DISTRIBUTOR AGREEMENT
QuickLinks -- Click here to rapidly navigate through this document
Exhibit 10.8
This AGREEMENT ("Agreement") dated as of December 9, 1998, by and between Ethicon Endo-Surgery, Inc., an Ohio corporation ("EES"), and ▇▇▇▇▇▇▇ Imaging Corporation, a Delaware corporation ("▇▇▇▇▇▇▇").
Preliminary Statement
▇▇▇▇▇▇▇ is in the business of manufacturing and selling, among other products, stereotactic X-ray equipment and desires to appoint a distributor regarding such products in the Exclusive Territory and the Non-Exclusive Territory (as defined below).
EES wishes to be appointed as a distributor of the Products in the Exclusive Territory and Non-Exclusive Territory as more fully described below.
Pursuant to an Agreement dated October 10, 1997, between EES and ▇▇▇▇▇▇▇ (the "1997 Agreement"), ▇▇▇▇▇▇▇ and EES set forth a commercial relationship including the grant by ▇▇▇▇▇▇▇ to EES of an option to distribute ▇▇▇▇▇▇▇'▇ stereotactic X-ray imaging products on a non-exclusive basis worldwide. To date, EES has not exercised this option but ▇▇▇▇▇▇▇ and EES wish to expand their current relationship through this Agreement.
ACCORDINGLY, the parties hereto agree as follows:
ARTICLE I
DEFINITIONS
As used herein, the following defined terms shall have the meanings set forth in this Article 1:
Section 1.01 "Affiliate" of a party hereto shall mean another party that controls, is controlled by, or is under common control with such party hereto, and for such purpose "control" shall mean the possession, direct or indirect, of 50% or more of voting power or beneficial interest in equity in the entity, whether through the ownership of voting securities, by contract or otherwise.
Section 1.02 "Exclusive Territory" shall mean the countries of Germany and Italy.
Section 1.03 "Initial Term" shall mean the period commencing on the date hereof and ending 24 months later. For the purposes of this Agreement, the Initial Term shall be divided into two parts, the First Half and the Second Half. The "First Half" shall mean the first 12 months of the Initial Term, and the "Second Half" shall mean the last 12 months of the Initial Term.
Section 1.04 "Non-Exclusive Territory" shall mean as of the date hereof the countries of the continent of Europe (excluding the countries of Germany and Italy), the countries of the region known as the Middle East and the country of South Africa, with such additions or deletions during the term of the Agreement as are mutually agreed.
Section 1.05 "Products" shall mean the products meeting the Specifications set forth in Exhibit A hereto and any or all improvements thereto.
Section 1.06 "Specifications" shall mean the specifications for the Products. The initial specifications for the Products are set forth in Exhibit A hereto.
Section 1.07 "Year 2000 Compliant" shall mean:
(a) a Product performs in a consistent manner and functions without interruptions regardless of the date in time on which the Product is delivered, used and/or further distributed, whether before, on or after January 1, 2000 and whether or not the dates are affected by leap years;
(b) a Product, if computerized, accepts, calculates, compares, sorts, extracts, sequences and otherwise processes date inputs and date values, and returns and displays date values and performs, in a consistent manner regardless of the dates used, whether before, on or after January 1, 2000;
(c) a Product, if computerized, accepts and responds to two-digit year-date input where applicable in a manner that resolves any ambiguities as to the century in a defined, predetermined and appropriate manner;
(d) a Product, if computerized, stores and displays date information in ways that are unambiguous as to the determination of the century; and
(e) a Product shall be delivered in a timely manner without interruptions caused by the date in time on which the product is ordered or is actually delivered or the services are scheduled or actually performed under normal procedures in the ordinary course, whether before, on or after January 1,2000.
ARTICLE II
APPOINTMENT
Section 2.01 Appointment. Subject to all of the terms and conditions of this Agreement, ▇▇▇▇▇▇▇ hereby appoints EES as, and EES hereby accepts such appointment, as:
(a) the authorized, independent Distributor of the Products in the Exclusive Territory. EES shall have exclusive distribution rights in the Exclusive Territory for the Products during the Initial Term. Such exclusive distribution rights shall continue:
(i) during the Second Half of the Initial Term so long as EES sells a minimum of ten new stereotactic tables in the Exclusive Territory and Non-Exclusive Territory combined during the First Half of the Initial Term, and
(ii) during each subsequent 12 month period of the remainder of the Term (as defined in Section 9.01 below) so long as EES sells a minimum of ten new stereotactic tables in the Exclusive Territory and Non-Exclusive Territory combined during the previous 12 month period.
If EES does not meet the requirements for exclusivity set forth above, the right of EES to distribute the Products in the Exclusive Territory shall be converted to non-exclusive rights during the subsequent 12 month period. While EES meets the requirements for exclusivity set forth above, ▇▇▇▇▇▇▇ and its Affiliates may not themselves, nor may they appoint a distributor or cause an existing distributor to, sell and distribute new or used Products in the Exclusive Territory.
(b) the authorized, independent distributor of the Products in the Non-Exclusive Territory. EES shall have non-exclusive distribution rights in the Non-Exclusive Territory for the Products during the Term. Except for any pre-existing agreements it is a party to, ▇▇▇▇▇▇▇ agrees during the Term of this Agreement that it will not offer to any other distributor or dealer in the Non-Exclusive Territory (or the Exclusive Territory during any period when the exclusivity requirements are not met) terms more favorable than those offered to EES in this Agreement.
2
Section 2.02 Payments to ▇▇▇▇▇▇▇. During the Term of the Agreement, EES agrees to pay ▇▇▇▇▇▇▇ the following amounts within seven days of the occurrence of the contingencies set forth below:
(i) $200,000 at signing of this Agreement,
(ii) $100,000 after written certification by ▇▇▇▇▇▇▇ that (A) all three of the individuals based in Germany with respect to the ▇▇▇▇▇▇▇ European Infrastructure (as referred to in Section 4.08 hereof) have commenced full-time employment with ▇▇▇▇▇▇▇, and (B) an authorized representative of EES Europe certifies that ▇▇▇▇▇▇▇ has a reasonable inventory of service spare parts located in Europe, and
(iii) $300,000 upon the 1st day of the Second Half of the Initial Term if an Event of Default has not occurred.
These amounts are one time payments.
Section 2.03 Relationship of the Parties. The relationship established hereby is that of independent contractors and not that of principal and agent.
Section 2.04 Price.
(a) From the date hereof through the expiration of the Initial Term, the transfer prices with respect to new Products to be distributed by EES pursuant to this Agreement shall be as set forth in Exhibit B.
(b) Six months prior to the expiration of the Initial Term, ▇▇▇▇▇▇▇ shall initiate discussions by written notice to EES for the parties to negotiate in good faith the prices for the Products, the Rebate (as defined in Section 2.07 below), if any, and other appropriate terms and conditions to be applicable for the remainder of the Term of the Agreement. The failure by the parties to agree on the prices for the Products, the Rebate or other appropriate terms and conditions for the remainder of the Term prior to the expiration of the Initial Term shall result in the automatic termination of this Agreement upon the expiration of the Initial Term.
(c) For a new table Product sold in the Non-Exclusive Territory, EES agrees to pay an extra $10,000 per table to be added to the transfer price to cover service warranty and installation by ▇▇▇▇▇▇▇ or its designee. Where the table is installed and the service warranty is performed by a designee of ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ agrees to pay the entire $10,000 payment to such designee.
Section 2.05 Applications Training. ▇▇▇▇▇▇▇ shall be responsible for all customer applications training ("Applications Training") for the Products as set forth in Exhibit C attached hereto. If EES desires ▇▇▇▇▇▇▇ to provide additional Applications Training, EES shall notify ▇▇▇▇▇▇▇ of such desire in writing. ▇▇▇▇▇▇▇ agrees to promptly provide such additional training to be billed at a reasonable cost to the relevant EES Affiliate.
