DATED 5 FEBRUARY 2019 AMENDMENT AND RESTATEMENT AGREEMENT relating to an agreement for the purchase and sale of source plasma originally dated 28 December 2018
THE SYMBOL “[**]” INDICATES MATERIAL WHERE CERTAIN IDENITIFIED INFORMATION HAS BEEN EXCLUDED BECAUSE IT IS BOTH (i) NOT MATERIAL AND (ii) WOULD LIKELY CAUSE COMPETITIVE HARM TO THE COMPANY IF PUBLICLY DISCLOSED.
DATED 5 FEBRUARY 2019
(1) GRIFOLS WORLDWIDE OPERATIONS LIMITED
(2) GRIFOLS S.A.
(3) BIOTEST PHARMACEUTICALS CORPORATION
and
(4) HAEMA AG
AMENDMENT AND RESTATEMENT AGREEMENT
relating to
an agreement for the purchase and sale of source plasma originally dated
28 December 2018
THIS AGREEMENT dated 5 February 2019
BETWEEN:
(1) GRIFOLS WORLDWIDE OPERATIONS LIMITED, a company duly organized and existing under the laws of the Republic of Ireland, having its registered office at Grange Castle Business Park, Grange Castle, Clondalkin, Xxxxxx 00, Xxxxxxx (“GWWO”);
(2) GRIFOLS S.A., a company validly incorporated and existing under the laws of Spain, with its principal place of business at Xxxxxxxx xx xx Xxxxxxxxxxx, 000, Xxxx empresarial Can Xxxx Xxxx, 08174 Sant Cugat del Xxxxxx, Barcelona (the “Guarantor”);
(3) BIOTEST PHARMACEUTICALS CORPORATION, a corporation existing and incorporated under the laws of the State of Delaware (USA) with its principal place of business at 000 Xxxxxx Xxxx, Xxxxx 000 Xxxx Xxxxx, XX 00000-0000, XXX (“Biotest”); and
(4) HAEMA AG, a corporation existing and incorporated under the laws of Germany with its principal place of business at Xxxxxxxxxxxxxxxxxx 0, 00000 Xxxxxxx, Xxxxxxx (“Haema” and, together with Biotest, the “Supplier”).
1. BACKGROUND
(a) This Agreement is supplemental to an agreement for the purchase and sale of source plasma originally dated 28 December 2018 and made between (1) GWWO, (2) the Guarantor, (3) Biotest and (4) Haema (the “Original PSA”).
(b) The Parties have agreed, subject to the terms of this Agreement, to make certain amendments to the Original PSA and for such amendments to take retrospective effect on and from 28 December 2018.
(c) With effect from the Effective Date, the Original PSA will be amended and restated so that it reads as if it were the Amended PSA and such amendment and restatement will be deemed to take retrospective effect from 28 December 2018.
2. DEFINITIONS AND INTERPRETATION
2.1 Definitions
In this Agreement:
“Amended PSA” means the agreement for the purchase and sale of source plasma in the form set out in schedule 3 (Amended PSA);
“Effective Date” means the date on which each Party to this Agreement has duly executed this Agreement; and
“Party” means a party to this Agreement.
2.2 Clauses
(a) In this Agreement any reference to a “clause” or “schedule” is, unless the context otherwise requires, a reference to a clause or schedule of this Agreement.
(b) Clause and schedule headings are for ease of reference only.
2.3 Continuing Obligations
Subject to the provisions of this Agreement:
(a) the Original PSA shall remain in full force and effect; and
(b) from the Effective Date, the Original PSA shall be read and construed as one document with this Agreement.
3. RESTATEMENT
3.1 Restatement
With effect from the Effective Date, the Original PSA shall be amended and restated so that it shall be read and be construed for all purposes as set out in schedule 3 (Amended PSA) and such amendment and restatement shall be deemed to take retrospective effect from 28 December 2018.
4. MISCELLANEOUS
4.1 Incorporation of Terms
The provisions of clauses 5.1 (Relationship of the Parties), 5.2 (Indemnification), 5.5 (Notices), 5.13 (Third Party Rights), 5.14 (Arbitration) and 5.16 (Confidentiality) of the Original PSA shall apply to this Agreement as if set out in full in this Agreement and as if references in those clauses to “this Agreement” are references to this Agreement.
4.2 Counterparts
This Agreement may be executed in any number of counterparts, and this has the same effect as if the signatures on the counterparts were on a single copy of this Agreement.
5. GOVERNING LAW AND ENFORCEMENT
This Agreement and any issues, disputes or claims arising out of or in connection with it (whether contractual or non-contractual in nature such as claims in tort, from breach of statute or regulation or otherwise) shall be governed by, and construed in accordance with, the laws of Ireland.
This Agreement has been entered into on the date stated at the beginning of this Agreement.
SCHEDULE 1: AMENDED PSA
EXECUTION PAGES
GRIFOLS WORLDWIDE OPERATIONS LIMITED
|
Name: |
/s/ Xxxxxxx Xxxxxx |
|
|
|
|
|
|
Title: |
CFO |
|
BIOTEST PHARMACEUTICALS CORPORATION
|
Name: |
/s/ Xxxxxxx Xxxxxxx |
|
|
|
|
|
|
Title: |
Authorized Signatory |
|
HAEMA AG
|
Name: |
/s/ Xxxxxxx Xxxxxxx |
|
|
|
|
|
|
Title: |
Authorized Signatory |
|
|
GRIFOLS S.A. |
GRIFOLS S.A. | |||
|
|
| |||
|
|
| |||
|
|
|
|
|
|
|
Name: |
/s/ Raimon Grifols |
|
Name: |
/s/ Xxxxxx Grifols Deu |
|
|
|
|
|
|
|
Title: |
Co-CEO |
|
Title: |
Co-CEO |
[Plasma – ARA to PSA Signatures Pages]
AGREEMENT FOR THE PURCHASE AND SALE
OF SOURCE PLASMA
This Agreement for the Purchase and Sale of Plasma (the “Agreement”) is made and entered into on 28 December 2018 as amended and restated on 5 February 2019 with effects as of 1 January 2019 (the “Effective Date”), by and between:
(i) GRIFOLS WORLDWIDE OPERATIONS LIMITED, a company duly organized and existing under the laws of the Republic of Ireland, having its registered office at Grange Castle Business Park, Grange Castle, Clondalkin, Xxxxxx 00, Xxxxxxx (“GWWO”),
(ii) GRIFOLS, S.A a company validly incorporated and existing under the laws of Spain, with with its principal place of business at Xxxxxxxx xx xx Xxxxxxxxxxx, 000. Parc empresarial Can Xxxx Xxxx 08174 Sant Cugat del Xxxxxx, Barcelona (“Guarantor”); and
(iii) BIOTEST PHARMACEUTICALS CORPORATION, a corporation existing and incorporated under the Laws of the State of Delaware (USA), with its principal place of business at 000 Xxxxxx Xxxx, Xxxxx 000 Xxxx Xxxxx, XX 00000-0000, XXX (“BIOTEST”).
(iv) HAEMA AG, a corporation existing and incorporated under the Laws of Germany, with its principal place of business at Xxxxxxxxxxxxxxxxxx 0, 00000 Xxxxxxx, Xxxxxxx (“HAEMA”, and collectively with BIOTEST, “SUPPLIER”).
This Agreement is governed by the following terms and conditions:
ARTICLE 1. DEFINITIONS
1.1 Left blank
1.2 [**].
1.3 [**].
1.2 Definition of Plasma. The term “Plasma” as used in this Agreement shall refer to the production of normal source plasma to be derived from automated plasmapheresis procedures conducted at the US Food and Drug Administration (“FDA”) and at European Authorities approved and IQPP-certified Plasma (when applicable) donor centers identified on Exhibit A attached hereto (the “Centers”), which Centers shall be operated by SUPPLIER and/or third parties and shall be subject to GWWO’s approval, all of which Plasma, collected or purchased and released by SUPPLIER shall comply with the specifications set forth herein, specifically those specifications set forth in the quality agreement attached hereto as Exhibit B (hereafter, the “Quality Agreement”). The Parties shall enter into the Quality Agreement as soon as possible and in any event within 20 days of the Effective Date. As used herein, the term “European Authorities” refers to and includes the European Medicines Agency, the MHRA, GHA and other constituent organizations with and through which the European Medicines Agency regulates plasma and plasma donor centers. Plasma shall further include various types of specialty plasma collected by SUPPLIER, including tetanus plasma, anti-D plasma, anti-HBs plasma, and rabies plasma.
1.3 Definition of Business Plan means the business plan in the form agreed between the parties prior to the original date of this Agreement.
1.4 Supplier’s Group means Scranton Plasma BV and its subsidiaries (including the Suppliers) and any entity (other than Scranton Enterprises) which is a holding company of Scranton Plasma BV
ARTICLE 2. TERMS OF SALE
2.1 Agreement to Purchase and Sell. Pursuant to and subject to the terms and conditions of this Agreement: (a) SUPPLIER agrees to supply fully tested Plasma in accordance with this Agreement; and (b) GWWO agrees to take delivery of all the Plasma collected and/or purchased by SUPPLIER from the Centers, subject to the terms and conditions set forth in the Quality Agreement.
2.2 Delivery Plan. Unless otherwise agreed to by the parties, each calendar year prior to November 15, GWWO and SUPPLIER’s management will discuss the upcoming year’s delivery schedule of Plasma (the “Delivery Plan”). GWWO shall provide SUPPLIER with a Delivery Plan for each calendar year no later than November 15 of the prior year. The Delivery Plan shall provide an estimated delivery schedule for the production of each Center’s during each month of the following year. During the Term (as defined below), SUPPLIER will have the right to add additional center(s) to the approved listing of Centers, but such additional center(s) must meet the Quality Agreement and will be subject to GWWO’s approval (acting reasonably). In this event, GWWO agrees to inspect any additional center(s) within ten (10) working days of notification, or within such reasonable time period as agreed in writing by the parties.
[**].
2.3 Price and Payment.
2.3.1 [**].
2.3.2 Payment Terms. Except for any invoice sums that are the subject of a good faith dispute between the parties, payment for Plasma received by GWWO shall be made to SUPPLIER within thirty (30) days from the date of issuance of the invoice from SUPPLIER, as such issuance date is referenced on the invoice. A late fee of twelve percent (12%) per annum will be applied to all late invoice payments.
2.3.3 Shipment Costs. GWWO agrees to bear all costs of shipment, freight, insurance and all governmental taxes and duties incurred during shipping of the Plasma sold hereunder from the Centers to facilities designated by
GWWO. It is agreed that the shipments from the Centers will be bi-weekly or weekly depending on the volume at each of the Centers, or as otherwise agreed to in writing between the parties.
2.4 Delivery and Risk of Loss. Title and risk of loss with respect to Plasma collected by SUPPLIER hereunder shall transfer to GWWO when the Plasma is loaded by SUPPLIER at its Centers for transportation by GWWO. With respect to Plasma purchased by SUPPLIER from a third-party, title and risk of loss shall transfer to GWWO when the Plasma reaches GWWO facilities. Upon request, SUPPLIER will provide GWWO with any reasonably requested production documentation related to any delivered Product.
2.5 Term of the Agreement. The term of this Agreement shall be of thirty (30) years, starting on the Effective Date [**]. [**].
Notwithstanding the foregoing, this Agreement may be terminated by either party as set forth under Section 5.3.1 herein.
ARTICLE 3. GENERAL PROVISIONS
3.1 Specifications. SUPPLIER warrants and agrees that all Plasma sold to GWWO hereunder will meet the terms and conditions of the Quality Agreement, as well as the following requirements and conditions:
The Centers must be approved plasma centers and licensed by the FDA and/or the European Authorities utilizing an approved system of procedures. SUPPLIER agrees that each Center shall follow the procedures as written in the Quality Agreement and any revisions to those procedures or the terms of the Quality Agreement as they may be issued or adopted by GWWO from time to time, as approved, when applicable, by the FDA or European Authorities, if such approval is required. SUPPLIER acknowledges that it has received full, complete and accurate copy of the specifications of the Plasma as in effect as of the Effective Date. SUPPLIER also agrees to have all of its Centers QPP certified (quality plasma program as defined by PPTA) and to maintain that certification for the Term. Any Center that is not QPP certified during any portion of this Agreement can be excluded from supplying Plasma, but the lost Plasma volume may be replaced from one or more other Centers that are FDA and European Authorities approved and QPP certified plasma centers and that meet the Quality Agreement.
SUPPLIER represents and warrants that all Plasma sold to GWWO under this Agreement will be collected, processed, tested, stored, packaged, labeled and shipped in strict accordance with the Quality Agreement and all applicable FDA and European Authorities regulations, will meet all requirements of cGMPs, and will be fit for the purpose and use intended by GWWO.
If any of the Centers are closed as a result of regulatory sanctions placed on such Centers by the FDA or European Authorities, or if any Center is found by GWWO to be clearly deficient, SUPPLIER will have thirty (30) working days to provide, in writing, a corrective action plan acceptable to GWWO. If the action plan is unacceptable, or if a Center cannot provide Plasma within sixty (60) days of a closure, this Agreement, at SUPPLIER’s option, can be modified to eliminate such Center and replace it with one or more other approved and QPP certified plasma Centers that meet GWWO’s approval and the terms of the Quality Agreement within sixty (60) days of any such modification of this Agreement. If SUPPLIER requests an additional Center to be added to this Agreement, GWWO agrees to inspect and approve, if the Center meets the terms of the Quality Agreement, any such additional Center within ten (10) working days. GWWO and SUPPLIER agree to give best efforts to complete all documentation necessary to effect the addition of such Center within the ten (10) day period. This provision for replacement Centers does not apply in the event of systemic deficiencies or problems that affect all or the majority of the Centers, and which event may be grounds for termination of this Agreement by GWWO with no further liability owing to SUPPLIER.
SUPPLIER or the relevant third parties shall operate each Center in accordance with its FDA and/or European Authorities approved Standard Operating Procedure (“SOP”). In the event SUPPLIER wishes to modify or deviate from any of the practices of the instructions for the collection and handling of Plasma as set forth in its SOP, relevant changes or significant deviations must be approved in writing by GWWO.
SUPPLIER shall collect, process, store, package and ship the Plasma in strict accordance with the Quality Agreement given to SUPPLIER by GWWO during the Term. These may be amended from time to time by GWWO or by an appropriate governmental agency during the Term. Any amendments by GWWO must be reasonable and meet industry standards.
SUPPLIER agrees to permit or cause to permit GWWO and any authorized representative of GWWO, the FDA, the European Authorities and any State or local governmental agency to conduct inspections of the Centers, testing facilities, and Corporate Offices at any time for the purposes of ensuring compliance with this Agreement or with all regulatory requirements, and will permit them to review all records kept by SUPPLIER regarding the Center’s quality systems and the collection, storage, processing and shipment of Plasma. GWWO, at its sole option and expense, shall have the right to make and retain copies of all such records kept by SUPPLIER. As used herein, the term “Corporate Offices” means and includes offices housing any quality assurance, quality control, and compliance functions with respect to the Centers.
SUPPLIER agrees to comply with the mandatory disclosure obligations set forth in the Quality Agreement.
In the event that the costs incurred by SUPPLIER in the collection, packaging, sampling, labeling, testing, processing or storage of Plasma are increased or decreased to any extent above or below the cost in effect as of the Effective Date as a result of a modification by GWWO of the Quality Agreement, then the purchase price per liter shall be increased or decreased to the extent properly allocable to the Plasma sold to GWWO under this Agreement, using generally accepted cost accounting principles. In the event a government mandated program significantly affects SUPPLIER’s costs, then the parties will negotiate how the costs or savings will be shared. All cost allocations are independent of Section 2.2 of this Agreement.
3.2 Testing. SUPPLIER shall bear all costs of testing, including EIA and PCR (NAT) testing. SUPPLIER agrees that samples for PCR (NAT) testing from any applicant donors will not be sent before first receiving negative serological test results. PCR samples from qualified donors can be sent on the day of donation. PCR results, with the exception of re-tests, are expected to be received within twenty-one (21) days of sample submission.
Any plasma, which is not produced in accordance with the Quality Agreement, or other FDA regulations or is otherwise not as warranted can be rejected by GWWO notwithstanding prior payment by GWWO and returned to SUPPLIER. In such event, SUPPLIER shall then reimburse GWWO for any payments made to SUPPLIER for such rejected Plasma, as well as any testing costs. At GWWO’s option, such reimbursement shall be reflected as an offset and deduction against any outstanding invoices, a credit towards a future invoice, or as a separate payment to GWWO. SUPPLIER shall bear any and all costs and expenses of returning or disposing of any such rejected Plasma. SUPPLIER shall have the right to instruct GWWO to destroy such rejected Plasma, at SUPPLIER’s cost and expense, in lieu of returning it to SUPPLIER.
GWWO shall not be obligated to buy or pay for any Plasma pursuant to this Agreement or any option under this Agreement, which does not, in all respects, comply with the Quality Agreement and the FDA or European Authorities regulations.
3.3 Shipping of Plasma. Plasma shall be packed by SUPPLIER in such a manner as to prevent damage to the Plasma or Plasma containers during shipping and shall be shipped by a transportation company designated by GWWO, subject to such other conditions set forth in the Quality Agreement and any other written instructions provided by GWWO. No Plasma shall be released for sale pursuant to this Agreement unless and until such Plasma fully complies with the Quality Agreement, and SUPPLIER shall be responsible for ensuring compliance with the terms of the Quality Agreement such and release protocols.
3.4 Donor Testing Results. Subject to the confidentiality obligations set forth in Section 5.16 of this Agreement, SUPPLIER agrees to arrange testing for each donor of Plasma as required by Title 21 of the Code of Federal Regulations and any other applicable law and make available test results of all such tests to GWWO. The method by which SUPPLIER will provide such test results, should GWWO require them, will be mutually agreed to between the SUPPLIER and GWWO.
3.5 Licenses, Permits and Approvals. SUPPLIER represents and warrants that it has obtained and shall continue to possess during the Term, a current, valid and unrevoked license from the United States Food and Drug Administration’s Center for Biologic Evaluation and Research (CBER) and/or European Authorities (depending on the Plasma origin) authorizing and qualifying SUPPLIER to conduct automated plasmapheresis, as well as any special programs to be pursued by SUPPLIER at any or all of the Centers identified herein, as well as a license permitting SUPPLIER to produce and ship Plasma related products derived from the automated operations conducted at the Centers. In addition, SUPPLIER represents and warrants that it shall maintain for the Term, all other local, state and federal licenses, permits and approvals required to operate the Center and, as a condition precedent to the commencement of GWWO’s performance of its obligations hereunder, SUPPLIER agrees to furnish a copy of all such licenses, permits, and approvals to GWWO.
ARTICLE 4. PERSONNEL, MATERIAL AND ADDITIONAL RELATED SERVICES TO BE
FURNISHED
4.1 SUPPLIER agrees to furnish all managerial, administrative, medical and professional personnel (including persons qualified to conduct plasmapheresis operation) required to produce the annual quantities of Plasma set forth in Section 2.1 hereof. In addition, SUPPLIER agrees to provide, at its own cost and expense, all facilities, equipment, materials and softgoods needed to produce Plasma at each Center. All personnel and equipment provided by SUPPLIER shall be under the management, supervision and control of SUPPLIER and not GWWO, at all times.
4.2 Further, Haema undertakes to assume and perform, on behalf of GWWO, all necessary services, tasks and dealings related or derived from the supply of its Plasma to any third party designated by GWWO, such as, without limitation, quality, regulatory and/or technical requirements, all in accordance with the instructions provided by GWWO from time to time and in compliance with law.
ARTICLE 5. MISCELLANEOUS PROVISIONS
5.1 Relationship of the Parties. The relationship between GWWO and SUPPLIER is, and during the Term shall be, that of buyer and seller. SUPPLIER is in no way the partner, legal representative or agent of GWWO for any purpose whatsoever and has no right or authority to incur, assume, or create, in writing or otherwise, any warranty, liability or obligation of any kind, expressed or implied, in the name of, or on behalf of GWWO. The terms of this Agreement shall not be construed as creating a dependent agency relationship for U.S. federal income tax purposes.
5.2 Indemnification. SUPPLIER and GWWO hereby indemnify and agree to hold harmless each other and its respective affiliates, agents, employees, officers and directors, from and against any and all claims, losses, liabilities, damages, attorney’s fees, costs and expenses which may be sustained by and/or claimed against the other party by virtue of the negligent performance of services rendered by the other party, the willful misconduct by the other party or its officers, employees or agents, or any representation or warranty contained in this Agreement being breached, untrue or materially misleading, by omission or otherwise. It being understood, however, that, save for GWWO’s obligation under Section 2.1 b), the financial liability under this section shall be limited to the extent of each party’s insurance coverage that shall be in force and maintained during the Term (which in the case of GWWO shall be the standard insurance cover of GRIFOLS and its Affiliates from time to time procured and maintained on a prudent basis and at least consistent with current and past practice as at the original date of this agreement. GWWO shall provide the SUPPLIER with confirmation of such insurance on an annual basis)
5.3 Default.
5.3.1 Rights and Remedies
In the event
(a) SUPPLIER or GWWO is in breach of any provision, or is in default of any obligation under this Agreement, or in the event of any representation or warranty contained in this Agreement is breached, untrue or materially misleading, by omission or otherwise, and such breach is not subject to remedy or curable, or
(b) [**],
then the non-breaching party (which in the case of 5.3.1 (b) shall only be SUPPLIER not GWWO) will be entitled to forthwith terminate the Agreement, by serving a written notice thereof to the breaching party. For the avoidance of doubt if GRIFOLS is in default of [**] and as a result SUPPLIER cannot perform any of its obligations under this Agreement, GWWO shall not be able to claim that SUPPLIER is in breach of this Agreement.
For any other type of breach, and upon written notice from the non-breaching party to the breaching party on the occurrence of the breach, the parties shall meet and discuss in good faith the remedies to apply in order to rectify the breach and the term in which such breach should be remedied (the “Remedial Plan”). Should the parties not reach an agreement on the Remedial Plan within a reasonable timeframe thereafter not to exceed thirty (90) days from the initial notice’s date issued by the non-breaching party, the breach shall be submitted to each party’s executives, who, within a term not to exceed ninety (90) days thereafter (or such longer term as the parties’ executives may agree to at that time), shall decide upon the Remedial Plan. In the event that each party’s executives do not reach a consensus on the Remedial Plan, the non-breaching party shall be entitled to terminate the Agreement.
Notwithstanding any of the above, the SUPPLIER may terminate this Agreement forthwith by written notice on Change of Control of the SUPPLIER.
5.3.2 Remedies Cumulative; No Waiver
The rights and remedies available to GWWO and SUPPLIER under this Agreement (including, without limitation, the remedies set forth in Sections 3.1, 3.2, 5.2, 5.3 and 5.4.1) or any other agreement among the parties are cumulative, and the exercise of any right or remedy shall not preclude or dismiss GWWO’s or SUPPLIER’s right to pursue any other or additional right or remedy, including, without limitation, specific performance, injunctive relief, termination, cancellation or rescission as permitted at law or in equity, and any claim for damages. The failure to exercise any right or remedy in the event of any breach or default shall not constitute a waiver or adversely affect GWWO’s or SUPPLER’s right to exercise any right or remedy for the same or any other breach or default.
5.3.3 Guarantee
The Guarantor hereby irrevocably and unconditionally guarantees to the SUPPLIER the prompt and full discharge by GWWO (and its Affiliates, where relevant) of all of GWWO’s (or, where relevant, its Affiliate) covenants, agreements, obligations and liabilities under this Agreement, including the due and punctual payment of all amounts which are or may become due and payable by GWWO hereunder, when and as the same shall become due and payable (collectively, the “GWWO Obligations”), in accordance with the terms hereof or thereof. The Guarantor acknowledges and agrees that, with respect to all GWWO Obligations to pay money, such guaranty shall be a guaranty of payment and performance and not of collection and shall not be conditioned or contingent upon the pursuit of any remedies against GWWO. If GWWO shall default in the due and punctual performance of any GWWO Obligation, including the full and timely payment of any amount due and payable pursuant to any GWWO Obligation, the Guarantor will forthwith perform or cause to be performed such GWWO Obligation and will forthwith make full payment of any amount due with respect thereto at its sole cost and expense.
The liabilities and obligations of the Guarantor pursuant to this Agreement are unconditional and absolute and, without limiting the generality of the foregoing, shall not be released, discharged or otherwise affected by:
(a) any acceleration, extension, renewal, settlement, compromise, waiver or release in respect of any GWWO Obligation by operation of law or otherwise;
(b) the invalidity or unenforceability, in whole or in part, of this Agreement;
(c) any modification or amendment of or supplement to this Agreement;
(d) any change in the corporate existence, structure or ownership of GWWO or the Guarantor or any insolvency, bankruptcy, reorganization or other similar proceeding affecting any of them or their assets; or
(e) any other act, omission to act, delay of any kind by any party hereto or any other person, or any other circumstance whatsoever that might, but for the provisions of this Section, constitute a legal or equitable discharge of the obligations of the Guarantor hereunder.
The Guarantor irrevocably and unconditionally agrees with the SUPPLIER that, if any obligation guaranteed by it is or becomes unenforceable, invalid or illegal, it will, as an independent and primary obligation, indemnify the SUPPLIER immediately on demand against any cost, loss or liability it incurs as a result of GWWO or the Guarantor not paying any amount which would, but for such unenforceability, invalidity or illegality, have been payable by it under this Agreement on the date which it would have been due.
The Guarantor hereby waives any right, whether legal or equitable, statutory or non-statutory, to require the SUPPLIER to proceed against or take any action against or pursue any remedy with respect to GWWO or any other person or make presentment or demand for performance or give any notice of non-performance before the SUPPLIER may enforce its rights hereunder against the Guarantor.
This guarantee is to be a continuing guarantee and accordingly the Guarantor’s obligations hereunder shall remain in full force and effect until the GWWO Obligations shall have been performed in full. If at any time any performance by any person of any GWWO Obligation is rescinded or must be otherwise restored or returned, whether upon the insolvency, bankruptcy or reorganization of GWWO or otherwise, the Guarantor’s obligations hereunder with respect to such GWWO Obligation shall be reinstated at such time as though such GWWO Obligation had become due and had not been performed.
5.4 Force Majeure. The performance of GWWO and SUPPLIER hereunder is subject to all contingencies except those beyond the direct control of GWWO and SUPPLIER including, without being limited to, the following:
5.4.1 Strikes, or other labor disputes or labor troubles of any kind;
5.4.2 Hurricanes, floods, earthquakes, droughts, or accidents;
5.4.3 Commotions, insurrections, riots, wars, or consequences of war;
5.4.4 Acts of God or perils of the sea;
5.4.5 Rules, laws, orders, actions, quotas, embargoes, seizures, regulations, restrictions, or actions of any governmental agency or divisions thereof, or rejections by inspectors or retentions of goods by customs authorities;
5.4.6 Breakdowns in manufacturing machinery, casualties, fires, loss of goods in public or private warehouses, provided, however, that such breakdowns, casualties, fires or losses are not the result of GWWO’s or SUPPLIER’s intentional acts;
5.4.7 In any such event, GWWO and SUPPLIER shall have the right, at its good faith and election and without incurring any liability for such occurrence or event to:
a. Notify other party of its intention and mutually agree between the parties to perform a modified or restricted agreement and perform the Agreement as so restricted or modified because of any of the foregoing contingencies. In the event the parties hereto mutually agree to perform a modified or restricted Agreement, the restricted or modified performance shall operate as a complete discharge of any obligations hereunder which are inconsistent with such modification or restriction; or
b. Perform the Agreement within a reasonable time after the causes of nonperformance or delay have terminated.
5.4.8 Notwithstanding any other provision of this Agreement, in the event of the occurrence of any of the events described in Section 5.4.6, GWWO shall be obligated to continue to perform each of its obligations under this Agreement, including the obligation to purchase the amounts of Plasma specified herein at the then prevailing price thereof (as determined pursuant to this Agreement), and all risk of economic loss relating thereto shall be for the account of GWWO.
5.5 Notices. All notices or other communications required or permitted to be given or made under this Agreement may be effected by personal delivery in writing, which shall then be deemed communicated the same day as the personal delivery thereof, or by registered or certified mail, postage prepaid, return receipt requested, which shall then be deemed communicated five (5) days from the mailing thereof (each a “Notice”). Notices shall be addressed to the parties at the address given below or at such address as the respective parties may hereafter designate to the other in writing:
If addressed to GWWO:
Grifols Worldwide Operations, Ltd.
Grange Castle Business Park
Grange Castle
Clondalkin
Xxxxxx 00
Xxxxxxx
If addressed to Guarantor:
Grifols SA
Avinguda de la Generalitat, 152
Parc empresarial Can Xxxx Xxxx
08174 Sant Cugat del Xxxxxx
Barcelona
Spain
If addressed to BIOTEST:
Biotest Pharmaceuticals Corporation
000 Xxxxxx Xxxx, Xxxxx 000
Xxxx Xxxxx
XX 00000-0000
XXX
If addressed to HAEMA:
Haema AG
Xxxxxxxxxxxxxxxxxx 0, 00000
Xxxxxxx
Xxxxxxx
5.6 Applicable Law. This Agreement and any issues, disputes or claims arising out of or in connection with it (whether contractual or non-contractual in nature such as claims in tort, from breach of statute or regulation or otherwise) shall be governed by, and construed in accordance with, the laws of Ireland.
5.7 Effectiveness of Agreement. This Agreement shall become effective only upon execution and acceptance by GWWO and SUPPLIER.
5.8 Multiple Originals. This Agreement may be executed simultaneously or in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
5.9 Partial Invalidation. In the event any provision of this Agreement shall for any reason be or become void or unenforceable, the remaining provisions shall continue in full force and effect, and under no circumstances shall an unenforceable provision have any effect upon any provision which is otherwise enforceable.
5.10 Paragraph Headings. The subject headings of the paragraphs and subparagraphs of this Agreement are included for the purposes of convenience only, and shall not affect the construction or interpretation of any of its provisions.
5.11 Assignability. Except as otherwise set forth herein, this Agreement shall not be assignable by either party hereto, either voluntarily or by operation of law or otherwise, without the prior written consent of the other party. Such prohibition on the assignment of rights under this Agreement shall be operable to the extent permitted by law. Any assignment without prior written consent is void. Consent shall not be unreasonably withheld. Notwithstanding the above, the Supplier may assign its rights under this Agreement (by way of security only) to any bank(s) and/or financial institution(s) lending money or making other banking facilities available to its parent corporation (or to its affiliate or subsidiary under common ownership or control) for the acquisition of the Supplier (“Lender”). On the enforcement of any security of a kind referred to in this Clause any person having the benefit of such security (including any administrative receiver) may assign any or all of the relevant rights to any person, but the continuing party’s liability to any assignee in respect of those rights shall not be greater than if no assignment had taken place.
5.12 Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties hereto, their successors and assigns, subject to the provisions of Section 5.11 above.
5.13 Third Party Rights. Except for such corporation(s), firm(s), partnership(s) or other legal entity(ies) affiliated with and under common ownership and control of GWWO or SUPPLIER, nothing in this Agreement, whether expressed or implied, is intended to confer any rights or remedies under or by reason of this Agreement on any persons other than the parties to it and their respective successors and assigns, nor is this Agreement intended to relieve or discharge the obligation or liability of any third persons to any party to this Agreement, nor shall any provision give any third persons any right of subrogation or action over or against any party to this Agreement.
5.14 Arbitration. Any dispute under, or in connection with, or arising out of this Agreement, including any dispute as to the existence or validity of this Agreement, shall be referred, by written notice from either party, (the “Referral”), to the decision of a single arbitrator selected by agreement of the parties, or failing agreement within ten (10) days of the date of the Referral, as may be nominated by the President of the Law Society of Ireland. Any such reference to arbitration will be a submission to arbitration within the meaning of the Arbitration Xxx 0000 or any Act amending or repealing same and shall be arbitration conducted in the city of Dublin (Ireland) in the English language and shall be governed by the Arbitration Xxx 0000 subject to the following provisions. The award rendered by the arbitrator shall be final and binding upon the parties hereto.
The parties agree that the courts of Ireland shall have power to make any order for third party discovery of documents and exclusive power to make any order relating to security for costs of the arbitration.
If an arbitrator declines the appointment or after appointment is removed by order of a competent court or is incapable of acting or dies and the parties do not, within twenty-eight (28) days of the vacancy arising, fill the vacancy, then the vacancy will be filled on the nomination of President of the Law Society of Ireland.
The existence, content and results of the arbitration and any rulings or awards shall be confidential to the parties and, without the prior written consent of the other party, neither party may disclose the existence, content, or results of any such arbitration, save as required by law, or for the purpose of presenting or preparing its case in the arbitration or for the purpose any legal proceedings regarding the arbitration or any rulings or awards.
5.15 Authority to Execute. SUPPLIER is not a party to, nor is it bound by any agreement which precludes or otherwise restricts the performance of its obligations hereunder. SUPPLIER represents and warrants that it has the right, legal capacity and authority to enter into this Agreement and that the execution of this Agreement has been duly authorized.
5.16 Confidentiality. GWWO and SUPPLIER and its employees and agents shall hold in confidence any and all documents, materials and information provided to SUPPLIER by GWWO, including but not limited to the terms and conditions of this Agreement include permission to disclose agreement and all related information to lenders]. SUPPLIER agrees that it will not disclose, except to their employees and agents on a need-to-know basis, any such information or documents described herein at any time during, or after termination of this Agreement, without the prior written consent of GWWO. The requirements of this Paragraph shall not apply to information which is publicly disclosed by GWWO. In addition to the above, both SUPPLIER and GWWO shall fully comply with the U.S. Department of Health and Human Services’ regulation on “Privacy Standards for Individually Identifiable Health Information” which comprises 45 C.F.R. §§ 160.101 through 164.534, promulgated pursuant to the Health Insurance Portability and Accountability Act of 1996.
5.17 Entire Agreement. This Agreement constitutes the entire agreement of the parties hereto with respect to the subject matter hereof and supersedes all prior agreements or understandings, both written and oral, with respect to such subject matter. No party hereto has made any representation or warranty or given any covenant to another party hereto except as set forth in this Agreement.
5.18 Order of Precedence
In the event of any conflict or inconsistency between the terms of any of the sections of this Agreement and any Exhibit (including the Quality Agreement) the terms of the section of the Agreement shall prevail and shall be the binding obligation of the Parties.
EXHIBIT A DONOR CENTERS 16

