COLLABORATION AND LICENSE Agreement
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit 10.1
COLLABORATION AND LICENSE Agreement
This Collaboration and License Agreement (the “Agreement”) is entered into as of July 8, 2020 (the “Effective Date”), by and between Ovid Therapeutics Inc., a Delaware company having an address at 0000 Xxxxxxxx, Xxxxx 00000 Xxx Xxxx, XX 00000, XXX (“Ovid”) and Xxxxxxxx Pharma Rare Diseases AG, a Swiss company under direction and control of Xxxxxxxx Pharma S.p.A. and having an address at Consulting GmbH Xxxxxxxxxxxx 00 0000 Xxxxxxxxxxx, Xxxxxx, Xxxxxxxxxxx (“Licensee”). Ovid and Licensee may be referred to herein individually as a “Party” or collectively as the “Parties”.
Recitals
Whereas, Ovid, a biopharmaceutical company, owns or controls certain patents, know-how, and other intellectual property relating to its proprietary compound known as OV101, a selective agonist of the GABAA receptor, including certain intellectual property rights in-licensed from X. Xxxxxxxx A/S, which Ovid has been developing for the treatment of Angelman syndrome and Fragile X syndrome and potentially other Rare Diseases indications;
Whereas, Licensee is a subsidiary, 100% under direction and control of Xxxxxxxx Pharma S.p.A., an Italian pharmaceutical company, which is experienced in the development and commercialization of pharmaceutical products; and
Whereas, Licensee and Ovid desire to form a collaboration for the continued development and commercialization of OV101, with the status of Orphan Medicine and with Final Label 1a, 1b and/or 2, all on the terms and conditions set forth below.
Agreement
Now, Therefore, in consideration of the foregoing premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Ovid and Licensee hereby agree as follows:
1.1“Additional Development Activities” has the meaning set forth in Section 4.3(a).
1.2“Additional Indications” means any Rare Diseases Indication other than the Initial Indication and the Second Indication that has been submitted to and approved by the CGB.
1.3“Additional Pivotal Trial” means the additional Phase 3 Clinical Trial of the Product for the Initial Indication, if required by the EMA or by Licensee at its own discretion, for the purpose of seeking or maintaining MAA Approval of the Product for the Initial Indication in the Licensee Territory, as described in Exhibit H.
1.4“Affiliate” means, with respect to any party, any entity that, directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with such party, but for only so long as such control exists. As used in this Section 1.4, “control” means
1
(a) to possess, directly or indirectly, the power to direct the management or policies of an entity, whether through ownership of voting securities, by contract relating to voting rights or corporate governance; or (b) direct or indirect beneficial ownership of more than fifty percent (50%) (or such lesser percentage which is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) of the voting share capital or other equity interest in such entity.
1.5“Allocated Quantity of Compound” means [***] of the Compound in Ovid’s control as of the Effective Date, which Ovid has agreed, under the Supply Agreement, to allocate exclusively for the purpose of fulfilling Licensee’s or Licensee Affiliate’s Purchase Orders under the Supply Agreement.
1.6“Allowable Increases” has the meaning set forth in Section 4.5(b).
1.7“Amended Development Budget” has the meaning set forth in Section 4.2.
1.8“Amended Development Plan” has the meaning set forth in Section 4.2.
1.9“Xxxxxxxx Commercially Reasonable Efforts” means, with respect to Licensee and its obligations under this Agreement, commercially reasonable efforts and resources as commonly used by a similar size pharmaceutical company to Develop and Commercialize a product controlled by Licensee or to which it has exclusive rights, which product is at similar stage in its development or product life and is of similar market potential taking into account efficacy, safety, approved and/or anticipated labelling, the competitiveness of alternative products sold by Third Parties in the marketplace, the patent and other proprietary position of the product, likelihood of regulatory approval given the regulatory structure involved, the profitability of the product, [***] and other relevant factors, including technical, legal, scientific and/or medical factors. Commercially Reasonable Efforts shall be determined on a country-by-country basis and indication-by-indication basis for a particular Product, and it is anticipated that the level of effort will change over the time, reflecting changes in the status of the Product and the country(s) involved. [***].
1.10“Applicable Laws” means the applicable provisions (including Data Protection Legislation) of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidance, ordinances, judgments, decrees, directives, injunctions, orders, permits (including XXXx) of or from any court, Regulatory Authority, or governmental agency or authority having jurisdiction over or related to the subject item.
1.11“Auditor” has the meaning set forth in Section 9.4.
1.12“Calendar Quarter” means each respective period of three (3) consecutive months ending on March 31, June 30, September 30, and December 31.
1.13“Calendar Year” means each respective period of twelve (12) consecutive months ending on December 31.
1.14“cGCP” means the current good clinical practice standards as set out in (a) ICH Harmonized Guidance on current Good Clinical Practice (CPMP/ICH/135/95), (b) U.S. 21 C.F.R.
2
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Parts 50, 54, 56, 58, 210, 211 and 312, and (c) the equivalent law or regulation in any other applicable jurisdiction in the Territory, each as may be amended from time to time.
1.15“cGLP” means current good laboratory practice standards promulgated or endorsed by the FDA, as defined in U.S. 21 C.F.R. Part 58 (or such other comparable regulatory standards in jurisdictions outside the U.S.), as may be amended from time to time.
1.16“cGMP” means the current standards for systems to assure the proper design, monitoring, and control of processes and facilities to be used for the manufacture, processing, packing, or holding of a drug as specified by applicable laws of the relevant countries at the time of manufacturing conducted in accordance with this Agreement, defined under (a) U.S. 21 C.F.R. Parts 210 and 211 and (b) the equivalent law or regulation in any other applicable jurisdiction in the Territory, each as may be amended from time to time.
1.17“Claim” has the meaning set forth in Section 12.3.
1.18“Clawback” means post sales clawback policies applied to manufacturers, requiring them to pass a part of their revenues in respect of a product to a national health service or other Governmental Authority.
1.19“Clinical Trial” or “Clinical Trials” means Phase 1 Clinical Trial, Phase 2 Clinical Trial, Phase 3 Clinical Trial, or Phase 4 Clinical Trial, as the context dictates.
1.20“Collaboration Governance Board” or “CGB” has the meaning set forth in Section 3.1.
1.21“Combination Product” means any pharmaceutical product containing the Compound as an active pharmaceutical ingredient in combination with one or more other therapeutically active ingredients, in any and all forms, presentations, dosages, and formulations.
1.22“Commercialization” means the conduct of all activities undertaken before and after Regulatory Approval relating to the promotion, sales, marketing, medical and patient support and services, and distribution (including importing, exporting, transporting, customs clearance, warehousing, invoicing, handling, and delivering Products to customers) of Products in the Field, including sales force efforts, detailing, advertising, market research, market access (including price and reimbursement activities), medical education and information services, publication, scientific and medical affairs, advisory and collaborative activities with opinion leaders and professional societies including symposia, marketing, sales force training, and sales (including receiving, accepting and filling Product orders) and distribution. “Commercialize” and “Commercializing” have correlative meanings.
1.23“Commercialization Plan” has the meaning set forth in Section 6.2.
1.24“Committee” means the CGB, JPT, or any other subcommittee established by the CGB, as applicable.
1.25“Committee for Medicinal Products for Human Use (CHMP)” means the EMA committee responsible for human medicines.
3
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
1.26“Competing Acquirer” means a Third Party that, as of the closing date of the applicable transaction, is engaged in [***] of products, other than Products, for [***].
1.27“Competing Product” means any product or compound, other than the Compound and Products and the Current Licensee Pipeline Products, (a) [***], or (b) [***].
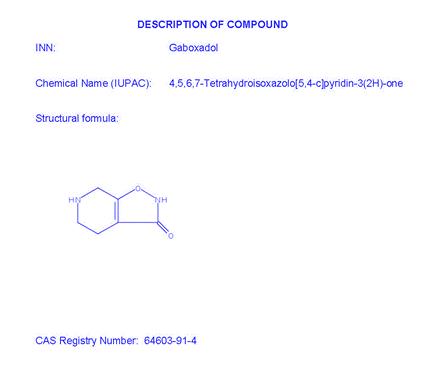
1.28“Compound” means the δ-selective extrasynaptic GABAA receptor agonist known internally by Ovid as OV101, having the chemical structure set forth in Exhibit A.
1.29“Compound Invention” has the meaning set forth in Section 10.1(b)(i).
1.30“Compounding” or “Compounding Activities” means [***].
1.31“Confidentiality Agreement” means that certain Confidential Disclosure Agreement between Ovid and Licensee dated as of July 8, 2019.
1.32“Confidential Information” means all Know-How and other proprietary scientific, marketing, financial, or commercial information or data that is generated by or on behalf of a Party or its Affiliates or which one Party or any of its Affiliates has supplied or otherwise made available to the other Party or its Affiliates, whether made available orally, in writing, or in electronic form, including information comprising or relating to concepts, discoveries, inventions, data, designs, or formulae in relation to this Agreement; provided that all Ovid Technology will be deemed Ovid’s Confidential Information, all Licensee Technology will be deemed Licensee’s Confidential Information, and all Joint Inventions and Joint Patents will be deemed both Parties’ Confidential Information.
1.33“Control” or “Controlled” means, with respect to any Know-How, Patents, or other intellectual property rights, the legal authority or right (whether by ownership, license, or otherwise, but without taking into account any rights granted by one Party to the other Party pursuant to this Agreement) of a Party to grant access, a license, or a sublicense of or under such Know-How, Patents, or other intellectual property rights to another Party, or to otherwise disclose proprietary or trade secret information to such other Party, without breaching the terms of any agreement with a Third Party, or misappropriating the proprietary or trade secret information of a Third Party.
1.34“Cost of Goods” means, with respect to the Drug Product, the fully burdened cost to manufacture and supply such Drug Product, which means: (a) in the case of products and services acquired from Third Parties, [***]; and (b) in the case of manufacturing services performed by a Party or its Affiliates, including manufacturing services to support products and services acquired from Third Parties as contemplated in subsection (a), [***].
1.35“Current Licensee Pipeline Products” means, in each case as of the Effective Date, Licensee’s pipeline for products related to (a) [***], (b) [***], and (c) [***], in each case as better defined in Exhibit I.
1.36“Data” means any and all scientific, technical, test, marketing, or sales data pertaining to any Product that is generated by or on behalf of Ovid, Licensee, and their respective
4
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Affiliates and (sub)licensees, including research data, clinical pharmacology data, pre-clinical data, clinical data, clinical study reports, or submissions made in association with an IND or MAA with respect to any Product.
1.37“Data Protection Legislation” means all applicable legislation relating to the protection and processing of personal data, data privacy and the privacy of electronic communications in any relevant jurisdiction, including the General Data Protection Regulation ((EU) 2016/679), and any national implementing laws, regulations and secondary legislation, as amended or updated from time to time.
1.38“Development” means all non-clinical and clinical drug development activities, including toxicology, pharmacological, and other non-clinical efforts, statistical analysis, the performance of Clinical Trials, including the Manufacturing of the Products for use in the Clinical Trials, Manufacturing Development or other activities reasonably necessary in order to obtain or maintain Regulatory Approval of Products in the Field in the Territory, as detailed in a Development Plan for the Products. “Development” shall exclude all Commercialization activities. When used as a verb, “Develop” means to engage in Development activities.
1.39“Development Budget” means the initial reasonably detailed budget estimated by the Parties for all Development activities set forth in the Development Plan.
1.40“Development Costs” means the costs incurred by a Party or for its account or by the Parties jointly, during the Term and pursuant to this Agreement, that are specifically directed (or reasonably allocable) to the Development of a Product. The Development Costs shall include amounts that a Party pays to Third Parties involved in the Development of a Product (at cost), and all internal costs (calculated on an FTE basis at the then-current FTE Rate) and out-of-pocket costs incurred by or on account of a Party in performing Development in accordance with the Development Plan. For clarity, [***].
1.41“Development Plan” means the initial plan of Development activities for the Product set forth in Exhibit C.
1.42“Drug Product” means the Compound, filled and finished into unit doses, but not packaged or labelled.
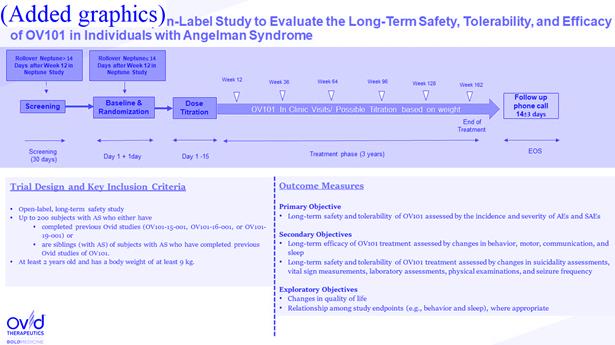
1.43“Elara Trial” means the open-label extension study for patients with Angelman Syndrome who have previously been enrolled in a Clinical Trial of the Product (including the Neptune Trial) as of the Effective Date, as further described in Exhibit J. [***].
1.44“EMA” means the European Medicines Agency, and any successor agency or authority having substantially the same function.
1.45“Executive Officers” means the [***] of Ovid and the [***] of Licensee.
1.46“Export Control Laws” means all applicable U.S. laws and regulations relating to (a) sanctions and embargoes imposed by the Office of Foreign Assets Control of the U.S. Department of Treasury or (b) the export or re-export of commodities, technologies, or services,
5
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
including the Export Administration Act of 1979, 24 U.S.C. §§ 2401-2420, the International Emergency Economic Powers Act, 50 U.S.C. §§ 1701-1706, the Trading with the Enemy Act, 50 U.S.C. §§ 1 et. seq., the Arms Export Control Act, 22 U.S.C. §§ 2778 and 2779, and the International Boycott Provisions of Section 999 of the U.S. Internal Revenue Code of 1986, in each case, as amended.
1.47“Extended Commercialization Term” means the period commencing on the expiration of the Royalty Term and continuing for so long as Ovid continues to supply to Licensee or Licensee Affiliate the Product under the terms of the Lundbeck License Agreement.
1.48“FCPA” means the U.S. Foreign Corrupt Practices Act (15 U.S.C. Section 78dd-1, et. seq.), as amended.
1.49“FDA” means the U.S. Food and Drug Administration, and any successor agency or authority having substantially the same function.
1.50“Field” means the treatment of (a) the Initial Indication, (b) the Second Indication (subject to Section 4.2(e)) and (c) any Additional Indication(s) for which the Parties agree to incorporate Additional Development Activities into the Development Plan and Development Budget in accordance with Section 4.3.
1.51“Final Label” refers to the information regarding an approved Indication and population target that is included with a prescription drug as approved by an applicable Regulatory Authority.
1.52“Final Label 1a” means the Final Label for a population from two (2) years of age up to twelve (12) years of age.
1.53“Final Label 1b” means the Final Label for a population from four (4) years of age up to twelve (12) years of age.
1.54“Final Label 2” means the Final Label for the population from twelve (12) years of age up to, at least seventeen (17) years of age or more.
1.55“First Commercial Sale” means, on a Product-by-Product and country-by-country basis, the first sale of such Product in such country by Licensee or its Affiliates or Sublicensees to a Third Party after Regulatory Approval for such Product has been obtained in such country.
1.56“FTE” means the equivalent of a full-time individual’s work for a twelve (12) month period, consisting of a total of [***] hours per year of dedicated effort. Any person who devotes more or less than [***] hours per year on the applicable activities shall be treated as an FTE on a pro-rata basis, based upon the actual number of hours worked by such person on such activities, divided by [***]. For clarity, the hours spent by temporary workers and contractors on applicable activities may be treated as FTE on a pro-rata basis.
1.57“FTE Rate” means an initial rate of (a) with respect to Ovid’s personnel, [***] per FTE per year and (b) with respect to Licensee’s personnel, [***] per FTE per year, which rates shall apply through [***]. Thereafter, the FTE Rate shall be changed annually on a Calendar Year
6
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
basis to reflect any year-to-year percentage increase or decrease (as the case may be) (i) with respect to Ovid, in the Consumer Price Index for All Urban Consumers for the U.S., as published by the U.S. Department of Labor, Bureau of Labor Statistics, and (ii) with respect to Licensee, in the Italy Consumer Price Index as published by the Italian National Statistical Institute (both changes based on the change from the most recent applicable index available as of the Effective Date to the most recent applicable index available as of the date of the calculation of such revised FTE Rate).
1.58“Generic Product” means, (a) any pharmaceutical product which has received Regulatory Approval [***] of the Product; and/or (b) any other pharmaceutical product, approved under an Abbreviated New Drug Application, or ANDA, in the United States, under Section 505(b)(2) of the Food Drug and Cosmetic Act, or similar regulatory pathways outside of the U.S., in any case, with the Product as the reference product.
1.59“Generic Product Competition” with respect to a Product, on a country-by-country basis, Generic Product Competition shall exist if [***] there are one or more Generic Products sold [***] in such country and the sales of such Generic Product(s) account for [***] or more of the sales revenue of the Product and its Generic Product(s) in the given country during such [***] as determined by reference to applicable sales data obtained from IMS Health, Verispan, or from such other reputable source for such sales data as may be used and relied upon by the Parties from time to time, provided however if sale of such Generic Product(s) [***], Generic Product Competition shall no longer exist.
1.60 “Governmental Authority” means any national, international, federal, state, provincial, or local government, or political subdivision thereof, or any multinational organization, or any authority, agency, or commission entitled to exercise any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power, or any court or tribunal (or any department, bureau or division thereof, or any governmental arbitrator or arbitral body).
1.61“ICH” means the International Conference on Harmonization (of Technical Requirements for Registration of Pharmaceuticals for Human Use).
1.62“IND” means an application filed with a Regulatory Authority for authorization to commence Clinical Trials, including (a) an Investigational New Drug Application or any successor application or procedure filed with the FDA, (b) a Clinical Trial Application or any successor application or procedure filed with the EMA, (c) any equivalent of the applications in (a) and (b) in countries or regulatory jurisdictions outside the U.S. and EU, and (d) all supplements, amendments, variations, extensions, and renewals thereof that may be filed with respect to the foregoing.
1.63“Indemnitee” has the meaning set forth in Section 12.3.
1.64“Indemnitor” has the meaning set forth in Section 12.3.
1.65“Independent Development Activities” has the meaning set forth in Section 4.3(d).
7
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
1.66“Independent Development Costs” has the meaning set forth in Section 8.2(b).
1.67“Indication” means a separate and distinct disease, disorder, illness, or health condition and all of its associated signs, symptoms, stages, or progression (including precursor conditions), in each case for which a separate MAA may be filed. Subpopulations or patients with a primary disease or condition, however stratified (including stratification by stages or progression, particular combinations of symptoms associated with the primary disease or condition, prior treatment courses, response to prior treatment, family history, clinical history, phenotype, or other stratification) shall not be deemed to be separate “Indications” for the purposes of this Agreement.
1.68“Initial Indication” means Angelman syndrome (AS).
1.69“International Transparency Reporting Requirements for Europe” means EFPIA consolidated Code for Pharmaceutical Companies to report transfers of value related to HCPs: donations and grants, contribution to costs related to events, fees for service and consultancy, research and development.
1.70“Inventions” means all inventions, whether or not patentable, discovered, made, conceived, or reduced to practice in the course of activities contemplated by this Agreement.
1.71“JPT” has the meaning set forth in Section 3.2.
1.72“Joint Development Activities” has the meaning set forth in Section 4.2(d).
1.73“Joint Inventions” has the meaning set forth in Section 10.1(b)(ii).
1.74“Joint Patents” has the meaning set forth in Section 10.1(b)(ii).
1.75“Know-How” means all technical and scientific information, know-how, and data, including inventions, discoveries, trade secrets, specifications, instructions, processes, formulae, compositions of matter, cells, cell lines, assays, animal models, and other physical, biological, or chemical materials, expertise, and other technology applicable to development, registration, use, or marketing or to methods of assaying or testing them, and including all biological, chemical, pharmacological, biochemical, toxicological, pharmaceutical, physical, and analytical safety, nonclinical, and clinical data, regulatory documents, data and filings, instructions, processes, formulae, expertise, and information relevant to the research, development, use, importation, offering for sale, or sale of, or which may be useful in studying, testing, developing, Products. Know-How excludes Patents.
1.76“Licensee Data” has the meaning set forth in Section 10.1(a).
1.77“Licensee Indemnitee” has the meaning set forth in Section 12.1.
1.78“Licensee Know-How” means all Know-How that Licensee or its Affiliate(s) Controls as of the Effective Date or during the Term, including any Joint Inventions, that is necessary or reasonably useful for the research, Development, manufacture, use, importation, offer for sale, or sale of the Compound or any Product in the Field. For clarity, subject to Section 8.2(b)
8
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
in the case of any Licensee Data generated as a result of Licensee’s Independent Development Activities, the Licensee Know‑How includes the Licensee Data.
1.79“Licensee Patents” means all Patents that Licensee or its Affiliate(s) Controls as of the Effective Date or during the Term (including any Joint Patents) that would be infringed, absent a license or other right to practice granted under such Patents, by the research, Development, manufacture, use, importation, offer for sale, or sale of any Compound or Product (considering patent applications to be issued with the then-pending claims and considering Joint Patents as if owned solely by Licensee or its Affiliate).
1.80“Licensee Technology” means the Licensee Know-How and the Licensee Patents, including Licensee’s interest in the Joint Inventions and Joint Patents.
1.81“Licensee Territory” or “Licensed Territory” means Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Russia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, and the United Kingdom.
1.82“Licensee Territory Development Activities” has the meaning set forth in Section 4.2(b).
1.83“Losses” has the meaning set forth in Section 12.1.
1.84“Lundbeck License Agreement” means the License Agreement by and between Ovid and X. Xxxxxxxx A/S (“Lundbeck”) dated 25 March, 2015, and amended on July 7, 2020 to clarify certain sights of sublicensees upon termination of such agreement (such amendment, the “Lundbeck Sublicense Amendment”).
1.85“MAA” means a marketing authorization application or equivalent application, and all amendments and supplements thereto, filed with the applicable Regulatory Authority in any country or jurisdiction. For clarity, MAA does not include any application for Pricing and Reimbursement Approval.
1.86“MAA Approval” means approval of an MAA by the applicable Regulatory Authority for marketing and sale of a Product in the applicable country or jurisdiction, but excluding any Pricing and Reimbursement Approval.
1.87“Major European Market” means [***].
1.88Manufacturing Development” means any of the following with respect to the Compound or a Product: manufacturing process development, process improvements and any analytical development or validation associated with such development or improvements.
1.89“Market Exclusivity” means any exclusive marketing rights or data exclusivity rights conferred by a Regulatory Authority with respect to a Product other than patents, including Directive 2001/83/EC and 2004/27/EC (as amended) in the EU and rights equivalent to those
9
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
conferred in the U.S. under the Xxxxx-Xxxxxx Act or the FDA Modernization Act of 1997 (including pediatric exclusivity).
1.90“Medical Affairs” or “Medical Affairs Activities” means activities designed to ensure or improve appropriate medical use of, conduct medical education of, or further research regarding, the Product, including by way of example: (a) activities of medical scientific liaisons/medical advisors who, among their other functions, may: (i) conduct service based medical activities including providing input and assistance with consultancy meetings, proposing investigators for clinical trials sponsored or co-sponsored by a Party or Affiliate, and providing input in the design of such trials and other research related activities; and/or (ii) deliver non-promotional communications and conduct non-promotional activities; (b) grants to support continuing medical education, symposia, or Third Party research related to the Product; (c) development, publication, and dissemination of original and literature review data relating to the Products; (d) medical information services provided in response to inquiries communicated via sales representatives or received by letter, phone call, or email; (e) conducting advisory board meetings, international advisory board activities, or other consultant programs, including the engagement of key opinion leaders and health care professional in individual or group advisory and consulting arrangements; and (f) the evaluation of applications submitted for support of investigator-initiated trials.
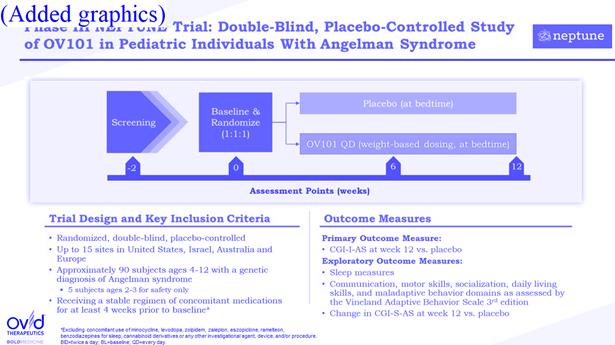
1.91“Neptune Trial” means the Phase 3 Clinical Trial of the Product for Angelman Syndrome ongoing as of the Effective Date and for which Ovid is the sponsor, as further described in Exhibit J.
