EX-10.147 3 a2219425zex-10_147.htm EX-10.147 SERIES B-2 CONVERTIBLE PREFERRED STOCK AND WARRANT PURCHASE AGREEMENT
SERIES B-2 CONVERTIBLE PREFERRED
STOCK AND WARRANT PURCHASE AGREEMENT
This Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement (this “Agreement”), dated this 14th day of February, 2014, is entered into by and among Radius Health, Inc., a Delaware corporation (the “Corporation”), and the persons listed on Schedule I attached hereto (the “Investors,” and each individually, an “Investor”).
WHEREAS, the Corporation and the Investors wish to provide for the issuance of shares of Series B-2 Preferred Stock (as defined below) and warrants to purchase Common Stock (as defined below), each as more specifically set forth hereinafter.
NOW, THEREFORE. in consideration of the mutual covenants and agreements herein contained, the parties hereto, intending to be legally bound, hereby agree as follows:
SECTION 1. Authorized Preferred Stock. Prior to the Initial Closing (as defined in Section 4.1 hereof), the Corporation shall have filed a Certificate of Designations of the Series B-2 Convertible Preferred Stock of the Corporation, in the form attached hereto as Exhibit A (the “Series B-2 Certificate”), with the Secretary of State of the State of Delaware, providing for the authorization of a series of preferred stock of the Corporation to be designated as Series B-2 Convertible Preferred Stock, par value $.0001 per share (the “Series B-2 Preferred Stock”).
SECTION 2. Authorization of Issuance and Sale of Series B-2 Preferred Stock and Warrants. Subject to the terms and conditions of this Agreement, the Corporation has authorized the following:
(a) the issuance and sale to the Investors, in one or more Closings, of up to an aggregate of 655,000 shares of Series B-2 Preferred Stock (the “Shares”);
(b) the issuance and sale to the Investors, in one or more Closings, of warrants to acquire up to an aggregate of 1,637,500 shares (the “Warrant Shares”) of the Corporation’s Common Stock, par value $.0001 per share (the “Common Stock”), with each such warrant to acquire Common Stock being in the form attached hereto as Exhibit B (individually, a “Warrant” and, collectively, the “Warrants”); and
(c) the reservation of 6,550,000 shares of Common Stock for issuance upon conversion of the Shares (the “Reserved Common Shares”) and 1,637,500 shares of Common Stock for issuance upon exercise of the Warrants.
SECTION 3. Sale and Delivery of Shares and Warrants.
3.1 Agreement to Sell and Purchase the Shares and Warrants. Subject to the terms and conditions hereof, the Corporation is selling to each Investor and each Investor is severally (but not jointly) purchasing from the Corporation, for the aggregate purchase price set forth opposite such Investor’s name under the heading “Aggregate Purchase Price” on Schedule I hereto: (i) the number of Shares set forth opposite the name of such Investor under the heading “Series B-2 Shares” on Schedule I hereto; and (ii) a Warrant to acquire the number of Warrant Shares set forth opposite the name of such Investor on Schedule I hereto under the heading
“Warrant Shares”.
3.2 Delivery of Shares and Warrants. At each Closing (as defined in Section 4.2), the Corporation shall deliver to each Investor (i) a certificate or certificates, registered in the name of such Investor, representing the number of Shares being purchased by such Investor at such Closing and (ii) a Warrant issued in the name of such Investor to acquire the number of Warrant Shares being purchased by such Investor, in each case in accordance with Section 3.1 above. In each case, delivery to each Investor of certificates representing Shares and of a Warrant shall be made against receipt by the Corporation of a check payable to the Corporation or a wire transfer to an account designated by the Corporation in the full amount of the aggregate purchase price set forth opposite such Investor’s name under the heading “Aggregate Purchase Price” on Schedule I hereto.
SECTION 4. The Closings.
4.1 Initial Closing. An initial closing (the “Initial Closing”) hereunder with respect to the transactions contemplated by Sections 2 and 3 hereof will take place on the date hereof concurrently with the execution and delivery of this Agreement by the Corporation and the Investors (such date sometimes being referred to herein as the “Initial Closing Date”). The Initial Closing will take place remotely by facsimile or electronic transmission of executed copies of the documents contemplated hereby.
4.2 Subsequent Closings. Subject to the terms and conditions of this Agreement, the Corporation may sell, on or before March 15, 2014 to such other persons and entities as are acceptable to the Corporation, up to the total number of Shares that were not issued and sold by the Corporation at the Initial Closing, together with Warrants for the purchase of a total number of shares of Common Stock equal to 25% of the number of shares issuable upon conversion of the Shares to be issued pursuant to this Section 4.2 (each sale and issuance of such remaining Shares and associated Warrants at any time after the Initial Closing Date being sometimes referred to herein as a “Subsequent Closing”). Any such sale shall be upon the same terms and conditions as those contained herein. Each such person or entity, by delivery of an executed Investor signature page to this Agreement, shall become a party to this Agreement and, as a condition to such sale, such person or entity shall become a party to the Stockholders’ Agreement (as defined in Section 5.2(b)) by executing and delivering to the Corporation an Instrument of Adherence substantially in the form attached to the Stockholders’ Agreement. Following the execution and delivery by such person or entity of an Investor signature page to this Agreement and of such Instrument of Adherence to the Stockholders’ Agreement, such person or entity shall become a party to this Agreement, shall have the rights and obligations of an Investor hereunder, and shall be added to Schedule I hereto (together with all relevant information regarding the number of Shares, Warrant Shares and aggregate purchase price) without any further action by the Corporation or the other Investors. Pursuant to Sections 2.3 and 11 of the Existing Stockholders’ Agreement (as defined below), the Investors party to this Agreement and the Stockholders’ Agreement who represent the Majority Investors under the Existing Stockholders’ Agreement hereby irrevocably waive, on behalf of all parties to the Existing Stockholders’ Agreement, any pre-emptive rights or rights of first offer they may possess now or hereafter with respect to sales of Shares and Warrants (and any related issuances of Reserved Common Shares and Warrant Shares) made pursuant to this Section 4.2. For
convenience of reference, each of the Initial Closing and each Subsequent Closing are sometimes hereinafter singly referred to as a “Closing” and, together, they are referred to as the “Closings”.
SECTION 5. Representations and Warranties of the Corporation to the Investors.
Except as set forth in the Corporation’s disclosure schedule dated as of the date hereof and delivered herewith (the “Corporation’s Disclosure Schedule”), which shall be arranged to correspond to the representations and warranties in this Section 5, or, in each case, as applicable to the relevant other Sections of this Agreement, and the disclosure in any portion of the Corporation’s Disclosure Schedule shall qualify the corresponding provision in this Section 5 and any other provision of this Agreement, including but not limited to the provisions of this Section 5, to which it is reasonably apparent on its face that such disclosure relates notwithstanding the lack of any explicit cross-reference, the Corporation hereby represents and warrants to the Investors as of the date hereof as follows, except as set forth in the Public Filings (as defined below):
5.1 Organization. The Corporation is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware and has all requisite corporate power and authority to own and lease its property and to carry on its Business (as defined in Section 5.6) as presently conducted and as proposed to be conducted as described in the Public Filings (as defined in Section 5.6). The Corporation is duly qualified to do business as a foreign corporation in the Commonwealth of Massachusetts. The Corporation does not own or lease property or engage in any activity in any other jurisdiction which would require its qualification in such jurisdiction and in which the failure to be so qualified would have a material adverse effect on the Business, properties, assets, liabilities, condition (financial or otherwise) or prospects of the Corporation (a “Corporation Material Adverse Effect”).
5.2 Capitalization.
(a) The authorized capital stock of the Corporation immediately prior to the Initial Closing consists of:
(i) 100,000,000 shares of Common Stock, of which:
(1) 879,370 shares are validly issued and outstanding, fully paid and nonassessable (including 1,753,357 shares issuable upon exercise of warrants to purchase Common Stock);
(2) 40,829,997 shares have been duly reserved for issuance upon conversion of the Series B-2 Preferred Stock, the Corporation’s Series B Convertible Preferred Stock, par value $.0001 per share (the “Series B Preferred Stock”), the Corporation’s Series A-1 Convertible Preferred Stock, par value $.0001 per share (the “Series A-1 Preferred Stock”), the Corporation’s Series A-2 Convertible Preferred Stock, par value $.0001 per share (the “Series A-2 Preferred Stock”), the Corporation’s Series A-3 Convertible Preferred Stock, par value $.0001 per share, (the “Series A-3 Preferred Stock”), the Corporation’s Series A-4 Convertible Preferred Stock, par value $.0001 per share (the “Series A-4 Preferred Stock”), the Corporation’s Series A-5 Convertible Preferred Stock, par value $.0001 per share (the “Series A-5 Preferred Stock”), and the
Corporation’s Series A-6 Convertible Preferred Stock, par value $.0001 per share (the “Series A-6 Preferred Stock” and together with the Series B Preferred Stock, Series A-1 Preferred Stock, the Series A-2 Preferred Stock, the Series A-3 Preferred Stock, the Series A-4 Preferred Stock and the Series A-5 Preferred Stock, the “Existing Preferred Stock”, and the Existing Preferred Stock together with the Series B-2 Preferred Stock, the “Preferred Stock”) (including 157,287 shares of Series A-1 Preferred Stock issuable upon exercise of warrants to purchase Series A-1 Preferred Stock); and
(3) 7,354,290 shares have been duly reserved for issuance in connection with options outstanding or available under the Corporation’s 2011 Equity Incentive Plan, as amended, or 2003 Long-Term Incentive Plan, as amended (collectively, the “Plan Option Shares”).
(ii) 10,000,000 shares of Preferred Stock, of which:
(1) 655,000 have been designated the Series B-2 Preferred Stock, none of which is issued or outstanding;
(2) 980,000 have been designated the Series B Preferred Stock, 701,235 of which are issued and outstanding, fully paid and nonassessable;
(3) 1,000,000 have been designated the Series A-1 Preferred Stock, 939,612 of which are issued and outstanding, fully paid and nonassessable;
(4) 983,213 have been designated the Series A-2 Preferred Stock, 983,208 of which are issued and outstanding, fully paid and nonassessable;
(5) 142,230 have been designated the Series A-3 Preferred Stock, 142,227 of which are issued and outstanding, fully paid and nonassessable;
(6) 4,000 have been designated the Series A-4 Preferred Stock, 3,998 of which are issued and outstanding, fully paid and nonassessable;
(7) 7,000 have been designated the Series A-5 Preferred Stock, 6,443 of which are issued and outstanding, fully paid and nonassessable; and
(8) 800,000 have been designated the Series A-6 Preferred Stock, none of which are issued or outstanding.
(b) Except (i) pursuant to the terms of this Agreement, (ii) at any time prior to the Initial Closing, pursuant to the terms of the Third Amended and Restated Stockholders’ Agreement, dated as of April 23, 2013, by and among the Corporation and the stockholders named therein, as amended to date (the “Existing Stockholders’ Agreement”), (iii) as of and at all times following the Initial Closing, pursuant to the terms of that certain Fourth
Amended and Restated Stockholders’ Agreement to be entered into in connection with the Initial Closing, as contemplated by Section 7.1(e), in the form attached hereto as Exhibit C (the “Stockholders’ Agreement”), and (iv) as set forth in Schedule 5.2 attached hereto, there are and, immediately following the Initial Closing, there will be: (1) no outstanding warrants, options, rights, agreements, convertible securities or other commitments or instruments pursuant to which the Corporation is or may become obligated to issue, sell, repurchase or redeem any shares of capital stock or other securities of the Corporation (other than the Plan Option Shares); (2) no preemptive, contractual or similar rights to purchase or otherwise acquire shares of capital stock of the Corporation pursuant to any provision of law, the Certificate of Incorporation of the Corporation (as amended and/or restated, including without limitation, the Series B-2 Certificate, the Certificate of Designations of the Series B Preferred Stock, filed by the Corporation with the Secretary of State of the State of Delaware on April 23, 2013 (the “Series B Certificate”), the Certificate of Designations of the Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, Series A-5 Preferred Stock and Series A-6 Preferred Stock filed by the Corporation with the Secretary of State of the State of Delaware on May 17, 2011, as amended to date and from time to time (the “Series A Certificate”), the “Charter”), the by-laws of the Corporation (the “by-laws”) or any agreement to which the Corporation is a party or may otherwise be bound; (3) no restrictions on the transfer of capital stock of the Corporation imposed by the Charter or by-laws of the Corporation, any agreement to which the Corporation is a party, any order of any court or any governmental agency to which the Corporation is subject, or any statute other than those imposed by relevant state and federal securities laws; (4) no cumulative voting rights for any of the Corporation’s capital stock; (5) no registration rights under the Securities Act of 1933, as amended (the “Securities Act”), with respect to shares of the Corporation’s capital stock; (6) to the Corporation’s Knowledge (as defined below), no options or other rights to purchase shares of capital stock from stockholders of the Corporation granted by such stockholders; and (7) no agreements, written or oral, between the Corporation and any holder of its securities, or, to the Corporation’s Knowledge, among holders of its securities, relating to the acquisition, disposition or voting of the securities of the Corporation.
