SUBSCRIPTION AGREEMENT
Exhibit 10.31
THE SECURITIES TO BE ISSUED PURSUANT TO THIS AGREEMENT HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (“SECURITIES ACT”), OR ANY OTHER APPLICABLE STATE SECURITIES LAWS AND MAY NOT BE OFFERED OR SOLD UNLESS REGISTERED THEREUNDER OR UNLESS AN EXEMPTION FROM SUCH REGISTRATION IS AVAILABLE.
Shuttle
Pharmaceuticals Holdings, Inc.
Xxx Xxxxxxxx Xxxxx, Xxxxx 000
Xxxxxxxxx, Xxxxxxxx 00000
| Attn: | Xxxxxxx Xxxxxxxxxx, M.D. |
| Chief Executive Officer |
Ladies and Gentlemen:
Subscription. The undersigned (sometimes referred to herein as the “Investor”) hereby subscribes for and agrees to purchase the principal amount of the Notes (as defined below) of Shuttle Pharmaceuticals Holdings, Inc., a Delaware corporation (the “Company”), for the purchase price (the “Purchase Price”) set forth on the signature page hereto, on the terms and conditions described herein and in Exhibits A, B, C, D, E, F, G, and H hereto (collectively, the “Offering Documents”). Terms not defined herein are as defined in the Offering Documents. The Company is seeking to raise, through a private placement of the Notes pursuant to Rule 506(b) promulgated under the Securities Act of 1933, as amended, a maximum of $2,000,000 (the “Maximum Offering Amount”) in this Offering. Boustead and the Company, in their sole discretion, may accept subscriptions in excess of the Maximum Offering Amount. The minimum amount of investment required from any one subscriber to participate in this Offering is $25,000, however, the Company reserves the right, in its sole discretion, to accept subscriptions less than this amount. All references to $ or “dollar(s)” means United States dollars. The undersigned acknowledges that the Company has engaged Boustead Securities, LLC (“Boustead” or “Placement Agent”) as its exclusive placement agent in connection with this offering.
1. Description of Securities; Description of Company and Risk Factors; Lock-Up.
| a. | Description of Securities. The Company is offering (the “Offering”) to the Investor in the minimum subscription amount of $25,000, however, the Company reserves the right, in its sole discretion, to accept subscriptions less than this amount, of the Company’s 6% convertible unsecured promissory notes due three years from the date of execution (the “Notes”). | |
| This Offering is being conducted in advance of the Company’s intended initial public offering (“IPO”) of our common stock, par value $0.00001 per share (the “Common Stock”), and listing our Common Stock for trading on the Nasdaq Capital Market or other national securities exchange. | ||
| The Notes issued herein may be converted at any time by the holders into Common Stock. In addition, in connection with an IPO, the Notes will automatically (and without any action on the part of the holders) be converted into shares of Common Stock of the Company at a conversion price (the “Conversion Price”) equal to the 50% of the public offering price per share of the Common Stock offered to the public in the IPO. For the avoidance of doubt if, for example, the initial per share offering price in the IPO is $5.00 per share, the conversion price would be $2.50 (50% of the $5.00 per share IPO price). |
| 1 |
| Under our engagement letter with Boustead, dated as of November 10, 2021 (the “Engagement Letter”), Boustead has been engaged as our exclusive financial advisor for the 18-month term of the Engagement Letter. In addition, Boustead has expressed its intent to enter into an Underwriting Agreement with the Company to act as the lead underwriter for the proposed IPO on a “firm commitment” basis. There can be no assurance that we and Boustead will be able to agree on the terms of such Underwriting Agreement or that our proposed IPO will be successfully consummated. | ||
| In the event that an IPO is not consummated, and if the Company (a) is acquired as a result of a “Sale of Control” (as defined below), (b) merges with a “SPAC” (as defined below) or (c) consummates a “Reverse Merger” (as defined below) (each, a “Liquidity Event”) prior to the maturity date of the Notes, the Notes will be convertible at the option of the holders into shares of common stock of any successor-in-interest to the Company at a price per share equal to 50% of the aggregate “Transaction Consideration” (as defined below), divided by the total number of outstanding shares of common stock of the acquiror resulting from the Liquidity Event. | ||
| As used herein, (i) the term “Sale of Control” shall mean a sale of all or substantially as of the capital stock or assets of the Company to any unaffiliated third Person, whether through share sale, asset sale, merger, consolidation or like combination, as a result of which the ability to control the board of directors of the Company shall pass to such third Person, (ii) the term “SPAC” shall mean a special purpose acquisition corporation listed on Nasdaq or other national securities exchange, and (iii) the term “Reverse Merger” shall mean a reverse merger of the Company with a fully-reporting public corporation without any significant business activities, including a special purpose acquisition corporation or “SPAC,” that is then trading on Nasdaq or the OTCQX platform of the OTC Market (“Pubco”; it being contemplated that in a transaction with a SPAC or a Reverse Merger, the stockholders of the Company will own a substantial majority of the equity securities of the SPAC or Pubco. As used herein, the term “Transaction Consideration” shall mean the dollar value placed on the total consideration paid to the Company including, but not limited to, (i) the value of the Liquidity Event, including consideration whether in cash, stock or in-kind, received by and/or paid by the Company, (ii) the total amount of indebtedness for borrowed funds, capitalized lease obligations and non-trade liabilities of the Company that are either assumed by the acquirer, redeemed or otherwise satisfied in connection with the Liquidity Event, or which remain outstanding after the Liquidity Event is consummated; (iii) the fair market value of any assets excluded from the Liquidity Event; (iv) the fair market value of any ownership interests which are retained by the Company’s shareholders or which remain outstanding after the Liquidity Event is consummated; and (v) the amount of any contingent payments, including, without limitation, earn-outs and future royalties payable in connection with the Liquidity Event. | ||
| Within one hundred and eighty-one (181) days or six calendar months, whichever is later, following the consummation of the IPO, the Company shall use its reasonable commercial efforts to file a registration statement on Form S-1 (the “Resale Registration Statement”) with the SEC in order to register for resale all of the shares of Common Stock of the Company or common stock of any successor-in-interest to the Company issued to all holders of the Notes upon automatic conversion of the Notes (the “Conversion Shares”), and will use its reasonable bests efforts to cause such Resale Registration Statement to be declared effective by the SEC within forty-five (45) business days from the date of its initial submission or filing; provided, that such Conversion Shares will continue to be subject to restrictions on resale for a period of six (6) months following consummation of the IPO. | ||
| In the event neither an IPO nor a Liquidity Event is consummated within twelve (12) months of the Closing of the Offering, the Company may elect either to (a) repay the Notes in whole or in part (subject to the conversion rights of the Holders), or (b) if the Company does not repay the Notes the unpaid principal amount of the Notes will automatically increase to 110% of the outstanding principal amount. The Company may also elect to prepay the Note at any time after March 31, 2022 upon 20 business days’ prior written notice to the Holder. |
| 2 |
| In the event that the Company shall elect to raise additional capital through a private placement of Common Stock or other securities that are convertible or exercisable for a price less than the “Optional Conversion Price,” as defined below, then and in such event the Conversion Price of the Notes shall be adjusted to reflect such lower amount. The “Optional Conversion Price” shall mean a price or conversion price that is equal to the price per share determined by dividing $50 million by the total number of outstanding shares of Common Stock of the Company. | ||
| Holders of the Notes will enter into an Investor Rights Agreement and Lock-Up Agreement. The Investor Rights Agreement will provide for typical “drag along” and “tag along” rights and will permit the holders to participate in subsequent securities offerings, including the IPO, in a percentage amount of such securities offering equal to 100% of the percentage invested by such Holder in the Notes. For the avoidance of doubt, if a holder purchases $100,000 of Notes, such holder has the right to invest in subsequent offerings no less than $100,000 in the subsequent offerings, including the IPO. | ||
| The form of Note is attached as Exhibit F hereto and is part of the Offering Documents. In addition, holders of the Notes will also enter into an Investor Rights and Lock-Up Agreement with the Company in the form of Exhibit G attached hereto which shall contain customary “tag along” and “drag along” rights. | ||
| For a more detailed description of the Notes see the Term Sheet attached hereto as Exhibit A. The Notes and the shares of Common Stock or common stock of a SPAC or Pubco (“Successor Common Stock”) into which the Notes are convertible are sometimes referred to herein as the “Securities.” The above referenced IPO, SPAC acquisition or Reverse Merger is sometimes hereinafter collectively referred to as a “Liquidity Event” and the Company Common Stock or Successor Common Stock into which the Notes are convertible are sometimes collectively referred to herein as the “Conversion Shares”). The Notes and the Conversion Shares are sometimes collectively referred to herein as the “Securities.” | ||
| b. | Risks Related to the Investment in the Securities. Investing in the Securities involves a high degree of risk. Before investing, Investors should carefully consider the summary description of our business annexed hereto as Exhibit B, the risks related to our business, as set forth in Exhibit C, the description of the business set forth in Exhibit D, and the investor presentation in Exhibit E, together with the other information contained in Offering Documents. | |
| c. | Lock-Up. In connection with this Offering, the Investor shall enter into an Investors Rights and Lock-up Agreement in the form of Exhibit G, pursuant to which the Investor shall agree that from and after the date hereof and until the 180th day after the first to occur of (i) consummation of an IPO, (ii) consummation of a transaction with a SPAC or, (iii) consummation of another form of Reverse Merger, as applicable (each, the “Lock-Up Trigger Date”), the Investor agrees not to sell, transfer or otherwise dispose of the Conversion Shares. |
2. Purchase.
| a. | I hereby agree to tender to Xxxxxx Securities, Inc. (the “Escrow Agent”), by check or wire transfer of immediately available funds (to a bank account and related wire instructions to be provided to me on my request) made payable to “Xxxxxx Securities, Inc., as Agent for the Investors in Shuttle Pharmaceuticals Holdings, Inc.” for the principal amount of the Note indicated on the signature page hereto, an executed copy of this Subscription Agreement and an executed copy of my Investor Questionnaire attached as Exhibit A hereto. Funds will be held in escrow, as set forth in more detail below (the “Escrow Account”), pending the Initial Closing. |
| 3 |
| b. | The Offering is for a maximum offering of the Maximum Offering Amount. All subscriptions to purchase Notes will be held in a noninterest-bearing escrow account (the “Escrow Account”) maintained by the Escrow Agent. The subscriptions will remain in the Escrow Account until the Company has accepted such subscriptions and the Company, in its sole discretion, may accept subscriptions in excess of the Maximum Offering Amount. | |
| c. | This Offering will continue until the earlier of (a) the sale Notes for the Maximum Offering Amount, (b) January 31, 2022, or such extension date agreed to, in their sole discretion, by the Company and Boustead (the “Termination Date”). Upon the earlier of a “Closing” (defined below) on my subscription or completion of the Offering, I will be notified promptly by the Company as to whether my subscription has been accepted by the Company. |
3. Acceptance or Rejection of Subscription.
| a. | I understand and agree that the Company reserves the right to reject this subscription for the Securities, in whole or in part, for any reason and at any time prior to the “Closing” (defined below) of my subscription. | |
| b. | In the event the Company rejects this subscription, my subscription payment will be promptly returned to me without interest or deduction and this Subscription Agreement shall be of no force or effect. In the event my subscription is accepted and the Offering is completed, the subscription funds submitted by me shall be released to the Company. |
4. Closing. The closing (“Closing”) of this Offering may occur at any time and from time to time on or before the Termination Date. The Company may conduct an initial Closing (the “Initial Closing”) at any time after the acceptance of an investor’s subscription, at which time the Initial Closing will be held and all funds will be released from the Escrow Account and paid to the Company, less professional fees and compensation paid to the Placement Agent and syndicate members, if any. Thereafter, additional Closings will be held as funds are received up to the earlier to occur of receipt of the Maximum Offering Amount or the Termination Date. Boustead and the Company, in their sole discretion, may accept subscriptions in excess of the Maximum Offering Amount. All subscriptions will be placed in escrow with the Escrow Agent. If, for any reason, at the Company’s sole discretion, an investors subscription is rejected the subscribers escrowed funds will be returned to subscribers, without interest or deduction. The Securities subscribed for herein shall not be deemed issued to or owned by me until one copy of this Subscription Agreement has been executed by me and countersigned by the Company and the Closing with respect to such Securities has occurred.
5. Disclosure. Because this offering is limited to accredited investors as defined in Section 2(15) of the Securities Act, and Rule 501 promulgated thereunder, in reliance upon the exemption contained in Section 4(a)(2) of the Securities Act and applicable state securities laws, the Securities are being sold without registration under the Securities Act. I acknowledge receipt of the Offering Documents and represent that I have carefully reviewed and understand the Offering Documents, including all exhibits attached hereto. I have received all information and materials regarding the Company that I have requested. I fully understand that the Company has a limited financial and operating history and that the Securities are speculative investments which involve a high degree of risk, including the potential loss of my entire investment. I fully understand the nature of the risks involved in purchasing the Securities and I am qualified to make such investment based on my knowledge of and experience in investing in securities of this type. I have carefully considered the potential risks relating to the Company and purchase of its Securities and have, in particular, reviewed each of the risks set forth in the Offering Documents. Both my advisors and I have had the opportunity to ask questions of and receive answers from representatives of the Company or persons acting on its behalf concerning the Company and the terms and conditions of a proposed investment in the Company and my advisors and I have also had the opportunity to obtain additional information necessary to verify the accuracy of information furnished about the Company. Accordingly, I have independently evaluated the risks of purchasing the Securities.
| 4 |
6. Investor Representations and Warranties. I acknowledge, represent and warrant to, and agree with, the Company as follows:
| a. | I am aware that my investment involves a high degree of risk as disclosed in the Offering Documents and have read carefully the Offering Documents, and I understand that by signing this Subscription Agreement I am agreeing to be bound by all of the terms and conditions of the Offering Documents. | |
| b. | I acknowledge and am aware that there is no assurance as to the future performance of the Company. | |
| c. | I acknowledge that there may be certain adverse tax consequences to me in connection with my purchase of Securities, and the Company has advised me to seek the advice of experts in such areas prior to making this investment. | |
| d. | I am purchasing the Securities for my own account for investment purposes only and not with a view to or for sale in connection with the distribution of the Securities, nor with any present intention of selling or otherwise disposing of all or any part of the foregoing securities. I agree that I must bear the entire economic risk of my investment for an indefinite period of time because, among other reasons, the Securities have not been registered under the Securities Act or under the securities laws of any state and, therefore, cannot be resold, pledged, assigned or otherwise disposed of unless they are subsequently registered under the Securities Act and under applicable securities laws of certain states or an exemption from such registration is available. I hereby authorize the Company to place a restrictive legend on the Securities that are issued to me. | |
| e. | I recognize that the Securities, as an investment, involve a high degree of risk including, but not limited to, the risk of economic losses from operations of the Company and the total loss of my investment. I believe that the investment in the Securities is suitable for me based upon my investment objectives and financial needs, and I have adequate means for providing for my current financial needs and contingencies and have no need for liquidity with respect to my investment in the Company. | |
| f. | I have been given access to full and complete information regarding the Company and have utilized such access to my satisfaction for the purpose of obtaining information in addition to, or verifying information included in, the Offering Documents, and I have either met with or been given reasonable opportunity to meet with officers of the Company for the purpose of asking questions of, and receiving answers from, such officers concerning the terms and conditions of the offering of the Securities and the business and operations of the Company and to obtain any additional information, to the extent reasonably available. | |
| g. | I have such knowledge and experience in financial and business matters as to be capable of evaluating the merits and risks of an investment in the Securities and have obtained, in my judgment, sufficient information from the Company to evaluate the merits and risks of an investment in the Company. I have not utilized any person as my purchaser representative as defined in Regulation D under the Securities Act in connection with evaluating such merits and risks. | |
| h. | I have relied solely upon my own investigation in making a decision to invest in the Company. |
| 5 |
| i. | I have received no representation or warranty from the Company or any of its officers, directors, employees or agents in respect of my investment in the Company and I have received no information (written or otherwise) from them relating to the Company or its business other than as set forth in the Offering Documents. I am not participating in the offer as a result of or subsequent to: (i) any advertisement, article, notice or other communication published in any newspaper, magazine or similar media or broadcast over television or radio or (ii) any seminar or meeting whose attendees have been invited by any general solicitation or general advertising. | |
| j. | I have had full opportunity to ask questions and to receive satisfactory answers concerning the offering and other matters pertaining to my investment and all such questions have been answered to my full satisfaction. | |
| k. | I have been provided an opportunity to obtain any additional information concerning the offering and the Company and all other information to the extent the Company possesses such information or can acquire it without unreasonable effort or expense. | |
| l. | I am an “accredited investor” as defined in Section 2(15) of the Securities Act and in Rule 501 promulgated thereunder and have attached the completed Accredited Investor Questionnaire to indicate my “accredited investor” status. I can bear the entire economic risk of the investment in the Securities for an indefinite period of time and I am knowledgeable about and experienced in making investments in the equity securities of non-publicly traded companies, including early stage companies. I am not acting as an underwriter or a conduit for sale to the public or to others of unregistered securities, directly or indirectly, on behalf of the Company or any person with respect to such securities. | |
| m. | I understand that (1) the Securities have not been registered under the Securities Act, or the securities laws of certain states, in reliance on specific exemptions from registration, (2) no securities administrator of any state or the federal government has recommended or endorsed this offering or made any finding or determination relating to the fairness of an investment in the Company, and (3) the Company is relying on my representations and agreements for the purpose of determining whether this transaction meets the requirements of certain exemptions from registration afforded by the Securities Act and certain state securities laws. | |
| n. | I understand that since neither the offer nor sale of the Securities has been registered under the Securities Act or the securities laws of any state, the Securities may not be sold, assigned, pledged or otherwise disposed of unless they are so registered or an exemption from such registration is available. | |
| o. | I have had the opportunity to seek independent advice from my professional advisors relating to the suitability of an investment in the Company in view of my overall financial needs and with respect to the legal and tax implications of such investment. | |
| p. | If the Investor is a corporation, company, trust, employee benefit plan, individual retirement account, Xxxxx Plan, or other tax-exempt entity, it is authorized and qualified to become an Investor in the Company and the person signing this Subscription Agreement on behalf of such entity has been duly authorized by such entity to do so. | |
| q. | The information contained in my Investor Questionnaire, as well as any information which I have furnished to the Company with respect to my financial position and business experience, is correct and complete as of the date of this Subscription Agreement and, if there should be any material change in such information prior to the Closing of the offering, I will furnish such revised or corrected information to the Company. I hereby acknowledge and am aware that except for any rescission rights that may be provided under applicable laws, I am not entitled to cancel, terminate or revoke this subscription and any agreements made in connection herewith shall survive my death or disability. |
| 6 |
7. Placement Agent. The Company has engaged Boustead Securities LLC, a broker-dealer licensed with FINRA (the “Placement Agent”), as placement agent for the Offering on a reasonable best-efforts basis. The Company anticipates that the Placement Agent and its sub-agents or syndicate members will be paid at each Closing from the proceeds in the Escrow Account, fees including and not to exceed: a cash commission of nine percent (9%) of the first $5,000,000 in gross Purchase Price paid by Subscribers in the Offering and seven percent (7%), thereafter; a non-accountable expense allowance for certain investors of one percent (1%) of the gross purchase price paid by Subscribers in the Offering; and will receive warrants to purchase a number of shares of Common Stock equal to ten percent (10%) of the Common Stock underlying the Notes sold in the Offering to investors, with a term of five (5) years from the relevant Closing Date, and at a per share exercise price equal to the conversion price of the Notes issued to the Subscribers herein (the “Placement Agent Warrants”). Any sub-agent or syndicate member of the Placement Agent that introduces investors to the Offering will be entitled to share in the cash fees and Placement Agent Warrants attributable to those investors as described above, pursuant to the terms of an executed sub-agent or selected dealer agreement. The Company will also pay certain expenses of the Placement Agent.
8. Representations and Warranties of the Company. When used in this Section 8, unless the context indicates otherwise, all references to the “Company” also mean and include the direct and indirect subsidiaries of the Company. The Company hereby represents and warrants to the Subscriber, as of the date hereof and on each Closing Date, the following:
| a. | Organization and Qualification. The Company and each of its subsidiaries, if any, is a corporation or other business entity duly organized, validly existing and in good standing under the laws of the jurisdiction of its formation, and has the requisite corporate power to own its properties and to carry on its business as now being conducted. The Company and each of its subsidiaries is duly qualified as a foreign corporation to do business and is in good standing in every jurisdiction in which the nature of the business conducted by it makes such qualification necessary, except to the extent that the failure to be so qualified or be in good standing would not have a material adverse effect on the assets, business, financial condition, results of operations or future prospects of the Company and its subsidiaries taken as a whole (a “Material Adverse Effect”). | |
| b. | Authorization, Enforcement, Compliance with Other Instruments. (i) The Company has the requisite corporate power and authority to enter into and perform its obligations under this Agreement, and each of the Offering Documents and to issue the Securities in accordance with the terms hereof, (ii) the execution and delivery by the Company of each of the Offering Documents and the consummation by it of the transactions contemplated hereby and thereby, including, without limitation, the issuance of the Securities have been, or will be at the time of execution of such Offering Document, duly authorized by the Company’s Board of Directors, and no further consent or authorization is, or will be at the time of execution of such Offering Document, required by the Company, its respective Board of Directors or its stockholders, (iii) each of the Offering Documents will be duly executed and delivered by the Company, (iv) the Offering Documents when executed and delivered by the Company and each other party thereto will constitute the valid and binding obligations of the Company enforceable against the Company in accordance with their terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of creditors’ rights and remedies. | |
| c. | Capitalization. The authorized capital stock of the Company consists of 120,000,000 shares of capital stock consisting of (a) 100,000,000 shares of Common shares each with a par value of $0.00001 per share (the “Common Stock”), and (b) 20,000,000 shares of preferred stock, of which have been issued the shares of Common stock, preferred stock and warrants set forth in the Exhibit H – Capitalization Table, and options to purchase Common Stock at fair market value, with exercise prices ranging from $5 to $7 per share, as well as restricted stock units, have been allotted and vest over time and upon the achievement of certain business goals such as the successful launch of the Company’s services, or subject to performance earnouts or vesting, also as set forth in the Exhibit H – Capitalization Table. The Company also issues Common Stock, stock options, restricted stock units or other forms of equity compensation from time to time in lieu of salary or services rendered to the Corporation at fair market value. |
| 7 |
| All of the outstanding shares of Common Stock of the Company and all of the share capital of each of the Company’s subsidiaries have been or will be, as of the Initial Closing, duly authorized, validly issued and are fully paid and nonassessable. No shares of capital stock of the Company or any of its subsidiaries will be subject to preemptive rights or any other similar rights or any liens or encumbrances suffered or permitted by the Company; (ii) aside from the outstanding Series A convertible preferred stock and warrants issuable in connection therewith (as set forth on Exhibit H – Capitalization Table), which has certain registration rights, there will be no agreements or arrangements under which the Company or any of its subsidiaries is obligated to register the sale of any of their securities under the Securities Act, and (iii) there are no securities or instruments of the Company or any of its subsidiaries containing anti-dilution or similar provisions, including the right to adjust the exercise, exchange or reset price under such securities, that will be triggered by the issuance of the Securities as described in this Agreement. Upon request, the Company will make available to the Subscriber true and correct copies of the Company’s Certificate of Incorporation, as amended and as in effect on the date hereof (the “Certificate of Incorporation”), and the Company’s By-laws, as amended as in effect on the date hereof (the “By-laws”), and the terms of all securities exercisable for Common Stock and the material rights of the holders thereof in respect thereto other than stock options issued to officers, directors, employees and consultants. | ||
| d. | Subsidiaries and Affiliates. The Company’s direct operating subsidiary is Shuttle Pharmaceuticals, Inc., a Maryland corporation. | |
| e. | Issuance of Securities. The Securities are duly authorized and, upon issuance in accordance with the terms hereof, shall be duly issued, fully paid and nonassessable, and will be free and clear of all taxes, liens and charges with respect to the issue thereof. | |
| f. | No Conflicts. The execution, delivery and performance of each of the Offering Documents by the Company, and the consummation by the Company of the transactions contemplated hereby and thereby will not (i) result in a violation of the Certificate of Incorporation or the By-laws (or equivalent constitutive document) of the Company or any of its subsidiaries or (ii) violate or conflict with, or result in a breach of any provision of, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company or any subsidiary is a party, except for those which would not reasonably be expected to have a Material Adverse Effect, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including U.S. federal and state securities laws and regulations) applicable to the Company or any subsidiary or by which any property or asset of the Company or any subsidiary is bound or affected except for those which could not reasonably be expected to have a Material Adverse Effect. Except those which could not reasonably be expected to have a Material Adverse Effect, neither the Company nor any subsidiary is in violation of any term of or in default under its constating documents. Except those which could not reasonably be expected to have a Material Adverse Effect, neither the Company nor any subsidiary is in violation of any term of or in default under any material contract, agreement, mortgage, indebtedness, indenture, instrument, judgment, decree or order or any statute, rule or regulation applicable to the Company or any subsidiary. The business of the Company and its subsidiaries is not being conducted, and shall not be conducted in violation of any law, ordinance, or regulation of any governmental entity, except for any violation which could not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect. Except as specifically contemplated by this Agreement and as required under the Securities Act and any applicable state securities laws, neither the Company nor any of its subsidiaries is required to obtain any consent, authorization or order of, or make any filing or registration with, any court or governmental agency in order for it to execute, deliver or perform any of its obligations under or contemplated by this Agreement or the other Offering Documents in accordance with the terms hereof or thereof. Neither the execution and delivery by the Company of the Offering Documents, nor the consummation by the Company of the transactions contemplated hereby or thereby, will require any notice, consent or waiver under any contract or instrument to which the Company or any subsidiary is a party or by which the Company or any subsidiary is bound or to which any of their assets is subject, except for any notice, consent or waiver the absence of which would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect and would not adversely affect the consummation of the transactions contemplated hereby or thereby. All consents, authorizations, orders, filings and registrations which the Company or any of its subsidiaries is required to obtain pursuant to the preceding two sentences have been or will be obtained or effected on or prior to the Closing. |
| 8 |
| g. | Absence of Litigation. There is no action, suit, claim, inquiry, notice of violation, proceeding (including any partial proceeding such as a deposition) or investigation before or by any court, public board, governmental or administrative agency, self-regulatory organization, arbitrator, regulatory authority, stock market, stock exchange or trading facility (an “Action”) now pending or, to the knowledge of the Company, threatened, against or affecting the Company or any of its subsidiaries, wherein an unfavorable decision, ruling or finding would (i) adversely affect the validity or enforceability of, or the authority or ability of the Company to perform its obligations under this Agreement or any of the other Offering Documents, or (ii) have a Material Adverse Effect. | |
| h. | Acknowledgment Regarding Subscriber’s Purchase of the Securities. The Company acknowledges and agrees that each Subscriber is acting solely in the capacity of an arm’s length purchaser with respect to the Offering Documents and the transactions contemplated hereby and thereby. The Company further acknowledges that each Subscriber is not acting as a financial advisor or fiduciary of the Company (or in any similar capacity) with respect to the Offering Documents and the transactions contemplated hereby and thereby and any advice given by such Subscriber or any of their respective representatives or agents in connection with the Offering Documents and the transactions contemplated hereby and thereby is merely incidental to such Subscriber’s purchase of the Securities. | |
| i. | No General Solicitation. Neither the Company, nor any of its “affiliates” (as defined in Rule 144 under the Securities Act), nor, to the knowledge of the Company, any person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation D) in connection with the offer or sale of the Securities. | |
| j. | No Integrated Offering. Neither the Company, nor any of its affiliates, nor to the knowledge of the Company, any person acting on its or their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would require registration of the Securities under the Securities Act or cause this offering of the Securities to be integrated with prior offerings by the Company for purposes of the Securities Act. | |
| k. | Employee Relations. Neither the Company nor any subsidiary is involved in any labor dispute nor, to the knowledge of the Company, is any such dispute threatened. Neither the Company nor any subsidiary is party to any collective bargaining agreement. The Company’s and/or its subsidiaries’ employees are not members of any union, and the Company believes that its and its subsidiaries’ relationship with their respective employees is good. |
| 9 |
| l. | Permits. The Company and its subsidiaries have all authorizations, approvals, clearances, licenses, permits, certificates or exemptions (including manufacturing approvals and authorizations, pricing and reimbursement approvals, labeling approvals, registration notifications or their foreign equivalent) issued by any regulatory authority or governmental agency (collectively, “Permits”) required to conduct their respective businesses as currently conducted except to the extent that the failure to have such Permits would not have a Material Adverse Effect. The Company or its subsidiaries have fulfilled and performed in all material respects their obligations under each Permit, and, as of the date hereof, to the knowledge of the Company, no event has occurred or condition or state of facts exists which would constitute a breach or default or would cause revocation or termination of any such Permit except to the extent that such breach, default, revocation or termination would not have a Material Adverse Effect. | |
| m. | Title. Each of the Company and its subsidiaries has good and marketable title to all of its real and personal property and assets, free and clear of any material restriction, mortgage, deed of trust, pledge, lien, security interest or other charge, claim or encumbrance which would have a Material Adverse Effect. With respect to properties and assets it leases, each of the Company and its subsidiaries is in material compliance with such leases and holds a valid leasehold interest free of any liens, claims or encumbrances which would have a Material Adverse Effect. | |
| n. | Rights of First Refusal. The Company is not obligated to offer the Securities offered hereunder on a right of first refusal basis or otherwise to any third parties including, but not limited to, current or former stockholders of the Company, underwriters, brokers, agents or other third parties. | |
| o. | Reliance. The Company acknowledges that the Subscriber is relying on the representations and warranties made by the Company hereunder and that such representations and warranties are a material inducement to the Subscriber purchasing the Securities. The Company further acknowledges that without such representations and warranties of the Company made hereunder, the Subscribers would not enter into this Agreement. | |
| p. | Brokers’ Fees. The Company does not have any liability or obligation to pay any fees or commissions to any broker, finder or agent with respect to the transactions contemplated by this Agreement, other than those set forth in Section 7 above. | |
| q. | Off-Balance Sheet Arrangements. There is no transaction, arrangement, or other relationship between the Company or any subsidiary and an unconsolidated or other off-balance sheet entity that is required to be disclosed by the Company in the Financial Statements and is not so disclosed or that otherwise would have a Material Adverse Effect. | |
| r. | Investment Company. The Company is not required to be registered as, and is not an affiliate of, and immediately following the Closing will not be required to register as, an “investment company” within the meaning of the Investment Company Act of 1940, as amended. | |
| s. | Reliance. The Company acknowledges that the Purchaser is relying on the representations and warranties made by the Company hereunder and that such representations and warranties are a material inducement to the Purchaser purchasing the Notes. The Company further acknowledges that without such representations and warranties of the Company made hereunder, the Purchaser would not enter into this Agreement. |
| 10 |
9. Indemnification. I hereby agree to indemnify and hold harmless the Company and its officers, directors, shareholders, employees, agents, advisors and counsel, and Boustead Securities, LLC and its officers, directors, shareholders, employees, agents, advisors and counsel, against any and all losses, claims, demands, liabilities and expenses (including reasonable legal or other expenses, including reasonable attorneys’ fees) incurred by each such person in connection with defending or investigating any such claims or liabilities, whether or not resulting in any liability to such person, to which any such indemnified party may become subject under the Securities Act, under any other statute, at common law or otherwise, insofar as such losses, claims, demands, liabilities and expenses (a) arise out of or are based upon any untrue statement or alleged untrue statement of a material fact made by me and contained in this Subscription Agreement or my Investor Questionnaire, or (b) arise out of or are based upon any breach by me of any representation, warranty, or agreement made by me contained herein or therein.