1.92“Net Sales” means, with respect to any Product, the aggregate gross amount invoiced by Licensee, its Affiliates, or Sublicensees for the sale of the Product to a Third Party, less the following deductions to the extent allowed and actually taken on such sales:
(a)transportation charges relating to the Product to the extent actually invoiced to Licensee’s, its Affiliate’s, or Sublicensee’s customers, including handling charges and insurance premiums relating thereto;
(b)sales taxes, excise taxes, use taxes, VAT, and duties paid by Licensee, its Affiliate, or Sublicensee in relation to the Product and any other equivalent governmental charges imposed on the importation, use, or sale of the Product;
(c)government-mandated rebates (including any Paybacks or Clawbacks charged with respect to the Product) and other rebates or fees;
(d)customary trade, quantity, and case discounts allowed on the Product;
(e)allowances or credits to customers on account of retrospective price reductions affecting the Product; and
(f)customary rebates (including confidential discount agreements Success Fee and Performance based Payments).
10
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
The transfer of Product between Licensee and its Affiliates and Sublicensees shall not be considered a sale unless the Affiliate or Sublicensee is a bona fide purchaser at fair market value for resale. If the Affiliate or Sublicensee is not an end user, Net Sales shall be determined based on the invoiced sale price by the Affiliate or Sublicensee to the first Third Party trade purchaser, less the deductions allowed under this Section 1.92. Disposal of Product for or use of Product in Clinical Studies or as free samples in quantities common in the industry for this sort of Product shall not give rise to any deemed sale under this definition.
Net Sales shall be calculated and accounted for in accordance with accounting standards during the Term (i.e., IFRS), consistently applied.
If Product is sold for other than cash payment, Net Sales of the Product shall be deemed to be the cash value of such other payment.
If the Product is sold as part of a Combination Product, Net Sales of such Product shall be deemed to be an amount equal to the following:
(X divided by Y) multiplied by Z,
where “X” is the average sales price during the applicable reporting period achieved for the relevant Product in the country in which such sale occurred when the Product contains only the Compound and no other active pharmaceutical ingredient;
“Y” is the sum of the average sales price as a single entity during the applicable reporting period achieved in that country (as applicable) of each product included in the Combination Product when such product is sold as a separate product and not as part of a Combination Product; and
“Z” is the single price at which the relevant Combination Product was actually sold.
In the event that no separate sale of either (a) the Product comprising the Compound as the sole active pharmaceutical ingredient, or (b) a product containing the other active pharmaceutical ingredient(s) included in the Combination Product, are made during the accounting period in which the sale was made or if the price for a particular therapeutically active ingredient cannot otherwise be determined for an accounting period, Net Sales allocable to the Product shall be determined by mutual agreement of the Parties prior to the end of the accounting period in question based on an equitable method of determining the same that takes into account, in the Territory, variations in potency, the relative contribution of each therapeutically active ingredient in the Combination Product, and relative value to the end user of each therapeutically active ingredient; provided, that if the Parties cannot reach mutual agreement prior to the end of the applicable accounting period, such matter shall be resolved in accordance with Article 15.
1.93“Orphan Drug Designation” means a status assigned to a medicine intended for use against a rare condition. The medicine must fulfil certain criteria for designation as an orphan medicine so that it can benefit from incentives such as protection from competition once on the market.
11
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
1.94“Orphan Drug Designation of the Product” means the EMA orphan drug designation obtained for the Product.
1.95“Orphan Medicine” means a medicine that continues to meet the criteria for maintaining its orphan status in parallel with the assessment of an application for marketing authorisation by the European Medicines Agency (EMA).
1.96“Ovid Commercially Reasonable Efforts” means, with respect to Ovid and its obligations under this Agreement, commercially reasonable efforts and resources as commonly used by a similar size pharmaceutical company to Develop and Commercialize a product controlled by Ovid or to which it has exclusive rights, which product is at similar stage in its development or product life and is of similar market potential taking into account efficacy, safety, approved and/or anticipated labelling, the competitiveness of alternative products sold by Third Parties in the marketplace, the patent and other proprietary position of the product, likelihood of regulatory approval given the regulatory structure involved, the profitability of the product, [***], and other relevant factors, including technical, legal, scientific and/or medical factors. Commercially Reasonable Efforts shall be determined on a country-by-country basis and indication-by-indication basis for a particular Product, and it is anticipated that the level of effort will change over the time, reflecting changes in the status of the Product and the country(s) involved.
1.97“Ovid Data” has the meaning set forth in Section 10.1(a).
1.98“Ovid Indemnitee” has the meaning set forth in Section 12.2.
1.99“Ovid Know-How” means all Know-How that Ovid Controls as of the Effective Date or, subject to Section 2.7(b), during the Term, including any Joint Inventions, that is necessary or reasonably useful for the Development, use, importation, offer for sale, or sale of any Compound or Product in the Field in the Licensee Territory. For clarity, any Ovid Data generated during Ovid’s Independent Development Activities will be included within the Ovid Know‑How following reimbursement by Licensee of its share of Development Costs in accordance with Section 8.2(b).
1.100“Ovid Ongoing Trials” means the Rocket Trial, Elara Trial, and Neptune Trial.
1.101“Ovid Patents” means all Patents in the Licensee Territory that Ovid Controls as of the Effective Date or, subject to Section 2.7(b), during the Term (including any Joint Patents) that would be infringed, absent a license or other right to practice granted under such Patents, by the Development, use, importation, offer for sale, or sale of any Compound or Product in the Field in the Licensee Territory (considering patent applications to be issued with the then-pending claims and considering Joint Patents as if owned solely by Ovid). The Ovid Patents existing as of the Effective Date are set forth in Exhibit B.
1.102“Ovid Technology” means the Ovid Know‑How and the Ovid Patents, including Ovid’s interest in the Joint Inventions and Joint Patents.
1.103“Ovid Territory” means the world outside the Licensee Territory.
12
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
1.104“Paediatric Investigational Plan (PIP)” is the development plan aimed at ensuring that the necessary data are obtained through studies in children, to support the authorisation of a medicine for use in children. Any Applicants must obtain EMA approval of the PIP before MAA.
1.105“Patents” means (a) all patents, certificates of invention, applications for certificates of invention, priority patent filings, provisional patent applications and patent applications, and (b) any renewals, divisions, or continuations (in whole or in part) of any of such patents, certificates of invention and patent applications, and any all patents or certificates of invention issuing thereon, and any and all reissues, reexaminations, extensions, supplementary protection certificates, divisions, renewals, substitutions, confirmations, registrations, revalidations, revisions, and additions of or to any of the foregoing.
1.106“Payback” means payback policies which require manufacturers to pay back a share of their revenue in respect of a product, if a prespecified budget ceiling for public pharmaceutical expenditures is exceeded.
1.107“Pharmacovigilance Agreement” has the meaning set forth in Section 5.4.
1.108“Phase 1 Clinical Trial” means a human clinical trial of a pharmaceutical product that satisfies the requirements for a Phase 1 study as defined in 21 CFR § 312.21(a) (or any amended or successor regulations), regardless of where such clinical trial is conducted.
1.109“Phase 2 Clinical Trial” means a human clinical trial of a pharmaceutical product that satisfies the requirements for a Phase 2 study as defined in 21 CFR § 312.21(b) (or any amended or successor regulations), regardless of where such clinical trial is conducted.
1.110“Phase 3 Clinical Trial” means a human clinical trial of a pharmaceutical product for an indication on a sufficient number of subject that is designed to establish that the pharmaceutical product is safe and efficacious for its intended use, and to determine warnings, precautions and adverse reactions that are associated with the pharmaceutical product in the dosage range to be described, and to support Regulatory Authority to market such Product in patients having the indication being studied and that satisfies the requirements for a Phase 3 study as defined in 21 CFR § 312.21(c) (or any amended or successor regulations), regardless of where such clinical trial is conducted.
1.111“Phase 4 Clinical Trial” means a product support clinical trial of a Product that is commenced after receipt of MAA Approval in the country where such trial is conducted. Phase 4 Clinical Trial may include epidemiological studies, modeling and pharmacoeconomic studies, and post-marketing surveillance trials.
1.112“PIP Compliance Check” means that PIP has been checked by EMA for compliance with all the measures mentioned in the PIP decision, including the timelines for the conduct of the studies or collection of the data.
1.113“Pricing and Reimbursement Approval” means, with respect to a Product, the approval, agreement, determination, or decision of any applicable Governmental Authority
13
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
establishing the price or reimbursement level for such Product, as required in a given country or jurisdiction prior to sale of such Product in such country or jurisdiction.
1.114“Product” means any pharmaceutical product containing the Compound as an active ingredient, alone or in combination with one or more other active pharmaceutical ingredients, in any form, presentation, dosage, or formulation, and for any mode of administration.
1.115“Product Infringement” has the meaning set forth in Section 10.3(a).
1.116“Promotional Materials” has the meaning set forth in Section 6.4(c).
1.117“Proposal” has the meaning set forth in Section 4.3.
1.118“Proposing Party” has the meaning set forth in Section 4.3.
1.119“Public Official or Entity” means (a) any officer, employee (including physicians, hospital administrators, or other healthcare professionals), agent, representative, department, agency, de facto official, representative, corporate entity, instrumentality, or subdivision of any government, military, or international organization, including any ministry or department of health or any state-owned or affiliated company or hospital, or (b) any candidate for political office, any political party, or any official of a political party.
1.120“Rare Disease” means a disease that affects one person per two thousand (2,000).
1.121“Recall” has the meaning set forth in Section 5.7.
1.122“Regulatory Approval” means, with respect to a country or jurisdiction, any and all approvals (including MAA Approval and, if required by Applicable Law, Pricing and Reimbursement Approval), licenses, registrations, permits, notifications and authorizations (or waivers) of any Regulatory Authority that are necessary for the manufacture, use, storage, import, transport, promotion, marketing, distribution, offer for sale, sale, or other commercialization of a Product in such country or jurisdiction.
1.123“Regulatory Authority” means any Governmental Authority that has responsibility in its applicable jurisdiction over the testing, development, manufacture, use, storage, import, transport, promotion, marketing, distribution, offer for sale, sale, or other commercialization of pharmaceutical products in a given jurisdiction. For countries where Pricing and Reimbursement Approval is required, Regulatory Authority shall also include any Governmental Authority whose grant of Pricing and Reimbursement Approval of the Product is required.
1.124“Regulatory Filing” means all applications, filings, submissions, approvals, licenses, registrations, permits, notifications, and authorizations (or waivers) with respect to the testing, Development, manufacture, or Commercialization of any Product made to or received from any Regulatory Authority in a given country, including INDs and XXXx.
1.125“Regulatory Meeting” has the meaning set forth in Section 5.1(c)(ii).
14
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
1.126“Regulatory Milestone Event” has the meaning set forth in Section 8.3(a).
1.127“Rocket Trial” means the Phase 2 Clinical Trial of the Product for Fragile X Syndrome ongoing as of the Effective Date and for which Ovid is the sponsor, as further described in Exhibit J.
1.128“Safety Data” means Data related solely to any adverse drug experiences and serious adverse drug experience as such information is reportable to Regulatory Authorities. Safety Data also includes “adverse events”, “adverse drug reactions”, and “unexpected adverse drug reactions” as defined in the ICH Harmonised Tripartite Guideline for Clinical Safety Data Management: Definitions and Standards for Expedited Reporting.
1.129“SEC” means the U.S. Securities and Exchange Commission, or any successor entity or its foreign equivalent, as applicable.
1.130“Safety Reason” has the meaning set forth in Section 4.15.
1.131“Sales Milestone Event” has the meaning set forth in Section 8.4(a).
1.132“Scientific Advice” means any report of the advice provided by EMA to medicine developers on the most appropriate way to generate robust evidence on a medicine’s benefits and risks.
1.133“Second Indication” means Fragile X syndrome (FXS).
1.134“Second Indication Opt-In” means the election by Licensee, following the Licensee’s exercise of the Second Indication Opt-Out, to resume the Licensee’s rights granted to Licensee according to Section 2.1 with respect to the Second Indication.
1.135“Second Indication Opt-Out” means the election by Licensee, in accordance with the terms hereof, not to participate in Development and/or Commercialization activities for the Product for the Second Indication.
1.136“Segregate” means, with respect to a Competing Product, to [***] segregate the Development, Manufacturing and Commercialization of such Competing Product in the Field from Development, Manufacture and Commercialization activities with respect to Compounds and Products under this Agreement, including [***]; provided that, [***].
1.137“Sponsor” means the Party that takes the ultimate responsibility for the initiation, performance, and management of, including financing or arranging the financing for, the applicable Clinical Trial.
1.138“Sublicensee” means a Third Party to whom Licensee grants a sublicense to Develop, use, import, promote, offer for sale, or sell any Product in the Field in the Licensee Territory. In no event shall Ovid or any of its Affiliates be deemed a Sublicensee.
1.139“Success Fee” or “Performance Based Payments” means the returned part of the Product-generated revenues for those cases where outcomes are not meeting pre-defined clinical
15
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
goals set by National Health Authority (e.g.QoL Gain, Survival, Progression, Course of Disease Modification).
1.140“Sunshine Reporting Laws” has the meaning set forth in Section 5.8.
1.141“Supply Agreement” means the supply agreement between Ovid and Licensee attached hereto as Exhibit K.
1.142“Take the Lead” means, with the respect to a particular Party and a particular activity, that such Party is primarily responsible for, and has the authority to make, all day-to-day operational decisions, in accordance with the Development Plan and Commercialization Plan and the terms of this Agreement; provided that [***]; and provided further that [***]. For clarity, [***].
1.143“Technology Transfer Completion Date” means the earlier of (a) the date on which the activities [***], as set forth in the Technology Transfer Plan, has been completed, but for clarity, excluding [***] and (b) the [***].
1.144“Technology Transfer Agreement” has the meaning set forth in Section 11.4(e).
1.145“Technology Transfer Plan” has the meaning set forth in Section 11.4(e).
1.146“Term” has the meaning set forth in Section 14.1.
1.147“Third Party” means any entity other than Ovid or Licensee or an Affiliate of Ovid or Licensee.
1.148“Threshold Price Sale” means, on a Product-by-Product and country-by-country basis, the first sale of such Product in such country (after Regulatory Approval for such Product has been obtained in such country) for which Licensee or its Affiliate or Sublicensee, as applicable, invoices a Third Party (a) [***], or (b) [***].
1.149“Total Supply Price” means, for any Product, [***].
1.150“U.S.” means the United States of America, including its territories and possessions (including Puerto Rico).
1.151“Valid Claim” means (a) a claim of an issued and unexpired patent that has not been revoked or held unenforceable, unpatentable, or invalid by a decision of a court or other governmental agency of competent jurisdiction that is not appealable or has not been appealed within the time allowed for appeal, and that has not been abandoned, disclaimed, denied, or admitted to be invalid or unenforceable through reissue, re-examination, or disclaimer or otherwise, or (b) a claim of a pending patent application that has not been cancelled, withdrawn, or abandoned or finally rejected by an administrative agency action from which no appeal can be taken.
16
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
2.1Licenses Granted to Licensee. Subject to the terms and conditions of this Agreement, Ovid hereby grants to Licensee, during the Term:
(a)an exclusive (even as to Ovid, except as expressly set forth herein), royalty-bearing license, with the right to grant sublicenses (through multiple tiers) solely as provided in Section 2.2, under the Ovid Technology to use and have used, sell, offer for sale, import, and otherwise Commercialize and have Commercialized (but not to make or have made, unless otherwise provided herein) the Products in the Field in the Licensee Territory;
(b)a non-exclusive license, with the right to grant sublicenses (through multiple tiers) solely as provided in Section 2.2, under the Ovid Technology to Develop (but not to make or have made unless otherwise provided herein) the Products on a worldwide basis in accordance with the Development Plan, and to use the Products for that purpose; and
(c)a non-exclusive, royalty-bearing license, with the right to grant sublicenses (through multiple tiers) solely as provided in Section 2.2, under the Ovid Technology to make or have made the Products solely for use in the Field in the Licensee Territory in accordance with the licenses granted in Sections 2.1(a) and 2.1(b), provided that, for clarity, Licensee’s rights under any Patents and Know-How Controlled by Lundbeck that are included within the Ovid Technology shall be subject to the completion of the Technology Transfer and the terms of the Lundbeck License Agreement; and
(d)solely as and to the extent provided in Section 14.5, an exclusive (even as to Ovid, except as expressly set forth herein), royalty-bearing license, with the right to grant sublicenses (through multiple tiers) solely as provided in Section 2.2, under the Ovid Technology to Develop, have developed, use and have used, sell, offer for sale, import, and otherwise Commercialize and have Commercialized the Products in the Field in the Licensee Territory.
2.2Sublicenses. Licensee shall have the right to grant sublicenses under the licenses granted in Section 2.1:
(a)to an Affiliate of Licensee without Ovid’s express prior written consent but with written notice to Ovid, provided that such sublicense will terminate if such sublicensee no longer qualifies as an Affiliate of Licensee.
(b)to a Third Party other than as set forth in Section 2.2(a) with Ovid’s express prior written consent (not to be unreasonably withheld, conditioned or delayed).
All sublicenses granted under the licenses granted in Section 2.1 shall be in writing and shall be subject to, and consistent with, the terms and conditions of this Agreement and shall provide that any such Sublicensee (including any distributor) shall not further sublicense except with the written consent of Licensee and Ovid. Licensee shall ensure that each agreement with a Sublicensee grants Ovid all rights with respect to Data, Inventions, and Regulatory Filings made or generated by such Sublicensee as if such Data, Inventions, and Regulatory Filings were made or generated by Licensee. Licensee shall be responsible for the compliance of its Affiliates,
17
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Sublicensees (including any distributors), and subcontractors with the terms and conditions of this Agreement. Licensee shall provide written notice to Ovid of each sublicense granted to a Third Party hereunder, specifying the name of the Sublicensee, the territory, and the duration of the sublicense.
2.3Reserved Rights. Ovid hereby expressly reserves:
(a)the right under the Ovid Technology to exercise its rights and perform its obligations under this Agreement, whether directly or through one or more licensees or subcontractors; and
(b)all rights to practice, and to grant licenses under, the Ovid Technology outside of the scope of the licenses granted in Section 2.1.
2.4Licenses Granted to Ovid. Subject to the terms and conditions of this Agreement, including Section 2.8, Licensee hereby grants to Ovid:
(a)a non-exclusive, royalty-free, fully paid-up license, with the right to sublicense (through multiple tiers), under the Licensee Technology to use, sell, offer for sale, import, and otherwise Commercialize the Products in the Ovid Territory; and
(b)a non-exclusive, worldwide, royalty-free, fully paid-up license, with the right to sublicense (through multiple tiers), under the Licensee Technology to (i) Develop the Compound and Products on a worldwide basis under the Development Plan, and (ii) to make and have made the Compound and Products anywhere in the world.
2.5No Implied Licenses; Negative Covenant. Except as set forth in this Agreement, neither Party shall acquire any license or other intellectual property interest, by implication or otherwise, under or to any Patents, Know-How, or other intellectual property owned or controlled by the other Party. Neither Party shall, nor shall it permit any of its Affiliates or (sub)licensees to, practice any Patents or Know-How licensed to it by the other Party outside the scope of the licenses granted to it under this Agreement.
2.6Disclosure of Know-How. For as long as the Parties are conducting Development activities under the Development Plan, upon Licensee’s written request no more frequently than [***], Ovid shall disclose and make available to Licensee, in electronic form where possible, all Ovid Know-How that comes into existence after the Effective Date and that was not previously provided to Licensee, promptly after the earlier of the development, making, conception, or reduction to practice of such Ovid Know-How. For as long as the Parties are conducting Development activities under the Development Plan, upon Ovid’s written request no more frequently than [***], Licensee shall, and shall cause its Affiliates to, disclose and make available to Ovid, in electronic form where possible, any Licensee Know-How not previously provided to Ovid, promptly after the earlier of the development, making, conception, or reduction to practice of such Licensee Know-How. The CGB shall establish a mechanism for the reciprocal disclosure of such Know-How.
18
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(a)Notice. Licensee shall promptly notify Ovid if it becomes aware of any Third Party Know-How or Patent that is necessary or reasonably useful to Develop, make, have made, use, sell, offer for sale, or import the Compound or Product in the Field in the Licensee Territory, and [***].
(b)Sublicense under Third Party License. If Ovid enters into any agreement with a Third Party after the Effective Date that includes a license from such Third Party to Ovid under any Know-How or Patents that are necessary to Develop, use, sell, offer for sale, or import the Products in the Field in the Licensee Territory, and Ovid has the right to grant a sublicense under such Know-How or Patents to Licensee, then Ovid shall notify Licensee and identify the relevant Know-How or Patents and provide Licensee with the substantive terms of the applicable Third Party license agreement to Licensee, in each case to the extent applicable to the rights that would be sublicensed to Licensee. Such Know-How and Patents, to the extent falling within the definition of Ovid Technology, will be sublicensed to Licensee only if Licensee provides Ovid with written notice in which (i) Licensee consents to adding such Patents and Know-How to the definition of Ovid Technology and (ii) Licensee acknowledges in writing that its sublicense under such license agreement is subject to the terms and conditions of such license agreement.
(c)Licensee Restriction. Except with the prior written consent of Ovid, Licensee shall not obtain a license to any Third Party Patent or Know-How that is necessary to Develop, make, have made, use, sell, offer for sale, or import the Products in the Ovid Territory.
(a)Exclusivity. Subject to Section 2.8(c), for the period starting from the Effective Date and for [***] in the Licensee Territory, neither Party shall, directly or indirectly (including through an Affiliate or a Third Party), develop or commercialize any Competing Product that is directed to the treatment of the Initial Indication or Second Indication in the Licensee Territory.
(b)Competing Program Exclusivity. If a Third Party becomes an assignee of this Agreement from a Party, or becomes an Affiliate of a Party after the Effective Date through merger, acquisition, consolidation, or other similar transaction, and such Third Party, as of the closing date of such transaction, is engaged in the development or commercialization of a Competing Product in the Licensee Territory (a “Competing Program”), then such Competing Program shall not be a breach of Section 2.8(a), so long as (i) such Third Party, or such Party or its Affiliates, [***], and (ii) [***] such Competing Product. [***] of such Competing Program.
(c)Territory Exclusivity. During the Term, Licensee shall not, directly or indirectly (including through an Affiliate or a Third Party), Commercialize the Product in the Ovid Territory. Ovid shall not, directly or indirectly (including through an Affiliate or a Third Party), Commercialize the Product in the Licensee Territory, except as provided in Section 2.3(a).
(d)Effect of Triggering Acquisition. In the event of an acquisition of Ovid by a Competing Acquirer that closes prior to [***] (a “Triggering Acquisition”), then Ovid shall
19
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
provide notice to Licensee of such Triggering Acquisition within [***] after the date upon which the Triggering Acquisition closes or otherwise becomes effective. On or before the date that is [***] after the date on which Licensee receives notice of a Triggering Acquisition, Licensee shall have the right to elect to Take the Lead on any or all then current Joint Development Activities and future Licensee activities in the Licensee Territory, [***] remaining in full force and effect. For sake of clarity, [***].
2.9Licensee’s access to Ovid Data, Know-How and Patents
(a)Ovid hereby agrees to provide to Licensee, within [***] following the Effective Date, a list of (a) [***] the Ovid Know-How [***] and (b) [[***] the Compound and/or Product necessary for Licensee’s Development, use, manufacture, and Commercialization of the Product in the Field in the Licensee Territory, [***].
(b)Promptly following the Effective Date, Ovid shall transfer to Licensee information in its possession and control that relate to [***].
(c)From time to time during the Term, and at no additional cost to Licensee (subject to Section 4.5), Ovid shall provide to Licensee [***] the Ovid Know-How solely relating to the Compound and/or the Product, reasonably requested by Licensee and necessary for Licensee’s Development, use, manufacture, and Commercialization of the Product.
(d)Ovid hereby agrees that, [***] following the Effective Date, Ovid will provide to Licensee the Ovid Know-How [***] for Licensee to Develop, manufacture, use, and Commercialize the Compound and the Product in the Field in the Licensee Territory in accordance with the terms of this Agreement. Licensee shall reimburse Ovid all out of pocket costs and reasonable, internal cost related to such technology transfer.
2.10Licensee’s Access to Ovid Personnel. The Parties acknowledge the significant contribution and experience of Ovid and Ovid employees to the Development, manufacture, use, and Commercialization of the Compound and Product prior the Effective Date and that these contributions and experience may be valuable to Licensee. Accordingly, Licensee is entitled to meet with employees of Ovid who hold significant experience or expertise in the Development activities, and manufacture, use, and Commercialization of the Compound and Product. Licensee shall provide reasonable notice to Ovid requesting such a meeting and Ovid’s approval of date and agenda of meeting shall not be unreasonably withheld. Each Party shall bear its own cost in relation to such meetings.
