Master Manufacturing Services Agreement
Exhibit 10.2
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Master Manufacturing Services Agreement
Effective Date: June 27, 2019
PARTIES
PATHEON UK LIMITED
a company existing under the laws of England and Wales, with its registered office at Xxxxxxxxxx Xxxxx, Xxxxxxxxx, Xxxxxxx, XX0 0XX ("Patheon"),
- and -
CARA THERAPEUTICS INC.
a corporation existing under the laws Delaware with its principal place of business at 4 Stamford Plaza, 000 Xxx Xxxxxx, 0xx Xxxxx, Xxxxxxxx, Xxxxxxxxxxx 00000, Xxxxxx Xxxxxx ("Client").
|
June 27, 2019 |
|
|
|
Confidential |
|
Page 1 of 47 |
|
1. |
4 |
|||
|
|
|
|
|
|
|
|
1.1 |
4 |
||
|
|
1.2 |
4 |
||
|
|
1.3 |
4 |
||
|
|
1.4 |
10 |
||
|
2. |
10 |
|||
|
|
2.1 |
10 |
||
|
|
2.2 |
11 |
||
|
3. |
11 |
|||
|
|
3.1 |
11 |
||
|
|
3.2 |
12 |
||
|
|
3.3 |
12 |
||
|
|
3.4 |
14 |
||
|
4. |
14 |
|||
|
|
4.1 |
14 |
||
|
|
4.2 |
14 |
||
|
|
4.3 |
15 |
||
|
5. |
15 |
|||
|
|
5.1 |
15 |
||
|
|
5.2 |
18 |
||
|
|
5.3 |
19 |
||
|
|
5.4 |
19 |
||
|
|
5.5 |
19 |
||
|
6. |
20 |
|||
|
|
6.1 |
20 |
||
|
|
6.2 |
21 |
||
|
|
6.3 |
22 |
||
|
7. |
22 |
|||
|
|
7.1 |
22 |
||
|
|
7.2 |
23 |
||
|
|
7.3 |
23 |
||
|
|
7.4 |
23 |
||
|
|
7.5 |
24 |
||
|
|
7.6 |
25 |
||
|
|
7.7 |
25 |
||
|
8. |
26 |
|||
|
|
8.1 |
26 |
||
|
|
8.2 |
26 |
||
|
|
8.3 |
28 |
||
|
|
8.4 |
29 |
||
|
9. |
29 |
|||
|
|
9.1 |
29 |
||
|
|
9.2 |
29 |
||
|
|
9.3 |
30 |
||
|
|
9.4 |
31 |
||
|
|
9.5 |
31 |
||
|
10. |
31 |
|||
|
|
10.1 |
31 |
||
|
|
10.2 |
32 |
||
|
|
10.3 |
33 |
||
|
|
10.4 |
33 |
||
|
|
10.5 |
34 |
||
|
|
10.6 |
34 |
||
|
11. |
34 |
|||
|
|
11.1 |
34 |
||
|
|
11.2 |
35 |
||
|
|
11.3 |
35 |
||
|
|
11.4 |
36 |
||
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 2 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
11.5 |
36 |
||
|
|
11.6 |
36 |
||
|
|
11.7 |
36 |
||
|
|
11.8 |
37 |
||
|
12. |
37 |
|||
|
|
12.1 |
37 |
||
|
|
12.2 |
37 |
||
|
13. |
38 |
|||
|
|
13.1 |
38 |
||
|
|
13.2 |
38 |
||
|
|
13.3 |
38 |
||
|
|
13.4 |
38 |
||
|
|
13.5 |
39 |
||
|
|
13.6 |
39 |
||
|
|
13.7 |
40 |
||
|
|
13.8 |
40 |
||
|
|
13.9 |
41 |
||
|
|
13.10 |
41 |
||
|
|
13.11 |
41 |
||
|
|
13.12 |
41 |
||
|
|
13.13 |
41 |
||
|
|
13.14 |
42 |
||
|
|
13.15 |
43 |
||
|
|
13.16 |
43 |
|
|
|
|
|
|
|
|
|
1 |
|
|||
|
|
|
|
|
|
|
|
3 |
|
||
|
|
|
|
|
|
|
1 |
|
|||
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
1 |
|
|
|
|
2 |
|
||
|
|
|
|
|
|
|
1 |
|
|||
|
|
|
|
|
|
|
|
0 |
|
||
|
|
1 |
|
||
|
|
2 |
|
||
|
|
|
|
|
|
|
1 |
|
|||
|
|
1 |
|
||
|
|
2 |
|
||
|
|
|
|
||
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 3 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
With effect from the date stated at the start of this Agreement (the “Effective Date”), the Parties have agreed to the following terms:
This Agreement establishes the general terms and conditions under which Patheon, or any Affiliate of Patheon that is agreeable to Client, may perform Manufacturing Services for Client or any Affiliate of Client. This master form of agreement is intended to allow the Parties, or any of their Affiliates, to contract for the manufacture of one or more Products through Patheon’s global network of manufacturing sites by entering into specific Product Agreements without having to re-negotiate the general terms and conditions that apply.
This Agreement is structured so that Product Agreements may be entered into by the parties (or their Affiliates) for the manufacture of one or more Products at any Patheon manufacturing site agreeable to Client. Each Product Agreement will be governed by and will incorporate by reference the terms and conditions of this Agreement, and in the case of any conflict between the terms of a Product Agreement and this Agreement, the terms of this Agreement shall control, except to the extent that the applicable Product Agreement specifically and expressly provides to the contrary, as to the specific conflicting terms in that Product Agreement that are to control. Unless otherwise agreed by the Parties, each Product Agreement will be substantially in the general form, and contain the information referred to, in Appendix 1.
The following capitalized terms will have the respective meanings set out below, and grammatical variations of these terms will have corresponding meanings:
"Affiliate" means, with respect to a particular Party, a business entity that, directly or indirectly, controls, is controlled by, or is under common control with such Party;
For this definition, the term "control" means (with correlative meanings for the terms “controlled by” and “under common control with”) that the applicable entity has the lawful right and actual ability to determine (by ownership of shares or otherwise) the election of the majority of directors (or equivalent managers) of the applicable Party, or otherwise has the actual ability (by contract or otherwise) to control and direct the business affairs of such Party;
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 4 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
"Annual Volume" means, for the purpose of the Price for a particular Product, Patheon’s assumed minimum volume of Product to be manufactured in any Year as set out in the “Annual Volume Forecast” section of Schedule A of the applicable Product Agreement for such Product;
“API” means the active pharmaceutical material as listed in the applicable Product Agreement (references to “Active Materials” or “Active Pharmaceutical Ingredient” in documents forming part of this Agreement or of a Product Agreement will mean “API”);
"API Credit Value" means the value of the API for certain purposes of this Agreement, as set out in the applicable Product Agreement;
"Applicable Laws" means: (i) for Patheon, the Laws of the jurisdiction where the Manufacturing Site is located; and (ii) for Client and the Products, the Laws of all jurisdictions where the Products are manufactured, distributed, and marketed as these are agreed by the parties in the applicable Product Agreement;
"Authority" means any governmental or regulatory authority, department, body or agency or any court, tribunal, bureau, commission or other similar body, whether federal, state, provincial, county or municipal, with competent jurisdiction over a Party, the Manufacturing Services, or the relevant Product (or its use);
"Business Day" means a day other than a Saturday, Sunday or a day that is a statutory holiday in Patheon’s resident jurisdiction, Client’s resident jurisdiction, or the jurisdiction where the applicable Manufacturing Site is located;
“Capital Equipment Agreement” means the separate agreement that the Parties may enter into that addresses the rights and responsibilities of the Parties regarding capital equipment and facility modifications that may be required to perform the Manufacturing Services under a particular Product Agreement;
"Certificate of Analysis" means, with respect to Product ordered Client and delivered by Patheon hereunder, a document signed by an authorized employee of the Patheon Quality organization stating and confirming that the Product to which such document refers has been manufactured in accordance with the Processing Instructions, and cGMPs.
"cGMPs" means, as applicable, current good manufacturing practices as described in:
|
|
(a) |
Parts 210 and 211 of Title 21 of the United States' Code of Federal Regulations; |
|
|
(b) |
Commission Directive (EU) 2017/1572 (art. 2); and |
|
|
(c) |
Division 2 of Part C of the Food and Drug Regulations (Canada); |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 5 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
together with current final industry-accepted Health Canada, FDA and EMA guidance documents pertaining to manufacturing and quality control practice, all as updated, amended and revised from time to time;
“Client Intellectual Property” means Intellectual Property generated, made, discovered, created, in-licensed or derived (b) by Client at any time prior to or during the term of this Agreement, or (b) by Patheon while performing any Manufacturing Services, which Intellectual Property is specific to, or dependent upon, a Product or the manufacture or use thereof;
“Client-Supplied Components” means those Components supplied or to be supplied to Patheon by or on behalf of Client as identified in Schedule A of a Product Agreement, for use by Patheon for performing the Manufacturing Services under such Product Agreement;
"Components" means, collectively, all packaging components, raw materials, ingredients, and other materials (including labels, product inserts and other labelling for the Products) required to manufacture the Product under a particular Product Agreement in accordance with the applicable Processing Instructions, other than the API;
“Confidential Information” has the meaning specified in Section 11.1;
“DEA” means the Drug Enforcement Administration of the United States Department of Justice;
“Deficient Product” has the meaning specified in Section 6.1(b)(i);
“Disclosing Party” has the meaning specified in Section 11.1;
"EMA" means the European Medicines Agency;
"FDA" means the United States Food and Drug Administration;
"Firm Order" has the meaning specified in Section 5.1(d);
"Health Canada" means the department of the Canadian Government known as Health Canada and includes, among other relevant branches, the Therapeutic Products Directorate and the Health Products and Food Branch Inspectorate;
“Initial Product Term” has the meaning specified in Section 8.1;
"Intellectual Property" means all legal rights in intellectual property, including all rights granted by or inherent in patents, patent applications, trademarks, trademark applications, trade-names, copyrights, industrial designs, trade secrets, and proprietary and confidential Inventions;
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 6 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
"Invention" means any innovation, improvement, development, discovery, computer program, device, trade secret, know-how, method, process, technique or the like, whether or not written or otherwise fixed in any form or medium, regardless of the media on which it is contained and whether or not patentable or copyrightable, and all information comprising any of the foregoing;
"Inventory" means all inventories of Components and work-in-process produced or held by or on behalf of Patheon (or its applicable Affiliate) for the manufacture of the applicable Product;
“Knowledge” means, with respect to a particular statement herein, that to the actual knowledge of the executive officers of Client or Patheon, as applicable, such officers are not aware of any facts or information that make such statement materially untrue.
"Laws" means all laws, statutes, ordinances, regulations, rules, by-laws, judgments, decrees or orders of any Authority;
“Local Currency” has the meaning specified in Appendix 4;
“Long Term Forecast” has the meaning specified in Section 5.1(a);
"Manufacturing Services" means, as to a particular Product, the manufacturing, quality control, quality assurance, stability testing, packaging, and related services for the manufacture of such Product, as set out in the relevant Product Agreement, for the manufacture and supply to Client of such Product;
"Manufacturing Site" means the facility identified in a Product Agreement where the Manufacturing Services for the applicable Product will be performed;
"Minimum Order Quantity" means, for each manufacturing campaign ordered by Client under a particular Product Agreement, the minimum number of units or batches of the relevant Product that Client must purchase under such campaign, as set out in Schedule A of such Product Agreement;
“Obsolete Stock” has the meaning specified in Section 5.2(b);
“Patheon Competitor” means a business entity that derives greater than 50% of its revenues from performing contract pharmaceutical or biopharmaceutical manufacturing services for clinical development or commercial use;
“Patheon Intellectual Property” means Intellectual Property generated, made, discovered, created, in-licensed or derived by Patheon or its Affiliates before performing any Manufacturing Services, developed by Patheon while performing the Manufacturing Services, or otherwise generated or derived by Patheon in its business which Intellectual Property is not specific to, or dependent upon, the Product or its manufacture or use, which may include Inventions and Intellectual Property that apply generally to manufacturing processes or the formulation or development of drug products or drug delivery systems that are unrelated to the specific requirements or manufacture of a Product;
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 7 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
“Price” means, with respect to a particular Product, the fees to be charged by Patheon for:
|
|
(a) |
performing the Manufacturing Services for such Product; |
|
|
(b) |
the cost of the applicable Components (other than Client-Supplied Components); and |
|
|
(c) |
any separate cost items and other fees applicable to the manufacture and supply of the Product under the applicable Product Agreement; |
all as set out in Schedule A of the applicable Product Agreement;
"Processing Instructions" means, for a particular Product, the set of documents relating to such Product as agreed by the Parties in writing to be the “processing instructions” for the manufacture and supply of such Product under the applicable Product Agreement, which shall include:
|
|
(a) |
quality control testing methods for the API and Components for such Product; |
|
|
(b) |
all manufacturing instructions, directions, standard operating procedures and processes for the manufacture, quality control and assurance, and packaging of such Product; |
|
|
(c) |
any storage requirements for such API and Components; |
|
|
(d) |
all environmental, health and safety information for the Product including material safety data sheets; and |
|
|
(e) |
the finished Product quality control testing methods, packaging instructions and shipping requirements for the Product, and the form of Certificate of Analysis for the Product; |
"Product" means the pharmaceutical product listed in Schedule A of a particular Product Agreement;
“Product Warranty” has the meaning set forth in Section 9.4.
“Product Agreement” means an agreement entered into between Patheon and Client (or their applicable Affiliates) substantially in the form set out in Appendix 1, under which Patheon will perform Manufacturing Services for a particular Product;
“Product Claims” has the meaning specified in Section 6.1(b)(i);
"Quality Agreement" means a separate agreement between the Parties that sets out the quality assurance standards and related obligations applicable to the performance of Manufacturing Services;
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 8 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
“Recall” has the meaning specified in Section 6.2(a);
“Recipient” has the meaning specified in Section 11.1;
“Regulatory Approval” has the meaning specified in Section 7.5(a);
"Regulatory Authority" means the FDA, EMA, and Health Canada and any other foreign regulatory agencies with the legal authority to grant marketing approvals for pharmaceutical or biopharmaceutical products, including the Products, in the applicable country or jurisdiction in the Territory;
“Release Date” means, in relation to a particular batch of Product manufactured under a particular Product Agreement, the scheduled date by which the Product will be released by Patheon’s quality department (by confirmation or certification) as agreed in the Quality Agreement and made available for shipment, and as confirmed by Patheon in a Firm Order;
“Representatives” means, as to a Party, all the directors, officers, employees, legal advisers, agents, consultants, subcontractors (excluding the other Party and its Affiliates), service partners, professional advisors, or legal representatives of such Party or its Affiliate;
“Rolling Forecast” has the meaning specified in Section 5.1(b);
"Technical Dispute" has the meaning specified in Appendix 2;
"Territory" means, as to a particular Product, the geographic area described in the relevant Product Agreement where such Product manufactured by Patheon will be distributed by or on behalf of Client or its Affiliate or licensees or distributors;
"Third Party Rights" means the Intellectual Property of any third party;
"VAT" has the meaning specified in Section 13.14; and
"Year" means, with respect to this Agreement or a particular Product Agreement: (a) in the first year of this Agreement or such Product Agreement, the time from the effective date of the applicable agreement up to and including December 31 of the same calendar year, and (b) after that will mean a calendar year.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 9 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
The division of this Agreement into Sections, Subsections, Appendices and Schedules, and the insertion of headings, are for convenience of reference only and will not affect the interpretation of this Agreement. Unless otherwise indicated, any reference in this Agreement to a Section, Appendix or Schedule refers to the specified Section, Appendix or Schedule to this Agreement. In this Agreement, the term "this Agreement" and similar expressions refer to this Agreement as a whole and not to any particular part, Section, Appendix or Schedule of this Agreement. Except as otherwise expressly stated or unless the context otherwise requires, all references to the singular will include the plural and vice versa. The term “including” or “includes” shall not be interpreted to limit the scope or breadth of the provisions preceding such term and shall be deemed to mean “including without limitation”.
