Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Triple asterisks denote omissions. RESEARCH COLLABORATION AND LICENSE AGREEMENT BY AND BETWEEN PFIZER INC. AND MACROGENICS, INC. OCTOBER [—], 2010
Exhibit 10.22
Execution Version
Confidential Materials omitted and filed separately with the Securities and Exchange Commission.
Triple asterisks denote omissions.
RESEARCH COLLABORATION AND LICENSE AGREEMENT
BY AND BETWEEN
PFIZER INC.
AND
MACROGENICS, INC.
OCTOBER [—], 2010
TABLE OF CONTENTS
| 1. | DEFINITIONS. | 1 | ||||||
| 2. | RESEARCH PROGRAM. | 15 | ||||||
| 2.1. | Selection of Research Project Targets. | 15 | ||||||
| 2.2. | Scope and Conduct of the Research Program. | 16 | ||||||
| 2.3. | Research Plans. | 17 | ||||||
| 2.4. | Research Program Antibodies and Passed MacroGenics Antibodies. | 18 | ||||||
| 2.5. | Governance of the Research Program. | 19 | ||||||
| 2.6. | Alliance Managers. | 21 | ||||||
| 2.7. | Conformance with Law. | 21 | ||||||
| 2.8. | MacroGenics Personnel Matters. | 21 | ||||||
| 2.9. | Debarment Certification. | 21 | ||||||
| 2.10. | Subcontractors. | 22 | ||||||
| 2.11. | Inspections. | 22 | ||||||
| 2.12. | Records. | 22 | ||||||
| 2.13. | Transfer and Use of Pfizer Proprietary Materials. | 23 | ||||||
| 3. | PRODUCT DEVELOPMENT, MANUFACTURING, COMMERCIALIZATION AND REGULATORY MATTERS. | 24 | ||||||
| 3.1. | General. | 24 | ||||||
| 3.2. | Diligence. | 24 | ||||||
| 3.3. | Regulatory Approvals. | 26 | ||||||
| 3.4. | Control of Commercialization Activities. | 26 | ||||||
| 3.5. | Manufacturing. | 26 | ||||||
| 3.6. | Progress Reporting. | 26 | ||||||
| 4. | LICENSES AND RELATED GRANTS OF RIGHTS. | 27 | ||||||
| 4.1. | Grants to Pfizer. | 27 | ||||||
| 4.2. | Grants to MacroGenics. | 29 | ||||||
| 4.3. | Reciprocal Non-Exclusive Research License for Disclosed Know-How and Confidential Information. | 29 | ||||||
| 4.4. | Retained Rights to Antibodies. | 30 | ||||||
| 4.5. | Exclusivity. | 30 | ||||||
| 4.6. | Section 365(n) of Bankruptcy Code. | 31 | ||||||
| 4.7. | No Implied Rights. | 31 | ||||||
| 5. | PAYMENTS TO MACROGENICS. | 31 | ||||||
| 5.1. | Upfront License Payment. | 31 | ||||||
| 5.2. | Research Support Funding. | 31 | ||||||
| 5.3. | Milestones. | 32 | ||||||
| 5.4. | Royalties. | 34 | ||||||
| 5.5. | Reports and Payments. | 36 | ||||||
| 5.6. | Maintenance of Records; Audits. | 37 | ||||||
| 5.7. | Late Payments. | 38 | ||||||
| 6. | INTELLECTUAL PROPERTY. | 39 | ||||||
| 6.1. | Inventions. | 39 | ||||||
| 6.2. | Patent Rights. | 39 | ||||||
i
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
| 6.3. | Recording. | 46 | ||||||
| 6.4. | Trademarks. | 47 | ||||||
| 7. | CONFIDENTIALITY | 47 | ||||||
| 7.1. | Confidentiality. | 47 | ||||||
| 7.2. | Authorized Disclosure. | 47 | ||||||
| 7.3. | Public Announcements; Publications. | 50 | ||||||
| 7.4. | Obligations in Connection with Change of Control. | 51 | ||||||
| 8. | REPRESENTATIONS AND WARRANTIES. | 51 | ||||||
| 8.1. | Mutual Representations and Warranties. | 51 | ||||||
| 8.2. | Representations and Warranties of MacroGenics. | 52 | ||||||
| 8.3. | Representations and Warranties of Pfizer. | 53 | ||||||
| 8.4. | MacroGenics Covenants. | 54 | ||||||
| 8.5. | Representation by Legal Counsel. | 54 | ||||||
| 8.6. | Disclaimer. | 55 | ||||||
| 9. | GOVERNMENT APPROVALS; TERM AND TERMINATION. | 55 | ||||||
| 9.1. | Government Approvals. | 55 | ||||||
| 9.2. | Term. | 55 | ||||||
| 9.3. | Termination by Either Party for Cause. | 55 | ||||||
| 9.4. | Termination by Pfizer without Cause. | 55 | ||||||
| 9.5. | Effects of Termination. | 56 | ||||||
| 9.6. | Disposition of Inventories of Products. | 59 | ||||||
| 9.7. | Survival of Certain Obligations. | 59 | ||||||
| 9.8. | Right to Termination of Research Project(s) or Research Program by Pfizer upon Change of Control of XxxxxXxxxxx. | 00 | ||||||
| 00. | LIMITATION ON LIABILITY, INDEMNIFICATION AND INSURANCE. | 60 | ||||||
| 10.1. | No Consequential Damages. | 60 | ||||||
| 10.2. | Indemnification by Pfizer. | 60 | ||||||
| 10.3. | Indemnification by MacroGenics. | 61 | ||||||
| 10.4. | Procedure. | 61 | ||||||
| 10.5. | Insurance. | 63 | ||||||
| 11. | MISCELLANEOUS. | 63 | ||||||
| 11.1. | Assignment. | 63 | ||||||
| 11.2. | Further Actions. | 63 | ||||||
| 11.3. | Force Majeure. | 63 | ||||||
| 11.4. | Notices. | 64 | ||||||
| 11.5. | Amendment. | 65 | ||||||
| 11.6. | Waiver. | 65 | ||||||
| 11.7. | Severability. | 65 | ||||||
| 11.8. | Descriptive Headings. | 65 | ||||||
| 11.9. | Dispute Resolution. | 65 | ||||||
| 11.10. | Governing Law. | 66 | ||||||
| 11.11. | Consent to Jurisdiction. | 66 | ||||||
| 11.12. | Entire Agreement. | 66 | ||||||
| 11.13. | Independent Contractors. | 66 | ||||||
| 11.14. | Counterparts. | 67 | ||||||
| 11.15. | No Third Party Rights or Obligations. | 67 | ||||||
ii
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
EXHIBITS
| Exhibit 2.3.1 | Form of Research Plans | |
| Exhibit 7.3.1 | Press Release |
i
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULES
| Schedule 1.19 | *** | |
| Schedule 1.34 | *** | |
| Schedule 1.80 | *** | |
| Schedule 1.125 | *** | |
| Schedule 2.10 | MacroGenics Subcontractors and Services | |
| Schedule 8.2.3 | Patent Rights of MacroGenics | |
| Schedule 8.2.8 | Funding Agreements | |
| Schedule 8.2.13 | Litigation |
ii
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
RESEARCH COLLABORATION AND LICENSE AGREEMENT
This Research Collaboration and License Agreement (the “Agreement”) is entered into as of October [—], 2010 (the “Effective Date”), by and among Pfizer, Inc., a corporation organized and existing under the laws of the State of Delaware and having a place of business at 000 Xxxxxx Xxxx, Xxxxxxxxxxxx, Xxxxxxxxxxxx 00000, Xxxxxx Xxxxxx (“Pfizer”) and MacroGenics, Inc., a corporation organized and existing under the laws of the State of Delaware and having a place of business at 0000 Xxxx Xxxx Xxxxx, Xxxxxxxxx, XX 00000 (“MacroGenics”). Pfizer and MacroGenics may each be referred to herein individually as a “Party” and collectively as the “Parties.”
WHEREAS, Pfizer is engaged in the research, development and commercialization of pharmaceutical and health care products;
WHEREAS, MacroGenics has developed a T-DART platform, which is focused on dual specificity “antibody-like” therapeutic proteins capable of targeting both immune effector cells and other cells with a single recombinant molecule, and certain intellectual property useful in connection with the application of such T-DART platform; and
WHEREAS, Pfizer and MacroGenics desire to collaborate to discover and research T-DARTs active against certain designated targets and to provide for Pfizer to further research, develop, manufacture and commercialize such T-DARTs and products containing such T-DARTs, as provided for herein.
NOW THEREFORE, in consideration of the mutual promises and covenants set forth below and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties hereby agree as follows:
| 1. | DEFINITIONS. |
When used in this Agreement, the following capitalized terms shall have the meanings set forth in this Section 1. Any terms defined elsewhere in this Agreement shall be given equal weight and importance as though set forth in Section 1.
| 1.1. | “Additional Third Party Licenses” is defined in Section 5.4.3(b). |
1.2. “Affiliate” means, with respect to any Person, any other Person that controls, is controlled by or is under common control with such Person. A Person shall be regarded as in control of another entity if it owns or controls at least fifty percent (50%) of the equity securities of the subject entity entitled to vote in the election of directors (or, in the case of an entity that is not a corporation, for the election of the corresponding managing authority), provided, however, that the term “Affiliate” shall not include subsidiaries or other entities in which a Party or its Affiliates owns a majority of the ordinary voting power necessary to elect a majority of the board of directors or other managing authority, but is restricted from electing such majority by contract or otherwise, until such time as such restrictions are no longer in effect.
1
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.3. “Agreement” is defined in the introduction to this Agreement.
1.4. “Agreement Product” means any product containing an Agreement T-DART.
1.5. “Agreement T-DART” means any T-DART that is identified during the course of the Research Program as binding to a Research Project Target, including any portion, fragment or subunit thereof, and any modified or derivative form of any of the foregoing, and the nucleic acid sequence encoding for any of the foregoing.
1.6. “Agreement T-DART IP” means any invention that constitutes or comprises the composition or formulation of, or any method of manufacture or use of, any Agreement T-DART or Agreement Product and that is invented by MacroGenics or any of its Affiliates, or any Subcontractor of MacroGenics or any of its Affiliates, solely or jointly with Pfizer or any of its Affiliates, or any Subcontractor of Pfizer or any of its Affiliates, in the conduct of the Research Program during the Research Term, and excluding any invention included in the Pfizer Material Improvements.
1.7. “Agreement T-DART Patent Right” means any Pfizer Patent Right filed after the Effective Date covering or claiming any invention assigned to Pfizer as Agreement T-DART IP pursuant to Section 4.1.2.
1.8. “Alliance Manager” is defined in Section 2.6.
1.9. “Annual Net Sales” means, with respect to any Agreement Product, the aggregate Net Sales by Pfizer, its Affiliates and its Sublicensees from the sale of such Agreement Product in the Territory during each Pfizer Year of the applicable Royalty Term for such Agreement Product.
1.10. “Antibody” means a molecule which comprises or contains: (a) one or more immunoglobulin variable domains; (b) fragments, variants, modifications or derivatives of such immunoglobulin variable domains irrespective of origin or source; or (c) the nucleic acid consisting of a sequence of nucleotides encoding (or complementary to a nucleic acid encoding) the foregoing molecules in (a) or (b). The term “Antibody” shall include any monospecific antibodies and less than full-length antibody forms such as Fv, Fab, and F(ab’).
1.11. “Applicable Law” means the laws, statutes, rules, regulations, guidelines, or other requirements (including Good Manufacturing Practices (“GMP”), Good Laboratory Practices and Good Clinical Practices), that may be in effect from time to time and apply to a Party’s activities to be performed under this Agreement, including any such laws, statutes, rules, regulations, guidelines, or other requirements of the FDA or the EMEA.
1.12. “Bankruptcy Code” is defined in Section 4.6.
1.13. “Binding Obligation” means, with respect to a Party (a) any oral or written agreement or arrangement that binds or affects such Party’s operations or property, including any assignment, license agreement, loan agreement, guaranty, or financing
2
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
agreement; (b) the provisions of such Party’s charter, bylaws or other organizational documents or (c) any order, writ, injunction, decree or judgment of any court or Governmental Authority entered against such Party or by which any of such Party’s operations or property are bound.
1.14. “Biosimilar Biologic Product” is defined in Section 5.4.3(b).
1.15. “Business Day” means a day other than a Saturday, a Sunday or a day that is a national holiday in the United States.
1.16. “Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 or December 31, for so long as this Agreement is in effect.
1.17. “Calendar Year” means each successive period of twelve (12) calendar months commencing on January 1 and ending on December 31.
1.18. “CD3” means the complex comprising the epsilon, delta and gamma subunits of the CD3 receptor complex (including any subtypes, alleles, and splice variants).
1.19. ***
1.20. “CPI” is defined in Section 1.43.
1.21. “Change of Control” means, with respect to a Party, (a) a merger, reorganization or consolidation of such Party with a Third Party which results in the voting securities of such Party outstanding immediately prior thereto ceasing to represent at least fifty percent (50%) of the combined voting power of the surviving or resulting entity immediately after such merger, reorganization or consolidation, (b) a Third Party becoming the beneficial owner of fifty percent (50%) or more of the combined voting power of the outstanding securities of such Party, or (c) the sale or other transfer to a Third Party of all or substantially all of such Party’s business or assets to which this Agreement relates; provided that, for purposes of subsections (a), (b) and (c), such Third Party or an Affiliate of such Third Party is engaged immediately prior to the Change of Control in the pharmaceutical or biotechnology business and is not a venture capital or other institutional investor. Change of Control shall not include any public offering of the shares of MacroGenics.
1.22. “Combination Product” means an Agreement Product containing an Agreement T-DART and one or more other therapeutically active ingredients.
1.23. “Commercialization” or “Commercialize” means activities directed to marketing, promoting, distributing, importing, exporting, using for commercial purposes or selling or having sold an Agreement Product. Commercialization shall not include any activities related to Manufacturing or Development.
3
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.24. “Commercially Reasonable Efforts” means, with respect to the efforts to be expended by a Party with respect to any objective, those reasonable, good faith efforts to accomplish such objective as such Party would normally use to accomplish a similar objective under similar circumstances. With respect to any efforts relating to the Development, Regulatory Approval or Commercialization of an Agreement T-DART or Agreement Product by a Party, generally or with respect to any particular country in the Territory, a Party will be deemed to have exercised Commercially Reasonable Efforts if such Party has exercised those efforts normally used by such Party, in the relevant country, with respect to a compound, product or product candidate, as applicable of similar modality owned or Controlled by such Party, or to which such Party has similar rights, which compound, product or product candidate is of similar market potential in such country, and is at a similar stage in its development or product life cycle as the Agreement T-DART or Agreement Product, taking into account all Relevant Factors in effect at the time such efforts are to be expended. To the extent that the performance of a Party’s obligations hereunder is adversely affected by the other Party’s failure to perform its obligations hereunder, the impact of such performance failure will be taken into account in determining whether such Party has used its Commercially Reasonable Efforts to perform any such affected obligations.
1.25. “Confidential Information” of a Party means all Know-How or other information, including proprietary information and materials (whether or not patentable) regarding such Party’s technology, products, business or objectives, that is communicated in any way or form by the Disclosing Party to the Receiving Party, either prior to or after the Effective Date of this Agreement (including any information disclosed pursuant to the Confidentiality Agreements), and whether or not such Know-How or other information is identified as confidential at the time of disclosure. The terms and conditions of this Agreement shall be considered the Confidential Information of each Party.
1.26. “Confidentiality Agreements” means the Confidentiality Agreements between the Parties dated ***.
1.27. “Control” or “Controlled” means, with respect to any (a) item of information, including Know-How, or (b) any other intellectual property right, the possession (whether by ownership interest or license, other than pursuant to this Agreement) by a Party of the ability to grant to the other Party access to or a license under such item or right, as provided herein, without violating the terms of any agreement or other arrangements with any Third Party; provided that any item of information or other intellectual property right that is licensed or acquired by a Party after the Effective Date and that would otherwise be considered to be under the Control of a Party shall not be deemed to be under the Control of such Party if the application of such definition in the context of any licenses or sublicenses granted to the other Party under this Agreement would require the granting Party to make any additional payments or royalties to a Third Party in connection with such license or sublicense grants, unless the other Party agrees to pay all such additional payments or royalties due to such Third Party.
4
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.28. “Develop” or “Development” means to discover, research or otherwise develop a product, including conducting any pre-clinical, non-clinical or clinical research and any drug development activity, including discovery, research, toxicology, pharmacology and other similar efforts, test method development and stability testing, manufacturing process development, formulation development, delivery system development, quality assurance and quality control development, statistical analysis, clinical studies (including pre- and post-approval studies), diagnostic assays in connection with clinical studies, and all activities directed to obtaining any Regulatory Approval, including any marketing, pricing or reimbursement approval. Development shall not include any activities related to Manufacturing or Commercialization.
1.29. “Development Milestone” is defined in Section 5.3.2.
1.30. “Development Milestone Payment” is defined in Section 5.3.2.
1.31. “Diligence Issue” is defined in Section 3.2.4.
1.32. “Disclosing Party” is defined in Section 7.1.
1.33. “Effective Date” is defined in the introduction to this Agreement.
1.34. ***
1.35. “EMEA” means the European Medicines Agency, or any successor agency thereto.
1.36. “FD&C Act” means the United States Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq.), as amended, and the rules and regulations promulgated thereunder.
1.37. “FDA” means the United States Food and Drug Administration or any successor agency thereto.
1.38. “Field” means all human and veterinary therapeutic, diagnostic, prophylactic and prognostic purposes, provided, however, that for so long as MacroGenics has obligations under, and solely to the extent required in order for MacroGenics not to be in violation of its obligations under, ***, the “Field” shall not include the Development or Commercialization of any Agreement T-DART or Agreement Product that binds to CD3 and is intended to be Developed or Commercialized *** ***
1.39. “First Commercial Sale” means, with respect to any Agreement Product and any country of the world, the first sale of such Agreement Product under this Agreement by Pfizer, its Affiliates or its Sublicensees to a Third Party in such country, after such Agreement Product has been granted Regulatory Marketing Approval by the competent Regulatory Authorities in such country. When used without reference to a specified Indication, First Commercial Sale means the First Commercial Sale for any Indication.
1.40. “First Research Project Target” is defined in Section 2.1.1.
5
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.41. “Foregone Agreement T-DART Patent Right” is defined 6.2.1(b).
1.42. “FTE” means a full time equivalent scientific person *** *** year, consisting of a minimum of a total of *** of scientific work directly related to and in support of the Research Program by an employee or full-time contractor or consultant of MacroGenics or any of its Affiliates.
1.43. “FTE Rate” means ***, increased or decreased annually by the percentage increase or decrease in the Consumer Price Index–Urban Wage Earners and Clerical Workers, United States City Average, All Items, 1982-84 = 100, published by the United States Department of Labor, Bureau of Labor Statistics (or its successor equivalent index) in the United States (“CPI”) as of December 31 of the then most recently ended Calendar Year over the level of the CPI on December 31, 2010 (i.e., the first such increase or decrease would occur on January 1, 2012).
1.44. “GAAP” means generally accepted accounting principles, consistently applied.
1.45. “GMP” is defined in Section 1.11.
1.46. “Generic Competition” is defined in Section 5.4.3(a).
1.47. “Governmental Authority” means any court, agency, department, authority or other instrumentality of any national, state, county, city or other political subdivision.
1.48. “IND” means an Investigational New Drug Application, as defined in the FD&C Act, that is required to be filed with the FDA before beginning clinical testing of an Agreement Product in human subjects, or an equivalent foreign filing.
1.49. “Indemnified Party” is defined in Section 10.4.1.
1.50. “Indemnifying Party” is defined in Section 10.4.1.
1.51. “Indication” means a disease, disease stage, discrete form of a disease or any precursor condition thereof or a line of treatment thereof ***, for which a separate Regulatory Approval Application has been obtained or is being sought.
1.52. “Initial Research Project Targets” is defined in Section 2.1.1.
1.53. “Initial Reserved Targets” is defined in Section 2.1.2.
1.54. “Joint Invention” is defined in Section 6.1.1(b).
1.55. “Joint Know-How” is defined in Section 6.1.1(b).
1.56. “Joint Patent Right” means any Patent Right claiming any Joint Invention.
1.57. “Joint Steering Committee” or “JSC” is defined in Section 2.5.1.
6
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.58. “Joint Technology” means, collectively, the Joint Patent Rights, the Joint Inventions and the Joint Know-How.
1.59. “Know-How” means any proprietary invention, discovery, data, information, process, method, technique, material, technology, result or other know-how, whether or not patentable.
1.60. “Liability” is defined in Section 10.2.
1.61. ***
1.62. ***
1.63. “Litigation Conditions” is defined in Section 10.4.2.