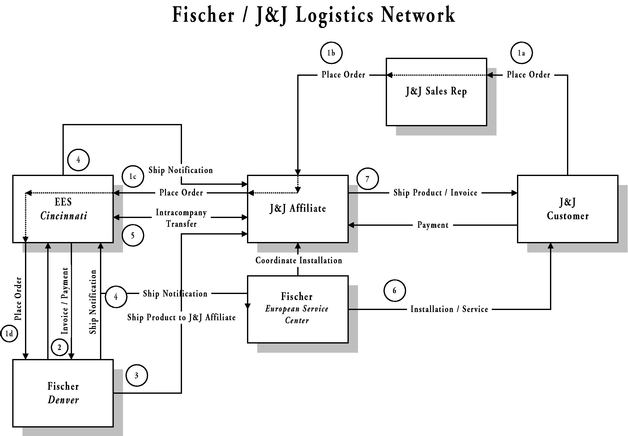
Section 2.06 Transmittal of Orders; Delivery.
(a) ▇▇▇▇▇▇▇ acknowledges that EES is relying on ▇▇▇▇▇▇▇ to make on time delivery of Products sold pursuant to this Agreement. ▇▇▇▇▇▇▇ shall use all reasonable efforts to ensure that orders for Products, when ordered by EES, are promptly filled but in any event within 60 days of receipt of the order or other specific delivery date as mutually agreed upon in writing. ▇▇▇▇▇▇▇ shall not be responsible for paying any financial penalties due to late delivery of an order unless it or an authorized representative specifically agreed in writing to the imposition of such penalty in advance of accepting the purchase order for such Product.
(b) Delivery of the orders is FOB ▇▇▇▇▇▇▇'▇ Denver manufacturing facility.
(c) After the Product or Products have been delivered to EES, ▇▇▇▇▇▇▇ shall invoice EES in its usual manner. EES agrees to pay ▇▇▇▇▇▇▇ 50% of the amount due within 30 days of the Product
3
being invoiced, and the remainder within 30 days after (i) installation of the Product, (ii) completion of Applications Training, and (iii) the obtaining of all site approvals; but in any event not longer than 60 days after invoice of the Product to EES.
Section 2.07 Rebate in Exclusive Territories.
(a) EES agrees that after the sale by it of an aggregate of 10 new tables in the Exclusive Territory in each of the two consecutive periods of the Initial Term, then with respect to the sale of any additional Mammotest Plus S tables in the Exclusive Territory with an Actual Selling Price (as defined below), greater than [****], EES shall pay to ▇▇▇▇▇▇▇ a rebate (the "Rebate").
(b) For purposes of this Section, the "Actual Selling Price" shall be calculated by adding together the combined customer invoice price of the table and any Options (as defined in Exhibit B) sold with the table and deducting from such sum the total of the following amounts to the extent they are actually paid by EES or its Affiliates: (i) any goods and service or value added taxes (by whatever name known), and (ii) the Option Rebate Calculation Price (as defined in Exhibit B) for each of the Options sold with the table.
(c) If the Actual Selling Price is greater than [****], EES shall pay 50% of the difference between the Actual Selling Price and [****] up to a maximum rebate of [****] to ▇▇▇▇▇▇▇. The Rebate is payable by EES 30 days after receipt by EES of the complete payment for the table from the customer, at which time the calculations above shall be converted into U.S. dollars at the prevailing spot market exchange rate to see if the Actual Selling Price exceeded [****].
****Represents material which has been redacted and filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
ARTICLE III
RESPONSIBILITIES OF EES
Section 3.01 Forecasts. In order to facilitate ▇▇▇▇▇▇▇'▇ timely manufacture of Product, within ninety (90) days after the execution and delivery of this Agreement, and at the beginning of every calendar quarter thereafter, EES shall submit on a quarterly basis a good faith, rolling twelve-month forecast of the estimated requirements of Product during the subsequent twelve (12) month period, it being agreed such forecasts shall not be legally binding.
Section 3.02 Promotional Efforts; Materials; Claims; Trademarks. EES agrees to promote the Products on customer calls and at trade shows conducted within the Exclusive Territory and the Non-Exclusive Territory to the extent set forth below. EES shall utilize the Demonstration Model Table (as defined in Article 7 below) to promote the Products at trade shows. ▇▇▇▇▇▇▇ agrees to cover all shipping and set-up expenses for the following trade shows on an annual basis: the European Congress of Radiology, and one other trade show in Europe to be decided at the discretion of EES which may be changed by it from year to year upon written notification to ▇▇▇▇▇▇▇. EES agrees to cover all shipping and set-up expenses for all other trade shows. EES shall not make any claims in the advertising and promotion of the Products other than those provided by ▇▇▇▇▇▇▇ or approved in advance in writing by ▇▇▇▇▇▇▇. EES shall acquire no rights in any trademarks, tradenames, or service marks of ▇▇▇▇▇▇▇, and shall not use any of the foregoing except to the extent included in advertising and promotional materials provided or approved by ▇▇▇▇▇▇▇.
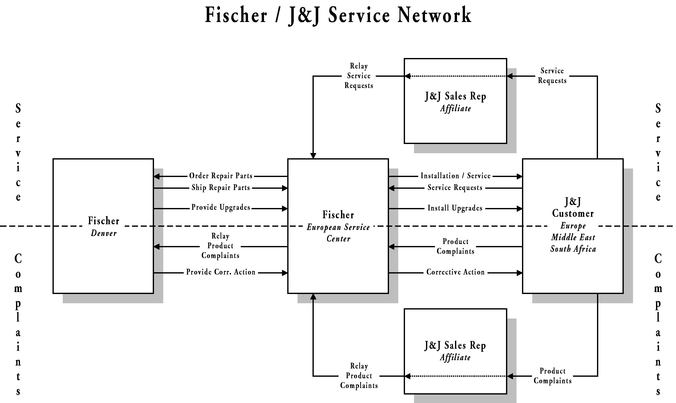
Section 3.03 Customer Technical Service. ▇▇▇▇▇▇▇ shall have exclusive responsibility for providing customer technical support and service. Such services shall be with respect to the operation and application of the Products. EES shall enable ▇▇▇▇▇▇▇ to perform such service by providing information regarding the customer to ▇▇▇▇▇▇▇ on a timely basis.
4
Section 3.04 Recalls. EES agrees to cooperate with ▇▇▇▇▇▇▇ to enable ▇▇▇▇▇▇▇ to satisfy its obligations as the licensed manufacturer of the Products with respect to any recall or corrective action relating to the Products.
Section 3.05 Customer Complaints. EES shall immediately notify ▇▇▇▇▇▇▇ in a manner reasonably requested by ▇▇▇▇▇▇▇ should it become aware, through customer complaint or otherwise, of any defect or condition which may render the Product in violation of any law or governmental regulatory requirement, or which may in any way deviate from the quality requirements (set forth in Section 4.01 below) or warranties for the Product.
ARTICLE IV
RESPONSIBILITIES OF ▇▇▇▇▇▇▇
Section 4.01 Quality. ▇▇▇▇▇▇▇ shall manufacture the Products in compliance with the Specifications and all applicable laws, rules and regulations and shall maintain the quality of the Product and attempt to upgrade such quality as necessary to maintain market competitiveness within the frame work of the Specifications.
Section 4.02 Translations. ▇▇▇▇▇▇▇ agrees to obtain translations of the operators manuals, and other appropriate labeling of the Products in compliance with the European Medical Device Directives and applicable national laws, such translations to be in German, French, Italian and a fourth language to be mutually agreed upon. Any further language translations shall be considered for joint funding based upon market analysis and mutual agreement.
Section 4.03 Market Support. ▇▇▇▇▇▇▇ shall make available to EES at its request reasonable quantities of brochures, catalogues, sales aids and other marketing material as ▇▇▇▇▇▇▇ shall have produced with respect to the Products and is then distributing generally to its sales personnel.
