PATENT LICENSE AGREEMENT
Exhibit 10.5
This Agreement is entered into as of November 9, 2007 (the “Effective Date”), by and between
Applera Corporation, a corporation of the State of Delaware, through its Applied Biosystems Group,
having an office at 000 Xxxxxxx Xxxxxx Xxxxx, Xxxxxx Xxxx, Xxxxxxxxxx 00000 (“AB”) on the one hand,
and Biotrove, Inc., having a place of business at 00 Xxxx Xxxxxx, Xxxxxx, XX 00000 (“Licensee”),
each of AB and BT hereafter referred to as a “Party” and, collectively, as “Parties”.
WHEREAS, AB owns or controls certain patents, as described herein, that pertain to real-time
thermal cyclers, through-hole arrays, assay loading devices and sample loading devices;
WHEREAS, the Parties have, contemporaneously with this Agreement, entered into that certain
Collaboration and Supply Agreement (the “Collaboration Agreement”) directed at the collaborative
development and commercialization of arrays loaded with certain assays, as well as assay loading
and sample loading devices for such arrays.
WHEREAS, Licensee desires and AB is willing to grant a license under such patents on the teens
and conditions set forth in this Agreement.
NOW, THEREFORE, in consideration of the recitals set forth above and the terms and conditions
set forth below, the Parties agree as follows:
1. Definitions
For the purpose of this Agreement the terms set forth hereinafter shall be defined as follows:
1.1 “Affiliate” shall mean a business entity directly or indirectly controlled by, controlling
or under common control with a Party to this Agreement. For purposes of this Section 1.1, the word
and root “control” means, in the case of a corporation, either: (a) the direct or beneficial
ownership of at least fifty percent (50%) of the shares of stock entitled to vote for Directors to
the Board of Directors of such corporation, or, (b) where the laws of a particular jurisdiction
limit the percentage ownership of domestic corporations by foreign corporations to less than fifty
percent (50%) of the shares of stock entitled to vote for Directors to the Board of Directors, the
maximum percentage ownership permitted by such jurisdiction, and provided that (in the case of both
(a) and (b)) the Party nevertheless has the ability to direct the activities of such entity related
to this Agreement.
1.2 “Array” shall mean, individually and collectively, Genotyping Arrays, Gene Expression
Arrays and any other BT Open Array, the manufacture, use, offer for sale, sale or importation of
which would, but for the license granted under this Agreement, infringe at least one Valid Claim of
any of the Licensed Patent Rights.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
1.3 “ABC Patent Rights” shall mean, individually and collectively, the Group A Patent Rights,
the Group B Patent Rights and the Group C Patent Rights.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
1.4 “Assay Loader” shall mean an apparatus or device for filling, depositing or transferring
reagents or assays onto BT Open Arrays the manufacture, use, offer for sale, sale or importation of
which would, but for the license granted under this Agreement, infringe at least one Valid Claim of
any of the ABC Patent Rights.
1.5 “Group A Patent Rights” shall mean U.S. Xxx. Nos. 7,211,148, 6,849,127, and 6,579,367 and
any patents and patent application claiming priority thereto and any foreign counterparts thereof.
1.6 “BT Open Arrays” shall mean through-hole array products (such as were, as of the Effective
Date, being commercialized by BT under the “BT Open ArrayTM” xxxx, as further described
on Exhibit I attached hereto, the manufacture, use, offer for sale, sale or importation of which
would, but for the license granted under this Agreement, infringe at least one Valid Claim of any
of the ABC Patent Rights.
1.7 “Early Access Period” shall mean the period that commences on the Effective Date and ends
upon the last day of the later to end of the Genotyping Early Access Period and the Gene Expression
Early Access Period.
1.8 “Field” shall mean all fields excluding Human In Vitro Diagnostics Field. Notwithstanding
the foregoing, the term “Field” shall include the Human In Vitro Diagnostics Field solely as to the
ABC Patent Rights but solely to the extent set forth in Section 2.10.
1.9 “Gene Expression” shall mean all analysis of ribonucleic acid (RNA) or other products of
transcription of DNA or translation of mRNA including, without limitation proteins.
1.10 “Gene Expression Arrays” means BT Open Arrays loaded with (a) AB TaqMan® gene expression
assays and related reagents and consumables; or (b) SYBR Xxxxx xxxx expression assays and related
reagents and consumables. However, to the extent any such TaqMan® or SYBR assays, reagents and
consumables are covered by patents owned or in-licensed by AB with the right to grant sublicenses,
the foregoing TaqMan® or SYBR Green assays, reagents and consumables that are covered by such
patents must either be manufactured and sold under a license agreement with AB (encompassing
applicable reagent, chemistry or method patents or patent applications) or purchased from a
supplier that is either licensed by AB or otherwise has valid rights under such patents (and is
therefore not infringing such patents) to sell the same to kit manufacturers or suppliers within
the Field.
1.11 “Gene Expression Commercialization Phase” shall have the meaning set forth in the
Collaboration Agreement.
1.12 “Gene Expression Early Access Period” shall mean the period that commences on the
Effective Date and ends upon AB’s decision, made in its sole discretion, that it is ready to
commence direct or indirect distribution or sales of Gene Expression Arrays.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
3
1.13 “Genotyping” shall mean all analysis of genomic deoxyribonucleic acid (DNA),
modifications or derivatives thereof.
1.14 “Genotyping Arrays” shall mean BT Open Arrays loaded with AB TaqMan® SNP genotyping
assays and related reagents and consumables. However, to the extent any such TaqMan® assays,
reagents and consumables are covered by patents owned or in-licensed by AB with the right to grant
sublicenses, the foregoing TaqMan® assays, reagents and consumables that are covered by such
patents must either be manufactured and sold under a license agreement with AB (encompassing
applicable reagent, chemistry or method patents or patent applications) or purchased from AB or a
supplier that is either licensed by AB or otherwise has valid rights under such patents (and is
therefore not infringing such patents) to sell the same to kit manufacturers or suppliers within
the Field.
1.15 “Genotyping Commercialization Phase” shall have the meaning set forth in the
Collaboration Agreement.
1.16 “Genotyping Early Access Period” shall mean the period that commences on the Effective
Date and ends upon AB’s decision, made in its sole discretion, that it is ready to commence direct
or indirect distribution or sales of Genotyping Arrays.
1.17 “Human In Vitro Diagnostic Field” shall mean the in vitro measurement, observation,
and/or determination of attributes, characteristics, diseases, traits or other conditions of a
human being for the medical management of such human being. The Human In Vitro Diagnostic Field
includes, but is not limited to, the use, sale or promotion of a real-time thermal cycler, such as
a Licensed Real-Time Thermal Cycler, or a through-hole array such as an Array, in connection with
analyte specific reagents in the United States or with any registered in vitro diagnostic product
worldwide. Without limiting the foregoing, any real-time thermal cycler, such as a Licensed
Real-Time Thermal Cycler, that is registered with any regulatory authority for use as a diagnostic
instrument or that bears a diagnostic use label (e.g. CE-IVD xxxx) shall be deemed within the Human
In Vitro Diagnostic Field.
1.18 “Licensed Patent Rights” shall mean, individually and collectively, the Real-Time
Apparatus Patent Rights, the Group A Patent Rights, the Group B Patent Rights and the Group C
Patent Rights.