Patheon will perform the Manufacturing Services as set out in the relevant Product Agreement for the Price and in accordance with the terms and conditions of this Agreement, such Product Agreement and the Quality Agreement. Subject to the preceding sentence, Patheon will convert API and Components into Product, and provide supportive Manufacturing Services such as quality assurance (for example quality controls, analytical testing, and stability programs), primary and secondary packaging, and any other related Manufacturing Services as agreed between the parties under the relevant Product Agreement. Patheon (and its applicable Affiliates) will perform all Manufacturing Services in accordance with all Applicable Laws.
For each Product for which the Parties (or their respective Affiliates) have entered into a Product Agreement, and unless otherwise expressly agreed in a Product Agreement, and except as otherwise provided in this Agreement, Patheon will be a non-exclusive manufacturer during the term of the applicable Product Agreement of the Products offered for sale by Client or its Affiliates, provided that, Client agrees to order and purchase from Patheon, and Patheon agrees to manufacture and supply hereunder, [***] of Client’s requirements for Products for commercial sale in the United States (as provided in Client’s rolling forecasts); provided further that, for clarity, the foregoing does not apply to manufacture of any API or Components.
Except as otherwise provided in the Product Agreement covering manufacture of particular Product for Client, for each Product that is covered by a Product Agreement during the term of this Agreement, Patheon shall maintain, commencing on the date that is twelve months after the launch of commercial sales of such Product in the Territory for such Product (or at such later date as agreed in writing, as to a particular Product), at least two separate and distinct manufacturing sites that are fully qualified and able to perform the Manufacturing Services set forth herein (“Manufacturing Redundancy”). The details of the allocation of the manufacturing services between such sites, for a Product, shall be as agreed reasonably by the Parties at the time of qualification. In the event Patheon fails to maintain, for a consecutive period of [***] in any twelve month period, the required Manufacturing Redundancy for a
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 10 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
particular Product covered by a Product Agreement, then Client shall no longer be required to use Patheon as the manufacturer of at least [***] of Client’s requirements for such Product under the relevant Product Agreement.
Patheon may subcontract the Manufacturing Services under a particular Product Agreement to one or more of its Affiliates, as agreed in writing in advance by Client (such agreement not to be unreasonably withheld) pursuant to the applicable Product Agreement. In the case of such subcontracting, such Affiliate shall be deemed to be bound by Patheon’s obligations under this Agreement, the Quality Agreement and the applicable Product Agreement, Patheon will remain liable to Client for any breach of this Agreement, the Quality Agreement or such Product Agreement by, or the negligence or recklessness of, by its Affiliates in the course of performing: (i) subcontracted Manufacturing Services under a Product Agreement; or (ii) obligations under the Quality Agreement. Patheon may also arrange for non-Affiliate subcontractors to perform specific services arising under any Product Agreement with the consent of Client (“Third Party Subcontractors”). Patheon also may subcontract to a Third Party Subcontractor certain Manufacturing Services under a particular Product Agreement, as agreed in writing in advance by Client pursuant to the applicable Product Agreement. Patheon will be liable to Client for any failure by any Third Party Subcontractor to perform any part of the subcontracted services in compliance with the obligations under this Agreement, the Quality Agreement, or the relevant Product Agreement, or the breach, negligence or recklessness of the Third Party Subcontractor in performing such services. But Patheon’s liability for Third Party Subcontractors will remain subject to all limitations on Patheon’s liability as set out in this Agreement. Patheon will have no liability arising from the performance of services by Third Party Subcontractors: (i) that are initially chosen by Client and required by Client to be used by Patheon; or (ii) that are suppliers or service providers not validated and utilized by Patheon prior to the date of this Agreement.
In consideration for Manufacturing Services actually performed and Product supplied to Client in accordance with the terms of this Agreement and the applicable Product Agreement, Client will pay Patheon the applicable Price in accordance with Sections 4 and 5. All cost items that are not included in the Price (as and to the extent specified in the applicable Product Agreement) are subject to additional fees to be paid by Client as provided in such Product Agreement.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 11 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Before the start of commercial manufacturing of Product under this Agreement and the applicable Product Agreement, Client will provide to Patheon a copy of the Processing Instructions for such Product manufacturing, which must be accompanied by the applicable specifications for the API, the applicable Components and the finished Product (if applicable, precisely matching the applicable specifications approved by the applicable Regulatory Authority). If the Processing Instructions or accompanying documents provided by Client are subsequently amended by or on behalf of Client, or no longer reflect those currently approved by the Regulatory Authority, then Client will give Patheon a copy of the revised Processing Instructions documents (if applicable, precisely matching the revised specifications approved by the applicable Regulatory Authority). All such Processing Instructions shall be deemed accepted by Patheon on the date received, unless Patheon provides to Client, within [***] of such receipt, with reasonable written objections (a “PI Objection”) to particular document(s) or instructions in such Processing Instructions and provides in such PI Objection the detailed reasons for why such document(s) or instructions are improper and not acceptable to Patheon. If, as to particular Processing Instructions provided by Client, Patheon timely provides a PI Objection to such Processing Instructions, then the Parties shall meet as soon as practicable thereafter and work reasonably and in good faith to agree as soon as practicable on mutually agreeable modifications to such Processing Instructions (which must comply with all Applicable Laws). If the Parties cannot agree on such modifications, such that the Processing Instructions (as modified) are mutually agreed and acceptable to the Parties, within [***] of the date such PI Objection is received, then such issue shall be a “Dispute” under the provisions of Appendix 2, and the Parties will proceed under the “Negotiations” paragraph of Appendix 2. If such Dispute is not resolved by the Senior Officers under the terms of such paragraph, then: (a) if Client reasonably believes that the Processing Instructions that are the subject of the PI Objection in such Dispute are reasonably required (either by the Applicable Laws or regulations in the applicable Territory, or due to other legitimate, material business concerns), then Client may terminate the applicable Product Agreement on [***] prior written notice (in which case section 8.3 shall apply), unless agreement can be reached in such 90 day period; or (b) as to all other such Disputes, the Parties shall proceed to seek to resolve such dispute pursuant to the other terms of Appendix 2. Upon acceptance (or deemed acceptance) of Processing Instructions (including all accompanying documents), as such Processing Instructions may be modified by the Parties (if applicable) according to the foregoing, Patheon will give Client a signed and dated receipt indicating Patheon’s acceptance of such Processing Instructions. At Patheon’s reasonable request, Client will provide evidence of the executed original documents comprising the Processing Instructions submitted by or on behalf of Client to the applicable Regulatory Authority.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 12 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
and Client-Supplied Components to the extent imported to the Manufacturing Site, and Client is responsible for compliance with Applicable Laws (and the cost of compliance) relating to that role as the importer. For API or Client-Supplied Components which may be subject to import or export to or from the United States, Client agrees that its vendors and carriers will comply with applicable requirements of the U.S. Customs and Border Protection Service and the Customs Trade Partnership Against Terrorism. |
|
|
(b) |
Unless otherwise agreed in writing between the Parties, the API and any Client-Supplied Components shall be delivered by the Client to the Manufacturing Site at least [***] before the scheduled manufacture start date for Product covered by a Firm Order in sufficient quantity to enable Patheon to manufacture the agreed quantities of Product, and if such delivery is not timely made, then Patheon is not obligated to initiate such manufacture campaign (but will use reasonable efforts to do so, if the applicable API and any Client-Supplied Components are delivered by Client at time that allows Patheon to commence such manufacturing at the scheduled date) until a revised date, such date to be agreed by the parties, both acting reasonably and in good faith, such date to be as soon as practicable after such delivery by Client, taking into account Patheon’s other business commitments. Patheon reserves the right to refuse to store any quantity of API materially in excess of the amount necessary for the particular Manufacturing Services covered by a Firm Order, at its sole discretion at any time. If Client does not deliver the API or Client-Supplied Components within the agreed time period and, making commercially reasonable efforts, Patheon is unable to manufacture Product on the scheduled date because of the delay in such delivery, the delivery date for the Products under such Firm Order will be considered a postponement of the Release Date by Client to a new Release Date based on the revised manufacturing initiation date agreed to by the Parties as referred to above, and Section 5.1(e) will apply. Patheon will reschedule manufacture under a replacement purchase order at Client’s request subject to Patheon’s manufacturing slot availability at the time of the request. |
|
|
(c) |
Patheon will control the unloading of API and Client-Supplied Components arriving at the Manufacturing Site, and Client will comply and ensure that its carrier complies with all related reasonable unloading directions of Patheon. The API and Client-Supplied Components will be held by Patheon on behalf of Client as set out in this Agreement. The API and Client-Supplied Components will at all times remain the property of Client. Any API and Client-Supplied Components received by Patheon will only be used by Patheon to perform the applicable Manufacturing Services. |
|
|
(d) |
Client will ensure that: (i) all delivered API meets the specifications for that API as set forth in the applicable Processing Instructions; and (ii) all shipments of API to Patheon are accompanied by the required documentation as specified in the applicable Quality Agreement. |
|
|
(e) |
If Client asks Patheon to qualify an additional supplier for the API or any Component, the Parties must agree on the scope of work to be performed by Patheon and the additional fees to be paid by Client for such work performed. For any such API or Component, this work at a minimum will include: (i) laboratory testing to confirm the API or Component meets existing |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 13 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
specifications therefor as set forth in the applicable Processing Instructions; (ii) manufacture of an experimental batch of Product using such API or Component that will be placed on three months accelerated stability; and (iii) manufacture of full-scale validation batches that will be placed on concurrent stability (one batch may be the registration batch if manufactured at full scale). |
|
|
(f) |
Patheon will promptly advise Client if it encounters API or Component supply problems, including delays and/or delivery of non-conforming API or Components from a Client designated additional supplier. In such event, the Parties will cooperate to reduce or eliminate any supply problems from these additional suppliers. If supply problems persist, Patheon may suspend the Manufacturing Services affected by the problems until it is satisfied that the Client has resolved the problems with its supplier or appointed an alternative supplier. Client will certify (according to typical industry standards) all Client designated additional suppliers on an annual basis at its expense and will provide Patheon with copies of these annual certifications. If Patheon agrees to certify a Client designated additional supplier on behalf of Client, it will do so for an additional fee agreed to and payable by Client. |
Client will be responsible for the cost of artwork development and approval of all artwork. Client will be responsible for changes to labels, product inserts, and other packaging for the Products, including obtaining all required approvals. Client will be responsible for the cost of labelling obsolescence as contemplated in Section 5.2. Patheon's name will not appear on the label or anywhere else on the Products unless: (i) required by any Applicable Laws; or (ii) Patheon consents in writing to the use of its name. At least [***] prior to the Release Date of Product for which new or modified artwork is required, Client will provide at no cost to Patheon and in accordance with the applicable specifications, final camera ready artwork for all packaging Components to be used in the manufacture of the Product. Client will be responsible for all costs associated with complying with any and all regulatory requirements for the labelling and tracking of the manufactured Product, including product serialisation, product data transfer and anti-counterfeiting requirements in the Territory.
The Price for each particular Product will be listed in Schedule A of the applicable Product Agreement, and a Price may be adjusted under this Section 4.
[***]
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 14 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
The Prices may be adjusted by Patheon at any time upon written notice to Client as follows:
|
|
(a) |
Extraordinary Increases in Component Costs. If the cost of a Component increases cumulatively by at least [***] as a result of market factors, then Patheon will be entitled to adjust the Price proportionately (to account solely for that actual increase in price of the Component) and as otherwise agreed in the Product Agreement. The revised Price will become effective with the first use of the higher cost Component in the manufacture of the Product. For a Price adjustment under this Section 4.3(a), Patheon will deliver to Client a revised Schedule A to the Product Agreement. In the event Patheon invokes the increase contemplated in this Section 4.3(a), Patheon shall review such Component costs every six months and in the event the price of such Component decreases by [***], Patheon shall reduce the price proportionately (to account solely for the actual decrease in price of the Component). In any case, Patheon shall provide Client the basis for the Price adjustment, which shall include the current and increased costs and inventory of existing Component available. |
|
|
(b) |
Changes. The scope of the Manufacturing Services is set by the agreed Processing Instructions, the Regulatory Approvals, the Quality Agreement and any assumptions, inclusions, exclusions and other parameters set out in the applicable Product Agreement. Changes to the scope of the Manufacturing Services and related changes to the Price must be agreed in writing by the Parties (using a “Change of Scope” agreement, or similar, setting out the agreed activities and costs of implementation) and are subject to the change control provisions of the Quality Agreement. Where Patheon requests a change to the Manufacturing Services, the change will be implemented following appropriate regulatory approvals and upon written approval of Client, which Client will not unreasonably withhold, condition or delay. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 15 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(c) |
Orders. On or before the tenth day of each month, Client will issue a new purchase order for the amount of Product required by Client. Each purchase order must meet the Minimum Order Quantity for the applicable month and specify the purchase order number, quantities by Product type, and requested release dates for the Product (which must occur at least [***] after the first day of the next month; each a “Release Date”). |
|
|
(i) |
for the first Year, by reference to the first Rolling Forecast; |
|
|
(ii) |
for the second Year: (1) if the Effective Date of the Product Agreement occurs after July 1, by reference to the first Rolling Forecast; and (2) otherwise, by reference to the Rolling Forecast applicable at July 1 of the previous Year; |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 16 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(iii) |
for each subsequent Year, by reference to the Rolling Forecast applicable at July 1 of the previous Year, |
with the applicable Rolling Forecast (as determined above) for a particular Year being the “Yearly Forecast Volume” for such Product for that Year.
At the end of each Year, if the aggregate actual volume of Product ordered by Client with a confirmed Release Date within the Year, taking into account any Product paid for but not ordered, (“Actual Yearly Volume”) is less than [***], then Patheon may invoice and Client will pay Patheon [***].
If the quantity of Product requested by Client in a Year (in purchase orders received by Patheon) exceeds [***] of the Yearly Forecast Volume for that Year, Patheon will use commercially reasonable efforts to supply the additional Product volumes (i.e., in excess of such [***] limitation) ordered; however, Patheon will not be considered to have accepted any purchase order to the extent such purchase order is for additional Product volumes above such [***] of the Yearly Forecast Volume for such Product without written confirmation of such acceptance.
|
|
(h) |
Controlled Substance Quota Requirements (if applicable). Client will give Patheon the information set out below for obtaining any required DEA or equivalent agency quotas (“Quota”) needed to perform the Manufacturing Services. Patheon will be responsible for routine management of Quota information in accordance with Applicable Laws. The Parties |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 17 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
will cooperate to communicate the information and to assist each other in Regulatory Authority information requirements related to the Product as follows: (i) by April 1 of each Year for the applicable Product, Client will provide to Patheon the next Year’s annual Quota requirements for the Product; (ii) by August 1 of each Year, Client will provide to Patheon any changes to the next Year’s Quota requirements; (iii) Client will pro-actively communicate any changes to the Quota requirements for the then-current Year in sufficient time to allow Patheon to file and finalize Regulatory Authority filings supporting the changes; (iv) upon Patheon receiving the necessary forecast information from Client in order to request additional Quota, Patheon will submit to the applicable Regulatory Authority, on a timely basis, all filings necessary to obtain Quotas for API and will use commercially reasonable efforts to secure sufficient Quota from the applicable Regulatory Authority so as to achieve Release Dates for Product as set out in applicable purchase orders and forecasts submitted to Patheon by Client or its designee; and (v) Patheon will not be responsible for any Regulatory Authority’s refusal or failure to grant sufficient Quota for reasons beyond the reasonable control of Patheon (including where Client fails to provide the required information in accordance with this Section 5.1(h)). |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 18 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
If: (i) Client does not take possession or arrange for the destruction of Obsolete Stock within [***] of receipt of written notice from Patheon identifying the Obsolete Stock; (ii) any equipment (other than existing Patheon equipment) is stored at the Manufacturing Site at any time prior to its use in the Manufacturing Services; or (iii) Product is not collected by Client within [***] of the Release Date notified by Patheon, Client will pay Patheon [***] after that for storing such Obsolete Stock, unused equipment or Product. Storage fees for Obsolete Stock or Product which contain controlled substances or require refrigeration will be charged at [***]. Storage fees are subject to a one pallet minimum charge per month. Patheon may ship Product held by it longer than [***] to Client at Client’s expense on [***] written notice to Client. If Patheon is unable to store any material due to capacity constraints, Patheon may use an Affiliate or third party storage provider to store (outside the Manufacturing Site) any material under this Agreement. After the limited storage periods stated above, Client will assume all risk of loss or damage to materials and Client will be responsible for having appropriate insurance coverage in place for this risk.
