LICENSE AND COLLABORATION AGREEMENT BETWEEN VISTAGEN THERAPEUTICS, INC. AND EVERINSIGHT THERAPEUTICS INC.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
EXHIBIT
10.2
BETWEEN
AND
EVERINSIGHT THERAPEUTICS INC.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-1-
This
LICENSE AND COLLABORATION AGREEMENT (this “Agreement”)
is made as of June 24, 2020 (“Effective Date”), by and
among VistaGen Therapeutics, Inc., a company organized under the
laws Nevada (“VistaGen”), and having an Affiliate of
the same name, and
EverInsight Therapeutics Inc., a company
incorporated under the laws of the British Virgin Islands
(“EverInsight”) and having a registered address at
Vistra Corporate Services Centre, Wickhams Cay II, Road Town,
Tortola, VG1110, British Virgin Islands. VistaGen and EverInsight
are referred to individually as a “Party” and
collectively as the “Parties.”
RECITALS
WHEREAS,
VistaGen owns or controls certain intellectual property and
associated data and materials relating to a pharmaceutical compound
known as PH94B, which is an intranasal synthetic neuroactive
steroid product being developed for the treatment of social anxiety
disorder and other anxiety-related disorders;
WHEREAS,
VistaGen wishes to grant a license to EverInsight, and EverInsight
wishes to take a license, under such intellectual property and
associated items to develop, manufacture and commercialize PH94B in
certain territories in accordance with the terms and conditions set
forth below;
NOW,
THEREFORE, in consideration of the foregoing premises and the
mutual covenants herein contained, the receipt and sufficiency
which are hereby acknowledged, the Parties hereby agree as
follows.
ARTICLE 1 DEFINITIONS
Unless
the context otherwise requires, the terms in this Agreement with
initial letters capitalized, shall have the meanings set forth
below, or the meaning as designated in the indicated places
throughout this Agreement.
1.1
“Active Pharmaceutical Ingredient”
or “API” means
any substance intended to be used in a pharmaceutical product that
when used becomes an active ingredient of that product intended to
exert a pharmacological, immunological or metabolic action with a
view to restoring, correcting or modifying physiological functions
in man or animal; but excluding formulation components such as
coatings, stabilizers, excipients or solvents, adjuvants or
controlled release technologies.
1.2
“Affiliate” means, with respect to
a Party, any Person that, directly or indirectly through one or
more intermediaries, controls, is controlled by, or is under common
control with that Party, but for only so long as such control
exists. For the purpose of this definition, “control”
(including, with correlative meaning, the terms “controlled
by” and “under common control”) means (a) to
possess, directly or indirectly, the power to direct the management
or policies of an entity, whether through ownership of voting
securities, by contract relating to voting rights or corporate
governance, or otherwise; or (b) direct or indirect beneficial
ownership of more than fifty percent (50%), or such lesser
percentage which is the maximum allowed to be owned by a foreign
corporation in a particular jurisdiction, of the voting share
capital or other equity interest in such entity; provided however
that, notwithstanding the foregoing, EverInsight’s Affiliates
shall not include CBC Group or any of its portfolio
companies.
1.3
“Applicable Laws” means the
applicable provisions of any and all national, supranational,
regional, federal, state and local laws, treaties, statutes, rules,
regulations, administrative codes, guidance, ordinances, judgments,
decrees, directives, injunctions, orders, permits (including XXXx)
of or from any court, arbitrator, Regulatory Authority or
Government Authority having jurisdiction over or related to the
subject item, including the FFDCA, DAL, and the Provisions for Drug
Registration of NMPA.
1.4
“Auditor” has the meaning set forth
in Section 8.10 (Audit Dispute).
1.5
“Business Day” means a day other
than a Saturday, Sunday or a bank or other public holiday in
Mainland China, Hong Kong or the State of California in the United
States.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-2-
1.6
“Calendar Quarter” means each
respective period of three (3) consecutive months ending on 31
March, 30 June, 30 September, and 31 December, except that the
first Calendar Quarter of the Term shall commence on the Effective
Date and end on the day immediately prior to the first 1 January, 1
April, 1 July or 1 October to occur after the Effective Date, and
the last Calendar Quarter shall end on the last day of the
Term.
1.7
“Calendar Year” means each
successive period of 12 calendar months commencing on 1 January and
ending on 31 December except that the first Calendar Year of the
Term shall commence on the Effective Date and end on 31 December of
the year in which the Effective Date occurs and the last Calendar
Year of the Term shall commence on 1 January of the year in which
the Term ends and end on the last day of the Term.
1.8
“CFR” means the U.S. Code of
Federal Regulations.
1.9
“Challenge” means to contest or
assist, directly or indirectly, in the contesting of the validity
or enforceability of any of the VistaGen Patents or EverInsight
Patents (as applicable), in whole or in part, in any court,
arbitration proceeding or other tribunal, including the United
States Patent and Trademark Office and the United States
International Trade Commission. For the avoidance of doubt, the
term “contest” includes: (a) filing an action under 28
U.S.C. §§ 2201-2202 seeking a declaration of invalidity
or unenforceability of any such Patents; (b) citation to the United
States Patent and Trademark Office pursuant to 35 U.S.C. § 301
of prior art patents or printed publications or statements of the
patent owner concerning the scope of any such Patents; (c) filing a
request under 35 U.S.C. § 302 for re-examination of any such
Patents; (d) filing, or joining in, a petition under 35 U.S.C.
§ 311 to institute inter parties review of any such Patents or
any portion thereof; (e) filing, or joining in, a petition under 35
U.S.C. § 321 to institute post-grant review of such Patents or
any portion thereof; (f) provoking or becoming a party to an
interference or a derivation proceeding with an application for any
such Patents pursuant to 35 U.S.C. § 135; (g) filing or
commencing any re-examination, opposition, cancellation, nullity or
similar proceedings against any such Patents in any country; or (h)
any foreign equivalents of subsection (a) through (g) applicable in
the Territory; provided however, notwithstanding the foregoing,
“Challenge” shall not include (i) any action taken
by a Party in response to an action by the other Party to enforce
such Patents against such Party, or (ii) any argument made by
a Party in the course of patent prosecution that distinguish the
inventions claimed in such Party’s Patents from those
inventions claimed in the other Party’s Patent.
1.10
“Claims” means all Third Party
demands, claims, actions, proceedings and liabilities (whether
criminal or civil, in contract, tort or otherwise) for losses,
damages, legal costs and other expenses of any nature.
1.11
“CMC” means chemistry,
manufacturing, and controls.
1.12
“Combination Product” means any
Licensed Product comprised of the following, either formulated
together (i.e., a fixed
dose combination), packaged together and sold for a single price,
or co-administered or jointly provided to patients, whether or not
packaged together: (a) the Compound, and (b) at least one other
API.
1.13
“Commercialization” means the
conduct of all activities undertaken before and after Regulatory
Approval has been obtained relating to the promotion, marketing,
sale and distribution (including importing, exporting, transporting
for commercial sales, customs clearance, warehousing, invoicing,
handling and delivering the Licensed Product to customers) of the
Compound or the Licensed Product, including: (a) sales force
efforts, detailing, advertising, medical education, planning,
marketing, sales force training, and sales and distribution; and
(b) scientific and medical affairs. For clarity, Commercialization
does not include any Development activities, whether conducted
before or after Regulatory Approval. “Commercialize”
and “Commercializing” have correlative
meanings.
1.14
“Commercialization Plan” has the
meaning set forth in Section 7.2 (Commercialization
Plan).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-3-
1.15
“Commercially Reasonable Efforts”
means, with respect to each Party’s obligations under this
Agreement relating to the Development, Manufacturing, and
Commercialization activities with respect to the Compound or the
Licensed Product, the carrying out of such activities using efforts
and resources that are consistent with the exercise of customary
scientific and business practices as applied in the
biopharmaceutical industry for a company of a similar stage and
size as the entity and having similar resources, for development,
regulatory, manufacturing and commercialization activities
conducted with respect to products at a similar stage of
development or commercialization and having similar commercial
potential, taking into account relative safety and efficacy,
product profile, the regulatory environment, payers’ policies
and regulations, competitiveness of the marketplace and the market
potential of such products, the nature and extent of market
exclusivity, including patent coverage and regulatory data
protection, and price and reimbursement status. The Parties hereby
agree that the level of effort may be different for different
markets and may change over time, reflecting changes in the status
of the aforementioned attributes and potential of the Compound and
the Licensed Product. When used regarding obligations under this
Agreement other than the Development, Manufacturing, and
Commercialization activities with respect to the Compound or the
Licensed Product, the term “Commercially Reasonable
Efforts” shall mean the carrying out of such activities using
commercially reasonable efforts and financial, personnel and other
resources that are consistent with the exercise of customary
business practices as applied in the carrying out of such
activities generally by and on behalf of biopharmaceutical
companies of a similar stage and size and having similar
resources.
1.16
“Compound” means PH94B, and all
salt, free acid/base, solvate, hydrate, prodrug, metabolite,
stereoisomer, and enantiomer thereof, and polymorphic forms
thereof.
1.17
“Confidential Information” of a
Party means all Know-How, Inventions, unpublished patent
applications and other information and data of a financial,
commercial, business, operational or technical nature of such Party
that is disclosed or made available by or on behalf of such Party
or any of its Affiliates to the other Party or any of its
Affiliates, whether made available orally, in writing or in
electronic or other form. The terms of this Agreement are the
Confidential Information of both Parties.
1.18
“Control” or “Controlled” means, with respect to
any Know-How, Patents, Regulatory Documentation or other
intellectual property rights, that a Party has the legal authority
or right (whether by ownership, license or otherwise, other than by
virtue of any license granted to such Party by the other Party
pursuant to this Agreement) to grant a license, sublicense, access
or other right (as applicable) under such Know-How, Patents,
Regulatory Documentation or other intellectual property rights to
the other Party on the terms and conditions set forth herein, in
each case without breaching the terms of any agreement with a Third
Party, infringing third party intellectual property, or
misappropriating third party trade secrets.
1.19
“Controlling Party” has the meaning
set forth in Section 9.6 (Invalidity or Unenforceability Defenses
or Actions).
1.20
“Corporate Names” has the meaning
set forth in Section 1.81 (Licensed Trademarks).
1.21
“Cost of Goods” means, with respect
to any Compound or any Licensed Product, [*****].
1.22
“CTA” means a Clinical Trial
Application that is required to initiate a clinical trial for
registering a drug product under the Drug Administration Law of the
People’s Republic of China and the Provisions for Drug
Registration of NMPA, and equivalents thereof under future Chinese
laws and regulations, and the laws and regulations of other
countries and jurisdictions in the Territory, in each as the same
may be amended from time to time.
1.23
“DAL” means the Drug Administration
Law of the People’s Republic of China and the equivalent laws
of other countries and jurisdictions in the Territory, in each as
the same may be amended from time to time.
1.24
“Develop” or “Development” means to develop
(including clinical, non-clinical and CMC development), analyze,
test and conduct preclinical, clinical and all other regulatory
trials for the Compound or Licensed Product, including all
post-approval clinical trials, as well as all related regulatory
activities and any and all activities pertaining to new
Indications, pharmacokinetic studies and all related activities
including work on new formulations, new methods of treatment and
CMC activities including new manufacturing methods.
“Developing” and “Development” have
correlative meanings.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-4-
1.25
“Development Plan” has the meaning
set forth in Section 4.2 (Development Plan).
1.26
“Disclosing Party” has the meaning
set forth in Section 10.1(a) (Duty of Confidence - subsection
(a)).
1.27
“Dispute” has the meaning set forth
in Section 14.10(a) (Dispute Resolution - subsection
(a)).
1.28
“Dollars” means U.S. dollars, and
“$” shall be interpreted accordingly.
1.29
“EverInsight Development Data”
means any non-clinical or clinical data that are generated by
EverInsight through the Development, Manufacture and
Commercialization of the Compound and Licensed Product under this
Agreement, Controlled by EverInsight, and related to the Compound
or any Licensed Product or otherwise included in, or filed in
support of, the Regulatory Documentation filed by EverInsight, its
Affiliates or Sublicensees in the Territory.
1.30
“EverInsight Know-How” means all
Know-How that is generated by EverInsight through the Development,
Manufacture and Commercialization of the Compound and Licensed
Product under this Agreement, Controlled by EverInsight as of the
Effective Date or during the Term, and necessary or reasonably
useful for the Development, Manufacture, Commercialization or other
Exploitation of any Compound or Licensed Product in the Licensed
Field, including EverInsight Sole Inventions, EverInsight’s
interest in any Joint Inventions, EverInsight Development Data and
EverInsight’s Regulatory Documentation.
1.31
“EverInsight Indemnitees” has the
meaning set forth in Section 13.1 (Indemnification by
VistaGen).
1.32
“EverInsight Patents” means
EverInsight Sole Invention Patents and EverInsight’s interest
in the Joint Patents, in each case necessary or reasonably useful
for the Development, Manufacture, Commercialization, or other
Exploitation of the Compound or any Licensed Product for use in the
Licensed Field.
1.33
“EverInsight Sole Inventions” means
any Inventions that are conceived and reduced to practice solely by
employees of, or consultants or service providers to, EverInsight
and its Affiliates, at any time during the Term of this
Agreement.
1.34
“EverInsight Sole Invention
Patents” means any Patents that contain one or more
claims that cover EverInsight Sole Inventions.
1.35
“EverInsight Technology” means the
EverInsight Patents and the EverInsight Know-How.
1.36
“Excluded Claim” has the meaning
set forth in Section 14.10(g) (Dispute Resolution - subsection
(g)).
1.37
“Executive Officers” has the
meaning set forth in Section 3.3(a) (JSC Decision Making -
subsection (a)).
1.38
“Exploit” means to make, have made,
import, use, sell or offer for sale, including to research,
Develop, Commercialize, register, Manufacture, have Manufactured,
hold or keep (whether for disposal or otherwise), have used,
export, transport, distribute, promote, market or have sold or
otherwise dispose of.
1.39
“Exploitation” means the act of
Exploiting the Compound, product or process.
1.40
“FDA” means the United States Food
and Drug Administration or any successor entity
thereto.
1.41
“FFDCA” means the United States
Federal Food, Drug, and Cosmetic Act, as amended from time to time,
together with any rules, regulations and requirements promulgated
thereunder (including all additions, supplements, extensions and
modifications thereto).
1.42
“First Commercial Sale” means, with
respect to any Licensed Product in any jurisdiction in the
Territory, the first arm’s length sale of such Licensed
Product by EverInsight, its Affiliates or Sublicensees to a Third
Party for monetary value for use or consumption of such Licensed
Product by the end user in the general public after Regulatory
Approval for such Licensed Product in such jurisdiction has been
granted. Sales prior to receipt of Regulatory Approval for such
Licensed Product, such as so-called “treatment IND
sales,” “named patient sales,” and
“compassionate use sales,” shall not be construed as a
First Commercial Sale.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-5-
1.43
“GAAP” means the then-current
Generally Accepted Accounting Principles or International Financial
Reporting Standards (IFRS), whichever is adopted as the standard
financial accounting guideline in the United States for public
companies, as consistently applied.
1.44
“Generic Competition” means
[*****].
1.45
“Generic Product” means, with
respect to a Licensed Product, any product that contains the same
Compound as such Licensed Product and that is sold under an
approved Marketing Authorization Application granted by a
Regulatory Authority to a Third Party that is not a Sublicensee of
EverInsight or its Affiliates and did not obtain such product in a
chain of distribution that includes any of EverInsight, its
Affiliates, or its Sublicensees.
1.46
“Good Manufacturing Practices” or
“GMP” shall mean
all applicable Good Manufacturing Practices standards, including,
as applicable, those standards required by any Regulatory Authority
in the Territory.
1.47
“Government Authority” means any
federal, state, national, state, provincial or local government, or
political subdivision thereof, or any multinational organization or
any authority, agency or commission entitled to exercise any
administrative, executive, judicial, legislative, police,
regulatory or taxing authority or power, any court or tribunal (or
any department, bureau or division thereof, or any governmental
arbitrator or arbitral body).
1.49
“IND” means a CTA or any other
investigational new drug application, clinical trial application,
clinical trial exemption or similar or equivalent application or
submission for approval to conduct human clinical investigation
filed with or submitted to the Regulatory Authority in the relevant
jurisdiction in conformance with the requirements of such
Regulatory Authority, including the FDA in the US and NMPA in
Mainland China.
1.50
“Indemnification Claim Notice” has
the meaning set forth in Section 13.3(a) (Notice of
Claim).
1.51
“Indemnified Party” has the meaning
set forth in Section 13.3(a) (Notice of Claim).
1.52
“Indemnifying Party” has the
meaning set forth in Section 13.3(a) (Notice of
Claim).
1.53
“Indication” means a separate and
distinct disease, disorder, illness or health condition for which a
separate MAA approval is required.
1.54
“Indirect Costs” means, with
respect to a multi-regional clinical trial, all Third Party costs
and expenses incurred by VistaGen or EverInsight to conduct such
multi-regional clinical trial that are not directly allocable to a
Party’s territory (or to clinical sites within a
Party’s territory), including, without limitation, fees,
costs and expenses for data management, clinical evaluation
committees, data safety monitoring boards, physician consulting,
investigator meetings, travel, document translation and other
technology solutions and services that are not specific to a
territory or a clinical site within a territory.
1.55
“Initiation” means, with respect to
a clinical trial, the first dosing (whether with investigational
drug, comparator drug or placebo) of the first subject in such
clinical trial.
1.56
“Initial Supply Agreement” has the
meaning set forth in Section 6.3 (Supply Agreement).
1.57
“In-License Agreement” has the
meaning set forth in Section 2.4(b) (In-License
Agreements).
1.58
“Invention” means any technical,
scientific and other know-how and information, trade secrets,
knowledge, technology, means, methods, processes, practices,
formulae, instructions, skills, techniques, procedures,
experiences, ideas, technical assistance, designs, drawings,
assembly procedures, computer programs, apparatuses,
specifications, data, results and other material, including:
biological, chemical, pharmacological, toxicological,
pharmaceutical, physical and analytical, pre-clinical, clinical,
safety, manufacturing and quality control data and information,
including study designs and protocols, assays and biological
methodology process, composition of matter, article of manufacture,
discovery or finding, that is or may be patentable, that is made,
generated, conceived or otherwise invented as a result of a Party
exercising its rights or carrying out its obligations under this
Agreement, whether directly or via its Affiliates, agents or
independent contractors, including all rights, title and interest
in and to the intellectual property rights therein. For clarity,
“Invention” does not include VistaGen Development Data
or EverInsight Development Data.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-6-
1.59
“Joint Steering Committee” or
“JSC” has the
meaning set forth in Section 3.1 (Joint Steering
Committee).
1.60
“Joint Inventions” means any
Inventions that are conceived and reduced to practice by employees
of, or consultants or service providers to, VistaGen or its
Affiliates, on the one hand, jointly with employees of, or
consultants or service providers to, EverInsight or its Affiliates,
on the other hand, at any time during the Term of this Agreement
and that are made, generated, conceived or otherwise invented as a
result of VistaGen and EverInsight exercising their rights or
carrying out their obligations under this Agreement, whether
directly or via their Affiliates, agents or independent
contractors.
1.61
“Joint Patents” means any Patents
that contain one or more claims that cover Joint
Inventions.
1.62
“Know-How” means any information,
including discoveries, improvements, modifications, processes,
methods, techniques, protocols, formulas, data, inventions,
know-how, trade secrets and results, patentable or otherwise,
including physical, chemical, biological, toxicological,
pharmacological, safety, and preclinical and clinical data, dosage
regimens, control assays, and product specifications, but excluding
any Patents.
1.63
“Licensed Field” means all uses in
humans.
1.64
“Licensed Know-How” means all
Know-How that VistaGen (or its Affiliates) Controls as of the
Effective Date or during the Term that is necessary or reasonably
useful for the Development, Manufacture, Commercialization or other
Exploitation of the Compound or any Licensed Product for use in the
Licensed Field in the Territory, including all VistaGen Sole
Inventions, VistaGen’s interest in any VistaGen Joint
Inventions in the Territory, VistaGen Development Data and
VistaGen’s Regulatory Documentation (with respect to Compound
or a Licensed Product).
1.65
“Licensed Manufacturing Know-How”
has the meaning set forth in Section 6.4 (Manufacturing Technology
Transfer).
1.66
“Licensed Patents” means all
Patents Controlled by VistaGen or its Affiliates as of the
Effective Date or during the Term that are necessary or reasonably
useful for the Development, Manufacture, Commercialization, or
other Exploitation of the Compound or any Licensed Product for use
in the Licensed Field in the Territory, including any VistaGen Sole
Invention Patents and VistaGen’s interest in the Joint
Patents in the Territory. [*****].
1.67
“Licensed Product” means any
pharmaceutical product that contains the Compound, alone or in
combination with one or more other molecules or agents in any
dosage form or formulation. For purposes of this Agreement, with
respect to a Licensed Product that has been approved for an initial
Indication, the approval of such License Product for one or more
additional Indications shall not constitute a new and separate
Licensed Product.
1.68
“Licensed Technology” means the
Licensed Patents and the Licensed Know-How.
1.69
“Licensed Trademarks” means any
corporate name or corporate logo (“Corporate Names”) of VistaGen or
its or Affiliates, and any Trademark that consists of or includes
any Corporate Name of VistaGen or its Affiliates, including the
Trademarks, names and logos identified on Exhibit B hereto and such
other Trademarks, names and logos as VistaGen may designate for
Licensed Product in a writing sent to EverInsight from time to time
during the Term.
1.70
“MAA” or “Marketing Authorization
Application” means an application to the appropriate
Regulatory Authority for approval to market a Licensed Product (but
excluding Pricing Approval) in any particular jurisdiction, and all
amendments, renewals and supplements thereto, including, without
limitation, an NDA filed with the FDA in the U.S. and an NDA (or
any future equivalent thereto as defined in the DAL and the
Provisions for Drug Registration) filed with the NMPA in Mainland
China.
1.71
“Mainland China” means the
People’s Republic of China, including Hainan Island, but
excluding Hong Kong, the Macau Special Administrative Region of the
People’s Republic of China and Taiwan.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-7-
1.72
“Manufacture” and
“Manufacturing”
means all activities related to the production, manufacture,
processing, filling, finishing, packaging, labeling, in-process and
finished testing, shipping, storing, or release of a product or any
ingredient or intermediate thereof, including process development,
process qualification and validation, scale-up, pre-clinical,
clinical and commercial manufacture and analytic development,
product characterization, test method development and stability
testing, formulation, quality assurance and quality control of the
any compound, product or intermediate, and regulatory affairs with
respect to the foregoing.
1.73
“Manufacturing Transfer Period” has
the meaning set forth in Section 6.2.
1.74
“Milestone Event” has the meaning
set forth in Section 8.2(a) - (8.2 Development and Regulatory
Milestone Payments - clause (a)).
1.75
“Milestone Payment” has the meaning
set forth in Section 8.2(a) - (8.2 Development and Regulatory
Milestone Payments - clause (a)).
1.76
“NDA” means a New Drug Application
(as more fully defined in 21 C.F.R. §314.5 et seq. or successor regulation) and
all amendments and supplements thereto filed with the FDA and any
other equivalent filing(s) in the Territory.
1.77
“Net Sales” means, [*****]
1.78
“NMPA” means the National Medical
Products Administration of the People’s Republic of China,
formerly known as the China Food and Drug Administration, or its
successor.
1.79
“Patent” means all patents and
patent applications, including all provisionals, divisionals,
reissues, reexaminations, renewals, continuations,
continuations-in-part, substitute applications, priority
applications and inventors’ certificates, extensions and
supplemental certificates and any and all foreign equivalents of
the foregoing.
1.80
“Payment” has the meaning set forth
in Section 8.8(b).
1.81
“Person” means any individual,
partnership, limited liability company, firm, corporation,
association, trust, unincorporated organization or other
entity.
1.82
“PH94B” means the compound known as
PH94B and having the chemical structure shown in Exhibit
C.
1.83
“Phase 1 Clinical Trial” means a
human clinical trial that would satisfy the requirements for a
Phase 1 study as defined in 21 CFR § 312.21(a) (or any amended
or successor regulations) or any equivalent regulations in
jurisdictions in the Territory, regardless of where such clinical
trial is conducted.