5.3 Authorization of this Agreement and the Stockholders’ Agreement. The execution, delivery and performance by the Corporation of this Agreement and the Stockholders’ Agreement and the consummation of the transactions contemplated hereby and thereby, including the filing of the Series B-2 Certificate, have been duly authorized by all requisite action on the part of the Corporation. Each of this Agreement and the Stockholders’ Agreement has been duly executed and delivered by the Corporation and constitutes a valid and binding obligation of the Corporation, enforceable in accordance with its respective terms. The execution, delivery and performance of this Agreement and the Stockholders’ Agreement, the filing of the Series B-2 Certificate and the compliance with the provisions hereof and thereof by the Corporation, will not:
(a) violate any provision of law, statute, ordinance, rule or regulation or any ruling, writ, injunction, order, judgment or decree of any court, administrative agency or other governmental body;
(b) conflict with or result in any breach of any of the terms, conditions or provisions of, or constitute (with due notice or lapse of time, or both) a default (or give rise to
any right of termination, cancellation or acceleration) under (i) any agreement, document, instrument, contract, understanding, arrangement, note, indenture, mortgage or lease to which the Corporation is a party or under which the Corporation or any of its assets is bound, which conflict, breach or default would have a Corporation Material Adverse Effect, (ii) the Charter, or (iii) the by-laws;
(c) result in the creation of any lien, security interest, charge or encumbrance upon any of the properties or assets of the Corporation; or
(d) conflict with any stockholder’s rights to participate in the transactions contemplated hereby, including but not limited to any rights to purchase Shares or Warrants hereunder.
5.4 Authorization of Shares, Warrants and Reserved Common Shares.
(a) The issuance, sale and delivery of the Shares and Warrants pursuant to the terms hereof have been duly authorized by all requisite action of the Corporation, and, when, in the case of the Shares, issued, sold and delivered in accordance with the terms of this Agreement or, in the case of the Warrants, exercised in accordance with the terms of the Warrants, the Shares and Warrant Shares, respectively, will be validly issued and outstanding, fully paid and nonassessable, with no personal liability attaching to the ownership thereof, and, except as may be set forth in the Stockholders’ Agreement (with respect to which the Corporation is in compliance with its obligations thereunder), not subject to preemptive or any other similar rights of the stockholders of the Corporation or others.
(b) The reservation, issuance, sale and delivery by the Corporation of the Reserved Common Shares and the Warrant Shares have been duly authorized by all requisite action of the Corporation, and the Reserved Common Shares and the Warrant Shares have been duly reserved in accordance with Section 2 of this Agreement. Upon the issuance and delivery of the Reserved Common Shares in accordance with the terms of this Agreement, the Reserved Common Shares will be validly issued and outstanding, fully paid and nonassessable and, except as may be set forth in the Stockholders’ Agreement, not subject to preemptive or any other similar rights of the stockholders of the Corporation or others.
5.5 Consents and Approvals. No authorization, consent, approval or other order of, or declaration to or filing with, any governmental agency or body (other than filings of a Form D required to be made under applicable federal securities laws and filings required to be made under applicable state securities laws) or any other person, entity or association is required for: (a) the valid authorization, execution, delivery and performance by the Corporation of this Agreement and the Stockholders’ Agreement; (b) the valid authorization, issuance, sale and delivery of the Shares; (c) the valid authorization, issuance, sale and delivery of the Warrants and Warrant Shares; (d) the valid authorization, reservation, issuance, sale and delivery of the Reserved Common Shares; or (e) the filing of the Series B-2 Certificate. The Corporation has obtained all other consents that are necessary to permit the consummation of the transactions contemplated hereby and thereby.
5.6 Business of the Corporation.
(a) Except as set forth in Schedule 5.6(a) of the Corporation’s Disclosure Schedule, the business of the Corporation (the “Business”) is described in the annual and other reports filed by the Corporation with the Securities and Exchange Commission (collectively, the “Public Filings”).
(b) All agreements or commitments to which the Corporation is a party or by which the Corporation or the Corporation’s assets and properties are bound that are material to the business of the Corporation as currently conducted are included as exhibits to the Public Filings. Without limitation of the foregoing, Schedule 5.6 of the Corporation’s Disclosure Schedule sets forth a list of all of the types of agreements or commitments set forth below that are not included as exhibits to the Public Filings (each such agreement included in the Public Filings and each agreement listed on Schedule 5.6 of the Corporation’s Disclosure Schedule being sometimes referred to herein as a “Material Agreement”):
(i) agreements which require future expenditures by the Corporation in excess of $250,000 or which might result in payments to the Corporation in excess of $250,000;
(ii) employment and consulting agreements, employee benefit, bonus, pension, profit-sharing, stock option, stock purchase and similar plans and arrangements;
(iii) agreements involving research, development, or the license of Intellectual Property (as defined in Section 5.12) (other than research, development, or license agreements which require future expenditures by the Corporation in amounts less than $250,000 or which might result in payments to the Corporation in amounts less than $250,000 in each case that do not grant to a third party or to the Corporation any rights in connection with the commercialization of any products), the granting of any right of first refusal, or right of first offer or comparable right with respect to any Intellectual Property or payment or receipt by the Corporation of milestone payments or royalties;
(iv) agreements relating to a joint venture, partnership, collaboration or other arrangement involving a sharing of profits, losses, costs or liabilities with another person or entity;
(v) distributor, sales representative or similar agreements;
(vi) agreements with any current or former stockholder, officer or director of the Corporation or any “affiliate” or “associate” of such persons (as such terms are defined in the rules and regulations promulgated under the Securities Act), including without limitation agreements or other arrangements providing for the furnishing of services by, rental of real or personal property from, or otherwise requiring payments to, any such person or entity;
(vii) agreements under which the Corporation is restricted from carrying on any business, or competing in any line of business, anywhere in the world;
(viii) indentures, trust agreements, loan agreements or notes that
involve or evidence outstanding indebtedness, obligations or liabilities for borrowed money;
(ix) agreements for the disposition of a material portion of the Corporation’s assets (other than for the sale of inventory in the ordinary course of business);
(x) agreements of surety, guarantee or indemnification;
(xi) interest rate, equity or other swap or derivative instruments;
(xii) agreements obligating Corporation to register securities under the Securities Act; and
(xiii) agreements for the acquisition of any of the assets, properties, securities or other ownership interests of the Corporation or another person or the grant to any person of any options, rights of first refusal, or preferential or similar rights to purchase any of such assets, properties, securities or other ownership interests.
(c) The Corporation has no present expectation or intention of not fully performing all of its obligations under each Material Agreement and, to the Corporation’s Knowledge, there is no breach or anticipated breach by any other party or parties to any Material Agreements.
(d) All of the Material Agreements are valid, in full force and effect and binding against the Corporation and to the Corporation’s Knowledge, binding against the other parties thereto in accordance with their respective terms. Neither the Corporation, nor, to the Corporation’s Knowledge, any other party thereto, is in default of any of its obligations under any of the Material Agreements, nor, to the Corporation’s Knowledge, does any condition exist that with notice or lapse of time or both would constitute a default thereunder. The Corporation has delivered or made available to each Investor or its representative true and complete copies of all of the foregoing Material Agreements or an accurate summary of any oral Material Agreements (and all written amendments or other modifications thereto).
(e) Except as provided in Schedule 5.6(e) of the Corporation’s Disclosure Schedule: (i) there are no actions, suits, arbitrations, claims, investigations or legal or administrative proceedings pending or, to the Corporation’s Knowledge, threatened, against the Corporation, whether at law or in equity; (ii) there are no judgments, decrees, injunctions or orders of any court, government department, commission, agency, instrumentality or arbitrator entered or existing against the Corporation or any of its assets or properties for any of the foregoing or otherwise; and (iii) the Corporation has not admitted in writing its inability to pay its debts generally as they become due, filed or consented to the filing against it of a petition in bankruptcy or a petition to take advantage of any insolvency act, made an assignment for the benefit of creditors, consented to the appointment of a receiver for itself or for the whole or any substantial part of its property, or had a petition in bankruptcy filed against it, been adjudicated a bankrupt, or filed a petition or answer seeking reorganization or arrangement under the federal bankruptcy laws or any other laws of the United States or any other jurisdiction.
(f) Except as set forth in Schedule 5.6(f) of the Corporation’s Disclosure Schedule, the Corporation is in compliance with all obligations, agreements and
conditions contained in any evidence of indebtedness or any loan agreement or other contract or agreement (whether or not relating to indebtedness) to which the Corporation is a party or is subject (collectively, the “Obligations”), the lack of compliance with which could afford to any person the right to accelerate any indebtedness or terminate any right of or agreement with the Corporation. To the Corporation’s Knowledge, all other parties to such Obligations are in compliance with the terms and conditions of such Obligations.
(g) Except for employment and consulting agreements set forth on Schedule 5.6 attached hereto and for agreements and arrangements relating to the Plan Option Shares and except as provided in Schedule 5.6(g) of the Corporation’s Disclosure Schedule, this Agreement and the Stockholders’ Agreement, there are no agreements, understandings or proposed transactions between the Corporation and any of its officers, directors or other “affiliates” (as defined in Rule 405 promulgated under the Securities Act).
(h) To the Corporation’s Knowledge, no employee of or consultant to the Corporation is in violation of any term of any employment contract, patent disclosure agreement or any other contract or agreement, including, but not limited to, those matters relating (i) to the relationship of any such employee with the Corporation or to any other party as a result of the nature of the Corporation’s Business as currently conducted, or (ii) to unfair competition, trade secrets or proprietary or confidential information.
(i) Each employee and director of or consultant to the Corporation, and each other person who has been issued shares of the Corporation’s Common Stock or options to purchase shares of the Corporation’s Common Stock is a signatory to, and is bound by, the Stockholders’ Agreement and, in the case of Common Stock issued to employees, directors and consultants, a stock restriction agreement, all with stock transfer restrictions and rights of first offer in favor of the Corporation in a form previously approved by the Board of Directors of the Corporation (the “Board of Directors”). In addition, each such stock restriction agreement contains a vesting schedule previously approved by the Board of Directors.
(j) The Corporation does not have any collective bargaining agreements covering any of its employees or any employee benefit plans.
(k) The Corporation has at all times complied with all provisions of its by-laws and Charter, and is not in violation of or default under any provision thereof, any contract, instrument, judgment, order, writ or decree to which it is a party or by which it or any of its properties are bound, and the Corporation is not in violation of any material provision of any federal or state statute, rule or regulation applicable to the Corporation.
5.7 Disclosure. None of this Agreement, the Stockholders’ Agreement or the Public Filings, nor any document, certificate or instrument furnished to any of the Investors or their counsel in connection with the transactions contemplated by this Agreement, contains or will contain any untrue statement of a material fact or omits or will omit to state a material fact necessary in order to make the statements contained herein or therein, in light of the circumstances under which they were made, not misleading. To the Corporation’s Knowledge, there is no fact which the Corporation has not disclosed to the Investors or their counsel which would reasonably be expected to result in a Corporation Material Adverse Effect.
5.8 Financial Statements. The Corporation has furnished or made available to each of the Investors (i) a complete and accurate copy of the audited balance sheet of the Corporation at December 31, 2012 and the related audited statements of operations and cash flows for the fiscal year then ended, and (ii) the unaudited balance sheet of the Corporation (the “Balance Sheet”) at November 30, 2013 (the “Balance Sheet Date”) and the related unaudited statements of operations and cash flows for the eleven month period then ended (collectively, the “Financial Statements”). The Financial Statements are in accordance with the books and records of the Corporation, present fairly in all material respects the financial condition and results of operations of the Corporation at the dates and for the periods indicated, and have been prepared in accordance with generally accepted accounting principles (“GAAP”) consistently applied, except, in the case of any unaudited Financial Statements, for the absence of footnotes normally contained therein and subject to normal and recurring year-end audit adjustments that are substantially consistent with prior year-end audit adjustments.
5.9 Absence of Undisclosed Liabilities. The Corporation has no liabilities of any nature (whether known or unknown and whether absolute or contingent), except for (a) liabilities shown on the Balance Sheet and (b) contractual and other liabilities incurred in the ordinary course of business which are not required by GAAP to be reflected on a balance sheet and which would not, either individually or in the aggregate, have or result in a Corporation Material Adverse Effect. The Corporation does not have any liabilities (and there is no basis for any present or, to the Corporation’s Knowledge, future proceeding against the Corporation giving rise to any liability) arising out of any personal injury and/or death or damage to property relating to or arising in connection with any clinical trials conducted by or on behalf of the Corporation.
5.10 Absence of Changes. Since the Balance Sheet Date and except as contemplated by this Agreement, there has been (i) no event or fact that individually or in the aggregate has had a Corporation Material Adverse Effect, (ii) no declaration, setting aside or payment of any dividend or other distribution with respect to, or any direct or indirect redemption or acquisition of, any of the capital stock of the Corporation, (iii) no waiver of any valuable right of the Corporation or cancellation of any debt or claim held by the Corporation, (iv) no loan by the Corporation to any officer, director, employee or stockholder of the Corporation, or any agreement or commitment therefor, (v) no increase, direct or indirect, in the compensation paid or payable to any officer, director, employee or agent Corporation and no change in the executive management of the Corporation or the terms of their employment, (vi) no material loss, destruction or damage to any property of the Corporation, whether or not insured, (vii) no labor disputes involving the Corporation, or (viii) no acquisition or disposition of any assets (or any contract or arrangement therefor), nor any transaction by the Corporation otherwise than for fair value in the ordinary course of business.
