EX-10.52 2 arna-ex1052_779.htm EX-10.52 ***Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Sections 200.80(b)(4) and 240.24b-2. Confidential Execution Version TRANSACTION...
Exhibit 10.52
***Text Omitted and Filed Separately
with the Securities and Exchange Commission.
Confidential Treatment Requested
Under 17 C.F.R. Sections 200.80(b)(4) and 240.24b-2.
Confidential Execution Version
This Transaction Agreement (this “Agreement”) is entered into as of December 28, 2016 (the “Effective Date”) by and among 356 Royalty Inc., a company organized under the laws of Delaware having a principal place of business at 0000 Xxxxx Xxxxx Xxxxx, Xxx Xxxxx, XX 00000 (“Arena”), Eisai Inc., a company organized under the laws of Delaware having a principal place of business at 000 Xxxx Xxxx., Xxxxxxxxx Xxxx, Xxx Xxxxxx 00000 (“ESI”), and Eisai Co., Ltd., a company organized under the laws of Japan having a principal place of business at 0-0-00 Xxxxxxxxxx Xxxxxx-xx, Xxxxx, Xxxxx, 000-00 (“ECL”). “Eisai” shall mean (a) ESI, with respect to all rights and obligations of Eisai under this Agreement with respect to the ESI Territory (as defined below) and (b) ECL, with respect to all rights and obligations of Eisai under this Agreement with respect to the ECL Territory (as defined below). Each of Arena and Eisai may be referred to in this Agreement individually as a “Party” and collectively as the “Parties”.
A.Arena, Arena US and Arena GmbH own or control certain patents, know-how and other intellectual property relating to products containing lorcaserin hydrochloride hemihydrate for weight loss or weight maintenance, among other potential indications;
B.Eisai is a pharmaceutical company with the ability to develop, manufacture, promote, market, sell and commercialize pharmaceutical products worldwide;
C.Arena GmbH and Eisai previously entered into a Marketing and Supply Agreement, dated as of July 1, 2010 (the “Original Agreement”); they subsequently amended and restated the Original Agreement by entering into the Amended and Restated Marketing and Supply Agreement, dated as of May 9, 2012 (the “Restated Agreement”), which superseded and replaced the Original Agreement and was amended by several written amendments; and they then entered into a Seconded Amended and Restated Marketing and Supply Agreement, dated as of November 7, 2013 (the “Existing Agreement”), which superseded and replaced the Restated Agreement and was amended by several written amendments, and under which Arena GmbH granted Eisai exclusive distribution rights for Products (as defined below) in the United States and other specified countries and Arena GmbH agreed to manufacture or have manufactured and sell to Eisai, and Eisai agreed to purchase from Arena GmbH, certain Products for such countries;
D.The Parties desire to enter into this Agreement to revise the Existing Agreement in its entirety (subject to certain terms that will survive as expressly set forth in this Agreement) and replace the rights and obligations in the Existing Agreement with the rights and obligations set forth in this Agreement and the Supply Agreement among Eisai and Arena GmbH effective as of the Effective Date (the “Supply Agreement”); and
1
E.Subject to the terms and conditions of this Agreement (and, in the case of Arena GmbH, the Supply Agreement), Arena, Arena US and Arena GmbH wish to license to Eisai the Arena Licensed IP and the Arena Licensed Records, to sell to Eisai the Purchased Assets, and to transfer the Assumed Liabilities to Eisai, and Eisai wishes to obtain such license and to purchase the Purchased Assets and to assume the Assumed Liabilities.
As used in this Agreement, the following capitalized terms have the meanings set out in this Article 1.
1.1“Affiliate” of a Party means any other Person that, directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with such Party, as the case may be, but for only so long as such control exists. As used in this definition, the term “control” (with correlative meanings for the terms “controlled by” and “under common control with”) means (a) direct or indirect beneficial ownership of more than 50% of the voting share capital or other equity interest in such Person able to elect the directors or management of such Person or (b) the power to direct the management and policies of such Person by contract or otherwise.
1.2“Agreement” has the meaning set forth in the opening paragraph hereto.
1.3“Applicable Laws” means the applicable provisions of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidance, ordinances, judgments, decrees, directives, injunctions, Orders, Permits (including Regulatory Approvals) of or from any court, arbitrator, Regulatory Authority or other governmental agency or authority having jurisdiction over or related to the subject activity or item as they may be in effect from time to time.
1.4“Apportioned Obligations” has the meaning set forth in Section 7.1(b).
1.5“Arena” has the meaning set forth in the opening paragraph hereto.
1.6“Arena GmbH” means Arena Pharmaceuticals GmbH.
1.7“Arena Indemnitees” has the meaning set forth in Section 12.2.
1.8“Arena Licensed IP” means the Arena Licensed Know-How and Arena Licensed Patents.
2
1.9“Arena Licensed Know-How” means all Know-How, excluding the Purchased Know-How, the Purchased Manufacturing Know-How and the Arena Licensed Manufacturing Know-How, that (a) is Controlled by Arena or any of its Affiliates (other than Arena GmbH) as of the Effective Date or at any time during the Term, (b) is necessary for, or is as of the Effective Date or was at any time during the 24-month period prior to the Effective Date used for, the development, manufacture or Commercialization of any Product in any country in the Territory in accordance with this Agreement, as such Product exists as of the Effective Date or existed prior thereto, and (c) is Confidential Information of Arena. Notwithstanding the foregoing, in the event of a Change of Control of Arena, the Arena Licensed Know-How shall not include any Know-How that is owned or Controlled by the acquiring Person described in the definition of “Change of Control,” directly or indirectly (other than indirectly through Arena or any of its Affiliates (other than Arena GmbH) existing as of the closing of such Change of Control), and that (i) exists prior to the closing of such Change of Control or (ii) is developed after such Change of Control without the use of the Arena Licensed Know-How.
1.10“Arena Licensed Manufacturing Know-How” has the meaning ascribed to the term “Arena Licensed Know-How” in the Supply Agreement.
1.11“Arena Licensed Patent” means any Arena Patent pending or issued in any country in the Arena Licensed Patent Territory. Notwithstanding the foregoing, in the event of a Change of Control of Arena, the Arena Licensed Patents shall not include any Patent that is owned or Controlled by the acquiring Person described in the definition of “Change of Control,” directly or indirectly (other than indirectly through Arena or any of its Affiliates existing as of the closing of such Change of Control), and that (x) exists prior to the closing of such Change of Control, (y) exists after the closing of such Change of Control and claims only inventions made prior to the closing of such Change of Control or (z) exists after the closing of such Change of Control and claims only inventions made after such Change of Control without the use of the Arena Licensed Know-How.
1.12“Arena Licensed Patent Territory” means the Territory excluding Brazil, China, Columbia, Israel, Japan, Mexico, South Africa, South Korea and Taiwan.
1.13“Arena Licensed Records” means all Records, other than the Purchased Records, Purchased Supply Records and Arena Licensed Supply Records, owned by Arena or any of its Affiliates (other than Arena GmbH).
1.14“Arena Licensed Supply Records” has the meaning ascribed to the term “Arena Licensed Records” in the Supply Agreement.
1.15“Arena Manufacturing Defect Losses” means Product Liability Losses attributable to a Product Liability Claim to the extent alleging defective manufacturing of a Product where such Product was manufactured by Arena GmbH or any successor or assign of Arena GmbH under the Supply Agreement, but excluding Product manufactured by any successor or assign of Arena GmbH under the Supply Agreement after the date that is six months after the closing of the transaction resulting in such Person becoming a successor or assign (a “Facility Acquisition”), if Eisai consented in writing to such Person.
3
1.16“Arena Patent” means any Patent pending or issued in any country in the Territory that is Controlled by Arena or any of its Affiliates as of the Effective Date or at any time during the Term, and that claims (a) the Compound, a Related Compound or a Product as a composition of matter, (b) a method of use of the Compound, a Related Compound or a Product, or (c) manufacture of the Compound, a Related Compound or a Product, in the case of clauses (a) or (c), as such Compound, Related Compound or Product exists as of the Effective Date or existed prior thereto, but, in the case of clauses (a), (b) and (c) excluding all claims of any such Patent that do not involve or relate to a Compound, a Related Compound or a Product or the development, manufacture or Commercialization thereof.
1.17“Arena Regulatory Approvals” means any and all (a) Regulatory Approvals in respect of the Products that have been issued to or received by Arena as of the Effective Date and (b) all applications, notifications or submissions for Regulatory Approvals in respect of the Products pending as of the Effective Date.
1.18“Arena Third Party Agreements” has the meaning set forth in Section 4.1(b).
1.19“Arena US” means Arena Pharmaceuticals, Inc., an Affiliate of Arena.
1.20“Assumed Liabilities” has the meaning set forth in Section 2.3(a).
1.21“Auditor” has the meaning set forth in Section 8.7(a).
1.22“Board of Directors” has the meaning set forth in the definition of “Change of Control”.
1.23“Business Day” means any day other than a Saturday or Sunday or a day on which banking institutions located in New York, New York or in Zofingen, Switzerland are permitted or required by Applicable Law to remain closed.
1.24“Calendar Quarter” means a period of three consecutive months during a Calendar Year beginning on and including January 1st, April 1st, July 1st or October 1st; provided, that the last Calendar Quarter shall end on the last day of the Term.
1.25“Calendar Year” means a period of 12 consecutive months beginning on and including January 1st; provided, that the first Calendar Year of the Term shall commence on the Effective Date and end on December 31 of the year in which the Effective Date occurs; provided, that the last Calendar Year shall end on the last day of the Term.
1.26“Change of Control” means, with respect to each Party, the occurrence of any of the following:
(a)any “person” or “group” (as such terms are defined below) is or becomes the “beneficial owner” (as defined below), directly or indirectly, in a transaction or series of related transactions, of shares of capital stock or other interests (including partnership or LLC membership interests) of such Party (or any of its Controlling Affiliates) then-outstanding and normally entitled (without regard to the occurrence of any contingency) to vote in the election of
4
the directors, managers or similar supervisory positions (“Voting Stock”) (or its Controlling Affiliate, as applicable) of such Party representing 50% or more of the total voting power of all outstanding classes of Voting Stock of such Party (or its Controlling Affiliate, as applicable); or
(b)such Party (or any of its Controlling Affiliates) enters into a merger, consolidation or other form of business combination, share exchange, reorganization, recapitalization or other similar extraordinary transaction with another Person (whether or not such Party (or its Controlling Affiliate, as applicable) is the surviving entity) and as a result of such merger, consolidation or other form of business combination, share exchange, reorganization, recapitalization or similar extraordinary transaction (i) the members of the board of directors or similar governing body of such Party (or its Controlling Affiliate, as applicable) (as the case may be, “Board of Directors”) immediately prior to such transaction constitute less than a majority of the members of the Board of Directors of such Party (or its Controlling Affiliate, as applicable) or, if not such Party (or its Controlling Affiliate, as applicable), such surviving Person immediately following such transaction or (ii) the Persons that beneficially owned, directly or indirectly, the shares of Voting Stock of such Party (or its Controlling Affiliate, as applicable) immediately prior to such transaction cease to beneficially own, directly or indirectly, shares of Voting Stock representing at least a majority of the total voting power of all outstanding classes of Voting Stock of the surviving Person immediately following such transaction; or
(c)such Party (or any of its Controlling Affiliates) sells or transfers to any Third Party, in one or more related transactions, properties or assets representing all or substantially all of the consolidated total assets of such Party and its Affiliates.
For the purpose of this definition: (x) “person” and “group” have the meanings given such terms under Section 13(d)(3) and 14(d)(2) of the Exchange Act and the term “group” includes any group acting for the purpose of acquiring, holding or disposing of securities within the meaning of Rule 13d-5(b)(1) under the Exchange Act; (y) “beneficial owner” shall be determined in accordance with Rule 13d-3 under the Exchange Act; and (z) the terms “beneficially owned” and “beneficially own” shall have meanings correlative to that of “beneficial ownership”.
1.27“Closing” has the meaning set forth in Section 2.4.
1.28“Commercialization” means marketing, promoting, detailing, offering for sale, selling, importing and distributing in the Territory the applicable Product, and other similar activities related to the commercial sale of the Product in the Territory, but excluding for clarity all activities relating to research, development, or manufacturing of any Product. When used as a verb, “Commercializing” means to engage in Commercialization and “Commercialize” and “Commercialized” have corresponding meanings.
1.29“Commercially Reasonable Efforts” means, with respect to a particular Party’s specific obligations under this Agreement with respect to a Product and a country in the Territory at the relevant point in time, that level of efforts and application of resources that is consistent with the usual practice followed by that Party in conducting similar activities, in the exercise of its reasonable scientific, business or regulatory judgment, but in no event less than the level of efforts and resources consistent with the commercially reasonable practices of the
5
research-based pharmaceutical industry in the applicable country in the Territory, relating to other prescription pharmaceutical products owned or licensed by it or to which it has exclusive rights that have a market potential and are at a stage of development or product life similar to the applicable Product, taking into account the anticipated or, if applicable, actual Patent coverage and the nature and extent of such Product’s market exclusivity (including Patent coverage and regulatory exclusivity), the likelihood of Regulatory Approval of such Product, the safety and efficacy of such Product, the cost to develop such Product, such Product’s profile, the competitiveness of the marketplace with respect to such Product, the proprietary position of such Product, the regulatory structure involved with respect to such Product, the profitability of such Product (including pricing and reimbursement status and the amounts of marketing and promotional expenditures), and other relevant factors, including comparative technical, legal, scientific, or medical factors. Commercially Reasonable Efforts shall be determined on a country-by-country basis. References in this Agreement to “commercially reasonable” and similar formulations shall be deemed to incorporate the standard set forth in this definition of “Commercially Reasonable Efforts.”
1.30“Competing Product” means (a) with respect to the United States, a pharmaceutical product, other than a Product, that is approved for sale in the United States by the applicable Regulatory Authorities for a weight loss, weight management or obesity Indication and (b) with respect to any country in the Territory, any branded version of (i) the combination product of naltrexone HCl and bupropion HCl (marketed in the U.S. on the Effective Date as Contrave) or (ii) the combination product of phentermine and topiramate extended release (marketed in the U.S. on the Effective Date as Qsymia).
1.31“Competing Program” has the meaning set forth in Section 4.7(b).
1.32“Compound” means the compound known as (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine, the structure of which is set forth in Exhibit A, in the hydrochloride hemihydrate form, or any other specific pharmaceutically acceptable salt, hydrate, solvate or crystalline polymorph of such compound.
1.33“Confidential Information” has the meaning set forth in Section 9.1.
1.34“Consent” means, with respect to a Third Party Distributor Agreement, any consent or approval of any Third Party which, in accordance with the terms of such Third Party Distributor Agreement, is required to be obtained for the assignment thereof to Eisai.
1.35“Constitutive Documents” means, with respect to a Person that is a legal entity, any constitutive document of such entity, including (a) with respect to a Person that is a corporation, such Person’s certificate or articles of incorporation and bylaws, (b) with respect to a Person that is a limited liability company, such Person’s certificate of formation and operating or limited liability company agreement, (c) with respect to a Person that is a partnership, such Person’s partnership agreement, (d) with respect to a Person that is a trust, such Person’s trust instrument or agreement, and (e) with respect to a Person that is a form of legal entity other than the types described in clauses (a) through (d), any document analogous to those described in clauses (a) through this clause (e).
6
1.36“Contract” means any agreement, bond, debenture, note, mortgage, indenture, guarantee, lease, contract, commitment, instrument, obligation, undertaking, license or legally binding arrangement or understanding, whether written or oral.
1.37“Control” (including any variations such as “Controlled” and “Controlling”), in the context of Materials, Patents, Know-How or regulatory filings (including specific Confidential Information), means that the applicable Party or its Affiliate owns or has a license (but excluding license rights granted to such Party by the other Party) to such Materials, Patents, Know-How or regulatory filings and has the ability to grant to the other Party the applicable license (or sublicense, as applicable) or right to use such Materials, Patents, Know-How or regulatory filings under this Agreement without violating the terms of an agreement with a Third Party.
1.38“Controlling Affiliate” means, with respect to a Party, an Affiliate of such Party that controls (within the meaning given under the definition of “Affiliate”) such Party.
1.39“Co-Promotion Partner” means any Person other than an Eisai Affiliate engaged by Eisai or by any other Co-Promotion Partner to provide promotional or marketing activities (including detailing to prescribers), in collaboration with and as prescribed by Eisai or such other Co-Promotion Partner, to assist in the promotion of sales of Product in a particular country (or countries) in the Territory (either on a co-promotion or co-marketing basis), but excluding Distributors and Sublicensees in the applicable country. “Promotional or marketing” as used herein does not include the right to sell or distribute, or to invoice or book Product sales. For clarity, any such Person engaged to provide promotional activities shall constitute a Co-Promotion Partner only during the term of such engagement.
1.40“CVOT” means the cardiovascular outcome study of the Initial Product being conducted in part to satisfy the FDA post-marketing requirement for assessment of long-term cardiovascular safety (study protocol number: APD356-G000-401; study title; A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Effect of Long-Term Treatment with BELVIQ (lorcaserin HCl) on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects with Cardiovascular Disease or Multiple Cardiovascular Risk Factors).
1.41“Delay to Onset of Diabetes Study” means the component of the CVOT relating to the co-primary objective of assessing whether or not treatment with the Initial Product reduces the incidence of conversion to type 2 diabetes mellitus (T2DM) compared to placebo, but excluding any component relating to the CVOT required by the FDA to be conducted in connection with the approval of the Initial Product. For clarity, if a component relates to assessing whether or not treatment with the Initial Product reduces the incidence of conversion to T2DM and some other component of the CVOT, it shall not be considered part of the Delay to Onset of Diabetes Study for purposes of this Agreement.
7
1.42“Development Data” means, with respect to clinical trials and other development work conducted on a Product, all data, results, information and other Know-How generated from or related to such clinical trials and development work, including preclinical, non-clinical and clinical data, reports and information, protocols, statistical analysis plans, methods, and batch records for all Products used in such work.
1.43“Disclosing Party” has the meaning set forth in Section 9.1.
1.44“Distributor” means any of the Third Party Distributors and any Third Party that Eisai or any Distributor appoints to market, promote, sell and distribute Product in a country (or countries) in the Territory, pursuant to the terms of Section 4.3, including any Third Party appointed as a sub-distributor under the Existing Agreement. For clarity, (a) any such Third Party appointed to market, promote, sell and distribute Product shall constitute a Distributor only during the term of such appointment and (b) Eisai is deemed to have appointed the Third Party Distributors as Distributors effective as of the Effective Date.
1.45“Domain Name” means a combination of alpha-numeric characters in combination with a top-level domain name.
1.46“ECL Territory” means all countries in the Territory other than the ESI Territory.
1.47“Effective Date” has the meaning set forth in the opening paragraph hereto.
1.48“Eisai” has the meaning set forth in the opening paragraph hereto.
1.49“Eisai Grantback Know-How” means, with respect to the Territory or a Terminated Territory, as applicable, that certain Know-How that (a) is Controlled by Eisai or any of its Affiliates as of the effective date of the applicable termination of this Agreement, (b) is necessary or useful for the development or Commercialization of the Compound, a Related Compound or a Product in the Territory or such Terminated Territory, as applicable, as the Compound, such Related Compound or such Product exists as of the effective date of such termination or existed prior thereto, and (c) is Confidential Information of Eisai.
1.50“Eisai Grantback Patent Rights” means, with respect to the Territory or a Terminated Territory, as applicable, any Patent pending or issued in any country in the Territory or such Terminated Territory, as applicable, that is Controlled by Eisai or any of its Affiliates as of the effective date of the applicable termination of this Agreement (and all Patents arising in the course of prosecution or maintenance of such Patents), and that claims (a) the Compound, a Related Compound or a Product as a composition of matter, or (b) a method of use or manufacture of the Compound, a Related Compound or a Product, as the Compound, such Related Compound or such Product exists as of the effective date of such termination or existed prior thereto, but excluding all claims of any such Patent that do not involve or relate to a Compound, a Related Compound or a Product or the development, manufacture or Commercialization thereof.
8
1.51“Eisai Indemnitees” has the meaning set forth in Section 12.3.
1.52“Eisai Related Party” means any Affiliate of Eisai or any Distributor or Sublicensee.
1.53“Eisai Related Party Indemnitees” means an Eisai Related Party, its Affiliates, and its and their respective directors, officers, stockholders and employees. For purposes of this definition of “Eisai Related Party Indemnitees”, the reference to “Party” in the definition of “Affiliate” shall be deemed a reference to the applicable “Eisai Related Party”.
1.54“ESI Territory” means each of the countries in North America, South America, Central America or the Caribbean.
1.55“European Union” means the organization of member states of the European Union, as it may be constituted from time to time; provided, that for the purposes of this Agreement the United Kingdom and any other country that is a member of the European Union on the Effective Date, shall be deemed to be a member of the European Union even if such country ceases to be a member of the European Union during the Term.
1.56“Exchange Act” means the Securities Exchange Act of 1934, as it may be amended from time to time.
1.57“Excluded Liabilities” has the meaning set forth in Section 2.3(b).
1.58“Excluded List” means any of the Department of Health and Human Service’s List of Excluded Individuals/Entities or the General Services Administration’s Lists of Parties Excluded from Federal Procurement and Non-Procurement Programs.
1.59“Existing Agreement” has the meaning set forth in the recitals to this Agreement.
1.60“Existing Agreement Audit Period” has the meaning set forth in Section 8.6(a).
1.61“Existing Agreement Product” has the meaning set forth in Section 15.1.
1.62“Existing Agreement Territory” means the Territory (as defined in the Existing Agreement).
1.63“Existing Arena Patents” has the meaning set forth in Section 11.2(c)(i).
1.64“Existing Eisai Know-How” means any Eisai Know-How (as defined in the Existing Agreement) owned by Eisai as of the Effective Date.
1.65Existing Eisai Patent” means any Patent that claims or covers any invention within the Existing Eisai Know-How.
1.66“Facility Acquisition” has the meaning set forth in Section 1.15.
9
1.67“FDA” means the United States Food and Drug Administration or its successor.
1.68“FDA Pediatric Studies” means the pediatric clinical trial for the Initial Product required by the FDA, in the FDA approval letter for the Initial Product NDA dated June 27, 2012, to be conducted after FDA approval of the Initial Product NDA as a condition to granting such approval, and related development activities.
1.69“FFDCA” means the United States Federal Food, Drug, and Cosmetic Act, 21 U.S.C. 301, et seq., as it may be amended from time to time, and the rules, regulations, guidances, guidelines, and requirements promulgated or issued thereunder.
1.70“First Commercial Sale” means, with respect to a particular Product in a country in the Territory, on a Product-by-Product and country-by-country basis, the first bona fide, arm’s length sale of the Product by Eisai or any Eisai Related Party to a Third Party (that is not an Eisai Related Party) in the particular country in the Territory. Sales of a Product for registration samples, compassionate use sales, named patient use, inter-company transfers to Affiliates of Eisai and the like shall not constitute a First Commercial Sale.
1.71“Force Majeure” has the meaning set forth in Section 15.2.
1.72“GAAP” means generally accepted accounting principles in the Territory, or internationally, as appropriate, consistently applied, and means international financial reporting standards (“IFRS”) at such time as IFRS becomes the generally accepted accounting standard and Applicable Laws require that a Party use IFRS.
1.73“Generic Version” means, with respect to a particular Product, a product sold (i) by a Third Party (who is not authorized by Eisai or any of its Affiliates and who neither Arena nor any of its Affiliates has authorized at Eisai’s request) or (ii) by Arena, any of its Affiliates or any Third Party authorized by Arena or any of its Affiliates that, in each case ((i) or (ii)), (a) contains as an active pharmaceutical agent the same Compound or Related Compound that such Product contains as an active pharmaceutical agent, and (b) (1) if sold in the United States, has been approved for sales introduction into commerce in the United States by reference to the Regulatory Approval for such Product in the United States pursuant to Section 505(b)(2) or 505(j) of the FFDCA (or the successor thereof) or (2) if sold in a country other than the United States, has been approved for sale in such country pursuant to an equivalent regulatory law or regulation, but excluding for clarity any Products sold by Eisai or any Eisai Related Party during the Term.
1.74“Good Clinical Practices” or “GCP” means the then-current standards, practices and procedures promulgated or endorsed by the FDA for designing, conducting, recording, analyzing and reporting clinical trials that involve the participation of human subjects, including as set forth in 21 C.F.R. parts 50, 54, 56 and 312 and in the ICH guidelines entitled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” and comparable regulatory standards, practices and procedures in other countries in the Territory outside of the United States, as they may be updated from time to time.
10
1.75“Good Laboratory Practices” or “GLP” means the then-current good laboratory practice standards promulgated or endorsed by the FDA for nonclinical laboratory studies that support or are intended to support applications to conduct research on human subjects or to obtain regulatory approval, including as set forth in 21 C.F.R. Part 58, and comparable regulatory standards in other countries in the Territory outside of the United States, as they may be updated from time to time.
1.76“Governmental Entity” means any nation, state, province, county, city or political subdivision, any supranational organization of sovereign states, and any official, agency, arbitrator, authority, court, department, commission, board, bureau, instrumentality or other governmental, quasi-governmental or Regulatory Authority thereof, whether domestic or foreign.
1.77“ICH” means the International Conference on Harmonization (of Technical Requirements for Registration of Pharmaceuticals for Human Use).
1.78“ICC” has the meaning set forth in Section 13.3(a).
1.79“IND” means an Investigational New Drug Application (including any amendments thereto) filed with the FDA pursuant to 21 C.F.R. §312 before commencement of clinical trials of a pharmaceutical product and its equivalent in other countries or regulatory jurisdictions outside the United States.
1.80“Indemnitee” has the meaning set forth in Section 12.7(a).
1.81“Indemnitor” has the meaning set forth in Section 12.7(a).
1.82“Indemnity Threshold” has the meaning set forth in Section 12.6(a).
1.83“Indication” means the diagnosis, treatment, prevention or amelioration of any disease or condition for which an NDA or similar regulatory filing may be filed and approved.
1.84“Initial Formulation” means the pharmaceutical product in solid, oral tablet form containing 10mg of the Compound as its sole active pharmaceutical agent as described in the Initial Product NDA as of the Effective Date.
1.85“Initial Product” means the Initial Formulation as indicated for the Indication(s) that, as of the Effective Date, is (are) the subject of the Initial Product NDA.
1.86“Initial Product NDA” means NDA22529.
1.87“Inventory” means the materials purchased by Eisai from Arena GmbH that are set forth on Exhibit A Part 1 of the Supply Agreement.
1.88“Know-How” means all tangible and intangible scientific, technical, trade, financial or business information and materials, including compounds, compositions of matter, formulations, techniques, processes, methods, trade secrets, formulae,
11
procedures, tests, data, results, analyses, documentation, reports, information (including pharmacological, toxicological, non-clinical (including chemistry, manufacturing and control)), and clinical test design, methods, protocols, data, results, analyses, and conclusions, quality assurance and quality control information, regulatory documentation, information and submissions pertaining to, or made in association with, filings with any Regulatory Authority, product life cycle management strategies, knowledge, know‑how, skill, and experience, and all other discoveries, developments, inventions (whether or not confidential, proprietary, patented or patentable), and tangible embodiments of any of the foregoing. For clarity, Know-How does not include Trademarks.
1.89“Knowledge” means, with respect to a particular statement to which such term is attributed, that none of the applicable Party’s or any of its Affiliates’ respective employees with the title of vice president or higher or in-house general counsel (and, solely with respect to Arena, the general manager or the co-general manager of the Plant) are aware of any facts or information that make such statement untrue after performing a reasonably diligent investigation with respect to such statement.
1.90“Legal Proceeding” means any action, suit, proceeding, claim, arbitration or investigation before any Governmental Entity or before any arbitrator or mediator or similar party, or any investigation or review by any Governmental Entity.