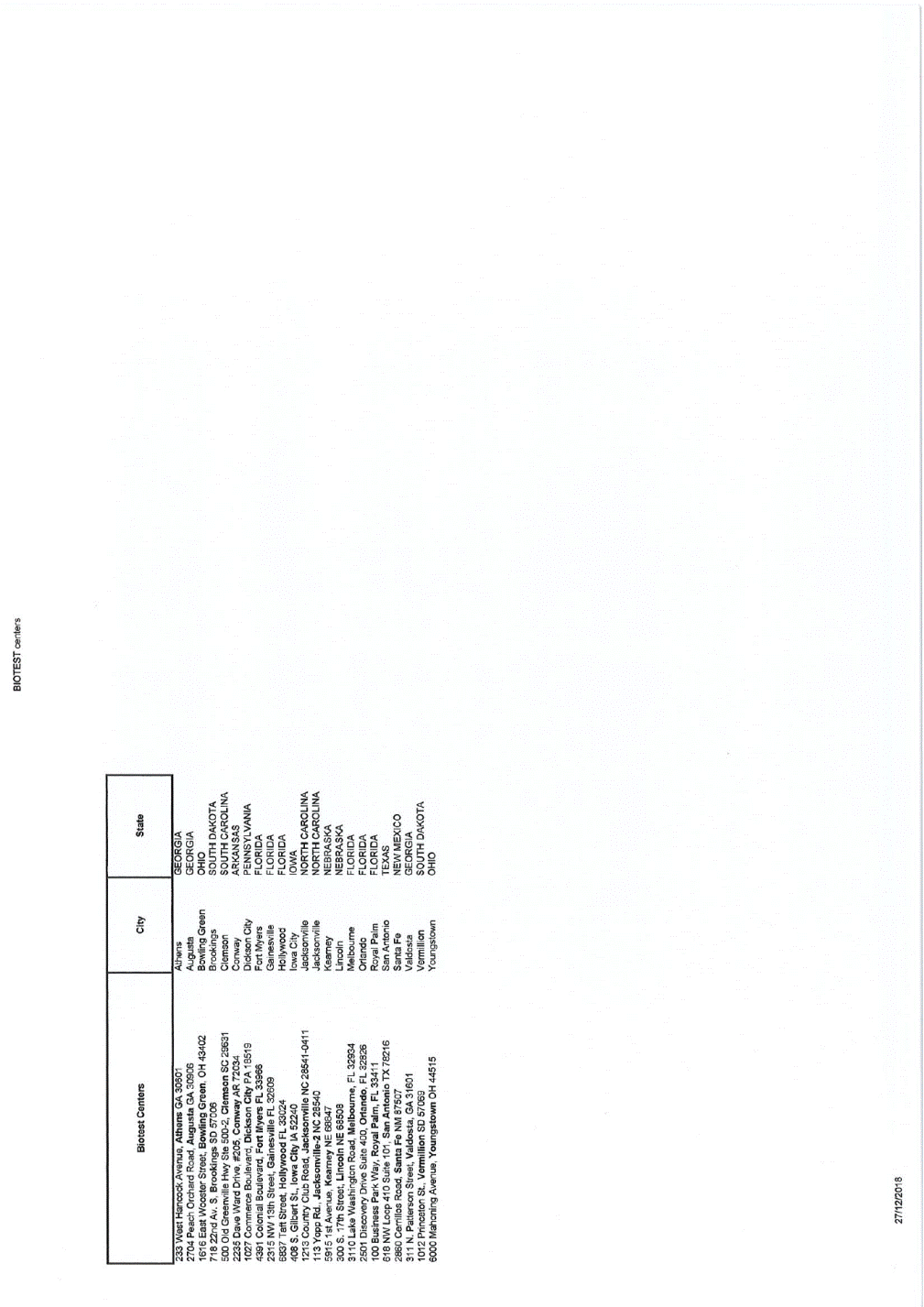
BIOTEST centers IL · I c ·: I 000 Xxxx Xxxxxxx Xxxxxx,"x""" ""'.ouov1 Athens Augusta GEORGIA 0000 Xxxxx Xxxxxxx Xxxx,Xxxxxxx XX 00000 0000 Xxxx Xxxxxxx XxxxxXxxxxxx Xxxxx,XX 00000 000 00xx Xx.X, Xxxxxxxxx XX 00000 000 Xxx Xxxxxxxxxx Xxx Xxx 000-0,XxxxxxxXX 00000 0000 Xxxx Xxxx Xxxxx, #000, Xxxxxx XX 00000 0000 Xxxxxxxx Xxxxxxxxx, Xxxxxxx Xxxx XX 00000 0000 Xxxxxxxx Xxxxxxxxx,Xxxx Xxxxx Xx00000 0000 XX 00xx Xxxxxx, Xxxxxxxxxxx X0x0000 0000 Xxxx Xxxxxx, Xxxxxxxxx X0x0000 000 X. Xxxxxxx Xx,Xxxx Xxxx xX 00000 0000 Xxxxxxx Xxxx Xxxx, Xxxxxxxxxxxx XX 285414111 000 Xxxx Xx., Xxxxxxxxxxxx-0 XX 00000 0000 0xx Xxxxxx, Xxxxxx XX 00000 000 X. 00xx Xxxxxx, Xxxxxxx XX 00000 0000 Xxxx Xxxxxxxxxx Xxxx, Xxxxxxxxx,Xx00000 0000Xxxxxxxxx Xxxxx Xxxxx 000,Xxxxxxx,XX 00000 000 Xxxxxxxx Xxxxx Xxx, Xxxxx Xxxx,X0x 0000 000 XX xxxx 000 Xxxxx 000,Xxx Xxxxxxx XX 00000 0000 Xxxxxxxxx Xxxx, Xxxxx Xx XX 00000 311N.Xxxxxxxxx XxxxxXxxxxxxx, XX 00000 0000 Xxxxxxxxx Xx, Xxxxxxxxxx XX 00000 XXXXXXX XXXX SOUTH DAKOTA SOUTH CAROLINA ARKANSAS PENNSYLVANIA FLORIDA FLORIDA FLORIDA IOWA NORTH CAROLINA NORTH CAROLINA NEBRASKA NEBRASKA FLORIDA FLORIDA FLORIDA TEXAS NEW MEXICO GEORGIA SOUTH DAKOTA OHIO Bowling Green Brookings Clemson Conway DicksonCity Fort Xxxxx Gainesville Hollywood Iowa City Jacksonville Jacksonville Keamey lincoln Melboume Orlando Royal Palm San Antonio Santa Fe Valdosta Xxxxxxxxxx Youngstown 0000 Xxxxxxxx Xxxxxx,Xxxxxxxxxx XX 00000 27/1212018
HaemaAG centres I I Center Center Location Post Code GERMANY 1 RaemaAG, BERLIN-GHARLOTTENBURG Xxxxxxxxxxxxx XxxxXx 00 00000 - 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Haema AG, BERLIN-HELLESDORF Haema AG, BERLIN-MARZAHN Haema AG, BERLIN-PRENZLAUER XXXX Xxxxx AG, BERLIN-TEGEL Haema AG, BERLIN-WEDDING Haema AG, BONN Haema AG, XXXXXXXXXXX/XXXXX Xxxxx AG, CHEMNITZ Haema AG, DORTMUND Haema AG, DRESDEN-FETSCHERPLATZ Haema AG, DRESDEN-WORL TRADE CENTER Haema AG, ERFURT Haema AG, ESSEN Haema AG,FRANKFURT/DOER Haema AG,XXXXXXXX Xxxxx AG,XXXX Xxxxx AG, GORLITZ Haema AG, GOTHA Haema AG, GRIMMA Haema AG, HALLE Haema AG, XXXX Xxxxx AG,XXXX Xxxxx AG, LEIPZIG-GONNEWITZ Haema AG, LEIPZIG-GOHLIS ARKADEN Haema AG, LEIPZIG-MARKT Haema AG, XXXXXX Xxxxx AG, MUNCHEN Haema AG, PLAUEN Haema AG, POTSDAM HaemaAG,REGENSBURG Haema AG, ROSTOCK Haema AG, XXXXXXXX Xxxxx AG, WEIMAR Haema AG, ZWICKAU Xxxxx-Xxxxx-Xxxxx 1 HavemannstraBe 12b Xxxxxxxxxxx Allee 117 12627 12689 10407 - - ----< Xxxxxxxx XxxxXx 00 XxxxxxxXx 4a XxxxxxxxXx 00, XxxxxxxxxxxxXx 1-2 Markt 5, 13507 13357 53111 14776 09111 44135 D-01307 D-01067 ---- Xxxxxxxxxxxx 00-00, Xxxxxxxxxxxxx 0 --Xxxxxxxxxxx 00 - -··· Xxxxxxxxxx 0, Xxxxxxxxx Xxx. 00, Kari-Marx-StraBe 2 XxxxxxxxxxxXx 00, Xxxxxxxxxxxxxxx&x 1-5, An der Frauenkirche12 Xxxxxxx&x 00, Xxxxx-Xxxxxxxx-XxxxXx 6, Xxxxxxxxxx Passage 17A, Westring2, Goethestra&e 3 A, 99084 45127 15230 09599 7545 02826 r--t I I I 99887 04668 06122 59065 07743 04277 D-04155 D-04109 23552 80335 8523 14480 93053 18057 19055 I I I --1-- ----Xxxx-Liebknecht-Stra&e 155, Xxxxxxxxxxxx 00 Xxxxx0 Xxxxxxx,0-00 Xxxxxxxx Xxx. 00, Xxxxxxxxxxxx-Xxxxxx Xxxxxxxx 00, NuthestraBe 1 ----0--------x----Xxxxxxxxx-xxx-Xxxxxx-XxxxXx 0, Xxxxxxxx MED Platz_2 -----r--- I Friedrichstr.18 XxxxxxxxxxxXx 00, XxxxxxxxxxXx 3, 99423 8056 27/1212018
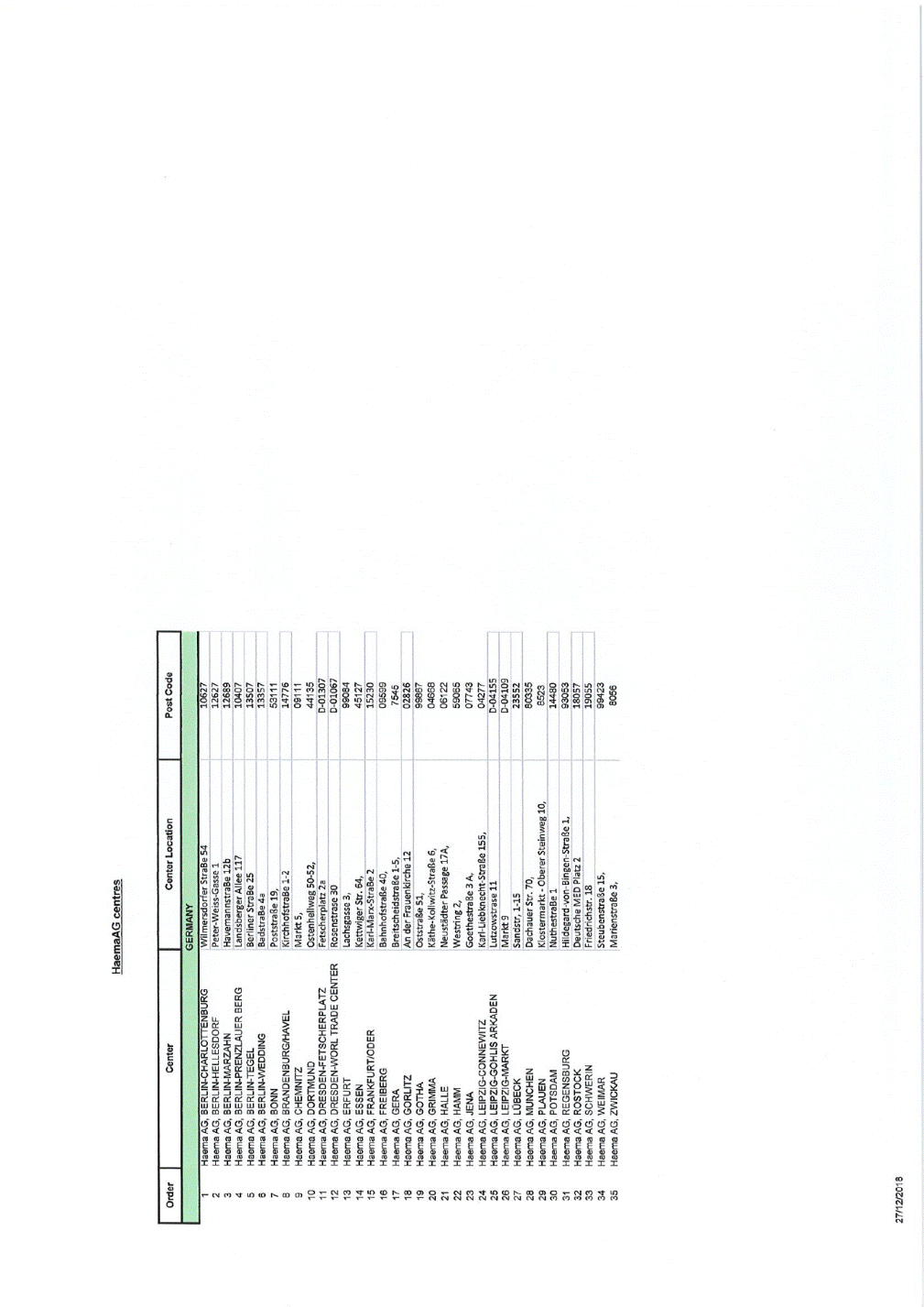
EXHIBIT B QUALITY AGREEMENT 17

QUALITY AGREEMENT SOURCE PLASMA INTENDED FOR THE MANUFACTURE OF MEDICINAL PRODUCTS FOR HUMAN USE This Quality Agreement is part of the Services Agreement dated 28-December-2018 by and between Grifols Worldwide Operations, Ltd. ("MANUFACTURER") with its principal place of business at Grange Castle Business Park, Grange Castle, Clondalkin, Xxxxxx 00, Xxxxxxx and Haema AG with its principal place of business at xxxxxxxxxxxxxxxxxx 0, 00000 xxxxxxx, Xxxxxxx ("HAEMA", and "PLASMA SUPPLIER"). The term of this Quality Agreement ("Agreement") commenced on the date set forth above (the "Effective Date") of signature of the Services Agreement. This Agreement document will be updated due to the continuous evolution of standards of quality and safety for the collection, testing, processing, storage and distribution of human plasma. Appendices to this Agreement may be revised without revision to this Agreement as long as appropriate approvals and proper signatures according controlled documentation systems for both parties are in place. 1. GENERAL PROVISIONS 1) MANUFACTURER is a company that manufactures medicinal products or intermediate fractions for further processing derived from human plasma and uses Source Plasma as starting material/raw material. 2) In connection with its medicinal products business, MANUFACTURER desires to obtain, and PLASMA SUPPLIER desires to provide, Source Plasma (including hyperimmune plasma) solely in accordance with the terms and conditions set forth in this Agreement covering the technical and quality requirements of Source Plasma as a raw material for further manufacturing at MANUFACTURER. Source Plasma is obtained and manufactured by PLASMA SUPPLIER from Health Authority licensed Plasma (Human) collection centers PLASMA SUPPLIER may also supply MANUFACTURER with Source Plasma obtained from other approved suppliers (contract and third party). PLASMA SUPPLIER is responsible for the assurance that the Source Plasma obtained from these suppliers (including cent§.rs, laboratory test, storage facilities or transport/transit companies) meets all of the requirements stipulated in this agreement and MANUFACTURER Specifications. A detailed list of centers, laboratories, storage facilities and transport/transit companies is enclosed in ATTACHMENT 1. PLASMA SUPPLIER will also provide additional services to the MANUFACTURER. PLASMA SUPPLIER will be responsible for the handling, storage, and transport of intermediate pastes and for the handling, storage, transport and release of plasma that is contracted to the MANUFACTURER for further processing. Such services must meet all of the applicable requirements for the materials in accordance with the terms and conditions set forth in this Agreement, the applicable regulations, and the MANUFACTURERS Specifications. 3) to PLASMA SUPPLIER agrees that all Source Plasma ("Plasma") supplied to MANUFACTURER as starting material produce medicinal products for human use hereunder will meet the following requirements, specifications and conditions.

