SUPPLY AGREEMENT BETWEEN BIOMEDICA Life Sciences, S.A. AND MOLECULAR INSIGHT PHARMACEUTICALS, INC. dated as of October 19, 2009
Exhibit 10.5
BETWEEN
BIOMEDICA Life Sciences, S.A.
AND
MOLECULAR INSIGHT PHARMACEUTICALS, INC.
dated as of
October 19, 2009
TABLE OF CONTENTS
| Article 1 |
2 | |
| Definitions |
2 | |
| Article 2 |
4 | |
| Supply of Compound and Product |
4 | |
| Article 3 |
6 | |
| Price, Purchase Commitment and Payment |
6 | |
| Article 4 |
8 | |
| Exclusivity and Non-Compete |
8 | |
| Article 5 |
8 | |
| Term and Termination |
8 | |
| Article 6 |
9 | |
| Confidentiality |
9 | |
| Article 7 |
10 | |
| Indemnification and Insurance |
10 | |
| Article 8 |
12 | |
| Dispute Resolution |
12 | |
| Article 9 |
12 | |
| General Provisions |
12 | |
2
This supply agreement (“Agreement”), dated this 19th day of October, 2009 (the “Effective Date”) is entered into by and between Molecular Insight Pharmaceuticals, Inc. (referred to herein as “MIP”), a corporation organized and existing under the laws of The Commonwealth of Massachusetts and having its principal office at 000 Xxxxxx Xxxxxx, Xxxxxxxxx, XX 00000 XXX, and BIOMEDICA Life Sciences S.A., a corporation organized and existing under the laws of Greece, with offices at 0 Xxxxxxxxxx Xxx., 00000 Xxxxxxxx, Xxxxxx, Xxxxxx (referred to herein as “BIOMEDICA”), with Greek Tax ID of EL 000000000, from the tax office of FAEE Athens; each a “Party” and collectively the “Parties” hereto.
WHEREAS the Parties have executed a Territory License Agreement dated September 1, 2009;
WHEREAS, BIOMEDICA desires MIP to source and manufacture certain products (defined below) and supply such products to BIOMEDICA in accordance with the Territory License Agreement;
WHEREAS, MIP agrees to source and/or manufacture the products (defined below) and supply such products to BIOMEDICA;
NOW, THEREFORE, in consideration of the mutual covenants hereinafter expressed and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties agree as follows:
1. DEFINITIONS
1.1. “Affiliate” shall mean any person, corporation, or other entity which controls, is controlled by, or is under common control with, a Party to this Agreement. A corporation or other entity shall be regarded as in control of another corporation or entity if it owns or directly or indirectly controls more than fifty percent (50%) of the voting stock or other ownership interest of the other corporation or entity, or if it possesses, directly or indirectly, the power to direct or cause the direction of the management and policies of the corporation or other entity or the power to elect or appoint fifty percent (50%) or more of the members of the governing body of the corporation or other entity.
3
1.2. “Agreement” shall mean this Agreement together with all schedules, and appendices attached to this Agreement, all as respectively amended, modified or supplemented by the Parties in accordance with the terms of this Agreement.
1.3. “Compound” shall mean a lyophilized kit comprising the DOTA-chelated somatostatin peptide analogue known as edotreotide plus excipients provided.
1.4. “Confidential Information” shall mean any information relating to the scientific and business affairs of a Party disclosed to the other Party in connection with this Agreement, regardless of whether such information is specifically designated as confidential and regardless of whether such information is in written, oral, electronic, or other form. Such Confidential Information may include, without limitation, trade secrets, know-how, Inventions, non-published Patent Rights, technical data or specifications, materials formulations, compositions, processes, information, testing methods and/or results, business or financial information and methods, research and development activities, production and marketing plans, and customer and supplier information.
1.5. “Dose” shall mean a unit consisting of one patient vial of Compound or Product.
1.6. “Effective Date” shall mean the date first above written, which date post-dates the beginning of the Term as such as defined herein.
1.7. “Good Clinical Practice” or “GCP” shall mean the generally accepted standard of Good Clinical Practice within the pharmaceutical industry for the design, conduct, performance, monitoring, auditing, recording, analyses and reporting of clinical trials that provides assurance that the data and reported results are clinical and accurate and that the rights, integrity and confidentiality of the trial subjects are protected.
1.8. “Good Manufacturing Practices” or “GMP” shall mean the then current Good Manufacturing Practices as such term is defined from time to time by the FDA or other relevant governmental authority having jurisdiction over the development, manufacture or sale of the Compound or the Product in the Territory.
1.9. “Inventory” shall mean Compound or Product.
1.10. “Product” shall mean the yttrium radiolabeled Compound for therapeutic use and indium-111 radiolabeled Compound for dosimetry purposes, both in a form ready for use in human clinical trials and/or by the ultimate consumer with the trademark of Onalta.
1.11. “Purchase Order” shall mean the order form to be completed and issued by BIOMEDICA to MIP or MIP’s designee for the purpose of ordering Compound or Product (Exhibit B).
1.12. “Qualified Manufacturer” shall mean a licensed manufacturer capable of manufacturing commercial scale radiopharmaceuticals, in accordance with cGMP requirements and all applicable national and European directives, laws and regulations.
4
1.13. “Specification(s)” shall mean the standards established for the characteristics, quality, and quality control testing of the Compound or the Product, and its constituents, components, and packaging, as described in the IMPD, Section 2.1 SS.4, 2.1 SK.4 and 2.1.P.5 and MIP product specification and as may be amended from time to time by the mutual written agreement of the Parties (Exhibit A).
1.14. “Term” shall mean the period commencing on the September 1, 2009 (the effective date of the Territory License Agreement) and continuing for 10 (ten) years thereafter, with a renegotiation of the terms during year five (5) for the second five (5) years. In no case shall the Term survive termination of the Territory License Agreement.
1.15. “Territory” shall mean those countries as of the Effective Date belonging to the European Union, the European Free Trade Association (Switzerland, Iceland, Lichtenstein and Norway), Eastern Europe, Russia and the other former Commonwealth of Independent States (CIS), the Middle East and Arabic States, Turkey, and the North Africa region. “Territory” shall specifically exclude the State of Israel unless and until such rights become available. For the sake of clarity, as of the Effective Date, the Territory shall mean the specifically enumerated countries listed in Exhibit F attached hereto.
