ACQUISITION AGREEMENT
between
HCI VIOCARE TECHNOLOGIES LIMITED
Centrum Offices, 00 Xxxxx Xxxxxx,
Xxxxxxx, X0 0XX, Xxxxxxxx XX
and
XXXXXXXX XXXXXXX
00 Xxxxxxxxx Xxxxx, Xxxxxxxxxx,
Xxxxxxx X00 0XX, Xxxxxxxx, XX
1
CONTENTS
|
Claus
|
Page No.
|
|
|
1.
|
Definitions
|
3
|
|
2.
|
Commencement and Duration
|
6
|
|
3.
|
The Project
|
6
|
|
4.
|
Good Faith
|
6
|
|
5.
|
Company’s Representative
|
6
|
|
6.
|
Acquisition and Consideration
|
7
|
|
7.
|
Intellectual Property Rights
|
7
|
|
8.
|
Infringement
|
8
|
|
9.
|
Exploitation
|
9
|
|
10.
|
Warranties and Representations
|
9
|
|
11.
|
Indemnities and Limitation of Liability
|
10
|
|
12.
|
Confidentiality
|
10
|
|
13.
|
Statements
|
11
|
|
14.
|
Protection of Business Interests
|
11
|
|
15.
|
Termination
|
12
|
|
16.
|
Consequences of Termination
|
13
|
|
17.
|
Entire Agreement
|
13
|
|
18.
|
Variation
|
13
|
|
19.
|
Waiver
|
13
|
|
20.
|
Severability
|
13
|
|
21.
|
Assignation
|
13
|
|
22.
|
Force Majeure
|
13
|
|
23.
|
Notices
|
14
|
|
24.
|
Governing Law
|
14
|
|
SCHEDULE
|
||
|
Part 1 – Details of the Project
|
15
|
|
|
Part 2 – Individual’s Background IPR
|
16
|
2
THIS AGREEMENT (the “Agreement”) dated February 12, 2014 (the “Effective Date”) is made by and between
HCI VIOCARE TECHNOLOGIES LIMITED, a company registered in the UK under number 467480, whose registered office is at Centrum Offices, 00 Xxxxx Xxxxxx, Xxxxxxx, X0 0XX, Xxxxxxxx XX (the “Company”); and
XXXXXXXX XXXXXXX, resident of 00 Xxxxxxxxx Xxxxx, Xxxxxxxxxx, Xxxxxxx X00 0XX, Xxxxxxxx XX (the “Individual”)
WHEREAS:
(A) The Individual is the proprietor of the Individual’s Background IPR, which the Company wishes to acquire in order to undertake the Project;
(B) the Individual is willing to assign the Individual’s Background IPR to the Company for the purposes of the Project and to enable the Exploitation of the results of the Project; and
(C) the Parties wish to record in writing the terms on which they have agreed to collaborate in the Project and in the Exploitation of the results of the Project.
NOW THEREFORE, it is hereby agreed as follows:
|
1.
|
Definitions
|
|
1.1.
|
In this Agreement, unless the context requires otherwise, the following words and phrases shall have the meanings set opposite them:
|
|
“Agreement”
|
means this Agreement together with the Schedule and recitals;
|
|
“Commencement Date”
|
means 16 January, 2014, notwithstanding the date or dates of execution of this Agreement;
|
|
“Confidential Information”
|
means any and all confidential and proprietary information of any of the parties, including, but not limited to, information relating to the business, finances, transactions and affairs of any of the parties, and any other information which is designated as confidential by a Party or which, because of its character or the circumstances or manner of its disclosure is evidently confidential;
|
|
“Consideration”
|
means the consideration as defined under Section 6.1 herein
|
3
|
“Documentation”
|
means all papers relating to the Individual’s Background IPR, their applications, designs, drawings, research production, prototypes and formulation details;
|
|
“Exploitation”
|
means the commercial exploitationofthe Individual's Background IPR, the Foreground IPR, and/or any Patent, and "Exploit" shall be construed accordingly;
|
|
“Exploitation Proceeds”
|
means any and all proceeds generated from the Exploitation of the Individual's Background IPR, the Foreground IPR and/or any Patent by way of licensing to a third party other than a Spin-out Company, which are received by the Company, whether in money or money's worth, including, for the avoidance of doubt, cash, or shares in a company other than a Spin-out Company;
|
|
“Foreground IPR”
|
means all and any IPR arising, created or developed in the course of and relating to the Project but excluding all the Individual’s Background IPR;
|
|
“Group Company”
|
means any undertaking which from time to time is: (a) a subsidiary of the Company; (b) apparent undertaking of the Company; or (c) a subsidiary of any such parent undertaking where "subsidiary undertaking" and "parent undertaking" have the meanings given to them in the Companies Xxx 0000 section 258;
|
|
“Individuals Background IPR”
|
means any and all IPR owned or available for use by the Individual, acquisition of which the Company determines is necessary or desirable for the purposes of the Project, including but not limited to that which is set out in Part 2 of the Schedule;
|
|
“IPR”
|
means any and all intellectual property or industrial rights of any description anywhere in the world including without limitation to the foregoing generality any patents, trademarks, domain names, registered designs, copyright (including without limitation to the foregoing generality rights in computers software, object and source code), rights in the nature of copyright, database rights, semi-conductor topography rights, unregistered design rights, rights in and to trade names, business names, product names and logos, inventions, databases, discoveries, specifications, formulae, processes, know how, trade secrets, confidential information and any analogous or similar right in any jurisdiction (whether any such rights referred to in this definition are registered, unregistered, registerable or not), and any applications or rights to apply for registration of any of them together with any registered rights resulting from any such applications or rights to apply for registration;
|
|
“Knowhow”
|
means all knowhow relating to the Individual’s Background IPR and the Project;
|
|
“New Individual IPR”
|
shall have the meaning given to it in Clause 6.3;
|
|
“Parties”
|
means the Company and the Individual and "Party" shall be construed accordingly;
|
|
“Patent(s)”
|
means any and all patent applications filed by the Parties in accordance with Clause 7.3 and any patents granted pursuant to such applications;
|
|
“Permitted Costs”
|
means those incurred by the Company and arising from the following:
a)the filing, prosecution, procurement and maintenance of any Patent(s) after the commencement of Exploitation;
b)proceedings before the Patent Office or other appropriate forum, including any appeals;