3.1Collaboration Governance Board. As of the Effective Date, the Parties have established a board to govern their collaboration under this Agreement (the “Collaboration Governance Board” or the “CGB”), composed of an equal number of up to [***] employees of each Party, to oversee and guide the strategic direction of the collaboration of the Parties under this Agreement. The CGB shall act as a joint consultative body and, to the extent expressly provided herein, a joint decision-making body. The CGB shall in particular:
20
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(a)review and discuss the strategy for the Development of the Product worldwide, coordinate and oversee the overall implementation of the Development Plan;
(b)provide a forum for discussion of the Development and Commercialization of the Compound and Products in the Licensee Territory;
(c)monitor performance of Development activities in and for the Licensee Territory, including performance against agreed budgets, and discuss and approve any budget overruns;
(d)oversee the activities of the JPT, and serve as a forum for resolution of disputes arising at the JPT;
(e)review and discuss any proposed amendments to the Development Plan, including corresponding budgets, and approve any proposed amendments to Joint Development Activities under the Development Plan;
(f)review, discuss, and approve Clinical Trial protocols for jointly-conducted Clinical Trials, and monitor the progress of all Clinical Trials in the Licensee Territory;
(g)review Clinical Trial Data from jointly-conducted Clinical Trials to determine whether progress to the next phase Clinical Trial is merited;
(h)review, discuss, and approve Proposals, including amendments to the Development Plan with respect to activities directed to Additional Indications;
(i)monitor and coordinate regulatory strategy and activities for the Licensee Territory, including activities directed to obtaining Regulatory Approval in Additional Indications;
(j)coordinating reporting of pharmacovigilance and safety matters for the Product worldwide (monitoring and coordinating of pharmacovigilance and safety matters will conducted in accordance with the pharmacovigilance agreement);
(k)oversee and coordinate Medical Affairs Activities for the Product in all Indications in the Licensee Territory;
(l)review and discuss the Commercialization Plan for the Licensee Territory, including any amendments proposed thereto, and oversee the Commercialization of Products in the Licensee Territory in accordance with the global commercialization strategy for Products;
(m)provide a forum for discussion regarding launch sequencing, pricing and reimbursement strategy for Products in the Licensee Territory and discuss potential international pricing reference by relevant Regulatory Authorities;
(n)oversee the manufacturing and supply strategy and monitor supply of Products for the Licensee Territory;
21
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(o)oversee and facilitate the Parties’ communications and activities with respect to publications under Section 13.4;
(p)establish joint subcommittees as it deems necessary or advisable to further the purpose of this Agreement, including as set forth in Section 3.7; and
(q)perform such other functions as appropriate to further the purposes of this Agreement, as expressly set forth in this Agreement or allocated to it by the Parties’ written agreement, including providing financial oversight of the activities conducted pursuant to this Agreement.
3.2Joint Project Team. No later than [***] following the Effective Date, the Parties will establish a cross-functional joint project team (the “JPT”), composed of an equal number of up to [***] employees of each Party, to (a) design and implement the Development Plan (subject to CGB review and approval), and (b) oversee the commercial strategy and Commercialization of the Products in the Licensee Territory. The JPT shall in particular:
(a)oversee the conduct of activities under the Development Plan in the Licensee Territory and report to the CGB on such activities;
(b)provide a forum for, and facilitate communications between the Parties with respect to the Development of Products in the Licensee Territory, including sharing of Data in accordance with Section 4.7;
(c)prepare and submit to the CGB for approval protocols for jointly-conducted Clinical Trials;
(d)prepare amendments to the Development Plan as needed (including corresponding changes to the Development Budget) and submit such amendments to the CGB for approval;
(e)review and discuss Proposals and make recommendations to the CGB with respect thereto;
(f)review and discuss the CMC components of the Regulatory Filings in the Licensee Territory, and oversee and coordinate the manufacture and supply of Drug Product to Licensee for use in Clinical Trials for which Licensee is the Sponsor in the Licensee Territory;
(g)prepare and submit to the CGB for approval a plan for manufacture and supply of Drug Product for commercialization in the Licensee Territory;
(h)review the Commercialization Plan prepared by Licensee for alignment with the global commercialization strategy for Products, and provide comments thereto;
(i)monitor the conduct of Commercialization of Products in the Licensee Territory; and
22
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(j)perform such other functions as may be appropriate to further the purposes of this Agreement with respect to the Development of Products.
3.3Committee Membership and Meetings.
(a)Committee Members. Each Committee representative shall have appropriate knowledge and expertise and sufficient seniority within the applicable Party to make decisions arising within the scope of such Committee’s responsibilities. Each Party may replace its representatives on a Committee on written notice to the other Party. The CGB chairperson shall be one of the representatives appointed by Ovid. The chairperson of the JPT shall alternate between one of the representatives appointed by Ovid and one of the representatives appointed by Licensee. The chairperson shall prepare and circulate agendas to Committee members at least [***] before each Committee meeting and shall direct the preparation of reasonably detailed notes for each such Committee meeting, which shall be approved by the chairperson and circulated to Committee members within [***] after such meeting. If not determined as of the Effective Date, the Parties shall determine their respective initial members of each Committee promptly following the Effective Date.
(b)Meetings. Each Committee shall hold meetings at such times as it elects to do so, but in no event shall meetings of the JPT be held less frequently than [***], and meetings of the CGB once every [***]. The first CGB meeting and first JPT meeting shall be held within [***] after the Effective Date. Committee meetings may be held in person or by audio or video teleconference. In-person Committee meetings shall be held at locations alternately selected by the Parties. Each Party shall be responsible for all of its own expenses of participating in any Committee meeting. No action taken at any Committee meeting shall be effective unless at least one (1) representative of each Party is participating. In addition, upon written notice to the other Party, either Party may request that a special ad hoc meeting of a Committee be convened for the purpose of resolving any disputes in connection with, or for the purpose of reviewing or making a decision pertaining to any material subject-matter within the scope of such Committee, the review or resolution of which cannot be reasonably postponed until the following scheduled Committee meeting. Such ad hoc meeting shall be convened at such time as may be mutually agreed by the Parties, but no later than [***] following the notification date of request that such meeting be held.
(c)Non-Member Attendance. Each Party may from time to time invite a reasonable number of participants, in addition to its representatives, to attend Committee meetings in a non‑voting capacity; provided that if either Party intends to have any Third Party (including any consultant) attend such a meeting, such Party shall provide reasonable prior written notice to the other Party and obtain the other Party’s approval for such Third Party to attend such meeting, which approval shall not be unreasonably withheld or delayed. Such Party shall ensure that such Third Party is bound by written confidentiality and non-use obligations consistent with the terms of this Agreement.
(a)All decisions of a Committee shall be made by unanimous vote, with each Party’s representatives collectively having one (1) vote. If after reasonable discussion and good faith consideration of each Party’s view on a particular matter, the representatives of the Parties
23
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
cannot reach an agreement as to such matter within [***] after such matter was first considered, then if such disagreement arose within the JPT, it shall be referred to the CGB for resolution. If the CGB cannot resolve such matter within a further [***], or if the disagreement first arose within the CGB, then prior to any formal dispute resolution process such issue shall be referred to the Executive Officers for resolution.
(b)If the Executive Officers cannot resolve such matter within [***] after such matter has been referred to them, then:
(i)Ovid shall have the final decision making authority, which shall be exercised in its reasonable discretion, with respect to (A) Ovid’s Independent Development Activities, (B) the Ovid Ongoing Trials, (C) all manufacturing matters outside the Licensee Territory, and (D) Joint Development Activities necessary for obtaining Regulatory Approvals for Products in any Indication in the Licensee Territory for which Ovid is the sponsor (including any necessary updates to the Development Plan and Development Budget pursuant to Section 4.2, but subject in all cases to Sections 3.4(b)(i)(1) and 3.4(b)(i)(2)), except for:
(1) the addition of new Clinical Trials to the Development Plan as Joint Development Activities (the cost of which would be shared by the Parties); and
(2)any material modification to a previously agreed upon Clinical Trial that is set forth in the Development Plan as Joint Development Activities (unless such modification is required by a Regulatory Authority or any local or regional IRB/ethics committee, or is reasonably necessary to protect patient safety); for the purpose of this Section 3.4(b)(i)(2), “material modification” means [***].
(ii)Licensee shall have the final decision making authority with respect to (A) Joint Development Activities necessary for obtaining Regulatory Approvals for Products in any Indication in the Licensee Territory for which Licensee is the sponsor (including any necessary updates to the Development Plan and Development Budget pursuant to Section 4.2, but subject in all cases to Sections 3.4(b)(i)(1) and 3.4(b)(i)(2)), (B) the determination of whether the Additional Pivotal Trial is necessary to obtain Regulatory Approval in the Licensee Territory (although [***] for the Additional Pivotal Trial) (C) Commercialization of the Product in the Licensee Territory, (D) Licensee Territory Development Activities and (E) Licensee’s Independent Development Activities in the Licensee Territory, and (F) Phase 4 Clinical Trials for the Licensee Territory (but subject in all cases to Sections 3.4(b)(i)(1) and 3.4(b)(i)(2)), in each case (A) through (F) provided that Licensee’s exercise of any such decision right does not adversely affect, the Development, manufacture, or Commercialization of the Product in the Ovid Territory, and complies with the terms and conditions of this Agreement. For clarity, [***].
(iii)Neither Party shall have the final decision making authority with respect to the matters in Sections 3.4(b)(i)(1) and (2), and the status quo shall persist with respect to such matter unless and until the Parties are able to agree.
24
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
3.5Limitations on Authority. Each Committee shall have only such powers as are expressly assigned to it in this Agreement, and such powers shall be subject to the terms and conditions of this Agreement. Without limiting the generality of the foregoing, no Committee will have the power to amend this Agreement, and no Committee decision may be in contravention of any terms and conditions of this Agreement.
3.6Discontinuation of the Committees. The activities to be performed by each Committee shall solely relate to governance under this Agreement and are not intended to be or involve the delivery of services. Each Committee shall continue to exist until the first to occur of (a) the Parties mutually agree to disband such Committee or (b) Ovid provides written notice to Licensee of its intention to disband and no longer participate in such Committee. Once the Parties mutually agree or Ovid has provided written notice to disband a Committee, such Committee shall have no further obligations under this Agreement and, thereafter, each Party shall designate a contact person for the exchange of information under this Agreement or such exchange of information shall be made through Alliance Managers, and decisions of such Committee shall be decisions as between the Parties, subject to the other terms and conditions of this Agreement. [***].
3.7Alliance Managers. Promptly after the Effective Date, each Party shall appoint an individual who shall be an employee of such Party having appropriate qualification and experience to act as the alliance manager for such Party (the “Alliance Manager”). Each Alliance Manager shall be responsible for coordinating and managing processes and interfacing between the Parties on a day-to-day basis throughout the Term. The Alliance Manager will ensure communication to the CGB of all relevant matters raised at the JPT and any other subcommittee. Each Alliance Manager shall be permitted to attend meetings of the CGB and JPT, in each case as appropriate and as non-voting participants. The Alliance Managers shall be the primary contact for the Parties regarding the activities contemplated by this Agreement and shall facilitate all such activities hereunder. Each Party may replace its Alliance Manager with an alternative representative at any time with prior written notice to the other Party. Any Alliance Manager may designate a substitute to temporarily perform the functions of that Alliance Manager. Each Party shall bear its own costs of its Alliance Manager, which costs shall be excluded from the Parties’ respective Development and manufacturing costs (including Cost of Goods) under this Agreement.
3.8Supply Contacts. Each Party shall designate one (1) qualified and experienced supply chain professional to serve as that Party’s primary supply contact regarding the supply of Drug Product under this Agreement (“Supply Contacts”). Each Party may replace its Supply Contact with an alternative representative at any time with prior written notice to the other Party. The Supply Contacts shall be responsible for facilitating information exchange and discussion between the Parties regarding the supply of Drug Product under this Agreement. The Supply Contacts shall have decision-making authority with respect to the supply of Drug Product under this Agreement within the guidance of the JPT and subject to the review and approval of the CGB. Each Party shall bear its own costs of its Supply Contact, which costs shall be excluded from the Parties’ respective Development and manufacturing costs (including Cost of Goods) under this Agreement.
25
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
4.1Overview. Subject to the terms and conditions of this Agreement, the Parties will collaborate with respect to the Development of the Compound and Products and share the Data resulting from such collaboration as provided in this Article 4 to facilitate the Development of the Compound and Products throughout the Licensee Territory and the Ovid Territory.
4.2Amendments to Development Plan. Any amendment to the Development Plan for the Compound and Products under this Agreement, including the Ovid Ongoing Trials, Licensee Territory Development Activities for registrational purposes, all Joint Development Activities, and all Independent Development Activities shall be conducted pursuant to a comprehensive written updated (from time to time) Development Plan (the “Amended Development Plan”), which shall be incorporated by reference into this Agreement. The Amended Development Plan will include Clinical Trials for the Initial Indications that the Parties have agreed to conduct jointly (unless modification is required by a Regulatory Authority or any local or regional IRB/ethics committee, or is reasonably necessary to protect patient safety), the Clinical Trials for the Second Indication that Ovid may conduct under its sole responsibility and cost if Licensee exercises the Second Indication Opt-Out and not the Second Indication Opt-In, as well as Clinical Trials for the Additional Indications that the Parties (through the CGB) mutually agree to conduct and include in accordance with Section 4.3. The Amended Development Plan will also include:
|
|
(1) |
any other Development activities approved by the CGB in accordance with Article 3; and |
|
|
(2) |
the PIP for the Initial Indication; and |
|
|
(3) |
the Additional Pivotal Trial, if applicable. |
The Amended Development Plan shall also set forth the amendments to the detailed budget of the anticipated costs for all Development activities (the “Amended Development Budget”) on a study-by-study or Clinical Trial-by-Clinical Trial basis. As of the Effective Date, the Parties have agreed upon an initial Development Plan and Development Budget, attached to this Agreement as Exhibit C. If the terms of the Development Plan contradict, or create inconsistencies or ambiguities with, the terms of this Agreement, then the terms of this Agreement shall govern. In addition, the following shall apply to the Development of the Compound and Products under the Development Plan:
(a)Neptune Trial, Rocket Trial and Elara Trial. Ovid shall be responsible for, and shall use Commercially Reasonable Efforts to conduct, at its own expense, (i) the Neptune Trial, (ii) the Rocket Trial, and (iii) the Elara Trial ((i)-(iii) collectively, the “Ovid Ongoing Trials”).
(b)PIP. Ovid shall be responsible for the full applying process to obtain EMA agreement for the Paediatric Investigational Plan in order to allow the Licensee to submit the MAA for the Product for the Initial Indication in the Licensee Territory. Ovid shall discuss and agree with the Licensee the PIP before moving forward with the application process.
26
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(c)Licensee Territory Development Activities for Initial Indication.
(i)Licensee shall be solely responsible for and shall use Xxxxxxxx Commercially Reasonable Efforts to conduct, at its sole expense, all Development activities that are exclusively for the benefit of one or more countries within the Licensee Territory for the Initial Indication, including (A) any and all Development activities required or recommended by a Regulatory Authority for the exclusive benefit of the Licensee Territory, including any Phase 4 Clinical Trials for the Initial Indication in the Licensee Territory, and (B) any and all Development activities required for any Pricing and Reimbursement Approval in the Licensee Territory.
(ii) Licensee shall be solely responsible for the Additional Pivotal Trial, and for the Phase 4 Clinical Trials of the Product in the Licensee Territory, but Licensee shall bear [***] and Ovid shall bear [***] of the Development Costs for the Development Activities for the Additional Pivotal Trial, up to a total amount of [***], and for any Additional Pivotal Trial Development Costs in excess of [***] Licensee shall bear [***] and Ovid shall bear [***].
(iii)The activities specified in the foregoing subsections (i) and (ii) shall be referred to collectively as the “Licensee Territory Development Activities”. Prior to commencing any Licensee Territory Development Activities, Licensee shall provide the CGB with a draft workplan therefor, and following review and approval of such Licensee Territory Development activities by the CGB, such Licensee Territory Development Activities shall be included in and conducted in accordance with the Development Plan, subject to the oversight of the CGB and JPT as set forth in Article 3. Notwithstanding anything to the contrary herein, Licensee shall consider in good faith and incorporate Ovid’s reasonable comments on any proposed Licensee Territory Development Activities.
(d)Joint Development Activities for Initial Indication and Second Indication. The Development Plan shall set forth the timeline and details ([***]) of all preclinical (if any) and clinical Development activities ([***]) to be conducted jointly by the Parties in order to generate Data sufficient to meet the common requirements of the FDA, EMA, and other Regulatory Authorities agreed upon by the Parties for MAA Approval of the Compound and Products for each of the Initial Indication and eventually and separately for the Second Indication, ([***]) (such activities, the “Joint Development Activities”). All Joint Development Activities shall be included in and conducted in accordance with the Development Plan, subject to the oversight of the CGB and JPT as set forth in Article 3. The Development Plan shall also set forth the allocation of responsibility for all Joint Development Activities as between the Parties. All Development Costs Associated with Joint Development Activities shall be borne in accordance with Section 8.2(a).
(e)Second Indication Opt-Out and Second Indication Opt-In.
(i)Second Indication Opt-Out. If Licensee determines, at its sole and absolute discretion, within [***], not to pursue further additional Development activities with respect to the Second Indication, Licensee shall have the right to exercise the Second Indication Opt-Out, exercisable by written notice to Ovid. The Second Indication Opt-Out shall be effective as of the date of such notice. If Licensee exercises the Second Indication Opt-Out, then the definition of Field shall automatically be deemed to not include the Second Indication, provided
27
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
that if Ovid undertakes to submit an MAA for the Product for the Second Indication in the Licensee Territory it shall do so with a trademark other than the Trademark for the Initial Indication.
(ii)Second Indication Opt-In. In any case, after Licensee has made the Second Indication Opt-Out, Licensee will have the right, at any time as below and for any reason, to exercise the Second Indication Opt-In, exercisable by written notice to Ovid [***]. The Second Indication Opt-In shall be effective as of the date of such notice. If Licensee makes the Second Indication Opt-In, then Licensee shall reimburse to Ovid an amount equal to [***] of all Development Costs incurred by Ovid in the conduct of Development activities for the Second Indication during the period of Licensee’s Second Indication Opt-Out, and [***] of all Development Costs incurred for such Development activities following the Second Indication Opt-In. Then the definition of Field shall automatically be deemed to include the Second Indication
(f)Regulatory Filings. The Development Plan shall include a coordinated Development and regulatory strategy, including the Parties’ respective roles in the development of the registration dossier and Regulatory Filings for the Products, and the countries in which Clinical Trials of the Products will occur. All costs associated with regulatory activities in connection with Regulatory Filings and obtaining MAA Approval for Products in the Licensee Territory shall be borne solely by Licensee.
(g)Updates. During the Term (at least on a [***] basis), the JPT shall review the Development Plan and prepare updates and amendments, as appropriate, to the then-current Development Plan, including budgets, and submit such updates and amendments to the CGB for review and approval. If the CGB determines that any pre-clinical studies or Clinical Trials not included in the Development Plan are required in order to obtain or maintain MAA Approval for a Product for the Initial Indication in one or more countries in the Licensee Territory, then the CGB shall review and approve an amendment to the Development Plan reflecting such additional studies, including associated budget. Such additional Development activities for the Initial Indication shall be either Licensee Territory Development Activities or Joint Development Activities and the costs of such additional studies shall be borne by the Parties as provided in Section 4.5(a).
(a)Proposal. If either Party (the “Proposing Party”) desires to pursue additional Development of a Product in order to seek Regulatory Approval of the Product in one or more Additional Indications for the benefit of (a) the Ovid Territory and Licensee Territory in the case of Ovid, or (b) the Licensee Territory in the case of Licensee, in each case beyond what is set forth in the then-current Development Plan, then such Party shall provide the other Party (the “Reviewing Party”) with a written detailed plan and budget for such additional work (the “Proposal”). Within [***] after the Reviewing Party’s receipt of the Proposal (or at such other time as the Parties may mutually agree), the JPT shall meet to review and discuss in good faith the Proposal and permit the Reviewing Party an opportunity to ask questions and request additional information from the Proposing Party related to the Proposal, including whether such Proposal is reasonably likely to have any adverse effect on the Development or Commercialization of the Product in the Reviewing Party’s territory. No work under any Proposal shall proceed unless and until (i) the CGB determines in its reasonable discretion that such Proposal is not likely to
28
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
adversely affect the Development or Commercialization of the Product in the Reviewing Party’s territory, and (ii) in the case of Licensee as the Proposing Party, Ovid consents in writing to such activities being included as Joint Development Activities, or to Licensee performing such additional Development as Licensee Territory Development Activities (where such Development Activities solely and specifically relate to the Licensee Territory), or as Independent Development Activities, such consent not to be unreasonably withheld. For clarity, (i) Licensee’s final decision right under Section 3.4(b)(ii) shall not apply to Licensee Territory Development Activities proposed in connection of initiation of Development for any Additional Indication, but once Development Activities have been commenced in such Additional Indication in and for the Licensee Territory, any further Licensee Territory Development Activities proposed by Licensee in respect of such Additional Indication shall be subject to the process set forth in Section 4.2(b) and (ii) Ovid will not develop in the Licensee Territory any Product in additional indications other than Rare Diseases without Licensee’s prior written consent. Following each such determination and, if applicable, consent, the CGB shall incorporate such additional Development activities and the corresponding budget into the Development Plan (the “Additional Development Activities”).
(b)Costs. If the Parties jointly agree to conduct Additional Development Activities, such Additional Development Activities shall be included in the Development Plan and conducted as Joint Development Activities, subject to the allocation of responsibility for leading such activities set forth in Section 4.3(c), and the costs of such Joint Development Activities shall be shared as further set forth in Section 8.2(a). Notwithstanding the foregoing, for Additional Development Activities that would otherwise be Joint Development Activities (if the Parties agreed to conduct such activities together), the Reviewing Party may elect, at its discretion, and by written notice delivered to the Proposing Party within [***] following the receipt of the Proposal, to opt out of funding its share of the Development Costs for such Additional Development Activities. Upon such an election, such Additional Development Activities shall be deemed the “Independent Development Activities” of the Proposing Party and the Proposing Party may pursue such work subject to the remainder of this Section 4.3, and the Development Costs with respect thereto shall be Independent Development Costs subject to Section 8.2(b).
(c)Conduct of Additional Development Activities. In general, except as the Parties may agree in an amendment to the Development Plan, including to allocate specific activities to Licensee in the Licensee Territory, (i) Ovid shall be the lead Party responsible for conducting Additional Development Activities that relate to both the Ovid Territory and the Licensee Territory, provided that such activities shall be subject to the oversight of the CGB to the extent such activities impact the Licensee Territory, and (ii) Licensee shall be the lead Party responsible for conducting Additional Development Activities that relate to the Licensee Territory and not the Ovid Territory.
(d)Independent Development Activities. The CGB shall amend the Development Plan to include any Additional Development Activities in the Rare Diseases indications that are Independent Development Activities, and thereafter the Proposing Party may conduct such Independent Development Activities, provided that: (i) the Proposing Party shall have the right to make the final decision in the event of any dispute regarding the conduct of the Independent Development Activities, except to the extent that, where the Proposing Party is Licensee, Ovid reasonably believes that Licensee’s exercise of such right would adversely impact Ovid’s Development and Commercialization of Products outside the Licensee Territory, (ii) the
29
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Proposing Party shall have the right to make the final decision in the event of any dispute regarding the conduct of the Independent Development Activities, except to the extent that, where the Proposing Party is Ovid, Licensee reasonably believes that Ovid’s exercise of such right would adversely impact Licensee’s Development and Commercialization of Products in the Licensee Territory, (iii) to the extent applicable to the Licensee Territory, Independent Development Activities shall be conducted in accordance with the amended Development Plan, (iv) the Proposing Party shall provide updates to the CGB with respect to such Independent Development Activities impacting the Licensee Territory at each regularly scheduled CGB meeting, and (v) neither Party shall conduct any Independent Development Activities in a manner that would have any adverse effect on the Development or Commercialization of the Product in either Party’s territory. The Investigator initiated trials (IIT) proposed to each Party for the Licensee Territory shall be discussed and agreed between the Parties before providing feedback to the proposing Investigator. The Party receiving the proposal will be responsible for the costs to support the IIT unless a different specific agreement is entered among the Parties. For sake of clarity, Independent Development Activities shall not include indications other than Rare Disease.