1.84
“Phase 3 Clinical Trial” means a
human clinical trial that would satisfy the requirements for a
Phase 3 study as defined in 21 CFR § 312.21(c) (or any amended
or successor regulations) or any equivalent regulations in
jurisdictions in the Territory, regardless of where such clinical
trial is conducted.
1.85
“Pricing Approval” means such
governmental approval, agreement, determination or decision
establishing prices for a Licensed Product that can be charged
and/or reimbursed in a regulatory jurisdiction where the applicable
Government Authority approves or determines the price and/or
reimbursement of pharmaceutical products and where such approval or
determination is necessary for the commercial sale of such Licensed
Product in such jurisdiction.
1.86
“Product Infringement” has the
meaning set forth in Section 9.4(a) (Notice).
1.87
“Product Trademarks” means the
Trademark(s) used or to be used by EverInsight or its Affiliates or
its or their Sublicensees for the Commercialization of Licensed
Product in the Licensed Field in the Territory and any
registrations thereof or any pending applications relating thereto
in the Territory (excluding, in any event, any Corporate Names of
EverInsight, its Affiliates or its or their Sublicensees and any
Licensed Trademarks that consist of or include any Corporate Name
of VistaGen or its Affiliates or (sub)licensees).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-8-
1.88
“Receiving Party” has the meaning
set forth in Section 10.1(a) (Duty of Confidence - subsection
(a)).
1.89
“Regulatory Approval” means, with
respect to a jurisdiction in the Territory, any and all approvals
(including approvals of Marketing Authorization Applications),
licenses, registrations or authorizations of any Regulatory
Authority necessary to commercially distribute, sell or market a
Licensed Product in such jurisdiction, including, where applicable:
(a) pricing or reimbursement approval in such jurisdiction; (b)
pre- and post-approval marketing authorizations (including any
prerequisite Manufacturing approval or authorization related
thereto); and (c) labelling approval.
1.90
“Regulatory Authority” means any
applicable Government Authority responsible for granting Regulatory
Approvals for any Licensed Product, including the FDA, the NMPA,
and any corresponding national or regional regulatory
authorities.
1.91
“Regulatory Documentation” means:
all (a) applications (including all Regulatory Filings, INDs, CTAs
and Marketing Authorization Applications), registrations, licenses,
authorizations and approvals (including Regulatory Approvals); (b)
correspondence and reports submitted to or received from Regulatory
Authorities (including minutes and official contact reports
relating to any communications with any Regulatory Authority) and
all supporting documents with respect thereto, including all
adverse event files and complaint files; and (c) clinical and other
data contained or relied upon in any of the foregoing; in each case
(a), (b) and (c)) relating to the Compound or a Licensed
Product.
1.92
“Regulatory Exclusivity” means any
exclusive marketing rights or data exclusivity rights conferred by
any Regulatory Authority with respect to a pharmaceutical product
other than Patents, and including, without limitation, orphan drug
exclusivity, new chemical entity exclusivity, data exclusivity or
pediatric exclusivity.
1.93
“Regulatory Filings” means, with
respect to the Compound or Licensed Product, any submission to a
Regulatory Authority of any appropriate regulatory application
specific to the Compound or Licensed Product, and shall include,
without limitation, any submission to a regulatory advisory board
and any supplement or amendment thereto. For the avoidance of
doubt, Regulatory Filings shall include any IND, CTA, NDA, MAA,
Regulatory Approval or the corresponding application in any other
country or jurisdiction.
1.94
“Representative” has the meaning
set forth in Section 10.1(c) (Duty of Confidence - Subsection
(c)).
1.95
“Respective Territory” means, in
the case of EverInsight, the Territory, and in the case of
VistaGen, all countries of the world outside the
Territory.
1.96
“Retained Rights” means, with
respect to the Compound and Licensed Product, the rights of
VistaGen, its Affiliates and its and their licensors,
(sub)licensees and contractors to:
(a) perform
VistaGen’s obligations under this Agreement;
(b) Manufacture
and have Manufactured (including CMC and manufacturing process
development work) the Compound or Licensed Product within the
Territory solely for Exploitation outside the
Territory;
(c) Develop
and have Developed the Compound and Licensed Product in the
Territory but only as part of a global Phase 3 Clinical Trial that
EverInsight elects to participate in pursuant to Section 4.4(b);
and
(d) Develop,
Manufacture, Commercialize and otherwise Exploit the Compound and
Licensed Product for any and all purposes outside the
Territory.
1.97
“Royalty Term” has the meaning set
forth in Section 8.4(b) (Royalty Term).
1.98
“SEC” has the meaning set forth in
Section 10.5 (Publicity/Use of Names - subsection
(a)).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-9-
1.99
“Sublicense” means a license or
sublicense granted by EverInsight (or a Sublicensee) to Develop,
make, use, import, promote, offer for sale or sell the Compound or
any Licensed Product, including any license given to any of the
rights granted to EverInsight under Section 2.1(Licenses to
EverInsight).
1.100
“Subcontractor” has the meaning set
forth in Section 2.8 (Subcontracting).
1.101
“Sublicensee” means a Third Party
to whom EverInsight or its Affiliate has granted a Sublicense in
accordance with the terms of this Agreement.
1.102
“Tax” or “Taxes” means any (a) all federal,
provincial, territorial, state, municipal, local, foreign or other
taxes, imposts, rates, levies, assessments and other charges in the
nature of a tax (and all interest and penalties thereon and
additions thereto imposed by any Government Authority), including
without limitation all income, excise, franchise, gains, capital,
real property, goods and services, transfer, value added, gross
receipts, windfall profits, severance, ad valorem, personal
property, production, sales, use, license, stamp, documentary
stamp, mortgage recording, employment, payroll, social security,
unemployment, disability, escheat, estimated or withholding taxes,
and all customs and import duties, together with all interest,
penalties and additions thereto imposed with respect to such
amounts, in each case whether disputed or not; (b) any liability
for the payment of any amounts of the type described in subsection
(a) as a result of being or having been a member of an affiliated,
consolidated, combined or unitary group; and (c) any liability for
the payment of any amounts as a result of being party to any tax
sharing agreement or arrangement or as a result of any express or
implied obligation to indemnify any other person with respect to
the payment of any amounts of the type described in subsection (a)
or (b).
1.103
“Term” has the meaning set forth in
Section 11.1 (Term).
1.104
“Territory” means Greater China
(Mainland China, Taiwan, Hong Kong and Macau), South Korea,
Southeast Asia (Singapore, Malaysia, Thailand, Indonesia,
Philippines, and Vietnam).
1.105
“Third Party” means any Person
other than a Party or an Affiliate of a Party.
1.106
“Third Party Infringement Claim”
has the meaning set forth in Section 9.5 (Infringement claims by
Third Parties).
1.107
“Trademark” means any word, name,
symbol, color, shape, designation or any combination thereof,
including any trademark, service mark, trade name, brand name,
sub-brand name, trade dress, product configuration rights, program
name, delivery form name, certification mark, collective mark,
logo, tagline, slogan, design or business symbol, that functions as
an identifier of source, origin or quality, whether or not
registered, and all statutory and common law rights therein and all
registrations and applications therefor, together with all goodwill
associated with, or symbolized by, any of the
foregoing.
1.108
“Transfer Tax” has the meaning set
forth in Section 8.8(c) (Transfer Tax).
1.109
“United States” or
“U.S.” means the
United States of America including its territories and
possessions.
1.110
“Valid Claim” means, with respect
to any jurisdiction in the Territory, a claim of an issued and
unexpired Licensed Patent (as may be extended through supplementary
protection certificate or patent term extension or the like) that
has not been cancelled, revoked, held invalid or unenforceable by a
decision of a patent office or other Government Authority of
competent jurisdiction from which no appeal can be taken (or from
which no appeal was taken within the allowable time period) and
which claim has not been disclaimed, denied or admitted to be
invalid or unenforceable through reissue, re-examination or
disclaimer or otherwise; provided that in any jurisdiction in the
Territory, a Valid Claim shall cease to be a Valid Claim in such
jurisdiction if its scope is such that it does not reasonably block
or prevent the entry, or Commercialization, of Generic
Products.
1.111
“VistaGen CMO” has the meaning set
forth in Section 6.2 (Manufacturing Technology
Transfer)
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-10-
1.112
“VistaGen Development Data” means
any nonclinical or clinical data that are Controlled by VistaGen
and related to the Compound or any Licensed Product or otherwise
included in, or filed in support of, the Regulatory Documentation
filed by VistaGen, its Affiliates, licensees or sublicensees
outside of the Territory.
1.113
“VistaGen Indemnitees” has the
meaning set forth in Section 13.2 (Indemnification by
EverInsight).
1.114
“VistaGen Sole Inventions” means
any Inventions that are conceived and reduced to practice solely by
employees of, or consultants or service providers to, VistaGen, at
any time during the Term of this Agreement and that are made,
generated, conceived or otherwise invented as a result of a Party
exercising its rights or carrying out its obligations under this
Agreement, whether directly or via its Affiliates, agents or
independent contractors.
1.115
“VistaGen Sole Invention Patents”
means any Patents that contain one or more claims that cover
VistaGen Sole Inventions.
1.116
Interpretation. In
this Agreement, unless otherwise specified:
(a)
“includes”
and “including” shall mean, respectively, includes
without limitation and including without limitation;
(b)
words denoting the
singular shall include the plural and vice versa and words denoting
any gender shall include all genders;
(c)
words such as
“herein”, “hereof”, and
“hereunder” refer to this Agreement as a whole and not
merely to the particular provision in which such words appear;
and
(d)
the Exhibits and
other attachments form part of the operative provision of this
Agreement and references to this Agreement shall include references
to the Exhibits and attachments.
ARTICLE 2 LICENSES
2.1
License to EverInsight.
(a)
Subject to the
terms and conditions of this Agreement, VistaGen hereby grants to
EverInsight an exclusive (even as to VistaGen), royalty-bearing
license and sublicense, as the case may be, under the Licensed
Technology solely to Exploit Licensed Product in the Licensed Field
in the Territory, with the right to grant sublicenses in accordance
with Section 2.3 (Sublicense Rights).
(b)
In addition,
VistaGen hereby grants to EverInsight a non-exclusive license and
sublicense, as the case may be, under the Licensed Technology to
Manufacture and have Manufactured the Compound and Licensed Product
outside the Territory solely for Exploitation in the Territory,
with the right to grant sublicenses in accordance with Section 2.3
(Sublicense Rights).
2.2
License to VistaGen. Subject to the
terms and conditions of this Agreement, EverInsight hereby grants
to VistaGen an exclusive (even as to EverInsight), royalty-free
license under the EverInsight Technology solely to Exploit Licensed
Product in the Licensed Field outside the Territory, with the right
to grant sublicenses in accordance with Section 2.3 (Sublicense
Rights).
2.3
Sublicense Rights.
(a)
Affiliates. Subject to the terms of this
Section 2.3 (Sublicense Rights), EverInsight may grant a sublicense
of the license granted in Section 2.1 (License to EverInsight)
through multiple tiers to Affiliates of EverInsight without prior
notice to or the prior consent of VistaGen; provided that (i)
Licensed Know-How may only be sublicensed along with the Licensed
Patents; (ii) EverInsight shall cause each Affiliate to comply with
the applicable terms and conditions of this Agreement, as if such
Affiliate were a Party to this Agreement; and (iii) EverInsight
shall be responsible for all actions, activities and obligations to
VistaGen of such Affiliate. Subject to the terms of this Section
2.3 (Sublicense Rights), VistaGen may grant a sublicense of the
license granted in Section 2.2 (License to VistaGen) through
multiple tiers to Affiliates of VistaGen without prior notice to or
the prior consent of EverInsight; provided that (i) EverInsight
Know-How may only be sublicensed along with the EverInsight
Patents; (ii) VistaGen shall cause each Affiliate to comply with
the applicable terms and conditions of this Agreement, as if such
Affiliate were a Party to this Agreement; and (iii) VistaGen shall
be responsible for all actions, activities and obligations to
EverInsight of such Affiliate.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-11-
(b)
Third Parties. Upon the prior written
consent of VistaGen, such consent not to be unreasonably withheld,
conditioned, or delayed, EverInsight may grant a sublicense of the
rights granted under the license in Section 2.1 (License to
EverInsight) through multiple tiers to any Third Party; provided
that (i) Licensed Know-How may only be sublicensed along with the
Licensed Patents (other than in the case of a sublicense to a
fee-for-service Subcontractor in the context of subcontracting
pursuant to Section 2.8 (Subcontracting)); (ii) each sublicense
granted to a Third Party shall be in writing, and shall incorporate
terms and conditions that are consistent with, and expressly made
subject to, the terms and conditions of this Agreement; (iii)
VistaGen shall be provided by EverInsight with a copy of such
sublicense agreement within thirty (30) days of execution, which
copy may redact any financial or other proprietary terms; and (iv)
EverInsight shall be responsible to VistaGen for a breach of this
Agreement due to the breach by such Third Party of such sublicense
agreement. EverInsight hereby waives any requirement that VistaGen
exhaust any right, power or remedy, or proceed against any such
sublicensee for any obligation or performance under this Agreement
prior to proceeding directly against EverInsight. Upon the prior
written consent of EverInsight, such consent not to be unreasonably
withheld, conditioned, or delayed, VistaGen may grant a sublicense
of the rights granted under the license in Section 2.2 (License to
VistaGen) through multiple tiers to any Third Party; provided that
(i) EverInsight Know-How may only be sublicensed along with the
EverInsight Patents (other than in the case of a sublicense to a
fee-for-service Subcontractor pursuant to Section 2.8
(Subcontracting)); (ii) each sublicense granted to a Third Party
shall be in writing, and shall incorporate terms and conditions
that are consistent with, and expressly made subject to, the terms
and conditions of this Agreement; (iii) EverInsight shall be
provided by VistaGen with a copy of such sublicense agreement
within thirty (30) days of execution, which copy may redact any
financial or other priority terms; and (iv) VistaGen shall be
responsible to EverInsight for a breach of this Agreement due to
the breach by such Third Party of such sublicense agreement.
VistaGen hereby waives any requirement that EverInsight exhaust any
right, power or remedy, or proceed against any sublicensee for any
obligation or performance under this Agreement prior to proceeding
directly against VistaGen.
2.4
VistaGen’s Retained Rights; Limitations
of License Grants.
(a)
Retained Rights.
(i)
Notwithstanding
anything to the contrary in this Agreement and without limitation
of any rights granted by or reserved to VistaGen pursuant to any
other term or condition of this Agreement, VistaGen hereby
expressly retains, on behalf of itself and its Affiliates (and on
behalf of its and their direct and indirect Third Party licensors
under any In-License Agreement, (sub)licensees and contractors) all
right, title and interest in and to the Licensed Patents, the
Licensed Know-How, VistaGen Development Data, VistaGen’s
interests in and to Joint Patents and Joint Know-How, Regulatory
Documentation of VistaGen and the Corporate Names of VistaGen and
their Affiliates, in each case, for purposes of performing or
exercising the Retained Rights.
(ii)
Notwithstanding
anything to the contrary in this Agreement and without limitation
of any rights granted by or reserved to EverInsight pursuant to any
other term or condition of this Agreement, EverInsight hereby
expressly retains, on behalf of itself and its Affiliates (and on
behalf of its and their direct and indirect Third Party licensors
under any In-License Agreement, (sub)licensees and contractors) all
right, title and interest in and to the EverInsight Patents, the
EverInsight Know-How, EverInsight Development Data,
EverInsight’s interests in and to Joint Patents and Joint
Know-How, Regulatory Documentation of EverInsight and the Corporate
Names of EverInsight and their Affiliates, in each case, for
purposes of performing its obligations or exercising its rights
under this Agreement, and also for purposes of Manufacturing or
having Manufactured the Compound and Licensed Product outside the
Territory solely for Exploitation in the Territory.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-12-
(b)
In-License Agreements.
(1)
If VistaGen or any
of its Affiliates negotiates with a Third Party at arms’
length to obtain a license to any Know-How or Patent that are
necessary or reasonably useful for the Development, Manufacture,
Commercialization or other Exploitation of the Compound or any
Licensed Product (such Know-How or Patent, “VistaGen Third Party IP”, such
license, an “In-License
Agreement”), then VistaGen shall promptly notify
EverInsight and identify the relevant VistaGen Third Party IP, with
a copy to the JSC. The applicable VistaGen Third Party IP shall be
included in the license granted to EverInsight under Section 2.1
(License to EverInsight) and considered VistaGen Patents and
VistaGen Know-How, respectively, only if VistaGen discloses the
substantive terms of the In-License Agreement to EverInsight, which
VistaGen hereby agrees to do, and XxxxXxxxxxx agrees in writing to
(A) comply with all the relevant obligations of such In-License
Agreement, and (B) pay [*****] of the portion
of all upfront, milestone, royalty and other payments under the
In-License Agreement that are allocable to the Development,
Manufacture or Commercialization of the Compound or any Licensed
Product in the Licensed Field in the Territory; provided, however,
that, such upfront, milestone, royalty and other payments should be
(x) at fair market value for such a license in the Territory; and
(y) directly attributable to the Development, Manufacture or
Commercialization of the Compound or any Licensed Product in the
Licensed Field in the Territory by EverInsight or any of its
Affiliates or any Sublicensees; and (z) for any such payment that
is applicable to the Respective Territories of both Parties (such
as upfront payment), such payment shall be allocated between the
Parties’ Respective Territories based on the relative value
of the market for the Licensed Product in each Party’s
Respective Territory, and EverInsight shall pay [*****] of the portion
allocable to the Territory (for clarity, VistaGen shall be solely
responsible for, and EverInsight shall have no obligation to pay
any portion of, all such payment that is not allocable to the
Territory, such as royalty payment for the sale of Licensed Product
outside the Territory). For the avoidance of doubt, if EverInsight
reasonably determines that such VistaGen Third Party IP under the
In-License Agreement is not necessary for the Development,
Manufacture or Commercialization of the Compound or any Licensed
Product in the Licensed Field in the Territory, EverInsight has the
right not to pay any costs associated with such In-License
Agreement, in which case such VistaGen Third Party IP shall not be
included in the license granted to EverInsight under Section 2.1
(License to EverInsight) and shall not be considered to be VistaGen
Patents and VistaGen Know-How.
(2)
If EverInsight or
any of its Affiliates or Sublicensees negotiates with a Third Party
at arms’ length to obtain a license to any Know-How or Patent
that are necessary or reasonably useful for the Development,
Manufacture, Commercialization or other Exploitation of the
Compound or any Licensed Product and actually applies such Know-How
or Patent in the Development, Manufacture, Commercialization or
other Exploitation of the Compound or any Licensed Product (such
Know-How or Patent, “EverInsight Third Party IP”, such license, an
“EverInsight
In-License
Agreement”), then EverInsight shall promptly notify
VistaGen and identify the relevant EverInsight Third Party IP, with
a copy to the JSC. The applicable EverInsight Third Party IP shall
be included in the license granted by EverInsight to VistaGen under
Section 2.2 (License to VistaGen) and considered EverInsight
Patents and EverInsight Know-How, respectively, only if EverInsight
discloses the substantive terms of such EverInsight In-License
Agreement to VistaGen, which EverInsight hereby agrees to do, and
VistaGen agrees in writing to (A) comply with all the relevant
obligations of such EverInsight In-License Agreement; (B) pay
[*****] of
the portion of all upfront, milestone, royalty and other payments
under the EverInsight In-License Agreement that are allocable to
the Development, Manufacture or Commercialization of the Compound
or any Licensed Product in the Licensed Field in the Territory,
which VistaGen hereby agrees to do; and (C) pay [*****] of the portion
of all upfront, milestone, royalty and other payments applicable to
the Development, Manufacture or Commercialization of the Compound
or any Licensed Product in the Licensed Field outside the
Territory; provided, however, that, such upfront, milestone,
royalty and other payments under clause (B) above should be (x) at
fair market value for such a license in the Territory; and (y)
directly attributable to the Development, Manufacture or
Commercialization of the Compound or any Licensed Product in the
Licensed Field in the Territory by EverInsight or any of its
Affiliates or any Sublicensees; and (z) for any such payment that
is applicable to the Respective Territories of both Parties (such
as upfront payment), such payment shall be allocated between the
Parties’ Respective Territories based on the relative value
of the market for the Licensed Product in each Party’s
Respective Territory, and VistaGen shall pay [*****] of the portion
allocable to the Territory (for clarity, pursuant to clause (C)
above, VistaGen shall be solely responsible for, and shall
reimburse EverInsight for, all such payment that is not allocable
to the Territory, such as royalty payment for the sale of Licensed
Product outside the Territory). For the avoidance of doubt, if
VistaGen reasonably determines that such EverInsight Third Party IP
is not necessary for the Development, Manufacture or
Commercialization of the Compound or any Licensed Product in the
Licensed Field outside the Territory, VistaGen has the right not to
pay the costs associated with such EverInsight In-License Agreement
outside the Territory under clause (C) above (for further clarity,
VistaGen shall remain obligated to pay its share of the costs
associated with such EverInsight In-License Agreement in the
Territory under clause (B) above), in which case such EverInsight
Third-Party IP shall not be included in the license granted to
VistaGen under Section 2.2 (License to VistaGen) and shall not be
considered to be EverInsight Patents and EverInsight Know-How. In
the event that VistaGen does agree to accept such Third-Party
license outside of the Territory, the provisions of clauses (3),
(4) and (5) of this Section 2.4(b) (In-License Agreements) shall
apply, mutatis mutandis, to any such Third Party
license.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-13-
(3)
Subject to this
Section 2.4(b) (In-License Agreements), the licenses granted by
VistaGen in Section 2.1 (License to EverInsight) include
sublicenses solely under the applicable license rights granted to
VistaGen or its Affiliates by Third Parties under the In-License
Agreements. Any Sublicense with respect to Know-How or Patents of a
Third Party hereunder and any right of EverInsight (if any) to
grant a further sublicense thereunder, shall be subject and
subordinate to the terms and conditions of the In-License Agreement
under which such sublicense is granted and shall be effective
solely to the extent permitted under the terms of such agreement.
Without limitation of the foregoing, in the event and to the extent
that any In-License Agreement requires that particular terms or
conditions of such In-License Agreement be contained or
incorporated in any agreement granting a sublicense thereunder,
such terms and conditions are hereby deemed to be incorporated
herein by reference and made applicable to the sublicense granted
herein under such In-License Agreement.
(4)
The Parties shall
cooperate with each other in good faith to support each other in
negotiating rights under EverInsight Third Party IP in order for
VistaGen to obtain such rights outside of the Territory and in
complying with VistaGen’s and its Affiliates’
obligations under each In-License Agreement. Without limitation to
the foregoing, (A) the Parties shall, from time to time, upon the
reasonable request of either Party, discuss the terms of an
In-License Agreement and agree upon, to the extent reasonably
possible, a consistent interpretation of the terms of such
In-License Agreement in order to, as fully as possible, allow
VistaGen and its Affiliates to comply with the terms of such
In-License Agreement; (B) to the extent there is a conflict between
any terms of this Agreement and any terms of any In-License
Agreement (including with respect to sublicensing rights, diligence
obligations, prosecution, maintenance, enforcement, defense, any
obligations for a counterparty to such In-License Agreement to
maintain a Party’s information as confidential and any
obligations for a Party to maintain as confidential the information
of a counterparty to such In-License Agreement), the terms of such
In-License Agreement shall control with respect to the relevant
Know-How, Patents or other rights granted to EverInsight hereunder;
and (C) EverInsight and its Affiliates and Sublicensees shall
comply with any applicable reporting and other requirements under
the In-License Agreements, and the provisions regarding currency
conversion, international payments and late payments, and any other
relevant definitions and provisions, of the relevant In-License
Agreements shall apply to the calculation of the payments due under
the relevant In-License Agreements.
(5)
On an In-License
Agreement-by-In-License Agreement basis, from and after the date on
which XxxxXxxxxxx agrees in writing pursuant to Section 2.4(b)(1)
to accept the Patents and Know-How covered by such In-License
Agreement as Licensed Technology under this Agreement, VistaGen
shall maintain such In-License Agreement in full force and effect,
shall not enter into any subsequent agreement with any other party
to such In-License Agreement that modifies or amends such
In-License Agreement in any way that would materially adversely
affect EverInsight’s rights or interest under this Agreement
without EverInsight’s prior written consent, which shall not
be unreasonably withheld, conditioned or delayed, and shall provide
EverInsight with a copy of all modifications to or amendments of
such In-License Agreement, regardless of whether
EverInsight’s consent was required with respect
thereto.