For shipments to Client of Product ordered by Client hereunder, Patheon will issue invoices to Client on or after the Release Date of the Product. Otherwise, Patheon will issue invoices for Manufacturing Services on completion or as agreed in the applicable Product Agreement. Patheon will also submit to Client, with each shipment of Products, a duplicate copy of the invoice covering the shipment. Invoices will be sent by email to the email address given by Client to Patheon in writing. Each invoice will, to the extent applicable, identify Client’s Manufacturing Services purchase order number, Product numbers, names and quantities, unit price, freight charges, and the total amount to be paid by Client. Client will pay the undisputed amounts in all invoices within [***] of the date of the invoice. If any portion of an invoice is disputed, Client will pay Patheon for the undisputed amount, and the Parties will use good faith efforts to reconcile the disputed amount as soon as practicable. Interest on undisputed past due accounts will accrue at 1.0% per month (or such lesser amount as required by Laws). If Client is more than [***] late in paying undisputed amounts on an invoice, and such past due amount is material, Patheon may, on giving [***] notice to Client, suspend all Manufacturing Services until all such undisputed past due invoices have been paid in full. Patheon will have no liability to Client if this suspension results in delayed performance of any Manufacturing Services or cancellation or rescheduling of any manufacturing slots.
Delivery of Products ordered by Client hereunder will be made EXW (Incoterms 2010) from Patheon’s Manufacturing Site for such Product, unless otherwise agreed in a Product Agreement. Subject to Section 8.3, risk of loss or of damage to Products will remain with Patheon until Patheon loads the Products onto the carrier’s vehicle for shipment at the shipping point, at which time risk of loss or damage will transfer to Client. Each delivery of Product shall be accompanied by a signed Certificate of Analysis for the batch of Product delivered, as well as any other documents required by the relevant Product Agreement and/or Quality Agreement (“Delivery Documents”). Client will notify Patheon in writing within [***] of receipt of such Delivery Documents if Client has identified a compliance issue that
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 19 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
will prevent shipment. Subject to Client’s acceptance of the Delivery Documents as set forth above, if Client does not collect, or arrange for the collection, of Product within one month after Client has received written notice from Patheon that it has been released for shipment by Patheon, Client will assume all risk of loss or damage to the released Product, and Patheon may, in accordance with Client’s instructions and as agent for Client, at Client’s risk, arrange for shipping (to Client or any third party nominated by Client) of such Product, to be paid by Client. Client will arrange for insurance and will select the freight carrier used by Patheon to ship Products and may monitor Patheon’s shipping and freight activity under this Agreement.
5.6Sale. Client agrees that it will not sell (itself or through a third party) Product supplied by Patheon hereunder in any market where Client (or its Affiliate or sublicensee or third party seller) does not have the permits or regulatory approvals (or registrations) required to sell such Product in such market.
|
|
(b) |
Product Claims. |
|
|
(i) |
Client may claim a remedy (a “Product Claim”) for any portion of any batch of Product that does not comply with the Product Warranty or for which Patheon did not perform the Manufacturing Services in accordance with the then-current Processing Instructions, cGMPs, or Applicable Laws (“Deficient Product”). Client will inspect Product manufactured by Patheon, or batch documentation provided by Patheon, promptly after receipt and will give Patheon written notice of all Product Claims within [***] after receipt or, in the case of any defects not reasonably susceptible to discovery upon receipt by such inspection process, within [***] after discovery by Client, but not after the expiration date of the Product. If Client does not provide a Product Claim within the applicable 30 day period, then the Product will be considered to have been accepted by Client on the 31st day of such period. Except as set forth in Section 10.3, Patheon will have no liability for any deficiency for which Client has not sent such notice within the applicable 30 day period. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 20 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
Manufacturing Services in accordance with the agreed Processing Instructions, cGMPs or Applicable Laws, or (2) not due to manufacturing defects that result from Patheon’s (or its Affiliate’s) negligence or other fault; (iii) due to a defect in the API or an incorporated Component that was not reasonably discoverable by Patheon using the test methods set out in the Processing Instructions; (iv) by actions of Client or third parties occurring after the Product is delivered by Patheon; (v) resulting from packaging design or labelling defects or omissions for which Patheon has no responsibility; (vi) by an unascertainable reason in the case where Patheon performed the Manufacturing Services for the Product in accordance with the Processing Instructions, cGMPs, and Applicable Laws; or (vii) due to any breach by Client of its obligations under this Agreement. If after a full investigation as set out in the Quality Agreement and this Section 6.1(b)(ii), it is determined that particular Product, which is believed by Client to be Deficient Product, was manufactured by Patheon in accordance with the then-current Processing Instructions, cGMPs, and Applicable Law, and, if it was delivered, such Product complied with the Product Warranty at the time of delivery by Patheon to Client (or its carrier) hereunder, then Client will pay Patheon the Price for such Product Patheon’s only liability for API loss or damage is set out in Appendix 3. |
|
|
(c) |
Determination of Deficiency. Upon receipt of a Product Claim, Patheon will have [***] to advise Client by notice in writing whether it disagrees with the contents of the Product Claim. If the Parties do not agree within [***] after Patheon's notice to Client as to whether any Product identified in the Product Claim was Deficient Product, the Parties will investigate the matter in accordance with the Quality Agreement. If, after joint testing or investigation has been performed, the Parties still cannot agree on whether the Product is Deficient Product, then the provisions of Appendix 2 will apply and, after the required negotiation, the dispute will be handled as a Technical Dispute. |
|
|
(d) |
Shortages and Price Disputes. Claims for shortages in the amount of Product shipped by Patheon or a Price dispute will be dealt with by reasonable agreement of the Parties. Any claim for a shortage or a Price dispute will be considered waived by Client if it has not been presented within [***] of the date of the relevant invoice. |
|
|
(a) |
Records and Notice. Each of the Parties will maintain records necessary to permit a Recall of any Product delivered to Client or customers of Client. Each Party will promptly notify the other of any information which might affect the marketability, safety or effectiveness of the Product or which might result in the Recall or seizure of the Product in accordance with the Quality Agreement. Upon receiving this notice or upon this discovery, the Parties will discuss in good faith the appropriate steps and whether it is appropriate to stop making any further shipments of such Product in its possession or control, until a decision has been made by Client whether a Recall or some other corrective action is necessary. The decision to initiate a Recall or to take some other corrective action, if any, will be made and implemented solely by Client. "Recall" will mean any action: (i) by (or on behalf of) Client or |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 21 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
its Affiliate or licensee to recover title to or possession of quantities of the Product sold or shipped to third parties (including, without limitation, the voluntary withdrawal of Product from the market); or (ii) by any Regulatory Authority to detain or destroy any of the Product. |
Client will not dispose of any damaged, defective, returned, or Deficient Products for which it intends to assert a Product Claim against Patheon without Patheon’s prior written authorization to do so, such authorization not to be unreasonably withheld. Patheon may instruct Client to return the Deficient Products to Patheon. Patheon will bear all costs of return, disposition and/or destruction of any Deficient Products that Patheon agrees are Deficient Product, or that is determined (as above) to be Deficient Product. In all other circumstances, Client will bear the cost of return and disposition, including all applicable fees for Manufacturing Services related to such Deficient Product. Patheon will pay for Client’s reasonable and actual third party storage fees (as documented) of all agreed (or determined to be) Deficient Products until such time as Patheon instructs Client to return or dispose of the Deficient Product.
Each Party will promptly after execution of this Agreement appoint one of its employees to be a relationship manager responsible for liaison between the Parties for the day to day interactions under this Agreement and the Product Agreements. The relationship managers will meet (which may be by telephone or video conference) on a frequency agreed between the Parties to review the current status of the business relationship and manage any issues that have arisen.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 22 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Subject to any restrictions in the Quality Agreement, and expect as otherwise provided below in this Section 7.2, Client shall have the sole right to communicate with any Regulatory Authority responsible for granting Regulatory Approval for the Products and any other relevant Authority regarding the Products. If, in the opinion of Patheon's counsel, Patheon must make a communication to such a Regulatory Authority regarding the manufacture of Product because such communication is necessary to comply with the terms of this Agreement or the requirements of the Authority or Applicable Laws, then Patheon must, subject to the timing requirements for such communications set out in Applicable Laws (which will take precedence), at least [***] prior to making any such communication, give notice to Client of such necessary communication and the reasons it is believed to be necessary, and in such case the Parties will consult with each other immediately in relation to such proposed regulatory communications relating to the Product, and Patheon shall not make such communication except with Client’s prior written approval, not to be unreasonably withheld. The Parties also will cooperate with each other regarding communications with Regulatory Authorities in accordance with the Quality Agreement.
Patheon will keep complete and accurate records of the manufacture, testing, and shipping of the Products, and retain samples of the Products as are necessary to comply with manufacturing regulatory requirements applicable to Patheon, Applicable Laws, cGMP and the Quality Agreement. Copies of the records and samples will be retained as and for the period specified in the Quality Agreement. Patheon reserves the right to destroy or return to Client, at Client’s sole expense, any document or samples for which the retention period has expired if Client fails to arrange for destruction or return within 30 days of receipt of notice from Patheon.
|
|
(a) |
Subject to the limits agreed in the Quality Agreement, Patheon will give Client reasonable access at agreed times to the areas of the Manufacturing Site in which the Products are manufactured, stored, handled, or shipped, all as needed to permit Client to verify that the Manufacturing Services are being performed in accordance with the Process Instructions, the terms of this Agreement and the applicable Product Agreement, cGMPs, and Applicable Laws. If Client wishes to audit Patheon beyond the agreed limits, except where the audit is required due to Patheon’s material breach, Client will pay to Patheon a fee of [***]. Under no circumstances will: (a) Client have a right of access to Patheon’s financial records; or (b) any Patheon Competitor be permitted access to the Manufacturing Site. |
|
|
(b) |
Patheon shall notify Client within the period specified in the Quality Agreement after it receives notice from a Regulatory Authority of an audit or inspection involving the Product, any component thereof, or any portion of Patheon’s facility used or likely to be used in connection with the activities of Patheon to be conducted under this Agreement and, to the extent reasonably practicable, shall allow Client to be present at the Manufacturing Facility to support Patheon during the audit or inspection, but not to participate. The responsibility for |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 23 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
conducting the audit or inspection rests with Patheon. In each such case, whether or not Client was present at the Manufacturing Facility such audit or inspection, Patheon shall provide to Client copies of any resulting document of action (e.g., FDA Form 483 inspection observation report, regulatory letters, etc.) resulting from such audits or inspections, which pertains to the Product, any component thereof, or any portion of Patheon’s facility used or likely to be used in connection with the activities of Patheon to be conducted under this Agreement, within the period specified in the Quality Agreement after receipt. Should either Patheon or Client receive any such document of action, it shall so notify the other within the period specified in the Quality Agreement after receipt and shall provide to the other an opportunity to the extent feasible under the circumstances, to provide input to any response to any such document of action. |
|
|
(b) |
Deficiencies. If Patheon reasonably determines that any Product regulatory information relating to manufacture of Product given by Client is inaccurate or deficient in any manner that may cause Patheon material regulatory or legal risk (the "Regulatory Deficiencies"), Patheon will notify Client in writing of the Regulatory Deficiencies and will provide all information regarding why it believes the information is inaccurate or deficient and creates risk for Patheon. In such event, the parties will work together to have the Regulatory Deficiencies resolved prior to the date of filing of the relevant regulatory approval application and in any event before any Product pre-approval inspection of the applicable Manufacturing Site or before the Product is placed on the market if a pre-approval inspection is not performed. |
|
|
(c) |
Inspection by Regulatory Authorities. If Client does not give Patheon the documents reasonably requested under this Section 7.5 or the Quality Agreement within the time specified and if Patheon reasonably believes that Patheon’s standing with a Regulatory Authority may be materially jeopardized due to such not having access to such documents, then Patheon may, in its sole discretion, delay or postpone any inspection by the Regulatory Authority of the applicable Manufacturing Site with respect to such Product until Patheon has reviewed the requested documents and is reasonably satisfied with their contents. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 24 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(d) |
Pharmacovigilance. Client will be responsible, at its expense, for all pharmacovigilance obligations for the Products in accordance with Applicable Laws and the monitoring and management of post-marketing complaints and queries at its cost (including, without limitation, the cost of assistance required of Patheon under the Quality Agreement). Unless required by Applicable Law, neither Party will be obliged to exchange with the other Party any information or data which it compiles in carrying out pharmacovigilance obligations or activities. |
|
|
(e) |
No Patheon Responsibility. Except as otherwise agreed in the Quality Agreement, Patheon will not assume any responsibility for: (a) the submission, accuracy or cost of any application for Regulatory Approval or related documentation (or the success of those applications); (b) any activity that is required by Applicable Laws for Regulatory Approval (including pharmacovigilance and complaints handling, and preparation and submission of any regular quality or other update); or (c) any dealings with the relevant Regulatory Authority on behalf of Client for Regulatory Approval. If a Regulatory Authority, or other governmental body, requires Patheon to incur fees, costs or activities in relation to the Products which Patheon considers unexpected and extraordinary, then Patheon will notify Client in writing and the parties will discuss in good faith appropriate mutually acceptable actions, including fee/cost sharing, or termination of all or any part of this Agreement or a Product Agreement. Patheon will be not be obliged to undertake these activities or to pay for the fees or costs until the Parties reach agreement on scope and fees for Patheon’s assistance, except as otherwise provided in the Quality Agreement (including required inspections by Regulatory Authorities of the Manufacturing Sites). |
The Parties agree that the release of the Products for sale or distribution under the applicable marketing approval for the Products will not by itself indicate compliance by Patheon with its obligations relating to the Manufacturing Services. Nothing in this Agreement will remove or limit the authority of the relevant quality function (as specified by the Quality Agreement) to determine whether the Products are to be released for sale or distribution.
