LICENSE AND COLLABORATION AGREEMENT by and between ALNYLAM PHARMACEUTICALS, INC. and GENZYME CORPORATION
Exhibit 10.49
| Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Double asterisks denote omissions. |
EXECUTION COPY |
LICENSE AND COLLABORATION AGREEMENT
by and between
and
GENZYME CORPORATION
TABLE OF CONTENTS
| 1. |
DEFINITIONS |
1 | ||||||
| 2. |
DEVELOPMENT COLLABORATION |
16 | ||||||
| 2.1 |
Overview. |
16 | ||||||
| 2.2 |
Development Plans. |
17 | ||||||
| 2.3 |
Responsibilities for Development Activities and Costs. |
18 | ||||||
| 2.4 |
Diligence. |
19 | ||||||
| 2.5 |
Records; Reports; Information Sharing. |
19 | ||||||
| 2.6 |
Third Parties. |
22 | ||||||
| 3. |
REGULATORY MATTERS. |
22 | ||||||
| 3.1 |
Regulatory Filings and Interactions. |
22 | ||||||
| 3.2 |
Costs of Regulatory Affairs. |
23 | ||||||
| 3.3 |
Right of Reference. |
23 | ||||||
| 3.4 |
Pharmacovigilance. |
24 | ||||||
| 4. |
COMMERCIALIZATION OF THE LICENSED PRODUCTS |
24 | ||||||
| 4.1 |
Responsibility, Cost and Diligence. |
24 | ||||||
| 4.2 |
Genzyme Commercialization Plan. |
24 | ||||||
| 4.3 |
Alnylam Commercialization Plan. |
25 | ||||||
| 4.4 |
Advertising and Promotional Materials. |
25 | ||||||
| 4.5 |
Reporting Obligations. |
26 | ||||||
| 4.6 |
Sales and Distribution. |
26 | ||||||
| 4.7 |
Recalls, Market Withdrawals or Corrective Actions. |
26 | ||||||
| 4.8 |
Ex-Territory Sales; Export Monitoring. |
26 | ||||||
| 5. |
COLLABORATION MANAGEMENT |
27 | ||||||
| 5.1 |
Joint Steering Committee. |
27 | ||||||
| 5.2 |
Appointment of Subcommittees, Project Teams and Collaboration Managers. |
27 | ||||||
| 5.3 |
Meetings. |
27 | ||||||
| 5.4 |
Minutes. |
28 | ||||||
| 5.5 |
Decision-Making. |
29 | ||||||
| 5.6 |
Term of JSC. |
30 | ||||||
| 5.7 |
Alnylam Third Party Partner. |
30 | ||||||
| 6. |
MANUFACTURE AND SUPPLY OF THE LICENSED PRODUCT |
32 | ||||||
| 6.1 |
Supply Agreements. |
32 | ||||||
Confidential
- i -
| 6.2 |
Transfer of Manufacturing Know-How. |
32 | ||||||
| 7. |
LICENSES |
32 | ||||||
| 7.1 |
License Grants to Genzyme. |
32 | ||||||
| 7.2 |
License Grants to Alnylam. |
34 | ||||||
| 7.3 |
Joint Collaboration IP. |
35 | ||||||
| 7.4 |
In-Licenses. |
35 | ||||||
| 7.5 |
Improvement Products Option. |
39 | ||||||
| 7.6 |
Alnylam Territory Right of First Negotiation. |
42 | ||||||
| 7.7 |
Bankruptcy. |
43 | ||||||
| 7.8 |
[**] |
|||||||
| 7.9 |
No Other Rights. |
44 | ||||||
| 8. |
CERTAIN FINANCIAL TERMS |
44 | ||||||
| 8.1 |
Upfront Fee. |
44 | ||||||
| 8.2 |
Development Milestone Fees. |
44 | ||||||
| 8.3 |
Royalties. |
46 | ||||||
| 8.4 |
Audits. |
50 | ||||||
| 8.5 |
Payment Exchange Rate. |
50 | ||||||
| 8.6 |
Late Payments. |
51 | ||||||
| 8.7 |
Blocked Payments. |
51 | ||||||
| 8.8 |
Taxes. |
51 | ||||||
| 9. |
CONFIDENTIALITY AND PUBLICATION |
52 | ||||||
| 9.1 |
Nondisclosure Obligation. |
52 | ||||||
| 9.2 |
Publication and Publicity. |
53 | ||||||
| 10. |
REPRESENTATIONS, WARRANTIES AND COVENANTS; INDEMNIFICATION |
55 | ||||||
| 10.1 |
Mutual Representations and Warranties. |
55 | ||||||
| 10.2 |
Representations and Warranties of Alnylam. |
55 | ||||||
| 10.3 |
Representations and Warranties of Genzyme. |
57 | ||||||
| 10.4 |
Warranty Disclaimer. |
57 | ||||||
| 10.5 |
Certain Covenants. |
58 | ||||||
| 11. |
INDEMNIFICATION; LIMITATION OF LIABILITY; INSURANCE |
58 | ||||||
| 11.1 |
General Indemnification by Genzyme. |
58 | ||||||
| 11.2 |
General Indemnification by Alnylam. |
59 | ||||||
| 11.3 |
Product Liability. |
59 | ||||||
| 11.4 |
Ongoing Litigation. |
59 | ||||||
| 11.5 |
[**] |
60 | ||||||
Confidential
- ii -
| 11.6 |
Indemnification Procedure. |
60 | ||||||
| 11.7 |
Limitation of Liability. |
60 | ||||||
| 11.8 |
Insurance. |
61 | ||||||
| 12. |
INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION AND RELATED MATTERS |
61 | ||||||
| 12.1 |
Inventorship. |
61 | ||||||
| 12.2 |
Ownership. |
61 | ||||||
| 12.3 |
Prosecution and Maintenance of Patent Rights. |
61 | ||||||
| 12.4 |
Third Party Infringement. |
63 | ||||||
| 12.5 |
Patent Term Extensions. |
65 | ||||||
| 12.6 |
Common Interest. |
66 | ||||||
| 12.7 |
Trademarks. |
66 | ||||||
| 12.8 |
Cooperative Research and Technology (CREATE) Act Acknowledgment. |
67 | ||||||
| 13. |
TERM AND TERMINATION |
67 | ||||||
| 13.1 |
Term. |
67 | ||||||
| 13.2 |
Termination Rights. |
67 | ||||||
| 13.3 |
Effect of Termination. |
69 | ||||||
| 13.4 |
Fundamental Breach of Alnylam’s Development Obligations. |
71 | ||||||
| 13.5 |
Effect of Expiration or Termination; Survival. |
71 | ||||||
| 14. |
MISCELLANEOUS |
71 | ||||||
| 14.1 |
Assignment. |
71 | ||||||
| 14.2 |
Governing Law. |
72 | ||||||
| 14.3 |
Jurisdiction. |
72 | ||||||
| 14.4 |
Venue. |
72 | ||||||
| 14.5 |
Entire Agreement; Amendments. |
72 | ||||||
| 14.6 |
Severability. |
73 | ||||||
| 14.7 |
Headings. |
73 | ||||||
| 14.8 |
Waiver of Rule of Construction. |
73 | ||||||
| 14.9 |
Interpretation. |
73 | ||||||
| 14.10 |
No Implied Waivers; Rights Cumulative. |
73 | ||||||
| 14.11 |
Notices. |
74 | ||||||
| 14.12 |
Compliance with Export Regulations. |
75 | ||||||
| 14.13 |
Force Majeure. |
75 | ||||||
| 14.14 |
Independent Contractors. |
75 | ||||||
Confidential
- iii -
| 14.15 |
Counterparts. |
75 | ||||||
| 14.16 |
Performance by Affiliates. |
75 | ||||||
| 14.17 |
Binding Effect; No Third Party Beneficiaries. |
76 | ||||||
Confidential
- iv -
SCHEDULES
| Schedule 1.4 | Additional Alnylam In-Licenses | |
| Schedule 1.7 | ALN-TTR02 | |
| Schedule 1.8 | ALN-TTRsc | |
| Schedule 1.9 | Alnylam Core Technology Patents | |
| Schedule 1.16 | Alnylam Product-Specific Patents | |
| Schedule 1.47 | Existing Alnylam In-Licenses | |
| Schedule 1.52 | First Phase II Success Objectives | |
| Schedule 2.2.1 | Global Development Strategy | |
| Schedule 2.2.2 | Alnylam Territory Development Plan | |
| Schedule 2.2.3 | Genzyme Territory Development Plan | |
| Schedule 6.1 | Supply Agreement Principles | |
| Schedule 7.4.3.2 | Baseball Arbitration Procedure Regarding JPAC/LP Milestone Allocation | |
| Schedule 9.2.2(a) | Joint Press Release | |
| Schedule 10.2 | Disclosure Schedule | |
Confidential
- v -
LICENSE AND COLLABORATION AGREEMENT
THIS LICENSE AND COLLABORATION AGREEMENT (this “Agreement”), effective as of October 18, 2012 (the “Effective Date”), by and between Alnylam Pharmaceuticals, Inc., a corporation organized and existing under the laws of the State of Delaware (“Alnylam”) and, Genzyme Corporation, a corporation organized and existing under the laws of the Commonwealth of Massachusetts (“Genzyme”).
RECITALS:
WHEREAS, Alnylam owns or controls certain fundamental intellectual property relating to RNA interference, and is developing proprietary therapeutic products in the Field (as defined below) targeting the human TTR gene known as ALN-TTR02 and ALN-TTRsc (each, as defined below);
WHEREAS, Genzyme desires to develop and commercialize such therapeutic RNA interference products in the Genzyme Territory (as defined below);
WHEREAS, Alnylam desires to continue to develop and to commercialize such therapeutic RNA interference products in the Alnylam Territory (as defined below); and
WHEREAS, Alnylam and Genzyme believe that a license and collaboration for such purpose on the terms and conditions of this Agreement would be desirable.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, the Parties hereby agree as follows:
1. DEFINITIONS
Unless specifically set forth to the contrary herein, the following terms, whether used in the singular or plural, shall have the respective meanings set forth below:
1.1 “Acquired Business” has the meaning set forth in Section 14.16.2.
1.2 “Acquirer” has the meaning set forth in Section 14.16.2.
1.3 “Action” has the meaning set forth in Section 14.3.
1.4 “Additional Alnylam In-Licenses” means the agreements set forth on Schedule 1.4.
1.5 “Affiliate” means, with respect to a Person, any other Person which controls, is controlled by, or is under common control with the applicable Person. For purposes of this definition, “control” shall mean: (a) in the case of corporate entities, direct or indirect ownership of at least fifty percent (50%) of the stock or shares (or such lesser percentage which is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) entitled to vote for the election of directors, or otherwise having the power to control or direct the affairs of such Person; and (b) in the case of non-corporate entities, direct or indirect ownership of at least fifty percent (50%) of the equity interest or the power to direct the management and policies of such non-corporate entities.
Confidential
1.6 “▇▇▇▇ ▇▇▇▇▇ Nanoparticle Formulation” means a lipid-based nanoparticle consisting of the following lipid species: [**].
1.7 “ALN-TTR02” means an siRNA Product Controlled by Alnylam, comprising the siRNA (#AD[**]) formulated in an ▇▇▇▇ ▇▇▇▇▇ Nanoparticle Formulation, as further described on Schedule 1.7.
1.8 “ALN-TTRsc” means an siRNA Product Controlled by Alnylam, comprising the siRNA (#AD[**]) conjugated to a Ga1NAc Conjugate, as further described on Schedule 1.8.
1.9 “Alnylam Core Technology Patents” means Patent Rights Controlled by Alnylam during the Term that are reasonably necessary or useful to Develop, Commercialize, and/or Manufacture Licensed Products, other than Alnylam Product-Specific Patents or Alnylam’s interest in Patent Rights included in Joint Collaboration IP. Alnylam Core Technology Patents include Patent Rights Controlled by Alnylam that claim [**], or (ii) subject matter applicable to siRNA or siRNA delivery in general. The Alnylam Core Technology Patents existing as of the Effective Date are identified on Schedule 1.9.
1.10 “Alnylam Developed siRNA Product” means an siRNA product with respect to which (a) Alnylam Controls Patent Rights Covering such siRNA product; provided that, once a product first satisfies the criterion set forth in this clause (a) such criterion shall be deemed satisfied at all times thereafter as to such product; and (b) Alnylam or an Affiliate of Alnylam plays(ed) a material role in the Development.
1.11 “Alnylam Development Candidate” means an siRNA Product for which Alnylam or its Affiliates have initiated IND-Enabling Toxicology Studies and as to which Alnylam and its Related Parties have not abandoned development and commercialization.
1.12 “Alnylam Indemnitees” has the meaning set forth in Section 11.1.
1.13 “Alnylam In-Licenses” means (a) the Existing Alnylam In-Licenses, and (b) any agreement between Alnylam and a Third Party entered into after the Effective Date pursuant to which Alnylam acquires Control of Know-How or Patent Rights that are necessary or useful to Develop, Manufacture or Commercialize Licensed Products in the Field in the Genzyme Territory, but in the case of any such agreement described in clause (b), solely to the extent that such agreement is designated as an Alnylam In-License pursuant to Section 7.4.2.2.
1.14 “Alnylam Know-How” means Know-How Controlled by Alnylam during the Term that is reasonably necessary or useful for Genzyme to Develop, Manufacture and/or Commercialize Licensed Products in the Field in the Genzyme Territory, other than Alnylam’s interest in Know-How included in Joint Collaboration IP.
Confidential
- 2 -
1.15 “Alnylam Patents” means Alnylam Core Technology Patents and Alnylam Product-Specific Patents.
1.16 “Alnylam Product-Specific Patents” means Patent Rights Controlled by Alnylam during the Term that claim (a) an siRNA targeting the human TTR gene contained in a Licensed Product, (b) during the Exclusivity Period, an siRNA targeting the human TTR gene not contained in a Licensed Product and not contained in an Alnylam Development Candidate, (c) the composition of matter of (i) a Licensed Product or (ii) an siRNA targeting the human TTR gene together with a formulation or compound that mediates delivery of an siRNA Product, which combination is contained in a Licensed Product, (d) methods of using a Licensed Product as a human therapeutic or prophylactic, methods of using a Licensed Product to modulate human TTR, or methods of using a Licensed Product to inhibit expression of human TTR, or (e) methods and materials specific to the synthesis or analysis of a Licensed Product; provided however, patents that include claims that are directed to subject matter applicable to siRNA or siRNA delivery in general will not be considered Alnylam Product Specific Patents but will be considered Alnylam Core Technology Patents. Notwithstanding the foregoing, for the purposes of clause (b) above, Alnylam Product-Specific Patents shall not include any Patent Right that claims any siRNA targeting the human TTR gene that (A) is contained in an Alnylam Development Candidate and (B) is not contained in a Licensed Product. The Alnylam Product-Specific Patents existing as of the Effective Date are identified on Schedule 1.16. Notwithstanding the foregoing, Alnylam Product-Specific Patents shall exclude Alnylam’s interest in Patent Rights included in Joint Collaboration IP.
1.17 “Alnylam Technology” means, collectively, Alnylam Know-How, Alnylam Patents and Alnylam’s interest in Joint Collaboration IP.
1.18 “Alnylam Territory” means all countries and territories of the world other than the Genzyme Territory.
1.19 “Alnylam Territory Commercialization Plan” has the meaning set forth in Section 4.3.
1.20 “Alnylam Territory Development Plan” has the meaning set forth in Section 2.2.2.
1.21 “Alnylam Trademark” has the meaning set forth in Section 12.7(b).
1.22 “ANDA” means an Abbreviated New Drug Application (or any successor application or procedure) as defined in regulations promulgated by the FDA under the FDCA, which ANDA is filed with or intended to be filed with the FDA (and, as applicable, any other analogous application filed with a Regulatory Authority in any country other than the U.S. in the Genzyme Territory) for regulatory approval for marketing and selling a Licensed Product in the Genzyme Territory
1.23 “ATTR” means the human disease TTR-mediated amyloidosis.
Confidential
- 3 -
1.24 “Backup Compound” means as to either of ALN-TTR02 or ALN-TTRsc, as applicable, a replacement product that (a) Alnylam brings forward as a substitute for ALN-TTR02 or ALN-TTRsc, as applicable, pursuant to Section 2.5.3.3; and (b) (i) in the case of such a replacement product for ALN-TTR02, is [**], (ii) in the case of such a replacement product for ALN-TTRsc, is [**], (iii) in the event that Alnylam discontinues the Development of ALN-TTR02 because an [**], then, notwithstanding clause (b)(i) above, is any siRNA Product in the Field that Alnylam advances into Clinical Studies (as evidenced by filing of an IND) within [**] years after the Effective Date or (iv) in the event that both ALN-TTR02 and ALN-TTRsc are discontinued prior to receipt of Regulatory Approval thereof, then, notwithstanding clauses (b)(i) and (b)(ii) above, is any siRNA Product in the Field that Alnylam advances into Clinical Studies (as evidenced by filing of an IND) within [**] years after the Effective Date.
1.25 “Bankrupt Party” has the meaning set forth in Section 7.7.
1.26 “Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31 of each Calendar Year; provided that (a) the first Calendar Quarter of the Term shall begin on the Effective Date and end on the first to occur of March 31, June 30, September 30 or December 31 thereafter and the last Calendar Quarter of the Term shall end on the last day of the Term and (b) the first Calendar Quarter of a Royalty Term for a Licensed Product in a country shall begin on the First Commercial Sale of a Licensed Product in such country and end on the first to occur of March 31, June 30, September 30 or December 31 thereafter and the last Calendar Quarter of a Royalty Term shall end on the last day of such Royalty Term.
1.27 “Calendar Year” means each successive period of twelve (12) months commencing on January 1 and ending on December 31; provided that (a) the first Calendar Year of the Term shall begin on the Effective Date and end on the first December 31 thereafter and the last Calendar Year of the Term shall end on the last day of the Term and (b) the first Calendar Year of a Royalty Term for a Licensed Product in a country shall begin on the First Commercial Sale of a Licensed Product in such country and end on the first December 31 thereafter and the last Calendar Year of the Term shall end on the last day of such Royalty Term.
1.28 [**]
1.29 “Clinical Study” means a Phase I Study, Phase II Study, Phase III Study, or Post-Marketing Commitment Study, as applicable; but excluding any Post-Approval Studies.
1.30 “Collaboration” means the collaboration of the Parties in the Development, Manufacture and Commercialization of Licensed Products under this Agreement.
1.31 “Collaboration Manager” has the meaning set forth in Section 5.2.
Confidential
- 4 -
1.32 “Commercialization” or “Commercialize” means any and all activities directed to marketing, promoting, distributing, importing, exporting, offering to sell and/or selling a product, including the conduct of Post-Approval Studies, and activities directed to obtaining pricing and reimbursement approvals, as applicable.
1.33 “Commercially Reasonable Efforts” means (a) with respect to each Party’s obligations under this Agreement that relate to a Licensed Product (including Development, Manufacture or Commercialization obligations), those efforts reasonably used by an entity in the biotechnology/pharmaceutical industry of similar resources and expertise as such Party, for such similar entity’s own products (including internally developed, acquired and in-licensed products) of similar market potential at a similar stage in development or product life, taking into account all relevant factors, including (i) issues of safety, tolerability and efficacy, (ii) product profile, (iii) difficulty in and costs of developing or manufacturing the Licensed Product, (iv) competitiveness of the Licensed Product and competitive products (but not taking into account Exempted Complementary TTR Products Controlled by the Party to which the efforts obligation applies) in the marketplace, (v) the extent of market exclusivity, (vi) the patent or other proprietary position of the Licensed Product, (vii) the regulatory structure involved, and (viii) the potential profitability of the Licensed Product, (but not taking into account the profitability relative to or the potential profitability of other products Controlled by the Party to which the efforts obligations applies); and (b) with respect to such Party’s other obligations under this Agreement, the carrying out of such obligations in a diligent, expeditious and sustained manner using efforts and resources, including reasonably necessary personnel and financial resources, that biopharmaceutical companies of comparable size and resources typically devote to similar tasks under similar circumstances.
1.34 “Competitive Infringement” has the meaning set forth in Section 12.4.1.
1.35 “Confidential Information” means any and all confidential or proprietary information and data, including Alnylam Technology, Genzyme Technology, and Joint Collaboration IP and all other scientific, pre-clinical, clinical, regulatory, manufacturing, marketing, financial and commercial information or data, whether communicated in writing or orally or by any other method, which is provided by one Party to the other Party in connection with this Agreement. Alnylam Technology is the Confidential Information of Alnylam. Genzyme Technology is the Confidential Information of Genzyme. Joint Collaboration IP and the terms of this Agreement are the Confidential Information of both Parties, subject to Section 9.2.2.
1.36 “Control”, “Controls” or “Controlled by” means, with respect to any Know-How, Patent Rights or other intellectual property right, the possession of (whether by ownership or license, other than pursuant to this Agreement), and the ability of a Person or its Affiliates to assign, transfer, or grant access to, or to grant a license or sublicense of, such item or right as provided for herein without violating the terms of any agreement or other arrangement with any Third Party existing at the time such Person would be required hereunder to assign, transfer or grant another Person such access or license or sublicense, provided that, with respect to rights to any Third Party Know-How, Patent Rights or other
Confidential
- 5 -
intellectual property right that are licensed to, or otherwise obtained by, (i) a Party pursuant to an agreement entered into by such Party after the Effective Date or (ii) Alnylam pursuant to any Additional Alnylam In-License, such Third Party Know-How, Patent Rights or other intellectual property right shall be deemed not to be under the Control of such Party or Alnylam, respectively, unless and until the agreement pursuant to which such rights are obtained becomes an In-License pursuant to Section 7.4.2.
1.37 “Cover,” “Covering” or “Covers” means, as to a product and Patent Rights, that, in the absence of a license granted under, or ownership of, such Patent Rights, the manufacture, use, offer for sale, sale, or importation of such product would infringe such Patent Rights or, as to a pending claim included in such Patent Rights, the manufacture, use, offer for sale, sale, or importation of such product would infringe such Patent Rights if such pending claim were to issue in an issued patent.
1.38 “CPI” shall mean the Consumer Price Index – Urban Wage Earners and Clerical Workers, U.S. City Average, All Items, 1982-84 = 100, published by the United States Department of Labor, Bureau of Labor Statistics (or its successor equivalent index) in the United States.
1.39 “Development,” “Developing” or “Develop” means the research and development activities related to the generation, characterization, optimization, construction, expression, use and production of Licensed Products, any other non-clinical, pre-clinical or clinical research and development activities related to the testing and qualification of Licensed Products, including toxicology studies, statistical analysis and report writing, pre-clinical testing, formulation development, Clinical Studies, regulatory affairs, product approval and registration activities, and all other activities necessary to seek, obtain and maintain Regulatory Approval, but excluding Post-Approval Studies.
1.40 “Development Plan” means (a) with respect to Alnylam, the Alnylam Territory Development Plan and (b) with respect to Genzyme, the Genzyme Territory Development Plan.
1.41 “[**].
1.42 “Effective Date” has the meaning set forth in the preamble.
1.43 “EMA” means the European Medicines Agency and any successor governmental authority having substantially the same function.
1.44 “EU” means the European Union, as its membership may be altered from time to time, and any successor thereto.
1.45 “Exclusivity Period” means, on a country-by-country basis within the Genzyme Territory, from the Effective Date until the First Commercial Sale of a Licensed Product in a country, and thereafter until the end of the last Royalty Term in such country to terminate or expire.
Confidential
- 6 -
1.46 “Exempted Complementary TTR Product” means a product for administration to ATTR patients that the JSC determines would not, if Commercialized in a country of the Genzyme Territory, reasonably be expected to adversely affect the Development or Commercialization of any Licensed Product in such country.
1.47 “Existing Alnylam In-Licenses” means (a) the Third Party agreements set forth on Schedule 1.47 and (b) any Additional Alnylam In-License included within the definition of Existing Alnylam In-Licenses pursuant to Section 7.4.2.3.
1.48 “FDA” means the United States Food and Drug Administration and any successor governmental authority having substantially the same function.
1.49 “FDCA” means the United States Federal Food, Drug, and Cosmetic Act of 1938, as amended from time to time, and the regulations and guidelines promulgated thereunder.
1.50 “Field” means the treatment and/or prevention of all human diseases, including treatment and/or prevention of ATTR, including familial amyloid polyneuropathy (FAP), familial amyloid cardiomyopathy (FAC) and senile systemic amyloidosis (SSA) in humans.
1.51 “First Commercial Sale” means, with respect to a country, the first sale for end use or consumption of the Licensed Product in such country after all Regulatory Approvals legally required for such sale have been granted by the Regulatory Authority of such country.
1.52 “First Phase II Success” means the completion of a Phase II Clinical Trial the results of which satisfy the criteria set forth in Schedule 1.52.
1.53 “FTE” means [**] hours of work devoted to or in support of the Development or Manufacture of a Licensed Product, that is carried out by one or more qualified scientific or technical employees of a Party or its Affiliates.
1.54 “FTE Cost” means, for any period, the FTE Rate multiplied by the number of FTEs in such period.
1.55 “FTE Rate” means [**] U.S. Dollars ($[**]) per FTE, increased annually beginning on January 1, 2014 and thereafter on January 1 of each succeeding year by the percentage increase in the CPI as of December 31 of the then most recently ended calendar year over the level of the CPI on December 31, 2012.
1.56 “Fundamental Breach” means a substantial abandonment of Alnylam’s Development obligations hereunder as a whole resulting from either (a) Alnylam’s willful failure to exercise Commercially Reasonable Efforts to perform such Development obligations, or (b) Alnylam’s lack of the financial resources necessary to perform such Development obligations. For the avoidance of doubt, a discontinuation of Development of ALN-TTR02 and/or ALN-TTRsc that is consistent with the exercise of Commercially
Confidential
- 7 -
Reasonable Efforts shall not constitute a Fundamental Breach, and Alnylam’s failure to Develop a Backup Compound or Improvement Product, or Alnylam’s failure to conduct any Post-Approval Study, shall not constitute a Fundamental Breach. For clarity, the Parties agree that a breach of Alnylam’s diligence obligations under Section 2.4.2 need not rise to the level of a Fundamental Breach to be considered a material breach of the Agreement.
1.57 “Future Alnylam In-License” has the meaning set forth in Section 7.4.3.1.
1.58 “GAAP” means generally accepted accounting principles as practiced in the United States or, to the extent applicable, IFRS (International Financial Reporting Standards), in each case, consistently applied.
1.59 [**].
1.60 “Generic Competition” means, with respect to a Licensed Product in any country in the Genzyme Territory in a given Calendar Quarter, that, during such Calendar Quarter, (a) [**] Generic Products are commercially available in such country, and (b) aggregate Net Sales of such Licensed Product in such country in such Calendar Quarter equal less than [**] percent ([**]%) of the average aggregate Net Sales of the Licensed Product over the four (4) consecutive Calendar Quarters immediately prior to the Calendar Quarter in which [**] Generic Products first became commercially available in such country.
1.61 “Generic Product” for a given country means a pharmaceutical product that (a) is sold by a Person that is not a Related Party of Genzyme under a marketing authorization granted by a Regulatory Authority to a Third Party, (b) contains the same active ingredient(s) as are contained in a Licensed Product, where the term “active ingredient” means the siRNA Product(s) responsible for the pharmacological or physiological action of the Licensed Product, and (c) is approved by the Regulatory Authority pursuant to an approval process that relies in part on pivotal safety and/or efficacy data in such Regulatory Authority’s previous grant of marketing authorization to a Licensed Product.
1.62 “Genzyme Collaboration IP” means (a) any Know-How, first identified, discovered or developed solely by employees of Genzyme or its Affiliates or other persons not employed by Alnylam acting on behalf of Genzyme, in the conduct of the Collaboration and (b) any Patent Rights that claim or cover such Know-How and are Controlled by Genzyme at any time during the Term. Genzyme Collaboration IP excludes Genzyme’s interest in Joint Collaboration IP, in each case, (a) and (b) other than Genzyme Manufacturing IP.
1.63 “Genzyme Disclosed Manufacturing Know-How” means Know-How (a) Controlled by Genzyme at any time during the Term that is useful in the Manufacture of a Licensed Product, and (b) that Genzyme, in its sole discretion, discloses in writing to Alnylam in the course of the Collaboration.
Confidential
- 8 -
1.64 “Genzyme Indemnitees” has the meaning set forth in Section 11.2.
1.65 “Genzyme In-Licenses” means (a) any agreement between Genzyme and a Third Party entered into after the Effective Date pursuant to which Genzyme Controls Know-How or Patent Rights, other than Genzyme Manufacturing IP, used to Develop, Manufacture or Commercialize Licensed Products in the Field and (b) that is designated as a Genzyme In-License pursuant to Section 7.4.2.2.
1.66 “Genzyme Know-How” means Know-How Controlled by Genzyme during the Term that is reasonably necessary or useful for Alnylam to Develop, Commercialize and/or Manufacture Licensed Products in the Field in the Alnylam Territory (other than Genzyme’s rights in Joint Collaboration IP, Genzyme Collaboration IP and Genzyme Manufacturing IP).
1.67 “Genzyme Manufacturing IP” means (a) any Know-How related to the Manufacture of Licensed Products (or oligonucleotides generally) Controlled by Genzyme at any time during the Term, and (b) any Patent Rights that claim or cover such or Know-How and are Controlled by Genzyme at any time during the Term.
1.68 “Genzyme Patent Rights” means those Patent Rights Controlled by Genzyme during the Term that are reasonably necessary or useful to Develop, Commercialize and/or Manufacture Licensed Products in the Field in the Alnylam Territory. Genzyme Patent Rights excludes Patent Rights included in Genzyme Collaboration IP, Genzyme’s interest in Joint Collaboration IP and Genzyme Manufacturing IP.
1.69 “Genzyme Technology” means, collectively, Genzyme Know-How and Genzyme Patent Rights, Genzyme Collaboration IP and Genzyme’s interest in Joint Collaboration IP, but excluding Genzyme Manufacturing IP.
1.70 “Genzyme Territory” means the countries of Australia, Bangladesh, Cambodia, China, East Timor, Hong Kong, India, Indonesia, Japan, Laos, Malaysia, Micronesia, Mongolia, Myanmar, Nepal, New Zealand, North Korea, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam.
1.71 “Genzyme Territory Commercialization Plan” has the meaning set forth in Section 4.2.
1.72 “Genzyme Territory Development Plan” has the meaning set forth in Section 2.2.3.
1.73 “Genzyme Trademark” has the meaning set forth in Section 12.7(b).
1.74 “Global Branding Strategy” has the meaning set forth in Section 4.4.1.
1.75 “Global Development Strategy” has the meaning set forth in Section 2.2.1.
Confidential
- 9 -
1.76 “Governmental Authority” means any applicable government authority, court, tribunal, arbitrator, agency, department, legislative body, commission or other instrumentality of (a) any government of any country or territory, (b) any nation, state, province, county, city or other political subdivision thereof or (c) any supranational body.
1.77 “Improvement Manufacturing Patent Right” means a Patent Right owned exclusively by Genzyme or its Affiliates that claims an invention related to the Manufacture of a Licensed Product that was made (a) by Genzyme or its Affiliates after the Effective Date in connection with Manufacturing Licensed Products and (b) using Alnylam Know-How that, at the time such Alnylam Know-How was disclosed to Genzyme or its Affiliates, constituted Alnylam’s Confidential Information under Section 9.1.
