License Agreement
Exhibit 10.9
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
by and between
Annji Pharmaceutical Co. Ltd.
and
Avenue Therapeutics, Inc
dated February 28, 2023
Table of Contents
| 1. | Definitions | 1 |
| 2. | License; Right of First Negotiation | 20 |
| 3. | Cost-Covering Study; Joint Steering Committee | 23 |
| 4. | Diligence and Performance | 27 |
| 5. | Clinical Supply | 33 |
| 6. | Financial Terms | 33 |
| 7. | Intellectual Property | 40 |
| 8. | Confidentiality | 44 |
| 9. | Representations & Warranties; Covenants | 47 |
| 10. | Indemnification; Insurance; Limitation of Liability | 50 |
| 11. | Term & Termination | 53 |
| 12. | Miscellaneous | 58 |
Schedule of Exhibits
| Exhibit A | – | Licensed Molecule |
| Exhibit B | – | Licensed Patents |
| Exhibit C | – | Charter |
| Exhibit D | – | Form of Subscription Agreement |
| Exhibit E | – | Form of Registration Rights Agreement |
| Exhibit F | – | Term Sheet for Clinical Supply Agreement |
| Exhibit G | – | Advisor Qualifications |
| Exhibit H | – | Commercial Supply Agreement Arbitration |
This License Agreement (this “Agreement”) is made and entered into effective as of February 28, 2023 (the “Effective Date”) by and between AnnJi Pharmaceutical Co. Ltd., a Taiwanese corporation having its principal office at B405, Xx. 00, Xxxxxx Xx., Xxxxxxxxxx Xxxx., Xxxxxx Xxxx 000, Xxxxxx (“Licensor”), and Avenue Therapeutics, Inc., a Delaware corporation having its principal office at 0 Xxxxxxxxxx Xxxxxx, 0xx Xxxxx, Xxx Xxxx XX 00000, USA (“Avenue”). Each of Licensor and Avenue may be referred to herein individually as a “Party,” and collectively as the “Parties.”
Recitals
Whereas, Licensor has developed the molecule known as JM17 (defined in more detail below as the “Licensed Molecule”) that activates Nrf1 and Nrf2 and enhances androgen receptor degradation; and
Whereas, the Parties desire to enter into this Agreement, pursuant to which Avenue wishes to obtain, and Licensor wishes to grant, a license under the Licensed IP (as defined below) with respect to the Licensed Molecule and Licensed Products in the Field and in the Territory (each, as defined below) on the terms and conditions set forth in this Agreement.
Now, therefore, in consideration of the foregoing and the mutual agreements set forth below, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
Agreement
|
1. |
Definitions |
When used and capitalized in this Agreement (other than in Section headings), including the foregoing recitals and any Exhibits or Schedules hereto, the following terms shall have the meanings assigned to them in this Section 1, and include the plural as well as the singular, and all participles of each such term, as applicable.
1.1 “Accelerated Assessment” means a scheme to reduce the timeframe for the MAA application reviewed by EMA Committee for Medicinal Products for Human Use to 150 days according to Article 14 (9) of Regulation (EC) No 726/2004.
1.2 “Accelerated Approval” means United States Regulatory Approval obtained pursuant to the FDA’s Accelerated Approval Program as set forth 21 C.F.R. § 314 Subpart H (as applicable) or any successor program, and any equivalent program outside of the United States, including Conditional Market Authorization.
1.3 “Accounting Standards” means: (a) United States Generally Accepted Accounting Principles (“GAAP”); or (b) to the extent that a Party adopts International Financial Reporting Standards (“IFRS”), IFRS, in either case ((a) or (b)), consistently applied.
1.4 “Affiliate” means any Person which, directly or indirectly through one (1) or more intermediaries, controls, is controlled by, or is under common control with a Party. For purposes of this Section 1.4 only, the term “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”) as used with respect to a Person means: (a) direct or indirect ownership of fifty percent (50%) or more of the voting securities or other voting interest of any Person (including attribution from related parties); or (b) the possession, directly or indirectly, of the power to direct, or cause the direction of, the management and policies of such Person, whether through ownership of voting securities, by contract, as a general partner, as a manager, or otherwise. For the avoidance of doubt, “Affiliate” includes Subsidiaries.
1.5 “Agreement” is defined in the preamble.
1.6 “AnnJi Regulatory Materials and Data” means all Regulatory Materials and Data, in each case to the extent: (a) Controlled by AnnJi or its Affiliates during the Term; and (b) relating specifically to Licensed Product or Licensed Molecule.
1.7 “Annual Net Sales” means, with respect to a particular Calendar Year, aggregate Net Sales in the Territory of all Licensed Products in such Calendar Year.
1.8 “Applicable Law” means all applicable laws, statutes, rules, regulations, orders, judgments, or ordinances having the effect of law of any national, multinational, federal, state, provincial, county, city, or other political subdivision, including, to the extent applicable but without limitation, GCP, GLP, and GMP, as well as all applicable data protection and privacy laws, rules, and regulations, including, to the extent applicable, the United States Department of Health and Human Services privacy rules under the Health Insurance Portability and Accountability Act (HIPAA) and the Health Information Technology for Economic and Clinical Health Act (HITECH) and the EU Data Protection Directive (Council Directive 95/46/EC) and applicable laws implementing the EU Data Protection Directive and the General Data Protection Regulation (2016/679).
1.9 “Auditor” is defined in Section 6.6(b).
1.10 “Avenue” is defined in the preamble.
1.11 “Avenue Indemnitees” is defined in Section 10.2.
1.12 “Avenue Regulatory Materials and Data” will mean any and all Regulatory Materials and Data, in each case to the extent: (a) Controlled by Avenue, its Affiliates, or Sublicensees during the Term; and (b) relating specifically to Licensed Product or Licensed Molecule.
1.13 “Avenue Sole Invention License Term” means a period commencing on the Effective Date and continuing until: (a) with respect to each Patent that claims Sole Inventions, the expiration of such Patent; or (b) with respect to Know-How comprising Sole Inventions, the termination or expiration of this Agreement.
1.14 “Business Day” means a day on which banking institutions in New York City, New York, are open for business, excluding any Saturday or Sunday.
1.15 “Breakthrough Designation” means a designation by the FDA of a drug as a breakthrough therapy as set forth in 21 U.S.C. § 356(a) and related FDA guidance, and equivalent rules, regulations and guidance outside of the United States, for example, PRIority MEdicine designation granted by EMA.
1.16 “Calendar Quarter” means each of the three (3)-month periods ending March 31, June 30, September 30, and December 31, provided that: (a) the first Calendar Quarter of the Term shall extend from the Effective Date to the end of the first complete such three (3)-month period thereafter; and (b) the final Calendar Quarter of the Term shall end on the last day of the Term.
1.17 “Calendar Year” means the period beginning on the Effective Date and ending on December 31 of the calendar year in which the Effective Date falls, and thereafter each successive period of twelve (12) consecutive calendar months beginning on January 1 and ending on December 31, provided that the final Calendar Year of the Term shall end on the last day of the Term.
1.18 “Clinical Supply Agreement” is defined in Section 5.1.
1.19 “Clinical Trial” means a human clinical trial of a Licensed Product, regardless of its controlled or uncontrolled status.
1.20 “Code” is defined in Section 11.5(b).
1.21 “Combination Product” means any pharmaceutical or biological product that includes or incorporates Licensed Product (with or without one or more such other active ingredients), in each such case when the Licensed Product and any of the foregoing are co-formulated, co-packaged or sold under one pricing scheme (whether payment of such price is paid to the same or to more than one seller).
1.22 “Commercial Agreement Request” is defined in Section 4.7.
1.23 “Commercial Supply Agreement” is defined in Section 4.7.
1.24 “Commercial Supply Agreement Arbitrator” is defined in Exhibit H. The Commercial Supply Agreement Arbitrator is also referred to as the “CSA Arbitrator.”
1.25 “Commercialization” means any and all activities directed to the commercialization of a product, including marketing, detailing, promotion, market research, distributing, order processing, handling returns and recalls, booking sales, customer service, administering, and commercially selling such product, importing, exporting, and transporting such product for commercial sale, and seeking Pricing Approval of a product (if applicable), whether before or after Regulatory Approval has been obtained, as well all regulatory compliance with respect to the foregoing. For clarity, “Commercialization” shall be deemed to include conducting medical affairs activities, and interacting with Regulatory Authorities regarding any of the foregoing; but does not include: (a) Manufacturing; or (b) any Clinical Trials and other trials commenced after Regulatory Approval. When used as a verb, “Commercialize” means to engage in Commercialization.
1.26 “Commercially Reasonable Efforts” means: (a) with respect to the efforts to be expended by any Party with respect to any objective, such reasonable, diligent, and good faith efforts as such Party would normally use to accomplish a similar objective under similar circumstances with respect to a Party in relation to an obligation under this Agreement with respect to a Licensed Product; and (b) with respect to any objective relating to Development or Commercialization of Licensed Product by Avenue, such efforts that are consistent with the efforts and resources normally used by a competent biotechnology or pharmaceutical company adequate for the appropriate performance and completion of such an obligation, as applicable, at a similar stage in its research, development, or commercial life as such Licensed Product, and that has commercial and market potential similar to such Licensed Product, on a market-by-market and product-by-product basis in view of conditions prevailing at the time, taking into account issues of intellectual property coverage, safety and efficacy, stage of development, product profile, competitiveness of the marketplace, proprietary position, regulatory exclusivity, anticipated or approved labeling, present and future market and commercial potential, the likelihood of receipt of Regulatory Approval, profitability (including pricing and reimbursement status achieved or likely to be achieved), the existence and developmental stages of alternative products and programs, and legal issues, but without consideration of any of the payments required to be made from Avenue to the other(s) under this Agreement. In determining whether Avenue has used Commercially Reasonable Efforts, neither the Royalty nor other payments made or required to be made from Avenue to the other(s) under this Agreement shall be considered in determining market potential (that is, Avenue may not apply lesser resources or efforts in support of a Licensed Product because it must pay the Royalty or make milestone or any other payments hereunder to Licensor or the Third Party).
1.27 “Committee” is defined in Section 3.2(a).
1.28 “Common Stock” is defined in Section 6.7(a).
1.29 “Competing Infringement” is defined in Section 7.4(a).
1.30 “Competing Product” shall mean any pharmaceutical product (other than Licensed Products) for the treatment of spinal and bulbar muscular atrophy.
1.31 “Conditional Market Authorization” means the market authorization of a medicine that addresses unmet medical needs of patients on the basis of less comprehensive data than normally required as set forth in Regulation (EC) No 507/2006.
1.32 “Confidential Information” of a Party means any information, including business intentions, business plans, regulatory matters, regulatory compliance matters, strategies, customers, vendors, pricing and other commercial terms, budgets, forecasts, projections, sales and other financial results, trade secrets, inventions or discoveries, proprietary information, formulae, processes, techniques and information relating to a Party’s past, present and future research, Development, manufacture or Commercialization activities of any compound, product or potential product or technology or any (other) Know-How that is disclosed by or on behalf of such Party to the other Party or any Affiliate thereof under this Agreement, in any form (whether written, oral, photographic, electronic, magnetic, or otherwise), or that otherwise becomes known to the other Party or any Affiliate thereof by virtue of this Agreement, irrespective of whether such information is patentable or not and irrespective of whether such information is marked as confidential by the disclosing Party or not.
1.33 “Control,” “Controls,” or “Controlled” means, with respect to any particular Patents, Know-How or Regulatory Material, possession by the Party granting the applicable right, license, access or release to the other Party as provided herein of the power and authority, whether arising by ownership, license, or other authorization, to disclose and deliver such Patents or Know-How, and to grant and authorize under such Patents, Know-How or Regulatory Materials, the right, license, access or release, as applicable of the scope granted to such other Party in this Agreement without giving rise to any violation of the term of any written agreement with any Third Party existing at the time such disclosure is first made or such right, license access or release first comes into effect hereunder. “Controlled” and “Controlling” have their correlative meanings.
1.34 “Cost-Covering Study” is defined in Section 3.1(a).
1.35 “Cost-Covering Study Know-How” is defined in Section 3.1(d).
1.36 “Cost-Covering Study Report” is defined in Section 3.1(e).
1.37 “Cost of Goods” means, with respect to Licensed Product in bulk form Manufactured for use as an active pharmaceutical ingredient or in finished final packaged and labeled product form, or in intermediate states, as the case may be, Manufactured by or on behalf of a Party under this Agreement, the reasonable, documented internal and external costs of such Party or any of its Affiliates incurred in Manufacturing such Licensed Product, including: (a) to the extent that such Licensed Product is Manufactured by such Party or any of its Affiliates, the cost of goods sold of such Licensed Product, consisting of direct materials and direct labor costs, plus Manufacturing overhead directly attributable to Licensed Product supplied (excluding facilities start-up costs, corporate administrative overhead, depreciation and costs associated with excess capacity), all calculated in accordance with the Accounting Practices of such Party or its Affiliates; (b) to the extent that such Licensed Product is Manufactured by a Third-Party manufacturer, the actual fees paid by such Party or any of its Affiliates to the Third Party for the Manufacture, supply, testing, packaging, labeling and shipping (including the cost of insuring such shipment) of such Licensed Product, and any reasonable out-of-pocket and direct labor costs actually incurred by such Party or any of its Affiliates in managing or overseeing the Third-Party relationship; and (c) subject to Avenue’s prior written consent, license or other fees paid by such Party or any of its Affiliates to Third Parties in respect of Manufacture of such Licensed Product.
1.38 “Cover” means, with respect to a Licensed Molecule or Licensed Product in a particular country, that the research, Development, Manufacture, use, sale, offer for sale, importation, or other Commercialization of such Licensed Molecule or Licensed Product, as applicable, in such country would, but for any licenses granted under any Licensed Patent, infringe a Valid Claim of such Licensed Patent (considering Valid Claims of patent applications to be issued as then pending). “Covering” has a corresponding meaning.
1.39 “Cure Period” is defined in Section 11.3(a).
1.40 “Damages” means all losses, costs, claims, damages, judgments, liabilities, and expenses (including reasonable attorneys’ fees and other reasonable and documented out-of-pocket costs in connection therewith).
1.41 “Data” means any and all raw scientific, technical or test data, including research data, clinical pharmacology data, CMC data (including analytical and quality control data and stability data), pre-clinical data, clinical data, pharmacovigilance and pharmacoeconomic data and all data in publications, presentations or submissions made in association with Regulatory Materials for a Licensed Product.
1.42 “Default” means: (a) any breach, violation, or default; (b) the existence of circumstances or the occurrence of an event that with the passage of time or the giving of notice or both would constitute a breach, violation, or default; or (c) the existence of circumstances or the occurrence of an event that, with or without the passage of time or the giving of notice or both, would give rise to a right of termination, renegotiation, acceleration, or material change of terms.
1.43 “Development” means: (a) research activities (including drug discovery, identification, or synthesis) with respect to a product; or (b) preclinical and clinical drug development activities and other development activities with respect to Licensed Product, including test method development and stability testing, toxicology, formulation, process development, qualification and validation, quality assurance, quality control, Clinical Trials (including the conduct of Clinical Trials and other trials commenced after Regulatory Approval), statistical analysis and report writing, the preparation and submission of INDs and XXXx, regulatory affairs with respect to the foregoing, and all other activities necessary or useful or otherwise requested or required by a Regulatory Authority or as a condition or in support of obtaining or maintaining a Regulatory Approval. For clarity, “Development” does not include Manufacturing. When used as a verb, “Develop” means to engage in Development.
1.44 “Development Plan” means any initial and subsequent plans established and updated in accordance with Section 4.4 setting forth the Development of the Licensed Product under this Agreement.
1.45 “DGCL” is defined in Section 6.7(d).
1.46 “Diligence Milestone” is defined in Section 4.2.
1.47 “Disclosing Party” is defined in Section 8.1.
1.48 “Dispute” is defined in Section 12.7(b).
1.49 “Dollars” or “$” means the legal tender of the United States.
1.50 “Effective Date” is defined in the preamble.
1.51 “EMA” means the European Medicines Agency (and any successor entity thereto).
1.52 “Encumbrance” means any claim, charge, equitable interest, hypothecation, lien, mortgage, pledge, security interest, license, adverse claim of ownership or use, reversion, violation, option, restriction on transfer, defect of title, covenant, restriction, rights of others, or any other encumbrance of any kind, whether imposed by agreement, understanding, law, equity or otherwise.
1.53 “EU” means all countries that are officially recognized as member states of the European Union as of the Effective Date.
1.54 “Exercise Notice” is defined in Section 2.5.
1.55 “Executive Officer(s)” means with respect to a Party, a senior officer of such Party having at least the level of authority which is customarily held by employees of such Party holding a title of “vice president.”
1.56 “Existing Indication” is defined in Section 1.83.
1.57 “Existing Product” means Licensor’s product identified as AJ201, which is Phase 1b/2a clinical stage product (Clinical Trial Identifier NCT05517603) in an oral suspension form.
1.58 “Extension Payment” is defined in Section 4.2(c).
1.59 “FDA” means the United States Food and Drug Administration, or any successor agency thereto.
1.60 “FD&C Act” means that federal statute entitled the Federal Food, Drug, and Cosmetic Act and enacted in 1938 as Public Law 75-717, as such may have been amended, and which is contained in Title 21 of the C.F.R. Section 301 et seq.
1.61 “Field” means all uses, including the prevention, treatment, diagnosis, detection, monitoring or predisposition testing of all diseases, states or conditions in humans or animals, except for androgenetic alopecia and Alzheimer’s Disease.
1.62 “First Commercial Sale” means, on an Licensed Product-by-Licensed Product and country-by-country basis, the first sale by Avenue, or its Affiliate or Sublicensee of such Licensed Product in such country for use or consumption by the general public (following receipt of all Regulatory Approvals that are required in order to sell such Licensed Product in such country) and for which a Selling Party has invoiced sales of Licensed Products in the Territory (if no such Regulatory Approval or similar marketing approval is required for a Licensed Product in a country, the date upon which such Licensed Product is first commercially sold in such country to end users), provided that the following shall not constitute a First Commercial Sale: (a) any sale or transfer to an Affiliate or Sublicensee, unless such Affiliate or Sublicensee is the last Person in the distribution chain of such Licensed Product; or (b) any transfer for use of such Licensed Product in Clinical Trials or non-clinical development activities with respect to such Licensed Product by or on behalf of a Selling Party, or transfer for use of such Licensed Product for a bona fide charitable purpose, compassionate use (which is different from Named Patient Sales), or samples. For the avoidance of doubt, Named Patient Sales in any country in Territory will not constitute the First Commercial Sale unless indicated otherwise.
1.63 “First EMA Approval” is defined in Table 6.2(a).
1.64 “First FDA Approval” is defined in Table 6.2(a).
1.65 “First Product EMA Label Expansion” is defined in Table 6.2(a).
1.66 “First Licensed Product sNDA” is defined in Table 6.2(a).
1.67 “Force Majeure Event” is defined in in Section 12.3.
1.68 “FPFD” means the administration of the first dose of a Licensed Product to the first patient (or volunteer, as relevant) participating in a Clinical Trial.
1.69 “GAAP” is defined in Section 1.3.
1.70 “GCP” means the applicable then-current ethical and scientific quality standards for designing, conducting, recording, and reporting Clinical Trials as are required by applicable Regulatory Authorities or Applicable Law in the relevant jurisdiction, including in the United States, Good Clinical Practices established through FDA guidances, and, outside the United States, Guidelines for Good Clinical Practice – ICH Harmonized Tripartite Guideline (ICH E6).
1.71 “Generic Product” is defined in Section 1.100.
1.72 “Generic Product Introduction Date” is defined in Section 1.100.
1.73 “GLP” means the applicable then-current good laboratory practice standards as are required by applicable Regulatory Authorities or Applicable Law in the relevant jurisdiction, including in the United States, those promulgated or endorsed by the FDA in U.S. 21 C.F.R. Part 58, or the equivalent thereof as promulgated or endorsed by the applicable Regulatory Authorities outside of the United States.
1.74 “GMP” means the applicable then-current good manufacturing practice standards relating for fine chemicals, intermediates, bulk products, or finished pharmaceutical, biological, or diagnostic products, as are required by applicable Regulatory Authorities or Applicable Law in the relevant jurisdiction, including, as applicable: (a) all applicable requirements detailed in the FDA’s current Good Manufacturing Practices regulations, U.S. 21 C.F.R. Parts 210 and 211; (b) all applicable requirements detailed in the EMA’s “The Rules Governing Medicinal Products in the European Community, Volume IV, Good Manufacturing Practice for Medicinal Products;” and (c) all Applicable Law promulgated by any Governmental Authority having jurisdiction over the manufacture of the applicable molecule, agent, compound, or pharmaceutical, biological, or diagnostic product, as applicable.
1.75 “Governmental Authority” means any: (a) federal, state, local, municipal, foreign, or other government; (b) governmental or quasi-governmental authority of any nature (including any agency, board, body, branch, bureau, commission, council, department, entity, governmental division, instrumentality, office, officer, official, organization, representative, subdivision, unit, and any court or other tribunal); (c) multinational governmental organization or body; or (d) entity or body exercising, or entitled to exercise, any executive, legislative, judicial, administrative, regulatory, police, military, or taxing authority or power of any nature.