c)professional advice (including consultant’s and advisers’ fee) on the Patent(s) and other intellectual property matters relating to the Individual's Background IPR or the Patent(s) or the Foreground IPRs;
d)proceedings by or against the Company in any court or tribunal for the enforcement or defence of any Patent or for revocation of or opposite onto any such Patent or for any other cause relating to the Individual's Background IPR or the Foreground IPRs (including any costs or sums awarded against the Company in any such proceedings);
e)agents’ commission;
f)marketing and public relations activities in relation to the Individual's Background IPR, the Patent(s) and/or any Foreground IPRs;
g)establishing and managing any Spin-out Company;
h)negotiating and managing any licence of the Individual's Background IPR, the Patent(s) and/or any Foreground IPRs;
i)arranging and maintaining any intellectual property insurance in respect of the Individual's Background IPR, the Patent(s) and/or any Foreground IPRs;
j)any tax, charges, duties, deductions or withholdings which the Company is required to pay or make by law; and
k)any other expenditure which may be agreed from time to time by the Individual and the Company;
|
4
|
“Project”
|
means the final development of the technology entitled ‘Socket Fit’ for the Company to take it to the market, and the funding of any ongoing and/or further development for the purposes of the Project, as more generally described in Part 1 of the Schedule;
|
|
“Schedule”
|
means the schedule in two (2) Parts annexed to this Agreement which shall be deemed incorporated herein;
|
|
“Spin-out Company”
|
means any separate legal entity established by the Company as a vehicle to Exploit the Foreground IPRs;
|
|
1.2.
|
In this Agreement:
|
|
|
1.2.1.
|
the use of the singular includes the plural and vice versa;
|
|
|
1.2.2.
|
references to gender include references to all genders;
|
|
|
1.2.3.
|
unless otherwise stated, any reference to a Clause or Schedule is to the relevant Clause or Schedule of or to this Agreement and any reference to a sub
|
|
|
1.2.4.
|
clause or paragraph is to the relevant sub-clause or paragraph of the Clause or Schedule in which it appears;
|
|
|
1.2.5.
|
the Clause headings are for reference only and will not affect the construction or interpretation of this Agreement; and
|
|
|
1.2.6.
|
references to statutes, any statutory instrument, regulation or order will be construed as a reference to such statute, statutory instrument, regulation or order as amended or re-enacted from time to time.
|
|
1.3.
|
In this Agreement, except where the context otherwise requires, any reference to:
|
|
|
1.3.1.
|
another agreement or any deed or other instrument or document will be construed as a reference to that other agreement, deed or other instrument or document as the same may have been, or may from time to time be, amended, varied, supplemented or notated;
|
|
|
1.3.2.
|
the words “include” or "including" are to be construed as meaning without limitation;
|
|
|
1.3.3.
|
a “day” means a period of 24 hours (or such other number of hours as may be relevant in the case of changes for daylight saving) ending at 12.00 midnight;
|
|
|
1.3.4.
|
a "week" means a period of 7 consecutive days;
|
|
|
1.3.5.
|
a "month" means a calendar month; and
|
|
|
1.3.6.
|
a "person" includes any individual, partnership, firm, company, corporation, joint venture, trust, association, organisation or other entity, in each case whether or not having a separate legal personality.
|
5
|
2.
|
Commencement and Duration
|
This Agreement shall commence on the Commencement Date and shall continue unless terminated in accordance with the provisions of this Agreement.
|
3.
|
The Project
|
|
3.1.
|
The Project will be undertaken by the Company.
|
|
3.2.
|
The Individual will at all times during the period of this Agreement use his best endeavours to promote the interests of the Company.
|
|
3.3.
|
The Individual warrants that he will not, as a consequence of entering into and performing his obligations under this Agreement, be in breach of any terms express or implied (whether concerning confidentiality, non-competition or otherwise) of any contract, agreement or other arrangement with, or any obligation to, any third party binding upon the Individual and that the Individual is under no obligation, covenant or restriction which would or might give rise to any conflict of interest between the Individual and the Company or any Group Company.
|
|
4.
|
Good Faith
|
|
4.1.
|
Subject to sub-clauses 4.2 and 4.3, the Individual may have a financial interest in or advise or act as a consultant to any business provided that the Individual will not during the period of his involvement in the Project (unless with the prior written consent of the Company) undertake any other activities or accept other engagements which may interfere with or detract from the Individual's obligations, whether as specified in this Agreement or otherwise. In the event of a conflict between the Individual's foregoing obligations and the Individual's obligations to any third party, the Individual’s foregoing obligations to the Company or any Group Company shall take precedence.
|
|
4.2.
|
The Individual will not use or otherwise turn to the Individual's advantage the Individual's knowledge of any connection or contact with any of the manufacturers used by customers of, or suppliers to the Company or any Group Company or any prospective manufacturers, customers or suppliers in a manner that is detrimental to the interests of the Company or any Group Company.
|
|
4.3.
|
The Individual will not receive or obtain directly or indirectly any discount, rebate, commission or other benefit in respect of any goods or services supplied to or acquired by the Company or any other business transacted by it and, if the Individual does receive any such discount, rebate, commission or other benefit, the Individual will account to the Company for it.
|
|
5.
|
Company’s Representative:
|
The person within the Company who will act as the main contact point and channel of communication for the Project during the period of this Agreement is Xxxxxxxx Xxxxxxxxxxx. The Company will inform the Individual of any change in the identity of such person.