2. QUALITY AND TECHNICAL REQUIREMENTS General 1) All Plasma supplied by PLASMA SUPPLIER under this Agreement must be derived from Source Plasma collected at plasma collection centers approved by competent authorities that have all necessary and regulatory approvals and permits,. All Plasma supplied by PLASMA SUPPLIER under this Agreement must be collected, processed, tested, stored, packaged, labeled, released and shipped in compliance with all applicable sections of the origin Country Regulation, including without limitation current Good Manufacturing Practices ("cGMP") A list of relevant regulations is enclosed in ATTACHMENT 2. 2) PLASMA SUPPLIER shall collect, process, test, store, package, label, release and ship the Plasma in accordance with MANUFACTURER Plasma Specifications ("MANUFACTURER Specifications"). A copy of the MANUFACTURER Specifications is enclosed as ATTACHMENT 3. PLASMA SUPPLIER hereby acknowledges receipt of a copy of MANUFACTURER Specifications and Shipping instructions, which are incorporated herein by reference as though fully set forth herein. These may be amended from time to time by MANUFACTURER or by updates due to new Quality or Regulatory requirements made by an appropriate governmental agency during the term of this Agreement, and upon receipt thereof, PLASMA SUPPLIER shall jointly review with MANUFACTURER, be in agreement and approve any such amendments and not unreasonably withheld or delay. 3) Plasma collection centers will screen all donors to ensure that each of them is under good health conditions and that plasma is not collected from a high risk population for blood-borne infections. Therefore, plasma centers with an active High Risk Donor collection program (donors known to be positive for HIV, HCV or HBV at the time of collection) will not be accepted by Grifols. 4) Plasma is for further manufacture use only and is not to be represented as suitable for use as a finished human drug. 5) The MANUFACTURER Specifications may be changed and/or modified by MANUFACTURER requirement in order to comply with any new legislation in force and/or new controls or test methods which are available in order to obtain the safest Plasma as possible. 6) PLASMA SUPPLIER, agrees to maintain regular plasma configuration and do not to alter the configuration of the normal donor panel population as starting material for all plasma-derived products without MANUFACTURER approval. Units from donors that have participated in a hyperimmune program cannot be used as Source Plasma without prior knowledge of the MANUFACTURER to avoid cluster of units going to same plasma pool. Plasma is for further manufacture use only and is not to be represented as suitable for use as a finished human drug. Quality Control 7) PLASMA SUPPLIER agrees to arrange testing for each donor of plasma in accordance with MANUFACTURER Specifications, as well as National Regulations, applicable European Directive, as well as any other applicable federal, state, and local or foreign competent authorities, governmental agencies regulations concerning donor testing, and agrees to furnish test results of all such tests to MANUFACTURER in agreed certificate format when required. Both parties shall ensure that all laboratories used are in compliance with cGMP and are qualified in all of the methodologies associated with plasma testing. All testing laboratories used to test Plasma supplied must be on the approved laboratory list as per Attachment 1. All testing methods must be validated and fulfil adequate levels of sensitivity and specificity according to their intended use, fulfilling all regulation in force. All validations, including protocols, reports and documentation shall be approved by the quality assurance function of the laboratory.

Tests and analysis of the Plasma shall be done in a laboratory approved by PLASMA SUPPLIER using licensed kits. AITACHMENT 4 includes a list of approved test kits and validated NAT platfonns. Plasma testing shall be perfonned according test kit manufacturer instructions. Submission of plasma samples to testing laboratories for any testing will be compliant with written procedures for submission of such samples. This includes, but is not limited to: use of approved containers and shippers, procedures for handling and shipping of samples, and the preparation and submission of appropriate documentation with samples. The laboratories shall be responsible to notify the centers of any paperwork or samples received that does not confonn to the requirements for sample paperwork, sample labeling and sample shipping. 8)Each unit of Plasma must meet the quality control specifications and the claimed and approved shelf-life for the Plasma at the maximum approved storage temperature as stated in the labeling specified in the MANUFACTURER Specifications. Subcontracted Operations 9) PLASMA SUPPLIER and MANUFACTURER shall jointly agree in writing on any contracted service (i.e., donor center collection, plasma storage, shipping, testing) to be completed by any third party per the MANUFACTURER Specifications. MANUFACTURER will have the right to audit any such facility and may jointly audit with the PLASMA SUPPLIER auditors. PLASMA SUPPLIER will provide a written report to MANUFACTURER confinning that the Plasma units have been collected, tested, shipped and stored properly and meet the requirements of the MANUFACTURER Specifications. Both parties shall ensure that all sites or services used are in compliance with cGMP and are qualified in all of the methodologies associated with plasma handling and its premises. All contract sites used for the collection, transport, testing or storage of the Plasma supplied must be on MANUFACTURER approved suppliers list. The contracted sites must show that methods are validated fulfilling regulation in force. All validations, including protocols, reports and documentation shall be approved by the quality assurance function of the third party organization. Any contracted organization must be approved and listed in ATIACHMENT 1 and testing must comply with ATIACHMENT 4. Traceability and look-Back Communication (Post-Collection Information) 10) PLASMA SUPPLIER shall communicate to MANUFACTURER in writing within (5) five calendar days all infonnation on a donor after a donation that fulfilled all MANUFACTURER Specifications is rejected for seroconversion, CJD/nvCJD or post donation infonnation that may become an infectious disease. Notification instructions and required notification information are detailed in MANUFACTURER Specifications (traceability requirements and look back). PLASMA SUPPLIER will notify MANUFACTURER as soon as practicable and in no case later than (5) five calendar days of discovery of significant deviations that may impact batch(s) or Plasma previously released and/or shipped to MANUFACTURER. 11) PLASMA SUPPLIER shall supply to the MANUFACTURER a report prior to product release on all post donation notifications received after pooling so that the MANUFACTURER can perfonn a viral risk assessment on the product. Change Control 12) All changes shall undergo a technical and cGMP impact assessment. PLASMA SUPPLIER will notify MANUFACTURER in advance of any planned changes reasonably foreseeable to affect the regulatory submissions to the Health Autorities involved (. PLASMA SUPPLIER will have responsibility for providing justification for the changes and validating (when appropriate) any such changes to cGMP standards. Change control procedures will include a

requirement for PLASMA SUPPLIER to obtain written approval from MANUFACTURER prior to the implementation of any change reasonably foreseeable to affect any regulatory submission, and any other change to parameters that are covered by the change control scope that could have an effect on the safety, purity, etc. of the Plasma collected. The scope of the change management process includes collecting, testing, labeling, storage and shipping processes of Plasma, as well as intermediate fractions, storage and shipping. The associated changes may relate to: the master batch records, xxxx of materials, analytical standards, test methods and Specifications; and any changes to validated facilities, utilities, computer systems, equipment or processes used in the cleaning, manufacturing, testing, storage or shipment with respect to Plasma or intermediate fractions. Grifols Source Plasma Specifications may be changed and/or modified by Grifols as required in order to comply with current European requirements. Grifols shall submit all proposed revisions to the Specifications to PLASMA SUPPLIER for review and approval, which cannot be unreasonably withheld. Grifols shall advise PLASMA SUPPLIER of all proposed revisions to the Specifications as promptly as practicable so as to provide PLASMA SUPPLIER with as much notice as possible. PLASMA SUPPLIER shall have thirty (30) business days to agree to implement or otherwise respond to Grifols proposed revisions to the Specifications;. PLASMA SUPPLIER response shall, if applicable, state PLASMA SUPPLIER reasons (which shall be commercially reasonable) for refusing to implement such revisions. PLASMA SUPPLIER and Grifols shall attempt in good faith to resolve any disputes as to the implementation of any requested revisions. Timeline for implementation of the change will be agreed by both companies. Quality Assurance 13) PLASMA SUPPLIER shall have a quality management system that ensures all critical processes involved in collection, processing, packaging, labelling, testing, storage, release and transportation are conducted in adequate facilities and according to current GMP principles as well as current legislation. PLASMA SUPPLIER shall maintain an active quality management system which at least shall review deviations and trends, ensure appropriate training of personnel, conduct internal audits on a periodic basis, with a follow up of deviations with a CAPA program and a change control system in force. The quality system shall apply to all operations which may impact on quality and safety of Plasma, from donor selection to Plasma delivery to the MANUFACTURER. 14) PLASMA SUPPLIER shall be responsible for creating the manufacturing and testing master documentation specific to Plasma collection. It shall be responsible for creating and maintaining at the Premises all SOPs ad other documentation required to support cGMP operations. 15) Any deviation from the process and SOPs during Plasma collection, storage or testing must be appropriately explained, investigated, documented and approved in the batch records or analytical records. The deviation documentation must identify the cause and the corrective action, where appropriate, and must assess the impact to the Plasma donor's safety, and Plasma's quality. PLASMA SUPPLIER shall develop appropriate procedures for deviation investigations. PLASMA SUPPLIER will notify MANUFACTURER as soon as practicable before shipment or in no case later than (5) five calendar days of discovery that may impact batch(s) or Plasma previously released and/or shipped. 16) PLASMA SUPPLIER shall ensure that adequate trained and qualified staff is conducting the activities with a clear description of employee responsibilities. Training must be conducted according to an approved training program. Initial and continuous training shall be performed as well as proper staff competency follow-up. Training records shall be retained and available for review. 17) Materials to be used in the collection of Plasma shall be approved in accordance with applicable legal requirements for Plasma collection. Upon receipt of such materials, PLASMA SUPPLIER shall confirm the proper condition of packaging, sterility certification, locks and seals and check that labels correspond with the shipping documents. PLASMA SUPPLIER shall implement an appropriate system to maintain certification of materials and chemicals used in the collection and testing of Plasma. For those specified materials of animal origin, if any, PLASMA

SUPPLIER shall assure each lot is in full compliance with licensed requirements, with National regulations and final approved by Grifols). 18) PLASMA SUPPLIER shall approve all vendors and suppliers of critical raw materials, reagents and components used in the collection and testing of Plasma and shall maintain an approved supplier list. PLASMA SUPPLIER shall ensure that all materials are received, stored, segregated and used correctly, and all materials are appropriate for their intended use. It also shall ensure appropriate separation and/or segregation of any materials that may present a potential hazard to the materials used in the production of Plasma units. PLASMA SUPPLIER shall be responsible for maintaining the relevant certificate of analysis, certificate of compliance and/or certification of origin (as appropriate) for the materials. Storage and Shipping 19) Storage and shipment of Plasma supplied by PLASMA SUPPLIER under this Agreement must be conducted in approved facilities that have all necessary and regulatory approvals. Storage facilities and shipping process must be properly validated. 20) Plasma must be frozen within twenty-four (24) hours of collection by cooling rapidly in conditions validated to ensure that a temperature of -25°C or below is attained at the core of each Plasma unit within twelve (12) hours of placing in the freezer apparatus. Plasma must be stored at -20°C or lower. Freezing, storage and shipping must be done according to WHO recommendations. 21) PLASMA SUPPLIER shall provide sufficient and suitable storage facilities that meet the storage specifications for Plasma units, as documented and agreed upon in the MANUFACTURER Specification, SOPs, applicable regulations related to Plasma storage, National Regulations for-Human Plasma for Fractionation. Any off-site Plasma storage facilities used must be approved by PLASMA SUPPLIER quality assurance and meet the same requirements. Mobile storage facilities shall only be used short tenn for emergency situations and must be appropriately validated to maintain correct temperatures. It shall be ensured that during storage of Plasma before shipping the plasma has been properly packaged and protected to avoid deterioration, interference, theft, product contamination or mixture with any other materials. PLASMA SUPPLIER will maintain proper segregation of the Plasma units. Details of any labeling requirements and shipping container sealing and integrity will be documented. The Plasma units shall be suitably packaged for transit, with each outer container being labeled as per written procedures. 22) Unreleased Plasma that is shipped to a PLASMA SUPPLIER quarantine location at the authorized plasma warehouse will be stored in a physically segregated area in a controlled, validated warehouse. Oversight of that area and the Plasma contained therein is the responsibility of PLASMA SUPPLIER quality assurance organization. Such unreleased Plasma must be controlled by standard operating procedures, including appropriate labeling and electronic material status. Such procedures will govern post unit donation removal, temperature deviation reporting requirements, unit and lot traceability, employee access and training, release of Plasma and final transfer to MANUFACTURER, regulatory filings and other such notifications and regulatory requirements not specifically covered by this Agreement whenever applicable. 23) Rejected Plasma must be properly segregated, identified and dispositioned under quality assurance control. Biological Product Devi ations and Recalls 24) PLASMA SUPPLIER shall be responsible for monitoring the manufacturing and release data of the Plasma and if detects a potentially reportable deviation, as applicable to Plasma sent to MANUFACTURER, this should be reported and discussed with MANUFACTURER within five (5 business day of confirmation. PLASMA SUPPLIER is responsible for Plasma unit deviation reporting to the appropriate regulatory authorities. Page 5 of 32

25) MANUFACTURER, as the medicinal product license holder, shall be responsible for reporting deviations pertaining to biological products to the regulatory authorities. PLASMA SUPPLIER will work collaboratively with MANUFACTURER on filing any such report thought to be due to the collection of Plasma. PLASMA SUPPLIER will promptly perfonn investigations for any issues that are thought to be Plasma unit based. Both parties shall collaborate in the development of the global reporting regulatory strategy for the event. PLASMA SUPPLIER shall provide necessary infonnation to MANUFACTURER if receives a warning letter, significant regulatory observation or BPDR follow up question(s). 26) PLASMA SUPPLIER is responsible for Plasma unit recall or withdrawal per the applicable regulations. MANUFACTURER shall be notified of any recall and of any problems thought to be due to Plasma quality within five(S) business day of confinnation. When requested by MANUFACTURER, PLASMA SUPPLIER will perform investigations for these problems. Investigation reports regarding the defect or cause of recall will be forwarded to MANUFACTURER within a mutually agreeable timeframe. MANUFACTURER is responsible for any final product or intennediate product recall or withdrawal for those products that are manufactured. Equipment, Computer and Facility Validation and/or Qualification Process 27) PLASMA SUPPLIER shall ensure that all process, equipment, systems and facilities are qualified and validated if applicable prior its use and after any significant change to ensure that processes are capable of consistently achieving the Plasma specifications. Documentation must be reviewed and approved. Calibration and preventive maintenance programs shall be established for equipment. It will maintain cGMP validation status of process procedures, computers and associated equipment with respect to the manufacturing, storage and shipping procedures that are used in connection with Plasma. PLASMA SUPPLIER shall be responsible for all equipment, computer and facility qualification activities associated with the Plasma collection, storage and shipment. Validations must meet National Regulations and further approved by Grifols, as applicable and will promptly, upon request by MANUFACTURER, make validation reports available for review. PLASMA SUPPLIER shall develop procedures and implementation plans to assure that all computer systems and software are compliant with required Regulations 28) PLASMA SUPPLIER will approve all process qualifications applicable to the Plasma collection, including, but not limited to, the freezer validation and shipping validation for test samples and Plasma. PLASMA SUPPLIER will provide copies of validation reports upon request. Validation reports may also be requested as part of an audit or an event review. Plasma Release 29) PLASMA SUPPLIER collection centers and third party suppliers shall all have at least one Qualified Person on the Premises to oversee the quality aspects of the Plasma collection, testing, shipment, evaluate deviations, etc., in order to assure compliance with cGMPs. 30) PLASMA SUPPLIER has designated a person for plasma release for further manufacturing of medicinal products in accordance with applicable regulations. PLASMA SUPPLIER and its suppliers shall release units by means of a corporate Qualified Person who will document and certify that each unit of Plasma has been collected and tested in approved facilities according to GMP and that the units fulfil quality and safety requirements of the MANUFACTURER Specifications. The Responsible Person and designee are named in this Agreement as the individuals authorised to release Plasma units. 3. LICENSES, PERMITS AND APPROVALS

1. All Plasma supplied by PLASMA SUPPLIER under this Agreement must be derived from Plasma collected at an approved plasma collection center located in XXXXXX that has all necessary and regulatory approvals and permits,. The plasma collection center(s) under this Agreement are set forth in ATTACHMENT 1. 2. PLASMA SUPPLIER represents and warrants that all their plasmapheresis donor centers in operation are certified under the Plasma Protein Therapeutics Association's International Quality Plasma Program ("PPTA IQPP"). PLASMA SUPPLIER must preserve these certifications without interruption for the remaining term of this Agreement. If PLASMA SUPPLIER fails to maintain such plasmapheresis donor centers certifications, then MANUFACTURER has to be immediately notified within one (1) business day to evaluate actions to be taken. 3. PLASMA SUPPLIER agrees to follow the mutually agreed upon MANUFACTURER Specifications and all other requirements or specifications issued in writing by MANUFACTURER, and any versions to those procedures, requirements or specifications as they may be issued or adopted by MANUFACTURER and agreed upon by PLASMA SUPPLIER, as approved by appropriate governmental regulatory agency or competent authority, if such approval is mandated by law or required by MANUFACTURER. 4. In the event of a suspension, revocation, or other administrative action adversely affecting or limiting any such license, permit or approval, MANUFACTURER shall be immediately notified within one (1) business day to take necessary actions. 5. PLASMA SUPPLIER may not relocate, open or close any plasmapheresis collection centers, testing laboratories or plasma storage facilities without prior written notification to the MANUFACTURER. Written approval by MANUFACTURER is required for inclusion of new centers, testing laboratories, plasma warehouses and transportation companies, which shall not be unreasonably withheld. 4. INSPECTIONS 1. Collection and storage of the Plasma shall be conducted in a suitable controlled environment and such Premises shall be regularly inspected and monitored to ensure compliance with cGMP, and other applicable manufacturing authorizations. 2. PLASMA SUPPLIER will be responsible for qualification and auditing of all vendors of supplies used in the collection and shipment of Plasma. All suppliers must appear on the PLASMA SUPPLIER approved supplier list. PLASMA SUPPLIER is responsible for handling complaints to the vendor for supplies and the interaction with the vendor to resolve those complaints. 3. MANUFACTURER shall have the right to conduct periodic audits of the approved plasmapheresis donor collection centres, testing laboratories, Corporate Quality Management Systems, plasma warehouse storage facilities and/or transport/transit and shipping companies and to review all procedures and records kept by such facilities related the collection, processing, testing, storage, packaging, labeling, release and shipping of plasma as well as all operations conducted in conformance with generally accepted industry practice. These audits may be done as joint audits with PLASMA SUPPLIER, at the discretion of the MANUFACTURER. MANUFACTURER will provide PLASMA SUPPLIER with not less than thirty (30) day notice prior to any of its audits, unless agreed otherwise by the Parties, and such audits shall be during normal business hours, shall be limited to matters reasonably related to this Quality Agreement, and shall be conducted in conformance with generally accepted cGMP. No audit shall continue in duration for more than three (3) business days unless such an extension is reasonably necessary. A maximum of three (3) auditors shall participate in any inspection unless otherwise agreed between PLASMA SUPPLIER and MANUFACTURER. Such audits shall occur periodically (at least, every two years) for facilities having an acceptable inspection I audit history unless otherwise necessary to inspect or audit as a result of requirements by regulatory authorities or a serious failure or material concern that either impacts or threatens to :fiJ% p,,, 7 of"

impact quality, safety, potency, or compliance with this Quality Agreement. In the event of such additional audit(s), MANUFACTURER shall provide PLASMA SUPPLIER reasonable written notice of its intent to conduct such additional audit(s). MANUFACTURER maintains the right to event reviews (directed audit), in addition to periodic audits, to address significant quality, performance or safety problems associated with the products.These events should be requested, scheduled and conducted at the first available date. 4. PLASMA SUPPLIER shall be entitled to perform at least one (1) standard cGMP compliance audit every year of the above facilities to encompass all cGMP operations (testing laboratories, plasma warehouses, transport companies, corporate offices). 5. PLASMA SUPPLIER Compliance Audit Team will provide a written report to MANUFACTURER of all observations. Within thirty (30) days of the audit date, PLASMA SUPPLIER will coordinate to provide a written response to all findings that details corrective action to be implemented. PLASMA SUPPLIER will ensure that all corrective actions are implemented and provide a report to MANUFACTURER upon implementation. In those instances where corrective action will require an extended period to implement, PLASMA SUPPLIER shall provide reports to MANUFACTURER on the progress of such corrective action at such intervals as mutually agreed upon by the parties. 6. MANUFACTURER shall have also the right to cause any authorized representative of the Public Health Service, any state, local, foreign competent regulatory authorities and governmental agencies, and any authorized third party as deemed necessary or desirable to carry out its business worldwide, to conduct periodic inspections of the approved plasmapheresis donor collection centers, testing laboratories, plasma warehouse storage facilities and/or transport/transit and shipping companies and to review all procedures and all records kept by such donor centers or such testing laboratories regarding the collection, processing, testing, storage, packaging, labeling, release and shipping of Plasma, and all operations conducted in conformance with generally accepted industry practices. MANUFACTURER shall have the right to make and retain copies of all such procedures and records kept by donor centers, as well as copies of all correspondence between donor centers and any governmental regulatory agencies regarding any such inspections. 7. MANUFACTURER will be responsible for ultimate approval of all Plasma centers and their status as approved or not approved to ship plasma to the MANUFACTURER. The information will be contained in the MANUFACTURER list of approved Plasma suppliers and the types of Plasma the center is approved to collect. 8. PLASMA SUPPLIER agrees to send MANUFACTURER written notice of any federal, state or local government for cause audit or relevant investigation of the donor centers, testing laboratories, storage, release and shipping facilities/companies with no more than ten (10) business day of the investigation's commencement. 9. PLASMA SUPPLIER agrees to communicate to MANUFACTURER within ten (10) business days of discovery of critical event (failure of a plasma collection center, storage facility, testing laboratory and/or transportation which may impact quality or safety of the Plasma supplied 10. PLASMA SUPPLIER will notify MANUFACTURER in a timely manner of any meetings or substantive discussions with r any other regulatory authority that directly relate to the manufacture, supply and/or control of Plasma supplied. 11. PLASMA SUPPLIER shall provide to MANUFACTURER within ten (10) business days an information of the following: (i) Update of critical findings related to the inspection performed, (ii) Notice of Intent to Revoke License Letter, (iii) License Suspension Letter, (iv) Seizure action regarding Corporate Offices, plasma collection centers, storage facilities, or testing laboratories. 12. In a quarterly basis, PLASMA SUPPLIER shall provide Grifols a summary of regulatory National authority inspection of any plasma collection centers, testing laboratories, storage facilities, and corporate offices, included in

Attachment 1 of the this Quality Agreement. This summary shall include information concerning inspections of National health authority. This summary will also include a list of critical observations and an overall evaluation of the regulatory inspections outcome including: • Identification of the facility • Identification of inspection date • Category (# of Minor, Major, Critical) • Result: pass/not pass • - Kind of Deficiencies (for example: Documentation, Storage, etc.). 13. In the event that during an inspection by MANUFACTURER (or PLASMA SUPPLIER as designee), self notification to PLASMA SUPPLIER by the donor center, test lab, warehouse or transportation company or by any authorized representative of the Public Health Service, or any state or local or foreign competent authorities and governmental agencies to corporate, donor centers, test labs, plasma warehouses or transportation company, critical or serious observations are found that may impact quality or safety of the plasma supplied PLASMA SUPPLIER shall give MANUFACTURER notice within five(5) business days and establish a timeframe to pass the inspection and resolve issues. In the event that PLASMA SUPPLIER has not corrected the failure, MANUFACTURER shall be immediately informed in order to take necessary actions, including termination of this Quality Agreement and the Supply Agreement in the event such breach remains uncured for a period of sixty (60) business days. 5. DOCUMENTATION AND RECORD RETENTION 1) PLASMA SUPPLIER shall make available upon request documents such as Site Master Files (Donor Centers, Testing Laboratories, Storage Facilities, Corporate Offices related to Quality Management Systems), floor plans, equipment validation, and other production information in order to ensure safety, purity, and potency of the product. MANUFACTURER, as the Plasma-derived product license holder, is responsible to prepare and submit any regulatory submission linked to in process and final product as required by applicable regulations,. PLASMA SUPPLIER shall provide all documentation requested by MANUFACTURER to support regulatory submissions. PLASMA SUPPLIER commits to provide to MANUFACTURER on a regular basis the relevant information and regulatory documentation that is necessary for Regulatory Submissions according to final product applicable regulations . Such documentation shall include at minimum, certificates of approval by competent health authorities for facilities, laboratories, plasma warehouses and transportation companies, inspection status information, donor suitability criteria, approved technical specifications for soft goods, main equipment validation protocol, methods and validations of testing platforms, proficiency studies and epidemiological data. 2) 3) 4) Epidemiology data and viral marker data must be supplied by PLASMA SUPPLIER in accordance with the current regulatory requirements. Data must be submitted using the standard forms found in the Source Plasma Specification. Should a PLASMA SUPPLIER donor center exceed (on an annual basis, Jan-December.) the Grifols and/or its affiliated or parent company established VM Alert Limits (Attachment 3): •PLASMA SUPPLIER will provide Grifols an investigation and corrective/preventive actions (when appropriate); and •Plasma will not be accepted from a donor center that has exceeded the Grifols established Viral Marker Alert Limits for three (3) consecutive years.. 5) PLASMA SUPPLIER shall keep complete and accurate records of equipment usage, cleaning, testing, temperature holds, and any maintenance/calibration performed and other parameters in accordance with the most stringent applicable legal requirements. 6) PLASMA SUPPLIER agrees to retain donor records according to all applicable sections and regulations issued or promulgated by Competent Authorities applicable federal, state, and local or foreign competent authorities and governmental agencies regulations. Page 9 of32