No later than [***] following completion or permanent cessation of the Manufacturing Services at the applicable Manufacturing Site, Client will: (a) ensure that any regulatory filings relating to the Product are withdrawn or amended to remove all references to the Manufacturing Site and, as applicable, Patheon and/or its Affiliates and their facilities (except in an historic context); and (b) provide to Patheon written confirmation of its compliance with this Section 7.7. If this time is not sufficient to meet the requirements of certain Regulatory Authorities, despite Client’s commercially reasonable efforts, then Patheon may agree to extend the period based on the written reassurances of Client.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 25 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
This Agreement will become effective as of the Effective Date and will continue until December 31 of the fifth Year of the Agreement (the "Initial Term"), unless terminated earlier by one of the Parties as provided below in this Article 8. This Agreement will automatically renew after the Initial Term (and as the term may be extended pursuant to this sentence) for successive terms of two Years each if there is a Product Agreement then in effect, unless either Party gives written notice to the other Party of its intention to terminate this Agreement at least 18 months prior to the end of the then current term. In any event, the legal terms and conditions of this Agreement will continue to govern any Product Agreement in effect. Each Product Agreement will have an initial term from the Effective Date of the Product Agreement until December 31 of the Year agreed to by the parties in the Product Agreement (each, an “Initial Product Term”). Product Agreements will automatically renew after the Initial Product Term (as such term is extended hereunder) for successive terms of two Years each unless either party gives written notice to the other party of its intention to terminate the Product Agreement at least 18 months prior to the end of the then current term.
|
|
(b) |
A Party may immediately terminate this Agreement or a Product Agreement upon written notice to the other Party if: (i) the other Party is declared insolvent or bankrupt by a court of competent jurisdiction; (ii) a voluntary petition of bankruptcy or insolvency is filed in any court of competent jurisdiction by the other Party and such petition is not dismissed within 90 days thereafter; or (iii) this Agreement or a Product Agreement is assigned by the other party for the benefit of creditors. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 26 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(c) |
Client may remove one or more countries or jurisdictions from the definition of Territory in a Product Agreement upon 90 days' prior written notice if any Authority takes any action, or raises any objection, that on its terms permanently prevents Client from selling the Product in that or those countries or jurisdictions in the Territory. |
|
|
(f) |
If, after the first full year of commercial sales of the Product, Client forecasts zero volume for twelve successive months during the term of a Product Agreement after obtaining Regulatory Approval of the Product in the U.S. or the EU, then Patheon may terminate the Product Agreement by providing 30 days prior written notice to Client. Within that period, Client may either: (i) withdraw the zero forecast and re-submit a reasonable volume forecast, after which the termination notice will be null and void; or (ii) negotiate other terms and conditions on which the Product Agreement will remain in effect. |
|
|
(g) |
Client may terminate a Product Agreement upon 90 day’s prior written notice if it determines that the manufacture or supply of Product under such Product Agreement, or the sale of such Product, more likely than not infringes Third Party Rights, provided that, (i) prior to issuing such termination notice, Client must provide its material evidence of the risk of infringement to Patheon; and (ii) Client must actually permanently withdraw the applicable Product from the relevant market in the Territory because of the risk of infringement, except that if Client subsequently determines to recommence sales of such Product in such market (e.g., because it obtains a license under the applicable Third Party rights, or determines that the risk of infringement is actually low), then Client shall cause such Product Agreement to be reinstated on written notice, and in such case the Parties shall negotiate and re-execute such Product Agreement (provided that Patheon has at that time sufficient manufacturing capacity to perform its obligations under the Product Agreement). If Patheon reasonably believes that the manufacture or sale to Client of Product under a Product Agreement likely infringes Third Party Rights, then Patheon may provide Client written notice of such believe, which notice shall include all material evidence of such infringement in Patheon’s (or its Affiliate’s or attorney’s) control or awareness. The Parties will meet promptly after any such Patheon notice and shall discuss, reasonably and in good faith, the infringement risk and any appropriate actions to be taken. If (w) the Parties do not agree on an acceptable resolution to such infringement risk, and (x) such infringement risk is not based solely on manufacturing steps used by Patheon that are not specified by the Processing Instructions, (y) Patheon reasonably demonstrates that the risk of infringement by the manufacture or supply by Patheon of the Product is significant, and (z) Client does not obtain a license (or other resolution) to the infringement risk, then Patheon may terminate such Product Agreement upon 60 day’s prior written notice. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 27 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
If a Product Agreement expires, or is terminated in whole or in part for any reason, then:
|
|
(a) |
Client will take delivery of and pay for all undelivered Products that are manufactured and packaged in accordance with this Agreement under a Firm Order for such Product, at the Price in effect at the time the Firm Order was placed; |
|
|
(b) |
Client will purchase all Inventory that was purchased (or will be purchased under existing unfulfilled orders for Components), maintained or produced by Patheon solely and specifically in contemplation of filling Firm Orders submitted by Client prior to notice of termination or expiration (as applicable) or in accordance with Section 5.2, at Patheon's actual cost (including all costs incurred by Patheon for the purchase, handling, and processing of the Inventory). If Patheon uses non-expired Components in the manufacture of third party products, Client will receive a credit for the cost of such Components to the extent paid by Client. Patheon shall use good faith, reasonable efforts minimize creation of Obsolete Stock. |
|
|
(c) |
Client, at its own expense, will remove from the Manufacturing Site, within [***] following the completion, termination, or expiration of the Product Agreement, all unused API and Client-Supplied Components, all applicable Inventory (whether current or obsolete), supplies, undelivered Product, chattels, equipment or other moveable property owned by Client, related to the Agreement and located at the Manufacturing Site or that is otherwise under Patheon’s care and control (“Client Property”). If Client does not remove such Client Property within the 30 day period, Client will pay Patheon [***] for any of Client Property that contains controlled substances, requires refrigeration or other special storage requirements) after that for storing Client Property and will assume any third party storage charges invoiced to Patheon regarding Client Property (which Patheon may incur at its discretion). Patheon may ship Client Property to Client or to an external warehouse at Client’s risk and expense. Patheon will invoice Client for these storage charges as set out in Section 5.3 of this Agreement. If Client fails to remove Client Property within [***] following the completion, termination, or expiration of the Product Agreement, Client will assume all risk of loss or damage to the stored Client Property and it will be Client’s responsibility to have appropriate insurance coverage in place for this risk. If Client asks Patheon to destroy any Client Property, Client will be responsible for the cost of destruction; and |
|
|
(d) |
any termination or expiration of this Agreement or a Product Agreement will not affect any prior outstanding obligations or payments due nor will it prejudice any other remedies that either of the Parties may have under this Agreement or a Product Agreement or any related Capital Equipment Agreement for breaches obligations occurring prior to the termination. Termination or expiration of this Agreement or of a Product Agreement for any reason will not affect the obligations and responsibilities of the Parties under Sections 5.1(e), 5.1(f), 5.4, 5.5, 8.3, 10, 11, 12, 13.14, 13.15 and 13.16, all of which survive any termination or expiration, as well as any other provisions that are by implication or otherwise intended to |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 28 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
survive any termination or expiration. Where Patheon has agreed to provide stability services beyond the final supply of Product, the relevant provisions of this Agreement will survive for the agreed duration of those stability services. |
Following expiration or termination of a Product Agreement for any reason, or if Client elects to utilize a second manufacturer as provided in Section 2.1, or in the event of a failure to supply pursuant to Section 5.1(g), or at Client’s request within twelve months before the end of the term of the Product Agreement, Patheon will provide all reasonable assistance to transfer to Client (or its designated contract manufacturer) the actual manufacturing process, know-how and analytical testing methodology for the applicable Product (a “Technology Transfer”) as reasonably needed for use by Client (or its contract manufacturer) to manufacture the Product. Patheon will also disclose to Client any Patheon Intellectual Property that is reasonably required to manufacture the Product. Patheon will, upon request of Client, prepare a written proposal to perform the Technology Transfer. Client will pay the agreed fees for the Technology Transfer performed by Patheon, which must be no more than a commercially reasonable compensation for the work involved.
Each Party represents, and warrants that it has the full right and authority to enter into this Agreement and that it is not aware of any impediment that would inhibit its ability to perform its obligations under this Agreement.
|
|
(a) |
Non-Infringement. Client represents and warrants to Patheon as of the Effective Date that: |
|
|
(i) |
the Processing Instructions (including the specifications therein) for each Product are its or its Affiliate's property and that Client may lawfully disclose the Processing Instructions to Patheon for use in accordance with this Agreement; |
|
|
(ii) |
any Client Intellectual Property used by Patheon in performing the Manufacturing Services (A) is Client’s or its Affiliate's unencumbered property (including as licensed to Client), (B) may be lawfully used as directed by Client and agreed in this Agreement, and (C) to the Knowledge of Client, the use of such Client Intellectual Property by Patheon to perform the requested Manufacturing Services does not infringe and will not infringe any Third Party Rights; |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 29 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(iii) |
to the Knowledge of Client, the performance of the Manufacturing Services by Patheon or the use or other disposition of any Product by Patheon as may be required to perform its obligations under this Agreement or any Product Agreement does not and will not infringe any Third Party Rights; and |
|
|
(iv) |
there are no actions or other legal proceedings by a Third Party against Client or its Affiliates that concerns the infringement of Third Party Rights based upon manufacture or use of Product or the practice any of the Processing Instructions (including the specifications therein), or the manufacture or use of any of the API or Client-Supplied Components, or the sale, use, or other disposition of any Product made in accordance with the Processing Instructions. |
|
|
(b) |
Quality and Compliance. Client represents and warrants as of the Effective Date that: |
|
|
(i) |
To the Knowledge of Client, the Processing Instructions (including the specifications therein) for all Products conform to all Applicable Laws; |
|
|
(ii) |
To the Knowledge of Client, the Products, if labelled and manufactured in accordance with the Processing Instructions and in compliance with applicable cGMPs and Applicable Laws, and if complying with the Product Warranty at the time of delivery: (i) may be lawfully sold and distributed in every jurisdiction in which Client markets the Products,; and |
|
|
(iii) |
on receipt by Patheon, the API, will conform to the specifications for the API that Client has given to Patheon and will be adequately contained, packaged, and labelled in accordance with Applicable Laws and will conform to the affirmations of fact on the container. |
Patheon represents, and warrants to Client that:
|
|
(a) |
it will perform the Manufacturing Services in accordance with the terms of this Agreement, the Quality Agreement and the applicable Product Agreement, the Processing Instructions, cGMPs, and Applicable Laws; |
|
|
(b) |
as of the Effective Date any Patheon Intellectual Property used by Patheon to perform the Manufacturing Services (i) is Patheon’s or its Affiliate's unencumbered property, (ii) may be lawfully used by Patheon, and (iii) to its Knowledge, does not infringe and will not infringe any Third Party Rights; |
|
|
(c) |
it will not in the performance of its obligations under this Agreement use the services of any person it knows is debarred or suspended under 21 U.S.C. §335(a) or (b); and |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 30 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(d) |
it does not currently have, and it will not hire, as an officer or an employee any person whom it knows has been convicted of a felony under the laws of the United States for conduct relating to the regulation of any drug product under the United States Federal Food, Drug, and Cosmetic Act. |
|
|
(a) |
Client will be solely responsible for obtaining or maintaining, on a timely basis, any permits or other regulatory approvals for the Products, Processing Instructions or specifications, including, without limitation, all marketing and post-marketing approvals, and any specific approvals referred to in the Quality Agreement. |
|
|
(b) |
Patheon will maintain at all relevant times when performing the Manufacturing Services all required governmental permits, licenses, approval, and authorities. |
other than the WARRANTIES OF PATHEON expressly set out in this Agreement, Patheon makes no OTHER warranty or condition of any kind, either expressed or implied, by fact or law, INCLUDING ANY GENERAL warranty or condition of fitness for a particular purpose OR merchantability for the products OR ANY GENERAL WARRANTY OF NON-INFRINGEMENT.
other than the WARRANTIES OF CLIENT expressly set out in this Agreement, CLIENT makes no OTHER warranty or condition of any kind, either expressed or implied, by fact or law, INCLUDING ANY GENERAL warranty or condition of fitness for a particular purpose, OR any warranty of merchantability for the products.
Under no circumstances whatsoever will either Party be liable to the other Party, whether in contract, tort, negligence, indemnity, breach of statutory duty, or otherwise, for: (i) any (direct or indirect) damages or penalty caused by delay, loss of profits, of anticipated savings, of business, of goodwill, or of use of the Product or costs of any substitute services; or (ii) any reliance damages, including costs or expenditures incurred to evaluate the viability of entering into this Agreement or to prepare for performance under this Agreement; or (iii) for any consequential, indirect, punitive or special damages incurred by the other party, or (iv) for any other liability, damages, costs, penalty, or expense of any kind of an indirect or consequential nature regardless of any notice of the possibility of these damages.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 31 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(i) |
replace the Product conforming to the Product Warranty and other obligations of this Agreement, as soon as reasonably practicable and at Patheon’s cost (after which Patheon may invoice for the replacement unless Client has paid for the original Deficient Product), if Patheon is able to manufacture the replacement Product at the Manufacturing Site and contingent upon the receipt from Client of all API and Client-Supplied Components required for the manufacture of the replacement Product; or |
|
|
(ii) |
refund 100% of the Price paid for the Deficient Product (by credit or offset against other amounts due to Patheon under the Product Agreement, as elected by Client). |
Except for the indemnity set out in Section 10.3 and any claim for expenses related to a Recall under Section 6.2(c), the remedies described in Section 6.2 and this Section 10.2 will be Client’s sole remedy in contract, tort, negligence, equity or otherwise, for Deficient Product.
The remedy under this Section 10.2, if applicable (including in the case of Recall), will apply only to the extent that the affected Deficient Product is unsold and returned, destroyed or otherwise disposed of by Client in accordance with this Agreement, or if sold, is demonstrated by reasonable means to be Deficient Product.
|
|
(b) |
API. Except as expressly set out in Appendix 3, under no circumstances whatsoever will Patheon be liable to Client in contract, tort, negligence, indemnity, breach of statutory duty, or otherwise for any loss or damage to the API. Patheon’s maximum aggregate liability for loss of or damage to the API will not exceed [***]. |
|
|
(c) |
Maximum Liability. In any Year, in addition to the specific remedies under Section 10.2(a) for Deficient Product (which are not limited by this subsection (c)), Patheon’s maximum aggregate liability to Client under or in connection with this Agreement or any Product Agreement (however arising, including contract, tort, negligence, indemnity, breach of statutory duty, losses of API, or otherwise) will not exceed on a per Product basis [***] of revenues (being payments of the Price) received by Patheon for that Product under the applicable Product Agreement during the previous Year (or, in the case of the first Year, the expected revenue for that Product if the agreed Yearly Forecast Volumes were ordered). |
|
|
(d) |
Death, Personal Injury and Fraudulent Misrepresentation. Nothing contained in this Agreement will act to exclude or limit either Party’s liability for personal injury or death caused by the negligence, recklessness or breach of such Party or fraudulent misrepresentation. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 32 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(a) |
Patheon agrees to defend and indemnify Client and its Affiliates, and their respective officers, agents and employees (the “Client Indemnitees”), against all losses, damages, costs, judgments and liability (“Losses”) suffered by a Client Indemnitee resulting from any claims, demands, subpoenas, and legal actions (“Claims”) by a Third Party against any Client Indemnitee for personal injury or property damage to the extent that the injury or damage is the result of (i) a failure by Patheon (or its Affiliate or contractor) to perform the Manufacturing Services in accordance with the Processing Instructions, cGMPs, and/or Applicable Laws, or other fault of Patheon (or its Affiliate) that causes Product to breach the Product Warranty at delivery; or (ii) a breach by Patheon (or its Affiliate or contractor) of this Agreement or the applicable Product Agreement (including breach of the Product Warranty; and except to the extent that the Losses and/or Claims are due to the negligence or wrongful act(s) of Client, its officers, employees, or Affiliates. |
|
|
(b) |
If a Third Party Claim against a Client Indemnitee occurs for which Client intends to seek indemnity under the above subsection (a), Client will: (i) promptly notify Patheon of the Claim; (ii) use commercially reasonable efforts to mitigate the effects of the Claim; (iii) reasonably cooperate with Patheon in the defense of the Claim; and (iv) permit, at Patheon’s option, Patheon to control the defense and settlement of the Claim, all at Patheon's cost and expense, provided that if Patheon does not defend such Claim using reasonable efforts and on a timely basis, Client may defend the Claim, at Patheon’s expense. |
|
|
(a) |
Client agrees to defend and indemnify Patheon and its Affiliates, and their respective officers, agents and employees (the “Patheon Indemnitees”) against all Losses of a Patheon Indemnity resulting from any Claim by a Third Party against a Patheon Indemnity to the extent that such Claim or Loss is the result of: (i) any claim of infringement of any Third Party Rights by (1) the Products or (2) the manufacture of the Product by a proprietary process disclosed by Client or by Patheon’s use of Client’s Intellectual Property to perform the Manufacturing Services, or any portion of them, or (ii) any claim of personal injury or property damage caused by sale or use of Product, excluding any Loss or Claim to the extent that the injury or damage or Claim or Losses arises from Deficient Product; and except to the extent that the Losses and/or Claims are due to the breach of this Agreement or a Product Agreement by, or negligence or wrongful act(s) of, Patheon, its officers, employees, contractors, or Affiliates. |
|
|
(b) |
If a Third Party Claim against a Patheon Indemnitee occurs for which Patheon intends to seem indemnity under the above subsection (a), Patheon will: (i) promptly notify Client of the claim; (ii) use commercially reasonable efforts to mitigate the effects of the Claim; (iii) reasonably cooperate with Client in the defense of the Claim; and (iv) permit, at Client’s option, Client to control the defense and settlement of the Claim, all at Client's cost and expense, provided that if Client does not defend such Claim using reasonable efforts and on a timely basis, Patheon may defend the Claim, at Patheon’s expense. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 33 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
This Agreement (including, without limitation, this Section 10) is reasonable and creates a reasonable allocation of risk for the relative profits the Parties each expect to derive from its activities on the Products. Patheon assumes only a limited degree of risk arising from the manufacture, distribution, and use of the Products because Client has developed and will hold the marketing approval for the Products, Client requires Patheon to manufacture and label the Products strictly in accordance with the Processing Instructions, and Client, not Patheon, is best positioned to inform and advise potential users about the circumstances and manner of use of the Products. Thus, Patheon shall bear risks based solely on its manufacturing obligations (and breaches thereof or negligence with respect thereto), subject to the liability limitations and exclusions set out in this Agreement.