1.78 “Improvement Product” means any siRNA Product Controlled by Alnylam, other than (a) ALN-TTR02, (b) ALN-TTRsc or (c) a Back-Up Compound that becomes a Licensed Product pursuant to Section 2.5.3.3.
1.79 “Improvement Product Development Budget” has the meaning set forth in Section 7.5.
1.80 “Improvement Product Development Costs” has the meaning set forth in Section 7.5.3.1.
1.81 “Improvement Product Development Plan” has the meaning set forth in Section 7.5.
1.82 “Incremental Amounts” has the meaning set forth in Section 11.4.2.
1.83 “IND” means an Investigational New Drug application, Clinical Trial Application or similar application or submission for approval to conduct human clinical investigations filed with or submitted to a Regulatory Authority in conformance with the requirements of such Regulatory Authority.
1.84 “IND-Enabling Toxicology Studies” means the pharmacokinetic and toxicology studies required to meet the requirements for filing an IND.
1.85 “Indemnitee” has the meaning set forth in Section 11.6.
1.86 “In-Licenses” means, collectively, the Alnylam In-Licenses and the Genzyme In-Licenses.
1.87 “Joint Collaboration IP” means, collectively, (a) any Know-How first identified, discovered or developed jointly by employee(s), agent(s) or consultant(s) acting on behalf of Alnylam or its Affiliates, on the one hand, and employee(s), agent(s) or consultant(s) acting on behalf of Genzyme or its Affiliates, on the other hand, in the conduct of the Collaboration that is Controlled by Alnylam and Genzyme, and (b) any Patent Rights that Cover such Know-How and is Controlled by Alnylam and Genzyme.
Confidential
- 10 -
1.88 “Joint Development Budget” has the meaning set forth in Section 2.3.3.
1.89 “Joint Clinical Study” has the meaning set forth in Section 2.3.3.
1.90 “Joint Development Costs” has the meaning set forth in Section 2.3.3.
1.91 “Joint Development Plan” has the meaning set forth in Section 2.3.3.
1.92 “Joint Steering Committee” or “JSC” means the joint steering committee as more fully described in Section 5.1.
1.93 “JPAC/LP Milestone” has the meaning set forth in Section 7.4.3.2(b).
1.94 “JPAC/LP-Specific Milestone” has the meaning set forth in Section 7.4.3.2(b).
1.95 “Know-How” means, all chemical or biological materials and other tangible materials, inventions, improvements, practices, discoveries, developments, data, information, technology, methods, protocols, formulas, knowledge, know-how, trade secrets, processes, assays, skills, experience, techniques and results of experimentation and testing, including pharmacological, toxicological and pre-clinical and clinical data and analytical and quality control data; provided, however, excluding in any event any Patent Right and Trademarks.
1.96 “Laws” means all applicable laws, statutes, rules, regulations, orders, judgments, injunctions, ordinances or other pronouncements having the binding effect of law of any Governmental Authority.
1.97 “Licensed Product” means any of the following siRNA Products: (a) ALN-TTR02, (b) ALN-TTRsc, (c) a Backup Compound that becomes a Licensed Product pursuant to Section 2.5.3.3 or other siRNA Product that becomes a Licensed Product pursuant to Section 2.5.3.4, or (d) an Improvement Product that becomes a Licensed Product pursuant to Section 7.5.
1.98 “Licensing Party” has the meaning set forth in Section 7.4.2.2.
1.99 “Lipid Nanoparticle Formulation” means a lipid-based nanoparticle comprising more than one lipid species designed to enable delivery of siRNAs to mammalian cells.
1.100 [**].
1.101 “Losses” has the meaning set forth in Section 11.1.
1.102 “Manufacturing” or “Manufacture” means, as applicable, all activities associated with the production, manufacture, process of formulating, processing, filling, finishing, packaging, labeling, shipping, importing, and storage of Licensed Products
Confidential
- 11 -
(including Bulk Drug Product, Bulk Drug Substance, and Finished Product, each as defined in the Supply Agreements), including process development, process validation, stability testing, manufacturing scale-up, pre-clinical, clinical and commercial manufacture and analytical development, product characterization, quality assurance and quality control development, testing and release.
1.103 “Manufacturing Claim” means a claim within a Patent Right directed solely to Manufacturing a Licensed Product.
1.104 “MC3” has the meaning set forth in Section 1.6.
1.105 “MHLW” has the meaning set forth in Section 1.129.
1.106 “MicroRNA” or “▇▇▇▇▇” means a structurally defined functional RNA molecule usually between 19 and 25 nucleotides in length, which is derived from an endogenous, genetically-encoded non-coding RNA which is predicted to be processed into a hairpin RNA structure that is a substrate for the double-stranded RNA-specific ribonuclease Drosha and subsequently is predicted to serve as a substrate for the enzyme Dicer, a member of the RNase III enzyme family.
1.107 “MicroRNA Mimic” means a single-stranded or double-stranded oligonucleotide with the same or substantially similar-base composition and sequence (including chemically modified bases) as a particular natural, ▇▇▇▇▇ and which is designed to mimic the activity of such ▇▇▇▇▇. For clarity, ▇▇▇▇▇ Mimic excludes a double-stranded oligonucleotide which functions or is designed to function as an siRNA.
1.108 “NDA” means a New Drug Application, Biologics License Application, Marketing Authorization Application or similar application or submission filed with a Regulatory Authority in a country or group of countries to obtain marketing approval for a biological, pharmaceutical or other therapeutic or prophylactic product in that country or in that group of countries.
1.109 “Net Sales” means the aggregate gross invoiced sales prices from sales of all units of Licensed Products sold by Genzyme and its Related Parties to independent Third Parties (other than a Sublicensee) after deducting, if not previously deducted, from the amount invoiced or received:
(b) trade, quantity and cash discounts, credits or allowances actually given;
(c) returns, rejections, recalls, rebates, chargebacks and other credits or allowances actually given;
(d) retroactive price reductions or billing corrections;
(e) value added, sales and use, excise and other similar taxes and surcharges, customary transportation and insurance, custom duties, and other governmental charges; and
Confidential
- 12 -
(f) amounts previously included in Net Sales of such Licensed Products that are adjusted or written-off by Genzyme or its Related Parties as bad debt or otherwise uncollectible in accordance with the standard practices of Genzyme or its Related Parties for writing off uncollectible amounts consistently applied; provided that if any such written-off amounts are subsequently collected, such collected amounts shall be included in Net Sales in the period in which they are subsequently collected.
Such amounts shall be determined from the books and records of Genzyme or its Related Parties, maintained in accordance with GAAP.
In the case of any sale or other disposal for value, such as barter or counter-trade, of Licensed Product, or part thereof, other than in an arm’s length transaction exclusively for cash, Net Sales shall be calculated as above on the value of the non-cash consideration received or the fair market price (if higher) of the Licensed Product in the country of sale or disposal, as determined in accordance with GAAP.
Notwithstanding the foregoing, the following will not be included in Net Sales: (1) sales between or among Genzyme and its Related Parties shall be excluded from the computation of Net Sales, (but Net Sales shall include sales to the first Third Party (other than a Sublicensee) by Genzyme or its Related Parties); and (2) Licensed Product used as samples to promote additional Net Sales, in amounts consistent with normal business practices of Genzyme.
1.110 “Non-Bankrupt Party” has the meaning set forth in Section 7.7.
1.111 “Ongoing Litigation” means (a) any litigation ongoing as of the Effective Date that was brought against Alnylam regarding or relating to any Alnylam Technology or (b) all related or similar actions arising from substantially the same underlying facts as any litigation described in clause (a), whether brought prior to, on or after the Effective Date.
1.112 “Option” has the meaning set forth in Section 7.5.
1.113 “Option Exercise Package” has the meaning set forth in Section 7.5.
1.114 “Option Exercise Period” has the meaning set forth in Section 7.5.
1.115 “Out-of-Pocket Costs” means, with respect to certain activities hereunder, direct expenses paid or payable by either Party or its Affiliates to Third Parties and specifically identifiable and incurred to conduct such activities for a Licensed Product, including payments to contract personnel.
1.116 “Party” means Genzyme and/or Alnylam.
1.117 “Patent Rights” means (a) all issued patents (extensions, restorations by existing or future extension or registration mechanism, including patent term adjustments, patent term extension, supplemental protection certificates or the equivalent thereof, substitutions, confirmations, re-registrations, re-examinations, and patents of addition), (b) patent applications (including all provisional applications, substitutions, requests for continuation, continuations, continuations-in-part, divisionals and renewals), (c) inventor’s certificates, and (d) and all equivalents of the foregoing in any country of the world.
Confidential
- 13 -
1.118 “Person” shall mean any natural person, corporation, unincorporated organization, partnership, association, sole proprietorship joint stock company, joint venture, limited liability company, trust or government, or any agency or political subdivision of any government, or any other similar entity.
1.119 “Phase I Study” means a study in humans which provides for the introduction into humans of a product, conducted in healthy volunteers or patients, to obtain initial information on product safety, tolerability, pharmacological activity or pharmacokinetics, as more fully defined in 21 C.F.R. § 312.21(a) (or the equivalent thereof outside the United States).
1.120 “Phase II Study” means a study in humans of the safety, dose ranging or efficacy of a product, as further defined in 21 C.F.R. § 312.21(b) (or the equivalent thereof outside the United States).
1.121 “Phase III Study” means a study in humans of the efficacy and safety of a product, which is prospectively designed to demonstrate statistically whether such product is effective and safe for use in a particular indication in a manner sufficient (alone or together with one or more other such studies) to file an application for Regulatory Approval for the product. For the avoidance of doubt, the Parties acknowledge that, because of the nature of ATTR, a Phase III Study for a Licensed Product may not include all of the elements that a Phase III Study for other kinds of drug products may include (e.g., a Phase III Study for a Licensed Product may be a single study and there may be no prospective plan to run a second pivotal clinical trial independent of such Phase III Study).
1.122 “PMDA” has the meaning set forth in Section 1.129.
1.123 “Positive Reimbursement Decision” means The National Health Insurance Bureau issues a pricing recommendation to MHLW in Japan.
1.124 “Post-Approval Study” means a human clinical study of a Licensed Product initiated after receipt of Regulatory Approval for such Licensed Product in a country or territory, other than a Post-Marketing Commitment Study.
1.125 “Post-Marketing Commitment Study” means a non-human or human clinical study of a Licensed Product initiated after receipt of Regulatory Approval for such Licensed Product in a country or territory that is required by the Regulatory Authority in such country or territory to maintain the Regulatory Approval for such Licensed Product in such country or territory.
1.126 “Product Trademark(s)” means the Trademarks for use in connection with the distribution, marketing, promotion and sale of the Licensed Products. Product Trademarks specifically excludes the corporate names and logos of the Parties and their Affiliates. Product Trademark includes both the Alnylam Trademarks and the Genzyme Trademarks.
Confidential
- 14 -
1.127 “Promotional Materials” has the meaning set forth in Section 4.4.2.
1.128 “Regulatory Approval” means any and all approvals, licenses, registrations or authorizations of any Regulatory Authority that are necessary for the marketing and sale of a product in a country or group of countries.
1.129 “Regulatory Authority” means any applicable government regulatory authority involved in granting approvals for the Development, Manufacturing, Commercialization, reimbursement and/or pricing of Licensed Products, including the FDA, the EMA, the Japanese Ministry of Health, Labour and Welfare (“MHLW”) and the Pharmaceuticals and Medical Devices Agency in Japan (“PMDA”).
1.130 “Regulatory Exclusivity” means, with respect to a Licensed Product in a country, any exclusive marketing right, data exclusivity right, orphan drug designation or other country-wide exclusive right conferred by any Governmental Authority with respect to such Licensed Product in such country, other than a Patent Right.
1.131 “Related Party” means a Party’s Affiliates, permitted Sublicensees and, with respect to Alnylam, licensees in the Alnylam Territory.
1.132 “Royalty Term” has the meaning set forth in Section 8.3.2.
1.133 “SDEA” has the meaning set forth in Section 3.4.
1.134 “siRNA” means a small interfering ribonucleic acid.
1.135 “siRNA Product” means an oligonucleotide composition of native or chemically modified RNA, designed to modulate the human TTR gene through activation of the RNA interference pathway which is not a MicroRNA, MicroRNA antagonist or MicroRNA Mimic.
1.136 “SPC” has the meaning set forth in Section 12.5.
1.137 “Sublicensee” means a Third Party to whom a Party grants a sublicense under any Alnylam Technology or Genzyme Technology, as the case may be, to Develop, Manufacture or Commercialize Licensed Product in the Field pursuant to Section 7.1.3 or Section 7.2.4.
1.138 “Supply Agreements” means the Clinical Supply Agreement and the Commercial Supply Agreement.
1.139 “Term” has the meaning set forth in Section 13.1.
Confidential
- 15 -
1.140 “Territory” means (a) with respect to Alnylam, the Alnylam Territory and (b) with respect to Genzyme, the Genzyme Territory.
1.141 “Third Party” means an entity other than a Party and its Affiliates.
1.142 “Third Party Collaboration Agreement” has the meaning set forth in Section 5.7.
1.143 “Third Party License Payment” means royalties, upfront fees, milestones or other amounts payable under an In-License.
1.144 “Third Party Partner” has the meaning set forth in Section 5.7.
1.145 “Trademark” means any trademark, trade name, service ▇▇▇▇, service name, brand, domain name, trade dress, logo, slogan or other indicia of origin or ownership, including the goodwill and activities associated with each of the foregoing.
1.146 “TTR” means the human TTR gene and its protein product, transthyretin. As of the Effective Date, the NCBI RefSeq code for the TTR gene is NM_000371.3 and the NCBI RefSeq code for the TTR protein product is NP_000362.1.
1.147 “United States” means the United States of America and its territories, possessions and commonwealths.
1.148 “Valid Claim” means a claim of: (a) an issued and unexpired patent, which claim has not been withdrawn, cancelled, abandoned, disclaimed, revoked or held unenforceable or invalid by an unappealable decision of a court or other governmental agency of competent jurisdiction, or has not been appealed within the time allowed for appeal, or by an appealed decision of a court or other governmental agency of competent jurisdiction where the appeal has been pending for more than [**] years (unless and until such decision is subsequently overturned on appeal) and which has not been abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination or disclaimer or otherwise, or (b) a patent application that has been pending less than [**] years from the date of filing of the earliest patent application from which such patent application claims priority, which claim has not been cancelled, withdrawn or abandoned or finally rejected by an administrative agency action from which no appeal can be taken.
2. DEVELOPMENT COLLABORATION
2.1 Overview. Prior to the Effective Date, Alnylam has been engaged in the Development of the Licensed Products. Under this Agreement, the Parties will collaborate in the further Development of Licensed Products, with Alnylam retaining primary responsibility for the Development of Licensed Products in the Alnylam Territory and Genzyme assuming primary responsibility for the Development of Licensed Products in the Genzyme Territory.
Confidential
- 16 -
2.2 Development Plans.
2.2.1 Global Development Strategy. The global Development strategy for the Licensed Products (“Global Development Strategy”) is attached hereto as Schedule 2.2.1. Alnylam may amend the Global Development Strategy from time to time by providing notice and a copy of such changes to the JSC. [**] will have final decision-making authority over all such amendments; provided, however, that [**] may not amend the Global Development Strategy in a manner that would [**] the Development of Licensed Products in the [**] Territory if [**] would [**] Development in the [**] Territory [**] Development in the [**] Territory. [**] will present any proposed amendments to the Global Development Strategy to the JSC reasonably in advance of implementing such amendments, and [**] shall have the right to review and comment thereon, and [**] shall consider in good faith [**] timely comments.
2.2.2 Alnylam Territory Development Plan. The Development activities and Post-Approval Studies to be undertaken with respect to each Licensed Product by or on behalf of Alnylam will be set forth in a written work plan and time table (each, a “Alnylam Territory Development Plan”), a draft framework of which is attached hereto as Schedule 2.2.2, and Alnylam will provide to Genzyme the first complete Alnylam Territory Development Plan for ALN-TTR02 and the then-current draft Development Plan for ALN-TTRsc by [**]. Each Alnylam Territory Development Plan will set forth a rolling written work plan and time table with respect to the Development of and Post-Approval Studies for a Licensed Product from the Effective Date until the later of (a) [**] years from date of such plan, and (b) receipt of Regulatory Approval for such Licensed Product. Each Alnylam Territory Development Plan shall subsequently be updated by Alnylam from time to time not later than [**] of each Calendar Year until such time as no further Development or Post-Approval Studies are occurring or expected to occur with respect to the applicable Licensed Product. Alnylam will have final decision-making authority over the Alnylam Territory Development Plans; provided, however, that the Alnylam Territory Development Plans shall at all times be materially consistent with the Global Development Strategy and shall not be amended in a manner that would have a material adverse effect on the Development of Licensed Products in the Genzyme Territory if such material adverse effect would have a disproportionately significant impact on Development in the Genzyme Territory as compared to any such impact on Development in the Alnylam Territory. Alnylam will present the Alnylam Territory Development Plans and any proposed amendments thereto to the JSC reasonably in advance of implementing such plans or amendments, and Genzyme shall have the right to review and comment thereon, and Alnylam shall consider in good faith Genzyme’s timely comments.
2.2.3 Genzyme Territory Development Plan. The Development activities and Post-Approval Studies to be undertaken with respect to each Licensed Product by or on behalf of Genzyme will be set forth in a written work plan and time table (each, a “Genzyme Territory Development Plan”), which will be prepared by Genzyme by [**] and following review and approval, subject to Section 5.5.3(a), by the JSC, will then be attached hereto as Schedule 2.2.3. Each Genzyme Territory Development Plan will set forth a rolling written work plan and time table with respect to the Development of and Post-Approval Studies for a Licensed Product from the Effective Date until the later of (a) [**] years from the date of such plan, and (b) receipt of Regulatory Approval for such Licensed Product. The Genzyme Territory Development Plans shall subsequently be updated by Genzyme from time to time not later than [**] of each Calendar Year until such time as no further Development or Post-Approval Studies are occurring
Confidential
- 17 -
or expected to occur with respect to the applicable Licensed Product in the Genzyme Territory. Genzyme will have final decision-making authority over the Genzyme Territory Development Plans; provided, however, that the Genzyme Territory Development Plans shall at all times be materially consistent with the Global Development Strategy and shall not be amended in a manner that would have a material adverse effect on the Development of Licensed Products in the Alnylam Territory; provided, however, that Genzyme may, in accordance with Section 5.5, amend the Genzyme Territory Development Plan in a manner that materially adversely affects the Development of Licensed Products in the Alnylam Territory if the Development activities contemplated by such amendment are reasonably required to obtain Regulatory Approval of a Licensed Product in the Genzyme Territory and there is no reasonable alternative to such activity that would similarly enable Genzyme or its Related Parties to obtain such Regulatory Approval in the Genzyme Territory with a lesser adverse effect on the Development of Licensed Products in the Alnylam Territory. Genzyme will present the Genzyme Territory Development Plans and any proposed amendments thereto to the JSC reasonably in advance of implementing such plans or amendments, and Alnylam shall have the right to review and comment thereon and Genzyme shall consider in good faith Alnylam’s timely comments.
2.3 Responsibilities for Development Activities and Costs.
2.3.1 Alnylam Development. Alnylam shall be primarily responsible for the global Development of Licensed Products and all Development activities the primary purpose of which is to obtain or maintain Regulatory Approval of Licensed Products in the Alnylam Territory. Except as otherwise provided in a Joint Development Plan with respect to Joint Development Costs agreed to pursuant to Section 2.3.3, Alnylam shall be responsible for one hundred percent (100%) of all Development costs with respect to Development activities that are conducted by Alnylam for the Licensed Products in the Alnylam Territory pursuant to the Alnylam Territory Development Plan. Alnylam will promptly provide Genzyme with written notice of any decision by Alnylam to commence the first [**] of a Licensed Product by Alnylam or any of its Related Parties, but in any event, Alnylam will provide such notice no later than [**] days prior to commencement thereof. Except as otherwise agreed by the Parties pursuant to Section 2.3.3 with respect to joint Development activities or pursuant to Section 7.5 with respect to the Development of an Improvement Product, Alnylam will conduct all Development of the Licensed Products for the Alnylam Territory solely in accordance with the Alnylam Territory Development Plan, as such Alnylam Territory Development Plan may be amended in accordance with this Agreement.
2.3.2 Genzyme Development. Genzyme shall be responsible for the Development of Licensed Products under the Genzyme Territory Development Plan. Except as otherwise provided in a Joint Development Plan with respect to Joint Development Costs agreed to pursuant to Section 2.3.3, Genzyme shall be responsible for one hundred percent (100%) of all Development costs with respect to Development activities that are conducted by Genzyme pursuant to the Genzyme Territory Development Plan. Except as otherwise agreed by the Parties pursuant to Section 2.3.3 with respect to joint Development activities or pursuant to Section 7.5 with respect to the Development of an Improvement Product, Genzyme will conduct all Development of the Licensed Products for the Genzyme Territory solely in accordance with the applicable Genzyme Territory Development Plan, as such Genzyme Territory Development Plan may be amended in accordance with this Agreement.
Confidential
- 18 -
2.3.3 Joint Development. In the event that the Parties mutually agree to conduct a Clinical Study of a Licensed Product specifically designed for the purpose of facilitating Regulatory Approval of a Licensed Product in either (a) both the Alnylam Territory and the Genzyme Territory or (b) solely the Genzyme Territory (a “Joint Clinical Study”), the Parties will (i) agree in writing to a written work plan and time table for conducting such Joint Clinical Study (the “Joint Development Plan”) and a mechanism for adopting amendments thereto, (ii) agree in writing to governance and management mechanisms for such Joint Clinical Study, including coordination of such Clinical Study through the JSC and (iii) negotiate in good faith a budget therefor (the “Joint Development Budget”), a mechanism for adopting amendments thereto, and an equitable allocation of costs (“Joint Development Costs”) between the Parties.
2.4 Diligence.
2.4.1 Genzyme Diligence. Genzyme will use Commercially Reasonable Efforts to (a) Develop at least [**] and obtain Regulatory Approval therefor in each of (i) Japan and (ii) any other country(ies) within the Genzyme Territory where Genzyme determines that it is commercially reasonable to seek Regulatory Approval (taking into account all relevant factors as provided in the definition of “Commercially Reasonable Efforts”), (b) perform the Development activities under the Genzyme Territory Development Plan and (c) if applicable as to Joint Clinical Studies, the activities allocated to Genzyme under the Joint Development Plan. For clarity, it may be consistent with Genzyme’s Commercially Reasonable Effort’s obligation to abandon Development of a Licensed Product in favor of another Licensed Product.
2.4.2 Alnylam Diligence. Alnylam will use Commercially Reasonable Efforts to (a) Develop at least [**] and obtain Regulatory Approval therefor by the FDA in the U.S. or by the EMA in the European Union, (b) perform the Development activities under the Alnylam Territory Development Plan and (c) if applicable as to Joint Clinical Studies, the activities allocated to Alnylam under the Joint Development Plan. For clarity, Alnylam’s election not to bring forward a Backup Compound or Improvement Product for Development as a Licensed Product shall not constitute a breach of Alnylam’s obligations under this Section 2.4.2.
2.5 Records; Reports; Information Sharing.
2.5.1 Development Activities. Each Party will periodically provide to the JSC, but in no event less than [**], or more frequently as reasonably requested by the other Party, an update regarding Development activities conducted by or on behalf of such Party, as well as any Post-Approval Studies conducted by the other Party. The Parties will periodically report to the JSC, but in no event less than [**], regarding their respective activities conducted under the Joint Development Plan.
2.5.2 Improvement Products. Alnylam will periodically provide to the JSC updates regarding the status of potential Improvement Products, if any, for discussion pursuant to Section 5.4.1(a).
Confidential
- 19 -
2.5.3 Backup Compounds.
2.5.3.1. Alnylam will periodically provide to the JSC updates regarding the status of potential Backup Products, if any, for discussion pursuant to Section 5.4.1(a).
2.5.3.2. In the event that Alnylam determines, following discussion pursuant to Section 5.4.1(a) and subject to its diligence obligations under Section 2.4.2, to discontinue Development of ALN-TTR02 or ALN-TTRsc prior to receipt of Regulatory Approval therefor in the Alnylam Territory, Alnylam will provide written notice thereof to Genzyme within [**] days following such determination.
2.5.3.3. In the event that Alnylam, at its election following discussion pursuant to Section 5.4.1(a), intends to bring forward a Backup Compound as a substitute Licensed Product for a product that has been discontinued under Section 2.5.3.2, Alnylam will provide Genzyme with written notice thereof, together with a statement detailing the results and data of any pre-clinical or Clinical Studies for such proposed Backup Compound. Genzyme will have [**] days following the receipt of such notice and statement to review the proposed Backup Compound and to decide whether to include such Backup Compound as a Licensed Product under this Agreement. In the event that Genzyme notifies Alnylam within such [**] day period that it desires to include such proposed Backup Compound as a Licensed Product under this Agreement, such proposed Backup Compound will thereafter become a Licensed Product for all purposes of this Agreement. Notwithstanding the foregoing, if Alnylam brings forward a Backup Compound as a substitute Licensed Product for a product that has been discontinued under Section 2.5.3.2, without providing Genzyme with the written notice and information required by this Section 2.5.3.3, such Backup Compound shall automatically become a Licensed Product for all purposes of this Agreement until such time as Genzyme notifies Alnylam in writing that it does not wish such Backup Compound to be a Licensed Product, in which case such Backup Compound shall cease to be a Licensed Product for all purposes of this Agreement from the date of such notice. If a Backup Compound becomes a Licensed Product pursuant to this Section 2.5.3, the Licensed Product for which Development has been discontinued that is being replaced by such Backup Compound shall cease to be a Licensed Product for the purposes of this Agreement.
2.5.3.4. In the event that Genzyme determines, subject to its obligations under Section 2.4.1, to discontinue Development of ALN-TTR02 prior to receipt of Regulatory Approval for ALN-TTR02 in the Genzyme Territory, but Alnylam has not discontinued Development of ALN-TTR02 in the Alnylam Territory prior to receipt of Regulatory Approval for ALN-TTR02 in the Alnylam Territory, Genzyme may provide Alnylam with written notice of its desire to abandon Development of ALN-TTR02 in the Genzyme Territory and to pursue an alternative siRNA Product that is an siRNA targeting the human TTR gene formulated in an ▇▇▇▇ ▇▇▇▇▇ Nanoparticle Formulation. After Alnylam’s receipt of such notice, Alnylam will provide Genzyme with any information in Alnylam’s possession that Genzyme may reasonably request regarding
Confidential
- 20 -
such siRNA Products. After its evaluation of such information, Genzyme may, by providing written notice to Alnylam, cause any one of such siRNA Products to become a Licensed Product for all purposes of this Agreement from the date of such notice, and may Develop such siRNA Product for the Genzyme Territory; provided, however, that if Genzyme causes such an siRNA Product to become a Licensed Product, Alnylam shall have no obligation to Develop such siRNA Product in the Alnylam Territory pursuant to this Agreement. If such an siRNA Product becomes a Licensed Product pursuant to this Section 2.5.3.4, then (a) the licenses granted by Alnylam to Genzyme with respect to ALN-TTR02 under this Agreement shall terminate; (b) without limiting Genzyme’s obligations under Sections 2.4.1 and 4.1.1 as to the Development and Commercialization of Licensed Products generally, Genzyme’s obligations to Develop and Commercialize ALN-TTR02 shall terminate, including all obligations under Section 2, Section 3, and Section 4 with respect to ALN-TTR02; (c) the development milestone payment under Section 8.2(iv) shall not be payable by Genzyme with respect to Alnylam’s or its Related Parties’ development of ALN-TTR02 if such development milestone payment has not become payable prior to Genzyme’s discontinuation of development of ALN-TTR02; (d) the JSC shall have no further responsibilities with respect to decision making or governance relating to the Development or Commercialization of ALN-TTR02, but Alnylam shall continue to provide informational updates to Genzyme regarding ALN-TTR02 to the extent such information is reasonably useful or necessary to Genzyme with regard to the Development or Commercialization of Licensed Products in the Genzyme Territory; (e) Alnylam’s continued Development of ALN-TTR02 shall continue to count toward the satisfaction of Alnylam’s obligations under Section 2.4.2; and (f) Alnylam’s obligations set forth in Section 5.7.1 shall cease to apply with respect to Alnylam’s licensing of ALN-TTR02.
2.5.4 Scientific Records. Each Party will maintain scientific records, in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes, which will fully and properly reflect all work done and results achieved in the performance of the Development activities and Post-Approval Studies with respect to Licensed Products by such Party.
2.5.5 Information Exchange and Development Assistance. During the Term, upon the reasonable request of the other Party, each Party shall provide to the other Party, without additional compensation (except as provided in Section 6.2) and in a commercially reasonable format, Know-How Controlled by such Party and/or its Related Parties that is licensed to the other Party under this Agreement (i.e. Know-How included in Genzyme Technology for Genzyme and Know-How included in Alnylam Technology for Alnylam) to the extent that it is reasonably necessary or useful for Developing a Licensed Product in the requesting Party’s Territory or for obtaining or maintaining Regulatory Approval for a Licensed Product in the requesting Party’s Territory, including copies of (a) all scientific information and data related to the Licensed Products (including all data made, collected or otherwise generated in the conduct of any pre-clinical studies, Clinical Studies, Post-Approval Studies or early access/named patient programs for the Licensed Products, as well as CMC information), and (b) protocols and investigator brochures, in each case, that are reasonably necessary for the other Party (or its Related Parties) to perform its obligations or exploit its rights under this Agreement with respect
Confidential
- 21 -
to Licensed Products. Notwithstanding the foregoing, Genzyme shall have no obligation to transfer or disclose to Alnylam any Know-How included in the Genzyme Manufacturing IP; provided however, that if Genzyme elects, in its sole discretion, to transfer or disclose any such Know-How to Alnylam in writing, it shall be “Genzyme Disclosed Manufacturing Know-How” under this Agreement and licensed to Alnylam in accordance with Section 7.2.3.
2.5.6 Personnel. Each Party may request, through the JSC or the other Party’s Collaboration Manager, if the JSC appoints Collaboration Managers, that the other Party reasonably make available for consultation regarding the Development of Licensed Products certain of its employees engaged in Development activities and Post-Approval Studies with respect to Licensed Products. The JSC or the Collaboration Managers will reasonably coordinate, upon reasonable notice during normal business hours and at their respective places of employment, consultation between the Parties on the progress of Licensed Product Development and Post-Approval Studies.
2.5.7 Confidentiality. All information exchanged by the Parties under this Section 2.5 will be deemed to be Confidential Information of the disclosing Party and maintained in accordance with Section 9.
2.6 Third Parties. The Parties shall be entitled to utilize the services of Third Party contract research and contract manufacturing organizations to perform their respective Development and Manufacturing activities under this Agreement; provided that (a) each Party shall require that such Third Party operates in a manner consistent with the terms of this Agreement and (b) each Party shall remain at all times fully liable for its respective responsibilities. Each Party shall require that any such Third Party agreement include confidentiality and non-use provisions that are no less stringent than those set forth in Section 9 of this Agreement and shall use Commercially Reasonable Efforts to obtain ownership of, and/or a fully sublicenseable license under and to, any Know-How and Patent Rights that are developed by such Third Party in the performance of such agreement and are reasonably necessary or useful to Develop, Manufacture and/or Commercialize Licensed Products in the Field.
