CERTAIN INFORMATION HAS BEEN OMITTED FROM THIS EXHIBIT IN PLACES MARKED “[***]” BECAUSE IT IS BOTH NOT MATERIAL AND WOULD LIKELY CAUSE COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED. LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT by...
Exhibit 10.28
CERTAIN INFORMATION HAS BEEN OMITTED FROM THIS EXHIBIT IN PLACES MARKED “[***]” BECAUSE IT IS BOTH NOT MATERIAL AND WOULD LIKELY CAUSE COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
by and between
BIOCRYST PHARMACEUTICALS, INC.
and
SHIONOGI & CO., LTD.
Dated as of February 28, 2007
LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
This LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT (this “Agreement”) is entered into as of February 28, 2007 by and between BIOCRYST PHARMACEUTICALS, INC., a corporation organized and existing under the laws of the State of Delaware having offices at 0000 Xxxxxxx Xxxx Xxxxx, Xxxxxxxxxx, Xxxxxxx 00000 (“BioCryst”), and SHIONOGI & CO., LTD., a corporation organized and existing under the laws of the Japan having offices at 1-8, Xxxxxxxxxx 0-xxxxx, Xxxx-xx, Xxxxx 000-0000, Xxxxx (“Shionogi”). BioCryst and Shionogi are each referred to herein by name or individually as a “Party” or collectively as the “Parties.”
BACKGROUND
WHEREAS, BioCryst owns or controls patents, know-how and other intellectual property related to a compound known as Peramivir.
WHEREAS, Shionogi has expertise in the discovery, development, manufacture and sale of pharmaceutical products in the Territory (as defined below).
WHEREAS, Shionogi wishes to obtain, and BioCryst wishes to grant, in the Territory only, rights and licenses under certain of BioCryst’s patents, know-how and trademarks to Shionogi so that Shionogi can obtain the necessary regulatory approvals to sell Licensed Products (as defined below) in the Territory.
NOW, THEREFORE, in consideration of the premises and mutual covenants herein below, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
ARTICLE 1
DEFINITIONS
1.1 Defined Terms. As used in this Agreement, the following terms shall have the meanings indicated:
(a) "Affiliate” means any corporation or other entity which is directly or indirectly controlling, controlled by or under common control of a Party, for so long as such control exists. For the purposes of this Section 1.1(a), “control” means direct or indirect ownership of fifty percent (50%) or more (or, if less than fifty percent (50%), the maximum ownership interest permitted by applicable Law) of the voting rights, shares or other equity or income interest of a Party.
(b) "BioCryst Know-How” means Know-How owned, developed or controlled by, or licensed to, BioCryst.
(c) "BioCryst Intellectual Property Rights” means all Intellectual Property Rights owned or controlled by BioCryst, including but not limited to BioCryst Know-How, the BioCryst Marks, and BioCryst Patents.
(d) "BioCryst Logo” means the company logo of BioCryst in a form provided, and approved in writing, by BioCryst from time to time.
(e) "BioCryst Marks” means the BioCryst Logo and any trademark, trade name or logo approved by BioCryst for use in connection with the Commercialization of the Licensed Product (whether or not owned by BioCryst).
(f) "BioCryst Patents” means those Patents owned, licensed or controlled by BioCryst which are filed in the Territory and which relate to the manufacture, use or sale of Licensed Products and/or Compound, which are set forth on Schedule 1.1(f).
(g) "Budget” means, individually, the applicable budget set forth in the Development Plan or Commercialization plan.
(h) "cGMPs” means the United States then-current good manufacturing practices and the equivalent standards of the Japanese government.
(i) "Change of Control” means, with respect to a Party, any of the following events: (i) any corporation or other entity is or becomes the “beneficial owner” (as such term is used in sections 12(d) and 13(d) of the Securities Exchange Act of 1934, as amended, except that a corporation or other entity shall be deemed to have “beneficial ownership” of all shares that any such corporation or other entity has the right to acquire, whether such right may be exercised immediately or only after the passage of time), of a majority of the total voting power represented by all classes of capital stock then outstanding of such Party normally entitled to vote in elections of directors of the Party; (ii) such Party consolidates with or merges into another corporation or entity, or any corporation or entity consolidates with or merges into such Party, other than (A) a merger or consolidation which would result in the voting securities of such Party outstanding immediately prior to such merger or consolidation continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or any parent thereof) a majority of the combined voting power of the voting securities of such Party or such surviving entity or any parent thereof outstanding immediately after such merger or consolidation, or (B) a merger or consolidation effected to implement a recapitalization of such Party (or similar transaction) in which no corporation or other entity becomes the beneficial owner, directly or indirectly, of voting securities of such Party representing a majority of the combined voting power of such Party’s then outstanding securities; or (iii) such Party conveys, transfers or leases all or substantially all of its assets to any corporation or other entity other than a wholly-owned subsidiary of such Party in one or more related transactions.
(j) “COGS” or “Cost of Goods Sold” means, [***].
(k) “Combination Product” means [***].
(l) "Commercialization” means, with respect to Licensed Product, any and all processes and activities conducted to establish and maintain sales for such Licensed Product, including offering for sale, detailing, selling (including launch), marketing (including education and advertising activities), promoting, manufacturing Licensed Product from Compound, but not manufacturing Compound itself), storing, transporting, supporting, distributing, and importing such product, but shall exclude development and manufacturing of Compound. “Commercialize” and “Commercializing” shall have their correlative meanings.
(m) "Compound” means the chemical compound known as “Peramivir” having the following chemical structure:
(1S,2S,3R,4R)-3-[(1S)-1-(acetylamino)-2-ethylbutyl] -4-[(aminoiminomethyl)amino]-2-hydroxy-cyclopentanecarboxylic acid, trihydrate
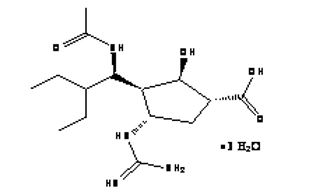
including the salts, esters, prodrugs, metabolites, tautomers, isomers, labeled compounds, conjugates, complexes, and other related compounds thereof.
(n) "Data” means any and all research, pharmacology, medicinal chemistry, chemistry, manufacturing and controls, nonclinical, clinical and other data (including investigator reports and clinical study reports (both preliminary and final), statistical analyses, expert opinions and reports, safety and other electronic databases), in each case specifically directed to, or used in the Development and Commercialization of, a Licensed Product and/or Compound.
(o) "Development” means, with respect to a Licensed Product, any and all processes and activities conducted to obtain Marketing Approvals for such product, including IND Enabling Studies and all other activities conducted thereafter, which may involve nonclinical studies, studies of chemistry, manufacturing and controls, clinical trials, quality of life assessments, pharmacoeconomics, post-marketing studies, label expansion studies, and further activities related to development of such product to a stage ready for Commercialization thereof. “Develop” and “Developing” shall have their correlative meanings.
(p) "Development Costs” means all costs and expenses to be incurred by a Party in the course of the Development of Licensed Product, including, but not limited, to the costs of conducting clinical trials, regulatory filing and maintenance fees, pricing and reimbursement filing and maintenance fees and costs relating to approval by the Regulatory Authority of the Licensed Product.
(q) "Development Plan” means the development plan pursuant to which Shionogi shall Develop the Licensed Product, which shall be prepared by the JSC within forty-five (45) days after the Effective Date, attached to this Agreement as Schedule 1.1(q) and made a part of this Agreement, and which may be modified at anytime, and from time to time by the JSC.
(r) “Diligent Efforts” means, [***].
(s) “Effective Date” means the date hereof.
(t) "FDA” means the United States Food and Drug Administration, or any successor entity thereto.
(u) "Field” means the prevention and/or treatment of all forms of influenza, in humans (including avian influenza) .
(v) "GAAP” means then-current generally accepted accounting principles in the United States as established by the Financial Accounting Standards Board or any successor entity or other entity generally recognized as having the right to establish such principles, in each case consistently applied.
(w) “Generic Product” means [***].
(x) "GLP” means the then-current good laboratory practice (or similar standards) for the performance of laboratory activities for pharmaceutical products as are required by any Regulatory Authority in the applicable jurisdiction.
(y) “Governmental Entity” means [***].
(z) "Guiding Principle” means in the timely Development of Licensed Products.
(aa) "IND” means an Investigation of New Drug filing (or the Japanese equivalent) with a Regulatory Authority in the Territory for purposes of obtaining permission to initiate human clinical testing in such jurisdiction.
(bb) "IND Enabling Studies” means studies which in each case are reasonably necessary to obtain approval of an IND, including GLP, ADME (absorption, distribution, metabolism and excretion), toxicology, pharmacology and safety pharmacology studies, or studies of chemistry, manufacturing and controls.
(cc) "Initiation” means, with respect to a particular clinical trial, the date of enrollment of the first subject or patient in such trial.
(dd) "Insolvency Event” means, with respect to any Party, the occurrence of any of the following: (i) such Party shall commence a voluntary case concerning itself under any bankruptcy, liquidation or insolvency code; (ii) an involuntary case is commenced against such Party under any bankruptcy, liquidation or insolvency code and the petition is not controverted within ten (10) business days, or is not dismissed within sixty (60) days, after commencement of the case; (iii) a custodian is appointed for, or takes charge of, all or substantially all of the property of such Party or such Party commences any other proceedings under any reorganization, arrangement, adjustment of debt, relief of debtors, dissolution, insolvency or liquidation or similar law of any jurisdiction whether now or hereafter in effect relating to such Party or there is commenced against such Party any such proceeding which remains undismissed for a period of sixty (60) days; (iv) any order of relief or other order approving any such case or proceeding is entered; (v) such Party is adjudicated insolvent or bankrupt; (vi) such Party suffers any appointment of any custodian, receiver or the like for it or any substantial part of its property to continue undischarged or unstayed for a period of sixty (60) days; (vii) such Party makes a general assignment for the benefit of creditors; (viii) such Party shall be unable to pay, its debts generally as they become due; (ix) such party shall call a meeting of its creditors with a view to arranging a compromise or adjustment of its debts; (x) such Party shall by any act or failure to act consent to, approve of or acquiesce in any of the foregoing; or (xi) any corporate, limited liability company, partnership or individual action, as applicable, is taken by such Party for the purpose of effecting any of the foregoing.
(ee) "Intellectual Property Rights” shall mean all Patent, copyright, trade secret, trademark and other proprietary and intellectual property rights, anywhere in the world.
(ff) “Japan” means the country of Japan.
(gg) “JSC” or “Joint Steering Committee” shall have the meaning set forth in Section 4.1.
(hh) "Know-How” means all scientific and technical information and know-how, trade secrets, Data and technology now or hereafter during the term of this Agreement (whether patented, patentable or not) owned, developed or acquired by a Party or any of its Affiliates or as to which such Party or any of its Affiliates has the right to license (without a payment obligation to any third party), which relates to the Licensed Product and/or Compound, including but not limited to (a) medical, clinical, toxicological or other scientific Data; and (b) processes and analytical methodology useful in the development, testing, formulation, analysis or packaging (but not manufacturing of Compound) of the Licensed Product and/or Compound.
