PAGE OF PAGES AWARDICONTRACT 1. THIS CONTRACT IS A RATED ORDER UNDER DPAS (15 CFR 350) 1 I 28 2. CONTRACT (Prsc. Insr. 1denr.l NO. 3..EFFECTIVE DATE 4. REQUlSlTlONlPURCHASE REQUESTIPROJECT NO. HHS’0100200700032c I 1 6. ISSUED BY CODE1 ADMINISTERED BY...
Exhibit
10.3
NOTE: THIS DOCUMENT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST PURSUANT TO
RULE 24B-2 UNDER THE SECURITIES EXCHANGE ACT OF 1934. PORTIONS
OF THIS DOCUMENT FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED HAVE BEEN REDACTED
AND ARE MARKED HEREIN BY
“ ”.
SUCH REDACTED INFORMATION HAS BEEN FILED SEPARATELY WITH THE COMMISSION PURSUANT TO THE
CONFIDENTIAL TREATMENT REQUEST.

| PAGE OF PAGES AWARDICONTRACT 1. THIS CONTRACT IS A RATED ORDER UNDER DPAS (15 CFR 350) 1 I 28 2. CONTRACT (Prsc. Insr. 1denr.l NO. 3..EFFECTIVE DATE 4. REQUlSlTlONlPURCHASE REQUESTIPROJECT NO. HHS’0100200700032c I 1 6. ISSUED BY CODE1 ADMINISTERED BY (If other than Item 5J CODE I HHS/OS/OPHEP/OPHEMC ▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇, ▇▇, ▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ 7. ▇▇▇▇▇▇▇ ADDRESS OF CONTRACTOR (~6...weer, corny, sure rnd ZIP codf?/ 8. DELIVERY ~io~rysPharmaceuticals Inc.t ’ aF o e oRlGlN OTHER (.See bniowl ▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇ ‘9. DISCOUNT FOR PROMPT PAYMENT Birmingham, AL 35244 N/ A 10. SUBMIT INVOICES ITEM (4 copies unless other- wise specified) TO THE JFACILITY CODE ADDRESS SHOWN IN. 5 CODE 1 1. SHIP TOlMARK FOR 12. PAYMENT WILL BE MADE BY CODE CODE 1 See Paragraph D.l. See Article G.4, .- .,. _____ , . .. , ,,..,. ....,.. . .. b13. AWHORITY FOR USING OTHER THAN FULL AND OPEN COMPETITION: . 114. ACCOUNTING AND APPROPRIATION DATA 0 10 U.S.C. 23041~)1 1 41 U.S.C. 2531~)I ) 158. SUPPLIESISERVICES 15C. QUANTITY 15D.UNIT 15E. UNIT PRIC~ 15F. AMOUNT 15A. ITEM NO. |
SECTION B—SUPPLIES OR SERVICES AND PRICES/COSTS
B.1. BRIEF DESCRIPTION OF SUPPLIES OR SERVICES
This project from the Department of Health and Human Services (HHS) through the Office of Public
Health Emergency Medical Countermeasures (OPHEMC) within the Office of Public Health Emergency
Preparedness (OPHEP) provides incremental multi-year funding for cost-reimbursable contracts for
the advanced development of prophylactic and therapeutic drugs against pandemic and seasonal
influenza viral pathogens leading towards U.S.-licensure. The antiviral drugs of interest may
include any compound or drug providing anti-influenza. These may include synthetic chemical
compounds, snRNAi, polyclonal/monoclonal antibody cocktails or other drugs, which could be used in
the treatment and/or prophylaxis to decrease the morbidity and mortality associated with seasonal
and pandemic influenza. The objective of this project is to facilitate the development and
U.S.-licensure of antiviral drugs effective as prophylactic/therapeutic agents against influenza
virus infection.
B.2. HHSAR 352.232-74 Estimated Cost and Fixed Fee — Incrementally Funded Contract (Apr 1984)
(a) It is estimated that the total cost to the Government for full performance of this contract
will be $102,661,429, of which the sum of represents the estimated reimbursable costs
and represents the fixed fee.
(b) Total funds currently available for payment and allotted to this contract are , of
which represents the estimated reimbursable costs and represents
the fixed-fee. For further provisions on funding, see the Limitation of Funds clause.
(c) It is estimated that the amount currently allotted will cover performance through .
(d) The Contracting Officer may allot additional funds to the contract without the concurrence of
the Contractor
B3. Cancellation Ceiling
(a) This clause does not apply when the contract is fully funded.
(b) The total funding in B.2 (c) includes an amount that covers the cancellation charge described
in FAR 52.217-2, Cancellation Under Multi-year Contracts. The cancellation charge shall not exceed
$ (insert dollar amount or express as a percentage of total funding under B.2 (b).
Page 2 of 27
B.4. CONTRACT LINE ITEM NUMBERS (CLINS)
| ITEM | SUPPLIES / SERVICES | QTY/UNIT | EST. COST | FIXED FEE | TOTAL EST. CPFF | |||||||
| 0001 | Product Development Plan (milestone 1) |
1 Job | ||||||||||
| 0002 | Clinical Development,
and Regulatory
Licensure Plans (milestone 2) |
1 Job | ||||||||||
| 0003 | Feasibility Plan (milestone 3) |
1 Job | ||||||||||
| 0004 | Contractor Defined
Milestones
(milestone 4); See
milestone 4 of the
Statement of Work for
key elements
|
1 Job | ||||||||||
| 0005 | Technical Progress
Reports and Executive
Summary
|
12 reports per year |
||||||||||
| 0006 | Security of Contract
Operations and
Information Technology
Security
|
Not Separately Priced (NSP) |
NSP | NSP | NSP | |||||||
| 0007 | Final Report
|
1 report | ||||||||||
Note: For the purposes of this contract, “United States” and “U.S.” are defined to include the 50
states, the District of Columbia, Puerto Rico.
Page 3 of 27
SECTION C — DESCRIPTION/SPECIFICATIONS
C.1 STATEMENT OF WORK
Purpose
The purpose of this contract is to support advanced stage development of new antiviral compounds
for the treatment and prophylaxis of pandemic and seasonal influenza leading toward submission of a
U.S. licensure application and development of required industrial capacity to support
implementation of the influenza antivirals at full production capacity at or before the onset of a
pandemic. Influenza antivirals shall be produced at one or more Food and Drug Administration
(FDA)-licensed manufacturing facilities and shall provide sufficient surge capacity to contribute
substantially to U.S. and ideally global antiviral needs during an influenza pandemic.
This advanced development contract is milestone-driven and funding is expected to occur in
phases. Periodic assessments of progress will be conducted by DHHS. Continuation of effort on
initial and subsequent milestones and associated funding will be based on contractor
performance, timeliness and quality of deliverables, availability of other antiviral drugs and
products deemed more advantageous to the USG, and consultations between the contractor, HHS, and
interagency working group members. This paragraph does not limit the Government’s rights under
contract clauses that include, but are not limited to, FAR 52.217-2, Cancellation Under
Multi-year contracts, and 52.249-6, Termination (Cost-Reimbursement).
STATEMENT OF WORK
Independently and not as an agent of the government, the contractor shall furnish all the necessary
services, qualified personnel, materials, equipment, and facilities not otherwise provided by the
government as needed to perform the work described below.
The proposal will include a Contractor Work Plan (CWP) that describes the activities to be
performed in response to the RFP requirements and a single ▇▇▇▇▇ chart to include all activities
described in the CWP with a time-phased and task-linked budget. The level of detail contained in
the CWP and the corresponding ▇▇▇▇▇ chart will be sufficient to facilitate management and execution
of the contract.