2.5
Transfer of Know-How. Within
[*****] days
following the Effective Date, VistaGen shall commence disclosing
and making available to EverInsight the Licensed Know-How
(including the VistaGen Development Data therein) necessary or
reasonably required for EverInsight to file a CTA covering a
Licensed Product and to Develop the Compound and Licensed Product
in the Licensed Field in the Territory. In addition, throughout the
Term of this Agreement, VistaGen shall promptly disclose and make
available to EverInsight any Licensed Know-How (including the
VistaGen Development Data therein) that has not previously been
provided to EverInsight, or is developed or generated or otherwise
comes into VistaGen’s Control after the Effective Date. Such
disclosure and transfer shall be made at no additional cost to
EverInsight and according to a timeline mutually agreed by
EverInsight and VistaGen, each of which shall cooperate with each
other in good faith to enable a smooth transfer of the Licensed
Know-How from VistaGen to EverInsight. Upon EverInsight’s
reasonable request during such transfer, VistaGen shall provide
reasonable technical assistance, at no additional cost to
EverInsight, including making appropriate employees available to
EverInsight at reasonable times, places and frequency, and upon
reasonable prior notice, for the purpose of assisting EverInsight
to understand and use the Licensed Know-How in connection with
EverInsight’s filing of such CTA covering such Licensed
Product and the Development of the Compound and Licensed Product in
the Licensed Field in the Territory.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-14-
2.6
No Implied Licenses; Negative Covenant.
Except as set forth herein, no Party shall acquire any license or
other intellectual property interest, by implication or otherwise,
under any Know-How, Patents, trademarks or other intellectual
property rights owned or Controlled by any other Party. EverInsight
hereby covenants not to practice, and not to permit or cause any of
its Affiliates or any Third Party to practice, any Licensed
Technology for any purpose other than as expressly authorized in
this Agreement.
2.7
Non-Diversion.
(a)
EverInsight hereby
covenants and agrees that it will not, and will ensure that its
Affiliates will not, and will ensure its Sublicensees and
subcontractors are bound by contractual obligations not to, either
directly or indirectly, promote, market, solicit, distribute,
import, sell or have sold Licensed Product outside the Territory.
In furtherance of the foregoing, EverInsight shall not and will
ensure that its Affiliates do not, and shall use Commercially
Reasonable Efforts to ensure that its or their Sublicensees or
distributors do not knowingly distribute, market, promote, offer
for sale or sell the Compound or any Licensed Product directly or
indirectly to any Person outside the Territory or to any Person
inside the Territory that EverInsight or any of its Affiliates or
any of its or their Sublicensees or distributors knows has directly
or indirectly distributed, marketed, promoted, offered for sale or
sold, or has reasonable grounds to believe intends to directly or
indirectly distribute, market, promote, offer for sale or sell, the
Compound or any Licensed Product for use outside the Territory. If
EverInsight or any of its Affiliates receives or becomes aware of
the receipt by it or any Sublicensee or distributor of any orders
for the Compound or any Licensed Product for use outside the
Territory, such Person shall refer such orders to
VistaGen.
(b)
VistaGen hereby
covenants and agrees that it will not, and shall ensure that its
Affiliates will not, and will ensure its licensees and sublicensees
(other than EverInsight, its Affiliates and Sublicensees) and
subcontractors are bound by contractual obligations not to, either
directly or indirectly, promote, market, solicit, distribute,
import, sell or have sold Licensed Product in the Territory. In
furtherance of the foregoing, VistaGen shall not and will ensure
that its Affiliates do not, and shall use Commercially Reasonable
Efforts to ensure that its or their licensees and sublicensees
(other than EverInsight, its Affiliates and Sublicensees) or
distributors do not knowingly distribute, market, promote, offer
for sale or sell the Compound or any Licensed Product directly or
indirectly to any Person in the Territory or to any Person outside
the Territory that VistaGen or any of its Affiliates or any of its
or their licensees or sublicensees (other than EverInsight, its
Affiliates and Sublicensees) or distributors knows has directly or
indirectly distributed, marketed, promoted, offered for sale or
sold, or has reasonable grounds to believe intends to directly or
indirectly distribute, market, promote, offer for sale or sell, the
Compound or any Licensed Product for use in the Territory. If
VistaGen or any of its Affiliates receives or becomes aware of the
receipt by it or any licensees, sublicensee (other than
EverInsight, its Affiliates and Sublicensees) or distributor of any
orders for the Compound or any Licensed Product for use in the
Territory, such Person shall refer such orders to
EverInsight.
2.8
Non-Compete. During the Term of this
Agreement, neither Party shall, and each Party shall cause its
Affiliates and their respective Sublicensees not to, directly or
indirectly, enable or assist any Person that is not a Party to this
Agreement to, Develop, Manufacture or Commercialize any intra-nasal
formulation of Androstadienol in the Territory for the treatment of
social anxiety disorder, other than the Compound and the Licensed
Product in accordance with this Agreement (the “Competing Product”). If
EverInsight requests a waiver of this Section with regard to a
particular product and/or a particular transaction, VistaGen will
in good faith give due consideration to such request.
Notwithstanding the foregoing, if EverInsight is acquired by or
merges or consolidates with a Third Party that, at the time of such
acquisition, is actively Developing, Manufacturing and/or
Commercializing a Competing Product in the Territory, then the
activities of EverInsight, its Affiliates and their respective
Sublicensees under and in accordance with the terms of such license
agreement and the activities of such Third Party acquirer for the
continued development, manufacturing and/or commercialization of
the Competing Product, respectively, shall not be deemed to breach
this Section 2.8.
2.9
Subcontracting. Notwithstanding Section
2.3 (Sublicense Rights), each Party may, without the other
Party’s consent, subcontract on a fee-for-service basis with
a Third Party to perform any or all of its obligations hereunder (a
“Subcontractor”), including by
appointing one or more distributors, and grant a sublicense to the
Subcontractor solely to the extent necessary to perform such
subcontracted obligations; provided that (a) no such permitted
subcontracting shall relieve the subcontracting Party of any
obligation hereunder (except to the extent satisfactorily performed
by such Subcontractor) or any liability and the subcontracting
Party shall be and remain fully responsible and liable therefor;
(b) the agreement pursuant to which the subcontracting Party
engages any Subcontractor must be consistent in all material
respects with this Agreement, including terms consistent with the
confidentiality, restrictions on use and intellectual property
provisions of this Agreement, and (c) the subcontracting Party
shall be responsible to the other Party for the breach of this
Agreement due to breach of any subcontracting agreement by its
Subcontractors. The subcontracting Party hereby waives any
requirement that the other Party exhaust any right, power or
remedy, or proceed against any Subcontractor for any obligation or
performance under this Agreement prior to proceeding directly
against the subcontracting Party.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-15-
2.10
Statements and Compliance with Applicable
Laws. Each Party shall and shall cause its Affiliates and
its and their respective licensees and Sublicensees to comply with
all Applicable Laws with respect to the Exploitation of Licensed
Product, including the extranational application of U.S. laws and
regulations as related, for example, to regulatory matters, export
controls and transfer of technology to certain countries and to
foreign corrupt practices. Each Party shall, and shall cause its
Affiliates to, and shall use Commercially Reasonable Efforts to
cause its and their licensees, Sublicensees, employees,
representatives, agents, and distributors to avoid taking, or
failing to take, any actions that such Party knows or reasonably
should know would jeopardize the goodwill or reputation of the
other Party or its Affiliates or the Licensed Product or any
Trademark associated therewith. Without limitation to the
foregoing, each Party shall in all material respects conform its
practices and procedures relating to the Commercialization of the
Licensed Product and educating the medical community in its
Respective Territory with respect to the Licensed Product to any
applicable industry association regulations, policies and
guidelines, as the same may be amended from time to time, and
Applicable Laws. Each Party agrees that in performing its
obligations under this Agreement, it will not employ or engage any
Person who has been debarred or disqualified by any Regulatory
Authority, or, to its knowledge, is the subject of debarment or
disqualification proceedings by a Regulatory
Authority.
2.11
Section 365(n). All rights and licenses
granted under or pursuant to this Agreement by VistaGen or
EverInsight are, and will otherwise be deemed to be, for the
purposes of Section 365(n) of the U.S. Bankruptcy Code, and any
similar law in the Territory, licenses of rights to
“intellectual property” as defined under Section 101 of
the U.S. Bankruptcy Code or any similar law in the Territory. The
Parties agree that each Party, as licensees of such rights under
this Agreement, will retain and may fully exercise all of its
rights and elections under the U.S. Bankruptcy Code or any similar
law in the Territory. The Parties further agree that, in the event
of the commencement of a bankruptcy proceeding by or against either
Party under the U.S. Bankruptcy Code or any similar law in the
Territory, the Party that is not a party to such proceeding will be
entitled to a complete duplicate of (or complete access to, as
appropriate) any such intellectual property and all embodiments of
such intellectual property, and same, if not already in their
possession, will be promptly delivered to them (a) upon any such
commencement of a bankruptcy proceeding upon their written request
therefor, unless the Party subject to such proceeding elects to
continue to perform all of its obligations under this Agreement, or
(b) if not delivered under (a) above, following the rejection of
this Agreement by or on behalf of the Party subject to such
proceeding upon written request therefor by the non-subject
party.
2.12
Technology Escrow. Promptly after the
Effective Date, VistaGen shall deposit all existing Licensed
Know-How (for clarity, including all Licensed Manufacturing
Know-How) with an escrow agent selected by XxxxXxxxxxx and
reasonably acceptable to VistaGen and pursuant to an escrow
agreement that requires the escrow agent to release the Licensed
Know-How to EverInsight upon the commencement of a bankruptcy
proceeding by or against VistaGen under the U.S. Bankruptcy Code or
any similar law in the Territory. Throughout the term of this
Agreement, VistaGen shall periodically (no less than annually)
update such technology escrow to include any new Licensed Know-How
that is developed or generated or otherwise comes into
VistaGen’s Control after the Effective Date. The Parties
shall share equally the cost of establishing and maintaining such
technology escrow.
ARTICLE 3 GOVERNANCE
3.1
Joint Steering Committee. As soon as
practicable after the Effective Date, the Parties shall establish a
joint steering committee (the “Joint Steering Committee” or the
“JSC”), composed
of equal number of representatives of VistaGen and representatives
of EverInsight, to coordinate the Development and Commercialization
of the Compound and Licensed Product in the Licensed Field in the
Territory. Each JSC representative shall have appropriate knowledge
and expertise and sufficient seniority within the applicable Party
to make decisions arising within the scope of the JSC’s
responsibilities. The JSC shall:
(a)
serve as a forum
for discussing Development of the Compound and Licensed Product in
the Licensed Field in the Territory, including by reviewing the
Development Plan and coordinating the conduct of the Development
activities;
(b)
serve as a forum
for discussing the Manufacture and supply of Compound and Licensed
Product in the Licensed Field in the Territory, including by
reviewing the Development strategy and Commercialization strategy
for the Territory and coordinating the conduct of the Manufacturing
and supply activities;
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-16-
(c)
serve as a forum
for discussing Development of the Compound and Licensed Product in
the Licensed Field in the Territory, including by (i) providing
EverInsight with a forum at each meeting to disclose
EverInsight’s, or its Affiliates’ or
Sublicensees’ activities with respect to achieving Regulatory
Approvals of Licensed Product in the Territory; material clinical
study results; and the Marketing Authorization Applications that
EverInsight or any of its Affiliates reasonably expect to make,
seek or attempt to obtain in the Territory; (ii) reviewing the
current Development Plan and, with the JSC’s approval, making
any amendments or updates to the Development Plan; and (iii)
coordinating the conduct of the Development
activities;
(d)
serve as a forum to
keep EverInsight updated on the Development of the Compound and
Licensed Product in the Licensed Field outside the Territory,
including material clinical study results and any Marketing
Authorization Application for the Licensed Product filed outside
the Territory;
(e)
coordinate the
activities of VistaGen and EverInsight under this
Agreement;
(f)
establish a Joint
Manufacturing Committee to enable regular information exchange on
CMC issues, discuss possible costs reductions and review potential
CMOs and prepare joint manufacturing plans, transfers and
selections of joint manufacturing partners, and a Joint
Commercialization Committee for discussing and coordinating the
launch activities for the Licensed Product (for clarity, neither
such subcommittee nor the JSC shall have any decision making
authority over commercialization of the Licensed Product anywhere
in the Territory); and
(h)
perform such other
functions as are set forth herein or as the Parties may mutually
agree in writing, except where in conflict with any provision of
this Agreement.
The JSC
shall have only such powers as are expressly assigned to it in this
Agreement, and such powers shall be subject to the terms and
conditions of this Agreement. For clarity, the JSC shall not have
any right, power or authority: (i) to determine any issue in a
manner that would conflict with the express terms and conditions of
this Agreement; or (ii) to modify or amend the terms and conditions
of this Agreement.
3.2
JSC Membership and
Meetings.
(a)
JSC Members. Each Party will designate
equal number (at least two) of representatives to the JSC within
thirty (30) days after the Effective Date. Each Party may replace
its JSC representatives on written notice to the other Party, but
each Party shall strive to maintain continuity. The Alliance
Managers shall jointly prepare and circulate the meeting agenda at
least five (5) Business Days in advance of each meeting, and shall
also promptly, but in no event later than thirty (30) days after
such meeting, prepare and circulate for review and approval of the
Parties the minutes of such meeting.
(b)
JSC Meetings. The JSC will hold its
first meeting within thirty (30) days of establishment of the JSC
pursuant to Section 3.1 (Joint Steering Committee). At this first
meeting, the JSC will address the initial transfer of Licensed
Know-How provided for in Section 2.5 (Transfer of Know-How) and any
other topics the Parties deem appropriate. Thereafter, the JSC
shall hold meetings at such times as it elects to do so, but in no
event shall such meetings be held less frequently than once per
Calendar Quarter. Meetings may be held in person, or by audio or
video teleconference; provided, that unless otherwise agreed by
VistaGen and EverInsight, at least one (1) meeting per year shall
be held in person, and all in-person JSC meetings shall be held at
locations mutually agreed upon by VistaGen and EverInsight. Each
Party shall be responsible for all of its own expenses of
participating in JSC meetings.
(c)
Non-Member Attendance. Each of VistaGen
and EverInsight may from time to time invite a reasonable number of
participants, in addition to its representatives, to attend JSC
meetings in a non‑voting capacity; provided, that if either
VistaGen or EverInsight intends to have any Third Party (including
any consultant) attend such a meeting, such Party shall provide at
least five (5) Business Days’ prior written notice to the
other Party and obtain the other Party’s approval for such
Third Party to attend such meeting, which approval shall not be
unreasonably withheld or delayed. Such Party shall ensure that such
Third Party is bound by confidentiality and non-use obligations
consistent with the terms of this Agreement. The Party inviting any
such Third Party shall be responsible for all of such Third
Party’s costs and expenses of participating in JSC meetings,
unless such invitation is mutually made by VistaGen and
EverInsight, in which case they shall equally share such costs and
expenses.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-17-
3.3
JSC Decision-Making. All decisions of
the JSC shall be made by unanimous vote, with VistaGen’s
representatives and XxxxXxxxxxx’s representatives each
collectively having one (1) vote. If after reasonable discussion
and good faith consideration of each of their views on a particular
matter before the JSC, the representatives of VistaGen and
EverInsight cannot reach an agreement as to such matter within
thirty (30) calendar days after such matter was brought to the JSC
for resolution, such disagreement shall:
(a)
be referred to the
Chief Executive Officer of VistaGen (or his or her designee) and
the Chief Executive Officer of EverInsight (or his or her designee)
(collectively, the “Executive
Officers”) for resolution, who shall use good faith
efforts to resolve such matter within forty-five (45) calendar days
after it is referred to them and, if such matter is resolved by the
Executive Officers, such resolution shall be implemented by and
binding on the Parties.
(b)
If the Executive
Officers are unable to reach consensus on any such matter during
such forty-five (45) calendar day period, then
(i) the
Chief Executive Officer of EverInsight shall have the right to make
the final decision if such matter (A) involves the Development of,
Regulatory Approval for, Commercialization or other Exploitation of
the Compound or a Licensed Product in the Territory and (B) is not
reasonably expected to have a material adverse effect on the
Development of, Regulatory Approval for, Commercialization or
Exploitation of the Compound or a Licensed Product outside the
Territory;
(ii)
the Chief Executive Officer of VistaGen shall have the right to
make the final decision if such matter (A) involves the Development
of, Regulatory Approval for, Commercialization or other
Exploitation of the Compound or a Licensed Product outside the
Territory, and (B) is not reasonably expected to have a material
adverse effect on the Development of, Regulatory Approval for, or
Commercialization or Exploitation of the Compound or a Licensed
Product in the Territory; or
(iii)
in all other cases, such matter will be resolved in accordance with
Section 14.10 (Dispute Resolution).
(c)
If the Parties
dispute whether a matter subject to the decision making mechanism
set forth above is reasonably expected to have a material adverse
effect on the Development of, Regulatory Approval for, or
Commercialization or Exploitation of the Compound or a Licensed
Product in a Party’s Respective Territory, such dispute shall
be resolved by an independent, impartial and conflicts-free Third
Party expert, who shall be experienced in the global aspects of the
development, manufacture and commercialization of pharmaceutical
products similar to the Licensed Product (the “Expert”). For clarity, such
dispute shall not be subject to the dispute resolution mechanism
set forth in Section 14.10. Within fifteen (15) days after a Party
alleges material adverse impact in its Respective Territory as set
forth above and the other Party disagrees with such allegation, the
Parties shall mutually agree upon the Expert and, as promptly as
possible thereafter, the Parties shall jointly retain the Expert.
If the Parties are unable to agree on a mutually acceptable Expert
within such fifteen (15) day period, each Party will select one (1)
Expert and those two (2) Party selected Experts will select a third
Expert within ten (10) days thereafter, and such third Expert shall
be the sole Expert to resolve such dispute in accordance with this
Section 3.3(c). Each Party shall bear its own costs associated such
Expert decision and share the costs of the Expert equally. The
determination of the Expert shall be binding on the Parties and the
Parties shall act in accordance with the Expert’s
decision.
3.4
Alliance Manager. Each Party will assign
an Alliance Manager, who will be a non-voting member of the JSC and
the primary contact for all non-technical matters of governance,
who will organize JSC meetings as reasonably necessary and lead the
drafting of minutes. Either Alliance Manager may also call for
ad-hoc meetings if one of the Parties deems that
necessary.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-18-
ARTICLE 4 DEVELOPMENT
4.1
General. Subject to the terms and
conditions of this Agreement (including without limitation the
Retained Rights), EverInsight shall be solely responsible for the
Development of the Compound and Licensed Product in the Licensed
Field in the Territory, including the performance of preclinical
and clinical studies of any Compound or any Licensed Product in the
Licensed Field in the Territory. Notwithstanding the foregoing,
VistaGen shall be solely responsible for conducting a six (6) month
rat toxicology study in China, which study will be conducted by
[*****] at
VistaGen’s own cost and expense.
4.2
Development Plan. EverInsight’s
initial plan for the Development of the Compound and Licensed
Product (the “Development
Plan”) is attached hereto as Exhibit D. The
Development Plan will include, among other things, critical
activities to be undertaken, certain timelines, go/no go decision
points and relevant decision criteria and certain allocations of
responsibilities between the Parties to facilitate the
registration, launch, and Commercialization of the Compound and
Licensed Product in the Territory. The Development Plan will be
focused on efficiently obtaining Regulatory Approval for a Licensed
Product in the Licensed Field in the Territory, with an emphasis on
Mainland China and South Korea. EverInsight shall conduct all
Development of the Compound and Licensed Product in the Licensed
Field in the Territory in accordance with the Development Plan. The
Development Plan also shall take into consideration Development,
Regulatory Approval, or commercial impacts on the Licensed Product
outside the Licensed Field and Territory. From time to time, but at
least once per Calendar Year, EverInsight will, with the assistance
of the JSC, update the Development Plan and submit such updated
plan to the JSC for review, discussion, and approval. Any
disagreement or dispute in the JSC regarding the Development Plan
shall be resolved in the manner set forth in Section 3.3 (JSC
Decision-Making). If any updated or new terms of the Development
Plan contradict, or create inconsistencies or ambiguities with, the
terms of this Agreement, then the terms of this Agreement shall
govern.
4.3
Diligence.
(a)
Commercially Reasonable Efforts by
EverInsight. EverInsight, directly and/or with or through
its Affiliates or Sublicensees, shall use Commercially Reasonable
Efforts to Develop and obtain Regulatory Approval for the Compound
and the Licensed Product in the Licensed Field in Mainland China
and South Korea in accordance with the Development
Plan.
(b)
Commercially Reasonable Efforts by
VistaGen. VistaGen, directly and/or with or through its
Affiliates or Sublicensees, shall use Commercially Reasonable
Efforts to Develop and obtain Regulatory Approval for the Compound
and the Licensed Product in the Licensed Field in the
U.S.
4.4
Development Costs.
(a)
As between the
Parties, XxxxXxxxxxx shall be solely responsible for the cost for
the Development of the Compound and the Licensed Product in the
Licensed Field in the Territory and VistaGen shall be solely
responsible for the cost for the Development of the Compound and
the Licensed Product in the Licensed Field outside the Territory,
except as otherwise provided in Section 4.1 and 4.4(b). For
clarity, VistaGen shall be responsible for the cost of the
toxicology study to be conducted in China as described in Section
4.1.
(b)
EverInsight shall
have the option, but not the obligation, to participate in global
Phase 3 Clinical Trial and long-term safety study in social anxiety
disorder conducted by VistaGen (or its Affiliates or
(sub)licensees) to support Regulatory Approval in the Territory.
VistaGen shall keep EverInsight informed on its global development
plan for the Compound and Licensed Product. Before initiating any
global Phase 3 Clinical Trial and long-term safety study for the
Licensed Product, VistaGen shall notify EverInsight and provide
EverInsight with relevant study plan and protocol for review and
consideration. If EverInsight elects to participate in such global
Phase 3 Clinical Trial or long-term safety study, then the Parties
shall ensure that sufficient number of subjects in the Territory
are enrolled in such clinical trial in order to support Regulatory
Approval in the Territory, and EverInsight shall (i) be responsible
for the conduct of, and all direct costs and expenses of
conducting, such clinical trial in the Territory (provided however
that if VistaGen requests in writing that EverInsight enrolls in
the Territory more subjects than the minimum number required for
Regulatory Approval in the Territory, then the Parties shall
discuss such request in good faith, and if EverInsight agrees to
enroll such excess subjects, VistaGen shall reimburse EverInsight
for the clinical trial cost for such excess subjects); and (ii) pay
or reimburse VistaGen for a pro
rata portion (based on the number of subject enrolled in the
Territory vs worldwide in such clinical trial) of all of the
Indirect Costs of such global clinical trial outside of the
Territory, not to exceed [*****] of the total
Indirect Costs of such global clinical trial. VistaGen shall
provide EverInsight with reasonable supporting documents (including
Third Party invoices) for the Indirect Costs of such global
clinical trial. For clarity and notwithstanding the foregoing, the
cost sharing in this subsection (b) shall not apply to the first
U.S. Phase 3 Clinical Trial, the cost of which shall be solely born
by XxxxxXxx.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-19-
4.5
Development Records and
Reports.
(a)
EverInsight shall,
and shall cause its Affiliates and its and their Sublicensees to,
maintain, in good scientific manner, complete and accurate records
and reports pertaining to Development of Licensed Product
hereunder, in sufficient detail for VistaGen to verify
EverInsight’s compliance with its obligations under this
Agreement. Such records and reports shall (i) be summarized in
English in sufficient detail for VistaGen to verify
EverInsight’s compliance with its obligations under this
Agreement and for VistaGen to properly use such records and reports
for patent and regulatory purposes, (ii) be appropriate for patent
and regulatory purposes; (iii) be in compliance with Applicable
Laws; (iv) properly reflect all work done and results achieved in
the performance of its Development activities hereunder; (v) record
only such activities and not include or be commingled with records
of activities outside the scope of this Agreement; and (vi) be
retained by EverInsight for at least five (5) years after the
expiration or termination of this Agreement in its entirety or for
such longer period as may be required by Applicable
Laws.
