LICENSE AGREEMENT
Exhibit 10.51
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
This LICENSE AGREEMENT (the “Agreement”) is made and effective as of either the date of execution by the last Party to sign below (the “Effective Date”), by and between EB Pharma, LLC.,a company organized and existing under the laws of the State of Delaware having a business address at ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇▇, ▇▇ ▇▇▇▇▇ (“EBP”), and ▇▇▇▇▇▇▇ Pharmaceutica NV, a company organized and existing under the laws of Belgium having a business address at ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇ (“▇▇▇▇▇▇▇”). EBP and ▇▇▇▇▇▇▇ are each referred to individually as a “Party” and together as the “Parties.”
RECITALS
WHEREAS, EBP has experience in researching and developing antiviral agents, including agents active against novel targets in the treatment of hepatitis;
WHEREAS, ▇▇▇▇▇▇▇ owns, directly and through its Affiliates, certain rights relating to its proprietary compounds known as tipifarnib (also known as R115777) and a related proprietary back-up compound (also known as R208176); and
WHEREAS, EBP wishes to obtain from ▇▇▇▇▇▇▇ certain rights to develop and commercialize tipifarnib for human use in the field of virology, and ▇▇▇▇▇▇▇ is willing to grant such rights in accordance with the terms and conditions of this Agreement.
NOW THEREFORE, in consideration of the mutual covenants and agreements contained herein, the Parties agree as follows:
1. DEFINITIONS AND INTERPRETATION
1.1 Definitions. Unless the context otherwise requires, the terms in this Agreement with initial letters capitalized, shall have the meanings described below, or the meaning as designated in the indicated places throughout this Agreement.
“AAA” means the American Arbitration Association.
“Accounting Standards” means Generally Accepted Accounting Principles in the United States or the International Financial Reporting Standards, as appropriate, as generally and consistently applied in compliance with Applicable Laws throughout the relevant Party’s organization at the relevant time.
“Affiliate” means, in reference to a particular Party, any corporation or other entity that directly or indirectly controls, is controlled by, or is under common control with such Party. For purposes of this definition, “control” or “controlled” means ownership, directly or indirectly, of more than fifty percent (50%) of the shares of stock entitled to vote for the election of directors in the case of a corporation, or more than fifty percent (50%) of the equity interest in the case of any other type of legal entity (or if the jurisdiction where such corporation or other entity is
Page 1 of 60
domiciled prohibits foreign ownership of such entity, the maximum foreign ownership interest permitted under such laws, provided that such ownership interest provides actual control over such entity), status as a general partner in any partnership, or any other arrangement whereby an entity controls or has the right to control the board of directors or equivalent governing body of the entity.
“Alliance Manager” shall have the meaning set forth in Section 3.2.
“Applicable Laws” means the applicable provisions of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidance, ordinances, judgments, decrees, directives, injunctions, orders, permits (including Marketing Authorizations) of or from any court, arbitrator, Regulatory Authority or governmental agency or authority having jurisdiction over or related to the subject item, including to the FCPA, Export Control Laws, and other laws and regulations pertaining to domestic or international corruption, commercial bribery, fraud, embezzlement, or money-laundering.
“EBP Indemnified Party” shall have the meaning set out in Section 12.2.
“EBP Patent Rights” means all Development Program Patent Rights Controlled by EBP or any of its Affiliates during the Term that include any claim Covering any Reverted Product or Compound therein for which ▇▇▇▇▇▇▇ exercises its option rights under Section 15.2(b), use of such a Reverted Product or a Compound therein in the Field, formulation, preparation or manufacture of such a Reverted Product or Compound therein, or material for formulating, preparing or manufacturing such a Reverted Product or Compound therein. For the sake of clarity, Eiger Patent Rights include all related Patent Rights arising in the course of Prosecution of the foregoing Patent Rights.
“EBP Sublicensee” means any of EBP’s Affiliates or any Third Party licensee or sublicensee of rights granted by ▇▇▇▇▇▇▇ to EBP under this Agreement, but not including any Third Party to the extent that it functions as a distributor.
“Bankruptcy” means, with respect to a Party, that: (a) the Party has been declared insolvent or bankrupt by a court of competent jurisdiction; or (b) a voluntary or involuntary petition in bankruptcy is filed in any court of competent jurisdiction against the Party and such petition has not dismissed within ninety (90) days after filing; or (c) the Party has made or executed an assignment of substantially all of its assets for the benefit of creditors.
“Bioequivalent” means, with respect one drug substance (or active pharmaceutical ingredient) contained in one pharmaceutical product in reference to the drug substance (or active pharmaceutical ingredient) of another pharmaceutical product, that: (i) the two substances are pharmaceutically equivalent to each other and their bioavailabilities (rate and extent of availability) after administration in the same molar dose are similar to such a degree that their
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 2 of 60
effects, with respect to both efficacy and safety, can be expected to be essentially the same; or (ii) the two substances are or would be recognized by a Regulatory Authority as being biologically equivalent in vivo.
“Breaching Party” shall have the meaning set out in Section 14.2.
“Business Day” means any day, other than Saturday or Sunday, on which the banks in New York, New York and San Francisco, California are generally open for business.
“Claims” shall have the meaning set out in Section 13.1.
“Combination Product” means (a) any Product containing or comprising a Compound and at least one (1) active ingredient that is not a Compound; or (b) any combination of a Product and another pharmaceutical product containing or comprising at least one (1) active ingredient that is not a Compound where the Product and such other product are not formulated together but are sold together and invoiced as one product.
“Commercialize” means, in reference to a Product, performing any activities directed to marketing, promoting, offering for sale, or selling a Product for use in the Field, including detailing and medical affairs activities, and distribution and importation activities in support thereof.
“Commercially Reasonable Efforts” means the carrying out of obligations or tasks in a commercially diligent manner consistent with the efforts that a similarly situated biotechnology company in the pharmaceutical industry would reasonably devote to a research, development or marketing program owned by such company or to which such company has exclusive rights, of similar market potential and at a similar stage of development, based on conditions then prevailing, and taking into account efficacy, safety, regulatory authority approved labeling, the competitiveness of alternative products in the marketplace, the patent and other proprietary position of the products, ability to finance the program, medical and clinical considerations, the likelihood of regulatory approval given the regulatory structure involved, the profitability of the products, including the royalties payable to licensors of patent or other rights, and the costs of development, manufacture and marketing.
“Compound” means: (a) the compound known as R115777 or tipifarnib, which has the structure shown in Exhibit 1, or the compound known as R208176, which has the structure shown in Exhibit 1; or (b) or a Bioequivalent of either such compound (such as a pharmaceutical salt, acid, base, hydrate, solvate, ester, polymorph, or stereoisomer thereof); or (c) an active metabolite, prodrug, or radiolabeled form of any of the foregoing defined in clause (a) or (b).
“Confidential Information” means any: (a) Know-How or other proprietary or unpublished business, scientific, technical, formulation, process, manufacturing, clinical, non-clinical, regulatory, marketing, financial or commercial information or data, which is generated by or on behalf of a Party or which one Party or any of its Affiliates has supplied or otherwise
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 3 of 60
made available to the other Party either during the Term for purposes contemplated by this Agreement or pursuant to the Confidentiality Agreement, whether made available orally, in writing, or in electronic form, including information comprising or relating to concepts, discoveries, inventions, data, designs or formulae in relation to this Agreement; or (b) sample of any compound, reagent, biological specimen, or other material which one Party or any of its Affiliates has supplied or otherwise made available to the other Party during the Term of this Agreement for purposes contemplated hereunder.
“Confidentiality Agreement” means the Confidential Disclosure Agreement between ▇▇▇▇▇▇▇ Research & Development, LLC (an Affiliate of ▇▇▇▇▇▇▇) and Eiger BioPharmaceuticals, Inc. dated June 12, 2014.
“Control” (and, with correlative meaning, “Controlled”) means, with respect to any Know-How, Patent Rights or other intellectual property rights, the legal authority or right (whether by ownership, license or otherwise, but without taking into account any rights granted by one Party to the other Party under the terms of this Agreement) of a Party to grant access, a license or a sublicense of or under Know-How, Patent Rights, or intellectual property rights to the other Party, or to otherwise disclose proprietary or trade secret information to the other Party, without breaching the terms of any agreement with a Third Party.
“Cover” means, with respect to a claim of any Patent Rights in reference to a specified invention or technology, reading on, or literally encompassing such invention or technology under principles of applicable patent law, whether generically or specifically.
“Date of Delivery” shall have the meaning set out in Section 2.2(b).
“Develop”means, in reference to a Product, performing any Pre-Phase I research, clinical trials (including Phase I Studies, Phase II Studies, Phase III Studies, and post-marketing studies), and other activities to study a drug candidate or product and develop it toward approval, and to maintain approval, for marketing or Commercialization of the Product in the Field, including toxicology and ADME tests, analytical method development, stability testing, process development and improvement, process validation, process scale-up, formulation development, delivery system development, quality assurance and quality control development, statistical analysis, pre- and post-approval clinical studies or trials, regulatory affairs, and regulatory activities.
“Development Plan” means the written plan of activities to be performed by or on behalf of EBP hereunder to Develop any Licensed Product for use in the Field, as such plan may be supplemented or otherwise amended from time to time.
“Development Program” means the activities of either or both of the Parties conducted hereunder after the Effective Date in Developing any Products for use in the Field.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 4 of 60
“Development Program Invention” means an invention (whether or not patentable) arising in the Development Program directly from any Development activities performed by or on behalf of EBP hereunder, which invention is necessary or useful for the Manufacturing or Development of any Compound or Product, or for the Commercialization of any Product, including any invention made in the Development Program pertaining to the Manufacture, preparation, formulation, administration, delivery, dosing, or use in the Field of any Compound or Product.
“Development Program IP” means the Development Program Know-How and Development Program Patent Rights, collectively.
“Development Program Know-How” means any and all Know-How generated or developed in the Development Program from any Development activities performed by or on behalf of EBP hereunder, which Know-How relates to any Compound or Product, including for purposes of illustration: any Development Program Inventions; clinical trial data or other information relating to any form of any Compound or Product, any method of using any Compound or Product, any process or material for Manufacturing, formulating, or delivering any Compound or Product, the use of any Compound in any Combination Product, any companion diagnostic for use in Developing or Commercializing a Product in the Field, any material or process for making any Compound or Product, any method of using, testing, or characterizing any Compound or Product; and any data and other information contained in any regulatory filings relating to any Product.
“Development Program Patent Right” means any Patent Right filed after the Effective Date, and Controlled by EBP, that includes (as filed or at any other time during its pendency in a Patent Office) any claim Covering (generally or specifically) any Development Program Invention. For purposes of illustration, exemplary Development Program Patent Rights may include one or more claims Covering any Compound or Product form, any method of using any Compound or Product, any process or material for manufacturing, formulating, or delivering any Compound or Product, any Combination Product to the extent it directly relates to a Compound hereunder (excluding, for the avoidance of doubt, Patent Rights directed to other active ingredients alone), or any companion diagnostic for use in Commercializing any Product in the Field.
“Dispute” means any dispute, claim, or controversy arising from or regarding this Agreement, including the interpretation, application, breach, termination or validity of any provision hereof.
“Effective Date” or “Execution Date”means the last date of execution by the Parties hereto.
“EMA” means the European Medicines Agency and any successor thereto.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 5 of 60
“Excluded Claim” means a dispute, controversy or claim that concerns (i) the validity, enforceability or infringement of a patent, trademark or copyright; or (ii) any antitrust, anti-monopoly or competition law or regulation, whether or not statutory.
“Exercise Notice” shall have the meaning set out in Section 2.2(c).
“Existing Third Party Agreements” means the agreements between ▇▇▇▇▇▇▇ or an Affiliate and a Third Party that are listed in Exhibit 5, as such agreements and Exhibit may be amended from time to time. For clarity, the Existing Third Party Agreements exclude the UT License.
“Export Control Laws” means all applicable U.S. laws and regulations relating to (a) sanctions and embargoes imposed by the Office of Foreign Assets Control of the U.S. Department of Treasury or (b) the export or re-export of commodities, technologies, or services, including, but not limited to, the Export Administration Act of 1979, 24 U.S.C. §§ 2401-2420, the International Emergency Economic Powers Act, 50 U.S.C. §§ 1701-1706, the Trading with the Enemy Act, 50 U.S.C. §§ 1 et. seq., the Arms Export Control Act, 22 U.S.C. §§ 2778 and 2779, and the International Boycott Provisions of Section 999 of the U.S. Internal Revenue Code of 1986 (as amended).
“FCPA” means the U.S. Foreign Corrupt Practices Act (15 U.S.C. Section 78dd-1, et. seq.) as amended.
“FDA” means the United States Food and Drug Administration and any successor thereto.
“Field” means all therapeutic and diagnostic uses in humans, for including the prevention, treatment, control or diagnosis of any human virology diseases, disorders or medical conditions, excluding any oncology diseases.
“First Commercial Sale” means the first arm’s length sale of a Product in a country in the Territory to a Third Party following receipt of Marketing Authorization in such country, if such Marketing Authorization is required.
“Generic Product” means, with respect to a Product, any pharmaceutical product (a) that is sold by a Person other than a Party or its Affiliates, or any licensee of such Party or its Affiliates, and who did not purchase such product in a chain of distribution that included such Party or its Affiliate or licensee of either of the foregoing, (b) contains the same Compound as such Product, and (c) whose Marketing Authorization Application is approved by a Regulatory Authority in reliance, in whole or in part, on the prior approval (or on safety or efficacy data submitted in support of the prior approval or on the finding by a Regulatory Authority of the safety or efficacy) of such Product, including any product authorized for sale (i) in the U.S. pursuant to Section 505(b)(2) or Section 505(j) of the Act (21 U.S.C. 355(b)(2) or 355(j), respectively), (ii) in the EU pursuant to a provision of Articles 10, 10a or 10b of Parliament and
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 6 of 60
Council Directive 2001/83/EC as amended (including an application under Article 6.1 of Parliament and Council Regulation (EC) No 726/2004 that relies for its content on any such provision) or (iii) in any other country or jurisdiction pursuant to all equivalents of such provisions.
“Good Clinical Practice” or “GCP” means the then-current good clinical practice standards applicable to the clinical Development of a Product under Applicable Law, including ICH guidelines, or in the event such standards are less stringent than the current U.S. Good Clinical Practice, then such term shall mean the then-current U.S. Good Clinical Practice.
“Good Laboratory Practice” or “GLP” means the then-current good laboratory practice standards applicable to the Development of a Product under Applicable Law, including 21 C.F.R. Part 58, or in the event such standards are less stringent than the current U.S. Good Laboratory Practice, then such term shall mean current U.S. Good Laboratory Practice.
“Good Manufacturing Practice” or “GMP” means the then-current good manufacturing practice standards applicable to the Manufacturing of a Product under Applicable Law, including 21 C.F.R. parts 210 and 211 and all applicable FDA rules, regulations, orders and guidances, or in the event such standards are less stringent than the current U.S. Good Manufacturing Practice, then such term shall mean the then-current U.S. Good Manufacturing Practice.
“IND”means an investigational new drug application filed with the FDA or the corresponding application filed with the Regulatory Authority in any other country, for authorization to proceed with the clinical investigation of a Product in any country or group of countries, as defined in the Applicable Laws.
“Indication” means an application or use set forth in labeling (including any package insert) for a Product as approved by a Regulatory Authority, identifying a specific therapeutic or prophylactic indication for which the Product may be used, where the approval is based on data from a pivotal clinical trial in the Development Program. For clarity, an Indication may be the initial one or a later-approved one such as for an expanded or additional patient population, or for using the Product in combination with another treatment or drug product.
“Indemnified Losses” shall have the meaning set out in Section 12.1.
“Indemnified Party” shall have the meaning set out in Section 12.3(a).
“Indemnifying Party” shall have the meaning set out in Section 12.3(a).
“▇▇▇▇▇▇▇ Indemnified Party” shall have the meaning set out in Section 12.1.
“▇▇▇▇▇▇▇ IP” means the ▇▇▇▇▇▇▇ Patent Rights and ▇▇▇▇▇▇▇ Know-How.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 7 of 60
“▇▇▇▇▇▇▇ Know-How” means the Know-How Controlled by ▇▇▇▇▇▇▇ as of the Effective Date that is specific to any Compound and contained in the records identified in Exhibit 3, as such Exhibit may be amended from time to time including such Know-How pertaining to: processes; techniques; toxicological, pharmacological, clinical, and chemical data; specifications; medical uses; adverse reactions; and manufacture and quality control methods.
