Exhibit 10.17
Confidential information redacted and filed separately with the Commission.
Omitted portions indicated by [***]
CONTRACT MANUFACTURING AND PACKAGING AGREEMENT
Agreement dated as of May 29, 2009 by and between Annie’s Enterprises, Inc.
(d/b/a Annie’s Naturals) (“Customer”) and Chelten House Products, Inc. (“Manufacturer”), each a “Party” and together the “Parties”.
1. Manufacture of Products.
Customer Responsibility. Each week Customer will provide Manufacturer with a [***] week rolling forecast for the products set forth on Schedule A
(the “Products”) and will issue purchase orders to the Manufacturer for the quantity of Products it wishes to be produced.
Annual Minimum Order Volume Forecast. The annual minimum order volume forecast (“Forecast”) is [***] million six count equivalent
cases (“SCE Cases”) in each year of the Term of this Agreement. The Forecast will be mutually negotiated by Parties for each renewal period. If Customer elects to utilize a secondary co-packer to produce any of the Products set
forth on Schedule A, and the actual SCE Cases of Products ordered from Manufacturer falls below the Forecast in any year of the Term of this Agreement, Customer will use its best efforts to move production from the secondary co-packer to
Manufacturer to increase Manufacturer’s production back to Forecast level.
Manufacturer Responsibility. Manufacturer shall have
full responsibility for timely procurement of and payment for all materials and necessary to produce and package Products for Customer under this Agreement (the “Materials”). Manufacturer shall use commercially reasonable efforts to
achieve purchasing synergies and reduce Materials costs. Manufacturer shall store all Materials in accordance with good manufacturing practices prevailing in the industry and in strict compliance with the Specifications and the terms of this
Agreement. Manufacturer shall purchase in quantities that it deems fit, but unless approved in writing, Customer shall be liable only for such quantities of Materials as would normally be used (based upon the rolling forecast provided by the
Customer at the time of the purchase) by Manufacturer within three months of such purchase. Manufacturer shall manufacture, package, store and ship to Customer the Products in the quantities and according to the terms and conditions set forth on
Customer’s purchase order and in accordance with the specifications set forth on Schedule B (the “Specifications”). Each week Manufacturer shall provide to Customer the production and shipping schedule for Customer’s
Products and will provide accurate reporting of production amounts.
New Product Development and Formula Changes. Manufacturer shall use its best
efforts to assist Customer in the development of new products and/or the modification of existing Products to reduce costs and/or improve quality. All product development activities conducted for Customer in Manufacturer’s laboratory will be
the Manufacturer’s responsibility excluding the cost of ingredients utilized. Production tests will be billed using the fee schedule set forth on Schedule A plus the cost of any Materials used in the test. Manufacturer will provide to Customer
an estimate of the number of full and/or 1/2 days
that will be required for any production test, prior to any scheduled test days. All such new Products or modifications shall be the sole property of the Customer.
Facility. Manufacturer shall produce and package the Products at its facility as set forth on Schedule A. Manufacturer represents and warrants that Manufacturer shall for the term of this Agreement
solely operate the facility and all processing equipment located in the facility. Unless there is a material change in the Products and/or Specifications, Manufacturer shall be responsible for any changes necessary to the facility and for the
purchase of any processing equipment necessary to produce the Products.
2. Rejection of Products. Customer may reject and refuse to
pay for Products within [***] days of delivery which (i) have been damaged during manufacturing, storage or handling while the Products were in Manufacturer’s custody, or (ii) are not in material compliance with the terms and
conditions of this Agreement. In addition, Customer may reject any Products that do not fully comply with the Specifications. If Customer has previously paid Manufacturer for Products that are later rejected by Customer for failure to fully comply
with Specifications, Customer shall invoice Manufacturer, including all supporting documentation, for the cost of such rejected Products including the costs of any Provided Materials and for any freight, handling or other disposition costs or
expenses incurred by Customer in connection with such rejected Products and shall receive credit from Manufacturer (or payment from Manufacturer, if no amounts are then due from Customer to Manufacturer) within 30 days of such invoice.
3. Compliance with Applicable Law. The Parties agree that Manufacturer will provide Products of consistent quality composed of safe and wholesome
ingredients, manufactured, packaged, stored, and shipped under conditions compliant with all applicable federal, state, and local requirements.
|
|
|
|
|
| Confidential Information Redacted |
|
1 |
|
|
Confidential
Treatment Requested
4. Quality and Inventory Control, Inspection and Notice.
Quality Control. Manufacturer shall manufacture, prepare, store and label according to procedures set forth on Schedule C, Manufacturer Quality
System Requirements, Testing and Records.
