EX-10.31 13 d255425dex1031.htm YALE EXCLUSIVE LICENSE AGREEMENT BETWEEN THE REGISTRANT AND YALE UNIVERSITY YALE EXCLUSIVE LICENSE AGREEMENT
Exhibit 10.31
YALE EXCLUSIVE LICENSE AGREEMENT
THIS AGREEMENT by and between YALE UNIVERSITY, a corporation organized and existing under, and by virtue of, a charter granted by the general assembly of the Colony and State of Connecticut and located in New Haven, Connecticut (“YALE”), and Rib-X Pharmaceuticals, Inc., a corporation organized and existing under the laws of the State of Delaware, with principal offices located in Guilford, CT (“LICENSEE”) shall be effective upon the date of final execution below (“EFFECTIVE DATE”).
ARTICLE 1 BACKGROUND
1.1 In the course of research conducted under YALE auspices, Drs. Xxxxxx X. Xxxxxx, a Xxxxxx Xxxxxx Medical Institute (HHMI) investigator at YALE, Xxxxx X. Xxxxx, Nenad Ban, Xxxx Xxxxxx and Xxxxxxx Xxxxxx in the Department of Molecular Biophysics and Biochemistry at YALE (Nenad Ban and Xxxxxxx Xxxxxx also employed by HHMI at YALE) (collectively the “INVENTORS”), have produced inventions entitled “Drug Design Using Atomic Structure of 50S Ribosomal Subunit and Bound Antibiotic” (the “OCR 1079 INVENTION”) and “Structure Based Design Method to Develop New Antibiotics that Inhibit Protein Synthesis on the Large Subunit of the Bacterial Ribosome” (the “OCR 1220 INVENTION” and, together with the OCR 1079 INVENTION, the “INVENTIONS”).
1.2 Pursuant to an assignment by the respective INVENTOR(S) and HHMI to YALE of all of each INVENTOR’s right, title and interest in and to the INVENTIONS and any resulting patents, YALE is the owner of the INVENTIONS, subject to rights reserved by the U.S. government and/or any other third party sponsors, including HHMI.
1.3 YALE wishes to have the INVENTIONS and any resulting patents commercialized to benefit the public good.
1.4 LICENSEE has represented to YALE to induce YALE to enter into this AGREEMENT that it plans to engage in the diligent development of the LICENSED INTELLECTUAL PROPERTY leading to the commercialization of LICENSED PRODUCTS and SERVICES so that public utilization and benefit shall result.
1.5 YALE is willing to grant an exclusive license to LICENSEE subject to the terms and conditions of this AGREEMENT.
1.6 In consideration of these statements and mutual promises, YALE and LICENSEE agree to the terms of this AGREEMENT.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
ARTICLE 2 DEFINITIONS
The following terms used in this AGREEMENT shall be defined as set forth below:
2.1 “AFFILIATE” means any corporation or other legal entity “controlled,” “controlling” or “under common control with,” another corporation or legal entity, where “control” means ownership, directly or indirectly, of more than fifty percent (50%) of the voting capital shares or similar voting securities of the LICENSEE.
2.2 “CLINICAL TRIAL” shall mean any clinical trial approved by the FDA, or foreign equivalent, where a LICENSED PRODUCT is tested in a number of either sick or healthy human or veterinary patients or for diagnostic purposes, data from which can then be used to establish the safety and/or efficacy of the LICENSED PRODUCT for the indication or use for which regulatory approval is sought.
2.3 “CONFIDENTIAL INFORMATION” shall mean all information including, but not limited to know-how and data, technical or non-technical, trade secrets or inventions, whether or not patentable, LICENSED INTELLECTUAL PROPERTY, LICENSED INFORMATION, and the research and business plan (“PLAN”), as further described in Article 7, which is not publicly known and is disclosed in writing clearly marked “CONFIDENTIAL” to one party by the other during the negotiation of or under this AGREEMENT or if orally disclosed, is reduced to writing clearly marked “CONFIDENTIAL” within thirty (30) days of such disclosure unless such information is subject to an exception described in Article 8.2.
2.4 “EARNED ROYALTY” is defined in Article 6.1.
2.5 “FAIR MARKET VALUE” shall mean the cash price that would be paid in an arm’s length transaction between two unrelated parties. The FAIR MARKET VALUE shall be determined by the mutual agreement of both parties based on information provided to YALE by LICENSEE, which shall include the terms or valuation upon which LICENSEE received the equivalent consideration in question.
2.6 “EFFECTIVE DATE” is defined in the introductory paragraph of this AGREEMENT.
2.7 “FIELDS OF USE” shall mean (1) pharmaceutical use in humans, (2) diagnostic applications in humans, and (3) pharmaceutical and diagnostic veterinary uses.
2.8 “IMPROVEMENT(S)” shall mean all inventions, whether patentable or not, including Xxxxxx and/or Xxxxx as named inventor(s), that are necessary to allow LICENSEE to make, have made, use, sell, have sold and import LICENSED PRODUCTS or offer SERVICES covered by the LICENSED INTELLECTUAL PROPERTY.
2.9 “IND” shall mean an investigational new drug application filed with the U.S. Food and Drug Administration prior to beginning CLINICAL TRIALS in humans in the United States or any comparable application filed with regulatory authorities in or for a country or group of countries other than the United States.
2.10 “INVENTIONS” are defined in Article 1.1.
2.11 “INVENTOR(S)” is defined in Article 1.1.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
2.12 “INSOLVENT” means that LICENSEE is insolvent as defined by U.S. Federal Bankruptcy Law.
2.13 “LICENSE” is defined in Article 3.1.
2.14 “LICENSED INFORMATION” shall mean tangible manifestations of know-how and information developed in the laboratories of Xxxxxx and/or Xxxxx that are unpublished when they are disclosed to LICENSEE relating directly to the LICENSED PATENTS (including, but not limited to, data sets, lab protocols and methods for production of ribosomes, and ribosome-specific crystallographic data analysis methodology) that are necessary to practice the LICENSED PATENTS and that are uniquely useful for this purpose and which are owned by YALE and in YALE’s possession and for which YALE has the rights to grant the LICENSE during the term of the AGREEMENT.
2.15 “LICENSED INTELLECTUAL PROPERTY” shall consist of LICENSED PATENTS and LICENSED INFORMATION.
2.16 “LICENSED PATENTS” shall mean the INVENTIONS as covered by the patent applications listed in Appendix A to this AGREEMENT, and as covered by patent application(s) to be filed, and corresponding foreign patent application(s), together with any continuations, continuations-in-part (to the extent the claims of such continuation-in-part applications are directed to subject matter specifically described in the applications listed in Appendix A), and any divisionals thereof, the resulting U.S. and foreign patents and substitute patents, any reissues or re-examinations of any such applications or patents, and any extension of the term of any such patent.
2.17 “LICENSED PRODUCTS” shall mean any products, methods, procedure, process, apparatus, kit, or component part thereof, the manufacture, use or sale of which, but for the LICENSE from YALE, would infringe any VALID CLAIM of the LICENSED PATENTS, and/or which makes material use of LICENSED INFORMATION.
2.18 “LICENSED TERRITORY” shall mean the whole world.
2.19 “NDA” shall mean a new drug application filed with the U.S. Food and Drug Administration to obtain marketing approval for a LICENSED PRODUCT in the United States or any comparable application filed with regulatory authorities in or for a country or group of countries other than the United States.
2.20 “NET SALES” shall include: gross sales revenues or FAIR MARKET VALUE of equivalent consideration from the sale, lease or other transfer of the LICENSED PRODUCTS to third parties or from SERVICES, which are received by LICENSEE or its AFFILIATES, less the following items, but only if they actually pertain to the disposition of the LICENSED PRODUCTS or SERVICES by LICENSEE or its AFFILIATES and are separately itemized:
| (a) | all discounts, credits and allowances on account of returns; |
| (b) | transportation and insurance; and |
| (c) | duties, taxes and other governmental charges. |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
No deductions shall be made for any other costs or expenses, including but not limited to, commissions to independents, agents or those on LICENSEE’s payroll or for the cost of collection.
2.21 “NET SALES” shall not include gross sales revenues or FAIR MARKET VALUE of equivalent consideration: (i) for LICENSED PRODUCTS sold, leased or otherwise transferred, or from SERVICES performed by LICENSEE, to any AFFILIATE unless such AFFILIATE is an end-user of any LICENSED PRODUCT or SERVICE and then such consideration shall be included at the average selling price charged to a third party during the same quarter; (ii) the transfer of a LICENSED PRODUCT or provision of a SERVICE to a third party without consideration in connection with the development, testing, marketing or promotion of a LICENSED PRODUCT or SERVICE; or (iii) for LICENSED PRODUCTS sold, leased or otherwise transferred to any sublicensee (but not to include sales, leases or transfers to distributors or sales agents) for incorporation into COMBINATION PRODUCTS (as defined below) and resale to end users, provided that revenue from sublicensee’s net sales is calculated in accordance with Article 4.3. However, LICENSED PRODUCTS sold, leased or otherwise transferred to a sublicensee for resale to end users shall be included in the calculation of NET SALES as defined in this Article 2.20, and shall be excluded from the calculation of net sales by sublicensees in Article 4.3. If the aforementioned sublicensee is an end-user of any LICENSED PRODUCT or SERVICE then such consideration shall be included in the calculation of NET SALES as defined in Article 2.20 at the average selling price charged to a third party during the same quarter.
2.22 “REASONABLE COMMERCIAL EFFORTS” shall mean reasonably documented efforts and resources commonly used in the research-based pharmaceutical industry for a company in a similar position as LICENSEE at such time for a product at a similar stage in its development or product life, as applicable, of similar market potential taking into account efficacy, safety, the competitiveness of alternative products in the marketplace or under development, the patent and other proprietary position of the product, and the likelihood of regulatory approval, the profitability of the product and alternative products and other relevant commercial or scientific factors.
2.23 “SERVICES” shall mean services performed for a third party using LICENSED INTELLECTUAL PROPERTY under a stand-alone contract having a fixed rate, time and materials, or other similar fee arrangement, where the third party does not receive any sublicense or marketing rights with respect to the LICENSED INTELLECTUAL PROPERTY.
2.24 “VALID CLAIM” shall mean a pending, issued or unexpired claim included among the LICENSED INTELLECTUAL PROPERTY so long as such claim shall not have been irrevocably abandoned or held to be invalid in an unappealable decision of a court or other authority of competent jurisdiction.
2.25 “FUNDING DATE” shall mean the date on which LICENSEE receives initial financing of at least Two Million US Dollars ($2,000,000), including any bridge loans.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
ARTICLE 3 LICENSE GRANT AND TERM
3.1 Subject to all the terms and conditions of this AGREEMENT, YALE hereby grants to LICENSEE an exclusive worldwide license, with the right to grant sublicenses, to practice LICENSED PATENTS and use LICENSED INFORMATION for purposes of research and development of products and to make, have made, use, sell, have sold and import LICENSED PRODUCTS or SERVICES in the FIELDS OF USE (the “LICENSE”).
3.2 The LICENSE is expressly made subject to a non-exclusive, irrevocable, royalty-free license already granted to the U.S. Government for OCR 1079 and OCR 1220, a copy of which is attached as Appendix B and incorporated into this AGREEMENT
3.3 The LICENSE is expressly made subject to YALE’ s and HHMI’s reservation of the right to make, use and practice the LICENSED INTELLECTUAL PROPERTY and any IMPROVEMENTS for their own non-commercial purposes. Nothing in this AGREEMENT shall be construed to grant by implication estoppel or otherwise any licenses under patents of YALE other than the LICENSED INTELLECTUAL PROPERTY.
3.4 Unless terminated earlier as provided in Article 13, the term of the LICENSE for the LICENSED PATENTS shall commence on the EFFECTIVE DATE and shall be for the life of the last to expire of the LICENSED PATENTS.
3.5 For a period of three (3) years from the EFFECTIVE DATE of the AGREEMENT, YALE shall grant LICENSEE a one-hundred and twenty (120) day option to negotiate to add IMPROVEMENT(S) in the FIELDS OF USE and relating to the atomic structures of ribosomes or ribosomal subunits and/or other factors interacting with ribosomes, to the definition of LICENSED PATENTS by amendment to the AGREEMENT, on non-equity based financial terms to be negotiated. If at the end of the one hundred twenty (120) day option period the parties are unable to reach agreement, despite the good faith efforts of both parties, then neither party shall be under any further obligation to the other with respect to the particular IMPROVEMENT(S). YALE shall provide LICENSEE with notice of invention disclosures for IMPROVEMENTS within ninety (90) days of receipt of the invention disclosure by YALE’s Office of Cooperative Research.
ARTICLE 4 SUBLICENSES
4.1 YALE hereby grants to LICENSEE the right to sublicense (and any sublicensee may be granted the right to further sublicense at any tier) to unaffiliated third parties under the LICENSED INTELLECTUAL PROPERTY the right to practice the LICENSED PATENTS and use LICENSED INFORMATION for purposes of research and development of products and to make, have made, use, sell, have sold and import LICENSED PRODUCTS and/or SERVICES in the FIELDS OF USE, provided this AGREEMENT is in effect.