1.19 “Licensed Real-Time Thermal Cycler” shall mean a thermal cycler instrument, specifically
adapted for use with a BT Open Array, whether such instrument is sold as an integrated product or
as one or more components or modules, the manufacture, importation, offer for sale, sale or use of
which such instrument would, but for the rights granted under this Agreement, infringe at least one
Valid Claim of the Real-Time Apparatus Patent Rights.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
4
1.20 “Licensed Product” shall mean, individually and collectively, any Licensed Real-Time
Thermal Cycler, Array, Assay Loader and Sample Loader.
1.21 “Net Sales shall mean
| (a) | in the case of an arm’s-length transaction to unrelated Third-Party end users not enjoying any special course of dealing with Licensee, its Affiliates or distributors, wherein the end user is not providing any nonmonetary consideration and the gross invoice price for a product or service that is subject to royalty obligation under Article 3 herein is not affected by any other purchase of goods or services or any license of intellectual property (other than the end user rights conveyed pursuant to Article 2 of this Agreement): the gross invoice price for such sale of such product (together with any associated warranty coverage sold) or service to an end user by Licensee, its Affiliates or distributors of such product, minus the following deductions where applicable: (i) discount allowed and taken, in amounts customary in the trade, and (ii) sales and/or use taxes and/or duties for particular sales. No allowance or deduction shall be made for commissions or collections, by whatever name known; and | ||
| (b) | in the case of a sale, loan, lease, consignment, distribution or transfer of products or services subject to royalty obligations under Article 3 herein that is not within the scope of Section 1.21(a): the Average Net Sales Price (as such term is defined below) for sales to end users for such product or services calculated under Section 1.21(a). The Net Sales Price for Licensed Real-Time Thermal Cyclers or components thereof that are used by Licensee in providing services to third parties shall, as per Section 3.1, equal the corresponding Average Net Sales Price (as defined below) that would apply for the sale of such Licensed Real-Time Thermal Cyclers or components. Without limiting the foregoing, Net Sales Price shall be calculated as set forth in this Section 1.21(b) in the event of any sale, loan, lease, consignment, gift, distribution, other transfer or use (including use of a Licensed Real-Time Thermal Cycler by Licensee as a service provider for third parties) (i) by or to an end user that is Licensee itself, an Affiliate or a distributor, (ii) to an end user that enjoys a special course of dealing with one or more of the Licensee, an Affiliate or distributor, or (iii) under a reagent rental agreement. As used herein the term “Average Net Sales Price” shall mean (x) the average Net Sales (as calculated for |
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
5
transfers under
Section 1.21(a)) over the two calendar quarter period, spanning the quarter in
which such transfer that is within this Section 1.21(b) occurred and the preceding quarter, of the same type
and model of product (as the case may be) and in the same country as such
transferee; (y) if such average Net Sales data is unavailable, then the
Licensee’s, Affiliate’s or distributor’s published list price of such
product for end users in such country as the transferee; or (z) or if data
is unavailable under part (x) or (y), then the fair market value (with
reference to the sales price of comparable products).
For any sales under which a [***] is [***] within [***] for [***], Licensee shall be entitled to a [***] and the [***] so [***] shall no longer be [***] or [***] hereunder.
In the event Licensee is unable to account for end user sales by any distributor, the Net
Sales shall be calculated as [***] (as determined in Section [***] or [***] margin [***].
1.22 “Real-Time Apparatus Patent Rights” shall mean (a) Applera’s United States Patent No.
6,814,934; (b) other United States patents and patent applications owned by Applera, directed to an
instrument, which patents and patent applications claim priority to United States patent
application Serial No. 07/695,201; (c) EP 872562 and all patent applications and patents claiming
priority thereto; and (d) and any Canadian counterparts of any of the foregoing that claim an
instrument (the patents and patent applications in part (a) through (d) above of this Section 1.22,
collectively, “Category I Real-Time Rights”). Without limiting the foregoing, the term “Real-Time
Apparatus Patent Rights” shall include claims 12 through 28 of U.S. Xxx. Nos. 5,928,907 (covering
real-time methods for instrumentation using a passive reference), 7,188,030 and 7,228,237 and
7,272,506 and all patent applications and patents claiming priority thereto. Notwithstanding
anything herein to the contrary, the term “Real-Time Apparatus Patent Rights” expressly excludes:
(i) any patents and patent applications that cover real-time chemistry, reagents,
reagent-containing kits, reagent-containing systems, and methods employing particular real-time
chemistry, reagents, reagent-containing kits, reagent-containing systems, that instrument users,
including Licensee, may wish or need for the performance of amplification and detection methods,
including without limitation real-time detection methods, utilizing Licensed Real-Time Thermal
Cyclers; and (ii) any patents and patent applications licensed under the TCSA. The Real-Time
Apparatus Patent Rights other than the Category I Real-Time Rights are referred to collectively as
the “Added Real-Time Rights”.
1.23 “Sample Loader” shall mean an apparatus or device for loading samples to be run on
(assayed on) Arrays, the manufacture, use, offer for sale, sale or importation of which apparatus
or device would, but for the license granted under this Agreement, infringe at least one Valid
Claim of any of the ABC Patent Rights.
1.24 “Group B Patent Rights” shall mean U.S. Pub. No. 2005/0220675 (pending) and any patents
and patent application claiming priority thereto and any foreign counterparts thereof.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
6
1.25 “Group C Patent Rights” shall mean U.S. Xxx. Nos. 6,090,251, 6,086,825, and 6,451,188 and any patents and patent application claiming priority thereto and
any foreign counterparts thereof.
1.26 “TCSA” shall mean that certain “Thermal Cycler Supplier Agreement” entered into between
the Parties effective as of December 22, 2004.
1.27 “Territory” shall mean the world.
1.28 “Valid Claim” shall mean a claim of an unexpired patent or pending application which has
not been held invalid or unenforceable by a decision of a court, Patent Office, or administrative
tribunal from which no appeal is available.
2. Grant
2.1 Licensed Real-Time Thermal Cyclers. AB hereby grants to Licensee a personal,
non-transferable, royalty-bearing, non-exclusive license under the Real-Time Apparatus Patent
Rights in the Field and in the Territory to make, but not have made, to use in providing services
to third parties, Licensed Real-Time Thermal Cyclers (and components, modules and software for such
Licensed Real-Time Thermal Cyclers), and to sell (directly or through distributors) the same solely
to end users, and solely in connection with the sale to such end-users of Genotyping Arrays and
Gene Expression Arrays. Upon commencement of the Genotyping Commercialization Phase, the foregoing
license shall exclude the Genotyping field and the foregoing right to sell Licensed Real-Time
Thermal Cycler’s shall be limited to sales to end users in connection with the sale of Gene
Expression Arrays. Upon commencement of the Gene Expression Commercialization Phase, the foregoing
license shall exclude the Gene Expression field (and if both the Genotyping Commercialization Phase
and the Gene Expression Commercialization Phase have commenced, the foregoing license shall be
suspended). Unless this Agreement has expired or been terminated, upon termination of the
Collaboration Agreement, the foregoing license shall resume in the Field (both as to Genotyping and
Gene Expression).
2.2 Genotyping Arrays. AB hereby grants to Licensee a personal, non-transferable,
royalty-bearing, non-exclusive license under the ABC Patent Rights in the Genotyping field and in
the Territory to make, but not have made, to use in providing services to third parties and sell
(directly or through distributors) Genotyping Arrays to end-users. Upon commencement of the
Genotyping Commercialization Phase, the foregoing license shall be suspended. Unless this Agreement
has expired or been terminated, upon termination of the Collaboration Agreement, the foregoing
license in this Section 2.2 shall resume.
