ASSIGNMENT AGREEMENT
Exhibit 10.3
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
THIS ASSIGNMENT AGREEMENT (the “Agreement”) is made effective as of August 20, 2012 (the “Effective Date”), by and between ACLARIS THERAPEUTICS, INC., a Delaware corporation, having an address of ▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ (“Assignee”), and ▇▇▇▇▇▇ ▇ ▇▇▇▇▇▇, ▇▇, of ▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇, ▇▇▇▇. #▇▇▇, ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇▇, as Personal Representative of the estate of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ (“▇▇▇▇▇▇ Estate”).
BACKGROUND
1. Mickey ▇▇▇ ▇▇▇▇▇▇ (▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I) was an inventor of certain inventions relating to high-concentration hydrogen peroxide and its use for certain dermatological conditions, and developed certain related data and know-how and obtained certain patents relating to the inventions;
2. ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I is deceased and, as personal representative for his estate, ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ represents that the. ▇▇▇▇▇▇ Estate holds all of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ Ps rights to such patent rights, data, and know-how;
3. Assignee is interested in acquiring rights to such patent rights, data, and know-how; and
4. ▇▇▇▇▇▇ Estate is willing to assign to Assignee, and Assignee is willing to purchase from ▇▇▇▇▇▇ Estate, such patent rights, data and know-how, all on the terms and conditions more particularly set forth below.
5. Prior to the Effective Date, the probate court having jurisdiction has determined that ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ and ▇▇▇▇▇▇ ▇▇▇▇ are the sole heirs of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I and his estate. Each such heir is signing, in his personal capacity, the Consent of Heir that is set forth in Exhibit C to this Agreement, in which each acknowledges ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ s right to sign for the estate, and consents to and agrees not to challenge the transaction contemplated by this Agreement, as more particularly provided for in the Consent of Heir. ▇▇▇▇▇▇ Estate shall deliver such Consents of Heir to Assignee within five (5) days after the Effective Date of this Agreement.
AGREEMENT
NOW, THEREFORE, in consideration of the foregoing premises and the covenants and obligations set forth in this Agreement, the Parties (defined below) hereby agree as follows:
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
ARTICLE 1
DEFINITIONS
As used herein, the following terms have the following meanings (with derivative forms being interpreted accordingly) and the words “include,” “including” and derivative forms of them shall be deemed followed by the phrase “without limitation”:
1.1 “$” and “Dollars” means United States dollars.
1.2 “Affiliate” means, with respect to a given legal entity, any other entity that, directly or indirectly, through one or more intermediaries, controls, is controlled by or is under common control with such first legal entity. For this purpose, “control” shall mean the ownership of fifty percent (50%) or more of the voting securities entitled to elect the directors or management of the entity, or the actual power to elect or direct the management or policies of the entity by law, contract, or otherwise.
1.3 “Business Day” means any Monday, Tuesday, Wednesday, Thursday or Friday that is not a national, statutory holiday in the United States.
1.4 “Claims” means, with respect to a particular item or product and a particular issued patent, that such issued patent claims the composition of such item or product or any of its ingredients or formulations; a method of making or using it or them; or an item used or present in the manufacture of such item or product (including chemical intermediates); such that, in each case, in the absence of ownership of a patent or a license granted thereunder, such item or product or its manufacture or use as and where actually practiced would infringe a Valid Claim of such issued patent.
1.5 “Confidential Information” means, subject to the limitations set forth in Section 8.1: (i) all information received by ▇▇▇▇▇▇ Estate, counsel to ▇▇▇▇▇▇ Estate, or any of the ▇▇▇▇▇▇ Estate Group pursuant to the Prior CDA or pursuant to this Agreement from Assignee, any person or entity who negotiated for the rights under this Agreement prior or on behalf of Assignee (including without limitation, Sciaderm, Inc., a Pennsylvania corporation and KPT Consulting, LLC), or any of the owners, investors and/or prospective investors of any of them; (ii) the Transferred Know-How; and (iii) the existence and terms of this Agreement and nature of the Products and the intellectual property assigned under this Agreement.
1.6 “Control” means, with respect to a particular item of Know-How or Patent, that the applicable Party has ownership of or a license to and has the ability to grant to the other Party access to and a license or sublicense under such Know-How or Patent.
1.7 “FDA” means the United States Food and Drug Administration, and any successor thereto.
1.8 “IND” means an Investigational New Drug Application as defined in the United States Food, Drug and Cosmetic Act and applicable regulations promulgated thereunder by the FDA or the equivalent application to the equivalent agency in any other country or group of
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
countries, the filing of which is necessary to commence clinical testing of Product in humans in a particular jurisdiction.
1.9 “Indication” means treatment, prophylaxis or diagnosis of any and all dermatological indications, including any and all diseases and conditions of the skin, whether or not mentioned, claimed or covered in the Transferred Patents as of the Effective Date, and whether or not a sub-indication of, or condition or symptom related to, those dermatological indications that are mentioned, claimed or covered in the Transferred Patents as of the Effective Date.
1.10 “Know-How” means any and all data, instructions, processes, methods, formulae, materials, expert opinions, inventions (whether or not patentable), biological materials (including cell lines, vectors and their progeny and derivatives), know-how, and information (including biological, chemical, pharmacological, toxicological, pharmaceutical, physical, analytical, clinical, safety, manufacturing and quality control data).
1.11 “Licensee” means any entity to which Assignee or an Affiliate of Assignee grants a license under the Transferred Patents and/or Transferred Know-How to make, have made, use, sell, offer for sale, import and/or export Product. The term “Licensee” also includes the sublicensees of those whom Assignee or its Affiliate has directly licensed under the Transferred Patents and/or Transferred Know-How. The term “Licensee” also includes assignees of the Transferred Patents (or any subset thereof) and their licensees and sublicensees of the Transferred Patents (or any subset thereof).
1.12 “▇▇▇▇▇▇ Estate Group” means ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ and ▇▇▇▇▇▇ ▇▇▇▇ and their respective spouses (if any), and any corporate entities controlled by any of the foregoing people (and/or any combination of them) that Control Technology and/or intellectual property rights in Technology.
1.13 “▇▇▇▇▇▇ I” means ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ who is a named inventor on the Transferred Listed Patents.
1.14 “Net Sales” means the gross revenues actually received by Assignee, or its Affiliates or Licensees, from the sale of Products to Third Parties, less deductions for: (i) transportation and insurance charges; (ii) sales and excise taxes, tax, tariff, duty or any other governmental charges or duties paid; (ii) normal and customary trade, quantity and cash discounts and rebates allowed or granted in whatever form (including those in the form of fees (or reverse fees) provided for in the distribution or selling contract); (iii) allowances on account of rejection or return by customers; (iv) credits, rebates, charge-backs, reimbursements, retroactive price adjustments, or similar payments actually granted or given to wholesalers and other distributors, buying groups, health care insurance carriers, governmental agencies and other institutions; (v) payments or rebates actually paid in connection with state or federal Medicare, Medicaid or similar programs.
To avoid any doubt, sales of Products among Assignee, its Affiliates and Licensees under the Transferred Patents are not taken into account in the calculation of Net Sales, but resales by
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
any of them to Third Parties (but specifically excluding transfers for use in clinical trials and/or provision free of charge as samples or for compassionate use) are taken into account in the calculation of Net Sales. In the case of Third-Party distributors, Net Sales occur on sale to the distributor, not the distributor’s resale.
Notwithstanding the foregoing definition of Net Sales, if Assignee in its agreement with a Licensee will also be receiving sales royalties, and agrees on a different definition of Net Sales with the Licensee that will govern such sales royalties, then Net Sales under this Agreement for purposes of the sales by such Licensee shall have the meaning given in such agreement between Assignee and the Licensee, rather than the definition given above.
1.15 “Party” means Assignee or ▇▇▇▇▇▇ Estate.
1.16 “Patent” means any patent application or patent, including all of the following kinds and their equivalents outside the United States (as applicable): provisional, converted provisional (or regular), divisional, continuation, continuation-in-part, and substitution applications; and regular utility, re-issue, re-examination, renewal and extended patents (including Supplementary Protection Certificates).
1.17 “Prior CDA” means that those confidentiality-related agreements set forth in Exhibit D.
1.18 “Products” means all product candidates and products (a) that includes Technology, (b) the manufacture of which includes Technology, and/or (c) the clinically investigated or Regulatorily Approved use of which includes Technology.
1.19 “Regulatory Agency” means a supranational, regional, federal, state, provincial or other local regulatory agency, department, bureau or other governmental authority with jurisdiction over Regulatory Approvals, including the FDA.
1.20 “Regulatory Approval” means, collectively with respect to a particular jurisdiction, all governmental approvals, product and/or establishment licenses, registrations or authorizations necessary for the manufacture, use, storage, import, export, transport, marketing and sale of a composition as a pharmaceutical product in such jurisdiction.
1.21 “Settlement Agreement” has the meaning given in Section 2.10.
1.22 “Technology” means (a) any composition containing hydrogen peroxide and having utility to treat any Indication (including any and all of the foregoing compositions and mentioned or covered in any Transferred Listed Patent); (b) all pharmaceutical and/or cosmeceutical formulations of such compositions (including reformulations created after the Effective Date by or for Assignee); (c) any method of use and/or delivery of any composition of clause (a) and/or (b) to treat any Indication (including dosing schedules and methods of application); (c) any device used in such a method; and (d) all methods of making any of the foregoing. To avoid doubt, the Technology includes any formulations described in the Transferred Listed Patents as they exist as of the Effective Date of the Agreement, which
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
formulations have previously been tested by or for ▇▇▇▇▇▇ Estate, whether or not such formulations are claimed in such Transferred Listed Patents.
1.23 “Third Party” means any entity or person other than Assignee, ▇▇▇▇▇▇ Estate, an Affiliate of either of them or of any other member of the ▇▇▇▇▇▇ Estate Group.
1.24 “Trademarks” means all Technology-related trademarks and tradenames owned, used or conceived of by ▇▇▇▇▇▇ Estate or any member of ▇▇▇▇▇▇ Estate Group on our before the Effective Date, including the trademark identified in Exhibit B.
1.25 “Transferred Know-How” means all Know-How related to or constituting Technology and developed in whole or in part or owned or Controlled by ▇▇▇▇▇▇ I or ▇▇▇▇▇▇ Estate on or before the Effective Date, including: (a) all preclinical and clinical data generated relating to Technology before the Effective Date by or on behalf of ▇▇▇▇▇▇ I; (b) all manufacturing information regarding the processes for Technology that ▇▇▇▇▇▇ Estate or any member of ▇▇▇▇▇▇ Estate Group has made or tested on or before the Effective Date (including the formula and master batch records for each Technology formulation that may have been tested); (c) such formulations; and (d) all information as to clinical investigators ▇▇▇▇▇▇ Estate knows to be currently (as of the Effective Date) exploring Technology or to have done so in the three (3) years prior to the Effective Date.
1.26 “Transferred Listed Patents” means (a) U.S. Patent Serial Number 7,381,427 and U.S. Patent Serial Number 7,138,146, and those ex-U S filings and U.S. provisional patent applications identified in the next paragraph; (b) all patent applications claiming common priority with or based on the foregoing, including all converted provisional or regular utility filings, divisionals, continuations, continuations-in-part and substitutions of any of the foregoing; (c) all patents issuing on any of the foregoing, and all reissues, reexaminations, renewals and extensions of any of the foregoing; (d) all counterparts to the foregoing in other countries; and (e) all Supplementary Protection Certificates and other similar rights of ▇▇▇▇▇▇ Estate based on any of the foregoing.
The ex-U.S. filings are set forth in Exhibit E-1. The Parties acknowledge that Exhibit E includes ex-U.S. active patents as well as patents and applications that have lapsed. The U.S. provisional patent applications are set forth in Exhibit E-2. The Parties acknowledge that these provisional patent applications have expired.
1.27 “Transferred Patents” means (a) the Transferred Listed Patents; (b) all Patents (currently pending or issued and/or that may be filed in the future) claiming Transferred Know-How, to the extent of any ownership interest therein based on the inventorship interest of any named inventor whose interest ▇▇▇▇▇▇ Estate conveys to Assignee under this Agreement; and (c) all other Patents owned by ▇▇▇▇▇▇ Estate or any member of the ▇▇▇▇▇▇ Estate Group during the term of this Agreement naming or that should properly name ▇▇▇▇▇▇ I as an inventor, and are directed to Technology.