1.76 “ICC” is defined in Section 12.7(c)(iii).
1.77 “ICC Rules” is defined in Exhibit H.
1.78 “IFRS” is defined in Section 1.3.
1.79 “IND” means an investigational new drug application (including any amendment or supplement thereto) submitted to the FDA pursuant to U.S. 21 C.F.R. Part 312, including any amendments thereto. References herein to IND shall include, to the extent applicable, any comparable filing(s) outside the U.S. for the investigation of any product in any other country or group of countries (such as a Clinical Trial Application in the EU).
1.80 “Indemnification Claim Notice” is defined in Section 10.3(a).
1.81 “Indemnitee” is defined in Section 10.3(a).
1.82 “Indemnitor” is defined in Section 10.3(a).
1.83 “Identified New License” is defined in Section 2.5.
1.84 “Indication” means a specific disease or medical condition in humans. For purposes of determining whether an Indication is distinct from another Indication, an Indication (“New Indication”) is distinct from an existing Indication (“Existing Indication”) if the Licensed Product could not be lawfully promoted for the treatment of the New Indication under the Regulatory Approval for the Existing Indication.
1.85 “Invention(s)” means any process, method, composition of matter, article of manufacture, discovery, or finding that is conceived or reduced to practice.
1.86 “Issued and Outstanding Equity Securities” means all of the issued and outstanding Common Stock. For the avoidance of doubt, “Issued and Outstanding Equity Securities” does not include preferred stock, options, restricted stock units, warrants, other convertible securities or any of the shares into which any such securities may be convertible or exchangeable.
1.87 “Joint Inventions” is defined in Section 7.1.
1.88 “Joint Patents” is defined in Section 7.1.
1.89 “JSC” is defined in Section 3.2(a).
1.90 “JSC Chair” is defined in Section 3.2(a).
1.91 “Know-How” means any scientific or technical information, results and data, protocols, regulatory filings, regulatory documentation (e.g., adverse event reports and CMC documentation), regulatory approvals, methods, processes, techniques, plans, formulations, formulae, data (including pharmacological, biological, chemical, toxicological, clinical and analytical information, quality control, trial and stability data), case reports forms, data analyses, reports, studies and procedures, designs for experiments and tests and results of experimentation and testing (including results of research or development), summaries and information contained in submissions to and information from ethical committees, the FDA or other regulatory authorities, and development information, results and data, whether or not patentable.
1.92 “Label Expansion” means, following first receipt of Regulatory Approval for a Licensed Product in an Indication, any subsequent receipt of Regulatory Approval for a New Indication.
1.93 “Licensed Know-How” means: (a) all Know-How Controlled by Licensor or its Affiliates as of the Effective Date and that is or would be reasonably necessary or useful to Develop, Commercialize, or otherwise use Licensed Products; and (b) all Cost-Covering Study Know-How.
1.94 “Licensed IP” means the Licensed Patents and the Licensed Know-How.
1.95 “Licensed Molecule” means the small molecule pharmaceutical compound with Licensor internal identifier JM17, the structure of which is set forth on Exhibit A.
1.96 “Licensed Patents” means: (a) any and all Patents that are Controlled by Licensor as of the Effective Date that Cover the Licensed Product, including the Patents set forth on Exhibit B; (b) any substitutions, divisionals, continuations, continuations-in-part, reissues, renewals, registrations, confirmations, re-examinations, extensions, supplementary protection certificates, and the like of any Patents included in clause (a) above; (c) any and all Patents issuing from the patent applications described in clauses (a) or (b); (d) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, re-examinations or any other post-grant proceedings and extensions (including any supplementary protection certificates and the line) of the Patents described in clauses (a), (b), or (c); and (e) foreign counterparts of any of the foregoing.
1.97 “Licensed Product” means any product in oral form, that constitutes, incorporates, comprises, or contains the Licensed Molecule as an active pharmaceutical ingredient, whether or not as the sole active ingredient or in combination with one or more other active pharmaceutical ingredients. Licensed Product includes the Existing Product. For the avoidance of doubt, Licensed Products under distinct XXXx will constitute distinct Licensed Products for purposes of Avenue’s diligence obligations pursuant to Section 4 and any Milestone Payment calculation (provided that in no event will an sNDA be deemed a distinct MAA from the applicable NDA).
1.98 “Licensed Product Annual Net Sales” is defined in Section 6.3(a).
1.99 “Licensor” is defined in the preamble.
1.100 “Licensor Indemnitees” is defined in Section 10.1.
1.101 “Loss of Market Exclusivity” means, with respect to a specified country in the Territory: (a) the reduction in Net Sales by 50% or more in any twelve (12)-month period following the First Commercial Sale in such country of any pharmaceutical product containing the Licensed Molecule which is marketed by any entity or entities other than Avenue, its Affiliates or any Sublicensees (any such product with respect to a given country, a “Generic Product” and the date of first commercial sale of the first such Generic Product in a given country, the “Generic Product Introduction Date”) in such country as compared with the twelve (12)-month period immediately prior to the Generic Product Introduction Date (as measured by reputable published data, (e.g., by reference to market share data collected by IQVIA or Symphony Health)); and (b) such reduction in Net Sales is reasonably attributable to sales of such Generic Product.
1.102 “LPLD” means the administration of the last dose of a Licensed Product to the last patient (or volunteer, as relevant) participating in a Clinical Trial.
1.103 “MAA” means a Marketing Authorization Application, NDA, or similar application, as applicable, and all amendments and supplements thereto, submitted to the FDA, EMA, or any equivalent filing in a country or regulatory jurisdiction other than the U.S. or EU with the applicable Regulatory Authority, to obtain marketing approval for a pharmaceutical, biological, or diagnostic product, in a country or in a group of countries.
1.104 “Manufacture,” or “Manufacturing” means all activities related to the manufacture and production of a Licensed Product, including the production of any of the following to the extent used in a Licensed Product: any drug substance produced in bulk form for use as an active pharmaceutical ingredient, drug product, compounded or finished final packaged and labeled form, and in intermediate states, including the following activities: reference standard preparation, purification, formulation, scale-up, packaging, disposition of product, quality assurance oversight, quality control testing (including in-process release and stability testing), storage of product or any component or ingredient thereof and validation activities directly related to all of the foregoing, and data management and recordkeeping related to all of the foregoing. References to a Person engaging in Manufacturing activities will include having any or all of the foregoing activities performed by a Third Party.
1.105 “Major Market Country” means each or any of the U.S., France, Germany, Great Britain, Italy, Spain, or the EU as a whole.
1.106 “Manufacturing Rights Payment” means Avenue’s payment to Licensor in accordance with Section 4.9(a), of an amount equal to: (a) if Licensor has experienced a Rejection Event in the two hundred ten (210) calendar days preceding the date of such payment, $7,500,000; or (b) if Licensor has not experienced a Rejection Event in the two hundred ten (210) calendar days preceding the date of such payment, $25,000,000.
1.107 “Manufacturing Rights Trigger” means the occurrence of:
(a) termination of the Clinical Supply Agreement in connection with Licensor’s uncured material breach thereof or default thereunder, provided that Avenue has a reasonable need to Manufacture the Licensed Product upon such termination (taking into consideration the scope of Avenue’s current inventory, existing and forecasted order, alternative sources, and other similar factors);
(b) termination of the Commercial Supply Agreement in connection with Licensor’s uncured material breach thereof or default thereunder, provided that Avenue has a reasonable need to Manufacture the Licensed Product upon such termination (taking into consideration the scope of Avenue’s current inventory, existing and forecasted order, alternative sources, and other similar factors); or
(c) (i) a Supply Failure, provided that Avenue, its Affiliates, or Sublicensees are not in uncured material breach of the Commercial Supply Agreement (including with respect to all payment obligations thereunder) as of such occurrence, or (ii) in the event that Avenue, its Affiliates, or Sublicensees are in uncured material breach of the Commercial Supply Agreement (including with respect to all payment obligations thereunder) upon the occurrence of a Supply Failure, the curing of such material breach within sixty (60) calendar days following the occurrence of such Supply Failure; provided that in either case ((i) or (ii)), Avenue provides Licensor with written notice of Avenue’s election to Manufacture within ninety (90) calendar days of the occurrence of such Supply Failure or the curing of such material breach, as applicable.
Notwithstanding the foregoing, written notice to Licensor pursuant to Section 1.107(c) shall only be required in connection with the first occurrence of an event described in clause (i) or (ii) of Section 1.107(c) (i.e., the second occurrence of an event described in clause (i) or (ii) of Section 1.107(c) shall automatically constitute a Manufacturing Rights Trigger without the provision of such notice.)
1.108 “Materials” means excipients and solvents and other chemicals and other raw materials and components, packaging and shipping materials (including vials) and other materials and supplies reasonably necessary to Manufacture Licensed Products.
1.109 “Milestone Event” is defined in Section 6.2.
1.110 “Milestone Payment” is defined in Section 6.2.
1.111 “Named Patient Sales” means the sale of a Licensed Product in a given country in the Territory prior to receipt of Marketing Approval of such Product in such country, directly or through an entity that is qualified to distribute unregistered pharmaceutical products in that country, on a “named-patient” basis to meet the special needs of particular patients under the order of a medical practitioner.
1.112 “NDA” means a New Drug Application submitted to the FDA, or any successor application or procedure, as more fully defined in 21 C.F.R. § 314.50 et. seq.
1.113 “Net Sales” means the gross amounts invoiced for Licensed Product sold by Avenue, its Affiliates, or its Sublicensees (each a “Selling Party”) in finished product form, packaged and labeled for sale in arm’s length transactions to Third Parties, less the following deductions from such gross amounts:
(a) normal and customary trade, cash and other discounts and allowances actually allowed by the Selling Party and taken by the customer;
(b) credits, price adjustments or allowances actually granted to the customer for damaged goods, returns or rejections of a Licensed Product;
(c) sales taxes or similar taxes, including duties or other governmental charges imposed on the sale of a Licensed Product (including value added taxes or other governmental charges, but excluding any income taxes), to the extent the Selling Party is not otherwise entitled to a credit or a refund for such taxes, duties or payments made;
(d) chargeback payments, rebates, fees, and other adjustments, including those granted on price adjustments, billing errors, reimbursements or similar payments granted or given to wholesalers or other distributors, buying groups, health insurance carriers or other institutions, including those paid in connection with such sales to any governmental entity;
(e) any invoiced amounts which are not collected by the Selling Party, including bad debts (provided that if any such bad debt is subsequently collected, such collected amount will be added to Net Sales);
(f) the portion of administrative fees paid during the relevant time period to group purchasing organizations or pharmaceutical benefit managers relating to such Licensed Product; and
(g) any invoiced freight, shipping, insurance and other transportation charges.
For purposes of determining Net Sales, a Licensed Product will be deemed to be sold when invoiced, and Net Sales does not include and shall be deemed zero with respect to transfers or dispositions provided as samples or Licensed Product for compassionate use, which is different from Named Patient Sales, indigent programs, or similar bona fide arrangements or for pre-clinical or clinical purposes. In the case of any sale of a Licensed Product for consideration other than cash, such as barter or countertrade, Net Sales shall be calculated on the fair market value of the consideration received as agreed by the Parties.
Net Sales, as set forth in this definition, will be calculated by applying the Selling Party’s standard accounting practices, in accordance with the Accounting Standards used by the Selling Party, as consistently applied in its respective audited financial statements.
(x) If, on a country-by country basis, any Licensed Product is, or is sold as part of, a Combination Product, Net Sales will be calculated by multiplying the total Net Sales (as described above) of the Combination Product by the fraction:
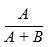
where “A” is the weighted average gross selling price in such country of such Licensed Product determined in accordance with the Accounting Standards, if sold separately in such country, and “B” is the weighted-average price in such country of such other product(s) containing such other active pharmaceutical or biological ingredients included in the Combination Product (and not the Licensed Molecule contained in such Licensed Product) determined in accordance with the Accounting Standards, if sold separately in such country.
(y) On a country-by country basis, in the event that: (i) the Licensed Product without the other active pharmaceutical or biological ingredients is sold separately in the same formulation and dosage; and (ii) the other active ingredients in the same formulation and dosage as in the Combination Product are not sold separately, then Net Sales will be calculated by multiplying the total Net Sales (as described above) of the Combination Product by the fraction:
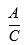
where “A” is the average per unit Net Sales in the applicable country in the Territory of the Licensed Product sold separately in the same formulation and dosage, and “C” is the average per unit Net Sales in the applicable country in the Territory of the Combination Product during the applicable Calendar Quarter.
(z) In the event that, in a particular country the circumstances in clauses (x) or (y) of this Section 1.112 do not apply: or (i) the Licensed Product without the other active ingredients or devices is not sold separately in the same formulation and dosage during the applicable quarter in such country; and (ii) the other active ingredients in the same formulation and dosage as in the Combination Product are not sold separately during the applicable quarter in such country, then Net Sales for such Combination Product for such country will be calculated by multiplying the total Net Sales (as described above) of the Combination Product by the fraction:
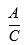
where “A” is the average per unit worldwide Net Sales of the Licensed Product sold separately in the same formulation and dosage, and “C” is the average per unit worldwide Net Sales of the Combination Product during the applicable Calendar Quarter.
1.114 “New Indication” is defined in Section 1.83.
1.115 “Non-Prosecuting Party” is defined in Section 7.3(b).
1.116 “Observer” is defined in Section 6.7(e).
1.117 “Open Terms” is defined in Section 4.7.
1.118 “Orange Book” means the FDA list of approved pharmaceuticals titled “Approved Drug Products with Therapeutic Equivalence Evaluations.”
1.119 “Parties” is defined in the preamble.
1.120 “Party” is defined in the preamble.
1.121 “Patents” means: (a) all patents and patent applications in any country or supranational jurisdiction worldwide; (b) any substitutions, divisionals, continuations, continuations-in-part, reissues, renewals, registrations, confirmations, re-examinations, extensions, supplementary protection certificates, and the like of any such patents or patent applications; (c) any and all patents that have issued or in the future issue from the foregoing patent applications described clauses (a) or (b), including utility models, xxxxx patents, innovation patents, design patents and certificates of invention; and (d) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, re-examinations or any other post-grant proceedings and extensions (including any supplementary protection certificates and the line) of the foregoing patents or patent applications ((a), (b) and (c)).
1.122 “Patent Proceeding” is defined in Section 11.6.
1.123 “Person” means any individual, partnership, joint venture, limited liability company, corporation, firm, trust, association, unincorporated organization, governmental authority or agency, or any other entity not specifically listed herein.
1.124 “Phase 1b/2a Clinical Trial” means a Clinical Trial of a Licensed Product for the principal purposes of gaining evidence of the safety and tolerability of, and potential pharmacological activity for, any product, as described in 21 C.F.R. § 312.21(a) (including any such clinical study in any country other than the United States), as further described in 21 C.F.R. § 312.21(b) (including any such clinical study in any country other than the United States).
1.125 “Phase 2/3 Clinical Trial” means a Clinical Trial which is: (a) initiated to determine the safety and efficacy in the target patient population, as described in 21 C.F.R. § 312.21(b) (as amended or any replacement thereof), or a similar clinical study prescribed by the Regulatory Authorities in a foreign country; and (b) converted to a Phase 3 Clinical Trial following an interim analysis of safety and efficacy data generated from the initial patents enrolled in such Clinical Trial
1.126 “Phase 3 Clinical Trial” means a Clinical Trial of a Licensed Product in any country that would satisfy the requirements of U.S. 21 C.F.R. § 312.21(c) and which is intended to: (a) establish that the product is safe and efficacious for its intended use; (b) define contraindications, warnings, precautions, and adverse reactions that are associated with the product in the dosage range to be prescribed; and (c) support Regulatory Approval for such product, or a similar clinical study prescribed by the relevant Regulatory Authorities in a country other than the United States.
1.127 “Price Per Share” means the dollar volume-weighted average price (the “VWAP”) for the Common Stock on the NASDAQ Stock Market during the period commencing ten (10) trading days prior to the Effective Date at 9:30:00 a.m., New York City time, and ending ten (10) trading days after the Effective Date at 4:00:00 p.m., New York City time (the “VWAP Period”), as reported by Bloomberg through its “Volume at Price” function or, if the foregoing does not apply, the VWAP of the Common Stock in the over-the-counter market on the electronic bulletin board for the shares of Common Stock during the VWAP Period, as reported by Bloomberg. If the VWAP cannot be calculated for the Common Stock on such date on any of the foregoing bases, the VWAP of such shares of Common Stock on such date shall be the fair market value per share of Common Stock as determined in good faith by Avenue’s Board of Directors after consultation with Licensor.
1.128 “Pricing Approval” means any approval, agreement, determination, or decision establishing prices that can be charged to consumers for a pharmaceutical or biological product or that will be reimbursed by Governmental Authorities for a pharmaceutical or biological product, in each case, in a country where Governmental Authorities approve or determine pricing for pharmaceutical or biological products for reimbursement or otherwise.
1.129 “PRIority MEdicine” means a scheme launched by the European Medicines Agency (EMA) to enhance support for the development of medicines that target an unmet medical need according to Recital 33 and Article 14(9) of Regulation (EC) No 726/2004.
1.130 “Priority Review” means a priority review of, and action upon, a human drug application by the FDA not later than six (6) months after the sixty (60)-day filing date, or eight (8) months after submission, of such application to the FDA, as defined in the FD&C Act and the performance goals set forth in the applicable Prescription Drug User Fee Act commitment letter, or any successor program, and any equivalent program outside of the United States, including Accelerated Assessment.
1.131 “Proposed Commercial Supply Agreement” is defined in Exhibit H.
1.132 “Prosecuting Party” is defined in Section 7.3(b).
1.133 “Prosecution and Maintenance” or “Prosecute and Maintain” means, with respect to a Patent, the preparation, filing, prosecution, and maintenance of such Patent, as well as re-examinations, reissues, appeals, and requests for patent term adjustments and patent term extensions with respect to such Patent, together with the initiation or defense of interferences, oppositions, post grant review, inter parties review, derivations, re-examinations, post-grant proceedings, and other similar proceedings (or other defense proceedings with respect to such Patent, but excluding the defense of challenges to such Patent as a counterclaim in an infringement proceeding) with respect to the particular Patent, and any appeals therefrom. For clarification, “Prosecution and Maintenance” or “Prosecute and Maintain” shall not include any other enforcement actions taken with respect to a Patent.
1.134 “Put Right” is defined in Section 6.7(d).
1.135 “Put Shares” is defined in Section 6.7(d).
1.136 “Receiving Party” is defined in Section 8.1.
1.137 “Registration Rights Agreement” is defined in Section 6.7(c).
1.138 “Registrational Trial” means: (a) any Clinical Trial that is intended by Avenue, its Affiliate, or a Sublicensee to be submitted for Regulatory Approval in the Territory; or (b) any Clinical Trial based on an agreement or other statement or guidance from the Regulatory Authority in such country that such Clinical Trial is designed to provide data on which a Regulatory Approval will be based.
1.139 “Regulatory Approval” means all approvals, licenses, and authorizations of the applicable Regulatory Authority necessary for the marketing and sale of a pharmaceutical, biological, or diagnostic product for a particular Indication in a country or region, including the approvals by the applicable Regulatory Authority of any expansion or modification of the label for such Indication.
1.140 “Regulatory Authority” means any national or supranational Governmental Authority, including the FDA in the U.S., the EMA in the EU, Medicines and Healthcare products Regulatory Agency in the United Kingdom, or any health regulatory authority in any country or region that is a counterpart to the foregoing agencies, in each case, that holds responsibility for development and commercialization of, and the granting of Regulatory Approval for, a pharmaceutical, biological, or diagnostic product in such country or region.
1.141 “Regulatory Cause” means the existence of regulatory, safety, technical, scientific, efficacy or other circumstances outside the reasonable control of a Party or its Affiliates, including without limitation difficulties associated with patient enrollment, that inhibit or preclude one or more of the steps necessary to allow the achievement of a particular obligation of such Party under this Agreement.
1.142 “Regulatory Exclusivity” means, on a country by country and Licensed Product by Licensed Product basis, the ability to exclude any other Person from manufacturing or commercializing a product that could compete with such Licensed Product in such country in the Territory either through data exclusivity rights, orphan drug designation or such other similar rights conferred by a Regulatory Authority in such country.
1.143 “Regulatory Materials” means, with respect to a Licensed Product: (a) all the regulatory registrations, applications, authorizations, licenses, and approvals (including approvals of XXXx, supplements and amendments, pre- and post-approvals, Pricing Approvals, and labeling approvals), Regulatory Approvals, and other submissions made to or with any Regulatory Authority for research, development (including the conduct of Clinical Trials), manufacture, or commercialization of a pharmaceutical, biological, or diagnostic product in a regulatory jurisdiction, together with (b) all related correspondence and reports to or from any Regulatory Authority and all documents referenced in the complete regulatory chronology for each MAA, including all drug master files (if any), INDs, BLAs, NDAs, adverse event files, and complaint files, and foreign equivalents of any of the foregoing, and (c) Data contained in any of the foregoing, in each case ((a)–(c)), only to the extent necessary to obtain Regulatory Approval of such Licensed Product.
1.144 “Rejection Event” is defined in Section 11.5(a).
