LICENSE AND ASSET PURCHASE AGREEMENT
EXHIBIT 10.1
EXECUTION COPY
LICENSE AND ASSET PURCHASE AGREEMENT
THIS LICENSE AND ASSET PURCHASE AGREEMENT (this “Agreement”) is made as of December 8, 2006 (the “Effective Date”) by and between Biovest International, Inc., a Delaware corporation, with its offices located at 000 X. Xxxx Xxxx Xxxxxx Xxxxx 000, Xxxxx XX 00000 (“Biovest”), and AutovaxID, Inc., a Florida corporation with offices located at 000 Xxxxxxxxxx Xx. Xxxxxxxxx, Xxxxxxxxxxxxx 00000 (“AutovaxID”). Together, AutovaxID and Biovest are collectively referred to as the “Parties,” and, individually Biovest and AutovaxID are referred to as a “Party”.
RECITALS
WHEREAS, Biovest has developed and owns all rights to the Autovax automated cell production instrument described in Exhibit A (as covered by the Licensed Patent rights owned by Biovest under the patent numbers described in Exhibit A of the License Agreement attached hereto as Exhibit B, together with any successor innovation thereto developed by Biovest or its affiliates, the “Autovax Instrument”);
WHEREAS, Biovest wishes to grant to AutovaxID pursuant to the terms and conditions hereof the exclusive, perpetual right to manufacture, market, sell and commercialize the Autovax Instrument in North America pursuant to the License Agreement attached hereto as Exhibit B;
WHEREAS, Biovest wishes to sell to AutovaxID at the Leasehold Occupancy date certain assets listed on Exhibit C hereto to be used in the manufacture of Autovax Instrument (the “Purchased Equipment”);
WHEREAS, AutovaxID desires to license the commercial rights to the Autovax Instrument as provided herein and to purchase the Equipment subject to the liens described in Exhibit D (the “Continuing Liens”) on the Leasehold Occupancy date; and
WHEREAS, AutovaxID has executed a certain Lease Agreement (“Lease”) as amended attached as Exhibit E with respect to portions of premises located at 0000 Xxxxxxxx Xxxxxx, Xx. Xxxxx, XX 00000 (the “AutovaxID Lease”) which requires completion of tenant improvements and a Certificate of Occupancy before occupancy can commence (“Leasehold Occupancy”); and
WHEREAS AutovaxID has provided its Business Plan outlining its anticipated operations in connection with the commercial production and distribution of the Autovax Instrument, which Business Plan is attached hereto as Exhibit F; and
WHEREAS, the parties wish to set forth the terms and conditions pursuant to which AutovaxID will serve as a contract manufacturer for Biovest for Autovax Instruments to be used by Biovest, as requested by Biovest to produce anti-cancer vaccine to be used by Biovest in its clinical trial and for commercial sale.
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants contained herein and other consideration, the receipt and sufficiency of which are hereby acknowledged, and intending to be legally bound, Biovax and Biovest hereby agree as follows:
1. License.
At Closing (as defined in Section 4), Biovest shall grant to AutovaxID the license to manufacture, market, sell and commercialize the Autovax Instrument in North America pursuant to the terms and conditions of the License Agreement attached hereto as Exhibit A. The License Agreement shall be duly executed by Biovest and delivered to AutovaxID at Closing. The license shall be non-exclusive prior to the achievement of Leasehold Occupancy (the “Operational Date”) and shall become an exclusive license on the Operational Date. Prior to the Operational Date, all Autovax Instruments shall continue to be manufactured by Biovest and AutovaxID shall have no right or interest in the instruments manufactured by Biovest prior to the Operational Date or the proceeds of the sale of such instruments.
2. Sale of Equipment.
At Leasehold Occupancy, AutovaxID shall purchase for fair market value the Equipment listed in Exhibit C, as is and where is, subject to the Continuing Liens listed in Exhibit D. The Equipment shall be conveyed by Xxxx of Sale subject to the right of the Continuing Liens.
3. Pricing and Payment Terms.
As the full purchase price for the License and related business opportunity, AutovaxID shall pay to Biovest Five Million Six Hundred Thousand Dollars ($5,600,000) (the “Purchase Price”) in cash at Closing.