1.28 “Valid Claim” means with respect to any country, a claim of any issued, unexpired patent in that country that has not been held revoked, unenforceable or invalid by a
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
decision of a court or governmental authority of competent jurisdiction, and has not lapsed or been abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue or disclaimer or otherwise.
ARTICLE 2
GRANTS OF RIGHTS
2.1 Assignment. ▇▇▇▇▇▇ Estate hereby irrevocably, perpetually and forever assigns and conveys to Assignee the entire right, title and interest in and to the Transferred Patents and Transferred Know-How, together with all powers, privileges, benefits, causes of action, remedies, and other rights relating, appertaining to and/or associated with the Transferred Patents and Transferred Know-How; provided, however, that such assignment is expressly conditioned upon and will only be effective upon payment of the Upfront Fee described in Section 4.1. Assignee hereby accepts such assignment.
2.2 Specific Rights and Privileges. Without limiting the generality of the assignment in Section 2.1, as owner of the Transferred Patents, Assignee shall have, and the assignment and conveyance pursuant to Section 2.1 includes, the following specific rights and privileges:
(a) Assignee shall have the sole and exclusive right, but not the duty, to file and prosecute pending and future applications within the Transferred Patents worldwide;
(b) Assignee shall have the sole and exclusive right, but not the duty, to maintain and enforce the Transferred Patents worldwide, except as and to the extent explicitly provided in Article 5, as regards the obligation to maintain Transferred Listed Patents that are issued in the U.S. as of the Effective Date;
(c) Assignee shall have the sole and exclusive right, but not the duty, to grant licenses (which licenses may include the right to grant sublicenses) under the Transferred Patents and to collect and retain royalty and/or other payments for such licenses;
(d) Assignee shall have the sole and exclusive right, but not the duty, to ▇▇▇ on the Transferred Patents, and to collect all damages and profits for any past, present and/or future infringements thereof; and
(e) Assignee shall have the sole and exclusive right to sell, assign or otherwise transfer to any other entity or entities any or all of the rights assigned and transferred to Assignee under this Agreement (Assignee must either make such payments as are required under this Agreement or require the assignee to do so).
Except as expressly provided in Article 4, Assignee shall not currently or in the future owe any further consideration to ▇▇▇▇▇▇ Estate for or in respect of Assignee’s exercise of the rights assigned to Assignee hereunder, including any amounts Assignee may collect on licenses it grants under the Transferred Patents; recover by enforcing the Transferred Patents against infringement; and/or receive for the sale or transfer of any of the rights assigned Assignee hereunder.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
2.3 Further Documentation to Perfect and Record. ▇▇▇▇▇▇ Estate shall sign and have notarized the short-form patent assignment document attached hereto as Exhibit A upon execution of this Agreement and/or within 30 days after requested in writing by Assignee. ▇▇▇▇▇▇ Estate shall further execute and deliver to Assignee and/or its representatives all other documents and instruments, to be prepared by Assignee, as Assignee reasonably requests, in order for Assignee to prosecute, perfect, record and/or enforce any of the rights that are granted to it under this Agreement, promptly after requested by Assignee. If Assignee is unable, after making reasonable inquiry, to obtain ▇▇▇▇▇▇ Estate’s signature on any such documents, then if and only if such documents are reasonably necessary due to ▇▇▇▇▇▇ Estate having previously been the assignee of record on the Transferred Patents, ▇▇▇▇▇▇ Estate hereby appoints Assignee as ▇▇▇▇▇▇ Estate’s attorney-in-fact for the sole purpose of executing and delivering such documents, which appointment is coupled with an interest.
2.4 Further Assurances. ▇▇▇▇▇▇ Estate and such inventors shall take reasonable further actions to execute and deliver all further documents that Assignee may reasonably require to further the purpose and intent of this Agreement.
2.5 Transferred Know-How Confidentiality Protection. ▇▇▇▇▇▇ Estate acknowledges that the Transferred Know-How is, as a result of the assignment of this Agreement, the commercially valuable confidential information of Assignee. Accordingly, ▇▇▇▇▇▇ Estate and the other members of the ▇▇▇▇▇▇ Estate Group shall treat such Know-How as the Confidential Information of Assignee. ▇▇▇▇▇▇ Estate acknowledges that, as between ▇▇▇▇▇▇ Estate and Assignee, Assignee shall have the sole right to file, prosecute, maintain and enforce Patents. Claiming Transferred Know-How (but Assignee shall have no obligation to do so).
2.6 Disclosure of Know-How. Commencing within fifteen (15) days after the Effective Date and to be completed over a period of thirty (30) days, ▇▇▇▇▇▇ Estate shall provide to Assignee true, complete and correct copies and/or originals of all tangibly documented Transferred Know-How in existence as of the Effective Date (including reports of all relevant preclinical and clinical data, to the extent not already provided to Assignee prior to the Effective Date) and to the extent not prohibited by the Settlement Agreement, or court order, and all laboratory notebooks or journals kept by ▇▇▇▇▇▇ I (if any) relating to Technology and/or its invention and/or development.
If at any time after such disclosure is believed complete, ▇▇▇▇▇▇ Estate discovers additional documentation of Transferred Know-How, it shall promptly transfer such documentation to Assignee.
2.7 Assignment of Trademarks. ▇▇▇▇▇▇ Estate hereby irrevocably, perpetually and forever assigns and conveys to Assignee all of ▇▇▇▇▇▇ Estate’s right, title and interest throughout the world in and to: (a) the Trademarks; (b) all renewals and extensions for registrations included in the Trademarks; and (c) all benefits, privileges, causes of action and remedies relating to or conferred by any of the foregoing, whether accrued before or after the Effective Date. Such benefits, privileges, causes of action and remedies include the exclusive rights to apply for and maintain all such registrations, renewals and/or extensions; to ▇▇▇ for all past, present or future infringements or other violations of any rights in the Trademark; and to settle and retain proceeds
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
from any such actions. Neither ▇▇▇▇▇▇ Estate nor any other member of the ▇▇▇▇▇▇ Estate Group retains any rights to use or to display the Trademarks. ▇▇▇▇▇▇ Estate and the other members of the ▇▇▇▇▇▇ Estate Group shall not challenge the validity of Assignee’s ownership in the Trademarks. ▇▇▇▇▇▇ Estate and all members of the ▇▇▇▇▇▇ Estate Group each hereby further agrees to execute and deliver all documents and instruments required to evidence or record such assignment or to enforce the assigned rights (and hereby appoints Assignee as ▇▇▇▇▇▇ Estate’s attorney-in-fact to execute and deliver such documents if unable after making reasonable inquiry to obtain. ▇▇▇▇▇▇ Estate’s signatures on any of them).
2.8 Rights of Reference. To the extent relevant, necessary or useful to support Assignee’s (and its Affiliates’ and the Licensees’) Product activities, Assignee (and its Affiliates and Licensees) shall have the right to reference and the right to access all INDs of ▇▇▇▇▇▇ Estate relating to Technology and in existence as of the Effective Date, if any.
2.9 Technology-Related Agreements. Based on counsel’s review of client files for ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ (I) and ▇▇▇▇▇▇ Estate, ▇▇▇▇▇▇ Estate indicates that as of the Effective Date it is not aware of any Third-Party agreements in effect between ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ (I), ▇▇▇▇▇▇ Estate, or any corporation controlled by either of them that relate to Technology and/or the disclosure thereof, other than the Settlement Agreement referred to in Section 2.10 and confidentiality agreements listed in Exhibit D. If any such agreement comes to the attention of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ (through counsel or otherwise), ▇▇▇▇▇▇ Estate shall promptly disclose the applicable agreement to Assignee and as and to the extent requested by Assignee in writing either assign such agreement to Assignee or if it is not assignable then reasonably cooperate to afford Assignee the benefits of such agreement at Assignee’s cost (meaning that Assignee would cover any related out-of-pocket costs of such cooperation on a pass-through basis; ▇▇▇▇▇▇ Estate will obtain approval of expenditures in advance so that Assignee can elect not to pursue the matter if the costs outweighed the benefits in its opinion).
2.10 Counsel Authorization and Instructions. One or more of the Assigned Listed Patents in existence as of the Effective Date has previously been the subject of a litigation relating to ownership. [***] (“Settlement Agreement”). As the new owner of the Assigned Listed Patents, Assignee’s interests are aligned with ▇▇▇▇▇▇ Estate’s interest in such litigation, and under the Settlement Agreement. As the new owner of the Assigned Listed Patents, Assignee may need to confer with counsel who represented ▇▇▇▇▇▇ I in the settled litigation, engage with such counsel, and obtain files and documentation in such counsel’s possession. ▇▇▇▇▇▇ Estate hereby authorizes all of the foregoing and agrees to provide any other written authorization that such counsel may require (including conflict waivers, if applicable) for the foregoing. The same shall apply with respect to transactional counsel to ▇▇▇▇▇▇ I for the Settlement Agreement, if different than such litigation counsel. Furthermore, it is understood and agreed that all papers and documentation relating to such litigation and/or any legal advice received in connection with it, that ▇▇▇▇▇▇ Estate has in its possession or has the ability to access other than through public records, shall be included in the transfer of documentation by ▇▇▇▇▇▇ Estate to Assignee under Section 2.6 to the extent not prohibited by the Settlement Agreement or court order. Without imposing any Settlement Agreement obligations on Assignee, ▇▇▇▇▇▇ Estate shall upon request assign the benefits of the Settlement Agreement to Assignee, and reasonably assist Assignee in any necessary enforcement of such Settlement Agreement.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
ARTICLE 3
DEVELOPMENT/COMMERCIALIZATION
3.1 Allocation of Rights for Development and Commercialization. As between the Parties, Assignee shall have the sole right to conduct all additional preclinical and clinical studies of Products, in order to be able to seek Regulatory Approval of and commercialize Products. As between the Parties, Assignee will be solely responsible for the costs of these activities. Assignee shall also have the exclusive right to commercialize Products and be exclusively responsible for the costs of such commercialization. Assignee shall be fully and freely entitled to engage Licensees, contractors and distributors in Product development and commercialization. Assignee shall have full and sole discretion over licensing, intellectual property transactions and use of contractors and distributors. As between the Parties, Assignee shall have the sole right and sole discretion to select (and use and own) the trademarks and tradenames for Products.
3.2 Assignee Responsibilities in Further Development and Commercialization; Diligence. Assignee shall devote Commercially Reasonable Efforts (defined below in this Section) to develop and commercialize at least one (1) Product for at least one (1) Indication for the United States market. Such obligation shall expire on expiration of the last Assigned Patent. To avoid doubt, the first sentence of this Section shall not be read to require Commercially Reasonable Efforts towards development and commercialization of more than one (1) Product, nor towards development and commercialization of that Product for more than one (1) Indication, nor development and commercialization for any market other than the U.S. market.
All development and commercialization activities performed by any Assignee Affiliate(s) and any Licensee(s), contractors and distributors shall inure to the benefit of Assignee for purposes of determining Assignee’s compliance with its obligation under this Section.
In the first four (4) and ten (10) years of the term of this Agreement (respectively), Assignee shall be deemed to have fulfilled its obligations under this Section 3.2 through the fourth (4th) anniversary of the Effective Date, if it files an IND for a Product to treat an Indication within four (4) years after the Effective Date, and Assignee shall be deemed to have fulfilled its obligations under this Section 3.2 through the tenth (10th) anniversary of the Effective Date, if it files an application in the U.S. for Regulatory Approval for a Product to treat an Indication within ten (10) years after the Effective Date.
If Assignee does not achieve either of the foregoing by its corresponding target date, but Assignee can demonstrate through documentary evidence or other competent proof that (i) it has diligently sought to be in a position to do so, (ii) the failure to do so by the corresponding timeline after the Effective Date was not caused by Assignee’s intentional delays but rather was caused by technical, scientific or regulatory events beyond Assignee’s control, and (iii) Assignee has a written plan setting forth specific objectives and goals to advance the research and development of the Product in order to achieve such objectives as soon as otherwise commercially reasonable, then Assignee shall be deemed to be in compliance with its obligations under this Section 3.2 as long as it devotes reasonable efforts to carry out such plan.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
No diligence obligations other than the ones set forth in this Section 3.2 and Section 5.1 (see second paragraph) shall be implied under or in connection with this Agreement, at law or in equity, and Assignee’s diligence obligations in relation to its rights under this Agreement shall be solely as set forth in this Section 3.2 and Section 5.1 (see second paragraph).