Milestones
| I. | Milestone 1: Within three (3) months of contract award, the Contractor shall provide
to the HHS for review and acceptance a milestone-driven Product Development Plan for
development of influenza antiviral drugs. This plan shall include: a) in vitro and in vivo
antiviral testing, b) pre-clinical studies (already completed) to support clinical evaluation;
c) process development and scale up manufacturing of antiviral compounds; d) clinical and
consistency lot manufacturing for FDA product licensure, e) general clinical development plan
including development and validation of clinical sample assays; f) product lot release assay
development and validation, and g) regulatory master plan for product licensure. Antiviral
agents used in these studies may be produced by the Contractor or a corporate partner. |
| II. | Milestone 2: Within six (6) months of contract award, the Contractor shall submit to
HHS for review and acceptance complete milestone-driven Clinical Development and
Regulatory Licensure Plans to initiate new antiviral development, clinical studies, as
appropriate based on the current stage of development and as outlined in the development,
testing, and manufacturing plan. |
| A. | A pre-clinical testing plan and results that are integrated with the
clinical testing and manufacturing plans using the most current and available
information including consultation with Center for Drug Evaluation and Research (CDER)
at FDA. As this stage of product
development should be completed, and then a detailed summary of the studies and results
should be incorporated as an appendix in the preliminary results section of the
technical proposal. |
Page 4 of 27
| B. | A clinical testing plan that is integrated with the pre-clinical
testing and manufacturing plans using the most current and available information
including consultation with FDA CDER. Clinical trials performed as a result of this
solicitation shall include any of Phase I and Phase 2, trials, as needed to achieve
U.S. licensure. Trials should include children, adults, and the elderly, as needed, to
support licensure for both low and high-risk populations. Given the duration, cost,
and importance of clinical trials, the schedule for each clinical trial should clearly
indicate key outcomes, populations, study sites and collaborators, analytic strategy,
sample size, timelines, and other key components. If one or more these stages of
product development have been completed, then a detailed summary of the studies and
results should be incorporated as an appendix in the preliminary results section of
the technical proposal. |
| C. | A regulatory plan that is integrated with all products and clinical
testing and manufacturing activities using the most current and available information
including consultation with FDA CDER. |
| III. | Milestone 3: Within twelve (12) months of contract award the Contractor shall provide
the USG with the following, as appropriate for the antiviral drug(s) being developed. A
feasibility plan comprehensive of all antiviral drug descriptions and studies for U.S.
licensure as follows: |
| (a) | Mechanism of action, antiviral activity in vitro, and resistance studies such
as drug resistance and cross resistance, immune response. |
| (b) | Pharmacokinetics studies like drug absorption and bioavailability,
distribution, metabolism, elimination, |
||
| (c) | Animal toxicology studies. |
| (d) | Drug interactions, carcinogenesis, mutagenesis and fertility impairment
studies. |
| (e) | Special population studies including pediatric, geriatric groups and groups
with impaired organ functions. |
||
| (f) | Treatment, prophylaxis, dosage, administration routes, adverse events. |
||
| (g) | Safety studies in different age group of people. |
||
| (h) | Seasonal influenza challenge studies |
| IV. | Milestone 4: Contractor defined milestones The Contractor shall provide a work breakdown structure including comprehensive and integrated timelines (▇▇▇▇▇ chart) and major milestones to complete the remaining the scope of work as relevant given the stage of antiviral development and evaluation toward product licensure. The Contractor shall propose milestones at which time data will be presented summarizing results of prior activities and new plans and protocols that will be submitted for review and approval in order to guide all subsequent activities. Potential milestones may include FDA acceptance of an IND application, production of investigational lots of antiviral drugs validation of QC lot release product methods, validation of manufacturing processes, stability study programs, consistency lot manufacturing, completion of clinical trials and progress to a new phase of antiviral drug evaluation, submission of a licensure application. |
Page 5 of 27
Security of Contract Operations and Information Technology
The work performed for development, manufacture, transport, storage and distribution will be
performed under a detailed security plan that ensures against theft, tampering or destruction of
the specific pertinent product-related material, equipment, documents, information, and data. The
Contractor shall develop a written Draft Security Plan, for the protection of physical facilities,
using, for example, fencing, controlled access, surveillance equipment, 2-person integrity rule,
tamper evident packaging, and armed guards. The Contractor shall submit the Draft Security Plan
to the Contracting Officer and Project Officer with the technical proposal. The Draft Security
Plan shall describe the procedures to be utilized to manage and
monitor the general internal operations of the firm and a description of the Contractor’s
facility(ies) in which the work will be performed and related activity conducted, including work by
any subcontractors and consultants. The Draft Security Plan shall also include the Contractor’s
procedures for screening and background investigations of all employees, subcontractors and
consultants who have access to the development, manufacturing, transport, storage, and distribution
of the product. Such background inquiries and screening should include, but not be limited to,
education, previous employment, fingerprints and complete criminal history (FBI, state, and local),
credit reports, civil actions, DMV, social security account number verification, drug testing, and
references. Screening data should include the employee’s full name, any aliases, date(s) of birth,
and Social Security numbers and other identifying numbers as appropriate, e.g., Passport number.
(At time of award) The US Government can audit and review at its discretion the Contractor’s
personnel records in order to confirm compliance with personnel screening and background
investigation requirements. Such access will also include interviews with relevant Contractor
human resources supervisory and hiring personnel.
This plan shall ensure confidentiality, integrity of, and timely access by authorized individuals
to data, information and information technology systems, consistent with OMB Circular A-130,
Appendix III. This plan should also address the Contractor’s security-related due diligence on
public information, marketing, advertising, including use of web site[s] impacting product and
supply chain security.
This plan shall also include the security measures to be used to protect the medical countermeasure
to be stored at the Contractor’s facility (e.g., refrigeration/freezer alarm systems, backup
electrical power generator systems, etc.), and the contingency plan to accommodate any
manufacturing and storage problems caused by natural or man-made disasters, power loss, refrigerant
loss, equipment failures, etc.
The Project Officer and the Information Protection and Systems Security (IPASS) Coordinator will
review the plan and submit comments to the Contractor within after receipt. The
Contractor shall revise the Security Plan, if required, and submit a Final Security Plan to the
Government within . Upon completion of all the required security measures, the
Contractor shall supply to the Project Officer a letter certifying compliance. Performance of work
under this contract shall be in accordance with this written Security Plan.
[END OF STATEMENT OF WORK]
Meetings and conferences:
The Contractor shall participate in regular meetings to coordinate and oversee the contract effort
as directed by the Project Officer. Such meetings may include, but are not limited to, meetings of
all Contractors and subcontractors to discuss clinical manufacturing progress, product
development, product assay development, scale up manufacturing development, clinical sample assays
development, preclinical/clinical study designs and regulatory issues,; meetings with individual
contractors and other HHS officials to discuss the technical, regulatory, and ethical aspects of
the program; and meetings with technical consultants to discuss technical data provided by the
Contractor. Monthly teleconferences with the Contractor and subcontractors with HHS officials
will be held at times and dates to be determined to review technical and product development
progress.
Page 6 of 27
C.2. REPORTING REQUIREMENTS
In addition to those reports required by other terms of this contract, the Contractor(s) shall
submit to the Contracting Officer and the Project Officer technical progress reports covering the
work accomplished during each reporting period on a periodical basis as established by the
Project Officer. These reports are subject to the technical inspection and requests for
clarification by the Project Officer. These reports shall be brief and factual and prepared in
accordance with the following format:
| I. | Technical Progress Reports: On the fifteenth of each month for the previous calendar month or
within fifteen days past the achievement of prescribed project milestones, the Contractor
shall submit to the Project Officer and the Contracting Officer. The frequency of Technical
Progress Reporting will be determined by the Contracting Officer and Project Officer after
contract award. The format and type of Technical Progress Report and Executive Summary will
be provided by the Project Officer. Technical Progress Reports will include project timelines
and milestones and summaries of product manufacturing, testing, and clinical evaluation. A
Technical Progress Report will not be required for the period when the Final Report is due.
The Contractor shall submit one copy of the Technical Progress Report electronically via
e-mail. Any attachments to the e-mail report shall be submitted in Microsoft Word or Word
Perfect, Microsoft Excel, Microsoft Project Manger, and/or Adobe Acrobat PDF files. Such
reports shall include the following specific information: |
| A. | Title page containing Technical Progress Report, the contract number and
title, the period of performance or milestone being reported, the contractor’s name,
address, and other contact information, the author(s), and the date of submission; |
| B. | Introduction/Background — An introduction covering the purpose and scope of
the contract effort; |
| C. | Progress — The report shall detail, document, and summarize the results of
work performed, test results, and milestones achieved during the period covered. Also
to be included is a summary of work planned for the next reporting period; |
| D. | Issues — Issues resolved, new issues, and outstanding issues are enumerated
with options and recommendations for resolution. An explanation of any difference
between planned progress and actual progress, why the differences have occurred, and,
if project activity is delinquent, then what corrective steps are planned. Revised
timelines are provided. |
||
| E. | Invoices — Summary of any invoices submitted during the reporting period. |
| F. | Action Items — Summary table of activities or tasks to be accomplished by a
certain date and by whom. |
| G. | Distribution List — A list of persons receiving the Technical Progress
report |
||
| H. | Attachments — Results on the project are provided as attachments |
| II. | The Executive Summary, which shall accompany each Technical Progress Report, will be
formatted in Microsoft Power Point presentations and include the following: |
| A. | Title page containing Executive Title, the contract number and title, the
period of performance or milestone being reported, the contractor’s name and the date
of submission; |
| B. | Project Progress presented as milestone events, test results, tasks, and
other activities achieved during the reporting period as talking point bullets; |
||
| C. | Project Issues presented headings and each item as a talking point bullet. |
| III. | Final Reports — By the expiration date of the contract, the Contractor shall submit a
comprehensive Final Report that shall detail, document, and summarize the results of the
entire contract work. The report shall explain comprehensively the results achieved. A draft
Final Report will be submitted to the Project Officer for review and revision, then the
original, four copies, and an electronic file containing the Final Report with revisions shall
be submitted to the Project Officer for distribution to the Contracting Officer and the
Program. |
Page 7 of 27
SECTION D—PACKAGING, MARKING AND SHIPPING
D.1. SHIPPING
I. Method of Delivery
Unless otherwise specified by the Contracting Officer or the Contracting Officer’s representative,
delivery of items, to be furnished to the government under this contract (including invoices),
shall be made by first class mail.