6
|
6.
|
Acquisition and Consideration
|
|
6.1.
|
In consideration for the acquisition, the Company shall cause the issuance of a total of five hundred thousand (500,000) common shares of the common stock of China Northern Medical Device, Inc., the parent corporation of the Company, to the Individual, which shares shall be issued on the execution of this Agreement and shall be subject to such restrictions as may be required by the requisite regulatory authorities (the “Consideration Shares”).
|
|
6.2.
|
The Individual hereby assigns exclusive, perpetual, irrevocable, transferable, royalty free, worldwide ownership of the Individual’s Background IPR and Knowhow to the Company for the purposes of using it to the extent necessary or desirable to further develop and exploit the Individual’s Background IPR, and to create, develop and exploit the Foreground IPR.
|
|
6.3.
|
The Individual shall, to the extent he has not already done so, disclose all of the Individual’s Background IPR to the Company and deliver all Documentation to the Company.
|
|
6.4.
|
If, following the Commencement Date, the Individual undertakes any research and development work based on or relating to the Individual’s Background IPR and IPR is created or arises as a result of such research and development work ("New Individual
|
|
6.5.
|
IPR") then from the date of its creation, such New Individual IPR shall form part of Individual’s Background IPR and shall be the Company’s exclusive property pursuant to Clause 6.1. The Individual shall disclose all such New Individual IPR to the Company promptly after its creation or arising.
|
|
6.6.
|
The Individual shall, at the request and expense of the Company, promptly execute any further document and perform any further action which may be reasonably required by the Company to provide the full benefit of this Clause 6 to the Company and to give full effect to the intention of the Parties to this Agreement.
|
|
7.
|
Intellectual Property Rights
|
|
7.1.
|
Subject to Clause 7.4, the Parties hereby acknowledge and agree that any and all Foreground IPR arising or created out of the Project shall be the exclusive property of the Company, and the Individual hereby agrees that he shall not acquire any right to any Foreground IPR.
|
|
7.2.
|
The Parties hereby acknowledge and agree that the Company shall be solely responsible for all decisions relating to the preparation, filing, and maintenance of any Patent comprised with the Individual’s Background IPR and/or the Foreground IPR.
|
|
7.3.
|
The Company shall procure that any Patent comprised within the Individual's Background IPR and/or the Foreground IPR shall be filed in the joint names of the Company and the Individual, and that the Individual shall be named as inventor in any such Patent.
|
|
7.4.
|
The Individual hereby assigns to the Company exclusive, perpetual, irrevocable, transferable, royalty free, worldwide ownership of the Individual’s rights in, to, and under any Patent.
|
|
7.5.
|
All costs, fees and expenses relating to the prosecution and maintenance of any Patent application (or application for other intellectual property) shall be borne by the Company.
|
7
|
8.
|
Infringement
|
|
8.1.
|
Each party shall promptly and fully notify the other of:
|
|
8.1.1.
|
any actual, threatened or suspected infringement which comes to its notice of any of the Individual’s Background IPR or of any Foreground IPR or of any of the Company’s Patents irrespective of when such infringement occurred; and
|
|
8.1.2.
|
any proceedings threatened or commenced against it in which the validity of any Individual’s Background IPR or of any Foreground IPR or any of the Company’s Patents is challenged irrespective of when such challenge arose.
|
|
8.2.
|
As soon as practicable after any such notification has been given pursuant to Clause 8.1, the Company shall notify the Individual whether or not it has decided that the proceedings shall be defended in the circumstances referred to in Clause 8.1.2 limited to any of the Individual’s Background IPR.
|
|
8.3.
|
In the event that the Company notifies the Individual that it has decided not to defend any proceedings pursuant to Clause 8.2., the Individual shall be entitled to take such action as it sees fit subject to informing the Company of its proposed action and advising the Company of the outcome.
|
|
8.4.
|
Any action taken pursuant to Clause 8.2 will be at the Company's sole expense and any action taken pursuant to Clause 8.3 will be at the Individual’s sole expense.
|
|
8.5.
|
As exclusive owner the Company shall be free to take such actions as contemplated by Clause 8.2 above (and irrespective of when the challenge occurred) in its own name provided that it consults reasonably with the Individual in this regard prior to such action being taken.
|
|
8.6.
|
Where the Company takes action as provided for in 8.2 above, any award, damages or settlement monies awarded shall accrue to the benefit of the Company, provided that any reasonable and properly validated costs incurred by the Individual in a court action shall be fully reimbursed by the Company.
|
|
8.7.
|
If for any reason (other than lack of funds) the Company cannot take such action as is contemplated by Clause 8.2 above but does wish to defend or commence proceedings or if the Company, as exclusive owner, is unable to pursue any proceedings unless the Individual is joined as a pursuer or defender, the Company can require the Individual to take such action in the Individual’s own name, subject to the following:
|
|
8.7.1.
|
the costs reasonably and necessarily incurred by the Individual shall be met by the Company as they fall due (and the Individual shall keep the Company fully advised of the extent and nature of all such costs as soon as the Individual becomes aware of the same);and
|
|
8.7.2.
|
any award, damages or settlement monies awarded to the Individual shall accrue to the benefit of the Company and, if received by the Individual, shall be held by the Individual as agent for the Company. For the avoidance of doubt, if the Individual takes such action pursuant to Clause 8.3., any award, damages, or settlement monies awarded to the Individual as a result of such action shall accrue to the benefit of the Individual.
|
|
8.8.
|
The Individual shall do all such things and execute all such documents as may reasonably be required of it by the Company for the purpose of assisting the Company in bringing or defending any proceedings as contemplated by Clause 8.2, and the Company shall bear the cost of the provision of such assistance.
|
|
8.9.
|
For the avoidance of doubt, in no circumstances can the Individual agree to the settlement of any such action as contemplated by Clause 8.2 above, without the prior written consent of the Company.
|
8
|
9.
