Contract
[ *** ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act Of 1933, as amended.
Exhibit 10.15
AMONG
REPLIDYNE, INC.
DAIICHI SUNTORY PHARMA CO., LTD.
AND
NIPPON SODA CO., LTD.
DATED: DECEMBER 20TH, 2004
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
TABLE OF CONTENTS
| Article | Title | Page | ||||
1
|
Definitions | 2 | ||||
2
|
Validation of Nihongi Facility | 2 | ||||
3
|
Manufacture and Supply of Drug Substance | 5 | ||||
4
|
Forecasts, Purchase Orders and Deliveries | 10 | ||||
5
|
Purchase Price and Payment | 15 | ||||
6
|
Drug Substance Quality | 21 | ||||
7
|
Regulatory Matters | 24 | ||||
8
|
Transfer of Manufacturing | 28 | ||||
9
|
Proprietary Rights | 31 | ||||
10
|
Secrecy | 31 | ||||
11
|
Duration and Termination | 32 | ||||
12
|
Effect of Termination | 34 | ||||
13
|
Hold Harmless and Insurance | 38 | ||||
14
|
Representations, Warranties and Covenants | 39 | ||||
15
|
Force Majeure | 40 | ||||
16
|
Non-Waiver | 40 | ||||
17
|
Modification | 41 | ||||
18
|
Miscellaneous | 41 | ||||
19
|
Applicable Law | 43 | ||||
20
|
Dispute Resolution | 43 | ||||
21
|
Notice | 44 | ||||
22
|
Publicity | 44 | ||||
23
|
Language | 45 | ||||
24
|
Execution in Counterparts | 45 | ||||
TABLE OF ATTACHMENTS
| Number | Title | |
1
|
Definitions | |
2.3
|
Timeline | |
3.3
|
Delay Compensation | |
4.1
|
Batch Sizes | |
4.2(a)
|
Initial Clinical Forecast | |
5.1
|
Purchase Prices | |
6.1
|
Drug Substance Specifications | |
7.9
|
Sensitive Manufacturing Information | |
8.2
|
Manufacturing Patents | |
11.1
|
Patents |
[ *** ] = Certain confidential information contained in this document, marked by brackets,
has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE i |
THIS SUPPLY AGREEMENT (“Agreement”) is made as of December 20th, 2004 (“Effective
Date”) among:
| (1) | REPLIDYNE, INC., a corporation organized and existing under the laws of the State of Delaware, U.S.A., having its principal business office at ▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇.▇.▇. (“Replidyne”); | |
| (2) | DAIICHI SUNTORY PHARMA CO., LTD., a corporation organized and existing under the laws of Japan and having its registered office at ▇-▇, ▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇ (“DSP”); and | |
| (3) | NIPPON SODA CO., LTD., a corporation organized and existing under the laws of Japan, having its registered office at ▇-▇, ▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇ (“Nisso”). |
DSP and Nisso are sometimes referred to collectively herein as the “Supplier”, and shall be
jointly and severally liable for the obligations of “Supplier” hereunder as provided in Section
18.5 (Independent Contractor) hereof. Replidyne, DSP and Nisso are sometimes referred to
collectively herein as the “Parties” or individually as a “Party”.
WITNESSETH:
WHEREAS, Replidyne and DSP have entered into the license agreement on March 15, 2004 pursuant
to which Replidyne has been granted certain licenses in the Territory (as defined herein) under the
patents and the know-how (“License Agreement”); and
WHEREAS, Replidyne, DSP and Nisso have entered into the Letter of Intent for Key Business
Terms Faropenem Daloxate Supply Agreement on the same date (“Supply LOI”) which require the
execution and delivery of this Agreement by the Parties; and
WHEREAS, Replidyne, DSP and Nisso entered into a letter agreement on November 15, 2004
(“Letter Agreement”) wherein Replidyne provided Nisso with an instruction to commence
validation activities for Nisso’s Nihongi Facility to manufacture certain quantities of Drug
Substance (hereinafter defined) and procurement of raw materials relating to such Drug Substance
and Nisso agreed to commence such validation activities; and
WHEREAS, Replidyne desires to purchase from DSP pre-clinical, clinical and commercial
quantities of the Drug Substance, and the Supplier desires to supply such Drug Substance to
Replidyne in accordance with the terms and conditions of this Agreement.
NOW, THEREFORE, for and in consideration of the premises and the provisions contained herein,
the Parties, intending to be legally bound, agree as follows:
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 1 |
ARTICLE 1: DEFINITIONS
Certain defined terms as used in this Agreement shall have the meanings respectively specified
in Attachment 1 hereto.
ARTICLE 2: PREPARATION OF NIHONGI FACILITY
| 2.1 | General. Nisso shall perform all necessary engineering work, equipment acquisition and commissioning, training, qualification and validation activities and other work required for the Nihongi Facility to manufacture and supply the Drug Substance meeting the Specifications in accordance with the then-current Drug Master File for the Drug Substance and all Regulations and Regulatory Approvals. | |
| 2.2 | Engineering Activities. Nisso shall undertake and subsequently complete all necessary engineering activities, equipment acquisition and commissioning, training and other activities required for the Nihongi Facility to manufacture and supply the Drug Substance meeting the Specifications in accordance with the then-current Drug Master File for the Drug Substance (excluding qualification and validation activities as provided in Section 2.3). Subject to Section 12.5(c) (Engineering Costs) hereof, all costs related to such activities shall be for the sole account of Nisso. It is acknowledged by the Parties that Nisso have already commenced such activities in accordance with the Letter Agreement. | |
| 2.3 | Validation Activities. Nisso shall undertake and complete all necessary qualification and validation activities for the Nihongi Facility to manufacture and supply commercial quantities of the Drug Substance meeting the Specifications in accordance with the then-current Drug Master File for the Drug Substance and all Regulations and Regulatory Approvals. Such activities shall be undertaken in accordance with the timeline and plan set forth in Attachment 2.3 hereto (“Timeline”). Subject to Section 2.4 (Purchase of Validation Lots of Drug Substance) hereof, all costs related to such activities shall be for the sole account of Nisso. It is acknowledged by the Parties that Nisso have already commenced a part of such activities in accordance with the Letter Agreement. | |
| 2.4 | Purchase of Validation Batches of Drug Substance. Replidyne shall purchase and take delivery of Drug Substance manufactured at Nihongi Facility during the validation activities specified in Section 2.3 (Validation Activities) as follows: |
| (a) | Number of Batches: | Four (4) batches as further specified in the protocol approved by Nisso and Replidyne (each a “Validation Batch”). | ||||
| (b) | Size of Batches: | [ *** ] kg ([ *** ] kg in the potency basis) for the first batch and [ *** ] kg ([ *** ] kg in the potency basis) for the second, third and fourth batches. Estimated total quantity is [ *** ] Kg ([ *** ]Kg in the potency basis). |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 2 |
| (c) | Purchase Price: | JP¥[ *** ]/kg (JP¥[ *** ]/kg in the potency basis), priced on the basis of Ex Works Nihongi (to be increased by mutual agreement to reflect delivery FCA Narita pursuant to Section 4.7 (Delivery, Title and Risk of Loss)). | ||||
| (d) | Delivery: | In accordance with the Timeline (shipment from Nihongi Facility by [ *** ]) and Section 4.7 (Delivery, Title and Risk of Loss). | ||||
| (e) | Payment Terms: | As set out in Section 5.3 (Payment), provided that [ *** ] percent ([ *** ]%) of the purchase price of JP¥[ *** ] which is calculated based on the estimated total quantity (JP¥[ *** ] x [ *** ]Kg) shall be paid by Replidyne in advance within five (5) days after Replidyne places its purchase orders for the relevant delivery to DSP. Within thirty (30) days after receipt of each of 1st, 2nd and 3rd Batches, Replidyne shall pay DSP the purchase price corresponding to [ *** ]percent ([ *** ]%) of the quantity of each Validation Batch actually delivered. Further, within thirty (30) days after receipt of the 4th Batch, Replidyne shall pay DSP the balance of the total purchase price which shall be calculated as follows: | ||||
| Payment for the 4th Validation Batch = [JP¥ [ *** ] x Total quantity (potency) actually delivered for 4 batches (1st through 4th Validation Batch) ] — [(Initial payment described above (a)) + (Total amount paid for the 1st, 2nd and 3rd Validation Batches described above (b))]. | ||||||
| (f) | Condition: | Only Drug Substance meeting the Specifications manufactured under a protocol approved by Replidyne. (For clarity, Replidyne shall not be required to purchase Drug Substance that results from experimental, pilot, scale-up or other batches that do not meet the Specifications.) |
Upon the execution of this Agreement, Replidyne shall deliver Supplier the purchase
order of the Validation Batch containing the above (a) to (f).
Nisso shall provide to Replidyne (i) written notice of its release of the relevant
Validation Batch, (ii) a certificate of analysis for such Validation Batch, (iii) the
results of all relevant testing data for such Validation Batch, and (iv) samples of such
Validation Batch for Replidyne’s independent testing, which shall be reasonably satisfactory
to Replidyne and Replidyne shall pay Nisso the reasonable shipping cost of such samples
separately. Within thirty (30) days after the date of Replidyne’s actual receipt of Nisso’s
notice, certificate, information and samples provided pursuant to this Section 2.4,
Replidyne shall review
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 3 |
such documents and, if desired, independently test such samples.
Replidyne, by written notice to Nisso, shall either accept such Validation Batch or reject
such Validation Batch with written reasonable reasons for such rejection. Nisso at its cost
shall create and retain all necessary and customary batch samples and records. Other than the Validation
Batches, Replidyne shall not be obligated to purchase quantities of Drug Substance
manufactured pursuant to Section 2.3 (Validation Activities) hereof.
| 2.5 | Replidyne Review. Nisso shall provide Replidyne with the opportunity to review and approve all plans and protocols for Validation Batches prior to commencing any such batch, such approval not to be unreasonably withheld or delayed, provided that Replidyne may withhold such approval if such plans or protocols fail to meet industry, FDA or ICH standards or cGMP or if Nisso’s equipment or process will not reasonably deliver the Drug Substance that would meet the warranties specified in Section 6.5 (Warranties) hereof. | |
| 2.6 | Inspections. Prior to use of the Nihongi Facility for the production of Drug Substance, and in addition to the provisions of Sections 7.3 (Inspections) and 7.6 (Access to Facilities), such facility shall have passed inspection and audit by Replidyne and all relevant Regulatory Authorities, including without limitation the FDA, for compliance with cGMPs, USP GMPs, ICH Guidelines or other guidelines issued by such Regulatory Authorities in the Territory or ICH. At the request of Replidyne or any relevant such Regulatory Authorities, Nisso shall correct any deficiency at the Nihongi Facility which would reasonably cause the production of the Drug Substance not to be in compliance with such requirements. If there is any difference in the opinion of Replidyne and Nisso with respect to the correction of the deficiencies identified in any inspection or audit of such facilities by Replidyne and Replidyne and Nisso are not able to agree upon a plan for correction of such deficiencies within thirty (30) days after inspection and audit by Replidyne, Replidyne and Nisso shall, without undue delay, reach an agreement on a Third Party expert who shall finally decide the issue regarding the correction of such deficiencies. The award issued by such Third Party expert shall be final and binding upon Replidyne and Nisso. Replidyne and Nisso shall share the cost of the Third Party expert equally. If Nisso agrees to make a correction or is obliged to make a correction of the deficiency in accordance with the award issued by the Third Party expert, Nisso shall bear all costs relating to such correction. | |
| 2.7 | Launch Go Date. When Replidyne decides in its sole discretion to Launch the Drug Product and place its first firm purchase order for scaled up production of Drug Substance for commercial use in such Launch, Replidyne shall so notify the Supplier in writing. The date of such notice to Supplier shall be referred to herein as the “Launch Go Date”. (For clarity, Replidyne may, at its option prior to the Launch Go Date, delay its decision to commercially sell a Drug Product, or determine to not commercially sell a Drug Product, or make a limited launch of a Drug Product, without liability to Supplier other than pursuant to Section 3.3 (Compensation to Nisso for Delay in Launch) or Section 12.5(c) (Engineering Costs) or Section 12.5(d) (Other Pre-Approved Reimbursable Costs) hereof.) Nisso shall use its commercially reasonable efforts to have the capability to |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 4 |
| commercially produce the Drug Substance at the Nihongi Facility in accordance with the Timeline. | ||
| 2.8 | Registration. Replidyne shall have the right to specify the Nihongi Facility as the site for production of the Drug Substance in all NDAs and Regulatory Approvals related to the Drug Products. Nisso shall register the Nihongi Facility with all relevant Regulatory Authorities, including, without limitation, with the FDA by (i) filing a FDA Form 2656 for the Nihongi Facility, and (ii) completing a foreign site listing with the registration of a U.S. agent for the Nihongi Facility. | |
| 2.9 | Available Capacity. From and after the Launch Go Date during the Term, Nisso shall at all times maintain an annual manufacturing capacity for Drug Substance of not less than [ *** ] tons in potency base at the Nihongi Facility. | |
| 2.10 | Stability Studies. Nisso shall undertake stability studies in accordance with a timeline and plan that shall be mutually agreed in writing between Replidyne and Nisso. Nisso shall provide Replidyne with the opportunity to review and approve all plans and protocols related to such stability studies, such approval shall not be unreasonably withheld or delayed, provided that Replidyne may withhold such approval if such plans or protocols fail to meet cGMPs, Regulations or other guidelines issued by Regulatory Authorities or ICH. |
ARTICLE 3: MANUFACTURE AND SUPPLY OF DRUG SUBSTANCE
| 3.1 | Supply of the Drug Substance. Subject to the provisions of this Agreement, during the Term, the Supplier shall exclusively supply Replidyne with all the Drug Substance which Replidyne, Replidyne’s Affiliates and its sublicensees, as authorized and directed in writing by Replidyne from time to time (hereinafter collectively called “Replidyne’s Designees”), require for their pre-clinical, clinical and commercial use in the Territory, and Replidyne shall exclusively purchase all of Replidyne’s and Replidyne’s Designees’ pre-clinical, clinical and commercial requirements of the Drug Substance in the Territory from DSP, which in turn shall obtain such supply from Nisso; provided, however, that such obligation of Replidyne shall cease to apply, and Replidyne may in its sole discretion manufacture or purchase any quantities of the Drug Substance from Third Parties, as provided in Section 3.2 (Minimum Purchase Quantities) hereof and Article 8 (Transfer of Manufacturing) hereof. Supplier hereby undertakes to supply such quantities of the Drug Substance as may be ordered by Replidyne, on the terms and conditions provided herein. Subject to Supplier’s prior written consent (such consent not to be unreasonably withheld), upon written request to Supplier by Replidyne, Nisso shall deliver the Drug Substance directly to Replidyne’s Designees. During the Term, all quantities of Drug Substance manufactured or supplied by Supplier and their Affiliates for use or sale in the Territory shall be exclusively for Replidyne. In the event that Supplier or their Affiliates manufacture or supply, or agree to manufacture or supply, any product which is, or is to be used for, a pharmaceutical formulation of faropenem daloxate to any Third Party on terms which are in any respect more favorable than the terms of this Agreement, then Supplier |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 5 |
| shall disclose such terms to Replidyne, and the Parties shall meet and negotiate in good faith commercially reasonable changes to this Agreement. | ||
| 3.2 | Minimum Purchase Quantities. |
| (a) | First Three Years. Within ninety (90) days after the Launch Go Date, Replidyne and Nisso shall negotiate in good faith and mutually agree in writing upon appropriate minimum purchase quantities of the Drug Substance for the three (3) year period (the “Three Year Period”) which commences on date of the first shipment of Drug Substance from Nisso for use in such Launch. The Parties agree that a minimum purchase quantity of [ *** ] metric tons of the Drug Substance during the Three Year Period is a reasonable requirement based on Nisso’s investment and costs associated with the production of the Drug Substance. Accordingly, the minimum purchase quantity for the Three Year Period shall be [ *** ] metric tons of the Drug Substance unless Replidyne determines that it is necessary to have a lower minimum based on results of the Phase III clinical studies, market conditions, reasonable sales projections and other relevant information relating to the commercialization of the Drug Products. In such event, Replidyne and Nisso shall negotiate in good faith in an effort to agree in writing on a lower minimum purchase quantity of the Drug Substance for the Three Year Period. If Replidyne and Nisso cannot agree on a lower minimum within such ninety (90) day period and Replidyne does not agree to the [ *** ] metric ton minimum within ten (10) days thereafter, then (i) Nisso shall have the right to terminate this Agreement and receive reimbursement from Replidyne pursuant to Section 12.5(c) (Engineering Costs) hereof, and (ii) Replidyne shall have the right to require Nisso to transfer the Manufacturing Technology of the Drug Substance to Replidyne or its designee (the “Manufacturing Designee”) pursuant to the terms and conditions stipulated in Sections 8.2, 8.3, 8.4 and 8.5 hereof. | ||
| (b) | Next Five Years. Within ninety (90) days prior to the end of the Three Year Period, Replidyne and Nisso shall negotiate in good faith and agree in writing upon appropriate minimum purchase quantities of the Drug Substance for the five (5) year period commencing on the date following the last day of the Three Year Period and ending on the fifth (5th) anniversary date thereof (the “Five Year Period”). The Parties agree that a minimum purchase quantity of [ *** ] metric tons per year of the Drug Substance during the Five Year Period is a reasonable requirement based on Nisso’s investment and costs associated with the production of the Drug Substance. Accordingly, the minimum purchase quantity for the Five Year Period shall be [ *** ] metric tons per year of the Drug Substance during the Five Year Period unless Replidyne determines that it is necessary to have a lower minimum based on market conditions, reasonable sales projections and other relevant information relating to the commercialization of the Drug Products. In such event, Replidyne and Nisso shall negotiate in good faith in an effort to agree in writing on a lower minimum purchase quantity of the Drug Substance for the Five Year Period. If Replidyne and Nisso cannot agree on a lower minimum within such ninety-day |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 6 |