(ii) "Law” means, individually and collectively, any and all laws, ordinances, rules, directives and regulations of any kind whatsoever of any governmental or regulatory authority within the applicable jurisdiction.
(jj) “Licensed Product” means [***].
(kk) "Marketing Approval” means, with respect to a particular product in a particular jurisdiction, all approvals, licenses, registrations or authorizations necessary for the Commercialization of such product in such jurisdiction. Marketing Approval shall be deemed to have been received upon first receipt by a Party or its designee of notice from the applicable Regulatory Authority that Commercialization of such product has been approved in such jurisdiction.
(ll) “Marketing Approval Application” or “MAA” means a filing with the applicable Regulatory Authority for purposes of obtaining Marketing Approval in a particular jurisdiction.
(mm) “Material Use” means [***].
(nn) "MHLW” means the Ministry of Health, Labour and Welfare of Japan or any successor entity thereto.
(oo) "NDA” means a New Drug Application (or the Japanese equivalent), including all supplements and amendments thereto, for the approval of the Licensed Product as a new drug by the MHLW or applicable Regulatory Authority in the Territory.
(pp) “Net Sales” means, [***].
(qq) "Patent” means any of the following, whether existing now or in the future anywhere in the world: (a) patents and patent applications; (b) continuations, continuations-in-part, divisionals and substitute applications with respect to any such patent application; (c) any patents issued based on or claiming priority to any such patent applications; (d) any reissue, reexamination, renewal or extension (including any supplemental patent certificate) of any such patents; and (e) any confirmation patent or registration patent or patent of addition based on any such patents.
(rr) "Phase I Clinical Trial” means a clinical trial of Licensed Product including small scale clinical trial in human subjects to obtain information on such Licensed Product’s safety, tolerability, pharmacological activity, pharmacokinetics and/or pharmacodynamics, and supporting Marketing Approval of such Licensed Product in the Field, as more fully defined in 21 CFR 312.21(a) or the equivalent statute or regulation in the Territory.
(ss) "Phase II Clinical Trial” means a well-controlled clinical trial of Licensed Product in patients, a principal purpose of which is to make a preliminary determination that such Licensed Product is safe for its intended use and to obtain sufficient information about such Licensed Product’s safety, as well as to obtain an indication of the dosage regimen required, to permit the design of further clinical studies, and supporting Marketing Approval of such Licensed Product in the Field, as more fully defined in 21 CFR 312.21(b) or the equivalent statute or regulation in the Territory.
(tt) "Phase III Clinical Trial” means a large scale clinical trial conducted in a sufficient number of patients that is designed to establish that the Licensed Product is safe and efficacious for its intended use, and to obtain warnings, precautions and adverse reactions that are associated with such Licensed Product in the dosage range to be prescribed, and supporting Marketing Approval of such Licensed Product in the Field, as more fully defined in 21 CFR 312.21(c) or the equivalent statute or regulation in the Territory.
(uu) “Plans” means, collectively, the Development Plan and the Commercialization Plan.
(vv) "Pre-Existing Third Party License” means the agreement dated as of November 23, 1994 by and between, on the one hand The UAB Research Foundation (“UAB”), and on the other hand BioCryst, as amended and may be amended from time to time.
(ww) "Promotional Material” means all Licensed Product packaging and labeling, and all written, printed, graphic, electronic, audio or video matter, including journal advertisements, sales visual aids, leave behind items, formulary binders, reprints, direct mail, direct-to consumer advertising, Internet postings, broadcast advertisements and sales reminder aids (for example, scratch pads, pens and other like items), in each case created by a Party or on its behalf and used or intended for use in connection with any promotion of a Licensed Product in the Territory.
(xx) "Regulatory Authority” means any federal, national, multinational, state, provincial or local regulatory agency, department, bureau or other governmental entity with authority over the Development, Commercialization or other use (including the granting of Marketing Approvals) of any Licensed Product in any jurisdiction, including the FDA, the MHLW and the Pharmaceuticals and Medical Devices Agency.
(yy) "Regulatory Filings” means all submissions, applications, filings and approvals by, with or from any Regulatory Authority.
(zz) "Sale”, “Sold” or “Sell” shall mean the sale, transfer or disposition of a Licensed Product for commercial or clinical purposes as provided in this Agreement, for value to a Third Party (whether an end user, wholesaler or otherwise) by Shionogi or any of its Affiliates.
(aaa) “Shionogi Know-How” means all Know-How owned, developed or acquired by or on behalf of Shionogi and its Affiliates.
(bbb) “Territory” means Japan.
(ccc) "Third Party” means any entity other than Shionogi or BioCryst, or their respective Affiliates.
(ddd) “U.S. Government” shall mean the federal government of the United States of America and any of its branches and instrumentalities, including its departments, agencies, bureaus, commissions, boards, courts, corporations, offices, and other entities, and any divisions or units thereof.
(eee) “Valid Claim” means a claim in any unexpired and issued BioCryst Patent that has not been revoked or held invalid by a final unappealable decision of a court or governmental agency of competent jurisdiction.
ARTICLE 2
LICENSE GRANT, RETAINED RIGHTS AND PROVISION OF DATA
2.1 License Grant; Reservation of Rights. Solely to the extent necessary for Shionogi to perform its obligations hereunder in accordance with the terms of this Agreement, and subject to all of the rights retained hereunder, BioCryst hereby grants Shionogi a personal, non-sublicensable, non-transferable, non-assignable right and license under the BioCryst Patents and BioCryst Know-How, to (i) exclusively Develop Licensed Products solely in the Field and in the Territory, and (ii) exclusively Commercialize Licensed Products solely in the Field and in the Territory. Other than as explicitly set forth in this Section 2.1, no other licenses to the BioCryst Intellectual Property Rights or otherwise (including but not limited to all rights in BioCryst Intellectual Property Rights outside the Field and outside the Territory) are granted in this Agreement. [***]
2.2 Manufacturing. [***].
2.3 Retained Rights; Government Rights. All rights granted to Shionogi hereunder are subject to rights reserved by and/or granted to UAB or the U. S. Government. Shionogi specifically understands and agrees that BioCryst shall have the unrestricted and fully unfettered right under the BioCryst Intellectual Property Rights outside of the Field in the Territory and outside of the Territory in the Field, including in connection with the testing, Development, manufacture and Commercialization of products covered by the BioCryst Patents and BioCryst Know-How.
2.4 BioCryst Logo. BioCryst hereby grants to Shionogi a personal, non-sublicensable, non-transferable, non-assignable right and license to use the BioCryst Logo on Licensed Products in the Field in the Territory in accordance with the terms of this Agreement. Shionogi agrees to xxxx (i) all packaging, labeling and package inserts for Licensed Product and (ii) such Promotional Material as shall be agreed upon by the Parties in writing, with the BioCryst Logo. All use of the BioCryst Logos shall be as directed by BioCryst and shall be in a form, style and prominence as directed by BioCryst, and all goodwill associated with the use of the BioCryst Logos shall inure to BioCryst.
2.5 Transfer of Data.
(a) BioCryst Existing Data. BioCryst shall transfer to Shionogi, [***] after the Effective Date, all Data (to the extent contractually permissible) possessed or controlled by BioCryst as of the Effective Date, including, but not limited to [***]. Certain Data (including the Future Data described below) specified by Shionogi shall be accompanied by a statement or certificate by an employee or agent of BioCryst in such form as mutually agreed upon by the Parties. Shionogi acknowledges and agrees that delivery of Data in electronic form shall be acceptable. BioCryst represents and warrants that, to its best knowledge, all studies, testings and clinical trials from which the Data were derived were conducted in accordance with then-applicable United States Laws.
(b) Shionogi Nonclinical Data and Phase I Data. Shionogi will promptly, [***] disclose to BioCryst all nonclinical Data and Data from Phase I Clinical Trials (whether or not such Data meets the criteria of “Future Data”, set forth below in Section 2.5(c)(i)) developed by or on behalf of Shionogi or which otherwise comes into Shionogi’s possession or control after the Effective Date.
(c) Future Data.
(i) Data Exchange. From time to time (including upon request by either Party) during the Term of this Agreement, each Party shall, [***], disclose to the other Party all previously undisclosed Data relating to the Licensed Product and/or Compound that (i) comes into such Party’s possession or control after the Effective Date and (ii) is necessary to obtain or maintain a Marketing Approval for a Licensed Product (“Future Data”). [***].
(ii) Material Use. [***].
(d) Raw Data. A Party generating the Data subject to exchange under Section 2.5(a) and (c)(ii) shall keep all of raw data from which the Data were derived in commercially usable condition in accordance with applicable Laws and shall allow the other Party access to such raw data upon the reasonable request by the other Party.
ARTICLE 3
COMMERCIAL MATTERS
3.1 General. Shionogi shall use Diligent Efforts to Develop Licensed Products in the Field in the Territory, including any IND, MAA, Marketing Approval and any approval for any product labeling or Promotional Materials and to maintain all such Regulatory Filings; and unless otherwise agreed or required by applicable Laws, all such approvals shall be owned by and be held in the name of Shionogi or its Affiliates. BioCryst shall cooperate with Shionogi in preparing all Regulatory Filings and correspondence with Regulatory Authorities in the Territory. Shionogi shall use Diligent Efforts to Commercialize the Licensed Products in the Territory. Notwithstanding the foregoing covenant to cooperate, the Parties acknowledge and agree that all responsibility for Regulatory Filings and exercising Diligent Efforts in the Territory shall be Shionogi’s.
3.2 BioCryst’s Participation.
(a) Protocols. Shionogi shall pay due consideration to the protocols and desired endpoints in the trials sponsored by BioCryst in preparing the protocols for the clinical trials to be conducted by or on behalf of Shionogi in the Territory. Shionogi shall provide BioCryst with the outline of the draft of protocols (in the English language) for clinical trials. Consistent with applicable Laws, Shionogi shall afford BioCryst an opportunity to comment on such protocols within fifteen (15) business days after receipt and shall consider in good faith such BioCryst’s comments with respect thereto.
(b) Filings and Correspondence. Shionogi shall promptly provide BioCryst with (i) copies of all Regulatory Filings relating to the Territory submitted by Shionogi (in the original language) and (ii) copies of material correspondence with Regulatory Authorities in the Territory (including minutes of meetings, telephone conferences and/or discussions with such Regulatory Authority) (in the original language). Shionogi agrees to assist BioCryst the English translation of such documents at BioCryst’s cost.
(c) Regulatory Meetings. Shionogi shall promptly provide BioCryst with reasonable advanced notice (to the extent practicable) of meetings, scheduled or unscheduled, with any Regulatory Authority that pertain to a Licensed Product, and, to the extent not prohibited by applicable Law, shall afford BioCryst’s representatives an opportunity to attend and participate in all such meetings with relevant Regulatory Authorities as observers, to the extent reasonably practicable under the circumstances. Likewise, BioCryst shall promptly provide Shionogi with reasonable advanced notice (to the extent practicable) of meetings, scheduled or unscheduled, with any Regulatory Authority that pertain to a Licensed Product developed by BioCryst (for clarity, BioCryst itself, and not licensees of BioCryst) outside the Territory, and, to the extent not prohibited by applicable Law, shall afford Shionogi’s representatives an opportunity to attend and participate in all such meetings with relevant Regulatory Authorities as observers, to the extent reasonably practicable under the circumstances.