“▇▇▇▇▇▇▇ Patent Rights” means the Patent Rights Controlled by ▇▇▇▇▇▇▇ identified in Exhibit 2(A) and Exhibit 2(B) as updated pursuant to Section 8.2(b), and any Patent Rights related thereto Controlled by ▇▇▇▇▇▇▇ that are filed or issued after the Effective Date.
“Joint Development Committee” or “JDC” means a joint committee established by the Parties pursuant to Section 3.3 to monitor and discuss Development of Product hereunder.
“Know-How” means all technical information, know-how and data, including: inventions, discoveries, trade secrets, specifications, instructions, processes, formulae, materials, expertise and other technology applicable to formulations, compositions or products or to their manufacture, development, registration, use or marketing or to methods of assaying or testing them or processes for their manufacture, formulations containing them or compositions incorporating or comprising them, and including all biological, chemical, pharmacological, biochemical, toxicological, pharmaceutical, physical and analytical, safety, quality control, manufacturing, preclinical and clinical data, instructions, processes, formulae, expertise and information, relevant to the development, manufacture, use or sale of and/or which may be useful in studying, testing, developing, producing or formulating products, or intermediates for the synthesis thereof.
“MAA” means an application for the authorization for marketing of a Product in any country or group of countries outside the United States, and all supplements, including all documents, data and other information concerning the Product, as defined in the Applicable Laws and filed with the Regulatory Authority of a given country or group of countries.
“Major Market Country” means each of the following countries: France, Germany, Italy, Spain, the United Kingdom, and the United States (including their respective territories and possessions).
“Manufacturing” means, in reference to a Product, performing any activities to manufacture the Product into final form for end use in the Field, including producing intermediates or building blocks used to manufacture the Compound of the Product, manufacturing such intermediates or building blocks into Compound (e.g., in bulk form), formulating the Compound into Product in finished dosage form, filling, finishing, packaging, labeling, performing quality assurance testing and release, and shipping and storing the packaged Product.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 8 of 60
“Marketing Authorization” means the grant of any and all approvals (including supplements, amendments, pre- and post-approvals, pricing and reimbursement approvals), licenses, registrations or authorizations of any national, supra-national (e.g., the European Commission or the Council of the European Union), regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, that are necessary for the manufacture, distribution, use and sale of a Product in a regulatory jurisdiction, including where required, pricing and reimbursement approvals.
“NDA” means a new drug application and all supplements filed with the FDA, including all documents, data and other information concerning a Product which are necessary for, or included in, a Marketing Authorization to use, sell, supply and market the Product in the United States.
“Net Sales” means the gross amounts invoiced on sales, or gross operating revenues earned for other commercial dispositions, of a Product by EB or any EB Sublicensee to a Third Party purchaser that is not an EB Sublicensee in an arms-length transaction, less the following customary deductions, determined in accordance with Accounting Standards, to the extent specifically and solely allocated to such Product and actually taken, paid, accrued, allowed, included or allocated based on good faith estimates in the gross sales prices with respect to such sales (and consistently applied as set forth below):
(a) normal and customary trade, cash and/or quantity discounts, allowances (including wholesaler allowances), and credits allowed or paid (including for returned, damaged or expired Product), in the form of deductions or credits actually allowed or fees actually paid with respect to sales of such Product (to the extent not already reflected in the amount invoiced) excluding commissions for commercialization;
(b) excise taxes, use taxes, tariffs, sales taxes and customs duties, and/or other government charges imposed on the sale of Product to the extent included in the price and separately itemized on the invoice price (but specifically excluding, for clarity, any income taxes assessed against the income arising from such sale) (including VAT, but only to the extent that such VAT taxes are not reimbursable or refundable);
(c) outbound freight, shipment and insurance costs to the extent included in the price and separately itemized on the invoice price;
(d) compulsory payments and cash rebates related to the sales of such Product paid to a governmental authority (or agent thereof) pursuant to Applicable Laws by reason of any national or local health insurance program or similar program, to the extent allowed and taken; including government levied fees as a result of healthcare reform policies;
(e) retroactive price reductions, credits or allowances actually granted upon rejections or returns of Product, including for recalls or damaged good and billing errors; and
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 9 of 60
(f) rebates, chargebacks, and discounts (or equivalent thereof) actually granted to managed health care organizations, pharmacy benefit managers (or equivalent thereof), federal, state/provincial, local or other governments, or their agencies or purchasers, reimbursers, or trade customers.
All aforementioned deductions shall only be allowable to the extent they are commercially reasonable and shall be determined, on a country-by-country basis, as incurred in the ordinary course of business in type and amount consistent with EB or the EB Sublicensee’s (as the case may be) business practices consistently applied across its product lines and in accordance with Accounting Standards and verifiable based on its sales reporting system. All such discounts, allowances, credits, rebates, and other deductions shall be fairly and equitably allocated to Product and other products of ▇▇▇▇▇▇▇ or the ▇▇▇▇▇▇▇ Sublicensee such that Product does not bear a disproportionate portion of such deductions.
In the event Product is sold as a Combination Product and the Third Party customer receives a specific discount for such “bundling” of products (for clarity, this situation describes bundling of two or more separate products, each in finished dosage form, and not a fixed combination of two active pharmaceutical ingredients), the Net Sales of such Combination Product, for the purposes of determining royalty payments due hereunder, shall be determined by multiplying the relevant Net Sales by the fraction A/(A+B), where A is the weighted (by sales volume) average sale price in a particular country of the Product in the previous Calendar Year when sold separately and B is the weighted average sale price in that country in the previous Calendar Year of the other product sold separately. In the event that such average sale price cannot be determined for either the Product or the other product it has been sold with, in combination, (1) for purposes of determining any royalties due hereunder, the bundling discount granted shall be considered as having been granted in its entirety with respect to the other product only and shall not be applied to the sales of any Product or (2) Net Sales for purposes of determining royalties due shall be multiplied by an adjustment factor which will be the fraction equal to one divided by the number of active ingredients in such Combination Product.
“Non-Breaching Party” shall have the meaning set out in Section 14.2.
“Option” shall have the meaning set forth in Section 2.2(a).
“Option Term” shall have the meaning set forth in Section 2.2(a).
“Paragraph IV Certification” shall have the meaning set forth in Section 8.3(a).
“Patent Expenses” means the actual out-of-pocket fees, expenses and disbursements (including payments made to Third Party agents) paid by a Party to any Third Party such as its outside patent counsel or agent, or any Patent Offices, in connection with the Prosecution of particular ▇▇▇▇▇▇▇ Patent Rights, including the costs of patent interference and opposition proceedings, reissues, and reexaminations.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 10 of 60
“Patent Office” means the United States Patent and Trademark Office, European Patent Office, or other government agency or office responsible for the examination of patent applications or granting of patents in a country, region, or supra-national jurisdiction.
“Patent Rights” means, with respect to a particular invention, any and all original (priority-establishing) patents and patent applications filed anywhere in the world including any claim covering the invention, including provisional and nonprovisional applications, and all related applications thereafter filed including any claim covering such invention or including a common priority right, including any continuations, continuations-in-part, divisional and substitute applications, any patents issued or granted from any such patent applications, and any reissues, renewals, reexaminations, extensions (including by virtue of any supplementary protection certificates) of any such patents, and any confirmation patents, inventor’s certificates or registration patents or patents of addition based on any such patents, and all foreign counterparts or equivalents in any country or jurisdiction of any of the foregoing.
“Patent Term Extension” means an extension of the term of any issued patent, or a right of protection equivalent to such an extension, granted under the U.S. Drug Price Competition and Patent Term Restoration Act of 1984, the Supplementary Certificate of Protection of the member states of the EU, or another similar law or regulation in any other country or jurisdiction. For clarity, a pediatric extension extending the term of any patent shall not be deemed a Patent Term Extension.
“POC Data Package” means a package of materials comprising copies of written reports providing all raw data (excluding, for the avoidance of doubt, any private patient data or any other information that cannot be provided under Applicable Law) from the POC Trial in Eiger’ possession and Control and other information, including summaries, analyses, findings, conclusions and other results from such clinical study in EBP possession and Control.
“POC Trial” means a Phase II Study of the Compound tipifarnib in patients for a hepatitis Indication in the Field, as more fully described in the Development Plan.
“Phase I Study” means a study in humans which provides for the first introduction into humans of a product, conducted in normal volunteers or patients to generate information on product safety, tolerability, pharmacological activity or pharmacokinetics, as more fully defined in Federal Regulation 21 C.F.R. §312.21(a) and its foreign equivalents.
“Phase II Study” means a study in humans of the safety, dose ranging and efficacy of a Product, which is prospectively designed to generate sufficient data (if successful) to commence a Phase III Study or to file for accelerated approval, as further defined in Federal Regulation 21 C.F.R. §312.21(b) and its foreign equivalents.
“Phase III Study” means a pivotal study in humans of the efficacy and safety of a Product, which is prospectively designed to demonstrate statistically whether such product is
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 11 of 60
effective and safe for use in a particular indication in a manner sufficient to file an NDA or MAA to obtain regulatory approval to market the product, as further defined in Federal Regulation 21 C.F.R. §312.21(c) and its foreign equivalents.
“Pre-Phase I” means the initial portion of a development program prior to initiation of a Phase I Study, which starts with the selection of a NME and includes initiation of GMP scale-up activities and GLP toxicological studies. For illustrative purposes, Pre-Phase I development activities typically include toxicological (full-scale GLP toxicology for obtaining approval from a Regulatory Authority to administer Product to humans in clinical trials), pharmacological and any other studies required for filing an IND, as well as Product formulation and manufacturing development necessary to obtain the permission of Regulatory Authorities to begin a Phase I Study.
“Product” means a product containing or comprising a Compound, alone or together with one or more active or inactive ingredients, including any such product in the form of a preparation, kit, article of manufacture, composition of matter, material, formulation, or dosage or administration form.
“Prosecuting” means, with regard to specified Patent Rights, preparing, filing, prosecuting, maintaining, and defending such Patent Rights in Patent Office proceedings or appeals therefrom, including with respect to any reexamination, reissue, interference, revocation, invalidation, protest, or opposition proceedings. For the avoidance of doubt, “Prosecuting” excludes any infringement suits or other legal proceedings to enforce the specified Patent Rights, regardless of whether or not such proceedings also involve the defense of the Patent Rights in suit.
“Regulatory Authority” means a federal, national, multinational, state, provincial or local regulatory agency, department, bureau or other governmental entity with authority over the testing, manufacture, use, storage, import, promotion, marketing or sale of a pharmaceutical product in a country or territory, including the FDA and the EMA.
“Regulatory Exclusivity” means a right granted by a Regulatory Authority in a country with respect to a Product affording the ability to preclude a Third Party from commercializing a product that could compete with such Product in such country, either through data exclusivity rights, new chemical entity designation, orphan drug designation, or such other rights conferred by a Regulatory Authority in such country, other than through Patent Rights.
“Regulatory Filing” means any documentation comprising or relating to or supporting any filing or application with any Regulatory Authority with respect to a Product, or its use or potential or investigative use in humans, including any documents submitted to any Regulatory Authority and all supporting data, including Drug Master Files, INDs, supportive documents enabling a clinical program, NDAs and ▇▇▇▇, and all correspondence with any Regulatory Authority with respect to any Licensed Product (including minutes of any meetings, telephone conferences or discussions with any Regulatory Authority).
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 12 of 60
“Reverted Products” shall have the meaning set out in Section 14.2(a).
“Royalty Term” shall have the meaning set forth in Section 6.3(b).
“Senior Officers” shall have the meaning set forth in Section 4.3(e).
“Taxes”means any present or future taxes, levies, imposts, duties, charges, assessments, or fees of any nature (including any interest thereon).
“Term” shall have the meaning set forth in Section 14.1.
“Territory” means the entire world.
“Third Party” means any entity other than ▇▇▇▇▇▇▇ or Eiger or an Affiliate of ▇▇▇▇▇▇▇ or Eiger.
“Third Party Infringement” shall have the meaning set forth in Section 8.3(a).
“United States” or “U.S.” means the United States of America and its territories and possessions.
“UT License” means the Non-Exclusive License Agreement between Board of Regents of the University of Texas System and ▇▇▇▇▇▇▇ Pharmaceutica NV dated as of March 5, 1998.
“Valid Claim” means, with respect to referenced Patent Rights, (a) a published and pending claim of a patent application that is included in the Patent Rights that is being Prosecuted diligently and in good faith, but has not been pending for more than a total of [ * ] years after the earliest priority date for such claim and has not been finally rejected through a binding decision without filing of an appeal or with loss of all right to appeal, or (b) an issued claim of any patent included in the Patent Rights in any country that (i) has not expired; (ii) has not been disclaimed; (iii) has not been canceled or superseded, or if canceled or superseded, has been reinstated; and (iv) has not been revoked, held invalid, or otherwise declared unenforceable or not allowable by a tribunal or patent authority of competent jurisdiction over such claim in such country from which no further appeal has or may be taken.
“ZARNESTRA ▇▇▇▇” means the trademark “ZARNESTRA”.
1.2 Interpretations. In this Agreement, unless the context requires otherwise:
(a) the headings are included for convenience only and shall not affect its construction;
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 13 of 60
(b) references to “persons” includes individuals, bodies corporate (wherever incorporated), unincorporated associations and partnerships;
(c) words denoting the singular shall include the plural and vice versa and words denoting any gender shall include all genders;
(d) the words “comprise”, “comprising”, “contain”, “containing”, “include” and “including” are used in their open, non-limiting form, and shall be understood to include the words “without limitation” even if not expressly stated;
(e) a Party includes its permitted assignees and/or the respective successors in title to substantially the whole of its undertaking;
(f) any reference to a specified enactment, statute, regulation, or other provision of any Applicable Law is a reference to it as it may have been, or may from time to time be amended, modified, consolidated or re-enacted at the relevant time;
(g) all references to “EURO” or “EUR” shall mean EUROS; and
(h) the Exhibits and other attachments form part of the operative provisions of this Agreement and references to this Agreement shall, unless the context otherwise requires, include references to the recitals and the Exhibits and attachments. In the event of any inconsistency between the Exhibits and the terms of the body of this Agreement, the terms of the body of this Agreement shall prevail.
2. GRANT OF RIGHTS
2.1 Grant of Commercial License to EBP.
(a) Under Compound IP. Subject to the terms and conditions of this Agreement (including Article 6), ▇▇▇▇▇▇▇ hereby grants to EBP an exclusive (even as to ▇▇▇▇▇▇▇, except for reservation of the Option pursuant to Section 2.2), sublicensable (subject to Sections 2.2 and 2.4), license during the Term, under the ▇▇▇▇▇▇▇ IP, to Develop, Manufacture, have Manufactured, offer for sale, sell, and otherwise Commercialize Compounds and Products in the Field throughout the Territory, and to make, have made, use, and import Compounds and Products throughout the world for such purposes.
(b) No License to ZARNESTRA® Trademark Rights. EBP acknowledges and agrees that this Agreement does not grant it any license or other rights to the ZARNESTRA ▇▇▇▇ or under any of ▇▇▇▇▇▇▇’▇ trademark rights pertaining thereto.
(c) Option for Sublicense under UT License. ▇▇▇▇▇▇▇, upon authorization by the Board of Regents of the University of Texas, grants EBP a non-exclusive option, exercisable by notice from EBP to ▇▇▇▇▇▇▇ at any time hereunder during the term of the UT
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 14 of 60
License, to be granted a non-exclusive authorization or sublicense, under the license rights then Controlled by ▇▇▇▇▇▇▇ under the UT License, solely for purposes of exercising any rights granted to EBP under Section 2.1(a) above to Develop or Commercialize Compounds and Products, provided that EBP agrees to and shall assume all responsibility for making all payments that become due to ▇▇▇▇▇▇▇’▇ licensor under the UT License on account of any activities by EBP or any EBP Sublicensees in exercise of its sublicense rights under the UT License. Promptly after EBP exercises such option, the Parties shall negotiate and execute a written sublicense agreement documenting the grant of sublicense rights under the UT License to EBP and EBP’s payment obligations as provided above.