1.91“Lien” means any lien, pledge, mortgage, encumbrance, or other security interest of any kind, whether arising by contract or by operation of Applicable Law.
1.92“Losses” has the meaning set forth in Section 12.2.
1.93“XXXX Plus Study” means the component of the CVOT relating to the co-primary objective of assessing whether or not treatment with the Initial Product reduces the incidence of major adverse cardiovascular events including in totality or in part the following events: stroke or myocardial infarction; cardiovascular death or hospitalization for unstable angina or heart failure; or any coronary revascularization compared to placebo (“XXXX Plus”), but excluding any component relating to the CVOT required by the FDA to be conducted in connection with the approval of the Initial Product. For clarity, if a component relates to assessing whether or not treatment with the Initial Product reduces the incidence of XXXX Plus and some other component of the CVOT, it shall not be considered part of the XXXX Plus Study for purposes of this Agreement.
1.94“Major Market” means each of the United States, the European Union, China and Japan.
1.95“Materials” has the meaning set forth in Section 5.7.
1.96 “Maximum Price Discount” means […***…]% for each of Argentina, Brazil, Chile, Columbia, Mexico, Peru, Venezuela and Uruguay and three percent for each other country in the Territory.
***Confidential Treatment Requested
12
1.97“NDA” means a New Drug Application (including an Abbreviated New Drug Application) as described in 21 C.F.R. § 314.50, et seq., and all amendments and supplements thereto, that is filed with the FDA, and its equivalent in other countries or regulatory jurisdictions outside the United States, in each case including all documents, data, and other information concerning the applicable product filed therewith.
1.98“Net Sales” means, with respect to a Product during any period, the gross invoiced sales price in US Dollars (as converted into US Dollars for sales made in other currency) for all quantities of such Product sold by Eisai or any Eisai Related Party to a Third Party (other than an Eisai Related Party) during such period, less the following deductions to the extent actually incurred, allowed, or paid with respect to such sale by the selling party, using GAAP applied on a consistent basis:
(a)sales taxes or other taxes included in the gross invoiced sales price;
(b)credits or allowances given or made for rejection, recall or return of previously sold Product, in amounts not exceeding usual and customary reductions, or billing errors with respect to such Product;
(c)Retroactive Price Discounts;
(d)costs of outbound freight, insurance, and other transportation charges directly related to the distribution of such Product to the purchaser, to the extent separately set forth in the applicable invoice;
(e)quantity, cash and other trade discounts, or inventory management fees, including those generated as a result of distributor service agreements, in amounts not exceeding usual and customary discounts and fees; and
(f)rebates, credits, and chargeback payments (or the equivalent thereof) granted to managed health care organizations, wholesalers, or to federal, state, local and other governments, including their agencies, purchasers, or reimbursers, or to trade customers, in amounts not exceeding usual and customary amounts and calculated in accordance with GAAP.
In no event shall any particular amount of deduction, identified above, be deducted more than once in calculating Net Sales (i.e., no “double counting” of reductions). Each of the above deductions shall be substantially consistent with Eisai’s or the applicable Eisai Related Party’s internal accounting policies as consistently applied by Eisai or such Eisai Related Party in the applicable country in the Territory across its products at the time of sale. In no event shall the deductions with respect to Retroactive Price Discounts in any country in the Territory in any Calendar Quarter exceed the applicable Maximum Price Discount for such country of the amount arrived at after deducting the items described in clauses (a), (b), (d), (e) and (f) above from the gross invoiced sales price in US Dollars (as converted into US Dollars for sales made in other currency) for all quantities of such Product sold by Eisai or the Eisai Related Parties to a Third Party (other than any Eisai Related Party) in such country in the Territory during such Calendar Quarter; provided, that any deductions for Retroactive Price Discounts not taken in any Calendar Quarter pursuant to this sentence shall be carried forward and applied in future Calendar Quarters. Eisai shall not, and shall cause the Eisai Related Parties not to, use any Product as a
13
loss leader or otherwise unfairly or inappropriately discount the gross invoiced sales price of a Product in a manner that is intended to benefit, or provide an incentive to enhance sales of, any other pharmaceutical product sold by Eisai or any Eisai Related Party. Sales of a Product between Eisai and any of the Eisai Related Parties for resale shall be excluded from the computation of Net Sales, but the subsequent resale of such Product to a Third Party (other than an Eisai Related Party) shall be included within the computation of Net Sales. Notwithstanding anything to the contrary herein, the transfer, disposal or use of Product, without consideration, for marketing, regulatory, development or charitable purposes, such as samples, clinical trials, preclinical trials, compassionate use, named patient use, or indigent patient programs, shall not be deemed a sale hereunder.
1.99“New Program Know-How” means any and all Know-How discovered, identified, conceived, reduced to practice or otherwise made, as necessary to establish authorship, inventorship or ownership under applicable United States law as such law exists as of the Effective Date irrespective of where such discovering, identifying, conception, reduction to practice or other making occurs, in the course of or as a result of or related to the activities under this Agreement or the Supply Agreement after the Effective Date, (a) solely by one or more employees of or consultants to Arena or any of its Affiliates, (b) solely by one or more employees of or consultants to Eisai or any of the Eisai Related Parties or Co-Promotion Partners, or (c) jointly by one or more employees of or consultants to Arena or any of its Affiliates, on the one hand, and one or more employees of or consultants to Eisai or any of the Eisai Related Parties or Co-Promotion Partners, on the other hand.
1.100“New Program Patent” means any Patent that claims or covers any invention within the New Program Know-How.
1.101“Non-Compete Period” has the meaning set forth in Section 4.7(a).
1.102“Once-Daily Product” means a once-daily oral tablet formulation that contains the Compound as its sole active pharmaceutical agent.
1.103“Order” means any writ, judgment, decree, injunction, settlement, or similar order of or approved by any Governmental Entity (in each case whether preliminary or final).
1.104“Ordinary Course of Business” means the ordinary course of business in substantially the same manner as presently conducted and consistent with past practice and in compliance with Applicable Law as determined from the perspective of an on-going owner-operator of the Purchased Assets.
1.105“Original Agreement” has the meaning set forth in the recitals to this Agreement.
1.106“Original Effective Date” means July 1, 2010.
1.107“Panel” has the meaning set forth in Section 13.3(b).
14
1.108“Paragraph IV Notice” has the meaning set forth in Section 10.3(e).
1.109“Party” and “Parties” has the meaning set forth in the opening paragraph of this Agreement.
1.110“Patent(s)” means (a) all patents, certificates of invention, applications for certificates of invention, priority patent filings and patent applications, including provisional patent applications, (b) any renewal, division, continuation (in whole or in part), or request for continued examination of any of such patents, certificates of invention and patent applications, and any all patents or certificates of invention issuing thereon, and any and all extensions, divisions, renewals, substitutions, confirmations, registrations, revalidations, revisions, and additions of or to any of the foregoing, and (c) any patents or patent applications that are the subject of administrative proceedings before a jurisdiction’s patent office, including reissues, reexaminations, oppositions, third party observations, post-grant reviews and inter partes review proceedings.
1.111“Patent Term Extension” means any term extensions, supplementary protection certificates, regulatory exclusivity and equivalents thereof offering patent protection beyond the initial term with respect to any issued Patents.
1.112“Payee Party” has the meaning set forth in Section 8.5.
1.113“Paying Party” has the meaning set forth in Section 8.5.
1.114“Payment” has the meaning set forth in Section 8.5.
1.115“Permit” means any permit, license, approval, certificate, consent, waiver, concession, exemption, order, injunction, judgment, decree, ruling, writ, assessment or arbitration award, registration, notice or other authorization from any Governmental Entity.
1.116“Permitted Lien” means the following: (a) statutory Liens for Taxes not yet due or payable, (b) Liens for assessments and other governmental charges or Liens of landlords, carriers, warehousemen, mechanics and repairmen incurred in the Ordinary Course of Business, in each case for sums not yet due and payable, or due but not delinquent, or being contested in good faith by appropriate proceedings, (c) any Liens under the terms of the Third Party Distributor Agreements, and (d) Liens incurred in the Ordinary Course of Business in connection with workers’ compensation, unemployment insurance and other types of social security.
1.117“Person” means any individual, corporation, partnership, limited liability company, trust, Governmental Entity, or other legal entity of any nature whatsoever.
1.118“Plant” means the manufacturing plant of Arena GmbH located at Xxxxxx Xxxxxxxxxxxx 0, 0000, Xxxxxxxx, Xxxxxxxxxxx, at which the Products are manufactured as of the date of this Agreement.
1.119“Post-Closing Tax Period” has the meaning set forth in Section 7.1(b).
15
1.120“Pre-Closing Tax Period” means (a) any Tax period ending on or before the Effective Date and (b) with respect to a Tax period that commences before but ends after the Effective Date, the portion of such period up to and including the Effective Date.
1.121“Product” means each of (a) the Initial Product, (b) the Once-Daily Product and (c) any pharmaceutical product (in any specific dosage form or mode of administration) that contains the Compound or a Related Compound as an active pharmaceutical agent (but excluding the Initial Product and the Once-Daily Product) (which product may also include one or more other active pharmaceutical agents, excluding an active pharmaceutical agent that is proprietary to Arena or any of its Affiliates and that is not a Compound or Related Compound).
1.122“Product Domain Name” means a Domain Name that contains in whole or in part (a) any Product Trademark, (b) the generic name for an active pharmaceutical ingredient in any Product (for example, “lorcaserin”), or (c) any other word, name, or xxxx confusingly similar to the foregoing, including a Domain Name containing an intentional misspelling.
1.123“Product Liability Claim” means any Third Party Claim brought against any Arena Indemnitee, Eisai Indemnitee or Eisai Related Party Indemnitee arising from, based on or occurring as a result of personal injury, death or property damage (to the extent resulting from personal injury or death) caused by or resulting from (or allegedly caused by or resulting from) the use of a Product sold, distributed, dispensed or otherwise administered in the Existing Agreement Territory after the Original Effective Date and prior to the Effective Date or in the Territory on or after the Effective Date and prior to the end of the Term.
1.124“Product Liability Defense Costs” means costs and expenses paid to counsel and other Third Parties, including Third Party experts and investigators, in connection with the defense of Product Liability Claims. For clarity, Product Liability Defense Costs shall not include Product Liability Losses.
1.125“Product Liability Losses” means, with respect to a Product Liability Claim, (a) amounts paid to the Third Party(ies) bringing such Product Liability Claim to satisfy a judgment in such Product Liability Claim, but excluding punitive damages, or (b) amounts paid to the Third Party(ies) bringing such Product Liability Claim in settlement of such Product Liability Claim. For clarity, Product Liability Losses shall not include Product Liability Defense Costs.
1.126“Product Trademark” has the meaning set forth in the Existing Agreement.
1.127“Purchase Price” means the amounts payable by Eisai to Arena pursuant to Article 8.
1.128“Purchased Assets” has the meaning set forth in Section 2.2(a).
16
1.129“Purchased Intellectual Property” means (a) all Know-How owned by Arena or any of its Affiliates (other than Arena GmbH) as of the Effective Date that is related solely to the Compound or Product, as such Compound or Product exists as of the Effective Date or existed prior thereto, including the composition, manufacture or use thereof (the “Purchased Know-How”), (b) all Know-How owned by Arena GmbH as of the Effective Date that is solely related to the Compound or Product as such Compound or Product exists as of the Effective Date or existed prior thereto, including the composition, manufacture or use thereof (the “Purchased Manufacturing Know-How”), (c) the Arena Patents (excluding the Arena Licensed Patents) owned by Arena or any of its Affiliates as of the Effective Date (the “Purchased Patents”), including the Patents set forth on Schedule 1.129(c) and (c) any and all Purchased Trademarks.
1.130“Purchased Know-How” has the meaning set forth in Section 1.129.
1.131“Purchased Manufacturing Know-How” has the meaning set forth in Section 1.129.
1.132“Purchased Patents” has the meaning set forth in Section 1.129.
1.133“Purchased Records” means those Records owned by Arena or any of its Affiliates (other than Arena GmbH) as of the Effective Date that are related solely to the Compound, Product, Inventory, Third Party Distributor Agreements, Arena Regulatory Approvals, Purchased Intellectual Property, Samples, Purchased Validation Materials or Product Domain Names, but excluding any Records to the extent including or referencing data and information relating to the performance of obligations or exercise of rights under any Third Party Distributor Agreement before the Effective Date or any claim or demand that a Third Party Distributor or Arena or its Affiliate may have against the other that relates to matters under any Third Party Distributor Agreement arising before the Effective Date.
1.134“Purchased Supply Records” has the meaning ascribed to the term “Purchased Records” in the Supply Agreement.
1.135“Purchased Trademarks” has the meaning ascribed to the term “Purchased Trademarks” in the Supply Agreement.
1.136“Purchased Validation Materials” has the meaning ascribed to the term “Purchased Validation Materials” in the Supply Agreement.
1.137“PV Agreement” means the Lorcaserin Pharmacovigilance Agreement for the Exchange of Drug Safety Information, dated as of May 13, 2014, entered into by Eisai and Arena GmbH, as amended from time to time.
1.138“Quarterly Report” has the meaning set forth in Section 8.3(c).
1.139“Receiving Party” has the meaning set forth in Section 9.1.
1.140“Recipient” has the meaning set forth in Section 9.1.
17
1.141“Records” means all books, records, files, documents, correspondence, and manuals, or portions thereof, in each case only to the extent data and information included or referenced therein relates to the Compound or any Product, the Inventory, Third Party Distributor Agreements, Arena Regulatory Approvals, Purchased Intellectual Property, Samples, Purchased Validation Materials or Product Domain Names (including regulatory, financial, research and development and expense records, correspondence and, to the extent not originals, complete and accurate copies of all files relating to the filing, prosecution, issuance, maintenance, enforcement or defense of any Patents, Patent applications, Trademarks, copyrights or other intellectual property rights within the Purchased Intellectual Property, including written Third Party correspondence, records and documents related to research and pre-clinical and clinical testing and studies for the Compound or the Products, including laboratory notebooks, procedures, tests, dosages, criteria for patient selection, safety and efficacy and study protocols, investigators brochures and all pharmacovigilance and other safety records) that are maintained by Arena or its Affiliates (other than Arena GmbH) on the Effective Date and necessary for, or are as of the Effective Date or were at any time during the 24-month period prior to the Effective Date used for, the development, manufacture or Commercialization of any Product in any country in the Territory, in all forms, including electronic, in which they are stored or maintained. For clarity, to the extent books, records, files, documents, correspondence and manuals, or portions thereof, include data and information unrelated to the Compound or any Product, the Inventory or any Third Party Distributor Agreements, any Arena Regulatory Approvals, Purchased Intellectual Property, Samples, Purchased Validation Materials or Product Domain Names, such unrelated data and information will not be considered Records. In addition, Records does not include any books, records, files, documents, correspondence or manuals, or portions thereof, that are subject to an attorney-client privilege or that are attorney work product.
1.142“Regulatory Approval” means, with respect to a Product to be sold for use in a particular country in the Territory: (a) as to the United States, approval by the FDA of the NDA covering such Product in the United States and, if applicable, all necessary approvals or authorizations by the U.S. Drug Enforcement Administration (or its successor) necessary to sell such Product in the United States; and (b) as to a country in the Territory other than the United States, all approvals, registrations, authorizations and licenses by the Regulatory Authorities in such country necessary to sell such Product in such country.
1.143“Regulatory Authority” means, as to a particular country, any national, regional, state or local regulatory agency, department, bureau, commission, council or other Governmental Entity whose review, approval or authorization is necessary for the manufacture, packaging, use, storage, import, export, distribution, promotion, marketing, offer for sale or sale of a Product in such country. In the event that governmental approval is required for pricing or reimbursement for a Product in a country in the Territory to be reimbursed by national health insurance (or its local equivalent), “Regulatory Authority” shall also include any national, regional, state or local regulatory agency, department, bureau, commission, council or other Governmental Entity whose approval or authorization of pricing or reimbursement is required.
18
1.144“Regulatory Filings” means all applications, approvals, licenses, notifications, registrations, submissions and authorizations made to or received from a Regulatory Authority in the Territory necessary for the development, manufacture or commercialization of a pharmaceutical product, including any INDs, NDAs and Regulatory Approvals.
1.145“Regulatory Strategy” means, with respect to a Product in a country in the Territory, the strategy for conducting the interactions with Regulatory Authorities needed to develop such Product for such country and to obtain and maintain Regulatory Approval of such Product in such country, including making Regulatory Filings (including INDs, NDAs, and amendments thereto) and developing and implementing risk evaluation and mitigation strategies.
1.146“Related Compound” means (a) any known prodrug, known metabolite (having similar physiological activity as the Compound), or racemate or other optically active form of the Compound (other than the Compound itself), (b) any free acid form or free base form of the Compound (other than the Compound itself), (c) any compound that is claimed by claim 1 of U.S. Patent No. 6,953,787 and acts primarily as a 5HT2C agonist and has physiological activity similar to the Compound, or (d) any compound that is claimed by International Patent Publication No. WO 2005/003096 (as such claims are published as of the Original Effective Date).
1.147“Related Documents” means, other than this Agreement, all agreements, certificates and documents signed and delivered by either Party in connection with the Closing under this Agreement, excluding the Supply Agreement.
1.148“Representatives” means, with respect to a Person, such Person’s legal, financial, internal and independent accounting and other advisors and representatives.
1.149“Restated Agreement” has the meaning set forth in the recitals to this Agreement.
1.150“Retroactive Price Discount” means, with respect to a Product, a discount off of the invoiced price for such Product provided for in a contract entered into by Eisai or any of the Eisai Related Parties during any period stipulating a discounted contract price for such Product that is effective for Product purchased prior to the execution of such contract.
1.151“Samples” means whole blood samples, sera, plasma, cells, bone marrow samples, other tissue samples, and other substances collected or generated in a non-clinical or clinical study with respect to the Compound, a Related Compound or a Product and any DNA, RNA, cells, proteins, and other biomaterials extracted or directly derived therefrom.
1.152“SEC” has the meaning set forth in Section 9.5(a).
1.153“Senior Executives” means the President of Arena and the President of Eisai.
19
1.154“Shadow Counsel” means, with respect to a particular Product Liability Claim, the counsel (if any) appointed by Arena in such Product Liability Claim to participate in and monitor (but not control) such Product Liability Claim.
1.155“Side Letter Agreement” means that certain letter agreement between Arena US and Eisai dated the Effective Date.
1.156“Specified Date” means July 1, 2016.
1.157“Sublicense” means a sublicense granted by Eisai under the license granted to it in this Agreement or in the Side Letter Agreement or Supply Agreement, or a license granted by Eisai under the Purchased Assets, to a Sublicensee or an Affiliate of Eisai, or granted by any Sublicensee or Affiliate of Eisai under the sublicense granted to such Person under the Arena Licensed IP, Arena Licensed Records, Arena Licensed Manufacturing Know-How or Arena Licensed Supply Records or the license granted to such Person under the Purchased Assets.
1.158“Sublicensee” means any Person other than Eisai and its Affiliates to whom Eisai or its Affiliate, or any Sublicensee, has granted a sublicense under the license granted to it in this Agreement, the Side Letter Agreement or the Supply Agreement or under any Sublicense, as applicable, or a license or sublicense under the Purchased Assets, with respect to any Product in any country (or countries) in the Territory, pursuant to the terms of Section 4.3 or the corresponding provision of the Supply Agreement. For clarity, any such Person shall constitute a Sublicensee only during the term of the sublicense granted to such Person.
1.159“Supply Agreement” has the meaning set forth in the recitals hereto.
1.160“Supply Records” has the meaning ascribed to the term “Records” in the Supply Agreement.
1.161“Survival Period” has the meaning set forth in Section 12.1.
1.162“Tax” or “Taxes” means any and all taxes, assessments, levies, tariffs, duties or other charges or impositions in the nature of a tax (together with any and all interest, penalties, additions to tax and additional amounts imposed with respect thereto) imposed by any Governmental Entity, including income, estimated income, gross receipts, profits, business, license, occupation, franchise, capital stock, real or personal property, sales, use, transfer, value added, employment or unemployment, social security, disability, alternative or add-on minimum, customs, excise, stamp, environmental, commercial rent or withholding taxes, and shall include any liability for Taxes of any other Person under Applicable Law, as a transferee or successor, by contract or otherwise.
1.163“Tax Return” means any return, declaration, report, claim for refund, information return or statement relating to Taxes, including any schedule or attachment thereto, filed or maintained, or required to be filed or maintained, in connection with the calculation, determination, assessment or collection of any Tax, including any amended returns required as a result of examination adjustments made by the Internal Revenue Service or other Tax authority.
20
1.164“Term” has the meaning set forth in Section 13.1.
1.165“Terminated Product Trademark” means, with respect to the Territory or a Terminated Territory, as applicable, the Trademark(s) used by Eisai or any Eisai Related Party for the development, manufacture or Commercialization of the Products in the Territory or such Terminated Territory, as applicable, and any registrations thereof or any pending applications relating thereto in the Territory or such Terminated Territory, as applicable (excluding, in any event, any Trademarks that include any corporate name or logo of Eisai or any Eisai Related Parties).
1.166“Terminated Territory” has the meaning set forth in Section 13.2(a).
1.167“Termination Dispute” has the meaning set forth in Section 13.3(a).
1.168“Territory” means all countries and territories of the world, excluding any Terminated Territory.
1.169“Third Party” means any Person other than Arena, Eisai, and their respective Affiliates.
1.170“Third Party Claim” has the meaning set forth in Section 12.2(e).
1.171“Third Party Distributor” means each of Abic Marketing Limited, CY Biotech Company Limited and Ildong Pharmaceutical Co., Ltd.
1.172“Third Party Distributor Agreement” means, as amended, supplemented or modified as of the Effective Date, each of (a) the Marketing and Supply Agreement by and between Arena GmbH and Abic Marketing Limited, dated July 21, 2014, (b) the Marketing and Supply Agreement by and between Arena GmbH and CY Biotech Company Limited, dated July 24, 2013, and (c) the Marketing and Supply Agreement by and between Arena GmbH and Ildong Pharmaceutical Co., Ltd., dated November 6, 2012.
1.173“Trademark” means any word, name, symbol, color, designation or device or any combination thereof, including any trademark, trade dress, brand xxxx, service xxxx, trade name, brand name, logo or business symbol, whether or not registered.
1.174“Transfer Taxes” has the meaning set forth in Section 7.1(a).
1.175“United States” means the United States of America and its territories and possessions, including Puerto Rico and the District of Columbia.
1.176“Validation Materials” has the meaning ascribed to the term “Validation Materials” in the Supply Agreement.
1.177“Voting Stock” has the meaning set forth in the definition of “Change of Control”.
21
2.1Purchase and Sale of Assets; Consideration. Pursuant to the terms and subject to the conditions of this Agreement, the Side Letter Agreement and the Supply Agreement, at the Closing, Arena, Arena US and Arena GmbH shall sell, convey, deliver, transfer and assign to Eisai or Eisai’s designee, free and clear of all Liens (other than Permitted Liens), and Eisai shall purchase, take delivery of and acquire from Arena, Arena US and Arena GmbH, all of Arena’s, Arena US’s and Arena GmbH’s right, title and interest in, to and under all of the Purchased Assets. In consideration of the sale, conveyance, delivery, transfer, and assignment of the Purchased Assets to Eisai, the license of the Arena Licensed IP, the Arena Licensed Records, the Arena Licensed Manufacturing Know-How and the Arena Licensed Supply Records and Arena’s, Arena US’s and Arena GmbH’s other covenants and obligations hereunder and under the Side Letter Agreement and Supply Agreement, pursuant to the terms and subject to the conditions hereof and thereof, Eisai shall make the payments specified in Article 8.
2.2Purchased Assets.
(a)The term “Purchased Assets” means:
(i)All of Arena’s and Arena US’s right, title and interest in, to and under the following:
|
|
(A) |
the Purchased Patents; |
(ii)All of Arena’s right, title and interest in, to and under the following:
|
|
(A) |
all Arena Regulatory Approvals; |
|
|
(B) |
all Samples; |
|
|
(C) |
all Purchased Records; and |
|
|
(D) |
all Purchased Know-How; and |
(iii)All of Arena GmbH’s right, title and interest in, to and under the following:
|
|
(A) |
all Purchased Manufacturing Know-How; |
|
|
(B) |
all Purchased Trademarks; |
|
|
(C) |
all Purchased Supply Records; |
|
|
(D) |
all rights in and to the Third Party Distributor Agreements, including all rights to assert claims and take other actions in respect of breaches or other violations thereof on or after the |
22
|
|
Effective Date (but for clarity excluding any rights to enforce (1) rights to indemnification from a Third Party Distributor relating to matters occurring in the period before the Effective Date, including in respect of claims that arise after the Effective Date with respect to such matters, or (2) breaches of the indemnification obligations of a Third Party Distributor relating to matters occurring in the period before the Effective Date, including in respect of claims that arise after the Effective Date with respect to such breaches); |
|
|
(E) |
all Purchased Validation Materials; and |
|
|
(F) |
the exclusive ownership of and all rights to (including the right to use) and in the Product Domain Names. |
(b)Eisai shall perform a good faith assessment of the Purchased Trademarks within 90 days after the Effective Date to determine which of such Trademarks Eisai is reasonably likely to use in connection with the Commercialization of Products. Promptly following such 90-day period, Eisai shall assign to Arena or its designee all of Eisai’s and its Affiliates’ right, title and interest in and to all of the Purchased Trademarks that Eisai determines during such 90-day period it is not reasonably likely to use in connection with the Commercialization of Products, and upon such assignment, such Trademarks shall cease to be Purchased Trademarks. Eisai shall be solely responsible for all of its own costs, and shall reimburse all reasonable, documented out-of-pocket costs incurred by Arena or its Affiliates, to effect the assignment of any such Trademarks to or from Arena or its Affiliates, within 30 days after receipt of each invoice from Arena for such costs.
(c)Eisai shall not acquire from Arena or any of its Affiliates pursuant to this Agreement, the Side Letter, and any Related Document or the Supply Agreement any assets of Arena or its Affiliates that are not specifically included in the Purchased Assets.
2.3Assumed Liabilities; Eisai Not Successor to Arena; Excluded Liabilities.
(a)Pursuant to the terms and subject to the conditions of this Agreement, the Supply Agreement and the Consents, at the Closing, Arena GmbH shall sell, convey, transfer and assign to Eisai, and Eisai shall assume from Arena GmbH, only the Assumed Liabilities. “Assumed Liabilities” means all liabilities, obligations and commitments under the Third Party Distributor Agreements accruing with respect to the period commencing on the Effective Date (excluding, however, any liability or obligation under any Third Party Distributor Agreement arising from or relating to the performance or non-performance by Arena or any of its Affiliates of any such Third Party Distributor Agreement prior to the Effective Date).
(b)Eisai shall not be the successor to Arena or its Affiliates, and Eisai expressly does not assume any liabilities, obligations or commitments of Arena or its Affiliates (other than Assumed Liabilities), whether accrued or fixed, absolute or contingent, known or unknown, determined or determinable, or otherwise (and whether due or to become due) (the
23
“Excluded Liabilities”). The preceding sentence shall not be construed to, and is not intended to, limit or otherwise affect Eisai’s indemnification obligations under Article 12.
Article 3 CLOSING DELIVERABLES
3.1Closing Deliverables of Arena. At the Closing, Arena shall deliver or caused to be delivered to Eisai:
(a)all Consents, each duly executed by Arena GmbH and the applicable Third Party Distributor;
(b)Bills of Sale, substantially in the form set forth in Exhibit B, duly executed by each of Arena, Arena US and Arena GmbH;
(c)the Side Letter Agreement, duly executed by Arena US;
(d)the Supply Agreement, duly executed by Arena GmbH; and
(e)assignments for the registered Purchased Intellectual Property substantially in the form set forth in Exhibit D, which shall be recordable in all jurisdictions in which such registrations have been made or such applications have been filed, including assignments with respect to the Product Domain Names.