|
Exploitation
|
|
9.1.
|
The Parties hereby acknowledge and agree that the Company shall be solely responsible for all decisions relating to the Exploitation of the Individual's Background IPR, the Foreground IPR and/or any Patent.
|
|
9.2.
|
In the event that the Individual's Background IPR, the Foreground IPR and/or any Patent is exploited by way of the Company establishing a Spin-out Company:
|
|
|
9.2.1.
|
the Company shall procure that the Spin-out Company is granted a licence or sub-licence of the Individual’s Background IPR, the Foreground IPR and any Patent on such terms as may be negotiated with the Spin-out Company;
|
|
|
9.2.2.
|
if required by the Spin-out Company or its prospective investors, the Company shall assign the Individual’s Background IPR to the Spin-out Company;
|
|
10.
|
Warranties and Representations
|
|
10.1.
|
The Individual represents and warrants to the Company that:
|
|
10.1.1.
|
to the best of his knowledge and belief, the Individual is the true and sole owner of the Individual’s Background IPR and there are and will be no liens, charges, encumbrances or restrictions which affect the Company’s ability to acquire the Individual’s Background IPR in accordance with this Agreement;
|
|
10.1.2.
|
the Individual has not assigned property or granted any licence of the Individual’s Background IPR to any other person, company or undertaking;
|
|
10.1.3.
|
the Individual is not aware that any third party owns or claims that it owns any rights which would be infringed by the acquisition of the Individual’s Background IPR by the Company in accordance with the provisions of this Agreement;
|
|
10.1.4.
|
to the best of the Individual’s knowledge and belief, no third party has infringed or threatened to infringe or is infringing or threatening to infringe the Individual’s Background IPR; and
|
|
10.1.5.
|
the Individual has full power and authority to enter into and perform his obligations under this Agreement without being in breach of any obligation or undertaking to any third party.
|
|
10.2.
|
The Company represents and warrants to the Individual that:
|
|
10.2.1.
|
the Company is a corporation duly organized, validly existing, and in good standing under the laws of Scotland, with full corporate power, authority and capacity to conduct its business as presently conducted, to own or use the properties and assets that it purports to own or use, and to perform all its obligations under any applicable contracts;
|
|
10.2.2.
|
the Company has all requisite corporate power and authority to execute and deliver the Agreement to be signed by the Company and to perform its obligations hereunder and to consummate the Acquisition. The execution and delivery of the Agreement by the Company and the consummation of the Acquisition have been duly authorized by the board of directors of the Company. Other than as set out in this Agreement, no other corporate or shareholder proceedings on the part of the Company are necessary to authorize the Agreement or to consummate the Acquisition. This Agreement has been, and the Consideration when issued and delivered by the Company as contemplated by this Agreement will be, duly executed and delivered by the Company and this Agreement is a valid and binding obligation of the Company enforceable in accordance with its respective terms; and
|
|
10.2.3.
|
the Consideration Shares to be issued to the Individual pursuant to this Agreement will, upon issuance, have been duly and validly authorized and, the Consideration Shares when so issued in accordance with the terms of this Agreement, will be duly and validly issued, fully paid and non-assessable shares in the capital of China Northern Medical Device Inc.
|
9
|
11.
|
Indemnities and Limitation of Liability
|
|
11.1.
|
The Company shall indemnify the Individual on demand in respect of all loss, damages, costs, claims, demands and expenses (including all legal and other professional fees and expenses on a full indemnity basis but always excluding all indirect losses) suffered or incurred by the Individual as a result of any breach by the Company of this Agreement or as a result of any negligent act or omission of the Company in connection with this Agreement.
|
|
11.2.
|
The Individual shall indemnify the Company on demand in respect of all loss, damages, costs, claims, demands and expenses (including all legal and other professional fees and expenses on a full indemnity basis but always excluding all indirect losses) suffered or incurred by the Company or any Group Company as a result of any breach by the Individual of this Agreement or as a result of any negligent act or omission of the Individual in connection with this Agreement.
|
|
11.3.
|
Subject to Clause 11.4, the aggregate liability of each Party to the other, whether in contract, delict (including negligence or breach of statutory duty) or otherwise arising in connection with this Agreement shall not exceed five (5) years.
|
|
11.4.
|
Nothing in Clause 11.3 shall exclude or limit the liability of a Party for death or personal injury caused by that Party's negligence, nor for any matter, in respect of which it would be illegal for that Party to exclude or limit, or attempt to exclude or limit, its liability.
|
|
12.
|
Confidentiality
|
|
12.1.
|
The Parties agree and undertake that during the term of this Agreement and thereafter they will keep confidential and will not use for their own purposes except for the performance of this Agreement nor, without the prior written consent of the Party who discloses the relevant Confidential Information, disclose to any third party any Confidential Information provided to them, unless:
|
|
12.1.1.
|
the receiving Party can show such information is public knowledge or already known to the receiving Party at the time of disclosure, or subsequently becomes public knowledge other than by breach of this Agreement; or
|
|
12.1.2.
|
the receiving Party can show that such information subsequently comes lawfully into the possession of the receiving Party from a third party without an obligation of confidentiality; or
|
|
12.1.3.
|
Clause 12.3 applies to such information, to the extent only as permitted by these Clauses.
|
|
12.2.
|
Any Confidential Information disclosed by any Party to any other Party prior to the execution of this Agreement and/or the Commencement Date shall be considered as having been disclosed under this Agreement.
|
|
12.3.
|
Any party may disclose Confidential Information to the extent it is required to do so by a statutory, legal, regulatory or parliamentary obligation placed upon the Party making the disclosure (including in the case of the Company, its obligations to Audit Scotland and any successor body to Audit Scotland), provided, to the extent practicable, that the Party obliged to make the disclosure will provide the Party to whom the Confidential Information belongs with a reasonable opportunity to make representations to the body compelling such disclosure as to the nature and extent of
|
|
12.4.