2.2 Reservation of Right of First Negotiation by ▇▇▇▇▇▇▇.
(a) Option Grant. Subject to the terms and conditions of this Agreement, EBP hereby grants to ▇▇▇▇▇▇▇ an exclusive option and first right to negotiate, during the Option Term, for an exclusive license back from EBP, under the Development Program IP and the ▇▇▇▇▇▇▇ IP, to Develop and Commercialize Compounds and Products in any or all countries of the Territory (the “Option”). ▇▇▇▇▇▇▇ may exercise the Option at any time during the [ * ] period following the Date of Delivery by EBP to ▇▇▇▇▇▇▇ of the POC Data Package (the “Option Term”). For the avoidance of doubt, until expiration of the Option Term, without ▇▇▇▇▇▇▇’▇ exercise of the Option, EBP shall not grant any Third Party any right to Develop (except as a subcontractor on EBP’s behalf) or Commercialize any Compounds in the Field. If a POC Trial is not initiated or completed within a reasonable time after the Effective Date, then upon a Party’s request to the other, the Parties shall confer and attempt to negotiate a redefinition of the Option Term that is reasonable in light of the circumstances. For clarity, nothing in this Section shall prohibit EBP from negotiating and completing any transaction for the sale of all or substantially all of its business or assets that includes the assignment of this Agreement pursuant to Section 15.1 (whether by merger, sale of stock, sale of assets, or otherwise), provided that any assignee shall assume all obligations of EBP hereunder, including with respect to the Option rights of ▇▇▇▇▇▇▇ herein.
(b) Delivery of POC Data Package. Following completion of the POC Trial of a Product under the Development Plan, EBP will provide ▇▇▇▇▇▇▇ with the POC Data Package. If, within [ * ] after the date EBP first provides the POC Data Package to ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ provides written notice to EBP requesting additional information that would reasonably be expected to be included in the POC Data Package, then EBP shall use Commercially Reasonable Efforts to provide ▇▇▇▇▇▇▇ such requested additional information. The date that EBP initially provides the POC Data Package or, if ▇▇▇▇▇▇▇ requests additional information in accordance with this Section, the date that EBP provides additional information for inclusion in the POC Data Package or advises ▇▇▇▇▇▇▇ in writing that such additional information cannot be provided after using Commercially Reasonable Efforts, as applicable, shall be the “Date of Delivery” of the POC Data Package.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 15 of 60
(c) Exercise of Option. Subject to the terms and conditions of this Agreement, ▇▇▇▇▇▇▇ may exercise the Option at any time during the Option Term by sending written notice of such exercise (“Exercise Notice”) to EBP.
(d) Effect of Expiration or Termination of Option. If ▇▇▇▇▇▇▇ does not exercise the Option during the Option Term by providing an Exercise Notice to EBP, then ▇▇▇▇▇▇▇’▇ Option shall terminate and EBP shall be free to grant rights to one or more Third Parties to Develop or Commercialize Compounds and Products in the Field. If ▇▇▇▇▇▇▇ gives EBP an Exercise Notice during the Option Term but the Parties do not enter into a definitive license agreement within [ * ] after the Exercise Notice (the “Negotiation Period”, as may be extended or shortened by written agreement of the Parties), then ▇▇▇▇▇▇▇’▇ Option shall terminate and EBP shall be free to grant sublicense rights to one or more Third Parties to Develop or Commercialize Compounds and Products in the Field, provided that [ * ], [ * ] any such rights to Develop or Commercialize any Compounds or Products in the Field [ * ].
2.3 Reservation of Rights. Subject to the Option and to the licenses and sublicenses that are or may be granted to each Party pursuant to Section 2.1 and/or 2.2 and the other terms and conditions of this Agreement, ▇▇▇▇▇▇▇ retains all rights under the ▇▇▇▇▇▇▇ IP that are not expressly licensed to EBP hereunder, including with respect to: (a) chemical compounds, other than Compounds, that are Covered by any claim of the ▇▇▇▇▇▇▇ Patent Rights; or (b) applications of Compounds and Products outside the Field. No right or license under any Patent Rights or Know-How of either Party is granted or shall be granted by implication. All rights or licenses under a Party’s intellectual property rights are or shall be granted only as expressly provided in the terms of this Agreement.
2.4 Sublicense. EBP shall have the right to grant sublicenses to its subcontractors to Develop and/or make the Compound or Product on EBP’s behalf at any time. Upon expiration of ▇▇▇▇▇▇▇’▇ Option rights pursuant to Section 2.2 above, EBP shall have the right to grant sublicenses of the Development and Commercialization rights granted to it under Section 2.1 of this Agreement to its Affiliates and to Third Parties, provided that:
(a) any sublicense agreement shall be in writing and be consistent with the terms and conditions of this Agreement, and the sublicensee shall not have the right to grant further sublicense;
(b) any such sublicense agreement shall provide for the termination of the sublicense upon termination of this Agreement; and
(c) EBP shall be liable for all acts or omissions of its sublicensees and shall at all times, and at its own cost, enforce compliance by the sublicensee with the terms of the sublicense agreement.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 16 of 60
3. ALLIANCE MANAGEMENT
3.1 General. Except as may otherwise be provided herein, the Parties acknowledge and agree that EBP is undertaking the responsibility for performance of the Development Program. If the Option is exercised by ▇▇▇▇▇▇▇ during the Option Term, the Parties shall negotiate a definitive agreement whereby EBP grants back to ▇▇▇▇▇▇▇ any rights to Develop or Commercialize any Compounds or Products in the Field pursuant to Section 2.2.
3.2 Alliance Managers. Within fifteen (15) days after the Effective Date, each Party will appoint a representative having a general understanding of pharmaceutical development and commercialization issues (“Alliance Manager”). The Alliance Managers will be primarily responsible for facilitating the flow of information and otherwise promoting routine communications between the Parties hereunder. Each Party may replace its Alliance Manager on written notice to the other Party.
3.3 Joint Development Committee.
(a) Establishment of JDC. Promptly after the Effective Date, the Parties shall establish a Joint Development Committee, composed of the Alliance Managers and one (1) additional representative from EBP and one (1) additional representative from ▇▇▇▇▇▇▇ as its members. Each Party will designate by written notice its initial members to serve on the JDC. Each Party may replace its representatives on the JDC by written notice to the other Party.
(b) JDC Responsibilities. The JDC, which will have no decision-making authority, will monitor the activities of EBP in the Development Program and serve as a forum for reviewing EBP progress and results of the Development Program.
(c) JDC Meetings. The JDC shall meet at least annually through completion of the POC Trial and at such other times as the Parties may agree. The first meeting of the JDC shall be held as soon as reasonably practicable. Meetings shall be held at such place or places as are mutually agreed or by teleconference or videoconference, provided that at least one representative of ▇▇▇▇▇▇▇ and one representative of EBP are present at any JDC meeting. Each Party may from time to time invite a reasonable number of participants, in addition to its representatives on the JDC, to attend JDC meetings on an ad hoc basis. The JDC meetings will be chaired by EBP. The chairperson shall set agendas for JDC meetings in advance. The Parties will rotate the responsibility for recording, preparing and, within a reasonable time, issuing draft minutes of each JDC meeting to each Party’s Alliance Manager for review, who upon their approval shall issue final minutes to the Parties.
(d) Expenses. Each Party shall bear all its own costs, including expenses incurred by its JDC members or by any additional non-member participants of such Party in connection with their attendance at JDC meetings and other activities related to the JDC.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 17 of 60
(e) POC Trial Design Input. Promptly after the Effective Date, EBP shall use Commercially Reasonable Efforts to provide the JDC with EBP initial Development Plan, which shall include a description of the clinical study design for the POC Trial. The Development Plan, and any amendments thereto, shall be discussed at a JDC meeting, and EBP shall reasonably consider the input from discussions at JDC meetings regarding the design of the POC Trial and any other plans for any Phase II Study or Phase III Study of any Compound or Product in the Development Program.
(f) Review of Plans and Results. In advance of each JDC meeting, EBP will provide the JDC representatives with a summary regarding the Development activities performed by or on behalf of EBP since the last JDC meeting (if any), including a description of data, results, and other information generated in, and any activities planned for, Developing any Compounds or Products. Without limiting the generality of the foregoing, such summaries shall include (a) the status and results of any Development activities, including, non-clinical and/or preclinical studies and activities (including toxicology and pharmacokinetic studies); and (b) the Regulatory Filings and Marketing Authorization applications with respect to any Compound and Product that EBP or any of its Affiliates or sublicensees have filed, sought, or obtained.
(g) No Authority to Modify Agreement. For the avoidance of doubt, the JDC shall have no authority to modify any provision set forth in the body or in any Exhibit of this Agreement, including any payment conditions or terms, periods for performance, or obligations of the Parties as set forth in this Agreement, which may be modified only by written agreement of the Parties.
4. DEVELOPMENT PROGRAM
4.1 Diligence. EBP (directly and through applicable EBP Sublicensees) shall use Commercially Reasonable Efforts to Develop at least one (1) Product through Marketing Authorization in one or more of the Major Market Countries. For the avoidance of doubt, the foregoing diligence requirement shall not be construed so as to necessitate that EBP seek Marketing Authorization in all Major Market Countries simultaneously.
4.2 Records. EBP shall, and shall require its subcontractors to, maintain in accordance with Applicable Law complete and accurate records in segregated laboratory notebooks of all work conducted in furtherance of the Development of Compounds and Products, including all raw data, observations, conclusions, and analyses. Such records shall be complete and accurate and shall fully and properly reflect all work done and results achieved in sufficient detail and in a manner appropriate for patent and regulatory purposes.
4.3 Use of Animals. In conducting any Development Program activities involving any animals, (i) the animals shall be provided with humane care and treatment in accordance with current generally accepted veterinary practice, and (ii) in accordance with ▇▇▇▇▇▇▇’▇
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 18 of 60
Guidelines on the Care & Use of Laboratory Research Animals appended to this Agreement as Exhibit 6.
4.4 Standards for Conduct. EBP shall use Commercially Reasonable Efforts to execute and to perform, or cause to be performed, Development activities in in good scientific manner and in compliance with Applicable Law, Good Clinical Practice, and Good Laboratory Practice.
4.5 Development Reports. EBP shall submit to ▇▇▇▇▇▇▇ annual written progress reports by [ * ] of each year of the Term covering EBP’s (and any of its Affiliates’, subcontractors’, and sublicensees’) activities related to the Development of each Product in the Field, the status of obtaining Marketing Authorization, and other activities undertaken in order to meet the diligence requirement set forth in Section 4.1, until First Commercial Sale of such Product in the Field in the United States, which reports will be again required if, and for so long as, all sales of such Product are suspended or discontinued in all countries during the Term. Upon ▇▇▇▇▇▇▇’▇ reasonable request, EBP shall supplement any such Development progress report with other information in its possession that is pertinent to the Development efforts with respect to Products in the Field for as long as the respective diligence obligation under Section 4.1 applies. For the avoidance of doubt, all information contained in such reports shall be deemed EBP’s Confidential Information.
4.6 Drug Supply for Development.
(a) Responsibility. Following the Effective Date, EBP will be solely responsible, itself and through its Affiliates and sublicensees at their own expense, for Manufacturing or having Manufactured Compound and Product for Development purposes, including for producing clinical supplies. The Manufacturing of supplies of Compound and Product for human use shall be performed in accordance with Applicable Law and Good Manufacturing Practice.
(b) No Supply from ▇▇▇▇▇▇▇’▇ Inventory. EBP further acknowledges that this Agreement does not grant it rights to any quantities of Compound or Product from ▇▇▇▇▇▇▇’▇ supply existing as of the Effective Date, and that there is no guarantee that there will be any amount available at any given time for transfer to EBP.
4.7 Know-How Transfer and Assistance. ▇▇▇▇▇▇▇ shall use commercially reasonable efforts to complete shipment (in one shipment or on a rolling basis), during the period running [ * ] from the Effective Date, at EBP expense for out-of-pocket (but not internal ▇▇▇▇▇▇▇) costs, to EBP or its designee, copies of all ▇▇▇▇▇▇▇ Know-How documentation listed in Exhibit 3 (which shall be treated as ▇▇▇▇▇▇▇’▇ Confidential Information) within ▇▇▇▇▇▇▇’▇ possession or control, including that relating to clinical Development and Manufacture of the Compound. ▇▇▇▇▇▇▇ will prioritize for shipment copies of Regulatory Filings within the ▇▇▇▇▇▇▇ Know-How documentation. For the period running [ * ] after EBP receipt of the complete copies of the
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 19 of 60
▇▇▇▇▇▇▇ Know-How and for no more than a cumulative of [ * ], ▇▇▇▇▇▇▇ will provide reasonable assistance requested by EBP to facilitate its understanding of the ▇▇▇▇▇▇▇ Know-How, by making one appropriately qualified representative of ▇▇▇▇▇▇▇ reasonably available for meetings or teleconferences regarding the content of the ▇▇▇▇▇▇▇ Know-How documentation. In addition, upon EBP’s written request ▇▇▇▇▇▇▇ will provide EBP with a right of cross-reference or access to or copies of any Regulatory Filings possessed or controlled by ▇▇▇▇▇▇▇, as appropriate and considering Applicable Law and any license rights outside of the Field granted by ▇▇▇▇▇▇▇ to Third Parties. For clarity, ▇▇▇▇▇▇▇ is not obligated to provide any other assistance beyond that which is set forth in Section 4.7, except as may be agreed upon by the Parties in a separate written services agreement.
4.8 Regulatory Submissions. EBP (directly or through its EBP Sublicensees) shall be responsible for submitting (or having submitted) all Regulatory Filings after the Effective Date, and for obtaining and maintaining all Marketing Authorizations for Products in the Field. EBP (directly or through its Sublicensees), shall use Commercially Reasonable Efforts to coordinate with ▇▇▇▇▇▇▇ or with any Third Party identified by ▇▇▇▇▇▇▇ to EBP as having been granted licensee rights to develop and commercialize Compounds and/or Products outside of the Field as necessary to compile, maintain, and report adverse event and other relevant safety data from use of Compounds and products as required by Applicable Laws.
5. COMMERCIALIZATION
5.1 Diligence. EBP (directly and through its EBP Sublicensees) shall use Commercially Reasonable Efforts to Commercialize Products in countries where Marketing Authorization has been obtained.
5.2 Legal Compliance. EBP agrees that in performing any Development, Manufacturing and Commercialization activities with respect to any Compounds or Products as contemplated hereunder, (a) it shall, and shall use reasonable measures to cause its Affiliates, sublicensees, and subcontractors to, comply with all applicable current international regulatory standards, including GMP, GLP, GCP and other Applicable Laws, and (b) it shall not, and shall use reasonable measures to cause its Affiliates, sublicensees, and subcontractors to not, knowingly employ or use any person that has been debarred under Section 306(a) or 306(b) of the U.S. Federal Food, Drug and Cosmetic Act.
5.3 Commercialization Reports. EBP shall submit to ▇▇▇▇▇▇▇ [ * ] written progress reports by [ * ] of each year of the Term covering EBP’s (and any EBP Sublicensees’) activities related to the Commercialization of each Product in the Field and other activities undertaken in order to meet the diligence requirement set forth in Section 5.1. Upon ▇▇▇▇▇▇▇’▇ reasonable request, EBP shall supplement any such Commercialization progress report with other information in its possession that is pertinent to the Commercialization efforts with respect to Products in the Field for as long as the respective diligence obligation under Section 5.1 applies.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 20 of 60
For the avoidance of doubt, all information contained in such reports shall be deemed EBP’s Confidential Information.
5.4 No Use of ZARNESTRA ▇▇▇▇. EBP shall not use the ZARNESTRA ▇▇▇▇ in connection with its Development or Commercialization of Product. For the avoidance of doubt, this Agreement does not ▇▇▇▇▇ ▇▇▇▇▇ any license or other rights to any trademarks, designs, logos, slogans, taglines, trade names, or trade dress that ▇▇▇▇▇▇▇ owns or otherwise controls.
6. FINANCIAL PROVISIONS
6.1 Milestone Payments. Each of the milestone payments identified in this Section 6.2 shall be due upon the achievement of the specified milestone event with respect to any Compound or Product.