3.2Closing Deliverables of Eisai. At the Closing, Eisai shall deliver or caused to be delivered to Arena:
(a)All Consents, each duly executed by Eisai;
24
(b)the Supply Agreement, duly executed by Eisai; and
(c)the Side Letter Agreement, duly executed by Eisai.
4.1Exclusive License for Products.
(a)Subject to the occurrence of the Closing and the other terms and conditions of this Agreement, Arena hereby grants to Eisai during the Term an exclusive (even as to Arena except as provided in Section 4.5), royalty-bearing license, with the right to grant Sublicenses and to appoint Co-Promotion Partners and Distributors through multiple tiers as provided in Section 4.3, under Arena’s rights in the Arena Licensed IP and Arena Licensed Records to develop, make, have made, use, import, offer for sale, sell and otherwise Commercialize Products in the Territory. Eisai shall have the exclusive right in the Territory during the Term to invoice and book all sales of Products. For clarity, Eisai may exercise any or all of its rights under this Section 4.1 through any Eisai Related Party. The rights granted in this Section 4.1 and other provisions of this Agreement to the extent applicable to the territory of any Third Party Distributor Agreement are subject to the rights and obligations set forth in the Third Party Distributor Agreements, as may be amended from time to time. Arena shall reasonably cooperate with Eisai to identify Arena Licensed Records which Eisai may need access to at any time during the Term. As soon as reasonably possible following request by Eisai, Arena shall provide to Eisai a copy of any Arena Licensed Records so requested by Eisai; provided, that Arena may redact any information therein not related to the Compound or any Product, the
25
Inventory or the Purchased Assets; and provided, further, that, Eisai shall reimburse Arena for Arena’s reasonable and documented out-of-pocket costs to provide such copies.
(b)Subject to confidentiality or other obligations owed by Arena or any of its Affiliates to a Third Party, Arena shall provide Eisai with copies of any and all agreements between Arena or any of its Affiliates and any Third Party pursuant to which Arena or any of its Affiliates Controls any Arena Licensed IP that is the subject of the license granted by Arena to Eisai pursuant to Section 4.1(a) or any Arena Licensed Manufacturing Know-How that is the subject of the licenses granted by Arena GmbH to Eisai pursuant to the Supply Agreement (“Arena Third Party Agreements”). Subject to the foregoing, (x) Eisai shall be responsible for (1) making any payments (including royalties, milestones and other amounts) payable by Arena or any of its Affiliates to any Third Parties under any such Arena Third Party Agreements owing as a result of the grant to Eisai of such license, or the exercise of such license by Eisai or any of its Affiliates or sublicensees, by making such payments directly to Arena or its applicable Affiliate, which payments shall be made in sufficient time to enable Arena or its applicable Affiliate to comply with its obligations to such Third Party and (2) complying with any other obligations included in the Arena Third Party Agreements that are applicable to the grant to Eisai of such license, or the exercise of such license by Eisai or any of its Affiliates or sublicensees, and (y) Arena shall be responsible for paying or providing to any such Third Party any payments or reports made or provided by Eisai under this Section 4.1(b). Notwithstanding the foregoing, upon written notice to Arena, Eisai, may, at any time and in its sole discretion, reject its rights under Section 4.1(a) to all Arena Licensed IP or its rights under the license granted by Arena GmbH in the Supply Agreement to all Arena Licensed Manufacturing Know-How, as applicable, that are the subject of an Arena Third Party Agreement, upon which rejection any such Know-How and Patents shall not be included as Arena Licensed IP for the purposes of Section 4.1(a) or as Arena Licensed Manufacturing Know-How for the purposes of the license granted by Arena GmbH in the Supply Agreement, as applicable, and Eisai shall have no further obligations to Arena with respect to such Arena Third Party Agreement (except for any amounts accrued prior to such notice).
(c)Arena shall promptly disclose to Eisai all Arena Licensed Patents Controlled by Arena that become included in the scope of the license granted to Eisai in Section 4.1(a) after the Effective Date.
4.3Sublicense Rights; Co-Promotion Partners and Distributors.
(a)Co-Promotion Partners, Sublicensees and Distributors. (i) Eisai shall have the right to appoint one or more Third Parties as Co-Promotion Partners to co-promote or co-market Products with Eisai in the Territory, to grant one or more Sublicenses in the Territory, or to appoint one or more Third Parties as Distributors to market, promote, sell and distribute the Products on Eisai’s behalf in the Territory; and (ii) each such Co-Promotion Partner shall have the right to appoint additional Co-Promotion Partners, each such Distributor shall have the right to appoint additional Distributors, and each such Sublicensee or Affiliate shall have the right to grant further Sublicenses, in each case (i) and (ii), as and to the extent set forth below in this Section 4.3(a). Any such Third Party described in this Section 4.3(a) shall be a “subcontractor” of Eisai for which Eisai shall be responsible as provided in Section 15.5(b).
26
(i)In the U.S. Neither Eisai nor any Eisai Related Party or Co-Promotion Partner shall grant a Sublicense in the United States or appoint a Distributor or Co-Promotion Partner in the United States during the first […***…] months after the Effective Date without Arena’s prior written consent, which consent Arena may grant or withhold in its sole discretion. After such […***…]-month period, Eisai shall have the right to appoint a Third Party as a Co-Promotion Partner in the United States, to grant a Sublicense in the United States, and to appoint one or more Third Parties as Distributors in the United States (which may include development work on a Product in the United States), without Arena’s prior written consent but on at least ten Business Days prior written notice to Arena. In addition, after such […***…]-month period, any Co-Promotion Partner shall have the right to appoint a Third Party as a Co-Promotion Partner in the United States, any Distributor shall have the right to appoint a Third Party as a Distributor in the United States, and any Sublicensee may grant further Sublicenses in the United States, in each case on at least ten Business Days prior written notice to Arena. During the first […***…] months after the Effective Date Eisai shall notify Arena if it or its Affiliate or any Distributor or Co-Promotion Partner desires to appoint any such Distributor or Co-Promotion Partner, or if it or any Affiliate or Sublicensee desires to grant any such Sublicense, and upon such notice the Parties shall discuss in good faith the qualifications of such proposed Co-Promotion Partner, Sublicensee or Distributor and whether and under what conditions Arena would grant the right to use such Third Party to co-promote or co-market Products in the United States, to grant a Sublicense to such Third Party or to appoint such Third Party as a Distributor.
(ii)Outside the U.S. Eisai shall have the right to appoint a Third Party as a Co-Promotion Partner outside the United States, to grant a Sublicense in any country in the Territory outside the United States, and to appoint one or more Third Parties as Distributors in any country in the Territory outside the United States (which may include development work on a Product in such country), without Arena’s prior written consent but on at least three Business Days prior written notice to Arena. In addition, any Co-Promotion Partner shall have the right to appoint a Third Party as a Co-Promotion Partner outside the United States, any Distributor shall have the right to appoint a Third Party as a Distributor outside the United States, and any Sublicensee may grant further Sublicenses outside the United States, in each case on at least three Business Days prior written notice to Arena.
(b)Assignment of Know-How. After the Closing, Eisai shall use Commercially Reasonable Efforts to cause each Co-Promotion Partner, Sublicensee or Distributor, and each Third Party manufacturing Compounds, Related Compounds or Products on behalf of Eisai or any Eisai Related Party, to assign (or license, if assignment cannot be achieved) to Eisai any and all Know-How discovered, identified, conceived, reduced to practice or otherwise made by such Co-Promotion Partner, Sublicensee, Distributor or other Third Party in the course of or as a result of or related to any development, manufacture or Commercialization activities with respect to Products and Patents claiming or covering such Know-How.
(c)Third Party Agreements. Any Sublicense grant by Eisai or any Affiliate or Sublicensee, and each agreement appointing a Distributor or Co-Promotion Partner (i) shall be consistent with the terms and conditions of this Agreement, and (ii) in the case of a Sublicense to an Affiliate, shall automatically terminate if such Person ceases to be an Affiliate of Eisai.
***Confidential Treatment Requested
27
4.5Arena’s Retained Rights. Arena and its Affiliates retain the exclusive right to (a) practice and license the Arena Licensed IP and use the Arena Licensed Records outside the scope of the licenses granted to Eisai under Section 4.1, (b) practice and license the Arena Licensed Manufacturing Know-How and use the Arena Licensed Supply Records outside the scope of the licenses granted to Eisai under the Supply Agreement and (c) use the Arena Licensed Records and Arena Licensed Supply Records in connection with any claim or demand that a Third Party Distributor or Arena or its Affiliate may have against the other that relates to matters under any Third Party Distributor Agreement occurring before the Effective Date.
4.6No Implied Licenses. Except as expressly set forth herein (including the license of the Arena Licensed IP and the Arena Licensed Records and the acquisition of the Purchased Intellectual Property), neither Party shall acquire any license or other right or interest, by implication or otherwise, under any intellectual property of the other Party. Each Party shall not, and shall not permit any of its Affiliates or sublicensees to, practice any Patents or Know-How licensed to it by the other Party outside the scope of the licenses granted to it under this Agreement.
(a)Mutual Covenant. Each Party shall not, and shall cause its Affiliates and (as to Eisai) Eisai Related Parties and Co-Promotion Partners not to, file an NDA, a BLA or any equivalent thereof for, market, promote, detail, offer for sale, sell or distribute, or conduct other similar activities related to the commercial sale of, a Competing Product in an applicable country in the Territory during the period commencing on the Original Effective Date and ending 12 years after the First Commercial Sale of the first Product in such country (the “Non-Compete Period” for such country).
(b)Arena Exception. Notwithstanding Section 4.7(a), Arena shall not be in breach of Section 4.7(a) by virtue of any Person filing an NDA, a BLA or any equivalent thereof for, marketing, promoting, detailing, offering for sale, selling or distributing, or conducting other similar activities related to the commercial sale of, any Competing Product in an applicable country in the Territory (a “Competing Program” in such country), which Person becomes an Affiliate of Arena through a Change of Control of Arena during the Non-Compete Period for such country; provided, that Arena notifies Eisai in writing promptly after the closing of such Change of Control of Arena.
(c)Eisai Exception. Notwithstanding Section 4.7(a), if Eisai would violate the provisions of Section 4.7(a) by virtue of (i) any Person having a Competing Program in an applicable country in the Territory becoming an Affiliate of Eisai during the applicable Non-
28
Compete Period through a Change of Control of Eisai, then Eisai shall, at its election: (A) terminate this Agreement either in its entirety or only with respect to such applicable country(ies) upon 90 days’ notice to Arena (which notice, if given, must be given within 60 days after such Change of Control) or (B) cease entirely, or cause its applicable Affiliate to cease entirely, such Competing Program (whether by a divestiture of such Competing Program in a transaction where Eisai and its Affiliates retain no interest in the divested Competing Program, or otherwise) within six months after such Change of Control; provided, that in any case Eisai or such Affiliate, as the case may be, shall be permitted to file an NDA, a BLA or any equivalent thereof for, market, promote, detail, offer for sale, sell or distribute, and conduct other similar activities related to the commercial sale of, the applicable Competing Product in such applicable country during such six-month period; and provided, further, that Eisai’s obligations under Article 6 with respect to such applicable country(ies) shall remain in effect during such six-month period, or (ii) (A) any Person having a Competing Program in an applicable country in the Territory becoming an Affiliate of Eisai during the applicable Non-Compete Period through an acquisition of such Person by Eisai or any of its Affiliates or a merger or consolidation with such Person (including merger by a subsidiary of such Person) by Eisai or any of its Affiliates, which transaction does not result in a Change of Control of Eisai or (B) the acquisition by Eisai or any of its Affiliates of all or substantially all of the assets of a Person having a Competing Program in an applicable country in the Territory, then in each case ((A) and (B)) Eisai shall cease entirely, or cause its applicable Affiliate to cease entirely, such Competing Program (whether by a divestiture of such Competing Program in a transaction where Eisai and its Affiliates retain no interest in the divested Competing Program, or otherwise) within six months after such transaction; provided, that in any case Eisai or such Affiliate, as the case may be, shall be permitted to file an NDA, a BLA or any equivalent thereof for, market, promote, detail, offer for sale, sell or distribute, and conduct other similar activities related to the commercial sale of, the applicable Competing Product in such applicable country during such six-month period; and provided, further, that Eisai’s obligations under Article 6 shall remain in effect during such six-month period, and in each case ((i) and (ii)) Eisai shall not be in breach of Section 4.7(a) if it complies with the terms of this Section 4.7(c). All of the exceptions applicable to Eisai and its Affiliates in this Section 4.7(c) shall also be applicable to each Eisai Related Party and Co-Promotion Partner.
Article 5 PRODUCT DEVELOPMENT AND REGULATORY ACTIVITIES
5.1Overview. Prior to the Effective Date, the Parties have conducted development and regulatory activities with respect to Products in the Territory in accordance with the Existing Agreement. During the Term, subject to the terms and conditions of this Agreement, Eisai shall have the exclusive right and responsibility to plan and implement all research and development of Products throughout the Territory, at its own cost and expense, including (a) conducting, or causing any Eisai Related Party (in the applicable country) to conduct, all regulatory activities, and (b) conducting, or causing any Eisai Related Party (in the applicable country) to conduct, all clinical and other development activities, in each case ((a) and (b)) with respect to each Regulatory Authority in the Territory for each Product in accordance with this Agreement. Eisai shall be solely responsible for all costs and expenses incurred in connection with the development of Products in the Territory under the Existing Agreement or this Agreement from and after the Specified Date.
29
5.2Conduct of Development and Regulatory Activities.
(a)Diligence. Eisai shall, or shall cause the applicable Eisai Related Party (in the applicable country) to (i) conduct all studies related to the Initial Product or the Once-Daily Product required by the FDA as a condition to obtain and maintain Regulatory Approval thereof in the United States (including the FDA Pediatric Studies and the CVOT, but excluding the XXXX Plus Study and the Delay to Onset of Diabetes Study), (ii) conduct the XXXX Plus Study until the earlier of February 1, 2018 and the completion of such study through determination of whether the primary endpoint is achieved, substantially consistent with the current trial objectives as of the Effective Date, (iii) conduct the Delay to Onset of Diabetes Study until the earlier of February 1, 2018 and the completion of such study through determination of whether the primary endpoint is achieved, substantially consistent with the current trial objectives as of the Effective Date and (iv) use Commercially Reasonable Efforts to develop and seek Regulatory Approval for a Product in each of China, Japan and the European Union. Notwithstanding the foregoing, if any Regulatory Authority or independent data safety committee in the Territory requires Eisai to suspend or terminate, or not to commence, the conduct of any study in any of the foregoing clauses (i)-(iii) for safety reasons, then Eisai shall be relieved of its obligation to conduct such study in the applicable regulatory jurisdiction, to the extent and for the duration of such required suspension or termination, but only for so long as Eisai uses Commercially Reasonable Efforts to address any relevant issues raised by the applicable Regulatory Authority or independent data safety committee if it would be commercially reasonable to continue such study after addressing any such issues.
(b)Information Regarding Development Activities. Eisai shall maintain, or cause to be maintained, records of the clinical trials and other development work, in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes, which shall fully and properly reflect all work done and results achieved by or on behalf of Eisai in the performance of such clinical trials and other development work under this Agreement or the Existing Agreement. Eisai shall retain such records during the Term and for at least five years after the Term, or for such longer period as may be required by Applicable Laws.
(c)Additional Development Considerations.
(i)Eisai (or the Eisai Related Party in the applicable country) shall hold the IND and be responsible for executing the Regulatory Strategy for the Products in all countries in the Territory.
(ii)Eisai (or the Eisai Related Party in the applicable country) will be responsible for all NDA and other filings for Regulatory Approval (including deciding whether an NDA shall be filed, subject to Section 5.2(a)) for all Products in all countries in the Territory. Eisai (or the Eisai Related Party in the applicable country) shall hold all NDAs and all Regulatory Approvals for all Products in all countries in the Territory.
(iii)Eisai (or the Eisai Related Party in the applicable country) shall be responsible for obtaining pricing and reimbursement approvals, as applicable, for all Products in all countries in the Territory at its expense.
30
5.3Product Regulatory Activities.
(a)Information Regarding Regulatory Communications. Eisai (or the Eisai Related Party in the applicable country that is responsible for executing the Regulatory Strategy for a Product in a country in the Territory) (and during a particular stage of development, if applicable) shall conduct all regulatory activities for such Product in such country in accordance with such Regulatory Strategy. Eisai shall update Arena following each Calendar Quarter in which there are any changes with respect to Regulatory Strategy and the status of labeling of each Product in any of the Major Markets, and if there are no such changes in any Calendar Year, Eisai shall notify Arena of such fact following such Calendar Year. Arena shall reasonably cooperate with Eisai on a timely basis with respect to all such activities, including responding promptly to all of Eisai’s reasonable requests for information and comments necessary for such regulatory activities.
(b)Regulatory Approvals and Applications. Eisai (or the Eisai Related Party in the applicable country) shall hold in the name of Eisai (or such Eisai Related Party) all applications for Regulatory Approval and all Regulatory Approvals in the Territory, and shall provide Arena copies of all such applications and approvals and all other material correspondence with respect to any Product with Regulatory Authorities in the Territory.
5.4Regulatory Compliance. Eisai shall, or shall cause the applicable Eisai Related Party (in the applicable country) to, conduct all regulatory activities in the Territory in compliance with all Applicable Laws.
5.5Regulatory Cooperation. Arena shall reasonably cooperate, at Eisai’s expense, with any reasonable requests for assistance from Eisai with respect to (i) Eisai’s (or any Eisai Related Party’s) conducting regulatory activities with respect to Products in the Territory, and (ii) maintaining any Regulatory Approval of a Product that is held by Eisai (or any Eisai Related Party), including by:
(a)making its employees, consultants and other staff reasonably available upon reasonable notice during normal business hours to attend meetings with Regulatory Authorities concerning the applicable Products; and
(b)disclosing and making available to Eisai, in a reasonable form as Eisai may reasonably request, all manufacturing and quality control data, chemistry, manufacturing and controls data and other information possessed by Arena or its Affiliates or subcontractors and related to the applicable Product and the manufacturing process therefor as is reasonably necessary or desirable to prepare, file, obtain and maintain any such Regulatory Approval.
31
Eisai shall reimburse Arena for all reasonable, documented out-of-pocket expenses incurred by Arena in providing such cooperation under this Section 5.5 within 30 days of the date of invoice provided by Arena. In addition, during the term of the Supply Agreement prior to a Facility Acquisition, for any activities conducted by Arena under this Section 5.5 in excess of […***…], Eisai will reimburse Arena for its fully-burdened internal costs to conduct such activities, at a rate reasonably determined by Arena in accordance with its customary accounting procedures consistently applied. After the earlier of a Facility Acquisition or expiration of the term of the Supply Agreement, for any activities conducted by Arena under this Section 5.5, Eisai will reimburse Arena at a rate of […***…] per hour of cooperation; provided, that commencing January 1, 2018, such hourly rate shall be adjusted annually, effective January 1 of the applicable Calendar Year, to reflect any year-to-year percentage increase or decrease (as the case may be) in the U.S. Bureau of Labor Statistics Employee Cost Index (“ECI”) (based on the change in the ECI from the most recent index available as of the Effective Date to the most recent index available as of the date of the calculation of such adjusted hourly rate). Eisai shall reimburse such costs within 30 days after the date of invoice therefor provided by Arena.
5.6Development Reports. Eisai shall provide Arena with a report, in reasonable detail and in substantially the form attached hereto as Exhibit E, detailing its and the Eisai Related Parties’ development of each Product and the results of such development at least once per six-month period.
5.7Materials Transfer. Either Party (or its Affiliate) may have provided to the other Party (or its Affiliate) pursuant to the Existing Agreement, the Restated Agreement or the Original Agreement or may provide pursuant to this Agreement to the other Party certain biological materials or chemical compounds (other than Compound or Product) Controlled by the supplying Party (collectively, “Materials”) for use by the other Party in furtherance of clinical trials or other development work contemplated by any such agreement. Except as otherwise provided for under this Agreement, all such Materials delivered to the other Party will remain the sole property of the supplying Party. Except as otherwise provided for under this Agreement, the receiving Party shall: (a) only use such Materials in furtherance of the clinical trials and other development work that were contemplated by the Existing Agreement, the Restated Agreement or the Original Agreement or are contemplated by this Agreement, (b) not use or deliver any Materials to or for the benefit of any Third Party, except for permitted subcontractors, without the prior written consent of the supplying Party, and (c) use the Materials in compliance with all Applicable Laws. The Parties shall use such Materials with prudence and appropriate caution in any experimental work because not all of their characteristics may be known. Except as otherwise expressly set forth in this Agreement, SUCH MATERIALS ARE PROVIDED “AS IS” AND WITHOUT ANY REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR OF FITNESS FOR ANY PARTICULAR PURPOSE OR ANY WARRANTY THAT THE USE OF THE MATERIALS WILL NOT INFRINGE OR VIOLATE ANY PATENT OR OTHER PROPRIETARY RIGHTS OF ANY THIRD PARTY.
***Confidential Treatment Requested
32
5.8Pharmacovigilance. Eisai or the Eisai Related Party in the applicable country shall be responsible, at its own expense, for all required safety reporting with respect to each Product in each country in the Territory. Eisai shall be responsible at its expense for maintaining the global safety database for each Product. Eisai shall be responsible for ensuring compliance by the Eisai Related Parties with respect to pharmacovigilance responsibilities under Applicable Law with respect to each Product in the applicable countries in the Territory.
6.1Commercialization Rights and Responsibility. Commencing upon the Effective Date, Eisai shall be solely responsible, and has the exclusive rights, for Commercializing all of the Products in the Territory, subject to the terms and conditions of this Agreement. In connection with such Commercialization, Eisai shall be solely responsible for manufacturing or having manufactured Products for all uses, subject to the Supply Agreement during the term of the Supply Agreement. Eisai may license, sublicense or subcontract its obligations with respect to Commercializing and manufacturing the Products in the Territory as set forth in Section 4.3.
6.4Commercialization Standards of Conduct. Eisai shall, and shall use Commercially Reasonable Efforts to cause the Eisai Related Parties and Co-Promotion Partners to, in all respects comply with all Applicable Laws in Commercializing the Products in the Territory.
33
recall or withdrawal and shall keep Arena reasonably informed of all actions taken in conducting such recall or withdrawal. During the term of the Supply Agreement prior to a Facility Acquisition, Eisai shall, to the extent practicable, discuss with Arena whether to recall or withdraw such Product in the Territory prior to making such decision; provided, that during the term of the Supply Agreement and thereafter, Eisai shall have the right to decide whether to recall or withdraw such Product in the Territory. Eisai shall bear any and all recall or withdrawal expenses, subject only to Arena GmbH’s obligations pursuant to the Supply Agreement to be responsible for recall expenses and subject to any indemnification obligation of Arena under Article 12 or Arena’s obligations with respect to Product Liability Claims under Section 12.8.
7.1Certain Tax Matters.
(a)Transfer Taxes. All recordation, transfer, documentary, excise, sales, value added, use, stamp, conveyance or other similar Taxes, duties or governmental charges, and all recording or filing fees or similar costs, imposed or levied by reason of, in connection with or attributable to this Agreement, the Supply Agreement and the Related Documents or the transactions contemplated hereby and thereby (collectively, “Transfer Taxes”) shall be borne by Eisai; provided, however, that Eisai and Arena shall reasonably cooperate with one another to lawfully minimize such Taxes. In the case of Transfer Taxes for which Arena is liable to the applicable taxing authority, at the Closing, Eisai shall pay to Arena the amount of such Transfer Taxes as reasonably estimated by Arena, with subsequent additional payments by Eisai to Arena or refunds by Arena to Eisai, as the case may be, of amounts previously paid by Eisai in the event it is subsequently determined that the amount of the subject Transfer Taxes was more or less than the estimated amounts.
(b)Allocation of Taxes. All personal property and similar ad valorem obligations levied with respect to the Purchased Assets for a taxable period that includes (but does not end on) the Effective Date (collectively, the “Apportioned Obligations”) shall be apportioned between Arena (on behalf of itself and Arena US and Arena GmbH) and Eisai based on the number of days of such taxable period during the Pre-Closing Tax Period and the number of days after the Effective Date (such portion of such taxable period, the “Post-Closing Tax Period”). Arena (on behalf of itself and Arena US and Arena GmbH) shall be liable for the proportionate amount of such Apportioned Obligations that is attributable to the Pre-Closing Tax Period, and Eisai shall be liable for the proportionate amount of such Apportioned Obligations that is attributable to the Post-Closing Tax Period.
34
(c)Payment of Taxes. Apportioned Obligations and Transfer Taxes shall be timely paid, and all applicable filings, reports and returns shall be filed, as provided by Applicable Law. The paying Party shall be entitled to reimbursement from the non-paying Party in accordance with Section 7.1(a) or Section 7.1(b), as the case may be. Upon payment of any such Apportioned Obligation or Transfer Tax, the paying Party shall present a statement to the non-paying Party setting forth the amount of reimbursement to which the paying Party is entitled under Section 7.1(a) or Section 7.1(b), as the case may be, together with such supporting evidence as is reasonably necessary to calculate the amount to be reimbursed. The non-paying Party shall make such reimbursement promptly but in no event later than ten days after the presentation of such statement.
(d)Cooperation and Exchange of Information. Each of Arena (on behalf of itself and Arena US and Arena GmbH) and Eisai shall (i) provide the other with such assistance as may reasonably be requested by the other Party in connection with the preparation of any Tax Return, audit or other examination by any taxing authority or Legal Proceedings relating to liability for Taxes in connection with the Purchased Assets, (ii) retain and provide the other with any records or other information that may be relevant to such Tax Return, audit or examination or Legal Proceedings, and (iii) provide the other with any final determination of any such audit or examination, Legal Proceedings or determination that affects any amount required to be shown on any Tax Return of the other for any period.
(e)Survival of Covenants. The covenants contained in this Section 7.1 shall survive until 60 days after the expiration of the applicable statute of limitations (including extensions thereof).
7.2Checks; Remittances and Refunds. After the Closing, if Arena, Arena US or Arena GmbH receives any payment, refund or other amount that is attributable to, results from or is related to a Purchased Asset or is otherwise properly due and owing to Eisai in accordance with the terms of this Agreement, Arena (on behalf of itself or Arena US or Arena GmbH) shall promptly remit, or cause to be remitted, such amount to Eisai. Arena, Arena US or Arena GmbH shall promptly endorse and deliver to Eisai any notes, checks, negotiable instruments, letters of credit or other documents received on account of, attributable to or otherwise relating to the Purchased Assets that are properly due and owing to Eisai in accordance with the terms of this Agreement, and Eisai shall have the right and authority to endorse, without recourse, the name of Arena, Arena US or Arena GmbH on any such instrument or document. After the Closing, if Eisai or any of its Affiliates receives any refund or other amount that is properly due and owing to Arena, Arena US or Arena GmbH in accordance with the terms of this Agreement, Eisai shall promptly remit, or cause to be remitted, such amount to Arena, Arena US or Arena GmbH.
7.3Cooperation in Litigation. Other than with respect to Product Liability Claims (which are governed by Section 12.8) and claims with respect to intellectual property (which are governed by Article 10), from and after the Effective Date, Eisai and Arena shall reasonably cooperate with each other in the defense or prosecution of any Legal Proceedings instituted prior to the Closing or that may be instituted thereafter against or by such Parties relating to or arising out of the conduct of the manufacture, Development and Commercialization of the Products prior to or after the Closing (other than litigation between Eisai and Arena or their respective Affiliates arising out of the transactions contemplated hereby or by the Supply
35
Agreement or the Related Documents). Subject to Article 12, the Party requesting such cooperation shall pay the reasonable and verifiable out-of-pocket costs and expenses of providing such cooperation (including legal fees and disbursements) incurred by the Party providing such cooperation and by its officers, directors, employees and agents, and any applicable Taxes in connection therewith, but shall not be responsible for reimbursing such Party or its officers, directors, managers, employees or agents for their time spent in such cooperation; provided, however, that the amount of such time is reasonable and consistent with such individual’s other obligations.