|
the disclosure required and the procedures for maintaining confidentiality of the Confidential Information to be disclosed.
|
10
|
13.
|
Statements
|
The Individual, will not at any time whether during the term of this Agreement or at any time thereafter make any public statement in relation to the Company or its businesses, affairs, customers or clients or officers and employees except when both the making of and terms of any such statement have been approved by the Company.
|
14.
|
Protection of Business Interests
|
|
14.1.
|
Since the Individual is likely, in the course of the Project, to have access to Confidential Information and dealings with the customers, clients and suppliers and other contacts of the Company or any Group Company, he hereby agrees, in order to safeguard such Confidential Information and the good will of the Company and any such Group Company that, in the event of the termination of this Agreement he shall not for a period of five (5) years (except where expressly stated otherwise in this Clause 14.1) from the date of such termination:
|
|
14.1.1.
|
in competition with the Company, entice or solicit, or endeavour to entice or solicit, away from the Company or any Group Company, the custom or business of any person, company or other undertaking, who or which is or has been a customer or client of the Company or such Group Company, and with whom or which the Individual has regularly dealt at any time during the five (5) years immediately prior to the date of such termination in the course of the Project or by reason of services rendered to or offices held by him in or his employment by the Company or any Group Company; or
|
|
14.1.2.
|
in competition with the Company, accept any business from any person, company, or other undertaking, who or which is or has been a customer or client of the Company or any Group Company and with whom or which the Individual has regularly dealt at any time during the five (5) years immediately prior to the date of such termination in the course of the Project or by reason of services rendered to or offices held by him in or his employment by the Company or any such Group Company, provided that this restriction shall not prohibit the Individual from having business dealings with or accepting business from any such person, company or other undertaking in respect of any business which is not in competition with the Company or any such Group Company as at the date of such termination and in which the Individual was concerned to a material extent during the five (5) years immediately prior to the date of such termination; or
|
|
14.1.3.
|
without the prior written consent of the Company, be engaged, interested or concerned in any business carried on, or about to be carried on, by any person, company or other undertaking which is in competition with any business carried on by the Company or any Group Company as at the date of such termination in any territory in which such business was carried on, provided that this restriction shall not prohibit (i) the Individual from being engaged, interested or concerned in any such business, company or other undertaking so
|
|
14.1.4.
|
far as his/her involvement therein or duties in connection therewith shall relate exclusively to work of a kind or nature with which the Individual was not concerned to a material extent during the five (5) years immediately prior to the date of such termination; or (ii) the Individual from being beneficially interested in any class of securities in any company, if such class of securities is listed or dealt in a recognized stock exchange where the Individual (together with his spouse and children) neither holds nor is interested in more than five percent (5%) of any single class of securities in that company; or
|
|
14.1.5.
|
entice or solicit, or endeavour to entice or solicit, away from the employment of the Company or any Group Company any person who was senior employer or consultant at anytime in the five (5) year period prior to the date of the termination of the Agreement and with whom the Individual regularly dealt or had contact in the course of the Project or by reason of services rendered to or offices held by him in or his employment by the Company or any such Group Company; or
|
|
14.1.6.
|
employ or engaged any person who was a senior employee of or consultant to the Company or any Group Company or any employee or consultant who has Confidential Information about a customer or client (as defined in Clause 5.1) of the Company or any Group Company for a period of five (5) years from the date on which this Agreement terminates; or
|
|
14.1.7.
|
at any time after the date of termination of this Agreement carry on business or trade under a name which is identical or similar to any name used by the Company or any Group Company; or
|
|
14.1.8.
|
at any time after the termination of the Agreement do or say anything which might reasonably be expected to be harmful to the reputation of the Company or any Group Company or which may lead any person to cease to do business with the Company or any Group Company on substantially the same terms to those previously offered or at all.
|
11
The restrictions set out in this Clause 14.1 shall apply to any action taken by the Individual in any capacity (whether as principal, agent, representative, partner, director, party to a joint venture, consultant or otherwise).
|
14.2.
|
On the termination of this Agreement (how so ever caused), the Individual shall not, at any time thereafter, represent himself still to be connected with the Company or any Group Company, except with the prior consent, or at the request, of the Company or to the extent that he shall be so connected as a result of being a shareholder or director of the Company or any Group Company.
|
|
14.3.
|
The Individual acknowledges and agrees that each of sub-paragraphs 14.1.1 to 14.1.7 (inclusive) of Clause 14.1 constitutes an entirely separate and independent restriction on him and that the duration, extent and application of each of such restrictions are no greater than is necessary for the protection of the legitimate interests of the Company and any Group Company for which he is required to perform duties.
|
|
14.4.
|
While the restrictions set out in sub-paragraphs 14.1.1 to 14.1.7 (inclusive) of Clause 14.1 are considered by the Parties to be reasonable in all the circumstances, it is acknowledged that restrictions of such a nature may fail or become invalid for
|
|
14.5.
|
technical reasons unforeseen or because of changing circumstances and, accordingly, the Parties agree that, if any of such restrictions shall adjudged to be void or ineffective as going beyond what is reasonable in all the circumstances for the protection of the interests of the Company or for any other reason but would be valid and effective if part of the wording thereof was deleted and/or any period or are a referred to therein reduced in time or scope, such restrictions shall apply with such deletions or modifications as may be necessary to make them valid and effective.
|
|
15.
|
Termination
|
|
15.1.
|
This Agreement may be terminated by written notice with immediate effect in any of the following events:
|
|
15.1.1.
|
by the Individual in the event of the liquidation (except for the purposes of a solvent reconstruction or amalgamation) or receivership of the Company or the appointment of an administrator of the Company or its ceasing or threatening to cease trading; or
|
|
15.1.2.
|
by the Individual or the Company in the event of the material breach by the other(s) of any of its or their obligations hereunder which is irremediable or if it can be remedied, remains without remedy on the expiry of thirty (30) days after receipt by the Party in breach of written notice from the Party serving notice specifying the breach and the action required to remedy same; or
|
|
15.1.3.