The following Exhibits attached to this Agreement are hereby incorporated by reference:
| Exhibit A: | Specifications |
| Exhibit B: | Purchase Order |
| Exhibit C: | Ordering and Fulfillment Process |
| Exhibit D: | Monthly Minimum Payment Obligation |
| Exhibit E: | Example of Over Achievement /Underachievement Adjustments |
| Exhibit F: | Listing of Countries in Territory |
2. SUPPLY OF COMPOUND AND PRODUCT
2.1 Engagement. During the Term and after the Effective Date of this Agreement, BIOMEDICA hereby engages MIP and MIP accepts such engagement as BIOMEDICA’s exclusive supplier of Compound and Product subject to the terms of this Agreement.
2.2 Product and Specifications. MIP agrees to supply, directly or through a MIP designee selected by MIP to be identified to BIOMEDICA immediately upon signing of this Agreement, the Compound or the Product to BIOMEDICA according to the Specifications (Exhibit A), in accordance with current good manufacturing practices (“GMP”) and in accordance with any other applicable standards imposed by law.
2.3 Orders of Compound or Product. BIOMEDICA shall submit to MIP or MIP’s designee a binding Purchase Order substantially in the form attached hereto as Exhibit B by
5
facsimile (or other suitable means) at least 10 Business Days in advance of intended shipment, (see Exhibit C Ordering and Fulfillment Process). Such Purchase Orders shall specify the quantity of the Compound or Product ordered, the delivery date, and shipping information. The Parties agree that the terms and conditions of this Agreement shall control as to a particular Purchase Order unless otherwise agreed to in writing by the Parties.
2.4 Prior to the first shipment of Compound or Product to any third party site, BIOMEDICA or its licensee shall obtain and forward to MIP from such third Party its license evidencing proper legal authority for the receipt and possession of the Compound or Product. If and to the extent necessary, BIOMEDICA shall further obtain all approvals, licenses and permits required to import Compound or Product into each country in the Territory as directed by BIOMEDICA.
2.5 MIP or MIP’s designee shall deliver Compound or Product in accordance with the quantities and requested delivery date(s) specified in the relevant order. MIP or MIP’s designee will pack the Compound or Product for shipment and storage in accordance with the applicable Specifications.
2.6 Delivery. MIP or MIP’s designee shall ship the Compound or the Product to BIOMEDICA’s facility (or as otherwise reasonably directed by BIOMEDICA in writing) using a carrier of BIOMEDICA’s selection, approved by MIP or MIP’s designee. MIP or its designee shall ship the Compound and the Products to BIOMEDICA or it’s designees in accordance with all appropriate regulations. BIOMEDICA shall be responsible for providing evidence of proper legal authority for the receipt and possession of Compound or Product in each country in the Territory. BIOMEDICA shall obtain all approvals, licenses appointing any customs brokers, and acquiring any necessary licenses or permits, including but not limited to obtaining the necessary import licenses from the appropriate governmental authorities. Delivery of Final Product as directed by BIOMEDICA shall be FCA (INCOTERMS) MIP or MIP designee’s facility shipping dock. Title and risk of loss for the goods shall pass to BIOMEDICA at point of delivery by MIP or MIP’s designee to the selected carrier. All transportation and packaging costs incurred to deliver Compound or Product ordered by BIOMEDICA shall be borne by BIOMEDICA.
2.7 Packaging Information. Packages will consist of one Dose per package. The package design for the Product will be IAEA compliant Type A packaging for international transportation. BIOMEDICA is solely responsible for compliance of the text of the package labels with applicable regulations in the Territory countries for both the Product and the Compound. It is the intent of the Parties that the package label will indicate BIOMEDICA as the Party for whom the Compound and/or Product is manufactured.
2.8 If either Party or its designee discovers that the Compound, Product and/or Dose does not comply with Specifications, then the discovering Party shall within 1 (one) business day inform the other Party of such non-compliance to determine a mutually agreed course of action. With respect to any such Compound, Product and/or Dose which do not comply with Specifications as a result of shortcomings in process or parameters under the direct control of
6
MIP or MIP’s designee, then MIP or MIP’s designee promptly will replace such Compound, Product and/or Dose at no additional cost to BIOMEDICA.
2.9 BIOMEDICA shall not modify the Compound or the Product, or use alternative radioisotopes with the Compound or Product. Yttrium-90 isotope supply must meet Specifications (Exhibit A) and be qualified and validated for the Product and/ or Compound. MIP will provide BIOMEDICA with two (2) qualified and validated suppliers of Yttrium-90. BIOMEDICA shall not be limited to these two suppliers to the extent additional suppliers of qualified and validated Ytrrium-90 may also be available.
2.10 During the period that precedes availability of the Product, MIP agrees to provide the Compound for purposes of radiolabeling by BIOMEDICA in connection with its Onalta compassionate use and clinical study activities in the Territory subject to BIOMEDICA first securing and providing MIP with evidence of receipt of all relevant licenses and regulatory approvals required in each relevant country in the Territory. The final formulation and doses of Compound will be compounded at the treatment center or designated site and coordinated by BIOMEDICA. BIOMEDICA shall be solely responsible for preparing or having prepared any Product and insuring compliance with GMP (Good Manufacturing Practices) and GCP (Good Clinical Practices) standards required or customary in the Territory.
2.10.1 Certificates of Manufacturing Compliance. At the request of BIOMEDICA and at BIOMEDICA’s expense, MIP shall provide or cause to be provided for such Compounds or Products purchased, a certificate of manufacturing compliance, containing the types of information reasonably agreed upon by BIOMEDICA and MIP. Such certificate shall certify that the Compounds or Products were manufactured in accordance with the specifications provided by BIOMEDICA and in compliance with all local laws in the country of manufacture.
2.10.2 Prior to the initial release and shipment of the Compound or Product in each country in the Territory, BIOMEDICA shall provide MIP or MIP’s designee with applicable radioactive license(s) and regulatory documentation authorizing the use and administration of the Compound or the Product in humans.