4.2 Any sublicense granted by LICENSEE or its sublicensees (or any further tiers of sublicensees) shall include substantially the same Definitions, and provisions on Confidentiality and Publicity, Reporting Requirements, Indemnification, Insurance and Warranties, Patent Notices and Use of YALE’s Name, as are agreed to in this AGREEMENT, and shall include due diligence provisions.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
4.3 For purposes of this Article: (i) sublicensees’ “net sales” shall have a parallel meaning to the definition of LICENSEE’S NET SALES in Article 2.20; and (ii) “Sublicense Milestones” shall mean all milestone payments LICENSEE receives from sublicensees in consideration for sublicense rights to the LICENSED INTELLECTUAL PROPERTY, but shall specifically exclude R&D reimbursements, sponsored research payments, and full-time equivalent scientist support. LICENSEE shall pay to YALE [***] percent ([***]%) of all non-milestone payments LICENSEE receives from sublicensees in consideration for sublicense rights to the LICENSED INTELLECTUAL PROPERTY, including sublicense issue fees, maintenance fees, royalties on sublicensee net sales (excluding sublicensees’ net sales of LICENSED PRODUCTS sold, leased or otherwise transferred by LICENSEE or its AFFILIATES to sublicensees as further defined in Article 2.20 above) and any premium paid for equity investments, but excluding research support, non-premium equity investments and loans. LICENSEE shall pay to YALE [***] percent ([***]%) of all Sublicense Milestones during the first two years after the EFFECTIVE DATE, unless LICENSEE has included in the license to the sublicensee new patent claim(s) or rights to patent applications not included in the LICENSED INTELLECTUAL PROPERTY and owned by Rib-X in such sublicense, in which case the percentage of Sublicense Milestones due YALE shall be [***] percent ([***]%); and [***] percent ([***]%) of all Sublicense Milestones after the second anniversary of the EFFECTIVE DATE. Notwithstanding the foregoing, under no circumstances, during any three-year consecutive period (the first period to be measured beginning on the date of LICENSEE’s first receipt of sublicensing revenue and ending on the third anniversary of that date, and then on a rolling basis thereafter), shall YALE receive cumulative sublicense payments of less than [***] percent ([***]%) of sublicensees’ net sales (“Sublicensing Minimum”). Within ninety (90) days after the end of each calendar year, LICENSEE shall provide an accounting from the EFFECTIVE DATE of the AGREEMENT of (i) cumulative payments to YALE from sublicensing of the LICENSED INTELLECTUAL PROPERTY and (ii) cumulative sublicensee net sales; provided that LICENSEE shall not be obligated to provide information regarding a particular sublicensee’s net sales until ten (10) days after LICENSEE’s receipt of any such information related to that sublicensee. If the cumulative sublicense payments to YALE over each consecutive three (3) year period beginning on the third anniversary of the date of LICENSEE’s first receipt of sublicense payments are greater than [***] percent ([***]%) of cumulative sublicensee net sales, no Sublicensing Minimum shall be due. If, however, the cumulative sublicense payments to YALE over the aforementioned three (3) year period are less than [***] percent ([***]%) of cumulative sublicensee net sales, LICENSEE shall pay YALE the difference between the cumulative payments and the Sublicensing Minimum for that three year period. Such payments by LICENSEE of the differential shall be included as cumulative sublicensing revenue payments to YALE in the accounting for future years to determine whether additional payments are due YALE to meet the Sublicensing Minimum.
4.4 LICENSEE agrees that it has sole responsibility to promptly:
(a) provide YALE with a copy of each sublicense granted by LICENSEE under this AGREEMENT and any amendments to such sublicense or termination thereof, provided that all material disclosed to YALE in relation hereto shall be considered as LICENSEE’s
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
CONFIDENTIAL INFORMATION and that LICENSEE may redact information related to the specific research and development plans of sublicensees to the extent that it is not necessary for YALE to monitor compliance with the terms and conditions of this AGREEMENT;
(b) guarantee to pay all payments due YALE from LICENSEE from sublicenses in accordance with Article 4.3; and
(c) summarize and deliver copies of all reports due to LICENSEE from sublicensees relating to the performance of this AGREEMENT.
ARTICLE 5 LICENSE ISSUE FEE AND MILESTONE PAYMENTS
5.1 In partial consideration for License rights granted hereunder to LICENSED INTELLECTUAL PROPERTY, LICENSEE will issue to YALE on the EFFECTIVE DATE 344,595 shares of LICENSEE’s common stock, $.001 par value per share, equal to 7.5% of all outstanding equity in LICENSEE pre-money and before allocation of equity for employee stock options. Such stock shall be delivered to YALE within ten (10) days after the EFFECTIVE DATE, subject to YALE executing the Stockholders Agreement.
5.2 LICENSEE shall pay the following milestone payments to YALE for the first three (3) LICENSED PRODUCTS developed by LICENSEE (and not by any of its sublicensees) in each of the FIELDS OF USE:
| [***]: | $[***] | |||
| [***]: | $[***] |
For purposes of clarification, in the case of development of LICENSED PRODUCTS by sublicensees, LICENSEE shall pay YALE the appropriate percentage of Sublicense Milestone payments made to LICENSEE as stipulated in Article 4.3 above; provided that, in the event that the aggregate of such payments for a given LICENSED PRODUCT developed by a sublicensee is less than the sum of all milestone payments listed in this Article 5.2 above that would have been paid by LICENSEE as of the date of NDA approval or termination of development of such LICENSED PRODUCT if such LICENSED PRODUCT had been developed by LICENSEE, then LICENSEE shall pay YALE the difference promptly following the date of NDA approval or termination of development of such LICENSED PRODUCT.
Furthermore, in the event that LICENSEE enters into a sublicense that does not provide for payments to LICENSEE of Sublicense Milestones, then, in lieu of any other payment owed pursuant to this Section 5.2 of this AGREEMENT, LICENSEE shall pay to YALE the sum of [***] US Dollars ($[***]) promptly upon approval of an NDA relating to a LICENSED PRODUCT by such sublicensee, and such LICENSED PRODUCT shall count toward the nine (9) LICENSED PRODUCTS for which milestone payments are owed by LICENSEE pursuant to this Section 5.2.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
| 5.3 | (a) No particular Milestone payment(s) shall be due for a LICENSED PRODUCT that is a replacement product for the same indication for a LICENSED PRODUCT that has been abandoned for which such particular Milestone payment(s) has already been paid. |
(b) No additional milestone payment(s) shall be due for a given LICENSED PRODUCT for which a particular milestone payment(s) has already been paid unless that LICENSED PRODUCT is further developed through CLINICAL TRIALS for a different indication(s). Each LICENSED PRODUCT(S) for which such additional milestone payments are paid for a different indication(s) (but for which a second $[***] is not due for the “Approval of IND” milestone) shall be considered one of the nine maximum LICENSED PRODUCTS to which Article 5.2 applies. If a LICENSED PRODUCT is approved for label extension without an additional CLINICAL TRIAL, no additional milestone payments shall be due, and LICENSED PRODUCTS that receive IND, diagnostic regulatory, NDA (or foreign equivalent of all) approval in different countries for the same indication shall not trigger multiple milestone payments for that particular LICENSED PRODUCT.
5.4 Neither the equity provision of Article 5.1 nor the milestone payments of Article 5.2 shall be credited against EARNED ROYALTIES payable under Article 6.
ARTICLE 6 ROYALTIES
6.1 As partial consideration for the LICENSE granted under this AGREEMENT LICENSEE shall pay to YALE an EARNED ROYALTY for the sale of LICENSED PRODUCTS and SERVICES by LICENSEE or its AFFILIATES of either (but never both for the same LICENSED PRODUCT or SERVICE):
(i) [***]% on any NET SALES of LICENSED PRODUCTS or SERVICES covered by a VALID CLAIM of the LICENSED PATENTS in the country where made, used or sold; or
(ii) [***]% on any NET SALES of LICENSED PRODUCTS or SERVICES not covered by any VALID CLAIM of the LICENSED PATENTS in the country where made, used or sold, but that make material use of LICENSED INFORMATION.
No multiple amounts shall be payable because any LICENSED PRODUCT or SERVICE, its manufacture, use, lease, sale or import are or shall be covered by more than one patent application or patent and/or more than one article of LICENSED INFORMATION within the LICENSED INTELLECTUAL PROPERTY.
6.2 LICENSEE shall pay all royalties accruing to YALE within sixty (60) days from the end of each calendar quarter (March 31, June 30, September 30 and December 31) in which NET SALES occur.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
6.3 During the term of this AGREEMENT, LICENSEE agrees to pay an annual License Maintenance Fee (“LMF”) of [***] US Dollars ($[***]) per year for the first two consecutive years, and [***] US Dollars ($[***]) per year for the next three consecutive years thereafter, starting two years after the FUNDING DATE, creditable to all payments due to YALE under this AGREEMENT, except not to be creditable against EARNED ROYALTIES or patent expense reimbursements due under Article 10 or late fees that may be due as per Article 6.5.
6.4 During the term of this AGREEMENT, LICENSEE agrees to pay YALE annual Minimum Royalty Payments (“MRP”), commencing after the calendar year in which LICENSEE first generates [***] US Dollars $[***] in annual NET SALES. The MRP shall be in the amount of [***] US Dollars ($[***]) per calendar year. Within sixty (60) days after the end of the calendar year, LICENSEE shall calculate EARNED ROYALTIES paid for the previous calendar year and shall pay YALE as MRP any deficit between [***] US Dollars ($[***]) and such EARNED ROYALTIES. YALE shall fully credit each MRP made against any EARNED ROYALTIES payable by LICENSEE in the same calendar year and future calendar years.
6.5 All royalties and other payments due under this AGREEMENT, shall be paid to YALE in U.S. Dollars. In the event that conversion from foreign currency is required in calculating a payment under this AGREEMENT, the exchange rate used shall be the Interbank rate quoted by Citibank at the end of the last business day of the quarter in which the EARNED ROYALTY was earned. If overdue, the royalties and any other payments due under this AGREEMENT, shall bear interest until payment at a per annum rate [***] percent ([***]%) above the prime rate in effect at Citibank on the due date. The payment of such interest shall not foreclose YALE from exercising any other right it may have as a consequence of the lateness of any payment.
6.6 Combination Product. For purposes of calculating running royalties, in the event that a LICENSED PRODUCT includes both component(s) that would infringe any VALID CLAIM of the LICENSED PATENTS or make material use of LICENSED INFORMATION (“LICENSED COMPONENT”) and a component which is diagnostically useable or therapeutically active alone or in a combination which does not require the Patented Component, and such component does not infringe any VALID CLAIM of the LICENSED PATENTS and does not make material use of LICENSED INFORMATION (“OTHER COMPONENT”), then NET SALES of the Combination Product shall be calculated using one of the following methods:
(i) By multiplying the NET SALES of the Combination Product during the applicable royalty ACCOUNTING PERIOD (“ACCOUNTING PERIOD”) by a fraction, the numerator of which is the aggregate gross selling price of the LICENSED COMPONENT(s) contained in the Combination Product if sold separately, and the denominator of which is the sum of the gross selling price of both the LICENSED COMPONENT(s) and the OTHER COMPONENT(s) contained in the Combination Product if sold separately; or
(ii) In the event that no such separate sales are made of the LICENSED COMPONENT(s) or the OTHER COMPONENT(s) during the applicable ACCOUNTING PERIOD, NET SALES for purposes of determining royalties payable hereunder shall be calculated by multiplying the NET SALES of the Combination Product by a fraction, the numerator of which is the fully allocated production cost of the LICENSED COMPONENT(s) and the denominator of which is the sum of the fully allocated production costs of the LICENSED COMPONENT(s) and the OTHER COMPONENT(s) contained in the Combination Product. Such fully allocated costs shall be determined by using LICENSEE’s standard accounting procedures, which procedures must conform to standard cost accounting procedures.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
6.7 If the total payments paid by LICENSEE to YALE and to a third party(s) in consideration for license rights to enable LICENSEE to practice the LICENSED INTELLECTUAL PROPERTY and make, have made, use, sell, have sold and import LICENSED PRODUCTS or SERVICES in the FIELDS OF USE at any time exceed a commercially reasonable percentage of revenue, which for purposes of this agreement shall be at least [***] ([***]%), LICENSEE shall promptly provide written notice to YALE and YALE and LICENSEE intend to convene to discuss the issue. Nothing in this Article 6.7 shall obligate YALE or LICENSEE to do more than discuss the issue during the period of thirty (30) days following receipt of written notice from LICENSEE; nor shall this Article obligate the parties to alter any term of this agreement unless such alteration is acceptable to each party in its discretion.
ARTICLE 7 DUE DILIGENCE
7.1 LICENSEE shall have designed a PLAN for developing and commercializing the LICENSED INTELLECTUAL PROPERTY. Such PLAN shall include, if appropriate, information related to research and development, testing, government approval, manufacturing, marketing and sale or lease of LICENSED PRODUCTS and/or SERVICES. Prior to executing this AGREEMENT, YALE has approved the PLAN, a copy of which is incorporated into this AGREEMENT as Appendix “C”.
7.2 LICENSEE shall use REASONABLE COMMERCIAL EFFORTS, within ninety (90) days after the EFFECTIVE DATE of this AGREEMENT, to begin implementation of the PLAN at its sole expense.
7.3 LICENSEE shall provide YALE with an updated and revised copy of the PLAN upon each anniversary date of the EFFECTIVE DATE, which shall be substituted into this AGREEMENT as Appendix “C,” and which shall indicate progress and problems to date in commercialization of the LICENSED INTELLECTUAL PROPERTY. YALE shall allow LICENSEE to make modifications to the PLAN from time to time after YALE has approved the PLAN under Article 7.1 and before the annually revised PLAN is due. LICENSEE shall provide YALE with a written copy of any such modifications.
7.4 If at any time LICENSEE abandons or suspends its intent to develop, employ or otherwise utilize the LICENSED INTELLECTUAL PROPERTY for a period exceeding one hundred eighty (180) days, LICENSEE shall immediately notify YALE giving reasons and a statement of its intended actions. If such abandonment or suspension is not cured, if capable of being cured within sixty (60) days, after written notice from YALE, then YALE shall be entitled to terminate this AGREEMENT in accordance with Article 13.5 (b).
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
7.5 (a) LICENSEE must meet the following due diligence milestones:
(i) the FUNDING DATE will be within one year of the EFFECTIVE DATE of this AGREEMENT; and
(ii) a cumulative total of [***] US Dollars ($[***]) will be raised within two years of the EFFECTIVE DATE of the AGREEMENT, including any bridge loans and the initial financing received on or before the FUNDING DATE; and
(iii) LICENSEE will provide YALE with a copy of LICENSEE’s written PLAN for commercialization and regulatory filing of diagnostic tests or kits in the U.S. by LICENSEE or a sublicensee within one (1) year of the EFFECTIVE DATE of the AGREEMENT. After reviewing the PLAN, YALE and LICENSEE will meet to mutually agree on acceptable due diligence milestones for diagnostic product development; and
(iv) an IND will be filed by LICENSEE or a sublicensee for a LICENSED PRODUCT or a product developed using the LICENSED PATENTS or LICENSED INFORMATION within six (6) years from the EFFECTIVE DATE of the AGREEMENT.