Section 4.04 Product Service. All customer product service and extended warranty or service contracts shall be the responsibility of ▇▇▇▇▇▇▇. ▇▇▇▇▇▇▇ agrees to make these service choices available to all customers who purchase Products from EES pursuant to the terms of this Agreement. The level of product service support to be offered for the NonExclusive Territory and the Exclusive Territory is detailed in Exhibit D. If for whatever reason, whether during or after the Term of this Agreement, ▇▇▇▇▇▇▇ can no longer provide product service to the customers of EES, abandons the market, or provides an inadequate level of product service support (to be quantitatively determined against the criteria detailed in Exhibit D), EES shall have the option of assuming (either directly or indirectly, through a contracted third party) the right to provide customers of EES with product service for the Products by giving 30 days advance written notice to ▇▇▇▇▇▇▇. If EES exercises such option, ▇▇▇▇▇▇▇ shall transfer to EES, with respect to the Products sold pursuant to this Agreement, (i) all service records, (ii) all service and operators manuals, (iii) a license to any software code or trademarks as solely required to extend service to EES customers, (iv) any product drawings required to render service, (v) all component specifications as may be required to provide service to existing customers of the Products, and with respect to said components grant hereby a license to make, have made, use or sell such components as solely required to provide service to existing customers of the Products, (vi) any other technical know-how necessary to service the Products, and (vii) access to the source of supply of any of the above. In addition, ▇▇▇▇▇▇▇ agrees to sell at its standard dealer net cost all spare parts on hand in Europe to EES, and provide access to EES to the suppliers of such parts to allow a continued source of supply.
Section 4.05 Sales Training. ▇▇▇▇▇▇▇ shall provide, at no charge to EES, initial Product training to the marketing and sales organizations of EES and its Affiliates with responsibility for distribution of the Products in the Non-Exclusive Territory and Exclusive Territory. Such product training shall include, but not be limited to, product features and benefit training, and other market position training. Such training shall not exceed six (6) full days and shall require no more than three (3) sessions in order to
5
facilitate training for 35 to 40 individuals in groups of 12 to 15 people. EES shall bear all expenses incurred by its personnel during such training.
Section 4.06 Customer Complaints, FDA audits, EES Inspections. ▇▇▇▇▇▇▇ shall immediately notify EES in writing should it become aware, through customer complaint or otherwise, of any defect or condition which may render the Products in violation of law or any government applicable regulations, or which may in any way deviate from the quality, specifications or warranties for the Products. In accordance with the Medical Device Reporting ("MDR") and Medical Devices Vigilance ("MDV") system of the United States Food and Drug Administration (FDA) and all equivalent/applicable EU Directives/national laws and regulations, ▇▇▇▇▇▇▇ shall maintain responsibility for (i) managing all customer complaints or product inquires, (ii) reporting to government agencies and (iii) all corrective action where appropriate. ▇▇▇▇▇▇▇ agrees to notify EES as soon as practical after ▇▇▇▇▇▇▇ becomes aware of any FDA audit (or of any audit from a regulatory agency in another country), or of any request for information from the FDA (or a regulatory agency in another Country) related to the manufacture or use of the Products. ▇▇▇▇▇▇▇ agrees that EES or its designated representative may at any time during business hours but subject to reasonable prior notice inspect the records referred to above or inspect the manufacturing facilities of ▇▇▇▇▇▇▇ with respect to the Products to ensure compliance with the provisions of this Agreement.
Section 4.07 Indemnification. Subject to the limitations set forth in Section 9.11, ▇▇▇▇▇▇▇ shall indemnify and hold EES and its affiliates, officers, directors, employees, sales representatives and agents (collectively, the "EES Agents") harmless against any and all claims, losses, damages, judgments, costs, awards, expenses (including reasonable attorney's fees and expenses) and liabilities of every kind (collectively "Losses" arising out of or resulting from (i) a claim from a third party that the manufacture, use and sale of existing Products infringes the proprietary rights of such third party, or (ii) the use of the Products; except to the extent such Loss is caused by the negligence or willful misconduct of EES or the EES Agents; provided that (i) EES informs ▇▇▇▇▇▇▇ promptly of any such Loss asserted against EES, (ii) ▇▇▇▇▇▇▇ shall have the right to control the defense against any such claim or action, and (iii) EES cooperates fully with ▇▇▇▇▇▇▇ in such defense.
Section 4.08 ▇▇▇▇▇▇▇ European Infrastructure. In order for ▇▇▇▇▇▇▇ to perform its obligations under this Agreement, ▇▇▇▇▇▇▇ agrees to employ a minimum of three employees to be based in Germany and one employee to be based in Italy (the "Infrastructure Employees"). The Infrastructure Employees in Germany shall include a Business/Technical Product Manager, an Applications Specialist, and a Service Engineer, and the Infrastructure Employee in Italy shall include a Service Engineer. The parties agree that while the final decision whether to hire an Infrastructure Employee rests with ▇▇▇▇▇▇▇, EES shall be consulted and kept informed of progress by ▇▇▇▇▇▇▇. It is understood that the four Infrastructure Employees are in addition to ▇▇▇▇▇▇▇'▇ existing Service Engineer based in Germany, and its Manager of Service Support based in Denmark (such employees, the "Existing Employees"). ▇▇▇▇▇▇▇ agrees to maintain the level of Infrastructure Employees and Existing Employees set forth above during the Initial Term, and to replace any such employee who leaves the employment of ▇▇▇▇▇▇▇ for whatever reason. After the expiration of the Initial Term, ▇▇▇▇▇▇▇ shall only be required to maintain the level of Infrastructure Employees and Existing Employees while EES is able to satisfy the requirements to maintain its exclusive distribution rights in the Exclusive Territory, as set forth in Section 2.01.
Section 4.09 Recalls. If ▇▇▇▇▇▇▇ shall request, or if any governmental agency having jurisdiction in the matter shall order, ▇▇▇▇▇▇▇ shall be required to institute and fund any recall, field corrective action, or the like.
Section 4.10 Enhancements, Upgrades. In the event ▇▇▇▇▇▇▇ modifies, enhances, upgrades or adds an option to a Product (including any "second-generation" or replacement product for a Product) (each, an "Upgrade") during the Initial Term or any extension of this Agreement, ▇▇▇▇▇▇▇ agrees to
6
provide EES with such Upgrade in accordance with its then prevailing pricing policy regarding supply of the Upgrade to its other distributors and the Upgrade shall be deemed added to the definition of Products and pricing of Products contained in Exhibits A and B of this Agreement, respectively.
Section 4.11 Changes Made to Products. If a change is contemplated to any of the Products listed in Exhibit "A" (as amended from time to time) that would effect the form, fit, or function of the product or could potentially effect the safety or efficacy of a Product, such change must be notified to EES in writing 60 days in advance of implementing the change. During the term of this Agreement, ▇▇▇▇▇▇▇ agrees to maintain the design of the Products in a manner which is compatible with the functionality and design of the Mammotome Biopsys system of EES and subsequent improvements thereto. Further, ▇▇▇▇▇▇▇ shall not, without the prior written consent of EES, design, develop, manufacture or distribute any Products that substantially conform to the specifications and intended functionality of the Mammotome Biopsys system.
Section 4.12 Regulatory Submissions. ▇▇▇▇▇▇▇ either directly or through a designated third party shall prepare and maintain at ▇▇▇▇▇▇▇'▇ expense, all technical files and submissions necessary to obtain all necessary approvals or concurrences required to sell the Product in the Exclusive Territory and the Non-Exclusive Territory. With respect to the Non-Exclusive Territory, it is understood that such technical files and submissions will be prepared only upon written request from the relevant EES Affiliate, based on specific tender-quotation and or installation requirements.
Section 4.13 Liability Insurance. ▇▇▇▇▇▇▇ shall at its own expense maintain a policy or policies of commercial general liability insurance including product liability and contractual liability insurance during the entire term of the agreement and for as long as the Products are distributed. Such insurance shall include commercial general liability insurance in an amount not less that $1 million per occurrence and aggregate and product liability and contractual liability insurance in an amount not less than $10 million per occurrence and aggregate. The policies shall name Ethicon Endo-Surgery, Inc. as additional insured and shall provide 30 days written notice of cancellation or material change. ▇▇▇▇▇▇▇ shall provide annual certificates of insurance reflecting the required coverage.