2.10.3 Compound Radiolabeling Tech Transfer Support. MIP will provide up to ****** hours for Compound radiolabeling tech transfer support to BIOMEDICA personnel without a labor charge but with reimbursement for all reasonable expenses incurred. At BIOMEDICA’s request, MIP will provide BIOMEDICA additional tech transfer support, for which support, BIOMEDICA agrees to reimburse MIP for at a daily rate range of ****** depending on the technical expertise and experience level required for the specific assistance requested by BIOMEDICA, plus reasonable expenses incurred. BIOMEDICA will provide MIP an advance notice of 30 days for requested support.
* Confidential Treatment Requested *
7
3. PRICING, PURCHASE COMMITMENT AND PAYMENT
3.1 Minimum Purchase Obligation. During the Term of this Agreement BIOMEDICA commits to purchase or otherwise pay MIP certain guaranteed monthly minimum amounts. Such guaranteed monthly minimum amounts shall commence at the beginning of Q3 2010 and continue through Q4 2013 inclusive. The cumulative guaranteed monthly minimum amount due in any given quarter shall define a target guaranteed quarterly minimum amount for calculating any underachievement adjustment or overachievement adjustment to be received by BIOMEDICA in an immediately subsequent quarter under the conditions set forth in 3.1.1 and 3.1.2.
3.1.1 Adjustments Underachievement Associated with Minimum Purchase Obligation. ****** of any payment made by BIOMEDICA to meet a Minimum Purchase Obligation amount in any particular quarter will be applied by MIP as an Underachievement Adjustment against any overachievement amount in excess of a subsequent quarter’s Guaranteed Quarterly Minimum Purchase Amount. In no case shall such an underachievement adjustment exceed any payment due by BIOMEDICA to MIP in excess of the minimum payment required in the subsequent quarter. In no case will the underachievement adjustment survive beyond such subsequent quarter. In no event will the underachievement adjustment reduce the subsequent quarter’s target Guaranteed Quarterly Minimum Amount to less than zero. ******
3.1.2 Adjustments Overachievement Associated with Minimum Purchase Obligation. ****** of any amount paid by BIOMEDICA in excess of the Guaranteed Quarterly Minimum Amount due in any particular quarter for Compound and/or Product, where such payment has taken into account any overachievement or underachievement adjustments, will be applied by MIP as an Overachievement Adjustment against any underachievement amount that may be owed by BIOMEDICA toward the subsequent quarter’s target Guaranteed Quarterly Minimum Amount. In no case will the overachievement adjustment survive beyond such subsequent quarter. In no event will the overachievement adjustment reduce the subsequent quarter’s target Guaranteed Quarterly Minimum Amount to less than zero. ******
3.1.3. For purposes of this section, “Material Change” shall mean an identifiable event or events that give rise to a significant and material adverse change in demand for Compound and/or Product in any given quarter by more than ****** of projected figures contained in the BIOMEDICA Business Plan attached and incorporated into the Territory License Agreement between the Parties dated September 1, 2009 for that quarter. A change in demand for either Compound or Product of less than ******, or a change in demand attributed substantially and directly to conduct within BIOMEDICA’S reasonable control, or where the cause of the event is not identifiable with reasonable certainty, shall not constitute a Material Change. Any increase beyond projections of Compound or Product shall not constitute a Material Change. BIOMEDICA and MIP
* Confidential Treatment Requested *
8
agree to account for Material Changes in the demand for Compound and/or Product only during the portion of the Term during which minimum purchase obligations are applicable.
3.1.3.1 MIP agrees that if BIOMEDICA can demonstrate that a Material Change outside of BIOMEDICA’s reasonable control has occurred, such as major regulatory, legal or reimbursement change, the Parties will promptly meet to discuss in good faith an appropriate reforecast of the minimums. During the period of good faith discussions, the Parties agree that the existing minimum monthly purchase obligations as set forth in this Agreement will continue to be paid by BIOMEDICA. Upon agreement on reforecasted minimums, any overpayment made by BIOMEDICA from the date on which the Material Change was demonstrated will be refunded to BIOMEDICA.
3.2 Pricing Commitment. The agreed upon pricing for the Compound and the Product shall be as follows:
3.2.1 Pricing ******
| • | Compound Transfer Price is set at ****** per Dose |
| • | Product for clinical trials is set at ****** per Dose |
| • | Product Transfer Price. The BIOMEDICA price per dose of the Product will be determined by the national competent authority of each country of the Territory in which the Product will be launched. If the price per dose for the Product by the national competent authority is set below ****** then the Parties will renegotiate in good faith the transfer price for Product in that country in the Territory. |
| Price Per Dose* |
Transfer Price |
Percentage of Onalta Price Per Dose** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** | ||
| ****** |
****** | ****** |
| * | Minimum transfer price is set at ****** for the initial five year period |
| ** | Governing equation |
* Confidential Treatment Requested *
9
| 3.2.2 | Pricing ****** Product Transfer Price. BIOMEDICA and MIP will negotiate new transfer prices after the ****** anniversary of the Supply Agreement during year ******, governing ******. One of the three mechanisms set forth below will be selected, at the choice of BIOMEDICA, to determine the re-negotiated transfer price for Product. BIOMEDICA will notify MIP in writing within ****** days of the ****** of the Supply Agreement of the mechanism BIOMEDICA has selected. |
| 3.2.2.1 | Comparator Pricing with BIOMEDICA. In the event that BIOMEDICA, or a BIOMEDICA affiliate, submits a bid as a Qualified Manufacturer, MIP will source bids from ****** other Qualified Manufacturers, for the manufacturing of Product for years ****** of this Agreement. In the event that BIOMEDICA’s bid is the lowest, such bid will be adjusted to be at least ****** of the lowest Qualified Manufacture bid secured by MIP. If BIOMEDICA selects this comparator pricing option, BIOMEDICA will offer MIP the option to match the quoted price of the vendor selected by BIOMEDICA, plus a ****** premium (i.e. MIP will continue to supply Product ****** of the lowest bid price secured by BIOMEDICA). |
| 3.2.2.2 | Comparator Pricing without BIOMEDICA. In the event that BIOMEDICA or a BIOMEDICA affiliate does not wish to provide a quotation to manufacture then BIOMEDICA needs ****** qualified manufacturer quotation to be provided to MIP. If BIOMEDICA selects this comparator pricing option, BIOMEDICA will offer MIP the option to match the quoted price of the vendor selected by BIOMEDICA, plus a ****** (i.e. MIP will continue to supply Product ****** of the lowest bid price secured by BIOMEDICA). Such transfer price will be amortized ******. |
| 3.2.2.3 | Best Price with Quantity Discount. BIOMEDICA may request as an alternative to the Comparator Pricing option above in 3.2.2.1 or 3.2.2.2 that MIP provide BIOMEDICA with a best price quotation, to continue to supply the Product during ******. |
* Confidential Treatment Requested *
10
3.3 Advance Payment on Inventory. BIOMEDICA shall provide MIP advance payments for Inventory on the following quarterly schedule, payable within 10 business days of the start of each quarter:
| • | Qtr-4 2009 ****** |
| • | Qtr-1 2010 ****** |
| • | Qtr-2 2010 ****** |
| • | Qtr-3 2010 ****** |
| • | Qtr-4 2010 ****** |
| • | Qtr-1 2011 ****** |
| • | Qtr-2 2011 ****** |
3.3.1 Advance Payment Refund.
| • | Upon cumulative payments from BIOMEDICA to MIP for Product transfer of ****** the refund will be ****** of the ****** advance payment ******. |
| • | Upon cumulative payments from BIOMEDICA to MIP for Product transfer of ****** an additional refund of ****** of the ****** advance inventory payment ****** will be made. |
| • | Upon cumulative payments from BIOMEDICA to MIP for Product transfer of ****** an additional refund of ****** of the ****** advance inventory payment ****** will be made. |
| • | Upon cumulative payments from BIOMEDICA to MIP for Product transfer of ****** the final refund of ****** of the ****** advance inventory payment ****** will be made. |
3.4 Payment Terms. MIP shall invoice BIOMEDICA as shipped based upon shipments of actual orders of Compound and Product as well as for any additionally required guaranteed minimum monthly payment amounts owed to MIP as provided for and agreed to in section 3.1 of this Agreement. BIOMEDICA shall pay such invoice(s) within 60 days of date of shipment with the following terms: ******. All payments for the Compound and Product shall be made in EURO.
4. EXCLUSIVITY AND NON-COMPETE
4.1 During the Term of the Supply Agreement, MIP is excluded from entering into any other supply agreements with other third parties for ****** therapeutic products that are similar to the Compound or Product within the defined Territory (Exhibit F).
* Confidential Treatment Requested *
11
4.2 During the Term of the Supply Agreement, BIOMEDICA is precluded from distributing any product similar to the Compound or Product in the Territory (Exhibit F), specifically products that are targeted to ****** using a ****** targeting molecule attached to a radionuclide.
5. TERM AND TERMINATION
5.1 Term. This Agreement shall commence on the Effective Date, and unless terminated sooner in accordance with its terms and conditions in this Agreement, shall continue in effect until expiration of the Term.
5.2 Termination for Breach. This Agreement may be terminated by either Party in the event of breach by the other Party of a material term or condition hereof; provided, however, the other Party shall first give to the breaching party written notice of the proposed termination of this Agreement (a “Breach Notice”), specifying the grounds of any alleged breach. Upon receipt of such Breach Notice, the breaching party shall have such time as necessary, but in any event not more than sixty (60) days to (i) cure such alleged breach, or (ii) dispute in writing the grounds of the alleged breach set out in the Breach Notice in good faith. Upon failure to cure or dispute by the breaching party, the non-breaching party may terminate the Agreement without prejudice to any other rights or remedies which may otherwise be available to the non-breaching party.
5.2.1 In the event of termination for Breach by MIP under this section, MIP shall be entitled to retain all amounts paid by BIOMEDICA to MIP as well as the full value of any Compound or Product ordered (and ready for delivery) by BIOMEDICA at the time of termination.
5.2.2 In the event of termination under such section by BIOMEDICA, BIOMEDICA shall be entitled to (i) recover the available balance of any advance payments under Article 3.3 received by MIP from BIOMEDICA and (ii) receive all Compound and Product which have been ordered but not delivered.
5.2.3 In the event of a timely received dispute by the non-breaching party in response to its Breach Notice to the breaching party, the non-breaching party may not unilaterally terminate this Agreement and shall only seek redress under the terms set out in Articles 8.1 and 9.10 of this Agreement regarding resolution of disputes by the Parties Neither Party may enter into an agreement with any manufacturer precluding such manufacturer from selling to the other Party at any time during the Term of this Agreement.
5.3 Termination for MIP Failure to Supply. With respect to the supply of Compound or Product by MIP or MIP’s designee pursuant to purchase orders placed in accordance with this Agreement, MIP or MIP’s designee failure to supply Compound or
* Confidential Treatment Requested *
12
Product in a timely manner and consistent with such Purchase Orders and the Specifications shall not be considered a material breach by MIP or MIP’s designee unless and until such failure by MIP or MIP’s designee occurs four (4) times in a consecutive 12 month period (a “Supply Breach”). It shall not be considered a Supply breach, in the event that (i) the failure to supply is attributable, in whole or in part, directly or indirectly, to BIOMEDICA, (ii) MIP or MIP’s designee is able to supply an additional replacement of Compound or Product meeting the Specifications in accordance with this Agreement within one (1) week of the delivery date of the originally scheduled order of Compound or Product, or (iii) if the Compound or Product failure is the result of a manufacturing deviation requested by BIOMEDICA. In the event of a Supply Breach, BIOMEDICA may terminate this Agreement upon thirty (30) days prior written notice to MIP provided it gives written notice of termination to MIP within sixty (60) days of the Supply Breach. Any failure by MIP or MIP’s designee to supply Compound or Product due to a Force Majeure shall not be a material breach or Supply Breach under this Agreement.
5.4 If MIP terminates this Agreement, under Articles 5.2 or 5.7, MIP, in addition to any claim for damages MIP may have, shall be entitled to retain all amounts paid by BIOMEDICA to MIP prior to such termination. BIOMEDICA shall further reimburse MIP for all costs and work necessarily and properly incurred by MIP in relation to the orderly cessation of the work and sums owing but not invoiced prior to the effective date of any such termination by MIP under this Agreement.
5.5 If BIOMEDICA terminates this Agreement, under Article 5.2, 5.3 or 5.7, in addition to any claim for damages BIOMEDICA may have, BIOMEDICA shall be entitled to within thirty (30) days of such termination at BIOMEDICA’s expense receive all Compound or Product which have been ordered but not delivered. BIOMEDICA shall reimburse MIP for all reimbursable costs and work necessarily and properly incurred in relation to the orderly cessation of the work and sums owing but not invoiced prior to the effective date of any such termination by BIOMEDICA under this Agreement.