4. Closing.
The closing shall take place at 000 Xxxxxxxxxx Xxxxxx, Xxxxxxxxx XX on December 8, 2006 at 10 am (the “Closing”). At the Closing, the parties shall deliver the following:
AutovaxID shall deliver to Biovest:
| 1. | Cash in payment of the Cash Portion of the Purchase Price |
| 2. | Executed License Agreement in the Form of Exhibit B (the “License Agreement”). |
| 3. | Acknowledgement of Assumed Liens |
Biovest shall deliver to AutovaxID:
| 1. | Executed License Agreement in the form of Exhibit B. |
| 2. | Upon Leasehold Occupancy the Xxxx of Sale to the Equipment listed in Exhibit D |
5. Contract Manufacturer.
As more fully described in the License Agreement, AutovaxID shall serve as a non-exclusive contract manufacturer to manufacture Autovax Instruments for Biovest to be used by Biovest for internal manufacturing purposes, including but not limited to producing anti-cancer vaccine for clinical trial or commercial sale and to produce other cell products including contract manufacturing of any description and for re-sale to customers outside North America, provided Biovest cannot purchase Autovax Instruments for resale to customers in or to be used in North America. Biovest shall have no minimum purchase requirements and all such purchases shall be as and when required by Biovest and shall be at the specifications submitted in writing by Biovest and accepted by AutovaxID. All Autovax Instruments manufactured by AutovaxID for Biovest shall be paid for at fair market value (which shall be no less than fully burdened manufactured cost). AutovaxID’s fully burdened cost includes all third party and overhead expenses, such as wages and salaries, lease payments, utilities, purchases of manufacturing materials, maintenance and repairs to equipment and leasehold, amortization, and other expenditures necessary or appropriate to operate the Lease Premises currently accrued, using the same methodology as currently used in Biovest financial accounting. Fully burdened costs do not include capital expenditures such as purchases of equipment and partially completed vaccines, expansion of facilities, leasehold improvements, and employee training. Biovest shall have the right to inspect and audit the calculation of full burdened manufactured cost upon reasonable notice to AutovaxID. Invoices shall be paid within 30 days after invoice. Nothing herein shall prohibit Biovest from purchasing Autovax Instruments from other manufacturing sources outside of North America.
6. Shared Support Services.
AutovaxID shall be entitled to, but shall not be obligated to, purchase support services, such as HR support, IT support, accounting support and legal support, from Biovest. The purchase price shall be an amount equal to the allocated cost to Biovest for providing such services purchased by AutovaxID in its discretion. Upon Leasehold Occupancy, Biovest shall provide as part of its shared resource assistance to AutovaxID in training newly hired employees to staff the manufacturing facility in St. Louis, MO. Xxxxx Xxxxxxx will serve as an employee of AutovaxID shared with Biovest. Xx. Xxxxxxx shall maintain his office in Worcester, MA in an area that is verified to be a qualified census tract for New Markets Tax Credits.
- 2 -
7. No Interest in Vaccine or Intellectual Property of Biovest.
AutovaxID will not acquire, by virtue hereof, any interest in any property of Biovest not expressly transferred hereunder. In expansion of, and not in limitation of, the forgoing, it is expressly agreed and acknowledged that AutovaxID shall have no interest in, right to use or ownership of Biovest’s anti-cancer vaccine, any patent or proprietary property of Biovest, the Investigational New Drug Applications owned by Biovest, the clinical trials being conducted by Biovest, and any other contractual rights or property of Biovest.
8. Representations and Warranties.
A. Representations and Warranties of Biovest. Biovest represents and warrants the following to AutovaxID with the intention that AutovaxID may rely upon the same and acknowledges that (except as otherwise indicated in the specific paragraph) the same shall be true on the date hereof, as of the Operational Date (as if made at the Closing), and as of the Closing, and shall survive the closing of this transaction.
1. Title. As of the Purchase Closing, Biovest will own the Autovaxid instrument business (the “Autovax License Rights”) and Purchased Equipment free and clear of any liens, claims, charges or other encumbrances other than the Continuing Liens.
2. Licenses and Permits. Biovest possesses all permits, licenses, approvals and notifications, governmental or otherwise, the absence of which would have a material adverse effect on Biovest’s Autovaxid Instrument business.
3. Litigation, Adverse Claims, and Related Matters. There is no pending or threatened litigation (nor, to Biovest’s knowledge, any claim which may lead to a threat of litigation), proceeding, or investigation (including any environmental, building or safety investigation) relating to any material aspect of the Autovax License Rights or the Purchased Equipment, nor is Biovest subject to any existing judgment, order or decree which would prevent, impede, or make illegal the consummation of the transactions contemplated in this Agreement or which would have a material adverse effect on the License Agreement.
4. Laws and Regulations. Biovest has complied, and is in compliance on the Closing Date, with all applicable laws, statutes, orders, rules, regulations and requirements promulgated by governmental or other authorities relating to the License Agreement or the Equipment, the failure of which would have a material adverse effect on the License Agreement. Biovest has not received any notice of any alleged violation of any such statute, order, rule, regulation or requirement.