(d) JSC Oversight. In addition to Section 3.2(a), (b) and (c) above with respect to Regulatory Filings and meetings with Regulatory Authorities, Shionogi’s Development and Commercialization activities, including the content and subject matter of, and strategy for, any MAA, all correspondence submitted to Regulatory Authorities related to clinical trial design, all proposed labeling and labeling discussions and decisions with Regulatory Authorities, and all post-Marketing Approval labeling discussions and decisions with Regulatory Authorities (including the final approved labeling), and post-Marketing Approval labeling changes or expansions, in each case relating in any way to Licensed Product, shall be subject to reasonable oversight by the JSC.
3.3 Cooperation. Each Party agrees to make its personnel reasonably available, upon reasonable notice by the other Party, at their respective places of employment to consult with the other Party on issues arising related to the activities conducted in accordance with this Article 3 or otherwise relating to regulatory matters involving the Licensed Product, including any request from any Regulatory Authority, including regulatory, scientific, technical and clinical testing issues, or otherwise. Each Party (the “Enabling Party”) agrees to cooperate with the other (the "Filing Party”), at its request, to comply with specific requests of a Regulatory Authority (such as requests to inspect clinical trial sites), with respect to Data supplied or to be supplied by the Enabling Party to the Filing Party for filing with such Regulatory Authority, or with respect to Licensed Product supplied by the Enabling Party. The Enabling Party shall ensure that its contractors likewise comply with this Section 3.3.
3.4 Use of Contractors. Subject to the terms of this Agreement, Shionogi shall have the right to use the services of Third Party contractors, including contract research organizations, contract sales forces and the like, to assist Shionogi in fulfilling its obligations and exercising its rights under this Agreement, provided that each such Third Party is bound by a written agreement, that is consistent with terms of this Agreement, including confidentiality and intellectual property ownership provisions consistent with those set forth therein. Shionogi shall provide BioCryst with quarter annual updates of the identity of all contractors who assist Shionogi in exercising its rights or fulfilling its obligations hereunder. For the purposes of clarity, Shionogi shall remain responsible for the performance by all such contractors.
3.5 Development Supply.
(a) From the Effective Date through [***], BioCryst will supply to Shionogi, at Shionogi‘s expense, and Shionogi agrees to purchase exclusively from BioCryst, (i) the Licensed Product (including its placebos if needed) for use in clinical studies to be conducted in the Territory by or on behalf of (subject to the terms of Section 3.4, above) Shionogi, and (ii) the Compound necessary for the Development of the Licensed Product.
(b) On [***], BioCryst will supply Shionogi with Compound (at Shionogi’s expense) and Shionogi will have established the necessary resources to formulate Licensed Product from Compound for clinical use. The Parties agree to evaluate in good faith the above arrangement on an ongoing basis to ensure the timely progression and development of the Licensed Product in the Territory.
(c) During the term of this Agreement, BioCryst shall supply to Shionogi, [***] of Compound (in such individual amounts and at such times as reasonably agreed upon by the Parties) for Shionogi to use Diligent Efforts to develop an optimized intramuscular formulation of the Compound for use by Shionogi in the Territory and for use by BioCryst outside the Territory pursuant to Section 10.2. In addition, if the Parties agree in writing that Shionogi may explore the possibility to Develop New Formulations under mutually agreed conditions, BioCryst shall also supply to Shionogi, [***] (but upon such additional terms and conditions as the Parties may agree), the Compound for Development of such New Formulation. Both Parties understand and agree that there are no assurances that Shionogi’s efforts will generate an optimized intramuscular formulation of the Compound or lead to the successful Development of New Formulations.
(d) All Licensed Product and Compound delivered by BioCryst to Shionogi shall be manufactured in accordance and in compliance with the specifications to be determined by BioCryst; provided, however that BioCryst shall give due consideration to revised specifications (if any) requested by Shionogi. BioCryst shall carry out its responsibilities hereunder in conformance with cGMPs and all other applicable Laws (all of the foregoing, in the United States). All supply of Licensed Product and Compound shall be subject to the terms and conditions set forth in this Section 3.5 and shall be subject to the terms and on prices as attached in Schedule 3.5 hereto.
(e) BioCryst shall transfer to Shionogi the formulation and manufacturing processes that (i) are maintained or subsequently developed or optimized by BioCryst and (ii) are designed to ensure the quality of Licensed Product. All transfer of such Know-How shall take place in Birmingham, Alabama unless otherwise agreed upon by the Parties. If the transfer is to occur wholly or partially outside of Birmingham, Alabama, then Shionogi shall promptly [***].
3.6 Commercial Supply. BioCryst will supply to Shionogi, at Shionogi’s expense, and Shionogi agrees to purchase exclusively from BioCryst, Compound in bulk powder form for purposes of Commercialization in the Territory. Such supply shall be subject to the terms and on prices as attached in Schedule 3.5 hereto. Shionogi shall be responsible for manufacturing Licensed Product from Compound provided by BioCryst to Shionogi.
3.7 Covenant and Manufacturing Option. For the avoidance of doubt, the Parties hereby agree that they intend that BioCryst supply, and Shionogi exclusively purchase from BioCryst, Compound for all uses contemplated in this Agreement. Shionogi hereby acknowledges and agrees that it has no rights to, and shall not (and it and its Affiliates shall not otherwise) manufacture or have manufactured or purchase from a Third Party Compound unless otherwise agreed in writing between BioCryst and Shionogi. However, Shionogi shall have the option to manufacture or have manufactured Compound for the Territory; [***]. In the event that Shionogi exercises such option to manufacture Compound, BioCryst shall transfer to Shionogi all BioCryst Know-How or other technologies relating to manufacture of the Compound. All transfer of such Know-How shall take place in Birmingham, Alabama unless otherwise agreed upon by the Parties. If the transfer is to occur wholly or partially outside of Birmingham, Alabama, then Shionogi shall [***]. In such event, all products Commercialized in the Field which derive from such Compound shall be deemed for all purposes hereunder to be a Licensed Product.
ARTICLE 4
GOVERNANCE
4.1 Joint Steering Committee. Promptly following the Effective Date, but no later than forty-five (45) days after the Effective Date, the Parties shall establish a joint steering committee (the "Joint Steering Committee” or "JSC") to oversee, review and coordinate the conduct and progress of the Development of Licensed Product in the Territory. The JSC shall be responsible for, among other things: annually reviewing and updating the Development Plan; monitoring the competitive landscape for the Licensed Product in the Territory; and undertaking such other matters as are specifically provided for the JSC under this Agreement. Shionogi shall keep the JSC fully informed of progress and results of its activities under the Development Plan through its members on the JSC and as otherwise provided herein.
4.2 Committee Membership. The JSC shall be comprised of an equal number of representatives from each of BioCryst and Shionogi. The exact number of such representatives shall initially be three (3) for each of BioCryst and Shionogi, or such other number as the Parties may agree. The initial members of the JSC shall be as set forth on Exhibit A. Either Party may replace its respective committee representatives at any time with prior written notice to the other Party. Unless otherwise agreed, the JSC shall have at least one representative with relevant decision-making authority from each Party such that the JSC is able to effectuate all of its decisions within the scope of its responsibilities. In the event a JSC member from either Party is unable to attend or participate in a JSC meeting, the Party who designated such representative may designate a substitute representative for the meeting in its sole discretion.
4.3 Subcommittees. From time to time, the JSC may establish subcommittees to oversee particular projects or activities, and such subcommittees will be constituted as the JSC approves (each, a “Subcommittee”). If any Subcommittee is unable to reach a decision on any matter after endeavoring in good faith for [***] to do so, such matter shall be referred to the JSC for resolution as provided in Section 4.6.
4.4 Committee Co-Chairs. Each Party shall appoint one of its members to the JSC to co-chair the JSC’s meetings (each, a “Co-Chair”). The Co-Chairs shall (i) ensure the orderly conduct of the JSC’s meetings, (ii) attend each JSC meeting (either in-person, videoconference or telephonically), and (iii) prepare and issue written minutes of each meeting within thirty (30) days thereafter accurately reflecting the discussions and decisions of such Committee. In the event the Co-Chair from either Party is unable to attend or participate in a JSC meeting, the Party who designated such Co-Chair may designate a substitute Co-Chair for the meeting in its sole direction.
4.5 Committee Meetings. The JSC shall meet quarterly, or as more or less often as otherwise agreed by the Parties, and such meeting may be conducted by telephone, videoconference or in person as determined by the Co-Chairs. As appropriate, other employee representatives of the Parties may attend JSC meetings as nonvoting observers if mutually agreed by the Parties. Each Party may also call for special meetings of the JSC to resolve particular matters requested by such Party and within the areas of responsibility of the JSC. Each Co-Chair shall ensure that its JSC members receive adequate notice of such meetings.
4.6 Decision Making. Decisions of the JSC shall be made by consensus of the members present in person or by other means (e.g., teleconference) at any meeting, with each Party having one vote. In order to make any decision, the JSC must have present (in person, videoconference or telephonically) at least one representative of each Party. All decisions of the JSC shall be consistent with the Guiding Principle. Notwithstanding anything herein to the contrary, the JSC shall have no authority to amend, modify or waive compliance with this Agreement. In the event that the JSC cannot reach agreement with respect to any matter that is subject to its decision-making authority, then the matter shall be resolved pursuant to the provisions set forth in Article 15 (“Dispute Resolution”).
4.7 Performance of Representatives. BioCryst and Shionogi shall cause each of their representatives on the JSC and any other committee (including Subcommittees) or team established under this Agreement to vote, and shall otherwise perform their respective activities under this Agreement, in a good faith manner consistent with the Guiding Principle.
4.8 Day-to-Day Decision-Making Authority. Shionogi shall have decision making authority with respect to the day-to-day operations of the Development and Commercialization of Licensed Product in the Territory, provided that such decisions are not inconsistent with the Plans or the Guiding Principle, other decisions of the JSC and any other committee (including Subcommittees) or team established under this Agreement within the scope of their authority specified therein, or the express terms and conditions thereof.
ARTICLE 5
DEVELOPMENT
5.1 General. Shionogi shall use Diligent Efforts to Develop in the Territory Licensed Product for use in the Field, all in accordance with the Development Plan. Shionogi shall be responsible for conducting, and shall use Diligent Efforts to conduct, the activities set forth in the Development Plan to progress and complete such activities within the timeframes set forth in the Development Plan. Shionogi agrees not to perform, directly or indirectly (or through any Third Party on behalf of Shionogi), any Development activities outside the Territory with respect to any Licensed Product, and not to perform any Development activities in or for use in the Territory with respect to any Licensed Product except in accordance with the Development Plan or, in each case, as otherwise provided herein. Shionogi shall pay due consideration to the protocols and desired endpoints in the trials sponsored by BioCryst in preparing the protocols for the clinical trials to be conducted by or on behalf of Shionogi in the Territory.
5.2 Product Development outside the Territory. BioCryst shall have sole decision-making authority with regard to the Development and Commercialization of Licensed Products outside the Territory (and no rights under this Agreement are granted to Shionogi outside the Territory).