(b)
Starting on
[*****],
EverInsight shall provide VistaGen with an annual written report
summarizing in sufficient detail for VistaGen to verify
EverInsight’s compliance with its obligations under this
Agreement (i) the Development activities conducted in the preceding
Calendar Year by it and its Affiliates and Sublicensees, and (ii)
the Development activities planned to be conducted in such Calendar
Year by it and its Affiliates and Sublicensees. If at any time
VistaGen’s representatives on the JSC are not fully able to
perform their rights and duties on the JSC in the absence of a
review of any of such books and records, EverInsight shall, upon
reasonable written request from such JSC representative, provide a
copy of such records to the JSC.
ARTICLE 5 REGULATORY
5.1
Regulatory Responsibilities. EverInsight
shall be responsible, at its cost and subject to the Retained
Rights and except as set forth in this ARTICLE 5, for all
regulatory activities necessary to prepare, obtain and maintain
Marketing Authorization Applications, Regulatory Filings and other
Regulatory Approvals for the Compound and Licensed Product in the
Licensed Field in the Territory. EverInsight shall keep VistaGen
informed of regulatory developments related to the Compound or
Licensed Product in the Licensed Field in the Territory via the
JSC.
5.2
Regulatory Reports. Starting on
[*****],
EverInsight shall provide VistaGen with an annual written report
summarizing the clinical data and safety results generated from the
regulatory activities performed in the preceding Calendar Year by
it and its Affiliates and Sublicensees, in sufficient detail for
VistaGen to verify EverInsight’s compliance with its
obligations under this Agreement and for VistaGen to properly use
data and results for patent and regulatory purposes.
5.3
Regulatory Cooperation.
(a)
EverInsight. EverInsight shall notify
VistaGen of all material Regulatory Documentation submitted or
received by EverInsight or its Affiliates or Sublicensees that are
related to any Licensed Product in the Territory reasonably prior
to such submission or reasonably after receipt. Moreover, with
respect to Regulatory Filings in the Territory, EverInsight will
provide VistaGen with the draft of such Regulatory Filings and an
English summary thereof reasonably prior to submission so that
VistaGen may have reasonable opportunity to review and comment on
them. EverInsight shall consider all comments of VistaGen in good
faith, taking into account the best interests of the Development,
Regulatory Approval and/or Commercialization of the Licensed
Product, but has no obligation to accept any comments of VistaGen,
except to the extent that ignoring such comment could reasonably be
expected to have a material adverse effect on the Development of,
Regulatory Approval for, or Commercialization or Exploitation of
the Compound or a Licensed Product outside the Territory. Material
submissions made by EverInsight to, or correspondence with,
Regulatory Authorities will be provided to VistaGen reasonably in
advance to enable translation by VistaGen, if any such submissions
or correspondence are not available in English. VistaGen shall not
provide any Regulatory Documentation of EverInsight, its
Affiliates, or Sublicensees to any of VistaGen’s sublicensees
who does not agree pursuant to Section 5.3(b) (VistaGen) to permit
its Regulatory Documentation to be shared with EverInsight, its
Affiliates, and its Sublicensees.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-20-
(b)
VistaGen. VistaGen shall provide or make
available to EverInsight copies of all material Regulatory
Documentation submitted or received by VistaGen or its Affiliates
that are related to any Licensed Product outside the Territory
reasonably after such submission or receipt. VistaGen shall use
Commercially Reasonable Efforts to negotiate an agreement with each
sublicensee to make available to EverInsight copies of all material
Regulatory Documentation that are related to any Licensed Product
outside the Territory that are Controlled by its such sublicensee.
Upon reasonable request, VistaGen will support EverInsight’s
regulatory filing efforts, as necessary, and in alignment with
VistaGen’s formal role as the global study sponsor. This may
include participation in certain meetings with regulatory
authorities, if requested by XxxxXxxxxxx, and signing or co-signing
the clinical study site contracts, if global sponsor’s
signature is required by the study site in the Territory. Due to
requirement by many leading clinical trial hospitals in China that
the global sponsor listed on the protocol is a party to the site
contracts, VistaGen agrees to accept this responsibility.
EverInsight shall indemnify VistaGen for such contractual
liabilities in the Territory.
(c)
Confidentiality. Any information of a
Party to which the other Party obtains access pursuant to this
Section 5.3 (Regulatory Cooperation) shall, subject to ARTICLE 10
(Confidentiality; Publication), be deemed the Confidential
Information of such first Party.
5.4
Rights of Reference.
(a)
Without any
additional consideration to VistaGen, VistaGen hereby grants to
EverInsight and its Affiliates and Sublicensees a Right of
Reference and Use, as that term is defined in 21 C.F.R. §
314.3(b) and any foreign counterpart to such regulation, to all
VistaGen Regulatory Documentation and the VistaGen Development Data
to the extent necessary or reasonably useful for EverInsight to
Exploit the Compound or Licensed Product in the Licensed Field in
the Territory.
(b)
Without any
additional consideration to EverInsight, EverInsight hereby grants
to VistaGen and its Affiliates, and any current or future direct or
indirect (sub)licensee of VistaGen with respect to the Compound or
a Licensed Product, a Right of Reference and Use, as that term is
defined in 21 C.F.R. § 314.3(b) and any foreign counterpart to
such regulation, to the EverInsight Development Data to the extent
necessary or reasonably useful for VistaGen to Exploit the
Compound, Licensed Product(s) in the Licensed Field outside of the
Territory.
(c)
Promptly after a
Party, its Affiliate or its or their licensees or Sublicensees
generate(s) any VistaGen Development Data or EverInsight
Development Data (as applicable), such Party shall provide the
other Party with copies of such data, and the other Party may use
such data pursuant to the license granted to it under Section 2.1
or 2.2 (as applicable).
(d)
Each Party will
provide a signed statement to this effect, if requested by the
other Party, that is consistent with the requirements of 21 C.F.R.
§ 314.50(g)(3) or any foreign counterpart to such regulation,
in the case of a request by either Party, for the limited purpose
described in this Section 5.4 (Rights of Reference).
(e)
Other than as
expressly set forth in this Section 5.4 (Rights of Reference),
nothing in this Section 5.4 shall require either Party to take, or
forbear to take, any action.
(f)
Any information of
a Party to which the other Party obtains access pursuant to this
Section 5.4 (Rights of Reference) shall, subject to Sections 10.1
(Duty of Confidence) and 10.2 (Exceptions), be deemed the
Confidential Information of such first Party. For avoidance of
doubt, a Party’s submission of information of the other Party
to which such Party obtains access pursuant to this Section 5.4
(Rights of Reference) to a Regulatory Authority shall be governed
by and subject to the terms of ARTICLE 10 (Confidentiality;
Publication).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-21-
5.5
Recalls, Suspensions or Withdrawals.
Each Party shall notify the other Party promptly following its
determination that any event, incident or circumstance has occurred
that would reasonably be expected to result in the need for a
recall, market suspension or market withdrawal of a Licensed
Product in the Licensed Field and shall include in such notice the
reasoning behind such determination and any supporting facts. As
between the Parties, EverInsight shall have the right to make the
final determination whether to voluntarily implement any such
recall, market suspension or market withdrawal in the Licensed
Field in the Territory; provided that prior to any implementation
of such a recall, market suspension or market withdrawal,
EverInsight shall consult with VistaGen and shall consider
VistaGen’s comments in good faith. If a recall, market
suspension or market withdrawal is mandated by a Regulatory
Authority in the Territory, as between the Parties, EverInsight
shall initiate such a recall, market suspension or market
withdrawal in compliance with Applicable Laws. For all recalls,
market suspensions or market withdrawals undertaken pursuant to
this Section 5.5 (Recalls, Suspensions or Withdrawals), as between
the Parties, EverInsight shall be solely responsible for the
execution thereof. Subject to ARTICLE 13 (Indemnification;
Liability), EverInsight shall be responsible for all costs and
expenses of any such recall, market suspension or market
withdrawal. Notwithstanding the foregoing, any recall, market
suspension or market withdrawal that relates to the Manufacture and
supply of a Compound or Licensed Product by VistaGen to EverInsight
shall be governed by the terms and conditions of the Initial Supply
Agreement.
5.6
Pharmacovigilance Agreement; Global Safety
Database. The Parties shall enter into a pharmacovigilance
agreement at least [*****] days prior to
the Initiation of any Clinical Trial of Licensed Product(s) by
EverInsight in the Territory providing for the terms pursuant to
which (i) VistaGen shall establish, hold and maintain (at
VistaGen’s sole cost and expense) the global safety database
for Licensed Product and (ii) the Parties will establish a mutually
agreed procedure for safety data sharing, adverse event reporting
and prescription events monitoring related to the Licensed
Product(s), which procedure shall be in accordance with, and enable
the Parties to fulfill, their respective regulatory reporting
obligations under, all applicable laws. Each Party shall be
responsible for reporting safety data, adverse events, quality
complaints related to the Products to the global safety database
and to the applicable Regulatory Authorities in its Respective
Territory, as well as responding to safety issues and to all
requests of Regulatory Authorities related to the Licensed Product
in its Respective Territory, in each case at its own cost. VistaGen
shall provide EverInsight with access to the global safety database
to allow EverInsight to comply with its regulatory reporting
obligations under applicable laws in the Territory.
5.7
Regulatory Inspections. If any
Regulatory Authority (i) contacts a Party, its Affiliates or their
respective licensees or Sublicensees with respect to the alleged
improper Development, Manufacture or Commercialization of any
Licensed Product; (ii) conducts, or gives notice of its intent to
conduct, an inspection at such Party’s, its Affiliate’s
or licensee’s or Sublicensee’s facilities used in the
Development or Manufacturing of Licensed Product or (iii) takes, or
gives notice of its intent to take, any other regulatory action
with respect to any activity of such Party, its Affiliates or
licensees or Sublicensees that could reasonably be expected to
materially adversely affect any Development, Manufacture or
Commercialization activities with respect to the Licensed Product,
whether in or outside the Territory, then such will promptly notify
the other Party of such contact, inspection or notice and shall
provide the other Party with copies of all materials,
correspondence, statements, forms and records filed with or
received from the Regulatory Authority in connection
therewith.
ARTICLE 6 SUPPLY
6.1
Supply. Subject to the first sentence of
Section 6.2(a), before the completion of the manufacturing
technology transfer under Section 6.2(b), EverInsight shall
exclusively obtain its supply of Licensed Product from VistaGen,
and VistaGen shall supply to EverInsight all the Licensed Product
requested by EverInsight for Development in the Territory
[*****].
Nothing will prevent VistaGen from manufacturing or having
manufactured all or any portion of the Licensed Product in the
Territory.
6.2
Potential Alternative
Suppliers.
(a)
The Parties will
collaborate and jointly explore opportunities for identification
and qualification of alternative suppliers of the Licensed Product
with the mutual intent of reducing substantially the Cost of Goods
for the supply of the Licensed Product, for EverInsight, its
Affiliates, licensees and sublicensees after Regulatory Approval in
the Territory and for VistaGen and its Affiliates, licensees and
sublicensees outside the Territory after Regulatory Approval. Upon
mutual identification and qualification of such alternative
supplier(s) capable of reducing substantially the Cost of Goods for
the supply of the Licensed Product, VistaGen shall, pursuant to
Section 6.4, conduct a technology transfer of all relevant
manufacturing process it possesses to such alternative supplier(s)
to allow the Parties to obtain supply of Licensed Product from such
alternative supplier(s) at reduced Cost of Goods.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-22-
(b)
If the Parties are
unable to agree on the alternative supplier(s) under Section 6.2(a)
before the [*****], then, at
EverInsight’s option and at no additional cost, VistaGen
will, pursuant to Section 6.4, conduct a technology transfer of all
relevant manufacturing processes it possesses to EverInsight or a
Third Party manufacturer(s) that has been chosen by EverInsight for
commercial supply in the Territory. VistaGen shall make a good
faith effort to complete such technology transfer within
[*****] days
of EverInsight’s selection of the manufacturer(s)
(“Manufacturing Transfer
Period”). The Parties agree on a timely and proactive
sharing of manufacturing data to facilitate such manufacture
technology transfer. This data sharing might be done through the
JSC or through a Joint Manufacturing Committee that may be
established by the Parties.
6.3
Supply Agreement.
(a)
Initial Supply Agreement. VistaGen and
EverInsight agree to negotiate in good faith within [*****] days after the
Effective Date a separate agreement concerning the short-term
supply of the Compound and Licensed Product for EverInsight’s
Development use (including preclinical and/or clinical use) (the
“Initial Supply
Agreement”), [*****]. Under this
Initial Supply Agreement, EverInsight shall provide written notice
to VistaGen with rolling forecasts (at least quarterly) promptly
following its decision on initiating preclinical experiments or
clinical trials. Notwithstanding the foregoing, nothing in this
Agreement nor the Initial Supply Agreement shall restrict, impair
or otherwise limit VistaGen’s ability to manufacture the
Compound or Licensed Product in the Territory for use outside the
Territory. The Initial Supply Agreement shall include language on
VistaGen’s Commercially Reasonable Efforts to reduce the
manufacturing costs and an audit right for EverInsight to review
such manufacturing costs
(b)
Commercial Supply Agreement. Upon
EverInsight’s request, VistaGen shall introduce EverInsight
to VistaGen’s contract manufacturer(s) and reasonably
cooperate with EverInsight in its negotiation of a commercial
supply agreement for the Licensed Product directly with such
contract manufacturer(s).
(c)
Quality Agreement. In connection with
negotiation of the Initial Supply Agreement, VistaGen and
EverInsight agree to negotiate in good faith a separate agreement
concerning the quality of the Compound and Licensed Product
supplied by VistaGen to EverInsight (the “Quality
Agreement”). The Quality Agreement might either be an
attachment of the Initial Supply Agreement or a
stand-alone-agreement. Such Quality Agreement will include language
about the acceptance criteria and ways to handle failures of the
quality criteria among other terms.
6.4
Manufacturing Technology Transfer. In
order to enable the Parties to have Manufactured the Compound and
Licensed Product by the mutually-designated Third-Party
manufacturer(s) consistent with the terms of Section 6.2(a)
(Potential Alternative Suppliers), or if such mutually agreed
Third-Party manufacturer cannot be found by the [*****], to enable
EverInsight to Manufacture or have Manufactured the Compound and
Licensed Product for the Territory pursuant to Section 6.2(b),
VistaGen shall (a) perform or facilitate technology transfer to
such mutually-designated Third Party manufacturer, EverInsight or
the Third Party manufacturer selected by EverInsight (the
“Designated
Manufacturer(s)”) as is necessary or reasonably useful
in the Manufacture of the Compound and Licensed Product and as of
such date are being used by VistaGen or VistaGen CMO (as defined
below) to Manufacture the Compound and Licensed Product (the
“Licensed Manufacturing
Know-How”) solely for the Designated Manufacturer(s)
to Manufacture the Compound and Licensed Products in accordance
with the terms and conditions of this Agreement; (b) identify in
writing all Subcontractors who Manufacture Compounds or Licensed
Product for VistaGen (each, an “VistaGen CMO”); and (c) provide
technical assistance (both on site and otherwise) in the transfer
and demonstration of the Licensed Manufacturing Know-How that is
necessary to Manufacture the Compound and Licensed Product. To the
extent that any Licensed Manufacturing Know-How is in the Control
of VistaGen but is in the possession of a VistaGen CMO (and is not
in VistaGen’s possession), then during the Manufacturing
Transfer Period, VistaGen will use Commercially Reasonable Efforts
to facilitate the transfer of such Licensed Manufacturing Know-How
from such VistaGen CMO to the Designated Manufacturer(s), and/or
cause such VistaGen CMO to make such Licensed Manufacturing
Know-How available to the Designated Manufacturer(s). VistaGen
shall be solely responsible for the cost and expense of such
technology transfer and no payment shall be due from EverInsight to
VistaGen or any Third Party (including VistaGen CMO) for such
technology transfer.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-23-
ARTICLE 7 COMMERCIALIZATION
7.1
General. Subject to the terms and
conditions of this Agreement and the Commercialization Plan,
EverInsight shall be responsible for all aspects of the
Commercialization of the Licensed Product in the Licensed Field in
the Territory, including: (a) developing and executing a commercial
launch and pre-launch plan, (b) negotiating with applicable
Government Authorities regarding the price and reimbursement status
of the Licensed Product and obtaining and maintaining Pricing
Approvals; (c) marketing, medical affairs, and promotion; (d)
booking sales and distribution and performance of related services;
(e) subject to the provisions of Section 5.5 (Recalls, Suspensions
or Withdrawals) handling all aspects of order processing, invoicing
and collection, inventory and receivables; (f) providing customer
support, including handling medical queries, and performing other
related functions; and (g) conforming its practices and procedures
to Applicable Laws relating to the marketing, detailing and
promotion of Licensed Product in the Licensed Field in the
Territory. As between the Parties, EverInsight shall be solely
responsible for the costs and expenses of Commercialization of the
Licensed Product in the Licensed Field in the
Territory.
7.2
Commercialization Plan. EverInsight
shall conduct all Commercialization of Compound and Licensed
Product in the Licensed Field in the Territory in accordance with a
commercialization plan (as amended from time to time in accordance
with this Agreement, the “Commercialization Plan”), the
initial version of which EverInsight will prepare and provide to
the JSC no later than [*****] prior to the
anticipated First Commercial Sale of Licensed Product in the
Licensed Field in the Territory and which initial Commercialization
Plan shall be subject to the review (but not approval) of the
Parties through the JSC. From time to time, but at least once every
Calendar Year, EverInsight will update the Commercialization Plan
and submit such updated plan to the JSC for review and discussion.
If any updated Commercialization Plan omits details that a VistaGen
representative reasonably believes is necessary for (i) the proper
functioning of the JSC or (ii) to verify EverInsight’s
compliance with its obligations under this Agreement, then
EverInsight shall take into reasonable consideration such comments
and, if necessary, further update such Commercialize Plan. If the
terms of the Commercialization Plan contradict, or create
inconsistencies or ambiguities with, the terms of this Agreement,
then the terms of this Agreement shall govern.
7.3
Commercial Diligence. Upon Regulatory
Approval of a Licensed Product in mainland China or South Korea,
EverInsight, directly and/or with or through Affiliates or
Sublicensees, shall use Commercially Reasonable Efforts to
Commercialize such Licensed Product in the Licensed Field in such
jurisdiction.
ARTICLE 8 FINANCIAL PROVISIONS
8.1
Upfront Payment.
(a)
As partial
consideration of the rights granted by XxxxxXxx to EverInsight
hereunder, within thirty (30) Business Days after the Effective
Date, EverInsight shall pay to VistaGen a one-time, non-refundable
and non-creditable upfront payment of five million Dollars
($5,000,000).
8.2
Regulatory Milestone
Payments.
(a)
As additional
consideration of the rights granted by VistaGen to EverInsight
hereunder, within [*****] calendar days
after the first achievement of the regulatory milestone events
below (“Regulatory
Milestone Events”) by
or on behalf of EverInsight or any of its Affiliates or
Sublicensees, EverInsight or its Affiliate or Sublicensee shall
notify VistaGen of the achievement of such Regulatory Milestone
Event. The Regulatory Milestone Event triggers the corresponding
milestone payment due to VistaGen (“Milestone Payment”) and VistaGen
shall invoice EverInsight for the applicable non-refundable,
non-creditable Milestone Payment corresponding to the Regulatory
Milestone Event as shown below, and EverInsight shall remit payment
within [*****] Business Days of
the receipt of such invoice, as described in Section 8.6 (Currency;
Exchange Rate; Payments). For clarity, each Regulatory Milestone
Payment set forth above shall be due and payable only once upon the
first achievement of the corresponding Regulatory Milestone Event,
regardless of how many times such Regulatory Milestone Event is
achieved in the Territory.
●
Regulatory
Milestone Event for Licensed Product Regulatory Milestone Payment
(in U.S. Dollars):
(1)
[*****].
(2)
[*****].
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-24-
8.3
Commercial Milestones.
(a)
Within [*****] calendar days
after the end of the first Calendar Year in which aggregate annual
Net Sales for that Calendar Year for the Licensed Product in the
Territory reach any threshold indicated in the Commercial Milestone
Events listed below, EverInsight shall notify VistaGen of the
achievement of such Commercial Milestone Event and VistaGen shall
invoice EverInsight for the corresponding non-refundable,
non-creditable Milestone Payment set forth below and EverInsight
shall remit payment to VistaGen within [*****] Business Days
after the receipt of the invoice, as described in Section 8.6
(Currency; Exchange Rate; Payments).
Annual
Net Sales Milestones for Licensed Product Milestone Payments (in
Dollars) (each a “Commercial
Milestone Event”):
(1).
[*****]
(2).
[*****]
(3).
[*****]
(4).
[*****]
(5).
[*****]
(b)
For the purposes of
determining whether a Net Sales Milestone Event has been achieved,
Net Sales of Licensed Product(s) in the Territory shall be
aggregated. For clarity, the annual Net Sales Milestone Payments
set forth in this Section 8.3 (Commercial Milestones) shall be
payable only once, upon the first achievement of the applicable
Commercial Milestone Event, regardless of how many times such
Commercial Milestone Event is achieved.
(c)
If a Commercial
Milestone Event in Section 8.3 (Commercial Milestones) is achieved
and payment with respect to any previous Commercial Milestone Event
in Section 8.3 has not been made, then such previous Commercial
Milestone Event shall be deemed achieved and EverInsight shall
notify VistaGen within fifteen (15) calendar days of such
achievement. VistaGen shall then invoice EverInsight for such
unpaid previous Commercial Milestone Event(s) and EverInsight shall
pay VistaGen such unpaid previous milestone payment(s) within
thirty (30) Business Days of receipt of such invoice.
(d)
In the event that,
VistaGen believes any Commercial Milestone Event under Section
8.3(a) has occurred but EverInsight has not given VistaGen the
notice of the achievement of such Commercial Milestone Event, it
shall so notify EverInsight in writing and shall provide to
EverInsight data, documentation or other information that supports
its belief. Any dispute under this Section 8.3(d) (Commercial
Milestones - subsection (d)) that relates to whether or not a
Commercial Milestone Event has occurred shall be referred to the
JSC to be resolved in accordance with ARTICLE 3 (Governance) and
shall be subject to resolution in accordance with Section 14.10
(Dispute Resolution). The Milestone Payments made for each
Commercial Milestone Event shall be non-creditable and
non-refundable.
8.4
Royalty Payments.
(a)
Royalty Rate. Subject to the terms and
conditions of this Agreement (including Section 8.5), in partial
consideration of the rights granted by VistaGen to EverInsight
hereunder, EverInsight shall pay to VistaGen non-refundable,
non-creditable royalties based on the aggregate Net Sales of all
Licensed Product sold by EverInsight, its Affiliates and/or its or
their respective Sublicensees in the Territory during a Calendar
Year at the rates set forth in the table below. The obligation to
pay royalties will be imposed only once with respect to the same
unit of a Licensed Product.
Calendar Year Net
Sales (in Dollars) for all Licensed Product in the Territory
Royalty Rates as a Percentage (%) of Net Sales
(1).
[*****]
(2).
[*****]
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-25-
(b)
Royalty Term. Royalties under this
Section 8.4 (Royalty Payments) shall be payable on a
jurisdiction-by-jurisdiction and Licensed Product-by-Licensed
Product basis from the First Commercial Sale of a Licensed Product
in a jurisdiction until the latest to occur of: (i) expiration of
the last‑to‑expire Valid Claim that effectively
provides market exclusivity of such Licensed Product in such
jurisdiction in the Territory; (ii) expiration of Regulatory
Exclusivity for such Licensed Product in such Jurisdiction in the
Territory; and (iii) ten (10) years after the First Commercial Sale
of the Licensed Product in such jurisdiction in the Territory (the
“Royalty Term”
for the Licensed Product in the relevant jurisdiction). After
expiration of the Royalty Term for a particular Licensed Product in
a particular jurisdiction, the license granted by VistaGen to
EverInsight hereunder shall continue and shall become fully
paid-up, royalty free, perpetual and irrevocable with respect to
such Licensed Product in such jurisdiction.