1.145 “Research” means any pre-clinical research activities (including drug discovery, identification or synthesis). When used as a verb, “Research” means to engage in Research.
1.146 “Right of First Negotiation” is defined in Section 2.5.
1.147 “ROFN Notice” is defined in Section 2.5.
1.148 “Royalty Term” means, with respect to a Licensed Product in the Territory, on a country-by-country basis, the period beginning on the date of First Commercial Sale of such Licensed Product in a country of the Territory and ending on the events occurs the latest: (a) expiration of the last-to-expire Valid Claim of a composition of matter Licensed Patent in such country that covers the composition and formulation of the Licensed Molecule; (b) expiration of the Regulatory Exclusivity in such country; or (c) such Licensed Product has experienced a Loss of Market Exclusivity in such country.
1.149 “Safety Agreement” is defined in Section 4.6.
1.150 “SBMA Product” means a Licensed Product for the treatment of spinal and bulbar muscular atrophy.
1.151 “SEC” is defined in Section 8.3(a)(i).
1.152 “Securities Regulators” is defined in Section 8.3(a)(i).
1.153 “Selling Party” is defined in Section 1.112.
1.154 “Sole Invention” is defined in Section 7.1.
1.155 “Sole Patent” is defined in Section 7.1.
1.156 “sNDA” means a supplemental New Drug Application, as defined in the FD&C Act and applicable regulations promulgated thereunder.
1.157 “Subcommittee” is defined in Section 3.2(a).
1.158 “Sublicense Date” is defined in Section 6.4.
1.159 “Sublicensee” means, with respect to Avenue, a Third Party or Avenue Affiliate who is granted a sublicense, either directly or indirectly, under the Licensed IP licensed to Avenue by Licensor in accordance with Section 2.2.
1.160 “Sublicense Revenue” means all consideration received by Avenue from a Sublicensee solely as consideration for the grant to such Sublicensee of a sublicense of the rights granted under Section 2.2, but would exclude: (a) with respect to a Calendar Quarter, any sales-based royalties paid by such Sublicensee in excess of the royalty owed to Licensor by Avenue with respect to such sales pursuant to Section 6.3; (b) fair market purchases of equity or debt of Avenue or its Affiliates; (c) fair market payments made in connection with research and development agreements, joint ventures, partnerships or collaboration agreements whereby Avenue or any of its Affiliates is obligated to perform research or development activities for Licensed Products; (d) payments or reimbursements of patent expenses actually incurred or paid by Avenue under this Agreement; (e) payments or reimbursements for documented sponsored research or development activities; and (f) other payments made by such Sublicensee as consideration for Avenue’s or its Affiliates’ performance of services or provision of goods that are not Licensed Products. By way of clarification, the principal amount of any loan or other extension of credit provided to Avenue or its Affiliates in connection with the grant of a sublicense by Avenue that is other than an arm’s-length credit relationship shall be deemed to constitute “Sublicense Revenue.”
1.161 “Sublicensing Revenue Payment” is defined in Section 6.4.
1.162 “Subscription Agreement” is defined in Section 6.7(a).
1.163 “Subsidiary” means, with respect to a Party, any corporation or other business entity: (a) in which such Party owns, directly or indirectly through one (1) or more intermediaries, fifty percent (50%) or more of the voting securities or other voting interest; (b) in which such Party possesses, directly or indirectly, the power to direct, or cause the direction of, the management and policies of such corporation or business entity, whether through ownership of voting securities, by contract, as a general partner, as a manager, or otherwise.
1.164 “Supply Expert” is defined in Section 4.10(a).
1.165 “Supply Issue” is defined in Section 1.166(a).
1.166 “Supply Failure” means:
(a) (i) any failure by Licensor, for two (2) consecutive Calendar Quarters, to supply at least eighty percent (80%) of products ordered by Avenue pursuant to the Commercial Supply Agreement, (ii) any failure by Licensor, for two (2) consecutive Calendar Quarters, to supply at least eighty percent (80%) of products supplied to Avenue pursuant to the Commercial Supply Agreement in conformance with all specifications applicable to such products as set forth in the Commercial Supply Agreement, or (iii) the occurrence of any other event identified as a “Supply Failure” in the Commercial Supply Agreement (each of (i)–(iii), a “Supply Issue”); and
(b) if the applicable Supply Issue is determined, pursuant to Section 4.10(a), to have occurred (i) due to Regulatory Cause or a Force Majeure Event, Licensor or its Affiliates (A) fail to resolve such Supply Issue and resume Manufacturing Licensed Products in compliance with the Commercial Supply Agreement within twenty-four (24) months of such determination by the Supply Expert, or (B) fail to continuously use Commercially Reasonable Efforts to resolve such Supply Issue and resume Manufacturing Licensed Products in compliance with the Commercial Supply Agreement within twenty-four (24) months of such determination by the Supply Expert, or (ii) due to reasons other than Regulatory Cause or a Force Majeure Event, Licensor fails to resolve such Supply Issue and resume Manufacturing Licensed Products in compliance with the Commercial Supply Agreement within two (2) Calendar Quarters of such determination by the Supply Expert.
1.167 “Term” is defined in Section 11.1.
1.168 “Terminated Country” is defined in Section 11.3(a).
1.169 “Terminated Product” is defined in Section 11.3(a).
1.170 “Territory” means United States of America, Canada, European Union, Great Britain, and Israel.
1.171 “Third Party” means any Person other than Licensor or Avenue that is not an Affiliate of Licensor or of Avenue. “Third-Party” has the corresponding meaning.
1.172 “Third-Party Claim” means any and all suits, claims, actions, proceedings, or demands brought by a Third Party.
1.173 “Third-Party Infringement” is defined in Section 7.5(a).
1.174 “Third-Party License” is defined in Section 6.3(c).
1.175 “Third-Party Royalties” is defined in Section 6.3(c).
1.176 “United States,” or “U.S.” means the United States of America, including its territories and possessions.
1.177 “Valid Claim” means a claim of a Patent that: (a) has issued and has not expired, lapsed, been cancelled, or abandoned, or been dedicated to the public, disclaimed, or held unenforceable, invalid, unpatentable, revoked, or cancelled by a court or administrative agency of competent jurisdiction in an order or decision from which no appeal has been or can be taken (with respect to U.S. Patents, other than by a petition to the United States Supreme Court for a writ of certiorari), including through opposition, reexamination, reissue, disclaimer, inter partes review, post grant review, post grant procedures, or similar proceedings; or (b) is in a pending patent application that has not been abandoned, disclaimed, canceled or finally disallowed without the possibility of appeal or refiling and which has been pending for no longer than seven (7) years and continues to be prosecuted in good faith.
1.178 “VWAP” is defined in Section 1.126.
1.179 “VWAP Period” is defined in Section 1.126.
|
2. |
License; Right of First Negotiation |
2.1 License to Avenue. Subject to the terms and conditions of this Agreement, Licensor hereby grants to Avenue, and Avenue hereby accepts, an exclusive, royalty-bearing, transferrable (pursuant to Section 12.4), and sublicensable (in accordance with Section 2.2) license, under the Licensed IP, to Develop, Commercialize, use, sell, offer for sale, and import and export the Licensed Products in the Field in the Territory.
2.2 Sublicensing.
(a) Avenue shall have the right to grant sublicenses, through multiple tiers of Sublicensees, under the licenses granted under Section 2.1, to Third Parties and Affiliates, provided that (i) any such sublicense shall not be inconsistent with the terms of this Agreement applicable to the exercise of rights under such sublicense or that are reasonably applicable to any Sublicensee, (ii) such sublicense shall include terms that are substantially equivalent to Sections 2.4, 4.1, 4.2 (but only if the Sublicensee is Developing the SBMA Product), 4.4, 4.5, 4.6, 4.8, 4.11, 4.12, 4.13, 4.14, 4.15, 6.6(a), 8, 11.5, 11.6, 11.7, and 11.8(c) of this Agreement, (iii) the grant of any sublicense shall not relieve Avenue of its obligations under this Agreement, (iv) Avenue shall remain responsible for the acts and omissions of its Sublicensees in connection with such Sublicense, and for such Sublicensee’s compliance of Sublicensees with all terms and conditions of this Agreement relevant to such sublicense; (v) a copy of any such executed sublicense agreements (and any amendment(s) thereto) with Sublicensee, irrespective of the tier of such Sublicensee, will be provided to Licensor promptly following execution within ten (10) calendar days thereof, which agreement may be redacted as to terms not reasonably applicable to determining Avenue’s compliance with its obligations under this Agreement; and (vi) Avenue may grant sublicenses under this Section 2.2 without such consent if (A) Avenue has achieved the Diligence Milestone set forth in Section 4.2(a), (B) the applicable Sublicensee is a public biotechnology or pharmaceutical company with a market capitalization in excess of five hundred million dollars ($500,000,000) as of the effective date of such sublicense, or (C) the applicable Sublicensee is a public biotechnology or pharmaceutical company with global annual revenue for its most recently completed fiscal year that is equal to or greater than two hundred million dollars ($200,000,000), based on most recent data collected or compiled by Evaluate Pharma (or a similar company to the extent Evaluate Pharma’s data is not available) and provided that in all instances any such (1) sublicenses shall be entered on an arms-length basis in a manner consistent with all applicable laws, and (2) in the event of a material breach by a Sublicensee of a sublicense hereunder which material breach is also a material breach of a term of this Agreement, Avenue shall enforce, in a commercially reasonable manner, the terms of such sublicense against such Sublicensee with respect to such breach.
(b) For the avoidance of doubt, Licensor shall be entitled to receive its applicable Milestone Payments or royalties based on Annual Net Sales of Licensed Products sold by all Sublicensees under this Agreement.
(c) Subject to the terms and conditions of this Agreement, Avenue, its Affiliates, and Sublicensees may: (i) subcontract Development activities with respect to this Agreement to Third Parties, including Third-Party contract research organizations; (ii) subcontract Commercialization activities with respect to this Agreement to Third Parties, including Third-Party contract sales organizations or distributors whose business includes the marketing, distribution or sale of pharmaceutical products; and (iii) subcontract Manufacturing activities with respect to this Agreement to Third Parties, including Third-Party contract development or manufacturing organizations whose business includes the Manufacturing of pharmaceutical products Avenue, its Affiliates, and Sublicensees shall be entitled to grant to its or their subcontractors such sublicenses under the licenses granted under this Agreement to perform the subcontracted activities and no such subcontractors shall be considered Sublicensees for purposes of this Agreement.
2.3 Limited Reservation of Rights. Notwithstanding the exclusive nature of the license granted to Avenue pursuant to Section 2.1, Licensor may conduct research activities pertaining to Licensed Product in the Field in the Territory, but only to the extent agreed upon a charter that will be appended to the Agreement as shown in Exhibit C (“Charter”). For all activities which are not included or captured in the Charter in the Agreement, Licensor shall seek prior approval in writing by Avenue.
2.4 Right of Reference. Avenue hereby grants Licensor a non-exclusive, royalty-free, sublicensable right to reference Avenue Regulatory Materials and Data for the purpose of obtaining and maintaining Regulatory Approvals of Licensed Products outside of the Territory. Licensor hereby grants Avenue a non-exclusive, royalty-free, sublicensable right to reference AnnJi Regulatory Materials and Data for the purpose of obtaining and maintaining Regulatory Approvals of Licensed Products in the Territory.
2.5 Right of First Negotiation. Licensor shall not enter into an agreement with any Third Party to license the Licensed IP with regard to the Development and Commercialization of Licensed Products in the Territory for any Indication or formulation not included in the Field, unless Licensor has first delivered to Avenue written notice offering Avenue to negotiate with Licensor the terms and conditions of an amendment to this Agreement to include such new Indication or formulation outside of the Field, which notice shall identify the specific Indication or formulation for which it would like to enter into such negotiation (Avenue’s such right to negotiate herein referred to as “Right of First Negotiation;” such notice, a “ROFN Notice;” such new indication or formulation, the “Identified New License”)). Avenue shall respond to Licensor within thirty (30) calendar days following its receipt of the ROFN Notice to indicate its interest in exercising its Right of First Negotiation (an “Exercise Notice”). If Avenue causes Licensor to receive such an Exercise Notice exercising its Right of First Negotiation within such thirty (30) calendar day period, then, for a period of up to ninety (90) calendar days following Licensor’s receipt of such Exercise Notice, the Parties shall use Commercially Reasonable Efforts to negotiate in good faith a reasonable agreement granting Avenue rights to Develop and Commercialize Licensed Products in the Identified New License on terms mutually acceptable to both Parties. In the event that: (a) Avenue does not provide an Exercise Notice within thirty (30) calendar days of receipt of the ROFN Notice; or (b) Avenue provides an Exercise Notice to Licensor within such thirty (30) calendar day period, but the Parties do not execute a definitive agreement granting Avenue rights to Develop and Commercialize Licensed Products in the Identified New License within the ninety (90) day negotiation period mentioned above, Avenue shall not have any further rights, and Licensor shall not have any further obligations, under this Section 2.5 and Licensor shall be free to enter into negotiations and/or execute a definitive agreement with any Third Party granting such Third Party the right to Develop and Commercialize Licensed Products in the Identified New License.
2.6 No Implied License. Except as expressly provided in this Section 2.6 or elsewhere in this Agreement, neither Party will be deemed under this Agreement to have been granted any license or other rights to the other Party’s Patent or Know-How, either expressly or by implication, estoppel or otherwise.
|
3. |
Cost-Covering Study; Joint Steering Committee |
3.1 Cost-Covering Study.
(a) Avenue agrees to collaborate with and accordingly requests the service support from Licensor for the performance of the: (i) Phase 1b/2a Clinical Trial (i.e., JM17-201-201 with clinical trial identifier NCT 05517603); and (ii) long-term chronic toxicity studies (six (6)-month GLP in rats (i.e., JM-17-03-23-001) and nine (9)-month GLP in dogs (i.e., JM-17-03-23-002) respectively conducted for the development of Licensed Product in the Territory (“Cost-Covering Study”).
(b) Avenue has approved and agreed to protocols and all operation plans of Cost- Covering Study, including standard operating procedure, provided by Licensor. Cost-Covering Study is projected be completed no later than March 31, 2025. Any extension request should be provided to Avenue with the reason of such request in writing before March 1, 2025.
(c) Unless otherwise provided in Agreement, Licensor shall conduct the Cost Covering Study until it is completed (i.e., issuance of the final clinical study report), and shall, unless otherwise approved by Avenue in writing, complete the Cost-Covering Study on or before March 1, 2025. Licensor shall not terminate conduct of the Cost Covering Study without Avenue’s prior written consent. Without limiting the generality of the foregoing, in connection with conducting the Cost Covering Study, License shall perform the following activities:
(i) For Phase 1b/2a Clinical Trial:
(A) Clinical project management, including:
(1) Vendor management (monitoring, supervising, and communicating with the vendors, which include CRO for Clinical operation, PD assays, PK bioanalysis, to ensure all activities are executed as planned;
(2) Negotiate & finalize contract and budget terms with each site and monitor spending;
(3) Sites management: maintaining open communication with PIs and site staff; conducting on-site visits to ensure site compliance and CRO performance;
(4) Patient engagement, recruitment, and retention;
(5) Overall logistics, such as management and transportation of investigational product, biological samples, and ancillary supplies, and;
(6) Risks detection, mitigation, and management;
(B) Conduct and/or supervise all trainings required;
(C) Develop and review study related documents/materials;
(D) Revise/amend study protocol when necessary and ensure compliance;
(E) Perform query resolution related to regulatory and site startup activities;
(F) Oversee the stability, shipment, and inventory of the investigational product, and;
(G) Review, confirm and ensure proper reporting any AE/SAE or subject safety issues.
(ii) For the GLP chronic toxicity studies:
(A) Protocol review & finalization; including protocol amendment if needed;
(B) Site inspection and/or audit throughout the study period;
(C) Regular monitoring and discussion of study progress and findings as needed;
(D) Analytical method transfers for TK sample analysis;
(E) Raw data review;
(F) Draft report review; and
(G) Report finalization.
(d) In the event that any Know-How is generated in connection with the Cost-Covering Study (“Cost-Covering Study Know-How”), Licensor shall promptly disclose such Cost-Covering Study Know-How to Avenue, including copies of any data, results, or reports generated in connection with the Cost-Covering Study.
(e) For the duration of the Cost-Covering Study, Licensor shall, within thirty (30) calendar days of the end of each Calendar Quarter, deliver a report to Avenue regarding the Cost-Covering Study (each such report, a “Cost-Covering Study Report”). Each Cost-Covering Study Report shall include (at minimum) the following, each in reasonable detail: (i) the status of the Cost-Covering Study and any progress made during the prior Calendar Quarter; (ii) to the extent not previously provided to Avenue, any data or results generated during the prior Calendar Quarter; (iii) any problems or issues with the Cost-Covering Study; and (iv) any other information regarding the Cost-Covering Study reasonably requested by Avenue. Each Cost-Covering Study Report will be presented for discussion at the next meeting of the JSC.
(f) The Cost-Covering Study will be a part of Development Plan, provided that in no event will the Cost-Covering Study be discontinued, suspended, terminated, amended, or otherwise modified except: (i) by the mutual written consent of the Parties; or (ii) to the extent required by a Regulatory Authority.
(g) Licensor shall notify Avenue immediately upon learning of the occurrence of any serious and unexpected adverse event or any other event that Licensor believes may suggest a significant risk to a Cost-Covering Study subject or that may impair the integrity or validity of the Cost-Covering Study. Any such notification shall contain a summary of the serious and unexpected adverse event, shall be made within seventy-two (72) hours of such event, and shall be delivered in accordance with Section 12.2. Licensor shall also provide such additional information as Avenue may reasonably request.
(h) Licensor will keep all scientific records and data related to Licensor’s performance of the Cost-Covering Study, in sufficient detail and in a good scientific and compliant manner appropriate for patent and regulatory purposes, and which will fully and properly reflect all work done and results achieved in the performance of the Cost-Covering Study. Such records will be kept for a period of at least five (5) years after their creation. During the Term, Avenue will have the right, at Avenue’s expense, to appoint an independent third party pre-approved by Licensor in writing, to inspect and copy (or request that Licensor or its Affiliates copy) all records maintained by Licensor or its Affiliates in connection with the work done and results achieved in the performance of the Cost-Covering Study, but solely to the extent to which such records are reasonably necessary to confirm Licensor’s compliance with this Agreement. Inspections pursuant to this Section 3.1(h) shall occur no more often than once per calendar year, shall last no longer than one (1) day, and shall occur during Licensor’s normal business hours.
(i) Licensor shall ensure that the facilities, including any equipment, for the Cost-Covering Study are adequate for the proper conduct of the Cost-Covering Study. Licensor may enter into agreements with third parties, such as laboratory service providers, as may be necessary to perform Cost-Covering Study, but only upon prior written notice to Avenue.
(j) In consideration of the Cost-Covering Study (including the supply of any Licensed Product required for Licensor’s performance of the Cost-Covering Study), Avenue shall pay Licensor the installment payments set forth in Table 3.2(h).
Table 3.2(h)
|
Installment
|
Amount |
Date |
|
1 |
$[***] |
Within 120 Calendar Days of Effective Date |
|
2 |
$[***] |
By December 31, 2023 |
|
3 |
$[***] |
By March 31, 2024 |
|
4 |
$[***] |
By June 30, 2024 |
|
5 |
$[***] |
By September 30, 2024 |
|
6 |
$[***] |
Within 30 calendar days of Avenue’s receipt of the final clinical study report for the Cost-Covering Study |
(k) Within thirty (30) calendar days of XxxXx’s receipt of the final clinical study report for the Cost-Covering Study, Licensor will assign, and hereby does assign, to Avenue the IND applicable to the Cost-Covering Study.
3.2 Joint Steering Committee.
(a) JSC Membership. Promptly, and in any event within sixty (60) calendar days following the Effective Date, the Parties will establish a joint steering committee (the “JSC”) to oversee and coordinate the activities of the Parties under this Agreement with respect to the Development and Commercialization plans of Licensed Product. The JSC shall comprise two (2) employee representatives of Licensor and three (3) representatives of Avenue. Subject to the foregoing, each Party shall appoint its respective representatives to the JSC from time to time, and may change its representatives, in its sole discretion, effective upon notice to the other Party designating such change. One (1) of the members of the JSC appointed by Avenue will be designated the JSC chairperson (the “JSC Chair”). The JSC Chair shall be responsible for calling meetings of the JSC, circulating agenda and performing administrative tasks required to assure efficient operation of the JSC. The JSC may from time to time establish one (1) or more subcommittees (each, a “Subcommittee”), to perform certain duties and exercise certain powers of the JSC as expressly set forth in this Agreement as delegated by the JSC to such Subcommittee (the JSC and any Subcommittee are each referred to herein as a “Committee”). The JSC and each Subcommittee will be promptly disbanded following the end of the last Milestone Event.