| period and Replidyne does not agree to the [ *** ] metric ton per year minimum within ten (10) days thereafter, then (i) Nisso shall have the right to terminate this Agreement, and (ii) Replidyne shall have the right to require Nisso to transfer the Manufacturing Technology of the Drug Substance to Replidyne or its Manufacturing Designee pursuant to the terms and conditions stipulated in Sections 8.2, 8.3, 8.4 and 8.5 hereof. | |||
| (c) | Remainder of the Term. For each year during the Term after the Five Year Period (the “Remaining Term”), Replidyne and Nisso shall negotiate in good faith and agree in writing upon an appropriate minimum purchase quantity of the Drug Substance on an annual basis (each such year shall be a “Remaining Term Year”). Within ninety (90) days prior to the end of the Five Year Period or the then-current Remaining Term Year, as applicable, Replidyne and Nisso shall meet or communicate for the purpose of establishing the minimum purchase quantity for the next Remaining Term Year. The Parties agree that a minimum purchase quantity of [ *** ] metric tons per year of the Drug Substance for each Remaining Term Year is a reasonable requirement based on Nisso’s investment and costs associated with the production of the Drug Substance. Accordingly, the minimum purchase quantity for each such Remaining Term Year shall be [ *** ] metric tons of the Drug Substance unless Replidyne determines that it is necessary to have a lower minimum based on market conditions, reasonable sales projections and other relevant information relating to the commercialization of the Drug Products. In such event, Replidyne and Nisso shall negotiate in good faith in an effort to agree in writing on a lower minimum purchase quantity of the Drug Substance for the next Remaining Term Year. If Replidyne and Nisso cannot agree on a lower minimum within such ninety (90) day period and Replidyne does not agree to the [ *** ] metric ton per year minimum within ten (10) days thereafter, then (i) Nisso shall have the right to terminate this Agreement, and (ii) Replidyne shall have the right to require Nisso to transfer the Manufacturing Technology of the Drug Substance to Replidyne or its Manufacturing Designee pursuant to the terms and conditions stipulated in Sections 8.2, 8.3, 8.4 and 8.5 hereof. | ||
| (d) | Effect of Non-Exclusive Rights. If for any reason the rights of Replidyne pursuant to the License Agreement become non-exclusive rather than exclusive, including without limitation pursuant to Section 8.3 of the License Agreement, then the minimum purchase quantities obligations of Replidyne pursuant to this Section 3.2 shall cease to apply. | ||
| (e) | Credit to Replidyne. For purposes of Replidyne’s performance of its minimum purchase obligations hereunder, in case, without Replidyne’s failure, although Replidyne is unable to receive all quantities of the Drug Substance from Supplier which Replidyne ordered in good faith pursuant to Section 4.3 (Purchase Orders) hereof, it shall be deemed that Replidyne has satisfied its corresponding minimum purchase obligation hereunder to the extent that the quantities not received by |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 7 |
| Replidyne would have caused Replidyne to meet or exceed the applicable minimum purchase quantities. | |||
| (f) | Termination of Purchase in the event of Transfer of Manufacturing. In the event of the transfer of the manufacturing of the Drug Substance pursuant to Section 8.1 (Termination of Exclusive Purchase), Replidyne’s obligation to complete the purchase of such minimum purchase quantities shall terminate, provided, however, that if the cause for such transfer of manufacturing is due to Section 8.1 (a) (Prices Not Competitive), then Replidyne’s obligation shall not terminate for the Three Year Period. | ||
| (g) | Security of Supply. If Replidyne has reasonably determined that for security of supply against the risk stipulated in Section 8.1(c) (Failure to Supply Requirements) and 8.1(d) (Force Majeure) it would be necessary or advisable to have alternative sources of the Drug Substance, and Replidyne has consulted with Supplier regarding the basis for such determination, and Supplier has not provided adequate assurances that are satisfactory to Replidyne related to Replidyne’s concerns, then Nisso shall disclose all Manufacturing Technology (excluding, however, the Sensitive Manufacturing Information (defined below)) to Replidyne and/or a Third Party designated by Replidyne for Replidyne’s use in the production of the Drug Substance and the Drug Product only. DSP and Nisso shall assist Replidyne and/or such Third Party designated by Replidyne as reasonably necessary to manufacture the Drug Substance; provided that such assistance shall be at Replidyne’s expense. | ||
| (h) | Remedies. The compensation provisions of Section 5.8 (Compensation to Supplier) and Section 12.5(b) (Minimum Purchase Obligations) shall be Supplier’s sole remedy for Replidyne’s failure to purchase the minimum purchase quantities of Drug Substance pursuant to this Section 3.2. For clarity, the provisions of this Section 3.2, Section 5.8 and Section 12.5(b) shall only apply from and after the Launch Go Date. | ||
| (i) | Low Dose Drug Product. It is expressly understood that the provisions of the minimum purchase quantities stipulated in this Section 3.2 shall not be applied in case Replidyne makes only a limited launch (and not the Launch) of the Drug Product in the Territory. |
| 3.3 | Compensation to Nisso for Delay. If the Launch Go Date has not occurred by January 1, 2007, then Replidyne shall compensate Nisso for a percentage of actual, documented and reasonable maintenance and depreciation costs incurred by Nisso relating to the Nihongi Facility (Nisso’s estimate of such costs are specified in Attachment 3.3 hereto) (the “Delay Compensation”) for the period (the “Delay Compensation Period”) beginning on July 1, 2007 and ending on the date on which this Agreement terminates or Replidyne has both (i) provided its Launch Go Date notice to Supplier pursuant to Section 2.7 (Launch Go Date) hereof and (ii) made payment for delivery of the first firm purchase order for scaled up |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 8 |
production of Drug Substance for commercial use in Launch contemplated by
the Launch Go Date, as follows:
| Portion of Delay Compensation Period | Percentage of Delay Compensation Payable | |
[ *** ]
|
[ *** ] percent ([ *** ]%) | |
([ *** ] to [ *** ]) |
||
[ *** ]
|
[ *** ] percent ([ *** ]%) | |
([ *** ] to [ *** ]) |
||
Remainder of Delay Compensation Period
|
[ *** ] percent ([ *** ]%) | |
(From and after [ *** ]) |
provided, that the Delay Compensation for any twelve month period (before the above
percentages are applied) shall not exceed JP¥[ *** ]. For clarity, it is expressly
understood that in case the Launch Go Date occurs between January 1, 2007 and June 30, 2007,
the Delay Compensation hereof shall not be applied. If Replidyne disagrees with the amount
of the Delay Compensation as calculated by Nisso, Replidyne and Nisso shall negotiate in
good faith to resolve any differences. If both Parties cannot reach an agreement on the
Delay Compensation after thirty (30) days, Replidyne and Nisso shall select a Third Party
accounting firm mutually agreed by both Parties to finally decide any differences in the
calculation of the Delay Compensation. The award issued by such Third Party accounting firm
shall be final and binding upon both Parties. Replidyne and Nisso shall share equally the
cost of the Third Party accounting firm. No other fees or compensation shall be payable to
Nisso related to such delay pursuant to this Agreement. Notwithstanding the foregoing, no
Delay Compensation shall be payable (i) if Nisso is not in material compliance with its
obligations under this Agreement, or (ii) Nisso or the Nihongi Facility is not on schedule
to manufacture and supply the Drug Substance in accordance with the provisions of this
Agreement and the Timeline and Nisso has not satisfied all material qualification,
validation and registration matters in the Territory, or (iii) for time periods after the
expiration or termination of this Agreement. The payment of the Delay Compensation shall be
made to the bank account designated by Nisso within thirty (30) days following (i) the end
of each calendar year during the Delay Compensation Period, and (ii) the end of the Delay
Compensation for the partial calendar year in which the Delay Compensation Period ends.
| 3.4 | Coordinators. During the Term, Replidyne, DSP and Nisso shall each appoint one or more authorized representatives (“Coordinators”) for the exchange of all communications, other than formal notices hereunder, related to the supply of the Drug Substance. Each Party shall provide notice to the other Parties as to the name and title of the individuals so appointed. Each Party may replace its Coordinators at any time for any reason by providing written notice to the other Parties. The Coordinators shall meet at least once in each calendar year to review each Party’s past and future performance hereunder. | |
| 3.5 | Drug Products Plans. Replidyne’s plans and schedule for arrangements to manufacture the Drug Substance into Drug Products shall be provided to Supplier on or before the Launch Go Date. Replidyne reserves the right to modify such plans, schedule and |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 9 |
| arrangements from time to time in its sole discretion, provided that Replidyne shall inform Supplier of material changes to such plans and schedule related to this Agreement. | ||
| 3.6 | Observance of Applicable Laws. The Parties shall exercise and perform their respective rights and obligations under this Agreement in accordance with all applicable laws, including, without limitation, all Applicable Laws and Regulations of Japan, the U.S. and Canada and each country in which Drug Substance manufacturing is being conducted or that pertain to the performance by the Supplier of their obligations in connection with the manufacturing of the Drug Substance. The Parties shall assist each other in complying with all Applicable Laws and Regulations. | |
| 3.7 | Protective Measures. Each Party acknowledges that the Drug Substance is a Class 3 product that presents significant risks for employee injury and environmental contamination, and accordingly each Party undertakes and agrees at its cost to take all necessary and advisable precautions to ensure, and each Party shall be solely liable for, (i) the health and safety of its employees and all other persons under its direction or control having contact with the Drug Substance, and (ii) the protection of the environment related to Drug Substance in its possession. | |
| 3.8 | Facilities. Nisso shall manufacture (i) pre-clinical and clinical quantities of the Drug Substance at the Takaoka Facility or the Nihongi Facility, as mutually agreed, and (ii) commercial quantities of the Drug Substance only at the Nihongi Facility. | |
| 3.9 | No Cross-Contamination. Nisso shall use the Nihongi Facility only for the manufacture of (i) faropenem drug substance (i.e. faropenem sodium and faropenem daloxate), and (ii) drug substance for other anti-biotic pharmaceuticals in the penem class. Nisso shall not produce any other drugs or products at the Nihongi Facility, except with the prior written consent of Replidyne, not to be unreasonably withheld. |
ARTICLE 4: FORECASTS, PURCHASE ORDERS AND DELIVERIES
| 4.1 | Batch Sizes. The permissible batch sizes for the purposes of forecasts and Purchase Orders pursuant to this Article 4 are as specified in Attachment 4.1 hereto. | |
| 4.2 | Forecasts. Replidyne shall provide DSP with the following forecasts for the purchase of quantities of the Drug Substance pursuant to this Agreement (which DSP will promptly transfer such forecasts to Nisso), which shall constitute non-binding estimates of expected orders for the Drug Substance so that Nisso may make plans for the manufacturing of the Drug Substance. At the request of Nisso or DSP, the Parties shall review and discuss forecasts provided by Replidyne to assist the Nisso in efficiently scheduling the manufacture of the Drug Substance to meet the requirements of Replidyne pursuant to this Agreement. |
| (a) | Clinical Forecasts. Replidyne’s initial plan for the purchase of quantities of the Drug Substance to manufacture Drug Product for clinical use on a quarterly basis during the twelve (12) month period following the Effective Date is attached as |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 10 |
| Attachment 4.2(a) hereto. Such forecast shall be reviewed and updated by Replidyne at least every six (6) months. | |||
| (b) | Planning Forecasts for Commercial Quantities. Twelve (12) months prior to the first anticipated commercial shipment of the Drug Substance, and on or before the first day of each calendar quarter thereafter, Replidyne shall provide the Supplier with a written rolling five (5) year forecast, beginning on such first day of such calendar quarter, on a calendar quarterly basis (each a “Planning Forecast”, which shall include the items specified in Section 4.3(i) and (ii) hereof, but need not include other items) of its expected orders of the Drug Substance for the commercial sale of Drug Products. | ||
| (c) | Purchase Forecasts for Commercial Quantities. Twelve (12) months prior to the first anticipated commercial shipment of the Drug Substance, and on or before the first day of each calendar quarter thereafter, Replidyne shall provide the Supplier with a written rolling twelve (12) month forecast, beginning on such first day of such calendar quarter, on a monthly basis (each a “Purchase Forecast”, which shall include the items specified in Section 4.3(i) and (ii) hereof, but need not include other items) of its expected orders of the Drug Substance for the commercial sale of the Drug Products. |
| 4.3 | Purchase Orders. Replidyne may submit purchase orders to DSP (which DSP will promptly transfer such purchase order to Nisso) (“Purchase Orders”) during the Term for Drug Substance, with delivery dates in accordance with the lead times specified in Section 4.4 (Lead Times). Purchase Orders shall, at a minimum, include: (i) identification of the specific Drug Substance ordered; (ii) quantity; (iii) delivery date; (iv) shipping instructions and shipping address; and (v) the Purchase Price of the Drug Substance ordered. Any Purchase Order placed by Replidyne shall be subject to acceptance by Nisso; provided, however, that all Purchase Orders issued in accordance with this Agreement for quantities up to [ *** ] percent ([ *** ]%) of the quantities of the Drug Substance set forth in the first three (3) months of the most recent Purchase Forecast shall be automatically confirmed and accepted by Nisso and shall be supplied by the Supplier. The Supplier shall not be obligated, but shall use commercially reasonable efforts, to ship more than such quantities if ordered by Replidyne. It is expressly understood that, after using commercially reasonable efforts, if the Supplier fails to supply such excess quantities of the Drug Substance ordered by Replidyne, such failure shall not be deemed as a breach of this Supply Agreement, and Section 8.1 (Termination of Exclusive Purchase) hereof shall not apply, unless Nisso had accepted Replidyne’s Purchase Order(s) for the excess quantities not supplied. Replidyne shall have the obligation to purchase at least [ *** ] percent ([ *** ]%) of the quantities of the Drug Substance set forth in the first three (3) months of the most recent Purchase Forecast. The terms and conditions of this Agreement shall govern and supersede any additional or contrary terms set forth in any Purchase Order of Replidyne or any acceptance, confirmation, invoice or other document of the Supplier or Replidyne unless duly signed by an officer of Replidyne, an officer of DSP and an officer of Nisso and expressly stating and identifying which specific additional or contrary terms shall supersede the terms and conditions of this Agreement. Notwithstanding the foregoing, |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 11 |
| Replidyne shall make the Purchase Order prior to the Launch Go Date no more than once every three (3) months. | ||
| 4.4 | Lead Times. Supplier acknowledges and agrees that time is of the essence in making deliveries of Drug Substance. The normal lead time for delivery of Drug Substance under any Purchase Order shall be [ *** ] months. Replidyne shall specify a delivery date in each Purchase Order, which shall not be a date that is less than [ *** ] months following the date of such Purchase Order, provided that: |
| (a) | Waivers by Nisso. This limitation shall be waived if Nisso confirms such Purchase Order, even if such delivery date is less than [ *** ] months following the date of such Purchase Order. | ||
| (b) | First Purchase Order. The lead time for Replidyne’s first Purchase Order for commercial quantities of Drug Substance (whether such Purchase Order is issued before or at the Launch Go Date) shall be [ *** ] months, provided that Replidyne and Nisso shall in good faith negotiate and agree shorter lead time for the first Purchase Order subject to Replidyne’s cooperation with Nisso to guarantee payment for the advance purchase of raw materials pursuant to Section 12.5(d) (Other Pre-Approved Reimbursable Costs) hereof. | ||
| (c) | Launch Go Date Purchase Order. Subject to Section 4.4(b) (First Purchase Order) hereof, the lead time for Replidyne’s Purchase Order submitted with Replidyne’s notice to Supplier of the Launch Go Date pursuant to Section 2.7 (Launch Go Date) hereof shall have a lead time equal to the sum of (i) [ *** ] months, plus (ii) the time period, not to exceed an additional [ *** ] months, during which Replidyne and Nisso reach agreement upon minimum purchase quantities of the Drug Substance for the Three Year Period pursuant to Section 3.2(a) (First Three Years) hereof. |
Nisso (i)shall confirm any Purchase Order with a delivery date that is more than [ *** ] months
(or other time periods specified above) following the date of such Purchase Order
subject to the provisions in Section 4.3 above, and (ii) may, but shall not be
obligated to, confirm any Purchase Order with a delivery date that is less than [ *** ]
months following the date of such Purchase Order. Nisso shall timely deliver the specified
quantities of the Drug Substance by the date that is specified in Replidyne’s Purchase
Order. If Nisso is unable, at any time, to supply Replidyne on a timely basis with the
quantity of Drug Substance ordered by Replidyne in accordance with Section 4.3 for
any reason, including the occurrence of a force majeure event under Article 15 (FORCE
MAJEURE), the Supplier shall immediately notify Replidyne of such inability to supply and
the estimated extent of such inability (including delay time and the quantity of Drug
Substance involved).