(a) Development Milestones. In further consideration of the license rights granted to EBP under Section 2.1, each of the milestone payments identified in this Section 6.2(a) shall be due upon the first achievement of the specified milestone event with respect to any Compound or Product. EBP shall promptly provide written notification to ▇▇▇▇▇▇▇, at Beerse (Belgium), ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇▇▇▇▇: Finance Manager (Maarten Van Looveren) upon achievement of each Development Milestone. Such notification shall indicate that the Development Milestone was achieved and request that ▇▇▇▇▇▇▇ send a written invoice for such milestone to a specific address, if such address is different than that indicated in Section 15.11: Notices. Within [ * ] of the receipt of the invoice for each of the corresponding Development Milestones listed below, EBP shall pay by wire transfer the amount listed in each invoice to ▇▇▇▇▇▇▇ to the bank account identified in Section 7.2. For clarity, each milestone payment shall be due and payable only one time upon the first achievement of the event specified.
| Development Milestone Event |
Milestone Payment (EUR) |
|||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
(b) Sales Milestones. In further consideration of the license rights granted to EBP under Section 2.1, solely upon the first occurrence during the Term of aggregate annual worldwide Net Sales of all Products surpassing the sales threshold identified below, EBP shall immediately provide written notification to ▇▇▇▇▇▇▇, at Beerse (Belgium), ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇▇▇▇▇: Finance Manager (Maarten Van Looveren) upon achievement of each Sales
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 21 of 60
Milestone. Such notification shall indicate that the one-time corresponding sales milestone was achieved and request that ▇▇▇▇▇▇▇ send a written invoice for such milestone to a specific address, if such address is different than that indicated in Section 15.2: Notices. [ * ] of the receipt of the invoice for each of the corresponding Sales Milestones listed below, EBP shall pay by wire transfer the amount listed in each invoice to ▇▇▇▇▇▇▇ to the bank account identified in Section 7.2. For the avoidance of doubt, if in the same reporting period multiple sales milestones are first attained, then the payments for all such milestones attained as specified below shall be due.
| Sales Threshold (aggregate annual worldwide Net Sales of Products) in EUR |
Milestone Payment (EUR) |
|||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
| [ * ] |
[ * ] | |||
(c) For the avoidance of doubt, different milestones as specified in this Section 6.2 may be achieved by the same or a distinct Compound or Product. Additionally, should a Compound or Product be replaced or backed up by another Compound or Product, no additional milestone payments shall be due under Section 6.2 for milestone events completed by the replacement or back-up Compound or Product for which corresponding milestone payments were previously made to EBP with respect to such replaced Compound or Product.
(d) Third Party Sublicense. In the event that EBP sublicenses any of its rights to Compounds and/or Products to any Third Party, EBP would pay ▇▇▇▇▇▇▇: (i) [ * ] of all monetary compensation received from the Third Party sublicensee, including upfront and lump-sum payments, milestone payments, and royalties; and (ii) such amounts otherwise due based on EBP’s milestone and royalty payment obligations under this Agreement. For example, (1) if EBP receives an upfront of EUR [ * ] from a Third Party sublicense, EBP would pay ▇▇▇▇▇▇▇ EUR [ * ]; (2) if EBP receives a milestone from a Third Party sublicense for a milestone event that is listed in Section 6.1, EBP would pay ▇▇▇▇▇▇▇ [ * ] of the milestone from the Third Party sublicensee and the milestone event otherwise payable to ▇▇▇▇▇▇▇ under Section 6.1; and (3) if EBP receives a milestone from a Third Party sublicense for a milestone event that is not listed in Section 6.1, EBP would pay ▇▇▇▇▇▇▇ [ * ] of the milestone from the Third Party sublicensee. EBP shall immediately provide written notification to ▇▇▇▇▇▇▇, at Beerse (Belgium), ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇▇▇▇▇: Finance Manager (Maarten Van Looveren) upon achievement of each Third Party Sublicense. Such notification shall indicate that a Third Party Sublicense was achieved and request that ▇▇▇▇▇▇▇ send a written invoice for such Third Party Sublicense amount to a specific address, if such address is different than that indicated in Section 15.2: Notices. [ * ] days of the receipt of the invoice for each of the Third Party Sublicense, EBP shall pay by wire transfer the amount listed in each invoice to ▇▇▇▇▇▇▇ to the bank account identified in Section 7. Any Third Party Sublicense monetary compensation received by EBP in a currency other than Euro shall be converted into their Euro equivalent using the closing exchange rate as published
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 22 of 60
by The Wall Street Journal, Western U.S. Edition for the day the Third Party Sublicense compensation was achieved by EBP.
6.2 Royalty Payments.
(a) Royalty Basis and Rate. In partial consideration of the license rights under Section 2.1, royalties shall be due from EBP on aggregate annual Net Sales of Products during the Royalty Term throughout the Territory, and royalties shall be determined on a Product-by-Product and country-by-country basis where either: (i) any Valid Claim of the ▇▇▇▇▇▇▇ Patent Rights Covers the applicable Product or Compound contained therein as a composition or any method of use of such Product or Compound in the Field in such country; (ii) Regulatory Exclusivity applies to such Product in such country; or (iii) [ * ] from First Commercial Sale (for the financial convenience of the parties, and considering ▇▇▇▇▇▇▇’▇ willingness to accept the very modest upfront and development milestone payments, there would be no stepdown of the royalty rate if there is no valid patent claim or regulatory exclusivity in a particular country). Royalties due each calendar year of the Royalty Term shall be calculated by multiplying the applicable incremental Net Sales of Products against the applicable royalty rate identified below, subject to any applicable adjustments or reductions provided for in Section 6.3(c), with each royalty rate referred to below applying only to that increment of annual Net Sales that falls within the incremental sales bracket for such royalty rate.
| Aggregate annual Net Sales of Products | Royalty Rate | |||
| Less than or equal to [ * ] |
[ * ]% | |||
| Greater than [ * ] up to and equal to [ * ] |
[ * ]% | |||
| Greater than [ * ] |
[ * ]% | |||
To illustrate, if, for example, cumulative annual worldwide Net Sales of Products upon which royalties are due and payable as provided in this Section 6.3 were EUR[ * ] during any year of the Royalty Term, then absent any adjustments or reductions pursuant to Section 6.3(c), the royalties due would be EUR[ * ] calculated as follows: [ * ]. For the avoidance of doubt, royalties due under this Section 6.3 shall be payable only once with respect to the same unit of Product, and different formulations (e.g., dosage strengths, delivery forms) of a Compound and Bioequivalents thereof shall be deemed the same Product.
(b) Royalty Term. Royalties due on Net Sales of Products will be payable on a Product-by-Product and country-by-country basis until the later of (a) the expiration of the last to expire Valid Claim of the ▇▇▇▇▇▇▇ Patent Rights Covering either the Product or the Compound contained therein as a composition or any method of use of such Product or Compound in the
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 23 of 60
Field in such country, (b) the expiration of any Regulatory Exclusivity with respect to such Product in such country, and (c) [ * ] from First Commercial Sale (the “Royalty Term”). Following the Royalty Term on a Product-by-Product and country-by-country basis, EBP’s licenses with respect to such Product in such country under Section 2.1 shall continue in effect, but become fully paid-up, royalty-free, perpetual and irrevocable.
(c) Adjustments to Royalties.
(i) Compulsory Licenses. If at any time in any country a Third Party shall, under the right of a compulsory license granted or ordered to be granted by a competent governmental authority in a given country (other than failure of a court to enjoin infringement as a remedy in a patent infringement proceeding), be granted a license, under any ▇▇▇▇▇▇▇ Patent Rights licensed to Eiger hereunder, to sell in such country, or manufacture for distribution or sale by or on behalf the government in such country, any Product with respect to which royalties are payable pursuant to Section 6.3(a) at a royalty rate that is less than the applicable royalty rate for a given tier of incremental annual Net Sales as provided in Section 6.3(a), and such Product is sold by such Third Party during any calendar quarter during the Royalty Term, then the royalty rate to be paid by EBP on Net Sales of such Product in such country during such quarter that are included in such tier of incremental annual Net Sales shall be reduced to the rate paid by the Third Party compulsory licensee for so long as such compulsory license remains in effect.
(ii) Generic Competition. In the event that one or more Third Parties (other than any EBP Sublicensee) markets a Generic Product in a given country from and after the first calendar quarter in which the total amount of gross sales of such Product by EBP and EBP Sublicensees in such country is less than [ * ] of the average amount of total quarterly gross sales of such Product by EBP and EBP Sublicensees for the [ * ] consecutive calendar quarters immediately prior to the launch of the first Generic Product in such country, as measured by reputable published marketing data for such country (e.g. by reference to sales data collected by IMS or other reputable source) and such percentage decrease in gross sales by EBP can be reasonably attributed to erosion due to the Generic Product sales and no other cause, such as other competition or reduction in promotional efforts, the royalties to be paid by EBP on Net Sales of such Product in such country during such quarter shall be reduced to [ * ] of the royalties otherwise due to ▇▇▇▇▇▇▇ in such quarter with respect to such Product in such country.
7. REPORTS AND PAYMENT TERMS
7.1 Payment Terms.
(a) Notice of Milestone Events and Milestone Payments. Written notice of achievement of each milestone event shall be provided as set forth in Section 6.1(a) or (b), as applicable. Payments for achieving milestones shall be made as set forth in Section 6.1(a) or (b), as applicable.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 24 of 60
(b) Invoices. Any payment for an amount due to ▇▇▇▇▇▇▇ under this Agreement shall be payable, except as otherwise expressly provided herein, within [ * ] after EBP receipt of an invoice from ▇▇▇▇▇▇▇ for such amount. Each invoice shall specifically refer to this Agreement.
(c) Royalty Reporting and Payments. Within [ * ] days after the end of each calendar quarter EBP shall submit to ▇▇▇▇▇▇▇ a sales report to the address listed in Section 15.11 (Attention: Finance Manager) setting forth, on a Product-by-Product and country-by-country basis, the Gross Sales, the deductions taken from Gross Sales, and the Net Sales of Product and a calculation of the amount of royalty payment due on such Net Sales. This report shall also include the exchange rates and other methodology used in converting Net Sales into Euros, from the currencies in which sales were made in order to determine the appropriate royalty tier and royalty payable. Royalty payments shall be made within [ * ] days from receipt by EBP of an invoice from ▇▇▇▇▇▇▇ for the amount reflected in the sales report under this Section 7.1(c).
7.2 Remittance. All payments shall be made in immediately available funds by electronic transfer, by EBP or an Affiliate on its behalf, to the bank account identified below or such other bank account as ▇▇▇▇▇▇▇ may designate in writing to EBP. Any payments due and payable under this Agreement on a date that is not a Business Day may be made on the next Business Day. If, at any time, legal restrictions prevent the prompt remittance of part of or all of the royalties due hereunder with respect to any country where Products are sold, EBP shall have the right and option to make such payments by depositing the amount thereof in local currency to ▇▇▇▇▇▇▇’▇ account in a bank or depository in such country or by using such lawful means or methods as EBP may determine.
Name of Bank: ING Belgium
Bank address: ▇▇▇▇▇▇▇▇▇▇ ▇▇
▇▇▇▇ ▇▇▇▇▇▇▇▇
▇▇▇▇▇▇▇
Company Name and Address: ▇▇▇▇▇▇▇ Pharmaceutica NV
▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇
▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇
[ * ]
7.3 Currency. All payments under this Agreement shall be payable in Euro. With respect to sales of a Product invoiced in a currency other than Euro, such amounts and the amounts payable hereunder shall be converted into their Euro equivalent using an exchange rate equal to the simple monthly period average of the rates of exchange for the currency on the first
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 25 of 60
and last day of each calendar month of the country from which such payments are payable as published by The Wall Street Journal, Western U.S. Edition, during the calendar quarter in which the applicable sales were made.
7.4 Taxes.
(a) EBP will make all payments to ▇▇▇▇▇▇▇ under this Agreement without deduction or withholding for Taxes except to the extent that any such deduction or withholding is required by Applicable Law in effect at the time of payment.
(b) Any Tax required to be withheld on amounts payable under this Agreement will be paid by Eiger on behalf of ▇▇▇▇▇▇▇ to the appropriate governmental authority, and EBP will furnish ▇▇▇▇▇▇▇ with proof of payment of such Tax. Any such Tax required to be withheld will be an expense of and borne by ▇▇▇▇▇▇▇.
(c) EBP and ▇▇▇▇▇▇▇ will cooperate with respect to all documentation required by any taxing authority or reasonably requested by EBP to secure a reduction in the rate of applicable withholding Taxes. On or before the Effective Date, ▇▇▇▇▇▇▇ will deliver to EBP an accurate and complete Internal Revenue Service Form [W-9] [W-8BEN-E certifying that ▇▇▇▇▇▇▇ is entitled to the applicable benefits under the Income Tax Treaty between Belgium and the United States].
7.5 Records and Audit Rights.
(a) Maintenance of Records. Each Party shall keep (and, in the case of EBP, EBP shall cause the EBP Sublicensees to keep) complete, true and accurate books and records in accordance with its Accounting Standards in sufficient detail for the other Party to determine the payments due and costs incurred under this Agreement, including with respect to Patent Expenses and royalties. Each Party will keep such books and records for at least [ * ] following the date of the payment to which they pertain.
(b) Audit Right. Upon the written request of ▇▇▇▇▇▇▇ with respect to payments made by EBP pursuant to Article 6, not more than once in each calendar year, EBP shall permit an independent certified public accounting firm of nationally recognized standing selected by ▇▇▇▇▇▇▇ and reasonably acceptable to EBP to have confidential access during normal business hours to such of the records of EBP and its applicable EBP Sublicensees as may be reasonably necessary to verify the accuracy of the payments made under this Agreement for any period ending not more than [ * ] prior to the date of such request. The accounting firm shall provide each Party a correct and complete copy of the report summarizing the final results of such audit, which shall be treated as EBP Confidential Information. ▇▇▇▇▇▇▇ shall obligate its accounting firm to keep EBP information confidential, and shall at the request of EBP cause ▇▇▇▇▇▇▇’▇ accounting firm to execute a reasonable confidentiality agreement prior to commencing any such audit.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 26 of 60
(c) Audit Fees and Results. The fees charged by such accounting firm shall be paid by ▇▇▇▇▇▇▇; provided, however, that if the audit uncovers an under- or over-payment in favor of EBP exceeding [ * ] of the total amount due in accordance with this Agreement, then the fees of such accounting firm shall be paid by EBP. Any underpayments discovered by such audit or otherwise will be paid to ▇▇▇▇▇▇▇ within [ * ] of the date that ▇▇▇▇▇▇▇ delivers to EBP such accounting firm’s written report, or as otherwise agreed upon by the Parties, plus interest calculated in accordance with Section 7.6. For any overpayments discovered by such audit EBP shall receive a credit equal to such overpayment against the royalty otherwise payable to ▇▇▇▇▇▇▇.
7.6 Late Payments. Interest shall be payable by EBP on any amounts payable to ▇▇▇▇▇▇▇ under this Agreement which are not paid by the due date for payment. All interest shall accrue and be calculated on a daily basis (both before and after any judgment) at the rate of [ * ] per annum above the then-current prime rate quoted by Citibank in New York City (but in no event in excess of the maximum rate permissible under Applicable Laws), for the period from the due date for payment until the date of actual payment. The payment of such interest shall not limit ▇▇▇▇▇▇▇ from exercising any other rights it may have as a consequence of the lateness of any payment.
8. INTELLECTUAL PROPERTY RIGHTS
8.1 Ownership. Ownership of all inventions arising in the course of the Development Program and Development Program Patent Rights shall be determined in accordance with inventorship pursuant to U.S. patent laws. Accordingly: (i) all Development Program Inventions made solely by employees or consultants of one Party or such Party’s Affiliate or subcontractor shall be owned by such Party; and (ii) all Development Program Inventions made jointly by one or more employees or consultants of EBP or its Affiliate or subcontractor and one or more employees or consultants of ▇▇▇▇▇▇▇ or its Affiliate or subcontractor shall be owned jointly by the Parties. The Parties acknowledge that they do not contemplate that there would be any Development Program Inventions owned solely by ▇▇▇▇▇▇▇ given that the Development Program will be conducted by EBP. Subject to the terms and conditions of this Agreement, and except as prohibited or limited by its express terms, each Party shall have the right to practice and use, and grant licenses to practice and use, each jointly owned Development Program Invention (if any) without the other Party’s consent and shall have no duty to account to the other Party for such permitted practice, use or license except as expressly provided hereunder (including in Article 6).
8.2 Patent Prosecution.
(a) ▇▇▇▇▇▇▇ Patent Rights.
(i) Prosecution Control. ▇▇▇▇▇▇▇ will have the right to control the Prosecution of the ▇▇▇▇▇▇▇ Patent Rights, using outside patent counsel directed by ▇▇▇▇▇▇▇, provided that EBP shall have the right to review and comment on drafts of substantive patent
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 27 of 60
submissions prior to their filing in Patent Offices, and ▇▇▇▇▇▇▇ shall consider EBP’s reasonable requests with respect to the Prosecution of the ▇▇▇▇▇▇▇ Patent Rights. ▇▇▇▇▇▇▇ shall keep EBP regularly and fully informed of the status of ▇▇▇▇▇▇▇ Patent Rights in the Territory and provide copies of all substantive documentation submitted to, or received from, the Patent Offices in connection therewith. After the Effective Date, ▇▇▇▇▇▇▇ shall not, without EBP’s prior written consent, forgo or discontinue Prosecution of any ▇▇▇▇▇▇▇ Patent Right in any country in the Territory prior to obtaining from the Patent Office having jurisdiction in such country allowance or issuance of at least one claim Covering a Compound being developed or commercialized by EBP in such country.