10. Severability. In the event any parts of this Subscription Agreement are found to be void, the remaining provisions of this Subscription Agreement shall nevertheless be binding with the same force and effect as though the void parts were deleted.
11. Choice of Law and Jurisdiction. This Subscription Agreement shall be governed by the laws of the State of New York as applied to contracts entered into and to be performed entirely within the State of New York. Any action arising out of this Subscription Agreement shall be brought exclusively in a court of competent jurisdiction in New York County, New York, and the parties hereby irrevocably waive any objections they may have to venue in New York County, New York.
12. Counterparts. This Subscription Agreement may be executed in one or more counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument. The execution of this Subscription Agreement may be by actual or facsimile signature.
13. Benefit. This Subscription Agreement shall be binding upon and inure to the benefit of the parties hereto.
14. Notices and Addresses. All notices, offers, acceptance and any other acts under this Subscription Agreement (except payment) shall be in writing, and shall be sufficiently given if delivered to the addresses in person, by Federal Express or similar courier delivery or by electronic facsimile delivered to the party’s email address, as follows:
| Investor: | At the address designated on the signature page of this Subscription Agreement. | |
| Or the email address on the signature page of the Subscription Agreement | ||
| The Company: | Shuttle Pharmaceuticals Holdings, Inc. Xxx Xxxxxxxx Xxxxx, Xxxxx 000 Xxxxxxxxx, Xxxxxxxx 00000 | |
| With a copy to xxxxxxx.xxxxxxxxxx@xxxxxxxxxxxxx.xxx |
or to such other address as any of them, by notice to the others may designate from time to time. The transmission confirmation receipt from the sender’s facsimile machine shall be conclusive evidence of successful facsimile delivery. Time shall be counted to, or from, as the case may be, the delivery in person or by mailing.
15. Entire Agreement. This Subscription Agreement, together with the Offering Documents, constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior oral and written agreements between the parties hereto with respect to the subject matter hereof. This Subscription Agreement may not be changed, waived, discharged, or terminated orally but, rather, only by a statement in writing signed by the party or parties against which enforcement or the change, waiver, discharge or termination is sought.
| 11 |
16. Section Headings. Section headings herein have been inserted for reference only and shall not be deemed to limit or otherwise affect, in any matter, or be deemed to interpret in whole or in part, any of the terms or provisions of this Subscription Agreement.
17. Survival of Representations, Warranties and Agreements. The representations, warranties and agreements of Investor contained herein shall survive the delivery of, and the payment for, the Securities.
18. Acceptance of Subscription. The Company may accept this Subscription Agreement at any time for all or any portion of the Securities subscribed for by executing a copy hereof as provided and notifying me within a reasonable time thereafter.
RESIDENTS OF ALL STATES: THE SECURITIES OFFERED HEREBY HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), OR THE SECURITIES LAWS OF ANY STATE OR OTHER JURISDICTION AND ARE BEING OFFERED AND SOLD IN RELIANCE ON EXEMPTIONS FROM THE REGISTRATION REQUIREMENTS OF SAID ACT AND SUCH LAWS. THE SECURITIES ARE SUBJECT TO RESTRICTIONS ON TRANSFERABILITY AND RESALE AND MAY NOT BE TRANSFERRED OR RESOLD EXCEPT AS PERMITTED UNDER SAID ACT AND SUCH LAWS PURSUANT TO REGISTRATION OR EXEMPTION THEREFROM. INVESTORS SHOULD BE AWARE THAT THEY WILL BE REQUIRED TO BEAR THE FINANCIAL RISKS OF THIS INVESTMENT FOR AN INDEFINITE PERIOD OF TIME. THE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SECURITIES AND EXCHANGE COMMISSION, ANY STATE SECURITIES COMMISSION OR OTHER REGULATORY AUTHORITY, NOR HAVE ANY OF THE FOREGOING AUTHORITIES PASSED UPON OR ENDORSED THE MERITS OF THIS OFFERING OR THE ACCURACY OR ADEQUACY OF THE OFFERING DOCUMENTS. ANY REPRESENTATION TO THE CONTRARY IS UNLAWFUL.
SALES IN FLORIDA: THE SECURITIES OFFERED HEREBY WILL BE SOLD, AND ACQUIRED, IN A TRANSACTION EXEMPT UNDER SECTION 517.061(11) OF THE FLORIDA SECURITIES AND INVESTOR PROTECTION ACT. THE SECURITIES HAVE NOT BEEN REGISTERED UNDER SAID ACT IN THE STATE OF FLORIDA. PURSUANT TO SECTION 517.061(11) OF THE FLORIDA SECURITIES AND INVESTOR PROTECTION ACT, WHEN SALES ARE MADE TO FIVE (5) OR MORE PERSONS IN THE STATE OF FLORIDA, ANY SALE IN THE STATE OF FLORIDA MADE PURSUANT TO SECTION 517.061(11) OF SUCH ACT IS VOIDABLE BY THE PURCHASER IN SUCH SALE (WITHOUT INCURRING ANY LIABILITY TO THE COMPANY OR TO ANY OTHER PERSON OR ENTITY) EITHER WITHIN THREE (3) DAYS AFTER THE FIRST TENDER OF CONSIDERATION IS MADE BY SUCH PURCHASER TO THE ISSUER, AN AGENT OF THE ISSUER, OR AN ESCROW AGENT OR WITHIN THREE (3) DAYS AFTER THE AVAILABILITY OF THAT PRIVILEGE IS COMMUNICATED TO SUCH PURCHASER, WHICHEVER OCCURS LATER. TO VOID HIS OR HER PURCHASE, THE PURCHASER NEED ONLY SEND A LETTER OR TELEGRAM TO THE COMPANY AT THE ADDRESS INDICATED HEREIN. ANY SUCH LETTER OR TELEGRAM SHOULD BE SENT AND POSTMARKED PRIOR TO THE END OF THE AFOREMENTIONED THREE (3) DAY PERIOD. IT IS PRUDENT TO SEND ANY SUCH LETTER BY CERTIFIED MAIL, RETURN RECEIPT REQUESTED, TO ASSURE THAT IT IS RECEIVED AND ALSO TO HAVE EVIDENCE OF THE TIME THAT IT WAS MAILED. SHOULD A PURCHASER MAKE THIS REQUEST ORALLY, THAT PURCHASER MUST ASK FOR WRITTEN CONFIRMATION THAT THE REQUEST HAS BEEN RECEIVED. IF NOTICE IS NOT RECEIVED WITHIN THE TIME LIMIT SPECIFIED HEREIN, THE FOREGOING RIGHT TO VOID THE PURCHASE SHALL BE NULL AND VOID.
(Remainder of Page left intentionally blank.)
| 12 |
THE AGGREGATE AMOUNT SUBSCRIBED FOR HEREBY IS:
$__________ principal Notes
Manner in Which Title is to be Held. (check one)
| — Individual Ownership | — Community Property |
| — Joint Tenant with Right of Survivorship (both parties must sign) | |
| — Partnership | — Tenants in common |
| — Corporation or Trust | — XXX or Xxxxx |
| — Other (please indicate) | |
| INDIVIDUAL INVESTORS | ENTITY INVESTORS | |||
| Name of entity, if any | ||||
| Signature (Individual) | By: | |||
| *Signature | ||||
| Its: | ||||
| Signature | (Joint) | Title: | ||
| (all record holders must sign) | ||||
| Name(s) Typed or Printed | Name Typed or Printed | |||
| Address to Which Correspondence Should be Directed | Address to Which Correspondence Should be Directed | |||
| City, State and Zip Code | City, State and Zip Code | |||
| Email address for notices | Email address for notices | |||
| Name(s) Typed or Tax Identification or Social Security Number | Name(s) Typed or Tax Identification or Social Security Number | |||
* If Securities are being subscribed for by any entity, the Certificate of Signatory on the below page must also be completed
| 13 |
The foregoing subscription is accepted and the Company hereby agrees to be bound by its terms on _____ day of _________________, 2021.
| Shuttle Pharmaceuticals Holdings, Inc. | ||
| Dated: | By: | |
| Name: | Xx. Xxxxxxx Xxxxxxxxxx | |
| Its: | Chief Executive Officer | |
| 14 |
CERTIFICATE OF SIGNATORY
(To be completed if Securities are being subscribed for by an entity)
I, _____________________________, the _____________________________________
(name of signatory) (title)
Of ______________________________________________(“Entity”), a _____________________________
(name of entity) (type of entity)
Organized under the laws of _______________, hereby certify that I am empowered and duly authorized by the Entity to execute the Subscription Agreement and to purchase the Securities and certify further that the Subscription Agreement has been duly and validly executed on behalf of the Entity and constitutes a legal and binding obligation of the Entity.
IN WITNESS WHEREOF, I have set my hand this ______ day of ___________, 2021.
| (Signature) |
| 15 |
INSTRUCTIONS FOR COMPLETION OF
INVESTOR REPRESENTATION
AND SUITABILITY QUESTIONNAIRE
| Item I: | Name and address information must be provided. Securities will be issued in the name(s) set forth in this Item and delivered to the address set forth in this Item. If two people are subscribing jointly, both people must provide their names and social security numbers. A telephone number must also be provided. |
| Item II: | If the securities are to be held in a different name than the investor and sent to a different address (i.e., an XXX or other account held at a brokerage firm), this Item must be completed. If the securities are to be issued and delivered directly to the entity listed in Item I, this Item need not be completed. |
| Item III: | This Item needs to be read by the investor, but nothing needs to be written here. The Securities are suitable for investment only by prospective investors who are “Accredited Investors.” |
| Item IV: | A. Only complete this Item by checking the appropriate line if you are an individual investor. |
| B. Only complete this Item if you are an entity investor. | |
| C. Only complete this Item if you are a trust investor. | |
| Item V: | This Item needs to be read by the investor, but nothing needs to be written here. |
| Item VI: | The USA Freedom Act requires us to collect information on the sources of funds. Please complete section 1, add the documents requested in section 2 only if funds did not come from an approved country (U.S. is approved), and complete section 3. |
| Item VII: | You must thoroughly complete the Suitability Questionnaire in order for the Company and the Managing Dealer to make a determination whether this is a suitable investment for you. |
| Item VIII: | You must sign and date here. |
| 16 |
INSTRUCTIONS FOR PAYMENT
Review
and complete the Investor Representation and Suitability Questionnaire and deliver it
to the email below, then send a wire transfer using the instructions below:
Xxxxxx Securities, Inc.
Email: xxxxxxxxx@xxxxxxxx0000.xxx
If you prefer to send a wire transfer instead of a check, please mail or deliver your completed Investor Representation and Suitability Questionnaire to the address above and send the wire transfer using these instructions:
Wiring Instructions
Bank
Name: Banc of California
Bank Address: 0 XxxXxxxxx Xx, Xxxxx Xxx, XX 00000
SWIFT Code:
Routing Number:
Account Name: Xxxxxx Securities Inc.
Account Number:
REF: Shuttle Pharma – [Investor Name]
If
you prefer to send a check instead of a wire transfer, please send a
check to the account name and address below:
Xxxxxx
Securities, Inc.
0 Xxxxxxx, Xxxxx 000
Xxxxxx, XX 00000
If you need assistance, please contact:
Contact: Xxxxxxx Xxxxxxxxxxxx
Email:
xxxxxxxxx@xxxxxxxx0000.xxx
Phone: 000-000-0000
| 17 |

| 18 |

| 19 |

| 20 |

| 21 |

| 22 |

| 23 |
| 24 |

| 25 |

| 26 |

| 27 |
| 28 |
| 29 |
Exhibit A – Terms of the Offering
Shuttle Pharmaceuticals Holdings, Inc.
TERM SHEET SUMMARY
This Term Sheet Summary (the “Term Sheet”) summarizes the terms on which you and other qualified accredited investors (the “Investors”) are invited to make an investment (the “Investment”) in Shuttle Pharmaceuticals Holdings, Inc. a Delaware corporation. This Term Sheet is merely a summary of the terms and provisions of the Subscription Agreement (the “Subscription Agreement”), the form of which will be provided to you. Accordingly, this Term Sheet is qualified in its entirety by reference, and is subject in all instances, to the terms and provisions of the Subscription Agreement. You are advised to carefully review the terms and provisions of the Subscription Agreement, as well as the risk factors attached thereto, before making a decision concerning the Investment.
| Issuer: | Shuttle Pharmaceuticals Holdings, Inc., a Delaware corporation (“Shuttle Pharma,” “we,” “our,” “us” or the “Company”). | |
| Business: | For more information about the Company and its current and intended operations, see the Business Summary attached as Exhibit B to the Subscription Agreement and the business description attached as Exhibit D and the investor presentation attached as Exhibit E to the Subscription Agreement. | |
| Placement Agent: | Boustead Securities, LLC, a California-based investment bank and Broker/Dealer regulated by the U.S. Financial Industry Regulatory Association (“FINRA”) and a Member of the Securities Investor Protection Corporation (“SIPC”) (“Boustead” and the “Placement Agent”) and other licensed brokers who may become part of the selling syndicate. |
| A-1 |
Exhibit A – Terms of the Offering
| Notes being Offered: | The Notes will mature three years from the date of execution (the “Maturity Date”). The Notes issued herein may be converted at any time by the holders into Company Common Stock. In addition, the Notes will automatically (and without any action on the part of the holders) be converted into shares of Common Stock of the Company at a conversion price equal to 50% of the public offering price per share of the Common Stock offered to the public in the IPO. For the avoidance of doubt if, for example, the initial per share offering price in the IPO is $5.00 per share, the conversion price would be $2.50 (50% of the $5.00 per share IPO price).
In the event that an IPO is not consummated, and if the Company (a) is acquired as a result of a “Sale of Control” (as defined below), (b) merges with a “SPAC” (as defined below) or (c) consummates a “Reverse Merger” (as defined below) (each, a “Liquidity Event”) prior to the maturity date of the Notes, the Notes will be convertible at the option of the holders into shares of common stock of any successor-in-interest to the Company at a price per share equal to 50% of the aggregate “Transaction Consideration” (as defined below), divided by the total number of outstanding shares of common stock of the acquiror resulting from the Change of Control.
As used herein, (i) the term “Sale of Control” shall mean a sale of all or substantially as of the capital stock or assets of the Company to any unaffiliated third Person, whether through share sale, asset sale, merger, consolidation or like combination, as a result of which the ability to control the board of directors of the Company shall pass to such third Person, (ii) the term “SPAC” shall mean a special purpose acquisition corporation listed on Nasdaq or other national securities exchange, and (iii) the term “Reverse Merger” shall mean a reverse merger of the Company with a fully-reporting public corporation without any significant business activities that is then trading on Nasdaq or the OTCQX platform of the OTC Markets (“Pubco”); it being contemplated that in a transaction with a SPAC or a Reverse Merger, the stockholders of the Company will own a substantial majority of the equity securities of the SPAC or Pubco. As used herein, the term “Transaction Consideration” shall mean the dollar value placed on the total consideration paid to the Company including, but not limited to, (i) the value of the Transaction, including consideration whether in cash, stock or in-kind, received by and/or paid by the Company, (ii) the total amount of indebtedness for borrowed funds, capitalized lease obligations and non-trade liabilities of the Company that are either assumed by the acquirer, redeemed or otherwise satisfied in connection with the transaction, or which remain outstanding after the transaction is consummated; (iii) the fair market value of any assets excluded from the transaction; (iv) the fair market value of any ownership interests which are retained by the Company’s shareholders or which remain outstanding after the transaction is consummated; and (v) the amount of any contingent payments, including, without limitation, earn-outs and future royalties payable in connection with the transaction.
Within one hundred eighty-one (181) days or six calendar months, whichever is later, following the consummation of the IPO, the Company shall use its reasonable commercial efforts to file a registration statement on Form S-1 (the “Resale Registration Statement”) with the SEC in order to register for resale all of the shares of Common Stock of the Company or common stock of any successor-in-interest to the Company issued to all holders of the Notes upon automatic conversion of the Notes (the “Conversion Shares”), and will use its reasonable bests efforts to cause such Resale Registration Statement to be declared effective by the SEC within forty-five (45) business days from the date of its initial submission or filing; provided, that such Conversion Shares will continue to be subject to restrictions on resale for a period of six (6) months following consummation of the IPO.
In the event an IPO or a Liquidity Event is not consummated within twelve (12) months of the Closing of the Offering, the Company may elect either to (a) repay the Notes in whole or in part (subject to the conversion rights of the Holders), or (b) if the Company does not repay the Notes the unpaid principal amount of the Notes will automatically increase to 110% of the outstanding principal amount. The Company may also elect to prepay the Note at any time after March 31, 2022 upon 20 business days’ prior written notice to the Holder.
|
| A-2 |
In the event that the Company shall elect to raise additional capital through a private placement of Common Stock or other securities that are convertible or exercisable for a price less than the “Optional Conversion Price” (as defined below), then and in such event the Conversion Price of the Notes shall be adjusted to reflect such lower amount. The “Optional Conversion Price” shall mean a price or conversion price that is equal to the price per share determined by dividing $50 million by the total number of outstanding shares of the Company.
Holders of the Notes will enter into an Investor Rights Agreement and Lock-Up Agreement in the form attached hereto as Exhibit G. The Investor Rights Agreement will provide for typical “drag along” and “tag along” rights and will permit the holders to participate in subsequent securities offerings, including the IPO, in a percentage amount of such securities offering equal to 100% of the amount invested by such Holder in the Notes. For the avoidance of doubt, if a holder purchases $100,000 of Notes, such holder has the right to invest in subsequent offerings no less than $100,000 in the subsequent offerings, including the IPO. | ||
| Minimum Investment: | USD$25,000. The Company may accept investments for less than the minimum investment amount in its sole discretion. | |
| Offering Size | USD$2,000,000. The Maximum Amount: USD$2,000,000. Boustead and the Company, in their sole discretion, may accept subscriptions in excess of the Maximum Amount. |
| A-3 |
| Plan of Offering: | The Notes are being offered through the Placement Agent and selling syndicate on a “best efforts” basis. The offering will continue until January 31, 2022 (the “Expiration Date”), unless extended by the Company and Boustead Securities, LLC (“Boustead”) in their sole discretion
The Placement Agent and selling syndicate will receive a success fee of nine percent (9%) of the first $5,000,000 in gross purchase price of the Notes sold at each closing and seven percent (7%) thereafter, payable in cash. In addition, the Placement Agent and selling syndicate will receive a non-accountable expense allowance of one percent (1%) of the gross purchase price of the Notes sold at each closing.
In addition to the above, at each closing, the Placement Agent and selling syndicate will receive a five-year warrant to purchase a number of shares of Common Stock of the Company in an amount not to exceed ten percent (10%) of the Common Stock underlying the Notes sold at each closing, exercisable on a cashless basis, with an exercise price equal to the Conversion Price of the Notes.
Affiliates of the Placement Agent and the Company (including their respective officers, directors, employees and affiliates) may purchase Notes in this Offering.