2.3 Gene Expression Arrays. AB hereby grants to Licensee a personal,
non-
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
7
transferable, royalty-bearing, non-exclusive license under the ABC Patent Rights in the Gene
Expression field and in the Territory to make, but not have made, to use in providing services to
third parties and sell (directly or through distributors) Gene Expression Arrays to end-users. Upon
commencement of the Gene Expression Commercialization Phase, the foregoing license shall be suspended. Unless this Agreement has
expired or been terminated, upon termination of the Collaboration Agreement, the foregoing license
in this Section 2.3 shall resume.
2.4 Other Arrays. AB hereby grants to Licensee a personal, non-transferable,
royalty-bearing, non-exclusive license under the ABC Patent Rights, within that portion of the
Field that is outside of both the Genotyping field and Gene Expression field, and in the Territory
to make, but not have made, to use in providing services to third parties and sell (directly or
through distributors) Arrays to end-users.
2.5 Assay Loaders. AB hereby grants to Licensee a personal, non-transferable,
royalty-bearing, non-exclusive license under the ABC Patent Rights in the Field and in the
Territory to make, have made and to use in providing services to third parties (but not sell) Assay
Loaders, solely for the purpose of making Arrays subject to Sections 2.2-2.4 above. Upon
commencement of the Genotyping Commercialization Phase, the foregoing license shall exclude the
Genotyping field and any use of Assay Loaders for the purpose of making Genotyping Arrays. Upon
commencement of the Gene Expression Commercialization Phase, the foregoing license shall exclude
the Gene Expression field and any use of the Assay Loader for the purpose of making Gene Expression
Arrays (and if both the Genotyping Commercialization Phase and the Gene Expression
Commercialization Phase have commenced, the foregoing license shall exclude both the Genotyping
field and the Gene Expression field). Unless this Agreement has expired or been terminated, upon
termination of the Collaboration Agreement, the foregoing license in this Section 2.5 shall resume
in its entirety in the Field (both as to Genotyping and Gene Expression).
2.6 Sample Loader. AB hereby grants to Licensee a personal, non-transferable,
royalty-bearing, non-exclusive license under the ABC Patent Rights in the Field and in the
Territory to make, have made, use in providing services to third parties and to sell (directly or
through distributors) Sample Loaders solely to end users, and solely in connection with the sale of
Arrays to such end-users and solely for such end-users’ use of such Sample Loaders to load samples
to be run on (and assayed using) such Arrays. Upon commencement of the Genotyping Commercialization
Phase, the foregoing license shall exclude the Genotyping field and the foregoing right to sell
Sample Loaders shall be limited to sales to end users in connection with the sales of Arrays that
are not sold in the Genotyping field. Upon commencement of the Gene Expression Commercialization
Phase, the foregoing license shall exclude the Gene Expression field (and if both the Genotyping
Commercialization Phase and the Gene Expression Commercialization Phase have commenced, the
foregoing license shall exclude both Genotyping and Gene Expression). Unless this Agreement has
expired or been terminated, upon termination of the Collaboration Agreement, the foregoing license
in this Section 2.6 shall resume in its entirety the Field (both as to Genotyping and Gene
Expression).
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
8
2.7 Sales Distributor Rights. Licensee’s rights, to the extent expressly permitted in
Sections 2.1 through 2.4 and Section 2.6 above, to sell through distributors shall be contingent upon Licensee continuing to perform its obligations hereunder (including,
royalty reporting and payment obligations) as well as such distributors not exceeding the scope of
the license granted hereunder to Licensee (e.g., by selling or promoting Licensed Products outside
the Field or in a manner that does not comport with the labeling and notice requirements applicable
under this Agreement to such Licensed Products). The licenses granted under Sections 2.1 through
2.4 and Section 2.6 to sell Licensed Products through distributors shall not include the use of
distributors who infringe the Licensed Patent Rights.
2.8 No Implied Rights. Except as expressly set forth in this Agreement, the licenses
granted under this Agreement include no right, immunity or license, either expressly or by
implication, under any patent or patent application that is not included in the definition of
Licensed Patent Rights. Nothing in this Agreement shall be construed to grant to Licensee, its
Affiliates, distributors or respective customers any right, immunity from suit or license under any
patent claim or patent application claim directed to an assay, reagent, chemistry, real-time
process or method (including, without limitation any TaqMan® or SYBR assay, reagent, chemistry,
process or method rights).
2.9 Personal, Non-Transferable License. The rights granted to Licensee under this
Agreement are personal to Licensee alone. Licensee shall have no right to sublicense, assign or
otherwise transfer or share its rights hereunder. Without limiting other acts or omissions
constituting material breach hereof or AB’s remedies or causes of action for the same: (i) subject
to Section 2.10, any use, sale or promotion by Licensee of Licensed Products (including, without
limitation, using or promoting the use of Licensed Real-Time Thermal Cyclers with assays or
reagents in the Human In Vitro Diagnostic Field) in the Human In Vitro Diagnostic Field shall be
deemed a material breach of this agreement; and (ii) any breach by the Licensee of Article 2
(including, without limitation, by Licensee exceeding the scope of the license herein) shall be
deemed a material breach by Licensee of this Agreement.
2.10 ABC Patent Rights for Human In Vitro Diagnostic Field. In the event that
Licensee shall during the term of this Agreement obtain rights from Applera Corporation or its
assignee under the Real-Time Apparatus Patent Rights to make, use and sell Licensed Real-Time
Thermal Cycler instruments for use in the Human In Vitro Diagnostic Field, AB shall expand the
Field of the licenses granted under the ABC Patent Rights in Sections 2.2 through 2.6, to the
extent that the Applied Biosystems group of Applera Corporation is able to grant such rights
[***], to include
the rights of the Applied Biosystems group of Applera Corporation under such ABC Patent Rights in
the Human In Vitro Diagnostic Field. AB agrees that it [***] and that provisions of Article 2 that
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
9
suspend the licenses granted hereunder or that exclude the Genotyping field or Gene Expression
field upon commencement of the Genotyping Commercialization Phase or Gene Expression
Commercialization Phase, respectively, shall not be apply with respect to Licensed Products that
are sold in the Human In Vitro Diagnostics Field if and to the extent Applera Corporation or its
assignee expands the Field hereunder to encompass the Human In Vitro Diagnostics Field. Without
limiting the foregoing, to the extent the Applied Biosystems group of AB has the right to convey such rights [***], solely as to Arrays,
Assay Loaders and Sample fillers that are not used, promoted for use with or intended for use with
a thermal cycler (whether such thermal cycler is a Licensed Real-Time Thermal Cycler or an
end-point PCR thermal cycler that is not covered by the Real-Time Apparatus Patent Rights), the
Field of the licenses granted under the ABC Patent Rights in Sections 2.2 through 2.6 shall include
the rights of the Applied Biosystems group of Applera Corporation under such ABC Patent Rights in
the Human In Vitro Diagnostic Field. Notwithstanding anything herein to the contrary, all other
terms and conditions of the license granted herein under the ABC Patent Rights, including without
limitation the royalties to be paid pursuant to Section 3.1 (b) and other restrictions placed on
Licensee with respect to activities in the Field, shall continue to apply to the such activities in
the Field (whether or subject to this Section 2.10 such activities are in the Human In Vitro
Diagnostic Field).
