PUBLIC HEALTH SERVICE SECOND AMENDMENT TO L-216-2000/0
Exhibit 6.8
PUBLIC HEALTH SERVICE
SECOND AMENDMENT TO L-216-2000/0
This is the second amendment (“Second Amendment”) of the agreement by and between the National Institutes of Health (“NIH”) or the Food and Drug Administration (“FDA”), hereinafter singly or collectively referred to as agencies of the United States Public Health Service (“PHS”) within the Department of Health and Human Services (“HHS”), and 20/20 GeneSystems, Inc. having an effective date of November 9, 2000, and having NIH Reference Number L-216-2000/0, as amended by the first amendment to the agreement, having an effective date of April 14, 2005, and having NIH reference Number L-216-2000/1 (“First Amendment”) (hereinafter collectively referred to as the “Agreement”). This Second Amendment, having NIH Reference Number L-216-2000/2, is made between the PHS through the Office of Technology Transfer, NIH, having an address at 0000 Xxxxxxxxx Xxxxxxxxx, Xxxxx 000, Xxxxxxxxx, Xxxxxxxx 00000-0000, U.S.A., and 20/20 GeneSystems, Inc., having an office at 0000 Xxx Xxxx Xxx. Xxxxxxxxx, XX 00000 (“Licensee”). This Second Amendment includes, in addition to the amendments made below, 1) a Signature Page, and 2) Attachment 1 (Royalty Payment Information).
WHEREAS, PHS and Licensee desire that the Agreement be amended a second time as set forth below in order to update the Patent(s) and Patent Application(s) covered by the Licensed Patent Rights (in cover page and Appendix A), to update to provide a schedule for reimbursing PHS for patent expenses incurred after the Agreement effective date.
NOW, THEREFORE, in consideration of the mutual covenants and promises contained herein, PHS and Licensee, intending to be bound, hereby mutually agree to the following:
| 1. | Replace Cover Page of the Agreement under “Serial Number(s) of Licensed Patent(s) and/or Patent Application(s)” heading, and Appendix A, Patent(s) and Patent Application(s) with the following: |
| Country | Serial Number | Filing Date | Patent Number | PHS reference | ||||
| PCT | PCT/US00/20354 | July 26, 2000 | X-000-0000/0-XXX-00 | |||||
| Xxxxxxxxx | 66091/00 | July 26, 2000 | 771499 | E-079-1999/0-AU-05 | ||||
| Canada | 2375034 | July 26, 2000 | Pending | E-079-1999/0-CA-06 | ||||
| Europea | 00953685.5 | July 26, 2000 | 1,218,743 | X-000-0000/0-XX-00 | ||||
| Xxxxx | 2001-512292 | July 26, 2000 | 4,137,444 | X-000-0000/0-XX-00 | ||||
| Xxxxxx Xxxxxx | 10/048,194 | July 26, 2000 | 7,214,477 | X-000-0000/0-XX-00 | ||||
| Xxxxxx Xxxxxx | 09/718,990 | November 20, 2000 | 6,602,661 | X-000-0000/0-XX-00 | ||||
| Xxxxxx Xxxxxx | 10/627,352 | July 25, 0000 | Xxxxxxx | X-000-0000/0-XX-00 | ||||
| XXX | XXX/XX00/00000 | August 1, 2003 | X-000-0000/0-XXX-00 | |||||
| Xxxxxx Xxxxxx | 10/522,663 | January 27, 2005 | Abandoned | E-059-2003/0-US-03 | ||||
| PCT | PCT/US03/02933 | January 31, 2003 | Abandoned | E-101-2001/0-PCT-02 | ||||
| PCT-Combinationb | PCT/US01/44009 | November 20, 2001 | X-000-0000/0-XXX-00 | |||||
| Xxxxxxxxx | 2002243236 | November 20, 2001 | 2002243236 | E-079-1999/3-AU-05 | ||||
| Canada | 2428441 | November 20, 2001 | Abandoned | X-000-0000/0-XX-00 | ||||
| Xxxxxx | 01989119.1 | November 20, 2001 | Abandoned | X-000-0000/0-XX-00 | ||||
| Xxxxx | 2002-549932 | November 20, 0000 | Xxxxxxxxx | X-000-0000/0-XX-00 | ||||
| Xxxxxx Xxxxxx | 10/432,423 | May 20, 2003 | 6,969,615 | X-000-0000/0-XX-00 | ||||
| Xxxxxx Xxxxxx | 11/277,227 | March 22, 2006 | 7,838,222 | X-000-0000/0-XX-00 | ||||
| Xxxxxx Xxxxxx | 12/626,405 | November 25, 2009 | Pending | E-059-2003/1-US-02 |
| a | Issued in Germany, France, United Kingdom, Italy, Spain, Ireland, Switzerland and Belgium |