Under our engagement letter with Boustead, originally entered into on November 10, 2021 (the “Engagement Letter”), Boustead has been engaged as our exclusive financial advisor for the 18-month term of the Engagement Letter. In addition, Boustead has expressed its intent to enter into an Underwriting Agreement with the Company to act as the lead underwriter for the proposed IPO on a “firm commitment” basis. There can be no assurance that we and Boustead will be able to agree on the terms of such Underwriting Agreement or that our proposed IPO will be successfully consummated |
| A-4 |
| Payment and Escrow; Offering Period: | The purchase price for the Notes is payable in U.S. dollars upon delivery of the completed Purchase Agreement and Investor Questionnaire. All subscription funds will be held in a non-interest bearing escrow account, for the benefit of the investors, in the Company’s name with the Placement Agent’s affiliate Xxxxxx Securities, Inc., or with such other escrow agent as may be appointed by the Placement Agent and the Company. In the event that the Company does not accept subscriptions on or before January 31, 2022 all subscription funds will be refunded, without interest thereon, and will return to each investor the subscription documents completed by each such investor. If the Company rejects a subscription, either in whole or in part (which decision is in the sole discretion of the Company), the rejected subscription funds, or the rejected portion thereof, will be returned promptly to such investor without interest thereon. In addition, all subscriptions will remain in escrow until the Company has accepted such subscriptions. After the Initial Closing and until the Company has offered in an aggregate the Maximum Amount of Notes in the offering, subsequent closings may occur at any date mutually agreed by the Company and the Placement Agent but no later than January 31, 2022, subject to extension in the discretion of the Placement Agent and the Company.
| |
| Eligible Investors: | The Notes which are offered by this Term Sheet will be sold to an unlimited number of “accredited investors” including qualified institutional buyers as such term is defined in Rule 501(a) of Regulation D as promulgated under the Securities Act of 1933, as amended (the “Securities Act”). The Securities may also be offered and sold to purchasers outside the United States in accordance with the rules of Regulation S promulgated under the Securities Act and/or such other rules and regulations, as may be applicable under the circumstances. Investors will be required to make certain representations with respect to their status and business experience and to represent, among other things, that they have received a copy of this Term Sheet, that they understand the terms and risks of this Offering, and that they are capable of withstanding a loss of their entire investment in the Notes.
| |
| Authorized and Issued Capital of the Company: | The authorized capital stock of the Company consists of 120,000,000 shares of capital stock consisting of (a) 100,000,000 shares of Common shares each with a par value of $0.00001 per share (the “Common Stock”), and (b) 20,000,000 shares of “blank check” preferred stock, par value $0.00001 per share (the “Preferred Stock”), of which have been issued the shares and warrants set forth in the Exhibit H – Capitalization Table, and stock options to purchase Common Stock at fair market value, with exercise prices ranging from $5 to $7 per share, and restricted stock units, which have been allotted and vest over time and upon the achievement of certain business goals such as the successful launch of the Company’s services, or subject to performance earnouts or vesting, also as set forth in the Exhibit H – Capitalization Table. The Company also issues Common Stock, warrants and stock options from time to time in lieu of salary or services rendered to the Corporation at fair market value.
| |
| Use of Proceeds: | The Company intends to use the net proceeds from the Offering to: expand its current operations, including its technology and intellectual property portfolio, and to fund the costs of the IPO. The Company intends to use any remaining proceeds from the Offering for working capital and other general corporate purposes. |
| A-5 |
Representations and Warranties |
The Company will make the representations and warranties contained in the Subscription Agreement. | |
| Covenants: | The Investor Package contains certain affirmative and negative covenants of the Company which are customary in a transaction of this nature. | |
| Conditions Precedent: | The Company will have taken such corporate and stockholder actions as are necessary to approve the definitive agreements and any other transactions contemplated thereby. | |
| Governing Law: | State of New York. | |
| Private Placement: | The Securities offered hereby are not being registered under the Securities Act in reliance upon the exemption from registration provided by Section 4(a)(2) thereof and Rule 506(b) of Regulation D promulgated thereunder, and pursuant to certain state securities laws. The Company may also offer the Securities in “offshore transactions” to non-U.S. persons made in compliance with the provisions of Regulation S promulgated under the Securities Act. Accordingly, the sale, transfer or other disposition of any of our securities, which are purchased pursuant hereto, may be restricted by applicable federal securities laws and/or the securities laws of one or more non-U.S. countries (depending on the residency of the Investor) and by the provisions of the Purchase Agreement executed by such Investor. See also “Lock-Up” above. | |
| Restrictions on Transferability: | None of the Notes have been registered under the Securities Act. As such, they constitute “restricted securities” under the Securities Act. Such Securities may not be sold or otherwise transferred unless they are registered under the Securities Act. | |
| Risk Factors: | The Securities being offered hereby involve a high degree of risk and should be considered only by persons who can afford the loss of their entire investment. See the Risk Factors attached as Exhibit C to the Subscription Agreement. | |
| Confidentiality: | You are requested to keep the Offering and the terms thereof, including but not limited to the provisions of this Term Sheet, in the strictest of confidence. Neither this Term Sheet nor any other information regarding the Offering should be disclosed by you other than to your advisors who need to know such information for purposes of evaluating an investment. | |
| Additional Information: | In addition to carefully considering the information contained herein, prospective Investors are urged to request from the Company additional information or copies of relevant documents as they may deem necessary or advisable in evaluating an investment, such as financial statements and the related management’s discussion and analysis. |
| Contact: | Boustead Securities, LLC 0 Xxxxxxx, Xxxxx 000 Xxxxxx, Xxxxxxxxxx 00000 XXX xxxxxxxxx@xxxxxxxx0000.xxx |
| A-6 |
Exhibit B – Business Summary of the Company
Shuttle Pharmaceuticalss Holdings, Inc.
Business Summary
Shuttle Pharmaceuticals Holdings, Inc., a Delaware corporation (“Shuttle” or the “Company”), is a clinical stage pharmaceutical company leveraging our proprietary technology to develop novel therapies designed to cure cancers. Our goal is to extend the benefits of cancer treatments by leveraging insights into current cancer therapy with surgery, radiation therapy, chemotherapy and immunotherapy. Radiation therapy (RT) is one of the most effective modalities for treating cancers. We are developing a pipeline of products designed to address limitations of the current cancer therapies as well as to extend to the new applications of radiation therapy. We believe that our product candidates will enable us to deliver cancer treatments that are safer, more reliable and at a greater scale than that of the current standard of care.
Our product candidates include Ropidoxuridine, Extended Bio-availability Ropidoxuridine (IPdR/TPI), and HDAC inhibitors (SP-1-161, SP-2-225 and SP-1-303). We have advanced Ropidoxuridine through a Phase I clinical trial using non-dilutive NIH SBIR contracts and are currently preparing a Phase II study to open in 2022. We also plan to submit investigational new drug applications (INDs) for the extended Bio-availability Ropidoxuridine with the goals of initiating Phase I clinical trials in 20223, leveraging the outcomes of the Phase I clinical study results of Ropidoxuridine. We have applied for and received FDA approval of Orphan designation for Ropidoxuridine and RT for treating brain cancer (glioblastoma). In addition, we plan to continue to develop our pre-clinical products SP-1-161, SP-2-225 and SP-1-303 with the goal of submitting INDs in 2022 and 2023. We believe our management team’s expertise in radiation therapy, combined modality cancer treatment and immuno-oncology will help drive the rapid development and, if approved, the commercialization of these potentially curative therapies for patients with aggressive cancers.
Radiation Oncology has gone through transformative technological innovation to better define tumors, allow improved shaping of radiation delivery and support dose escalation with shorter courses of treatment. Furthermore, achieving higher dose distributions within tumor volumes has reached a practical plateau, since cancers are frequently integrated with or surrounded by more sensitive normal tissues and further dose increases risk tissue necrosis. To increase cancer cures at maximally tolerated radiation doses, pharmacological and biological modifications of cells are needed to sensitize cancers, protect normal tissues, and stimulate the immune system to react against antigens produced by irradiated, damaged cancer cells. Drugs that show sensitizing properties, or the ability to make cancer cells more sensitive to radiation, offer a solution to this problem. Currently, such drugs are used off-label and many have inherent toxicities since they were designed for direct cancer treatments and not for sensitization.
Our products address the unmet need in cancer treatment for a commercially marketable radiation response modifier solution that leads to greater sensitivity of cancer cells to ionizing radiation therapy. The goal of our products is to increase the therapeutic index for patients receiving radiation and to decrease radiation-related toxicities in patients with solid tumors. Our products operate across three areas related to the treatment of cancer with RT:
| 1. | Sensitization of growing cancer cells, rendering them more susceptible to the effects of radiation therapy. |
| 2. | Activation of the DNA damage response pathway to kill cancer cells and protect adjacent normal cells. |
| 3. | Activation of the immune system to kill any remaining cells after RT. |
Our platform technology allows for the creation of an inventory of products for radiation sensitizing, immune modulation, and protection of healthy tissue.
The Management Team is led by Xx. Xxxxxxx Xxxxxxxxxx, former Chairman of the Department of Radiation Medicine at Georgetown University School of Medicine and Chief of Radiation Oncology at MedStar-Georgetown University Hospital. Xx. Xxxxxxxxxx has also served as Medical Director of Georgetown University Hospital, Interim Director of the NCI-funded Xxxxxxxx Comprehensive Cancer Center, and as a co-founder of the biotech company, Oncomed (Neopharm, Inc). Our Scientific Officers include the following individuals: Xx. Xxxx Xxxx, a radiation biologist and molecular biologist is a Professor of Radiation Medicine at Georgetown University. She provides cellular, molecular biology and small animal model expertise needed for testing newly discovered drugs and serves as the Chief Scientific Officer. Our Clinical Director, Xx. Xxxxx Xxxx, has conducted clinical research at the University of Texas’s M.D. Xxxxxxxx Cancer Center and the University of Virginia. He has served as the chair of the GI committee for the Radiation Therapy Oncology Group’s (RTOG) national prospective trials utilizing fluoropyrimidine radiation sensitization in rectal and pancreatic cancers.
| B-1 |
Exhibit C – Risk Factors
Risk Factors
An investment in our common stock involves a high degree of risk. You should carefully consider the following risk factors and all the other information in this prospectus before you decide to buy our common stock. If any of the following risks related to our business actually occurs, our business, financial condition, operating results, and prospects would be adversely affected. The market price of our common stock could decline due to any of these risks and uncertainties related to our business, or related to an investment in our common stock, and you may lose part or all of your investment.
Risks Related to Our Business
Our success is primarily dependent on the successful development, regulatory approval and commercialization of our product candidates, all of which are in the early stages of development.
We currently have a clinical stage product candidate, Ropidoxuridine, which is in the early stages of development. Ropidoxuridine has undergone an SBIR funded Phase 1 clinical trial at Lifespan/Rhode Island Hospital. We also have an HDAC inhibitor small molecule platform. The 3-lead drug candidate molecules are in preclinical phases of development. none of our product candidates have gained marketing approval for sale in the United States or any other country, and we cannot guarantee that we will ever have marketable products. To date, we have invested substantially all of our efforts and financial resources in the research and development and commercial planning for our current product candidate and our HDAC small molecule delivery platform. Our near-term prospects, including our ability to finance our Company and generate revenue, as well as our future growth, will depend heavily on the successful development, marketing approval and commercialization of our product candidates. The clinical and commercial success of product candidates will depend on a number of factors, including the following:
| ● | obtaining favorable results from our Phase 1 clinical trial for IPdR and proceeding to Phase I(b), Phase II and Phase III clinical trials, which may be slower or cost more than we currently anticipate; |
| ● | even if our clinical trials are successful, there can be no assurance that the FDA will agree that we have satisfactorily demonstrated safety or efficacy or that the FDA will not raise new issues regarding the design of our clinical trials; |
| ● | our ability to demonstrate the safety and efficacy of our product candidates to the satisfaction of the FDA; |
| ● | whether we are required by the FDA to conduct additional clinical trials to support the approval of our product candidates; |
| ● | the acceptance by the FDA of our proposed parameters for regulatory approval, including our proposed indication, endpoints and endpoint measurement tools relating to our product candidates; |
| ● | the incidence, duration and severity of adverse side effects; |
| ● | the timely receipt of necessary marketing approvals from the FDA; |
| ● | whether we are able to secure collaborations for completing the development and, if approved, commercialization of our product candidates; |
| ● | the effectiveness of our and our potential collaborators’ marketing, sales and distribution strategy and operations of product candidates that are approved; |
| ● | our success in educating physicians and patients about the benefits, administration and use of our product candidates; |
| ● | the ability of our third-party manufacturers and potential collaborators to manufacture clinical trial and commercial supplies of our product candidates to remain in good standing with regulatory bodies, and to develop, validate and maintain commercially viable manufacturing processes that are compliant with current Good Manufacturing Practices (“cGMP”) regulations; |
| C-1 |
| ● | our ability to successfully commercialize our product candidates, if approved for marketing; |
| ● | our ability to enforce our intellectual property rights; |
| ● | our ability to avoid third-party patent interference or patent infringement claims; |
| ● | acceptance of our product candidates as safe and effective by patients and the medical community; and |
| ● | a continued acceptable safety profile of our product candidates following approval. |
Many of the above-listed factors are beyond our control. Accordingly, we cannot assure you that we will ever be able to generate revenue through the sale of our product candidates. Any one of these factors or other factors discussed in this prospectus could affect our ability to successfully commercialize product candidates, which could impact our ability to earn sufficient revenues to transition from a developmental stage company and continue our business. If we are not successful in obtaining marketing approval of and commercializing our product candidates, or are significantly delayed in doing so, our business will be materially harmed. We have a limited operating history and have incurred significant losses since our inception, and we anticipate that we will continue to incur losses for the foreseeable future and may never achieve or maintain profitability.
We are a Phase I clinical stage pharmaceutical company with a limited operating history upon which you can evaluate our business and prospects. Specialty pharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We do not currently have any product candidates in advanced clinical trials or approved for sale, and we continue to incur significant research and development and general and administrative expenses related to our operations. In addition, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the specialty pharmaceutical industry. We have not generated any revenue and have incurred losses in each year since our founding in December 2012. We expect to continue to incur significant losses for the foreseeable future. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
We have a limited operating history and have incurred significant losses since our inception, and we anticipate that we will continue to incur losses for the foreseeable future and may never achieve or maintain profitability.
We currently have no source of product sales revenue.
We have not generated any revenues from commercial sales of our product candidates. Our ability to generate product revenue depends upon our ability to successfully develop and commercialize products, including any of our current product candidates or other product candidates that we may develop, in-license or acquire in the future. We do not anticipate generating revenue from the sale of products for the foreseeable future. Our ability to generate future product revenue from our current or future product candidates also depends on a number of additional factors, including our ability to:
| ● | complete research and clinical development of current and future product candidates, either directly or through collaborative relationships; |
| ● | establish and maintain supply and manufacturing relationships with third parties, and ensure adequate and legally compliant manufacturing of bulk drug substances and drug products to maintain that supply; |
| ● | obtain regulatory approval from relevant regulatory authorities in jurisdictions where we intend to market our product candidates, either directly or through collaborative relationships; |
| ● | launch and commercialize future product candidates for which we obtain marketing approval, if any, through collaborative partners; |
| ● | obtain coverage and adequate product reimbursement from third-party payors, including government payors; |
| C-2 |
| ● | achieve market acceptance for our products, if any; |
| ● | establish, maintain and protect our intellectual property rights; and |
| ● | attract, hire and retain qualified personnel. |
In addition, because of the numerous risks and uncertainties associated with clinical product development, including that our product candidates may not advance through development or achieve the endpoints of applicable clinical trials, we are unable to predict the timing or amount of any potential future product sales revenues. Our expenses also could increase beyond expectations if we decide to or are required by the FDA, or comparable foreign regulatory authorities, to perform studies or trials in addition to those that we currently anticipate. Even if we complete the development and regulatory processes described above, we anticipate incurring significant costs associated with launching and commercializing these products.
The market may not be receptive to our product candidates based on our novel therapeutic modality, and we may not generate any future revenue from the sale or licensing of product candidates.
Even if approval is obtained for a product candidate, we may not generate or sustain revenue from sales of the product due to factors such as whether the product can be sold at a competitive cost and otherwise accepted in the market. The product candidates that we are developing are based on a new delivery platform therapeutic approaches (there currently is no drug which has FDA approval for indications of radiation sensitization). Market participants with significant influence over acceptance of new treatments, such as physicians and third-party payors, may not accept our delivery platform, and we may not be able to convince the medical community and third-party payors to accept and use, or to provide favorable reimbursement for, any product candidates developed by us. Market acceptance of our product candidates will depend on, among other factors:
| ● | timing of our receipt of any marketing and commercialization approvals; | |
| ● | terms of any approvals and the countries in which approvals are obtained; | |
| ● | safety and efficacy of our product candidates; | |
| ● | prevalence and severity of any adverse side effects associated with our product candidates; | |
| ● | warnings contained in any labeling approved by the FDA or other regulatory authority; | |
| ● | convenience and ease of administration of our product candidates; | |
| ● | success of our physician education programs; | |
| ● | availability of adequate government and third-party payor reimbursement; | |
| ● | pricing of our products, particularly as compared to alternative treatments; and | |
| ● | availability of alternative effective products for indications our product candidates are intended to treat. |
We will require substantial additional financing in order to obtain marketing approval of our product candidates and commercialize our product candidates; a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our product development, other operations or commercialization efforts.
Since our inception, substantially all of our resources have been dedicated to the preclinical and clinical development of our HDAC small molecule delivery platform and our initial product candidate, Ropidoxuridine. Our capital needs to date have been met by contributions from existing shareholders, as well as through private offerings of our securities and our SBIR contracts. We believe that we will continue to expend substantial resources for the foreseeable future on the completion of clinical development and regulatory preparedness of our product candidates, preparations for a commercial launch of our product candidates, if approved, and development of any other current or future product candidates we may choose to further develop. These expenditures will include costs associated with research and development, conducting preclinical studies and clinical trials, obtaining marketing approvals, and, if we are not able to enter into planned collaborations, manufacturing and supply as well as marketing and selling any products approved for sale. In addition, other unanticipated costs may arise. Because the outcome of any drug development process is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our current product candidates, if approved, or future product candidates, if any.
| C-3 |
We estimate that our net proceeds from this private placement offering will be up to approximately $2,000,000, less offering expenses payable by us. Following this private placement offering, we intend to pursue an underwritten public offering of our Common Stock. There is no guarantee that we will be successful in completing this private placement or our planned initial public offering. In addition, our operating plan may change as a result of factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings or other sources, such as strategic collaborations. Such financing may result in dilution to shareholders, imposition of debt covenants and repayment obligations, or other restrictions that may adversely affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements depend on many factors, including:
| ● | the scope, progress, results and costs of researching and developing our current product candidates, future product candidates and conducting preclinical and clinical trials; |
| ● | the cost of commercialization activities if our current product candidates and future product candidates are approved for sale, including securing collaborative ventures for completing development of, securing marketing approval for and ultimately marketing, selling and distributing our product candidates, if approved or building a corporate infrastructure if we have to undertake these activities directly; |
| ● | our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
| ● | the number and characteristics of any additional product candidates we may develop or acquire; |
| ● | any product liability or other lawsuits related to our products or commenced against us; |
| ● | the expenses needed to attract and retain skilled personnel; |
| ● | the costs associated with being a public company; |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
| ● | the timing, receipt and amount of sales of, or royalties on, any future approved products, if any. |
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to:
| ● | delay, limit, reduce or terminate preclinical studies, clinical trials or other development activities for our current product candidates or future product candidates, if any; |
| ● | delay, limit, reduce or terminate our research and development activities; or |
| ● | delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our current or future product candidates. |
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic collaborations and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a shareholder. The incurrence of indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic collaborations and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates or grant licenses on terms unfavorable to us.
| C-4 |
Our product candidates are in the early stages of development and may fail in development or suffer delays that materially adversely affect their commercial viability.
We have no products on the market and all of our product candidates are in the early stages of development. Our ability to achieve and sustain profitability depends on obtaining regulatory approvals, including institutional review board (“IRB”) approval, for and successfully commercializing our product candidates, either alone or with third parties. Before obtaining regulatory approval for the commercial distribution of our product candidates, we or one of our collaborators must conduct extensive preclinical tests and clinical trials to demonstrate the safety and efficacy in humans of our product candidates. Preclinical testing and clinical trials are expensive, difficult to design and implement, can take many years to complete and are uncertain as to outcome. The start or end of a clinical study is often delayed or halted due to changing regulatory requirements, manufacturing challenges, required clinical trial administrative actions, slower than anticipated patient enrollment, changing standards of care, availability or prevalence of use of a comparative drug or required prior therapy, clinical outcomes or financial constraints. For instance, delays or difficulties in patient enrollment or difficulties in retaining trial participants can result in increased costs, longer development times or termination of a clinical trial. Clinical trials of a new product candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the product candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, including the size of the patient population, the eligibility criteria for the clinical trial, the age and condition of the patients, the stage and severity of disease, the nature of the protocol, the proximity of patients to clinical sites and the availability of effective treatments for the relevant disease.
A product candidate can unexpectedly fail at any stage of preclinical and clinical development. The historical failure rate for product candidates is high due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. The results from preclinical testing or early clinical trials of a product candidate may not predict the results that will be obtained in later phase clinical trials of the product candidate. We, the FDA or other applicable regulatory authorities may suspend clinical trials of a product candidate at any time for various reasons, including a belief that subjects participating in such trials are being exposed to unacceptable health risks or adverse side effects. We may not have the financial resources to continue development of, or to enter into collaborations for, a product candidate if we experience any problems or other unforeseen events that delay or prevent regulatory approval of, or our ability to commercialize, product candidates, including:
| ● | negative or inconclusive results from our clinical trials or the clinical trials of others for product candidates similar to ours, leading to a decision or requirement to conduct additional preclinical testing or clinical trials or abandon a program; |
| ● | serious and unexpected drug-related side effects experienced by participants in our clinical trials or by individuals using drugs similar to our product candidates; |
| ● | delays in submitting an Investigational New Drug application (“IND”) or delays or failure in obtaining the necessary approvals from regulators to commence a clinical trial, or a suspension or termination of a clinical trial once commenced; |
| ● | conditions imposed by the FDA or comparable foreign authorities regarding the scope or design of our clinical trials; |
| ● | delays in enrolling research subjects in clinical trials; |
| ● | high drop-out rates of research subjects; |
| ● | greater than anticipated clinical trial costs; |
| ● | poor effectiveness of our product candidates during clinical trials; |
| C-5 |
| ● | unfavorable FDA or other regulatory agency inspection and review of a clinical trial site; |
| ● | failure of our third-party contractors or investigators to comply with regulatory requirements or otherwise meet their contractual obligations in a timely manner, or at all; |
| ● | delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight around clinical testing generally or with respect to our technology in particular; or |
| ● | varying interpretations of data by the FDA and similar foreign regulatory agencies. |
If third parties on which we depend to conduct our preclinical studies, or any future clinical trials, do not perform as contractually required, fail to satisfy regulatory or legal requirements or miss expected deadlines, our development program could be delayed with materially adverse effects on our business, financial condition, results of operations and prospects.
We are relying on third party collaborators to conduct our efficacy clinical trials for Ropidoxuridine and plan to rely on third party clinical investigators, contract research organizations (“CROs”), clinical data management organizations and consultants to design, conduct, supervise and monitor preclinical studies of our product candidates and will do the same for any clinical trials. Because we plan to largely rely on third parties and do not have the ability to conduct preclinical studies or clinical trials independently, we have less control over the timing, quality and other aspects of preclinical studies and clinical trials than we would if we conducted them on our own. These investigators, CROs and consultants are not our employees and we have limited control over the amount of time and resources that they dedicate to our programs. These third parties may have contractual relationships with other entities, some of which may be our competitors, which may draw time and resources from our programs. The third parties with whom we contract might not be diligent, careful or timely in conducting our preclinical studies or clinical trials, resulting in the preclinical studies or clinical trials being delayed or unsuccessful.
If we cannot contract with acceptable third parties on commercially reasonable terms, or at all, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for the conduct of preclinical studies or clinical trials or meet expected deadlines, our clinical development programs could be delayed and otherwise adversely affected. In all events, we are responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. The FDA requires clinical trials to be conducted in accordance with good clinical practices, including for conducting, recording and reporting the results of preclinical studies and clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of clinical trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Any such event could have a material adverse effect on our business, financial condition, results of operations and/or prospects.
Because we rely on third party manufacturing and supply partners, our supply of research and development, preclinical and clinical development materials may become limited or interrupted or may not be of satisfactory quantity or quality.
We rely on third party supply and manufacturing partners to supply the materials and components for, and manufacture, our research and development, preclinical and clinical trial drug supplies. We do not own manufacturing facilities or supply sources for such components and materials. There can be no assurance that our supply of research and development, preclinical and clinical development drugs and other materials will not be limited, interrupted, restricted in certain geographic regions or of satisfactory quality or continue to be available at acceptable prices. In particular, any replacement of any drug product formulation manufacturer we may use could require significant effort and expertise in the event there are a limited number of qualified replacements for a particular product candidate.
| C-6 |
The manufacturing process for a product candidate is subject to FDA and foreign regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as Current Good Manufacturing Practice (or CGMP). In the event that any of our suppliers or manufacturers fail to comply with such requirements or to perform its obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to manufacture the materials ourselves, for which we currently do not have the capabilities or resources, or enter into an agreement with another third party, which we may not be able to do on reasonable terms, if at all. In some cases, the technical skills or technology required to manufacture our product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third party and a feasible alternative may not exist. These factors would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third party manufacture our product candidates. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop product candidates in a timely manner or within budget.
We expect to continue to rely on third party manufacturers if we receive regulatory approval for any product candidate. To the extent that we have existing or future manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially reasonable terms, we may not be able to develop and commercialize our product candidates successfully. Our or a third party’s failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
| ● | an inability to initiate or continue clinical trials of product candidates under development; |
| ● | delay in submitting regulatory applications, or receiving regulatory approvals, for product candidates; |
| ● | loss of the cooperation of a collaborator; |
| ● | subjecting our product candidates to additional inspections by regulatory authorities; |
| ● | requirements to cease distribution or to recall batches of our product candidates; and |
| ● | in the event of approval to market and commercialize a product candidate, an inability to meet commercial demands for our products. |
We may be unsuccessful in engaging in strategic transactions which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expense and present significant distractions to our management.
From time to time, we may consider strategic transactions, such as collaborations, acquisitions of companies, asset purchases and out- or in- licensing of product candidates or technologies. In particular, we will evaluate and, if strategically attractive, seek to enter into additional collaborations, including with major biotechnology or pharmaceutical companies to complete development and marketing of our product candidates, if approved. The competition for collaborators is intense, and the negotiation process is time-consuming and complex. Any proposed collaboration may be on terms that are not optimal for us, and we may not be able to maintain any new or existing collaboration if, for example, development or approval of a product candidate is delayed, sales of an approved product candidate do not meet expectations or the collaborator terminates the collaboration. Any such collaboration, or other strategic transaction, may require us to incur non-recurring or other charges, increase our near- and long-term expenditures and pose significant integration or implementation challenges or disrupt our management or business. These transactions would entail numerous operational and financial risks, including exposure to unknown liabilities, disruption of our business and diversion of our management’s time and attention in order to manage a collaboration or develop acquired products, product candidates or technologies, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, impairment of relationships with key suppliers, manufacturers or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business. Accordingly, although there can be no assurance that we will undertake or successfully complete any transactions of the nature described above, any transactions that we do complete may be subject to the foregoing or other risks and have a material adverse effect on our business, results of operations, financial condition and prospects. Conversely, any failure to enter into any collaboration or other strategic transaction that would be beneficial to us could delay the development and potential commercialization of our product candidates and have a negative impact on the competitiveness of any product candidate that reaches market.
| C-7 |
We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours. If these companies develop technologies or product candidates more rapidly than we do or their technologies, including delivery technologies, are more effective, our ability to develop and successfully commercialize product candidates may be adversely affected.
The development and commercialization of drugs is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as with universities and other research institutions which are developing new technology. Our competitors have developed, are developing or will develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community and any new treatments that enter the market. We believe that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which we may try to develop product candidates.
Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources or experience than we have. If we successfully obtain approval for any product candidate, we will face competition based on many different factors, including the safety and effectiveness of our products, the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration, the timing and scope of regulatory approvals for these products, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position. Competing products could present superior treatment alternatives, including by being more effective, safer, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or noncompetitive before we recover the expense of developing and commercializing our product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan.
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of certain key management and other specialized personnel, including Xxxxxxx Xxxxxxxxxx, M.D., our chief executive officer, Xxxx Xxxx, Ph.D., our chief scientific officer for biology, Xxxxxxx Xxxxxx Hoek, our chief financial officer and vice president operations and regulatory, and Xxxxx Xxxxxxxxxx, our president and chief operating officer. The loss of one or more members of our management team or other key employees or advisors could delay our research and development programs and materially harm our business, financial condition, results of operations and prospects. The relationships that our key managers have cultivated within our industry make us particularly dependent upon their continued employment with us. We are dependent on the continued service of our technical personnel because of the highly technical nature of our product candidates and technologies and the specialized nature of the regulatory approval process. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty. We do not maintain key person life insurance policies on any of our management team members or key employees. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations.
If our product candidates advance into Phase II and Phase III clinical trials, we may experience difficulties in managing our growth and expanding our operations.