1.64. “MacroGenics” is defined in the introduction to this Agreement.
1.65. “MacroGenics Derived Terminated Agreement T-DART” is defined in Section 9.5.2(e)(i).
1.66. “MacroGenics Excluded Target Schedule” is defined in Section 2.1.1.
1.67. “MacroGenics Indemnified Party” is defined in Section 10.2.
1.68. “MacroGenics Know-How” means any Know-How comprised in the MacroGenics Technology, excluding any Joint Know-How.
1.69. “MacroGenics Patent Right” means any Patent Right comprised in the MacroGenics Technology, excluding any Joint Patent Rights.
1.70. “MacroGenics Technology” means any Patent Right or Know-How that (a) is Controlled by MacroGenics or any Affiliate of MacroGenics as of the Effective Date or that comes into the Control of MacroGenics or any of its Affiliates at any time during the Term and (b) is necessary or useful to Develop, Manufacture or Commercialize Agreement T-DARTs and/or Agreement Products.
1.71. “MacroGenics Third Party Agreement” means any agreement between MacroGenics and any Third Party under which MacroGenics obtains rights in or to any MacroGenics Technology.
1.72. “MacroGenics-Derived Terminated Agreement T-DART” is defined in Section 9.5.2(e)
1.73. “Major EU Market Country” means any of ***
1.74. “Major Market Country” means any ***
7
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.75. “Manufacturing” or “Manufacture” means activities directed to making, producing, manufacturing, processing, filling, finishing, packaging, labeling, quality assurance testing and release, shipping or storage of a product. Manufacturing shall not include any activities related to Development or Commercialization.
1.76. “Marginal Royalty Rates” is defined in Section 5.4
1.77. “Milestone Payment” means the Technical Milestones, Development Milestones, and Sales Milestones.
1.78. “Net Sales” means:
1.78.1. with respect to an Agreement Product that is not a Combination Product, gross receipts from sales by Pfizer and its Affiliates and Sublicensees (each, a “Selling Person”) of such Agreement Product to Third Parties in the Territory, less in each case, to the extent reasonably allocable to Agreement Products in accordance with GAAP, sales returns and allowances actually paid, granted or accrued, including trade, quantity and cash discounts and any other adjustments, including those granted on account of price adjustments, billing errors, rejected goods, damaged or defective goods, recalls, returns, rebates, chargeback rebates, reimbursements or similar payments granted or given to wholesalers or other distributors, buying groups, health care insurance carriers, chain pharmacies, mass merchandisers, staff model HMO’s, pharmacy benefit managers or other institutions, adjustments arising from consumer discount programs or other similar programs, customs or excise duties, sales tax, consumption tax, value added tax, and other taxes (except income taxes) or duties relating to sales, any payment in respect of sales to the United States government, any state government or any foreign government, or to any other Governmental Authority, or with respect to any government-subsidized program or managed care organization, and freight and insurance (to the extent that Pfizer bears the cost of freight and insurance for the Agreement Product) (the deductions described above are referred to collectively herein as “Permitted Deductions”); and
1.78.2. in the event an Agreement T-DART is sold as a Combination Product in any country, the Net Sales of the Combination Product, for the purposes of determining royalty payments, shall be determined by multiplying the Net Sales (as defined above in this Section) of the Combination Product by the fraction, A/(A+B) where A is the weighted (by sales volume) average sale price in such country of the Agreement T-DART when sold separately in finished form, and B is the aggregate weighted average sale price in such country of the other therapeutically active ingredients included in such Combination Product when sold separately in finished form. In the event that such average sale price cannot be determined for both the Agreement T-DART and the other product(s) included in the Combination Product, Net Sales for purposes of determining royalty payments shall be agreed by the Parties based on the relative value contributed by each component, such agreement not to be unreasonably withheld or delayed.
8
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
Sales between Pfizer and its Affiliates or Sublicensees shall be excluded from the computation of Net Sales and no payments will be payable on such sales except where such Affiliates or Sublicensees are end users, but Net Sales shall include the subsequent final sales to Third Parties by such Affiliates or Sublicensees. Net Sales shall be determined from books and records maintained in accordance with GAAP, as consistently applied by Pfizer with respect to sales of the Agreement Product. For clarity, in calculating Net Sales, except pursuant to Section 5.4.3, neither Party shall be entitled to deduct any payments to Third Parties by way of royalties or similar payments.
The Parties acknowledge that Pfizer does not currently intend to Commercialize any Agreement Product solely for diagnostic purposes and that the Parties anticipate that any sales of any Agreement Product for diagnostic purposes will occur only in connection with or in support of sales of an Agreement Product for therapeutic purposes. Notwithstanding the foregoing, in the event Pfizer, its Affiliates or Sublicensees Commercialize any Agreement Product for diagnostic purposes, sales of such Agreement Product for diagnostic purposes shall be included in the calculation of Net Sales provided that Pfizer and MacroGenics will negotiate in good faith a reasonable royalty applicable to Net Sales of any such Agreement Product for diagnostic purposes during the applicable Royalty Term, which royalty shall be no greater than the Marginal Royalty Rates otherwise set forth for Agreement Products under this Agreement.
1.79. “Non-Disclosing Party” is defined in Section 7.3.2.
1.80. ***
1.81. “Notice of Dispute” is defined in Section 11.9.1.
1.82. “Party” and “Parties” is defined in the introduction to this Agreement.
1.83. “Passed MacroGenics Antibody” means any proprietary (to MacroGenics) Research Program Antibody contributed to the Research Program by MacroGenics which the JSC has determined is no longer under Development or consideration for Development under the Research Program.
1.84. “Patent Rights” means any and all (a) patents, (b) pending patent applications, including all provisional applications, substitutions, continuations, continuations-in-part, divisions and renewals, and all patents granted thereon, (c) all patents-of-addition, reissues, reexaminations and extensions or restorations by existing or future extension or restoration mechanisms, including patent term extensions, supplementary protection certificates or the equivalent thereof, (d) inventor’s certificates, (e) any other form of government-issued right substantially similar to any of the foregoing and (f) all United States and foreign counterparts of any of the foregoing. The Patent Rights owned by either Party include any Patent Right assigned to such party pursuant to the provisions of this Agreement.
9
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.85. “Permitted Deduction” is defined in Section 1.78.1.
1.86. “Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, incorporated association, joint venture or similar entity or organization, including a government or political subdivision or department or agency of a government.
1.87. “Pfizer” is defined in the introduction to this Agreement.
1.88. “Pfizer Collaboration Patent Right” is defined in Section 9.5.2(e)(i).
1.89. “Pfizer Diligence Obligations” means Pfizer’s diligence obligations under Sections 3.2.1 and 3.2.2, collectively.
1.90. “Pfizer Indemnified Party” is defined in Section 10.3.
1.91. “Pfizer Know-How” means any Know-How comprised in the Pfizer Technology, excluding any Joint Know-How.
1.92. “Pfizer Patent Right” means any Patent Right comprised in the Pfizer Technology, excluding any Joint Patent Rights.
1.93. “Pfizer Proprietary Material Improvement” means all intellectual property rights constituting, comprising or covering any improvement or enhancement to, or any derivative or modification of, or any invention directly related to, any Pfizer Proprietary Material, which improvement, enhancement, derivative, modification or invention is conceived, discovered, invented, developed, created, made or reduced to practice or tangible medium by MacroGenics or any of its Affiliates, solely or jointly with Pfizer or any of its Affiliates, in the course of performing activities under this Agreement. For the avoidance of doubt, Pfizer Proprietary Material Improvement shall not include any Patent Right or Know-How that is directed to T-DARTs and any such Know-How or Patent Right that would otherwise of have been included as a Pfizer Proprietary Material Improvement shall instead be included in Agreement T-DART IP or the Agreement T-DART Patent Rights.
1.94. “Pfizer Proprietary Materials” means any and all biological and other materials Controlled by Pfizer and supplied by Pfizer to MacroGenics under this Agreement.
1.95. “Pfizer Quarter” means each of the four thirteen week periods (a) with respect to the United States, commencing on January 1 of any Pfizer Year and (b) with respect to any country in the Territory other than the United States, commencing on December 1 of any Pfizer Year.
1.96. “Pfizer Technology” means any Patent Right or Know-How that is (a) Controlled by Pfizer or any Affiliate of Pfizer as of the Effective Date or that comes into the Control of Pfizer or any of its Affiliates at any time during the Term and (b) is necessary or useful to Develop, Manufacture or Commercialize Agreement T-DARTs and/or Agreement Products.
10
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.97. “Pfizer Year” means the twelve (12) month fiscal periods observed by Pfizer (a) commencing on January 1 with respect to the United States and (b) commencing on December 1 with respect to any country in the Territory other than the United States.
1.98. “Phase I Clinical Study” means a study of an Agreement Product in human subjects or patients with the endpoint of determining initial tolerance, safety, metabolism or pharmacokinetic information and clinical pharmacology of such product as and to the extent defined for the United States in 21 C.F.R. § 312.21(a), or its successor regulation, or the equivalent regulation in any other country. A so-called Phase I/II Clinical Study shall be deemed to be a Phase I Clinical Study unless such study, when completed, allows Pfizer to proceed directly to a Pivotal Study.
1.99. “Phase II Clinical Study” means a study of an Agreement Product in human patients to determine the safe and effective dose range in a proposed therapeutic Indication as and to the extent defined for the United Sates in 21 C.F.R. § 312.21(b), or its successor regulation, or the equivalent regulation in any other country.
1.100. “Phase III Clinical Study” means a study of an Agreement Product in human patients with a defined dose or a set of defined doses of an Agreement Product designed to (a) ascertain efficacy and safety of such Agreement Product for its intended use; (b) define warnings, precautions and adverse reactions that are associated with the Agreement Product in the dosage range to be prescribed; and (c) support preparing and submitting applications for Regulatory Marketing Approval to the competent Regulatory Authorities in a country of the world, as and to the extent defined for the United States in 21 C.F.R.§ 312.21(c), or its successor regulation, or the equivalent regulation in any other country.
1.101. “Pivotal Study” means (a) a Phase III Clinical Study, or (b) a Phase II Clinical Study, or a combination Phase II Clinical Study and Phase III Clinical Study, if Pfizer has determined at the time of first dosing that the data generated in such trial, if successful, will be sufficient, without data from further studies, to support the filing of a Regulatory Marketing Approval Application for an Agreement Product.
1.102. “Receiving Party” is defined in Section 7.1.
1.103. “Regulatory Approval” means any technical, medical, scientific or other license, registration, authorization or approval of any Regulatory Authority (including any approval of a New Drug Applications or Biologic License Applications) necessary for the Development, Manufacture or Commercialization of a pharmaceutical product in any regulatory jurisdiction.
1.104. “Regulatory Approval Application” means any application submitted to an appropriate Regulatory Authority seeking any Regulatory Approval.
11
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.105. “Regulatory Authority” means, with respect to any national, supra-national, regional, state or local regulatory jurisdiction, any agency, department, bureau, commission, council or other governmental entity involved in the granting of a Regulatory Approval for such jurisdiction.
1.106. “Regulatory Exclusivity” means the ability to exclude Third Parties from Commercializing an Agreement Product or a Biosimilar Biologic Product with respect to any Agreement Product in a country, either through data exclusivity rights, orphan drug designation, or such other rights conferred by a Regulatory Authority in such country, other than through Patent Rights.
1.107. “Regulatory Marketing Approval” means, with respect to any pharmaceutical product and any Indication, Regulatory Approval (including any supplement thereto) to sell such pharmaceutical product for such Indication, including, in any jurisdiction other than the United States, to the extent required for any sale in such country, all pricing and reimbursement approvals to be obtained from the Regulatory Authority granting such Regulatory Approval or any affiliated Regulatory Authority.
1.108. “Regulatory Marketing Approval Application” means any Regulatory Approval Application submitted to an appropriate Regulatory Authority seeking any Regulatory Marketing Approval.
1.109. “Relevant Factors” means all relevant factors that may affect the Development, Regulatory Approval, Manufacture, or Commercialization of an Agreement Product, including (as applicable): actual and potential issues of safety, efficacy and/or stability; product profile (including product modality, category and mechanism of action); stage of development or life cycle status; actual and projected Development, Regulatory Approval, Manufacturing, and Commercialization costs, timelines and budgets; any issues regarding the ability to Manufacture or have Manufactured the Agreement Product; the likelihood of obtaining Regulatory Approvals (including satisfactory reimbursement or pricing approvals); the timing of such approvals; labeling or anticipated labeling; the then-current competitive environment and the likely competitive environment at the time of projected entry into the market; past performance of the product or similar products; present and future market potential; existing or projected pricing, sales, reimbursement and profitability; proprietary position, strength and duration of patent protection and anticipated exclusivity; other relevant scientific, technical, operational and commercial factors.
1.110. “Representatives” is defined in Section 7.2.1.
1.111. “Research Plan” is defined in Section 2.3.1.
1.112. “Research Plan Services” is defined in Section 2.3.3.
1.113. “Research Program” is defined in Section 2.2.
1.114. “Research Program Antibody” is defined in Section 2.4.1.
12
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.115. “Research Project” is defined in Section 2.3.1.
1.116. “Research Project Target” means a cancer cell Target that has been selected for a Research Project, including the Initial Research Project Targets and any Reserved Target substituted for an existing Research Project Target in accordance with Section 2.1.4.
1.117. “Research Term” means the period commencing on the Effective Date and continuing until three (3) years after the Effective Date, or until such later date as may be mutually agreed to by the Parties.
1.118. “Reserved Target” means any Target designated as a Reserved Target pursuant to Section 2.1.2 or 2.1.3 of this Agreement, including the Initial Reserved Targets.
1.119. “Review Period” is defined in Section 7.3.2.
1.120. “Royalty Term” is defined in Section 5.4.2.
1.121. “Sales Milestone” is defined in Section 5.3.3.
1.122. “Sales Milestone Payment” is defined in Section 5.3.3.
1.123. “Sales Threshold” is defined in Section 5.3.3.
1.124. “SEC” means the United States Securities and Exchange Commission.
1.125. ***
1.126. “Second Research Project Target” is defined in Section 2.1.1.
1.127. “Selling Person” is defined in Section 1.78.1.
1.128. “Senior Executives” is defined in Section 2.5.5(b).
1.129. “Sole Invention” is defined in Section 6.1.1(a).
1.130. “Sole Know-How” is defined in Section 6.1.1(a).
1.131. “Subcontractors” is defined in Section 2.10.1.
1.132. “Subcontractor Information” is defined in Section 2.11.
1.133. “Sublicensee” means any Third Party to whom Pfizer grants or has granted, directly or indirectly, a sublicense of rights licensed by MacroGenics to Pfizer under this Agreement, in accordance with the provisions of this Agreement.
1.134. “Subject Patent Right” is defined in Section 6.2.1(d).
13
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.135. “T-DART” means a dual affinity re-targeting molecule that binds with one arm to a Target expressed on an immune effector cell *** *** and with the second arm to a different Target expressed on another non-immune effector cell.
1.136. “Target” means (a) a specific biological molecule that is identified by a GenBank accession number or other public database accession identifier or by its amino acid or nucleic acid sequence; (b) all amino acid and nucleic acid variant forms of any biological molecule disclosed in clause (a), including naturally occurring variants, mutants, transcriptional and post-transcriptional isoforms (e.g., alternative splice variants), and post-translational modification variants (e.g., protein processing, maturation and glycosylation variants); and (c) truncated forms (including fragments thereof) which have a biological function substantially similar to that of any biological molecules disclosed in clause (a) or clause (b).
1.137. “Target Designation Date” means, with respect to any Research Project Target or Reserved Target, the date on which a Target becomes a Research Project Target or Reserved Target, as provided in Section 2.1. With respect to the Initial Research Project Targets and the Initial Reserved Targets, the Target Designation Date shall be deemed to be the Effective Date.
1.138. “Targeting” means, when used to describe the relationship between a molecule and a Target, that the molecule (a) binds to the Target (or a portion thereof) and (b) is designed or being developed to exert its biological effect in whole or in part through binding to such Target (or such portion thereof).
1.139. “Technical Milestone” is defined in Section 5.3.1.
1.140. “Technical Milestone Payment” is defined in Section 5.3.1.
1.141. “Term” is defined in Section 9.2.
1.142. “Terminated Agreement Product” is defined in Section 9.5.1(b).
1.143. “Terminated T-DART” is defined in Section 9.5.1.
1.144. “Territory” means the entire world.
1.145. “Third Party” means any Person other than Pfizer, MacroGenics or their respective Affiliates.
1.146. “Third Party Claim” is defined in Section 10.4.1.
1.147. “Trademark” means any trademark, trade dress, design, logo, slogan, house xxxx or name Controlled by Pfizer or its Affiliates or Sublicensees and used in connection with the Commercialization of any Agreement Product by Pfizer or its Affiliates or Sublicensees hereunder, including any registration or application for registration of any of the foregoing. For purposes of clarity, “Trademark” shall not include any trademark, trade dress, design, logo, slogan, house xxxx or name of MacroGenics, including “MacroGenics” or “DART”.
14
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
1.148. “Up-Front Payment” is defined in Section 5.1.
1.149. “Valid Claim” means (a) a claim of any unexpired United States or foreign issued patent that shall not have been dedicated to the public, disclaimed nor held invalid or unenforceable by a court or government agency of competent jurisdiction in an unappealed or unappealable decision or (b) ***
1.150. Construction. Except where the context expressly requires otherwise, (a) the use of any gender herein shall be deemed to encompass references to either or both genders, and the use of the singular shall be deemed to include the plural (and vice versa), (b) the words “include”, “includes” and “including” shall be deemed to be followed by the phrase “without limitation,” (c) the word “will” shall be construed to have the same meaning and effect as the word “shall,” (d) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (e) any reference herein to any Person shall be construed to include the Person’s successors and assigns, (f) the words “herein”, “hereof” and “hereunder”, and words of similar import, shall be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (g) all references herein to sections or exhibits shall be construed to refer to sections or exhibits of this Agreement, and references to this Agreement include all exhibits hereto, (h) the word “notice” means notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement, (i) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging), (j) references to any specific law, rule or regulation, or article, section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement or successor law, rule or regulation thereof, (k) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), and (l) the term “or” shall be interpreted in the inclusive sense commonly associated with the term “and/or.”
| 2. | RESEARCH PROGRAM. |
2.1. Selection of Research Project Targets.
2.1.1. Selection of Initial Research Project Target. Pfizer hereby designates *** as the Research Project Target for the first Research Project (the “First
15
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
Research Project Target”) and *** as the Research Project Target for the second Research Project (the “Second Research Project Target and together with the First Research Project Target, the “Initial Research Project Targets”).
2.1.2. Selection of Initial Reserved Targets. Pfizer hereby designates *** and *** as the initial reserved targets (“Initial Reserved Targets”).
2.1.3. Substitution of Targets. Pfizer may request that additional Targets be substituted on a one-for-one basis for the Second Research Project Target or any Reserved Target in accordance with this Section 2.1.3.
(a) At any time within *** following the Effective Date, Pfizer may substitute, without limitation on the number of substitutions and on a one-for-one basis, for the Second Research Project Target and the Reserved Targets.
(b) At any time beginning *** after the Effective Date and continuing until the earlier of (i) ***, and (ii) the allowance by MacroGenics of the second new Target to be substituted for a Reserved Target under this Section 2.1.3(b), Pfizer may request that additional Targets be substituted on a one-for-one basis for any existing Reserved Targets.
(c) MacroGenics will allow such substitutions set forth in (a) and (b), except that it may decline a proposed Target substitution based on (i) ***
2.1.4. Elevation of Reserved Targets. At any time in the *** following the Effective Date, Pfizer may submit a written notice to MacroGenics that up to two Reserved Targets will be substituted for any then-designated Research Project Target on a one-for-one basis. Any such Reserved Target substituted for an existing Research Project Target will then be deemed to be included within the definition of Research Project Target.
2.1.5. Former Reserved Targets. Any Reserved Targets shall cease to be a Reserved Target for all purposes under this Agreement (including under Section 4.5) upon the earlier of (a) replacement of such Reserved Target with a new Target in accordance with Section 2.1.3, or ***
2.1.6. Former Research Project Targets. Any Research Project Target, to the extent that it has been substituted by a Reserve Target, shall cease to be a Research Project Target for all purposes of this Agreement (including under Section 4.5).