Article 8 FINANCIAL PROVISIONS
8.1Shared Payment. Arena and Eisai shall each be entitled to […***…] payment to be made by Ildong Pharmaceutical Co., Ltd., net of any applicable withholding taxes, to Arena or its Affiliate. Such payment represents a milestone payment being paid in connection with the addition of BELVIQ XR (lorcaserin HCl extended-release) 20 mg tablets as an Additional Product within the scope of the Marketing and Supply Agreement, dated as of November 6, 2012, by and between Arena GmbH and Ildong Pharmaceutical Co., Ltd., pursuant to Amendment No. Two, dated December 15, 2016, to such agreement. Ildong Pharmaceutical Co., Ltd. is making no other milestone or other payments to Arena or any of its Affiliates in connection with the matters referred to in the immediately previous sentence. Arena or such Affiliate shall pay […***…] of such amount received from Ildong Pharmaceutical Co., Ltd. to Eisai or its designee, within 30 days following Arena’s or such Affiliate’s receipt of such payment from Ildong Pharmaceutical Co., Ltd.; provided that each Party shall advise Ildong Pharmaceutical Co., Ltd. to make such payment to Arena or its Affiliate (rather than Eisai), and if such payment is made to Eisai or its Affiliate, then Eisai shall pay […***…] of the amount it receives to Arena or its designee within 30 days following Eisai’s or such Affiliate’s receipt of such payment from Ildong Pharmaceutical Co., Ltd.
***Confidential Treatment Requested
36
|
Milestone Event |
Milestone Payment |
|
(a) Upon the occurrence of the date that is 15 days after the end of the month in which aggregate Net Sales in the Territory for a Calendar Year first exceed US$250,000,000 |
US$25,000,000 |
|
(b) Upon the earlier of Regulatory Approval or First Commercial Sale of Product in Brazil |
US$1,000,000 |
8.3Royalty Payments for Products.
(a)Royalties. Subject to the other terms of this Section 8.3, Eisai shall make royalty payments to Arena on the Net Sales of all Products sold in the Territory in each Calendar Quarter during the Term as calculated by multiplying the applicable royalty rate set forth below by the corresponding amount of incremental, aggregated Net Sales of all Products sold in the Territory in the applicable Calendar Year; provided, however, that Net Sales shall exclude sales of Existing Agreement Products for which the Product Purchase Price (as defined in the Supply Agreement) is paid pursuant to the Existing Agreement. Subject to Section 12.5, the payments set forth in this Section 8.3 shall not be refundable or creditable against any other payments owed or payable by Eisai to Arena or any of its Affiliates under this Agreement, the Existing Agreement or other written agreement between Arena or any of its Affiliates and Eisai.
|
Annual Net Sale of all Products in the Territory |
Royalty Rate |
|
For that portion of annual Net Sales less than or equal to $175,000,000 |
9.5% |
|
For that portion of annual Net Sales greater than $175,000,000 but less than or equal to $500,000,000 |
13.5% |
|
For that portion of annual Net Sales greater than $500,000,000 |
18.5% |
37
(b)Royalty Reduction. If, during a Calendar Quarter, there have been sales of a Generic Version of a Product in a country and the aggregate units of all Generic Versions of such Product sold in such country in such Calendar Quarter exceed […***…]% of the aggregate units of such Product and all Generic Versions of such Product sold in such country in such Calendar Quarter, then the royalties payable on Net Sales of such Product in such country for such Calendar Quarter will be reduced to […***…]% of the royalties otherwise payable under Section 8.3(a). Such royalty reduction will be calculated by (i) determining the portion of total Net Sales of all Products in the Territory in a Calendar Quarter that is attributable to the Product and country to which the reduction applies, (ii) determining the total royalties payable on Net Sales of all Products in the Territory without applying a reduction, (iii) determining the portion of such total royalties that is attributable to the Product and country to which the reduction applies (based on the Net Sales calculation under clause (i)) and (iv) reducing such portion of total royalties attributable to the applicable Product and country to […***…]% of such portion.
(c)Royalty Reports and Payment. Within 30 days after each Calendar Quarter, Eisai shall provide Arena with a report that contains the following information for the applicable Calendar Quarter, on a Product-by-Product and country-by-country basis (the “Quarterly Report”): (i) the amount of gross sales of the Products, (ii) an itemized calculation of Net Sales in the Territory showing separately each type of deduction provided for in the definition of Net Sales, (iii) a calculation of the royalty payment due, including the application of any reduction made in accordance with Section 8.3(b), and (iv) the exchange rate for such country. Concurrent with the delivery of the applicable Quarterly Report, Eisai shall pay Arena all royalties owed with respect to Net Sales for such Calendar Quarter; provided, that with respect to Net Sales by any Third Party Distributor for such Calendar Quarter, if such Third Party Distributor fails to pay Eisai all amounts payable to Eisai under the applicable Third Party Distributor Agreement with respect to such Net Sales by the due date, and Eisai uses Commercially Reasonable Efforts to enforce such Third Party Distributor Agreement to obtain such payment, then Eisai shall not have any obligation to pay Arena any amount pursuant to this Section 8.3 with respect to such Net Sales unless and until such Third Party Distributor has paid Eisai all amounts payable to Eisai under the applicable Third Party Distributor Agreement with respect to such Net Sales. Eisai will ensure that its agreements with Eisai Related Parties permit the information required under this section to be disclosed to Arena in accordance with the terms of this section, and further permit audit rights for Arena consistent with this Agreement.
(d)Basis for Royalties. The Parties acknowledge and agree that the royalties payable under this Section 8.3 are based on blended royalty rates that reflect combined consideration for the Purchased Assets, the New Program Know-How and New Program Patents and the rights granted under the Arena Licensed IP, Arena Licensed Records, Arena Licensed Manufacturing Know-How and Arena Licensed Supply Records. In establishing this payment structure, the Parties recognize, and Eisai acknowledges, the substantial value of the Purchased Assets, the New Program Know-How and New Program Patents and Arena Licensed IP, Arena Licensed Records, Arena Licensed Manufacturing Know-How and Arena Licensed Supply Records and the Parties agree that as a result the royalties set forth above are appropriate for the duration of the Term.
***Confidential Treatment Requested
38
8.4Currency. All payments to the Payee Party under this Agreement shall be made by bank wire transfer in immediately available funds to an account in the name of the Payee Party designated in writing by the Payee Party. Payments hereunder shall be considered to be made as of the day on which they are received by the Payee Party’s designated bank. Unless otherwise expressly stated in this Agreement, all amounts specified to be payable under this Agreement are in United States Dollars and shall be paid in United States Dollars.
8.5Taxes. The milestone payments, royalty payments and other amounts payable by one Party (the “Paying Party”) to the other Party (the “Payee Party”) pursuant to this Agreement (each, a “Payment”) shall not be reduced on account of any taxes except to the extent of amounts required to be withheld by the Paying Party by Applicable Laws, if any. The Payee Party alone shall be responsible for paying any and all taxes (other than withholding taxes required by Applicable Laws to be withheld from Payments and remitted by the Paying Party) levied on account of, or measured in whole or in part by reference to, any Payments it receives. Without limiting the above, the Paying Party shall not withhold from the Payments any taxes except to the extent that it is required to do so by Applicable Laws. Notwithstanding the foregoing, if the Payee Party is entitled under any applicable tax treaty to a reduction of rate of, or the elimination of, the applicable withholding tax, it may deliver to the Paying Party or the appropriate governmental authority (with the assistance of the Paying Party to the extent that this is reasonably required and is expressly requested in writing) the prescribed forms necessary to reduce the applicable rate of withholding or to relieve the Paying Party of its obligation to withhold tax, and the Paying Party shall apply the reduced rate of withholding, or dispense with withholding, as the case may be; provided, that the Paying Party has received evidence, in a form reasonably satisfactory to the Paying Party, of the Payee Party’s delivery of all applicable forms (and, if necessary, its receipt of appropriate governmental authorization) at least 15 days prior to the time that the applicable Payment is due. If, in accordance with the foregoing, the Paying Party withholds any amount, it shall pay to the Payee Party the balance when due, make timely payment to the proper taxing authority of the withheld amount and send to the Payee Party proof of such payment within 10 days following such payment. In the event any taxes are withheld on any Payment, the Paying Party shall promptly pay the Payee Party an amount equal to […***…]% of the withheld amount (less any additional required withholding). If and to the extent that a Payee Party reasonably believes (in good faith) that (a) it has actually realized a reduction in its current liability as a result of tax withholdings on any Payment and, as a result, it has actually paid a lesser amount to a tax authority or (b) it has actually received a refund of any taxes withheld on any Payment, the Payee Party shall promptly pay to the Paying Party an amount equal to the lesser of (i) […***…]% of such lesser amount paid as a result of the reduction in current tax liability or refund and (ii) the amount paid by the Paying Party to the Payee Party with respect to such taxes withheld on the applicable Payment pursuant to the preceding sentence. Notwithstanding the foregoing, (A) if as a result of any action by Arena or any of its Affiliates, including any assignment, sublicense, change of place of incorporation or failure to comply with Applicable Laws or filing or record retention requirements, a higher percentage is required to be withheld on Payments to Arena or its successor or assign than would have been withheld without such action, Eisai shall have no obligation to pay to Arena or its successor or assign any amounts in respect of withheld amounts above those which would have been withheld had such action not been taken and (B) if as a result of any action by Eisai or any of its Affiliates, including any assignment, sublicense, change of place of incorporation or failure to comply with Applicable Laws or filing or record retention requirements, a higher percentage is withheld on Payments to Arena than would have been withheld without such action, Eisai shall pay to Arena any withheld amounts above those which would have been withheld had such action not been taken.
***Confidential Treatment Requested
39
(a)Eisai. Eisai shall keep, and cause the Eisai Related Parties to keep, complete, true and accurate books of accounts and records for the purpose of determining the amounts payable to Arena pursuant to Section 8.2 and 8.3. Such books and records shall be kept for such period of time required by Applicable Laws, but no less than at least five years following the end of the Calendar Quarter to which they pertain. Eisai shall also keep, and cause its Affiliates and Distributors to keep, complete, true and accurate books of accounts and records for the purpose of determining the amounts payable to Arena under the Existing Agreement during the five-year period ending with the Specified Date (“Existing Agreement Audit Period”). Such records shall be subject to inspection in accordance with Section 8.7.
(b)Arena. Arena shall keep, and cause its Affiliates to keep, complete, true and accurate books of accounts and records for the purpose of determining the amounts payable to Eisai or payable by Eisai under the Existing Agreement during the Existing Agreement Audit Period, including the Finished Product COGS (as defined in the Existing Agreement). Such records shall be subject to inspection in accordance with Section 8.7.
(a)Audit of Eisai. Upon not less than 60 days’ prior written notice, Eisai shall permit an independent, certified public accountant of international recognition (for the purposes of this Section 8.7, the “Auditor”) selected by Arena and reasonably acceptable to Eisai, which acceptance shall not be unreasonably conditioned, withheld or delayed, to audit or inspect those books and records of Eisai and the Eisai Related Parties that relate to (i) Net Sales for the sole purpose of verifying the royalty payments made to Arena, and (ii) payments made by Eisai to Arena GmbH pursuant to the Existing Agreement during the Existing Agreement Audit Period for the sole purpose of verifying such payments made to Arena GmbH.
(b)Audit of Arena. Upon not less than 60 days’ prior written notice, Arena shall permit an Auditor selected by Eisai and reasonably acceptable to Arena, which acceptance shall not be unreasonably conditioned, withheld or delayed, to audit or inspect those books or records of Arena and its Affiliates that relate to (i) Finished Product COGS for the sole purpose of verifying the amounts invoiced by Arena GmbH pursuant to the Existing Agreement and (ii) payments made by Arena GmbH pursuant to the Existing Agreement during the Existing Agreement Audit Period, for the sole purpose of verifying such payments made to Eisai.
(c)Audit Procedures. The audited Party shall not be obligated to provide the Auditor any records until the Auditor executes a confidentiality agreement in a form reasonably acceptable to the audited party. The Auditor shall disclose to the auditing Party only whether any reports made or amounts invoiced under this Agreement or the Supply Agreement or, as applicable, the Existing Agreement are correct and details concerning any discrepancies. The Auditor shall send a copy of the report to the other Party at the same time it is sent to the auditing Party. Such audits or inspections may be made no more than once each Calendar Year (unless an audit or inspection reveals a material inaccuracy in reports made or amounts invoiced under this Agreement or, as applicable, the Existing Agreement, in which case it may be repeated within such Calendar Year), during normal business hours. If such report shows that the amounts paid by a Party for the period audited are less than the amounts actually payable by such
40
Party to the other Party during the period audited, then (absent manifest error or fraud in such audit report) the underpaying Party shall pay to the other Party the amount of such underpayment plus interest under Section 8.8, from the date such amounts were originally owed until payment is made, within 30 days of receipt of such audit. If such report shows that the amounts paid by a Party for the period audited exceed the amounts actually owed by such Party to the other Party for the period audited, then (absent manifest error or fraud in such audit report) the overpaying Party shall deliver to the other Party an invoice for such excess amount, and the other Party shall pay such invoiced excess amount within 30 days of receipt of such invoice. Such records for any particular Calendar Quarter shall be subject to no more than one audit or inspection and no audit or inspection with respect to any Calendar Quarter may be initiated later than five years after the end of such Calendar Quarter. Audits and inspections conducted under this Section 8.7 shall be at the expense of the auditing Party, unless a variation or error producing (i) with respect to an audit or inspection pursuant to subsection (a), an underpayment in amounts payable exceeding an amount equal to 5% of the amount paid for a period covered by the audit or inspection is established, in which case all reasonable and verifiable costs relating to the audit or inspection for such period and any unpaid amounts that are discovered shall be paid by Eisai and (ii) with respect to an audit or inspection pursuant to subsection (b), an overpayment in amounts payable by Eisai or an underpayment in amounts payable by Arena pursuant to the Existing Agreement during the Existing Agreement Audit Period exceeding an amount equal to 5% of the amount paid for a period covered by the audit or inspection is established, in which case all reasonable and verifiable costs relating to the audit or inspection for such period and any unpaid amounts that are discovered shall be paid by Arena. The auditing Party shall endeavor in such audit not to unreasonably disrupt the normal business activities of the audited party.
8.8Payment Due Dates; Late Payments. If any Payment is due on a day when banks in New York, New York are generally closed, then such Payment shall not be considered late if made on the next day on which such banks are generally open. In the event that any Payment due under this Agreement is not made when due, such Payment shall accrue interest from the date due at a rate per annum equal to 4% above the U.S. Prime Rate (as set forth in The Wall Street Journal, Eastern Edition) for the date on which payment was originally due until the date such Payment plus accrued interest hereunder is actually made, calculated daily on the basis of a 365-day year, or similar reputable data source; provided, that in no event shall such rate exceed the maximum legal annual interest rate. The payment of such interest shall not limit the Party entitled to receive such payment from exercising any other rights it may have as a consequence of the lateness of any Payment.
41
Article 9 CONFIDENTIALITY; STANDSTILL
9.1Confidential Information. Except to the extent expressly authorized by this Agreement or the Supply Agreement or otherwise agreed in writing by the Parties, the Parties agree that the receiving Party (the “Receiving Party”) shall keep confidential and not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement or the Supply Agreement any Know-How, information or materials, patentable or otherwise, in any form (written, oral, photographic, electronic, magnetic, or otherwise) that is disclosed to it by the other Party (the “Disclosing Party”) pursuant to this Agreement or the Supply Agreement including all information concerning the Initial Product, the Once-Daily Product or any other Product or any Compound or Related Compound and any other technical or business information of whatever nature concerning the Disclosing Party or its technology or business and all Confidential Information (as defined in the Original Agreement, the Restated Agreement or Existing Agreement) (collectively, “Confidential Information” of the Disclosing Party), except that the Receiving Party may disclose Confidential Information of the Disclosing Party to its Affiliates and its and its Affiliates’ respective officers, directors, employees, agents, subcontractors (including, in the case of Eisai, Eisai Related Parties and Co-Promotion Partners) and consultants with a need to know such Confidential Information to assist the Receiving Party with the activities contemplated or required of it by this Agreement or the Supply Agreement (and who shall be advised of the Receiving Party’s obligations hereunder and who are bound by confidentiality obligations with respect to such Confidential Information no less onerous than those set forth in this Agreement) (each, a “Recipient”). For the purposes of this Article 9, the term “Disclosing Party” shall include each Party and its Affiliates and its and their respective officers, directors, employees, agents, subcontractors and consultants who are directed to disclose such Party’s or its Affiliate’s Confidential Information, and the term “Receiving Party” shall include each Party and its Affiliates. For clarity, all Program Know-How (as defined in the Existing Agreement) (except any Program Know-How included in the Purchased Assets) is deemed to be the Confidential Information of Arena and shall be deemed to have been disclosed by Arena to Eisai for purposes of Section 9.2. It is understood and agreed that with respect to any Confidential Information included in the Purchased Assets, from and following the Effective Date, Eisai shall be the Disclosing Party with respect to any such Confidential Information. Notwithstanding the foregoing, the Parties acknowledge the practical difficulty of policing the use of information in the unaided memory of the Receiving Party or its Recipients, and as such each Party agrees that the Receiving Party shall not be liable for the use by any of its Recipients of specific Confidential Information of the Disclosing Party that is retained in the unaided memory of such Recipient; provided, that (a) such Recipient is not aware that such Confidential Information is the confidential information of Disclosing Party at the time of such use; (b) the foregoing is not intended to grant, and shall not be deemed to grant, the Receiving Party, its Affiliates, or its Recipients (i) a right to disclose the Disclosing Party’s Confidential Information or (ii) a license under any Patents or other intellectual property right of the Disclosing Party; and (c) such Recipient has not intentionally memorized such Confidential Information for use outside this Agreement.
42
9.2Exceptions. Notwithstanding Section 9.1, Confidential Information shall not include any Know-How, information or materials that, in each case as demonstrated by competent evidence:
(a)was already known to the Receiving Party or any of its Recipients, other than under an obligation of confidentiality, at the time of disclosure;
(b)was generally available to the public or was otherwise part of the public domain at the time of its disclosure to the Receiving Party;
(c)became generally available to the public or otherwise part of the public domain after its disclosure by the Disclosing Party and other than through any act or omission of the Receiving Party or any of its Recipients in breach of this Agreement or the Existing Agreement;
(d)was subsequently lawfully disclosed to the Receiving Party or any of its Recipients by a Person other than the Disclosing Party, and who, to the knowledge of the Receiving Party or such Recipient, did not directly or indirectly receive such information from the Disclosing Party or any of its Affiliates under an obligation of confidence; or
(e)was developed by the Receiving Party or any of its Recipients without use of or reference to any information or materials disclosed by the Disclosing Party.
Information specific to the use of certain compounds, methods, conditions or features shall not be deemed to be within the foregoing exceptions merely because such information is embraced by general disclosures in the public domain or in the possession of the Receiving Party or its Recipients. In addition, a combination of information will not be deemed to fall within the foregoing exceptions, even if all of the components fall within an exception, unless the combination itself and its significance are in the public domain or in the possession of the Receiving Party prior to the disclosures hereunder. Notwithstanding anything to the contrary herein, neither the act of using information in a clinical trial nor the filing of information with a governmental authority shall, for the purpose of this Article 9, in and of itself be deemed to place such information in the public domain.
9.3Permitted Disclosures. Notwithstanding the provisions of Section 9.1, the Receiving Party may disclose Confidential Information of the Disclosing Party, as expressly permitted by this Agreement or the Supply Agreement or if and to the extent such disclosure is reasonably necessary or useful in the following instances:
(a)the performance by the Receiving Party of its obligations or exercise of its rights as contemplated by this Agreement or the Supply Agreement; provided, that wherever reasonable and practicable in the circumstances the recipient of any such Confidential Information shall be subject to obligations of confidentiality and non-use with respect to such Confidential Information substantially similar to the obligations of confidentiality and non-use of the Receiving Party pursuant to this Article 9;
(b)filing or prosecuting Patents as permitted by this Agreement;
43
(c)seeking, obtaining or maintaining any Regulatory Approval as permitted by this Agreement; provided, that the Receiving Party shall take reasonable measures to assure confidential treatment of such Confidential Information, to the extent such treatment is available;
(d)prosecuting or defending litigation with respect to a Party or its Affiliates, and with respect to Eisai, Eisai Related Parties and Co-Promotion Partners, as permitted by this Agreement;
(e)complying with Applicable Laws; and
(f)disclosure to Third Parties in connection with due diligence or similar investigations by or on behalf of a Third Party in connection with a potential marketing, distribution or supply agreement with, or license to, or collaboration with such Third Party (including as to Eisai, a potential Distributor or Sublicensee, and as to Arena, a potential sublicensee under the licenses granted to Arena under Section 4.4) or a potential merger or acquisition by such Third Party, or in connection with performance of any such license, collaboration or merger agreement, and disclosure to potential Third Party investors in confidential financing documents; provided, in each case, that any such Third Party agrees to be bound by obligations of confidentiality and non-use substantially similar to the obligations of confidentiality and non-use of the Receiving Party pursuant to this Article 9.
Notwithstanding the foregoing, in the event the Receiving Party or a Recipient is required to make a disclosure of the Disclosing Party’s Confidential Information pursuant to Section 9.3(d) or (e) to comply with a subpoena or other legal order, it shall, except where impracticable, give reasonable advance notice to the Disclosing Party of such disclosure and give the Disclosing Party a reasonable opportunity to quash such subpoena or order and to obtain a protective order requiring that the Confidential Information and documents that are the subject of such subpoena or order be held in confidence by such court or agency or, if disclosed, be used only for the purposes for which such subpoena or order was issued; and provided, further, that if such subpoena or order is not quashed or a protective order is not obtained, the Confidential Information disclosed in response to such subpoena or order shall be limited to the Disclosing Party’s Confidential Information that is legally required to be disclosed in response to such subpoena or order and shall still be subject to the restrictions on use set forth in this Article 9.
9.4Confidentiality of Agreements and Their Terms. Except as otherwise provided in this Article 9, each Party agrees not to disclose to any Third Party the existence of this Agreement, the Supply Agreement, the Original Agreement, the Restated Agreement or the Existing Agreement or the terms and conditions of any such agreement without the prior written consent of the other Party, except that each Party may disclose the terms and conditions of any such agreement that are not otherwise made public as contemplated by Section 9.5 or were not otherwise made public in accordance with the Original Agreement, the Restated Agreement or the Existing Agreement, as permitted under Section 9.3.
(a)As soon as practicable following the Effective Date, each Party shall issue a mutually agreed to press release announcing the entry into this Agreement and the Supply
44
(b)Prior to the Effective Date, redacted versions of the Original Agreement, Restated Agreement and Existing Agreement were filed with the SEC. The Parties shall coordinate in advance with each other in connection with the filing of this Agreement and the Supply Agreement (including, if applicable, redaction of certain provisions of this Agreement and the Supply Agreement) with the SEC or any other governmental agency, the NASDAQ stock exchange or any other stock exchange on which securities issued by a Party or any of its Affiliates are traded, and each Party shall use reasonable efforts to seek confidential treatment for the terms reasonably requested by the other Party to be redacted; provided, that each Party shall ultimately retain control over what information to disclose to the SEC or any other governmental agency, the NASDAQ stock exchange or any other stock exchange, as the case may be, and nothing in this Agreement shall prevent a Party from taking all actions it reasonably considers necessary to comply with Applicable Laws with respect to any such filings or disclosures; and provided, further, that the Parties shall use their reasonable efforts to file redacted versions with any governing bodies that are consistent with redacted versions previously filed with any other governing bodies. Except as provided in the preceding sentence, neither Party nor any of their respective Affiliates shall be obligated to consult with or obtain approval from the other Party with respect to any filings to the SEC, the NASDAQ stock exchange or any other stock exchange or governmental agency.
9.6Use of Name. Neither Party shall use the name, insignia, symbol, Trademark, trade name or logotype of the other Party (or any abbreviation or adaptation thereof) in any publication, press release or marketing and promotional material or other form of publicity without the prior written approval of such other Party in each instance, which approval shall not be unreasonably conditioned, withheld or delayed, or except as expressly permitted in this Agreement or the Supply Agreement. The restrictions imposed by this Section 9.6 shall not prohibit either Party from making any disclosure (a) identifying the other Party or its Affiliate as a counterparty to this Agreement and the Supply Agreement to its investors, (b) that is required by Applicable Laws or the requirements of a national securities exchange or another similar regulatory body (provided, that any such disclosure shall be governed by this Article 9), (c) that is necessary for the performance by Eisai or Arena or Arena GmbH of its obligations or exercise of its rights as contemplated by this Agreement or the Supply Agreement or (d) with respect to which written consent has previously been obtained. Further, the restrictions imposed on each
45
Party under this Section 9.6 are not intended, and shall not be construed, to prohibit a Party from identifying the other Party in its internal business communications; provided, that any Confidential Information in such communications remains subject to this Article 9.
9.7Publication of Information Related to the Products. As between the Parties, Eisai shall have the sole right to publicly present or submit for written or oral publication a manuscript, abstract or the like that includes information relating to any Compound, Related Compound or Product (without Arena’s review or consent). The contribution of each Party shall be noted in all publications or presentations by acknowledgment or co-authorship, as appropriate.
(a)Certain Restrictions on Eisai. During the Term and for two years thereafter, except with the written consent of Arena or Arena US (which may be withheld by Arena or Arena US at the sole discretion of its board of directors) or by way of stock dividends or other distributions made to Arena’s or any of its Affiliates’ stockholders generally, neither Eisai nor any of its Affiliates shall, in any manner, directly or indirectly: (i) make, effect, initiate, cause or participate in (A) any acquisition of beneficial ownership of any securities of Arena or any securities of any Affiliate of Arena (in excess of 5% of the total outstanding securities of Arena or any such Affiliate of Arena at the time of any such acquisition), (B) other than purchase of any Purchased Assets and the Products under the Supply Agreement, any acquisition of any material assets of Arena or any material assets of any Affiliate of Arena, (C) any tender offer, exchange offer, merger, business combination, recapitalization, reorganization, restructuring, liquidation, dissolution or extraordinary transaction involving Arena or any Affiliate of Arena, or involving any securities of Arena or any securities of any Affiliate of Arena, or (D) any “solicitation” of “proxies” (as those terms are used in Regulation 14A of the Exchange Act) or consents with respect to any securities of Arena or any Affiliate of Arena; (ii) form, join or participate in a “group” (as defined in the Exchange Act and the rules promulgated thereunder) with respect to the beneficial ownership of any securities of Arena or any Affiliate of Arena in excess of the amounts permitted under subclause (i)(A); (iii) act, alone or in concert with others, to seek to control the management, board of directors or policies of Arena or any Affiliate of Arena; (iv) take any action that could reasonably be expected to require Arena or any Affiliate of Arena to make a public announcement regarding any of the types of matters set forth in clause “(i)” of this sentence; (v) agree or offer to take, or knowingly encourage or propose (publicly or otherwise) the taking of, any action referred to in clause “(i)”, “(ii)”, “(iii)” or “(iv)” of this sentence; (vi) induce or knowingly encourage any other person or entity to take any action of the type referred to in clause “(i)”, “(ii)”, “(iii)”, “(iv)” or “(v)” of this sentence; (vii) enter into any discussions, negotiations, arrangement or agreement with any other Person relating to any of the foregoing or (viii) request or propose that Arena or any Affiliate of Arena amend, waive or consider the amendment or waiver of any provision set forth in this Section 9.8(a).