(ii) Patent Costs. EBP shall reimburse ▇▇▇▇▇▇▇ for [ * ] of all Patent Expenses incurred by ▇▇▇▇▇▇▇ in the Prosecution of ▇▇▇▇▇▇▇ Patent Rights in the Territory after the Effective Date. Notwithstanding the foregoing in this Section 8.2(a), EBP may terminate its license rights under Section 2.1 under any particular ▇▇▇▇▇▇▇ Patent Rights by written notice to ▇▇▇▇▇▇▇ identifying each such ▇▇▇▇▇▇▇ Patent Right for which EBP is electing to terminate its license rights hereunder. Effective as of the date of ▇▇▇▇▇▇▇’▇ receipt of such notice, EBP’s license rights under each such ▇▇▇▇▇▇▇ Patent Right shall terminate and EBP shall not be responsible for any Patent Expenses incurred by ▇▇▇▇▇▇▇ in the Prosecution of any such ▇▇▇▇▇▇▇ Patent Rights after such license termination.
(b) Protection of Privileged Advice Shared for Common Interest. For the avoidance of doubt, any opinions or other advice of any qualified legal personnel (whether a patent attorney or other counsel) representing a Party hereunder communicated to the other Party or both Parties, directly by such legal personnel or indirectly such as through a patent liaison for common interest purposes contemplated hereunder (including under Section 8.3), shall be held in strict confidence to protect the privileged nature thereof, and not disclosed to any Third Party without the prior written consent of both Parties, each under the advice of its respective legal counsel.
8.3 Patent Infringement.
(a) Notice. During the Term, each Party will promptly notify the other of (i) any actual or threatened infringement by a Third Party of any ▇▇▇▇▇▇▇ Patent Rights of which it becomes aware, including any certification filed by a Third Party pursuant to 21 U.S.C. §355(b)(2)(A)(iv) or 355(j)(2)(A)(vii)(IV) or any notice under comparable U.S. or foreign law (a “Paragraph IV Certification”), which references the foregoing; or (ii) any actual or threatened challenge to any ▇▇▇▇▇▇▇ Patent Rights by a Third Party (collectively, “Third Party Infringement”). The Parties will consult with each other through each Party’s patent attorneys to attempt to agree on a joint program of action in response to any Third Party Infringement.
(b) Action Against Third Parties. If the Parties fail to agree on a joint program of action with respect to Third Party Infringement of any ▇▇▇▇▇▇▇ Patent Rights, then [ * ] bring and control any legal action (including by initiating any lawsuit or other proceeding) as it
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 28 of 60
reasonably determines appropriate in connection with the Third Party Infringement with respect to ▇▇▇▇▇▇▇ Patent Rights, and [ * ] shall have the right, at its own expense, to be represented in any such action by counsel of its own choice.
(c) Conduct of Enforcement Action. [ * ] shall have full control over the conduct of an action under this Section 8.3, including settlement thereof; provided, however, that in no event shall [ * ], through any such action, enter into any settlement arrangement or make any admission of invalidity of, or otherwise impair [ * ] any ▇▇▇▇▇▇▇ Patent Rights without [ * ] prior written consent.
(d) Assistance. At the request and expense of [ * ], [ * ] shall provide reasonable assistance in connection with a Third Party Infringement action with respect to ▇▇▇▇▇▇▇ Patent Rights under this Section 8.3, including by executing any required documents, participating in discovery (including producing documentation and providing access to employees or relevant persons), and joining as a party to the action if required. [ * ] shall [ * ] providing such assistance within [ * ].
(e) Allocation of Awards. Unless otherwise agreed to by the Parties as part of any cost-sharing arrangement, any recoveries resulting from an action under this Section 8.3 relating to a claim of Third Party Infringement with respect to ▇▇▇▇▇▇▇ Patent Rights (after payment of costs and expenses relating to such action incurred by each Party) will be retained by [ * ]; provided, however, that, if any portion of such recovery (after payment of each Party’s costs and expenses related to such action) is attributable to [ * ], [ * ] shall pay to [ * ] an amount equal to [ * ] under this Agreement.
8.4 Trademarks. Subject to Sections 2.1(b) and 5.4, EBP and the EBP Sublicensees shall have the right to brand, at their discretion, the Products using trademarks and trade names (other than the ZARNESTRA ▇▇▇▇) selected at their discretion and registered at their discretion in their own names.
8.5 Patent Term Extensions. EBP acknowledges that nothing herein prohibits ▇▇▇▇▇▇▇ from licensing any Third Party rights under the ▇▇▇▇▇▇▇ IP to any Compound or Products for use outside the Field, and that such a Third Party licensee of ▇▇▇▇▇▇▇ may receive Marketing Authorization for a Product outside the Field in a given country before EBP receives Marketing Authorization for a Product in the Field in the same country. Upon EBP reasonable request after it receives applicable Marketing Authorization for a Product in the Field in a given country, ▇▇▇▇▇▇▇ shall use reasonable efforts to apply for a Patent Term Extension in such country of a relevant ▇▇▇▇▇▇▇ Patent Right, and ▇▇▇▇▇▇▇ shall thereafter provide all reasonable assistance to EBP, including permitting EBP to proceed with the application for such Patent Term Extension in the name of ▇▇▇▇▇▇▇, if so required under Applicable Law.
8.6 Patent Marking; No Endorsement. Any patent markings on any Product made, used or sold by or on behalf of EBP or any EBP Sublicensee (or when the character of the
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 29 of 60
Product precludes marking, the package containing any such Product) shall be made in accordance with all Applicable Laws relating to patent marking.
9. CONFIDENTIALITY
9.1 Confidentiality Obligation. All Confidential Information disclosed or made available by a Party (directly or through its Affiliates) to the other Party will be maintained in confidence and otherwise safeguarded by the recipient Party. The recipient Party may only use the Confidential Information of the other Party and its Affiliates for the purposes expressly permitted by this Agreement. Each Party shall hold as confidential such Confidential Information of the other Party and its Affiliates in the same manner and with the same protection as such recipient Party maintains its own confidential information, but no less than a reasonable standard of care. A recipient Party may only disclose Confidential Information of the other Party and its Affiliates to investors, potential investors, lenders, potential lenders, employees, agents, contractors, consultants and advisers of the recipient Party and its Affiliates, licensees and sublicensees and to Third Parties to the extent reasonably necessary for the purposes of, and for those matters undertaken pursuant to, this Agreement; provided that such persons and entities are bound to maintain the confidentiality of the Confidential Information in a manner consistent with the confidentiality provisions of this Agreement.
9.2 Exceptions. The obligations under Section 9.1 shall not apply to any information within the Confidential Information to the extent the recipient Party can demonstrate by competent evidence that such information:
(a) is (at the time of disclosure) or becomes (after the time of disclosure) known to the public or part of the public domain through no breach of this Agreement by the recipient Party or its Affiliates;
(b) was known to, or was otherwise in the possession of, the recipient Party or its Affiliates prior to the time of disclosure by the disclosing Party;
(c) is disclosed to the recipient Party or any of its Affiliates on a non-confidential basis by a Third Party who is entitled to disclose it without breaching any confidentiality obligation to the disclosing Party or any of its Affiliates; or
(d) is independently developed by or on behalf of the recipient Party or its Affiliates, as evidenced by its written records, without reference to the Confidential Information disclosed by the disclosing Party or its Affiliates under this Agreement.
9.3 Authorized Disclosures.
(a) Authorized Disclosures. In addition to disclosures allowed under Section 9.1, a Party may disclose information within the Confidential Information of the other Party and its Affiliates to the extent such disclosure is necessary in the following instances:
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 30 of 60
(i) for Prosecuting Patent Rights as permitted by this Agreement; (ii) for making regulatory filings for Products the recipient Party has a license or right to develop hereunder; (iii) for prosecuting or defending litigation as permitted by this Agreement; (iv) for complying with applicable court orders or governmental regulations; (v) in the case of ▇▇▇▇▇▇▇, for disclosing in confidence to Third Parties to the extent required to comply with Existing Third Party Agreements; and (vi) for disclosing in confidence to actual or bona-fide potential Third Party investors or other Third Party transactional partners and to their bankers, lawyers, accountants, agents, provided, in each case that each such Third Party investor or other transactional partner or advisor thereof is bound to maintain the confidentiality of the Confidential Information in a manner consistent with the confidentiality provisions of this Agreement.
(b) Notification of Patent Filings. In the event a recipient Party or any of its Affiliates discloses to a Patent Office any Confidential Information of the other Party in connection with the Prosecution of any Patent Rights, the recipient Party shall notify the other Party of such disclosure, and, if requested, provide a copy of such disclosure as filed (which shall, to the extent it includes non-redacted information in addition to the Confidential Information of the other Party, be considered the recipient Party’s Confidential Information).
(c) Disclosure Required by Applicable Laws.
(i) In the event the recipient Party is required to disclose Confidential Information of the other Party by Applicable Laws, including to comply with any order of any court or governmental or regulatory authority, such disclosure shall not be a breach of this Agreement; provided that the recipient Party (i) informs the other Party as soon as reasonably practicable of the required disclosure, (ii) limits the disclosure to that reasonably required for the legal purpose and seeks protective treatment as available under Applicable Laws, and (iii) at the other Party’s request and expense, reasonably assists in its attempt to intervene to directly limit or protect the disclosure of its Confidential Information.
(ii) In the event a Party seeks to make a disclosure of this Agreement or any terms hereof to a government or regulatory authority as required by United States SEC regulations or other Applicable Laws applying to securities or by the rules of any recognized stock exchange or quotation system, the other Party shall reasonably cooperate with respect to the timing, form and content of such required disclosure to the extent practicable under the circumstances, and, if so requested by it, the Party subject to such disclosure obligation shall use commercially reasonable efforts to obtain an order protecting to the maximum extent possible the confidentiality of such provisions of this Agreement as reasonably requested by the other Party. If the other Party does not provide consent as to the form or content of the required disclosure, such disclosure shall be limited to the minimum required, as reasonably determined by the disclosing Party in consultation with its legal counsel.
(d) Regardless of any obligation of confidentiality hereunder, a Party may publish information regarding any of its clinical trials of Products in accordance with its policy
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 31 of 60
regarding public disclosure of such information consistently applied, and shall register information relating to clinical studies of Products as required by applicable law (e.g., with ▇▇▇.▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ when required by United States law).
9.4 Duration of Obligations. The obligations with respect to maintaining the confidentiality of and restrictions on use of Confidential Information shall apply during the Term of this Agreement and continue for a period running [ * ] thereafter.
10. PUBLICATIONS AND PUBLICITY
10.1 Scientific Publications. Any proposed oral or written publications (such as any abstracts, manuscripts, posters, slide presentations or other materials) of any activities or results relating to the Development Program shall not be made without the review of the other Party prior to their submission to the publisher or other release. For the avoidance of doubt, this Section 10.1 shall not apply to public disclosures required by Applicable Laws or the rules of any recognized stock exchange or quotation system as applicable, which are governed by Section 9.3(c)(ii) above. Each Party shall have the right to review and comment on a draft of any such material proposed for publication by the other Party, including for purposes of ensuring that none of its Confidential Information is disclosed without its permission. The Party proposing any such publication shall deliver a complete draft to the other Party at least [ * ] prior to submitting the material to a publisher or initiating any other release. Such other Party shall review any such material and give its comments to the Party proposing publication within [ * ] of the delivery of such draft to such other Party. The publishing Party shall comply with the other Party’s request to: delete from any such proposed publication material prior to its submission or release any references to the other Party and/or any of its Confidential Information; and/or delay any submission or release for a period of up to an additional [ * ] to permit the other Party to prepare and file, or have prepared and filed, any patent applications for any Development Program Inventions as contemplated hereunder.
10.2 Publicity. ▇▇▇▇▇▇▇ hereby consents to EBP’s issuance of the press release attached hereto as Exhibit 8 after execution of this Agreement. No other press release, announcement, or other public statement, whether oral or written, disclosing the existence of this Agreement, any terms hereof, or any information relating to this Agreement or performance hereunder shall be made, either directly or indirectly, by a Party without the prior written consent of the other Party, except as may be legally required by Applicable Laws or judicial order, without first obtaining the consent of the other Party as to the nature, text, and timing of such announcement, which consent shall not be unreasonably withheld. A Party desiring to make any such public announcement shall provide the other Party with a draft thereof at least [ * ] prior to the date on which such Party would like to make the public announcement. For the avoidance of doubt, this Section 10.2 shall not prohibit either Party from making any public statement as required to comply with any duty of disclosure it may have pursuant to Applicable Laws or the applicable rules of any recognized stock exchange or quotation system as applicable. A Party may reissue a press release or public announcement or make such other public statement if the
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 32 of 60
contents of such press release, public announcement or public statement have previously been made public other than through a breach of this Agreement by the issuing Party or its Affiliates.
10.3 Use of Names. Nothing contained in this Agreement will be construed as conferring any right to a Party to use in advertising, publicity or other promotional activities any name, trade name, trademark or other designation of the other Party or any of its Affiliates (including a contraction, abbreviation or simulation of any of the foregoing).
11. REPRESENTATIONS, WARRANTIES AND COVENANTS; DISCLAIMERS
11.1 Representations and Warranties by Each Party. Each Party represents and warrants to the other Party as of the Execution Date that:
(a) it is duly organized, validly existing, and in good standing under the laws of its jurisdiction of formation;
(b) it has full corporate power and authority to execute, deliver, and perform this Agreement, and has taken all corporate action required by law and its organizational documents to authorize the execution and delivery of this Agreement and the consummation of the transactions contemplated by this Agreement;
(c) this Agreement constitutes a valid and binding agreement enforceable against it in accordance with its terms (except as the enforceability thereof may be limited by bankruptcy, bank moratorium or similar laws affecting creditors’ rights generally and laws restricting the availability of equitable remedies and may be subject to general principles of equity whether or not such enforceability is considered in a proceeding at law or in equity); and
(d) the execution and delivery of this Agreement and all other instruments and documents required to be executed pursuant to this Agreement, and the consummation of the transactions contemplated hereby do not and shall not (i) conflict with or result in a breach of any provision of its organizational documents, (ii) result in a breach of any agreement to which it is a party; or (iii) to its knowledge, violate any Applicable Laws.
11.2 Additional Representations and Warranties by ▇▇▇▇▇▇▇. ▇▇▇▇▇▇▇ represents and warrants to EBP as of the Execution Date that:
(a) Exhibit 2(A) lists all Patent Rights existing as of the Execution Date that are owned by ▇▇▇▇▇▇▇ or any of its Affiliates and include any claim Covering any Compounds or their manufacture or use, or any Product in clinical development as of the Execution Date or its formulation or use; and Exhibit 2(B) lists all sublicensable Patent Rights that are licensed by ▇▇▇▇▇▇▇ or any of its Affiliates and include any claim Covering any Compounds or their manufacture or use, or any Product in clinical development as of the Execution Date or its formulation or use. and to the knowledge of ▇▇▇▇▇▇▇, neither ▇▇▇▇▇▇▇ nor any of Affiliates owns or otherwise controls any Patent Rights necessary or reasonably useful to Develop, Manufacture,
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 33 of 60
use, import, offer for sale, sell, or otherwise Commercialize any Compound or Product as formulated by ▇▇▇▇▇▇▇ for its clinical trials in the Field in the Territory other than those listed on Exhibit 2(A) and Exhibit 2(B);
(b) ▇▇▇▇▇▇▇ or an Affiliate thereof is the sole and exclusive owner of the Patent Rights listed in Exhibit 2(A), except as otherwise noted therein;
(c) to the knowledge of ▇▇▇▇▇▇▇, the ▇▇▇▇▇▇▇ Know-How contained in the records listed in Exhibit 3, which will be updated within [ * ] of the Effective Date, includes all Know-How in ▇▇▇▇▇▇▇’▇ possession and Control as of the Execution Date that is necessary or reasonably useful for to the Development and Commercialization of a Compound;
(d) ▇▇▇▇▇▇▇ has provided to EBP true and complete copies of the UT License as in effect on the Effective Date (excluding the financial terms), the UT License is in full force and effect, and ▇▇▇▇▇▇▇ has complied with all terms of the UT License material to this Agreement;
(e) to the knowledge of ▇▇▇▇▇▇▇, the records listed in Exhibit 5 includes all Existing Third Party Agreements material to the Development or Commercialization of any Compound in the Field in the Territory;
(f) ▇▇▇▇▇▇▇ has the right to grant the licenses and other rights to EBP as purported to be granted pursuant to this Agreement, including the right to use and disclose and to enable EBP to use and disclose (in each case under appropriate conditions of confidentiality) the ▇▇▇▇▇▇▇ Know-How, and ▇▇▇▇▇▇▇ has not granted any license or other rights to any Third Party that is inconsistent with the licenses and rights granted to EBP hereunder;
(g) to the knowledge of ▇▇▇▇▇▇▇ and except to the extent not yet due, all necessary and material application, registration, maintenance and renewal fees in respect of the pending or extant ▇▇▇▇▇▇▇ Patent Rights listed in Exhibit 2(A) in existence as of the Effective Date have been paid and, except to the extent not yet due, all necessary documents and certificates have been filed with the relevant Patent Offices for the purpose of maintaining such ▇▇▇▇▇▇▇ Patent Rights;
(h) to the knowledge of ▇▇▇▇▇▇▇, there are no claims, judgments or settlements against ▇▇▇▇▇▇▇ relating to the ▇▇▇▇▇▇▇ Patent Rights listed in Exhibit 2(A);
(i) to the knowledge of ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ has not received any notice from any Third Party asserting or alleging that tipifarnib (also known as R115777) or its method of use in clinical trials by or on behalf of ▇▇▇▇▇▇▇ before the Execution Date infringed or misappropriated any intellectual property rights of any Third Party; and
(j) to the knowledge of ▇▇▇▇▇▇▇, there is no actual infringement of any ▇▇▇▇▇▇▇ Patent Rights by any Third Party.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 34 of 60
11.3 Covenants.
(a) No Conflict. ▇▇▇▇▇▇▇ shall not grant any right or enter into any agreement with any Third Party that would conflict with any of EBP rights or ▇▇▇▇▇▇▇’▇ obligations under this Agreement. EBP shall not grant any right or enter into any agreement with any Third Party that would conflict with any of ▇▇▇▇▇▇▇’▇ rights or EBP obligations under this Agreement.