We have limited experience in drug development and have not begun clinical trials for any of our product candidates, other than a Phase 1 clinical trial for Ropidoxuridine. As our product candidates enter and advance through preclinical studies and any clinical trials, we will need to expand our development, regulatory and manufacturing capabilities or contract with other organizations to provide these capabilities for us. In the future, we expect to have to manage additional relationships with collaborators or partners, suppliers and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. We may not be able to implement improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
| C-8 |
If any of our product candidates are approved for marketing and commercialization and we are unable to develop sales, marketing and distribution capabilities on our own or enter into agreements with third parties to perform these functions on acceptable terms, we will be unable to commercialize successfully any such future products.
We currently have no sales, marketing or distribution capabilities or experience. If any of our product candidates is approved, we plan to enter into collaborations with third parties to sell, market and distribute our products. In the alternative, we would have to develop internal sales, marketing and distribution capabilities to commercialize any approved product, which would be expensive and time-consuming, or, as is more likely, enter into collaborations with third parties to perform these services. If we rely on third parties with sales, marketing and distribution capabilities to market our products or decide to co-promote products with collaborators, we will need to establish and maintain marketing and distribution arrangements with third parties, and there can be no assurance that we will be able to enter into such arrangements on acceptable terms, if, at all. In entering into third-party marketing or distribution arrangements, any revenue we receive will depend upon the efforts of the third parties and there can be no assurance that such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance of any approved product. If we decide to market our products directly, we will need to commit significant financial and managerial resources to develop a marketing and sales force with technical expertise and supporting distribution, administration and compliance capabilities. If we are not successful in commercializing any product approved in the future, either on our own or through third parties, our business, financial condition, results of operations and prospects could be materially adversely affected.
If we fail to comply with U.S. and foreign regulatory requirements, regulatory authorities could limit or withdraw any marketing or commercialization approvals we may receive and subject us to other penalties that could materially harm our business.
Even if we receive marketing and commercialization approval of a product candidate, there can be no assurance we will not be subject to future or continuing regulatory review, including in relation to adverse patient experiences with the product and clinical results that are reported after a product is made commercially available, both in the U.S. and any foreign jurisdiction in which we seek regulatory approval. The FDA has significant post-market authority, including the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate safety risks related to the use of a product or to require withdrawal of the product from the market. The FDA also has the authority to require a risk evaluation and mitigation strategies (“REMS”) plan after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug. The manufacturer and manufacturing facilities we use to make a future product, if any, will also be subject to periodic review and inspection by the FDA and other regulatory agencies, including for continued compliance with CGMP requirements. The discovery of any new or previously unknown problems with our third-party manufacturers, manufacturing processes or facilities may result in restrictions on the product, manufacturer or facility, including withdrawal of the product from the market. If we rely on third-party manufacturers, we will not have control over compliance with applicable rules and regulations by such manufacturers. Any product promotion and advertising will also be subject to regulatory requirements and continuing regulatory review. If we or our collaborators, manufacturers or service providers fail to comply with applicable continuing regulatory requirements in the U.S. or foreign jurisdictions in which we seek to market our products, we or they may be subject to, among other things, fines, warning letters, holds on clinical trials, refusal by the FDA to approve pending applications or supplements to approved applications, suspension or withdrawal of regulatory approval, product recalls and seizures, refusal to permit the import or export of products, operating restrictions, injunction, civil penalties and criminal prosecution.
Our business entails a significant risk of product liability and our ability to obtain sufficient insurance coverage could have a material effect on our business, financial condition, results of operations or prospects.
Our business exposes us to significant product liability risks inherent in the development, testing, manufacturing and marketing of therapeutic treatments. Product liability claims could delay or prevent completion of our development programs. If we succeed in marketing products, such claims could result in an FDA investigation of the safety and effectiveness of our products, our manufacturing processes and facilities or our marketing programs and potentially a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may also result in decreased demand for our products, injury to our reputation, costs to defend the related litigation, a diversion of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our stock price. We currently have product liability insurance that we believe is appropriate for our stage of development and may need to obtain higher levels prior to marketing any of our product candidates. Any insurance we have or may obtain may not provide sufficient coverage against potential liabilities. Furthermore, clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable cost to protect us against losses caused by product liability claims that could have a material adverse effect on our business.
| C-9 |
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we may establish, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. While we make an effort to maintain strict employee work processes and oversight, employee misconduct could expose us to liability through the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. Furthermore, it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Our internal computer systems, or those of our CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
Despite the implementation of cyber security measures, our internal computer systems and those of our CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruptions of our operations. For example, the loss of preclinical data or data from any future clinical trial involving our product candidates could result in delays in our development and regulatory filing efforts and significantly increase our costs. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
Our proprietary information, or that of our customers, suppliers and business partners, may be lost or we may suffer security breaches.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, clinical trial data, our proprietary business information and that of our customers, suppliers and business partners, and personally identifiable information of our customers, clinical trial subjects and employees, in our data centers and on our networks. The secure processing, maintenance and transmission of this information is critical to our operations. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Although to our knowledge we have not experienced any such material security breach to date, any such breach could compromise our network, or the networks of our CROs or other third party service providers, and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, regulatory penalties, disrupt our operations, damage our reputation, and cause a loss of confidence in our products and our ability to conduct clinical trials, which could adversely affect our business and reputation and lead to delays in gaining regulatory approvals for our drugs. Although we maintain business interruption insurance coverage, our insurance might not cover all losses from any future breaches of our systems.
| C-10 |
Failure of our information technology systems could significantly disrupt the operation of our business.
Our business increasingly depends on the use of information technologies, which means that certain key areas such as research and development, production and sales are to a large extent dependent on our information systems or those of third-party providers. Our ability to execute our business plan and to comply with regulatory requirements with respect to data control and data integrity, depends, in part, on the continued and uninterrupted performance of our information technology systems, or IT systems and the IT systems supplied by third-party service providers. These systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and backup measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite the precautionary measures we and our third-party service providers have taken to prevent unanticipated problems that could affect our IT systems, sustained or repeated system failures or problems arising during the upgrade of any of our IT systems that interrupt our ability to generate and maintain data, and in particular to operate our proprietary technology platform, could adversely affect our ability to operate our business.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development and manufacturing involve the use of hazardous materials and various chemicals. We maintain quantities of various flammable and toxic chemicals in our facilities in Gaithersburg, Maryland that are required for our research, development and manufacturing activities. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. We believe our procedures for storing, handling and disposing these materials in our Gaithersburg facilities comply with the relevant guidelines of Gaithersburg, the State of Maryland and the Occupational Safety and Health Administration of the U.S. Department of Labor. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards mandated by applicable regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of animals and biohazardous materials. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials. Additional federal, state and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate any of these laws or regulations.
Our information technology systems could face serious disruptions that could adversely affect our business.
Our information technology and other internal infrastructure systems, including corporate firewalls, servers, leased lines and connection to the Internet, face the risk of systemic failure that could disrupt our operations. A significant disruption in the availability of our information technology and other internal infrastructure systems could cause interruptions in our collaborations with our partners and delays in our research and development work.
Changes in accounting rules and regulations, or interpretations thereof, could result in unfavorable accounting charges or require us to change our compensation policies.
Accounting methods and policies for pharmaceutical companies, including policies governing revenue recognition, research and development and related expenses and accounting for stock-based compensation are subject to review, interpretation and guidance from relevant accounting authorities, including the SEC. Changes to accounting methods or policies, or interpretations thereof, may require us to reclassify, restate or otherwise change or revise our financial statements, including those contained in this prospectus.
| C-11 |
Risks Related to Intellectual Property
If we are not able to obtain and enforce patent protection for our technologies or product candidates, development and commercialization of our product candidates may be adversely affected.
Our success depends in part on our ability to obtain and maintain patents and other forms of intellectual property rights, including in-licenses of intellectual property rights of others, for our product candidates, methods used to manufacture our product candidates and methods for treating patients using our product candidates, as well as our ability to preserve our trade secrets, to prevent third parties from infringing upon our proprietary rights and to operate without infringing upon the proprietary rights of others. As of the date of this prospectus, we have filed five patent applications with the U.S. Patent and Trademark Office (the “USPTO”) with respect to various aspects of our HDAC inhibitor small molecule delivery platform and Ropidoxuridine, our lead product candidate. However, we may not be able to apply for patents on certain aspects of our product candidates or delivery technologies in a timely fashion or at all. To date, three patents have been granted. There is no guarantee that any of our pending patent applications will result in issued or granted patents, that any of our issued, granted or licensed patents will not later be found to be invalid or unenforceable or that any issued, granted or licensed patents will include claims that are sufficiently broad to cover our product candidates or delivery technologies or to provide meaningful protection from our competitors. Moreover, the patent position of specialty pharmaceutical companies can be highly uncertain because it involves complex legal and factual questions. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our current and future proprietary technology and product candidates are covered by valid and enforceable patents or are effectively maintained as trade secrets. If third parties disclose or misappropriate our proprietary rights, it may materially and adversely impact our position in the market.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other requirements during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case. The standards applied by the USPTO and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in pharmaceutical patents. As such, we do not know the degree of future protection that we will have on our proprietary products and technology. While we will endeavor to try to protect our product candidates with intellectual property rights such as patents, as appropriate, the process of obtaining patents is time-consuming, expensive and sometimes unpredictable.
Once granted, patents may remain open to opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings for a given period after allowance or grant, during which time third parties can raise objections against such initial grant. In the course of such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims thus attacked, or may lose the allowed or granted claims altogether. In addition, there can be no assurance that:
| ● | others will not or may not be able to make, use or sell compounds that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we own or license; |
| ● | we or our licensors, collaborators or any future collaborators are the first to make the inventions covered by each of our issued patents and pending patent applications that we own or license; |
| ● | we or our licensors, collaborators or any future collaborators are the first to file patent applications covering certain aspects of our inventions; |
| ● | others will not independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| ● | A third party may not challenge our patents and, if challenged, a court may not hold that our patents are valid, enforceable and infringed; |
| ● | any issued patents that we own or have licensed will provide us with any competitive advantages, or will not be challenged by third parties; |
| ● | we may develop additional proprietary technologies that are patentable; |
| C-12 |
| ● | the patents of others will not have an adverse effect on our business; and |
| ● | our competitors do not conduct research and development activities in countries where we do not have enforceable patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets. |
We intend to license patent rights from third-party owners or licensees. If such owners or licensees do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, or if they retain or license to others any competing rights, our competitive position and business prospects may be adversely affected.
We may not be able to protect our intellectual property rights throughout the world.
Obtaining a valid and enforceable issued or granted patent covering our technology in the U.S. and worldwide can be extremely costly. In jurisdictions where we have not obtained patent protection, competitors may use our technology to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where it is more difficult to enforce a patent as compared to the U.S. Competitor products may compete with our future products in jurisdictions where we do not have issued or granted patents or where our issued or granted patent claims or other intellectual property rights are not sufficient to prevent competitor activities in these jurisdictions. The legal systems of certain countries, particularly certain developing countries, make it difficult to enforce patents and such countries may not recognize other types of intellectual property protection, particularly that relating to biopharmaceuticals. This could make it difficult for us to prevent the infringement of patents or marketing of competing products in violation of our proprietary rights generally in certain jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We generally file a provisional patent application first (a priority filing) at the USPTO. A U.S. utility application and international application under the Patent Cooperation Treaty (PCT) are usually filed within twelve months after the priority filing. Based on the PCT filing, national and regional patent applications may be filed in the European Union, Japan, Australia and Canada and, depending on the individual case, also in any or all of, inter alia, China, India, South Korea, Singapore, Taiwan and South Africa. We have so far not filed for patent protection in all national and regional jurisdictions where such protection may be available. In addition, we may decide to abandon national and regional patent applications before grant. Finally, the grant proceeding of each national or regional patent is an independent proceeding which may lead to situations in which applications might in some jurisdictions be refused by the relevant registration authorities, while granted by others. It is also quite common that depending on the country, various scopes of patent protection may be granted on the same product candidate or technology. The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the U.S., and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we or our licensors encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions. Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position in the relevant jurisdiction may be impaired and our business and results of operations may be adversely affected.
| C-13 |
We or our licensors, or any future collaborators or a strategic partners may become subject to third party claims or litigation alleging infringement of patents or other proprietary rights or seeking to invalidate patents or other proprietary rights, and we may need to resort to litigation to protect or enforce our patents or other proprietary rights, all of which could be costly, time consuming, delay or prevent the development and commercialization of our product candidates, or put our patents and other proprietary rights at risk.
We or our licensors, or any future collaborators or strategic partners may be subject to third-party claims for infringement or misappropriation of patent or other proprietary rights. We are generally obligated under our license or collaboration agreements to indemnify and hold harmless our licensors or collaborator for damages arising from intellectual property infringement by us. If we or our licensors, or any future collaborators or strategic partners are found to infringe a third party patent or other intellectual property rights, we could be required to pay damages, potentially including treble damages, if we are found to have willfully infringed. In addition, we or our licensors, collaborators or any future strategic partners may choose to seek, or be required to seek, a license from a third party, which may not be available on acceptable terms, if at all. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we or our collaborator, or any future collaborator, may be unable to effectively market product candidates based on our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. In addition, we may find it necessary to pursue claims or initiate lawsuits to protect or enforce our patent or other intellectual property rights. The cost to us in defending or initiating any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s attention. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could delay our research and development efforts and limit our ability to continue our operations.
If we were to initiate legal proceedings against a third party to enforce a patent covering one of our products or our technology, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights.
Intellectual property rights of third parties could adversely affect our ability to commercialize our product candidates, and we might be required to litigate or obtain licenses from third parties in order to develop or market our product candidates. Such litigation or licenses could be costly or not available on commercially reasonable terms.
Our competitive position may suffer if patents issued to third parties or other third party intellectual property rights cover our products or elements thereof, or our manufacture or uses relevant to our development plans. In such cases, we may not be in a position to develop or commercialize products or product candidates unless we successfully pursue litigation to nullify or invalidate the third party intellectual property right concerned, or enter into a license agreement with the intellectual property right holder, if available on commercially reasonable terms.
Third party intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in marketing our products. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing any of our product candidates that are held to be infringing. We might, if possible, also be forced to redesign product candidates so that we no longer infringe the third party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
| C-14 |
If we fail to comply with our obligations under any license, collaboration or other agreements, we may be required to pay damages and could lose intellectual property rights that are necessary for developing and protecting our product candidates and delivery technologies or we could lose certain rights to grant sublicenses.
Our current licenses impose, and any future licenses we enter into are likely to impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement, and other obligations on us. If we breach any of these obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages and the licensor may have the right to terminate the license, which could result in us being unable to develop, manufacture and sell products that are covered by the licensed technology or enable a competitor to gain access to the licensed technology. Moreover, our licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights. In addition, while we cannot currently determine the amount of the royalty obligations we would be required to pay on sales of future products, if any, the amounts may be significant. The amount of our future royalty obligations will depend on the technology and intellectual property we use in products that we successfully develop and commercialize, if any. Therefore, even if we successfully develop and commercialize products, we may be unable to achieve or maintain profitability.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent protection for certain aspects of our product candidates and delivery technologies, we also consider trade secrets, including confidential and unpatented know-how important to the maintenance of our competitive position. We protect trade secrets and confidential and unpatented know-how, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to such knowledge, such as our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants that obligate them to maintain confidentiality and assign their inventions to us. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time- consuming, and the outcome is unpredictable. In addition, some courts in the U.S. and certain foreign jurisdictions are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We may be subject to claims that we or our employees or consultants have wrongfully used or disclosed alleged trade secrets of our employees’ or consultants’ former employers or their clients. These claims may be costly to defend and if we do not successfully do so, we may be required to pay monetary damages and may lose valuable intellectual property rights or personnel.
Many of our employees were previously employed at universities or biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper our ability to commercialize, or prevent us from commercializing, our product candidates, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected.
| C-15 |
Risks Related to Government Regulation
We may be unable to obtain U.S. or foreign regulatory approval and, as a result, unable to commercialize our product candidates.
Our product candidates are subject to extensive governmental regulations relating to, among other things, research, testing, development, manufacturing, safety, efficacy, approval, recordkeeping, reporting, labeling, storage, packaging, advertising and promotion, pricing, marketing and distribution of drugs. Rigorous preclinical testing and clinical trials and an extensive regulatory approval process are required to be successfully completed in the U.S. and in many foreign jurisdictions before a new drug can be marketed. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. It is possible that none of the product candidates we may develop will obtain the regulatory approvals necessary for us or our collaborators to begin selling them.
We have very limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA. The time required to obtain FDA and other approvals is unpredictable but typically takes many years following the commencement of clinical trials, depending upon the type, complexity and novelty of the product candidate. The standards that the FDA and its foreign counterparts use when regulating us are not always applied predictably or uniformly and can change. Any analysis we perform of data from preclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We may also encounter unexpected delays or increased costs due to new government regulations, for example, from future legislation or administrative action, or from changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. It is impossible to predict whether legislative changes will be enacted, or whether FDA or foreign regulations, guidance or interpretations will be changed, or what the impact of such changes, if any, may be.
Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues from the particular product candidate for which we are seeking approval. Furthermore, any regulatory approval to market a product may be subject to limitations on the approved uses for which we may market the product or the labeling or other restrictions. In addition, the FDA has the authority to require a Risk Evaluation and Mitigation Strategy (REMS) plan as part of an NDA or biologics license application (BLA) or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug or biologic, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. These limitations and restrictions may limit the size of the market for the product and affect reimbursement by third-party payors.
If we or our collaborators, manufacturers or service providers fail to comply with healthcare laws and regulations, we or they could be subject to enforcement actions, which could affect our ability to develop, market and sell our products and may harm our reputation.
We and our collaborators are subject to federal, state, and foreign healthcare laws and regulations pertaining to fraud and abuse and patients’ rights. These laws and regulations include:
| ● | the U.S. federal healthcare program anti-kickback law, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid; |
| ● | the U.S. federal false claims law, which prohibits, among other things, individuals or entities from knowingly presenting or causing to be presented, claims for payment by government funded programs such as Medicare or Medicaid that are false or fraudulent, and which may apply to us by virtue of statements and representations made to customers or third parties; |
| ● | the U.S. federal Health Insurance Portability and Accountability Act (HIPAA) and Health Information Technology for Economic and Clinical Health (HITECH) Act, which prohibit executing a scheme to defraud healthcare programs, impose requirements relating to the privacy, security, and transmission of individually identifiable health information, and require notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information; |
| ● | the federal Open Payments regulations under the National Physician Payment Transparency Program have been issued under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, and will require that manufacturers of pharmaceutical and biological drugs covered by Medicare, Medicaid, and Children’s Health Insurance Programs report all consulting fees, travel reimbursements, research grants, and other payments or gifts with values over $10 made to physicians and teaching hospitals; and |
| ● | state laws comparable to each of the above federal laws, such as, for example, anti-kickback and false claims laws applicable to commercial insurers and other non-federal payors, requirements for mandatory corporate regulatory compliance programs, and laws relating to patient data privacy and security. |
| C-16 |
If our operations are found to be in violation of any such requirements, we may be subject to penalties, including civil or criminal penalties, monetary damages, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, any of which could adversely our financial results. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
If we or our collaborators, manufacturers or service providers fail to comply with applicable federal, state or foreign laws or regulations, we could be subject to enforcement actions, which could affect our ability to develop, market and sell our products successfully and could harm our reputation and lead to reduced acceptance of our products by the market. These enforcement actions include, among others:
| ● | adverse regulatory inspection findings; |
| ● | warning letters; |
| ● | voluntary or mandatory product recalls or public notification or medical product safety alerts to healthcare professionals; |
| ● | restrictions on, or prohibitions against, marketing our products; |
| ● | restrictions on, or prohibitions against, importation or exportation of our products; |
| ● | suspension of review or refusal to approve pending applications or supplements to approved applications; |
| ● | exclusion from participation in government-funded healthcare programs; |
| ● | exclusion from eligibility for the award of government contracts for our products; |
| ● | suspension or withdrawal of product approvals; |
| ● | product seizures; |
| ● | injunctions; and |
| ● | civil and criminal penalties and fines. |
Any drugs we develop may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drugs vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. Although we intend to monitor these regulations, our programs are currently in the early stages of development and we will not be able to assess the impact of price regulations for a number of years. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product and negatively impact the revenues we are able to generate from the sale of the product in that country.
| C-17 |
Our ability to commercialize any products successfully also will depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Even if we succeed in bringing one or more products to the market, these products may not be considered cost-effective, and the amount reimbursed for any products may be insufficient to allow us to sell our products on a competitive basis. Because our programs are in the early stages of development, we are unable at this time to determine their cost effectiveness or the likely level or method of reimbursement. Increasingly, the third-party payors who reimburse patients or healthcare providers, such as government and private insurance plans, are requiring that drug companies provide them with predetermined discounts from list prices and are seeking to reduce the prices charged or the amounts reimbursed for pharmaceutical products. If the price we are able to charge for any products we develop, or the reimbursement provided for such products, is inadequate in light of our development and other costs, our return on investment could be adversely affected.
Our current product candidates will need to be administered under the supervision of a physician on an outpatient basis. Under currently applicable U.S. law, certain drugs that are not usually self-administered (including injectable drugs) may be eligible for coverage under the Medicare Part B program if:
| ● | they are incident to a physician’s services; |
| ● | they are reasonable and necessary for the diagnosis or treatment of the illness or injury for which they are administered according to accepted standards of medical practice; and |
| ● | they have been approved by the FDA and meet other requirements of the statute. |
There may be significant delays in obtaining coverage for newly-approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA. Moreover, eligibility for coverage does not imply that any drug will be reimbursed in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim payments for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement may be based on payments allowed for lower- cost drugs that are already reimbursed, may be incorporated into existing payments for other services and may reflect budgetary constraints or imperfections in Medicare data. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement rates. Our inability to promptly obtain coverage and adequate reimbursement rates from both government-funded and private payors for new drugs that we develop and for which we obtain regulatory approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our financial condition.
We believe that the efforts of governments and third-party payors to contain or reduce the cost of healthcare and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. A number of legislative and regulatory changes in the healthcare system in the U.S. and other major healthcare markets have been proposed in recent years, and such efforts have expanded substantially in recent years. These developments have included prescription drug benefit legislation that was enacted and took effect in January 2006, healthcare reform legislation enacted by certain states, and Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (the “ACA”), a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending and enhance remedies against fraud and abuse. The ACA also contains provisions that will affect companies in the pharmaceutical industry and other healthcare related industries by imposing additional costs and changes to business practices. Provisions affecting pharmaceutical companies include the following:
| ● | mandatory rebates for drugs sold into the Medicaid program have been increased, and the rebate requirement has been extended to drugs used in risk-based Medicaid managed care plans; |
| C-18 |
| ● | the 340B Drug Pricing Program under the Public Health Services Act has been extended to require mandatory discounts for drug products sold to certain critical access hospitals, cancer hospitals and other covered entities; |
| ● | pharmaceutical companies are required to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “Donut Hole”; and |
| ● | pharmaceutical companies are required to pay an annual non-tax deductible fee to the federal government based on each company’s market share of prior year total sales of branded products to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs and Department of Defense. Since we expect our branded pharmaceutical sales to constitute a small portion of the total federal health program pharmaceutical market, we do not expect this annual assessment to have a material impact on our financial condition. |
Moreover, we cannot predict what healthcare reform initiatives may be adopted in the future. Further federal and state legislative and regulatory developments are likely, and we expect ongoing initiatives in the U.S. to increase pressure on drug pricing. Such reforms could have an adverse effect on anticipated revenues from product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop product candidates.
Our ability to obtain services, reimbursement or funding from the federal government may be impacted by possible reductions in federal spending.
U.S. federal government agencies currently face potentially significant spending reductions. Under the Budget Control Act of 2011, the failure of Congress to enact deficit reduction measures of at least $1.2 trillion for the years 2013 through 2021 triggered automatic cuts to most federal programs. These cuts would include aggregate reductions to Medicare payments to providers of up to two percent per fiscal year, starting in 2013. Under the American Taxpayer Relief Act of 2012, which was enacted on January 1, 2013, the imposition of these automatic cuts was delayed until March 1, 2013. Certain of these automatic cuts have been implemented. The full impact on our business of these automatic cuts is uncertain. If federal spending is reduced, anticipated budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA or the National Institutes of Health to continue to function at current levels. Amounts allocated to federal grants and contracts may be reduced or eliminated. These reductions may also impact the ability of relevant agencies to timely review and approve drug research and development, manufacturing, and marketing activities, which may delay our ability to develop, market and sell any products we may develop.
If any of our product candidates receives marketing approval and we or others later identify undesirable side effects caused by the product candidate, our ability to market and derive revenue from the product candidates could be compromised.
In the event that any of our product candidates receive regulatory approval and we or others identify undesirable side effects caused by one of our products, any of the following adverse events could occur, which could result in the loss of significant revenue to us and materially and adversely affect our results of operations and business:
| ● | regulatory authorities may withdraw their approval of the product or seize the product; |
| ● | we may be required to recall the product or change the way the product is administered to patients; |
| ● | additional restrictions may be imposed on the marketing of the particular product or the manufacturing processes for the product or any component thereof; |
| ● | we may be subject to fines, injunctions or the imposition of civil or criminal penalties; |
| ● | regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication; |
| ● | we may be required to create a Medication Guide outlining the risks of such side effects for distribution to patients; |
| C-19 |
| ● | we could be sued and held liable for harm caused to patients; |
| ● | the product may become less competitive; and |
| ● | our reputation. |
Risks Related to our Common Stock and this Offering
You will experience immediate and substantial dilution as a result of this Offering and may experience additional dilution in the future.
If you purchase common stock in this Offering, you will incur immediate and substantial dilution of $[ ] per share, representing the difference between the assumed initial public offering price of $[ ] per share and our pro forma net tangible book value per share after giving effect to this of Offering. In addition, we can offer no assurance that you will not experience substantial dilution in the future.
The future issuance of equity or of debt securities that are convertible into Common Stock will dilute our share capital.
We may choose to raise additional capital in the future, depending on market conditions, strategic considerations and operational requirements. To the extent that additional capital is raised through the issuance of shares or other securities convertible into shares of our Common Stock, our stockholders will be diluted. Future issuances of our common stock or other equity securities, or the perception that such sales may occur, could adversely affect the trading price of our Common Stock and impair our ability to raise capital through future offerings of shares or equity securities. No prediction can be made as to the effect, if any, that future sales of Common Stock or the availability of Common Stock for future sales will have on the trading price of our Common Stock.
The offering price of the shares and the other terms of this Offering have been arbitrarily determined by the Company.