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF ▇▇▇▇▇▇▇
▇▇▇▇▇▇▇ represents and warrants to EES that:
Section 5.01 Manufacturer of Product; Non-Infringement. ▇▇▇▇▇▇▇ shall manufacture all Products in compliance with the Specifications and all applicable laws, rules and regulations, including good manufacturing practices (GMPs) of the FDA, and equivalent practices of regulatory agencies in other countries. All Products shall be free from defects in materials, workmanship or design and that to its best knowledge the manufacture, use and sale of the existing Products do not infringe the proprietary rights of any third party. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, ▇▇▇▇▇▇▇ MAKES NO WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, IN RESPECT OF THE PRODUCTS.
Section 5.02 Due Organization. ▇▇▇▇▇▇▇ is a corporation duly organized and validly existing under the laws of the State of Delaware and is duly qualified to carry on its business in each country, state or other jurisdiction where the failure to so qualify would have a material adverse effect on its business or properties taken as a whole or the performance of its obligations contemplated hereby.
Section 5.03 Binding Obligations. ▇▇▇▇▇▇▇ has full power, authority and legal right to enter into and perform its obligations hereunder. This Agreement has been duly authorized, executed, and delivered by ▇▇▇▇▇▇▇ and constitutes the legal, valid and binding obligation of ▇▇▇▇▇▇▇ enforceable against ▇▇▇▇▇▇▇ in accordance with its terms.
7
Section 5.04 No Conflict. The execution, delivery and performance of this Agreement and compliance with its terms by ▇▇▇▇▇▇▇ shall not result in a breach of any of the terms or conditions of, or result in the imposition of any lien, charge or encumbrance upon, any properties of ▇▇▇▇▇▇▇ pursuant to, or constitute a default under, any indenture, agreement or other instrument to which ▇▇▇▇▇▇▇ may be a party or by which ▇▇▇▇▇▇▇ may be bound.
Section 5.05 Quality and Customer Service. During the term of this Agreement, ▇▇▇▇▇▇▇ shall endeavor to maintain industry leading quality standards with respect to the manufacture, delivery and service of the Products sold pursuant to the terms of this Agreement.
Section 5.06 Year 2000.
(a) ▇▇▇▇▇▇▇'▇ Products are fully Year 2000 compliant, or it shall be able to demonstrate Year 2000 compliance and compliance in full production versions of such products, with accompanying documentation, no later than December 31, 1998;
(b) ▇▇▇▇▇▇▇'▇ information systems and other business systems for estimates, performance schedules, orders, confirmations, manufacture and delivery, invoicing, crediting of payments and other business operations shall accept and properly process input for dates before, on or after January 1, 2000 no later than December 31, 1998;
(c) the receipt of Year 2000 Compliant products and/or services shall be provided to EES under the Agreements in a timely and efficient manner without interruption and/or disruption at no additional fee or charge of any kind (including without limitation any installation, freight, or other costs or fees) to EES; and
(d) ▇▇▇▇▇▇▇ shall promptly provide to EES, (i) in response to EES' periodic requests for updates, information concerning its Year 2000 compliance program to the extent it affects performance of the Agreement and might impair its relationship with EES, and (ii) upon discovery by ▇▇▇▇▇▇▇ of any problems relating to Year 2000 compliance by the Products, written notice of the problem and ▇▇▇▇▇▇▇'▇ suggested response thereto.
ARTICLE VI
REPRESENTATIONS AND WARRANTIES OF EES
EES represents and warrants to ▇▇▇▇▇▇▇ that:
Section 6.01 Due Organization. EES is a corporation duly organized and validly existing under the laws of the State of Ohio and is duly qualified to carry on its business in each state or jurisdiction where the failure to so qualify would have a material adverse effect on its business or properties taken as a whole or the performance of its obligations contemplated hereby.
Section 6.02 Binding Obligations. EES has full power, authority and legal right to enter into and perform its obligations hereunder. This Agreement has been duly authorized, executed, and delivered by EES and constitutes the legal, valid and binding obligation of EES enforceable against EES in accordance with its terms.
Section 6.03 No Conflict. The execution, delivery and performance of this Agreement and compliance with its terms by EES shall not result in a breach of any of the terms or conditions of, or result in the imposition of any lien, charge or encumbrance upon, any properties of EES pursuant to, or constitute a default under, any indenture, agreement or other instrument to which EES or any of its officers, employees or agents may be a party or by which EES or any of its officers, employees or agents may be bound.
8
ARTICLE VII
DEMONSTRATION TABLE
Section 7.01 Demonstration Model Table; Security Interest.
(a) ▇▇▇▇▇▇▇ agrees to provide EES with a demonstration model of the Mammotest Plus S table (the "Demo Table") and maintain it in like new condition, subject to the payment of a deposit of $40,000 by EES (the "Deposit"). Such table shall be fully functional except that it shall not have functional X-ray capability. It is the intention of the parties that ▇▇▇▇▇▇▇ may utilize the Deposit during the term of this Agreement provided that upon termination of this Agreement, ▇▇▇▇▇▇▇ shall be required to repay the full amount of the Deposit to EES without interest. The Deposit is repayable to EES promptly upon termination of this Agreement. The Demonstration Model Table shall be made available to EES within 60 days of the date of signature of this agreement.
(b) In order to secure the repayment of the Deposit, ▇▇▇▇▇▇▇ hereby pledges, assigns, hypothecates, mortgages, conveys and transfers to EES a continuing first security interest in (a) the demo table, (b) all of ▇▇▇▇▇▇▇'▇ rights to collect, repossess, seize and foreclose on the foregoing property, (c) all of ▇▇▇▇▇▇▇'▇ insurance maintained upon and protecting the foregoing property, and (d) all products and proceeds (including insurance proceeds) of the foregoing property. ▇▇▇▇▇▇▇ shall, at any time and from time to time, upon the request of EES, and at the sole expense of ▇▇▇▇▇▇▇, promptly and duly execute and deliver to EES financing statements, continuation statements, mortgages, pledges and any and all other instruments and documents as specified by, and in form and substance acceptable to, EES to perfect the security interest hereby granted.
(c) ▇▇▇▇▇▇▇ hereby irrevocably appoints EES as its attorney-in-fact with respect to the security interest in the Demonstration Model Table, with full irrevocable power and authority to act in the place and stead of ▇▇▇▇▇▇▇ in the name of ▇▇▇▇▇▇▇ or in its own name upon the occurrence of an Event of Default or a Failure to Supply Products (as defined in Articles 8 and 9 hereof), in order to secure the repayment of the Deposit, and for the purpose of carrying out the terms of this Agreement, including without limitation to make collections and to otherwise preserve and protect EES' security interest in the foregoing property.
ARTICLE VIII
FAILURE TO SUPPLY PRODUCTS
Section 8.01 Failure to Supply Products; Termination
(a) In the event ▇▇▇▇▇▇▇ fails to supply a Product within 60 days of receipt of an order or other specific delivery date as mutually agreed upon in writing (in accordance with Section 2.06(a)) and ▇▇▇▇▇▇▇ shall not have cured such failure within 120 days from the date of a written notice from EES, then such failure shall be deemed a "Failure to Supply" for purposes of this Agreement. The parties acknowledge that it would be impossible or very difficult to determine actual damages (the "Damages") to EES in the event of a Failure to Supply. ▇▇▇▇▇▇▇ agrees that the sum of $600,000 is the reasonable, documented and proven damages to be paid to EES as a result of a Failure to Supply. This amount shall immediately become due and payable upon the occurrence of a Failure to Supply. ▇▇▇▇▇▇▇ agrees to waive any legal defense or doctrine which would have the effect of limiting the amount of the Damages. Upon the payment of the Damages to EES, this Agreement shall be terminated subject to the terms and conditions of Section 9.01.