(b) JSC Meetings. The JSC will meet once every Calendar Quarter or as otherwise mutually agreed by the Parties. Unless otherwise agreed by the JSC, the JSC shall meet by means of videoconference or other similar means. The JSC Chair or his/her designee will keep minutes of each JSC meeting that record in writing all decisions made, action items assigned or completed and other appropriate matters. The JSC Chair or his/her designee will send meeting minutes to all members of the JSC promptly after a meeting for review. Each member will have five (5) Business Days from receipt in which to comment on and to approve or provide comments to the minutes (such approval not to be unreasonably withheld, conditioned or delayed). If a member, within such time period, does not notify the JSC Chair that he/she does not approve of the minutes, the minutes will be deemed to have been approved by such member. Each Party’s JSC members may designate another staff member of such Party, who will coordinate the administrative work surrounding JSC, including sending the notice of holding JSC meetings, creating the draft of minutes or distributing the minutes.
(c) JSC Functions. The JSC’s responsibilities are as follows:
(i) Overseeing the Development and Commercialization of the Licensed Product(s) hereunder;
(ii) Resolving matters presented to it by any Subcommittee that are within the scope of responsibilities delegated to such Subcommittee by the JSC or otherwise pursuant to this Agreement; and
(iii) Fulfilling such other responsibilities as may be allocated to the JSC under this Agreement or by mutual written agreement of the Parties.
(d) If, despite using reasonable efforts, the JSC does not reach consensus on any matter within its decision-making authority within a period of fourteen (14) days (or such other period as the Parties may agree in writing) after it has met and attempted to reach such consensus, then Avenue shall have the final decision-making authority with respect to such matter.
(e) Scope of Committee Authority. For clarity and notwithstanding the creation of the JSC, each Party will retain the rights, powers and discretion granted to it hereunder, and none of the JSC will be delegated or vested with such rights, powers or discretion unless such delegation or vesting is expressly provided herein, or the Parties expressly so agree in writing. The JSC will not have the power to: (a) resolve any Dispute regarding the existence or amount of any payment owed under this Agreement; or (b) amend, waive or modify any term of this Agreement, and no decision of the JSC will be in contravention of any terms and conditions of this Agreement. It is understood and agreed that issues to be formally decided by the JSC are strictly limited to those specific issues that are expressly provided in Section 3.2(c) and the Disputes which relate to subjects other than those set forth in Section 3.2(c) will be handled according to Section 12.7(c)(iii).
(f) Once a Committee is disbanded, such Committee will have no further obligations under this Agreement, and thereafter, each Party will designate a contact person for the exchange of information under this Agreement. In the event a Committee is disbanded, any decisions that are designated under this Agreement as being subject to the review or approval of such Committee will be made by the Parties directly, subject to the other terms and conditions of this Agreement.
|
4. |
Diligence and Performance |
4.1 Generally. Avenue will use Commercially Reasonable Efforts to Develop and Commercialize the SBMA Product in the Territory, unless stated otherwise herein.
4.2 Milestones for the SBMA Product. Avenue will achieve the following for the SBMA Product (each, a “Diligence Milestone”):
(a) administration of the first dose of SBMA Product to the first patient in a Phase 2/3 Clinical Trial in the United States within four (4) years following the Effective Date;
(b) filing of an MAA for the SBMA Product within six (6) years following the Effective Date; and
(c) First Commercial Sale of the SBMA Product within one (1) year following the first Regulatory Approval of the SBMA Product in any Major Market Country.
If Avenue believes in good faith that it will be unable to timely achieve a specific Diligence Milestone after: (i) consultation with objective, qualified and competent advisor that meets the qualifications set forth in Exhibit G, subject to the approval of each Party, such approval not to unreasonably withheld or delayed; and (ii) such approved advisor’s determination of the existence of Regulatory Cause that inhibits or precludes one or more of the steps necessary to allow the achievement of such Diligence Milestone, then Avenue shall promptly provide Licensor with reasonable documentation supporting the Regulatory Cause, and all Diligence Milestones will automatically be extended by one (1) year after Licensor’s receipt of such documentation. Avenue may elect to further extend the deadline for the achievement of all Diligence Milestones (other than the Diligence Milestone specified in Section 4.2(c)) by up to two (2) successive twelve (12)-month extension periods by making a lump-sum payment to Licensor of $500,000 (an “Extension Payment”) per each such extension period prior to the commencement of each such extension period if (a) Avenue can demonstrate the existence of Regulatory Cause; and (b) Avenue continues to use Commercially Reasonable Efforts to Develop and (subsequent to the First Commercial Sale of the SBMA Product and solely with respect to the Diligence Milestone described in Section 4.2(c)) Commercialize Licensed Products.
4.3 Development of Licensed Products. Subject to the terms and conditions of this Agreement, Avenue shall undertake Development activities to Develop at least one Licensed Product in the Field pursuant to the Development Plans.
4.4 Development Plans. Avenue, its Affiliates, or its Sublicensees shall prepare and update Development Plans for Licensed Products in the Field under this Agreement and Avenue shall share such plans with JSC. Except for the first Development Plan, which shall be provided three (3) calendar months after the Effective Date, an updated Development Plan will be presented by Avenue to the JSC for approval by the JSC at least two (2) months prior to the end of each Calendar Year. Each Development Plan will set forth the plan for Development of each Licensed Product in the Field over at least three (3) Calendar Years and will include: (a) strategies and timelines for Developing and obtaining Approvals for the Licensed Products in the Field in the Territory; and (b) the allocation of responsibilities for Development activities between Avenue, or Third-Party service providers to the extent provided by the applicable Development Plan. For clarity, nothing in this Section 4.4 shall create any rights for Affiliates or Sublicensees to participate in or become members of the JSC.
4.5 Development Reports.
(a) Within sixty (60) calendar days after the end of each Calendar Quarter, Avenue shall provide the JSC with a written report summarizing the material activities undertaken during such Calendar Quarter in connection with each Development Plan, as applicable. Avenue shall cause its Sublicensees to provide all information necessary for such Development reports in accordance with this Section 4.5.
(b) Upon First Commercial Sale of the SBMA Product in the U.S. or EU, Section 4.5(a) shall be automatically amended by replacing each instance of the words “Calendar Quarter” with the words “Calendar Year.”
(c) Avenue’s obligation to provide reports under this Section 4.5 shall terminate upon such time as there are no longer any ongoing Clinical Trials with respect to any Licensed Product.
4.6 Safety Agreement. No later than one year before the First Commercial Sale, the Parties will enter into a written agreement setting forth worldwide safety and pharmacovigilance procedures for the Parties with respect to the Licensed Products (the “Safety Agreement”). The Safety Agreement will describe the obligations of both Parties with respect to the coordination of collection, investigation, reporting, and exchange of information between the Parties concerning any adverse event experienced by a subject or, in the case of non-clinical studies, an animal in a toxicology study, and the seriousness thereof, whether or not determined to be attributable to any Licensed Product, including any such information received by either Party from a Third Party (subject to receipt of any required consents from such Third Party) and will be sufficient to permit each Party and its Affiliates, licensees, or Sublicensees (as applicable) to comply with its legal obligations with respect thereto, including the obligations as the owner or holder of Regulatory Approvals and Pricing Approvals for such Licensed Product as applicable. The Safety Agreement will also detail each Party’s responsibilities with respect to recalls and withdrawals of the Licensed Products inside and outside of the Territory. If required by changes in Applicable Law, the Parties will make appropriate updates to the Safety Agreement. Each Party will comply with its respective obligations under the Safety Agreement and cause its Affiliates, licensees, and Sublicensees to comply with such obligations. Notwithstanding any provision to the contrary in this Agreement or the Safety Agreement, each Party and its Affiliates, licensees, and Sublicensees will have the right to disclose information related to the safety of one or more Licensed Products to the extent that such disclosure is required for such Party to comply with its obligations under Applicable Law or the safety requirements of the applicable Regulatory Authorities. The Parties will cooperate with each other to address any safety-related inquiries or requests for safety assessment by any Regulatory Authority, including providing any necessary data or information in a timely manner. To the extent that there is a conflict between the terms of this Agreement and the terms of the Safety Agreement, the terms of the Safety Agreement will govern with respect to the subject matter set forth therein.
4.7 Commercial Supply. Upon Avenue’s written request, which shall be no sooner than one hundred eighty (180) calendar days prior to the date Avenue, its Affiliates, and Sublicensees reasonably expects to file its first MAA for a Licensed Product (the “Commercial Agreement Request”), the Parties shall negotiate in good faith and enter into a supply agreement (the “Commercial Supply Agreement”) pursuant to which Licensor shall Manufacture and supply to Avenue, its Affiliates and Sublicensees commercial quantities of finished, packaged Licensed Products for sale and distribution in the Territory. Such Commercial Supply Agreement shall: (a) provide that the purchase price for Licensed Product supplied thereunder shall be no more than one hundred thirty percent (130%) of Licensor’s Cost of Goods; and (b) contain reasonable and customary commercial supply terms, including provisions addressing forecasting and ordering, delivery, payment, acceptance and rejection procedures, regulatory assistance, warranties, indemnification, limitations of liability and quality assurance and control. In the event that the Parties fail to execute the Commercial Supply Agreement within one hundred eighty (180) calendar days of the Commercial Agreement Request, then any terms of the inchoate Commercial Supply Agreement on which the Parties have not mutually agreed (such terms, “Open Terms”) shall be submitted for resolution via binding arbitration upon the expiration of such one hundred eighty (180)-day period, as described in Exhibit H.
4.8 Commercial Launch Plan. Prior to the First Commercial Sale of a Licensed Product in a Major Market Country, Avenue or its Sublicensees will furnish Licensor with a plan summarizing Avenue or its Sublicensees’ launch strategy for such Licensed Product in such Major Market Country.
4.9 Manufacturing Rights.
(a) Upon: (i) Avenue’s payment of the Manufacturing Rights Payment, which payment shall be made upon six (6) months’ prior written notice to Licensor, and which notice may be provided by Avenue at any time after the filing of an MAA for a Licensed Product by Avenue, its Affiliate, or Sublicensees; or (ii) the occurrence of a Manufacturing Rights Trigger, in either case ((i) or (ii)), this Agreement will automatically be amended as set forth in Section 4.9(b) and Section 4.9(c), and Licensor will provide (at Avenue’s request and expense) reasonable assistance in enabling Avenue or its Affiliates, Sublicensees, or vendors to Manufacture Licensed Product, including by transferring all applicable Know-How relating to Manufacturing with additional one-time cash payment of $5,000,000. For clarity, Licensor shall have no obligation to commence any such transfer of Know-How prior to receipt of the Manufacturing Rights Payment, if applicable, and such $5,000,000 payment pursuant to this Section 4.9(a).
(b) Upon: (i) Avenue’s payment of the Manufacturing Rights Payment; or (ii) the occurrence of a Manufacturing Rights Trigger, in either case ((i) or (ii)), Section 2.1 shall be amended and restated in its entirety as follows: “Subject to the terms and conditions of this Agreement, Licensor hereby grants to Avenue, and Avenue hereby accepts, an exclusive, royalty-bearing, transferrable (pursuant to Section 12.4), and sublicensable (in accordance with Section 2.2) license, under the Licensed IP, to Develop, Commercialize, Manufacture, use, sell, offer for sale, and import and export the Licensed Products in the Field in the Territory.”
(c) Upon: (i) Avenue’s payment of the Manufacturing Rights Payment; or (ii) the occurrence of a Manufacturing Rights Trigger, in either case ((i) or (ii)), Section 1.92 shall be amended and restated in its entirety as follows: “Licensed Know-How” means (a) all Know-How Controlled by Licensor or its Affiliates as of the Effective Date and that is or would be reasonably necessary or useful to Develop, Commercialize, or otherwise use Licensed Products; (b) all Cost-Covering Study Know-How; and (c) all Know How Controlled by Licensor or its Affiliates as of the occurrence of the events described clauses (i) or (ii) of Section 4.9(c), as applicable, that is necessary to Manufacture the Licensed Products.”
4.10 Supply Issues.
(a) Upon the occurrence of any Supply Issue, the Parties will meet as promptly as possible, but in any event within fifteen (15) calendar days, to determine whether the occurrence of such Supply Issue was due to either: (i) Regulatory Cause; or (ii) reasons other than Regulatory Cause. If the Parties cannot mutually agree on such determination within thirty (30) Business Days, then Avenue shall appoint an independent expert on the manufacturing of pharmaceutical products in the applicable jurisdiction(s) (the “Supply Expert”), which appointment shall be subject to Licensor’s reasonable approval, and the Supply Expert shall promptly make such determination. The Parties will cooperate with any reasonable request of the Supply Expert in making such determination, and such determination shall be final and binding upon the Parties.
(b) If the Supply Expert determines pursuant to Section 4.10(a) that the occurrence of a Supply Issue was due to reasons other than Regulatory Cause, then the Supply Expert shall monitor and advise both Parties on Licensor’s efforts to rectify such Supply Issue for at least two (2) Calendar Quarters.
(c) If the Parties mutually agree pursuant to Section 4.10(a) that the occurrence of a Supply Issue was due to reasons other than Regulatory Cause, then Avenue shall appoint a Supply Expert, which appointment shall be subject to Licensor’s reasonable approval, and the Supply Expert shall monitor and advise both Parties on Licensor’s efforts to rectify such Supply Issue for at least two (2) Calendar Quarters.
(d) With respect to any monitoring by the Supply Expert pursuant to Sections 4.10(b) or 4.10(c), Licensor shall: (i) reasonably cooperate with the Supply Expert; (ii) allow the Supply Expert access to Licensor’s facilities and personnel; (iii) upon the Supply Expert’s request, cause any relevant third parties to allow the Supply Expert the same level of access and information to which Licensor is be entitled; and (iv) reasonably consider any suggestions of the Supply Expert regarding the resolution of the Supply Issue.
(e) The appointment of the Supply Expert will be conditioned upon the Supply Expert’s execution of a customary nondisclosure agreement, and all communications between either Party and the Supply Expert shall be deemed the Confidential Information of both Parties. Licensor shall be responsible for the reasonable costs of retaining the Supply Expert pursuant to this Section 4.10.
4.11 Restriction on Bundling in the Territory. If Avenue, its Affiliates, or its Sublicensees sell a Licensed Product in the Field in the Territory to a customer who also purchases other products or services from any such entity, Avenue agrees not to, and to require its Affiliates and Sublicensees not to, bundle or include any Licensed Product as part of any multiple product offering or discount or price the Licensed Products in a manner that is reasonably likely to disadvantage a Licensed Product in order to benefit sales or prices of other products offered for sale by a Party or its Affiliates to such customer.
4.12 Market Exclusivity Extensions. Avenue, its Affiliates, or its Sublicensees shall use Commercially Reasonable Efforts to maintain, and, to the extent available, legally extend, the period of time during which, in any Major Market Country or Canada: (a) any Party has the exclusive legal right, whether by means of a patent right or through other rights granted by a Governmental Authority in such country, to Commercialize a Licensed Product in the Field in such country; and (b) no Generic Product is marketed in such country.
4.13 Restriction on Competition.
(a) During the Term, neither Avenue nor any of its respective Subsidiaries or Sublicensees, either alone or through any Third Party directly or indirectly, shall Develop or Commercialize any Competing Product in the Field in the Territory without Licensor’s written consent. In the event that the Agreement terminates for any legitimate reason, then for a period of eighteen (18) months after such termination, Avenue, its Subsidiaries, or Sublicensees shall not, directly or indirectly, Develop or Commercialize any Competing Product in the Field in any part of the Territory.
(b) During the Term, Licensor, its Affiliates, or its Sublicensees shall not, directly or indirectly, Develop or Commercialize the Licensed Product in Alzheimer’s Disease or androgenic alopecia in the Territory without Avenue’s written consent. In the event that the Agreement terminates for any legitimate reason, then for a period of eighteen (18) months after such termination, Licensor, its Affiliates, or its Sublicensees shall not, directly or indirectly, Develop or Commercialize the Licensed Product in Alzheimer’s Disease or androgenic alopecia in the Territory.
4.14 Restriction on Parallel Import. Avenue shall neither, and shall require its Affiliates and Sublicensees to neither, whether directly or indirectly through a Third Party: (a) sell or promote a Licensed Product outside of the Field or outside of the Territory; nor (b) export or distribute a Licensed Product outside of the Territory other than for Commercialization in the Field in the Territory.
4.15 Records and Information. Avenue and its Affiliates will keep scientific records related to Avenue’s Development and (if applicable) Manufacturing efforts with respect to Licensed Product in the Field, in sufficient detail and in a good scientific and compliant manner appropriate for patent and regulatory purposes, and which will fully and properly reflect all work done and results achieved in the performance of the Development and (if applicable) Manufacturing with respect to the Licensed Product in the Field under this Agreement. Such records will be kept for a period of at least five (5) years after their creation, or such longer period as required by Applicable Law. During the Term, Licensor will have the right, at Licensor’s expense, to appoint an independent third party pre-approved by Avenue in writing, to inspect and copy (or request that Avenue or its Affiliates copy) all records maintained by Avenue or its Affiliates in connection with the work done and results achieved in the performance of Development and (if applicable) Manufacturing under this Agreement, but solely to the extent to which such records are reasonably necessary to confirm Avenue’s compliance with this Agreement. Inspections pursuant to this Section 4.15 shall occur no more often than once per calendar year, shall last no longer than one (1) day, and shall occur during Avenue’s normal business hours.
|
5. |
Clinical Supply |
5.1 Purchase and Sale of Product. During the Term of this Agreement, Licensor shall (a) have Licensed Product Manufactured; and (b) sell and deliver to Avenue one hundred percent (100%) of the total requirements for Licensed Products in the Territory of Avenue, its Affiliates and Sublicensees for any Development activities after Phase 1b/2a Clinical Trial, including, Phase 2/3 Clinical Trial and Phase 3 Clinical Trial. The Parties agree that additional details regarding such clinical supply will be agreed in a separate clinical supply agreement, consistent with the terms and conditions set forth in Exhibit F, to be negotiated in good faith and executed by the Parties no later than March 31, 2024 (the “Clinical Supply Agreement”).
|
6. |
Financial Terms |
6.1 Upfront Payment. Avenue shall pay to Licensor: (a) $2,000,000 within sixty (60) calendar days of the Effective Date; and (b) $1,000,000 within one hundred eighty (180) calendar days following the Effective Date.
6.2 Milestones. Subject to the terms of this Section 6.2 and Section 6.5, upon the achievement of each milestone event set forth in the columns labeled “Milestone Event” in Table 6.2(a) and Table 6.2(b) (each such event, a “Milestone Event”), with respect to the Licensed Product achieving such Milestone Event under this Agreement, Avenue shall provide Licensor with a written notice within ten (10) calendar days of the achievement of a Milestone Event. Avenue shall pay to Licensor the corresponding amounts set forth beside such Milestone Event in the columns labeled “Milestone Payment” in Table 6.2(a) and Table 6.2(b) (each such amount, a Milestone Payment”). Notwithstanding the foregoing, in the event that any Milestone Events occur prior to the execution of the Clinical Supply Agreement, the corresponding Milestone Payments shall not be owed or payable to Licensor unless and until the Parties execute the Clinical Supply Agreement, whereupon any and all such Milestone Payments will be due to Licensor within thirty (30) days of such execution.
(a) Development Milestones.
Table 6.2(a)
|
Milestone Event |
Milestone |
|
(i) LPLD of a Clinical Trial in the United States sponsored by or on behalf of Avenue |
$[***] |
|
(ii) Thirty (30) days after filing an IND with the FDA for the first Registrational Trial in the United States |
$[***] |
|
(iii) FPFD of a Registrational Trial in the United States sponsored by or on behalf of Avenue |
$[***] |
|
(iv) FPFD of a Registrational Trial in the European Union sponsored by or on behalf of Avenue |
$[***] |
|
(v) First submission by Avenue or Sublicensee of an NDA for a Licensed Product to the FDA |
$[***] |
|
(vi) Following First FDA Approval, the first Submission by Avenue or Sublicensee of an sNDA to the FDA with respect to a New Indication and/or new target population (the “First Licensed Product sNDA”) |
$[***] |
|
(vii) First FDA approval of an NDA submitted by Avenue or Sublicensee with respect to a Licensed Product (“First FDA Approval”) |
$[***] |
|
(viii) FDA approval of the First Licensed Product sNDA |
$[***] |
|
(ix) FDA approval of an NDA submitted by Avenue or Sublicensee with respect to a second Licensed Product |
$[***] |
|
(x) First submission by Avenue or Sublicensee of an MAA for a Licensed Product to the EMA |
$[***] |
|
(xi) Following First EMA Approval, the first Submission by Avenue or Sublicensee of an MAA to the EMA for a Label Expansion (“First Product EMA Label Expansion”) |
$[***] |
|
(xii First EMA approval of an MAA submitted by Avenue or Sublicensee with respect to a Licensed Product (“First EMA Approval”) |
$[***] |
|
(xiii) EMA approval of the Licensed Product EMA Label Expansion |
$[***] |
|
(xiv EMA approval of an MAA by Avenue or Sublicensee with respect to a second Licensed Product |
$[***] |
|
(xv) EMA approval of an MAA or FDA approval of an NDA, in each case by Avenue or Sublicensee, for which Avenue or its Sublicensee has received Breakthrough Designation, Accelerated Approval, or Priority Review |
$[***] |
(b) Commercial Milestones.