3. REGULATORY MATTERS.
3.1 Regulatory Filings and Interactions.
3.1.1 Responsibilities. Except as otherwise provided in the Joint Development Plan, (a) each Party will own the INDs, the NDAs and related regulatory documents submitted to the applicable Regulatory Authorities in its Territory with respect to Licensed Products and (b) each Party will, as to Licensed Products in its Territory (i) oversee, monitor and coordinate all regulatory actions, communications and filings with, and submissions to, each Regulatory Authority, (ii) be responsible for interfacing, corresponding and meeting with each Regulatory Authority and(iii) be responsible for maintaining all regulatory filings.
3.1.2 Communications. Each Party will, as to Licensed Products in its Territory, notify the JSC in writing, including a brief description in English of the principal issues raised, of all material communications from Regulatory Authorities within [**] days, provide the JSC with a summary translation of such material communications in English as soon as reasonably
Confidential
- 22 -
possible but in any event within [**] days, and provide a full translation of such material communications in English as soon as reasonably possible thereafter. Each Party will provide the complete copies of the original correspondence in their native language to the other Party upon request.
3.1.3 Meetings. Each Party shall provide the other Party with reasonable advance notice of all substantive meetings with the Government Authorities in its Territory pertaining to any Licensed Products, or with as much advance notice as practicable under the circumstances. Each Party shall use Commercially Reasonable Efforts, to the extent reasonably practicable, to permit the other Party to have, at the other Party’s expense, one (1) mutually acceptable representative of the other Party to attend, solely as a non-participating observer, material, substantive meetings with the Governmental Authorities within such Party’s Territory pertaining to any Licensed Product; provided, however, that neither Party shall be obligated to change the schedule of such a meeting in order to accommodate the schedule of the other Party’s representative.
3.1.4 Submissions. In addition, each Party shall provide the other Party with written notice of (a) all filings and submissions for Regulatory Approval (including orphan drug applications and designations) regarding any Licensed Product in such Party’s Territory in a timely manner; (b) all Regulatory Approvals obtained or denied; and (c) the filing of any IND for any Licensed Product within [**] days after such event; provided, however, that in all circumstances, each Party shall inform the other Party of such event prior to public disclosure of such event by such Party.
3.2 Costs of Regulatory Affairs. Unless otherwise agreed to pursuant to Section 2.3.3, each Party shall be responsible for all costs incurred by such Party connection with applying for Regulatory Approval with respect to Licensed Products in its Territory, and related regulatory affairs activities.
3.3 Right of Reference. Each Party hereby grants to the other Party, and at the request of the other Party will grant to the other Party’s Related Parties, a “Right of Reference,” as that term is defined in 21 C.F.R. § 314.3(b) (or any successor rule or analogous Law recognized outside of the United States), to, and a right to copy, access, and otherwise use, all information and data (including all CMC information as well as data made, collected or otherwise generated in the conduct of any Clinical Studies or Post-Approval Studies or early access/named patient programs for the Licensed Products) included in any regulatory filing, Regulatory Approval, drug master file or other regulatory documentation (including orphan drug applications and designations) owned or controlled by such Party or its Related Parties that relates to (a) any Licensed Product or (b) with respect to such information and materials provided to Genzyme, to the extent necessary or useful to obtain Regulatory Approval of a Licensed Product in the Genzyme Territory, the siRNA Product Controlled by Alnylam and known as ALN-TTR01, and such Party shall provide a signed statement to this effect, if requested by the other Party, in accordance with 21 C.F.R. § 314.50(g)(3) (or any successor or analogous Law outside of the United States).
Confidential
- 23 -
3.4 Pharmacovigilance. At least [**] months prior to Genzyme obtaining the authorization to conduct any Genzyme sponsored study, the Parties will negotiate a Safety Data Exchange Agreement (“SDEA”) to be agreed upon in writing, that will define the pharmacovigilance responsibilities of the Parties and include safety data exchange procedures governing the coordination of collection, investigation, reporting, and exchange of information concerning any adverse experiences, and any product quality and product complaints associated with adverse experiences, related to Licensed Products, sufficient to enable each Party (and their respective Related Parties, if any) to comply with its legal and regulatory obligations.
4. COMMERCIALIZATION OF THE LICENSED PRODUCTS
4.1 Responsibility, Cost and Diligence.
4.1.1 Genzyme. Genzyme shall be solely responsible, at its expense, for all Commercialization activities relating to Licensed Products in the Field in the Genzyme Territory. Genzyme shall use Commercially Reasonable Efforts to Commercialize Licensed Products in each country within the Genzyme Territory where Genzyme has obtained Regulatory Approval for a Licensed Product. For clarity, it may be consistent with Genzyme’s Commercially Reasonable Effort’s obligation to abandon Commercialization of a Licensed Product in favor of another Licensed Product.
4.1.2 Alnylam. Alnylam shall be solely responsible, at its expense, for all Commercialization activities relating to Licensed Products in the Field in the Alnylam Territory.
4.1.3 Joint Commercialization. In the event that the Parties mutually agree to conduct any joint Commercialization activities regarding a Licensed Product, the Parties will (a) agree in writing to a written work plan and time table for conducting such activities, (b) agree in writing to management and governance mechanisms for such joint activities, including coordination of such activities through the JSC and (c) negotiate in good faith a budget therefor and an equitable allocation of costs between the Parties.
4.2 Genzyme Commercialization Plan. No less than [**] months in advance of [**] in the Genzyme Territory with respect to a Licensed Product, and on [**] basis thereafter, Genzyme shall prepare and deliver to the JSC for review a reasonable written plan that summarizes the Commercialization activities to be undertaken with respect to Licensed Products in the Genzyme Territory in the [**] and, to the extent commercially reasonable, Genzyme’s plans to obtain further Regulatory Approvals and Commercialize Licensed Products in countries in the Genzyme Territory in which Genzyme is not then Commercializing Licensed Products, and the dates by which such activities are targeted to be accomplished (the “Genzyme Territory Commercialization Plan”). The Genzyme Territory Commercialization Plan shall subsequently be updated and modified by Genzyme, from time to time at its discretion and no less frequently than [**], based upon, among other things, Genzyme’s Commercialization activities with respect to Licensed Products in the Genzyme Territory, a copy of which updated plan Genzyme will provide to the JSC. Notwithstanding the foregoing, in the event of any disagreement between the Parties regarding the Genzyme Territory Commercialization Plan pursuant to Section 5.5, the Genzyme representatives on the JSC shall have final decision-making authority over the preparation and updating of the Genzyme Territory Commercialization Plan; provided that, such decisions do not materially adversely affect the Commercialization of a Licensed Product in the Alnylam Territory.
Confidential
- 24 -
4.3 Alnylam Commercialization Plan. Reasonably in advance of the expected [**] in the Alnylam Territory with respect to a Licensed Product, and on [**] basis promptly as it becomes available, Alnylam will deliver to Genzyme its written plan, generated by Alnylam for its internal purposes, that summarizes the Commercialization activities to be undertaken with respect to Licensed Products in the Alnylam Territory in the [**] (the “Alnylam Territory Commercialization Plan”).
4.4 Advertising and Promotional Materials.
4.4.1 Global Branding. Alnylam shall have the right, from time to time during the Term, to develop (and thereafter modify and update) a global branding strategy (including global positioning, messages, logo, colors and other visual branding elements) for Licensed Products for use in the Field throughout the world (the “Global Branding Strategy”), which the JSC shall, in accordance with Sections 5.4.2(b) and 5.5, review and approve, and which the Parties shall, following such review and approval, implement. Alnylam will submit the Global Branding Strategy to the JSC at least [**] (or more frequently if reasonably requested by Genzyme). Alnylam shall consider in good faith any timely comments Genzyme may have with respect to the Global Branding Strategy, but shall have final decision-making authority with respect to such Global Branding Strategy.
4.4.2 Alnylam. Alnylam will be responsible for the creation, preparation, production, reproduction and filing with the applicable Regulatory Authorities, of relevant written sales, promotion and advertising materials relating to Licensed Products (“Promotional Materials”) for use in the Alnylam Territory. All such Promotional Material will be (a) compliant with applicable Law, and (b) if applicable, consistent in all material respects with the Global Branding Strategy. Alnylam will submit representative samples of its Promotional Materials developed by it for use in the Alnylam Territory to the JSC, at least [**] (or more frequently if reasonably requested by Genzyme). Alnylam shall consider in good faith any timely comments Genzyme may have with respect to such Promotional Materials, but shall have final decision-making authority with respect to such Promotional Materials.
4.4.3 Genzyme. Genzyme will be responsible for the creation, preparation, production, reproduction and filing with the applicable Regulatory Authorities, of relevant Promotional Materials for use in the Genzyme Territory. All such Promotional Materials will be (a) compliant with applicable Law, (b) consistent in all material respects with the Genzyme Territory Commercialization Plan, and (c) if applicable, consistent in all material respects with the Global Branding Strategy. Genzyme will submit representative samples of its Promotional Materials developed by it for use in the Genzyme Territory to the JSC at least [**] thereafter (or more frequently if reasonably requested by Alnylam). Genzyme shall consider in good faith any timely comments Alnylam may have with respect to such Promotional Materials, but shall have final decision-making authority with respect to such Promotional Materials.
Confidential
- 25 -
4.5 Reporting Obligations. Genzyme shall report to the JSC in writing, by no later than each [**] following the first Regulatory Approval of a Licensed Product in the Field in the Genzyme Territory (for the period ending December 31 of the prior Calendar Year), summarizing in reasonable detail Genzyme’s Commercialization activities for Licensed Products performed to date (or updating such report for activities performed since the last such report was given hereunder, as applicable). In addition, Genzyme shall provide Alnylam with written notice of the First Commercial Sale of each Licensed Product in the Genzyme Territory within [**] days after such event; provided, however, that in all circumstances, Genzyme shall inform Alnylam of such event prior to public disclosure of such event by Genzyme. Each Party shall provide such other information to the JSC as the other Party may reasonably request and shall keep the JSC reasonably informed of such Party’s Commercialization activities with respect to Licensed Products.
4.6 Sales and Distribution. Each Party and its Related Parties shall be responsible for booking sales and shall warehouse and distribute Licensed Products in its Territory. If a Party receives any orders for any Licensed Product in the other Party’s Territory, it shall refer such orders to the other Party. Moreover, each Party and its Related Parties shall be solely responsible for handling all returns of Licensed Product sold in its Territory, as well as all aspects of Licensed Product order processing, invoicing and collection, distribution, inventory and receivables of Licensed Products sold in its Territory.
4.7 Recalls, Market Withdrawals or Corrective Actions. In the event that any Regulatory Authority issues or requests a recall or takes a similar action in connection with the Licensed Product in a Territory, or in the event either Party determines that an event, incident or circumstance has occurred that may result in the need for a recall or market withdrawal in its own Territory, the Party notified of such recall or similar action, or the Party that desires such recall or similar action, shall within [**] advise the other Party thereof by telephone, facsimile or e-mail. Each Party, in consultation with the other Party, shall decide whether to conduct a recall in its own Territory and the manner in which any such recall shall be conducted (except in the case of a government mandated recall, when such Party may act without such advance notice but shall notify the other Party as soon as possible). Each Party shall bear the expense of any such recall in its own Territory. Each Party will make available all of its pertinent records that may be reasonably requested by the other Party in order to effect a recall in the other Party’s Territory.
4.8 Ex-Territory Sales; Export Monitoring.
4.8.1 Ex-Territory Sales. Subject to applicable Law, neither Party shall engage in any advertising or promotional activities relating to any Licensed Product directed primarily to customers or other buyers or users of such Licensed Product located outside its Territory or accept orders for Licensed Products from or sell Licensed Products into such other Party’s Territory for its own account, and if a Party receives any order for Licensed Products in the other Party’s Territory, it shall refer such orders to the other Party.
4.8.2 Export Monitoring. Each Party and its Related Parties will use Commercially Reasonable Efforts to monitor and prevent exports of Licensed Products from its own Territory for Commercialization in the other Party’s Territory using methods commonly used in the industry for such purpose, and shall promptly inform the other Party of any such exports of
Confidential
- 26 -
Licensed Products from its Territory, and the actions taken to prevent such exports. Each Party agrees to take reasonable actions requested in writing by the other Party that are consistent with Law to prevent exports of Licensed Products from its Territory for Commercialization in the other Party’s Territory.
5. COLLABORATION MANAGEMENT
5.1 Joint Steering Committee. The Parties hereby establish a joint steering committee (the “JSC”) to facilitate the Collaboration as follows:
5.1.1 Composition of the Joint Steering Committee. The Collaboration shall be conducted under the oversight of the JSC, which shall comprise [**] representatives of each Party. Each Party shall appoint its respective representatives to the JSC from time to time, and may substitute one or more of its representatives, in its sole discretion, effective upon notice to the other Party of such change. Each Party shall have at least one JSC representative who is a senior employee [**], and all JSC representatives shall have appropriate expertise and ongoing familiarity with the Collaboration. Additional representatives or consultants may from time to time, by mutual consent of the Parties, be invited to attend JSC meetings, subject to such representatives and consultants undertaking confidentiality obligations, whether in a written agreement or by operation of law, no less stringent than the requirements of Section 9.1.
5.1.2 JSC Chairperson. The JSC chairperson shall rotate every [**] months between a JSC representative of Alnylam and a JSC representative of Genzyme. The initial JSC chairperson shall be a representative of [**]. The JSC chairperson’s responsibilities shall include (a) scheduling meetings at least [**], but more frequently if the JSC determines it necessary; (b) setting agendas for meetings with solicited input from other members; (c) coordinating the delivery of draft minutes to the JSC for review and final approval; and (d) conducting meetings, including ensuring that objectives for each meeting are set and achieved.
5.2 Appointment of Subcommittees, Project Teams and Collaboration Managers. The JSC shall be empowered to create such subcommittees of itself and project teams as it may deem appropriate or necessary, such as for specific oversight of Development, Manufacturing, Commercialization or intellectual property issues. Each such subcommittee and project team shall report to the JSC, which shall have authority to approve or reject recommendations or actions proposed thereby subject to the terms of this Agreement. The JSC may direct each Party to designate a Collaboration manager (the “Collaboration Manager”) to serve as a primary point of contact for the other Party under the Collaboration. Each Party may change its Collaboration Manager at any time in its sole discretion with written notice to the other Party.
5.3 Meetings. The JSC shall meet in accordance with a schedule established by mutual written agreement of the Parties, but no less frequently than [**] during the Term, with the location for such meetings alternating between Alnylam and Genzyme facilities (or such other locations as are mutually agreed by the Parties). Alternatively, the JSC may meet by means of teleconference, videoconference or other similar communications equipment, but at least [**] meetings per [**] shall be conducted in person. All proceedings for the JSC shall take place in English. Each Party shall bear its own expenses relating to attendance at such meetings by its representatives.
Confidential
- 27 -
5.4 Minutes. A secretary shall be appointed for each meeting of the JSC and shall prepare minutes of the meeting, which shall provide a description in reasonable detail of the discussions held at the meeting and a list of any actions, decisions or determinations approved by the JSC.
5.4.1 JSC Development Responsibilities. The JSC shall have the following responsibilities with respect to the Development of Licensed Products pursuant to the Collaboration:
(a) reviewing and discussing updates to be provided by Alnylam pursuant to Section 2.5.1 and Section 7.5 regarding the Development status of potential Backup Compounds and Improvement Products;
(b) reviewing and approving, subject to Section 5.5.3(a), the initial Genzyme Territory Development Plan and reviewing and discussing updates provided by each Party regarding the Development of Licensed Products in such Party’s Territory, including updates to the Global Development Strategy, the Genzyme Territory Development Plan or Alnylam Territory Development Plan, as applicable, provided by the applicable Party in accordance with Section 2.2, and providing each Party with feedback regarding the same;
(c) monitoring and coordinating Development activities in the Genzyme Territory and the Alnylam Territory for Licensed Products, and regulatory and pharmacovigilance requirements and matters;
(d) monitoring, planning and coordinating any Joint Clinical Studies in accordance with any Joint Development Plan, Joint Development Budget, and written agreement between the Parties with respect to the governance and management of such Joint Clinical Studies;
(e) overseeing the manufacturing and supply relationship between the Parties with respect to the Manufacture of Licensed Products for Development activities (subject to the terms of the Clinical Supply Agreement); and
(f) performing such other activities as the Parties agree in writing shall be the responsibility of the JSC.
5.4.2 JSC Commercialization Responsibilities. The JSC shall have the following responsibilities with respect to the Commercialization of Licensed Products pursuant to the Collaboration, to the extent permissible under applicable Laws:
(a) reviewing and discussing updates to be provided by each Party regarding the Commercialization of Licensed Products in such Party’s Territory;
(b) reviewing and approving, subject to Section 5.5.3(b), any Global Branding Strategy developed by Alnylam;
Confidential
- 28 -
(c) reviewing and discussing the Genzyme Territory Commercialization Plan and updates thereto provided by Genzyme pursuant to Section 4.2;
(d) reviewing and discussing Promotional Material of both Parties in accordance with Sections 4.4.2 and 4.4.3;
(e) providing a forum for the Parties to discuss the Commercialization of Licensed Products in the Field worldwide, including coordination regarding Licensed Product positioning and messaging, key opinion leader relationship management, medical affairs, and marketing and selling materials;
(f) providing a forum for the Parties to discuss collaborating on commercial activities that can be leveraged for both Parties’ respective Territories and how the Parties would share the costs of such mutually agreed joint Commercialization activities;
(g) overseeing the manufacturing and supply relationship between the Parties with respect to the Manufacture of Licensed Products for Commercialization activities (subject to the terms of the Commercial Supply Agreement); and
(h) performing such other activities as the Parties agree in writing shall be the responsibility of the JSC.
5.5 Decision-Making.
5.5.1 With respect to decisions of the JSC, the representatives of each Party shall have collectively one vote on behalf of such Party. For each meeting of the JSC, at least [**] representatives of each Party shall constitute a quorum. Action on any matter may be taken at a meeting, by teleconference, videoconference or by written agreement. The JSC shall attempt to resolve any and all disputes before it for decision by consensus.
5.5.2 If the JSC is unable to reach a consensus with respect to a dispute for a period in excess of [**] days, then the dispute shall be submitted to the Chief Executive Officers, or his or her designee (any such designee to be a senior member of the designating Chief Executive Officer’s management team), of Alnylam and Genzyme for resolution.
5.5.3 If such dispute cannot be resolved for a period in excess of [**] days following escalation, then:
(a) subject to Section 5.5.4, the Chief Executive Officer of Genzyme or his or her designee shall have the deciding vote on any matter involving the Development or Commercialization of Licensed Products in the Field in the Genzyme Territory, including the Genzyme Territory Development Plan and the Genzyme Territory Commercialization Plan; and
(b) subject to Section 5.5.4, the Chief Executive Officer of Alnylam or his or her designee shall have the deciding vote on any matter involving the Development or Commercialization of Licensed Products in the Field in the Alnylam Territory, including the Alnylam Territory Development Plan and the Alnylam Territory Commercialization Plan, and on any matter involving the content of the Global Branding Strategy.
Confidential
- 29 -
5.5.4 Neither Party shall have the deciding vote on, and the JSC shall not have decision-making authority regarding, any of the following matters:
| (i) | the allocation of milestone payments to Third Parties under Future Alnylam In-Licenses pursuant to Section 7.4.3.2; |
| (ii) | the imposition of any requirements on the other Party to undertake obligations beyond those for which it is responsible, or forgo any rights, under this Agreement or the Supply Agreements; |
| (iii) | any matters that would cause a material increase in costs that the other Party is required to expend, as determined in the reasonable discretion of such other Party; |
| (iv) | the imposition of any requirements that the other Party take or decline to take any action that would result in a violation of any Law or any agreement with any Third Party or the infringement of intellectual property rights of Third Parties; |
| (v) | any matters that would excuse such Party from any of its obligations specifically enumerated under this Agreement or the Supply Agreements; or |
| (vi) | modifying the terms of this Agreement or any Supply Agreement or taking any action to expand or narrow the responsibilities of the JSC. |
5.6 Term of JSC. After the [**] anniversary of the Effective Date Alnylam shall have the right to terminate its participation in the JSC. Following any such termination, the Parties will discuss in good faith and agree upon reasonable processes regarding decision making and information sharing in order to provide each Party with substantially the same rights and information as such Party enjoyed during the period during which the JSC was constituted.
5.7 Alnylam Third Party Partner. If, at any time during the Term, Alnylam enters into an agreement (a “Third Party Collaboration Agreement”) with one or more Third Party(ies) (the “Third Party Partner”) to Develop and/or Commercialize a Licensed Product in the Field in the Alnylam Territory, Alnylam shall ensure that such agreement is consistent with the terms and conditions of this Agreement.
5.7.1 Required Terms. Without limitation, Alnylam shall negotiate terms in the Third Party Collaboration Agreement (a) regarding intellectual property necessary to permit Alnylam to license or sublicense to Genzyme any Patent Rights and Know-How developed in the course of activities pursuant to the Third Party Collaboration Agreement that are necessary for Genzyme to Develop, Manufacture and Commercialize Licensed Products in accordance with this Agreement, if any, (b) providing Genzyme with a right of reference as set forth in Section 3.3 (Right of Reference), (c) requiring such Third Party Partner to maintain records substantially as set forth in Section 2.5.4 (Scientific Records), (d) enabling Alnylam to provide Genzyme with
Confidential
- 30 -
copies of data and reports, including Clinical Study reports, in a commercially reasonable format, made, collected or otherwise generated by or on behalf of the Third Party Partner in the conduct of any pre-clinical studies, Clinical Studies or early access/named patient programs for the Licensed Products (excluding, for clarity, information generated in Post-Approval Studies and CMC information), and protocols and investigator brochures related to the Licensed Product, in each case that are reasonably necessary for Genzyme (or its Related Parties) to Develop and obtain Regulatory Approval for the Licensed Products in the Genzyme Territory, (e) enabling Alnylam to provide Genzyme with information with respect to matters in the Third Party Partner’s territory as are required to be provided to Genzyme under Sections 2.5.1 (Development Activities), 3.1.2 (Communications), to the extent requiring a brief description in English of principal issues raised in material communications from Regulatory Authorities and otherwise to the extent reasonably necessary to assist Genzyme with obtaining Regulatory Approvals of Licensed Products, and 3.1.4 (Submissions), (f) regarding pharmacovigilance that are consistent with Section 3.4 and the SDEA, and (g) regarding recalls that are consistent with Section 4.6.
5.7.2 Terms Subject to Commercially Reasonable Efforts. In addition, Alnylam shall use Commercially Reasonable Efforts to negotiate terms in the Third Party Collaboration Agreement (a) related to intellectual property that would give Genzyme substantially similar rights with respect to intellectual property developed in the course of activities under the Third Party Collaboration Agreement as Genzyme would have received with respect to intellectual property developed in the course of the Collaboration, and (b) enabling Alnylam to provide Genzyme with reciprocal information, rights and benefits with respect to the Development of Licensed Products and the conduct of Post Approval Studies for the Third Party Partner’s territory as are provided to Alnylam by Genzyme in this Agreement.
5.7.3 Global Coordination Committee. Alnylam shall use reasonable efforts to include in any Third Party Collaboration Agreement relating to rights licensed to a Third Party Partner in the United States and/or major markets in Europe, the discretionary participation by such Third Party Partner with Genzyme and Alnylam in a global coordination committee, which shall include representatives of Alnylam, Genzyme and the Third Party Partner and which shall be responsible for facilitating discussions between the parties with respect to the global Development, Manufacture, and Commercialization of Licensed Products.
5.7.4 Notice. Alnylam shall promptly provide Genzyme with a copy of any fully executed Third Party Collaboration Agreement, which may be redacted to remove terms and conditions which are not necessary to monitor compliance with this Section 5.7 and such Third Party Collaboration Agreement will be Alnylam Confidential Information for the purposes of Section 9 (Confidentiality and Publication).
5.7.5 Sublicenses. In addition, if the Third Party Collaboration Agreement grants the Third Party Partner a sublicense under the Genzyme Technology, Alnylam shall ensure that such Third Party Collaboration agreement complies with Section 7.2.4 (Sublicensing Terms).
5.7.6 Breach; Responsibility. If Alnylam becomes aware of a material breach of the terms of any Third Party Collaboration Agreement by a Third Party Partner compliance with which is necessary for Alnylam’s compliance with the terms of this Agreement, Alnylam shall
Confidential
- 31 -
promptly notify Genzyme of the particulars of the same and use Commercially Reasonable Efforts to cause the Third Party Partner to comply with all the terms of the Third Party Collaboration Agreement necessary for Alnylam’s compliance with the terms of this Agreement. Notwithstanding any Third Party Collaboration Agreement, Alnylam shall remain primarily liable to Genzyme for the performance of all of Alnylam’s obligations under, and Alnylam’s compliance with all terms and conditions of, this Agreement with respect to the entire Alnylam Territory.
6. MANUFACTURE AND SUPPLY OF THE LICENSED PRODUCT
6.1 Supply Agreements. Within [**] months after the Effective Date, the Parties will negotiate in good faith and enter into a supply agreement for clinical supply of Licensed Product (the “Clinical Supply Agreement”), which will be consistent with the terms set forth on Schedule 6.1. In addition, at the request of Genzyme reasonably in advance of the first commercial launch of a Licensed Product in the Genzyme Territory, the Parties will negotiate in good faith and enter into a supply agreement for commercial supply of Licensed Product (the “Commercial Supply Agreement”), which will also be consistent with the terms set forth on Schedule 6.1.
6.2 Transfer of Manufacturing Know-How. At Genzyme’s request at any time during the Term, Alnylam shall transfer to Genzyme, a Genzyme Related Party, or a Third Party manufacturer, all Alnylam Know-How necessary or useful to enable the Manufacture of the Licensed Products and not previously transferred to Genzyme, a Genzyme Related Party, or such Third Party manufacturer, in bulk and/or finished form (as requested by Genzyme), by providing copies or samples of relevant documentation, materials and other embodiments of such Know-How and by making available its qualified technical personnel on a reasonable basis to consult with Genzyme, its Related Party, or such Third Party manufacturer with respect to such Know-How. If requested by Genzyme, such Know-How transfer shall be conducted pursuant to a mutually-agreed technology transfer plan developed by the parties for the purpose of ensuring the complete and timely transfer of such Know-How that is consistent with Genzyme’s then current internal Technology Transfer Corporate Standard (or equivalent policy) that have been provided to Alnylam. Such Know-How transfer shall be conducted at Alnylam’s sole expense for the first [**] hours of Alnylam employees’ time, and thereafter at Genzyme’s expense at the cost of Alnylam employees’ time at the FTE Rate. Genzyme will reimburse Alnylam for its Out-of-Pocket Costs incurred in the course of such transfer, provided that such Out-of-Pocket Costs are approved in advance by Genzyme and itemized in Alnylam’s invoice. Genzyme shall pay Alnylam such FTE Rate and reimburse Alnylam for such Out-of-Pocket Costs within [**] days after receipt of an invoice therefor.
7. LICENSES
7.1 License Grants to Genzyme.
7.1.1 Development and Commercialization License in the Genzyme Territory. Subject to the terms and conditions of this Agreement, Alnylam hereby grants Genzyme a non-transferable (except as provided in Section 14.1), sublicensable (subject to Section 7.1.3)
Confidential
- 32 -
exclusive (even as to Alnylam) license under Alnylam Technology to Develop and Commercialize Licensed Products in the Field in the Genzyme Territory. Such license shall be royalty-bearing for the Royalty Term applicable to each Licensed Product in each country in the Genzyme Territory, and, after the Royalty Term applicable to such Licensed Product in such country, shall convert to a fully-paid license to Develop and Commercialize such Licensed Product in the Field in such country.
7.1.2 Manufacturing License. Subject to the terms and conditions of this Agreement, Alnylam hereby grants Genzyme a non-transferable (except as provided in Section 14.1), sublicensable (subject to Section 7.1.3), worldwide, exclusive license under Alnylam Technology to Manufacture Licensed Products inside or outside of the Genzyme Territory solely for Development and Commercialization in the Genzyme Territory and, to the extent permitted pursuant to Section 2.3.3, for Development in the Alnylam Territory. Notwithstanding the foregoing, Alnylam retains the exclusive right under the Alnylam Technology, with the right to grant licenses through multiple tiers without restriction, to Manufacture Licensed Products anywhere in the world (i) for Development and Commercialization in the Alnylam Territory and, to the extent permitted pursuant to Section 2.3.3 for Development in the Genzyme Territory, and (ii) to supply Genzyme pursuant to the Supply Agreements to be agreed by the Parties pursuant to Section 6.1.
7.1.3 Sublicensing Terms.
(a) Genzyme shall have the right to sublicense any of its rights under Section 7.1.1 and 7.1.2 to any of its Affiliates or to any Third Party (which sublicensed rights may be further sublicensable through multiple tiers) without the prior consent of Alnylam, subject to the requirements of this Section 7.1.3. Notwithstanding the foregoing, Genzyme shall not have the right to sublicense to a Third Party either (i) its primary Development or Commercialization rights in Japan; or (ii) all or substantially all of its rights under this Agreement.
(b) Each sublicense granted by Genzyme pursuant to this Section 7.1.3 shall be subject and subordinate to the terms and conditions of this Agreement and shall contain terms and conditions consistent with those in this Agreement. Genzyme shall promptly provide Alnylam with a copy of the fully executed sublicense agreement covering any sublicense granted hereunder (which copy may be redacted to remove terms and conditions which are not necessary to monitor compliance with this Section 7.1.3), and each such sublicense agreement shall contain the following provisions: (i) a requirement that the Sublicensee comply with the confidentiality and non-use provisions of Section 9 with respect to Alnylam’s Confidential Information, (ii) if such sublicense agreement contains a sublicense of Licensed Product Commercialization rights, such sublicense agreement shall also contain the following provisions: (x) a requirement that the Sublicensee submit applicable sales or other reports to Genzyme to the extent necessary or relevant to the reports required to be made or records required to be maintained under this Agreement; and (y) the audit requirement set forth in Section 8.4; and (iii) a requirement that the Sublicensee comply with the applicable provisions under any Alnylam In-License.
Confidential
- 33 -
(c) If Genzyme becomes aware of a material breach of the terms of any sublicense by any Genzyme Sublicensee, compliance with which is necessary for Genzyme’s compliance with the terms of this Agreement, Genzyme shall promptly notify Alnylam of the particulars of the same and use Commercially Reasonable Efforts to cause the Sublicensee to comply with all the terms of the sublicense necessary for Genzyme’s compliance with the terms of this Agreement. In the event that (i) the Sublicensee has failed to cure a material breach within [**] days after notice of such breach and (ii) such material breach also constitutes a breach of this Agreement, Genzyme shall terminate the sublicense at the request of Alnylam. Notwithstanding any sublicense, Genzyme shall remain primarily liable to Alnylam for the performance of all of Genzyme’s obligations under, and Genzyme’s compliance with all terms and conditions of, this Agreement.
7.2 License Grants to Alnylam.
7.2.1 Development and Commercialization License in the Alnylam Territory. Genzyme hereby grants Alnylam a non-transferable (except as provided in Section 14.1), sublicensable (subject to Section 7.2.4), exclusive, royalty-free license, under (a) Genzyme Collaboration IP, Genzyme’s interest in Joint Collaboration IP and (b) any Genzyme Technology that is produced, generated, conceived and reduced to practice as a result of, or used by Genzyme or its Related Parties in, the Development or Commercialization activities of Genzyme and its Related Parties under this Agreement, in each case of (a) and (b), to Develop and Commercialize Licensed Products in the Field in the Alnylam Territory.