(e)Ovid’s Right to Develop. Notwithstanding anything to the contrary herein, Ovid shall have the right to conduct any Development activities with respect to the Compound or Product in or relating to the Ovid Territory outside the scope of the Development Plan. Such Development activities shall be at Ovid’s expense and Licensee shall have the right of reference to the Safety Data generated in such Development activities as necessary for regulatory purposes without any reimbursement obligation to Ovid.
4.4Annual Update to Development Budget. The CGB shall review, discuss, and, with respect to Joint Development Activities, agree upon the subsequent year’s Development Budget on an annual basis no later than [***] of each year.
(a)Licensee Territory Development Costs. Licensee shall be solely responsible for all Development Costs incurred in connection with Licensee Territory Development Activities, except as provided in Section 4.2(c)(ii) with respect to the Additional Pivotal Study.
(b)Joint Development Costs. The Development Costs associated with all Joint Development Activities that are shared by the Parties in accordance with Section 8.2(a) shall include Allowable Increases. “Allowable Increases” means increased Development Costs resulting from [***].
(c)Independent Development Costs. The Party conducting Independent Development Activities pursuant to Section 4.3 shall be solely responsible for all Independent Development Costs as provided in Section 8.2(b).
4.6Development Responsibilities. Ovid shall be responsible for, and shall use Ovid Commercially Reasonable Efforts in connection with, the conduct of the Ovid Ongoing Trials. Licensee shall be responsible for the conduct of the Licensee Territory Development Activities, other than Additional Pivotal Study which shall be shared by the Parties as set forth in Section
30
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
4.2(c)(ii). The Parties shall each be responsible for the conduct of the Joint Development Activities, and the allocation of such agreed responsibility therefore (including which Party shall be the Sponsor for each applicable Clinical Trial), shall be set forth in the Development Plan. Each Party shall have the operational responsibility and be the Sponsor for its own Independent Development Activities.
(a)General. With respect to all Joint Development Activities, Licensee Territory Development Activities, and Independent Development Activities (but subject to Section 4.7(c)), each Party shall promptly provide the other Party with [***]. The Parties shall cooperate [***] to facilitate the sharing of reports, Data, and other information on a routine basis.
(b)Joint Development Activities Data. Each Party shall have the right to use and reference, without additional consideration, any and all Data generated by or on behalf of the other Party (including by any Sublicensee) in the performance of Joint Development Activities for obtaining and maintaining Regulatory Approval for the Products and Commercializing the Products in its territory in accordance with the terms of this Agreement. Notwithstanding the foregoing, should Licensee fail to obtain the foregoing use and reference rights from any Sublicensee, Licensee shall not have the right to grant use and access or other rights to such Sublicensee to any Data or other documentation provided to Licensee by Ovid pursuant to Section 4.7(a).
(c)Independent and Licensee Territory Development Activities Data. The Party receiving Data resulting from the other Party’s Independent Development Activities shall have the right to use such Data only to the extent reasonably necessary for the receiving Party to comply with its regulatory reporting and compliance obligations, including safety reporting obligations. Licensee shall have the right to use such Data only to support its own Development, Regulatory Approval, or Commercialization of the Product in the Field in such Party’s territory for the Initial Indication unless Licensee reimburses Ovid for Licensee’s share of Development Costs pursuant to Section 8.2(b). Ovid shall have the right to use Data resulting from the Licensee Territory Development Activities as necessary for Ovid or its Affiliates or licensees (other than Licensee) to comply with its regulatory reporting and compliance obligations, including safety reporting obligations, and to support its own Development, Regulatory Approval, or Commercialization of the Product in the Ovid Territory.
(d)Any data transfer between the Parties under this Agreement shall comply with the applicable data privacy laws of the EU and USA.
4.8Diligence. Each Party shall use its Commercially Reasonable Efforts to perform the Development activities assigned to such Party under and in accordance with the Development Plan and the Amended Development Plan. In addition, Licensee shall use Commercially Reasonable Efforts to perform any Independent Development Activities of Licensee, and Licensee Territory Development Activities and file XXXx and seek and maintain Regulatory Approval (including Pricing and Reimbursement Approval, as applicable) for the Products throughout the Licensee Territory.
31
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
4.9Compliance. Each Party shall perform Development activities for the Compound and Products in and for the Licensee Territory in compliance with all Applicable Laws, including good scientific and clinical practices under the Applicable Laws of the country in which such activities are conducted.
4.10Development Records. Each Party shall maintain complete, current, and accurate records of all Development activities conducted by it hereunder, and all data and other information resulting from such activities. Such records shall fully and properly reflect all work done and results achieved in the performance of the Development activities in good scientific manner appropriate for regulatory and patent purposes. Each Party shall document all non-clinical studies and Clinical Trials and/or Studies in formal written study reports according to Applicable Laws and national and international guidelines (e.g., ICH, GCP, GLP, and GMP).
4.11Development Reports. At each regularly scheduled JPT meeting, each Party shall provide the JPT with regular reports detailing its Development activities for the Products under this Agreement, and the results of such activities. In addition, after the completion of any Clinical Trial or other study of the Products, the Party responsible for the conduct of such Clinical Trial or study shall promptly provide the other Party with a data package consisting of, at a minimum, [***], as well as any other Data specified in the Development Plan or otherwise agreed by the Parties. The Parties shall discuss the status, progress, and results of each Party’s Development activities under this Agreement at such JPT meetings.
4.12Use of Subcontractors. Each Party may perform its Development activities under this Agreement through one or more subcontractors, provided that (a) such Party will remain responsible for the work allocated to, and payment to, such subcontractors to the same extent it would if it had done such work itself, (b) each subcontractor undertakes in writing obligations of confidentiality and non-use regarding Confidential Information that are substantially the same as those undertaken by the Parties pursuant to Article 13, (c) each subcontractor agrees in writing to assign all intellectual property developed in the course of performing any such work to such Party (or, in the event such assignment is not feasible, a license to such intellectual property with the right to sublicense to such other Party), and (d) in the case of Licensee engaging any subcontractor to conduct significant activities under this Agreement (e.g., a contract research organization to manage a Clinical Trial), Licensee will be responsible for the selection of the subcontractor and will inform Ovid on the selection process including the criteria applied and shall first obtain Ovid’s written consent to such subcontractor, such consent not to be unreasonably withheld. The Parties may also subcontract work on terms other than those set forth in this Section 4.12 with the prior approval of the CGB.
4.13Restrictions. During the Term, neither Party nor any of its Affiliates or Sublicensees shall, directly or through any Third Party, sponsor, conduct, cause to be conducted, otherwise assist in, supply any Product for use in connection with, or otherwise fund any research or Development of any Product in the Licensee Territory outside the scope of the Development Plan. For clarity and without limiting the foregoing, if Licensee wishes to perform or sponsor any study or test on the Compound or Products, including any pre-clinical or non-clinical study, toxicology study, CMC-related study, or comparator study, Licensee shall first prepare and provide to Ovid for Ovid’s approval a Proposal detailing such study in accordance with Section 4.3.
32
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
4.14Materials Transfer. In order to facilitate the Development activities contemplated by this Agreement, either Party may provide to the other Party certain biological materials or chemical compounds Controlled by the supplying Party (collectively, “Materials”) for use by the other Party in furtherance of such Development activities. Except as otherwise provided for under this Agreement, all such Materials delivered to the other Party will remain the sole property of the supplying Party, and will be used only in furtherance of the Development activities conducted in accordance with this Agreement, will not be used or delivered to or for the benefit of any Third Party, except to subcontractors, without the prior written consent of the supplying Party, and will be used in compliance with all Applicable Laws. The Materials supplied under this Agreement must be used with prudence and appropriate caution in any experimental work because not all of their characteristics may be known. Except as expressly set forth in this Agreement, THE MATERIALS ARE PROVIDED “AS IS” AND WITHOUT ANY REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTY OF MERCHANTABILITY OR OF FITNESS FOR ANY PARTICULAR PURPOSE OR ANY WARRANTY THAT THE USE OF THE MATERIALS WILL NOT INFRINGE OR VIOLATE ANY PATENT OR OTHER PROPRIETARY RIGHTS OF ANY THIRD PARTY.
4.15Suspension and Termination for Safety Reasons. Each Party shall have the right, at any time, to request that the CGB meet promptly to discuss whether to suspend the continued Development and/or Commercialization of the Products in the Field in the Licensee Territory for a period of [***] (the “Suspension”), upon providing written notice to the other Party, if such Party reasonably determines in good faith that the Compound or any other Product caused or is likely to cause a safety issue (a “Safety Reason”). If the CGB does not come to a consensus with regard to such Safety Reason during the Suspension, either Party may terminate the Agreement (with the understanding that the other Party shall have the continuing right to Develop and Commercialize the Product; provided, however, that if the other Party is Licensee and Licensee wishes to continue the Development and Commercialization of the Product, this Agreement shall remain in force and effect, but the provisions of Section 14.5 shall apply) with immediate effect, upon written notice to the other Party if: (i) the Executive Officers meet (in person or otherwise) within [***] to resolve the dispute in good faith; and (ii) such Executive Officers are unable to resolve the dispute.
5.1Regulatory Responsibilities.
(a)General.
(i)The Development Plan shall set forth the regulatory strategy for seeking Regulatory Approval for the Compound and Products by the appropriate Regulatory Authorities in the Licensee Territory and Ovid Territory. Subject to the oversight of the CGB and except as otherwise set forth in the Development Plan and the remainder of this Section 5.1(a)(i), each Party shall be responsible for implementing such regulatory strategy in its territory. The Development Plan shall also specify which Party shall apply for and hold Regulatory Filings in each country with respect to the conduct of Development activities, provided that (1) Ovid shall apply for and hold all Regulatory Filings and Regulatory Approvals for the Ovid Ongoing Trials
33
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
and Ovid’s Independent Development Activities and (2) Licensee shall apply for and hold all Regulatory Filings for the Licensee Territory arising from the Licensee Territory Development Activities, Licensee’s Independent Development Activities, and Joint Development Activities. Except as otherwise provided herein or in the Development Plan or required by Applicable Law, each Party shall (A) be responsible for the preparation and submission of any and all Product registrations and XXXx in its territory and (B) own and hold all such Regulatory Filings (including Regulatory Approvals). For the avoidance of doubt, in no event shall Licensee submit any Regulatory Filing for the Product in the Ovid Territory.
(ii)Each Party shall be responsible for the costs of all regulatory activities in its territory, except that any costs incurred by Ovid in connection with regulatory activities in the Licensee Territory pursuant to Ovid’s Independent Development Activities shall be Independent Development Costs of Ovid and subject to Section 8.2(b).
(iii)Licensee acknowledges that Ovid may be required from time to time to communicate with Regulatory Authorities in the Licensee Territory as a result of Development and manufacturing activities in such territory. Ovid shall notify Licensee as soon as reasonably practicable of such communication with Regulatory Authorities in the Licensee Territory.
(iv)Ovid will provide to Licensee [***]. Licensee shall have the right to use such information to [***] for the Product for the Initial Indication [***] under this Agreement.
(v)Prior to [***], Ovid will not, without Licensee’s input, (A) withdraw the IND for the Product in the Licensee Territory for any reason other than a Safety Reason, (B) file an MAA for the Product in the Licensee Territory, or (C) finalize the PIP for the Initial Indication. Prior to [***], Ovid shall notify Licensee of any scheduled meeting with a Regulatory Authority in the Licensee Territory that relates to the any of the Ovid Ongoing Trials and, to the extent permitted by Applicable Law and the relevant Regulatory Authority, shall permit a representative of Licensee to attend such meeting, at Licensee’s request and expense.
(vi)Ovid will, upon Licensee’s reasonable request, cooperate with Licensee in [***] Product in the Initial Indication in the Licensee Territory.
(vii)Ovid shall [***] for the Compound [***]. Licensee shall have the right to perform a Scientific Advice with the applicable Regulatory Authorities in the Licensee Territory.
(b)Regulatory Filing Right of Reference. Except as set forth in Section 5.1(c), Ovid shall grant and hereby grants to Licensee a right of reference and access to all Regulatory Approvals and Regulatory Filings Controlled by Ovid in the Licensee Territory for the Compound and Product, in each case to the extent necessary for Licensee to submit Regulatory Filings and obtain MAA Approvals for Products in the Initial Indication in the Licensee Territory. For the purposes of this Agreement, “right of reference” means the “right of reference or use” as defined in 21 C.F.R. §314.3(b) and any equivalent regulation outside the U.S., as each may be amended.
34
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(c)Licensee Regulatory Information Sharing and Right of Reference.
(i)Licensee shall promptly provide Ovid with copies of any Regulatory Filings prepared (including any drafts), submitted, or received by Licensee in the Licensee Territory pertaining to the Compound and Products, and Ovid shall have the right to comment on drafts of such Regulatory Filings. Licensee shall share with Ovid the following communications/correspondence with any Regulatory Authority: (1) [***], (2) [***], and (3) [***] relating to the Compound or Product. If any Regulatory Filing to be provided under this Section 5.1(c) was originally created in a language other than the English language, then Licensee shall provide an English translation along with the original document to Ovid. Licensee shall use Commercially Reasonable Efforts to grant to Ovid access and rights to use any such communications with any Regulatory Authority generated by or on behalf of any Sublicensee. Should Licensee fail to obtain such access and rights from any Sublicensee despite the application of Commercially Reasonable Efforts, Licensee shall not have the right to grant access or rights to such Sublicensee to any Regulatory Filing or right of reference granted to Licensee by Ovid pursuant to Section 5.1(b).
(ii)Licensee hereby grants to Ovid a right of reference to all Regulatory Filings for the Compound and Products submitted by or on behalf of Licensee. Ovid may use such right of reference to seek, obtain, and maintain Regulatory Approval of the Products in the Ovid Territory, except that Ovid may use such right of reference to any Regulatory Filings based on Data resulting from Licensee’s Independent Development Activities only to comply with its safety reporting obligations, unless Ovid reimburses Licensee for such work as set forth in Section 8.2(b).
5.2Meetings with Regulatory Authorities. On a current and ongoing basis, each Party (through the JPT) shall provide the other Party with a list and schedule of any in-person meeting or material teleconference with Regulatory Authorities (or related advisory committees) in the Licensee Territory planned for [***] that relates to the Development of the Compound and Products under the Development Plan in the Licensee Territory (each, a “Regulatory Meeting”). In addition, each Party shall notify the other Party as soon as reasonably possible if such Party becomes aware of any additional Regulatory Meetings that become scheduled for such Calendar Quarter and will keep the other Party informed of any significant interface or communication with any Regulatory Authority which might affect efforts to obtain Regulatory Approval for the Product in the Licensee Territory. Each Party shall be solely responsible for any communications with any Regulatory Authorities occurring or required in connection with performing its regulatory responsibilities set forth in this Article 5 with respect to the Product in the Licensee Territory. With respect to Regulatory Meetings for which Licensee is the responsible Party, Ovid shall have the right to comment in preparation for all such Regulatory Meetings and the right, but not the obligation, to have its representatives attend any such Regulatory Meetings.
5.3Regulatory Inspections. Licensee shall permit the Regulatory Authority(ies) in the Ovid Territory to conduct inspections of Licensee, its Affiliates, and its Sublicensees and subcontractors (including Clinical Trial sites) relating to the Development of the Product under the Development Plan, and shall ensure that such Affiliates and Sublicensees and subcontractors permit such inspections. In addition, Licensee shall promptly notify Ovid of any such inspection and shall supply Ovid with all information pertinent thereto. Ovid shall have the right to have a representative attend any such inspection. Ovid shall permit the Regulatory Authority(ies) in the
35
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Licensee Territory to conduct inspections of Licensee, its Affiliates, and its Sublicensees and subcontractors (including Clinical Trial sites) relating to the Development of the Product in the Licensee Territory under the Development Plan, and shall ensure that such Affiliates and Sublicensees and subcontractors permit such inspections. In addition, Ovid shall promptly notify the Licensee of any such inspection in the Licensee Territory and shall supply the Licensee with all information pertinent thereto. The Licensee shall have the right to have a representative attend any such inspection.
5.4Adverse Event Reporting; Pharmacovigilance Agreement. [***] after the Effective Date, the Parties shall enter into a pharmacovigilance agreement setting forth the worldwide pharmacovigilance procedures for the Parties with respect to the Products, such as Safety Data sharing, adverse event reporting, and safety signal and risk management (the “Pharmacovigilance Agreement”), which agreement shall be amended by the Parties from time to time as necessary to comply with any changes in Applicable Laws or any guidance received from Regulatory Authorities. Such procedures shall be in accordance with, and enable the Parties to fulfill, local and national regulatory reporting obligations under Applicable Laws (including, to the extent applicable, those obligations contained in ICH guidelines) to monitor patients’ safety. Ovid has established, and shall continue to hold (either by itself or through a vendor engaged by Ovid) the global safety database for the Products, and shall maintain such global safety database for so long as such Product is under Development or Commercialization by the Parties. The Parties envision that Ovid will separately maintain the Product global safety database and Licensee will maintain its own Product safety database with respect to the Licensee Territory and the Parties will synchronize the Product databases in accordance with the Pharmacovigilance Agreement so that they each maintain all Product safety data; however, the Parties agree that the Ovid global safety database will be the source for all periodic reports. Ovid shall [***] from its database and Licensee will maintain [***] its own Product safety database. The CGB shall establish a safety subcommittee to draft the Pharmacovigilance Agreement to define the process for exchanging adverse event reports using the Ovid global safety database, as well as periodic reports, regulatory communication, and other key elements. The Parties will collaboratively agree on data cut points for periodic safety reports and Ovid will review and approve all such reports. The Parties will jointly review and approve such reports before submission to Regulatory Authorities in the Licensee Territory as required. Such safety subcommittee shall implement the Pharmacovigilance Agreement and coordinate with respect to any Safety Data reporting for the Products to the Regulatory Authorities in the Licensee Territory including, responding to safety issues, communicating with Regulatory Authorities related to the Products under any MAA or Regulatory Approval for the Product held by such Party and filed with such Regulatory Authorities, including maintaining the qualified person for Pharmacovigilance and individual case safety report processing, in each case at its own cost. Each Party agrees to comply with its respective obligations under the Pharmacovigilance Agreement and to cause its Affiliates, licensees, and Sublicensees to comply with such obligations.
5.5No Harmful Actions. If a Party reasonably believes that the other Party is taking or intends to take any action with respect to a Product that could reasonably be expected to have a material adverse impact upon the regulatory status of such Product in such Party’s territory, then such Party may bring the matter to the attention of the CGB and the Parties shall discuss in good faith to promptly resolve such concern.
36
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
5.6Notification of Threatened Action. Each Party shall notify the other Party within twenty-four (24) hours of any information it receives regarding any threatened or pending action, inspection, or communication by any Regulatory Authority which may affect the safety or efficacy claims of any Product or the continued Development or Commercialization of any Product. Upon receipt of such information, the Parties shall promptly consult with each other in an effort to arrive at a mutually acceptable procedure for taking appropriate action.
5.7Recalls. In the event that a recall, withdrawal, or correction (including the dissemination of relevant information) of any Product in a Party’s territory is required by a Regulatory Authority of competent jurisdiction, or if any Regulatory Authority requires or advises either Party or such Party’s Affiliates or sublicensees to distribute a “Dear Doctor” letter or its equivalent regarding use of such Product in a Party’s territory, or if a recall, withdraw, or correction of a Product in its territory is deemed advisable by such Party in its sole discretion, such Party shall so notify the other Party no later than [***] in advance of the earlier of (a) [***], or (b) [***]. Any such recall, withdrawal, correction, or dissemination of information (e.g., “Dear Doctor” letter) shall be referred to herein as a “Recall”. Promptly after being notified of a Recall, each Party shall provide the other Party with such assistance in connection with such Recall as may be reasonably requested by such other Party. All costs and expenses in connection with a Recall [***]. Each Party shall handle exclusively the organization and implementation of all Recalls of Products in its territory. Notwithstanding the foregoing, any Recall related to the manufacture and supply of the Product by Ovid to Licensee shall be governed by the terms and conditions of the Supply Agreement.
5.8Sunshine Reporting Laws and the International Transparency Reporting Requirements for Europe. Each Party acknowledges that the other Party may be subject to federal, state, local, and international laws, regulations, and rules related to the tracking and reporting of payments and transfers of value provided to health care professionals, health care organizations, and other relevant individuals and entities, including, as applicable, International Transparency Reporting Requirements for Europe (collectively, “Sunshine Reporting Laws”), and agrees to provide the other Party with all information regarding such payments or transfers of value by such Party as necessary for such other Party to comply in a timely manner with its reporting obligations under the Sunshine Reporting Laws.
6.1General. Subject to the terms and conditions of this Agreement, including this Article 6, Licensee shall have the sole and exclusive responsibility, at its own expense, for all aspects of the Commercialization of the Products in the Licensee Territory, including (a) developing and executing a commercial launch and pre-launch plan, (b) negotiating with applicable Governmental Authorities and other payors regarding the price and reimbursement status of the Products, (c) marketing and promotion, (d) booking sales and distribution and performance of related services, (e) handling all aspects of order processing, invoicing and collection, inventory and receivables, (f) providing customer support, including handling medical queries, and performing other related functions, and (g) conforming its practices and procedures to Applicable Laws applying to the promotion, sales and marketing, access, and distribution of the Products in the Licensee Territory.
37
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
6.2Commercialization Plan. As soon as reasonably practicable, but no later than [***], Licensee shall prepare and present to the CGB a reasonably detailed plan for the Commercialization of the Product in the Licensee Territory (the “Commercialization Plan”). The Commercialization Plan will include specific information on a country-by-country basis, as applicable, and shall be consistent with the global commercialization plan for branding and messaging. Licensee shall update and amend the Commercialization Plan [***] following the First Commercial Sale of the Product in the Licensee Territory and present such updates and any amendments to the CGB for review and discussion. Subject to the terms of this Agreement and compliance with the Commercialization Plan, Licensee shall have full Control and authority with respect to the day-to-day Commercialization of the Products and implementation of the Commercialization Plan.
(a)General. During the Term, Licensee shall use Commercially Reasonable Efforts to Commercialize the Products for each and every Indication that receives Regulatory Approval in the Licensee Territory.
(b)Product Launch. Licensee shall use Xxxxxxxx Commercially Reasonable Efforts to launch the Product for each Indication that has received Regulatory Approval and, if required by Applicable Law, Pricing and Reimbursement Approval in the Licensee Territory (including any Indication that received Regulatory Approval as a result of Ovid’s Independent Development Activities). As applicable, Licensee shall obtain all necessary Pricing and Reimbursement Approvals necessary to list and to launch such Product for such Indication following receipt of MAA Approval of such Product in a country. Without limiting the generality of the foregoing, Licensee shall use Xxxxxxxx Commercially Reasonable Efforts to launch the Product in each country in the Licensee Territory within [***] after receiving Regulatory Approval, or, where required by Applicable Law, after the publication of the Pricing and Reimbursement Approval, of the Product for an Indication from the applicable Regulatory Authority in such country. Thereafter, Licensee shall utilize Commercially Reasonable Efforts in the ongoing support for such Product in such country.
(c)Commercial Updates. Licensee shall update the CGB on [***] basis regarding its Commercialization activities with respect to the Products in the Licensee Territory. Each such update shall be in a form to be agreed by the CGB and shall summarize Licensee’s and its Affiliates’ and Sublicensees’ significant Commercialization activities with respect to the Products in the Licensee Territory, and shall contain at least such information at a level of detail reasonably required by Ovid to determine Licensee’s compliance with its diligence obligations set forth in this Section 6.3. Such updates shall include Licensee’s sales activities, sales forecasts for at least the next [***], marketing activities, and Medical Affairs Activities.
6.4Coordination of Commercialization Activities.
(a)Generally. The Parties, through the CGB (or JPT or other designated team), shall update each other on Commercialization strategies for the Product (e.g., for market and payor research, branding and messaging, international congresses, advisory boards) in their respective territories, and the Parties shall work together to identify and take advantage of any
38
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
potential global strategies and messaging. The foregoing shall not be construed as requiring either Party to seek the other Party’s consent in connection with such first Party establishing or implementing any sales, marketing, or medical affairs practices in such first Party’s territory.
(b)Pricing. Following the CGB discussion and processes, Licensee shall keep Ovid timely informed on the status of any application for Pricing and Reimbursement Approval or material updates to an existing Pricing and Reimbursement Approval in the Licensee Territory, including any discussion with a Regulatory Authority with respect thereto. Licensee and its Affiliates and Sublicensees shall [***] include a Product, [***] of the Product [***] and the Product [***].