5. Breaches of Contracts; Required Consents. Neither the execution and delivery of this Agreement by Biovest, nor compliance by Biovest with the terms and provisions of this Agreement will:
(a) Conflict with or result in a breach of (i) any judgment, order, decree or ruling to which Biovest is a party, (ii) any injunction of any court or governmental authority to which Biovest is subject, (iii) any agreement, contract or commitment which is material to the License Agreement, or (iv) any of the terms, conditions or provisions of the Articles of Incorporation, Bylaws or other governing instruments of Biovest; or
(b) Require the affirmative consent or approval of any third party other than Laurus Master Fund, Ltd.
6. Organization; Binding Obligation. Biovest is a corporation duly organized, validly existing, and in good standing under the laws of the State of Delaware. Biovest has all requisite power and authority to own properties and assets and to conduct business as it is presently conducted. This Agreement constitutes the legal, valid and binding obligation of Biovest in accordance with the terms hereof. Biovest has all requisite corporate power and authority, including the approval of its shareholders and Board of Directors, to execute, perform, carry out the provisions of and consummate the transactions contemplated in this Agreement.
7. Completeness of Disclosure. The Business Plan attached as Exhibit F is not intended to be a full or complete disclosure of the business or risks of the Autovax Instrument. The Business Plan is not a material
- 3 -
representation or warrant hereunder. The Business Plan does not contemplate use of the Autovax Instrument for the purposes of producing stem cells or therapeutics, and AutovaxID may not use or sublicense the Autovax Instrument for such purposes.
8. Valuation. The Valuation attached hereto as Exhibit G was prepared by the independent firm of The Financial Valuation Group and Biovest makes no representation or warranty regarding Exhibit G.
B. Representations of AutovaxID
1. Licenses and Permits. AutovaxID possesses or shall obtain following the Closing Date all permits, licenses, approvals and notifications, governmental or otherwise, the absence of which would have a material adverse effect on AutovaxID’s ability to assume operation of the Autovax License Rights.
2. Litigation, Adverse Claims, and Related Matters. There is no pending or threatened litigation (nor, to AutovaxID’s knowledge, any claim which may lead to a threat of litigation), proceeding, or investigation (including any environmental, building or safety investigation) relating to any material aspect of AutovaxID’s business, nor is AutovaxID subject to any existing judgment, order or decree which would prevent, impede, or make illegal the consummation of the transactions contemplated in this Agreement or which would have a material adverse effect on AutovaxID’s business.
3. Laws and Regulations. AutovaxID has complied, and is in compliance on the Closing Date, with all applicable laws, statutes, orders, rules, regulations and requirements promulgated by governmental or other authorities, the failure of which would have a material adverse effect on AutovaxID’s business. AutovaxID has not received any notice of any alleged violation of any such statute, order, rule, regulation or requirement.
4. Breaches of Contracts; Required Consents. Neither the execution and delivery of this Agreement by AutovaxID, nor compliance by AutovaxID with the terms and provisions of this Agreement will:
(a) Conflict with or result in a breach of (i) any judgment, order, decree or ruling to which AutovaxID is a party, (ii) any injunction of any court or governmental authority to which AutovaxID is subject, (iii) any agreement, contract or commitment which is material to AutovaxID’s business, or (iv) any of the terms, conditions or provisions of the Articles of Incorporation, Bylaws or other governing instruments of AutovaxID; or
(b) Require the affirmative consent or approval of any third party, other than Laurus Master Fund, Ltd.
5. Organization; Binding Obligation. AutovaxID is a corporation duly organized, validly existing, and in good standing under the laws of the State of Florida. AutovaxID has all requisite power and authority to own properties and assets and to conduct business as it is presently conducted. This Agreement constitutes the legal, valid and binding obligation of Autovaxid in accordance with the terms hereof. AutovaxID has all requisite corporate power and authority, including the approval of its shareholders and Board of Directors, to execute, perform, carry out the provisions of and consummate the transactions contemplated in this Agreement.
6. Completeness of Disclosure. No representation in this Agreement contains any untrue statement of a material fact or omits to state any material fact the omission of which would be misleading.
9. Exclusion of Warranty.
BIOVEST MAKES NO WARRANTIES, EXPRESS OR IMPLIED, WITH RESPECT TO THE PURCHASED EQUIPMENT, AND HEREBY SPECIFICALLY DISCLAIMS ALL IMPLIED AND STATUTORY WARRANTIES, BY OPERATION OF LAW OR OTHERWISE, INCLUDING WITHOUT LIMITATION WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, AND NON-INFRINGEMENT OF A PATENT, TRADEMARK, OR OTHER INTELLECTUAL PROPERTY RIGHT. NO REPRESENTATION OR OTHER AFFIRMATION OF FACT, INCLUDING BUT NOT LIMITED TO STATEMENTS REGARDING CAPACITY,
- 4 -
SUITABILITY FOR USE OR PERFORMANCE OF THE CUSTOMIZED SYSTEM, WHETHER MADE BY BIOVEST EMPLOYEES OR OTHERWISE, WHICH IS NOT CONTAINED IN THIS AGREEMENT, SHALL BE DEEMED TO BE A WARRANTY BY BIOVEST FOR ANY PURPOSE OR GIVE RISE TO ANY LIABILITY OF BIOVEST WHATSOEVER.