“Commercially Reasonable Efforts” means a reasonable level of efforts, commensurate with the efforts that a venture-backed start-up company similarly situated to Assignee would devote to a product of similar potential and having similar commercial advantages and disadvantages as the Product, taking into account all relevant commercial factors, such as, but not limited to: (1) the intellectual property landscape and level of intellectual property exclusivity available for the product, (2) technical, scientific and clinical results and developments, (3) the competitive landscape and maturity of the marketplace, (4) the regulatory framework and hurdles, (5) pricing, (6) cost of goods, and (7) all other similarly relevant commercial factors.
If Assignee is acquired (whether through merger, reverse merger, sale of assets or other fond of transaction) (“M&A Transaction”), or any successor entity under this Agreement undergoes such an event, this Section 3.2 shall survive such acquisition. However, under no circumstances shall the surviving entity or Assignee’s successor under this Agreement be required, in order to be in compliance with this Agreement, to put forth a greater level of effort or conduct more activities or conduct activities on any faster timeline than set forth in Assignee’s development plan for the lead Product as such plan is in effect and approved by Assignee’s Board of Directors immediately prior to the closing of the M&A Transaction.
3.3 Authorization of and Non-Interference with Consulting and/or Advisory Relationships. It is understood and agreed that Assignee may wish to engage one (1) or more of the clinical investigators and/or other collaborators of the inventor on the Transferred Patents in a consulting, advisory, or other contract relationship. Both Parties recognize this may be beneficial for Product progress. ▇▇▇▇▇▇ Estate — to the extent its permission, waiver or other act would be required — hereby agrees that Assignee and any of the inventors may enter into such a relationship, and hereby provides all permissions and waivers and agrees to perform such other acts as may be required to permit this. Assignee will contact ▇▇▇▇▇▇ Estate or its counsel prior to contacting the inventors; but ▇▇▇▇▇▇ Estate and its counsel shall have no veto right, and shall have no intermediary role except as may be mutually agreed by the Parties in the future.
3.4 Non-Competition for Protection of Transferred Trade Secrets and Confidential Information. Recognizing that such activities would necessarily entail use of the Transferred Know-How and/or Confidential Information of Assignee reported to ▇▇▇▇▇▇ Estate in connection with this Agreement, ▇▇▇▇▇▇ Estate hereby covenants that it shall not during the term of this Agreement research, develop, make, have made, offer to sell, sell, import or export any Products. ▇▇▇▇▇▇ Estate hereby acknowledges on behalf of itself that the foregoing covenant is legally enforceable and is reasonable, necessary and appropriate to protect Assignee’s Confidential Information.
3.5 Assistance with Patent Activities. In accordance with Article 2, Assignee has the sole right to file, prosecute, conduct interferences of and enforce the Transferred Patents
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
(including any Patents that may be filed on the Transferred Know-How). ▇▇▇▇▇▇ Estate shall assist, and to the extent within its power shall cause any living inventors named in the Transferred Patents to reasonably assist, Assignee in all of the foregoing activities, promptly upon each reasonable request by Assignee, and at Assignee’s expense (on a pass-through basis with no markup).
3.6 Improvements. It is understood and agreed that the Transferred Patents themselves, including those that may be filed and/or prosecuted to issuance after the Effective Date, are royalty-bearing under this Agreement, and the royalties under this Agreement extend to all Valid Claims of the Transferred Patents, not just those Valid Claims of the Transferred Listed Patents that are issued as of the Effective Date (all of the foregoing at the applicable rates and under the conditions more particularly provided in Section 4.2). Accordingly, if Assignee files on improvements previously made but not filed on by ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ (I), or that he filed on but for which the provisional patent applications have lapsed, but ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ is properly named as an inventor on the patent filing, then the pending claims therein and any resulting Valid Claims shall support a royalty to the extent and at the applicable rate provided in Section 4.2. Furthermore, the presence of any Assignee Technology (defined in the next Section) in (or covering or used by) a Product, including a reformulated Product, shall not negate or lessen the royalty obligations of Section 4.2 for Products covered by the Transferred Patents during their applicable Royalty Terms.
3.7 Assignee Technology. Assignee shall as between the Parties have the right to own all enhancements, improvements, modifications, derivatives and amendments (including Know-How and published patentable or patented inventions) to that Technology that is in existence as of the Effective Date, which enhancements, improvements, modifications, derivatives and amendments are made, conceived, developed, reduced to practice or acquired by or for Assignee (including under any consulting or employment agreement between Assignee or its Affiliate and any inventor on any Transferred Listed Patent) (“Assignee Technology”) and all Patents on the Assignee Technology (the “Assignee Technology Patents”). To avoid doubt, the Assignee Technology Patents are not considered Transferred Patents, and are not royalty-bearing to ▇▇▇▇▇▇ Estate under this Agreement. However, it is understood and agreed that any applicability of the Assignee Technology Patents to Products (including reformulations) that are otherwise royalty-bearing under this Agreement shall not negate or lessen the royalty obligations at the applicable rates and on the conditions set forth in Section 4.2.
ARTICLE 4
FINANCIAL TERMS
4.1 Flat Fees.
(a) Upfront Fee. Assignee shall pay ▇▇▇▇▇▇ Estate a fee equal to Four Hundred Five Thousand Dollars ($405,000). Such amount shall be payable in two (2) installments.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
(i) The first installment, in the amount of Five Thousand Dollars ($5,000) shall be due within three (3) days after the Effective Date. (This amount shall help defray patent filing, prosecution and maintenance expenses incurred since the death of ▇▇▇▇▇▇ ▇▇▇▇▇▇ I.) Within seven (7) days after the Effective Date, the ▇▇▇▇▇▇ Estate shall make the Transferred Know-How (including any and all clinical data in ▇▇▇▇▇▇ Estate’s possession, but excluding patient names and addresses) available for inspection by Assignee at ▇▇▇▇▇▇ Estate’s or its counsel’s premises as described in Section 6.2(1). If within ten (10) days after the Transferred Know-How is made available, Assignee’s inspection shows that the Transferred Know-How (including such data) is to Assignee’s satisfaction, then the second installment shall be due on the timing set forth in the next subsection and this Agreement shall continue in full force and effect. If Assignee’s inspection shows that the Licensed Know-How (including such data) are not to Assignee’s satisfaction, then Assignee shall provide notice within such ten (10) days. In that case, this Agreement shall terminate, ▇▇▇▇▇▇ Estate shall be entitled to retain the five thousand dollar ($5,000) payment already (at that time) made under this Agreement, no further payments shall be due hereunder, ▇▇▇▇▇▇ Estate will retain ownership of the Transferred Patents and Transferred Know-How, and Assignee’s rights in the Transferred Patents and Transferred Know-How shall be fully cancelled.
(ii) The second installment (assuming no termination under Section 4.1(a)(i)), in the amount of Four Hundred Thousand Dollars ($400,000) shall be due on or before August 30, 2012. Time is of the essence regarding the payment installments of the Upfront Fee, and the notice and cure provision of Section 9.2(a) will not apply to such payment; provided, however, that if, for any reason, Assignee has not received its Series A funding by the deadline for paying the second installment of the Upfront Fee, Assignee will have the right, upon giving notice to the ▇▇▇▇▇▇ Estate before such deadline, to extend the deadline for up to two weeks.
(iii) Between the Effective Date and the date that the second installment is due, ▇▇▇▇▇▇ Estate shall not in any way alienate title to the Transferred Patents nor the Transferred Know-How, nor grant any license, lien or other right therein to any other party. ▇▇▇▇▇▇ Estate shall assure that its representations and warranties in Section 6.2 remain true as of the date the second installment is paid or due, otherwise, at Assignee’s option exercisable by written notice, the last sentence of Section 4.1(a)(i) shall fully apply.
(b) Milestone Fee. Assignee shall pay ▇▇▇▇▇▇ Estate a fee equal to two hundred thousand Dollars ($200,000) as a milestone payment within thirty (30) days after the end of the calendar month in which the first human subject is first dosed with a Product in the first human clinical trial sponsored by or on behalf of Assignee, its Affiliate or a Licensee. Such milestone payment shall be payable a maximum of one (1) time only under this Agreement, even if multiple clinical trials of Product are conducted under this Agreement.
(c) Patent Expense Reimbursement. To the extent that expenses incurred by ▇▇▇▇▇▇ Estate or ▇▇▇▇▇▇ ▇▇▇▇▇▇ II since the death of ▇▇▇▇▇▇ ▇▇▇▇▇▇ I for the foreign filing, prosecution, maintenance and revival of Transferred Listed Patents have exceeded five thousand dollars ($5,000), then within thirty (30) days after receiving an invoice from ▇▇▇▇▇▇ Estate itemizing the costs and the date they were incurred, Assignee shall reimburse to ▇▇▇▇▇▇ Estate such costs up to a maximum of five thousand dollars ($5,000) (such that the total recovered costs
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
are the five thousand dollars ($5,000) of the first payment under Section 4.1(a), plus the up to five thousand dollars ($5,000) under this Section 4.1(c)). ▇▇▇▇▇▇ Estate shall provide its invoice under this Section no later than sixty (60) days after the Effective Date. To avoid doubt, the reimburseable expenses under this Section are patent filing, prosecution, maintenance and revival expenses, not probate-related or transactional expenses.
4.2 Royalty Rates. Assignee shall pay ▇▇▇▇▇▇ Estate royalties on Net Sales of Products as follows:
(a) For Net Sales of Products sold to Third Parties in countries where the Product sold is Claimed by an issued Valid Claim of the Transferred Patents, at the rate of [***] of Net Sales. This royalty is due on Net Sales sold during the “Royalty Term” applicable to the particular Product in the particular country, defined as the time from such Product’s receiving Regulatory Approval in such country for the first Indication for which it is approved, until the date that there is no longer any Valid Claim of the Transferred Patents in such country Claiming such Product.
(b) For Net Sales on which no royalty is due per Section 4.2(a) (i.e. Net Sales of Product not Claimed by an issued Valid Claim of the Transferred Patents), but that are sold on or before the fifth (5th) anniversary of Regulatory Approval of the first Product in a given country, Assignee shall pay to ▇▇▇▇▇▇ Estate royalties on Net Sales of Product in such country at the rate of [***] of such Net Sales for so long as (and only for so long as) all of the following apply: (a) there is at least one (1) pending claim of a Transferred Patent that Claims the Product (determined country-by-country), (b) the pending patent claim of the Transferred Patents is no older than five (5) years old (looking to first priority date), and (c) the pending patent claim has not been abandoned, has not lapsed, and has not been finally rejected. This provision shall not be read to imply that Assignee has any further patent filing, prosecution, or maintenance obligation than as set forth in Section 5.1, second paragraph.
(c) If the Net Sale of any Product covered by Sections 4.2(a) or 4.2(b) requires Assignee (or its Affiliate or a Licensee) to make payments to a Third Party(ies) under intellectual property license(s), the aggregate royalties under which exceed [***] of Net Sales of Product, then the excess over [***] in aggregate across all such Third Parties shall be the “Excess Third-Party Royalties,” and with respect to the Net Sales on which Excess Third-Party Royalties are due, Assignee will be entitled to deduct up to [***] of the Excess Third-Party Royalties from the royalty owed by Assignee to ▇▇▇▇▇▇ Estate, but will not be allowed to reduce the royalty owed to ▇▇▇▇▇▇ Estate to below [***] of the royalties that would otherwise have been due to ▇▇▇▇▇▇ Estate in any calendar quarter. Any amounts of Excess Third-Party Royalties that Assignee is unable to credit due to the foregoing [***] limitation on the reduction in ▇▇▇▇▇▇ Estate’s royalties as applied in any calendar quarter shall carry forward to future calendar quarters, subject always to such [***] limitation on the reduction in ▇▇▇▇▇▇ Estate’s royalties as applied in such future calendar quarters.
As examples of how the foregoing clause operates:
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
(X) FIRST EXAMPLE: Assume a country in which a Valid Claim of the Transferred Patents that Claims the Product sold is in force, so that the royalty rate under this Agreement is [***], and Third-Party intellectual property licenses in the country total to [***] of Net Sales. In this example, because the Third-Party royalty obligation is less than [***], Assignee has no right offset any portion of it against the royalty of this Agreement. The royalty paid under this Agreement in this example will be [***], and the Third-Party royalties borne solely by Assignee will also be [***].