|
by the Company if the Individual is in breach of any of the warranties set out in Clause 11; or
|
|
15.1.4.
|
by the Company if it decides at any point to terminate the Project for any reason.
|
|
15.2.
|
Termination of this Agreement shall not affect any rights or obligations of the Parties in respect of the period up to the date of termination nor shall it affect any rights or obligations of the Parties which, due to the nature thereof, are due to be performed or observed following such termination, including but not limited to Clauses 4.2, 4.3, 6.1, 6.2, 6.4, 6.5, 7, 8.8, 8.9, 9.1, 10, 11, 12, 13, 15, 16 and 24.
|
12
|
16.
|
Consequences of Termination
|
In the event of termination of this Agreement by the Individual under Clause 15.1.1 or by the Company under Clause 15.1.4, the Company shall in good faith negotiate with the Individual a renunciation of the ownership of IPR assigned to the Company under this Agreement and an assignation of the Company’s interest in the Foreground IPR and any Patent(s).
|
17.
|
Entire Agreement
|
This Agreement constitutes the entire agreement and understanding between the Parties with respect to the subject matter hereof and cancels, terminates and supersedes any prior agreement or understanding entered into between the Parties, provided that nothing in this Clause 17 shall have effect to exclude liability of any Party for fraud or fraudulent misrepresentation.
|
18.
|
Variation
|
None of the provisions of this Agreement may be varied, waived, extended or modified except expressly in writing and signed by both Parties.
|
19.
|
Waiver
|
Any omission by either Party to exercise any right or remedy available to that Party under the terms of this Agreement shall not be taken to signify acceptance of the event giving rise to the right to exercise such right or remedy and shall be without prejudice to the future exercise of any such right or remedy and/or to the rights or remedies of either Party which may arise in the future.
|
20.
|
Severability
|
If any provision of this Agreement is declared to be void or unenforceable by any judicial or administrative authority in any jurisdiction in which this Agreement is effective, such provision will be deemed to be severable and the validity and force of the remainder of the provisions of this Agreement shall not be affected thereby. The Parties shall each use their reasonable endeavours in good faith to modify this Agreement so that the intent of the void and/or unenforceable provision of this Agreement can be carried out legally.
Any void or unenforceable provision shall be construed as per the Governing law defined hereinafter.
|
21.
|
Assignation
|
|
21.1.
|
Subject to Clauses 6.1, 7.3, and 21.2, neither Party shall be entitled to assign the benefit or burden of this Agreement without the prior written consent of the other (such consent not to be unreasonably withheld or delayed).
|
|
21.2.
|
The Company shall be entitled to assign or transfer any of its rights or obligations under this Agreement to any Spin-out Company or Group Company.
|
|
22.
|
Force Majeure
|
Neither of the Parties shall be liable to the other nor be held to be in breach of this Agreement to the extent that they are prevented, hindered or delayed in the performance or observance of their obligations hereunder by any cause or contingency whatsoever beyond their reasonable control.
13
|
23.
|
Notices
|
Any notices required to be given pursuant to this Agreement shall be in writing and shall be hand delivered or sent by certified e-mail (return receipt requested) and by confirming letter sent by first class registered mail (or its equivalent) posted within twenty four (24) hours of the said e-mail to the address (and e-mail address) and for the attention of the relevant Party, as set out below (or otherwise notified). Any such notice shall be deemed to have been received twenty-four (24) hours after the time of dispatch to the e-mail in question.
|
The Company:
|
For the attention of: Xxxxxxxx Xxxxxxxxxxx
Centrum Offices, 00 Xxxxx Xxxxxx,
Xxxxxxx, X0 0XX, Xxxxxxxx XX
E-mail: xxxxxx@xxxxxxx.xx
|
|
The Individual:
|
For the attention of: Xxxxxxxx Xxxxxxx
00 Xxxxxxxxx Xxxxx, Xxxxxxxxxx,
Xxxxxxx X00 0XX, Xxxxxxxx XX
E-mail: x.xxxxxxx@xxxxxxxxxx-xxxxxxxxxxx.xxx
|
|
24.
|
Governing Law
|
This Agreement shall be governed by, and is to be construed in accordance with, the law of Scotland, and the Scottish Courts will have exclusive jurisdiction to deal with any dispute which has arisen or may arise out of or in connection with this Agreement.
IN WITNESS whereof the Parties have caused this Agreement to be signed as follows:
HCi VIOCARE TECHNOLOGIES LIMITED
By:/s/ Xxxxxxxx Xxxxxxxx /s/ Xxxxxxxx Xxxxxxx
Name: Xxxxxxxx Xxxxxxxx XXXXXXXX XXXXXXX
Title: Attorney-in-Fact
14
SCHEDULE
PART 1
Details of the Project
The number of amputees world-wide is estimated to be 20 million, and for most amputees finding a well fitted prosthesis is far from easy. Traditional methods of design, manufacture and fitting of a prosthetic socket are typically carried out by “artisan” techniques but unfortunately often result in ill-fitting devices that make wearing a prosthesis almost intolerable for a large number of amputees.
A prosthetic socket is a custom-made “cone” that connects the rest of the prosthesis (foot, shank and knee) to an amputee’s residual limb. Sockets generally need to be replaced once or twice a year. While many significant technological advances have been made with the design and manufacture of prosthetic components, such as electronic knee assemblies and feet, socket design has not kept up. Over the past 20 years a great deal of research has been undertaken to automate the process of socket design and manufacturing, but it has met with limited success. Most sockets continue to be created with hand-sculpted plaster moulds made by the Prosthetist or a technician hours or days after examining the amputee. The result is that, typically, one in four sockets are discarded because of their poor fit.