Inventory Control. Manufacturer shall store and safeguard the inventory of Customer’s
Products to protect it at all times from theft, damage or other loss in accordance with best practices in the industry and in strict compliance with the Specifications and terms of this Agreement. Customer shall have the right at any time upon
reasonable notice and during regular business hours (unless otherwise agreed) and at Customer’s expense to inspect and perform a physical count of the inventory of Customer’s Products using such personnel of Customer or Customer’s
representatives (including auditors) as Customer determines to use in its sole discretion.
Quality Control Records and Samples.
Manufacturer shall prepare and submit to Customer quality control records and reports and shall retain one (1) sample for quality control purposes from each production batch of each of the Products for the period of time specified on Schedule
C. Customer may request production samples from Manufacturer at any time, and Manufacturer shall send samples to Customer at Customer’s cost unless otherwise agreed upon in writing.
Inspection Reports. Manufacturer shall make available to Customer, at the request of Customer, the results of all federal, state and local inspection reports and sanitation audits, conducted from
[***] days before to [***] days after the Term of this Agreement, including any renewals.
Notice. Manufacturer shall notify Customer
immediately by telephone and in writing of any such inspections or audits, or any other information, that indicate that presence of any bacteriological agent or any substance that is considered by health authorities as being indicative of either
unsanitary practices or of public health concern or if any of Manufacturer’s facilities, processes, inventories or equipment are in an unsanitary condition as defined by 21 CFR Section 110 et seq. Manufacturer shall notify Customer within
24 hours of any adulteration or potential misbranding of the Products.
5. Price and Payment Terms
Price. Prices per case for each Product shall be F.O.B. factory as set forth on Schedule A. The price per case shown on Schedule A must be
detailed and include Material Cost, Handling Upcharge and Conversion Cost.
Any dunnage costs including the cost for Grocery Manufacturers Associates #1, soft wood pallets should be included with the Material Costs. The Parties shall review Material Costs on a quarterly
basis to adjust for increases or decreases in the cost of raw materials and packaging materials of the Products. Upon request of the Customer, Manufacturer will provide documentation of Materials costs including source and in-bound freight.
The conversion costs per case for each year of the initial term of this Agreement are set forth on Schedule A. [***] days prior to renewal of
this Agreement, Manufacturer shall provide a notice of the proposed conversion costs for the renewal term with sufficient supporting documentation and Manufacturer will allow Customer to inspect and verify the documentation during normal business
hours upon reasonable notice to the Manufacturer. The Parties shall negotiate the conversion costs for the new term in good faith. In the event mutually acceptable conversion costs cannot be reached within [***] days of renewal, Customer may elect
to exclude a specific Product from the revised Exhibit A or either Party may elect to terminate this Agreement on [***] days’ notice. All orders during the [***] day notice period will reflect the revised Exhibit A pricing proposed by the
Manufacturer.
The Material Handling Upcharge rate set forth on Schedule A shall be fixed for the term of this Agreement, including any
renewals.
Invoices/Payment Terms. Manufacturer will invoice Products upon their release from an automatic production process quality
assurance hold. Payment terms shall be [***] days from the date of electronic invoicing, or if mailed, the date the invoice is actually received by Customer. Payment shall be made to Manufacturer by way of wire transfer or Automated Clearing House
transaction.
Records Retention. All books and records in connection with this Agreement, including receipts, documentation of labor
and other costs, and other supporting data as Customer may reasonably request to verify the computation of all amounts invoiced shall be maintained by Manufacturer for the period of time specified on Schedule C.
6. Term. The initial Term of this Agreement shall be three (3) years from the date that Parties mutually agree in writing that formula
qualification is materially complete and production has commenced, unless earlier canceled or terminated as provided in Section 11. This Agreement shall thereafter renew automatically each year for a period of one (1) year unless either
Party provides written notice of non-renewal to the other Party at least ninety (90) days prior to the expiration of the then-current term. In the event that either Party gives a non-renewal notice, Customer shall have the right to require
Manufacturer to continue to manufacture Products for up to 90 days following the expiration date on the same terms as in effect prior to expiration.
Confidential Information
Redacted
2
Confidential
Treatment Requested
7. Confidentiality.
Recipes/Formulas. The Parties agree that the specific recipes and/or formulas provided by Customer and used by Manufacturer in accordance with the Specifications for the Products (the
“Formulas”) will remain exclusive to the Customer and will not be disclosed by the Manufacturer to other customers of the Manufacturer or to any other person or entity. Customer hereby grants to Manufacturer a limited,
non-exclusive, non-transferable license to use the Formulas for the purpose of allowing Manufacturer to perform its obligations under this Agreement.
Confidential Information. During the Term of this Agreement and at all times thereafter, neither Party will disclose or use, either for itself or for the benefit of any third party any confidential
information (“Confidential Information”) of the other Party (the “Disclosing Party”) except in furtherance of the purposes of this Agreement. The provisions of this paragraph, however, shall not prevent the Party.