II. Addressees — For all contract deliverables.
Project Officer
|
Contracting Officer | |
HHS/OPHEP/OPHEMC
|
HHS/OPHEP/OPHEMC | |
▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇
|
▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇ | |
Room G640
|
Room G640 | |
Washington, D.C. 20201
|
Washington, D.C. 20201 |
Page 8 of 27
SECTION E—INSPECTION AND ACCEPTANCE
The Contracting Officer or the duly authorized representative will inspect and accept materials and
services to be delivered under the contract. Contractor inspector is ▇▇▇▇▇▇ noted, as the Project
Officer and place of inspection will be the contractor’s facilities. In addition, the following
clause is incorporated by reference:
FAR Clause No.52.246-9, INSPECTION OF RESEARCH AND DEVELOPMENT (SHORT FORM) (APR 1984)
Page 9 of 27
SECTION F—DELIVERIES OR PERFORMANCE
F.1. PERIOD OF PERFORMANCE
The period of performance of this contract is from the date of contract award to
_____
after
contract award.
Delivery will be required F.O.B. Destination as set forth in FAR 52.247-35, F.O.B. DESTINATION,
WITHIN CONSIGNEE’S PREMISES (APR 1984).
F.2. Technical Report Requirements
| Item | Deliverable | Quantity | Due Date | |||
1.
|
Technical Progress Report | Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
1st Report due on/before ; thereafter, due on/before the 15th of the month or milestone following each reporting period. Not due when Final is due. | |||
2.
|
Executive Summary | Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
1st Report due on/before ; thereafter, due on/before the 15th of the month following each anniversary date of the contract. Not due when Final is due. | |||
3.
|
Final Report | Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
Due on/before the completion date of the contract. |
Page 10 of 27
F.3. Contract Deliverables
| Milestones | Deliverable | Quantity | Due Date | |||||
1.
|
Product Development Plan (milestone 1) |
Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
. | |||||
2.
|
Clinical Development, and Regulatory Licensure Plans (milestone 2) | Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
||||||
3.
|
Feasibility Plan (milestone 3) |
Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
||||||
4.
|
Contractor Defined Milestones (milestone 4) |
Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
||||||
5.
|
Final Security Plan | Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
after Governments Final Comments | |||||
6.
|
Technical Progress Reports and Executive Summary | Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
See section C.2.Reporting requirements. | |||||
7.
|
Final Report | Original — C.O. 2 Copies — P.O. 1 Electronic Copy — P.O. |
See section C.2.Reporting requirements. | |||||
F.4. STOP WORK ORDER
The following clause is incorporated by reference:
FAR CLAUSE 52.242-15, STOP WORK ORDER (AUG 1989) with ALTERNATE I (APR 1984)
Page 11 of 27
SECTION G.—CONTRACT ADMINISTRATION DATA
G.1. CONTRACTING OFFICER
1) The Contracting Officer is the only individual who can legally commit the Government to the
expenditure of public funds. No person other than the Contracting Officer can make any changes to
the terms, conditions, general provisions or other stipulations of this contract.
2) The Contracting Officer is the only person with authority to act as agent of the Government
under this contract. Only the Contracting Officer has authority to: (1) direct or negotiate any
changes in the statement of work; (2) modify or extend the period of performance; (3) change the
delivery schedule; (4) authorize reimbursement to the Contractor any costs incurred during the
performance of this contract; or (5) otherwise change any terms and conditions of this contract.
3) No information, other than that which may be contained in an authorized modification to this
contract, duly issued by the Contracting Officer, which may be received from any person employed by
the United States Government, or otherwise, shall be considered grounds for deviation from any
stipulation of this contract.
G.2. PROJECT OFFICER
The Government’s Project Officer(s) will be identified by modification upon contract execution.
The Project Officer is responsible for: (1) monitoring the Contractor’s technical progress,
including the surveillance and assessment of performance and recommending to the Contracting
Officer changes in requirements; (2) interpreting the statement of work and any other technical
performance requirements; (3) performing technical evaluation as required; (4) performing technical
inspections and acceptances required by this contract; and (5) assisting in the resolution of
technical problems encountered during performance.
G.3. KEY PERSONNEL
Pursuant to HHSAR Clause 352.270-5, Key Personnel, incorporated in Section I of this contract, the
following individuals are considered to be essential to the work being performed hereunder:
| Name | Title | |
Prior to diverting any of the specified individuals to other programs, the Contractor shall notify
the Contracting Officer reasonably in advance and shall submit justification (including proposed
substitutions) in sufficient detail to permit evaluation of the impact on the program. No
diversion shall be made by the Contractor without the written consent of the Contracting Officer;
provided, that the Contracting Officer
may ratify in writing such diversion and such ratification shall constitute the consent of the
Contracting Officer. The contract may be modified from time to time during the course of the
contract to either add or delete personnel, as appropriate
Page 12 of 27
G. 4. INVOICE SUBMISSION
Invoices will be submitted in accordance with “Invoice/Financing Request Instructions” attached to
this contract.
G. 5. CONTRACT FINANCIAL REPORT
Financial reports will be submitted to the address specified in Block 7 of face page of the
contract. Normally, reports are due quarterly. Examples of the cost elements to be reported
include the following:
Expenditure Category
| 1. | Direct Labor |
| a. | Principal Investigator |
||
| b. | Co-Principal Investigator |
| 2. | Personnel — Other |
||
| 3. | Fringe Benefits |
||
| 4. | Materials/Supplies |
||
| 5. | Travel |
||
| 6. | Consultant Costs |
||
| 7. | Subcontract Costs |
||
| 8. | Other Direct Costs |
||
| 9. | Clinical Trials Costs |
||
| 10. | Indirect Cost |
||
| 11. | Fee |
||
| 12. | Total Cost |
G. 6. INDIRECT COST RATES
Profit making organizations will negotiate provisional and/or final indirect cost rates with their
cognizant Government Audit Agency.
G. 7. POST AWARD EVALUATION OF PAST PERFORMANCE
Interim and final evaluations of contractor performance shall be conducted on this contract in
accordance with FAR 42.15. The final performance evaluation shall be completed at the time of
completion of work. Interim and final evaluations will be submitted to the Contractor as soon as
practicable. The Contractor will be permitted thirty days to review the document and to submit
additional information or a rebutting statement.
Page 13 of 27
G.8. GOVERNMENT PROPERTY
| a. | In addition to the requirements of the clause, GOVERNMENT PROPERTY, incorporated in SECTION I
of this contract, the Contractor shall comply with the provisions of HHS Publication,
“Contractor’s Guide for Control of Government Property,” which is incorporated into this
contract byby reference. This document can be accessed at: ▇▇▇▇://▇▇▇.▇▇▇▇▇▇▇.▇▇▇.▇▇▇/▇▇▇/▇▇▇▇▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇.. Among other issues, this publication provides a summary of the Contractor’s responsibilities regarding purchasing authorizations and inventory and reporting requirements under the contract. A copy of this publication is available upon request to the Contracts Property Administrator. |
| b. | Notwithstanding the provisions outlined in the HHS Publication, “Contractor’s Guide for
Control of Government Property,” which is incorporated in this contract in paragraph a. above,
the contractor shall use the form entitled, “Report of Government Owned, Contractor Held
Property” for performing annual inventories required under this contract. This form is
included as an attachment in SECTION J of this contract. |
Page 14 of 27
SECTION H—SPECIAL CONTRACT REQUIREMENTS
H. 1. HUMAN SUBJECTS
Research involving human subjects shall not be conducted under this contract until the protocol
developed in has been approved by DHHS, written notice of such approval has been
provided by the Contracting Officer, and the Contractor has provided to the Contracting Officer a
properly completed Optional Form 310 certifying Internal Review Board (IRB) review and approval of
the protocol. The human subject certification can be met by submission of the Contractor’s self
designated form, provided that it contains the information required by the Optional Form 310.
H. 2. HUMAN MATERIALS
It is understood that the acquisition and supply of all human specimen material (including fetal
material) used under this contract will be obtained by the Contractor in full compliance with
applicable State and Local laws and the provisions of the Uniform Anatomical Gift Act in the United
States and that no undue inducements, monetary or otherwise, will be offered to any person to
influence their donation of human material.
H. 3. ANIMAL WELFARE ASSURANCE
The Contractor shall obtain, prior to the start of any work under this contract, an approved Animal
Welfare Assurance from the Office of Protection from Research Risks (OPRR), Office of the Director,
NIH, as required by Section I-43-30 of the Public Health Service Policy on Humane Care and Use of
Laboratory Animals. The Contractor shall maintain such assurance for the duration of this contract,
and any subcontractors performing work under this contract involving the use of animals shall also
obtain and maintain an approved Animal Welfare Assurance.