5.3 Development Reports. Within thirty (30) days after the end of each calendar quarter, Shionogi shall prepare and provide to BioCryst a written report that (i) summarizes the progress of the Development activities performed by Shionogi hereunder during the preceding calendar quarter, (ii) identifies any issues or circumstances of which it is aware that may prevent or adversely affect in a material manner the activities under the Development Plan in the then-current calendar quarter, and, to the extent reasonably practicable, (iii) identifies steps that may be taken, or changes that may be made, to resolve such issues. Shionogi shall maintain records in sufficient detail as will properly reflect all work done in the performance of activities arising out of, in conducting, or otherwise in connection with its Licensed Product Development activities. Likewise, BioCryst shall prepare and provide to Shionogi a quarterly written report summarizing the Development performed by BioCryst outside the Territory in reasonable detail. To the extent known by BioCryst and permitted by its licensees (and not otherwise prohibited by Law), BioCryst shall provide the foregoing information to Shionogi relating to BioCryst’s licensees outside of the Territory, provided that Shionogi shall keep such information strictly confidential pursuant to Section 11.2. BioCryst shall also provide Shionogi with its development plan and any amendment thereto in a timely manner.
5.4 Interactions Between Committees and Internal Teams. The Parties recognize that while they will establish the various committees and teams for the purposes hereof, each Party maintains internal structures (including its own committees, teams and review boards) that will be involved in administering such Party’s activities under this Agreement. The Parties shall establish procedures (including the appointment of alliance managers) to facilitate communications between the various committees and teams hereunder and the relevant internal committee, team or board within the Party in order to maximize the efficiency of the Parties’ activities pursuant to this Agreement. In addition, each of the Joint Steering Committee and any subcommittee shall coordinate with each other as appropriate.
5.5 Development Costs. Development Costs relating to the Licensed Product in the Territory shall be borne 100% by Shionogi, subject to Section 2.5(c).
5.6 Clinical Milestone Events. Shionogi shall use Diligent Efforts to achieve the events set forth in the table below (each a “Milestone Event”) by the date set forth in the table below (each a “Milestone Date”) in furtherance of the Development of the Licensed Products. The Parties agree that the JSC shall have forty-five (45) days from the Effective Date to review, and comment on the Milestone Events contained herein and shall use such Milestone Events contained herein as a framework to create other more detailed steps it feels necessary to Develop the Licensed Products. Any changes or additions to the Milestone Events or Milestone Dates made by the JSC shall be made in good faith and shall be consistent with the Guiding Principles and shall be included in the Development Plan.
|
Milestone Event |
Milestone Date |
|||
|
Submission of the first Phase II Clinical Trial protocol to MHLW or the applicable Regulatory Authority in the Territory |
[***] |
|||
|
Submission of the first Phase III Clinical Trial protocol to MHLW or the applicable Regulatory Authority in the Territory |
[***] |
|||
|
Submission of NDA to MHLW or the applicable Regulatory Authority in the Territory |
[***] |
|||
ARTICLE 6
COMMERCIALIZATION
6.1 General. Shionogi undertakes that it will Commercialize the Licensed Products in the Territory and carry out its obligations hereunder in compliance with all applicable Laws.
6.2 Commercialization Plan. Shionogi shall, beginning [***] prior to the anticipated date of Marketing Approval for a Licensed Product in the Territory and continuing until the expiration of the term of this Agreement, prepare and submit to BioCryst for its review and comment (which comments Shionogi shall consider reasonably and in good faith), a Territory-wide plan for the Commercialization of such Licensed Product in the Field following receipt of the requisite Marketing Approval (a “Commercialization Plan”) covering in detail (to the extent available) the [***] period prior to the first anticipated date on which such Licensed Product would be first shipped in commercial quantities for commercial sale to Third Parties in the Territory, and providing general plans (with an estimated Budget) for the [***] period following such anticipated shipping date. On or before December 15 of each calendar year, Shionogi shall update each Commercialization Plan to include detailed plans for the following calendar year (with an estimated Budget), and shall submit such updated Commercialization Plan to BioCryst for its review and comment (which comments Shionogi shall reasonably consider in good faith). Each Commercialization Plan shall include a detailed description of each Commercialization activity to be conducted in the Territory thereunder, including the following, as applicable:
(a) [***];
(b) [***];
(c) [***];
(d) [***];
(e) [***];
(f) [***];
(g) [***];
(h) [***];
(i) [***]
(j) [***].
6.3 Amendments. Shionogi shall review the Commercialization Plans on a regular basis during each calendar year and shall promptly submit any significant modifications of such plans to BioCryst for review and comment (which comments Shionogi shall reasonably consider in good faith).
6.4 Promotional Material. No later than [***] prior to the expected date of National Health Insurance (NHI) price listing for the first Licensed Product in the Territory, Shionogi shall provide BioCryst with a representative example of its proposed major Promotional Material, and BioCryst shall have the right to make comments or observations thereon within [***] of its receipt thereof. Thereafter, Shionogi shall provide BioCryst with a representative example of its Promotional Material as soon as practicable after BioCryst’s written request, such a request shall not be made more than once each calendar year, and BioCryst shall have the right to make comments or observations thereon within [***] of its receipt thereof. Notwithstanding BioCryst’s right to make comments or observations, and other than with respect to the BioCryst Logos (with respect to which BioCryst shall have sole decision-making power, even with respect to Shionogi’s Promotional Literature) all other decisions with respect to Shionogi’s Promotional Material shall be made by Shionogi in its sole discretion after in good faith taking into consideration BioCryst’s comments and observations.
6.5 Costs of Commercialization. Shionogi shall be responsible for all costs associated with the Commercialization of Licensed Products within the Territory.
6.6 Trademark. Shionogi shall have the right to select the trademark, from among the stocks of trademarks of Shionogi or BioCryst to be used in connection with the Commercialization of the Licensed Product in the Territory after paying due consideration of the opinions of the JSC. If Shionogi selects a registered trademark owned by BioCryst in the Territory, BioCryst shall grant to Shionogi a royalty-free license to use such trademark for the Licensed Product in the Field and in the Territory for the term of this Agreement upon such additional terms as BioCryst may request. If Shionogi selects its own registered trademark for use on Licensed Products in the Territory (the “Licensed Product Xxxx”), the ownership of such Licensed Product Xxxx shall remain in Shionogi and such Licensed Product Xxxx shall not be included in the BioCryst Marks.
6.7 Outside of Territory. Shionogi shall ensure that no Licensed Products are Commercialized outside of the Territory. Shionogi shall ensure that no Licensed Products are manufactured outside of the Territory, except as specifically provided herein.
ARTICLE 7
ADVERSE EVENT AND PRODUCT COMPLAINT REPORTING
7.1 By Shionogi. Shionogi will promptly (a) provide BioCryst with all Licensed Product complaints, adverse event information and safety data from clinical studies and Commercialization in its control; and (b) report all such adverse events in the Territory in accordance with Laws, and provide such information to BioCryst in such a manner and time so as to enable BioCryst to comply with all applicable Laws outside the Territory. Shionogi shall maintain a Territory-wide adverse event database for the Licensed Products and shall generate adverse event reports for BioCryst’s use. BioCryst shall have free and unfettered access to all data in such database. Shionogi shall be responsible for submitting adverse events reports to the applicable Regulatory Authorities in the Territory. Shionogi shall bear 100% of the costs of adverse events reporting and of maintaining the Territory-wide adverse events database.
7.2 By BioCryst. BioCryst will promptly (a) provide Shionogi with all Licensed Product complaints, adverse event information and safety data from clinical studies and Commercialization in its control; and (b) report all such adverse events outside the Territory in accordance with Laws, and provide such information to Shionogi in such a manner and time so as to enable Shionogi to comply with all applicable Laws in the Territory. BioCryst shall, at its own cost, maintain a global adverse event database for the Licensed Products and shall generate adverse event reports outside the Territory for Shionogi’s use. Shionogi shall have free and unfettered access to all data in such database. BioCryst shall be responsible for submitting adverse events reports to the applicable Regulatory Authorities outside the Territory, with respect to which BioCryst shall bear 100% of the costs.
7.3 Adverse Events and Reporting. As soon as reasonably practicable, but in no event later than three (3) months after the Effective Date, the Parties shall jointly establish, and mutually agree upon adverse event and complaint reporting procedures which each Party must adhere to and shall execute a separate agreement relating thereto. Such procedures shall at all times include any measures necessary for each Party to fully comply with applicable Laws and such procedures may be amended with the Parties’ mutual consent from time to time. Such operating procedures and any material revisions to them shall be provided to the JSC for review and comment before execution of the aforesaid agreement. In addition, each Party shall promptly notify the other if such Party becomes aware of any information or circumstance that is likely to have a material adverse effect on the Development or Commercialization of the Licensed Products.
ARTICLE 8
INSURANCE
8.1 Shionogi shall obtain and maintain, during the term of this Agreement, comprehensive general liability insurance, including products liability insurance and coverage for clinical trials, with reputable and financially secure insurance carriers in a form and at levels, respectively, that are reasonable and customary in the pharmaceutical industry for companies of comparable size and activities, but in any event shall be a minimum of [***] per occurrence with an annual aggregate limit of not less than [***]. The premium of any insurance will be borne by Shionogi. Such liability insurance shall be maintained on an occurrence basis to provide such protection for [***] after expiration or termination of this Agreement. Shionogi shall furnish to BioCryst on request certificates issued by the insurance company setting forth the amount of the liability insurance (or evidence of self insurance). BioCryst shall receive thirty (30) days written notice prior to termination or material reduction to the level of Shionogi’s insurance policy as required by this Article 8.
ARTICLE 9
PAYMENTS
9.1 Signing Fee. Within [***] of the Effective Date, in partial consideration for the licenses and rights granted to Shionogi under this Agreement, Shionogi shall pay or cause to be paid, a non-refundable, non-creditable payment of Fourteen Million U.S. Dollars ($14,000,000) to BioCryst as a signing fee (the “Signing Fee”).
9.2 Milestone Payments. As additional partial consideration for the licenses and rights granted by BioCryst to Shionogi herein, Shionogi shall pay to BioCryst the following one-time, non-refundable, non-creditable payments:
(a) [***]. Notwithstanding the foregoing, in the event of bona fide extraordinary circumstances relating to the profile of the Licensed Product which first arose during the Phase [***] Trial [***], the Parties will discuss in good faith extending the period for payment under Section 9.2(a)(i), above, which in no event shall exceed the [***] of receipt of the final case report form from the Phase [***] Trial for a Licensed Product in the Territory. If such bona fide safety concern is related to a formulation and Shionogi notifies BioCryst of its good faith decision to re-conduct a Phase [***] Trial with a newly developed formulation, then the payment set forth in this Subsection 9.2 (a) shall be made no later than the earlier of [***]. If such newly developed formulation again demonstrates in Phase [***] Trial a bona fide safety concern after re-conducting Phase [***] Trial and Shionogi has again made the good faith decision to repeat Phase [***] Trial(s) with another newly developed formulation, [***].
(b) [***] in the Territory for a Licensed Product.
(c) [***] in the Territory.