9. Indemnification. Manufacturer shall indemnify, defend, and hold harmless Customer, along with its employees, dealers, distributors,
affiliates, and other agents (collectively, the “Indemnitees”) from and against any claim asserted by any third party, including any governmental agency, arising out of any error, omission, violation of law, or action taken by Manufacturer
in connection with this Agreement.
10. Insurance. Manufacturer shall maintain commercial general liability insurance (including
products liability and contractual liability), with limits of not less than $[***] combined single limit, for bodily injury or death to any person or persons and loss or damage to any property. Such insurance shall be written by an insurance carrier
rated A or better by Best’s Guide Insurance Rating, and shall name Customer as an additional insured. Products Recall Liability insurance shall be carried by Manufacturer with a limit of $[***] per occurrence and include the Customer as
additional insured.
11. Termination.
Manufacturer may terminate this Agreement upon ninety (90) days’ written notice if Customer (i) fails to make any payment due under this Agreement and such nonpayment continues 30 days
after written notice from Manufacturer or (ii) fails to perform any other obligation under this Agreement and such failure continues thirty (30) days after written notice from Manufacturer.
Customer may terminate this Agreement upon giving
receiving the Confidential Information (the “Receiving Party”) from use or disclosure of
Confidential Information (i) that is in the public domain (other than information in the public domain as a result of a violation of this Agreement by the Receiving Party), (ii) that the Receiving Party can demonstrate that it acquired
outside of its relationship with the Disclosing Party from a third party in rightful possession of such Confidential Information and who was not prohibited from disclosing such Confidential Information, (iii) disclosure of which is required by
law or court order, or (iv) that is developed independently by personnel of the Receiving Party who have not had access to Confidential Information.
8. Trademarks. Trademarks and tradenames owned by Customer are set forth on Schedule D. Nothing in this Agreement shall give Manufacturer any right, title or interest in Customer trademarks. In
addition, Manufacturer shall not adopt any trademark, trade name, trade dress, labeling or packaging which is deceptively similar to or likely to cause confusion with respect to any of Customer trademarks or with respect to Products.
thirty (30) days’ written notice identifying specifically the basis for such notice for breach of a material term or condition of this
Agreement, provided that Manufacturer shall not have cured such breach within the thirty (30) day period; and provided further that a termination for contamination or adulteration of a Product, unless otherwise specified, shall be immediate
(i.e., without cure period).
Each Party reserves the right upon a breach of this Agreement by the other Party to pursue all of its legal and
equitable remedies.
Either Party hereto may terminate this Agreement effective immediately upon written notice to the other party if the
other party: (i) files a voluntary petition in bankruptcy or has an involuntary bankruptcy petition filed against it, which is not dismissed within ninety (90) days after its institution, (ii) is adjudged as bankrupt,
(iii) becomes insolvent, (iv) has a receiver, trustee, conservator or liquidator appointed for all or a substantial part of its assets, (v) ceases to do business, (vi) commences any dissolution, liquidation or winding up, or
(vii) makes an assignment of its assets for the benefit of its creditors.
Either Party may terminate this Agreement at any time on or
after the third anniversary (i.e. during any renewal Term) without payment of any termination fee upon giving ninety (90) days’ written notice to other Party. If Manufacturer elects to terminate this agreement, Customer shall have the
right to require Manufacturer to continue to manufacture Products for up to 120 days following the termination date on the same terms as in effect prior to termination.
Confidential Information
Redacted
3
Confidential
Treatment Requested
13. Miscellaneous.
Amendments. No modification or amendment of this Agreement shall be effective unless in writing and executed by a duly authorized representative of each Party.
Assignability. This Agreement shall be binding upon and be for the benefit of the Parties and their legal representatives, successors, and
assigns. Neither Party may assign this Agreement without the prior written consent of the other; provided, that notwithstanding the foregoing, either Party may assign this Agreement without such consent to the purchaser of all or substantially all
of such Party’s business and assets, and may otherwise assign this Agreement by operation of law to any successor of such Party due to merger or reorganization.
Notices. Any notice required or permitted under this Agreement shall be given in writing and shall be given by personal delivery, via overnight courier requiring a signature for delivery, or by
certified mail, return
receipt requested. All notices shall be delivered to the addresses last specified in writing by the Parties.
Partial Invalidity. If any provision hereof is found invalid or unenforceable pursuant to a final judicial decree or decision, the remainder of this Agreement will remain valid and enforceable
according to these terms.
Waiver. The failure of either Party to assert any of its rights under this Agreement shall not be deemed to
constitute a waiver of that Party’s right thereafter to enforce each and every provision of this Agreement in accordance with its terms.
