AMENDED AND RESTATED COLLABORATIVE RESEARCH, DEVELOPMENT AND MARKETING AGREEMENT Between MITOTIX, INC. and THE DUPONT MERCK PHARMACEUTICAL COMPANY dated as of June 2, 1997
Exhibit 10.05
Confidential treatment has been requested for portions of this Exhibit. The copy filed herewith omits the information subject to the confidentiality request. Omissions are designated as ***. A complete version of this Exhibit has been filed separately with the Securities and Exchange Commission.
EXECUTION COPY
AMENDED AND RESTATED
COLLABORATIVE RESEARCH, DEVELOPMENT
AND MARKETING AGREEMENT
Between
MITOTIX, INC.
and
THE DUPONT MERCK PHARMACEUTICAL COMPANY
dated as of June 2, 1997
TABLE OF CONTENTS
| ARTICLE 1. |
DEFINITIONS | 1 | ||||
| 1.1 |
“Affiliate” | 1 | ||||
| 1.2 |
“Allowable Expense” | 2 | ||||
| 1.3 |
“Alternate UBC Plan” | 2 | ||||
| 1.4 |
“Antisense” | 2 | ||||
| 1.5 |
“Annual CDK Research Plan” | 2 | ||||
| 1.6 |
“Annual Research Plan and Budget” | 2 | ||||
| 1.7 |
“Calendar Quarter” | 2 | ||||
| 1.8 |
“Calendar Year” | 2 | ||||
| 1.9 |
“Cdc 27/Cdc 16 License” | 2 | ||||
| 1.10 |
“CDK Collaboration” | 2 | ||||
| 1.11 |
“CDK Research Operating Committee” | 2 | ||||
| 1.12 |
“CDK Development Compounds” | 2 | ||||
| 1.13 |
“CDK-D Development Compounds” | 2 | ||||
| 1.14 |
“CDK-non-D Development Compounds” | 2 | ||||
| 1.15 |
“CDK Field” | 3 | ||||
| 1.16 |
“CDK-D Field” | 3 | ||||
| 1.17 |
“CDK-non-D Field” | 3 | ||||
| 1.18 |
“CDK Patent Rights” | 3 | ||||
| 1.19 |
“CDK Products” | 3 | ||||
| 1.20 |
“CDK Research Program” | 3 | ||||
| 1.21 |
“CDK Targets” | 3 | ||||
| 1.22 |
“CDK-D Targets” | 3 | ||||
| 1.23 |
“CDK-non-D Targets” | 4 | ||||
| 1.24 |
“Collaboration” | 4 | ||||
| 1.25 |
“Collaborative Policy Setting Committee” | 4 | ||||
| 1.26 |
“Cyclin E License” | 4 | ||||
| 1.27 |
“Cyclin D License” | 4 | ||||
| 1.28 |
“Competitive Product” | 4 | ||||
| 1.29 |
“Development Partner” | 4 | ||||
| 1.30 |
“Distributor” | 4 | ||||
| 1.31 |
“DuPont Merck Inventions” | 4 | ||||
| 1.32 |
“Effective Date” | 4 | ||||
| 1.33 |
“Extended UBC Collaboration” | 5 | ||||
| 1.34 |
“Extension Notice” | 5 | ||||
| 1.35 |
“First Commercial Sale” | 5 | ||||
| 1.36 |
“Gene Therapy” | 5 | ||||
| 1.37 |
“Immunoassay Product” | 5 | ||||
| 1.38 |
“IND” | 5 | ||||
| 1.39 |
“Initial UBC Term” | 5 | ||||
| 1.40 |
“Inventions” | 5 | ||||
| 1.41 |
“IPO” | 5 | ||||
| 1.42 |
“Know-how” | 5 | ||||
| 1.43 |
“Mitotix Inventions” | 5 | ||||
(i)
| 1.44 |
“Mitotix Pending License Agreements” | 5 | ||||
| 1.45 |
“Mitotix License Agreements” | 5 | ||||
| 1.46 |
“Mitotix Product” | 6 | ||||
| 1.47 |
“Net Sales” | 6 | ||||
| 1.48 |
“New UBC Target” | 7 | ||||
| 1.49 |
“NDA” | 7 | ||||
| 1.50 |
“Percentage Contribution” | 7 | ||||
| 1.51 |
“pl6 License” | 7 | ||||
| 1.52 |
“PRAD1 License” | 7 | ||||
| 1.53 |
“Product Patent Rights” | 7 | ||||
| 1.54 |
“Radiopharmaceutical” | 7 | ||||
| 1.55 |
“Radiopharmaceutical Product” | 7 | ||||
| 1.56 |
“Research Operating Committees” | 7 | ||||
| 1.57 |
“Research Year” | 8 | ||||
| 1.58 |
“Royalty-Bearing Products” | 8 | ||||
| 1.59 |
“Royalty Term” | 8 | ||||
| 1.60 |
“Strategic Countries” | 8 | ||||
| 1.61 |
“Target” | 8 | ||||
| 1.62 |
“Third Party” | 8 | ||||
| 1.63 |
“UBC Collaboration” | 8 | ||||
| 1.64 |
“UBC Deferral Payment” | 8 | ||||
| 1.65 |
“UBC Development Compounds” | 8 | ||||
| 1.66 |
“UBC Extension Payment” | 8 | ||||
| 1.67 |
“UBC Field” | 9 | ||||
| 1.68 |
“UBC Operational Disengagement Plan” | 9 | ||||
| 1.69 |
“UBC Operating Profit” | 9 | ||||
| 1.70 |
“UBC Patent Rights” | 9 | ||||
| 1.71 |
“UBC Pricing Date” | 9 | ||||
| 1.72 |
“UBC Products” | 9 | ||||
| 1.73 |
“UBC Research, Development and Pre-Launch Marketing Costs” | 9 | ||||
| 1.74 |
“UBC Research Operating Committee” | 9 | ||||
| 1.75 |
“UBC Research Program” | 9 | ||||
| 1.76 |
“UBC Targets” | 9 | ||||
| 1.77 |
“UBC9 License” | 10 | ||||
| 1.78 |
“Unique Product” | 10 | ||||
| 1.79 |
“Valid Patent Claim” | 10 | ||||
| ARTICLE 2. |
SCOPE AND STRUCTURE OF THE COLLABORATIONS | 10 | ||||
| ARTICLE 3. |
CDK COLLABORATION-CDK RESEARCH PROGRAM | 10 | ||||
| 3.1 |
Conduct of the CDK Research Program | 10 | ||||
| 3.1.1 | General Terms | 10 | ||||
| 3.1.2 | Annual Research Plan | 11 | ||||
| 3.1.3 | Transfer of Technical Information and Know-How | 11 | ||||
| 3.1.4 | DuPont Merck Compounds | 11 | ||||
| 3.1.5 | Subcontracts | 12 | ||||
| 3.1.6 | Data | 12 | ||||
(ii)
| 3.1.7 |
Quarterly Reports by Mitotix and DuPont Merck | 12 | ||||
| 3.2 |
Funding of the CDK Research Program | 13 | ||||
| 3.2.1 | Annual Payment | 13 | ||||
| 3.2.2 | Incentive Research Funding | 13 | ||||
| 3.3 |
Expiration or Termination of the CDK Research Program and Result of | |||||
| Such Termination |
14 | |||||
| ARTICLE 4. |
CDK COLLABORATION: CDK DEVELOPMENT PROGRAM | 14 | ||||
| 4.1 |
Designation of CDK Development Compounds | 14 | ||||
| 4.2 |
Clinical Development and Marketing | 14 | ||||
| 4.3 |
Development Information | 14 | ||||
| ARTICLE 5. |
GENERAL DESCRIPTION OF THE UBC COLLABORATION | 15 | ||||
| 5.1 |
Scope of UBC Collaboration | 15 | ||||
| 5.2 |
Initial Term and Option to Extend | 15 | ||||
| 5.2.1 | Initial Term | 15 | ||||
| 5.2.2 | Option to Extend; UBC Extension Payment | 15 | ||||
| 5.2.3 | UBC Deferral Payment | 16 | ||||
| 5.3 |
UBC Research Program | 16 | ||||
| 5.4 |
Research and Development Funding Commitments | 16 | ||||
| 5.4.1 | First Three Research Years | 16 | ||||
| 5.4.2 | Fourth Research Year and Thereafter | 17 | ||||
| 5.4.3 | Changes to Mitotix’s Funding Commitment | 17 | ||||
| 5.4.4 | Alternate UBC Plan | 17 | ||||
| 5.4.5 | Invoicing | 18 | ||||
| 5.4.6 | Records | 19 | ||||
| 5.5 |
Termination of UBC Collaboration if DuPont Merck Does Not Extend the UBC Collaboration and Result of Such Termination | 19 | ||||
| 5.6 |
Certain Royalty Rights | 20 | ||||
| 5.7 |
UBC Operational Disengagement Plan | 20 | ||||
| 5.8 |
New UBC Targets | 22 | ||||
| 5.9 |
Terms of Equity Investment | 22 | ||||
| 5.9.1 | General Provisions Relating to Price of Equity Investment | 22 | ||||
| 5.9.2 | Investment Prior to an Initial Public Offering | 23 | ||||
| 5.9.3 | Investment After an Initial Public Offering | 23 | ||||
| ARTICLE 6. |
THE UBC COLLABORATION - UBC RESEARCH PROGRAM | 24 | ||||
| 6.1 |
Conduct of the UBC Research Program | 24 | ||||
| 6.1.1 | General Terms | 24 | ||||
| 6.1.2 | Annual UBC Research Plan and Budget | 24 | ||||
| 6.1.3 | DuPont Merck Compounds | 25 | ||||
| 6.1.4 | Subcontracts | 25 | ||||
| 6.1.5 | Data | 25 | ||||
| 6.1.6 | Quarterly Reports by Mitotix and DuPont Merck | 26 | ||||
| 6.2 |
Expiration or Termination of the UBC Research Program and Result of Such Termination | 26 | ||||
| 6.2.1 | Termination of Initial UBC Term | 26 | ||||
(iii)
| 6.2.2 | Expiration Following Extension of the UBC Collaboration and Commencement of Alternate UBC Plan | 26 | ||||
| 6.2.3 | Termination of the UBC Research Program Following Extension of the UBC Collaboration and Prior to the Commencement of the Alternate UBC Plan | 26 | ||||
| 6.2.4 | Termination of the UBC Research Program Following Commencement of the UBC Operational Disengagement Plan | 28 | ||||
| ARTICLE 7. |
UBC COLLABORATION: UBC DEVELOPMENT PROGRAM | 28 | ||||
| 7.1 |
Description of UBC Development Compounds | 28 | ||||
| 7.2 |
Clinical Development and Marketing | 28 | ||||
| 7.2.1 | When the Alternate UBC Plan is Not in Effect | 28 | ||||
| 7.2.2 | Clinical Development and Marketing Under the Alternate UBC Plan | 28 | ||||
| 7.3 |
Development Information | 29 | ||||
| ARTICLE 8. |
MITOTIX’S RIGHT TO CO-PROMOTE | 29 | ||||
| 8.1 |
Mitotix’s Co-promotion Option | 29 | ||||
| ARTICLE 9. |
MANAGEMENT OF THE COLLABORATIONS | 30 | ||||
| 9.1 |
Collaborative Policy Setting Committee | 30 | ||||
| 9.1.1 | General | 30 | ||||
| 9.1.2 | Chair | 30 | ||||
| 9.1.3 | Minutes | 30 | ||||
| 9.2 |
Research Operating Committees | 30 | ||||
| 9.2.1 | Chairs | 31 | ||||
| 9.2.2 | Minutes | 31 | ||||
| 9.3 |
Disagreements | 31 | ||||
| 9.4 |
Project Leaders | 32 | ||||
| 9.5 |
Availability of Employees | 32 | ||||
| 9.6 |
Visit of Facilities | 32 | ||||
| ARTICLE 10. |
LICENSE GRANTS AND RIGHTS OF FIRST NEGOTIATION | 32 | ||||
| 10.1 |
Grant of License Rights to DuPont Merck in CDK Field | 32 | ||||
| 10.1.1 | Exclusive License in CDK Field | 32 | ||||
| 10.1.2 | Conversion to Non-Exclusive | 33 | ||||
| 10.1.3 | Election to Terminate License Grant | 33 | ||||
| 10.1.4 | Additional In-Licensed CDK Technology | 33 | ||||
| 10.1.5 | Release of Certain CDK Targets and Certain Royalty Payments | 33 | ||||
| 10.2 |
Grant of License Rights to DuPont Merck in the UBC Field | 34 | ||||
| 10.2.1 | Exclusive License in the UBC Field | 34 | ||||
| 10.2.2 | Conversion to Non-Exclusive | 34 | ||||
| 10.2.3 | Election to Terminate License Grant | 34 | ||||
| 10.2.4 | Additional In-Licensed UBC Technology | 34 | ||||
| 10.3 |
Reservation of Rights | 36 | ||||
| 10.4 |
Grant of License Rights to Mitotix | 36 | ||||
| 10.4.1 | License to Perform Obligations | 36 | ||||
(iv)
| 10.4.2 | License to DuPont Merck Inventions | 36 | ||||
| 10.5 |
DuPont Merck Rights of First Negotiation | 37 | ||||
| 10.5.1 | Right of First Negotiation with Respect to Antisense and/or Gene Therapy | 37 | ||||
| 10.5.2 | Right of First Negotiation with Respect to CDK-non-D Targets | 37 | ||||
| ARTICLE 11. |
MILESTONES AND ROYALTIES | 38 | ||||
| 11.1 |
Milestone Payments | 38 | ||||
| 11.1.1 | Development Events | 39 | ||||
| 11.1.2 | Notice of Achievement of Milestones | 41 | ||||
| 11.1.3 | Radiopharmaceutical Products and Immunoassay Products | 41 | ||||
| 11.1.4 | Non-Strategic Countries | 41 | ||||
| 11.2 |
Royalties Payable to Mitotix on Net Sales | 41 | ||||
| 11.2.1 | Royalty Payable to Mitotix on Net Sales in Strategic Countries | 42 | ||||
| 11.2.2 | Conditions on Royalty Payments | 42 | ||||
| 11.3 |
Royalties Payable to Mitotix on Net Sales to Distributors in Non-Strategic Countries | 43 | ||||
| 11.4 |
Royalties Payable to Mitotix on Net Sales by Development Partners and other Sublicensees in Japan and Other Non-Strategic Countries | 43 | ||||
| 11.5 |
Profit Sharing on Sales of UBC Products | 43 | ||||
| ARTICLE 12. |
ROYALTIES PAYABLE TO DUPONT MERCK | 44 | ||||
| 12.1 |
General | 44 | ||||
| 12.2 |
Royalties Payable to DuPont Merck on Net Sales | 44 | ||||
| 12.2.1 | Royalty Payable to DuPont Merck on Net Sales in Strategic Countries | 44 | ||||
| 12.2.2 | Conditions on Royalty Payments | 45 | ||||
| 12.3 |
Royalties Payable to DuPont Merck on Net Sales to Distributors in Non-Strategic Countries | 45 | ||||
| 12.4 |
Royalties Payable to DuPont Merck on Net Sales by Development Partners and other Sublicensees in Japan and Other Non-Strategic Countries | 45 | ||||
| ARTICLE 13. |
PAYMENT TERMS | 46 | ||||
| 13.1 |
General | 46 | ||||
| 13.2 |
Royalty Reports, Exchange Rates | 46 | ||||
| 13.3 |
Payment of Profit Sharing Amounts | 46 | ||||
| 13.4 |
Audits | 47 | ||||
| 13.5 |
Payment Terms | 48 | ||||
| 13.6 |
Exchange Controls | 48 | ||||
| 13.7 |
Payment Method | 48 | ||||
| 13.8 |
Interest on Late Payments | 48 | ||||
| 13.9 |
Overriding Provisions of License Agreements | 49 | ||||
| ARTICLE 14. |
PATENTS | 50 | ||||
| 14.1 |
Ownership of Inventions | 50 | ||||
(v)
| 14.2 |
Provisions Concerning the Filing, Prosecution and Maintenance of CDK Patent Rights, UBC Patent Rights and Inventions | 51 | ||||
| 14.2.1 | CDK Patent Rights, UBC Patent Rights and Inventions Owned by Each Party | 51 | ||||
| 14.2.2 | Joint Inventions Relating to CDK Targets and UBC Targets | 51 | ||||
| 14.2.3 | Joint Inventions Relating to CDK Products and UBC Products | 51 | ||||
| 14.2.4 | Consultation Regarding Joint Inventions | 51 | ||||
| 14.2.5 | Patent Costs | 52 | ||||
| 14.3 |
Cooperation | 52 | ||||
| 14.4 |
No Other Technology Rights | 52 | ||||
| 14.5 |
Enforcement of Patent Rights | 52 | ||||
| 14.6 |
Defense of Individual Infringement Actions Involving Use of the Targets | 53 | ||||
| 14.7 |
Defense of Joint Infringement Actions Involving Use of the Targets | 53 | ||||
| 14.8 |
Contribution | 54 | ||||
| 14.9 |
Third Party Patents | 54 | ||||
| 14.10 |
Infringement of Mitotix Inventions or Joint Inventions Covering CDK Products or UBC Products | 54 | ||||
| ARTICLE 15. |
CONFIDENTIALITY | 55 | ||||
| 15.1 |
Nondisclosure Obligations | 55 | ||||
| 15.2 |
Samples | 56 | ||||
| 15.3 |
Terms of this Agreement and Existence of UBC Collaboration | 56 | ||||
| 15.4 |
Publications | 57 | ||||
| ARTICLE 16. |
REPRESENTATIONS AND WARRANTIES | 57 | ||||
| 16.1 |
Authorization | 57 | ||||
| 16.2 |
License Agreements with Third Parties | 58 | ||||
| 16.3 |
Patent Validity | 58 | ||||
| 16.4 |
Exclusivity and Freedom-to-operate | 58 | ||||
| ARTICLE 17. |
INDEMNITY | 58 | ||||
| 17.1 |
DuPont Merck Indemnity Obligations | 58 | ||||
| 17.2 |
Mitotix Indemnity Obligations | 59 | ||||
| 17.3 |
Procedure | 59 | ||||
| 17.4 |
Insurance | 60 | ||||
| ARTICLE 18. |
TERM AND TERMINATION | 60 | ||||
| 18.1 |
Expiration | 60 | ||||
| 18.2 |
Termination for Cause | 60 | ||||
| 18.2.1 | Bankruptcy | 60 | ||||
| 18.2.2 | Material Breach | 60 | ||||
| 18.2.3 | Failure to Retain Qualified Scientists | 60 | ||||
| 18.3 |
Effect of Termination | 60 | ||||
| 18.4 |
Failure to Pursue | 61 | ||||
| ARTICLE 19. |
MISCELLANEOUS | 61 | ||||
(vi)
| 19.1 |
Force Majeure |
61 | ||||
| 19.2 |
Assignment |
62 | ||||
| 19.3 |
Severability |
62 | ||||
| 19.4 |
Notices |
62 | ||||
| 19.5 |
Applicable Law |
63 | ||||
| 19.6 |
Dispute Resolution |
63 | ||||
| 19.7 |
Entire Agreement |
63 | ||||
| 19.8 |
Headings |
64 | ||||
| 19.9 |
Independent Contractors |
64 | ||||
| 19.10 |
Agreement Not to Solicit Employees |
64 | ||||
| 19.11 |
Waiver |
64 | ||||
| 19.12 |
Counterparts |
64 | ||||
| 19.13 |
Definition and Effect of Change of Control |
64 | ||||
(vii)
APPENDICES
| Appendix A: |
CDK Patent Rights and Certain Mitotix License Agreements | |
| Appendix B: |
CDK Work Plan | |
| Appendix C: |
CDK Primary and Secondary Screens | |
| Appendix D: |
UBC Patent Rights and Certain Mitotix License Agreements | |
| Appendix E: |
Mitotix Pending License Agreements | |
| Appendix F: |
UBC Work Plan | |
| Appendix G1: |
Co-promotion of CDK Products and Co-promotion of UBC Products when the Alternate UBC Plan has been put into Effect | |
| Appendix G2: |
Co-promotion of UBC Products when the Alternate UBC Plan is not in Effect | |
| Appendix H: |
UBC Products - Definition of Percentage Contribution | |
| Appendix I: |
Definition of UBC Operating Profit | |
| Appendix J: |
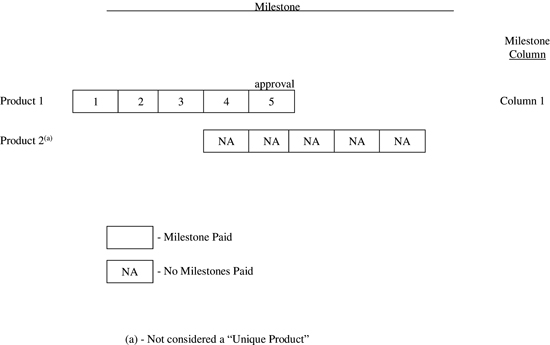
Examples of Milestone Payment Obligations | |
(viii)
AMENDED AND RESTATED
COLLABORATIVE RESEARCH, DEVELOPMENT
AND MARKETING AGREEMENT
THIS AMENDED AND RESTATED COLLABORATIVE RESEARCH, DEVELOPMENT AND MARKETING AGREEMENT dated as of June 2, 1997 (the “Agreement”) is made between MITOTIX, INC., a Delaware corporation having its principal place of business at ▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ (“Mitotix”), and THE DUPONT MERCK PHARMACEUTICAL COMPANY, a Delaware general partnership having its principal place of business at ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇ (“DuPont Merck”).
R E C I T A L S
Mitotix and DuPont Merck are parties to a Collaborative Research, Development and Marketing Agreement, dated as of December 6, 1995 (the “Initial Agreement”), pursuant to which Mitotix and DuPont Merck agreed, inter alia, (i) to utilize certain biological targets in order to identify and develop small molecules that can be used as active agents in therapeutic products for the treatment of diseases resulting from inappropriate cell division, proliferation, or longevity; and (ii) to collaborate on research with respect to such biological targets and on the discovery, worldwide development, and commercialization of CDK Products and UBC Products (both as defined below) for therapeutic purposes.
Mitotix and DuPont Merck desire to amend and restate the Initial Agreement to extend the Initial UBC Term (as defined below) and to make certain other changes as provided herein.
NOW THEREFORE, in consideration of the foregoing and of the covenants herein contained, the parties hereto mutually agree to amend and restate the Initial Agreement as follows:
ARTICLE 1. DEFINITIONS
The terms in this Agreement with initial letters capitalized, whether used in the singular or the plural, shall have meaning set forth below or, if not listed below, the meaning as designated in places throughout this Agreement.
1.1 “Affiliate” shall mean any corporation or other entity which controls, is controlled by, or is under common control with a party to this Agreement. A corporation or other entity shall be regarded as in control of another corporation or entity if it owns or directly or indirectly controls more than fifty percent (50%) of the voting stock or other ownership interest of the other corporation or entity, or if it possesses, directly or indirectly,
the power to direct or cause the direction of the management and policies of the corporation or other entity or the power to elect or appoint more than fifty percent (50%) of the members of the governing body of the corporation or other entity. For purposes of this Agreement, DuPont Merck shall not be deemed to be an Affiliate of ▇.▇. ▇▇▇▇▇▇ de Nemours & Co. or Merck & Co., Inc. or any of their subsidiaries.
1.2 “Allowable Expense” shall have the meaning set forth in Appendix I.
1.3 “Alternate UBC Plan” shall mean the research, development and marketing plan for the UBC Products that is described in Section 5.4.4.
1.4 “Antisense” shall mean inhibiting or preventing in vivo expression of a gene product in a human or animal through the use of an oligonucleotide or modified oligonucleotide which binds to RNA or DNA.
1.5 “Annual CDK Research Plan” shall have the meaning set forth in Section 3.1.2 below.
1.6 “Annual Research Plan and Budget” shall have the meaning set forth in Section 6.1.2 below.
1.7 “Calendar Quarter” shall mean the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31.
1.8 “Calendar Year” shall mean each successive period of twelve (12) months commencing on January 1 and ending on December 31.
1.9 “Cdc 27/Cdc 16 License” shall mean the license agreement dated as of July 12, 1995 between Mitotix and Harvard University.
1.10 “CDK Collaboration” shall have the meaning set forth in Section 2 below.
1.11 “CDK Research Operating Committee” shall mean the joint committee composed of representatives of Mitotix and DuPont Merck described in Section 9.2 of this Agreement.
1.12 “CDK Development Compounds” shall mean, collectively, the CDK-D Development Compounds and the CDK-non-D Development Compounds.
1.13 “CDK-D Development Compounds” shall mean compounds selected by DuPont Merck through the use of the CDK-D Targets for clinical development in the CDKD Field.
1.14 “CDK-non-D Development Compounds” shall mean compounds selected by DuPont Merck through the use of the CDK-non-D Targets for clinical development in the CDK-non-D Field.
2
1.15 “CDK Field” shall mean, collectively, the CDK-D Field and the CDK-non-D Field.
1.16 “CDK-D Field” shall mean the use of CDK-D Targets for the discovery, identification and development of CDK-D Development Compounds for all therapeutic indications and the use, manufacture, distribution, marketing and sale for all indications of therapeutic and Radiopharmaceutical agents incorporating CDK-D Development Compounds. The use of the CDK-D Targets for Antisense, Gene Therapy and all non-Radiopharmaceutical diagnostic applications is specifically excluded from the CDK-D Field.
1.17 “CDK-non-D Field” shall mean the use of CDK-non-D Targets for the discovery, identification and development of CDK-non-D Development Compounds for oncology indications and the use, manufacture, distribution, marketing and sale for oncology indications of therapeutic and Radiopharmaceutical agents incorporating CDK-non-D Development Compounds. The use of CDK-non-D Targets for Antisense, Gene Therapy and all non-Radiopharmaceutical diagnostic applications is specifically excluded from the CDK-non-D Field.
1.18 “CDK Patent Rights” shall mean those United States patents and patent applications and the international patent applications owned or licensed by Mitotix which may be useful in the CDK Field and which are identified in Appendix A and any division, continuation, continuation-in-part thereof, any foreign patent applications corresponding to any such applications or any United States or foreign patents or the equivalent thereof issuing thereon or any reissue or extension thereof. CDK Patent Rights shall also include those United States patents and patent applications and the international patent applications which may be useful in the CDK Field for which Mitotix may acquire, from a Third Party during the term of the CDK Research Program, license rights and the right to grant sublicenses (including rights acquired pursuant to the Mitotix Pending License Agreements), and any division, continuation, continuation-in-part thereof, any foreign patent applications corresponding to any such applications or any United States or foreign patents or the equivalent thereof issuing thereon or any reissue or extension thereof.
1.19 “CDK Products” shall mean pharmaceutical and Radiopharmaceutical compositions incorporating CDK-D Development Compounds or CDK-non-D Development Compounds whether such compounds are selected for development, developed, marketed or sold during the term of the CDK Research Program or thereafter.
1.20 “CDK Research Program” shall mean the research program described generally in Article 3 below and in the research work plan set forth in Appendix B hereto, as revised from time to time as provided in this Agreement.
1.21 “CDK Targets” shall mean collectively Targets included within the CDK-D Targets and the CDK-non-D Targets. CDK Targets shall specifically exclude cdc25 phosphatase and complexes thereof and all Targets included within the UBC Targets.
1.22 “CDK-D Targets” shall mean the following Targets: all cyclin Ds, associated cyclin dependent kinases, complexes thereof, and functional equivalents (all homologues of
3
cyclin Ds and associated cyclin dependent kinases) thereof; physically direct biochemical modulators of cyclin Ds or homologues thereof and of associated cyclin dependent kinases; and any targets of modulation of p16, functional homologues of p16, or other natural cell cycle modulators of cyclin Ds, their functional homologues, associated cyclin dependent kinases or complexes thereof.
1.23 “CDK-non-D Targets” shall mean all non-D cyclins, associated cyclin dependent kinases, complexes thereof, and functional equivalents (all non-D cyclin homologues and associated cyclin dependent kinases) thereof; physically direct biochemical modulators of non-D cyclins or homologues thereof and of associated cyclin dependent kinases; and any targets of modulation of natural cell cycle modulators of non-D cyclins, their functional homologues, associated cyclin dependent kinases or complexes thereof.
1.24 “Collaboration” shall have the meaning set forth in Section 2 below.
1.25 “Collaborative Policy Setting Committee” shall mean the joint committee composed of representatives of Mitotix and DuPont Merck described in Section 9.1 of this Agreement.
1.26 “Cyclin E License” shall mean the license agreement dated as of September 5, 1995 between Mitotix and ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ Cancer Research Center.
1.27 “Cyclin D License” shall mean the license agreement dated as of October 22, 1992 between Mitotix and Cold Spring Harbor Laboratory.
1.28 “Competitive Product” means a product directed at the same Target and having the same mechanism of action as a Royalty-Bearing Product or a Mitotix Product and which together with all other such products sold in a particular country, achieves sales in any Calendar Quarter equal to *** of the total gross sales or gross unit sales in such quarter of the Royalty-Bearing Product or Mitotix Product, as the case may be.
1.29 “Development Partner” shall mean a Third Party that is either (i) licensed by DuPont Merck to conduct all or a significant portion of the clinical development with respect to a particular Royalty-Bearing Product and to market and sell such Royalty Bearing-Product in a non-Strategic Country or (ii) licensed by Mitotix to conduct all or a significant portion of the clinical development with respect to a particular Mitotix Product and to market and sell such Mitotix Product in a non-Strategic Country.
1.30 “Distributor” shall mean a Third Party that is either (i) engaged by DuPont Merck to market and distribute Royalty-Bearing Products or (ii) engaged by Mitotix to market and distribute Mitotix Products.