H. 4. CONFIDENTIALITY OF INFORMATION
The following information is covered by HHSAR 352.224-70, confidentiality of Information (APR
1984):
[redacted]
H. 5. REVIEW AND APPROVAL
The Contractor shall not release any reports, manuscripts, press releases, or abstracts about the
work being performed under this contract without written approval in advance from the Government.
H. 6. IDENTIFICATION AND DISPOSITION OF DATA
The Contractor will be required to provide certain data generated under this contract to the
Department of Health and Human Services (DHHS). DHHS reserves the right to review any other data
determined by DHHS to be relevant to this contract. The contractor shall keep copies of all data
required by the Food and Drug Administration (FDA) relevant to this contract for the time specified
by the FDA.
H. 7. EPA ENERGY STAR REQUIREMENTS
In compliance with Executive Order 12845 (requiring Agencies to purchase energy efficient computer
equipment) all microcomputers, including personal computers, monitors, and printers that are
purchased using Government funds in performance of a contract shall be equipped with or meet the
energy efficient low-power standby feature as defined by the EPA Energy Star program unless the
equipment always meets
EPA Energy Star efficiency levels. The microcomputer, as configured with all components, must be
Energy Star compliant.
Page 15 of 27
This low-power feature must already be activated when the computer equipment is delivered to the
agency and be of equivalent functionality of similar power managed models. If the equipment will be
used on a local area network, the vendor must provide equipment that is fully compatible with the
network environment. In addition, the equipment will run commercial off-the-shelf software both
before and after recovery from its energy conservation mode.
H.8. REPORTING MATTERS INVOLVING FRAUD, WASTE AND ABUSE
Anyone who becomes aware of the existence or apparent existence of fraud, waste and abuse in DHHS
funded programs is encouraged to report such matters to the HHS Inspector General’s Office in
writing or on the Inspector General’s Hotline. The toll-free number is ▇-▇▇▇-▇▇▇-▇▇▇▇
(▇-▇▇▇-▇▇▇-▇▇▇▇). All telephone calls will be handled confidentially. The e-mail address is
▇▇▇▇▇@▇▇.▇▇▇▇.▇▇▇.
Office of Inspector General
Department of Health and Human Services
TIPS HOTLINE
P.O. Box 23489
Washington, DC 20026
Department of Health and Human Services
TIPS HOTLINE
P.O. Box 23489
Washington, DC 20026
H.9. ACKNOWLEDGMENT OF FEDERAL FUNDING
| A. | Section 507 of P.L. 104-208 mandates that contractors funded with Federal dollars, in whole
or in part, acknowledge Federal funding when issuing statements, press releases, requests for
proposals, bid solicitations and other documents. Contractors are required to state (1) the
percentage and dollar amounts of the total program or project costs financed with Federal
money, and (2) the percentage and dollar amount of the total costs financed by nongovernmental
sources. |
|
This requirement is in addition to the continuing requirement to provide an acknowledgment of
support and disclaimer on any publication reporting the results of a contract funded activity. |
||
| B. | Publication and Publicity |
|
The Contractor shall acknowledge the support of the Department of Health and Human Service,
Office of Public Health Emergency Preparedness, Office of Research and Development Coordination
whenever publicizing the work under this contract in any media by including an acknowledgment
substantially as follows: |
||
“This project has been funded in whole or in part with Federal funds from the Office of Public
Health Emergency Preparedness, Office of Public Health Emergency Medical Countermeasures, under
Contract No. HHSO100200700032C. |
||
| C. | Press Releases |
|
Pursuant to Section 508 of Public Law 109-49, the contractor shall clearly state, when issuing
statements, press releases, requests for proposals, bid solicitations and other documents
describing projects or programs funded in whole or in part with Federal money that: (1) the
percentage of the total costs of the program or project which will be financed with Federal
money; (2) the dollar amount of Federal funds for the project or program; and (3) the
percentage and dollar amount of the total costs of the project or program that will be financed
by nongovernmental sources. |
Page 16 of 27
H.10. NEEDLE EXCHANGE
Pursuant to Section 505 of Public Law 109-49, contract funds shall not be used to carry out any
program of distributing sterile needles or syringes for the hypodermic injection of any illegal
drug. Section 505, however, is subject to the condition stated in Section 506. Specifically,
Section 506 states that after March 31, 1998, a program for exchanging needles and syringes for
used hypodermic needles and syringes may be carried out in a community if: (1) the Secretary of
Health and Human Services determines that exchange projects are effective in preventing the spread
of HIV and do not encourage the use of illegal drugs; and (2) the project is operated in accordance
with criteria established by the Secretary for preventing the spread of HIV and for ensuring that
the project does not encourage the use of illegal drugs.
H.11 PRESS RELEASES
Pursuant to Section 508 of Public Law 109-49, the contractor shall clearly state, when issuing
statements, press releases, requests for proposals, bid solicitations and other documents
describing projects or programs funded in whole or in part with Federal money that: (1) the
percentage of the total costs of the program or project which will be financed with Federal money;
(2) the dollar amount of Federal funds for the project or program; and (3) the percentage and
dollar amount of the total costs of the project or program that will be financed by nongovernmental
sources.
H.12. PROHIBITION ON CONTRACTOR INVOLVEMENT WITH TERRORIST ACTIVITIES
The Contractor acknowledges that U.S. Executive Orders and Laws, including but not limited to
Executive Order 13224 and Public Law 107-56, prohibit transactions with, and the provisions of
resources and support to, individuals and organizations associated with terrorism. It is the legal
responsibility of the contractor to ensure compliance with these Executive Orders and Laws. This
clause must be included in all subcontracts issued under this contract.
H.13. MANUFACTURING STANDARDS
The Current Good Manufacturing Practice Regulations (cGMP) (21 CFR Parts 210-211) will be the
standard to be applied for manufacturing, processing and packing of this therapeutic product.
If at any time during the life of the contract, the Contractor fails to comply with cGMP in
the manufacturing, processing and packaging of this therapeutic product and such failure results in
a material adverse effect on the safety, purity or potency of this therapeutic product (a material
failure) as identified by CBER and CDER, the Contractor shall have thirty (30) calendar days from
the time such material failure is identified to cure such material failure. If the Contractor
fails to take such an action within the thirty (30) calendar day period, then the contract may be
terminated.
H.14. ANTI-LOBBYING PROVISIONS
The contractor is hereby notified of the restrictions on the use of Department of Health and Human
Service’s funding for lobbying of Federal, State and Local legislative bodies.
Section 1352 of Title 10, United States Code (Public Law 101-121, effective 12/23/89), among other
things, prohibits a recipient (and their subcontractors) of a Federal contract, grant, loan, or
cooperative agreement from using appropriated funds (other than profits from a federal contract) to
pay any person for influencing or attempting to influence an officer or employee of any agency, a
Member of Congress, an officer or employee of Congress, or an employee of a Member of Congress in
connection with any of the following covered Federal actions; the awarding of any Federal contract;
the making of any Federal grant;
the making of any Federal loan; the entering into of any cooperative agreement; or the modification
of any Federal contract, grant, loan, or cooperative agreement. For additional information of
prohibitions against lobbying activities see FAR Subpart 3.8 and FAR Clause 52.203-12.
Page 17 of 27
In addition, the current Department of Health and Human Services Appropriations Act provides that
no part of any appropriation contained in this Act shall be used, other than for normal and
recognized executive-legislative relationships, for publicity or propaganda purposes, for the
preparation, distribution, or use of any kit, pamphlet, booklet, publication, radio, television, or
video presentation designed to support, or defeat legislation pending before the Congress, or any
State or Local legislature except in presentation to the Congress, or any State or Local
legislative body itself.
The current Department of Health and Human Services Appropriations Act also provides that no part
of any appropriation contained in this Act shall be used to pay the salary or expenses of any
contract or grant recipient, or agent acting for such recipient, related to any activity designed
to influence legislation or appropriations pending before the Congress, or and State or Local
legislature.
H.15. POSSESSION, USE AND TRANSFER OF SELECTED BIOLOGICAL AGENTS OR TOXINS
The contractor shall not conduct work involving select agents or toxins under this contract until
it and any associated subcontractor(s) comply with the following:
For prime or subcontract awards to domestic institutions that possess, use, and/or transfer Select
Agents under this contract, the institution must comply with the provisions of 42 CFR part 73, 7
CFR part 331, and/or 9 CFR part 121
(▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇-▇▇-▇▇.▇▇▇), as required,
before using DHHS funds for research involving Select Agents. No DHHS funds can be used for
research involving Select Agents if the final registration certificate is denied.
For prime or subcontract awards to foreign institutions that possess, use, and/or transfer Select
Agents under this contract, before using DHHS funds for any work directly involving the Select
Agents, the foreign institution must provide information satisfactory to the DHHS that safety,
security, and training standards equivalent to those described in 42 CFR part 73, 7 CFR part 331,
and/or 9 CFR part 121 at:
(▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇-▇▇-▇▇.▇▇▇)
are in place and will be administered on behalf of all Select Agent work sponsored by these funds.