Many prosthetists, (if not all), are frustrated by the lack of an objective method that would ensure an optimum fit of the socket. This ‘need’ for assistance instigated the advent of industrial sector technologies to be taken up in the field, even though these systems, in reality, lack the necessary ‘ingredient’ to allow the optimum socket to be produced. They merely attempt to replicate current practices in a digital manner (CAD-CAM systems), without taking into account the biomechanical or anatomical characteristics of the residual limb.
The key element of the project is work done by Xx. Xxxxxxxx Xxxxxxx to improve the nature of the data used in socket modeling software. Finite element analysis has been used widely in a variety of applications, including prosthetics. But its prosthetics applications have suffered from the fact that only external boundary data and limited information on the nature of the internal tissue was provided. The results were not promising. By including far more data on the nature of the internal anatomy as well as, for the first time ever (to our knowledge) data on the bio-mechanical properties of the tissue to the FEA, a system can be created that enables prosthetists to build a socket that evenly distributes weight, provides enhanced comfort, and raises the bar across the industry on socket creation.
SocketFit is a digital system for assessing an amputee’s residual limb and for the production of truly functional and comfortable prosthetic sockets. It takes account of the external and internal geometry of the amputee’s xxxxx, the biomechanical properties of the each individual soft tissue layer i.e. skin, fat, muscle and bone, and the boundary and loading conditions of a complete prosthesis to generate a virtual 3D model of the residual limb. It is then possible to produce an accurate, functional and comfortable prosthetic socket.
15
PART 2
Individual’s Background IPR
Existing techniques of socket production can neither provide information relating to the internal geometry of the residual limb nor offer information on the biomechanical properties of living tissue. The introduction of a system that is capable of providing the Prosthetist with on-line information concerning a detailed mapping of the residual limb and the pressure distribution at the xxxxx/socket interface, as well as the maximum stresses in the tissue, is an innovation in this field and offers the following key advantages over existing techniques:
|
|
·
|
Provides optimally fitted prosthesis.
|
|
|
·
|
Provides the Prosthetist with on-line information concerning the detailed mapping of the residual limb (external and internal geometry and structure).
|
|
|
·
|
Real diagnosis of residual limb problems.
|
|
|
·
|
Accelerates the rehabilitation of the patient.
|
|
|
·
|
Improves the quality of prosthetic socket design.
|
|
|
·
|
Reduces costs by reducing the number of visits to the Prosthetist by the patient.
|
|
|
·
|
Reduces costs by reducing materials and time wastage due to poor fitted prostheses.
|
|
|
·
|
Improves the physical and mental well-being of the patients and therefore increase the usage of their prosthesis as they will be more satisfied and feel more comfortable wearing them.
|
|
|
·
|
The system requires minimum training and familiarisation by the Prosthetists.
|
|
|
·
|
Compact, mobile, and cost-effective.
|
Xx Xxxxxxxx Xxxxxxx has carried out extensive research in designing a new system to aid the creation of sockets for prosthetic limbs. This research differed from other research as it aimed to acquire and utilise not only the external shape of a residual limb but also:
|
|
·
|
The internal anatomy of the limb.
|
16
|
|
·
|
Bio-mechanical properties of each individual tissue layer of the residual limb.
|
|
|
·
|
Known boundary conditions (i.e. socket template information and the effects of internal and external forces on the residual limb).
|
SocketFit is a digital system for assessing an amputee’s residual limb and for the production of truly functional and comfortable prosthetic sockets. It takes account of the external and internal geometry of the amputee’s xxxxx, the biomechanical properties of the each individual soft tissue layer i.e. skin, fat, muscle and bone, and the boundary and loading conditions of a complete prosthesis to generate a virtual 3D model of the residual limb. It is then possible to produce an accurate, functional and comfortable prosthetic socket. By minimising the time and cost of socket production and by reducing the number of faulty sockets (it has been reported that a quarter of all prostheses are currently rejected due to poor fit), there will be a reduction in costs incurred by health services and insurance companies worldwide as well as great benefits to the amputee.
Socket-Fit consists out of three modules:
|
|
1.
|
The Ring - a device to scan the residual limb and export data
|
|
|
2.
|
Data Tools – software to analyse and collate the data into intelligible information.
|
|
|
3.
|
Socket Modelling – FEA simulation procedures that combine scanned information with known boundary conditions to create a model of a socket for the specific amputee.
|
The system consists of a “ring” attached on a vertical axis, capable of moving vertically along this axis as well as spinning, with the use of a stepper motor and a liner stepper slider. The “ring” is equipped with ultrasound transducers and load transducers as well as with rotary position sensors. An ultrasound transducer and a load transducer are placed on top of each other at the tip of a protruding (spring-supported) arm at the internal side of the ring. A rotary position sensor is also attached on the base of the arm. Two such arms are present on the device.
The external geometry of the medium under examination, is acquired by the position data from the stepper instruments and the use of the rotary position sensors; as the ring moves vertically, spinning at the same time, the spring-supported arms comes in contact with the entire residual limb, mapping every detail and transmitting it to a PC.
The internal geometry is acquired with the use of the on-board ultrasound transducers. Information on the mechanical properties of the tissues are also been captured.
All the data are telemeter to the control box for onward transmission to the PC. Software generates a 3D image of the xxxxx’x external and internal geometry. FEA and optimisation software generate the optimum design for the socket in order to obtain the best pressure distribution, and therefore the most comfortable prosthesis for the amputee. (Figures 1 and 2).
Figure 1, Basic elements of the SocketFit System.
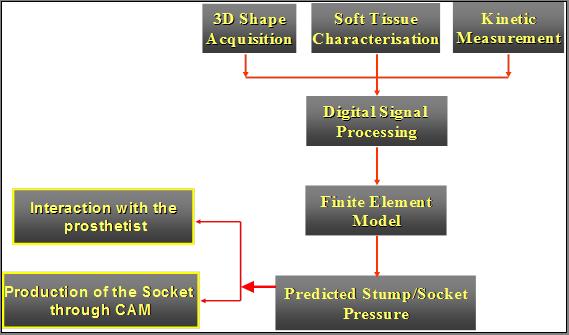
17
Figure 2, Schematic diagram of the system.