2.2. Scope and Conduct of the Research Program. Under the terms and conditions set forth herein, MacroGenics and Pfizer shall collaborate during the Research Term to conduct discovery and pre-clinical Development activities to screen and identify Antibodies to the two (2) Research Project Targets (or the Reserved Targets that become Research Project Targets pursuant to Section 2.1.4) for construction of T-DARTs (the
16
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
“Research Program”). The Research Program shall be conducted in accordance with the Research Plan for each Research Project (as more fully provided in Section 2.3 below), and each Party shall use its Commercially Reasonable Efforts to perform all activities assigned to it and fulfill all of its obligations under each Research Plan. In addition, each Party shall conduct its activities under the Research Plan(s) in accordance with Applicable Law. MacroGenics will not, without first providing prior written notice to Pfizer, take any action with respect to conducting the Research Program that would require MacroGenics to make any payments to a Third Party such that any item of information or other intellectual property would be excluded from the definition of “Control” under this Agreement. For avoidance of doubt, upon expiration or termination of the Research Term, the Research Program and all Research Projects shall automatically terminate.
2.3. Research Plans.
2.3.1. Adoption of Research Plans. The Parties shall adopt a research plan (each a “Research Plan”) for all activities conducted under the Research Program during the Research Term, including a Research Plan that covers each Research Project Target; a “Research Project” shall mean the work to be performed pursuant to such a Research Plan. The form for each Research Plan for each Research Project Target is attached as Exhibit 23.1 The Research Plan for each Research Project Target shall be prepared by the JSC and adopted within *** after the Target Designation Date for such Research Project Target by the JSC. Each Research Plan shall reference this Agreement and shall be subject to all of the provisions of this Agreement, in addition to the specific details set forth in such Research Plan. To the extent any provisions of a Research Plan conflict or are inconsistent with the provisions of this Agreement, the provisions of this Agreement shall control. Unless otherwise expressly stated in a Research Plan, the provisions of each Research Plan shall be independent of and shall not affect the provisions of any other Research Plan. If the Parties are unable to agree on a Research Plan within the specified time period, the JSC may specify the Research Plan, and all disputes regarding the preparation or modification of any Research Plan (including the approval of any amendments thereto) shall be resolved by the JSC.
2.3.2. Researchers. During the *** of the Research Term the Parties will dedicate *** to the Research Program and this number cannot be changed without agreement of the Parties. The Parties currently expect to dedicate an average of *** to the Research Program for the remainder of the Research Term. The number of FTEs will be reviewed by the JSC on a semi-annual basis and may be updated as necessary by the JSC.
17
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
2.3.3. Responsibilities. Each Research Plan shall set forth the services and the obligations and responsibilities assigned to each Party (collectively the “Research Plan Services”), and shall include the following minimum terms:
(a) For each Research Project, one or both of the Parties shall generate or provide Antibodies that bind to the Research Project Target for such Research Project.
(b) In accordance with Section 2.4, Pfizer shall be entitled to select up to *** Antibodies to be investigated by MacroGenics for possible construction of a T-DART to the extent Antibodies are generated under a Research Plan.
(c) MacroGenics will investigate each Antibody selected by Pfizer in accordance with the applicable Research Plan.
(d) MacroGenics will support the ***
(e) Pfizer will support additional in vivo modeling, completion of IND-enabling studies, clinical Development, GMP-Manufacturing, and Commercialization.
(f) The Parties may jointly decide to expand MacroGenics’s research and Development responsibilities to include additional ***
(g) In no event shall MacroGenics be required to commit more than *** to support the Research Program during any Calendar Year during the Research Term.
2.4. Research Program Antibodies and Passed MacroGenics Antibodies.
2.4.1. For each Research Project Target and prior to initiation of in vivo modeling (as defined in the related Technical Milestone under Section 5.3.1) for such Research Project Target, Pfizer may identify in writing to MacroGenics up to *** Antibodies which Pfizer intends to evaluate for the relevant Research Project (each a “Research Program Antibody”).
2.4.2. Upon MacroGenics’s request, the JSC will promptly determine whether any proprietary (to MacroGenics) Research Program Antibody contributed to the Research Program by MacroGenics is no longer under Development or consideration for Development under the Research Program and has become a Passed MacroGenics Antibody.
2.4.3. Other than as set forth in the Research Plan for the Initial Research Project Target that is attached as Exhibit 2.3.1 or as subsequently agreed by the Parties and set forth in a Research Plan, MacroGenics shall have no obligation to provide Pfizer with any Antibodies developed or acquired by MacroGenics outside of this Agreement.
18
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
2.5. Governance of the Research Program.
2.5.1. Formation of the Joint Steering Committee. MacroGenics and Pfizer shall establish a “Joint Steering Committee” (or “JSC”) to oversee and coordinate the activities of the Parties under this Agreement in regard to the Research Program. The Joint Steering Committee shall also serve as a forum to facilitate communications between the Parties regarding the Research Program. The Joint Steering Committee shall be comprised of three (3) representatives from each Party as appointed by such Party. The Joint Steering Committee may change its size from time to time by mutual consent of its members. A Party may replace one or more of its representatives from time to time upon written notice to the other Party. Each Party, respectively, shall designate its initial members of the Joint Steering Committee within thirty (30) days after the Effective Date. The Joint Steering Committee shall exist until expiration of the Research Term, unless the Parties otherwise agree in writing.
2.5.2. Co-Chairpersons and Secretary of the Joint Steering Committee. Each Party shall designate a co-chairperson of the Joint Steering Committee and a secretary of the Joint Steering Committee shall be designated in accordance with Section 2.6 below. A Party may change the designation of its co-chairperson from time to time upon written notice to the other Party. The co-chairpersons shall be responsible for scheduling meetings of the Joint Steering Committee, preparing agendas for meetings and sending to all Joint Steering Committee members notices of all regular meetings and agendas for such meetings at least five (5) Business Days before such meetings. The co-chairpersons shall solicit input from both Parties regarding matters to be included on the agenda, and any matter either Party desires to have included on the agenda shall be included for discussion. Nothing herein shall be construed to prohibit the Joint Steering Committee from discussing or acting on matters not included on the applicable agenda. The secretary shall record the minutes of the meeting, circulate copies of meeting minutes to the Parties and each Joint Steering Committee member promptly following the meeting for review, comment and approval by the Joint Steering Committee members and finalize approved meeting minutes. The co-chairpersons shall be members of the Joint Steering Committee but the secretary need not be a member of the Joint Steering Committee.
2.5.3. Meetings. The Joint Steering Committee shall meet at least once each Calendar Quarter until it has been terminated in accordance with Section 2.5.1 at dates and times mutually agreed by the Joint Steering Committee, unless otherwise mutually agreed by the Parties. The initial meeting of the Joint Steering Committee shall be held within ninety (90) days after the Effective Date. Either Party may call a special meeting of the Joint Steering Committee on fifteen (15) days prior written notice to the other Party’s members of the Joint Steering Committee (or upon such shorter notice as exigent circumstances may require). Such written notice shall include an agenda for the special meeting. In-person meetings, including special meetings, of the Joint Steering Committee shall alternate between the offices of the Parties, unless otherwise agreed upon by the members of the Joint Steering Committee. Meetings of the Joint Steering
19
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
Committee may be held telephonically or by video conference; provided, however, that at least two (2) meetings per year shall be held in-person. Meetings of the Joint Steering Committee shall be effective only if at least one representative of each Party is in attendance or participating in the meeting. Members of the Joint Steering Committee shall have the right to participate in and vote at meetings held by telephone or video conference. In addition, the Joint Steering Committee may act on any matter or issue without a meeting if it is documented in a written consent signed by each member of the Joint Steering Committee.
2.5.4. Responsibilities of the Joint Steering Committee. The JSC shall be responsible for (a) planning and supervising research and development under this Agreement, including establishing, reviewing and recommending modifications and updates to the Research Plans; (b) receiving and reviewing all data and other information obtained by either Party in connection with the Research Program and monitoring and reporting to the Parties on activities conducted pursuant to the Research Plans; (c) documenting and approving initiation and completion of each Research Project and the achievement of any Technical Milestones, Development Milestones and Sales Milestones; (d) evaluating FTE requirements for the performance of the Research Plans; and (e) such other functions as expressly specified hereunder or as agreed by the Parties.
2.5.5. Decisions by Consensus.
(a) All decisions of the Joint Steering Committee shall be made by unanimous agreement of both Parties’ representatives, with each Party having a single vote, irrespective of the number of JSC representatives in attendance at a meeting.
(b) If, in accordance with Section 2.5.5(a), the JSC does not resolve any matter within its purview by it within *** after the matter is first considered by it, the matter may be referred by either Party to MacroGenics’s *** to be resolved by negotiation in good faith as soon as practicable but in no event later than *** after referral. Such resolution, if any, of a referred issue by the *** shall be final and binding on the Parties.
(c) If a dispute referred to the *** has not been resolved in accordance with Section 2.5.5(b), then, subject to Section 2.5.5(d), ***. Any decisions made by Pfizer under this Section 2.5.5(c) shall be deemed a decision of the JSC for purposes of this Agreement.
(d) Notwithstanding Section 2.5.5(c), *** shall not have the right to exercise such decision-making authority (i) in a manner that excuses *** from any of its obligations specifically enumerated under this Agreement; (ii) in a manner that negates any consent rights or other rights specifically allocated to MacroGenics under this Agreement; (iii) to resolve any
20
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
dispute regarding whether a Technical Milestone, Development Milestone or Sales Milestone has been achieved; (iv) in a manner that would require *** (A) *** will not reimburse *** costs (except as expressly set forth in this Agreement ); (B) which would require *** to perform a category of services not previously contemplated as being performed by *** under the Research Program and which *** does not have the existing capability to perform; or (C) which require *** to acquire from any Third Party any Know-How, Patent Right or other technology not contemplated in a Research Plan and that is not readily available from such Third Party or readily capable of being developed internally by ***; or (vi) in a manner that would require *** to perform any act that it reasonably believes to be inconsistent with any Applicable Law or any approval, order, policy, guidelines of a Regulatory Authority or ethical requirements or ethical guidelines.
2.6. Alliance Managers. In addition to the foregoing governance provisions, each of the Parties shall appoint a single individual to serve as that Party’s alliance manager (“Alliance Manager”). The role of each Alliance Manager shall be to facilitate the relationship between the Parties as established by this Agreement. The Alliance Managers shall attend meetings of the Joint Steering Committee and support the respective co-chairpersons of such committee in the discharge of their responsibilities. Unless otherwise determined by the Joint Steering Committee, Pfizer’s Alliance Manager shall serve as secretary at each meeting of the Joint Steering Committee. Alliance Managers shall be non-voting participants in such committee meetings. A Party may replace its Alliance Manager from time to time upon written notice to the other Party.
2.7. Conformance with Law. Each Party shall perform and discharge its obligations under this Agreement and the Research Program in conformance with (a) professional standards and practices, (b) this Agreement and the Research Plan(s) and (c) all Applicable Laws. Without limiting the generality of the foregoing, each Party shall retain all records relating to its performance of this Agreement and the Research Plan(s) for the time periods required by Applicable Laws.
2.8. MacroGenics Personnel Matters. MacroGenics acknowledges and agrees that it is solely responsible for the compensation of the personnel assigned to the Research Plan Services, and as employer shall be responsible for withholding all national, state, local or other applicable taxes and similar items. MacroGenics also shall be responsible for all other employer related obligations, including providing appropriate insurance coverage and employee benefits, and making all other deductions required by law affecting the gross wages of each employee. MacroGenics personnel assigned to the Research Plan Services are not nor shall they be deemed to be employees of Pfizer.
2.9. Debarment Certification. Neither Party nor any Person employed or retained to perform services by either Party has been debarred under Section 306(a) or (b) of the FD&C Act or any comparable provision of foreign law and no debarred Person shall in the future be employed or retained to perform services by either Party in connection with
21
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
any work to be performed for or on behalf of the other Party. If, at any time after execution of this Agreement, either Party becomes aware that such Party or any Person employed or retained to perform services by such Party in connection with any work performed for or on behalf of such Party is, or is in the process of being, debarred, such Party shall so notify the other Party immediately.
2.10. Subcontractors.
2.10.1. MacroGenics may not engage any contractor, subcontractor or other vendor (a “Subcontractor”) to perform any Research Plan Services or Research Program activities not specifically contemplated or set forth in the applicable Research Plan without Pfizer’s prior written consent; provided, however, that Pfizer hereby provides its prior written consent to MacroGenics’s engagement of the Subcontractors set forth on Schedule 2.10 attached hereto for performance of the Research Plan Services set forth on such Schedule 2.10. MacroGenics shall be responsible for the management of all permitted Subcontractors. The engagement by MacroGenics of any Subcontractor in compliance with this Section 2.10 shall not relieve MacroGenics of its obligations under this Agreement or any applicable Research Plan. Any agreement between MacroGenics and a permitted Subcontractor pertaining to the Research Plan Services shall be consistent with the provisions of this Agreement. Furthermore, unless otherwise agreed by Pfizer in writing, prior to or at the time of engagement of any Subcontractor to perform any obligations hereunder, MacroGenics shall cause such Subcontractor to agree in writing to be bound by substantially equivalent obligations of confidentiality and use of proprietary materials as those in this Agreement.
2.10.2. ***
2.11. Inspections. Pfizer authorized representative(s), and Regulatory Authorities to the extent required by law and applicable to the scope of the Research Plan Services performed, may, during regular business hours and, to the extent legally possible, at times arranged in advance with MacroGenics, audit, inspect and copy all data, records and work products, and audit and inspect all facilities, relating to the Research Plan Services and MacroGenics’s performance under this Agreement and the applicable Research Plan(s) (including all data, records, work products and facilities of Subcontractors); provided, however, that MacroGenics may limit the scope of any such audit or inspection to prevent the disclosure of confidential information of MacroGenics or the disclosure of confidential information of MacroGenics’s Subcontractors (“Subcontractor Information”), unless such confidential information or Subcontractor Information is directly related to the performance of the Research Plan Services and the disclosure is required for purposes of the audit or inspection.
2.12. Records. Each Party shall prepare, maintain and retain complete and accurate written records, accounts, notes, reports and data of the Research Plan Services and its performance under this Agreement and the Research Plan(s), in a form and of quality reasonably acceptable to both Parties. All such information generated by MacroGenics
22
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
shall be treated as Confidential Information of both Parties for the purpose of this Agreement and all such information generated by Pfizer shall be treated as Confidential Information of Pfizer for the purposes of this Agreement.
2.13. Transfer and Use of Pfizer Proprietary Materials.
2.13.1. Transfer. From time to time, pursuant to a Research Plan, or otherwise, Pfizer may provide MacroGenics with Pfizer Proprietary Materials. Pfizer represents and warrants to MacroGenics that Pfizer has the right to provide the Pfizer Proprietary Materials to MacroGenics for the uses authorized herein. Except as expressly set forth in the preceding sentence, the Pfizer Proprietary Materials are provided by Pfizer on an “as-is” basis without representation or warranty of any type, express or implied, including any representation or warranty of merchantability, non-infringement, title or fitness for a particular purpose, each of which is hereby disclaimed by Pfizer.
2.13.2. Use of Pfizer Proprietary Materials. MacroGenics shall use the Pfizer Proprietary Materials solely in connection with conducting the specific activities under this Agreement for which such Pfizer Proprietary Materials are provided to MacroGenics, including, if applicable, the provisions of any specific Research Plan under which such Pfizer Proprietary Materials are provided, and for no other purpose. Without limiting the generality of the foregoing, except as expressly set forth in this Agreement or in any applicable Research Plan, MacroGenics shall not make or attempt to make analogues, progeny or derivatives of, or modifications to, the Pfizer Proprietary Materials and MacroGenics shall not use the Pfizer Proprietary Materials for the benefit of any Third Party or of its own internal research programs outside of the Research Program. MacroGenics shall not administer any of the Pfizer Proprietary Materials to any human. MacroGenics shall comply with all Applicable Laws regarding the handling and use of the Pfizer Proprietary Materials. MacroGenics agrees to retain possession over the Pfizer Proprietary Materials and not to provide the Pfizer Proprietary Materials to any Third Party without Pfizer’s prior written consent, except as required to perform the Research Program.
2.13.3. Unauthorized Use of Materials. In the event that MacroGenics uses the Pfizer Proprietary Materials for any purpose other than the purposes authorized herein, the results of such unauthorized research, and any discoveries or inventions that arise from such unauthorized research, whether patentable or not, shall belong solely and exclusively to Pfizer. If required in order to perfect or enforce Pfizer’s rights to such results, discoveries or inventions, MacroGenics hereby assigns and agrees to assign to Pfizer all of its right, title and interest in and to all such results, discoveries or inventions. MacroGenics agrees to cooperate with Pfizer, and to execute and deliver any and all documents that Pfizer deems reasonably necessary, to perfect and enforce its rights hereunder.
23
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
2.13.4. Title to Pfizer Proprietary Materials. All right, title and interest in the Pfizer Proprietary Materials shall remain the sole property of Pfizer notwithstanding the transfer to and use by MacroGenics of the same.
2.13.5. Return of Pfizer Proprietary Materials. Upon completion of the activities for which the Pfizer Proprietary Materials have been provided, or upon expiration or termination of this Agreement or the applicable Research Plan, if earlier, MacroGenics shall, at Pfizer’s option and expense, either destroy or return to Pfizer all unused Pfizer Proprietary Materials.
2.13.6. Assignment. MacroGenics hereby assigns to Pfizer all of MacroGenics’s right, title and interest in and to any Pfizer Proprietary Material Improvements. During and after the Term, MacroGenics shall promptly notify Pfizer of any Pfizer Proprietary Material Improvement developed by MacroGenics and shall cooperate fully in obtaining patent and other proprietary protection for such Pfizer Proprietary Material Improvement. Such protection shall be obtained in the name of Pfizer and at Pfizer’s cost and expense, and MacroGenics, without limitation, shall execute and deliver all requested applications, assignments and other documents, and take such other measures, as Pfizer shall reasonably request, in order to perfect and enforce Pfizer’s rights in any Pfizer Proprietary Material Improvement. MacroGenics appoints Pfizer its attorney to execute and deliver any such documents and take such actions on MacroGenics’s behalf in the event MacroGenics fails to do so.
| 3. | PRODUCT DEVELOPMENT, MANUFACTURING, COMMERCIALIZATION AND REGULATORY MATTERS. |
3.1. General. Except as expressly set forth in Section 2, Pfizer shall have sole authority over and control of the Development, Manufacture and Commercialization of Agreement Products.
3.2. Diligence.
3.2.1. Development Diligence. Pfizer will use Commercially Reasonable Efforts to Develop (including to seek Regulatory Approval for) each Agreement Product in at least one Indication in the ***: (i) ***. Pfizer may determine in its sole discretion the order in which it seeks any such Regulatory Approval. Pfizer will have no other diligence obligations with respect to the Development (including to seek Regulatory Approval of) of Agreement Products under this Agreement. For the avoidance of doubt, any actions taken by Pfizer’s Affiliates or Sublicensees under this Agreement shall be treated as actions taken by Pfizer in regard to satisfaction of the requirements of this Section 3.2.
3.2.2. Commercial Diligence. Pfizer will use Commercially Reasonable Efforts to Commercialize a given Agreement Product in each Major Market Country in the Field in the Territory where Pfizer or its Affiliates have received Regulatory
24
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
Approval for such Agreement Product. Pfizer will have no other diligence obligations with respect to the Commercialization of Agreement Products under this Agreement.
3.2.3. Exceptions to Diligence Obligations. Notwithstanding any provision of this Agreement to the contrary, Pfizer will be relieved from and will have no obligation to undertake any efforts with respect to any Pfizer Diligence Obligation in the event that:
(a) Pfizer or MacroGenics receives or generates any safety, tolerability or other data reasonably indicating, as measured by Pfizer’s safety and efficacy evaluation criteria and methodology, or signaling that an Agreement Product has or would have an unacceptable risk-benefit profile or is otherwise not reasonably suitable for initiation or continuation of clinical trials in humans; or
(b) Pfizer or MacroGenics receive any notice, information or correspondence from any applicable Regulatory Authority, or any applicable Regulatory Authority takes any action, that reasonably indicates that an Agreement Product is unlikely to receive Regulatory Approval.
3.2.4. Assertion of Pfizer Diligence Obligation Claims. If MacroGenics becomes aware of facts that might form a reasonable basis to allege that Pfizer has failed to meet any Pfizer Diligence Obligation, then MacroGenics will notify Pfizer in writing of such potential alleged performance failure (each such potential alleged performance failure, a “Diligence Issue”). Promptly upon Pfizer’s receipt of any notice of a Diligence Issue pursuant to this Section 3.2.3, the Pfizer Alliance Manager will contact the MacroGenics Alliance Manager to discuss the specific nature of such Diligence Issue and seek to identify an appropriate corrective course of action. If, no later than thirty (30) days after Pfizer’s receipt of such a notice, (a) the Parties have not reached consensus regarding whether Pfizer has failed to satisfy the Pfizer Diligence Obligations and (b) the Parties’ respective Alliance Managers have not agreed upon an appropriate corrective course of action for such Diligence Issue, then such Diligence Issue will be escalated and resolved pursuant to the dispute resolution provisions set forth in Section 11.9. Failure by MacroGenics to provide notice of a Diligence Issue pursuant to this Section 3.2.4 shall not result in a waiver by MacroGenics of its rights under Section 3.2.5 of this Agreement.