The process for making this determination includes inspection of the foreign laboratory facility
by an HHS representative. During this inspection, the foreign institution must provide the
following information: concise summaries of safety, security, and training plans; names of
individuals at the foreign institution who will have access to the Select Agents and procedures
for ensuring that only approved and appropriate individuals, in accordance with institution
procedures, will have access to the Select Agents under the contract; and copies of or links to
any applicable laws, regulations, policies, and procedures applicable to that institution for the
safe and secure possession, use, and/or transfer of select agents. An DHHS-chaired committee of
U.S. federal employees (including representatives of select DHHS grants/contracts and scientific
program management, CDC, Department of Justice and other federal intelligence agencies, and
Department of State) will ultimately assess the results of the laboratory facility inspection, and
the regulations, policies, and procedures of the foreign institution for equivalence to the U.S.
requirements described in 42 CFR part 73, 7 CFR part 331, and/or 9 CFR part 121
(▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇-▇▇-▇▇.▇▇▇). The committee
will provide recommendations to the OPHEMC Director, DHHS. The Director (or designee) will make
the approval decision and notify the Contracting Officer. The Contracting Officer will inform the
prime contractor of the approval status of the foreign institution. No DHHS funds can be used for
research involving Select Agents at a foreign institution until DHHS grants this approval.
Listings of HHS select agents and toxins, and overlap select agents or toxins as well as
information about the registration process for domestic institutions, are available on the Select
Agent Program Web site at
▇▇▇▇://▇▇▇.▇▇▇.▇▇▇/▇▇/▇▇▇/ and ▇▇▇▇://▇▇▇.▇▇▇.▇▇▇/▇▇/▇▇▇/▇▇▇▇/▇▇▇▇▇▇.▇▇▇.
Listings of USDA select agents and toxins as well as information about the registration process for
domestic institutions are available on the APHIS/USDA website at:
▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/▇▇▇▇▇.▇▇▇▇ and
▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇_▇▇▇▇▇.▇▇▇▇ .
For foreign institutions, see the NIAID Select Agent Award information:
▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇.▇▇▇/▇▇▇/▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇_▇▇▇▇▇▇▇▇▇▇.▇▇▇
▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇.▇▇▇/▇▇▇/▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇_▇▇▇▇▇▇▇▇▇▇.▇▇▇
Page 18 of 27
PART II — CONTRACT CLAUSES
SECTION I — CONTRACT CLAUSES
ARTICLE I.1. GENERAL CLAUSES FOR A COST-REIMBURSEMENT RESEARCH AND DEVELOPMENT CONTRACT — FAR
52.252-2, CLAUSES INCORPORATED BY REFERENCE (FEBRUARY 1998)
This contract incorporates the following clauses by reference, with the same force and effect as if
they were given in full text. Upon request, the Contracting Officer will make their full text
available. Also, the full text of a clause may be accessed electronically at this address:
▇▇▇▇://▇▇▇.▇▇▇▇▇▇▇▇▇▇▇.▇▇▇/▇▇▇▇/▇▇▇/▇▇▇▇▇.▇▇▇▇.
I.1. GENERAL CLAUSES
General Clauses for a Cost-Reimbursement Research and Development Contract
FAR
|
52.202-1 | Jul 2004 | Definitions | |||
FAR
|
52.203-3 | Apr 1984 | Gratuities (Over $100,000) | |||
FAR
|
52.203-5 | Apr 1984 | Covenant Against Contingent Fees (Over $100,000) | |||
FAR
|
52.203-6 | Sep 2006 | Restrictions on Subcontractor Sales to the Government (Over $100,000) | |||
FAR
|
52.203-7 | Jul 1995 | Anti-Kickback Procedures (Over $100,000) | |||
FAR
|
52.203-8 | Jan 1997 | Cancellation, Rescission, and Recovery of Funds for Illegal or Improper Activity (Over $100,000) | |||
FAR
|
52.203-10 | Jan 1997 | Price or Fee Adjustment for Illegal or Improper Activity (Over $100,000) | |||
FAR
|
52.203-12 | Sep 2003 | Limitation on Payments to Influence Certain Federal Transactions (Over $100,000) | |||
FAR
|
52.204-4 | Aug 2000 | Printed or Copied Double-Sided on Recycled Paper (Over $100,000) | |||
FAR
|
52.204-7 | Oct 2003 | Central Contractor Registration | |||
FAR
|
52.209-6 | Sep 2006 | Protecting the Government’s Interests When Subcontracting With Contractors Debarred, Suspended, or Proposed for Debarment (Over $25,000) | |||
FAR
|
52.215-2 | Jun 1999 | Audit and Records — Negotiation (Over $100,000) | |||
FAR
|
52.215-8 | Oct 1997 | Order of Precedence — Uniform Contract Format | |||
FAR
|
52.215-10 | Oct 1997 | Price Reduction for Defective Cost or Pricing Data | |||
FAR
|
52.215-12 | Oct 1997 | Subcontractor Cost or Pricing Data (Over $500,000) | |||
FAR
|
52.215-14 | Oct 1997 | Integrity of Unit Prices (Over $100,000) |
Page 19 of 27
FAR
|
52.215-15 | Oct 2004 | Pension Adjustments and Asset Reversions | |||
FAR
|
52.215-18 | Jul 2005 | Reversion or Adjustment of Plans for Post-Retirement Benefits (PRB) other than Pensions | |||
FAR
|
52.215-19 | Oct 1997 | Notification of Ownership Changes | |||
FAR
|
52.215-21 | Oct 1997 | Requirements for Cost or Pricing Data or Information Other Than Cost or Pricing Data — Modifications | |||
FAR
|
52.216-7 | Dec 2002 | Allowable Cost and Payment | |||
FAR
|
52.216-8 | Mar 1997 | Fixed Fee | |||
FAR
|
52.217-2 | Oct 1997 |
Cancellation Under Multi-Year Contracts | |||
FAR
|
52.219-8 | May 2004 | Utilization of Small Business Concerns (Over $100,000) | |||
FAR
|
52.219-9 | Sep 2006 | Small Business Subcontracting Plan (Over $500,000) | |||
FAR
|
52.219-16 | Jan 1999 | Liquidated Damages — Subcontracting Plan (Over $500,000) | |||
FAR
|
52.222-2 | Jul 1990 | Payment for Overtime Premium (Over $100,000) (Note: The dollar amount in paragraph (a) of this clause is $0 unless otherwise specified in the contract.) | |||
FAR
|
52.222-3 | Jun 2003 | Convict Labor | |||
FAR
|
52.222-21 | Feb 1999 | Prohibition of Segregated Facilities | |||
FAR
|
52.222-26 | Apr 2002 | Equal Opportunity | |||
FAR
|
52.222-35 | Sep 2006 | Equal Opportunity for Special Disabled Veterans, Veterans of the Vietnam Era, and Other Eligible Veterans | |||
FAR
|
52.222-36 | Jun 1998 | Affirmative Action for Workers with Disabilities | |||
FAR
|
52.222-37 | Sep 2006 | Employment Reports on Special Disabled Veterans, Veterans of the Vietnam Era, and Other Eligible Veterans | |||
FAR
|
52.223-6 | May 2001 | Drug-Free Workplace | |||
FAR
|
52.223-14 | Aug 2003 | Toxic Chemical Release Reporting (Over $100,000) | |||
FAR
|
52.225-1 | Jun 2003 | Buy American Act — Supplies | |||
FAR
|
52.225-13 | Feb 2006 | Restrictions on Certain Foreign Purchases | |||
FAR
|
52.227-1 | Jul 1995 | Authorization and Consent, Alternate I (Apr 1984) | |||
FAR
|
52.227-2 | Aug 1996 | Notice and Assistance Regarding Patent and Copyright Infringement (Over $100,000) | |||
FAR
|
52.227-11 | Jan 1997 | Patent Rights — Retention by the Contractor (Short Form) (Note: In accordance with FAR 27.303(a)(2), paragraph (f) is modified to include the requirements in FAR 27.303(a)(2)(i) through (iv). The frequency of reporting in (i) is annual. |
Page 20 of 27
FAR
|
52.227-14 | Jun 1987 | Rights in Data — General | |||
FAR
|
52.232-9 | Apr 1984 | Limitation on Withholding of Payments | |||
FAR
|
52.232-17 | Jun 1996 | Interest (Over $100,000) | |||
FAR
|
52.232-18 | Apr 1984 | Availability of Funds | |||
FAR
|
52.232-20 | Apr 1984 | Limitation of Cost (applies when contract is fully funded) | |||
FAR
|
52.232-22 | Apr 1984 | Limitation of Funds (applies when contract is incrementally funded) | |||
FAR
|
52.232-23 | Jan 1986 | Assignment of Claims | |||
FAR
|
52.232-25 | Oct 2003 | Prompt Payment, Alternate I (Feb 2002) | |||
FAR
|
52.232-33 | Oct 2003 | Payment by Electronic Funds Transfer—Central Contractor Registration | |||
FAR
|
52.233-1 | Jul 2002 | Disputes | |||
FAR
|
52.233-3 | Aug 1996 | Protest After Award, Alternate I (Jun 1985) | |||
FAR
|
52.233-4 | Oct 2004 | Applicable Law for Breach of Contract Claim | |||
FAR
|
52.242-1 | Apr 1984 | Notice of Intent to Disallow Costs | |||
FAR
|
52.242-3 | May 2001 | Penalties for Unallowable Costs (Over $650,000) | |||
FAR
|
52.242-4 | Jan 1997 | Certification of Final Indirect Costs | |||
FAR
|