10. Limitation of Liability.
AutovaxID acknowledges that the Equipment is sold “As is/Where is” and that in no event will Biovest retain any liability for the Purchased Equipment sold under the terms of this agreement. AutovaxID expressly acknowledges the Continuing Liens and the rights of the lienors. AutovaxID agrees to execute such subordination and other agreements as Biovest may reasonably request from time to time in order to effectuate the purposes of this Agreement.
11. Governing Law.
This Agreement shall be construed and enforced in accordance with the laws of the State of Delaware without regard for its provisions with respect to conflict of laws.
12. Jurisdiction
Any claim, suit, action or proceeding initiated by any Party (whether in contract, tort or otherwise) arising out of this Agreement or the relationship of the parties hereunder, shall be filed and prosecuted only in the state or federal courts in the State of Delaware, which the Parties agree shall have exclusive jurisdiction over such matters and the Parties agree to submit to the jurisdiction of such courts. Each of the Parties, to the extent permitted by applicable law, hereby waives and agrees not to assert, by way of motion, as a defense or otherwise, in any such suit, action or proceeding, any claim that it is not subject personally to the jurisdiction of the above-referenced courts, that its property is exempt or immune from attachment or execution, that the suit, action or proceeding is brought in an inconvenient forum, that the venue of the suit, action or proceeding is improper or that this Agreement or the subject matter hereof may not be enforced in or by such court. Each of the Parties hereby agrees that its submission to jurisdiction is made for the express benefit of the other Party hereto.
13. Termination
This Agreement may, by notice given prior to its performance, be terminated upon the earlier of (the “Termination Date”):
a. mutual consent of the Parties; or
b. notice to the other Party by either Party, if the Closing has not occurred (other than through the failure of such Party to comply with its obligations under this Agreement) by the close of business on December 20, 2006.
In the event of termination of this Agreement pursuant hereto, this Agreement shall forthwith become void and of no further force and effect and the parties hereto shall be released from any and all obligations hereunder.
14. Binding Effect.
This Agreement shall be binding upon the Parties hereto and their respective successors and assigns; provided, however, that neither this Agreement nor any Party’s rights or obligations hereunder may be assigned, or held in trust for a third-party, by any Party hereto without the prior written consent of the other Party, except that Biovest may assign or transfer this Agreement to any affiliate or subsidiary of Biovest.
- 5 -
15. Waiver of Conditions; Exhibits.
Any Party hereto may waive any condition provided in this Agreement for its benefit. All of the Exhibits attached to this Agreement are hereby incorporated herein and made a part hereof.
| 16. | Notices. |
Any notice, authorization, request or demand required or permitted to be given hereunder shall be in writing and shall be deemed to have been duly given on the earlier of (i) the date when received by the addressee, if sent by overnight courier or delivered personally, (ii) when confirmation is confirmed by the sender’s fax machine, if sent by facsimile, provided that a copy thereof is also contemporaneously sent to the addressee by a method described in clauses (i) or (iii) of this Section 16, or (iii) the fifth business day after the date when posted, if sent by registered or certified mail, in any such case to the respective addresses specified in the introductory paragraph of this Agreement.
Any Party may, by written notice delivered in accordance with this Section 16, change the address to which any notices, authorizations, requests or demands are to be delivered hereunder.
17. Counterparts.
This Agreement may be executed in two or more counterparts, each of which shall be binding as of the date first written above. Each such copy shall be deemed an original, and it shall not be necessary in making proof of this Agreement to produce or account for more than one such counterpart.
18. Amendment.
This Agreement may be amended at any time pursuant to a written instrument signed by the Parties.
19. Interpretation.
Unless the context of this Agreement clearly requires otherwise, (a) references to the plural include the singular, the singular the plural, the part the whole, (b) references to any gender include all genders, (c) “including” has the inclusive meaning frequently identified with the phrase “but not limited to,” (d) the word “or” shall be deemed to be inclusive, and (e) references to “hereunder” or “herein” relate to this Agreement. The section and other headings contained in this Agreement are for reference purposes only and shall not control or affect the construction of this Agreement or the interpretation thereof in any respect. Section, subsection, and Exhibit references are to this Agreement unless otherwise specified. Capitalized terms set forth in the Exhibits attached hereto shall have the same meanings as set forth in this Agreement, unless defined otherwise in such Exhibit.