(b) ▇▇▇▇▇▇▇ agrees for itself, its successors and assigns, that following a Failure to Supply, it will not directly or indirectly make, have made, use or sell the Products or Product Equivalents, or otherwise compete with EES with respect to the Products or Product Equivalents, in the Exclusive Territory and Non-Exclusive Territory for a period of three years after a Failure to Supply unless
9
▇▇▇▇▇▇▇ is supplying the Products or Product Equivalents to EES on the same basis, including price, as ▇▇▇▇▇▇▇ is supplying the Product or Product Equivalents to its customers or distributors (by whatever name known) in the Exclusive Territory and the Non-Exclusive Territory. For purposes of this clause, a "Product Equivalent" is a product which performs substantially the same functions as the Products. ▇▇▇▇▇▇▇ agrees that damages for a breach of this Section is inadequate at law and that EES would be irreparably harmed by its inability to sell the Products or Product Equivalents in the Exclusive Territory and Non-Exclusive Territory during such three year period. For purposes of clarification, it is the intention of the parties that any reference to ▇▇▇▇▇▇▇ in this Section 8.01(b) shall include ▇▇▇▇▇▇▇'▇ successors and assigns.
ARTICLE IX
MISCELLANEOUS
Section 9.01 Term; Termination.
(a) Unless earlier terminated as provided below, this Agreement shall continue in effect until October 10, 2002 (the "Term").
(b) In addition to any other right of termination under this Agreement, EES may terminate this Agreement at any time after the expiration of the Initial Term by giving ▇▇▇▇▇▇▇ 120 days prior written notice of its intention to so terminate.
(c) The non-defaulting party may terminate this Agreement in the event of an Event of Default (as defined below) by giving written notice of its intent to terminate and stating the grounds therefor. The party receiving such notice shall have 60 days from the date of such notice to cure the Event of Default. If the Event of Default is cured during such 60 days, the notice shall be of no effect. If the Event of Default is not cured during such period, then this Agreement may, at the option of the non-defaulting party, exercised by further written notice to the defaulting party, terminate at the end of said 60-day period. For purposes of this paragraph, an "Event of Default" shall mean the breach of or a failure to perform any material obligation, representation or agreement hereunder, or under the 1997 Agreement or any Addendum thereto. In the event EES provides a notice of an Event of Default or a Failure to Supply Product to ▇▇▇▇▇▇▇, EES shall not be required to make any further payments referenced in Section 2.02 until such time, if any, as the Event of Default or a Failure to Supply Product is cured.
(d) In the event of a Change in Control of ▇▇▇▇▇▇▇, EES may but shall not be obligated to terminate this Agreement by giving ▇▇▇▇▇▇▇ 60 days prior written notice. In the event of a Change of Control of ▇▇▇▇▇▇▇ and this Agreement is not so terminated by EES, and ▇▇▇▇▇▇▇, its assigns or successors shall be unable, unwilling or otherwise fail to supply the Products or otherwise prevent EES from distributing the Products, then ▇▇▇▇▇▇▇, its successors and assigns shall be bound by the provisions of Section 8.01. A "Change in Control" shall mean (i) the liquidation or dissolution of ▇▇▇▇▇▇▇ or the sale or other transfer by ▇▇▇▇▇▇▇ (excluding transfers to subsidiaries) of all or substantially all of its assets; or (ii) the occurrence of a tender offer, stock purchase, other stock acquisition, merger, consolidation, recapitalization, reverse split, sale or transfer of assets or other transaction, as a result of which any person, entity or group (a) becomes the beneficial owner, directly or indirectly, of securities of ▇▇▇▇▇▇▇ representing more than 50% of the ordinary shares of ▇▇▇▇▇▇▇ or representing more than 50% of the combined voting power with respect to the election of directors (or members of any other governing body) of ▇▇▇▇▇▇▇'▇ then outstanding securities, (b) obtains the ability to appoint a majority of the Board of Directors (or other governing body) of ▇▇▇▇▇▇▇, or obtains the ability to direct the operations or management of ▇▇▇▇▇▇▇ or any successor to ▇▇▇▇▇▇▇'▇ business; provided, however, that Change in Control shall not include the issuance by a party of equity to the public through a public offering or offerings.
10
(e) In the event ▇▇▇▇▇▇▇ receives any FDA or other equivalent international regulatory agency action after the date of signing of this agreement which in the reasonable opinion of EES adversely effects or puts at risk the Products, EES may but shall not be obligated to terminate this agreement at any time by written notice to ▇▇▇▇▇▇▇.
(f) Termination of this Agreement for any reason shall not affect the rights and obligations of the parties through the effective date, including without limitation the continuing indemnification provisions related to Products sold pursuant hereto prior to termination. Upon termination of this Agreement, nothing shall be deemed to restrict EES from marketing any products in the Exclusive Territory or the Non-Exclusive Territory, including products directly competitive with the Products. Sections 4.04, 4.07, and 8.01(b) and this Article 9 shall survive termination of this Agreement.
Section 9.02 Confidential Information. From time to time during the term of this Agreement, either party may provide to the other party papers, memoranda, customer lists, price lists, bulletins and other data of a confidential or proprietary nature pertaining to the business of the other party and marked "Confidential" (collectively, the "Information"). Each party shall during the term of this Agreement and for a period of three years after termination hereof: (i) maintain the Information in confidence, (ii) not to disclose or make a copy of the Information or any portion of the information available to any third party, to whom disclosure is not permitted on the terms set forth herein, and (iii) not use the Information or any portion or copy of it for any purpose not directly related to performance of such party's obligations under this Agreement. The obligations of this Section shall not apply to any Information which is or which becomes generally known to the public by publication or by means other than a breach of duty by the disclosing party, or which becomes otherwise available to the disclosing party which, to the best of such party's knowledge, has the right to make such disclosure; or which is conceived or developed by the disclosing party independently of the Information. Either party shall disclose the Information only to those officers, employees and agents bound by similar terms of confidentiality to those imposed herein. Upon termination of this Agreement for any reason whatsoever, each shall either return to the other all Information and copies thereof and all samples, demonstration units and like materials then in the possession of such party or elect to destroy any or all of such Information and certify as to such destruction; provided that each may retain one copy thereof in their law department files solely for evidentiary and regulatory purposes.
Section 9.03 No Assignment. Neither party shall transfer or assign this Agreement, in whole or in part, without the prior written consent of the other; except that EES may, without such consent, assign this Agreement to any Affiliate. Subject to the foregoing restrictions on assignment, this Agreement shall inure to the benefit of and bind the successors and assigns of each of the parties.
Section 9.04 Notices. Notices and other communication required or called for under this Agreement shall be in writing, shall be transmitted by certified mail, postage prepaid, and shall be deemed delivered upon mailing.
In the case of EES, such communications shall be addressed to:
Ethicon
Endo-Surgery, Inc.
▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇-▇▇▇▇
Attention: President
with copies to:
Ethicon
Endo-Surgery (Europe) GmbH
European Headquarters
Hummelsbütteler ▇▇▇▇▇▇▇▇▇ ▇▇
▇-▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇
Attention: President
11
And:
▇▇▇▇▇▇▇ &
▇▇▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇ & ▇▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇
Attention: Office of General Counsel
In the case of ▇▇▇▇▇▇▇, such communications shall be addressed to:
▇▇▇▇▇▇▇
Imaging Corporation
▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Attention: Vice President Sales, Marketing & Service
with a copy to:
▇▇▇▇▇▇▇
Imaging Corporation
▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Attention: Office of Corporate Secretary
or to the attention of such other individual or to such other address as either party may give to the other in writing.
Section 9.05 Inconsistency. In the event of any inconsistency between the terms of this Agreement and the terms typed, stamped or printed on any purchase order submitted hereunder, the terms of this Agreement shall govern. Any different or additional terms contained in any purchase order shall not be binding unless specifically and expressly assented to in a writing signed by both parties.
Section 9.06 No Waiver. The failure of either party to enforce at any time for any period any provision hereof shall not be construed to be a waiver of such provision or of the right of such party thereafter to enforce each such provision.
Section 9.07 Publicity. No party to this Agreement shall originate any publicity, news release or other public announcement, written or oral, whether relating to this Agreement or the existence of any arrangement between the parties, without the prior written consent of the other party, except where such publicity, news release or other public announcement is required by law; provided that in such event, the party issuing same shall still be required to consult with the other party or parties a reasonable time (being not less than 48 hours) prior to its release to allow the named party or parties to comment on the use of its name and, after its release, shall provide the named party or parties with a copy thereof.