5.6 Termination For Insolvency. Either Party may terminate this Agreement immediately upon delivery of written notice to the other Party (a) upon the institution by or against the other Party of insolvency, receivership or bankruptcy proceedings or any other proceedings for the settlement of the other Party’s debts; provided, however with respect to involuntary proceedings, that such proceedings are not dismissed within one hundred and twenty (120) days; (b) upon the other Party’s making an assignment for the benefit of creditors; (c) upon the other Party’s dissolution or ceasing to do business.
6. CONFIDENTIALITY
6.1. Confidentiality and Exceptions. During the Term of this Agreement and for a period of ten (10) years thereafter, each party hereto shall maintain in confidence and not use or disclose to others for any purpose, other than to its employees or agents (which agents shall enter into a confidentiality agreement incorporating similar terms as set forth herein
13
or be otherwise reasonably acceptable to the other party) with a need to know such information to perform such party’s obligations under this Agreement or other than as expressly authorized in this Agreement, the content of the transactions contemplated herein and other information disclosed to such party by the other party which is identified as “Confidential Information” by the disclosing party (collectively “Confidential Information”). This obligation of confidentiality shall not apply to the extent that it can be established by the party in receipt of such information, that the information:
| 6.1.1. | was already known to the receiving party at the time of disclosure; |
| 6.1.2. | was generally available to the public or otherwise part of the public domain at the time of its disclosure; |
| 6.1.3. | became generally available to the public or otherwise part of the public domain after its disclosure to the receiving party through no act or omission of the receiving party; |
| 6.1.4. | was disclosed to the receiving party by a third party who was not known to the receiving party to have obligations restricting disclosure of such information; or |
| 6.1.5. | was independently developed by the receiving party without any use of Confidential Information of the disclosing party. |
6.2 Each party, and its employees and agents shall protect and keep confidential and shall not use, publish or otherwise disclose to any third party, except as permitted by this Agreement, or with the other party’s written consent, the other party’s Confidential Information.
6.3 All Confidential Information supplied by one party to the other to assist in carrying out the obligations hereunder shall remain the property of such party and shall be returned to the other party upon termination or expiration of this Agreement.
7. INDEMNIFICATION AND INSURANCE
7.1 Indemnification by BIOMEDICA. BIOMEDICA agrees to indemnify, defend and hold MIP and its Affiliates and their respective directors, officers, employees and agents, harmless from and against any damages, claims, liabilities and expenses (including, but not limited to, reasonable attorney’s fees) resulting from claims or suits (“General Claims Against MIP”) arising out of (a) BIOMEDICAS’ or a third party’s use, handling or shipping of the Compound or Product (including in the event that MIP or MIP’s designee makes shipping arrangements on behalf of BIOMEDICA), (b) BIOMEDICAS’ breach of any of its material obligations, warranties or representations hereunder, (c) BIOMEDICAS’ negligent acts or omissions or willful misconduct. Notwithstanding the foregoing, BIOMEDICA will not be required to indemnify, defend and hold MIP and its Affiliates and their respective directors, officers, employees and
14
agents harmless from and against any General Claims Against MIP to the extent that such claims arise out of (i) MIP’s breach of any of its obligations, warranties or representations hereunder; (ii) MIP’s negligent acts or omissions or willful misconduct; (iii) any failure of MIP to supply (except to the extent labels and/or content thereof are provided by BIOMEDICA) or prepare for shipment of the Compound or Product in accordance with this Agreement, cGMPs or any other applicable laws, rules, regulations or other requirements of any applicable governmental entity; or (iv) any failure of MIP to supply Compound or Product consistent with the Specifications and requirements set forth herein.
7.2 Indemnification by MIP. MIP agrees to indemnify, defend and hold BIOMEDICA and its Affiliates and their respective directors, officers, employees and agents, harmless from and against any damages, claims, liabilities and expenses (including, but not limited to, reasonable attorney’s fees) resulting from claims or suits (“General Claims Against BIOMEDICA”) arising out of (a) MIP’s supply (except to the extent that labels and/or content thereof is provided by BIOMEDICA) or preparation for shipment of the Compound or Product; (b) MIP’s breach of any of its material obligations, warranties or representations hereunder; (c) MIP’s negligent acts or omissions or willful misconduct; or (d) any failure of the Compound or Product to meet the Specifications. Notwithstanding the foregoing, MIP will not be required to indemnify, defend and hold BIOMEDICA and its Affiliates and their respective directors, officers, employees and agents harmless from and against any General Claims Against BIOMEDICA to the extent that such claims arise out of (i) BIOMEDICAS’ breach of any of its obligations, warranties or representations hereunder; (ii) BIOMEDICAS’ negligent acts or omissions or willful misconduct; (iii) BIOMEDICAS’ or third party’s use, labeling, handling or shipment of the Compound or Product.
7.3 Conditions of Indemnification. A Party or any of its Affiliates or their respective employees or agents (the “Indemnitee”) that intends to claim indemnification under this Article 6 shall promptly notify the other Party (the “Indemnitor”) of any Liability in respect of which the Indemnitee intends to claim such indemnification reasonably promptly after the Indemnitee is aware thereof, and the Indemnitor shall assume the defense of any related third party action, suit or proceeding with counsel mutually satisfactory to the Parties; provided, however, that an Indemnitee shall have the right to retain its own counsel and participate in the defense thereof at its own cost and expense. Indemnity shall not apply to amounts paid in settlement of any claim, loss, damage or expense if such settlement is effected without the consent of the Indemnitor, which consent shall not be withheld unreasonably. The failure of an Indemnitee to deliver notice to the Indemnitor within a reasonable time after becoming aware of any such matter, if prejudicial to the Indemnitor’s ability to defend such action, shall relieve the Indemnitor of any liability to the Indemnitee under this Article 6. The Indemnitee under this Article 6 and its employees and agents shall cooperate fully with the Indemnitor and its legal representatives in the investigation and defense of any matter covered by this indemnification.
15
7.4 Insurance. Each Party shall obtain and maintain insurance reasonably sufficient to cover its potential liability under this Agreement and shall provide evidence of such insurance to the other Party upon request.