7) The following items will have to be recorded and stored appropriately by PLASMA SUPPLIER in order to secure the quality and safety of the partially processed biological materials to comply with the current regulation identified in this Quality Agreement in order to comply with applicable period as may be required by any law or regulation governing the distribution of biological products manufactured from such Plasma o o o traceability records donorcard health record of each donor (including health history and physical examination of each donor, which are maintained in the donor management system) donor questionnaires (i.e., AIDS,...) donor number whose plasma was used for production pooling. NAT and serological test results, which are kept in the donor management system. o o o 6. CONSEQUENCES OF TERMINATION Upon termination of the supply contract: PLASMA SUPPLIER shall continue to comply with its obligation under this Agreement if and to the extent it continues to have the responsibility for post donation information and look back information after the expiry or termination of the Supply Agreement. All obligation srelating to compliance with cGMP in respect of the plasma shall continue in force according to the requirements of cGMP, including but not limited to: Record Retention and availability . Regulatory inspections and data requested Post donation information and look back information. Epidemiological Data for the year after the termination of the supply agreement. 7. CONTACT PERSONS In the event of serious failure, regulatory issues, update or change in specifications: PLASMA SUPPLIER If addressed to HAEMA: HaemaAG Xxxxxxxxxxxxxxxxxx 0, 00000 Xxxxxxx Xxxxxxx MANUFACTURER Grifols Worldwide Operations, Ltd. Xxxxxx Xxxxxx Xxxxxxxx Xxxx Xxxxxx Xxxxxx Xxxxxxxxxx Xxxxxx 00 Ireland Page 10 of 32

1) Each party shall notify the other party in writing of any significant change in reporting relationships involving the quality department or quality functions within five (5) business days of such change and proceed according the Change Control requirement process outlined in this agreement. 2) Each party shall provide written notice to the other party of any change in the management of a critical operation that is applicable to plasma (e.g., production, quality, new center set up and validation) wHhin five (3) business days of such change and proceed according the Change Control requirement process outlined in this agreement.. ACCEPTANCE SIGNATURES: PLASMA SUPPLIER MANUFACTURER HaemaAG Approved by: h.t{_ ! . /'\ Approved by:--\:::. '--- 0 f . U /i. L0 ) 1 7· <;_'-'"'-- l :J Date: Date: Ut (...e.-k "CS -\"-'.e._( Haema o Name: Name: """' Dr. med. Xxxxxxx Xxxxxxx-Xxxxxxx HaemaAG Title: Title: Medizinischer Vorstand Page 11 of 29
ATTACHMENT 1 LIST OF CENTRES, TEST LABORATORIES, PLASMA WAREHOUSES, TRANSPORTATION COMPANIES HAEMA centers Xxxx:AG ccntrns Po tCodc wumersdorter Stra!lt Sli Xxxxx-\Velss·Gasse 1 H vcrn nnstra5e l2b Xxxxxxxxxxx Xxxxx 000 00xxxxxx XxxxXx 25 Bnd tr.rlle 4• Xxxxxxx.xXx 00, XxxxxxxxxxxxXx0-0 11.1crkl5, OsiQnhe!lwos SD-52, Fetscherplatl ZD ltosenstr..se 30 t.ach ; $Se3, Kettwiger Str.64, !!:arl-1\larx-strcBe 2 11a hnhofstrafl. Sreits:heid51ra(l,ct 1-5, Xxxx Frauenkirche 12 Osllitro8eSl, Xxxxx-Kollwitt·StraSe 5, Xxxxxxxxxx Passage 17A. Wcstring 2, G cth<:$tr Be 3 A, !t31l-liebknecllt·Sirne 155, Lutiow.>trase ll Xxxxx0 Xxxxxxx, x-00 0 0 0 Xx XX, XXXXXX-XXXXXXXXXXXXX Hcema AG, BERLIN·HELLI:SDORF Haema AG, BERLIN·MARZAHN Hne!rm AG, BERLIN-PRENZLAUER XXXX Xxxxx AG, BERLIN-TEGEL li 'na IIG, BERLIN-V.JCDOING Hoe.Tru AG, BONN H uAG,XXXXXXXXXXX VE H.oa.'llA; G, CHEMNilZ Haem:a AG, DORTMUND Haeme AG, ORESDEN-FETSCHERPLATZ Hl!!!ma AG, DRESDEN-WORL TRADE CENTER HoomAG, ERFURT H<!"..ma AG, ESSEN Haema AG, FRANKFURT/DOER Haome AG, XXXXXXXX Haamo AG, XXXX H ma AG, GORUTZ H&"..ma AG, GOTHrll Haa:na AG, GRIMMA HH1no AG, HALLE Hseme AG, 1-'.AMM Ha m.a AG, JENA Haama AG, LElPZIG·CONNEWITZ Haem:; AG, LEIPZIG-GOHLIS ARi<AOEN H.,om:1 AG, LEIPZIC-l.lARKT HaemA; G, XXXXXX Hsemoe AG, MUNCHEN Haem:e AG, PLAUEN H;,eme AG, POTSDAM 1-'.aemc AG, REGENSBURG Xxxx::; AG, ROSTOCK Haemf;l..G, SCH\1\'XXXX H:leme AG, WEIMAR Haem<! AG, ZWICKAU 105Z7 12627 12689 i0407 13507 13357 53111 14776 09111 44135 D-0130i 0·01057 55034 45127 15230 Ge593 75 02826 5EE67 041!89 06122 5!0055 0774:3 C-4277 D-4155 0.()4109 23552 eons 6523 14480 3 18057 19055 23 BOSS "' 5 e 7 e 9 10 11 12 13 14 15 16 17 1e 19 20 21 22 23 24 25 2o 27 :ze 23 3 31 32 33 34 35 DacheuerStr.70, Kltctrmorllt-c-xxxxx Xxxxxxxx 00, XxxxxxxxxXx 0 Xxxxxxxxxx-\·xx Xxx.xxxXxxxXx 0. DeutsChe MEO Xxxxx 0 Xxxxxxxxxxxx.00 X ubensll'oBc 15, M ricn trn3e 3, Haema Central Donor Laboratory, Xxxxxxxx XXxxXx 00, 00000 Xxxxxx Xxx r Center Center Location GERJIIANY
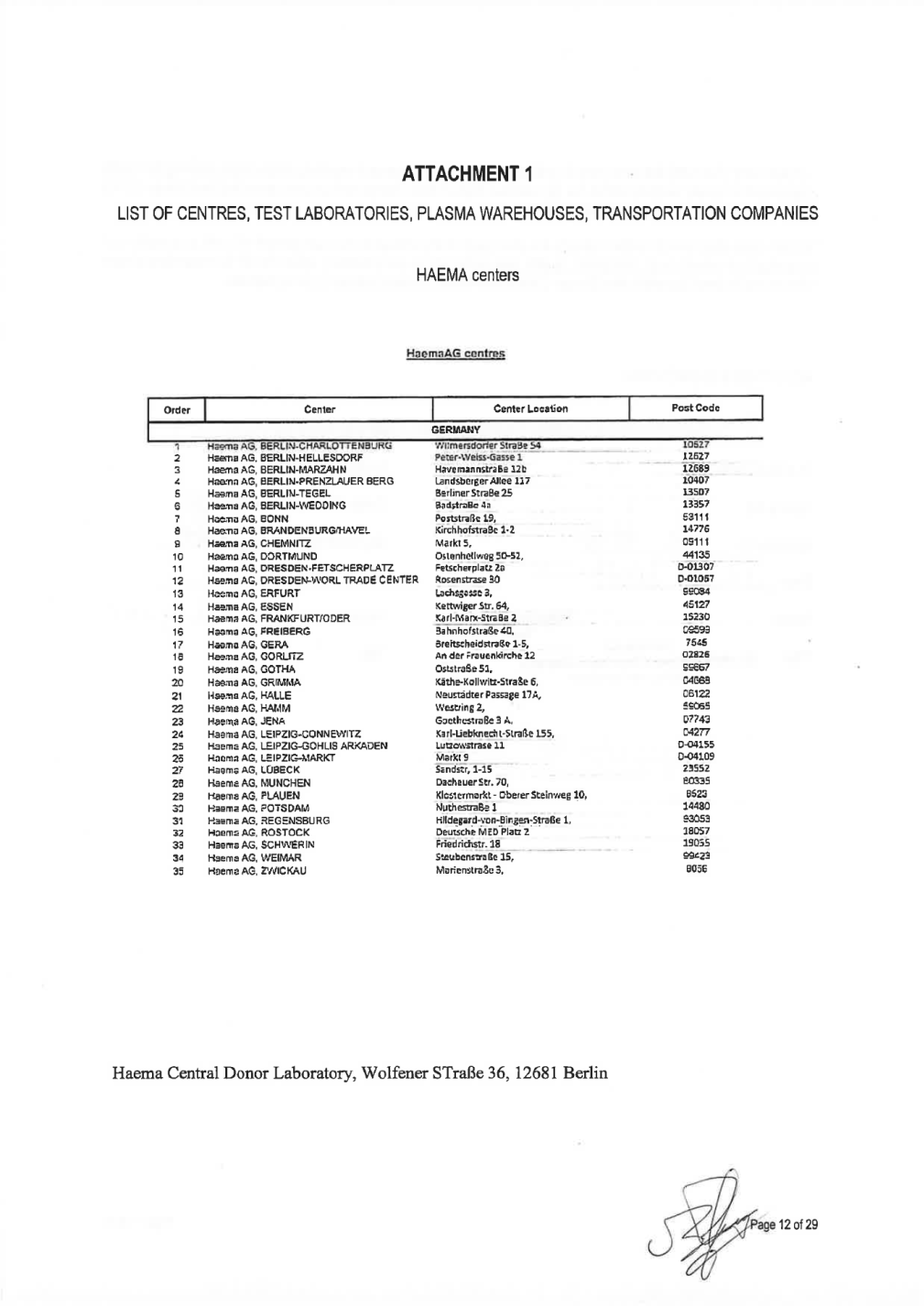
ATTACHMENT 2 RELEVANT REGULATIONS o EU Good Manufacturing Practices for Medicinal Products for Human use, all as amended from time to time o Council of Europe: Guide to the preparation use and quality assurance of blood components (current version). o European Pharmacopeia monographs ('EP') requirements particularly in respect of blood or blood components as starting material for the manufacture of proprietary medicinal products. o Recommendations of the World Health Organization (WHO). o Comission Directive 2005/61/EC of 30 September ·2005 implementing Directive 2002/98/EC as regards traceability requirements and notification of serious adverse reactions and events. o Comission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC, regarding donor suitability requirements. o Directive 2001/83/EC, GMP Annex 14 and certain recommendations of the Council of Europe, as well as any other applicable international, federal, state and local regulations concerning the collection, processing, testing, packaging, labeling, release and shipping of Plasma. storage, o Guideline on plasma-derived medicinal products (EMA/CPMP/BWP, current version). Page 13 of29

ATTACHMENT 3 SPECIFICATIONS 1.-PURPOSE To provide and describe the conditions required for Source Plasma to be accepted by Grifols for the procurement of Source Plasma intended for manufacture of hemoderivate products. This specification is intended to assure that incoming plasma meets all Grifols requirements, in addition to establish domestic and international regulations, 2.-SCOPE These specifications cover all source plasma intended for fractionation, defined as the fluid portion ofhwnan blood collected by plasmapheresis. Grifols can only use plasma for fractionation that fulfils all the Grifols requirements for all of the components that are part of Plasma Supply Chain, i.e.: Plasma collection centers Plasma testing facilities and Plasma testing methods Plasma storage facilities Plasma transportation companies. Every one of them has to be approved by Grifols and previously authorized by competent Health Authorities. 3.-RELATED DOCUMENTS o o o o o PPTA Viral Marker Standard PPTA Qualified Donor Standard PPTA NAT Testing Standard EUEU Pharmacopeia monograph for Hwnan Plasma for Fractionation Revision to Annex 14 to EU Guide to GMP: Manufacture of products derived from hwnan blood or plasma EUEMEA/CHMP/BWP/3794/03 Guideline on the Scientific Data Requirements for a Plasma Master File EUEMEA/CHMP/BWP/125/04 Guideline on Epidemiological Data on Blood Transmissible Infections eucouncil of Europe: Guide to the preparation use and quality assurance ofblood components EUOirective 2002/98/CE EUOirective 2004/33/CE o o o o o EUfor plasma intented to be used as raw material for Hemoderivates Products for European Market SPfor plasmatic raw material intented to be processed in Spanish Manufacturing Plant facilities :ff#t-""'"of29

4.-PROCEDURE All plasma units shall be collected by approved suppliers and collection facilities, and tested by approved laboratories using approved kits, and stored and transported using approved establishments in compliance with Plasma types will be defined according to plasma center involved, laboratories involved, testing methods involved, specific test for final product destination requirement, warehouse involved and plasma carrier involved in which is transported. The Supplier will keep updated a quality assurance system that will include, at least, review and investigation of deviations and detection of possible trending, performance of internal compliance audits with corresponding follow-up of corrective and preventive actions and implementation of an internal Change Control procedure. The quality assurance system will be applied along the collection, processing, testing, storage, and control procedures. All activities, functions and/or documentation practices will be performed according to cGMP (current Good Manufacturing Practices). 4.1. PLASMA COLLECTION Plasmapheresis Centers Plasmapheresis centers shall be approved by the Health Authorities of plasma origin and be in compliance with Epidemiological data established by Manufacturing company.(IQPP certified). Plasma collection centers will screen all donors to ensure that each of them is under good health conditions and that plasma is not collected from a high risk population. Management and control of donors should be done using an IT system. In addition for plasma intended for use as raw material in hemoderivate products for European market, plasma centers have to be approved by European Health Authorities. Plasma Supplier will supply Grifols for corresponding evaluation and for each center Epidemiological Data of viral markers (HBV, HCV & HIV), annually in EMA format and half yearly in PPTA format. All alert notifications and their correspondinginvestigations aswell as their corrective/preventive actions (if applicable) should be included in the continuous revision of epidemiological data. Centers participating in specific programs of collecting plasma from donors tested reactive/positive in viral markers cannot be Grifols' suppliers. Donor selection Donor selection procedures shall fulfill with competent authorities Regulations. In addition for plasma intended for use as raw material in hemoderivate products for European market, Donor selection process has to be in compliance with those established by European Health Authorities. All plasma donations shall be from qualified donors. Page 15 of 29

Sterility Plasma is separated from cells by an aseptic method using a sterile system designed to prevent micro-organism contamination. Furthermore, the plasma containers are closed, with the aim to prevent the possibility of contamination. 4.2 PLASMA TESTING The laboratory shall be approved by competent authorities. The laboratory will keep approved and updated SOPs, containing the necessary working instructions. All analytical methods shall be validated and shall have an optimum level of sensitivity and specificity and shall fulfill with current legislation. All the validations, including protocols, reports and related documentation will be approved by Quality Assurance area. The laboratory will join in certification programs (proficiency testing) to ensure suitability of methods and techniques in use. Plasma shall be tested by facilities approved by competent authorities (additionally approved by a competent Health Authority Likewise, all analytical methods used shall be approved by competent authorities (additionally approved by a competent Health Authority and shall be included as part of the approved specifications. For plasma intended to be used as raw material for hemoderivate products for European Market, testing facilities and analytical methods used have to be approved by European Health Authorities. Plasma testing requirements Prior to use, the test kit manufacturer, test kit methodology and testing facility must be approved by Grifols. • All donors shall be screened for all the biochemical parameters required by national authorities as control of the donor and for indirect xxxxxx reactivity in the first donation and every 90 days, and the obtained result must be non-reactive. • All donors shall be screened for excluding Syphilis presence as required by national regulations. Donor test is performed every 4 months. • All plasma units must be tested and found non-reactive for Hepatitis B surface antigen (HBsAg) by a validated test method approved by competent authorities. • All plasma units must be tested and found non-reactive for HIV-1 and HIV-2 antibodies by a validated test method approved by competent authorities. • All plasma units must be tested and found non-reactive for Hepatitis C antibodies by a validated test method approved by competent authorities. • Additionally, all plasma units must be tested and found non-reactive by means of pools consisting of not more than 512 individual units for HBV-DNA, HCV-RNA and HIV-RNA

by a validated test method approved by competent authorities and for HAY-RNA and Parvovirus B19-DNA by a validated test method. The cut-off of Parvovirus B19-DNA determination must be such to ensure that plasma manufacturing pools do not exceed a titer of lx104 IU/ml. • All plasma units must be tested for all viral or non-viral markers as required by the plasma Supplier's Health Authorities. • For plasma intended to be used as raw material for hemoderivate products for European Market, analytical methods used have to be approved by European Health Authorities. Hyperimmune plasma For hyperimmune plasma all donors shall be immunized using Competent authorities approved immunizing program and shall contain at least the titers agreed with the Supplier. These are described in corresponding registers (IG_REG-000683 and IG_REG-000686). Traceability and look Back Requirements While fully respecting confidentiality, there is a system in place which enables the path taken by each donation to be traced, both forward from the donor and back from the finished medicinal product, including but not limited to plasma center, plasma donor number, analytical testing and results, plasma manufacturing pool testing, Data of Manufacturing process and final product identification. It's a system by which we can identify and remove look back and PDI units. A look-back unit is a unit of plasma that complies with all the established requirements but collected from a donor, from whom a subsequent donation has been rejected for any reason, related with an infection or with behavior that may involve belonging to a risk group (Post Donation Information), Creutzfeldt-Jakob disease (CJD) or new variant Creutzfeldt-Jakob disease (nvCJD). HEY-NAT testing will be performed exclusively for donors of medicinal products. In case of an HEY positive result, the corresponding blood components will be discarded and a look back report will include all donations (including plasma for fractionation) within 6 months before the reactive one. In case of reasons related to infection or belonging to risk group behaviour (PDI), Plasma Suppliers shall communicate to Grifols manufacturing companies all the plasma units drawn from a donor within twelve months prior to the date of the last negative tested donation for anti HCY and HCY-RNA, for HBsAg and HBY-DNA and for anti-HIY 1/2 and HIY-RNA. In case of CJD and nvCJD risk factors and/or diagnosis, communications shall include all the units sent to any Grifols manufacturing company from that donor. Look-back units shall be notified to any Grifols manufacturing company in written form as quickly as possible and not more than three (5) calendar days from the event that caused the procedure. Page 17 of 29

4.3. PLASMA PROCESSING Physical conditions of plasma units and samples Each plasma unit shall be collected from a single donor. All plasma units must be placed in the freezer within one hour of collection and maintained at - 20° C or colder. In case of plasma intended for used as raw material in hemoderivate products for European Market, it has to be in compliance with European Pharmacopeia requirements: Plasma intended for the recovery of labile proteins in plasma is frozen within 24h after collection by cooling rapidly in conditions validated to ensure that a temperature of - 25°C or below is attained at the core of each plasma unit within 12h after placing in the freezing apparatus, ensuring the preservation from activity oflabile coagulation factors. Plasma intended for the recovery of not labile proteins in plasma is frozen by cooling at - 20°C or colder as soon as possible and at the latest within 24h after collection. All plasma units shall be accompanied by a plasma sample of not less than 2 ml and both shall be identified with the bleed number and bar code. Both unit and sample of plasma shall exhibit neither hemolytic nor lipemia signs when visually inspected, nor both plasma bottle and sample tube shall not have any fissure or be broken. Storage The warehouse shall be approved by Grifols and by competent authorities. All plasma units and their corresponding samples shall be kept at -20 oc or colder, unless more restrictive conditions are required by the competent Health Authorities. Temperature of all shipments will be monitored using validated temperature recorders in order to ensure that plasma units and their corresponding samples have been kept at temperatures according to specification (S-20°C). Any deviation shall be investigated and approved. In case of plasma intended for used as raw material in hemoderivate products for European Market, the warehouse has to be also approved by European Health Authorities. Quarantine Plasma pool will be ready for fractionation once completed mtrumum volume needed for processing a production lot and prior minimum quarantine of 2 or 3 months from collection date for all units included on it. The quarantine period (2 or 3 months) will be determined depending on fractionation product destination and it will be controlled by computer management system.

Shelf-life Source plasma has a shelf-life of 36 months in all manufacturing plants in which plasma is processed. Plasma must not be older than 30 months when shipped to Grifols Manufacturing company. For units older than 30 months, it must be necessary a previous authorization by Qualified person of Grifols Manufacturing company. 4.4. PLASMA SHIPPING Shipment All carriers used to transport Source Plasma must be approved by Grifols. The temperature of the transport trailer must be -25°C or colder prior to loading the plasma shipment. The shipping procedure has to be designed to keep all plasma units and samples at -20 oc or colder during the whole transportation process according to Regulationos of Plasma Origin Country. In addition for plasma intended for use in hemoderivate products for European market the shipping procedure has to be in compliance with Ph. Eur. Monography, 'Human Plasma for Fractionation' Temperature of all shipments will be monitored using validated temperature recorders, in order to ensure that plasma units and their corresponding samples have been kept at temperatures according to specification ( -20°C). Any deviation shall be investigated to analyze cause and potential impact before to accept and approved plasma involved in it. All documents and plasma pool samples (if required) shall be sent in advance to any Grifols manufacturing company for shipment confirmation. For plasma intended as raw material for use in hemoderivate products for European market, all carriers used as raw material for used to transport Source Plasma must be approved by competent European Health Authorities. Identification of the shipping boxes The plasma Supplier shall identify boxes as following: -Supplier name. -Shipment number. -Total boxes by shipment. -Box/boxes (number of order regarding total number of boxes). Label All plasma units have to be labeled with at least the following information: 1-Supplier name. 2-Product name. 3-Bleed number (code bar). iff·"''

4-Volume or weight of the unit. 5-Date of collection. 6-Storage temperature. 7-Type and volume of anticoagulant. 8-Results of viral testing. Bleed Documentation Bleed documentation must contain the data displayed below: 1-Supplier name. 2-Product name. 3-Bleed number. 4-Donor number. 5-Shipment number or date of shipment. 6-Date of collection. 7-Test result for each one of the viral markers tested. The bleed number must be the same, as per numbers, prefixes and suffixes, in: -The list of donations. -The plasma unit label. -The plasma unit bar code reading. -The plasma sample label. -The plasma sample bar code reading. -The Alert notification of the look-back units. 4.5 QUA LITY ASSURANCE Inspections All plasma Suppliers, as well as laboratories, warehouses and transport companies, shall inform Grifols of the result of the inspections of the competent Health Authorities (Europe, other). All plasma Suppliers laboratories, warehouses and transport companies, may be audited periodically by Grifols staff or personnel authorized by Grifols. During these audits all procedures and reports of plasma suppliers, laboratories, warehouses and transport companies related to collection, processing, testing, storage, packaging, labelling and shipment of plasma may be reviewed. Serious incidences Plasma Supplier shall inform within 5 working days, any serious incidence produced at center, laboratory, and warehouse facilities and/or during transport process, which may affect the quality and safety of the provided plasma. Changes Control Plasma Supplier shall inform any Grifols Manufacturing company of any planned changes that may have an impact in the quality and safety of plasma during collection, processing, testing,

storage, packaging, labeling and shipment. Those changes requiring license amendment from Health Authorities (variations to the approved registry) need prior written approval of Grifols manufacturing company before their implementation. Documentation and Records An approved system of documentation and proper control systems of review and authorization will be established. Documentation shall be updated annually if necessary. All donor .records shall be retained by the Supplier for at least 30 years from the date of collection of the last donation for each donor. If necessary, Grifols and plasma Supplier will agree on additional donor records, health information, donor questionnaires, donor number and testing if requested by competent Health Authorities. All donor testing records shall be retained by the supplier for at least 30 years from the date of the shipment of each unit to Grifols. Plasma Supplier will provide upon request by Grifols copies of documents related to collection, receipt, storage, handling and distribution of products (i.e. recorder temperatures, maintenance records, etc...) Plasma Supplier will keep updated and available upon request by Grifols, documents such as SMF (Site Master File), map and flows, chart, equipment and process validation and any other production documents that ensure the quality and safety of plasma. Plasma Supplier will provide annually -or upon request by Grifols-the necessary documentation to keep updated the Plasma Master File. Training and qualified personnel There should be a sufficient number of qualified personnel and Center shall provide training in accordance with an approved program. Initial and continuing training will be performed and effectiveness assessed. Training records shall be kept for all personnel. Premises and Equipment (qualification and maintenance). Materials Equipment, systems, premises and instruments shall be qualified or validated before use or after any relevant change. Related qualification and validation documentation shall be reviewed and approved. A calibration program and preventive maintenance program for equipment and instruments shall be established. Critical materials shall come from approved Suppliers that meet the documented requirements and specifications. 4.6.PLASMA RELEASE Plasma units release: Certificate of Compliance The Supplier shall only release plasma units to its delivery to any Grifols manufacturing company as long as the Responsible Person states in a Certificate of Compliance that: Page 21 of 29

• All source plasma units fulfill the requirements and Source Plasma Specifications in accordance with the Quality Agreement in force. • All plasma units of the shipment have been tested and found non-reactive for viral markers, specifying the test kits and/or reagents used in each case for: o HBsAg, anti-HIV 1/2, anti-HCV, tested in individual units o Luetic serology, initially tested and every 4 months o Indirect Xxxxxx, initially tested and at least every 3 months o HAV-RNA, HBV-DNA, HCV-RNA, HIV-RNA and Parvovirus B19-DNA, tested by means of pools consisting of not more than 512 individual units. • All collection, processing, testing, quality control records review, storage, packing and labeling operations have been performed under current Good Manufacturing Practices, The certificate must be issued by the Responsible Person of the Plasma Supplier. The Supplier will provide Grifols with a list of names of the Responsible Persons and authorized designees and their corresponding signatures and responsibilities.