3. Fees, Royalties, Records and Reports
3.1 Licensee shall make the following payments to AB in consideration of the licenses granted
under Article 2 of this Agreement:
| (a) | Issuance Fees. Licensee shall pay to AB the following non-refundable license issuance fees (that shall not be creditable against royalties). Such issuance fees shall be payable in six (6) installments as described below: |
| (i) | [***] in consideration of issuance of the license herein under the [***] due and payable in six (6) quarterly installments of [***], with the first quarterly installment due and payable within ten (10) business days after the Effective Date and each subsequent payment due and payable by the first day of each subsequent calendar quarter (by the following January 1, April 1, July 1 and October 1 respectively); and | ||
| (ii) | [***] (the “ABC Fee”) in consideration of issuance of the licenses herein under the ABC Patent Rights. Licensee shall receive a credit, not to exceed five hundred thousand dollars ($500,000), against such ABC Fee payment obligation which credit shall equal the following sum: the price [***] (as set forth in Section [***] of the Collaboration Agreement) [***] (as defined in the Collaboration Agreement) that are supplied [***] by Licensee to AB pursuant to the Collaboration Agreement (such credit, the “In-Kind Credit”). [***] of the foregoing ABC Fee shall be due and payable in six quarterly installments of [***], with the first such payment due and payable within ten (10) business days after the Effective Date and each subsequent payment due and payable by the first day of each subsequent calendar quarter (by the following January 1, April 1, July 1 and October 1 respectively). To the extent that the foregoing in-kind consideration provided by Licensee through the one year anniversary of the Effective Date of this Agreement is such that the In-Kind Credit is [***] the following sum shall be due within thirty (30) days after such one year anniversary: [***] minus the In-Kind Credit. |
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
10
In the event this Agreement is terminated pursuant to Article 6, the foregoing payment
obligations in this Section 3.1(a) shall accelerate and become due immediately upon the effective
date of any termination of this Agreement.
| (b) | Licensed Real-Time Cyclers Royalties. Licensee shall pay to AB the following non-refundable royalties on Net Sales of Licensed Real-Time Thermal Cyclers (including without limitation, all associated modules, components, software and computers): |
| (i) | a royalty for each Licensed Real-Time Thermal Cycler, including, without limitation, all modules, components, software and computers therein (whether sold as a complete system or sold separately for assembly of or for adding onto or installation on such Licensed Real-Time Thermal Cyclers) and associated warranty sold, delivered, invoiced or otherwise provided (or used in providing services to third parties) by any of Licensee, its Affiliates or distributors after the Effective Date, [***]; and | ||
| (ii) | a royalty for each repair or replacement component of a Licensed Real-Time Thermal Cycler, including any replacement modules and components, including software and computers, sold, delivered, invoiced or otherwise transferred (or used in providing services to third parties) by any of Licensee, its Affiliates or distributors after the Effective Date, [***], except that parts supplied free of charge under warranty, or repair, not amounting to reconstruction, of a Licensed Real-Time Thermal Cycler for which a royalty already has been paid under this Agreement, including replacement of components by identical components shall not be subject to royalty hereunder unless the component itself would directly or contributorily infringe the Real-Time Apparatus Patent Rights. |
However, so long as Licensee remains a licensee [***], for each royalty-bearing
item under Section (3.1) for which Licensee pays inter alia, a percentage of the [***], those percentage royalties [***] payments under Section [***] will be fully
[***] solely from such [***] that accrue after [***], under Section [***] of this Agreement for the very same item (i.e., the same unit) of [***].
| (c) | Array Royalties. Licensee shall pay to AB a royalty of [***] of Net Sales of each Array sold, delivered, invoiced or otherwise provided by any of Licensee, its Affiliates or distributors after the Effective Date; |
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
11
| (d) | Assay Loader. Licensee shall pay to AB a royalty of [***] of Net Sales of each Array sold, delivered, invoiced or otherwise provided by any of Licensee, its Affiliates or distributors after the Effective Date that is loaded, filled or reformatted with reagents or assays using an Assay Loader. The royalties paid under Section 3.1(c) for a given Array shall be credited against royalty obligations under this Section 3.1(d) for such Array such that the cumulative royalties under this Agreement for such Array shall not exceed [***] of Net Sales of such Array; | ||
| (e) | Sample Loader. Licensee shall pay to AB a royalty of [***] of Net Sales of each Sample Loader sold, delivered, invoiced or otherwise provided by any of Licensee, its Affiliates or distributors after the Effective Date; and | ||
| (f) | Services. Licensee shall pay to AB a royalty of [***] of Net Sales of services using Arrays, Assay Loader or Sample Loaders. In addition, Licensee shall pay to AB a royalty of [***] of the Average Net Sales Price (calculated in the same manner per Section 1.21(Net Sales) as a non-arms length sale of such products occurring on the date such services are rendered) of any Licensed Real-Time Thermal Cyclers, and repair or replacement components thereof, that are used by Licensee in providing services. For the avoidance of doubt, royalties on a given Licensed Real-Time Thermal Cycler, and repair or replacement component thereof, that are used by Licensee in rendering services shall only be due and payable once even if such Licensed Real-Time Thermal Cycler, and repair or replacement component thereof, are used subsequently in rendering additional services. |
3.2 Currency. All amounts payable hereunder shall be payable in United States
dollars. Any payments due hereunder on Net Sales outside of the United States will be paid to AB in
U.S. Dollars using the New York rate of exchange as quoted in the Wall street Journal for the
market close on the last business Thursday of the month preceding the month in which the Licensed
Product is sold or otherwise disposed of. If such rate of exchange is not so published, the Parties
may agree in writing on a substitute publication. In the event there is no comparable publication,
the applicable exchange rate for such date by the appropriate governmental agency in such country
(outside of the United States) shall apply.
3.3 Records. Licensee shall keep, and shall require its Affiliates and distributors,
as applicable, to keep, full, true and accurate records containing all particulars necessary to
show the amount payable to AB under this Agreement and to demonstrate Licensee’s (and its
Affiliates and distributors’) compliance with its obligations under this Agreement. Licensee shall
keep records sufficient to show
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
12
shipments (model, shipping date, quantity) to each Third-Party
licensee under the second paragraph of Section 2.1, which records shall be supplied to AB as part
of the quarterly royalty report to be delivered in accordance with Section 3.5 herein. Such records
and the supporting data shall be open at all reasonable times, for [***] (and access shall not be
denied thereafter, if reasonably available), to the inspection of an independent certified public
accounting firm retained by AB. Such accounting firm will hold such records and supporting data in
strict confidence, except as necessary to consult with and report to AB and Licensee on Licensee’s
compliance with this Agreement. If in dispute, such records shall be kept until the later of [***]
or the date the dispute is settled. Inspection shall be at AB’s expense, unless the inspector concludes that the amount payable that is stated in a
report is understated by [***] or more, in which case expenses shall be paid by Licensee.
3.4 Reporting. Licensee shall within [***] after the first of January, April, July
and October deliver (by U.S. mail or nationally recognized courier service with a text copy in
excel format (or other reasonable format requested in writing by AB) of such report transmitted by
email to [***]) to AB a true and accurate royalty accounting report. This report shall be on a
country-by-country basis and shall give such particulars of the business conducted by Licensee in
each country during the preceding three (3) calendar months as are pertinent to accounting under
this Agreement, and shall be in accordance with, and include all information specified in, the
royalty report form attached hereto as Exhibit II, as may be amended by AB in writing from time to
time.
The correctness and completeness of each report shall be attested to in writing by the
responsible financial officer of Licensee or by Licensee’s external auditor.
3.5 Payment & Reporting Address. Simultaneously with the delivery of each royalty
account report (as per the schedule set forth in Section 3.4), Licensee shall pay to AB the monies
then due under this Agreement for the period covered by the report. Each report and payment shall
be sent by the due date to the following address:
Applied Biosystems
000 Xxxxxxx Xxxxxx Xxxxx
Xxxxxx Xxxx, Xxxxxxxxxx, 00000 U.S.A.