(c)Sharing of Promotional Materials. Licensee shall, at its own expense, prepare, develop, produce, or otherwise obtain and utilize sales, promotional, advertising, marketing, website, educational, and training materials (the “Promotional Materials”) to support its Commercialization activities in the Licensee Territory, and shall ensure that such Promotional Materials, as well as all information contained therein, comply with all Applicable Laws and are consistent with the Regulatory Approvals obtained for the Product in the applicable jurisdiction in the Licensee Territory. At Ovid’s request, Licensee shall share samples of and updates to Promotional Materials with respect to the Commercialization of the Products with Ovid. If Ovid has such promotional materials available for the Product for the Ovid Territory prior to the commercial launch of such Product in the Licensee Territory, Ovid will share such materials with the Licensee upon its request.
(d)Commercialization in Ovid Territory. For clarity, Ovid shall have the exclusive right to Commercialize the Product in the Ovid Territory at its own expense, with or without Third Party(ies).
6.5Medical Affairs Activities.
(a)Coordination of Global Medical Affairs Activities. Ovid shall be responsible for all Medical Affairs Activities for the Product in the Ovid Territory in accordance with the medical affairs portion of the Development Plan. Licensee shall be responsible for Medical Affairs Activities in the Licensee Territory in accordance with the medical affairs portion of the Development Plan, provided, however, that Ovid shall have the right, but not the obligation, to also conduct Medical Affairs Activities in the Licensee Territory in global support of the Product, consistent with the medical affairs portion of the Development Plan and in coordination and agreement with Licensee, such agreement not to be unreasonably withheld, conditioned, or delayed. Licensee will not undertake Medical Affairs Activities in the Ovid Territory without Ovid’s prior written consent, to be given on a case-by-case basis in Ovid’s sole discretion.
(b)Advisory Panels. To the extent practicable, each Party shall give the other Party written notice at least [***] in advance of any major market or international level advisory panel meetings with key opinion leaders with respect to the Commercialization of the Products in the Licensee Territory and the Ovid Territory that are held, sponsored, or attended by either Party or its Affiliate or sublicensee, and each Party shall have the right to attend and participate in such meetings with the consent of the other Party.
39
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(c)Medical Information. Ovid will provide Licensee with any relevant materials developed by Ovid for the Product in the Ovid Territory for medical information, including reports of collected and answered inquires, which Licensee may use in connection with medical affairs activities in the Licensee Territory. For clarity, Ovid shall provide such materials in the form used by Ovid in the Ovid Territory.
(d)Scientific Training. At times reasonably agreed by the Parties, Ovid will deliver the onboarding scientific training to Licensee’s medical and commercial team and to share updates with respect to the Commercialization of the Product. Each Party shall bear its own cost in relation to such meetings.
6.6Diversion. To enforce the Parties’ respective rights and obligations set forth in Sections 2.1, 2.4, and 2.8 of the Agreement, to the extent permitted by Applicable Law, each Party hereby covenants and agrees that it and its Affiliates shall not, and it shall contractually obligate (and use Commercially Reasonable Efforts to enforce such contractual obligation) its sublicensees not to, directly or indirectly, promote, market, distribute, import, sell, or have sold any Product, including via the Internet or mail order, to any Third Party or to any address or Internet Protocol address or the like in the other Party’s territory. Neither Party shall engage, nor permit its Affiliates and sublicensees to engage, in any advertising or promotional activities relating to any Product for use directed primarily to customers or other buyers or users of such Product located in any country or jurisdiction in the other Party’s territory, or solicit orders from any prospective purchaser located in any country or jurisdiction in the other Party’s territory. If a Party or its Affiliates or sublicensees receives any order for a Product for use from a prospective purchaser located in a country or jurisdiction in the other Party’s territory, such Party shall immediately refer that order to such other Party and shall not accept any such orders. Neither Party shall, nor permit its Affiliates and sublicensees to, deliver or tender (or cause to be delivered or tendered) any Product for use in the other Party’s territory.
6.7Ovid’s Right to Commercialize. Notwithstanding anything to the contrary herein, if Ovid conducts Development activities for the Product in the Licensee Territory as provided in Section 2.3(a) for any Additional Indication(s) for which Licensee has elected to not share Development Costs as provided in Section 8.2(a) and/or Section 8.2(b), as applicable, then prior to filing for Regulatory Approval for the Product in any Additional Indication, the Parties shall discuss in good faith whether Licensee wishes to include such Additional Indication within the Field and to Commercialize such Product in such Additional Indications. For clarity, neither Party may file for Regulatory Approval or Commercialize Products in (i) any Additional Indication for which Licensee has elected not to share Development Costs or (ii) any additional indication other than Rare Diseases unless the Parties mutually agree to do so.
|
7. |
Manufacture and Supply |
7.1Upon Licensee’s request, Ovid will manufacture and supply, itself or through a Third Party contract manufacturer, all Drug Product for use in the Development and Commercialization of the Products under this Agreement and according to the Supply Agreement. All Drug Product supplied by Ovid to Licensee or Licensee Affiliate for use for Development and Commercial purposes shall be supplied at [***], provided that [***] (“Total Supply Price Threshold”). In case the Total Supply Price will be [***], the Cost of Good for such Drug Product
40
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
will be [***] the Total Supply Price Threshold. For clarity, the Total Supply Price Threshold [***]. Payment shall be due within [***] after Licensee’s receipt from Ovid of an invoice for such Drug Product. Drug Product shall be delivered EXW (Incoterms 2020) at supplier facility (or that of its Third Party contract manufacturer), and Licensee shall be responsible for all costs of freight, insurance, taxes and duties associated with shipment of Drug Product to Licensee’s designated delivery point. Licensee shall be responsible, at its expense, for the final packaging and labeling of the Product for all countries in the Licensee Territory. Licensee shall also be responsible, at its sole expense, for any specific manufacturing requirements, such as stability studies or development of finished Product presentations, necessary to obtain MAA Approval of the Product in the Licensee Territory. If Licensee is manufacturing Drug Product following the Technology Transfer Completion Date, Ovid may request, and Licensee or Licensee Affiliate will supply Drug Product to Ovid, on the same terms as set forth above, pursuant to a mutually agreed supply agreement.
8.1Upfront and Technology Transfer Financials.
(a)Upfront Payment. Licensee shall make a one-time, non-refundable, non‑creditable upfront payment to Ovid of twenty million dollars ($20,000,000) within five (5) business days after the Effective Date.
(b)Technology Transfer and Compound Delivery Payment.
(i)Within [***] following (A) the transfer to Licensee of [***], (B) the delivery ([***]) to Licensee or to Licensee Affiliate of [***] and (C) [***], Licensee shall pay to Ovid a one-time, non-refundable, non-creditable payment of (1) [***] if [***], or (2) [***] if [***]. Licensee shall use Xxxxxxxx Commercially Reasonable Efforts to cooperate with Ovid and Lundbeck to implement the foregoing transfers within the timelines set forth in this Section 8.1(b)(i).
(ii)Within [***] following the Technology Transfer Completion Date, Licensee shall make a one-time, non-refundable, non-creditable technology transfer payment of [***]. Licensee shall use Xxxxxxxx Commercially Reasonable Efforts to cooperate with Ovid and Lundbeck to implement the Technology Transfer Agreement within [***] after the Effective Date.
8.2Sharing/Reimbursements of Development Costs.
(a)Shared Development Costs. With respect to Joint Development Activities conducted pursuant to Section 4.2(b) or Section 4.3 directed to obtaining Regulatory Approval for any Indication, excluding the Ovid Ongoing Trials, Ovid shall bear [***] and Licensee shall bear [***] of all Development Costs for such Joint Development Activities. No later than [***] after the beginning of each Calendar Quarter during which a Party will perform any Joint Development Activities pursuant to Section 4.2(b) or Section 4.3 in such Calendar Quarter, such Party shall submit to the other Party a statement setting forth the Development Costs incurred, including the other Party’s share (calculated in accordance with the foregoing sentence) of (i) estimated Development Costs for the then current quarter; (ii) variances from prior invoiced estimates and
41
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
actual Development Costs; and (iii) Development Costs incurred by or on account of such Party in the past quarter not previously invoiced. Such invoice shall include a reasonably detailed report for such Development Costs, including reasonable supporting documents. The other Party shall pay the amount invoiced within [***] after the receipt of the invoice, subject to the other Party’s right to audit the invoicing Party’s records and books related to such costs as provided in Section 9.4. If both Parties will perform Joint Development Activities pursuant to Section 4.2(b) or Section 4.3 under the Development Plan in such Calendar Quarter, the Parties shall consolidate the payments for such Calendar Quarter into a single payment from one Party to the other Party, as applicable.
(b)Independent Development Costs. In general, each Party shall bear all Development Costs incurred by or on account of such Party in performing its own Independent Development Activities (the “Independent Development Costs”). After the completion of such Independent Development Activities, such Party shall provide the other Party (the “Non-Funding Party”) with a report of such Independent Development Costs. If a Non-Funding Party desires to submit any portion of the Data, or reference any portion of a Regulatory Filing, resulting from Independent Development Activities conducted by the other Party to support any Regulatory Approval in the Non-Funding Party’s territory, then such Non-Funding Party may notify the other Party in writing of such request at any time following the completion of such Independent Development Activities. Within [***] after its receipt of such notice, the Party having conducted such Independent Development Activities shall submit to the other Party a reasonably detailed invoice for, [***] of the Independent Development Costs incurred [***] in connection with the performance of such Independent Development Activities, with such invoiced amount representing the Non-Funding Party’s base share of Development Costs ([***]), had such Party originally opted in, plus [***], provided that in no event will either Party be required to pay, as a result of the application of such premium, more than [***] the costs actually incurred in conducting such Independent Development Activities. If the Non-Funding Party subsequently uses or references such Data, in whole or in part to support Regulatory Approval of the Product in the applicable Indication in its territory, then such Party shall notify the conducting Party in writing of such decision and pay the amount set forth in the invoice within [***] after its submission of a Regulatory Filing including or referencing such Data.
(c)Internal Development Cost. Each Party shall record and calculate its Development Costs for the Development Activities implemented from the Effective Date on an FTE basis at the applicable FTE Rate. The Parties agree that [***] from the Effective Date.
8.3Development Milestone Payments.
(a)Development Milestones. Subject to the remainder of this Section 8.3, Licensee shall pay to Ovid the one-time, non-refundable, non-creditable payments set forth in the table below upon the achievement of the applicable milestone event (whether by or on behalf of Licensee or its Affiliates or Sublicensees or Ovid or its Affiliates or licensee(s) (other than Licensee)).
42
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
Regulatory Milestone Amount |
|
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
(b)Notice and Payment. Each Party shall notify the other Party in writing within [***] after the achievement of any milestone event set forth in this Section 8.3 by such Party or its Affiliates or Sublicensees. Licensee shall pay to Ovid the applicable development milestone payment within [***] after the delivery or receipt of such notice.
(c)One-time Payment. Each of the above Regulatory Milestone amounts shall only be payable once no matter how many times the corresponding Regulatory Milestone Event is achieved.
(d)For clarity, Licensee shall pay to Ovid each Regulatory Milestone amount the first time that the corresponding Regulatory Milestone Event is achieved.
(a)Licensee shall pay to Ovid the one-time, non-refundable, non-creditable payments set forth in the table below when the aggregated Net Sales of all Products in the Licensee Territory in any Calendar Year first reach the values indicated in the table below. For clarity, each payment in this Section 8.4 shall be payable once only upon first achievement of the applicable milestone event, regardless of the number of times such milestone is subsequently achieved.
|
Aggregate Net Sales of all Products in the Licensee Territory in a Calendar Year |
Sales Milestone Payment Amount |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
43
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(b)Notice and Payment. As part of the report in Section 9.1, Licensee shall provide written notice to Ovid if the aggregated Net Sales of all Products in the Licensee Territory in any Calendar Year first reach the values set forth in Section 8.4(a), and Licensee shall pay to Ovid the corresponding Net Sales milestone payment along with its payment of royalties in accordance with Section 9.1, following the end of the Calendar Quarter in which such Net Sales milestone is achieved.
(c)One-time Payment. Each of the above Sales Milestone amounts shall only be payable once during the Term of this Agreement no matter how many times the corresponding Sales Milestone Event is achieved. For clarity, Licensee shall pay to Ovid each Sales Milestone amount the first time that the corresponding Sales Milestone Event is achieved.
8.5Royalty During the Royalty Term.
(a)Royalty Rate. Subject to the remainder of this Section 8.5, during the Royalty Term, Licensee shall make quarterly non-refundable, non-creditable royalty payments to Ovid on the annual Net Sales of all Products sold in the Licensee Territory at the applicable rate to the relevant portion of annual Net Sales as set forth below:
|
Annual Net Sales of all Products in the Licensee Territory |
Royalty Rate |
|
Portion of annual Net Sales up to [***] |
[***]% |
|
Portion of annual Net Sales greater than [***] and less than or equal to [***] |
[***]% |
|
Portion of annual Net Sales greater than [***] and less than or equal to [***] |
[***]% |
|
Portion of annual Net Sales greater than [***] |
[***]% |
For example purposes only, [***].
(b)Royalty Term. Royalties shall be paid on a Product-by-Product and country-by-country basis in the Licensee Territory from the First Commercial Sale of such Product in such country by or on behalf of Licensee, its Affiliates, or Sublicensees, until the last to occur of (i) the expiration of the last‑to‑expire Valid Claim of the Ovid Patents (ii) the expiration of all Market Exclusivity covering such Product in such country, and (iii) fifteen (15) years after the First Commercial Sale of such Product in such country (the “Royalty Term”).
(c)Royalty Stacking Reduction. If it is necessary for Licensee to obtain a license from a Third Party under any Patent in a particular country in the Licensee Territory in order to sell the Compound or the Compound incorporated into a Product for the Initial Indication or, if Licensee does not exercise the Second Indication Opt-Out, the Second Indication in such country and Licensee obtains such a license, then solely during the Royalty Term, Licensee may
44
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
deduct from the royalty payment that would otherwise have been due pursuant to Section 8.5(a) with respect to Net Sales of such Product in such country in a particular Calendar Quarter an amount equal to [***] of the royalties paid by Licensee to such Third Party pursuant to such license on account of the sale of such Product in such country during such Calendar Quarter.
(d)Royalty Floor. Notwithstanding the foregoing Sections 8.5(c) and Section 8.7 with respect to any Product in any Calendar Quarter, the royalties that would otherwise have been due under Section 8.5(a) with respect to Net Sales of such Product in the applicable country(ies) during such Calendar Quarter shall not be reduced by more than [***] as a result of all such reductions.
8.6Royalties During the Extended Commercialization Term. During the Extended Commercialization Term, Licensee shall pay to Ovid a quarterly non-refundable, non-creditable royalty of [***] on the annual Net Sales of all Products sold in the Licensee Territory, as calculated by multiplying the foregoing royalty rate by the amount of Net Sales of the Product in the Territory in the applicable Calendar Quarter.
8.7Royalty Reduction for Generic Competition. If at any time Generic Product Competition exists in a given country with respect to a Product, then the royalty rate set forth in Section 8.5(a) with respect to sales of such Product in such country shall be reduced by [***]. For clarity, if Generic Product Competition ceases to exist, then the royalty rate shall no longer be reduced.
8.8Upstream License Agreement Payments. Any and all payments that become due under the Lundbeck License Agreement as a result of activities under this Agreement, whether by or on behalf of Ovid or by Licensee or its Affiliate or Sublicensee, shall be the responsibility of Ovid and Ovid shall make such payment in accordance with the terms of the Lundbeck License Agreement.
9.1Payment; Reports. All royalty payments due under this Agreement shall be accompanied by a report setting forth, on a country-by-country basis, Net Sales of the Products by Licensee and its Affiliates and Sublicensees in the Licensee Territory in sufficient detail to permit confirmation of the accuracy of the royalty payment made, including, for each country, the number of Products sold, the gross sales and Net Sales of Products, including the deductions from gross sales to arrive at Net Sales, the royalty payable, the exchange rates used, any adjustments to the royalty paid during the Royalty Term pursuant to Section 8.5(c), and whether any Net Sales milestone under Section 8.4 has been achieved. Within [***] following the end of each Calendar Quarter during the Royalty Term and Extended Commercialization Term, Licensee shall provide Ovid with the foregoing report and the payment due for such Calendar Quarter. For clarity, royalty payments during the Extended Commercialization Term shall not be subject to any offsets or reductions whatsoever, including those set forth in Section 8.5(c). Prior to the First Commercial Sale of the Product in the Licensee Territory, the Parties will agree on the form of royalty report. Licensee shall submit a single report for all Net Sales during a Calendar Year, including all of Licensee’s and its Affiliates’ and Sublicensees’ Net Sales, but shall separately identify the Net Sales and other information applicable to each entity.
45
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
9.2Exchange Rate; Manner and Place of Payment. All references to dollars and “$” herein shall refer to U.S. dollars. Unless otherwise specified herein, all payments due under this Agreement shall be payable in U.S. dollars. When conversion of Net Sales from any currency other than U.S. dollars is required, such conversion shall be at the exchange rate equal to the conversion rate for the U.S. dollar for the currency of the country in which the applicable Net Sales were made as published by [***]. All payments owed under this Agreement shall be made by wire transfer in immediately available funds to a bank and account designated in writing by Ovid, unless otherwise specified in writing by Ovid.
(a)Taxes on Income. Each Party shall be solely responsible for the payment of all taxes imposed on its share of income arising directly or indirectly from the activities of the Parties under this Agreement.
(b)Tax Cooperation. The Parties agree to cooperate with one another and use reasonable efforts to avoid or reduce tax withholding or similar obligations in respect of the milestone payments, royalty payments, and other payments made by Licensee to Ovid under this Agreement. To the extent that Licensee is required by Applicable Laws to deduct and withhold taxes on any payment to Ovid, Licensee shall withhold the amount of such taxes otherwise payable to Ovid and, if and when necessary pay the amounts of such taxes to the proper Governmental Authority in a timely manner and promptly transmit to Ovid an official tax certificate or other evidence of such payment sufficient to enable Ovid to claim such payment of taxes. Ovid shall provide Licensee any tax forms that may be reasonably necessary in order for Licensee to not withhold tax or to withhold tax at a reduced rate under an applicable bilateral income tax treaty, to the extent legally able to do so. Ovid shall use reasonable efforts to provide any such tax forms to Licensee in advance of the due date. Licensee shall provide Ovid with reasonable assistance to enable the recovery, as permitted by Applicable Laws, of withholding taxes or similar obligations resulting from payments made under this Agreement, such recovery to be for the benefit of Ovid. Licensee shall have the right to deduct any such tax, levy, or charge actually paid from payment due to Ovid. Each Party agrees to assist the other Party in claiming exemption from such deductions or withholdings under double taxation or similar agreement or treaty from time to time in force and in minimizing the amount required to be so withheld or deducted.
(c)Taxes Resulting From Licensee’s Action. If a Party takes any action of its own discretion (not required by a Regulatory Authority and without consent of the other Party), including any assignment, sublicense, change of place of incorporation, or failure to comply with Applicable Laws or filing or record retention requirements, which results in a withholding or deduction obligation (a “Withholding Tax Action”), then such Party shall pay the sum associated with such Withholding Tax Action. For clarity, if Licensee undertakes a Withholding Tax Action, then the sum payable by Licensee (in respect of which such deduction or withholding is required to be made) shall be increased to the extent necessary to ensure that Ovid receives a sum equal to the sum which it would have received had no such Withholding Tax Action occurred. If a change in Applicable Laws results in a withholding or deduction obligation absent either Party taking a Withholding Tax Action, then the amount of such withholding or deduction obligation shall be paid by Licensee to the applicable Governmental Authority on behalf of Ovid, provided that Licensee shall assist Ovid in minimizing or recovering such withholding or deduction obligation.
46
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
The Parties shall use commercially reasonable efforts to invoke the application of any applicable bilateral income tax treaty that would reduce or eliminate otherwise applicable taxes with respect to payments payable pursuant to this Agreement.
9.4Records; Audit. Each Party shall maintain complete and accurate records in sufficient detail in relation to this Agreement to permit the other Party to confirm the accuracy of the amount of Development Costs to be reimbursed or shared, achievement of Net Sales milestones, and the amount of royalty and other payments payable under this Agreement. Each Party will keep such books and records for at least [***] following the Calendar Year to which they pertain. Upon reasonable prior notice, such records shall be inspected during regular business hours at such place or places where such records are customarily kept by an independent certified public accountant (the “Auditor”) selected by the auditing Party and reasonably acceptable to the audited Party for the sole purpose of verifying for the auditing Party the accuracy of the financial reports furnished by the audited Party pursuant to this Agreement or of any payments made, or required to be made, by or to the audited Party pursuant to this Agreement. Before beginning its audit, the Auditor shall execute an undertaking acceptable to each Party by which the Auditor agrees to keep confidential all information reviewed during the audit. Such audits may occur no more often than [***]. Each Party shall only be entitled to audit the books and records from the [***] in which the audit request is made. The Auditor shall not disclose the audited Party’s Confidential Information to the auditing Party, and shall only verify the accuracy or inaccuracy of the financial reports furnished by the audited Party or the amount of payments by such Party under this Agreement, and, in the case of any inaccuracy, the amount of such inaccuracy. In the event that the final result of the inspection reveals an undisputed underpayment or overpayment, the underpaid or overpaid amount shall be settled within [***] after the Auditor’s report. The auditing Party shall bear the full cost of such audit unless such audit reveals an overpayment to, or an underpayment by, the audited Party, which underpayment or overpayment was more than [***], in which case the audited Party shall reimburse the auditing Party for the costs for such audit. For clarity, the foregoing audit rights may also be exercised by Ovid on behalf of Lundbeck pursuant to the terms of the Lundbeck License Agreement.
9.5Late Payments. In the event that any payment due under this Agreement is not paid when due in accordance with the applicable provisions of this Agreement, the payment shall accrue interest from the date due [***]; provided, however, that in no event shall such rate exceed the maximum legal annual interest rate. The payment of such interest shall not limit the Party entitled to receive payment from exercising any other rights it may have as a consequence of the lateness of any payment.
(a)Data. All Data generated in connection with any Development or Commercial activities with respect to any Product conducted solely by or on behalf of Ovid and its Affiliates and licensees (other than Licensee) (the “Ovid Data”) shall be the sole and exclusive property of Ovid or such Affiliates or licensees, as applicable. All Data generated in connection with any Development or Commercial activities with respect to any Product conducted solely by or on behalf of Licensee or its Affiliates or Sublicensees (the “Licensee Data”) shall be the sole
47
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
and exclusive property of Licensee or such Affiliates or Sublicensees, as applicable. All Data generated in connection with any Joint Development Activities or joint Commercial activities with respect to any Product and for which the Parties are sharing Development Costs pursuant to Section 8.2(a) shall be jointly owned by the Parties. For clarity, each Party shall have access and right to use and reference the other Party’s Data as and to the extent set forth in this Agreement.
(b)Inventions. Inventorship of any Inventions will be determined in accordance with the standards of inventorship and conception under U.S. patent laws. The Parties will work together to resolve any issues regarding inventorship or ownership of Inventions. Ownership of Inventions will be allocated as follows:
(i)Ovid shall solely own all data, Inventions, and Patents claiming such Inventions that relate to the composition, formulation, manufacture, dosing, new indications or method of use of the Compound, or any improvement of any such composition, manufacture, or use, including in combination with other agents or components (each, a “Compound Invention”). All Compound Inventions will be included in the Ovid Know-How, and Patents in the Licensee Territory claiming such Inventions will be included in the Ovid Patents. To the extent that any Compound Invention is made by Licensee, whether solely or jointly with Ovid, Licensee shall, and hereby does, transfer and assign to Ovid, without additional consideration, all of its right, title, and interest in such Compound Invention. To effectuate the foregoing assignment, Licensee shall ensure that all entities and individuals that perform any Development under this Agreement are under a written or other legally enforceable obligation to assign all of its right, title, and interest in such Invention to Licensee or to an entity that is obligated to assign all right, title, and interest to Licensee.
(ii)Except for Compound Inventions, each Party shall solely own any Inventions made solely by it and its Affiliates’ employees, agents, or independent contractors, and the Parties shall jointly own any Inventions that are made jointly by employees, agents, or independent contractors of one Party and its Affiliates together with employees, agents, or independent contractors of the other Party and its Affiliates (“Joint Inventions”). All Patents claiming patentable Joint Inventions shall be referred to herein as “Joint Patents”. Except to the extent either Party is restricted by the licenses granted to the other Party under this Agreement, each Party shall be entitled to practice, license, assign, and otherwise exploit its interest under the Joint Inventions and Joint Patents without the duty of accounting or seeking consent from the other Party.
10.2Patent Prosecution and Maintenance.