5.11 Payment of Taxes. The Corporation has prepared and filed within the time prescribed by, and in material compliance with, applicable law and regulations, all federal, state and local income, excise or franchise tax returns, real estate and personal property tax returns, sales and use tax returns, payroll tax returns and other tax returns required to be filed by it, and has paid or made provision for the payment of all accrued and paid taxes and other charges to which the Corporation is subject and which are not currently due and payable. The federal income tax returns of the Corporation have never been audited by the Internal Revenue Service.
Neither the Internal Revenue Service nor any other taxing authority is now asserting nor is threatening to assert against the Corporation any deficiency or claim for additional taxes or interest thereon or penalties in connection therewith, and the Corporation does not know of any such deficiency or basis for such deficiency or claim.
5.12 Intellectual Property.
(a) Schedule 5.12(a) lists each patent, patent application, copyright registration or application therefor, mask work registration or application therefor, and trademark, trademark application, trade name, service xxxx and domain name registration or application therefor owned by the Corporation, licensed by the Corporation or otherwise used by the Corporation (collectively, the “Listed Rights”). For each of the Listed Rights set forth on Schedule 5.12(a), an assignment to the Corporation of all right, title and interest in the Listed Right (or license to practice the Listed Right if owned by others) has been executed. All employees of and consultants to the Corporation have executed an agreement providing for the assignment to the Corporation of all right, title and interest in any and all inventions, creations, works and ideas made or conceived or reduced to practice wholly or in part during the period of their employment or consultancy with the Corporation, including all Listed Rights, to the extent described in any such agreement and providing for customary provisions relating to confidentiality and non-competition.
(b) Except as set forth on Schedule 5.12(b), the Listed Rights comprise all of the patents, patent applications, registered trademarks and service marks, trademark applications, trade names, registered copyrights and all licenses that have been obtained by the Corporation, and which, to the Corporation’s Knowledge, are necessary for the conduct of the Business of the Corporation as now being conducted and as proposed to be conducted in the Public Filings. Except as set forth on Schedule 5.12(b), the Corporation owns all of the Listed Rights and Intellectual Property, as hereinafter defined, free and clear of any valid and enforceable rights, claims, liens, preferences of any party against such Intellectual Property. To the Corporation’s Knowledge, except as set forth in Schedule 5.12(b), the Listed Rights and Intellectual Property are valid and enforceable rights and the practice of such rights does not infringe or conflict with the rights of any third party.
(c) To the Corporation’s Knowledge, the Corporation owns or has the right to use all Intellectual Property necessary (i) to use, manufacture, market and distribute the Customer Deliverables (as defined below) and (ii) to operate the Internal Systems (as defined below). The Corporation has taken all reasonable measures to protect the proprietary nature of each item of Corporation Intellectual Property (as defined below), and to maintain in confidence all trade secrets and confidential information that it owns or uses. To the Corporation’s Knowledge no other person or entity has any valid and enforceable rights to any of the Corporation Intellectual Property owned by the Corporation (except as set forth in Schedule 5.12(c)), and no other person or entity is infringing, violating or misappropriating any of the Corporation Intellectual Property.
(d) To the Corporation’s Knowledge, none of the Customer Deliverables, or the manufacture, marketing, sale, distribution, importation, provision or use thereof, infringes or violates, or constitutes a misappropriation of, any valid and enforceable
Intellectual Property rights of any person or entity; and, to the Corporation’s Knowledge neither the marketing, distribution, provision or use of any Customer Deliverables currently under development by the Corporation will, when such Customer Deliverables are commercially released by the Corporation, infringe or violate, or constitute a misappropriation of, any valid and enforceable Intellectual Property rights of any person or entity that exist today. To the Corporation’s Knowledge, none of the Internal Systems, or the use thereof, infringes or violates, or constitutes a misappropriation of, any valid and enforceable Intellectual Property rights of any person or entity.
(e) There is neither pending nor overtly threatened, or, to the Corporation’s Knowledge, any basis for, any claim or litigation against the Corporation contesting the validity or right to use any of the Listed Rights or Intellectual Property, and the Corporation has not received any notice of infringement upon or conflict with any asserted right of others nor, to the Corporation’s Knowledge, is there a basis for such a notice. To the Corporation’s Knowledge, no person, corporation or other entity is infringing the Corporation’s rights to the Listed Rights or Intellectual Property. Schedule 5.12(e) lists any complaint, claim or notice, or written threat thereof, received by the Corporation alleging any such infringement, violation or misappropriation, and the Corporation has provided to the Investors complete and accurate copies of all written documentation in the possession of the Corporation relating to any such complaint, claim, notice or threat. The Corporation has provided to the Investors complete and accurate copies of all written documentation in the Corporation’s possession relating to claims or disputes known to each of the Corporation concerning any Corporation Intellectual Property.
(f) Except as otherwise provided in Schedule 5.12(f), the Corporation, to the Corporation’s Knowledge has no obligation to compensate others for the use of any Listed Right or any Intellectual Property, nor has the Corporation granted any license or other right to use, in any manner, any of the Listed Rights or Intellectual Property, whether or not requiring the payment of royalties. Schedule 5.12(f) identities each license or other agreement pursuant to which the Corporation has licensed, distributed or otherwise granted any rights to any third party with respect to any Corporation Intellectual Property. Except as described in Schedule 5.12(f), the Corporation has not agreed to indemnify any person or entity against any infringement, violation or misappropriation of any Intellectual Property rights with respect to any Corporation Intellectual Property.
(g) Schedule 5.12(g) identifies each item of Corporation Intellectual Property that is owned by a party other than the Corporation, and the license or agreement pursuant to which the Corporation uses it (excluding off-the-shelf software programs licensed by the Corporation pursuant to “shrink wrap” licenses).
(h) The Corporation has not disclosed the source code for any software developed by it, or other confidential information constituting, embodied in or pertaining to such software, to any person or entity, except pursuant to the agreements listed in Schedule 5.12(h), and the Corporation has taken reasonable measures to prevent disclosure of any such source code.
(i) All of the copyrightable materials incorporated in or bundled with
the Customer Deliverables have been created by employees of the Corporation within the scope of their employment by the Corporation or by independent contractors of the Corporation who have executed agreements expressly assigning all right, title and interest in such copyrightable materials to the Corporation. Except as listed in Schedule 5.12(i), no portion of such copyrightable materials was jointly developed with any third party.
(j) To the Corporation’s Knowledge, the Customer Deliverables and the Internal Systems are free from significant defects or programming errors and conform in all material respects to the written documentation and specifications therefor.
(k) For purposes of this Agreement, the following terms shall have the following meanings:
(i) “Customer Deliverables” shall mean (a) the products that the Corporation (i) currently manufactures, markets, sells or licenses or (ii) currently plans to manufacture, market, sell or license in the future and (b) the services that the Corporation (i) currently provides or (ii) currently plans to provide in the future.
(ii) “Internal Systems” shall mean the internal systems of each of the Corporation that are presently used in its Business or operations, including, computer hardware systems, software applications and embedded systems.
(iii) “Intellectual Property” shall mean all: (A) patents, patent applications, patent disclosures and all related continuation, continuation-in-part, divisional, reissue, reexamination, utility model, certificate of invention and design patents, design patent applications, registrations and applications for registrations, including Listed Rights; (B) trademarks, service marks, trade dress, internet domain names, logos, trade names and corporate names and registrations and applications for registration thereof; (C) copyrights and registrations and applications for registration thereof; (D) mask works and registrations and applications for registration thereof; (E) computer software, data and documentation; (F) inventions, trade secrets and confidential business information, whether patentable or nonpatentable and whether or not reduced to practice, know-how, manufacturing and product processes and techniques, research and development information, copyrightable works, financial, marketing and business data, pricing and cost information, business and marketing plans and customer and supplier lists and information; (G) other proprietary rights relating to any of the foregoing (including remedies against infringements thereof and rights of protection of interest therein under the laws of all jurisdictions); and (H) copies and tangible embodiments thereof.
(iv) “Corporation Intellectual Property” shall mean the Intellectual Property owned by or licensed to the Corporation and incorporated in, underlying or used in connection with the Customer Deliverables or the Internal Systems.
(v) “Corporation’s Knowledge” shall mean (a) with respect to matters relating directly to the Corporation and its operations, the knowledge of Xxxxxx X. Xxxx, Xxxxxxxx Xxxxxx, and Xxxx Xxxxxxxxxx (the “Officers”) as well as other knowledge which such Officers would have possessed had they made diligent inquiry of appropriate employees and agents of the Corporation with respect to the matter in question; provided, that such Officers
shall not be obligated to inquire further with respect to any list herein or in any schedule hereto, and (b) with respect to external events or conditions, the actual knowledge of the Officers.
5.13 Securities Laws. Neither the Corporation nor anyone acting on its behalf has offered securities of the Corporation for sale to, or solicited any offers to buy the same from, or sold securities of the Corporation to, any person or organization, in any case so as to subject the Corporation, its promoters, directors and/or officers to any Liability under the Securities Act, the Securities and Exchange Act of 1934, as amended, or any state securities or “blue sky” law (collectively, the “Securities Laws”). The offer, grant, sale and/or issuance of the following were not, are not, or, as the case may be, will not be, in violation of the Securities Laws when offered, sold and issued in accordance with (i) this Agreement, (ii) the Series A-1 Convertible Preferred Stock Purchase Agreement, dated as of April 25, 2011, as amended, by and among the Corporation and the Investors listed therein, (iii) the Amended and Restated Stock Issuance Agreement, dated May 16, 2011, by and among the Corporation and Nordic Bioscience Clinical Development VII A/S, as amended, (iv) the 2003 Long-Term Incentive Plan, as amended, and (v) the 2011 Equity Incentive Plan, as amended: (a) the Shares, Warrants, Warrant Shares and Reserved Common Shares, as contemplated by this Agreement and the Exhibits and Schedules hereto, and in partial reliance upon the representations and warranties of the Investors set forth in Section 6 hereof; (b) the Existing Preferred Stock; (c) the Common Stock issuable upon the conversion of the Existing Preferred Stock; and (d) the Plan Option Shares and stock options covering such shares.
5.14 Title to Properties.
(a) The Corporation has valid title to, or in the case of leased properties and assets, valid leasehold interests in, all of its properties and assets, necessary to conduct the Business in the manner in which it is currently conducted (in each case, free and clear of all liens, security interests, charges and other encumbrances of any kind, except liens for taxes not yet due and payable), including without limitation, all rights under any investigational drug application of the Corporation filed in the United States and in foreign countries, all rights pursuant to the authority of the FDA (as hereinafter defined) and any foreign counterparts to conduct clinical trials with respect to any investigational drug application filed with such agency relating to biologics or drugs relating to the Business and all rights, if any, to apply for approval to commercially market and sell biologics or drugs and none of such properties or assets is subject to any lien, security interest, charge or other encumbrance of any kind, other than those the material terms of which are described in Schedule 5.14(a).
(b) The Corporation does not own any real property or any buildings or other structures, nor have options or any contractual obligations to purchase or acquire any interest in real property. Schedule 5.14(b) lists all real property leases to which the Corporation is a party and each amendment thereto. All such current leases are in full force and effect, are valid and effective in accordance with their respective terms, and there is not, under any of such leases, any existing default or event of default (or event that with notice or lapse of time, or both, would constitute a default). The Corporation, in its capacity as lessee, is not in violation of any zoning, building or safety ordinance, regulation or requirement or other law or regulation applicable to the operation of its leased properties, nor has it received any notice of violation with which it has not complied.
(c) The equipment, furniture, leasehold improvements, fixtures, vehicles, any related capitalized items and other tangible property material to the Business are in good operating condition and repair, ordinary wear and tear excepted.
5.15 Investments in Other Persons. Except as indicated in Schedule 5.15 attached hereto, (a) the Corporation has not made any loan or advance to any person or entity which is outstanding on the date hereof nor is it committed or obligated to make any such loan or advance, and (b) the Corporation has never owned or controlled and does not currently own or control, directly or indirectly, any subsidiaries and has never owned or controlled and does not currently own or control any capital stock or other ownership interest, directly or indirectly, in any corporation, association, partnership, trust, joint venture or other entity.
5.16 ERISA. Except as set forth in Schedule 5.16, neither the Corporation nor any entity required to be aggregated with the Corporation under Sections 414(b), (c), (m) or (n) of the Code (as hereinafter defined), sponsors, maintains, has any obligation to contribute to, has any liability under, or is otherwise a party to, any Benefit Plan. For purposes of this Agreement, “Benefit Plan” shall mean any plan, fund, program, policy, arrangement or contract, whether formal or informal, which is in the nature of (i) any qualified or non-qualified employee pension benefit plan (as defined in Section 3(2) of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”)) or (ii) an employee welfare benefit plan (as defined in section 3(1) of ERISA). With respect to each Benefit Plan listed in Schedule 5.16, to the extent applicable:
(a) Each such Benefit Plan has been maintained and operated in all material respects in compliance with its terms and with all applicable provisions of ERISA, the Internal Revenue Code of 1986, as amended (the “Code”), and all statutes, orders, rules, regulations, and other authority which are applicable to such Benefit Plan;
(b) All contributions required by law to have been made under each such Benefit Plan (without regard to any waivers granted under Section 412 of the Code) to any fund or trust established thereunder in connection therewith have been made by the due date thereof:
(c) Each such Benefit Plan intended to qualify under Section 401(a) of the Code is the subject of a favorable unrevoked determination letter issued by the Internal Revenue Service as to its qualified status under the Code, which determination letter may still be relied upon as to such tax qualified status, and no circumstances have occurred that would adversely affect the tax qualified status of any such Benefit Plan;
(d) The actuarial present value of all accrued benefits under each such Benefit Plan subject to Title IV of ERISA did not, as of the latest valuation date of such Benefit Plan, exceed the then current value of the assets of such Benefit Plan allocable to such accrued benefits, all as based upon the actuarial assumptions and methods currently used for such Benefit Plan;
(e) None of such Benefit Plans that are “employee welfare benefit plans” as defined in Section 3(1) of ERISA provides for continuing benefits or coverage for any
participant or beneficiary of any participant after such participant’s termination of employment, except as required by applicable law; and
(f) Neither the Corporation nor any trade or business (whether or not incorporated) under common control with the Corporation within the meaning of Section 4001 of ERISA has, or at any time has had, any obligation to contribute to any “multiemployer plan” as defined in Section 3(37) of ERISA.