1.31 “DuPont Merck Inventions” shall have the meaning set forth in Section 14.1 below.
1.32 “Effective Date” shall mean December 6, 1995.
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
4
1.33 “Extended UBC Collaboration” shall have the meaning set forth in Section 5.2 below.
1.34 “Extension Notice” shall have the meaning set forth in Section 5.2.2 below.
1.35 “First Commercial Sale” shall mean the first sale for use or consumption by the general public of a product in a country after required marketing and pricing approval has been granted by the governing health authority of such country.
1.36 “Gene Therapy” shall mean the introduction of a gene, together with associated regulatory elements, if any, into human cells (whether in vivo or ex vivo) in order to treat or prevent disease through the endogenous expression of the gene product in humans. Gene shall mean a DNA or RNA sequence of human or other origin that encodes a protein or other molecule.
1.37 “Immunoassay Product” shall mean an assay which contains a Development Compound or a metabolite derived therefrom as a standard reference and is sold by DuPont Merck, its Affiliates, Development Partners, Distributors or sublicensees in connection with a particular CDK Product or UBC Product for use in monitoring the level of the CDK Product or UBC Product, as the case may be, administered to a patient. All Immunoassay Products shall be Royalty Bearing Products.
1.38 “IND” shall mean an investigational new drug application filed with the United States Food and Drug Administration prior to beginning clinical trials in humans.
1.39 “Initial UBC Term” shall have the meaning set forth in Section 5.2 below.
1.40 “Inventions” shall have the meaning set forth in Section 14.1 below.
1.41 “IPO” shall have the meaning set forth in Section 5.9.2 below.
1.42 “Know-how” shall mean all confidential technical information in the possession of Mitotix or DuPont Merck during the term of this Agreement relating to the CDK Targets, the UBC Targets or relating to the discovery, development, manufacture, marketing and sale of the CDK Products or the UBC Products.
1.43 “Mitotix Inventions” shall have the meaning set forth in Section 14.1 below.
1.44 “Mitotix Pending License Agreements” shall mean those agreements under negotiation between Mitotix and a Third Party as of the Effective Date under which Mitotix will license patent rights or know-how that may be useful in the CDK Field or the UBC Field. The Mitotix Pending License Agreements are identified in Appendix E. Upon execution, such Mitotix Pending License Agreements shall be deemed to be Mitotix License Agreements.
1.45 “Mitotix License Agreements” shall mean those license agreements entered into by Mitotix and a Third Party on or prior to the Effective Date under which Mitotix has
5
exclusively licensed patent rights or know-now that may be useful in the CDK Field or the UBC Field. The Mitotix License Agreements are listed in Appendix A and Appendix D.
1.46 “Mitotix Product” shall mean (i) any Antisense or Gene Therapy product sold by Mitotix for oncology indications incorporating a compound that was discovered or selected for development by Mitotix through the use of the CDK Targets or the UBC Targets and was first identified within *** years following the termination of the CDK Research Program (with respect to the use of the CDK Targets) or within *** years following the termination of the UBC Research Program (with respect to the use of the UBC Targets), and (ii) any products meeting the description of Mitotix Products set forth in Section 5.6, Section 5.8, Section 6.2 3(a)(i) or in Section 10.1.5.
1.47 “Net Sales” with respect to any product (UBC Product, Royalty-Bearing Product or Mitotix Product) shall mean the gross invoiced sales price of such product sold to independent Third Party customers, including but not limited to Distributors, in bona fide, arms-length transactions, less actual (to the extent not already deducted in the amount invoiced):
(a) quantity, cash, or other trade discounts actually accrued or taken;
(b) bad debt expense;
(c) custom duties, surcharges and taxes and other governmental charges incurred, if any, directly related to the sale;
(e) amounts repaid or credited by reason of rejections, return of goods, or retroactive price reductions;
(f) amounts incurred resulting from governmental, or an agency thereof, mandated rebate programs;
(g) third party rebates and chargebacks actually accrued or allowed;
(h) freight and insurance costs incurred in transporting such product to such customers; and
(i) as agreed by the parties, any other specifically identifiable amounts included in gross sales that were or ultimately will be credited and that are substantially similar to those listed herein above.
The amount of Net Sales for any period shall be determined on the basis of sales recorded in such period in accordance with generally accepted accounting principles. The transfer of any product by DuPont Merck or Mitotix, or one of their Affiliates, to another Affiliate of such party shall not be considered a sale; in such cases, Net Sales shall be determined based on the invoiced sales price by the Affiliate to an independent Third Party customer, less the deductions allowed under this Section.
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
6
In the case of a Combination Product (said Combination Product being a pharmaceutical or Radiopharmaceutical product which contains one or more pharmacologically active ingredients in addition to the Royalty-Bearing Product, UBC Product, or Mitotix Product as the case may be) only, Net Sales shall be calculated on the basis of the invoice price for the product (Royalty-Bearing Product, UBC Product, or Mitotix Product as the case may be) containing the same weight of product and sold alone without other active ingredients. In the event that the product is not sold alone without other active ingredients, then Net Sales shall be the Net Sales of the Combination Product, determined as set forth above, multiplied by the fraction A/(A+B) where A is the seller’s cost of acquiring or manufacturing the product (Royalty-Bearing Product, UBC Product, or Mitotix Product as the case may be) and B is the seller’s cost of acquiring or manufacturing the other active ingredient(s) in the Combination Product, determined in accordance with generally accepted accounting principles.
1.48 “New UBC Target” shall mean a UBC Target that was not identified by Mitotix, DuPont Merck or a Third Party prior to the Effective Date and for which a novel screen and selectivity assays have been developed and validated for a specific therapeutic program as part of the UBC Collaboration. A Target shall not be a New UBC Target until approved by the Collaborative Policy Setting Committee as a New UBC Target as described in Section 5.8.
1.49 “NDA” shall mean a new drug application filed with the United States Food and Drug Administration after completion of human clinical trials to obtain marketing approval for a Royalty-Bearing Product, or the corresponding application for authorization for marketing for a Royalty-Bearing Product filed in any other country in accordance with the applicable laws and regulations of that country.
1.50 “Percentage Contribution” shall have the meaning set forth in Section 5.4.1 and Appendix H.
1.51 “p16 License” shall mean the license agreement dated as of July 1, 1995 between Mitotix and Cold Spring Harbor Laboratory.
1.52 “PRAD1 License” shall mean the license agreement dated as of August 25, 1995 between Mitotix and the General Hospital Corporation.
1.53 “Product Patent Rights” shall have the meaning set forth in Section 14.10.
1.54 “Radiopharmaceutical” shall mean any use for human in vivo medical imaging purposes.
1.55 “Radiopharmaceutical Product” shall mean any CDK Product or UBC Product used for human in vivo medical imaging purposes.
1.56 “Research Operating Committees” shall mean collectively the CDK Research Operating Committee and the UBC Research Operating Committee.
7
1.57 “Research Year” shall mean each twelve-month period during the Collaboration, with the first Research Year beginning on January 1, 1996.
1.58 “Royalty-Bearing Products” shall mean (i) all CDK Products, (ii) all Radiopharmaceutical Products that are UBC Products, (iii) effective upon the initiation of the Alternate UBC Plan, all UBC Products; (iv) effective upon the deemed commencement of the Alternate UBC Plan for financial purposes following the commencement of the UBC Operational Disengagement Plan pursuant to Section 5.7, all UBC Products and (v) all Immunoassay Products; provided, however, that Royalty Bearing Products shall not include: (i) CDK Products incorporating compounds that are first identified more than *** years following the earlier of (X) the termination of the license grant set forth in Section 10.1.1 pursuant to Section 10.1.3 or (Y) the termination of the Agreement; and (ii) UBC Products incorporating compounds that are first identified more than *** years following the earlier of (X) the termination of the license grant set forth in Section 10.2.1 pursuant to Section 10.2.3 or (Y) the termination of this Agreement. In addition, any product meeting the description of a Royalty Bearing Product set forth in Section 6.2.3(a)(ii) shall be a Royalty Bearing Product.
1.59 “Royalty Term” shall mean, with respect to each product in each country, the period of time equal to the longer of (a) ten (10) years from the date of the First Commercial Sale of such product in such country or (b) if the manufacture, use or sale of such product in such country is covered by a Valid Patent Claim owned by or exclusively licensed to the party responsible for the relevant royalty payment, the term for which such Valid Patent Claim or any new Valid Patent Claim remains in effect.
1.60 “Strategic Countries” shall mean the United States of America, Canada, France, Germany, Italy, the United Kingdom and Spain.
1.61 “Target” shall mean a specific, identified biomolecule, including a protein, polynucleotide, carbohydrate, lipid, or any combination thereof.
1.62 “Third Party” shall mean any person or entity other than Mitotix or DuPont Merck and their respective Affiliates.
1.63 “UBC Collaboration” shall have the meaning set forth in Articles 2 and 5.
1.64 “UBC Deferral Payment” shall have the meaning set forth in Section 5.2.3.
1.65 “UBC Development Compounds” shall mean compounds selected by the Collaborative Policy Setting Committee through the use of UBC Targets for clinical development in the UBC Field, unless the Alternate UBC Plan is in effect, in which case “UBC Development Compounds” shall mean compounds selected by DuPont Merck through the use of UBC Targets for clinical development in the UBC Field.
1.66 “UBC Extension Payment” shall have the meaning set forth in Section 5.2.3.
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
8
1.67 “UBC Field” shall mean use of the UBC Targets for the discovery, identification and development of UBC Development Compounds and the use, manufacture, distribution, marketing and sale for all indications of therapeutic and Radiopharmaceutical agents incorporating UBC Development Compounds. The use of the UBC Targets for Antisense, Gene Therapy and all non-Radiopharmaceutical diagnostic applications is specifically excluded from the UBC Field.
1.68 “UBC Operational Disengagement Plan” shall have the meaning set forth in Section 5.7.
1.69 “UBC Operating Profit” shall have the meaning set forth in Appendix I hereof.
1.70 “UBC Patent Rights” shall mean those United States patents and patent applications and the international patent applications owned or licensed by Mitotix which may be useful in the UBC Field and which are identified in Appendix D and any division, continuation, continuation-in-part thereof, any foreign patent applications corresponding to any such applications or any United States or foreign patents or the equivalent thereof issuing thereon or any reissue or extension thereof. UBC Patent Rights shall also include those United States patents and patent applications and the international patent applications which may be useful in the UBC Field for which Mitotix may acquire, from a Third Party during the term of the UBC Research Program, license rights and the right to grant sublicenses, and any division, continuation, continuation-in-part thereof, any foreign patent applications corresponding to any such applications or any United States or foreign patents or the equivalent thereof issuing thereon or any reissue or extension thereof.
1.71 “UBC Pricing Date” shall have the meaning set forth in Section 5.9.1.
1.72 “UBC Products” shall mean pharmaceutical and Radiopharmaceutical compositions incorporating UBC Development Compounds whether such compounds are selected for development, developed, marketed or sold during the term of the UBC Research Program or thereafter.
1.73 “UBC Research, Development and Pre-Launch Marketing Costs” shall have the meaning set forth in Appendix H.
1.74 “UBC Research Operating Committee” shall mean the joint committee composed of representatives of Mitotix and DuPont Merck described in Section 9.2 of this Agreement.
1.75 “UBC Research Program” shall mean the research program described generally in Section 6.1 and in the research workplan set forth in Appendix F hereto, as revised from time to time as provided in this Agreement.
1.76 “UBC Targets” shall mean the following Targets: (i) all ubiquitin-mediated proteolytic pathways, including enzymes and ligands involved in the ubiquitin-mediated degradation of proteins and (ii) all HPV-mediated ubiquitinylation, ▇▇, ▇▇▇▇, binding ligands thereof, associated complexes thereof, and direct biochemical modulators thereof. In
9
the event that cdc25 proves to be a candidate for a New UBC Target as set forth in Section 1.44, it will be presented to the Collaborative Policy Setting Committee as such for evaluation as described in Section 5.8.
1.77 “UBC9 License” shall mean the license agreement dated as of March 8, 1995 between Mitotix and Harvard University.
1.78 “Unique Product” shall have the meaning set forth in Section 11.1.1.
1.79 “Valid Patent Claim” shall mean a claim of an issued and unexpired patent which has not been revoked or held unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, and which has not been admitted to be invalid or unenforceable through reissue or disclaimer or otherwise.
ARTICLE 2. SCOPE AND STRUCTURE OF THE COLLABORATIONS
Mitotix and DuPont Merck wish to establish two collaborative alliances both with the objective and intent of discovering, developing and marketing compounds that therapeutically or for Radiopharmaceutical use affect or bind to the defined Targets within the defined research and development fields. One collaboration will focus on research and development with respect to the CDK Targets (the “CDK Collaboration”) and the other will focus on research and development with respect to the UBC Targets (the “UBC Collaboration”). During the course of the collaborations, Mitotix and DuPont Merck will communicate regularly and will assume different rights and responsibilities for the discovery, development and commercialization of CDK Products and UBC Products, based on the phase of development and commercialization and the territory involved, all as more specifically described below and in the Appendices hereto. Throughout this Agreement the CDK Collaboration and the UBC Collaboration shall be referred to collectively as “the Collaboration.”
ARTICLE 3. CDK COLLABORATION-CDK RESEARCH PROGRAM
3.1 Conduct of the CDK Research Program.
3.1.1 General Terms. The CDK Research Program will be conducted in good scientific manner, and in compliance with all applicable good laboratory practices, and applicable legal requirements, to attempt to achieve efficiently and expeditiously the objectives described in the work plan set forth in Appendix B hereto. Throughout the CDK Research Program, including any extensions thereof as provided in Section 3.3, Mitotix shall assign eight full-time equivalent qualified scientists to perform the work set forth in each Annual CDK Research Plan (as defined below). The name, curriculum vitae, and percentage of time devoted to working on the CDK Research Program for each scientist comprising such eight full-time equivalent scientists shall be provided to DuPont Merck within thirty (30) days of the Effective Date and not later than sixty (60) days prior to the start of each subsequent
10
Research Year. The mixture of skills and levels of expertise represented by such scientists shall be appropriate to the scientific objectives of the CDK Research Program at any point in time and shall not, without the consent of DuPont Merck, differ substantially from the mixture of skills and levels of expertise among the full-time equivalent scientists assigned to Mitotix proprietary research projects having similar objectives. The names of such scientists and the percentage of time to be devoted by each to working on the CDK Research Program shall be identified in each Annual CDK Research Plan. The selection of such scientists shall be subject to DuPont Merck approval, such approval not to be unreasonably withheld. Mitotix and DuPont Merck shall proceed diligently with the work set out in each Annual CDK Research Plan by using their respective good faith efforts considering, in the case of Mitotix, the funding received from DuPont Merck hereunder.
3.1.2 Annual Research Plan. The CDK Research Program will be conducted under an annual research plan which describes the work to be pursued by Mitotix and DuPont Merck during the Research Year (the “Annual CDK Research Plan”). The first Annual CDK Research Plan shall be prepared by the CDK Research Operating Committee and approved by DuPont Merck with the concurrence of the Collaborative Policy Setting Committee within forty-five (45) days after the Effective Date. Subsequent Annual CDK Research Plans shall be prepared by the CDK Research Operating Committee, for submission to, and approval by, DuPont Merck with the concurrence of the Collaborative Policy Setting Committee, not later than sixty (60) days prior to the start of each Research Year.
3.1.3 Transfer of Technical Information and Know-How. Within three (3) months after the Effective Date, Mitotix shall provide DuPont Merck with technical information, Know-how, and upon reasonable request by DuPont Merck, materials that are not otherwise available, necessary to enable DuPont Merck to utilize the primary and secondary screens for CDK Targets that have been validated by Mitotix as of such date; such primary and secondary screens are identified in Appendix C attached hereto. As additional primary and secondary screens for the CDK Targets become available and are validated, Mitotix shall promptly provide DuPont Merck with additional technical information, Know-how, and upon reasonable request by DuPont Merck, materials that are not otherwise available, to enable DuPont Merck to utilize such additional primary and secondary screens. All such technical information and Know-how provided by Mitotix under this Section shall be treated as “Information” under Article 15.
3.1.4 DuPont Merck Compounds. The Collaborative Policy Setting Committee shall determine which party shall be responsible for conducting the primary screening of compounds. Following primary screening, compounds which meet the specifications set forth by the CDK Research Operating Committee for suitable candidates for continued preclinical development shall be provided to Mitotix. Mitotix shall conduct secondary screening of such compounds and conduct additional preclinical work with respect to such compounds, all as set forth in the applicable Annual CDK Research Plan. Mitotix will promptly provide to DuPont Merck all data from such screening and preclinical work in accordance with Section 3.1.7 and as otherwise reasonably requested by DuPont Merck in support of the development of CDK Development Compounds. Such compounds provided by DuPont Merck will be used by Mitotix solely for screening and preclinical work within the CDK Field, as approved by DuPont Merck, and for no other purpose. Any know-how
11
incidentally developed at Mitotix outside of the CDK Field using a DuPont Merck compound shall be promptly disclosed to DuPont Merck. Any know-how and patent rights developed by Mitotix using a DuPont Merck compound provided hereunder shall be considered Know-how and CDK Patent Rights, respectively. DuPont Merck shall at all times own all rights to the compounds it provides to Mitotix hereunder, including without limitation, all rights with respect to the manufacture, use, or sale of such compounds. In accordance with the foregoing, Mitotix agrees to (i) disclose to DuPont Merck any inventions it conceives or makes with respect to the manufacture, use or sale of compounds provided by DuPont Merck to Mitotix hereunder; (ii) assign to DuPont Merck all patent rights with respect to such inventions and (iii) execute any necessary papers and otherwise reasonably cooperate with DuPont Merck in securing patents on such inventions. Upon expiration or termination of the CDK Research Program, Mitotix shall, upon the request of DuPont Merck, return or destroy all compounds provided to Mitotix by DuPont Merck in the performance of the CDK Research Program.
3.1.5 Subcontracts. Subject to the provisions of Article 15 and, if applicable, the p16 License, and as set forth in the Annual CDK Research Plan and Budget or otherwise approved by the Collaborative Policy Setting Committee, Mitotix and DuPont Merck may subcontract portions of the CDK Research Program to be performed by them in the normal course of their business to a Third Party upon prior written notice to the other; provided, however, that such Third Party has entered into an appropriate confidentiality agreement with Mitotix and/or DuPont Merck obligating such Third Party to be bound by the confidentiality obligations contained in this Agreement, or such subcontracting would not require the transfer of confidential information to the Third Party.
3.1.6 Data. Mitotix and DuPont Merck shall each maintain records in sufficient detail and in good scientific manner appropriate for patent purposes and as will properly reflect all work done and results achieved in the performance of the CDK Research Program (including all data in the form required to be maintained under any applicable governmental regulations). Such records shall include books, records, reports, research notes, charts, graphs, comments, computations, analyses, recordings, photographs, computer programs and documentation thereof, computer information storage means, samples of materials and other graphic or written data generated in connection with the CDK Research Program. Mitotix and DuPont Merck shall each provide the other the right to inspect such records, and shall provide copies of all requested records, to the extent reasonably required for the performance of the requesting party’s obligations under this Agreement; provided, however, that each party shall maintain such records and the information of the other contained therein in confidence in accordance with Article 15 below and shall not use such, records or information except to the extent otherwise permitted by this Agreement.
3.1.7 Quarterly Reports bv Mitotix and DuPont Merck. Within thirty (30) days following the end of each Calendar Quarter, Mitotix and DuPont Merck shall each provide to the members of the CDK Research Operating Committee a written report summarizing in reasonable detail the preclinical work performed by it under the CDK Research Program during the preceding Calendar Quarter.
12
3.2 Funding of the CDK Research Program.
3.2.1 Annual Payment.
(a) In consideration of Mitotix’s performance of its obligations under the CDK Research Program, DuPont Merck shall pay Mitotix a research fee at the rate of *** Dollars *** per Research Year during the term of the CDK Research Program, including any extension thereof pursuant to Section 3.3; provided, however, that no research fee shall be due to Mitotix from DuPont Merck during the six month period from January 1, 2000 through June 30, 2000. The parties acknowledge that such funding is intended to cover the cost of the eight full-time equivalent scientists that Mitotix has committed to the CDK Research Program at the rate of *** Dollars *** per scientist. The research fee for each Research Year shall be paid in equal quarterly payments throughout the Research Year (or any portion thereof), with the first payment of ***Dollars ***to be paid to Mitotix on the Effective Date. Such research funding by DuPont Merck in support of the CDK Research Program shall be contingent upon Mitotix providing and retaining the eight qualified full-time equivalent scientists described in Section 3.1.1 and Mitotix making a good faith effort in accordance with industry standards to achieve the goals of the CDK Collaboration as set forth annually in the Annual CDK Research Plan.
(b) Without limiting DuPont Merck’s rights or remedies, DuPont Merck shall have the right to reduce the level of funding described above to reflect the failure by Mitotix to provide or retain the eight qualified full-time equivalent scientists or to make a good faith effort in accordance with industry standards to achieve the goals of the CDK Collaboration. DuPont Merck may exercise such right to reduce funding upon six (6) months’ prior written notice to Mitotix of its intention to do so, stating the reasons why DuPont Merck believes Mitotix has either failed to provide or retain the required scientists or to make the required effort, provided that Mitotix has not taken good faith commercially reasonable steps during such six (6) month period to diligently correct such deficiencies. If such funding is discontinued completely as a result of this Section 3.2.l(b), then the CDK Research Program shall be deemed to have terminated and the provisions of Section 3.3 shall apply.
(c) In the event of a Change of Control (defined in Section 19.13) and the failure of Mitotix (or its successor) to adhere to the provisions of Section 19.13, as reasonably determined by DuPont Merck, DuPont Merck may elect to discontinue payment of the research funding to Mitotix upon three (3) months prior written notice to Mitotix. Upon such discontinuation of funding, Mitotix shall no longer be obligated to provide research support for the CDK Research Program, and the CDK Research Program shall be deemed to have terminated.
(d) 3.2.2 Incentive Research Funding. In the event safety assessment studies with respect to a CDK Development Compound are completed with a result that human clinical trials for such CDK Development Compound commence on or before December 31, 1997, then DuPont Merck shall pay Mitotix *** upon the commencement of such human clinical trials, in support of the CDK Research Program. DuPont Merck shall use
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
13
commercially reasonable efforts to commence such human clinical trials on or before such date.
3.3 Expiration or Termination of the CDK Research Program and Result of Such Termination. Unless sooner terminated pursuant to Section 3.2.l(b) or Section 3.2.l(c) above, the term of the CDK Research Program shall commence on January 1, 1996 and shall continue through June 30, 2000. DuPont Merck shall have the option to extend the CDK Research Program for up to three (3) additional consecutive one-year periods. In order to exercise its option to extend the CDK Research Program, DuPont Merck shall provide Mitotix with written notice of the extension of the CDK Research Program at least six (6) months prior to the commencement of each such additional one-year period. Upon the expiration or termination of the CDK Research Program the terms of Sections 10.1.2 and 10.1.3 shall apply.
ARTICLE 4. CDK COLLABORATION: CDK DEVELOPMENT PROGRAM
4.1 Designation of CDK Development Compounds. Based upon the preclinical work conducted by Mitotix and DuPont Merck, DuPont Merck shall determine, with collaborative input from Mitotix through the Collaborative Policy Setting Committee, whether to designate a compound as a CDK Development Compound. DuPont Merck shall provide Mitotix prompt written notice of its decision. DuPont Merck will be responsible for directing the preclinical and clinical development of compounds in the CDK Field in the CDK Collaboration.
4.2 Clinical Development and Marketing. The clinical development of a CDK Development Compound starting with the commencement of human clinical trials for such CDK Development Compound and continuing through obtaining regulatory approvals of any resulting CDK Product shall be the sole responsibility of DuPont Merck and shall be conducted at the sole expense of DuPont Merck, with input from Mitotix through the Collaborative Policy Setting Committee. Subject to Mitotix’s co-promotion option set forth in Article 8, DuPont Merck shall have sole responsibility for and shall bear all expenses associated with the marketing and sale of all CDK Products. For each CDK Development Compound, DuPont Merck shall use its good faith commercially reasonable efforts to evaluate and develop such compound or CDK Product in a timely manner in accordance with industry standards and DuPont Merck internal practices and shall advise the Collaborative Policy Setting Committee as to the status of the development of each CDK Development Compound. Consistent with industry standards and DuPont Merck internal practices, DuPont Merck shall use its good faith commercially reasonable efforts to conduct at its own expense, such human clinical trials as are necessary to obtain all regulatory approvals to manufacture and market CDK Products and to seek and obtain all necessary regulatory approvals to market and promptly commence worldwide marketing of each CDK Product.
4.3 Development Information. DuPont Merck shall keep Mitotix informed as to the progress of DuPont Merck in the development and testing of all CDK Development Compounds and CDK Products and the preparing, filing and obtaining of the approvals necessary for marketing. Within thirty (30) days following the end of each Calendar Quarter
14
following the designation of a CDK Development Compound, DuPont Merck shall provide to Mitotix a reasonably detailed report which shall describe the progress of the development and testing of the CDK Products incorporating such CDK Development Compounds. DuPont Merck may satisfy its reporting obligation hereunder by providing an oral report to the Collaborative Policy Setting Committee within such thirty (30) day period, provided that a written report of the oral presentation is delivered to Mitotix no later than thirty (30) days after the oral report is made to the Collaborative Policy Setting Committee. In addition, DuPont Merck shall provide Mitotix with a minimum of three (3) months’ advance notice of the contemplated filing of an IND or an NDA. DuPont Merck shall provide Mitotix with a report on all material regulatory submissions at least forty-five (45) days prior to the date of such submissions, or any major amendments or supplements thereto within ten (10) business days of filing thereof. DuPont Merck shall provide to Mitotix in a timely manner copies of any material regulatory submission, or amendments thereof, reasonably requested by Mitotix or, alternatively shall provide Mitotix reasonable access to each such material regulatory submission or amendments thereof at DuPont Merck’s offices, for review and copying by Mitotix.
ARTICLE 5. GENERAL DESCRIPTION OF THE UBC COLLABORATION
5.1 Scope of UBC Collaboration. In the performance of the UBC Collaboration hereunder, DuPont Merck and Mitotix will collaborate in research on the UBC Targets and on the discovery and development of therapeutic agents which act on such UBC Targets, as set forth in this Agreement. The goal of the UBC Collaboration is to discover and develop products efficiently while allowing Mitotix to grow development capabilities in the United States in accordance with the specific terms of this Agreement. It will be the responsibility of the Collaborative Policy Setting Committee to ensure that the division of research and development efforts are consistent with this goal. The financial goal of the UBC Collaboration is for each party to share in the UBC Operating Profit as outlined in Section 11.5 and Appendix I in proportion to the contribution each party has made to the UBC Research, Development and Pre-Launch Marketing Costs of such UBC Products, as set forth in this Agreement and described in Appendix H.
5.2 Initial Term and Option to Extend.
5.2.1 Initial Term. The UBC Collaboration shall commence on January 1, 1996 and continue in effect through April 30, 2000 (the “Initial UBC Term”).
5.2.2 Option to Extend; UBC Extension Payment. DuPont Merck shall have the option to extend the term of the UBC Collaboration by (i) providing Mitotix written notice of its intention to do so (the “Extension Notice”) and (ii) making a *** Dollar *** equity investment in Mitotix prior to the expiration of the Initial UBC Term, payable as set forth in Section 5.2.3 and upon the terms set forth in Section 5.9. If Mitotix receives the Extension Notice and the *** Dollar *** equity investment by DuPont Merck prior to the termination of the Initial UBC Term, the UBC Collaboration shall continue as the “Extended UBC Collaboration.”
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
15
5.2.3 UBC Deferral Payment. The Initial Agreement provided that the Initial UBC Term would continue in effect through April 30, 1998. In consideration of Mitotix’s agreement to extend the Initial UBC Term by two years, DuPont Merck shall pay Mitotix an aggregate of *** Dollars *** (the “UBC Deferral Payment”). The UBC Deferral Payment shall be paid in nine equal quarterly installments, with the first payment of *** Dollars *** to be received by Mitotix within ten (10) business days of January 1, 1998; provided, however, that DuPont Merck’s obligation to pay each installment shall be contingent on Mitotix providing and retaining the ten qualified full-time equivalent scientists described in Section 6.1.1 and Mitotix making a good faith effort in accordance with industry standards to achieve the goals of the UBC Collaboration; and provided, further, that if Mitotix has reduced the number of qualified scientists devoted to working on the UBC Research Program at the written request of DuPont Merck, then DuPont Merck shall be required nonetheless to make each installment of the UBC Deferral Payment. Upon DuPont Merck’s election to extend the UBC Collaboration as set forth in Section 5.2.2, all installments of the UBC Deferral Payment received prior to the date of such extension shall be applied toward the *** Dollar *** equity investment in Mitotix described in Section 5.2.2 and the balance of such equity investment (the “UBC Extension Payment”) shall be paid by wire transfer to an account designated by Mitotix. If DuPont Merck elects not to extend the UBC Collaboration or fails to pay the balance of the equity investment, any prior installments of the UBC Deferral Payment shall be deemed to be research funding and shall not result in the issuance of equity to DuPont Merck. The UBC Deferral Payment shall not be included in calculating the amount of UBC Research, Development and Pre-Launch Marketing Costs funded by DuPont Merck pursuant to Section 5.4, but costs incurred by Mitotix in providing and retaining the qualified full-time equivalent scientists described in Section 6.1.1 shall be included in calculating the amount of UBC Research, Development and Pre-Launch Marketing Costs funded by Mitotix.