| 4.5 | Modification of Purchase Orders. No Purchase Order shall be modified or canceled except upon the mutual written agreement of Replidyne and the Supplier, except as provided in this Section 4.5. Mutually agreed change orders shall be subject to all |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 12 |
provisions of this Agreement, whether or not the changed Purchase Order so states. Notwithstanding the
foregoing, any Purchase Order may be canceled by Replidyne as to any quantity of Drug
Substance which is not delivered by or on the delivery date specified by Replidyne in the
relevant Purchase Order (as modified if applicable). Replidyne shall have the right to modify
all forecasts provided pursuant to Section 4.2 (Forecasts) hereof but shall not have
the right to modify or cancel the Purchase Order, if for any reason DSP decides to alter the
exclusive license granted to Replidyne under the License Agreement to become a non-exclusive
license, including without limitation pursuant to Section 8.3 of the License Agreement,
provided, however, that DSP shall postpone such alteration of the exclusive license to a
non-exclusive license until the Drug Substance of such Purchase
Order is used up for the manufacturing of the Drug Product and such Drug Product has been
sold by Replidyne in the ordinary course of its business, but such postpone period shall not
exceed twelve (12) months.
| 4.6 | Release Documentation. All Drug Substance shall meet the Specifications and shall be subjected to a quality control inspection and final release by Nisso. Nisso, at its cost, shall complete the manufacture, inspect and complete all necessary testing and other functions for each batch of the Drug Substance to demonstrate conformance to the warranties provided in Section 6.5 (Warranties) hereof prior to the release of the Drug Substance for shipment, all in accordance with Nisso’s quality control standards and in compliance with cGMPs. Nisso shall provide to Replidyne prompt written notice of such release, and shall include a packing list and documents in each shipment of the Drug Substance providing the following information and such other information as may be reasonably requested by Replidyne from time to time (“Release Documentation”): (i) Purchase Order number; (ii) Drug Substance code; (iii) quantity; (iv) Nisso lot number; (v) a certificate of analysis for each batch of the Drug Substance (which shall contain, at a minimum, analytical tests and results, date of manufacture, date of re-control (or shelf-life of the batch), batch size (total amount produced), a release statement, and a certification that the analytical tests are performed in accordance with the United States Pharmacopoeia); (vi) a certificate of compliance for each batch of the Drug Substance (confirming that the Drug Substance was manufactured in accordance with cGMPs); (vii) the results of all relevant testing data for each batch of the Drug Substance; and (viii) if required by Replidyne, samples of each batch of the Drug Substance (which shall be included in the quantities ordered by Replidyne under the relevant Purchase Order) for Replidyne’s independent testing pursuant to Section 4.8 (Inspection, Shortages and Defects) hereof (the reasonable shipping costs of such samples shall be for the account of Replidyne), which shall be reasonably satisfactory to Replidyne. Nisso shall also mail (i) copies of such packing list and documents to the shipping destination and to Replidyne, and (ii) such samples to Replidyne, for each shipment at the time of shipment. Nisso shall also notify Replidyne of rejected batches of the Drug Substance with the reasons for such rejection, and upon Replidyne’s request shall provide copies of Nisso’s internal documents related to deviation investigations. | |
| 4.7 | Delivery, Title and Risk of Loss. All Drug Substance shall be shipped by the Supplier FCA (“Free Carrier”), INCOTERMS 2000, Narita airport (“Shipping Point”) after its |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 13 |
release by Nisso at the time notified by Replidyne to the Supplier. The Parties
acknowledge and agree that the sale of Drug Substance to Replidyne under this Agreement is an
export sale which should not be subject to Japanese Consumption Tax; in the event that any
Japanese tax authority asserts such a tax liability against Replidyne, Supplier shall
cooperate with Replidyne, including without limitation providing any necessary documentation
and properly reforming this Agreement, to confirm that the sales of Drug Substance hereunder
are export sales from Japan. All Drug Substance shall be properly stored by Nisso in
accordance with the Specifications, cGMPs and Replidyne’s reasonable instructions. Physical
delivery of the Drug Substance and the Release Documentation to Replidyne may be made directly
by Nisso.
| 4.8 | Inspection, Shortages and Defects. |
| (a) | Inspection. As between the Parties, Replidyne and its representatives shall have the right, but not the obligation, to inspect the Drug Substance and, if desired, independently test the samples (using such testing methods as Replidyne may determine) sent to Replidyne pursuant to Section 4.6 (Release Documentation) hereof, provided, however, that Replidyne acknowledges that Replidyne is subject to the inspection requirements, as applicable, for the Drug Substance in accordance with cGMPs, USP GMPs, ICH Guidelines or other guidelines issued by such Regulatory Authorities in the Territory or ICH, including, but not limited to, the inspection of the Release Documentation. Replidyne shall provide notice to Supplier if it discovers any shortage in quantity or Defect in quality pursuant to this Section 4.8. | ||
| (b) | Shortages. After the receipt of each shipment of the Drug Substance, Replidyne or its authorized representatives shall carry out a quantity count of such shipment. Replidyne shall notify Supplier in writing of any obvious shortage in quantity of any shipment of Drug Substance within its possession within forty (40) days after receipt by Replidyne and/or Replidyne’s Designees. A shipment of the Drug Substance is to be considered to have fulfilled the quantity specified in the Purchase Orders if Replidyne or its representatives does not notify the Supplier about any objections within such time periods. | ||
| (c) | Defects. Upon notification to Supplier, Replidyne shall have the right to reject any lot in whole or in part that contains Drug Substance which is damaged or has a Defect (“Defective Product”) which should reasonably be discovered in the inspection stipulated in Section 4.8 (a) (Inspection) and quantity count of the shipment stipulated in Section 4.8 (b) (Shortages) above, provided, however, that such notification shall be provided to the Supplier within forty (40) days following the receipt of the Drug Substance by Replidyne and/or Replidyne’s Designees, and provided, further, that such notification shall be provided to the Supplier within one hundred eighty (180) days following the receipt of the Defective Product with respect to latent Defect which Replidyne cannot reasonably discover through the inspection stipulated in Section 4.8(a) (Inspection) above. For purposes of this |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 14 |
| Section 4.8 (but not for purposes of any other provision of this Agreement, including without limitation Sections 6.5 (Warranties) and 13.2 (Hold Harmless by Supplier) hereof), a shipment of the Drug Substance is to be considered to have fulfilled the Specifications, and Replidyne shall not request a replacement or credit for such shipment, if Replidyne or its representatives does not notify the Supplier about any Defects within such time periods. Replidyne shall provide Supplier with information as to the reason for the rejection of the Defective Product, including a description of the test procedure and results, if any, on which the rejection is based. | |||
| (d) | Disposal or Return. Supplier shall instruct Replidyne as to the disposal or return of Defective Product. If Supplier instructs Replidyne to dispose of Defective Product, Supplier shall be responsible for such disposal costs. If Supplier instructs Replidyne to return Defective Product, Supplier shall be responsible for such return shipping charges. | ||
| (e) | Replacement or Credit. At Replidyne’s option, Supplier shall either (i) immediately replace shortages of the Drug Substance or Defective Product, without additional cost to Replidyne, permit Replidyne to issue a debit memorandum to Supplier for the Purchase Price for shortages of the Drug Substance or Defective Product, and re-invoice Replidyne for the Drug Substance shipped to replace such shortages or Defective Product at the time of shipment of the replacement Drug Substance; or (ii) credit Replidyne for the Purchase Price for such shortages or Defective Product after receipt of Replidyne’s debit memorandum related to such shortage of the Drug Substance or Defective Product. | ||
| (f) | Exceptions. The provisions of this Section 4.8 shall not apply to any Drug Substance which has a Defect due solely to (i) storage, handling, formulation, labeling or shipping by Replidyne, its Affiliate, sublicensees or customers, or (ii) Defects first discovered after the expiry of its shelf life. | ||
| (g) | Disputes. In the event of a dispute regarding whether any Drug Substance has a Defect which Supplier and Replidyne are unable to resolve, a sample of such Drug Substance shall be submitted by Replidyne to an independent laboratory reasonably acceptable to Nisso and Replidyne for testing and the test results obtained by such laboratory shall be final and controlling. The fees and expenses of such laboratory testing and all additional shipping and transportation costs incurred as a result of the dispute shall be borne entirely by the party against whom such laboratory’s findings are made. |
ARTICLE 5: PURCHASE PRICE AND PAYMENT
| 5.1 | Purchase Prices. |
| (a) | General. As full compensation for the purchase by Replidyne of the Drug Substance and all other obligations to be performed by Supplier hereunder (other than as provided in Sections 3.3 (Compensation to Nisso for Delay), 5.8 |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 15 |
| (Compensation to Supplier) and 12.5 (Compensation to Nisso) hereof), Replidyne shall pay DSP the prices specified in Attachment 5.1 hereto (the “Purchase Prices”) for quantities of the Drug Substance actually delivered by Nisso pursuant to Section 4.7 (Delivery, Title and Risk of Loss) hereof, subject to adjustment pursuant to this Article 5. No other fees shall apply. | |||
| (b) | Reconciliation. The Purchase Price to be paid by Replidyne for the Drug Substance is based solely upon quantities of the Drug Substance purchased during any given calendar year. Based on the Purchase Forecast of Replidyne for the relevant year, an estimated Purchase Price for each Purchase Order in such year shall be calculated by the Parties. Within thirty (30) days following the end of each calendar year, the Supplier shall submit to Replidyne a reconciliation of the total amount that should have been paid by Replidyne for all quantities of the Drug Substance purchased during such calendar year in accordance with the actual Purchase Price against the total amounts actually billed by Supplier and paid for by Replidyne for such quantities based on the estimated Purchase Price for such calendar year. Replidyne shall within a period of thirty (30) calendar days after receipt of any reconciliation prepared by the Supplier by written notice either issue its confirmation of the amount identified therein as an overpayment or underpayment or state that it disagrees therewith with reasons set out therein. If no such written objection is delivered to Supplier by the end of such thirty (30) day period, then the overpayment or underpayment amount identified in such reconciliation shall be deemed to be accepted and approved by Replidyne and shall be final and binding on the Parties. If a written objection is delivered to the Supplier within such thirty (30) day period, then the matter shall be resolved in accordance with Article 20 (Dispute Resolution) hereof. If such reconciliation, as accepted or as so finally determined, shows that Replidyne has overpaid for such purchases, then the Supplier shall within ten (10) calendar days of the reconciliation being finally determined (the “Final Reconciliation”), at Replidyne’s election, either refund such overpayment or credit such overpayment against future purchases of the Drug Substance. If such reconciliation shows that Replidyne has underpaid for such purchases, then Replidyne shall remit the balance so determined to be due to the Supplier within ten (10) calendar days of the Final Reconciliation. |
| 5.2 | Adjustment of Purchase Prices. If Nisso reasonably decides that an adjustment of the Purchase Price is commercially necessary due to an increase in the aggregate direct manufacturing costs of Nisso (including labor costs and costs of materials, but excluding indirect administrative and other allocated or fixed costs) for the Drug Substance, which can be documented by Nisso (after taking into account items of cost decreases), the Parties shall negotiate with each other in good faith a reasonable mechanism for increasing the Purchase Prices that would apply during the following calendar year taking into account net increases and decreases in the aggregate in such direct manufacturing costs experienced by Nisso or a general inflationary factor equal to the increase or decrease in the wholesale price index (“WPI”) as published by the Bank of Japan, in both cases from the Effective Date (or, if a previous adjustment to the Purchase Prices has been made |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 16 |
| pursuant to this Section 5.2, from the date of such adjustment); provided, however, that in no event will the cumulative increase in the Purchase Prices during the Term exceed [ *** ] percent ([ *** ]%) of the prices specified in Attachment 5.1 hereto. Any claims of increased direct manufacturing costs of Nisso for an increase in the Purchase Prices pursuant to this Section 5.2 shall (i) be accompanied by a detailed statement with reasonable supporting documentation from Nisso, evidencing the changes in all direct manufacturing cost items from the direct manufacturing costs as of the Effective Date (or, if a previous adjustment to the Purchase Prices has been made pursuant to this Section 5.2, from the date of such adjustment), and (ii) be subject to audit by independent auditors pursuant to Section 5.5 (Audits) hereof. Nisso warrants that it shall provide to an independent certified public accountant or attorney nominated by Nisso and approved by Replidyne under confidentiality the details of the actual cost for each direct manufacturing cost item of the Drug Substance within thirty (30) days after the Effective Date, which shall be verified by such independent certified public accountant or attorney and then made available to independent auditors appointed by Replidyne in connection with audits undertaken pursuant to Section 5.5 (Audits) hereof to verify changes in Purchase Prices pursuant to this Section 5.2. | ||
| 5.3 | Payment. Amounts payable pursuant to this Article 5 shall be paid by Replidyne to DSP within forty five (45) days of receipt of the Supplier’s invoice (which shall not be issued by the Supplier prior to the release and delivery of the relevant Drug Substance) by wire transfer in immediately available funds to the following bank account of DSP: |
| Sumitomo Mitsui Banking Co. | ||
| Tokyo-Chuo Branch | ||
| ▇-▇-▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇-▇▇, ▇▇▇▇▇, ▇▇▇-▇▇▇▇ ▇▇▇▇▇ | ||
| SWIFT : ▇▇▇▇▇▇▇▇ | ||
| Account Number: 246171 |
| If any amount payable to DSP under this Agreement is not paid when due and payable, DSP shall notify Replidyne of such non-payment, stipulating that payment must be made within seven (7) days from the date of notice. If Replidyne fails to make payment by the end of such notice period, then Replidyne shall pay to DSP interest on such amount at the rate that is three percent (3%) per annum above the rate 30 day U.S. Dollar LIBOR rate, as published in The Financial Times effective on the date such payment is due, such rate not to exceed the maximum interest rate permitted by Applicable Laws, from the date when due and payable until paid in full. Such interest rate shall change to a new thirty (30) day U.S. Dollar LIBOR date period (as applicable), shall be compounded monthly and shall be due and payable by Replidyne to DSP at the end of each calendar month that the amount remains unpaid until the date when such amount and all interest is paid in full. | ||
| 5.4 | Taxes. Replidyne shall be responsible for and shall pay all taxes, duties, import, deposits, sales taxes and value added taxes that are: (i) associated with the payment of any amounts by Replidyne to DSP pursuant to this Agreement, or (ii) based on the sale or use of the Drug Substance following delivery of the Drug Substance to Replidyne; provided, |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule
406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 17 |
| however, that Replidyne shall not be responsible for (A) taxes imposed on the Supplier by the country of manufacture of the Drug Substance or (B) taxes imposed on the Supplier’s income. Supplier shall be responsible for and shall pay all taxes, duties, import deposits, sales tax or value added taxes prior to delivery of the Drug Substance to Replidyne. If Applicable Laws require that taxes be withheld on any payment made under this Agreement, the paying Party shall be entitled to (i) deduct those taxes from the payment, (ii) pay the taxes to the proper taxing authority, and (iii) send evidence of the obligation together with proof of payment to the other Party hereto. |
| 5.5 | Audits. |
| (a) | By Replidyne. Nisso shall permit an independent auditor appointed by Replidyne subject to the reasonable approval of Nisso, and who shall enter into a confidentiality agreement in favor of Nisso, during the Term and for a period of two (2) years after the Term to examine and audit the books and records of Nisso related to Section 3.3 (Compensation to Nisso for Delay), Section 5.1 (Purchase Prices), Section 5.2 (Adjustment of Purchase Prices), Section 5.4 (Taxes), Section 5.9 (Cost Reduction Opportunities) or Section 12.5 (Compensation to Nisso), for the preceding three (3) year period, at a mutually convenient time within normal business hours to the extent necessary to determine the accuracy of any calculations for payments due to Nisso hereunder. The costs of such examination and audit shall be borne by Replidyne; provided, however, that if any such examination and audit discloses an overpayment by Replidyne of five percent (5%) or more for any relevant time period due to Nisso’s fault and/or negligence, Nisso shall reimburse Replidyne for the reasonable costs of such examination and audit. The amount of any such overpayments shall be reimbursed to Replidyne. |
| (b) | By Nisso. Effective upon the occurrence of any of the circumstances specified in Section 8.1 (Termination of Exclusive Purchase), Replidyne shall permit an independent auditor appointed by Nisso subject to the reasonable approval of Replidyne, and who shall enter into a confidentiality agreement with Replidyne, for a period commencing from the date on which Replidyne or Manufacturing Designee begins the manufacturing of the Drug Substance and ending two (2) years after such manufacturing ceases, to examine and audit the books and records of Replidyne related to Section 8.3 (Royalty), for the preceding three (3) year period, at a mutually convenient time within normal business hours to the extent necessary to determine the accuracy of any calculations for payments due from Replidyne under such Section 8.3. The costs of such examination and audit shall be borne by Nisso; provided, however, that if any such examination and audit discloses an underpayment by Replidyne of five percent (5%) or more for any relevant time period due to Replidyne’s fault and/or negligence, Replidyne shall reimburse Nisso for the reasonable costs of such examination and audit. The amount of any such underpayments shall be reimbursed to Nisso. |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 18 |