7.2.2 License to Improvement Manufacturing Patent Rights. Subject to the terms and conditions of this Agreement, Genzyme hereby grants Alnylam a non-transferable (except as provided in Section 14.1), sublicensable (subject to Section 7.2.4), worldwide, non-exclusive license under the Improvement Manufacturing Patent Rights, to Manufacture (a) Alnylam Developed siRNA Products targeting any human gene; and (b) Licensed Products for Development and Commercialization in the Alnylam Territory and, to the extent permitted pursuant to Section 2.3.3, for Development in the Field in the Genzyme Territory.
7.2.3 License to Genzyme Disclosed Manufacturing Know-How. Subject to the terms and conditions of this Agreement, Genzyme hereby grants Alnylam a non-transferable (except as provided in Section 14.1), sublicensable (subject to Section 7.2.4), worldwide, non-exclusive license under the Genzyme Disclosed Manufacturing Know-How, to Manufacture Licensed Products for Development and Commercialization in the Alnylam Territory and, to the extent permitted pursuant to Section 2.3.3, for Development in the Field in the Genzyme Territory.
7.2.4 Sublicensing Terms.
(a) Alnylam shall have the right to sublicense any of its rights under Section 7.2.1, 7.2.2 and 7.2.3 (which sublicensed rights may be further sublicensable through multiple tiers) to any of its Affiliates or to any Third Party without the prior consent of Genzyme, and subject to the requirements of this Section 7.2.4; provided that Alnylam’s right to sublicense the rights granted to it under Section 7.2.2(a) to Third Parties shall be limited to licensees of Alnylam that
Confidential
- 34 -
have been granted the right to develop and/or commercialize Alnylam Developed siRNA Products and have been granted the right to use Alnylam manufacturing technology to manufacture Alnylam Developed siRNA Products.
(b) Each sublicense granted by Alnylam pursuant to this Section 7.2.4 shall be subject and subordinate to the terms and conditions of this Agreement and shall contain terms and conditions consistent with those in this Agreement. Alnylam shall promptly provide Genzyme with a copy of the fully executed sublicense agreement covering any sublicense granted hereunder (which copy may be redacted to remove terms and conditions which are not necessary to monitor compliance with this Section 7.2.4), and each such sublicense agreement shall contain the following provisions: (i) a requirement that the Sublicensee comply with the confidentiality and non-use provisions of Section 9 with respect to Genzyme’s Confidential Information and (ii) a requirement that the Sublicensee comply with the applicable provisions under any Genzyme In-License.
(c) If Alnylam becomes aware of a material breach of any sublicense by any Alnylam Sublicensee, compliance with which is necessary for Alnylam’s compliance with the terms of this Agreement, Alnylam shall promptly notify Genzyme of the particulars of the same and use Commercially Reasonable Efforts to cause the Sublicensee to comply with all the terms of the sublicense necessary for Alnylam’s compliance with the terms of this Agreement. In the event that (i) the Sublicensee has failed to cure a material breach within [**] days after notice of such breach and (ii) such material breach also constitutes a breach of this Agreement, Alnylam shall terminate the sublicense at the request of Genzyme. Notwithstanding any sublicense, Alnylam shall remain primarily liable to Genzyme for the performance of all of Alnylam’s obligations under, and Alnylam’s compliance with all terms and conditions of, this Agreement.
7.3 Joint Collaboration IP. Subject to the rights and licenses granted to, and the obligations (including royalty obligations) of, each Party under this Agreement, either Party is entitled to practice Joint Collaboration IP for all purposes on a worldwide basis and license without consent of and without a duty of accounting to the other Party. Each Party will grant and hereby does grant all permissions, consents and waivers with respect to, and all licenses under, the Joint Collaboration IP, throughout the world, necessary to provide the other Party with such rights of use and exploitation of the Joint Collaboration IP, and will execute documents as necessary to accomplish the foregoing.
7.4 In-Licenses.
7.4.1 Compliance with In-Licenses. All licenses and other rights granted to Genzyme under this Section 7 are subject to the rights and obligations of Alnylam under the Alnylam In-Licenses. All licenses and other rights granted to Alnylam under this Section 7 are subject to the rights and obligations of Genzyme under the Genzyme In-Licenses. Each Party shall comply with all applicable terms and conditions of the In-Licenses, and shall perform and take such actions as may be required to allow the Party that is party to such In-License to comply with its obligations thereunder, including obligations relating to sublicensing, patent matters, confidentiality, reporting, audit rights, indemnification and diligence. Without limiting the foregoing, each Party shall prepare and deliver to the other Party any additional reports required
Confidential
- 35 -
under the applicable In-Licenses and requested by such other Party, in each case sufficiently in advance to enable the Party that is party to such In-License to comply with its obligations under the applicable In-Licenses. Each Party agrees, upon the other Party’s request, to provide the other Party with copies of any In-Licenses to which it is a party. Confidential Information of the providing Party or its counterparty may be redacted from such copies, except to the extent that such information is required in order to enable the other Party to comply with its obligations to the providing Party under this Agreement with respect to such In-License or in order to enable the providing Party to ascertain compliance with the provisions of this Agreement.
7.4.2 New In-Licenses; Additional Alnylam In-Licenses.
7.4.2.1. Alnylam Negotiation of Future Alnylam In-Licenses. In the event that Alnylam determines to enter into an agreement with a Third Party after the Effective Date pursuant to which Alnylam would acquire a license under Patent Rights that are necessary or useful to Develop, Manufacture or Commercialize Licensed Products in the Field in the Alnylam Territory, Alnylam shall, if counterparts of such Patent Rights exist in the Genzyme Territory and a license under such Patent Rights is available for the Genzyme Territory, also obtain a comparable license under such Patent Rights with respect to Licensed Products in the Genzyme Territory.
7.4.2.2. Acceptance of Future In-Licenses. In the event that a Party (the “Licensing Party”) enters into an agreement with a Third Party after the Effective Date that meets the criteria set forth in clause (a) of the definition of Genzyme In-License (in the case of Genzyme) or that meets the criteria set forth in clause (b) of the definition of Alnylam In-License (in the case of Alnylam), then the Licensing Party will promptly provide the other Party with notice and a copy of the applicable Third Party agreement. Within [**] days following receipt of such notice, such other Party will decide, in its sole discretion, whether to accept the applicable Third Party agreement as a Genzyme In-License or Alnylam In-License, as applicable, and provide notice of such other Party’s decision to the Licensing Party. In the event that such other Party declines to accept such agreement as a Genzyme In-License or Alnylam In-License, as applicable, any rights granted to such Party thereunder will not be deemed to be “Controlled” by the Licensing Party or licensed to such other Party under this Agreement, and will not be subject to the payment provisions under this Agreement relating to In-Licenses. In the event that such other Party accepts such Third Party agreement as a Genzyme In-License or Alnylam In-License, as applicable, such agreement will thereafter be included within the definition of Genzyme In-License or Alnylam In-License, respectively, and any rights granted to the Licensing Party thereunder will be deemed to be “Controlled” by the Licensing Party and sublicensed to such other Party under this Agreement. Notwithstanding the foregoing, in the event that Genzyme enters into a Third Party agreement that meets the criteria set forth in clause (a) of the definition of Genzyme In-License and such Agreement relates solely to the Genzyme Territory, then such agreement shall automatically be included within the definition of Genzyme In-License.
7.4.2.3. Additional Alnylam In-Licenses. In the event that a Patent Right licensed to Alnylam under an Additional Alnylam In-License issues that actually is
Confidential
- 36 -
or will be infringed by Genzyme’s Commercialization of Licensed Products in the Genzyme Territory or Genzyme’s Manufacture or Development of Licensed Products in accordance with this Agreement, then such agreement will thereafter be included within the definition of Existing Alnylam In-Licenses, and all rights granted to Alnylam thereunder will be deemed to be “Controlled” by Alnylam and sublicensed to Genzyme under this Agreement effective as of the later of the date the applicable Patent Right issues or the date that Genzyme’s Commercialization of Licensed Products in the Genzyme Territory or Genzyme’s Manufacture or Development of Licensed Products in accordance with this Agreement would infringe such Patent Right in the absence of a license thereunder from Alnylam.
7.4.3 Payments Under In-Licenses.
7.4.3.1. Existing Alnylam In-Licenses and Alnylam In-Licenses Related to Existing Products. Subject to Sections 7.4.2 (New In-Licenses-Additional Alnylam In-Licenses), 7.4.4 (Amendments to In-Licenses), 7.5.4 (Improvement Product - Royalty Adjustments) and 8.3.5 (Royalty Floor), Alnylam shall bear one hundred percent (100%) of any Third Party License Payments under (a) the Existing Alnylam In-Licenses, and (b) Alnylam In-Licenses entered into after the Effective Date (“Future Alnylam In-Licenses”) in which Alnylam obtains licenses under Patent Rights that Cover ALN-TTR02 or ALN-TTRsc, which Third Party License Payments become payable based on the licensing or sublicensing to, or exercise by, Genzyme or its Related Parties of rights thereunder with respect to Licensed Products.
7.4.3.2. Future Alnylam In-Licenses Related to New Products. Subject to Section 7.4.4 (Amendments to In-Licenses), 7.5.4 (Improvement Products - Royalty Adjustments) and 8.3.5 (Royalty Floor), with respect to Future Alnylam In-Licenses accepted by Genzyme pursuant to Sections 7.4.2 (New In-Licenses; Additional Alnylam In-Licenses) that do not include licenses under Patent Rights that Cover ALN-TTR02 or ALN-TTRsc, (x) Alnylam shall bear one hundred percent (100%) of any Third Party License Payments under such Future Alnylam In-Licenses other than milestone payments and (y) milestone payments under Future Alnylam In-Licenses that do not include licenses under Patent Rights that Cover ALN-TTR02 or ALN-TTRsc shall be allocated between Genzyme and Alnylam as follows:
(a) Base Rule – Alnylam Pays 100%. Except as allocated to Genzyme in this Section 7.4.3.2 below, Alnylam shall bear one hundred percent (100%) of such milestone payments.
(b) [**]% of JPAC/LP-Specific Milestones. Genzyme shall, subject to its right to apply credits against royalties as set forth in Section 7.4.3.2(e), pay [**] percent ([**]%) of milestone payments that become payable based on (i) [**] for a Licensed Product in the Genzyme Territory, (ii) [**] of a Licensed Product in the Genzyme Territory or (iii) [**] of a Licensed Product in the Genzyme Territory (any such milestone ((i) through (iii)) a “JPAC/LP Milestone”), but only if such JPAC/LP Milestone could only have been triggered by a Licensed Product in the Genzyme Territory (such a JPAC/LP Milestone, a “JPAC/LP-Specific Milestone”). For clarity, a JPAC/LP Milestone is a JPAC/LP-Specific Milestone (and allocated
Confidential
- 37 -
one hundred percent (100%) to Genzyme) only if the JPAC/LP Milestone could not have been triggered, in whole or in part, (A) by a product other than a Licensed Product (e.g., in the case of a milestone for [**] in Japan, if such milestone was first achieved by a Licensed Product, but could have been achieved by a product other than a Licensed Product in Japan, or, in the case of a sales milestone, if sales in the Genzyme Territory of products other than Licensed Products could have contributed to the achievement of such milestone), or (B) by the occurrence of an event outside the Genzyme Territory (e.g., in the case of a milestone for [**] Japan and countries outside the Genzyme Territory, if [**] Japan, or, in the case of a sales milestone, if worldwide sales could have contributed to the achievement of such milestone).
(c) [**] Non-Specific JPAC/LP Milestones. With respect to JPAC/LP Milestones that are not JPAC/LP-Specific Milestones (and therefore are not [**] percent ([**]%) payable by Genzyme under subsection 7.4.3.2(b) above), the [**] of such JPAC/LP Milestone payments between the Parties, and Genzyme shall, subject to crediting against royalties as set forth in Section 7.4.3.2(e), pay the portion of such milestone payments allocated by the JSC to Genzyme. In the event that [**] or if the Parties cannot agree as to whether a JPAC/LP Milestone payment is or is not a JPAC/LP-Specific Milestone following escalation pursuant to Section 5.5, the Parties shall submit the matter to “baseball” style arbitration in accordance with the arbitration procedure set forth in Schedule 7.4.3.2.
(d) Ceiling on Genzyme Share of JPAC/LP Milestones. Notwithstanding clauses (b) and (c) of this Section 7.4.3.2, if the amount of any JPAC/LP Milestone payment payable under a Future Alnylam In-License exceeds the amount payable for the achievement of a comparable milestone in either the United States or the EU under such Future Alnylam In-License, then the amount of such JPAC/LP Milestone payment that is allocable to [**] pursuant to this Section 7.4.3.2(b) and 7.4.3.2(c) shall be deemed to be the lowest amount that would be payable under such Future Alnylam In-License for achievement of the comparable milestone in either the United States or the EU.
(e) Genzyme Credit for JPAC/LP Milestones. Subject to Sections 7.5.4 (Improvement Products - Royalty Adjustments) and 8.3.5 (Royalty Floor), Genzyme shall be entitled to credit against the royalties due to Alnylam on Net Sales of Licensed Products to which the relevant Alnylam In-License applies the amount [**] of any JPAC/LP Milestone paid by Genzyme pursuant to this Section 7.4.3.2, and Genzyme shall have the right to carry forward unused portions of such credit amounts for application against royalties payable to Alnylam with respect to Net Sales of such Licensed Products to future royalty periods until such credit amounts have been fully applied against such royalty obligations.
7.4.3.3. Genzyme In-Licenses. Genzyme shall bear one hundred percent (100%) of any Third Party License Payments under the Genzyme In-Licenses that become payable based on the licensing or sublicensing to or exercise by Genzyme or its Related Parties of rights thereunder with respect to Licensed Products in or for the Genzyme Territory. If Alnylam accepts such Genzyme In-License pursuant to Section 7.4.2.2, then Alnylam will pay for one hundred percent (100%) of any portion of Third Party License Payments that become payable based on the licensing or sublicensing to or exercise by Alnylam of rights thereunder in the Alnylam Territory.
Confidential
- 38 -
7.4.4 Amendments to In-Licenses. If a Party amends an In-License to which it is a Party in a manner that increases the royalty payable by the other Party under such In-License without the prior written consent of the other Party, such Party shall be solely responsible for any such additional payments due under such In-License.
7.4.5 Stand-by Licenses. Upon Genzyme’s reasonable request during the Term, Alnylam shall reasonably cooperate in good faith with Genzyme’s efforts to obtain stand-by license agreements with Alnylam In-License licensors pursuant to which, upon a termination of such Alnylam In-License, Genzyme would receive a direct license from such licensor of the Patent Rights and Know-How licensed to Alnylam pursuant to such Alnylam In-License that are sublicensed to Genzyme pursuant to this Agreement. Such stand-by agreement will be in a form approved in advance by Alnylam. Any costs incurred by Alnylam in cooperating with Genzyme’s efforts to obtain any such stand-by license agreement shall be reimbursed by Genzyme.
7.4.6 Breach or Termination of In-Licenses. In the event that (a) a Party receives notice of an alleged breach by such Party under an In-License Agreement to which it is a party, or (b) a Party intends to terminate an In-Licensed Agreement to which it is a party, then such Party will promptly, but in no event less than [**] days thereafter, provide written notice thereof to the other Party.
7.5 Improvement Products Option.
7.5.1 Option Grant. During the Term, Alnylam will provide the JSC with timely updates regarding Alnylam’s development plans and progress developing, or having developed, any Improvement Product. So long as the Exclusivity Period in Japan continues, Genzyme shall have the option to designate any particular Improvement Product as a Licensed Product under this Agreement (the “Option”) as provided in this Section 7.5. Following the completion of the [**] of an Improvement Product and prior to the commencement of the [**] of such Improvement Product, Alnylam shall provide Genzyme with a written notice to such effect accompanied by copies of the development plan for development through Regulatory Approval for such Improvement Product in the Alnylam Territory (the “Improvement Product Development Plan”) and development budget for such Improvement Product for the Alnylam Territory (the “Improvement Product Development Budget”), a statement detailing all Improvement Product Development Costs incurred by Alnylam with respect to the Development of such Improvement Product from the date of commencement of [**] for such Improvement Product to the present date, and the [**], including a copy of the [**], for such Improvement Product (such notice and accompanying information, the “Option Exercise Package”). At any time prior to the completion of the [**] of an Improvement Product but after the [**] for such Improvement Product, Genzyme may request that Alnylam provide reasonable information, of the type to be included in the Option Exercise Package, regarding the Development of an Improvement Product. Alnylam will use good faith efforts to provide such information, and Alnylam will thereafter permit Genzyme to exercise its Option with respect to such Licensed Product prior to the Option Exercise Period; provided, however, that, if Genzyme exercises the Option prior to the Option Exercise Period, the cost sharing provisions of Section 7.5.3 shall apply commencing upon Genzyme’s early exercise of the Option, but the budgeting process in
Confidential
- 39 -
Section 7.5.3 shall not apply during the period commencing with Genzyme’s early exercise of the Option and ending on the [**]. Such request will not relieve Alnylam of its obligation to provide the Option Exercise Package as set forth above if Genzyme does not exercise the Option early with respect to such Improvement Product. Genzyme may exercise the Option by providing written notice of exercise to Alnylam at any time following Genzyme’s receipt of the Option Exercise Package until [**] days following Genzyme’s receipt of the Option Exercise Package (the “Option Exercise Period”). During such Option Exercise Period, Alnylam will promptly provide any patent, regulatory, or other information that Genzyme may reasonably request regarding such Improvement Product to assist Genzyme in deciding whether to exercise the Option for such Improvement Product.
7.5.2 Option Not Exercised. If Genzyme does not exercise the Option as to an Improvement Product during the Option Exercise Period applicable to such Improvement Product, Genzyme’s Option with respect to such Improvement Product shall expire, Genzyme’s rights under this Section 7.5 shall lapse and Alnylam shall thereafter be free to develop and/or commercialize, alone or with one or more Third Parties, such Improvement Product without further obligation to Genzyme with respect to such Improvement Product; provided that, the restrictions set forth in Section 10.5.1 as to such Improvement Products, if applicable, shall continue to apply in accordance with their terms.
7.5.3 Option Exercised. If Genzyme exercises the Option as to an Improvement Product during the Option Exercise Period applicable to such Improvement Product, such Improvement Product shall become a Licensed Product hereunder and the following shall apply:
7.5.3.1. Genzyme shall, within [**] days after Alnylam’s invoice therefor, pay Alnylam [**] percent ([**]%) of Alnylam’s worldwide Development costs and expenses (including Alnylam’s Out-of-Pocket Costs and FTE Costs) incurred by Alnylam with respect to such Improvement Product (“Improvement Product Development Costs”) from the date of commencement of [**] for such Improvement Product to the present date of Genzyme’s exercise of the Option.
7.5.3.2. Additionally, following Genzyme’s exercise of the Option, the Development of such Improvement Product for the Alnylam Territory shall be included in the Alnylam Territory Development Plan and the Development of such Improvement Product for the Genzyme Territory shall be included in the Genzyme Territory Development Plan. Genzyme shall pay [**] percent ([**]%) and Alnylam shall pay [**] percent ([**]%) of the Improvement Product Development Costs incurred by the Parties with respect to Development of the Improvement Product pursuant to the Alnylam Territory Development Plan (which costs shall be reflected in the Improvement Product Development Budget).
7.5.3.3. Genzyme shall pay [**] percent ([**]%) of the Improvement Product Development Costs incurred by the Parties with respect to such Improvement Product for the Genzyme Territory pursuant to the Genzyme Territory Development Plan.
Confidential
- 40 -
7.5.3.4. Alnylam will provide Genzyme with updates to the Improvement Product Development Budget for such Improvement Product (a) prior to the commencement of [**] that Alnylam plans to conduct concurrently for such Improvement Product following the [**] conducted concurrently following Genzyme’s exercise of the Option for such Improvement Product and (b) otherwise with each update to the Alnylam Territory Development Plan that Alnylam provides in accordance with Section 2.2.2. The Improvement Product Development Budget provided to Genzyme prior to its exercise of the Option shall be binding on the Parties with respect to the Improvement Product Development Costs budgeted for the [**] to be conducted concurrently for such Improvement Product following such Option exercise, and each updated Improvement Product Development Budget provided by Alnylam to Genzyme in accordance with clause (a) above for each subsequent [**] to be conducted concurrently for such Improvement Product shall be binding on the Parties with respect to such [**] for such Improvement Product; provided, however that to the extent that a Party (or its Related Parties) incurs Improvement Product Development Costs for any such [**] for which such Party is responsible under the Improvement Product Development Plan that exceed the amounts set forth in the Improvement Product Development Budget for such activities by [**] percent ([**]%) or less, then such overage amount shall automatically be included in the Improvement Product Development Budget and the Parties shall share such costs as set forth in this Section 7.5.3. Following Genzyme’s receipt of each updated Improvement Product Development Budget as provided in clause (a) above, Genzyme shall have the right, exercisable within [**] days after receipt of such Improvement Product Development Budget, to opt-out of the Development of such Improvement Product (other than with respect to then-ongoing [**], for which Genzyme will remain obligated to continue to participate in funding) by providing Alnylam written notice thereof, in which case such Improvement Product shall no longer be a Licensed Product for the purposes of this Agreement.
7.5.4 Improvement Products - Royalty Adjustments. Notwithstanding Section 7.4.3.2 (Future Alnylam In-Licenses Related to New Products), Section 8.3.1 (Royalties Payable on Licensed Products), Section 8.3.3 (No Alnylam Patents or Regulatory Exclusivity), and Section 8.3.4 (Royalty Adjustments for Generic Products) (which Sections 8.3.3 and 8.3.4 will apply in part as expressly provided for below), in no event will the royalties payable by Genzyme to Alnylam on Net Sales of Improvement Products in the Genzyme Territory for any Calendar Quarter during the applicable Royalty Term for such Improvement Product be less than the sum of (a) the amount necessary to fully cover Alnylam’s obligations to pay sales milestones and royalties due under Alnylam In-Licenses with respect to sales of such Improvement Product in the Genzyme Territory during such Calendar Quarter in accordance with Section 7.4.3 and (b) [**] percent ([**]%) of Net Sales of such Improvement Product, subject to adjustment of the component set forth in this clause (b) as provided in Sections 8.3.3 and 8.3.4. Furthermore, in no event will the royalties payable by Genzyme to Alnylam on Net Sales of an Improvement Product in any country in the Genzyme Territory for any Calendar Quarter after the applicable Royalty Term for such Improvement Product, after all reductions to such royalties are applied, be less than the royalty floor established in clause (b) of Section 8.3.5. For the avoidance of doubt, Genzyme will not be entitled to apply any credit pursuant to Section 7.4.3.2(e) against the royalty payment payable by Genzyme to Alnylam on Net Sales of an Improvement Product in
Confidential
- 41 -
the Genzyme Territory for any Calendar Quarter to the extent such credit would reduce the amount of such royalty payment to an amount less than the applicable minimum payment established by the two immediately preceding sentences. Notwithstanding the foregoing, no portion of any sales milestone payment under any Future Alnylam In-License paid by Genzyme pursuant to Section 7.4.3.2 shall be taken into account for the purpose of calculating the royalty floor under this Section 7.5.4 (i.e., no double-counting). The following two examples are provided for illustrative purposes only:
(x) Example #1. In the event that (i) Net Sales of an Improvement Product by made Genzyme or its Related Parties in the Genzyme Territory are less than [**] dollars ($[**]) in the first Calendar Quarter of a Calendar Year and therefore the royalty percentage due under Section 8.3.1 with respect to such Improvement Product would be [**] percent ([**]%), (ii) Alnylam is responsible for paying aggregate royalties equal to [**] percent ([**]%) of Net Sales of such Improvement Product in the Genzyme Territory under Alnylam In-License Agreements, (iii) Genzyme is not entitled to credit any amounts against such royalties pursuant to Section 7.4.3.2(e) (Genzyme Credit for JPAC/LP Milestones), and (iv) no reductions are applicable pursuant to Section 8.3.3 (No Alnylam Patents or Regulatory Exclusivity) or 8.3.4 (Royalty Adjustment for Generic Products), then the royalty due under this Agreement on such Net Sales, as adjusted based on this Section 7.5.4, would be [**] percent ([**]%) plus [**] percent ([**]%), or [**] percent ([**]%) of such Net Sales.
(y) Example #2. In the event that (i) Net Sales of an Improvement Product by made Genzyme or its Related Parties in the Genzyme Territory are less than [**] dollars ($[**]) in the first Calendar Quarter of a Calendar Year and therefore the royalty percentage due under Section 8.3.1 with respect to such Improvement Product would be [**] percent ([**]%), (ii) Alnylam is responsible for paying aggregate royalties equal to [**] percent ([**]%) of Net Sales of such Improvement Product in the Genzyme Territory under Alnylam In-License Agreements, (iii) Genzyme is not entitled to credit any amounts against such royalties pursuant to Section 7.4.3.2(e) (Genzyme Credit for JPAC/LP Milestones), and (iv) a reduction is applicable pursuant to Section 8.3.4 (Royalty Adjustment for Generic Products), then the royalty due under this Agreement on such Net Sales, as adjusted based on this Section 7.5.4 would be [**]% plus [**]% (i.e., [**]%), or [**]% of such Net Sales.
7.5.5 Improvement Products. All other provisions of this Agreement applicable to Licensed Products, including without limitation the royalty provisions in Section 8.3, shall apply to such Improvement Product.
7.6 Alnylam Territory Right of First Negotiation. If, at any time during the Term, Alnylam desires to grant any Third Party rights to Develop and/or Commercialize one or more Licensed Product(s) in the Field in any portion of the Alnylam Territory, Alnylam shall notify Genzyme in writing of its intent. Genzyme shall have [**] days from receipt of such written notice to notify Alnylam in writing as to whether Genzyme desires to negotiate for such rights in such territory, and if Genzyme so notifies Alnylam that it does desire to negotiate for such rights in such territory, Genzyme shall have the exclusive right for [**] days from the date of such notification to Alnylam to negotiate with Alnylam and to make one or more written non-binding offers to Alnylam concerning the acquisition of such rights in such territory by Genzyme.
Confidential
- 42 -
Genzyme shall have the exclusive right for [**] days (or such longer period as may be mutually agreed by the Parties) after such [**] day period, to finalize and enter into a definitive agreement with Alnylam for such rights in such territory; provided that, if either Genzyme does not provide such written notice within such [**] day period or Genzyme does provide such written non-binding offer within such subsequent [**] day period, or Genzyme provides such notice of interest and such written offer but for any reason Genzyme and Alnylam do not enter into a definitive agreement within the [**] day negotiation period, Alnylam shall be free to enter into an agreement with a Third Party(ies) relating to such rights in such territory, without further obligation to Genzyme. Alnylam shall not, during the exclusive [**] and [**] day negotiating periods described above, enter into discussions, exchange information, or otherwise negotiate with any Third Party with respect to an agreement with respect to the Development and/or Commercialization of a Licensed Product in the Field in the Alnylam Territory. Notwithstanding the foregoing, during the period of [**] months after the termination of any such negotiation that does not result in a definitive agreement between Alnylam and Genzyme, Alnylam shall not enter into a transaction with respect to such rights in such territory with any Third Party on terms that in the aggregate are materially more favorable to the Third Party than the last terms offered in writing by Genzyme to Alnylam unless Alnylam first re-offers such transaction to Genzyme on such more favorable terms and Genzyme does not accept such offer and enter into such transaction with Alnylam within [**] days after such re-offer. For clarity, prior to the exclusive negotiating periods described above, Alnylam shall be free to engage in discussions and exchange information with Third Parties with respect to such Licensed Product rights, but shall not enter into any binding agreement with any Third Party with respect to such rights.
7.7 Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by a Party to the other, including those set forth in Sections 7.1, 7.2, and 3.3, are and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the Parties and their respective Sublicensees, as sublicensees of such rights under this Agreement, shall retain and may fully exercise all of their rights and elections under the U.S. Bankruptcy Code and any foreign counterpart thereto. The Parties further agree that that upon commencement of a bankruptcy proceeding by or against a Party (the “Bankrupt Party”) under the Bankruptcy Code, the other Party (the “Non-Bankrupt Party”) will be entitled to a complete duplicate of, or complete access to (as the Non-Bankrupt Party deems appropriate), all such intellectual property and all embodiments of such intellectual property. Such intellectual property and all embodiments of such intellectual property will be promptly delivered to the Non-Bankrupt Party (a) upon any such commencement of a bankruptcy proceeding and upon written request by the Non-Bankrupt Party, unless the Bankrupt Party elects to continue to perform all of its obligations under this Agreement, or (b) if not delivered under (a) above, upon the rejection of this Agreement by or on behalf of the Bankrupt Party and upon written request by the Non-Bankrupt Party. Without limiting the foregoing, Alnylam hereby grants to Genzyme a right of access to and to obtain possession of (a) copies of research data, (b) laboratory samples, (c) samples of Licensed Product, (d) formulas, (e) laboratory notes and notebooks, (f) data and results related to clinical trials, (g) regulatory filings and approvals, (h) rights of reference in respect of regulatory filings and approvals, (i) pre-clinical research data and results, (j) marketing, advertising and promotional materials, all of which (in clauses (a) through (j)) constitute “embodiments” of intellectual property pursuant to Section 365(n) of the Bankruptcy
Confidential
- 43 -
Code and (k) all other embodiments of such intellectual property, and in respect of each of the foregoing clauses (a) through (k), solely for the purpose of the exercise of Genzyme’s rights and licenses under this Agreement, whether any of the foregoing are in Alnylam’s possession or control or in the possession and control of Third Parties. The Bankrupt Party (in any capacity, including debtor-in-possession) and its successors and assigns (including any trustee) agrees not to interfere with the exercise by Non-Bankrupt Party or its Related Parties of its rights and licenses to such intellectual property and such embodiments of intellectual property in accordance with this Agreement, and agrees to assist the Non-Bankrupt Party and its Related Parties in obtaining such intellectual property and such embodiments of intellectual property in the possession or control of Third Parties as reasonably necessary or desirable for the Non-Bankrupt Party to exercise such rights and licenses in accordance with this Agreement. The foregoing provisions are without prejudice to any rights the Non-Bankrupt Party may have arising under the Bankruptcy Code or other Laws.
7.8 [**].
7.9 No Other Rights. Except as otherwise expressly provided in this Agreement, under no circumstances shall a Party, as a result of this Agreement, obtain any ownership interest or other right in any Know-How, Patent Rights or other intellectual property rights of the other Party, including items owned, controlled or developed by the other Party, or provided by the other Party to the receiving Party at any time pursuant to this Agreement.
8. CERTAIN FINANCIAL TERMS
8.1 Upfront Fee. In consideration for the rights, licenses and options granted by Alnylam to Genzyme under this Agreement, within [**] days after the Effective Date, Genzyme shall pay Alnylam a non-creditable initial payment of Twenty-Two Million Five Hundred Thousand U.S. Dollars ($22,500,000).