(b)Exception to Standstill Provisions.
(i)The provisions of Section 9.8(a) shall cease to apply: (A) if Arena or any Affiliate of Arena publicly announces or otherwise engages in a process designed to solicit offers relating to transactions that, if consummated, would result in (1) a Third Party
46
acquiring beneficial ownership of 50% or more of the outstanding securities of Arena or such Affiliate, as applicable, immediately after such transaction, (2) a sale of all or substantially all of the consolidated assets of Arena and all its Affiliates, or (3) a merger, consolidation or any similar extraordinary transaction involving Arena or any Affiliate of Arena pursuant to which all or substantially all of the consolidated assets of Arena and all its Affiliates would, after the closing of such transaction, be under the control of a Person that did not, prior to such transaction, control Arena or any of its Affiliates, in each case ((1), (2) and (3)) from the time of such announcement or the commencement of such process and continuing until such time, if any, as the board of directors of Arena or the applicable Affiliate publicly announces that such process has terminated; or (B) if the board of directors of Arena or any Affiliate of Arena adopts a plan of liquidation or dissolution.
(ii)Notwithstanding Section 9.8(a), (A) in the event a Third Party makes a bona fide public offer or proposal that, if consummated, would result in such Third Party, together with its affiliates and other members of any group of which such Third Party is a member, beneficially owning 50% or more of the outstanding shares of Arena or any Affiliate of Arena or all or substantially all of the assets of Arena or any Affiliate of Arena, from the time such offer or proposal is made public and continuing until such offer or proposal expires or is publicly rescinded or (B) from and after the 10th day following the filing of a preliminary proxy statement by any Third Party with respect to the commencement of a bona fide proxy or consent solicitation subject to Section 14 of the Exchange Act to elect or remove more than one-half of the directors of Arena or any Affiliate of Arena, then either case ((A) or (B)) during the applicable time frame above Eisai or any of its Affiliates shall have the right to submit a confidential, non-public proposal to the board of directors of Arena or any Affiliate of Arena or any executive officer thereof with respect to any transaction of the type referred to in Section 9.8(a)(i), and in connection with such a proposal Eisai and its Affiliates may consult on a confidential basis with third party advisors with respect to any such proposal.
(iii)Nothing in Section 9.8(a) shall prohibit Eisai or any of its Affiliates from acquiring beneficial ownership of securities of Arena or any Affiliate of Arena by or through (A) a diversified mutual or pension fund managed by an independent investment adviser or pension plan established for the benefit of the employees of Eisai or any of its Affiliates, (B) any employee benefit plan of Eisai or any of its Affiliates or (C) any stock portfolios not controlled by Eisai or any of its Affiliates that invest in Arena or any Affiliate of Arena among other companies; provided, that Eisai or any of its Affiliates does not, directly or indirectly, request the trustee or administrator or investment adviser of such fund, plan or portfolio to acquire beneficial ownership of such securities. Further, nothing herein shall prevent Eisai or any of its Affiliates from acquiring securities of another pharmaceutical or biotechnology company or other Person that beneficially owns any securities of Arena or any Affiliate of Arena; provided, that such Person beneficially does not own, at the time of the consummation of such acquisition of securities by Eisai or any of its Affiliates, more than 10% of any class of outstanding securities of Arena or any Affiliate of Arena.
9.9Confidentiality under the Supply Agreement. Eisai and Arena agree that this Article 9 will govern the confidentiality and use restrictions of the Parties to the Supply Agreement with respect to all Confidential Information disclosed pursuant to the Supply
47
Agreement prior to Facility Acquisition. Thereafter, Section 14.1 of the Supply Agreement will govern information disclosed under the Supply Agreement.
10.1Ownership of Intellectual Property.
(a)Arena Intellectual Property Rights. Arena and its Affiliates have, and shall retain, all right, title and interest in and to the Arena Licensed IP, Arena Licensed Records, Arena Licensed Manufacturing Know-How, Arena Licensed Supply Records and any other intellectual property owned by Arena or its Affiliates as of the Effective Date, (other than the Purchased Intellectual Property) or developed by Arena or its Affiliates outside the scope of this Agreement during the Term.
(b)Eisai Intellectual Property Rights. Eisai and its Affiliates have, and shall retain, all right, title and interest in and to the Existing Eisai Know-How and Existing Eisai Patents, any other intellectual property owned by Eisai or its Affiliates as of the Effective Date or developed by Eisai or its Affiliates during the Term, including any New Program Know-How or New Program Patents and, from the Effective Date, any Purchased Intellectual Property.
(c)New Program Intellectual Property Rights. Eisai shall have and own the entire right, title and interest in and to all New Program Know-How and New Program Patents and shall have and retain the right to use, disclose and exploit the New Program Know-How and New Program Patents for any and all purposes, including the right to disclose the New Program Know-How to its Affiliates. Each Party has disclosed to the other Party in writing the discovery, identification, conception, reduction to practice or other making of any Program Know-How (as defined in the Existing Agreement) prior to the Effective Date, and Arena shall disclose to Eisai in writing the discovery, identification, conception, reduction to practice or other making of any New Program Know-How or New Program Patents from and after the Effective Date. Arena shall, and hereby does, assign, and shall cause its Affiliates to so assign, to Eisai or an Affiliate of Eisai designated by Eisai in writing, without additional compensation, all of its right, title and interest in and to any New Program Know-How and New Program Patents as well as any intellectual property rights with respect thereto to fully effect the ownership by Eisai provided for in this Section 10.1(c). Arena and its Affiliates shall execute all documents and take all actions reasonably requested by Eisai to fully effect the ownership by Eisai provided for in this Section 10.1(c).
10.2Patent Prosecution and Maintenance.
(a)Arena Licensed Patents. During the Term, Eisai shall be responsible for the preparation, filing, prosecution and maintenance of all Arena Licensed Patents, at Eisai’s own expense and at its discretion. During the Term, Eisai shall keep Arena informed of progress with regard to the preparation, filing, prosecution and maintenance of Arena Licensed Patents in the Territory in a timely manner, but not less frequently than once per Calendar Quarter. To that end, Eisai shall: (i) provide Arena with a copy of the final draft of any proposed application for the Arena Licensed Patents at least 30 days prior to filing the same in the Territory, unless
48
otherwise agreed by patent counsel for each Party, and Eisai shall consider in good faith any comments or revisions suggested by Arena or its counsel; (ii) promptly provide Arena with a copy of each patent application as filed, together with a notice of its filing date and serial number; (iii) provide Arena with a copy of any action, communication, letter, or other correspondence issued by the applicable patent office within at least 10 days of receipt thereof, and Eisai shall consult with Arena regarding responding to the same and shall consider in good faith any comments, strategies, and the like proposed by Arena or its counsel; (iv) provide Arena with a copy of any response, amendment, paper, or other correspondence filed with the applicable patent office within 10 days of Eisai’s receipt of the as-filed document; and (v) promptly notify Arena of the allowance, grant, or issuance of such Arena Licensed Patents in the Territory. Arena shall, and shall cause its Affiliates to, assist and cooperate with Eisai, as Eisai may reasonably request from time to time, in the preparation, filing, prosecution and maintenance of the Arena Licensed Patents under this Agreement, including that Arena shall, and shall cause its Affiliates to, provide access to relevant documents and other evidence and make its employees available at reasonable business hours. If Eisai elects to abandon or cease prosecution or maintenance of any Arena Licensed Patent in the Territory, Eisai shall provide reasonable prior written notice to Arena of such intention to abandon (which notice shall, to the extent possible, be given no later than 90 calendar days prior to the next deadline for any action that must be taken with respect to any such Arena Licensed Patent). In such case, to the extent consistent with Eisai’s global intellectual property strategy for the Products, upon written notice to Eisai, Arena may elect to continue prosecution or maintenance of any such Arena Licensed Patent at its own expense, and Eisai shall take such actions, at Eisai’s expense, as may be reasonably necessary to enable Arena to do so. If Arena elects to continue prosecution or maintenance of any such Patent that constitutes an Arena Licensed Patent, then such Patent shall be excluded from the Arena Licensed Patents and no longer subject to this Agreement.
(b)Eisai Patents. Eisai shall be solely responsible for the preparation, filing, prosecution and maintenance of all Patents owned by Eisai or any of its Affiliates throughout the world, at Eisai’s own expense and at its discretion, including any Purchased Patents, the Existing Eisai Patents and any New Program Patents. Arena shall, and shall cause its Affiliates to, assist and cooperate with Eisai, as Eisai may reasonably request from time to time, in the preparation, filing, prosecution and maintenance of the Purchased Patents, including that Arena shall, and shall cause its Affiliates to, provide reasonable access to relevant documents and other evidence and make its employees reasonably available at reasonable business hours.
10.3Infringement by Third Parties.
(a)Notice. During the Term, in the event that either Arena or Eisai becomes aware of any infringement or threatened infringement by a Third Party of any Arena Licensed Patents, it shall notify the other Party in writing to that effect. Any such notice shall include any evidence that such notifying Party has in its possession and is legally able to disclose that supports such allegation of infringement or threatened infringement by such Third Party.
(b)Enforcement Procedures. Subject to the following provisions, during the Term, Eisai shall have the first right to bring and control any action or proceeding with respect to any infringement of any Arena Licensed Patent by a Third Party in the Territory, at its own expense as it reasonably determines appropriate, and Arena shall have the right to be represented in
49
any such action at its own expense by counsel of its choice. If Eisai does not bring such action or proceeding within the earlier of (i) 60 days after the notice provided pursuant to Section 10.3(a) or pursuant to the Existing Agreement, or (ii) 10 days before the expiration date for bringing such action or proceeding, then Arena shall have the right, to the extent consistent with Eisai’s global intellectual property strategy for the Products, to bring and control any action or proceeding with respect to such infringement of any Arena Licensed Patent by a Third Party in the Territory, at its own expense as it reasonably determines appropriate, and Eisai shall have the right to be represented in any such action at its own expense by counsel of its choice.
(c)Cooperation. During the Term, each Party shall cooperate fully with the other Party with respect to such actions or proceedings described in Section 10.3(b), or similar actions or proceedings brought under the Existing Agreement and ongoing during the Term, including being joined as a party plaintiff or joining the other Party as a party plaintiff in such action or proceeding and providing access to relevant documents and other evidence and making its employees available at reasonable business hours.
(d)Recoveries. Any monetary recovery resulting from such actions or proceedings described in Section 10.3(b), or similar actions or proceedings brought under the Existing Agreement and ongoing during the Term, or any actions or proceedings by Eisai under Section 10.3(f) to enforce any Purchased Patents or any New Program Patents, will be allocated as follows: each of Eisai and Arena first will be reimbursed, out of such recovery, for its reasonable and verifiable costs and expenses with respect to such action or proceeding (such reimbursement to be pro-rata based on the Parties’ relative costs and expenses if the recovery is not sufficient to reimburse both Parties fully) with any remainder retained by Eisai and deemed Net Sales, subject to royalty payments from Eisai to Arena under Section 8.3.
(e)Paragraph IV Notices. During the Term, if either Party receives a notice under 21 U.S.C. §355(b)(2)(A)(iv) or 355(j)(2)(A)(vii)(IV) concerning an Arena Licensed Patent, or any similar notice under the Applicable Laws of a country in the Arena Licensed Patent Territory outside of the United States (each, a “Paragraph IV Notice”), then it shall provide a copy of such notice to the other Party within five days after its receipt thereof. Patent infringement litigation based on a Paragraph IV Notice concerning an Arena Licensed Patent or any similar notice received prior to the Effective Date shall be brought as provided in Section 10.3(b).
(f)Eisai Patents. Eisai shall have the exclusive right to enforce Patents owned by Eisai or any of its Affiliates throughout the world, at Eisai’s own expense and at its discretion, including any Purchased Patents, Existing Eisai Patents and New Program Patents. Arena shall, and shall cause its Affiliates to, assist and cooperate with Eisai, as Eisai may reasonably request from time to time, in the enforcement of the Purchased Patents, including that Arena shall, and shall cause its Affiliates to, provide reasonable access to relevant documents and other evidence and make its employees reasonably available at reasonable business hours.
50
10.4Infringement of Third Party Rights. During the Term, each Party shall promptly notify the other in writing of any allegation by a Third Party that the activity of either of the Parties pursuant to this Agreement infringes or may infringe the intellectual property rights of such Third Party.
(a)Eisai. With respect to any claim alleging that (i) the Commercialization of Products in the Territory pursuant to this Agreement, the Original Agreement, the Restated Agreement or the Existing Agreement, (ii) the making or having made, or importing or selling in the Territory of any Compound, Related Compound or Product or intermediate thereof, or (iii) the conduct of development activities by or on behalf of Eisai or Eisai Related Parties pursuant to this Agreement, the Original Agreement, the Restated Agreement or the Existing Agreement, in each case ((i), (ii) and (iii)) infringes the intellectual property rights of any Third Party, Eisai shall have the sole right, subject to Section 12.7, to control any defense of any such claims at its own expense (including outside counsel fees and all amounts payable by Eisai as a judgment based on such claim or in settlement of such claim) and by counsel of its own choice.
(b)Arena. With respect to any claim alleging that (i) the discovery or development prior to the Original Effective Date of any Compound or Product, or (ii) any activity by Arena or its Affiliates prior to the Effective Date outside the Territory (as it existed under the Existing Agreement), infringes or may infringe the intellectual property rights of any Third Party, in each case ((i) - (ii)) Arena shall have the sole right, subject to Section 12.7, to control any defense of any such claims at its own expense (including outside counsel fees and all amounts payable by Arena as a judgment based on such claim or in settlement of such claim) and by counsel of its own choice.
10.5Invalidity or Unenforceability Defenses or Actions.
(a)Third Party Defense or Counterclaim. During the Term, if a Third Party asserts, as a defense or as a counterclaim in any action or proceeding described in Section 10.3(b), or any similar action or proceeding brought under the Existing Agreement and ongoing during the Term, that any Arena Licensed Patent is invalid or unenforceable, then the Party that brought such action or proceeding pursuant to Section 10.3(b) or the Existing Agreement shall respond to such defense or defend against such counterclaim (as applicable), at its own expense.
(b)Third Party Declaratory Judgment or Similar Action. During the Term, if a Third Party asserts, in a declaratory judgment action or similar action or claim filed by such Third Party in the Territory, that any Arena Licensed Patent is invalid or unenforceable, then the Party first becoming aware of such action or claim shall promptly give written notice to the other Party. With respect to the Arena Licensed Patents, Eisai shall have the first right to defend against such action or claim, at its own expense as it reasonably determines appropriate, and Arena shall have the right to be represented in any such action at its own expense by counsel of its choice. If Eisai determines not to defend against such action or claim, it will so notify Arena sufficiently in advance of the deadline for response to allow Arena to respond, and Arena shall have the right to defend against such action or claim, at its own expense as it reasonably determines appropriate, and Eisai shall have the right to be represented in any such action at its own expense by counsel of its choice. Each Party shall cooperate fully with the other Party with
51
respect to such defense, including being joined as a Party defendant or joining the other Party as a Party defendant in such defense and providing access to relevant documents and other evidence and making its employees available at reasonable business hours.
10.6Consent for Settlement. Neither Party shall enter into any settlement or compromise of any action or proceeding under Section 10.3 or Section 10.5 without the prior written consent of the other Party, which consent shall not be unreasonably conditioned, withheld or delayed. The defending Party may enter into a settlement or compromise of any action or proceeding under Section 10.4 without the consent of the other Party; provided, that such settlement or compromise would not reasonably be expected to materially adversely affect such other Party or its Affiliates.
10.7Patent Term Extensions. The Parties shall discuss and recommend for which, if any, of the Arena Licensed Patents the Parties should seek Patent Term Extensions in the Arena Licensed Patent Territory. After the Effective Date, Eisai shall have the final decision-making authority with respect to seeking and obtaining any such Patent Term Extensions in the Arena Licensed Patent Territory for the Arena Licensed Patents, and shall act with reasonable promptness in light of the development stage of each Product to apply for any such Patent Term Extensions, where it so elects; provided, that Eisai shall consult with Arena in good faith to determine which such Arena Licensed Patents should be the subject of efforts to obtain a Patent Term Extension. Arena shall cooperate fully with Eisai in making such filings or actions, for example and without limitation, by making available all required regulatory data and information in Arena’s possession or Control and executing any required authorizations to apply for such Patent Term Extension. All expenses incurred in connection with activities of Eisai with respect to the Arena Licensed Patent for which Eisai seeks Patent Term Extensions pursuant to this Section 10.7 shall be entirely borne by Eisai.
10.8Orange Book Listings. Eisai shall fully involve Arena in the planning and decisions regarding listing the applicable Arena Licensed Patent with the applicable Regulatory Authorities in the Arena Licensed Patent Territory for each Product and Eisai shall consult in good faith with Arena regarding such listing, including all so called “Orange Book” listings required under the Xxxxx-Xxxxxx Act in the United States and equivalent listings in other countries in the Arena Licensed Patent Territory; provided, that Eisai shall have final decision-making authority regarding which Arena Licensed Patents to list and shall maintain such listings in each applicable country (and shall make such filings). Arena shall use reasonable efforts to provide any needed cooperation, including to (a) provide to Eisai a correct and complete list of Arena Licensed Patents in such country covering such Product and any other information that is Controlled by Arena or any of its Affiliates, and is, to the Knowledge of Arena, otherwise necessary or reasonably useful to enable Eisai to make such decision and filings with Regulatory Authorities in such country with respect to the applicable Arena Licensed Patents, and (b) cooperate with Eisai’s reasonable requests in connection therewith, including meeting any submission deadlines, in each case, to extent required or permitted by Applicable Laws. Eisai shall pay the costs of all “Orange Book” (and any equivalent) filings with respect to any Product in the Territory.
52
10.10Intellectual Property under the Supply Agreement. Eisai and Arena agree that prior to a Facility Acquisition, Section 10.1 will govern the ownership of New Program Know–How discovered, identified, conceived, reduced to practice or otherwise made in the course of or as a result of activities under the Supply Agreement.
Article 11 REPRESENTATIONS, WARRANTIES AND COVENANTS
11.1Mutual Representations, Warranties and Covenants. As of the Effective Date, each Party hereby represents and warrants to the other Party and covenants as follows:
(a)Duly Organized. Such Party and Arena US (i) is a corporation or limited liability company, with restricted liability, duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization and (ii) is qualified to do business and is in good standing as a foreign corporation or organization in each jurisdiction in which the conduct of its business or the ownership of its properties requires such qualification and failure to have such qualification would prevent such Party from performing its obligations under this Agreement.
(b)Due Authorization; Binding Agreement. The execution, delivery and performance of this Agreement and the Related Documents by such Party have been duly authorized by all necessary corporate or organizational action. This Agreement and the Related Documents are legal and valid obligations binding on such Party and enforceable in accordance with their respective terms subject to the effects of bankruptcy, insolvency or other laws of general application affecting the enforcement of creditor rights and judicial principles affecting the availability of specific performance and general principles of equity, whether enforceability is considered in the proceeding at law or equity.
(c)No Conflicts. The execution, delivery and performance of this Agreement, the Supply Agreement and the Related Documents by such Party, Arena GmbH or Arena US, as applicable, does not: (i) violate any Applicable Law; (ii) conflict with or result in a breach of the Constitutive Documents of such Party, Arena GmbH or Arena US, as applicable; or (iii) conflict with, constitute a default under or give rise to any right of termination, cancellation, modification or acceleration under, any agreement, instrument or understanding, oral or written, to which such Party, Arena GmbH or Arena US, as applicable, is a party or by which it, or any of its material assets or properties, including in the case of Arena, the Purchased Assets and Inventory, is bound, except, in the case of clause (iii) for any conflicts or defaults that would not, individually or in the aggregate, materially affect the ability of such Party, Arena GmbH or Arena US to perform its obligations under this Agreement, the Supply Agreement and the Related Documents or otherwise prevent or materially impede, interfere with, hinder or delay the consummation of the transactions contemplated by this Agreement, the Supply Agreement and the Related Documents.
(d)No Conflicting Grant of Rights. Such Party and Arena GmbH have the right to grant (or cause its Affiliates to grant) the rights granted by such Party to the other Party under this Agreement and the Supply Agreement and have not granted any rights to any Person that are in conflict with the rights granted by such Party or Arena GmbH to the other Party under this Agreement or the Supply Agreement.
53
(e)Debarment. Such Party is not debarred under the FFDCA or listed on either Excluded List and it does not, and shall during the Term not, employ or use the services of any Person who is debarred or listed on either Excluded List, in connection with the development, manufacture or Commercialization of the Initial Product, the Once-Daily Product or any other Product. In the event that either Party becomes aware of the debarment or threatened debarment of, or listing or threatened listing on either Excluded List of, any Person providing services to such Party, including the Party itself and its Affiliates, contractors, licensees, or distributors, that directly or indirectly relate to activities under this Agreement or the Supply Agreement, the other Party shall be immediately notified in writing.
(f)Development. Such Party and its Affiliates have conducted, and to the Knowledge of such Party, their respective contractors and consultants have conducted, all development with respect to the Compound and the Initial Product that they have conducted prior to the Effective Date, in accordance with GLP and GCP and other Applicable Laws, except to the extent any noncompliance would not materially negatively affect the likelihood of obtaining Regulatory Approval of the Initial Product or the commercial viability of the Initial Product in any country in the Territory.
(g)Legal Compliance.
(i)With respect to Regulatory Filings for which such Party or GmbH was responsible under the Existing Agreement, such Party or GmbH prepared, maintained and retained all such Regulatory Filings that are required to be maintained or retained as of the Effective Date, pursuant to and in accordance with GLP, GCP and other Applicable Laws.
(ii)Neither such Party nor its Affiliates received any written notice or to the Knowledge of such Party, any oral notice, that indicates that any of the INDs for the Initial Product are not currently in good standing with the FDA.
(iii)With respect to INDs for which such Party or Arena GmbH was responsible under the Existing Agreement, such Party or Arena GmbH filed with the FDA all required notices, supplemental applications and annual or other reports or documents, including adverse experience reports, with respect to each such IND that was material to the continued development of the Initial Product.
(iv)Except for a failure which would not have a material adverse effect on the Products or such Party’s or Arena GmbH’s business pertaining to the Products, (1) the business and operations of such Party and Arena GmbH are, and for the past six years have been, in compliance with all Applicable Laws and (2) neither such Party nor Arena GmbH has received a notice or other communication alleging a possible violation by such Party or Arena GmbH of any Applicable Law relating to the Products or such Party’s or Arena GmbH’ business pertaining to the Products.
54
11.2Representations, Warranties and Covenants of Arena. As of the Effective Date, Arena represents and warrants to Eisai and hereby (where applicable) covenants to Eisai:
(a)Consents. No material notice to, filing with, authorization of, exemption by, or consent of, any Person, including any Governmental Entity, including any foreign Governmental Entity, is required for Arena or its Affiliates to transfer the Purchased Assets or the Inventory to Eisai and otherwise consummate the transactions contemplated hereunder or under the Supply Agreement, except for the Consents.
(i)Arena has good and marketable title to, (1) the Arena Regulatory Approvals, (2) the Samples, and (3) the Purchased Records free and clear of all Liens (other than Permitted Liens);
(ii)Arena GmbH has good and marketable title to, or valid contract rights to, (1) the Purchased Trademarks, (2) the Purchased Supply Records, (3) the Purchased Validation Materials, (4) the Product Domain Names, (5) the Third Party Distributor Agreements and (6) the Inventory, free and clear of all Liens (other than Permitted Liens);
(iii)Arena has the complete and unrestricted power and unqualified right (except as described in the final proviso of Section 3.3) to sell, convey, deliver, transfer and assign to Eisai, as applicable, (1) the Arena Regulatory Approvals, (2) the Samples and (3) the Purchased Records;
(iv)Arena GmbH has the complete and unrestricted power and unqualified right (subject to the Consents with respect to the Third Party Distributor Agreements) to sell, convey, deliver, transfer and assign to Eisai, as applicable, (1) the Purchased Trademarks, (2) the Purchased Supply Records, (3) the Purchased Validation Materials, (4) the Product Domain Names, (5) the Third Party Distributor Agreements and (6) the Inventory;
(v)Arena or Arena US has good and marketable title to all of the Purchased Patents and Purchased Know-How free and clear of all Liens (other than Permitted Liens);
(vi)Arena GmbH has good and marketable title to all of the Purchased Manufacturing Know-How free and clear of all Liens (other than Permitted Liens);
(vii)Arena and Arena US have the complete and unrestricted power and unqualified right to sell, convey, deliver, transfer and assign to Eisai, as applicable, the Purchased Patents and the Purchased Know-How;
(viii)Arena GmbH has the complete and unrestricted power and unqualified right to sell, convey, deliver, transfer and assign to Eisai, as applicable, the Purchased Manufacturing Know-How;
55
(ix)to the Knowledge of Arena, there are no adverse claims of ownership to the Purchased Assets (excluding the Purchased Intellectual Property) or the Inventory;
(x)Arena has not received written, or to the Knowledge of Arena oral, notice that any Person has asserted a claim of ownership or right of possession or use in or to any of the Purchased Assets or to the Inventory, except as previously disclosed to Eisai prior to the Effective Date; and
(xi)at the Closing, Eisai will acquire from Arena, Arena US or Arena GmbH, good and marketable title to, or valid contract rights to, as applicable, all of the Purchased Assets and the Inventory, free and clear of any Liens (other than Permitted Liens), license or other restriction or limitation regarding use or disclosure.
(c)Intellectual Property.
(i)Patent Rights. The Arena Patents existing as of the Effective Date are set forth on Exhibit C attached hereto (“Existing Arena Patents”) and are all Patents issued or pending in any country in the Territory that, as of the Effective Date, Arena or any of its Affiliates owns or has a license to that claim the Compound, the Initial Product, the Once-Daily Product or any other Product or a method of use or manufacture of the Compound, a Related Compound, the Initial Product, the Once-Daily Product or any other Product, as such Compound, Related Compound, Initial Product, Once-Daily Product or other Product exists as of the Effective Date or existed prior thereto, in the Territory, and Arena and Arena US are the exclusive owners of the Existing Arena Patents. The Existing Arena Patents, the Purchased Know-How, the Purchased Manufacturing Know-How and the Purchased Trademarks are not subject to any Liens (other than Permitted Liens) or, to the Knowledge of Arena, claims of ownership, by any Third Party that would prevent the grant of the rights granted to Eisai under this Agreement or the Supply Agreement or materially interfere with Arena’s performance of its obligations under this Agreement or Arena GmbH’s performance of its obligations under the Supply Agreement or materially prevent Eisai from exercising its rights under this Agreement. True, complete and correct copies of the file wrapper relating to the prosecution and maintenance of the Existing Arena Patents filed in the United States have been made available to Eisai prior to the Effective Date. Except as previously disclosed to Eisai prior to the Effective Date, the Existing Arena Patents in the United States have been diligently prosecuted before the United States patent office in accordance with Applicable Laws, and to the Knowledge of Arena, the Existing Arena Patents in the countries of the Territory outside the United States in which such Patents have been filed have been diligently prosecuted before the applicable patent offices in accordance with Applicable Laws. Except as previously disclosed to Eisai prior to the Effective Date, the Existing Arena Patents in the United States have been filed and maintained in accordance with Applicable Laws, and to the Knowledge of Arena, the Existing Arena Patents in the countries of the Territory outside the United States in which such Patents have been filed have been filed and maintained in accordance with Applicable Laws. Except as previously disclosed to Eisai prior to the Effective Date, all applicable fees owed with respect to the prosecution and maintenance of the Existing Arena Patents have been paid on or before the due date for payment to the extent necessary to prevent the abandonment of the Existing Arena Patents. With respect to any issued patents included in the Existing Arena Patents in the United
56
States, Arena or one of its Affiliates has presented all prior art material to the patentability of the claims of such applications of which it and the inventors are aware to the relevant Patent Examiner at the United States patent office, to the extent required and in accordance with Applicable Laws.