(i)Subject to the remainder of this Section 10.2(a), and as between the Parties, Ovid shall have the sole right, but not the obligation, to control the preparation, filing, prosecution, and maintenance (including any interferences, reissue proceedings, reexaminations, inter partes review, patent term extensions, applications for supplementary protection certificates, oppositions, invalidation proceedings and defense of validity or enforceability challenges) of the Ovid Patents (other than Joint Patents) worldwide, using counsel of its own choice. Subject to the terms of the Lundbeck License Agreement, Ovid shall (A) keep Licensee informed of material
48
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
progress with regard to the preparation, filing, prosecution, and maintenance of the Ovid Patents in the Licensee Territory, sufficiently in advance for Licensee to be able to review any material documents, including content, timing, and jurisdiction of the filing of such Ovid Patents in the Licensee Territory, (B) consult with, and consider in good faith the requests and suggestions of, Licensee with respect to strategies for filing, prosecuting, and defending, if any, the Ovid Patents in the Licensee Territory, and (C), [***].
(ii)In the event that Ovid desires to abandon or cease prosecution or maintenance of any Ovid Patent in any country in the Licensee Territory, Ovid shall provide reasonable prior written notice to Licensee of such intention to abandon (which notice shall, to the extent possible, be given no later than [***] prior to the next deadline for any action that must be taken with respect to any such Ovid Patent in the relevant patent office). In such case, upon Licensee’s written election provided no later than [***] after such notice from Ovid, Ovid shall continue prosecution and maintenance of such Ovid Patent at Licensee’s direction and expense. If Licensee does not provide such election within [***] after such notice from Ovid, Ovid may, in its sole discretion, continue prosecution and maintenance of such Ovid Patent or discontinue prosecution and maintenance of such Ovid Patent.
(i)Subject to the remainder of this Section 10.2(b), Licensee shall have the first right, but not the obligation, to control the preparation, filing, prosecution and maintenance (including any interferences, reissue proceedings, reexaminations, patent term extensions, applications for supplementary protection certificates, oppositions, invalidation proceedings, and defense of validity or enforceability challenges) of all Licensee Patents (other than Joint Patents) worldwide, at its sole cost and expense and by counsel of its own choice in the Licensee Territory and by counsel mutually agreed to by the Parties in the Ovid Territory. Licensee shall keep Ovid informed of the status of filing, prosecution, maintenance, and defense, if any, of the Licensee Patents, and Licensee shall consult with, and consider in good faith the requests and suggestions of, Ovid with respect to strategies for filing, prosecuting, and defending the Licensee Patents.
(ii)In the event that Licensee desires to abandon or cease prosecution or maintenance of any Licensee Patent, Licensee shall provide reasonable prior written notice to Ovid of such intention to abandon (which notice shall, to the extent possible, be given no later than [***] prior to the next deadline for any action that must be taken with respect to any such Licensee Patent in the relevant patent office). In such case, upon Ovid’s written election provided no later than [***] after such notice from Licensee, Ovid shall have the right to assume prosecution and maintenance of such Licensee Patent at Ovid’s expense and Licensee shall assign to Ovid all of its rights, title, and interest in and to such Licensee Patent. If Ovid does not provide such election within [***] after such notice from Licensee, Licensee may, in its sole discretion, continue prosecution and maintenance of such Licensee Patent or discontinue prosecution and maintenance of such Licensee Patent.
(i)Subject to the remainder of this Section 10.2(c), Ovid shall have the first right, but not the obligation, to prepare, file, prosecute, and maintain (including any
49
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
interferences, reissue proceedings, reexaminations, patent term extensions, applications for supplementary protection certificates, oppositions, invalidation proceedings, and defense of validity or enforceability challenges) Joint Patents using a patent counsel selected by Ovid in the Ovid Territory and counsel mutually agreed to by the Parties in the Licensee Territory.. Ovid shall keep Licensee informed of material progress with regard to the preparation, filing, prosecution, maintenance, and defense, if any, of the Joint Patents, including content, timing, and jurisdiction of the filing of such Joint Patents, and Ovid shall consult with, and consider in good faith the requests and suggestions of, Licensee with respect to filing, prosecuting, and defending of the Joint Patents in the Licensee Territory.
(ii)In the event that Ovid desires to abandon or cease prosecution or maintenance of any Joint Patent in any country in the Licensee Territory, Ovid shall provide reasonable prior written notice to Licensee of such intention to abandon (which notice shall, to the extent possible, be given no later than sixty (60) days prior to the next deadline for any action that must be taken with respect to any such Joint Patent in the relevant patent office). In such case, at Licensee’s sole discretion, upon written notice from Licensee to Ovid, Licensee may elect to continue prosecution or maintenance of any such Joint Patent at its own expense, and Ovid shall execute such documents and perform such acts, at Licensee’s expense, as may be reasonably necessary to allow Licensee to continue the prosecution and maintenance of such Joint Patent in such country in the Licensee Territory. Any such assignment shall be completed in a timely manner to allow Licensee to continue prosecution and maintenance of any such Joint Patent and any such Patent so assigned shall cease to be either a Joint Patent or a Licensee Patent and shall no longer be subject to the licenses and other rights granted by Licensee to Ovid under this Agreement
(d)Cooperation. Each Party agrees to cooperate fully in the preparation, filing, prosecution, maintenance, and defense, if any, of Patents under Section 10.2 and in the obtaining and maintenance of any patent term extensions and supplementary protection certificates and their equivalents, at its own cost (except as expressly set forth otherwise in this Section 10). Such cooperation includes (i) executing all papers and instruments, or requiring its employees or contractors, to execute such papers and instruments, so as enable the other Party to apply for and to prosecute patent applications in any country as permitted by Section 10.2; and (ii) promptly informing the other Party of any matters coming to such Party’s attention that may affect the preparation, filing, prosecution, or maintenance of any such patent application and the obtaining of any patent term extensions or supplementary protection certificates or their equivalents.
(a)Notice. Each Party shall notify the other within [***] of becoming aware of any alleged or threatened infringement by a Third Party of any of the Ovid Patents (including Joint Patents) in the Licensee Territory, which infringement adversely affects or is reasonably expected to adversely affect any Product, including any declaratory judgment, opposition, or similar action alleging the invalidity, unenforceability, or non-infringement of any of the Ovid Patents (collectively, “Product Infringement”).
(b)Enforcement Right. Ovid shall have the first right to use Ovid Commercially Reasonable Efforts to bring and control any legal action in connection with such
50
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Product Infringement at its own expense as it reasonably determines appropriate. If Ovid (i) decides not to bring such legal action against a Product Infringement (the decision of which Ovid shall inform Licensee promptly) or (ii) Ovid otherwise fails to bring such legal action against a Product Infringement within [***] after first becoming aware of such Product Infringement, subject to the terms of the Lundbeck License Agreement, Licensee shall have the right to bring and control any legal action in connection with such Product Infringement at its own expense as it reasonably determines appropriate after [***] Ovid.
(c)Collaboration. Each Party shall provide to the enforcing Party reasonable assistance in such enforcement, at such enforcing Party’s request and expense, including to be named in such action if required by Applicable Laws to pursue such action. The enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts, shall reasonably consider the other Party’s comments on any such efforts, including determination of litigation strategy and filing of material papers to the competent court. The non-enforcing Party shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall at all times cooperate fully with the enforcing Party.
(d)Expense and Recovery.
(i)Except as set forth in Section 10.3(d)(ii), the enforcing Party shall be solely responsible for any cost and expenses incurred by such Party as a result of such enforcement action. If such Party recovers monetary damages in such enforcement action, such recovery shall be allocated [***].
(ii)Notwithstanding the foregoing, if [***] to bring such action. If [***], then [***].
(e)Other Infringement. Except for Product Infringement as set forth above, each Party shall have the exclusive right to enforce its own Patents against any infringement anywhere in the world. For clarity, as between the Parties, Ovid shall have the exclusive right to enforce (i) the Ovid Patents against any infringement in the Licensee Territory that is not a Product Infringement, and (ii) the Ovid Patents and Joint Patents against any infringement in the Ovid Territory, in each case at its own expense as it reasonably determines appropriate, and subject to the terms of the Lundbeck License Agreement. The Parties shall discuss global enforcement strategy for the Ovid Patents and Licensee Patents, including the defense of validity and enforceability challenges arising from any enforcement action.
10.4Infringement of Third Party Rights. If any Product used or sold by Licensee, its Affiliates, or Sublicensees becomes the subject of a Third Party’s claim or assertion of infringement of any intellectual property rights in a jurisdiction within the Licensee Territory, Licensee shall promptly notify Ovid and the Parties shall promptly meet to consider the claim or assertion and the appropriate course of action and may, if appropriate, agree on and enter into a “common interest agreement” wherein the Parties agree to their shared, mutual interest in the outcome of such potential dispute. Absent any agreement to the contrary, and subject to claims for indemnification under Article 12, each Party may defend itself from any such Third Party claim at its own cost and expense, provided that [***]. If [***].
51
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(a)Product Trademarks. Ovid shall develop and adopt trademarks, including trade names, trade dresses, branding, and logos, to be used for the Products (the “Product Marks”). Ovid shall own all Product Marks throughout the world and all goodwill in the Product Marks shall accrue to Ovid. The Parties shall collaborate to have a global, worldwide trademark to be used on the Product. In the event Ovid is unable to obtain or maintain the Product Marks for the Product in the Licensee Territory or in some countries in the Licensee Territory, the Parties shall collaborate to select such other Product Marks as may be available for registration and marketing of the Product in those countries. Ovid shall be responsible for the registration, maintenance, defense and enforcement of the Product Marks using counsel of its own choice in the Ovid Territory and counsel mutually agreed to by the Parties in the Licensee Territory. Ovid shall keep Licensee informed of material progress with regard to the registration, prosecution, maintenance and defense, if any, of the Product Trademarks in the Licensee Territory, including content, timing, and jurisdiction of the filing of such Product Trademarks in the Licensee Territory, sufficiently in advance for Licensee to be able to review any material documents, and Ovid shall consult with, and consider in good faith the requests and suggestions of, Licensee with respect to strategies for filing, prosecuting and defending the Product Trademarks in the Licensee Territory.
(b)Trademark License. Licensee shall use the Product Marks to Commercialize the Product in the Licensee Territory. In addition, unless prohibited by Applicable Laws, Licensee shall use Commercially Reasonable Efforts to include Ovid’s corporate trademark on the packaging and product information of the Products sold in the Licensee Territory to indicate that the Product is licensed from Ovid. Ovid hereby grants to Licensee a limited, royalty-free license to use Ovid’s corporate trademark and Product Marks solely in connection with the Commercialization of the Product in the Licensee Territory under this Agreement. All use of the Product Marks and Ovid’s corporate trademark shall comply with Applicable Laws and shall be subject to Ovid’s review and approval. For clarity, Licensee shall also include its (or its Affiliate’s or Sublicensee’s, as applicable) corporate logo in the Product sold in the Licensee Territory.
(c)Product Domain Names. In the Licensee Territory, Licensee shall have the exclusive right to register Product Marks as domain names, including all internet domain names under all existing top level domains. This includes as example top level domains such as .com, .net., .info and country code top level domains such as .dk, .uk and .it.
|
11. |
Representations and Warranties |
11.1Mutual Representations and Warranties. Each Party represents and warrants to the other that, as of the Effective Date: (a) it is duly organized and validly existing under the laws of its jurisdiction of incorporation or formation, and has full corporate or other power and authority to enter into this Agreement and to carry out the provisions hereof, (b) it is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder, and the person or persons executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate or partnership action, (c) this Agreement is legally binding upon it, enforceable in accordance with its terms, and does not conflict with any agreement, instrument, or understanding, oral or written, to which it is a Party or by which it may be bound, nor violate any material law or regulation of any court, governmental body, or administrative or other agency having jurisdiction
52
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
over it, (d) it has the right to grant the licenses granted by it under this Agreement; (e) the execution and delivery of this Agreement and the performance of such Party’s obligations under this Agreement do not and will not result in the breach of, constitute a default, cause the acceleration of performance, require any consent under, or result in the creation of any lien, charge or encumbrance upon any of its property or assets pursuant to any material instrument or agreement to which it is a party, or by which it or its properties may be bound or affected, as of the Effective Date; and (f) it is not a party to any agreement, arrangement or understanding with any Third Party which in any significant way prevents it from fulfilling any its material obligations under the terms of this Agreement.
(a)Employees, Consultants, and Contractors. Each Party covenants that it has obtained or will obtain written agreements from each of its employees, consultants, and contractors who perform Development activities pursuant to this Agreement, which agreements will obligate such persons to obligations of confidentiality and non-use and to assign (or, in the case of contractor, grant a license under) Inventions, in each case in a manner consistent with the provisions of this Agreement.
(b)Debarment. Each Party represents, warrants, and covenants to the other Party that it is not debarred or disqualified under the U.S. Federal Food, Drug and Cosmetic Act, as may be amended, or comparable laws in any country or jurisdiction other than the U.S., and it does not, and will not during the Term, employ or use the services of any person who is debarred or disqualified, in connection with activities relating to the Compound or any Product. In the event that either Party becomes aware of the debarment or disqualification or threatened debarment or disqualification of any person providing services to such Party, including the Party itself or its Affiliates or Sublicensees, that directly or indirectly relate to activities contemplated by this Agreement, such Party shall immediately notify the other Party in writing and such Party shall cease employing, contracting with, or retaining any such person to perform any such services.
(c)Compliance. Licensee covenants as follows:
(i)In the performance of its obligations under this Agreement, Licensee shall comply and shall cause its and its Affiliates’ employees and contractors to comply with all Applicable Laws.
(ii)Licensee and its and its Affiliates’ employees and contractors shall not, in connection with the performance of their respective obligations under this Agreement, directly or indirectly through Third Parties, pay, promise, or offer to pay, or authorize the payment of, any money or give any promise or offer to give, or authorize the giving of anything of value to a Public Official or Entity or other person for purpose of obtaining or retaining business for or with, or directing business to, any person, including, Licensee (and Licensee represents and warrants that as of the Effective Date, Licensee, and to its knowledge, its and its Affiliates’ employees and contractors, have not directly or indirectly promised, offered, or provided any corrupt payment, gratuity, emolument, bribe, kickback, illicit gift, or hospitality or other illegal or unethical benefit to a Public Official or Entity or any other person in connection with the performance of Licensee’s obligations under this Agreement, and Licensee covenants that it and
53
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
its Affiliates’ employees and contractors shall not, directly or indirectly, engage in any of the foregoing).
(iii)Licensee and its Affiliates, and their respective employees and contractors, in connection with the performance of their respective obligations under this Agreement, (1) shall not violate or cause the violation of the FCPA, Export Control Laws, or any other Applicable Laws, or otherwise cause any reputational harm to Ovid, and (2) shall immediately notify Ovid if Licensee has any information or suspicion that there may be a violation of any of the foregoing in connection with activities under this Agreement.
(iv)In connection with the performance of its obligations under this Agreement, Licensee shall comply and shall cause its and its Affiliates’ employees and contractors to comply with Licensee’s own anti-corruption and anti-bribery policy, a copy of which has been provided to Ovid prior to the Effective Date.
(v)Ovid will have the right, upon reasonable prior written notice and during Licensee’s regular business hours, to conduct at its own cost and expenses inspections of and to audit Licensee’s books and records in the event of a suspected violation or to ensure compliance with the representations, warranties, and covenants of this Section 11.2(c); provided, however, that in the absence of good cause for such inspections and audits, Ovid may exercise this right no more than annually.
(vi)In the event that Licensee has violated or been suspected of violating any of the representations, warranties, or covenants in this Section 11.2(c), Licensee will cause its or its Affiliates’ personnel or others working under its direction or control to submit to periodic training that Licensee will provide on anti-corruption law compliance.
(vii)Licensee will, at Ovid’s request, annually certify to Ovid in writing Licensee’s compliance, in connection with the performance of Licensee’s obligations under this Agreement, with the representations, warranties, or covenants in Section 11.2(c), which certification shall be issued by Licensee’s global commercial head for the Product.
(viii)Ovid shall have the right to suspend or terminate this Agreement in its entirety where there is a credible finding, after a reasonable investigation, that Licensee, its Affiliates, or its Sublicensees, in connection with performance of Licensee’s obligations under this Agreement, has engaged in chronic or material violations of the FCPA.
11.3Additional Ovid Representations and Warranties. Ovid represents, warrants, and covenants, on behalf of itself and its Affiliates, as applicable, to Licensee that, as of the Effective Date:
(a)to the extent applicable to the performance of its obligations under this Agreement, Ovid shall comply and shall cause its and its Affiliates’ employees and contractors to comply with all Applicable Laws;
54
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(b)Exhibit B lists all of the Ovid Patents as of the Effective Date that are necessary or useful for the Development, registration, use or Commercialization of the Product in the Licensee Territory;
(c)to Ovid’s knowledge, Ovid has the right to grant all rights and licenses it purports to grant to Licensee with respect to the Ovid Technology under this Agreement;
(d)as set forth above, Ovid is not a party to any agreement, arrangement or understanding with any Third Party which in any manner prevents it from fulfilling or affects its ability to perform any of its obligations under the terms of this Agreement to a material extent;
(e)Ovid and its Affiliates have not granted any liens or security interests on the Ovid Technology;
(f)Ovid and its Affiliates have not received any written notice from a Third Party that the Development of any Product conducted by Ovid prior to the Effective Date has infringed any Patents of any Third Party;
(g)Ovid and its Affiliates have not as of the Effective Date, and will not during the Term, grant any right to any Third Party under the Ovid Technology that would conflict with or derogate the licenses or other rights granted to Licensee hereunder;
(h)no claim or action has been brought or, to Ovid’s knowledge, threatened in writing, by any Third Party alleging that the Ovid Patents are invalid or unenforceable, and no Ovid Patent is the subject of any interference, opposition, cancellation, or other protest proceeding;
(i)to Ovid’s knowledge, no Third Party is infringing or misappropriating or has materially infringed or misappropriated the Ovid Technology in the Licensee Territory;
(j)to Ovid’s knowledge, all research and Development of the Product conducted by or on behalf of Ovid prior to the Effective Date has been conducted in compliance with all Applicable Laws;
(k)to Ovid’s knowledge, it has disclosed to Licensee the clinical and non-clinical data in Ovid’s Control that is material to the evaluation of the safety, efficacy, and manufacturing process of the Product;
(l)to Ovid’s knowledge, there are no issues or information, which to Ovid’s knowledge and reasonable opinion, are reasonably likely to have a material impact on the Development of the Product that have not been fully disclosed to Licensee in the course of Licensee’s due diligence;
(m)to Ovid’s knowledge, the Ovid Technology includes all intellectual property rights which are reasonably necessary for the Development, registration, manufacture, use and Commercialization of the Product in the Licensee Territory as contemplated by the terms of this Agreement;
55
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(n)no litigation has been brought against Ovid or any of its Affiliate, or threatened in writing, which would adversely affect the rights granted to Licensee under the Ovid Technology;
(o)neither Ovid nor any of its Affiliates has granted to any Third Party any right (including any license or option or other right to obtain a license) to develop or commercialize Product in the Field in the Licensee Territory that would conflict with the rights granted to Licensee under this Agreement;
(p)as set forth above, Ovid has not granted any Third Party any license, option or other right under or with respect to the Ovid Technology that would conflict with or derogate from the licenses, options and other rights granted to Licensee hereunder;
(q)Ovid has the right to grant the License under Ovid Know-How specified herein free and clear of any liens or encumbrances which would have prevent or impair the grant of such rights;
(r)as set forth above, to Ovid’s knowledge, it has the right to grant the License specified in Section 2.1 free and clear of any liens or encumbrances which would prevent or impair the grant of such rights;
(s)as set forth above, to its knowledge, Ovid has not and will not enter into any agreement that is or would be inconsistent with the obligations, rights and licenses granted to Licensee in this Agreement.
(t)it will use Ovid Commercially Reasonable Efforts in the conduct of the Development and Commercialization of the Compound and/or Product under this Agreement and in compliance with Applicable Law; and
(u)as set forth above, it has to not and will not enter into any agreement that is or would be inconsistent with the obligations, rights and licenses granted to Licensee under this Agreement.
(v)Ovid: (i) has provided or made available to Licensee all material relevant documents and written communications and materials in its and its Affiliates’ possession or Control from and to any Regulatory Authority in the Licensee Territory related to the Product; (ii) as of the Effective Date, Ovid and its Affiliates have not received any oral or written communication (including any notice of inspection, deficiency letter, warning letter or similar notice) from any Regulatory Authority alleging that the Development of Product by or on behalf of Ovid and its Affiliates is not currently materially in compliance with any and all applicable laws or regulations implemented by any Regulatory Authority; and (iii) to Ovid’s knowledge, Ovid and its Affiliates have not made, with respect to the Product, any untrue statement of material fact to any Regulatory Authority, or failed to disclose a material fact required to be disclosed to such Regulatory Authority.
11.4Ovid Covenants Regarding the Lundbeck License Agreement. Ovid shall make any and all payments that become due under the Lundbeck License Agreement as a result of any
56
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
activity under this Agreement, whether by or on behalf of Ovid or by Licensee or its Affiliate or Sublicensee, in each case in accordance with the terms of the Upstream License Agreement.
(a)Ovid is in compliance in all material respects with the Lundbeck License Agreement, and, to Licensor’s knowledge, the other party to the Lundbeck License Agreement is not in breach or default in any respect of the Lundbeck License Agreement pertaining to the Product.
(b)In the event that Ovid receives a notice or other communication alleging it is in breach (including a notice or other communication threatening termination) of the Lundbeck License Agreement, Ovid shall promptly provide Licensee with a copy of such notice.
(c)Ovid shall not agree to any amendment or other modification (including termination) to the Lundbeck License Agreement in a manner that materially adversely affects the rights sublicensed to Licensee under this Agreement without Licensee’s prior written consent, such consent not to be unreasonably withheld or delayed.
(d)Ovid and Licensee have negotiated with Lundbeck a technology transfer agreement (the “Technology Transfer Agreement”) for the transfer from Lundbeck to Licensee of Know-How, information, and data owned or controlled by Lundbeck that is necessary or reasonably useful for the manufacture of the Compound and Drug Product in or for the Licensee Territory and according to the technology transfer plan (the “Technology Transfer Plan”) as attached to the Technology Transfer Agreement. Such Technology Transfer Agreement shall provide such transfer to be finalized over a period of [***] starting from the Effective Date, provided that such time period shall be extended to the extent of any delay caused by Licensee or any other factor outside Ovid’s reasonable control, including delays caused by any force majeure event as described in Section 16.8 of the Agreement.
11.5Additional Licensee Representations, Warranties, and Covenants. Licensee represents, warrants, and covenants to Ovid that, as of the Effective Date, Licensee has not granted, and will not grant during the Term, any right to any Third Party under the Licensee Technology that would conflict with the rights granted to Ovid hereunder. Licensee further represents, warrants, and covenants to Ovid that, as of the Effective Date, Licensee does not own or control any Licensee Patents. Licensee further represents, warrants, and covenants to Ovid that Licensee has the personnel, expertise, capacity, resources, equipment, and facilities necessary to implement the Technology Transfer Plan in accordance with the timelines specified therein so that such plan can be completed over a period of [***] following the date such technology transfer is commenced.
11.6Disclaimer. Except as expressly set forth in this Agreement, THE TECHNOLOGY AND INTELLECTUAL PROPERTY RIGHTS PROVIDED BY EACH PARTY HEREUNDER ARE PROVIDED “AS IS” AND EACH PARTY EXPRESSLY DISCLAIMS ANY AND ALL WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING THE WARRANTIES OF DESIGN, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NONINFRINGEMENT OF THE INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES, OR ARISING FROM A COURSE OF DEALING, USAGE OR TRADE PRACTICES, IN ALL CASES WITH RESPECT THERETO. Without limiting the foregoing, (a) neither Party represents or warrants that any data obtained from conducting Clinical
57
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Trials in one country or jurisdiction will comply with the laws and regulations of any other country or jurisdiction, and (b) neither Party represents or warrants the success of any study or test conducted pursuant to this Agreement or the safety or usefulness for any purpose of the technology it provides hereunder.
12.1Indemnification by Ovid. Ovid hereby agrees to defend, indemnify, and hold harmless Licensee and its Affiliates and their respective directors, officers, employees, and agents (each, a “Licensee Indemnitee”) from and against any and all liabilities, expenses, and losses including any product liability, personal injury, property damage, including reasonable legal expenses and attorneys’ fees (collectively, “Losses”) to which any Licensee Indemnitee may become subject as a result of any claim, demand, action, or other proceeding by any Third Party to the extent such Losses arise out of or result from: (a) the Development, use, handling, storage, Commercialization, or other disposition of any Compound or Product by Ovid or its Affiliates or licensees or the contractors of any of them (excluding any activities by or on behalf of Licensee or its Affiliates or Sublicensees), (b) the negligence or willful misconduct of any Ovid Indemnitee, or (c) the breach by Ovid of any warranty, representation, covenant, or agreement made by Ovid in this Agreement; except, in each case (a)-(c), to the extent such Losses arise out of any activities for which Licensee is obligated to indemnify any Ovid Indemnitee(s) under Section 12.2.