Table 6.2(b)
|
Milestone Event |
Milestone |
|
(i) Annual Net Sales equal to or greater than $75,000,000 and less than $125,000,000 |
$[***] |
|
(ii) Annual Net Sales equal to or greater than $125,000,000 and less than or equal to $200,000,000 |
$[***] |
|
(iii) Annual Net Sales equal to or greater than $200,000,000 and less than or equal to $300,000,000 |
$[***] |
|
(iv) Annual Net Sales equal to or greater than $300,000,000 and less than or equal to $500,000,000 |
$[***] |
|
(v) Annual Net Sales equal to or greater than $500,000,000 and less than or equal to $750,000,000 |
$[***] |
|
(vi) Annual Net Sales equal to or greater than $750,000,000 |
$[***] |
In the event more than one of the Milestone Events set forth in Table 6.2(b) is met during a single Calendar Year, the highest milestone will be paid in that Calendar Year and other earned but unpaid milestones will be accrued and paid out in the subsequent Calendar Year.
(c) Each Milestone Payment shall be payable a maximum of one (1) time as set forth in Table 6.2(a) and Table 6.2(b), regardless of the number of times the applicable Milestone Event is achieved (i.e., a maximum of fifteen (15) Milestone Payments may be made pursuant to Section 6.2(a) and a maximum of six (6) Milestone Payments may be made pursuant to Section 6.2(b), and no Milestone Payment shall be due hereunder for subsequent or repeated achievement of any such Milestone Event).
(d) Payment of Milestone Payments. Avenue shall pay Licensor a Milestone Payment within thirty (30) calendar days following the date that the applicable Milestone Event has been achieved.
6.3 Royalties.
(a) Royalty Rates. Subject to the terms of this Section 6.3 and Section 6.5, Avenue shall pay Licensor royalties on Annual Net Sales during the applicable Royalty Term equal to the Annual Net Sales of the applicable Licensed Product multiplied by the applicable royalty rate in the column titled “Royalty Rate” in Table 6.3(a) which is set forth beside such portion of Annual Net Sales during the applicable Royalty Term for each such Licensed Product (the “Licensed Product Annual Net Sales”), which royalties shall be paid in accordance with Section 6.3(d).
Table 6.3(a)
|
Licensed Product Annual Net Sales |
Royalty |
|
(i) Aggregate Annual Net Sales less than or equal to $50,000,000 |
[***]% |
|
(ii) Aggregate Annual Net Sales equal to or greater than $50,000,000 and less than $150,000,000 |
[***]% |
|
(iii) Aggregate Annual Net Sales equal to or greater than $150,000,000 and less than $300,000,000 |
[***]% |
|
(iv) Aggregate Annual Net Sales equal to or greater than $300,000,000 |
[***]% |
(b) Royalty Term. Avenue’s royalty obligations to Licensor under Section 6.3(a) shall apply, on a country-by-country and Licensed Product-by-Licensed Product basis, only during the applicable Royalty Term for such Licensed Product in such country. Following the expiration of the applicable Royalty Term for a given Licensed Product in a given country: (a) no further royalties shall be payable with respect to sales of such Licensed Product in such country; and (b) Section 11.2 shall apply with respect to such Licensed Product in such country.
(c) Royalty Reductions. In the event Avenue reasonably determines that the research, development, commercialization or other exploitation of a Licensed Product under this Agreement would infringe or misappropriate a Third Party’s Patents or Know-How absent a license from such Third Party (each such Third-Party license is referred to herein as a “Third-Party License”), Avenue shall have the right to deduct [***] percent ([***]%) of any royalties or similar payments paid to any such Third Party for a license to such Patents or Know-How (such consideration, “Third-Party Royalties”) from any payments due to Licensor under this Agreement, provided that such amounts payable to Licensor would not be reduced, with respect to any Calendar Quarter, below [***] percent ([***]%) of the amounts otherwise due to Licensor with respect to such Calendar Quarter without such offset. Any Third-Party Royalties not deducted in a Calendar Quarter would be carried forward to subsequent Calendar Quarters until deducted.
(d) Payment of Royalties and Sublicense Revenue Report. Avenue shall: (a) within sixty (60) calendar days following the end of each Calendar Quarter in which a royalty payment pursuant to Section 6.3(a) or Section 6.4 accrues, provide to Licensor a report specifying, for such Calendar Quarter (i) the Annual Net Sales of Licensed Products that are subject to such royalty, (ii) the applicable royalty rate under Section 6.3(a), (iii) the royalty calculation and royalties payable in Dollars, (iv) any reduction(s) to the royalty applied by Avenue pursuant to Section 6.3(c), and (v) the Sublicensing Revenue generated during such Calendar Quarter (if any) and a calculation of the Sublicensing Revenue Payment owed by Avenue as a result; (b) make the royalty payments owed to Licensor that are attributable to Avenue’s Net Sales within sixty (60) calendar days from the end of the Calendar Quarter in which such payment accrues; and (c) make the royalty payments owed to Licensor that are attributable to Net Sales by Sublicensees, and Sublicensing Revenue Payments owed to Licensor under this Agreement in accordance with such royalty report, in arrears, within fifteen (15) calendar days after Avenue’s receipt of such amounts from its Sublicensees, but in no event more than ninety (90) calendar days after the expiration of such Calendar Quarter.
6.4 Sublicense Revenue. Subject to the terms of this Section 6.4 and Section 6.5, Avenue shall pay Licensor the applicable percentage of Sublicense Revenue received from a Sublicensee based on the effective date (the “Sublicense Date”) of the agreement under which Sublicense Revenue is received by Avenue, as set forth in Table 6.4 (each such payment, a “Sublicensing Revenue Payment”).
Table 6.4
|
Sublicense Date |
Sublicensing |
|
(a) Prior to First Commercial Sale of any Licensed Product in the U.S. or EU |
10% |
|
(b) On or after First Commercial Sale of any Licensed Product in the U.S. or EU and prior to the fifth (5th) anniversary of the First Commercial Sale of any Licensed Product in the U.S. or EU |
5% |
|
(c) On or after the fifth (5th) anniversary of First Commercial Sale of any Licensed Product in the U.S. or EU |
2.5% |
6.5 Additional Payment Terms.
(a) Currency; Conversion. All payments hereunder shall be made in Dollars by wire transfer to a bank designated in writing by Licensor no later than the date by which the applicable payment must be made. Conversion of sales recorded in local currencies to Dollars shall be performed at the exchange rate stated in The Wall Street Journal, New York Edition at the close of the last Business Day of the Calendar Quarter to which such royalty payment relates.
(b) Taxes; Withholding.
(i) Generally. Each Party shall pay any and all income taxes levied on account of all payments it receives under this Agreement, except as otherwise provided in this Section 6.5(b).
(ii) Tax Withholding. Each Party shall be entitled to deduct and withhold from any amounts payable under this Agreement such taxes as are required to be deducted or withheld therefrom under any provision of Applicable Law. The Party that is required to make such withholding shall: (A) deduct those taxes from such payment; (B) timely remit the taxes to the proper taxing authority; and (C) send evidence of the obligation, together with proof of tax payment, to the other Party on a timely basis following such tax payment. Each Party shall reasonably cooperate with the other Party in claiming refunds or exemptions from such deductions or withholdings under any relevant agreement or treaty which is in effect to ensure that any amounts required to be withheld pursuant to this Section 6.5(b)(ii) are reduced in amount to the fullest extent permitted by Applicable Law. In addition, the Parties shall cooperate in accordance with Applicable Law to minimize indirect taxes (such as value added tax, sales tax, consumption tax, and other similar taxes) in connection with this Agreement.
(c) Late Payments. Avenue agrees that all payments or portions thereof required to be paid by Avenue to Licensor under this Agreement which are not made when due shall accrue simple interest, to the extent permitted by Applicable Law, from the date due until paid, at a rate equal to the prime rate, as quoted in The Wall Street Journal (New York Edition) on the date on which the payment was due, plus two percent (2%), in each case, calculated on the number of days such payment is delinquent, compounded monthly.
(d) Foreign Currency Exchange. For any currency conversion from the currency of one country in which Licensed Products are sold into United States dollars (or another currency if applicable) required in determining the amount of Net Sales or any Royalties due hereunder, such conversion shall be calculated at the conversion rate as reported in The Wall Street Journal (New York Edition) (or if that is no longer published, the interbank rate quoted by Citibank, N.A.) on the last Business Day of the applicable quarterly period in which the Net Sales are determined.
6.6 Records; Audit Rights.
(a) Records. Avenue shall keep complete, true, and accurate books and records in accordance with its Accounting Standards in relation to this Agreement in relation to Net Sales, royalties, Sublicense Revenue, and Milestone Payments for at least four (4) years following the Calendar Year to which they pertain or for such longer period of time as required under any Applicable Law. Avenue shall cause each Selling Party to keep complete, true, and accurate books and records in accordance with its Accounting Standards in relation to this Agreement in relation to Net Sales, royalties, Sublicense Revenue, and Milestone Payments for at least three (3) years following the Calendar Year to which they pertain or for such longer period of time as required under any Applicable Law.
(b) Audit Rights. Subject to the other terms of this Section 6.6(b), during the Term and for a period of four (4) years thereafter, at the request of Licensor, which shall not be made more frequently than one (1) time per Calendar Year other than for cause, upon at least thirty (30) days’ prior written notice from Licensor, and at the expense of Licensor, Avenue shall permit an independent, nationally-recognized certified public accountant selected by Licensor and reasonably acceptable to Avenue (each, an “Auditor”) to inspect, during regular business hours, the relevant records required to be maintained by Avenue under Section 6.6(a). Prior to its inspection, the Auditor shall enter into a confidentiality agreement with both Parties having obligations of confidentiality and non-use with respect to the Confidential Information no less restrictive than those set forth in Section 8 and limiting the disclosure and use of such information by the Auditor to authorized representatives of the Parties and the purposes germane to Section 6.6(a). Results of any such review shall be binding on both Parties absent manifest error. Licensor shall treat the results of any Auditor’s review of Avenue’s records as Confidential Information of Avenue subject to the terms of Section 8. In the event such audit leads to the discovery of a discrepancy to Licensor’s detriment, Avenue shall, within thirty (30) days after receipt of such report from the Auditor, pay any undisputed amount of the discrepancy. Licensor shall pay the full cost of the audit unless the underpayment of amounts due by Avenue is greater than five percent (5%) of the amount due for the entire period being examined, in which case Avenue shall pay the reasonable cost charged by the Auditor for such review. Any undisputed overpayments by Avenue revealed by an examination shall be paid by Licensor within thirty (30) calendar days of Licensor’s receipt of the applicable report. This Section 6.6(b) shall survive any expiration or termination of this Agreement.
6.7 Equity.
(a) Avenue shall, within thirty (30) days after the Effective Date, issue to Licensor a number of shares of Avenue common stock (the “Common Stock”) equal to fifteen percent (15%) of the Issued and Outstanding Equity Securities of Avenue, determined as of the Effective Date, subject to Licensor’s execution of a subscription agreement in the form attached hereto as Exhibit D (the “Subscription Agreement”).
(b) Within sixty (60) days following enrollment of the eighth (8th) patient in the first Phase 1b/2a Clinical Trial in the United States, Avenue shall issue to Licensor a number of shares of Common Stock equal to four and ninety-nine hundredths percent (4.99%) of the Issued and Outstanding Equity Securities of Avenue, as determined as of the Effective Date, subject to Licensor’s execution of the Subscription Agreement.
(c) In connection with the issuance of the shares of Common Stock pursuant to this Agreement, Avenue and Licensor shall enter into a registration rights agreement (the “Registration Rights Agreement”) in the form attached hereto as Exhibit E. For the avoidance of doubt, the Registration Rights Agreement shall provide for the registration of all shares of Common Stock issuable by Avenue to Licensor pursuant to this Agreement and any other shares of capital stock of Avenue distributed to Licensor as a result of their ownership thereof.
(d) In the event that the shares of Common Stock cease to be traded on the NASDAQ Stock Market or another national securities exchange at any time following the Effective Date, Licensor shall have the right, which shall not be exercised more than two (2) times, but not the obligation, to cause Avenue to purchase or compensate Licensor for all or a portion of the shares of Common Stock held by Licensor in accordance with this Section 6.7(d) (the “Put Right”) within thirty (30) days of such cessation of trading that is continuing, by providing Avenue with written notice of Licensor’s exercise of the Put Right, which notice must specify the number of shares of Common Stock Licensor desires to sell (the “Put Shares”). Provided that Licensor uses commercially reasonable efforts to sell all Put Shares at prevailing prices within the ninety (90)-day period following delivery of such notice, Avenue shall, upon the expiration of such ninety (90)-day period: (i) purchase any remaining Put Shares held by Licensor at a price per share equal to the greater of (A) the Price Per Share, or (B) $2.10 per share, provided in either case ((A) or (B)) that such purchase of Put Shares shall be paid solely in cash and shall be subject to the execution of a customary purchase agreement with representations and warranties from Licensor limited to power, title and due authority; (ii) with respect to any Put Shares sold by Licensor during such ninety (90)-day period at a price of less than $2.10 per share, pay to Licensor a cash amount equal to difference between (A) the aggregate consideration Licensor would have received had all such Put Shares been sold at a price of $2.10 per share, and (B) the aggregate consideration actually received by Licensor for the sale of all such Put Shares; and (iii) reimburse Licensor for reasonable, documented, out-of-pocket expenses actually incurred by Licensor in connection with the sale of Put Shares during such ninety (90)-day period, up to a maximum of 6% of the aggregate consideration actually received by Licensor for the sale of such Put Shares. Notwithstanding the foregoing, Avenue shall have no liability to Licensor, and shall not be obligated to repurchase any shares, under this Section 6.7(d) if such repurchase would (x) cause the Company to violate Section 160 of the Delaware General Corporation Law (the “DGCL”) or (y) result in the imposition of any liability on any director or officer of the Company under Sections 172 or 174 of the DGCL.
(e) For so long as Licensor holds shares of Common Stock greater than or equal to three percent (3%) of the Issued and Outstanding Equity Securities of Avenue as of any given time, Avenue shall, subject to the Observer, as defined herein, executing a confidentiality agreement with Avenue: (i) permit one (1) individual nominated by Licensor (the “Observer”) to attend all meetings of the Board of Directors of Avenue (excluding any committee or subcommittee meetings thereof); and (ii) concurrently with delivery to the Board of Directors of Avenue, give the Observer copies of all notices, minutes, consents, and other material that Avenue provides to its directors, except that the Observer may be excluded from access to any material or meeting or portion thereof if the Board of Directors of Avenue reasonably determines in good faith, upon advice of counsel, that such exclusion is reasonably necessary to preserve the attorney-client privilege, to protect highly confidential proprietary information, or for other similar reasons. Upon reasonable notice and at a scheduled meeting of the Board of Directors of Avenue or such other time, if any, as the Board of Directors of Avenue may determine in its sole discretion, such Observer may address the Board of Directors of Avenue with respect to Observer’s concerns regarding significant business issues facing Avenue.
7. Intellectual Property
7.1 Ownership. Subject to Section 2.1, ownership of all Inventions arising from the Parties’ activities under this Agreement, including any Patents covering such Inventions, will be determined by inventorship. As between Licensor and Avenue, each Party shall own any Inventions made solely by its own employees, agents, or independent contractors in the course of conducting any activities under this Agreement (all such Inventions, “Sole Inventions” and all Patents that claim such Sole Inventions, “Sole Patents”). All such Inventions conceived or reduced to practice jointly by the Parties will be jointly owned (all such Inventions, “Joint Inventions” and all Patents that claim such Joint Inventions, “Joint Patents”). The determination of whether information and Inventions are conceived, discovered, developed, or otherwise made by a Party for the purpose of allocating proprietary rights (including Patent, copyright, Know-How, etc.) therein, shall, for purposes of this Agreement, be made in accordance with Applicable Law in the United States as such law exists as of the Effective Date irrespective of where such conception, discovery, development or making occurs. Subject to the terms and conditions of this Agreement, Avenue hereby grants to Licensor, and Licensor hereby accepts, a non-exclusive, royalty-free, fully paid-up, transferrable (solely in connection with a permitted assignment or transfer of this Agreement pursuant to Section 12.4), and fully sublicensable license during the Avenue Sole Invention License Term to use and practice any Sole Inventions and Sole Patents Controlled by Avenue to the extent reasonably necessary or useful to sell, offer for sale, import and export, distribute and otherwise Manufacture, Develop, and Commercialize products, provided that such products are not offered in the Field. With respect to any sublicense of the license granted to Licensor pursuant to this Section 7.1: (a) such sublicense must be consistent with the terms of this Agreement applicable to the exercise of rights under such sublicense; (b) the grant of any sublicense shall not relieve Licensor of its obligations under this Agreement; and (c) Licensor or its Affiliates shall remain responsible for the compliance of sublicensees with all terms and conditions of this Agreement relevant to such sublicense. The license granted to Licensor pursuant to this Section 7.1 shall terminate upon the expiration or earlier termination of this Agreement.
7.2 Disclosure of Inventions. Each Party shall promptly disclose to the other Party any Inventions that such Party believes are patentable Joint Inventions.
7.3 Prosecution and Maintenance.
(a) As between the Parties, Avenue shall use Commercially Reasonable Efforts to handle all Prosecution and Maintenance of the Licensed Patents, at its sole cost and expense and through counsel of its choice, provided that, with respect to all validity, enforceability and other material prosecution matters (including decisions related to nullity, reissue, opposition and re-examination proceedings), Avenue shall provide Licensor with a reasonable opportunity to review and comment on material communications from any patent authority regarding the Licensed Patents and drafts of any material filings or responses to be made to such patent authorities in advance of submitting such filings or responses. Avenue shall consider Licensor’s comments regarding such communications and drafts in good faith. If, as between the Parties, Avenue decides not to Prosecute and Maintain a Licensed Patent in a country in the Territory, Avenue shall provide reasonable prior written notice to Licensor of such intention explicitly and clearly at least sixty (60) days prior to the date upon which such Patent will lapse or become abandoned and, Licensor shall thereupon have the right, in its sole discretion, to assume the Prosecution and Maintenance of such Licensed Patent at its sole cost and expense in such country.
(b) Each Party shall assist the other Party at the reasonable request of the other Party from time to time in connection with its Prosecution and Maintenance activities set forth in Section 7.3(a). The Party that has the right to Prosecute and Maintain a Licensed Patent (the “Prosecuting Party”) shall: (i) keep the other Party (the “Non-Prosecuting Party”) informed of all steps to be taken in the preparation and prosecution of all applications filed by it pursuant to Section 7.3(a); (ii) furnish the Non-Prosecuting Party with copies of such applications for Patents, amendments thereto and other related correspondence to and from patent offices, including correspondence relating to any office actions; and (iii) to the extent reasonably practicable, permit the Non-Prosecuting Party an opportunity to offer its comments on such applications, amendments and other correspondence before making a submission to a patent office, which comments the Prosecuting Party shall consider in good faith. The Non-Prosecuting Party shall offer its comments, if any, promptly.
(c) Except as otherwise provided in this Section 7.3(c), Avenue shall have the primary right and responsibility to prepare, file, prosecute and maintain Joint Patents on a worldwide basis, and such costs shall be shared equally between the Parties. The Parties shall confer and mutually agree to a filing strategy, including the jurisdictions in which to Prosecute and Maintain a patent application, and Avenue shall provide Licensor a reasonable opportunity to review and comment on material communications from any patent authority regarding the Joint Patents and drafts of any material filings or responses to be made to such patent authorities in advance of submitting such filings or responses. Avenue shall consider Licensor’s comments regarding such communications and drafts in good faith. In the event that Avenue elects not to continue the Prosecution and Maintenance of a Joint Patent in any country, then Avenue shall provide Licensor with written notice of such determination at least sixty (60) calendar days prior to the date upon which such Joint Patent will lapse or become abandoned and, Licensor shall thereupon have the right, in its sole discretion, to assume the Prosecution and Maintenance of such Joint Patent at its sole cost and expense in such country.
7.4 Enforcement.
(a) Each Party shall promptly notify the other Party in writing if it becomes aware of infringement or a Patent challenge by a Third Party of any Licensed Patent or Joint Patent in the Territory, including any declaratory judgment, opposition, post grant review, inter partes review, or similar action alleging the invalidity, unenforceability, unpatentability, or non-infringement with respect to such Licensed Patent, including under any regulatory filing based on Section 351(k) of the Public Health Service Act (42 U.S.C. § 262), or Section 10(4) of the Directive 2001/83/EC, or any other similar regulation promulgated by the FDA, EMA, or by other applicable similar Governmental Authority or other actual or potential infringement or Patent challenge by a generic, or potential generic competitor anywhere in the Territory (collectively, “Competing Infringement”).