| b | Application S/Ns 09/718,990 (E-079-1999/1-US-01); 09/753,574 (E-079-1992/2-US-01); 60/304,031 (E-037-2002/0-US-01); and 60/286,258 (E-285-2003/0-US-01) combined with USSN 60/296,475 (E-114-2002/0-US-01) in PCT-Combo application. |
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
Above Patent(s) and Patent Application(s) originate from PHS Technology references:
E-079-1999/0
E-079-1999/1
E-079-1999/2 (abandoned, combined into PCT)
E-079-1999/3
E-037-2002/0 (abandoned, combined into PCT)
E-059-2003/0
E-101-2001/0 (abandoned)
E-097-2004/0
E-113-2004/0 (abandoned)
E-114-2002/0 (abandoned, combined into PCT)
E-285-2003/0 (abandoned, combined into PCT
| 2. | Replace entire Appendix E of the Agreement with new Appendix E – Benchmarks and Performance as follows: |
Licensee agrees to the following Benchmarks for its performance under this Agreement and, within thirty (30) days of achieving a Benchmark, shall notify PHS that the Benchmark has been achieved.
Licensed Product(s) using Specific or Universal Capture Membranes
| a. | Development of Licensed Product(s) for research applications. |
| Activity Description | Benchmark | ||
| Offering of Services utilizing Licensed Product(s) for tissue analysis | Completed October 1, 2010, but currently inactive | ||
| Offering of Kits that incorporate Licensed Products(s) | July 1, 2012 | ||
| (Research Use Only) for tissue analysis |
| b. | Development of Licensed Product(s) for clinical applications. |
| Activity Description | Benchmark | ||
| Commercial Sale of Licensed Product for clinical applications/diagnostic – 1st application | October 2012 | ||
| Commercial Sale of Licensed Product for clinical applications/diagnostic – 2nd application | April,2013 | ||
| Commercial Sale of Licensed Product for clinical applications/diagnostic – 3rd application | October 2013 |
| 3. | Append the following to section B of Appendix F: Commercial Development Plan of the Agreement: |
Research, Development and Testing
Since 2007 Licensee has conducted over $2.5 million in R&D directed to the development and testing of various embodiments of Licensed Products that can effectively identify multiple biomarkers in tissue sections. These Licensed Products are now referred to as “Layered Immunohistochemistry” or “L-IHC” to signal to the marketplace that they can be used as an alternative to the established IHC technology when multiplex histological analysis is indicated.
Between 2007 and 2009 this R&D primarily focused on a version of Licensed Products that utilized specific capture membranes. Those membranes were coated with oligonucleotides that were complimentary to oligonucleotides conjugated to antibody probes. (The NIST-ATP program funded much of this work.) Despite significant effort, oligonucleotide coated membranes posed significant technical challenges from the standpoint of forming the basis of a commercially viable product.
Licensee has transitioned the bulk of its R&D efforts to refining and perfecting a version of Licensed Products that formed the basis of much of its work prior to 2007, namely, universal capture membranes. Aided by several technical improvements to transfer protocols, membrane coating, process optimization, etc. we are now getting excellent results. In short, we are now getting robust, reproducible results with several solid tumor types (kidney, breast) using panels of biomarkers associated with disease prognosis and therapy selection.
| a. | Clinical Diagnostics |
“Layered-IHC”1 now reliably permits 10 or more biomarkers to be measured simultaneously from a single slice of biopsied tissue while maintaining morphology. No other marketed technology has this dual capability. 20/20 is applying this technology to develop a series of companion diagnostic products to predict the efficacy of molecularly targeted therapies in cancer.