(c)
Royalty Reports and Payment. Within
ninety (90) calendar days after each Calendar Quarter of each
Calendar Year, commencing with the Calendar Quarter during which
the First Commercial Sale of any Licensed Product is made anywhere
in the Territory, EverInsight shall provide VistaGen with a report
that contains the following information for the applicable Calendar
Quarter, on a jurisdiction-by-jurisdiction basis: (A) Net Sales in
the Territory; (B) a calculation of the royalty payment due on Net
Sales in the Territory; and (C) the exchange rates used. After the
receipt of such royalty report, VistaGen shall invoice EverInsight
for the royalty payment set forth in such royalty report. Within
thirty (30) Business Days after the receipt of the invoice,
EverInsight will pay VistaGen all royalties owed with respect to
Net Sales for such Calendar Quarter. If, during the following
Calendar Quarter, XxxxXxxxxxx discovers that it reported an
incorrect amount of Net Sales in the Territory and/or the amounts
payment due on such Net Sales in the immediately preceding Calendar
Quarter, then EverInsight may, subject to review by VistaGen,
adjust and reconcile any such calculation of Net Sales and/or any
such underpayment or overpayment of royalty payments due, and shall
timely report the same within thirty (30) calendar days after such
following Calendar Quarter.
8.5
Royalty Adjustments. Except as otherwise
set forth in this Agreement, royalties due hereunder are subject to
adjustment as set forth below (such adjustments to be prorated for
the Calendar Quarter in which the adjustment becomes
applicable):
(a)
Royalty Adjustment for Patent
Expiration. In the event that in any jurisdiction in the
Territory in any Calendar Quarter during the Royalty Term for a
Licensed Product, there is no Valid Claim that provides effective
market exclusivity for such Licensed Product (or the Compound
contained in such Licensed Product) in such jurisdiction in such
Calendar Quarter, then the royalty rate set forth in Section 8.4(a)
(Royalty Rate) with respect to such Licensed Product in such
jurisdiction in such Calendar Quarter shall be reduced by
[*****];
(b)
Royalty Adjustment for Generic
Competition. In the event that in any jurisdiction in the
Territory in any Calendar Quarter during the Royalty Term for a
Licensed Product, there is Generic Competition for such Licensed
Product in such jurisdiction in such Calendar Quarter, then the
royalty rate set forth in Section 8.4(a) (Royalty Rate) with
respect to such Licensed Product in such jurisdiction in such
Calendar Quarter shall be reduced by [*****] (provided
however that the royalty reduction under Section 8.5(a) shall not
apply to such Licensed Product in such jurisdiction in such
Calendar Quarter if the royalty reduction under Section 8.5(b)
applies).
8.6
Currency; Exchange Rate; Payments. All
payments required to be made by EverInsight under this Agreement
shall be made in Dollars. All payments payable to, or invoiced from
or on behalf of, VistaGen shall be paid bank wire transfer in
immediately available funds to one or more bank accounts of
VistaGen as designated in written notice from VistaGen. If any
currency conversion shall be required in connection with any
payment hereunder, such conversion shall be made by using the
exchange rates at the closing on the last Business Day of the
Calendar Quarter to which such payment relates as reported in The
Wall Street Journal on the following day.
8.7
Late Payments. Any payments or portions
thereof due hereunder that are not paid on the date such payments
are due under this Agreement shall bear interest at an annual rate
equal to two (2) percentage points above the prime rate as
published by The Wall Street Journal or any successor thereto on
the first day of each Calendar Quarter in which such payments are
overdue calculated on the number of days such payment is
delinquent.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-26-
8.8
Taxes.
(a)
Taxes on Income. Notwithstanding
anything else set forth in this Section 8.8 (Taxes), each Party
shall solely bear and pay all Taxes imposed on such Party’s
net income or gain (however denominated) arising directly or
indirectly from the activities of the Parties under this
Agreement.
(b)
Tax Payments. The upfront payment,
milestone payments, royalties, and any other payment payable by
EverInsight to VistaGen pursuant to this Agreement (each, a
“Payment”) shall
be paid free and clear of any and all Taxes (which, for clarity,
shall be the responsibility of EverInsight), except for any Taxes
required by Applicable Laws to be withheld or deducted. The Parties
agree to cooperate with one another and use reasonable efforts to
avoid or reduce Tax withholding or similar obligations in respect
of the payments made under this Agreement. To the extent
EverInsight is required to deduct and withhold Taxes on any payment
to VistaGen, EverInsight shall deduct those Taxes from the
remittable payment, pay the Taxes to the proper tax authority in a
timely manner, and promptly send proof of payment to VistaGen.
VistaGen shall provide EverInsight any tax forms that may be
reasonably necessary in order for EverInsight to not withhold tax
or to withhold tax at a reduced rate under an applicable bilateral
income tax treaty. VistaGen shall use reasonable efforts to provide
any such tax forms to EverInsight in advance of the due
date.
8.9
Financial Records and Audit. EverInsight
shall (and shall ensure that its Affiliates and Sublicensees will)
maintain complete and accurate books and records pertaining to the
Commercialization of Licensed Product hereunder, including books
and records of invoiced sales and Net Sales of Licensed Product, in
sufficient detail to calculate and verify all amounts payable
hereunder and in sufficient detail to permit VistaGen to confirm
the accuracy of any royalty payments, other amounts paid or payable
under this Agreement and to verify the achievement of Milestone
Events under this Agreement. EverInsight shall and shall cause its
Affiliates and its and their Sublicensees to, retain such books and
records until the later of (a) three (3) years after the end of the
period to which such books and records pertain; (b) the expiration
of the applicable tax statute of limitations (or any extensions
thereof); and (c) for such period as may be required by Applicable
Laws. Upon at least thirty (30) Business Days’ prior notice,
such records shall be open for examination, during regular business
hours, for a period of three (3) Calendar Years from the end of the
Calendar Year to which such records pertain, and not more often
than once each Calendar Year, by an independent and internationally
recognized certified public accountant selected by VistaGen and
reasonably acceptable to EverInsight, for the sole purpose of
verifying for VistaGen the accuracy of the financial reports
furnished by EverInsight under this Agreement or of any payments
made, or required to be made, by EverInsight to VistaGen pursuant
to this Agreement. The independent public accountant shall disclose
to VistaGen only (x) the accuracy of Net Sales reported and the
basis for royalty, Milestone Payments and any other payments made
to VistaGen under this Agreement and (y) the difference, if any, by
which such reported and paid amounts vary from amounts determined
as a result of the audit and the details concerning such
difference. Except as required by Applicable Laws, no other
information shall be provided to VistaGen. No record may be audited
more than once. VistaGen shall bear the full cost of such audit
unless such audit reveals an underpayment by EverInsight of more
than one hundred thousand Dollars ($100,000) or five percent (5%)
of the amount actually due (whichever is greater) for any Calendar
Year being audited, in which case EverInsight shall reimburse
VistaGen for the reasonable costs and expenses for such audit.
Unless disputed pursuant to Section 8.10 (Audit Dispute),
EverInsight shall pay to VistaGen any underpayment discovered by
such audit within thirty (30) days after the accountant’s
report, plus interest (as set forth in Section 8.7 (Late Payments))
from the original due date. If the audit reveals an overpayment by
EverInsight, then EverInsight may take a credit for such
overpayment against any future payments due to VistaGen and, if
there will be no future payment due, VistaGen shall promptly refund
such overpayment to EverInsight.
8.10
Audit Dispute. If EverInsight disputes
the results of any audit conducted pursuant to Section 8.9
(Financial Records and Audit), the Parties shall work in good faith
to resolve the disagreement. If the Parties are unable to reach a
mutually acceptable resolution of any such dispute within thirty
(30) days, the dispute shall be submitted for resolution to a
certified public accounting firm jointly selected by each
Party’s certified public accountants or to such other Person
as the Parties shall mutually agree (the “Auditor”). The decision of the
Auditor shall be final and the costs of such procedure shall be
borne between the Parties in such manner as the Auditor shall
determine. If the Auditor determines that there has been an
underpayment by EverInsight, EverInsight shall pay to VistaGen the
underpayment within thirty (30) days after the Auditor’s
decision, plus interest (as set forth in Section 8.7 (Late
Payments)) from the original due date. If the Auditor determines
that there has been an overpayment by EverInsight, then EverInsight
may take a credit for such overpayment against any future payments
due to VistaGen and, if there will be no future payment due,
VistaGen shall promptly refund such overpayment to
EverInsight.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-27-
ARTICLE 9 INTELLECTUAL PROPERTY RIGHTS
9.1
Ownership
of Intellectual Property
(a)
Ownership of Technology. As between the
Parties:
(1)
VistaGen shall
solely own on a worldwide basis all right, title and interest in
and to any and all VistaGen Sole Inventions, whether or not
patented or patentable, and any and all VistaGen Sole Invention
Patents; and
(2)
EverInsight shall
solely own on a worldwide basis all right, title and interest in
and to any and all EverInsight Sole Inventions, whether or not
patented or patentable, and any and all EverInsight Sole Invention
Patents.
For
clarity, each Party shall own on a worldwide basis and retain all
right, title and interest in and to any and all Know-How,
Inventions, Patents and other intellectual property rights that are
owned or otherwise Controlled (other than pursuant to the license
grants set forth in Section 2.1 (Licenses to EverInsight) and 2.2
(License to VistaGen)) by such Party or its Affiliates or its or
their (sub)licensees (or Sublicensees) (as applicable) outside of
this Agreement.
(b)
Ownership of Joint Patents and Joint
Inventions. As between the Parties:
(1)
Each of VistaGen
and EverInsight shall own an equal, undivided interest in any and
all Joint Inventions and Joint Invention Patents; and
(2)
Each of VistaGen
and EverInsight shall promptly disclose to the other in writing,
and shall cause its Affiliates and its and their respective
Sublicensees to so disclose, the development, making, conception or
reduction to practice of any Joint Inventions. Subject to the
licenses granted under Section 2.1 (License to EverInsight) and
Section 2.2 (License to VistaGen), each of VistaGen and EverInsight
shall have the right to Exploit the Joint Inventions and Joint
Invention Patents without the duty of accounting or seeking consent
from the other Party.
(c)
United States Law. The determination of
whether Inventions, Know-How and other intellectual property rights
are conceived, discovered, developed or otherwise made by a Party
for the purpose of allocating proprietary rights (including Patent,
copyright or other intellectual property rights) therein, shall,
for purposes of this Agreement, be made in accordance with
Applicable Laws in the United States as such law exists as of the
Effective Date irrespective of where or when such conception,
discovery, development or making occurs; provided that if the
application of such United States Applicable Laws prevents or
materially impairs the proper prosecution or maintenance of Patent
Rights in any jurisdiction in the Territory, then the Parties shall
mutually agree to the application of an appropriate Applicable Laws
in order to best advance and maintain the prosecution and
maintenance of such Patents in such jurisdiction in the Territory.
Each of VistaGen and EverInsight shall, and does hereby, assign,
and shall cause its Affiliates and its and their (sub)licensees and
Sublicensees to so assign, to the other Party, without additional
compensation, such right, title and interest in and to any
Inventions, Know-How, Patents and other intellectual property
rights with respect thereto, as is necessary to fully effect, as
applicable, the sole or joint ownership as provided for in Section
9.1(a) (Ownership of Technology) or 9.1(b) (Ownership of Joint
Patents and Joint Inventions); subject to the license granted under
this Agreement.
(d)
Assignment Obligation. Each Party shall
cause all Persons who perform Development activities, Manufacturing
activities or regulatory activities for such Party under this
Agreement or who conceive, discover, develop or otherwise make any
Inventions, Know-How or other intellectual property rights by or on
behalf of either Party or its Affiliates or its or their
(sub)licensees (or Sublicensees) under or in connection with this
Agreement to be under an obligation to assign to such Party their
rights in any Inventions, Know-How, Patents and other intellectual
property to the extent related to the Compound or Licensed Product,
except where Applicable Laws requires otherwise and except in the
case of governmental, not-for-profit and public institutions that
have standard policies against such an assignment and except in the
case of generally applicable (i.e., applicable generally to
products other than the Licensed Product) Inventions, Know-How,
Patents and other intellectual property (in each case, a suitable
license or right to obtain such a license, shall be
obtained).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-28-
(e)
Ownership of Product Trademarks. Subject
to Section 11.3 (Effect of Termination), as between the Parties,
(i) EverInsight shall own all right, title and interest in and to
the Product Trademarks in the Territory, (ii) EverInsight shall
have the right to market the Licensed Product in the Licensed Field
in the Territory under the Product Trademarks and all goodwill
associated therewith will inure to the benefit of EverInsight and
(iii) VistaGen may not use the Product Trademarks without obtaining
a proper trademark license from EverInsight (except to the extent
necessary to perform its obligations under this
Agreement).
(f)
Ownership of Corporate Names. As between
the Parties, each Party shall retain all right, title and interest
in and to its Corporate Names.
(g)
Ownership of Development Data. Subject
to ARTICLE 2 (Licenses) and Section 11.3 (Effect of Termination),
EverInsight shall own EverInsight Development Data and VistaGen
shall own VistaGen Development Data.
9.2
Patent Prosecution and
Maintenance.
(a)
VistaGen shall have
the first right, but not the obligation, to control the
preparation, filing, prosecution (including any interferences,
reissue proceedings and reexaminations) and maintenance of all
Licensed Patents and Joint Patents, both in and outside the
Territory, by counsel of its own choice, except that such counsel
in the Territory shall be reasonably acceptable to EverInsight
(such acceptance not to be unreasonably withheld, delayed or
conditioned). VistaGen shall consult with EverInsight and keep
EverInsight informed of the status of such Patents in the Territory
and also in the US and EU, and shall promptly provide EverInsight
with all material correspondence received from any patent authority
in the Territory and also in the US and EU in connection therewith.
In addition, VistaGen shall promptly provide EverInsight with
drafts of all proposed material filings and correspondence to any
patent authority in the Territory and also in the US and EU with
respect to such Patents for EverInsight’s review and comment
at least thirty (30) days prior to the submission of such proposed
filings and correspondence. VistaGen shall confer with EverInsight
and consider in good faith EvxxXxxxxxx’s comments prior to
submitting such filings and correspondence, provided that
EverInsight provides such comments within fifteen (15) days (or a
shorter period reasonably designated by VistaGen if fifteen (15)
days is not practicable given the filing deadline) of receiving the
draft filings and correspondence from VistaGen. VistaGen shall also
keep EverInsight informed as to the payment schedule for patent
maintenance fee for the Licensed Patents and Joint Patents.
VistaGen shall be responsible for the costs and expenses incurred
by VistaGen for the preparation, filing, prosecution and
maintenance of the Licensed Patents and Joint Patents both in and
outside the Territory. For the avoidance of doubt, VistaGen shall
be responsible for all costs incurred prior to the Effective Date
with respect to the prosecution and maintenance of any Licensed
Patents. If EverInsight reasonably determines that a Licensed
Patent added after the Effective Date (other than Patent Rights
added by an In-License Agreement that EverInsight has accepted
pursuant to Section 2.4(b)(1) (In-License Agreements)) or Joint
Patent that EverInsight subsequently determines is of low value to
EverInsight, then EverInsight has the right upon at least sixty
(60) days’ prior written notice to remove such Licensed
Patent or Joint Patent from the Licensed Technology hereunder, in
which case, following delivery of such notice to VistaGen, (1) the
license of Licensed Technology to EverInsight under Section 2.1
(License to EverInsight) as to such Licensed Patent or Joint Patent
shall be terminated; (2) the claims of such Licensed Patent or
Joint Patent, as the case may be, shall be excluded from Valid
Claim; and (3) if requested by VistaGen, EverInsight shall assign,
and shall cause its Affiliates and its and their (sub)licensees and
Sublicensees to so assign, to VistaGen, without additional
compensation, EverInsight’s right, title and interest in and
to the relevant Joint Patent (provided that EverInsight shall
retain a non-exclusive, fully paid, royalty free, sublicenseable
(through multiple tiers), perpetual and irrevocable license and
right under the Joint Patent assigned to VistaGen).
(b)
In the event that
VistaGen desires to abandon or cease prosecution or maintenance of
any Licensed Patent in the Territory (or any jurisdiction therein)
or any Joint Patent anywhere in the world, VistaGen shall provide
reasonable prior written notice to EverInsight of such intention to
abandon (which notice shall, to the extent possible, be given no
later than thirty (30) days prior to the next deadline for any
action that must be taken with respect to any such Patent in the
relevant patent office). In such case, upon EvxxXxxxxxx’s
written election provided no later than twenty (20) days after such
notice from VistaGen, EverInsight shall have the right to assume
prosecution and maintenance of such Licensed Patent or Joint Patent
at EverInsight’s sole cost and expense. If EverInsight does
not provide such election within twenty (20) days after such notice
from VistaGen, VistaGen may, in its sole discretion, abandon or
cease prosecution and maintenance of such Patent in the Territory
(or the relevant jurisdiction).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-29-
(c)
EverInsight shall
have the sole right, but not the obligation, to control the
preparation, filing, prosecution (including any interferences,
reissue proceedings and reexaminations) and maintenance of all
EverInsight Patents throughout the world, at EverInsight’s
own cost and expense.
9.3
Cooperation of the Parties. Each Party
agrees to cooperate fully in the preparation, filing, prosecution
and maintenance of Patents under Section 9.2 (Patent Prosecution
and Maintenance), at its own cost. Such cooperation includes: (a)
executing all papers and instruments, or requiring its employees or
contractors, to execute such papers and instruments, so as enable
the applicable Party to apply for and to prosecute patent
applications in any country as permitted by Section 9.2 (Patent
Prosecution and Maintenance); and (b) promptly informing the other
Party of any matters coming to such Party’s attention that
may affect the preparation, filing, prosecution or maintenance of
any such patent applications.
9.4
Infringement by Third
Parties.
(a)
Notice. In the event that either
VistaGen or EverInsight becomes aware of any infringement or
threatened infringement by a Third Party of any Licensed Patent or
Joint Patent in and/or outside the Territory, which infringing
activity involves the using, making, importing, offering for sale
and/or selling of a Licensed Product or any product that falls
within the scope of the Licensed Patents (regardless of whether or
not EverInsight and/or VistaGen is currently Developing using,
making, importing, offering for sale, selling, and/or otherwise
Commercializing the same Licensed Product), or the submission to a
Party or a Regulatory Authority in and/or outside the Territory of
an application for a product referencing a Licensed Product, or any
declaratory judgment or equivalent action challenging any Licensed
Patent or Joint Patent in and/or inside the Territory in connection
with any such infringement (each, a “Product Infringement”), it will
promptly notify the other Party in writing to that effect. Any such
notice shall include evidence to support an allegation of
infringement or threatened infringement, or declaratory judgment or
equivalent action, by such Third Party.
(b)
Enforcement of Licensed Patents and Joint
Patents. To the extent permitted by the Pherin License,
EverInsight shall have the first right, as between VistaGen and
EverInsight, but not the obligation, to bring an appropriate suit
or take other action against any Person or entity engaged in, or to
defend against, such Product Infringement in the Territory of any
Licensed Patent or Joint Patent, at its own expense and by counsel
of its own choice. VistaGen shall have the right, at its own
expense, to be represented in any such action in the Territory by
counsel of its own choice, and EverInsight and its counsel will
reasonably cooperate with VistaGen and its counsel in strategizing,
preparing and prosecuting any such action or proceeding. If
EverInsight fails to bring an action or proceeding in the Territory
with respect to such Product Infringement of any Licensed Patent or
Joint Patent within (A) ninety (90) days following the notice of
alleged infringement or declaratory judgment or (B) sixty (60) days
before the time limit, if any, set forth in the Applicable Laws for
the filing of such actions, whichever comes first, VistaGen shall
have the right, but not the obligation, to bring and control any
such action in the Territory at its own expense and by counsel of
its own choice, and EverInsight shall have the right, at its own
expense, to be represented in any such action by counsel of its own
choice. Except as otherwise agreed by the Parties as part of a
cost-sharing arrangement, any recovery or damages realized as a
result of such action or proceeding with respect to Product
Infringement of any Licensed Patent or Joint Patent, or settlement
of the same, shall be used (A) first, to reimburse the
Parties’ documented out-of-pocket legal expenses relating to
the action or proceeding; and (B) any remainder after such
reimbursement is made shall be retained by the enforcing Party,
provided, that if EverInsight is the enforcing Party, then to the
extent that any award or settlement (whether by judgment or
otherwise) with respect to any Licensed Patent or Joint Patent is
attributable to loss of sales or profits with respect to a Licensed
Product in the Licensed Field in the Territory, such amounts
(except punitive damages) that may be recovered or realized by
EverInsight after reimbursement of enforcement cost shall be
considered Net Sales and subject to the royalty obligations under
Section 8.4 (Royalty Payments) and the commercial Milestone Payment
obligations under Section 8.3 (Commercial Milestones) (provided
that such amount shall be evenly spread (on Calendar Quarterly
basis) over the time period during which the lost sales or profits
occurred for the purpose of determine aggregate annual Net Sales,
royalty tiers and achievement of commercial milestones).
Notwithstanding anything to the contrary in this Article 9, in the
event that patent enforcement or patent defense litigation
regarding the Licensed Patents occurs in multiple countries, within
or outside the Territory, then VistaGen shall have the first right
but not the obligation to bring an appropriate suit or take other
appropriate action.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-30-
(c)
Cooperation. In the event a Party brings
an action in accordance with this Section 9.4 (Infringement by
Third Parties), the other Party shall cooperate fully at its own
expense, including, if required to bring such action, and providing
access to relevant documents and other evidence including, without
limitation, making its employees available at reasonable business
hours to the Party’s counsel for all pre-trial and trial
proceedings, as well as the furnishing of a power of attorney or
being named as a party to such action as may reasonably be
necessary.
(d)
Other Infringement. VistaGen shall have
the sole right, but not the obligation, to bring and control, at
its own cost and expense, any legal action in connection with any
Product Infringement of any Licensed Patent or Joint Patent outside
the Territory and any legal action in connection with any
infringement of any Licensed Patent that is not a Product
Infringement; provided, however, that such legal action is not
combined with a legal action involving a Product Infringement. The
Parties shall jointly control any legal action in connection with
any infringement of any Joint Patent anywhere in the world that is
not a Product Infringement and is not combined with a Product
Infringement legal action. Any recovery or damages realized as a
result of such action or proceeding with respect to Product
Infringement of any Licensed Patent or Joint Patent shall be used
(A) first, but only if a Joint Patent was the subject of such legal
action, to reimburse the Parties’ documented out-of-pocket
legal expenses relating to such action or proceeding; and (B) any
remainder after such reimbursement, if applicable, shall be
retained by the Party initiating such action or proceeding (or, in
the case of Joint Patent, shared by the Parties
equally).
(e)
Effect of Pherin License. The Parties
acknowledge that provisions of the Pherin License may affect the
standing and ability of a Party to bring and control infringement
litigation, notwithstanding the contemplated allocation of
litigation-related enforcement rights as between the Parties in
this Agreement.
9.5
Infringement Claims by Third Parties. If
the Exploitation of a Licensed Product in the Licensed Field in the
Territory pursuant to this Agreement results in, or is reasonably
expected to result in, any claim, suit or proceeding by a Third
Party against EverInsight or any of its Affiliates or Sublicensees
alleging infringement by EverInsight or any of its Affiliates or
its or their Sublicensees, distributors or customers (a
“Third Party Infringement
Claim”), including any defense or counterclaim in
connection with a Product Infringement action initiated pursuant to
Section 9.4(b) (Enforcement of Licensed Patents and Joint Patents),
the Party first becoming aware of such alleged infringement shall
promptly notify the other Party thereof in writing. As between the
Parties, subject to ARTICLE 13 (Indemnification; Liability): (a)
VistaGen shall be responsible for defending any such claim, suit or
proceeding at its sole cost and expense, using counsel of
VistaGen’s choice; (b) EverInsight may participate in any
such claim, suit or proceeding with counsel of its choice at its
sole cost and expense; provided that VistaGen shall retain the
right to control such claim, suit or proceeding; (c)EverInsight
shall, and shall cause its Affiliates to, assist and co-operate
with VistaGen, as VistaGen may reasonably request from time to
time, in connection with its activities set forth in this Section
9.5 (Infringement Claims by Third Parties), including where
necessary, furnishing a power of attorney solely for such purpose
or joining in, or being named as a necessary party to, such action,
providing access to relevant documents and other evidence and
making its employees available at reasonable business hours;
provided that VistaGen shall reimburse EverInsight for its
reasonable and verifiable out-of-pocket costs and expenses incurred
in connection therewith; (d) VistaGen shall keep EverInsight
reasonably informed of all material developments in connection with
any such claim, suit or proceeding; (e) VistaGen agrees to provide
EverInsight with copies of all material pleadings filed in such
action and to allow EverInsight reasonable opportunity to
participate in the defense of the Claims; and (f) any damages, or
awards, including royalties, incurred or awarded in connection with
any Third Party Infringement Claim defended under this Section 9.5
(Infringement Claims by Third Parties) shall be borne by VistaGen,
and VistaGen shall indemnify and hold EverInsight Indemnitee
harmless from such Third Party Infringement Claim pursuant Section
13.1(d).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-31-
9.6
Invalidity or Unenforceability Defenses or
Actions. Each Party shall promptly notify the other Party in
writing of any alleged or threatened assertion of invalidity or
unenforceability of any of the Licensed Patents, Joint Patents or
EverInsight Patents worldwide, by a Third Party and of which such
Party becomes aware. As between the Parties: (a) VistaGen and
EverInsight shall coordinate with each other to defend and control
the defense of the validity and enforceability of any Joint Patents
in the Territory and share the cost and expense thereof; (b)
VistaGen shall have the first right, but not the obligation, to
defend and control the defense of the validity and enforceability
of any Licensed Patents, at its sole cost and expense, using
counsel of VistaGen’s choice; (c) EverInsight shall have the
first right, but not the obligation, to defend and control the
defense of the validity and enforceability of any EverInsight
Patents, at its sole cost and expense, using counsel of
EverInsight’s choice; provided however that, notwithstanding
the foregoing, Section 9.4 shall control with respect to any such
claim that is a Product Infringement or is a counter claim in an
enforcement action against a Project Infringement. For purposes of
this Section 9.6 (Invalidity or Unenforceability Defenses or
Actions), the Party defending and controlling the defense of the
validity and enforceability pursuant to the foregoing sentence with
respect to a Patent shall be the “Controlling Party”.