9.3 Royalty Payments. In partial consideration for the licenses and rights granted to Shionogi under this Agreement, Shionogi shall pay to BioCryst the following royalty payments, which shall be paid within [***] after the end of each calendar quarter:
(a) for sales of Licensed Products to any Governmental Entity ( “Non-Commercial Sales"), the greater of [***]. For the purposes of this Agreement, “Adjusted Net Sales” shall mean [***].
(b) for sales of Licensed Products to non-Governmental Entity parties (“Commercial Sales”), royalty payments on incremental Net Sales according to the following rates for the following ranges of Net Sales:
(i) [***].
(ii) [***].
By way of example [***]:
|
Amount of Net Sales |
Royalty Rate |
Royalty Payment |
||||||
|
[***] |
[* |
**]% |
[* |
**] |
||||
|
[***] |
[* |
**]% |
[* |
**] |
||||
|
[***] |
[* |
**] |
||||||
(c) Term. The term for the obligations to pay royalties under this Section 9.3 shall expire on the date that is the later of (i) [***]. If the royalty obligations in this Section 9.3(c) are prohibited by applicable Law, then the royalty obligations shall continue until such time as the obligation is prohibited by applicable Law.
(d) Patent Coverage Adjustment. If there is no Valid Claim that, but for this Agreement would be infringed by the manufacture, use or sale of Licensed Product in the Territory, then the royalty obligations from Shionogi to BioCryst shall be reduced by [***]. If there is a Valid Claim, and if
(i)
[***] , then [***]
(ii)
[***]
Where:
|
• |
GPS = the number of units of Generic Products sold in the Territory for a given period; and |
||
|
• |
LPS = the number of units of Licensed Products sold in the Territory for a given period. |
For purposes of this Section 9.3(d) the number of “units” sold shall be appropriately adjusted to account for units of varying volumes.
(e) Third Party Rights.
(i) New Formulations. In the event Third Party Intellectual Property Rights are necessary (or desired by Shionogi) in order to Develop or Commercialize New Formulations of Licensed Products in the Territory and BioCryst desires to obtain a license to such Third Party Intellectual Property Rights for outside the Territory, Shionogi shall procure a worldwide license to such Intellectual Property Rights from such Third Party (each, a “Third Party New Formulations License”). Shionogi agrees (a) to keep BioCryst apprised of and involved in the negotiations of such license, (b) to take into consideration BioCryst’s requests regarding the same, and (c) not to execute any agreement for a Third Party New Formulations License with such Third Party without obtaining BioCryst’s prior consent on the terms and conditions of such agreement which relate to the license outside the Territory. If BioCryst obtains rights under the Third Party New Formulations License outside the Territory, BioCryst shall bear royalties and other payments owed to the licensing Third Party of such Third Party Intellectual Property Rights outside the Territory. The Parties agree that no royalty offset (described in Section 9.3(e)(ii), below) shall be available to Shionogi for payments made under Third Party New Formulations Licenses.
(ii) Royalty Offset. In the event that, Shionogi, in order to exploit the licenses and rights granted to it under Section 2.1 hereof, actually makes royalty or other payments to one or more Third Parties (“Third Party Payments”) as consideration for a license to Intellectual Property Rights of such Third Parties, in the absence of which the importation or use of Compound or manufacture, use or sale of Licensed Product could not legally be made in the Territory due to the infringement of valid claims in such Intellectual Property Rights of such Third Parties, then Shionogi shall have the right to reduce the royalties otherwise due to BioCryst pursuant to this Section 9.3 for such Licensed Product by [***] of such Third Party Payments. Notwithstanding the foregoing, the offset set forth in this Section 9.3(e) (ii) shall in no event reduce the royalty for Licensed Product in the Territory by [***] of the royalty rate otherwise due to BioCryst pursuant to this Section 9.3.
(f) Royalty Reports. All royalty payments shall be accompanied by a written report from Shionogi to BioCryst, showing for the calendar quarter for which such payment applies, in U.S. Dollars, all information required by BioCryst to verify the royalty payments payable hereunder, including but not limited to the information set forth on Schedule 9.3(f) and any other information customary with industry standards of the Territory.
9.4 One-time Net Sales Milestone Payments for Sales of Licensed Products. In partial consideration for the licenses and rights granted to Shionogi under this Agreement, Shionogi shall pay or cause to be paid, to BioCryst within [***] of the first achievement of the following milestones, the following one-time, non-refundable, non-creditable payments in the amounts set forth next to such milestone:
|
Cumulative Calendar Year Net Sales |
||
|
including both Non-Commercial Sales |
||
|
and Commercial Sales |
Payment (in U.S. Dollars) |
|
|
• [***] |
• [***] |
|
|
• [***] |
• [***] |
|
|
• [***] |
• [***] |
|
|
• [***] |
• [***] |
|
|
Total Commercial Sales Milestone Payments: |
$95 million |
9.5 Payments for Clinical and Commercial Supply. In consideration for the supply of Licensed Product and Compound for Development and commercial use, Shionogi shall pay to BioCryst an amount equal to [***].
9.6 Payments; Foreign Exchange. All amounts referenced herein are in United States Dollars. Unless otherwise specified, all payments under this Agreement shall be made within thirty (30) days of the date of invoice, in U.S. Dollars, by wire transfer to a bank and to an account designated by BioCryst. Any payment amount, or any component used to calculate a payment amount, computed in a currency other than the U.S. Dollar shall be converted into U.S. Dollars at the exchange rate for transfers from such currency to U.S. Dollars as quoted by [***] on the business day immediately prior to the payment day. Any payments or portions thereof due hereunder which are not paid when due shall bear interest equal to the lesser [***]. This Section 9.6 shall in no way limit any other remedies available to either Party. Other than as set forth herein or except in the case of overpayment, all payments hereunder shall be non-refundable and non-creditable.
9.7 Taxes. The Parties agree that all amounts due by Shionogi to BioCryst under this Agreement shall be treated as “royalties” for purposes of the U.S. Japan Income Tax Treaty. Accordingly, all payments hereunder shall be made free and clear, and without deduction or withholding, of any present or future taxes, duties, levies and other similar charges, including related interest, additions to tax and penalties (“Taxes”). [***].
9.8 Audit Rights. Each Party shall have the right, at its own expense, to inspect the other Party’s relevant financial books and records through an independent certified public accountant designated by the auditing Party and reasonably acceptable to the Party being audited upon at least fifteen (15) days advance written notice for the purpose of confirming the audited Party’s compliance with the terms herein. Each Party and its Affiliates shall keep full, true and accurate books of account containing all particulars that may be necessary for the purpose of calculating all royalties and other payments payable under this Agreement and shall retain such books of account for a minimum of [***] after the applicable reporting period. Audit results and findings shall be shared by the auditing Party with the audited Party. If the audit reveals an underpayment, the audited Party shall make up such underpayment within thirty (30) days, plus interest at the rate of [***]. If the audit reveals an overpayment, the auditing Party shall return such overpayment within thirty (30) days. If the audit reveals an underpayment or overpayment in the amount of [***] for any calendar quarter, then the audited Party shall reimburse the auditing Party for the costs of the audit. Notwithstanding the foregoing, any audit of BioCryst shall be limited to the books and records necessary in order to verify the calculation of COGS.
ARTICLE 10
INTELLECTUAL PROPERTY
10.1 Prosecution and Maintenance of BioCryst Patents. BioCryst shall prosecute and maintain the BioCryst Patents as BioCryst reasonably determines. As between BioCryst and Shionogi, BioCryst shall determine whether, where and when to maintain any of the BioCryst Patents and to file any patent applications included in the BioCryst Patents and, if it determines to take any action, it shall do so at its own cost and expense. However, with respect to the BioCryst Patent Japanese [***] (“Core Patent”), BioCryst undertakes to prosecute and maintain such Core Patent in the Territory and shall provide Shionogi with copies of all material communications received from or filed in patent office(s) in the Territory. In the event that BioCryst determines not to continue to prosecute or maintain any of the BioCryst Patents, BioCryst shall notify Shionogi not less than [***] before any relevant deadlines. Thereafter, Shionogi shall have the right to pursue at its sole cost and discretion the prosecution or maintenance of such patent application or patent. The Parties shall reasonably cooperate with each other in gaining patent term extension(s) or the like applicable to the BioCryst Patents in the Territory.
10.2 Inventions. The ownership of any improvements to the BioCryst Intellectual Property Rights (including any Patents and any other BioCryst Intellectual Property Rights, whether patentable or not) that include, are based on, or are derived from, Compound or Licensed Product (or the Development or Commercialization thereof), BioCryst Patents or the BioCryst Know-How, including but not limited to the Shionogi Know-How (“Agreement Improvements”, which for the avoidance of doubt shall include Intellectual Property Rights underlying New Formulations and shall include the Licensed Product Xxxx) shall be determined in accordance with the laws of inventorship of the United States. Shionogi hereby grants to BioCryst an irrevocable, exclusive, worldwide, perpetual, royalty-free, fully paid up, transferable and sublicensable right under Shionogi’s interest in Agreement Improvements (including without limitation to Shionogi’s interest in joint inventions made with BioCryst) to make, have made, use, sell, import, have imported, and otherwise fully commercially exploit such inventions outside of the Territory in all fields and inside the Territory outside of the Field (it being understood that the Licensed Product Xxxx shall not be used by BioCryst inside the Territory). Notwithstanding the foregoing, in the case that Agreement Improvements are relevant to Compound or Licensed Products (including New Formulations) and also other products, technology or applications, then the foregoing license grant shall be deemed to be non-exclusive.
10.3 Infringement by Third Party.
(a) Each Party shall notify the other Party promptly of any conduct on the part of Third Parties that it deems to be a potential infringement, misappropriation, act of unfair competition, dilution or other violation of the BioCryst Intellectual Property Rights.
(b) BioCryst will have the first right, in its sole discretion and expense, to take any and all action it deems necessary to stop such violation, including the bringing of an action based on the BioCryst Intellectual Property Rights or for unfair competition with respect thereto. BioCryst will exclusively control the prosecution or settlement of any such action and will bring such action in the name of BioCryst only or in the name of both BioCryst and Shionogi. Shionogi shall have the right (but not obligation) to participate in such action through its own counsel at Shionogi’s cost. If BioCryst does not take any action to stop such violation within sixty (60) days from the date when either of BioCryst or Shionogi notifies the other Party of such violation, Shionogi shall have the right (but not obligation) to take any and all action it deems necessary to stop such violation. In either case, the Parties shall provide all reasonable assistance to each other and reasonably cooperate to prosecute or settle such action. Each of BioCryst and Shionogi shall recover their respective actual out-of-pocket expenses or equitable proportions thereof associated with any such action or settlement thereof from any monetary proceeds, damages and other relief obtained by BioCryst and/or Shionogi. Any excess amount shall be retained by the Party in charge of the enforcement action; provided, however, that if such party is Shionogi, all such proceeds shall be deemed to be “Net Sales” under the terms of this Agreement, for which a royalty shall be paid to BioCryst.