Governing Law and Venue. This Agreement shall be governed by, and any dispute arising hereunder shall be determined in accordance with, the laws
of State of New York, without giving effect to conflict of laws principles. The Parties hereto irrevocably submit to the jurisdiction and venue of the state and federal courts sitting in New York, New York.
|
|
|
|
|
|
|
|
|
| ANNIE’S ENTERPRISES, INC. |
|
|
|
CHELTEN HOUSE PRODUCTS, INC. |
|
|
|
|
|
| By: |
|
/s/ Xxxxxx Xxxxxxx |
|
|
|
By: |
|
/s/ Xxxxx Xxxxxx |
|
|
Xxxxxx Xxxxxxx, Treasurer |
|
|
|
|
|
Xxxxx Xxxxxx, CEO |
4
Confidential
Treatment Requested
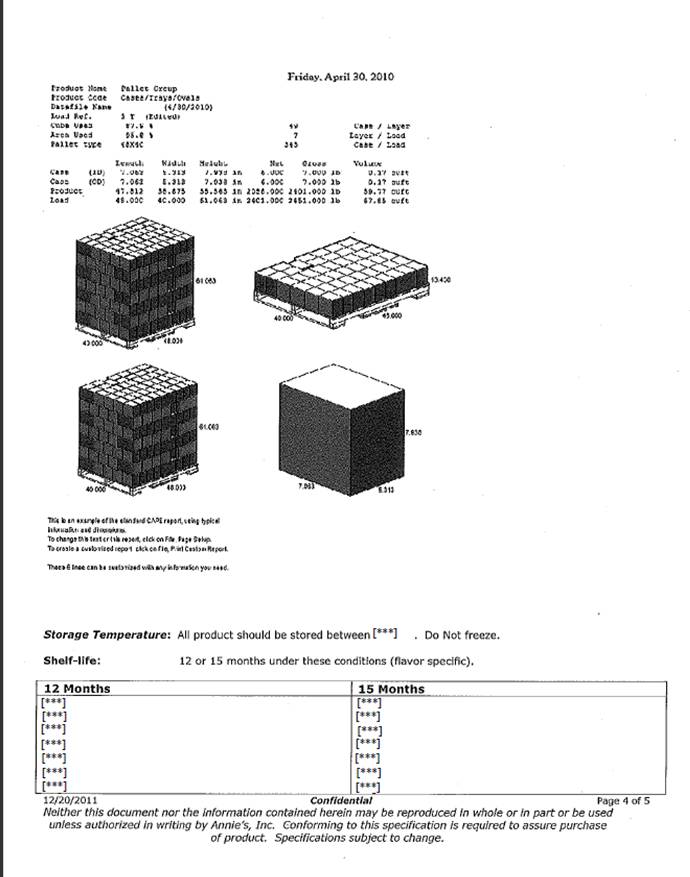
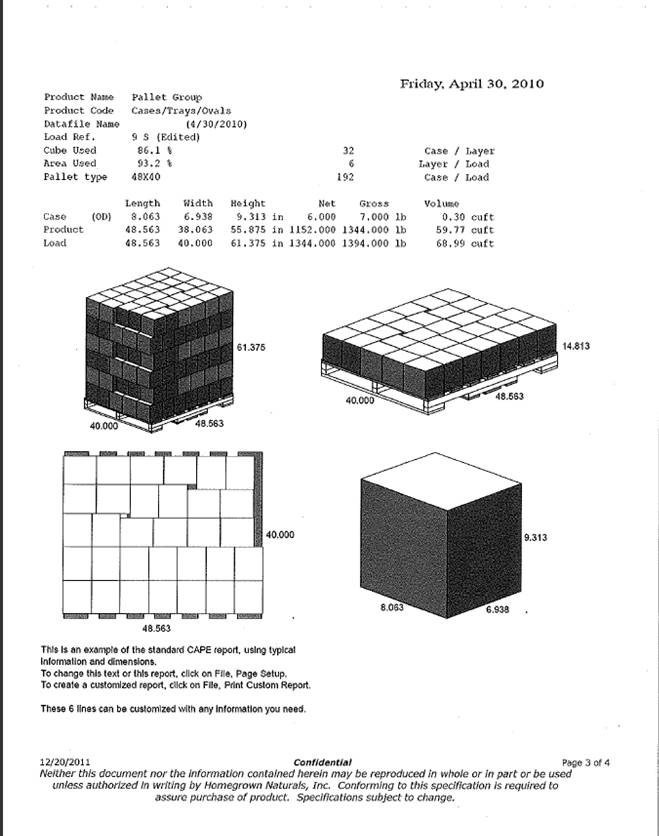
Schedule A
Confidential
Products
Material Handling Upcharge
[***]% of Manufacturer Purchased Materials Costs
Conversion Costs per Case
|
|
|
|
|
|
|
|
|
|
|
|
|
| Pack |
|
Year 1 |
|
|
Year 2 |
|
|
Year 3 |
|
| 6/8oz |
|
|
$[***] |
|
|
|
$[***] |
|
|
|
$[***] |
|
| 12/8 oz |
|
|
$[***] |
|
|
|
$[***] |
|
|
|
$[***] |
|
| 6/16 oz |
|
|
$[***] |
|
|
|
$[***] |
|
|
|
$[***] |
|
| 6/12 oz Round |
|
|
$[***] |
|
|
|
$[***] |
|
|
|
$[***] |
|
| 12/12 oz |
|
|
$[***] |
|
|
|
$[***] |
|
|
|
$[***] |
|
| 12/5 oz |
|
|
$[***] |
|
|
|
$[***] |
|
|
|
$[***] |
|
| 6/1 Liter |
|
|
$[***] |
|
|
|
$[***] |
|
|
|
$[***] |
|
Facility
000 Xxxxx Xxxxx
Xxxxxxxxxx, XX 00000
Production Test Fees
1/2 Day $[***]
Full Day $[***]
([***])
|
|
|
|
|
| Confidential Information Redacted |
|
5 |
|
|
Confidential
Treatment Requested
Schedule B
Confidential
Specifications
Confidential Treatment Requested

Confidential Information
Redacted
7
Confidential
Treatment Requested

8
Confidential
Treatment Requested
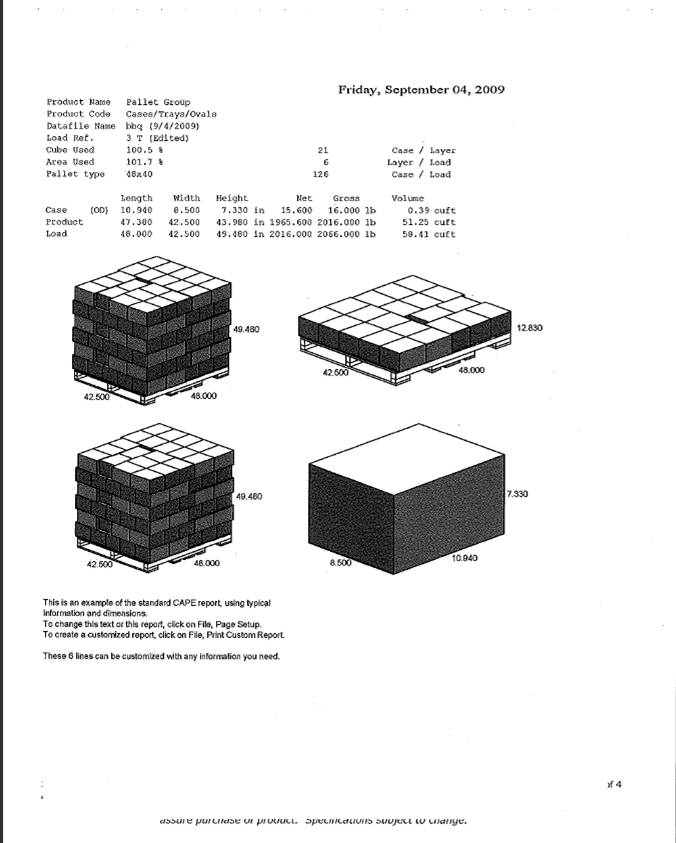
9
Confidential
Treatment Requested

Confidential Information
Redacted
10
Confidential
Treatment Requested

Confidential Information
Redacted
11
Confidential
Treatment Requested

12
Confidential
Treatment Requested
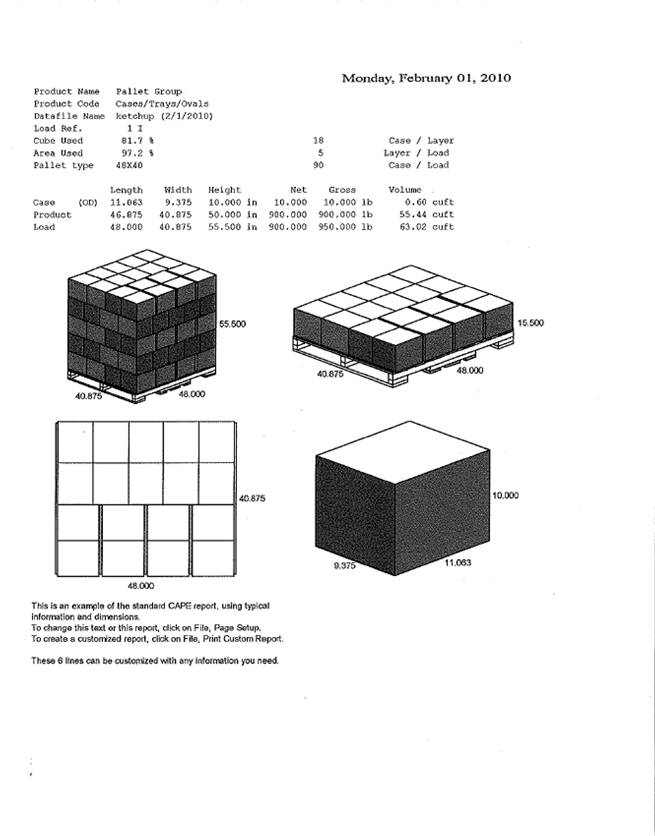
13
Confidential
Treatment Requested

Confidential Information
Redacted
14
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
15 |
|
|
Confidential
Treatment Requested

16
Confidential
Treatment Requested
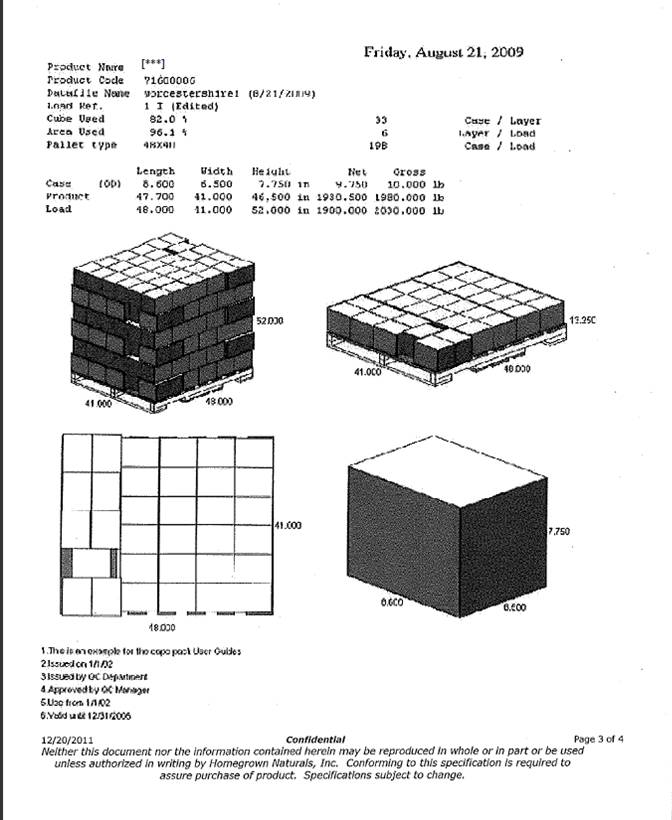