The offering price of the shares and other terms of this Offering have been arbitrarily determined by the Company and bear no relationship to the Company’s assets, book value, potential earnings or any other recognized criterion of value. In addition, no investment banker, appraiser, or other independent third party has been consulted concerning the offering price for the shares or the fairness of the offering price used for the shares.
An active trading market for our common stock may not develop.
Prior to this offering, there has been no public market for our common stock. Following this offering, we intend to complete an underwritten public offering and apply to have the shares of common stock listed on Nasdaq, subject to our sale of a sufficient number of shares in such offering to meet the listing requirements of Nasdaq. There can be no assurance that we will be successful in completion of an initial public offering, whether underwritten or otherwise, or that an application for listing the shares on Nasdaq or on any other market will be approved. Accordingly, an active trading market for our shares may never develop or be sustained following this offering. If an active market for our common stock does not develop, it may be difficult for you to sell any shares issuable upon conversion of the note you purchase in this offering without depressing the market price for the shares or at all.
Because our management will have broad discretion over the use of the net proceeds from this Offering, you may not agree with how we use them and the proceeds may not be invested successfully.
We intend to use the net proceeds to us from this offering to fund offering to fund our business operations and pay the fees necessary to complete an underwritten initial public offering. In addition, we require funds to fund preclinical and clinical trials of product candidates, Ropidoxuridine and Doranidazole, new formulations of Ropidoxuridine with Tipiracil, O-18 containing molecules for proton radiation sensitization, continued HDAC technology platform development, working capital and general corporate purposes, including the costs of operating as a public company, as well as potential acquisition or in-licensing activities. Therefore, our management will have broad discretion as to the use of the offering proceeds. Accordingly, you will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that the proceeds will be invested in a way that does not yield a favorable, or any, return for our company.
| C-20 |
If we are able to complete an initial public offering, if securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
At present, we are a private company and there is no market for our common stok. However, if we are successful in completing an initial public offering, the trading market for our common stock will then be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our target studies and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Our board of directors has the authority, without shareholder approval, to issue preferred stock with terms that may not be beneficial to holders of our common stock and such issuance could potentially affect adversely shareholder voting power and perpetuate their control over us.
Our Certificate of Incorporation allow us to issue shares of preferred stock without any vote or further action by our shareholders. Our board of directors has the authority to fix and determine the relative rights and preferences of any preferred stock. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of shares of our common stock. These rights and preferences could negatively affect the holders of our common stock.
The ability of our executive officers and directors, who are our principal shareholders, to control our business may limit or eliminate the ability of minority shareholders to influence corporate affairs.
Our executive officers and directors, who are our principal shareholders, own and will continue to own approximately seventy-three percent (73%%) of our issued and outstanding common stock. Accordingly, they will be able to effectively control the election of directors, as well as all other matters requiring shareholder approval. The interests of our principal shareholders may differ from the interests of other shareholders with respect to the issuance of shares, business transactions with or sales to other companies, selection of other directors and other business decisions. The minority shareholders have no way of overriding decisions made by our principal shareholders. This level of control may also have an adverse impact on the market value of our shares because our principal shareholders may institute or undertake transactions, policies or programs that result in losses and may not take any steps to increase our visibility in the financial community and/or may sell sufficient numbers of shares to significantly decrease our price per share.
Our Articles of Incorporation and Bylaws provide for indemnification of officers and directors at the expense of the Company and limit their liability that may result in a major cost to us and hurt the interests of our shareholders because corporate resources may be expended for the benefit of officers and/or directors.
Our Articles of Incorporation and Bylaws provide for the indemnification of our officers and directors. We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under federal securities laws is against public policy as expressed in the Securities Act and is therefore, unenforceable.
We do not expect to pay cash dividends in the foreseeable future.
We have never paid cash dividends on our common stock. We do not expect to pay cash dividends on our common stock at any time in the foreseeable future. The future payment of dividends directly depends upon our future earnings, capital requirements, financial requirements and other factors that our board of directors will consider. Since we do not anticipate paying cash dividends on our common stock, return on your investment, if any, will depend solely on an increase, if any, in the market value of our common stock.
| C-21 |
Provisions in our amended and restated certificate of incorporation and bylaws and Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the market price of our common stock.
Our amended and restated certificate of incorporation and bylaws contain provisions that could depress the market price of our common stock by acting to discourage, delay or prevent a change in control of our company or changes in our management that the stockholders of our company may deem advantageous. These provisions among other things:
| ● | establish a classified board of directors so that not all members of our board are elected at one time; |
| ● | permit the board of directors to establish the number of directors; |
| ● | provide that directors may only be removed “for cause” and only with the approval of 66 2/3 percent of our stockholders; |
| ● | require super-majority voting to amend some provisions in our amended and restated certificate of incorporation and bylaws; |
| ● | authorize the issuance of “blank check” preferred stock that our board could use to implement a stockholder rights plan (also known as a “poison pill”); |
| ● | eliminate the ability of our stockholders to call special meetings of stockholders; |
| ● | prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders; |
| ● | provide that the board of directors is expressly authorized to make, alter or repeal our bylaws; and |
| ● | establish advance notice requirements for nominations for election to our board or for proposing matters that can be acted upon by stockholders at annual stockholder meetings. |
In addition, Section 203 of the Delaware General Corporation Law may discourage, delay or prevent a change in control of our company. Section 203 imposes certain restrictions on merger, business combinations and other transactions between us and holders of 15 percent or more of our common stock.
| C-22 |
Exhibit D – Business Description of the Company
THIS IS A PRIVATE OFFERING OF SECURITIES OF SHUTTLE PHARMACEUTICALS HOLDINGS, INC. THAT IS BEING MADE PURSUANT TO RULE 506(B) UNDER THE SECURITIES ACT OF 1933, AS AMENDED AND IS BEING OFFERED ONLY TO ACCREDITED INVESTORS AS DEFINED IN RULE 501 UNDER THE ACT. PAST PERFORMANCE IS NOT INDICATIVE OF FUTURE RESULTS.
NEITHER THIS BUSINESS SUMMARY NOR THE BUSINESS DESCRIPTION NOR THE ACCOMPANYING INVESTOR PRESENTATION MAY BE SHOWN OR GIVEN TO ANY PERSON OTHER THAN THE PERSON TO WHOM IT WAS DIRECTLY PROVIDED BY THE COMPANY AND MAY NOT BE PRINTED, REPRODUCED OR DISSEMINATED IN ANY MANNER WHATSOEVER. FAILURE TO COMPLY WITH THIS DIRECTIVE CAN RESULT IN A VIOLATION OF APPLICABLE LAWS, INCLUDING THE U.S. SECURITIES ACT OF 1933, AS AMENDED, AND/OR THE U.S. SECURITIES EXCHANGE ACT OF 1934, AS AMENDED, INCLUDING REGULATION FD. ANY FURTHER DISTRIBUTION OR REPRODUCTION OF THESE MATERIALS, IN WHOLE OR IN PART, OR THE DIVULGENCE OF ANY OF THE CONTENTS BY AN INVESTOR IS UNAUTHORIZED AND STRICTLY PROHIBITED.
BUSINESS SUMMARY
Shuttle Pharmaceuticals Holdings, Inc. is a clinical stage pharmaceutical company leveraging our proprietary technology to develop novel therapies designed to cure cancers. Our goal is to extend the benefits of cancer treatments by leveraging insights into current cancer therapy with surgery, radiation therapy, chemotherapy and immunotherapy. Radiation therapy (RT) is one of the most effective modalities for treating cancers. We are developing a pipeline of products designed to address limitations of the current cancer therapies as well as to extend to the new applications of radiation therapy. We believe that our product candidates will enable us to deliver cancer treatments that are safer, more reliable and at a greater scale than that of the current standard of care.
Our product candidates include Ropidoxuridine, Extended Bio-availability Ropidoxuridine (IPdR/TPI), and HDAC inhibitors (SP-1-161, SP-2-225 and SP-1-303). We have advanced Ropidoxuridine through a Phase I clinical trial using non-dilutive NIH SBIR contracts and are currently preparing a Phase II study to open in 2022. We also plan to submit investigational new drug applications (INDs) for the extended Bio-availability Ropidoxuridine with the goals of initiating Phase I clinical trials in 20223, leveraging the outcomes of the Phase I clinical study results of Ropidoxuridine. We have applied for and received FDA approval of Orphan designation for Ropidoxuridine and RT for treating brain cancer (glioblastoma). In addition, we plan to continue to develop our pre-clinical products SP-1-161, SP-2-225 and SP-1-303 with the goal of submitting INDs in 2022 and 2023. We believe our management team’s expertise in radiation therapy, combined modality cancer treatment and immuno-oncology will help drive the rapid development and, if approved, the commercialization of these potentially curative therapies for patients with aggressive cancers.
Radiation Oncology has gone through transformative technological innovation to better define tumors, allow improved shaping of radiation delivery and support dose escalation with shorter courses of treatment. Furthermore, achieving higher dose distributions within tumor volumes has reached a practical plateau, since cancers are frequently integrated with or surrounded by more sensitive normal tissues and further dose increases risk tissue necrosis. To increase cancer cures at maximally tolerated radiation doses, pharmacological and biological modifications of cells are needed to sensitize cancers, protect normal tissues, and stimulate the immune system to react against antigens produced by irradiated, damaged cancer cells. Drugs that show sensitizing properties, or the ability to make cancer cells more sensitive to radiation, offer a solution to this problem. Currently, such drugs are used off-label and many have inherent toxicities since they were designed for direct cancer treatments and not for sensitization.
| D-1 |
Our products address the unmet need in cancer treatment for a commercially marketable radiation response modifier solution that leads to greater sensitivity of cancer cells to ionizing radiation therapy. The goal of our products is to increase the therapeutic index for patients receiving radiation and to decrease radiation-related toxicities in patients with solid tumors. Our products operate across three areas related to the treatment of cancer with RT:
| 1. | Sensitization of growing cancer cells, rendering them more susceptible to the effects of radiation therapy. |
| 3. | Activation of the DNA damage response pathway to kill cancer cells and protect adjacent normal cells. |
| 4. | Activation of the immune system to kill any remaining cells after RT. |
Our platform technology allows for the creation of an inventory of products for radiation sensitizing, immune modulation, and protection of healthy tissue.
The Management Team is led by Xx. Xxxxxxx Xxxxxxxxxx, former Chairman of the Department of Radiation Medicine at Georgetown University School of Medicine and Chief of Radiation Oncology at MedStar-Georgetown University Hospital. Xx. Xxxxxxxxxx has also served as Medical Director of Georgetown University Hospital, Interim Director of the NCI-funded Xxxxxxxx Comprehensive Cancer Center, and as a co-founder of the biotech company, Oncomed (Neopharm, Inc). Our Scientific Officers include the following individuals: Xx. Xxxx Xxxx, a radiation biologist and molecular biologist is a Professor of Radiation Medicine at Georgetown University. She provides cellular, molecular biology and small animal model expertise needed for testing newly discovered drugs and serves as the Chief Scientific Officer. Our Clinical Director, Xx. Xxxxx Xxxx, has conducted clinical research at the University of Texas’s M.D. Xxxxxxxx Cancer Center and the University of Virginia. He has served as the chair of the GI committee for the Radiation Therapy Oncology Group’s (RTOG) national prospective trials utilizing fluoropyrimidine radiation sensitization in rectal and pancreatic cancers.
| D-2 |
Our Pipeline
We are currently developing a pipeline of radiation sensitizers and immune response regulating drugs. Our most advanced product candidate is Ropidoxuridine, an orally available halogenated pyrimidine with strong cancer radiation sensitizing properties. In addition to our clinical study-ready candidate, we have a significant pipeline of complimentary product candidates that we are developing to address a host of solid tumor cancer indications. Our pipeline is represented in the diagram below:
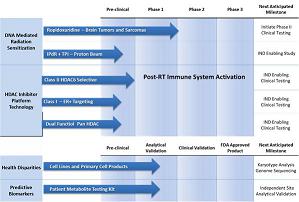
Figure 2. Time-line for clinical phase (Ropidoxuridine) and pre-clinical phase (HDAC inhibitors) pipeline. Health disparities research reagents and predictive biomarkers are developed by NIH funded grants.
Our lead product candidates include:
| ● | Ropidoxuridine (IPdR) is the lead candidate radiation sensitizer for use in combination with RT to treat brain tumors (glioblastoma) and sarcomas. Phase I clinical trial results supported by Shuttle Pharmaceuticalss and the NCI (CTEP) were reported at the 30th EORTC-NCI-AACR Symposium in November 2018 and in a full report in the medical journal, Clinical Cancer Research, in July 2019, by our SBIR subcontractor. Eighteen patients completed dose escalations to 1,800 mg/ day for 30 days, establishing the maximum tolerated dose (MTD) of 1,200 mg/day in combination with RT. Four partial responses, nine stable disease and one progressive disease in target lesions were reported. These results support the safety and potential efficacy in combination with radiation and provide the foundation for design of a Phase Ib/II clinical trials in brain tumors and Phase II clinical trials in sarcomas or un-resectable pancreatic cancers, all three disease sites are eligible for orphan disease designations. |
| ● | Ropidoxuridine and Tipracil (IPdR/TPI) is a new combination formulation demonstrating extended bio-availability after oral administration in an animal model system. The IPdR/TPI formulation will be developed for use as a radiation sensitizer of stage II and stage III rectal cancers with an endpoint of pathologic complete response rate (pCR) of greater than 40% as the therapeutic target. The pCR is recognized as a surrogate of survival in patients with solid tumors. Other potential clinical indications for IPdR/TPI may include un-resectable pancreatic cancers. |
| ● | SP-1-161 is Shuttle’s candidate lead, pre-clinical HDAC inhibitor product. This pan HDAC inhibitor initiates the mutated in ataxia-telangiectasia (ATM) response pathway. Using rational drug design, we discovered HDAC inhibitors and ATM activators capable of radiation sensitizing cancer cells and protecting normal cells. These candidate drugs may serve as direct chemotherapeutic agents or as radiation sensitizers for improving the outcomes of cancer treatment in breast cancers. |
| D-3 |
| ● | SP-2-225 is a class IIb selective HDAC inhibitor that affects histone deacetylase HDAC6. SP-2-225 has effects on the regulation of the immune system. The interactions of RT with the immune response to cancers are of great current interest, offering insight into potential mechanisms for primary site and metastatic cancer treatment. With the introduction of check-point inhibitors, CAR-T therapies and personalized medicine in cancer, regulation of the immune response following RT is of significant clinical and commercial interest. |
| ● | SP-1-303 is a selective Class I HDAC inhibitor that preferentially affects histone deacetylases HDAC1 and HDAC3 and is a member of the class I HDAC family. SP-1-303 has shown a pronounced, direct cellular toxicity in ER positive breast cancer cells. Furthermore, SP-1-303 increases the PD-L1 expression level in a time-dependent manner, suggesting that a combination of SP-1-303 with an immune checkpoint blocker may enhance the therapeutic efficacy in hormone responsive breast cancer. |
Our Approach
We believe that we have established a leadership position in radiation sensitizer development. In approximately six years of research, we have identified two clinical phase product candidates and discovered new pre-clinical molecules using our proprietary platform technologies to increase the therapeutic index for patients receiving radiation for treatment of solid tumors. Our development strategy has four key pillars: (1) to improve the efficacy of RT by demonstrating improved disease-free survival rates in patients who undergo radiation therapy, (2) reduce the amount of radiation needed for a favorable tumor response; thereby, limiting the potential for radiation related toxicities to healthy cells, (3) decrease the extent of surgery needed to remove cancers and improve quality of life, and (4) leverage our next generation technologies to create drugs that regulate the immune response assisting immune checkpoint and CAR-T therapies and other personalized medicines targeting cancers.
We propose to perform Phase I and Phase II clinical trials to advance our clinical product candidates. Candidate HDAC inhibitor molecules will be tested and IND-enabling studies will be performed to prepare for Phase I clinical trials.
We have been awarded three SBIR contracts from the NIH to:
| ● | develop IPdR as a radiation sensitizer for the treatment of gastro-intestinal cancers, in combination with radiation therapy; and |
| ● | develop prostate cancer cell lines from African-American men, with donor matched normal prostate cells, with the goal of establishing 50 prostate cancer cell lines for accelerating research to reduce prostate cancer health disparities in African-American men; and |
| ● | develop predictive biomarkers for determining outcomes for prostate cancer patients following treatment with SBRT. |
The NIH SBIR program is designed to encourage small businesses to engage in Federal Research/Research and Development (“R/R&D”) that has the potential for commercialization.
Our History and Team
Shuttle Pharmaceuticals was originally founded in 2012 as Shuttle Pharmaceuticals, LLC (“Shuttle Pharma”), a Maryland limited liability company, for the discovery, development and commercialization of innovative drugs for sensitizing cancers to radiation therapy (RT). The Company’s founders are Xxxxxxx Xxxxxxxxxx, MD, a radiation oncologist, Xxxxxx Xxxxx, MD, PhD, a medicinal chemist, and Xxxx Xxxx, PhD, a cellular and molecular radiation biologist. The founding team members have worked together in research for more than 15 years. At the time of its formation, Shuttle Pharma’s three founders were all faculty members of Georgetown University with extensive experience in radiation oncology, radiation biology and medicinal chemistry. Shuttle Pharma’s objectives are to develop and commercialize drugs to sensitize cancers and protect normal tissues to improve patient outcomes in clinical radiation oncology. Shuttle Pharma presently has five employees, a board of directors and a board of scientific advisors with expertise in radiation oncology and cancer biology.
| D-4 |
In July 2017, Shuttle Pharma converted into a Maryland C corporation and changed its name to Shuttle Pharmaceuticals, Inc. In June 2018, Shuttle entered into a share exchange agreement with Shuttle Pharma Acquisition Corp., a Delaware corporation (the “Company”), pursuant to which Shuttle Pharma became a wholly-owned subsidiary of the Company. Following the share exchange, the Company changed its name to Shuttle Pharmaceuticals Holdings, Inc. Currently, Shuttle Pharma is funded by private investors, government grants and the founding members hold ownership positions. The Company conducts business under the name Shuttle Pharmaceuticals, Inc.
Drawing on research experience in drug discovery and development, Shuttle Pharma’s founders initiated a rational drug design strategy to discover molecules capable of sensitizing cancers to radiation therapy while protecting normal tissues from radiation injury. Halogenated thymidine analogs, nitroimidazole nucleoside analogs and inhibitors of histone deacetylases (HDAC) provided the platform of technologies for design of novel, small molecules, leading to company owned intellectual property. The common goal of developing these molecules focuses on sensitizing cancers to RT. Intellectual property for therapeutic molecules has been developed exclusively within the company.
Our Strategy
Our goal is to maintain and build upon our leadership position in radiation sensitization. We plan to rapidly develop and, if approved, commercialize our product candidates for the treatment of cancers that is safer, more reliable, and at a greater scale than the existing standard of care. We believe achieving this goal could result in radiation sensitizer and immunotherapy products that could become the standard of care in cancer treatment, enabling us to make potentially curative therapies more readily accessible to more patients throughout the world. Key elements of our strategy include:
| ● | Capitalize on our first mover advantage of having potentially the first-in-class drug approved as a radiation sensitizer by the FDA. To date, there is no drug approved by the FDA specifically as a radiation sensitizer. If successful, Ropidoxuridine will become the first-in-class sensitizer and would be in position to displace selected, currently used, ‘off-label’ drugs for radiation sensitization. |
| ● | Expand our leadership position within radiation sensitizers. In addition to our traditional radiation sensitizers, we plan to advance our near-term pipeline to include radiation sensitizers for proton therapy. Proton Therapy is growing worldwide as a form of radiation therapy due to its unique beam shaping characteristics. As a result, this new technology offers a major opportunity for Shuttle to develop a first-in-class drug for proton therapy sensitization. |
| ● | Execute a disciplined business development strategy to strengthen our portfolio of product candidates. We have built our current product pipeline through in-house development, partnerships with leading academic institutions and through successful in-licensing deals. We will continue to evaluate new in-licensing opportunities and collaboration agreements with leading academic institutions and other biotechnology companies around programs that seek to address areas of high unmet need and for which we believe there is a high probability of clinical success, including programs beyond our target franchise areas and current technology footprint. |
| ● | Invest in our HDAC platform technology and maximize its utility across cancer therapies. We are initially applying the platform to develop drugs for cancer radiation sensitization and normal tissue radiation protection. In addition, we have data suggesting these drugs also have potential immune regulatory properties. We intend to invest to improve our platform technology in order to develop more efficacious therapeutics. |
| ● | Enter into collaborations to realize the full potential of our platform. The breadth of our HDAC technology platform enables its application to a number of therapeutic cancer treatments, including radiation therapy and immune therapy. We intend to form collaborations around certain aspects of our platform as we believe we will benefit from the resources and capabilities of other organizations in the manufacture, development and commercialization of diverse immunotherapies. |
| D-5 |
Exhibit E – Investor Presentation
Shuttle Pharmaceuticals Holdings, Inc. Investor Presentation
AUTIONARY STATEMENT CONCERNING FORWARD LOOKING STATEMENTS
This document contains forward-looking statements. In addition, from time to time, we or our representatives may make forward-looking statements orally or in writing. We base these forward-looking statements on our expectations and projections about future events, which we derive from the information currently available to us. Such forward-looking statements relate to future events or our future performance, including: our financial performance and projections; our growth in revenue and earnings; and our business prospects and opportunities. You can identify forward-looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” or “hopes” or the negative of these or similar terms. In evaluating these forward-looking statements, you should consider various factors, including: our ability to change the direction of the Company; our ability to keep pace with new technology and changing market needs; and the competitive environment of our business. These and other factors may cause our actual results to differ materially from any forward-looking statement. Forward-looking statements are only predictions. The forward-looking events discussed in this document and other statements made from time to time by us or our representatives, may not occur, and actual events and results may differ materially and are subject to risks, uncertainties and assumptions about us. Examples of such risks and uncertainties that could cause our actual results to differ materially from the forward-looking statements made by the Company include, but are not limited to, the risk that the Company will be unable to raise any amounts pursuant to this Offering, or otherwise raise sufficient funds to execute our business strategies and plans. We are not obligated to publicly update or revise any forward-looking statement, whether as a result of uncertainties and assumptions, the forward-looking events discussed in this document and other statements made from time to time by us or our representatives might not occur.
| E-1 |
Exhibit F – Form of Convertible Note
Form of Note
NEITHER THIS NOTE NOR THE SECURITIES INTO WHICH THIS NOTE IS CONVERTIBLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE. THESE SECURITIES HAVE BEEN SOLD IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS.
SHUTTLE PHARMACEUTICALS HOLDINGS, INC.
CONVERTIBLE NOTE
| Issuance Date: ____________ __, 2021 | Original Principal Amount: $_____________ | |
| Note No. __ |
FOR VALUE RECEIVED, Shuttle Pharmaceuticals Holdings, Inc., a Delaware corporation (“Shuttle Pharma” or the “Maker”), hereby promises to pay to the order of _________________________ (the “Subscriber”), or registered assigns (together with the Subscriber, the “Holder”), the amount set out above as the Original Principal Amount, as reduced pursuant to the terms hereof pursuant to redemption, conversion or otherwise (the “Principal”), when due, whether upon the Maturity Date (as defined below), acceleration, redemption or otherwise (in each case in accordance with the terms hereof) and to pay interest (“Interest”) on any outstanding Principal at the applicable Interest Rate from the date set out above as the Issuance Date (the “Issuance Date”) until the same becomes due and payable, upon the Maturity Date or acceleration, conversion, redemption or otherwise (in each case in accordance with the terms hereof).
The Original Principal Amount is _________________________ Dollars ($__________). For purposes hereof, the term “Outstanding Balance” means the Original Principal Amount, as reduced or increased, as the case may be, pursuant to the terms hereof for conversion, breach hereof or otherwise, plus any accrued but unpaid interest, collection and enforcements costs, and any other fees or charges incurred under this Note provided that, in the event of an optional or mandatory conversion of the Note into shares of Common Stock (as provided herein), all accrued interest on the Principal subject to such conversion shall be waived.
This Note is being issued pursuant to the terms of a subscription agreement dated as of ____ __, 2021 between the Maker and the Subscriber and exhibits thereto (collectively, the “Transaction Documents”). Unless otherwise defined herein, all capitalized terms, when used in this Note, shall have the same meaning as they are defined in the Transaction Documents.
1. GENERAL TERMS
(a) Payment of Principal. Unless previously converted into shares of the common stock, $0.00001par value, of Shuttle Pharma or the common stock of any successor in interest to the Maker (each the “Common Stock”) as contemplated hereby, this Note, together with all accrued interest hereon at the Interest Rate, shall be due and payable on December 31, 2024 (the “Maturity Date”). In the event that within 12 months of the Issuance Date, the Maker shall not have consummated an initial public offering of its Common Stock and the listing or trading of its Common Stock on a “Qualified Securities Market”, as defined below (the “IPO”) or other “Liquidity Event” (hereinafter defined), the Maker may elect either (a) up on thirty (30) days prior written notice to the Holder, elect to prepay all of the principal amount of the Note and accrued interest hereon, subject to the Holder’s right to convert the Note into Common Stock during such thirty (30) day period, or (b) if the Maker does not prepay the entire principal amount of the Note or the remaining principal amount of the Note, this Note will automatically increase to 110% of the original or unpaid portion of the outstanding principal amount.
| F-1 |
Exhibit F – Form of Convertible Note
(b) Interest. Interest shall accrue from the Issuance Date on the Original Principal Amount or other outstanding Principal at an annual rate of six percent (6%) (the “Interest Rate”) and all accrued interest shall be fully paid on the Maturity Date (or sooner as provided herein) to the Holder or its assignee in whose name this Note is registered on the records of the Maker regarding registration and transfers of Notes in cash. However, in the event of an optional or mandatory conversion of the Note into shares of Common Stock (as provided herein), all accrued interest on the Principal subject to such conversion shall be waived.