7.5 Limitation of Damages. Neither Party nor its affiliates shall have any liability for any special, incidental, or consequential damages, including, but not limited to the loss of opportunity, revenue or profit, in connection with or arising out of this Agreement, even if it shall have been advised of the possibility of such damages.
8. DISPUTE RESOLUTION
8.1 Dispute Resolution. The Parties recognize that disputes as to certain matters may from time to time arise which relate to either Party’s rights and/or obligations hereunder. The Parties hereby agree that they will attempt in good faith to resolve any controversy, claim or dispute (collectively, a “Dispute”) arising out of or relating to this Agreement promptly by negotiations. Any such Dispute which is not settled by the Parties within fifteen (15) days after notice of such Dispute is given by one Party to the other in writing shall be referred to a senior executive of MIP and of BIOMEDICA who are authorized to settle such Disputes on behalf of their respective companies (“Senior Executives”) and who, if possible, are not involved in the Dispute. The Senior Executives will meet for negotiations within thirty (30) days of the end of the 15-day negotiation period referred to above, at a time and place mutually acceptable to both Senior Executives. If the Dispute has not been resolved within thirty (30) days after the end of the 15-day negotiation period referred to above (which period may be extended by mutual agreement), the other Party can immediately bring an action relating to the Dispute before a court of competent jurisdiction in accordance with Article 9.10.
9. GENERAL PROVISIONS
9.1 Notices. All notices given under this Agreement shall be in writing and shall be personally delivered or mailed by certified first class mail return receipt requested or a reputable express delivery service to the Party for which it is intended at its address as set forth below, or at such other address as the addressee may have designated to the other Party in writing. Any notice shall be deemed given only upon actual delivery thereof at the proper address.
All notices to MIP shall be addressed to:
If to MIP, at:
Molecular Insight Pharmaceuticals, Inc.
000 Xxxxxx Xxxxxx
Xxxxxxxxx, XX 00000 XXX
Attn: Xxxx Xxxxxxx, General Counsel
If to BIOMEDICA at:
16
BioMedica Life Sciences, Ltd.
0 Xxxxxxxxxx Xxx.,
00000 Xxxxxxxx, Xxxxxx, Xxxxxx
Attn: Xxxxxxx Xxxxxxxx, CEO
9.2 Entire Agreement. This Agreement sets forth the entire understanding of the Parties with respect to the subject matter hereof and supersedes all prior agreements, written and oral, between the Parties. No modification of any of the terms of this Agreement shall be deemed to be valid unless it is in writing and signed by the Party against whom enforcement is sought. No course of dealing or usage of trade shall be used to modify the terms and conditions herein.
9.3 Force Majeure. “Force Majeure” shall mean an occurrence arising from unforeseen circumstances beyond a party’s reasonable control which prevents, delays or interferes with the performance by such party of any of its obligations hereunder including without limitation an event that occurs by reason of any act of God, flood, power failure, fire, explosion, casualty or accident, failure of suppliers or usual suppliers to have available for supply sufficient raw materials, equipment or machinery, or war, revolution, civil commotion, acts of public enemies, act of terrorism, blockage or embargo, interruption of or delay in transportation, strike or labor disruption. Neither party shall be liable to the other for failure to perform or delay in performing its obligations under this Agreement by virtue of the occurrence of an event of Force Majeure.
9.3.1 In the event of Force Majeure, the party affected shall promptly notify the other and shall exert commercially reasonable efforts to eliminate, cure or overcome such event and to resume performance of its obligations.
9.3.2 MIP shall, upon signing of this Agreement, qualify ****** suppliers of Yttrium-90. All costs and expenses, including but not limited to qualifying and validating, associated with qualifying a back up supply from the second MIP qualified supplier shall be borne equally by both MIP and BIOMEDICA.
9.3.3. In the event such Force Majeure affecting either party continues for more than ninety (90) days the party not subject of the Force Majeure may, upon thirty (30) days written notice, terminate this Agreement.
9.4 Waiver. No waiver by either Party of any default, right or remedy shall be effective unless in writing, nor shall any such waiver operate as a waiver of any other or of the same default, right or remedy, respectively, on a future occasion.
9.5 Assignment. This Agreement may not be assigned or otherwise transferred by either Party without the prior written consent of the other Party; provided, however, that either Party may assign this Agreement, without the consent of the other Party, (i) to any
* Confidential Treatment Requested *
17
of its Affiliates, if the assigning Party guarantees the full performance of its Affiliates’ obligations hereunder or (ii) in connection with the transfer or sale of all or substantially all of its assets or business or in the event of its merger or consolidation with another company. In all cases the assigning Party shall provide the other Party with prompt notice of any such assignment. Any purported assignment in contravention of this article shall, at the option of the non assigning Party, be null and void and of no effect. No assignment shall release either Party from responsibility for the performance of any accrued obligation of such Party hereunder.
9.6 Binding Effect. This Agreement shall inure to the benefit of and be binding on the Parties, their Affiliates and permitted assigns.
9.7 Severability. It is the intention of the Parties to comply with all applicable laws domestic or foreign in connection with the performance of its obligations hereunder. In the event that any provision of this Agreement, or any part hereof, is found invalid or unenforceable, the remainder of this Agreement will be binding on the Parties hereto, and will be construed as if the invalid or unenforceable provision or part thereof had been deleted, and the Agreement shall be deemed modified to the extent necessary to render the surviving provisions enforceable to the fullest extent permitted by law.
9.8 Headings; Interpretation. The headings used in this Agreement are for convenience only.
9.9 Independent Parties. This Agreement shall not be deemed to create any partnership, joint venture, or agency relationship between the Parties.
9.10 Governing Law. The construction, validity and performance of this Agreement will be governed in all respects by the laws of Switzerland. All disputes arising out of or affecting this Agreement which cannot be resolved amicably shall be submitted to the exclusive jurisdiction of the courts of the Commonwealth of Massachusetts, U.S.A. if the defendant is MIP, or to the exclusive jurisdiction of the courts of Athens, Greece, should BIOMEDICA be the defendant.
9.11 Execution by Counterparts; Exchange by Facsimile. This Agreement may be executed by the Parties in one or more counterparts. Such counterparts may be exchanged by facsimile (provided that each executed counterpart is transmitted in one complete transmission). Where there is an exchange of executed counterparts, each Party shall be bound by the Agreement notwithstanding that original copies of the Agreement may not be exchanged immediately. The Parties shall cooperate after execution of the Agreement and exchange by facsimile to ensure that each Party obtains an original executed copy of this Agreement.
