity of packagings depends on the size and/or type of products packaging
Attachment 5
Standard Operating Procedures
[to be mutually agreed in accordance with the procedure specified in Section 5.4 prior to shipment of the first Products to Company for commercial sale]
Attachment 6
Product Warranty Periods
|
PRODUCT |
WARRANTY PERIOD |
|
Mammary Implants including: -Inflatable Mammary Implant (textured surface Posterior Valve) -Inflatable Mammary Implant (textured surface Anterior Valve) -Anatomical Inflatable Mammary Implant -Anatomical Mammary Implant (silicone gel polyurethane foam coated) -Anatomical Mammary Implant (silicone gel textured surface) -Mammary Implant (silicone gel smooth surface) -Mammary Implant (silicone gel polyurethane foam coated surface) -Mammary Implant (silicone gel textured surface) -Smooth Surface Mammary Implant (round shape) -Textured Surface Mammary Implant (round shape) -Nuance® Mammary Implant/Enhance® Mammary Implant -Quartzo® Mammary Implant -Pitanguy/Xxxxxxx Mammary Implant -Inferior Pole Anatomical System |
10 years from manufacturing date |
|
|
|
|
Contour Implants including: -Calf Implant (filled) -Calf Implant -Gluteal Implant -- Round and Oval bases --4 Designs -- Round and Oval bases --5 Designs -- Quartzo -Pectoral Implant -- Silicone Smooth Surface/Texture Surface -- Silicone Texture Only |
10 years from manufacturing date |
|
|
|
|
Tissue Expanders -Tissue Expanders -Anatomical Tissue Expanders (breast) -Gingival Expander |
5 years from manufacturing date |
|
|
|
|
Facial Implants including: -Eyelid Suspensor Implant -Ear Implant -Medgel Nasal Splint -Nostril Retainer -Anatomical Chin Implant -Chin Implant -Anatomical Malar Implant -Malar Implant -Implant for Nasal Dorsum -Nasal Implant in “L” Shape -Zygomatic Implant |
10 years from manufacturing date |
|
|
|
|
Hand Surgery Products including: -Tendon Spacer -Implant for the First Intermetacarpal Space |
5 years from manufacturing date |
|
|
|
|
Urology Products including: -Inflatable Periurethral Constrictor -Vesical Conformer -Tube for Hypospadius -Testicular Implant -Malleable Penile Implant -Adjustable Penile Implant -Vaginal Stent |
5 years from manufacturing date |
|
|
|
|
Bariatric Products (Bands) including: -Adjustable gastric band |
10 years from manufacturing date |
|
Bariatric Products (Balloons) including: -Gastric Balloon |
1 year from manufacturing date |
|
|
|
|
Miscellaneous Products including: -SILICAT Chemotherapy Kit -Guide for Canula -Suspension Sheet for Mammoplasty -Medgel -Silicone Sheets and Blocks |
None |
Manufacturer will include a certificate showing the date of manufacture with each shipment of each Product. No Product will be supplied more than 180 days following the date of Manufacture.