Where Products are manufactured by Patheon (or any of its Affiliates) under a separate pharmaceutical development or technology transfer agreement (the “Development Agreement”) and then released by Patheon for commercial sale or distribution by Client, the performance of the applicable pharmaceutical development or technology transfer services including the manufacture of the Product will be governed by the terms of the Development Agreement and will not be subject to the terms and conditions of this Agreement. The terms of this Agreement and the applicable Product Agreement will apply to any Product after quality release by Patheon.
“Confidential Information” means, with respect to a particular Party (the “Disclosing Party”, as to such information), any information disclosed by the Disclosing Party to the Recipient (whether disclosed in oral, written, electronic or visual form) that is non-public, confidential or proprietary, which may include information relating to the Disclosing Party’s patent and trademark applications, process designs, process models, products, manufacturing methods, drawings, plans, designs, data, databases and extracts therefrom, formulae, methods, know-how and other Intellectual Property, its clients and its clients’ confidential information, finances, marketing, products and processes and all price quotations, manufacturing or professional services proposals, information relating to composition, proprietary technology, and all other information relating to manufacturing capabilities and operations. In addition, all analyses, compilations, studies, reports or other documents prepared by any Recipient's Representatives containing Confidential Information of the Disclosing Party will be considered Confidential Information of the Disclosing Party, and all Client Intellectual Property will be considered the Confidential Information of Client. All Patheon Intellectual Property will be considered the Confidential Information of Patheon. Samples or materials provided under this Agreement as well as any and all information derived from the approved analysis of the samples or materials will also constitute Confidential Information. A Party’s rights and obligations under this Section 11 will apply to any Confidential Information that is disclosed by or received by that Party’s Representatives. For the purposes of this Section 11, a Party receiving Confidential Information of the other Party under this Agreement (including through its Representatives) is
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 34 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
a “Recipient” as to such information, and a Party disclosing Confidential Information under this Agreement (including through its Representatives) is the “Disclosing Party” as to such information. The existence, parties to, and terms of this Agreement or of any Product Agreement will be considered Confidential Information of each Party.
The Recipient will use the Confidential Information of the Disclosing Party solely for the purpose of exercising its rights and meeting its obligations under this Agreement, and will not use such Confidential Information for any other purpose. The Recipient will keep the Confidential Information of the Disclosing Party strictly confidential and will not disclose such Confidential Information in any manner whatsoever, in whole or in part, other than as otherwise permitted in this Agreement, or to those of its Representatives who (i) have a need to know the Confidential Information for the purpose of this Agreement; (ii) have been advised of the confidential nature of the Confidential Information and (iii) have obligations of confidentiality and non-use to the Recipient no less restrictive than those of this Agreement. Recipient will protect the Confidential Information of the Disclosing Party by using all reasonable precautions to prevent the unauthorized disclosure, dissemination or use of the Confidential Information, which precautions will not be less than those exercised by Recipient for its own confidential or proprietary Confidential Information of a similar nature.
A Recipient’s obligations of confidentiality, non-disclosure and non-use in this Section 11 will not apply to particular Confidential Information of the other Party to the extent that such Confidential Information:
|
|
(a) |
is or becomes publicly known through no breach of this Agreement or fault of the Recipient or its Representatives; |
|
|
(b) |
is in the Recipient's possession at the time of disclosure by the Disclosing Party other than as a result of the Recipient's breach of any legal obligation; |
|
|
(c) |
is or becomes known to the Recipient on a non-confidential basis through disclosure by sources, other than the Disclosing Party, having the legal right to disclose the Confidential Information, if the other source is not known by the Recipient to be bound by any obligations (contractual, legal, fiduciary, or otherwise) of confidentiality to the Disclosing Party for the Confidential Information; |
|
|
(d) |
is independently developed by the Recipient without use of or reference to the Disclosing Party's Confidential Information as evidenced by Recipient’s written records; or |
|
|
(e) |
is expressly authorized for release by the written authorization of the Disclosing Party. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 35 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Any combination of information which comprises part of the Confidential Information is not exempt from the obligations of confidentiality merely because individual parts of that Confidential Information are covered by exceptions in this Section 11.3, unless the combination itself is covered by any of those exceptions.
Neither Party will take any photographs or videos of the other Party’s facilities, equipment or processes, nor use any other audio or visual recording equipment (such as camera phones) while at the other Party’s facilities, without that Party’s express written consent.
Notwithstanding any other provision of this Agreement, the Recipient may disclose Confidential Information of the Disclosing Party to the extent required, as advised by counsel, in response to a valid order of a court or other governmental body or as required by law, regulation or stock exchange rule, provided that the Recipient will advise the Disclosing Party reasonably in advance of the disclosure and limit the required disclosure to the extent practicable and permissible by the order, law, regulation or stock exchange rule and any other applicable law, will reasonably cooperate with the Disclosing Party, if required, in seeking an appropriate protective order or other remedy, and will otherwise continue to perform its obligations of confidentiality set out in this Agreement. If any public disclosure is required by law, the Parties will consult concerning the form of announcement prior to the public disclosure being made.
The Disclosing Party will use reasonable efforts to summarize in writing the content of any oral disclosure or other non-tangible disclosure of Confidential Information within [***] of the disclosure, but failure to provide this summary will not affect the nature of the Confidential Information disclosed if the Confidential Information was identified as confidential or proprietary when disclosed orally or in any other non-tangible form.
Upon the written request of the Disclosing Party, the Recipient will promptly return the Confidential Information to the Disclosing Party or, if the Disclosing Party directs, destroy all Confidential Information disclosed in or reduced to tangible form including any copies, summaries, compilations, analyses or other notes derived from the Confidential Information except for one copy which may be maintained by the Recipient in its archival records solely for use in complying with the terms of this Article 11. The retained copy will remain subject to all confidentiality provisions contained in this Agreement. Client will not unreasonably require the return of Confidential Information that is necessary or useful to perform the Manufacturing Services.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 36 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Each of the Parties acknowledges that monetary damages may not be sufficient to remedy a breach by a Party of this Section 11 and agrees that the non-breaching Party will be entitled to seek specific performance, injunctive and/or other equitable relief to prevent breaches of this Section 11 and to specifically enforce Section 11 in addition to any other remedies available at law or in equity. These remedies will not be the exclusive remedies for breach of this Section 11 but will be in addition to any and all other remedies available at law or in equity.
|
|
(a) |
For the term of this Agreement, Client grants to Patheon a non-exclusive, paid-up, royalty-free, non-transferable license under applicable Client’s Intellectual Property that Patheon must use in order to perform the Manufacturing Services, solely to perform the Manufacturing Services for Client under Product Agreements, and for no other purpose. |
|
|
(b) |
All Client Intellectual Property will be the exclusive property of Client. Patheon shall disclose to Client and hereby assigns to Client the entire rights, title and interest in and to all Client Intellectual Property created, made, discovered or obtained by Patheon or its Affiliate under this Agreement or a Product Agreement. |
|
|
(c) |
All Patheon Intellectual Property will be the exclusive property of Patheon. Unless Patheon identifies in advance any specific Patheon Intellectual Property that will be subject to a separate licensing agreement between the Parties, Patheon grants to Client a non-exclusive, perpetual, paid-up, royalty-free, transferable license of the Patheon Intellectual Property used by Patheon in the manufacture of the Product for use in relation to manufacturing that Product only. |
|
|
(d) |
Each Party will be solely responsible for the costs of filing, prosecution, and maintenance of patents and patent applications on its own Inventions. |
|
|
(e) |
Either Party will give the other party written notice, as promptly as practicable, of all Inventions which can reasonably be considered to be improvements or other modifications of the Products, processes or technology owned or otherwise controlled by the party. |
Neither party has, nor will it acquire, any interest in any of the other party’s Intellectual Property unless otherwise expressly agreed to in writing. Neither party will use any Intellectual Property of the other party, except as specifically authorized by the other party or as required for the performance of its obligations under this Agreement.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 37 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Each party will maintain commercial general liability insurance, including blanket contractual liability insurance covering the obligations of that party under this Agreement through the term of this Agreement and for a period of three years after that. This insurance will have policy limits of not less than: (i) EURO 5,000,000/USD 5,000,000 for each occurrence for personal injury or property damage liability; and (ii) EURO 5,000,000/USD 5,000,000 in the aggregate per annum for product and completed operations liability. If reasonably requested each party will give the other a certificate of insurance evidencing the above and showing the name of the issuing company, the policy number, the effective date, the expiration date, and the limits of liability. The insurance certificate will further provide for a minimum of [***] written notice to the insured of a cancellation of, or material change in, the insurance. If a party is unable to maintain the insurance policies required under this Agreement through no fault of its own, then the party will without delay notify the other party in writing and the parties will in good faith negotiate appropriate amendments to the insurance provision of this Agreement in order to provide adequate assurances.
The parties are independent contractors, and this Agreement and any Product Agreement does not create between the parties any other relationship such as, by way of example only, that of employer and employee, principal and agent, joint-venturers, co-partners, or any similar relationship, the existence of which is expressly denied by the parties.
Neither party's failure to require the other party to comply with any provision of this Agreement or any Product Agreement will be considered a waiver of the provision or any other provision of this Agreement or any Product Agreement, with the exception of Sections 6.1 and 8.2 of this Agreement.
|
|
(a) |
Patheon may not assign this Agreement or any Product Agreement or any of its associated rights or obligations without the written consent of Client, this consent not to be unreasonably withheld. |
|
|
(b) |
Client may assign this Agreement or any Product Agreement or any of its associated rights or obligations without approval from Patheon, provided that this assignment right shall not disable in any way Patheon’s rights under Section 8.2(e). Client will give Patheon prior written notice of any assignment, and any such assignee will covenant in writing with Patheon to be bound by the applicable assigned terms of this Agreement or the Product Agreement in the stead of Client. Prior to any such assignment, Client may inquire of Patheon whether Patheon believes that the proposed assignee is a Patheon Competitor, or |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 38 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
a party with less than $500 million in market capitalization, (such that Patheon would have termination rights in Section 8.2(e)), and Patheon shall answer such inquiry truthfully (according to its knowledge at the time) and in writing within [***] of such inquiry, and if such inquiry answer is in the negative, such answer shall be binding on Patheon. Where necessary, Client shall assist Patheon by providing reasonable, non-privileged information in its possession regarding the assignee to enable Patheon to form this belief. If Client makes a partial assignment (i.e., assigns only certain of its rights and/or obligations under the Agreement or a Product Agreement), then Patheon may conduct a cost review of the impact of the assignment on the manufacturing costs for the assigned Products, and if Patheon determines that such costs actually will increase based on such assignment, Patheon may terminate this Agreement or any Product Agreement or any assigned part of them, on 12 months’ prior written notice to Client and the assignee, if good faith discussions between the Parties do not lead to agreement on amended Manufacturing Service fees to reflect such actual increase in costs, within a reasonable time after such Patheon determination.Client will reimburse Patheon for any costs incurred by Patheon in connection with the partial assignment including any expenses incurred by Patheon for any due diligence audits in connection with the partial assignment. |
|
|
(c) |
Despite the preceding provisions of this Section 13.4, either party may assign this Agreement or any Product Agreement to any of its Affiliates or to a successor to or purchaser of all or substantially all of its business, but the assignee must provide a written commitment to the non-assigning Party whereby the assignee agrees to be bound by the obligations of this Agreement owed to the non-assigning Party. |
Neither party will be liable for the failure to perform its obligations under this Agreement or any Product Agreement if the failure is caused by an event beyond that party's reasonable control, including, but not limited to, strikes or other labor disturbances, lockouts, riots, quarantines, communicable disease outbreaks, wars, acts of terrorism, cyber-attacks, fires, floods, storms, interruption of or delay in transportation, defective equipment, lack of or inability to obtain fuel, power or components, or compliance with any order, regulation, or enforcement decision of any government entity (a "Force Majeure Event"). A party claiming a right to excused performance under this Section 13.5 will immediately notify the other party in writing of the extent of its inability to perform, which notice will specify the event beyond its reasonable control that prevents the performance. Neither party will be entitled to rely on a Force Majeure Event to relieve it from an obligation to pay money (including any interest for delayed payment) which would otherwise be due and payable under this Agreement or any Product Agreement.
Additional Products may be added to, or existing Products deleted from, any Product Agreement by amendment to the Product Agreement including its Schedules as applicable. If Client requests services other than those expressly set out in this Agreement or in any Product Agreement (such as
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 39 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
qualification of a new packaging configuration or shipping studies, or validation of alternative batch sizes), or any cost items that are specifically excluded from the Price, Patheon will provide a written quote of the fee for the additional services and Client will advise Patheon whether it wishes to have the additional services performed by Patheon. The scope of work and fees will be agreed in writing by the parties.
Unless otherwise agreed in a Product Agreement, any notice, approval, instruction or other written communication required or permitted under this Agreement will be sufficient if made or given to the other party by personal delivery or confirmed receipt email or by sending the same by first class mail, postage prepaid to the respective addresses or email addresses set out below:
If to Client:
CARA THERAPEUTICS INC.
0 Xxxxxxxx Xxxxx, 000 Xxx Xxxxxx, 0xx Xxxxx,
Xxxxxxxx, Xxxxxxxxxxx 00000, Xxxxxx Xxxxxx
Attention: Frederique Menzaghi, Senior VP, Research and Development
Email address: [***]
Mandatory copy to: Xxxxx Xxxxxxxxxx, General Counsel, Secretary & Chief Compliance Officer
Email address: [***]
If to Patheon:
Patheon UK Limited
Xxxxxxxxxx Xxxxx
Xxxxxxxxx
Xxxxxxx
XX0 0XX
Xxxxxx Xxxxxxx
Attention: Legal Director
Email address: [***]
or to any other addresses or email addresses given to the other party in accordance with the terms of this Section 13.7. Notices or written communications made or given by personal delivery, or email will be considered to have been sufficiently made or given when sent (receipt acknowledged), or if mailed, five days after being deposited in the United States, Canada, or European Union mail, postage prepaid or upon receipt (supported by reasonable written evidence), whichever is sooner.