(b) Intellectual Property Ownership and Confidentiality. Each Party shall require that all of its and its Affiliates’ employees, consultants, contractors and agents involved in the Development, Manufacture or Commercialization of Compounds or Products have entered into written confidentiality and invention assignment agreements that are consistent with the terms of this Agreement and pursuant to which they assign any rights they may have in any inventions relating to Compounds or Products made during such work to such Party; provided, however, that such invention assignment requirement shall not apply with respect to a contractor or consultant that is a university or other non-profit research institution or academic collaborator if a non-exclusive license (with or without any right to obtain an exclusive license), with right to grant sublicenses, to any such inventions relating to Compounds or Products made during work performed by such contractor or consultant and to corresponding Patent Rights thereon is granted to such Party so as to preserve each Party’s ability to exercise its rights as provided hereunder without any payment obligation to any such contractor or consultant.
(c) Compliance with Law. Each Party shall perform its obligations under this Agreement in accordance with all Applicable Laws, including FCPA. No Party shall, or shall be required to, undertake any activity under or in connection with this Agreement which violates, or which it believes, in good faith, may violate, any Applicable Laws. Without limiting the foregoing, each Party agrees that it shall, and shall cause its Affiliates and sublicensees to, (a) comply with all applicable international, national, state regional and local laws and regulations, including FCPA, in performing its obligations and/or exercising its rights hereunder, including with respect to any use, manufacture, sale or import of Products, (b) observe all applicable United States and foreign laws with respect to the transfer of Products and related technical data to countries other than the United States, including all Export Control Laws, and (c) manufacture Products in compliance with applicable government importation laws and regulations of a particular country for Products made outside the particular country in which such Products are used, sold or otherwise exploited. In furtherance of the foregoing, each Party and its subcontractors and sublicensees shall conduct their activities hereunder in accordance with the guidelines set forth in Exhibit 7 (Compliance with Laws and the FCPA).
11.4 Debarment. EBP shall not use in conducting any applicable Development activities under this Agreement any person who has been:
(a) debarred, or proposed to be debarred under Section 306(a) or 306(b) of the United States Federal Food, Drug and Cosmetic Act, as amended from time to time, and the rules, regulations and guidelines promulgated thereunder, or under 42 U.S.C. Section 1320-7;
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 35 of 60
(b) sanctioned by, suspended, debarred, excluded or otherwise ineligible to participate in any federal or state health care program, including Medicare and Medicaid or in any federal procurement or non-procurement programs; or
(c) charged with or convicted of any felony or misdemeanor under 42 U.S.C. Section 1320a-7(a) or 42 U.S.C. Section 1320a-7(b)(1)-(3), or otherwise proposed for exclusion.
EBP will promptly inform ▇▇▇▇▇▇▇, but in no event later than five (5) Business Days, if EBP becomes aware that its or any of its Affiliates or sublicensees or subcontractors, or any employee of EBP or any of its Affiliates or sublicensees or subcontractors, in each case performing any Development activities under this Agreement or in support of the Marketing Authorizations, is not in compliance with any of the criteria set forth in this Section 11.4 on or after the Effective Date.
11.5 Limitations. Notwithstanding anything contained in this Agreement, ▇▇▇▇▇▇▇ gives no warranty and makes no representation that any patent application within the ▇▇▇▇▇▇▇ Patent Rights shall proceed to grant or that any patent within the ▇▇▇▇▇▇▇ Patent Rights will be valid and enforceable. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, EACH PARTY EXPRESSLY DISCLAIMS ANY AND ALL REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING ANY WARRANTIES OF NON-INFRINGEMENT OR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY, EITHER EXPRESS OR IMPLIED, THAT ANY OF THE DEVELOPMENT AND/OR COMMERCIALIZATION EFFORTS WITH REGARD TO ANY PROGRAM COMPOUND OR PRODUCT WILL BE SUCCESSFUL.
12. INDEMNIFICATION; INSURANCE
12.1 Indemnification by EBP. EBP shall, and shall require the EBP Sublicensees to, indemnify and hold harmless ▇▇▇▇▇▇▇ and its Affiliates, and their respective officers, directors, employees, contractors, agents and assigns (each, a “▇▇▇▇▇▇▇ Indemnified Party”), from and against any losses, damages and liability, including reasonable legal expense and attorneys’ fees (collectively, “Indemnified Losses”), incurred by any ▇▇▇▇▇▇▇ Indemnified Party as a result of any Third Party demands, claims or actions, including product liability claims (collectively, “Claims”) against any ▇▇▇▇▇▇▇ Indemnified Party arising or resulting from: (a) the negligence or willful misconduct of EBP in performing EBP’s obligations or exercising EBP’s rights under this Agreement; (b) the breach of any of the covenants, warranties and representations made by EBP to ▇▇▇▇▇▇▇ under this Agreement; (c) Development Program activities conducted by or on behalf of EBP; or (d) the Development, Manufacture, use, sale, offer for sale, other Commercialization or importation of any Compounds or Products in the Field in the Territory by EBP or any of its Affiliates, licensees or sublicensees (other than ▇▇▇▇▇▇▇, if applicable). Notwithstanding the foregoing, EBBP shall not be responsible for the indemnification of any ▇▇▇▇▇▇▇ Indemnified Party to the extent that the Indemnified Losses of such ▇▇▇▇▇▇▇ Indemnified Party were caused
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 36 of 60
by: (i) the negligence or willful misconduct of such ▇▇▇▇▇▇▇ Indemnified Party; or (ii) any breach by ▇▇▇▇▇▇▇ of its covenants, obligations, warranties or representations pursuant to this Agreement.
12.2 Indemnification by ▇▇▇▇▇▇▇. ▇▇▇▇▇▇▇ shall indemnify and hold harmless EBP and its Affiliates and Sublicensees, and their respective officers, directors, employees, contractors, agents and assigns (each, an “EBP Indemnified Party”), from and against Indemnified Losses incurred by any EBP Indemnified Party as a result of any Claims against any EBP Indemnified Party arising or resulting from: (a) the research, development, manufacture or commercialization of any Compounds and/or Products by or on behalf of ▇▇▇▇▇▇▇, its Affiliates or any ▇▇▇▇▇▇▇ Sublicensee; (b) the negligence or willful misconduct of ▇▇▇▇▇▇▇ in performing ▇▇▇▇▇▇▇’▇ obligations or exercising ▇▇▇▇▇▇▇’▇ rights under this Agreement; or (c) the breach of any of the covenants, warranties and representations made by ▇▇▇▇▇▇▇ to EBP under this Agreement. Notwithstanding the foregoing, ▇▇▇▇▇▇▇ shall not be responsible for the indemnification of any EBP Indemnified Party to the extent that the Indemnified Losses of such EBP Indemnified Party were caused by: (i) the negligence or willful misconduct of such EBP Indemnified Party; or (ii) any breach by EBP of its covenants, obligations, warranties or representations pursuant to this Agreement.
12.3 Indemnification Procedure.
(a) Notification. Any ▇▇▇▇▇▇▇ Indemnified Party or EBP Indemnified Party seeking indemnification hereunder (“Indemnified Party”) shall notify the Party against whom indemnification is sought (“Indemnifying Party”) in writing reasonably promptly after the assertion against the Indemnified Party of any Claim in respect of which the Indemnified Party intends to base a claim for indemnification hereunder, but the failure or delay so to notify the Indemnifying Party shall not relieve the Indemnifying Party of any obligation or liability that it may have to the Indemnified Party, except to the extent that the Indemnifying Party demonstrates that its ability to defend or resolve such Claim is adversely affected thereby.
(b) Indemnifying Party Right to Handle Claims. Subject to the provisions of Section 12.3(d) and (e) below, the Indemnifying Party shall have the right, upon written notice given to the Indemnified Party within thirty (30) days after receipt of the notice from the Indemnified Party of any Claim, to assume the defense and handling of such Claim at the Indemnifying Party’s sole expense, in which case the provisions of Section 12.3(c) below shall govern.
(c) Indemnifying Party Handling of Claims. The Indemnifying Party shall select counsel reasonably acceptable to the Indemnified Party in connection with conducting the defense and handling of such Claim, and the Indemnifying Party shall defend or handle the same in consultation with the Indemnified Party, and shall keep the Indemnified Party timely apprised of the status of such Claim. The Indemnifying Party shall not, without the prior written consent of the Indemnified Party, agree to a settlement of any Claim which could lead to liability or
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 37 of 60
create any financial or other obligation on the part of the Indemnified Party for which the Indemnified Party is not entitled to indemnification hereunder, or would involve any admission of wrongdoing on the part of the Indemnified Party. The Indemnified Party shall cooperate with the Indemnifying Party, at the request and expense of the Indemnifying Party, and shall be entitled to participate in the defense and handling of such Claim with its own counsel and at its own expense. Notwithstanding the foregoing, in the event the Indemnifying Party fails to conduct the defense and handling of any Claim in good faith after having assumed such, then the provisions of Section 12.3(e) below shall govern.
(d) Right of Indemnified Party to Assume Handling of Claims. If the Indemnifying Party does not give written notice to the Indemnified Party, within thirty (30) days after receipt of the notice from the Indemnified Party of any Claim, of the Indemnifying Party’s election to assume the defense and handling of such Third Party Claim, the provisions of Section 12.3(e) below shall govern.
(e) Indemnified Party Handling of Claims. Unless Section 12.3(c) applies, the Indemnified Party may, at the Indemnifying Party’s expense, select counsel reasonably acceptable to the Indemnifying Party in connection with conducting the defense and handling of such Claim and defend or handle such Claim in such manner as it may deem appropriate, provided, however, that the Indemnified Party shall keep the Indemnifying Party timely apprised of the status of such Claim and shall not settle such Claim without the prior written consent of the Indemnifying Party, which consent shall not be unreasonably withheld. If the Indemnified Party defends or handles such Claim, the Indemnifying Party shall cooperate with the Indemnified Party, at the Indemnified Party’s request but at no expense to the Indemnified Party, and shall be entitled to participate in the defense and handling of such Claim with its own counsel and at its own expense.
12.4 Insurance. Each Party, at its own expense, shall maintain liability insurance in an amount consistent with industry standards during the Term, but in no event shall such insurance be in an amount less than [ * ] per occurrence/annual aggregate during the Term. In addition, during the term of commercialization and for a period of at least [ * ] thereafter, EBP shall maintain product liability insurance in an amount not less than [ * ] per occurrence and annual aggregate. A Party responsible for the conduct any clinical studies hereunder shall maintain clinical trial insurance in compliance with all Applicable Law pertaining to the jurisdictions in which such clinical studies are conducted. Each Party shall provide a certificate of insurance evidencing such coverage to the other Party upon its written request. Each Party shall notify the other thirty (30) days in advance of cancelation of any such insurance.
12.5 Materials Provided As Is. EEBP acknowledges that compounds, reagents, and other materials supplied by ▇▇▇▇▇▇▇ hereunder are experimental in nature and provided as is, without any warranties as to merchantability or fitness for a particular purpose. EBP further acknowledges that all of such materials’ properties or characteristics are not known, and agrees that it shall use such materials with reasonable care and shall assume responsibility for any losses
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 38 of 60
or injuries incurred by it or its Affiliates or subcontractors or sublicensees through use of such materials.
13. TERM AND TERMINATION
13.1 Term. The term of this Agreement (the “Term”) will commence on the Effective Date and, subject to earlier termination in accordance herewith, shall expire on the last to occur of: (a) the expiry of the last-to-expire patent term, or conclusion of Prosecution of the last-to-be-Prosecuted, of the ▇▇▇▇▇▇▇ Patent Rights; or (b) the expiration of the last-to-expire Royalty Term.
13.2 Termination for Cause by Either Party.
(a) By ▇▇▇▇▇▇▇ for EBP’s Lack of Diligence. In the event that EBP fails to use Commercially Reasonable Efforts to Develop and Commercialize at least one Product with respect to a Major Market Country as described in Sections 4.1 and 5.1, then (without limiting ▇▇▇▇▇▇▇’▇ right to seek termination of the entire Agreement pursuant to Section 13.2(b) below if such breach by EBP is material to the Agreement in its entirety) ▇▇▇▇▇▇▇ may terminate EBP’s license rights under this Agreement with respect to such Major Market Country upon written notice to EBP, provided that EBP will have a period of [ * ] following receipt of such notice to demonstrate to ▇▇▇▇▇▇’▇ reasonable satisfaction that EBP has not failed to use Commercially Reasonable Efforts in accordance with Section 4.1 or 5.1. Notwithstanding anything to the contrary in this Agreement, EBP’s and the EBP Sublicensees’ collective efforts and resources expended toward Developing and Commercializing any Products throughout the Territory shall be considered in determining whether EBP has met its diligence obligations under Sections 4.1 and 5.1 with respect to any particular Major Market Country.
(b) By Either Party for the Other Party’s Material Breach. If either ▇▇▇▇▇▇▇ or EBP (in such capacity, the “Breaching Party”) is in material breach of this Agreement (excluding any breach described in Section 13.2(a), in which case such provision shall govern), the other Party (in such capacity, the “Non-Breaching Party”) may give written notice to the Breaching Party specifying the claimed particulars of such breach, and in such event, if the breach is not cured within [ * ] after such notice ([ * ] in the event of failure to make any payment when due), the Non-Breaching Party shall have the right thereafter to terminate this Agreement by giving written notice to the Breaching Party to such effect, provided, however that if such breach (other than failure to make any payment when due) is capable of being cured but cannot be cured within such [ * ] period and the Breaching Party initiates actions to cure such breach within such period and thereafter diligently pursues such actions, the Breaching Party shall have an additional [ * ] to cure such breach.
(c) Suspension of Time Periods for Curing Breach. From the date of initiation of any measures under Section 15.6 to resolve a Dispute pertaining to an alleged breach under Section 13.2(a) or (b) and until such time as such Dispute has been finally resolved under
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 39 of 60
Section 15.6, the running of the time periods under this Section 13.2 as to which a Party must cure a breach of this Agreement shall be suspended as to the subject matter of the Dispute.
(d) By Either Party for the Other Party’s Bankruptcy. In the event of the Bankruptcy of a Party (or its successor in interest in the event this Agreement is assigned as permitted hereunder), the other Party may terminate this Agreement by notice to the bankrupt Party.
13.3 Termination Without Cause by EBP. EBP may terminate this Agreement at any time after the [ * ] upon [ * ] prior written notice to ▇▇▇▇▇▇▇.
14. EFFECT OF TERMINATION
14.1 Effect of Termination of Rights in Particular Country. Upon any early termination with respect to a Major Market Country under Section 13.2(a), any licenses and sublicenses granted by ▇▇▇▇▇▇▇ to EBP with respect to such Major Market Country will terminate and revert to ▇▇▇▇▇▇▇, and the Territory shall be redefined to exclude such Major Market Country from the scope of the Territory, and the terms of Section 14.2 below shall apply mutatis mutandi with respect to such Major Market Country.