1 referred to at the time of the license as “Layered Expression Scanning” (“LES”)
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
More specifically Licensee is now developing a series of companion diagnostic tests for cancer therapeutic agents targeted at the mTOR pathway. The mTOR pathway is an important pathway in tumorigenesis and currently the target of several drugs on the market for kidney cancer as well as a number of compounds in development for multiple tumor types. It has been demonstrated that a more complete characterization of the mTOR pathway is required to best determine the appropriateness of a specific therapy and as such measurement of a panel of multiple biomarkers will be required. Thus 20/20’s L-IHC system is a most appropriate system for the development of mTOR companion diagnostics. Our ongoing SBIR Bridge award is focused on the development and characterization of L-IHC for the profiling of kidney and breast cancer related biomarkers and has focused on the feasibility, reproducibility and accuracy of L-IHC for the measurement of these markers. To date, we have successfully imported most of the key mTOR pathway biomarkers into the L-IHC system.
Tests to help patients that have been diagnosed with cancer select optimum treatments is a defined market that can be successfully addressed by a small company. For the predicTOR® product line we will hire several dedicated technical sales professionals to call on the major secondary cancer centers (Memorial Xxxxx, Xxxxx Xxxxxxx, etc.). The primary customer is the oncologist who will order the test and request that the pathology laboratory send tissue sections to the 20/20 in-house reference lab. Our first test, predicTOR® for predicting response of kidney tumors to AFFINITOR (Novartis) and TORISEL (Pfizer), is expected to be available before the end of 2012.
In addition to drugs that target the mTOR pathway we are also developing L-IHC based tests to aid in the management of kidney cancer based on feedback from treating physicians. Specifically these include (i) a test to predict recurrence of early stage renal cancers (RecurRenTM) and (ii) a test to predict therapeutic response to the drug SUTENT® that was approved for treating kidney cancer in 2005.
The following flowchart illustrates how this family of L-IHC based tests fit together in the context of kidney cancer management. (The green diamonds identify specific L-IHC tests in development.)
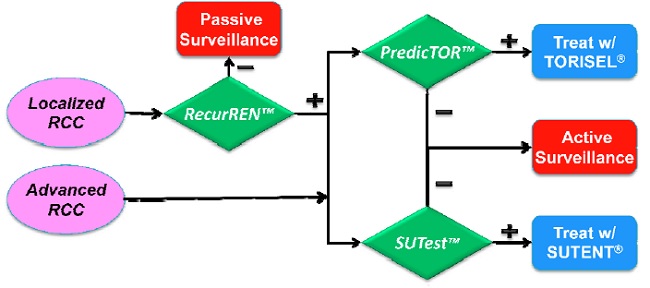
Importantly, we anticipate the development of other L-IHC based tests for various solid tumors including breast cancer. These can be developed by 20/20 or licensed third parties.
By 2015 we expect annual revenue based on the L-IHC technology to be approaching $12 million.
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
| b. | Research Products and Services |
Over the years Licensee has contemplated various approaches to making the L-IHC (LES) technology widely available to the life science research community.
In 2003 we launched a kit for creating multiple blots of 2-D electrophoresis gels. That product, sold through Kodak Scientific Imaging, was ultimately removed from the market largely due to pricing issues. Specifically, the low cost of conventional Western blotting made it hard for us to price the Multi-Blot kit competitively.
Since, 2007 we have focused L-IHC solely on tissue profiling applications since the competitive advantages are more compelling due to the limitations of IHC for multiplex biomarker analysis. In 2010 we began on a limited basis to offer services to drug companies using L-IHC. It is not yet apparent whether there is sufficient demand for such a service, whether it can be operated profitably or whether Licensee could provide such a service without distracting from its diagnostic development priorities.
At the BIO International Convention in June 2011 we were approached by a leading life science tools companies with interest in developing and marketing a L-IHC based Research Use Only kit for tissue analysis. Discussions with this company are expected to commence in July 2011.
| 4. | Paragraph 6.02 of the Agreement shall be deleted and replaced with the following new Paragraph 6.02: |
Licensee agrees to pay to PHS a non-refundable minimum annual royalty as set forth in Appendix C. The minimum annual royalty is due and payable on January 1 of each calendar year from 2003 through 2011. After 2011, further payment of the minimum annual royalty will be deferred until January 1 of the calendar year following the first calendar year Licensee achieves annual Net Sales of Licensed Product equal to or greater than One Million US Dollars ($1,000,000). The minimum annual royalty will continue to be due January 1 of each calendar year thereafter. The minimum annual royalty paid in any calendar year may be credited against earned royalties due for Licensed Product sales made in that year.