(b) Avenue, its Affiliate, or its Sublicensee shall have the first right, but not the obligation, to bring and control any legal action or take such other actions as it reasonably deems appropriate (including responding to Third-Party notice letters and controlling settlements subject to the terms in this Section 7.4), which may include the granting of licenses and authorizing the launch of generic products in connection with any Competing Infringement of any Licensed Patent, at its cost and expense with counsel of its choice. Prior to commencing any such action, Avenue, its Affiliate, or its Sublicensee shall consult with Licensor and shall give due consideration to Licensor’s recommendations regarding the proposed action. Avenue shall cause Licensor to receive timely notice of any proposed settlement of any such action instituted by Avenue or the others and shall not, without the prior written consent of Licensor (which shall not be unreasonably withheld or delayed), enter into any settlement that would: (i) adversely affect the validity, enforceability or scope of any of the Licensed Patent or Joint Patent; (ii) give rise to liability of Licensor or its Affiliates; (iii) admit non-infringement of any Licensed Patent or Joint Patent; or (iv) otherwise impair Licensor’s rights in any Licensed Patent, Joint Patent, or under this Agreement. At the request and expense of Avenue, Licensor shall provide reasonable assistance in connection with Avenue’s legal or other actions in connection with any such Competing Infringement, including by executing reasonably appropriate documents, cooperating in discovery, and joining as a party to the action if required.
(c) If: (i) within ninety (90) calendar days of receiving notice or becoming aware, as applicable, of such Competing Infringement; or (ii) ninety (90) calendar days before the expiration date for filing such actions, Avenue does not initiate proceedings or take other measures to address such Competing Infringement, then Licensor shall have the sole and exclusive right (but not the obligation) to assume or control the enforcement thereof at Licensor’s own cost and expense with counsel of its choice. At the request and expense of Licensor, Avenue, its Affiliate, or its Sublicensee shall provide reasonable assistance in connection with Licensor’s legal or other actions in connection with any such Competing Infringement, including by executing reasonably appropriate documents, cooperating in discovery, and joining as a party to the action if required.
7.5 Defense.
(a) Each Party shall promptly notify the other Party in writing after becoming aware of any claim alleging that the Development, Manufacture, or Commercialization of any Licensed Molecule or Licensed Product in the Territory infringes, misappropriates, or otherwise violates any Patents, Know-How, or other intellectual property rights of any Third Party (“Third-Party Infringement”). In any such instance, the Parties shall as soon as practicable thereafter discuss in good faith the most appropriate response to such notice of Third-Party Infringement.
(b) Without limiting either party’s indemnification obligations under Section 10, Avenue shall have the first right to defend and take such other actions as it reasonably deems appropriate with respect to any such claim of Third-Party Infringement, at Avenue’s sole discretion, cost and expense, provided that, if Avenue does not commence actions to defend such claim of Third-Party Infringement within thirty (30) days after it becomes aware of such claim or within thirty (30) days before the expiration date for responding to such claim, whichever is earlier, then Licensor shall have the sole and exclusive right to control the defense of such claim with counsel of its choice and at its sole discretion, cost, and expense, to the extent allowed by Applicable Laws. The defending Party shall keep the non-defending Party reasonably informed of the status of such claim of Third-Party Infringement and shall cause the non-defending Party to receive timely notice of any proposed settlement of any such action arising from such claim of Third-Party Infringement and shall not, without the prior written consent of the non-defending Party (which shall not be unreasonably withheld or delayed), enter into any settlement that would give rise to liability of the non-defending Party or its Affiliates or would otherwise impair the non-defending Party or its Affiliates’ rights in any Licensed Products or Licensed Molecule, or under this Agreement. At the request and expense of the defending Party, the non-defending Party shall provide reasonable assistance in connection with the defending Party’s legal or other actions in connection with the defense of such claim of Third-Party Infringement, including by executing reasonably appropriate documents, cooperating in discovery, and joining as a party to the action if required.
7.6 Recovery. Any recovery received as a result of any action under Section 7.4 or Section 7.5 shall be allocated in the following order: (a) to reimburse the enforcing/defending Party for the reasonable costs and expenses (including attorneys’ and professional fees) that the enforcing/defending Party incurred in connection with such action, to the extent not previously reimbursed; (b) to reimburse the non-enforcing/defending Party, where it joins a legal action as provided under Section 7.4 or Section 7.5 (as applicable), for the reasonable costs and expenses (including attorneys’ and professional fees) that the non-enforcing/defending Party incurred in connection with such action, to the extent not previously reimbursed; and (c) the remainder of the recovery shall be retained by the enforcing/defending Party and, if the enforcing/defending Party is Avenue, with respect to any portion of such recovery constituting compensatory damages for lost sales of Licensed Products, the amount determined to be lost sales of Licensed Product hereunder shall be treated as Net Sales for the purposes of Section 6.3.
7.7 Orange Book Listing. Subject to Section 6.3, Avenue, its Affiliates, or its Sublicensees shall be solely responsible for listing and maintaining all appropriate Licensed Patents and Joint Patents in the Orange Book in the United States or the Patent List in Canada, including payment of all expenses related to such listing and maintenance incurred after the Effective Date.
|
8. |
Confidentiality |
8.1 Nondisclosure. Each Party agrees that a Party (the “Receiving Party”) which receives the Confidential Information of the other Party (the “Disclosing Party”) pursuant to this Agreement shall: (a) maintain in confidence such Confidential Information using not less than the efforts that such Receiving Party uses to maintain in confidence its own proprietary information, but in no event less than a reasonable degree of efforts; (b) not disclose such Confidential Information to any Third Party without first obtaining the prior written consent of the Disclosing Party, except for disclosures expressly permitted pursuant to this Section 8; (c) cause its Affiliates, advisors or other Persons that receive any Confidential Information from or at the direction of the Receiving Party to abide by the confidentiality and non-use provisions of this Agreement, including but not limited to the terms and conditions of this Section 8; and (d) not use such Confidential Information for any purpose except those permitted under this Agreement, including, in the case of Avenue, the exercise of the rights and licenses granted to Avenue hereunder. The obligations of confidentiality, non-disclosure, and non-use under this Section 8 shall remain in full force and effect from the Effective Date until five (5) years following the Term. The Receiving Party shall return all copies of, or destroy the Confidential Information of the Disclosing Party disclosed or transferred to it by the other Party pursuant to this Agreement, promptly, but in any event within thirty (30) days after the termination (but not the expiration) of this Agreement, provided that subject to the other provisions of this Section 8, a Party may retain: (x) Confidential Information of the other Party to exercise rights and licenses which expressly survive such termination or expiration pursuant to this Agreement; (y) one (1) copy of all other Confidential Information in archives solely for the purpose of establishing the contents thereof; and (z) the Disclosing Party’s Confidential Information contained in the Receiving Party’s electronic back-up files that are created in the normal course of business pursuant to the Receiving Party’s standard protocol for preserving its electronic records; provided, however, that any such Confidential Information retained by the Receiving Party pursuant to clauses (x), (y), or (z) of this Section 8.1 shall continue to be subject to the terms and conditions of this Section 8, until such time that the Receiving Party no longer retains such Confidential Information pursuant to clauses (x), (y), or (z) of this Section 8.1.
8.2 Exceptions. Section 8.1 shall not apply with respect to any portion of the Confidential Information of the Disclosing Party to the extent that such Confidential Information:
(a) was known to the Receiving Party or any of its Affiliates, as evidenced by written records, prior to disclosure by the Disclosing Party;
(b) is subsequently disclosed to the Receiving Party or any of its Affiliates by a Third Party lawfully in possession thereof and without any obligation to keep it confidential or any restriction on its use, provided that such Third Party is not and was not prohibited from disclosing such Confidential Information to the Receiving Party by a legal, fiduciary or contractual obligation owing to the Disclosing Party;
(c) is published by a Third Party or otherwise becomes publicly available or enters the public domain, either before or after it is disclosed to the Receiving Party, without any breach by the Receiving Party of its obligations hereunder; or
(d) is independently developed by or for the Receiving Party or any of its Affiliates, as evidenced by written records, without reference to, use of, or reliance upon the Disclosing Party’s Confidential Information.
Any combination of features or disclosures shall not be deemed to fall within the foregoing exclusions merely because individual features are published or available to the general public or in the rightful possession of the Receiving Party unless the combination itself and principle of operation are published or available to the general public or in the rightful possession of the Receiving Party.
8.3 Authorized Disclosure and Use.
(a) Disclosure. Notwithstanding Section 8.1, the Receiving Party may disclose Confidential Information belonging to the Disclosing Party in the following instances:
(i) subject to Section 8.5, to comply with Applicable Law (including the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”) or any national securities exchange in any jurisdiction) (collectively, the “Securities Regulators”) or with judicial process (including prosecution or defense of litigation), if, in the reasonable opinion of the Receiving Party’s counsel, such disclosure is necessary for such compliance or for such judicial process (including prosecution or defense of litigation);
(ii) disclosure to governmental or other regulatory agencies in order to obtain Patents, to obtain or maintain approval to conduct Clinical Trials, or to market the Licensed Molecule or Licensed Products under this Agreement, in each case, in accordance with this Agreement, provided that reasonable steps are taken to ensure confidential treatment of such Confidential Information to the extent available;
(iii) disclosure to (A) any of its officers, directors, employees, consultants, agents, or Affiliates, (B) in the case of Avenue, any actual or potential collaborators or Sublicensees, (B) in the case of either Party, to such Party’s permitted subcontractors for the purpose of such subcontractors performing obligations of such Party under this Agreement, and (D) in the case of either Party, to such Party’s actual or potential acquirers, provided that, prior to any such disclosure, each such disclosee is bound by reasonable and customary written obligations of confidentiality, non-disclosure, and non-use, including, in the case of disclosure to Third Parties, obligations that are consistent with the obligations set forth in this Section 8, provided that, in each of the above situations described in this Section 8.3(a)(iii), the Receiving Party shall be liable to the Disclosing Party for any failure by any Person who receives Confidential Information from such Receiving Party pursuant to this Section 8.3(a)(iii) to treat such Confidential Information as required under this Section 8;
(iv) disclosure to any actual or potential acquirer, or prospective investment bankers, investors, lenders, or other financial partners, provided that (A) prior to any such disclosure, each such disclosee is bound by written obligations of confidentiality, non-disclosure, and non-use consistent with the obligations set forth in this Section 8, (B) such disclosure shall solely be in the form of the redacted version of this Agreement, which version has been agreed upon by the Parties in good faith, including any such redacted version that has been agreed upon for actual or potential filing to the SEC, and (C) it being understood and agreed that only after negotiations between the Receiving Party and any such Third Party have progressed so that the Receiving Party reasonably and in good faith believes that consummation of the proposed transaction with such Third Party is imminent (e.g., commencement of Third Party’s due diligence in an M&A transaction), the Receiving Party may provide an unredacted version of this Agreement to such Third Party; and
(v) disclosure to its advisors (including attorneys and accountants) in connection with activities under this Agreement, provided that prior to any such disclosure, each such disclosee is bound by written obligations of confidentiality, non-disclosure, and non-use that are at least as stringent with respect to the disclosure and non-use of Confidential Information as the obligations set forth in this Section 8 (provided that in the case of legal advisors, no written agreement shall be required), to maintain the confidentiality thereof and not to use such Confidential Information except as expressly permitted by this Agreement, provided that in each of the above situations in this Section 8.3(a)(v), the Receiving Party be liable to the Disclosing Party for any failure by any Person who receives Confidential Information from such Receiving Party pursuant to this Section 8.3(a)(v) to treat such Confidential Information as required under this Section 8.
(b) Use. Each Party shall have the right to use the Confidential Information of the other Party to fulfill its obligations and exercise its rights under this Agreement, including with respect to Licensor the use of Confidential Information that is deemed to be Avenue’s to issue the press releases described in Section 8.7.
(c) Terms of Disclosure. If and whenever any Confidential Information is disclosed in accordance with this Section 8.3, such disclosure shall not cause any such information to cease to be Confidential Information, except to the extent that such disclosure results in a public disclosure of such information other than by breach of this Agreement.
8.4 Terms of this Agreement. The Parties agree that this Agreement, the terms hereof, the transactions contemplated hereby, and any other agreement, document, instrument or certificate contemplated by this Agreement, shall be deemed to be Confidential Information of both Licensor and Avenue, and each Party agrees not to disclose this Agreement or any terms hereof without obtaining the prior written consent of the other Party, provided that each Party may disclose this Agreement or any terms hereof in accordance with the provisions of Section 8.3, Section 8.5, or Section 8.7, as applicable.
8.5 Securities Filing; Disclosure Under Applicable Law. Each Party acknowledges and agrees that the other Party may submit this Agreement to, or file this Agreement with, the Securities Regulators or other Persons as may be required by Applicable Law, and if a Party submits this Agreement to, or files this Agreement with, any Securities Regulator or other Person as may be required by Applicable Law, such Party agrees to consult with the other Party with respect to the preparation and submission of a confidential treatment request for this Agreement. Notwithstanding the foregoing, if a Party is required by any Securities Regulator or other Person as may be required by Applicable Law to make a disclosure of the terms of this Agreement in a filing or other submission as required by such Securities Regulator or such other Person, and such Party has: (a) provided copies of the disclosure to the other Party reasonably in advance under the circumstances of such filing or other disclosure; (b) promptly notified the other Party in writing of such requirement and any respective timing constraints; and (c) given the other Party reasonable time under the circumstances from the date of provision of a copy of such disclosure to comment upon and request confidential treatment for such disclosure, then such Party shall have the right to make such disclosure at the time and in the manner reasonably determined by its counsel to be required by the Securities Regulator or the other Person. Notwithstanding the foregoing, if a Party seeks to make a disclosure as required by a Securities Regulator or other Person as may be required by Applicable Law as set forth in this Section 8.5 and the other Party provides comments in accordance with this Section 8.5, the Party seeking to make such disclosure or its counsel, as the case may be, shall use good-faith efforts to incorporate such comments.
8.6 Use of Name. Except as expressly provided herein, neither Party shall mention or otherwise use the name, logo, or trademark of the other Party or any of its Affiliates (or any abbreviation or adaptation thereof) in any publication, press release, marketing and promotional material, or other form of publicity without the prior written approval of such other Party in each instance. The restrictions imposed by this Section 8.6 shall not prohibit either Party from making any disclosure identifying the other Party that is required by Applicable Law or pursuant to Section 8.5.
8.7 Publicity. The Parties shall make a joint public announcement of the execution of this Agreement which shall be issued at a time to be mutually agreed by the Parties. The Parties intend the final content must be mutually agreed upon by both Parties prior to being issued by either party. Each Party agrees not to issue any other press release or other public statement (except to the extent required by Applicable Law or pursuant to Section 8.5) disclosing other information relating to this Agreement or the transactions contemplated hereby that contains information not previously publicly disclosed in accordance with this Section 8.7 without the prior written consent of the other Party, not to be unreasonably withheld, conditioned or delayed. Each Party shall cause the other Party to be notified within twenty-four (24) hours prior to any major public release relating to this Agreement and required by Applicable Law or applicable securities exchange rules.
|
9. |
Representations & Warranties; Covenants |
9.1 Representations and Warranties of Each Party. Each Party hereby represents and warrants to the other Party, as of the Effective Date, that:
(a) such Party is duly organized, validly existing, and in good standing under the Applicable Law of the jurisdiction of its formation and has full requisite power and authority, corporate or otherwise, to enter into this Agreement and to carry out the provisions hereof;
(b) such Party has taken all necessary corporate action on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder;
(c) this Agreement has been duly executed and delivered on behalf of such Party and constitutes a legal, valid, and binding obligation, enforceable against it in accordance with its terms, except to the extent that enforcement of the rights and remedies created hereby is subject to (i) bankruptcy, insolvency, reorganization, moratorium, and other similar laws of general application affecting the rights and remedies of creditors, or (ii) laws governing specific performance, injunctive relief, and other equitable remedies;
(d) the execution, delivery, and performance of this Agreement by such Party does not and will not breach, violate or conflict with such Party’s charter documents, bylaws or other organizational documents, any agreement or any provision thereof, or any instrument or understanding, oral or written, to which such Party (or any of its Affiliates) is a party or by which such Party (or any of its Affiliates) is bound, nor violate any Applicable Law of any Governmental Authority having jurisdiction over such Party (or any of its Affiliates);
(e) no government authorization, consent, approval, license, exemption of or filing or registration with any court or governmental department, commission, board, bureau, agency, or instrumentality, domestic or foreign, under any Applicable Law currently in effect, is or shall be necessary for, or in connection with, the transaction contemplated by this Agreement, or for the performance by it of its obligations under this Agreement, except: as may be required to conduct Clinical Trials or to seek or obtain Regulatory Approvals or applicable Regulatory Materials;
(f) it has obtained all necessary authorizations, consents, and approvals of any Third Party that is required to be obtained by it for, or in connection with, the transactions contemplated by this Agreement, or for the performance by it of its obligations under this Agreement, except as may be required to seek or obtain Regulatory Approvals or applicable Regulatory Materials; and
(g) it has not been debarred or is subject to debarment and it will not use in any capacity, in connection with the services to be performed under this Agreement, any Person who has been debarred pursuant to Section 306 of the FD&C Act or who is the subject of a conviction described in such section. It will inform the other Party in writing promptly if it or any such Person who is performing services hereunder is debarred or is the subject of a conviction described in Section 306 or if any action, suit, claim, investigation or legal or administrative proceeding is pending, relating to the debarment or conviction of it or any such Person performing services hereunder.
9.2 Representations and Warranties of Licensor. Licensor hereby represents and warrants to Avenue, as of the Effective Date, that:
(a) all the Licensed Patents in existence as of the Effective Date in the Territory are listed in Exhibit B;
(b) Licensor Controls the Patents listed in Exhibit B;
(c) Licensor has the right to grant the license and rights herein to Avenue, and has not entered into any agreement that conflicts with the license and rights granted herein to Avenue;
(d) there are no claims, actions, demands, suits, proceedings, arbitrations, grievances, citations, summonses, subpoenas, inquiries, investigations, judgments or settlements against or owed by Licensor relating to the Licensed Molecule, or to the Licensed Patents or Licensed Know-How in the Territory for which Licensor has received written notice, including notice of any claims or demands alleging that the Licensed Patents are invalid or unenforceable;
(e) Licensor owns the entire right, title, and interest in the Licensed IP free and clear of all Encumbrances;
(f) to the actual knowledge of Licensor, no Third Party has challenged the extent, validity or enforceability of the Licensed Patents (including, by way of example, through the institution or threat of institution of interference, opposition, post-grant review, reexamination, nullity or similar invalidity proceedings before a United States or foreign court, the United States Patent and Trademark Office or any analogous foreign entity);
(g) to the actual knowledge of Licensor, the conception, development and reduction to practice of Licensed IP existing as of the Effective Date have not constituted or involved the misappropriation or infringement of Patents, trade secrets, or other intellectual property rights of any Third Party in any manner that would conflict with the license granted to Avenue hereunder;
(h) to the actual knowledge of Licensor, each of the patents and patent applications included within the Licensed Patents properly identifies each and every inventor of the claims thereof as determined in accordance with the laws of the jurisdiction in which such patent is issued or such patent application is pending;
(i) none of Licensor’s Affiliates Controls any Patent Covering the Licensed Molecule; and
9.3 Representations and Warranties of Avenue. Avenue hereby represents and warrants to Licensor, as of the Effective Date, that:
(a) Avenue has all rights necessary to grant the rights granted to Licensor pursuant to Section 2.4 and Section 7.1;
(b) Avenue (i) does not own or license any Patents or Patent applications that cover a Competing Product, and (ii) is not currently engaging in any Development or Commercialization of any Competing Product; and
(c) there are no Patents or Patent applications owned by Avenue or its Affiliates that claim the subject matters of the Licensed Patents as listed in Exhibit B.
9.4 Additional Covenants.
(a) To the extent permissible under Applicable Law, each Party shall ensure that its Affiliates and its and their employees performing activities under this Agreement shall be under an obligation to assign all right, title and interest in and to their Inventions, whether or not patentable, and intellectual property rights therein, to such Party or its Affiliate(s) as the sole owner thereof. Neither Party shall have any obligation to contribute to any remuneration of any inventor employed or previously employed by the other Party or any of its Affiliates in respect of any such Inventions, Information and discoveries and intellectual property rights therein that are so assigned to such other Party or its Affiliate(s). Each Party will pay all such remuneration, if any, due to inventors performing activities under this Agreement on behalf of such Party or its Affiliates with respect to such Inventions and other Know-How and intellectual property rights therein.
(b) In performing its obligations under this Agreement, each Party shall, and shall cause its Affiliates and Sublicensees to comply with: (i) all Applicable Laws, including any applicable anti-corruption or anti-bribery laws or regulation, of any Governmental Authority with jurisdiction over the activities performed by such Party or its Affiliates or sublicensees in furtherance of such obligations; and (ii) the Pharmaceutical Research and Manufacturers of America (PhRMA) guidelines in the U.S., European Federation of Pharmaceutical Industries and Associations’ Code of Practice, and International Federation of Pharmaceutical Manufacturers & Associations’ Code of Practice.
(c) Each Party shall not, and shall ensure that its Affiliates and Sublicensees shall not, without the prior written consent of Licensor, solicit, induce, encourage, or participate in soliciting, inducing, or encouraging any employee of the other Party or any of its Affiliates who has been as of, or becomes after the Effective Date, involved in the discussion leading to this Agreement, or the Development, Manufacture, or Commercialization of the Licensed Molecule or Licensed Products to terminate such employee’s relationship with such other Party or its Affiliates.
(d) Each Party shall notify the other Party promptly after becoming aware of any Person’s actual or threatened infringement or misappropriation of any Joint Patents.