9.12 Survival. Except where explicitly provided elsewhere herein, termination of this Agreement for any reason, or expiration of this Agreement, will not affect: (i) obligations, including the payment of any sums, which have accrued as of the date of
18
termination or expiration, and (ii) rights and obligations which, from the context thereof, are intended to survive termination or expiration of this Agreement.
—Signature Page to Follow—
19
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be executed by their duly authorized representatives as of the day and year first above written.
| BioMedica Life Sciences S.A. | Molecular Insight Pharmaceuticals Inc. | |||||
| Name: | /s/ Yannis Vitsaras | Name: | /s/ Xxxxxxx Xxxxxxxx | |||
| Title: | CEO | Title: | Chief Financial Officer | |||
20
21
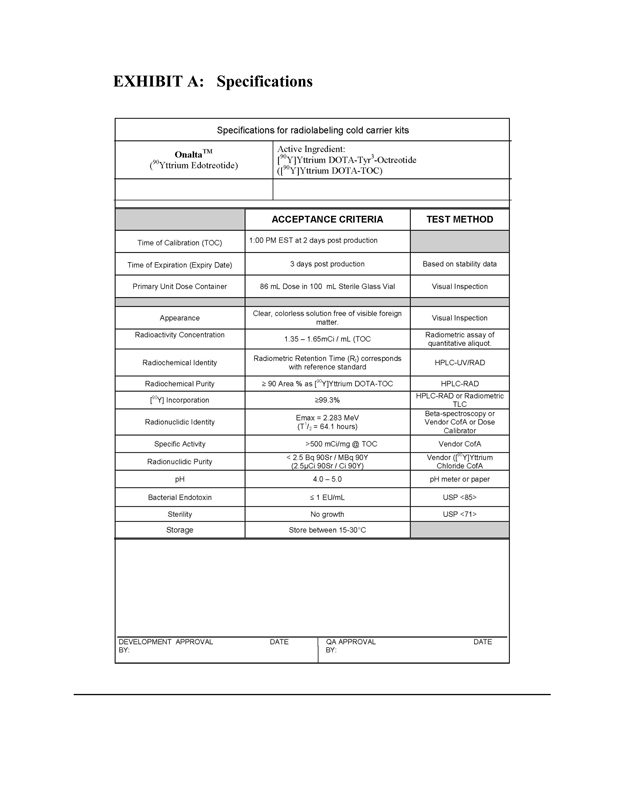
EXHIBIT A:Specifications
Specifications for radiolabeling cold carrier kits
OnaltaTM Active Ingredient: [90Y] Yttrium DOTA-Tyr3-Octreotide (90Y Yttrium Edotreotide) ([90Y] Yttrium DOTA-TOC)
ACCEPTANCE CRITERIA
TEST METHOD
Time of Calibration (TOC)
1:00 PM EST at 2 days post production
Time of Expiration (Expiry Date)
3 days post production
Based on stability data
Primary Unit Dose Container
86 mL Dose in 100 mL Sterile Glass Vial
Visual Inspection
Appearance
Clear, colorless solution free of visible foreign matter.
Visual Inspection
Radioactivity Concentration
1.35 – 1.65mCi/mL(TOC
Radiometric assay of quantitative aliquot.
Radiochemical Identity
Radiometric Retention Time (Rt) corresponds with reference standard
HPLC-UV/RAD
Radiochemical Purity
> 90 Area % as [90Y] Yttrium DOTA-TOC
HPLC-RAD
[90Y] Incorporation
> 99.3%
HPLC-RAD or Radiometric TLC
Radionuclidic Identity
Emax = 2.283 MeV (T1/2 = 64.1 hours)
Beta-spectroscopy or Vendor CofA or Dose Calibrator
Specific Activity
>500 mCi/mg @ TOC
Vendor CofA
Radionuclidic Purity
< 2.5 Bq 90Sr / MBq 90Y (2.5uCi 90Sr / Ci 90Y)
Vendor ([90Y]Yttrium Chloride CofA
pH
4.0 – 5.0
pH meter or paper
Bacterial Endotoxin
< 1 EU/mL
USP <85>
Sterility
No growth
USP <71>
Storage
Store between 15-30°C

22
PerkinElmer*
precisely.
PerkinElmer Life and Analytical Sciences 000 Xxxxxx Xxxxxx Xxxxxx, XX 00000
TechnicaI Data
Caution: For Labortatory Use. A research chemical for research purposes only.
YTTRIUM-90
LOT SPECIFIC INFORMATION
Catalog # NEZ-306
Order# 1505466587 2
Lot# C11082207
Production date/time: 22-Aug-07/Noon ET
Activity. 301 mCi
Volume: 614 µL
Acid: 0.05 M HCl
Vial: P-2 glass vial
Product Specifications (as of Production date)
Sr-90/Y-90 Activity Ratio
Chemical Purity Ca Zn Al Cu Fe Cr Pb P
Zn+Fe+Cu+Pb Specific Activity
Product Release:
as of
Calibration date/time: 27-Aug-07/Noon ET Expiration date/time: l-Sep-07/Noon ET
Value at Prod.
No carrier added
Date:
0.7E-07 Ci/Ci
1-5 µg/Ci
PHYSICAL DATA
Half Life:
Maximum Beta Energy: Maximum Range of Beta in Air: Maximum Range of Beta in water
64.0 Hours 2.28MeV(100%)(1)) 9m(30reet)(2) 11 mm(0.43 inch)(2)
STORAGE CONDITIONS 20°-25° C; excursions permitted to 15°-30° C Warning: This product contains a chemical known to the state of California to cause cancer.
This document contains general Information designed to provide a basic understanding of radiation safely. While we believe the Information to be accurate, regulatory requirements may change and Information contained herein Is not tailored to Individual needs. A radiation protection specialist should be consulted for specific applications.
Certificate of Analysis
Parameter
Value at Cal.