Attention: Director of Licensing
000 Xxxxxxx Xxxxxx Xxxxx
Xxxxxx Xxxx, Xxxxxxxxxx, 00000 U.S.A.
Attention: Director of Licensing
or to any address that AB may advise in writing four (4) days prior to its becoming effective.
3.6 Interest. If Licensee shall fail to pay any amount owing under this Agreement by
the due date, the amount owed shall bear interest at [***] from the due date until paid, provided,
however, that if this interest rate is held to be unenforceable for any reason, the interest rate
shall be the maximum rate allowed by law at the time the payment is due.
3.7 Payment Default. Failure of Licensee to pay any amount specified under
this Agreement within thirty (30) days after the due date will allow AB to automatically terminate this
Agreement under Section 6.5.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
13
3.8 [***], Licensee acknowledges and agrees that the license herein reflects a [***]. In particular, rather than a [***] that is [***] to [***] of licensed patents and patent application [***], and also to avoid [***], Licensee has [***] reflected in this Article 3 whereby royalties are due on [***] so long as
the [***] is covered in any [***] by at least one [***] of the patents and patent applications licensed pursuant to [***] herein.
3.9 Acknowledgment. Licensee acknowledges that, until the date of last of the Valid
Claims of the Category I Real-Time Rights (or other applicable Real-Time Apparatus Patent Rights to
the extent such other Real-Time Apparatus Patent Rights also cover such apparatus), an apparatus
for conducting nucleic acid amplification reactions which apparatus is capable of alternately
heating and/or cooling samples contained in one or more reaction vessels and which apparatus is further capable of detecting reaction products
during the amplification reactions without opening the one or more reaction vessels (irrespective
of any hardware or software control or operational functionalities used in connection with the
apparatus for enabling or disabling selected functionalities of the apparatus) is covered by the
Real-Time Apparatus Patent Rights and is within the definition of Licensed Real-Time Thermal
Cycler. The foregoing shall not limit the definition of Licensed Real-Time Thermal Cycler.
Without limiting the foregoing, the following products have the hardware capability of
conducting real-time PCR analysis (irrespective of any instrument control or operational hardware
or software used in connection with the instrument is installed on such instrument or whether such
software is enabled or disabled) and are therefore within the scope of the Valid Claims of the
Real-Time Apparatus Patents and subject to the royalty obligations hereunder, subject to Section
3.8, as a Licensed Real-Time Thermal Cycler: (a) Licensee’s Open ArrayTM Genotyping
System (regardless of whatever other names under such instrument is now or in future sold and
whether sold or promoted solely for one or for both Genotyping or Gene Expression applications);
and (b) any other Licensee instrument (regardless of whether such instrument is sold assembled or
sold in separate components, such as a Reader and a separate thermal cycler that can be coupled to
such Reader, that are promoted for assembly or use together) that runs reactions on the Open
ArrayTM platform that provides or is adapted to provide functionality for substantially
contemporaneous sample amplification and detection of such amplification products. Without limiting
the foregoing, if after the Effective Date, Licensee sells, transfers, licenses or leases
instrument component or module (whether hardware or software) that confers the capability to
perform real-time amplification and detection (as described above) to a customer that had purchased
an instrument without such capability before the Effective Date which instrument (with such added
capability), if sold after the Effective Date, would be deemed a Licensed Real-Time Thermal Cycler
then such transaction with respect to such component or module shall, as of the date of such
transaction, be treated in the same manner as a sale or transfer that is not at arm’s-length (as
per Section 1.21 (Net Sales) of a Real-Time Thermal Cycler and shall be subject to royalties (at
the rate in Section 3.1(b)(i) herein) calculated on the Average Net Sales Price of a complete
Real-Time Thermal Cycler system (including, without limitation, all modules, components, software and computers therein).
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
14
3.10 Challenges. Without limiting Section 6.7 herein, if Licensee, directly or
indirectly, challenges or aids a third party in challenging the validity, enforceability or
priority (whether by or through a declaratory judgment action, interference, reexamination,
opposition, nullity or other action or proceeding) of any of the Licensed Patent Rights (in whole
or in part) or participates in, or supports in any way, any such action or proceeding with respect
thereto (each of the foregoing a “Challenge”), then any [***] that are subject to [***] that are still covered by one or more [***] after the [***] shall be subject, from the date of such [***] and thereafter, to [***] of the respective royalty rates set forth in Section 3.2 herein,
reflecting the [***] that has [***]. For the purposes of this Section 3.10, a [***] shall be deemed to be [***] at the time of [***], or,
if applicable, at the time the [***].
This Section 3.10 shall not apply with respect to Licensee [***] to the extent Licensee is [***] or other [***] in a [***] or other [***] by a third party that is not an
Affiliate of Licensee provided that Licensee has not [***] or otherwise provided such third party [***].
4. Past Sales and Activities
4.1 Past Sales and Activities. Within thirty
(30) days after the Effective Date Licensee shall submit to AB a royalty accounting report that conforms with the requirements of
Section 3.4 herein that shall detail (and that Licensee represents and warrants is accurate in all respects) all of the pre-Effective
Date activities that, had they occurred after the Effective Date of this Agreement, would be within the scope of the licenses and
subject to the royalty obligations of this Agreement (such pre-Effective Date activities “Past Activities”). Subject to the following,
such Past Activities shall be deemed licensed under this Agreement. Although such Past Activities shall be subject to the same royalty obligations
as the corresponding activities would accrued after the Effective Date, Licensee shall have the right to post-pone payment of such royalties
for such Past Activities for the term of this Agreement (without interest accruing on such payment obligation) for so long as Licensee remains
in good-standing under this Agreement and does not materially breach this Agreement or otherwise engage in conduct that would trigger
AB’s right to terminate this Agreement. Such payment obligation for Past Activities shall be waived upon expiration of this Agreement
(provided that for the term of the Agreement Licensee remained in good-standing under neither materially breached this Agreement nor otherwise
engage in conduct that would trigger AB’s right to terminate this Agreement). The royalties on Past Activities shall be due and payable
within five (5) business days of written notice by AB in the event this Agreement is terminated by Licensee prior to the expiration date prior
to the Effective Date or in the event Licensee materially breaches this Agreement or otherwise engages in conduct that would trigger AB’s
right to terminate this Agreement. The foregoing will not limit Licensee’s obligations to pay royalties due under Article 3 herein for
post-Effective Date royalty-bearing activities.
4.2 Notices to Past Customers. Licensee at its cost shall use reasonable efforts to
send to the original end-user customers of the Licensed Real-Time Thermal Cyclers that are the
subject of Section 4.1, license notices in accord with Section 5.1 with a means reasonably
satisfactory to AB to relate each such notice to the appropriate Licensed Real-Time Thermal Cycler.
Such notices shall limit the end-user’s rights to use within the Fields in a manner consistent with
this Agreement. Any such Licensed Real-Time Thermal Cycler not having such a license notice within
one hundred and eighty (180) days after the Effective Date of this Agreement shall cease to be a
Licensed Real-Time Thermal Cycler. Any of Licensee’s costs of enforcing such field limitations
shall be borne by Licensee.