With respect to any such claim, suit or proceeding in the Territory
under this Section 9.6 (Invalidity or Unenforceability Defenses or
Actions), the non-Controlling Party may participate in such claim,
suit or proceeding with counsel of its choice at its sole cost and
expense; provided that the Controlling Party shall retain control
of the defense in such claim, suit or proceeding. If the
Controlling Party elects not to defend the applicable Patents in a
suit, then the Controlling Party shall notify the non-Controlling
Party of such election at least sixty (60) days before the time
limit, if any, set forth in Applicable Laws for defending such
actions, with the proviso that if the Controlling Party is
VistaGen, then, to the extent permitted under the Pherin License,
EverInsight shall have the right, but not the obligation, for any
such Invalidity or Unenforceability Defenses or Actions, to assume
control of the defense of any such claim, suit or proceeding at its
sole cost and expense. The non-Controlling Party in such an action
shall, and shall cause its Affiliates to, assist and co-operate
with the Controlling Party, as such Controlling Party may
reasonably request from time to time. in connection with its
activities set forth in this Section 9.6 (Invalidity or
Unenforceability Defenses or Actions), including where necessary,
furnishing a power of attorney solely for such purpose or joining
in, or being named as a necessary party to, such action, providing
access to relevant documents and other evidence and making its
employees available at reasonable business hours; provided that the
Controlling Party shall reimburse the non-Controlling Party for its
reasonable and verifiable out-of-pocket costs and expenses incurred
in connection therewith. In connection with any activities with
respect to a defense, claim or counterclaim relating to the
Licensed Patents, EverInsight Patents or Joint Patents licensed
under Section 2.1 (License to EverInsight) or Section 2.2 (License
to VistaGen), the Controlling Party shall (i) consult with the
non-Controlling Party as to the strategy for such activities, (ii)
consider in good faith any comments from the non-Controlling Party
and (iii) keep the non-Controlling Party reasonably informed of any
material steps taken and provide copies of all material documents
filed, in connection with such defense, claim or
counterclaim.
9.7
Consent for Settlement. Neither Party
shall unilaterally enter into any settlement or compromise of any
action or proceeding under this ARTICLE 9 (Intellectual Property
Rights) that would in any manner alter, diminish, or be in
derogation of the other Party’s rights under this Agreement
or otherwise without the prior written consent of such other Party,
which shall not be unreasonably withheld, conditioned or
delayed.
9.8
Common Ownership under Joint Research
Agreement. Notwithstanding anything to the contrary in this
ARTICLE 9, no Party shall have the right to make an election under
35 U.S.C. 102(c) when exercising its rights under this ARTICLE 9
without the prior written consent of the other Party. With respect
to any such permitted election, the Parties shall co-ordinate their
activities with respect to any submissions, filings or other
activities in support thereof. The Parties acknowledge and agree
that this Agreement is a “joint research agreement” as
defined in 35 U.S.C. 100(h).
9.9
Patent Extensions. VistaGen and
EverInsight shall jointly, following consultation with each other,
have decision making authority regarding, and they shall cooperate
with each other, in obtaining, patent term restoration,
supplemental protection certificates or their equivalents, and
patent term extensions with respect to the Licensed Patents, Joint
Patents, and EverInsight Patents in the Territory where applicable.
If mutually agreed, XxxxXxxxxxx shall file for such extensions at
the Parties’ shared cost and expense. If the Parties cannot
agree, the matter will be referred to the JSC for decision pursuant
to Section 3.3 (JSC Decision Making).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-32-
9.10
Trademarks. VistaGen and EverInsight
shall provide to the other Party prompt written notice of any
actual or threatened infringement of the Product Trademarks or
Licensed Trademarks in the Territory and of any actual or
threatened Claim that the use of the Product Trademarks or Licensed
Trademarks in the Territory violates the rights of any Third Party,
in each case, of which such Party becomes aware. EverInsight shall
have the right to select and register, and shall own and be
responsible for, at its expense, all Product Trademarks, trade
names, branding or logos related to the Compound or Licensed
Product in the Licensed Field in the Territory. EverInsight shall
have the sole right to take such action as EverInsight deems
necessary against a Third Party based on any alleged, threatened or
actual infringement, dilution, misappropriation or other violation
of or unfair trade practices or any other like offense relating to,
the Product Trademarks by a Third Party in the Territory at its
sole cost and expense and using counsel of its own choice and
EverInsight shall retain any damages or other amounts collected in
connection therewith.
9.11
Licensed Trademarks. If EverInsight is lawfully
required by any Regulatory Authority or otherwise desires to use
any of the Licensed Trademarks or any other Trademark used by
VistaGen (either in connection with or in lieu of Product
Trademarks selected by EverInsight) to market, promote, distribute
and/or sell any Licensed Product in the Licensed Field outside the
Territory for the purpose of Commercialization of the relevant
Licensed Product in a jurisdiction in the Territory, EverInsight
shall promptly notify VistaGen, and VistaGen shall immediately
grant EverInsight an exclusive, fully-paid, royalty-free and
sublicensable license to use such Licensed Trademark or such other
Trademark solely in connection with the Commercialization of the
relevant Licensed Product in the Licensed Field in such
jurisdiction in the Territory; provided that any such license shall
automatically terminate upon the expiration or termination of this
Agreement with respect to such Licensed Product in such
jurisdiction.
ARTICLE 10 CONFIDENTIALITY; PUBLICATION
10.1
Duty of Confidence. Subject to the other
provisions of this ARTICLE 10 (Confidentiality;
Publication):
(a)
all Confidential
Information disclosed by a Party (the “Disclosing Party”) or its
Affiliates under this Agreement will be maintained in confidence
and otherwise safeguarded by the recipient Party (the
“Receiving
Party”) and its Affiliates using at least the same
standard of care as the Receiving Party uses to protect its own
proprietary or Confidential Information (but in no event less than
reasonable care);
(b)
the Receiving
Party, its Affiliates and Representatives may only use any such
Confidential Information for the purposes of performing its
obligations or exercising its rights under this Agreement;
and
(c)
the Receiving Party
may disclose Confidential Information of the Disclosing Party only
to: (i) the Receiving Party’s Affiliates; and (ii) employees,
directors, agents, contractors, Subcontractors, consultants and
advisers of the Receiving Party and its Affiliates and, in the case
of EverInsight as the Receiving Party, its Sublicensees, in each
case to the extent reasonably necessary for the purposes of, and
for those matters undertaken pursuant to, this Agreement
(collectively, the “Representatives”); provided, that
such Representatives are bound to maintain the confidentiality, and
not to make any unauthorized use, of the Confidential Information
in a manner consistent with this ARTICLE 10 (Confidentiality;
Publication).
10.2
Exceptions. The foregoing obligations as
to particular Confidential Information of a Disclosing Party shall
not apply to the extent that the Receiving Party can demonstrate by
competent evidence that such Confidential Information:
(a)
is known by the
Receiving Party at the time of its receipt, and not through a prior
disclosure by the Disclosing Party, as demonstrated by
documentation or other competent proof of the Receiving Party, but
excluding Joint Inventions or the terms of this
Agreement;
(b)
is in the public
domain by use and/or publication before its receipt from the
Disclosing Party, or thereafter enters the public domain through no
fault of, or breach of this Agreement by, the Receiving
Party;
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-33-
(c)
is subsequently
disclosed to the Receiving Party on a non-confidential basis by a
Third Party who, to the Receiving Party’s knowledge after
reasonable inquiry, may lawfully do so and is not under an
obligation of confidentiality to the Disclosing Party;
or
(d)
is developed by the
Receiving Party independently and without use of or reference to
any Confidential Information disclosed to, or materials provided
to, it by or on behalf of the Disclosing Party, as shown by
contemporaneous written documents of the Receiving
Party.
10.3
Authorized Disclosures. Notwithstanding
the obligations set forth in Section 10.1 (Duty of Confidence), the
Receiving Party may disclose Confidential Information of the
Disclosing Party and the terms of this Agreement to the extent such
disclosure is reasonably necessary for such Disclosing Party to
perform its obligations or exercise its rights under this
Agreement, in the following instances:
(a)
filing or
prosecuting of Patents as permitted by this Agreement;
(b)
enforcing the
Receiving Party’s rights under this Agreement or performing
the Receiving Party’s obligations under this
Agreement;
(c)
in Regulatory
Filings for Licensed Product that such Party has the right to file
under this Agreement;
(d)
prosecuting or
defending litigation as permitted by this Agreement;
(e)
to the Receiving
Party’s Representatives and actual or potential Sublicensees
(in the case of EverInsight), in each case, who have a need to know
such Confidential Information in order for the Receiving Party to
exercise its rights or fulfill its obligations under this
Agreement; provided, in each case, that any such Person agrees to
be bound by terms of confidentiality and non-use (or, in the case
of the Receiving Party’s attorneys and independent
accountants, such Person is obligated by applicable professional or
ethical obligations) at least as restrictive as those set forth in
this ARTICLE 10 (Confidentiality; Publication);
(f)
to actual or
potential investors, investment bankers, lenders, other financing
sources or acquirers (and attorneys and independent accountants
thereof) in connection with potential investment, acquisition,
collaboration, merger, public offering, due diligence or similar
investigations by such Third Parties or in confidential financing
documents; provided, in each case, that any such Third Party agrees
to be bound by terms of confidentiality and non-use (or, in the
case of the Receiving Party’s attorneys and independent
accountants, such Third Party is obligated by applicable
professional or ethical obligations) that are no less stringent
than those contained in this Agreement (except to the extent that a
shorter confidentiality period is customary in the industry);
and
(g)
such disclosure is
required by court order, judicial or administrative process or
Applicable Laws; provided that in such event the Receiving Party
shall promptly inform the Disclosing Party of such required
disclosure and provide the Disclosing Party an opportunity to
challenge or limit the disclosure obligations. Confidential
Information that is disclosed as required by court order, judicial
or administrative process or Applicable Laws shall remain otherwise
subject to the confidentiality and non-use provisions of this
ARTICLE 10 (Confidentiality; Publication), and the Receiving Party
shall take all steps reasonably necessary, including seeking of
confidential treatment or a protective order, to ensure the
continued confidential treatment of such Confidential
Information.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-34-
10.4
Publication. Prior to publishing or
presenting the results of any studies carried out under this
Agreement or otherwise related to the Compound or Licensed Product,
the publishing or presenting Party shall submit the draft of the
publication or presentation to the other Party no later than
forty-five (45) calendar days prior to the planned submission for
publication or presentation for the other Party’s review and
comment. The publishing or presenting Party shall: (a) consider in
good faith any comments thereto provided by the other Party within
such review period; and (b) remove any Confidential Information of
the other Party if requested by the other Party. The other Party
shall be deemed to have consented to such publication or
presentation if it has not sent any response to the publishing or
presenting Party’s request within thirty (30) calendar days
of receipt of the draft publication or presentation from the
publishing or presenting Party. The other Party may reasonably
request a reasonable delay in publication or presentation in order
to protect patentable information. If the other Party reasonably
requests a delay, then the publishing or presenting Party shall,
and shall ensure that its Affiliate(s) or the Sublicensee(s) shall,
delay submission or presentation for a period of sixty (60)
calendar days (or such shorter period as may be mutually agreed by
the Parties) to enable the other Party to file patent applications
protecting the other Party’s rights in such
information.
10.5
Publicity/Use of Names. The Parties
intend to agree upon the content of one (1) or more press releases,
the release of which the Parties shall coordinate in order to
accomplish such release promptly upon execution of this Agreement.
Other than as set forth in the prior sentence, no other disclosure
of the existence, or the terms, of this Agreement may be made by
either Party or its Affiliates, and neither Party shall use the
name, trademark, trade name or logo of the other Party, its
Affiliates or their respective employee(s) in any publicity,
promotion, news release or disclosure relating to this Agreement or
its subject matter, without the prior express written permission of
the other Party, except as may be required by Applicable Laws.
Notwithstanding the above, each Party and its Affiliates may
disclose on its website, in news releases, its promotional
materials and other disclosures relating to this Agreement that the
other Party is a development and commercialization partner of such
Party for the Licensed Product in the Territory and may use the
other Party’s name and logo in conjunction with such
disclosure. Notwithstanding the foregoing:
(a)
A Party may
disclose this Agreement and its terms, and material developments or
material information generated under this Agreement, in news
releases and securities filings with the U.S. Securities and
Exchange Commission (“SEC”) (or equivalent foreign
agency) to the extent required by Applicable Laws after complying
with the procedure set forth in this Section 10.5 (Publicity/Use of
Names). In such event, the Party seeking to make such disclosure
will prepare a draft of such disclosure together with, if
applicable, a confidential treatment request to request
confidential treatment for this Agreement and proposed redacted
version of this Agreement, and the other Party agrees to promptly
(and in any event, no less than three (3) Business Days after
receipt of such request for disclosure required for 8-K and no less
than five (5) Business Days for other disclosure, including, if
applicable, proposed redactions) give its input in a reasonable
manner in order to allow the Party seeking disclosure to file its
request within the time lines prescribed by applicable SEC
regulations. The Party seeking such disclosure shall exercise
Commercially Reasonable Efforts to obtain confidential treatment of
this Agreement from the SEC as represented by the redacted version
reviewed and agreed upon in good faith by the other
Party.
(b)
Further, each Party
acknowledges that the other Party may be legally required, or may
be required by the listing rules of any exchange on which the other
Party’s or its Affiliate’s securities are traded or
advised by its counsel, to make public disclosures (including in
filings with the SEC or other agency) of certain material
developments or material information generated under this Agreement
and agrees that each Party may make such disclosures as required by
law, listing rules or advice; provided that the Party seeking such
disclosure shall provide the other Party with a copy of the
proposed text of such disclosure sufficiently in advance of the
scheduled release to afford such other Party a reasonable
opportunity to review and comment thereon.
(c)
If either Party
desires to issue a press release or make a public announcement
concerning the material terms of this Agreement or the Development,
Commercialization or Exploitation of the Compound or the Licensed
Product under this Agreement, such as the achievement of Regulatory
Approvals of the Licensed Product or data from a clinical trial,
such Party shall provide the other Party with the proposed text of
such announcement for prior review and, except to the extent such
press release or public announcement is permitted by subsection (a)
or (b) above, approval by such other Party.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-35-
(d)
The Parties agree
that after a public disclosure has been made or a press release or
other public announcement has been issued in compliance with
subsection (a), (b) or (c) hereof, each Party may make subsequent
public disclosures or issue press releases or other public
announcements disclosing the same content without having to obtain
the other Party’s prior consent and approval.
10.6
Reporting of Financial Information. From
and after the Effective Date, to the extent required by the SEC (or
equivalent foreign agency) in connection with EverInsight or an
Affiliate of EverInsight registering securities in a public
offering, VistaGen shall (a) cooperate with EverInsight or its
Affiliates and their respective accountants and auditors by
providing copies of books, and records related to the Licensed
Product as EverInsight may reasonably request in connection with
the preparation by EverInsight or its Affiliates of historical and
pro forma financial statements related to the Licensed Product as
may be required to be included in any filing made by EverInsight or
any of its Affiliates under the Securities Act of 1933, as amended,
or the Securities Exchange Act of 1934, as amended, and the
regulations promulgated thereunder, including Regulation S-X (or
equivalent foreign laws and regulations) and (b) without limiting
the foregoing, shall provide EverInsight with such information as
is required for EverInsight or its Affiliates to prepare audited
“carve out” financial statements related to the
Licensed Product, for the three (3) Calendar Years prior to the
Effective Date (or such shorter period as agreed to by EverInsight)
and information requested by EverInsight and reasonably necessary
to prepare any applicable pro forma financial information required
to be filed by EverInsight with the SEC (or equivalent foreign
agency). EverInsight may also derive such “carve out”
financial statements from VistaGen’s historical financial
statements and accurately present in all material respects the
financial position of the Licensed Product in the Licensed Field in
the Territory as of the dates thereof. EverInsight shall (i) submit
to VistaGen any proposed filing containing or incorporating by
reference any financial statements provided to EverInsight under
this Section 10.6 (Reporting of Financial Information) as far in
advance as reasonably practicable (and in no event, unless
inconsistent with Applicable Laws, less than fifteen (15) days
prior to the anticipated date of filing) so as to provide VistaGen
a reasonable opportunity to comment thereon and (ii) in good faith
consider incorporating such comments. All information of VistaGen
obtained by or on behalf of EverInsight under this Section 10.6
(Reporting of Financial Information) shall be deemed Confidential
Information of VistaGen.
10.7
Privileged Communications. In
furtherance of this Agreement, it is expected that the Parties may,
from time to time, disclose to one another privileged
communications with counsel, including opinions, memoranda, letters
and other written, electronic and verbal communications. Such
disclosures are made with the understanding that they shall remain
confidential in accordance with this ARTICLE 10 (Confidentiality;
Publication), that they will not be deemed to waive any applicable
attorney-client or attorney work product or other privilege and
that they are made in connection with the shared community of legal
interests existing between VistaGen and EverInsight, including the
community of legal interests in avoiding infringement of any valid,
enforceable patents of Third Parties and maintaining the validity
of the Licensed Patents, EverInsight Patents and Joint Patents. In
the event of any litigation (or potential litigation) with a Third
Party related to this Agreement or the subject matter hereof, the
Parties shall, upon either Party’s request, enter into a
reasonable and customary joint defense or common interest
agreement. In any event, each Party shall consult in a timely
manner with the other Party before engaging in any conduct (e.g.,
producing Information or documents) in connection with litigation
or other proceedings that could conceivably implicate privileges
maintained by the other Party. Notwithstanding anything contained
in this Section 10.7 (Privileged Communications), nothing in this
Agreement shall prejudice a Party’s ability to take discovery
of the other Party in disputes between them relating to the
Agreement and no information otherwise admissible or discoverable
by a Party shall become inadmissible or immune from discovery
solely by this Section 10.7 (Privileged
Communications).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-36-
ARTICLE 11 TERM AND TERMINATION
11.1
Term. Unless earlier terminated as
permitted by this Agreement, the term of this Agreement will
commence upon the Effective Date and continue in full force and
effect, on a jurisdiction-by-jurisdiction and Licensed
Product-by-Licensed Product basis, until expiration of the Royalty
Term for such Licensed Product in such jurisdiction the Territory
(the “Term”).
Following the expiration (but not the earlier termination) of the
Royalty Term for a Licensed Product in a jurisdiction in the
Territory, the grants in Section 2.1 (Licenses to EverInsight)
shall continue and become exclusive, fully-paid, royalty-free, and
irrevocable for such Licensed Product (existing at the time of such
expiration) in such jurisdiction. For clarity, (a) upon the
expiration (but not the earlier termination) of the Term, the
grants in Section 2.1 (Licenses to EverInsight) shall become
exclusive, fully-paid, royalty-free, and irrevocable in their
entirety solely as to the Licensed Product in the Territory at the
time of such expiration and (b) upon the expiration (but not the
earlier termination) of the Term, the grant in Section 2.2 (License
to VistaGen) shall become an exclusive, perpetual, fully- paid,
royalty-free and irrevocable license under the EverInsight
Technology to Exploit the Licensed Product (existing at the time of
such expiration) in the Licensed Field outside the Territory, in
each case with the right to grant sublicenses.
11.2
Termination.
(a)
Automatic Termination for Nonpayment of Upfront
Payment. If XxxxXxxxxxx fails to pay VistaGen the upfront
payment set forth in Section 8.1 (Upfront Payment) within thirty
(30) Business Days after the Effective Date; then, in any such
case, this Agreement will automatically and immediately
terminate.
(b)
Termination by EverInsight for Convenience. At any
time, EverInsight may terminate this Agreement (either in its
entirety or on a Licensed Product-by-Licensed Product and
jurisdiction-by-jurisdiction basis), at its sole discretion and for
any reason or no reason, by providing written notice of termination
to VistaGen, which notice includes an effective date of termination
at least [*****] after the date
of the notice.
(c)
Termination for Cause. If either
VistaGen or XxxxXxxxxxx believes that the other Party is in
material breach of its obligations hereunder, then the
non-breaching Party may deliver notice of such breach to the other
Party. The allegedly breaching Party shall have (i) [*****] Business Days in
the case of a payment breach and or (ii) [*****] Business Days in
the case of a non-payment breach, to cure such breach from the
receipt of the notice. If the allegedly breaching Party fails to
cure that breach within the applicable period set forth above, or
has not undertaken reasonable steps to cure the breach if a
complete cure is not reasonably to be expected within such period,
then the Party originally delivering the notice of breach may
terminate this Agreement on written notice of termination. Any
right to terminate this Agreement under this Section 11.2(c)
(Termination for Cause) shall be stayed and the applicable cure
period tolled in the event that, during such cure period, the Party
alleged to have been in material breach shall have initiated
dispute resolution in accordance with Section 14.10 (Dispute
Resolution) with respect to the alleged breach, which stay and
tolling shall continue until such dispute has been resolved in
accordance with Section 14.10 (Dispute Resolution). If a Party is
determined to be in material breach of this Agreement, the other
Party may terminate this Agreement if the breaching Party fails to
cure the breach within thirty (30) Business Days after the
conclusion of the dispute resolution procedure (and such
termination shall then be effective upon written notification from
the notifying Party to the breaching Party).
(d)
Termination for Patent Challenge.
VistaGen may terminate this Agreement immediately upon prior
written notice to EverInsight if EverInsight or its Affiliates or
its or their Sublicensees, individually or in association with any
other person or entity, directly or indirectly, commences or
participates in a Challenge to the validity or enforceability of
any Licensed Patents, unless EverInsight, such Affiliate or
Sublicensee dismisses or withdraws such Challenge within
[*****]
days, or in the case of a Challenge by a Sublicensee, EverInsight
terminates the sublicense agreement with such Sublicensee within
[*****]
days. EverInsight may terminate the license granted by EverInsight
to VistaGen this Agreement (but retain the license granted by
VistaGen to EverInsight hereunder) immediately upon prior written
notice to VistaGen if VistaGen or its Affiliates or its or their
Sublicensees, individually or in association with any other person
or entity, directly or indirectly, commences or participates in a
Challenge to the validity or enforceability of any EverInsight
Patents, unless VistaGen, such Affiliate or Sublicensee dismisses
or withdraws such Challenge within [*****] days, or in the
case of a Challenge by a Sublicensee, VistaGen terminates the
sublicense agreement with such Sublicensee within [*****]
days.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-37-
(e)
Termination for Bankruptcy. This
Agreement may be terminated at any time during the Term by either
Party upon the other Party’s filing or institution of
bankruptcy, reorganization, liquidation or receivership
proceedings, or upon an assignment of a substantial portion of the
assets for the benefit of creditors by the other Party; provided
that in the case of any involuntary bankruptcy proceeding such
right to terminate shall only become effective if the Party
consents to the involuntary bankruptcy or such proceeding is not
dismissed within [*****] days after the
filing thereof.