8.2 Development Milestone Fees. Genzyme shall make the non-creditable milestone payments to Alnylam set forth below, each payable once, no later than [**] days after either the earliest date on which the corresponding milestone event has first been achieved with respect to a Licensed Product, with respect to milestone events achieved by Genzyme or its Related Parties, or the date on which Genzyme receives notice from Alnylam of completion of the milestone event, with respect to milestone events achieved by Alnylam or its Related Parties.
| Milestone Event |
Milestone Payment | |||
| (i) First Phase II Success by Alnylam or any of its Related Parties |
$ | 7,000,000 | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
Confidential
- 44 -
(a) If following the completion of a Phase II Study the Parties either (I) do not agree whether First Phase II Success has been achieved or (II) agree that the First Phase II Success has not been achieved, then, in each case of either (I) or (II):
| (i) | Genzyme shall have the right to terminate this Agreement by providing forty-five (45) days prior written notice to Alnylam. Genzyme shall have no obligation to pay to Alnylam the milestone payment for the milestone event described in Section 8.2(i) if Genzyme so terminates prior to the later of (1) Alnylam’s or any of its Related Parties’ commencement of the first [**] with respect to a Licensed Product, or (2) forty-five (45) days following the date on which Alnylam delivers written notice pursuant to Section 2.3.1 to Genzyme of its intent to commence such a [**] with respect to a Licensed Product. |
| (ii) | If Genzyme does not terminate this Agreement in accordance with Section 8.2(a)(i) above prior to the later of (1) [**] with respect to the Licensed Product conducted by Alnylam or any of its Related Parties, or (2) forty-five (45) days following the date on which Alnylam delivers written notice pursuant to Section 2.3.1 to Genzyme of its intent to commence such a [**] with respect to a Licensed Product, then the milestone payment for milestone event described in Section 8.2(i) shall be paid by Genzyme to Alnylam as follows: (x) [**] U.S. Dollars ($[**]) shall be paid together with the milestone payment described in Section 8.2(ii) and (y) [**] U.S. Dollars ($[**]) shall be paid upon the earliest of (A) the first filing by Alnylam or any of its Related Parties of [**] in (I) the United States, (II) in the EU (with the EMA), or (III) in any one of the following countries: France, Germany, Portugal, Sweden and the United Kingdom, or (B) the first filing by Genzyme or any of its Related Parties of [**] in a country in the Genzyme Territory or the [**] for the Licensed Product in Japan. For clarity, payment under (x) and (y) above is in addition to payments due under 8.2(ii) and 8.2(iii). |
(b) If Alnylam or any of its Related Parties [**], but (i) Genzyme or any of its Related Parties [**] as contemplated by the Genzyme Territory Development Plan, and, (ii) Alnylam or any of its Related Parties [**], then the milestone payment for the milestone event described in Section 8.2(iv) shall be paid by Genzyme to Alnylam within [**] days following the events described in both clause (i) and (ii) of this paragraph being satisfied.
Confidential
- 45 -
(c) Each milestone payment by Genzyme to Alnylam hereunder shall be payable only once, regardless of the number of times achieved with respect to a Licensed Product.
(d) Genzyme shall provide Alnylam with written notice of the achievement by Genzyme or any of its Related Parties of any milestone event set forth in Section 8.2(iii), (v) and (vi) and Alnylam shall provide Genzyme with written notice of the achievement by Alnylam or any of its Related Parties of any milestone event set forth in Section 8.2(i), (ii) and (iv), in each case within [**] days after such event; provided, however, that the notifying Party shall inform the other Party of such event prior to any public disclosure of such event by the notifying Party.
8.3 Royalties.
8.3.1 Royalties Payable on Licensed Products. Subject to the terms and conditions of this Agreement, Genzyme shall pay to Alnylam royalties on aggregate Net Sales by Genzyme and its Related Parties of all Licensed Products in the Genzyme Territory, as follows:
| Aggregate Calendar Year Net Sales in the Genzyme Territory |
Royalty (as a percentage of Net Sales) |
|||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| Greater than $[**] |
[** | ] | ||
By way of example only, if Genzyme receives [**] U.S. Dollars ($[**]) in Net Sales in the Genzyme Territory during a given Calendar Year, then the royalties payable by Genzyme under Section 8.3.1 during such Calendar Year would be calculated as follows:
[**]
Royalties on aggregate Net Sales shall be paid at the rate applicable to the portion of such aggregate Net Sales within each of the Net Sales levels above during such Calendar Year.
If (i) more than one Licensed Product is sold in the Genzyme Territory during any Calendar Quarter, (ii) all portions of aggregate Net Sales during such Calendar Quarter fall within the same Net Sales level during such Calendar Quarter, (iii) such Licensed Products are subject to different royalty rates pursuant to Section 8.3.3 or 8.3.4, and (iv) the aggregate Net Sales of such Licensed Products during the Calendar Year fall within different Net Sales levels, then the Net Sales of each Licensed Product shall be deemed to be distributed proportionally on a percentage basis relative to the Net Sales of each Licensed Product in the Calendar Year, according to such differing royalty rates. For example, if:
| • | [**] U.S. Dollars ($[**]) of aggregate Net Sales occur for two Licensed Products during the first Calendar Quarter of a Calendar Year during which aggregate Net Sales total [**] U.S. Dollars ($[**]), and |
Confidential
- 46 -
| • | [**] percent ([**]%) of aggregate Net Sales during such Calendar Year are attributed to the first Licensed Product and [**] percent ([**]%) to the second Licensed Product, and |
| • | the first Licensed Product is subject to a full royalty and the second is subject to a [**] percent ([**]%) royalty pursuant to Section 8.3.4, |
then royalties during such Calendar Quarter will be calculated as follows:
[**]
If (i) more than one Licensed Product is sold in the Genzyme Territory during any Calendar Quarter, (ii) portions of aggregate Net Sales during such Calendar Quarter fall within more than one of the above Net Sales levels, (iii) such Licensed Products are subject to different royalty rates pursuant to Section 8.3.3 or 8.3.4, and (iv) the aggregate Net Sales of such Licensed Products during the Calendar Year fall within different Net Sales levels, then the Net Sales of each Licensed Product shall be deemed to be distributed proportionally on a percentage basis relative to the Net Sales of each Licensed Product in the Calendar Year, according to such differing royalty rates and between the applicable Net Sales levels. For example, if:
| • | [**] U.S. Dollars ($[**]) of aggregate Net Sales occur for two Licensed Products during the fourth Calendar Quarter of a Calendar Year during which aggregate Net Sales total [**] U.S. Dollars ($[**]), |
| • | [**] percent ([**]%) of aggregate Net Sales during such Calendar Year are attributed to the first Licensed Product and [**] percent ([**]%) to the second Licensed Product, |
| • | [**] U.S. Dollars ($[**]) of aggregate Net Sales during such Calendar Quarter fall into the $[**] Net Sales level and [**] U.S. Dollars ($[**]) fall into the $[**] Net Sales level, and |
| • | and the first Licensed Product is subject to a full royalty and the second is subject to a [**] percent ([**]%) royalty pursuant to Section 8.3.4, |
then royalties during such Calendar Quarter will be calculated as follows:
[**]
8.3.2 Royalty Term. Subject to Section 8.3.5, the period during which the royalties set forth in Section 8.3.1 shall be payable, on a Licensed Product-by-Licensed Product and country-by-country basis, shall commence with the First Commercial Sale of the Licensed
Confidential
- 47 -
Product in such country and continue until the latest of (a) the expiration of the last Valid Claim of the Alnylam Patents or any Patent Right included in the Joint Collaboration IP Covering the Manufacture, use, offer for sale, sale or importation of the Licensed Product in the country of sale, (b) the expiration of Regulatory Exclusivity for such Licensed Product in such country, or (c) subject to Section 8.3.3, the twenty-fifth (25th) anniversary of the First Commercial Sale of the Licensed Product in such country (each such period, a “Royalty Term”). For purposes of the foregoing clause (a), following the twelfth (12) anniversary of the First Commercial Sale of a Licensed Product in a country, Manufacturing Claims shall no longer constitute Valid Claims with respect to such Licensed Product in such country. Notwithstanding the foregoing, at any time during the Royalty Term for a Licensed Product in a country following the latest of (a) the expiration of the last Valid Claim of the Alnylam Patents or any Patent Right included in the Joint Collaboration IP Covering the Manufacture, use, offer for sale, sale or importation of such Licensed Product in such country, (b) the expiration of Regulatory Exclusivity for such Licensed Product in such country, or (c) the twelfth (12th) anniversary of the First Commercial Sale of such Licensed Product in such country, Genzyme may terminate the Royalty Term with respect to such Licensed Product in such country (and its obligation to pay royalties under this Section 8.3 with respect to such Licensed Product in such country) by providing written notice to Alnylam, which termination will be effective commencing in the next full Calendar Quarter commencing at least thirty (30) days following the date of such notice.
8.3.3 No Alnylam Patents or Regulatory Exclusivity. The royalties to be paid by Genzyme to Alnylam pursuant to Section 8.3.1 shall be reduced to [**] percent ([**]%) of the amounts otherwise payable pursuant to Section 8.3.1 with respect to Net Sales in a country of the Genzyme Territory of Licensed Products as to which both (i) the Manufacture, use, offer for sale, sale or importation of which is not Covered by any Valid Claim in any Alnylam Patent or in any Patent Right included in the Joint Collaboration IP in such country and (ii) there is no applicable Regulatory Exclusivity in such country.
8.3.4 Royalty Adjustments for Generic Products. If, during a given Calendar Quarter when a Licensed Product is being Commercialized by or on behalf of Genzyme in a particular country in the Genzyme Territory, there is Generic Competition in such country with respect to a Licensed Product, then, subject to Section 8.3.5, the royalties payable pursuant to Section 8.3.1 on the Net Sales of such Licensed Product in such country shall thereafter be reduced to [**] percent ([**]%) of the amounts otherwise payable pursuant to Section 8.3.1 with respect to such Licensed Product in such country for such Calendar Quarter for so long as such Generic Competition remains.
8.3.5 Royalty Floor. Notwithstanding Section 7.4.3 or the foregoing provisions of this Section 8.3 (a) in no event during the applicable Royalty Term for a Licensed Product in a country of the Genzyme Territory shall the royalties payable to Alnylam hereunder for such Licensed Product in such country for any Calendar Quarter be reduced pursuant to Sections 8.3.3 and 8.3.4 to less than [**] percent ([**]%) of the royalties payable pursuant to Section 8.3.1 as to such Licensed Product in such country for such Calendar Quarter and (b) in no event shall the royalties payable to Alnylam hereunder for a Licensed Product in a country of the Genzyme Territory for any Calendar Quarter, whether or not reduced pursuant to Section 8.3.3 or 8.3.4, and whether or not during or after the applicable Royalty Term for such Licensed Product in
Confidential
- 48 -
such country, be less than the amount necessary to fully cover Alnylam’s obligations to pay royalties and sales milestones due under Alnylam In-Licenses with respect to sales of such Licensed Product in such country during such Calendar Quarter in accordance with Section 7.4.3. If the royalties payable to Alnylam hereunder for a Licensed Product in a country of the Genzyme Territory for any Calendar Quarter would otherwise not be sufficient to fully cover Alnylam’s obligations to pay royalties and sales milestones due under Alnylam In-Licenses with respect to sales of such Licensed Product in such country during such Calendar Quarter in accordance with Section 7.4.3, Genzyme shall pay Alnylam such additional royalty amounts with respect to sales of such Licensed Product in such country for such Calendar Quarter as are necessary to fully cover Alnylam’s obligations to pay such royalties and sales milestones and Genzyme shall not be entitled to credit any amount pursuant to Section 7.4.3 against royalties payable to Alnylam on sales of such Licensed Product in such country during such Calendar Quarter. If the royalties payable to Alnylam hereunder for a Licensed Product in a country of the Genzyme Territory for any Calendar Quarter exceed the amount necessary to fully cover Alnylam’s obligations to pay royalties and sales milestones with respect to sales of such Licensed Product in such country during such Calendar Quarter in accordance with Section 7.4.3, Genzyme shall be entitled to credit amounts pursuant to Section 7.4.3 with respect to sales of such Licensed Product in such country during such Calendar Quarter against the portion of such royalties payable to Alnylam that exceeds Alnylam’s obligations to pay royalties and sales milestones due under Alnylam In-Licenses with respect to sales of such Licensed Product in such country during such Calendar Quarter. Notwithstanding the foregoing, no portion of any sales milestone that is a JPAC/LP Milestone payment under any Future Alnylam In-License paid by Genzyme pursuant to Section 7.4.3.2 shall be taken into account for the purpose of calculating the royalty floor under this Section 8.3.5 (i.e., no double-counting).
8.3.6 Validation Information. At Genzyme’s request, Alnylam will provide Genzyme with such information as Genzyme may reasonably request to validate the amount of the royalty floor described in Section 8.3.5.
8.3.7 Reports; Payment of Royalty. During the Term, following the First Commercial Sale of the Licensed Product in the Genzyme Territory, Genzyme shall furnish to Alnylam a written report within [**] days after the end of each Calendar Quarter showing, on a Licensed Product-by-Licensed Product and country-by-country basis, the Net Sales of each Licensed Product in each country of the Genzyme Territory, royalties payable under Genzyme In-Licenses with respect to such Net Sales and the royalties payable under this Agreement. Quarterly reports shall be due no later than the [**] day following the end of each Calendar Quarter. Royalties shown to have accrued by each royalty report shall be due and payable on the date such royalty report is due. In addition Genzyme shall prepare and deliver to Alnylam (a) any additional reports as required under the Alnylam In-Licenses and requested by Alnylam; and (b) an annual report no later than [**] of each Calendar Year listing gross sales and Net Sales of Licensed Products in each Calendar Quarter in the prior Calendar Year (A) in Japan and (B) in all other countries in the Genzyme Territory in the aggregate. Upon Alnylam’s reasonable request no more than [**], Genzyme shall prepare a report detailing the deductions from gross sales (itemized by deduction category) included in the calculation of Net Sales for each Licensed Product in Japan and the Genzyme Territory as a whole. Genzyme and its Related Parties involved in Commercializing Licensed Products shall keep complete and accurate records in sufficient detail to enable the royalties and other payments payable hereunder and by Alnylam to Third Parties under the Alnylam In-Licenses to be determined.
Confidential
- 49 -
8.4 Audits.
8.4.1 Upon the written request of a Party and not more than [**], the other Party and its Related Parties shall permit an independent certified public accounting firm of internationally-recognized standing selected by the requesting Party and reasonably acceptable to the other Party, at the requesting Party’s expense except as set forth below, to have access during normal business hours to such of the records of the other Party as may be reasonably necessary to verify the accuracy of the royalty, Improvement Product Development Cost, and other amounts payable or reports under this Agreement for any year ending not more than [**] years prior to the date of such request for the sole purpose of verifying the basis and accuracy of payments made under Sections 6, 7.5 and 8.
8.4.2 If such accounting firm identifies a discrepancy made during such period, the appropriate Party shall pay the other Party the amount of the discrepancy, together with late-payment interest in accordance with Section 8.6, within [**] days after the date the requesting Party delivers to the other Party such accounting firm’s written report so concluding, or as otherwise agreed by the Parties in writing. The fees charged by such accounting firm shall be paid by the requesting Party, unless such discrepancy represents an underpayment by the other Party of at least five percent (5%) of the total amounts due hereunder in the audited period, in which case such fees shall be paid by the other Party.
8.4.3 Each Party shall comply with all applicable audit requirements in the In-Licenses and shall include in each sublicense granted by it pursuant to this Agreement a provision requiring the Sublicensee to make reports to the Party that is party to such In-License, to keep and maintain records of sales made pursuant to such sublicense and to grant access to such records by the independent accountant of the Party that is party to such In-License to the same extent required of a Party under this Agreement.
8.4.4 Unless an audit for such year has been commenced prior to and is ongoing upon the [**] anniversary of the end of such year, the calculation of royalties, expense reimbursement and other payments payable with respect to such year shall be binding and conclusive upon both Parties, and each Party and its Related Parties shall be released from any further liability or accountability with respect to such royalties or expense reimbursement for such year.
8.4.5 Each Party shall treat all financial information subject to review under this Section 8.4 or under any sublicense agreement in accordance with the confidentiality and non-use provisions of this Agreement, and shall cause its accounting firm to enter into a confidentiality agreement with the other Party and/or its Related Parties obligating it to retain all such information in confidence pursuant to such confidentiality agreement, which terms shall be no less stringent than the provisions of Section 9.
8.5 Payment Exchange Rate. All payments to be made under this Agreement shall be made in United States dollars and shall be paid by bank wire transfer in immediately available funds to such bank account in the United States as may be designated in writing by the receiving
Confidential
- 50 -
Party from time to time. In the case of Net Sales made or expenses incurred by a Party and its Related Parties in currencies other than United States dollars during a Calendar Quarter, the rate of exchange to be used in computing the amount of United States dollars due shall be the rate of exchange utilized by such Party in its worldwide accounting system and calculated in accordance with GAAP.
8.6 Late Payments. Any amount owed by a Party to the other Party under this Agreement that is not paid on or before the date such payment is due shall bear interest at a rate per annum equal to the lesser of (a) the then current one (1) month London Inter-Bank Offering Rate for US Dollars, as quoted on the British Banker’s Association’s website currently located at ▇▇▇.▇▇▇.▇▇▇.▇▇ (or such other source as may be mutually agreed by the Parties) plus [**] percentage points per annum or (b) the highest rate permitted by Law, calculated on the number of days such payments are paid after such payments are due and compounded monthly.
8.7 Blocked Payments. If, by reason of Laws in any jurisdiction in a Party’s Territory, it becomes impossible or illegal for a Party to transfer milestone payments, royalties or other payments under this Agreement to the other Party, the payor shall promptly notify the payee. During any such period described above, the payor shall deposit such payments in local currency in the relevant jurisdiction to the credit of the payee in a recognized banking institution designated by the payee or, if none is designated by the payee within a period of [**] days, in a recognized banking institution selected by the payor and identified in a written notice given to the payee.
8.8 Taxes. If a timely and appropriately completed and executed Internal Revenue Service Form W-9 is provided by the receiving Party to the paying Party, the Parties acknowledge and agree that no United States tax withholding shall be applied with respect to the payments due under Sections 8.1 and 8.2. Each Party shall use reasonable efforts to minimize tax withholding on payments made to the other Party. Notwithstanding such efforts, if such Party concludes that tax withholdings under the Laws of any country are required with respect to payments to the other Party, such Party shall first notify the other Party and provide such Party with [**] days to determine whether there are actions such receiving Party can undertake to avoid such withholding. During this notice period, the paying Party shall refrain from making such payment until the receiving Party instructs the paying Party that (a) the receiving Party intends to take actions (satisfactory to both Parties) that will obviate the need for such withholding, in which case the paying Party shall make such payment only after it is instructed to do so by the receiving Party, or (b) the paying Party should make such payment and withhold the required amount and pay it to the appropriate governmental authority. In such case, the withholding Party shall promptly provide the other Party with copies of receipts or other evidence reasonably required and sufficient to allow the other Party to document such tax withholdings adequately for purposes of claiming foreign tax credits and similar benefits. The Parties will cooperate reasonably in completing and filing documents required under the provisions of any applicable tax laws or under any other applicable law, in connection with the making of any required tax payment or withholding payment, or in connection with any claim to a refund of or credit for any such payment. The Parties will cooperate to minimize such taxes in accordance with applicable Laws, including using reasonable efforts to access the benefits of any applicable treaties. Notwithstanding the foregoing, if, as a result of (i) the assignment of this
Confidential
- 51 -
Agreement by Genzyme to an Affiliate or a Third Party outside of the United States or (ii) the exercise by Genzyme of its rights under this Agreement through an Affiliate or Third Party outside of the United States (or the direct exercise of such rights by an Affiliate of Genzyme outside of the United States), foreign withholding tax in excess of the foreign withholding tax amount that would have been payable in the absence of such assignment or exercise of rights becomes payable with respect to amounts due to Alnylam hereunder, such amount due to Alnylam will be increased so that the amount actually paid to Alnylam equals the amount that would have been payable to Alnylam in the absence of such excess withholding (after withholding of the excess withholding tax and any additional withholding tax on such increased amount). However, if a similar assignment or exercise of rights described in (i) or (ii) of the preceding sentence by Alnylam results in foreign withholding tax in excess of the foreign withholding tax amount that would have been payable in the absence of such assignment or exercise of rights, any amount due to Alnylam will not be increased for such excess withholding and, subject to the terms of this Agreement, the required amount will be withheld and submitted to the appropriate governmental authority.
9. CONFIDENTIALITY AND PUBLICATION
9.1 Nondisclosure Obligation. (a) All Confidential Information disclosed by one Party to the other Party hereunder shall be maintained in confidence by the receiving Party and shall not be disclosed to a Third Party or used for any purpose except as set forth herein without the prior written consent of the disclosing Party, except to the extent that such Confidential Information:
| (i) | is known by the receiving Party at the time of its receipt, and not through a prior disclosure by the disclosing Party, as documented by the receiving Party’s business records; |
| (ii) | is known to the public before its receipt from the disclosing Party, or thereafter becomes generally known to the public through no breach of this Agreement by the receiving Party; |
| (iii) | is subsequently disclosed to the receiving Party by a Third Party who is not known by the receiving Party to be under an obligation of confidentiality to the disclosing Party; or |
| (iv) | is developed by the receiving Party independently of Confidential Information received from the disclosing Party, as documented by the receiving Party’s business records. |
(b) Notwithstanding the obligations of confidentiality and non-use set forth above and in Section 9.1(c) below, a receiving Party may provide Confidential Information disclosed to it, and disclose the existence and terms of this Agreement as may be reasonably required in order to perform its obligations and to exploit its rights under this Agreement, and specifically to (i) Related Parties, and their employees, directors, agents, consultants, advisors and/or other Third Parties for the performance of its obligations hereunder (or for such entities to determine their interest in performing such activities) in accordance with this Agreement in each case who are
Confidential
- 52 -
under an obligation of confidentiality with respect to such information that is no less stringent than the terms of this Section 9.1(b); (ii) governmental or other Regulatory Authorities in order to obtain patents or perform its obligations or exploit its rights under this Agreement; provided that such Confidential Information shall be disclosed only to the extent reasonably necessary to do so, (iii) the extent required by Law, including by the rules or regulations of the United States Securities and Exchange Commission or similar regulatory agency in a country other than the United States or of any stock exchange or listing entity, (iv) any bona fide actual or prospective acquirers, underwriters, investors, lenders or other financing sources and any bona fide actual or prospective collaborators or strategic partners and to consultants and advisors of such Party, in each case who are under an obligation or confidentiality with respect to such information that is no less stringent than the terms of this Section 9.1(b) and (v) to Third Parties to the extent a Party is required to do so pursuant to the terms of an Existing Alnylam In-License. If a Party is required by Law to disclose Confidential Information that is subject to the non-disclosure provisions of this Section 9.1(b) or Section 9.1(c), such Party shall promptly inform the other Party of the disclosure that is being sought in order to provide the other Party an opportunity to challenge or limit the disclosure obligations. Confidential Information that is required to be disclosed by Law shall remain otherwise subject to the confidentiality and non-use provisions of this Section 9.1(b) and Section 9.1(c). If either Party concludes that a copy of this Agreement must be filed with the United States Securities and Exchange Commission or similar regulatory agency in a country other than the United States, such Party will provide the other Party with a copy of this Agreement showing any provisions hereof as to which the Party proposes to request confidential treatment, will provide the other Party with an opportunity to comment on any such proposed redactions and to suggest additional redactions, and will take such Party’s reasonable comments into consideration before filing the Agreement.
(c) Alnylam recognizes the value of the license granted in this Agreement depends, in part, on Alnylam protecting the secrecy of the Alnylam Know-How. Therefore, without limiting Alnylam’s right to license the Alnylam Know-How in any way it chooses, Alnylam shall use Commercially Reasonable Efforts to protect the confidentiality of the Alnylam Know-How as determined by Alnylam in its reasonable business judgment.
9.2 Publication and Publicity.
9.2.1 Publication. Genzyme and Alnylam each acknowledge the other Party’s interest in publishing certain key results of the Collaboration. Each Party also recognizes the mutual interest in obtaining valid patent protection and in protecting trade secret information. Consequently, except for disclosures permitted pursuant to Section 9.1 and 9.2.2(a), either Party wishing to make a publication or public presentation that contains the Confidential Information of the other Party shall deliver to the other Party a copy of the proposed written publication or presentation at least [**] days prior to submission for publication or presentation. The reviewing Party shall have the right (a) to propose modifications to the publication or presentation for patent reasons, trade secret reasons or business reasons, and the publishing Party will remove all Confidential Information of the other Party if requested by the reviewing Party, and (b) to request a reasonable delay in publication or presentation in order to protect patentable information. If the reviewing Party requests a delay, the publishing Party shall delay submission or presentation for a period of [**] days (or such shorter period as may be mutually agreed by the
Confidential
- 53 -
Parties) to enable the non-publishing Party to file patent applications protecting such Party’s rights in such information in accordance with Section 12. With respect to any proposed publications or disclosures by investigators or academic or non-profit collaborators, such materials shall be subject to review under this Section 9.1(c) to the extent that Genzyme or Alnylam, as the case may be, has the right and ability (after using Commercially Reasonable Efforts to obtain such right and ability) to do so.
9.2.2 Publicity. Except as set forth in Section 9.1 above and Section 9.2.2(a) and 9.2.2(b) below, the terms of this Agreement may not be disclosed by either Party, and no Party shall use the name, trademark, trade name or logo of the other Party or its employees in any publicity, news release or disclosure relating to this Agreement, its subject matter, or the activities of the Parties hereunder without the prior express written permission of the other Party, except as may be required by Law or expressly permitted by the terms hereof.
(a) Following the execution of this Agreement, the Parties shall issue a joint press release in the form set forth in Schedule 9.2.2(a). After such initial press release, except as provided in Section 9.2.2(b), neither Party shall issue a press release or public announcement relating to this Agreement without the prior written approval of the other Party, which approval shall not be unreasonably withheld, conditioned or delayed, except that a Party may (i) once a press release or other public statement is approved in writing by both Parties, make subsequent public disclosure of the information contained in such press release or other written statement without the further approval of the other Party, and (ii) issue a press release or public announcement as required, in the reasonable judgment of such Party, by Law, including by the rules or regulations of the United States Securities and Exchange Commission or similar regulatory agency in a country other than the United States or of any stock exchange or listing entity.
(b) Notwithstanding anything in this Section 9.2.2 to the contrary, either Party may issue a press release or make a public disclosure relating to such Party’s Development, Manufacturing or Commercialization activities with respect to Licensed Products in such Party’s Territory, provided that such press release or public disclosure does not disclose Confidential Information of the other Party. Furthermore, either Party may issue a press release or make a public disclosure relating to the other Party’s Development, Manufacturing or Commercialization activities with respect to the Licensed Products in the other Party’s Territory, provided that (i) such press release or public disclosure does not disclose Confidential Information of the other Party and (ii) such press release or public disclosure merely repeats subject matter previously disclosed by the other Party in a manner that is substantially consistent with such prior disclosure. Prior to making any such disclosure, however, the Party making the disclosure shall provide the other Party with a draft of such proposed disclosure a reasonable time prior to disclosure to allow the other Party to review and comment prior to making any such disclosure, for the other Party’s review and comment, and the disclosing Party shall consider in good faith any timely comments provided by the other Party.
Confidential
- 54 -
10. REPRESENTATIONS, WARRANTIES AND COVENANTS; INDEMNIFICATION
10.1 Mutual Representations and Warranties. Each Party represents and warrants to the other Party that as of the Effective Date:
10.1.1 It is duly organized and validly existing under the laws of its jurisdiction of incorporation or formation, and has full corporate or other power and authority to enter into this Agreement, and to carry out the provisions hereof.
10.1.2 It is duly authorized to execute and deliver this Agreement, and to perform its obligations hereunder, and the person or persons executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate action.
10.1.3 This Agreement is legally binding upon it and enforceable in accordance with its terms. The execution, delivery and performance of this Agreement by it does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party and by which it may be bound, or with its charter or by-laws.
10.1.4 It has not granted, and will not grant, during the Term, any right to any Third Party that would conflict with the rights granted to the other Party hereunder.
10.1.5 Neither Party nor any of its Affiliates has been debarred or is subject to debarment and neither Party nor any of its Affiliates will use in any capacity, in connection with the Collaboration or the performance of its obligations under this Agreement, any person or entity that has been debarred pursuant to Section 306 of the United States Federal Food, Drug, and Cosmetic Act, as amended, or that is the subject of a conviction described in such section. Each Party agrees to inform the other Party in writing immediately if it or any person or entity that is performing activities in the Collaboration or under this Agreement, is debarred or is subject to debarment or is the subject of a conviction described in Section 306, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to the best of the notifying Party’s knowledge, is threatened, relating to the debarment or conviction of the notifying Party or any person or entity used in any capacity by such Party or any of its Affiliates in connection with the Collaboration or the performance of its other obligations under this Agreement.
10.2 Representations and Warranties of Alnylam. Except as provided in Schedule 10.2, Alnylam represents and warrants to Genzyme that as of the Effective Date:
10.2.1 Alnylam is the sole and exclusive owner of, or otherwise Controls pursuant to an Alnylam In-License (or will Control pursuant to an Additional Alnylam In-License at such time that such Additional Alnylam In-License is included as an Alnylam In-License pursuant to Section 7.4.2), the Alnylam Technology, and all of the Alnylam Technology licensed to Genzyme hereunder in the Genzyme Territory that is solely and exclusively owned by Alnylam is free and clear of liens, charges or encumbrances other than licenses granted to Third Parties that are not inconsistent with the rights and licenses granted to Genzyme under this Agreement.
Confidential
- 55 -
10.2.2 Alnylam has sufficient legal and/or beneficial title and ownership of, or sufficient license rights under, the Alnylam Technology to grant the licenses to such Alnylam Technology granted to Genzyme pursuant to this Agreement.
10.2.3 (a) Schedule 1.9 and Schedule 1.16 collectively set forth a complete and accurate list of the Alnylam Patents owned, either solely or jointly, by Alnylam, and to Alnylam’s knowledge, Schedule 1.9 and Schedule 1.16 collectively set forth a complete and accurate list of the Alnylam Patents licensed, either exclusively or nonexclusively, to Alnylam, (b) to Alnylam’s knowledge, each issued Alnylam Patent remains in full force and effect and (c) Alnylam or its Affiliates have timely paid all filing and renewal fees payable with respect to such Alnylam Patents for which Alnylam controls prosecution and maintenance. Schedule 1.9 and Schedule 1.16 indicate whether each Alnylam Patent is owned exclusively by Alnylam, is owned jointly by Alnylam and one or more Third Parties, or is licensed to Alnylam. For each Alnylam Patent Right that is owned, but not owned exclusively, by Alnylam, or that is licensed to Alnylam, Schedule 1.9 and Schedule 1.16 identify the Third Party owner(s) and, if applicable, the Alnylam In-License pursuant to which Alnylam Controls such Alnylam Patent. For each Alnylam Product-Specific Patent that is licensed, but not exclusively licensed, to Alnylam, Schedule 1.16 indicates the non-exclusive nature of the license. For each Alnylam Core Technology Patent family (other than Patent Rights licensed from Isis Pharmaceuticals, Inc.) that is licensed, but not exclusively licensed, to Alnylam, Schedule 1.9 indicates the non-exclusive nature of the license. Alnylam is the sole and exclusive owner of all Patent Rights identified in Schedule 1.9 and Schedule 1.16 as being owned exclusively by Alnylam and Controls all other Patent Rights identified on such schedules.
10.2.4 To Alnylam’s knowledge, the Alnylam Product-Specific Patents, are, or, upon issuance, will be, valid and enforceable patents and no Third Party has challenged or threatened to challenge the scope, validity or enforceability of any Alnylam Product-Specific Patent (including, by way of example, through opposition or the institution or written threat of institution of interference, nullity or similar invalidity proceedings before the United States Patent and Trademark Office or any analogous foreign Governmental Authority).