5. License Notice
5.1 Notice. Licensee agrees to include prominently in the front of the user’s manual,
and affix a corresponding label statement to the corresponding product, for each Licensed Real-Time
Thermal Cycler sold worldwide, and for no other Thermal Cycler or Temperature Cycling Instrument, a
Notice as specified from time to time by Applera.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
15
Unless and until Applera reasonably instructs differently, the Notice shall be:
| (a) | For a Licensed Real-Time Thermal Cycler sold as an integrated system containing at least a real time detector and a Thermal Cycler: |
NOTICE TO PURCHASER
This real-time thermal cycler is licensed under U.S. Patent No. 6,814,934 and corresponding claims
in any foreign counterpart patent thereof owned by Applera Corporation, and data analysis software
for analyzing data generated from such real-time thermal cycler is licensed under U.S. Patent Nos.
7,188,030, 7,272,506, and 7,228,237 and corresponding claims in any foreign counterpart patent
thereof owned by Applera Corporation, in all cases solely for research and development and all
other applied fields except human in vitro diagnostics and in all cases solely for use with Open
ArrayTM through-hole array products that are licensed under U.S. Patent No. 6,086,825, 6,090,251, and
6,451,188 and corresponding claims in any foreign counterpart patent thereof owned by Applera
Corporation. No rights are conveyed expressly, by implication or estoppel to any patents on
real-time methods, including but not limited to 5’ nuclease assays, or to any patent claiming a
reagent or kit with the exception of U.S. Patent No. 5,928,907, claims 12-28, covering real-time
methods for instrumentation using a passive reference. This real-time thermal cycler may be covered
by at least one of U.S. Patent No. 6,105,674, 5,333,675, 5,656,493, 5,038,852, 5,475,610 (claims
160-163 and 167), 5,602,756, 6,703,236 (claims 1-6), and 7,238,517. For further information on
purchasing additional rights, contact the Director of Licensing at Applied Biosystems, 000 Xxxxxxx
Xxxxxx Xxxxx, Xxxxxx Xxxx, Xxxxxxxxxx, 00000, XXX.
| (b) | For Arrays: |
NOTICE
TO PURCHASER
This Open ArrayTM through-hole array product is licensed under U.S. Patent No.
6,086,825, 6,090,251, and 6,451,188 and corresponding claims in any foreign counterpart patent
thereof owned by Applera Corporation solely for research and development and all other applied
fields except human in vitro diagnostics and where interrogation of such array product requires
thermal cycling of the samples such array product contains then this array product is licensed
solely for use with thermal cyclers (whether real-time or basis thermal cyclers) that are either
not be covered by patents owned by Applera Corporation or, if covered by such patents, then such
thermal cyclers must be purchased from a licensed supplier of such thermal cyclers. No rights are
conveyed expressly, by implication or estoppel to any other patent claim (such as apparatus or
system claims of U.S. Patent No. 6,814,934). No rights are conveyed expressly, by implication or
estoppel to any patents on real-time methods, including but not limited to 5’ nuclease assays, or
to any patent claiming a reagent or kit with the exception of U.S. Patent No. 5,928,907, claims
12-28, covering real-time methods for instrumentation using a passive reference.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
16
For further
information on purchasing additional rights, contact the Director of Licensing at Applied
Biosystems, 000 Xxxxxxx Xxxxxx Xxxxx, Xxxxxx Xxxx, Xxxxxxxxxx, 00000, XXX.
| (c) | For Sample Loaders: |
NOTICE TO PURCHASER
This sample loader and methods of loading is licensed under apparatus and method claims of U.S.
Patent 7,211,148, 6,849,127, and 6,579,367 and method claims of U.S. Patent Application No.
2005/022675 and 2005/0226782 and corresponding claims in any foreign counterpart patent thereof
owned by Applera Corporation solely for research and development and all other applied fields
except human in vitro diagnostics and in all cases solely for use with Open ArrayTM
through-hole array products that are licensed under U.S. Patent No. 6,086,825, 6,090,251, and
6,451,188 and corresponding claims in any foreign counterpart patent thereof owned by Applera
Corporation. No rights are conveyed expressly, by implication or estoppel to any other patent claim
(such as apparatus or system claims of U.S. Patent No. 6,814,934). For further information on purchasing
additional rights, contact the Director of Licensing at Applied Biosystems, 000 Xxxxxxx Xxxxxx
Xxxxx, Xxxxxx Xxxx, Xxxxxxxxxx, 00000, XXX.
6. Term and Termination
6.1 Termination; Expiration. This Agreement (and the licenses and royalty obligations
hereunder), unless sooner terminated pursuant to Article 6 herein, shall continue until the date of
expiration of the last-to-expire of the patents under which rights are granted in this Agreement.
6.2 Termination by Licensee At Will. Licensee may terminate this Agreement for any
reason by giving written notice to AB and ceasing to label, advertise or promote any instruments as
Licensed Real-Time Thermal Cyclers. Such termination shall be effective thirty (30) days after said
notice or cessation, whichever is later.
6.3 Insolvency. This Agreement shall terminate upon (i) an adjudication of Licensee as
bankrupt or insolvent, or Licensee’s admission in writing of its inability to pay its obligations
as they mature; (ii) an assignment by Licensee for the benefit of creditors; (iii) the appointment
of, or Licensee’s applying for or consenting to the appointment of, a receiver, trustee or similar
officer for a substantial part of its property; or (iv) the institution of or any act of Licensee
instituting any bankruptcy, insolvency arrangement, or similar proceeding. This Agreement shall
terminate immediately and automatically without any requirement of notice or any other action by AB
upon the occurrence of any of the following unless cured by Licensee within a reasonable period
following such process: the issuance or levy of any judgment, writ, warrant or attachment or
execution or similar process against a substantial part of the property of Licensee. A termination
pursuant to this Section 6.3 shall be deemed to have occurred immediately prior to the events set forth in this Section triggering such termination.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
17
6.4 Change of Control. AB may terminate this Agreement immediately on notice upon any
change in the ownership or control of Licensee. For such purposes, a “change in ownership or
control” shall mean that, forty percent (40%) or more of the voting stock of Licensee becomes subject to the ownership or
control of a person or entity or any related group of persons or entities acting in concert, which
persons or entities did not own or control such portion of voting stock on the Effective Date
hereof. AB shall have the same right to terminate upon any transfer of forty percent (40%) or more of the assets of
Licensee. Notwithstanding the foregoing, a change in ownership or control shall not be deemed to
have occurred as a result of the acquisition of forty percent (40%) or more of the voting stock of Licensee by purely
financial investors (who are not operating business entities) in an initial public offering of
Licensee’s voting stock on a national stock exchange.
6.5 Breach and Cure. In the event Licensee materially breaches this Agreement, then
without limiting AB’s legal or equitable remedies, this Agreement shall terminate automatically
sixty (60) days after AB provides notice thereof to Licensee provided that Licensee fails to cure such
material breach within such sixty (60) days period. Any failure by Licensee to perform any payment obligation as and when due under this Agreement shall be
deemed a material breach of this Agreement.
6.6 Survival of Certain Rights. Subject to Section 6.7, termination or expiration of
this Agreement for any reason shall be without prejudice to any rights that shall have accrued to
the benefit of a Party prior to or on account of such termination or expiration. All remedies
provided hereunder or elsewhere are cumulative. Licensee’s obligations to report and pay royalties
as to activities under this Agreement, and AB’s right to audit under Section 3.4, shall survive
termination or expiration. Without limiting the survival of other Sections herein that expressly
survive termination or expiration, Articles 1, 6, 7, 8, 10 and 11 shall survive any termination or
expiration of this Agreement. Notwithstanding anything herein to the contrary, upon expiration or
termination of this Agreement, all licenses and rights granted to Licensee herein shall terminate.