| 5.6 | Third Party Royalties. Except as provided in the License Agreement, the Purchase Prices are inclusive of all royalty payment obligations to Third Parties, which shall be for the sole account of and paid by DSP, including without limitation royalties due to Tanabe Seiyaku for U.S. Patent No. 5,260,438 and all counterparts thereto in Japan and the Territory. |
| 5.7 | Exchange Rate Risk. The Purchase Prices specified in Attachment 5.1 shall be adjusted for currency fluctuation based on this Section 5.7. For any month in which the Suppler issues an invoice to Replidyne under Section 5.3 (Payment) hereof, the Supplier shall include in such invoice the calculation of the average of the closing Yen/U.S. Dollar exchange rate as published in The Financial Times for the tenth (10th) and the last business day of each month for the immediately preceding six (6) months from the invoice date (“Averaged 6 Month Rate”). |
| (a) | If the Average 6 Month Rate is greater than the exchange rate benchmark of [ *** ] ¥/US$ (“Benchmark”) by more than [ *** ] percent ([ *** ]%), the Purchase Price shall be revised as follows: | ||
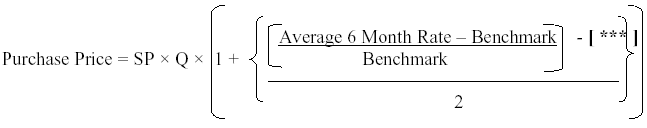 |
|||
| provided, however, that if the Average 6 Month Rate is greater than [ *** ] ¥/US$, then the figure [ *** ] ¥/US$ shall be used in place of the Average 6 Month Rate for purposes of this Section 5.7(a). | |||
| SP: Supply Price Stipulated in Attachment 5.1 Q: Quantities of the Drug Substance delivered by Nisso |
|||
| (b) | If the Average 6 Month Rate is less than the Benchmark by more than [ *** ] percent ([ *** ]%) but not more than [ *** ] percent ([ *** ]%), the Purchase Price shall be revised as follows: | ||
 |
SP: Supply Price Stipulated in Attachment 5.1
Q: Quantities of the Drug Substance delivered by Nisso
Q: Quantities of the Drug Substance delivered by Nisso
| For example, if the Average 6 Month Rate increases to [ *** ] ¥/US$ (+[ *** ]%), then the Purchase Prices will increase by [ *** ]%; and if the Average 6 Month Rate decreases to [ *** ] ¥/US$ (-[ *** ]%), then the Purchase Prices will decrease by [ *** ]%. |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 19 |
| (c) | If the Average 6 Month Rate fluctuates by [ *** ] percent ([ *** ]%) or less, then the Purchase Prices stipulated in Attachment 5.1 shall continue to apply. | ||
| (d) | If the Average 6 Month Rate decreases by more than [ *** ] percent ([ *** ]%) below the Benchmark (that is, the exchange rate is less than [ *** ] ¥/US$), then Replidyne may propose, in writing, to confer with the Parties regarding an adjustment of the Purchase Price, and the Parties shall discuss in good faith the amendment of the Purchase Prices. If the Parties cannot agree on the amendment of the Purchase Prices within sixty (60) days after commencing such discussions, Replidyne shall have the right to (i) terminate this Agreement and (ii) require Nisso to transfer the Manufacturing Technology of the Drug Substance to Replidyne or the Manufacturing Designee pursuant to the terms and conditions stipulated in Sections 8.2, 8.3, 8.4 and 8.5 hereof. Pending an agreement by the Parties on an adjustment of the Purchase Price or if the Parties cannot agree on a new Purchase Price in connection with the decrease in the exchange rate of more than [ *** ] percent ([ *** ]%), the Parties shall apply the exchange rate of [ *** ] ¥/US$ with respect to all Drug Substance shipped to Replidyne during such pending period. |
| 5.8 | Compensation to Supplier for Minimum Purchase Obligation. In the event that Replidyne fails to purchase the applicable minimum purchase quantities of Drug Substance as required by Section 3.2 (Minimum Purchase Quantities) at the end of the Three Year Period or at the end of applicable calendar year in the Five Year Period or at the end of then-current Remaining Term Year (but not thereafter), whichever is applicable, then as Supplier’s sole and exclusive remedy for the failure of such purchase Replidyne shall compensate Supplier in an amount equal to [ *** ] percent ([ *** ]%) of the aggregate Purchase Price pursuant to this Article 5 for such minimum purchase quantities which have not been purchased by Replidyne within thirty (30) days after the end of the Three Year Period, the applicable year in the Five Year Period and/or the then-current Remaining Term Year. |
| 5.9 | Cost Reduction Opportunities. |
| (a) | Alternative AOSA Supplies. If at any time (i) a Third Party vendor or Replidyne provides a firm quote to supply, for a specific period and a specific quantity, AOSA meeting the Specifications, or modified Specifications as accepted by Replidyne pursuant to Section 6.1 (Drug Substance Specifications) hereof, and specifying in detail the quantity and the validity period of such offer, at a lower cost than the greater of (A) Nisso’s current actual cost to manufacture AOSA (assuming an annual volume of Drug Substance of at least [ *** ] metric tons), or (B) JP¥[ *** ] per kilogram (the greater of such two amounts for purposes of this clause shall be “Nisso’s Cost”), (ii) such Third Party vendor or Replidyne agrees to commit in writing to supply, for a specific period and a specific quantity, Nisso’s requirements for AOSA to manufacture the Drug Substance for Replidyne’s requirements, (iii) an independent Third Party consultant who is knowledgeable in the field reasonably verifies that such Third Party vendor or Replidyne is able to |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 20 |
| meet Nisso’s requirements for AOSA, then Nisso shall be required to purchase and accept AOSA from such Third Party vendor or Replidyne. All of the costs and all of the benefits of such supply shall be for the account of Replidyne, provided that Nisso shall not be liable for failure or delay of supply of the Drug Substance by Nisso due to the failure or delay of the supply of AOSA by such Third Party vendor or Replidyne or any defect in the AOSA supplied by such Third Party vender or Replidyne. In such case, each purchase of AOSA from the Third Party vendor shall be made by Replidyne or be manufactured by Replidyne and Replidyne shall transfer such AOSA to Nisso at the same price per kilogram as Replidyne’s cost for such AOSA (the “Alternative AOSA Price”). The Purchase Prices for the Drug Substance, per kilogram, shall be reduced by an amount equal to the product of the Cost Difference times the AOSA Consumption. For such calculation, “Cost Difference” means the difference between Nisso’s Cost and the Alternative AOSA Price, and “AOSA Consumption” means the ratio (which is greater than 1:1) of the average quantity of AOSA used to manufacture one (1) kilogram of the Drug Substance, as specified in the then-current Drug Master File. Replidyne shall be responsible for any incremental costs associated with obtaining AOSA from such alternative Third Party vendor or Replidyne, including, but not limited to, reasonable validation costs. Notwithstanding the foregoing, Nisso may meet the Alternative AOSA Price in stead of accepting to purchase AOSA from such Third Party vender or Replidyne. In such event, the Purchase Price shall be reduced in the same manner as described above in this Section 5.9 (a). The provisions of Section 5.5 (Audits) hereof shall apply to permit Replidyne or its representatives to confirm that such cost reductions are so passed through to Replidyne, if requested by Replidyne. |
| (b) | Other Cost Reductions. For all cost reduction opportunities other than as provided in Section 5.9(a) (Alternative AOSA Supplies), Nisso shall use good faith efforts to identify and implement such opportunities to reduce the costs of the manufacture and supply of the Drug Substance, provided that Replidyne shall not share in the costs or benefits of such opportunities (except as provided in Section 5.2 (Adjustment of Purchase Prices)), unless if (i) Replidyne makes concrete proposal to reduce such costs and presents such proposal to Nisso, and (ii) Nisso concurs that such proposal is desirable or implements such proposal, then Nisso and Replidyne shall agree in writing to jointly pursue such proposal made by Replidyne and the costs and benefits of their efforts shall be equally shared. |
ARTICLE 6: DRUG SUBSTANCE QUALITY
| 6.1 | Drug Substance Specifications; DMF. The specifications (“Specifications”), including without limitation the route of synthesis (“Route of Synthesis”) which is included within the Specifications, for the Drug Substance are specified in Attachment 6.1 hereto. The Drug Substance shall comply with the requirements of the Specifications at the time of purchase. The current Specifications have been jointly established by the Parties (attached hereto as Attachment 6.1) and may only be amended or supplemented from time to time pursuant to the provisions below. No changes, modifications, deviations or exceptions to |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 21 |
| the Specifications, place of manufacture, materials or fabrication manufacturing or packaging processes may be made without the prior written approval of Replidyne, which approval may be provided or withheld by Replidyne in its sole discretion. |
| (a) | Significant Changes by Nisso. If Nisso intends to make any changes to its Route of Synthesis, major raw materials or supply sources thereof, manufacturing locations or analytical methods or any other amendment to the Drug Master File or Specifications which have the potential to impact Replidyne’s Regulatory Approvals (each a “Significant Change”), Nisso shall obtain Replidyne’s prior written approval before making such Significant Change. For any proposed Significant Changes that Replidyne does not approve, Replidyne shall notify the Supplier of its non-approval within three (3) months of Replidyne’s receipt of the notice of change and supporting documents from Nisso. Nisso shall not make a Significant Change until after Replidyne has given its written approval. | ||
| (b) | Other DMF Changes by Nisso. For other changes or updates to Nisso’s Drug Master File, Nisso shall notify Replidyne thirty (30) days prior to the filing of making such change or updates so that the Parties may discuss such changes or updates. In addition, Nisso shall provide Replidyne with written notification of Nisso’s Drug Master File updates and of any changes to the Drug Master File thereafter. | ||
| (c) | Changes by Replidyne. In the event that Replidyne requests Nisso to make changes to the Specifications, raw materials, fabrication, manufacturing or packaging processes, Nisso shall (i) make such change if related to a regulatory or safety concern, or (ii) reasonably consider such request in good faith if related to any other concern. In the event that raw materials costs, fabrication costs, manufacturing costs or packaging costs are materially increased or decreased by any such change requested by Replidyne and approved by Nisso, then Replidyne shall receive the benefit and obligation, as applicable, of such increase or decrease of the cost and the Parties shall adjust the Purchase Price accordingly; provided, however, that if additional engineering work, equipment acquisition and commissioning, training, qualification and validation activities and/or other works are required to implement any such changes requested by Replidyne, Replidyne shall bear all such costs. | ||
| (d) | Certain Costs and Benefits for Changes by Nisso. In the event that Nisso makes changes to the Specifications, raw materials, fabrication, manufacturing or packaging processes in accordance with Section 6.1 (a) and/or (b) above, (i) the Purchase Prices shall be reduced as provided in Section 5.9 (Cost Reduction Opportunities) if such changes result in cost reductions, and (ii) Nisso shall bear cost increases resulting from such changes, without an increase in the Purchase Prices, provided that Replidyne shall be compensated for any reasonable costs incurred by Replidyne for updates to its Regulatory Approvals and costs to manufacture Drug Products. In addition, if additional engineering work, equipment acquisition and commissioning, training, qualification and validation activities and |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 22 |
other work are required to implement any such changes, Nisso shall bear all such
costs.
| (e) | Certain Costs for Changes Required by Regulatory Authorities. Changes or modifications required to address the requirements of Regulatory Authorities shall be (i) borne and paid for by Replidyne if related to Drug Substance Specifications or Drug Product Specifications, or (ii) be borne and paid for by Nisso if related to the manufacturing or packaging processes. For clarity, Nisso shall promptly (and in any event within the time period specified by the applicable Regulatory Authority, or if no such time period is specified, within thirty (30) calendar days unless another suitable time period is mutually agreed by the Parties) make any change in the Specifications as required to address the requirements of the Regulatory Authorities approved in writing by Replidyne. |
| 6.2 | Packaging and Labeling. Replidyne shall, in its sole discretion, have control of and shall deliver the text of all packaging and labeling for the Drug Substance, and Nisso shall incorporate all reasonable changes directed in writing by Replidyne. If requested by Replidyne, Nisso shall provide Replidyne with a cost quote for any such change prior to Replidyne’s final authorization to make the change. Replidyne shall pay Nisso its actual costs of changes to packaging and labeling that are requested by Replidyne. | |
| 6.3 | Subcontracts and Suppliers. Commercial quantities of the Drug Substance shall be manufactured at and supplied from the Nihongi Facility. Nisso shall not use any subcontractor or supplier which (i) is not expressly specified in the then-current Specifications, or (ii) approved in writing by Replidyne. | |
| 6.4 | Minimum Shelf Life. All Drug Substance, as of the date shipped to Replidyne pursuant to Section 4.7 (Delivery, Title and Risk of Loss) hereof, shall have a remaining shelf life of not less than the longer of (i) eighteen (18) months, or (ii) the period that is a half of the duration of stability reasonably confirmed by Nisso in the ordinary course of its stability testing program, not to exceed four (4) years, provided, that Replidyne shall store the Drug Substance at a warehouses at a constant temperature of lower than ten (10) degrees centigrade and follow storage instructions in the Technical Agreement. | |
| 6.5 | Warranties. The Supplier represents, warrants and covenants to Replidyne as follows: |
| (a) | Drug Substance. The Drug Substance, at the time of delivery to Replidyne by Nisso pursuant to Section 4.7 (Delivery, Title and Risk of Loss) hereof: |
| (i) | shall conform to the Specifications, as then in effect; | ||
| (ii) | shall be free from defects in processing, materials and workmanship; and |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 23 |
| (iii) | shall have been manufactured, packaged, tested and stored in conformity with the Drug Master File and Applicable Laws including without limitation then-current cGMPs. |
| (b) | Conduct. Nisso shall perform all services, including storage and delivery, hereunder in accordance with the applicable professional standards of the pharmaceutical industry and in accordance with provision of Section 4.7 (Delivery, Title and Risk of Loss) and reasonable written instructions given by Replidyne. | ||
| (c) | Adherence to Specifications. The Supplier shall produce the Drug Substance strictly in accordance with the Specifications. | ||
| (d) | Compliance with Applicable Laws. The Supplier shall fully comply with, and the Drug Substance shall be manufactured, packaged, tested and stored by Supplier in conformity with, all Applicable Laws. | ||
| (e) | Qualified Personnel. The Supplier shall engage and employ only professionally qualified personnel to perform the services contemplated hereunder. The Supplier shall not in the performance of its obligations under this Agreement use the services of any person debarred or suspended pursuant to any Applicable Law, including without limitation Sections 302(a) and (b) or 335 (a) or (b) of the Act. The Supplier does not as of the Effective Date have, and covenants that it will not during the Term hire, as an officer or an employee, any person who has been convicted of a crime, including without limitation a felony under the laws of the United States of America, for conduct relating to the regulation of any drug product under the Act. | ||
| (f) | Necessary Rights. The Supplier shall have all rights of any nature whatsoever required to perform its obligations under this Agreement. To the Supplier’s best knowledge as of the Effective Date, the manufacture and sale to Replidyne of the Drug Substance hereunder shall not interfere with, infringe upon, misappropriate, or otherwise come into conflict with any intellectual property rights of Third Parties. All Drug Substance sold to Replidyne pursuant to this Agreement shall not be subject to any liens, security interests or any other encumbrances. |
| 6.6 | Reprocessing. Nisso shall not, without the prior written consent of Replidyne, reprocess or “rework” any Drug Substance or components thereof. If Replidyne provides such written consent, such consent to not be unreasonably withheld (provided that it shall be reasonable for Replidyne to withhold such consent for regulatory concerns), Nisso ensure that such reprocess or rework meets the Specifications and cGMPs and is fully validated under all Applicable Laws. | |
| 6.7 | Complaints. In the event that any Party receives any complaint regarding the Drug Substance, it shall notify the other Parties promptly. Nisso shall evaluate each such complaint and respond to Replidyne in writing. Replidyne may also evaluate each such complaint. Replidyne shall have the sole right to manage all communication with Third Parties, provided that the parties shall cooperate to permit Supplier to defend against Third |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 24 |
| Party claims in accordance with Section 13.2 (Hold Harmless by Supplier) from and after such time as Replidyne asserts an indemnification claim thereunder. |
ARTICLE 7: REGULATORY MATTERS
| 7.1 | Technical Agreement. The Parties shall agree to the allocation of certain regulatory responsibilities as are customarily contained in a Technical Agreement for the manufacture of active pharmaceutical ingredients. In the event of any conflict between the provisions of this Agreement and such Technical Agreement, the provisions of this Agreement shall govern. | |
| 7.2 | Establishment Registrations. The Supplier shall maintain all registrations as are required by Applicable Laws to perform its obligations hereunder. | |
| 7.3 | Inspections. Nisso shall promptly notify Replidyne of any inspection or notice of inspection of the Nihongi Facility, the Takaoka Facility or any other facilities used in the manufacturing or processing the Drug Substance by the FDA or any other Regulatory Authority which relates to the manufacture or processing of the Drug Substance and provide Replidyne with information about the progress and outcome of such inspection, including, without limitation, copies of any notice of observations or warnings, requests for remedial action, corrective actions or other adverse findings. Nisso shall permit such inspections pursuant to Section 7.6 (Access to Facilities) hereof. Nisso at its cost shall promptly address and correct any deficiencies identified in any inspection or audit of such facilities by the FDA or any other Regulatory Authorities in an appropriate manner. | |