|
|
|
|
|
| Confidential Information Redacted |
|
17 |
|
|
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
18 |
|
|
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
19 |
|
|
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
20 |
|
|
Confidential
Treatment Requested

21
Confidential
Treatment Requested
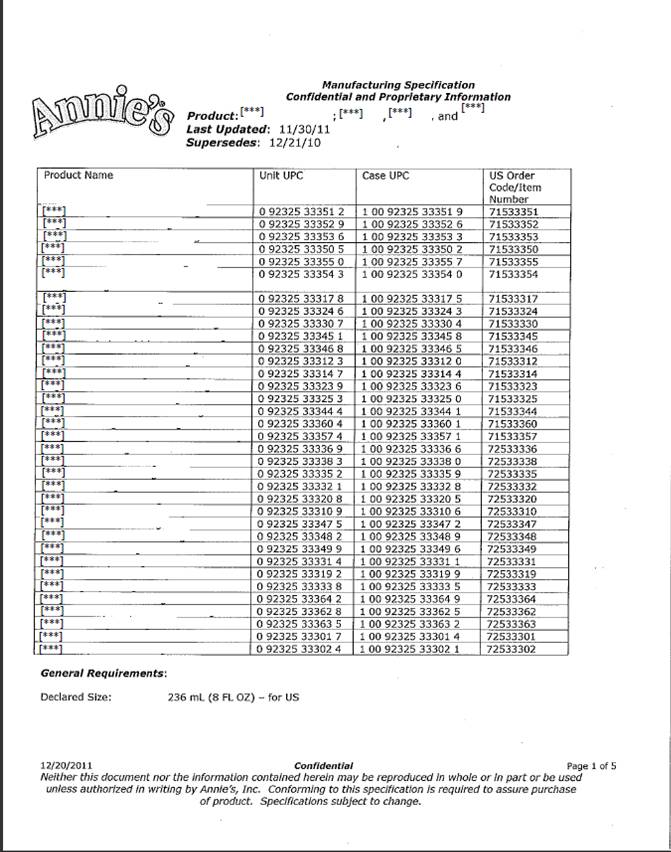
|
|
|
|
|
| Confidential Information Redacted |
|
22 |
|
|
Confidential
Treatment Requested

23
Confidential
Treatment Requested

24
Confidential
Treatment Requested
|
|
|
|
|
| Confidential Information Redacted |
|
25 |
|
|
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
26 |
|
|
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
27 |
|
|
Confidential
Treatment Requested

28
Confidential
Treatment Requested
29
Confidential
Treatment Requested
|
|
|
|
|
| Confidential Information Redacted |
|
30 |
|
|
Confidential
Treatment Requested
|
|
|
|
|
| Confidential Information Redacted |
|
31 |
|
|
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
32 |
|
|
Confidential
Treatment Requested
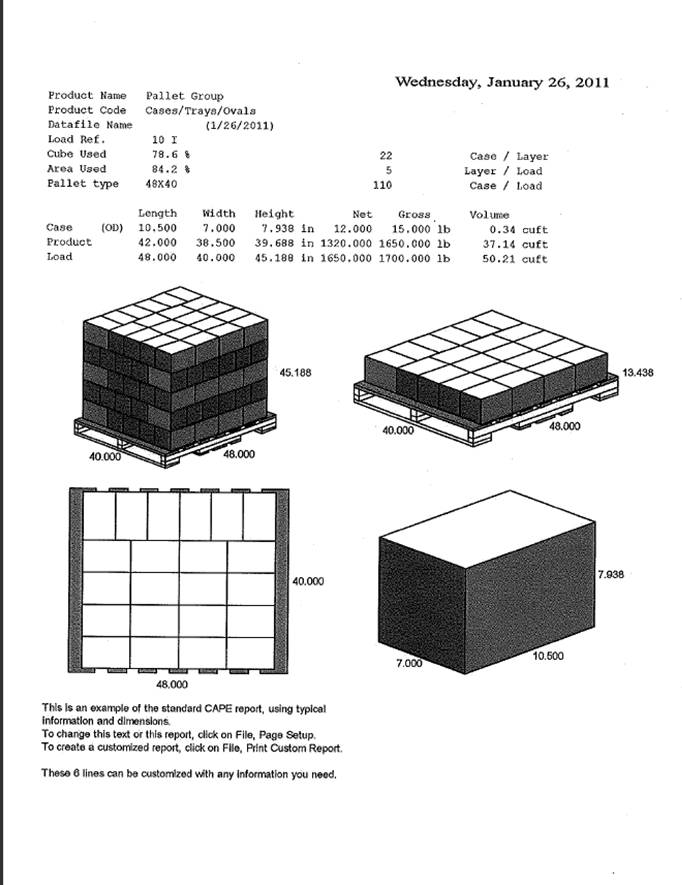
33
Confidential
Treatment Requested

|
|
|
|
|
| Confidential Information Redacted |
|
34 |
|
|
Confidential
Treatment Requested
Schedule C
Manufacturer Quality System Requirements, Testing and Records
|
|
|
| Applicable laws, regulations, and requirements |
|
All Products shall be of consistent quality composed of safe and wholesome ingredients, manufactured, prepared, packed, held, labeled, and shipped under conditions compliant with
all applicable U.S. and Canadian federal, state, and local requirements including but not limited to applicable laws, regulations, and guidelines adopted by (i) the Food and Drug Administration (“FDA”) pursuant to the Federal Food, Drug,
and Cosmetic Act, as amended (the “Act”) and the Public Health Security and Bioterrorism Preparedness and Response Act (the “Bioterrorism Act”), including but not limited to the food safety, composition, labeling, registration,
and manufacturing provisions and current industry good manufacturing practices; (ii) the United States Department of Agriculture (“USDA”) and Food Safety Inspection Service (“FSIS”), including but not limited to the National