(Y) SECOND EXAMPLE: Assume a country in which a Valid Claim of the Transferred Patents that Claims the Product sold is in force, so that the royalty rate under this Agreement is [***], and Third-Party intellectual property licenses in the country total to [***] of Net Sales. In this example, [***] of the Third-Party royalty burden is considered Excess Third-Party Royalties, and so one and [***] is eligible to be offset against the royalty of this Agreement. However, because offsetting the entire [***] against the [***] royalty of this Agreement would reduce the royalty of this Agreement by more than half, only [***] of the Excess Third-Party Royalties can be offset. Accordingly, the royalty paid to ▇▇▇▇▇▇ Estate in this example will be [***], and the Third-Party royalties borne exclusively by licensee will be [***]).
4.3 Recoveries on Infringement of Transferred Patents. In accordance with Sections 2.1 and 2.2, Assignee has the sole right to enforce the Transferred Patents against infringement. Any recoveries on such infringement suits in excess of Assignee’s (or its Affiliate’s or Licensee’s) costs in connection with such infringement suits (including all outside counsel costs and a reasonable allocation of the costs of internal counsel) shall be deemed Net Sales under this Agreement, and shall be deemed sold in a country in which the Product is Claimed by an issued Valid Claim of the Transferred Patents, in the calendar quarter in which the recovery over costs is actually received, and shall bear a royalty under Section 4.2.
4.4 Quarterly Payment Timings. All royalties due under Section 4.2 shall be paid quarterly, on a country-by-country basis, within the following timelines:
(a) If Assignee or its Affiliate is the marketing party for the underlying Product Net Sales, then payment shall be made thirty (30) days after the end of the relevant calendar quarter for which royalties are due, in the case of U.S. Net Sales; the time period shall be sixty (60) days after the end of the relevant calendar quarter for ex-U.S. Net Sales; and
(b) If a Licensee unaffiliated with Assignee is such marketing party, then Assignee shall make the royalty payments due hereunder within ten (10) Business Days after receiving royalties on the same Net Sales from the Licensee.
Payments due under Section 4.3 shall be paid within thirty (30) days after receipt of the underlying funds by Assignee.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
4.5 Royalty Payment Reports. With respect to each calendar quarter, within thirty (30) days after the end of the calendar quarter, Assignee shall provide to ▇▇▇▇▇▇ Estate a written report stating the number and description of all Products sold by or on behalf of Assignee during the relevant calendar quarter; the gross sales associated with such sales; and the calculation of Net Sales on such sales, including the amount of any deduction provided for in the definition of Net Sales in Article 1. The report shall provide all such information on a country-by-country and Product-by-Product basis. The quarterly report will be certified as accurate by a duly authorized officer of Assignee. Beginning with the calendar quarter in which Regulatory Approval is achieved for the first Product, such quarterly reports will be furnished to the ▇▇▇▇▇▇ Estate regardless of whether any Products were shipped during the relevant month or whether any actual payment is owed. The receipt or acceptance by the ▇▇▇▇▇▇ Estate of any report or payment will not prevent the ▇▇▇▇▇▇ Estate from subsequently challenging the validity or accuracy of such report or payment.
4.6 Payment Method. All payments due under this Agreement to ▇▇▇▇▇▇ Estate shall be made by bank wire transfer in immediately available funds to an account designated by ▇▇▇▇▇▇ Estate in writing. Once ▇▇▇▇▇▇ Estate has designated a bank account, it may only be changed on ten (10) Business Days advance written notice, unless Assignee consents to a shorter time frame in writing. All payments hereunder shall be made in the legal currency of the United States of America. For the purposes of payment of the Upfront Fee of Section 4.1(a) and the reimbursement expense of Section 4.1(c), ▇▇▇▇▇▇ Estate designates the trust account of ▇▇▇▇▇▇▇ & Bosco, P.A. as the designated bank account.
4.7 Taxes. Assignee shall be responsible to withhold from payments otherwise to be made to ▇▇▇▇▇▇ Estate under this Agreement any taxes required to be withheld by Assignee under applicable law. If any such taxes are levied on such payments due hereunder (“Withholding Taxes”), Assignee shall (i) deduct the Withholding Taxes from the payment amount, (ii) pay all applicable Withholding Taxes to the proper taxing authority, and (iii) send evidence of the obligation together with proof of tax payment to ▇▇▇▇▇▇ Estate with the next royalty report under Section 4.5.
4.8 Foreign Exchange. If any currency conversion shall be required in connection with the calculation of amounts payable hereunder, such conversion shall be made using the average of the exchange rates for the purchase and sale of U.S. dollars, as reported by Bank of America in San Francisco, California (or its successor entity) on the last business day of the calendar quarter to which such payment pertains. With any payment in relation to which a currency conversion is performed to calculate the amount of payment due, Assignee shall provide to ▇▇▇▇▇▇ Estate a true, accurate and complete copy of the exchange rates used in the calculation.
4.9 Late Payments. Any payment due under this Article 4 that is not paid on or before the date such payment is due shall bear interest at a rate equal to the lesser of: ten percent (10%) per year; or the maximum rate permitted by law, calculated based on the number of days that payment is delinquent until full payment has been made, less a 15 calendar-day grace period in the case of payments under Sections 4.2 and 4.3.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
4.10 Records and Audit. Assignee shall keep (or cause to be kept) complete and accurate records pertaining to Net Sales of Products and the payments due under this Agreement, in sufficient detail to permit ▇▇▇▇▇▇ Estate to confirm the accuracy of all payments due under this Agreement. ▇▇▇▇▇▇ Estate shall have the right, at its expense, to cause an independent, certified public accountant to audit such records as necessary to confirm Assignee’s payments for the preceding year. Such independent, certified public accountant shall be legally bound by written confidentiality and non-use obligations running directly to Assignee. It shall be nationally recognized in the United States. Such audit rights may be exercised no more often than once a year, once only with respect to records regarding any given accounting period, within three (3) years after the year to which such records relate, upon reasonable advance notice to Assignee and during normal business hours. The terms of this Section shall survive any termination or expiration or termination of this Agreement for a period of one (1) year.
In the event that such audit reveals an underpayment by Assignee of the actual amount owed the ▇▇▇▇▇▇ Estate, Assignee will pay the difference, plus interest calculated at the rate of ten percent (10%) per year. If such underpayment is more than ten percent (10%) for any calendar month, Licensee will also reimburse the ▇▇▇▇▇▇ Estate for the cost of such audit. If the audit reveals that Assignee overpaid, then Assignee may credit the overpaid amounts against future payments due hereunder, or require reimbursement of the overpaid amounts within thirty (30) days after the audit.
All books and records relative to Licensee’s obligations hereunder will be maintained by Licensee at Licensee’s address set forth in this Agreement (which will be in the United States) for at least three (3) years after the end of the calendar year to which they relate, including after termination of this Agreement as applicable.
In the case of records held by Assignee’s Licensees, it shall suffice if Assignee obtains an audit right for itself similar to Assignee’s audit right above, and the right to share the results of its own audits with ▇▇▇▇▇▇ Estate; Assignee shall not be required to obtain a direct right for ▇▇▇▇▇▇ Estate to audit a Licensee.
ARTICLE 5
PATENT PROSECUTION, MAINTENANCE AND ENFORCEMENT
5.1 Patent Prosecution, Maintenance and Reports. Assignee shall have the right to prosecute (including by conducting interferences, oppositions, reissues, reexaminations and other similar proceedings), maintain (including the timely payment of all maintenance fees, renewal fees and other applicable fees), and extend the Transferred Patents. All of the foregoing shall be at Assignee’s sole expense. At least once each calendar year, Assignee will provide to the ▇▇▇▇▇▇ Estate an update on the status and plans relating to the prosecution and maintenance of Patents relating to the Technology. In addition, upon at least 15 days’ notice to Assignee, the ▇▇▇▇▇▇ Estate may request a second update during any calendar year, provided, however, that such request may not be made within three months of the previous update.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
Assignee shall have the obligation at its own expense to pay any maintenance fees that come due after the Effective Date on the Transferred Listed Patents that have issued as of the Effective Date in the United States. If Assignee fails to pay the maintenance fees timely and this leads to a loss of a Transferred Listed Patents Valid Claim that had previously issued and would have supported a royalty obligation at the [***] rate under Section 4.2, then as liquidated damages for such failure to maintain, Assignee shall be required to pay such royalty on Net Sales as if the maintenance fee had been paid and the Valid Claim had remained in place. This calculation of liquidated damages shall thereafter occur quarterly, taking account of whether and to what extent any other Valid Claim or pending claim of a Transferred Patent already independently causes royalties to be due (i.e., if another Valid Claim already causes a [***] royalty on the applicable Net Sales, then no liquidated damages shall be due on those Net Sales; if a pending claim causes a [***] royalty on the applicable Net Sales, then an additional [***] royalty shall be due on Net Sales that are subject to the pending claim [***] royalty but would instead have been royalty-bearing at the [***] rate if the lapsed Valid Claim had continued in force).
Other than the foregoing obligation at its own expense to maintain the Transferred Listed Patents that have issued as of the Effective Date in the United States, Assignee shall have no obligation to file, prosecute, maintain or extend any other Transferred Patent, express or implied, at law or in equity.
5.2 Enforcement. Without limiting Assignee’s rights in Article 2, it is understood and agreed that if in connection with any enforcement of Transferred Patents Assignee requests documentation that ▇▇▇▇▇▇ Estate may have in its possession or testimony from ▇▇▇▇▇▇ Estate or any member of ▇▇▇▇▇▇ Estate Group, ▇▇▇▇▇▇ Estate shall reasonably cooperate and assist Assignee in all reasonable ways at Assignee’s expense (meaning that Assignee would cover any related out-of-pocket expenses of such cooperation, including without limitation hotel and travel expenses, on a pass-through basis; provided that the ▇▇▇▇▇▇ Estate will obtain approval of expenditures in advance).
Licensee will fully comply with the patent marking provisions of the patent laws of the United States and any applicable foreign countries, to the extent in accordance with then-customary practices in the pharmaceutical industry for dermatology products, and to the extent in accordance with regulatory requirements relating to labeling of pharmaceutical products.
ARTICLE 6
REPRESENTATIONS AND WARRANTIES
6.1 Reciprocal Representations and Warranties. Each Party hereby represents and warrants to the other Party that as of the Effective Date the representing and warranting Party has the full legal right, power and authority to enter into and perform this Agreement; that this Agreement has been authorized by all requisite action within such representing and warranting Party (in the case of a corporate entity, and all applicable or required legal process to bind the estate of ▇▇▇▇▇▇ I, in the case of ▇▇▇▇▇▇ Estate); and that this Agreement is legally binding upon such representing and warranting Party.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
6.2 ▇▇▇▇▇▇ Estate Representations and Warranties. ▇▇▇▇▇▇ Estate represents and warrants to Assignee as follows:
(a) Sole Owner. Immediately prior to the assignment hereunder becoming effective, ▇▇▇▇▇▇ Estate was the sole and lawful owner of the entire right, title, and interest in and to the Transferred Listed Patents and Transferred Know-How.
(b) No Liens. There are as of the Effective Date no outstanding liens, security interests, pledges, charges, mortgages, restrictions, interests and/or encumbrances burdening any of the Transferred Patents nor the Transferred Know-How.
(c) No Licenses or Encumbrances. ▇▇▇▇▇▇ I and ▇▇▇▇▇▇ Estate each has not granted, expressly or otherwise, any assignment, license or other extension of rights, covenant not to ▇▇▇ or other similar interest or benefit, exclusive or otherwise, to, under or in the Transferred Patents or the Transferred Know-How.
(d) No Inconsistent Agreements. ▇▇▇▇▇▇ I, ▇▇▇▇▇▇ Estate and the ▇▇▇▇▇▇ Estate Group have not executed, and ▇▇▇▇▇▇ Estate further covenants that ▇▇▇▇▇▇ Estate and the ▇▇▇▇▇▇ Estate Group shall not execute, any agreements inconsistent with this Agreement or to the detriment of the Transferred Patents or the Transferred Know-How.