(c) In the event that any action is brought against BioCryst or Shionogi or any Affiliate of either Party alleging the violation of the Intellectual Property Rights of a Third Party by reason of the Development, manufacture or Commercialization of Compound and/or Licensed Product in the Field and in the Territory, Shionogi shall have the first right, but not the obligation, to defend itself and BioCryst in such action at its sole expense. BioCryst shall have the right to participate in such action through its own counsel at BioCryst’s cost. The Parties shall provide all reasonable assistance to each other and reasonably cooperate to defend or settle such action. Neither Party shall assert counterclaims based on the BioCryst Intellectual Property Rights, or compromise, settle or otherwise dispose of any such action without the other Party’s advice and prior consent, provided that the Party not defending the action shall not unreasonably withhold its consent to any settlement which does not have a material adverse effect on its business.
ARTICLE 11
PUBLICITY; CONFIDENTIALITY
11.1 Publicity.
(a) Oversight by Communications Subcommittee. The JSC shall constitute a Communications Subcommittee. Prior to communicating or disclosing any publications, abstracts, scientific presentations, websites, press releases or other disclosures relating to the relationship of the Parties, each Party shall submit to the Communications Subcommittee a copy of such communication or disclosure for review in accordance with this Agreement and guidelines established by the Communications Subcommittee. Such guidelines shall include among other things a process for ensuring submission of all such communications and disclosures by the Parties to the Communications Subcommittee reasonably in advance of disclosure to allow sufficient time for review, including the preparation of a communications calendar that anticipates disclosures expected to be made during the following calendar quarter. If the Communications Subcommittee is unable to agree upon the acceptability of a public disclosure after endeavoring to do so in good faith, BioCryst’s Co-Chair shall have the right to cast the deciding vote.
(b) Prior Review. Each Party may disclose results and significant developments regarding Licensed Product and other activities in connection with this Agreement from time to time only with the approval of the other Party, which approval shall not be unreasonably withheld, conditioned or delayed. Such disclosures may include achievement of significant events in the research, Development (including regulatory process), manufacture or Commercialization of Licensed Product under this Agreement or the receipt of material payments. When a Party (the “Requesting Party”) elects to make any such public disclosure under this Section 11.1(b), it will give the other Party (the “Cooperating Party”) through its Communications Subcommittee representatives, a copy of any such statement and at least five (5) business days to review and comment on such statement, it being understood that if the Cooperating Party does not notify the Requesting Party in writing within such five (5) business day period of any objections, such disclosure shall be deemed approved, and in any event the Cooperating Party shall work diligently and reasonably to agree on the text of any proposed disclosure in an expeditious manner. The principles to be observed in such disclosures shall be accuracy, compliance with applicable Law and regulatory guidance documents, and reasonable sensitivity to potential negative reactions of the FDA and/or the MHLW or the applicable Regulatory Authority in the Territory (and their foreign counterparts).
(c) Publications. Except as required by applicable Law or court order, any publication or presentation of Confidential Information (as defined below), including studies or clinical trials carried out by a Party or the Parties under this Agreement shall be subject to the oversight and guidelines of the Communications Subcommittee. The Communications Subcommittee shall establish, promptly after the Effective Date, guidelines that (i) allow for each Party’s timely review of all such publications or presentations, (ii) provide for protection of Confidential Information and ensure coordination with other applicable joint-committees prior to any disclosure of protectable subject matter, and (iii) ensure that all such publications and presentations are consistent with good scientific practice and accurately reflect work done and the contributions of the Parties. Unless otherwise mutually agreed upon by the Parties, (A) the Party desiring to publish or present any (the “Publishing Party”) shall transmit to the other Party (the “Reviewing Party”) for review and comment a copy of the proposed publication or presentation, at least [***] prior to the submission of the proposed publication or presentation to any Third Party; (B) the Publishing Party shall postpone the publication or presentation for up to an additional [***] upon request by the Reviewing Party in order to allow the Reviewing Party to consider appropriate patent applications or other protection to be filed on information contained in the publication or presentation; (C) upon request of the Reviewing Party, the Publishing Party shall remove all Confidential Information of the Reviewing Party from the information intended to be published or presented; and (D) the Publishing Party shall consider all reasonable comments made by the Reviewing Party to the proposed publication or presentation. For the avoidance of doubt, no restriction set forth in this Section 11.1 shall apply to BioCryst’s actions outside the Territory.
11.2 Confidential Information; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, the Parties agree that the receiving Party shall keep confidential and shall not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement any confidential and proprietary information or materials of the other Party furnished to it by the other Party or learned by it from or through its exercise of its rights pursuant to this Agreement (collectively, “Confidential Information”) during the term hereof and for a period of five (5) years following the termination of this Agreement; provided, however, that the obligation to keep a Party’s trade secrets confidential shall survive for such time as such information remains a protected trade secret. For the avoidance of doubt, Agreement Improvements shall be deemed to be the Confidential Information of both Parties. Notwithstanding the foregoing, Confidential Information shall not include any information to the extent that it can be established by written documentation of the receiving Party that such information:
(a) was already known to the receiving Party, other than under an obligation of confidentiality (except to the extent such obligation has expired or an exception is applicable under the relevant agreement pursuant to which such obligation established), at the time of disclosure;
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement; or
(d) was disclosed to the receiving Party, other than under an obligation of confidentiality (except to the extent such obligation has expired or an exception is applicable under the relevant agreement pursuant to which such obligation established), by a Third Party who had no obligation to the disclosing Party not to disclose such information to others.
11.3 This Agreement. Each of the Parties agrees not to disclose to any Third Party the terms and conditions of this Agreement without the prior approval of the other Party, except (i) to advisors (including attorneys and accountants) on a need to know basis, in each case under circumstances that reasonably ensure the confidentiality thereof, or (ii) under circumstances that reasonably ensure the confidentiality of the information, to the extent necessary to comply with the terms of agreements with Third Parties existing as of the Effective Date; provided, however, that if a Party is required by Law to make any such disclosure of the terms or conditions of this Agreement, it will give reasonable advance notice to the other Party of such disclosure requirement and will use its reasonable efforts to secure confidential treatment of the terms and conditions which the Parties agree should be maintained as confidential. In addition to the foregoing, with respect to complying with the disclosure requirements of the U.S. Securities and Exchange Commission, the Financial Services Agency of Japan (collectively the “Securities Authorities”) in connection with any required filing with any of the Securities Authorities of this Agreement, the filing Party shall provide to the other Party a copy of the proposed filing and the Parties shall work cooperatively in good faith, taking into consideration the other Party’s suggestions, regarding the text of the disclosure as well as information for which the filing Party will seek to obtain confidential treatment. Notwithstanding the foregoing, the Parties shall agree upon and release a mutual press release to announce the effectiveness of this Agreement together with a corresponding Question & Answer outline for use in responding to inquiries about the Agreement; thereafter, the Parties may each disclose to Third Parties the information contained in such press release and Question & Answer outline without the need for further approval by the other.
11.4 Authorized Disclosure. Except as expressly provided otherwise in this Agreement, each Party may use and disclose Confidential Information of the other Party as follows: (i) under appropriate confidentiality provisions substantially equivalent to those in this Agreement, in connection with the performance of its obligations or as reasonably necessary or useful in the exercise of its rights under this Agreement in complying with the terms of agreements with Third Parties existing as of the Effective Date; (ii) to the extent such disclosure is reasonably necessary in filing or prosecuting patent, copyright and trademark applications in accordance with this Agreement, prosecuting or defending litigation, complying with applicable governmental regulations, obtaining regulatory approval or fulfilling post-approval regulatory obligations, or otherwise required by Law, provided, however, that if a Party is required by Law to make any such disclosure of the other Party’s Confidential Information it will, except where impracticable for necessary disclosures (for example, in the event of medical emergency), give reasonable advance notice to the other Party of such disclosure requirement and, except to the extent inappropriate in the case of patent applications, will use its reasonable efforts to secure confidential treatment of such Confidential Information required to be disclosed; (iii) in communication with advisors (including lawyers and accountants) on a need to know basis, in each case under appropriate confidentiality provisions substantially equivalent to those of this Agreement; or (iv) to the extent mutually agreed to by the Parties. Notwithstanding the foregoing and for the avoidance of doubt, Shionogi acknowledges and agrees that BioCryst may disclose to the U.S. Government or other Regulatory Authority all Data received from Shionogi; provided, however, that in the event BioCryst intends to disclose the Data or information (including databases) under a different process than the process applied by Shionogi in its protocol, CSR and/or analytical report, then BioCryst shall obtain Shionogi’s consent prior to such disclosure.
ARTICLE 12
SHIONOGI OPTION TO LICENSE OTHER PRODUCTS
12.1 Right of First Negotiation. BioCryst hereby grants [***].
12.2 [***].
ARTICLE 13
REPRESENTATIONS, WARRANTIES AND COVENANTS
13.1 By BioCryst. BioCryst hereby represents and warrants to, and covenants with, Shionogi as follows:
(a) BioCryst is duly organized and validly existing under the Laws of its jurisdiction of incorporation and has full corporate power and authority, and has taken all corporate action necessary, to enter into and perform its obligations under this Agreement.
(b) This Agreement is a legal, valid and binding obligation of BioCryst, enforceable against BioCryst in accordance with its terms. Neither the execution and delivery of this Agreement by BioCryst, nor the performance by BioCryst of its obligations hereunder, conflicts with any agreement, instrument or understanding, oral or written, by which BioCryst is bound.
(c) To BioCryst’s knowledge, no authorization, consent, approval, license, exemption of or filing or registration with any court or governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, under any applicable Law currently in effect, is required in connection with the execution and delivery of this Agreement by BioCryst, or the performance by BioCryst of its obligations hereunder.
(d) BioCryst has sufficient right in and to BioCryst Patents and BioCryst Know-How to enable it to carry out its obligations under this Agreement. To its best knowledge, BioCryst has not committed and shall not commit any breach of the Pre-Existing Third Party License which will lead to a forfeiture of the rights granted under this Agreement.
(e) BioCryst has not been debarred or the subject of debarment proceedings by any Regulatory Authority. BioCryst shall not knowingly use in connection with the Development of Licensed Product any employee, consultant or investigator that has been debarred or the subject or debarment proceedings by any Regulatory Authority.
(f) BioCryst shall use diligent efforts in carrying out its obligations pursuant to this Agreement, consistent with all applicable United States Laws and highest industry standards.
13.2 By Shionogi. Shionogi hereby represents and warrants to, and covenants with, BioCryst as follows:
(a) Shionogi is duly organized and validly existing under the Laws of its jurisdiction of incorporation and has full corporate power and authority, and has taken all corporate action necessary, to enter into and perform its obligations under this Agreement.
(b) This Agreement is a legal, valid and binding obligation of Shionogi, enforceable against Shionogi in accordance with its terms. Neither the execution and delivery of this Agreement by Shionogi, nor the performance by Shionogi of its obligations hereunder, conflicts with any agreement, instrument or understanding, oral or written, by which Shionogi is bound.
(c) To Shionogi’s knowledge, no authorization, consent, approval, license, exemption of or filing or registration with any court or governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, under any applicable Law currently in effect, is required in connection with the execution and delivery of this Agreement by Shionogi, or the performance by Shionogi of its obligations hereunder.
(d) Neither Shionogi nor any of its Affiliates have been debarred or the subject of debarment proceedings by any Regulatory Authority. Neither Shionogi nor any of its Affiliates shall use in connection with the Development of Licensed Product any employee, consultant or investigator that has been debarred or the subject or debarment proceedings by any Regulatory Authority.