11.3
Effect of Termination. Upon termination
of this Agreement by either Party, the following consequences shall
apply and shall be effective as of the effective date of such
termination (if this Agreement is terminated on a Licensed
Product-by-Licensed Product and jurisdiction-by-jurisdiction basis,
then this Section 11.3 shall only apply to the terminated Licensed
Product in the terminated jurisdiction):
(a)
EverInsight’s
license under Section 2.1 (License to EverInsight) shall terminate
and all milestone and any other payments accruing prior to the
effective date of termination will be paid by EverInsight on or
before the termination date and all reports and accountings that
are due prior to the effective date of termination shall be
submitted by EverInsight on or before the termination
date.
(b)
If this Agreement
is terminated in its entirety by VistaGen pursuant to Section
11.2(c) (Termination for Cause), 11.2(d) (Termination for Patent
Challenge), or 11.2(e) (Termination for Bankruptcy), or if this
Agreement is terminated by EverInsight in its entirety pursuant to
Section 11.2(b) (Termination by EverInsight for Convenience), then
EverInsight hereby grants to VistaGen, effective only upon such
termination, an exclusive (even as to EverInsight), royalty-free,
fully-paid, perpetual and irrevocable license, with the right to
grant sublicenses through multiple tiers, under the EverInsight
Technology, EverInsight Development Data and EverInsight Regulatory
Documentation, to Develop, make, have made, use, import, offer for
sale, sell and otherwise Commercialize or Exploit the Compound and
any product containing the Compound anywhere in the world in all
fields of use. During a reasonable period of time (but no more than
six (6) months) after termination, EverInsight shall reasonably
cooperate with VistaGen to facility the transfer of the Development
and regulatory activities for the Compound and Licensed Product to
VistaGen.
(c)
If this Agreement
is terminated by EverInsight pursuant to Section 11.2(c)
(Termination for Cause), or 11.2(e) (Termination for Bankruptcy),
then VistaGen may request, within [*****] days of such
termination, that EverInsight enter into good faith negotiations
for no more than [*****] days concerning
the terms of an agreement with EverInsight granting to VistaGen an
exclusive (even as to EverInsight) license under the EverInsight
Technology, EverInsight Development Data and EverInsight Regulatory
Documentation. If no agreement is reached, then the license to
VistaGen under Section 2.2 (License to VistaGen) shall
terminate.
(d)
If this Agreement
is terminated in its entirety, VistaGen shall be solely responsible
for all future worldwide Development, Manufacture and
Commercialization of the Compound and Licensed Product in the
Licensed Field, at its sole cost and expense.
(e)
If this Agreement
is terminated in its entirety, each Party shall return to the other
Party or destroy, at the other Party’s election, all
Confidential Information of the other Party, including all copies
thereof and all materials, substances and compositions delivered or
provided by or on behalf of the other Party; except that (i) each
Party may retain one copy of the other Party’s Confidential
Information for legal archival purpose, and neither Party shall be
required to delete or destroy any electronic back-up tapes or other
electronic back-up files that have been created solely by automatic
or routine archiving and back-up procedures; and (ii) if the
Parties reach agreement with respect to a license grant by
EverInsight to VistaGen under clause (c) or VistaGen has license
rights under clause (b), then VistaGen shall not be required to
return or destroy EverInsight’s Confidential Information to
the extent VistaGen has the right to use such Confidential
Information solely as necessary to practice such
license.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-38-
(f)
If VistaGen
automatically has license rights under clause 11.3(b) or the
Parties reach agreement with respect to a license grant by
EverInsight to VistaGen under clause 11.3(c) then:
(i)
EverInsight shall
deliver to VistaGen all Regulatory Filings and Regulatory Approvals
for the Compound and any Licensed Product, all EverInsight
Development Data and all EverInsight Know-How.
(ii)
EverInsight shall
(1) disclose to VistaGen all EverInsight Know-How, EverInsight
Development Data and all Joint Inventions to the extent not already
known to VistaGen, which may be necessary or reasonably useful for
VistaGen to continue to Develop, Manufacture and Commercialize the
Compound and Licensed Product in the Licensed Field; and (2), at
VistaGen’s request, provide reasonable technical assistance
and transfer all EverInsight Know-How, EverInsight Development Data
and Joint Inventions necessary to Manufacture the Compound or
Licensed Product to VistaGen or its designee; provided that
VistaGen shall reimburse EverInsight for the reasonable cost and
expense of such technical assistance.
(iii)
EverInsight shall,
at VistaGen’s request and election, introduce VistaGen to
EverInsight’s Third Party providers of clinical research,
manufacturing and/or distribution services and assign any contracts
with such entities to VistaGen to the extent such contracts (or
portions thereof, such as a work order under a master services
agreement) relate solely to the Licensed Product and are assignable
to VistaGen.
(iv)
EverInsight shall
transfer to VistaGen all units of the Compound and the Licensed
Product in its possession, provided that VistaGen shall reimburse
EverInsight for the Cost of Goods of such units.
(v)
EverInsight shall,
and hereby does, effective on such termination, assign to VistaGen
all of EverInsight’s and its Affiliates’ right, title
and interest in and to any and all Product Trademarks and other
trademarks used by EverInsight and its Affiliates in the Territory
in connection with its Development, Manufacture or
Commercialization of Licensed Product (excluding any such
trademarks that include, in whole or part, any corporate name or
logo of EverInsight or its Affiliates), including all goodwill
therein, and EverInsight shall promptly take such actions and
execute such instruments, assignments and documents as may be
necessary to effect, evidence, register and record such
assignment.
11.4
Survival. Expiration or termination of
this Agreement shall not relieve any Party of any obligation
accruing prior to such expiration or termination, nor shall
expiration or any termination of this Agreement preclude either
Party from pursuing all rights and remedies it may have under this
Agreement, at law or in equity, with respect to breach of this
Agreement. In addition, the provisions of ARTICLE 1 (Definitions),
subclauses (b) through (d) of Section 5.4 (Rights of Reference),
Section 8.8 (Taxes), Section 8.9 (Financial Records and Audit),
Section 8.10 (Audit Dispute), Section 9.1 (Ownership of
Intellectual Property); ARTICLE 10 (Confidentiality; Publicity),
Section 11.3 (Effect of Termination), this Section 11.4 (Survival),
ARTICLE 13 (Indemnification; Liability), and ARTICLE 14 (General
Provisions) hereof shall survive the expiration or termination of
this Agreement. In addition, in the event that the this Agreement
is terminated by EverInsight pursuant to Section 11.2(c)
(Termination for Cause) and, pursuant to Section 11.3 (Effect of
Termination), either VistaGen does not timely request that
EverInsight enter into good faith negotiations concerning the terms
of an agreement with VistaGen granting VistaGen a license under the
EverInsight Technology and EverInsight Development Data, or if no
agreement is timely reached, then the provisions of Sections 9.2
through 9.9 of ARTICLE 9 (Intellectual Property), solely with
respect to Joint Inventions, shall also survive the expiration or
termination of this Agreement.
11.5
Termination Not Sole Remedy. Termination
is not the sole remedy under this Agreement and, whether or not
termination is effected, and notwithstanding anything contained in
this Agreement to the contrary, all other remedies will remain
available except as agreed to otherwise herein.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-39-
11.6
Alternative Remedy for VistaGen’s
Breach. In the event EverInsight would be entitled to
terminate this Agreement pursuant Section 11.2(c) for
VistaGen’s uncured material breach, but if EverInsight does
not desire to exercise such termination right, then, EverInsight
may, in its sole discretion and without waiving or releasing any
right, claim or remedy for such breach, elect to maintain this
Agreement in full force and effect and reduce all future payments
due to VistaGen hereunder by [*****].
ARTICLE 12 REPRESENTATIONS AND WARRANTIES
12.1
Representations and Warranties of Each
Party. Each Party represents and warrants to each other
Parties as of the Effective Date that:
(a)
it has the full
right, power and authority to enter into this Agreement, to perform
its obligations hereunder;
(b)
this Agreement has
been duly executed by it and is legally binding upon it,
enforceable in accordance with its terms, and does not conflict
with any agreement, instrument or understanding, oral or written,
to which it is a party or by which it may be bound, nor violate any
material law or regulation of any court, governmental body or
administrative or other agency having jurisdiction over
it;
(c)
this Agreement is a
legal, valid and binding obligation of such Party enforceable
against it in accordance with its terms and conditions, subject to
the effects of bankruptcy, insolvency or other laws of general
application affecting the enforcement of creditor rights, judicial
principles affecting the availability of specific performance and
general principles of equity (whether enforceability is considered
a proceeding at law or equity);
(d)
it is not under any
obligation, contractual or otherwise, to any Person that conflicts
with or is inconsistent in any material respect with the terms of
this Agreement or that would impede the diligent and complete
fulfillment of its obligations hereunder; and
12.2
Mutual Covenants.
(a)
Employees, Consultants and Contractors.
Each Party covenants that it has obtained or will obtain written
agreements from each of its employees, consultants and contractors
who perform Development activities pursuant to this Agreement,
which agreements will obligate such persons to obligations of
confidentiality and non-use and to assign Inventions in a manner
consistent with the provisions of this Agreement.
(b)
Debarment. Each Party represents,
warrants and covenants to the other Parties that it is not debarred
or disqualified under the FFDCA, as may be amended, or comparable
laws in any country or jurisdiction other than the U.S., and it has
not employed or used, does not, and will not during the Term,
employ or use the services of any person who is debarred or
disqualified, in connection with activities relating to the
Compound or any Licensed Product. In the event that any Party
becomes aware of the debarment or disqualification or threatened
debarment or disqualification of any person providing services to
such Party, including the Party itself or its Affiliates, that
directly or indirectly relate to activities contemplated by this
Agreement, such Party shall immediately notify the other Parties in
writing and such Party shall cease employing, contracting with, or
retaining any such person to perform any such
services.
(c)
Compliance. Each Party covenants as
follows:
(1)
In the performance
of its obligations under this Agreement, such Party shall comply
and shall cause its and its Affiliates’ employees and
contractors to comply with all Applicable Laws, including all
export control, anti-corruption and anti-bribery laws and
regulations, and shall not cause such other Party to be in
violation of any Applicable Laws.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-40-
(2)
Such Party and its
and its Affiliates’ employees and contractors shall not, in
connection with the performance of their respective obligations
under this Agreement, directly or indirectly through Third Parties,
pay, promise or offer to pay, or authorize the payment of, any
money or give any promise or offer to give, or authorize the giving
of anything of value to a Public Official or Entity or other person
for purpose of obtaining or retaining business for or with, or
directing business to, any person, including, without limitation,
either Party. Each Party represents and warrants that as of the
Effective Date, such Party, and to its knowledge, its and its
Affiliates’ employees and contractors, have not directly or
indirectly promised, offered or provided any corrupt payment,
gratuity, emolument, bribe, kickback, illicit gift or hospitality
or other illegal or unethical benefit to a Public Official or
Entity or any other person in connection with the performance of
such Party’s obligations under this Agreement, and each Party
covenants that it and its Affiliates’ employees and
contractors shall not, directly or indirectly, engage in any of the
foregoing.
(3)
Each Party shall
have the right to suspend or terminate this Agreement in its
entirety where there is a credible finding, after a reasonable
investigation, that the other Party, in connection with performance
of such other Party’s obligations under this Agreement, has
materially violated any anti-corruption or anti-bribery laws or
regulations.
(4)
Each Party shall
not, during the Term, assign, transfer, convey or otherwise
encumber its right, title and interest in (A) Licensed Technology,
in the case of VistaGen, in a manner that is inconsistent with the
exclusive license granted to EverInsight under Section 2.1
(Licenses to EverInsight) or (B) EverInsight Technology, in the
case of EverInsight, in a manner that is inconsistent with the
exclusive license granted to VistaGen under Section 2.2 (License to
VistaGen), in each case without the prior written consent of the
other Party (which consent shall not be unreasonably withheld,
conditioned or delayed)
(5)
Each Party shall
not grant any right to any Third Party under the (A) Licensed
Technology (in the case of VistaGen) that would conflict with the
rights granted to EverInsight hereunder, or (B) EverInsight
Technology (in the case of EverInsight) that would conflict with
the rights granted to VistaGen hereunder.
12.3
Representations and Warranties by
XxxxxXxx. VistaGen represents and warrants to EverInsight as
of the Effective Date that:
(a)
The patents and
patent applications listed on Exhibit A constitute all Licensed
Patents existing as of the Effective Date (the “Existing Licensed
Patents”);
(b)
Except for
[*****],
VistaGen is the sole and exclusive owner of all Licensed
Technology, free and clear from any mortgages, pledges, liens,
security interests, conditional and installment sales agreements,
encumbrances, charges or claims of any kind, and has the right to
grant the license to EverInsight as purported to be granted under
this Agreement;
(c)
The Licensed
Technology is complete, accurate, effective and capable of
achieving the Development and Manufacturing of the Compound and the
Licensed Product. The Parties hereby irrevocably agree that the
Licensed Technology shall be deemed to be complete, accurate,
effective and capable of achieving the Development and
Manufacturing of the Compound and the Licensed Product (and the
foregoing representation and warranty shall be satisfied) if, after
the completion of relevant technology transfer, EverInsight (or its
contractor) is able to produce the Compound or the Licensed
Products (as the case may be) in a manner that (1) complies with
the specifications contained in (i) the technical documents
VistaGen provided to EverInsight for evaluation and (ii) IND(s)
submitted to the applicable Regulatory Authority(ies) and (2) does
not infringe or misappropriate any intellectual property of any
Third Party.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-41-
(d)
VistaGen has not
received any notice from a Third Party that the Development or
Manufacture of the Compound or any Licensed Product conducted by or
on behalf of VistaGen prior to the Effective Date has infringed any
Patents of any Third Party or infringed or misappropriated any
other intellectual property of any Third Party. Based on
VistaGen’s understanding as of the Effective Date of the
Compound and the Licensed Product and their intended use as
disclosed to EverInsight as of the Effective Date, the Development,
Manufacture, use or sale of any Compound or any Licensed Product
pursuant to this Agreement does not and will not, to the knowledge
of VistaGen, (y) infringe any Patents of any Third Party or (z)
infringe or misappropriate any other intellectual property of any
Third Party.
(e)
To the knowledge of
VistaGen, the use of Licensed Trademark in connection with
Commercialization of the Licensed Product will not violate the
rights of any Third Party. No claim or action has been brought or,
to VistaGen’s knowledge, threatened in writing, by any
Governmental Authority or Third Party (i) that any Licensed
Trademark violates the rights of a Third Party or (ii) currently
challenging the enforceability or validity of any Licensed
Trademark;
(f)
VistaGen has not as
of the Effective Date granted any right to any Third Party under
the Licensed Technology or Licensed Trademark that would conflict
with the rights granted to EverInsight hereunder;
(g)
VistaGen has no
knowledge as of the Effective Date of any Third Party that is
infringing or misappropriating any of the Licensed Technology or
Licensed Trademark;
(h)
no claim or action
has been brought or, to VistaGen’s knowledge, threatened in
writing by any Third Party involving any Compound, Licensed Product
and/or Licensed Technology, including any claim or action alleging
that the issued patents in the Licensed Patent Rights are invalid
or unenforceable, and any interference, opposition, cancellation or
other protest proceeding involving any Licensed Patents anywhere in
the world;
(i)
to VistaGen’s
knowledge, as of the Effective Date, there is no Know-How owned or
controlled by VistaGen that is necessary for the Development of the
Compound that is not within the Licensed Know-How; and
(j)
to VistaGen’s
knowledge, (x) all development works for the Compound and Licensed
Product, including clinical trials, conducted by VistaGen or its
Affiliates (including their contractors) prior to the Effective
Date have been in compliance in all material respects with all
Applicable Laws, and (y) no data or other information generated or
otherwise received from such clinical trials conducted up to the
Effective Date has, or is reasonably expected to have, any
materially negative impact on the Exploitation of any Licensed
Product in the Territory.
(k)
To the knowledge of
VistaGen, VistaGen has obtained all necessary government approvals
required for the grant of the license and the transfer of the
Licensed Know-How to EverInsight, including such approvals required
by applicable technology export control laws, and VistaGen will do
and execute or procure to be done and executed all such further
acts, things, agreements and other documents as may be necessary to
give effect to the terms of this Agreement, including to comply
with the applicable technology import and export laws and
regulations in the United States and the Territory;
(l)
Except for the
Pherin License, there is no agreement between VistaGen or its
Affiliates and any Third Party pursuant to which VistaGen or its
Affiliates have obtained any right or license to the Compound,
Licensed Product or Licensed Technology. VistaGen has provided
EverInsight with a copy of the Pherin License that is complete with
regard to the relevant provisions of this Agreement. The Pherin
License is in full force and effect. No notice of default or
termination has been received or given under the Pherin License.
There is no act or omission by VistaGen that would provide a right
to terminate the Pherin License;
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-42-
(m)
During the Term of
this Agreement, VistaGen shall maintain [*****] each In-License
Agreement in full force and effect and shall not terminate, amend,
waive or otherwise modify (or consent to any of the foregoing) its
rights under [*****] any In-License
Agreement in any manner that materially diminishes the rights or
licenses granted to EverInsight hereunder, without
EverInsight’s express written consent, which shall not be
unreasonably withheld, conditioned or delayed, and VistaGen shall
provide EverInsight with a copy of all modifications to or
amendments thereto, regardless of whether EverInsight’s
consent was required with respect thereto. In the event of any
notice of breach of [*****] any In-License
Agreement by VistaGen, VistaGen shall immediately notify
EverInsight in writing, and if VistaGen fails to cure such breach
in a timely manner, EverInsight shall have the right, but not the
obligation, to cure such breach and to seek reimbursement of or
offset any reasonable amount incurred or paid by EverInsight in
connection with the cure against amount payable to VistaGen
hereunder. In the event of any notice of breach of [*****] any In-License
Agreement by the applicable Third Party in a manner that will or is
likely to materially affect EverInsight’s rights or
obligations under this Agreement, VistaGen shall immediately notify
EverInsight in writing and take such actions as reasonably
requested by EverInsight to enforce the [*****] In-License
Agreement; and
(n)
All information
provided by VistaGen to EverInsight for due diligence purposes in
relation to this Agreement is complete and accurate in all material
respects. Without limiting the foregoing, VistaGen has disclosed or
made available to EverInsight for review all material non-clinical
and clinical data for the Compound and Licensed Product, and all
other material information (including relevant correspondence with
the FDA and other Regulatory Authorities) relating to the Compound
and Licensed Product, in each case that would be material for
EverInsight to assess the safety and efficacy of the Compound and
Licensed Product.
12.4
Representations and Warranties by
EverInsight. EverInsight represents and warrants to VistaGen
as of the Effective Date that:
(a)
EverInsight has not
previously assigned, transferred, conveyed or otherwise encumbered
its right, title and interest in EverInsight Technology in a manner
that is inconsistent with the exclusive license granted to VistaGen
under Section 2.2 (License to VistaGen);
(b)
EverInsight has not
as of the Effective Date, and will not during the Term, grant any
right to any Third Party under the EverInsight Technology that
would conflict with the rights granted to VistaGen
hereunder;
(c)
EverInsight has no
knowledge as of the Effective Date of any Third Party that is
infringing or misappropriating any of the EverInsight
Technology;
(d)
no claim or action
has been brought or, to EverInsight’s knowledge, threatened
in writing by any Third Party alleging that the EverInsight Patents
are invalid or unenforceable, and no EverInsight Patent is the
subject of any interference, opposition, cancellation or other
protest proceeding; and
(e)
as of the Effective
Date, EverInsight reasonably believes it has or will have the
capability and sufficient access to the financial resources
necessary to perform its obligations under this Agreement,
including without limitation, its obligations to (i) use
Commercially Reasonable Efforts to Develop, Exploit, Commercialize
and obtain Regulatory Approval for the Compounds and each Licensed
Product in the Licensed Field in the Territory and (ii) make the
required payments to VistaGen hereunder.
12.5
No Other Warranties. EXCEPT AS EXPRESSLY
SET FORTH IN THIS AGREEMENT, NO PARTY MAKES, AND EACH PARTY
EXPRESSLY DISCLAIMS, ANY AND ALL WARRANTIES OF ANY KIND, EXPRESS OR
IMPLIED, INCLUDING THE WARRANTIES OF DESIGN, MERCHANTABILITY,
FITNESS FOR A PARTICULAR PURPOSE, VALIDITY OF PATENTS,
NON-INFRINGEMENT OF THE INTELLECTUAL PROPERTY RIGHTS OF THIRD
PARTIES, OR ARISING FROM A COURSE OF DEALING, USAGE OR TRADE
PRACTICES.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-43-
ARTICLE 13 INDEMNIFICATION; LIABILITY
13.1
Indemnification by VistaGen. VistaGen
shall indemnify, defend and hold EverInsight, its Affiliates, and
their respective officers, directors, agents and employees
(“EverInsight
Indemnitees”) harmless from and against any Claims
against them to the extent arising or resulting from:
(a)
the material breach
by VistaGen of this Agreement;
(b)
the gross
negligence or willful misconduct on the part of VistaGen or its
Affiliates or its or their respective officers, directors, agents
or employees in performing its obligations under this Agreement;
or
(c)
the Exploitation by
VistaGen or any of its Affiliates or its or their sublicensees or
its or their distributors or contractors of the Compound or the
Licensed Product outside the Territory; or
(d)
any Third Party
Infringement Claim that VistaGen is responsible for defending
pursuant to Section 9.5;
except,
in each case (a), (b) and (c) above, for those Claims for which
EverInsight has an obligation to indemnify VistaGen pursuant to
Section 13.2 (Indemnification by EverInsight) hereof or, to the
extent such Claims result from the material breach by EverInsight
of any covenant, representation, warranty or other agreement made
by EverInsight in this Agreement or the negligence or willful
misconduct of any EverInsight Indemnitee. Notwithstanding the
above, VistaGen will have no obligation to defend or indemnify
EverInsight or its Affiliates for any claim brought by a
shareholder or a class of shareholders of EverInsight or its
Affiliates including, but not limited to, securities fraud claims,
shareholder direct claims, and shareholder derivative claims,
except to the extent resulting from the gross negligence or willful
misconduct on the part of VistaGen or any Affiliate.
13.2
Indemnification by EverInsight.
EverInsight shall indemnify, defend and hold VistaGen, its
Affiliates, and their respective officers, directors, agents and
employees (“VistaGen
Indemnities”) harmless from and against any Claims
arising under or related to this Agreement against them to the
extent arising or resulting from:
(a)
the material breach
by XxxxXxxxxxx of this Agreement;
(b)
the gross
negligence or willful misconduct on the part of EverInsight or its
Affiliates or its or their respective officers, directors, agents
or employees in performing its obligations under this Agreement;
or
(c)
the Exploitation by
EverInsight or any of its Affiliates or its or their Sublicensees
or its or their distributors or contractors of the Compound or the
Licensed Product in the Territory;
except,
in each case (a), (b) and (c) above, those Claims for which
VistaGen has an obligation to indemnify EverInsight pursuant to
Section 13.1 (Indemnification by VistaGen) hereof or, to the extent
such Claims result from the material breach by VistaGen of any
covenant, representation (other than the representation set forth
in Section 12.3(d), warranty or other agreement made by VistaGen in
this Agreement or the negligence or willful misconduct of any
VistaGen Indemnitee. Notwithstanding the above, EverInsight will
have no obligation to defend or indemnify VistaGen or its
Affiliates for any claim brought by a shareholder or a class of
shareholders of VistaGen or its Affiliates including, but not
limited to, securities fraud claims, shareholder direct claims, and
shareholder derivative claims, except to the extent resulting from
the gross negligence or willful misconduct on the part of
EverInsight or any Affiliate.
13.3
Indemnification
Procedure.