5.17 Use of Proceeds. The net proceeds received by the Corporation from the sale of the Series B-2 Preferred Stock shall be used by the Corporation generally for the purposes set forth on Schedule 5.17 attached hereto.
5.18 Permits and Other Rights; Compliance with Laws. The Corporation has all permits, licenses, registrations, certificates, accreditations, orders, authorizations or approvals from any Governmental Entity (“Permits”) issued to or held by the Corporation. Other than the Permits listed on Schedule 5.18, there are no Permits, the loss or revocation of which would result in a Corporation Material Adverse Effect. The Corporation has all Permits necessary to permit it to own its properties and to conduct its Business as presently conducted and as proposed to be conducted. Each such Permit is in full force and effect and, to the Corporation’s Knowledge, no suspension or cancellation of such Permit is threatened and there is no basis for believing that such Permit will not be renewable upon expiration. The Corporation is in compliance in all material respects under each such Permit, and the transactions contemplated by this Agreement will not cause a violation under any of such Permits. The Corporation is in compliance in all material respects with all provisions of the laws and governmental rules and regulations applicable to its Business, properties and assets, and to the products and services sold by it, including, without limitation, all such rules, laws and regulations relating to fair employment practices and public or employee safety. The Corporation is in compliance with the Clinical Laboratories Improvement Act of 1967, as amended.
5.19 Insurance. Schedule 5.19 sets forth a true and complete list of all policies or binders of fire, theft, liability, product liability, workmen’s compensation, vehicular, directors’ and officers’ and other insurance held by or on behalf of the Corporation. Such policies and binders are in full force and effect, are in the amounts not less than is customarily obtained by corporations of established reputation engaged in the same or similar business and similarly situated and are in conformity with the requirements of all leases or other agreements to which the Corporation is a party and are valid and enforceable in accordance with their terms. The Corporation’s product liability insurance covers its clinical trials. The Corporation is not in default with respect to any provision contained in such policy or binder nor has the Corporation failed to give any notice or present any claim under any such policy or binder in due and timely fashion. There are no outstanding unpaid claims under any such policy or binder. The Corporation has not received notice of cancellation or non-renewal of any such policy or binder.
5.20 Board of Directors. Except as provided in Schedule 5.20 attached hereto, the Corporation has not extended any offer or promise or entered into any agreement, arrangement, understanding or otherwise, whether written or oral, with any person or entity by which the Corporation has agreed to allow such person or entity to participate, in any way, in the affairs of the Board of Directors, including without limitation, appointment or nomination as a
member, or right to appear at, or receive the minutes of a meeting of the Board of Directors.
5.21 Books and Records. The minute books of the Corporation contain complete and accurate records of all meetings and other corporate actions of the stockholders and Boards of Directors and committees thereof. The stock ledger of the Corporation is complete and accurate and reflects all issuances, transfers, repurchases and cancellations of shares of capital stock of the Corporation.
5.22 Environmental Matters.
(a) The Corporation has not used, generated, manufactured, refined, treated, transported, stored, handled, disposed, transferred, produced, processed or released (together defined as “Release”) any Hazardous Materials (as hereinafter defined) in any manner or by any means in violation of any Environmental Laws (as hereinafter defined). To the Corporation’s Knowledge, neither the Corporation nor any prior owner or tenant of the Property (as hereinafter defined) has Released any Hazardous Material or other pollutant or effluent into, on or from the Property in a way which can pose a risk to human health or the environment, nor is there a threat of such Release. As used herein, the term “Property” shall include, without limitation, land, buildings and laboratory facilities owned or leased by the Corporation or as to which the Corporation now has any duties, responsibilities (for clean-up, remedy or otherwise) or liabilities under any Environmental Laws, or as to which the Corporation or any subsidiary of the Corporation may have such duties, responsibilities or liabilities because of past acts or omissions of the Corporation or any such subsidiary or their predecessors, or because the Corporation or any such subsidiary or their predecessors in the past was such an owner or operator of, or some other relationship with, such land, buildings and/or laboratory facilities, all as more fully described in Schedule 5.22(a) of the Corporation’s Disclosure Schedule. The term “Hazardous Materials” shall mean (A) any chemicals, materials or substances defined as or included in the definition of “hazardous substances,” “hazardous wastes,” “hazardous materials,” “extremely hazardous wastes,” “restricted hazardous wastes,” “toxic substances,” “toxic pollutants,” “hazardous air pollutants,” “contaminants,” “toxic chemicals,” “toxins,” “hazardous chemicals,” “extremely hazardous substances,” “pesticides,” “oil” or related materials as defined in any applicable Environmental Law, or (B) any petroleum or petroleum products, oil, natural or synthetic gas, radioactive materials, asbestos-containing materials, urea formaldehyde foam insulation, radon, and any other substance defined or designated as hazardous, toxic or harmful to human health, safety or the environment under any Environmental Law.
(b) No notice of lien under any Environmental Laws has been filed against any Property of the Corporation.
(c) The use of the Property complies with lawful, permitted and conforming uses in all material respects under all applicable building, tire, safety, subdivision, zoning, sewer, environmental, health, insurance and other such laws, ordinances, rules, regulations and plan approval conditions of any governmental or public body or authority relating to the use of the Property.
(d) Except as described in Schedule 5.22(d) of the Corporation’s Disclosure Schedule, to the Corporation’s Knowledge, the Property does not contain: (i) asbestos
in any form; (ii) urea formaldehyde foam insulation; (iii) transformers or other equipment which contain dialectic fluid containing levels of polychlorinated biphenyls; (iv) radon; or (v) any other chemical, material or substance, the exposure to which is prohibited, limited or regulated by a federal, state or local government agency, authority or body, or which, even if not so regulated, to the Corporation’s Knowledge after reasonable investigation, may or could pose a hazard to the health and safety of the occupants of the Property or the owners or occupants of property adjacent to or in the vicinity of the Property.
(e) The Corporation has not received written notice that the Corporation is a potentially responsible party for costs incurred at a cleanup site or corrective action under any Environmental Laws. The Corporation has not received any written requests for information in connection with any inquiry by any Governmental Authority (as defined hereinafter) concerning disposal sites or other environmental matters. As used herein, “Governmental Authority” shall mean any nation or government, any federal, state, municipal, local, provincial, regional or other political subdivision thereof and any entity or person exercising executive, legislative, judicial, regulatory or administrative functions of, or pertaining to, government, Schedule 5.22(e) of the Corporation’s Disclosure Schedule identifies all locations where Hazardous Materials used in whole or in part by the Business of the Corporation or resulting from the Business, facilities or Property of the Corporation have been stored or disposed of by or on behalf of the Corporation. As used herein, “Environmental Laws” shall mean all applicable federal, state and local laws, ordinances, rules and regulations that regulate, fix liability for, or otherwise relate to, the handling, use (including use in industrial processes, in construction, as building materials, or otherwise), storage and disposal of hazardous and toxic wastes and substances, and to the discharge, leakage, presence, migration, threatened Release or Release (whether by disposal, a discharge into any water source or system or into the air, or otherwise) of any pollutant or effluent. Without limiting the preceding sentence, the term “Environmental Laws” shall specifically include the following federal and state laws, as amended:
FEDERAL
Comprehensive Environmental Response Compensation and Liability Act of 1980, as amended by the Superfund Amendments and Reauthorization Act of 1986, 42 U.S.C. § 9601 et seq.; the Emergency Planning and Community Xxxxx-xx-Xxxx Xxx, 00 X.X.X. § 00000 et seq.; the Resource Conservation and Recovery Act, 42 U.S.C. § 6901 et seq.; the Federal Water Pollution Control Act, 33 U.S.C. § 1251 et seq.; the Federal Insecticide, Fungicide and Rodenticide Act, 7 U.S.C. § 136 et seq.; the Toxic Substance Control Act, 15 U.S.C. § 2601 et seq.; the Oil Pollution Act of 1990, 33 U.S.C. § 1001 et seq.; the Hazardous Materials Transportation Act, as amended, 49 U.S.C. § 1801 et seq.; the Atomic Energy Act, as amended 42 U.S.C. § 2011 et seq.; the Occupational Safety and Health Act, as amended, 29 U.S.C. § 651 et seq.; the Federal Food, Drug and Cosmetic Act, as amended 21 U.S.C. § 301 et seq. (insofar as it regulates employee exposure to Hazardous Substances); the Clean Air Act, 42 U.S.C. 7401 et. seq.
STATE
MASSACHUSETTS ENVIRONMENTAL STATUTES
Massachusetts Clean Waters Act, Mass. Gen. L. Ch. 21, Section 26, et. seq., and regulations thereto; Massachusetts Solid Waste Disposal Laws. Mass. Gen. L. Ch. 16, Section 18, et. seq., and Ch. 111, Section 1 05A, and regulations thereto; Massachusetts Oil and Hazardous Materials Release Prevention and Response Act, Mass. Gen. L., Ch. 21 E, Section 1, et. seq., and regulations thereto; Massachusetts Solid Waste Facilities Law, Mass. Gen. L., Ch. 21H, Section 1, et. seq., and regulations thereto; Massachusetts Toxic Use Reduction Act, Mass. Gen. L., Ch. 211, Section 1, et. seq., and regulations thereto; Massachusetts Litter Control Laws, Mass. Gen. L. Ch. 111. Section 1 50A, et. seq., and regulations thereto; Massachusetts Wetlands Protection Laws, Mass. Gen. L., Ch. 130, Section 105, et. seq., and regulations thereto; Massachusetts Environmental Air Pollution Control Law, Mass. Gen. L.. Ch. 101, Section 2B, et. seq., and regulations thereto; Massachusetts Environmental Policy Act, Mass. Gen. L. Ch. 30, Section 61, et. seq., and regulations thereto; and Massachusetts Hazardous Waste Laws, Mass. Gen. L. Ch. 21C, Section 1, et. seq., and regulations thereto.
(f) The Corporation has maintained all environmental and operating documents and records substantially in the manner and for the time periods required by the Environmental Laws and any other laws, regulations or orders and has never conducted an environmental audit except as disclosed in Schedule 5.22(f) of the Corporation’s Disclosure Schedule. For purposes of this Section 5.22(f), an environmental audit shall mean any evaluation, assessment, study or test performed at the request of or on behalf of a Governmental Authority, including, but not limited to, a public liaison committee, but does not include normal
or routine inspections, evaluations or assessments which do not relate to a threatened or pending charge, restraining order or revocation of any permit, license, certificate, approval, authorization, registration or the like issued pursuant to the Environmental Laws and any other law, regulation or order.
(g) To the Corporation’s Knowledge, no part of the Property of the Corporation is (i) located within any wetlands area, (ii) subject to any wetlands regulations, or (iii) included in or is proposed for inclusion in, or abuts any property included in or proposed for inclusion in, the National Priority List or any similar state lists.
5.23 FDA Matters.
(a) The Corporation has (i) complied in all material respects with all applicable laws, regulations and specifications with respect to the manufacture, design, sale, storing, labeling, testing, distribution, inspection, promotion and marketing of all of the Corporation’s products and product candidates and the operation of manufacturing facilities promulgated by the U.S. Food and Drug Administration (the “FDA”) or any corollary entity in any other jurisdiction and (ii) conducted, and in the case of any clinical trials conducted on its behalf, caused to be conducted, all of its clinical trials with reasonable care and in compliance in all material respects with all applicable laws and the stated protocols for such clinical trials.
(b) All of the Corporation’s submissions to the FDA and any corollary entity in any other jurisdiction, whether oral, written or electronically delivered, were true, accurate and complete in all material respects as of the date made, and remain true, accurate and complete in all material respects and do not misstate any of the statements or information included therein, or omit to state a fact necessary to make the statements therein not materially misleading.
(c) The Corporation has not committed any act, made any statement or failed to make any statement that would breach the FDA’s policy with respect to “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” set forth in 56 Fed. Reg. 46191 (September 10, 1991) or any similar laws, rules or regulations, whether under the jurisdiction of the FDA or a corollary entity in any other jurisdiction, and any amendments or other modifications thereto. Neither the Corporation nor, to the Corporation’s Knowledge, any officer, employee or agent of the Corporation has been convicted of any crime or engaged in any conduct that would reasonably be expected to result in (i) debarment under 21 U.S.C. Section 335a or any similar state or foreign law or regulation or (ii) exclusion under 42 U.S.C. Section 1320a 7 or any similar state or foreign law or regulation, and neither the Corporation nor, to the Corporation’s Knowledge, any such person has been so debarred or excluded.