Organic Program and the organic food regulations adopted pursuant to the Federal Organic Foods Production Act; (iii) applicable state and local authorities responsible for regulating the manufacture, preparation, packing, storage, labeling, and
shipment of food products and establishments, including without limitation the California Organic Foods Act, as amended and all applicable organic food certification programs; and (iv) Health Canada pursuant to the Food and Drugs Act, as amended,
and the Canadian Food Inspection Agency pursuant to the Canada Agricultural Products Act and the Organic Products Regulations. |
|
|
| Production Records |
|
Facility and production records must be maintained including, but not limited to, pest control, receiving and inspection, batch records, processing, weight control, calibration,
sanitation audits and procedures, and training. |
|
|
| Organic Program (If Applicable) |
|
Facility must be certified Organic by a third party agency accredited by the USDA National Organic Program. All Organic policies and procedures must be followed as outlined in 7
CFR Part 205. All documentation and records must be maintained for five years. |
|
|
| Quality Testing |
|
Incoming ingredient and in process checks must be conducted to ensure that the manufacturing process is in control and that finished Products meet all Specifications. Testing
shall include (as applicable) pH, titratable acidity, batching and processing validation, sensory, packaging checks, and net contents. Finished Products will be tested (as applicable) for pH, %Titratable Acidity, and Microbiological tests, or other
tests determined necessary to ensure that they meet specifications. |
|
|
| Record Retention |
|
Manufacturing and Quality records must be retained for a minimum of [***] years for non-organic products, and [***] years for organic products. |
|
|
| Certificate of Analysis |
|
Customer may request a Certificate of Analysis that would document and summarize the in-process and finished product testing. |
|
|
| Retain Samples |
|
Representative samples from a production run must be collected and submitted to Customer per the frequency outlined in the