(e) Non-infringement of Third Party Rights. As of the Effective Date to ▇▇▇▇▇▇ Estate’s actual knowledge, after making a review of those files of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I in ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ II’s possession (and/or in the possession of counsel), no published Patents or trade secret rights owned or controlled by a Third Party, would be infringed or misappropriated by the manufacture, use, sale, offer for sale or importation of any Products in topical applications for Indications. ▇▇▇▇▇▇ Estate and the other members of the ▇▇▇▇▇▇ Estate Group have received no written claims relating to any claims of such infringement or misappropriation.
(f) Claims. There are no claims, actions, suits or proceedings commenced or pending, or to ▇▇▇▇▇▇ Estate’s knowledge threatened, against it, ▇▇▇▇▇▇ I, or any other member of the ▇▇▇▇▇▇ Estate Group, as of the Effective Date, that could affect the rights and benefits granted to Assignee under this Agreement. As of the Effective Date, ▇▇▇▇▇▇ Estate has not received verbal or written notice that any third party is challenging or intends to challenge the patentability, validity or ownership of the Transferred Listed Patents, other than those allegations that culminated in Physicians Choice of Arizona Inc. v. ▇▇▇▇▇▇ ▇▇▇▇▇▇, et al., CV2003-020242, in the Superior Court of Maricopa County. All of the claims and allegations giving rise to such case were finally settled in the Settlement Agreement. As of the Effective Date, ▇▇▇▇▇▇ Estate and the other members of the ▇▇▇▇▇▇ Estate Group have no knowledge of prior art relevant to the Transferred Patents not cited in the file wrappers of the Transferred Listed Patents.
(g) Settlement Agreement. The copy of the Settlement Agreement that ▇▇▇▇▇▇ Estate has disclosed to Assignee and its representatives prior to the Effective Date is a true, accurate and complete copy, and nothing has been redacted or omitted therefrom except exactly as indicated by the blackened areas shown in that copy; none of the redacted information in those blackened areas changes the meaning of the remainder of the Settlement Agreement; and
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
there is nothing in such blackened areas that is necessary to disclose in order to make the disclosure of the remainder of the Settlement Agreement not misleading. The Settlement Agreement is in full force and effect as of the Effective Date. ▇▇▇▇▇▇ Estate knows of no breach thereof by any party to such agreement and has received no written notice of such a breach. ▇▇▇▇▇▇ Estate does not know of any loss or diminution of rights that would occur under the Settlement Agreement as a result of the transaction contemplated under this Agreement. There are no payments currently due or due in the future under the Settlement Agreement. ▇▇▇▇▇▇ Estate has the right to assign the benefits of the Settlement Agreement to Licensee in accordance with this Agreement.
(h) Estate Bound and Agreement Approved Through Probate Process. Exhibit F to this Agreement is a true and correct copy of the Letters of Administration issued by the Superior Court of the State of Arizona and appointing Mickey ▇▇▇ ▇▇▇▇▇▇ II as the personal representative of the ▇▇▇▇▇▇ Estate. The ▇▇▇▇▇▇ Estate, and all heirs claiming through such estate, is and are legally bound by this Agreement; A.R.S. § 14-3711 gives ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇, as personal representative of the ▇▇▇▇▇▇ Estate, the same power over the title to property of the ▇▇▇▇▇▇ Estate that an absolute owner would have, which power may be exercised without notice, hearing or order of the court. Exhibit G to this Agreement is a true and correct copy of the Order of Intestacy and Determination of Heirs issued by the Superior Court of the State of Arizona naming ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ and ▇▇▇▇▇▇ ▇▇▇▇ as the sole heirs of the Mickey J, ▇▇▇▇▇▇ I. ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ knows of no people other than himself and ▇▇▇▇▇▇ ▇▇▇▇ who are claiming to be heirs under the ▇▇▇▇▇▇ Estate, and knows of no one who has challenged or is planning to challenge the probate court finding that he and ▇▇▇▇▇▇ ▇▇▇▇ are the sole heirs of the estate or that has challenged or is planning to challenge the ▇▇▇▇▇▇ Estate’s ownership as of the Effective Date (and before the effects of this Agreement are carried out) of the assets transferred to Assignee under this Agreement. Fully and properly signed Consents of Heir from each of the two (2) heirs shall be delivered to Assignee within five (5) days after the Effective Date.
(i) Third-Party Activities; Grounds. As of the Effective Date and to ▇▇▇▇▇▇ Estate’s actual knowledge without any special enquiry, there are no (i) activities by Third Parties that would constitute infringement or misappropriation of the Transferred Listed Patents (in the case of pending claims, evaluating them as if issued), nor (ii) grounds currently existing on which any claims, actions, suits or proceedings might be commenced against ▇▇▇▇▇▇ Estate or Assignee with respect to the manufacture, use or sale of Products for Indications and/or practice of the Transferred Listed Patents.
(j) Patents. The Transferred Listed Patents are the only Patents that ▇▇▇▇▇▇ Estate or any other member of the ▇▇▇▇▇▇ Estate Group owns or Controls, as of the Effective Date, that claim or are directed to Technology.
(k) Trademarks. Exhibit B contains a complete list of all trademarks that ▇▇▇▇▇▇ Estate or any other member of the ▇▇▇▇▇▇ Estate Group owns or Controls, as of the Effective Date, that are associated with Technology or have been registered for use with Technology. (It is acknowledged by Assignee that Exhibit B lists no trademarks.)
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
(l) Data. Within seven (7) days after the Effective Date, ▇▇▇▇▇▇ Estate will disclose to Assignee (which disclosure may occur by making available for inspection at ▇▇▇▇▇▇ Estate’s premises or the premises of ▇▇▇▇▇▇ Estate’s counsel) all data and information (including preclinical and clinical data and information) generated by, disclosed to and/or known to ▇▇▇▇▇▇ Estate or any other member of the ▇▇▇▇▇▇ Estate Group regarding Technology and any information required to fairly and accurately interpret such data and information and make ▇▇▇▇▇▇ Estate’s disclosures thereof to Assignee complete, accurate and not misleading; provided that such disclosure does not violate the terms of the Settlement Agreement, any other agreement set forth in Exhibit D or any court order. It is understood that ▇▇▇▇▇▇ Estate is entitled to redact patient names and addresses so as to ensure that such disclosure does not violate the terms of the Settlement Agreement. ▇▇▇▇▇▇ Estate represents and warrants that the disclosures under this Section shall be true and accurate in all material respects, and shall not omit to disclose any information known to ▇▇▇▇▇▇ Estate (other than patient names and addresses) necessary to make the information that is disclosed to Assignee under this Section complete, accurate and not misleading.
(m) No Debarment. In the course of developing Technology and any products based on it, to ▇▇▇▇▇▇ Estate’s actual knowledge without any special enquiry, ▇▇▇▇▇▇ Estate and ▇▇▇▇▇▇ Estate’s predecessors-in-interest has and have not engaged any person who has been debarred by the FDA or to ▇▇▇▇▇▇ Estate’s knowledge is the subject of debarment proceedings by the FDA.
(n) Affiliates of ▇▇▇▇▇▇ Estate. As of the Effective Date, ▇▇▇▇▇▇ Estate has no Affiliates and the ▇▇▇▇▇▇ Estate Group does not have any Affiliates holding rights to any Technology.
6.3 ▇▇▇▇▇▇ Estate Covenants. ▇▇▇▇▇▇ Estate hereby covenants that, without limiting Assignee’s right set forth elsewhere in this Agreement (including in Section 6.2(d)), ▇▇▇▇▇▇ Estate and the other members of the ▇▇▇▇▇▇ Estate Group shall not purport to convey to any Third Party any Transferred Patent and/or Transferred Know-How.
6.4 Disclaimer of Warranties. EXCEPT FOR THE REPRESENTATIONS AND WARRANTIES EXPLICITLY SET FORTH IN SECTIONS 6.1 AND 6.2 EACH OF ASSIGNEE AND ▇▇▇▇▇▇ ESTATE HEREBY EXPRESSLY DISCLAIMS ALL REPRESENTATIONS AND WARRANTIES, EXPRESS, STATUTORY OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY, NON-INFRINGEMENT OR FITNESS FOR A PARTICULAR PURPOSE.
ARTICLE 7
INDEMNIFICATION
7.1 Indemnification by Assignee. Assignee shall indemnify, hold harmless and defend ▇▇▇▇▇▇ Estate, the other members of the ▇▇▇▇▇▇ Estate Group, and their respective officers, directors, members, employees and agents (the “▇▇▇▇▇▇ Estate Indemnitees”) from and against any and all losses, damages, liabilities, judgments, fines, amounts paid in settlement, expenses
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
and costs of defense (including reasonable attorneys’ fees and witness fees) (collectively “Losses”) resulting from any demand, claim, action or proceeding brought or initiated by a Third Party (each a “Third-Party Claim”) against any ▇▇▇▇▇▇ Estate Indemnitees(s) to the extent that such Third-Party Claim arises out of (i) the breach or alleged breach of any representation, warranty or covenant by Assignee in this Agreement; (ii) the negligence or willful misconduct of any Assignee Indemnitee (defined in Section 7.2); or (iii) the development, manufacture, storage, handling, use, sale, offer for sale, import, export or distribution of Products by or for Assignee and its Affiliates and Licensees on or after the Effective Date; provided that (a) the ▇▇▇▇▇▇ Estate Indemnitees comply with the procedure set forth in Section 7.3; and (b) such indemnity shall not apply to the extent ▇▇▇▇▇▇ Estate has an indemnification obligation pursuant to Section 7.2 for such Loss. To avoid doubt, Third-Party Claims shall exclude and claims brought by heirs of the estate of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I amongst themselves and/or against the estate.
7.2 Indemnification by ▇▇▇▇▇▇ Estate. ▇▇▇▇▇▇ Estate shall indemnify, hold harmless and defend Assignee, its Affiliates, the Licensees, the investors in Assignee, Sciaderm, Inc. and the investors in Sciaderm, Inc., and the respective officers, directors, employees and agents of each of the foregoing (the “Assignee Indemnitees”) from and against any and all Losses resulting from any Third-Party Claim(s) against any Assignee Indemnitee(s) to the extent that such Third-Party Claim(s) arises out of (i) the breach or alleged breach of any representation, warranty or covenant by ▇▇▇▇▇▇ Estate in this Agreement; (ii) the negligence or willful misconduct of any ▇▇▇▇▇▇ Estate Indemnitee; or (iii) disputes amongst the heirs of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I; provided that (a) the Assignee Indemnitees comply with the procedure set forth in Section 7.3; and (b) such indemnity shall not apply to the extent Assignee has an indemnification obligation pursuant to Section 7.1 for such Loss.
7.3 Mechanics. A Party whose Assignee Indemnitee or ▇▇▇▇▇▇ Estate Indemnitee is entitled to be indemnified pursuant to this Article 7 (the “Indemnified Party”) shall give prompt notice of the Third Party Claim to the other Party (the “Indemnifying Party”) and the Indemnifying Party shall defend against such Third Party Claim with the reasonable cooperation of the Indemnified Party; provided that the Indemnifying Party shall not settle any such Third-Party Claim for anything other than money damages without the prior written consent of the Indemnified Party, which consent shall not be unreasonably withheld, conditioned or delayed. The Indemnified Party’s Indemnitees must tender defense of the applicable Third-Party Claim and provide all reasonable cooperation and assistance in such defense, in order to remain eligible to be indemnified and held harmless; provided, however, that where Assignee is the Indemnified Party, unless ▇▇▇▇▇▇ Estate has adequate insurance to cover the alleged potential Losses and is tendering defense to such insurer who has indicated in writing that they will fully assume the defense and cover any resulting Losses, the Assignee Indemnitees shall not be required to tender defense in order to remain eligible to be indemnified and held - harmless and instead notwithstanding anything express or implied in this Section 7.3 Assignee and/or the Assignee Indemnitees may do so and be indemnified under this Agreement. The Indemnified Party shall have the right to be present in person or through counsel at substantive legal proceedings relating to the Third-Party Claim giving rise to the Indemnified Party’s right to indemnification hereunder. If the Parties cannot agree as to the application of Sections 7.1 and 7.2 to any Loss or Third-Party Claim, the Parties may conduct separate defenses of such Third-Party Claim. In such
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
case, each Party further reserves the right to claim indemnity from the other upon resolution of such underlying Third-Party Claim.