(ii)Patent Status. As of the Effective Date, (i) all issued Arena Patents are in full force and effect and subsisting, and, to Arena’s Knowledge, are not invalid or unenforceable; (ii) except as previously disclosed to Eisai prior to the Effective Date, none of the Arena Patents is currently involved in any interference, reissue, reexamination, or opposition proceeding; and (iii) except as previously disclosed to Eisai prior to the Effective Date, neither Arena nor any of its Affiliates has received any written notice from any Person, or has Knowledge, of any such actual or threatened proceeding.
(iii)Non-Infringement of Third Party Rights. To the Knowledge of Arena, the discovery, identification, conception, reduction to practice or other making of any inventions claimed in the Arena Patents existing as of the Effective Date have not constituted or involved the misappropriation of trade secrets of any Third Party. Except as previously disclosed to Eisai prior to the Effective Date, neither Arena nor any of its Affiliates has received any written notice from any Third Party, nor does Arena have any Knowledge of any actual or threatened claim or assertion by a Third Party, that the manufacture, use, sale or import of the Compound or the Initial Product in the Territory infringes or misappropriates the intellectual property rights of a Third Party.
(iv)Non-Action or Claim. As of the Effective Date, there are no pending or threatened in writing, or to Knowledge of Arena, threatened adverse actions, suits, claims, or formal governmental investigations by or against Arena or any of its Affiliates in or before any court, Regulatory Authority or other governmental authority in the Territory with respect to the Compound or the Initial Product, including in connection with the conduct of any clinical trials or manufacturing activities with respect thereto. As of the Effective Date, there are no material unsatisfied judgments or outstanding orders, injunctions, decrees, stipulations or awards (whether rendered by a court, an administrative agency or by an arbitrator) against Arena (or any of its Affiliates) in the Territory with respect to the Compound or the Initial Product.
(v)No Conflicting Agreement. Except for the Third Party Distributor Agreements, neither Arena nor any of its Affiliates has entered into any Contract that granted any Third Party the right to develop, promote, market or sell in the Territory the Compound, the Initial Product, the Once-Daily Product or any other Product or otherwise assigned, transferred, licensed, conveyed or otherwise encumbered in a manner that would prevent Eisai from exercising its rights under this Agreement, its right, title or interest in or to, the Arena Patents or the Regulatory Filings in the Territory with respect to the Initial Product, the Once-Daily Product or any other Product (including by granting any covenant not to xxx with respect thereto) and during the Term it will not enter into any such agreements or grant any such right, title or interest to any Person that conflicts with the rights granted to Eisai under this Agreement. There are no Contracts pursuant to which Arena or any of its Affiliates has granted any Third Party rights to any portion of the Purchased Intellectual Property, other than any nondisclosure agreements and material transfer agreements entered into in the ordinary course of business, the Third Party Distributor Agreements, agreements with service providers granting a license to the Purchased Intellectual Property solely to the extent
57
necessary to perform the service provider’s duties under such agreement, investigator-initiated study agreements and clinical trial agreements granting investigators and institutions a license to the Purchased Intellectual Property solely for non-commercial research purposes and educational purposes, and agreements providing consent or limiting use in the Purchased Trademarks that have been previously disclosed to Eisai or that will not materially adversely affect the Purchased Trademarks. The Purchased Know-How, the Purchased Manufacturing Know-How, the Arena Licensed Know-How and the Arena Licensed Manufacturing Know-How together constitute all Know-How that, as of the Effective Date, Arena or any of its Affiliates owns or has a license to and that (a) is necessary for, or is as of the Effective Date or was at any time during the 24-month period prior to the Effective Date used for, the development, manufacture or Commercialization of any Product in any country in the Territory, as such Product exists as of the Effective Date or existed prior thereto and (b) is Confidential Information of Arena. No Affiliate of Arena, other than Arena US, has any right, title or interest (including beneficial ownership and any economic interest) in any of the Purchased Know-How, Arena Licensed Know-How, Purchased Patents and Arena Licensed Patents. No Affiliate of Arena GmbH has any right, title or interest (including beneficial ownership and any economic interest) in any of the Purchased Trademarks, Purchased Manufacturing Know How or Arena Licensed Manufacturing Know-How.
(d)Third Party Distributor Agreements.
(i)Arena has made available to Eisai complete and accurate copies of all Third Party Distributor Agreements, including all amendments, modifications and waivers relating thereto.
(ii)Each Third Party Distributor Agreement is in full force and effect and constitutes a legal, valid and binding agreement of each party thereto, enforceable in accordance with its terms; and neither Arena GmbH nor, to the Knowledge of Arena, any other party to a Third Party Distributor Agreement, is or is alleged to be, or has received notice that it is or is alleged to be, in violation or breach of or default under any such Third Party Distributor Agreement (or with notice or lapse of time or both, would be in violation or breach of or default under any such Third Party Distributor Agreement). Except as previously disclosed to Eisai, no Third Party Distributor has made any demands for renegotiation of any amount to be paid or payable to or by Arena GmbH under a Third Party Distributor Agreement. There are no disputes under any Third Party Distributor Agreement and Arena GmbH has not received any notice that any Third Party Distributor intends to cancel or terminate any Third Party Distributor Agreement. Neither Arena GmbH, nor to Arena’s Knowledge, any Third Party Distributor, has taken any action that would cause any Third Party Distributor Agreement to terminate or fail to renew.
(e)Inventory. All of the Inventory has not been adulterated (except to the extent where adulteration was solely due to the expiration of the Product) and has been manufactured, handled, maintained and stored at all times in accordance with the specifications set forth in the relevant Arena Regulatory Approvals, in substantial compliance with all requirements of relevant Good Manufacturing Practices, and in substantial compliance with all requirements of relevant Governmental Entities.
(f)Legal Proceedings. Except as previously disclosed to Eisai, (i) there are no Legal Proceedings or Orders pending or, to the Knowledge of Arena, threatened against the
58
Purchased Assets, the Arena Licensed IP, the Arena Licensed Manufacturing Know-How, the Arena Licensed Supply Records, the Arena Licensed Records or the Inventory, or against Arena or any of its Affiliates that could reasonably be expected to adversely affect any of the Purchased Assets, the Arena Licensed IP, the Arena Licensed Manufacturing Know-How, the Arena Licensed Supply Records, the Arena Licensed Records or the Inventory, and (ii) there are no Legal Proceedings pending by Arena or any of its Affiliates, or that Arena or such Affiliate intends to initiate, against any other Person that would adversely affect the Purchased Assets, the Arena Licensed IP, the Arena Licensed Manufacturing Know-How, the Arena Licensed Supply Records, the Arena Licensed Records or the Inventory.
(g)Taxes.
(i)Arena, Arena US and Arena GmbH have timely paid all Taxes that will have been required to be paid by it, the non-payment of which would result in a Lien on any Purchased Asset or the Inventory or would result in Eisai becoming liable or responsible therefor.
(ii)Arena, Arena US and Arena GmbH have established, in accordance with GAAP, adequate reserves for the payment of, and will timely pay, all Taxes that arise from or with respect to the Purchased Assets or Inventory and are incurred or attributable to the Pre-Closing Tax Period, the non-payment of which would result in a Lien on any Purchased Asset or the Inventory or would result in Eisai becoming liable therefor.
(h)Regulatory Matters.
(i)No governmental authority (including the FDA) has commenced or, to the Knowledge of Arena, threatened to initiate any action to enjoin production of the Initial Product at any facility, nor has Arena or any of its Affiliates or, to the Knowledge of Arena, any of its contractors, received any written notice thereof.
(ii)At no time when Arena GmbH was responsible for Regulatory Filings under the Existing Agreement did Arena or any of its Affiliates, or any of its or their respective officers, employees, or agents, make an untrue statement of material fact or fraudulent statement to the FDA or any other Regulatory Authority with respect to the development of the Initial Product, fail to disclose a material fact required to be disclosed to the FDA with respect to the development of the Initial Product, or commit an act, make a statement, or fail to make a statement with respect to the development of the Initial Product that could reasonably be expected to provide a basis for the FDA to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities”, set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto.
(iii)Regulatory Approvals. Arena GmbH has not requested that any of the Third Party Distributors transfer any Regulatory Approvals to Arena or any of its Affiliates.
(iv)Clinical Studies. Arena and its Affiliates are not conducting any clinical studies or trials related to the Compound or any of the Products.
59
(i)Brokers. No broker, finder, financial advisor, investment banker or other Person is or will be entitled to any brokerage, finder’s, financial advisor’s or other similar fee or commission in connection with the transactions contemplated under this Agreement and the Supply Agreement based upon arrangements made by or on behalf of Arena or any of its Affiliates, for which Arena or Eisai could be liable, or by or on behalf of Arena or any of its Affiliates.
(j)Suppliers. Schedule 11.2(j) specifies as of the Effective Date the names of the suppliers of each of the direct materials (i.e., only the API, excipients, packaging materials and precursor materials 4CPE and ZP3) used in the manufacture of the Products. None of such suppliers has given Arena or its Affiliates written notice terminating, canceling or threatening to terminate or cancel any Contract with Arena or its Affiliates relating to the applicable component of the Products supplied to Arena.
(k)Scientific and Technical Information.
(i)Arena or Arena GmbH has heretofore disclosed or made available to Eisai (or Eisai otherwise has access to) (i) all material scientific and technical information known to any of its or its Affiliates’ respective employees with the title of vice president or higher or in-house general counsel relating to (A) the safety and efficacy of the Compound and the Initial Product, including the results of any material nonclinical studies required for filing an IND or clinical trials with respect to the foregoing, (B) the drug quality, including stability, variability, impurities and delivery performance, of the Compound and the Initial Product and (C) the status of the Initial Product under the Controlled Substances Act, as it may be amended from time to time, and the rules, regulations, guidances, guidelines, and requirements promulgated or issued thereunder and (ii) all material Regulatory Filings submitted to, or filed with, or listed by a Regulatory Authority and the status of all material discussions with Regulatory Authorities, in each case, in respect of the Compound and the Initial Product in the Territory.
(ii)To the Knowledge of Arena, (x) no serious adverse event information has come to the attention of Arena or any of its Affiliates with respect to the Compound or the Initial Product that is materially different with respect to the incidence, severity or nature of such serious adverse events than the information that was filed as safety updates to any Regulatory Filings for the Compound or the Initial Product, and (y) all written data summaries that were included in any such Regulatory Filings based on clinical trials conducted or sponsored by Arena or any of its Affiliates accurately summarize in all material respects the raw data underlying such summaries.
(iii)To the Knowledge of Arena, the manufacturing of the Initial Product to be provided by Arena GmbH to Eisai pursuant to the Supply Agreement, as such manufacturing is conducted as of the Effective Date, does not and will not infringe the Patents of a Third Party that are granted in the Territory as of the Effective Date.
(l)Purchase Orders. Ildong Pharmaceutical Co., Ltd. has not made any payments to Arena or any of its Affiliates in respect of any unfulfilled purchase orders issued by Ildong Pharmaceutical Co., Ltd. to Arena GmbH under the Marketing and Supply Agreement by
60
and between Arena GmbH and Ildong Pharmaceutical Co., Ltd., dated November 6, 2012, as amended. Arena agrees that from the Effective Date, the right to payment from Ildong Pharmaceutical Co., Ltd. in respect of the fulfillment of any such unfulfilled purchase orders or future purchase orders shall belong to Eisai.
11.3Representations, Warranties and Covenants of Eisai. As of the Effective Date, Eisai represents and warrants to Arena and hereby (where applicable) covenants to Arena:
(a)Consents. No material notice to, filing with, authorization of, exemption by, or consent of, any Person, including any Governmental Entity, including any foreign Governmental Entity, is required for Eisai to purchase the Purchased Assets or the Inventory from Arena and otherwise consummate the transactions contemplated hereunder or under the Supply Agreement.
(b)Brokers. No broker, finder, financial advisor, investment banker or other Person is or will be entitled to any brokerage, finder’s, financial advisor’s or other similar fee or commission in connection with the transactions contemplated under this Agreement or the Supply Agreement based upon arrangements made by or on behalf of Eisai.
(c)Non-Action or Claim. As of the Effective Date, there are no pending or threatened in writing, or to Knowledge of Eisai, threatened adverse actions, suits, claims, or formal governmental investigations by or against Eisai or any of its Affiliates in or before any court, Regulatory Authority or other governmental authority in the Territory with respect to Eisai’s marketing, promotion or sale of pharmaceutical products in the Territory that would materially negatively affect Eisai’s ability to perform its obligations under this Agreement or the Supply Agreement.
(d)Investigations. Eisai shall notify Arena within 30 days after it becomes the subject of an investigation by any governmental authority with respect to its marketing practices or marketing conduct with respect to pharmaceutical products in the Territory (including any Product).
(e)No Blocking Patents. To Eisai’s Knowledge, Eisai does not own or control any Patents as of the Effective Date in the Territory that would be infringed by Arena’s or Arena GmbH’s conduct of the development activities contemplated to be conducted under this Agreement or manufacturing activities contemplated to be conducted under the Supply Agreement as of the Effective Date.
(f)Regulatory Matters. At no time when Eisai was responsible for Regulatory Filings under the Existing Agreement did Eisai or any of its Affiliates, or any of its or their respective officers, employees, or agents, make an untrue statement of material fact or fraudulent statement to the FDA or any other Regulatory Authority with respect to the development of the Initial Product, fail to disclose a material fact required to be disclosed to the FDA with respect to the development of the Initial Product, or commit an act, make a statement, or fail to make a statement with respect to the development of the Initial Product that could reasonably be expected to provide a basis for the FDA to invoke its policy respecting “Xxxxx,
00
Xxxxxx Xxxxxxxxxx of Material Facts, Bribery, and Illegal Gratuities”, set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto.
(g)Scientific and Technical Information. To the Knowledge of Eisai, (x) no serious adverse event information has come to the attention of Eisai or any of its Affiliates with respect to the Compound or the Initial Product that is materially different with respect to the incidence, severity or nature of such serious adverse events than the information that was filed as safety updates to any Regulatory Filings for the Compound or the Initial Product, and (y) all written data summaries that were included in any such Regulatory Filings based on clinical trials conducted or sponsored by Eisai or any of its Affiliates accurately summarize in all material respects the raw data underlying such summaries.
(h)Excluded Liabilities. To the Knowledge of Eisai, as of the Effective Date, there are no facts or circumstances, and there have been no allegations by a Third Party, that are reasonably expected to result in any Excluded Liability for which Eisai would incur Losses.
Article 12 INDEMNIFICATION; LIABILITY
12.1Survival; Expiration. All representations and warranties contained in this Agreement or any Related Document shall survive the Closing and shall expire on the second anniversary of the Effective Date; provided, however, that the representations and warranties contained in Sections 11.1(a), 11.1(b), 11.2(b) and 11.2(g) and Section 4(a) of the Side Letter Agreement shall survive the Closing until the expiration of any applicable statute of limitations (after giving effect to any extensions or waivers) (each applicable period, the “Survival Period”). No claim for indemnification hereunder for breach of any such representations or warranties may be made after the expiration of the applicable Survival Period, except in the case of fraud, willful breach or intentional misrepresentation; provided, that, such obligations to indemnify, hold harmless and reimburse shall not terminate with respect to any Losses as to which the Indemnitee shall have, on or prior to such date, previously made a claim by delivering a written notice of such claim to the Indemnitor. All covenants and agreements in this Agreement shall survive the Closing until fully performed or as further specified in this Agreement. Each Party shall give prompt written notice to the other Party of (x) any event, circumstance or condition that constitutes a breach of, or makes inaccurate, any representation and warranty of such Party hereunder, or (y) the non-fulfillment of any covenant, agreement or obligation of such Party hereunder.
62
(a)any breach or inaccuracy of any representation or warranty of Eisai in this Agreement or any Related Document;
(b)any failure by Eisai to duly and timely perform or fulfill any of its covenants or agreements required to be performed by Eisai under this Agreement or any Related Document;
(c)any Transfer Taxes or Apportioned Obligations allocated to Eisai pursuant to Section 7.1;
(d)any Assumed Liability; and
(e)any claims, actions, suits or proceedings brought by a Third Party (each, a “Third Party Claim”) against any Arena Indemnitee to the extent arising from, based upon or occurring as a result of: (i) the actual or alleged (A) negligence or willful misconduct of, (B) violation of Applicable Laws by, in each case ((A) and (B)), Eisai or any of its Affiliates or other Eisai Related Party, Co-Promotion Partner or other subcontractors in performing any activity contemplated by this Agreement, or (C) breach of this Agreement or the PV Agreement by Eisai or any Eisai Related Party or Co-Promotion Partner; (ii) the regulatory activities, development, manufacture, use, handling, storage, sale, Commercialization or other exploitation of any Compound, Related Compound or Product by Eisai or any Eisai Related Party or Co-Promotion Partner or its or their collaborators or other subcontractors at any time after the Effective Date; (iii) any investigation by a Governmental Entity of Eisai’s or any Eisai Related Party’s or Co-Promotion Partner’s marketing, promotion, detailing or similar activities with respect to Products in the Territory; or (iv) any alleged or actual infringement arising from, based on or occurring as a result of the use by Eisai, any Eisai Related Party or Co-Promotion Partner of any Product Trademark, Non-Branded Trademark or any Development Trademark (each as defined in the Existing Agreement); except that the foregoing indemnification obligations shall not apply (A) to Product Liability Claims and (B) to the extent any such Third Party Claim is based on or result from matters within the scope of the indemnification obligations of Arena set forth in Section 12.3 or in Section 12.10(b), as to which Third Party Claim each Party shall indemnify the other Party to the extent of its liability with respect to the Losses applicable to such Third Party Claim.
12.3Indemnification of Eisai. Arena shall defend, indemnify and hold harmless each of Eisai, its Affiliates, and its and their respective directors, officers, stockholders and employees (collectively, the “Eisai Indemnitees”) from and against any and all Losses from:
(a)any breach or inaccuracy of any representation or warranty of Arena or Arena US in this Agreement or any Related Document;
63
(b)any failure by Arena or Arena US to duly and timely perform or fulfill any of its covenants or agreements required to be performed by Arena under this Agreement or any Related Document;
(c)any Excluded Liability; except that the indemnification obligation in this clause (c) shall not apply (i) to Product Liability Claims or (ii) to any matters within the scope of the indemnification obligations of Eisai set forth in Section 12.2 or in Section 12.10(a), as to which matter each Party shall indemnify the other Party to the extent of its liability with respect to the Losses applicable to such matter;
(d)any Apportioned Obligations allocated to Arena pursuant to Section 7.1; and
(e)any Third Party Claims against any Eisai Indemnitee to the extent arising from, based on or occurring as a result of: (i) the regulatory activities, development, manufacture, use, handling, storage, sale or other exploitation of any Compound, Related Compound or Product by Arena, any of its Affiliates or any other subcontractor, licensee, distributor or collaborator of Arena or any of its Affiliates (excluding in all cases Eisai, its Affiliates and their respective licensees, distributors and subcontractors) at any time after the Effective Date, but excluding any activities under the Supply Agreement; (ii) the actual or alleged (A) negligence or willful misconduct of or (B) violation of Applicable Laws by, in each case ((A) and (B)), Arena or any of its Affiliates or its or their respective other subcontractors in performing any activity contemplated by this Agreement; or (iii) the actual or alleged breach of this Agreement or the PV Agreement by Arena or any of its Affiliates; except that the foregoing indemnification obligations shall not apply (A) to Product Liability Claims and (B) to the extent any such Third Party Claim is based on or result from matters within the scope of the indemnification obligations of Eisai set forth in Section 12.2 or in Section 12.10(a), as to which Third Party Claim each Party shall indemnify the other Party to the extent of its liability with respect to the Losses applicable to such Third Party Claim.
12.4Calculation of Losses. Any indemnity payment hereunder shall be treated as an adjustment to the Purchase Price to the extent permitted by Applicable Law.
12.5Set-Off Rights. Until the date that is […***…] following the Effective Date, Eisai shall have the right to set-off, against any amounts that would otherwise be payable by Eisai to Arena pursuant to Section 7.2, Section 8.2 or Section 8.3 or otherwise under this Agreement, the amount required to be paid by Arena at the time of set-off (but not any amounts not yet due and payable) pursuant to Section 12.3 or Section 12.8.
(a)No Eisai Indemnitee shall be entitled to be indemnified pursuant to Section 12.3(a) unless the aggregate of all Losses under Section 12.3(a) to which the Eisai Indemnitees would, but for this Section 12.6(a), be entitled to indemnification exceeds on a cumulative basis […***…] (the “Indemnity Threshold”), at which point each Eisai Indemnitee shall be entitled to be indemnified for the aggregate amount of all Losses and not just amounts in excess of the Indemnity Threshold (except that this Section 12.6(a) shall not apply to any breach of the representations and warranties set forth in Sections 11.1(a), 11.1(b), 11.2(b) or 11.2(g), Section 4(a) of the Side Letter Agreement or any actual fraud, intentional misrepresentation or willful misconduct as determined under common law).
***Confidential Treatment Requested
64
(b)Arena shall have no liability pursuant to Section 12.3(c) for (i) any Losses above […***…] arising from any single claim, action, suit or proceeding, (ii) any Losses above […***…] in the aggregate (except that the limitations in clauses (i) and (ii) shall not apply to Losses from or relating to any Third Party Distributor Agreement prior to the Effective Date), (iii) any Losses arising from Product Liability Claims or (iv) any Losses arising from any claim, action, suit or proceeding brought after the date that is […***…] months after the Effective Date.
(c)No Arena Indemnitee shall be entitled to be indemnified pursuant to Section 12.2(a) unless the aggregate of all Losses under Section 12.2(a) to which the Arena Indemnitees would, but for this Section 12.6(c), be entitled to indemnification exceeds the Indemnity Threshold, at which point each Arena Indemnitee shall be entitled to be indemnified for the aggregate Losses and not just amounts in excess of the Indemnity Threshold (except that this Section 12.6(c) shall not apply to any breach of the representations and warranties set forth in Sections 11.1(a) or 11.1(b) or any actual fraud, intentional misrepresentation or willful misconduct as determined under common law).
(d)To the fullest extent permitted by Applicable Law, the indemnities set forth in this Article 12 shall be the exclusive monetary remedies of the Eisai Indemnitees against Arena and the Arena Indemnitees against Eisai, as applicable, for any breach of representation or warranty or breach of any covenant or agreement contained in this Agreement or any Related Document, except in the case of fraud, intentional misrepresentation or willful misconduct or in the case of equitable remedies.
(e)The representations, warranties, agreements, covenants and obligations of Arena and Arena US, and the rights and remedies that may be exercised by the Eisai Indemnitees, shall not be limited or otherwise affected by or as a result of any information furnished to, or any investigation made by or knowledge of, any of the Eisai Indemnitees or any of their Representatives. The representations, warranties, agreements, covenants and obligations of Eisai, and the rights and remedies that may be exercised by the Arena Indemnitees, shall not be limited or otherwise affected by or as a result of any information furnished to, or any investigation made by or knowledge of, any of the Arena Indemnitees or any of their Representatives.
***Confidential Treatment Requested
65
(a)Notice and Right to Assume. A Party that intends to exercise its rights to defense, indemnity or hold harmless under this Article 12 (the “Indemnitee”) shall promptly notify the indemnifying Party (the “Indemnitor”) in writing of any Third Party Claim in respect of which the Indemnitee intends to exercise such rights. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim shall only relieve the Indemnitor of its obligations under this Article 12 if and to the extent the Indemnitor is actually prejudiced thereby. The Indemnitee shall provide the Indemnitor with reasonable assistance, at the Indemnitor’s expense, in connection with the defense of the Third Party Claim. The Indemnitor shall have the right to assume and conduct the defense of the Third Party Claim with counsel of its choice. The Indemnitee may participate in and monitor such defense with counsel of its choice, which shall be at its own expense. The Indemnitor shall not settle any Third Party Claim without the prior written consent of the Indemnitee, not to be unreasonably conditioned, withheld or delayed, unless the settlement involves only the payment of money by the Indemnitor and does not involve any admission of liability or wrongdoing on the part of any Arena Indemnitees or Eisai Indemnitees, as applicable. So long as the Indemnitor is defending the Third Party Claim, the Indemnitee shall not settle any such Third Party Claim without the prior written consent of the Indemnitor.
(b)Indemnitor Conducts Defense. The assumption of a defense by the Indemnitor shall not be deemed an admission that the Indemnitor has an obligation to defend, indemnify or hold harmless an Arena Indemnitee or Eisai Indemnitee, as applicable, from and against any Loss from a Third Party Claim. If the Indemnitor assumes and conducts the defense of a Third Party Claim as provided above, and if it is ultimately determined that the Indemnitor was not obligated to indemnify, defend, or hold harmless an Arena Indemnitee or Eisai Indemnitee, as applicable, from and against any Loss from such Third Party Claim, the Indemnitee shall reimburse the Indemnitor for any and all reasonable and verifiable costs and expenses (including attorneys’ fees and costs of suit) and all other Losses incurred by the Indemnitor in connection with such Third Party Claim.
(c)Indemnitor Does Not Conduct Defense. If the Indemnitor does not assume and conduct the defense of a Third Party Claim as provided above, (i) the Indemnitee may defend against such Third Party Claim; provided, that the Indemnitee shall not settle any Third Party Claim without the prior written consent of the Indemnitor, not to be unreasonably conditioned, withheld or delayed and (ii) if it is ultimately determined that the Indemnitor was obligated to indemnify, defend, or hold harmless an Arena Indemnitee or Eisai Indemnitee, as applicable, from and against any Loss from such Third Party Claim, the Indemnitor shall reimburse the Indemnitee for any and all reasonable and verifiable costs and expenses (including attorneys’ fees and costs of suit) and all other Losses incurred by the Indemnitee in connection with such Third Party Claim.
66
(a)All Product Liability Claims brought prior to the date that is […***…] months after the Effective Date will be governed by the terms of Section 11.4 of the Existing Agreement (with Arena substituted for Arena GmbH in such terms). All Product Liability Claims brought on or after the date that is […***…] months after the Effective Date will be governed by Sections 12.8(b) and 12.8(c).
(b)Except as set forth in Section 12.8(c) with respect to Arena Manufacturing Defect Losses, for any Product Liability Claim brought on or after the date that is […***…] months after the Effective Date, Eisai shall bear 100% of the Product Liability Defense Costs and shall bear 100% of all Product Liability Losses.
(c)If a Product Liability Claim brought on or after the date that is […***…] months after the Effective Date includes allegations of Arena Manufacturing Defect Losses, Arena may participate in and monitor the defense of such Product Liability Claim defense with Shadow Counsel appointed by Arena, and the attorneys’ fees and costs for such Shadow Counsel shall be borne solely by Arena. Eisai shall not settle, or consent to the settlement of, any Product Liability Claim brought on or after the date that is […***…] months after the Effective Date that would require the payment to a Third Party of Arena Manufacturing Defect Losses without the prior written consent of Arena, such consent not to be unreasonably withheld, conditioned or delayed. Each of Arena and Eisai shall bear […***…]% of all Arena Manufacturing Defect Losses.
(d)Notwithstanding anything to the contrary in this Agreement, if after a Facility Acquisition, Eisai is actually indemnified by Arena GmbH or its successor or assign against any Losses under the Supply Agreement, Arena shall have no obligation to also indemnify Eisai in respect of such Losses and may credit any Losses for which Arena GmbH or its successor or assign indemnified Eisai against any Arena Manufacturing Defect Losses for which Arena is responsible under Section 12.8. Prior to a Facility Acquisition, Arena GmbH’s obligations to indemnify Eisai under the Supply Agreement will not apply to any Product Liability Claims, Product Liability Defense Costs and Product Liability Losses, which will instead be governed by the terms of this Section 12.8.