3.2.5. Remedies for Breach of Pfizer Diligence Obligations. If Pfizer materially breaches any Pfizer Diligence Obligation and fails to remedy such breach within *** of Pfizer’s receipt of notice of such breach from MacroGenics, then MacroGenics may, in its sole discretion, elect to either (a) terminate this Agreement with the effects of the provisions of Section 9.5.2 on an Agreement Product-by-Agreement Product and country-by-country basis, but only to the
25
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
extent that an Agreement Product in a given country in the Territory is directly and adversely impacted by such uncured material breach, or (b) convert any exclusive licenses granted to Pfizer under this Agreement with respect to an Agreement Product in a given country in the Territory into non-exclusive licenses, but only to the extent that such Agreement Product in such country is directly and adversely impacted by such uncured material breach. In the event MacroGenics elects Section 3.2.5(b) as a remedy to address a Diligence Issue, Pfizer shall continue to have the same obligations to pay all milestones, FTE costs and Net Sales royalties for each Agreement T-DART and Agreement Product affected by MacroGenics election. MacroGenics acknowledges and agrees that the elections set forth in this Section 3.2.5 (i) have been negotiated by the Parties to fully address any harm that MacroGenics may incur as a result of Pfizer’s material breach of any Pfizer Diligence Obligation and (ii) constitute MacroGenics’s sole and exclusive remedies with respect to any breach by Pfizer of the Pfizer Diligence Obligations.
3.3. Regulatory Approvals. Pfizer or its designated Affiliate(s) shall file, in its own name, all Regulatory Approval applications for Agreement Products where, subject to the Pfizer Diligence Obligations, Pfizer, in its sole discretion, determines it is commercially advantageous to do so. Pfizer, or its designated Affiliate(s), shall have the sole responsibility for, and sole authority with respect to, communications with any Regulatory Authority regarding any Regulatory Approval application or any Regulatory Approval for an Agreement Product once granted. Except to the extent necessary to fulfill its obligations under Section 3.2.1, neither Pfizer nor any of its Affiliates shall have any obligation to seek Regulatory Approval for any Agreement Product.
3.4. Control of Commercialization Activities.
3.4.1. General. Subject to the Pfizer Diligence Obligations, Pfizer shall have sole and exclusive control over all matters relating to the Commercialization of Agreement Products.
3.4.2. Branding. Pfizer shall select and own all Trademarks, including all goodwill associated therewith. Neither MacroGenics nor its Affiliates shall use or seek to register, anywhere in the world, any trademarks which are confusingly similar to any Trademarks.
3.5. Manufacturing. Subject to Section 2.3.3(f), Pfizer shall have the exclusive right to Manufacture Agreement Products itself or through one or more Affiliates or Third Parties selected by Pfizer. Pfizer shall have no diligence obligations with respect to the Manufacture of Agreement Products except to the extent necessary to fulfill the Pfizer Diligence Obligations.
3.6. Progress Reporting. Pfizer shall provide MacroGenics with semi-annual written reports on Pfizer’s activities to Develop and Commercialize Agreement Products. Any information or written report provided by Pfizer to MacroGenics pursuant to this Section 3.6
26
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
shall be deemed to be Pfizer’s Confidential Information subject to the provisions of Section 7. MacroGenics shall have the opportunity to reasonably seek further explanation or clarification of matters covered in such reports, and Pfizer shall provide such explanation or clarification. Furthermore, if after receiving such a report MacroGenics wishes to meet with Pfizer to discuss such report, Pfizer shall meet (in person or via teleconference) with MacroGenics at a site reasonably requested by MacroGenics within thirty (30) days after MacroGenics requests such meeting.
| 4. | LICENSES AND RELATED GRANTS OF RIGHTS. |
4.1. Grants to Pfizer.
4.1.1. Research License. Subject to the terms and conditions of this Agreement and during the Research Term, MacroGenics hereby grants to Pfizer a non-exclusive, worldwide, royalty-free license under the MacroGenics Technology to (a) use, have used, make and have made any T-DART directed to any Research Project Target for any and all uses in the Field, (b) conduct preclinical studies in vitro and in vivo in any non-human species with any T-DART directed to the Research Project Target for any and all uses in the Field and (c) otherwise perform the activities assigned to Pfizer under the Research Plans. The foregoing license shall be sublicenseable by Pfizer as provided in Section 4.1.4.
4.1.2. Assignment of Agreement T-DART IP. MacroGenics shall assign and hereby does assign to Pfizer all of MacroGenics’s and its Affiliates’ right, title and interest in and to the Agreement T-DART IP (including the right in and to any data, writings (irrespective of whether in written or electronic form) and information (tangible and intangible) covering the foregoing). MacroGenics shall execute, and cause its employees, agents and subcontractors to execute (directly or through assignment to MacroGenics and assignment by MacroGenics to Pfizer), assignments to Pfizer of all right, title and interest in and to any such Agreement T-DART IP. Subject to the terms of Section 9.5.2(a) and Section 9.5.2(b), any Agreement T-DART IP shall be the sole and exclusive property of Pfizer and shall constitute Confidential Information of Pfizer. MacroGenics will cooperate with Pfizer to execute and deliver any and all documents that Pfizer deems reasonably necessary to perfect and enforce Pfizer’s rights under this Section 4.1.2. Pfizer shall not grant any license under or other right with respect to any rights in any Agreement T-DART IP subject to potential reassignment to MacroGenics under Section 9.5.2 , that would prevent, survive or be otherwise inconsistent with the reassignment of such Agreement T-DART IP to MacroGenics if required by Section 9.5.2 free and clear of any such license or other right and shall take no action with respect to any other Agreement T-DART IP that is inconsistent with Pfizer’s obligations under Section 9.5.2.
4.1.3. Exclusive License. Subject to the terms and conditions of this Agreement and effective on the Effective Date, MacroGenics hereby grants to Pfizer an exclusive (even as to MacroGenics, except to the extent necessary for
27
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
MacroGenics to perform its obligations under the Research Program), license under the MacroGenics Technology and MacroGenics’s interest in the Joint Technology to use, have used, Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized, import, have imported, export and have exported Agreement T-DARTs and Agreement Products in the Territory for use in the Field, with the right to sublicense as provided in Section 4.1.4.
4.1.4. Right to Sublicense. Pfizer shall have the right to grant sublicenses under the licenses granted to it (a) under Section 4.1.1 and 4.1.3 . to Affiliates of Pfizer at any time, and (b) (i) under Section 4.1.1 to Third Party contractors solely for the provision of services to Pfizer in connection with the performance of activities assigned to Pfizer under the Research Plans; and (ii) under Section 4.1.3 to Third Parties; provided that any sublicense granted to a Third Party under this Agreement shall be pursuant to a written agreement that subjects such sublicensee to all relevant restrictions and limitations set forth in this Agreement. Pfizer shall provide MacroGenics notice of any Third Party sublicense within *** after the execution thereof, including the name and address of each permitted sublicensee, the date of the grant of the sublicense and a description of the rights granted. Pfizer shall be jointly and severally responsible with its sublicensees to MacroGenics for failure by its sublicensees to comply with, and Pfizer guarantees the compliance by each of its sublicensees with, all such applicable restrictions and limitations in accordance with the terms and conditions of this Agreement. Pfizer shall remain responsible for the payment to MacroGenics of all Sales Milestones and royalties payable with respect to Net Sales made by any of Pfizer’s Affiliates and Sublicensees.
4.1.5. Direct License to Affiliates. Pfizer may at any time request and authorize MacroGenics to grant licenses directly to Affiliates of Pfizer by giving written notice designating to which Affiliate a direct license is to be granted. Upon receipt of any such notice, MacroGenics shall enter into and sign a separate direct license agreement with such designated Affiliate of Pfizer. All such direct license agreements shall be consistent with the terms and conditions of this Agreement, except for such modifications as may be required by the laws and regulations in the country in which the direct license will be exercised. The Parties further agree to make any amendments to this Agreement that are necessary to conform the combined terms of such direct license agreements and this Agreement to the terms of this Agreement as set forth on the Effective Date. In countries where the validity of such direct license agreements requires prior governmental approval or registration, such direct license agreements shall not become binding between the parties thereto until such approval or registration is granted, which approval or registration shall be obtained by Pfizer. All costs of making such direct license agreement(s), including MacroGenics’s reasonable attorneys’ fees, under this Section 4.1.5 shall be borne by Pfizer.
28
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
4.1.6. Right of Reference. To the extent that MacroGenics does not breach any contractual obligations it has with Third Parties, MacroGenics hereby grants to Pfizer a “Right of Reference,” as that term is defined in 21 C.F.R. § 314.3(b), to any data Controlled by MacroGenics or its Affiliates (a) that relates to the Agreement T-DARTS, the Agreement Products or preclinical studies with respect to the Agreement Products and (b) that Pfizer reasonably believes may be necessary or useful to the Development, Manufacturing or Commercialization of any Agreement T-DART or any Agreement Product pursuant to this Agreement, and MacroGenics will provide a signed statement to the foregoing effect, if so requested by Pfizer in accordance with 21 C.F.R. § 314.50(g)(3).
4.2. Grants to MacroGenics.
4.2.1. Research License. Subject to the terms and conditions of this Agreement and during the Research Term, Pfizer hereby grants to MacroGenics a non-exclusive, worldwide, royalty-free license under the Pfizer Technology solely to the extent necessary to conduct activities assigned to MacroGenics under the Research Plan, with the right to sublicense as provided in Section 4.2.1(a).
(a) Right to Sublicense. MacroGenics may sublicense the foregoing license but only to any one or more of MacroGenics’ Affiliates or subcontractors.
4.2.2. Grant Back License with Respect to Agreement T-DART IP Assigned to Pfizer. Subject to the terms and conditions of this Agreement, Pfizer hereby grants to MacroGenics a non-exclusive, worldwide, royalty-free license under the Agreement T-DART IP and the Agreement T-DART Patent Rights to use, have used, Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized, import, have imported, export and have exported any T-DART or other bi-specific targeting molecule (other than (a) any T-DART Targeting any Research Project Target or any Reserved Target or (b) any other bi-specific targeting molecule that consists of or comprises any Research Program Antibody (other than a Passed MacroGenics Antibody) Targeting any Research Project Target or any Reserved Target), either alone or as part of any pharmaceutical or other product, with the right to sublicense.
4.3. Reciprocal Non-Exclusive Research License for Disclosed Know-How and Confidential Information. Without limiting any other license granted to either Party under this Agreement:
4.3.1. MacroGenics hereby grants to Pfizer and Pfizer’s Affiliates a non-exclusive, irrevocable, perpetual, royalty-free, fully paid-up, worldwide license to use any and all MacroGenics Know-How or MacroGenics Confidential Information disclosed to Pfizer during the Term solely for internal research purposes.
29
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
4.3.2. Pfizer hereby grants to MacroGenics and MacroGenics Affiliates a non-exclusive, irrevocable, perpetual, royalty-free, fully paid-up, worldwide license to use any and all Pfizer Know-How or Pfizer Confidential Information (other than any information regarding the identity of or Pfizer’s reasons for selecting any Research Project Target or Reserved Target, which shall only be disclosed by MacroGenics to MacroGenics personnel as necessary to comply with the terms of this Agreement) disclosed to MacroGenics during the Term solely for internal research purposes.
4.3.3. Notwithstanding the foregoing, neither Pfizer nor MacroGenics shall have any right under this Section 4.3 to make any use of any physical material supplied by the other Party for use in the Research Program other than for use in the Research Program.
4.4. Retained Rights to Antibodies. For the avoidance of doubt, except as expressly provided in regard to the assignments and licenses contained in this Section 4, neither Party will have any rights in the other Party’s Antibodies and each Party will retain ownership of all of its Pfizer Technology, MacroGenics Technology or Joint Technology, as applicable, covering any Antibody that such Party contributes to the collaboration.
4.5. Exclusivity.
4.5.1. Exclusivity Covenant. During the Research Term and for *** after the Research Term, except to the extent required for MacroGenics to fulfill its obligations under this Agreement, neither MacroGenics nor any of its Affiliates will (i) (either directly or with or through a Third Party) Develop, Manufacture or Commercialize any T-DART or Research Program Antibody (other than a Passed MacroGenics Antibody) Targeting any Research Project Target or any Reserved Target or (ii) license or otherwise grant any right to any Third Party to Develop, Manufacture or Commercialize any T-DART or Research Program Antibody (other than a Passed MacroGenics Antibody) Targeting any Research Project Target or any Reserved Target.
4.5.2. Other Pfizer Programs. MacroGenics understands and acknowledges that Pfizer may have present or future initiatives or opportunities, including initiatives or opportunities with its Affiliates or Third Parties, involving similar products, programs, technologies or processes that are similar to or that may compete with a product, program, technology or process covered by this Agreement. MacroGenics acknowledges and agrees that nothing in this Agreement will be construed as a representation, warranty, covenant or inference that Pfizer will not itself Develop, Manufacture or Commercialize or enter into business relationships with one or more of its Affiliates or Third Parties to Develop, Manufacture or Commercialize products, programs, technologies or processes that are similar to or that may compete with any product, program, technology or process covered by this Agreement.
30
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
4.6. Section 365(n) of Bankruptcy Code. All rights and licenses now or hereinafter granted by a Party to the other Party under or pursuant to any section of this Agreement, including Sections 4.1.1 , 4.1.3, 4.2.1 and 4.2.2 are rights to “intellectual property” (as defined in Section 101(35A) of Title 11 of the United States Code, as amended (such Title 11, the “Bankruptcy Code”)). The Parties hereto acknowledge and agree that the payments provided for under Sections 5.1, 5.2 and 5.3 and all other payments by Pfizer to MacroGenics under this Agreement, other than royalty payments pursuant to Section 5.4, do not constitute royalties within the meaning of Section 365(n) of the Bankruptcy Code or relate to licenses of intellectual property under this Agreement.
4.7. No Implied Rights. Except as expressly provided in this Agreement, neither Party shall be deemed by estoppel, implication or otherwise, to have granted the other Party any license or other right with respect to any intellectual property of such Party.
| 5. | PAYMENTS TO MACROGENICS. |
5.1. Upfront License Payment. Within *** days after the Effective Date, Pfizer shall pay to MacroGenics the non-creditable, non-refundable amount of Five Million Dollars ($5,000,000) (the “Up-Front Payment”).
| 5.2. | Research Support Funding. |
5.2.1. FTE Reimbursement. During the Research Term, Pfizer shall reimburse MacroGenics for the costs of the FTEs for the Research Program at the FTE Rate per FTE per Calendar Year. ***
5.2.2. Out-of-Pocket Expenses. During the Research Term, Pfizer shall reimburse MacroGenics’s out-of-pocket costs incurred by MacroGenics and paid to Third Parties in connection with executing the Research Plans, but only to the extent contemplated in the Research Plans or otherwise approved by Pfizer in advance in writing.
5.2.3. Other Expenses. Except as expressly set forth in Section 5.2.1 and Section 5.2.2, MacroGenics shall be solely responsible for all expenses it incurs in performing its obligations under the Research Program.
5.2.4. Reimbursement Payments. Pfizer shall reimburse MacroGenics for expenses pursuant to Section 5.2 within *** days after receipt of an invoice issued by MacroGenics within *** days after the end of each Calendar Quarter.
5.2.5. Audit Rights. During the Research Term and for a period of thirty-six (36) months thereafter, MacroGenics shall keep and maintain accurate and complete records showing the time devoted and activities performed by each FTE in performing MacroGenics’s obligations under the Research Program. Upon thirty (30) days prior written notice from Pfizer, MacroGenics shall permit an independent certified public accounting firm of internationally recognized standing selected by Pfizer and reasonably acceptable to MacroGenics, to
31
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
examine, at Pfizer’s sole expense, the relevant books and records of MacroGenics as may be reasonably necessary to verify the accuracy of the invoices submitted to Pfizer under Section 5.2.4 for the number of FTEs applied to the performance of MacroGenics’s obligations under the Research Program. An examination by Pfizer under this Section 5.2.5 shall occur not more than once in any Calendar Year and shall be limited to the pertinent books and records for any Calendar Year ending not more than thirty-six (36) months before the date of the request. Such examination shall be conducted during MacroGenics’s normal business hours at MacroGenics’s facility(ies) where such books and records are normally kept. MacroGenics may require the accounting firm to sign a standard non-disclosure agreement before providing the accounting firm access to MacroGenics’s facilities or records. The accounting firm shall provide both MacroGenics and Pfizer a written report disclosing whether the invoices submitted by MacroGenics are correct or incorrect and the specific details concerning any discrepancies. No other information shall be provided to Pfizer. If the accounting firm determines the number of FTEs actually utilized by MacroGenics was materially less than the number funded by Pfizer during the period covered by the audit ***, MacroGenics shall, at Pfizer’s sole discretion, either (a) refund the excess payments to Pfizer within *** after its receipt of the auditor’s report so concluding or (b) immediately offset all such excess payments against any outstanding or future amounts payable by Pfizer to MacroGenics under this Agreement until Pfizer has received full credit for all such overpayments. Additionally, if the amount to be refunded exceeds more than five percent (5%) of the amount that was properly payable, MacroGenics shall reimburse Pfizer for the cost of the audit.
5.2.6. Underpayments/Overpayments. If such accounting firm concludes that MacroGenics under-billed Pfizer for such FTEs, Pfizer shall reimburse MacroGenics for such costs within *** after its receipt of the auditor’s report.
5.3. Milestones
5.3.1. Technical Milestones. Pfizer shall pay to MacroGenics the amount set forth below within *** following the first occurrence of each event (each, a “Technical Milestone”) described below for each Research Project Target, each such amount (a “Technical Milestone Payment”) to be payable only once with respect to each different Research Project Target regardless of how many Agreement T-DARTs binding to such Research Project Target achieve such Technical Milestone.
| Technical Milestone |
Technical Milestone Payment | |
| *** |
*** | |
| *** |
*** | |
| *** |
*** |
32
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
If the Technical Milestone set forth in Section 5.3.1(c) is achieved prior to the achievement of the Technical Milestone set forth in Section 5.3.1(a) and/or 5.3.1(b), then the unpaid Technical Milestone Payment set forth in Section 5.3.1(a) and/or 5.3.1(b) shall be due and payable simultaneously with the payment for achievement of the milestone set forth in Section 5.3.1(c). For the avoidance of doubt, in the event that the Technical Milestone set forth in Section 5.3.1(b) is achieved prior to the achievement of the Technical Milestone set forth in Section 5.3.1(a), the Technical Milestone Payment set forth in Section 5.3.1(a) shall not be due or payable simultaneously with the Technical Milestone Payment for achievement of the Technical Milestone set forth in Section 5.3.1(b), but instead shall only be due and payable upon achievement of Technical Milestone Payment set forth in Section 5.3.1(a) or Section 5.3.1.(c).
5.3.2. Development Milestones. Pfizer shall pay to MacroGenics the amount set forth below within *** days following the first occurrence of each event (each, a “Development Milestone”) described below for each Research Project Target, each such amount (a “Development Milestone Payment”) to be payable only once with respect to each different Research Project Target regardless of how many Agreement T-DARTs binding to such Research Project Target achieve such Development Milestone.
| Development Milestone |
Development Milestone Payment | |
| *** |
*** | |
| *** |
*** | |
| *** |
*** | |
| *** |
*** | |
| *** |
*** | |
| *** |
*** |
If the Development Milestone set forth in Section 5.3.2(b) is achieved prior to the achievement of the milestone set forth in Section 5.3.2(a), then the Development Milestone Payment set forth in Section 5.3.2(a) shall be due and payable simultaneously with the Development Milestone Payment for achievement of the Development Milestone set forth in Section 5.3.2(b); if the Development Milestone set forth in Section 5.3.2(c) is achieved prior to the achievement of the Development Milestone set forth in Section 5.3.2(a) and/or 5.3.2(b), then the unpaid Development Milestone Payment set forth in Section 5.3.2(a) and/or 5.3.2(b) shall be due and payable simultaneously with the payment for achievement of the milestone set forth in Section 5.3.2(c); if the Development Milestone set forth in Section 5.3.2(d), (e) or (f) is achieved prior to the achievement of the Development Milestone set forth in Section 5.3.2(a), 5.3.2(b) and/or 5.3.2(c), then the unpaid Development Milestone Payment set forth in Section 5.3.2(a), 5.3.2(b) and/or 5.3.2(c) shall be due and payable simultaneously with the Development Milestone Payment for achievement of the milestone set forth in Section 5.3.2(d), (e) or (f).