14.2 Effect of Termination by ▇▇▇▇▇▇▇ under Section 13.2(b) or by EBP under Section 13.3. Upon any early termination of this Agreement in its entirety by ▇▇▇▇▇▇▇ pursuant to 13.2(b) or by EBP pursuant to Section 13.3:
(a) The licenses and sublicenses granted by ▇▇▇▇▇▇▇ to EBP will terminate and revert to EBP (except any license in any country that has become perpetual and irrevocable as provided in Section 6.3(b)).
(b) If EBP has initiated clinical development of, or obtained Marketing Authorization for, any Compounds or Products or Commercialized any Products (each a “Reverted Product”), EBP shall promptly provide to ▇▇▇▇▇▇▇ a summary of the status of the Development and Commercialization of any such Reverted Products up to such termination and: (i) ▇▇▇▇▇▇▇ shall have, and EBP hereby grants to ▇▇▇▇▇▇▇, a paid-up, exclusive option, during the [ * ] running from termination of this Agreement, to elect to develop and commercialize any such Reverted Products; and (ii) during such option period, prior to notice of ▇▇▇▇▇▇▇’▇ election decision or upon EBP reasonable request, ▇▇▇▇▇▇▇ shall permit EBP to undertake activities to wind down in a commercially reasonable manner any ongoing development or commercialization activities with respect to each such Product for which EBP license rights under this Agreement have been terminated (subject to EBP obligation under Sections 6.2 and 6.3 to pay any milestones and royalties that may accrue during such wind-down period on account of Net Sales of such Reverted Products from the supply on hand as of the termination). Promptly after EBP receipt of a notice within the [ * ] option exercise period of ▇▇▇▇▇▇▇’▇ election to take over development and commercialization of such a Reverted Product, the Parties
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 40 of 60
shall negotiate in good faith and enter into a written confirmatory agreement under which: (x) EBP shall ▇▇▇▇▇ ▇▇▇▇▇▇▇ a worldwide, exclusive, sublicenseable right and license to develop and commercialize such Reverted Product under the EBP Patent Rights (if any) and applicable Know-How (including data submitted to Regulatory Authorities) Controlled by EBP (directly or through its Affiliates or sublicensees) that was developed by or on behalf of EBP in its development of the Product; and (y) ▇▇▇▇▇▇▇ shall pay EBP a royalty on Net Sales of such Reverted Product [ * ], with provisions parallel to those set forth in Sections 6.3 and 7 hereof applicable mutatis mutandi to ▇▇▇▇▇▇▇’▇ royalty payments. Moreover, if ▇▇▇▇▇▇▇ reasonably requests in the notice of its exercise of such option rights under this Section that EBP also ▇▇▇▇▇ ▇▇▇▇▇▇▇ rights to trademarks Controlled by EBP that are directly associated with the Reverted Product, or to any valuable core or platform technology utilized by EBP to manufacture or commercialize the Product that is Covered by Patent Rights Controlled by EBP, the confirmatory agreement shall specify the terms (including any agreed-upon transfer cost payments from ▇▇▇▇▇▇▇ to EBP) under which EBP would transfer to such requested rights in trademarks associated with the Reverted Product and/or licenses under such Patent Rights (solely to the extent necessary for the development and/or commercialization of the Reverted Product), which terms will be commercially reasonable and fair considering the particular reason for termination. For clarification, any license granted to ▇▇▇▇▇▇▇ as described in this Section 14.2(b) will include the right to use clinical and regulatory data and information generated by EBP for regulatory purposes relating to the Reverted Products. In connection with any exclusive license to Reverted Products granted under this Section 14.1(b), EBP shall transfer and assign to ▇▇▇▇▇▇▇ all of its right, title and interest in and to all U.S. and foreign Marketing Authorizations with respect to the Reverted Products and all drug master files and drug dossiers with respect to the Reverted Products (other than those related to manufacturing facilities).
(c) EBP or EBP Sublicensees shall continue, to the extent that EBP or EBP Sublicensees continue to have stocks of usable Reverted Products, to fulfill orders received for Products in the Territory until [ * ] following the date of termination. For Reverted Products sold by EBP or EBP Sublicensees after the effective date of a termination, EBP shall continue to pay sales milestones and royalties pursuant to Sections 6.2(b) and 6.3. Prior to the end of such [ * ] period, EBP shall provide ▇▇▇▇▇▇▇ written notice of an estimate of the quantity of Reverted Products and shelf life remaining in the inventory of EBP or EBP Sublicensees and ▇▇▇▇▇▇▇ shall have the right, upon its election to take an exclusive license to Reverted Products under Section 14.2(b), to purchase any such quantities of Reverted Products from EBP and EBP Sublicensees at a price mutually agreed by the Parties. In addition, EBP shall use commercially reasonable efforts to transition to ▇▇▇▇▇▇▇ upon ▇▇▇▇▇▇▇’▇ request any arrangement with any contractor from which EBP had arranged to obtain supplies of Reverted Products (or the Compounds therein), to the extent permitted under any such agreement with such contractor. In the event that Reverted Products are manufactured by EBP or its Affiliate, then, upon request by ▇▇▇▇▇▇▇, EBP shall continue to provide ▇▇▇▇▇▇▇ with such materials at a price to be agreed by the Parties for not longer than [ * ].
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 41 of 60
(d) In the event that EBP has any Development activities with regard to any Reverted Products ongoing, the Parties shall negotiate in good faith and adopt a plan to wind-down the development activities in an orderly fashion or, at ▇▇▇▇▇▇▇’▇ election of exclusive license rights pursuant to Section 14.2(b), promptly transition such Development activities for any Reverted Products to ▇▇▇▇▇▇▇ or its designee, with due regard for patient safety and the rights of any subjects that are participants in any clinical trials of any Reverted Product and take any actions it deems reasonably necessary or appropriate to avoid any human health or safety problems and in compliance with all Applicable Laws.
(e) The provisions of this Section 14.2 shall survive such termination for so long as ▇▇▇▇▇▇▇ or any of its Affiliates, licensees or sublicensees Develops or Commercializes any Reverted Product hereunder.
(f) Except as provided in this Section 14.2, Eiger will immediately cease to use, distribute, or market the Reverted Products.
(g) Upon ▇▇▇▇▇▇▇’▇ request, EBP will promptly return, or at ▇▇▇▇▇▇▇’▇ option, destroy, any ▇▇▇▇▇▇▇ Know-How or any materials containing the ▇▇▇▇▇▇▇ Know-How or any Confidential Information of ▇▇▇▇▇▇▇ in EBP’s possession, except for one archival copy to safekeep for legal purposes and such records as may be required to be retained by EBP by Applicable Laws, all of which shall continue to be subject to the confidentiality and non-use obligations in Article 9.
14.3 Effect of Termination by EBP under Section 13.2. Upon termination of this Agreement by EBP pursuant to Section 13.2:
(a) The licenses and sublicenses granted by ▇▇▇▇▇▇▇ to EBP will terminate and revert to ▇▇▇▇▇▇▇ (except any license in any country that has become perpetual and irrevocable as provided in Section 6.3(b).
(b) EBP or EBP Sublicensees shall continue, to the extent that EBP or EBP Sublicensees continue to have stocks of usable Reverted Products, to fulfill orders received for Reverted Products in the Field until [ * ] following the date of termination. For Products sold by EBP or EBP Sublicensees after the effective date of a termination, EBP shall continue to pay sales milestones and royalties pursuant to Sections 6.2(b) and 6.3. Except as provided in this Section 14.2(b), EBP will cease to use, distribute, or market the Products.
(c) Following the period set forth in Section 14.2(b), each Party will promptly return, or at the other Party’s option, destroy any Know-How of such other Party or any materials containing such Know-How or any Confidential Information of such other Party in its or its Affiliates’ possession, except for one archival copy to safekeep for legal purposes and such records as may be required to be retained by such Party by Applicable Laws, all of which shall continue to be subject to the confidentiality and non-use obligations in Article 10.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 42 of 60
14.4 Survival. Expiration or termination of this Agreement shall not relieve the Parties of any obligation (including any payment obligations in Article 6) accruing prior to such expiration or termination, nor affect in any way the survival of any other right, duty or obligation of the Parties which is expressly stated elsewhere in this Agreement to survive such termination or expiry. Without limiting the foregoing, the provisions of Articles 1, 9, 14 (including the additional sections referenced therein) and 15 and Sections 7.5, 8.1, 10.2, 10.3, 11.5, 12.1, 12.2, 12.3 and 12.5, and any other provisions that should survive as apparent from the express terms thereof in the context of this Agreement, shall survive expiration or termination of this Agreement.
14.5 Exercise of Right to Terminate. The exercise by either Party of an early termination right provided for under Article 14 shall not give rise to the payment of damages or any other form of compensation or relief to the other Party on account of such exercise.
14.6 Damages; Relief. Subject to Section 14.5, early termination of this Agreement under Article 14 shall not preclude either Party from claiming any other damages, compensation or relief that it may be entitled to upon such termination.
14.7 Rights in Bankruptcy. All rights and licenses and sublicenses granted under or pursuant to this Agreement by a Party to the other are, and will otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code (or comparable provisions of laws of other jurisdictions), licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code (or comparable provisions of laws of other jurisdictions). The Parties agree that the Parties, as licensees of such rights under this Agreement, will retain and may fully exercise all of their rights and elections under the U.S. Bankruptcy Code (and comparable laws of other jurisdictions). The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against either Party under the U.S. Bankruptcy Code (and comparable laws of other jurisdictions), the Party that is not a party to such proceeding will be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, and same, if not already in their possession, will be promptly delivered to them (a) upon any such commencement of a bankruptcy proceeding upon their written request therefor, unless the Party subject to such proceeding elects to continue to perform all of its obligations under this Agreement, or (b) if not delivered under subsection (a) above, following the rejection of this Agreement by or on behalf of the Party subject to such proceeding upon written request therefor by the non-subject Party. All rights, powers and remedies granted hereunder to a Party as a licensee of any intellectual property rights as provided in this Section 14.7 are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity, in the event of the commencement of a Bankruptcy case by or against the granting Party under Applicable Law, and the licensee Party, in addition to the rights, powers and remedies expressly provided herein, shall be entitled to exercise all other such rights and powers and resort to all other such remedies as may now or hereafter exist at law or in equity in such event.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 43 of 60
15. GENERAL PROVISIONS
15.1 Assignment. Neither Party may assign its rights and obligations under this Agreement without the other Party’s prior written consent, except that (a) either Party may assign its rights and obligations under this Agreement or any part hereof to one or more of its Affiliates without the consent of any other Party, provided that the Party assigning to an Affiliate any part of this Agreement shall remain liable and responsible to the non-assigning Party for the performance and observance of all such duties and obligations by such Affiliate; and (b) either Party may assign this Agreement in its entirety to a successor to all or substantially all of its business relating to Compounds and Products, whether by merger, sale of stock, sale of assets or otherwise, provided that in the event of a transaction (whether this Agreement is actually assigned or is assumed by the acquiror by operation of law (e.g., in the context of a reverse triangular merger)), intellectual property rights of the acquiror to such transaction (if other than one of the Parties to this Agreement) existing before such transaction, or arising after such transaction through activities conducted in good faith separately and independently by such acquiror or its Affiliates and without use of any Confidential Information of the acquired Party, as can be demonstrated by adequate evidence, shall not become subject to this Agreement. The assigning Party shall provide the other Party with prompt written notice of any such assignment. This Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and permitted assigns. Any attempted assignment in contravention of the foregoing shall be void.
15.2 Performance by Affiliates; EBP Performance by Subcontractor. Subject to the terms and conditions of this Agreement, any obligation of a Party under or pursuant to this Agreement may be satisfied, met or fulfilled, in whole or in part, either by such Party directly or by any Affiliate of such Party that such Party causes to satisfy, meet or fulfill such obligation, in whole or in part. Each Party shall remain liable for the performance of all actions, agreements and obligations to be performed by any Affiliates of such Party under the terms and conditions of this Agreement. Subject to the terms and conditions of this Agreement, EBP shall have the right to engage subcontractors for the purpose of performing its obligations under this Agreement.
15.3 Severability. Should one or more of the provisions of this Agreement become void or unenforceable as a matter of law, then this Agreement shall be construed as if such provision were not contained herein and the remainder of this Agreement shall be in full force and effect, and the Parties will use their commercially reasonable efforts to substitute for the invalid or unenforceable provision a valid and enforceable provision which conforms as nearly as possible with the original intent of the Parties.
15.4 Special, Indirect and Other Losses. IN NO EVENT SHALL EITHER PARTY OR ANY OF ITS AFFILIATES BE LIABLE FOR SPECIAL, INDIRECT, INCIDENTAL, PUNITIVE OR CONSEQUENTIAL DAMAGES OR FOR ANY ECONOMIC LOSS OR LOSS OF PROFITS SUFFERED BY THE OTHER PARTY, EXCEPT FOR LIABILITY FOR BREACH OF ARTICLE 9 OR TO THE EXTENT ANY SUCH DAMAGES ARE REQUIRED
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 44 of 60
TO BE PAID TO A THIRD PARTY AS PART OF A CLAIM SUBJECT TO INDEMNIFICATION PURSUANT TO ARTICLE 13. PAYMENTS ACCRUED AND PAYABLE UNDER ARTICLE 6 AND NOT PAID WHEN OWED SHALL BE TREATED AS GENERAL DAMAGES (NOT SPECIAL, INDIRECT, INCIDENTAL, PUNITIVE OR CONSEQUENTIAL DAMAGES OR ECONOMIC LOSSES OR LOST PROFITS).
15.5 Governing Law. This Agreement shall be governed by and construed under the laws of the State of New York, U.S., without reference to its conflicts of law principles with the exception of sections 5-1401 and 5-1402 of New York General Obligations Law (without limiting the Parties’ rights and obligations under Section 15.6). The United Nations Conventions on Contracts for the International Sale of Goods shall not be applicable to this Agreement.
15.6 Dispute Resolution.
(a) Resolution of Disputes. The Parties shall negotiate in good faith and use reasonable efforts to settle any Dispute arising from or related to this Agreement or the breach thereof. If the Parties cannot resolve the Dispute within [ * ] of a written request by either Party to the other Party, the Parties agree to hold a meeting, attended by the Senior Officers (or their designee with executive authority), as appropriate in light of the subject matter of the Dispute, to attempt in good faith to negotiate a resolution of the Dispute prior to pursuing other available remedies. If, within [ * ] after such written request, the Parties have not succeeded in negotiating a resolution of the Dispute, and a Party wishes to pursue the matter, each such Dispute that is not an Excluded Claim shall be resolved by binding arbitration in accordance with the Commercial Arbitration Rules and Supplementary Procedures for Large Complex Disputes of the American Arbitration Association (AAA) as then in effect, and judgment on the arbitration award may be entered in any court having jurisdiction thereof. The decision rendered in any such arbitration will be final and not appealable. If either Party intends to commence binding arbitration of such Dispute, such Party will provide written notice to the other Party informing the other Party of such intention and the issues to be resolved. Within [ * ] after the receipt of such notice, the other Party may by written notice to the Party initiating binding arbitration, add additional issues to be resolved.
(b) Arbitration Panel. The arbitration shall be conducted by a panel of three (3) neutral arbitrators, none of whom is a current or former employee or director, or a then-current stockholder, of either Party or their respective Affiliates. Unless otherwise agreed by the Parties, each of the arbitrators will be a lawyer with at least fifteen (15) years of experience with a law firm or corporate law department or who was a judge of a court of general jurisdiction, and who has reasonable experience in arbitrating contract disputes within the pharmaceutical and biotechnology sector. Within [ * ] after receipt of the original notice of binding arbitration (the “Notice Date”), each Party shall select one person to act as arbitrator and the two Party-selected arbitrators shall select a third arbitrator within [ * ] of their appointment. If the arbitrators selected by the Parties are unable or fail to agree upon the third arbitrator, the third arbitrator
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 45 of 60
shall be appointed by the AAA. The place of arbitration shall be New York, New York, and all proceedings and communications shall be in English.
(c) Limited Discovery. It is the intention of the Parties that discovery, although permitted as described herein, will be limited except in exceptional circumstances. The arbitrators will permit such limited discovery necessary for an understanding of any legitimate issue raised in the arbitration, including the production of documents. No later than [ * ] after selection of the third arbitrator, the Parties and their representatives shall hold a preliminary meeting with the arbitrators, to mutually agree upon and thereafter follow procedures seeking to assure that the arbitration will be concluded within [ * ] from such meeting. Failing any such mutual agreement, the arbitrators will design and the Parties shall follow procedures to such effect.
(d) Governing Law. The arbitrators will, in rendering their decision, apply the governing law set forth in Section 15.5.