| 7.4 | Tests; Retained Samples and Records. Nisso shall perform, or cause to be performed, tests on each lot of the Drug Substance, including all samples thereof, manufactured pursuant to this Agreement before delivery to Replidyne. Such tests shall include required assay and stability testing and the testing of the Drug Substance for compliance with the Specifications and applicable registrations of and approvals for the Drug Substance and Applicable Laws. Nisso, at its cost, shall maintain and retain for five (5) years following the end of the applicable calendar year in which a lot is released (or such longer period as may be required by Applicable Laws or prudent industry standards, which at a minimum shall be the shelf life of the relevant Drug Product plus one year), (i) true and accurate books and records relating to its performance under this Agreement, including without limitation, the master batch records, the batch record specifications, the test records for the Drug Substance and other records of the manufacture, testing and shipping of the Drug Substance, and (ii) all retained samples of the Drug Substance and of the raw materials for the Drug Substance as are necessary to (A) comply with regulatory requirements applicable to the Supplier or Replidyne, and (B) assist with resolving product complaints and other similar investigations, provided that procedures for retained samples for raw materials shall be as mutually agreed in writing between Replidyne and Nisso from time to time on a case-by-case basis. Replidyne or an independent testing laboratory nominated by Replidyne may inspect and test Nisso’s samples relating to the Drug Substance in accordance with standard industry practice upon reasonable written request. |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 25 |
| 7.5 | Manufacturing Compliance. Nisso shall manufacture the Drug Substance, including all samples thereof, in accordance with the Specifications, the Drug Master File, cGMPs and applicable registrations of and approvals for the Drug Substance. Nisso shall promptly notify Replidyne of any out-of-specification manufacturing events, shall investigate such events, and shall provide Replidyne with a report with full supporting data of such investigation and corrective actions, which Replidyne may comment upon and dispute. As requested from time to time, Nisso shall provide Replidyne with a letter certifying that the manufacture of Drug Substance is in compliance with cGMPs, including without limitation, as required by the CFR. |
| 7.6 | Access to Facilities. Upon the reasonable prior written request of Replidyne, Replidyne and its authorized representatives shall have the right to inspect the facilities where the Drug Substance is being manufactured, processed, tested or stored, as the case may be, during regular business hours, to ascertain compliance with cGMPs, the Specifications and Nisso’s obligations hereunder. In addition, Nisso shall permit all Regulatory Authorities to inspect the Facilities where the Drug Substance is being manufactured, processed or stored, as the case may be, at reasonable times and within reasonable limits and in a reasonable manner. If (i) Nisso agrees to make a correction requested by Replidyne, or (ii) Nisso is obligated to make a correction to ensure its compliance with this Agreement or the requirements of Regulatory Authorities, including without limitation as identified in any such inspection by a Regulatory Authority, then Nisso, at its cost, shall promptly address and correct any deficiencies in an appropriate manner to ensure its compliance with this Agreement and such requirements. | |
| 7.7 | Regulatory Correspondence, Responses and Communications. Supplier shall promptly (and in all cases within five (5) calendar days) provide to Replidyne copies of all Supplier correspondence to or from the FDA or any other Regulatory Agency relating to the Drug Substance, including without limitation, FDA Form 483 letters, deficiency letters and warning letters. Supplier shall promptly respond to all such correspondence in compliance with the applicable time frames which are required or advisable under pharmaceutical industry standards, provided that all such responses shall be subject to the review and comments of Replidyne (with Replidyne having at least five (5) business days to review the draft response proposed by Supplier) prior to submission. Supplier shall consider all such comments in good faith. Supplier shall consult with Replidyne prior to any communication or meeting with any Regulatory Agency relating to the Drug Substance, and shall permit an authorized representative of Replidyne to attend any such communication (if by telephone call) or meeting. | |
| 7.8 | Additional Information. Nisso shall provide to Replidyne in a timely manner, but in no event less than sixty (60) days prior to the due date of Replidyne’s annual report to the FDA or any other Regulatory Authority with respect to the Drug Product, all information (in written form) which Replidyne reasonably requests regarding the Drug Substance in order to comply with Applicable Laws. | |
| 7.9 | Ownership of Regulatory Approvals. Nisso and Replidyne shall cooperate as necessary to obtain and maintain Regulatory Approvals. Replidyne shall own and control all |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 26 |
| Regulatory Approvals and applications, amendments or supplements underlying any such Regulatory Approvals; provided that it is expressly understood and agreed that the Drug Master File for the Drug Substance shall be registered and controlled by Nisso. Nisso shall, without additional compensation, (i) collaborate with Replidyne to prepare and file the Drug Master File in “Common Technical Document” format, which shall be in form and substance satisfactory to both Nisso and Replidyne prior to filing by Nisso with the FDA, (ii) maintain such Drug Master File in good standing with all applicable Regulatory Authorities, including the payment of all required fees and the filing of all required reports (including without limitation an annual report filed with the FDA within thirty (30) days of the anniversary date of original Drug Master File submission date of each year), supplements and amendments, (iii) give a reference letter to the Regulatory Authorities in connection with Replidyne’s efforts in seeking Regulatory Approvals and submitting any such applications, amendments, or supplements for Drug Products, and (iv) only make or permit filings, submissions, changes, reports, supplements, modifications, deviations or exceptions to such Drug Master File in accordance with Section 6.1 (Drug Substance Specifications; DMF) hereof. Replidyne acknowledges and agrees that Nisso may maintain as confidential and not disclose to Replidyne the manufacturing information specified in Attachment 7.9 hereto that will be included in the Drug Master File (the “Sensitive Manufacturing Information”), provided that Replidyne shall have the right to engage a mutually agreeable third party to verify the suitability of such information for filing with the FDA in “Common Technical Document” format. Nisso shall cooperate with Replidyne in the coordination of supplements and annual reports for the Drug Master File and Regulatory Approvals, including without limitation classification of changes as “minor”, “moderate” and “major” for reporting purposes. Replidyne shall have the right, but not the obligation, to list all patents of the Supplier which are relevant to the Drug Substance in the FDA’s “Orange Book” and similar documents for other Regulatory Authorities with respect to each Drug Product. Upon the occurrence of any of the circumstances specified in Section 8.1 (Termination of Exclusive Purchase) hereof, Nisso shall promptly provide Replidyne with a complete copy of all Drug Master File documents, and Replidyne shall have the right to prepare and file a new drug master file and use all information in or related to the Drug Master File. | ||
| 7.10 | Adverse Events. The Parties shall promptly provide each other with necessary information and data relating to adverse events, regardless of causality, associated with the use of the Drug Substance or the Drug Products or other forms of faropenem, received by or reported to the Parties from any sources anywhere in the world during the Term of this Agreement, in accordance with the written reporting procedure to be separately agreed upon by the Parties. | |
| 7.11 | Recalls. Each Party shall notify the other of any information, whether received directly or indirectly, which might affect the marketability, safety or effectiveness of the Drug Substance and/or which might result in the Recall or seizure of the Drug Substance or the Drug Products incorporating the Drug Substance. The Parties shall take all appropriate corrective actions, and shall cooperate in the investigations, related to a Recall. In the event that a Recall results from a Defect in Nisso’s manufacture, storage or handling of the Drug Substance which constitutes a breach by Nisso of this Agreement, in addition to all |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 27 |
other remedies available to Replidyne, Nisso shall be responsible for all documented
out-of-pocket expenses of Replidyne related to such Recall; provided, however, that the
Parties acknowledge and agree that Replidyne shall use commercially reasonable efforts to
mitigate such out-of-pocket expenses. For purposes of this Agreement, the out-of-pocket
expenses of Recall shall include the expenses of notification and destruction or return of
the Recalled products, all other documented out-of-pocket costs incurred in connection with
such Recall and the replacement of the Recalled products. In the event that the Recall does
not result from a Defect in Nisso’s manufacturing, storage or handling of the Drug Substance
which constitutes a breach by Nisso of this Agreement, Replidyne shall be responsible for
all costs and expenses of the Recall.
| 7.12 | Further Cooperation. The Supplier shall cooperate with Replidyne as may be reasonably necessary or appropriate to satisfy all governmental requirements and obtain all needed permits, approvals and licenses with respect to the development, manufacture, storage, packaging, marketing and sale of the Drug Products incorporating the Drug Substance. Such cooperation may include communicating with regulatory authorities and making available as promptly as practicable all information, documents and other materials which result from the performance by the Supplier of its services hereunder which Replidyne is required to submit or which Replidyne may otherwise reasonably request in connection with governmental filings relating to the Drug Substance or the Drug Products incorporating the Drug Substance. Replidyne shall reimburse the Supplier for its reasonable out-of-pocket costs and expenses of such cooperation, if applicable, as quoted in writing by Supplier and approved in writing by Replidyne in advance. |
ARTICLE 8: TRANSFER OF MANUFACTURING
| 8.1 | Termination of Exclusive Purchase. Notwithstanding Sections 3.1 (Supply of the Drug Substance) and 3.2 (Minimum Purchases Quantities) hereof, Replidyne may, in its sole discretion, (i) itself manufacture any quantities of the Drug Substance, (ii) purchase any quantities of the Drug Substance from Third Parties, and/or (iii) transfer and license the Manufacturing Technology and all other relevant documents, know-how, intellectual property and other rights as provided in this Article 8 to Replidyne or one or more Third Parties, and Replidyne shall not thereafter be obligated to purchase the Drug Substance exclusively from Supplier, under any one or more of the following circumstances: |
| (a) | Prices Not Competitive. After an NDA is filed for a Drug Product in the United States, if all events (i) through (iii) have occurred: |
| (i) | the Purchase Prices cause the retail prices for Drug Products to not be competitive (i.e., the retail prices of Drug Products would be at a competitive disadvantage to the prices of competitive, single active ingredient (excluding combination products), branded community anti-biotic products in oral formulation in the relevant market in the Territory for treatment for a similar set of indications, including but not limited to Acute Sinusitis and Community Acquired Pneumonia, as for the Drug Product, taking into consideration all other relevant costs and a normal |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed
separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as
amended.
| SUPPLY AGREEMENT | PAGE 28 |
| profit margin to Replidyne), or render the commercialization of Drug Product not viable; | |||
| (ii) | the Parties are unable to agree after discussions over a six (6) month period of time on revised Purchase Prices that addresses the issues specified in Section 8.1(a)(i) hereof; and | ||
| (iii) | Replidyne can demonstrate with documentary evidence that Replidyne is more likely itself or with a Third Party than with Supplier to be able to realize a lower supply price for the Drug Substance as required by Replidyne to address the issues specified in Section 8.1(a)(i) hereof over the course of a commercially reasonable period of time following the transfer of the manufacturing to Replidyne or Replidyne’s then-current Manufacturing Designee. |
| (b) | Failure to Timely Validate the Nihongi Facility. Nisso has not completed, or it becomes reasonably evident that Nisso will not be able to complete, its obligations pursuant to Article 2 (VALIDATION OF NIHONGI FACILITY) hereof in accordance with the Timeline. | ||
| (c) | Failure to Supply Requirements. Except for an occurrence of a force majeure event pursuant to Article 15 (FORCE MAJEURE), the Supplier is unable or unwilling, during a consecutive six (6) month period, to deliver at least eighty percent (80%) in the aggregate of the quantities of the Drug Substance without Defects ordered pursuant to Purchase Orders. (For example, this Section 8.1(c) would apply if Replidyne orders 40 tons for delivery in a six month period and Supplier only delivers 31 tons in such period. (40 tons x 80% = 32 tons)) | ||
| (d) | Force Majeure. An occurrence of a force majeure event pursuant to Article 15 (FORCE MAJEURE) hereof prevents or can reasonably be foreseen to prevent the Supplier from delivering fifty percent (50%) in the aggregate of the quantities of the Drug Substance without Defects ordered pursuant to Purchase Orders during a consecutive six (6) month period; provided that such quantities are consistent with the Purchase Forecasts. | ||
| (e) | Termination or Non-Renewal. A notice of termination of this Agreement has been properly provided by any Party to the other Parties pursuant to Article 11 (TERM AND TERMINATION) hereof (other than termination pursuant to Sections 11.2(b) (Material Breach by Replidyne), 11.2(d) (Bankruptcy Event for Replidyne), or 11.2(h) (Abandonment of Development or Commercialization ), or termination of the License Agreement due to the material breach or bankruptcy event of Replidyne), or the expiration of this Agreement due to Nisso declining after reasonable negotiation to extend the Term of this Agreement for the next Renewal Term. | ||
| (f) | Bankruptcy Event. Either DSP or Nisso is the subject of a Bankruptcy Event. |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 29 |
| 8.2 | Grant of License. Effective upon the occurrence of any of the circumstances specified in Section 8.1 (Termination of Exclusive Purchase) hereof, the Supplier hereby grants to Replidyne a non-exclusive, royalty-bearing, worldwide license to manufacture the Drug Substance, with the right to sublicense to the Manufacturing Designee, under all patents, know-how and other intellectual property rights, including, without limitation, the Manufacturing Technology and the patents and patent applications listed in Attachment 8.2 hereof and all foreign counterparts, that are owned or controlled by Supplier (excluding DSP’s patents and all other intellectual property rights licensed to Replidyne pursuant to the License Agreement) to (i) research, develop, make, have made and use the Drug Substance and Drug Products anywhere in the world, and (ii) promote, offer to sell, sell, have sold and otherwise commercialize the Drug Substance and Drug Products in the Territory. |
| 8.3 | Royalty. In compensation for the license granted pursuant to Section 8.2 (Grant of License) hereof, Replidyne shall pay: |
| (a) | a royalty to Supplier, in amounts equal to [ *** ] percent ([ *** ]%) of the transfer price (net of transportation costs, insurance costs, taxes and duties, and royalties payable to Third Parties) for the Drug Substance purchased from a Third Party manufacturer by Replidyne or Replidyne’s Designees for a period of [ *** ] years commencing from the date on which Replidyne or Manufacturing Designee begins the manufacturing of the Drug Substance; and | ||
| (b) | royalties properly payable to Tanabe Seiyaku, if applicable. |
| If Replidyne or its Affiliates manufacture the Drug Substance by themselves, the Parties shall negotiate in good faith and agree on the formula to decide appropriate royalty amount for the compensation of the license granted pursuant to Section 8.2 (Grant of License) hereof which shall approximate the royalties that would be payable to Nisso if Replidyne would have sourced the Drug Substance from a Third Party manufacturer. | ||
| Nisso and DSP shall discuss and agree separately with respect to the sharing of the royalty payments received in accordance with Section 8.3 (a) above by Nisso and DSP. If Replidyne requests a (sub)license under the relevant Tanabe Seiyaku patents, DSP and Nisso shall either (i) grant a sublicense at the same rate DSP/Nisso pays, if the Tanabe Seiyaku agreement permits sublicensing, or (ii) [ *** ]. | ||
| 8.4 | Disclosure of Manufacturing Technology. Effective upon the occurrence of any of the circumstances specified in Section 8.1 (Termination of Exclusive Purchase) hereof, Nisso shall disclose all Manufacturing Technology to Replidyne and/or the Manufacturing Designee, for Replidyne’s use in the production of the Drug Substance and the Drug Product only. DSP and Nisso shall assist Replidyne and/or the Manufacturing Designee as reasonably necessary to effectively enable Replidyne or the Manufacturing Designee to manufacture the Drug Substance. In the event this Section 8.4 applies due to Section 8.1 (a) or (d) above, such assistance shall be at Replidyne’s expense. In the event this Section 8.4 applies due to Section 8.1 (b), (c), (e) or (f) above, such assistance shall be at the |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 30 |
| Supplier’s expense for the first three (3) months and at Replidyne’s reasonable expense thereafter. In no event shall the term for such technical assistance exceed two (2) years from the date of Replidyne’s notice invoking provisions of Section 8.1 (Termination of Exclusive Purchase) hereof. In case Replidyne requires Nisso to disclose the Manufacturing Technology to the Manufacturing Designee pursuant to this Section 8.4, Replidyne shall be responsible to have such Manufacturing Designee observe the terms and conditions of this Agreement. | ||
| 8.5 | Continued Supply. In the event this Article 8 (TRANSFER OF MANUFACTURING) applies due to Section 8.1 (a), (e) or (f) above, at Replidyne’s election and request, the Supplier shall continue to supply such quantities of the Drug Substance as Replidyne may request in an uninterrupted manner (or, in the case of Section 8.1(b), (c) or (d) above, use commercially reasonable efforts to do so), until such time as Replidyne’s or the Manufacturing Designee’s facility is certified by the applicable Regulatory Authorities and able to meet Replidyne’s requirements for the Drug Substance, provided, however, that such term shall not exceed two (2) years from the date of Replidyne’s notice invoking the provisions of Section 8.1 (Termination of Exclusive Purchase) hereof. The supply prices pursuant to this Section 8.5 shall be the Purchase Prices in accordance with Article 5 (PURCHASE PRICE AND PAYMENT) hereof. At such time as Replidyne’s or Manufacturing Designee’s facility is certified by the applicable Regulatory Authorities and is able to meet Replidyne’s requirements for the Drug Substance, the Supplier’s obligations to supply the Drug Substance pursuant to this Section 8.5 (Continued Supply) shall terminate. | |
| 8.6 | Escrow of Manufacturing Technology. Upon Replidyne’s reasonable request, Nisso shall deposit documentation of the Manufacturing Technology in accordance with the provisions of that certain Manufacturing Technology Escrow Agreement of even date herewith, which among other things provides that Replidyne shall reimburse Nisso for its reasonable costs to provide such documentation. From time to time after such request and deposit during the term hereof as such Manufacturing Technology changes, and in any event promptly upon the request of Replidyne, Nisso shall update and supplement, but shall not delete, the contents of the Manufacturing Technology pursuant to such Manufacturing Technology Escrow Agreement. |