7.4 Limitation of Liability. IN NO EVENT SHALL EITHER PARTY OR ITS RESPECTIVE AFFILIATES (OR IN THE CASE OF ▇▇▇▇▇▇ ESTATE, ANY RESPONSIBLE ▇▇▇▇▇▇ ESTATE GROUP MEMBERS) BE LIABLE FOR SPECIAL, EXEMPLARY, CONSEQUENTIAL OR PUNITIVE DAMAGES, WHETHER IN CONTRACT, WARRANTY, TORT, STRICT LIABILITY OR OTHERWISE, EXCEPT TO THE EXTENT SUCH PARTY MAY BE REQUIRED TO INDEMNIFY THE OTHER PARTY FROM SUCH DAMAGES CLAIMED BY THIRD PARTIES UNDER THIS ARTICLE 7.
ARTICLE 8
CONFIDENTIALITY
8.1 Confidential Information; Exceptions. At all times before the effectiveness of the assignment under this Agreement of the Transferred Patents and Transferred Know-How: (i) each Party that has received Confidential Information from the other (the “Receiving Party”) shall maintain all such Confidential Information in trust and confidence and shall not disclose any such Confidential Information to any Third Party (except as expressly provided below) or use any such Confidential Information for any purposes other than for performance under or determining compliance with and administering this Agreement; and (ii) the Receiving Party shall not disclose such Confidential Information to any employee, agent, attorney, consultant, or Affiliate who does not have a reasonable need for such information for the foregoing purposes. Disclosures to such persons with a reasonable need for the information are only permitted to the extent the person is subject to binding obligations of confidentiality and limited use at least as restrictive in scope and as long in duration as those of this Article 8. The Receiving Party shall use at least the same standard of care as it uses to protect its own confidential information of a similar nature to prevent unauthorized disclosures or uses of the Confidential Information, but no less than reasonable care. The Receiving Party shall promptly notify the other Party upon discovery of any unauthorized use or disclosure of the Confidential Information.
After the assignment hereunder of the Transferred Patents and Transferred Know-How becomes effective and the ▇▇▇▇▇▇ Confidential Information becomes Assignee’s Confidential Information protected under this Article, the confidentiality obligations of this Article shall apply to the ▇▇▇▇▇▇ Estate to protect Assignee’s Confidential Information, but shall no longer restrict the Assignee.
Confidential Information shall not include any information which, as shown by ▇▇▇▇▇▇ Estate through competent proof:
(a) is now, or hereafter becomes, through no act or failure to act on the part of the receiving Party in breach hereof, generally known or available;
(b) is known by the receiving Party at the time of receiving such information, as shown by contemporaneous written records — but other than the Transferred Know-How and
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
any documentation thereof; the Transferred Know-How and any documentation thereof is deemed Confidential Information under this Agreement;
(c) is independently developed by the Receiving Party without the aid, application or use of Confidential Information, as shown by written records; or
(d) is hereafter furnished to the receiving Party by a Third Party, as a matter of right, without breach of any confidentiality agreement, and without restriction on disclosure.
8.2 Authorized Disclosure. Notwithstanding any other provision of this Agreement, the Receiving Party may disclose Confidential Information to the extent and to the persons and entities required by an applicable governmental law, rule, regulation or order; provided, however, that the Receiving Party shall first have given prompt notice to the other Party to enable such other Party to seek any available exemptions from or limitations on such disclosure requirement and shall reasonably cooperate in any such efforts by such other Party.
8.3 Return of Confidential Information. If this Agreement is terminated for breach according to the provisions of Section 9.2, the Receiving Party shall use diligent efforts to return all of the other Party’s Confidential Information. The Receiving Party will be allowed to keep one archival copy of any Confidential Information for record-keeping purposes only.
8.4 Use of Names. A Party shall not use any of the other Party’s names, trademarks, logos, employee names, investor names or symbols in any publicity, promotion or similar public disclosure, without the advance written withholdable consent of such other Party, except as may be required by law or stock exchange requirement.
ARTICLE 9
TERM AND TERMINATION
9.1 Term. The term of this Agreement shall commence upon the Effective Date and, unless sooner terminated as provided in this Article 9, shall expire upon the expiration of the last-to-expire Valid Claim of the Transferred Patents, but in any event no sooner than fifteen (15) years after the Effective Date and/or if later the expiration of the last pending patent claim of a Transferred Patent with the potential to become a Valid Claim.
9.2 Termination for Breach.
(a) Right to Terminate for Material Breaches. Either Party may terminate this Agreement for the other’s material breach of this Agreement, unless the material breach is cured within ninety (90) days of the allegedly breaching Party receiving written notice from the other Party specifying in detail what the material breach of this Agreement is and stating explicitly that the notice is a breach and potential termination notice under this Section 9.2(a). The notice and cure period shall be thirty (30) days in the case of a payment failure breach. In the case of a material breach of this Agreement that is incapable of cure within ninety (90) days, and is not a failure to make payment, but is capable of cure in a longer reasonable period, then the allegedly breaching Party shall within such ninety (90) day notice period provide a reasonable written plan
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
to cure the breach, and shall have a reasonable time to cure the breach without losing rights under this Agreement (it shall cure as soon as practicable).
(b) Mechanics. If a Party gives notice of termination under Section 9.2(a) and the other Party disputes whether such notice was proper, then the issue of whether this Agreement has been terminated shall be resolved in accordance with Section 10.1. If as a result of such dispute resolution process it is finally determined that the notice of termination was proper, then such termination shall become effective as of the date of such final determination; provided, however, that the breaching Party fails thereafter to cure the underlying breach in accordance with the determination made in the resolution process under Section 10.1 within the time period set forth in this Section 9.2 for the applicable breach following such determination (meaning it must either cure within ninety (90) days after such final determination or provide within such time period a reasonable written plan for cure; in the case where the cure would be payment of monies, the time period for cure shall be thirty (30) days after such final determination). If, however, as a result of such dispute resolution process it is determined that the notice of termination was improper, then no termination shall have occurred and this Agreement shall remain in effect.
9.3 Effects of Termination.
(a) Survival of Licensee’s Rights and Obligations. If ▇▇▇▇▇▇ Estate terminates this Agreement pursuant to Section 9.2(a), then notwithstanding anything in this Agreement, at law, or in equity, if at the time of termination there are any Licensees, such Licensees’ rights to the Transferred Patents, Transferred Know-How and Trademarks shall not be affected by the termination, and each such Licensee shall pay directly to ▇▇▇▇▇▇ Estate (or, if the ▇▇▇▇▇▇ Estate is closed, to the entity designated to receive payment as set forth in Section 10.5) any payments coming due under Section 4.2 or 4.3 of this Agreement after the effective date of termination as a result of its own practice of its surviving rights to the Transferred Patents, Transferred Know-How and Trademarks.
(b) After Assignee Terminates for ▇▇▇▇▇▇ Estate’s Breach. If Assignee terminates this Agreement pursuant to Section 9.2(a), then, in addition to those provisions that survive any expiration or termination of this Agreement as set forth in Section 9.3(c), the following shall survive and apply: Sections 3.4, 3.5, 3.6, and 3.7; and Article 4. Article 5 shall not survive such a termination. Section 3.2 shall not survive such a termination (nor shall Assignee have any diligence obligation under this Agreement, express or implied, at law or in equity, after such a termination). To avoid doubt, in this scenario, as between the Parties, Assignee retains title to the Transferred Know-How and Transferred Patents.
(c) General Survival. Expiration or termination of this Agreement for any reason shall not affect any accrued rights or obligations of the Parties, and the following Articles shall survive any expiration or termination of this Agreement: Articles 1, 2 and 7-10.
9.4 Elective Termination after Certain Obligations. Assignee shall have the right to terminate its obligations under Sections 3.2 and Article 5 without cause at any time after the payments of Section 4.1(a) and 4.1(c) (in the latter case, if any) have been fully paid. In this case,
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
only Assignee’s obligations Sections 3.2 and Article 5 shall be terminated, and the remainder of the Parties’ rights and obligations under this Agreement shall remain in full force and effect.
ARTICLE 10
MISCELLANEOUS
10.1 Dispute Resolution.
(a) Initial Dispute Resolution. The Parties recognize that disputes may from time to time arise between the Parties during the term of this Agreement. It is the objective of the Parties to establish procedures to facilitate the resolution of disputes arising under this Agreement in an expedient manner by mutual cooperation and without resort to litigation. To accomplish this objective, the Parties agree to follow the procedures set forth in this Section 10.1 to resolve any dispute arising under this Agreement. If such a dispute between the Parties arises, then either Party, by written notice to the other Party, may have such dispute referred to the Parties’ respective executive officers designated below or their successors, for attempted resolution by good faith negotiations within thirty (30) days after such notice is received. Said designated officers are as follows:
Assignee: President and CEO
▇▇▇▇▇▇ Estate: ▇▇▇▇▇▇ ▇▇▇▇▇▇ II
(b) Preliminary Relief. A Party is entitled to seek interlocutory relief and/or a preliminary injunction without first following the procedure of this Section 10.1; provided that it also invokes the procedure of this Section 10.1 in parallel. Each Party hereby irrevocably waives its right to jury trial of any and all disputes arising under this Agreement, and consents to have such disputes decided instead by a judge or justice.
(c) Arbitration. Except as otherwise set out in this Section 10.1, any dispute that cannot be settled amicably by agreement of the Parties pursuant to Section 10.1(a) shall be finally settled by an arbitration administered by JAMS applying its most applicable procedural rules (and the substantive laws of the State of California) provided that the appointed arbitrator(s) shall have appropriate experience in the pharmaceutical industry (or if no such person is available then in the biopharmaceutical industry or the closest industry possible). The place of arbitration shall be San Francisco, California. The language to be used in the arbitration proceedings shall be English. The award rendered in any arbitration shall be final and binding upon both Parties. The judgment rendered by the arbitrator(s) may include costs of arbitration, reasonable legal fees and reasonable costs for any expert and other witnesses. Nothing in this Agreement shall be deemed as preventing either Party from seeking injunctive relief (or any other provisional remedy) from any court having jurisdiction over the Parties and the subject matter of the dispute as necessary to protect either Party’s name, Confidential Information (in the case of Assignee) or intellectual property. Judgment upon the award may be entered in any court having jurisdiction, or application may be made to such court for judicial acceptance of the award and/or an order of enforcement as the case may be. Notwithstanding the foregoing, either Party shall be free to submit any dispute relating to the scope, validity, enforceability or other
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
like matter regarding intellectual property to any court having jurisdiction over the Parties and the subject matter of the dispute and to seek such relief and remedies as are available in that court.
10.2 Jurisdiction. Both Parties consent to the exclusive personal jurisdiction of all courts sitting within San Francisco, California for resolving all disputes arising out of or in connection with this Agreement. Each Party hereby waives any and all defenses it may have to the jurisdiction and venue of such courts, including a defense that such a court may not assert personal jurisdiction over such Party, or of forum non conveniens.
10.3 Governing Law. This Agreement is made in accordance with and shall be governed and construed under the laws of the State of California excluding its choice of law principles.
10.4 No Agency, Joint Venture or Partnership. Neither Party is, nor will be deemed to be, an employee, agent or legal representative of the other Party for any purpose. Neither Party will be entitled to enter into any contracts in the name of, or on behalf of the other Party, nor will a Party be entitled to pledge the credit of the other Party in any way or hold itself out as having authority to do so. The parties are independent contractors, this Agreement is for an arm’s-length transaction, and the relationship that it governs shall not be construed to be or create any agency, joint venture or partnership.
10.5 Assignment. Except as explicitly provided for in this Agreement, neither Party shall have the right or power to assign any rights or obligations under this Agreement without the consent of the other Party, except that Assignee may assign one or more times to an Affiliate or to a successor to substantially all of the business or assets of Assignee to which this Agreement relates (whether through merger, sale of stock, sale of assets or other transaction). This Agreement shall be binding upon and inure to the benefit of the successors and explicitly permitted assigns of the Parties. Any assignment of this Agreement not made in accordance with this Agreement is prohibited hereunder and shall be null and void. Any assignee must certify in writing to the non-assigning Party, within ninety (90) days after the requested in writing by the non-assigning Party, that such assignee agrees to the terms and conditions of this Agreement going forward from the date of assignment.