33
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
5.3.3. Sales Milestones. Pfizer shall pay to MacroGenics the following one-time payments (each, a “Sales Milestone Payment”) when aggregate Annual Net Sales of all Agreement Product in a Pfizer Year first reach the respective threshold (a “Sales Threshold”) indicated below (each, a “Sales Milestone”):
| Total Annual Net Sales |
Sales Milestone Payment | |
| Total Annual Net Sales exceeding *** |
*** | |
| Total Annual Net Sales exceeding *** |
*** | |
| Total Annual Net Sales exceeding *** |
*** | |
| Total Annual Net Sales exceeding *** |
*** |
If more than one unmet Sales Threshold is achieved with respect to the same Pfizer Year, payment will be made with respect to the higher or highest Sales Threshold achieved in such Pfizer Year and all other previously unmet Sales Thresholds achieved with respect to such Pfizer Year will remain eligible to be met in future Pfizer Years. Any Sales Milestone Payment shall be payable by Pfizer with respect to any Pfizer Year shall be payable within sixty (60) days of the end of such Pfizer Year in the United States.
5.4. Royalties. Subject to the provisions of Section 5.4.3, Pfizer shall pay MacroGenics royalties in the amount of the rates (“Marginal Royalty Rates”) set forth below of Annual Net Sales of any Agreement Product during the Royalty Term:
| Annual Net Sales |
Marginal Royalty Rate (% of the Annual Net Sales) | |
| Annual Net Sales of such Agreement Product during a given Pfizer Year above $0, up to and including *** |
*** | |
| Annual Net Sales of such Agreement Product during a given Pfizer Year above ***, up to and including *** |
*** | |
| Annual Net Sales of such Agreement Product during a given Pfizer Year above ***, up to and including *** |
*** | |
| Annual Net Sales of such Agreement Product during a given Pfizer Year above *** |
*** |
5.4.1. Marginal Royalty Rate Application. Each Marginal Royalty Rate set forth in the table above shall apply only to that portion of the Annual Net Sales of a given Agreement Product in the Territory during a given Pfizer Year that falls within the indicated range.
34
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
5.4.2. Royalty Term. “Royalty Term” means, with respect to any Agreement Product and any country in the Territory, the period of time from the First Commercial Sale of such Agreement Product in such country until the later of (a) ***
5.4.3. Royalty Adjustments. The following adjustments shall be made, on Agreement Product-by-Agreement Agreement Product and country-by-country basis, to the royalties payable pursuant to this Section 5.4:
(a) Generic Competition. Royalties payable following establishment of Generic Competition with respect to the sale by a Third Party of product that is a Biosimilar Biologic Product to such Agreement Product in such country shall be payable at *** of the otherwise applicable rate prior to application of this Section 5.4.3(a) “Generic Competition” means with respect to a given Calendar Year with respect to an Agreement Product in any country, that during such Calendar Year, *** have received Regulatory Approval to sell in such country a Biosimilar Biologic Product, such Biosimilar Biologic Product shall be commercially available in such country and such Biosimilar Biologic Product shall have, in the aggregate, *** or more market share of the aggregate of such Agreement Product and Biosimilar Biologic Product (based on data provided by IMS International, or if such data is not available, such other reliable data source as reasonably determined by Pfizer and agreed by MacroGenics (such agreement not to be unreasonably withheld)) as measured by sales. In the event IMS International data (or such other agreed data source) is not sufficient to determine the percentage market share for each country in the EU, the percent market share for the EU countries for which data is not available will be deemed to be the average percent market share for those EU countries in which the data is available. A product shall be a “Biosimilar Biologic Product” with respect to an Agreement Product if such product (1) has been licensed as a biosimilar or interchangeable product by FDA pursuant to Section 351(k) of the Public Health Service Act (42 U.S.C. 262(k)), as may be amended, or any subsequent or superseding law, stature or regulation, (2) has been licensed as a similar biological medicinal product by EMEA pursuant to Directive 2001/83/EC, as may be amended, or any subsequent or superseding law, stature or regulation, or (3) has otherwise achieved analogous Regulatory Marketing Approval from another applicable Regulatory Authority.
(b) Third Party Patents. If it is necessary for Pfizer to license one or more Patent Rights from one or more Third Parties in order to Develop, Manufacture or Commercialize or use any Agreement Product, whether directly or through any Pfizer Affiliate or Sublicensee, then Pfizer may, in its sole discretion, negotiate and obtain a license under such Patent Right(s) (each such Third Party license referred to herein as an “Additional Third Party License”). Any royalty otherwise payable to
35
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
MacroGenics under this Agreement with respect to Net Sales of any Agreement Product by Pfizer, its Affiliates or Sublicensees shall be reduced by *** of the royalties payable to Third Parties that are reasonably allocable to Agreement Products pursuant to any Additional Third Party Licenses, such reduction to continue until all such amounts have been expended, provided that in no event shall the total royalty payable to MacroGenics for any Agreement Product be less than *** of the royalty amounts otherwise payable for such Agreement Product.
(c) No Adjustment for MacroGenics Third Party Agreements. MacroGenics shall be solely responsible for all obligations (including any royalty or other obligations that relate to the MacroGenics Technology) under its agreements with Third Parties that are in effect as of the Effective Date or that MacroGenics enters into during the Term.
5.4.4. Fully Paid-Up, Royalty Free License. After expiration of the Royalty Term for any Agreement Product in a country in the Territory, no further royalties shall be payable in respect of sales of such Agreement Product in such country and thereafter, the license granted to Pfizer under Section 4.1.3 with respect to such Agreement Product in such country shall be a fully paid-up, perpetual, non-exclusive, irrevocable, royalty-free license.
5.5. Reports and Payments.
5.5.1. Cumulative Royalties. The obligation to pay royalties under Section 5.4 shall be imposed only once with respect to a single unit of an Agreement Product regardless of how many Valid Claims in Patent Rights included within the MacroGenics Patent Rights would, but for this Agreement, be infringed by the use or sale of such Agreement Product in the country in which such Agreement Product is used or sold.
5.5.2. Royalty Statements and Payments. Within *** after the end of each Pfizer Quarter, Pfizer shall deliver to MacroGenics a report setting forth for such Calendar Quarter the following information, on an Agreement Product-by-Agreement Product basis: (a) the Net Sales of each Agreement Product, (b) the basis for any adjustments to the royalty payable for the sale of each Agreement Product and (c) the royalty due hereunder for the sale of each Agreement Product. No such reports shall be due for any Agreement Product before the First Commercial Sale of such Agreement Product in the Territory. The total royalty due for the sale of Agreement Products during such Pfizer Quarter shall be remitted at the time such report is delivered to MacroGenics.
5.5.3. Taxes and Withholding. It is understood and agreed between the Parties that any payments made this Agreement are inclusive of any value added tax imposed upon such payments. In addition, in the event any of the payments made by Pfizer pursuant to this Agreement become subject to withholding taxes under
36
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
the laws of any jurisdiction, Pfizer shall deduct and withhold the amount of such taxes for the account of MacroGenics, to the extent required by law, such amounts payable to MacroGenics shall be reduced by the amount of taxes deducted and withheld, and Pfizer shall pay the amounts of such taxes to the proper Governmental Authority in a timely manner and promptly transmit to MacroGenics an official tax certificate or other evidence of such tax obligations together with proof of payment from the relevant Governmental Authority of all amounts deducted and withheld sufficient to enable MacroGenics to claim such payment of taxes. Any such withholding taxes required under applicable Law to be paid or withheld shall be an expense of, and borne solely by, MacroGenics. Pfizer will provide MacroGenics with reasonable assistance to enable MacroGenics to recover such taxes as permitted by Law.
5.5.4. Currency. All amounts payable and calculations hereunder shall be in United States dollars. As applicable, Net Sales and any royalty deductions shall be converted into United States dollars in accordance with Pfizer’s customary and usual conversion procedures, consistently applied.
5.5.5. Additional Provisions Relating to Payments. MacroGenics acknowledges and agrees that nothing in this Agreement (including any schedules and exhibits hereto) shall be construed as representing an estimate or projection of either (a) the number of Agreement Products that shall or may be successfully Developed or Commercialized or (b) anticipated sales or the actual value of any Agreement Product. PFIZER MAKES NO REPRESENTATION OR WARRANTY, EITHER EXPRESS OR IMPLIED, THAT IT SHALL BE ABLE TO SUCCESSFULLY DEVELOP OR COMMERCIALIZE ANY PRODUCT OR, IF COMMERCIALIZED, THAT IT WILL ACHIEVE ANY PARTICULAR SALES LEVEL OF SUCH PRODUCT(S), PROVIDED THAT THE FOREGOING SHALL NOT LIMIT PFIZER’S OBLIGATIONS UNDER THIS AGREEMENT.
5.6. Maintenance of Records; Audits.
5.6.1. Record Keeping. Pfizer shall keep, and shall cause its Affiliates and Sublicensees to keep, accurate books of account and records in connection with the sale of Agreement Products, in sufficient detail to permit accurate determination of all figures necessary for verification of royalties to be paid hereunder. Pfizer shall maintain, and shall cause its Affiliates and Sublicensees to maintain, such records for a period of at least three (3) years after the end of the Calendar Year in which they were generated.
5.6.2. Audits. Upon thirty (30) days prior written notice from MacroGenics, Pfizer shall permit an independent certified public accounting firm of internationally recognized standing selected by MacroGenics and reasonably acceptable to Pfizer to examine, at MacroGenics’s sole expense, the relevant books and records of Pfizer during the period covered by such examination, as
37
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
may be reasonably necessary to verify the accuracy of the reports submitted by Pfizer in accordance with Section 5.5 and the payment of royalties hereunder. An examination by MacroGenics under this Section 5.6.2 shall occur not more than once in any Calendar Year and shall be limited to the pertinent books and records for any Calendar Year ending not more than three (3) years before the date of the request. The accounting firm shall be provided access to such books and records at Pfizer’s or its Affiliates’ facilities where such books and records are kept and such examination shall be conducted during Pfizer’s normal business hours. Pfizer may require the accounting firm to sign a standard non-disclosure agreement before providing the accounting firm access to Pfizer’s facilities or records. Upon completion of the audit, the accounting firm shall provide both Pfizer and MacroGenics a written report disclosing whether the reports submitted by Pfizer are correct or incorrect, whether the royalties paid are correct or incorrect and, in each case, the specific details concerning any discrepancies. No other information shall be provided to MacroGenics.
5.6.3. Underpayments/Overpayments. If such accounting firm concludes that additional royalties were due to MacroGenics, Pfizer shall pay to MacroGenics the additional royalties within thirty (30) days after the date Pfizer receives such accountant’s written report so concluding. If such underpayment exceeds five percent (5%) of the royalties that were to be paid to MacroGenics, Pfizer also shall reimburse MacroGenics for all reasonable charges of such accountants for conducting the audit. If such accounting firm concludes that Pfizer overpaid royalties to MacroGenics, Pfizer shall be entitled to offset all such overpayments against any outstanding or future amounts payable by Pfizer to MacroGenics under this Agreement until Pfizer has received full credit for all such overpayments.
5.6.4. Confidentiality. All financial information of Pfizer which is subject to review under this Section 5.6 shall be deemed to be Pfizer’s Confidential Information subject to the provisions of Section 7 hereof, and MacroGenics shall not disclose such Confidential Information to any Third Party or use such Confidential Information for any purpose other than verifying payments to be made by Pfizer to MacroGenics hereunder, provided, however, that such Confidential Information may be disclosed by MacroGenics to Third Parties only to the extent necessary to enforce MacroGenics’s rights under this Agreement.
5.7. Late Payments. If a Party shall fail to make a timely payment pursuant to the terms of this Agreement, interest shall accrue on the past due amount at the ***, computed for the actual number of days the payment was past due (but in no event in excess of the maximum rate permissible under Applicable Law).
38
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
| 6. | INTELLECTUAL PROPERTY. |
6.1. Inventions.
6.1.1. Ownership.
(a) Except for inventions and Know-How subject to Section 2.13.6 or Section 4.1.2, each Party shall own all inventions and Know-How conceived or generated solely by it and its Affiliates and their respective employees, agents and independent contractors in the course of performing research or development activities under this Agreement (a “Sole Invention” or “Sole Know-How,” respectively).
(b) Except for inventions and Know-How subject to Section 2.13.6 or Section 4.1.2, all inventions and Know-How that are conceived or generated jointly by employees, Affiliates, agents, or independent contractors of each Party in the course of performing research or development activities under this Agreement shall be owned jointly by the Parties (a “Joint Invention” or “Joint Know-How,” respectively). During and after the Term, either Party may exploit (including by license, sublicense, assignment of such Party’s interest or otherwise) any Joint Technology without accounting to or obtaining consent from the other Party, subject to the rights and obligations of the Parties with respect to Joint Technology under this Agreement, including the exclusive license of MacroGenics’s interest in the Joint Technology granted to Pfizer under Section 4.1.3, the Joint Patent Right prosecution and maintenance provisions set forth in Section 6.2 and Pfizer’s obligation to pay royalties on Agreement Products under Section 5.4.
(c) All determinations of inventorship under this Agreement shall be made in accordance with the patent law of the United States.
6.1.2. Disclosure. Each Party shall promptly disclose to the other Party any Joint Invention, Sole Invention, Agreement T-DART IP or Pfizer Proprietary Material Improvement, including all invention disclosures or other similar documents submitted to such Party by its or its Affiliates’, employees, agents or independent contractors describing such foregoing invention, and shall cooperate to promptly determine each Party’s inventive contribution thereto. The Parties shall cooperate with each other with respect to the timing, scope, and filing of patent applications and patent claims relating to any of the foregoing inventions to enhance the patent protection for Agreement T-DARTs and Agreement Products, and their manufacture and use.
6.2. Patent Rights.
6.2.1. Filing, Prosecution and Maintenance of Patent Rights.
(a) Pfizer Patent Rights. Pfizer, at its own expense, shall have the sole right, but not the obligation, to prepare, file, prosecute and maintain, throughout the world, any Pfizer Patent Right other than any Agreement T-DART Patent Right.
39
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
(b) Agreement T-DART Patent Rights.
(i) Pfizer shall have the first right, but not the obligation, to file, prosecute and maintain, at Pfizer’s expense, any Agreement T-DART Patent Right. Pfizer will provide MacroGenics with an opportunity to review the text of the initial application with respect to any Agreement T-DART Patent Right before filing, shall consult MacroGenics with respect thereto, shall not unreasonably refuse to address any of MacroGenics’ comments, and shall provide MacroGenics promptly with a copy of the application as filed, together with notice of its filing date and serial number. Pfizer will keep MacroGenics advised of the status of the Agreement T-DART Patent Rights annually or more often at MacroGenics’ reasonable request. Pfizer shall either provide MacroGenics with copies of, or require that Pfizer’s outside counsel copy MacroGenics on, substantive official correspondence received from all patent offices related to the filing, prosecution and maintenance of the patent filings in those offices (e.g., office actions and any other correspondence of a similar nature), and of Pfizer’s proposed responses to such correspondence or other substantive filings (e.g., responses to office actions, elections and any other responses or filings of a similar nature), in each case, reasonably in advance of Pfizer’s proposed responses or other filings to allow MacroGenics to comment.
(ii) Pfizer shall file, prosecute and maintain each Agreement T-DART Patent Right in all Major Market Countries and in such other countries as MacroGenics may request, provided, however, that if Pfizer at any time wishes not to file, prosecute or maintain any Agreement T-DART Patent Right in any country (a “Foregone Agreement T-DART Patent Right”), Pfizer shall provide MacroGenics with *** prior written notice to such effect, provided that an Agreement T-DART Patent Right shall not constitute a Foregone Agreement T-DART Patent Right if Pfizer’s discontinuation of filing, prosecution or maintenance of such Agreement T-DART Patent Right is in order to effect a settlement or to avoid an interference, opposition or other proceeding in which the validity of such Agreement T-DART Patent Right may be determined or because another Agreement T-DART Patent Right of similar claim scope is being prosecuted or has been issued. For any such Foregone Agreement T-DART Patent Right for which Pfizer gives notice in accordance with the foregoing sentence and of which MacroGenics, its Affiliates, or
40
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
Subcontractors of MacroGenics or any of its Affiliates are the sole inventors, MacroGenics shall have the right to request assignment within *** after MacroGenics’ receipt of the notice provided by Pfizer and Pfizer shall assign to MacroGenics all of Pfizer’s right, title and interest in and to any such Foregone Agreement T-DART Patent Right so requested within *** of such request. Upon assignment of such Foregone Agreement T-DART Patent Right, Pfizer shall have no responsibility for any further prosecution or maintenance of or expenses incurred in connection with such Foregone Agreement T-DART Patent Right. Pfizer shall execute, and cause its employees, agents and subcontractors to execute, at MacroGenics’ expense, all documents necessary for MacroGenics to continue prosecution and maintenance of such Foregone Agreement T-DART Patent Right. Any Foregone Agreement T-DART Patent Right assigned to MacroGenics will be included in MacroGenics Patent Rights for the purposes of this Agreement.
(iii) If, upon a timely request by MacroGenics in connection with an Agreement T-DART Patent Right of which MacroGenics, its Affiliates, or Subcontractors of MacroGenics or any of its Affiliates are the sole inventors, Pfizer declines to file a continuation or divisional application to pursue claims of a broader scope than those that have been allowed or are being or will be sought by Pfizer, MacroGenics shall have the right to file, in Pfizer’s name, such continuation or divisional application (but not a continuation-in-part) and Pfizer shall have no responsibility for any further prosecution or maintenance of or expenses incurred in connection with such application. Pfizer shall execute, and cause its employees, agents and subcontractors to execute, at MacroGenics’ expense, all documents necessary for MacroGenics to continue prosecution and maintenance of such application. The second, third and fourth sentences of this Section 6.2.1(b) shall apply to MacroGenics’ filing and prosecution, provided that each Party’s name in those sentences shall be substituted with the other Party’s name.
(c) MacroGenics Patent Rights Other than Subject T-DART Patent Rights. Except as provided in the Section 6.2.1(d) in regard to Subject Patent Rights, MacroGenics, at its own expense, shall have the sole right, but not the obligation, to prepare, file, prosecute and maintain, throughout the world, any MacroGenics Patent Right.
(d) Subject Patent Rights. MacroGenics shall have the first right, but not the obligation, to file, prosecute and maintain, at MacroGenics’s expense, any Subject Patent Right. “Subject Patent Right” means any MacroGenics Patent Right covering the composition of, or any method of
41
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
making or method of using, any Agreement T-DART or Agreement Product. MacroGenics shall keep Pfizer advised of the status of Subject Patent Rights (once published) annually or more often at Pfizer’s reasonable request. MacroGenics shall consider in good faith any comments provided by Pfizer concerning the prosecution of Subject Patent Rights, and MacroGenics shall not use any information generated in the Research Program in connection with prosecution of any Subject Patent Rights without Pfizer’s prior written consent, which shall not be unreasonably withheld or delayed. MacroGenics shall file, prosecute and maintain each Subject Patent Right in all Major Market Countries, provided, however, that if MacroGenics at any time wishes not to file, prosecute or maintain any Subject Patent Right in any country, MacroGenics shall provide Pfizer with *** prior written notice to such effect. Unless MacroGenics’ discontinuation of filing, prosecution or maintenance of such Subject Patent Right is in order to effect a settlement or to avoid an interference, opposition or other proceeding in which the validity of such Subject T-DART Patent Right may be determined or because another Subject T-DART Patent Right of similar claim scope is being prosecuted or has been issued, Pfizer shall have the first right to file, prosecute and maintain such Subject Patent Right (in MacroGenics’ name) in each such country, in which event the Subject Patent Right will no longer be deemed to be included in the MacroGenics Patent Rights for the purpose of determining the Royalty Term with respect to any Agreement Product.
(e) Joint Patent Rights. In the event the Parties make any Joint Invention, the Parties shall promptly meet to discuss and determine, based on mutual consent, whether to seek patent protection thereon and how prosecution, maintenance and enforcement of any Patent Right covering such Joint Invention will be handled. Neither Party will file any Patent Right covering or claiming a Joint Invention without the consent of the other Party.
6.2.2. Enforcement of Patent Rights.
(a) Notice. If either Pfizer or MacroGenics becomes aware of any infringement, anywhere in the world, of any issued Subject Patent Right or Agreement T-DART Patent Right, such Party shall promptly notify the other Party in writing to that effect.
(b) Infringement of Subject Patent Rights.
(1) If any infringement of a Subject Patent Right by a Third Party arises from the Development, Manufacture or Commercialization of a product that competes with an Agreement Product, Pfizer shall have the first right, but not the obligation, to
42
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
take action to obtain a discontinuance of infringement or bring suit against a Third Party infringer of such Subject Patent Right within *** from the date of notice and to join MacroGenics as a party plaintiff.