20. Construction.
The Parties agree and acknowledge that they have jointly participated in the negotiation and drafting of this Agreement. In the event of an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties and no presumptions or burdens of proof shall arise favoring any Party by virtue of the authorship of any of the provisions of this Agreement.
21. No Third Party Rights.
Nothing in this Agreement shall be deemed to create any right on the part of any person or entity not a party to this Agreement.
22. Waiver of Jury Trial.
The Parties specifically waive any right to trial by jury in any court with respect to any contractual, tortious or statutory claim, counterclaim or cross-claim against the other arising out of or
- 6 -
connected in any way to this Agreement because the parties hereto, both of whom are represented by counsel, believe that the complete commercial aspect of their dealing with one another make a jury determination neither desirable nor appropriate.
[THIS SPACE INTENTIONALLY LEFT BLANK]
- 7 -
IN WITNESS WHEREOF, the parties hereto have duly executed this Agreement as of the date first above written.
| AutovaxID, Inc. | ||
| By: | /s/ Xxxxxx Xxxxxxx | |
| Name: | Xxxxxx Xxxxxxx, M.D. | |
| Title: | Chairman & CEO | |
| Hereunto duly authorized | ||
| Biovest International, Inc. | ||
| By: | /s/ Xxxxxx Xxxxxxx | |
| Name: | Xxxxxx Xxxxxxx, M.D. | |
| Title: | Chairman & CEO | |
| Hereunto duly authorized | ||
Signature Page to License and Asset Purchase Agreement
EXHIBIT A

The AutovaxID - A breakthrough technology in the production of biologics for the Biotechnology industry
The AutovaxID is a self-contained automated cell growth instrument.
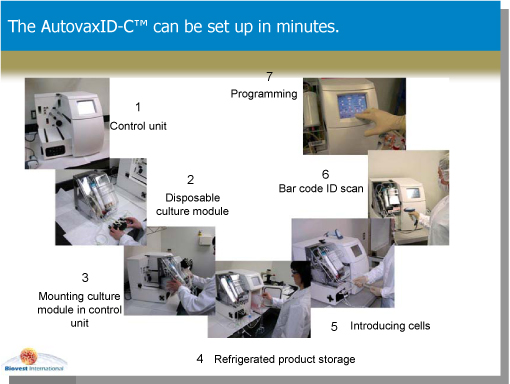
| • | Two component design - a base or control module (1) – (numbers refer to photos in figure below)- and a disposable culture unit (2). |
| • | Functionally replaces conventional cell growth xxxxxxxx that would take up over ten times the space. |
The AutovaxID is a first-of-its kind automated modular cell growth instrument intended for research, biotechnology and pharmaceutical applications. The instrument is ideally suited for those seeking to produce any cell product including monoclonal antibodies, as well as those seeking to grow and expand a wide variety of cells. The instrument results in dramatic savings in space, manpower and consumable cost compared to conventional cell culture approaches.
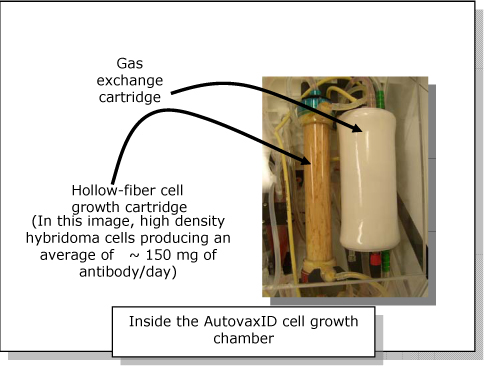
The AutovaxID’s base, or control module, contains the unit’s mechanics and electronics. The disposable culture unit is a single -use disposable element containing a hollow-fiber cell growth chamber and a gas exchange cartridge. The culture unit is an integrated sealed module that snaps easily (3) into the base unit housing. Once the starter cells are introduced into the unit through an access port (4), the unit is sealed and the cells expand and grow in a temperature and CO2-regulated environment optimized for their specific needs. Cells expand in number within the growth chamber and grow for as long as required—days, weeks or longer. During this time little or no operator intervention is required.
In conventional cell culture systems in which cells are grown in flasks or bottles, the cells have to be diluted one or more times per week, and fed fresh growth medium. These manipulations are labor intensive and must be done by trained technicians. If the cells or their products are to be used for therapeutic purposes, these manipulations must done in costly clean-room facilities with sophisticated air handling systems. By contrast, cells in an AutovaxID require no splitting and are fed automatically with fresh medium. Because the cell growth units are sterile and sealed from the environment, the units can be housed in relatively inexpensive laboratory facilities.