(d) The Corporation has not sold or marketed any products prior to receiving any required or necessary approvals or consents from any federal or state governmental authority, including but not limited to the FDA under the Food, Drug & Cosmetics Act of 1976, as amended, and the regulations promulgated thereunder, or any corollary entity in any jurisdiction. The Corporation has not received any notice of, nor is the Corporation aware of any, actions, citations, warning letters or Section 305 notices from the FDA or any corollary entity.
5.24 Compliance with Privacy Laws
(a) For purposes of this Agreement:
(i) “Foreign Privacy Laws” shall mean (a) the Directive 95/46/EC of the Parliament and of the Council of the European Union of 24 October 1995 on the protection of individuals with regard to the collection, use, disclosure, and processing of personal data and on the free movement of such data, (b) the corresponding national rules, regulations, codes, orders, decrees and rulings thereunder of the member states of the European Union and (c) any rules, regulations, codes, orders, decree, and rulings thereunder related to privacy, data protection or data transfer issues implemented in other countries.
(ii) “US Privacy Laws” shall mean any rules, regulations, codes, orders, decrees, and rulings thereunder of any federal, state, regional, county, city, municipal or local government of the United States or any department, agency, bureau or other administrative or regulatory body obtaining authority from any of the foregoing that relate to privacy, data protection or data transfer issues, including all implementing laws, ordinances or regulations, including, without limitation, the Health Insurance Portability and Accountability Act of 1996, as amended; the Children’s Online Privacy Protection Act (COPPA) of 1998, as amended; the Financial Modernization Act (Xxxxxx-Xxxxx-Xxxxxx Act) of 2000, as amended; the Fair Credit Reporting Act of 1970, as amended; the Privacy Act of 1974, as amended; the Family Education Rights and Privacy Act of 1974, as amended; the Right to Financial Privacy Act of 1978, as amended; the Privacy Protection Act of 1980, as amended; the Cable Communications Policy Act of 1984, as amended; the Electronic Communications Privacy Act of 1986, as amended; the Video Privacy Protection Act of 1988, as amended; the Telephone Consumer Protection Act of 1991, as amended; the Driver’s Privacy Protection Act of 1994, as amended; the Communications Assistance for Law Enforcement Act of 1994, as amended; the Telecommunications Act of 1996, as amended; and any implementing regulations related thereto;
(b) The Corporation is currently and has been at all times in compliance in all material respects with all Foreign Privacy Laws and US Privacy Laws; and the Corporation has not received notice (in writing or otherwise) regarding violation of such Foreign Privacy Laws or US Privacy Laws.
(c) No action, suit, proceeding, investigation, charge, complaint, claim, demand, or notice has been filed or commenced against the Corporation, nor to the Corporation’s Knowledge threatened against the Corporation, relating to Foreign Privacy Laws and US Privacy Laws; nor has the Corporation incurred any material liabilities (whether accrued, absolute, contingent or otherwise) under any Foreign Privacy Laws or US Privacy Laws.
(d) Health Insurance Portability and Accountability Act of 1996. The Corporation (i) has assessed the applicability of the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations (collectively, “HIPAA”) to the Corporation, including the fully insured and self-insured health plans that the Corporation sponsors or has sponsored or contributes to or has contributed to and health care provider activities, if any, in which the Corporation engages, (ii) has complied in all relevant respects with
HIPAA, including 45 C.F.R. Part 160 and Subparts A and E of Part 164 (the “HIPAA Privacy Rule”), including but not limited to HIPAA Privacy Rule requirements relating to health information use and disclosure, notices of privacy rights, appointment of a Privacy Officer, adoption of a privacy policy, amendment of plan documents, and implementation of employee training as to the handling of protected health information, and (iii) if required under the HIPAA Privacy Rule, has entered into business associate agreements on behalf of the Corporation’s health plans covering the handling of protected health information with vendors and others categorized under HIPAA as business associates of the Corporation’s health plans.
(e) Other Health Information Laws. Without limiting the generality of Section 5.24(a) through Section 5.24(d),
(i) the Corporation is currently, and has been at all times since its incorporation, in compliance in all material respects with all applicable health insurance, health information security, health information privacy, and health information transaction format Laws (each a “Health Information Law”), including, without limitation, any rules, regulations, codes, orders, decrees, and rulings thereunder of any federal, state, regional, county, city, municipal or local government, whether foreign or domestic, or any department, agency, bureau or other administrative or regulatory body obtaining authority from any of the foregoing; and
(ii) no action, suit, proceeding, investigation, charge, complaint, claim, demand, or notice has been filed or commenced against the Corporation nor to the Corporation’s Knowledge threatened against the Corporation, alleging any failure to comply with any Health Information Law; nor has the Corporation incurred any material liabilities (whether accrued, absolute, contingent or otherwise) under any Health Information Law.
5.25 Health Care and Affiliated Transactions; Xxxxx and Anti-Kickback Laws.
(a) For purposes of the Xxxxx II law and implementing regulations, if applicable, none of the directors or officers of the Corporation, or physicians employed by the Corporation, any other affiliates of the Corporation, or any of their respective immediate family members is (i) to the Corporation’s Knowledge, a partner or stockholder or has any other economic interest in any customer or supplier of the Corporation; (ii) a party to any transaction or contract with the Corporation; or (iii) indebted to the Corporation. The Corporation has not paid, or incurred any obligation to pay, any fees, commissions or other amounts to and is not a party to any agreement, business arrangement or course of dealing with any firm of or in which any of directors, officers or affiliates of the Corporation, or any of their respective immediate family members, is a partner or stockholder or has any other economic interest, other than ownership of less than one percent (1%) of a publicly traded corporation. No physician or family member of a physician has a financial relationship with the Corporation in violation of Section 1877 of the Social Security Act. The Corporation has made all filings required by Section 1877 of the Social Security Act.
(b) The Corporation has complied with all applicable state and federal “anti-kickback,” fraud and abuse, false claims and related statutes and regulations. The Corporation has received no notice of nor is otherwise aware of any inquiries, audits, subpoenas
or other investigations involving Corporation by the U.S. Department of Health and Human Services, the U.S. Office of Inspector General, any U.S. Attorney’s Office or any other federal or state agency with jurisdiction over such statutes or regulations.
SECTION 6. Representations and Warranties of the Investors to the Corporation.
Each of the Investors, as to itself, represents and warrants to the Corporation as follows:
(a) It is acquiring the Shares and Warrants and, in the event it should acquire Reserved Common Shares upon conversion of the Series B-2 Preferred Stock or Warrant Shares upon the exercise of its Warrant, it will be acquiring such Reserved Common Shares or Warrant Shares, for its own account, for investment and not with a view to the distribution thereof within the meaning of the Securities Act.
(b) It is an “accredited investor” as such term is defined in Rule 501(a) promulgated under the Securities Act.
(c) It agrees that the Corporation may place a legend on the certificates and Warrants delivered hereunder stating that the Shares, Warrants and any Reserved Common Shares and Warrant Shares have not been registered under the Securities Act, and, therefore, cannot be offered, sold or transferred unless they are registered under the Securities Act or an exemption from such registration is available and that the offer, sale or transfer of the Shares, Warrants and any Reserved Common Shares and Warrant Shares is further subject to any restrictions imposed by this Agreement and the Stockholders’ Agreement.
(d) The execution, delivery and performance by it of this Agreement have been duly authorized by all requisite action of it.
(e) It further understands that the exemptions from registration afforded by Rule 144 and Rule 144A (the provisions of which are known to it) promulgated under the Securities Act depend on the satisfaction of various conditions, and that, if applicable, Rule 144 may afford the basis for sales only in limited amounts.
(f) It has such knowledge and experience in business and financial matters and with respect to investments in securities of privately-held companies so as to enable it to understand and evaluate the risks of its investment in the Shares and Warrants and form an investment decision with respect thereto. It has been afforded the opportunity during the course of negotiating the transactions contemplated by this Agreement to ask questions of, and to secure such information from, the Corporation and its officers and directors as it deems necessary to evaluate the merits of entering into such transactions.
(g) If it is a natural person, it has the power and authority to enter into this Agreement. If it is not a natural person, it is duly organized and validly existing and has the power and authority to enter into this Agreement. Any Investor which is a corporation, partnership or trust represents that it has not been organized, reorganized or recapitalized specifically for the purpose of acquiring the securities of the Corporation.
(h) It has adequate net worth and means of providing for its current
needs and personal contingencies to sustain a complete loss of its investment in the Corporation. The Investors understand that the foregoing representations and warranties shall be deemed material and to have been relied upon by the Corporation.
SECTION 7. Closing Conditions.
7.1 Deliveries; Conditions Precedent to the Initial Closing. The several obligations of each Investor to purchase and pay for the Shares and Warrants under this Agreement at the Initial Closing are subject to the satisfaction or waiver by such Investor or any waiver adopted or implemented pursuant to Section 17 of the following conditions precedent:
(a) All proceedings to have been taken and all waivers and consents to be obtained in connection with the transactions contemplated by this Agreement shall have been taken or obtained, and all documents incidental thereto shall be satisfactory to each Investor and its counsel, and each Investor and its counsel shall have received copies (executed or certified, as may be appropriate) of all documents which such Investor or its counsel may reasonably have requested in connection with such transactions.
(b) All legal matters incident to the purchase or acquisition of the Shares and Warrants shall be satisfactory to each Investor’s counsel, and the Investors shall have received from Xxxxxx & Xxxxxxx LLP a legal opinion addressed to the Investors and dated the date of the Initial Closing in a form reasonably acceptable to the Investors.
(c) All consents, permits, approvals, qualifications and/or registrations required to be obtained or effected under any applicable securities or “Blue Sky” laws of any jurisdiction shall have been obtained or effected.
(d) Except as set forth in the Disclosure Schedules hereto, on the Initial Closing Date, the representations and warranties of the Corporation contained herein shall be true and correct on and as of the date of such Closing with the same force and effect as though such representations and warranties had been made on and as of such date (other than any representation or warranty made as of a particular date which shall be true and correct as of such date).
(e) A duly executed Series B-2 Certificate in the form of Exhibit A hereto shall have been filed with and accepted by the Secretary of State of Delaware and shall be effective under the laws of the State of Delaware, and a Stockholders’ Agreement in form and substance attached hereto as Exhibit C shall have been executed by the Corporation and the requisite stockholders of the Corporation such that the Stockholders’ Agreement amends and restates in its entirety the Existing Stockholders’ Agreement, and such executed Stockholders’ Agreement shall have been delivered to the Investors.
(f) The Corporation shall have delivered to the Investors a certificate or certificates, dated as of the Initial Closing Date, of the Secretary of the Corporation certifying as to (i) the resolutions of the Corporation’s Board of Directors and stockholders authorizing the execution and delivery of this Agreement and the delivery to the Investors of the Shares and Warrants, such other documents and instruments as may be required by this Agreement, and the consummation of the transactions contemplated hereby and thereby, (ii) that such resolutions
were duly adopted and have not been rescinded or amended as of said date, (iii) the name and the signature of the officers of the Corporation authorized to sign, as appropriate, this Agreement and the other documents and certificates to be delivered pursuant to this Agreement by either the Corporation or any of its officers, (iv) the Charter and (v) the Corporation’s by-laws.
(g) The Corporation shall have delivered to the Investors a certificate or certificates, dated as of the Initial Closing Date, of the President and Chief Executive Officer of the Corporation certifying as to the accuracy and completeness of the representations and warranties made by the Corporation pursuant to this Agreement as of each of the date of this Agreement and the date of the Initial Closing.
(h) The Corporation shall have entered into confidentiality and, to the extent allowable under arrangements or agreements between a consultant and any relevant institution with which he may be an employee, assignment of inventions agreements with all employees and consultants of the Corporation satisfactory in form and substance to the Investors and their counsel.
(i) The Board of Directors of the Corporation shall be comprised of the following individuals (collectively, the “Director Individuals”): Xxxx Xxxxxxxx, Xxxxxxx Xxxxxxx, Xxxx Xxxxxx, Xxxx Xxxxxx, Xxxxxx Xxxxx-Xxxxxxxxx, Xxxxxx Muenchbach, Xxxxxxxxx Xxxxxx, and Xxxxxx X. Xxxx.
(j) The Corporation shall have executed and delivered to each Investor so requesting a management rights letter in a form acceptable to such Investor.
(k) Stockholders comprising the Senior Majority (as defined in the Series A Certificate) and the Requisite Majority (as defined in the Series B Certificate) shall have consented to the adoption and filing of the Series B-2 Certificate, including the creation of the Series B-2 Preferred pursuant to the Series B-2 Certificate.
(l) Stockholders comprising the Majority Investors (as defined in the Existing Stockholders’ Agreement) shall have consented to the entry into this Agreement and the Stockholders’ Agreement by the Corporation, and the transactions contemplated hereby, and shall have waived all rights of first refusal (i.e., preemptive rights) under the Existing Stockholders’ Agreement (including the notice requirements set forth therein).