(2) Pfizer shall bear all the expenses of any suit brought by it claiming infringement of any such Subject Patent Right. MacroGenics shall cooperate with Pfizer in any such suit and shall have the right to consult with Pfizer and to participate in and be represented by independent counsel in such litigation at its own expense. Pfizer shall incur no liability to MacroGenics as a consequence of such litigation or any unfavorable decision resulting therefrom, including any decision holding any such Subject Patent Right invalid or unenforceable; provided, however, that Pfizer shall not, without MacroGenics’ prior written consent, enter into any settlement or consent decree that requires any payment by or admits or imparts any other liability to MacroGenics or admits the invalidity or unenforceabilty of any such Subject Patent Right.
(3) If Pfizer has not obtained a discontinuance of infringement by, or filed suit against, any such Third Party infringer within the *** period set forth in subsection (i) above, then MacroGenics shall have the right, but not the obligation, to bring suit against such Third Party infringer, at MacroGenics’ sole expense. provided that (x) Pfizer will not be obligated to join such suit unless Applicable Law requires that Pfizer must be a party for MacroGenics to maintain the suit, and (y) MacroGenics shall indemnify Pfizer against any damages, penalties, attorney fees or other recoveries assessed against Pfizer in or as a result of such suit. Pfizer may, at its sole discretion, elect to consult with MacroGenics and to participate in and be represented by independent counsel in such litigation at its own expense. MacroGenics shall incur no liability to Pfizer as a consequence of such litigation or any unfavorable decision resulting therefrom, including any decision holding any such Subject Patent Right invalid or unenforceable; provided, however, that MacroGenics shall not, without Pfizer’s prior written consent, enter into any settlement or consent decree that requires any payment by or admits or imparts any other liability to Pfizer.
43
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
(4) The enforcing Party shall keep the other Party reasonably informed of all material developments in connection with any such suit. Any recoveries obtained by either Party as a result of any proceeding against such a Third Party infringer shall be allocated as follows:
(A) Such recovery shall first be used to reimburse each Party for all out-of-pocket litigation costs in connection with such litigation paid by that Party; and
(B) With respect to any remaining portion of such recovery, if Pfizer was the enforcing Party, MacroGenics shall receive either (1) if the reward to Pfizer is based on lost profits, an amount equal to the royalty that would be payable, pursuant to Section 5.4, on the corresponding amount (as determined by the court) of lost Net Sales of the relevant Agreement Product(s) in the country(ies) where such infringement occurred; or (2) if the reward is based on reasonable royalty payments, such reward due to Pfizer (as determined by the court) shall be considered as Net Sales subject to the applicable royalty in accordance with Section 5.4, unless MacroGenics is separately awarded its own royalty award, and Pfizer shall receive any remaining portion of such recovery; or
(C) With respect to any remaining portion of such recovery, if MacroGenics was the enforcing Party, MacroGenics shall receive any remaining portion of such recovery.
(c) Other Infringement of MacroGenics Patent Rights. If the infringement of any MacroGenics Patent Right does not fall within the category of infringements covered by the first sentence of Section 6.2.2(b), MacroGenics shall have the sole right, but not the obligation, to take action against such infringement.
(d) Infringement of Agreement T-DART Patent Rights.
(1) Pfizer shall have the sole right but not the obligation to bring an action against a Third Party infringer of an Agreement T-DART Patent Right or otherwise address such alleged infringement. MacroGenics shall cooperate with Pfizer, at Pfizer’s expense, in any such suit brought by Pfizer and shall have the right to consult with Pfizer concerning the status and prosecution of such litigation Pfizer shall incur no liability to MacroGenics as a consequence of such litigation or any unfavorable decision resulting therefrom, including any decision holding any such Agreement T-DART Patent Right invalid or unenforceable.
44
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
(2) Any recoveries obtained by Pfizer as a result of any proceeding against a Third Party infringer shall be allocated as follows:
(A) Such recovery shall first be used to reimburse each Party for all out-of-pocket litigation costs in connection with such litigation paid by that Party; and
(B) With respect to any remaining portion of such recovery, MacroGenics shall receive either (1) if the reward to Pfizer is based on lost profits, an amount equal to the royalty that would be payable, pursuant to Section 5.4, on the corresponding amount (as determined by the court) of lost Net Sales of the relevant Agreement Product(s) in the country(ies) where such infringement occurred; or (2) if the reward is based on reasonable royalty payments , such reward due to Pfizer (as determined by the court) shall be considered as Net Sales subject to the applicable royalty in accordance with Section 5.4, and Pfizer shall receive any remaining portion of such recovery.
6.2.3. Infringement of Third Party Patent Rights. If the Development, Manufacture or Commercialization of any Agreement T-DART and/or Agreement Product is alleged by a Third Party to infringe a Third Party’s patent or other intellectual property rights, the Party becoming aware of such allegation shall promptly notify the other Party. The Party that is alleged to infringe the Third Party’s patent or intellectual property shall have the right to take such action as it deems appropriate in response to such allegation, and shall be solely responsible for all damages, costs and expenses in connection therewith, subject to Section 10.
6.2.4. Patent Invalidity Claim. Each Party shall promptly notify the other in the event of any legal or administrative action by any Third Party against any Subject Patent Right or Agreement T-DART Patent Right of which it becomes aware, including any opposition, interference, nullity, revocation, reexamination or compulsory license proceeding. Such action shall be treated as part of the prosecution of such Patent Right under Section 6.2.1 unless such action is a declaratory judgment action or counterclaim in response to an assertion of infringement, in which cases such action shall be treated as an enforcement action under Section 6.2.2.
6.2.5. Biosimilar Applications. Each Party shall immediately give written notice to the other of any notice received from a Third Party of an application for FDA approval under the Biologics Price Competition and Innovation Act of 2009 (or any amendment or successor statute thereto) of a Biosimilar Biologic Product referencing an Agreement Product or any certification under a similar statutory or regulatory requirement in any non-United States country in the Territory claiming that a Subject Patent Right or an Agreement T-DART Patent Right covering any Agreement Product is invalid or that infringement will not arise from the Development, Manufacture or Commercialization of a proposed Biosimilar
45
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
Biologic Product by a Third Party. Upon the giving or receipt of such notice, Pfizer shall have the first right (or the sole right, in the case of an Agreement T-DART Patent Right) but not the obligation, to bring an infringement action against such Third Party in connection with such certification. In the case of a Subject Patent Right, Pfizer shall notify MacroGenics at least *** to the date set forth by statute or regulation of its intent to exercise, or not exercise, this right. Any infringement action against a Third Party arising under this Section 6.2.5 shall be governed by the provisions of Section 6.2.2(b).
6.2.6. Patent Term Restoration and Extension. Pfizer shall have the first right, but not the obligation, to seek, in MacroGenics’s name if so required, patent term extensions, and supplemental protection certificates and the like available under Applicable Law, including 35 U.S.C. § 156 and applicable foreign counterparts, in any country in the Territory in relation to any Subject Patent Right and Agreement Products. MacroGenics and Pfizer shall cooperate in connection with all such activities. Pfizer, its agents and attorneys will give due consideration to all suggestions and comments of MacroGenics regarding any such activities, but in the event of a disagreement between the Parties, Pfizer will have the final decision-making authority. If Pfizer does not do so, MacroGenics may seek to extend any Subject Patent Right in relation to Agreement Products, including through the use of supplemental protection certificates and the like, unless in Pfizer’s reasonable legal determination such Subject Patent Right may not be extended under Applicable Law in relation to Agreement Products without limiting Pfizer’s right to extend any other Patent Right in relation to Agreement Products.
6.2.7. Joint Research Agreement. This Agreement shall be understood to be a joint research agreement under 35 U.S.C. § 103(c)(3) entered into for the purpose of researching, identifying and Developing Agreement T-DARTs and Agreement Products.
6.2.8. Orange Book Information. Pfizer shall be responsible for all submissions of patent information pertaining to each Agreement Product pursuant to 21 U.S.C. § 355(b)(1)(G) (or any amendment or successor statute thereto), any similar statutory or regulatory requirement enacted in the future regarding biologic products, or any similar statutory or regulatory requirement in any non-United States country or other regulatory jurisdiction.
6.3. Recording. If Pfizer deems it necessary or desirable to register or record this Agreement or evidence of this Agreement with any patent office or other appropriate government authorities in one or more jurisdictions in the Territory, then Pfizer shall submit to MacroGenics any proposed evidence of such recording and the Parties will comply with the terms of Section 7.2.3 in respect of such filing. MacroGenics shall, at Pfizer’s expense, execute and deliver to Pfizer any documents necessary or desirable, in Pfizer’s reasonable judgment, to complete such registration or recordation in accordance with the terms of Section 7.2.3; provided that such registration or recordation specifies
46
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
the applicable limitations of such license, and provided further that such registration shall have no effect on the allocation of patent prosecution, maintenance and enforcement rights and obligations set forth in this Section 6. In the event the licenses granted to Pfizer under this Agreement are terminated, Pfizer shall promptly take such actions and execute such documents as are reasonably requested by MacroGenics to cancel such registration(s) or recordation(s) in the applicable countries with respect to the terminated license grants.
6.4. Trademarks. Pfizer shall, in its sole discretion, select and own all Agreement Product-related Trademarks and copyrights to be used in connection with the Commercialization of any Agreement Product hereunder. MacroGenics shall neither use nor seek to register, anywhere in the world, any Trademarks that are confusingly similar to any Trademark used by or on behalf of Pfizer or its Affiliates or Sublicensees in connection with any Agreement Product; provided, however, that nothing in this Section 6.4 shall be construed to prevent MacroGenics from enforcing its own trademark rights or grant Pfizer any license or right in and to any MacroGenics trademark or house xxxx.
| 7. | CONFIDENTIALITY |
7.1. Confidentiality. Except to the extent expressly authorized by this Agreement, the Parties agree that, during the Term and for ***, each Party (the “Receiving Party”) receiving any Confidential Information of the other Party (the “Disclosing Party”) hereunder shall: (a) keep the Disclosing Party’s Confidential Information confidential; (b) not disclose, or permit the disclosure of, the Disclosing Party’s Confidential Information; and (c) not use, or permit to be used, the Disclosing Party’s Confidential Information for any purpose; provided, however, that a Receiving Party may disclose Confidential Information of the Disclosing Party to the extent that such Confidential Information (i) was already known by the Receiving Party (other than under an obligation of confidentiality to the Disclosing Party) at the time of disclosure by the Disclosing Party; (ii) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party; (iii) became generally available to the public or otherwise part of the public domain after its disclosure to the Receiving Party, other than through any act or omission of the Receiving Party in breach of its obligations under this Agreement; (iv) was disclosed to the Receiving Party, other than under an obligation of confidentiality, by a Third Party who had no obligation to the Disclosing Party not to disclose such information to the Receiving Party; or (v) was independently discovered or developed by or on behalf of the Receiving Party without the use of any Confidential Information of the Disclosing Party. The terms and conditions of this Agreement shall be considered Confidential Information of each Party.
7.2. Authorized Disclosure.
7.2.1. Disclosure to Party Representatives. Notwithstanding the foregoing provisions of Section 7.1, the Receiving Party may disclose Confidential Information belonging to the Disclosing Party to the Receiving Party’s, its Affiliates’ and its Sublicensees’ officers, directors, employees, consultants,
47
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
contractors and agents (collectively, “Representatives”) who (a) have a need to know such Confidential Information in connection with the performance of the Receiving Party’s obligations or the exercise of the Receiving Party’s rights under this Agreement and (b) have agreed in writing to non-disclosure and non-use provisions with respect to such Confidential Information that are at least as restrictive as those set forth in this Section 7.
7.2.2. Disclosure to Third Parties.
(a) Notwithstanding the foregoing provisions of Section 7.1, each Party may disclose Confidential Information belonging to the other Party:
(i) to Governmental Authorities (i) to the extent reasonably necessary to obtain or maintain INDs or Regulatory Approvals for any Agreement T-DART or Agreement Product within the Territory, and (ii) in order to respond to inquiries, requests or investigations relating to this Agreement;
(ii) to outside consultants, contractors, advisory boards, managed care organizations, and non-clinical and clinical investigators, in each case to the extent reasonably necessary to develop, register or market any Agreement T-DART or Agreement Product; provided that the Receiving Party shall obtain the same confidentiality obligations from such Third Parties as it obtains with respect to its own similar types of confidential information;
(iii) to the extent reasonably necessary in connection with filing or prosecuting Patent Rights as permitted by this Agreement;
(iv) to the extent reasonably necessary in connection with prosecuting or defending litigation as permitted by this Agreement;
(v) subject to Section 7.3.2, in connection with or included in scientific presentations and publications relating to Agreement T-DARTs or Agreement Products, including abstracts, posters, journal articles and the like, and posting results of and other information about clinical trials to xxxxxxxxxxxxxx.xxx or PhRMA websites; and
(vi) to the extent necessary or desirable in order to enforce its rights under this Agreement.
(b) Notwithstanding anything to the contrary in this Section 7, MacroGenics may disclose Pfizer’s Confidential Information to: (i) Governmental Authorities in order to respond to inquiries, requests or investigations relating to this Agreement and (ii) to the extent necessary or desirable in order to enforce its rights under this Agreement. In the event a
48
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
Party deems it reasonably necessary to disclose Confidential Information belonging to the other Party pursuant to this Section 7.2, the Disclosing Party shall to the extent possible give reasonable advance written notice of such disclosure to the other Party and take all reasonable measures to ensure confidential treatment of such information.
7.2.3. SEC Filings and Other Disclosures. Notwithstanding any provision of this Agreement to the contrary:
(a) Either Party may disclose the terms of this Agreement to the extent required, in the reasonable opinion of such Party’s legal counsel, to comply with Applicable Law, including the rules and regulations promulgated by the SEC or any equivalent governmental agency in any country in the Territory. Notwithstanding the foregoing, before disclosing this Agreement or any of the terms hereof pursuant to this Section 7.2.3, the Parties will consult with one another on the terms of this Agreement to be redacted in making any such disclosure. Further, if a Party discloses this Agreement or any of the terms hereof in accordance with this Section 7.2.3, such Party shall, at its own expense, seek such confidential treatment of confidential portions of this Agreement and such other terms, as may be reasonably requested by the other Party.
(b) Either Party may disclose the existence and terms of this Agreement in confidence:
(i) (A) to its attorneys, professional accountants, and auditors, and (B) bankers or other financial advisors in connection with an initial public offering, other strategic transaction, or corporate valuation for internal purposes; provided that any such disclosure to such professional accountants, auditors, bankers or other financial advisors is under an agreement to keep the terms of confidentiality and non-use no less rigorous than the terms contained in this Agreement and to use such information solely for the applicable purpose permitted pursuant to this Section 7.2.3(b)(i);
(ii) to potential acquirers (and their respective attorneys and professional advisors), in connection with a potential merger, acquisition or reorganization; provided that (A) the Party making the disclosure has a bona fide offer from such Third Party for such a transaction, and (B) such disclosure is under an agreement to keep the terms of confidentiality and non-use no less rigorous than the terms contained in this Agreement and to use such information solely for the purpose permitted pursuant to this Section 7.2.3(b)(i);
49
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
(iii) to existing or potential investors, lenders or permitted assignees of such Party (and their respective attorneys and professional advisors); provided that such disclosure is under an agreement to keep the terms of confidentiality and non-use no less rigorous than the terms contained in this Agreement; and
(iv) to potential licensees or sublicensees of such Party (and their respective attorneys and professional advisors); provided that (A) such disclosure shall not include any financial terms or identify any Research Project Targets or Reserved Targets; and (B) such disclosure is under an agreement to keep the terms of confidentiality and non-use no less rigorous than the terms contained in this Agreement.
7.3. Public Announcements; Publications.
7.3.1. Announcements. Except as may be expressly permitted under Section 7.2.3, neither Party will make any public announcement regarding this Agreement without the prior written approval of the other Party. For the sake of clarity, nothing in this Agreement shall prevent (a) either Party from making any public disclosure relating to this Agreement if the contents of such public disclosure has previously been made public other than through a breach of this Agreement by the issuing Party or its Affiliates; (b) Pfizer from making any scientific publication (in accordance with Section 7.3.2) or public announcement with respect to any Agreement Product under this Agreement; or (c) MacroGenics from making any public announcement approved in advance by Pfizer with respect to any Milestone Payment received by MacroGenics hereunder, which approval may not be unreasonably withheld or delayed; provided, however, that, except as permitted under Section 7.2, Pfizer shall not disclose any of MacroGenics’s Confidential Information in any such publication or announcement without obtaining MacroGenics’s prior written consent to do so. The Parties agree that MacroGenics may release the announcement attached hereto as Exhibit 7.3.1 regarding the signing of this Agreement following the Effective Date.
7.3.2. Publications. During the Term, each Party shall submit to the other Party (the “Non-Disclosing Party”) for review and approval any proposed academic, scientific and medical publication or public presentation which contains the Non-Disclosing Party’s Confidential Information. In addition, MacroGenics shall submit to Pfizer for review and approval any proposed publication or public presentation relating to the Research Program. In both instances, such review and approval will be conducted for the purposes of preserving the value of the MacroGenics Technology, the Pfizer Technology, the Joint Technology, the rights granted to Pfizer hereunder and determining whether any portion of the proposed
50
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
publication or presentation containing the Non-Disclosing Party’s Confidential Information should be modified or deleted. Written copies of such proposed publication or presentation required to be submitted hereunder shall be submitted to the Non-Disclosing Party no later than *** before submission for publication or presentation (the “Review Period”). The Non-Disclosing Party shall provide its comments with respect to such publications and presentations within *** after its receipt of such written copy. The Review Period may be extended for an additional *** in the event the Non-Disclosing Party can, *** after receipt of the written copy, demonstrate reasonable need for such extension including for the preparation and filing of patent applications. MacroGenics and Pfizer will each comply with standard academic practice regarding authorship of scientific publications and recognition of contribution of other parties in any publication governed by this Section 7.3.2.
7.4. Obligations in Connection with Change of Control. If MacroGenics is subject to a Change of Control, MacroGenics will, and it will cause its Affiliates and Representatives to, ensure that no Confidential Information of Pfizer (except as may be expressly permitted under Section 7.2.3 with respect to the terms of this Agreement and with respect to any royalty statements provided by Pfizer pursuant to Section 5.5.2) is released to (a) any Affiliate of MacroGenics that becomes an Affiliate as a result of the Change of Control or (b) any Representatives of MacroGenics (or of the relevant surviving entity of such Change of Control) who become Representatives as a result of the Change of Control, unless such Representatives have signed individual confidentiality agreements which include equivalent obligations to those set out in this Section 7. If any Change of Control of MacroGenics occurs, MacroGenics shall promptly notify Pfizer, ***
| 8. | REPRESENTATIONS AND WARRANTIES. |
8.1. Mutual Representations and Warranties. Each of MacroGenics and Pfizer hereby represents and warrants to the other Party that:
8.1.1. it is duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization;
8.1.2. the execution, delivery and performance of this Agreement by such Party has been duly authorized by all requisite action under the provisions of its charter, bylaws and other organizational documents, and does not require any action or approval by any of its shareholders or other holders of its voting securities or voting interests;
8.1.3. it has the power and authority to execute and deliver this Agreement and to perform its obligations hereunder
8.1.4. this Agreement has been duly executed and is a legal, valid and Binding Obligation on each Party, enforceable against such Party in accordance with its terms; and
51
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
8.1.5. the execution, delivery and performance by such Party of this Agreement and its compliance with the terms and provisions hereof does not and will not conflict with or result in a breach of or default under any Binding Obligation existing as of the Effective Date.