Section 9.08 Severance. If any provision of this Agreement should be or become fully or partly invalid or unenforceable for any reason whatsoever or violate any applicable law, this Agreement is to be considered divisible as to such provision and such provision is to be deleted from this Agreement, and the remainder of this Agreement shall be deemed valid and binding as if such provision were not included. A suitable provision which, as far as legally possible, comes nearest to what the parties desired according to the sense and purpose of this Agreement had this point been considered when concluding this Agreement shall be substituted for any such provision deemed to be deleted.
12
Section 9.09 Force Majeure. Neither ▇▇▇▇▇▇▇ nor EES shall be liable for failure due to force majeure to perform its duties under this Agreement. As used herein, force majeure means acts of God; acts, regulations or laws of any government; war, civil commotion; strike, lock-out or labor disturbance; destruction of production facilities or materials by fire, earthquake or storm; failure of public utilities or common carriers; and any other causes beyond the reasonable control of that party.
Section 9.10 Arbitration. Except as expressly provided otherwise in this Section, any controversy or claim arising out of or relating to this Agreement or the validity, inducement, or breach thereof (each such controversy or claim is hereinafter referred to as a "Dispute") shall be settled as follows:
(a) Officers at or above the Vice President level of each party shall attempt to resolve any Dispute prior to commencing the procedures set forth below.
(b) If after seven days the officers are unable to resolve the Dispute, the Chief Executive Officer and/or the highest ranking officer of each party shall submit to nonbinding mediation which shall take place for a period of one day in Cincinnati, Ohio before a mediator that is knowledgeable about the subject matter of the Dispute and that is mutually acceptable to the parties. If the parties are unable to agree on the selection of a mediator, a mediator shall be chosen by an arbitrator selected pursuant to the rules of the American Arbitration Association (AAA) who shall then select such mediator from a list of distinguished neutral mediators maintained by the AAA. The mediator shall confer with the parties to design procedures to conclude the mediation within no more than 45 days after initiation. Under no circumstances shall the commencement of arbitration under paragraph (c) below be delayed more than 45 days by the mediation process specified in this paragraph (b). Each party has the right to pursue any provisional relief from the appropriate court, such as attachment, preliminary injunction, replevin, etc. to avoid irreparable harm, maintain the status quo, or preserve the subject matter of the arbitration, even though mediation has not been commenced or completed.
(c) If during such one-day mediation the parties are unable to resolve the Dispute, the Dispute shall be settled by arbitration before a single arbitrator in accordance with the Commercial Arbitration Rules of the AAA then pertaining, except where those rules conflict with this provision, in which case this provision controls. The parties hereby consent to the jurisdiction of the Federal District Court for the Northern District of Ohio for the enforcement of these provisions and the entry of judgment on any award rendered hereunder. Should such court for any reason lack jurisdiction, any court with jurisdiction shall enforce this clause and enter judgment on any award. The arbitrator shall be an attorney specializing in business litigation who has at least 15 years of experience with a law firm or corporation of over 25 lawyers or was a judge of a court of general jurisdiction. The arbitration shall be held in Cincinnati, Ohio and the arbitrator shall apply the substantive law of New York, except that the interpretation and enforcement of this arbitration provision shall be governed by the Federal Arbitration Act. Within 30 days of initiation of arbitration, the parties shall reach agreement upon and thereafter follow procedures assuring that the arbitration shall be concluded and the award rendered within no more than six months from selection of the arbitrator. Failing such agreement, the AAA shall design and the parties shall follow such procedures. The parties agree neither to request or seek to enforce any punitive, exemplary or special damages from the arbitrator and the arbitrator shall not be empowered to grant any such damages. In addition, the parties agree neither to request or seek to enforce any consequential damages from the arbitrator and the arbitrator shall not be empowered to grant any such damages from the other unless (i) the foreseeability of such damages at the time of this Agreement, and (ii) the amount of such damages are proven by clear and convincing evidence. The arbitrator shall issue written findings of fact and conclusions of law. Either party may appeal issues of law to the appropriate court, including the application of law to the facts.
13
(d) The arbitrator shall be bound by the express terms of this Agreement and may not amend or modify such terms in any matter. Any award rendered by the arbitrator shall be consistent with the terms of this Agreement, and such terms shall control the rights and obligations of the parties. The proceedings shall be confidential and the arbitrators shall issue appropriate protective orders to safeguard both parties' confidential information.
Section 9.11 Limitation of Liability. Under no circumstances shall either party hereto be liable to the other for any indirect damages (whether incidental, consequential, special or otherwise) resulting from any errors or delays in the shipment or delivery of any products hereunder (unless previous agreed to by such party in writing), or from any breach of the product warranty.
Section 9.12 Governing Law. All matter affecting the interpretation, validity and performance under this Agreement shall be governed by the internal laws of the State of New York, without regard for its conflict of laws principles.
Section 9.13 Headings. The Article and Section headings included in this Agreement are for reference purposes only and shall not affect the meaning or interpretation of this Agreement.
Section 9.14 Counterparts. This Agreement may be executed in any number of counterparts with the same effect as if all parties had signed the same document. All such counterparts shall be deemed an original, shall be construed together and shall constitute the same instrument.
Section 9.15 Entire Agreement. The parties intend to provide certainty as to their future rights and remedies against each other by defining the extent of their undertakings. The parties have in this Agreement incorporated all representations, warranties, covenants, commitments and understandings on which they have relied in entering into this Agreement. Except as expressly provided, neither party makes any covenant or other commitment to the other concerning its future action. Accordingly, this Agreement (i) constitutes the entire Agreement and understanding between the parties with respect to the Exclusive Territory and the Non-Exclusive Territory and no promises, representations, conditions, provisions or terms other than those set forth in this Agreement exist for such territories and (ii) supersedes all previous understandings, agreements and representations between the parties, written or oral with respect to its subject matter in the Exclusive Territory and the Non-Exclusive Territory, including the 1997 Agreement and any Addendum thereto. This Agreement may not be changed, modified or amended except by a writing signed by both parties.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement by their officers thereunto duly authorized as of the date first-above written.
| |
|
|
||
|---|---|---|---|---|
| ETHICON ENDO-SURGERY, INC. | ||||
| By: | /s/ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ |
|||
| Name: | ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ | |||
| Title: | Vice President Breast Care Management Europe | |||
▇▇▇▇▇▇▇ IMAGING CORPORATION |
||||
| By: | /s/ ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ |
|||
| Name: | ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ | |||
| Title: | Chairman/CEO | |||
14
PRODUCTS AND SPECIFICATIONS
A. NEW PRODUCTS
85200G-2 MAMMOTEST® PLUS BIOPSY AND LOCALIZATION SYSTEM
The MAMMOTEST PLUS system is designed expressly for needle biopsy of the breast. The system consists of an elevating, floating tabletop on which the patient lies prone with one breast suspended through an aperture in the tabletop. Beneath the tabletop, a specially designed mammographic X-ray tube takes stereoscopic images of the breast. The information is captured by a digital camera and automatically processed to provide the spatial coordinates of the suspected lesion. An automated punction device mounted beneath the tabletop aims the needle to the precise spatial coordinates and the operator may proceed with the biopsy. Accuracy is within 1 mm. A dedicated generator provides power for the X-ray procedures.
MAMMOTEST PLUS SYSTEM
Consisting of:
- •
- MAMMOTEST
PLUS biopsy table enabling 360° needle access to the breast
- •
- Mammographic
x-ray tube with 0.1/0.3 mm focal spots and molybdenum anode;
- •
- Two
procedural lamps
- •
- Automatic
breast compression with footswitch
- •
- Universal
stereo cassette tunnel with grid (79 lines/cm, 3.5:1), for use with either DIN or Min-R cassettes
- •
- Small
and large biopsy compression paddles, and one frame
- •
- 18 × 24
cm compression paddle and frame
- •
- 5 × 5
cm Digital aperture
- •
- 18 × 24
cm aperture
- •
- Dedicated
high frequency generator, two-tube, REF: 85490G-4
- •
- Transparent
operator shield, REF: 80712G-4
- •
- 240
VAC kit, REF: 77180G-1
- •
- Universal
localization needle holder for wire localization systems
- •
- One
case of 10 sterile disposable procedure trays
- •
- One case of 10 disposable procedure drapes
AUTOGUIDE™ MOTORIZED NEEDLE GUIDANCE SYSTEM, REF: 85705G-2
An automated polar coordinate needle positioner with depth stop, needle guide holder, disposable needle guides (one case of ten 14 gauge guides and one case of ten 20 gauge guides), and storage case.