It is understood and agreed that ▇▇▇▇▇▇ Estate may divide the proceeds due to it under this Agreement amongst the heirs to the estate, and commit to the heirs to do so as regards future payments. It may distribute to the heirs or enter into written agreements with the heirs for the distribution of such proceeds, and the heirs may further transfer or assign their rights to such proceeds. Any such written agreement or activity shall not be considered in breach of and is hereby explicitly allowed under this Section 10.5. It is understood and agreed, however, that whatever the distribution between the ▇▇▇▇▇▇ Estate and the heirs, Assignee’s sole responsibility with respect to each payment due is to make that payment to ▇▇▇▇▇▇ Estate in accordance with this Agreement, or if the estate has been closed, then to ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ or the single legal entity designated by him in writing under the next paragraph. Assignee shall not be required to split payments among different heirs nor to deal with more than one representative of ▇▇▇▇▇▇ Estate and/or designee for receipt of payment if the estate has been closed.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
It is understood and agreed that during the Willi of this Agreement the estate of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I is likely to be closed because probate will not remain open over the life of this Agreement. If the ▇▇▇▇▇▇ Estate is closed prior to any payment coming due under this Agreement, then notwithstanding anything express or implied in this Agreement, after the date that the ▇▇▇▇▇▇ Estate is closed Assignee shall make all subsequent payments that would have been due to ▇▇▇▇▇▇ Estate, instead, to ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ in his personal capacity, or to such other single legal entity as may be specified by ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ in writing no later than ten (10) Business Days prior to the payment due date. As among the Parties and the heirs of the ▇▇▇▇▇▇ Estate, it shall be ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ II’s responsibility to distribute those payments to any and all heirs of the estate and/or their successors, or, in the case that ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ has designated a different single legal entity to receive payment, it shall be that entity’s responsibility to do so. Assignee shall have no responsibility whatsoever to the heirs, nor to their successors, and Assignee’s sole responsibility is to make payment to ▇▇▇▇▇▇ Estate, ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇, or the single legal entity designated in writing by him to receive payment as provided for above, as applicable.
10.6 Amendment. No amendment or modification hereof shall be valid or binding upon the Parties unless made in writing and signed by authorized officers of both Parties.
10.7 Notices. Any notice or other communication required or permitted to be given to either Party hereto shall be in writing unless otherwise specified and shall be deemed to have been properly given and to be effective (a) on the date of delivery if delivered in person; (b) the date of electronically confirmed facsimile transmission if during the recipient’s normal business hours, or otherwise on the next Business Day; (c) two (2) Business Days after sending for next Business Day delivery by internationally recognized expedited courier service for no later than next-possible-business-day delivery; and (d) upon actual receipt by the recipient of an email, in the case of an emailed notice:
In the case of Assignee:
▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇▇▇ ▇▇▇
▇▇▇▇▇▇▇ ▇▇ ▇▇▇▇▇
Attn: ▇▇. ▇▇▇▇ ▇▇▇▇▇▇
Fax — [To be provided by written notice by Aclaris within 60 days after the Effective Date.]
Email — ▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
With required copies to:
▇▇▇▇▇▇▇▇▇▇ Life Sciences PC
▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇ ▇▇▇ ▇▇▇
▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attn: ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇
Fax — ▇▇▇ ▇▇▇ ▇▇▇▇
Email — ▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
In the case of ▇▇▇▇▇▇ Estate:
▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇, ▇▇
▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇, ▇▇▇. #▇▇▇
▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇▇
Fax: None
Email - ▇▇▇▇▇▇▇@▇▇▇.▇▇▇
With a required copy to:
▇▇▇▇▇▇▇ & ▇▇▇▇▇, P.A.
Third Floor Camelback Esplanade II
▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
Attn: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇
Fax ▇▇▇-▇▇▇-▇▇▇▇
Email - ▇▇▇@▇▇▇▇▇.▇▇▇
In the case of (c) (expedited courier service), the Party providing the notice shall as a courtesy additionally provide the notice by a facsimile in accordance with (b). Either Party may change its address for communications by a notice to the other Party in accordance with this Section 10.7.
10.8 No Implied Licenses. Except as otherwise expressly set forth in this Agreement, nothing in this Agreement shall give either Party any right, title or interest in or to any Patents or other intellectual property owned by or licensed to the other Party.
10.9 Force Majeure. Any delay in or failure of performance by any Party under this Agreement shall not be considered a breach of this Agreement if and to the extent caused by occurrences beyond the reasonable control of the Party affected, including acts of God, embargoes, governmental restrictions, strikes or other acts of workers, fire, flood, earthquake, explosion, riots, wars, acts of terrorism, civil disorder, rebellion or sabotage and technical events beyond the Party’s reasonable control; provided, however, the payment of any value due and owing hereunder shall not be delayed by the payor because of a force majeure affecting the payer, unless such force majeure specifically precludes the payment process. The Party suffering such occurrence shall notify the other Party and any time for performance hereunder shall be extended by the actual time of delay caused by the occurrence.
10.10 Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original but all of which together shall constitute a single instrument.
10.11 Captions. All section titles or captions contained in this Agreement, in any Exhibit referred to herein and the table of contents, if any, to this Agreement are for convenience only, shall not be deemed a part of this Agreement and shall not affect the meaning or interpretation of this Agreement.
10.12 Draftsmanship. Each Party acknowledges that it has participated in, and has been represented by counsel in, the drafting of this Agreement. Any applicable rule of construction to
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
the effect that ambiguities are to be resolved against the drafting party will not be applied in connection with the construction or interpretation of this Agreement.
10.13 No Third Party Rights or Obligations. Except for the rights of the ▇▇▇▇▇▇ Estate Indemnitees and the Assignee Indemnitees as expressly provided in Article 7 of this Agreement, no provision of this Agreement shall be deemed or construed in any way to result in the creation of any rights or obligation in any Third Party.
10.14 Severability. If any term, condition or provision of this Agreement is held to be invalid or unenforceable for any reason by any court of competent jurisdiction form which no appeal can be or is taken, or in arbitration proceedings between the Parties as set forth in Article 10 of this Agreement, it shall, if possible, be narrowed, shortened, or interpreted to achieve the intent of the Parties to this Agreement to the extent legally possible rather than voided or if not to any extent legally possible be deemed severed from this Agreement. In any event, all other terms, conditions and provision of this Agreement shall be deemed valid and enforceable to the full extent.
10.15 Compliance with Laws. Each Party shall carry out its activities pursuant to this Agreement in compliance with all applicable supranational, national, state, provincial and other local laws, rules, regulations and guidelines.
10.16 Cumulative Rights. The rights, powers and remedies hereunder shall be in addition to, and not in limitation of, all rights, powers and remedies provided at law or in equity, or under any other agreement between the Parties. All of such rights, powers and remedies shall be cumulative, and may be exercised successively or cumulatively.
10.17 Waiver. No failure or delay on the part of either Party to exercise any power, right, privilege or remedy under this Agreement will operate as a waiver thereof. No single or partial exercise of any such power, right, privilege or remedy will preclude any other or further exercise thereof or of any other power, right, privilege or remedy. Waivers of powers, rights, privileges and remedies under this Agreement may only be waived in a writing executed by a duly authorized officer of the waiving Party.
10.18 Net Liability. Notwithstanding any provision of this Agreement, every liability of Assignee to ▇▇▇▇▇▇ Estate is subject to and conditioned upon the recoupment of any and all liabilities owing from ▇▇▇▇▇▇ Estate to Assignee, so as to establish a net liability. However, Assignee shall not reduce the amounts of its payments under Article 4 based on its net liability unless (i) this Agreement is properly terminated pursuant to Section 9.2 for ▇▇▇▇▇▇ Estate’s uncured material breach of the Agreement, or (ii) a dispute resolution is pending with regard to whether Assignee has the right to terminate pursuant to Section 9.2 for ▇▇▇▇▇▇ Estate’s uncured material breach of the Agreement,. In the case that such a dispute resolution is pending, Assignee will deposit and maintain in a separate account the amount of its damages and deduct the deposited amounts from payments to ▇▇▇▇▇▇ Estate; the separate account shall belong to Assignee and be used to pay any back amounts due if Assignee does not prevail in dispute resolution.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
10.19 Costs. Each Party shall bear its own legal costs of and incidental to the preparation, negotiation and execution of this Agreement.
10.20 Entire Agreement. This Agreement embodies the entire understanding of the Parties with respect to the subject matter hereof and shall supersede all previous communications, representations or understandings, either oral or written, between the Parties relating to the subject matter of this Agreement. To be clear, this Agreement supersedes the Prior CDA with respect to Confidential Information and the Parties’ rights and obligations with respect thereto.
10.21 Attorney’s Fees. The prevailing party in any arbitration proceeding or litigation between the Parties arising as a result of any breach or dispute under this Agreement will have a right to reasonable attorneys’ fees incurred in connection with such arbitration or litigation from the other party.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
IN WITNESS WHEREOF, both Assignee and ▇▇▇▇▇▇ Estate have executed this Agreement by their respective officers hereunto duly authorized.
|
|
▇▇▇▇▇▇ ESTATE | |||
|
|
|
| ||
|
By |
/s/ ▇▇▇▇ ▇▇▇▇▇▇ |
|
By: |
/s/ ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇, ▇▇ |
|
|
|
|
|
|
|
Name: |
▇▇▇▇ ▇▇▇▇▇▇ |
|
Name: |
▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇, ▇▇ |
|
|
|
|
|
|
|
Title: |
President and CEO |
|
Title: |
Personal Representative |
|
|
|
|
|
|
|
Date: |
8/21/12 |
|
Date: |
8/20/12 |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBITS LIST
Exhibit A - Form of Recordation Document
Exhibit B - Trademarks
Exhibit C - Consent of Heirs
Exhibit D - Prior Confidentiality Agreements
Exhibit E-1 - Ex-U.S. filings of the Transferred Listed Patents
Exhibit E-2 - U.S. Provisional filings of the Transferred Listed Patents
Exhibit F - Estate of Mickey ▇▇▇ ▇▇▇▇▇▇ Letters of Administration
Exhibit G - Estate of Mickey ▇▇▇ ▇▇▇▇▇▇ Order of Intestacy and Determination of Heirs
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT A
FORM OF RECORDATION DOCUMENT
SHORT-FORM PATENT ASSIGNMENT
▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I was heretofore the owner of the entire right, title and interest in the patent applications referred to in Exhibit A to this Short-Form Patent Assignment (“Assigned Families”). ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I is deceased, and his estate therefore became the owner of his interest in the Assigned Families, which was the sole ownership interest therein (by prior assignment of the co-inventor), as evidence by the ruling of the probate court reproduced in Exhibit B to this Short-Form Patent Assignment. ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ is Personal Representative of the estate of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I (in such capacity of Personal Representative of the estate, “ASSIGNOR”), as evidenced by the appointment attached to this Short-Form Patent Assignment as Exhibit B to Short-Form Patent Assignment.
By prior assignment pursuant to that certain Assignment Agreement executed between ASSIGNOR and Inc., a Delaware corporation, effective , 2012, ASSIGNOR transferred, assigned and conveyed to Assignee, the entire right, title, and interest in and to the Assigned Families and Letters Patent that may be issued on any of the Assigned Families in the United States, Australia, Canada, Japan, the countries in the European Patent Organisation, and everywhere else in the world.
NOW, THEREFORE, ASSIGNOR hereby acknowledges that, in consideration of the foregoing and the good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, set forth in such Assignment Agreement, ASSIGNOR has heretofore transferred, assigned and conveyed to Assignee all right, title and interest in and to the Assigned Families and Letters Patent that may be issued on any of the Assigned
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
Families in the United States, Australia, Canada, Japan, the countries in the European Patent Organisation, the PCT, its participating countries, and everywhere else in the world.
[CONTINUES ON NEXT PAGE]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
ASSIGNOR authorizes and requests the Commissioner of Patents and Trademarks of the United States and of Australia, Canada, Japan, the countries in the European Patent Organisation, the PCT, its participating countries, and anywhere else in the world to issue any Letters Patent granted on the Assigned Families, whether on any subsequently filed division, continuation, continuation-in-part, reexamination, or reissue application, to Assignee, its successors and assigns, as the assignee of the entire interest in the Assigned Families.