5.3 UBC Research Program. During the UBC Collaboration, the parties shall conduct the UBC Research Program as set forth in Article 6 below. As set forth in Section 6.2 below, the term of the UBC Research Program shall commence on January 1, 1996 and shall terminate as of the expiration of the Initial UBC Term, unless DuPont Merck exercises its option to extend the UBC Collaboration pursuant to Section 5.2. As set forth in Section 6.2, if DuPont Merck exercises its option to extend the UBC Collaboration pursuant to Section 5.2, then the UBC Research Program shall continue for the remaining Research Year in which DuPont Merck exercises its option to extend and continue for an additional three (3) Research Years, (unless, as set forth in Section 6.2.3, where the Alternate UBC Plan is not in effect, the Collaborative Policy Setting Committee determines that the UBC Research Program should terminate earlier), after which DuPont Merck shall have the option to extend the UBC Research Program for up to three (3) additional consecutive one-year Research Years.
5.4 Research and Development Funding Commitments.
5.4.1 First Three Research Years. During the first three (3) Research Years after the Effective Date, each party shall fund an agreed upon amount of all UBC Research, Development and Pre-Launch Marketing Costs set forth in the Annual UBC Research Plan
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
16
and Budget; provided, however, Mitotix shall fund not less than thirty percent (30%) and DuPont Merck shall fund not more than seventy percent (70%) of all UBC Research, Development and Pre-Launch Marketing Costs set forth in the Annual UBC Research Plan and Budget. Each party shall use good faith commercially reasonable efforts to meet its funding commitment set forth herein. The percentage share of the total amount of UBC Research, Development and Pre-Launch Marketing Costs incurred by a party at any point in time during the UBC Collaboration shall hereafter be referred to as a party’s “Percentage Contribution”. The Percentage Contribution at any point in time shall be calculated based on the net present value of the UBC Research, Development and Pre-Launch Marketing Costs incurred from the Effective Date, as set forth in Appendix H. At the end of each Research Year the parties shall calculate the Percentage Contribution. At the end of the third Research Year it is the expectation of the parties that Mitotix’s Percentage Contribution shall not be less than thirty percent (30%) and DuPont Merck’s Percentage Contribution shall not be more than seventy percent (70%).
5.4.2 Fourth Research Year and Thereafter. Commencing with the fourth Research Year and continuing for each Research Year thereafter, Mitotix will fund at a level to maintain its Percentage Contribution between twenty percent (20%) and thirty percent (30%), and DuPont Merck will fund at a level to maintain its Percentage Contribution between seventy percent (70%) and eighty percent (80%) of all UBC Research, Development and Pre-Launch Marketing Costs for each Research Year, with the goal of achieving as described in Appendix H, a 30% and 70% Percentage Contribution by Mitotix and DuPont Merck, respectively, by the time of First Commercial Sale of a UBC Product.
5.4.3 Changes to Mitotix’s Funding Commitment. In Mitotix’s sole discretion, it may reduce its funding commitment during any Research Year in order to decrease its Percentage Contribution from thirty percent (30%) to a minimum of twenty percent (20%). However, if after any Research Year, Mitotix allows its Percentage Contribution to fall below thirty percent (30%), Mitotix’s Percentage Contribution calculated at the end of the Research Year in which it decreased its funding commitment shall become the new target Percentage Contribution for Mitotix and Mitotix shall not be permitted to increase its Percentage Contribution beyond such new target amount.
5.4.4 Alternate UBC Plan.
(a) Mitotix may, at any time prior to the entry of the last patient into the pivotal clinical efficacy trial on which the NDA filing for the first UBC Product would be based, provide DuPont Merck with written notice of its election to commence the Alternate UBC Plan, and the Alternate UBC Plan shall commence upon DuPont Merck’s receipt of such written notice. In addition, if at any time Mitotix permits its Percentage Contribution to decrease to less than twenty percent (20%), then effective upon the end of the Research Year in which Mitotix’s Percentage Contribution falls below twenty percent (20%), the Alternate UBC Plan shall commence.
(b) Upon the commencement of the Alternate UBC Plan (i) the UBC Research Program shall continue and all future planning and funding with respect to the UBC Research, Development and Pre-Launch Marketing Costs shall be the sole responsibility of
17
DuPont Merck, (ii) Mitotix shall provide research and development support of the UBC Research Program (up to a level of eight qualified full time equivalent scientists and substantially similar to the corresponding level of support provided by Mitotix under the CDK Research Program) in accordance with the Annual UBC Research Plan and Budget, and subject to DuPont Merck’s funding of such research and development support, and Mitotix shall invoice DuPont Merck, in accordance with the terms set forth in Section 5.4.5, for all UBC Research, Development and Pre-Launch Marketing Costs incurred by Mitotix in performing its obligations under the Annual UBC Research Plan and Budget (as revised as necessary to reflect the commencement of the Alternate UBC Plan), (iii) all UBC Products shall be deemed to be Royalty-Bearing Products, (iv) the provisions of Article 6 and 7 shall remain in effect, except that the decision-making power of the parties shall be as set forth under the CDK Collaboration, and (v) DuPont Merck shall reimburse Mitotix on a straight dollar for dollar basis (i.e. not time adjusted) for all UBC Research, Development and Pre-Launch Marketing Costs incurred by Mitotix from the Effective Date until the commencement of the Alternate UBC Plan; provided, however, that Mitotix shall not be entitled to such reimbursement under clause (v) above if (a) Mitotix permits its Percentage Contribution to decrease to less than twenty percent (20%) after the entry of the last patient into the pivotal clinical efficacy trial on which the NDA filing for the first UBC Product is based, or (b) no UBC Product is ever sold. Such reimbursement under clause (v) shall be made in equal quarterly payments over a three (3) year period commencing with the First Commercial Sale of a UBC Product, provided, however, that no such quarterly payment shall exceed thirty percent (30%) of the Net Sales of the UBC Product in that quarter.
5.4.5 Invoicing. At the beginning of the fourth and each subsequent Research Year, based upon the tasks assigned to each party by the Collaborative Policy Setting Committee pursuant to the Annual UBC Research Plan and Budget and the amount of the annual budget that is expected to be spent by each party in connection with the performance of its obligations under the Annual UBC Research Plan and Budget, the parties shall mutually determine in writing whether it is necessary for either party to invoice the other so that each party funds the UBC Research, Development and Pre-Launch Marketing Costs for the Research Year in accordance with the Percentage Contribution targets set forth above. In addition, at the beginning of each Research Year following the commencement of the Alternate UBC Plan, based upon the tasks assigned to Mitotix by the Collaborative Policy Setting Committee pursuant to the Annual UBC Research Plan and Budget and the amount of the annual budget that is expected to be spent by Mitotix in connection with the performance of its obligations under the Annual UBC Research Plan and Budget, Mitotix will invoice DuPont Merck for the UBC Research Development and Pre-Launch Marketing Costs it is expected to incur during the Research Year. The amount that is to be invoiced to any one party, if any, shall be invoiced in four equal quarterly amounts throughout the Research Year. Within fifteen (15) days of a receipt of an invoice from a party hereunder, the receiving party shall make payment in full under the invoice in United States dollars by check, wire transfer of funds, or other means acceptable to the party to whom payment is to be made. Adjustments as necessary to reflect the expenses actually incurred by each party shall be made as promptly as possible following the end of each Research Year quarter and shall be reflected in the determination of invoice amounts for the subsequent quarter.
18
5.4.6 Records. Each party shall keep complete and accurate records of the UBC Research, Development and Pre-Launch Marketing Costs incurred by it in each Research Year, which records shall be retained for three (3) years after the end of the Research Year to which they relate. The records shall conform to generally accepted accounting principles consistently applied and shall be in accordance with procedures to be agreed upon between the parties, as outlined in Appendix H and Appendix I. Each party shall have the right at its own expense during the term of this Agreement and during the subsequent three-year period to appoint an independent certified public accountant reasonably acceptable to the other party to inspect said records. Upon reasonable notice from a party, the other party shall make its records available during regular business hours for inspection by the independent certified public accountant at the place or places where such records are customarily kept, to the extent reasonably necessary to verify the occurrence of the expenditures. The right of inspection shall not be exercised more than once in any Calendar Year. The parties agree to hold in strict confidence all information concerning such expenditures and all information learned in the course of any audit or inspection, except to the extent necessary for a party to enforce any rights it may have pursuant to this Agreement or if disclosure is required by law. The failure of either party to request verification of any expenditures during the three (3) year period the parties are required to retain the records for any Research Year shall be considered acceptance of the accuracy of the reports concerning such expenditures.
5.5 Termination of UBC Collaboration if DuPont Merck Does Not Extend the UBC Collaboration and Result of Such Termination. If DuPont Merck does not exercise the option to extend the UBC Collaboration pursuant to Section 5.2, the UBC Collaboration will terminate as of the expiration of the Initial UBC Term. In the event of such termination of the UBC Collaboration:
(a) the license granted to DuPont Merck under Section 10.2.1 shall immediately terminate and be of no further force and effect,
(b) all Mitotix rights to the UBC Targets, including any New UBC Targets shall immediately revert to Mitotix,
(c) Mitotix shall have, without any further action by the parties, a worldwide, exclusive license in the UBC Field, with the right to sublicense, to any and all rights in (a) any Know-how and DuPont Merck Inventions gained or discovered by DuPont Merck during the UBC Collaboration relating specifically to the UBC Targets, including any New UBC Targets; and (b) all Joint Inventions in the UBC Field relating specifically to UBC Targets; provided however, that such exclusive license shall become non-exclusive after the expiration of a ten (10) year period following the termination of the Initial UBC Term,
(d) Mitotix shall have, without any further action by the parties, a worldwide non- exclusive license in the UBC Field to any Know-how and DuPont Merck Inventions gained or developed by DuPont Merck during the UBC Collaboration relating to assays or otherwise useful in the UBC Field,
19
(e) any Confidential Information delivered to DuPont Merck by Mitotix for use in the UBC Collaboration shall be promptly returned to Mitotix,
(f) notwithstanding the foregoing, DuPont Merck shall retain all rights to, and no rights are granted to Mitotix with respect to, compounds delivered to Mitotix pursuant to Section 6.1.3 or otherwise disclosed to Mitotix, including without limitation all DuPont Merck know-how and patent rights relating to such compounds,
(g) Mitotix shall promptly return to DuPont Merck all compounds delivered to Mitotix pursuant to Section 6.1.3 and any information delivered to Mitotix by DuPont Merck relating to DuPont Merck compounds for use in the UBC Collaboration shall be promptly returned to DuPont Merck, and
(h) DuPont Merck shall not, either individually, or with an Affiliate or Third Party pursue any research, development, manufacturing, sales or marketing efforts in the UBC Field for a period of twenty four (24) months following the termination of the UBC Collaboration.
5.6 Certain Royalty Rights. If Mitotix or a Third Party licensee of Mitotix, sells a therapeutic or Radiopharmaceutical product in the UBC Field that incorporates a compound that was:
(a) (x) first identified within ten (10) years after the termination of the UBC Collaboration through the use of the UBC Targets and (y) discovered or selected for development in reliance upon the Know-how or DuPont Merck Inventions exclusively licensed to Mitotix pursuant to Section 5.5(c) above, or
(b) (x) first identified within four (4) years after the termination of the UBC Collaboration through the use of the UBC Targets and (y) discovered or selected for development in reliance upon the Know-how in existence prior to the termination of the UBC Collaboration,
then such product shall be a Mitotix Product and Mitotix shall pay the royalties set forth in Article 12 upon the sale of such Mitotix Product.
5.7 UBC Operational Disengagement Plan.
In the event of a Change of Control (defined in Section 19.13) and the failure of Mitotix (or its successor) to adhere to the provisions of Section 19.13, as reasonably determined by DuPont Merck, (i) DuPont Merck may elect (subject to the restrictions in Section 19.13) to commence the UBC Operational Disengagement Plan effective upon three (3) months prior written notice to Mitotix or (ii) if the Change of Control occurs prior to the expiration of DuPont Merck’s option to extend the UBC Collaboration under Section 5.2, DuPont Merck may, effective upon three (3) months prior written notice to Mitotix, elect to terminate the UBC Collaboration and such termination shall have the effect set forth in Section 5.5. If DuPont Merck elects to commence the UBC Operational Disengagement Plan, DuPont Merck’s option to extend the UBC Collaboration under Section 5.2 shall
20
remain in effect. If DuPont Merck does not exercise the option to extend the UBC Collaboration pursuant to Section 5.2, the terms of Section 5.5 shall apply.
Upon commencement of the UBC Operational Disengagement Plan, the Collaborative Policy Setting Committee shall dissolve with respect to the UBC Collaboration and all research and development activities under the UBC Collaboration shall become the sole responsibility of DuPont Merck. Under the UBC Operational Disengagement Plan, DuPont Merck will assume responsibility for all operational elements of the UBC Collaboration, but the financial elements of the UBC Collaboration shall not be altered.
Following commencement of the UBC Operational Disengagement Plan, upon request by DuPont Merck, the UBC Research Program may be continued under DuPont Merck’s direction and Mitotix shall provide research and development support for the UBC Research Program in accordance with an Annual UBC Research Plan and Budget and subject to DuPont Merck’s funding of such research and development support. In such event, such research and development support by Mitotix shall be provided up to a level of eight qualified full time equivalent scientists and substantially similar to the corresponding level of support provided by Mitotix under the CDK Research Program. Upon commencement of the UBC Operational Disengagement Plan, upon request by DuPont Merck, Mitotix shall discontinue its operational activities in support of the UBC Collaboration.
Following commencement of the UBC Operational Disengagement Plan, upon request by DuPont Merck, Mitotix shall provide DuPont Merck with all materials, technical information and know-how necessary or useful to enable DuPont Merck to continue the UBC Research Program (to the extent not transferred earlier) and shall provide DuPont Merck with reasonable assistance in transferring assays to DuPont Merck. Upon request by DuPont Merck, all compounds and information in tangible form received by Mitotix from DuPont Merck shall be returned to DuPont Merck.
Following the commencement of the UBC Operational Disengagement Plan, Mitotix shall continue to have the right to fund UBC Research, Development and Pre-Launch Marketing Costs incurred by DuPont Merck so as to maintain Mitotix’s Percentage Contribution above twenty percent (20%) in accordance with Section 5.4, and if Mitotix does so, Mitotix shall continue to have the right to receive a share of the UBC Operating Profit in accordance with Section 11.5 and Appendices H and I. In the event that Mitotix permits its Percentage Contribution to decrease to less than twenty percent (20%), the Alternate UBC Plan shall be deemed to have commenced, for purposes of the financial provisions only, such that (i) all UBC Products shall be deemed to be Royalty-Bearing Products, and (ii) DuPont Merck shall reimburse Mitotix on a straight dollar for dollar basis (i.e. not time adjusted) for all UBC Research, Development and Pre-Launch Marketing Costs incurred by Mitotix from the Effective Date until the deemed commencement of the Alternate UBC Plan hereunder; provided, however, that Mitotix shall not be entitled to such reimbursement under clause (ii) above if (a) the entry of the last patient into the pivotal clinical efficacy trial on which the NDA filing for the first UBC Product would be based did not occur prior to the Change of Control and Mitotix permits its Percentage Contribution to decrease to less than twenty percent (20%) after the commencement of the UBC Operational Disengagement Plan and after the entry of such patient, or (b) no UBC Product is ever sold. Such reimbursement
21
under clause (ii) shall be made in equal quarterly payments over a three (3) year period commencing with the First Commercial Sale of a UBC Product, provided, however, that no such quarterly payment shall exceed thirty percent (30%) of the Net Sales of the UBC Product in that quarter.
5.8 New UBC Targets. The parties agree that the UBC Collaboration will focus on an area of science that is relatively undefined as of the Effective Date, and that it is possible that more than one New UBC Target may be discovered during the course of the UBC Collaboration. Within ninety (90) days after the Collaborative Policy Setting Committee has accepted a new target as a New UBC Target it shall notify the parties in writing of its determination as to whether such New UBC Target shall be pursued as part of the UBC Collaboration. If the Collaborative Policy Setting Committee determines to include a New UBC Target within the UBC Collaboration, the New UBC Target shall become a UBC Target for the purposes of this Agreement. If the Collaborative Policy Setting Committee determines in writing not to include a particular New UBC Target within the UBC Collaboration, Mitotix shall have all rights to use such New UBC Target either independently or in collaboration with a Third Party within the UBC Field, and such New UBC Target will not be included in the definition of UBC Target and will not be subject to the license grant set forth in Section 10.2.1. If Mitotix, either independently or in collaboration with a Third Party, uses such New UBC Target to discover or select for development a compound that is first identified within four (4) years following the release of such New UBC Target by DuPont Merck and that is ultimately incorporated within a therapeutic or Radiopharmaceutical product, then such product shall be a Mitotix Product and Mitotix shall pay the royalties set forth in Article 12 upon any sale of such Mitotix Product. Any royalty payments made by Mitotix to DuPont Merck under this Section shall not be deemed to be a UBC Research, Development and Pre-Launch Marketing Cost for purposes of Section 5.4 hereof.
5.9 Terms of Equity Investment.
5.9.1 General Provisions Relating to Price of Equity Investment. The number of shares of Mitotix stock to be issued to DuPont Merck in exchange for the UBC Deferral Payment shall be calculated as of the date Mitotix receives each installment of the UBC Deferral Payment. The number of shares of Mitotix stock to be issued to DuPont Merck in exchange for the UBC Extension Payment shall be calculated as of the date Mitotix receives the Extension Notice, provided that Mitotix receives the UBC Extension Payment no later than thirty (30) days after it receives the Extension Notice. If Mitotix receives the UBC Extension Payment more than thirty (30) days following receipt of the Extension Notice, then the number of shares to be issued in exchange for the UBC Extension Payment shall be calculated as of the date Mitotix receives the UBC Extension Payment. Each calculation date referred to in the preceding three sentences is hereinafter referred to as the applicable “UBC Pricing Date.” In any case where the number of shares issuable to DuPont Merck is determined by reference to prices paid by investors in Mitotix or reported trading prices, such prices shall be appropriately adjusted to reflect stock splits, stock dividends, combinations, mergers and other recapitalizations affecting shares of Mitotix capital stock.
22
5.9.2 Investment Prior to an Initial Public Offering. If Mitotix has not completed an initial public offering of its common stock (an “IPO”) at the time the UBC Extension Payment is received by Mitotix, then DuPont Merck shall receive, in exchange for the UBC Deferral Payment and the UBC Extension Payment, redeemable, convertible shares of the next available class of preferred stock with rights, preferences and privileges substantially similar to the rights, preferences and privileges attached to the Series C Convertible Preferred Stock of Mitotix, except that the liquidation preference associated with such new class of stock will be junior to all prior series of preferred stock. With respect to each UBC Pricing Date, the number of shares of preferred stock issuable to DuPont Merck shall be determined by dividing the amount of the UBC Deferral Payment made on that date or the UBC Extension Payment by the price at which Mitotix most recently (as of such UBC Pricing Date) sold shares of preferred stock to investors, provided, however, that if Mitotix did not receive gross proceeds of at least *** from such most recent sale of stock to investors, then the price shall be the weighted average purchase price of all shares of stock sold by Mitotix during the period beginning on the Effective Date (including the shares sold to DuPont Merck on the Effective Date) and ending on the applicable UBC Pricing Date, excluding shares of stock and options to purchase shares of stock issued to employees and directors of and consultants to Mitotix and shares issued to non-profit institutions in exchange for technology rights in arms-length negotiated transactions in which no other material investor in Mitotix has any direct or indirect interest in the shares issued to the non-profit institution; and provided, further, that the price of such shares shall in no event be less than $3.00 per share nor more than $7.00 per share (subject to appropriate adjustment for stock splits, stock dividends, combinations, mergers and other recapitalizations affecting shares of Mitotix capital stock.)
5.9.3 Investment After an Initial Public Offering. If Mitotix has completed an IPO before the UBC Extension Payment is received by Mitotix, then DuPont Merck shall receive, in exchange for the UBC Deferral Payment and the UBC Extension Payment, shares of Mitotix common stock. With respect to each UBC Pricing Date occurring prior to an IPO, the number of shares of common stock issuable to DuPont Merck shall be determined by dividing the amount of the UBC Deferral Payment made on that date by the price determined pursuant to Section 5.9.2. With respect to each UBC Pricing Date occurring after an IPO, the number of shares shall be determined by dividing the amount of the UBC Deferral Payment made on that date or the UBC Extension Payment by the average of the closing prices reported for Mitotix’s common stock during the thirty (30) trading days ending on the second trading day prior to the applicable UBC Pricing Date. The shares issued to DuPont Merck pursuant to this Section 5.9.3 shall be afforded registration rights substantially similar to those granted to DuPont Merck with respect to shares purchased by it under and pursuant to the Series C Convertible Preferred Stock Purchase Agreement, entered into among Mitotix, DuPont Merck and others (the “Purchase Agreement”), except that, (i) in addition to the registration rights granted DuPont Merck under Sections 7.4 and 7.5 of the Purchase Agreement, DuPont Merck may require registration of all or part (but at least 50%) of the shares purchased by it under this Section 5.9.3 for sale in a firm commitment underwritten offering by an underwriter selected by DuPont Merck and reasonably acceptable to Mitotix, in which offering all registration expenses are borne by Mitotix and all underwriting discounts and selling commissions are borne by DuPont Merck, (ii) supplementing DuPont Merck’s rights under Section 7.4 of the Purchase Agreement in the
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
23
case of a “piggy-back” registration in which the managing underwriter in such offering is entitled to exclude shares held by selling shareholders from the offering, Mitotix will use good faith efforts to cause all other holders of “piggy-back” registration rights to agree that their shares will be subject to exclusion from the offering before any shares held by DuPont Merck are excludable. The additional rights to be granted to DuPont Merck under clause (i) of the preceding sentence will expire when the shares purchased by DuPont Merck under this Section 5.9.3 become eligible for sale pursuant to Rule 144(k) under the Securities Act of 1933, or any successor provision.
ARTICLE 6. THE UBC COLLABORATION - UBC RESEARCH PROGRAM
6.1 Conduct of the UBC Research Program.
6.1.1 General Terms. The UBC Research Program shall be conducted in good scientific manner, and in compliance with all applicable good laboratory practices, and applicable legal requirements, to attempt to achieve efficiently and expeditiously the objectives described in the work plan set forth in Appendix F hereto. Throughout the UBC Research Program, Mitotix and DuPont Merck shall each assign a minimum of four full-time equivalent qualified scientists to perform the work set forth in the Annual UBC Research Plan and Budget (as defined below); provided, however, that DuPont Merck shall not be obligated to pay any installment of the UBC Deferral Payment unless Mitotix has assigned, throughout the Initial UBC Term, a minimum of ten full-time equivalent qualified scientists to perform such work; and provided, further, that if Mitotix has reduced the number of qualified scientists devoted to working on the UBC Research Program at the written request of DuPont Merck, then DuPont Merck shall be required nonetheless to make each installment of the UBC Deferral Payment. The name and percentage of time devoted to working on the UBC Research Program for each scientist comprising such requisite number of full-time equivalent scientists for each party shall be provided in each Annual UBC Research Plan and Budget. The mixture of skills and levels of expertise of such scientists shall be appropriate to the scientific objectives of the UBC Research Program. Mitotix and DuPont Merck shall proceed diligently with the work set out in the Annual UBC Research Plan and Budget by using their respective good faith efforts considering the funding commitments of both parties hereunder.
6.1.2 Annual UBC Research Plan and Budget. The UBC Research Program shall be conducted under an annual UBC research plan and budget which describes the work to be pursued by Mitotix and DuPont Merck during the Research Year (the “Annual UBC Research Plan and Budget”). The first Annual UBC Research Plan and Budget shall be prepared by the UBC Research Operating Committee and approved by the Collaborative Policy Setting Committee within forty-five (45) days after the Effective Date. Subsequent Annual UBC Research Plans and Budgets shall be prepared by the UBC Research Operating Committee, for submission to, and approval by, the Collaborative Policy Setting Committee, not later than sixty (60) days prior to the start of each Research Year. The Collaborative Policy Setting Committee shall determine which party shall be responsible for conducting the primary screening of compounds, continued biological discovery, target validation, biochemical and cell-based assay refinement, and new assay development and screening
24
activities for UBC Targets, and where such activities will be conducted. Mitotix and DuPont Merck will share in the chemistry efforts, in vivo model development and animal pharmacology within the UBC Field.
6.1.3 DuPont Merck Compounds. Following primary screening, compounds which meet the specifications set forth by the UBC Research Operating Committee for suitable candidates for continued preclinical evaluation shall be provided to Mitotix. Mitotix shall conduct secondary screening of such compounds, and shall conduct additional preclinical work with respect to such compounds, all as set forth in the Annual UBC Research Plan and Budget. Mitotix will promptly provide to DuPont Merck all data from such screening and preclinical work in accordance with Section 6.1.6 and as otherwise reasonably requested by DuPont Merck in support of the development of UBC Development Compounds. Such compounds provided by DuPont Merck will be used by Mitotix solely for screening and other preclinical work within the UBC Field in accordance with the Annual UBC Research Plan and Budget and for no other purpose. Any know-how incidentally developed by Mitotix outside of the UBC Field using a DuPont Merck compound provided hereunder shall be promptly disclosed to DuPont Merck. Any know-how and patent rights developed by Mitotix using a DuPont Merck compound shall be considered to be Know-how and UBC Patent Rights, respectively. DuPont Merck shall at all times own all rights to the compounds it provides to Mitotix hereunder, including without limitation, all rights with respect to the manufacture, use, or sale of such compounds. In accordance with the foregoing, Mitotix agrees to (i) disclose to DuPont Merck any inventions it conceives or makes with respect to the manufacture, use or sale of compounds provided by DuPont Merck to Mitotix hereunder; (ii) assign to DuPont Merck all patent rights with respect to such inventions and (iii) execute any necessary papers and otherwise reasonably cooperate with DuPont Merck in securing patents on such inventions. Upon expiration or termination of the UBC Research Program, Mitotix shall, upon the request of DuPont Merck, return or destroy all compounds provided to Mitotix by DuPont Merck in the performance of the UBC Research Program.
6.1.4 Subcontracts. Subject to the provisions of Article 15, and as set forth in the Annual UBC Research Plan and Budget or otherwise approved by the Collaborative Policy Setting Committee, Mitotix and DuPont Merck may subcontract portions of the UBC Research Program to be performed by them in the normal course of their business to a Third Party upon prior written notice to the other; provided, however, that such Third Party has entered into an appropriate confidentiality agreement with Mitotix and/or DuPont Merck obligating such Third Party to be bound by the confidentiality obligations contained in this Agreement, or such subcontracting would not require the transfer of confidential information to the Third Party.
6.1.5 Data. Mitotix and DuPont Merck shall each maintain records in sufficient detail and in good scientific manner appropriate for patent purposes and as will properly reflect all work done and results achieved in the performance of the UBC Research Program (including all data in the form required to be maintained under any applicable governmental regulations). Such records shall include books, records, reports, research notes, charts, graphs, comments, computations, analyses, recordings, photographs, computer programs and documentation thereof, computer information storage means, samples of
25
materials and other graphic or written data generated in connection with the UBC Research Program. Mitotix and DuPont Merck shall each provide the other the right to inspect such records, and shall provide copies of all requested records, to the extent reasonably required for the performance of the requesting party’s obligations under this Agreement; provided, however, that each party shall maintain such records and the information of the other contained therein in confidence in accordance with Article 15 below and shall not use such records or information except to the extent otherwise permitted by this Agreement.
6.1.6 Quarterly Reports by Mitotix and DuPont Merck. Within thirty (30) days following the end of each Calendar Quarter, Mitotix and DuPont Merck shall each provide to the members of the UBC Research Operating Committee a written report summarizing in reasonable detail the work performed by it under the UBC Research Program during the preceding Calendar Quarter.
6.2 Expiration or Termination of the UBC Research Program and Result of Such Termination.
6.2.1 Termination of Initial UBC Term. The term of the UBC Research Program shall commence on January 1, 1996 and shall terminate as of the expiration of the Initial UBC Term, unless DuPont Merck exercises its option to extend the UBC Collaboration pursuant to Section 5.2. Upon the termination of the UBC Research Program pursuant to this Section 6.2.1, the provisions of Section 5.5 shall apply.
6.2.2 Expiration Following Extension of the UBC Collaboration and Commencement of Alternate UBC Plan. If DuPont Merck exercises its option to extend the UBC Collaboration and the Alternate UBC Plan is in effect in accordance with the terms of Section 5.4.4, then the UBC Research Program shall continue for the remaining Research Year and continue for an additional three (3) Research Years. DuPont Merck shall have the option to extend the UBC Research Program for up to three (3) additional consecutive one- year Research Years. In order to exercise its option to extend the UBC Research Program, DuPont Merck shall provide Mitotix with written notice of extension of the UBC Research Program at least six (6) months prior to the commencement of each such additional one-year period. Upon the expiration of the UBC Research Program pursuant to this Section 6.2.2, the terms of Sections 10.2.2 and 10.2.3 shall apply.