52.242-13 | Jul 1995 | Bankruptcy (Over $100,000) | |||
FAR
|
52.243-2 | Aug 1987 | Changes — Cost Reimbursement, Alternate V (Apr 1984) | |||
FAR
|
52.244-2 | Aug 1998 | Subcontracts, Alternate II (Aug 1998) *If written consent to subcontract is required, the identified subcontracts are listed in ARTICLE B, Advance Understandings. | |||
FAR
|
52.244-5 | Dec 1996 | Competition in Subcontracting (Over $100,000) | |||
FAR
|
52.244-6 | Sep 2006 | Subcontracts for Commercial Items | |||
FAR
|
52.245-5 | May 2004 | Government Property (Cost-Reimbursement, Time and Material, or Labor-Hour Contract) | |||
FAR
|
52.246-23 | Feb 1997 | Limitation of Liability (Over $100,000) | |||
FAR
|
52.249-6 | May 2004 | Termination (Cost-Reimbursement) | |||
FAR
|
52.249-14 | Apr 1984 | Excusable Delays |
Page 21 of 27
FAR
|
52.253-1 | Jan 1991 | Computer Generated Forms | |||
HHSAR
|
352.202-1 | Jan 2001 | Definitions — with Alternate paragraph (h) (Jan 2001) | |||
HHSAR
|
352.216-72 | Oct 1990 | Additional Cost Principles | |||
HHSAR
|
352.228-7 | Dec 1991 | Insurance — Liability to Third Persons | |||
HHSAR
|
352.232-9 | Apr 1984 | Withholding of Contract Payments | |||
HHSAR
|
352.233-70 | Apr 1984 | Litigation and Claims | |||
HHSAR
|
352.242-71 | Apr 1984 | Final Decisions on Audit Findings | |||
HHSAR
|
352.270-5 | Apr 1984 | Key Personnel | |||
HHSAR
|
352.270-6 | Jul 1991 | Publications and Publicity | |||
HHSAR
|
352.270-7 | Jan 2001 | Paperwork Reduction Act |
I.2. AUTHORIZED SUBSTITUTIONS OF CLAUSES
ARTICLE I.1. of this SECTION is hereby modified as follows:
FAR Clauses 52.219-9, Small Business Subcontracting Plan (September 2006), and 52.219-16,
Liquidated Damages—Subcontracting Plan (January 1999) are deleted in their entirety.
FAR Clause 52.232-20, Limitation of Cost, is deleted in its entirety and FAR Clause 52.232-22,
Limitation of Funds (APRIL 1984) is substituted therefore. [Note: When this contract is fully
funded, FAR Clause 52.232-22, Limitation of Funds will no longer apply and FAR Clause 52.232-20,
Limitation of Cost will become applicable.]
I.3. ADDITIONAL CONTRACT CLAUSES
This contract incorporates the following clauses by reference, with the same force and effect, as
if they were given in full text. Upon request, the contracting officer will make their full text
available.
52.215-17 Waiver of Facilities Capital Cost of Money (October 1997)
52.243-2, Changes—Cost Reimbursement (August 1987)
Page 22 of 27
I.4 Additional Contract Clauses of SECTION I — Added in full text
52.222-39 Notification of Employee Rights Concerning Payment of Union Dues or Fees.
Notification of Employee Rights Concerning Payment of Union Dues or Fees (Dec 2004)
(a) Definition. As used in this clause—
“United States” means the 50 States, the District of Columbia, Puerto Rico, the Northern Mariana
Islands, American Samoa, Guam, the U.S. Virgin Islands, and Wake Island.
(b) Except as provided in paragraph (e) of this clause, during the term of this contract, the
Contractor shall post a notice, in the form of a poster, informing employees of their rights
concerning union membership and payment of union dues and fees, in conspicuous places in and about
all its plants and offices, including all places where notices to employees are customarily posted.
The notice shall include the following information (except that the information pertaining to
National Labor Relations Board shall not be included in notices posted in the plants or offices of
carriers subject to the Railway Labor Act, as amended (45 U.S.C. 151-188)).
Notice to Employees
Under Federal law, employees cannot be required to join a union or maintain membership in a union
in order to retain their jobs. Under certain conditions, the law permits a union and an employer to
enter into a union-security agreement requiring employees to pay uniform periodic dues and
initiation fees. However, employees who are not union members can object to the use of their
payments for certain purposes and can only be required to pay their share of union costs relating
to collective bargaining, contract administration, and grievance adjustment.
If you do not want to pay that portion of dues or fees used to support activities not related to
collective bargaining, contract administration, or grievance adjustment, you are entitled to an
appropriate reduction in your payment. If you believe that you have been required to pay dues or
fees used in part to support activities not related to collective bargaining, contract
administration, or grievance adjustment, you may be entitled to a refund and to an appropriate
reduction in future payments.
For further information concerning your rights, you may wish to contact the National Labor
Relations Board (NLRB) either at one of its Regional offices or at the following address or toll
free number:
National Labor Relations Board
Division of Information
▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇, ▇.▇.
Washington, DC 20570
▇-▇▇▇-▇▇▇-▇▇▇▇
▇-▇▇▇-▇▇▇-▇▇▇▇ (TTY)
To locate the nearest NLRB office, see NLRB’s website at ▇▇▇▇://▇▇▇.▇▇▇▇.▇▇▇.
Division of Information
▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇, ▇.▇.
Washington, DC 20570
▇-▇▇▇-▇▇▇-▇▇▇▇
▇-▇▇▇-▇▇▇-▇▇▇▇ (TTY)
To locate the nearest NLRB office, see NLRB’s website at ▇▇▇▇://▇▇▇.▇▇▇▇.▇▇▇.
(c) The Contractor shall comply with all provisions of Executive Order 13201 of February 17, 2001,
and related implementing regulations at 29 CFR Part 470, and orders of the Secretary of Labor.
(d) In the event that the Contractor does not comply with any of the requirements set forth in
paragraphs (b), (c), or (g), the Secretary may direct that this contract be cancelled, terminated,
or suspended in whole or in part, and declare the Contractor ineligible for further Government
contracts in accordance with procedures at 29 CFR Part 470, Subpart B—Compliance Evaluations,
Complaint Investigations and Enforcement Procedures. Such other sanctions or remedies may be
imposed as are provided by 29 CFR Part 470, which implements Executive Order 13201, or as are
otherwise provided by law.
(e) The requirement to post the employee notice in paragraph (b) does not apply to—
(1) Contractors and subcontractors that employ fewer than 15 persons;
(2) Contractor establishments or construction work sites where no union has been formally
recognized by the Contractor or certified as the exclusive bargaining representative of the
Contractor’s employees;
(3) Contractor establishments or construction work sites located in a jurisdiction named in the
definition of the United States in which the law of that jurisdiction forbids enforcement of
union-security agreements;
(4) Contractor facilities where upon the written request of the Contractor, the Department of Labor
Deputy Assistant Secretary for Labor-Management Programs has waived the posting requirements with
respect to any of the Contractor’s facilities if the Deputy Assistant Secretary finds that the
Contractor has demonstrated that—
(i) The facility is in all respects separate and distinct from activities of the Contractor related
to the performance of a contract; and
(ii) Such a waiver will not interfere with or impede the effectuation of the Executive order; or
(5) Work outside the United States that does not involve the recruitment or employment of workers
within the United States.
Page 23 of 27
(f) The Department of Labor publishes the official employee notice in two variations; one for
contractors covered by the Railway Labor Act and a second for all other contractors. The Contractor
shall—
(1) Obtain the required employee notice poster from the Division of Interpretations and Standards,
Office of Labor-Management Standards, U.S. Department of Labor, ▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇, ▇▇▇▇
▇-▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇, or from any field office of the Department’s Office of
Labor-Management Standards or Office of Federal Contract Compliance Programs;
(2) Download a copy of the poster from the Office of Labor-Management Standards website at
▇▇▇▇://▇▇▇.▇▇▇▇.▇▇▇.▇▇▇; or
(3) Reproduce and use exact duplicate copies of the Department of Labor’s official poster.