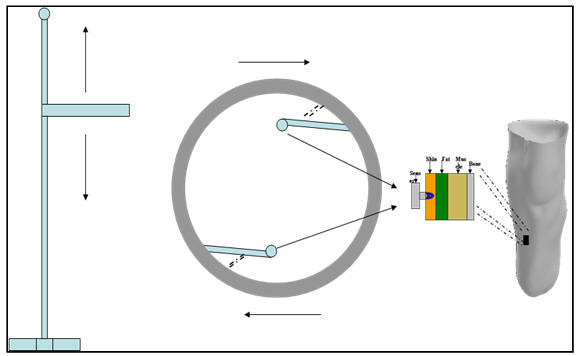
The software
Automation software that controls the hardware, and collects and pre-processes the data.
The automation software calibrates all the sensors and initialises them, controls the motion of both the stepper motor and the slider and acquires the scanning data. These are then saved in a series of ASCI format files and are passed to the appropriate analysis software.
The 1D ultrasonic data are processed by dedicated signal analysis software, which by incorporating a number of advanced analysis methods, such as deconvolution, spectral analysis and speckle registration, produces 2D images/maps of the medium under investigation. It also calculates the displacement caused to the different tissue layers inside the medium by the load applied by the contact “arms”.
18
The displacement data supplied by the ultrasonic analysis software, together with the pressure data collected by the pressure sensor, are fed in a custom made bio-mechanical properties calculation software. The data, in the form of stress vs. strain are processed and the bio-mechanical properties of the tissue layers in the medium under examination calculated.
The final step of the analysis is the Finite Elements Analysis software, or FEA.
A 3D geometry is generated by the 2D images supplied by the ultrasonic analysis software and the appropriate bio-mechanical properties are input into the FE model. The FEA software then generates the shape a prosthetic socket that applies even pressure at the entire surface of the specific medium, residual limb, apart of course from specific points that cannot take any pressure.
The final data from the FEA analysis will be transferred, by means of email or uploaded on data cloud to a 3D xxxxxx or a 3D printer where the prosthetic socket, or in the case of a xxxxxx the negative mould, will be created. The amputee will then wear the prosthesis and continue with his/her way of living.
1. Control and Acquisition Software
The vertical and rotational movement of the Ring is controlled by the control software. Based upon thorough testing of the device the optimum resolution for rotational movement was calculated to be 1 degree and for vertical movement 1 mm.
The software then acquires and saves the scanning data for later use in the Ultrasonic Analysis and Bio-mechanical Properties Software. Data is saved in a series of ASCI format files and then passed to the appropriate analysis software. Stored data includes:
|
|
·
|
pressure data
|
|
|
·
|
displacement data
|
|
|
·
|
positional data
|
|
|
·
|
coordinates data
|
|
|
·
|
ultrasound data
|
19
2. Ultrasonic Analysis Software
The Ultrasonic Analysis Software is a set of signal and image processing algorithms that have been combined to analyse and process the scanned data.
The algorithms were initially developed and used very successfully in the seismic exploration industry. The algorithms are been adapted and optimised to work with Ultrasound for the first time.
The 1D ultrasonic data derived from the scan is processed by dedicated signal analysis software, which by incorporating a number of advanced analysis methods, such as deconvolution, spectral analysis and speckle registration, produces 2D images/maps of the medium under investigation.
Additionally it also calculates the displacement caused to the different tissue layers inside the medium by the load applied by the contact “arms”.
2D Images will only include the following anatomical information:
|
|
·
|
Bones (including exact positional information)
|
|
|
·
|
Fat (note we are only interested in the fat surrounding the muscle and not any fat in between muscles in the centre of the leg)
|
|
|
·
|
Other Materials (all other materials will be grouped together as one, i.e. different muscle groups, etc.)
|
The displacement data supplied by the ultrasonic analysis software, together with the pressure data collected by the pressure sensor, are fed to a custom-made bio-mechanical properties calculation software.
3. Volume Rendering
This module will take each of the 2D slices and generate a 3D representation of the limb. This representation will be fed into the Socket Modeling module.
There is still some debate around the best tools to use for this module. Options include:
|
|
·
|
developing a bespoke application
|
|
|
·
|
use an existing application
|
|
|
·
|
use existing facilities within FEA
|
20
4. Bio-Mechanical Tools
The Bio-Mechanical Tools are a set of algorithms that use Bio-Mechanical data derived from the scans to generate stress-strain curves for the tissue within the limb.
This software can predict the stress distribution at any point inside the residual limb, by just knowing the surface pressure applied. The calculated stress, together with the known strain that is calculated by the ultrasonic analysis software from tissue displacement data, produce actual stress vs. strain curves which characterise the tissue layers derived from.
This information will also be fed into the Socket Modelling module.
Socket Modelling
The final step of the analysis is the Finite Elements Analysis software, or FEA. The FEA module incorporates all the information output from the Data Tools (external and internal geometry and bio-mechanical properties) against known Boundary Information to create a model of a customised “uniform pressure” socket for the amputee.
Boundary Information will include:
|
|
1.
|
Standard Socket Information – template sockets exist which are modified to suit individuals.
|
|
|
2.
|
Information regarding the impact of external and internal forces (e.g. weight of the amputee).
|
The FEA software then generates the shape a prosthetic socket that applies even pressure at the entire surface of the specific medium, residual limb, apart of course from specific points that cannot take any pressure.
The final data from the FEA analysis will be transferred, by means of email, CDs or any other media, to a 3D xxxxxx or a 3D printer where the prosthetic socket, or in the case of a xxxxxx the negative mould, will be created. The amputee will then wear the prosthesis and continue with his/her way of living.