(e) Shionogi shall use Diligent Efforts in carrying out its obligations pursuant to this Agreement, consistent with all applicable Laws and highest industry standards.
13.3 Disclaimer. ALL PATENTS, KNOW-HOW, DATA AND OTHER INTELLECTUAL PROPERTY RIGHTS, AND ALL LICENSED PRODUCT AND COMPOUND PROVIDED HEREUNDER IS PROVIDED AS-IS. NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY WITH REGARD TO ANY PATENT, KNOW-HOW, DATA, LICENSED PRODUCT, COMPOUND OR OTHERWISE IN CONNECTION WITH THIS AGREEMENT EXCEPT AS SPECIFICALLY SET FORTH IN THIS AGREEMENT. EXCEPT AS EXPRESSLY PROVIDED IN THIS AGREEMENT, EACH PARTY DISCLAIMS, AND WAIVES ALL WARRANTIES OF AND TO, THE OTHER, EXPRESS OR IMPLIED, ARISING BY LAW OR OTHERWISE, WITH RESPECT TO ANY LICENSED PRODUCT, BIOCRYST INTELLECTUAL PROPERTY RIGHTS OR OTHERWISE IN CONNECTION WITH THIS AGREEMENT, INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, IMPLIED WARRANTY ARISING FROM COURSE OF PERFORMANCE, COURSE OR DEALING OR USAGE OF TRADE, AND ANY IMPLIED WARRANTY OF NONINFRINGEMENT.
OTHER THAN IN CONNECTION WITH A PARTY’S INDEMNITY OBLIGATIONS, A BREACH OF THE LICENSE GRANTS TO SHIONOGI OR IN CONNECTION WITH AN INDEMNIFYING PARTY’S INDEMNITY OBLIGATIONS UNDER THIS AGREEMENT, IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER PARTY FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, EXEMPLARY, SPECIAL OR PUNITIVE DAMAGES WHATSOEVER RESULTING OR ARISING FROM ANY CAUSE OR CLAIM WHATSOEVER, WHETHER BY TORT, OR CONTRACT OR OTHERWISE, INCLUDING BUT NOT LIMITED TO LOSS OF PROFITS AND LOSS OF SAVINGS, BUSINESS DATA, OR GOODWILL. SHIONOGI’S SOLE AND EXCLUSIVE REMEDY FOR DAMAGES RELATING TO LICENSED PRODUCT OR COMPOUND SUPPLIED BY BIOCRYST SHALL BE REPLACEMENT BY BIOCRYST OF ANY NON-CONFORMING MATERIAL.
13.4 Compliance with Laws. Shionogi understands and acknowledges that BioCryst is subject to regulation by agencies of the U.S. Government, including, but not limited to, the U.S. Department of Commerce and the U.S. Treasury Department’s Office of Foreign Assets Control, both of which regulate the import, export and diversion of certain products and technology from and to certain countries. Any and all obligations of BioCryst to provide the Compound or Licensed Product, as well as any other technical information or assistance, and all rights on the part of Shionogi to perform its obligations hereunder, shall be subject in all respects to such United States laws and regulations as shall from time to time govern the license and delivery of technology and products abroad by persons subject to the jurisdiction of the United States, including but not limited to regulations promulgated under Executive Order No. 12924 of August 19, 1994 issued pursuant to the President’s authority under the International Emergency Economic Powers Act, Title 50 U.S. C., Chapter 35, Section 1701 et seq. and those contained in Title 31, Part 500 of the U.S. Code of Federal Regulations. Shionogi agrees to cooperate with BioCryst including, without limitation, providing required documentation, in order to comply with any and all applicable United States Laws and regulations. Shionogi warrants that it shall comply with all United States Laws and regulations governing exports in effect from time to time that are applicable to BioCryst as if such laws and regulations were applicable to Shionogi. In the event any rights or obligations hereunder are or become illegal or the subject of sanctions or restrictions, then BioCryst shall have the right, in its sole discretion, to terminate, without penalty and immediately upon written notice, the provisions of this Agreement which in BioCryst’s sole discretion relate to such restrictions.
ARTICLE 14
TERM AND TERMINATION
14.1 Term. The term of this Agreement shall commence on the Effective Date and shall continue unless terminated pursuant to this Article 14.
14.2 Termination By BioCryst. Without limiting any other rights or remedies either Party may have under this Agreement or otherwise, BioCryst shall have the right to terminate this Agreement upon notice to Shionogi at any time in the event that the Pre-Existing Third Party License is terminated (or modified to an extent that BioCryst cannot perform its obligations hereunder), or if any of the following shall occur:
(a) if Shionogi breaches, in any material respect, any of its representations, warranties or obligations under this Agreement, and, if curable, such breach is not cured within thirty (30) days after Shionogi’s receipt of written notice of such breach; or
(b) if Shionogi suffers an Insolvency Event.
14.3 Termination By Shionogi.
(a) Termination for Breach. Without limiting any other rights or remedies either Party may have under this Agreement or otherwise, Shionogi shall have the right to terminate this Agreement upon written notice to BioCryst, if BioCryst breaches, in any material respect, any of its representations, warranties or obligations under this Agreement, and, if curable, such breach is not cured within thirty (30) days after BioCryst’s receipt of written notice of such breach.
(b) Termination without Cause. Shionogi shall have the right to terminate this Agreement at any time at its discretion by providing BioCryst with [***] prior written notice. Shionogi agrees to terminate this Agreement pursuant to this Section 14.3(b) in the event that, at any time, Shionogi does not plan to exercise, or has not exercised, or will not exercise, Diligent Efforts to Develop or Commercialize Licensed Products.
14.4 Effect of Termination.
(a) The termination or expiration of this Agreement shall not affect any payment of any debts or obligations accruing prior to such date of termination or expiration. Sections [***] shall survive the termination or expiration of this Agreement.
(b) Furthermore, upon termination of this Agreement all licenses and rights granted to Shionogi shall terminate, and Shionogi shall terminate all activities related to the Development and Commercialization of Licensed Products, cease all use of the BioCryst Intellectual Property Rights, and shall return to BioCryst all documents (including copies) of any kind concerning the Compound, BioCryst Intellectual Property Rights or the Licensed Products and other Confidential Information received from BioCryst or otherwise created in the course of performing this Agreement. Shionogi shall promptly destroy all Compound and Licensed Product which it holds in stock at the time of such termination or expiration of this Agreement. Shionogi shall promptly and diligently provide to BioCryst all assistance reasonably necessary in order to assist BioCryst in transitioning and assigning to BioCryst all aspects of the Parties’ relationship hereunder, including but not limited to all work in progress, regulatory submissions, Agreement Improvements and Shionogi Know-How to BioCryst. For the avoidance of doubt, and in consideration for BioCryst’s granting the New NI Compound option to Shionogi, Shionogi hereby assigns to BioCryst all Intellectual Property Rights in the Agreement Improvements (to the extent that such Agreement Improvements are solely related to the Compound and/or Licensed Product; and to the extent such Agreement Improvements are not solely related to the Compound and/or Licensed Product, Shionogi will and hereby does grant to BioCryst an irrevocable, non-exclusive, worldwide, perpetual, royalty-free, fully paid up, transferable and sublicensable right under Shionogi’s interest in such Agreement Improvements), and agrees to take all further actions reasonably requested by BioCryst to perfect such assignment and vest the rights assigned to BioCryst in BioCryst or its designee(s). Shionogi shall pay to BioCryst any and all amounts already due under this Agreement and transfer and assign to BioCryst any Regulatory Filings relating to the Licensed Product that are in Shionogi’s possession (including and but not limited to any IND, MAA, Marketing Approval or any approval for any product labeling or Promotional Materials owned by or held in the name of Shionogi or its Affiliates), including the ownership thereof.
(c) In the event of termination of this Agreement by a Party for the other Party’s uncured material breach, such termination shall not affect the terminating Party’s right to claim damages against the breaching Party for such breach. In the event the non-breaching Party waives its right under Section 14.3 to terminate, such non-breaching Party shall not be prevented from seeking damages for a material breach by the breaching Party during the Term of this Agreement.
ARTICLE 15
DISPUTE RESOLUTION
15.1 General. Any dispute or disagreement between the Parties arising out of, under or in connection with this Agreement shall be settled in accordance with this Article 15.
15.2 Informal Mediation. In the event any dispute or disagreement between the Parties arises out of, under or in connection with this Agreement, either Party shall submit the dispute to the following executives for resolution: for BioCryst, Senior Executive Officer responsible for corporate development (or such successor as may be named by BioCryst); for Shionogi: General Manager of License Department responsible for alliance management (or such successor as may be named by Shionogi). Such executives shall work together in good faith for a period of [***] to resolve the dispute.
15.3 Escalation. In the event that a dispute is not resolved pursuant to the provisions of Section 15.2, above, the dispute or disagreement shall be submitted to the Senior Officers (defined below) for resolution. In such event, either Party, by written notice to the other Party, may formally request that the dispute be resolved by the Senior Officers, specifying the nature of the dispute with sufficient specificity to permit adequate consideration by the Senior Officers. The Parties shall cause their respective Senior Officers to use commercially reasonable efforts to resolve the referred dispute in good faith within [***] of receiving such written notification, including, without limitation, by means of a face-to-face meeting if requested by either Party. “Senior Officers” means, for Shionogi, the Senior Executive Officer or similar ranking officer, and for BioCryst, the CEO or similar ranking officer.
15.4 Arbitration. Any disputes, controversies between the Parties arising under or in connection with this Agreement not resolved through the procedures set out in the preceding clauses of this ARTICLE 15 shall be finally settled by arbitration without the right to appeal, in New York City, if requested by Shionogi, or in Osaka, if requested by BioCryst, before a panel of three (3) arbitrators under the Rules of the International Chamber of Commerce (“ICC Rules”), which Rules are deemed to be incorporated by reference to this clause. Each Party shall nominate an arbitrator, and the Party-nominated arbitrators shall agree upon the third arbitrator who will be the chair of the arbitrate tribunal. If the two Party-nominated arbitrators are unable to agree upon the chair, the chair shall be selected as provided in the ICC Rules. The arbitration award shall be binding upon the Parties and enforceable by any court of competent jurisdiction. The arbitration award shall include an award as to costs including attorney fees. These provisions shall not prevent a Party from making application to any court of competent jurisdiction seeking equitable relief in case of urgency.
15.5 No Arbitration of Intellectual Property Issues. Notwithstanding anything to the contrary contained herein, unless otherwise agreed by the Parties, disputes relating to Intellectual Property Rights shall not be subject to arbitration, and shall be submitted to a court of competent jurisdiction.
ARTICLE 16
INDEMNIFICATION
16.1 Indemnification by Shionogi. Shionogi shall indemnify, defend, and hold harmless BioCryst, the Affiliates of BioCryst, and their respective officers, directors, managers, members, partners, owners, employees, licensees, successors, and assigns (collectively, the "BioCryst Indemnitees”) from and against all actions, causes of action, suits, debts, obligations, losses, damages, amounts paid in settlement, liabilities, costs, and expenses whatsoever, including reasonable attorneys’ fees (collectively, “Losses”), whether arising out of a claim involving a Third Party or between the Parties, resulting to, imposed upon, asserted against, or incurred by any of BioCryst Indemnitees in connection with, or arising out of or relating to this Agreement, including without limitation, the Licensed Products and the Development or Commercialization thereof (including but not limited to product liability claims and recalls in the Territory), and/or any breach by Shionogi of a representation, warranty or covenant under this Agreement.