(g) The Contractor shall include the substance of this clause in every subcontract or purchase
order that exceeds the simplified acquisition threshold, entered into in connection with this
contract, unless exempted by the Department of Labor Deputy Assistant Secretary for
Labor-Management Programs on account of special circumstances in the national interest under
authority of 29 CFR 470.3(c). For indefinite quantity subcontracts, the Contractor shall include
the substance of this clause if the value of orders in any calendar year of the subcontract is
expected to exceed the simplified acquisition threshold. Pursuant to 29 CFR Part 470, Subpart
B—Compliance Evaluations, Complaint Investigations and Enforcement Procedures, the Secretary of
Labor may direct the Contractor to take such action in the enforcement of these regulations,
including the imposition of sanctions for noncompliance with respect to any such subcontract or
purchase order. If the Contractor becomes involved in litigation with a subcontractor or vendor, or
is threatened with such involvement, as a result of such direction, the Contractor may request the
United States, through the Secretary of Labor, to enter into such litigation to protect the
interests of the United States.
52.227-14, Rights in Data Alternate II (June 1987)
(g)(2) Notwithstanding paragraph (g)(1) of this clause, the contract may identify and
specify the delivery of limited rights data, or the Contracting Officer may require by written
request the delivery of limited rights data that has been withheld or would otherwise be
withholdable. If delivery of such data is so required, the Contractor may affix the following
“Limited Rights Notice” to the data and the Government will thereafter treat the data, subject
to the provisions of paragraphs (e) and (f) of this clause, in accordance with such Notice:
Limited Rights Notice (June 1987)
| (a) | These data are submitted with limited rights under Government Contract No.
HHSO10020070032C and subcontracts. These data may be reproduced and used by the
Government with the express limitation that they will not, without written permission
of the Contractor, be used for purposes of manufacture nor disclosed outside the
Government; except that the Government may disclose these data outside the Government
for the following purposes, if any; provided that the Government makes such disclosure
subject to prohibition against further use and disclosure: |
(i) Use (except for manufacture) by support service contractors.
| (b) | This Notice shall be marked on any reproduction of these data, in whole or in
part. |
(End of Clause)
Page 24 of 27
I. 5. Department of Health and Human Services Acquisition Regulations (HHSAR)
(48 CFR Chapter 3) Clauses: Full text of these clauses can be found at
▇▇▇▇://▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇/▇▇▇/▇▇▇▇▇.▇▇▇▇/
▇▇▇▇://▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇/▇▇▇/▇▇▇▇▇.▇▇▇▇/
352.223-70, Safety and Health (January 2001)
352.224-70, Confidentiality of Information (April 1984)
352.270-5, Key Personnel (April 1984)
352.270-8, Protection of Human Subjects (January 2001)
Note: The Office for Human Research Protections (OHRP), Office of the Secretary (OS), Department
of Health and Human Services (DHHS) is the office responsible for oversight of the Protection of
Human Subjects and should replace Office for Protection from Research Risks (OPRR), National
Institutes of Health (NIH) wherever it appears in this clause.
352.270-9, Care of Live Vertebrate Animals (January 2001)
Page 25 of 27
PART III — LIST OF DOCUMENTS, EXHIBITS AND OTHER ATTACHMENTS
SECTION J — LIST OF ATTACHMENTS
Invoice/Financing Request Instructions (1 page)
Report of Government owned Contractor held property (1 page)
Contractor defined milestones (December 11, 2006, 7 pages)
Page 26 of 27
SECTION K — REPRSENTATIONS AND CERTIFICATIONS
The following documents are incorporated by reference in this contract:
Annual Representations and Certifications completed and located at the Online Representations and
Certifications Application (ORCA) website. [This includes the changes if any identified in
paragraph (b) of the FAR provision 52.204-8, Annual Representations and Certifications, contained
in the contractor’s proposal.]
Page 27 of 27
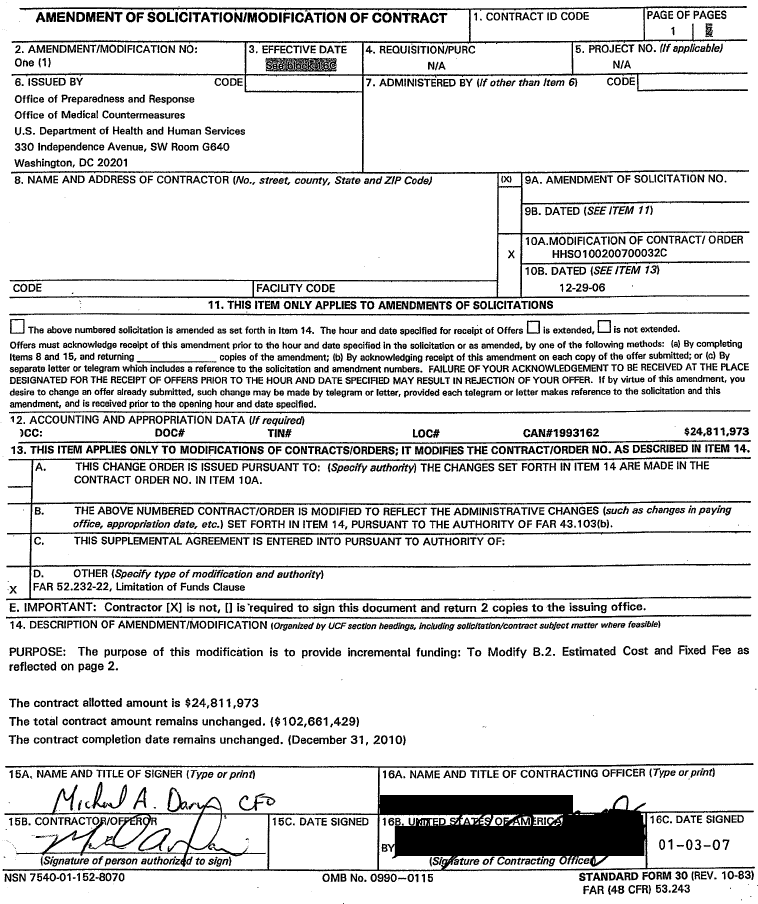
| AMENDMENT OF SOL ’CITATIONIMODIFICAT OF CON R)TRACTI 11 CONTRACT ID CODE PAGE OF PAGES 1 1 , —1 I I 2. AMENDMENTIMODIFICATION NO: 3 . EFFECTIVE DATE 4. REQUISITIONIPURC ‘ ‘ 5. PROJECT NO. flf applicable) Ohe (1) NIA NIA 6. ISSUED BY CODE 7. ADMINISTERED BY (If other than Item 6) CODEI Office of Preparedness and Response I Office of Medical Countermeasures I U.S. Department of Health and Human Services ▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ 8. NAME AND ADDRESS OF CONTRACTOR (No., street, county, State andZlP Code) 9A. AMENDMENT OF SOLICITATION NO. I 198. DATED (SEE ITEM I 1) 10A.MODIFICATION OF CONTRACT1 ORDER X HHS0100200700032C 10B. DATED (SEE ITEM 13) CODE . 1 FACILITY CODE 12-29-06 ‘ 11. THIS ITEM ONLY APPLIES TO AMENDMENTS OF SOLICITATIONS The above numbered solicitation is amended as set forth in Item 14. The hour and date specified for receia of Offers is extended, is not extended. Offers must acknowledge receipt of this amendment prior to the hour and date specified in the solicitation or as amended, by one of the following methods: (a) BY Completing Items 8 and 15, and returning copies of the amendment; (b) By acknowledging receipt of this amendment on each copy of the offer submitted: or (c) By separate letter or telegram which includes a reference to the solicitation and amendment numbers. FAILURE OF YOUR ACKNOWLEDGEMENT TO BE RECEIVED AT THE PLACE DESIGNATED FOR THE RECEIPT OF OFFERS PRIOR TO THE HOUR AND DATE SPECIFIED MAY RESULT IN REJECTION OF YOUR OFFER. If by virtue of this amendment, YOU desire to change an offer already submitted, such change may be made by telegram or letter, provided each telegram or letter makes reference to the Solicitation and this amendment, and is received prior to the opening hour and date specified. 