6.7 Termination Upon Challenge. AB shall have the right to terminate this Agreement
in all countries in the Territory where such termination is legally permissible and enforceable
under applicable law, effective immediately upon written notice of termination to Licensee, in the
event that Licensee or any of its Affiliates either directly or indirectly, initiates, engages,
assists others or participates in a challenge to the validity, enforceability or priority (whether
by or through a declaratory judgment action, interference, reexamination, opposition, nullity or
other action or proceeding) of any of the Licensed Patent Rights in any country in the Territory.
Without limiting the foregoing or Licensee’s royalty obligations hereunder, Thermal Cycler Supplier
shall not be entitled to recover royalties paid during the course of a legal challenge or contest
to the validity, enforceability or priority of any of the Licensed Patent Rights even if such
challenge or contest should be successful. This Section 6.7 shall not apply with respect to
Licensee responding (e.g., by testifying or producing documents legally required) to the extent
Licensee is legally required to do so to subpoena or other involuntary legal process in a
litigation or other action by a third party that is not an Affiliate of Licensee provided that
Licensee has not encouraged, aided or suggested such litigation or other action or otherwise
provided such third party any assistance beyond what was legally required after service upon
License of such subpoena or other involuntary legal process in such litigation or action.
6.8 Court Decision. Should a court or administrative body find AB liable or culpable
due to Licensee’s manufacture of any Licensed Products or components thereof, covered by this
Agreement or due to the sale or distribution of such products by Licensee, its or its distributors,
ABI shall have the right to terminate this Agreement immediately upon written notice without Licensee having a right to cure.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
18
7. Confidentiality — Publicity
7.1 Advertising. Unless and until AB reasonably instructs differently, in
advertisements, catalogs, brochures, sales literature and promotional literature for Licensed
Real-Time Thermal Cyclers, Licensee, Affiliates and distributors shall bear licensing statements
prominently displayed that are consistent with the notice requirements set forth in Article 5
herein.
7.2 Changes to Certain References. With respect to Licensee’s distribution of any
written information to Third Parties, including but not limited to advertising, brochures,
catalogs, promotional and sales material, and public relations material, AB shall have the right to
require changes regarding references to, or descriptions of: Applera, Applied Biosystems, the
patents under which rights are granted in this Agreement or this Agreement. Licensee agrees to
comply with AB’s reasonable requirements.
7.3 Confidentiality. Except as provided in Sections 7.1 and 7.2, Licensee shall
maintain the confidentiality of the provisions of this Agreement and shall refrain from disclosing the terms of this Agreement without the prior written consent of AB, except to
the extent such disclosure is required under applicable law or regulation provided that (i) such
disclosure is limited to the information that must be so disclosed under applicable law or
regulation; and (ii) Licensee notifies AB of such requirement and the text of the proposed
disclosure at least (30) days before such proposed disclosure is required or in any event as far in
advance of the date of disclosure as is reasonably possible and allows ABG a reasonable opportunity
to comment upon, object or seek a protective order or other injunctive relief to prevent or limit
such disclosure. Licensee shall be permitted to issue a press release, reasonably acceptable to AB,
that announces the grant of the license hereunder.
8. Compliance and Quality
8.1 Compliance With Applicable Laws. In the exercise of any and all rights and in
performance hereunder, it shall be the duty of Licensee, not AB, to comply fully with all
applicable laws, regulations and ordinances and to obtain and keep in effect licenses, permits and
other governmental approvals (federal, state or local) necessary or appropriate to carry on
activities hereunder.
8.2 Non-Endorsement. AB does not approve or endorse any Licensed Product of Licensee
in any way or for any purpose, including for real-time PCR. Quality and quality control with
respect to suitability for real-time PCR, according to standards and requirements that may exist in
the marketplace from time to time, are the sole responsibility of Licensee.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
19
9. Assignment
9.1 Non-Assignability by Licensee. This Agreement (and the licenses granted hereunder)
is personal in nature. This Agreement and the rights hereunder are non-delegable and non-assignable
by the Licensee. Without limiting the foregoing, this Agreement cannot be assumed or assumed and
assigned by a trustee or debtor-in-possession in bankruptcy as set forth in Section 365(c)(1) of
the United States Bankruptcy Code or any similar provisions of state or federal law. This Agreement
shall not be assigned or transferred by Licensee (including without limitation by any attempted
assignment or transfer that would arise from a sale or transfer of Licensee’s business or assets or
acquisition of all of the equity or any change of control of Licensee) except as permitted under
this Section 9.1. Any such assignment or transfer or attempted assignment or transfer (except as
permitted under this Section 9.1) shall be void ab initio and result in the immediate and automatic
termination of this Agreement without any requirement of notice or other action by AB.
9.2 Assignability by ABI. Notwithstanding the foregoing, AB may assign or transfer
all or any part of its rights and obligations under this Agreement at any time without the consent
of Licensee. Licensee agrees to execute such further acknowledgements or other instruments as AB
may reasonably request in connection with such assignment.
10. Negation of Warranties and Indemnity
10.1 Disclaimer of Certain Representations, Warranties and Covenants. Nothing in this
Agreement shall be construed as: a) a warranty or representation by AB as to the validity,
enforceability or scope of any patent; b) a warranty or representation that anything made, used,
sold, or otherwise disposed of pursuant to this Agreement is or will be free from infringement of
patents or other intangible rights of third parties; or a warranty or representation that the
practice (or making, using, selling, offering, importing or transferring products licensed under
the Real-Time Apparatus Patent Rights) under the Real-Time Apparatus Patent Rights is or will be
free from infringement of patents of Third Parties; c) an obligation by AB to file any patent
application, secure any patent, or maintain any patent in force; d) any obligations of AB to
prosecute, enforce or sublicense its patent rights to (or against) third party infringers; e)
except as expressly set forth herein, conferring upon Licensee the right to use in advertising,
publicity or otherwise, in any form, the name of, or any trademark or trade name of, AB or any of
its affiliates; f) granting by implication, estoppel, or otherwise, any license, immunity or rights
under patents, trade secrets, know-how, copyrights, or other intangible rights of AB other than the
express licenses granted under the Real-Time Apparatus Patent Rights pursuant to Article 2
regardless of whether such patent is dominant or subordinate to the patents under which rights are
granted in this Agreement; g) an obligation to furnish any know-how; or h) creating any agency,
partnership, joint venture or similar relationship between AB and Licensee. Without limiting the
foregoing, nothing herein shall be construed to convey to Licensee any rights, licenses or
immunities from suit under any of the Licensed Patent Rights outside of the Field.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
20
10.2 Disclaimer of Express or Implied Warranties; Consequential Damages. EXCEPT AS
EXPRESSLY STATED HEREIN, AB MAKES NO EXPRESS OR IMPLIED WARRANTIES OF ANY KIND (INCLUDING, WITHOUT
LIMITATION, ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NONINFRINGEMENT)
WITH RESPECT TO THE REAL-TIME APPARATUS PATENT RIGHTS, THE PRACTICE OF THE LICENSE HEREUNDER OR THE
MAKING, USING OR SELLING OF PRODUCTS LICENSED HEREUNDER. IN NO EVENT SHALL AB BE LIABLE FOR ANY
SPECIAL, PUNITIVE, INCIDENTAL OR CONSEQUENTIAL DAMAGES (INCLUDING, WITHOUT LIMITATION LOST
PROFITS).