2. EVENTS OF DEFAULT.
Whenever used herein, an “Event of Default” means the occurrence and continuation of any one of the following events, whatever the reason, and whether it shall be voluntary or involuntary, or effected by operation of law or pursuant to any judgment, decree or order of any court, or any order, rule or regulation of any administrative or governmental body:
(a) The Maker’s failure to pay to the Holder any amount of Principal, Interest, or other amounts when and as due under this Note; or
(b) A Conversion Failure as defined in Section 3(d)(ii); or
(c) A material breach by Shuttle Pharma of any material representation, warranty or covenant contained in the Transaction Documents or a material breach by Shuttle Pharma of any material representation, warranty or covenant contained in the Purchase Agreement, that, if capable of cure, is not cured within 30 days from the date such breach has occurred; or
(d) The Maker or any subsidiary of the Maker shall commence, or there shall be commenced against the Maker or any subsidiary of the Maker under any applicable bankruptcy or insolvency laws as now or hereafter in effect or any successor thereto, or the Maker or any subsidiary of the Maker commences any other proceeding under any reorganization, arrangement, adjustment of debt, relief of debtors, dissolution, insolvency or liquidation or similar law of any jurisdiction whether now or hereafter in effect relating to the Maker or any subsidiary of the Maker or there is commenced against the Maker or any subsidiary of the Maker any such bankruptcy, insolvency or other proceeding which remains undismissed for a period of ninety-one (91) days; or the Maker or any subsidiary of the Maker is adjudicated insolvent or bankrupt; or any order of relief or other order approving any such case or proceeding is entered; or the Maker or any subsidiary of the Maker suffers any appointment of any custodian, private or court appointed receiver or the like for it or any substantial part of its property which continues undischarged or unstayed for a period of ninety-one (91) days; or the Maker or any subsidiary of the Maker makes a general assignment for the benefit of creditors; or the Maker or any subsidiary of the Maker shall fail to pay, or shall state that it is unable to pay, or shall be unable to pay, its debts generally as they become due; or the Maker or any subsidiary of the Maker shall call a meeting of its creditors with a view to arranging a composition, adjustment or restructuring of its debts; or the Maker or any subsidiary of the Maker shall by any act or failure to act expressly indicate its consent to, approval of or acquiescence in any of the foregoing; or any corporate or other action is taken by the Maker or any subsidiary of the Maker for the purpose of effecting any of the foregoing.
3. CONVERSION OF NOTE. This Note shall be convertible into shares of Common Stock, on the terms and conditions set forth in this Section 3.
(a) Certain Definitions. As used in this Note, the following capitalized terms shall have the meaning set forth below:
(i) “Alternative Liquidity Event” shall mean any one of a Sale of Control, a SPAC Acquisition, or a Reverse Merger.
| F-2 |
Exhibit F – Form of Convertible Note
(ii) “Alternative Liquidity Event Conversion Price” shall mean a conversion price that is equal to 50% of the aggregate “Transaction Consideration” (as defined) divided by the total number of outstanding shares of common stock of the acquiror resulting from a Sale of Control, the merger with a SPAC or the successor in interest “Pubco” (as defined) in connection with a Reverse Merger.
(iii) “Common Stock” shall mean, as applicable, the individual or collective reference to the Common stock, $0.00001 par value per share, of the Maker or the common stock of any acquiror in a Sale of Control, SPAC or Pubco resulting from a Sale of Control, SPAC Acquisition or Reverse Merger.
(iv) “Conversion Shares” shall mean the aggregate number of shares of Common Stock of the Maker, the Acquiror in a Sale of Control the SPAC or Pubco, as applicable (each an “Issuer”) that are issuable to the Holder in connection with any mandatory conversion (set forth in Section 3(b)) or optional conversion (set forth in Section 3(c)) of this Note.
(v) “IPO” shall mean an initial public offering of Common Stock of the Maker pursuant to a registration statement on Form S-1 that is declared effective by the Securities and Exchange Commission.
(vi) “IPO Conversion Price” shall mean a conversion price equal to 50% of the initial public offering price per share of the Common Stock offered to the public in the IPO.
(vii) “Liquidity Event” shall mean any one of an IPO, a Sale of Control, a SPAC Acquisition or a Reverse Merger.
(viii) “Optional Conversion Price” shall mean a conversion price that is equal to the price per share determined by dividing $50 million by the total number of outstanding shares of Common Stock of the Maker.
(ix) “Pre-Money Valuation” shall mean the dollar value placed on the total number of outstanding shares of Common Stock and Preferred Stock of the Company immediately prior to a Liquidity Event.
(x) “Preferred Stock” means the Series A convertible preferred stock, par value $0.00001 per share, of the Company, of which there are 1,212.5 shares outstanding.
(xi) “Pubco” means a fully-reporting public corporation under the Securities Exchange Act of 1934, as amended, that does not have any significant business activities and is trading on Nasdaq or the OTCQX platform of the OTC Market.
(xii) “Qualified Securities Market” shall mean any one of the Nasdaq Stock Exchange (including the Nasdaq Capital Market), the NYSE:Amex Exchange, the New York Stock Exchange or the OTCQX platform of the OTC Markets.
(xiii) “Reverse Merger” means a merger of the Maker with or the acquisition of the Maker by Pubco, as a result of which such transaction, the stockholders of the Maker will own a substantial majority of the equity securities of Pubco.
(xiv) “Sale of Control” shall mean a sale of all or substantially as of the capital stock or assets of the Company to any unaffiliated third Person, whether through share sale, asset sale, merger, consolidation or like combination, as a result of which the ability to control the board of directors of the Company shall pass to such third Person.
| F-3 |
Exhibit F – Form of Convertible Note
(xv) “SPAC” means a special purpose acquisition corporation whose securities are listed on Nasdaq or the New York Stock Exchange.
(xvi) “SPAC Acquisition” means a merger of the Maker with or the acquisition of the Maker by a SPAC or its subsidiary, as a result of which such transaction, the stockholders of the Maker will own a majority of the equity securities of the SPAC.
(xvii) “Transaction Consideration” shall mean the dollar value placed on the total consideration paid to the Company including, but not limited to, (i) the value of the Transaction, including consideration whether in cash, stock or in-kind, received by and/or paid by the Company, (ii) the total amount of indebtedness for borrowed funds, capitalized lease obligations and non-trade liabilities of the Company that are either assumed by the acquirer, redeemed or otherwise satisfied in connection with the transaction, or which remain outstanding after the transaction is consummated; (iii) the fair market value of any assets excluded from the transaction; (iv) the fair market value of any ownership interests which are retained by the Company’s shareholders or which remain outstanding after the transaction is consummated; and (v) the amount of any contingent payments, including, without limitation, earn-outs and future royalties payable in connection with the transaction.
(b) Mandatory Conversion. In the event that prior to the Maturity Date of this Note, the Maker shall consummate an IPO and its Common Stock shall be approved for listing or trading on any Qualified Securities Market, the entire Outstanding Balance of this Note shall automatically, and without any further consent or approval of the Holder, be converted into Common Stock of the Maker at the IPO Conversion Price. In the event that prior to the Maturity Date, the Maker shall consummate an Alternative Liquidity Event, the Holder may elect at his or its option to convert the outstanding and unpaid Outstanding Balance of this Note into Common Stock of the Maker at the Alternative Liquidity Event Conversion Price. The IPO Conversion Price and the Alternative Liquidity Event Conversion Price (either, the “Mandatory Conversion Price”) shall be subject to adjustment, as provided for in Section 3(f) below.
(c) Optional Conversion. At any time, at the Holder’s option, such Holder may convert the outstanding and unpaid Outstanding Balance of this Note into fully paid and nonassessable shares of Common Stock in accordance with this Section 3(c), at the Optional Conversion Price, subject to adjustment as provided in Section 3(f) below. If the issuance would result in the issuance of a fraction of a share of Common Stock, Shuttle Pharma shall round such fraction of a share of Common Stock up to the nearest whole share. Shuttle Pharma shall pay any and all transfer agent fees, legal fees, costs and any other fees or costs that may be incurred or charged in connection with the issuance and legend removal of shares of Common Stock to the Holder arising out of or relating to the conversion of this Note up to a maximum of five thousand dollars ($5,000).
(d) Mechanics of Conversion.
(i) Optional Conversion. To convert the Note pursuant to an optional conversion into shares of Common Stock on any date (a “Conversion Date”), the Holder shall (A) transmit by email, facsimile (or otherwise deliver), for receipt on or prior to 11:59 p.m., New York, NY Time, a copy of an executed notice of conversion in the form attached hereto as Exhibit A (the “Conversion Notice”) to Shuttle Pharma. On or before the tenth (10th) Business Day following the date of receipt of a Conversion Notice (the “Share Delivery Date”), Shuttle Pharma shall (A) if legends are not required to be placed on certificates of Common Stock pursuant to the then existing provisions of Rule 144 of the Securities Act of 1933 (“Rule 144”) and provided that the Transfer Agent is participating in the Depository Trust Company (“DTC”) Fast Automated Securities Transfer Program, credit such aggregate number of shares of Common Stock to which the Holder shall be entitled to the Holder’s or its designee’s balance account with DTC through its Deposit Withdrawal Agent Commission system or (B) if the Transfer Agent is not participating in the DTC Fast Automated Securities Transfer Program, issue and deliver to the address as specified in the Conversion Notice, a certificate, registered in the name of the Holder or its designee, for the number of shares of Common Stock to which the Holder shall be entitled which certificates shall not bear any restrictive legends unless required pursuant the Rule 144. The Person or Persons entitled to receive the shares of Common Stock issuable upon a conversion of this Note shall be treated for all purposes as the record holder or holders of such shares of Common Stock upon the transmission of a Conversion Notice.
| F-4 |
Exhibit F – Form of Convertible Note
(ii) Issuer’s Failure to Timely Convert. If within ten (10) business days after a Liquidity Event or (in the case of an optional conversion) Shuttle Pharma receipt of the facsimile or email copy of a Conversion Notice together with documentation satisfactory to the Transfer Agent that the Conversion Shares are eligible for such electronic issuance, the Issuer shall fail to issue and deliver to Holder via “DWAC/FAST” electronic transfer (assuming that such shares are “DWAC/FAST” eligible) the number of Conversion Shares to which the Holder is entitled upon such holder’s conversion of any Conversion Shares (a “Conversion Failure”), the Outstanding Balance of the Note shall increase by 0.05% per day until such time as the Issuer of the Conversion Shares issues and delivers a certificate to the Holder or credit the Holder’s balance account with DTC for the number of Conversion Shares to which the Holder is entitled upon such mandatory or optional conversion. The Issuer of the Conversion Shares will not be subject to any penalties once its transfer agent processes the shares to the DWAC system. If the issuer fails to deliver shares in accordance with the timeframe stated in this Section, resulting in a Conversion Failure, the Holder, at any time prior to selling all of those Conversion Shares, may rescind any portion, in whole or in part, of that particular conversion attributable to the unsold shares and have the rescinded conversion amount returned to the Outstanding Balance with the rescinded Conversion Shares returned to the applicable Issuer.
(iii) Book-Entry. Notwithstanding anything to the contrary set forth herein, in connection with any optional or mandatory conversion of this Note in accordance with the terms hereof, the Holder shall not be required to physically surrender this Note to Shuttle Pharma unless and until such time as the Holder has converted his or her shares in full. Upon a partial or full conversion, Holder shall receive either (i) one or more stock certificates, or a book entry account statement, evidencing the Conversion Shares (in the event Shuttle Pharma’s Common Stock is not yet DTC eligible) or (ii) physical evidence from the Issuer’s transfer agent that the Holder’s balance account with DTC showing that the Conversion Shares have been credited for the number of Conversion Shares to which the Holder is entitled upon such mandatory or optional conversion. The Holder and the Issuer shall maintain records showing the Outstanding Balance converted and the dates of such conversions or shall use such other method reasonably satisfactory to the Holder and Issuer, so as not to require physical surrender of this Note upon conversion, unless so requested by Shuttle Pharma.
(e) Limitations on Conversions or Trading.
If at any time after the Closing, the Holder shall or would receive Conversion Shares or shall purchase additional shares of Common Stock of an Issuer, so that the Holder would, together with other shares of Common Stock held by it or its Affiliates, own or beneficially own by virtue of such action or receipt of additional shares of Common Stock a number of shares exceeding 9.99% of the number of shares of Common Stock outstanding on such date (the “Maximum Percentage”), the Issuer shall not be obligated and shall not issue to the Holder Conversion Shares which would exceed the Maximum Percentage, but only until such time as the Maximum Percentage would no longer be exceeded by any such receipt of shares of Common Stock by the Holder. Upon delivery of a written notice to the applicable Issuer the Holder may from time to time increase (with such increase not effective until the sixty-first (61st) day after delivery of such notice) or decrease the Maximum Percentage to any other percentage not in excess of 9.99% as specified in such notice; provided that (i) any such increase in the Maximum Percentage will not be effective until the sixty-first (61st) day after such notice is delivered to Shuttle Pharma and (ii) any such increase or decrease will apply only to the Holder and its Affiliates. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity with the terms of this Section 3(e) to the extent necessary to correct this paragraph (or any portion of this paragraph) which may be defective or inconsistent with the intended beneficial ownership limitation contained in this Section 3(e) or to make changes or supplements necessary or desirable to properly give effect to such limitation. The limitation contained in this paragraph may not be waived and shall apply to a successor holder of the Note.
(f) Adjustment of Conversion Price. In the event that a Liquidity Event prior to the December 31, 2024 Maturity Date of this Note, the Maker shall raise additional capital through a private placement of Common Stock or other securities that are convertible or exercisable for Common Stock, in either case, at a price less than the Optional Conversion Price, then and in such event the Conversion Price of the Notes shall be adjusted to reflect such lower amount.
(g) Other Provisions.
| F-5 |
Exhibit F – Form of Convertible Note
(i) Share Reservation. Shuttle Pharma shall at all times reserve and keep available out of its authorized Common Stock a number of shares equal to at least the full number of shares of Common Stock issuable upon conversion of all outstanding amounts under this Note.
(ii) Prepayment. This Note may not be prepaid by Shuttle Pharma until March 31, 2022. Thereafter, the Note may either be prepaid by the Company in whole or in part without penalty, fees or premium upon not less than twenty (20) business days prior written notice to the Holder (the “Prepayment Notice”) which shall set forth the date on which the Note shall be prepaid (the “Prepayment Date”), subject to the Holder’s right to convert all or any portion of this Note into Conversion Shares at the Optional Conversion Price prior to the Prepayment Date.
(iii) All calculations under this Section 3 shall be rounded up to the nearest whole share.
(iv) Nothing herein shall limit a Holder’s right to pursue actual damages or declare an Event of Default pursuant to Section 2 herein for Shuttle Pharma’s failure to deliver certificates or credit entries representing shares of Common Stock upon conversion within the period specified herein and such Holder shall have the right to pursue all remedies available to it at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief, in each case without the need to post a bond or provide other security. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
(v) The Maker shall use its best efforts to assist the Holder to obtain a legal opinion for the removal of any restrict legend in connection with any shares converted from this Note.
(vi) This Note is one of the Convertible Notes issued on or about the date of this Note by the Maker in an aggregate principal amount of up to $2,000,000, (the “Notes”). Each of the Notes shall rank equally without preference or priority of any kind over one another, and all payments and recoveries under the Notes payable on account of principal and interest on the Notes shall be paid and applied ratably and proportionately on the balance of all outstanding Notes on the basis of their original principal amount.
4. REISSUANCE OF THIS NOTE.
Upon receipt by the Maker of evidence reasonably satisfactory to the Maker of the loss, theft, destruction or mutilation of this Note, and, in the case of loss, theft or destruction, of any indemnification undertaking by the Holder to the Maker in customary form and, in the case of mutilation, upon surrender and cancellation of this Note, the Maker shall execute and deliver to the Holder a new Note representing the outstanding Principal.
5.
NOTICES. Any notices, consents, waivers or other communications required or permitted to
be given under the terms shall be handled according to the Notice clause in the Subscription Agreement.
The addresses for such communications shall be:
If to the Maker:
Xx. Xxxxxxx Xxxxxxxxxx, CEO
Shuttle Pharmaceuticals Holdings, Inc.
Xxx Xxxxxxxx Xxxxx, Xxxxx 000
Xxxxxxxxx,
XX 00000
Email: [ ]@xxxxxxxxxxxxx.xxx
If to the Holder:
| F-6 |
Exhibit F – Form of Convertible Note
6. APPLICABLE LAW AND VENUE. This Note shall be governed by and construed in accordance with the laws of the State of New York, without giving effect to conflicts of laws thereof. Any action brought by either party against the other concerning the transactions contemplated by this Agreement shall be brought only in the state courts of New York or in the federal courts located in New York County, in the State of New York. Both parties and the individuals signing this Agreement agree to submit to the jurisdiction of such courts.
7. WAIVER. Any waiver by the Holder of a breach of any provision of this Note shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other provision of this Note. The failure of the Holder to insist upon strict adherence to any term of this Note on one or more occasions shall not be considered a waiver or deprive that party of the right thereafter to insist upon strict adherence to that term or any other term of this Note. Any waiver must be in writing.
8. MISCELLANEOUS
(a) Lawful Money; Costs of Collection. All amounts payable hereunder are payable in lawful money of the United States. Shuttle Pharma agrees to pay all costs of collection when incurred, including reasonable attorneys’ fees and costs, whether or not a suit or action is instituted to enforce this Note, including but not limited to court costs, appraisal fees, the cost of searching records, obtaining title reports and title insurance and trustee’s fees, to the extent permitted by applicable law.
(b) No Offset; Holder in Due Course. All payments under this Note made by or on behalf of Shuttle Pharma shall be made without setoff or counterclaim and free and clear of, and without deduction or withholding for or on account of, any federal, state, or local taxes. Shuttle Pharma waives any right of offset it now has or may hereafter have against Agent or Holder and its successors and assigns as to this Note (but retains any such rights as to any other prior or future transaction between these parties), and agrees to make the payments called for hereunder in accordance with the terms hereof. The holder hereof and all successors thereof shall have all the rights of a holder in due course as provided in the Delaware Uniform Commercial Code and other laws of the State of Delaware.
(c) Waivers. Shuttle Pharma and any endorsers, guarantors or sureties hereof severally waive presentment and demand for payment, notice of intent to accelerate maturity, protest or notice of protest or non-payment, bringing of suit and diligence in taking any action to collect any sums owing hereunder or in proceeding against any of the rights and properties securing payment hereunder; expressly agree that this Note, or any payment hereunder, may be extended from time to time; and consent to the acceptance of further security or the release of any security for this Note, all without in any way affecting the liability of Shuttle Pharma and any endorsers or guarantors hereof. No extension of time for the payment of this Note, or any installment hereof, made by agreement by the holder hereof with any person now or hereafter liable for the payment of this Note, shall affect the original liability under this Note of Shuttle Pharma, even if Shuttle Pharma (or any entity comprising Shuttle Pharma) is not a party to such agreement.
(d) Usury Protection. The parties hereto intend to conform strictly to the applicable usury laws. In no event, regardless of any provisions contained therein or in any other document executed or delivered in connection herewith, shall the holder hereof ever be deemed to have contracted for or be entitled to receive, collect or apply as interest on this Note, any amount in excess of the maximum amount permitted by applicable law (the “Maximum Rate”). In no event, whether by reason of demand for payment, prepayment, acceleration of the maturity hereof or otherwise, shall the interest contracted for, charged or received by the holder hereunder or otherwise exceed the Maximum Rate. If for any circumstance whatsoever interest would otherwise be payable to the holder in excess of the maximum lawful amount, the interest payable to the holder shall be reduced automatically to the Maximum Rate and any payment received in excess of such amount shall be applied to the outstanding principal balance of the Note.
(e) Entire Agreement. This Note, the other Transaction Documents, and all other documents and instruments contemplated hereby and thereby together constitute the entire agreement between and among the parties pertaining to the subject matter hereof. No supplement, modification or amendment of this Note shall be binding unless executed in writing by the parties. No waiver shall be binding unless executed in writing by the party making the waiver. No provision of this Note shall be interpreted for or against the drafting party.
| F-7 |
Exhibit F – Form of Convertible Note
(f) Commercial Purpose. Shuttle Pharma agrees that no funds advanced under this Note shall be used for personal, family or household purposes, and that all funds advanced hereunder shall be used solely for business, commercial, investment or other similar purposes.
(g) Successors and Assigns. All the terms and provisions of this Note shall be binding upon and inure to the benefit of the parties to this Note and their respective successors and assigns.
(h) Assignment. Shuttle Pharma may not, voluntarily or involuntarily, directly or indirectly, by operation of law or otherwise, sell, transfer, assign, hypothecate, pledge or in any way alienate this Note or any right or interest in this Note (each a “Transfer”) without Holder’s prior written consent, which Holder may withhold in its sole and absolute discretion. Any consent by Holder to any Transfer shall not constitute consent to any other Transfer. Holder may freely Transfer its interest, rights, or title in or to this Note or the other Transaction Documents in Holder’s sole and absolute discretion.
(i) Construction. Whenever used in this Note, the terms “including,” “include,” “includes” and the like are not intended as terms of limitation, and, hence, shall be deemed to be followed by “without limitation.”
(j) Severability. If any provision of this Note, as applied to any party or to any circumstance, shall be found by a court of competent jurisdiction to be void, invalid or unenforceable, the same shall in no way affect any other provision of this Note, the application of any such provision in any other circumstance, or the validity or enforceability of this Note, and any provision which is found to be void, invalid or unenforceable shall be curtailed and limited only to the extent necessary to bring such provision within the requirements of the law.
(k) Survival of Terms. The terms and provisions of this Note shall survive the Maturity Date until full payment of all amounts due hereunder.
(l) Preferential Payment. If at any time any payment made pursuant to this Note is deemed to have been a voidable preference, fraudulent conveyance or other similar conveyance or preferential payment under any bankruptcy, insolvency or other debtor relief or similar law, then the obligation to make such payment shall survive any cancellation or satisfaction of this Note or return of this Note to Shuttle Pharma and shall not be discharged or satisfied with any such payment or cancellation. Such payment shall instead remain a valid and binding obligation enforceable in accordance with the terms of this Note and shall be immediately due and payable.
(m) Relief From Stay. As an additional inducement to and material consideration for Holder agreeing to execute this this Note and the other Transaction Documents, Shuttle Pharma agrees that in the event a Bankruptcy or Judicial Action (as hereinafter defined in this Section 8(n)) is commenced which subjects Holder to any stay in the exercise of Holder’s rights and remedies under this Note or the other Transaction Documents, including, but not limited to, the automatic stay imposed by Xxxxxxx 000 xx xxx Xxxxxx Xxxxxx Bankruptcy Code (individually and collectively, “Stay”), then Shuttle Pharma irrevocably consents and agrees that such Stay shall automatically be lifted and released against Holder, and Holder shall thereafter be entitled to exercise all of its rights and remedies against Shuttle Pharma that is or could be subject any Stay under this Note or the other Transaction Documents. Nothing contained herein shall limit or prevent Holder from exercising all of its rights and remedies against Shuttle Pharma that is not the subject any Stay under this Note or the other Transaction Documents. Shuttle Pharma acknowledges that it is knowingly, voluntarily, and intentionally waiving its rights to any Stay and agrees that the benefits provided to Shuttle Pharma under the terms of this Note are valuable consideration for such waiver. As used in this Section 8(n), the term “Bankruptcy or Judicial Action” shall mean any voluntary or involuntary case filed by or against a Shuttle Pharma under the United States Bankruptcy Code, or any voluntary or involuntary petition in composition, readjustment, liquidation, or dissolution, or any state and federal bankruptcy law action filed by or against a Shuttle Pharma, any action where a Shuttle Pharma is adjudicated as bankrupt or insolvent, any action for dissolution of a Shuttle Pharma, or any action in furtherance of any of the foregoing, or any other action, case, or proceeding that has the effect of staying (or in which a stay is being obtained against) the enforcement by Holder of its rights and remedies under the this Note or the other Transaction Documents.
| F-8 |
Exhibit F – Form of Convertible Note
Except to enforce the terms of the Transaction Documents, Shuttle Pharma shall not take any action and shall not fail to take any action which such action or omission will or might tend to interfere with, delay, enjoin or otherwise prohibit the commencement, continuation or completion of efforts by Holder to enforce its remedies under this Note or the other Transaction Documents, or applicable law. Without limiting the generality of the foregoing and except to enforce the terms of the Transaction Documents, each Shuttle Pharma waives its, his, or her rights, if any, to seek or obtain a stay, injunction or other form of order prohibiting in any way any act necessary or appropriate for the commencement or completion of Holder’s enforcement of its remedies under the this Note or the other Transaction Documents, or applicable law (without limiting the generality of the foregoing, such waiver extends to such rights which may exist under any statute or rule relating to bankruptcy cases, including, without limitation, 11 U.S.C. § 105, 11 U.S.C. § 301, 11 U.S.C. § 302, 11 U.S.C. § 303, 11 U.S.C. § 304, 11 U.S.C. § 362, 11 U.S.C. § 348, 11 U.S.C. § 706, 28 U.S.C. § 157, 28 U.S.C. § 158, Federal Rule of bankruptcy Procedure (“FRBP”) 3007, FRBP 3008, FRBP 3012, FRBP 8005, FRBP 9023, FRBP 9024, or FRBP 9029).
9. AMENDMENT AND WAIVER OF RIGHTS. This Note may be amended and the observance of any term hereof may be waived (either generally or in a particular instance either retroactively or prospectively) only by a written instrument executed by the Maker and the Holder.
10. WAIVER OF RIGHT TO TRIAL BY JURY.
EACH PARTY TO THIS NOTE HEREBY EXPRESSLY WAIVES ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION, OR CAUSE OF ACTION (1) ARISING UNDER THIS NOTE, THE OTHER TRANSACTION DOCUMENTS, OR ANY OTHER INSTRUMENT, DOCUMENT, OR AGREEMENT EXECUTED OR DELIVERED IN CONNECTION THEREWITH, OR (2) IN ANY WAY CONNECTED WITH OR RELATED OR INCIDENTAL TO THE DEALINGS OF THE PARTIES HERETO OR ANY OF THEM WITH RESPECT TO THIS NOTE OR ANY OTHER INSTRUMENT, DOCUMENT, OR AGREEMENT EXECUTED OR DELIVERED IN CONNECTION HEREWITH, OR THE TRANSACTIONS RELATED HERETO OR THERETO, IN EACH CASE WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER SOUNDING IN CONTRACT OR TORT OR OTHERWISE; AND EACH PARTY HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION, OR CAUSE OF ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A JURY. THE PARTIES HERETO HEREBY AGREE THAT THE PROVISIONS CONTAINED HEREIN HAVE BEEN FAIRLY NEGOTIATED ON AN ARM’S-LENGTH BASIS, WITH BOTH SIDES AGREEING TO THE SAME KNOWINGLY AND BEING AFFORDED THE OPPORTUNITY TO HAVE THEIR RESPECTIVE LEGAL COUNSEL CONSENT TO THE MATTERS CONTAINED HEREIN. ANY PARTY TO THIS NOTE MAY FILE AN ORIGINAL COUNTERPART OR A COPY OF THIS SECTION WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY AND THE AGREEMENTS CONTAINED HEREIN REGARDING THE APPLICATION OF JUDICIAL REFERENCE IN THE EVENT OF THE INVALIDITY OF SUCH JURY TRIAL WAIVER.