ARTICLE 9: PROPRIETARY RIGHTS
| 9.1 | Replidyne Property. Replidyne shall own and retain all right, title and interest in and to the Replidyne Property under this Agreement. The Supplier shall have no right or license to the Replidyne Property under this Agreement except as required to perform their obligations hereunder during the Term. | |
| 9.2 | Supplier’s Work Product and Improvements. Nisso shall own and retain all right, title and interest in and to Nisso’s Improvements. Replidyne and its Representatives shall have no right or license to Nisso’s Improvements, except as provided in this Agreement and to research, develop, make, have made, use, promote, offer to sell, sell, have sold and otherwise commercialize the Drug Substance and the Drug Products for the Territory. |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 31 |
ARTICLE 10: SECRECY
| 10.1 | Secrecy. Each Party shall keep secret and confidential any information, data and know-how of the other Parties received from each other Party after the date of the Supply LOI or under this Agreement (“Confidential Information”) and shall not use such Confidential Information for any purpose other than for the purposes permitted in this Agreement, except as otherwise expressly authorized herein, provided that a Party shall have no obligation to maintain the secrecy of Confidential Information which: |
| (i) | at the time of disclosure by the disclosing Party is in the public domain; | ||
| (ii) | after disclosure by the disclosing Party enters the public domain through no improper conduct of the receiving Party; | ||
| (iii) | prior to disclosure by the disclosing Party was already in the possession of the receiving Party as evidenced by the receiving Party’s written records; | ||
| (iv) | subsequent to disclosure hereunder is obtained by the receiving Party from Third Parties who are lawfully in possession of such information, data and know-how and are not subject to an obligation to refrain from disclosing such information, data and know-how to others; or | ||
| (v) | is required to be revealed under compulsion of law; provided, that the Party under a legal compulsion to disclose the Confidential Information makes every effort to preserve the confidentiality of the information and also provides the disclosing Party sufficient prior notice of the disclosure, so that such disclosing Party shall have an opportunity to take whatever action it deems necessary or desirable to protect its Confidential Information. |
| 10.2 | Exceptions. Notwithstanding the provisions of the preceding Section 10.1 (Secrecy) hereof, a Party shall be entitled to disclose Confidential Information for the purpose of implementing this Agreement to any of the following: |
| (i) | the Party’s Representatives who have a need to know for the purposes of the performance of this Agreement; provided, that the recipients have been informed of and are bound to the secrecy obligations of this Agreement; | ||
| (ii) | Replidyne’s Affiliates, Replidyne’s Designees, the Manufacturing Designee, actual or potential investors, contract manufacturers, contract research organizations, professional service providers, distributors, sublicensees and the like, or Supplier’s Affiliates or their employees, who have a need to know, to the extent they are involved in the development, manufacturing, sale and/or marketing of the Drug Products or the manufacturing of the Drug Substance; provided, that the recipients have been informed of and are bound to secrecy obligations substantially similar to those in this Agreement; and |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 32 |
| (iii) | Regulatory Authorities which have been advised of the confidential status of the Confidential Information, provided all necessary procedures are followed to preserve confidentiality. |
| It is expressly understood that Nisso may, at its choice, not provide Sensitive Manufacturing Information to Third Party manufacturers pursuant to Section 3.2(g) (Security of Supply) hereof. In addition, prior to the commercial sale of the Drug Products, the Parties shall mutually discuss and agree upon the reasonable disclosure of any Confidential Information necessary to be disclosed in connection with Replidyne’s, its Affiliates’ and Replidyne’s Designees’ pre-launch sales and marketing activities. |
ARTICLE 11: DURATION AND TERMINATION
| 11.1 | Term. The term of this Agreement (“Term”) shall become effective on the Effective Date and shall, unless sooner terminated as hereinafter provided in this Agreement, continue in full force and effect, on a country-by-country basis, in each country in the Territory until the latest of: |
| (i) | the expiration date of all valid claims of all patents specified in Attachment 11.1 licensed to Replidyne pursuant to the License Agreement and all periods of extended commercial exclusivity for Drug Products granted under any laws or regulations in such country in the Territory for Drug Products actually manufactured, used or sold by Replidyne; or | ||
| (ii) | the date that is twelve (12) years after the date of the first commercial sale of a Drug Product (other than the Drug Product of which Replidyne makes only a limited launch and not the Launch) in such country in the Territory. |
| If Replidyne desires to continue to purchase Drug Substance from Supplier following the expiration of this Agreement, the Parties shall also discuss in good faith the terms and conditions for the extension of this Agreement, which such negotiation shall commence no later than one (1) year prior to the expiration of this Agreement. |
| 11.2 | Termination. The Party respectively specified below, as the case may be, may terminate this Agreement forthwith by notice in writing to the other Parties upon the occurrence of any of the following events: |
| (a) | Termination of License Agreement. Replidyne may terminate this Agreement in the event of the expiration or termination, for whatever reason, of the License Agreement. | ||
| (b) | Material Breach by Replidyne. DSP or Nisso may terminate this Agreement if Replidyne commits a material breach of this Agreement, which in the case of a breach shall not have been remedied within ninety (90) days of the receipt by Replidyne of a notice identifying the breach and requiring its remedy or such longer |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 33 |
| time as Replidyne may demonstrate to DSP or Nisso is necessary to remedy the breach using its reasonable efforts to do so. |
| (c) | Material Breach by DSP or Nisso. Replidyne may terminate this Agreement if DSP or Nisso commits a material breach of this Agreement, which in the case of a breach shall not have been remedied within ninety (90) days of the receipt by DSP or Nisso of a notice identifying the breach and requiring its remedy or such longer time as DSP or Nisso may demonstrate to Replidyne is necessary to remedy the breach using its reasonable efforts to do so. | ||
| (d) | Bankruptcy Event for Replidyne. DSP or Nisso may terminate this Agreement if Replidyne is the subject of a Bankruptcy Event. | ||
| (e) | Bankruptcy Event for DSP or Nisso. Replidyne may terminate this Agreement if DSP or Nisso is the subject of a Bankruptcy Event. | ||
| (f) | Force Majeure Event for Replidyne. DSP or Nisso may terminate this Agreement if Replidyne is unable to perform its obligations under this Agreement by an event of Force Majeure for more than six (6) months. | ||
| (g) | Force Majeure Event for DSP or Nisso. Replidyne may terminate this Agreement if DSP or Nisso is unable (or it is reasonably foreseeable that DSP or Nisso will be unable) to deliver [ *** ] percent ([ *** ]%) of quantities of the Drug Substance without Defects ordered pursuant to Purchase Orders during a six (6) month period, provided that such quantities are consistent with the Purchase Forecasts, by an event of Force Majeure. | ||
| (h) | Abandonment of Development or Commercialization. Replidyne may terminate this Agreement if Replidyne determines in its sole discretion to abandon the development and/or commercialization of Drug Products. | ||
| (i) | Intolerable Delay. Nisso shall have the right to terminate this Agreement any time following July 1 2009, at sole discretion, if Replidyne does not notify Supplier of the Launch Go Date on or before July 1, 2009, provided, that if Nisso exercises such right, Replidyne shall have the right to require Nisso to transfer the Manufacturing Technology of the Drug Substance to Replidyne or its Manufacturing Designee pursuant to the terms and conditions stipulated in Section 7.9 (Ownership of Regulatory Approvals) and Article 8 (Transfer of Manufacturing) hereof. |
ARTICLE 12: EFFECT OF TERMINATION
| 12.1 | Survival. The provisions of Sections 3.7 (Protective Measures), 4.8 (Inspection, Shortages and Defects), 5.4 (Taxes), 5.5 (Audits), 6.5 (Warranties), 6.7 (Complaints), 7.4 (Tests; Retained Samples and Records), 7.10 (Adverse Events), 7.11 (Recalls), 13.1 (Hold Harmless by Replidyne), 13.2 (Hold Harmless by Supplier) and Articles 9 (Proprietary |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 34 |
| Rights), 10 (Secrecy), 12 (Effect of Termination), 19 (Applicable Law), 20 (Dispute Resolution), 21 (Notice) and 22 (Publicity) shall survive the expiration or termination of this Agreement for any reason. In addition, any other provisions including Section 3.2 (Minimum Purchase Quantities), 8.2 (Grant of License), 8.3 (Royalty), 8.4 (Disclosure of Manufacturing Technology) and Section 13.3 (Insurance) which are required to interpret and enforce the Parties’ rights and obligations under this Agreement shall also survive such expiration or termination to the extent required for the full observation and performance of this Agreement by the Parties. |
| 12.2 | Accrued Rights and Obligations. Expiration or termination of this Agreement for whatever reason shall not affect the accrued rights and obligations of any Party arising under this Agreement. | |
| 12.3 | Extension of Supply. Upon expiration of this Agreement, if Replidyne desires to continuously purchase the Drug Substance from the Supplier following the expiration of this Agreement, the Parties shall also discuss in good faith of the terms and conditions for the extension of this Agreement which such negotiation shall commence no later than one (1) year prior to the expiration of this Agreement. | |
| 12.4 | Fulfillment of Purchase Orders. If this Agreement is terminated as provided in Section 11.2(a) (Termination of License Agreement) other than the termination of the License Agreement due to the material breach or bankruptcy event of Replidyne, Section 11.2(c) (Material Breach by DSP or Nisso), 11.2(e) (Bankruptcy Event for DSP or Nisso) or 11.2 (g) (Force Majeure Event for DSP or Nisso) by Replidyne, then (i) Replidyne may cancel without liability (other than for reimbursement as provided in Section 12.5(a) (Reimbursement for Purchase Orders) hereof) all outstanding Purchase Orders for which delivery has not been completed, as determined by Replidyne in its sole discretion, and (ii) such termination, except the termination as provided in Section 11.2 (g) (Force Majeure Event for DSP or Nisso), shall not relieve the Supplier of its obligation to deliver Drug Substance ordered by Replidyne prior to the effective date of termination (except to the extent Purchase Orders are so cancelled by Replidyne). |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 35 |
| 12.5 | Compensation to Nisso. |
| (a) | Reimbursement for Purchase Orders. In the event of the termination of this Agreement pursuant to Section 11.2(b) (Material Breach by Replidyne) or Section 11.2(d) (Bankruptcy Event for Replidyne) or Section 11.2(h) (Abandonment of Development or Commercialization) or in the event of the termination of this Agreement caused by the termination of the License Agreement due to the material breach or bankruptcy event of Replidyne, but not in the event of any other termination or the expiration of this Agreement, within sixty (60) days after the effective date of such termination of this Agreement, Replidyne shall reimburse Nisso for reasonable costs and expenses incurred or which cannot be avoided or cancelled for quantities of Drug Substance which are the subject of Purchase Orders delivery of which will not be completed pursuant to Section 12.4 (Fulfillment of Purchase Orders) hereof. | ||
| (b) | Minimum Purchase Obligations. In the event (i) of the termination of this Agreement pursuant to Section 11.2(b) (Material Breach by Replidyne) or Section 11.2(d) (Bankruptcy Event for Replidyne) or Section 11.2(h) (Abandonment of Development or Commercialization) or in the event of the termination of this Agreement caused by the termination of the License Agreement due to the material breach or bankruptcy event of Replidyne, but not in the event of any other termination or the expiration of this Agreement, and (ii) that the Launch Go Date has occurred (but only if the Launch Go Date has occurred), Replidyne shall pay Nisso, at Replidyne’s election, either: |
| (A) | within sixty (60) days after the effective date of termination of this Agreement, an amount equal to the net present value (using as the discount factor the rate that is one and half percent (1.5%) per annum above the 30 day U.S. Dollar LIBOR rate, as published in The Financial Times for the effective date of termination, compounded) of an amount equal to [ *** ] percent ([ *** ]%) of the aggregate Purchase Price pursuant to Article 5 for the applicable minimum purchase quantities of Drug Substance which Replidyne has failed to purchase as required by Section 3.2 (Minimum Purchase Quantities) for the Three Year Period, the applicable calendar year in the Five Year Period or the then-current Remaining Term Year (but not thereafter), whichever is applicable (for clarity, the period that is applicable is only the period in which such termination occurs, and not prior or subsequent periods); or | ||
| (B) | on an annual basis, after the effective date of termination of this Agreement, by March 1st of each such year, the amount of the compensation due to Supplier pursuant to Section 5.8 (Compensation to Supplier) hereof; |
| to the extent not already compensated by Replidyne, and for such minimum purchase quantities, if any, which have not been purchased by Replidyne as of the effective date of termination or will not be purchased by Replidyne pursuant to |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 36 |
| Section 12.3 (Extension of Supply) or Section 12.4(ii) (Fulfillment of Purchase Orders) hereof, as follows: |
| (i) | if such termination occurs in the Three Year Period, Replidyne’s obligation pursuant to this Section 12.5(b) shall only apply to the minimum purchase quantities applicable pursuant to Section 3.4(a) (First Three Years) hereof; or | ||
| (ii) | if such termination occurs in the Five Year Period, Replidyne’s obligation pursuant to this Section 12.5(b) shall only apply to the minimum purchase quantities applicable for the then-current year in the Five Year Period pursuant to Section 3.4(b) (Next Five Years) hereof; or | ||
| (iii) | if such termination occurs in the Remaining Term, Replidyne’s obligation pursuant to this Section 12.5(b) shall apply to the minimum purchase quantities applicable only for the Remaining Term Year in which such termination occurs prorated to the effective date of termination for such year. |
| The foregoing shall be Supplier’s sole remedy for Replidyne’s failure to purchase the minimum purchase quantities of Drug Substance pursuant to Section 3.4 (Minimum Purchase Quantities) hereof due to the termination of this Agreement, but shall only apply if the Launch Go Date has occurred. |
| (c) | Engineering Costs. In the event (i) of the termination of this Agreement pursuant to Section 11.2(b) (Material Breach by Replidyne) or Section 11.2(d) (Bankruptcy Event for Replidyne) or Section 11.2(h) (Abandonment of Development or Commercialization) or Section 11.2(i) (Intolerable Delay of Launch Go Date) or in the event of the termination of this Agreement caused by the termination of the License Agreement due to the material breach or bankruptcy event of Replidyne, but not in the event of any other termination or the expiration of this Agreement, and (ii) that the Launch Go Date has NOT occurred, within thirty (30) days after the effective date of termination of this Agreement, Replidyne shall reimburse Nisso for [ *** ] percent ([ *** ]%) of all actual costs incurred by Nisso (including, without limitation, all materials and labor costs), but excluding overhead expenses, relating to engineering activities (but not qualification or validation activities) undertaken by Nisso, and approved by Replidyne pursuant to Section 2.5 (Replidyne Review) hereof, in connection with Sections 2.2 (Engineering Activities) in the event that this Agreement is terminated at any time after the Effective Date but prior to the Launch Go Date; provided that Replidyne’s payment obligation to Nisso pursuant to this Section 12.5(c) shall not exceed JP¥[ *** ]. | ||
| (d) | Other Pre-Approved Reimbursable Costs. In the event (i) of the termination of this Agreement pursuant to Section 11.2(b) (Material Breach by Replidyne) or Section 11.2(d) (Bankruptcy Event for Replidyne) or Section 11.2(h) (Abandonment of Development or Commercialization) or Section 11.2(i) |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 37 |
| (Intolerable Delay of Launch Go Date) or in the event of the termination of this Agreement caused by the termination of the License Agreement due to the material breach or bankruptcy event of Replidyne, but not in the event of any other termination or the expiration of this Agreement, and (ii) that the Launch Go Date has NOT occurred, within thirty (30) days after the effective date of termination of this Agreement, Replidyne shall reimburse Nisso for such costs and expenses as may be mutually agreed in writing from time to time prior to the Launch Go Date to be reimbursed by Replidyne by express reference to this Section 12.5(d). For example purposes, such costs may include by such written agreement the cost of a quality control laboratory or the advance purchase of raw materials pursuant to Section 4.4(b) (First Purchase Order) hereof. |
| 12.6 | Payment Assurance. To secure Replidyne’s payment obligations of or under (i) Section 12.5(b) (Minimum Purchase Obligations) and/or (ii) Sections 12.5(c) (Engineering Costs) and 12.5(d) (Other Pre-Approved Reimbursable Costs) if and when those obligations cumulate to more than [ *** ] U. S. dollars (US$[ *** ]) and/or (iii) Section 5.3 (Payment) if and when those obligations prior to the Launch Go Date cumulate to more than [ *** ] U. S. dollars (US$[ *** ]) (not only for the excess of [ *** ] U. S. dollars (US$[ *** ]), but for the full amount of such obligations) and/or (iv) Section 5.3 (Payment) if and when those obligations after the Launch Go Date for the period starting from the shipment of the Drug Substance pursuant to Section 4.7 (Delivery, Title and Risk of Loss) and ending at making the payment by Replidyne on any purchase order pursuant to Section 5.3 (Payment) exceeding [ *** ] U. S. dollars (US$[ *** ]) (not only for the excess of [ *** ] U. S. dollars (US$[ *** ]) but for the full amount of such purchase order), Replidyne shall, independently or together with one or more of Replidyne’s Designees, establish a commercially reasonable mechanism for securing Replidyne’s financial ability to make payment of, or obtain an insurance for, such obligations in accordance with the following time line: |
Minimum Purchase Obligations:
|
Upon agreement of the minimum purchase quantities | |
Engineering & Other:
|
No later than thirty (30) days prior to the date on which it is reasonably foreseen that accumulated amount stipulated in Section 12.5 (c) (Engineering Costs) and other payment obligations of Replidyne stipulated in Section 12.5 (d) (Other Pre-Approved Reimbursable Costs) will exceed [ *** ] U. S. dollars (US$[ *** ]). (The cost for raw materials for the Drug Substance for the first purchase order shall be included for the purpose of the calculation of such amount, regardless of whether or not Replidyne requests Nisso to shorten the delivery time.) | |
Certain Purchase Orders |
||
Before the Launch Go Date:
|