If any provision of this Agreement or any Product Agreement is determined by a court of competent jurisdiction to be invalid, illegal, or unenforceable in any respect, that determination will not impair or affect the validity, legality, or enforceability of the remaining provisions, because each provision is separate, severable, and distinct.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 40 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
This Agreement, together with its Appendices, the applicable Product Agreement, Capital Equipment Agreement (if any) and the Quality Agreement, constitutes the full, complete, final and integrated agreement between the parties relating to the subject matter of the Agreement and supersedes all previous written or oral negotiations, commitments, representations, agreements, transactions, or understandings concerning the subject matter of this Agreement. The basis of the parties’ agreement is set out expressly and they have not been induced by or relied on any statement or representation that is not set out in this Agreement. Any modification, amendment, or supplement to this Agreement or any Product Agreement must be in writing and signed by authorized representatives of both parties. In case of conflict, the prevailing order of documents will be this Agreement, Product Agreement, and the Quality Agreement (except that the Quality Agreement will prevail in relation to quality matters) as further described in Section 1.2.
No terms, provisions or conditions of any purchase order or other business form or written authorization used by the parties will have any effect on the rights, duties, or obligations of the parties under or otherwise modify this Agreement or any Product Agreement, regardless of any failure of a party to object to the terms, provisions, or conditions unless the document specifically refers to this Agreement or the applicable Product Agreement and is signed by both parties.
Nothing in this Agreement or any Product Agreement will confer or be construed as conferring on any third party any benefit or the right to enforce any express or implied term of this Agreement or any Product Agreement (except that Patheon Affiliates acting as subcontractors under this Agreement may enforce Sections 10.1 and 10.2). The rights of the parties to terminate, rescind or agree any variation, waiver or settlement under this Agreement are not subject to the consent of any other person.
This Agreement and any Product Agreement may be executed in two or more counterparts, by original or electronic (including “pdf”) signature, each of which will be considered an original, but all of which together will constitute one and the same instrument.
Neither party may use the other party’s name, trademarks or logo or any variations of them, alone or with any other word or words, without the prior written consent of the other party. Despite this, Client agrees that Patheon may include Client’s name and logo in customer lists or related marketing and promotional material for the purpose of identifying users of Patheon’s Manufacturing Services.
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 41 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(a) |
VAT. |
[[Any payment due to Patheon under this Agreement in consideration for the provision of Manufacturing Services to Client by Patheon is exclusive of value added taxes (“VAT”), turnover taxes, sales taxes or similar taxes, including any related interest and penalties (together referred to as "Transaction Tax"). If any Transaction Tax is payable on a Manufacturing Service supplied by Patheon to Client under this Agreement, this Transaction Tax will be added to the invoice amount and will be for the account of (and reimbursable to Patheon by) Client.
If any Transaction Tax on the supplies by Patheon is payable by Client under a reverse charge or withholding procedure (i.e., shifting of liability, accounting or payment requirement to recipient of supplies), Client will ensure that Patheon will not effectively be held liable for this Transaction Tax by the relevant taxing authorities or other parties.
Where applicable, Patheon will use its reasonable commercial efforts to ensure that its invoices to Client are issued in a way to meet the requirements for deduction of input VAT by Client, if Client is permitted by law to do so.
Each Party will provide the other with reasonable assistance to enable the recovery, as permitted by Applicable Laws, of Transaction Tax resulting from payments made under this Agreement, this recovery to be for the benefit of the Party bearing the Transaction Tax.
If Patheon is acting as Client’s buying agent, Patheon will always charge to Client the Transaction Tax in the relevant territory in addition to the amount paid by Patheon to supplier.
For the avoidance of doubt, reference to the Manufacturing Services in this Section also includes any element (or the entirety) of the Manufacturing Services characterized as a supply of goods by Patheon, its subcontractors or any tax authority for Transaction Tax purposes.
|
|
(b) |
Duties. Client will bear the cost of all duties, levies, tariffs and similar charges (and any related interest and penalties) (together “Duties”) however designated, arising from the performance of the Manufacturing Services by Patheon, including (without limitation) those imposed as a result of the shipping of materials (including drug substance, materials, components and finished Product) to, from or between Patheon site(s). If these Duties are incurred by Patheon, then Patheon will be entitled to invoice Client for these Duties at the time that they are incurred. |
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 42 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
(c) |
Withholding Tax. |
Where any sum due to be paid to Patheon hereunder is subject to any withholding or similar tax, Client will pay the withholding or similar tax to the appropriate Government Authority without deduction from or offset of the amount then due to Patheon. The Parties agree to cooperate with one another and use reasonable efforts to reduce or eliminate or enable the recovery of any tax withholding or similar obligations in respect of royalties, milestone payments, and other payments made by Client to Patheon under this Agreement.
Patheon will provide Client any tax forms that may be reasonably necessary in order for Client not to withhold tax or to withhold tax at a reduced rate under an applicable bilateral income tax treaty.
Each Party will provide the other with reasonable assistance to enable the recovery, as permitted by Applicable Laws, of withholding taxes, or similar obligations resulting from payments made under this Agreement, this recovery to be for the benefit of the Party bearing the withholding tax.
|
|
(d) |
No Offset. Any Transaction Tax, Duty, Withholding Tax or other tax that Client pays, or is required to pay, but which Client believes should properly be paid by Patheon under this Agreement may not be offset against sums due by Client to Patheon whether due under this Agreement or otherwise. |
This Agreement and any Product Agreement, and any related contractual or non-contractual causes of action, disputes and claims, will be governed by and construed in accordance with the laws of State of New York, USA, and irrevocably subject to the exclusive jurisdiction of the courts of the State of New York, USA. The UN Convention on Contracts for the International Sale of Goods will not apply to this Agreement.
All disputes that arise under or in connection with this Agreement will be resolved in accordance with Appendix 2.
[Signature page to follow]
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 43 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
This Agreement is signed by the authorized representatives of the parties on the dates shown below and will take effect from the Effective Date.
|
PATHEON UK LIMITED |
|
CARA THERAPEUTICS INC. |
||||
|
By: |
/s/ X. Xxxxxxxx |
|
By: |
/s/ Xxxxx Xxxxxxxx |
||
|
|
|
|
|
|
|
|
|
Name: |
X. Xxxxxxxx |
|
|
Name: |
Xxxxx Xxxxxxxx |
|
|
|
|
|
|
|
|
|
|
Title: |
Director |
|
|
Title: |
CEO |
|
|
|
|
|
|
|
|
|
|
Date: |
08 July 2019 |
|
|
Date: |
June 27, 2019 |
|
|
|
|
|
|
|
||
|
June 27, 2019 |
Master Manufacturing Services Agreement between Patheon and Cara Therapeutics Inc. |
|
|
Confidential |
|
Page 44 of 47 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
APPENDIX 1 – Form of Product Agreement
Product Agreement for [INSERT PRODUCT NAME]
This Product Agreement (this “Product Agreement”) is issued under the Master Manufacturing Services Agreement dated [INSERT DATE] between [PATHEON ENTITY] and [CLIENT ENTITY] (the “Master Agreement”), and is entered into between [PATHEON ENTITY], a corporation existing under the laws of [England] [or applicable founding jurisdiction for Patheon entity], having a principal place of business at [Kingfisher Drive, Covingham, Swindon, SN3 5BZ, England] (“Patheon”) and [CLIENT ENTITY] [insert Client name, legal entity, founding jurisdiction and address] (“Client”). For the purpose of this Product Agreement, references in the Master Agreement to “Patheon” and “Client” mean the entities defined respectively as Patheon and Client in this Product Agreement.
The terms and conditions of the Master Agreement are incorporated into this Product Agreement except to the extent this Product Agreement expressly modifies specific provisions in the Master Agreement. All capitalized terms that are used but not defined in this Product Agreement will have the respective meanings given to them in the Master Agreement.
Initial Product Term: will be from the Effective Date until December 31, 20[ ]
Manufacturing Site: The Manufacturing Services will be performed at the following Manufacturing Site(s): [ ]
Notices: (if different to Section 13.7 of the Master Agreement): [insert contact details]
API Name: [insert API name]
API Credit Value: Client’s actual cost for API not to exceed [ ] per kilogram. API value to be provided by Client and supported by such reasonable evidence as Patheon requests.
Local Currency: [insert currency]
Billing Currency: [insert currency]
Initial Exchange Rate: [1.00 Local Currency] to [1.00 Billing Currency]
Inflation Index: [if different from the Master Agreement]
Governing Law: [if different from the Master Agreement]]
Modifications to the Master Agreement (if any): [insert here]
|
Month DD, 20YY |
|
Product Agreement |
|
Confidential |
|
Page 1 of 3 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Schedule A – Commercial Supply Pricing Proposal: Description of the Manufacturing Services and related terms of this Product Agreement, which may include: Product Features and Assumptions, Key Assumptions to be Finalized, Annual Volume Forecasts, Pricing Tables, Costs Included in Price, Costs Not Included in Price, Equipment Requirements (if applicable), Manufacturing Parameters, Packaging Parameters, Testing Conditions, Supply Chain.
In case of conflict between Schedule A and the other parts of this APPENDIX 1, those other parts will prevail.
This Agreement is signed by the authorized representatives of the parties on the dates shown below and will take effect from the later of those dates (the “Effective Date”).
|
[PATHEON ENTITY] |
|
[CLIENT ENTITY] |
||||
|
By: |
|
|
By: |
|
||
|
|
|
|
|
|
|
|
|
Name: |
|
|
|
Name: |
|
|
|
|
|
|
|
|
|
|
|
Title: |
|
|
|
Title: |
|
|
|
|
|
|
|
|
|
|
|
Date: |
|
|
|
Date: |
|
|
|
|
|
|
|
|
||
|
Month DD, 20YY |
|
Product Agreement |
|
Confidential |
|
Page 2 of 3 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Schedule A – Commercial Supply Pricing Proposal
[Insert Commercial Supply Pricing Proposal]
[End of Product Agreement]
|
Month DD, 20YY |
|
Product Agreement |
|
Confidential |
|
Page 3 of 3 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
APPENDIX 2 – Dispute Resolution
If any dispute or issue between the Parties arises under or out of this Agreement or any Product Agreement (a “Dispute”), the Parties will first try to resolve the Dispute amicably by good faith, reasonable discussions. In the case of any Dispute, either Party may send a notice of the Dispute to the other, and each Party will appoint, within [***] from receipt of the notice, an appropriate single representative having full power and authority to discuss and seek to resolve the Dispute. Such representatives will meet promptly and as necessary in order to resolve the Dispute. If the representatives are unable to resolve the Dispute within one month from their appointment (which resolution will be reflected in a written document signed by authorized senior representatives of each Party), or if a Party fails to appoint a representative as required above, then: (a) for Technical Disputes, the expert determination procedure (as set forth below) may be started by either Party; and for all other Disputes, either Party will refer the Dispute immediately to the Chief Operating Officer or equivalent (or another senior manager as he/she may designate) (“Senior Officers”) who will meet and discuss reasonably and in good Faith as necessary to try to resolve the Dispute amicably.
If, as to an unresolved Dispute that has been referred to the Senior Officers as provided in the above paragraph, the Senior Officers do not resolve the Dispute within [***] of that referral, the Parties may (on written agreement of each Party) l attempt in good faith to settle the Dispute promptly by confidential mediation under the then current CPR Mediation Procedure, before resorting to litigation. If the Parties agree, such mediation procedure shall be initiated prior to the expiration of the applicable negotiation periods. The mediator will be chosen with the assistance of CPR (and CPR’s choice will be accepted by the parties in the absence of conflict or bias), unless the parties agree a specific mediator in writing within [***] of the referral to mediation. The mediation will take place in New York, New York and the language of the mediation will be English. Unless otherwise agreed, the parties will select a mediator from the CPR Panels of Distinguished Neutrals. If one Party does not agree to participate in such settlement negotiations using such mediation procedure, then either Party may initiate litigation in an applicable court with jurisdiction to resolve the Dispute in accordance with Section 13.15.
Except where proceedings are required for the purpose of an interim injunction or other interim equitable relief or to preserve a parties legal position pending the outcome of negotiation or mediation, neither Party may commence any court proceedings in relation to a Dispute until the required discussions under “Negotiation” paragraph above has concluded without resolving that Dispute or the other Party fails to participate in those discussions. Where a Party decides not to take part in mediation under this Appendix 2, it will send written notice of that decision to the other Party.
|
Month DD, 20YY |
|
Product Agreement |
|
Confidential |
|
Page 1 of 2 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
If a Dispute arises between the Parties that is exclusively related to technical aspects of the manufacturing, packaging, labelling, quality control testing, handling, storage, or other activities under this Agreement, including conformance of Product to applicable specifications (a "Technical Dispute"), the Parties will use all reasonable efforts to resolve the Dispute by amicable negotiations as provided in the “Negotiation” paragraph above. If the Parties are unable to resolve a Technical Dispute by such negotiations, by the end of the applicable time periods, then the Technical Dispute will, at the written request of either Party, be referred for determination to a an expert in the following manner:
|
|
(a) |
Appointment of Expert. Within [***] after the written request, the Parties will together agree on and appoint a single expert who (i) has significant experience and expertise in the subject matter of the Technical Dispute and in the operation of manufacturing agreements similar to this Agreement; and (ii) is neutral and has no relations or affiliation with either Party or its Affiliates. As a condition of any expert’s appointment, the Parties will ensure that the expert discloses any actual or potential conflicts of interest promptly as they arise. If the parties fail to agree the appointment within that period, then either party may request that a neutral from the International Institute of Conflict Prevention and Resolution appoints a suitable expert (and both parties will accept that appointment in the absence of evident conflict or bias). |
|
|
(b) |
Procedure. The expert shall establish a reasonable schedule for briefing on the technical dispute at issue, and shall provide each Party reasonable briefing on such issue(s) and for providing any needed testimony before the expert. The Parties will require the expert to provide an opinion on each referred issue (with reasonably detailed reasoning) within [***] after the conclusion of all such briefing and testimony (or as otherwise agreed by the Parties with the expert). Each party will give to the expert all the evidence and information within their respective possession or control relating to the technical issue as the expert may reasonably request, which they will disclose promptly and in any event within [***] of a written request from the expert to do so. At all times the parties will co-operate in good faith and seek to narrow and limit the issues to be determined. |
|
|
(c) |
Final and Binding. The determination of the expert will, except for fraud or manifest error or where an unapproved conflict of interest is discovered, be final and binding upon the parties with respect to the referred Technical Dispute. |
|
|
(d) |
Costs. Each party will bear its own costs for any matter referred to an expert under this Appendix 2 and, in the absence of express agreement to the contrary, the costs and expenses of the expert will be shared equally by the parties. |
|
Month DD, 20YY |
|
Product Agreement |
|
Confidential |
|
Page 2 of 2 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
APPENDIX 3 – API Yield Calculation
Reconciliation: For each Year, Patheon will prepare an annual reconciliation of API including the calculation of the Actual Annual Yield as set forth below.
“Actual Annual Yield” means the percentage of the Quantity Dispensed that was converted to Products for the Product at the Manufacturing Site in that Year and is calculated as follows:

“Quantity Dispensed” means the API received and dispensed in commercial manufacturing of Products, calculated as follows:
The total quantity of API that complies with the specifications and is received at the Manufacturing Site during the Year added to the inventory of API that complies with the specifications held at the start of the Year, minus the inventory of API that complies with the specifications held at the end of the Year.
The Quantity Dispensed includes API lost in the warehouse prior to and during dispensing but excludes (i) API retained by Patheon as samples; (ii) API contained in Product retained as samples; (iii) API used in testing (if applicable); (iv) API contained in Product that is rejected for specific market related requirements such as visual inspection of the Product that is not part of normal processing and (v) API received or dispensed in technical transfer activities or development activities, including without limitation, any regulatory, stability, validation or test batches manufactured during the applicable period.
“Quantity Converted” means the total amount of API contained in the Products manufactured with the Quantity Dispensed (including any additional Products supplied as a replacement remedy), released for delivery, and not rejected as Deficient Product in accordance with Section 6.1. The quantity of API contained in Deficient Product will be included in the Quantity Dispensed but not in the Quantity Converted.
Target Yield and Credit Calculation
[***]
|
Month DD, 20YY |
|
API Yield Calculation |
|
Confidential |
|
Page 1 of 2 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Shortfall Credit. If there is a Shortfall for a Product in a Year, then Patheon will credit Client’s account for the amount of the Shortfall not later than [***] after the end of each Year.