ATTACHMENT 4 TEST KITS FOR EIA/NAT TEST KITS FOR EIAINAT Viral Marker Test Kits of Individual Donors Re1dstration Number 00011 00021 00079 NAT: Name of test Analytical Technique . - NAT-HCV N T-HIV NAT-HE!_V NAT-HAV NAT-PVB19 TaqMan Real-Time Multiplex RT PCR Cobas MPX-Test for cobas 6800/8800 TaqMan Real-Time Duplex RT-PCR Cobas DPX-Test for cobas 6800/8800 Test kit Manufacturer Roche Diagnostics GmbH XX-Xxxx: yes 23of29 Parameter Test Method/ Name of the Test Kit Manufacturer License I Establishment Anti-HCV ChLWABBOTI PRISM HCV) Xxxxxx Laboratories 0088/0964174/00012 HIV-p24-Antigen, Anti-HIV-I+ Anti-HIV-2 ChLWABBOTI Prism HlV A'i/ Ab Combo Xxxxxx Laboratories 0088/0964174/ 00074 HBsAg ChLWABBOTI PRISM HBsAg Xxxxxx Laboratories 0088/0964 I 74/ Anti-HCV ChLWARCHITEcr Anti-HCV Reagent Ki Xxxxxx Laboratories 0088/0964174/ H!V-p24-Antigen, Anti-H!V-1 +Anti-HIV-2 ChLWARCHITEcr HIV A'i/Ab Combo ReagentKit Xxxxxx Laboratories 0088/0964174/ HBsAg ChLWARCHITEcr HBsAg Qualitative II Xxxxxx Laboratories CE0843

ATTACHMENT 5 TABLE OF RESPONSIBILITIES Summary Table and Division of Quality Responsibilities of PLASMA SUPLIERIMANUFACTURER 1.1. The responsible Party is denoted by X DESCRIPTION PLASMA SUPPLIER MANUFACTURER 1 GENERAL PROVISIONS Negotiate the Quality Agreement X X Notification of change in reporting relationship involving Contact Persons X X Assure that Plasma obtained from Source Plasma (Human) collection centers from Haema meet all the requirements stipulated in this Agreement and the MANUFACTURER Specifications X Responsible for the handling, storage, and transport of intennediates pastes and plasma that is contracted to the MANUFACTURER in accordance with the tenns and conditions set forth in this Agreement, the applicable regulationsand the MANUFACTURER Specifications X Approve any new contract or third party Suppliers of Plasma X X 2 QUALITY AND TECHNICAL REQUIREMENTS Collect, process, test, store, package, label, release Plasma in accordance with MANUFACTURER Specifications X ship Plasma in accordance with MANUFACTURER Specifications X QUALITY CONTROL Arrange testing lor each donor of plasma in accordance with MANUFACTURER Specifications, as well as applicable regulations X Ensure all laboratories are in compliance wilh cGMP and are qualified in all the methodologies associated with Plasma and the Premises X Perform testing and analysis of the Plasma in approved laboratories using licensed kits included in the EU Plasma Master File if plasma is intended for EU X
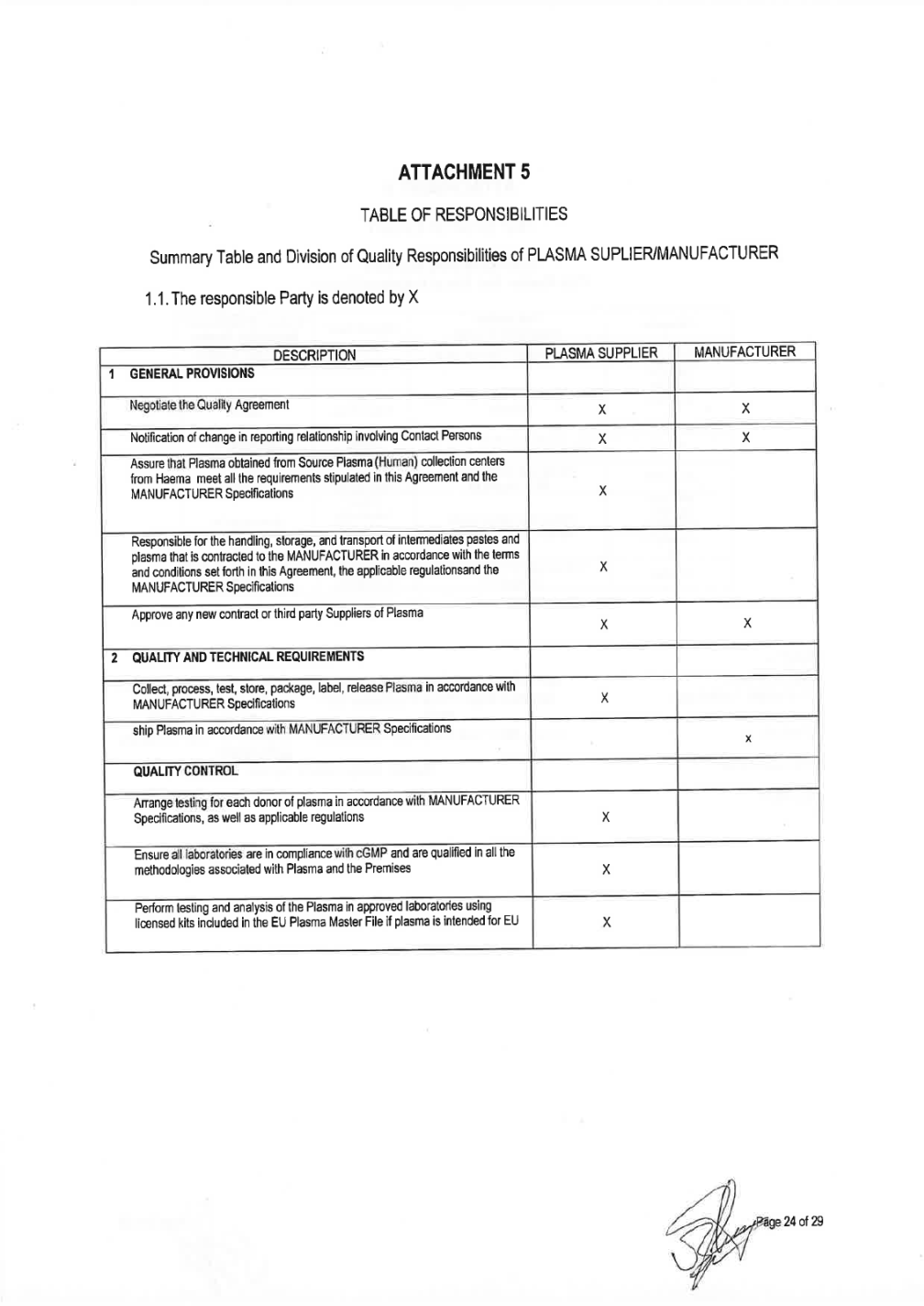
age 25 of29 DESCRIPTION PLASMA SUPPLIER MANUFACTURER SUBCONTRACTING OPERATIONS Agree in writing on any contracted service (i.e., storage, testing) to be completed by third party per the MANUFACTURER Specifications X X Perform audits of third party contractors X X Provide a written report to MANUFACTURER confirming that the Plasma units have been collected, tested, shipped and stored properly and meet the requirements of the MANUFACTURER Specifications X Ensure all sites or services used are in compliance with cGMP and are qualified in all of the methodologies associated with Plasma and the Premises. X X TRACEABILITY AND LOOK BACK COMMUNICATION (POST-DONATION COLLECTION INFORMATION Communicate to MANUFACTURER in writing within 5 calendar days all information per this Agreement on a donor after a donation that fulfilled all MANUFACTURER Specifications is rejected for seroconversion or post donation information that may become an infectious disease (Notifications per the MANUFACTURER Specifications) X Notify the MANUFACTURER no later than 5 calendar days of discovery of significant deviations that may impact batches or Plasma previously released and/or shipped to the MANUFACTURER. X Supply a report prior to product release on all post donation notifications received after pooling so that the MANUFACTURER can perform a viral risk assessment on the product X CHANGE CONTROL Notify MANUFACTURER in advance of any planned changes reasonably foreseeable to affect the regulatory submissions to regulatory authorities including EU Plasma Master File variations and/or available supply of the Plasma-Obtain written approval from MANUFACTURER prior to the implementation of such changes and any other change that are covered by the change control scope of this Agreement that could have any effect on the safety, purity, etc. of the Plasma collected X Provide justification for the changes and validate any changes to cGMP standards X Provide written approval for change controls per this Agreement X
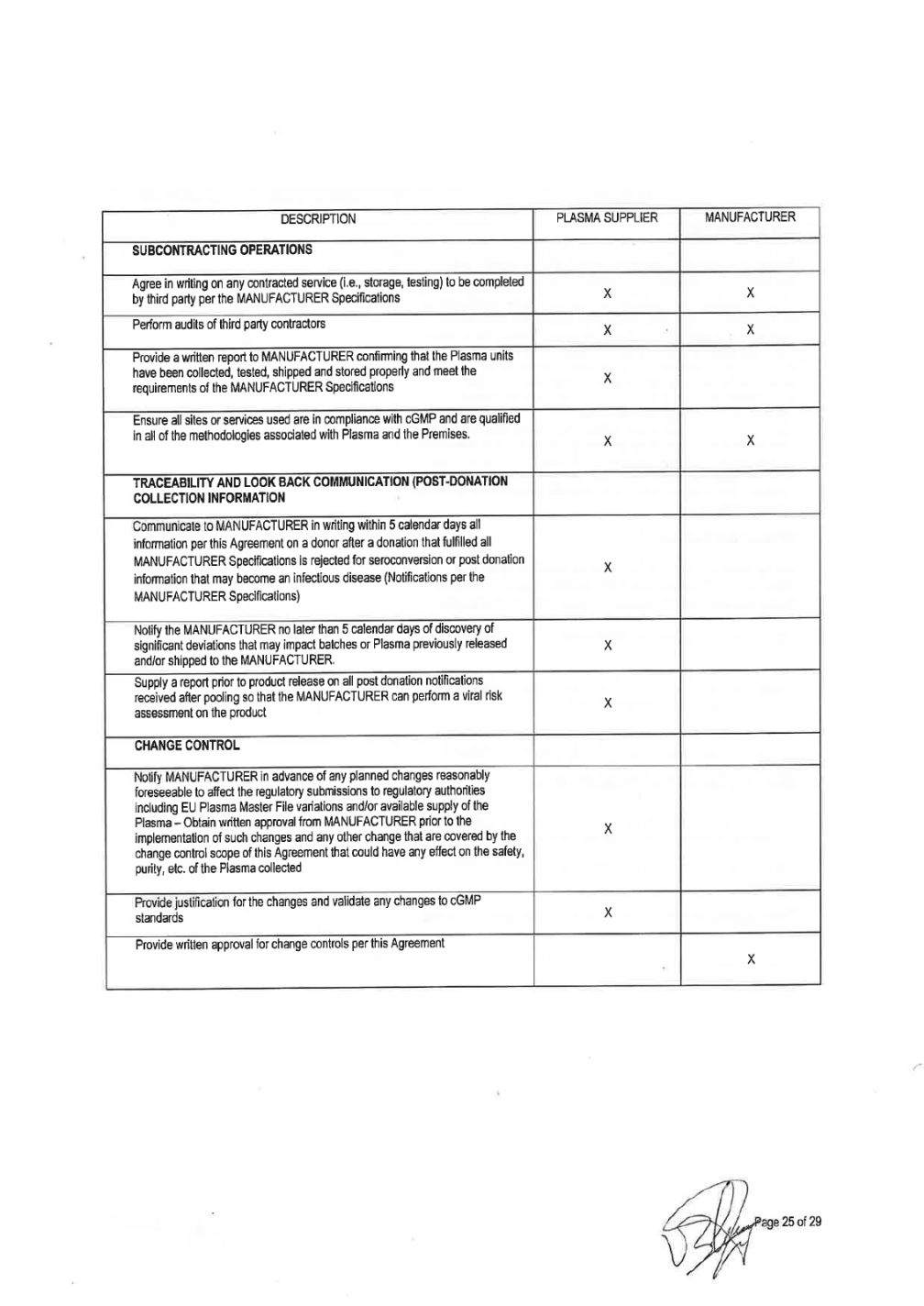
DESCRIPTION PLASMA SUPPLIER MANUFACTURER QUALITY ASSURANCE Maintain an active quality management system, which shall apply to all operations which may impact on the quality and safety of the Plasma X Document and investigate deviations from the processes and procedures per this Agreement and notify MANUFACTURER as soon as possible before shipment or in no case later than 5 calendar days of discovery that a deviation may impact a batch or Plasma previously released and/or shipped X Approve the use of materials for the collection of Plasma; confirm the proper condition of the packaging, sterility certification, locks/seals, shipping documentation; ensure that each lot is in full compliance with licensed requirements and with applicable regulations X Approve all vendors and Suppliers of critical raw materials, reagents and components used in the collection and testing of Plasma; Ensure materials are received, stored, segregated and used correctly and all materials are appropriate for their intended use;Maintain relevant certificates of analysis, certificates of compliance, certificates of origin for such materials, as appropriate; Maintain an approved vendor list X STORAGE AND SHIPPING Provide storage conditions for the Plasma that meets the agreed upon MANUFACTURER Specifications, SOPs, applicable regulations related to Plasma storage and the EP monograph-Human Plasma to Fractionation X Maintain proper segregation of the Plasma units X Provide quality assurance oversight of unreleased Plasma received into quarantine and stored in a controlled, validated warehouse X Provide quality assurance oversight to segregate, identify and disposition rejected plasma X BIOLOGICAL PRODUCT DEVIATIONS AND RECALLS Monitor the manufacturing and release data of the Plasma to detect potentially reportable deviations within 5 business day of confirmation X Plasma unit deviation reporting to the appropriate regulatory authorities X Biological product deviation reporting to the appropriate regulatory authorities X X

Page 27of29 DESCRIPTION PLASMA SUPPLIER MANUFACTURER Perform investigations for any issues thought to be Plasma unit based and provide information to MANUFACTURER X Responsible for Plasma unit recall or withdrawal; Provide notification to MANUFACTURER within 5 business day of confinmation and perfonm investigations for these problems and forward investigation report to MANUFACTURER within mutually agreeable timeframe X Responsible for final product or intermediate product recall or withdrawal X EQUIPMENT, COMPUTER AND FACILITY VALIDATION AND/OR QUALIFICATION PROCESS Ensure all processes, equipment, systems and facilities are qualified and validated, as applicable, per this Agreement X Approve all process qualifications appUcabte to the Plasma collection, including but not limited to, the freezer validation and Haema-shipping validation for test samples and Plasma X Approve all process qualiffcaUons applicable to the Plasma shipping validation for test samples and Plasma X PLASMA RELEASE Have at least one Qualified Person on the Premises to oversee the quality aspects of the Plasma collection, testing, shipment and evaluate deviations, In order to ensure compliance with cGMP; the Responsible Person will certify that .each unit of Plasma has been collected and tested In approved facilities according to GMP and fulfil quality and safety requirements according to the MANUFACTURER Specifications X 3 LICENSES, PERMITS AND APPROVALS Represents and warrants that all their plasmapheresis donor centers in operation are currently certified under the PPTA IQPP; such certification must be preserved without interruption during the tenm of this Agreement; if plasmapheresis donor center fails to remain certified, notification to MANUFACTURER immediately, or within five business dav X Follow al requirements and specifications for exporting products to Europe X Notify MANUFACTURER within five business day in the event of a suspension, revocation or other administrative action adversely affecting or limiting license, permit or approval X Provide prior writlen notification to MANUFACTURER prior to relocating, opening or closing a plasmapheresis center; Obtain wrillen approval from MANUFACTURER for indusion of new centers, testing laboratories,plasma warehouses and transportation companies X
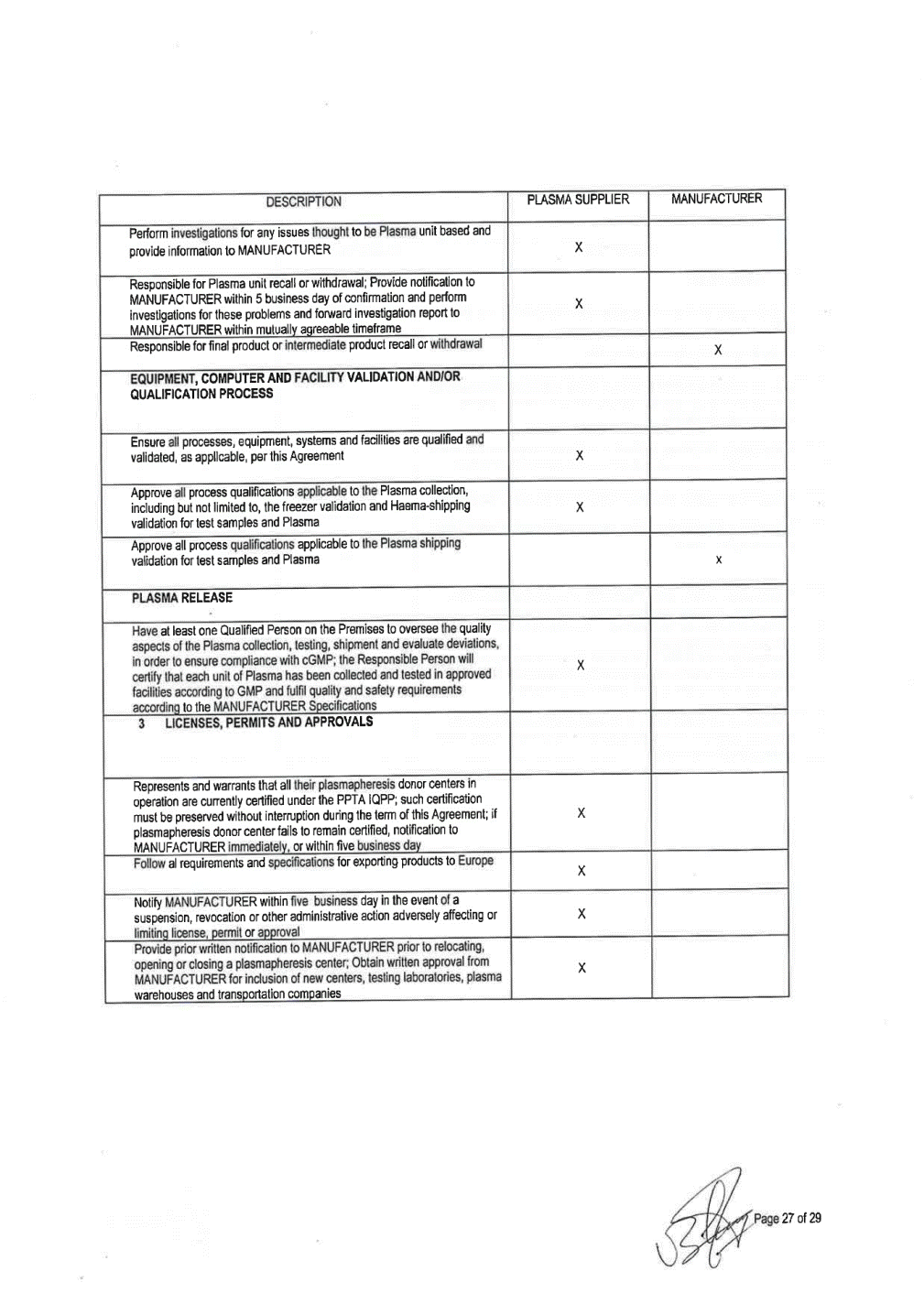
DESCRIPTION PLASMA SUPPLIER MANUFACTURER 4 INSPECTIONS Audit and qualify all vendors of supplies used in the collection and shipment of Plasma X Handle complaints to the vendor of supplies X Allow the MANUFACTURER to conduct annual inspections of the approved plasmapheresis donor collection centers, testing laboratories, plasma warehouse storage facilities X Responsible for the ultimate approval of all Plasma centers and their status as approved or not approved to ship Plasma to the MANUFACTURER X Provide written report to MANUFACTURER within 30 days of the audit date X Ensure that all the corrective actions are implemented and provide a report to the MANUFACTURER X Provide written notice to MANUFACTURER of any investigations of the donor centers, testing laboratorieS, storage, release and shipping facilities/companies with no more than 10 business days of the investiaation's commencement X Communicate to MANUFACTURER within 5 business day any event or serious failure of a center, testing laboratory, storage and/or transportation which may impact quality or safety of the Plasma supplied X Notify MANUFACTURER of any meetings/substantive discussions with regulatory authorities that directly relate to the manufacture, supply and/or control of the Plasma supplied X

DESCRIPTION PLASMA SUPPLIER MANUFACTURER 5DOCUMENTATION AND RECORD RETENTION Responsible for preparing and submitting regulatory submissions linked to the process and final product as required by applicable regulations X Provide all the documentation requested to support regulatory submissions, annual update for the EU Plasma Master File, epidemiology date/viral marker data X Keep complete and accurate records of equipment usage, cleaning, testing, temperature holds, and any maintenance/calibration performed, per this Agreeement X Retain donor records per applicable regulations and per this Agreement X
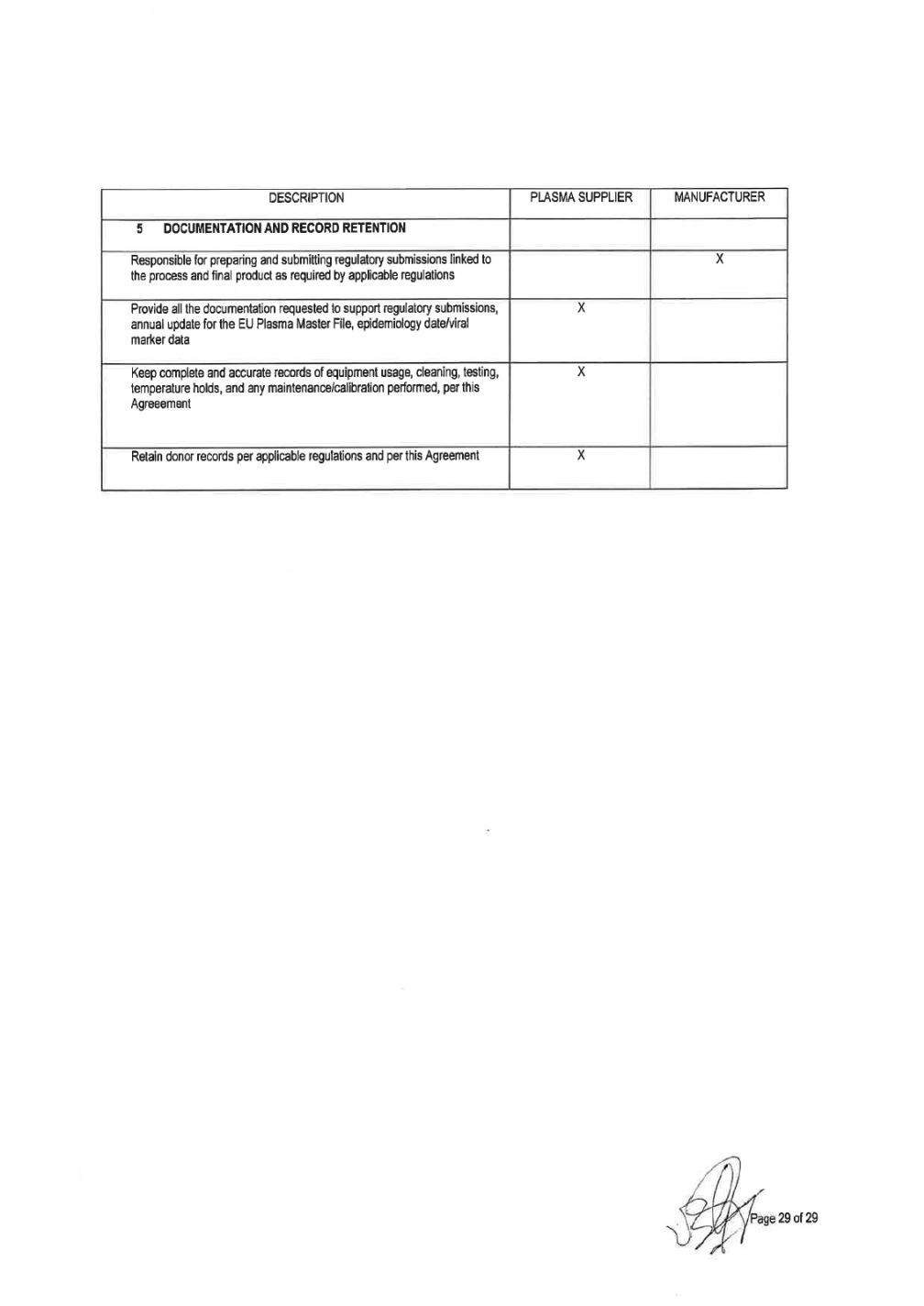
QUALITY AGREEMENT SOURCE PLASMA INTENDED FOR THE MANUFACTURE OF MEDICINAL PRODUCTS FOR HUMAN USE This Quality Agreement ("Agreement") is part of the Services Agreement dated 28-December-2018 by and between Grifols Worldwide Operations, Ltd. ("MANUFACTURER"} with its principal place of business at Grange Castle Business Park, Grange Castle, Clondalkin, Xxxxxx 00, Xxxxxxx, and Biotest Pharmaceuticals Corporation with its principal place of business at 000 Xxxxxx Xxxx, Xxxxx 000 Xxxx Xxxxx, XX 00000-0000, XXX ("PLASMA SUPPLIER"} and collectively "parties or Parties"}. The term of this Agreement commenced on the date set forth above (the "Effective Date") of signature of the Services Agreement. This Agreement document will be updated due to the continuous evolution of standards of quality and safety for the collection, testing, processing, storage and distribution of human plasma. Appendices to this Agreement may be revised without revision to this Agreement as long as appropriate approvals and proper signatures according controlled documentation systems for both parties are in place. 1. GENERAL PROVISIONS 1} MANUFACTURER is a company that manufactures medicinalproducts or intermediate fractions for further processing derived from human plasma and uses as starting material/raw material (hereinafter "Source Plasma"). 2) In connection with its medicinal products business, MANUFACTURER desires to obtain, and PLASMA SUPPLIER desires to provide, Source Plasma (including hyperimmune plasma) solely in accordance with the terms and conditions set forth in this Agreement covering the technical and quality requirements of Source Plasma as a raw material for further manufacturing at MANUFACTURER. Source Plasma is obtained and manufactured by PLASMA SUPPLIER from Health Authority Licensed Plasma (Human) collection centers PLASMA SUPPLIER may also supply MANUFACTURER with Source Plasma obtained from other approved suppliers (contract and third party). PLASMA SUPPLIER is responsible for the assurance that the Source Plasma obtained from these suppliers (including centers, laboratory test, storage facilities or transport/transit companies) meets all of the requirements stipulated in this Agreement and MANUFACTURER Specifications. A detailed Jist of centers, laboratories, storage facilities and transport/transit companies can be found on ATTACHMENT 1. 3) PLASMA SUPPLIER agrees that all plasma ("Plasma") derived from Source Plasma supplied to MANUFACTURER as starting material to produce medicinal products for human use hereunder meet the following requirements, specifications and conditions.