| 5. | Licensee agrees to reimburse PHS for patent expenses incurred after the effective date of the Agreement and through August 31, 2011 as follows: |
| a. | Five Thousand US Dollars ($5,000) due within sixty (60) days of executing this Second Amendment. |
| b. | Ten Thousand US Dollars ($10,000) due January 1st of each calendar year beginning on January 1, 2013 until Licensee has achieved Five Hundred Thousand US Dollars ($500,000) in annual Net Sales of Licensed Products. |
| c. | Twenty Thousand US Dollars ($20,000) due January 1st of each calendar year beginning on the first calendar year after Licensee has achieved Five Hundred Thousand US Dollars ($500,000) in annual Net Sales of Licensed Products and until Licensee has achieved One Million US Dollars ($1,000,000) in annual Net Sales of Licensed Products. |
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
| d. | The balance of any remaining unreimbursed patent expenses incurred by PHS after the effective date of the Agreement through August 31, 2011 shall be due January 1st of the first calendar year after Licensee achieves One Million US Dollars ($1,000,000) in annual Net Sales of Licensed Products or upon license termination or expiration, whichever comes first. |
For clarification, from the effective date of the Agreement through August 31, 2011, PHS paid One Hundred Eighty Four Thousand Six Hundred Fifty Four US Dollars and Twenty Cents ($184,654.20) in patent expenses not yet reimbursed by Licensee. This amount includes the Seventy Three Thousand Six Hundred Twenty Four Dollars and Ninety Six Cents ($73,624.96) in patent expenses identified in amended Appendix C under paragraph 2. h. of the First Amendment.
While the above schedule of payments for reimbursement of patent expenses is in progress, PHS will also submit annual requests to Licensee for reimbursement of patent expenses incurred after August 31, 2011 and expect payment within sixty (60) days per Paragraph 6.10 of the Agreement.
| 6. | In the event any provision(s) of the Agreement is/are inconsistent with Attachment 1, such provision(s) is/are hereby amended to the extent required to avoid such inconsistency and to give effect to the payment information in such Attachment 1. |
| 7. | All terms and conditions of the Agreement not herein amended remain binding and in effect. |
| 8. | The terms and conditions of this Second Amendment shall, at PHS’ sole option, be considered by PHS to be withdrawn from Licensee’s consideration and the terms and conditions of this Second Amendment, and the Second Amendment itself, to be null and void, unless this Second Amendment is executed by Licensee and a fully executed original is received by PHS within sixty (60) days from the date of PHS signature found at the Signature Page. |
| 9. | This Second Amendment is effective on July 25, 2011 upon execution by all parties. |
SIGNATURES BEGIN ON NEXT PAGE
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
SECOND AMENDMENT TO L-216-2000/0
SIGNATURE PAGE
In Witness Whereof, the parties have executed this Second Amendment on the dates set forth below. Any communication or notice to be given shall be forwarded to the respective addresses listed below.
| For PHS: | |||
| /s/ Xxxxxxx X. Xxxxxxxxx | 12-23-11 | ||
| Xxxxxxx X. Xxxxxxxxx | Date | ||
| Director, Division of Technology Development and Transfer Office of Technology Transfer | |||
| National Institutes of Health | |||
| Mailing Address or E-mail Address for Agreement notices and reports: | |||
| Chief, Monitoring & Enforcement Branch, DTDT | |||
| Office of Technology Transfer | |||
| National Institutes of Health | |||
| 0000 Xxxxxxxxx Xxxxxxxxx, Xxxxx 000 | |||
| Xxxxxxxxx, Xxxxxxxx 00000-0000 X.X.X. | |||
E-mail: XxxxxxxXxxxxxx_Xxxxxxx@xxxx.xxx.xxx
For Licensee (Upon information and belief, the undersigned expressly certifies or affirms that the contents of any statements of Licensee made or referred to in this document are truthful and accurate.):
| Signature of Authorized Official | Date |
Name: Xxxxxxxx Xxxxx
Title: President and CEO
| I. | Official and Mailing Address for Agreement notices: |
| Xxxxxxxx Xxxxx | ||
| Name | ||
| President and CEO | ||
| Title | ||
| Mailing Address: | ||
| 20/20 GeneSystems, Inc. 0000 Xxx Xxxx Xxx. Xxxxxxxxx, XX 00000 |
| Email Address: | xxxxxx@0000xxxx.xxx | |
| Phone: | 0000000000 |
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
| II. | Official and Mailing Address for Financial notices (Licensee’s contact person for royalty payments): |
| Xxxxxxxx Xxxxx | ||
| Name | ||
| President and CEO | ||
| Title | ||
| Mailing Address: | ||
| 20/20 GeneSystems, Inc. 0000 Xxx Xxxx Xxx. Xxxxxxxxx, XX 00000 |
| Email Address: | xxxxxx@0000xxxx.xxx | |
| Phone: | 0000000000 |
Any false or misleading statements made, presented, or submitted to the Government, including any relevant omissions, under this Agreement and during the course of negotiation of this Agreement are subject to all applicable civil and criminal statutes including Federal statutes 31 U.S.C. §§3801-3812 (civil liability) and 18 U.S.C. §1001 (criminal liability including fine(s) or imprisonment).