MAMMOVISION® PLUS COD DIGITAL IMAGING SYSTEM
A fiber-optically coupled 1024 × 1024 charge coupled device (CCD) based digital imaging system. MAMMOVISION PLUS allows the operator to acquire digital images, manipulate images on screen
15
for contrast enhancement and magnification, localize lesions for needle biopsy or preoperative wire placement, and produce hardcopy output. The camera is removable and can be shared with the new HF-X PIus™ diagnostic mammography unit.
CCD Camera/Image Receptor
- •
- Fiber
optically coupled 1024 × 1024 pixel COD
- •
- 50
mm × 50 mm imaging area (48p~m pixels)
- •
- 12
bit analog-to-digital converter (4096 shades of ▇▇▇▇)
- •
- Limiting
spacial resolution: 10 lp/mm
- •
- Needle placement accuracy: ±1mm
Workstation REF: 77206-4
- •
- ▇▇▇
▇▇▇ ▇▇▇▇▇▇▇ computer
- •
- 32MBRAM
- •
- 2.1
GB Hard Drive that allows temporary storage of approximately 900 images
- •
- Image
display time: approximately 4 seconds
- •
- 24"
diagonal monochrome 1280 × 1024 pixel video display monitor
- •
- Trackball
- •
- Keyboard
LATERAL APPROACH BIOPSY SYSTEM, REF: 85450G
Lateral arm device for orthogonal access to compressed breast. Includes targeting software with user-friendly graphical presentation.
PHANTOMS
Scatter
Phantom, REF: 77330G-1
Target Verification Phantom, REF: 85460G
Sony SME-E511 5.25" Multi-Functional Optical Disk Drive, REF: 77255G-2
Includes one 2.6 GB disk, PCB, and cabling to interface with MAMMOVISION. The optical disk drive system alone does not provide hard copy output.
PLAN
Room planning and drawings
WARRANTY
Includes twelve (12) months parts and labor, excluding glassware which is manufacturer's pro-rated warranty.
85201 G-1 MAMMOTEST® PLUS "5" BIOPSY AND LOCALIZATION SYSTEM
The MAMMOTEST PLUS "5" system is designed expressly for needle biopsies and needle localizations of the breast. The system consists of an elevating, floating tabletop on which the patient
16
lies prone with one breast suspended through an aperture in the tabletop. Beneath the tabletop, a specially designed mammographic X-ray tube takes stereoscopic images of the breast. The information is captured by a digital COD camera and automatically processed to provide the spatial coordinates of the suspected lesion. An automated punction device called an Autoguide™ is mounted beneath the tabletop and has a dual state-of-the-art electro luminescent digital display and message center. The Autoguide aims the needle to the precise spatial coordinates given and the operator may proceed with the biopsy. Accuracy is within 1mm. A dedicated high frequency generator provides power for the X-ray procedures.
MAMMOTEST PLUS "S" SYSTEM
Consisting of:
- •
- MAMMOTEST
PLUS "5" biopsy table enabling 360° needle access to the breast
- •
- Mammographic
x-ray tube with 0.1/0.3 mm focal spots and molybdenum anode;
- •
- Two
procedural lamps
- •
- Automatic
breast compression with footswitch
- •
- Small
and large biopsy compression paddles
- •
- 18 × 24
cm compression paddle and frame
- •
- 5 × 5
cm Digital aperture
- •
- 18x24
cm aperture
- •
- 18 × 24
cm scout film tunnel
- •
- Universal
localization needle holder for wire localization systems
- •
- Dedicated
high frequency generator, two tube capability, REF: ▇▇▇▇▇▇-4
- •
- Transparent
operator shield, REF: 80712G-4
- •
- 240 VAC kit, REF: 771BOG-2
AUTOGUIDE™ GUIDANCE AND MESSAGE CONTROL SYSTEM, REF: 85705G-1
An automated polar coordinate needle positioner with depth stop, digital display and motion control at tableside. The Autoguide contains a stateof-the-art electro luminescent digital touch control and message center, that is conveniently located at the working end of the table. This feature eliminates the need for the hand held targeting controls, giving instantaneous targeting and informational readouts for the automated polar coordinate Needle Guidance System. The ergonomic design allows new and more versatile functions for the future.
MAMMOVISION® PLUS COD DIGITAL IMAGING SYSTEM
A fiber-optically coupled 1024 × 1024 charge coupled device (COD) based digital imaging system. MAMMOVISION PLUS allows the operator to acquire digital images, manipulate images on screen for contrast enhancement and magnification, localize lesions for needle biopsy or preoperative wire placement, and produce hardcopy output. The camera is removable and can be shared with the HF-X PLUS diagnostic mammography unit.
COD Camera/Image Receptor
- •
- Fiber
optically coupled 1024 × 1024 pixel COD
17
- •
- 50
mm × 50 mm imaging area (48pm pixels)
- •
- 12
bit analog-to-digital converter (4096 shades of ▇▇▇▇)
- •
- Needle
placement accuracy: ± 1 mm
Workstation, REF: 77206-4
- •
- 233
MHz Intel Pentium PC
- •
- 32MBRAM
- •
- 2.1
GB Hard Drive that allows temporary storage of approximately 900 images
- •
- 24"
diagonal monochrome 1280 × 1024 pixel video display monitor
- •
- Trackball
- •
- Keyboard
LATERAL APPROACH BIOPSY SYSTEM
Lateral arm device for orthogonal access to compressed breast. Includes targeting software with user-friendly graphical presentation.
PHANTOMS
- •
- Scatter
Phantom, REF: 77330G-1
- •
- Target Verification Phantom, REF: 85460G
ARCHIVAL DEVICE, REF: 77255G-2
Sony
SME-E51 1 5.25" Multi-Functional Optical Disk Drive
Includes one 2.6 GB disk, PCB, and cabling to interface with MAMMOVISION.
The optical disk drive system alone does not provide hard copy output.
PLAN
Room planning and drawings
WARRANTY
Includes twelve (12) months parts and labor, excluding glassware which is manufacturer's pro-rated warranty.
OPTIONS:
85600G-1 HF-X PLUS DIAGNOSTIC MAMMOGRAPHY SYSTEM
The HF-X Plus is a complete diagnostic, high frequency mammographic system designed to perform high quality, low dose breast examinations quickly, easily and efficiently. The HF-X Plus consists of a cabinet housing a microprocessor controlled high frequency X-ray generator, and a vertical column supporting a U-arm with X-ray tube and image receptor. A full length transparent shield protects the operator. Also, the HF-X Plus has the ability to accept the Mammovision Plus COD Camera.