12. ACCOUNTING AND APPROPRIATION DATA (Ifreauired) K C : DOC# TIN# LOC# CAN#1993162 $24,811,973 13. THlS ITEM APPLIES ONLY TO MODIFICATIONS OF CONTRACTSIORDERS; IT MODIFIES THE CONTRACTIORDER NO. AS DESCRIBED IN ITEM 14. A. THIS CHANGE ORDER IS ISSUED PURSUANT TO: (Specify authority) THE CHANGES SET FORTH IN ITEM 1 4 ARE MADE IN THE — CONTRACT ORDER NO. IN ITEM 10A. 1 16. THE ABOVE NUMBERED CONTRACTIORDER IS MODIFIED TO REFLECT THE ADMINISTRATIVE CHANGES (such as changes inpaying office, appropriation date, etc.) SET FORTH IN ITEM 14, PURSUANT TO THE AUTHORITY OF FAR 43.103(b). C. THlS SUPPLEMENTAL AGREEMENT IS ENTERED INTO PURSUANT TO AUTHORITY OF: D. OTHER (Specify type o fmodification and authority) IX FAR 52.232-22, Limitation of Funds Clause E. IMPORTANT: Contractor [XI is not, [I is’required t o sign this document and return 2 copies t o the issuing office. 1 4. DESCRlPTlON OF AMENDMENTIMODIFICATION (Organizedby UCF section headings, includhg so ioit tlortcontract subject matter where feasjble) PURPOSE: The purpose o f this modification is t o provide incremental funding: To Modify 8.2. Estimated Cost and Fixed Fee as reflected on page 2. The contract allotted amount is $24,811,973 The total contract amount remains unchanged. ($1 02,661,429) The contract completion date remains unchanged. (December 31, 2010) 15A, NAME AND TITLE OF SIGNER (Type or print) ( 16A. NAME AND TITLE OF CONTRACTING OFFICER (Type or,printl FAR (48 CFR) 53.243 |
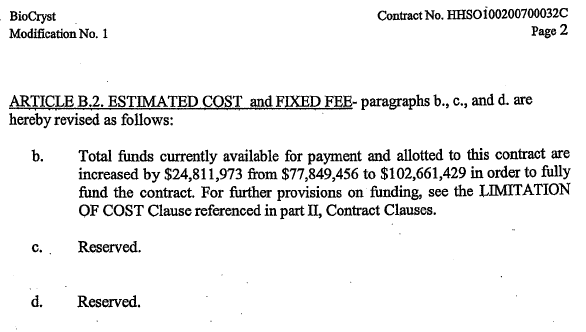
| BioCryst Contract No. 0100200700032C Modification No. 1 Page 2 mTICLE B12. ESTIMATED COST and FIXED FEE- paragraphs b., c., and d. are hereby revised as follows: b. Total funds currently available for payment and allotted to this contract are increased by $24,811,973 from $77,849,456 to $102,661,429 in order to fully fund the contract. For further provisions on funding, see the LIMITATION OF COST Clause referenced in part 11, Contract Clauses. c. . Reserved. d. Reserved. |
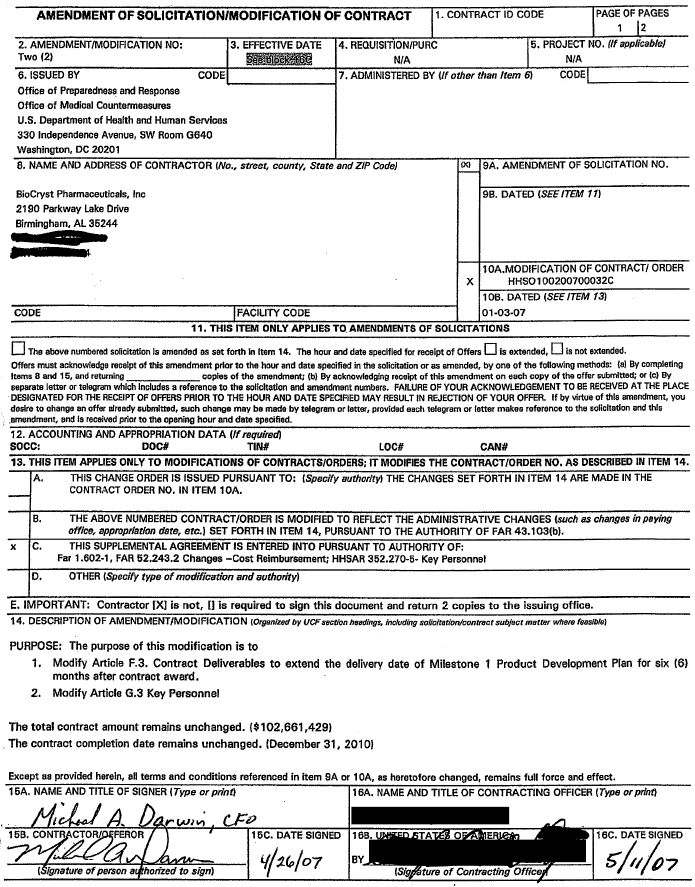
| AMENDMENT OF SOLlClTATlONlMODlFlCATlON OF CONTRACT 1. CONTRACT ID CODE PAGE OF PAGES 1 12 I I I 2. AMENDMENT/MODlFlCATlON NO: 13. EFFECTIVE DATE 14. REQUISITIONIPURC 15. PROJECT NO. (If applicablel Office of Medical Countermeasures U.S. Department of Health and Human Services ▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇ Room 0 6 4 0 Washington, DC 20201 8. NAME AND ADDRESS OF CONTRACTOR (No., sweet, county, Slate and ZIP Code) 9A. AMENDMENT OF SOLICITATION NO. BioCryst Pharmaceuticals, Inc 9B. DATED (SEE ITEM 7 71 21 9 0 Parkway Lake Drive Binninaharn. AL 36244 I 1OA.MODIFICATION OF CONTRACT1 ORDER X HHS0100200700032C 100. DATED (SEE ITEM 131 CODE FACILITY CODE 0 1-03-07 11. THlS ITEM ONLY APPLIES TO AMENDMENTS OF SOLICITATIONS The above numbered solicitation is amended as set foRfl in Item 14. The how and date specified for receipt of Offers is extended, Is not extended. Offers must acknowledge recelpt of this amendment prior to the hour and date specified in the solicitation or as amended, by one of the following methods: la1 BY completing Items 8 and 15, and returning copies of the amendment; (b) By acknowledging receipt of this amendment on each copy of the offer submitted; 01 (c) BY separate letter or telegram whlch includes a reference to the sollcitatlon and amendment numbers. FAILURE OF YOUR ACKNOWLEDGEMENT TO BE RECEIVED AT THE PLACE DESIGNATED FOR M E RECEIPT OF OFFERS PRIOR TO THE HOUR AND DATE SPECIFIED MAY RESULT IN REJECTION OF YOUR OFFER. If by virtue of this amendment, You desire to change an offer already submlmd, such change may be made by telegram or letter, provided each telegram or letter makes reference to the solicitatlon and thls .amendment, end is received prior to the opening hour and dam specified. 12. ACCOUNTING AND APPROPRIATION DATA (If required) SOCC:-. -. DOC#- — -. TIN#...... LOC#— CAN#-. . .- . . 13. THlS ITEM APPLIES ONLY TO MODIFICATIONS OF CONTRACTSIORDERS; IT MODIFIES THE CONTRACTIORDER NO. AS DESCRIBED IN ITEM 14. IA. THlS CHANGE ORDER IS ISSUED PURSUANT TO: (Specify authorifyl THE CHANGES SET FORTH I N ITEM 14 ARE MADE IN THE 4 CONTRACT ORDER NO. IN ITEM IOA. B. THE ABOVE NUMBERED CONTRACTIORDER IS MODIRED TO REFLECT THE ADMINISTRATIVE CHANGES (such as changes in paying offlce, approprlarlon date, etc.1 SET FORTH IN ITEM 14, PURSUANT TO THE AUTHORITY OF FAR 43.103(bl. x C. THlS SUPPLEMENTAL AGREEMENT IS ENTERED INTO PURSUANT TO AUTHORITY OF: Far 1.602-1, FAR 52.243.2 Changes -Cost Reimbursement; HHSAR 352.270-5- Key Personnel D. OTHER (Specify type of modification and authority) E. IMPORTANT: Contractor [XI is not, 11 is required to sign this document and return 2 copies to the issuing office. 14. DESCRIPTION OF AMENDMENT/MODIFICATION (Organizedby UCFsection heedings, inc/▇▇▇▇▇ solicitarion/contmctsubjact matter when, feasible) PURPOSE: The purpose of this modification is to 1. Modify Article F.3. Contract Deliverables to extend the delivery date of Milestone 1 Product Development Plan for six (6) months after contract award. 2. Modify Article 0.3 Key Personnel The total contract amount remains unchanged. ($102,661,429) The contract completion date remains unchanged. (December 31, 2010) Except as provided herein, all terms and conditions referenced in item 9A or 10A, as heretofore changed, remains full force and effect. 16A. NAME AND TITLE OF SIGNER (Type orprint) 116A. NAME AND TITLE OF CONTRACTING OFFICER (Type orpdnt) $Pure Compliance (RR) Centerhead |

| NSN 7540-01-1 52-8070 OMB NO. 0990-01 15 STANDARD FORM 30 (REV. 10-83) Article F.3 Contract Deliverables, are hereby revised as follows Milestones Deliverable Quantity Due Date 1 Product Development Plan Original -C.O. Six (6) months after (milestone 1) 2 Copies -P.0 contract award 1 Electronic Copy -P.0 Article 6.3 Key Personnel, are hereby revised as follows: |