10.3 Indemnity. Licensee shall assume full responsibility for its operation under the
patents under which rights are granted in this Agreement, the manufacture of Licensed Real-Time
Thermal Cyclers and the use thereof and shall defend, indemnify and hold AB harmless from and
against all liability, demands, damages, expenses (including attorneys’ fees) and losses arising or
resulting from any and all third party claims (including, without limitation, any claims for
infringement or misappropriation of third party intellectual property rights, death, personal
injury, illness, property damage or any other injury or damage, including any damages or expenses
arising in connection with state or federal regulatory action) resulting from or arising in connection with
Licensee’s exercise of the licenses hereunder or Licensee’s, its agents, Affiliates or distributors
commercialization or promotion of any product licensed hereunder.
10.4 Representation of Ownership. AB represents and warrants to Licensee that AB is
the sole and exclusive owner of all right, title and interest in the Licensed Patent Rights, and
such Licensed Patent Rights are not subject to any encumbrance, lien or claim of ownership by any
third party that would encumber the rights granted to Licensee in this Agreement
11. General
11.1 Entire Agreement. This Agreement together with the Collaboration Agreement
constitutes the entire agreement between the Parties as to the subject matter hereof, and all prior
negotiations, representations, agreements and understandings are merged into, extinguished by and
completely expressed by it. The TCSA shall remain in effect in accordance with the terms and
conditions therein. This Agreement may be modified or amended only by a writing executed by
authorized officers of each of the Parties.
11.2 Notices. Any notice required or permitted to be given by this Agreement shall be
given by postpaid, first class, registered or certified mail, or by overnight courier or facsimile,
properly addressed to the other Party at the respective address as shown
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
21
below:
If to AB:
Applied Biosystems
000 Xxxxxxx Xxxxxx Xxxxx
Xxxxxx Xxxx, Xxxxxxxxxx, X.X.X. 00000
Attention: Director of Licensing
Fax No.: (000) 000-0000
000 Xxxxxxx Xxxxxx Xxxxx
Xxxxxx Xxxx, Xxxxxxxxxx, X.X.X. 00000
Attention: Director of Licensing
Fax No.: (000) 000-0000
If to Licensee:
Either Party may change its address by providing notice to the other. A notice shall be deemed
given four (4) full business days after the day of mailing, or one full day after the date of
delivery to the courier, or the date of facsimile transmission, as the case may be.
11.3 Governing Law and Venue. This Agreement shall be deemed made in the
State of California, and it shall be construed and enforced in accordance with the law of the
State of California. The Parties agree that the exclusive jurisdiction and venue for any dispute or
controversy arising from this Agreement shall be in the state or federal courts in California.
11.4 Severability. If any provision hereof should be held invalid, illegal or
unenforceable in any respect, then, to the fullest extent permitted by applicable law: (a) all
other provisions hereof shall remain in full force and effect and shall be liberally construed in
order to carry out the intent of the Parties as nearly as may be possible, and (b) the Parties
agree to negotiate in good faith a provision, in replacement of the provision held invalid, illegal
or unenforceable, that is consistent with applicable law and accomplishes, as nearly as possible,
the original intention of the Parties with respect thereto. To the fullest extent permitted by
applicable law, each Party hereby waives any provision of law that would render any provision
hereof prohibited or unenforceable in any respect.
11.5 Construction. Except where the context otherwise requires, wherever used, the
singular shall include the plural and the word “or” is used in the inclusive sense. The captions
and headings of this Agreement are for convenience of reference only and in no way define,
describe, extend or limit the scope or intent of this Agreement or the intent of any provision
contained in this Agreement. Each Party hereto and its counsel
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
22
have participated fully in the
review and negotiation of this Agreement. Both parties have participated equally in the formation
of this Agreement; the language of this Agreement shall not be presumptively construed against
either Party.
11.6 Independent Contractors. It is expressly agreed that the Parties, shall be
independent contractors and that the relationship between the Parties shall not constitute a
partnership, joint venture or agency.
11.7 Waiver. The waiver by either Party hereto of any right hereunder or the failure
to perform or a breach by the other Party shall not be deemed a waiver of any other right hereunder
or of any other breach or failure by said other Party whether of a similar nature or otherwise.
11.8 Execution in Counterparts. This Agreement may be executed (including via
facsimile or other reliable electronic means of transmitting signed copies such as via e-mail of
signed copies that are scanned as .pdf file attachments) in two (2) or more counterparts, each of
which shall be deemed an original, but all of which together shall constitute one and the same
instrument.
11.9 No Third Party Beneficiary. The representations, warranties, covenants, rights
and obligations set forth in this Agreement are for the sole benefit of the Parties and their
successors and permitted assigns, and they shall not be construed as conferring any rights on any
third parties.
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
23
IN WITNESS WHEREOF, the Parties hereto have duly executed this AGREEMENT as of the Effective
Date.
By
|
/s/ Xxxx X. Xxxxxxxxx
|
By | /s/ Xxxxxx X Xxxxxxx
|
|||||||
Name:
|
Xxxx X. Xxxxxxxxx | Name: | Xxxxxx X. Xxxxxxx | |||||||
Title:
|
Executive Vice President Applied Biosystems |
Title: | President & Chief Executive Officer |
| Approve for Signature | ||||
By:
|
/s/ Xxxxxx X. Xxxxxxxx | |||
Name:
|
Xxxxxx X. Xxxxxxxx | |||
Date:
|
11/9/07 | |||
| Applied Biosystems Legal Department | ||||
| Approve for Signature | ||||
By:
|
/s/ Xxxx Xxx | |||
Name:
|
Xxxx Xxx | |||
Date:
|
11/9/07 | |||
| Applied Biosystems Finance Department | ||||
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
24
EXHIBIT I
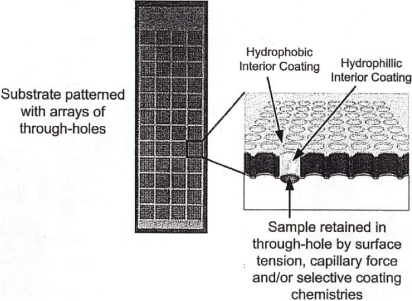
BT Open Array products commercialized and subject to this Agreement are each formed as a generally
planar substrate having opposing surfaces into which a multiplicity of through-holes are patterned
and extend through the substrate surfaces. Through-holes are sized so as to retain desired fluid
volumes introduced and retained therein. Fluid retention within each through-hole results at least
in part from one of: surface tension, capillary force, or use of selected coating chemistries such
as hydrophilic and hydrophobic coatings. Each through-hole may serve as a discrete low-volume
reaction chamber when filled with an appropriate sample and reaction chemistries. BT Open Arrays
may be adapted for use in genetic, genomic, proteomic, biochemical, or cellular sample analysis.
BT Open Array products may also serve as a carrier or holder for discrete sample localization and
distribution whereby through-holes are filled with selected samples, processed as desired, and such
samples later withdrawn for use in other processes or analysis protocols.
Exemplary OpenArrayTM Product
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
25
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
26
for the Period: to for Sales in the country of
Licensee: Effective Date: Royalty/Fee Rate
| o | Check here if there were no sales for this period. |
I hereby certify the information set forth above is correct and complete with respect to the
amounts due under this License Agreement.
By Title
Date
Name (please print)
Applera Corporation Send report to: Director of Licensing, Applied Biosystems, 000 Xxxxxxx Xxxxxx
Xxxxx, Xxxxxx Xxxx, XX 00000 XXX with an Excel-compatible copy sent via email to [***]
Portions of this Exhibit were omitted and have been filed separately with the Secretary of the
Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act.
27