6.2.3 Termination of the UBC Research Program Following Extension of the UBC Collaboration and Prior to the Commencement of the Alternate UBC Plan. If DuPont Merck exercises its option to extend the UBC Collaboration under Section 5.2 and the Alternate UBC Plan is not in effect, then the UBC Research Program shall continue for the remaining Research Year following DuPont Merck’s exercise of such option and for an additional three (3) Research Years unless the Collaborative Policy Setting Committee determines in writing that the UBC Research Program should terminate earlier. DuPont Merck shall have the option to extend the UBC Research Program for up to three (3) additional consecutive one-year Research Years. In order to exercise its option to extend the UBC Research Program, DuPont Merck shall provide Mitotix with written notice of extension of the UBC Research Program at least six (6) months prior to the commencement of each such additional one-year period. Upon the expiration of the UBC Research Program
26
pursuant to this Section 6.2.3, the terms of Section 10.2.2 and 10.2.3 shall apply, in addition to the following:
(a) Upon termination of the UBC Research Program under this Section 6.2.3, neither party shall engage in further research activities in the UBC Field without first notifying the other party of the UBC Targets it wishes to pursue. Within ninety (90) days after receipt of such notice, the recipient shall notify the other party if it wishes to collaborate on such research, in which event the parties will perform the research together as part of the UBC Collaboration in accordance with Articles 5 and 6. If the recipient does not respond in writing within such ninety (90) day period or responds in writing that it does not wish to collaborate, then the party who wishes to continue research may do so on the following basis:
(i) In the case of continuation of the research by Mitotix, Mitotix shall have all rights to use the UBC Targets that were so identified to DuPont Merck by Mitotix, either independently or in collaboration with a Third Party, within the UBC Field, or otherwise, and such UBC Targets will not be included in the definition of UBC Target and will not be subject to the license grant set forth in Section 10.2.1. If Mitotix, either independently or in collaboration with a Third Party, uses such UBC Targets to discover or select for development a compound that is first identified within four (4) years following expiration of the UBC Research Program and that is ultimately incorporated within a therapeutic or Radiopharmaceutical product, then such product shall be a Mitotix Product and Mitotix shall pay the royalties set forth in Article 12 upon any sale of such Mitotix Product. Mitotix’s costs of performing such research and any royalty payments made by Mitotix to DuPont Merck under this paragraph shall not be deemed to be a UBC Research, Development and Pre-Launch Marketing Cost or Allowable Expense.
(ii) In the case of continuation of the research by DuPont Merck, DuPont Merck’s costs of performing such research shall not be deemed to be a UBC Research, Development and Pre-Launch Marketing Cost or Allowable Expense and Mitotix will not be entitled to share in UBC Operating Profits from sales of any UBC Products developed from use of such UBC Targets by DuPont Merck. If DuPont Merck, either independently or in collaboration with a Third Party, uses such UBC Targets to discover or select for development a compound that is first identified within four (4) years following the earlier of (x) the termination of the license grant set forth in Section 10.2.1 pursuant to Section 10.2.3 or (y) the termination of this Agreement and that is ultimately incorporated within a UBC Product, then such product shall be a Royalty-Bearing Product and DuPont Merck shall pay the royalties set forth in Article 11 upon any sale of such Royalty-Bearing Product. Notwithstanding the inclusion of such product within the definition of Royalty-Bearing Products, DuPont Merck shall not be obligated to pay any milestone payments to Mitotix under Article 11 with respect to such product. DuPont Merck’s costs of performing such research and any royalty payments made by Mitotix to DuPont Merck under this paragraph shall not be deemed to be a UBC Research, Development and Pre-Launch Marketing Cost or Allowable Expense.
(b) Upon conversion of the license granted to DuPont Merck in Section 10.2.1 to a non-exclusive license pursuant to Section 10.2.2, the provisions of paragraph (a)
27
above shall no longer apply as to any additional research that a party wishes to pursue in the UBC Field.
6.2.4 Termination of the UBC Research Program Following Commencement of the UBC Operational Disengagement Plan. If DuPont Merck elects to commence the UBC Operational Disengagement Plan and at any time thereafter determines not to request that Mitotix provide research and development support for the UBC Research Program as set forth in Section 5.7, the UBC Research Program shall be deemed to have terminated and the provisions of Sections 10.2.2 and 10.2.3 shall apply.
ARTICLE 7. UBC COLLABORATION: UBC DEVELOPMENT PROGRAM
7.1 Description of UBC Development Compounds. Based upon the preclinical work conducted by Mitotix and DuPont Merck and the recommendation of the UBC Research Operating Committee, (i) unless the Alternate UBC Plan is in effect, the Collaborative Policy Setting Committee shall determine whether to designate a compound as a UBC Development Compound, and (ii) if the Alternate UBC Plan is in effect, DuPont Merck shall determine whether to designate a compound as a UBC Development Compound.
7.2 Clinical Development and Marketing.
7.2.1 When the Alternate UBC Plan is Not in Effect.
(a) If the Alternate UBC Plan is not in effect, the clinical development of a UBC Development Compound outside of the United States following a decision by the Collaborative Policy Setting Committee to designate a compound as a UBC Development Compound and continuing through obtaining regulatory approvals of any resulting UBC Product shall be the sole responsibility of DuPont Merck with input from Mitotix through the Collaborative Policy Setting Committee. With respect to the clinical development of a UBC Development Compound in the United States, the Collaborative Policy Setting Committee shall determine the participation of Mitotix and DuPont Merck; provided, however, that Mitotix shall have the right, at its discretion, to participate in such development in a manner consistent with Mitotix’s then current Percentage Contribution and its co-promotion rights set forth in Appendix G2, and DuPont Merck shall be responsible for at least seventy percent (70%) of the development activities. It is agreed that Mitotix’s personnel expenses in support of the clinical development of a UBC Development Compound shall not exceed the lesser of (i) Mitotix’s Percentage Contribution times the total personnel expenses in support of the clinical development of such UBC Development Compound or (ii) thirty percent (30%) of such total personnel expenses, unless otherwise determined by Collaborative Policy Setting Committee. The personnel provided by Mitotix in support of the UBC Collaboration shall possess the experience and qualifications appropriate to the objectives of the UBC Collaboration in accordance with industry standards.
7.2.2 Clinical Development and Marketing Under the Alternate UBC Plan. If the Alternate UBC Plan is in effect, then DuPont Merck shall have sole responsibility for conducting, at its own expense, all clinical, regulatory, marketing, and sales work associated
28
with a UBC Development Compound, subject to Mitotix’ co-promotion rights in the United States, as set forth in Appendix G1. For each such UBC Development Compound, DuPont Merck shall use good faith commercially reasonable efforts to evaluate and develop such compound in a timely manner in accordance with industry standards and DuPont Merck internal practices and shall advise the Collaborative Policy Setting Committee as to the status of the development of each UBC Development Compound. Consistent with industry standards and DuPont Merck internal practices, DuPont Merck shall use its good faith commercially reasonable efforts to conduct at its expense such human clinical trials as are necessary to obtain all regulatory approvals to manufacture and market UBC Products which are subject to the Alternate UBC Plan and to seek and obtain all necessary regulatory approvals to market and promptly commence worldwide marketing of each such UBC Product.
7.3 Development Information. If the Alternate UBC Plan is in effect and/or as to all clinical development of a UBC Development Compound outside the United States, (i) DuPont Merck shall keep Mitotix informed as to the progress of DuPont Merck in the development and testing of all UBC Development Compounds and UBC Products and the preparing, filing and obtaining of the approvals necessary for marketing, (ii) within thirty (30) days following the end of the first month of each Calendar Quarter following the designation of a UBC Development Compound, DuPont Merck shall provide to Mitotix a reasonably detailed report which shall describe the progress of the development and testing of the UBC Products incorporating such UBC Development Compounds. DuPont Merck may satisfy its reporting obligation hereunder by providing an oral report to the Collaborative Policy Setting Committee within such thirty (30) day period, provided that a written report of the oral presentation is delivered to Mitotix no later than thirty (30) days after the oral report is made to the Collaborative Policy Setting Committee. In addition, DuPont Merck shall provide Mitotix with a minimum of three (3) months’ advance notice of the contemplated filing of an IND or an NDA, and DuPont Merck shall provide Mitotix with a report on all material regulatory submissions at least forty-five (45) days prior to the date of such submissions, or any major amendments or supplements thereto within ten (10) business days of filing thereof. DuPont Merck shall provide to Mitotix in a timely manner copies of any material regulatory submission, or amendments thereof, reasonably requested by Mitotix or, alternatively shall provide Mitotix reasonable access to each such material regulatory submission or amendments thereof at DuPont Merck’s offices, for review and copying by Mitotix.
ARTICLE 8. MITOTIX’S RIGHT TO CO-PROMOTE
8.1 Mitotix’s Co-promotion Option. Mitotix shall have the option to co-promote each CDK Product and UBC Product in the United States under the terms set forth in Appendix Gl and Appendix G2. respectively. In the event that Mitotix determines to exercise its option to co-promote a CDK Product or UBC Product in the United States, Mitotix and DuPont Merck will negotiate, execute and deliver a co-promotion agreement containing the principles of agreement set forth in Appendix Gl and Appendix G2, respectively, and such other terms and conditions as DuPont Merck and Mitotix may
29
mutually agree upon. If the Alternate UBC Plan is in effect, Mitotix’s co-promotion rights will be limited to such rights described for CDK Products in Appendix G1.
ARTICLE 9. MANAGEMENT OF THE COLLABORATIONS
9.1 Collaborative Policy Setting Committee.
9.1.1 General. A joint committee comprised of not more than four named senior representatives of DuPont Merck and not more than four named senior representatives of Mitotix (the “Collaborative Policy Setting Committee”) shall be appointed and shall meet not less than semi-annually during the term of the Collaboration. Each representative shall be appointed by the applicable party. The first meeting will be held no later than forty-five (45) days after the Effective Date. Meetings shall be at times and places agreed to by Mitotix and DuPont Merck, alternating between Cambridge, Massachusetts and Wilmington, Delaware, or such other locations or in such other form (e.g., telephone or video conference) as the members of the Collaborative Policy Setting Committee shall agree. At such meetings, the principal function of the Collaborative Policy Setting Committee will be to discuss the CDK Collaboration and the UBC Collaboration and to set priorities thereunder, to approve the Annual CDK Research Plan and the Annual UBC Research Plan and Budget, to discuss the compounds chosen for development, and to discuss the quality and productivity of the personnel assigned to each project. The Collaborative Policy Setting Committee may also discuss at such meetings the preclinical or clinical development of CDK Development Compounds and UBC Development Compounds, as well as any anticipated regulatory filings with respect to possible CDK or UBC Products. Members of the Collaborative Policy Setting Committee may be represented at any meeting by another member of the Collaborative Policy Setting Committee, or by a deputy. Any approval, determination or other action agreed to by all of the members of the Collaborative Policy Setting Committee or their deputies present at the relevant Collaborative Policy Setting Committee meeting shall be the approval, determination or other action of the Collaborative Policy Setting Committee, provided that at least two representatives of each party are present at such meeting.
9.1.2 Chair. The Collaborative Policy Setting Committee shall be jointly chaired by a DuPont Merck and a Mitotix representative to the Committee. Each chair shall be appointed by the applicable party, rather than by a vote of the committee.
9.1.3 Minutes. The Collaborative Policy Setting Committee shall keep accurate minutes of its deliberations which record all proposed decisions and all actions recommended or taken. The responsibility for drafting the minutes shall rotate between the joint chairs. Draft minutes shall be sent to all members of the Collaborative Policy Setting Committee within thirty (30) working days after each meeting. Draft minutes shall then be edited by the chair who drafted the minutes and shall be issued to the members of the Collaborative Policy Setting Committee in final form. All records of the Collaborative Policy Setting Committee shall be available to both parties.
9.2 Research Operating Committees. A CDK Research Operating Committee and a UBC Research Operating Committee, each comprised of not more than four representatives
30
of DuPont Merck and not more than four representatives of Mitotix shall be appointed and each committee shall meet as it determines necessary, but not less than three times during each Research Year. Each representative shall be appointed by the applicable party. Over time, it is anticipated that the UBC Research Operating Committee will begin to act as a development committee as it takes on responsibility for overseeing the day-to-day development of the UBC Development Compounds and the UBC Products. Meetings shall be held at times and places agreed to by Mitotix and DuPont Merck, alternating between Cambridge, Massachusetts and Wilmington, Delaware, or such other locations or in such other form (e.g., telephone or video conference) as the members of the committee shall agree. The Research Operating Committees will be responsible for overseeing the day-to-day implementation of goals of the respective Research Programs as determined by the Collaborative Policy Setting Committee and preparing the Annual CDK Research Plan and the Annual UBC Research Plan and Budget. Members of each Research Operating Committee may be represented at any meeting by another member of the respective Research Operating Committee or by a deputy. Any approval, determination or other action agreed to by all members of a Research Operating Committee or their deputies present at the relevant Research Operating Committee shall be the approval, determination or other action of such Research Operating Committee, provided that at least a majority of the representatives of each party are present at such meeting.
9.2.1 Chairs. The CDK Research Operating Committee shall be jointly chaired by a representative of Mitotix and a representative of DuPont Merck. During the Initial UBC Term, the UBC Research Operating Committee shall be chaired by a Mitotix representative to the committee. During the Extended UBC Collaboration, the UBC Research Operating Committee shall be jointly chaired by a representative of Mitotix and a representative of DuPont Merck. Each chair shall be appointed by the applicable party, rather than by a vote of the committee.
9.2.2 Minutes. Each Research Operating Committee shall keep accurate minutes of its deliberations which record all proposed decisions and all actions recommended or taken. The chairs shall be responsible for the preparation of draft minutes, and when a Research Operating Committee is jointly chaired, the responsibility for drafting the minutes shall rotate between the joint chairs. Draft minutes shall be sent to all members of the Research Operating Committee within thirty (30) working days after each meeting. Draft minutes shall be edited by the chair who drafted the minutes and shall be issued to the members of the Research Operating Committee and the members of the Collaborative Policy Setting Committee in final form. All records of each Committee shall at all times be available to both parties.
9.3 Disagreements. All disagreements within the Collaborative Policy Setting Committee or either of the Research Operating Committees shall be subject to the following:
(a) the representatives of the committee will negotiate in good faith for a period of not less than sixty (60) days to attempt to resolve the dispute; disagreements within either of the Research Operating Committees shall be presented to the Collaborative Policy Setting Committee and shall be negotiated in good faith by the Collaborative Policy Setting Committee to attempt to resolve the dispute;
31
(b) failing resolution at the above level, the representatives of the disputing Collaborative Policy Setting Committee shall promptly present the disagreement to the Chief Executive Officer of Mitotix and the President, DuPont Merck Research Laboratories, and such executives shall meet or discuss in a telephone or video conference each party’s view and make a good faith effort to resolve the disagreement;
(c) failing resolution at the above level, the parties shall attempt to resolve the disagreement by promptly presenting the disagreement to the Chairman of Mitotix and the Chief Executive Officer of DuPont Merck, and such executives shall meet or discuss in a telephone or video conference and make a good faith effort to resolve the disagreement.
9.4 Project Leaders. At least once a year Mitotix and DuPont Merck shall provide each other with the names of the key employees within their organizations who are working on the UBC Collaboration and the CDK Collaboration. In addition, each party shall appoint a project leader for the CDK Collaboration and a project leader for the UBC Collaboration. Project leaders shall be the primary contact between the parties with respect to each collaboration and shall serve on the respective Research Operating Committee. Each party shall notify the other within thirty (30) days of the date of this Agreement of the appointment of its project leader and shall notify the other party as early as practicable regarding any change in the appointment.
9.5 Availability of Employees. Each party agrees to make its employees and consultants reasonably available to consult with the other party on issues arising during the Collaboration and in connection with any request from any regulatory agency, including regulatory, scientific, technical and clinical testing issues.
9.6 Visit of Facilities. Representatives of Mitotix and DuPont Merck may, upon reasonable notice and at times reasonably acceptable to the other party, visit the facilities where the CDK Research Program or the UBC Research Program is being conducted, and consult informally, during such visits and by telephone, with personnel of the other party performing work on the respective Research Program. If requested by the other party, Mitotix and DuPont Merck shall cause appropriate individuals working on the CDK Research Program or the UBC Research Program or on CDK or UBC Products to be available for meetings at the location of the facilities where such individuals are employed at times reasonably convenient to the party responding to such request.
ARTICLE 10. LICENSE GRANTS AND RIGHTS OF FIRST NEGOTIATION
10.1 Grant of License to DuPont Merck in CDK Field.
10.1.1 Exclusive License in CDK Field. Except as otherwise provided, herein, Mitotix hereby grants DuPont Merck an exclusive worldwide right and license (with the right to sublicense) under the CDK Pastent Rights, Mitotix Inventions, Joint Inventions, and Know-how (i) utilize the CDK-D Targets in the CDK-D-Field, (ii) to develop, make have made, use and sell CDK Products and Immunoassay Products in the CDK-D Field, (iii) utilize the CDK-non-D Targets in the CDK-non-D Field and (iv) develop, make have made,
32
use and sell CDK Products and Immunoassay Products in the CDK-non-D Field. No license is granted to DuPont Merck under this Section 10.1.1 to utilize the CDK-D Targets or the CDK-non-D Targets for Antisense, Gene Therapy or non-Radiopharmaceutical diagnostic applications.
10.1.2 Conversion to Non-Exclusive. Unless sooner terminated, the license granted to DuPont Merck in Section 10.1.1(i) and (iii) shall become a non-exclusive license without any further action on the part of Mitotix or DuPont Merck seven (7) years after the expiration of the CDK Research Program, including any extensions thereof. Following the conversion of such license to a non-exclusive license, Mitotix shall have the right to develop, make, have made, use and sell products in the CDK Field and in the CDK non-D Field.
10.1.3 Election to Terminate License Grant. Upon written notice to Mitotix, DuPont Merck may elect to terminate the license granted to DuPont Merck in Section 10.1.1 at any time following the expiration or termination of the CDK Research Program pursuant to Sections 3.2.l(b), 3.2.l(c) or 3.3. above; provided, however, that upon such election, DuPont Merck shall retain an exclusive license under the Mitotix Inventions, Joint Inventions and applicable Know-how solely to develop, make, have made, use and sell CDK Products incorporating compounds that at the time of termination of the CDK Research Program have been designated as CDK Development Compounds.
10.1.4 Additional In-Licensed CDK Technology. If, from time to time during the term of the CDK Research Program, DuPont Merck shall determine that it is necessary or desirable and in the best interest of the CDK Collaboration to license certain patent rights or know-how from a Third Party for use in the CDK Field, DuPont Merck shall so notify the Collaborative Policy Setting Committee and Mitotix in writing. DuPont Merck shall promptly commence negotiations with such Third Party to determine the cost of licensing such patent rights and/or know-how. In the event that DuPont Merck enters into a license agreement with a Third Party which includes a license grant under such patent rights and/or know-how to use CDK Targets for Antisense, Gene Therapy and non- Radiopharmaceutical diagnostic applications, and/or to use CDK-non-D Targets outside the CDK-non-D Field, DuPont Merck shall sublicense the patent rights and know-how for such uses to Mitotix, and such sublicense shall survive termination of this Agreement. All costs and expenses incurred by DuPont Merck in obtaining any license referred to in this Section, including but not limited to upfront license payments, milestone payments, royalty payments and legal fees and disbursements, shall be borne by DuPont Merck, unless such costs and expenses are attributable to applications sublicensed to Mitotix pursuant to the provisions above, in which case, such costs and expenses shall be the responsibility of Mitotix.
10.1.5 Release of Certain CDK Targets and Certain Royalty Payments. If at any time during the CDK Collaboration, the Collaborative Policy Setting Committee determines that a specific CDK Target will not be pursued as part of the CDK Collaboration, DuPont Merck, at its sole discretion, may release the CDK Target to Mitotix, and shall provide Mitotix with written notice of its decision. If DuPont Merck releases such CDK Target to Mitotix, the CDK Target will no longer be included in the definition of CDK Targets and will no longer be subject to the license grant set forth in Section 10.1.1. Thereafter, Mitotix or a Third Party licensee of Mitotix shall be permitted to use and license
33
Third Parties to use the released CDK Target without restriction; provided, however, that if any product is ultimately sold by Mitotix or its licensee within the CDK Field that incorporates a compound first identified within four (4) years following the release of the CDK Target by DuPont Merck and discovered or selected for development through the use of such released CDK Target, then such product shall be a Mitotix Product and Mitotix shall pay the royalties set forth in Article 12 upon any sale of such Mitotix Product.
10.2 Grant of License Rights to DuPont Merck in the UBC Field.
10.2.1 Exclusive License in the UBC Field. Except as otherwise provided herein, Mitotix hereby grants DuPont Merck an exclusive worldwide right and license (with the right to sublicense) under the UBC Patent Rights, Mitotix Inventions, Joint Inventions and the Know-how to (i) utilize the UBC Targets in the UBC Field and (ii) to develop, make, have made, use and sell UBC Products and Immunoassay Products in the UBC Field. No license is granted to DuPont Merck under this Section 10.2.1 to utilize the UBC Targets for Antisense, Gene Therapy or non-Radiopharmaceutical diagnostic applications.
10.2.2 Conversion to Non-Exclusive. Unless sooner terminated, the license granted to DuPont Merck in Section 10.2.1 (i) shall become a non-exclusive license without any further action on the part of Mitotix or DuPont Merck seven (7) years after the expiration of the UBC Research Program, including any extensions thereof. Following the conversion of such license to a non-exclusive license, Mitotix shall have the right to develop, make, have made, use and sell products in the UBC Field. If DuPont Merck does not exercise its option to commence the Extended UBC Collaboration, the license granted to DuPont Merck in Section 10.2.1 shall terminate as provided in Section 5.5.
10.2.3 Election to Terminate License Grant. Upon written notice to Mitotix, DuPont Merck may elect to terminate the license granted to DuPont Merck in Section 10.2.1 at any time following the expiration or termination of the UBC Research Program pursuant to Sections 6.2.1, 6.2.2 or 6.2.3 above; provided, however, that upon such election, DuPont Merck shall retain an exclusive license under the Mitotix Inventions, Joint Inventions and applicable Know-how solely to develop, make, have made, use and sell UBC Products incorporating compounds that at the time of termination of the UBC Research Program have been designated as UBC Development Compounds.
10.2.4 Additional In-Licensed UBC Technology.
(a) If, from time to time during the term of the UBC Research Program prior to DuPont Merck’s exercise of its option to commence the Extended UBC Collaboration, Mitotix shall determine that it is necessary or desirable and in the best interest of the UBC Collaboration to license certain patent rights or know-how from a Third Party for use in the UBC Field, Mitotix shall so notify DuPont Merck and the Collaborative Policy Setting Committee in writing. Mitotix shall promptly commence negotiations with such Third Party to determine the cost of licensing such patent rights and/or know-how. Mitotix shall enter into a license agreement with such Third Party upon terms and conditions approved in writing by the Collaborative Policy Setting Committee and shall sublicense its rights thereunder within the UBC Field to DuPont Merck. All costs and expenses incurred
34
by Mitotix in obtaining any license referred to in this Section, including but not limited to up front license payments, milestone payments, royalty payments and legal fees and disbursements, shall be treated as UBC Research, Development and Pre-Launch Marketing Costs or Allowable Expenses, as appropriate, unless the Alternate UBC Plan is in effect, in which case, such costs shall be promptly reimbursed to Mitotix by DuPont Merck; provided, however, that any such costs and expenses incurred by Mitotix with respect to such license agreements attributable to applications outside the UBC Field shall be paid by Mitotix and shall not be treated as UBC Research, Development and Pre-Launch Marketing Costs or Allowable Expenses and shall not be reimbursed by DuPont Merck. Mitotix will not be required to issue stock or rights to acquire stock in connection with any such license, except upon terms acceptable to Mitotix in its sole discretion.
(b) After DuPont Merck’s exercise of its option to commence the Extended UBC Collaboration and prior to the commencement of the Alternate UBC Plan, this Section 10.2.4(b) shall apply. If the Collaborative Policy Setting Committee determines that it is necessary or desirable and in the best interest of the UBC Collaboration for Mitotix or DuPont Merck to license certain patent rights or know-how from a Third Party for use in the UBC Field, the Collaborative Policy Setting Committee shall so notify Mitotix and DuPont Merck in writing and shall instruct one of the parties to promptly commence negotiations with such Third Party to determine the cost of licensing such patent rights and/or know-how. Upon written approval of the Collaborative Policy Setting Committee such party may enter into a license agreement with such Third Party upon terms and conditions approved in writing by the Collaborative Policy Setting Committee. If Mitotix is the licensee, Mitotix shall exclusively sublicense its rights thereunder within the UBC Field to DuPont Merck. If DuPont Merck is the licensee, DuPont Merck shall exclusively sublicense its rights thereunder with respect to uses of UBC Targets for Antisense, Gene Therapy and non-Radiopharmaceutical diagnostic applications to Mitotix, and such sublicenses shall survive termination of this Agreement; provided, however, such sublicense shall not survive termination of this Agreement by DuPont Merck if such termination is under Section 18.2.2 hereof. All costs and expenses paid to Third Parties by the licensing party in obtaining any license referred to in this Section 10.2.4(b), including but not limited to upfront license payments, milestone payments, royalty payments and legal fees and disbursements, shall be treated as UBC Research, Development and Pre-Launch Marketing Costs or Allowable Expenses, as appropriate, provided, however, that (i) any such costs and expenses incurred by Mitotix with respect to such license agreements attributable to applications outside the UBC Field shall be the responsibility of Mitotix and shall not be treated as UBC Research, Development and Pre-Launch Marketing Costs or Allowable Expenses and shall not be reimbursed by DuPont Merck and (ii) any such costs and expenses incurred by DuPont Merck with respect to such license agreements attributable to applications outside of the UBC Field shall be the sole responsibility of DuPont Merck and shall not be treated as UBC Research, Development and Pre-Launch Marketing Costs or Allowable Expenses unless such costs and expenses are attributable to applications sublicensed to Mitotix pursuant to the provisions above, in which case, such costs and expenses shall be the responsibility of Mitotix and shall not be treated as UBC Research, Development and Pre-Launch Marketing Costs or Allowable Expenses and shall not be reimbursed by DuPont Merck. Neither party will be required to issue stock or rights to acquire stock in connection with any such license, except upon terms acceptable to such party in its sole discretion.
35
(c) After DuPont Merck’s exercise of its option to commence the Extended UBC Collaboration and after the Alternate UBC Plan is in effect, this Section 10.2.4(c) shall apply. If DuPont Merck shall determine that it is necessary or desirable and in the best interest of the UBC Collaboration for DuPont Merck to license certain patent rights or know-how from a Third Party for use in the UBC Field, DuPont Merck shall so notify the Collaborative Policy Setting Committee and Mitotix in writing. DuPont Merck shall promptly commence negotiations with such Third Party to determine the cost of licensing such patent rights and/or know-how. In the event that DuPont Merck enters into a license agreement with a Third Party which includes a license grant to use such patent rights and/or know-how for Antisense, Gene Therapy and non-Radiopharmaceutical diagnostic applications, DuPont Merck shall sublicense the patent rights and know-how for such uses to Mitotix, and such sublicenses shall survive termination of this Agreement; provided, however, such sublicenses shall not survive termination of this Agreement by DuPont Merck if such termination is under Section 18.2.2 hereof. All costs and expenses incurred by DuPont Merck in obtaining any license under this Section 10.2.4(c) shall be borne by DuPont Merck, unless such costs and expenses are attributable to applications sublicensed to Mitotix pursuant to the provisions above, in which case, such costs and expenses shall be the responsibility of Mitotix.
10.3 Reservation of Rights. Notwithstanding the license grants set forth in Sections 10.1 and 10.2 above, (i) Mitotix at all times reserves a non-exclusive license under the CDK Patent Rights, UBC Patent Rights, Mitotix Inventions, Joint Inventions and Know-how solely to permit Mitotix to perform its obligations and exercise its rights under this Agreement, including but not limited to its rights of co-promotion with respect to the CDK Products and the UBC Products as set forth in Article 8, and (ii) Mitotix at all times reserves its rights under the CDK Patent Rights, UBC Patent Rights, Mitotix Inventions, Joint Inventions and Know-how to use the CDK Targets and UBC Targets outside of the CDK Field and the UBC Field, respectively, including but not limited to uses relating to Antisense, Gene Therapy and non-Radiopharmaceutical diagnostic applications.
10.4 Grant of License Rights to Mitotix.
10.4.1 License to Perform Obligations. DuPont Merck hereby grants Mitotix a non-exclusive worldwide license under the DuPont Merck Inventions and Know-how solely to permit Mitotix to perform its obligations and exercise its rights under this Agreement, including but not limited to Mitotix’s obligation to conduct screening of the CDK Development Compounds and the UBC Development Compounds. Mitotix specifically agrees that it will use compounds delivered by DuPont Merck under this Agreement (see Sections 3.1.4 and 6.1.3) solely for the purpose of performing its obligations under this Agreement and for no other purpose. Such DuPont Merck compounds will not be transferred to any Third Party without the prior written approval of DuPont Merck.
10.4.2 License to DuPont Merck Inventions. DuPont Merck hereby grants Mitotix an exclusive (except as to DuPont Merck) worldwide license (with the right to sublicense) under any DuPont Merck Inventions and Joint Inventions solely to permit Mitotix to use the CDK Targets and the UBC Targets to discover, develop, make, have made, use and sell Antisense or Gene Therapy products in the oncology field.
36
10.5 DuPont Merck Rights of First Negotiation.
10.5.1 Right of First Negotiation with Respect to Antisense and/or Gene Therapy. Mitotix shall provide DuPont Merck advance written notice, (the “Antisense/Gene Therapy Notice”) of its intention to grant a license to any Third Party to (i) use the CDK Targets or the UBC Targets for Antisense, Gene Therapy and non-Radiopharmaceutical diagnostic applications and/or (ii) to discover, develop, make, have made, use and/or sell Antisense, Gene Therapy or non-Radiopharmaceutical diagnostic products incorporating compounds selected through the use of the CDK Targets or the UBC Targets. DuPont Merck shall have a period of thirty (30) days from its receipt of the Antisense/Gene Therapy Notice to provide Mitotix with written notice of its desire to enter negotiations with Mitotix regarding such a license. Upon (i) the failure of DuPont Merck to provide such written notice of its desire to enter negotiations with Mitotix in response to the Antisense/Gene Therapy Notice within thirty (30) days of DuPont Merck’s receipt thereof, (ii) the failure of DuPont Merck and Mitotix to agree upon a memorandum of intent within one hundred twenty (120) days of DuPont Merck’s receipt of the Antisense/Gene Therapy Notice, or (iii) the failure of Mitotix and DuPont Merck to execute a definitive agreement within one hundred eighty (180) days of DuPont Merck’s receipt of the Antisense/Gene Therapy Notice, Mitotix shall be free to license a Third Party; provided, however, if Mitotix offers terms and conditions which are, taken as a whole, equal to or less favorable to Mitotix than the last offer made by Mitotix to DuPont Merck, then Mitotix shall offer such terms and conditions to DuPont Merck under the procedure described above.