18
- •
- Microprocessor
controlled, 5 kW, with LED readouts
- •
- Automatic
exposure control and self-diagnostic program
- •
- Automatic
or manual selection of kV, mA and mAs
- •
- kV:
20 to 39 in steps of 0.5 kV
- •
- mA:
Small focal spot—15,23 and 30 mA Large focal spot—100 and 125 mA
- •
- mAs:
1 to 750 mAs
- •
- Automatic
line compensation
- •
- High
frequency output: 40 kHz
- •
- Input
power: 208-240 VAC, 30A, single phase
- •
- Automatic
interlocks for Bucky and magnification stand
HF-X PLUS DIAGNOSTIC MAMMOGRAPHIC SYSTEM
High Frequency, Constant Potential Generato
- •
- Computerized
non-linear density tracking
- •
- Solid
state detector; 6 photocell positions
- •
- 11
density stations with 12.5% change per station
- •
- Automated or manual selection of kV, mA and back-up mAs
Computrac II™ Automatic Exposure Control
- •
- Variable
aperture collimator with 5 independently adjustable blades
- •
- Rectangular,
"D" shaped or irregular fields
Collimating Cone with Full Field Illumination
- •
- Quick
release bar
- •
- 1
8x24 cm and 24 × 30 cm polycarbonate compression paddles included
- •
- Adjustable
pressure scale footswitch
Independent Automatic and Manual Breast Compression
- •
- 0.05/0.3mm
focal spots, 0.8/3.5 kW
- •
- 71mm
molybdenum anode, grounded metal center section, beryllium window
- •
- 300,000
HU target capacity, 60,000 HU/minute cooling, 500,000 HU housing capacity
- •
- 25ìm molybdenum filter
Heavy Duty Rotating Anode X-Ray Tube
- •
- +180°
to -140° with electromagnetic tube locks
- •
- 66cm
Source to Image Distance
U-Arm Assembly
19
- •
- Patient
hand holds
- •
- Vertical travel range: 39" (100 cm), electromagnetic lock
- •
- Bucky
drive mechanism and control electronics
- •
- 18x24cmplattorm
- •
- 24 × 30
cm platform
- •
- Cassette clamp included (specify DIN or Min-R)
- •
- 24 × 72"
(61 × 183 cm) leaded plastic with 0.3mm Pb equivalent
- •
- 18 × 24
cm Bucky assembly with 5:1 ratio, carbon-fiber covered grid, 31 lines/cm, fiber interspaced, carbon-fiber front cover with soft edges, REF:
81460-1
- •
- 24 × 30
cm Bucky assembly with 5:1 ratio, carbon-fiber covered grid, 31 lines/cm, fiber interspaced, carbon-fiber front cover with soft edges, REF:
81 460-2
- •
- Combination
1 .8X and 1 .5X magnification stand with 15 × 19 cm compression paddle, REF: 70518G
- •
- Magnification
spot compression paddle: 3 × 6" (7.5 × 15 cm), REF: 70802G
- •
- Magnification
spot compression paddle: 3" (7.5 cm) diameter, with straight front edge, REF: 70439G
- •
- Spot
compression paddle: 3" (7.5cm) diameter, with straight front edge, REF: 70440G
- •
- Spot
compression paddle: 4 × 6" (10 × 15 cm), REF: 70803G
- •
- Exposed
cassette storage rack, REF: 70550G-2
- •
- Pair of accessory storage racks, REF: 70509G
Image Receptor
Transparent Shield
77257-1 IV4600 Series D Multi-Format Camera:
Two-on-one format on single sheet of 8" × 10" film. Includes cabling to interface with MAMMOVISION.
77271 Digital Interface to Laser Printer:
(Consists of MAMMOVISION interface only. Does not include any digital interfaces required on laser imager itself. Please contact manufacturers local laser imager representative for additional requirements for laser imager interfacing.)
77265G ACR-NEMA PACS Network Interface:
Enables
users to send images over the ACR NEMA
DICOM 3.0 PACS network
20
85599-1 Site Select Holder
ACE 1000i UPS
VAR uninterruptable power supply for MAMMOTEST® system. Model ACE 1000i provides battery back-up capacity for MAMMOTEST® system.
B. USED PRODUCTS
Used equipment shall include the MAMMOTEST®, MAMMOTEST® Plus™, and the MAMMOTEST® Plus™ S breast biopsy systems. Detailed specifications shall be determined on case by case basis.
21
A. NEW PRODUCTS
Mammotest® Plus™: [****]*
Mammotest® Plus™ 5: [****]*
Options: The pricing for new options with respect to the products listed above are the list price of ▇▇▇▇▇▇▇ as of the date of this Agreement less 20%. As new options become available, the pricing shall be set as list price of ▇▇▇▇▇▇▇ less 20%:
| |
|
Transfer Price for Initial Term |
Option Rebate Calculation Price** |
|||||
|---|---|---|---|---|---|---|---|---|
| a. | Laser camera interface | $ | [****] | $ | [****] | |||
| b. | Dicom interface | $ | [****] | $ | [****] | |||
| c. | Multi format camera | $ | [****] | $ | [****] | |||
| d. | HFX two bucky system | $ | [****] | $ | [****] | |||
| e. | Site Select Adapter: | $ | [****] | $ | [****] | |||
| f. | Mammotome® Driver | N/A | $ | [****] | ||||
| g. | ACE 1000i UPS | $ | [****] | $ | [****] | |||
- *
- The
Mammotest Plus and Mammotest Plus S systems shall include an initial service warranty (parts only) of 12 months, installation, and applications training of 4 full days, all
included in the above transfer prices. For tables in the Exclusive Territory, the 12 month service warranty above shall also include labor. For tables in the Non-Exclusive
Territory, EES agrees to pay an additional $10,000 per table to be added to the transfer prices for the service warranty including labor and installation to be performed by ▇▇▇▇▇▇▇ or ▇▇▇▇▇▇▇'▇
distributor in accordance with Section 2.04(c) of the Agreement.
- **
- For purposes of the Rebate calculation in Section 2.07 of the Agreement only.
B. USED PRODUCTS
All pricing and terms for Used Products shall be determined on a case by case basis.
***Represents material which has been redacted and filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
22
APPLICATIONS TRAINING
The Application personnel will cover instructional review for use of all ▇▇▇▇▇▇▇ equipment sold with the exception of the Mammotome breast biopsy device. The Mammotome instructional use will be covered by the EES Representative
▇▇▇▇▇▇▇ Applications training will be responsible for teaching the Customer the following:
Nuances and functionality of the ▇▇▇▇▇▇▇ equipment pertaining to the Mammotest® Plus or the Mammotest® Plus S prone stereotactic breast biopsy system. This will include:
- 1.
- Set-up
procedures. Preparing the MammoVision workstation with all pertinent information that is needed to perform breast biopsies.
- 2.
- Audit
start-up procedures for "Daily Calibrations" of the equipment.
- 3.
- During
the "Daily Calibrations", Angle Trimming (needle verification testing), will be covered. This will include information on trimming angles from the frontal approach as well as
the Lateral Arm approach.
- 4.
- Helpful
tips on prone positioning of a patient will be covered.
- 5.
- The
Customer will be shown how to access the "Demonstration Program" within the MammoVision workstation, so that practice exams can be performed.
- 6.
- Target
on Scout software and targeting practices will be reviewed.
- 7.
- Needle
localization procedures will be reviewed from the frontal approach as well as from the Lateral Arm approach.
- 8.
- Formatting
the Optical diskettes will be covered.
- 9.
- Printing
to optional hard copy devices will be covered if applicable.
- 10.
- Patient
procedures for optimizing stereotactic operations in coordination with the EES Representative providing applications training for the Mammotome®.
- 11.
- MammoVision
operations will be reviewed that pertain to image manipulation. This will cover not only the image post-processing features, but will also include deleting
images from the hard drive, deleting patient database and annotation on an image.
- 12.
- Shutdown procedures for the equipment.
23
Product Service Support
- 1.
- For
both the Exclusive Territory and the Non-Exclusive Territory, a European based customer service response phone number will exist for customers who have questions or
problems regarding the use of the Products, and this phone will be answered by a live person during normal business hours.
- 2.
- For
the Exclusive Territories, the same level of service response will be offered to customers as is offered in the U.S. This will include but not be limited to, a return phone call by
a Service Engineer within 30 minutes of a call by a customer requesting assistance, and a Service Engineer on site within 24 hours of a customer's request for assistance if required to solve a
problem.
- 3.
- For
the Non-Exclusive Territory, ▇▇▇▇▇▇▇ will ensure, either directly or through a designated third party service organization that, a reasonable service response time will
be provided to a customer requesting assistance.
- 4.
- For customers in both the Exclusive Territory and the Non-Exclusive Territory, once an assessment of the problem has been made, the time required to resolve the problem will be consistent with the expectations for problem resolution in the U.S. including equal access and availability of repair parts.
24
25
26
DISTRIBUTOR AGREEMENT