Surplus Credit. If there is a Surplus for a Product in a Year, then Patheon will be entitled to apply the amount of the Surplus as a credit against any Shortfall for that Product which may occur in the next Year. If there is no Shortfall in the next Year the Surplus credit will expire.
Each credit under this paragraph will be summarized in an annual reconciliation report. Upon expiration or termination of a Product Agreement, any remaining Shortfall credit amount owing under this paragraph will be paid to Client.
A Shortfall caused by rejected Deficient Product (including in the case of Recall) will only result in a Shortfall Credit to the extent the affected Product is unsold and returned, destroyed or otherwise disposed of by Client in accordance with the terms of this Agreement.
Any payable reimbursement (within the maximum liability limits) for lost API will be made at the API Credit Value.
|
Month DD, 20YY |
|
API Yield Calculation |
|
Confidential |
|
Page 2 of 2 |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.

APPENDIX 4 – Price Adjustments
Price Adjustment Calculation Due To Inflation
Refer to Section 4.2(a)
Definitions:
“Inflation Index” means the overall harmonised Index of Consumer Prices (HICP) published by the European Central Bank (xxx.xxx.xxxxxx.xx/xxxxx/xxxxxx/xxxx/xxxx/xxxxx.xx.xxxx) for Manufacturing Sites in Europe, and the Producer Price Index pcu32541235412 for Pharmaceutical Preparation Manufacturing (PPI) published by the United States Department of Labor, Bureau of Labor Statistics (hyperlink) for Manufacturing Sites in North America.
“Inflation Percentage” means the average of the monthly annual percentage changes in the Inflation Index from September of the preceding Year to August of the then current Year. For example, at the end of 2019 the new Inflation Percentage would be calculated as follows (figures are for illustration only):
|
From: Month - Year |
To: Month - Year |
Annual Percentage Change |
|
September - 2017 |
September - 2018 |
0.7 % |
|
October - 2017 |
October - 2018 |
1.1 % |
|
November - 2017 |
November - 2018 |
1.0 % |
|
December - 2017 |
December - 2018 |
0.8 % |
|
January - 2018 |
January - 2019 |
0.8 % |
|
February - 2018 |
February - 2019 |
1.1 % |
|
March - 2018 |
March - 2019 |
1.1 % |
|
April - 2018 |
April - 2019 |
1.5 % |
|
May - 2018 |
May - 2019 |
1.7 % |
|
June - 2018 |
June - 2019 |
1.4 % |
|
July - 2018 |
July - 2019 |
1.2 % |
|
August - 2018 |
August - 2019 |
1.1 % |
|
Inflation Percentage |
1.13% |
|
Calculation:

CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Price Adjustment Calculation Due To Currency Fluctuation
Refer to Section Error! Reference source not found.
Definitions:
“Billing Currency” means the currency in which the Manufacturing Services will be invoiced and paid as specified in the Product Agreement.
“Local Currency” means the currency that is used in the country where the Manufacturing Site is located as specified in the Product Agreement.
“Initial Exchange Rate” means the initial exchange rate set out in the Product Agreement to convert one unit of the Patheon Manufacturing Site Local Currency into the Billing Currency for the first Year of the Product Agreement.
“Current Year Exchange Rate” means the exchange rate calculated as of the current Year of the Product Agreement (starting from the second year), and is calculated as the average interbank exchange rate for conversion of one unit of the Patheon Manufacturing Site Local Currency into the Billing Currency during the period (September 1st of the preceding year to August 31th of the current year) as published by XXXXX.xxx under the heading “Average Exchange Rates” at xxx.xxxxx.xxx/xxxxxxxx/xxxxxxx.
“Preceding Year Exchange Rate” means the exchange rate calculated in the previous Year to the then current Year of the Product Agreement.
Calculation:
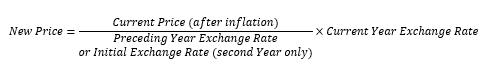
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 2 of 3 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
For example:
|
Billing Currency |
USD |
|
Local Currency |
EURO |
|
Current Price (after inflation) |
1.50 USD |
|
Preceding Year Exchange Rate |
1.2 (1 EURO to 1.2 USD) |
|
Current Year Exchange Rate |
1.1 (1 EURO to 1.1 USD) |
|
New Price |
|
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 3 of 3 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Product Agreement for CR 845 Sterile Liquid Vials
This Product Agreement (this “Product Agreement”) is issued under the Master Manufacturing Services Agreement dated June 27, 2019 between Patheon UK Limited and Cara Therapeutics Inc (the “Master Agreement”), and is entered into on 28 June 2019 (the “Effective Date“) between Patheon Manufacturing Services LLC, a corporation existing under the laws of Delaware, having a principal place of business at 0000 Xxxxxx Xxxxxx Xxxx Xx. Xxxxxxx, Xxxxxxxxxx, XX 00000, XXX (“Patheon”) and Cara Therapeutics Inc a company existing under the laws of Delaware with its principal place of business at 4 Stamford Plaza, 000 Xxx Xxxxxx, 0xx Xxxxx, Xxxxxxxx, Xxxxxxxxxxx 00000, Xxxxxx Xxxxxx (“Client”). For the purpose of this Product Agreement, references in the Master Agreement to “Patheon” and “Client” mean the entities defined respectively as Patheon and Client in this Product Agreement.
The terms and conditions of the Master Agreement are incorporated into this Product Agreement except to the extent this Product Agreement expressly modifies specific provisions in the Master Agreement. All capitalized terms that are used but not defined in this Product Agreement will have the respective meanings given to them in the Master Agreement.
Initial Product Term: will be from the Effective Date until December 31, 2024
Manufacturing Site: The Manufacturing Services will be performed at 0000 Xxxxxx Xxxxxx Xxxx Xx. Xxxxxxx, Xxxxxxxxxx, XX 00000, XXX
Notices: (if different to Section 13.7 of the Master Agreement): See Section 13.7 of the Master Agreement
API Name: CR845
API Credit Value: Client’s actual cost for API not to exceed [***]. API value to be provided by Client and supported by such reasonable evidence as Patheon requests.
Local Currency: USD
Billing Currency: USD
Initial Exchange Rate: 1.00 Euro to 1.15 USD.
Inflation Index: See Appendix 4 of the Master Agreement
Governing Law: See Section 13.15 of the Master Agreement
Modifications to the Master Agreement (if any): None
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Schedule A – Commercial Supply Pricing Proposal: Description of the Manufacturing Services and related terms of this Product Agreement.
In case of conflict between Schedule A and the other parts of this Product Agreement, those other parts will prevail.
This Agreement is signed by the authorized representatives of the parties on the dates shown below and will take effect from the later of those dates (the “Effective Date”).
|
PATHEON MANUFACTURING SERVICES LLC |
|
CARA THERAEPEUTICS INC. |
||||
|
By: |
/s/ Xxxxx Xxxxx |
|
By: |
/s/ Xxxxx Xxxxxxxx |
||
|
|
|
|
|
|
|
|
|
Name: |
Xxxxx Xxxxx |
|
|
Name: |
Xxxxx Xxxxxxxx |
|
|
|
|
|
|
|
|
|
|
Title: |
Director, POS |
|
|
Title: |
CEO |
|
|
|
|
|
|
|
|
|
|
Date: |
09 Jul 2019 |
|
|
Date: |
June 27, 2019 |
|
|
|
|
|
|
|
||
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 2 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Schedule A – Commercial Supply Pricing Proposal

|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 3 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Revision History
|
Proposal Number |
Date Issued |
Key Revisions |
|
C-MNC-62877-R2 |
March 30, 2018 |
2018 commercial pricing |
Contents
|
|
4 |
|
||
|
1. |
|
5 |
|
|
|
2. |
|
5 |
|
|
|
3. |
|
6 |
|
|
|
4. |
|
8 |
|
|
|
|
9 |
|
||
|
1. |
|
9 |
|
|
|
2. |
|
9 |
|
|
|
3. |
|
10 |
|
|
|
4. |
|
11 |
|
|
|
5. |
|
11 |
|
|
|
|
12 |
|
||
|
1. |
|
12 |
|
|
|
2. |
|
12 |
|
|
|
3. |
|
13 |
|
|
|
4. |
|
14 |
|
|
|
Part D: Business Terms |
|
|
|
|
Part A:
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 4 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Project Overview
Patheon is providing updated commercial pricing for CR845 Sterile Liquid Vials out of Patheon’s Monza and Greenville facilities.
Patheon will be responsible for the following core services:
|
|
1.1 |
Supply of raw materials and packaging components, with the exception of the Active Pharmaceutical Ingredient (“API”), which is to be furnished by Cara Therapeutics. |
|
|
1.2 |
Bulk manufacturing, filling, sealing and bulk packaging of CR845 sterile liquid vials. |
|
|
1.3 |
API identification test and all QC testing requirements for raw materials, packaging components and finished product. |
Patheon’s Monza Operations, features four separate sterile areas. One sterile area houses a state of the art pre-filled syringe and cartridge filling line capable of filling a range of nested syringes and cartridges from 0.5mL to 20mL in size, and with a fill range of between 0.1mL and 20mL. The remaining three sterile areas house seven lyophilization units ranging in size from 312 ft² (29 m²) to 431 ft² (40 m²) and offering a combined lyophilization production capacity of 2562 ft² (238 m²). These state-of-the-art lyophilization systems are ideal for the commercial manufacture of biopharmaceuticals and small molecules. The facility is also designed for the aseptic filling of small volume parenterals (SVP).
|
|
|
Monza Commercial Operations is a centre of excellence, offering extensive technology transfer and commercial manufacturing experience in sterile liquid products in vials, pre-filled syringes and cartridges and also sterile lyophilized vials. Monza supplies all the major international markets, including US, EU and Japan.The facility also provides high-volume manufacturing capabilities for a range of conventional solid dosage forms such as tablets, capsules, and granules. All manufacturing services are fully supported with comprehensive in-house analytical and bio-analytical capabilities. Monza also offers compliance with controlled drug regulations in Europe and US as well as disposable manufacturing technologies and fully integrated secondary packaging services. |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 5 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Site Regulatory History
|
Date of Inspection |
Regulatory Authority |
Inspection Type |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
Patheon’s Greenville Operations, North Carolina USA, supports both commercial manufacturing and pharmaceutical development services (PDS) in both sterile and oral solid dosage forms. The Sterile North facility has 340,519 square feet of floor space and features five separate CTM & Commercial manufacturing suites housing ten lyophilization units ranging in size from 24ft² (2.23m²) to 640ft² (59m²). Additionally, two 8ft² (0.74m²) lyophilization units are utilized for development and lyo cycle optimization activities. These state-of-the-art lyophilization systems are ideal for the large-scale production of biopharmaceuticals and small molecules, and the facility is also designed for the aseptic filling of small volume parenterals (SVP).
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 6 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Greenville Operations also serves as a Center of Excellence for sterile products, immediate and sustained release solid oral dosage forms, as well as controlled substances (Sterile and OSD). With U.S. DEA manufacturing registrations (Schedule I to V), analytical registrations (Schedule I to V), and distribution registrations (Schedule II to IV), the facility is equipped to fully accommodate controlled drug product requirements.
Site Regulatory History
|
Date of Inspection |
Regulatory Authority |
Inspection Type |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 7 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
|
4. Product Features and Assumptions 4.1 API: CR845 • Patheon’s preliminary categorisation: Category 3A |
|
|
4.2 |
Key product parameter overview: |
|
Product |
Vial Size |
Fill Volume |
Packaging Configuration |
|
CR845 sterile liquid vials |
2R |
1.5mL |
Bulk unlabeled vials |
|
|
4.3 |
Territories –U.S.A. |
|
|
4.4 |
Commercial launch date: [***] |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 8 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Cara Therapeutics has provided a (from 2017) projected annual volume forecast as outlined in the table below.
|
Product |
Annual Volume Forecast (Vials)* |
||||
|
2020 |
2021 |
2022 |
2023 |
2024 |
|
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
*Note: Reported for total units for EU-US.
The forecast presented above is a critical driver for important parameters such as batch size, campaign length, equipment train and site selection, as well as influencing the business model outlined within this proposal. Adjustments to the forecast will likely have a material impact on unit pricing and other business considerations described herein, leading to a review by Patheon and revision of the proposal.
|
|
2.1 |
Monza Bulk Vial Prices: |
|
Product |
Batch Size (Vials) |
Campaign Length (Batches) |
Filling Shifts |
Price Per Vial (Bulk) |
||
|
Material Price |
Conversion Price |
Bulk Price |
||||
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
*Note: Patheon intends to include both batch sizes for manufacturing flexibility and with 3 Shifts Patheon can increase the batch size up to [***].
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 9 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
2.2 |
Greenville Bulk Vial Prices: |
|
Product |
Batch Size (Vials) |
Campaign Length (Batches) |
Price Per Vial (Bulk) |
||
|
Material Price |
Conversion Price |
Bulk Price |
|||
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
|
|
3.1 |
Product manufactured, tested and packaged according to the approved and registered specifications. |
|
|
3.2 |
Monza Pricing: Estimated material costs. Material costs included in the proposal are best estimates based on Patheon’s current standards and specifications and do not include any extraordinary or custom raw materials. Final material costs will be provided after confirmation of specifications and formal quotations have been received from the suppliers. The cost of materials to Cara Therapeutics will be Patheon’s direct costs plus [***] markup as a handling fee. |
|
|
3.3 |
Greenville Pricing: Estimated material costs (excluding API). Materials to be purchased by Patheon will be supplied by Patheon approved suppliers. |
|
|
3.4 |
Procurement, storage, inventory control and Quality Control (“QC”) testing of all required raw materials & packaging materials to supply the CR845 sterile liquid vials , including storage of client-supplied components to meet firm order requirements or such time as agreed between the parties to accommodate long lead time items. |
|
|
3.5 |
Qualification and auditing of all raw material and component suppliers (with the exception of client-supplied raw material and component suppliers). |
|
|
3.6 |
Active Pharmaceutical Ingredients (API) identity test according to Patheon standard incoming process. If Cara Therapeutics stipulates a vendor, Cara Therapeutics will audit and approve the Vendor and ensure cGMP compliance. If Patheon is to release an API or other client stipulated material based on “ID only,” Cara Therapeutics will ensure the required verification testing by an independent laboratory has been completed. |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 10 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
3.7 |
Batch Record copies for validation batches, first ten commercial batches, and one commercial batch per Year thereafter. |
|
|
3.8 |
First Product Approval Inspection (“PAI”) and copy of FDA Report. Additional PAI support will be subject to additional fees. |
|
|
3.9 |
Continued process verification (CPV) Data Collection, Data Analysis, Reporting (one set of analysis and report per year). |
|
|
4.1 |
API, reference standards, and client-supplied materials to be supplied by Cara Therapeutics at no cost to Patheon. |
|
|
4.2 |
API complete QC testing or special API testing requests. NOTE this testing will be mandatory if Cara Therapeutics has not performed the verification testing specified in 3.6. |
|
|
4.3 |
Stability testing program − Patheon can store and test in accordance with an agreed protocol and ICH guidelines. |
|
|
4.4 |
Any additional data or report requested by Cara Therapeutics beyond the scope of cGMPs and customary FDA or other regulatory agencies requirements will be subject to an additional fee to be agreed upon between Patheon and Cara Therapeutics. |
|
|
4.5 |
Regulatory support (such as preparation of Annual Report and Chemistry, Manufacturing, and Controls (“CMC”) files). Regulatory support work is subject to an additional fee and will be charged at a rate of [***]. |
|
|
4.6 |
Label copy change and batch record changes initiated by Cara Therapeutics. |
|
|
4.7 |
Any specific visual inspection of the bulk or of the finished products outside of standard release testing. |
|
|
4.8 |
Testing required to support OOS results or stability failures, testing required in support of complaint investigations and testing of products which exceeds routine testing that are not related to Patheon’s performance. |
|
|
4.9 |
Copy of the Product Quality Review Report. Pricing of this service will depend on the level of complexity required by Cara Therapeutics. |
Greenville capital requirements have been provided in the CTM/Registration Proposal for Cara Therapeutics, Inc. (P-GRP-92560-R4).