(a)
Notice of Claim. All indemnification
claims in respect of a Party, its Affiliates or their respective
directors, officers, employees and agents shall be made solely by
such Party to this Agreement (the “Indemnified Party”). The
Indemnified Party shall give the other Party (the
“Indemnifying
Party”) a prompt written notice (an
“Indemnification Claim
Notice”) of any Claims or discovery of fact upon which
such Indemnified Party intends to base a request for
indemnification under this ARTICLE 13 (Indemnification; Liability)
within [*****] days from
written receipt of such Claim or discovery of facts that that might
give rise to such Claim. Each Indemnification Claim Notice must
contain a description of the Claim and the nature and amount of
such Claim (to the extent that the nature and amount of such Claim
is known at such time).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-44-
(b)
Control of Defense. The Indemnifying
Party shall have the right to assume the defense of any Claim by
giving written notice to the Indemnified Party within [*****] days
after the Indemnifying Party’s receipt of an Indemnification
Claim Notice. The assumption of the defense of a Claim by the
Indemnifying Party shall not be construed as an acknowledgment that
the Indemnifying Party is liable to indemnify the Indemnified Party
in respect of the Claim, nor shall it constitute a waiver by the
Indemnifying Party of any defenses it may assert against the
Indemnified Party’s claim for indemnification. Upon assuming
the defense of a Claim, the Indemnifying Party may appoint as lead
counsel in the defense of the Claim any legal counsel selected by
the Indemnifying Party; provided that it obtains the prior written
consent of the Indemnified Party (which consent shall not be
unreasonably withheld, conditioned or delayed). In the event the
Indemnifying Party assumes the defense of a Claim, upon the
Indemnifying Party’s relevant notice the Indemnified Party
shall immediately deliver to the Indemnifying Party all original
notices and documents (including court papers) received by the
Indemnified Party in connection with the Claim. Should the
Indemnifying Party assume the defense of a Claim, except as
provided in Section 13.3(c) (Right to Participate in Defense), the
Indemnifying Party shall not be liable to the Indemnified Party for
any legal expenses subsequently incurred by such Indemnified Party
in connection with the analysis, defense or settlement of the Claim
unless specifically requested and approved in writing by the
Indemnifying Party. In the event that it is ultimately determined
that the Indemnifying Party is not obligated to indemnify, defend
or hold harmless the Indemnified Party from and against the Claim,
the Indemnified Party shall reimburse the Indemnifying Party for
any and all reasonable and verifiable out-of-pocket costs and
expenses (including attorneys’ fees and costs of suit)
incurred by the Indemnifying Party in accordance with this ARTICLE
13 (Indemnification; Liability) in its defense of the
Claim.
(c)
Right to Participate in Defense. Any
Indemnified Party shall be entitled to participate in the defense
of such Claim and to employ counsel of its choice for such purpose;
provided, however, that such employment shall be at the Indemnified
Party’s sole cost and expense unless (i) the employment
thereof has been specifically authorized in writing in advance by
the Indemnifying Party (in which case, the defense shall be
controlled as provided in Section 13.3(b) (Control of Defense),
with such provisions applying mutatis mutandis; (ii) the
Indemnifying Party has failed to assume the defense and employ
counsel in accordance with Section 13.3(b) (Control of Defense) (in
which case the Indemnified Party shall control the defense, with
the reasonable out-of-pocket expense with respect thereto borne by
the Indemnifying Party); or (iii) the interests of the indemnitee
and the Indemnifying Party with respect to such Claim are
sufficiently adverse to prohibit the representation by the same
counsel of both Parties under Applicable Laws, ethical rules or
equitable principles (in which case, the Indemnified Party shall
control its defense, with the reasonable out-of-pocket expense with
respect thereto borne by the indemnifying Party).
(d)
Settlement. With respect to any Claims
relating solely to the payment of money damages in connection with
a Claim that shall not result in the applicable indemnitee(s)
becoming subject to injunctive or other relief or otherwise
adversely affect the business or interests of the Indemnified Party
in any manner and as to which the Indemnifying Party shall have
acknowledged in writing the obligation to indemnify the applicable
indemnitee hereunder, the Indemnifying Party shall have the sole
right to consent to the entry of any judgment, enter into any
settlement or otherwise dispose of such Claim, on such terms as the
Indemnifying Party, in its sole discretion, shall deem appropriate.
With respect to all other Claims in connection with Claims, where
the Indemnifying Party has assumed the defense of the Claim in
accordance with Section 13.3(b) (Control of Defense), the
Indemnifying Party shall have authority to consent to the entry of
any judgment, enter into any settlement or otherwise dispose of
such Claim; provided, it obtains the prior written consent of the
Indemnified Party (which consent shall not be unreasonably
withheld, conditioned or delayed). If the Indemnifying Party does
not assume and conduct the defense of a Claim as provided above,
the Indemnified Party may defend against such Claim; provided, that
the Indemnified Party shall not settle any Claim without the prior
written consent of the Indemnifying Party (which consent shall not
be unreasonably withheld, conditioned or delayed).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-45-
(e)
Cooperation. If the Indemnifying Party
chooses to defend or prosecute any Claim, the Indemnified Party
shall and shall cause each indemnitee to, cooperate in the defense
or prosecution thereof and furnish such records, information and
testimony, provide such witnesses and attend such conferences,
discovery proceedings, hearings, trials and appeals as may be
reasonably requested by the indemnifying Party in connection
therewith. Such cooperation shall include access during normal
business hours afforded to the Indemnifying Party to and reasonable
retention by the Indemnified Party of, records and information that
are reasonably relevant to such Claim and making the Indemnified
Party, the indemnitees and other employees and agents available on
a mutually convenient basis to provide additional information and
explanation of any material provided hereunder and the Indemnifying
Party shall reimburse the Indemnified Party for all of its, its
Affiliates’ and its and their sublicensees’ or their
respective directors’, officers’, employees’ and
agents’, as applicable, reasonable and verifiable
out-of-pocket expenses in connection therewith.
(f)
Expenses. Except as provided above, the
costs and expenses, including fees and disbursements of counsel,
incurred by the Indemnified Party and its Affiliates and its and
their sublicensees and their respective directors, officers,
employees and agents, as applicable, in connection with any Claim
shall be reimbursed on a Calendar Quarter basis by the Indemnifying
Party, without prejudice to the Indemnifying Party’s right to
contest the Indemnified Party’s right to indemnification and
subject to refund in the event the Indemnifying Party is ultimately
held not to be obligated to indemnify the Indemnified
Party.
13.4
Mitigation of Loss. Each Indemnified
Party will take and will procure that its Affiliates take all such
reasonable steps and actions as are reasonably necessary or as the
Indemnifying Party may reasonably require in order to mitigate any
Claims (or potential losses or damages) under this ARTICLE 13
(Indemnification; Liability). Nothing in this Agreement shall or
shall be deemed to relieve any Party of any common law or other
duty to mitigate any losses incurred by it.
13.5
Special, Indirect and Other Losses.
EXCEPT IN THE EVENT OF A BREACH OF SECTION 2.7 (NON-DIVERSION),
SECTION 2.8 (NON-COMPETE) OR ARTICLE 10 (CONFIDENTIALITY;
PUBLICATION), NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE
OTHER PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE
DAMAGES IN CONNECTION WITH THIS AGREEMENT OR ANY LICENSE GRANTED
HEREUNDER; provided, however, that this Section 13.5 shall not be
construed to limit either Party’s indemnification obligations
under Section 13.1 (Indemnification by VistaGen) or Section 13.2
(Indemnification by EverInsight), as applicable.
13.6
Insurance. Each Party, at its own
expense, shall maintain product liability and other appropriate
insurance in an amount consistent with sound business practice and
reasonable in light of its obligations under this Agreement during
the Term. Each Party shall provide a certificate of insurance
evidencing such coverage to the other Party upon
request.
ARTICLE 14 GENERAL PROVISIONS
14.1
Governing Law. This Agreement shall be
governed by and construed in accordance with the law of Hong Kong
without reference to its conflicts of laws principles.
14.2
Assignment.
(a)
Except as expressly
provided hereunder, neither this Agreement nor any rights or
obligations hereunder may be assigned or otherwise transferred by
either Party without the prior written consent of the other Party
(which consent shall not be unreasonably withheld); provided that
either Party may assign or otherwise transfer this Agreement and
its rights and obligations hereunder without the other
Party’s consent: (a) in connection with the transfer or sale
of all or substantially all of the business or assets of such Party
to which this Agreement relates to a Third Party, whether by
merger, consolidation, divesture, restructure, sale of stock, sale
of assets or otherwise; provided that in the event of any such
transaction (whether this Agreement is actually assigned or is
assumed by the acquiring party by operation of law (e.g., in the
context of a reverse triangular merger)), intellectual property
rights of the acquiring party to such transaction (if other than
one of the Parties to this Agreement) and its Affiliates existing
prior to the transaction shall not be included in the technology
licensed hereunder; or (b) to an Affiliate, provided that the
assigning Party shall remain liable and responsible to the
non-assigning Party hereto for the performance and observance of
all such duties and obligations by such Affiliate; and provided,
further, that in any such case the assigning Party shall provide
written notice to the other Party within five (5) calendar days
after such assignment or transfer. The rights and obligations of
the Parties under this Agreement shall be binding upon and inure to
the benefit of the successors and permitted assigns of the Parties,
and the name of a Party appearing herein will be deemed to include
the name of such Party’s successors and permitted assigns to
the extent necessary to carry out the intent of this section. Any
assignment not in accordance with this Section 14.2 (Assignment)
shall be null and void.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-46-
(b)
The rights to
Information, materials and intellectual property, shall, in each of
cases (1) and (2) below, be automatically excluded from the rights
licensed or granted to the other Party under this
Agreement:
(1)
Rights to
Information, materials and intellectual property controlled by a
Third Party permitted assignee of a Party that immediately prior to
such assignment (other than as a result of a license or other grant
of rights, covenant or assignment by such Party or its Affiliates
to, or for the benefit of, such Third Party); or
(2)
Rights to
Information, materials and intellectual property controlled by an
Affiliate of a Party that becomes an Affiliate through any Change
of Control of such Party that was Controlled by such Affiliate (and
not such Party) immediately prior to such Change of Control (other
than as a result of a license or other grant of rights, covenant or
assignment by such Party or its other Affiliates to, or for the
benefit of, such Affiliate).
14.3
Entire Agreement; Modification. This
Agreement is both a final expression of the Parties’
agreement and a complete and exclusive statement with respect to
all of its terms. This Agreement supersedes all prior and
contemporaneous agreements and communications, whether oral,
written or otherwise, concerning any and all matters contained
herein. No amendment, modification, release or discharge shall be
binding on the Parties unless in writing and duly executed by
authorized representatives of each of VistaGen and EverInsight;
provided that, pursuant to the definition of “Licensed
Trademarks” herein, VistaGen may designate in a writing to
EverInsight from time to time such other Trademarks, names and
logos as VistaGen may reasonably determine. In the event of any
inconsistencies between this Agreement and any schedules or other
attachments hereto, the terms of this Agreement shall
control.
14.4
Relationship among the Parties. The
Parties’ relationship with one another, as established by
this Agreement, is solely that of independent contractors. This
Agreement does not create any partnership, joint venture or similar
business relationship between the Parties. Neither Party is a legal
representative of the other Party. Neither Party can assume or
create any obligation, representation, warranty or guarantee,
express or implied, on behalf of the other Party for any purpose
whatsoever. All persons employed by a Party shall be employees of
such Party and not of the other Party and all costs and obligations
incurred by reason of any such employment shall be for the account
and expense of such first Party.
14.5
Non-Waiver. The failure of a Party to
insist upon strict performance of any provision of this Agreement
or to exercise any right arising out of this Agreement shall
neither impair that provision or right nor constitute a waiver of
that provision or right, in whole or in part, in that instance or
in any other instance. Any waiver by a Party of a particular
provision or right shall be in writing, shall be as to a particular
matter and, if applicable, for a particular period of time and
shall be signed by such Party. The rights and remedies provided
herein are cumulative and do not exclude any other right or remedy
provided by Applicable Law or otherwise available except as
expressly set forth herein.
14.6
Force Majeure. Neither Party shall be
held liable or responsible to the other Party or be deemed to have
defaulted under or breached this Agreement for failure or delay in
fulfilling or performing any term of this Agreement (other than an
obligation to make payments unless the force majeure event affects
the payment process itself, such as bank closure or government
closure that affects the review and approval of the payment) when
such failure or delay is caused by or results from events beyond
the reasonable control of the non- performing Party, including
fires, floods, earthquakes, hurricanes, embargoes, shortages,
epidemics, quarantines, war, acts of war (whether war be declared
or not), terrorist acts, insurrections, riots, civil commotion,
strikes, lockouts or other labor disturbances (whether involving
the workforce of the non-performing Party or of any other Person),
acts of God or acts, omissions or delays in acting by any
governmental authority (including expropriation, seizure of works,
requisition, nationalization, exercise of march-in rights or
compulsory licensing, except to the extent such delay results from
the breach by the non-performing Party or any of its Affiliates of
any term or condition of this Agreement) and any material change in
the Applicable Laws of a Regulatory Authority that results in a
development, clinical or regulatory delay [*****]. The
non-performing Party shall notify the other Party of such force
majeure within thirty (30) days after such occurrence by giving
written notice to the other Party stating the nature of the event,
its anticipated duration and any action being taken to avoid or
minimize its effect. The suspension of performance shall be of no
greater scope and no longer duration than is necessary and the
non-performing Party shall use Commercially Reasonable Efforts to
remedy its inability to perform.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-47-
14.7
Export Control. This Agreement is made
subject to any restrictions concerning the export of products or
technical information from the United States or other countries
that may be imposed on the Parties from time to time. Each Party
agrees that it will not export, directly or indirectly, any
technical information acquired from the other Party under this
Agreement or any products using such technical information to a
location or in a manner that at the time of export requires an
export license or other governmental approval, without first
obtaining the written consent to do so from the appropriate agency
or other governmental entity in accordance with Applicable Laws.
VistaGen hereby undertakes to use Commercially Reasonable Efforts
to obtain necessary licenses (if required) for exporting the
Compound, the Licensed Product and the Licensed Technology from the
United States or other countries.
14.8
Severability. If any provision of this
Agreement is held to be illegal, invalid or unenforceable under any
present or future law and if the rights or obligations of either
Party under this Agreement will not be materially and adversely
affected thereby: (a) such provision shall be fully severable; (b)
this Agreement shall be construed and enforced as if such illegal,
invalid or unenforceable provision had never comprised a part
hereof; (c) the remaining provisions of this Agreement shall remain
in full force and effect and shall not be affected by the illegal,
invalid or unenforceable provision or by its severance herefrom;
and (d) in lieu of such illegal, invalid or unenforceable
provision, there shall be added automatically as a part of this
Agreement a legal, valid and enforceable provision as similar in
terms to such illegal, invalid or unenforceable provision as may be
possible and reasonably acceptable to the Parties. To the fullest
extent permitted by Applicable Laws, each Party hereby waives any
provision of law that would render any provision hereof illegal,
invalid or unenforceable in any respect.
14.9
Notices. Any notice to be given under
this Agreement must be in writing and delivered either (a) in
person or (b) by overnight courier, to the Party to be notified at
its address(es) given below for convenience, or at any address such
Party may designate by prior written notice to the other. Notice
shall be deemed sufficiently given for all purposes upon the date
of actual receipt.
If to
VistaGen:
VistaGen
Therapeutics, Inc.
000
Xxxxxxxx Xxxxxx
South
San Francisco, CA 94080
United
States of America
Attention:
CEO
with a
mandatory copy (which shall not constitute notice) to:
Xxxx
Xxxxx, X.X.
Capital
Technology Law Group
0000
Xxxxxxxxx Xxx., X.X., Xxxxx 000
Washington, DC
20015
United
States of America
If to
EverInsight:
EverInsight
Therapeutics Inc.
Vistra
Corporate Services Centre
Wickhams
Cay II, Road Town
Tortola,
VG1110
British
Virgin Islands
ATTN:
CEO / General Counsel
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-48-
with a
mandatory copy to (which shall not constitute notice)
to:
Xxxxxx
LLP
0000
Xxxxxxx Xxxxxx
Palo
Alto, CA 94304-1130
ATTN:
Xxxx Hope,
Ph.D.
14.10
Dispute Resolution.
(a)
Except as provided
in Section 3.3(b)(i), (b)(ii), (c) or Excluded Claims as set forth
in subsection 14.10(g) below, if a dispute arises within the JSC
with respect to any decision under the jurisdiction of the JSC that
remains unresolved pursuant to Section 3.3 (JSC Decision-Making) or
otherwise between the Parties in connection with or relating to
this Agreement or any document or instrument delivered in
connection herewith (collectively, a “Dispute”), then either Party shall
have the right to refer such Dispute to the Executive Officers for
attempted resolution by good faith negotiations during a period of
forty-five (45) days. Any final decision mutually agreed to in
writing by the Executive Officers shall be conclusive and binding
on the Parties.
(b)
The Executive
Officers shall negotiate in good faith and use reasonable efforts
to settle any Dispute arising from or related to this Agreement or
the breach thereof within such forty-five (45) day period. Subject
to Section 14.10(h) (Dispute Resolution - subsection (h)), in the
event the Executive Officers cannot fully resolve or settle such
Dispute within such period, and a Party wishes to pursue the matter
further, each such Dispute that is not an Excluded Claim (defined
in Section 14.10(g) (Dispute Resolution - subsection (g)) below)
shall be finally resolved by binding arbitration administered by
the Hong Kong International Arbitration Centre (“HKIAC”) in accordance with its
arbitration rules then in effect.
(c)
The arbitration
shall be conducted by a panel of three (3) neutral arbitrators
experienced in the pharmaceutical business, none of whom shall be a
current or former employee or director, or a current stockholder,
of either Party or any of their respective Affiliates or any
Sublicensee. Within thirty (30) days after initiation of
arbitration, each Party shall select one (1) person to act as
arbitrator and the two (2) Party-selected arbitrators shall select
a third arbitrator within thirty (30) days of their appointment. If
the arbitrators selected by the Parties are unable or fail to agree
upon the third arbitrator, the third arbitrator shall be appointed
by the HKIAC (or its successor entity) in accordance with the
then-current HKIAC arbitration rules, except as modified in this
Agreement. The place of arbitration shall be in Hong Kong, and all
proceedings and communications shall be in English. The procedures
for the taking of evidence shall be governed by the HKIAC. The
decision or award rendered by the arbitrators shall be final,
binding, conclusive and non-appealable, and judgment may be entered
upon it in accordance with Applicable Laws in the Hong Kong or any
other court of competent jurisdiction.
(d)
Either Party may
apply to the arbitrators for interim injunctive relief until the
arbitration award is rendered or the controversy is otherwise
resolved. The arbitrators’ authority to award punitive or any
other type of damages not measured by a Party’s compensatory
damages shall be subject to the limitation set forth in Section
13.5 (Special, Indirect and Other Losses). Each Party shall bear
its own costs and expenses and attorneys’ fees and an equal
share of the arbitrators’ fees and any administrative fees of
arbitration.
(e)
Except to the
extent necessary to confirm or enforce an award or as may be
required by law, neither Party nor an arbitrator may disclose the
existence, content, or results of an arbitration without the prior
written consent of the other Party. In no event shall an
arbitration be initiated after the date when commencement of a
legal or equitable proceeding based on the dispute, controversy or
claim would be barred by the applicable Hong Kong statute of
limitations.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-49-
(f)
The Parties agree
that, in the event of a dispute over the nature or quality of
performance under this Agreement, neither Party may terminate this
Agreement until final resolution of the dispute through arbitration
or other judicial determination. The Parties further agree that any
payments made pursuant to this Agreement pending resolution of the
dispute shall be refunded if an arbitrator or court determines that
such payments are not due.
(g)
As used in this
Section, the term “Excluded Claim” means a dispute,
controversy or claim that concerns the construction, scope,
validity, enforceability, inventorship or infringement of a patent,
patent application, trademark or copyright.
(h)
Nothing contained
in this Agreement shall deny either Party the right to seek
injunctive or other equitable relief from a court of competent
jurisdiction in the context of a bona fide emergency or prospective
irreparable harm, and such an action may be filed and maintained
notwithstanding any ongoing discussions between the Parties or any
ongoing arbitration proceeding. In addition, either Party may bring
an action in any court of competent jurisdiction to resolve
disputes pertaining to the construction, scope, validity,
enforceability, inventorship or infringement of a patent, patent
application, trademark or copyright, and no such claim shall be
subject to arbitration pursuant to subsections (b) and (c) of this
Section 14.10 (Dispute Resolution). Both Parties agree to waive any
requirement that the other (i) post a bond or other security as a
condition for obtaining any such relief; or (ii) show irreparable
harm, balancing of xxxxx, consideration of the public interest or
inadequacy of monetary damages as a remedy.
14.11
Performance by Affiliates. Each Party
may discharge any obligations and exercise any rights hereunder
through any of its Affiliates. Each Party hereby guarantees the
performance by its Affiliates of such Party’s obligations
under this Agreement and shall cause its Affiliates to comply with
the provisions of this Agreement in connection with such
performance. Any breach by a Party’s Affiliate of any of such
Party’s obligations under this Agreement shall be deemed a
breach by such Party, and the other Party may proceed directly
against such Party without any obligation to first proceed against
such Party’s Affiliate.
14.12
Headings. The captions to the several
Articles, Sections and subsections hereof are not a part of this
Agreement but are merely for convenience to assist in locating and
reading the several Articles and Sections hereof.
14.13
Waiver of Rule of Construction. Each
Party has had the opportunity to consult with counsel in connection
with the review, drafting and negotiation of this Agreement.
Accordingly, the rule of construction that any ambiguity in this
Agreement shall be construed against the drafting Party shall not
apply.
14.14
Business Day Requirements. In the event
that any notice or other action or omission is required to be taken
by a Party under this Agreement on a day that is not a Business Day
then such notice or other action or omission shall be deemed to
require to be taken on the next occurring Business
Day.
14.15
English Language. This Agreement has
been prepared in the English language, and the English language
shall control its interpretation. In addition, all notices required
or permitted to be given hereunder, and all written, electronic,
oral or other communications between the Parties regarding this
Agreement shall be in the English language
14.16
No Benefit to Third Parties. Except as
provided in ARTICLE 13 (Indemnification; Liability), the covenants
and agreements set forth in this Agreement are for the sole benefit
of the Parties hereto and their successors and permitted assigns
and they shall not be construed as conferring any rights on any
other Persons.
14.17
Further Assurances. Each Party shall
duly execute and deliver, or cause to be duly executed and
delivered, such further instruments and do and cause to be done
such further acts and things, including the filing of such
assignments, agreements, documents and instruments, as may be
necessary or as the other Party may reasonably request in
connection with this Agreement or to carry out more effectively the
provisions and purposes hereof or to better assure and confirm unto
such other Party its rights and remedies under this
Agreement.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-50-
14.18
Counterparts. This Agreement may be
executed in two or more counterparts, each of which shall be deemed
an original, but all of which together shall constitute one and the
same instrument.
IN WITNESS WHEREOF, the Parties intending to be bound have
caused this License Agreement to be executed by their duly
authorized representatives.
|
EverInsight
Therapeutics Inc.
|
|
VistaGen
Therapeutics, Inc.
|
|
|
|
|
|
|
| By: |
/s/ Xxx
Xx
|
By:
|
/s/ Xxxxx X.
Xxxxx
|
|
Name:
|
Xxx
Xx
|
Name:
|
Xxxxx X. Xxxxx,
X.X.
|
|
Title:
|
Director of
EverInsight Therapeutics Inc.
|
Title
|
Chief Executive
Officer
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-51-
LIST
OF EXHIBITS
Exhibit
A:
Licensed Patents
Existing as of the Effective Date
Exhibit
B:
Licensed
Trademarks
Exhibit
C:
PH94B Chemical
Structure
Exhibit
D:
Initial Development
Plan
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-52-
Exhibit
A: Licensed Patents in the
Territory as of the Effective Date
[*****]
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-53-
Exhibit
B: Licensed Trademarks as
of the Effective Date
VISTAGEN®,
United States Registration # 2787886 and international counterparts
in the Territory to be obtained in due course
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-54-
Exhibit
C: PH94B Chemical
Structure
(3b)-androsta-4,16-dien-3-ol
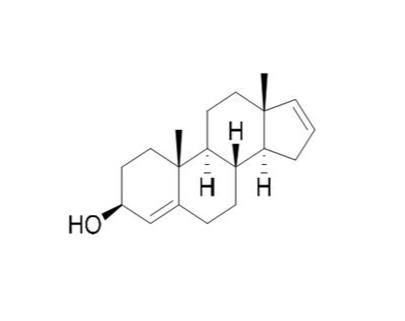
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-55-
Exhibit
D
Initial
Development Plan for Acute Treatment of SAD in the
Territory
[*****]
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED
BY [*****], HAS BEEN OMITTED BECAUSE VISTAGEN THERAPEUTICS, INC.
HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD
LIKELY CAUSE COMPETITIVE HARM TO VISTAGEN THERAPEUTICS,
INC. IF PUBLICLY DISCLOSED.
-56-