(e) Interim Relief. Either Party may apply to the arbitrators for interim injunctive relief until the arbitration award is rendered or the controversy is otherwise resolved. Either Party also may, without waiving any remedy under this Agreement, seek from any court having jurisdiction any injunctive or provisional relief necessary to protect the rights or property of that Party pending the arbitration award. The arbitrators shall have no authority to award punitive or any other non-compensatory damages, except as may be permitted by Section 15.4. Each Party shall bear its own costs and expenses and attorneys’ fees and an equal share of the arbitrators’ and any administrative fees of arbitration.
(f) No Disclosure. Except to the extent necessary to confirm or enforce an award or as may be required by Applicable Laws, neither a Party nor an arbitrator may disclose the existence, content, or results of an arbitration without the prior written consent of both Parties. In no event shall an arbitration be initiated after the date when commencement of a legal or equitable proceeding based on the Dispute would be barred by the applicable New York statute of limitations.
(g) Enforcement of Arbitration Award. The Parties consent to the jurisdiction of any appropriate court for the venue in which the arbitration is held for the enforcement of these provisions and the modification, vacation or affirmation of judgment on any award rendered hereunder. Should such court for any reason lack jurisdiction, any court with jurisdiction shall act in the same fashion. Each Party has the right before or, if the arbitrators cannot hear the matter within an acceptable period, during the arbitration to seek from the appropriate court provisional remedies such as preliminary injunction, to avoid irreparable harm, maintain the status quo, or preserve the subject matter of the arbitration. Each Party hereto waives its right to trial of any issue by jury.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 46 of 60
15.7 Injunctive Relief. Notwithstanding the provisions of Section 15.6, each Party acknowledges that, in the event of a breach of an obligation under Article 9 to maintain in confidence the other Party’s Confidential Information, the other Party shall have the right, in addition to any other rights available under Applicable Laws, to seek from any court of competent jurisdiction injunctive relief to restrain any breach or threatened breach of, or otherwise to specifically enforce, any covenant or obligation of such Party under such provisions.
15.8 Force Majeure. Neither Party shall be responsible to the other for any failure or delay in performing any of its obligations under this Agreement or for other non-performance hereunder (excluding, in each case, the obligation to make payments when due) if such delay or non-performance is caused by strike, fire, flood, earthquake, accident, war, act of terrorism, act of God or of the government of any country or of any local government, or by cause unavoidable or beyond the control of any Party hereto. In such event, the Party affected will use commercially reasonable efforts to resume performance of its obligations.
15.9 Waivers and Amendments. The failure of any Party to assert a right hereunder or to insist upon compliance with any term or condition of this Agreement shall not constitute a waiver of that right or excuse a similar subsequent failure to perform any such term or condition by the other Party. No waiver shall be effective unless it has been given in writing and signed by the Party giving such waiver. No provision of this Agreement, including any of its Exhibits or other attachments, may be amended or modified other than by a written document signed by authorized representatives of each Party.
15.10 Relationship of the Parties. Nothing contained in this Agreement shall be deemed to constitute a partnership, joint venture, or legal entity of any type between EBP and ▇▇▇▇▇▇▇, or to constitute one as the agent or employer of the other. Each Party shall act solely as an independent contractor, and nothing in this Agreement shall be construed to give any Party the power or authority to act for, bind, or commit the other.
15.11 Notices. All notices, consents, waivers, and other communications under this Agreement must be in writing and will be deemed to have been duly given when (a) delivered by hand (with written confirmation of receipt), (b) sent by fax (with written confirmation of receipt), provided that a copy is sent by an internationally recognized overnight delivery service (with delivery tracking and confirmation), or (c) when received by the addressee, if sent by an internationally recognized overnight delivery service (with delivery tracking and confirmation), in each case to the appropriate addresses and fax numbers set forth below (or to such other addresses and fax numbers as a Party may designate by notice):
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 47 of 60
If to EBP:
▇▇▇▇▇ ▇▇▇▇
President and CEO, EB Pharma, LLC.
▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇ ▇▇▇▇▇, ▇▇ ▇▇▇▇▇
With a copy to:
▇▇▇▇ ▇▇▇▇
Corporate Counsel, EB Pharma, LLC
▇▇▇▇▇▇ LLP
▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇
If to ▇▇▇▇▇▇▇:
Attn: Chairman
▇▇▇▇▇▇▇ Pharmaceutica NV
▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇
▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇▇
With a copy to:
Chief Intellectual Property Counsel
▇▇▇▇▇▇▇ & ▇▇▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇ & ▇▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇, ▇.▇.▇.
Fax: ▇▇▇-▇▇▇-▇▇▇▇
15.12 Further Assurances. ▇▇▇▇▇▇▇ and EBP each hereby covenants and agrees, without the necessity of any further consideration, to execute, acknowledge and deliver any and all such other documents and take any such other action as may be reasonably necessary to carry out the intent and purposes of this Agreement.
15.13 No Third Party Beneficiary Rights. This Agreement is not intended to and shall not be construed to give any Third Party any interest or rights (including, without limitation, any third party beneficiary rights) with respect to or in connection with any agreement or provision contained herein or contemplated hereby.
15.14 Entire Agreement. This Agreement, including its Exhibits and any other attachments, sets forth the entire agreement and understanding of the Parties as to the subject matter hereof and supersedes all proposals, oral or written, and all other communications
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 48 of 60
between the Parties with respect to such subject matter, including the Confidentiality Agreement. In the event of any conflict between any provisions of the body of this Agreement and any Exhibit or other attachment hereto, the provisions of the body of this Agreement shall prevail.
15.15 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Signatures provided by facsimile transmission or in Adobe™ Portable Document Format (PDF) sent by electronic mail shall be deemed to be original signatures.
15.16 Expenses. Each Party shall pay its own costs, charges and expenses incurred in connection with the negotiation, preparation and completion of this Agreement.
15.17 English Language. This Agreement is in the English language, and the English language shall control its interpretation. In addition, all notices required or permitted to be given under this Agreement, and all written, electronic, oral or other communications between the Parties regarding this Agreement, shall be in the English language.
15.18 Additional Agreements. Each Party further agrees that it has not entered into this Agreement in reliance upon any representation, warranty or undertaking of the other Party which is not expressly set out in this Agreement or the Equity Agreement.
15.19 Effect of Laws. Nothing in this Agreement shall operate to:
(a) exclude any provision implied into this Agreement by law that may not be excluded by law; or
(b) limit or exclude any liability, right or remedy to a greater extent than is permissible under law.
15.20 Government Approvals.
(a) Each Party will use commercially reasonable efforts to obtain any government approval required in its country of domicile to enable this Agreement to become effective, or to enable any payment hereunder to be made, or any other obligation hereunder to be observed or performed. Each Party will keep the other informed of progress in obtaining any such government approval, and will cooperate with the other Party in any such efforts, and notwithstanding anything to the contrary herein, this Agreement shall become effective upon obtaining any such required government approval.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 49 of 60
IN WITNESS WHEREOF, the Parties intending to be bound have caused this Agreement to be executed by their duly authorized representatives.
| ▇▇▇▇▇▇▇ PHARMACEUTICA NV | EB PHARMA LLC | |||||||
| By: | /s/ ▇▇▇ ▇▇▇▇▇▇ |
By: | /s/ ▇▇▇▇▇ ▇▇▇▇ | |||||
| Name: | ▇▇▇ ▇▇▇▇▇▇ | Name: | ▇▇▇▇▇ ▇. ▇▇▇▇ | |||||
| Title: | Managing Director, Chairman | Title: | President and CEO | |||||
| Date: | December 15, 2014 | Date: | December 15, 2014 | |||||
SIGNATURE PAGE TO COLLABORATION, OPTION AND LICENSE AGREEMENT
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 50 of 60
EXHIBIT 1
Structures of R115777 and R208176
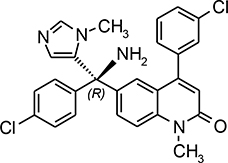
| R115777 | ||
| [ * ] | ||
| R208176 |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 51 of 60
EXHIBIT 2(A)
▇▇▇▇▇▇▇ Patent Rights Owned by ▇▇▇▇▇▇▇ or an Affiliate as of the Execution Date
[[ * ]]
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 52 of 60
EXHIBIT 2(B)
▇▇▇▇▇▇▇ Patent Rights Licensed by ▇▇▇▇▇▇▇ or an Affiliate as of the Execution Date
US Patent No. [*]
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 53 of 60
EXHIBIT 3
Records of ▇▇▇▇▇▇▇ Know-How as of the Execution Date
On Appended DVD(s) with a label including the following: “Exhibit 3 to Tipifarnib License Agreement”
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 54 of 60
EXHIBIT4
Existing Third Party Agreements as of the Execution Date
On Appended DVD with a label including the following: “Exhibit 4 to Tipifarnib License Agreement”
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 55 of 60
EXHIBIT 5
Guidelines on Care and Use of Service Animals
| • | All laboratory research animals housed or used in connection with the Development Program will be treated humanely. They will be housed and cared for in compliance with the Applicable Law governing animal care and use for research (e.g., the Animal Welfare Act (7 USC 2131), the National Research Council Guide for the Care and Use of Laboratory Animals, the EU Commission, or the Japanese Ministry of Health and Welfare). |
| • | No laboratory animal will be subjected to unnecessary pain and/or distress. Where pain and/or distress are unavoidable, appropriate analgesics, anesthetics and tranquilizers will be used except where their use will interfere with the scientific results. Exceptions should be reviewed and approved on a case-by-case basis by the Institutional Animal Care and Use Committee (IACUC) or the Ethics Committee on Animal Experiments. |
| • | Only humane and appropriate methods of euthanasia will be used, as described by the American Veterinary Medical Association Guidelines on Euthanasia (current version) and the EU Commission. |
| • | Prolonged physical restraint will be used only after alternative procedures have been considered and found inadequate. |
| • | Vivaria are or will be accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). |
| • | Purpose-bred animals will be used. In those geographic regions of the world where purpose-bred animals are not available, animals must be obtained through regulated dealers that meet reasonable criteria for the humane care and use of laboratory research animals. |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 56 of 60
EXHIBIT 6
Compliance with Laws and the FCPA
| 1.1. | Each Party shall comply with all laws and regulations concerning its efforts in the Development Program where it is providing work under the Agreement. Each Party shall become familiar with the FCPA, its prohibitions and purposes, and shall not undertake any actions that may violate the FCPA. Accordingly, each Party hereby agrees that: |
| (i) | no person shall be employed by it is an official or employee of any government or any department, agency or instrumentality thereof (including, but not limited to, any health or medical providers owned or controlled by the government); |
| (ii) | no payment or offer to pay, or the giving or offering to give, anything of value to an official or employee of any department, agency or instrumentality thereof (including, but not limited to, any health or medical providers owned or controlled by the government), or to any political party or any candidate for political office, shall be made with the purpose of influencing any decisions favorable to either Party or its Affiliates in contravention of the FCPA or the laws of the country in which it is providing work; |
| (iii) | it not pay, nor offer or agree to pay, nor caused to be paid, directly or indirectly, any political contributions, fees or commissions to any governmental employee or representative (including, but not limited to, any employee of any health or medical provider owned or controlled by the government) that could cause a violation of the FCPA; |
| (iv) | it will not, directly or indirectly, in connection with the Agreement and the business resulting therefrom, offer, pay, promise to pay, or authorize the giving of money or anything of value to any governmental official or representative, to any political party or official thereof, or to any candidate for political office, or to any person, while knowing or being aware of the probability that all or any portion of such money or thing of value will be offered, given, or promised, directly or indirectly, to any government official, to any political party or official thereof, or to any candidate to political office, for the purpose of: |
| a. | influencing any act or decisions of such official, political party, party official, or candidate in its official capacity, including a decision to fail to perform official functions; or |
| b. | inducing such official, political party, party official, or candidate to use influence with the government or instrumentality thereof to affect or influence any act or decision of such government or instrumentality, in order to assist either Party in obtaining or retaining business for or with, or directing business to, any third party. |
| (v) | Each Party will immediately notify the other Party if it becomes aware of any apparent violation of the FCPA in connection with its activities hereunder. |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 57 of 60
| 1.2. | Each Party shall provide the other Party and its agents and representatives (collectively, “Agents”), as well as any regulatory authorities having regulatory oversight of the Party or its Affiliates, with access to its facilities, records (financial and otherwise), and supporting documentation as may be requested by any Agents in order to document or verify compliance with the provisions of this Exhibit. Each Party acknowledges that the provisions of this Exhibit granting the other Party certain audit rights shall in no way relieve the Party of any of its obligations under the Agreement, nor shall such provisions require the other Party to conduct any such audits. |
| 1.3. | Each Party shall maintain true and accurate records necessary to demonstrate compliance with the Agreement (including the requirements of this Exhibit). |
| 1.4. | If a Party fails to comply with any of the provisions of this Exhibit (irrespective of the size, nature or materiality of such violation), such failure may be treated by the other Party as a material breach. |
| 1.5. | Notwithstanding anything to the contrary in the Agreement, a Party may disclose its terms and conditions (including any financial terms) to any government authority that it determines in good faith has a legitimate need for access to such information (including, but not limited to, any governmental authorities in the U.S. or those in the country where research is being provided). |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 58 of 60
EXHIBIT 7
EBP Press Release
EB Pharma Announces License Agreement for Investigational Drug, Tipifarnibfrom ▇▇▇▇▇▇▇ Pharmaceutica for Development in Hepatitis Delta (HDV)
Palo Alto, November X, 2014 /PRNewswire/ — EB Pharma, LLC., a subsidiary of Eiger BioPharmaceuticals, Inc., today announced that it has executed an agreement with ▇▇▇▇▇▇▇ Pharmaceutica NV, (“▇▇▇▇▇▇▇”), for an exclusive license, , to tipifarnib in the field of virology and a related, clinical stage back-up compound. EB Pharma is conducting clinical studies in patients infected with Hepatitis Delta (HDV) and will assess the efficacy and tolerability of tipifarnib as a potential new therapy.
“This novel approach to treating HDV is the culmination of decades of research”, said ▇▇▇▇▇▇▇ ▇▇▇▇▇, MD, PhD, Scientific Founder and Associate Professor of Medicine, Stanford University. “I think it has the potential to change the treatment paradigm for the worst form of human viral hepatitis, and offers new hope for these patients.”
“HDV is the least common but has the poorest outcome of all forms of viral hepatitis”, said ▇▇▇▇▇ ▇▇▇▇, President and Chief Executive Officer of Eiger. “We are excited to license tipifarnib from ▇▇▇▇▇▇▇ and study a potential new therapy for this life threatening disease.”
About Tipifarnib
Tipifarnib is a well-characterized, late stage, orally active inhibitor of farnesyl transferase, an enzyme involved in modification of proteins through a process called prenylation. HDV uses this host cell process inside liver cells to complete a key step in its life cycle. Tipifarnib inhibits the prenylation step of HDV replication inside liver cells and blocks the ability of the virus to multiply. Since prenylation is a host process, not under control of HDV, and tipifarnib inhibits prenylation, there is also a theoretical higher barrier to resistance with tipifarnib therapy. Virus mutation, a common pathway to drug resistance, is not expected to be a potential pathway to tipifarnib resistance by HDV.
Tipifarnib is not approved for sale for any indication.
About HDV
Hepatitis Delta is caused by infection with the hepatitis D virus (HDV) and is considered to be the most severe form of viral hepatitis in humans. Hepatitis D occurs only as a co-infection in individuals with hepatitis B (HBV), leads to more severe liver disease than HBV alone, and is associated with accelerated liver fibrosis, liver cancer, and liver failure. HDV is a disease with a significant impact on global health affecting ~15 million people worldwide. The prevalence of HDV varies
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 59 of 60
between different parts of the world. HDV meets criteria for Orphan Designation in the United States (less than 200,000 people), Europe (less than 5 in 100,000 people), and Japan (less than 50,000 people). Globally, HDV infection is reported to be present in approximately 4% - 6% of chronic hepatitis B carriers. In some parts of the world, including certain areas of China, Russia, Central Asia, Turkey, Africa, and South America, prevalence as high as 40% has been reported in HBV infected patients.
About EB Pharma
EB Pharma is a privately held subsidiary of Eiger BioPharmaceuticals, Inc., focused on the research, development and commercialization of innovative therapies in viral hepatitis. The company will focus on developing tipifarnib for the treatment of Hepatitis Delta Virus (HDV), the most severe form of viral hepatitis. Tipifarnib is not approved for sale. EB Pharma’s research programs are focused on the discovery of targeted, small-molecule therapeutics and biomarkers to treat and monitor serious liver diseases.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Page 60 of 60