8.2. Representations and Warranties of MacroGenics. MacroGenics hereby represents and warrants to Pfizer that as of the Effective Date:
8.2.1. MacroGenics is the sole and exclusive owner of the MacroGenics Technology, all of which is free and clear of any claims, liens, charges or encumbrances;
8.2.2. it has and will have the full right, power and authority to grant all of the right, title and interest in the licenses and other rights granted or to be granted to Pfizer or Pfizer’s Affiliates under this Agreement;
8.2.3. (a) Schedule 8.2.3 sets forth a true and complete list of all Patent Rights owned or otherwise Controlled by MacroGenics or its Affiliates that relate to the MacroGenics Technology, (b) each such Patent Right remains in full force and effect and (c) MacroGenics or its Affiliates have timely paid all filing and renewal fees payable with respect to such Patent Rights;
8.2.4. to its best knowledge: (a) the MacroGenics Patent Rights, are, or, upon issuance, will be, valid and enforceable patents and (b) no Third Party (i) is infringing any MacroGenics Patent Right or (ii) has challenged or threatened to challenge the extent, validity or enforceability of any MacroGenics Patent Right (including, by way of example, through the institution or written threat of institution of interference, nullity or similar invalidity proceedings before the United States Patent and Trademark Office or any analogous foreign Governmental Authority);
8.2.5. to its best knowledge, it has complied with all Applicable Laws, including any disclosure requirements, in connection with the filing, prosecution and maintenance of the MacroGenics Patent Rights;
8.2.6. MacroGenics has independently developed all MacroGenics Know-How or otherwise has a valid right to use, and to permit Pfizer, Pfizer’s Affiliates and Pfizer’s Sublicensees to use, the MacroGenics Know-How for all permitted purposes under this Agreement;
8.2.7. it has obtained from all inventors of MacroGenics Technology existing as of the Effective Date, valid and enforceable agreements assigning to MacroGenics each such inventor’s entire right, title and interest in and to all such MacroGenics Technology;
52
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
8.2.8. except as expressly disclosed in Schedule 8.2.8, no MacroGenics Technology existing as of the Effective Date is subject to any funding agreement with any government or Governmental Authority;
8.2.9. neither MacroGenics nor any of its Affiliates are subject to any agreement or obligation that limits any ownership or license right granted to Pfizer or its Affiliates under this Agreement, including any right granted to Pfizer or its Affiliates to access, practice, grant any licenses or sublicenses under, or provide Pfizer’s Sublicensees with access to any intellectual property right or material (including any Patent Right, Know-How or other data or information), in each case, that would, but for such agreement or obligation, be included in the rights licensed or assigned to Pfizer or its Affiliates pursuant to this Agreement;
8.2.10. (a) there are no MacroGenics Third Party Agreements and (b) no Third Party has any right, title or interest in or to, or any license under, any MacroGenics Technology;
8.2.11. it has disclosed to Pfizer all provisions of *** relevant to determining the extent of any and all limitations on Pfizer’s rights under this Agreement necessitated by MacroGenics’s obligations under ***;
8.2.12. except as disclosed to Pfizer prior to the Effective Date in written form, referencing this Section 8.2.12 of the Agreement, to the best of its knowledge, the use, practice or application by MacroGenics or Pfizer (or their respective Affiliates or Sublicensees) as contemplated under this Agreement of any MacroGenics Technology relating to T-DARTs (a) does not and will not infringe any issued patent of any Third Party or (b) would not infringe the claims of any published Third Party patent application when and if such claims were to issue;
8.2.13. except as expressly disclosed in Schedule 8.2.13, there is no (a) claim, demand, suit, proceeding, arbitration, inquiry, investigation or other legal action of any nature, civil, criminal, regulatory or otherwise, pending or, to the best knowledge of MacroGenics, threatened against MacroGenics or any of its Affiliates or (b) judgment or settlement against or owed by MacroGenics or any of its Affiliates, in each case in connection with the MacroGenics Technology or relating to the transactions contemplated by this Agreement.
8.3. Representations and Warranties of Pfizer. Pfizer hereby represents and warrants to MacroGenics that as of the Effective Date:
8.3.1. there is no (a) claim, demand, suit, proceeding, arbitration, inquiry, investigation or other legal action of any nature, civil, criminal, regulatory or otherwise, pending or, to the best knowledge of Pfizer, threatened against Pfizer or any of its Affiliates or (b) judgment or settlement against or owed by Pfizer or any of its Affiliates, in each case in connection with the Pfizer Proprietary Materials or relating to the transactions contemplated by this Agreement.
53
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
8.4. MacroGenics Covenants. In addition to the covenants made by MacroGenics elsewhere in this Agreement, and subject to the provisions of Section 6, MacroGenics hereby covenants to Pfizer that, from the Effective Date until expiration or termination of this Agreement:
8.4.1. it will (a) not enter into any MacroGenics Third Party Agreement that is inconsistent with (i) the rights granted to Pfizer or Pfizer’s Affiliates hereunder or (ii) MacroGenics’s ability to fully perform its obligations hereunder; (b) not amend or otherwise modify any MacroGenics Third Party Agreement (including any Disclosed Third Party Agreement) or consent or waive rights with respect thereto in any manner that (i) is inconsistent with the rights granted to Pfizer or Pfizer’s Affiliates hereunder or (ii) MacroGenics’s ability to fully perform its obligations hereunder; (c) promptly furnish Pfizer with copies of all (i) amendments to the Disclosed Third Party Agreements and (ii) MacroGenics Third Party Agreements and related amendments executed following the Effective Date; (d) remain, and cause its Affiliates to remain, in compliance in all material respects with all MacroGenics Third Party Agreements (including Disclosed Third Party Agreements); and (e) furnish Pfizer with copies of all notices received by MacroGenics or its Affiliates relating to any alleged breach or default by MacroGenics or its Affiliates under any MacroGenics Third Party Agreement (including and Disclosed Third Party Agreement) within fifteen (15) Business Days after receipt thereof;
8.4.2. it will not enter into or otherwise allow itself or its Affiliates to be subject to any agreement or arrangement which limits the ownership rights of Pfizer or its Affiliates with respect to, or limits the ability of Pfizer or its Affiliates to grant a license, sublicense or access, or provide or provide access or other rights in, to or under, any intellectual property right or material (including any Patent Right, Know-How or other data or information), in each case, that would, but for such agreement or arrangement, be included in the rights licensed or assigned to Pfizer or its Affiliates pursuant to this Agreement;
8.4.3. it will maintain valid and enforceable agreements with all Persons acting by or on behalf of MacroGenics or its Affiliates under this Agreement which require such Persons to assign to MacroGenics their entire right, title and interest in and to all MacroGenics Technology and Joint Technology; and
8.4.4. in the conduct of any Research Project, unless MacroGenics receives prior written consent from Pfizer, MacroGenics shall neither use funding from any Government Authority nor use CHO-S cells.
8.5. Representation by Legal Counsel. Each Party hereto represents that it has been represented by legal counsel in connection with this Agreement and acknowledges that it has participated in the drafting hereof. In interpreting and applying the terms and provisions of this Agreement, the Parties agree that no presumption shall exist or be implied against the Party which drafted such terms and provisions.
54
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
8.6. Disclaimer. THE FOREGOING REPRESENTATIONS AND WARRANTIES OF EACH PARTY ARE IN LIEU OF ANY OTHER REPRESENTATIONS AND WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED WARRANTIES OF MERCHANTABILITY, ANY IMPLIED WARRANTY OF NON-INFRINGEMENT OR ANY IMPLIED WARRANTIES OF FITNESS FOR A PARTICULAR PURPOSE, ALL OF WHICH ARE HEREBY SPECIFICALLY EXCLUDED AND DISCLAIMED.
| 9. | GOVERNMENT APPROVALS; TERM AND TERMINATION. |
9.1. Government Approvals. Each of MacroGenics and Pfizer shall cooperate with the other Party and use Commercially Reasonable Efforts to make all registrations, filings and applications, to give all notices and to obtain as soon as practicable all governmental or other consents, transfers, approvals, orders, qualifications authorizations, permits and waivers, if any, and to do all other things necessary or desirable for the consummation of the transactions as contemplated hereby.
9.2. Term. The term of this Agreement shall commence on the Effective Date and shall extend, unless this Agreement is terminated earlier in accordance with this Section 9, until the expiration of the last-to-expire of all Royalty Terms with respect to all Agreement Products (the “Term”).
9.3. Termination by Either Party for Cause. Either Party may terminate this Agreement, in its entirety or, at the terminating Party’s option, on an Agreement T-DART-by-Agreement T-DART basis, at any time during the Term by giving written notice to the other Party if the other Party commits a material breach of its obligations under this Agreement and such breach remains uncured for ***, measured from the date written notice of such breach is given to the breaching Party; provided, however, that if such breach (other than breach of an undisputed payment obligation) is not susceptible of cure within the stated period and the breaching Party uses diligent, good faith efforts to cure such breach, the stated period shall be extended by an *** Notwithstanding the foregoing, a Party shall have the right to terminate this Agreement pursuant to this Section 9.3 in part with respect to an individual Agreement T-DART only if the other Party’s material breach giving rise to such termination right relates solely to such Agreement T-DART.
9.4. Termination by Pfizer without Cause. Pfizer shall have the right to terminate the Agreement at any time for any or no reason, on a Research Project Target-by-Research Project Target basis, by providing *** advance written notice of such termination to MacroGenics; provided that, during the ***, Pfizer will retain at least one Research Project Target under the Research Program. ***, Pfizer shall have the right, exercisable upon *** prior written notice to MacroGenics, to terminate this Agreement at any time for any or no reason either (a) in its entirety or (b) on a Research Project Target-by-Research Project Target basis.
55
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
9.5. Effects of Termination.
9.5.1. Effect of Termination by Pfizer for Cause. If Pfizer terminates this Agreement with respect to any or all Agreement T-DARTS pursuant to Section 9.3 (each, a “Terminated T-DART”):
(a) all work under the applicable Research Plan(s) with respect to each Terminated T-DART shall cease;
(b) (i) all licenses granted to Pfizer with respect to such Terminated T-DART and any Agreement Product incorporating such Terminated T-DART (“Terminated Agreement Product”), including licenses under Section 4.1, shall continue and shall become perpetual, irrevocable licenses; (ii) Pfizer’s rights under Section 4.1 shall remain in full force and effect with respect to any such Terminated T-DART or Terminated Agreement Product; and (iii) Pfizer shall have no further obligations to MacroGenics under this Agreement with respect to any such Terminated T-DART or Terminated Agreement Product, other than (x) obligations to pay Milestones and royalties with respect to each Terminated Target and Net Sales of Agreement Products incorporating each Terminated T-DART, as applicable, in an amount equal to *** of the amount that would otherwise have been payable under this Agreement, and (y) those other obligations that expressly survive termination in accordance with Section 9.7 shall survive;
(c) MacroGenics shall remain entitled to receive payments that accrued before the effective date of such termination;
(d) nothing in this Section 9.5.1 shall limit any other remedy Pfizer may have for MacroGenics’s breach of this Agreement; and
(e) the rights and obligations of the Parties with respect to all Agreement T-DARTs other than any such Terminated T-DART shall remain in full force and effect.
9.5.2. Effect of Termination by MacroGenics for Cause or by Pfizer without Cause.
(a) If MacroGenics terminates this Agreement with respect to any Agreement T-DARTs pursuant to Section 9.3, or if Pfizer terminates this Agreement with respect to any Agreement T-DARTs pursuant to Section 9.4: (i) all licenses granted by MacroGenics to Pfizer under Section 4.1 with respect to any such Agreement T-DART and any Agreement Product containing such Agreement T-DART shall terminate and (ii) on MacroGenics’s written request within *** of such termination, Pfizer shall reassign to MacroGenics all of the right, title and interest in Agreement T-DART IP that was assigned by MacroGenics to Pfizer pursuant to Section 4.1.2 with respect to such Agreement T-DART, and in any Agreement
56
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
T-DART Patent Rights relating thereto that have been filed (unless another alternative approach has been agreed by the Parties in view of prosecution exigencies), any such assignment to be completed within *** of such request. After such assignment, or if MacroGenics declines to request assignment or such *** period expires without a request by MacroGenics, Pfizer will have no further obligations to prosecute or maintain such Agreement T-DART Patent Rights or to pay any costs related thereto. All rights and obligations with respect to all other Agreement T-DARTs and Agreement Products shall remain in full force and effect (and for avoidance of doubt, any Agreement T-DART IP and Agreement T-DART Patent Rights assigned to MacroGenics pursuant to this Section 9.5.2(a) will be included in MacroGenics Technology and MacroGenics Patent Rights, respectively, for the purposes of such other Agreement T-DARTs and Agreement Products). MacroGenics shall have a fully paid up, royalty free non-exclusive license under any such Agreement T-DART Patent Right not assigned to MacroGenics pursuant to the provisions of this Section 9.5.2(a).
(b) If MacroGenics terminates this Agreement in its entirety pursuant to Section 9.3, or if Pfizer terminates this Agreement in its entirety pursuant to Section 9.4: (i) all licenses granted by MacroGenics to Pfizer under Section 4.1 shall terminate, (ii) on MacroGenics’s written request within thirty (30) days of such termination, Pfizer shall reassign to MacroGenics all of MacroGenics’ right, title and interest in Agreement T-DART IP that was assigned by MacroGenics to Pfizer pursuant to Section 4.1.2, and in any Agreement T-DART Patent Rights relating thereto that have been filed (unless another alternative approach has been agreed by the Parties in view of prosecution exigencies), any such assignment to be completed within *** of such request, and MacroGenics shall have no further obligations to Pfizer under this Agreement other than those obligations that expressly survive termination in accordance with Section 9.7. After assignment of any Agreement T-DART Patent Rights pursuant to this Section 9.5.2(b), or if MacroGenics declines to request assignment or *** expires without a request by MacroGenics, Pfizer will have no further obligations to prosecute or maintain such Agreement T-DART Patent Rights or to pay any costs related thereto. MacroGenics shall have a fully paid up, royalty free non-exclusive license under any Agreement T-DART Patent Right not assigned to MacroGenics pursuant to the provisions of this Section 9.5.2(b).
(c) For the avoidance of doubt, if MacroGenics terminates this Agreement with respect to any Agreement T-DARTs pursuant to Section 9.3, or if Pfizer terminates this Agreement with respect to any Agreement T-DARTs pursuant to Section 9.4, in each case including all Agreement T-DARTs in the event that this Agreement is terminated in its entirety, (i) all rights granted by Pfizer to MacroGenics under Section 4.2
57
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
shall survive such termination and (ii) any such Agreement T-DART will no longer be considered to be an Agreement T-DART for the purpose of this Agreement.
(d) Nothing in this Section 9.5.3 shall limit MacroGenics’s right to receive payments that accrued before the effective date of termination or limit any other remedy MacroGenics may have for Pfizer’s breach of this Agreement.
(e) At MacroGenics’s request within thirty (30) days of any termination of this Agreement, Pfizer shall negotiate with MacroGenics regarding the possible grant to MacroGenics of some or all of the following rights, it being understood and agreed that neither Party shall have any obligation to enter into any such arrangement and that if agreement is not reached within *** after such request there shall be no further obligation on either Party to conduct further negotiations:
(i) Grant Back License with Respect to MacroGenics-Derived Terminated Agreement T-DARTs. The grant by Pfizer to MacroGenics of an exclusive or non-exclusive, worldwide, royalty-bearing license under the Pfizer Collaboration Patent Rights to use, have used, Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized, import, have imported, export and have exported any MacroGenics-Derived Terminated Agreement T-DART and, in each case in the form in which such MacroGenics-Derived Terminated Agreement T-DART or related Agreement Product exists as of termination, for a commercially reasonable royalty rate and on terms and conditions regarding payment provisions based on the provisions of this Agreement regarding such matters as royalty term, adjustments, reports, audits and the like). “Pfizer Collaboration Patent Right” means any Pfizer Patent Right covering or claiming any invention made solely by Pfizer or any of its Affiliates in the course of performing the Research Program and “MacroGenics-Derived Terminated Agreement T-DART” means any Agreement T-DART for which (A) this Agreement is terminated by MacroGenics pursuant to Section 9.3, or by Pfizer pursuant to Section 9.4 and (B) MacroGenics provided Antibodies to Pfizer for the Research Project for such Agreement T-DART.
(ii) Inventory. MacroGenics obtaining Pfizer’s inventory of any such MacroGenics-Derived Terminated Agreement T-DART and/or related Agreement Product.
58
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
(iii) Supply. Pfizer supplying MacroGenics with quantities of such MacroGenics-Derived Terminated Agreement T-DART and/or related Agreement Product for a limited period of time.
(iv) Trademarks. Pfizer providing with MacroGenics with certain rights in and to the Trademark(s) used in connection with any such MacroGenics-Derived Terminated Agreement T-DART and/or related Agreement Product (but not any Pfizer house marks or any trademark containing the word “Pfizer”).
(v) Regulatory Filings and Approvals. Pfizer providing MacroGenics with certain rights in and regulatory filings and approvals with respect to and such MacroGenics-Derived Terminated Agreement T-DART and/or related Agreement Product.
(vi) No Obligation. For the avoidance of doubt, the description here of these possible arrangements shall not imply any obligation on either Party to enter into any such arrangement.
9.5.3. Satisfaction of Obligations During Notice Period. During the period from providing a notice of termination pursuant to Section 9.4 through the termination of the Agreement, the Parties shall continue to perform their obligations under this Agreement.
9.5.4. Pending Dispute Resolution. If a Party gives notice of termination under Section 9.3 and the other Party disputes whether such notice was proper, then the issue of whether this Agreement has been terminated shall be resolved in accordance with Section 11.9 and each Party shall continue to perform its obligations hereunder pending the conclusion of such dispute resolution proceeding. If as a result of such dispute resolution process it is determined that the notice of termination was proper, then such termination shall be effective immediately. If as a result of such dispute resolution process it is determined that the notice of termination was improper, then no termination shall have occurred and this Agreement shall remain in effect.
9.6. Disposition of Inventories of Products. Following termination of this Agreement with respect to one or more Agreement T-DARTs, Pfizer, its Affiliates and its Sublicensees shall have the right to continue to sell their existing inventories of Agreement Product(s) incorporating such Agreement T-DART(s) that have received Regulatory Marketing Approval prior to such termination for a period not to exceed *** after the effective date of such termination or expiration and Pfizer shall pay any royalties payable in connection with such sales in accordance with Sections 5.4.4 and 5.5.
9.7. Survival of Certain Obligations. Expiration or termination of this Agreement shall not relieve the Parties of any obligation that accrued before such expiration or
59
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
termination. The following provisions shall survive expiration or termination of this Agreement: Sections 1, 2.11, 2.12, 2.13.3, 2.13.4, 2.13.5, 4.3, 5.2.5, 5.6, 5.7, 6.1.1, 6.2.1(e), 7, 8.6, 9.5, 9.6, 9.7, 9.8, 10 and 11.
9.8. Right to Termination of Research Project(s) or Research Program by Pfizer upon Change of Control of MacroGenics. If a Change of Control of MacroGenics is consummated during the Research Term, Pfizer shall have the right to terminate any Research Project or the Research Program in its entirety, at any time, upon written notice to MacroGenics within 30 days after consummation of such Change of Control of MacroGenics. Such termination of any Research Project or the Research Program (a) shall not constitute termination of this Agreement, (b) shall not affect the Parties’ rights and obligations under this Agreement other than those relating to such Research Project or the Research Program and (c) shall not relieve either Party of any obligation that arose prior to such termination. Following any such termination of any Research Project or the Research Program, as applicable, Pfizer shall have no further funding obligation under Section 2 with respect to such Research Project or the Research Program, as applicable, other than that which may have accrued prior to such termination. For the avoidance of doubt, in the event that Pfizer terminates a Research Project or the Research Program in accordance with this Section 9.8, such termination will not be deemed to be a termination for cause under Section 9.3 or a termination without cause under Section 9.4, and the only effects of such termination are as set forth in this Section 9.8.
| 10. | LIMITATION ON LIABILITY, INDEMNIFICATION AND INSURANCE. |
10.1. No Consequential Damages. Except with respect to liability arising from a breach of Section 7, or to the extent such Party may be required to indemnify the other Party under this Section 10, in no event will either Party, its Affiliates, its Sublicensees or any of its, its Affiliates’ or its Sublicensees’ respective Representatives be liable under this Agreement for any special, indirect, incidental, consequential or punitive damages, whether in contract, warranty, tort, negligence, strict liability or otherwise, including loss of profits or revenue suffered by either Party or any of its respective Affiliates, agents or representatives. Without limiting the generality of the foregoing, “consequential damages” will be deemed to include, and neither Party will be liable to the other Party or any of such other Party’s Affiliates, Representatives or stockholders for any damages based on or measured by loss of projected or speculative future sales of the Agreement Products, any Milestone Payment due upon any unachieved event under Section 5.2, any unearned royalties under Section 5.4 or any other unearned, speculative or otherwise contingent payments provided for in this Agreement.
60
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
10.2. Indemnification by Pfizer. Pfizer will indemnify, defend and hold harmless MacroGenics, each of its Affiliates, and each of its and its Affiliates’ employees, officers, directors and agents (each, a “MacroGenics Indemnified Party”) from and against any and all liability, loss, damage, expense (including reasonable attorneys’ fees and expenses) and cost (collectively, a “Liability”) that the MacroGenics Indemnified Party may be required to pay to one or more Third Parties resulting from or arising out of:
10.2.1. the Development, Manufacture, Commercialization or use of any Agreement T-DART or Agreement Product by, on behalf of, or under the authority of, Pfizer (other than by any MacroGenics Indemnified Party), other than claims for which MacroGenics is required to indemnify Pfizer pursuant to Section 10.3; or
10.2.2. the material breach by Pfizer of any of its representations, warranties or covenants set forth in Section 2.13.1, 8.1 or 8.3;
except, in each case, to the extent caused by the negligence, recklessness or intentional acts of MacroGenics or any MacroGenics Indemnified Party.