16.2 Indemnification by BioCryst. BioCryst shall indemnify, defend, and hold harmless Shionogi, the Affiliates of Shionogi, and their respective officers, directors, managers, members, partners, owners, employees, licensees, successors, and assigns (collectively, the “Shionogi Indemnitees”) from and against all Losses, whether arising out of a claim involving a Third Party or between the Parties, resulting to, imposed upon, asserted against, or incurred by any of Shionogi Indemnitees in connection with, or arising out of or relating to (a) any material breach of this Agreement by BioCryst or (b)the gross negligence or willful misconduct on the part of BioCryst.
16.3 Indemnification Procedures. If any claim, demand, action or proceeding is made or commenced by any Third Party (a “Third-Party Claim”) against any BioCryst Indemnitee or Shionogi Indemnitee that is entitled to be indemnified with respect thereto under this ARTICLE 16 (the "Indemnified Party”), the Indemnified Party shall give the other Party (the “Indemnifying Party”) prompt written notice thereof; the failure to give such written notice shall not affect the liability of the Indemnifying Party under this Agreement except to the extent such failure materially and adversely affects the ability of the Indemnifying Party to defend the Third-Party Claim. The Indemnifying Party shall have the right to assume the defense and resolution of the Third-Party Claim, provided that (i) the Indemnified Party shall have the right to participate in the defense of the Third-Party Claim at its own expense through counsel of its choice (control of the defense will remain with the Indemnifying Party), (ii) the Indemnifying Party shall not consent to the entry of any judgment or enter into any settlement that would require any act or forbearance on the part of the Indemnified Party or which does not unconditionally release the Indemnified Party from all liability in respect of the Third-Party Claim without the prior written consent of the Indemnified Party, which consent shall not be unreasonably withheld, conditioned or delayed, and (iii) the Indemnified Party may undertake the defense of the Third-Party Claim, at the Indemnifying Party’s expense, if the Indemnifying Party fails promptly to assume and diligently to prosecute the defense.
ARTICLE 17
MISCELLANEOUS
17.1 Assignment. This Agreement and any rights granted hereunder are personal to each Party and shall not be sold, assigned, sublicensed, encumbered or otherwise transferred (each a "Transfer”), directly or indirectly, by operation of law or otherwise, by either Party without the prior written consent of the other Party, which consent may be granted or withheld in such other Party’s sole discretion; provided, however, that BioCryst, without notice and at any time for any reason, may Transfer this Agreement in whole or in part to (i) any of its Affiliates who agree to be bound by the terms and conditions of this Agreement or (ii) to any successor of BioCryst by merger, sale of all or substantially all of its business assets to which this Agreement relates or otherwise. Any attempted Transfer of this Agreement or any of the rights granted hereunder in violation of this Section 17.1 shall be void ab initio. The consent by any Party to any Transfer shall not constitute a waiver of the necessity for such consent in any subsequent Transfer. Notwithstanding anything to the contrary contained in this Section 17.1, either Party shall have the right to assign its rights under this Agreement in connection with a Change of Control of such Party to the successor in interest to such Party; provided, however, that the Party effecting a Change of Control shall give written notice of such Change of Control to the other Party within ten (10) days of such Change of Control, and such Transfer shall not relieve such assigning Party of its obligations under this Agreement except to the extent any permitted assignee assumes in writing the obligations of the assigning Party under this Agreement.
17.2 Section 365(n). All licenses granted under this Agreement are deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined in Section 101 of such Code. In addition, if Shionogi desires to register license granted hereunder with the Japanese Patent Office, BioCryst shall provide reasonable cooperation to this end.
17.3 Governing Law; Venue. This Agreement shall be governed by and construed and enforced in accordance with the laws of the State of New York without regard to choice-of-law principles of the State of New York. All actions arising under this Agreement which are not arbitrable shall be brought in the State and Federal Courts located in New York County, New York. The Parties hereby irrevocably submit to the jurisdiction of such courts.
17.4 Severability. If any one or more of the provisions of this Agreement shall be held to be invalid, illegal or unenforceable, the validity, legality or enforceability of the remaining provisions hereof shall not in any way be affected or impaired thereby. In the event any provisions shall be held invalid, illegal or unenforceable, the Parties shall use best efforts to substitute a valid, legal and enforceable provision, which, insofar as practical, implements the purposes hereof.
17.5 Notices. All notices, requests, demands and other communications hereunder shall be given in writing and shall be: (a) personally delivered; (b) sent by telecopier, facsimile transmission or other electronic means of transmitting written documents; or (c) sent to the Parties at their respective addresses indicated herein by registered or certified U.S. mail, return receipt requested and postage prepaid, or by private overnight mail courier service. The respective addresses to be used for all such notices, demands or requests are as follows:
If to BioCryst, to:
BIOCRYST PHARMACEUTICALS, INC.
0000 Xxxxxxx Xxxx Xxxxx
Xxxxxxxxxx, Xxxxxxx 00000
XXX
Attention: CEO
Facsimile No.: x0-000-0000
with a copy to:
Proskauer Rose LLP
0000 Xxxxxxxx
Xxx Xxxx, Xxx Xxxx 00000-0000
XXX
Attention: Xxxxx Xxxxxxxx, Esq.
Telephone: x0-000-000-0000
Facsimile: x0-000-000-0000
or to such other person or address as BioCryst shall furnish to Shionogi in writing.
If to Shionogi, to:
SHIONOGI & CO., LTD.
00-0, Xxxxxx 0 xxxxx
Xxxxxxxxx-xx, Xxxxx 000-0000
Xxxxx
Attention: General Manager, License Department
Telephone: x00-0-0000-0000
Facsimile: x00-0-0000-0000
or to such other person or address as Shionogi shall furnish to BioCryst in writing.
If personally delivered, such communication shall be deemed delivered upon actual receipt; if electronically transmitted pursuant to this paragraph, such communication shall be deemed delivered on the day transmitted unless it is received after 5:00 p.m., local time, or on a day which is not a business day, in which case it shall be deemed delivered on the next business day after transmission (and sender shall bear the burden of proof of delivery); if sent by overnight courier pursuant to this paragraph, such communication shall be deemed delivered upon receipt; and if sent by mail pursuant to this paragraph, such communication shall be deemed delivered as of the date of delivery indicated on the receipt issued by the relevant postal service; or, if the addressee fails or refuses to accept delivery, as of the date of such failure or refusal. Either Party may change its address for the purposes of this Agreement by giving notice thereof in accordance with this Section 17.4.
17.6 No Waiver. None of the provisions of this Agreement can be waived except in a writing signed by the Party granting the waiver. No failure by a Party to exercise any right under this Agreement shall operate as a waiver of such right, nor shall any single or partial exercise of any right preclude any other or further exercise of that right or the exercise of any other rights. The waiver by any Party of any breach of this Agreement shall not be deemed a waiver of any prior or subsequent breach. All remedies of either Party shall be cumulative and the pursuit of one remedy shall not be deemed a waiver of any other remedy.
17.7 Further Assurances. Each Party shall execute, acknowledge and deliver, without additional consideration, such further assurances, instruments and documents, and shall take such further actions, as the other Party shall reasonably request in order to fulfill the intent of this Agreement and the transactions contemplated hereby.
17.8 No Third-Party Beneficiaries. Nothing in this Agreement is intended or shall be construed to give any other person or entity any legal or equitable right, remedy or claim under or in respect of this Agreement or any provision contained herein, other than BioCryst Indemnitees, Shionogi Indemnitees and any assignee permitted under Section 17.1 above.
17.9 Relationship of the Parties. The relationship of the Parties under this Agreement shall be solely that of independent contractors and nothing herein shall be construed to create or imply any relationship of employment, agency, joint venture, partnership or any relationship other than that of independent contractors. BioCryst and Shionogi acknowledge and agree that each of them is engaged in a separate and independent business and neither shall state, represent or imply any interest in or control over the business of the other.
17.10 Government Funding. BioCryst’s obligations under this Agreement have been funded in whole or in part with Federal funds from the Office of Public Health Emergency Preparedness, Office of Public Health Emergency Medical Countermeasures, under Contract No, HHSO100200700032C.
17.11 Cost. Unless otherwise specified, each Party shall bear the full Cost of its compliance with the terms of this Agreement and its respective obligations hereunder. For purposes of this Agreement, the term “Costs” when used herein means the fully allocated costs including but not limited to the fully allocated cost of goods and services and manufacturing overhead directly related to Licensed Product, and allocation of all administrative and general expenses directly related to Licensed Product. Costs shall be determined by generally accepted accounting principles, applied on a consistent basis.
17.12 Interpretation. The captions and headings to this Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions of this Agreement. Unless specified to the contrary, references to Articles, Sections, Schedules or Exhibits mean the particular Articles, Sections, Schedules or Exhibits to this Agreement and references to this Agreement include all Exhibits and Schedules attached hereto. Each accounting term used herein that is not specifically defined herein shall have the meaning given to it under GAAP, but only to the extent consistent with its usage and the other definitions in this Agreement. Unless context otherwise clearly requires, whenever used in this Agreement: (i) the words "include” or “including” shall be construed as incorporating, also, “but not limited to” or "without limitation;” (ii) the word “day”, “month” or “year” means a calendar day, month or year unless otherwise specified; (iii) the word “notice” means notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement; (iv) the words “hereof,” “herein,” “hereby” and derivative or similar words refer to this Agreement (including any Exhibits and Schedules); (v) provisions that require that a Party, the Parties or any committee or team hereunder “agree,” "consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise; (vi) words of any gender include the other gender; and (vii) references to any specific Law or article, section or other division thereof shall be deemed to include the then-current amendments thereto or any replacement Law thereof.
17.13 No Modifications. Unless otherwise specified herein and the Exhibits attached hereto, nothing contained in this Agreement shall affect the rights and obligations of the Parties under the other License Documents, and the terms and conditions of all such agreements shall remain in full force and effect.
17.14 Entire Agreement. This Agreement and the Exhibits and Schedules attached hereto constitute the entire understanding between the Parties relating to the subject matter hereof, and no amendment or modification to this Agreement shall be valid or binding upon the Parties unless designated as such, made in writing and signed by the representatives of such Parties
17.15 Counterparts. This Agreement may be executed in one or more counterparts, each of which shall be deemed an original, and all of which together, shall constitute one and the same instrument.
[The remainder of this page intentionally left blank]
IN WITNESS WHEREOF, the Parties have executed and delivered this Agreement in duplicate originals by their duly authorized representatives as of the date and year first above written.
|
BIOCRYST PHARMACEUTICALS, INC. |
SHIONOGI & CO., LTD. |
|
|
By: /s/XXX X. XXXXXXXXXX |
By: /s/MOTOZO SHIONO |
|
|
Name: XXX X. XXXXXXXXXX |
Name: MOTOZO SHIONO |
|
|
Title: Chief Executive Officer |
Title: President |