10.5.2 Right of First Negotiation with Respect to CDK-non-D Targets. During the term of the CDK Research Program, including any extensions thereof, Mitotix shall provide DuPont Merck advance written notice (the “CDK-non-D Notice”) of its intention to grant a license to any Third Party to (i) to use the CDK-non-D Targets outside the CDK-non-D Field and/or (ii) to discover, develop, make, have made, use and/or sell therapeutic products incorporating compounds selected through the use of the CDK-non-D Targets for indications other than oncology indications. DuPont Merck shall have a period of thirty (30) days from its receipt of the CDK-non-D Notice to provide Mitotix with written notice of its desire to enter negotiations with Mitotix regarding such a license. Upon (i) the failure of DuPont Merck to provide such written notice of its desire to enter negotiations with Mitotix in response to the CDK-non-D Notice within thirty (30) days of DuPont Merck’s receipt thereof, or (ii) the failure of DuPont Merck and Mitotix to agree upon a memorandum of intent within one hundred twenty (120) days of DuPont Merck’s receipt of the CDK-non-D Notice, or (iii) the failure of Mitotix and DuPont Merck to execute a definitive agreement within one hundred eighty (180) days of DuPont Merck’s receipt of the CDK-non-D Notice, Mitotix shall be free to license a Third Party, provided, however, if Mitotix offers terms and conditions to a Third Party which are, taken as a whole, equal to or less favorable to Mitotix than the last offer made by Mitotix to DuPont Merck, then Mitotix shall offer such terms and conditions to DuPont Merck under the procedure described above.
37
ARTICLE 11. MILESTONES AND ROYALTIES PAYABLE TO MITOTIX
11.1 Milestone Payments.
(a) In consideration of Mitotix’s work on the CDK Research Program, and if the Alternate UBC Plan is initiated or deemed to be initiated for financial purposes following commencement of the UBC Operational Disengagement Plan, in consideration of Mitotix’s work on the UBC Research Program, and the licenses granted to DuPont Merck hereunder, DuPont Merck shall pay the amounts set forth in the chart below to Mitotix upon the achievement of each of the following milestones; provided, however, that no milestone payments shall be due on (i) CDK Products incorporating compounds that are first identified more than two (2) years following the earlier of (x) the termination of the license grant set forth in Section 10.1.1 pursuant to Section 10.1.3 or (y) the termination of this Agreement, and (ii) UBC Products incorporating compounds that are first identified more than two (2) years following the earlier of (x) the termination of the license grant set forth in Section 10.2.1 pursuant to Section 10.2.3 or (y) the termination of this Agreement.
(b) No milestone payments shall be due with respect to UBC Products prior to commencement of the Alternate UBC Plan. If the Alternate UBC Plan becomes effective, or is deemed to be effective for financial purposes following commencement of the UBC Operational Disengagement Plan, after any of the following milestones have been achieved with respect to any UBC Product, then no milestone payment shall be due for such previously achieved milestone with respect to such UBC Product.
38
11.1.1 ***
| Milestone |
Column 1 (1) |
Column 2 (2) | ||
| 1. IND Filing in the United States |
*** | *** | ||
| 2. Enrollment of at least 50% of the predicted number of patients for the first clinical trial for efficacy evaluation involving fewer than 100 patients per trial |
*** | *** | ||
| 3. Enrollment of 50% of the predicted number of patients for the first clinical trial for efficacy evaluation involving more than 100 patients per trial |
*** | *** | ||
| 4. Filing of the first NDA in the United States |
*** | *** | ||
| 5. Approval of the first NDA in the United States |
*** | *** | ||
| (1) | DuPont Merck shall pay Mitotix the amounts set forth in this column upon the achievement of the stated milestone for the first Royalty-Bearing Product CDK Product to achieve such milestone and, if the Alternate UBC Plan is activated or is deemed to be activated for financial purposes following commencement of the UBC Operational Disengagement Plan, upon the achievement of the stated milestone for the first Royalty-Bearing Product UBC Product to achieve such milestone. |
| (2) | DuPont Merck shall pay Mitotix the amounts set forth in this column upon the achievement of the stated milestone for each subsequent Royalty-Bearing Product CDK Product which is a Unique Product (as defined below) to achieve such milestone and, if the Alternate UBC Plan is activated or is deemed to be activated for financial purposes following commencement of the UBC Operational Disengagement Plan, upon the achievement of the stated milestone for each subsequent Royalty-Bearing Product UBC Product to achieve such milestone which is a Unique Product. |
| (3) | If an NDA for the first Royalty-Bearing Product is filed in any non-U.S. Strategic Country before an NDA is filed in the United States for the Royalty-Bearing Product, then DuPont Merck shall pay Mitotix the sum of *** for each NDA filed in a non-U.S. Strategic Country for the Royalty-Bearing Product; provided, however, that |
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
▇▇
▇▇▇▇▇▇ ▇▇▇▇▇ shall not be obligated to pay Mitotix more than a total of *** with respect to the filing of NDAs worldwide for the first Royalty-Bearing Product.
| (4) | If an NDA for the subsequent Royalty-Bearing Product is filed in any non-U.S. Strategic Country before an NDA is filed in the United States for the Royalty-Bearing Product, then DuPont Merck shall pay Mitotix the sum of *** for each NDA filed in a non-U.S. Strategic Country for the Royalty-Bearing Product; provided, however, that DuPont Merck shall not be obligated to pay Mitotix more than a total of $2,000,000 with respect to the filing of NDAs worldwide for the subsequent Royalty- Bearing Product. |
| (5) | If an NDA for the first Royalty-Bearing Product is approved in any non-U.S. Strategic Country before an NDA is approved in the United States for the Royalty-Bearing Product then DuPont Merck shall pay Mitotix the sum of *** for each NDA approved in a non-U.S. Strategic Country for the Royalty-Bearing Product; provided, however, that DuPont Merck shall not be obligated to pay Mitotix more than a total of *** with respect to the approval of NDAs worldwide for the first Royalty-Bearing Product. |
| (6) | If an NDA for the subsequent Royalty-Bearing Product is approved in any non-U.S. Strategic Country before an NDA is approved in the United States for the Royalty- Bearing Product then DuPont Merck shall pay Mitotix the sum of *** for each NDA approved in a non-U.S. Strategic Country for the Royalty-Bearing Product; provided, however, that DuPont Merck shall not be obligated to pay Mitotix more than a total of *** with respect to the approval of NDAs worldwide for the subsequent Royalty-Bearing Product. |
The guiding principle behind the payment of milestone payments shall be that for each Unique Product in the CDK Collaboration one, and only one, milestone payment shall be paid for each milestone event achieved and if the Alternate UBC Plan is in effect or is deemed to be in effect for financial purposes following the commencement of the UBC Operational Disengagement Plan, for each Unique Product in the UBC Collaboration, one, and only one, milestone payment shall be paid for each milestone event achieved. A “Unique Product” refers to a Royalty-Bearing Product that the Collaborative Policy Setting Committee agrees has a distinctly different Target and mechanism of action relative to any Royalty-Bearing Product for which a milestone payment has been previously paid to Mitotix by DuPont Merck. Thus, if two or more Royalty-Bearing Products are in development simultaneously, or one is subsequently dropped from clinical development, that utilize the same Target and mechanism of action all such Royalty-Bearing Products will be treated as one Unique Product and only one milestone will be paid for each milestone event achieved. If two or more Royalty-Bearing Products are in development simultaneously, but utilize a different Target and mechanism of action, each of these products will be treated as a separate Unique Product and each will be eligible for milestone payments.
In addition, DuPont Merck shall pay a milestone payment in the amount of *** upon NDA approval for each Royalty-Bearing Product which has the same Target and mechanism of action as a Unique Product for which milestone payments have
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
40
previously been paid, but is approved for a new indication relative to such Unique Product. As used in the preceding sentence, “new indication” refers to a new and distinct primary disease (for example, cancer versus psoriasis) and does not, for example, refer to types or classes of diseases (for example, colon versus breast cancer) or the subsequent approval of a claim for an expanded or different patient population with the same primary disease as for the initially approved claim.
In addition, DuPont Merck shall pay a milestone payment in the amount of *** upon NDA approval for each Royalty-Bearing Product which has the same Target and mechanism of action as a Unique Product for which milestone payments have previously been paid, but is approved for a new mode of delivery (i.e., PO versus IV) relative to such Unique Product.
All milestone payments will be paid according to column 2 in the above table, except that the first Royalty-Bearing Product CDK Product which is a Unique Product and, if the Alternate UBC Plan is in effect or is deemed to be in effect for financial purposes following the commencement of the UBC Operational Disengagement Plan, the first Royalty-Bearing Product UBC Product which is a Unique Product to reach any of the five milestone events is to be paid in accordance with column 1 above.
Appendix J sets forth examples of how the milestone payments are to be made in accordance with this Section 11.1.
11.1.2 Notice of Achievement of Milestones. DuPont Merck shall notify Mitotix in writing within ten (10) business days following the occurrence of each event described in Section 11.1.1 above and shall, within thirty (30) days following the occurrence of such event, pay to Mitotix in United States dollars by wire transfer or other means acceptable to Mitotix, the amounts due upon the occurrence of such events. The milestone payments made to Mitotix pursuant to Section 11.1.1 are not refundable under any circumstances and will not be credited against royalty payments due Mitotix under Section 11.2.
11.1.3 Radiopharmaceutical Products and Immunoassav Products. Subject to Section 13.9, DuPont Merck shall not be obligated to pay milestone payments under this Section 11.1 with respect to any Royalty-Bearing Products which are Radiopharmaceutical Products or Immunoassay Products.
11.1.4 Non-Strategic Countries. DuPont Merck shall have no milestone payment obligations to Mitotix with respect to non-Strategic Countries.
11.1.5 Additional Obligations. DuPont Merck shall also be responsible for additional milestone and royalty payments, if any, under the Mitotix License Agreements, but only to the extent set forth in Section 13.9.
11.2 Royalties Payable to Mitotix on Net Sales. All royalties payable under this Section 11.2 shall be paid throughout the Royalty Term.
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
41
11.2.1 Royalty Payable to Mitotix on Net Sales in Strategic Countries. In consideration of the licenses granted to DuPont Merck hereunder, DuPont Merck shall pay to Mitotix annual royalties for each Calendar Year on (i) Net Sales by DuPont Merck, its Affiliates and sublicensees of each Royalty-Bearing Product in the Strategic Countries, and (ii) Net Sales by DuPont Merck and its Affiliates of each Royalty-Bearing Product in the non-Strategic Countries where DuPont Merck does not use a Distributor or sublicensee to conduct such sale. Such royalty shall be payable based upon the annual Net Sales in a Calendar Year of each individual Royalty-Bearing Product at the rates and in the amounts set forth below:
(a) *** percent (***%) of aggregate Net Sales of the Royalty-Bearing Product, for annual Net Sales of such Royalty-Bearing Product of up to four hundred million dollars ($400,000,000);
(b) *** percent (***%) of the aggregate Net Sales of the Royalty-Bearing Product, for annual Net Sales of such Royalty-Bearing Product of between four hundred million dollars ($400,000,000) and seven hundred million dollars ($700,000,000);
(c) *** percent (***%) of aggregate Net Sales of the Royalty-Bearing Product, for annual Net Sales of such Royalty-Bearing Product above seven hundred million dollars ($700,000,000).
Notwithstanding the above, (i) if (x) a royalty is owed under this Section 11.2.1 with respect to sales of a Royalty-Bearing Product in any country in which the Royalty-Bearing Product is not covered by a Valid Patent Claim owned by DuPont Merck or exclusively licensed to DuPont Merck and (y) a Competitive Product is sold in such country, then the royalty payable pursuant to this Section 11.2.1 based upon sales of the Royalty-Bearing Product in such country shall be *** percent (***%); and (ii) if a Third Party Competitive Product is sold in such country, then the royalty rates set forth above shall be reduced by *** percent (***%), but in no event shall such royalty rates fall below *** percent (***%).
11.2.2 Conditions on Royalty Payments. Royalties on each Royalty-Bearing Product at the rate set forth above shall be payable on a country-by-country basis during the Royalty Term subject to the following conditions:
(a) only one royalty shall be due with respect to the same unit of Royalty- Bearing Product.
(b) no royalties shall be due upon the sale or other transfer among DuPont Merck and its Affiliates, but in such cases the royalty shall be due and calculated upon DuPont Merck’s or its Affiliate’s Net Sales of Royalty-Bearing Product to a Third Party;
(c) no royalties shall accrue on the disposition of Royalty-Bearing Product in reasonable quantities by DuPont Merck or its Affiliates as bona fide samples or as donations to non-profit institutions or government agencies for non-commercial purposes;
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
42
(d) notwithstanding the above royalty rates, upon DuPont Merck request, the parties agree to discuss in good faith a reduction of such royalty rate in any given country in the event the level of development, patent protection, or general commercial environment affects the commercial viability of the Royalty-Bearing Product under such royalty rate; and
(e) if DuPont Merck utilizes one of its general partners or any of their subsidiaries as a Distributor and the prices charged to such general partner or subsidiary of a general partner are less than those ordinarily charged to other Third Party Distributors of the same product, then any sales to such partner or subsidiary of a partner shall be treated as sales to an Affiliate rather than as sales to a Distributor.
11.3 Royalties Payable to Mitotix on Net Sales to Distributors in Non-Strategic Countries. In consideration of the licenses granted to DuPont Merck hereunder, DuPont Merck shall pay to Mitotix a royalty equal to *** percent (***%) of the Net Sales of all Royalty Bearing Products sold to its Distributors in each non-Strategic Country where DuPont Merck uses a Distributor to conduct such sale.
11.4 Royalties Payable to Mitotix on Net Sales by Development Partners and other Sublicensees in Japan and Other Non-Strategic Countries. DuPont Merck agrees to use good faith commercially reasonable efforts to enter into a license agreement with a Third Party in Japan pursuant to which the Third Party will perform as Development Partner for one or more Royalty-Bearing Products in Japan and will market and sell such Royalty-Bearing Product(s) in Japan. In connection with such arrangement and other similar arrangements that DuPont Merck may pursue in non-Strategic Countries, DuPont Merck shall pay Mitotix *** percent (***%) of all royalties that DuPont Merck receives from such Development Partners or other sublicensees on sales of the Royalty-Bearing Product(s). DuPont Merck shall not owe any amount to Mitotix based upon DuPont Merck’s receipt from a Development Partner or other sublicensees of upfront license payments or milestone payments unless such amounts are creditable against future royalties owed DuPont Merck from such Development Partner or sublicensee. In such cases, Mitotix shall be entitled to receive *** percent (***%) of such amount as it is credited against royalties earned. DuPont Merck shall not owe any amount to Mitotix based upon DuPont Merck’s receipt of equity payments from a Development Partner or sublicensee. Notwithstanding the above, if DuPont Merck engages a Development Partner in a country in which DuPont Merck maintains a development organization and a pharmaceutical sales force with the reasonable capability to develop and market the Royalty-Bearing Product, DuPont Merck shall pay royalties to Mitotix on the Net Sales made by such Development Partner as if the Royalty- Bearing Product had been sold by DuPont Merck itself.
11.5 Profit Sharing on Sales of UBC Products. Unless the Alternate UBC Plan is in effect or is deemed to be in effect for financial purposes following the commencement of the UBC Operational Disengagement Plan, Mitotix and DuPont Merck shall share in the UBC Operating Profits (defined in Appendix I) from sales of UBC Products worldwide in accordance with their respective Percentage Contributions as set forth in Appendices H and I hereof. In order to effectuate such profit sharing with respect to sales of UBC Products, Mitotix and DuPont Merck agree that prior to the First Commercial Sale of such UBC
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
43
Products one or more partnerships or similar entities will be formed if required to facilitate and support the allocation of the UBC Operating Profit, in accordance with applicable tax laws and regulations.
ARTICLE 12. ROYALTIES PAYABLE TO DUPONT MERCK
12.1 General. All royalties payable under this Article 12 shall be paid throughout the Royalty Term.
12.2 Royalties Payable to DuPont Merck on Net Sales.
12.2.1 Royalty Payable to DuPont Merck on Net Sales in Strategic Countries. In consideration of the licenses granted to Mitotix hereunder, Mitotix shall pay to DuPont Merck annual royalties for each Calendar Year on (i) Net Sales by Mitotix, its Affiliates and sublicensees of each Mitotix Product in the Strategic Countries and (ii) Net Sales by Mitotix and its Affiliates of each Mitotix Product in the non-Strategic Countries where Mitotix does not use a Distributor or sublicensee to conduct such sale. Such royalty shall be payable based upon the annual Net Sales in a Calendar Year of each individual Mitotix Product at the rates and in the amounts set forth below:
(a) *** percent (***%) of aggregate Net Sales of the Mitotix Product, for annual Net Sales of such Mitotix Product of up to four hundred million dollars ($400,000,000);
(b) *** percent (***%) of the aggregate Net Sales of the Mitotix Product, for annual Net Sales of such Mitotix Product of between four hundred million dollars ($400,000,000) and seven hundred million dollars ($700,000,000);
(c) *** percent (***%) of aggregate Net Sales of the Mitotix Product, for annual Net Sales of such Mitotix Product above seven hundred million dollars ($700,000,000).
Notwithstanding the above, (i) if (x) a royalty is owed under this Section 12.2.1 with respect to sales of a Mitotix Product in any country in which the Mitotix Product is not covered by a Valid Patent Claim owned by Mitotix or exclusively licensed to Mitotix and (y) a Competitive Product is sold in such country, then the royalty payable pursuant to this Section 12.2.1 based upon sales of the Mitotix Product in such country shall be *** percent (***%); and (ii) if a Third Party Competitive Product is sold in such country, then the royalty rates set forth above shall be reduced by *** percent (***%), but in no event shall such royalty rates fall below *** percent (***%).
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
44
12.2.2 Conditions on Royalty Payments. Royalties on each Mitotix Product at the rate set forth above shall be payable on a country-by-country basis during the Royalty Term subject to the following conditions:
(a) only one royalty shall be due with respect to the same unit of Mitotix Product;
(b) no royalties shall be due upon the sale or other transfer among Mitotix and its Affiliates, but in such cases the royalty shall be due and calculated upon Mitotix’s or its Affiliate’s Net Sales of Mitotix Product to a Third Party;
(c) no royalties shall accrue on the disposition of Mitotix Product in reasonable quantities by Mitotix or its Affiliates as bona fide samples or as donations to non-profit institutions or government agencies for non-commercial purposes; and
(d) notwithstanding the above royalty rates, upon Mitotix request, the parties agree to discuss in good faith a reduction of such royalty rate in any given country in the event the level of development, patent protection, or general commercial environment affects the commercial viability of the Mitotix Product under such royalty rate.
12.3 Royalties Payable to DuPont Merck on Net Sales to Distributors in Non-Strategic Countries. In consideration of the licenses granted to Mitotix hereunder, Mitotix shall pay to DuPont Merck a royalty equal to *** percent (***%) of the Net Sales of all Mitotix Products sold to Distributors in each non-Strategic Country where Mitotix uses a Distributor to conduct such sale.
12.4 Royalties Payable to DuPont Merck on Net Sales by Development Partners and other Sublicensees in Japan and Other Non-Strategic Countries. Mitotix agrees to use good faith commercially reasonable efforts to enter into a license agreement with a Third Party in Japan pursuant to which the Third Party will perform as Development Partner for one or more Mitotix Products in Japan and will market and sell such Mitotix Product(s) in Japan. In connection with such arrangement and other similar arrangements that Mitotix may pursue in non-Strategic Countries, Mitotix shall pay DuPont Merck *** percent (***%) of all royalties that Mitotix receives from such Development Partners or other sublicensees on sales of the Mitotix Product(s). Mitotix shall not owe any amount to DuPont Merck based upon Mitotix’s receipt from a Development Partner or other sublicensees of upfront license payments or milestone payments unless such amounts are creditable against future royalties owed Mitotix from such Development Partner or sublicensee. In such cases, Mitotix shall be entitled to receive *** percent (***%) of such amount as it is credited against royalties earned. Mitotix shall not owe any amount to DuPont Merck based upon Mitotix’s receipt of equity payments from a Development Partner or sublicensee. Notwithstanding the above, if Mitotix engages a Development Partner in a country in which Mitotix maintains a development organization and a pharmaceutical sales force with the reasonable capability to develop and market the Mitotix Product, Mitotix shall pay royalties to DuPont Merck on the Net Sales made by such Development Partner as if the Mitotix Product had been sold by Mitotix itself.
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
45
ARTICLE 13. PAYMENT TERMS
13.1 General. Where relevant, the following payment terms and conditions shall apply to all payments made by one party to the other under this Agreement, whether such payments are made as milestone payments, royalty payments or profit sharing payments.
13.2 Royalty Reports, Exchange Rates. During the term of this Agreement following the First Commercial Sale of a Royalty-Bearing Product or a Mitotix Product, as the case may be, the party owing royalties as a result of such First Commercial Sale (the “Paying Party”) shall furnish to the party having a right to receive royalties as a result of such First Commercial Sale (the “Receiving Party”) a written quarterly report showing, on a country by country basis, (i) the gross sales of all Royalty-Bearing Products or Mitotix Products, as the case may be, sold to Third Party customers, including Distributors, by the Paying Party, its Affiliates and sublicensees, during the reporting period and the calculation of Net Sales from such gross sales; (ii) the royalty payments received from a Paying Party Development Partner and the royalty payments which would have been due from a Paying Party Development Partner except for the credit such Paying Party Development Partner received for upfront payments, milestone payments or other payments made to the Paying Party during the reporting period; (iii) the royalties and other payments payable in United States dollars which shall have accrued hereunder in respect of the items described in (i) and (ii) above; (iv) withholding taxes, if any, required by law to be deducted in respect of the items described in (i) and (ii) above; (v) the dates of the First Commercial Sales of any Royalty Bearing Product or Mitotix Product, as the case may be, during the reporting period; and (vi) the exchange rates used in determining the amount of United States dollars due. All payments to be made under this Agreement shall be made in United States dollars. In the case of sales outside the United States, the rate of exchange to be used in computing the amount of currency equivalent in United States dollars due shall be the Calendar Quarter-end exchange rate, which is the exchange rate utilized by DuPont Merck in its worldwide accounting system and reflects the average exchange rate for the Calendar Quarter in which the sales are recorded. Reports and any amounts payable shall be due on the forty fifth (45th) day following the close of each Calendar Quarter. If no royalty is due for any royalty period hereunder, the Paying Party shall so report. The Paying Party shall keep complete and accurate records in sufficient detail to properly reflect all gross sales and Net Sales and to enable the royalties and other amounts payable hereunder to be determined.
13.3 Payment of Profit Sharing Amounts. This Section 13.3 will not apply to UBC Products if the Alternate UBC Plan is in effect. Within sixty (60) days of the end of each Calendar Quarter following the First Commercial Sale of a UBC Product, DuPont Merck shall report to Mitotix its Net Sales of UBC Products during such Calendar Quarter and each party shall report to the other party its Allowable Expenses incurred and any sublicense revenues received by such party during such quarter. Within thirty (30) days after exchange of such reports, each party shall present to the other its calculation of the UBC Operating Profit for such quarter and the payment, if any, to be made by DuPont Merck to Mitotix in order to achieve a sharing of the UBC Operating Profit that is equal to each party’s Percentage Contribution, in accordance with and as set forth in Appendices H and I hereof. In the event a partnership or similar entity is formed pursuant to Section 11.5, such allocation of UBC Operating Profit and corresponding payments will be made by the
46
partnership in accordance with the applicable tax laws and regulations. With respect to the sales of UBC Products and such profit sharing, the party receiving payments or receiving reports shall be the “Receiving Party” and the party making payment or submitting reports shall be the “Paying Party”.
13.4 Audits. Upon the written request of the Receiving Party, the Paying Party shall permit an independent certified public accounting firm of nationally recognized standing selected by the Receiving Party, and acceptable to the Paying Party, which acceptance shall not be unreasonably withheld, to have access during normal business hours to such of the records of the Paying Party as may be reasonably necessary to verify the accuracy of the royalty reports aad profit sharing reports hereunder in respect of any Calendar Year ending not more than thirty-six (36) months prior to the date of such request. All such verifications shall be conducted at the Receiving Party’s expense and not more than once in each Calendar Year. These rights with respect to any Calendar Year shall terminate three (3) years after the end of any such Calendar Year.
In the event such accounting firm concludes that additional royalties or profit sharing payments were owed during such period, the additional amount shall be paid within thirty (30) days of the date the Receiving Party delivers to the Paying Party such accounting firm’s written report so correctly concluding. The fees charged by such accounting firm shall be paid by the Receiving Party unless the audit discloses that the payments payable by the Paying Party for the audited period are more than one hundred ten percent (110%) of the payments actually made for such period, in which case the Paying Party shall pay the reasonable fees and expenses charged by the accounting firm.
The Paying Party shall include in each sublicense agreement granted by it pursuant to this Agreement a provision requiring the sublicensee to make reports to the Paying Party, to keep and maintain records of sales made pursuant to such sublicense agreement and to grant access to such records by the Receiving Party’s independent accountant to the same extent required of the Paying Party under this Agreement. Upon the expiration of thirty-six (36) months following the end of any Calendar Year, the calculation of royalties payable with respect to such Calendar Year shall be binding and conclusive upon the Receiving Party; and the Paying Party and its sublicensees shall be released from any liability or accountability with respect to royalties for such Calendar Year.
The Receiving Party agrees that all information subject to review under this Section 13.4 or under any sublicense agreement entered into pursuant to this Agreement is confidential and will be subject to the confidentiality provisions of this Agreement, and that the Receiving Party shall cause its accounting firm to enter into an acceptable confidentiality agreement with the Receiving Party obligating it to retain all such information in confidence.
In addition, pursuant to the terms of the pl6 License, DuPont Merck shall maintain at its principal office usual books of account and records showing its actions under this Agreement. Once per Calendar Year, upon reasonable notice, such books and records shall be open to inspection and copying, during usual business hours, by an independent certified public accounting firm of nationally recognized standing selected by Cold Spring Harbor Laboratory and to whom DuPont Merck has no reasonable objection, for two years after the
47
Calendar Quarter to which they pertain, for purposes of verifying the accuracy of the amounts paid to Cold Spring Harbor Laboratory by Mitotix or DuPont Merck as a result of sales of Royalty Bearing Products deemed to be Licensed Products or Ultimate Products (each as defined in the pl6 License). If it is correctly determined that Mitotix’s payment to Cold Spring Harbor Laboratory with respect to such sales is less than the amount owing by more than ten percent (10%) of the total amount of the amount owing as a result of DuPont Merck’s underpayment to Mitotix, DuPont Merck shall pay the amount owing plus ten percent (10%) of the deficiency and shall pay the reasonable fees and expenses charged by the accounting firm, including travel expenses. Payment of any deficiency in the amount owing, any penalty, or any cost shall be made within ten days after the DuPont Merck receives written notice of such accounting firm’s written report so correctly concluding.
13.5 Payment Terms. Payments shown to have accrued by each royalty report (Section 13.2) or profit sharing report (Section 13.3) provided for under this Agreement shall be due and payable on the date such report is due. Payments hereunder in whole or in part may be made in advance of such due date. Payments determined to be owing, and any overpayments to be credited, with respect to any Calendar Quarter shall be added, together with interest thereon accruing under this Agreement from the date of the report for the Calendar Quarter for which such amounts are owing, or credited, as the case may be, to the next quarterly payment hereunder.
13.6 Exchange Controls. Except as hereinafter provided in this section, all license fees, research payments, milestone payments, royalties, profit sharing payments and other payments due under this Agreement shall be paid in United States dollars. If at any time legal restrictions prevent the prompt remittance of part or all royalties or profit sharing amounts with respect to any country where a Royalty-Bearing Product, a Mitotix Product or UBC Product, as the case may be, is sold, payment shall be made through such lawful means or methods as the Paying Party may determine. When in any country the law or regulations prohibit both the transmittal and deposit of royalties on sales in such a country, royalty payments shall be suspended for as long as such prohibition is in effect, and as soon as such prohibition ceases to be in effect, all royalties that the Paying Party would have been obligated to transmit or deposit, but for the prohibition, shall forthwith be deposited or transmitted promptly to the extent allowable, as the case may be. If the royalty rate specified in this Agreement should exceed the permissible rate established in any country, the royalty rate for sales in such country shall be adjusted to the highest legally permissible or government-approved rate.
13.7 Payment Method. Except as otherwise provided in this Agreement or agreed to by the parties, all payments under this Agreement shall be made by electronic bank transfer in funds available on the date due to such account as the Receiving Party specifies to the Paying Party before such payment is due.
13.8 Interest on Late Payments. Any payments by a Paying Party that are not paid within ten (10) days of the date such payments are due under this Agreement shall bear interest, to the extent permitted by applicable law, at two percentage points above the base rate of interest declared from time to time by The First National Bank of Boston in Boston, Massachusetts, calculated on the number of days payment is delinquent.