(b) YALE shall be entitled to terminate this AGREEMENT in accordance with Article 13.5 (b) in the event of LICENSEE’ s material breach of subsections (i), (ii) or (iv) of Article 7.5(a) above. In the event of LICENSEE’ s material breach of subsection (iii), or of any subsequently agreed due diligence milestones for diagnostic tests, YALE shall be entitled to terminate the LICENSE in the FIELD OF USE of diagnostic applications in humans and this AGREEMENT shall otherwise remain in effect.
ARTICLE 8 CONFIDENTIALITY, PUBLICATION AND PUBLICITY
8.1 YALE and LICENSEE each recognize that the other’s CONFIDENTIAL INFORMATION constitutes highly valuable information. Subject to the parties’ rights and obligations pursuant to this AGREEMENT, including without limitation Article 10.5, YALE and LICENSEE agree that during the term of this AGREEMENT and for five years thereafter, they:
(a) will keep confidential and will cause their AFFILIATES to keep confidential, the other’s CONFIDENTIAL INFORMATION, by taking whatever action it would take to preserve the confidentiality of its own CONFIDENTIAL INFORMATION, but no less than reasonable efforts; and
(b) will only disclose that part of the other’s CONFIDENTIAL INFORMATION that is necessary for those officers, employees or agents who need to know to carry out its responsibilities under this AGREEMENT; and
(c) will not disclose the other’s CONFIDENTIAL INFORMATION to any third parties (except that LICENSEE may disclose in connection with sublicensing, raising funding and technical development activities under terms of confidentiality at least as restrictive as those set forth in this AGREEMENT) under any circumstance without advance, written permission from the other party; and
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
(d) will, within sixty (60) days of the request of the other party upon termination of this AGREEMENT, return all the CONFIDENTIAL INFORMATION disclosed to the other party pursuant to this AGREEMENT except for one copy, which may be retained by the Recipient for monitoring compliance with Article 8.
8.2 The obligations of confidentiality described above shall not pertain to that part of the CONFIDENTIAL INFORMATION which:
(a) was known to the Recipient prior to the disclosure by the Disclosing Party; or
(b) is or becomes publicly known through no fault or omission attributable to the Recipient; or
(c) is rightfully given to the Recipient from sources independent of the Disclosing Party; or
(d) is required to be disclosed by law in the opinion of Recipient’s attorney, but only after prompt written notice to the owner of the CONFIDENTIAL INFORMATION and opportunity to seek a protective order or to agree to such disclosure.
8.3 Notwithstanding Articles 8.1 and 8.2 above, YALE and HHMI investigators shall retain the right to pursue research, including non-commercial academic collaborations, with regard to the LICENSED INTELLECTUAL PROPERTY and may publish the results of their own non-commercial research and collaboration efforts without restriction. Should YALE or HHMI investigator desire to make a public disclosure, in writing or by oral presentation related directly to the LICENSED INTELLECTUAL PROPERTY, YALE shall provide LICENSEE with a copy of any manuscript or presentation no less than thirty (30) days before the date of publication or presentation (the “Review Period”). In the event that LICENSEE determines that such publication or disclosure contains proprietary information of LICENSEE or any LICENSED INTELLECTUAL PROPERTY or IMPROVEMENT(S), LICENSEE may request that YALE either (a) delay the publication or other public disclosure for up to thirty (30) additional days after a written request by LICENSEE that is received by YALE within the Review Period, as necessary to preserve U.S. or foreign patent rights for LICENSED INTELLECTUAL PROPERTY or IMPROVEMENT(S) or (b) delete any LICENSEE proprietary information from the proposed publication or public disclosure.
8.4 Publicity. Except as required by law, neither party shall issue any press release or make any public disclosure, announcement, comment or statement concerning the existence or the terms and conditions of this AGREEMENT, the PLAN or the transactions contemplated hereby without the prior written consent of the other party, such consent not to be unreasonably withheld or delayed, except as may be necessary or appropriate in the reasonable opinion of the disclosing party to comply with applicable laws or regulations or the rules or policies of any securities exchange on which such party’s securities are traded in which event the party shall give the other party as much notice as is reasonably possible under the circumstances and shall, in such event, seek to limit the disclosure to the extent reasonably possible consistent with the foregoing.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
ARTICLE 9 REPORTS, RECORDS AND INSPECTIONS
9.1 LICENSEE shall, within sixty (60) days after the calendar year in which NET SALES first occur, and within sixty (60) days after each calendar quarter (March 31, June 30, September 30 and December 31) thereafter, provide YALE with a written report detailing all NET SALES, if any, made of LICENSED PRODUCTS or SERVICES during the preceding calendar quarter and calculating the payments due pursuant to Article 6; provided, that LICENSEE shall not be obligated to provide information regarding a particular sublicensee’s net sales until ten (10) days after LICENSEE’ s receipt of any such information from that sublicensee. Each such report shall be signed by an officer of LICENSEE (or the officer’s designee), and must include:
(a) the number of LICENSED PRODUCTS and SERVICES manufactured and sold by LICENSEE and by all sublicensees, and
(b) the prices of the LICENSED PRODUCTS and SERVICES sold, and
(c) any deductions made, and
(d) total royalties or other payment due, and
(e) names and addresses of all sublicensees.
9.2 LICENSEE shall, and shall require its sublicensees, to keep and maintain complete and accurate records and books containing an accurate accounting of all data using methods conforming to good accounting principles as applied to a similar company similarly situated, to enable verification of EARNED ROYALTIES and other payments under this AGREEMENT. LICENSEE shall preserve such books and records for three (3) years after the sales recorded were actually made. Such books and records shall be open to inspection by YALE or an independent certified public accountant, at YALE’s expense, at the place or places where such records are customarily kept, during normal business hours upon ten (10) days prior written notice, for the purpose of verifying the accuracy of the reports and computations rendered by LICENSEE. All reports provided by LICENSEE under Article 9.1, and all information learned in the course of any audit or inspection, shall be deemed to be CONFIDENTIAL INFORMATION, except to the extent that it is necessary for YALE to reveal the information to its legal and/or financial advisors in order to enforce any rights it may have pursuant to this AGREEMENT or if such disclosure is required by law. The failure of YALE to request verification of any EARNED ROYALTIES after the three (3) year period shall be considered acceptance by YALE of the accuracy of the EARNED ROYALTY calculations, and LICENSEE shall have no obligation to maintain any records pertaining to such report or statement beyond such three (3) year period, unless they have been disputed by YALE within such three (3) year period.
ARTICLE 10 PATENT PROTECTION
10.1 The patent applications contained in the LICENSED INTELLECTUAL PROPERTY and the dates they were filed are listed in Appendix “A.” It is understood that as of the EFFECTIVE DATE, no patent protection exists in the U.S. or any foreign country but only the potential to realize the same.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
10.2 LICENSEE shall be responsible for all costs of filing, prosecution and maintenance of all U.S. patent applications contained in the LICENSED INTELLECTUAL PROPERTY, incurred prior to and after the EFFECTIVE DATE. Any and all such U.S. patent applications, and resulting patents shall remain the property of YALE.
10.3 LICENSEE shall be responsible for all costs of filing, prosecution and maintenance of all foreign patent applications, and patents contained in the LICENSED INTELLECTUAL PROPERTY, incurred prior to and after the EFFECTIVE DATE, in the countries selected by LICENSEE or YALE and agreed to by LICENSEE. All such applications or patents shall remain the property of YALE.
10.4 The costs mentioned in Articles 10.2, 10.3 and 10.5 shall include, but are not limited to any taxes, annuities, working fees, maintenance fees, and renewal and extension charges. Within thirty (30) days of the FUNDING DATE, and upon receipt of an itemized invoice, payment of such costs incurred prior to the EFFECTIVE DATE of this AGREEMENT shall be made by direct reimbursement to YALE. YALE estimates these past costs to be approximately $46,000.00. LICENSEE shall make payment of costs incurred after the EFFECTIVE DATE of this AGREEMENT directly to Patent Counsel. LICENSEE shall make payment directly to the appropriate party within thirty (30) days of receiving their invoice.
10.5 After the EFFECTIVE DATE, LICENSEE will be primarily responsible for the preparation, filing, prosecution and maintenance of all patent applications and patents covering the LICENSED INTELLECTUAL PROPERTY. Such patent applications and patents shall be prepared, prosecuted, filed and maintained by LICENSEE’s counsel of choice, currently Xxxxx, Xxxxxxx & Xxxxxxxxx, LLP, unless otherwise agreed by YALE and LICENSEE. LICENSEE shall instruct patent counsel to keep both YALE and LICENSEE fully informed of the progress of all patent applications and patents, and to give both YALE and LICENSEE reasonable opportunity to comment on the type and scope of useful claims and the nature of supporting disclosures. LICENSEE shall retain substantive control of and responsibility for direct preparation, prosecution, filing and maintenance; however, any submission that may materially adversely affect the scope of the LICENSED PATENTS, or the claims contained therein, shall be subject to the prior written approval of YALE, such approval not to be unreasonably withheld or delayed. YALE agrees to cooperate with LICENSEE and to use reasonable efforts to obtain the cooperation of its employees, including the INVENTOR(S), as might reasonably be requested in connection with the preparation, filing and prosecution of such patent applications. LICENSEE may not abandon any patent application, pending claim, issued patent or issued patent claim under the LICENSED INTELLECTUAL PROPERTY without ninety (90) days prior written notice to YALE to allow YALE to assume the prosecution of such patent application at its own expense. If LICENSEE does not agree to pay or fails to pay the expenses of filing, prosecuting or maintaining a patent application in the United States or any country outside the United States or if LICENSEE elects to abandon any patent application in any country, then LICENSEE’s rights under this AGREEMENT may be terminated by YALE only with respect to that patent or patent application in that country after sixty (60) days’ notice from YALE, if not cured by LICENSEE within said sixty (60) day notice period. On the EFFECTIVE DATE, LICENSEE will take over payment of all such costs invoiced by Xxxxx, Xxxxxxx & Xxxxxxxxx, LLP. The party directing prosecution shall have no liability to the other party for damages, whether direct, indirect or incidental, consequential or otherwise, allegedly arising
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
from its good faith decisions, actions and omissions in connection with such prosecution, except as provided in this Article 10. LICENSEE will have the right, but not the obligation, to defend and enforce the LICENSED INTELLECTUAL PROPERTY in any interference proceedings.
10.6 LICENSEE shall apply, and shall require sublicensees to apply, the patent marking notices required by the law of any country where LICENSED PRODUCTS and SERVICES are made, sold or used, and in accordance with the applicable patent laws of that country.
ARTICLE 11 INFRINGEMENT AND LITIGATION
11.1 Each party shall promptly notify the other in writing in the event that it obtains knowledge of infringing activity by third parties, or issued or threatened with an infringement suit in any country as a result of activities that concern the LICENSED INTELLECTUAL PROPERTY and shall supply the other party with documentation of the infringing activities that it possesses.
11.2 During the term of this AGREEMENT,
(a) Subject to sub-paragraph (d) below, LICENSEE shall have the first right, but not the obligation, to enforce the LICENSED INTELLECTUAL PROPERTY against infringement or interference by third parties. This right includes, without limitation, entering into settlement negotiations or bringing any legal action for infringement and defending any counterclaim of invalidity or action of a third party for declaratory judgment for non-infringement or non-interference. LICENSEE may use the name of YALE as party plaintiff for such purposes. LICENSEE shall have sole control over such settlement negotiations and litigation and may, in its sole discretion, settle such suits solely in its own name and solely at its own expense and through counsel of its own selection, provided that LICENSEE may not enter into a settlement that relates to the practice or use of the LICENSED INTELLECTUAL PROPERTY, without the prior written consent of YALE, which consent shall not be unreasonably withheld or delayed. LICENSEE shall bear the expense of such legal actions and shall obtain all the benefits from it. However, YALE shall recover a percentage of any excess recovery over LICENSEE’s out-of-pocket expenses (including reasonable attorneys’ and experts’ fees and court costs) equal to the EARNED ROYALTY percent set forth in Article 6.1.
(b) Except for providing reasonable assistance, at the request and expense of LICENSEE, YALE shall have no obligation regarding the legal actions described in Article 11.2(a) unless (i) required to participate by law or (ii) if YALE is named as a party plaintiff, in which cases YALE will cooperate at the request and expense of LICENSEE. However, YALE shall have the right to participate in any such action through its own counsel and at its own expense.
(c) In the event LICENSEE fails to initiate or participate in the actions described in Article 11.2(a) within six (6) months of notification of an infringing activity, YALE shall have the right to initiate such legal action to enforce the LICENSED INTELLECTUAL PROPERTY at YALE’s expense. YALE shall have sole control over such litigation and may, in its sole discretion, settle such suits solely in its own name and solely at its own expense and through counsel of its own selection. YALE shall bear the expense of such legal actions and shall obtain all the benefits from it.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
(d) LICENSEE shall have the sole right in accordance with the terms and conditions herein to sublicense any alleged infringer for future use of the LICENSED INTELLECTUAL PROPERTY.
11.3 If LICENSEE receives notice of a claim or action by a third party alleging infringement of such third party’s rights in connection with the manufacture, use, sale or import of the LICENSED PRODUCTS, SERVICES and/or LICENSED INTELLECTUAL PROPERTY by LICENSEE or it AFFILIATES, permitted assignees or sublicensees, LICENSEE shall have the right, but not the obligation, to enter into settlement or license negotiations or to conduct the legal defense and, to enter into any disposition (including a license) with respect thereto. All costs of LICENSEE’s defense, including its attorneys’ fees and court costs, and any damages awarded or amounts paid in settlement in any such claim or action shall be the sole responsibility of LICENSEE. YALE shall reasonably cooperate with LICENSEE in its settlement or defense of such infringement claim or Action at LICENSEE’s sole expense.