<1.8E-07 Ci/Ci
<100 µg/Ci
<20 µg/Ci
<20 µg/Ci
<20 µg/Ci
<20 µg/Ci
<20 µg/Ci
<20 µg/Ci
<100 µg/Ci
<20 µg/Ci
<1.0E-07 Ci/Ci
0.4 µg/Ci
<0.3 µg/Ci
<0.8 µg/Ci
<0.3 µg/Ci
<0.2 µg/Ci
<2.0 µg/Ci
<10 µg/Ci
<20 µg/Ci
<3.7E-07 Ci/Ci
1.5 µg/Ci
8-27-07
RECEIVED

23
EXHIBIT B FORM OF PURCHASE ORDER
Xxxxxx & Xxxxxxx Nuclitec XxxxX
Xxxxxxxxx 0
00000 Xxxxxxxxxxxx, Xxxxxxx
Tel x00 0000 000-000
Mobile x00 000 0000000
Fax x00 0000 000-000
Attn: Xxxxxx Xxxxxxxxx
Product and Sales Manager
xxxxxx.xxxxxxxxx@xxxx.xxx
BIOMED1CA Life Sciences 0 Xxxxxxxxxx Xxx.,
00000 Xxxxxxxx, Xxxxxx, Xxxxxx
hereinafter referred to as “Purchaser”.
DESCRIPTION OF ORDER: Compound and Product
SALE OF Compound and Product
Purchaser hereby agrees to purchase from Manufacturer, and Manufacturer agrees
to sell to Purchaser [specify quantity] of the compound and/or Product.
The Compound or Product shall be delivered to
(the “Place of Destination”).
Manufacturer shall provide all compound and Product FCA (INCOTERMS 2000) to Place of
Destination unless otherwise specified
Manufacturer shall use commercially reasonable efforts to ensure the Compound and/or Product are
received by the Purchaser by [insert delivery date] .
EZN
By: Name: Title: Date:
By: Name: Title: Date:
BIOMED1CA
hereinafter referred to as “Manufacturer”;
EXHIBIT C: Ordering and Fulfillment Process

24
EXHIBIT D: Monthly Minimum Payment Obligation
******
* Confidential Treatment Requested *
25
EXHIBIT E: Example of Over Achievement / Under Achievement Adjustment
******
* Confidential Treatment Requested *
26
Exhibit F: Listing of Countries in Territory
| Table of Countries by Territory |
| European Union | 1 | Albania | European Free Trade |
00 | Xxxxxxxxxxxx | Xxxxxx Xxxx & Xxxxxx Xxxxxx |
62 | Mauritania | ||||||||||||
| 2 | Andorra | 36 | Norway | 63 | Morocco | |||||||||||||||
| 3 | Austria | Eastern Europe |
37 | Belarus | 00 | Xxxx | ||||||||||||||
| 0 | Xxxxxxx | X/X | Xxxxxxx* | 65 | Qatar | |||||||||||||||
| 5 | Boznia and Xxxxxxxxxxx | X/X | Xxxxxx* | 00 | Xxxxx Xxxxxx | |||||||||||||||
| 6 | Bulgaria | N/A | Lithuania* | |||||||||||||||||
| 7 | Croatia | 38 | Xxxxxxx | 00 | Somalia | |||||||||||||||
| 8 | Cyprus | 39 | Xxxxxxx | 00 | Xxxxx | |||||||||||||||
| 0 | Xxxxx Xxxxxxxx | Russia & CIS |
40 | Russia | 69 | Syria | ||||||||||||||
| 10 | Xxxxxxx | 00 | Xxxxxxx | 70 | Tunisia | |||||||||||||||
| 11 | Xxxxxxx | 00 | Xxxxxxxxxx | 00 | XXX | |||||||||||||||
| 00 | Xxxxxxx | N/A | Belarus* | 72 | Western Sahara | |||||||||||||||
| 13 | France | 43 | Georgia | |||||||||||||||||
| 14 | Germany | 44 | Xxxxxxxxxx | 00 | Xxxxx | |||||||||||||||
| 00 | Xxxxxx | 45 | Xxxxxxxxxx | Xxxxxx | 00 | Xxxxxx | ||||||||||||||
| 00 | Xxxxxxx | X/X | Xxxxxxx* | Xxxxx Xxxxxx |
X/X | Xxxxxxx* | ||||||||||||||
| 00 | Xxxxxxx | 46 | Tajikistan | N/A | Egypt* | |||||||||||||||
| 00 | Xxxxx | 00 | Xxxxxxxxxxxx | X/X | Xxxxx* | |||||||||||||||
| 00 | Xxxxxx | N/A | Ukraine* | N/A | Morocco* | |||||||||||||||
| 20 | Lithuania | 48 | Uzbekistan | N/A | Sudan* | |||||||||||||||
| 00 | Xxxxxxxxxx | Xxxxxx Xxxx & Xxxxxx |
49 | Algeria | N/A | Tunisia* | ||||||||||||||
| 22 | Xxxxx | 00 | Xxxxxxx | X/X | Xxxxxxx Xxxxxx* | |||||||||||||||
| 00 | Xxxxxxxxxxx | 51 | Chad | Europe: Non-Sovereign Locations |
75 | Åland | ||||||||||||||
| 24 | Poland | 52 | Comoros | 76 | Akrotiri and Dhekelia | |||||||||||||||
| 25 | Portugal | 53 | Dijibouti | 77 | Faroe Islands | |||||||||||||||
| 26 | Romania | 54 | Egypt | 78 | Gibraltar | |||||||||||||||
| 27 | Xxxxxxxx | 00 | Xxxxxxx | 00 | Xxxxxxxx | |||||||||||||||
| 00 | Xxxxxxxx | 56 | Iran | 80 | Isle of Man | |||||||||||||||
| 29 | Spain | 81 | Jersey | |||||||||||||||||
| 30 | Sweden | 57 | Iraq | Europe: Partially Recognized Locations | 00 | Xxxxxxxx | ||||||||||||||
| 00 | Xxxxxx Xxxxxxx | 58 | Jordan | 83 | Kosovo | |||||||||||||||
| 32 | Vatican City | 00 | Xxxxxx | 00 | Xxxxxxxx Xxxxxx | |||||||||||||||
| European Free Trade Assoc. | 33 | Switzerland | 60 | Lebanon | 85 | South Ossetia | ||||||||||||||
| 34 | Iceland | 61 | Libya | Europe: Unrecognized Locations | 86 | Nagorno-Karabakh | ||||||||||||||
| 87 | Transnistria |
| * | Countries are represented twice in two different territories, only counted once |
27