7.2 Conditions to Obligations of the Corporation. It shall be a condition precedent to the obligations of the Corporation hereunder to be performed at each Closing, as the case may be, as to each Investor severally, but not jointly, that (a) the representations and warranties contained herein of each of the Investors hereunder shall be true and correct as of the date of each such Closing with the same force and effect as though such representations and warranties had been made on and as of such date, (b) each Investor who is an individual shall have completed, executed and delivered to the Corporation an accredited investor questionnaire, in a form provided by and to the reasonable satisfaction of the Corporation and (c) such Investor shall be, or shall have executed and delivered a counterpart signature page or an Instrument of Adherence to become, a party to the Stockholders’ Agreement in the capacity of an Investor thereunder.
SECTION 8. Expenses and Fees.
Each party shall pay all of its own costs and other expenses incurred in connection with the agreements and conditions contained herein or contemplated hereby. The Corporation further agrees that it will pay, and hold each of the Investors harmless from, any and all liability with respect to any stamp or similar taxes which may be determined to be payable in connection with the execution and delivery of this Agreement or any modification, amendment or alteration of the terms or provisions of this Agreement and that it will similarly pay, and hold each of the Investors harmless from, all issue taxes in respect of the issuance of the Shares and Warrants to each of the Investors. Either at or as soon as reasonably practicable following the Initial Closing, the Corporation shall pay up to $20,000 of the reasonable and documented fees and expenses of Xxxxxxx XxXxxxxxx LLP, counsel to the Investors, incurred in connection with the review and negotiation of this Agreement, all documents and agreements related hereto and the transactions contemplated hereby.
SECTION 9. Brokers or Finders.
The Corporation represents and warrants to each of the Investors, and each of the Investors, as to itself, represents and warrants to the Corporation, that no person or entity has or will have, as a result of the transactions contemplated by this Agreement, any right, interest or valid claim against or upon the Corporation or the Investors for any commission, fee or other compensation as a finder or broker because of any act or omission by the Corporation or the Investors or by any agent of the Corporation or the Investors.
SECTION 10. Exchanges Lost. Stolen or Mutilated Certificates.
Upon surrender by any Investor to the Corporation of shares of Series B-2 Preferred Stock or Reserved Common Shares issued upon conversion of any Series B-2 Preferred Stock purchased or acquired by such Investor hereunder, the Corporation, at its expense, will issue in exchange therefor, and deliver to such Investor, a new certificate or certificates representing such shares in such denominations as may be requested by such Investor. Upon receipt of evidence satisfactory to the Corporation of the loss, theft, destruction or mutilation of any certificate representing any shares of Common Stock or Series B-2 Preferred Stock purchased or acquired by any Investor hereunder and, in case of any such loss, theft or destruction, upon delivery of any indemnity agreement satisfactory to the Corporation, or in case of any such mutilation, upon surrender and cancellation of such certificate, the Corporation, at its expense, will issue and deliver to such Investor a new certificate for such shares of Common Stock or Series B-2 Preferred Stock, as applicable, of like tenor, in lieu of such lost, stolen or mutilated certificate.
SECTION 11. Survival of Representations and Warranties.
The representations and warranties set forth in Sections 5 and 6 hereof shall survive the Closings indefinitely.
SECTION 12. Indemnification.
The Corporation shall indemnify, defend and hold each of the Investors harmless against any and all liabilities, loss, cost or damage, together with all reasonable costs and expenses
related thereto (including legal and accounting fees and expenses), arising from, relating to, or connected with the untruth, inaccuracy or breach of any statements, representations, warranties or covenants of the Corporation contained herein, including, but not limited to, all statements, representations, warranties or covenants concerning environmental matters.
SECTION 13. Remedies.
In case any one or more of the representations, warranties, covenants and/or agreements set forth in this Agreement shall have been breached by any party hereto, the party or parties entitled to the benefit of such representations, warranties, covenants or agreements may proceed to protect and enforce its or their rights either by suit in equity and/or action at law, including, but not limited to, an action for damages as a result of any such breach and/or an action for specific performance of any such covenant or agreement contained in this Agreement. The rights, powers and remedies of the parties under this Agreement are cumulative and not exclusive of any other right, power or remedy which such parties may have under any other agreement or law. No single or partial assertion or exercise of any right, power or remedy of a party hereunder shall preclude any other or further assertion or exercise thereof.
SECTION 14. Successors and Assigns.
Except as otherwise expressly provided herein, this Agreement shall bind and inure to the benefit of the Corporation and each of the Investors and the respective permitted successors and assigns of each of the Investors and the permitted successors and assigns of the Corporation. Subject to the provisions of Sections 3.1, 3.2, 3.3 and 3.10 of the Stockholders’ Agreement, this Agreement and the rights and duties of the Investors set forth herein may be freely assigned, in whole or in part, by the Investors. Neither this Agreement nor any of the rights or duties of the Corporation set forth herein shall be assigned by the Corporation, in whole or in part, without having first received the written consent of the Investors holding at least seventy percent (70%) of the shares of Series B-2 Preferred Stock then held by all of the Investors (the “Series B-2 Majority Investors”).
SECTION 15. Entire Agreement.
This Agreement, together with the other writings referred to herein, including the Series B-2 Certificate and the Stockholders’ Agreement, or delivered hereunder and which form a part hereof, contains the entire agreement among the parties with respect to the subject matter hereof and amends, restates and supersedes all prior and contemporaneous arrangements or understandings, whether written or oral, with respect thereto.
SECTION 16. Notices.
All notices, requests, consents and other communications hereunder to any party shall be deemed to be sufficient if contained in a written instrument delivered in person or duly sent by first class registered, certified or overnight mail, postage prepaid, or telecopied or e-mailed with a confirmation copy by regular mail, addressed, telecopied or e-mailed, as the case may be, to such party at the address, telecopier number or e-mail address, as the case may be, set forth below or such other address, telecopier number or e-mail address, as the case may be, as may hereafter be designated in writing by the addressee to the addressor listing all parties:
(i) if to the Corporation. to:
Radius Health, Inc.
000 Xxxxxxxx
Xxxxx Xxxxx
Xxxxxxxxx, XX 00000
Attention: B. Xxxxxxxx Xxxxxx
Telecopier: (000) 000-0000 E-mail: xxxxxxxx@xxxxxxxxxxx.xxx
with a copy to:
Xxxxxx & Xxxxxxx LLP
Xxxx Xxxxxxx Tower, 20th Floor
000 Xxxxxxxxx Xxxxxx
Xxxxxx, XX 00000
Attention: Xxxxx X. Xxxxxxxxx, Esq.
Telecopier: (000) 000-0000 E-mail: xxxxx.xxxxxxxxx@xx.xxx
(ii) if to Investors, as set forth on Schedule 1.
All such notices, requests, consents and other communications shall be deemed to have been received: (a) in the case of personal delivery, on the date of such delivery; (b) in the case of mailing, on the third business day following the date of such mailing; (c) in the case of overnight mail, on the first business day following the date of such mailing; (d) in the case of facsimile transmission, when confirmed by facsimile machine report; or (e) in the case of e-mail delivery, when confirmed by the sender’s e-mail system.
SECTION 17. Changes.
The terms and provisions of this Agreement may not be modified or amended, or any of the provisions hereof waived, temporarily or permanently, except pursuant to a writing executed by a duly authorized representative of the Corporation and the Series B-2 Majority Investors. Notwithstanding the foregoing, any modification or amendment to this Agreement that would adversely affect one Investor in a manner that is directed specifically to such Investor, rather than to all Investors, shall be subject to the approval of each such Investor. It is understood that this
separate consent would not be required if any such adverse effect results from the application of criteria uniformly to all Investors even if such application may affect Investors differently.
SECTION 18. Counterparts.
This Agreement may be executed in any number of counterparts, and each such counterpart shall be deemed to be an original instrument, but all such counterparts together shall constitute but one agreement.
SECTION 19. Headings.
The headings of the various sections of this Agreement have been inserted for convenience of reference only and shall not be deemed to be a part of this Agreement.
SECTION 20. Nouns and Pronouns.
Whenever the context may require, any pronouns used herein shall include the corresponding masculine, feminine or neuter forms, and the singular form of names and pronouns shall include the plural and vice-versa.
SECTION 21. Severability.
Any provision of this Agreement that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction.
SECTION 22. Further Assurances.
The parties shall cooperate reasonably with each other in connection with any steps required to be taken as part of their respective obligations under this Agreement, and shall furnish upon request to each other such further information, execute and deliver to each other such other documents, and do such other acts and things, all as the other party may reasonably request for purposes of carrying out the intend of this Agreement and consummating the transactions contemplated hereby.
SECTION 23. Governing Law.
This Agreement shall be governed by and construed in accordance with the laws of the Commonwealth of Massachusetts, excluding choice of laws rules thereof.
(Remainder of Page Left Intentionally Blank.)
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
|
|
THE CORPORATION: | ||
|
|
| ||
|
|
RADIUS HEALTH, INC. | ||
|
|
|
| |
|
|
By: |
/s/ Xxxxxx X. Xxxx | |
|
|
|
Name: |
Xxxxxx X. Xxxx |
|
|
|
Title: |
President & Chief Executive Officer |
|
|
| ||
|
|
| ||
|
INVESTORS: |
| ||
|
|
| ||
|
F2 BIOSCIENCE IV L.P. |
| ||
|
|
| ||
|
By: F2 Bioscience IV GP Ltd., General Partner |
| ||
|
|
|
| |
|
By: |
/s/ Xxxxxx Xxxxx-Embiricos |
| |
|
Name: |
Dr. Xxxxxx Xxxxx-Embiricos |
| |
|
Title: |
Authorized Signatory |
| |
|
|
|
| |
|
|
|
| |
|
F2 BIO VENTURES V L.P. |
| ||
|
|
| ||
|
By: F2 Bio Ventures GP Ltd., General Partner |
| ||
|
|
|
| |
|
By: |
/s/ Xxxxxx Xxxxx-Embiricos |
| |
|
Name: |
Dr. Xxxxxx Xxxxx-Embiricos |
| |
|
Title: |
Authorized Signatory |
|
|
BB BIOTECH VENTURES II, L.P. |
| ||
|
|
|
| |
|
By: |
BB Biotech Ventures GP (Guernsey) Limited |
| |
|
|
As General Partner to BB Biotech |
| |
|
|
Ventures II, L.P. |
| |
|
|
|
| |
|
|
|
| |
|
By: |
/s/ Xxxxxx Xxxxxxx |
| |
|
Name: |
Xxxxxx Xxxxxxx |
| |
|
Title: |
Director |
| |
|
BIOTECH GROWTH N.V. |
| |
|
|
| |
|
|
|
|
|
By: |
/s/ H.J. van Neutegem |
|
|
Name: |
H.J. van Neutegem |
|
|
Title: |
Managing Director |
|
(Investor Signature Page for Subsequent Closing under Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement)
INVESTOR:(1)
|
Legal Name of Investor: |
| ||||
|
| |||||
|
| |||||
|
Signature: |
| ||||
|
| |||||
|
Name of Signatory: |
| ||||
|
| |||||
|
Title of Signatory (if applicable): |
| ||||
|
| |||||
|
Date: |
| ||||
(1) An Investor participating in any Subsequent Closing may provide a specific signature block to use in executing this signature page in lieu of the blank signature block provided here.
Schedule I
|
Name of Investor |
|
Address of Record |
|
Series B-2 Shares |
|
Warrant Shares |
|
Aggregate Purchase Price |
| |
|
F2 Bioscience IV X.X. |
|
Xxxxxx Xxxxx Xxxxx Xxxxxx Xxxxxx PO Box 309 Xxxxxx Town KY1-1104 Grand Cayman Cayman Islands |
|
227,938 |
|
569,845 |
|
$ |
13,999,951.96 |
|
|
|
|
|
|
|
|
|
|
|
| |
|
F2 Bio Ventures V X.X. |
|
Xxxxxx Xxxxx Xxxxx Xxxxxx Xxxxxx PO Box 309 Xxxxxx Town KY1-1104 Grand Cayman Cayman Islands |
|
110,713 |
|
276,783 |
|
$ |
6,799,992.46 |
|
|
|
|
|
|
|
|
|
|
|
| |
|
BB Biotech Ventures II, L.P. |
|
Trafalgar Court Les Banques St. Xxxxx Port Guernsey Channel Islands GY1 3QL
With copies to Xxxxxx Xxxxxxxxx Bellevue Asset Management Xxxxxxxxxx 00 0000 Xxxxxxxx Xxxxxxxxxxx |
|
12,211 |
|
30,528 |
|
$ |
749,999.62 |
|
|
|
|
|
|
|
|
|
|
|
| |
|
Biotech Growth N.V. |
|
Xxxxxxx 00 Xxxxxxx |
|
65,125 |
|
162,813 |
|
$ |
3,999,977.50 |
|
|
TOTAL: |
|
|
|
415,987 |
|
1,039,969 |
|
$ |
25,549,921.54 |
|
Exhibit A
Form of Certificate of Designations of Series B-2 Convertible Preferred Stock
CERTIFICATE OF DESIGNATIONS
OF THE
SERIES B-2 CONVERTIBLE PREFERRED STOCK
OF
RADIUS HEALTH, INC.
Radius Health, Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the “Corporation”), hereby certifies that the following resolution pertaining to this Certificate of Designations of the Series B-2 Convertible Preferred Stock of the Corporation (this “Certificate of Designations” or this “Certificate”) was adopted by the Board of Directors of the Corporation (the “Board of Directors” or the “Board”) as required by Section 151 of the General Corporation Law of the State of Delaware at a meeting of the Board held on January 30, 2014. Capitalized terms used but not defined herein shall have the respective meanings ascribed thereto in the Corporation’s Certificate of Incorporation (the “Certificate of Incorporation”), the Series B Certificate (as defined below) or the Series A Certificate (as defined below), as applicable.