(e)For the avoidance of doubt, notwithstanding any other provision of this Agreement, any claims, actions, suits or proceedings based on or occurring as a result of personal injury, death or property damage (to the extent resulting from personal injury or death) caused by or resulting from (or allegedly caused by or resulting from) the use of a Product sold, distributed, dispensed or otherwise administered in any country outside of the Existing Agreement Territory prior to the Effective Date and all Losses related thereto shall, as between Eisai and Arena, be the exclusive responsibility of Arena and constitute Excluded Liabilities hereunder.
***Confidential Treatment Requested
67
12.9Insurance; Capitalization.
(a)Each Party, at its own expense, shall maintain appropriate insurance with an insurance carrier that has a minimum rating of A.M. Best’s rating of A-7 in an amount consistent with industry standards, for a company in a similar position to such Party, which shall include, (a) during the Term, in the case of Arena and Eisai, general liability insurance in the minimum amount of US$1 million per occurrence, US$2 million in the aggregate, and US$10 million umbrella coverage, (b) during the Term, in the case of Eisai, product liability insurance (including clinical trial insurance) in the minimum amount of US$10 million per occurrence and in the aggregate, and (c) until the first anniversary of the Effective Date, in the case of Arena, product liability insurance in the minimum amount of US$/10 million per occurrence and in the aggregate. Eisai shall maintain such product liability insurance at the same level for not less than five years after termination of this Agreement, and shall maintain such clinical trial insurance at the same level for five years after the last clinical trial for a Product conducted by or on behalf of Eisai. Each Party shall provide the other Party with written notice at least 30 days prior to any cancellation, nonrenewal or material change in the insurance described in clause (a) and Eisai shall provide Arena with written notice at least 30 days prior to any cancellation, nonrenewal of material change in the insurance described in clause (b) above, and each Party shall name the other Party as an additional insured with respect to such insurance. Each Party shall provide a certificate of insurance evidencing such coverage to the other Party upon request. It is understood that such insurance shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligations under this Article 12.
(b)Arena shall at all times during the Term hold cash in an amount of no less than […***…].
(a)Indemnification of Arena under the Supply Agreement. Eisai shall defend, indemnify and hold harmless the Arena Indemnitees from and against any and all Losses from any Third Party Claims against any Arena Indemnitee to the extent arising from, based upon or occurring as a result of: (i) the actual or alleged (A) negligence or willful misconduct of or (B) violation of Applicable Laws by, in each case ((A) and (B)), Eisai or any Eisai Related Party or other subcontractor under the Supply Agreement in performing any activity contemplated by the Supply Agreement or the Quality Agreements (as defined in the Supply Agreement); or (ii) any actual or alleged breach by Eisai (or any Eisai Related Party or other subcontractor under the Supply Agreement) of the Supply Agreement or the Quality Agreements; except that the foregoing indemnification obligations shall not apply to the extent any such Third Party Claim is based on or result from matters within the scope of the indemnification obligations of Arena set forth in Section 12.3 of 12.10(b), as to which Third Party Claim each Party shall indemnify the other Party to the extent of its liability with respect to the Losses applicable to such Third Party Claim.
***Confidential Treatment Requested
68
(b)Indemnification of Eisai under the Supply Agreement. Arena shall defend, indemnify and hold harmless the Eisai Indemnitees from and against any and all Losses from any Third Party Claims against any Eisai Indemnitee to the extent arising from, based on or occurring as a result of: (i) the actual or alleged (A) negligence or willful misconduct of or (B) violation of Applicable Laws by, in each case ((A) and (B)), Arena or any of its Affiliates or subcontractors under the Supply Agreement in performing any activity contemplated by the Supply Agreement or the Quality Agreements, but excluding Product Liability Claims; (ii) any actual or alleged breach by Arena (or any of its Affiliates or subcontractors under the Supply Agreement) of the Supply Agreement or the Quality Agreements (as defined in the Supply Agreement), but excluding Product Liability Claims, except as expressly provided in Section 12.8; or (c) any actual or alleged breach by Eisai of a Third Party Distributor Agreement to the extent resulting from an act or omission of Arena except to the extent Arena was acting in accordance with Eisai’s written instructions or resulting from Eisai’s failure to pay any amounts due under the Supply Agreement; except that the foregoing indemnification obligations shall not apply to the extent any such Third Party Claim is based on or result from matters within the scope of the indemnification obligations of Eisai set forth in Section 12.2 or 12.10(a), as to which Third Party Claim each Party shall indemnify the other Party to the extent of its liability with respect to the Losses applicable to such Third Party Claim.
Article 13 TERM AND TERMINATION
13.1Term. This Agreement shall commence on the Effective Date and shall continue in full force and effect until termination of this Agreement with respect to all countries in the Territory (such period, the “Term”).
13.2Post-Closing Termination.
(a)By Arena for Eisai’s Failure to Develop or Commercialize. Arena may terminate this Agreement upon written notice to Eisai, (i) with respect to a Major Market (such Major Market, a “Terminated Territory”), if (A) prior to obtaining Regulatory Approval of a Product in such Major Market, Eisai permanently ceases development of all Products for such Major Market or (B) after obtaining Regulatory Approval of a Product in such Major Market, Eisai permanently ceases Commercialization of all Products in such Major Market or (ii) with respect to this Agreement in its entirety, if Eisai permanently ceases development and Commercialization of all Products in the Territory. For purposes of this Section 13.2(a), the normal pauses or gaps between or following clinical trials or other studies for the analysis of data, preparation of reports and design of future clinical trials or preparation of regulatory filings and other customary development functions not constituting clinical trials do not constitute a cessation of development and launch preparation activities shall constitute Commercialization.
(b)By Eisai for Non-Compete Reasons. Eisai may terminate this Agreement in its entirety or with respect to one or more countries in the Territory pursuant to Section 4.7(c).
69
(c)Other Arena Termination Rights.
(i)Arena shall have the right to terminate this Agreement immediately upon written notice to Eisai if Eisai or any of its Affiliates commences, or knowingly and materially assists or encourages any Third Party to commence or conduct, any interference, re-examination or opposition proceeding with respect to, challenges the validity or enforceability of, or opposes any extension of or the grant of a supplementary protection certificate with respect to, any Arena Licensed Patent.
(ii)Arena shall have the right to terminate this Agreement immediately upon written notice to Eisai if Eisai is debarred under the FFDCA or listed on either Excluded List.
(iii)Arena shall have the right to terminate this Agreement on five days written notice to Eisai if Eisai breaches its obligations under Section 9.8.
13.3Adjudication of Disputes Regarding Cessation of Development or Commercialization.
(a)In the event of any dispute, controversy or claim regarding whether or not Arena has a right to terminate this Agreement in its entirety or with respect to a Major Market pursuant to Section 13.2 (a “Termination Dispute”), the Parties shall attempt to resolve such Dispute in accordance with Section 14.1. If such Termination Dispute is not resolved in accordance with Section 14.1 and Arena wishes to pursue such termination, such Termination Dispute shall be resolved by binding arbitration in accordance with the Rules of Arbitration of the International Chamber of Commerce (the “ICC”) as then in effect as such rules may be modified by this Section 13.3 or agreement of the Parties, and judgment on the arbitration award may be entered in any court having jurisdiction thereof. The decision rendered in any such arbitration will be final and not appealable, absent manifest error. If Arena intends to commence binding arbitration of such Termination Dispute, Arena shall file a request for arbitration with the ICC and provide written notice to Eisai informing Eisai of such intention.
(b)The arbitration shall be conducted by a panel of three arbitrators experienced in the pharmaceutical business, each of whom shall not be a current or former employee or director, or a then-current stockholder, of either Party or any of its Affiliates (the “Panel”). Within 30 days after receipt of the original notice of binding arbitration, each Party shall nominate one arbitrator for the ICC’s confirmation (with the right to nominate a replacement arbitrator until an arbitrator nominated by such Party is confirmed by the ICC) and such two arbitrators shall jointly nominate the third arbitrator for the ICC’s confirmation; provided, that if the two arbitrators nominated by the Parties are unable or fail to agree upon the third arbitrator within such period, the third arbitrator shall be appointed by the ICC. The place of arbitration shall be New York, New York.
(c)Within 30 days after the appointment and selection of the Panel, the Parties shall reach an agreement upon and thereafter shall follow the arbitration procedures, including limits on discovery, ensuring that the arbitration will be concluded and the award rendered as expeditiously as possible, but in any event within eight months from appointment
70
and selection of the Panel. In the event the Parties fail to reach an agreement on procedures, procedures meeting such time limits shall be determined by the Panel and adhered to by the Parties.
(d)All rulings of the Panel shall be in writing and shall be delivered to the Parties within seven days of the conclusion of the arbitration.
(e)The Panel shall, in rendering its decision, apply the substantive law of the laws of the State of New York, United States, without reference to its conflicts of law principles with the exception of sections 5-1401 and 5-1402 of New York General Obligations Law, and without giving effect to any rules or laws relating to arbitration.
(f)The Panel, in rendering its decision, must only determine whether or not Arena has a right to terminate this Agreement in its entirety or with respect to the applicable Major Market pursuant to Section 13.2 and may not award any other relief or take any other action.
(g)Either Party may apply to the Panel for interim injunctive relief until the arbitration award is rendered or the controversy is otherwise resolved. Either Party also may, without waiving any remedy under this Agreement, seek from any court having jurisdiction any injunctive or provisional relief necessary to protect the rights or property of that Party pending the arbitration award. Subject to Section 12.7, each Party shall bear its own costs and expenses and attorneys’ fees, and the non-prevailing Party shall pay the full costs of the Panel’s fees and any administrative fees of arbitration.
(h)All proceedings and decisions of the Panel shall be deemed Confidential Information of each of the Parties, and shall be subject to Article 9. Except to the extent necessary to confirm or enforce an award or as may be required by Applicable Laws, neither a Party nor any member of the Panel may disclose the existence, content, or results of an arbitration without the prior written consent of both Parties.
13.4Accrued Obligations. The termination of this Agreement, in its entirety or with respect to a Terminated Territory, for any reason shall not release either Party from any liability or obligation that, at the time of such termination, has already accrued to such Party or that is attributable to a period prior to such termination, nor will any termination of this Agreement preclude either Party from pursuing all rights and remedies it may have under this Agreement, at law or in equity, with respect to breach of this Agreement.
13.5Effects of Termination in Entirety. If this Agreement is terminated in its entirety by Arena pursuant to Section 13.2(a) or (c) or by Eisai pursuant to Section 13.2(b), the following shall apply (in addition to any other rights and obligations under this Agreement with respect to such termination):
(a)License. Eisai shall and hereby does, and shall cause its Affiliates to, effective as of the effective date of termination, grant Arena and its Affiliates a perpetual, irrevocable, exclusive, royalty-free license, with the right to grant multiple tiers of sublicenses, in and to the Eisai Grantback Know-How and the Eisai Grantback Patent Rights to develop, make, have made, use, import, offer for sale, sell and otherwise Commercialize Products in the Territory.
71
(b)Assignment of Purchased Assets. Eisai hereby assigns, and shall cause its Affiliates to assign, to Arena or its designee all of its and its Affiliates’ right, title and interest in and to the Purchased Assets, free and clear of all Liens (other than Permitted Liens).
(c)Winding-Down of Development Activities. In the event there are any on-going clinical trials or other development work with respect to a Product in the Territory:
(i)The Parties shall work together in good faith to adopt a plan to wind-down such clinical trials or other development work in an orderly fashion, provided at Arena’s election Eisai will promptly transition such clinical trials or other development work activities to Arena or its designee, including the transfer to Arena of any Development Data then in Eisai’s or its Affiliate’s possession that has not previously been transferred (or developed) by Arena, with due regard for patient safety and the rights of any subjects that are participants in any clinical trials of a Product, and take any actions it deems reasonably necessary or appropriate to avoid any human health or safety problems and in compliance with all Applicable Laws; and
(ii)If Arena elects to wind-down the clinical trials or other development work with respect to a Product, all costs and expenses incurred from the effective date of the termination notice shall be borne 100% by Eisai. If Arena elects to transition the clinical trials or other development work with respect to a Product to Arena, Eisai will bear its own costs and expenses incurred from the effective date of the termination notice but Arena will bear any other costs or expenses incurred in connection with such transition, including any Third Party costs or expenses.
(d)Inventory. Arena shall have the right, but not obligation, on written notice to Eisai, to purchase from Eisai and its Affiliates and Eisai Related Parties quantities of Product remaining in inventory as of the termination date at the applicable Estimated Price (as defined in the Existing Agreement), if purchased under the Existing Agreement, or Product Purchase Price (as defined in the Supply Agreement), if purchased under the Supply Agreement, or the price charged by the manufacturer in an arms-length transaction, if purchased from such a manufacturer after the conclusion of the Continuation Period (as defined in the Supply Agreement), paid by Eisai or such Eisai Related Party for such Product.
(e)Assignment of Regulatory Filings (including Regulatory Approvals). Upon Arena’s request and to the extent permitted by Applicable Laws, Eisai shall assign or cause to be assigned to Arena or its designees (or to the extent not so assignable, Eisai shall take all reasonable actions to make available to Arena or its designee the benefits of) all Regulatory Filings (including INDs, NDAs and Regulatory Approvals) for the Products in the Territory, including any such Regulatory Filings made or owned by Eisai or any Eisai Related Party, at no cost to Arena. Eisai shall provide a complete copy of all Regulatory Filings assigned (or made available), as well as copies of all correspondence with Regulatory Authorities not already provided to Arena, pertaining to Products in the Territory.
(f)Transition. Eisai shall, at Arena’s cost and written request, use Commercially Reasonable Efforts to cooperate with Arena or its designee to effect a smooth and orderly transition in the development and Commercialization of the Products in the Territory. To the extent applicable, Arena shall use, identify and finalize an agreement or other arrangement
72
with a Third Party in relation to the Products and the transfer of the Regulatory Filings (including INDs, NDAs and Regulatory Approval) into the name of Arena or Arena’s designee so that the transition occurs as promptly as reasonably possible.
(g)Customer Agreements. Upon the completion of the rights and obligations defined in this Section 13.5, at the written request of Arena, Eisai shall assign to Arena or its designee any Third Party distribution agreements and Sublicense agreements that solely relate to the Products, to the extent permitted under each such agreement. In the event such assignment is not requested by Arena or is not permitted under any such agreement, then the rights of such Third Party with respect to each Product shall terminate upon termination of Eisai’s rights with respect thereto. Eisai shall use its good faith efforts to include provisions requiring compliance with the foregoing provision in the agreements with applicable Third Parties.
(h)Terminated Product Trademarks. Upon Arena’s request, Eisai shall assign or cause to be assigned to Arena or its designees (or to the extent not so assignable, Eisai shall take all actions to make available to Arena or its designee the benefits of) all Terminated Product Trademarks, at no cost to Arena. Eisai shall provide a list of all Terminated Product Trademarks used in Commercialization of the Products within 30 days of Arena’s request.
13.6Effects of Termination With Respect to a Terminated Territory. If this Agreement is terminated with respect to one or more Terminated Territories by Arena pursuant to Section 13.2(a), the following shall apply (in addition to any other rights and obligations under this Agreement with respect to such termination):
(a)Licenses. All licenses granted by Arena hereunder shall automatically be deemed to be amended to exclude, if applicable, the right to develop, make, have made, use, import, offer for sale, sell and otherwise Commercialize Products in such Terminated Territory other than to develop, make or have made Product in such Terminated Territory solely for the purpose of furthering any Commercialization of the Products in the remaining Territory.
(b)Certain Effects of Termination. The effects of termination set forth in Section 13.5 shall apply solely with respect to the activities and matters specific to such Terminated Territory.
73
Article 14 DISPUTE RESOLUTION AND GOVERNING LAW
14.2Governing Law; Litigation; Exclusive Venue and Service. This Agreement and all questions regarding its existence, validity, interpretation, breach or performance, shall be governed by, and construed and enforced in accordance with, the laws of the State of New York, United States, without reference to its conflicts of law principles with the exception of sections 5-1401 and 5-1402 of New York General Obligations Law. Subject to
74
Section 13.3, the Parties hereby irrevocably and unconditionally consent to the exclusive jurisdiction of the courts of the State of New York and the United States District Court for the Southern District of New York for any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement, and agree not to commence any action, suit or proceeding (other than appeals therefrom) related thereto except in such courts. The Parties irrevocably and unconditionally waive their right to a jury trial. The Parties further hereby irrevocably and unconditionally waive any objection to the laying of venue of any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement in the courts of the State of New York or in the United States District Court for the Southern District of New York, and hereby further irrevocably and unconditionally waive and agree not to plead or claim in any such court that any such action, suit or proceeding brought in any such court has been brought in an inconvenient forum. Each Party further agrees that service of any process, summons, notice or document by registered mail to its address set forth in Section 15.10 shall be effective service of process for any action, suit or proceeding brought against it under this Agreement in any such court.
15.1Existing Agreement. As of the Effective Date, the Existing Agreement is revised in its entirety and replaced with the provisions set forth in this Agreement, and the PV Agreement is hereby terminated; provided, however, that (a) the provisions regarding Product Purchase Price in Section 7.4(a) of the Existing Agreement shall survive and continue to apply only to (i) Product delivered for which payment has been made or is due as of the Specified Date, except as included in the reconciliation report dated as of March 31, 2016 provided by Eisai under Section 7.4(c) of the Existing Agreement, and (ii) Product ordered (but not yet delivered) prior to the Specified Date (collectively, the “Existing Agreement Product”), (b) the Product Purchase Price paid for the Existing Agreement Product shall not be subject to adjustment or reconciliation pursuant to Section 7.4(b), (c), (d) or (e) or Section 7.5 of the Existing Agreement and (c) Section 11.4 of the Existing Agreement shall survive and continue to apply only to Product Liability Claims required to be governed by the terms of Section 11.4 of the Existing Agreement pursuant to Section 12.8 hereof. Nothing in this Section 15.1 shall relieve either Party of any liability for any breach of the Existing Agreement or any indemnification obligations or any other remedies existing under the Existing Agreement, in each case, prior to the Effective Date.
15.2Force Majeure. If the performance of any part of this Agreement by a Party (other than making payment when due) is prevented, restricted, interfered with or delayed by any reason or cause beyond the reasonable control of such Party (including: fire, flood, volcano, embargo, power shortage or failure, acts of war, insurrection, riot, terrorism, strike, lockout or other labor disturbance, shortage of raw materials, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, or storm or like catastrophe, acts of God or any acts, omissions or delays in acting of the other Party) or by compliance with any injunction, law, order, proclamation, regulation, ordinance, demand or requirement of any government or of any subdivision, authority or representative of any such government (including changes in the requirements of a Regulatory Authority), whether or not it is later held to be invalid, except to the extent any such injunction,
75
law, order, proclamation, regulation, ordinance, demand or requirement operates to delay or prevent the non-performing Party’s performance as a result of any breach by such Party or any of its Affiliates of any term or condition of this Agreement, the PV Agreement or the Quality Agreement or any breach of Applicable Laws (an event of “Force Majeure”), the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such Force Majeure event; provided, that the affected Party shall use its substantial, good faith efforts to avoid or remove such causes of non-performance and shall continue performance with the utmost dispatch whenever such causes are removed or it is otherwise able (with Commercially Reasonable Efforts) to perform its obligations.
(a)Notification. If either Party becomes aware that such an event of Force Majeure has occurred, is imminent or likely, it shall immediately notify the other Party.
(b)Keeping the Other Informed. The Party subject to an event of Force Majeure shall keep the other Party informed as to the progress of overcoming or avoiding the effects of such an event of Force Majeure and of recommencing performing the affected obligation.
15.3Waiver of Breach. Any condition or term of this Agreement may be waived at any time by the Party that is entitled to the benefit thereof. No such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the waiving Party. No delay or waiver by either Party of any condition or term of this Agreement in any one or more instances shall be construed as a further or continuing waiver of such condition or term or of another condition or term of this Agreement.
15.4Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to perform all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
15.5Performance by Affiliates or Subcontractors.
(a)To the extent that this Agreement imposes obligations on Affiliates of a Party, such Party agrees to cause its Affiliates to perform such obligations. Either Party may contract with one or more of its Affiliates to perform its obligations hereunder; provided, that the Parties shall remain liable hereunder for the prompt payment and performance of all of their respective obligations hereunder.
(b)Each Party may subcontract some of its obligations under this Agreement to the extent expressly permitted under this Agreement; provided, that with respect to all subcontractors: (i) none of the other Party’s rights hereunder are materially diminished or otherwise materially adversely affected as a result of such subcontracting; (ii) the subcontractor undertakes in writing reasonable and customary obligations of confidentiality and non-use; (iii) the subcontractor does not have the right to further subcontract such obligation unless agreed by the other Party; (iv) the subcontracting Party shall remain responsible and liable for the performance by any subcontractor of its obligations under this Agreement; and (v) such permitted subcontracting shall not relieve the subcontracting Party of any liability or obligation under this Agreement, except to the extent satisfactorily performed by such subcontractor. In the event a Party performs any of its obligations under this Agreement through a subcontractor, then
76
such Party shall at all times be fully responsible for the performance and payment of such subcontractor. The termination of the engagement of, or termination of the appointment of, any subcontractor of a Party shall not release such Party from any liability or obligation that, at the time of such termination, has already accrued to such Party with respect to the subcontractor, nor will any such termination of such an engagement or termination of an appointment preclude the other Party from pursuing all rights and remedies it may have under this Agreement, at law or in equity, with respect to the subcontractor and its acts and omissions.
15.6Modification. No amendment or modification of any provision of this Agreement shall be effective unless in a prior writing signed by authorized officers of both Parties. No provision of this Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance, or any other matter not set forth in an agreement in writing and signed by authorized officers of both Parties.
15.7Severability. In the event any provision of this Agreement is held invalid, illegal or unenforceable in any jurisdiction, to the fullest extent permitted by Applicable Laws, (a) the Parties shall negotiate, in good faith and enter into a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and (b) if the rights and obligations of either Party will not be materially and adversely affected, all other provisions of this Agreement shall remain in full force and effect in such jurisdiction. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction.
15.9Language. The language of this Agreement is English. Any translation of this Agreement in another language shall be deemed for convenience only and shall never prevail over the original English version.
77
15.10Notices. Any notice or communication required or permitted under this Agreement shall be in writing in the English language, delivered personally, sent by facsimile or sent by internationally-recognized overnight courier to the following addresses of the Parties (or such other address for a Party as may be at any time thereafter specified by like notice):
|
To Arena: 356 Royalty Inc. 0000 Xxxxx Xxxxx Xxxxx Xxx Xxxxx, XX 00000 XXX Facsimile: (000) 000-0000 Attention: President and Chief Executive Officer
|
To Eisai: Eisai Inc. 000 Xxxx Xxxx. Xxxxxxxxx Xxxx, Xxx Xxxxxx 00000 Facsimile: (000) 000-0000 Attention: General Counsel |
|
with a copy to: Arena Pharmaceuticals, Inc. 0000 Xxxxx Xxxxx Xxxxx Xxx Xxxxx, XX 00000 XXX Facsimile: (000) 000-0000 Attention: General Counsel |
with a copy to: Eisai Inc. 000 Xxxx Xxxx. Xxxxxxxxx Xxxx, Xxx Xxxxxx 00000 Facsimile: (000) 000-0000 Attention: Chief Strategy Officer, Neurology Business Group |
Any such notice shall be deemed to have been given: (a) when delivered if personally delivered, (b) on the third day after dispatch if sent by confirmed facsimile, or (c) on the sixth day after dispatch if sent by internationally-recognized overnight courier. This Section 15.10 is not intended to govern the day-to-day business communications necessary between the Parties in performing their obligations under this Agreement.
15.11Assignment. This Agreement shall not be assignable or otherwise transferred, nor may any right or obligations hereunder be assigned or transferred (except as otherwise expressly stated in this Agreement), by either Party to any Third Party without the prior written consent of the other Party; except that (a) Arena may assign its rights to receive royalty payments and other payments under this Agreement to a Third Party without Eisai’s consent and (b) either Party may assign or otherwise transfer this Agreement without the consent of the other Party to a successor in interest that acquires all or substantially all of the business or assets of the assigning Party to which this Agreement relates, whether by merger, acquisition or otherwise; provided, that the successor in interest assumes this Agreement in writing or by operation of law; provided, that Eisai shall not have the right to assign this Agreement under the preceding clause prior to the expiration of the first 18 months after the Effective Date without Arena’s prior written consent, which may be granted or withheld in Arena’s sole discretion. In addition, either Party shall have the right to assign, sublicense, subcontract or delegate this Agreement or any or all of its obligations or rights hereunder to an Affiliate upon written notice to the other Party; provided, that the assigning, sublicensing, subcontracting or delegating Party hereby guarantees and shall remain fully and unconditionally obligated and responsible for the
78
full and complete performance of this Agreement by such Affiliate and in no event such assignment, sublicensing, subcontracting or delegation be deemed to relieve such Party’s liabilities or obligations to the other Party under this Agreement. The other Party shall, at the request and expense of the assigning, sublicensing, subcontracting or delegating Party, enter into such supplemental agreements with the applicable Affiliates as may be necessary or advisable to permit such Affiliates to avail itself of any rights or perform any obligations of the assigning, sublicensing, subcontracting or delegating Party hereunder. Subject to the foregoing, this Agreement shall inure to the benefit of each Party, its successors and permitted assigns. Any assignment of this Agreement in contravention of this Section 15.11 shall be null and void.
15.12No Partnership or Joint Venture. Each Party is an independent contractor under this Agreement. Nothing contained herein shall be deemed to create an employment, agency, joint venture or partnership relationship between the Parties or any of their agents or employees, or any other legal arrangement that would impose liability upon one Party for the act or failure to act of the other Party. The Parties shall operate their own businesses separately and independently and they shall hold themselves out as, act as, and constitute independent contractors in all respects and not as principal and agent, partners or joint venturers. The Parties shall each be responsible for fulfilling their own obligations under this Agreement, and they shall not have control or responsibility over the actions of the other Party. The Parties shall make and receive only such payments as are required under this Agreement, and shall not share in, or participate in, the business operations of the other Party. Neither Party shall have any express or implied power to enter into any contracts or commitments or to incur any liabilities in the name of, or on behalf of, the other Party, or to bind the other Party in any respect whatsoever.
15.13Interpretation. The captions to the several Articles and Sections of this Agreement are not a part of this Agreement but are included for convenience of reference and shall not affect its meaning or interpretation. In this Agreement: (a) the word “including” shall be deemed to be followed by the phrase “without limitation” or like expression; (b) “hereof”, “hereto”, “hereby”, “herein” and “hereunder” and words of similar import when used in this Agreement refer to this Agreement as a whole and not to any particular provision of this Agreement; (c) “extent” in the phrase “to the extent” means the degree to which a subject or other thing extends, and such phrase does not mean simply “if”; (d) the singular shall include the plural and vice versa; (e) references to a Person are also to its permitted successors and assigns; (f) masculine, feminine and neuter pronouns and expressions shall be interchangeable; (g) except where the context requires otherwise, “or” has the inclusive meaning represented by the phrase “and/or”; (h) references to an Applicable Law include any amendment or modification to such Applicable Law and any rules or regulations issued thereunder, whether such amendment or modification is made, or issuance of such rules or regulations occurs, before or after the Effective Date; and (i) a reference to any agreement includes any supplements and amendments to such agreement. Each accounting term used herein that is not specifically defined herein has the meaning given to it under GAAP consistently applied, but only to the extent consistent with its usage and the other definitions in this Agreement. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties and no rule of strict construction shall be applied against either Party. As used in this Agreement, the words “previously disclosed to Eisai prior to the Effective Date” means during a teleconference between counsel to Arena (which may include inside or outside counsel) and counsel to Eisai (which may include inside or outside counsel) at least one Business Day prior to the Effective Date, with such call beginning
79
with a statement indicating that items disclosed on such call constitute disclosure under this Agreement.