No later than sixty (60) days prior to shipment in case then outstanding aggregate purchase amount of the |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 38 |
| Drug Substance prior to Launch Go Date exceeds [ *** ] U. S. dollars (US$[ *** ]). | ||
Certain Purchase Orders |
||
After the Launch Go Date:
|
No later than thirty (30) days prior to shipment; provided that Supplier agrees to bear any third party costs associated with the financial assurance mechanism for the period starting from the shipment of the Drug Substance pursuant to Section 4.7 (Delivery, Title and Risk of Loss) and ending at making the payment by Replidyne on any purchase order pursuant to Section 5.3 (Payment), such as customary and reasonable bank fees for a letter of credit, escrow, or other arrangement. |
ARTICLE 13: HOLD HARMLESS AND INSURANCE
Notwithstanding and in addition to Sections 18.1 (Hold Harmless by Replidyne) and 18.2 (Hold
Harmless by DSP) of the License Agreement, the Parties hereto agree as follows:
| 13.1 | Hold Harmless by Replidyne. Replidyne shall indemnify, defend and hold the Supplier and the Supplier’s Affiliates and their respective officers, directors, employees, partners and agents (“Supplier Indemnitees”) harmless from and against any and all liability, damages, cost or expenses (including reasonable attorneys’ fees and disbursements) (“Damages”) incurred as a result of any claim made or suit brought by a Third Party against a Supplier Indemnitee arising out of or related to (i) the development, manufacture, handling, sale, storage, transportation, testing, labeling, packaging, marketing or use of the Drug Products by Replidyne, or any of Replidyne’s Designees, (ii) the manufacture, handling, sale, storage, transportation, testing, labeling, packaging, marketing or use of the Drug Substance by Replidyne or the Manufacturing Designee or (iii) the negligence or intentional misconduct or breach of this Agreement by Replidyne, except to the extent that such Damages arise out of or relate to (a) any Defect in the Drug Substance supplied by Nisso hereunder existing at the time the Drug Substance is delivered to Replidyne, and/or (b) the negligence or intentional misconduct or breach of this Agreement by the Supplier Indemnitees. Upon receipt of any such claim or suit by any of the Supplier Indemnitees, the Supplier or such Supplier Indemnitees shall promptly notify Replidyne in writing of such claim or suit, and shall permit Replidyne to defend against and control the defense of such claim or suit; provided, that Replidyne shall not compromise or settle such claim or suit in a manner materially and adversely affecting the Supplier without the written approval of the Supplier and the Supplier shall have the right to participate in the defense of such claim or suit at its own expense. The Supplier or any Supplier Indemnitee shall not compromise or settle such claim or suit without the prior written approval of Replidyne. |
| 13.2 | Hold Harmless by Supplier. The Supplier shall indemnify, defend and hold Replidyne and Replidyne’s Affiliates and their respective officers, directors, employees, contractors, partners and agents (“Replidyne Indemnitees”) harmless from and against any and all |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 39 |
| Damages incurred as a result of any claim made or suit brought by a Third Party against a Replidyne Indemnitee arising out of or related to (i) any Defect in the Drug Substance supplied to Replidyne hereunder, and/or (ii) the negligence or intentional misconduct or breach of this Agreement by the Supplier except to the extent that such Damages arise out of or relate to the negligence or intentional misconduct or breach of this Agreement by the Replidyne Indemnitees. Upon receipt of any such claim or suit by any of the Replidyne Indemnitees, Replidyne or such Replidyne Indemnitees shall promptly notify the Supplier in writing of such claim or suit and shall permit the Supplier to defend against and control the defense of such claim or suit; provided, that the Supplier shall not compromise or settle such claim or suit in a manner materially and adversely affecting Replidyne without the written approval of Replidyne and Replidyne shall have the right to participate in the defense of such claim or suit at its own expense. Replidyne or any Replidyne Indemnitee shall not compromise or settle such claim or suit without the prior written approval of the Supplier. | ||
| 13.3 | Insurance. Each Party shall, at its sole cost and expense, obtain and keep in force during the Term of this Agreement and for a period of not less than seven (7) years after termination or expiration of this Agreement the following insurance: |
| (a) | Nisso. Nisso shall have general liability insurance, including product liability insurance and blanket contractual liability coverage with bodily injury, death and property damage limits of $[ *** ] per occurrence and $[ *** ] in the aggregate. | ||
| (b) | DSP. DSP has already bought, and will obtain and keep in force, during the term of the License Agreement, the following insurance, under the License Agreement: general liability insurance and blanket contractual liability coverage with bodily injury, death and property damage limits of $[ *** ] per occurrence and $[ *** ] in the aggregate; provided, however, that DSP’s obligation to have such insurance shall not be required until commencement of the production of the Drug Substance for use in clinical use and commercialization of the Drug Products. | ||
| (c) | Replidyne. Replidyne has already bought, and will obtain and keep in force, during the term of the License Agreement, the following insurance, under the License Agreement: (i) general liability insurance, including blanket contractual liability coverage with bodily injury, death and property damage limits of $[ *** ] per occurrence and $[ *** ] in the aggregate; and (ii) clinical studies and product liability insurance with bodily injury, death and property damage limits of not less than $[ *** ] per occurrence and $[ *** ] in the aggregate; provided, however, that Replidyne’s obligation to have such insurance shall not be required until commencement of the Development (as defined in the License Agreement). |
| After the execution of this Agreement, and upon the other Party’s request thereafter, each Party shall furnish the other with a certificate of insurance signed by an authorized representative of such Party’s insurance underwriter evidencing the insurance coverage required by this Agreement. Each Party shall use its commercially reasonable efforts to cause Third Parties engaged by such Party to perform its obligations under this Agreement |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 40 |
| to maintain such types of insurance coverage and for such period of time as are customary for such Third Parties given the nature of the services to be provided. |
ARTICLE 14: REPRESENTATIONS, WARRANTIES AND COVENANTS
| 14.1 | Corporate Power. Each Party hereby represents, warrants and covenants that, as of the Effective Date, such Party is duly organized, validly existing under the laws of its state or country of incorporation and has full corporate power and authority to enter into this Agreement and carry out the provisions hereof. | |
| 14.2 | Due Authorization. Each Party hereby represents and warrants that, as of the Effective Date, such Party is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder. | |
| 14.3 | Binding Obligations/No Conflict. Each Party hereby represents, warrants and covenants that, as of the Effective Date: (i) this Agreement is a legal and valid obligation binding upon it and is enforceable in accordance with its terms; and (ii) the execution, delivery and performance of this Agreement by such Party does not, and will not during the Term of this Agreement, conflict with any agreement, instrument or understanding to which it is a party or by which it is bound, nor to the best knowledge of each Party as of the Effective Date will such execution, delivery and performance violate any Applicable Laws. Without limiting the generality of the foregoing, Nisso represents, warrants and covenants that during the Term Nisso shall own the Nihongi Facility, free and clear of all liens, claims and encumbrances, including without limitation any obligation to the Bayer group. | |
| 14.4 | Debarment. During the Term of this Agreement, each Party shall not knowingly use any employee, representative, agent, assistant or associate who has been debarred by the FDA pursuant to 21 U.S.C. Section 335 (a) or (b) of the Act in connection with any of the activities to be carried out under this Agreement. Each of DSP and Nisso represents and warrants that, as of the Effective Date, it has not engaged in any conduct or activity which could lead to any debarment action. |
ARTICLE 15: FORCE MAJEURE
No Party hereto shall be liable to the other Parties for any losses or damages attributable to
a default in or breach of this Agreement which is the result of any cause beyond the reasonable
control of such Party, including but not limited to war (whether declared or undeclared), acts of
God, revolution, terrorism, civil commotion, strike or labor disruption, fire, earthquake, flood,
pestilence, explosion, riot, enactment or change of laws and regulations, accident(s), labor
trouble, or shortage of or inability to obtain services, material, equipment or transport. The
performance of obligations hereunder shall be suspended during, but no longer than, the existence
of such cause mentioned in this Article. In the event that a cause mentioned in this Article
prevents or will prevent implementation of this Agreement for more than six (6) months, the
additional rights of the Parties specified in Sections 8.1(d), 11.2(f) (Force Majeure
Event for Replidyne) and 11.2(g) (Force Majeure Event for DSP or Nisso) hereof shall
apply.
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 41 |
ARTICLE 16: NON-WAIVER
A Party’s failure to exercise and/or delay in exercising, any right, remedy, power or
privilege hereunder, shall not operate as a waiver thereof; nor shall any single or partial
exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise
thereof or the exercise of any other right, remedy, power or privilege.
ARTICLE 17: MODIFICATION
No modification, extension, or waiver of any provision of this Agreement shall be valid unless
the same is in writing signed by the duly authorized officers of all Parties hereto.
ARTICLE 18: MISCELLANEOUS
| 18.1 | Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATIONS OR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT. |
| 18.2 | Liquidated Damages. Except with respect to a breach of Article 10 (Confidentiality) and respect to each Party’s obligation to indemnify the other Parties for indemnification liability to a Third Party under Article 13 (Hold Harmless and Insurance) and the provisions of this Section 18.2, no Party shall be liable for any special, consequential, indirect, incidental or punitive damages, under any cause of action, whether under any contract, negligence, strict liability or other legal or equitable theory, with respect to any subject matter of this Agreement and whether or not such Party or its agents have been advised of the possibility of such damage. This limitation shall apply notwithstanding any failure of the essential purpose of any limited remedy provided herein. Notwithstanding the foregoing, as liquidated damages and not as a penalty, Nisso shall pay to Replidyne an amount equal to Replidyne’s Net Margin for Lost Sales. For purposes of this Section 18.2: |
| (i) | “Supply Interruption” means any period during the Term after Nisso has failed to timely deliver to Replidyne more than twenty percent (20%) of quantities of the Drug Substance ordered by Replidyne for delivery in a ninety (90) day period conforming to the applicable Purchase Orders, warranties, Specifications and Applicable Laws as required by this Agreement, including without limitation due to Supplier’s failure to perform its obligations under Article 2 hereof in accordance with the Timeline, other than due to (i) a material breach by Replidyne of its obligations hereunder, or (ii) Force Majeure. (For example, if Replidyne ordered 100 kilograms of the Drug Substance for delivery in a 90 day period and Nisso only delivered 79 kilograms (or 79% of the quantities ordered) in such period, then a Supply Interruption would have occurred.) |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 42 |
| (ii) | “Lost Sales” means, determined on a quarterly basis for each calendar quarter or portion thereof during a Supply Interruption, the quantity of sales that Replidyne would have reasonably realized from Third Parties for Drug Products which Replidyne cannot fill because it is out-of-stock due to a Supply Interruption. | ||
| (iii) | “Net Margin” shall mean the gross invoice amount for Drug Products ordinarily invoiced by Replidyne for the relevant Third Party, less (i) promotional and trade discounts; (ii) sales and excise taxes, value-added and other taxes, shipping costs and insurance premiums and duties which are billed to such Third Party as separate items on invoices; (iii) allowances for short shipments, claims for returned goods and price adjustments; (iv) contracts charge-backs and government rebates; and (v) the cost of the Drug Products to Replidyne. Also deducted from Net Margin shall be out-of-pocket costs to Replidyne related to the commercialisation of Drug Products that are reasonably cancellable by Replidyne without penalty. |
| Replidyne shall invoice Nisso quarterly for such payments, and Nisso shall pay each such invoice not later than forty five (45) calendar days after its receipt thereof. Each such invoice shall be accompanied by documentation reasonably satisfactory to Nisso setting forth the Net Margin for Lost Sales covered by the invoice. |
| 18.3 | Severability. In the event that any one or more of the provisions of this Agreement should for any reason be held by the competent authorities to be invalid, illegal or unenforceable, to the extent practicable such provision or provisions shall be reformed or renegotiated to as nearly approximate the original reasonable intent of the Parties as possible and the validity, legality or enforceability of the remaining provisions shall in no way be affected or impaired thereby. |
| 18.4 | Accrued Obligation. Termination of this Agreement for any reason shall not release any Party hereto from any liability which at the time of such termination has already accrued to such Party or which is attributable to a period prior to such termination, nor preclude any Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement. | |
| 18.5 | Independent Contractor. The relationship among DSP, Nisso and Replidyne is that of independent contractors. DSP, Nisso and Replidyne are not joint venturers, partners, principal and agent, employer and employee, and have no other relationship other than independent contracting Parties. No Party shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on another Party, without the prior written consent of such other Party. Notwithstanding the foregoing, DSP and Nisso shall be jointly and severally liable for the obligations of the “Supplier” hereunder but only where it is explicitly specified as the “Supplier” in this Agreement and Replidyne shall be entitled to pursue any claim for such obligations of the Supplier hereunder against either of DSP or Nisso or both of them. Subject to the foregoing, the obligations and liabilities of each Party hereunder (except those of DSP and Nisso as “Supplier”) are several only and are not joint or joint and several. |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 43 |
| 18.6 | Assignment. This Agreement shall not be assignable by any Party to any Third Party without the prior written consent of the other Parties hereto, such consent not to be unreasonably withheld, except (i) in connection with any acquisition or merger of such Party or sale of all or substantially all of its assets, or (ii) by Replidyne, in connection with a permitted assignment of the License Agreement. Any attempted assignment in breach of this Section 18.6 (Assignment) shall be null and void. This Agreement including any amendment and/or supplement hereto shall inure to the benefit of and be binding upon the Parties hereto and their respective successors and permitted assigns. |
| 18.7 | Entire Agreement. This Agreement and the Attachments hereto and the purchase agreement dated May 25, 2004 (“Purchase Agreement”) constitute the entire agreement among all of the Parties hereto concerning the subject matter hereof and any representation, promise or condition in connection therewith, not incorporated in this Agreement or the Purchase Agreement shall not be binding upon any Party, except that the Parties may amend such agreement(s) by a written agreement executed by all of the Parties. The Supply LOI and Letter Agreement are hereby superceded and terminated completely. |
| 18.8 | Captions. The captions used in this Agreement are for convenience only, and this Agreement shall not be construed or interpreted by reference to such captions. |
| 18.9 | Guarantee on Mergers and Acquisitions. In case any Party (“Acquired Party”) is merged with or acquired by or has sold all or substantially all of its assets to a Third Party (“Acquiring Party”), the Acquired Party shall obtain from the Acquiring Party a written assurance addressed to the other Parties stating that the Acquiring Party agrees be to bound by and perform and comply with all of the rights and obligations of the Acquired Party under this Agreement. In case any such assurance is not obtained within six (6) months of the effective date of such merger, acquisition or sale, such other Parties shall have the right to terminate this Agreement at any time within six (6) months after the expiration of such six (6) month period, by ninety (90) days’ notice in writing to the Acquired Party. |
| 18.10 | Cumulative Remedies. Except as expressly provided herein, the rights and remedies afforded to each Party pursuant to any provision of this Agreement are in addition to and do not in any way limit any other rights or remedies afforded to any Party by any other provision of this Agreement or by law. All such rights and remedies are cumulative and may be exercised singularly or concurrently. |
ARTICLE 19: APPLICABLE LAW
This Agreement and any additional agreements, documents or instruments to be executed in
connection herewith between the Parties hereto shall be prepared in English language. This
Agreement shall be governed by and construed in accordance with the laws of the State of New York
unless the mandatory law of any country of the Territory is applicable.
ARTICLE 20: DISPUTE RESOLUTION
The Parties shall endeavor to resolve all conflicts and disputes arising out of or in any way
in connection with this Agreement amicably between themselves. In the event the Parties are
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 44 |
unable to amicably resolve any such conflict or dispute among themselves, any such conflict or
dispute shall be finally settled by arbitration according to the rules of the International Chamber
of Commerce (“ICC”). If a Party intends to commence arbitration to resolve such dispute,
it shall give the other Parties written notice (“Arbitration Notice”) of such intention and
the issues to be resolved. The arbitration shall take place in Tokyo, Japan if Replidyne demands
arbitration, or in Denver, Colorado U.S.A., if DSP or Nisso demands arbitration. The award shall
be final and binding upon the Parties hereto. The arbitration shall be conducted by a panel of
industry experts experienced in the issues comprising any such dispute, selected in such a manner
that DSP and Nisso are considered to be one Party, and Replidyne considered to be one Party,
pursuant to such rules. In no event shall the arbitration proceeding extend more than twelve (12)
months from the date of filing of the Arbitration Notice with the ICC. Each Party shall bear its
own expense in the arbitration and share equally the fees of the arbitration panel, unless such
panel determines that its fees are to be paid by the non-prevailing Party. The Panel shall have
the authority to award any remedy allowed by law or in equity, excluding however, punitive damages
and those damages described in Section 18.2 (Liquidated Damages) hereof.