2. QUALITY AND TECHNICAL REQUIREMENTS 1) All Plasma supplied by PLASMA SUPPLIER under this Agreement must be derived from Source Plasma collected at plasma collection centers approved by competent authorities that have all necessary and regulatory approvals and permits. All Plasma supplied by PLASMA SUPPLIER under this Agreement must be collected, processed, tested, stored, packaged, labeled, released and shipped in compliance with all applicable sections of the origin Country Regulation, including without limitation current Good Manufacturing Practices ("cGMP"). A list of relevant regulations can be found on ATTACHMENT 2. 2) PLASMA SUPPLIER shall collect, process, test, store, package, label, release and ship the Plasma in accordance with MANUFACTURER Plasma Specifications ("MANUFACTURER Specifications"). A copy of the MANUFACTURER Specifications can be found on ATTACHMENT 3. PLASMA SUPPLIER hereby acknowledges receipt of a copy of MANUFACTURER Specifications and Shipping instructions, which are incorporated herein by reference as though fully set forth herein. These may be amended from time to time by MANUFACTURER or by updates due to new Quality or Regulatory requirements made by an appropriate governmental agency during the term of this Agreement, and upon receipt thereof, PLASMA SUPPLIER shall jointly review with MANUFACTURER, be in agreement and approve any such amendments with regard to updated Quality or Regulatory Requirements and such approval of the amendments shall not be unreasonably withheld or delayed. 3) Plasma collection centers will screen all donors to ensure that each of them is under good health conditions and that plasma is not collected from a high risk population for blood-borne infections. Therefore, plasma centers with an active High Risk Donor collection program (donors known to be positive for HIV, HCV or HBV at the time of collection) will not be accepted by MANUFACTURER. 4) Plasma is for further manufacture use only and is not to be represented as suitable for use as a finished human drug. 5) The MANUFACTURER Specifications may be changed and/or modified by MANUFACTURER requirements in order to comply with any new legislation in force and/or new controls or test methods which are available in order to obtain the safest Plasma as possible. 6) PLASMA SUPPLIER, agrees to maintain regular plasma configuration to not alter the configuration of the normal donor panelpopulation as starting materialfor all plasma-derived products without MANUFACTURER approval. Units from donors that have participated in a hyperimmune program cannot be used as Source Plasma without prior kno,wledge of the MANUFACTURER to avoid cluster c1f units going to same plasma pool. Plasma is for further manufacture use only and is not to be represented as suitable for use as a finished human drug. Quality Control 7) PLASMA SUPPLIER agrees to arrange testing for each donor of plasma in accordance with MANUFACTURER Specifications, as well as NationalRegulations, applicable European Directive, as well as any other applicable federal, state, and local or foreign competent authorities, governmental agencies regulations concerning donor tesling, and agrees to furnish test results of all such tests to MANUFACTURER in an agreed certificate format when required. Both parties shall ensure that all laboratories used are in compliance with cGMP and are qualified in all of the methodologies associated with plasma testing. All testing laboratories used to test Plasma supplied must be on the approved laboratory list as per Attachment 1. All testing methods must be validated and fulfil adequate levels of sensitivity and specificity according to their intended use, fulfilling all regulations in force. All validations, including protocols, reports and documentation shall be approved by the quality assurance function of the laboratory.

Tests and analysis of the Plasma shall be done in a laboratory approved by PLASMA SUPPLIER using licensed kits. ATTACHMENT 4 includes a list of approved test kits and validated NAT platforms. Plasma testing shall be performed according to test kit manufacturer instructions. Submission of plasma samples to testing laboratories for any testing will be compliant with written procedures for submission of such samples. This includes, but is not limited to: use of approved containers and shippers, procedures for handling and shipping of samples, and the preparation and submission of appropriate documentation with samples. The laboratories shall be responsible to notify the centers of any paperwork or samples received that do not conform to the requirements for sample paperwork, sample labeling and sample shipping. 8) Each unit of Plasma must meet the quality control specifications and the claimed and approved shelf-life for the Plasma at the maximum approved storage temperature as stated in the labeling specified in the MANUFACTURER Specifications. Subcontracted Operations 9) PLASMA SUPPLIER and MANUFACTURER shall jointly agree in writing on any contracted service (i.e., donor center collection, plasma storage, shipping, testing) to be completed by any third party per the MANUFACTURER Specifications. MANUFACTURER will have the right to audit any such facility and may jointly audit with the PLASMA SUPPLIER's auditors. PLASMA SUPPLIER will provide a written report to MANUFACTURER confirming that the Plasma units have been collected, tested, shipped and stored properly and meet the requirements of the MANUFACTURER Specifications. Both parties shall ensure that all sites or services used are in compliance with cGMP and are qualified in all of the methodologies associated with plasma handling and its premises. All contract sites used for the collection, transport, testing or storage of the Plasma supplied must be on MANUFACTURER's approved suppliers list. The contracted sites must show that methods are validated fulfilling regulations in force. All validations, including protocols, reports and documentation shall be approved by the quality assurance function of the third party organization. Any contracted organization must be approved and listed on ATTACHMENT 1 and testing must comply with ATTACHMENT 4. Traceability and Look-Back Communication {Post-Collection Information) 10) PLASMA SUPPLIER shall communicate to MANUFACTURER in writing within three (3) calendar days all information on a donor after a donation that fulfilled all MANUFACTURER Specifications is rejected for seroconversion, CJD/nvCJD or post donation information that may become an infectious disease. Notification instructions and required notification information are detailed in MANUFACTURER Specifications (traceability requirements and look back). Pl.ASMA SUPPLIER will notify MANUFACTURER as soon as practicable and in no case later than three (3) calendar days of discovery of significant deviations that may impact batch(s) or Plasma previously released and/or shipped to MANUFACTURER. 11) PLASMA SUPPLIER shall supply to the MANUFACTURER a report prior to product release on all post donation notifications received after pooling so that the MANUFACTURER can perform a viral risk assessment on the product. Change Control 12) All changes shall undergo a technical and cGMP impact assessment. PLASMA SUPPLIER will notify MANUFACTURER in advance of any planned changes reasonably foreseeable to affect the regulatory submissions to the Health Authorities involved. PLASMA SUPPLIER will have responsibility for providing justification for the changes and validating (when appropriate) any such changes to cGMP standards. Change control procedures will include a requirement for PLASMA SUPPLIER to obtain written approval from MANUFACTURER prior to the implementation of any change reasonably foreseeable to affect any regulatory submission, and any other change to

parameters that are covered by the change control scope that could have an effect on the safety, purity, etc. of the Plasma collected. The scope of the change management process includes collecting, testing, labeling, storage and shipping processes of Plasma, as well as intermediate fractions, storage and shipping. The associated changes may relate to: the master batch records, xxxx of materials, analytical standards, test methods and Specifications; and any changes to validated facilities, utilities, computer systems, equipment or processes used in the cleaning, manufacturing, testing, storage or shipment with respect to Plasma. MANUFACTURER Source Plasma Specifications may be changed and/or modified by MANUFACTURER as required in order to comply with current European requirements. MANUFACTURER shall submit all proposed revisions to the Specifications to PLASMA SUPPLIER for review and approval, which cannot be unreasonably withheld. MANUFACTURER shall advise PLASMA SUPPLIER of all proposed revisions to the Specifications as promptly as practicable so as to provide PLASMA SUPPLIER with as much notice as possible. PLASMA SUPPLIER shall have thirty (30) business days to agree to implement or otherwise respond to MANUFACTURER proposed revisions to the Specifications. PLASMA SUPPLIER response shall, if applicable, state PLASMA SUPPLIER reasons (which shall be commercially reasonable) for refusing to implement such revisions. PLASMA SUPPLIER and MANUFACTURER shall attempt in good faith to resolve any disputes as to the implementation of any requested revisions. Timeline for implementation of the change will be agreed by both companies. Quality Assurance 13) PLASMA SUPPLIER shall have a quality management system that ensures all critical processes involved in collection, processing, packaging, labelling, testing, storage, release and transportation are conducted in adequate facilities and according to current GMP principles as well as current legislation. PLASMA SUPPLIER shall maintain an active quality management system which at least shall review deviations and trends, ensure appropriate training of personnel, conduct internal audits on a periodic basis, with a follow up of deviations with a CAPA program and a change control system in force. The quality system shall apply to all operations which may impact on quality and safety of Plasma, from donor selection to Plasma delivery to the MANUFACTURER. 14) PLASMA SUPPLIER shall be responsible for creating the manufacturing and testing master documentation specific to Plasma collection. It shall be responsible for creating and maintaining at the Premises all standard operating procedures ("SOPs") and other documentation required to support cGMP operations. 15) Any deviation from the process and SOPs during Plasma collection, storage or testing must be appropriately explained, investigated, documented and approved in the batch records or analytical records. The deviation documentation must identify the cause and the corrective action, where appropriate, and must assess the impact to the Plasma donor's safety, and Plasma's quality. PLASMA SUPPLIER shall develop appropriate procedures for deviation investigations. PLASMA SUPPLIER will notify MANUFACTURER as soon as practicable before shipment or in no case later than three (3) calendar days of discovery that may impact batch(s) or Plasma previously released and/or shipped. 16) PLASMA SUPPLIER shall ensure that adequate trained and qualified staff is conducting the activities with a clear description of staff responsibilities. Training must be conducted according to an approved training program. Initial and continuous training shall be pertormed as well as proper staff competency follow-up. Training records shall be retained and available for review. 17) Materials to be used in the collection of Plasma shall be approved in accordance with applicable legal requirements for Plasma collection. Upon receipt of such materials, PLASMA SUPPLIER shall confirm the proper condition of packaging, sterility certification, locks and seals and check that labels correspond with the shipping documents. PLASMA SUPPLIER shall implement an appropriate system to maintain certification of materials and chemicals used in the collection and testing of Plasma. For those specified materials of animal origin, if any, PLASMA SUPPLIER

shall assure each lot is in full compliance with licensed requirements, with National regulations and final approved by MANUFACTURER. 18) PLA.SMA SUPPLIER shall approve all vendors and suppliers of critical raw materials, reagents and components used in the collection and testing of Plasma and shall maintain an approved supplier list. PLASMA SUPPLIER shall ensure that all materials are received, stored, segregated and used correctly, and all materials are appropriate for their intended use. It also shall ensure appropriate separation and/or segregation of any materials that may present a potential hazard to the materials used in the production of Plasma units. PLASMA SUPPLIER shall be responsible for maintaining the relevant certificate of analysis, certificate of compliance and/or certification of origin (as appropriate) for the materials. Storage and Shipping 19) Storage and shipment of Plasma supplied by PLASMA SUPPLIER under this Agreement must be conducted in approved facilities that have all necessary and regulatory approvals. Storage facilities and shipping process must be properly validated. 20) Plasma must be frozen within twenty-four (24) hours of collection by cooling rapidly in conditions validated to ensure that a temperature of -25°C or below is attained at the core of each Plasma unit within twelve (12) hours of placing in the freezer apparatus. Plasma must be stored at -20°C or lower. Freezing, storage and shipping must be done according to WHO recommendations. 21) PLASMA SUPPLIER shall provide sufficient and suitable storage facilities that meet the storage specifications for Plasma units, as documented and agreed upon in the MANUFACTURER Specification, SOPs, applicable regulations related to Plasma storage, National Regulations for-Human Plasma for Fractionation. Any off-site Plasma storage facilities used must be approved by PLASMA SUPPLIER quality assurance and meet the same requirements. Mobile storage facilities shall only be used short term for emergency situations and must be appropriately validated to maintain correct temperatures. It shall be ensured that during storage of Plasma before shipping the plasma has been properly packaged and protected to avoid deterioration, interference, theft, product contamination or mixture with any other materials. PLA.SMA SUPPLIER will maintain proper segregation of the Plasma units. Details of any labeling requirements and shipping container sealing and integrity will be documented. The Plasma units shall be suitably packaged for transit, with each outer container being labeled as per written procedures. 22) Unreleased Plasma that is shipped to a PLASMA SUPPLIER quarantine location at the authorized plasma warehouse will be stored in a physically segregated area in a controlled, validated warehouse. Oversight of that area and the Plasma contained therein is the responsibility of PLASMA SUPPLIER quality assurance organization. Such unreleasedPlasma must be controlled by standard operating procedures, including appropriate labeling and electronic material status. Such procedures will govern post unit donation removal, temperature deviation reporting requirements, unit and lot traceability, employee access and training, release of Plasma and final transfer to MANUFACTURER, regulatory filings and other such notifications and regulatory requirements not specifically covered by this Agreement whenever applicable. 23) Rejected Plasma must be properly segregated, identified and dispositioned under quality assurance control. Biol ogical Product Deviations and Recalls 24) PLASMA SUPPLIER shall be responsible for monitoring the manufacturing and release data of the Plasma and if detects a potentially reportable deviation, as applicable to Plasma sent to MANUFACTURER, this should be reported and discussed with MANUFACTURER within one (1) business day of confirmation. PLASMA SUPPLIER is responsible for Plasma unit deviation reporting to the appropriate regulatory authorities. Page 5 of29 -----·---

25) MANUFACTURER, as the medicinal product license holder, shall be responsible for reporting deviations pertaining to biological products to the regulatory authorities. PLASMA SUPPLIER will work collaboratively with MANUFACTURER on filing any such report thought to be due to the collection of Plasma. PLASMA SUPPLIER will promptly perform investigations for any issues that are thought to be Plasma unit based. Both parties shall collaborate in the development of the global reporting regulatory strategy for the event. PLASMA SUPPLIER shall provide necessary information to MANUFACTURER if it receives a warning letter, significant regulatory observation or BPDR follow up question(s). 26) PLASMA SUPPLIER is responsible for Plasma unit recall or withdrawal per the applicable regulations. MANUFACTURER shall be notified of any recall and of any problems thought to be due to Plasma quality within one (1) business day of confirmation. When requested by MANUFACTURER, PLASMA SUPPLIER will perform investigations for these problems. Investigation reports regarding the defect or cause of recall will be forwarded to MANUFACTURER within a mutually agreeable timeframe. MANUFACTURER is responsible for any final product or intermediate product recall or withdrawal for those products that are manufactured. Equipment. Computer and Facility Validation and/or Qualification Process 27) PLASMA SUPPLIER shall ensure that all process, equipment, systems and facilities are qualified and validated if applicable prior its use and after any significant change to ensure that processes are capable of consistently achieving the Plasma specifications. Documentation must be reviewed and approved. Calibration and preventive maintenance programs shall be established for equipment. It will maintain cGMP validation status of process procedures, computers and associated equipment with respect to the manufacturing, storage and shipping procedures that are used in connection with Plasma. PLASMA SUPPLIER shall be responsible for all equipment, computer and facility qualification activities associated with the Plasma collection, storage and shipment. Validations must meet National Regulations and further approved by MANUFACTURER, as applicable and will promptly, upon request by MANUFACTURER, make validation reports available for review. PLASMA SUPPLIER shall develop procedures and implementation plans to assure that all computer systems and software are compliant with required Regulations. 28) PLASMA SUPPLIER will approve all process qualifications applicable to the Plasma collection, including, but not limited to, the freezer validation and shipping validation for test samples and Plasma. PLASMA SUPPLIER will provide copies of validation reports upon request. Validation reports may also be requested as part of an audit or an event review. Plasma Release 29) PLASMA SUPPLIER collection centers and third party suppliers shall all have at least one quality Responsible person on the Premises to oversee the quality aspects of the Plasma collection, testing, shipment, evaluate deviations, etc., in order to assure compliance with cGMPs. 30) PLASMA SUPPLIER has designated a person for plasma release for further manufacturing of medicinal products in accordance with applicable regulations PLASMA SUPPLIER and its suppliers shall release units by means of a corporate Responsible Person who will document and certify that each unit of Plasma has been collected and tested in approved facilities according to GMP and that the units fulfil quality and safety requirements of the MANUFACTURER Specifications. The Responsible Person and designee are named in this Agreement as the individuals authorised to release Plasma units. 3. LICENSES. PERMITS AND APPROVALS 1. All Plasma supplied by PLASMA SUPPLIER under this Agreement must be derived from Plasma collected at an approved plasma collection center located in XXXXXX that has all necessary and regulatory approvals and permits.,. The plasma collection center(s) under this Agreement are set forth on AITACHMENT 1. ag' e 6 of 29

2. PlASMA SUPPLIER represents and warrants that all their plasmapheresis donor centers in operation are certified under the Plasma Protein Therapeutics Association's International Quality Plasma Program ("PPTA IQPP"). PlASMA SUPPLIER must preserve these certifications without interruption for the remaining term of this Agreement. If PlASMA SUPPLIER fails to maintain such plasmapheresis donor centers certifications, then MANUFACTURER has to be immediately notified within one (1) business day to evaluate actions to be taken. 3. PlASMA SUPPLIER agrees to follow the mutually agreed upon MANUFACTURER Specifications and all other requirements or specifications issued in writing by MANUFACTURER, and any versions to those procedures, requirements or specifications as they may be issued or adopted by MANUFACTURER and agreed upon by PlASMA SUPPLIER, as approved by appropriate governmental regulatory agency or competent authority, if such approval is mandated by law or required by MANUFACTURER. 4. In the event of a suspension, revocation, or other administrative action adversely affecting or limiting any such license, permit or approval, MANUFACTURER shall be immediately notified within one (1) business day to take necessary actions. 5. PlASMA SUPPLIER may not relocate, open or close any plasmapheresis collection centers, testing laboratories or plasma storage facilities without prior written notification to the MANUFACTURER. Written approval by MANUFACTURER is required for inclusion of new centers, testing laboratories, plasma warehouses and transportation companies, which shall not be unreasonably withheld. 4. INSPECTIONS 1. Collection and storage of the Plasma shall be conducted in a suitable controlled environment and such Premises shall be regularly inspected and monitored to ensure compliance with cGMP, and other applicable manufacturing authorizations. 2. PlASMA SUPPLIER will be responsible for qualification and auditing of all vendors of supplies used in the collection and shipment of Plasma. All suppliers must appear on the PlASMA SUPPLIER approved supplier list. PlASMA SUPPLIER is responsible for handling complaints to the vendor for supplies and the interaction with the vendor to resolve those complaints. 3. MANUFACTURER shall have the right to conduct periodic audits of the approved plasmapheresis donor collection centers, testing laboratories, Corporate.Quality Management Systems, plasma warehouse storage facilities and/or transport/transit and shipping companies and to review all procedures and records kept by such facilities related the collection, processing, testing, storage, packaging, labeling, release and shipping of plasma as well as all operations conducted in conformance with generally accepted industry practice. These audits may be done as joint audits with PlASMA SUPPLIER, at the discretion of the MANUFACTURER. MANUFACTURER will provide PlASMA SUPPLIER with not less than thirty (30) days' notice prior to any of its audits, unless agreed otherwise by the Parties, and such audits shall be during normal business hours, shall be limited to matters reasonably related to this Quality Agreement, and shall be conducted in conformance with generally accepted cGMP. No audit shall continue in duration for more than three (3) business days unless such an extension is reasonably necessary. A maximum of three (3) auditors shall participate in any inspection unless otherwise agreed between PlASMA SUPPLIER and MANUFACTURER. Such audits shall occur periodically (at least, every two years) for facilities having an acceptable inspection I audit history unless otherwise necessary to inspect or audit as a result of requirements by regulatory authorities or a serious failure or material concern that either impacts or threatens to impact quality, safety, potency, or compliance with this Quality Agreement. In the event of such additional audit(s), MANUFACTURER shall provide PLASMA SUPPLIER reasonable written notice of its intent to conduct such additional audit(s). Page 7 of 29

MANUFACTURER maintains the right to event reviews (directed audit), in addition to periodic audits, to address significant quality, performance or safety problems associated with the products. These events should be requested, scheduled and conducted at the first available date. 4. PLASMA SUPPLIER shall be entitled to perform at least one (1) standard cGMP compliance audit every year of the above facilities to encompass all cGMP operations (testing laboratories, plasma warehouses, transport companies, corporate offices). 5. PLASMA SUPPLIER Compliance Audit Team will provide a written report to MANUFACTURER of all observations. Within thirty (30) days of the audit date, PLASMA SUPPLIER will coordinate to provide a written response to all findings that details corrective action to be implemented. PLASMA SUPPLIER will ensure that all corrective actions are implemented and provide a report to MANUFACTURER upon implementation. In those instances,. where corrective action will require an extended period to implement, PLASMA SUPPLIER shall provide reports to MANUFACTURER on the progress of such corrective action at such intervals as mutually agreed upon by the parties. 6. MANUFACTURER shall have also the right to cause any authorized representative of the Public Health Service, any state, local, foreign competent regulatory authorities and governmental agencies, and any authorized third party as deemed necessary or desirable to carry out its business worldwide, to conduct periodic inspections of the approved plasmapheresis donor collection centers, testing laboratories, plasma warehouse storage facilities andfor transportftransit and shipping companies and to review all procedures and all records kept by such donor centers or such testing laboratories regarding the collection, processing, testing, storage, packaging, labeling, release and shipping of Plasma, and all operations conducted in conformance with generally accepted industry practices. MANUFACTURER shall have the right to make and retain copies of all such procedures and records kept by donor centers, as well as copies of all correspondence between donor centers and any governmental regulatory agencies regarding any such inspections. 7. MANUFACTURER will be responsible for ultimate approval of all Plasma centers and their status as approved or not approved to ship plasma to the MANUFACTURER. The information will be contained in the MANUFACTURER's list of approved plasma suppliers and the types of Plasma the center is approved to collect. 8. PLASMA SUPPLIER agrees to send MANUFACTURER written notice of any federal, state or local government for cause audit or relevant investigation of the donor centers, testing laboratories, storage, releae and shipping facilitiesfcompanies with no more than three (3) business day of the investigation's commencement. 9. PLASMA SUPPLIER agrees to communicate to MANUFACTURER within ten (10) business days of discovery of critical event (failure of a plasma collection center, storage facility, testing laboratory andfor transportation which may impact quality or safety of the Plasma supplied 10. PLASMA SUPPLIER will notify MANUFACTURER in a timely manner of any meetings or substantive discussions with r any other regulatory authority that directly relate to the manufacture, supply and/or control of Plasma supplied. 11. PLASMA SUPPLIER shall provide to MANUFACTURER within ten (10) business days any information of the following: (i) Update of critical findings related to the inspection performed, (ii) Notice of Intent to Revoke License Letter, (iii) License Suspension Letter, (iv) Seizure action regarding Corporate Offices, plasma collection centers, storage facilities, or testing laboratories. 12. On a quarterly basis, PLASMA SUPPLIER shall provide MANUFACTURER, a summary of regulatory national authority inspections of any plasma collection centers, testing laboratories, storage facilities, and corporate offices, included on Attachment 1 of this Quality Agreement. This summary shall include information concerning inspections of National health authority. This summary will also include a list of critical observations and an overall evaluation of the regulatory inspections outcome including:

• Identification of the facility • Identification of inspection date • Category (#of Minor, Major, Critical) • Result: pass/not pass • Kind of Deficiencies (for example: Documentation, Storage, etc.). 13. In the event that during an inspection by MANUFACTURER (or PLASMA SUPPLIER as designee), self-notification to PLASMA SUPPLIER by the donor center, test lab, warehouse or transportation company or by any authorized representative of the Public Health Service, or any state or local or foreign competent authorities and governmental agencies to corporate, donor centers, test labs, plasma warehouses or transportation company, critical or serious observations are found that may impact quality or safety of the plasma supplied, PLASMA SUPPLIER shall give MANUFACTURER notice within one (1) business days and establish a timeframe to pass the inspection and resolve issues. In the event that PLASMA SUPPLIER has not corrected the failure, MANUFACTURER shall be immediately informed in order to take necessary actions, including termination of this Quality Agreement and the Supply Agreement in the event such breach remains uncured for a period of sixty (60) business days. 5. DOCUMENTATION AND RECORD RETENTION 1) PLASMA SUPPLIER shall make available upon request documents such as Site Master Files (Donor Centers, Testing Laboratories, Storage Facilities, Corporate Offices related to Quality Management Systems), floor plans, equipment validation, and other production information in order to ensure safety, purity, and potency of the product. 2) MANUFACTURER, as the Plasma-derived product license holder, is responsible to prepare and submit any regulatory submission linked to in process and final product as required by applicable regulations. PLASMA SUPPLIER shall provide all documentation requested by MANUFACTURER to support regulatory submissions. 3) PLASMA SUPPLIER commits to provide to MANUFACTURER on a regular basis, the relevant information and regulatory documentation that is necessary for Regulatory Submissions according to final product applicable regulations. Such documentation shall include at minimum, certificates of approval by competent health authorities for facilities, laboratories, plasma warehouses and transportation companies, inspection status information, donor suitability criteria, approved technical specifications for soft goods, main equipment validation protocol, methods and validations of testing platforms, proficiency studies and epidemiological data. 4) Epidemiology data and viral marker data must be supplied by PLASMA SUPPLIER in accordance with the current regulatory requirements. Data must be submitted using the standard forms found in the Source Plasma Specification. Should a PLASMA SUPPLIER donor center exceed (on an annual basis, Jan-December.) the MANUFACTURER and/or its affiliated or parent company established VM Alert Limits (Attachment 3): •PLASMA SUPPLIER will provide MANUFACTURER an investigation and corrective/preventive actions (when appropriate); and •Plasma will not be accepted from a donor center that has exceeded the MANUFACTURER established Viral Marker Alert Limits for three (3) consecutive years. 5) PLASMA SUPPLIER shall keep complete and accurate records of equipment usage, cleaning, testing, temperature holds, and any maintenance/calibration performed and other parameters in accordance with the most stringent applicable legal requirements. 6) PLASMA SUPPLIER agrees to retain donor records according to all applicable sections and regulations issued or promulgated by Competent Authorities applicable federal, state, and local or foreign competent authorities and governmental agencies regulations. 7) The following items will have to be recorded and stored appropriately by PLASMA SUPPLIER in order to secure the quality and safety of the partially processed biological materials to comply with the current regulation identified in this Page 9 of29

Quality Agreement in order to comply with applicable pertod as may be required by any law or regulation governing the distrtbution of biological products manufactured from such Plasma: o o o traceability records donor card health record of each donor (including health history and physical examination of each donor, which are maintained in the donor management system) donor questionnaires (i.e., AIDS,) donor number whose plasma was used for production pooling. NAT and serological test results, which are kept in the donor management system. o o o 6. CONSEQUENCES OF TERMINATION Upon termination of the supply contract: PlASMA SUPPLIER shall continue to comply with its obligation under this Agreement if and to the extent it continues to have the responsibility for post donation information and look back information after the expiry or termination of the Supply Agreement. All obligations relating to compliance with cGMP in respect of the plasma shall continue in force according to the requirements of cGMP, including but not limited to: Record Retention and availability Regulatory inspections and data requested Post donation information and look back information. Epidemiological Data for the year after the termination of the supply agreement. 7. CONTACT PERSONS In the event of serious failure, regulatory issues, update or change in specifications: PLASMA SUPPLIER If addressed to BIOTEST: Biotest Pharmaceuticals Corporation 000 Xxxxxx Xxxx, Xxxxx 000 Xxxx Xxxxx XX 00000-0000 XXX MANUFACTURER Grifols Worldwide Operations, Ltd. Grange Castle Business Park Grange Castle Clondalkin Dublin 22 Ireland 1) Each party shall notify the other party in wrtting of any significant change in reporting relationships involving the quality department or quality functions within five (5) business days of such change and proceed according the Change Control requirement process oullined In this agreement. age 10 of 29 - --- --------

2) Each party shall provide written notice to the other party of any change in the management of a critical operation that is applicable to plasma (e.g., production, quality, new center set up and validation) within five (3) business days of such change and proceed according the Change Control requirement process outlined in this agreement. 3) PLASMA SUPPLIER shall communicate to MANUFACTURER within one (1) business day of notification or discovery, whichever is first, of debarment, under Section 306(a) or 306(b) of the Federal Food, Drug and Cosmetic Act [21 USC 335a] of any of PLASMA SUPPLIER employees or agents involved in the collection or control of the Plasma. ACCEPTANCE SIGNATURES: PLASMA SUPPLIER MANUFACTURER Biotest Xxxxx ceu 'cals CorP?ration Approved by: -'--:7.>::::..::;4/- Approved b•'.f::;:;;:;: ======--- Date: 6>/kftr 1· '-Llt.. Cj Date: I ' Name: Ileana Ca isle lS\.---\" <i/11--/ Name: \}c._e._e. Title: Title: CEO/President Page 11 of 29
ATTACHMENT 1 LIST OF CENTERS, TEST LABORATORIES, PLASMA WAREHOUSES, TRANSPORTATION COMPANIES

BIOTEST PHARMACEUTICALS PLASMA CENTERS 210<1 PoarAI Orclul<dRoad. oAuguStl GA 30l10il 1616 Ea•t Wacster Stroll!. 8ci'Niing Green, OHG102 000 00xx XX X. Xxxxxx!xxx Xxx X0XXX .AI.igmla Xx.xxx Qrec Brocki1195 Clenlaon Co WQY OiQu;QnCily Fort Xxxxx Gllil'll.lMiille HOII]'XXXx xx\o.'!i City Jadls!:lnvllla Jacl<sllnv le Kl!'limay t...ncoln Molbeumc 01lando Royal PCIItl Antonio SanlsFt: Val:lo$:a Vmmll an 'I'CIWlg$lcwn GEO GIA 01-110 souru DAJ<OTA 5:lO Old Gnserwlb! Hny Sle SOC*'-n $C 2!1631 2235 Xxxx xxxx xxx e,mfi,COIIYI'By AR 7'2034 1027 Coomen:e Baul• ani, Di ksm ClPA tSSI 9 391 CpiQn8et.S van;l, Fl>lt Mfl3S9Ge :2315 NW 13111 Slr<l*Gainesville FL32&'J!I 6837 TSfHt Ollywood Flll3024 SOUlli CAROUNA. ARKANSAS PENNSYLVANIA FLORIDA FLORillA FLOIUOA IOWA I«>RTH XXXX XXX NORTH CAROUNA NEBRASKA NEBRASKA FLORIDA FLORIDA FLO XXX TI5)(A.S NEWr.tE>QCO GEORGIA SOUTH DAKOTA OHIO ..r.oe S. Glb:ut St•• IIIWil City tA 522.W 1'21$ Counlly tl\lb Road. nviHt NC ::18541-'1411 113 Y pp.JK caonYUieNC :285110 59151dA\'E!<'tllB, Kellmty NE 58$47 3CO S.17111S1reet t.mciiiJ!liiE6BS08 0000 Xxxx 'xx'Xxxxxxxxx Xxxx, Xxxxxxxxx,XX 32<134 2501 Dit o;o el}' Drive Sui! co.Or'.amlo, Ft. 32e2D 1110Buslncs:;P, r!<Wfi'!, Royal Pelm, 1'1.33411 616 1('1/l.a>p 410 Suc 101,a;m MIOilko TX 71!216 2/leQ Cerrii':J& Road,Sana! Fe NM 87607 311 N. Pa .!fSQnSireet, V.,DStl!l, GA31M1 1012 Prin::o!Dn St., VNI'II'illiOn SD 57C69 6000 falla'ling A.....,ue. YDUng IDwn Oli+IS15 Illoint Ccmimo City 5altl> \1\' an;.ocK A\IOl'IVO, A!I'IMIS GA 305U1A_\lla<n;GEOI(GIA

ATTACHMENT 2 RELEVANT REGULATIONS o 21 CFR Parts 210, 211, 600. o EU Good Manufacturing Practices for MedicinalProducts for Human use, all as amended from time to time o Council of Europe: Guide to the preparation use and quality assurance of blood components (current version). o European Pharmacopeia monographs ('EP') requirements particularly in respect of blood or blood components as starting material for the manufacture of proprietary medicinal products. o Recommendations of the World Health Organization (WHO). o Commission Directive 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC as regards traceability requirements and notification of serious adverse reactions and events. o Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002198/EC, regarding donor suitability requirements. o Directive 2001/83/EC, GMP Annex 14 and certain recommendations of the Council of Europe, as well as any other applicable international, federal, state and local regulations concerning the collection, processing, testing, storage, packaging, labeling, release and shipping of Plasma. o Guideline on plasma-derived medicinal products (EMA/CPMP/BWP, current version).

ATIACHMENT3 SPECIFICATIONS 1.-PURPOSE To provide and describe the conditions required for Source Plasma to be accepted by MANUFACTURER for the procurement of Source Plasma intended for manufacture of hemoderivate products. This specification is intended to assure that incoming plasma meets all MANUFACTURER requirements, in addition to establish domestic and international regulations, 2.-.SCOPE These specifications cover all source plasma intended for fractionation, defined as the fluid portion of human blood collected by plasmapheresis. MANUFACTURER can only use plasma for fractionation that fulfils all the MANUFACTURER requirements for all of the components that are part of Plasma Supply Chain, i.e.: Plasma collection centers Plasma testing facilities and Plasma testing methods Plasma storage facilities Plasma transportation companies. Every one of them has t9 be approved by MANUFACTURER and previously authorized by competent Health Authorities. 3.-RELATED DOCUMENTS FDA:21 Code Federal Regulation all applicable sections o 21 CFR 210-Current Good Manufacturing Practice in Manufacturing, Processing, Packing or Holding of Drugs; General. 21 CFR 211 - Good Manufacturing Practice for Finished Pharmaceuticals. 21 CFR 600 - Biological Products General 21 CFR 606 - Current Good Manufacturing Practices for Blood and Blood Components 21 CFR 610-General Biological Products Standards 21 CFR 640-Additional Standards for Human Blood and Blood Products, Plasma and Source Plasma 42 CFR 493 - CLIA regulations FDA approved Standard Operating Procedures Manuals All current FDA guidance documents related to collecting, testing, processing, storing or transporting Source Plasma PPTA Viral Marker Standard PPTA Qualified Donor Standard PPTA NAT Testing Standard EUEU Pharmacopeia monograph for Human Plasma for Fractionation Revision to Annex 14 to EU Guide to GMP: Manufacture of products derived from human blood or plasma EUEMEA/CHMP/BWP/3794/03 Guideline on the Scientific Data Requirements for a Plasma Master File o o o o o o o o o o o o o o

o EUEMEA/CHMP/BWP/125/04 Guideline on Epidemiological Data on Blood Transmissible Infections EUCouncil of Europe: Guide to the preparation use and quality assurance of blood components EUDirective 2002/98/CE EUDirective 2004/33/CE sPReal Decreta 1088/2005 o o o o EUFor plasma intended to be used as raw material for Hemoderivates Products for European Market sPFor plasmatic raw material intended to be processed in Spanish Manufacturing Plant facilities 4.-PROCEDURE All plasma units shall be collected by approved suppliers and collection facilities, and tested by approved laboratories using approved kits, and stored and transported using approved establishments in compliance with all applicable sections of US Code of Federal Regulations (US CFR Tille 21) and all applicable requirements related to final product destination (i.e.: European Regulations) if it's the intended for use in. Plasma types will be defined according to plasma center involved, laboratories involved, testing methods involved, specific test for final product destination requirement, warehouse involved and plasma carrier involved in which is transported. The PLASMA SUPPLIER will keep updated a quality assurance system that will include, at least, review and investigation of deviations and detection of possible trending, performance of internal compliance audits with corresponding follow-up of corrective and preventive actions and implementation of an internal Change Control procedure. The quality assurance system will be applied along the collection, processing, testing, storage, and control procedures. All activities, functions and/or documentation practices will be performed according to cGMP (current Good Manufacturing Practices). 4.1. PLASMA COLLECTION Plasmapheresis Centers Plasmapheresis centers shall be approved by the Health Authorities of plasma origin and be in compliance with Epidemiological data established by Manufacturing company (IQPP certified). Plasma collection centers will screen all donors to ensure that each of them is under good health conditions and that plasma is not collected from a high risk population. Management and control of donors should be done using an IT system. In addition, for plasma intended for use as raw material in hemoderivate products for European market, plasma centers have to be approved by European Health Authorities. PLASMA SUPLIER will supply MANUFACTURER for corresponding evaluation and for each center Epidemiological Data of viral markers (HBV, HCV & HIV), annually in EMA format and half-yearly in PPTA format. All alert notifications and their corresponding investigations as well as their corrective/preventive actions (if applicable) should be included in the continuous revision of epidemiological data. Centers participating in specific programs of collecting plasma from donors tested reactive/positive in viral markers cannot be MANUFACTURER's suppliers.

Donor selection Donor selection procedures shall fulfill with competent authorities Regulations. In addition, for plasma intended for use as raw material in hemoderivate products for European market, Donor selection process has to be in compliance with those established by European Health Authorities. All plasma donations shall be from qualified donors. Sterility Plasma is separated from cells by an aseptic method using a sterile system designed to prevent micro-organism contamination.Furthermore, the plasma containers are closed, with the aim to prevent the possibility of contamination. 4.2 PLASMA TESTING The laboratory shall be approved by competent authorities. The laboratory will keep approved and updated SOPs, containing the necessary working instructions. All analytical methods shall be validated and shall have an optimum level of sensitivity and specificity and shall fulfill with current legislation. All the validations, including protocols, reports and related documentation will be approved by Quality Assurance area. The laboratory will join in certification programs (proficiency testing) to ensure suitability of methods and techniques in use. Plasma shall be tested by facilities approved by competent authorities (additionally approved by a competent Health Authority (i.e. EMA). Likewise, all analytical methods used shall be approved by competent authorities (additionally approved by a competent Health Authority (i.e. EMA) and shall be included as part of the approved specifications. For plasma intended to be used as raw material for hemoderivate products for European Market, testing facilities and analytical methods used have to be approved by European Health Authorities. Plasma testing requirements Prior to use,the test kit manufacturer, test kit methodology and testing facility must be approved by MANUFACTURER. • All donors shall be screened for all the biochemical parameters required by national authorities as control of the donor and for indirect xxxxxx reactivity in the first donation and every 90 days, and the obtained result must be non-reactive. • All donors shall be screened for excluding Syphilis presence as required by national regulations. Donor test is performed every 4 months. • All plasma units must be tested and found non-reactive for Hepatitis B surface antigen (HBsAg) by a validated test method approved by competent authorities. • All plasma units must be tested and found non-reactive for HIV-1 and HIV-2 antibodies by a validated test method approved by competent authorities. • All plasma units must be tested and found non-reactive for Hepatitis C antibodies by a validated test method approved by competent authorities. • Additionally, all plasma units must be tested and found non-reactive by means of pools consisting of not more than 512 individual units for HCV-DNA, HCV-RNA and HIV-RNA by a validated test method approved by

competent authorities and for HAV-RNA and Parvovirus 819-DNA by a validated test method. The cut-off of Parvovirus 819-DNA detennination must be such to ensure that plasma manufacturing pools do not exceed a titer of 1x1()4 IU/ml. • All plasma units must be tested for all viral or non-viral markers as required by the plasma Supplies Health Authorities. • For plasma intended to be used as raw material for hemoderivate products for European Market, analytical methods used have to be approved by European Health Authorities. Hyperimmune plasma For hyperimmune plasma all donors shall be immunized using Competent authorities approved immunizing program and shall contain at least the titers agreed with the Supplier. These are described in corresponding registers (IG_REG-000683 and IG_REG-000686). Traceability and look Back Requirements While fully respecting confidentiamy, there is a system in place which enables the path taken by each donation to be traced, both forward from the donor and back from the finished medicinal product, including but not limited to plasma center, plasma donor number, analytical testing and results, plasma manufacturing pool testing, Data of Manufacturing process and final product identification. lfs a system by which we can identify and remove look back and POl units. A look-back unit is a unit of plasma that complies with all the established requirements but collected from a donor, from whom a subsequent donation has been rejected for any reason, related with an infection or with behavior that may involve belonging to a risk group (Post-Donation Information), Creutzfeldt-Jakob disease (CJD) or new variant Creutzfeldt-Jakob disease (nvCJD). In case of reasons related to infection or belonging to risk group behavior (POl), Plasma Suppliers shall communicate to MANUFACTURER's manufacturing companies all the plasma units drawn from a donor within twelve months prior to the date of the last negative tested donation for anti-HCV and HCV-RNA, for HBsAg and HBV-DNA and for anti-HIV 1/2 and HIV-RNA. In case of CJD and nvCJD risk factors and/or diagnosis, communications shall include all the units sent to any MANUFACTURER's manufacturing company from that donor. Look-back units shall be notified to any MANUFACTURER's manufacturing company in written form as quickly as possible and not more than three (3) calendar days from the event that caused the procedure. 4.3. PLASMA PROCESSING Physical conditions of plasma units and samples Each plasma unit shall be collected from a single donor. All plasma units must be placed in the freezer within one hour of collection and maintained at -20° C or colder. In case of plasma intended for used as raw material in hemoderivate products for European Market, it has to be in compliance with European Phannacopeia requirements: Plasma intended for the recovery of labile proteins in plasma is frozen within 24h after collection by cooling rapidly in conditions validated to ensure that a temperature of -25°C or below is attained at the core of each plasma unit within 12h after placing in the freezing apparatus, ensuring the preservation from activity of labile coagulation factors.

Plasma intended for the recovery of not labile proteins in plasma is frozen by cooling at -20°C or colder as soon as possible and at the latest within 24h after collection. All plasma units shall be accompanied by a plasma sample of not less than 2 ml and both shall be identified with the bleed number and bar code. Both unit and sample of plasma shall exhibit neither hemolytic nor lipemia signs when visually inspected, nor both plasma bottle and sample tube shall not have any fissure or be broken. Storage The warehouse shall be approved by MANUFACTURER and by competent authorities. All plasma units and their corresponding samples shall be kept at -20 oc or colder, unless more restrictive conditions are required by the competent Health Authorities. Temperature of all shipments will be monitored using validated temperature recorders in order to ensure that plasma units and their corresponding samples have been kept at temperatures according to specification ( -20°C). Any deviation shall be investigated and approved. In case of plasma intended for used as raw material in hemoderivate products for European Market, the warehouse has to be also approved by European Health Authorities. Quarantine Plasma pool will be ready for fractionation once completed minimum volume needed for processing a production lot and prior minimum quarantine of 2 or 3 months from collection date for all units included on it. The quarantine period (2 or 3 months) will be determined depending on fractionation product destination and it will be controlled by computer management system. Shelf-life Source plasma has a shelf-life of 36 months in all manufacturing plants in which plasma is processed. Plasma must not be older than 30 months when shipped to MANUFACTURER's manufacturing company. For units older than 30 months, it must be necessary a previous authorization by Qualified person of MANUFACTURER 'S manufacturing company. 4.4. PLASMA SHIPPING Shipment All carriers used to transport Source Plasma must be approved by MANUFACTURER. The temperature of the transport trailer must be -25°C or colder prior to loading the plasma shipment. The shipping procedure has to be designed to keep all plasma units and samples at -20 °C or colder during the whole transportation process according to Regulations of Plasma Origin Country. In addition, for plasma intended for use in hemoderivate products for European market the shipping procedure has to be in compliance with Ph. Eur. Monography, 'Human Plasma for Fractionation' Page 19 of 29

Temperature of all shipments will be monitored using validated temperature recorders, in order to ensure that plasma units and their corresponding samples have been kept at temperatures according to specification (:S-20°C). Any deviation shall be investigated to analyze cause and potential impact before to accept and approved plasma involved in it. All documents and plasma pool samples (if required) shall be sent in advance to any MANUFACTURER's manufacturing company for shipment confirmation. For plasma intended as raw material for use in hemoderivate products for European market, all carriers used as raw material for used to transport Source Plasma must be approved by competent European Health Authorities. Identification of the shipping boxes The plasma Supplier shall identify boxes as following: -Supplier name. -Shipment number. -Total boxes by shipment. -Box/boxes (number of order regarding total number of boxes). Label All plasma units have to be labeled with at least the following information: 1-Supplier name. 2-Product name. 3-Bleed number (code bar). 4-Volume or weight of the unit. 5-Date of collection. 6-Storage temperature. 7-Type and volume of anticoagulant. 8-Results of viral testing. Bleed Documentation Bleed documentation must contain the data displayed below: 1-Supplier name. 2-Product name. 3-Bleed number. 4-Donor number. 5-Shipment number or date of shipment. 6-Date of collection. 7-Test result for each one of the viral markers tested. The bleed number must be the same, as per numbers, prefixes and suffixes, in: -The list of donations. -The plasma unit label. -The plasma unit bar code reading. -The plasma sample label. -The plasma sample barcode reading. -The Alert notification of the look-back units

4.5 QUALITY ASSURANCE Inspections All plasma Suppliers, as well as laboratories, warehouses and transport companies, shall inform MANUFACTURER of the result of the inspections of the competent Health Authorities (US FDA, Europe, other). All plasma Suppliers laboratories, warehouses and transport companies, may be audited periodically by MANUFACTURER's staff or personnel authorized by MANUFACTURER. During these audits all procedures and reports of plasma suppliers, laboratories, warehouses and transport companies related to collection, processing, testing, storage, packaging, labelling and shipment of plasma may be reviewed. Serious incidences PLASMA SUPPLIER shall inform within three ("3") working days, any serious incidence produced at center, laboratory, and warehouse facilities and/or during transport process, which may affect the quality and safety of the provided plasma. Changes Control PLASMA SUPPLIER shall inform any MANUFACTURER manufacturing company of any planned changes that may have an impact in the quality and safety of plasma during collection, processing, testing, storage, packaging, labeling and shipment. Those changes requiring license amendment from Health Authorities (variations to the approved registry) need prior written approval of MANUFACTURER's manufacturing company before their implementation. Documentation and Records An approved system of documentation and proper control systems of review and authorization will be established. Documentation shall be updated annually if necessary. All donor records shall be retained by the Supplier for at least 40 years from the date of collection of the last donation for each donor. If necessary, MANUFACTURER and PLASMA SUPPLIER will agree on additional donor records, health information, donor questionnaires, donor number and testing if requested by competent Health Authorities. All donor testing records shall be retained by the supplier for at least 40 years from the date of the shipment of each unit to MANUFACTURER. PLASMA SUPPLLIER will provide upon request by MANUFACTURER'S copies of documents related to collection, receipt, storage, handling and distribution of products (i.e. recorder temperatures, maintenance records, etc...) PLASMA SUPPLIER will keep updated and available upon request by MANUFACTURER's, documents such as SMF (Site Master File), map and flows, chart, equipment and process validation and any other production documents that ensure the quality and safety of plasma. PLASMA SUPPLIER will provide annually -or upon request by MANUFACTURER-the necessary documentation to keep updated the Plasma Master File. Training and qualified personnel There should be a sufficient number of qualified personnel and Center shall provide training in accordance with an approved program. Initial and continuing training will be performed and effectiveness assessed. Training records shall be kept for all personnel.

Premises and Equipment (qualification and maintenance). Materials Equipment. systems, premises and instruments shall be qualified or validated before use or after any relevant change. Related qualification and validation documentation shall be reviewed and approved. A calibration program and preventive maintenance program for equipment and instruments shall be established. Critical materials shall come from approved suppliers that meet the documented requirements and specifications. 4.6. PLASMA RELEASE Plasma units released: Certificate of Compliance The PLASMA SUPPLIER shall only release plasma units to its delivery to any MANUFACTURER's manufacturing company as long as the Responsible Person states in a Certificate of Compliance that: • All source plasma units fulfill the requirements and Source Plasma Specifications in accordance with the Quality Agreement in force. • All plasma units of the shipment have been tested and found non-reactive for viral markers, specifying the test kits and/or reagents used in each case for: .o HBsAg, anti-HIV 1/2, anti-HCV, tested in individual units o Luetic serology, initially tested and every 4 months o Indirect Xxxxxx, initially tested and at least every 3 months o HAV-RNA, HCV-DNA, HCV-RNA, HIV-RNA and Parvovirus 819-DNA, tested by means of pools consisting of not more than 512 individual units. • All collection, processing, testing, quality control records review, storage, packing and labeling operations have been performed under current Good Manufacturing Practices, The certificate must be issued by the Re.sponsible Person of the Plasma Supplier. The PLASMA SUPPLIER will provide MANUFACTURER with alist of names of the Responsible Persons and authorized designees and their corresponding signatures and responsibililies.