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 11 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Part C:Key Technical Parameters
The following technical parameters apply to the production of CR845 sterile liquid vials and the materials used therein.
|
Manufacturing Parameters |
||
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
[***] |
[***] |
|
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
|
2.1 |
Primary packaging components: |
|
Component |
Specification |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
|
2.2 |
Secondary packaging – Patheon standard bulk packaging. |
|
|
2.3 |
Tertiary packaging – According to Patheon’s standard shipment preparations. |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 12 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
3.1 |
Testing for raw materials, packaging components and finished product are based on information provided by Cara Therapeutics and Patheon’s best estimates. |
|
|
3.2 |
It is assumed that the API would only require ID testing |
|
|
3.3 |
It is assumed that QC test methods are fully validated and robust at the time of commercial manufacture. |
|
Testing Requirements |
|
|
In-Process Controls |
Finished Product Testing |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
|
3.4 |
Micro testing on the finished product has been included. |
|
|
3.5 |
Testing labour may be subject to change after the final agreement on testing specifications and requirements. |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 13 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
4.1 |
Patheon will procure components and excipients for the manufacture of CR845 sterile liquid vials from Patheon qualified suppliers. Should Cara Therapeutics require Patheon to source any materials from specified suppliers, then these suppliers will remain under the quality audit control of Cara Therapeutics unless an agreement is reached for Patheon to take on this responsibility. |
|
|
4.2 |
Components and excipients will be supplied by Patheon in accordance with the specifications agreed. Patheon will issue formal Patheon specifications for each material. |
|
|
4.3 |
Each lot of incoming components and excipients will be sampled and tested according to the agreed specifications. |
|
|
4.4 |
The API will be provided free issue/released to Patheon by Cara Therapeutics or its qualified supplier. |
|
|
4.5 |
Monza only: Patheon assumes the API will be delivered from within the EU. Patheon will not act as the importer of record for API imported into the EU. |
|
|
4.6 |
The API and all excipients used for the manufacture will be GMP grade and from TSE/BSE certified sources. |
|
|
4.7 |
Finished product will be made available at Patheon’s proposed manufacturing site (supplied EXW according to Incoterms® 2010). |
[End of Product Agreement]
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 14 of 14 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Product Agreement for CR 845 Sterile Liquid Vials
This Product Agreement (this “Product Agreement”) is issued under the Master Manufacturing Services Agreement dated June 27, 2019 between Patheon UK Limited and Cara Therapeutics Inc (the “Master Agreement”), and is entered into on 28 June 2019 (the “Effective Date“) between Patheon UK Limited, a corporation existing under the laws of England, having a principal place of business at Xxxxxxxxxx Xxxxx, Xxxxxxxxx, Xxxxxxx, XX0 0XX, Xxxxxxx (“Patheon”) and Cara Therapeutics Inc a company existing under the laws of Delaware with its principal place of business at 4 Stamford Plaza, 000 Xxx Xxxxxx, 0xx Xxxxx, Xxxxxxxx, Xxxxxxxxxxx 00000, Xxxxxx Xxxxxx (“Client”). For the purpose of this Product Agreement, references in the Master Agreement to “Patheon” and “Client” mean the entities defined respectively as Patheon and Client in this Product Agreement.
The terms and conditions of the Master Agreement are incorporated into this Product Agreement except to the extent this Product Agreement expressly modifies specific provisions in the Master Agreement. All capitalized terms that are used but not defined in this Product Agreement will have the respective meanings given to them in the Master Agreement.
Initial Product Term: will be from the Effective Date until December 31, 2024
Manufacturing Site: The Manufacturing Services will be performed at Vtiale XX Xxxxxxx,000, I-20052 Monza Italy
Notices: (if different to Section 13.7 of the Master Agreement): See Section 13.7 of the Master Agreement
API Name: CR845
API Credit Value: Client’s actual cost for API not to exceed [***]. API value to be provided by Client and supported by such reasonable evidence as Patheon requests.
Local Currency: EURO
Billing Currency: USD
Initial Exchange Rate: 1.00 Euro to 1.15 USD.
Inflation Index: See Appendix 4 of the Master Agreement
Governing Law: See Section 13.15 of the Master Agreement
Modifications to the Master Agreement (if any): None
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 1 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Schedule A – Commercial Supply Pricing Proposal: Description of the Manufacturing Services and related terms of this Product Agreement.
In case of conflict between Schedule A and the other parts of this Product Agreement, those other parts will prevail.
This Agreement is signed by the authorized representatives of the parties on the dates shown below and will take effect from the later of those dates (the “Effective Date”).
|
PATHEON UK LIMITED |
|
CARA THERAPEUTICS INC. |
||||
|
By: |
/s/ X. Xxxxxxxx |
|
By: |
/s/ Xxxxx Xxxxxxxx |
||
|
|
|
|
|
|
|
|
|
Name: |
Xxxxxx Xxxxxxxx |
|
|
Name: |
Xxxxx Xxxxxxxx |
|
|
|
|
|
|
|
|
|
|
Title: |
Director |
|
|
Title: |
CEO |
|
|
|
|
|
|
|
|
|
|
Date: |
08 July 2019 |
|
|
Date: |
June 27, 2019 |
|
|
|
|
|
|
|
||
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 2 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Schedule A – Commercial Supply Pricing Proposal

Patheon a healthier world delivered CR845 Sterile Liquid Vials March 30, 2018 Commercial Supply Pricing Proposal for Cara Therapeutics. Inc CMNC 62877 R2
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 3 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Revision History
|
Proposal Number |
Date Issued |
Key Revisions |
|
C-MNC-62877-R2 |
March 30, 2018 |
2018 commercial pricing |
Contents
|
|
4 |
|
||
|
1. |
|
5 |
|
|
|
2. |
|
5 |
|
|
|
3. |
|
7 |
|
|
|
4. |
|
9 |
|
|
|
|
9 |
|
||
|
1. |
|
9 |
|
|
|
2. |
|
10 |
|
|
|
3. |
|
11 |
|
|
|
4. |
|
12 |
|
|
|
5. |
|
12 |
|
|
|
|
13 |
|
||
|
1. |
|
13 |
|
|
|
2. |
|
13 |
|
|
|
3. |
|
14 |
|
|
|
4. |
|
15 |
|
|
|
Part D: Business Terms |
|
|
|
|
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 4 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Project Overview
Patheon is providing updated commercial pricing for CR845 Sterile Liquid Vials out of Patheon’s Monza and Greenville facilities.
Patheon will be responsible for the following core services:
|
|
1.1 |
Supply of raw materials and packaging components, with the exception of the Active Pharmaceutical Ingredient (“API”), which is to be furnished by Cara Therapeutics. |
|
|
1.3 |
API identification test and all QC testing requirements for raw materials, packaging components and finished product. |
Patheon’s Monza Operations, features four separate sterile areas. One sterile area houses a state of the art pre-filled syringe and cartridge filling line capable of filling a range of nested syringes and cartridges from 0.5mL to 20mL in size, and with a fill range of between 0.1mL and 20mL. The remaining three sterile areas house seven lyophilization units ranging in size from 312 ft² (29 m²) to 431 ft² (40 m²) and offering a combined lyophilization production capacity of 2562 ft² (238 m²). These state-of-the-art lyophilization systems are ideal for the commercial manufacture of biopharmaceuticals and small molecules. The facility is also designed for the aseptic filling of small volume parenterals (SVP).
 Monza Commercial Operations is a centre of excellence, offering extensive technology transfer and commercial manufacturing experience in sterile liquid products in vials, pre-filled syringes and cartridges and also sterile lyophilized vials. Monza supplies all the major international markets, including US, EU and Japan. The facility also provides high-volume manufacturing capabilities for a range of conventional solid dosage forms such as tablets, capsules, and granules. All manufacturing services are fully supported with comprehensive in-house analytical and bio-analytical capabilities. Monza also offers compliance with controlled drug regulations in Europe and US as well as disposable manufacturing technologies and fully integrated secondary packaging services.
Monza Commercial Operations is a centre of excellence, offering extensive technology transfer and commercial manufacturing experience in sterile liquid products in vials, pre-filled syringes and cartridges and also sterile lyophilized vials. Monza supplies all the major international markets, including US, EU and Japan. The facility also provides high-volume manufacturing capabilities for a range of conventional solid dosage forms such as tablets, capsules, and granules. All manufacturing services are fully supported with comprehensive in-house analytical and bio-analytical capabilities. Monza also offers compliance with controlled drug regulations in Europe and US as well as disposable manufacturing technologies and fully integrated secondary packaging services.
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 5 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Site Regulatory History
|
Regulatory Authority |
Inspection Type |
|
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 6 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
|
Patheon’s Greenville Operations, North Carolina USA, supports both commercial manufacturing and pharmaceutical development services (PDS) in both sterile and oral solid dosage forms. The Sterile North facility has 340,519 square feet of floor space and features five separate CTM & Commercial manufacturing suites housing ten lyophilization units ranging in size from 24ft² (2.23m²) to 640ft² (59m²). Additionally, two 8ft² (0.74m²) lyophilization units are utilized for development and lyo cycle optimization activities. These state-of-the-art lyophilization systems are ideal for the large-scale production of biopharmaceuticals and small molecules, and the facility is also designed for the aseptic filling of small volume parenterals (SVP). |
Greenville Operations also serves as a Center of Excellence for sterile products, immediate and sustained release solid oral dosage forms, as well as controlled substances (Sterile and OSD). With U.S. DEA manufacturing registrations (Schedule I to V), analytical registrations (Schedule I to V), and distribution registrations (Schedule II to IV), the facility is equipped to fully accommodate controlled drug product requirements.
Site Regulatory History
|
Date of Inspection |
Regulatory Authority |
Inspection Type |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 7 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 8 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
4. Product Features and Assumptions
4.1 API: CR845
• Indication: pruritus
• Patheon’s preliminary categorisation: Category 3A
|
|
4.2 |
Key product parameter overview: |
|
Product |
Vial Size |
Fill Volume |
Packaging Configuration |
|
CR845 sterile liquid vials |
2R |
1.5mL |
Bulk unlabeled vials |
|
|
4.3 |
Territories –U.S.A. |
|
|
4.4 |
Commercial launch date: [***] |
Cara Therapeutics has provided a (from 2017) projected annual volume forecast as outlined in the table below.
|
Product |
Annual Volume Forecast (Vials)* |
||||
|
2020 |
2021 |
2022 |
2023 |
2024 |
|
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
*Note: Reported for total units for EU-US.
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 9 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
The forecast presented above is a critical driver for important parameters such as batch size, campaign length, equipment train and site selection, as well as influencing the business model outlined within this proposal. Adjustments to the forecast will likely have a material impact on unit pricing and other business considerations described herein, leading to a review by Patheon and revision of the proposal.
|
Product |
Batch Size (Vials) |
Campaign Length (Batches) |
Filling Shifts |
Price Per Vial (Bulk) |
||
|
Material Price |
Conversion Price |
Bulk Price |
||||
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
*Note: Patheon intends to include both batch sizes for manufacturing flexibility and with 3 Shifts Patheon can increase the batch size up to [***].
|
|
2.2 |
Greenville Bulk Vial Prices: |
|
Product |
Batch Size (Vials) |
Campaign Length (Batches) |
Price Per Vial (Bulk) |
||
|
Material Price |
Conversion Price |
Bulk Price |
|||
|
CR845 sterile liquid vials |
[***] |
[***] |
[***] |
[***] |
[***] |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 10 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
3.1 |
Product manufactured, tested and packaged according to the approved and registered specifications. |
|
|
3.3 |
Greenville Pricing: Estimated material costs (excluding API). Materials to be purchased by Patheon will be supplied by Patheon approved suppliers. |
|
|
3.5 |
Qualification and auditing of all raw material and component suppliers (with the exception of client-supplied raw material and component suppliers). |
|
|
3.7 |
Batch Record copies for validation batches, first ten commercial batches, and one commercial batch per Year thereafter. |
|
|
3.8 |
First Product Approval Inspection (“PAI”) and copy of FDA Report. Additional PAI support will be subject to additional fees. |
|
|
3.9 |
Continued process verification (CPV) Data Collection, Data Analysis, Reporting (one set of analysis and report per year). |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 11 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
4.1 |
API, reference standards, and client-supplied materials to be supplied by Cara Therapeutics at no cost to Patheon. |
|
|
4.2 |
API complete QC testing or special API testing requests. NOTE this testing will be mandatory if Cara Therapeutics has not performed the verification testing specified in 3.6. |
|
|
4.3 |
Stability testing program − Patheon can store and test in accordance with an agreed protocol and ICH guidelines. |
|
|
4.5 |
Regulatory support (such as preparation of Annual Report and Chemistry, Manufacturing, and Controls (“CMC”) files). Regulatory support work is subject to an additional fee and will be charged at a rate of [***]. |
|
|
4.7 |
Any specific visual inspection of the bulk or of the finished products outside of standard release testing. |
|
|
4.8 |
Testing required to support OOS results or stability failures, testing required in support of complaint investigations and testing of products which exceeds routine testing that are not related to Patheon’s performance. |
|
|
4.9 |
Copy of the Product Quality Review Report. Pricing of this service will depend on the level of complexity required by Cara Therapeutics. |
Greenville capital requirements have been provided in the CTM/Registration Proposal for Cara Therapeutics, Inc. (P-GRP-92560-R4).
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 12 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
Part C:Key Technical Parameters
The following technical parameters apply to the production of CR845 sterile liquid vials and the materials used therein.
|
Manufacturing Parameters |
||
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
[***] |
[***] |
|
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
|
|
|
2.1 |
Primary packaging components: |
|
Component |
Specification |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 13 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
2.2 |
Secondary packaging – Patheon standard bulk packaging. |
|
|
2.3 |
Tertiary packaging – According to Patheon’s standard shipment preparations. |
|
|
3.1 |
Testing for raw materials, packaging components and finished product are based on information provided by Cara Therapeutics and Patheon’s best estimates. |
|
|
3.2 |
It is assumed that the API would only require ID testing |
|
|
3.3 |
It is assumed that QC test methods are fully validated and robust at the time of commercial manufacture. |
|
Testing Requirements |
|
|
In-Process Controls |
Finished Product Testing |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
|
3.4 |
Micro testing on the finished product has been included. |
|
|
3.5 |
Testing labour may be subject to change after the final agreement on testing specifications and requirements. |
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 14 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
|
|
4.2 |
Components and excipients will be supplied by Patheon in accordance with the specifications agreed. Patheon will issue formal Patheon specifications for each material. |
|
|
4.3 |
Each lot of incoming components and excipients will be sampled and tested according to the agreed specifications. |
|
|
4.4 |
The API will be provided free issue/released to Patheon by Cara Therapeutics or its qualified supplier. |
|
|
4.5 |
Monza only: Patheon assumes the API will be delivered from within the EU. Patheon will not act as the importer of record for API imported into the EU. |
|
|
4.6 |
The API and all excipients used for the manufacture will be GMP grade and from TSE/BSE certified sources. |
|
|
4.7 |
Finished product will be made available at Patheon’s proposed manufacturing site (supplied EXW according to Incoterms® 2010). |
[End of Product Agreement]
|
Proposal # C-MNC-62877-R2 |
Cara Therapeutics. Inc. |
|
March 30, 2018 |
CR845 sterile liquid vials |
|
Confidential |
|
|
Page 15 of 15 |
|
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE CARA THERAPEUTICS, INC. HAS DETERMINED THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO CARA THERAPEUTICS, INC. IF PUBLICLY DISCLOSED.
 =1.50USD*1.1/1.2 =1.375USD
=1.50USD*1.1/1.2 =1.375USD