15.14References. Unless otherwise specified, (a) references in this Agreement to any Article, Section or Exhibit means references to such Article, Section or Exhibit of this Agreement and (b) references in any section to any clause are references to such clause of such section.
15.15Counterparts; Electronic Signature Pages. This Agreement may be executed in any number of counterparts each of which shall be deemed an original, and all of which together shall constitute one and the same instrument. This Agreement may be executed by facsimile or other electronic signatures and such signatures shall be deemed to bind each Party as if they were original signatures.
15.16Limitation of Liability. EXCEPT FOR LIABILITY FOR BREACH OF ARTICLE 9, NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE OTHER PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES IN CONNECTION WITH THIS AGREEMENT OR ANY RELATED DOCUMENT OR ANY LICENSE GRANTED HEREUNDER OR THEREUNDER; provided, that this Section 15.16 shall not be construed to limit either Party’s indemnification obligations under ARTICLE 12.
15.17Equitable Relief; Specific Performance. The Parties acknowledge and agree that the obligations and restrictions set forth in Article 9 are reasonable and necessary to protect the legitimate interests of the other Party and that such other Party would not have entered into this Agreement in the absence of such obligations and restrictions, and that any breach or threatened breach of any provision of Article 9 will result in irreparable injury to such other Party for which there will be no adequate remedy at law. In the event of a breach or threatened breach of any provision of Article 9 the non-breaching Party shall be authorized and entitled to obtain from any court of competent jurisdiction injunctive relief, whether preliminary or permanent, and an equitable accounting of all earnings, profits and other benefits arising from such breach, which rights shall be cumulative and in addition to any other rights or remedies to which such non-breaching Party may be entitled in law or equity. Each Party hereby waives any requirement that the other Party post a bond or other security as a condition for obtaining any such relief. Nothing in this Section 15.17 is intended, or should be construed, to limit either Party’s right to equitable relief or any other remedy for a breach of any other provision of this Agreement.
80
Article 16 COMPLIANCE WITH LAW
16.1Generally. Each Party covenants that during the Term it shall, and shall cause its Affiliates to, comply with Applicable Laws with respect to performing its obligations or exercising its rights under this Agreement.
16.2Securities Laws. Each of the Parties acknowledges that it is aware that the securities laws of the Territory and other countries prohibit any Person who has material non-public information about a publicly listed company from purchasing or selling securities of such company or from communicating such information to any person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities.
16.3Certain Payments. Each of the Parties acknowledges that it is aware that the United States and other countries have stringent laws that prohibit persons directly or indirectly to make unlawful payments to, and for the benefit of, government officials and related parties to secure approvals or permission for their activities.
[Signature Page Follows]
81
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the Effective Date.
|
356 ROYALTY INC. |
|
EISAI INC. | ||||
|
|
|
| ||||
|
By: |
|
/s/ Xxxx Xxxxxx |
|
By: |
|
/s/ Xxxxx Xxxxxxx |
|
|
|
|
|
|
|
|
|
Name: |
|
Xxxx Xxxxxx |
|
Name: |
|
Xxxxx Xxxxxxx |
|
|
|
|
|
|
|
|
|
Title: |
|
President and Chief Executive Officer |
|
Title: |
|
President and COO |
|
|
|
| ||||
|
EISAI CO., LTD. |
|
|
|
| ||
|
|
|
|
|
|
|
|
|
By: |
|
/s/ Xxxx Xxxxxx |
|
|
|
|
|
|
|
|
|
|
|
|
|
Name: |
|
Xxxx Xxxxxx |
|
|
|
|
|
|
|
|
|
|
|
|
|
Title: |
|
Corporate Officer and Senior Vice President |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[Signature Page to Transaction Agreement]
Confidential Execution Version
EXHIBIT A
Compound Structure
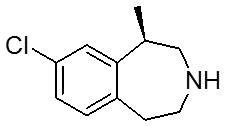
(R)-8-Chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine
EXHIBIT B
Xxxx of Sale
[See Attached]
Exhibit B-1
FORM OF Xxxx of Sale (ARENA)
This Xxxx of Sale (this “Xxxx of Sale”) is made as of this 28th day of December, 2016, is given by 356 Royalty Inc., a Delaware corporation having a principal place of business at 0000 Xxxxx Xxxxx Xxxxx, Xxx Xxxxx, XX 00000 (“Seller”) to Eisai Inc., a company organized under the laws of Delaware having a principal place of business at 000 Xxxx Xxxx., Xxxxxxxxx Xxxx, Xxx Xxxxxx 00000 and to (“ESI”), and Eisai Co., LTD., a company organized under the laws of Japan having a principal place of business at 0-0-00 Xxxxxxxxxx Xxxxxx-xx, Xxxxx, Xxxxx, 000-00 (“ECL”). “Buyer” shall mean (a) ESI, with respect to all rights of Eisai under this Agreement with respect to the ESI Territory and (b) ECL, with respect to all rights of Eisai under this Agreement with respect to the ECL Territory.
WHEREAS, Buyer and Seller, have entered into that certain Transaction Agreement, dated as of the date hereof (the “Transaction Agreement”); and
WHEREAS, pursuant to the Transaction Agreement, Seller has agreed to sell, deliver, convey, assign and transfer certain of the Purchased Assets to Buyer, and Buyer has agreed to purchase, take delivery of and acquire such Purchased Assets from Seller.
|
|
1. |
Definitions. Unless otherwise specifically provided herein, capitalized terms used in this Xxxx of Sale and not otherwise defined herein shall have the respective meanings ascribed thereto in the Transaction Agreement. |
|
|
2. |
Conveyance, Assignment and Transfer. In accordance with the provisions of the Transaction Agreement, Seller hereby sells, delivers, conveys, assigns and transfers to Buyer all of Seller’s right, title and interest in and to the Purchased Patents, the Arena Regulatory Approvals, the Samples, the Purchased Records and the Purchased Know-How. The assets referred to in the immediately preceding sentence related to or held in any country in Xxxxx Xxxxxxx, Xxxxx Xxxxxxx, Xxxxxxx Xxxxxxx and the Caribbean, shall be sold, delivered , conveyed, assigned or transferred to ESI and the assets referred to in the immediately preceding sentence related to or held in any other country shall be sold, delivered , conveyed, assigned or transferred to ECL. |
|
|
3. |
Transaction Agreement Controls. Notwithstanding any other provision of this Xxxx of Sale to the contrary, nothing contained herein shall in any way supersede, modify, replace, amend, change, rescind, waive, exceed, expand, enlarge or in any way affect the provisions, including warranties, covenants, agreements, conditions, representations or, in general any of the rights and remedies, or any of the obligations of Buyer or Seller set forth in the Transaction Agreement. This Xxxx of Sale is subject to and governed entirely in accordance with the terms and conditions of the Transaction Agreement. Nothing contained herein is intended to modify or supersede any of the provisions of the Transaction Agreement. |
|
|
4. |
Further Assurances. In accordance with Section 15.4 of the Transaction Agreement, for no further consideration, Seller shall execute, acknowledge and deliver such further instruments, and perform all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Xxxx of Sale or the Transaction Agreement. |
|
|
5. |
Assignment. This Xxxx of Sale and the rights and obligations of the Seller hereunder may not be assigned or delegated by the Seller. This Xxxx of Sale shall be binding upon and shall inure to the benefit of the Buyer and its successors and assigns. |
[Signature page follows]
2
IN WITNESS WHEREOF, Seller has executed this Xxxx of Sale as of the date first above written.
|
356 ROYALTY INC. | ||
|
| ||
|
By: |
|
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
Title: |
[Signature Page to Xxxx of Sale]
Exhibit B-2
FORM OF Xxxx of Sale (ARENA GMBH)
This Xxxx of Sale (this “Xxxx of Sale”) is made as of this 28th day of December, 2016, is given by Arena Pharmaceuticals GmbH, a company organized under the laws of Switzerland, having a principal place of business at Xxxxxx Xxxxxxxxxxxx 0, 0000, Xxxxxxxx, Xxxxxxxxxxx (“Seller”) to Eisai Inc., a company organized under the laws of Delaware having a principal place of business at 000 Xxxx Xxxx., Xxxxxxxxx Xxxx, Xxx Xxxxxx 00000 and to (“ESI”), and Eisai Co., LTD., a company organized under the laws of Japan having a principal place of business at 0-0-00 Xxxxxxxxxx Xxxxxx-xx, Xxxxx, Xxxxx, 000-00 (“ECL”). “Buyer” shall mean (a) ESI, with respect to all rights of Eisai under this Agreement with respect to the ESI Territory and (b) ECL, with respect to all rights of Eisai under this Agreement with respect to the ECL Territory.
|
|
1. |
Definitions. Unless otherwise specifically provided herein, capitalized terms used in this Xxxx of Sale and not otherwise defined herein shall have the respective meanings ascribed thereto in the Supply Agreement. |
|
|
2. |
Conveyance, Assignment and Transfer. In accordance with the provisions of the Supply Agreement, Seller hereby sells, delivers, conveys, assigns and transfers to Buyer all of Seller’s right, title and interest in and to the Purchased Assets. The assets referred to in the immediately preceding sentence related to or held in any country in Xxxxx Xxxxxxx, Xxxxx Xxxxxxx, Xxxxxxx Xxxxxxx and the Caribbean, shall be sold, delivered , conveyed, assigned or transferred to ESI and the assets referred to in the immediately preceding sentence related to or held in any other country shall be sold, delivered , conveyed, assigned or transferred to ECL. |
|
|
3. |
Supply Agreement and Transaction Agreement Control. Notwithstanding any other provision of this Xxxx of Sale to the contrary, nothing contained herein shall in any way supersede, modify, replace, amend, change, rescind, waive, exceed, expand, enlarge or in any way affect the provisions, including warranties, covenants, agreements, conditions, representations or, in general any of the rights and remedies, or any of the obligations of Buyer or Seller set forth in the Supply Agreement or any of the obligations of any party to the Transaction Agreement set forth in the Transaction Agreement. This Xxxx of Sale is subject to and governed entirely in accordance with the terms and conditions of the Supply Agreement. Nothing contained herein is intended to modify or supersede any of the provisions of the Supply Agreement and the Transaction Agreement. |
|
|
4. |
Further Assurances. In accordance with Section 17.6 of the Supply Agreement, for no further consideration, Seller shall execute, acknowledge and deliver such further instruments, and perform all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Xxxx of Sale or the Supply Agreement. |
|
|
5. |
Assignment. This Xxxx of Sale and the rights and obligations of the Seller hereunder may not be assigned or delegated by the Seller. This Xxxx of Sale shall be binding upon and shall inure to the benefit of the Buyer and its successors and assigns. |
[Signature page follows]
2
IN WITNESS WHEREOF, Seller has executed this Xxxx of Sale as of the date first above written.
|
ARENA PHARMACEUTICALS GMBH | ||
|
| ||
|
By: |
|
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
Title: |
[Signature Page to Xxxx of Sale]
Exhibit B-3
FORM OF Xxxx of Sale (ARENA US)
This Xxxx of Sale (this “Xxxx of Sale”) is made as of this 28th day of December, 2016, is given by Arena Pharmaceuticals Inc., a Delaware corporation having a principal place of business at 0000 Xxxxx Xxxxx Xxxxx, Xxx Xxxxx, XX 00000 (“Seller”) to Eisai Inc., a company organized under the laws of Delaware having a principal place of business at 000 Xxxx Xxxx., Xxxxxxxxx Xxxx, Xxx Xxxxxx 00000 and to (“ESI”), and Eisai Co., LTD., a company organized under the laws of Japan having a principal place of business at 0-0-00 Xxxxxxxxxx Xxxxxx-xx, Xxxxx, Xxxxx, 000-00 (“ECL”). “Buyer” shall mean (a) ESI, with respect to all rights of Eisai under this Agreement with respect to the ESI Territory and (b) ECL, with respect to all rights of Eisai under this Agreement with respect to the ECL Territory.
|
|
1. |
Definitions. Unless otherwise specifically provided herein, capitalized terms used in this Xxxx of Sale and not otherwise defined herein shall have the respective meanings ascribed thereto in the Transaction Agreement. |
|
|
2. |
Conveyance, Assignment and Transfer. In accordance with the provisions of the Letter Agreement, Seller hereby sells, delivers, conveys, assigns and transfers to Buyer all of Seller’s right, title and interest in and to the Purchased Intellectual Property. The assets referred to in the immediately preceding sentence related to or held in any country in Xxxxx Xxxxxxx, Xxxxx Xxxxxxx, Xxxxxxx Xxxxxxx and the Caribbean, shall be sold, delivered , conveyed, assigned or transferred to ESI and the assets referred to in the immediately preceding sentence related to or held in any other country shall be sold, delivered , conveyed, assigned or transferred to ECL. |
|
|
3. |
Letter Agreement and Transaction Agreement Control. Notwithstanding any other provision of this Xxxx of Sale to the contrary, nothing contained herein shall in any way supersede, modify, replace, amend, change, rescind, waive, exceed, expand, enlarge or in any way affect the provisions, including warranties, covenants, agreements, conditions, representations or, in general any of the rights and remedies, or any of the obligations of Buyer or Seller set forth in the Letter Agreement or any of the obligations of any party to the Transaction Agreement set forth in the Transaction Agreement. This Xxxx of Sale is subject to and governed entirely in accordance with the terms and conditions of the Letter Agreement. Nothing contained herein is intended to modify or supersede any of the provisions of the Letter Agreement and the Transaction Agreement. |
|
|
4. |
Further Assurances. In accordance with Section 15.4 of the Transaction Agreement, for no further consideration, Seller shall execute, acknowledge and deliver such further instruments, and perform all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Xxxx of Sale or the Transaction Agreement. |
|
|
5. |
Assignment. This Xxxx of Sale and the rights and obligations of the Seller hereunder may not be assigned or delegated by the Seller. This Xxxx of Sale shall be binding upon and shall inure to the benefit of the Buyer and its successors and assigns. |
[Signature page follows]
2
IN WITNESS WHEREOF, Seller has executed this Xxxx of Sale as of the date first above written.
|
ARENA PHARMACEUTICALS INC. | ||
|
| ||
|
By: |
|
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
Title: |
[Signature Page to Xxxx of Sale]
EXHIBIT C
Existing Arena Patents
[See Attached]
EXHIBIT C Existing Arena Patents (as of 12/21/2016)
[Pages 1 through 8 of this exhibit have been redacted and omitted pursuant to a confidential treatment request filed with the Securities and Exchange Commission.]
***Confidential Treatment Requested
EXHIBIT D
Form of Intellectual Property Assignments
[See Attached]
Exhibit D-1 to the Transaction Agreement
FORM OF
PATENT ASSIGNMENT (ARENA)1
This Patent Assignment (this “Assignment”) is made as of the __ day of ____, [ ], by 356 Royalty Inc., a Delaware corporation having a principal place of business at 0000 Xxxxx Xxxxx Xxxxx, Xxx Xxxxx, XX 00000 (“Assignor”) to [ ], having its place of business at _______________ (“Assignee”).
Assignor hereby requests the Commissioner of Patents and Trademarks and the corresponding entities or agencies in any other applicable countries to record Assignee as the assignee and owner of said Patents, and to issue any and all letters patent thereon to Assignee, as assignee of the entire right, title and interest in, to and under the same, for the sole use and enjoyment of Assignee, its successors, assigns or other legal representatives.
|
|
1 |
Note to Draft: Local forms outside the U.S. to be updated to comply with applicable law. |
|
2 |
Note to Draft: To be conformed to local jurisdictions that would require filing of a referenced agreement. |
[Assignor acknowledges and agrees that the representations, warranties, covenants, agreements and indemnitees contained in the Transaction Agreement shall not be superseded hereby, but shall remain in full force and effect to the full extent provided therein. To the extent that any provision of this Assignment is inconsistent or conflicts with the Transaction Agreement, the provisions of the Transaction Agreement shall control.]1 The parties may execute this Assignment in multiple counterparts, any one of which need not contain the signature of more than one party, but all such counterparts taken together shall constitute one and the same instrument. Any counterpart may be executed by facsimile or PDF signature and such facsimile or PDF signature shall be deemed an original. The terms and conditions of this Assignment shall inure to the benefit of Assignee, its successors, assigns and other legal representatives, and shall be binding upon Assignor, its successors, assigns and other legal representatives. Except to the extent that U.S. federal law preempts state law with respect to the matters covered hereby, this Assignment shall be governed by and construed in accordance with the laws of the State of New York, without giving effect to the principles of conflicts of law thereof.
|
|
1 |
Note to Draft: To be conformed to local jurisdictions that would require filing of a referenced agreement. |
|
|
Assignor | |
|
|
| |
|
|
356 Royalty INC. | |
|
|
| |
|
|
By |
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
Title: |
|
|
Assignee | |
|
|
| |
|
|
[●] | |
|
|
| |
|
|
By |
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
Title: |
[Signature Page to Patent Assignment]
Exhibit A – The Patents
[To be inserted]
Exhibit D-2 to the Transaction Agreement
FORM OF
PATENT ASSIGNMENT (ARENA US)1
Assignor hereby requests the Commissioner of Patents and Trademarks and the corresponding entities or agencies in any other applicable countries to record Assignee as the assignee and owner of said Patents, and to issue any and all letters patent thereon to Assignee, as assignee of the entire right, title and interest in, to and under the same, for the sole use and enjoyment of Assignee, its successors, assigns or other legal representatives.
[Assignor acknowledges and agrees that the representations, warranties, covenants, agreements and indemnitees contained in the Letter Agreement and the Transaction Agreement
|
|
1 |
Note to Draft: Local forms outside the U.S. to be updated to comply with applicable law. |
|
2 |
Note to Draft: To be conformed to local jurisdictions that would require filing of a referenced agreement. |
Exhibit D-2 to the Transaction Agreement
(the “Transaction Agreement”), dated December 28, 2016, between 356 Royalty Inc. and [Eisai] shall not be superseded hereby, but shall remain in full force and effect to the full extent provided therein. To the extent that any provision of this Assignment is inconsistent or conflicts with the Letter Agreement or the Transaction Agreement, the provisions of the Letter Agreement or the Transaction Agreement, as applicable, shall control.]1 The parties may execute this Assignment in multiple counterparts, any one of which need not contain the signature of more than one party, but all such counterparts taken together shall constitute one and the same instrument. Any counterpart may be executed by facsimile or PDF signature and such facsimile or PDF signature shall be deemed an original. The terms and conditions of this Assignment shall inure to the benefit of Assignee, its successors, assigns and other legal representatives, and shall be binding upon Assignor, its successors, assigns and other legal representatives. Except to the extent that U.S. federal law preempts state law with respect to the matters covered hereby, this Assignment shall be governed by and construed in accordance with the laws of the State of New York, without giving effect to the principles of conflicts of law thereof.
|
|
1 |
Note to Draft: To be conformed to local jurisdictions that would require filing of a referenced agreement. |
|
Assignor | |
|
| |
|
ARENA PHARMACEUTICALS, INC. | |
|
| |
|
By |
|
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
|
Assignee | |
|
| |
|
[●] | |
|
| |
|
By |
|
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
[Signature Page to Patent Assignment]
Exhibit A – The Patents
[To be inserted]
Exhibit D-3 to the Transaction Agreement
FORM OF
TRADEMARK ASSIGNMENT1
This Trademark Assignment (this “Assignment”) is dated as of ____________, [ ] (the “Effective Date”), and is made from Arena Pharmaceuticals GmbH, a company organized under the laws of Switzerland, having a principal place of business at Xxxxxx Xxxxxxxxxxxx 0, 0000, Xxxxxxxx, Xxxxxxxxxxx (“Assignor”) to [ ], a _________________ (“Assignee”).
WHEREAS, Assignor is the sole and exclusive owner of the Marks, and
Assignor does hereby irrevocably assign, transfer, convey and deliver to Assignee effective as of the Effective Date, and Assignee hereby accepts, all of Assignor’s worldwide right, title and interest in and to the Marks, including any common law, statutory and other rights associated therewith, together with the goodwill of the business associated with the use of and symbolized by the Marks related to a country in the [ESI][ECL] Territory (as defined in the Transaction Agreement), and all the registration applications and registrations therefor, and all rights to (i) bring an action, whether at law or in equity, for past, present or future infringement, dilution, misappropriation, misuse or other violation of said Marks against any third party, (ii) any proceeds, benefits, privileges, causes of action, and remedies relating to said Marks and (iii) recover damages, profits and injunctive relief for all past, present or future infringement, dilution, misappropriation, misuse, or other violation of said Marks.
Effective upon the Effective Date, Assignee shall be responsible for and shall pay all costs relating to the registration, maintenance and prosecution of said Marks, including payment of any associated fees therefor, for the notarization, authentication, legalization or consularization of the signatures hereof, and for the recording of such assignment documents with the appropriate governmental authorities.
|
|
1 |
Note to Draft: Local forms outside the U.S. to be updated to comply with applicable law. |
|
2 |
Note to Draft: To be conformed to local jurisdictions that would require filing of a referenced agreement. |
Assignor hereby requests the Commissioner of Patents and Trademarks and the corresponding entities or agencies in any other applicable countries to record Assignee as the assignee and owner of said Marks.
The parties acknowledge that certain of said Marks may not yet have been used in commerce prior to the Effective Date (each, an “Unused Xxxx”) and an application for registration for such Unused Xxxx xxx have been filed based on an intent to use it (an “ITU Application”) in one or more jurisdictions. With respect to the United States, the parties acknowledge and agree that the transfer contemplated by the Supply Agreement constitutes an assignment of a portion of the business of Assignor to which said Marks (including any Unused Marks) pertain, which business is ongoing and existing, as contemplated by Section 10 of the Xxxxxxxxx Xxx, 00 X.X.X. §0000, such that any such Unused Xxxx xxx be (and hereby is) included in this Assignment. To the extent that any applicable jurisdiction prohibits assignment of an ITU Application and/or Unused Xxxx xxxxx to use (even where, as here, transfer of a portion of the applicable business has occurred), and Assignor has not filed an allegation of use prior to the Effective Date, then the parties shall take such steps and file such documents, at Assignee’s request and expense, as may be necessary and appropriate to: (a) maintain such ITU Application; (b) enable Assignee to use such Unused Xxxx as Assignor’s licensee; (c) upon use of the Unused Xxxx, file an allegation of use in the appropriate jurisdiction; and (d) effect the assignment of such Unused Xxxx pursuant to terms comparable to the terms of this Assignment.
In addition to the any of the foregoing actions that may be necessary or appropriate, Assignor, at Assignee’s request and expense, shall execute, acknowledge and deliver to Assignee such other instruments of conveyance and transfer and will take such other actions and execute and deliver such other documents, certifications and further assurances as Assignee may reasonably require in order to vest title more effectively in Assignee, or to put Assignee more fully in possession of, any of said Marks. Both of the parties hereto shall cooperate with one another and execute and deliver to the other such other instruments and documents and take such other actions as may be reasonably requested from time to time by the other party hereto as necessary to carry out, evidence and confirm the intended purposes of this Assignment.
[Assignor acknowledges and agrees that the representations, warranties, covenants, agreements and indemnitees contained in the Supply Agreement or the Transaction Agreement (the “Transaction Agreement”), dated as of December 28, 2016, by and between 356 Royalty Inc. and [Eisai], shall not be superseded hereby but shall remain in full force and effect to the full extent provided therein. To the extent that any provision of this Assignment is inconsistent or conflicts with the Supply Agreement or the Transaction Agreement, the provisions of the Supply Agreement or the Transaction Agreement, as applicable, shall control.]1
This Assignment is executed by Assignor and shall be binding upon Assignor, its successors and assigns, for the uses and purposes above set forth and referred to and shall inure to the benefit of Assignee, its successors and assigns.
This Assignment may be executed in one or more counterparts, each of which shall be deemed an original, and together shall constitute one and the same agreement and shall become
|
|
1 |
Note to Draft: To be conformed to local jurisdictions that would require filing of a referenced agreement. |
effective when one or more counterparts have been signed by each of the parties and delivered to the other party, it being understood that the parties need not sign the same counterpart. This Assignment, following its execution, may be delivered via electronic mail or other form of electronic delivery, which shall constitute delivery of an execution original for all purposes.
Any claims and causes of action arising with respect to this Assignment shall be governed by and construed in accordance with the laws of the State of New York, without regard to the conflicts of law provisions thereof.
[Remainder of page intentionally left blank; signature page follows]
|
Assignor | |
|
| |
|
ARENA PHARMACEUTICALS GMBH | |
|
| |
|
By |
|
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
|
ASSIGNEE | |
|
| |
|
[●] | |
|
| |
|
By |
|
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
[Signature Page to Trademark Assignment]
Exhibit A – The Marks
[To be inserted]
Exhibit D-4 to the Transaction Agreement
FORM OF
DOMAIN NAME ASSIGNMENT
This Domain Name Assignment (this “Assignment”) is dated as of [ ] (the “Effective Date”), and is made from Arena Pharmaceuticals GmbH, a company organized under the laws of Switzerland, having a principal place of business at Xxxxxx Xxxxxxxxxxxx 0, 0000, Xxxxxxxx, Xxxxxxxxxxx (“Assignor”) to [ ] (“Assignee”).
WHEREAS, Assignor is the owner of the Domain Names; and
|
1. |
|
2. |
NY: 1026595
|
3. |
|
(a) |
Assignor acknowledges and agrees that the representations, warranties, covenants, agreements and indemnitees contained in the Supply Agreement and the Transaction Agreement shall not be superseded hereby but shall remain in full force and effect to the full extent provided therein. To the extent that any provision of this Assignment is inconsistent or conflicts with the Supply Agreement or the Transaction Agreement, the provisions of the Supply Agreement or Transaction Agreement, as applicable, shall control. |
|
(b) |
This Assignment is executed by Assignor and shall be binding upon Assignor, its successors and assigns, for the uses and purposes above set forth and referred to and shall inure to the benefit of Assignee, its successors and assigns. |
|
(c) |
This Assignment may be executed in one or more counterparts, each of which shall be deemed an original, and together shall constitute one and the same agreement and shall become effective when one or more counterparts have been signed by each of the parties and delivered to the other party, it being understood that the parties need not sign the same counterpart. This Assignment, following its execution, may be delivered via electronic mail or other form of electronic delivery, which shall constitute delivery of an execution original for all purposes. |
|
(d) |
Any claims and causes of action arising with respect to this Assignment shall be governed by and construed in accordance with the laws of the State of New York, without regard to the conflicts of law provisions thereof. |
[Remainder of page intentionally left blank; signature page follows]
2
|
ASSIGNOR | |
|
| |
|
ARENA PHARMACEUTICALS GMBH | |
|
| |
|
By: |
|
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
|
ASSIGNEE | |
|
| |
|
[●] | |
|
| |
|
By |
|
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
[Signature Page to Domain Name Assignment]
Annex A – The Domain Names
[See attached]
EXHIBIT E
Form of Development and Commercialization Report
[See Attached]
BELVIQ® Bi-Annual Report
Date
|
1. |
Executive Summary |
|
|
• |
Summary of major commercial, regulatory and development activities |
|
2. |
Global Commercial Performance […***…] |
|
3. |
Global Medical Affairs Activities […***…] |
|
4. |
Global Regulatory Activities […***…] |
***Confidential Treatment Requested
Bi-Annual Report: BEL-1
|
5. |
Global Development Status […***…] |
|
6. |
Partnership Activities |
End of Document
***Confidential Treatment Requested
Bi-Annual Report: BEL-2
Schedule 1.129(C) Purchased Patents (as of 12/21/2016)
[Pages 1 through 3 of this schedule have been redacted and omitted pursuant to a confidential treatment request filed with the Securities and Exchange Commission.]
***Confidential Treatment Requested
Schedule 11.2(j)
|
[…***…] |
|
***Confidential Treatment Requested
|
|
Business Classification: Confidential |
Page 1/1 |