12.2Indemnification by Licensee. Licensee hereby agrees to defend, indemnify, and hold harmless Ovid, its Affiliates, and licensees and their respective directors, officers, employees, and agents (each, a “Ovid Indemnitee”) from and against any and all Losses to which any Ovid Indemnitee may become subject as a result of any claim, demand, action, or other proceeding by any Third Party to the extent such Losses arise out of: (a) the Development, use, handling, storage, Commercialization, or other disposition of any Compound or Product by Licensee or its Affiliates or Sublicensees or the contractor of any of them, (b) the negligence or willful misconduct of any Licensee Indemnitee, or (c) the breach by Licensee of any warranty, representation, covenant, or agreement made by Licensee in this Agreement; except, in each case (a)-(c), to the extent such Losses arise out of any activities for which Ovid is obligated to indemnify any Licensee Indemnitee(s) under Section 12.1.
12.3Procedure. A party that intends to claim indemnification under this Article 12 (the “Indemnitee”) shall promptly notify the indemnifying Party (the “Indemnitor”) in writing of any Third Party claim, demand, action, or other proceeding (each, a “Claim”) in respect of which the Indemnitee intends to claim such indemnification, and the Indemnitor shall have sole control of the defense or settlement thereof. The Indemnitee may participate at its expense in the Indemnitor’s defense of and settlement negotiations for any Claim with counsel of the Indemnitee’s own choice. The indemnity arrangement in this Article 12 shall not apply to amounts paid in settlement of any action with respect to a Claim if such settlement is effected without the consent of the Indemnitor, which consent shall not be unreasonably withheld or delayed. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim shall only relieve the Indemnitor of its indemnification obligations under this Article 12 if and to the extent the Indemnitor is actually prejudiced thereby. The Indemnitee shall cooperate fully with the Indemnitor and its legal
58
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
representatives in the investigation of any action with respect to a Claim covered by this indemnification.
12.4Insurance. Each Party, at its own expense, hereby agrees and undertakes to maintain insurance policies with respect to the activities to be made by and obligations assumed by each of the Party in accordance to the provisions of this Agreement. In this respect the Parties agree to carry out at their sole expense and with a commercially reasonable insurance company, the following liability policies, in an amount consistent with sound business practice and reasonable in light of its obligations under this Agreement during the Term:
(a)Clinical Trial liability policies reasonable for each specific phase and country in which a Party is conducting activities under this Agreement, with limits and warranties no less than those required by Applicable Law; and
(b)product liability policies with limits at a minimum equivalent to [***] per each occurrence and per year.
The Public and Product liability policies above shall start from the Effective Date and last for a period of [***] after the expiration or the earlier termination of the Agreement. Each Party shall provide a certificate of insurance (or evidence of self-insurance) evidencing such coverages to the other Party upon request. It is understood that such policies shall not be construed to create any limit of either Party’s obligations or liabilities with respect to its indemnification obligations hereunder. In the event of use by either Party of subcontractors, sublicensees, or any Third Party in the performance of such Party’s obligations under the Agreement, such Party shall ensure that its subcontractor, sublicensee, or Third Party has a proper and adequate general liability or professional liability insurance to cover its risks with respect to the other Party for damages mentioned above.
12.5Limitation of Liability. NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE OTHER PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL, OR PUNITIVE DAMAGES, INCLUDING LOST PROFITS, IN CONNECTION WITH THIS AGREEMENT OR ANY LICENSE GRANTED HEREUNDER, REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES; provided, however, that this Section 12.5 shall not be construed to limit either Party’s indemnification obligations under this Article 12 or damages available as a result of a breach of a Party’s EXCLUSIVITY obligations under SECTION 2.8 or CONFIDENTIALITY OBLIGATIONS UNDER Article 13.
13.1Confidential Information. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, the Parties agree that, during the Term and for [***] thereafter, the receiving Party shall keep confidential and shall not publish or otherwise disclose and shall not use for any purpose other than as expressly provided for in this Agreement any Confidential Information of the other Party, and both Parties shall keep confidential and, subject to the remainder of this Article 13, shall not publish or otherwise disclose
59
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
the terms of this Agreement. Each Party may use the other Party’s Confidential Information only to the extent required to accomplish the purposes of this Agreement, including exercising its rights or performing its obligations under this Agreement. Each Party will use at least the same standard of care as it uses to protect proprietary or confidential information of its own (but no less than reasonable care) to ensure that its employees, agents, consultants, contractors, and other representatives do not disclose or make any unauthorized use of the Confidential Information of the other Party. Each Party will promptly notify the other upon discovery of any unauthorized use or disclosure of the Confidential Information of the other Party.
13.2Exceptions. The obligations of confidentiality and restriction on use under Section 13.1 will not apply to any information that the receiving Party can prove by competent written evidence: (a) is at the time of disclosure, or thereafter becomes, through no act or failure to act on the part of the receiving Party, generally known or available to the public; (b) is known by the receiving Party at the time of receiving such information, other than by previous disclosure of the disclosing Party, or its Affiliates, employees, agents, consultants, or contractors; (c) is disclosed to the receiving Party without restriction by a Third Party who has no obligation of confidentiality or limitations on use with respect thereto; or (d) is independently discovered or developed by the receiving Party without the use of or reference to the Confidential Information belonging to the disclosing Party.
13.3Authorized Disclosure. Each Party may disclose Confidential Information belonging to the other Party as expressly permitted by this Agreement or if and to the extent such disclosure is reasonably necessary in the following instances:
(a)filing, prosecuting, and maintaining Patents as permitted by this Agreement;
(b)submitting and maintaining Regulatory Filings for Products that such Party has a license or right to Develop or Commercialize under this Agreement in a given country or jurisdiction;
(c)prosecuting and defending litigation as permitted by this Agreement;
(d)complying with applicable court orders or governmental regulations, including regulations promulgated by securities exchanges; and
(e)disclosure to its and its Affiliates’ employees, consultants, contractors, agents, licensees, and sublicensees, in each case on a need-to-know basis in connection with the Development, manufacture, or Commercialization of the Compound and Products in accordance with the terms of this Agreement, in each case under written obligations of confidentiality and non-use at least as stringent as those herein;
(f)disclosure to actual and bona fide potential investors, acquirors, licensees, and other financial or commercial partners solely for the purpose of evaluating or carrying out an actual or potential investment, acquisition, collaboration, or other business relationship, in each case under written obligations of confidentiality and non-use at least as
60
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
stringent as those herein, provided that the disclosing Party redacts the financial terms and other provisions of this Agreement that are not reasonably required to be disclosed in connection with such potential investment, acquisition, collaboration, or business transaction which redaction shall be prepared in consultation with the other Party; and
(g)disclosure to Third Party licensors of intellectual property that is related to the Compound or any Product (e.g., the Third Party that is party to the Lundbeck License Agreement) for the purpose of meeting reporting obligations under such Third Party agreement.
Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to Section 13.3(c) or 13.3(d), it will, except where impracticable, give reasonable advance notice to the other Party of such disclosure, and to the extent possible, at least [***] notice, and use the same diligent efforts to secure confidential treatment of such Confidential Information as such Party would use to protect its own confidential information, but in no event less than reasonable efforts. In any event, the Parties agree to take all reasonable action to avoid disclosure of Confidential Information hereunder. Any information disclosed pursuant to Section 13.3 shall remain Confidential Information and subject to the restrictions set forth in this Agreement, including the foregoing provisions of this Section 13.
(a)Ovid shall have the right to review and comment on any material proposed for disclosure or publication by Licensee regarding results of and other information regarding Licensee’s Development activities during the Term with respect to the Compound and Product, whether by oral presentation, manuscript, or abstract. Before any such material is submitted for publication, or presentation of any such material is made, Licensee shall deliver a complete copy of the material proposed for disclosure to Ovid at least [***] prior to submitting the material to a publisher or initiating any other disclosure, or as close to these time frames as reasonably possible. Ovid shall review any such material and give its comments to Licensee within [***] of the receipt of such material. With respect to oral presentation materials and abstracts, Ovid shall make reasonable efforts to expedite review of such materials and abstracts, and shall return such items as soon as practicable to Licensee with comments, if any. Subject to Section 13.4(b), following the expiration of the applicable time period for review, Licensee shall be free to submit such proposed manuscript for publication or presentation materials for public disclosure, and does not need to follow this process for subsequent publications or presentations of the same data.
(b)If Ovid notifies Licensee within the applicable time period set forth in Section 13.4(a) that such publication or presentation, in Ovid’s reasonable judgment:
(i)contains an invention for which Ovid desires to obtain patent protection, Licensee shall delay such publication or presentation for a period of up to [***] (or such other time period agreed by the Parties in writing) to permit the preparation and filing of a patent application for such invention, or
(ii)contains any Confidential Information of Ovid, or could be expected to have an adverse effect on the commercial value of any Confidential Information disclosed by
61
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Ovid to Licensee, the Parties shall attempt to agree on revisions to the applicable disclosure so as to preserve both the commercial value of such Confidential Information and the scientific merit of such disclosure, provided that if and to the extent the Parties are unable to agree, Licensee shall delete such Confidential Information from the proposed publication or presentation.
13.5Publicity; Public Disclosures and Public Announcements. Save as permitted in this Section 13.5, neither Party shall make any public announcement or statement to the public containing Confidential Information of the other Party without the prior consent of such other Party. On or promptly after the Effective Date, the Parties shall issue a joint public announcement, or either Party may issue its own public announcement, regarding the execution of this Agreement with information substantially consistent with the information contained in the form of press release attached hereto as Exhibit D. It is understood that each Party may desire or be required to issue subsequent press releases relating to this Agreement or activities hereunder. The Parties agree to consult with each other reasonably and in good faith with respect to the text and timing of such press releases prior to the issuance thereof, to the extent practicable, and in the case of any press release Licensee wishes to issue, Licensee shall first obtain Ovid’s written approval of such release. Notwithstanding the foregoing, neither Party may unreasonably withhold, condition, or delay consent to such releases by more than [***], and either Party may issue such press releases or make such disclosures to the SEC or other applicable agency as it determines, based on advice of counsel, is reasonably necessary to comply with Applicable Laws or for appropriate market disclosure. Each Party shall provide the other Party with advance notice of legally required disclosures to the extent practicable, and to the extent possible, at least [***] prior to such disclosure. The Parties will consult with each other on the provisions of this Agreement to be redacted in any filings made by a Party with the SEC or as otherwise required by Applicable Laws; provided that each Party shall have the right to make any such filing as it reasonably determines necessary under Applicable Laws. In addition, following the initial joint press release announcing this Agreement, either Party shall be free to disclose, without the other Party’s prior written consent, the existence of this Agreement, the identity of the other Party, and those terms of the Agreement which have already been publicly disclosed in accordance with.
13.6Prior Confidentiality Agreement. As of the Effective Date, the terms of this Article 13 shall supersede any prior non-disclosure, secrecy, or confidentiality agreement between the Parties (or their Affiliates) relating to the subject of this Agreement, including the Confidentiality Agreement. Any information disclosed pursuant to any such prior agreement shall be deemed Confidential Information under this Agreement.
13.7Equitable Relief. Given the nature of the Confidential Information and the competitive damage that a Party would suffer upon unauthorized disclosure, use, or transfer of its Confidential Information to any Third Party, the Parties agree that monetary damages may not be a sufficient remedy for any breach of this Article 13. In addition to all other remedies, a Party shall be entitled to seek specific performance and injunctive and other equitable relief as a remedy for any breach or threatened breach of this Article 13.
14.1Term. This Agreement shall commence on the Effective Date and shall continue until terminated as provided in this Article 14 (the “Term”). Notwithstanding anything herein, on
62
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
a Product-by-Product and country-by-country basis, upon the expiration of the Royalty Term the licenses granted to Licensee in Section 2.1 shall become perpetual and fully paid-up with respect to such Product in such country, subject to Licensee’s payment obligations under Article 8 during the Extended Commercialization Term, but thereafter shall be on a non-exclusive basis.
(a)Material Breach. Each Party shall have the right to terminate this Agreement immediately in its entirety upon written notice to the other Party if such other Party materially breaches this Agreement and has not cured such breach to the reasonable satisfaction of the other Party within [***] after notice of such breach from the non-breaching Party. In the event of any dispute, the Agreement shall remain in place during the pendency of any such dispute.
(i)Either Party may terminate this Agreement in its entirety upon providing written notice to the other Party on or after the time that such other Party makes a general assignment for the benefit of creditors, files a petition for relief in bankruptcy, petitions for or acquiesces in the appointment of any receiver, trustee or similar officer to liquidate or conserve its business or any substantial part of its assets, commences under the laws of any jurisdiction any proceeding involving its insolvency, bankruptcy, reorganization, adjustment of debt, dissolution, liquidation or any other similar proceeding for the release of financially distressed debtors, or becomes the involuntary subject of any proceeding or action of the type described above and such proceeding or action remains un-dismissed or un-stayed for a period of more than [***].
(ii)All rights and licenses granted under or pursuant to this Agreement are, and shall otherwise be deemed to be, for purposes of Section 365(n) of Title 11 of the United States Code and other similar laws in any other jurisdiction outside of the Territory (collectively, the “Bankruptcy Laws”), licenses of rights to “intellectual property” as defined under the Bankruptcy Laws. If a case is commenced during the Term by or against a Party under Bankruptcy Laws then, unless and until this Agreement is rejected as provided pursuant to such Bankruptcy Laws, such Party (in any capacity, including debtor-in-possession) and its successors and assigns (including a Title 11 trustee) shall perform all of the obligations in this Agreement intended to be performed by such Party. If a case is commenced during the Term by or against a Party under the Bankruptcy Laws, this Agreement is rejected as provided for under the Bankruptcy Laws, and the non-bankrupt Party elects to retain its rights hereunder as provided for under the Bankruptcy Laws, then the Party subject to such case under the Bankruptcy Laws (in any capacity, including debtor-in-possession) and its successors and assigns (including a Title 11 trustee), shall provide to the non-bankrupt Party copies of all Patents and Information necessary for the non-bankrupt Party to [***] enjoy its rights under the terms of this Agreement. All rights, powers and remedies of the non-bankrupt Party as provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the Bankruptcy Laws) in the event of the commencement of a case by or against a Party under the Bankruptcy Laws. [***].
(c)Patent Challenge. Ovid shall have the right to terminate this Agreement immediately in its entirety upon written notice to Licensee if Licensee or any of its
63
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Affiliates or Sublicensees directly, or indirectly through any Third Party, commences any interference or opposition proceeding with respect to, challenges the validity or enforceability of, or opposes any extension of or the grant of a supplementary protection certificate with respect to, any Ovid Patent.
14.3Termination without Cause.
(a)Prior to the First Commercial Sale. Prior to the First Commercial Sale of the Product in the Licensee Territory, Licensee shall have the right to terminate this Agreement in its entirety without cause [***].
(b)After the First Commercial Sale. Following the First Commercial Sale of the Product in the Licensee Territory, Licensee shall have the right to terminate this Agreement in its entirety without cause [***].
14.4Effects of Termination. Upon any termination of this Agreement by either Party, the following Sections 14.4(a) through 14.4(g) will apply. For clarity, during the pendency of any termination notice period, all of the terms and conditions of this Agreement shall remain in effect and the Parties shall continue to perform all of their respective obligations hereunder.
(a)Licenses. Except in the event of (i) termination by Licensee for an uncured material breach by Ovid, or (ii) termination by Licensee in the circumstances set forth in Section 14.2(b)(i) with respect to Ovid, or (iii) Ovid’s termination for Safety Reason, the licenses granted by Licensee to Ovid shall survive such termination and shall automatically become worldwide. All of the licenses granted by Ovid to Licensee shall be automatically terminated.
(b)Regulatory Materials; Data. Except in the event of (i) termination by Licensee for an uncured material breach by Ovid, or (ii) termination by Licensee in the circumstances set forth in Section 14.2(b)(i) with respect to Ovid, or (iii) Ovid’s termination for Safety Reason (where Licensee elects to continue the Development and Commercialization of the Product), within [***] after the effective date of such termination, Licensee shall transfer and assign to Ovid, at no cost to Ovid, all Regulatory Filings and Regulatory Approvals for the Products, Data from all pre-clinical, non-clinical, and clinical studies of the Product conducted by or on behalf of Licensee, its Affiliates, or Sublicensees, and all pharmacovigilance data (including all adverse event data) on the Products. In addition, at Ovid’s request, Licensee shall provide Ovid with reasonable assistance with any inquiries and correspondence with Regulatory Authorities regarding the Product in the Licensee Territory, such assistance shall be limited to a period of [***] after such termination.
(c)Development Wind-Down. Except in the event of (i) termination by Licensee for an uncured material breach by Ovid, or (ii) termination by Licensee in the circumstances set forth in Section 14.2(b)(i) with respect to Ovid, or (iii) Ovid’s termination for Safety Reason (where Licensee elects to continue the Development and Commercialization of the Product), Licensee shall either, as directed by Ovid, (i) wind-down any ongoing Development activities (including any Clinical Trials) of Licensee or its Affiliates and Sublicensees with respect to any Product in the Licensee Territory in an orderly fashion or (ii) promptly transfer such
64
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Development activities to Ovid or its designee, in each case in compliance with all Applicable Laws.
(d)Cost of Ongoing Trials. Except in the event of (i) termination by Licensee for an uncured material breach by Ovid, or (ii) termination by Licensee in the circumstances set forth in Section 14.2(b)(i) with respect to Ovid, or (iii) Ovid’s termination for Safety Reason, if there is any ongoing Clinical Trial of the Product under the Development Plan for which the Parties are sharing costs, then Licensee shall continue to share the cost of such Clinical Trial until the effective date of termination.
(e)Commercial Wind-Down. Except in the event of (i) termination by Licensee for an uncured material breach by Ovid, or (ii) termination by Licensee in the circumstances set forth in Section 14.2(b)(i) with respect to Ovid, or (iii) Ovid’s termination for Safety Reason (where Licensee elects to continue the Development and Commercialization of the Product), Licensee shall, as directed by Ovid, (A) continue certain ongoing Commercial activities of Licensee and its Affiliates and Sublicensees with respect to any Product in the Licensee Territory for a period of up to [***] after the effective date of termination, as determined by Ovid, and (B) handoff such Commercial activities to Ovid or its designee, on a timetable to be set by Ovid, not to exceed [***] after the effective date of termination, and in compliance with all Applicable Laws. During such commercial wind-down period, Licensee shall continue to book sales and pay royalties to Ovid in accordance with Section 8.5. Except as necessary to conduct the foregoing activities as directed by Ovid, Licensee shall immediately discontinue its (and shall ensure that its Affiliates and Sublicensees immediately discontinue their) promotion, marketing, offering for sale, and servicing of the Product and its use of all Product Marks. In addition, Licensee shall immediately deliver to Ovid (at Licensee’s expense) all samples, demonstration equipment, sales materials, catalogs, and literature of Ovid in Licensee’s possession or control.
(f)Transition Assistance. Except in the event of (i) termination by Licensee for an uncured material breach by Ovid, or (ii) termination by Licensee in the circumstances set forth in Section 14.2(b)(i) with respect to Ovid, or (iii) Ovid’s termination for Safety Reason (where Licensee elects to continue the Development and Commercialization of the Product), Licensee shall, at no cost to Ovid, provide reasonable consultation and assistance for a period of no more than [***] after the effective date of termination for the purpose of transferring or transitioning to Ovid all Licensee Know-How not already in Ovid’s possession and, at Ovid’s request, all then-existing commercial arrangements, customer lists, and similar information relating to the Products that Licensee is able, using Commercially Reasonable Efforts, to transfer or transition to Ovid or its designee, in each case, to the extent reasonably necessary for Ovid to continue the Development or Commercialization of the Compound and Products in the Licensee Territory. If any such contract between Licensee and a Third Party is not assignable to Ovid or its designee (whether by such contract’s terms or because such contract does not relate specifically to the Products) but is otherwise reasonably necessary for Ovid to continue the Development or Commercialization of the Compound and Products in the Licensee Territory, or if Licensee is performing such work for the Compound and Product itself (and thus there is no contract to assign), then Licensee shall reasonably cooperate with Ovid to negotiate for the continuation of such services for Ovid from such entity, or Licensee shall continue to perform such work for Ovid, as applicable, for a reasonable period (not to exceed [***]) after the effective date of termination at Ovid’s cost until Ovid establishes an alternate, validated source of such services.
65
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
(g)Remaining Inventories. Except in the event of (i) termination by Licensee for an uncured material breach by Ovid, or (ii) termination by Licensee in the circumstances set forth in Section 14.2(b)(i) with respect to Ovid, or (iii) Ovid’s termination for Safety Reason (where Licensee elects to continue the Development and Commercialization of the Product), Ovid shall have the right, at its discretion, to obtain from Licensee any or all of the inventory of the Products held by Licensee as of the date of termination, free of charge. Ovid shall notify Licensee within [***] after the effective date of termination whether Ovid elects to exercise such right.
14.5Licensee Continuation. If (a) Licensee has the right to terminate this Agreement in its entirety for an uncured material breach by Ovid, but elects to continue this Agreement in its entirety as modified by this Section 14.5, or (b) Licensee has the right to terminate in the circumstances set forth in Section 14.2(b)(i), but elects to continue this Agreement as modified by this Section 14.5, or (c) Ovid elects to terminate this Agreement in its entirety for Safety Reason, but Licensee elects to continue this Agreement as modified by this Section 14.5, then, except as provided below, effective as of the date Licensee delivers notice of such election to Ovid, the following shall apply:
|
|
i. |
Licensee shall have the right to terminate the Supply Agreement, and if Licensee terminates the Supply Agreement, Ovid shall cooperate with Licensee for smooth technology transfer of the manufacturing process if termination occurs prior to the Technology Transfer Completion Date. |
|
|
ii. |
Notwithstanding anything contained in this Agreement to the contrary, Ovid shall cease any and all Development/Regulatory activities with respect to the Product in the Field in the Licensee Territory (other than the Ovid Ongoing Trials if still ongoing); |
|
|
iii. |
All Licensee payment obligations provided in this Agreement shall continue, including those that are accrued and unpaid as of the date of such election by Licensee, [***]; |
|
|
iv. |
After the effective date of such election by Licensee, the licenses granted by Ovid to Licensee in Section 2.1 under the Product Marks, Ovid Know-How and Ovid Patents for the research, the Development, the use, and the Commercialization of the Product in the Field in the Licensee Territory shall continue and the Licensee shall have such rights to Develop, Manufacture and Commercialize any Product in the Field in the Licensee Territory at Licensee’s sole discretion; |
66
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
provide a transfer of such material information and documents. At Licensee’s request, Ovid shall use reasonable efforts to assign to Licensee any and all agreements to which Ovid, or its Affiliate, and a Third Party are parties, and that solely cover or govern the Development, Commercialization and Manufacturing activities conducted in connection with a Product in the Field in the Territory prior to such election by Licensee, or if such assignment is not permitted under the relevant agreement, (A) grant to Licensee other reasonable rights to provide to Licensee the benefit of such non-assignable agreement, at Licensee’s expense, to the extent permitted under the terms of such non-assignable agreement; or (B) to the extent not permitted under the terms of such non-assignable agreement, the Parties shall discuss in good faith an alternative solution to enable Licensee to receive, at Licensee’s expense, the benefit of the terms of such non-assignable agreement. Ovid shall take such other reasonable actions and execute such other instruments, assignments and documents as may be necessary to effect the transfer of rights to such Product(s) as described in this Section 14.5(v) hereunder to Licensee; |
|
|
vi. |
Ovid shall transfer to Licensee any and all Regulatory Materials directly and solely related to the Product in the Field in the Licensee Territory, and, upon Licensee’s request, shall make available to Licensee any other relevant information reasonably requested by Licensee related to such Regulatory Materials; |
|
|
vii. |
As long as Licensee is not in breach of this Agreement or the Lundbeck License Agreement at the effective date of such election, Licensee shall have the rights of a non-breaching sublicensee set forth in the Lundbeck License Agreement (as amended by the Lundbeck Sublicense Amendment) from and after such election; |
|
|
viii. |
Licensee shall have no further obligation to use Xxxxxxxx Commercially Reasonable Efforts with respect to its obligations hereunder; |
|
|
ix. |
Ovid shall be obliged to bear the costs of the Ovid Ongoing Trials, if still ongoing; and |
14.6Confidential Information. Upon expiration or termination of this Agreement in its entirety, except to the extent that a Party obtains or retains the right to use the other Party’s Confidential Information, each Party shall promptly return to the other Party, or delete or destroy,
67
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
all relevant records and materials in such Party’s possession or control containing Confidential Information of the other Party; provided that such Party may keep one copy of such materials for archival purposes only subject to continuing confidentiality obligations. All Licensee Data and Regulatory Filings assigned to Ovid upon termination of this Agreement will be deemed Ovid’s Confidential Information and no longer Licensee’s Confidential Information.