48
13.9 Overriding Provisions of License Agreements.
(a) Notwithstanding any provision of Article 13, if the terms of any license agreement (including the Mitotix License Agreements) under which rights are sublicensed to a party pursuant to this Agreement require the licensee to make reports or royalty payments to the original licensor at different times than those specified herein, then the Paying Party shall make its royalty payments and reports hereunder and at such times and with such reporting details as will enable the licensee to comply with its obligations to the original licensor, if so required by the original licensor. In addition, the provisions of Sections 14.2 through 14.9 of this Agreement are expressly subject to any countervailing provision of a Mitotix License Agreement. In the event of any conflict between the provisions of Sections 14.2 through 14.9 of this Agreement and the provisions of any Mitotix License Agreement, the Mitotix License Agreement shall control. In addition, (i) to the extent that the material obligations of the Cyclin E License are not substantially set forth in this Agreement, DuPont Merck agrees, when and where applicable, to be bound by the terms of the Cyclin E License, (ii) DuPont Merck agrees to comply, when and where applicable, with the terms of the pl6 License, and (iii) DuPont Merck acknowledges that this Agreement, when and where applicable, shall be subject to and subordinate to the terms and conditions of the UBC9 License.
(b) Except as specifically otherwise provided hereunder, Mitotix shall be responsible for all costs and expenses with respect to the Mitotix License Agreements, including, but not limited to, any upfront payments, license maintenance fees, milestone payments, royalty payments and legal fees and disbursements.
(c) In the case of products developed by DuPont Merck that trigger a royalty payment pursuant to the pl6 License, DuPont Merck shall share equal responsibility for royalty payments on such products which may be owed to Cold Spring Harbor Laboratory under the pl6 License to the extent that such product is a Royalty-Bearing Product and the Royalty Term has ended with respect to such Royalty-Bearing Product. One-half of such payment which is due shall be paid by DuPont Merck directly to Cold Spring Harbor Laboratory and the remaining one-half of such payment shall be paid by Mitotix. DuPont Merck’s reporting obligations under Section 13.2 and Mitotix’s right to audit under Section 13.4 shall be in effect with respect to such payments to Cold Spring Harbor Laboratory under this paragraph.
(d) In the case of products developed by DuPont Merck that trigger a royalty payment pursuant to the pl6 License, DuPont Merck shall be responsible for royalty payments on such products which may be owed to Cold Spring Harbor Laboratory under the pl6 License, but only to the extent that DuPont Merck is not obligated to make any royalty or profit-sharing payments to Mitotix with respect to such product. Any such payment which is due shall be paid by DuPont Merck directly to Cold Spring Harbor Laboratory.
(e) In the case of products developed by DuPont Merck that trigger a royalty payment pursuant to the Cyclin E License, DuPont Merck shall be responsible for royalty payments on such products which may be owed to ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ Cancer Research Center under the Cyclin E License, but only to the extent that DuPont Merck is not obligated
49
to make any royalty or profit-sharing payments to Mitotix with respect to such product. Any such payment which is due shall be paid by DuPont Merck directly to ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ Cancer Research Center.
(f) Subject to Section 13.9(i), in the case of products developed by DuPont Merck that trigger a milestone payment pursuant to the pl6 License, DuPont Merck shall be responsible for milestone payments on such products which may be owed to Cold Spring Harbor Laboratory under the pl6 License, but only to the extent that the pl6 License requires a milestone payment for NDA filing with respect to such product and DuPont Merck is not obligated to make any milestone payments to Mitotix with respect to such product. Any such payment which is due shall be paid by DuPont Merck directly to Cold Spring Harbor Laboratory.
(g) Subject to Section 13.9(i), in the case of products developed by DuPont Merck that trigger a milestone payment pursuant to the Cyclin E License, DuPont Merck shall be responsible for milestone payments on such products which may be owed to ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ Cancer Research Center under the Cyclin E License, but only to the extent that DuPont Merck is not obligated to make any milestone payments to Mitotix with respect to such product. Any such payment which is due shall be paid by DuPont Merck directly to ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ Cancer Research Center.
(h) Subject to Section 13.9(i), in the case of products developed by DuPont Merck that trigger a milestone payment pursuant to the UBC9 License, DuPont Merck shall be responsible for milestone payments on such products which may be owed to Harvard University under the UBC9 License, but only to the extent that DuPont Merck is not obligated to make any milestone payment to Mitotix with respect to such product. Any such payment which is due shall be paid by DuPont Merck directly to Harvard University.
(i) Notwithstanding the foregoing, any and all milestone and royalty payments which may be owed to Mitotix’s licensor under the Mitotix License Agreements with respect to UBC Products which are subject to profit sharing under Section 11.5 shall be paid by Mitotix. Such milestone and royalty payments shall be UBC Research Development and Pre-Launch Marketing Costs or Allowable Expenses, as appropriate.
ARTICLE 14. PATENTS
14.1 Ownership of Inventions. All right, title and interest in all writings, inventions, discoveries, improvements and other technology, whether or not patentable or copyrightable, and any patent applications, patents or copyrights based thereon (collectively, the “Inventions”) that are made or conceived during and as a result of the Collaboration solely by employees of Mitotix or others acting on behalf of Mitotix (“Mitotix Inventions”) shall be owned by Mitotix. All Inventions that are made or conceived during and as a result of the Collaboration solely by employees of DuPont Merck or others acting on behalf of DuPont Merck (“DuPont Merck Inventions”) shall be owned by DuPont Merck. All Inventions that are made or conceived during and as a result of the Collaboration, jointly by employees or others acting on behalf of Mitotix and DuPont Merck (“Joint Inventions”) shall
50
be owned jointly by DuPont Merck and Mitotix. Each party shall promptly disclose to the other party the making, conception or reduction to practice of Inventions by employees or others acting on behalf of such party. Each party represents and agrees that all employees and other persons acting on its behalf in performing its obligations under this Agreement shall be obligated under a binding written agreement to assign to such party, or as such party shall direct, all Inventions made or conceived by such employee or other person, or in the case of non-employees working for other companies or institutions on behalf of a party, Mitotix or DuPont Merck, as applicable, shall have the right to license all Inventions made by such non-employees on behalf of Mitotix or DuPont Merck, as applicable, in accordance with the policies of said company or institution. Mitotix and DuPont Merck agree to enforce such agreements (including, where appropriate, by legal action) considering, among other things, the commercial value of such Inventions.
14.2 Provisions Concerning the Filing, Prosecution and Maintenance of CDK Patent Rights, UBC Patent Rights and Inventions.
14.2.1 CDK Patent Rights, UBC Patent Rights and Inventions Owned by Each Party. Mitotix shall be responsible for filing, prosecution and maintenance of patent applications covering CDK Patent Rights and UBC Patent Rights owned or licensed by Mitotix, to the extent permitted by the Mitotix License Agreements. Each party shall be responsible for filing, prosecution and maintenance of patent applications covering Inventions owned by such party. If a party does not file a patent application covering an Invention owned by such party within six months of the creation or discovery of such Invention, then the other party shall have the right to file a patent covering such Invention in the name of the party that created or discovered such Invention.
14.2.2 Joint Inventions Relating to CDK Targets and UBC Targets. Mitotix shall have the right to file, prosecute and maintain patent applications covering Joint Inventions relating to CDK Targets and UBC Targets in the names of both parties. If Mitotix does not file an application covering such Joint Invention within six months of the creation or discovery of such Joint Invention, then DuPont Merck shall have the right to file a patent covering such Joint Invention relating to CDK Targets and UBC Targets in the names of both parties.
14.2.3 Joint Inventions Relating to CDK Products and UBC Products. DuPont Merck shall have the right to file, prosecute and maintain patent applications covering Joint Inventions relating to CDK Products and UBC Products, including but not limited to patents covering compounds, formulations, pharmaceutical compositions, methods of use, and methods of synthesis, in the names of both parties. If DuPont Merck does not file an application covering such Mitotix Invention or Joint Invention within six months of the creation or discovery of such Joint Invention, then Mitotix shall have the right to file a patent covering such Joint Invention in the names of both parties.
14.2.4 Consultation Regarding Joint Inventions. A party filing a patent application with respect to Joint Inventions shall consult with the other as to the preparation and filing, prosecution and maintenance of such patent application and patents, and such party shall furnish to the other party copies of documents relevant to such preparation, filing,
51
prosecution or maintenance sufficiently prior to filing such document or making any payment due thereunder to allow for review and comments by the non-filing party.
14.2.5 Patent Costs. Mitotix shall bear all costs associated with filing, prosecuting and maintaining patent applications covering CDK Patent Rights and UBC Patent Rights owned or licensed by Mitotix, unless such costs are being borne by a licensor under a Mitotix License Agreement. Each party shall bear all costs associated with filing, maintaining and prosecuting any patents or patent applications relating to Inventions owned by such party or filed in the name of the other party. Each party shall bear fifty percent (50%) of all costs associated with filing, maintaining or prosecuting patents or patent applications relating to Joint Inventions. If either party elects at any time not to continue to und its portion of the costs associated with filing, maintaining or prosecuting patents or patent applications relating to Joint Inventions, the party shall so notify the other party in sufficient time for the other party to assume all such costs; provided, however, that the electing party shall remain obligated to pay for or to reimburse the other party for any costs with respect to patents or patent applications relating to the Joint Inventions incurred prior to such election. The electing party will relinquish all rights it may have in the Joint Invention and will execute any and all documents reasonably necessary to permit the party assuming such costs to file, maintain and prosecute patents or patent applications relating to the Joint Invention in its own name.
14.3 Cooperation. Each party shall make available to the other party or to the other party’s authorized attorneys, agents or representatives, its employees, agents or consultants to the extent necessary or appropriate to enable the appropriate party to file, prosecute and maintain patent applications and resulting patents with respect to Inventions and for periods of time sufficient for such party to obtain the assistance it needs from such personnel. Where appropriate, each party shall sign or cause to have signed all documents relating to said patent applications or patents at no charge to the other.
14.4 No Other Technology Rights. Except as otherwise expressly provided in this Agreement, under no circumstances shall a party hereto, as a result of this Agreement, obtain any ownership interest in or other right to any technology, know-how, patents, pending patent applications, compounds, products, antibodies, cell lines or cultures, or animals of the other party, including items owned, controlled or developed by the other party, or transferred by the other party to said party at any time pursuant to this Agreement.
14.5 Enforcement of Patent Rights. Mitotix and DuPont Merck shall promptly notify the other in writing of any alleged or threatened infringement of CDK Patent Rights in the CDK Field or of UBC Patent Rights in the UBC Field of which they become aware. Mitotix and DuPont Merck shall then confer and may agree jointly to prosecute any such infringement. Mitotix shall control the joint litigation in the event of any dispute between the parties with respect to any aspect of the litigation. If the parties do not agree on whether or how to proceed with enforcement activity within (i) ninety (90) days following the notice of alleged infringement or (ii) ten (10) business days before the time limit, if any, set forth in the appropriate laws and regulations for the filing of such actions, whichever comes first, then either party may act in its own name to commence litigation with respect to the alleged or threatened infringement. In the event a party brings an infringement action, the other
52
party shall cooperate fully, including, if required to bring such action, the furnishing of a power of attorney. Neither party shall have the right to settle any patent infringement litigation under this Section in a manner that diminishes the rights or interests of the other party without the express written consent of such other party.
The costs of any joint litigation commenced pursuant to this Section, including attorneys’ fees and expenses, shall be borne equally by the parties (unless they agree to a different cost sharing arrangement in any particular matter), with such costs to be accounted for by equalizing payments to be made on a quarterly basis. Only out-of-pocket costs shall be accounted for and reimbursed under this Section, without any allocation for internal resources devoted to the litigation. Except as otherwise agreed to by the parties as part of a cost sharing arrangement, any recovery realized as a result of such joint litigation shall be shared equally by the parties (unless they agree beforehand to a different sharing of such recovery).
14.6 Defense of Individual Infringement Actions Involving Use of the Targets. If Mitotix or DuPont Merck, or any of their respective Affiliates, shall be individually named as a defendant in a legal proceeding by a Third Party for infringement of a patent because of the use of CDK Targets in the CDK Field or the use of the UBC Targets in the UBC Field, the party which has been sued shall promptly notify the other party in writing of the institution of such suit. The party which has been sued shall be entitled to control the defense of such suit, and all out-of-pocket expenses including out-of-pocket costs, outside attorney fees, adverse judgments or settlement amounts incurred on account of DuPont Merck or Mitotix as the case may be shall be paid as set forth under Section 14.8 below. For Royalty-Bearing Products, DuPont Merck may deduct its share of such out-of-pocket expenses, for the purpose of determining the amount of Net Sales of Royalty-Bearing Products sold in the country where such infringement action is maintained. Such deduction shall continue from year-to-year until such out-of-pocket expenses have been deducted in full. The controlling party may not settle such suit or otherwise consent to an adverse judgment in such suit that diminishes the rights or interests of the non-controlling party without the express written consent of the non-controlling party. The party which has been sued shall keep the other party at all times reasonably informed as to the status of the suit. The party which is not controlling such legal proceedings shall have the right to be represented by advisory counsel of its own selection (and such counsel’s opinion shall be reasonably considered by the controlling party), at its own expense, and shall cooperate fully in the defense of such suit and reasonably cooperate in furnishing to the party controlling such legal proceedings all evidence and assistance in its control.
14.7 Defense of Joint Infringement Actions Involving Use of the Targets. If Mitotix and DuPont Merck, or any of their respective Affiliates, shall be jointly named as defendants in a legal proceeding by a Third Party or shall be joined in the same litigation for infringement of a patent because of the use of CDK Targets in the CDK Field or use of UBC Targets in the UBC Field, DuPont Merck shall be entitled to control the defense of such suit, and all out-of-pocket expenses including out-of-pocket costs, outside attorney fees, adverse judgments or settlement amounts incurred on account of DuPont Merck or Mitotix or both shall be paid as set forth under Section 14.8 below. For Royalty-Bearing Products, DuPont Merck may deduct its share of such out-of-pocket expenses, for the purpose of determining
53
the amount of Net Sales of Royalty-Bearing Products sold in the country where such infringement action is maintained. Such deduction shall continue from year-to-year until such out-of-pocket expenses have been deducted in full. Mitotix shall have the right to be represented by counsel of its own selection, but at its sole expense, and shall cooperate fully in the defense of such suit and reasonably cooperate in furnishing to DuPont Merck all evidence and assistance in its control. DuPont Merck shall, however, not be entitled to settle such suit or otherwise consent to an adverse judgment in such suit without the express written consent of Mitotix if such settlement or adverse judgment diminishes any right or interest of Mitotix hereunder.
14.8 Contribution. With respect to all such out-of-pocket expenses (including out- of-pocket costs, outside attorneys fees, judgments, or settlement amounts) payable with respect to legal proceedings covered by Sections 14.6 and 14.7, Mitotix shall contribute fifty percent (50%) of the amount owed or expended and DuPont Merck shall pay the remainder.
14.9 Third Party Patents. If DuPont Merck, in its reasonable judgment, is required to obtain a license from any Third Party under any patent in order to use the CDK Targets in the CDK Field or use the UBC Targets in the UBC Field, and to pay a royalty under such license, and the infringement of such patent cannot reasonably be avoided by DuPont Merck without such a license, DuPont Merck’s obligation to pay royalties for a Royalty-Bearing Product based on such use under Article 12 hereof shall be reduced by the amount of the royalty payable by DuPont Merck under such additional license, provided, however, that the royalties otherwise payable under Article 12 hereof shall not be reduced in any such event by more than one-half (1/2). If DuPont Merck is required by a court of competent jurisdiction to pay a royalty to any Third Party under any patent with respect to use of the CDK Targets in the CDK Field or use of the UBC Targets in the UBC Field, DuPont Merck’s obligation to pay royalties for a Royalty-Bearing Product based on such use under Article 12 hereof shall be reduced by the amount of the royalty payable by DuPont Merck to such Third Party, provided, however, that the royalties otherwise payable under Article 12 hereof shall not be reduced in any such event by more than one-half (1/2).
14.10 Infringement of Mitotix Inventions or Joint Inventions Covering CDK Products or UBC Products.
(a) Mitotix and DuPont Merck each shall immediately give notice to the other of any potential infringement by a Third Party of any patent rights covering Mitotix Inventions and Joint Inventions relating to CDK Products or UBC Products, including but not limited to patents covering compounds, formulations, pharmaceutical compositions, methods of use, and methods of synthesis, (collectively, “Product Patent Rights”) of which they become aware or of any certification of which they become aware filed under the United States “Drug Price Competition and Patent Term Restoration Act of 1984” claiming that Product Patent Rights covering a CDK Product or UBC Product are invalid or unenforceable or that infringement will not arise from the manufacture, use or sale of a CDK Product or UBC Product by a Third Party.
(b) DuPont Merck as exclusive licensee of such Product Patent Rights will have the right to settle with the infringer or to bring suit or other proceeding at its expense
54
against the infringer in its own name or in the name of Mitotix where necessary, after consultation with Mitotix. Mitotix shall be kept advised at all times of such suit or proceedings brought by DuPont Merck. Mitotix may, in its discretion, join with DuPont Merck as party to the suit or other proceeding, provided that DuPont Merck shall retain control of the prosecution of such suit or proceedings in such event. Mitotix agrees to cooperate with DuPont Merck in its efforts to protect such Product Patent Rights, including joining as a party where necessary.
(c) If DuPont Merck does not settle with the infringer of such Product Patent Rights or bring suit or other proceeding against the infringer, upon DuPont Merck’s prior written approval Mitotix may in its discretion, bring suit or other proceeding at its expense against the infringer. Mitotix shall first consult with DuPont Merck as to whether such act(s) by a Third Party reasonably constitute infringement and whether it is commercially advisable to bring such suit or proceeding as reasonably determined by DuPont Merck. In the event that Mitotix brings such suit or other proceeding, DuPont Merck shall be kept advised at all times of such suit or proceedings. In such event, DuPont Merck may, in its discretion, join with Mitotix as party to the suit or other proceeding, provided that Mitotix shall retain control of the prosecution of such suit or proceedings. In the event that Mitotix brings such suit or proceeding, DuPont Merck agrees to cooperate with Mitotix in its efforts to protect Product Patent Rights, including joining as a party where necessary.
(d) With respect to any suit or other proceeding against an infringer of the Product Patent Rights covering a CDK Product or a UBC Product when the Alternate UBC Plan is in effect, this Section 14.10(d) shall apply. Each party will bear its own expenses with respect to any suit or other proceeding against an infringer of the Product Patent Rights. Any recovery in connection with such suit or proceeding will first be applied to reimburse Mitotix and DuPont Merck for their out-of-pocket expenses, including attorney’s fees. The balance of any recovery shall be distributed as follows: (i) as to ordinary damages, DuPont Merck shall receive an amount equal to its lost profits or a reasonable royalty on the sales of the infringer (whichever measure of damages the court shall have applied), less a reasonable approximation of the royalties that DuPont Merck would have owed to Mitotix on sales of products that DuPont Merck lost to the infringer, which amount shall be received by Mitotix, and (ii) as to special or punitive damages, such amount shall be allocated between the parties in the same proportion as ordinary damages are allocated pursuant to clause (i).
(e) With respect to any suit or other proceeding against an infringer of the Product Patent Rights covering a UBC Product when the Alternate UBC Plan is not in effect, this Section 14.10(e) shall apply. Each party will bear its own expenses with respect to any suit or other proceeding against an infringer of the Product Patent Rights and such expenses shall be Allowable Expenses. Any recovery in connection with such suit or proceeding will be shared in accordance with the party’s respective Percentage Contribution.
ARTICLE 15. CONFIDENTIALITY
15.1 Nondisclosure Obligations. Except as otherwise provided in this Article 15 and subject to Section 19.13, during the term of this Agreement and for a period of ten (10)
55
years thereafter, both parties shall maintain in confidence and use only for purposes permitted by this Agreement (i) confidential information and data received from the other party resulting from or related to the use of CDK Targets or UBC Targets for the development of CDK Development Compounds or UBC Development Compounds, respectively; and (ii) all information and data not described in clause (i) above but supplied by the other party under this Agreement marked “Confidential.”
For purposes of this Article 15, information and data described in clause (i) or (ii) shall be referred to as “Information.” To the extent it is reasonably necessary or appropriate to fulfil its obligations or exercise its rights under this Agreement, a party may disclose Information it is otherwise obligated under this Section not to disclose to its Affiliates, sublicensees, consultants, outside contractors and clinical investigators, and in the case of DuPont Merck, the DuPont Merck Partnership Board on a need-to-know basis on condition that such Affiliates and Third Parties agree to keep the Information confidential for the same time periods and to the same extent as such party is required to keep the Information confidential under this Agreement; and a party or its sublicensees may disclose such Information to government or other regulatory authorities to the extent that such disclosure is reasonably necessary to obtain patents or authorizations to conduct clinical trials with and to commercially market any product. The obligation not to disclose Information shall not apply to any part of such Information that (i) is properly in the public domain; (ii) is disclosed to the receiving party by a Third Party who may lawfully do so and is not under an obligation of confidentiality to the disclosing party; (iii) is known by the receiving party at the time of its receipt, and not through a prior disclosure by the disclosing party, as documented by business records; or (iv) can be shown by written documents to have been independently developed by the receiving party without reference to the Information received from the disclosing party and without breach of any of the provisions of this Agreement.
15.2 Samples. Samples of compounds synthesized, purified or developed in the course of the CDK Research Program or the UBC Research Program shall not be supplied or sent by either party to any Third Party unless protected by an appropriate materials transfer and confidentiality agreement in accordance with the provisions and objectives of this Agreement.
15.3 Terms of this Agreement and Existence of UBC Collaboration. Mitotix and DuPont Merck each agrees (i) not to disclose any terms or conditions of this Agreement to any Third Party without the prior consent of the other party, except as required by applicable law and (ii) not to disclose to any Third Party that Mitotix and DuPont Merck are collaborating in the UBC Field unless the Extended UBC Collaboration is in effect, or unless the parties agree otherwise. Notwithstanding the foregoing, (i) DuPont Merck and Mitotix shall agree in writing upon the substance of information that can be used in a press release to describe the terms of this transaction, and DuPont Merck and Mitotix may disclose such information, as modified by prior written mutual agreement from time to time; provided that the disclosing party shall provide the other party with a copy of each such press release, (ii) each party shall be entitled to issue press releases or to otherwise to announce significant achievements in preclinical research, regulatory approval and/or the development process for CDK Products or UBC Products; provided, however, that such disclosures shall be subject to prior written approval by the other party, (iii) Mitotix may disclose the terms of this
56
Agreement to licensors of rights sublicensed hereunder, to potential licensees of rights that Mitotix has the power to grant under this Agreement and to potential investors in Mitotix (and professional advisors of such investors) who require such information for due diligence purposes, provided that in each case where disclosure is made under this clause (iii), Mitotix shall take all reasonable steps to minimize the extent of such disclosure, shall make such disclosures under appropriate confidentiality restrictions and shall inform DuPont Merck of the disclosures made.
15.4 Publications. During the term of this Agreement, Mitotix and DuPont Merck each acknowledge the other party’s interest in publishing certain of its results to obtain recognition within the scientific community and to advance the state of scientific knowledge. Each party also recognizes the mutual interest in obtaining valid patent protection and in protecting business interests and trade secret information. Consequently, either party, its employees or consultants wishing to make a publication (including any oral disclosure made without obligation of confidentiality) relating to work performed by such party as part of the Collaboration (the “Publishing Party”) shall deliver to the other party (the “Reviewing Party”) a copy of the proposed written publication at least sixty (60) days prior to submission for publication or presentation, or an abstract of such oral disclosure at least thirty (30) days prior to submission of the abstract or the oral disclosure, whichever is earlier. The Reviewing Party shall have the right (a) to propose modifications to the publication to protect proprietary information and (b) to request a reasonable delay in publication or presentation in order to protect proprietary information.
If the Reviewing Party requests such a delay, the Publishing Party shall delay submission or presentation of the publication for a period of ninety (90) days to enable patent applications protecting each party’s rights in such information to be filed or to otherwise revise the publication to protect proprietary information. If no request for delay or modification of the proposed publication is made within the applicable sixty (60) or thirty (30) day period referred to in the preceding paragraph, the Publishing Party shall be free to proceed with the publication or presentation. If the Reviewing Party requests modifications to the publication, the Publishing Party shall edit such publication to prevent disclosure of trade secret or proprietary business information prior to submission of the publication or presentation. In addition, the contributions of the parties to the work described in the publication shall be expressly noted in such publications or other public disclosures by acknowledgment or co-authorship, whichever is appropriate.
ARTICLE 16. REPRESENTATIONS AND WARRANTIES
16.1 Authorization. Each party warrants and represents to the other that it has the legal right and power to enter into this Agreement, to extend the rights and licenses granted to the other in this Agreement, and to fully perform its obligations hereunder, and that it has not made nor will it make any commitments to others in conflict with or in derogation of such rights or this Agreement. Except as otherwise disclosed, each party further represents to the other that it is not aware of any legal obstacles, including patent rights of others, which could prevent it from carrying out the provisions of this Agreement.
57
16.2 License Agreements with Third Parties. Mitotix warrants and represents to DuPont Merck that each Mitotix License Agreement authorizes a sublicensee thereunder to perform under the license agreement in place of Mitotix, or provides for the assignment of the sublicense to the Third Party licensor, in the event that Mitotix were to breach its obligations under the license agreement or to file for bankruptcy, and in such case the license agreement will remain in effect between the sublicensee and the Third Party licensor provided that DuPont Merck is not in breach of this Agreement or any terms of the applicable Mitotix License Agreement. Mitotix shall use good faith commercially reasonable efforts to negotiate each Mitotix Pending License Agreement to include a provision which authorizes a sublicensee thereunder to perform under the license agreement in place of Mitotix in the event that Mitotix were to breach its obligations under the license agreement or to file for bankruptcy, and in such case the license agreement will remain in effect between the sublicensee and the Third Party licensor. If DuPont Merck shall be required to make payment directly to a Third Party licensor in order to preserve the rights sublicensed to DuPont Merck under a Mitotix License Agreement, DuPont Merck shall be entitled to reduce any payments otherwise due Mitotix under this Agreement, by an amount equal to what DuPont Merck is obligated to pay to the Third Party licensor. Mitotix represents and warrants that Appendices A, D and E are accurate and complete and identify all Mitotix License Agreements and Mitotix Pending License Agreements, respectively. Mitotix shall use all good faith commercially reasonable efforts to successfully complete at its own expense the Mitotix Pending License Agreements. Mitotix warrants and represents that it shall use all good faith efforts to fully perform all of its obligations under the Mitotix License Agreements. Mitotix shall provide prompt notice to DuPont Merck of any dispute involving the Mitotix License Agreements.
16.3 Patent Validity. Nothing in this Agreement shall be construed as a warranty or representation by either party as to the validity or scope of any CDK Patent Rights or UBC Patent Rights. Mitotix represents and warrants that Appendix A and Appendix D are accurate and complete and identify all patent rights owned or licensed to Mitotix as of the Effective Date which are necessary or useful for the use of the CDK Targets and UBC Targets in accordance with the objectives of this Agreement.
16.4 Exclusivity and Freedom-to-operate. Mitotix represents and warrants that, except for certain material transfer agreements with academic institutions under which Mitotix has the first option to license any research results, no other person or organization presently has any effective option or license from Mitotix to use the CDK Patent Rights or UBC Patent Rights, or is authorized to use the Mitotix Know-how, in the CDK Field or UBC Field. Mitotix represents and warrants that, to the best of its knowledge and belief, the use of the CDK Patent Rights, UBC Patent Rights, and Mitotix Know-how in the CDK Field and UBC Field as contemplated in this Agreement does not infringe any Third Party patents or other rights.
ARTICLE 17. INDEMNITY
17.1 DuPont Merck Indemnity Obligations. In the absence of Mitotix’s negligence or a breach of warranty by Mitotix, DuPont Merck agrees to defend, indemnify and hold
58
Mitotix, its Affiliates and their respective employees and agents harmless from all claims, losses, damages or expenses arising as a result of (a) actual or asserted violations of any applicable law or regulation by DuPont Merck, its Affiliates or sublicensees by virtue of which CDK Products or UBC Products manufactured, distributed or sold shall be alleged or determined to be adulterated, misbranded, mislabeled or otherwise not in compliance with any applicable law or regulation; (b) claims for bodily injury, death or property damage attributable to the manufacture, distribution, sale or use of CDK Products or UBC Products by DuPont Merck, its Affiliates or sublicensees; (c) a CDK Product or UBC Product recall ordered by a governmental agency or required by a confirmed CDK Product or UBC Product failure as reasonably determined by the parties hereto; or (d) claims that any CDK Product or UBC Product infringes the intellectual property rights of a Third Party.
17.2 Mitotix Indemnity Obligations. In the absence of DuPont Merck’s negligence or a breach of warranty by DuPont Merck, Mitotix agrees to defend, indemnify and hold DuPont Merck, its Affiliates and parent companies and their respective employees and agents harmless from all claims, losses, damages or expenses relating to Mitotix’s performance under this Agreement and arising as a result of the negligence, unlawful act, or willful misconduct of Mitotix or its subcontractors or agents, and not caused by the negligence of DuPont Merck.
17.3 Procedure. Should a party or any of its Affiliates or its employees or agents (the “Indemnitee”) intend to claim indemnification under this Article 17, such Indemnitee shall promptly notify the other party (the “Indemnitor”) in writing of any loss, claim, damage, liability or action in respect of which the Indemnitee intends to claim such indemnification, and the Indemnitor shall be entitled to assume the defense thereof with counsel selected by the Indemnitor and approved by the Indemnitee, such approval not to be unreasonably withheld; provided, however, that if representation of Indemnitee by such counsel first selected by the Indemnitor would be inappropriate due to a conflict of interest between such Indemnitee and any other party represented by such counsel, then Indemnitor shall select other counsel for the defense of Indemnitee, with the fees and expenses to be paid by the Indemnitor, such other counsel to be approved by Indemnitee and such approval not to be unreasonably withheld. The indemnity agreement in this Article 17 shall not apply to amounts paid in settlement of any loss, claim, damage, liability or action if such settlement is effected without the consent of the Indemnitor, which consent shall not be withheld unreasonably. The failure to deliver notice to the Indemnitor within a reasonable time after the commencement of any such action, if prejudicial to its ability to defend such action, shall relieve such Indemnitor of any liability to the Indemnitee under this Article 17, but the omission so to deliver notice to the Indemnitor will not relieve it of any liability that it may have to any Indemnitee otherwise than under this Article 17. The Indemnitee under this Article 17, its employees and agents, shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action, claim or liability covered by this indemnification. In the event that each party claims indemnity from the other and one party is finally held liable to indemnify the other, the Indemnitor shall additionally be liable to pay the reasonable legal costs and attorneys’ fees incurred by the Indemnitee in establishing its claim for indemnity.