ATTACHMENT 4 TEST KITS FOR EIA/NAT TEST KITS FOR EIA/NAT Viral Marker Test Kits of Individual Donors Ucenst I Establishment Page23of29 Parameter Test Method/ Name of tht Te;t Kit Manufacturer u.s. Ret!istration Number Anti-HCV ABBOTI PRISM HCV Hepatitis C Virus Encoded Antigens (Recombinant cl 00-3. HCr43. NS5) Xxxxxx Laboratories U.S License 43 Anti-HIV-1/2 ABBOTI Prism HN 0 Plus Virus Types I and 2 (E. coli, B, megalerum, Recombinant) Antigen and Synthetic Peptide Abboll Laboratories U.S. License 43 HBsAg ABBOTI PRISM HBsAg, Antibody to Hepatitis B Surface Antigen (Mouse MonoclonallgM) Abboll Laboratories U.S. License 43 Anti-HCV Hepntitis C Virus Encoded Antigen ORTHO HCV v3.0 ELISA TSystem (Recombinant c22-3. c200. NS5) Ortho-Ciinical Diagnostics, Inc. U.S. License 43 Anti-HIV-1/2 GS HIV-1/HIV-2 Plus 0 EIA Bio-Rad Laboratories U.S. License 43 HBsAg GS HBsAg EIA 3.0 Bio-Rad Laboratories U.S. License 43
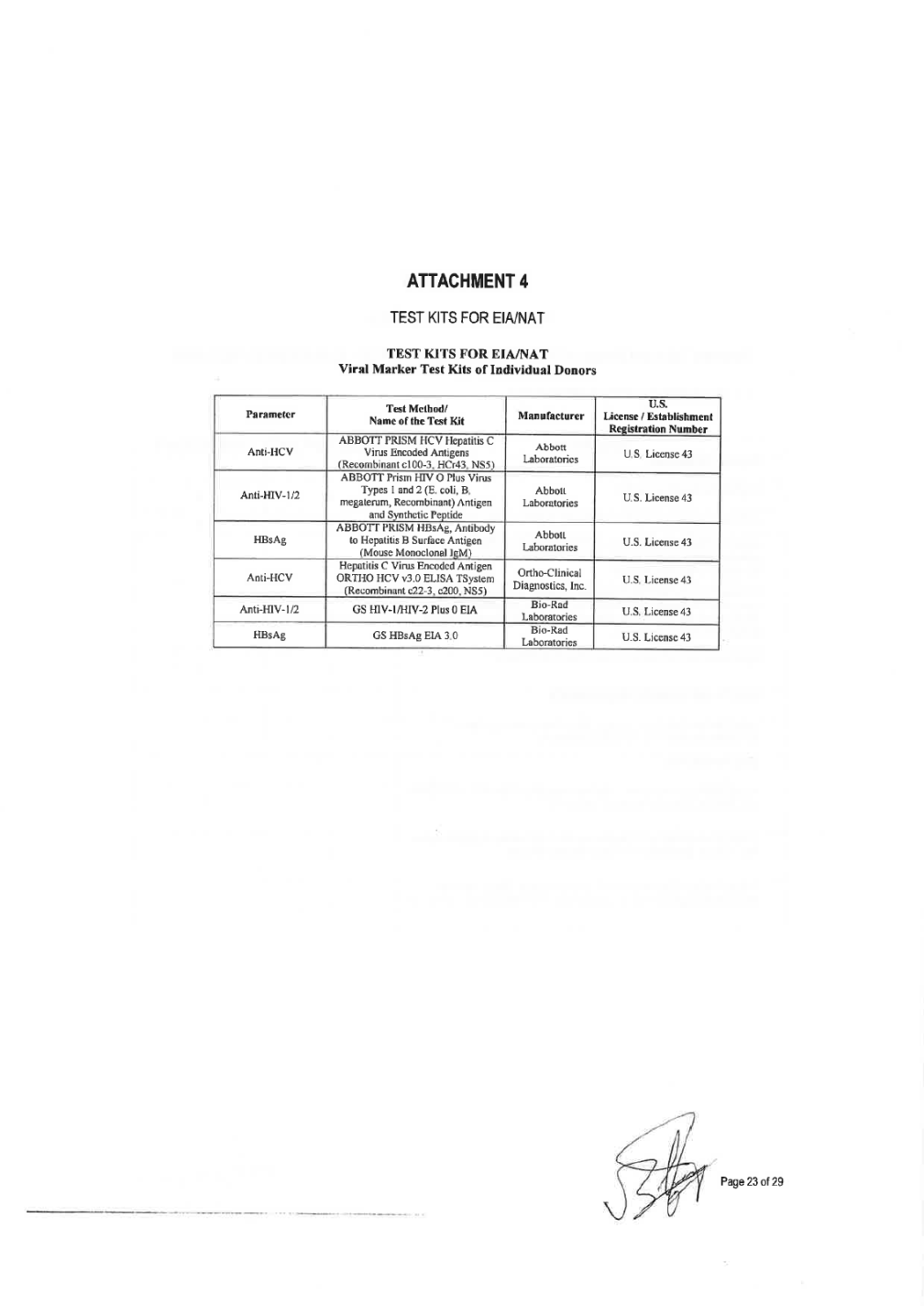
ATIACHMENT 5 TABLE OF RESPONSIBILITIES Summary Table and Division of Quality Responsibilities of PLASMA SUPLIERIMANUFACTURER 1.1.The responsible Party is denoted by X Page 24 of29 DESCRIPTION PLASMA SUPPLIER MANUFACTURER GENERAL PROVISIONS NegoUale the Quality Agreement X X Notification of change in reporting relationship involving Contact Persons X X Assure that Plasma obtained from US FDA licensed Source Plasma (Human) collection centers meet all the requirements stipulated in this Agreement and the MANUFACTURER Specifications X Responsible for the handling, storage, and transport of intermediates pastes and plasma that is contracted to the MANUFACTURER in accordance with the terms and conditions set forth in this Agreement, the applicable regulations and the MANUFACTURER Specifications X Approve any new contract or third party Suppliers of Plasma X X QUALITY AND TECHNICAL REQUIREMENTS Collect, process, test, store, package, label, release and ship Plasma in accordance with MANUFACTURER Specifications X QUALITY CONTROL Arrange testing for each donor of plasma in accordance with MANUFACTURER Specifications, as well as applicable regulations X Ensure all laboratories are in compliance with cGMP and are qualified in all the methodologies associated with Plasma and the Premises X Perform testing and analysis of the Plasma in approved laboratories using licensed kits included in the EU Plasma Master File if plasma is intended for EU X
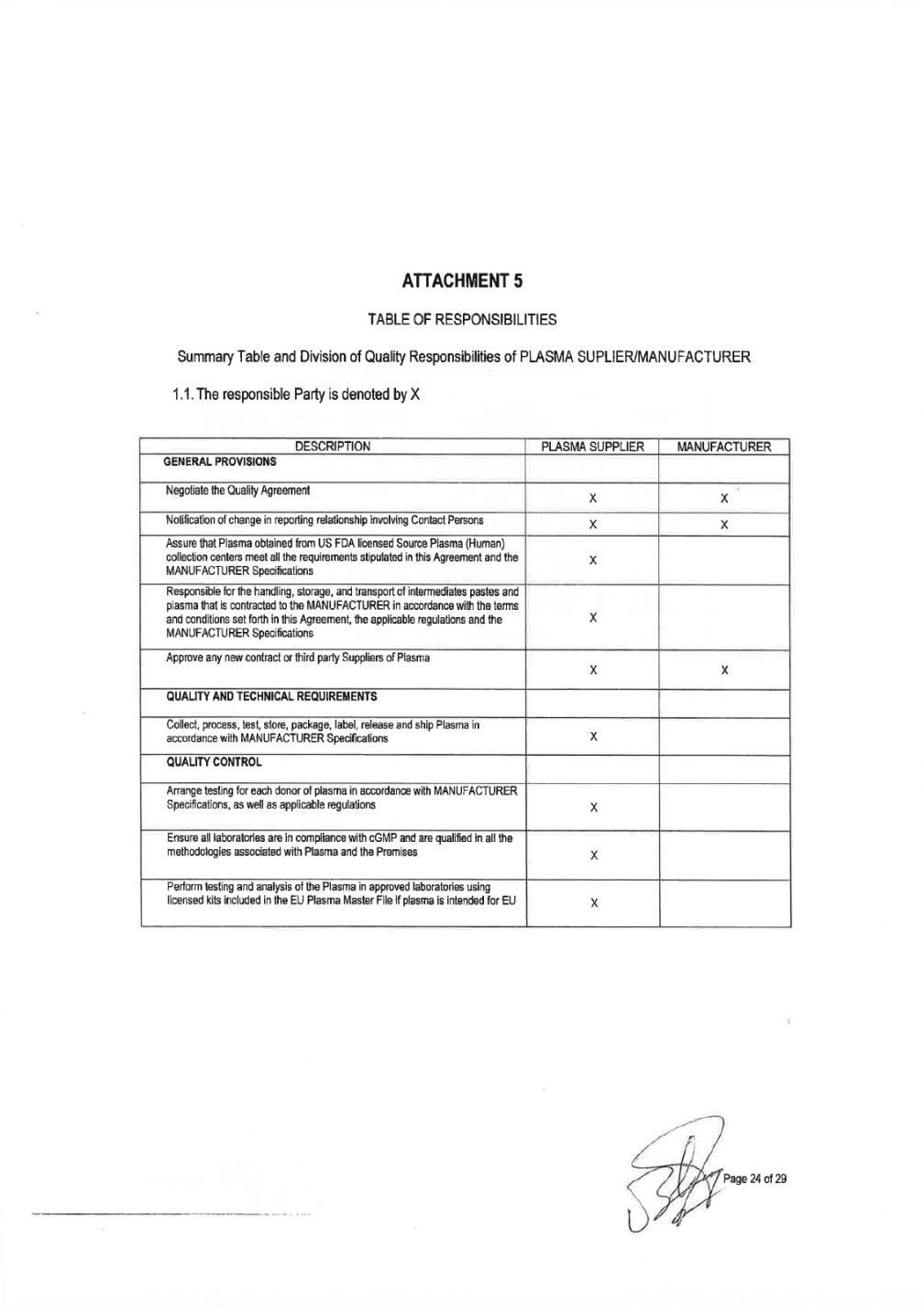
Page 25 of29 DESCRIPTION PLASMA SUPPLIER MANUFACTURER SUBCONTRACTING OPERATIONS Agree in writing on any contracted service (i.e., storage, testing) to be completed by third party per the MANUFACTURER Specifications X X Perform audits of third party contractors X X Provide a written report to MANUFACTURER confirming that the Piasa units have been collected, tested, shipped and stored property and meet the requirements of the MANUFACTURER Specifications X Ensure all sites or services used are in compliance with cGMP and are qualified in all of the methodologies associated with Plasma and the Premises. X X TRACEABILITY AND LOOK BACK COMMUNICATION (POST-DONATION COLLECTION INFORMATION Communicate to MANUFACTURER in writing within 3 calendar days all information per this Agreement on a donor after a donation that fulfilled all MANUFACTURER Specifications is rejected for seroconversion or post donation information that may become an infectious disease (Notifications per the MANUFACTURER Specifications) X Notify the MANUFACTURER no later than 3 calendar days of discovery of significant deviations that may impact batches or Plasma previously released and/or shipped to the MANUFACTURER. X Supply a report prior to product release on all post donation notifications received after pooling so that the MANUFACTURER can perform a viral risk assessment on the product X CHANGE CONTROL Notify MANUFACTURER in advance of any planned changes reasonably foreseeable to affect the regulatory submissions to regulatory authorities including EU Plasma Master File variations and/or available supply of the Plasma-Obtain written approval from MANUFACTURER prior to the implementation of such changes and any other change that are covered by the change control scope of this Agreement that could have any effect on the safely, purity, etc. of the Plasma collected X Provide justification for the changes and validate any changes to cGMP standards X Provide written approval for change controls per this Agreement X

DESCRIPTION PLASMA SUPPLIER MANUFACTURER QUALITY ASSURANCE Maintain an active quality management system, which shall apply to all operations which may impact on the quality and safety of the Plasma X Document and investigate deviations from the processes and procedures per this Agreement and notify MANUFACTURER as soon as possible before shipment or in no case later than three ('3") calendar days of discovery that a deviation may impact a batch or Plasma previously released and/or shipped X Approve the use of materials for the collection of Plasma; confirm the proper condition of the packaging, sterility certification, locks/seals, shipping documentation; ensure that each lot is in full compliance with licensed requirements and with applicable regulations X Approve all vendors and Suppliers of critical raw materials, reagents and components used in the collection and testing of Plasma;Ensure materials are received, stored, segregated and used correctly and all materials are appropriate for their intended use; Maintain relevant certificates of analysis, certificates of compliance, certificates of origin for such materials, as appropriate; Maintain an approved vendor list X STORAGE AND SHIPPING Provide storage conditions for the Plasma that meets the agreed upon MANUFACTURER Specifications,SOPs, applicable regulations related to Plasma storage and the EP monograph-Human Plasma to Fractionation X Maintain proper segregation of the Plasma units X Provide quality assurance oversight of unreleased Plasma received into quarantine and stored in a controlled, validated warehouse X Provide quality assurance oversight to segregate, identify and disposition rejected plasma X BIOLOGICAL PRODUCT DEVIATIONS AND RECALLS Monitor the manufacturing and releasetata af the Plasma to detect potentially reportable deviations within one (" 1") business day of confirmation X Plasma unit deviation reporting to the appropriate regulatory authorities X Biological product deviation reporting to the appropriate regulatory authorities X X

Page 27 of29 DESCRIPTION PLASMA SUPPLIER MANUFACTURER Perform investigations for any issues thought to be Plasma unit based and provide information to MANUFACTURER X Responsible for Plasma unit recall or withdrawal; Provide notification to MANUFACTURER within one ('1') business day of confirmation and perform investigations for these problems and forward investigation report to MANUFACTURER within mutually a!jreeable timeframe X Responsible for final product or intermediate product recall or withdrawal X EQUIPMENT, COMPUTER AND FACILITY VALIDATION AND/OR QUALIFICATION PROCESS Ensure all processes, equipment, systems and facilities are qualified and validated, as applicable, per this Agreement X Approve all process qualifications applicable to the Plasma collection, including but not limited to, the freezer validation and shipping validation for test samples and Plasma X PLASMA RELEASE Have at least one quality Responsible Person on the Premises to oversee the quality aspects of the Plasma collection, testing, shipment and evaluate deviations, in order to ensure compliance with cGMP; the Responsible Person will certify that each unit of Plasma has been collected and tested in approved facilities according to GMP and fulfil quality and safety requirements according to the MANUFACTURER Specifications X 1 LICENSES, PERMITS AND APPROVALS Represents and warrants that all their plasmapheresis donor centers in operation are currently certified under the PPTA IQPP; such certification must be preserved without interruption during the term of this Agreement; if plasmapheresis donor center fails to remain certified, notification to MANUFACTURER immediately, or within one {"1') business day X Follow al requirements and specifications for exporting products to Europe X Notify MANUFACTURER within one business day in the event of a suspension, revocation or other administrative action adversely affecting or limiting license, permit or approval X Provide prior written notification to MANUFACTURER prior to relocating, opening or closing a plasmapheresis center; Obtain written approval from MANUFACTURER for inclusion of new centers, testing laboratories, plasma warehouses and transportation companies X
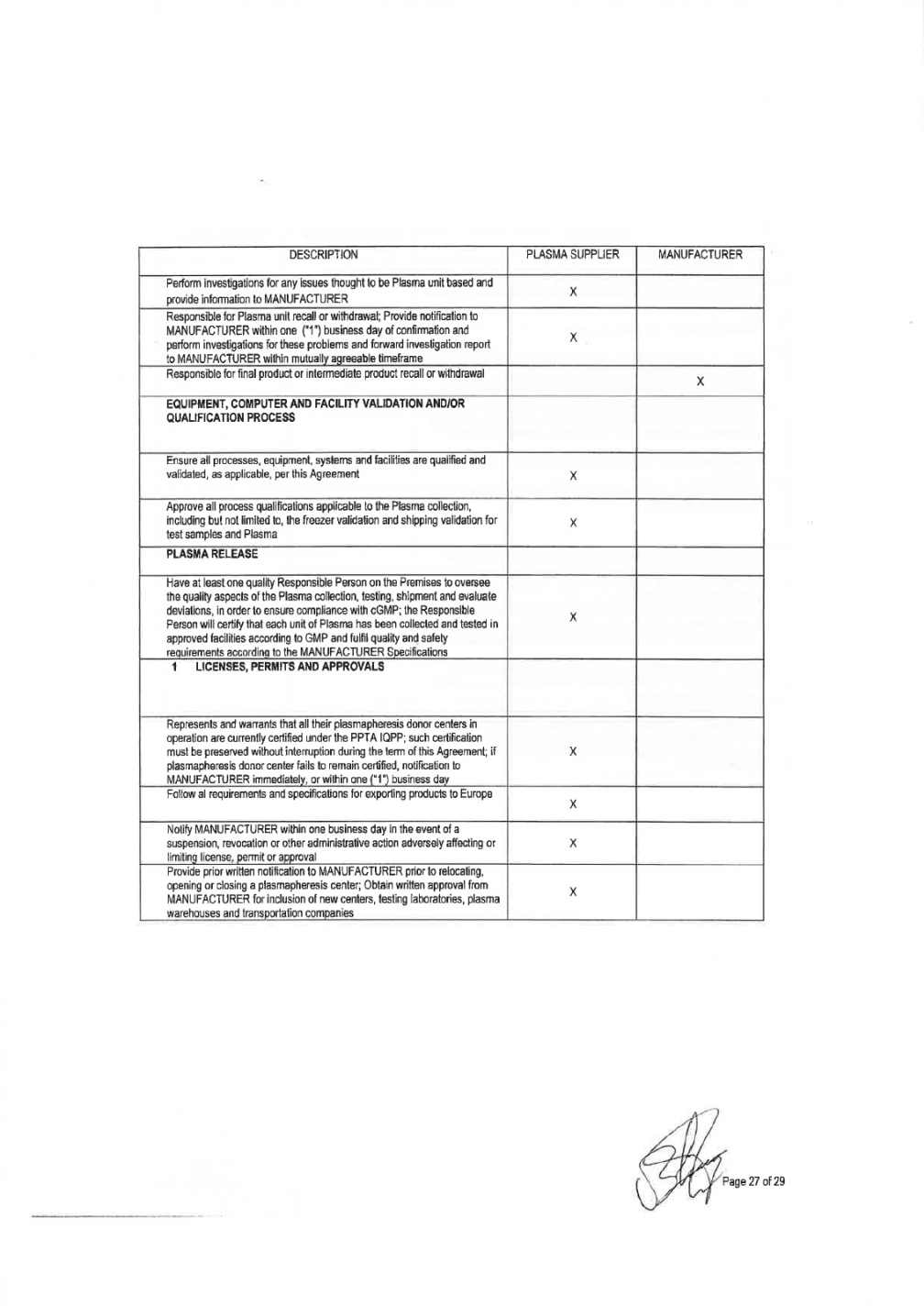
DESCRIPTION PLASMA SUPPLIER MANUFACTURER 2 INSPECTIONS Audit and qualify all vendors of supplies used in the collection and shipment of Plasma X Handle complaints to the vendor of supplies X Allow the MANUFACTURER to conduct annual inspections of the approved plasmapheresis donor collection centers, testing laboratories, plasma warehouse storage facilities and/or transport/transit and shipping companies and the BIOMAT USA,Inc. quality systems per this Agreement X Responsible for the ulllmale approval of all Plasma centers and their status as approved or not approved to ship Plasma to the MANUFACTURER X Provide written report to MANUFACTURER within thirty ('30') days of the audit date X Ensure that all the corrective actions are implemented and provide a report to the MANUFACTURER X Provide wrltlen notice to MANUFACTURER of any investigations ofthe donor centers, testing laboratories, storage, release and shipping facilities/companies with no more than three (' 3') business days of the investiqallon·s commencement X Communicate to MANUFACTURER within one ("1') business day any event or serious failure of a center, testing laboratory, storage and/or transportation which may impact quafi(y or safely of !he Plasma supplied X Notify MANUFACTURER of any meetings/substantive discussions with regulatory authorities that direclly relate to the manufacture, supply and/or control of !he Plasma supplied X Provide to MANUFACTURER a copy of the following within one ('1")business day of receipt: (i) List of Observations Form FDA 483, (ii) Information Letter, (iii) Warning Letter, (iv) Notice of Intent to Revoke License Letter, (v) Suspension Letter, Seizure Letter or the like issued by n qulatory authority X

DESCRIPTION PLASMA SUPPLIER MANUFACTURER 3 DOCUMENTATION AND RECORD RETENTION Responsible for preparing and submitting regulatory submissions linked to the process and final product as required by applicable regulations X Provide alllhe documentation requested to support regulatory submissions, annual update for the EU Plasma Master File, epidemiology xxxx/viral marker data X Keep complete and accurate records of equipment usage, cleaning, testing, temperature holds, and any maintenance/calibration performed, per this Agreement X Retain donor records per applicable regulations and per this Agreement X
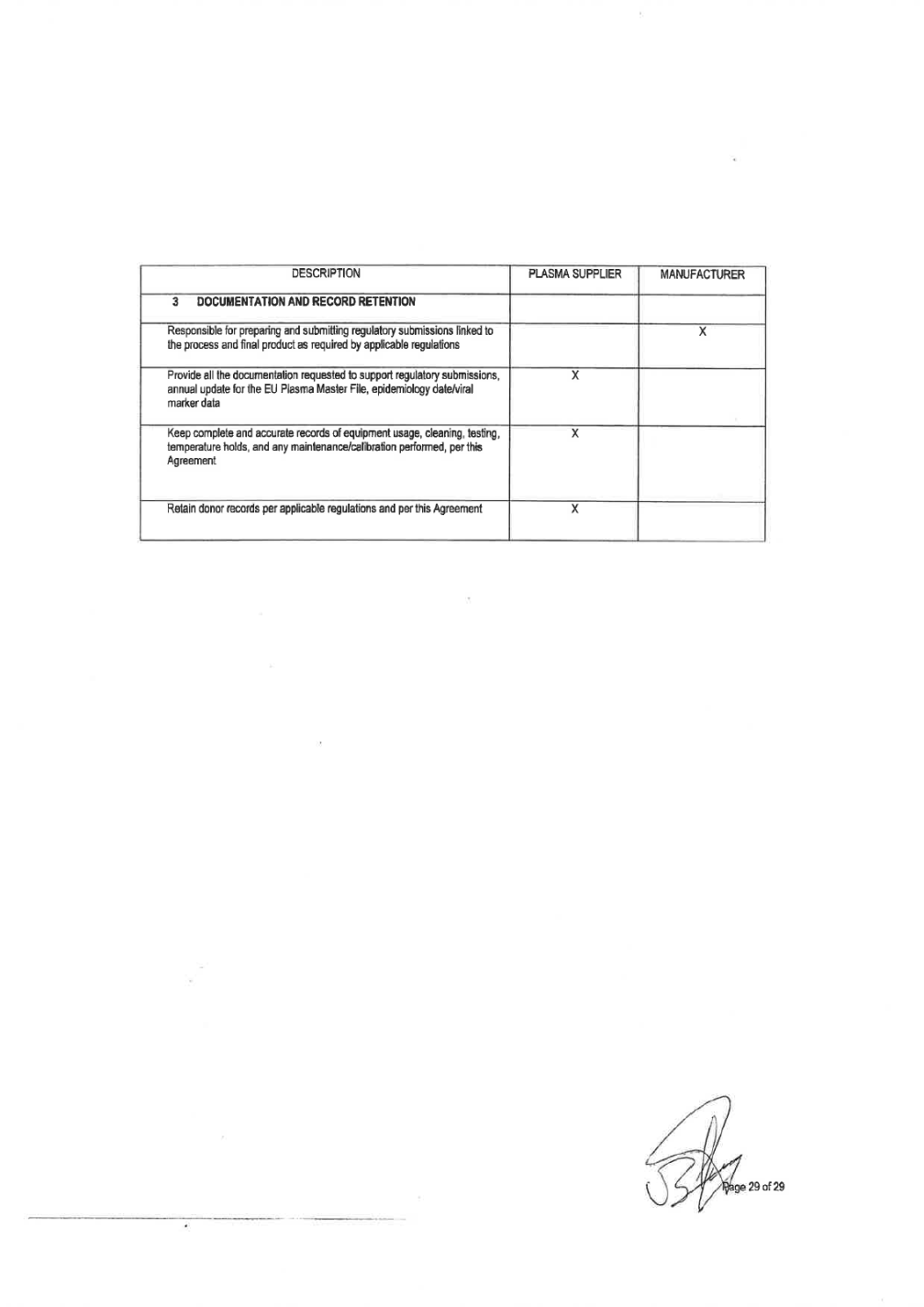
EXHIBIT C ANNUAL PLAN 18

EXHIBIT C ANNUAL PLAN