11.4 In the event LICENSEE is permanently enjoined from exercising its license right granted under this AGREEMENT pursuant to an infringement action brought by a third party, or if both LICENSEE and YALE elect not to undertake the defense or settlement of a suit alleging infringement for a period of six (6) months from notice of such suit, then the LICENSEE shall have the right to terminate this AGREEMENT in the country where the suit was filed with respect to the particular LICENSED PATENT(s) following thirty (30) days written notice to YALE and in accordance with the terms of Article 13.
11.5 If litigation, settlement and/or licensing in connection with the defense or enforcement of the LICENSED INTELLECTUAL PROPERTY by LICENSEE pursuant to this Article 11 at any time exceeds a commercially reasonable percentage of revenue, which for purposes of this agreement shall be at least [***] percent ([***]%), LICENSEE shall promptly provide written notice to YALE and YALE and LICENSEE intend to convene to discuss the issue. Nothing in this Article 11.5 shall obligate YALE or LICENSEE to do more than discuss the issue during the period of thirty (30) days following receipt of written notice from LICENSEE; nor shall this Article obligate the parties to alter any term of this agreement unless such alteration is acceptable to each party in its discretion.
ARTICLE 12 USE OF YALE’S AND LICENSEE’S NAMES
| 12.1 | (a) Except as required by law and reasonable use by LICENSEE in raising funding and market development initiatives in confidential documents, LICENSEE shall not use the name “Yale” or “Yale University”, nor any trademark or adaptation of it, nor the names of any of its employees, for any purpose without prior written consent obtained from YALE and/or said employee in each instance, such consent not to be unreasonably withheld or delayed, except that LICENSEE may state that it is licensed by YALE under one or more of the patents and/or applications comprising the LICENSED INTELLECTUAL PROPERTY. LICENSEE acknowledges that under HHMI policy, |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
LICENSEE may not use the name of HHMI or of any HHMI employee (including Xx. Xxxxxx Xxxxxx) in a manner that reasonably could constitute an endorsement of a commercial product or service; but that use for other purposes, even if commercially motivated, is permitted provided that (1) the use is limited to accurately reporting factual events or occurrences, and (2) any reference to the name of HHMI or any HHMI employees in press releases or similar materials intended for public release is approved by HHMI in advance. Notwithstanding the foregoing, LICENSEE may identify HHMI employees (by name and affiliation) in its press releases as inventors of the technology licensed hereunder, without obtaining advance approval from HHMI, if no further information about or quotes from such HHMI employees are included. No advance approval is needed for use of the name of HHMI or any HHMI employee in materials filed with regulatory authorities.
(b) Except as required by law, YALE shall not use LICENSEE’s name, nor any trademark or adaptation of it, nor the names of any of its employees, for any purpose without prior written consent obtained from LICENSEE or said employee in each instance, except that YALE may state that LICENSEE has licensed one or more YALE patents and/or applications.
ARTICLE 13 TERMINATION
13.1 Upon termination of this AGREEMENT, for any reason, all rights and licenses granted to LICENSEE under the terms of this AGREEMENT are terminated, except that, in the event the AGREEMENT terminates because the last of the LICENSED PATENTS has expired, the exclusive LICENSE to LICENSED INFORMATION shall convert to a fully paid-up, irrevocable, nontransferable, perpetual, non-exclusive license, without the right to sublicense. Upon termination of this AGREEMENT for any reason, each sublicensee in good standing shall have a right to obtain a license directly from YALE on the same terms and conditions as those embodied by their sublicense from LICENSEE, provided that YALE’ s obligations shall be no greater than its obligations in this AGREEMENT. Upon such termination LICENSEE shall cease to develop, employ or otherwise utilize the LICENSED PATENTS; except that so long as termination shall not have been due to a material breach by LICENSEE, LICENSEE and any sublicensee may, after the effective date of termination, complete all outstanding contracts for SERVICES, sell inventory and complete LICENSED PRODUCTS in the process of manufacture at the time of termination and sell the same within one year of the effective date of termination, provided that LICENSEE shall pay EARNED ROYALTIES and submit required reports. Within sixty (60) days of the effective date of termination LICENSEE shall:
(a) return to YALE or destroy (with written certification of destruction) all CONFIDENTIAL INFORMATION relating to the LICENSED PATENTS;
(b) send YALE the last report due; and
(c) remit all payments to YALE incurred up to the effective date of termination.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
13.2 Termination of this AGREEMENT shall not affect any rights or obligations accrued prior to the effective date of such termination and specifically LICENSEE’s obligation to pay all royalties and other payments specified by Articles 4, 5, 6 and 10 up to the effective date of termination and thereafter pursuant to Article 13.1 continuing activities, if any. Articles 2, 3.4, and 8, the preservation and inspection obligations of Article 9, Article 11, 13.1, 13.2 and the indemnification obligations of Article 14 and HHMI’ s third-party beneficiary provision of Article 17.5 all survive any such termination. Claims giving rise to indemnification may arise after the term or termination of the License granted herein.
13.3 The rights provided in this Article 13 shall be in addition and without prejudice to any other rights which the parties may have with respect to any breach or violations of the provisions of this AGREEMENT.
13.4 Waiver by either party of one or more defaults or breaches shall not deprive such party of the right to terminate because of any subsequent default or breach.
13.5 YALE may terminate this AGREEMENT, and such termination shall be automatically effective at the end of sixty (60) days after written notice, in the event LICENSEE:
(a) fails to make any material payment whatsoever due and payable pursuant to this AGREEMENT unless LICENSEE shall make all such payments within said sixty (60) day period; or
(b) commits a material breach of any other obligation of this AGREEMENT which is not cured (if capable of being cured) within the sixty (60) day period set by the notice; or
(c) fails to comply with the insurance requirements of Articles 14.4 and 14.5; or
(d) shall cease to carry on its business, becomes INSOLVENT or, a petition in bankruptcy is filed against LICENSEE and is consented to, acquiesced in or remains undismissed for 60 days; or makes a general assignment for the benefit of creditors, or a receiver is appointed for LICENSEE then YALE may terminate this AGREEMENT upon sixty (60) days notice to LICENSEE.
13.6 LICENSEE shall have the right to terminate this AGREEMENT:
(a) at any time upon ninety (90) days notice to YALE, and upon payment of all amounts due YALE throughout the effective date of termination. Upon written notice from YALE, LICENSEE shall provide YALE with copies of any and all LICENSED INFORMATION and files relating to LICENSED PATENTS (including LICENSED PATENTS on IMPROVEMENTS); or
(b) in the event of YALE’s material breach of any of the provisions of this AGREEMENT, upon sixty (60) days advance written notice to YALE, if said breach is not cured (if capable of being cured) within said sixty (60) day period.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
ARTICLE 14 INDEMNIFICATION; INSURANCE; NO WARRANTIES
14.1 LICENSEE shall defend, indemnify and hold harmless YALE, its trustees, directors, officers, employees, and agents and their respective successors, heirs and assigns against any liability, claims, demands, damages, judgments, losses and expenses of any nature, including legal expenses and attorney’s fees arising out of any theory of product liability (including tort, warranty, or strict liability) and the death, personal injury, or illness of any person or out of damage to any property, to third parties awarded in final settlement or in a final judgment of a court or other tribunal from which no appeal can be or is taken resulting from the production, manufacture, sale, use, lease, or other disposition or consumption or advertisement of the LICENSED PRODUCTS or SERVICES by LICENSEE or its AFFILIATES; or in connection with any representation or warranty of LICENSEE or its AFFILIATES with respect to the LICENSED PRODUCTS or SERVICES. Except as provided in Article 14.2, LICENSEE, however, shall not be required to defend, indemnify and hold harmless YALE, or any other indemnified party, to the extent such liability, claims, demands, damages, judgments, losses and/or expenses are determined with finality by a court of competent jurisdiction to have resulted from the negligent, reckless or intentional act or omission of YALE or any other indemnified party. Such indemnity obligation is contingent on prompt written notice of any claim, action or demand for which indemnity is claimed, reasonable cooperation (at LICENSEE’s expense) of YALE in such defense, and complete control of the defense and settlement thereof, except that LICENSEE will not have the right to make any monetary settlement or take any other action which would include any admission of liability on the part of YALE, its trustees, directors, officers, employees, and agents and their respective successors, heirs and assigns without the prior written consent of the party involved, which consent shall not be unreasonably withheld or delayed.
| 14.2 | (a) The Xxxxxx Xxxxxx Medical Institute (“HHMI”), and its trustees, officers, employees, and agents (collectively, “HHMI Indemnitees”), will be indemnified, defended by LICENSEE’S counsel reasonably acceptable to HHMI, and held harmless by LICENSEE from and against any claim, liability, cost, expense, damage, deficiency; loss, or obligation, of any kind or nature (including, without limitation, reasonable attorneys’ fees and other costs and expenses of defense) (collectively, “Claims”), based upon, arising out of, or otherwise relating to this AGREEMENT, including without limitation any cause of action relating to product liability, but excluding the defense or enforcement of the LICENSED INTELLECTUAL PROPERTY which shall be handled in accordance with Article 11. The previous sentence will not apply to any Claim that is determined with finality by a court of competent jurisdiction to result solely from the gross negligence or reckless or willful misconduct of an HHMI Indemnitee. |
(b) An HHMI Indemnitee shall provide Licensee with prompt notice of any claim for which indemnification may be sought pursuant to this AGREEMENT, such notice to be given reasonably promptly following actual receipt of written notice thereof by an officer or attorney of HHMI. Notwithstanding the foregoing, the delay or failure of any HHMI Indemnitee to give reasonably prompt notice to Licensee, of any such claim shall not affect the rights of such HHMI Indemnitee unless, and then only to the extent that, such delay or failure is prejudicial to or otherwise adversely affects Licensee.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
(c) LICENSEE agrees not to settle any Claim against an HHMI Indemnitee without HHMI’s written consent, where (a) such settlement would include any admission of liability on the part of any HHMI Indemnitee, (b) such settlement would impose any restriction on any HHMI Indemnitee’s conduct of any of its activities, or (c) such settlement would not include an unconditional release of all HHMI Indemnities from all liability for claims that are the subject matter of the settled Claim.
| 14.3 | (a) YALE makes NO REPRESENTATIONS or WARRANTIES that any LICENSED INTELLECTUAL PROPERTY claims, issued or pending are valid, or that the manufacture, use, sale or other disposal of the LICENSED PRODUCTS or SERVICES does NOT infringe upon any patent or other rights NOT vested in YALE |
(b) YALE DISCLAIMS ALL WARRANTIES WHATSOEVER WITH RESPECT TO THE LICENSED INTELLECTUAL PROPERTY AND THE LICENSED PRODUCTS OR SERVICES EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO WARRANTIES OR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. LICENSEE shall make no statements, representation or warranties whatsoever to any third parties which are inconsistent with such disclaimer by YALE.
14.4 On or before the date any LICENSED PRODUCT is tested or used on humans, LICENSEE shall purchase and maintain in effect and shall require its sublicensees to purchase and maintain in effect a policy of commercial, general liability insurance to protect LICENSEE, YALE and HHMI with respect to events described in Article 14.1. Such insurance shall:
(a) list “YALE, HHMI, their trustees, officers and employees” as additional insureds under the policy;
(b) provide that such policy is primary and not excess or contributory with regard to other insurance YALE may have;
(c) be endorsed to include product liability coverage in amounts no less than $1 Million Dollars per incident and $5 Million Dollars annual aggregate; and
(d) be endorsed to include contractual liability coverage for LICENSEE’s indemnification under Article 14.1; and
(e) the minimum amount of insurance coverage required under this Articlel4.4 (c) shall not be construed to create a limit of LICENSEE’s liability with respect to its indemnification under Article 14.1.
14.5 By signing this AGREEMENT, LICENSEE certifies that the requirements of Article 14.4 will be met on or before the date any LICENSED PRODUCT is tested or used on humans. Upon YALE’s request, LICENSEE shall furnish a Certificate of Insurance and a copy of the current Insurance Policy to YALE. LICENSEE shall give thirty (30) days written notice to YALE prior to any cancellation of or material change to the policy.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
ARTICLE 15 NOTICES, PAYMENTS
15.1 Any payment, notice or other communication required by this AGREEMENT shall be in writing and sent by Registered or Certified first class U.S. Mail, postage prepaid, and shall be deemed delivered within five (5) days if sent to the following addresses or to such other address as such party shall designate by written notice to the other party:
| FOR YALE: | FOR LICENSEE: | |
| Director YALE UNIVERSITY Office of Cooperative Research X.X. Xxx 000000 000 Xxxxxx Xxxxxx, 0xx Xxxxx Xxx Xxxxx, XX 00000-0000 | Xxxxx Xxxxxxxxx, PhD CEO & President RIB-X PHARMACEUTICALS, INC. 00 Xxxxxxx Xxxx Xxxxxxxx, XX 00000 |
ARTICLE 16 LAWS AND REGULATIONS
16.1 This AGREEMENT shall be governed by and in accordance with the laws of the state of Connecticut except where the federal laws of the United States are applicable and have precedence.
16.2 LICENSEE shall comply with all foreign and United States federal, state, and local laws, regulations, rules and orders applicable to the testing, production, transportation, packaging, labeling, export, sale and use of the LICENSED PRODUCTS or SERVICES. In particular, LICENSEE shall be responsible for assuring compliance with all U.S. export laws and regulations applicable to this license and LICENSEE’s activities under this AGREEMENT.
ARTICLE 17 MISCELLANEOUS
17.1 Binding Effect. This AGREEMENT shall be binding upon and inure to the benefit of the parties and their respective legal representatives, successors and permitted assigns.
17.2 Entire Agreement. This AGREEMENT constitutes the entire agreement of the parties relating to the subject matter hereof, and all prior representations and understandings, written or oral, are merged into it and are superseded by this AGREEMENT.
17.3 Severability. The provisions of this AGREEMENT shall be deemed separable. If any part of this AGREEMENT is rendered void, invalid, or unenforceable, such determination shall not affect the validity or enforceability of the remainder of this AGREEMENT unless the part or parts which are void, invalid or unenforceable shall substantially impair the value of the entire Agreement as to either party, in which case the parties shall renegotiate the terms of this Agreement.