RESOLVED, that pursuant to the authority expressly granted to and vested in the Board in accordance with the provisions of the Certificate of Incorporation, the Board hereby creates a series of preferred stock of the Corporation to be designated as Series B-2 Convertible Preferred Stock, par value $.0001 per share (the “Series B-2 Stock”), and hereby states the number of shares, powers, terms, conditions, designations, preferences and privileges, relative, participating, optional and other special rights, and qualifications, limitations and restrictions thereof as follows:
1. Series B-2 Designation and Amount; Existing Preferred Stock. The number of authorized shares of the Series B-2 Stock is six hundred fifty-five thousand (655,000). The number of shares, powers, terms, conditions, designations, preferences and privileges, relative, participating, optional and other special rights, and qualifications, limitations and restrictions, if any, of (i) the Corporation’s Series B Convertible Preferred Stock, par value $.0001 per share (the “Series B Stock”) are as set forth in that certain Certificate of Designations of the Series B Convertible Preferred Stock of the Corporation filed by the Corporation with the Secretary of State of the State of Delaware on April 23, 2013, as amended from time to time (the “Series B Certificate”), and (ii) the Corporation’s Series A-1 Convertible Preferred Stock, par value $.0001 per share (the “Series A-1 Stock”), Series A-2 Convertible Preferred Stock, par value $.0001 per share (the “Series A-2 Stock”), Series A-3 Convertible Preferred Stock, par value $.0001 per share, (the “Series A-3 Stock” and together with the Series B-2 Stock, the Series B Stock, the Series A-1 Stock and the Series A-2 Stock, the “Participating Preferred Stock”), Series A-4 Convertible Preferred Stock, par value $.0001 per share (the “Series A-4 Stock”), Series A-5 Convertible Preferred Stock, par value $.0001 per share (the “Series A-5 Stock”) and Series A-6 Convertible Preferred Stock, par value $.0001 per share (the “Series A-6 Stock” and together with the Series B-2 Stock, the Series B Stock, the Series A-1 Stock, the Series A-2 Stock, the Series A-3 Stock, the Series A-4 Stock and the Series A-5 Stock, the “Preferred Stock”) are as set forth in that certain Certificate of Designations of the Series A-1 Convertible Preferred Stock,
Series A-2 Convertible Preferred Stock, Series A-3 Convertible Preferred Stock, Series A-4 Convertible Preferred Stock, Series A-5 Convertible Preferred Stock and Series A-6 Convertible Preferred Stock of the Corporation filed by the Corporation with the Secretary of State of the State of Delaware on May 17, 2011, as amended to date and from time to time (the “Series A Certificate”).
2. Ranking. As to dividends (other than with respect to the payment of the Series A-5 Special Accruing Dividend (as defined in the Series A Certificate) which shall rank senior in payment to any other dividends payable on any and all series of Preferred Stock) and upon Liquidation (as defined in Section 4(b) hereof) or an Event of Sale (as defined in Section 5 hereof), each share of Series B-2 Stock shall rank equally with each other share of Series B Stock and Series B-2 Stock, senior to all shares of Series A-1 Stock, Series A-2 Stock, Series A-3 Stock, Series A-4 Stock, Series A-5 Stock and Series A-6 Stock and senior to all shares of Common Stock and all other classes or series of stock not authorized by this Certificate, the Series B Certificate or the Series A Certificate as of the date hereof (the “Effective Time”), except as otherwise approved by the affirmative vote or consent of (i) the holders of at least 70% of the then outstanding shares of Series B Stock, (ii) the holders of at least 70% of the then outstanding shares of Series B-2 Stock and (iii) the Senior Majority (as defined in the Series A Certificate) (the stockholders referenced in the immediately preceding clauses (i), (ii) and (iii) being referred to herein collectively as the “Required Investor Majority”).
3. Dividend Provisions.
(a) The holders of shares of Series B-2 Stock shall be entitled to receive a per share dividend at the rate of 8% of the Series B-2 Original Purchase Price (as defined in Section 8 hereof) per annum, compounding annually (the “Series B-2 Accruing Dividend”), and which will accrue on a daily basis, whether or not declared, commencing on the date of issuance of such share of Series B-2 Stock. The holders of Series B-2 Stock shall be entitled to receive dividends prior in right to the payment of dividends and other distributions (whether in cash, property or securities of the Corporation, including subscription or other rights to acquire securities of the Corporation) on the Series A-1 Stock, Series A-2 Stock, Series A-3 Stock, Series A-4 Stock, Series A-5 Stock, Series A-6 Stock and Common Stock, but not with respect to (i) the payment of the Series B Accruing Dividend, as set forth in Section 3(a) of the Series B Certificate, which shall rank equally in payment to any dividends payable with respect to the Series B-2 Stock and (ii) the payment of the Series A-5 Special Accruing Dividend, as set forth in Section 3(d) of the Series A Certificate, which shall rank senior in payment to any dividends payable with respect to the Series B-2 Stock. Any dividends with respect to the Series B-2 Stock shall be payable, at the sole discretion of the Board of Directors, in cash or the issuance of that number of shares of Common Stock equal to the quotient obtained by dividing (x) the amount of such accrued and unpaid dividends thereon by (y) the Current Market Price (as defined in Section 7(e)(viii) hereof) of a share of Common Stock, when as and if declared or paid by the Board of Directors and, as accrued, on any Liquidation (as defined in Section 4(b) hereof) or Event of Sale (as defined in Section 5 hereof). Dividends with respect to the Series B-2 Stock shall be payable in shares of Common Stock (calculated based upon the then effective Series B-2 Conversion Price, as defined in Section 7(e) hereof), as accrued, upon the conversion of the Series B-2 Stock into Common Stock. Whenever any dividend may be declared or paid on any share of Series B-2 Stock, the Board of Directors shall also declare and
pay a dividend on the same terms, at the same rate and in like kind upon each other share of the Series B-2 Stock then outstanding, so that all outstanding shares of Series B-2 Stock will participate equally with each other and ratably per share (calculated as provided in Section 3(b) hereof). Whenever any dividend or other distribution, whether in cash or property or in securities of the Corporation (or subscription or other rights to purchase or acquire securities of the Corporation), may be declared or paid on: (i) any shares of the Common Stock, the Board of Directors shall also declare and pay a dividend on the same terms, at the same rate and in like kind upon each share of the Series B-2 Stock then outstanding so that all outstanding shares of Series B-2 Stock will participate in such dividend ratably with such shares of Common Stock (calculated as provided in Section 3(b) hereof); or (ii) any shares of any other series of Preferred Stock (other than the Series B Accruing Dividend, the Series A-1 Accruing Dividend, the Series A-2 Accruing Dividend, the Series A-3 Accruing Dividend, and the Series A-5 Special Accruing Dividend), the Board of Directors shall also declare and pay a dividend on the same terms, at the same or equivalent rate upon each share of the Series B-2 Stock then outstanding so that all outstanding shares of Series B-2 Stock will participate in such dividend ratably with such shares of such other series of Preferred Stock (based on the number of shares of Common Stock into which each share of Series B-2 Stock and each share of such other series of Preferred Stock is then convertible, if applicable, or, otherwise, the relative liquidation preference per share, of such other series of Preferred Stock as compared with the Series B-2 Stock then outstanding).
(b) In connection with any dividend declared or paid hereunder, each share of Series B-2 Stock shall be deemed to be that number of shares (including fractional shares) of Common Stock into which it is then convertible, rounded up to the nearest one-tenth of a share. No fractional shares of capital stock shall be issued as a dividend hereunder. The Corporation shall pay a cash adjustment for any such fractional interest in an amount equal to the fair market value thereof on the last Business Day (as defined in Section 8 hereof) immediately preceding the date for payment of dividends as determined by the Board of Directors in good faith.
4. Liquidation Rights.
(a) As to rights upon any Liquidation (as defined in Section 4(b) hereof) or an Event of Sale (as defined in Section 5 hereof), each share of Series B-2 Stock shall rank equally with each share of Series B Stock and each other share of Series B-2 Stock and senior to all shares of Series A-1 Stock, Series A-2 Stock, Series A-3 Stock, Series A-4 Stock, Series A-5 Stock, Series A-6 Stock, Common Stock and all other classes or series of stock not authorized by this Certificate, the Series B Certificate or the Series A Certificate as of the Effective Time, except as otherwise approved by the affirmative vote or consent of the Required Investor Majority.
(b) In the event of any liquidation, dissolution or winding-up of the affairs of the Corporation (collectively, a “Liquidation”): (i) the holders of shares of Series B-2 Stock then outstanding (the “Series B-2 Stockholders”) shall be entitled to receive, ratably with each other, out of the assets of the Corporation legally available for distribution to its stockholders, whether from capital, surplus or earnings, before any payment shall be made to the holders of shares of Series A-1 Stock then outstanding (such holders, the “Series A-1 Stockholders”), the holders of shares of Series A-2 Stock then outstanding (such holders, the “Series A-2 Stockholders”), the
holders of shares of Series A-3 Stock then outstanding (such holders, the “Series A-3 Stockholders”), the holders of shares of Series A-4 Stock then outstanding (such holders, the “Series A-4 Stockholders”), the holders of shares of Series A-5 Stock then outstanding (such holders, the “Series A-5 Stockholders”) or the holders of shares of Series A-6 Stock then outstanding (such holders, the “Series A-6 Stockholders” and collectively with the Series A-1 Stockholders, Series A-2 Stockholders, Series A-3 Stockholders, Series A-4 Stockholders and the Series A-5 Stockholders, the “Preferred Stockholders”), or the holders of shares of Common Stock or any other class or series of stock ranking on Liquidation junior to such Series B-2 Stock and at the same time as any payment made to the holders of Series B Stock then outstanding (the “Series B Stockholders”) under Section 4(b) of the Series B Certificate or any other series of stock on Liquidation junior to or on parity with such Series B-2 stock, an amount per share equal to the sum of (A) the product of 1.5 and the Series B-2 Original Purchase Price (as defined in Section 8 hereof), plus (B) an amount equal to any declared or accrued but unpaid dividends thereon, calculated pursuant to Section 3(a) hereof; and (ii) after the distribution to the Series B Stockholders and the Series B-2 Stockholders and to the holders of any other class or series of capital stock ranking on Liquidation on parity with the Series B-2 stock of the full amount to which they are entitled to receive pursuant to this Section 4(b) or any other section of this Certificate as in effect from time to time, the Preferred Stockholders and the holders of shares of Common Stock or any other class or series of stock ranking on Liquidation junior to the Series B Stock and the Series B-2 Stock, shall be entitled to receive, out of the assets of the Corporation legally available for distribution to its stockholders, whether from capital, surplus or earnings, an amount, if any, as determined in accordance with the terms of the Series A Certificate, subject to the terms of Section 4(d) of this Certificate and Section 4(d) of the Series B Certificate.
(c) If, upon any Liquidation, the assets of the Corporation available for distribution to its stockholders shall be insufficient to pay the Series B Stockholders and the Series B-2 Stockholders the full amount to which each of them shall be entitled pursuant to Section 4(b) of the Series B Certificate or Section 4(b) above, as the case may be, then the Series B Stockholders and the Series B-2 Stockholders shall, prior to the Preferred Stockholders, the holders of shares of Common Stock or any other class or series of stock ranking on Liquidation junior to such Series B Stock and Series B-2 Stock, share ratably in any distribution of assets according to the respective amounts which would be payable to them in respect of the shares of Series B Stock or Series B-2 Stock, as the case may be, held upon such distribution if all amounts payable on or with respect to such shares were paid in full.
(d) In the event of any Liquidation, after payments shall have been made first to the Preferred Stockholders and to the holders of any class or series of capital stock that is senior to or on parity with the Preferred Stock, or any series thereof, as in effect from time to time, and to the holders of any class or series of capital stock that is junior to or on parity with the Preferred Stock but senior to the Common Stock, of the full amount to which they each shall be entitled as aforesaid, the holders of Common Stock, as a class, shall be entitled to share ratably with the holders of Participating Preferred Stock (as provided in the last sentence in this Section 4(d)) in all remaining assets of the Corporation legally available for distribution to its stockholders. For purposes of calculating the amount of any payment to be paid upon any such Liquidation pursuant to the participation feature described in this Section 4(d), each share of such Participating Preferred Stock shall be deemed to be that number of shares (including
fractional shares and any shares attributable to the payment of accrued and unpaid dividends upon conversion of such Participating Preferred Stock pursuant to Section 7(b)) of Common Stock into which it is then convertible, rounded to the nearest one-tenth of a share.