manufacturing specification. Retain samples must also be collected and stored at the manufacturing location for the duration of the product
shelf-life plus six (6) months. |
|
|
| Third Party Audits |
|
Facility must annually undergo and successfully pass one of the following recommended Food Safety/Quality Systems Audits: FPA/GMA-SAFE, Silliker GMP and Food Safety Systems
Audit, NSF Xxxx & Xxxxxxx Food Safety, Quality and Food Defense Audit, SQF Audit, or USDA Total Quality Systems Audit. Alternate audits permitted with Customer management approval. Corrective Action plans must be developed and implemented for
all significant deficiencies identified in the audit. Customer may request copies of detailed findings and/or corrective action plans of Manufacturer’s audit. Results of audit must be submitted to Customer when available, and posted on
Manufacturer’s iCiX account. |
|
|
|
|
|
| Confidential Information Redacted |
|
35 |
|
|
Confidential
Treatment Requested
|
|
|
| Customer Audits |
|
Customer has the right to inspect manufacturing and warehouse facilities where machines are in operation, and where finished Product, ingredients, and packaging materials are
stored. During the course of the audit, Customer’s designated representatives have the authority to stop production of Products, reject Products for sale or shipment, and implement withdrawal of Product that do not meet Customer Specifications
at the expense of the Manufacturer. On occasion, random samples of raw materials, ingredients, and finished product may be collected by Customer personnel and forwarded to an independent laboratory for analysis. The purpose is to survey Product to
assure compliance to microbiological, physical, or chemical specifications. |
|
|
| Regulatory Inspections |
|
If at any time the FDA, State, OSHA, CFIA, Health Canada, or any governmental regulatory agency enters the Manufacturer’s
facility to inspect Customer Product, Customer’s designated representatives MUST be notified immediately. See “Notice” in Section 4 of the Agreement.
Regulatory Samples: If samples are collected by a regulatory official during manufacture or from storage, a triplicate sample is to be taken by the factory.