The AutovaxID brings sophisticated hollow fiber technology within the reach of every laboratory
Each AutovaxID culture unit functionally replaces approximately 100 conventional T flasks, or a smaller number of roller bottles. This is made possible by the high surface area of the hollow fiber cartridge in which cells are grown. Hollow fiber cell culture is a proven technology for growing cells at high density in a compact space with reduced costs. Hollow fiber technology dramatically reduces the need for costly growth factors and media supplements and results in secreted product concentrations up to ten times higher than can be achieved with conventional cell culture techniques.
As an example, a typical 30 day growth run of hybridoma cells in an AutovaxID can yield up to 1 gram of monoclonal antibody in approximately 1-2 litres, 10-30 times more concentrated than could be achieved with conventional cell culture approaches. During the growth period in the AutovaxID the antibody- rich medium is continually collected in a sealed container stored a refrigerated compartment (4) integrated into the AutovaxID base unit. Typically, at the end of the run, the cell growth chamber is discarded and the product-rich medium is retained. For other applications, the cells also could be harvested and used for other purposes.
- 10 -
The National Cell Culture Center has utilized Biovest’s proprietary hollow fiber cartridge technology for the production of hundreds of cell lines for NIH sponsored researchers over the past 15 years.
The AutovaxID is ideal for laboratories that need to produce gram amounts of cell products such as
monoclonal antibodies.
A lab developing and testing many different antibodies could operate dozens of AutovaxIDs simultaneously, each growing a different cell line. All of this could be done in a modest sized room monitored by one or two (compared to 10-12) technicians, saving time and keeping costs at a minimum.
For ease of record keeping the AutovaxID contains an on-board computer that stores all of the operational parameters of each production run. Each run can be uniquely associated with the disposable growth chamber used via the integrated bar code scanner and unique bar code associated with each culture module (6). Finally, because different cell lines may require different growth conditions, the AutovaxID is fully user programmable. Frequently used programs can be stored in the system memory after being entered through the touch-screen display (7).
The AutovaxID can also be used in applications in which the cells which are grown in the unit are collected instead of or in addition to any materials produced by the cells. Applications for the AutovaxID operated in this way include cell expansion for research or therapeutic purposes.
Advantages of the AutovaxID over conventional cell culture methods
| Process/Parameter |
Manual Cell Culture |
AutovaxID | ||
| 1. Product concentration | Variable, no ability to regulate. Typically 100mg in 1-10 L | Controlled by medium flow rate through hollow fiber reactor. Typically greater than 100mg in 1L | ||
| 2. Culture-xxxx | Multiple manual transfers using large numbers of disposable units (flasks, etc) | Single integrated disposable culture unit. No transfers needed during production period | ||
| 3. Growth conditions | Change over time as nutrients are depleted and waste products accumulate | Maintained constant over time through automated feedback loop. | ||
| 4. Contamination | Possible at multiple steps | Virtually eliminated by sealed sterile culture-xxxx | ||
| 5. Culture Oxygenation | Achieved via passive diffusion or sparging. May limit O2 availability and damage cells. | Continuously regulated via gas-exchange cartridge in media recirculation loop. | ||
| 6. Batch record generation and documentation | Data must be recorded manually at intervals | In-process parameters automatically recorded and stored for record generation. | ||
| 7. Product harvest | Manual harvest in batch mode, contaminated by cells and debris | Continuous automated harvest of filtered material | ||
| 8. Process monitoring | Requires multiple monitoring and in-process measurement steps | Automated process monitoring with alarm alerts for out-of specification conditions | ||
| 9. Scalability | Increasing production requires increasing space and manpower | Because of automation and small footprint, production can typically be scaled up in existing space with minimal additional manpower | ||
- 11 -

For additional information about the AutovaxID contact:
Xxxxx Xxxxxxx
General Manager
Biovest Advanced Instrumentation Division
Biovest International
000 Xxxxxxxxxx Xx.
Xxxxxxxxx, XX 00000
000 000 0000 x 000
xxxxxxxx@xxxxxxx.xxx
The AutovaxID: Changing the world of cell culture
• Automated cell growth instrument
• Sterile, disposable cell growth chamber
• GMP-compliant
• Rapid set-up
• Touch-screen programming
• Automated batch record generation
• Small foot-print
- 12 -
EXHIBIT B
LICENSE AGREEMENT
[Attached]
- 13 -
EXHIBIT C
PURCHASED EQUIPMENT
| AutovaxID Manufacturing Assets |
8/20/2006 | |
| AutovaxID Instrument |
||
| Item # | Item Subtotal | |||||||||
| 1 | Assemby Fixtures | |||||||||
| 900022-000 | FIXTURE* LCD ALIGNMENT | 1710 | ||||||||
| 900023-000 | FIXTURE* STALL SENSOR ALIGNMENT, CCI | 1260 | ||||||||
| 900028-000 | FIXTURE* .068 INCH SPACER | 100 | ||||||||
| 900029-000 | FIXTURE8ROTARY VALVE TORQUE | 1000 | ||||||||
| 900032-000 | FIXTURE*GASSING MANIFOLD | 2320 | ||||||||
| 900033-000 | FIXTURE*INSTR/CW INTERFACE | 6300 | ||||||||
| 900035-000 | READER/WRITER, CARD, COMPO BNC (F) | 300 | ||||||||
| 900052-000 | FIXTURE*ASSY,TUBING,GASSING, CCI | 300 | ||||||||
| 900061-000 | AY* GREASE GUN, MODIFIED | 1000 | ||||||||
| 900062-000 | FIXTURE* PUMP DRIVE | 1000 | ||||||||
| 900065-000 | FIXTURE* ALIGNMENT, TRANSDUCER PIN | 1500 | ||||||||
| 900066-000 | FIXTURE*FLOW SENSOR, LIQUID | 2000 | ||||||||
| 900067-000 | FIXTURE* PUMP, BELT TENSION | 3620 | ||||||||
| 900068-000 | FIXTURE*POSITION ENCODER TEST | 3240 | ||||||||
| 900069-000 | FIXTURE* UV LIGHT GUIDE | 1960 | ||||||||
| 900070-000 | FIXTURE* CABLE, MINI DOUBLE BANANA TO US STANDARD | 270 | ||||||||
| 27880 |
| Item # | Item Subtotal | |||||||||
| 2 | Test Fixtures | |||||||||
| 900036-000 | FIXTURE*PUMP CARTRIDGE W/NEW TUBING,CCI | 300 | ||||||||
| 900038-000 | FIXTURE*ADAPTER PLUG, PH | 300 | ||||||||
| 900039-000 | FIXTURE*SENSOR, CO2 WITH READOUT | 1000 | ||||||||
| 900040-000 | FIXTURE*BOARD PROGRAMMING INTERFACE | 2000 | ||||||||
| 900041-001 | FIXTURE*TEMP. CAL. RES. 64.6 C, CCI | 300 | ||||||||
| 900041-002 | FIXTURE*TEMP. CAL. RES. 44.6 C, CCI | 300 | ||||||||
| 900041-003 | FIXTURE*TEMP. CAL. RES. 37.0 C, CCI | 300 | ||||||||
| 900041-004 | FIXTURE*TEMP. CAL. RES. 25.0 C, CCI | 300 | ||||||||
| 900041-005 | FIXTURE*TEMP. CAL. RES. 4.9 C, CCI | 300 | ||||||||
| 900041-006 | FIXTURE*TEMP. CAL. RES. - 5.0 C, CCI | 300 | ||||||||
| 900042-000 | FIXTURE*CABLE, BOARD POWER, CCI | 300 | ||||||||
| 900043-000 | FIXTURE*CABLE, BOARD REFRIG POWER, CCI | 200 | ||||||||
| 900044-000 | FIXTURE*HEATER & FAN INDICATOR, CCI | 200 | ||||||||
| 900045-000 | FIXTURE*CIRCULATION MOTOR W/BRACKET, CCI | 200 | ||||||||
| 900046-000 | FIXTURE*VALVE MOTOR, CCI | 1000 | ||||||||
| 900047-000 | FIXTURE*PINCH CLAMP INDICATOR, CCI | 500 |
C-2
| Item # | Item Subtotal | |||||||||
| 900048-000 | FIXTURE*SMB FEMALE TO MULTIMETER | 200 | ||||||||
| 900049-000 | FIXTURE*TEST LED W/RESISTOR, CCI | 200 | ||||||||
| 900050-000 | FIXTURE*ADAPTER SMB (M) TO BNC (F) | 200 | ||||||||
| 900053-000 | FIXTURE*4-MOTOR TEST, CCI | 2000 | ||||||||
| 900054-000 | FIXTURE* PUMP TORQUE | 500 | ||||||||
| 900056-000 | FIXTURE*ETHERNET HUB | 200 | ||||||||
| 900057-000 | FIXTURE*KEYBOARD, PS2 | 200 | ||||||||
| 900058-000 | FIXTURE*CABLE, ETHERNET | 200 | ||||||||