10.2.5 Alnylam has complied with all applicable Laws, including any duties of candor to applicable patent offices, in connection with the filing, prosecution and maintenance of the Alnylam Patent Rights.
10.2.6 Alnylam owns or Controls all Know-How that is or has been used by Alnylam in the Development and Manufacture of the Licensed Products, and has sufficient legal or beneficial title and ownership of, or sufficient license rights under such Know-How to transfer Know-How to Genzyme as provided in Section 6.2.
10.2.7 Schedule 1.47 sets forth a complete and accurate list of the Existing Alnylam In-Licenses. Alnylam Controls all Know-How and Patent Rights licensed to Alnylam under the Existing Alnylam In-Licenses that is necessary or useful for Genzyme to Develop, Manufacture and/or Commercialize Licensed Products in the Field in the Genzyme Territory. Without limiting the generality of the foregoing, Alnylam has obtained all necessary consents and fulfilled all necessary conditions, if any, to sublicense to Genzyme under this Agreement such Know-How
Confidential
- 56 -
and Patent Rights licensed to Alnylam under Existing Alnylam In-Licenses. At such time that an Additional Alnylam In-License is included as an Alnylam In-License pursuant to Section 7.4.2, Alnylam will Control all Know-How, if any, and Patent Rights licensed to Alnylam under such Additional Alnylam In-License that is necessary or useful for Genzyme to Develop, Manufacture and/or Commercialize Licensed Products in the Field in the Genzyme Territory.
10.2.8 To Alnylam’s knowledge, neither Alnylam nor its Affiliates are in breach or default under any Existing Alnylam In-License or Additional Alnylam In-License, and neither Alnylam nor its Affiliates have received any written notice of breach or default with respect to any Existing Alnylam In-License or Additional Alnylam In-License.
10.2.9 Alnylam has obtained from all inventors of Alnylam Technology owned by Alnylam valid and enforceable agreements assigning to Alnylam each such inventor’s entire right, title and interest in and to all such Alnylam Technology.
10.2.10 No Alnylam Technology existing as of the Effective Date is subject to any funding agreement with any government or Governmental Authority.
10.2.11 To Alnylam’s knowledge, the use, Development, Manufacture or Commercialization by Alnylam or Genzyme (or their respective Related Parties) of any Licensed Product as formulated and manufactured as of the Effective Date, or as intended to be formulated and manufactured as of the Effective Date (a) does not and will not infringe any issued patent of any Third Party or (b) will not infringe the claims of any published Third Party patent application when and if such claims were to issue in their current form.
10.2.12 There is no (a) claim, demand, suit, proceeding, arbitration, inquiry, investigation or other legal action of any nature, civil, criminal, regulatory or otherwise, pending or, to Alnylam’s knowledge, threatened against Alnylam or any of its Affiliates or (b) judgment or settlement against or owed by Alnylam or any of its Affiliates, in each case in connection with the Alnylam Technology or any Licensed Product.
10.3 Representations and Warranties of Genzyme. Genzyme represents and warrants to Alnylam as of the Effective Date that is not a party to any agreement with a Third Party under which it Controls Know-How or Patent Rights that are sublicensed to Alnylam under this Agreement.
10.4 Warranty Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, TO THE OTHER PARTY WITH RESPECT TO ANY TECHNOLOGY, LICENSED PRODUCT, GOODS, SERVICES, RIGHTS OR OTHER SUBJECT MATTER OF THIS AGREEMENT AND HEREBY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NON-INFRINGEMENT WITH RESPECT TO ANY AND ALL OF THE FOREGOING. EACH PARTY HEREBY DISCLAIMS ANY REPRESENTATION OR WARRANTY THAT THE DEVELOPMENT, MANUFACTURE OR COMMERCIALIZATION OF THE LICENSED PRODUCT PURSUANT TO THIS AGREEMENT WILL BE SUCCESSFUL OR THAT ANY PARTICULAR SALES LEVEL WITH RESPECT TO THE LICENSED PRODUCT WILL BE ACHIEVED.
Confidential
- 57 -
10.5 Certain Covenants.
10.5.1 Exclusivity.
(a) During the Exclusivity Period in a country in the Genzyme Territory, Genzyme will not, alone or with an Affiliate or Third Party, Develop or Commercialize in such country any product, including any Improvement Product, for the treatment of ATTR, other than a Licensed Product or an Exempted Complementary TTR Product, without the prior written agreement of Alnylam.
(b) During the Exclusivity Period in a country in the Genzyme Territory, Alnylam will not, alone or with an Affiliate or Third Party, Develop or Commercialize in such country any product, including any Improvement Product, for the treatment of ATTR, other than a Licensed Product or an Exempted Complementary TTR Product, without the prior written agreement of Genzyme.
(c) Alnylam shall not grant any license or other right under the Alnylam Technology that is inconsistent with the exclusivity granted to Genzyme with respect thereto under Section 7.1.1 and 7.1.2. Without limiting the generality of the foregoing, during the Exclusivity Period in a country in the Genzyme Territory, Alnylam shall also not, alone or with an Affiliate or Third Party, Develop (except as permitted herein for or on behalf of Genzyme) or Commercialize any Licensed Product in such country without the prior written agreement of Genzyme.
10.5.2 Compliance. Each Party and its Related Parties shall conduct the Collaboration and the Development, Manufacture and Commercialization of the Licensed Product in accordance with all Laws, including current governmental regulations concerning good laboratory practices, good clinical practices and good manufacturing practices.
10.5.3 Conflicting Transactions. During the Term, Alnylam shall not (a) transfer or assign any of its rights, title or interests in the Alnylam Technology other than as part of a transaction pursuant to which this Agreement is also assigned and assumed in accordance with Section 14.1, or (b) enter into any agreement granting a license or other right under the Alnylam Technology that is inconsistent with the terms of this Agreement.
11. INDEMNIFICATION; LIMITATION OF LIABILITY; INSURANCE
11.1 General Indemnification by Genzyme. Genzyme shall indemnify, hold harmless, and defend Alnylam, its Related Parties, and their respective directors, officers, employees and agents (“Alnylam Indemnitees”) from and against any and all Third Party claims, suits, losses, liabilities, damages, costs, fees and expenses (including reasonable attorneys’ fees and litigation expenses) (collectively, “Losses”) arising out of or resulting from, directly or indirectly, (a) any breach of, or inaccuracy in, any representation or warranty made by Genzyme in this Agreement, or any breach or violation of any covenant or agreement of Genzyme in or in the performance of this Agreement, or (b) the negligence or willful misconduct by or of Genzyme and its Related Parties, and their respective directors, officers, employees and
Confidential
- 58 -
agents in the performance of Genzyme’s obligations under this Agreement. Genzyme shall have no obligation to indemnify the Alnylam Indemnitees to the extent that the Losses arise out of or result from, directly or indirectly, any breach of, or inaccuracy in, any representation or warranty made by Alnylam in this Agreement, or any breach or violation of any covenant or agreement of Alnylam in or in the performance of this Agreement, or the negligence or willful misconduct by or of any of the Alnylam Indemnitiees, or matters for which Alnylam is obligated to indemnify Genzyme under Section 11.2 or 11.3 or 11.4.
11.2 General Indemnification by Alnylam. Alnylam shall indemnify, hold harmless, and defend Genzyme, its Related Parties and their respective directors, officers, employees and agents (“Genzyme Indemnitees”) from and against any and all Losses arising out of or resulting from, directly or indirectly, (a) any breach of, or inaccuracy in, any representation or warranty made by Alnylam in this Agreement, or any breach or violation of any covenant or agreement of Alnylam in or in the performance of this Agreement, or (b) the negligence or willful misconduct by or of Alnylam and its Related Parties, and their respective directors, officers, employees and agents in the performance of Alnylam’s obligations under this Agreement. Alnylam shall have no obligation to indemnify the Genzyme Indemnitees to the extent that the Losses arise out of or result from, directly or indirectly, any breach of, or inaccuracy in, any representation or warranty made by Genzyme in this Agreement, or any breach or violation of any covenant or agreement of Genzyme in or in the performance of this Agreement, or the negligence or willful misconduct by or of any of the Genzyme Indemnitees, or matters for which Genzyme is obligated to indemnify Alnylam under Section 11.1 or 11.3.
11.3 Product Liability. Any Losses arising out of Third Party product liability claims arising from the Development or Commercialization of Licensed Products shall be (a) borne by Genzyme, to the extent such Losses were incurred with respect to the Development or Commercialization of the Licensed Product in or for the Genzyme Territory by or on behalf of Genzyme and its Related Parties, and (b) be borne by Alnylam, to the extent such Losses were incurred with respect to Development or Commercialization of the Licensed Product in or for the Alnylam Territory by or on behalf of Alnylam and its Related Parties. The Party bearing such Losses in accordance with the immediately preceding sentence shall indemnify, hold harmless and defend the other Party and its Related Parties and their respective directors, officers, employees and agents from and against such Losses.
11.4 Ongoing Litigation. Without limiting Alnylam’s obligations in Section 11.2 or 11.3:
11.4.1 Alnylam shall indemnify, hold harmless and defend the Genzyme Indemnitees from and against any and all Losses and, subject to Section 11.6, any other amounts paid by Genzyme (such as through settlement) arising out of or resulting from any claim asserted (whether in the Ongoing Litigation or otherwise, but solely to the extent substantially related to the subject matter of the Ongoing Litigation) by the plaintiffs in the Ongoing Litigation.
11.4.2 In the event that Alnylam agrees (whether as part of any settlement or otherwise), or is required as part of any judgment or order, to pay to one or more plaintiffs in the Ongoing Litigation amounts that result in the aggregate amount of Third Party License Payments
Confidential
- 59 -
under an Existing Alnylam In-License exceeding, in duration or amount, the amount that Alnylam would be required to pay under such Existing Alnylam In-License based on Alnylam’s assertions in the Ongoing Litigation (any such excess, “Incremental Amounts”), Alnylam shall be fully responsible for such Incremental Amounts. Without limitation, Genzyme shall not be responsible for any Incremental Amounts pursuant to Section 7.4.3 and Incremental Amounts shall not be taken into account when calculating the royalty floor pursuant to Section 8.3.5.
11.5 [**].
11.5.1 [**].
11.5.2 [**].
11.6 Indemnification Procedure. In the event of any such claim against any Genzyme Indemnitee or Alnylam Indemnitee (individually, an “Indemnitee”), the indemnified Party shall promptly notify the other Party in writing of the claim and the indemnifying Party shall manage and control, at its sole expense, the defense of the claim and its settlement. The Indemnitee shall cooperate with the indemnifying Party and may, at its option and expense, be represented in any such action or proceeding. The indemnifying Party shall not be liable for any settlements, litigation costs or expenses incurred by any Indemnitee without the indemnifying Party’s written authorization. Notwithstanding the foregoing, if the indemnifying Party believes that any of the exceptions to its obligation of indemnification of the Indemnitees set forth in Sections 11.1, 11.2, 11.3 or 11.4 may apply, the indemnifying Party shall promptly notify the Indemnitees, which shall then have the right to be represented in any such action or proceeding by separate counsel at their expense; provided that the indemnifying Party shall be responsible for payment of such expenses if the Indemnitees are ultimately determined to be entitled to indemnification from the indemnifying Party for the matters to which the indemnifying Party notified the Indemnitees that such exception(s) may apply.
11.7 Limitation of Liability. NEITHER PARTY HERETO WILL BE LIABLE FOR SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES ARISING OUT OF THIS AGREEMENT OR THE EXERCISE OF ITS RIGHTS HEREUNDER, INCLUDING LOST PROFITS ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT, REGARDLESS OF ANY NOTICE OF SUCH DAMAGES, EXCEPT AS A RESULT OF A PARTY’S WILLFUL MISCONDUCT OR A BREACH OF SECTION 7.6 (ALNYLAM TERRITORY RIGHT OF FIRST NEGOTIATION), SECTION 9 (CONFIDENTIALITY AND PUBLICATION), OR SECTION 10.5.1 (EXCLUSIVITY). NOTHING IN THIS SECTION 11.7 IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF EITHER PARTY. NOTWITHSTANDING THE FOREGOING, ALNYLAM SHALL NOT BE LIABLE FOR ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES ARISING OUT OF ANY BREACH OF SECTION 7.6 (ALNYLAM TERRITORY RIGHT OF FIRST NEGOTIATION) UNLESS GENZYME BRINGS AN ACTION SEEKING SUCH DAMAGES WITHIN SIX (6) MONTHS AFTER THE EARLIER OF THE DATE ON WHICH THE TRANSACTION BETWEEN ALNYLAM AND A THIRD PARTY ENTERED INTO IN BREACH OF SECTION 7.6 FIRST BECOMES PUBLICLY KNOWN OR THE DATE ON WHICH ALNYLAM NOTIFIES GENZYME OF SUCH TRANSACTION.
Confidential
- 60 -
11.8 Insurance. Each Party shall maintain insurance during the Term and for a period of at least [**] years after the last commercial sale of any Licensed Product under this Agreement, with a reputable, solvent insurer in an amount appropriate for its business and products of the type that are the subject of this Agreement, and for its obligations under this Agreement. Specifically, each Party shall maintain product liability insurance and clinical trial liability insurance with limits of at least [**] U.S. Dollars ($[**]) per occurrence and in annual aggregate. Upon request, each Party shall provide the other Party with evidence of the existence and maintenance of such insurance coverage.
12. INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION AND RELATED MATTERS
12.1 Inventorship. Inventorship for inventions and discoveries first made during the course of the performance of activities pursuant to this Agreement shall be determined in accordance with United States patent Laws for determining inventorship.
12.2 Ownership. Alnylam shall own the entire right, title and interest in and to all inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered solely by employees or consultants of Alnylam or acquired solely by Alnylam in the course of conducting the Collaboration. Genzyme shall own the entire right, title and interest in and to all inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered solely by employees or consultants of Genzyme or acquired solely by Genzyme in the course of conducting the Collaboration. The Parties shall jointly own any inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered jointly in the course of conducting the Collaboration.
12.3 Prosecution and Maintenance of Patent Rights.
12.3.1 Genzyme Technology.
(a) Subject to Section 12.3.1(b) Genzyme has the sole responsibility to, at Genzyme’s discretion, file, prosecute, and maintain (including the defense of any interference or opposition proceedings), all Patent Rights comprising Genzyme Technology (other than Joint Collaboration IP), in Genzyme’s name.
(b) In the event that Genzyme elects not to seek or continue to seek or maintain patent protection on any Genzyme Collaboration IP in the Alnylam Territory, subject to the terms and conditions of any applicable Genzyme In-License, Alnylam shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain in any country patent protection on such Genzyme Collaboration IP in the name of Genzyme. Genzyme shall use Commercially Reasonable Efforts to make available to Alnylam its authorized attorneys, agents or representatives, such of its employees as are reasonably necessary to assist Alnylam in obtaining and maintaining the patent protection described under this Section 12.3.1(b). Genzyme shall sign or use Commercially Reasonable Efforts to have signed all legal documents necessary to file and prosecute such patent applications or to obtain or maintain such patents.
Confidential
- 61 -
12.3.2 Alnylam Technology.
(a) Subject to Section 12.3.2(c), Alnylam has the sole responsibility to, at Alnylam’s discretion, file, conduct prosecution, and maintain (including the defense of any interference or opposition proceedings), all Patent Rights comprising Alnylam Technology (other than Joint Collaboration IP), in Alnylam’s name. Alnylam agrees to use Commercially Reasonable Efforts to prosecute and maintain the Alnylam Patents in the Genzyme Territory.
(b) Notwithstanding the foregoing Section 12.3.2(a), Alnylam shall consult with Genzyme on the preparation, filing, prosecution, and maintenance of all Alnylam Product-Specific Patents in the Genzyme Territory and the Alnylam Territory. Alnylam shall furnish Genzyme with copies of proposed filings and documents received from or filed with the relevant patent offices with respect to Alnylam Product-Specific Patents and such other documents directly related to the prosecution and maintenance of Alnylam Product-Specific Patents reasonably necessary for Genzyme to exercise its rights under this Section 12.3.112.3.1(b), and as applicable in sufficient time prior to filing such document or making any payment due thereunder to allow for review and comment by Genzyme and shall consider in good faith timely comments from Genzyme thereon. Furthermore, Alnylam shall provide Genzyme with copies of documents received from or filed with the relevant national patent offices with respect to the filing, prosecution, and maintenance of Alnylam Core Technology Patents in the Genzyme Territory within a reasonable time after the filing of such documents.
(c) In the event that Alnylam elects not to seek or continue to seek or maintain patent protection on any Alnylam Product-Specific Patent in the Genzyme Territory or the Alnylam Territory, subject to the terms and conditions of any applicable Alnylam In-License, Genzyme shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain in any country patent protection on such Alnylam Product-Specific Patent in the Genzyme Territory. If Genzyme exercises such right, Alnylam shall thereafter and hereby does assign all right, title and interest in and to such Patent Rights to Genzyme. Alnylam shall use Commercially Reasonable Efforts to make available to Genzyme its authorized attorneys, agents or representatives, such of its employees as are reasonably necessary to assist Genzyme in obtaining and maintaining the patent protection described under this Section 12.3.2(b). Alnylam shall sign or use Commercially Reasonable Efforts to have signed all legal documents necessary to file and prosecute such patent applications or to obtain or maintain such patents.
(d) Alnylam shall, as provided in Section 12.3.2(b), consult with Genzyme prior to its filing of any patent application which would, if patented, be included in the definition of Alnylam Product-Specific Patents. Furthermore, (a) promptly after filing, any provisional or non-provisional patent application that is included in Alnylam Product Specific Patents and (b) promptly after publication, any non-provisional patent application that is included in the Alnylam Core Technology Patents that Cover a Licensed Product or an siRNA Product that Alnylam has identified to Genzyme as a potential Improvement Product, Alnylam shall promptly provide a copy of such patent application to Genzyme. All information provided by the Alnylam under this Section 12.3.3 will be deemed to be Confidential Information of Alnylam and maintained in accordance with Section 9.
Confidential
- 62 -
12.3.3 Joint Collaboration IP.
(a) Alnylam shall have the first right to, at Alnylam’s discretion, file, prosecute and maintain (including the defense of any interference or opposition proceedings), all Patent Rights comprising Joint Collaboration IP, in the names of both Alnylam and Genzyme. Alnylam shall provide Genzyme an opportunity to review and comment on material documents related to such filing, prosecution and maintenance in accordance with Section 12.3.4, which comments Alnylam will consider in good faith. Each Party shall sign, or use Commercially Reasonable Efforts to have signed, all legal documents necessary to file and prosecute patent applications or to obtain or maintain patents in respect of such Joint Collaboration IP, at its own cost.
(b) Notwithstanding the foregoing Section 12.3.3(a), Alnylam shall consult with Genzyme on the preparation, filing, prosecution, and maintenance of all Patent Rights included within the Joint Collaboration IP to be filed or pending in the Genzyme Territory. Alnylam shall furnish Genzyme with copies of documents relevant to such preparation, filing, prosecution, and maintenance in sufficient time prior to filing such document or making any payment due thereunder to allow for review and comment by Genzyme and shall consider in good faith timely comments from Genzyme thereon.
(c) In the event that Alnylam elects not to file or continue to prosecute or maintain patent protection on any Joint Collaboration IP, Genzyme shall have the right (but not the obligation) to file, prosecute and maintain Patent Rights comprising Joint Collaboration IP in the names of both Alnylam and Genzyme. If Genzyme exercises such right, Alnylam shall use Commercially Reasonable Efforts to make available to Genzyme its authorized attorneys, agents or representatives, such of its employees as are reasonably necessary to assist Genzyme in obtaining and maintaining the patent protection described under this Section 12.3.3(c). Alnylam shall sign or use Commercially Reasonable Efforts to have signed all legal documents necessary to file and prosecute such patent applications or to obtain or maintain such patents.
(d) The Parties shall share equally the out-of-pocket patent filing, prosecution and maintenance expenses incurred with respect to Patent Rights comprising Joint Collaboration IP.
12.3.4 Cooperation. Each Party hereby agrees: (a) to make its employees, agents and consultants reasonably available to the other Party (or to the other Party’s authorized attorneys, agents or representatives), to the extent reasonably necessary to enable such Party to undertake patent prosecution; (b) to provide the other Party with copies of all material correspondence pertaining to prosecution with the patent offices; (c) to cooperate, if necessary and appropriate, with the other Party in gaining patent term extensions wherever applicable to Patent Rights licensed under this Agreement; and (d) to endeavor in good faith to coordinate its efforts with the other Party to minimize or avoid interference with the prosecution and maintenance of the other Party’s patent applications.
12.4 Third Party Infringement.
12.4.1 Notices. Each Party shall promptly report in writing to the other Party any (a) known or suspected infringement of any Alnylam Technology, Genzyme Technology or Joint Collaboration IP or (b) unauthorized use or misappropriation of any Confidential Information or
Confidential
- 63 -
Know-How of a Party by a Third Party of which it becomes aware, in each case to the extent such infringing, unauthorized or misappropriating activities involve, as to a Licensed Product, a competing product in the Field (“Competitive Infringement”), and shall provide the other Party with all available evidence of such infringement, unauthorized use or misappropriation.
12.4.2 Rights to Enforce.
(a) Genzyme Technology. Subject to the provisions of any In-License, Genzyme shall have the sole and exclusive right to initiate an infringement or other appropriate suit anywhere in the world against any Third Party as to any infringement, or suspected infringement of, any Patent Rights, or of any use or suspected use without proper authorization of any Know-How, comprising Genzyme Patent Rights, Genzyme Know-How, or Genzyme Collaboration IP. Genzyme will consider in good faith any request from Alnylam to initiate an infringement or other appropriate suit against any Third Party with respect to Competitive Infringement in the Alnylam Territory of Genzyme Patent Rights, Genzyme Know-How or Genzyme Collaboration IP licensed to Alnylam under Section 7, however Genzyme shall not be required to initiate any such suit or permit Alnylam to initiate any such suit.
(b) Alnylam Technology. Subject to the provisions of any In-License, Genzyme shall have the first right to initiate an infringement or other appropriate suit anywhere in the world against any Third Party with respect to any Competitive Infringement in the Genzyme Territory of any Alnylam Product-Specific Patent or Joint Collaboration IP, or, with Alnylam’s prior written consent, Alnylam Core Technology Patent or Alnylam Know-How. Alnylam will consider in good faith any request from Genzyme to initiate an infringement or other appropriate suit against any Third Party with respect to a Competitive Infringement in the Genzyme Territory of any Alnylam Core Technology Patent; provided, however, that Alnylam shall not be required to initiate any such suit or permit Genzyme to initiate any such suit.
(c) Step-In Right.
| (i) | If within [**] days (or such shorter period of time as required by applicable Law to avoid loss of material enforcement rights) after Genzyme’s receipt of a notice of a Competitive Infringement with respect to any Alnylam Product-Specific Patent or Joint Collaboration IP, Genzyme does not take any action as described in Section 12.4.2(b) and permitted hereunder against such Competitive Infringement in the Genzyme Territory, Alnylam may in its sole discretion, bring and control any legal action in connection therewith at its sole expense. |
| (ii) | If (A) there are no Alnylam Product-Specific Patents or Patent Rights included in Joint Collaboration IP that can be asserted against a Competitive Infringement in the Genzyme Territory, (B) there are Alnylam Core Technology Patent(s) that can reasonably be asserted, but Alnylam refuses to either permit Genzyme to assert or itself assert at least one of such Alnylam Core Technology Patent(s) that can reasonably be asserted against a Competitive Infringement in the Genzyme Territory and (C) Genzyme and Alnylam are unable to stop the |
Confidential
- 64 -
| Competitive Infringement through enforcement of any other Patent Rights or Know-How Controlled by either Party, then the royalties to be paid by Genzyme to Alnylam pursuant to Section 8.3 with respect to the applicable Licensed Product shall be reduced throughout the Genzyme Territory as specified in Section 8.3.3 (regardless of whether any Alnylam Patent or Patent Right included in the Joint Collaboration IP Covers the Licensed Product) during the period after the conditions in the foregoing clauses (A), (B) and (C) exist when such Competitive Infringement continues. |
12.4.3 Procedures; Expenses and Recoveries. The Party having the right to initiate any infringement suit under Section 12.4.2 above shall have the sole and exclusive right to select counsel for any such suit and shall pay all expenses of the suit, including attorneys’ fees and court costs and reimbursement of the other Party’s reasonable Out-of-Pocket expense in rendering assistance requested by the initiating Party. If required under applicable Law in order for the initiating Party to initiate and/or maintain such suit, or if either Party is unable to initiate or prosecute such suit solely in its own name or it is otherwise advisable to obtain an effective legal remedy, in each case, the other Party shall join as a party to the suit and will execute and cause its Affiliates to execute all documents necessary for the initiating Party to initiate litigation to prosecute and maintain such action. In addition, at the initiating Party’s request, the other Party shall provide reasonable assistance to the initiating Party in connection with an infringement suit at no charge to the initiating Party except for reimbursement by the initiating Party of reasonable Out-of-Pocket expenses incurred in rendering such assistance. The non-initiating Party shall have the right to participate and be represented in any such suit by its own counsel at its own expense. If the Parties obtain from a Third Party, in connection with such suit, any damages, license fees, royalties or other compensation (including any amount received in settlement of such litigation), such amounts shall be allocated in all cases as follows:
| (i) | first, to reimburse each Party for all expenses of the suit incurred by the Parties, including attorneys’ fees and disbursements, court costs and other litigation expenses; |
| (ii) | second, [**] percent ([**]%) of the balance to be paid to the Party initiating the suit; and |
| (iii) | third, the remainder to the other Party. |
Notwithstanding the foregoing, in the event that Alnylam elects to itself assert an Alnylam Core Technology Patent against a Competitive Infringement in the Genzyme Territory, the Parties shall each be entitled to [**] percent ([**]%) of the balance of any recovery therefrom after reimbursement of expenses as described in clause (i) above.
12.5 Patent Term Extensions. Subject to the provisions of any Alnylam In-License, Alnylam shall use Commercially Reasonable Efforts to obtain all available supplementary protection certificates (“SPC”) and other extensions of Alnylam Product-Specific Patents in the Genzyme Territory. If more than one Alnylam Product-Specific Patent is eligible for extension or patent term restoration in the Genzyme Territory, Genzyme will determine, in its sole discretion, a strategy that will be designed to maximize patent protection and commercial value for the Licensed Product, and the Parties, subject to the provisions of any In-License, will seek
Confidential
- 65 -
patent term extensions, restorations and SPCs for Alnylam Product-Specific Patents in the Genzyme Territory in accordance with that strategy. If Genzyme determines not to so file for any SPC for any Alnylam Product-Specific Patent in the Genzyme Territory, it will give notice of such determination to Alnylam at least [**] days prior to the date on which such a filing must be made or the right to do so is lost, and Alnylam will have the right to make such filing. Where required under national law, Alnylam will make the filings for such extensions, restorations and SPCs for Alnylam Product-Specific Patents in the Genzyme Territory as directed by Genzyme. Each Party will execute such authorizations and other documents and take such other actions as may be reasonably requested by the other Party to obtain any such extensions, restorations and SPCs for Alnylam Product-Specific Patents in the Genzyme Territory.
12.6 Common Interest. All information exchanged between the Parties representatives regarding the preparation, filing, prosecution, maintenance, or enforcement of the Patents Rights under this Section 12 will be deemed Confidential Information. In addition, the Parties acknowledge and agree that, with regard to such preparation, filing, prosecution, maintenance, and enforcement of the Patents Rights under this Section 12, the interests of the Parties as collaborators and licensor and licensee are to obtain the strongest patent protection possible, and as such, are aligned and are legal in nature. The Parties agree and acknowledge that they have not waived, and nothing in this Agreement constitutes a waiver of, any legal privilege concerning the Patents Rights under this Section 12, including privilege under the common interest doctrine and similar or related doctrines.
12.7 Trademarks.
(a) Each Party has the right to use any trademark it owns or controls for Licensed Products in its Territory at its sole discretion, and each Party and its Affiliates shall retain all right, title and interest in and to its and their respective corporate names and logos.
(b) Genzyme will develop and propose, and the JSC shall review and comment on, one or more Product Trademark(s) for use by Genzyme and its Related Parties throughout the Genzyme Territory. Such Product Trademark(s) considered by the JSC may include, in Genzyme’s sole discretion, the Product Trademark(s) developed and/or used by Alnylam with respect to the Commercialization of Licensed Products in the Alnylam Territory (the “Alnylam Trademarks”), but may not include other trademarks owned or controlled by Alnylam. Any Product Trademark(s) (other than the Alnylam Trademarks) that are used by Genzyme to promote and sell Licensed Products in the Genzyme Territory are hereinafter referred to as the “Genzyme Trademarks.” Alnylam (or its Related Parties, as appropriate) shall own all rights to Alnylam Trademarks, and all goodwill associated therewith, throughout the Alnylam Territory and the Genzyme Territory. Genzyme (or its Related Parties, as appropriate) shall own all rights to Genzyme Trademarks and all goodwill associated therewith, throughout the Genzyme Territory. Alnylam shall also own rights to any Internet domain names incorporating the applicable Alnylam Trademarks or any variation or part of such Alnylam Trademarks used as its URL address or any part of such address; and Genzyme shall also own rights to any Internet domain names incorporating the applicable Genzyme Trademarks or any variation or part of such Genzyme Trademarks used as its URL address or any part of such address.
Confidential
- 66 -
(c) If Genzyme determines to use Alnylam Trademarks to promote and sell any Licensed Product in the Genzyme Territory, then Alnylam and Genzyme shall enter into a separate trademark license agreement containing commercially reasonable and customary terms pursuant to which Alnylam will grant Genzyme an exclusive, royalty-free license to use the applicable Alnylam Trademark(s) to Commercialize Licensed Products in the Genzyme Territory.
(d) In the event either Party becomes aware of any infringement of any Product Trademark by a Third Party, such Party shall promptly notify the other Party and the Parties shall consult with each other and jointly determine the best way to prevent such infringement, including by the institution of legal proceedings against such Third Party.
(e) For the avoidance of doubt, neither Party shall have any right to use the other Party’s or the other Party’s Affiliates’ corporate names or logos in connection with Commercialization of Licensed Products.
12.8 Cooperative Research and Technology (CREATE) Act Acknowledgment. It is the intention of the Parties that this Agreement is a “joint research agreement” as that phrase is defined in Section 35 U.S.C. 103(c).
13. TERM AND TERMINATION
13.1 Term. This Agreement shall be effective as of the Effective Date and, unless terminated earlier pursuant to Section 13.2, this Agreement shall continue in effect on a Licensed Product-by-Licensed Product and country-by-country basis until expiration of the last Royalty Term to expire under this Agreement (“Term”). Upon expiration of the Term, all licenses of the Parties under Section 7 then in effect shall become fully paid-up, perpetual, exclusive licenses.
13.2 Termination Rights.
13.2.1 Termination for Convenience. Genzyme shall have the right to terminate this Agreement in its entirety at any time after the Effective Date on six (6) months prior written notice to Alnylam.