IN TESTIMONY WHEREOF, the undersigned has executed this instrument on the day of 2012.
|
|
ASSIGNOR | |
|
|
| |
|
|
By: |
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
Title: |
|
State of
County of
On before me, ,
personally appeared
o personally known to me — OR - o proved to me on the basis of satisfactory evidence to be the person whose name is subscribed to the within instrument and acknowledged to me that he executed the same in his authorized capacity, and that by his signature on the instrument the person, or the entity upon behalf of which the person acted, executed the instrument.
|
|
WITNESS my hand and official seal. | ||
|
|
| ||
|
|
|
|
|
|
|
|
Signature of Notary |
|
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT A TO SHORT-FORM PATENT ASSIGNMENT
[To be completed prior to recordation.]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT B TO SHORT-FORM PATENT ASSIGNMENT
Estate of Mickey ▇▇▇ ▇▇▇▇▇▇ - Letters of Administration
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT B
TRADEMARKS
None.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT C
FORM OF CONSENT OF HEIR
I, , and individual residing as of at , have reviewed the assignment agreement attached as Schedule 1 to this Consent of Heir (“Assignment Agreement”).
I am an heir of the ▇▇▇▇▇▇ Estate referred to in the Assignment Agreement.
I hereby acknowledge that ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ has been appointed as the personal representative of the ▇▇▇▇▇▇ Estate and as such is authorized to enter into such Assignment Agreement on behalf of the estate.
I hereby acknowledge that upon his signature to the Assignment Agreement the ▇▇▇▇▇▇ Estate and my interests as an heir thereto shall be legally bound.
Without implying that my consent is an any way required, I hereby consent to the Assignment Agreement and waive any and all rights to challenge it.
I hereby covenant that, having consented to such Assignment Agreement, I shall not in any way challenge the legal effectiveness of the Assignment Agreement, assert ownership to any assets transferred thereby, assert a right to direct payment in my personal capacity under the Assignment Agreement, or otherwise challenge the transaction contemplated in the Assignment Agreement, nor the rights of Assignee thereunder. I further covenant that I have not executed any agreements that are inconsistent with the Assignment Agreement or to that are to the detriment of the Transferred Patents or the Transferred Know-How.
I waive any right that I may have to challenge such Assignment Agreement or the Assignee thereunder, known or unknown, present or future.
[▇▇▇▇▇▇ ▇▇▇▇: I agree to look only to the estate or ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ for payment; I recognize that the counterparty to the Assignment Agreement will make payment to ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ and will have no direct obligation to me as an heir to the estate.]
[▇▇▇▇▇▇ ▇ ▇▇▇▇▇▇ ▇▇: While I am currently personal representative for the Estate of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ (I), I am signing this Consent of Heir in my personal capacity, and agreeing to be responsible to distribute payments received by me in my capacity as personal representative of the estate currently, and in my personal capacity once the estate is closed, between myself and the other heir in the manner determined in probate or otherwise agreed in writing amongst the heirs.]
I have had the opportunity, whether or not I have chosen to use it, to consult with counsel with respect to the effect of this Consent of Heir and my waivers, agreements, acknowledgements and covenants set forth in this Consent of Heir.
[Pagination to be checked and state “remainder of page intentionally blank” if applicable]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
|
|
Signed: |
| |
|
|
| ||
|
|
[▇▇▇▇▇▇ ▇▇▇▇]/[▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇, ▇▇] | ||
|
|
| ||
|
|
Date: |
| |
State of
County of
On before me,
personally appeared
o personally known to me — OR - o proved to me on the basis of satisfactory evidence to be the person whose name is subscribed to the within instrument and acknowledged to me that he executed the same in his authorized capacity, and that by his signature on the instrument the person, or the entity upon behalf of which the person acted, executed the instrument.
|
|
WITNESS my hand and official seal. | ||
|
|
| ||
|
|
|
|
|
|
|
|
Signature of Notary |
|
Schedule 1 to Consent of Heir
[PDF of final version of Assignment Agreement to be attached.]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT D
PRIOR CONFIDENTIALITY AGREEMENTS
[***]
[***]
[***]
[***]
Note to Exhibit: The foregoing list omits one CDA that was signed with a party to cover licensing discussions that did not come to fruition. ▇▇▇▇▇▇ Estate does not have the right to disclose the name of the counterparty and has not done so, and Assignee acknowledges that it is not requiring the ▇▇▇▇▇▇ Estate to do so.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT E-1
EX-U.S. FILINGS OF THE TRANSFERRED LISTED PATENT
Active Patents
|
Matter |
|
Matter Name |
|
Matter No. |
|
India |
|
IN ▇▇▇ Acrochordon Alleviation |
|
[ *** ] |
|
Mexico |
|
MX ▇▇▇ Acrochordon Alleviation |
|
[ *** ] |
|
New Zealand |
|
NZ ▇▇▇ Acrochordon Alleviation |
|
[ *** ] |
|
Singapore |
|
SG ▇▇▇ Acrochordon Alleviation |
|
[ *** ] |
|
Australia |
|
AU Div ▇▇▇ Acrochordon Alleviation |
|
[ *** ] |
Lapsed Patents
|
Matter |
|
Matter Name |
|
Matter No. |
|
China |
|
CN ▇▇▇ Acrochordon Alleviation |
|
[ *** ] |
|
Europe - DE |
|
DE, FR and GB Patents (formerly EPO App) Acrochordon Alleviation |
|
[ *** ] |
|
Israel |
|
IL ▇▇▇ Acrochordon Alleviation |
|
[ *** ] |
|
Singapore |
|
Composition for the Treatment of Skin Conditions (Div) |
|
[ *** ] |
Expired/Lapsed Patents/Applications
|
Matter |
|
Matter Name |
|
Matter No. |
|
Brazil |
|
BR ▇▇▇ App Acrochordon Alleviation |
|
[ *** ] |
|
Canada |
|
CA ▇▇▇ App Acrochordon Alleviation |
|
[ *** ] |
|
Hong Kong |
|
Hong Kong ▇▇▇ App Acrochordon Alleviation |
|
[ *** ] |
|
Japan |
|
JP ▇▇▇ App Acrochordon Alleviation |
|
[ *** ] |
|
Singapore |
|
Composition for the Treatment of Skin Conditions (Div) |
|
[ *** ] |
|
Mexico |
|
MX ▇▇▇ App Treatment of Skin Conditions |
|
[ *** ] |
|
Australia |
|
AU ▇▇▇ App Acrochordon Alleviation (lapsed when divisional 12018-020 was filed) |
|
[ *** ] |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT E-2
U.S. PROVISIONAL PATENT APPLICATIONS OF THE TRANSFERRED LISTED PATENT
|
Date Filed |
|
Title |
|
Inventor |
|
Not filed (but dated October 9, 2003) |
|
Basal Cell Carcinoma Treatment |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Filing status unknown (but dated January 8, 2004) |
|
Age Spot Alleviation |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Filing status unknown |
|
Age Spot Cosmetic Regimen |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
July 24, 2007 |
|
Basal Cell Carcinoma Treatment |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Filing status unknown |
|
Video Microscope Assisted Seborrheic Keratosis Treatment |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Filing status unknown |
|
Age Spot Alleviation |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Apparently filed on March 17, 2009 |
|
Peroxide Treatment of Skin. Afflictions |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Apparently filed on September 23, 2009 |
|
Basal Cell Carcinoma Treatment |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
November 2, 2009 |
|
Single Application Age Spot Removal |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Filing status unknown |
|
Skin Lightening Cosmetic |
|
Mickey ▇▇▇ ▇▇▇▇▇▇ |
|
Filing status unknown |
|
Age Spot Formulations and Methods |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT F
ESTATE OF MICKEY ▇▇▇ ▇▇▇▇▇▇ LETTERS OF ADMINISTRATION
[ *** ]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT WHICH INCLUDE THIS AND TWO ADDITIONAL PAGES OF OMISSIONS. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
EXHIBIT G
ESTATE OF MICKEY ▇▇▇ ▇▇▇▇▇▇ ORDER OF INTESTACY AND DETERMINATION OF HEIRS
[SEE ATTACHED PDF]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2

CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
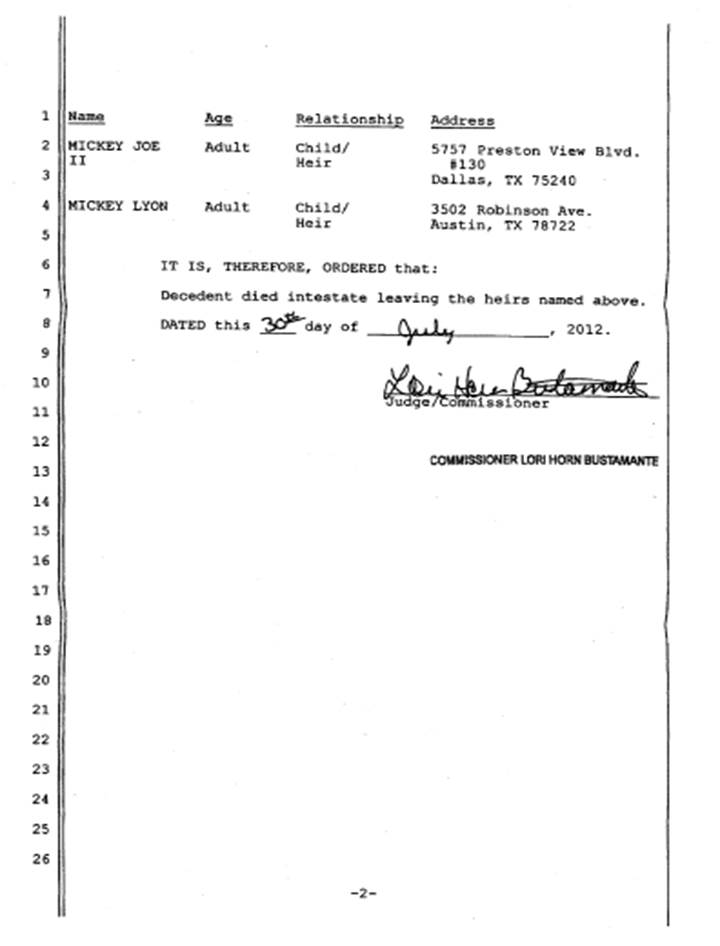
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
FORM OF CONSENT OF HEIR
I, ▇▇▇▇▇▇ ▇▇▇▇, an individual residing as of August 20, 2012, at , have reviewed the assignment agreement attached as Schedule 1 to this Consent of Heir (“Assignment Agreement”).
I am an heir of the ▇▇▇▇▇▇ Estate referred to in the Assignment Agreement.
I hereby acknowledge that ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇ has been appointed as the personal representative of the ▇▇▇▇▇▇ Estate and as such is authorized to enter into such Assignment Agreement on behalf of the estate.
I hereby acknowledge that upon his signature to the Assignment Agreement the ▇▇▇▇▇▇ Estate and my interests as an heir thereto shall be legally bound.
Without implying that my consent is in any way required, I hereby consent to the Assignment Agreement and waive any and all rights to challenge it.
I hereby covenant that, having consented to such Assignment Agreement, I shall not in any way challenge the legal effectiveness of the Assignment Agreement, assert ownership to any assets transferred thereby, assert a right to direct payment in my personal capacity under the Assignment Agreement, or otherwise challenge the transaction contemplated in the Assignment Agreement, nor the rights of Assignee thereunder. I further covenant that I have not executed any agreements that are inconsistent with the Assignment Agreement or to that are to the detriment of the Transferred Patents or the Transferred Know-How.
I waive any right that I may have to challenge such Assignment Agreement or the Assignee thereunder, known or unknown, present or future.
I agree to look only to the estate of ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ I for payment; I recognize that the counterparty to the Assignment Agreement will make payment to the ▇▇▇▇▇▇ Estate, ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ ▇▇, or the single legal entity designated in writing by him to receive payment as provided for in the Assignment Agreement and will have no direct obligation to me as an heir to the estate.
Remainder of page intentionally blank.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2
I have had the opportunity, whether or not I have chosen to use it, to consult with counsel with respect to the effect of this Consent of Heir and my waivers, agreements, acknowledgements and covenants set forth in this Consent of Heir.
|
|
Signed: |
|
|
|
▇▇▇▇▇▇ ▇▇▇▇ | |
|
|
| |
|
|
Date: |
|
|
State of |
|
|
|
|
|
|
|
County of |
|
|
|
On August , 2012, before me, |
|
|
personally appeared ▇▇▇▇▇ ▇▇▇▇,
o personally known to me — OR - o proved to me on the basis of satisfactory evidence to be the person whose name is subscribed to the within instrument and acknowledged to me that he executed the same in his authorized capacity, and that by his signature on the instrument the person, or the entity upon behalf of which the person acted, executed the instrument.
|
WITNESS my hand and official seal. | ||
|
|
|
|
|
|
|
|
|
|
Signature of Notary |
|
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.