10.3. Indemnification by MacroGenics. MacroGenics will indemnify, defend and hold harmless Pfizer, its Affiliates, Sublicensees, contractors, distributors and each of its and their respective employees, officers, directors and agents (each, a “Pfizer Indemnified Party”) from and against any and all Liabilities that the Pfizer Indemnified Party may be required to pay to one or more Third Parties resulting from or arising out of the material breach by MacroGenics of any of its representations, warranties or covenants set forth in Section 8.1 or 8.2, except to the extent caused by the negligence, recklessness or intentional acts of Pfizer or any Pfizer Indemnified Party.
10.4. Procedure.
10.4.1. Notice. Each Party will notify the other Party in writing in the event it becomes aware of a claim for which indemnification may be sought hereunder. In the event that any Third Party asserts a claim or other proceeding (including any governmental investigation) with respect to any matter for which a Party (the “Indemnified Party”) is entitled to indemnification hereunder (a “Third Party Claim”), then the Indemnified Party shall promptly notify the Party obligated to indemnify the Indemnified Party (the “Indemnifying Party”) thereof; provided, however, that no delay on the part of the Indemnified Party in notifying the Indemnifying Party shall relieve the Indemnifying Party from any obligation hereunder unless (and then only to the extent that) the Indemnifying Party is prejudiced thereby.
10.4.2. Control. Subject to Pfizer’s right to control any actions described in Sections 6.2 (even where MacroGenics is the Indemnifying Party), the Indemnifying Party shall have the right, exercisable by notice to the Indemnified Party within *** after receipt of notice from the Indemnified Party of the commencement of or assertion of any Third Party Claim, to assume direction and control of the defense, litigation, settlement, appeal or other disposition of the Third Party Claim (including the right to settle the claim solely for monetary consideration) with counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnified Party; provided that (a) the Indemnifying Party has sufficient financial resources, in the reasonable judgment of the Indemnified Party, to satisfy the amount of any adverse monetary judgment that is sought, and
61
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
(b) the Indemnifying Party expressly agrees in writing that as between the Indemnifying Party and the Indemnified Party, the Indemnifying Party shall be solely obligated to satisfy and discharge the Third Party Claim in full (the conditions set forth in clauses (a) and (b) above are collectively referred to as the “Litigation Conditions”). Within *** after the Indemnifying Party has given notice to the Indemnified Party of its exercise of its right to defend a Third Party Claim, the Indemnified Party shall give notice to the Indemnifying Party of any objection thereto based upon the Litigation Conditions. If the Indemnified Party reasonably so objects, the Indemnified Party shall continue to defend the Third Party Claim, at the expense of the Indemnifying Party, until such time as such objection is withdrawn. If no such notice is given, or if any such objection is withdrawn, the Indemnifying Party shall be entitled, at its sole cost and expense, to assume direction and control of such defense, with counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnified Party. During such time as the Indemnifying Party is controlling the defense of such Third Party Claim, the Indemnified Party shall cooperate, and shall cause its Affiliates and agents to cooperate upon request of the Indemnifying Party, in the defense or prosecution of the Third Party Claim, including by furnishing such records, information and testimony and attending such conferences, discovery proceedings, hearings, trials or appeals as may reasonably be requested by the Indemnifying Party. In the event that the Indemnifying Party does not satisfy the Litigation Conditions or does not notify the Indemnified Party of the Indemnifying Party’s intent to defend any Third Party Claim within ten *** after notice thereof, the Indemnified Party may (without further notice to the Indemnifying Party) undertake the defense thereof with counsel of its choice and at the Indemnifying Party’s expense (including reasonable, out-of-pocket attorneys’ fees and costs and expenses of enforcement or defense). The Indemnifying Party or the Indemnified Party, as the case may be, shall have the right to join in (including the right to conduct discovery, interview and examine witnesses and participate in all settlement conferences), but not control, at its own expense, the defense of any Third Party Claim that the other Party is defending as provided in this Agreement.
10.4.3. Settlement. The Indemnifying Party shall not, without the prior written consent of the Indemnified Party, enter into any compromise or settlement that commits the Indemnified Party to take, or to forbear to take, any action or declare that any Patent Right or Know-How is invalid or not properly owned or licensed by the Indemnified Party. The Indemnified Party shall have the sole and exclusive right to settle any Third Party Claim, on such terms and conditions as it deems reasonably appropriate, to the extent such Third Party Claim involves equitable or other non-monetary relief, but shall not have the right to settle such Third Party Claim to the extent such Third Party Claim involves monetary damages without the prior written consent of the Indemnifying Party. Each of the Indemnifying Party and the Indemnified Party shall not make any admission of liability in respect of any Third Party Claim without the prior written consent of the other Party, and the Indemnified Party shall use reasonable efforts to mitigate Liabilities arising from such Third Party Claim.
62
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
10.5. Insurance. Each Party shall obtain and maintain, during the Term, commercial general liability insurance, including products liability insurance, with reputable and financially secure insurance carriers (or pursuant to a program of self-insurance reasonably satisfactory to the other Party) to cover its indemnification obligations under Section 10.2 or Section 10.3, as applicable, in each case with limits of not less than *** and in the aggregate. Insurance shall be procured with carriers having an A.M. Best Rating of A-VII or better. The provisions of this Section 10.5 will survive for ***.
| 11. | MISCELLANEOUS. |
11.1. Assignment. MacroGenics may not assign this Agreement or any interest hereunder without the prior written consent of Pfizer, which consent will not be unreasonably withheld or delayed. Pfizer may not assign this Agreement or any interest hereunder without the prior written consent of MacroGenics, which consent will not be unreasonably withheld or delayed. Notwithstanding the foregoing, this Agreement may be assigned as follows: (a) either Party may assign its rights and obligations under this Agreement by way of sale of itself or the sale of the portion of its business to which this Agreement relates, through merger, sale of assets or sale of stock or ownership interest, provided that the assignee shall expressly agree to be bound by such Party’s obligations under this Agreement and that such sale is not primarily for the benefit of its creditors and (b) either Party may assign its rights and obligations under this Agreement to any of its Affiliates, provided that the assignee shall expressly agree to be bound by such Party’s obligations under this Agreement and that such Party shall remain liable for all of its rights and obligations under this Agreement. This Agreement shall be binding upon the successors and permitted assigns of the Parties and the name of a Party appearing herein shall be deemed to include the names of such Party’s successors and permitted assigns to the extent necessary to carry out the intent of this Agreement. Any assignment not in accordance with this Section 11.1 shall be void.
11.2. Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of the Agreement.
11.3. Force Majeure. Each Party shall be excused from the performance of its obligations under this Agreement to the extent that such performance is prevented by force majeure (defined below) and the nonperforming Party promptly provides notice of the prevention to the other Party. Such excuse shall be continued so long as the condition constituting force majeure continues and the nonperforming Party takes Commercially Reasonable Efforts to remove the condition. For purposes of this Agreement, “force majeure” shall include conditions beyond the control of the Parties, including an act of God, voluntary or involuntary compliance with any regulation, Law or order of any government, war, act of terror, civil commotion, labor strike or lock-out, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe.
63
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
11.4. Notices. Any notice or notification required or permitted to be provided pursuant to the terms and conditions of this Agreement (including any notice of force majeure, breach, termination, change of address, etc.) shall be in writing and shall be deemed given upon receipt if delivered personally or by facsimile transmission (receipt verified), five (5) days after deposited in the mail if mailed by registered or certified mail (return receipt requested) postage prepaid, or on the next Business Day if sent by overnight delivery using a nationally recognized express courier service and specifying next Business Day delivery (receipt verified), to the Parties at the following addresses or facsimile numbers (or at such other address or facsimile number for a Party as shall be specified by like notice, provided, however, that notices of a change of address shall be effective only upon receipt thereof):
All correspondence to Pfizer shall be addressed as follows:
Pfizer Worldwide Research & Development
000 Xxxx 00xx Xxxxxx
Xxx Xxxx, XX 00000
Attention: ***
with a copy to:
Pfizer Inc.
000 Xxxxxxx Xxxxx Xxxx, XX 0000-00
Groton, CT
Attention: ***
All correspondence to MacroGenics shall be addressed as follows:
MacroGenics, Inc.
0000 Xxxx Xxxx Xxxxx Xxxxxxxxx, XX 00000
Attention: ***
Facsimile: ***
with a copy to:
Xxxxxx Xxxxxx Xxxxxxxxx Xxxx and Xxxx LLP
000 Xxxx Xxxxxx
Xxx Xxxx, XX 00000
Attention: ***
Facsimile: ***
64
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
11.5. Amendment. No amendment, modification or supplement of any provision of this Agreement shall be valid or effective unless made in writing and signed by a duly authorized officer of each Party.
11.6. Waiver. No provision of this Agreement shall be waived by any act, omission or knowledge of a Party or its agents or employees except by an instrument in writing expressly waiving such provision and signed by a duly authorized officer of the waiving Party. The waiver by either of the Parties of any breach of any provision hereof by the other Party shall not be construed to be a waiver of any succeeding breach of such provision or a waiver of the provision itself.
11.7. Severability. If any clause or portion thereof in this Agreement is for any reason held to be invalid, illegal or unenforceable, the same shall not affect any other portion of this Agreement, as it is the intent of the Parties that this Agreement shall be construed in such fashion as to maintain its existence, validity and enforceability to the greatest extent possible. In any such event, this Agreement shall be construed as if such clause of portion thereof had never been contained in this Agreement, and there shall be deemed substituted therefor such provision as will most nearly carry out the intent of the Parties as expressed in this Agreement to the fullest extent permitted by Applicable Law.
11.8. Descriptive Headings. The descriptive headings of this Agreement are for convenience only and shall be of no force or effect in construing or interpreting any of the provisions of this Agreement.
11.9. Dispute Resolution. If any dispute or disagreement arises between Pfizer and MacroGenics in respect of this Agreement, they shall follow the following procedures in an attempt to resolve the dispute or disagreement:
11.9.1. The Party claiming that such a dispute exists shall give notice in writing (“Notice of Dispute”) to the other Party of the nature of the dispute.
11.9.2.*** after receipt of a Notice of Dispute, the Pfizer Alliance Manager and the MacroGenics Alliance Manager shall meet in person or by teleconference and exchange written summaries reflecting, in reasonable detail, the nature and extent of the dispute, and at this meeting they shall use their reasonable endeavors to resolve the dispute.
11.9.3. If the Alliance Managers are unable to resolve the dispute during the meeting described in Section 11.9.2 or if for any reason such meeting does not take place within the period specified in Section 11.9.2, then the dispute will be referred to the JSC which shall meet no later than *** following the initial receipt of the Notice of Dispute and use reasonable endeavors to resolve the dispute.
11.9.4. If the JSC is unable to resolve the dispute during the meeting described in Section 11.9.3 or if for any reason such meeting does not take place within the period specified in Section 11.9.3, then the Senior Executives shall meet at a mutually agreed-upon time and location for the purpose of resolving such dispute.
65
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
11.9.5. If, within *** initial receipt of the Notice of Dispute, the dispute has not been resolved, or if, for any reason, the meeting described in Section 11.9.4 has not been held within *** following initial receipt of the Notice of Dispute, then the Parties agree that either Party may initiate litigation to resolve the dispute.
11.9.6. Notwithstanding any provision of this Agreement to the contrary, either Party may immediately initiate litigation in any court of competent jurisdiction seeking any remedy at law or in equity, including the issuance of a preliminary, temporary or permanent injunction, to preserve or enforce its rights under this Agreement. The provisions of this Section 11.9 will survive for *** from the date of termination or expiration of this Agreement.
11.10. Governing Law. This Agreement, and all claims arising under or in connection therewith, shall be governed by and interpreted in accordance with the substantive laws of ***, without regard to conflict of law principles thereof.
11.11. Consent to Jurisdiction. Each Party to this Agreement, by its execution hereof, (a) hereby irrevocably submits to the exclusive jurisdiction of the state courts of the State *** of any and all actions, suits or proceedings arising in whole or in part out of, related to, based upon or in connection with this Agreement or the subject matter hereof, (b) hereby waives to the extent not prohibited by Applicable Law, and agrees not to assert, by way of motion, as a defense or otherwise, in any such action, any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune from attachment or execution, that any such action brought in one of the above-named courts should be dismissed on grounds of forum non conveniens, should be transferred to any court other than one of the above-named courts, or should be stayed by reason of the pendency of some other proceeding in any other court other than one of the above-named courts, or that this Agreement or the subject matter hereof may not be enforced in or by such court, and (c) hereby agrees not to commence any such action other than before one of the above-named courts nor to make any motion or take any other action seeking or intending to cause the transfer or removal of any such action to any court other than one of the above-named courts whether on the grounds of inconvenient forum or otherwise.
11.12. Entire Agreement. This Agreement constitutes and contains the complete, final and exclusive understanding and agreement of the Parties and cancels and supersedes any and all prior negotiations, correspondence, understandings and agreements, whether oral or written, between the Parties respecting the subject matter hereof and thereof, including the Confidentiality Agreement which is hereby terminated effective as of the Effective Date.
11.13. Independent Contractors. Both Parties are independent contractors under this Agreement. Nothing herein contained shall be deemed to create an employment, agency,
66
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
joint venture or partnership relationship between the Parties hereto or any of their agents or employees, or any other legal arrangement that would impose liability upon one Party for the act or failure to act of the other Party. Neither Party shall have any express or implied power to enter into any contracts or commitments or to incur any liabilities in the name of, or on behalf of, the other Party, or to bind the other Party in any respect whatsoever.
11.14. Counterparts. This Agreement may be executed in two counterparts, each of which shall be an original and both of which shall constitute together the same document. Counterparts may be signed and delivered by facsimile or PDF file, each of which shall be binding when received by the applicable Party.
11.15. No Third Party Rights or Obligations. No provision of this Agreement shall be deemed or construed in any way to result in the creation of any rights or obligation in any Person not a Party to this Agreement. However, either Party may decide, in its sole discretion, to use one or more of its Affiliates to perform its obligations and duties hereunder, provided that such Party shall remain liable hereunder for the performance by any such Affiliates of any such obligations.
(The remainder of this page has been intentionally left blank. The signature page follows.)
67
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
IN WITNESS WHEREOF, duly authorized representatives of the Parties have duly executed this Agreement to be effective as of the Effective Date.
| PFIZER INC. | MACROGENICS, INC. | |||||||
| By: | /s/ Xx. Xxxxxx Xxxxxxx |
By: | /s/ Xxxxx Xxxxxx | |||||
| Name: | Mikael Dolsten | Name: | Xxxxx Xxxxxx | |||||
| Title: | President, Worldwide Research & Development | Title: | CEO | |||||
Signature Page to Research Collaboration and License Agreement
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
EXHIBIT 2.3.1
FORM OF RESEARCH PLANS
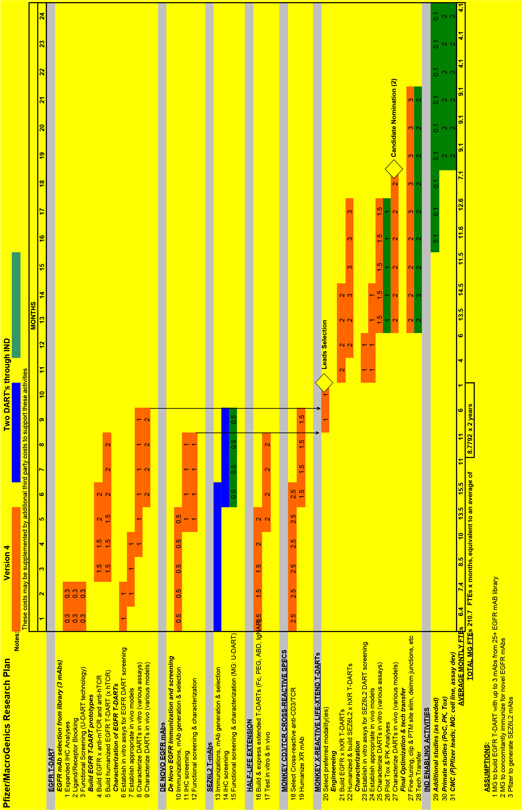
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
EXHIBIT 7.3.1
PRESS RELEASE
DRAFT
MacroGenics Enters Global Research Collaboration
and License Agreement with Pfizer
ROCKVILLE, MD , 2010 /PRNewswire/ — MacroGenics, Inc, a leader in next-generation antibody platforms and therapeutics, announced today that it has entered into a global research collaboration and license agreement with Pfizer Inc. to discover, develop and commercialize Dual-Affinity Re-Targeting (DART™) products directed at two undisclosed cancer targets. MacroGenics’ DART technology is a proprietary, bi-specific antibody platform in which a single recombinant molecule is able to target two different antigens. These DART proteins are amenable to several applications and can potentially be used to redirect the body’s cell-destroying, immune effector cells against tumor cells.
“MacroGenics’ DART candidates represent a promising new approach to potentially expand treatment options for cancer patients, and we look forward to a collaborative partnership with MacroGenics,” stated Xx. Xxxxxx Xxxxxxx, Senior Vice President & President, Worldwide Research and Development, Pfizer. “MacroGenics has established the versatility of its novel DART platform by generating a large array of DART proteins against a variety of different targets.”
“We are delighted to establish this strategic collaboration with Pfizer,” said Xx. Xxxxx Xxxxxx, MacroGenics’ President and Chief Executive Officer. “As we continue to make significant progress in the development of our pipeline of best-in-class product candidates for cancer, autoimmune disease, and infectious disease, this collaboration with Pfizer further validates the promise of our DART platform.”
Under the terms of the agreement, MacroGenics will receive an upfront cash payment and research funding. In addition, MacroGenics will be eligible to receive escalating preclinical, clinical, regulatory and commercial milestone payments as well as tiered royalties on sales of products resulting from the collaboration. Further details of the agreement have not been disclosed.
DART Background
MacroGenics’ DART technology enables the generation of highly stable antibody-based therapeutic molecules that can simultaneously target two different antigens. DART therapeutics can accommodate virtually any variable region sequence in a “plug-and-play” fashion, are potent, and have very favorable manufacturing properties. To date, the company has engineered over 65 different DART proteins and has completed multiple in vitro and in vivo proof-of-concept studies in a variety of disease models. The company has been able to produce DART
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
proteins in both bacterial and mammalian expression systems. DARTs also have been engineered with an Fc domain, which confers them with additional properties, such as Fc receptor binding and extended half-life. This functionality can be further expanded with the inclusion of MacroGenics’ proprietary Fc domain portfolio. MacroGenics has established and continues to expand a significant patent estate around its DART technology.
About MacroGenics
MacroGenics is a private, venture-backed biotechnology company that focuses on the discovery, development and delivery to patients of novel biologics for autoimmune disorders, cancer and infectious diseases. Since its founding in 2000, the company has built a fully-integrated set of capabilities in antibody-based product development. The company has generated a proprietary pipeline of innovative product candidates by leveraging its three core technology platforms. These proprietary platforms include: (1) cancer stem-like cells; (2) DART technology, which allows the company to incorporate multiple specificities within a single molecule; and (3) Fc optimization, which enhances antibody-dependent effector functions. The company’s lead program, teplizumab, is an anti-CD3 antibody. Teplizumab is being investigated in Phase 3 trials for the treatment of autoimmune diseases in collaboration with Xxx Lilly and Company. For more information about MacroGenics, please visit xxx.xxxxxxxxxxx.xxx.
Statements made in this news release that are not historical facts are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “expects,” “believes,” “intends,” and similar expressions are intended to identify forward-looking statements. Actual results may differ materially from those projected in any forward-looking statement. Specifically, there are a number of important factors that could cause actual results to differ materially from those anticipated, such as the Company’s ability to raise additional capital, and risks related to the Company’s ability to initiate, and enroll patients in, planned clinical trials. You should not place undue reliance on any forward-looking statements. The Company assumes no obligation to update any forward-looking statements as a result of new information, future events or developments, except as required by law.
Contacts:
***
###
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.19 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.19 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.34 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.34 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.34 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.80 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.80 ***
***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.80 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.80 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.80 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.80 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.125 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.125 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 1.125 ***
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 2.10
SUBCONTRACTORS***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 8.2.3
Patent Rights of MacroGenics
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** | ||||
| *** |
*** | *** | *** | *** |
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 8.2.8
FUNDING AGREEMENTS
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.
SCHEDULE 8.2.13
LITIGATION
***
*** = Portions of this exhibit have been omitted pursuant to a request for confidential treatment. An unredacted
version of this exhibit has been filed separately with the Commission.