IN WITNESS WHEREOF, each of the Maker has caused this Note to be duly executed by a duly authorized officer as of the date set forth above.
| Shuttle Pharmaceuticals Holdings, Inc. | ||
| By: | ||
| Name: | Xx. Xxxxxxx Xxxxxxxxxx | |
| Title: | Chief Executive Officer | |
Note No. [ ]
| F-9 |
EXHIBIT A
NOTICE OF CONVERSION
Shuttle
Pharmaceuticals Holdings, Inc.
Xxx Xxxxxxxx Xxxxx, Xxxxx 000
Xxxxxxxxx,
XX 00000
Email: [ [@xxxxxxxxxxxxx.xxx
The undersigned hereby elects to convert $[ ] of the $[ ] Convertible Note (Note No. [ ]) issued to [ ] on [ ], 2021 into Shares of Common Stock of Shuttle Pharmaceuticals Holdings, Inc. according to the conditions set forth in such Note as of the date written below.
If the number of shares to be delivered represents more than 4.99% of the common stock outstanding, this conversion notice shall immediately automatically extinguish and Holder must be immediately notified.
| Date of Conversion: | |
| Optional Conversion Amount: | |
| Conversion Price: | |
| Shares to be Delivered: | |
| Shares delivered in name of: |
| HOLDER | ||
| [ ] | ||
| By: | ||
| Title: | ||
Exhibit G – Investor Rights and Lock-Up Agreement
INVESTOR RIGHTS AND LOCK-UP AGREEMENT
This INVESTOR RIGHTS AND LOCK-UP AGREEMENT (this “Agreement”) is made and entered into as of ____________ ____, 2021 by and among Shuttle Pharmaceuticals Holdings, Inc., a Delaware corporation (the “Company”) and the investor on the signature page hereto.
RECITALS
A. The Investors have agreed to purchase from the Company, and the Company has agreed to sell to the Investors, 6% convertible notes of the Company due December 31, 2024 (the “Notes”) on the terms and conditions set forth in that certain Subscription Agreement, dated as of [_____________] by and among the Company and the Investors, as amended from time to time (the “Subscription Agreement” and together with the related Exhibits to the Subscription Agreement, and the Notes, collectively, the “Transaction Documents”); and
B. Unless otherwise defined in this Agreement all capitalized terms when used herein shall have the same meaning as they are defined in the Subscription Agreement and the Notes.
C. It is a condition to the closing of the sale of the Notes that the parties hereto execute and deliver this Agreement.
NOW, THEREFORE, in consideration of the foregoing recitals and the mutual promises hereinafter set forth, the parties hereto agree as follows:
1. COVENANTS OF THE COMPANY
1.1 Information Rights.
(a) Basic Financial Information. The Company will furnish to each Investor and any owner of 5% or more of the outstanding shares of Common Stock (“Qualifying Owner”):
(i) as soon as practicable, but no later than 120 days after the end of each fiscal year of the Company, (A) a balance sheet as of the end of such fiscal year, (B) a profit and loss statement as of the end of such fiscal year, (C) a statement of cash flows of the Company as of the end of such fiscal year, and (D) a statement of stockholders’ equity as of the end of such fiscal year, all prepared in accordance with generally accepted accounting principles and practices (“GAAP”) and audited and certified by an recognized accounting firm that is a PCAOB qualified auditor, commencing with the 2021 fiscal year;
(ii) as soon as practicable, but not later than 45 days after each fiscal quarter of the Company, quarterly reports of management of the Company generally describing material Company events from that quarter (except that such reports may (A) be subject to normal year-end auditing adjustments, and (B) not contain all notes thereto that may be required in accordance with GAAP, as required);
(iii) as soon as practicable, after a change of more than ten percent (10%) of the stock ownership of the Company, a statement showing the number of shares of each class and series of capital stock and securities convertible into or exercisable for shares of capital stock outstanding at the end of the period, the Common Stock issuable upon conversion or exercise of any outstanding securities convertible or exercisable for Common Stock and the exchange ratio or exercise price applicable thereto, and the number of shares of issued stock options and stock options not yet issued but reserved for issuance, if any, all in sufficient detail as to permit the Holders to calculate their respective percentage equity ownership in the Company, and certified by the Chief Executive Officer or senior finance officer of the Company as being true, complete, and correct;
(iv) as soon as practicable, but in any event by December 1 of each calendar year, the officers of the Company shall prepare and present an annual budget (the “Budget”) for the Company and each of its subsidiaries for the upcoming year, which Budget shall include, without limitation, all expense and capital spending expectations for the Company;
| G-1 |
(v) as soon as practicable, but in any event by March 15 after the end of the fiscal year of the Company, all tax information necessary for the Investors to file their respective state and federal tax filings;
(vi) at the option of an Investor holding a majority of the outstanding Notes (the “Majority Investor”), and up to two times annually, certain officers of the Company, as selected by the Majority Investor (which may include, among others, the Chief Executive Officer and/or senior finance officer), shall provide an in-person presentation to the Investors at the Company’s corporate headquarters or by Video teleconference covering, among any other topic(s) selected by the Investor or Qualifying Owner, the performance of (past and forecasted), recent developments relating to, and material risks facing, the Company; and
(vii) such other information relating to the financial condition, business, prospects, or corporate affairs of the Company as the Majority Investor may from time-to-time reasonably request.
If, for any period, the Company has any subsidiary whose accounts are consolidated with those of the Company, then in respect of such period the financial statements delivered pursuant to the foregoing sections shall be the consolidated and consolidating financial statements of the Company and all such consolidated subsidiaries.
Notwithstanding anything else in this Section 1.1 to the contrary, the Company may cease providing the information set forth in this Section 1.1 during the period starting with the date thirty (30) days before the Company’s good faith estimate of the date of filing of a registration statement in accordance with the Securities Act of 1933, as amended or the Securities Exchange Act of 1934, as amended; provided that (i) the Company’s covenants under this Section 1.1 shall be reinstated at such time as the Company is no longer actively employing its commercially reasonable efforts to cause such registration statement to become effective or such registration statement is withdrawn.
(b) Inspection Rights. At all times while the Notes remain outstanding, the Company shall cause to be maintained full and accurate books of account, which shall reflect all Company transactions and be appropriate and adequate for the Company’s business. The books and records of the Company shall be maintained at the principal office of the Company. Each Investor shall have the right during ordinary business hours and upon reasonable notice to inspect and copy all books and records of the Company.
2. RESTRICTIONS ON TRANSFER.
2.1 Each of the Holders hereby covenant and agree that except as set forth below, they shall not sell, transfer, convey or assign (collectively “Transfer”) any Conversion Shares to any Person, other than to members of their immediate family (children, spouse or parents, any entity wholly-owned by such Holder or trusts for the benefit of the Holder or members of his or its family (each a “Permitted Transferee”). As a condition to each Transfer to a Permitted Transferee, such Permitted Transferee shall agree to execute a joinder or related agreement pursuant to which he, she or it shall agree to be bound by the terms of this Agreement.
2.2 From and after the date hereof and until the 180th day after the first to occur of (a) consummation of an IPO, (b) consummation of a sale to a SPAC, or (c) consummation of another form of Reverse Merger, as applicable (each , the “Lock-Up Trigger Date”), the Holder and each Permitted Transferee agrees not to sell, transfer or otherwise dispose of the Conversion Shares or common stock of any successor-in-interest to the Company. After the 180th day following the Lock-Up Trigger Date, the Holder will be entitled to sell all or any portion of the Conversion Shares or other common stock without restriction.
3. TAG-ALONG RIGHTS. If a majority of the holders of the Company’s outstanding voting equity (collectively, the “Majority Stockholders”) want to consummate a transaction that constitutes a Sale of Control (a “Sale of Control Transaction”), then the Majority Stockholder(s) shall notify the other Investors of such proposed Sale of Control Transaction by a date which shall be not later than fifteen (15) days prior to the Company or any such Majority Stockholder(s) entering into any definitive binding agreement in respect thereof (the “Sale Notice’). Thereafter, each other Investor or Stockholder (each a “Tag-Along Stockholder”) may cause the Company or such Majority Stockholders to effect a Transfer of such other Stockholder’s Stock; in each case, only pursuant to and in accordance with the following provisions of this Section 3:
| G-2 |
(a) The Tag-Along Stockholders shall have the right, but not the obligation, to participate in the Proposed Sale of Control Transaction on the terms and conditions herein stated (the “Tag-Along Option”), which right shall be exercisable upon written notice (the “Acceptance Notice”) to the Company and/or the Majority Stockholders, as the case may be, within ten (10) days of receipt of the Sale Notice. Each Acceptance Notice shall indicate the maximum amount of Notes or number of Conversion Shares that the Tag-Along Stockholder wishes to sell on the terms and conditions stated in the Sale Notice.
(b) Each Tag-Along Stockholder shall have the right to sell a portion of its Notes or Conversion Shares pursuant to the Sale of Control Transaction which is equal to that percentage equal of the Common Stock that is being sold by the Majority Stockholders in the Sale of Control Transaction.
(c) Within ten (10) days after the date by which a Tag-Along Stockholder notifies the Company or the Majority Stockholders of its intent to exercise the Tag-Along Option, the Company or the Majority Stockholders shall notify such Tag-Along Stockholder of the amount of Notes and number of Conversion Shares held by such Tag-Along Stockholder that will be included in the sale and the date on which the Sale of Control Transaction will be consummated, which shall be no later than the later of (i) twenty (20) days after the date by which each Holder was required to notify the Company or the Majority Stockholders of its intent to exercise the Tag-Along Option and (ii) five (5) days after the satisfaction of any governmental approval or filing requirements, if any.
(d) Each Tag-Along Stockholder may effect its participation in any Sale of Control Transaction, and as part of its participation in the Sale of Control Transaction pursuant to a duly exercised Tag-Along Option, shall deliver to the Proposed Transferee at a closing to be held at the offices of the Company (or such other place as the parties agree), one or more Notes or certificates, properly endorsed for transfer, which represent all of the Notes or Conversion Shares owned by such Tag-Along Stockholder which is to be transferred in connection with the Sale of Control Transaction, and each Tag-Along Stockholder shall make such representations and warranties, and shall enter into such agreements, as are customary and reasonable in the context of the proposed Sale of Control Transaction, including, without limitation, representations and warranties (and indemnities with respect thereto) that the Proposed Transferee of the Notes or Conversion Shares (or interests therein) is receiving good and marketable title to such Notes or Conversion Shares (or interests therein), free and clear of all pledges, security interests, or other liens; provided, however, that with respect to any matter as to which a Tag-Along Stockholder shall agree to provide indemnification (other than its own title to such Notes or Conversion Shares), such Tag-Along Stockholder shall in no event be required to provide indemnification in an amount that would exceed its pro rata portion of the total liability for which such indemnification is sought, which pro rata portion shall be determined on the basis of the percentage of the total Notes or Conversion Shares involved in such transfer that are represented by the Notes or Conversion Shares owned by such Tag-Along Stockholder. In addition, each Tag-Along Stockholder and the Majority Stockholders shall reasonably cooperate and consult with each other in order to effect the Sale of Control Transaction, and each Tag-Along Stockholder shall provide reasonable assistance to the Majority Stockholders in connection with the preparation of disclosure schedules relating to representations and warranties to be made to the Proposed Transferee in connection with such Sale of Control Transaction and in the determination of the appropriate scope of, or limitations or exceptions to, such representations and warranties. At the time of consummation of the Sale of Control Transaction, the Proposed Transferee shall remit directly to each such Tag-Along Stockholder that portion of the sale proceeds to which such Tag-Along Stockholder is entitled by reason of its participation therein (less any adjustments due to the conversion of any convertible securities or the exercise of any exercisable securities)
4. DRAG ALONG RIGHTS. If the Company or one or more of Majority Stockholders (collectively, the “Drag-Along Sellers”) wants to consummate a Sale of Control Transaction, the Company or the Drag-Along Sellers, as the case may be, shall have the right (but not the obligation) to require the other Investors owning Notes or Conversion Shares (each a “Drag-Along Investor”) to Transfer all of their Notes or Conversion Shares to the Proposed Transferee for the same consideration per share and otherwise on the same terms and conditions upon which the Drag-Along Sellers are selling their Common Stock pursuant to the provisions set forth below (subject to any adjustments due to the conversion of any convertible securities or the exercise of any exercisable securities) (the “Drag-Along Right”). The Company and the Drag-Along Sellers may not exercise the right set forth in this Section 4 unless it or they hold not less than fifty percent (50%) of the Company Fully-Diluted Common Stock.
| G-3 |
(a) Prior to making the Transfer, the Drag-Along Sellers shall first send an Offer Notice and copies of all documentation, including relevant agreements, relating to the Transfer. Within fifteen (15) days following the date of the Offer Notice. Each Drag-Along Investor shall effect its participation in any Sale of Control Transaction, and as part of its participation in the Sale of Control Transaction pursuant to a duly exercised Drag-Along Right, shall deliver to the Proposed Transferee at a closing to be held at the offices of the Company (or such other place as the parties agree), one or more certificates, properly endorsed for transfer, which represent all of the Notes or Conversion Shares owned by such Drag-Along Investor which is to be transferred in connection with the Sale of Control Transaction, and each Drag-Along Investor shall make such representations and warranties, and shall enter into such agreements, as are customary and reasonable in the context of the proposed Sale of Control Transaction, including, without limitation, representations and warranties (and indemnities with respect thereto) that the Proposed Transferee of the Notes or Conversion Shares (or interests therein) is receiving good and marketable title to such Notes or Conversion Shares (or interests therein), free and clear of all pledges, security interests, or other liens; provided, however, that with respect to any matter as to which a Tag-Along Stockholder shall agree to provide indemnification (other than its own title to such Stock), such Drag-Along Investor shall in no event be required to provide indemnification in an amount that would exceed its pro rata portion of the total liability for which such indemnification is sought, which pro rata portion shall be determined on the basis of the percentage of the total Stock involved in such transfer that are represented by the Notes or Conversion Shares owned by such Drag-Along Investor. In addition, each Drag-Along Investor and the Drag-Along Sellers shall reasonably cooperate and consult with each other in order to effect the Sale of Control Transaction, and each Drag-Along Investor shall provide reasonable assistance to the Drag-Along Sellers in connection with the preparation of disclosure schedules relating to representations and warranties to be made to the Proposed Transferee in connection with such Sale of Control Transaction and in the determination of the appropriate scope of, or limitations or exceptions to, such representations and warranties. If any Drag-Along Investor should fail to deliver such certificates and instruments of transfer to the Drag-Along Sellers (or their designee), the Company shall cause its books and records to show that such shares of Notes or Conversion Shares are bound by the provisions of this Section 4 and that such Notes or Conversion Shares shall have been transferred to the Proposed Transferee, and all certificates or other evidence of ownership of the Notes or Conversion Shares subject to this Section 4 shall be deemed to be cancelled.
(b) Simultaneously with the consummation of the Sale of Control, pursuant to this Section 4, the Company shall notify the Drag-Along Investors and the other Company stockholders of the consummation of the sale, and shall cause the Proposed Transferee to remit directly to the Drag-Along Investors and other Company stockholders (including the Drag-Along Sellers) the total sales price, based on each stockholder’s pro rata Share ownership, of the Sale of Control or consideration paid pursuant thereto and shall furnish such other evidence of the completion and time of completion of such sale or other disposition and the terms thereof as may be reasonably requested.
5. PARTICIPATION RIGHT.
5.1 General. Each of the Investors (individually and collectively, the “Participation Right Holders”) has the right to co-invest and to purchase such Participation Right Holder’s Pro Rata Share (as defined below) of all (or any part) of any New Securities (including Common Stock being sold to the public in the IPO) that the Company may from time to time issue after the date of this Agreement (the “Participation Right”), provided, however, such Participation Right Holder shall have no right to purchase any such New Securities and exercise such Participation Right if such New Securities are being issued in a private placement pursuant to Regulation 506(b) under the Securities Act and such Participation Right Holder cannot demonstrate to the Company’s reasonable satisfaction that such Participation Right Holder is, at the time of the proposed issuance of such New Securities, an “accredited investor” as such term is defined in Regulation D under the Securities Act. A Participation Right Holder’s “Pro Rata Share” for purposes of this participation and co-investment right is a percentage of any New Securities (including Common Stock sold in the IPO) equal to one hundred (100%) of the amount of all Notes sold in the Offering to each Investor’s Note. For the avoidance of doubt, if for example, an Investor purchased a $100,000 principal amount of this Note, representing 10% of all $1,000,000 of the Notes held by all Investors and $10,000,000 of New Securities (including Common Stock sold in the IPO) are issued prior to termination of this Agreement, then and in such event such Investor’s Pro Rata Share of the New Securities would be $100,000.
| G-4 |
5.2 Procedures. In the event that the Company proposes to undertake an issuance of New Securities, it shall give to each Participation Right Holder a written notice of its intention to issue New Securities (the “Participation Right Notice”), describing the type of New Securities and the price and the general terms upon which the Company proposes to issue such New Securities. Each Participation Right Holder shall have twenty (20) days from the date such Participation Right Notice is given, to agree in writing to purchase such Participation Right Holder’s Pro Rata Share of such New Securities for the price and upon the general terms specified in the Notice by giving written notice to the Company and stating therein the quantity of New Securities to be purchased (not to exceed such Participation Right Holder’s Pro Rata Share).
5.3 Failure to Exercise. In the event that the Participation Right Holders fail to exercise in full the pa within such twenty (20) day period, then the Company shall have one hundred twenty (120) days thereafter to sell the New Securities with respect to which the Participation Right Holders’ Participation Right was not exercised, at a price not more favorable and upon general terms not materially more favorable to the purchasers thereof than specified in the Participation Right Notice to the Participation Right Holders. In the event that the Company has not issued and sold the New Securities within such one hundred twenty (120) day period, then the Company shall not thereafter issue or sell any New Securities without again first offering the Participation Right in such New Securities to the Participation Right Holders pursuant to this Section 5.
6. REGISTRATION RIGHTS. The Company covenants and agrees as follows:
6.1 Demand Registration.
(a) Resale Registration Statement. Within one hundred and eighty-one (181) days or six calendar months, whichever is later, following the consummation of the IPO, the Company shall use its reasonable commercial efforts to file a registration statement on Form S-1 (the “Resale Registration Statement”) with the SEC in order to register for resale all of the shares of Common Stock of the Company or common stock of any successor-in-interest to the Company issued to all holders of the Notes upon automatic conversion of the Notes (the “Conversion Shares”), and will use its reasonable bests efforts to cause such Resale Registration Statement to be declared effective by the SEC within forty-five (45) business days from the date of its initial submission or filing; provided, that such Conversion Shares will continue to be subject to restrictions on resale for a period of six (6) months following consummation of the IPO.
(b) Form S-1 Demand. In the event that, for any reason, the Company is unable to comply with the provisions of Section 6.1(a), at any time after one hundred eighty (180) days from the effective date of the Form S-1 registration statement in connection with the IPO, the Company receives a request from the Majority Investor(s) (the “Initiating Investors”) that the Company file a Form S-1 registration statement with respect to the Conversion Shares then outstanding having an anticipated aggregate offering price, net of selling expenses, of at least five million dollars ($5,000,000), then the Company shall (x) within ten (10) days after the date such request is given, give notice thereof (the “Demand Notice”) to all Investors other than the Initiating Investors; and (y) as soon as practicable, and in any event within sixty (60) days after the date such request is given by the Initiating Investors, file a Form S-1 registration statement under the Securities Act covering all Conversion Shares that the Initiating Investors requested to be registered and any additional Conversion Shares requested to be included in such registration by any other Investors, as specified by notice given by each such Holder to the Company within twenty (20) days of the date the Demand Notice is given, and in each case, subject to the limitations of Section 6.1(d) and Section 6.3, provided, however, that the Initiating Investors may not invoke this right more than twice.
(c) Deferral of Registration. Notwithstanding the foregoing obligations, if the Company furnishes to Investors requesting a registration pursuant to Section 6.1(b) above a certificate signed by the Company’s chief executive officer stating that in the good faith judgment of the Board it would be materially detrimental to the Company and its stockholders for such registration statement to either become effective or remain effective for as long as such registration statement otherwise would be required to remain effective, because such action would (i) materially interfere with a significant acquisition, corporate reorganization, or other similar transaction involving the Company; (ii) require premature disclosure of material information that the Company has a bona fide business purpose for preserving as confidential; or (iii) render the Company unable to comply with requirements under the Securities Act or Exchange Act, then the Company shall have the right to defer taking action with respect to such filing, and any time periods with respect to filing or effectiveness thereof shall be tolled correspondingly, for a period of not more than sixty (60) days after the request of the Initiating Investors is given; provided, however, that the Company may not invoke this right more than once in any twelve (12) month period; and provided further that the Company shall not register any securities for its own account or that of any other stockholder during such sixty (60) day period other than an Excluded Registration.
| G-5 |
(d) Deferral for Company-Initiated Registration. The Company shall not be obligated to effect, or to take any action to effect, any registration pursuant to Section 6.1(b) (i) during the period that is sixty (60) days before the Company’s good faith estimate of the date of filing of, and ending on a date that is one hundred eighty (180) days after the effective date of, a Company-initiated registration, provided, that the Company is actively employing in good faith commercially reasonable efforts to cause such registration statement to become effective; or (ii) after the Company has effected one registration pursuant to Section 6.1(b). A registration shall not be counted as “effected” for purposes of this Section 6.1(d) until such time as the applicable registration statement has been declared effective by the SEC, unless the Initiating Investors withdraw their request for such registration, elect not to pay the registration expenses therefor, and forfeit their right to one demand registration statement pursuant to Section 6.6, in which case such withdrawn registration statement shall be counted as “effected” for purposes of this Section 6.1(d).
6.2 Piggyback Registration. If, following its IPO, the Company proposes to register under the Securities Act (including, for this purpose, a registration of Common Stock effected by the Company or for the benefit of selling stockholders other than the Investors in connection with the public offering of such securities solely for cash (other than in an Excluded Registration), unless the Conversion Shares shall have been previously registered for resale, the Company shall, at such time, promptly give each Holder notice of such registration. Upon the request of each Holder given within twenty (20) days after such notice is given by the Company, the Company shall, subject to the provisions of Section 6.3, cause to be registered all of the Conversion Shares that each such Holder has requested to be included in such registration. The Company shall have the right to terminate or withdraw any registration initiated by it under this Section 6.2 before the effective date of such registration, whether or not any Holder has elected to include Conversion Shares in such registration. The expenses (other than Selling Expenses) of such withdrawn registration shall be borne by the Company in accordance with Section 6.6.
6.3 Underwriting Requirements.
(a) In connection with any offering involving an underwriting of shares of the Company’s capital stock pursuant to Section 6.1(b), Section 6.1(c) or Section 6.2, the Company shall not be required to include any of the Investors’ Conversion Shares in such underwriting unless the Investors accept the terms of the underwriting as agreed upon between the Company and its underwriters, and then only in such quantity, if any, as the underwriters in their sole discretion determine will not jeopardize the success of the offering by the Company. If the total number of securities, including Conversion Shares, requested by stockholders to be included in such offering exceeds the number of securities to be sold (other than by the Company) that the underwriters in their reasonable discretion determine is compatible with the success of the offering, then the Company shall be required to include in the offering only that number of such securities, including Conversion Shares, which the underwriters and the Company in their sole discretion determine will not jeopardize the success of the offering. If the underwriters determine that less than all of the Conversion Shares requested to be registered can be included in such offering, then the Conversion Shares that are included in such offering shall be allocated among the selling Investors in proportion (as nearly as practicable to) the number of Conversion Shares owned by each selling Holder or in such other proportions as shall mutually be agreed to by all such selling Investors. To facilitate the allocation of shares in accordance with the above provisions, the Company may in its sole discretion round the number of shares allocated to the Holder to the nearest 100 shares. Notwithstanding the foregoing, in no event shall (i) the number of Conversion Shares included in the offering be reduced unless all other securities (other than securities to be sold by the Company) are first entirely excluded from the offering, or (ii) the number of Conversion Shares included in the offering be reduced below twenty percent (20%) of the total number of securities included in such offering, unless such offering is the IPO, in which case the selling Investors may be excluded further if the underwriters make the determination described above and no other stockholder’s securities are included in such offering or (iii) notwithstanding (ii) above, any Conversion Shares which are not Conversion Shares of the Key Owner be excluded from such underwriting unless all Conversion Shares of the Key Owner are first excluded from such offering. For purposes of the provision in this Section 6.3(b) concerning apportionment, for any selling Holder that is a partnership, limited liability company, or corporation, the partners, members, retired partners, retired members, stockholders, and Affiliates of such Holder, or the estates and Immediate Family Members of any such partners, retired partners, members, and retired members and any trusts for the benefit of any of the foregoing Persons, shall be deemed to be a single “selling Holder,” and any pro rata reduction with respect to such “selling Holder” shall be based upon the aggregate number of Conversion Shares owned by all Persons included in such “selling Holder,” as defined in this sentence.
| G-6 |
(b) For purposes of Section 6.1, a registration shall not be counted as “effected” if, as a result of an exercise of the underwriter’s cutback provisions in Section 6.3(a), fewer than fifty percent (50%) of the total number of Conversion Shares that Investors have requested to be included in such registration statement are actually included.