| 900064-001 | AY*JUMPER,GASSING | 500 | ||||||||
| 900064-002 | AY*JUMPER,GASSING | 500 | ||||||||
| 900064-003 | AY*JUMPER,GASSING | 500 | ||||||||
| 900071-000 | FIXTURE*MODE PROGRAMMER,CO2 SENSOR | 300 | ||||||||
| 13300 | ||||||||||
| 3 | Software | |||||||||
| Revision “PreA-1 thru PreA-47” | 9270 hours X $60/hr burdened | 556200 | ||||||||
| 4 | Manufacturing Documentation | |||||||||
| 700371-000 | MANUAL*OPERATIONS,AUTOVAX | 1200 | ||||||||
| 700386-000 | SOP*PREPOWER OF AUTOVAXID-C INSTRUMENT | 1200 | ||||||||
| 700387-000 | SOP*POWER-UP OF AUTOVAXID-C INSTRUMENT | 1200 | ||||||||
| 700388-000 | FORM*MASTER LOG AUTOVAXID-C INSTRUMENT | 1200 | ||||||||
| 700389-000 | SOP*AUTOVAXID-C INST 72-HOUR BURN-IN OUR BURN-IN | 1200 | ||||||||
C-3
| Item # | Item Subtotal | |||||||||
| 700390-000 | SOP*CHASSIS CONTROL BOARD, CCI | 1200 | ||||||||
| 000000-000 | SOP*VALVE DRIVER BOARD, CCI | 1200 | ||||||||
| 000000-000 | SOP*4-POSITION PUMP BOARD, CCI | 1200 | ||||||||
| 700393-000 | SOP*GASSING MANIFOLD BOARD, CCI | 1200 | ||||||||
| 700394-000 | SOP*GASSING MANIFOLD W/PCB, CCI | 1200 | ||||||||
| 700395-000 | SOP* GASSING CHASSIS ASSEMBLY | 1200 | ||||||||
| 700396-000 | SOP* LOADING S/W IN PHILIPS 80C31 MICROCONTROLLER | 1200 | ||||||||
| 700398-000 | SOP*AUTOVAXID-C INST FINAL OPERATIONS INSPECTION-C,MANUFACTURING | 1200 | ||||||||
| 700401-000 | SOP* GASSING MANIFOLD INSPECTION | 1200 | ||||||||
| 700402-000 | SOP* PUMP MODULE ASSEMBLY | 1200 | ||||||||
| 700403-000 | SOP*LIQUID FLOW SENSOR, INSPECTION | 1200 | ||||||||
| 700406-000 | SOP*TEST PROCEDURE,POSITION ENCODER | 1200 | ||||||||
| 700407-000 | SOP* PUMP TUBING STOP INSPECTION AND BOND TEST | 1200 | ||||||||
| 700409-000 | SOP*TEST PROCEDURE,CO2 SENSOR | 1200 | ||||||||
| 700411-000 | FORM* REPAIR/UPGRADE LOG | 1200 | ||||||||
| 700412-000 | SOP* REPAIR/UPGRADE RECORD KEEPING | 1200 | ||||||||
| 700413-000 | SOP*WIRE ROUTING, AUTOVAXID-C INSTRUMENT ID-C INSTRUMENT | 1200 | ||||||||
| 26400 |
C-4
| Item # | Item Subtotal | |||||||||
| 5 | ComponentAssembly Dwg&Specifications | |||||||||
| 6 | Tooling | |||||||||
| Cabinet | 140970 | |||||||||
| AutovaxID Cultureware and Factor Bag Assemby | ||||||||||
| 7 | Assemby Fixtures | |||||||||
| 900027-000 | FIXTURE* AY, BACK PANEL, | 9700 | ||||||||
| 900060-000 | FIXTURE*AUTOVAXID C/W | 3000 | ||||||||
| 900063-000 | FIXTURE*C/W, FLOW SENSOR, O-RING | 2600 | ||||||||
| 15300 | ||||||||||
| 8 | Test Fixtures | |||||||||
| 900051-000 | FIXTURE*PRESSURE TEST, AUTOVAXID-C, CULTUREWARE | 3000 | ||||||||
| 900055-000 | FIXTURE* PRESSURE TEST, AUTOVAXID-C | 2000 | ||||||||
| 5000 | ||||||||||
| 9 | Manufacturing Documentation | |||||||||
| 700397-000 | SOP* ADHESIVE BONDING | 1200 | ||||||||
| 700399-000 | SOP* TEST, PRESSURE, AUTOVAXID-C CULTUREWARE | 1200 | ||||||||
| 700408-000 | SOP* pH ELECTRODE | 1200 | ||||||||
| 3600 | ||||||||||
| 10 | Tooling | |||||||||
| Cultureware Cover | 10850 | |||||||||
| Cultureware Backpanel | 63800 | |||||||||
| Pump Cassette -to date | 32800 | |||||||||
| 107450 | ||||||||||
| Total | 896100 | |||||||||
C-5
EXHIBIT D
CONTINUING LIENS
Lien on all assets held by Laurus Master Fund, Ltd.
EXHIBIT E
LEASE AGREEMENT
[Attached]
EXHIBIT F
BUSINESS PLAN
(attached)
EXHIBIT G
VALUATION
[Attached]