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
ATTACHMENT 1 – ROYALTY PAYMENT OPTIONS
The XXX License Number MUST appear on payments, reports and correspondence.
Automated Clearing House (ACH) for payments through U.S. banks only
The NIH encourages our licensees to submit electronic funds transfer payments through the Automated Clearing House (ACH). Submit your ACH payment through the U.S. Treasury web site located at: xxxxx://xxx.xxx.xxx. Locate the “NIH Agency Form” through the Xxx.xxx “Agency List”.
Electronic Funds Wire Transfers
The following account information is provided for wire payments. In order to process payment via Electronic Funds Wire Transfer sender MUST supply the following information within the transmission:
Drawn on a U.S. bank account via FEDWIRE should be sent directly to the following account:
| Beneficiary Account: | Federal Reserve Bank of New York or TREAS NYC | |
| Bank: | Federal Reserve Bank of New York | |
| ABA# | 000000000 | |
| Account Number: | 00000000 | |
| Bank Address: | 00 Xxxxxxx Xxxxxx, Xxx Xxxx, XX 00000 | |
| Payment Details: | License Number (L-XXX-XXXX) | |
| Name of Licensee |
Drawn on a foreign bank account should be sent directly to the following account. Payment must be sent in U.S. Dollars (USD) using the following instructions:
| Beneficiary Account: | Federal Reserve Bank of New York/ITS or FRBNY/ITS | |
| Bank: | Citibank N.A. (New York) | |
| SWIFT Code: | XXXXXX00 | |
| Account Number: | 00000000 | |
| Bank Address: | 000 Xxxxxxxxx Xxxxxx, Xxx Xxxx, XX 00000 | |
| Payment Details (Line 70): | NIH 75080031 | |
| License Number (L-XXX-XXXX) | ||
| Name of Licensee | ||
| Detail of Charges (line 71a): | Charge Our |
CONFIDENTIAL | Final - 20/20 GeneSystems, Ic. | 12/23/2011 L-216-2000/2 |
Checks
All checks should be made payable to “NIH Patent Licensing”
Checks drawn on a U.S. bank account and sent by US Postal Service should be sent directly to the following address:
National Institutes of Health (NIH)
X.X. Xxx 000000
Xx. Xxxxx, XX 00000-0000
Checks drawn on a U.S. bank account and sent by overnight or courier should be sent to the following address:
US Bank
Government Lockbox SL-MO-C2GL
0000 Xxxxxxxxxx Xxxxx
Xx. Xxxxx, XX 00000
Phone: 000-000-0000
Checks drawn on a foreign bank account should be sent directly to the following address:
National Institutes of Health (NIH)
Office of Technology Transfer
Royalties Administration Xxxx
0000 Xxxxxxxxx Xxxxxxxxx
Xxxxx 000, XXX 0000
Xxxxxxxxx, Xxxxxxxx 00000
| CONFIDENTIAL | ||
| Second Amendment of L-216-2000/0 | Final - 20/20 GeneSystems, Ic. | 12/23/2011 |
| Model 09-2006 (updated 8-2010) | Page 9 of 9 | L-216-2000/2 |