6.4 Obligations of the Company. Whenever required under this Section 6 to effect the registration of any Conversion Shares, the Company shall, as expeditiously as reasonably possible:
(a) prepare and file with the SEC a registration statement with respect to such Conversion Shares and use its commercially reasonable efforts to cause such registration statement to become effective and, upon the request of the Investors of a majority of the Conversion Shares registered thereunder, keep such registration statement effective for a period of up to one hundred eighty (180) days following the termination of the lock-up agreement entered into in connection with the IPO or, if earlier, until the distribution contemplated in the registration statement has been completed; provided, however, that (i) such one hundred eighty (180) day period shall be extended for a period of time equal to the period the Holder refrains, at the request of an underwriter of Common Stock (or other securities) of the Company, from selling any securities included in such registration, and (ii) in the case of any registration of Conversion Shares on Form S-1 that are intended to be offered on a continuous or delayed basis, subject to compliance with applicable SEC rules, such one hundred eighty (180) day period shall be extended for up to sixty (60) days, if necessary, to keep the registration statement effective until all such Conversion Shares are sold;
(b) prepare and file with the SEC such amendments and supplements to such registration statement, and the prospectus used in connection with such registration statement, as may be necessary to comply with the Securities Act in order to enable the disposition of all securities covered by such registration statement;
(c) furnish to the selling Investors such numbers of copies of a prospectus, including a preliminary prospectus, as required by the Securities Act, and such other documents as the Investors may reasonably request in order to facilitate their disposition of their Conversion Shares;
(d) use its commercially reasonable efforts to register and qualify the securities covered by such registration statement under such other securities or blue-sky laws of such jurisdictions as shall be reasonably requested by the selling Investors; provided that the Company shall not be required to qualify to do business or to file a general consent to service of process in any such states or jurisdictions, unless the Company is already subject to service in such jurisdiction and except as may be required by the Securities Act;
(e) in the event of any underwritten public offering, enter into and perform its obligations under an underwriting agreement, in usual and customary form, with the underwriter(s) of such offering;
(f) use its commercially reasonable efforts to cause all such Conversion Shares covered by such registration statement to be listed on a national securities exchange or trading system and each securities exchange and trading system (if any) on which similar securities issued by the Company are then listed;
(g) provide a transfer agent and registrar for all Conversion Shares registered pursuant to this Agreement and provide a CUSIP number for all such Conversion Shares, in each case not later than the effective date of such registration;
(h) promptly make available for inspection by the selling Investors, any underwriter(s) participating in any disposition pursuant to such registration statement, and any attorney or accountant or other agent retained by any such underwriter or selected by the selling Investors, all financial and other records, pertinent corporate documents, and properties of the Company, and cause the Company’s officers, directors, employees, and independent accountants to supply all information reasonably requested by any such seller, underwriter, attorney, accountant, or agent, in each case, as necessary or advisable to verify the accuracy of the information in such registration statement and to conduct appropriate due diligence in connection therewith;
| G-7 |
(i) notify each selling Holder, promptly after the Company receives notice thereof, of the time when such registration statement has been declared effective or a supplement to any prospectus forming a part of such registration statement has been filed; and
(j) after such registration statement becomes effective, notify each selling Holder of any request by the SEC that the Company amend or supplement such registration statement or prospectus.
In addition, the Company shall ensure that, at all times after any registration statement covering a public offering of securities of the Company under the Securities Act shall have become effective, its xxxxxxx xxxxxxx policy shall provide that the Company’s directors may implement a trading program under Rule 10b5-1 of the Exchange Act.
6.5 Furnish Information. It shall be a condition precedent to the obligations of the Company to take any action pursuant to this Section 4 with respect to the Conversion Shares of any selling Holder that such Holder shall furnish to the Company such information regarding itself, the Conversion Shares held by it, and the intended method of disposition of such securities as is reasonably required to effect the registration of such Holder’s Conversion Shares.
6.6 Expenses of Registration. All expenses (other than Selling Expenses) incurred in connection with registrations, filings, or qualifications pursuant to Section 4, including all registration, filing, and qualification fees; printers’ and accounting fees; fees and disbursements of counsel for the Company; and the reasonable fees and disbursements of one counsel for the selling Investors (“Selling Holder Counsel”), shall be borne and paid by the Company; provided however, that the Company shall not be required to pay for any expenses of any registration proceeding begun pursuant to Section 6.1 if the registration request is subsequently withdrawn at the request of the Investors of a majority of the Conversion Shares to be registered (in which case all selling Investors shall bear such expenses pro rata based upon the number of Conversion Shares that were to be included in the withdrawn registration), unless the Investors of a majority of the Conversion Shares agree to forfeit their right to one registration pursuant to Section 6.1 (a) or Section 6.1(b), as the case may be; provided further that if, at the time of such withdrawal, the Investors shall have learned of a material adverse change in the condition, business or prospects of the Company from that known to the Investors at the time of their request and have withdrawn the request with reasonable promptness after learning of such information then the Investors shall not be required to pay any of such expenses and shall not forfeit their right to one registration pursuant to Section 6.1(a) or Section 6.1(b). All Selling Expenses relating to Conversion Shares registered pursuant to this Section 5 shall be borne and paid by the Investors pro rata on the basis of the number of Conversion Shares registered on their behalf.
6.7 Delay of Registration. No Holder shall have any right to obtain or seek an injunction restraining or otherwise delaying any registration pursuant to this Agreement as the result of any controversy that might arise with respect to the interpretation or implementation of this Section 6.
6.8 Indemnification. If any Conversion Shares are included in a registration statement under this Section 6:
(a) To the extent permitted by law, the Company will indemnify and hold harmless each selling Holder, and the partners, members, officers, directors, and stockholders of each such Holder; legal counsel and accountants for each such Holder; any underwriter (as defined in the Securities Act) for each such Holder; and each Person, if any, who controls such Holder or underwriter within the meaning of the Securities Act or the Exchange Act, against any Damages, only to the extent such Damages arise out of or are based upon any untrue statement or alleged untrue statement of a material fact made by the Company or arise out of or are based upon any omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein not misleading, and the Company will pay to each such Holder, underwriter, controlling Person, or other aforementioned Person any legal or other expenses reasonably incurred thereby in connection with investigating or defending any claim or proceeding from which Damages may result, as such expenses are incurred; provided, however, that the indemnity agreement contained in this Section 6.8(a) shall not apply to amounts paid in settlement of any such claim or proceeding if such settlement is effected without the consent of the Company, which consent shall not be unreasonably withheld, nor shall the Company be liable for any Damages to the extent that they arise out of or are based upon actions or omissions made in reliance upon and in conformity with written information furnished by or on behalf of any such Holder, underwriter, controlling Person, or other aforementioned Person expressly for use in connection with such registration.
| G-8 |
(b) To the extent permitted by law, each selling Holder, severally and not jointly, will indemnify and hold harmless the Company, and each of its directors, each of its officers who has signed the registration statement, each Person (if any), who controls the Company within the meaning of the Securities Act, legal counsel and accountants for the Company, any underwriter (as defined in the Securities Act), any other Holder selling securities in such registration statement, and any controlling Person of any such underwriter or other Holder, against any Damages, in each case only to the extent that such Damages arise out of or are based upon actions or omissions made in reliance upon and in conformity with written information furnished by or on behalf of such selling Holder expressly for use in connection with such registration; and each such selling Holder will pay to the Company and each other aforementioned Person any legal or other expenses reasonably incurred thereby in connection with investigating or defending any claim or proceeding from which Damages may result, as such expenses are incurred; provided, however, that the indemnity agreement contained in this Section 6.8(b) shall not apply to amounts paid in settlement of any such claim or proceeding if such settlement is effected without the consent of the Holder, which consent shall not be unreasonably withheld; and provided, further that in no event shall the aggregate amounts payable by any Holder by way of indemnity or contribution under Section 6.8(b) and 6.8(d) exceed the proceeds from the offering received by such Holder (net of any Selling Expenses paid by such Holder), except in the case of fraud or willful misconduct by such Holder.
(c) Promptly after receipt by an indemnified party under this Section 6.8 of notice of the commencement of any action (including any governmental action) for which a party may be entitled to indemnification hereunder, such indemnified party will, if a claim in respect thereof is to be made against any indemnifying party under this Section 6.8, give the indemnifying party notice of the commencement thereof. The indemnifying party shall have the right to participate in such action and, to the extent the indemnifying party so desires, participate jointly with any other indemnifying party to which notice has been given, and to assume the defense thereof with counsel mutually satisfactory to the parties; provided, however, that an indemnified party (together with all other indemnified parties that may be represented without conflict by one counsel) shall have the right to retain one separate counsel, with the fees and expenses to be paid by the indemnifying party, if representation of such indemnified party by the counsel retained by the indemnifying party would be inappropriate due to actual or potential differing interests between such indemnified party and any other party represented by such counsel in such action. The failure to give notice to the indemnifying party within a reasonable time of the commencement of any such action shall relieve such indemnifying party of any liability to the indemnified party under this Section 6.8, to the extent that such failure materially prejudices the indemnifying party’s ability to defend such action. The failure to give notice to the indemnifying party will not relieve it of any liability that it may have to any indemnified party otherwise than under this Section 6.8.
(d) To provide for just and equitable contribution to joint liability under the Securities Act in any case in which either (i) any party otherwise entitled to indemnification hereunder makes a claim for indemnification pursuant to this Section 6.8 but it is judicially determined (by the entry of a final judgment or decree by a court of competent jurisdiction and the expiration of time to appeal or the denial of the last right of appeal) that such indemnification may not be enforced in such case, notwithstanding the fact that this Section 6.8 provides for indemnification in such case, or (ii) contribution under the Securities Act may be required on the part of any party hereto for which indemnification is provided under this Section 6.8, then, and in each such case, such parties will contribute to the aggregate losses, claims, damages, liabilities, or expenses to which they may be subject (after contribution from others) in such proportion as is appropriate to reflect the relative fault of each of the indemnifying party and the indemnified party in connection with the statements, omissions, or other actions that resulted in such loss, claim, damage, liability, or expense, as well as to reflect any other relevant equitable considerations. The relative fault of the indemnifying party and of the indemnified party shall be determined by reference to, among other things, whether the untrue or allegedly untrue statement of a material fact, or the omission or alleged omission of a material fact, relates to information supplied by the indemnifying party or by the indemnified party and the parties’ relative intent, knowledge, access to information, and opportunity to correct or prevent such statement or omission; provided, however, that, in any such case, (x) no Holder will be required to contribute any amount in excess of the public offering price of all such Conversion Shares offered and sold by such Holder pursuant to such registration statement, and (y) no Person guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) will be entitled to contribution from any Person who was not guilty of such fraudulent misrepresentation; and provided, further that in no event shall a Holder’s liability pursuant to this Section 6.8(d), when combined with the amounts paid or payable by such Holder pursuant to Section 6.8(b), exceed the proceeds from the offering received by such Holder (net of any Selling Expenses paid by such Holder), except in the case of willful misconduct or fraud by such Holder.
| G-9 |
(e) Notwithstanding the foregoing, to the extent that the provisions on indemnification and contribution contained in the underwriting agreement entered into in connection with the underwritten public offering are in conflict with the foregoing provisions, the provisions in the underwriting agreement shall control.
(f) Unless otherwise superseded by an underwriting agreement entered into in connection with the underwritten public offering, the obligations of the Company and Investors under this Section 6.8 shall survive the completion of any offering of Conversion Shares in a registration under this Section 6.8, and otherwise shall survive the termination of this Agreement.
6.9 Reports Under Exchange Act. With a view to making available to the Investors the benefits of SEC Rule 144 and any other rule or regulation of the SEC that may at any time permit a Holder to sell securities of the Company to the public without registration, the Company shall:
(a) make and keep available adequate current public information, as those terms are understood and defined in SEC Rule 144, at all times after the effective date of the registration statement filed by the Company for the IPO;
(b) use commercially reasonable efforts to file with the SEC in a timely manner all reports and other documents required of the Company under the Securities Act and the Exchange Act (at any time after the Company has become subject to such reporting requirements); and
(c) furnish to any Holder, so long as the Holder owns any Conversion Shares, forthwith upon request (i) to the extent accurate, a written statement by the Company that it has complied with the reporting requirements of SEC Rule 144 (at any time after ninety (90) days after the effective date of the registration statement filed by the Company for the IPO), the Securities Act, and the Exchange Act (at any time after the Company has become subject to such reporting requirements), or that it qualifies as a registrant whose securities may be resold pursuant to Form S-3 (at any time after the Company so qualifies); (ii) a copy of the most recent annual or quarterly report of the Company and such other reports and documents so filed by the Company; and (iii) such other information as may be reasonably requested in availing any Holder of any rule or regulation of the SEC that permits the selling of any such securities without registration (at any time after the Company has become subject to the reporting requirements under the Exchange Act) or pursuant to Form S-3 (at any time after the Company so qualifies to use such form).
6.10 Limitations on Subsequent Registration Rights. From and after the date of this Agreement, the Company shall not, until such time as the Conversion Shares are registered and without the prior written consent of the Investors of a majority of the Conversion Shares then outstanding, enter into any agreement with any holder or prospective holder of any securities of the Company that (i) would allow such holder or prospective holder to include such securities in any registration unless, under the terms of such agreement, such holder or prospective holder may include such securities in any such registration only to the extent that the inclusion of such securities will not reduce the number of the Conversion Shares of the Investors that are included; or (ii) allow such holder or prospective holder to initiate a demand for registration of any securities held by such holder or prospective holder. Upon the registration of the Conversion Shares, this paragraph 6.10 shall no longer be applicable.
| G-10 |
6.11 “Market Stand-off” Agreement. Each Holder hereby agrees that it will not, without the prior written consent of the managing underwriter, during the period commencing on the date of the final prospectus relating to the registration by the Company of shares of its Common Stock and ending on the date specified by the Company and the managing underwriter (such period not to exceed one hundred and eighty (180) days in the case of the IPO), (i) lend; offer; pledge; sell; contract to sell; sell any option or contract to purchase; purchase any option or contract to sell; grant any option, right, or warrant to purchase; or otherwise transfer or dispose of, directly or indirectly, any shares of Common Stock or any securities convertible into or exercisable or exchangeable (directly or indirectly) for Common Stock or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of such securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of Common Stock or other securities, in cash, or otherwise. The foregoing provisions of this Section 6.11 shall apply only to the IPO, shall not (A) prohibit any Holder from buying registered shares of Common Stock in the IPO or in the aftermarket or selling such shares of Common Stock, or (B) apply to the sale of any shares to an underwriter pursuant to an underwriting agreement, and shall be applicable to the Investors only if all officers and directors are subject to the same restrictions. The underwriters in connection with such registration are intended third party beneficiaries of this Section 6.11 and shall have the right, power, and authority to enforce the provisions hereof as though they were a party hereto. Each Holder further agrees to execute such agreements as may be reasonably requested by the underwriters in connection with such registration that are consistent with this Section 6.11 or that are necessary to give further effect thereto. Any discretionary waiver or termination of the restrictions of any or all of such agreements by the Company or the underwriters shall apply pro rata to all Investors subject to such agreements based on the number of shares subject to such agreements.
6.12 Termination of Registration Rights. The right of any Holder to request registration or inclusion of Conversion Shares in any registration pursuant to Section 6.1 or Section 6.2 shall terminate upon the termination of the Restricted Period on Transfers set forth in Section 2.2 above and the ability of each Holder to sell all of his or its remaining Conversion Shares in any broker transaction under Rule 144 and without any volume or percentage limitations on such sales.
7. ADDITIONAL INVESTOR RIGHTS. The Company shall use commercially reasonable efforts to cause the Conversion Shares, within the meaning of Section 1202(f) of the Internal Revenue Code (the “Code”), to constitute “qualified small business stock” as defined in Section 1202(c) of the Code; provided, however, that such requirement shall not be applicable if the Board determines, in its good faith business judgment, that such qualification is inconsistent with the best interests of the Company. The Company shall submit to its stockholders (including the Investors) and to the Internal Revenue Service any reports that may be required under Section 1202(d)(1)(C) of the Code and the regulations promulgated thereunder. In addition, within twenty (20) business days after any Investor’s written request therefor, the Company shall, at its option, either (i) deliver to such Investor a written statement indicating whether (and what portion of) such Investor’s interest in the Company constitutes “qualified small business stock” as defined in Section 1202(c) of the Code or (ii) deliver to such Investor such factual information in the Company’s possession as is reasonably necessary to enable such Investor to determine whether (and what portion of) such Investor’s interest in the Company constitutes “qualified small business stock” as defined in Section 1202(c) of the Code.
8. GENERAL PROVISIONS.
8.1 Amendment and Waiver of Rights. This Agreement may be amended or terminated, and the observance of any term hereof may be waived (either generally or in a particular instance either retroactively or prospectively) only by a written instrument executed by (a) the Company; (b) the Key Owner (as defined below) and (c) the Majority Investor(s) (as defined below). Notwithstanding the foregoing:
(a) any provision hereof may be waived by the waiving party on such party’s own behalf, without the consent of any other party; and
(b) no such amendment, modification or waiver shall amend, modify or waive (i) any provision of this Agreement granting any personal rights to a specific Investor or Key Owner (as opposed to the Investors or the holders of a specific class of stock generally), without the prior written consent of such Investor or Key Owner; or (ii) any rights of any Investor or Key Owner in a manner that materially adversely affects the rights of such Investor or Key Owner, unless approved in writing by such Investor or Key Owner.
For the purposes of this section, “Key Owner” shall mean Xxxxxxx Xxxxxxxxxx and Xxx Xxxxxxxxxx and/or an entity or entities controlled by him or her, and “Majority Investor(s)” shall mean an Investor or the Investors holding a majority of the outstanding Notes.
| G-11 |
The Company shall give prompt written notice of any amendment, termination or waiver hereunder to any party that did not consent in writing thereto. Any amendment, termination or waiver effected in accordance with this Subsection 7.1 shall be binding on each party and all of such party’s successors and permitted assigns, whether or not any such party, successor or assignee entered into or approved such amendment, termination or waiver. For purposes of this Subsection 7.1, the requirement of a written instrument may be satisfied in the form of an action by written consent of the Investors circulated by the Company and executed by the Holder parties specified, whether or not such action by written consent makes explicit reference to the terms of this Agreement.
8.2 Notices. All notices and other communications given or made pursuant to this Agreement shall be in writing and shall be deemed effectively given upon the earlier of actual receipt or: (a) personal delivery to the party to be notified, (b) when sent, if sent by electronic mail during normal business hours of the recipient, and if not sent during normal business hours, then on the recipient’s next business day, (c) five (5) days after having been sent by registered or certified mail, return receipt requested, postage prepaid, or (d) one (1) business day after deposit with a nationally recognized overnight courier, freight prepaid, specifying next business day delivery, with written verification of receipt. All communications shall be sent to the respective parties at their address as set forth on the signature page hereto, or to such address as subsequently modified by written notice given in accordance with this Section 8.2.
8.3 Entire Agreement. This Agreement and the documents referred to herein, together with all the Exhibits hereto, constitute the entire agreement and understanding of the parties with respect to the subject matter of this Agreement, and supersede any and all prior understandings and agreements, whether oral or written, between or among the parties hereto with respect to the specific subject matter hereof.
8.4 Governing Law. This Agreement shall be governed by, and construed in accordance with, the laws of the State of Delaware, regardless of the laws that might otherwise govern under applicable principles of conflicts of law.
8.5 Severability The invalidity or unenforceability of any provision hereof shall in no way affect the validity or enforceability of any other provision.
8.6 Third Parties. Nothing in this Agreement, express or implied, is intended to confer upon any person, other than the parties hereto and their successors and assigns, any rights or remedies under or by reason of this Agreement.
8.7 Successors and Assigns. This Agreement, and any and all rights, duties and obligations hereunder, shall not be assigned, transferred, delegated or sublicensed by any party without the prior written consent of the other parties. Any attempt by a party without such permission to assign, transfer, delegate or sublicense any rights, duties or obligations that arise under this Agreement shall be void. Subject to the foregoing, and except as otherwise provided herein, this Agreement, and the rights and obligations of the parties hereunder, will be binding upon and inure to the benefit of their respective successors, assigns, heirs, executors, administrators and legal representatives.
8.8 Titles and Headings. The titles, captions and headings of this Agreement are included for ease of reference only and will be disregarded in interpreting or construing this Agreement. Unless otherwise specifically stated, all references herein to “sections” and “exhibits” will mean “sections” and “exhibits” to this Agreement.
8.9 Counterparts. This Agreement may be executed in any number of counterparts, each of which when so executed and delivered will be deemed an original, and all of which together shall constitute one and the same agreement.
8.10 Costs and Attorneys’ Fees. In the event that any action, suit or other proceeding is instituted concerning or arising out of this Agreement or any transaction contemplated hereunder, the prevailing party shall recover all of such party’s costs and attorneys’ fees incurred in each such action, suit or other proceeding, including any and all appeals or petitions therefrom.
| G-12 |
8.11 Adjustments for Stock Splits, Etc. Wherever in this Agreement there is a reference to a specific number of shares of Common Stock of the Company of any class or series, then, upon the occurrence of any subdivision, combination or stock dividend of such class or series of stock, the specific number of shares so referenced in this Agreement shall automatically be proportionally adjusted to reflect the effect on the outstanding shares of such class or series of stock by such subdivision, combination or stock dividend.
8.12 Further Assurances. The parties agree to execute such further documents and instruments and to take such further actions as may be reasonably necessary to carry out the purposes and intent of this Agreement.
8.13 Electronic Signatures. This Agreement may be executed and delivered by electronic signature (such as .pdf or Docusign) and upon such delivery the electronic signature will be deemed to have the same effect as if the original signature had been delivered to the other party.
8.14 Termination of Agreement. Except for the provisions of Section 1 (Information Rights) and Section 5 (Participation Rights) of this Agreement which shall terminate upon the consummation of the Company’s IPO of Common Stock pursuant to an effective registration statement filed under the Securities Act and listing of such Common Stock on a Qualified Securities Market, following the Company’s IPO, this Agreement and all of the other rights and obligations of the parties hereunder shall continue to survive and remain in full force and effect. Notwithstanding anything to the contrary herein, this Agreement (excluding any then-existing obligations) shall terminate upon the Investors ceasing to hold Notes or Conversion Shares or upon the Company ceasing to have more than one Holder.
8.15 Dispute Resolution. Each party (a) hereby irrevocably and unconditionally submits to the jurisdiction of the federal or state courts located in New York County, New York for the purpose of any suit, action or other proceeding arising out of or based upon this Agreement or the Transaction Documents, (b) agrees not to commence any suit, action or other proceeding arising out of or based upon this Agreement or the Transaction Documents except in the federal or state courts located in New York County, New York, and (c) hereby waives and agrees not to assert, by way of motion, as a defense, or otherwise, in any such suit, action or proceeding, any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune from attachment or execution, that the suit, action or proceeding is brought in an inconvenient forum, that the venue of the suit, action or proceeding is improper or that this Agreement, the Transaction Documents or the subject matter hereof and thereof may not be enforced in or by such court.
9. DEFINITIONS. Except as otherwise noted herein, for purposes of this Agreement:
“Affiliate” means, with respect to any specified Person, any other Person who, directly or indirectly, controls, is controlled by, or is under common control with such Person, including without limitation any general partner, managing member, officer or director of such Person or any venture capital fund now or hereafter existing that is controlled by one or more general partners or managing members of, or shares the same management company with, such Person.
“Common Stock” has the meaning as defined in the Note.
“Common Stock Equivalents” shall mean any shares of Common Stock issuable upon conversion of any securities (other than the Notes) convertible into shares of Common Stock or any warrants or other rights (other than options or restricted stock units issued under the Company’s Incentive Stock Plan) entitling the holder to purchase Common Stock upon the exercise thereof.
“Conversion Shares” means (i) the Common Stock issuable or issued upon conversion of the Notes, (ii) any Common Stock issued or issuable (directly or indirectly) upon conversion and/or exercise of any other securities of the Company, acquired by the Investors after the date hereof; and (iii) any Common Stock issued as (or issuable upon the conversion or exercise of any warrant, right, or other security that is issued as) a dividend or other distribution with respect to, or in exchange for or in replacement of, the shares referenced in clauses (i) and (ii) above; excluding in all cases, however, any Conversion Shares sold by a Person in a transaction in which the applicable rights under this Agreement are not assigned pursuant to Section 8.7, and excluding for purposes of Section 5 any shares for which registration rights have terminated pursuant to Section 6.12 of this Agreement.
| G-13 |
“Damages” means any loss, damage, claim or liability (joint or several) to which a party hereto may become subject under the Securities Act, the Exchange Act, or other federal or state law, insofar as such loss, damage, claim or liability (or any action in respect thereof) arises out of or is based upon (i) any untrue statement or alleged untrue statement of a material fact contained in any registration statement of the Company, including any preliminary prospectus or final prospectus contained therein or any amendments or supplements thereto; (ii) an omission or alleged omission to state therein a material fact required to be stated therein, or necessary to make the statements therein not misleading; or (iii) any violation or alleged violation by the indemnifying party (or any of its agents or Affiliates) of the Securities Act, the Exchange Act, any state securities law, or any rule or regulation promulgated under the Securities Act, the Exchange Act, or any state securities law.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Excluded Registration” means (i) a registration relating to the sale of securities to employees of the Company or a subsidiary pursuant to a stock option, stock purchase, or similar plan; (ii) a registration relating to an SEC Rule 145 transaction; (iii) a registration on any form that does not include substantially the same information as would be required to be included in a registration statement covering the sale of the Conversion Shares; or (iv) a registration in which the only Common Stock being registered is Common Stock issuable upon conversion of debt securities that are also being registered.
“Form S-1” means such form under the Securities Act as in effect on the date hereof or any successor registration form under the Securities Act subsequently adopted by the SEC.
“Form S-3” means such form under the Securities Act as in effect on the date hereof or any registration form under the Securities Act subsequently adopted by the SEC that permits incorporation of substantial information by reference to other documents filed by the Company with the SEC.
“Holder” means collectively, each Investor owning Notes or Conversion Shares and each Permitted Transferee of such Holder.
“Immediate Family Member” means a child, stepchild, grandchild, parent, stepparent, grandparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, including adoptive relationships, of a natural person referred to herein.
“Initiating Investors” means, collectively, Holders who properly initiate a registration request under this Agreement.
“IPO” means the Company’s first underwritten public offering of its Common Stock under the Securities Act.
“New Securities” means any Common Stock, whether now authorized or not, and rights, options or warrants to purchase such Common Stock, and securities of any type whatsoever that are, or may become, convertible or exchangeable into such Common Stock that are issued for cash consideration; provided, however, that the term “New Securities” does not include any (a) New Securities issued as part of the consideration in connection with any acquisition of the assets or capital stock of any other Person, or (b) any options or other securities issued pursuant to the Incentive Stock Plan of the Company or any successor in interest to the Company.
“Person” means any individual, corporation, partnership, trust, limited liability company, association or other entity.
“Qualified Securities Market” shall mean any one of the Nasdaq Stock Exchange (including the Nasdaq Capital Market), the NYSE:Amex Exchange, the New York Stock Exchange or the OTCQX platform of the OTC Markets.
“SEC” means the Securities and Exchange Commission.
“SEC Rule 144” means Rule 144 promulgated by the SEC under the Securities Act.
“SEC Rule 145” means Rule 145 promulgated by the SEC under the Securities Act.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Selling Expenses” means all underwriting discounts, selling commissions, and stock transfer taxes applicable to the sale of Conversion Shares, and fees and disbursements of counsel for any Holder, except for the fees and disbursements of the Selling Holder Counsel borne and paid by the Company as provided in Section 6.6.
[Remainder of Page Intentionally Left Blank]
| G-14 |
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the day and year first written above.
| THE COMPANY: | ||
| Shuttle Pharmaceuticals Holdings, Inc. | ||
| By: | ||
| Name: | Xx. Xxxxxxx Xxxxxxxxxx | |
| Title: | Chief Executive Officer | |
| Address: | Xxx Xxxxxxxx Xxxxx, Xxxxx 000 Xxxxxxxxx, XX 00000 |
|
| Investor: | |
| [Name] | |
| [Name] |
| G-15 |
Exhibit H – Capitalization Table
| H-1 |