(e) Each Party shall promptly notify the other Party upon becoming aware of any suit, litigation, or written claim in the Territory brought or threatened by any Person alleging that the Development, Manufacture, or Commercialization of the Licensed Products infringes a Patent owned by such Person and shall cause its Affiliates and Sublicensees, as applicable, to provide notice to such Party to enable such Party to provide notice to the other Party in accordance with this Section 9.4(e).
9.5 Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED HEREIN, NEITHER PARTY MAKES ANY REPRESENTATIONS OR EXTENDS ANY WARRANTIES OF ANY KIND, EXPRESSOR IMPLIED, AND EACH PARTY HEREBY EXPRESSLY DISCLAIMS ANY AND ALL REPRESENTATIONS AND WARRANTIES NOT EXPRESSLY PROVIDED IN THIS AGREEMENT, INCLUDING WITH RESPECT TO ANY PATENTS OR KNOW-HOW, INCLUDING ANY AND ALL WARRANTIES OF VALIDITY, ENFORCEABILITY, MERCHANTABILITY, FITNESS FOR A PARTICULAR USE OR PURPOSE, PERFORMANCE, OR NON-INFRINGEMENT OF ANY THIRD-PARTY PATENT OR OTHER INTELLECTUAL PROPERTY RIGHT. NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, THAT IT WILL BE ABLE TO SUCCESSFULLY DEVELOP, MANUFACTURE, OR COMMERCIALIZE ANY LICENSED MOLECULE OR LICENSED PRODUCT OR, IF COMMERCIALIZED, THAT ANY PARTICULAR SALES LEVEL OF SUCH LICENSED PRODUCT WILL BE ACHIEVED.
|
10. |
Indemnification; Insurance; Limitation of Liability |
10.1 Indemnification by Avenue. Avenue shall indemnify, defend, and hold harmless Licensor, its Affiliates, and its and their respective directors, officers, employees, agents, successors, and assigns (collectively, the “Licensor Indemnitees”) from and against any and all Damages to the extent arising out of or relating to, directly or indirectly, any Third-Party Claim based upon:
(a) the Development, Manufacture, or Commercialization of any Licensed Molecule or Licensed Product in the Field in the Territory by Avenue, its Affiliates, or its Sublicensees;
(b) the gross negligence or willful misconduct of Avenue or its Affiliates or its or their respective directors, officers, employees, consultants, subcontractors, or agents, in connection with Avenue’s performance of its obligations under this Agreement, the Subscription Agreement, or the Registration Rights Agreement; or
(c) any breach by Avenue of any of its representations, warranties, covenants, agreements or obligations under this Agreement, the Subscription Agreement, or the Registration Rights Agreement.
Notwithstanding the foregoing, Avenue’s obligation to indemnify pursuant to this Section 10.1 shall not apply to any Third-Party Claims for which Licensor is required to indemnify Avenue pursuant to Section 10.2.
10.2 Indemnification by Licensor. Licensor shall indemnify and hold harmless Avenue, its Affiliates, and its and their respective directors, officers, employees, agents, successors, and assigns (collectively, the “Avenue Indemnitees”), from and against any and all Damages to the extent arising out of or relating to, directly or indirectly, any Third-Party Claim based upon:
(a) the Development, Manufacture, or Commercialization of any Licensed Molecule or Licensed Product prior to the Effective Date;
(b) the Manufacture of any Licensed Molecule or Licensed Product for Licensor, its Affiliates or a Third Party (other than a Sublicensee);
(c) the Manufacture of any Licensed Molecule or Licensed Product by Licensor or its Affiliates for Avenue, its Affiliates or Sublicensees, or the supply of such Licensed Molecules or Licensed Products to Avenue or its Affiliates or Sublicensees, except to the extent such Third-Party Claim arises from Licensor’s compliance with Avenue’s (or its Affiliates’ or any of its Sublicensees’) unique technical specifications or unique technical requirements that are requested in writing by Avenue;
(d) the Development, Manufacture, or Commercialization of any Licensed Molecule or Licensed Product outside of the Field or outside of the Territory by or on behalf of Licensor or its Affiliates or licensees on or after the Effective Date;
(e) the gross negligence or willful misconduct of Licensor or its Affiliates or its or their respective directors, officers, employees, consultants, subcontractors or agents, in connection with Licensor’s performance of its obligations under this Agreement, the Subscription Agreement, or the Registration Rights Agreement; or
(f) any breach by Licensor of any of its representations, warranties, covenants, agreements or obligations under this Agreement, the Subscription Agreement, or the Registration Rights Agreement, or of applicable law, including U.S. securities laws governing xxxxxxx xxxxxxx.
Notwithstanding the foregoing, Licensor’s obligation to indemnify pursuant to this Section 10.2 shall not apply to any Third-Party Claims for which Avenue is required to indemnify Licensor pursuant to Section 10.1.
10.3 Indemnification Procedure.
(a) If a Party is seeking indemnification pursuant to Section 10.1 or Section 10.2, as applicable (such Party, the “Indemnitee”), it shall inform the other Party (the “Indemnitor”) of the claim giving rise to the obligation to indemnify pursuant to Section 10.1 or Section 10.2, as applicable, as soon as reasonably practicable after receiving notice of or otherwise becoming aware of the claim (an “Indemnification Claim Notice”), provided that any delay or failure to provide such notice shall not constitute a waiver or release of, or otherwise limit, the Indemnitee’s rights to indemnification under Section 10.1 or Section 10.2, as applicable, except to the extent that such delay or failure prejudices the Indemnitor’s ability to defend against the relevant claims or results in increased Damages to the Indemnitor.
(b) The Indemnitor shall have the right, upon written notice given to the Indemnitee within thirty (30) days after receipt of the Indemnification Claim Notice (and, where the Indemnitor is Licensor, subject to receipt of Avenue’s prior written consent), to assume the defense of any such claim for which the Indemnitee is seeking indemnification pursuant to Section 10.1 or Section 10.2, as applicable. The Indemnitee shall cooperate with the Indemnitor and the Indemnitor’s insurer as the Indemnitor may reasonably request, and at the Indemnitor’s cost and expense. The Indemnitee shall have the right to participate, at its own expense, and with counsel of its choice, in the defense of any claim or suit that has been assumed by the Indemnitor.
(c) The Indemnitor shall not settle any claim to which it is subject pursuant to Section 10.1 or Section 10.2, as applicable, without first obtaining the prior written consent of the Indemnitee, not to be unreasonably withheld, conditioned, or delayed, provided that the Indemnitor shall not be required to obtain such consent if the settlement: (i) involves only the payment of money and shall not result in the Indemnitee (or other Licensor Indemnitees or Avenue Indemnitees, as applicable) becoming subject to injunctive, equitable, or other similar type of relief, including any restrictions on the operations of the business of the Indemnitee; (ii) does not require an admission by the Indemnitee (or other Licensor Indemnitees or Avenue Indemnitees, as applicable); (iii) does not adversely affect the rights or licenses granted to the Indemnitee (or its Affiliate) under this Agreement; and (iv) includes a general release of all Third-Party Claims against the Indemnitee by the applicable Third Party, if any, for which the Indemnitor is obligated to indemnify the Indemnitee pursuant to this Section 10. The Indemnitee shall not settle or compromise any such claim without first obtaining the prior written consent of the Indemnitor.
(d) If the Parties cannot agree as to the application of Section 10.1 or Section 10.2, as applicable, with respect to any claim, pending the resolution of the dispute pursuant to Section 12.7 (Governing Law; Dispute Resolution; Jurisdiction), the Parties may conduct separate defenses of such claims, with each Party retaining the right to claim indemnification from the other Party in accordance with Section 10.1 or Section 10.2, as applicable, upon resolution of the underlying claim. In each case, the Indemnitee shall reasonably cooperate with the Indemnitor and shall make available to the Indemnitor all pertinent information under the control of the Indemnitee, which information shall be subject to Section 8.
10.4 Insurance. During the Term and for a period of three (3) years thereafter, each Party shall maintain, at its cost, a program of insurance against liability and other risks associated with its activities and obligations under this Agreement (including with respect to its Clinical Trials), and its indemnification obligations hereunder, in such amounts, subject to such deductibles and on such terms as are customary for such Party for the activities to be conducted by it under this Agreement. At a minimum, Avenue’s insurance shall include: (a) during the Term, comprehensive general liability, including broad form and contractual liability, in a minimum amount of $3,000,000.00 combined single limit per occurrence and in the aggregate, written on an occurrence-basis, with no deductible, containing a separation of insureds provision, with additional coverage for broad form and contractual liability, completed operations; and (b) prior to the commencement of Clinical Trials involving Licensed Products, clinical trials coverage in a minimum amount of $10,000,000.00 combined single limit per occurrence and in the aggregate; and (c) prior to the First Commercial Sale of the first Licensed Product, product liability coverage, in a minimum amount of $15,000,000 combined single limit per occurrence and in the aggregate. Such insurance shall not be construed to create a limit on either Party’s liability with respect to its indemnification obligations under this Section 10 or otherwise.
10.5 Limitation of Liability. NEITHER LICENSOR NOR AVENUE, NOR ANY OF THEIR RESPECTIVE AFFILIATES, WILL BE LIABLE TO THE OTHER PARTY OR ITS AFFILIATES UNDER OR IN CONNECTION WITH THIS AGREEMENT FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, PUNITIVE, OR EXEMPLARY DAMAGES (INCLUDING LOST PROFITS OR LOST REVENUES), REGARDLESS OF WHETHER LIABILITY IS ASSERTED IN CONTRACT, TORT (INCLUDING NEGLIGENCE AND STRICT PRODUCT LIABILITY), INDEMNITY, CONTRIBUTION, OR OTHERWISE, AND REGARDLESS OF WHETHER SUCH PARTY OR ITS REPRESENTATIVES HAS BEEN ADVISED OF OR OTHERWISE ANTICIPATED THE POSSIBILITY OF ANY SUCH LOSS OR DAMAGE. NOTWITHSTANDING THE FOREGOING, THIS SECTION 10.5 SHALL NOT APPLY TO: (a) EITHER PARTY’S INDEMNIFICATION OBLIGATIONS UNDER SECTION 10; (b) DAMAGES ARISING FROM AVENUE’S USE OR EXPLOITATION OF THE LICENSED IP IN ANY MANNER THAT MATERIALLY VIOLATES OR EXCEEDS THE LICENSE GRANTED IN SECTION 2.1, EXCLUDING ANY SUCH VIOLATION OR EXCESS ARISING OUT OF AVENUE’S NEGLIGENCE; OR (c) DAMAGES ARISING FROM EITHER PARTY’S GROSS NEGLIGENCE, INTENTIONAL MISCONDUCT, FRAUD, OR BREACH OF SECTION 8. NEITHER PARTY’S LIABILITY FOR INDEMNIFICATION OBLIGATIONS PURSUANT TO SECTION 10 SHALL EXCEED THREE HUNDRED MILLION DOLLARS ($300,000,000).
|
11. |
Term & Termination |
11.1 Term. This Agreement shall become effective on the Effective Date and, unless earlier terminated in accordance with this Section 11, shall expire on a country-by-country and Licensed Product-by-Licensed Product basis upon the expiration of the Royalty Term under this Agreement with respect to such Licensed Product in such country (the “Term”).
11.2 Effect of Expiration. Upon the expiration of the Term in a given country and for a given Licensed Product pursuant to Section 11.1, the licenses set forth in Section 2.1 with respect to such Licensed Product in such country shall become fully paid-up, perpetual, irrevocable and royalty-free.
11.3 Termination for Material Breach.
(a) This Agreement may be terminated in its entirety or on a country-by-country (each, a “Terminated Country”) or Licensed Product-by-Licensed Product (each, a “Terminated Product”) basis by a Party for the material breach by the other Party of this Agreement, provided that the breaching Party has not cured such breach within sixty (60) calendar days after the date of written notice to the breaching Party of such breach (the “Cure Period”), which notice shall describe such breach in reasonable detail and shall state the nonbreaching Party’s intention to terminate this Agreement (in its entirety or on a Terminated Product by Terminated Product or Terminated Country-by-Terminated Country basis); provided that, if the breaching Party is exercising Commercially Reasonable Efforts to cure such breach, the Cure Period shall be automatically extended for so long as such breaching Party is exercising commercially reasonable efforts to cure such breach; provided, further, that in no event shall the Cure Period exceed ninety (90) calendar days in the aggregate. In the event that a material breach relates solely to a given Terminated Product or to a given Terminated Country, then the non-breaching Party may only exercise its termination right under this Section 11.3 with respect to such Terminated Product or such Terminated Country. Any such termination of this Agreement (in its entirety or on a Terminated Product-by-Terminated Product or Terminated Country-by-Terminated Country basis) under this Section 11.3 shall become effective at the end of the Cure Period, unless the breaching Party has cured such breach prior to the expiration of such Cure Period, or, if such breach is not susceptible to cure within the Cure Period. Avenue’s failure to use Commercial Reasonable Efforts to develop Licensed Product in accordance with Section 4.1 shall constitute a material breach for the purposes of this Section 11.3. In the event of Avenue’s material breach of Sections 3.1(j), 4.2, 6.2, 6.3, 6.4, 6.7, Licensor may, subject to Avenue’s right to cure, exercise its termination right to terminate the Agreement in its entirety. Notwithstanding the foregoing: (i) in the event that Avenue breaches Avenue’s payment obligations set forth in Sections 3.1(j), 6.1,6.2(d), 6.3(d), 6.7(a), 6.7(b), or 6.7(d), the Cure Period for such breach shall be thirty (30) days; and (ii) in the event that (A) Avenue breaches Section 4.2 by failing to achieve a Diligence Milestone, and (B) the timeline for Avenue’s achievement of the Diligence Milestones has, prior to such breach, been extended pursuant to Section 4.2, then there shall be no Cure Period for such breach.
(b) If the Parties reasonably and in good faith disagree as to whether there has been a material breach, including whether such breach was material, the Party that disputes whether there has been a material breach may contest the allegation in accordance with Section 12.7(b). Notwithstanding anything to the contrary contained in Section 12.7(b), the Cure Period for any Dispute shall run from the date that written notice was first provided to the breaching Party by the non-breaching Party through the resolution of such Dispute pursuant to Section 12.7(b), and it is understood and acknowledged that, during the pendency of a Dispute pursuant this Section 11.3(b), all of the terms and conditions of this Agreement shall remain in effect, and the Parties shall continue to perform all of their respective obligations under this Agreement.
11.4 Termination for Convenience. Avenue may terminate this Agreement at will, in its sole discretion, in its entirety or on a Licensed Product-by-Licensed Product or country-by-country basis, at any time (but in no event earlier than one full year following the Effective Date), upon ninety (90) calendar days’ prior written notice to Licensor. For clarity, each such terminated Licensed Product shall automatically become a Terminated Product and each such terminated country shall automatically become a Terminated Country at the expiration of such ninety (90) calendar days.
11.5 Termination for Bankruptcy.
(a) If either Party makes a general assignment for the benefit of, or an arrangement or composition generally with, its creditors, appoints or suffers appointment of an examiner or of a receiver, custodian, liquidator, trustee or similar person over all or substantially all of its property, passes a resolution for its winding up, liquidation, dissolution, or reorganization or similar process, or files a petition or commences a proceeding under any bankruptcy or insolvency act or law or has any such petition filed, or proceeding commenced, against it and such party or any other person seeks to reject or disaffirm this Agreement, (each, a “Rejection Event”), the other Party may terminate this Agreement in its entirety, effective immediately upon written notice to such Party.
(b) For purposes of Section 365(n) of the U.S. Bankruptcy Code (the “Code”) and any similar laws in any country other than the U.S., all rights and licenses granted under or pursuant to this Agreement are rights to “intellectual property” (as defined in Section 101(35A) of the Code). The Parties agree that the licensee of such rights under this Agreement will retain and may fully exercise all of its protections, rights, and elections under the Code and any similar laws in any country other than the U.S. Each Party hereby acknowledges that: (i) copies of research data; (ii) laboratory samples; (iii) product samples; (iv) formulas; (v) laboratory notes and notebooks; (vi) data and results related to Clinical Trials; (vii) regulatory filings and Regulatory Approvals; (viii) rights of reference in respect of regulatory filings and Regulatory Approvals; (ix) pre-clinical research data and results; and (x) marketing, advertising, and promotional materials, in each case ((i) – (x)), that relate to such intellectual property, constitute “embodiments” of such intellectual property pursuant to Section 365(n) of the Code, and that the licensee will be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, and the same, if not already in its possession, will be promptly delivered to it upon its written request therefor and election under Section 365(n)(1)(B) of the Code to retain the licenses granted by Licensor to Avenue hereunder in the event of Licensor’s rejection of this Agreement, unless Licensor elects to continue to perform all of its obligations under this Agreement. The provisions of this Section 11.5(b) are without prejudice to any rights the non-subject Party may have arising under the Code, laws of other jurisdictions governing insolvency and bankruptcy, or other Applicable Law.
(c) The Parties agree that they intend the following rights to extend to the maximum extent permitted by Applicable Law, including for purposes of the Code and any similar laws in any country other than the U.S.: (i) the right of access to any intellectual property (including all embodiments thereof) of Licensor, or any Third Party with whom Licensor contracts in accordance with this Agreement to perform an obligation of Licensor under this Agreement which is necessary or useful for the Development, Manufacture, or Commercialization of any Licensed Molecule or Licensed Product; (ii) the right to contract directly with any Third Party described in clause (i) to complete such contracted work; and (iii) the right to cure any Default under any such agreement with a Third Party and set off the costs thereof against amounts payable to such Licensor under this Agreement.
11.6 Termination for Patent Challenge.
(a) Licensor may terminate the entire Agreement at any time upon written notice to Avenue, if Avenue, or any of Avenue’s Affiliates, directly, or indirectly through assistance of a Third Party, commences any interference or opposition proceeding, challenges the validity or enforceability of, or opposes any extension of or the grant of a supplementary protection certificate with respect to (a “Patent Proceeding”) any Licensed Patent owned or controlled by Licensor that claims or discloses the composition of matter or the method of making or using the Licensed Product anywhere in the Territory except for a country in the Territory in which this Agreement has been terminated prior to the commencement of any such Patent Proceeding.
(b) In the event that a Sublicensee, directly or indirectly through the assistance of a Third Party, commences a Patent Proceeding, then upon becoming aware of such Patent Proceeding, Avenue will promptly terminate the applicable sublicense.
11.7 Termination for Cessation Business. Licensor may terminate this Agreement in its entirety upon the written notice to Avenue if Avenue, its Affiliates, or its Sublicensees at any time fail to conduct any material Development or Commercialization activities with respect to the Licensed Product for a continuous period of longer than twelve (12) months, and such suspension of activity is not: (a) contemplated in a Development Plans or Territorial Commercialization Plan or otherwise by written agreement of the Parties; (b) a result of Avenue’s reasonable response to written guidance from or action by a Regulatory Authority in the Territory (such as a clinical hold, or a recall or withdrawal); (c) due to Licensor’s failure to supply the Licensed Product in accordance with the terms of a Clinical Supply Agreement or Commercial Supply Agreement; or (d) due to a Force Majeure Event.
11.8 General Effects of Termination. The provisions of this Section 11.8 shall apply upon any expiration or termination of this Agreement.
(a) Payment Obligation. All applicable payment obligations hereunder shall terminate, other than those that are accrued and unpaid as of the effective date of the termination of this Agreement.
(b) Return of Confidential Information. Promptly, but in no event later than thirty (30) days after the effective date of the termination of this Agreement, each Party shall either destroy or return or cause to be returned to the other Party all Confidential Information in tangible form received from such other Party and all copies thereof and all materials substances or compositions delivered or provided by the other Party as instructed by the other Party, provided that: (i) Avenue may retain any such Confidential Information or materials as reasonably necessary for Avenue’s continued practice under any license under this Agreement that remains effective after such termination; and (ii) each Party may keep one (1) copy of Confidential Information received from the other Party in its confidential files for archival purposes. Notwithstanding the foregoing, neither Party shall be required to delete or destroy any electronic back-up files that have been created solely by the automatic or routine archiving and back-up procedures of such Party, to the extent created and retained in a manner consistent with its or their standard archiving and back-up procedures. For the avoidance of doubt, each of the foregoing exceptions to the Parties’ obligations to return or destroy Confidential Information upon termination of this Agreement are subject to the terms and conditions set forth in Section 8, including the Parties’ obligations with respect to the retention of Confidential Information set forth in Section 8.1.
(c) Sale of Existing Inventory. For a period of one hundred eighty (180) calendar days following the effectiveness of termination of this Agreement, Avenue may sell existing inventory of Licensed Product owned by Avenue or any of its Affiliates as of the effective date of such termination, provided that Avenue pays to Licensor royalties owing thereon pursuant to Section 6.3(a) and such sale complies with all Applicable Laws in the Territory.