13.2.2 Termination for Cause. This Agreement may be terminated at any time during the Term upon written notice by either Party if the other Party is in material breach of its obligations hereunder and has not cured such breach within [**] days in the case of a payment breach, or within [**] days in the case of all other breaches, after notice requesting cure of the breach; provided, however, that if any breach other than a payment breach is not reasonably curable within [**] days and if a Party is making a bona fide effort to cure such breach, such termination shall be delayed for a time period to be agreed by both Parties, not to exceed an additional [**] days, in order to permit such Party a reasonable period of time to cure such breach; provided, further, that in the event that the breach relates to a dispute between the Parties regarding Genzyme’s obligations to use Commercially Reasonable Efforts in Developing or Commercializing the Licensed Products and Genzyme disputes whether it has breached such obligation or whether such breach gives Alnylam the right to terminate this agreement and initiates a legal action against Alnylam to resolve such dispute within the foregoing [**] day cure
Confidential
- 67 -
period, then this Agreement shall not terminate during the pendency of such legal action; provided that if Genzyme is found in an unappealable decision by a court of competent jurisdiction or an appealable decision of a court of competent jurisdiction that has not been appealed in the time allowed for an appeal in such legal action to have materially breached this Agreement or if Genzyme admits in such legal action or settlement thereof that it has materially breached this Agreement then this Agreement shall terminate immediately following the Parties’ receipt of such decision or immediately following such admission, as applicable.
13.2.3 No First Phase II Success. Genzyme may also terminate this Agreement in accordance with Section 8.2(a)(i).
13.2.4 Challenges of Patent Rights. If during the Term Genzyme or any of its Affiliates (a) commences or participates in any action or proceeding (including any patent opposition or re-examination proceeding), or otherwise asserts any claim, challenging or denying the validity or enforceability of any Alnylam Patent or any claim thereof or (b) actively assists any other person or entity in bringing or prosecuting any action or proceeding (including any patent opposition or re-examination proceeding) challenging or denying the validity or enforceability of any of such Patent Rights or any claim thereof (each of (a) and (b), a “Patent Challenge”), then, to the extent permitted by the applicable Laws, Alnylam shall have the right, exercisable within [**] days following receipt of notice regarding such Patent Challenge, in its sole discretion, to give notice to Genzyme that Alnylam may terminate this Agreement ninety (90) days following such notice (or such longer period as Alnylam may designate in such notice), and, unless Genzyme or such Affiliate withdraws or causes to be withdrawn all such challenge(s) (or in the case of ex-parte proceedings, multi-party proceedings, or other Patent Challenges that Genzyme or Genzyme’s Affiliates do not have the power to unilaterally withdraw or cause to be withdrawn, Genzyme and Genzyme’s Affiliates cease actively assisting any other party to such Patent Challenge and, to the extent Genzyme or a Genzyme Affiliate is a party to such Patent Challenge, it withdraws from such Patent Challenge) within such ninety (90)-day period, Alnylam will have the right to terminate this Agreement by providing written notice thereof to Genzyme. The foregoing sentence shall not apply (i) with respect to any Alnylam Patent that Alnylam first asserts against Genzyme or any of its Affiliates where the Patent Challenge is made in defense of such assertion, (ii) with respect to any Patent Challenge commenced by a Third Party that after the Effective Date acquires or is acquired by Genzyme or its Affiliates or its or their business or assets, whether by stock purchase, merger, asset purchase or otherwise, but only with respect to Patent Challenges commenced prior to the closing of such acquisition.
13.2.5 Abandonment of Development. At any time on or after the tenth (10th) anniversary of the Effective Date, either Party may terminate this Agreement in its entirety if (a) no Licensed Product has received Regulatory Approval anywhere in the Genzyme Territory, (b) no Licensed Product is being Developed in the Genzyme Territory, (c) no Genzyme Territory Development Plan is in effect for any Licensed Product, provided that, if Genzyme provides Alnylam with a good faith Genzyme Territory Development Plan pursuant to Section 2.2.3 during the termination notice period set forth below in this Section 13.2.5, then the condition set forth in this clause (c) shall be deemed not satisfied at such time and the applicable termination notice shall be deemed not to have effect, and (d) the absence of Development in the Genzyme Territory or a good faith Genzyme Development Plan is not the result of a breach by the Party
Confidential
- 68 -
seeking to terminate this Agreement of its obligations under Section 2.4. In order to terminate this Agreement pursuant to this Section 13.2.5, the terminating Party must provide at least six (6) months’ prior written notice to the other Party referencing this Section 13.2.5 and specifying a termination date on or after the tenth (10th) anniversary of the Effective Date; provided that. for a termination by Alnylam, such notice must also include an update regarding the current status of Alnylam’s development activities with respect to Licensed Products, Backup Compounds and Improvement Products.
13.3 Effect of Termination.
13.3.1 Termination by Genzyme for Alnylam Breach. Without limiting any other legal or equitable remedies that either Party may have, if this Agreement is terminated by Genzyme pursuant to Section 13.2.2, then:
(a) all license grants in this Agreement from either Party to the other shall immediately terminate; and
(b) Genzyme shall as promptly as practicable transfer to Alnylam or Alnylam’s designee (i) possession and ownership of all Regulatory Approvals and pricing and reimbursement approvals relating to the Development, Manufacture or Commercialization of the Licensed Product, and (ii) copies of all non-clinical and clinical data and material regulatory correspondence relating to the Licensed Products.
13.3.2 Termination by Alnylam for Genzyme Breach or by Genzyme for Convenience. Without limiting any other legal or equitable remedies that either Party may have, if this Agreement is terminated by Genzyme pursuant to Section 13.2.1 or 13.2.3 or by Alnylam under Section 13.2.2 or 13.2.4, then:
(a) the license grants to Alnylam in Section 7.2 shall survive and shall be expanded to include the Genzyme Territory,
(b) Genzyme shall as promptly as practicable transfer to Alnylam or Alnylam’s designee (i) possession and ownership of all governmental or regulatory correspondence, conversation logs, filings and approvals (including all Regulatory Approvals and pricing and reimbursement approvals) relating to the Development, Manufacture or Commercialization of the Licensed Product and all Genzyme Trademarks, (ii) copies of all data, reports, records and materials, and other sales and marketing related information in Genzyme’s possession or Control to the extent that such data, reports, records, materials or other information relate to the Development, Manufacture or Commercialization of the Licensed Product, including all non-clinical and clinical data relating to the Licensed Product, and customer lists and customer contact information and all adverse event data in Genzyme’s possession or Control; provided that Genzyme shall use Commercially Reasonable Efforts to obtain for Alnylam the right to access all such data, reports, records, materials, and other sales and marketing related information, and (iii) all records and materials in Genzyme’s possession or Control containing Confidential Information of Alnylam. Genzyme shall further appoint Alnylam as Genzyme’s and/or Genzyme’s Related Parties’ agent for all Licensed Product-related matters involving Regulatory Authorities in the Genzyme Territory until all Regulatory Approvals and other regulatory filings have been transferred to Alnylam or its designee,
Confidential
- 69 -
(c) if the effective date of termination is after First Commercial Sale, then Genzyme shall appoint Alnylam as its exclusive distributor of the Licensed Product in the Genzyme Territory and grant Alnylam the right to appoint sub-distributors, until such time as all Regulatory Approvals in the Genzyme Territory have been transferred to Alnylam or its designee,
(d) if Genzyme or its Related Parties are Manufacturing Finished Product, at Alnylam’s option, supply the Finished Product to Alnylam in the Genzyme Territory on terms no less favorable than those on which Genzyme supplied the Finished Product prior to such termination to its most favored distributor in the Genzyme Territory, until the earlier of (i) such time as all Regulatory Approvals in the Genzyme Territory have been transferred to Alnylam or its designee, Alnylam has obtained all necessary manufacturing approvals and Alnylam has procured or developed its own source of Finished Product supply or (ii) [**] months following the effective date of such termination,
(e) if Alnylam so requests, and to the extent permitted under Genzyme’s obligations to Third Parties at the time of termination, Genzyme shall transfer to Alnylam any Third Party agreements relating solely and exclusively to the Development, Manufacture or Commercialization of the Licensed Product to which Genzyme is a party, subject to any required consents of such Third Party, which Genzyme shall use Commercially Reasonable Efforts to obtain promptly,
(f) Genzyme shall promptly transfer and assign to Alnylam all of Genzyme’s and its Affiliates’ rights, title and interests in and to the Genzyme Trademark(s) (but not any Genzyme house marks or any trademark containing the word “Genzyme” owned by Genzyme and used for the Licensed Products in the Field in the Genzyme Territory) owned by Genzyme and used for the Licensed Products in the Field in the Genzyme Territory
(g) Genzyme shall transfer to Alnylam any inventory of Licensed Products Controlled by Genzyme or its Affiliates as of the termination date at the actual price paid by Genzyme for such supply,
(h) Genzyme shall provide any other assistance reasonably requested by Alnylam for the purpose of allowing Alnylam or its designee to proceed expeditiously with the Development, Manufacture and Commercialization of Licensed Products in the Genzyme Territory, and
(i) Genzyme shall execute all documents and take all such further actions as may be reasonably requested by Alnylam in order to give effect to the foregoing clauses.
13.3.3 Termination for Abandonment of Development. If this Agreement is terminated by either Party pursuant to Section 13.2.5, then the license granted to Alnylam under Section 7.2.2 shall survive and all other licenses granted in this Agreement from either Party to the other shall immediately terminate.
Confidential
- 70 -
13.4 Fundamental Breach of Alnylam’s Development Obligations. Without limiting Genzyme’s rights under Section 13.2.2 with respect to other material breaches, in the event that Alnylam commits a Fundamental Breach of its Development obligations under Section 2.4.2 and Genzyme does not terminate this Agreement for cause pursuant to Section 13.2.2, then Genzyme may elect to receive the following remedies for such Fundamental Breach:
13.4.1 Genzyme’s obligation to pay development milestone fees under Section 8.2 shall automatically terminate;
13.4.2 Genzyme will receive a credit, which Genzyme may offset against royalties due to Alnylam pursuant to Section 8.3, in an amount equal to [**] percent ([**]%) of all costs (i) incurred by Genzyme to Develop the Licensed Products for the Genzyme Territory that are in excess of the costs budgeted by Genzyme in connection with the Genzyme Territory Development Plan in effect at the time of Alnylam’s Fundamental Breach of Section 2.4.2 and (ii) are incurred by Genzyme as a direct consequence of Alnylam’s Fundamental Breach of Section 2.4.2;
13.4.3 Genzyme may, in its discretion, terminate any or all of the following provisions of this Agreement (or any subsection thereof): Section 2.3.3, Section 3.1.3, Section 4.1.3, Section 4.2, Section 4.3, Section 4.4, Section 4.5, and Section 5; and
13.4.4 Without limiting Genzyme’s remedies under this Agreement or otherwise with respect to breaches of Alnylam’s Development obligations under Section 2.4.2(a) other than Fundamental Breaches, if Genzyme elects to receive the remedies set forth in this Section 13.4, such remedies shall be Genzyme’s sole and exclusive remedies with respect to such Fundamental Breach and Genzyme shall have no right to seek any further remedies or damages against Alnylam and its Affiliates with respect to such Fundamental Breach by Alnylam of Section 2.4.2(a).
13.5 Effect of Expiration or Termination; Survival. Expiration or termination of this Agreement shall not relieve the Parties of any obligation accruing prior to such expiration or termination. Any expiration or termination of this Agreement shall be without prejudice to the rights of either Party against the other accrued or accruing under this Agreement prior to expiration or termination, including the obligation to pay royalties for the Licensed Product sold prior to such expiration or termination. The provisions of Sections 1, 9, 11 and 14 and Sections 10.4, 12.1, 12.2, 13.3, and 13.5 shall survive any expiration or termination of this Agreement. Sections 8.3.5 shall survive any expiration of this Agreement or any termination with respect to royalties accruing prior to such termination. Section 8.3.7 (Reports; Payment of Royalty) and Section 8.4 (Audits) will survive for so long as any royalties are due under this Agreement plus three (3) years. Except as otherwise set forth in this Section 13, upon termination or expiration of this Agreement all rights and obligations of the Parties under this Agreement cease.
14. MISCELLANEOUS
14.1 Assignment. Except as provided in this Section 14.1, this Agreement may not be assigned or otherwise transferred, nor may any right or obligation hereunder be assigned or transferred, by either Party without the written consent of the other Party. However, either Party
Confidential
- 71 -
may, without the other Party’s written consent, assign this Agreement and its rights and obligations hereunder in whole or in part to an Affiliate or to a party that acquires, by or otherwise in connection with, merger, sale of assets or otherwise, all or substantially all of the business of the assigning Party to which the subject matter of this Agreement relates. The assigning Party shall remain responsible for the performance by its assignee of this Agreement or any obligations hereunder so assigned. An assignment to an Affiliate shall terminate, and all rights so assigned shall revert to the assigning Party, if and when such Affiliate ceases to be an Affiliate of the assigning Party. Any purported assignment in violation of this Section 14.1 shall be void.
14.2 Governing Law. This Agreement shall be construed and the respective rights of the Parties determined in accordance with the substantive Laws of the Commonwealth of Massachusetts, notwithstanding any provisions of Massachusetts Law or any other Law governing conflicts of laws to the contrary, and the patent Laws of the relevant jurisdiction without reference to any rules of conflict of laws.
14.3 Jurisdiction. Each Party by its execution hereof, (a) hereby irrevocably submits to the jurisdiction of the United States District Court and state courts located in Boston, Massachusetts for the purpose of any dispute arising between the Parties in connection with this Agreement (each, an “Action”) and (b) hereby waives to the extent not prohibited by applicable Law, and agrees not to assert, by way of motion, as a defense or otherwise, in any such Action, any claim that it is not subject personally to the jurisdiction of the above-named court, that its property is exempt or immune from attachment or execution, that any such Action brought in the above-named court should be dismissed on grounds of forum non conveniens, should be transferred or removed to any court other than the above-named court, or should be stayed by reason of the pendency of some other proceeding in any other court other than the above-named court, or that this Agreement or the subject matter hereof may not be enforced in or by such court and (c) hereby agrees not to commence any such Action other than before the above-named court. Notwithstanding the previous sentence a Party may commence any Action in a court other than the above-named court solely for the purpose of enforcing an order or judgment issued by the above-named court.
14.4 Venue. Each Party agrees that for any Action between the Parties arising in whole or in part under or in connection with this Agreement, such Party bring Actions only in the state and federal courts of the United States of America located in Boston, Massachusetts and any appellate court having jurisdiction over appeals from such courts. Each Party further waives any claim and will not assert that venue should properly lie in any other location within the selected jurisdiction.
14.5 Entire Agreement; Amendments. This Agreement contains the entire understanding of the Parties with respect to the subject matter hereof, and supersedes all previous arrangements with respect to the subject matter hereof, whether written or oral. This Agreement (including the Schedules hereto) may be amended, or any term hereof modified, only by a written instrument duly-executed by authorized representatives of both Parties hereto.
Confidential
- 72 -
14.6 Severability. If any provision hereof should be held invalid, illegal or unenforceable in any respect in any jurisdiction, the Parties hereto shall substitute, by mutual consent, valid provisions for such invalid, illegal or unenforceable provisions, which valid provisions in their economic effect are sufficiently similar to the invalid, illegal or unenforceable provisions that it can be reasonably assumed that the Parties would have entered into this Agreement with such valid provisions. In case such valid provisions cannot be agreed upon, the invalid, illegal or unenforceable of one or several provisions of this Agreement shall not affect the validity of this Agreement as a whole, unless the invalid, illegal or unenforceable provisions are of such essential importance to this Agreement that it is to be reasonably assumed that the Parties would not have entered into this Agreement without the invalid, illegal or unenforceable provisions.
14.7 Headings. The captions to the Sections hereof are not a part of this Agreement, but are merely for convenience to assist in locating and reading the several Sections hereof.
14.8 Waiver of Rule of Construction. Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, the rule of construction that any ambiguity in this Agreement shall be construed against the drafting Party shall not apply.
14.9 Interpretation. Except where the context expressly requires otherwise, (a) the use of any gender herein shall be deemed to encompass references to either or both genders, and the use of the singular shall be deemed to include the plural (and vice versa), (b) the words “include”, “includes” and “including” shall be deemed to be followed by the phrase “without limitation” and shall not be interpreted to limit the provision to which it relates, (c) the word “will” shall be construed to have the same meaning and effect as the word “shall”, (d) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (e) any reference herein to any Person shall be construed to include the Person’s successors and assigns, (f) the words “herein”, “hereof” and “hereunder”, and words of similar import, shall be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (g) all references herein to Sections, Exhibits or Schedules shall be construed to refer to Sections, Exhibits or Schedules of this Agreement, and references to this Agreement include all Exhibits and Schedules hereto, (h) the word “notice” means notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement, (i) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging), (j) references to any specific law, rule or regulation, or article, section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement or successor law, rule or regulation thereof, and (k) the term “or” shall be interpreted in the inclusive sense commonly associated with the term “and/or.”
14.10 No Implied Waivers; Rights Cumulative. No failure on the part of Alnylam or Genzyme to exercise, and no delay in exercising, any right, power, remedy or privilege under
Confidential
- 73 -
this Agreement, or provided by statute or at Law or in equity or otherwise, shall impair, prejudice or constitute a waiver of any such right, power, remedy or privilege or be construed as a waiver of any breach of this Agreement or as an acquiescence therein, nor shall any single or partial exercise of any such right, power, remedy or privilege preclude any other or further exercise thereof or the exercise of any other right, power, remedy or privilege.
14.11 Notices. All notices which are required or permitted hereunder shall be in writing and sufficient if delivered personally, sent by facsimile (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), sent by nationally-recognized overnight courier or sent by registered or certified mail, postage prepaid, return receipt requested, addressed as follows:
| If to Alnylam, to: |
▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ Attention: Vice President - Legal Facsimile No.: (▇▇▇) ▇▇▇-▇▇▇▇ | |
| With a copy to: | WilmerHale LLP ▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ Attention: ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇, Esq. Facsimile No.: (▇▇▇) ▇▇▇-▇▇▇▇ | |
| If to Genzyme, to: | Genzyme Corporation ▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ Attention: President of Rare Disease Facsimile No.: (▇▇▇) ▇▇▇-▇▇▇▇ | |
| With a copy to: | Genzyme Corporation ▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ Attention: General Counsel Facsimile: (▇▇▇) ▇▇▇-▇▇▇▇ | |
| And to: | Ropes & ▇▇▇▇ LLP Prudential Tower ▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇ Attention: ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇, Esq. Facsimile No.: (▇▇▇) ▇▇▇-▇▇▇▇ | |
or to such other address as the Party to whom notice is to be given may have furnished to the other Party in writing in accordance herewith. Any such notice shall be deemed to have been
Confidential
- 74 -
given: (a) when delivered if personally delivered or sent by facsimile on a business day (or if delivered or sent on a non-business day, then on the next business day); (b) on receipt if sent by overnight courier; and/or (c) on receipt if sent by mail.
14.12 Compliance with Export Regulations. Neither Party shall export any technology licensed to it by the other Party under this Agreement except in compliance with U.S. export Laws and regulations.
14.13 Force Majeure. Neither Party shall be held liable to the other Party nor be deemed to have defaulted under or breached this Agreement for failure or delay in performing any obligation under this Agreement to the extent that such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party, potentially including embargoes, war, acts of war (whether war be declared or not), insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, fire, floods, or other acts of God. The affected Party shall notify the other Party of such force majeure circumstances as soon as reasonably practical, and shall promptly undertake all reasonable efforts necessary to cure such force majeure circumstances.
14.14 Independent Contractors. It is expressly agreed that Alnylam and Genzyme shall be independent contractors and that the relationship between Alnylam and Genzyme shall not constitute a partnership, joint venture or agency. Alnylam shall not have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on Genzyme, without the prior written consent of Genzyme, and Genzyme shall not have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on Alnylam without the prior written consent of Alnylam.
14.15 Counterparts. The Agreement may be executed in two or more counterparts, including by facsimile or PDF signature pages, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
14.16 Performance by Affiliates.
14.16.1 Each Party acknowledges and accepts that the other Party may exercise its rights and perform its obligations under this Agreement either directly or through one or more of its Affiliates. A Party’s Affiliates will have the benefit of all rights (including all licenses) of such Party under this Agreement. Accordingly, in this Agreement “Genzyme” will be interpreted to mean “Genzyme and/or its Affiliates” and “Alnylam” will be interpreted to mean “Alnylam and/or its Affiliates” where necessary to give each Party’s Affiliates the benefit of the rights provided to such Party in this Agreement; provided, however, that in any event each Party will remain responsible for the acts and omissions, including financial liabilities, of its Affiliates.
14.16.2 Notwithstanding Section 14.16.1 or anything to the contrary in this Agreement, in the event of (a) an acquisition of a Party or its business or assets by a Third Party (an “Acquirer”) or (b) an acquisition by a Party of the business or assets of a Third Party that includes any program(s) of the acquired Third Party that, but for this Section 14.16.2, would violate Section 10.5.1, which program(s) represent less than [**] percent ([**]%) of the value of
Confidential
- 75 -
the acquired business or assets (an “Acquired Business”), in each case of (a) and (b) whether by merger, asset purchase or otherwise, then (i) as to any such Acquirer, the non-acquired Party shall not obtain rights, licenses, options or access to any Patent Rights, Know-How or products that are held by the Acquirer or any Affiliate of the Acquirer that becomes an Affiliate of the acquired Party as a result of such acquisition and that were not generated through any use or access to the Know-How or Patent Rights of the acquired Party and are not used by the acquired Party in connection with a Licensed Product; and (ii) as to any such Acquirer or Acquired Business, the Acquirer or Acquired Business and any Affiliate of the Acquirer or Acquired Business that becomes an Affiliate of the acquired or acquiring Party as a result of such acquisition shall not be subject to the restrictions in Section 10.5.1 as to programs for products of that such Acquirer, Acquired Business or Affiliate of such Acquirer or Acquired Business in existence prior to the closing date of such acquisition, or for the subsequent development and commercialization of such products following the closing date of such acquisition.
14.17 Binding Effect; No Third Party Beneficiaries. As of the Effective Date, this Agreement shall be binding upon and inure to the benefit of the Parties and their respective permitted successors and permitted assigns. Except as expressly set forth in this Agreement, no person or entity other than the Parties and their respective Affiliates and permitted assignees hereunder shall be deemed an intended beneficiary hereunder or have any right to enforce any obligation of this Agreement.
[THE REMAINDER OF THIS PAGE HAS BEEN LEFT INTENTIONALLY BLANK]
Confidential
- 76 -
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the Effective Date.
| GENZYME CORPORATION | ALNYLAM PHARMACEUTICALS, INC. | |||||||
| BY: | /s/ ▇▇▇▇▇ ▇▇▇▇▇▇ |
BY: | /s/ ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇ | |||||
| NAME: | ▇▇▇▇▇ ▇▇▇▇▇▇, M.D. | NAME: | ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇, Ph.D | |||||
| TITLE: | President and Chief Executive Officer | TITLE: | Chief Executive Officer | |||||
Confidential
Signature page to License and Collaboration Agreement
by and between Alnylam Pharmaceuticals, Inc. and Genzyme Corporation,
dated as of October 18, 2012
SCHEDULE 1.4
ADDITIONAL ALNYLAM IN-LICENSES
| • | The License Agreement between Cancer Research Technology Ltd. and Alnylam, dated July 18, 2003. |
| • | The agreement between the Board of Trustees of the ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ Junior University and Alnylam, dated September 17, 2003. |
Confidential
SCHEDULE 1.7
▇▇▇-▇▇▇▇▇
▇▇▇-▇▇▇▇▇ is an investigational ribonucleic acid interference (RNAi) therapeutic agent that is comprised of active pharmaceutical ingredient ALN-[**] (see sequence & diagram below), a synthetic small interfering RNA (siRNA) that is targeted to the TTR messenger RNA (mRNA) formulated in the AF-011 lipid nanoparticle (see description below).
[**]
AF-011 lipid nanoparticle Description:
AF-011 is a multi-component particle comprised of:
MC3/DSPC/Cholesterol/PEG-DMG (descriptions & diagrams below) in the following ratio:
50%/10%/38.5%/1.5%
MC3
(6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino)butanoate
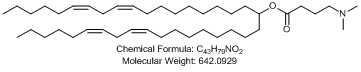
CAS Name: (10Z,13Z)-1-(9Z,12Z)-9,12-octadecadien-1-yl-10,13-nonadecadien-1-yl-4 (dimethylamino)-butanoic acid ester
CAS Registry Number: 1224606-06-7
DSPC
1,2-Distearoyl-sn-glycero-3-phosphatidylcholine
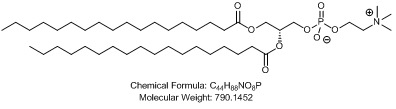
CAS Name: 1,2-Dioctadecanoyl-sn-glycero-3-phosphocholine
Confidential
CAS Registry Number: 816-94-4
Cholesterol
CAS Name: (3b)-Cholest-5-en-3-ol
CAS Registry Number: 57-88-5
PEG-DMG (mPEG2000-DMG; mPEG-DOMG)
(R)-2,3-bis(tetradecyloxy)propyl 1-(methoxy poly(ethylene glycol)2000)propylcarbamate

a-[3-[[[(2R)-2,3-Bis(tetradecyloxy)propoxy]carbonyl]amino]propyl]-w-(2-methoxyethoxy)- poly(oxy-1,2-ethanediyl) polymer
CAS Formula: (C2 H4 O)n C38 H77 N O6
CAS Registry Number: 1301751-57-4
Confidential
SCHEDULE 1.8
ALN-TTRsc
ALN-TTRsc is an investigational ribonucleic acid interference (RNAi) therapeutic agent that is comprised of active pharmaceutical ingredient ALN-[**] (see sequence & diagram below), a synthetic small interfering RNA (siRNA) that is targeted to the TTR messenger RNA (mRNA) and covalently linked on the 3’ end of the sense strand to a triantennary N-acetylgalactosamine (GalNAc3) ligand (see diagram below).
[**]
[**]
Confidential
SCHEDULE 1.9
ALNYLAM CORE TECHNOLOGY PATENTS
See attached.
Confidential
1.9 Lipid-formulation
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
Confidential materials omitted and filed separately with the Securities and Exchange Commission. A total of 18 pages were omitted. [**]
Confidential
1.9 Lipid-formulation
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
Confidential
1.9 Lipid-formulation
| CaseNumber | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] | ||||||||||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | ||||||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| CaseNumber | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] | ||||||||||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
Confidential
1.9 Linker-Conjugate
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
Confidential
1.9 Manufacturing
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
Confidential
1.9 PBL
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] |
Confidential
1.9 PBL
| DocketNumber |
Country |
Application/Patent Number |
Application Date |
Title |
Issue Date | |||||
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 29 pages were omitted. [**]
Confidential
SCHEDULE 1.16
ALNYLAM PRODUCT-SPECIFIC PATENTS
See attached.
Confidential
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | ||||||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
Confidential
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | ||||||||||
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
| [**] | ||||||||||||||||||||
| Our Ref. No. | Country | Case Type |
Status | Application No. |
Filing Date |
Publication No. |
Patent No. |
Title | Alnylam Rights |
In License | ||||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||||||
Confidential
SCHEDULE 1.47
EXISTING ALNYLAM IN-LICENSES
A. As of the Effective Date, Existing Alnylam In-Licenses includes the following Third Party agreements:
| 1. | Amended and Restated Strategic Collaboration and License Agreement between Isis Pharmaceuticals, Inc. and Alnylam Pharmaceuticals, Inc., dated April 28, 2009, as amended by letter agreement dated August , 2012. |
| 2. | Co-Exclusive License Agreement between ▇▇▇ ▇▇▇▇▇▇ Innovation GmbH (formerly Garching Innovation GmbH) and Alnylam Pharmaceuticals, Inc., dated December 20, 2002, as amended by Amendment dated July 2, 2003, the Requirements Amendment effective June 15, 2005, the Waiver Amendment effective August 9, 2007 and the Amendment to the Alnylam Co-Exclusive License Agreement dated as of March 14, 2011, by and between Alnylam Pharmaceuticals, Inc., on the one hand, and ▇▇▇▇▇▇▇▇▇ Institute for Biomedical Research, Massachusetts Institute of Technology and Max-▇▇▇▇▇▇-Innovation GmbH, on the other hand; and Co-Exclusive License Agreement between ▇▇▇ ▇▇▇▇▇▇ Innovation GmbH (formerly Garching Innovation GmbH) and Alnylam Europe AG (formerly Ribopharma AG), dated June 30, 2003. |
| 3. | Amended and Restated Cross-License Agreement between Alnylam Pharmaceuticals, Inc. and Protiva Biotherapeutics Inc., effective May 30, 2008, as amended by Amendment No. 1 effective October 25, 2010. |
| 4. | Amended and Restated License and Collaboration Agreement between Tekmira Pharmaceuticals Corporation and Alnylam Pharmaceuticals, Inc., effective May 30, 2008. |
| 5. | Sponsored Research Agreement among Alnylam Pharmaceuticals, Inc., The University of British Columbia, and AlCana Technologies, Inc., dated July 27, 2009, as amended by Amendment No. 1 dated July 27, 2011, and as supplemented by the Supplemental Agreement among Alnylam Pharmaceuticals, Inc., Tekmira Pharmaceuticals Corporation, Protiva Biotherapeutics Inc., The University of British Columbia, and AlCana Technologies, Inc., effective July 27, 2009. |
Confidential
SCHEDULE 1.52
FIRST PHASE II SUCCESS OBJECTIVES
[**]
Confidential
SCHEDULE 2.2.1
GLOBAL DEVELOPMENT STRATEGY
See attached.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 2 pages were omitted. [**]
| Confidential |

|
SCHEDULE 2.2.2
ALNYLAM TERRITORY DEVELOPMENT PLAN
See attached.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 5 pages were omitted. [**]
| Confidential |

|
SCHEDULE 2.2.3
GENZYME TERRITORY DEVELOPMENT PLAN
(To be attached when developed pursuant to Section 2.2.3.)