14.7Survival. Expiration or termination of this Agreement shall not relieve the Parties of any obligation or right accruing prior to such expiration or termination. Except as set forth below or elsewhere in this Agreement, the obligations and rights of the Parties under the following provisions of this Agreement shall survive expiration or termination of this Agreement.
14.8Exercise of Right to Terminate. All rights and obligations of a Party accrued prior to the effective date of a termination (including the rights to receive reimbursement for costs incurred prior to the effective date of such termination and payments accrued or due prior to the effective date of such termination) shall survive such termination.
15.1General. Except as provided in Sections 3.4 and 9.4, any dispute between the Parties in connection with or relating to this Agreement or any document or instrument delivered in connection herewith (a “Dispute”) shall be resolved pursuant to this Section 15.
15.2Executive Officers. Any Dispute shall first be referred to the Executive Officers of the Parties, who shall confer in good faith on the resolution of the issue. Any final decision mutually agreed to by the Executive Officers shall be conclusive and binding on the Parties.
15.3Intellectual Property Disputes. If the Executive Officers are not able to agree on the resolution of a Dispute within thirty (30) days (or such other period of time as mutually agreed by the Executive Officers) after such Dispute was first referred to them and such Dispute is with respect to the validity, scope, enforceability, inventorship, or ownership of any Patent, trademark, or other intellectual property right (“IP Dispute”), then, if a Party wishes to pursue further resolution of such IP Dispute, an action, claim, or proceeding to resolve such IP Dispute shall be brought in any court of competent jurisdiction in any country or jurisdiction in which such intellectual property rights apply.
15.4Arbitration. If the Executive Officers are not able to agree on the resolution of a Dispute within thirty (30) days (or such other period of time as mutually agreed by the Executive Officers) after such Dispute was first referred to them, then, except as otherwise set forth in Section 15.3, if a Party wishes to pursue further resolution of such Dispute, such Dispute shall be finally resolved by binding arbitration in accordance with this Section 15.4. Such Dispute shall be referred to and finally resolved by arbitration under the International Chamber of Commerce (“ICC”) Rules, as then in effect, by a tribunal of three (3) arbitrators. The seat and legal place of the arbitration shall be [***]. Each Party shall nominate one arbitrator and the third arbitrator shall be nominated by the two Party-nominated arbitrators within [***] after the second arbitrator’s appointment. If a Party does not nominate its arbitrator within [***] following the expiry of the allotted period, then such arbitrator shall be appointed by the ICC in accordance with its rules. Any arbitrator appointed by the ICC shall have at least [***] experience in the pharmaceutical
68
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
industry. The arbitration shall be conducted, and all documents submitted to the arbitrators shall be, in English. Each Party shall bear its own legal costs for its counsel and other expenses, and the Parties shall equally share the costs of the arbitration; provided that the arbitral tribunal shall have the discretion to provide that the losing party is responsible for all or a portion of such arbitration and legal costs, in such case the arbitral award will so provide. The arbitrators shall have no power to award damages excluded pursuant to Section 12.5. In no event shall the arbitrators assign a value to any issue greater than the greatest value for such issue claimed by either Party or less than the smallest value for such issue for such item claimed by either Party. The award shall be final and binding upon the Parties and the Parties undertake to carry out any award without delay. Judgment on the award may be entered in any court of competent jurisdiction. Except to the extent necessary to confirm, enforce, or challenge an award of the arbitration, to protect or pursue a legal right, or as otherwise required by Applicable Law or regulation or securities exchange, neither Party nor any arbitrator may disclose the existence, content, or results of any arbitration hereunder without the prior written consent of both Parties. Notwithstanding anything to the contrary in the foregoing, in no event shall an arbitration be initiated after the date when commencement of a legal or equitable proceeding based on the dispute, controversy, or claim would be barred by the applicable New York statute of limitations. Any disputes concerning the propriety of the commencement of the arbitration shall be finally settled by the arbitral tribunal.
15.5Interim Relief. Notwithstanding anything herein to the contrary, nothing in this Article 15 shall preclude either Party from seeking interim or provisional relief, including a temporary restraining order, preliminary injunction, or other interim equitable relief concerning a Dispute in any court of competent jurisdiction before or after the initiation of an arbitration as set forth in Section 15.4, if necessary to protect the interests of such Party. This Section 15.5 shall be specifically enforceable.
16.1Governing Law. This Agreement, and all questions regarding the existence, validity, interpretation, breach, or performance of this Agreement, shall be governed by, and construed and enforced in accordance with, the laws of the State of New York, United States, without reference to its conflicts of law principles.
16.2Entire Agreement; Modification. This Agreement, including the exhibits, is both a final expression of the Parties’ agreement and a complete and exclusive statement with respect to all of its terms. This Agreement supersedes all prior and contemporaneous agreements and communications, whether oral, written, or otherwise, concerning any and all matters contained herein. This Agreement may only be modified or supplemented in a writing expressly stated for such purpose and signed by the Parties to this Agreement.
16.3Relationship Between the Parties. The Parties’ relationship, as established by this Agreement, is solely that of independent contractors. This Agreement does not create any partnership, joint venture, or similar business relationship between the Parties, including for all tax purposes. Neither Party is a legal representative of the other Party, and neither Party can assume or create any obligation, representation, warranty, or guarantee, express or implied, on behalf of the other Party for any purpose whatsoever. The Parties (and any successor, assignee, transferee,
69
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
or Affiliate of a Party) shall (a) use commercially reasonable efforts to structure the arrangement and activities contemplated by this Agreement to avoid the arrangement contemplated by this Agreement being treated as a partnership that is engaged in a “United States trade or business” for United States tax purposes and (b) not treat or report the relationship between the Parties arising under this Agreement as a partnership for United States tax purposes, without the prior written consent of the other Party unless required by a final “determination” as defined in Section 1313 of the United States Internal Revenue Code of 1986, as amended.
16.4Non-Waiver. The failure of a Party to insist upon strict performance of any provision of this Agreement or to exercise any right arising out of this Agreement shall neither impair that provision or right nor constitute a waiver of that provision or right, in whole or in part, in that instance or in any other instance. Any waiver by a Party of a particular provision or right shall be in writing, shall be as to a particular matter and, if applicable, for a particular period of time and shall be signed by such Party.
16.5Assignment. Neither Party may assign or transfer this Agreement or any rights or obligations hereunder without the prior written consent of the other Party, such consent not to be unreasonably withheld. Any successor or assignee of rights and obligations permitted hereunder shall, in writing to the other Party, expressly assume performance of such rights and obligations. Notwithstanding the foregoing, either Party may assign this Agreement and its rights and obligations hereunder without the other Party’s consent:
(a) in connection with the transfer or sale, through whatever means, of all or substantially all of its assets or the business of such Party to which this Agreement relates (whatever by assignment, conveyance, reorganization, merger, assumption, purchase and sale of stock, by contract or by operation of law, provided that the intellectual property rights of the acquiring party to such transaction (if other than one of the Parties) shall not be included in the technology licensed under this Agreement; or
(b)to an Affiliate, provided that if the entity to which this Agreement is assigned ceases to be an Affiliate of the assigning Party, the Agreement shall be automatically assigned back to the assigning Party or its successor.
Any permitted assignment shall be binding on the successors and the permitted assigns of a Party. Any assignment or attempted assignment by either Party in violation of the terms of this Section 16.5 shall be null, void and of no legal effects.
16.6Severability. If, for any reason, any part of this Agreement is adjudicated invalid, unenforceable, or illegal by a court of competent jurisdiction, such adjudication shall not, to the extent feasible, affect or impair, in whole or in part, the validity, enforceability, or legality of any remaining portions of this Agreement. All remaining portions shall remain in full force and effect as if the original Agreement had been executed without the invalidated, unenforceable, or illegal part.
16.7Notices. Any notice to be given under this Agreement must be in writing and delivered either in person, by (a) air mail (postage prepaid) requiring return receipt, (b) overnight courier, or (c) facsimile confirmed thereafter by any of the foregoing, to the Party to be notified at
70
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
its address(es) given below, or at any address such Party may designate by prior written notice to the other in accordance with this Section 16.7. Notice shall be deemed sufficiently given for all purposes upon the earliest of: (i) the date of actual receipt, (ii) if air mailed, five (5) days after the date of postmark, (iii) if delivered by overnight courier, the next day the overnight courier regularly makes deliveries, or (iv) if sent by facsimile, the date of confirmation of receipt if during the recipient’s normal business hours, otherwise the next business day.
If to Licensee, notices must be addressed to:
Xxxxxxxx Pharma Rare Diseases AG
Consulting GmbH Xxxxxxxxxxxx 00 0000
Xxxxxxxxxxx, Xxxxxx
Xxxxxxxxxxx
Attention: Chief Executive Officer
With a copy to:
Xxxxxxxx Pharma S.p.A.
Xxxxx Xxxxxx 00, 00000
Xxxx, Xxxxx
Attention: Enza Xxxxx Xxxxxxxx Xxxxx, Global Pharma General Counsel
mail: [***]
If to Ovid, notices must be addressed to:
0000 Xxxxxxxx, Xxxxx 00000
Xxx Xxxx, XX 00000
XXX
Attention: Chief Executive Officer
And a copy to:
Attention: General Counsel
With a copy to (which shall not constitute notice):
Xxxxxx LLP
0000 Xxxxxxx Xxxxxx
Xxxx Xxxx, XX 00000
XXX
Attention: Xxxxx Xxxxxxx
16.8Force Majeure. Each Party shall be excused from liability for the failure or delay in performance of any obligation under this Agreement (other than failure to make payment when due) by reason of any event beyond such Party’s reasonable control including Acts of God, fire, flood, explosion, earthquake, pandemic (including the COVID-19 pandemic), quarantine or similar restrictions, or other natural forces, war, civil unrest, acts of terrorism, accident, destruction
71
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
or other casualty, any lack or failure of transportation facilities, any injunction, order, proclamation, demand, or requirement of any Governmental Authority [***], any lack or failure of supply of raw materials, or any other event similar to those enumerated above. Such excuse from liability shall be effective only to the extent and duration of the event(s) causing the failure or delay in performance and provided that the Party has not caused such event(s) to occur and uses reasonable efforts to overcome such event. Notice of a Party’s failure or delay in performance due to force majeure must be given to the other Party within [***] after its occurrence. All delivery dates under this Agreement that have been affected by force majeure shall be tolled for the duration of such force majeure. In no event shall any Party be required to prevent or settle any labor disturbance or dispute.
16.9Interpretation. The headings of clauses contained in this Agreement preceding the text of the sections, subsections, and paragraphs hereof are inserted solely for convenience and ease of reference only and shall not constitute any part of this Agreement, or have any effect on its interpretation or construction. All references in this Agreement to the singular shall include the plural where applicable. Unless otherwise specified, references in this Agreement to any Article shall include all Sections, subsections, and paragraphs in such Article, references to any Section shall include all subsections and paragraphs in such Section, and references in this Agreement to any subsection shall include all paragraphs in such subsection. The word “including” and similar words means including without limitation. The word “or” means “and/or” unless the context dictates otherwise because the subjects of the conjunction are, or are intended to be, mutually exclusive. The words “herein”, “hereof”, and “hereunder” and other words of similar import refer to this Agreement as a whole and not to any particular Section or other subdivision. All references to days in this Agreement mean calendar days, unless otherwise specified. Ambiguities and uncertainties in this Agreement, if any, shall not be interpreted against either Party, irrespective of which Party may be deemed to have caused the ambiguity or uncertainty to exist. This Agreement has been prepared in the English language and the English language shall control its interpretation. In addition, all notices required or permitted to be given hereunder, and all written, electronic, oral, or other communications between the Parties regarding this Agreement shall be in the English language.
16.10Counterparts; Electronic or Facsimile Signatures. This Agreement may be executed in any number of counterparts, each of which shall be an original, but all of which together shall constitute one instrument. This Agreement may be executed and delivered electronically or by facsimile and upon such delivery such electronic or facsimile signature will be deemed to have the same effect as if the original signature had been delivered to the other Party.
72
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
In Witness Whereof, the Parties hereto have caused this Collaboration and License Agreement to be executed and entered into by their duly authorized representatives as of the Effective Date.
|
By: /s/ Xxxxxx Xxxxx Name: Xxxxxx Xxxxx Title: Chief Executive Officer |
Xxxxxxxx Pharma Rare Diseases AG
By: /s/ Xxxxxxxxx Xxxxxxxxx Name: Xxxxxxxxx Xxxxxxxxx Title: Chairman of the BoD |
73
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
List of Exhibits and Schedules
Exhibit E: Technology Transfer Plan
Exhibit F: Documents to be transferred according to Section 8.1(b)(i)
Exhibit G: Other Documents to be transferred
Exhibit H: Description of Additional Pivotal Trial
Exhibit I: Current Licensee Pipeline Products
Exhibit J: Descriptions of Elara Trial, Neptune Trial, and Rocket Trial
Exhibit L: Finished Product Documentation
74
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
75
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit B
Ovid Patents
[***]
76
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Development Plan
77
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Press Release


Ovid Therapeutics and Xxxxxxxx Pharma Enter into Exclusive License Agreement to Develop, Manufacture and Commercialize OV101 for the Treatment of Angelman Syndrome in Europe
|
|
• |
Ovid Therapeutics will receive an upfront payment of $20 million and additional payments of up to $212.5 million upon the achievement of development, manufacturing and sales milestones, in addition to double-digit royalties on net sales if successfully commercialized |
|
|
• |
Xxxxxxxx Pharma will execute the agreement through its new affiliate Xxxxxxxx Pharma Rare Diseases AG |
NEW YORK and ROME, July XX, 2020 -- Ovid Therapeutics Inc. (NASDAQ: OVID, hereinafter “Ovid”), a biopharmaceutical company committed to developing medicines that transform the lives of people with rare neurological diseases, and Xxxxxxxx Pharma S.p.A. (hereinafter “Xxxxxxxx Pharma”), an Italian family-owned pharmaceutical company committed to helping patients with a constant and prevalent focus on Mental Health, Rare Diseases and Consumer Health, announced an agreement in which Xxxxxxxx Pharma will be responsible to develop, manufacture and commercialize OV101 (gaboxadol) for the potential treatment of Angelman syndrome in the European Union, other countries in the European Economic Area, Switzerland, Turkey and United Kingdom. Xxxxxxxx Pharma will execute the agreement through its new affiliate Xxxxxxxx Pharma Rare Diseases AG. OV101 is believed to be the only delta (δ)-selective GABAA receptor agonist in development, and is currently being evaluated in the pivotal Phase 3 NEPTUNE trial in Angelman syndrome, with topline results expected in the fourth quarter of 2020.
Under the terms of the agreement, Ovid will receive an upfront payment of $20 million and is eligible to receive up to an additional $212.5 million in payments upon the achievement of development, manufacturing and sales milestones for the initial indication (Angelman syndrome), as well as double-
78
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
digit royalties on net sales if OV101 is successfully commercialized. Ovid will retain all U.S. and rest-of-world commercial rights to OV101.
“We are excited to enter into a strategic collaboration with Xxxxxxxx Pharma with the goal of bringing OV101, if approved, to the Angelman community in Europe as quickly as possible. Xxxxxxxx Pharma is an ideal partner for Europe as they have deep regional knowledge, an established infrastructure with a history of successful product launches, and a commitment to improving the quality of life of the patient communities they serve,” said Xxxxxx Xxxxx, DPhil, MB, BChir, Chairman and Chief Executive Officer of Ovid Therapeutics. “Finding the right partners to bring OV101 to the Angelman community as rapidly as possible is a core part of our global strategy. We believe this partnership with Xxxxxxxx will help to maximize the potential commercial value of OV101 and achieve our strategic objectives in this important geography."
“Today is a day that we will remember. Through our collaboration with Ovid Therapeutics, we are laying the foundation to developing innovative health solutions for rare diseases, in line with Xxxxxxxx Pharma’s new strategy,” said Xxxxxxxxx Xxxxxxxxx, Xxxxxxxx Pharma CEO. “The new business unit Xxxxxxxx Pharma Rare Diseases AG will contribute to the development, registration, production and, if approved, commercialization in Europe of OV101, Ovid Therapeutics’ very promising drug being evaluated in a Phase 3 clinical trial for the treatment of Angelman syndrome. As of now, there is no effective treatment for this rare genetic disease, characterized by severe psychomotor disability, which manifests itself from childhood. Delivering on our commitment makes us proud both from a scientific perspective and its social impact”.
"As shareholders and executives of Xxxxxxxx Holding we continue to invest in the pharma area, which today represents half of our Group's turnover,” commented the executive vice president Thea Xxxxx Xxxxxxxx and the CEO Xxxxxx Xxxxxxx di Condojanni. “Our global development and internationalization strategy focuses on business areas with high growth potential. Particularly, we look closely at all the opportunities that can open up, not only in healthcare, but also in the consumer and machinery sector."
About Angelman Syndrome
Angelman syndrome is a rare genetic condition that is characterized by a variety of signs and symptoms. Characteristic features of this condition include delayed development, intellectual disability, severe speech impairment, problems with movement and balance, seizures, sleep disorders and anxiety. The most common cause of Angelman syndrome is the loss of function of the gene that codes for ubiquitin protein ligase E3A (UBE3A), which plays a critical role in nerve cell communication, resulting in impaired tonic inhibition. Individuals with Angelman syndrome typically have normal lifespans but are unable to live independently. Therefore, they require constant support from a network of specialists and caregivers. Angelman syndrome affects approximately 1 in 12,000 to 1 in 20,000 people globally.
There are no approved therapies by the U.S. Food and Drug Administration (FDA), European Medicines Agency or rest–of-world for Angelman syndrome, and treatment primarily consists of behavioral interventions and pharmacologic management of symptoms.
Angelman syndrome is associated with a reduction in tonic inhibition, a function of the delta (δ)-selective GABAA receptor that allows a human brain to decipher excitatory and inhibitory neurological signals correctly without being overloaded. If tonic inhibition is reduced, the brain becomes inundated with signals and loses the ability to separate background noise from critical information.
79
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
OV101 is believed to be the only delta (δ)-selective GABAA receptor agonist in development and the first investigational drug to specifically target the disruption of tonic inhibition, a central physiological process of the brain that is thought to be the underlying cause of certain neurodevelopmental disorders. OV101 has demonstrated in laboratory studies and animal models to selectively activate the δ-subunit of GABAA receptors, which are found in the extrasynaptic space (outside of the synapse), and thereby impact neuronal activity through modulation of tonic inhibition.
Ovid is developing OV101 for the treatment of Angelman syndrome and Fragile X syndrome to potentially restore tonic inhibition and thereby address several core symptoms of these conditions. In both these syndromes, the underlying pathophysiology includes disruption of tonic inhibition modulated through the δ-subunit of GABAA receptors. In preclinical studies, it was observed that OV101 improved symptoms of Angelman syndrome and Fragile X syndrome. This compound has also previously been tested in more than 4,000 patients (more than 1,000 patient-years of exposure) and was observed to have favorable safety and bioavailability profiles. Ovid is conducting a pivotal Phase 3 clinical trial with OV101 in Angelman syndrome (NEPTUNE) and has completed a Phase 2 signal-finding clinical trial with OV101 in Fragile X syndrome (ROCKET).
OV101 has received Rare Pediatric Disease Designation from the FDA for the treatment of Angelman syndrome. The FDA has also granted Orphan Drug and Fast Track designations for OV101 for both the treatment of Angelman syndrome and Fragile X syndrome. In addition, the European Commission (EC) has granted orphan drug designation to OV101 for the treatment of Angelman syndrome. The U.S. Patent and Trademark Office has granted Ovid patents directed to methods of treating Angelman syndrome and Fragile X syndrome using OV101. The issued patents expire in 2035 without regulatory extensions.
About Ovid Therapeutics
Ovid Therapeutics Inc. is a New York-based biopharmaceutical company using its BoldMedicine® approach to develop medicines that transform the lives of patients with rare neurological disorders. Ovid has a broad pipeline of potential first-in-class medicines. The Company’s most advanced investigational medicine, OV101 (gaboxadol), is currently in clinical development for the treatment of Angelman syndrome and Fragile X syndrome. Ovid is also developing OV935 (soticlestat) in collaboration with Takeda Pharmaceutical Company Limited for the potential treatment of rare developmental and epileptic encephalopathies (DEE). For more information on Ovid, please visit xxx.xxxxxx.xxx.
About Xxxxxxxx Pharma
Xxxxxxxx Pharma, owned by Xxxxxxxx Holding, is a pharmaceutical Company committed to helping patients with a constant and prevalent focus on Mental Health, including Pain, Rare Diseases and Consumer Health. Xxxxxxxx Pharma has an extensive and recognized R&D programs, "World Class" production plants and international commercialization activities of active ingredients and market-leading drugs. For further information, please visit xxx.xxxxxxxxxxxxxx.xxx
About Xxxxxxxx Holding
Xxxxxxxx Holding is the parent company of an international group operating in the pharmaceutical and consumer goods sectors. Founded in Italy in 1919, today Xxxxxxxx group operates in 17 countries with a staff of 5,600 and a turnover of €1,7 billion. In addition to the Pharmaceutical sector, Xxxxxxxx group operates in Personal and Home Care business area through Fater, a joint venture with Procter & Xxxxxx, in the Machinery field, again in joint venture with P&G, with the group operating in automation
80
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
and robotics for the consumer goods industry Fameccanica, in Perfumery and Skincare and Suncare with Xxxxxxxx Beauty and in the Wine sector through Bertani Domains. Xxxxxxxx Holding has recently entered the Baby food market as well through MadreNatura, a joint venture with Hero Group, which offers 100% organic baby food products.
Ovid Therapeutics Forward-Looking Statements
This press release includes certain disclosures that contain “forward-looking statements,” including, without limitation, statements regarding: advancing development of and commercializing OV101, the potential benefits and value of OV101; the anticipated reporting schedule of clinical data for OV101; and the potential benefits and outcome from this collaboration. You can identify forward-looking statements because they contain words such as “will,” “appears,” “believes” and “expects.” Forward-looking statements are based on Ovid’s current expectations and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that may differ materially from those contemplated by the forward-looking statements, which are neither statements of historical fact nor guarantees or assurances of future performance. Important factors that could cause actual results to differ materially from those in the forward-looking statements include uncertainties in the development and regulatory approval processes, and the fact that initial data from clinical trials may not be indicative, and are not guarantees, of the final results of the clinical trials and are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and/or more patient data become available. Additional risks that could cause actual results to differ materially from those in the forward-looking statements are set forth in Ovid’s filings with the Securities and Exchange Commission under the caption “Risk Factors”. Such risks may be amplified by the COVID-19 pandemic and its potential impact on Ovid’s business and the global economy. Ovid assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
Ovid Therapeutics Contacts
Investors and Media:
Investor Relations & Public Relations
xxxx@xxxxxx.xxx
Or
Investors:
Xxxxx Xxxxx
Xxxxx XxXxxxxxx, Inc.
xxxxxx@xxxxxxx.xxx
(000) 000-0000
Media:
Xxxxx Xxxxxxxx
1AB
xxxxx@0xxxxxxx.xxx
Xxxxxxxx Pharma Contact:
81
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Head of Global Communications Xxxxxxxx Pharma
x00 000 0000000
xxxxxxx.xxxxxx@xxxxxxxxxxxxxx.xxx
Xxxxxxxx Holding Contact:
Institutional & External Relations Director Xxxxxxxx Holding
x00 000 0000000
xxxxxxxxxx.xxxxxxx@xxxxxxxxxxxxxxx.xxx
82
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Technology Transfer Plan
83
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Documents to be transferred according to Section 8.1(b)(i)
[***]
84
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Other Documents to be transferred
85
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit H
Description of Additional Pivotal Trial
[To be completed if and when the Additional Pivotal Trial is required by the EMA or by Licensee at its own discretion.]
86
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit I
Current Licensee Pipeline Products
[***]
87
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit J
Descriptions of Elara Trial, Neptune Trial, and Rocket Trial
88
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
89
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
[***]
|
|
|
|
|
|
|
|
90
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit A:The Compound
Exhibit B:Mandatory Estimated Forecast
Exhibit C:Initial Purchase Order for Allocated Quantity of Compound
91
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.

92
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit B:Mandatory Estimated Forecast
[***]
93
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit C:Initial Purchase Order for Allocated Quantity of Compound
94
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Finished Product Documentation
[***]
95
[***] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.