17.4 Headings. Paragraph headings are inserted for convenience of reference only and do not form a part of this AGREEMENT.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
17.5 Third Party Beneficiaries. HHMI is not a party to this Agreement and has no liability to any licensee, sublicensee, or user of any technology covered by this Agreement, but HHMI is an intended third-party beneficiary of this Agreement and Articles 12.1(a) as it relates to HHMI and 14.2 are for the benefit of HHMI and are enforceable by HHMI in its own name. Other than HHMI, no person not a party to this AGREEMENT, including any employee of any party to this AGREEMENT, shall have or acquire any rights by reason of this AGREEMENT. Nothing contained in this AGREEMENT shall be deemed to constitute the parties partners with each other or any third party.
17.6 Amendment; Assignment. This AGREEMENT may not be amended except by written agreement executed by each of the parties, and shall not be assigned by LICENSEE except with the written consent of YALE, provided that LICENSEE may assign this AGREEMENT to a purchaser in connection with a merger, consolidation, or reorganization, sale or transfer of all or substantially all of its assets or equity, or of the entire business, equity and assets to which this AGREEMENT relates if the purchaser agrees to be bound by all of the terms and conditions set forth herein and if, prior to assignment to such a purchaser, LICENSEE notifies YALE of the assignment, including the name and address of the purchaser.
17.7 Force Majeure. Neither YALE nor LICENSEE shall be liable for failure of or delay in performing obligations set forth in this AGREEMENT, and neither shall be deemed in breach of its obligations, if such failure or delay is due to natural disasters or any causes reasonably beyond the control of YALE or LICENSEE.
(remainder of this page left intentionally blank)
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
IN WITNESS to their Agreement, the parties have caused this AGREEMENT to be executed in duplicate originals by their duly authorized representatives.
| Yale University | Rib-X Pharmaceuticals, Inc. | |||||||
| By: | /s/ Xxx Xxxxxxxxxx | By: | /s/ Xxxxx Xxxxxxxxx | |||||
| Name: | Xxx Xxxxxxxxxx | Name: | Xxxxx Xxxxxxxxx | |||||
| Title: | Managing Director | Title: | President + CEO | |||||
| Office of Cooperative Research | Date: | 12/06/01 | ||||||
| Date: | 12/6/01 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
APPENDIX A
| Confidential | Appendix A: Patent Application Family | Confidential | ||
| For | ||||
| The Crystal Structure of the 50s Ribosomal Subunit |
[***]
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
APPENDIX B
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
License to the United States Government
Sign and Fax this to (000) 000-0000
Invention Title: Drug Design Using Atomic Structure of 50S Ribosomal Subunit and Bound Antibiotic
Inventor(s): Nenad Ban, Xxxxxxx X. Xxxxxx, Xxxxx X. Xxxxx, Xxxx Xxxxxx, Xxxxxx Xxxxxx
U.S. Filing/Issue Date: [***]
Patent or Application Serial No.: [***]
Grant/Contract Number(s): [***]
Foreign Applications filed/intended in (countries):
The invention identified above is a Subject Invention under 35 U.S.C. 200, et seq., and the Standard Patent Rights clause at 37 CFR 401.14, FAR 52.227-11 or FAR 52.227-12 (if applicable) which are included among the terms of the above identified grant or contract award from the United State Government. This document is confirmatory of:
| 1. | The nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States the invention described in any patent application and in any and all divisions, continuations, and continuations in part, and in any and all patents and re-issues granted thereon throughout the world; and |
| 2. | All other rights acquired by the Government by reason of the above identified grant/contract award and the laws and regulations that are applicable to the award. |
The Government is hereby granted an irrevocable power to inspect and make copies of the above-identified patent application.
| Signed 4th day of June, 2007. | ||||||||
| By | Xxxx Xxxxx Xxxxxxx | /s/ Xxxx Xxxxxxx | ||||||
| (Name of Grantee/Contractor Official | (Signature) | |||||||
| Title License & Compliance Manager | ||||||||
| For Yale University | ||||||||
| (Grantee/Contractor Organization) | ||||||||
| At 000 Xxxxxx Xx. Xxx Xxxxx, XX 00000 XX | ||||||||
| (Business Address) |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
License to the United States Government
Sign and Fax this to (000) 000-0000
Invention Title: Drug Design Using Atomic Structure of 50S Ribosomal Subunit and Bound Antibiotic
Inventor(s): Xxxxxx Xxxxxx, Xxxx Xxxxxx, Xxxxx X. Xxxxx, Xxxxxxx X. Xxxxxx, Nenad Ban
U.S. Filing/Issue Date: [***]
Patent or Application Serial No.: [***]
Grant/Contract Number(s): [***]
Foreign Applications filed/intended in (countries):
The invention identified above is a Subject Invention under 35 U.S.C. 200, et seq., and the Standard Patent Rights clause at 37 CFR 401.14, FAR 52.227-11 or FAR 52.227-12 (if applicable) which are included among the terms of the above identified grant or contract award from the United State Government. This document is confirmatory of:
| 1. | The nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States the invention described in any patent application and in any and all divisions, continuations, and continuations in part, and in any and all patents and re-issues granted thereon throughout the world; and |