SCHEDULE 6.1
SUPPLY AGREEMENT TERMS
| 1. | Overview. Both the Clinical Supply Agreement and the Commercial Supply Agreement will provide for the manufacture and supply of Bulk Drug Substance, Bulk Drug Product, and/or Finished Product forms of Licensed Products (each a “Product” and collectively “Products”) on commercially reasonable terms customary to Third Party contract manufacturing organization supply agreements for pharmaceuticals that are consistent with the principles set forth below. Genzyme may elect, on the terms to be set forth in one or more Clinical Supply Agreements and one or more Commercial Supply Agreements to be negotiated by the Parties, which shall be consistent with the principles set forth in the Agreement and in this Schedule 6.1, for Alnylam to supply, either itself or through an Affiliate or Third Party manufacturer (including, in cases where a Third Party manufacturer is being used, facilitating arrangements where Genzyme may order directly from the Third Party manufacturer in lieu of ordering through Alnylam, if appropriate), any Product that Alnylam is manufacturing or having manufactured for the Alnylam Territory. Genzyme may also elect for Alnylam to provide technology transfer on the terms set forth in Section 6.2 of the Agreement. |
| a. | “Bulk Drug Substance” means an siRNA Product in bulk form manufactured for use as an active pharmaceutical ingredient in a Licensed Product. For the avoidance of doubt, (a) as to ALN-TTR02, Bulk Drug Substance is siRNA duplex AD [**], which may be separately manufactured and therefore may be separately supplied to Genzyme by Alnylam under a Supply Agreement, and (b) as to ALN-TTRsc, Bulk Drug Substance is siRNA duplex AD [**] conjugated to a GalNAc Conjugate. |
| b. | “Bulk Drug Product” means a formulated Bulk Drug Substance, in bulk form prior to filling and finishing. For the avoidance of doubt, (a) as to ALN-TTR02, Bulk Drug Product is siRNA duplex AD [**] formulated in an AF▇▇ ▇▇▇▇▇ ▇anoparticle Formulation, and (b) as to ALN-TTRsc, Bulk Drug Product is siRNA Duplex AD [**] conjugated to a Ga1NAc Conjugate, in water for injection. |
| c. | “Finished Product” means a Licensed Product in Bulk Drug Product form, filled into unit packages including final labeling and packaging. |
| 2. | Assumptions. This Schedule 6.1 is based on the assumption that Alnylam will be performing or managing the manufacture and supply of Product for use and sale throughout the Alnylam Territory. If Alnylam is not sourcing a Product for the Alnylam Territory, Alnylam shall not be obligated 2to establish a supply chain solely for Genzyme’s benefit, but in such case Alnylam will cooperate with Genzyme’s efforts to establish its own supply chain as set forth in Section 6.2 of the Agreement. The Parties also assume that Alnylam’s Cost of Goods for Bulk Drug Product will not be greater than |
| $[**] per gram from [**] and not greater than $[**] per gram from [**] and thereafter. If these assumptions are not correct, the Parties will re-evaluate the applicable terms set forth herein. Notwithstanding the foregoing, Alnylam makes no guarantee as to Alnylam’s Cost of Goods and, unless otherwise agreed by the Parties in an applicable Clinical Supply Agreement or Commercial Supply Agreement, the Supply Price shall be determined as described below. For the avoidance of doubt, the intent of the Parties is that any Clinical Supply Agreement or Commercial Supply Agreement that the Parties enter into in which Alnylam may be obligated to supply Genzyme will be structured so that Alnylam does not bear any liability to Genzyme for a Third Party manufacturer’s failure to meet its obligations beyond the actual amounts (net of Alnylam’s enforcement costs), if any, recovered by Alnylam from such Third Party for such failure. |
| 3. | Supply of Licensed Product. |
| a. | Supply Obligation. At Genzyme’s election, Alnylam will supply Bulk Drug Substance, Bulk Drug Product, and/or Finished Product to Genzyme in accordance with the Supply Agreement(s) to be negotiated by the Parties consistent with the principles set forth in this Schedule 6.1. |
| b. | Supply Price. |
| i. | Genzyme shall pay to Alnylam a per unit “Supply Price” with respect to supply of Product, which Supply Price will be equivalent to the following: |
| 1. | Product that Alnylam Does Not Manufacture Itself. Any Product manufactured by Alnylam’s Third Party manufacturers and supplied by Alnylam to Genzyme will be supplied at a price equal to the Product’s Cost of Goods plus [**]%. |
| 2. | Product that Alnylam Does Manufacture Itself. Any Product manufactured by Alnylam or its Affiliates and supplied to Genzyme will be supplied at a price equal to the Product’s Cost of Goods plus [**]%. |
Agreement Mechanics. The Supply Agreement will contain a mechanism by which the Parties determine and agree upon a per unit Supply Price for each Product for the upcoming Calendar Year (“Estimated Supply Price”). At the end of each Calendar Year, the Parties will compare the Estimated Supply Prices with the Supply Prices as determined by Alnylam’s actual Cost of Goods for the Products during such Calendar Year (the “Actual Supply Price”). Genzyme or Alnylam, as applicable, will thereafter make a payment to the other in order to reconcile the Estimated Supply Price paid with the Actual Supply Price during that Calendar Year. The Supply Agreement will also contain appropriate audit provisions, including to verify the amounts and calculations described in this Section.
| ii. | “Cost of Goods” means, with respect to the supply of Product, the reasonable internal and external costs Alnylam and its Affiliates incurred |
| in Manufacturing or having Manufactured such Product, including: (a) to the extent that such Product is Manufactured by Alnylam or its Affiliates, the fully allocated cost of Manufacture of such Product, consisting of direct material and direct labor costs, plus Manufacturing overhead attributable to such Product (including facilities start-up costs, all directly incurred Manufacturing variances and a reasonable allocation of related Manufacturing administrative and facilities costs and depreciation for such Product, but excluding corporate administrative overhead and/or costs associated with excess capacity), all calculated strictly in accordance with GAAP, and (b) to the extent that such Product is Manufactured by a Third Party manufacturer, the actual fees paid by Alnylam and its Affiliates to the Third Party for the Manufacture, supply and packaging of such Product. |
| c. | Genzyme Territory Process Development Costs. If Genzyme requests any change to the specifications or Manufacturing process for the supply of Product for the Genzyme Territory, Alnylam will use Commercially Reasonable Efforts to make such changes, and if the changes are not also required for the Alnylam Territory, Genzyme will bear one hundred percent (100%) of such costs. |
| d. | Forecasts. On a Calendar Quarterly basis Genzyme will provide rolling [**] Calendar Quarter forecasts for its orders of supply of Product. The first [**] Calendar Quarters of any forecast will constitute a firm obligation for Genzyme to issue purchase orders for the forecasted amount of Product. Notwithstanding the foregoing, to the extent that Alnylam is supplying through a Third Party manufacturer, Genzyme shall be required to comply with the forecasting and ordering provisions of Alnylam’s agreement with the Third Party manufacturer. |
| e. | Specifications. The Supply Agreements will contain mutually agreed written specifications for the Bulk Drug Substance, Bulk Drug Product and Finished Product, as applicable. |
| 4. | Third Party Manufacturing Agreements. The Clinical Supply Agreement(s) and Commercial Supply Agreement(s) shall include provisions (a) requiring Alnylam to reasonably consult with Genzyme through the JSC regarding proposed supply agreements to be entered into between Alnylam and Third Party Manufacturers to the extent Products to be supplied to Genzyme will be manufactured under such Third Party supply agreements; and (b) requiring Alnylam to use commercially reasonable efforts to include in applicable supply agreements with Third Party manufacturers rights for Genzyme to enforce such agreements against such Third Parties as such agreements relate to the supply of Product(s) for the Genzyme Territory, including by identifying Genzyme as a third party beneficiary under such agreements, and if Alnylam is not able to negotiate such third party beneficiary rights for Genzyme’s benefit, provisions requiring Alnylam to enforce such Third Party supply agreements at Genzyme’s direction and expense as such agreements relate to the supply of the applicable Product(s) for the Genzyme Territory. |
| 5. | Term and Termination. The Clinical Supply Agreement will continue in effect through the completion of Clinical Studies in the Genzyme Territory. The Commercial Supply Agreement will have a term of at least three (3) years with yearly renewals at Genzyme’s option term for two (2) additional years, each such renewal option to be exercised by providing Alnylam at least twelve (12) months written notice prior to the expiration of the then-current term. The Supply Agreements will contain customary termination provisions. Genzyme will have the right to terminate each Supply Agreement for convenience on at least twelve (12) months advanced written notice. Alnylam will have the right to terminate each Supply Agreement on at least twelve (12) months advanced written notice (or such longer period of time as reasonably necessary to avoid a supply disruption) if Alnylam determines to cease sourcing the applicable Product for the Alnylam Territory, but in such case Alnylam will cooperate with Genzyme to enable Genzyme to establish its own source for the Product (including, to the extent requested by Genzyme and within Alnylam’s ability to do so, by transferring Alnylam’s applicable Third Party manufacturing relationships to Genzyme). The Supply Agreements will terminate immediately upon termination of the Agreement. Notwithstanding the foregoing, to the extent that Alnylam is supplying through a Third Party manufacturer, Genzyme’s termination rights may be subject to limitations on termination set forth in Alnylam’s agreement with the Third Party manufacturer. Unless otherwise agreed by the Parties, following termination Genzyme shall remain obligated to purchase any orders of Product that have become firm orders prior to termination. |
| 6. | Compliance, Warranties, Acceptance, Recalls, Indemnification and Limitations of Liability. The Supply Agreements will contain terms and conditions regarding compliance with Laws (including cGMPs and the Regulatory Approvals for the Licensed Products) and specifications for the Licensed Products, delivery, acceptance, recalls, indemnification and limitations of liability that are customary in Third Party contract manufacturing agreements. In any event, Alnylam shall not be required to provide warranties or indemnification that are more extensive than Third Party contract manufacturers typically provide and, with respect to Products not manufactured by Alnylam, Alnylam shall not have obligations to Genzyme under any Supply Agreement for such Product that are greater than the obligations that Alnylam’s supplier of such Product has to Alnylam. |
| 7. | Shortages, Inventory, Backup Suppliers. The Supply Agreements will provide that in the event Alnylam is unable to supply Product to meet the demand in the combined Alnylam Territory and Genzyme Territory, then it will allocate Product between the two Territories on a pro rata basis based on forecasts of demand in the two Territories existing at the time of the shortfall. The Supply Agreements may also contain provisions requiring Alnylam to maintain backup suppliers and appropriate levels of Product inventory as agreed by the Parties; provided that, Alnylam may require that Genzyme purchase safety stock of Product in exchange for Alnylam’s agreement to maintain such safety stock in inventory. |
| 8. | Miscellaneous. The Supply Agreements will contain other customary terms and provisions for agreements of their type as mutually agreed by the Parties. |
SCHEDULE 7.4.3.2
BASEBALL ARBITRATION PROCEDURE REGARDING JPAC/LP MILESTONE
ALLOCATION
Selection of Expert and Submission of Positions. The Parties shall select and agree upon a mutually acceptable independent Third Party expert who is neutral, disinterested and impartial, and has experience relevant to the valuation of pharmaceutical and biotechnology industry license agreements (the “Expert”). If the Parties are unable to mutually agree upon an Expert within [**] days following the delivery of the Expert Resolution Notice, then upon request by either Party, the Expert shall be an arbitrator appointed by Judicial and Mediation Services (“JAMS”), which arbitrator need not have the above-described experience. Once the Expert has been selected, each Party shall within [**] days following selection of the Expert provide the Expert and the other Party with a written report setting forth its position with respect to the substance of the dispute and may submit a revised or updated report and position to the Expert within [**] days of receiving the other Party’s report. If so requested by the Expert, each Party shall make oral submissions to the Expert based on such Party’s written report, and each Party shall have the right to be present during any such oral submissions.
JAMS Supervision. In the event the Expert is a JAMS arbitrator selected by JAMS as provided in this Schedule 7.4.3.2 above, the matter shall be conducted as a binding arbitration in accordance with JAMS procedures, as modified by this Schedule 7.4.3.2 (including that the arbitrator shall adopt as his or her decision the position of one Party or the other, as described below). In such event, the arbitrator may retain a Third Party expert with experience relevant to the valuation of pharmaceutical and biotechnology industry license agreements to assist in rendering such decision, and the expenses of any such expert shall be shared by the Parties as costs of the arbitration as provided in this Schedule 7.4.3.2 below.
Determination by the Expert. The Expert shall, no later than [**] days after the last submission of the written reports and, if any, oral submissions, select one of the Party’s positions as his or her final decision, and shall not have the authority to modify either Party’s position or render any substantive decision other than to so select the position of either Genzyme or Alnylam as set forth in their respective written report (as initially submitted, or as revised in accordance with this Schedule 7.4.3.2 above, as applicable). The Parties agree that the decision of the Expert shall be the sole, exclusive and binding remedy between them regarding any the allocation of JPAC/LP Milestone payments, and the Expert’s decision shall become the decision of the JSC on the matter.
Location; Costs. Unless otherwise mutually agreed upon by the Parties, the in-person portion (if any) of such proceedings shall be conducted in Boston, Massachusetts. The Parties agree that they shall share equally the costs and fees of the Expert in connection with any proceeding under this Schedule 7.4.3.2, including the cost of the arbitration filing and hearing fees, the cost of any independent expert retained by the arbitrator and the cost of the arbitrator and administrative fees of JAMS if applicable. Each Party shall bear its own costs and attorneys’ and witnesses’ fees and associated costs and expenses incurred in connection with any proceeding under this Schedule 7.4.3.2.
Timetable for Completion in [**] Days. The Parties shall use, and shall direct the Expert to use, commercially reasonable efforts to resolve a dispute within [**] days after the selection of the Expert, or if resolution within [**] days is not reasonably achievable, as determined by the Expert, then as soon thereafter as is reasonably practicable.
SCHEDULE 9.2.2(A)
JOINT PRESS RELEASE

| Contacts: | ||
| Alnylam Pharmaceuticals, Inc. ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Vice President, Investor Relations and Corporate Communications ▇▇▇-▇▇▇-▇▇▇▇ |
Genzyme ▇▇▇▇ ▇▇▇▇▇▇ Director Corporate Communications ▇▇▇-▇▇▇-▇▇▇▇ | |
| ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ (Media) Spectrum ▇▇▇-▇▇▇-▇▇▇▇ ▇▇▇▇▇ |
||
Alnylam and Genzyme Form Alliance to Develop and Commercialize
RNAi Therapeutics in Asia
- Genzyme to Advance ALN-TTR02 and ALN-TTRsc Programs as Breakthrough Therapies for
Patients with ATTR in Japan and in the Broader Asian Market -
- Alnylam to Receive $22.5 Million in Upfront Payment in Addition to Milestone Payments
and Royalties on Product Sales;
Alnylam Maintains All Rights in U.S., Europe, and Rest of World -
Cambridge, Mass., October 22, 2012, 2012 - Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY) and Genzyme, a Sanofi company (EURONEXT: SAN and NYSE: SNY), announced today that they have formed an exclusive alliance to develop and commercialize RNAi therapeutics targeting transthyretin (TTR) for the treatment of transthyretin-mediated amyloidosis (ATTR) in Japan and other Asia-Pacific countries. ATTR is a rare, debilitating, hereditary disease that damages the nervous system and heart, resulting in a life expectancy of 5 to 15 years.
“Our ALN-TTR program holds promise as a breakthrough therapy for the treatment of ATTR, a debilitating orphan disease. As the lead program in our ‘Alnylam 5x15’ product strategy, we also view this program as a key part of building Alnylam for the future,” said ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, Ph.D., Chief Executive Officer of Alnylam. “In this important collaboration, Genzyme will advance our ALN-TTR program with their proven capabilities in the Japanese and broader Asian market, while we maintain our plans to develop and commercialize this potential breakthrough medicine in the U.S., Europe, and rest of world. In addition, a key part of the value proposition in this alliance for Alnylam is the potential for significant royalty payments on sales of products.”
Confidential
ATTR is an endemic disease in Japan, with a significant number of patients carrying the V30M TTR mutation which leads to onset of a severe form of ATTR known as familial amyloidotic polyneuropathy (FAP). Together, Alnylam and Genzyme intend to maximize the value of ALN-TTR worldwide by developing the program in FAP and other ATTR indications, such as familial amyloidotic cardiomyopathy (FAC) and senile systemic amyloidosis (SSA). Alnylam’s ALN-TTR program currently includes ALN-TTR02, which is in a Phase II clinical trial, and ALN-TTRsc, a subcutaneously administered RNAi therapeutic in late stage pre-clinical development.
Under the terms of the agreement, Genzyme will make an upfront cash payment of $22.5 million to Alnylam. The agreement also includes development milestone payments and tiered royalties expected to yield an effective rate in the mid-teens to mid-twenties on Genzyme’s sales of ALN-TTR products in their territory. In addition, each party will be responsible for the development and commercialization activities in their respective territories.
“We are encouraged by Alnylam’s progress with their ALN-TTR program and are excited by the potential for this innovative drug candidate to make a difference in the lives of patients with ATTR. The results to date demonstrate impressive clinical activity and support advancement of this promising therapeutic into pivotal studies and toward the market,” said ▇▇▇▇▇ ▇▇▇▇▇▇, M.D., President and Chief Executive Officer of Genzyme. “As we work to build our pipeline through both internal research and development, and through external collaborations, we look forward to working with Alnylam on this important program.”
Recently, Alnylam presented positive clinical results from its ALN-TTR02 Phase I trial demonstrating robust and unprecedented knockdown of serum TTR protein levels of up to 94%; the overall results were highly significant (p<0.00001 by ANOVA). Suppression of TTR, the disease-causing protein in ATTR, was found to be rapid, dose dependent, durable, and specific after just a single dose. The drug was generally safe and well tolerated in this Phase I study. Alnylam is currently enrolling patients in a Phase II multi-dose study of ALN-TTR02 in ATTR patients and aims to initiate a Phase III pivotal study of ALN-TTR02 by the end of 2013.
About ATTR
Transthyretin (TTR)-mediated amyloidosis (ATTR) is a hereditary, systemic disease caused by mutations in the TTR gene. TTR protein is produced primarily in the liver and is normally a carrier for thyroid hormones and retinol binding proteins. Mutations in TTR cause abnormal amyloid proteins to accumulate and damage body organs and tissue, such as the peripheral nerves and heart, resulting in intractable peripheral sensory neuropathy, autonomic neuropathy, and/or cardiomyopathy. ATTR represents a major unmet medical need with significant morbidity and mortality; FAP affects approximately 10,000 people worldwide and FAC affects at least 40,000 people worldwide. FAP patients have a mean life expectancy of five to 15 years from symptom onset, and the only treatment options for early stage disease are liver transplantation and tafamidis (approved in Europe). As a result, there is a significant need for novel therapeutics to treat patients who have inherited mutations in the TTR gene.
About RNA Interference (RNAi)
RNAi (RNA interference) is a revolution in biology, representing a breakthrough in understanding how genes are turned on and off in cells, and a completely new approach to drug discovery and development. Its discovery has been heralded as “a major scientific breakthrough that happens once every decade or so,” and represents one of the most promising and rapidly advancing frontiers in biology and drug discovery today which was awarded the 2006 Nobel Prize for Physiology or Medicine. RNAi is a natural process of gene silencing that occurs in organisms ranging from plants to mammals. By harnessing the natural biological process of RNAi occurring in our cells, the creation of a major new class of medicines, known as RNAi therapeutics, is on the horizon. Small interfering RNA (siRNA), the molecules that mediate RNAi and comprise Alnylam’s RNAi therapeutic platform, target the cause of diseases by potently silencing specific mRNAs, thereby preventing disease-causing proteins from being made. RNAi therapeutics have the potential to treat disease and help patients in a fundamentally new way.
About Alnylam Pharmaceuticals
Alnylam is a biopharmaceutical company developing novel therapeutics based on RNA interference, or RNAi. The company is leading the translation of RNAi as a new class of innovative medicines with a core focus on RNAi therapeutics for the treatment of genetically defined diseases, including ALN-TTR for the treatment of transthyretin-mediated amyloidosis (ATTR), ALN-AT3 for the treatment of hemophilia, ALN-PCS for the treatment of severe hypercholesterolemia, ALN-HPN for the treatment of refractory anemia, and ALN-TMP for the treatment of hemoglobinopathies. As part of its “Alnylam 5x15TM” strategy, the company expects to have five RNAi therapeutic products for genetically defined diseases in clinical development, including programs in advanced stages, on its own or with a partner by the end of 2015. Alnylam has additional partnered programs in clinical or development stages, including ALN-RSV01 for the treatment of respiratory syncytial virus (RSV) infection, ALN-VSP for the treatment of liver cancers, and ALN-HTT for the treatment of Huntington’s disease. The company’s leadership position on RNAi therapeutics and intellectual property have enabled it to form major alliances with leading companies including Merck, Medtronic, Novartis, Biogen Idec, Roche, Takeda, Kyowa Hakko Kirin, Cubist, Ascletis, Monsanto and Genzyme. In addition, Alnylam and Isis co-founded Regulus Therapeutics Inc., a company focused on discovery, development, and commercialization of microRNA therapeutics; Regulus has formed partnerships with GlaxoSmithKline, Sanofi, AstraZeneca and Biogen Idec. Alnylam has also formed Alnylam Biotherapeutics, a division of the company focused on the development of RNAi technologies for applications in biologics manufacturing, including recombinant proteins and monoclonal antibodies. Alnylam’s VaxiRNA™ platform applies RNAi technology to improve the manufacturing processes for vaccines; GlaxoSmithKline is a collaborator in this effort. Alnylam scientists and collaborators have published their research on RNAi therapeutics in over 100 peer-reviewed papers, including many in the world’s top scientific journals such as Nature, Nature Medicine, Nature Biotechnology, and Cell. Founded in 2002, Alnylam maintains headquarters in Cambridge, Massachusetts. For more information, please visit ▇▇▇.▇▇▇▇▇▇▇.▇▇▇.
Alnylam Forward-Looking Statements
Various statements in this release concerning Alnylam’s future expectations, plans and prospects, including without limitation, statements regarding Alnylam’s views with respect to the
potential for RNAi therapeutics, including the potential for ALN-TTR02 and ALN-TTRsc, its expectations regarding the receipt upfront, and potential development milestone and royalty payments under the Genzyme agreement, its expectations regarding the market opportunity for ALN-TTR, including in Japan, its expectations with respect to the timing and success of its clinical trials for ALN-TTR02, including the possible initiation of a Phase III pivotal trial, and the expected timing of an IND filing for ALN-TTRsc, and Alnylam’s expectations regarding its “Alnylam 5x15” product strategy, constitute forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including, without limitation, Genzyme’s ability to successfully advance ALN-TTR02 and/or ALN-TTRsc in Japan and other Asian countries including China, Australia, and India, as well as Alnylam’s ability to develop ALN-TTR02 and/or ALN-TTRsc in the rest of the world, resulting in the potential payment of development milestones and royalties to Alnylam, Alnylam’s ability to successfully demonstrate the efficacy and safety of its drug candidates, the pre-clinical and clinical results for these product candidates, which may not support further development of such product candidates, actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials for such product candidates, obtaining, maintaining and protecting intellectual property, obtaining regulatory approval for products, competition from others using technology similar to Alnylam’s and others developing products for similar uses, and Alnylam’s ability to establish and maintain strategic business alliances, including its collaboration with Genzyme, and new business initiatives, as well as those risks more fully discussed in the “Risk Factors” section of its most recent quarterly report on Form 10-Q on file with the Securities and Exchange Commission. In addition, any forward-looking statements represent Alnylam’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Alnylam does not assume any obligation to update any forward-looking statements.
About Genzyme, a Sanofi Company
Genzyme has pioneered the development and delivery of transformative therapies for patients affected by rare and debilitating diseases for over 30 years. We accomplish our goals through world-class research and with the compassion and commitment of our employees. With a focus on rare diseases and multiple sclerosis, we are dedicated to making a positive impact on the lives of the patients and families we serve. That goal guides and inspires us every day. Genzyme’s portfolio of transformative therapies, which are marketed in countries around the world, represents groundbreaking and life-saving advances in medicine. As a Sanofi company, Genzyme benefits from the reach and resources of one of the world’s largest pharmaceutical companies, with a shared commitment to improving the lives of patients. Learn more at ▇▇▇.▇▇▇▇▇▇▇.▇▇▇.
Sanofi Forward Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar
expressions. Although Sanofi’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group’s ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment policies and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2011. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
SCHEDULE 10.2
DISCLOSURE SCHEDULE
Section 10.2.1:
- Reference is made to certain claims alleged by the plaintiffs in the action entitled:
[**]
- Reference is made to certain claims alleged by the plaintiff in the action entitled:
[**]
Section 10.2.2:
- Reference is made to certain claims alleged by the plaintiffs in the [**] Litigation.
- Reference is made to certain claims alleged by the plaintiff in the [**] Litigation.
Section 10.2.4:
- Reference is made to certain claims alleged by the plaintiffs in the [**] Litigation.
Section 10.2.6:
- Reference is made to certain claims alleged by the plaintiffs in the [**] Litigation.
- Reference is made to certain claims alleged by the plaintiff in the [**] Litigation.
Section 10.2.7:
- Reference is made to certain claims alleged by the plaintiffs in the [**] Litigation.
- Reference is made to certain claims alleged by the plaintiff in the [**] Litigation.
Section 10.2.8:
- Reference is made to certain claims alleged by the plaintiffs in the [**] Litigation.
Section 10.2.11:
- Reference is made to certain claims alleged by the plaintiffs in the [**] Litigation.
Confidential
Section 10.2.12(a):
- Reference is made to certain claims alleged by the plaintiffs in the [**] Litigation.
- Reference is made to certain claims alleged by the plaintiff in the [**] Litigation.
Section 10.2.12(b):
- Reference is made to the [**].
Confidential
AMENDMENT TO LICENSE AND COLLABORATION AGREEMENT
THIS AMENDMENT TO LICENSE AND COLLABORATION AGREEMENT (this “Amendment”) is entered into as of February 19, 2013 (the “Amendment Effective Date”), by and between ALNYLAM PHARMACEUTICALS, INC., a Delaware corporation (“Alnylam”) and GENZYME CORPORATION, a Massachusetts corporation (“Genzyme”).
BACKGROUND
A. Alnylam and Genzyme are parties to a License and Collaboration Agreement dated as of October 18, 2012 (the “Collaboration Agreement”).
B. Following the Effective Date, Alnylam resolved certain disputes regarding intellectual property and technology that relate to the subject matter of the Collaboration Agreement.
C. As part of that resolution, Alnylam terminated the Amended and Restated Cross-License Agreement between Alnylam and Protiva Biotherapeutics Inc., (“Protiva”) effective May 30, 2008, as amended by Amendment No. 1 effective October 25, 2010 (the “Protiva License”) and the Amended and Restated License and Collaboration Agreement between Tekmira Pharmaceuticals Corporation (“Tekmira”) and Alnylam effective May 30, 2008 (the “Tekmira Collaboration Agreement”), and Alnylam, Protiva and Tekmira terminated, solely as among and between each other, the Sponsored Research Agreement among Alnylam, The University of British Columbia (“UBC”), and AlCana Technologies, Inc. (“AlCana”), dated July 27, 2009, as amended by Amendment No. 1 dated July 27, 2011, and as supplemented by the Supplemental Agreement among Alnylam, Tekmira, Protiva, UBC, and AlCana, effective July 27, 2009 (collectively with the Protiva License and the Tekmira Collaboration Agreement, the “Terminated In-Licenses”).
D. In replacement of the Terminated In-Licenses, Alnylam, Tekmira and Protiva entered into a Cross-License Agreement dated as of November 12, 2012 (the “New Cross License”).
E. Alnylam and Genzyme now wish to amend the Collaboration Agreement as set forth in this Amendment.
AMENDMENT
Alnylam and Genzyme hereby agree, and the Collaboration Agreement is hereby amended, as of the Amendment Effective Date, as follows:
1. Definitions from the Collaboration Agreement. Initially capitalized terms used but not defined in this Amendment shall have the meanings ascribed to them in the Collaboration Agreement.
2. Deletion of Section 1.41 of the Collaboration Agreement. Section 1.41 of the Collaboration Agreement is hereby amended and restated in its entirety to read as follows: “1.41 Reserved.”
3. Deletion of Section 11.5 of the Collaboration Agreement. Section 11.5 of the Collaboration Agreement is hereby amended and restated in its entirety to read as follows: “11.5 Reserved.”
4. Amendment to Schedule 1.47 (Existing Alnylam In-Licenses). Schedule 1.47 of the Agreement is hereby amended to read in its entirety as set forth in the Schedule 1.47 attached to this Amendment.
5. Amendment to Schedule 1.9 (Alnylam Core Technology Patents). Schedule 1.9 is hereby amended to reflect that the Patent Rights identified on Schedule 1.9 as the “▇▇▇▇▇▇/▇▇▇▇▇▇▇” Patent Rights are licensed to Alnylam pursuant to the Sublicense Agreement dated January 8, 2007 between Alnylam Pharmaceuticals, Inc. and Tekmira Pharmaceuticals Corporation (as successor in interest to Inex Pharmaceuticals Corporation), rather than pursuant to the Tekmira Amended and Restated Cross-License, as originally indicated.
6. No Loss of Rights; Incremental Amounts. Alnylam hereby represents and warrants that the termination of the Terminated In-Licenses and the entry into the New Cross License in replacement thereof has not resulted in any Loss of Rights and Alnylam continues to Control all Know-How and Patent Rights licensed to Alnylam under the Terminated In-Licenses that are necessary or useful for Genzyme to Develop, Manufacture and/or Commercialize Licensed Products in the Genzyme Territory. In addition, Alnylam hereby represents and warrants that the aggregate amount of Third Party License Payments required to be paid under the New Cross License does not exceed, in duration or amount, the amount that Alnylam would have been required to pay under the Terminated In-Licenses (had they not been terminated) based on Alnylam’s assertions in the Ongoing Litigation, or, alternatively, Alnylam agrees that to the extent it does exceed such amount, such excess shall be Incremental Amounts for all purposes of the Collaboration Agreement. Alnylam represents and warrants that it has provided Genzyme with an accurate and complete and unredacted copy of the New Cross License.
7. Future Termination of In-Licenses. Following the Amendment Effective Date, neither Party will voluntarily amend or terminate an In-License to which it is a party in a manner that terminates any rights sublicensed to the other Party that are necessary or useful for the other Party to Develop, Manufacture and/or Commercialize Licensed Products under the Collaboration Agreement with respect to the other Party’s Territory, without the prior written consent of the other Party.
8. Miscellaneous. As amended hereby, the Collaboration Agreement remains in full force and effect.
[SIGNATURE PAGE FOLLOWS]
2
IN WITNESS WHEREOF, the Parties have by duly authorized persons executed this Amendment as of the Amendment Effective Date.
| ALNYLAM PHARMACEUTICALS, INC.: | GENZYME CORPORATION: | |||||||
| By: | /s/ ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇ | By: | /s/ ▇▇▇▇▇ ▇▇▇▇▇▇ | |||||
| Title: Chief Executive Officer
Date: February 19, 2013 |
Title: President & Chief Executive Officer
Date: February 19, 2013 | |||||||
3
SCHEDULE 1.47
EXISTING ALNYLAM IN-LICENSES
Existing Alnylam In-Licenses includes the following Third Party agreements:
1. Amended and Restated Strategic Collaboration and License Agreement between Isis Pharmaceuticals, Inc. and Alnylam Pharmaceuticals, Inc., dated April 28, 2009, as amended by letter agreement dated August 27, 2012.
2. Co-Exclusive License Agreement between ▇▇▇ ▇▇▇▇▇▇ Innovation GmbH (formerly Garching Innovation GmbH) and Alnylam Pharmaceuticals, Inc., dated December 20, 2002, as amended by Amendment dated July 2, 2003, the Requirements Amendment effective June 15, 2005, the Waiver Amendment effective August 9, 2007 and the Amendment to the Alnylam Co-Exclusive License Agreement dated as of March 14, 2011, by and between Alnylam Pharmaceuticals, Inc., on the one hand, and ▇▇▇▇▇▇▇▇▇ Institute for Biomedical Research, Massachusetts Institute of Technology and Max-▇▇▇▇▇▇-Innovation GmbH, on the other hand; and Co-Exclusive License Agreement between ▇▇▇ ▇▇▇▇▇▇ Innovation GmbH (formerly Garching Innovation GmbH) and Alnylam Europe AG (formerly Ribopharma AG), dated June 30, 2003.
3. Sponsored Research Agreement among Alnylam Pharmaceuticals, Inc., The University of British Columbia, and AlCana Technologies, Inc., dated July 27, 2009, as amended by Amendment No. 1 dated July 27, 2011, and as supplemented by the Supplemental Agreement among Alnylam Pharmaceuticals, Inc., Tekmira Pharmaceuticals Corporation, Protiva Biotherapeutics Inc., The University of British Columbia, and AlCana Technologies, Inc., effective July 27, 2009.
4. Sublicense Agreement dated January 8, 2007 between Alnylam Pharmaceuticals, Inc. and Tekmira Pharmaceuticals Corporation (as successor in interest to Inex Pharmaceuticals Corporation).
5. Cross-License Agreement dated November 12, 2012 among Alnylam Pharmaceuticals, Inc., Tekmira Pharmaceuticals Corporation, and Protiva Biotherapeutics Inc.
4