ARTICLE 21: NOTICE
All notice, given by one Party hereto to the other Parties hereunder, shall be in writing and
made by registered or certified air mail, facsimile, express overnight courier or delivered
personally to the following addresses of the respective Parties:
| If to DSP: | Daiichi Suntory Pharma Co., Ltd. | |||
| General Manager, | ||||
| Business Planning & Development Dept. | ||||
| ▇-▇, ▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇ | ||||
| ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇ | ||||
| Facsimile Number: ▇▇▇-▇-▇▇▇▇-▇▇▇▇ | ||||
| If to Replidyne: | Replidyne, Inc. | |||
| Chief Executive Officer | ||||
| ▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇ | ||||
| ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇ | ||||
| ▇.▇.▇. | ||||
| Facsimile Number: (▇▇▇) ▇▇▇ ▇▇▇▇ | ||||
| If to Nisso: | Nippon Soda Co., Ltd. | |||
| General Manager | ||||
| Pharma-Chemicals Business Group | ||||
| ▇-▇, ▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇ | ||||
| ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇ | ||||
| Facsimile Number: ▇▇▇-▇-▇▇▇▇-▇▇▇▇ |
The notice, unless otherwise provided, shall be deemed to be effective: (a) upon receipt if
personally delivered or by facsimile with evidence of transmission; (b) on the tenth (10th)
business day following the date of mailing if sent by registered or certified air mail; or (c) on
the second
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 45 |
business day following the date of transmission or delivery to the overnight courier if sent
overnight courier. A Party may change its address listed above by sending notice to the other
Parties.
ARTICLE 22: PUBLICITY
No Party, without the prior written approval of the other Parties, shall originate any
publicity, news release or public announcement, written or oral, whether to the public press,
stockholders or otherwise, relating to this Agreement, including, without limitation, its
existence, the subject matter to which it relates, performance under it or any of its terms, to any
amendment hereto or performance hereunder, (“Announcement”), except to the extent such
Announcement contains only information authorized for release in a previous Announcement, or such
an Announcement is required by Applicable Laws to be made, as determined by counsel for the Party
making such Announcement. Other than Announcements required by law, a Party will give the other
Parties at least fifteen (15) business days’ advance written notice of the text of any proposed
Announcement so that the other Parties will have an opportunity to comment upon the Announcement.
For an Announcement required by law, each Party will endeavor to give the other Parties reasonable
notice, which presumptively shall be fifteen (15) days notice, unless circumstances require that
the Announcement be released sooner.
ARTICLE 23: LANGUAGE
All notices, plans, protocols, reports and other documents to be provided by the Supplier to
Replidyne or by Replidyne to the Supplier under this Agreement, whether for approval or for
information, shall be provided in the English language.
ARTICLE 24: EXECUTION IN COUNTERPARTS
This Agreement may be executed in any number of counterparts, each of which shall be deemed an
original but all of which together shall constitute one and the same instrument.
[Remainder of page intentionally left blank]
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 46 |
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be executed by
their duly authorized officers effective as of the Effective Date.
| DAIICHI SUNTORY PHARMA CO., LTD. | REPLIDYNE, INC. | |||||||
Signature:
|
/s/ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ | Signature: | /s/ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ | |||||
Name:
|
▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ | Name: | ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ | |||||
Title:
|
President | Title: | President and | |||||
| Chief Executive Officer | ||||||||
Date:
|
27/4/2005 | Date: | 5/31/05 | |||||
| NIPPON SODA CO., LTD. | ||||||||
Signature:
|
/s/ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇ | |||||||
Name:
|
▇▇▇▇▇▇▇▇ ▇▇▇▇▇ | |||||||
Title:
|
Board of Directors | |||||||
| General Manager | ||||||||
| Specialty & Performance Chemicals Div. | ||||||||
Date:
|
19/5/2005 | |||||||
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT | PAGE 47 |
Attachment 1
Definitions
“Act” means the United States Federal Food Drug and Cosmetic Act (21 U.S.C. Section
301 et seq.), as amended from time to time, and similar laws and regulations in the Territory.
“Affiliates” means any entity which directly or indirectly controls, is controlled by,
or is under common control with a specified entity. For purposes of this definition, an entity is
deemed to be in control of another entity if the former has the direct or indirect power to control
the management and policies of the latter, whether through ownership of voting securities, by
contract, or otherwise.
“Applicable Law(s)” means the Act, Regulations and all other applicable laws, rules,
regulations and guidelines that apply to the import, export, development, marketing, distribution
or sale of the Drug Products in the Territory or the performance of any Party’s obligations under
this Agreement, to the extent applicable and relevant, including without limitation cGMPs and
current Good Clinical Practices standards or similar guidelines promulgated by the Regulatory
Authorities.
“AOSA” means the chemical substance identified by the following chemical formula:
(3R,4R)-4-acetoxy-3-[(R)-1-tert-butyldimethylsiloxyethyl]-2-azetidinone.
“Bankruptcy Event” means if a Party: (i) passes any resolution for or permits any
proceedings for its winding up; or (ii) makes a general assignment for the benefit of creditors; or
(iii) has filed against it or files a petition in bankruptcy or insolvency or is declared bankrupt
or insolvent or declares that it is bankrupt or insolvent; or (iv) has filed against it or files
any petition or answer seeking reorganization, readjustment, or arrangement of its business or
debts and such action remains undismissed or unstayed for a period of more than sixty (60) days.
“CFR” means the United States Code of Federal Regulations, as amended from time to
time.
“cGMPs” means current good manufacturing practices as required by any applicable
regulatory authority in the Territory and all other laws and regulations relating to the
manufacture of the Drug Substance, including, but not limited to, the Current Good Manufacturing
Practices as specified in the CFR and established under the Act and the Regulations, the EU Good
Manufacturing Guidelines, the International Conference on Harmonization Guidelines and any other
Applicable Laws, guidelines and/or regulations, together with the latest FDA and other applicable
guidance documents pertaining to manufacturing and quality control practice, all as updated,
amended and revised from time to time.
“Defect” means a non-conformity of the Drug Substance with the Specifications, the
warranties provided in Section 6.5 (Warranties) hereof or Applicable Laws, or the
contamination of the Drug Substance with other substances and/or active ingredients.
[ *** ] = Certain confidential information contained in this document, marked by brackets, is filed with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
“Drug Master File” means the drug master file, as defined in 21 CFR Section 314.420 or
successor provision, filed with the FDA with respect to the Drug Substance, and other filings in
other countries in the Territory similar to such drug master file (including without limitation
“Common Technical Documents”) which permit Nisso to authorize others to rely on the information in
such file to support an application for Regulatory Approval of the Drug Products.
“Drug Product(s)” means finished pharmaceutical preparations for human use in all
dosage forms containing the Drug Substance as a sole active ingredient or containing one or more
other therapeutically active ingredients in addition to the Drug Substance.
“Drug Substance” means the chemical substance identified as faropenem daloxate as its
generic name (code number: SUN A0026) and having the chemical structure: (5-Methyl-2-oxo-1,
3-dioxolen-4-yl) methyl (5R, 6S)-6-[(R)-1-hydroxyethyl]-2-[(R)-2-tetrahydro-furyl]
penem-3-carboxylate.
“Facilities” means the Nihongi Facility and the Takaoka Facility.
“FDA” means the United States Food and Drug Administration, or any successor entity
thereto.
“Field” means all uses of the Drug Product to treat, ameliorate or prevent infectious
diseases in humans.
“Good Clinical Practices” means the then current standards for the performance of
clinical trials for pharmaceuticals, as set forth in the Act, applicable Regulations and guidance
documents promulgated thereunder, as amended from time to time.
“ICH Guidelines” means all relevant guidelines promulgated from time to time by the
International Conference on Harmonisation of Technical Requirements for Registration of
Pharmaceuticals for Human Use.
“Launch” means the first invoiced sale of a Drug Product by Replidyne, its Affiliates
or sublicensees, on a commercial basis to a Third Party (other than a Replidyne’s sublicensee) in
a country of the Territory after Regulatory Approval has been granted by the Regulatory Authority
of that country as part of a comprehensive, nationwide (covering more than half number of the
states in the United States of America, and not just in Canada) commercial introduction of a Drug
Product in a manner comparable to the commercial introduction of other community antibiotics in the
pharmaceutical industry.
“Manufacturing Technology” means all information and documentation necessary or
advisable to enable Replidyne or the Manufacturing Designee to manufacture the Drug Substance,
including without limitation, Nisso’s standard operating procedures, Nisso’s Improvements, and the
Drug Master File sections relevant to chemical manufacturing, testing and release, Sensitive
Manufacturing Information, and all updates thereto.
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
“NDA” means a New Drug Application filed with the FDA for Regulatory Approval, along
with any supplements and/or amendments to any NDA, or any other equivalent application in any
country in the Territory other than the U.S.
“Nihongi Facility” means that portion of the manufacturing facility of Nisso located
at Nihongi, Japan in which Drug Substance is manufactured, tested or stored.
“Nisso’s Improvements” means all information, documents and tangible and intangible
materials which result from or are related to the performance by Nisso of the services contemplated
by this Agreement (including, without limitation, data, test results, measurements, quantitative
and qualitative analyses, processes, samples, inventions, discoveries, improvements, intellectual
property, derivative works, technology and/or any other work product, whether patentable or not)
developed hereunder by Nisso or on behalf of Nisso by any of its Representatives relating to the
Drug Substance or the Drug Products.
“Recall” means any action: (i) by Replidyne to recover title to or possession of
quantities of Drug Products incorporating the Drug Substance sold or shipped to third parties
(including, without limitation, the voluntary withdrawal of Drug Products incorporating the Drug
Substance from the market), (ii) by any regulatory authorities to detain or destroy any Drug
Products incorporating the Drug Substance, or (iii) by a court of competent jurisdiction to order
such a recall. “Recall” shall also include the election by Replidyne to refrain from
selling or shipping quantities of such products to Third Parties which would have been subject to a
Recall if sold or shipped.
“Regulations” means regulations, statutes, rules, guidelines and procedures
promulgated by the FDA and any other Regulatory Authority pursuant to the Act or other law
applicable to the manufacture, use or sale of the Drug Products in the Territory, including without
limitation those regulations currently contained in Title 21 of the CFR.
“Regulatory Approval” means authorization granted by a Regulatory Authority to market
and sell the Drug Products in a country in the Territory that is required before the Drug Products
may be commercially marketed and sold in such country, including without limitation any pricing
and/or reimbursement approval(s) which must be obtained before placing a Drug Product on the market
in any country in the Territory in which such approval(s) is required.
“Regulatory Authority(ies)” means any regulatory agency, department, bureau, or other
governmental entity, including without limitation the FDA, which is responsible for issuing
approvals, licenses, registrations, clearances, or authorizations necessary for the manufacture,
use, storage, import, transport, marketing or sale of Drug Substance and/or Drug Products in a
country in the Territory.
“Replidyne Improvement” means any and all developments, enhancements, modifications,
inventions or discoveries in the Field relating to the Drug Products (but excluding those related
to the Drug Substance) for use in the Field and under the control of Replidyne that are developed
or created by or on behalf of Replidyne at any time during the Term of this Agreement, whether
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
patentable or not, including, but not be limited to, developments, inventions or discoveries
intended to enhance the safety or efficacy of the Drug Products, and all intellectual property
rights thereto which are necessary or useful for Replidyne to exercise the rights licensed to it
under the License Agreement.
“Replidyne Property” means (i) all information, documents and tangible and intangible
materials owned or controlled by Replidyne, which Replidyne provides to Supplier in connection with
the performance of services hereunder, and (ii) any data, inventions, discoveries, improvements,
Replidyne Improvements, intellectual property, derivative works and/or any other work product,
whether patentable or not, developed hereunder by Replidyne or on behalf of Replidyne by any of its
Representatives.
“Representatives” means, in respect of a Party, its Affiliates, licensees,
sublicensees, and their respective employees, agents, consultants, subcontractors and other
representatives. Without limiting the generality of the foregoing, Replidyne’s Representatives
shall include the a Third Party engaged by or on behalf of Replidyne to produce the Drug Product
from the Drug Substance, other contractors, clinical research organizations and vendors, and other
collaborators/business partners with respect to the Drug Products.
“Takaoka Facility” means that portion of the manufacturing facility of Nisso located
at Takaoka, Japan in which the Drug Substance is manufactured, tested or stored.
“Territory” means the United States of America and Canada.
“Third Party” means any entity other than DSP, Nisso or Replidyne or an Affiliate of
DSP, Nisso or Replidyne.
“U.S.” or “United States of America” means the fifty (50) states, the District
of Columbia, all territories, possessions and commonwealths of the United States, Puerto Rico, Guam
and the U.S. Virgin Islands.
“USP GMPs” means Good Manufacturing Practices as promulgated from time to time by the
United States Pharmacopoeia.
* * * * *
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 2.3
Timeline
[ *** ]
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 3.3
Delay Compensation
The cost items and their costs of the delay compensation for twelve month period are estimated as
follows:
| Cost Item | Cost per twelve months | |
[ *** ]
|
JPY[ *** ] | |
[ *** ]
|
JPY[ *** ] | |
[ *** ]
|
JPY[ *** ] | |
[ *** ]
|
JPY[ *** ] | |
Total
|
JPY[ *** ] |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 4.1
Batch Sizes
The batch size of Nihongi commercial production is planned as follows:
| - | The first batch of each campaign production [ *** ]Kg as is. Control range is [ *** ]Kg to [ *** ]Kg |
||
| - | The second batch and then after [ *** ]Kg as is. Control range is [ *** ]Kg to [ *** ]Kg |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 4.2(a)
Initial Clinical Forecast
| 1. | The quantity of Drug Substance purchased and delivered pursuant to that certain Purchase Agreement dated as of May 25, 2004 among the parties. | |
| 2. | The Validation Batches to be purchased pursuant to Section 2.4 (Purchase of Validation Batches of Drug Substance) hereof. |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 5.1
Purchase Prices
Clinical Quantities Pricing
To be negotiated by the Parties on a case-by-case basis.
Commercial Quantities Pricing
| Annual Quantity | Supply Price from DSP to Replidyne | |
[ *** ]t-[ *** ]t |
To be negotiated by the Parties on a case-by-case basis. | |
[ *** ]t-[ *** ]t |
[ *** ] JP¥/kg | |
[ *** ]t-[ *** ]t |
[ *** ] JP¥/kg | |
[ *** ]t-[ *** ]t |
[ *** ] JP¥/kg | |
[ *** ]t-[ *** ]t |
[ *** ] JP¥/kg | |
[ *** ]t-[ *** ]t |
[ *** ] JP¥/kg | |
[ *** ]t-[ *** ]t |
[ *** ] JP¥/kg | |
[ *** ]t-[ *** ]t |
[ *** ] JP¥/kg* | |
> [ *** ]t |
[ *** ] |
t = metric tons of the Drug Substance in the potency basis
Potency basis means the quantity of the Drug Substance divided by one point three nine
three (1.393), representing the quantity of active faropenem.
* = The price for the Annual Quantity of [ *** ]t-[ *** ]t in the potency basis as of the Effective
Date shall be [ *** ]JP¥/kg. However, Replidyne and Nisso shall engage in cost reduction programs
aiming to lower the future price at this level of quantity through the works stipulated in
Section 5.9 (Cost Reduction Opportunities).
Only one price tier in the table above will apply for all purchases in any given year, based upon a
Purchase Forecast of total demand for the year.
* * * * *
| SUPPLY AGREEMENT |
Attachment 6.1
Drug Substance Specifications
Release Specification
[ *** ]
[ *** ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406
of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 7.9
Sensitive Manufacturing Information
Sensitive Manufacturing Information is the detailed technical information with regard to the
production of Drug Substance that provides Nisso with competitive advantages relative to other
manufacturers but which is not necessary in order for Replidyne and/or its manufacturing designee
to produce Drug Substance in accordance with the Specifications and Applicable Laws (including
regulatory history and requirements specific to faropenem daloxate). For example:
| - | Process Description of Drug Substance written in the DMF | ||
| - | Process Diagram | ||
| - | Engineering drawing of Nihongi facility | ||
| - | Batch records | ||
| - | Document of production instruction |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406
of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 8.2
Manufacturing Patents
[ *** ]
[ *** ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406
of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |
Attachment 11.1
Patents
Composition of matter of faropenem daloxate:
| Country | Application Date | Patent No. | Registration Date | Expiration Date | ||||
U.S.A |
1991.08.16 | 5830889 | 1998.11.03 | 2015.11.03 | ||||
U.S.A (divisional) |
1991.08.16 | 5885981 | 1999.03.23 | 2015.11.03 | ||||
Canada |
1991.08.16 | 2089368 | 2002.08.06 | 2011.08.16 |
Sustained Release Formulation
The patent application filed in the US on March 3, 2004 which is the Application Number in Japan
2004 ▇▇▇▇▇▇▇.
6-substituted penem compounds
| Country | Application Date | Patent No. | Expiration Date | |||
U.S.A |
1980.11.18 | 4692442 | 2004.09.08 |
6-substituted thia-aza compounds
| Country | Application Date | Patent No. | Expiration Date | |||
U.S.A |
1989.08.21 | 4952690 | 2007.08.28 |
6-substituted thia-aza compounds
| Country | Application Date | Patent No. | Expiration Date | |||
Canada |
1979.01.31 | 1340273 | 2015.12.15 |
[ *** ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406
of the Securities Exchange Act of 1933, as amended.
| SUPPLY AGREEMENT |