1. Sampling equipment utilized by plant personnel, such as plastic bags,
sterile plastic bags, paper bags, sterile utensils, etc. should be identical to that which the Inspector uses during collection.
2. Sufficient sampling equipment should be available, properly prepared, and
readily attainable for both the Inspector’s and factory use. 3. It is imperative that samples be collected in exactly the same manner as the Inspector uses. The material should be handled with an instrument similar to that used by
the Inspector.
4. Collect three samples in addition to the one taken by the inspector.
The triplicate product should be taken from the closest proximity to the regulatory sample as possible (e.g., the next package produced). The amount of sample taken should be approximately three (3) times as much as the Inspector samples.
5. Samples must be labeled or otherwise adequately identified with the
following minimum information:
a. Date and Time of Day.
b. Location: Line pallet number, etc.
c. Lot
number or production code.
d. Name of substance: Commodity code, product code or other identification
number.
e. Name and address of supplier if
appropriate |
36
Confidential
Treatment Requested
|
|
|
| Regulatory Inspections (continued) |
|
f. Finished Product manufactured if a component is
sampled
g. Name of Inspectors and Regulatory agency
h. Any
peculiarities or pertinent observations of the sampling technique (e.g.: floor scrapings, aseptic collection into a sterile container, non-aseptic collection into a sterile container)
6. The
regulatory official should be requested to initial all samples retained by the Factory.
7. Customer’s designated representatives must be notified and will
provide directions for sending the samples to a testing laboratory. 8. Selected samples must be properly stored and retained pending results from the inspecting agency’s laboratory and authorization from Customer.
9. Customer will advise the Manufacturer whether it is necessary to withhold
Product or ingredient pending analytical results. |
37
Confidential
Treatment Requested

38
Confidential
Treatment Requested

39
Confidential
Treatment Requested