| 2. | All other rights acquired by the Government by reason of the above identified grant/contract award and the laws and regulations that are applicable to the award. |
The Government is hereby granted an irrevocable power to inspect and make copies of the above-identified patent application.
| Signed 4th day of June, 2007. | ||||||||
| By | Xxxx Xxxxx Xxxxxxx | /s/ Xxxx Xxxxxxx | ||||||
| (Name of Grantee/Contractor Official | (Signature) | |||||||
| Title License & Compliance Manager | ||||||||
| For Yale University | ||||||||
| (Grantee/Contractor Organization) | ||||||||
| At 000 Xxxxxx Xx. Xxx Xxxxx, XX 00000 XX | ||||||||
| (Business Address) |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
License to the United States Government
Sign and Fax this to (000) 000-0000
Invention Title: Drug Design Using Atomic Structure of 50S Ribosomal Subunit and Bound Antibiotic
Inventor(s): Xxxxxx Xxxxxx, Xxxx Xxxxxx, Xxxxx X. Xxxxx, Xxxxxxx X. Xxxxxx, Nenad Ban
U.S. Filing/Issue Date: [***]
Patent or Application Serial No.: [***]
Grant/Contract Number(s): [***]
Foreign Applications filed/intended in (countries):
The invention identified above is a Subject Invention under 35 U.S.C. 200, et seq., and the Standard Patent Rights clause at 37 CFR 401.14, FAR 52.227-11 or FAR 52.227-12 (if applicable) which are included among the terms of the above identified grant or contract award from the United State Government. This document is confirmatory of:
| 1. | The nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States the invention described in any patent application and in any and all divisions, continuations, and continuations in part, and in any and all patents and re-issues granted thereon throughout the world; and |
| 2. | All other rights acquired by the Government by reason of the above identified grant/contract award and the laws and regulations that are applicable to the award. |
The Government is hereby granted an irrevocable power to inspect and make copies of the above-identified patent application.
| Signed 4th day of June, 2007. | ||||||||
| By | Xxxx Xxxxx Xxxxxxx | /s/ Xxxx Xxxxxxx | ||||||
| (Name of Grantee/Contractor Official | (Signature) | |||||||
| Title License & Compliance Manager | ||||||||
| For Yale University | ||||||||
| (Grantee/Contractor Organization) | ||||||||
| At 000 Xxxxxx Xx. Xxx Xxxxx, XX 00000 XX | ||||||||
| (Business Address) |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
License to the United States Government
Sign and Fax this to (000) 000-0000
Invention Title: Drug Design Using Atomic Structure of 50S Ribosomal Subunit and Bound Antibiotic
Inventor(s): Nenad Ban, Xxxxxxx X. Xxxxxx, Xxxxx X. Xxxxx, Xxxx Xxxxxx, Xxxxxx Xxxxxx
U.S. Filing/Issue Date: [***]
Patent or Application Serial No.: [***]
Grant/Contract Number(s): [***]
Foreign Applications filed/intended in (countries):
The invention identified above is a Subject Invention under 35 U.S.C. 200, et seq., and the Standard Patent Rights clause at 37 CFR 401.14, FAR 52.227-11 or FAR 52.227-12 (if applicable) which are included among the terms of the above identified grant or contract award from the United State Government. This document is confirmatory of:
| 1. | The nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States the invention described in any patent application and in any and all divisions, continuations, and continuations in part, and in any and all patents and re-issues granted thereon throughout the world; and |
| 2. | All other rights acquired by the Government by reason of the above identified grant/contract award and the laws and regulations that are applicable to the award. |
The Government is hereby granted an irrevocable power to inspect and make copies of the above-identified patent application.
| Signed 4th day of June, 2007. | ||||||||
| By | Xxxx Xxxxx Xxxxxxx | /s/ Xxxx Xxxxxxx | ||||||
| (Name of Grantee/Contractor Official | (Signature) | |||||||
| Title License & Compliance Manager | ||||||||
| For Yale University | ||||||||
| (Grantee/Contractor Organization) | ||||||||
| At 000 Xxxxxx Xx. Xxx Xxxxx, XX 00000 XX | ||||||||
| (Business Address) |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
APPENDIX C
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.




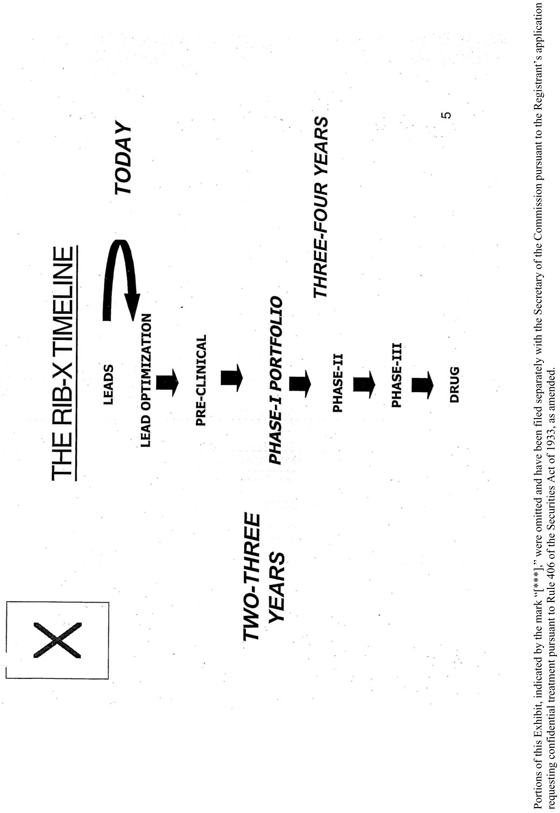











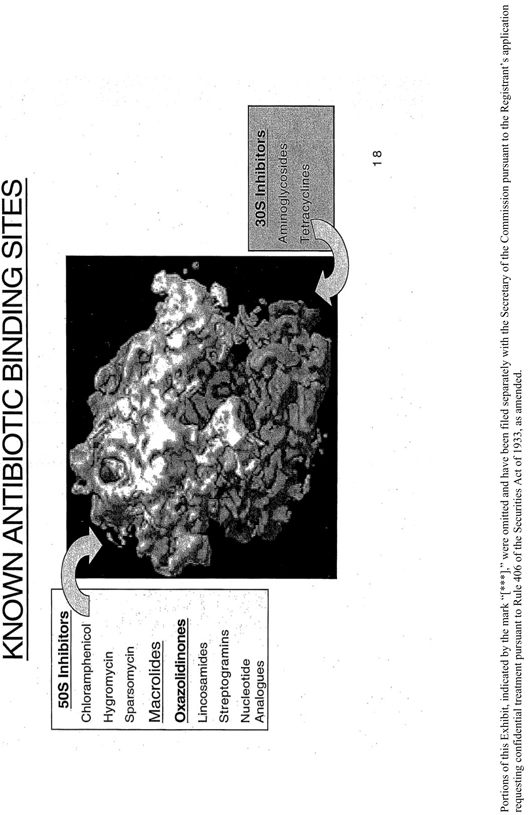

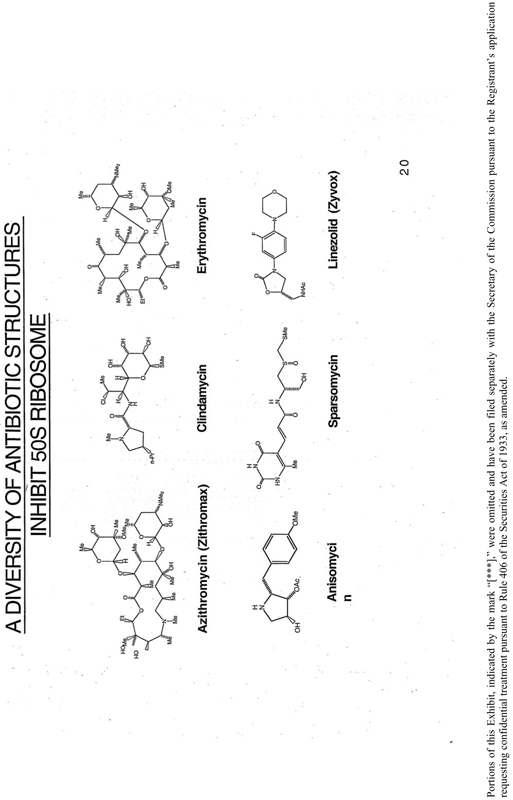
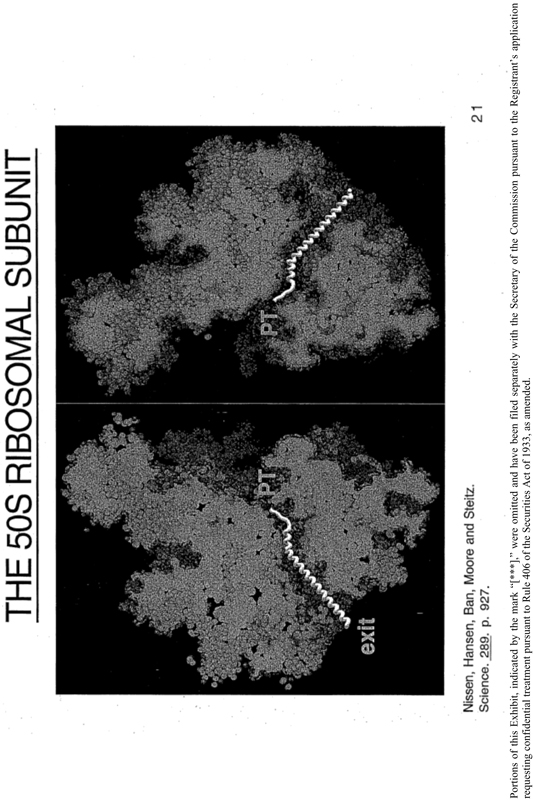

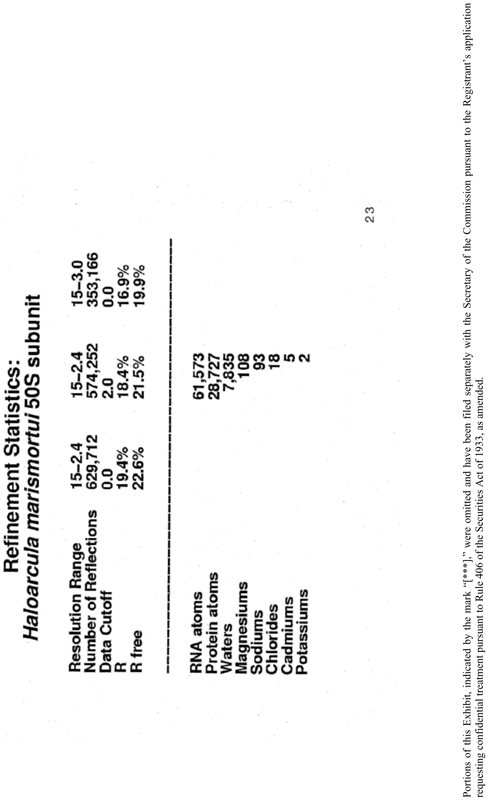


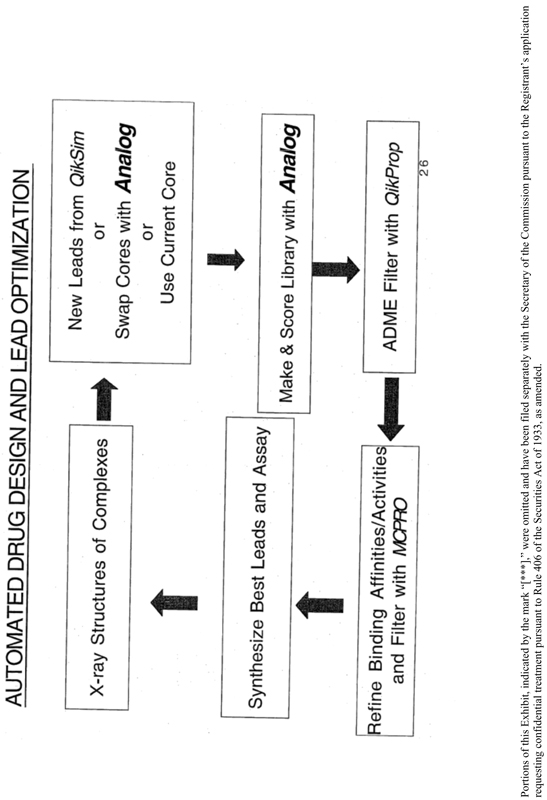



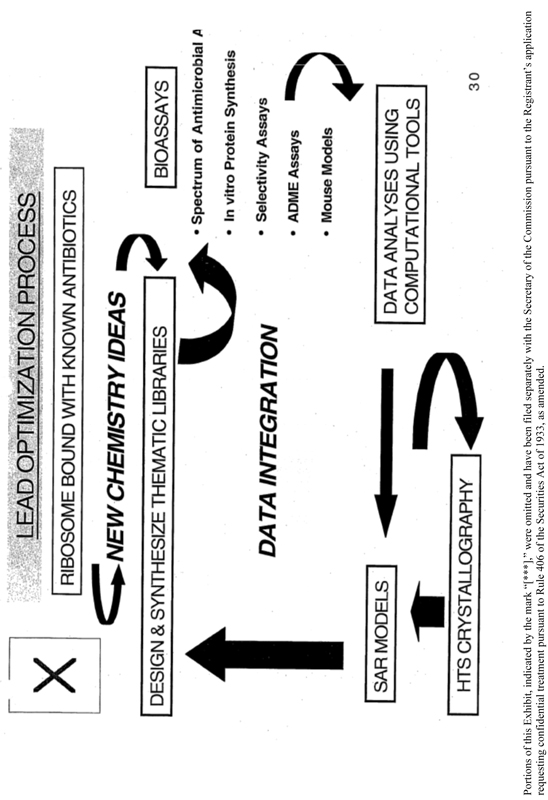
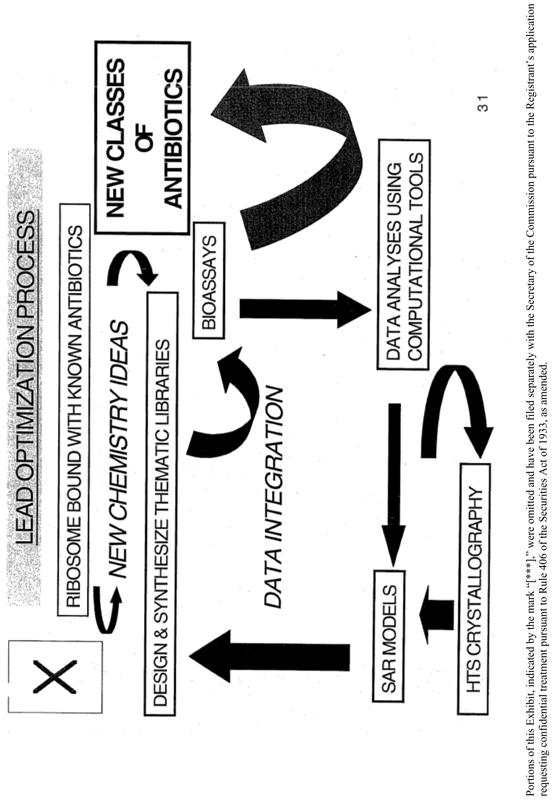
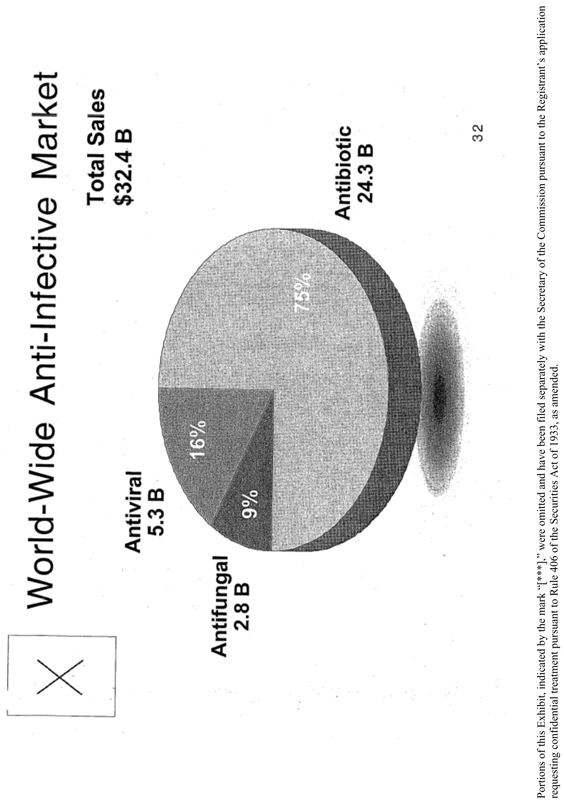

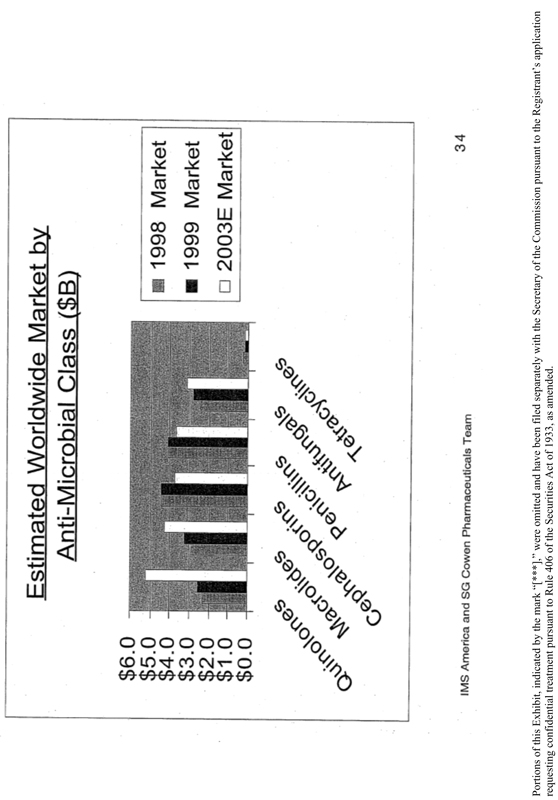
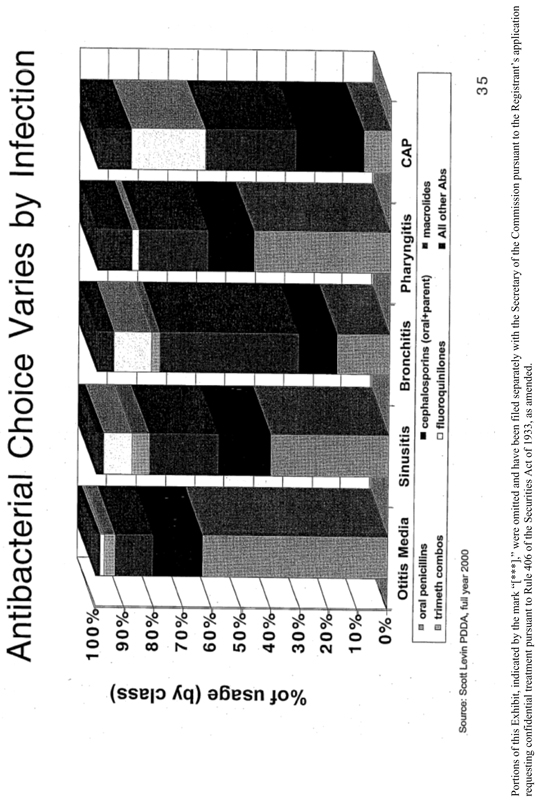
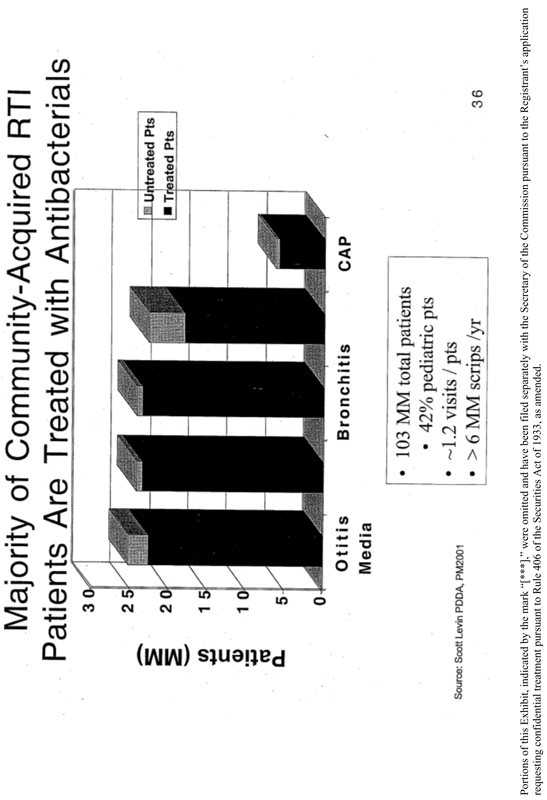
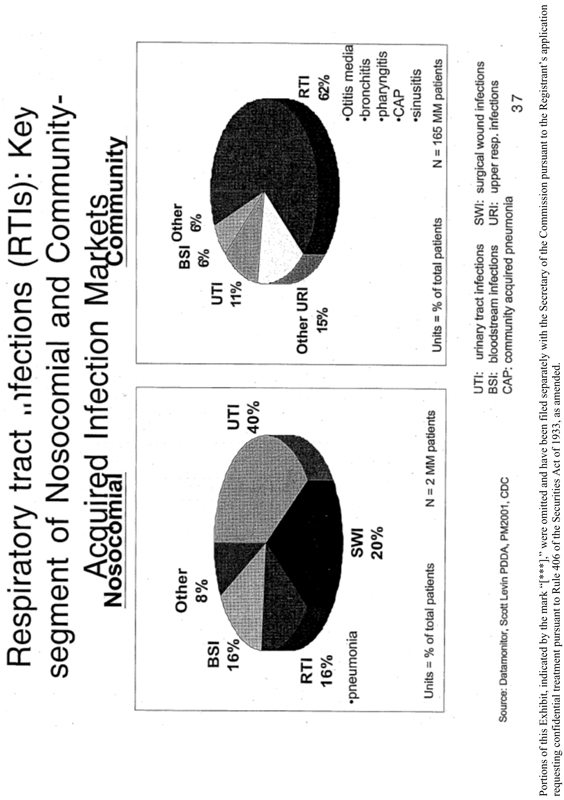
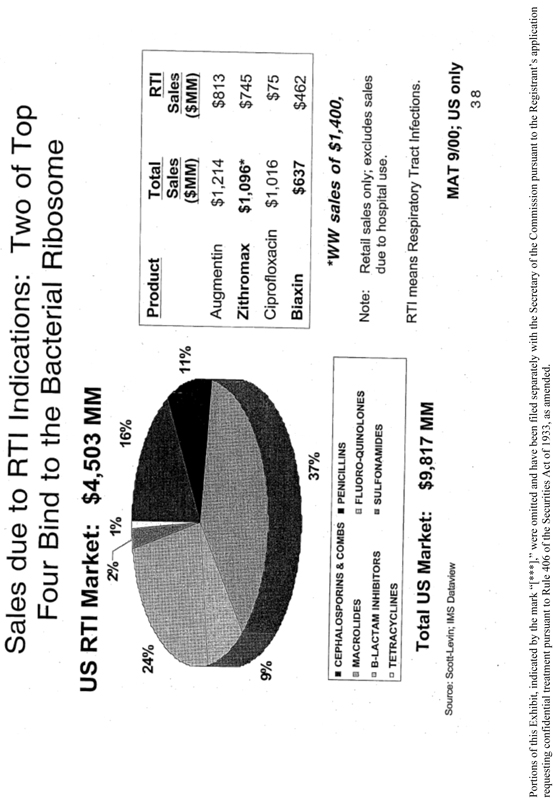










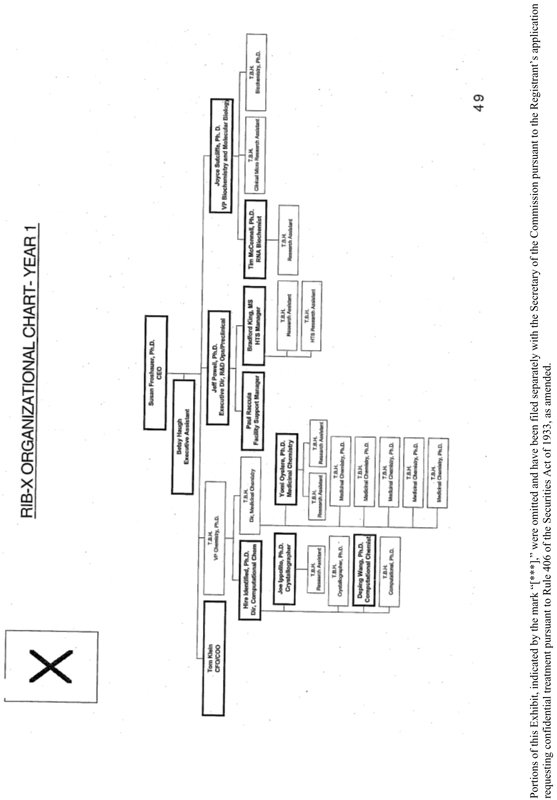

AMENDMENT NO. 1 TO YALE EXCLUSIVE LICENSE AGREEMENT
THIS AMENDMENT to the YALE EXCLUSIVE LICENSE AGREEMENT having an EFFECTIVE DATE of December 6, 2001, by and between YALE UNIVERSITY, a corporation organized and existing under, and by virtue of, a charter granted by the general assembly of the Colony and State of Connecticut (“YALE”), and Rib-X Pharmaceuticals, Inc., a corporation organized and existing under the laws of the State of Delaware (“LICENSEE”), shall be effective upon the date of final execution below.