59
17.4 Insurance. DuPont Merck and Mitotix shall each maintain product liability insurance with respect to development, manufacture and sales of CDK Products or UBC Products by DuPont Merck or Mitotix Products by Mitotix, respectively, in such amount as DuPont Merck or Mitotix, respectively, customarily maintains with respect to sales of its other products. DuPont Merck or Mitotix, as applicable, shall maintain such insurance for so long as it continues to manufacture or sell any CDK Products, UBC Products or Mitotix Products, and thereafter for so long as DuPont Merck or Mitotix, as applicable, maintains insurance for itself covering such manufacture or sales. In the case of DuPont Merck, such insurance may be self-insurance.
ARTICLE 18. TERM AND TERMINATION
18.1 Expiration. Unless terminated earlier pursuant to Section 18.2 below, this Agreement shall expire on the expiration of all royalty and profit sharing obligations under this Agreement.
18.2 Termination for Cause. This Agreement may be terminated upon the occurrence of any of the following.
18.2.1 Bankruptcy. Either party may terminate this Agreement upon or after the bankruptcy, insolvency, dissolution or winding up of the other party (other than dissolution or winding up for the purposes of reconstruction or amalgamation).
18.2.2 Material Breach. Either party may terminate this Agreement upon or after the breach of any material provision of this Agreement by the other party if the breaching party has not commenced to cure such breach within thirty (30) days after notice thereof by the other party and has not thereafter proceeded diligently to cure such breach within a reasonable time. In no event shall such reasonable time to cure such breach exceed one hundred eighty (180) days from the date of such notice.
18.2.3 Failure to Retain Qualified Scientists. DuPont Merck may terminate this Agreement in the event that Mitotix is unable to retain sufficient qualified scientists to provide commercially reasonable support for the CDK Collaboration or UBC Collaboration or to fulfill its obligations in the CDK Research Program, as described in Sections 3.1.1 and 3.2; provided, however, that DuPont Merck shall not have the right to terminate under this Section 18.2.3 unless (a) Mitotix is given six (6) months prior written notice by DuPont Merck of DuPont Merck’s intent to terminate, stating the reasons and justification for such termination, and (b) Mitotix has not taken good faith commercially reasonable steps during such six (6) months period to correct such stated deficiencies.
18.3 Effect of Termination. Expiration or termination of this Agreement shall not relieve the parties of any obligation accruing prior to such expiration or termination. Without limiting the foregoing, in the event of expiration or termination of this Agreement, for any reason (i) all licenses granted to DuPont Merck hereunder will terminate, except that DuPont Merck shall retain an exclusive license under the Mitotix Inventions, Joint Inventions and applicable Know-how solely to develop, make, have made, use and/or sell any CDK
60
Product or UBC Product incorporating compounds that at the time of termination or expiration of the Agreement have been designated as a Development Compound (ii) the provisions of Articles 11 and 12 will survive termination of the Agreement such that DuPont Merck will continue to have milestone and royalty obligations to Mitotix with respect to Royalty-Bearing Products where applicable; and Mitotix and DuPont Merck will continue to share in UBC Operating Profits in accordance with Section 11.5 where applicable; and Mitotix will continue to have royalty obligations to DuPont Merck with respect to Mitotix Products; (iii) the provisions of Section 13.9 will survive termination of the Agreement such that DuPont Merck will continue to have milestone and royalty obligations to certain Mitotix licensors where applicable, and (iv) the provisions of Section 5.5 will survive termination of the Agreement.
18.4 Failure to Pursue. If DuPont Merck is not diligently pursuing the discovery or development of either CDK Products or UBC Products using good faith commercially reasonable efforts in accordance with industry standards and consistent with the usual practice followed by DuPont Merck in pursuing the discovery or development of its other similar pharmaceutical products, then Mitotix shall have the right to terminate the license right granted to DuPont Merck pursuant to Section 10.1.1 (in the event that DuPont Merck is not diligently pursuing the discovery or development of any CDK Products) or Section 10.2.1 (in the event that DuPont Merck is not diligently pursuing the discovery or development of any UBC Products). Mitotix shall not have the right to terminate under this Section 18.4 unless (a) DuPont Merck is given six (6) months prior written notice by Mitotix of Mitotix’s intent to terminate, stating the reasons and justification for such termination and recommending steps which DuPont Merck should take in such development, and (b) DuPont Merck has not taken good faith commercially reasonable steps during such six (6) month period to diligently pursue the discovery or development of such CDK Products or UBC Products. Notwithstanding the foregoing, Mitotix shall in no event have the right to terminate such license if the development of such product is not being pursued on the basis that a competitive product is being diligently developed by DuPont Merck pursuant to this Agreement, or DuPont Merck reasonably determines in good faith and consistent with industry standards that such product is unlikely to yield satisfactory results in clinical trials or regulatory submissions, or that such product is commercially unattractive. Upon the termination of any such license grants, all rights licensed to DuPont Merck thereunder shall be returned to Mitotix. Upon Mitotix’s termination of one or more of the above-referenced licenses, and at the request of Mitotix, DuPont Merck shall enter into good faith negotiations with Mitotix regarding the grant of a license to Mitotix to pursue further development of the Development Compound Mitotix asserts is not being diligently pursued by DuPont Merck in the CDK Field or the UBC Field.
ARTICLE 19. MISCELLANEOUS
19.1 Force Majeure. Neither party shall be held liable or responsible to the other party nor be deemed to have defaulted under or breached this Agreement for failure or delay in fulfilling or performing any term of this Agreement when such failure or delay is caused by or results from causes beyond the reasonable control of the affected party including but not limited to fire, floods, embargoes, war, acts of war (whether war is declared or not),
61
insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, acts of God or acts, omissions or delays in acting by any governmental authority or the other party. Either party shall provide the other party with prompt written notice of any delay or failure to perform that occurs by reason of force majeure and the parties shall discuss in good faith any solution to such situation.
19.2 Assignment. This Agreement may not be assigned or otherwise transferred by either party without the consent of the other party; provided, however, that either Mitotix (subject to Section 19.13) or DuPont Merck may, without such consent, assign this Agreement and its rights and obligations hereunder to its Affiliates or in connection with the transfer or sale of all or substantially all of its business, or in the event of its merger or consolidation or change in control or similar transaction. Any purported assignment in violation of the preceding sentences shall be void. Any permitted assignee shall assume all obligations of its assignor under this Agreement.
19.3 Severabilitv. Each party hereby agrees that it does not intend to violate any public policy, statutory or common laws, rules, regulations, treaty or decision of any government agency or executive body thereof of any country or community or association of countries. Should one or more provisions of this Agreement be or become invalid, the parties hereto shall substitute, by mutual consent, valid provisions for such invalid provisions which valid provisions in their economic effect are sufficiently similar to the invalid provisions that it can be reasonably assumed that the parties would have entered into this Agreement with such valid provisions. In case such valid provisions cannot be agreed upon, the invalidity of one or several provisions of this Agreement shall not affect the validity of this Agreement as a whole, unless the invalid provisions are of such essential importance to this Agreement that it is to be reasonably assumed that the parties would not have entered into this Agreement without the invalid provisions.
19.4 Notices. Any consent, notice or report required or permitted to be given or made under this Agreement by one of the parties hereto to the other shall be in writing, and delivered personally or by facsimile (and promptly confirmed by personal delivery or overnight courier) or by a nationally-recognized overnight courier, addressed to such other party at its address indicated below, or to such other address as the addressee shall have last furnished in writing to the addressor and shall be effective upon receipt by the addressee.
| If to | ||
| Mitotix: | Mitotix, Inc. | |
| ▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇ | ||
| ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | ||
| Attention: President | ||
| Telephone: (▇▇▇) ▇▇▇-▇▇▇▇ | ||
| Telecopy: (▇▇▇) ▇▇▇-▇▇▇▇ |
62
| with a copy to: |
▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇, Esquire ▇▇▇▇▇▇ & Dodge ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ Telephone: (▇▇▇) ▇▇▇-▇▇▇▇ Telecopy: (▇▇▇) ▇▇▇-▇▇▇▇ |
| If to |
||
| DuPont Merck: | The DuPont Merck Pharmaceutical Company Experimental Station, ▇▇▇▇ ▇▇▇▇▇ ▇▇▇ ▇▇▇ ▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇ Attention: President, DuPont Merck Research Laboratories Telephone: ▇▇▇-▇▇▇-▇▇▇▇ Fax: ▇▇▇-▇▇▇-▇▇▇▇ |
| with a copy to: |
Associate General Counsel Legal Department The DuPont Merck Pharmaceutical Company ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇ Fax: ▇▇▇-▇▇▇-▇▇▇▇ |
19.5 Applicable Law. This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware without reference to any rules of conflict of laws, except that all questions concerning the construction or effect of patent rights will be construed in accordance with the laws of the country granting those rights.
19.6 Dispute Resolution. Subject to Section 9.3, any disputes arising between the parties relating to, arising out of or in any way connected with this Agreement or any term or condition hereof, or the performance by either party of its obligations hereunder, whether before or after termination of this Agreement, shall be promptly presented to the Chief Executive Officers of Mitotix and DuPont Merck for resolution. Such chief executive officers shall attempt in good faith to promptly resolve such dispute. If such officers are unable to resolve such dispute, any litigation instituted by DuPont Merck shall, unless otherwise agreed to in writing by Mitotix, be filed in a Massachusetts federal or state court located in Boston and any litigation instituted by Mitotix shall, unless otherwise agreed to in writing by DuPont Merck, be filed in a Delaware federal or state court located in Wilmington. Each party hereby irrevocably consents to the personal jurisdiction of such courts.
19.7 Entire Agreement. This Agreement, including the Appendices and Exhibits attached hereto, contains the entire understanding of the parties with respect to the subject matter hereof. All express or implied agreements and understandings, either oral or written, heretofore made are expressly merged in and made a part of this Agreement. This Agreement may be amended, or any term hereof modified, only by a written instrument duly executed by both parties hereto.
63
19.8 Headings. The captions to the several Articles and Sections hereof are not a part of this Agreement, but are merely guides or labels to assist in locating and reading the several Articles and Sections hereof.
19.9 Independent Contractors. It is expressly agreed that Mitotix and DuPont Merck shall be independent contractors and that the relationship between the two parties shall not constitute a partnership, joint venture or agency, except in the event and to the extent that a partnership or similar entity is formed between the parties pursuant to Section 11.5 Neither Mitotix nor DuPont Merck shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other, without the prior consent of the other party to do so.
19.10 Agreement Not to Solicit Employees. During the term of this Agreement and for a period of two (2) years following the termination of this Agreement, Mitotix and DuPont Merck agree not to seek to persuade or induce any employee of the other company to discontinue his or her employment with that company in order to become employed by or associated with any business, enterprise or effort that is associated with its own business.
19.11 Waiver. The waiver by either party hereto of any right hereunder or the failure to perform or of a breach by the other party shall not be deemed a waiver of any other right hereunder or of any other breach or failure by said other party whether of a similar nature or otherwise.
19.12 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
19.13 Definition and Effect of Change of Control. In the event that, any time before three (3) years after the termination or expiration, for any reason, of the UBC Research Program, any of the following events occur: (i) the acquisition of fifty percent (50%) or more of the voting stock or other ownership interest in Mitotix, by a Third Party that DuPont Merck, in good faith, determines to be a competitor of DuPont Merck, or (ii) the acquisition, directly or indirectly, of the power to direct or cause the direction of the management and policies of Mitotix or the power to elect or appoint fifty percent (50%) or more of the members of the governing body of Mitotix, by a Third Party that DuPont Merck, in good faith, determines to be a competitor of DuPont Merck, or (iii) the acquisition of all or substantially all of Mitotix’s assets employed or used in the Collaboration by a Third Party that DuPont Merck, in good faith, determines to be a competitor of DuPont Merck (any of the events discussed above shall be referred to herein as a “Change of Control”), then DuPont Merck shall, prior to the effective date of such Change of Control, or if such Change of Control occurs without the approval of the Mitotix Board of Directors, within thirty (30) days after the first public announcement thereof, notify Mitotix of its determination that such acquisition will constitute a Change in Control.
Upon receipt of such notification, Mitotix (or its successor) shall put into place appropriate safeguards to prevent the disclosure to the competitor of DuPont Merck Information or Know-how and technical Mitotix Information or Mitotix Know-how relating
64
specifically to the Collaboration. Such safeguards will include the maintenance of the facilities and personnel used in Mitotix’s performance under this Agreement separate from the operations and facilities of the DuPont Merck competitor. Without limitation, in no event shall chemical structures relating to the Collaboration be disclosed to the DuPont Merck competitor.
Mitotix (or its successor) shall prepare and present to DuPont Merck within thirty (30) days after receipt of notification from DuPont Merck of its determination that such acquisition will constitute a Change of Control, a written plan of operation under which Mitotix or its successor will establish and maintain the safeguards required by this Section and continue to perform all of its obligations under this Agreement. Within thirty (30) days after its receipt of such plan, DuPont Merck will either approve such plan or respond in writing with reasonable suggestions for modification of the plan. The plan will be modified to include such reasonable suggestions. Following a Change of Control, Mitotix (or its successors) shall continue to make good faith commercially reasonable efforts in support of the CDK and UBC Collaboration and shall make good faith efforts to continue the CDK and UBC Collaboration and cooperate with DuPont Merck in the same manner as before the Change of Control.
In the event of a Change of Control, DuPont Merck shall have the rights set forth in Section 3.2.l(c) and 5.7 above.
IN WITNESS WHEREOF, the parties have executed this Agreement as of the date first set forth above.
| MITOTIX, INC. | ||
| By: | /s/ ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ | |
| ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ | ||
| Title: Chairman | ||
| THE DUPONT MERCK PHARMACEUTICAL COMPANY | ||
| By: | /s/ Paul GV ▇▇▇▇▇ | |
| ▇▇▇▇ ▇▇ ▇▇▇▇▇ | ||
| Title: President, CEO | ||
65
APPENDIX A
CDK PATENT RIGHTS AND CERTAIN
MITOTIX LICENSE AGREEMENTS
| Cvclin D/p16 | ||||
| (1) |
U.S.S.N.: | 7/701,514 | ||
| Filed: | May 16, 1991 | |||
| Title: | “D-Type Cyclin and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (2) |
U.S.S.N.: | 7/888,178 | ||
| Filed: | May 26, 1992 | |||
| Title: | “D-Type Cyclin and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (3) |
U.S.S.N.: | 8/324,526 | ||
| Filed: | May 26, 1992 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (4) |
U.S.S.N.: | 7/963,308 | ||
| Filed: | October 16, 1992 | |||
| Title: | “D-Type Cyclin and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (5) |
U.S.S.N.: | 7/991,997 | ||
| Filed: | December 17, 1992 | |||
| Title: | “Cyclin Complex Rearrangement and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al. | |||
| (6) |
U.S.S.N.: | 8/154,915 | ||
| Filed: | November 18,1993 | |||
| Title: | “Cyclin Complex Rearrangement and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al. | |||
| (7) |
U.S.S.N.: | 8/227,371 | ||
| Filed: | April 14, 1994 | |||
| Title: | “Cell Cycle Regulatory Protein, and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al. | |||
| 1 | Cyclin D License |
| 2 | pl6 License |
| 3 | Mitotix owned Patents |
| 4 | PRADl License |
| 5 | Cyclin E License |
Appendix A-Page 1
| (8) |
U.S.S.N.: | 08/246,36 | ||
| Filed: | May 19, 1994 | |||
| Title: | “D.-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (9) |
U.S.S.N.: | 08/248,812 | ||
| Filed: | May 25, 1994 | |||
| Title: | “Cell Cycle Regulatory Proteins, and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al. | |||
| (10) |
U.S.S.N.: | 08/253,155 | ||
| Filed: | June 2, 1994 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇▇ | |||
| (11) |
U.S.S.N.: | 08/306,511 | ||
| Filed: | September 14, 1994 | |||
| Title: | “Cell Cycle Regulatory Protein, and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al. | |||
| (12) |
U.S.S.N.: | 08/346,147 | ||
| Filed: | November 29,1994 | |||
| Title: | “Cell Cycle Regulatory Protein, and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al. | |||
| (13) |
U.S.S.N.: | 08/464,517 | ||
| Filed: | June 5, 1995 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (14) |
U.S.S.N.: | 08/463,772 | ||
| Filed: | June 5,1995 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (15) |
U.S.S.N.: | 08/466,679 | ||
| Filed: | June 6, 1995 | |||
| Title: | “CDC37 Cell-Cycle Regulatory Protein, and Uses Related Thereto”3 | |||
| Inventor: | ▇▇▇▇ ▇▇▇▇▇▇, et al. | |||
| 1 | Cyclin D License |
| 2 | p16 License |
| 3 | Mitotix owned Patents |
| 4 | PRAD1 License |
| 5 | Cyclin E License |
Appendix A-Page 2
| (16) |
U.S.S.N.: | 08/497,214 | ||
| Filed: | June 30, 1995 | |||
| Title: | “Cell Cycle Regulatory Proteins, and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al | |||
| (17) |
U.S.S.N.: | tba | ||
| Filed: | October 31,1995 | |||
| Title: | “Inhabitors of Cyclin-Depenent Kinases”2 | |||
| Inventor: | ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇, et al. | |||
| (18) |
App. No.: | PCT/US92/04146 | ||
| Filed: | May 18, 1992 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (19) |
App. No.: | 2,103,161 (Canada) | ||
| Filed: | May 18, 1992 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (20) |
App. No.: | 92 913100.1 (EPO) | ||
| Filed: | May 18, 1992 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (21) |
App. No.: | 5-500242 (Japan) | ||
| Filed: | May 18, 1992 | |||
| Title: | “D-Type Cyclins and Uses Related Thereto”1 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach | |||
| (22) |
App. No.: | PCT/US93/09945 | ||
| Filed: | October 18, 1993 | |||
| Title: | “Cyclin Complex Rearrangement and Uses”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al. | |||
| (23) |
App. No.: | 54440/94 (Australia) | ||
| Filed: | October 18, 1993 | |||
| Title: | “Cyclin Complex Rearrangements and Uses”2 | |||
| Inventor: | DavidH. Beach, et al. | |||
| 1 | Cyclin D License |
| 2 | p16 License |
| 3 | Mitotix owned Patents |
| 4 | PRADl License |
| 5 | Cyclin E License |
Appendix A-Page 3
| (24) |
App. No.: | 2,146,965 (Canada) | ||
| Filed: | October 18, 1993 | |||
| Title: | “Cyclin Complex Rearrangements and Uses”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al | |||
| (25) |
U.S.S.N.: | 93 924946.2 (EPO) | ||
| Filed: | October 18, 1993 | |||
| Title: | “Cyclin Complex Rearrangements and Uses”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al | |||
| (26) |
App. No.: | 510328/94 (Japan) | ||
| Filed: | October 18, 1993 | |||
| Title: | “Cyclin Complex Rearrangements and Uses”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al | |||
| (27) |
App. No.: | 701512/95 (Korea) | ||
| Filed: | October 18, 1993 | |||
| Title: | “Cyclin Complex Rearrangements and Uses”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al | |||
| (28) |
App. No.: | PCT/US95/04636 | ||
| Filed: | April 14, 1995 | |||
| Title: | “Cell Cycle Regulatory Proteins, and Uses Related Thereto”2 | |||
| Inventor: | ▇▇▇▇▇ ▇. Beach, et al | |||
| (29) |
App. No.: | PCT/US95/071 | ||
| Filed: | June 2, 1995 | |||
| Title: | CDK4 Binding Proteins”3 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇▇ | |||
| PRAD1 |
||||
| (1) |
U.S.S.N.: | 07/667,711 | ||
| Filed: | March 11, 1991 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (2) |
U.S.S.N.: | tba | ||
| Filed: | June 2, 1995 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| 1 | Cyclin D License |
| 2 | p16 License |
| 3 | Mitotix owned Patents |
| 4 | PRAD1 License |
| 5 | Cyclin E License |
Appendix A-Page 4
| (3) |
U.S.S.N.: | 08/460,694 | ||
| Filed: | June 2, 1995 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (4) |
U.S.S.N.: | tba | ||
| Filed: | June 2, 1995 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (5) |
U.S.S.N.: | 08/460,655 | ||
| Filed: | June 2, 1995 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (6) |
U.S.S.N.: | 08/460,744 | ||
| Filed: | June 2, 1995 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (7) |
▇▇▇.▇▇.: | PCT/US92/01925 | ||
| Filed: | March 11, 1992 | |||
| Title: | Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (8) |
▇▇▇.▇▇.: | 92909340.0 (EPO) | ||
| Filed: | March 11, 1992 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (9) |
▇▇▇.▇▇.: | 2,104,871 (Canada) | ||
| Filed: | March 11, 1992 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (10) |
▇▇▇.▇▇.: | tba (Japan) | ||
| Filed: | March 11, 1992 | |||
| Title: | “Pradl Cyclin and its cDNA”4 | |||
| Inventor: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| 1 | Cyclin D License |
| 2 | pl6 License |
| 3 | Mitorix owned Patents |
| 4 | PRADl License |
| 5 | Cyclin E License |
Appendix A-Page 5
| Cvclin E |
||||
| (1) |
U.S.S.N.: | 07/764,309 | ||
| Date Filed: | September 20, 1991 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | ▇▇▇▇▇▇▇, Cross | |||
| (2) |
U.S.S. N.: | 07/947,311 | ||
| Filed: | September 16, 1992 | |||
| Date Issued: | September 12, 1995 | |||
| Patent No.: | 5,499,755 | |||
| Title: | “Human Cyclin E “5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (3) |
App. No.: | PCT/US92/07866 | ||
| Filed: | September 16, 1992 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (4) |
App. No.: | 1993-506220 (Japan) | ||
| Filed: | September 16, 1992 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (5) |
App. No.: | 92 920511.0 (EPO) | ||
| Filed: | September 16, 1992 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (6) |
App. No.: | 26663/92 (Australia) | ||
| Filed: | September 16, 1992 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (7) |
App. No.: | 2,119,443 (Canada) | ||
| Filed: | September 16, 1992 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (8) |
U.S.S. N.: | 08/522,166 | ||
| Filed: | June 7,1995 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| l | Cyclin D License |
| 2 | pl6 License |
| 3 | Mitorix owned Patents |
| 4 | PRAD1 License |
| 5 | Cyclin E License |
Appendix A-Page 6
| (9) |
U.S.S.N.: | 08/485,859 | ||
| Filed: | June 7, 1995 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (10) |
U.S.S.N.: | 08/488,382 | ||
| Filed: | June 7, 1995 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| (11) |
U.S.S.N.: | 08/480,912 | ||
| Filed: | June 7, 1995 | |||
| Title: | “Human Cyclin E”5 | |||
| Inventor: | Roberts, Koff, Ohtsubo, Cross | |||
| 1 | Cyclin D License |
| 2 | p16 License |
| 3 | Mitotix owned Patents |
| 4 | PRADl License |
| 5 | Cyclin E License |
Appendix A-Page 7
APPENDIX B
CDK WORK PLAN
The guiding principle behind the proposed workplan is that Mitotix and DuPont Merck with collaborate on the identification and development of CDK Development Compounds to be used as therapeutic agents. The Annual CDK Research Plan will initially focus on ***. The collaborative Policy Setting Committee will broaden the focus of the CDK Research Program as it determines necessary and appropriate and will describe any new areas of focus in subsequent Annual CDK Research Plans. The first Annual CDK Research Plan shall be prepared by the CDK Research Operating Committee and approved by DuPont Merck with the conurrence of the Collaborative Policy Setting Committee within forty-five (45) days after the Effective Date. Subsequent Annual CDK Research Plans shall be prepared by the CDK Research Operating Committee, for submission to, and approval by, DuPont Merck with the concurrence of the Collaborative Policy Setting Committee, not later than sixty (60) days prior to the start of each Research Year. Each Annual CDK Research Plan should include, without limitations, the following:
| (i) | Goals and objectives for the CDK Collaboration |
| (ii) | Names and percentage of time to be working on CDK Research Program by Mitotix FTEs |
Mitotix will continue biological discovery, target vbalidation, biochemical and cell-based assay refinement, and new assay development with respect to the CDK Targets. New assay development will include both (i) selectivity assays to screen out other activity ***, subject to the direction of the Collaborative Policy Setting Committee, and (ii) genetic engineering of ceil lines to test potential combination drug regimens. The Collaborative Policy Setting Committee shall determine which party shall be responsible for conducting the primary screening of compounds. Following primary screening, compounds which meet the specifications set forth by the CDK Research Operating Committee for suitable condidates for continued preclinical development shall be provided to Motitx. Mitotix will have primary responsibility for conducting secondary screening of compounds with respect to CDK Targets in the CDK Field. DuPont Merck will have primary responsibility for medicinal and strucutral chemistry of small-molecule leads, and will provide access to its chemical libraries (and other chemical libraries that it may access). Mitotix and DuPont Merck will work together to develop any necessary in vivo and animal pharmacology models for further testing of the compounds.
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Appendix B-Page 1
APPENDIX C
CDK PRIMARY AND SECONDARY SCREENS
Primary and Secondary Screens in the CDK Collaboration that have been validated by Mitorix as of the Effective Date:
***
| • | Actives will be tested against the following related kinase secondary screens: |
***
***
***
| • | Any active leads which remain after these assays will be tested in the pharmacology enzyme panel. The enzymes in the pharmacology panel are |
***
***
***
***
***
***
| • | Any active leads which remain after these assays will be tested in the tertiary cell line screens. |
***
| • | Actives will be tested against the following secondary screens: |
***
***
| • | Any active leads which remain after these assays will be tested in the enzyme pharmacology panel and the tertiary cell line screens. |
***
| • | Actives will be tested again the following secondary screens: |
***
***
***
| • | Any active leads which remain after these assays will be tested in the enzyme pharmacology panel and the tertiary cell line screens. |
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Appendix C-Page 1
APPENDIX D
UBC PATENT RIGHTS AND CERTAIN
MITOTIX LICENSE AGREEMENTS
| (1) |
U.S.S.N.: |
08/176,937 | ||
| Filed: |
January 4, 1994 | |||
| Title: |
“Assay and Reagents for Detecting Inhibitors of Ubiquitin- | |||
| dependent Degradation of Cell Cycle Regulatory Protein”1 | ||||
| Inventor: |
▇▇▇▇ ▇▇▇▇▇, et al. | |||
| (2) |
U.S.S.N.: |
08/247,904 | ||
| Filed: |
May 23, 1994 | |||
| Title: |
“Human Ubiquitin Conjugating Enzyme”1 | |||
| Inventor: |
▇▇▇▇ ▇▇▇▇▇, et al | |||
| (3) |
U.S.S.N.: |
08/305,520 | ||
| Filed: |
September 13, 1994 | |||
| Title: |
“Ubiquitin Conjugating Enzymes”1 | |||
| Inventor: |
▇▇▇▇ ▇▇▇▇▇, et al. | |||
| (4) |
U.S.S.N.: |
08/350,468 | ||
| Filed: |
December 7, 1994 | |||
| Titled: |
“Ubiquitin Conjugating Enzymes”2 | |||
| Inventor: |
▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇ Yew, ▇▇▇▇▇▇▇ ▇▇▇▇ | |||
| (5) |
U.S.S.N.: |
08/425,299 | ||
| Filed |
April 20, 1995 | |||
| Titled: |
“Assays and Reagents for Detecting Inhibitors of Ubiquitin- Dependent Degradation of Cell Cycle Regulatory Proteins”3 | |||
| Inventor: |
▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇, Jan-▇▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||
| (6) |
U.S.S.N.: |
08/486,663 | ||
| Filed: |
June 7,1995 | |||
| Title: |
“Ubiquitin Conjugating Enzymes”1 | |||
| Inventor: |
▇▇▇▇ ▇▇▇▇▇, et al. | |||
| (7) |
App. No.: |
PCT/US95/00164 | ||
| Filed |
January 4, 1995 | |||
| Title: |
“Ubiquitin Conjugating Enzymes”1 | |||
| Inventor: |
▇▇▇▇ ▇▇▇▇▇, et al. | |||
| 1 | Mitotix owned Patents |
| 2 | UBC9 License |
| 3 | Cdc27/cdc 16 License |
Appendix D-Page 1
APPENDIX E
MITOTIX PENDING LICENSE AGREEMENTS
The following Mitotix Pending License Agreements relating to technologies that may be useful in the CDK Field or UBC Field are under negotiation by Mitotix as of the Effective Date:
| (1) | Mitotix is negotiating an exclusive license to *** with ***. |
| (2) | Mitotix has applied for an exclusive license to *** from ***. |
| (3) | Mitotix is negotiating an exclusive license to *** from ***. |
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Appendix E–Page 1
APPENDIX F
UBC WORK PLAN
The guiding principle behind the proposed workplan and budget is that Mitotix and DuPont Merck will collaborate on the identification and development of UBC Development Compounds to be used as therapeutic agents. The Annual UBC Research Plan and Budget will initially focus on ***. The Collaborative Policy Setting Committee will broaden the focus of the UBC Research Program as it determines necessary and appropriate and will describe any new areas of focus in subsequent Annual UBC Research Plans and Budgets. The first Annual UBC Research Plan and Budget shall be prepared by the UBC Research Operating Committee and approved by the Collaborative Policy Secting Committee within forty-five (45) days after the Effective Date. Subsequent Annual UBC Research Plans and Budgets shall be prepared by the UBC Research Operating Committee, for submission to, and approval by the Collaborative Policy Setting Committee not later than sixty (60) days prior to the start of each Research Year.