11.9 Specific Effects of Termination. Upon termination of this Agreement in its entirety (or with respect to the applicable Terminated Country(ies) or Terminated Product(s)) by Avenue pursuant to Section 11.4, or by Licensor pursuant to Sections 11.3, 11.5, or 11.6:
(a) the rights and licenses granted to Avenue under Section 2.1 with respect to such Terminated Country(ies) and Terminated Product(s) shall be terminated and all such rights shall revert to Licensor, except to the extent and for so long as necessary for Avenue to fulfil its responsibilities under the surviving terms of this Agreement as provided in Section 11.10, it being agreed that all such activities shall be discontinued and ceased (unless otherwise agreed or required under Applicable Law) by transitioning such activities and responsibilities to Licensor as promptly as possible, subject to Applicable Law. Avenue’s failure to cease the Development, Manufacture (if applicable), and/or Commercialization of the Licensed Products upon the expiration or earlier termination of this Agreement may result in immediate and irreparable damage to Licensor. Avenue acknowledges that no adequate remedy at law exists for such failure, and Avenue agrees that Licensor may be entitled to seek an injunction or other equitable relief to prevent a breach of this Agreement by Avenue;
(b) upon Licensor’s written instruction, Avenue shall as soon as reasonably practicable transfer and assign (to the extent permitted) to Licensor all Regulatory Materials Controlled by Avenue, in each case, to the extent solely related to the Terminated Product(s) and necessary for Developing, Manufacturing, or Commercializing such Terminated Product(s) in the Field in the Territory;
(c) any and all sublicense agreements entered into by Avenue or any of its Affiliates with a Sublicensee pursuant to this Agreement with respect to the Terminated Product(s) and Terminated Country(ies), as applicable, shall survive such termination of this Agreement, remain in full force and effect and automatically be assigned to Licensor, with Licensor as each such Sublicensee’s direct licensor, respect to the Licensed Patents and Licensed Know-How, provided that (i) such Sublicensee’s payment obligations with respect to its exercise of its surviving rights to the Licensed Patents and Licensed Know-How (but not with respect to its exercise or enjoyment of any other rights or assets) shall, in lieu of any payment obligations set forth in applicable sublicense agreement, be the corresponding payment obligations set forth in this Agreement, and (ii) such Sublicensee observes the same or higher diligence standards set forth in Section 4 of this Agreement;
(d) each Party shall have the right to use the other Party’s Confidential Information solely to the extent necessary to exercise any surviving rights and fulfill any surviving obligations under this Agreement; subject to the terms and conditions of Section 8 of this Agreement; and
(e) if at the effective date of termination, Avenue, its Affiliate, or its Sublicensee is Manufacturing Licensed Product(s), then, if Licensor requests in writing to Avenue within sixty (60) calendar days of the effective date of termination, subject to the Parties’ negotiation and execution of a reasonable and customary transitional supply and quality agreement, and provided this Agreement is not terminated pursuant to Section 11.3, Avenue agrees to cause itself, its Affiliates, or its Sublicensee to Manufacture and supply such Licensed Product(s) to Licensor for a reasonable transitional period, not to exceed three hundred (300) days from the effective date of termination, pursuant to which such Licensed Products shall be supplied at one hundred thirty percent (130%) of the Cost of Goods as of the effective date of such transitional supply and quality agreement as further defined.
11.10 Surviving Provisions.
(a) Accrued Rights; Remedies. The expiration or termination of this Agreement for any reason shall be without prejudice to any rights that shall have accrued to the benefit of any Party prior to such expiration or termination, and any and all damages or remedies (whether at law or in equity) arising from any breach hereunder, each of which shall survive expiration or termination of this Agreement. Such expiration or termination shall not relieve any Party from obligations which are expressly indicated to survive expiration or termination of this Agreement. Except as otherwise expressly set forth in this Agreement, the termination provisions of this Section 11 are in addition to any other relief and remedies available to either Party under this Agreement, at law or in equity.
(b) Survival. Without limiting the provisions of Section 11.10(a), the rights and obligations of the Parties set forth in the following Sections shall survive the expiration or termination of this Agreement, in addition to those other terms and conditions that are expressly stated to survive termination or expiration of this Agreement: Sections 1 (Definitions) (to the extent the definitions are used in other surviving provisions); 8 (Confidentiality) 10 (Indemnification; Insurance; Limitation of Liability); 11 (Term & Termination); and 12 (Miscellaneous).
|
12. |
Miscellaneous |
12.1 Severability. If one (1) or more of the terms or provisions of this Agreement is held by a court of competent jurisdiction to be void, invalid, or unenforceable in any situation in any jurisdiction, such holding shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the void, invalid or unenforceable term or provision in any other situation or in any other jurisdiction, and such term or provision shall be considered severed from this Agreement solely for such situation and solely in such jurisdiction, unless the void, invalid, or unenforceable term or provision is of such essential importance to this Agreement that it is to be reasonably assumed that the Parties would not have entered into this Agreement without the void, invalid, or unenforceable term or provision. If the final judgment of such court declares that any term or provision hereof is void, invalid, or unenforceable, the Parties agree to: (a) reduce the scope, duration, area, or applicability of the term or provision or to delete specific words or phrases to the minimum extent necessary to cause such term or provision as so reduced or amended to be enforceable; and (b) make a good-faith effort to replace any void, invalid, or unenforceable term or provision with a valid and enforceable term or provision such that the objectives contemplated by the Parties when entering this Agreement may be realized.
12.2 Notices. Any notice required or permitted to be given by this Agreement shall be in writing and in English and shall be: (a) delivered by hand or by overnight courier with tracking capabilities; or (b) mailed postage prepaid by first class, registered, or certified mail, in each case, addressed as set forth below unless changed by notice so given.
| Address for notices to Licensor: | AnnJi Pharmaceutical Co. Ltd. |
| Attn: Xxxxx Xxxxx | |
| [***] | |
| with a copy to (which copy shall not constitute notice hereunder): | |
| Xxxxxxxxx Xxxxxxx, LLP | |
| Attn: [***] | |
| Address for notices to Licensor: | Avenue Therapeutics, Inc. |
| Attn: CEO | |
| 0 Xxxxxxxxxx Xxxxxx, 0xx Floor | |
| New York NY 10014, USA | |
| [***] | |
| with a copy to (which copy shall not constitute notice hereunder): | |
| Avenue Therapeutics, Inc. | |
| Attn: Legal | |
| 0 Xxxxxxxxxx Xxxxxx, 0xx Floor | |
| New York NY 10014, USA | |
| [***] |
Any such notice shall be deemed given on the date received, except any notice received after 5:30 p.m. (in the time zone of the receiving Party) on a Business Day or received on a non-Business Day shall be deemed to have been received on the next Business Day. A Party may add, delete, or change the person or address to which notices should be sent at any time upon written notice delivered to the other Parties in accordance with this Section 12.2.
12.3 Force Majeure. A Party shall not be liable for delay or failure in the performance of any of its obligations hereunder if such delay or failure is due to a cause beyond the reasonable control of such Party (each, a “Force Majeure Event”), including acts of God, fires, earthquakes, acts of war, terrorism, or civil unrest, or hurricane or other inclement weather, provided that the affected Party: (a) promptly notifies the other Party; and (b) shall use its commercially reasonable efforts to avoid or remove such causes of non-performance and to mitigate the effect of such occurrence, and shall continue performance in accordance with the terms of this Agreement whenever such causes are removed. When such circumstances arise and persist for more than ninety (90) days, the Parties shall negotiate in good faith any modifications of the terms of this Agreement that may be necessary or appropriate in order to arrive at an equitable solution.
12.4 Assignment.
(a) Generally. Except as expressly permitted herein, this Agreement may not be assigned or transferred by any Party, nor may any Party assign or transfer any rights or obligations created by this Agreement, except as expressly permitted hereunder without first obtaining the prior written consent of the other Party, which consent shall not be unreasonably withheld, conditioned, or delayed. Notwithstanding the foregoing, either Party may assign this Agreement, without such consent, to its successor in interest in connection with a transaction or series of transactions with one or more Third Parties that is: (i) a merger, share exchange, or other reorganization of the assigning Party; (ii) the sale, by one or more stockholders or holders of equity securities, of stock or equity securities representing a majority of the voting power of the assigning Party; or (iii) the sale or exclusive license of all or substantially all of the assets of the assigning Party, or a sale of all or substantially all of the assigning Party’s business or assets relating to the subject matter of this Agreement; or (iv) the acquisition of majority control of the board of directors or equivalent governing body of the assigning Party, for which, in the case of the foregoing clauses (i) or (ii), the stockholders or holders of other equity securities of the assigning Party prior to such transaction do not own a majority of the voting power of the acquiring, surviving, or successor entity, as the case may be.
(b) Notice of Assignment by Avenue. In the event of any assignment by Avenue pursuant to Section 12.4(a), Avenue shall: (i) notify Licensor of such assignment, which notice shall include the identity of the assignee; (ii) upon Licensor’s reasonable request, promptly provide any background information regarding such assignment which is in Avenue’s possession; and (iii) cause assignee to agree in writing to be legally bound by this Agreement.
(c) All Other Assignments Null and Void. The terms of this Agreement shall be binding upon and shall inure to the benefit of the successors, heirs, administrators and permitted assigns of the applicable Party. Any purported assignment in violation of this Section 12.4 shall be null and void ab initio.
12.5 Waivers; Modifications. The failure of any Party to insist on the performance of any obligation hereunder shall not be deemed to be a waiver of such obligation. Waiver of any breach of any provision hereof shall not be deemed to be a waiver of any other breach of such provision or any other provision on such occasion or any succeeding occasion. No waiver, modification, release, or amendment of any obligation under or provision of this Agreement shall be valid or effective unless in writing and signed by the Parties.
12.6 Export Control. This Agreement is made subject to any restrictions concerning the export of Licensed Products or technical information from the United States or other countries that may be imposed on the Parties from time to time. Each Party agrees that it shall not export, directly or indirectly, any technical information acquired from the other Party under this Agreement or any Licensed Products using such technical information to a location or in a manner that at the time of export requires an export license or other governmental approval, without first obtaining the written consent to do so from the appropriate agency or other governmental authority or Regulatory Authority in accordance with Applicable Law.
12.7 Governing Law; Dispute Resolution; Jurisdiction.
(a) Governing Law. This Agreement shall be governed by, enforced, and construed in accordance with the laws of the State of New York without reference to any rules of conflict of laws and excluding the United Nations Convention on Contracts for the International Sales of Goods.
(b) Dispute Resolution. The Parties agree that the procedures set forth in Section 12.7(c) shall be the exclusive mechanism for resolving any dispute (whether in contract, tort or otherwise), controversy, or claim between the Parties arising out of or in connection with this Agreement, any Party’s rights or obligations under this Agreement, breach of this Agreement, or the transactions contemplated by this Agreement (each, a “Dispute”).
(c) Resolution Process.
(i) Good Faith Negotiations. In the event of Dispute between the Parties arising out of or relating to the terms of the Agreement, or the breach, termination, or invalidity thereof, a Party seeking to resolve such Dispute will, by written notice to the other Party, have such Dispute referred to their respective Executive Officers, for attempted resolution by good faith negotiations within fifteen (15) Business Days after such notice is received.
(ii) Mediation. If the Parties are unable to resolve a Dispute through good faith negotiations pursuant to Section 12.7(c)(i), the Parties agree to submit such Dispute to non-binding mediation using an industry expert mutually acceptable to the Parties. Any such mediation will be conducted in New York, New York, under the then-current rules of Judicial Arbitration and Mediation Services. The costs of any such mediation shall be shared by the Parties equally.
(iii) If all good faith attempts to resolve a Dispute through negotiations and mediation pursuant to Sections 12.7(c)(i) and 12.7(c)(ii) fail, then sixty (60) Business Days after notice is provided pursuant to Section 12.7(c)(i), upon request of either Party, such Dispute shall be submitted to arbitration according to the Rules of Arbitration of the International Chamber of Commerce (“ICC”) and shall be finally settled under such rules by a panel of three (3) arbitrators. Each Party shall nominate one (1) arbitrator and shall obtain its nominee’s acceptance of such nomination within thirty (30) days after delivery of the request for arbitration. In the event a Party fails to nominate an arbitrator within this time period upon request of any Party, such arbitrator shall instead be appointed by the ICC in accordance with its rules within thirty (30) days of receiving such request. The third arbitrator, who shall act as chairman of the arbitration panel, shall be nominated by the two (2) arbitrators nominated by the Parties. If such third arbitrator is not so nominated within thirty (30) days of the date of nomination of the later of the two (2) party-nominated arbitrators, such third arbitrator shall be chosen in accordance with the ICC rules by the ICC. The place of arbitration shall be the Republic of Singapore. The language of the arbitration shall be English. The scope of the authority of the arbitrators shall be limited to the strict application of law. For the avoidance of doubt, the arbitrators shall not have the right to award any punitive damages. Except as may be required by Applicable Law, neither a Party nor its representatives nor a witness nor an arbitrator may disclose the existence, content, or results of any arbitration hereunder without the prior written consent of both Parties. Neither Party shall be required to give general discovery of documents, but may be required only to produce specific, identified documents or types of documents, which are relevant or considered relevant by the arbitrators to the dispute. Each of the Parties hereto irrevocably and unconditionally waives trial by jury in any legal action or proceeding relating to this Agreement. Each Party participating in an arbitration pursuant to the terms of this Agreement shall, subject to the award of the arbitrators, pay an equal share of the arbitrator’s fees. The arbitrators shall have the power to award recovery of all costs (including reasonable attorney’s fees, administrative fees, arbitrator’s fees and court costs) to the prevailing Party. The arbitrators’ award will be final and binding. The Parties expressly exclude any and all rights to appeal, set aside or otherwise challenge an award by the arbitrators, insofar as such exclusion can validly be made. It shall be a condition precedent to submitting a Dispute for arbitration that the Parties have sought to resolve the Dispute by either Party notifying the other Party in writing for resolution to the Executive Officers who shall meet (whether in person or via teleconference) within fifteen (15) Business Days of such notice to seek resolution in good faith. If the Executive Officers are unable to resolve the Dispute at such meeting, either Party may pursue any remedy available to such Party at law or in equity, subject to the terms and conditions of this Agreement, including this Section 12.7(c).
(iv) Notwithstanding the provisions of Section 12.7(c)(iii), either Party may, without waiving any remedy under this Agreement, seek from any court having jurisdiction any equitable relief, including any injunctive or provisional relief and specific performance to protect the rights or property of that Party. Such remedies shall not be deemed to be the exclusive remedies for a breach of this Agreement but shall be in addition to all other remedies available at law or in equity.
(v) Until final resolution of the Dispute pursuant to Section 12.7(c)(iii): (A) this Agreement shall remain in full force and effect; and (B) the time periods for cure as to any termination shall be tolled. The Parties further agree that any payments made pursuant to this Agreement pending resolution of the Dispute shall be refunded if the arbitrators determine that such payments are not due.
12.8 Relationship of the Parties. Licensor and Avenue are independent contractors under this Agreement. Nothing contained herein is intended or is to be construed so as to constitute either Party as a partner, agent, or joint venture of the other Party. No Party will incur any debts or make any commitments for the other Party, except to the extent, if at all, specifically provided therein. Neither Licensor nor Avenue, respectively, shall have any express or implied right or authority to assume or create any obligations on behalf of or in the name of Licensor and Avenue, respectively, or to bind Licensor and Avenue, respectively, to any contract, agreement, or undertaking with any Third Party.
12.9 Fees and Expenses. Except as otherwise specified in this Agreement, each Party shall bear its own costs and expenses (including investment banking and legal fees and expenses) incurred in connection with this Agreement and the transactions contemplated hereby.
12.10 Third-Party Beneficiaries. There are no express or implied Third-Party beneficiaries hereunder. The provisions of this Agreement are for the exclusive benefit of the Parties, and no other Person or entity shall have any right or claim against any Party by reason of these provisions or be entitled to enforce any of these provisions against any Party, except for with respect to the applicable indemnification provisions set forth in Sections 10.1, 10.2, and 10.3.
12.11 Entire Agreement. This Agreement (together with any Exhibits or Schedules attached hereto) contains the entire agreement by the Parties with respect to the subject matter hereof and supersedes any prior express or implied agreements, understandings, and representations, either oral or written, which may have related to the subject matter hereof in any way, including any and all term sheets relating to the transactions contemplated by this Agreement and exchanged between the Parties prior to the Effective Date. In the event of any conflict, this Agreement shall prevail.
12.12 Signatures; Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via email in “.pdf” form with any electronic signature complying with the U.S. federal ESIGN Act of 2000 (e.g., DocuSign), or via other transmission method.
12.13 Equitable Relief; Cumulative Remedies. Notwithstanding anything to the contrary herein, the Parties shall be entitled to seek equitable relief, including injunction and specific performance as a remedy for any breach of this Agreement. Such remedies shall not be deemed to be the exclusive remedies for a breach of this Agreement but shall be in addition to all other remedies available at law or in equity. The Parties further agree not to raise as a defense or objection to the request or granting of such relief that any breach of this Agreement is or would be compensable by an award of money damages. No remedy referred to in this Agreement is intended to be exclusive, but each shall be cumulative and in addition to any other remedy referred to in this Agreement or otherwise available under Applicable Law.
12.14 Interpretation.
(a) Generally. This Agreement has been diligently reviewed by and negotiated by and between the Parties, and in such negotiations each of the Parties have been represented by competent (in-house or external) counsel, and the final agreement contained herein, including the language whereby it has been expressed, represents the joint efforts of the Parties and their counsel. Accordingly, in interpreting this Agreement or any provision hereof, no presumption shall apply against any Party as being responsible for the wording or drafting of this Agreement or any such provision, and ambiguities, if any, in this Agreement and shall not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision.
(b) Definitions; Interpretation.
(i) The definitions of the terms herein shall apply equally to the singular and plural forms of the terms defined and, where a word or phrase is defined herein, each of its other grammatical forms shall have a corresponding meaning.
(ii) Whenever the context may require, any pronoun shall include the corresponding masculine, feminine, and neuter forms.
(iii) The word “will” shall be construed to have the same meaning and effect as the word “shall.”
(iv) The words “including,” “includes,” “include,” “for example,” and “e.g.,” and words of similar import, shall be deemed to be followed by the words “without limitation.”
(v) The word “or” shall be interpreted to mean “and/or,” unless the context requires otherwise.
(vi) The words “hereof,” “herein,” and “herewith,” and words of similar import, shall, unless otherwise stated, be construed to refer to this Agreement as a whole and not to any particular provision of this Agreement.
(vii) Unless expressly stated otherwise or required by the applicable context: (A) all references herein to Sections, Schedules, or Exhibits shall be construed to refer to Sections, Schedules, and Exhibits of this Agreement; and (B) all references in any Section to any clause or subclauses shall be construed to refer to the clauses or subclauses of such Section.
(c) Subsequent Events. Unless the context requires otherwise: (i) any definition of or reference to any agreement, instrument, or other document herein shall be construed as referring to such agreement, instrument, or other document as from time to time amended, supplemented, or otherwise modified (subject to any restrictions on such amendments, supplements, or modifications set forth herein); (ii) any reference to any Applicable Law herein shall be construed as referring to such Applicable Law as from time to time enacted, repealed, or amended; and (iii) subject to Section 12.4, any reference herein to any Person shall be construed to include the Person’s successors and assigns.
(d) Headings. Section headings, captions, and the like are for convenience only and shall not be used in the interpretation or construction of this Agreement.
(e) Independent Significance. Although the same or similar subject matter may be addressed in different provisions of this Agreement, the Parties intend that, except as reasonably apparent on the face of the Agreement or as expressly provided in this Agreement, each such provision shall be read separately, be given independent significance, and not be construed as limiting any other provision of this Agreement (whether or not more general or more specific in scope, substance, or content).
12.15 Further Assurances. Each Party shall execute, acknowledge, and deliver such further instruments, and do all such other ministerial, administrative, or similar acts, as may be reasonably necessary or appropriate in order to carry out the expressly stated purposes and the clear intent of this Agreement.
12.16 Extension to Affiliates. Subject to Sections 2.2 (Sublicensing), 6.5(b) (Taxes; Withholding), and 12.4 (Assignment), Each Party may extend (but not assign) the rights, licenses, immunities, and obligations granted in this Agreement to one (1) or more of its Affiliates, provided that each Party shall be liable for the acts and omissions of its Affiliates. All applicable terms and provisions of this Agreement shall apply to any such Affiliate to which this Agreement has been extended to the same extent as such terms and provisions apply to each Party.
[Signature Page Follows]
In witness whereof , the Parties have executed this License Agreement by the signature of their duly authorized representatives, effective as of the Effective Date.
| AnnJi Pharmaceutical Co. Ltd. | |||
| By: | /s/ Xxxxx Xxxxx | ||
| Name: | Xxxxx Xxxxx | ||
| Title: | Chair and President | ||
| Avenue Therapeutics, Inc. | |||
| By: | /s/ Xxxxxxxxx XxxXxxx | ||
| Name: | Xxxxxxxxx XxxXxxx | ||
| Title: | Chief Executive Officer | ||
Exhibit A
Licensed Molecule
Exhibit B
Licensed Patents
Exhibit C
Charter
Exhibit D
Form of Subscription Agreement
[Exhibit D to AnnJi – Avenue License Agreement] D-1
Exhibit E
Form of Registration Rights Agreement
[Exhibit E to AnnJi – Avenue License Agreement] E-1
Exhibit F
Term Sheet for Clinical Supply Agreement
[Exhibit F to AnnJi – Avenue License Agreement] F-1
Exhibit G
Advisor Qualifications
[Exhibit G to AnnJi – Avenue License Agreement] G-1
Exhibit H
Commercial Supply Agreement Arbitration