WHEREAS, YALE and LICENSEE desire to update Appendix A to the YALE EXCLUSIVE LICENSE AGREEMENT to include patent applications for LICENSED PATENTS filed after the EFFECTIVE DATE.
NOW THEREFORE, in consideration of one U.S. dollar and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, YALE and LICENSEE hereto agree as follows:
| 1. | To replace Appendix A entitled, “Patent Application Family For `The Crystal Structure of the 50s Ribosomal Subunit, [***]” attached to the YALE EXCLUSIVE LICENSE AGREEMENT on the EFFECTIVE DATE, with the updated Appendix A attached hereto; |
| 2. | Except as specified in the previous paragraph, all other terms and provisions of the YALE EXCLUSIVE LICENSE AGREEMENT shall remain in full force and effect; and |
| 3. | This AMENDMENT may be executed in counterparts, each of which shall constitute an original but both of which shall together constitute one and the same instrument. |
IN WITNESS WHEREOF, the parties have caused this AMENDMENT to be executed in duplicate originals by their duly authorized representatives.
| Yale University | Rib-X Pharmaceuticals, Inc. | |||||||
| By: | /s/ Xxx Xxxxxxxxxx | By: | /s/ Xxxxx Xxxxxxxxx | |||||
| Name: | Xxx Xxxxxxxxxx | Name: | Xxxxx Xxxxxxxxx | |||||
| Title: | Managing Director, OCR | Title: | President and CEO | |||||
| Date: | 11 Sept 2003 | Date: | Sept. 4, 2003 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
APPENDIX A – Patent Rights
| U.S. Patent Rights | Foreign Counterpart | |||||||||||||||||||||
| Serial No. | Filing Date | Patent No. | Issue Date | THT Ref. No. | Yale OCR. No. | Country | Serial No. | Filing Date | Patent No. | Issue Date | THT Ref. No. | |||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | Australia | [***] | [***] | [***] | |||||||||||||||
| Canada | [***] | [***] | [***] | |||||||||||||||||||
| Europe | [***] | [***] | [***] | |||||||||||||||||||
| Israel | [***] | [***] | [***] | |||||||||||||||||||
| Japan | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | Australia | [***] | [***] | [***] | |||||||||||||||
| Canada | [***] | [***] | [***] | |||||||||||||||||||
| Europe | [***] | [***] | [***] | |||||||||||||||||||
| Israel | [***] | [***] | [***] | |||||||||||||||||||
| Japan | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] | |||||||||||||||||||
| [***] | [***] | [***] | [***] |
| * | U.S. provisional application lapsed by operation of law. |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
AMENDMENT NO. 2 TO YALE EXCLUSIVE LICENSE AGREEMENT
THIS AMENDMENT to the YALE EXCLUSIVE LICENSE AGREEMENT having an EFFECTIVE DATE of December 6, 2001, by and between YALE UNIVERSITY, a corporation organized and existing under, and by virtue of, a charter granted by the general assembly of the Colony and State of Connecticut (“Yale”) and Rib-X Pharmaceuticals, Inc., a corporation organized and existing under the laws of the State of Delaware (“LICENSEE”), shall be effective upon the date of final execution below.
WHEREAS, YALE AND LICENSEE desire to enter into an exclusive license agreement and material transfer agreement with respect to the following Yale technology development (see also Appendix 2.1 of this Amendment):
“Methods for Improving the Resolution of Diffraction from Crystals of the Large Ribosomal Subunit of Haloarcula marismortui”(Yale OCR Number 1726), including but not limited to PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating mutant ribosomes from same organism.
WHEREAS, YALE AND LICENSEE desire to amend the YALE EXCLUSIVE LICENSE AGREEMENT to grant to LICENSEE an exclusive license to said Yale development and associated materials.
NOW THEREFORE, in consideration of the monies and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, YALE and LICENSEE hereto agree and acknowledge that the License Agreement shall be amended as follows:
1.1 Article 1 shall be amended by adding the following:
“INVENTIONS” shall be expanded to include “A Method for Improving the Resolution of Diffraction from Crystals of the Large Ribosomal Subunit of Haloarcula marismortui”, including but not limited to PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating mutant ribosomes from same organism, all as in existence as of the effective date of this Amendment (the “OCR 1726 INVENTION”).
2.16 Article 2.16 shall be amended by adding the following:
“LICENSED PATENTS” shall also include know-how and materials associated with the “OCR 1726 INVENTION”.
3.1 Article 3.1 shall be amended only with respect to the subject matter of this Amendment No. 2 to the Yale Exclusive License Agreement and as described in Appendix 2.1 attached, by adding the following:
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
“The exclusive worldwide license to practice know-how and materials associated with the Yale OCR Number 1726 Invention is an exclusive commercial license that does not preclude sharing of such know-how and materials, whether before or after publication, with academic or other non-profit institutions for non-commercial research or educational purposes.”
8.3 Section 8.3 shall be amended only with respect to the subject matter of this Amendment No. 2 to the Yale Exclusive License Agreement and as described in Appendix 2.1 attached, by adding the following:
“For the avoidance of doubt, LICENSED INTELLECTUAL PROPERTY and IMPROVEMENTS shall not be considered proprietary or confidential information of LICENSEE for purposes of Article 8. In addition, the pre-publication review provision of this Section 8.3 shall not apply to presentations or publications by Xx. Xxxxxx Xxxxxx of his research results relating to methods for improving the resolution of diffraction from crystals of the large ribosomal subunit of Haloarcula marismortui, including but not limited to PDB files, raw data sets, and methodology for generating mutant ribosomes from the same organism. Notwithstanding any other provisions of the Agreement, biological materials and data described in such publications may be shared by Xx. Xxxxxx with other academic scientists for research use, in accordance with HHMI’s policy on sharing publication-related materials, data, and software (available at xxxx://xxx.xxxx.xxx/xxx/Xxxxxxx.xxx).”
For the sake of clarity, the amendments made to Sections 3.1 and 8.3 contemplated above relate only to the subject matter described in Amendment No. 2 to the Yale Exclusive License Agreement and in no way is meant to modify any portion of the Yale Exclusive License Agreement with respect to the original subject matter or any additions not included specifically in this Amendment No. 2.
In partial consideration for amendment of the License agreement to include a grant to the OCR 1726 INVENTION, LICENSEE shall pay to YALE a one-time fee of $15,000.00 (Fifteen Thousand Dollars).
The transfer of materials shall be subject to the terms and conditions of a Material Transfer Agreement (attached as Appendix D).
IN WITNESS WHEREOF, the parties have caused this AMENDMENT to be executed in duplicate originals by their duly authorized representatives.
| YALE UNIVERSITY | RIB-X PHARMACEUTICALS, INC. | |||||||
| By: | /s/ E. Xxxxxxxx Xxxxxxxxxx | By: | /s/ Xxxxx Xxxxxxxxx | |||||
| E. Xxxxxxxx Xxxxxxxxxx | Name: | Xxxxx Xxxxxxxxx | ||||||
| Managing Director Office of Cooperative Research | Title: | President and CEO | ||||||
| Date: | 17 Sept 04 | Date: | 9/24/04 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
Appendix 2.1 of Amendment No. 2 to the Yale Exclusive License Agreement
Materials to be Transferred under the Material Transfer Agreement dated September 16, 2004
Methods for Improving the Resolution of Diffraction from Crystals of the Large Ribosomal Subunit of Haloarcula Marismortui (Yale OCR Number 1726 including but not limited to PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating mutant ribosomes from same organism.
Specific Materials will include:
1. With respect to mutant technology:
| • | L22, L4, G2099A and other mutants in which one or more regions of the 23S rRNA have been modified in addition to methods for making mutants and producing crystals with such mutants. |
| • | Methods and vectors for transformation used to create new H. marismortui mutant strains |
| • | H. marismortui strain(s) with one or two 23S rRNA alleles and other strains used to construct mutants. |
2. With respect to 50S crystal improvement technology
| • | Methods for routine improvement of H. marismortui crystals and the datasets collected to date under these improvements |
| • | High resolution datasets (HKL files and pdb coordinates) for any and all ribosome structures in either the presence or absence of antibiotics solved under improved conditions using either normal or mutant H. marismortui ribosomes. |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
Appendix 2.2 of Amendment No. 2 to the Yale Exclusive License Agreement
(also referred to as “Appendix D”)
MATERIAL TRANSFER AGREEMENT
In response to your request for material from the laboratories of Xx. Xxxxxx Xxxxxx, a Xxxxxx Xxxxxx Medical Institute investigator at Yale University, and Dr. Xxxxx Xxxxx (“the Yale Investigators”), YALE will make the following available to your company, Rib-X, Inc. and its affiliates. (“RECIPIENT”):
Yale Materials: Shall include Methods for Improving the Resolution of Diffraction from Crystals of the Large Ribosomal Subunit of Haloarcula Marismortui (Yale OCR Number 1726) including but not limited to PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating mutant ribosomes from same organism as further detailed in Appendix 2.1 to this Material Transfer Agreement.
1. The material described above (“MATERIAL”) is made available to RECIPIENT for all research and commercial purposes. The term “Commercial Purposes” shall mean the sale or other commercial provision of the MATERIAL to third parties. THE MATERIAL WILL NOT BE USED IN HUMANS UNDER ANY CIRCUMSTANCES.
2. RECIPIENT acknowledges that the MATERIAL is experimental in nature, and that YALE MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AS TO THE MERCHANTABILITY OR FITNESS OF THE MATERIAL FOR A PARTICULAR PURPOSE, or that the use of the MATERIAL or any product or process derived therefrom will not infringe any patent, copyright or other rights of third parties. In no event shall YALE be liable for any damages, direct, indirect or consequential, resulting from any use, storage or handling of the MATERIAL or any derivatives therefrom by the RECIPIENT or any other party.
The Xxxxxx Xxxxxx Medical Institute (“HHMI”), and its trustees, officers, employees, and agents (collectively, “HHMI Indemnitees”), will be indemnified, defended by counsel acceptable to HHMI, and held harmless by RECIPIENT from and against any claim, liability, cost, expense, damage, deficiency, loss, or obligation, of any kind or nature (including, without limitation, reasonable attorneys’ fees and other costs and expenses of defense) (collectively, “Claims”), based upon, arising out of, or otherwise relating to this agreement, including without limitation any cause of action relating to product liability. The previous sentence will not apply to any Claim that is determined with finality by a court of competent jurisdiction to result solely from the gross negligence or willful misconduct of an HHMI lndemnitee. The provisions of this paragraph 4 will survive termination of this agreement. HHMI is not a party to this agreement and has no liability to any licensee or user of the MATERIAL, but HHMI is an intended third-party beneficiary of this agreement and the foregoing indemnification provision is for the benefit of HHMI and is enforceable by HHMI in its own name.