Each Annual UBC Research Plan and Budget should include without limitation the following:
| (i) | Goals and objectives of the UBC Collaboration |
| (ii) | Specific responsibilities for each party including the names and percentage of time of Scientists to be working on the UBC Research Program |
| (iii) | Annual Budget and Estimated Percentage Contribution |
Mitotix will assign at least four full-time equivalent qualified scientists to work on the UBC Research Program.
DuPont will assign at least four full-time equivalent qualified scientists to work on the UBC Research Program.
The Collaborative Policy Setting Committee shall determine which Party shall be responsible for conducting the primary screening of compounds. Following primary screening, compounds which meet the specifications set forth by the UBC Research Operating Committee for suitable candidates for continued preclinical development shall be provided to Mitotix for secondary screening.
| *** | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Appendix F–Page 1
APPENDIX G
CO-PROMOTION OF CDK PRODUCTS AND CO-PROMOTION OF UBC PRODUCTS
WHEN THE ALTERNATE UBC PLAN HAS BEEN PUT INTO EFFECT
For purposes of this Appendix Gl, the term “Product” shall include CDK Products and, when the Alternate UBC Plan has been put into effect, UBC Products.
1. Timing of Mitotix’s Election to Co-Promote. DuPont Merck shall promptly notify Mitotix in writing of the filing of an NDA in the U.S. for a Product. DuPont Merck will also furnish Mitotix with such clinical and commercial information relating to the marketing strategy for the Product, which is reasonably available to DuPont Merck at the time of NDA filing and to the extent not provided to Mitotix earlier, as is reasonably necessary to enable Mitotix to determine whether to exercise its co-promotion option. Mitotix shall inform DuPont Merck whether or not it wishes to exercise its option to co-promote the Product within sixty (60) days after receipt of such written notice and related information from DuPont Merck.
2. Co-Promotion Territory. The United States of America.
3. Co-Promotion Option. Mitotix’s right to co-promote Products shall consist solely of the right to perform personal detailing to appropriate target audiences, likely to be limited to physicians, at up to ten percent (10%) of the total detailing effort at any given time for that Product in the United States. Mitotix will not be involved in any other marketing, promotion, or selling activities with regard to Products.
4. Negotiation of Co-Promotion Agreement. If Mitotix exercises its option to co-promote a Product, the parties shall negotiate in good faith and enter into a “Co-Promotion Agreement” not less than one (1) year prior to the anticipated First Commercial Sale of the Product in the United States, as determined by DuPont Merck in good faith. The Co-Promotion Agreement shall provide for the co-promotion of such Product by Mitotix to physicians on a fee-per-detail basis, up to a level of details per year which shall not exceed ten percent (10%) of the total number of details for such Product in a given year. The fee-per-detail will be at a fair market value, based on industry standards for similar fee-per-detail contract detailing arrangements.
In the event that the parties cannot agree on the allocation of Mitotix’ maximum 10% detailing effort, the matter will be taken to the Vice President, Marketing & Sales of each of the parties for resolution. If the issue remains unresolved at this level, the matter shall be taken to the President and/or CEO of each of the parties for final resolution.
“Detail” or “Detailing” as used in this Agreement shall mean, with respect to a drug product, the activity undertaken by a sales representative during a sales call on physicians in their offices, at hospitals or at other locations, including at hospital displays (excluding medical convention exhibits and displays and other forms of communication not involving face-to-face contact by a sales representative and a physician), of describing the FDA-
Appendix G1–Page 1
approved indicated uses, safety, effectiveness, contraindications, side effects, warnings and other relevant characteristics of such drug product, in a fair and balanced manner consistent with the requirements of the Food Drug Cosmetic Act, as amended, and regulations promulgated thereunder from time to time, and using, as necessary or desirable, the applicable promotional materials for the drug product, in an effort to increase physician prescribing preferences of the drug product for its FDA-approved indicated uses.
Appendix G1–Page 2
APPENDIX G2
CO-PROMOTION OF UBC PRODUCTS
WHEN THE ALTERNATE UBC PLAN IS NOT IN EFFECT
1. Timing of Mitotix’s Election to Co-Promote. DuPont Merck shall promptly notify Mitotix in writing of the filing of an NDA in the U.S. for a UBC Product. DuPont Merck will also furnish Mitotix with such clinical and commercial information relating to the marketing strategy for the Product, which is reasonably available to DuPont Merck at the time of NDA filing and to the extent not provided earlier to Mitotix, as is reasonably necessary to enable Mitotix to determine whether to exercise its co-promotion option. Mitotix shall inform DuPont Merck whether or not it wishes to exercise its option to co-promote the UBC Product within sixty (60) days after receipt of such written notice and related information from DuPont Merck.
2. Co-Promotion Territory. The United States of America.
3. Co-Promotion Option. Mitotix’s right to co-promote UBC Products when the Alternate UBC Plan is not in effect shall consist solely of the right to perform a percentage of the total Selling Effort (defined below) for the UBC Product, such percentage not to exceed the Mitotix Percentage Contribution at the time of launch of the UBC Product in the United States. Such percentage shall remain fixed unless the parties mutually agree otherwise. The costs of each party’s Selling Effort will be classified as Field Selling Expense in accordance with Appendix J.
4. Negotiation of Co-Promotion Agreement. If Mitotix exercises its option to co-promote a UBC Product, the parties shall negotiate in good faith and enter into a “Co-Promotion Agreement” not less than one (1) year prior to the anticipated First Commercial Sale of the UBC Product, as determined by DuPont Merck in good faith. The Co-Promotion Agreement shall set forth terms and conditions governing the co-promotion of the product which are commercially reasonable in light of information and assumptions pertaining at that time to the following (based on data contained in the NDA): (i) indication(s) for the product; (ii) dosage forms for the product; (iii) target audience (e.g, institutional and physician specialty); and (iv) each company’s strengths with the target audience and appropriate managed health care audiences. Unless mutually agreed otherwise during negotiation of the Co-Promotion Agreement, the Co-Promotion Agreement shall include the following provisions:
4.1. DuPont Merck shall be solely responsible for the pricing of the product.
4.2. The parties shall detail a single brand of the product using a trademark, branding strategy, promotional strategy, promotional investment and promotional mix selected and controlled by DuPont Merck.
4.3. To the extent not prohibited by applicable laws or regulations, DuPont Merck and Mitotix shall enjoy relative corporate exposure proponional to each parry’s Percentage Contribution (e.g., proponional prominence given to the DuPont Merck and Mitotix names on
Appendix G2–Page 1
the product, product samples, promotional items, exhibits, advertisements, etc.) during the co-promotion effort.
4.4. DuPont Merck will oversee the day-to-day marketing plan for the products, subject to guidance from a Collaborative Marketing Committee, which will consist of as many DuPont Merck representatives as deemed necessary by DuPont Merck and two representatives of Mitotix. The Collaborative Marketing Committee will make strategic and major operational recommendations to DuPont Merck on sales, marketing, promotion and advertising, including the target subscribers whom Mitotix shall detail and the number and manner of details.
4.5. “Selling Effort”, to be further defined in the Co-Promotion Agreement, shall mean personal sales detailing efforts mutually established between the parties and described in the marketing plan for the product. Examples of “Selling Effort” to be considered by the parties and counted as a detail are (i) personal detailing to appropriate target audiences, (ii) broad-based technical approaches (i.e., in-service training programs), and (iii) structured group presentations to pre-screened audiences. DuPont Merck shall, in consultation with Mitotix through the Collaborative Marketing Committee, determine the overall level and types of Selling Effort for the product which will maximize sales of the product. The parties will then agree, within the limits of Mitotix’s contractual rights, on the allocation of the types of Selling Effort to achieve optimal utilization of each parry’s field force. The parties recognize the rapidly changing health environment in the United States and acknowledge that parameters currently utilized for determining Selling Efforts and the results obtained therefrom may change over time, and each will take such changes into account and adopt such changes as appropriate to maximize sales of a product. In the event that the parties cannot agree on the allocation of types of Selling Effort needed to meet the above objectives, the matter will be taken to the Vice President, Marketing & Sales of each of the parties for resolution. If the issue remains unresolved at this level, the matter shall be taken to the President and/or CEO of each of the parties for final resolution.
Appendix G2–Page 2
APPENDIX H
UBC PRODUCTS
DEFINITION OF PERCENTAGE CONTRIBUTION
In summary, UBC Operating Profit (as defined in Appendix I) is to be shared between the parties based on each party’s contribution toward the funding of the expense of UBC Research, Development and Pre-Launch Marketing Costs. The mechanism by which the Percentage Contribution will be calculated is as described below:
Target Date: The Target Date is the date the first UBC Product is expected to be launched in the first Strategic Country. The expectation is that the parties will manage to and achieve the target 70% / 30% Percentage Contribution by the Target Date.
Set Date: The Set Date refers to the date when the first UBC Product has been launched in all Strategic Countries.
Percentage Contribution: The Percentage Contribution refers to the percentage share of each party’s Cumulative NPV Contribution relative to the combined Cumulative NPV Contribution for both parties.
Cumulative NPV Contribution: The Cumulative NPV Contribution is determined at any time by summing the Net Present Value (NPV) of each year’s UBC Research, Development and Pre-Launch Marketing Costs funded by each party.
Net Present Value: The Net Present Value (NPV) for each year’s UBC Research, Development and Pre-Launch costs is determined by discounting the full year’s expenses to the Effective Date at a discount rate of 10% per annum in accordance with standard financial methodology.
For all UBC Products, the Percentage Contribution shall be calculated up through the Set Date based on each party’s continued contribution to Research, Development, and Pre-Launch Marketing Costs. On the Set Date, a final calculation of the Percentage Contribution will be performed fixing the percentage share each party will receive of worldwide earnings from the Set Date onward for the first UBC Product as well as all subsequent UBC Products.
Appendix H - Page 1
Prior to the Set Date, each party will share in worldwide earnings based on the Percentage Contribution calculated at the end of the preceding year or estimated Percentage Contribution from the prior quarter if materially different. It is the expectation of both parties that the Percentage Contribution target of 70% / 30% will be managed to and achieved by the Target Date. The following illustrates the Percentage Contribution calculation for all UBC Products up through the Set Date:
| Annual Cost |
Cumulative NPV |
% Contribution |
||||||||||||
| DuPont/ Merck |
Mitotix |
DuPont/ Merck |
Mitotix |
DuPont / Merck |
Mitotix |
|||||||||
| Year 1 |
2.0 | 2.0 | 1.8 | 1.8 | 50 | % | 50 | % | ||||||
| Year 2 |
1.5 | 1.5 | 3.1 | 3.1 | 50 | % | 50 | % | ||||||
| Year 3 |
3.8 | 1.3 | 5.9 | 4.0 | 60 | % | 40 | % | ||||||
| Year 4 |
2.3 | 0.8 | 7.4 | 4.5 | 62 | % | 38 | % | ||||||
| Year 5 |
5.3 | 1.8 | 10.7 | 5.6 | 66 | % | 34 | % | ||||||
| Target Date |
21.0 | 7.0 | 22.5 | 9.5 | 70 | % | 30 | % | ||||||
| Year 7 |
2.7 | 0.3 | 23.9 | 9.7 | 71 | % | 29 | % | ||||||
| Set Date |
22.5 | 7.5 | 34.4 | 13.2 | 72 | % | 28 | % | ||||||
If the Percentage Contribution changes between the Target Date and the Set Date, as shown above, the share of worldwide earnings will likewise change and will be based on the Percentage Contribution calculated at the end of the prior year or estimated Percentage Contribution from the prior quarter if materially different. The following illustrates this concept: [Note: Costs included in determining the Percentage Contribution on UBC Products are not to be included as an Allowable Expense in the calculation of UBC Operating Profit as defined in Appendix I and vice versa.
| (Target Date) Launch in Europe |
(Year T+1) Launch in Canada |
(Set Date) Launch in U.S. |
(S + 1)All Thereafter |
|||||||||
| Worldwide Earnings (a) |
||||||||||||
| Cumulative NPV Contribution % |
5 | 8 | 20 | 25 | ||||||||
| - DuPont Merck |
66 | % | 70 | % | 71 | % | 72 | % | ||||
| - Mitotix |
34 | % | 30 | % | 29 | % | 29 | % | ||||
| Share of Worldwide Earnings |
||||||||||||
| - DuPont Merck |
3.3 | 5.6 | 14.2 | 18 | ||||||||
| - Mitotix |
1.7 | 2.4 | 5.8 | 7 |
| (a) | Excludes all Research, Development and Pre-launch Marketing Costs included in determining the Percentage Contribution. |
Appendix H - Page 2
UBC RESEARCH, DEVELOPMENT
AND PRE-LAUNCH MARKETING COSTS:
For purposes of calculating the Percentage Contribution, UBC Research, Development and Pre-Launch Marketing Costs shall be compiled annually on a worldwide basis from the Effective Date up through the Set Date. After the Set Date, all UBC Research, Development and Pre-Launch Marketing Costs will be included as Allowable Expenses (defined in Appendix J) in the determination of UBC Operating Profit. Costs included in determining the Percentage Contribution are not to be included as an Allowable Expense in the calculation of UBC Operating Profit as defined in Appendix I and vice versa.
The definitions of expenses listed below are representative of today’s conditions and business experience. These conditions may change over time as a result of structural changes in the respective companies, the pharmaceutical industry, or the UBC Collaboration. The parties agree to negotiate in good faith all necessary modifications to these definitions to reflect the actual experience of the parties at that time. Modifications to any cost standard will be set at the lowest cost for each activity that is or could be provided by either party, if the level and quality of service between the two parties is comparable. If the level of service is not comparable between the two parties, the cost standard for that service will reflect the cost of each party providing the service, as determined by the Collaborative Policy Setting Committee.
UBC Research, Development and Pre-Launch Marketing Costs shall include the following:
Discovery Costs: Discovery costs will be billed at a rate of S225,000(a) per full-time-equivalent (FTE) scientist working within the UBC field, plus all related extramural (out-of-pocket) expenses including payments to third parties for contract research and outside consultants.
Development Costs: Upon nomination into development, Development Costs will include the direct costs as tracked by each party’s project accounting system. These direct costs will include, but are not limited to, salaries, benefits, lab supplies, travel expenses, plus all related extramural (out-of-pocket) expenses including payments to third parties for contract research and outside consultants, clinical grant costs, bulk drug, contract manufacturing costs, clinical supplies and FDA user fees. In addition, an appropriate share of facilities costs, Information Resources, R&D administration and cash (or cash equivalent) incentive compensation performance bonus costs will also be allocated based on a reasonable allocation methodology agreed to by both parties.
Appendix H - Page 3
Post Registration Studies: Includes the cost of the Post Registration Studies 1) required by the FDA or other regulatory agencies as part of the NDA approval, 2) required to broaden the approved indications or 3) to improve the approved labeling. The direct costs for this support will be as tracked by each party’s internal systems. These direct costs will include, but are not limited to, salaries, benefits, lab supplies, travel expenses, plus all related extramural (out-of-pocket) expenses including payments to third parties for contract research and outside consultants, clinical grant costs, bulk drug, contract manufacturing costs, clinical supplies and FDA user fees. In addition, an appropriate share of facilities costs, Information Resources, R&D administration and cash (or cash equivalent) incentive compensation performance bonus costs will also be allocated. The total cost applied for Mitotix will include the cost of personnel who spend part of their time working with DuPont Merck on UBC Products. The cost of such personnel will be determined by taking their FTE cost ($ 125,000)(a) times the percentage of their time spent on UBC Products.
Pre-Launch Expenses: Pre-Launch expenses include, but are not limited to, all direct and indirect marketing costs incurred up through the date of launch including grant money, payments to advertising agencies, preliminary launch materials to FDA, symposia and opinion leader development activities, and use of DuPont Merck Clinical Liasons. The cost for these activities will be as compiled by DuPont Merck internal systems. The cost of each Clinical Liason will be determined by taking their FTE cost ($125,000)(a) times the percentage of their time spent on UBC Products. In addition, the cost of two marketing personnel of Mitotix who spend part of their time on the Collaborative Marketing Committee (as defined in Section 4.4 of Appendix G2) will be included by taking their FTE cost ($125,000)(a) times the percentage of their time spent on UBC Products plus travel expenses.
Other Expenses: All other expenses of a similar nature to the extent agreed by the parties.
| (a) | The annual FTE cost is to be adjusted annually for inflation using the average CPI for each succeeding year beginning with 1996. |
Appendix H - Page 4
APPENDIX I
DEFINITION OF UBC OPERATING PROFIT
The definitions of sales and expenses embodied within are representative of today’s conditions and business experience. These conditions may change over time as a result of structural changes in the respective companies, the pharmaceutical industry, or the UBC Collaboration. The parties agree to negotiate in good faith all necessary modifications to these definitions to reflect the actual experience of the parties at that time. Modifications to any cost standard will be set at the lowest cost for each activity that is or could be provided by either party, if the level and quality of service between the two parties is comparable. If the level of service is not comparable between the two parties, the cost standard for that service will reflect the cost of each party providing the service, as determined by the Collaborative Policy Setting Committee.
UBC Operating Profit: UBC Operating Profits shall mean the profits or losses resulting from the commercialization of all UBC Products worldwide and shall be equal to (i) worldwide Net Sales of all UBC Products less worldwide Allowable Expenses (defined below) incurred by Mitotix and DuPont Merck plus (ii) all revenues received from Third parties as consideration for sublicensing of the manufacture, distribution, use and sale of UBC Products. UBC Operating Profits will be shared based on each party’s Percentage Contribution as defined in Appendix H. The following table illustrates how UBC Operating Profits are calculated and how each party’s share of the UBC Operating Profits (assumed here to be split 70% / 30%) are realized via an earnings true-up payment.
CALCULATION OF EARNINGS SPLIT FOR UBC PRODUCTS
| DuPont/ Merck |
Mitotix |
UBC Worldwide Earnings |
||||||||||
| Net Sales |
100 | 100 | ||||||||||
| Allowable Expenses |
(40 | ) | (10 | ) | (50) | |||||||
| UBC Operating Profits |
60 | (10 | ) | 50 | ||||||||
| Earnings True-Up |
(25 | ) | 25 | |||||||||
| UBC Operating Profits |
35 | 15 | 35 | 15 | ||||||||
| 70 DMPC |
% % |
30 Mitotix |
% % | |||||||||
Appendix I - Page 1
Allowable Expenses: Allowable Expenses shall include on a country-by-country basis only those expenses incurred subsequent to commercial sale in that country (excluding Post Registration Studies unless after the Set Date). Expenses incurred prior to commercial sale in each country shall be classified as UBC Research, Development and Pre-Launch Marketing Costs and shall be included in the calculation of Percentage Contribution. All expenses incurred after the Set Date are classified as Allowable Expenses. The following table and Exhibit 1 show how different expenses are classified at different times relative to the launch and Set Date.
Classification of Expenses
| Prior To Launch |
After Launch / Prior to Set Date |
After Set Date | ||||
| R&D and Pre-Launch |
R&D (a) | R&D (a) | Allowable Expense (b) | |||
| Post Registration |
R&D (a) | R&D (a) | Allowable Expense (b) | |||
| Operating Expenses (c) |
N/A | Allowable Expense (b) |
Allowable Expense (b) | |||
| (a) | Used in the calculation of Percentage Contribution |
| (b) | Used in the calculation of UBC Operating Profit |
| (c) | Includes Manufacturing, Selling, Marketing, Distribution, Admin etc. |
Prior to the Set Date, Allowable Expenses shall include (i) Manufacturing Cost of Goods, (ii) Third party Royalties, (iii) Finished Product Distribution Expense, (iv) Field Selling Expense, (v) Direct Promotion Expense, (vi) Marketing Overhead, (vii) Regulatory Support, (viii) Medical Affairs, (ix) Administration and Other Support Services, and (x) Working Capital Charge. Subsequent to the Set Date, Allowable Expenses shall include all of the above plus (i) Discovery Costs (ii) Development Costs, (iii) Post Registration Studies, and (iv) Pre-Launch Expenses. [Note: Costs included in Allowable Expenses are not to be included in the Percentage Contribution as defined in Appendix H.
Appendix I - Page 2
The individual components of Allowable Expenses are defined as follows:
Manufacturing Cost of Goods: Manufacturing Cost of Goods shall include the sum of variable standard manufacturing costs plus manufacturing cost variances plus an allocated share of fixed manufacturing expenses including other support functions necessary to support the technology incurred by the party performing the work. It is the intent that to the extent possible, the amount of the allocated share of fixed and other manufacturing expense will be determined in the same manner as DuPont Merck normally employs in allocating such cost to all its production. The methodology shall give recognition to such factors as the use of direct labor, throughput, number of batches and other relevant measures of consumption of resources. A final definition of Manufacturing Cost of Goods should be agreed to by both parties when production of commercial quantities in a manufacturing facility is commencing.
Third party Royalties: Third party Royalties shall mean royalties or other fees or costs payable to a Third party in connection with UBC Products.
Finished Product Distribution Expense: Finished Product Distribution Expense (FPDE) includes all distribution expenses including transportation, customs duties, insurance, fees paid to warehouses, packaging and labeling expenses. The cost of FPDE will be set at 2% of Net Sales unless actual expenditures are found to be materially lower or in excess of this amount due to special handling etc.
Field Selling Expense: Field Selling Expense will be calculated by multiplying the number of details times the Selling Cost Per Physician Detail in a calendar year.
Selling Cost Per Physician Detail: The Selling Cost Per Physician Detail for DuPont Merck and Mitotix details will be set at a fair market value, and will be in the range defined by other co-promotion agreements DuPont Merck will have entered into in the five years prior.
Direct Promotion Expense: Includes all product specific promotion expenses incurred in the promotion of UBC Products including, but not limited to, journal or direct to consumer advertising, agency fees, samples, speaker programs and other costs incurred in the promoting and marketing of UBC Products.
Marketing Overhead: Marketing Overhead shall include all costs incurred by the highest ranking member of DuPont Merck’s marketing management and his organization’s related support for UBC Products including marketing administration, clinical development and education, FDA user fees, sales information, health economics studies, samples, marketing research, customer service, telemarketing, bad debt expense and patent and trademark maintenance. The total cost for DuPont Merck will be applied at 6% of Net Sales, unless actual expenditures are found to be materially lower or in excess of this amount. The total cost applied for Mitotix will include the cost of two marketing personnel who spend part of their time on the Joint
Appendix I - Page 3
Marketing Team. The cost of the marketing personnel will be determined by taking their FTE cost ($125,000)(a) times the percentage of their time spent on UBC Products plus travel expenses.
Regulatory Support: Regulatory Support for DuPont Merck includes all the external and internal support necessary to maintain the worldwide product registration for UBC Products including stability testing, preparation of annual reports, answering regulatory agency questions, preparing expert reports as required by regulatory agencies, and will involve other groups in development as needed. The direct costs for this support will be as tracked by DuPont Merck’s project accounting system. These direct costs will include, but are not limited to, salaries, benefits, travel expenses, plus all related extramural (out-of-pocket) expenses including payments to outside consultants, and FDA and other regulatory agency user fees. In addition, an appropriate share of facilities costs, Information Resources, R&D administration and cash (or cash equivalent) incentive compensation performance bonus costs will also be allocated. The total cost applied for Mitotix will include the cost of Regulatory personnel who spend part of their time working with DuPont Merck on UBC Products plus travel expenses. The cost of each Regulatory personnel will be determined by taking their FTE cost ($125,000)(a) times the percentage of their time spent on UBC Products.
Medical Affairs: Medical Affairs includes support necessary for adverse experience documentation and reporting, response to health professional and consumer inquiries about the product including the preparation and maintenance of the product database, administration of Phaes IV study grants and supplies (with the exception of Post-Registration Studies as defined in Appendix I), the monitoring and review of medical literature concerning the product(s), evaluation of the need for labeling changes, coordination with Marketing to manage promotional material review, and medical review and oversight of each of these activities. The cost for this support will be based on effort expended for UBC Products as tracked by DuPont Merck’s internal systems and will include, but not limited to salaries, benefits, travel expenses, plus all related extramural (out-of-pocket) expenses including payments to third parties for contract research and outside consultants. In addition, an appropriate share of facilities costs, Information Resources, R&D administration and cash (or cash equivalent) incentive compensation performance bonus costs will also be allocated.
No costs will be incurred by Mitotix since DuPont Merck will have sole responsibility for oversight for this service.
| (a) | The annual FTE cost is to be adjusted annually for inflation using the average CPI for each succeeding year beginning with 1996. |
Appendix I - Page 4
Administration and Other Support Services: Includes all DuPont Merck support services such as Finance, Legal, Information Resources, Human Resources, Public and Government Affairs. The total cost for these services will be applied at 5% of Net Sales. All claims, damages, liabilities, costs and expenses with respect to suits, claims or other legal proceedings resulting from, arising out of or in connection with this agreement will be in addition to the 5% of Net Sales, unless actual expenditures are found to be materially lower or in excess of this amount. Administration and Other Support Service costs provided by Mitotix will include the FTE cost of Legal and Human Resource personnel ($125,000 per FTE)(a) times the percentage of their time spent on UBC Products.
Working Capital Charge: Reflects DuPont Merck’s financing cost of working capital and will be calculated at a rate of 10% times the average annual Accounts Receivable and Inventory balance less related payables (estimated at 25% of receivables and inventory).
| (a) | The annual FTE cost is to be adjusted annually for inflation using the average CPI for each succeeding year beginning with 1996. |
Appendix I - Page 5
EXHIBIT 1 - to Appendix I
ILLUSTRATION
EXPENSES USED IN DETERMINING THE PERCENTAGE CONTRIBUTION
VERSUS EXPENSES USED IN DETERMINING UBC OPERATING PROFIT
| R&D and Pre-launch Expenses: | - Expenses qualified for inclusion in determining the Percentage Contribution. | |
| Allowable Expense: | - Expenses qualified for inclusion in determining worldwide earnings. | |
| Target Date: | Date when the first UBC Product will be launched in the first Strategic Country. The intention is that the target 70% : 30% Percentage Contribution will have been achieved by this date | |
| Set Date: | Date when first UBC Product has been launched in all Strategic Countries. On the Set Date, the Cumulative NPV Percentage Contribution is fixed. | |
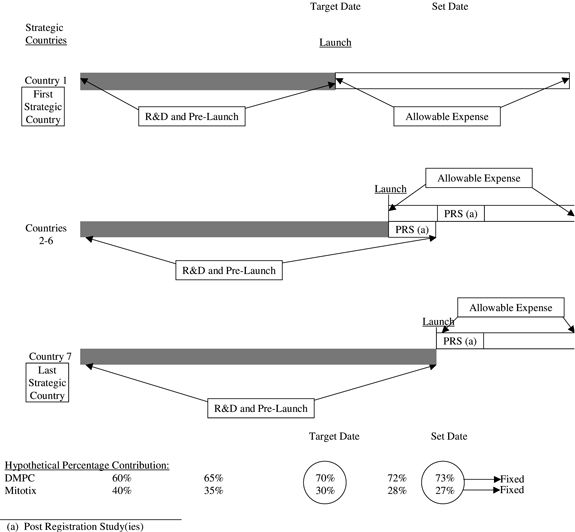
Appendix I - Page 6
APPENDIX J
The following examples illustrate the mechanics of the milestone payments as applied to Royalty-Bearing Products.
Example A: Product 1 is followed into clinical trials by back-up Product 2 after Product 1 has been approved. Product 1 attains all milestones and is paid in accordance with column 1 for First Royalty-Bearing Product. Product 2 attains all milestones, but no milestones are paid as it is not a Unique Product.
Example B: Product 1 has attained the first three milestones when clinical development is halted. Product 2 begins clinical development after the clinical failure of Product 1. Product 1 is paid the first three milestones from column 1. The first milestone for which Product 2 is eligible is milestone 4, which will be paid in accordance with column 1. Assuming Product 3 is also not a Unique Product, Product 3 would only be paid a milestone on any milestone events not previously paid for Products 1 & 2. If Product 2 were to achieve milestones 4&5 before Product 3 achieved these same milestone events, then no milestones would be paid for Product 3.
Example C: Product 1 is and Product 2 is not a Unique Product. Each achieves only the first two milestones. Product 1 being the first product receives milestone payments from column 1 for the first two milestone events. No milestones are paid for Product 2. Product 3 is a Unique Product and is approved. As a Unique Product, Product 3 receives all of the milestone payments from column 2, and in lieu of milestone payments 3,4 & 5 from column 2, it receives milestone payments from column 1 since it is the first product to achieve the latter milestones.
Example D: Product 1 has attained the first two milestone events when clinical development is halted. Product 2 which is one year behind is a Unique Product and likewise only achieves the first two milestone events. Since Product 1 is the first product to achieve the first two milestone events, it is paid milestones from column 1. Since Product 2 is a Unique Product, milestones are also paid for the first two milestone events, however, these milestone payments are from column 2. Product 3 (a back-up to Product 2) is not a Unique Product but achieves milestone events 3,4 &5. Since Product 3 is not a Unique Product, milestones are only paid on the milestone events not yet achieved by Product 2. Since no other CDK Product has yet to achieve these same milestone events, the milestone payments for milestones 3,4 & 5 will be paid from column 1. Had a previous CDK Product achieved these same milestones, all milestone payments for Product 3 would have been from column 2.
Appendix J–Page 1
Example A