3. RECIPIENT shall acknowledge YALE and the YALE INVESTIGATOR in any publication, as the provider of the MATERIAL.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
4. The exact person and address the MATERIAL will be sent to is: Xx. Xxxxxx Xxxxxxxx
5. This document represents the final agreement concerning transfer of the MATERIAL between the parties.
6. RECIPIENT represents that it is under no obligation inconsistent with the terms and conditions of this Agreement.
To be valid, this document must be signed by the Director of the Yale Office of Cooperative Research. Please have RECIPIENT indicate agreement with the above terms by having both enclosed originals signed by a duly authorized official, and return one to me at the above address. Upon receipt of a signed original and the fee, the MATERIAL will be forwarded to you.
| Yale University: | Rib-X | |||||||
| By: | /s/ E. Xxxxxxxx Xxxxxxxxxx | By: | /s/ Xxxxx Xxxxxxxxx | |||||
| E. Xxxxxxxx Xxxxxxxxxx | Xxxxx Xxxxxxxxx | |||||||
| Managing Director, Office of Cooperative Research | President and CEO | |||||||
| Date: | 17 Sept 04 | Date: | 9/24/04 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
AMENDMENT NO. 3 TO YALE EXCLUSIVE LICENSE AGREEMENT
THIS AMENDMENT to the YALE EXCLUSIVE LICENSE AGREEMENT having an EFFECTIVE DATE of December 3, 2001, by and between YALE UNIVERSITY, a corporation organized and existing under, and by virtue of, a charter granted by the general assembly of the Colony and State of Connecticut (“Yale”) and Rib-X Pharmaceuticals, Inc., a corporation organized and existing under the laws of the State of Delaware (“LICENSEE”), shall be effective upon the date of final execution below.
WHEREAS, YALE AND LICENSEE desire to enter into an exclusive license agreement and material transfer agreement with respect to the following Yale technology development (see also Appendix 1):
Yale Technology: Shall include the technology as described in the submitted manuscript, “The structures of the anti-Tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome” (Yale OCR Number 5322), including but not limited to Thermus thermophilus 70S ribosomes (including bare crystals and crystals complexed with viomycin and capreomycin), PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating ribosomes from same organism as further detailed in Appendix 1 to this Material Transfer Agreement.
WHEREAS, YALE AND LICENSEE desire to amend the YALE EXCLUSIVE LICENSE AGREEMENT to grant to LICENSEE an exclusive license to said Yale development and associated materials.
NOW THEREFORE, in consideration of the monies and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, YALE and LICENSEE hereto agree and acknowledge that the License Agreement shall be amended as followsl.1 Article 1 shall be amended by adding the following:
“INVENTIONS” shall be expanded to include “The structures of the anti-Tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome”, including but not limited to PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating ribosomes from Thermus thermophilus organism (the “OCR 5322 INVENTION”).
2.16 Article 2.16 shall be amended by adding the following:
“LICENSED PATENTS” shall also include know-how and materials associated with the “OCR 5332 INVENTION”.
5. This document represents the final agreement concerning transfer of the MATERIAL between the parties.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
6. RECIPIENT represents that it is under no obligation inconsistent with the terms and conditions of this Agreement.
To be valid, this document must be signed by the Director of the Yale Office of Cooperative Research. Please have RECIPIENT indicate agreement with the above terms by having both enclosed originals signed by a duly authorized official, and return one to me at the above address. Upon receipt of a signed original and the fee, the MATERIAL will be forwarded to you.
| Yale University: | Rib-X Pharmaceuticals, Inc. | |||||||
| By: | /s/ Xxx Xxxxxxxxxx | By: | /s/ Xxxxx Xxxxxxxxx, Ph.D. | |||||
| Name | Xxx Xxxxxxxxxx | Xxxxx Xxxxxxxxx, Ph.D. | ||||||
| Title | Managing Director | President and CEO | ||||||
| Office of Cooperative Research | ||||||||
| Date: | 3 Dec. 2009 | Date: | 12/03/09 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
Appendix E
MATERIAL TRANSFER AGREEMENT
In response to your request for material from the laboratories of Xx. Xxxxxx Xxxxxx, a Xxxxxx Xxxxxx Medical Institute investigator at Yale University, and Dr. Xxxxx Xxxxx (“the Yale Investigators”), YALE will make the following available to your company, Rib-X Pharmaceuticals, Inc. and its affiliates. (“RECIPIENT”):
Yale Materials: Shall include materials as described in the submitted manuscript, “The structures of the anti-Tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome” (Yale OCR Number 5322), including but not limited to Thermus thermophilus 70S ribosomes (including bare crystals and crystals complexed with viomycin and capreomycin), PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating ribosomes from same organism as further detailed in Appendix 1 to this Material Transfer Agreement.
Thermus thermophilus 70S ribosomes
1. The material described above (“MATERIAL”) is made available to RECIPIENT for all research and commercial purposes. The term “Commercial Purposes” shall mean the sale or other commercial provision of the MATERIAL to third parties. THE MATERIAL WILL NOT BE USED IN HUMANS UNDER ANY CIRCUMSTANCES.
2. RECIPIENT acknowledges that the MATERIAL is experimental in nature, and that YALE MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AS TO THE MERCHANTABILITY OR FITNESS OF THE MATERIAL FOR A PARTICULAR PURPOSE, or that the use of the MATERIAL or any product or process derived therefrom will not infringe any patent, copyright or other rights of third parties. In no event shall YALE be liable for any damages, direct, indirect or consequential, resulting from any use, storage or handling of the MATERIAL or any derivatives therefrom by the RECIPIENT or any other party.
The Xxxxxx Xxxxxx Medical Institute (“HHMI”), and its trustees, officers, employees, and agents (collectively, “HHMI lndemnitees”), will be indemnified, defended by counsel acceptable to HHMI, and held harmless by RECIPIENT from and against any claim, liability, cost, expense, damage, deficiency, loss, or obligation, of any kind or nature (including, without limitation, reasonable attorneys’ fees and other costs and expenses of defense) (collectively, “Claims”), based upon, arising out of, or otherwise relating to this agreement, including without limitation any cause of action relating to product liability. The previous sentence will not apply to any Claim that is determined with finality by a court of competent jurisdiction to result solely from the gross negligence or willful misconduct of an HHMI Indemnitee. The provisions of this paragraph 4 will survive termination of this agreement. HHMI is not a party to this agreement and has no liability to any licensee or user of the MATERIAL, but HHMI is an intended third-party beneficiary of this agreement and the foregoing indemnification provision is for the benefit of HHMI and is enforceable by HHMI in its own name.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
3. RECIPIENT shall acknowledge YALE and the YALE INVESTIGATOR in any publication, as the provider of the MATERIAL.
4. The exact person and address the MATERIAL will be sent to is:
Xx. Xxxxxx Xxxxxxxx
Rib-X Pharmaceuticals, Inc.
000 Xxxxxx Xx., Xxxxx 000
Xxx Xxxxx, XX 00000
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
Appendix 1
Materials to be Transferred under the Material Transfer Agreement dated December 3, 2009
Yale Materials: Shall include materials as described in the submitted manuscript, “The structures of the anti-Tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome” (Yale OCR Number 5322), including but not limited to Thermus thermophilus 70S ribosomes (including bare crystals and crystals complexed with viomycin and capreomycin), PDB files and raw data sets derived from synchrotron experiments for structures determined, as well as methodology and materials for generating ribosomes from same organism.
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
In partial consideration for amendment of the License agreement to include a grant to the OCR 5322 INVENTION, LICENSEE shall pay to YALE a one-time fee of $15,000.00 (Fifteen Thousand Dollars).
The transfer of materials shall be subject to the terms and conditions of a Material Transfer Agreement (attached as Appendix E).
IN WITNESS WHEREOF, the parties have caused this AMENDMENT to be executed in duplicate originals by their duly authorized representatives.
| YALE UNIVERSITY | RIB-X PHARMACEUTICALS, INC. | |||||||
| By: | /s/ E. Xxxxxxxx Xxxxxxxxxx | By: | /s/ Xxxxx Xxxxxxxxx, Ph.D. | |||||
| Managing Director | Name: | Xxxxx Xxxxxxxxx, Ph.D. | ||||||
| Office of Cooperative Research | Title: | President & CEO | ||||||
| Date: | 3 Dec. 2009 | Date: | 12/03/09 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
AMENDMENT NO. 4 TO YALE EXCLUSIVE LICENSE AGREEMENT
THIS AMENDMENT to the YALE EXCLUSIVE LICENSE AGREEMENT (the”AGREEMENT”) having an EFFECTIVE DATE of December 6, 2001, by and between YALE UNIVERSITY, a corporation organized and existing under, and by virtue of, a charter granted by the general assembly of the Colony and State of Connecticut (“Yale”) and Rib-X Pharmaceuticals, Inc., a corporation organized and existing under the laws of the State of Delaware (“LICENSEE”), shall be effective upon the date of final execution below.
WHEREAS, LICENSEE has entered into a Collaboration and License Agreement dated as of June 28, 2011 with Sanofi with respect to LICENSEE’s RX-04 Program (the “Sanofi Parntership”), wherein Sanofi may attain certain rights to the LICENSED TECHNOLOGY and/or LICENSED PRODUCTS as defined in the AGREEMENT.
WHEREAS, LICENSEE currently has identified and is currently developing [***] under LICENSEE’s RX-04 Program which have been partnered with Sanofi.
WHEREAS, LICENSEE, within the scope of its agreement with Sanofi, may design and optimize additional [***] that bind in the same ribosomal site as the [***] RX-04 [***].
WHEREAS, YALE AND LICENSEE desire to amend the YALE EXCLUSIVE LICENSE AGREEMENT to clarify payments from Sanofi that are either exclusions from sublicense revenue as defined in the AGREEMENT, or shall be considered as sublicense revenue as defined in the AGREEMENT.
NOW THEREFORE, YALE and LICENSEE hereby agree and acknowledge that the AGREEMENT shall be amended as follows:
1. “ARTICLE 4 SUBLICENSES” shall be amended by adding the following new Section 4.5:
4.5 “LICENSEE and YALE hereby agree that over the first three years of the Sanofi Partnership, the $10 million upfront payment and the first $[***] in research milestones shall be considered as research reimbursement from Sanofi to LICENSEE for [***] of LICENSEE’s FTEs committed to the Sanofi Partnership, and as such, these payments totaling $[***] shall be excluded from sublicense revenue. All other payments received by LICENSEE with respect to the [***] related to the RX-04 program [or “covered by the LICENSED PATENTS (as defined in the AGREEMENT)”], pursuant to the Sanofi Partnership, shall be considered to be Sublicense Milestones without any further
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
exclusions, exceptions, or deductions, and LICENSEE shall pay YALE per the following schedule within 30 days of receiving each of the corresponding Sublicense Milestones:
| Sublicense Milestone | Amount | Payment to Yale ([***]%) | ||||||||
| Upfront | $ | 10,000,000 | 0 | FTE reimbursement | ||||||
| [***] | [***] | 0 | FTE reimbursement | |||||||
| [***] | [***] | 0 | FTE reimbursement | |||||||
| [***] | [***] | 0 | FTE reimbursement | |||||||
| [***] | [***] | 0 | FTE reimbursement | |||||||
| [***] | [***] | 0 | FTE reimbursement | |||||||
| [***] | [***] | 0 | FTE reimbursement | |||||||
| [***] | [***] | [***] | per compound | |||||||
| [***] | [***] | [***] | per compound | |||||||
| [***] | [***] | [***] | per compound | |||||||
| [***] | [***] | [***] | per compound | |||||||
| [***] | [***] | [***] | per compound | |||||||
| [***] | [***] | [***] | per compound | |||||||
| [***] | [***] | [***] | per compound |
The [***] RX-04 [***] are listed in Appendix D, which is hereby added to the AGREEMENT. For the avoidance of doubt, should LICENSEE, within the scope of the Sanofi Partnership, design and optimize additional [***] that bind in the same ribosomal site as the [***] RX-04 [***], no payment would be due YALE for the [***] and [***] Milestones only, as payments from Sanofi to LICENSEE for such milestones shall be considered research reimbursements for LICENSEE’s FTEs. All other milestone payments from Sanofi to LICENSEE, with respect to such additional [***], shall be considered to be sublicense revenue without any further exclusions, exceptions, or deductions, and LICENSEE shall pay YALE [***] percent ([***]%) of such payments as set forth above.
2. “ARTICLE 7 DUE DILIGENCE” shall be amended by adding the following new paragraph to Section 7.3:
“The annual PLAN shall include a detailed table listing research programs or products that are subject to the LICENSED INTELLECTUAL PROPERTY, indicating whether each is covered by one or more VALID CLAIMS under LICENSED PATENTS, subject to the use of LICENSED INFORMATION, or both, and the status of development of each program or product. As of the date of this AMENDMENT,
| AMENDMENT NO. 4 | 2 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
LICENSEE represents the following programs are subject to the LICENSED INTELLECTUAL PROPERTY:
| Program | Subject to the Yale LICENSED INTELLECTUAL PROPERTY? | Status | ||
| Radezolid | Yes: covered by LICENSED PATENTS | Clinical - IND
milestone paid | ||
| RX-02 | Yes: covered by LICENSED PATENTS | Preclinical | ||
| RX-04 | Yes: covered by LICENSED PATENTS | Preclinical/Discovery | ||
| RX-05 | Yes: covered by LICENSED PATENTS | Discovery | ||
| RX-06 | Yes: covered by LICENSED PATENTS | Discovery |
IN WITNESS WHEREOF, the parties have caused this AMENDMENT to be executed in duplicate originals by their duly authorized representatives.
| YALE UNIVERSITY | RIB-X PHARMACEUTICALS, INC. | |
| By: /s/ E. Xxxxxxxx Xxxxxxxxxx | By: /s/ Xxxx Xxxxxxxxxxxxxx | |
| E. Xxxxxxxx Xxxxxxxxxx, Ph.D | Xxxx Xxxxxxxxxxxxxx | |
| Managing Director | President & CEO | |
| Office of Cooperative Research | Rib-X Pharmaceuticals, Inc. | |
| Date: March 2, 2012 | Date: March 1, 2012 |
| AMENDMENT NO. 4 | 3 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.
Appendix D
[***] RX-04 [***]
| [***] | [***] Name | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] |
| AMENDMENT NO. 4 | 4 |
Portions of this Exhibit, indicated by the xxxx “[***],” were omitted and have been filed separately with the Secretary of the Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 406 of the Securities Act of 1933, as amended.