(e) (i) In the event of and simultaneously with the closing of an Event of Sale, the Corporation shall, unless waived by the affirmative vote or consent of the holders of at least 70% of the then outstanding shares of Series B-2 Stock (the “Series B-2 Majority”) or otherwise prevented by law, redeem all of the shares of Series B-2 Stock then outstanding for a cash amount per share determined as set forth in Sections Section 4(a) through (d) hereof (the “Series B-2 Special Liquidation Price,” said redemption being referred to herein as a “Series B-2 Special Liquidation”). In the event the Event of Sale involves consideration that does not consist of cash, then the Series B-2 Special Liquidation Price may be paid with such consideration having a value equal to the Series B-2 Special Liquidation Price. To the extent there is any cash consideration in connection with an Event of Sale, at the option of the Series B-2 Majority, the cash consideration will first (i) be applied to satisfy the Series B-2 Special Liquidation Price payable to the Series B-2 Stockholders, the Series B Special Liquidation Price (as defined in the Series B Certificate) payable to the Series B Stockholders and the liquidation preference payable to the holders of any other class or series of capital stock that is senior to or on parity with the Series B-2 Stock as to Liquidation; and then (ii) be applied, pursuant to, and in accordance with, the terms of the Series A Certificate, to satisfy the Special Liquidation Price (as defined in the Series A Certificate) payable to the Preferred Stockholders and the liquidation preference payable to the holders of any other class or series of capital stock that is junior to the Series B-2 Stock but senior to or on parity with any other series of Preferred Stock as to Liquidation. For all purposes of this Section 4(e), the Series B-2 Special Liquidation Price shall be equal to that amount per share which would be received by each Series B-2 Stockholder if, in connection with an Event of Sale, all the consideration paid in exchange for the assets or the shares of capital stock (as the case may be) of the Corporation were actually paid to and received by the Corporation and the Corporation were immediately thereafter liquidated and its assets distributed pursuant to Sections 4(a) through (d) hereof. To the extent that the Series B-2 Special Liquidation, the Series B Special Liquidation under Section 4(e) of the Series B Certificate and/or Special Liquidations (as defined in the Series A Certificate) are occurring concurrently, the (i) Series B-2 Special Liquidation under this Section 4(e), and (ii) the Series B Special Liquidation under Section 4(e) of the Series B Certificate shall be deemed to occur simultaneously with each other and prior to any Special Liquidations (as defined in the Series A Certificate). The date upon which the Series B-2 Special Liquidation shall occur is sometimes referred to herein as the “Series B-2 Special Liquidation Date”.
(ii) In the absence of an applicable waiver pursuant to Section 4(e) above, at any time on or after the Series B-2 Special Liquidation Date, a Series B-2 Stockholder shall be entitled to receive the Series B-2 Special Liquidation Price for each such share of Series B-2 Stock owned by such holder. Subject to the provisions of Section 4(e)(iii) hereof, payment of the Series B-2 Special Liquidation Price will be made to each such holder upon actual delivery to the Corporation or its transfer agent of the certificate of such holder representing such shares of Series B-2 Stock, as the case may be, or an affidavit of loss as to the same.
(iii) If on the Series B-2 Special Liquidation Date less than all the shares of Series B-2 Stock then outstanding may be legally redeemed by the Corporation, the Series B-2 Special Liquidation shall be made first as to the Series B-2 Stock (and any other class or series of capital stock that is senior to or on parity with the Series B-2 Stock as to Liquidation, including the Series B Stock), pro rata with respect to such Series B-2 Stock (or such other class or series of capital stock that is senior to or on parity with the Series B-2 Stock as to Liquidation, including the Series B Stock) based upon the number of outstanding shares of Series B-2 Stock (or such other class or series of capital stock that is senior to or on parity with the Series B-2 Stock as to Liquidation, including the Series B Stock) then owned by each such holder thereof until such holders are satisfied in full.
(iv) On and after any Series B-2 Special Liquidation Date, all rights in respect of the shares of Series B-2 Stock to be redeemed shall cease and terminate except the right to receive the applicable Series B-2 Special Liquidation Price as provided herein, and such shares of Series B-2 Stock shall no longer be deemed to be outstanding, whether or not the certificates representing such shares of Series B-2 Stock have been received by the Corporation; provided, however, that, if the Corporation defaults in the payment of the Series B-2 Special Liquidation Price with respect to any Series B-2 Stock, the rights of the holder(s) thereof with respect to such shares of Series B-2 Stock shall continue until the Corporation cures such default.
(v) Anything contained herein to the contrary notwithstanding, all or any of the provisions of this Section 4(e) may be waived by the Required Investor Majority, by delivery of written notice of waiver to the Corporation prior to the closing of any Event of Sale.
(vi) Any notice required to be given to the holders of shares of Series B-2 Stock pursuant to Section 7(g) hereof in connection with an Event of Sale shall include a statement by the Corporation of (A) the Series B-2 Special Liquidation Price which each Series B-2 Stockholder shall be entitled to receive upon the occurrence of a Series B-2 Special Liquidation under this Section 4(e) and (B) the extent to which the Corporation will, if at all, be legally prohibited from paying each holder of Series B-2 Stock the Series B-2 Special Liquidation Price.
5. Definition of “Event of Sale”. For purposes of this Certificate of Designations, an “Event of Sale” shall mean: (A) the sale by the stockholders of voting control of the Corporation, (B) the merger, consolidation or reorganization with or into any other corporation, entity or person or any other corporate reorganization, in which (I) the capital stock of the Corporation immediately prior to such merger, consolidation or reorganization represents less than 50% of the voting power of the surviving entity (or, if the surviving entity is a wholly owned subsidiary, its parent) immediately after such merger, consolidation or reorganization or (II) the surviving entity (or, if the surviving entity is a wholly owned subsidiary, its parent) has a class of securities that is (or has been within 90 days prior to such transaction) tradeable on any public market or exchange or (C) the sale, exclusive license or other disposition of all or substantially all of the assets or intellectual property of the Corporation in a single transaction or series of related transactions.
6. Voting.
(a) Subject to any separate voting rights provided for herein or otherwise required by law, the holders of Series B-2 Stock shall be entitled to vote, together with the holders of Common Stock and the holders of other Preferred Stock as one class, on all matters as to which holders of Common Stock shall be entitled to vote, in the same manner and with the same effect as such holders of Common Stock. In any such vote, each share of Series B-2 Stock shall entitle the holder thereof to the number of votes per share that equals the number of shares of Common Stock (including fractional shares) into which each such share of Series B-2 Stock is then convertible, rounded up to the nearest one-tenth of a share, but not including any shares of Common Stock issuable upon conversion of any dividends accrued on such Series B-2 Stock.
(b) Except as otherwise expressed, implied or contemplated in this Certificate or the Series B-2 Purchase Agreement, the Corporation shall not, directly or indirectly, through a merger, consolidation, reorganization or otherwise, without the affirmative approval of the Required Investor Majority acting separately from the holders of Common Stock or any other securities of the Corporation, given by written consent in lieu of a meeting or by vote at a meeting called for such purpose, for which meeting or approval by written consent timely and specific notice in the manner provided in the by-laws of the Corporation shall have been given to each Series B-2 Stockholder, do any of the following:
(i) authorize, create, designate, issue or sell any class or series of capital stock (including any shares of treasury stock) or rights, options, warrants or other securities convertible into or exercisable or exchangeable for capital stock which by its terms is convertible into or exchangeable for any equity security, other than Excluded Stock (as defined in Section 7(e)(ii) of this Certificate), which, as to the payment of dividends or distribution of assets, including without limitation distributions to be made upon a Liquidation, is senior to or on a parity with the Series B-2 Stock; or
(ii) amend, alter or repeal any provision of this Certificate, the Series B Certificate or the Series A Certificate; or
(iii) permit, approve or agree to any Liquidation, Event of Sale, dissolution or winding up of the Corporation.
The foregoing approval shall be obtained in addition to any approval required by law.
(c) The Corporation shall obtain the consent of the Board of Directors before it may authorize or issue any additional shares of capital stock of the Corporation or any of its subsidiaries.
(d) Unless a different vote is specified in this Certificate, any of the rights, powers, preferences and other terms of the Series B-2 Stock set forth herein may be waived on behalf of all holders of Series B-2 Stock by the affirmative written consent or vote of the Series B-2 Majority.
7. Conversion.
(a) Any Series B-2 Stockholder shall have the right, at any time or from time to time, to convert any or all of its shares of Series B-2 Stock into that number of fully paid and nonassessable shares of Common Stock for each share of Series B-2 Stock so converted equal to the quotient of the Series B-2 Original Purchase Price divided by the Series B-2 Conversion Price (as defined in Section 7(e) hereof) for such share of Series B-2 Stock, as last adjusted and then in effect; provided, however, that cash shall be paid in lieu of the issuance of fractional shares of Common Stock, as provided in Section 7(d) hereof.
(b) (i) Any Series B-2 Stockholder who exercises the right to convert shares of Series B-2 Stock into shares of Common Stock pursuant to this Section 7 shall be entitled to payment of all accrued dividends, whether or not declared and all declared but unpaid dividends payable with respect to such Series B-2 Stock pursuant to Section 3 herein, up to and including the Conversion Date (as defined in Section 7(b)(iii) hereof).
(ii) Any Series B-2 Stockholder may exercise the right to convert such shares into Common Stock pursuant to this Section 7 by delivering to the Corporation during regular business hours, at the office of the Corporation or any transfer agent of the Corporation or at such other place as may be designated by the Corporation, the certificate or certificates for the shares to be converted (the “Series B-2 Certificate”), duly endorsed or assigned in blank to the Corporation (if required by it) or an affidavit of loss as to the same.
(iii) Each Series B-2 Certificate shall be accompanied by written notice stating that such holder elects to convert such shares and stating the name or names (with address) in which the certificate or certificates for the shares of Common Stock (the “Common Certificate”) are to be issued. Such conversion shall be deemed to have been effected on the date when such delivery is made, and such date is referred to herein as the “Conversion Date.”
(iv) As promptly as practicable thereafter, the Corporation shall issue and deliver to or upon the written order of such holder, at the place designated by such holder, (A) a Common Certificate for the number of full shares of Common Stock to which such holder is entitled and (B) a check or cash in respect of any fractional interest in shares of Common Stock to which such holder is entitled, as provided in Section 7(d) hereof, payable with respect to the shares so converted up to and including the Conversion Date.
(v) The person in whose name the Common Certificate or Certificates are to be issued shall be deemed to have become a holder of record of Common Stock on the applicable Conversion Date, unless the transfer books of the Corporation are closed on such Conversion Date, in which event the holder shall be deemed to have become the stockholder of record on the next succeeding date on which the transfer books are open, provided that the Series B-2 Conversion Price upon which the conversion shall be executed shall be that in effect on the Conversion Date.
(vi) Upon conversion of only a portion of the number of shares represented by a Series B-2 Certificate, the Corporation shall issue and deliver to or upon the written order of the holder of such Series B-2 Certificate, at the expense of the Corporation, a new certificate covering the number of shares of Series B-2 Stock representing the unconverted portion of the shares of Series B-2 Stock represented by the Series B-2 Certificate, which new certificate shall entitle the holder thereof to all the rights, powers and privileges of a holder of such Series B-2 Stock.
(c) If a Series B-2 Stockholder shall surrender more than one share of Series B-2 Stock for conversion at any one time or holds more than one share of Series B-2 Stock that is automatically converted, including without limitation pursuant to a Special Mandatory Conversion, then the number of full shares of Common Stock issuable upon conversion thereof shall be computed on the basis of the aggregate number of shares of Series B-2 Stock so surrendered or held.
(d) No fractional shares of Common Stock shall be issued upon conversion of Series B-2 Stock. The Corporation shall instead pay a cash adjustment for any such fractional interest in an amount equal to the Current Market Price thereof on the Conversion Date, as determined in accordance with Section 7(e)(viii) hereof.
(e) For all purposes of this Certificate of Designations, the initial conversion price of the Series B-2 Stock shall be $6.142, subject to adjustment from time to time as follows (the conversion price of the Series B-2 Stock is referred to herein as the “Series B-2 Conversion Price”):
(i) Subject to Section 7(e)(ii), 7(e)(x) and 9(a) below, if the Corporation shall, at any time or from time to time after the Series B-2 Original Issuance Date, issue or sell any shares of Common Stock (which term, for purposes of this Section 7(e)(i), including all subsections thereof, shall be deemed to include all other securities convertible into, or exchangeable or exercisable for, shares of Common Stock (including, but not limited to, Preferred Stock)) or options to purchase or other rights to subscribe for such convertible or exchangeable securities, in each case other than Excluded Stock (as defined in Section 7(e)(ii) below), for a consideration per share less than the Series B-2 Conversion Price in effect immediately prior to the issuance of such Common Stock or other securities (a “Dilutive Issuance”), then the Series B-2 Conversion Price in effect immediately prior to each such Dilutive Issuance shall automatically be reduced to a price equal to the product obtained by multiplying such Series B-2 Conversion Price by a fraction, the numerator of which shall be the number of shares of Common Stock outstanding immediately prior to such issuance (including, without limitation, shares of Common Stock issued or issuable upon conversion of the outstanding Series B-2 Stock, but excluding shares of Common Stock issuable upon conversion of any dividends accrued on such Series B-2 Stock) plus the number of shares of Common Stock that the aggregate consideration received by the Corporation for the additional stock so issued would purchase at such Series B-2 Conversion Price as in effect immediately prior to such issuance, and the denominator of which shall be the number of shares of Common Stock outstanding immediately prior to such issuance (including, without limitation, shares of Common Stock issued or issuable upon conversion of the outstanding Series B-2 Stock, but excluding
shares of Common Stock issuable upon conversion of any dividends accrued on such Series B-2 Stock) plus the number of shares of additional stock so issued. For purposes of this Section 7(e)(i), the number of shares of Common Stock deemed issuable upon conversion of such outstanding shares of Series B-2 Stock shall be determined without giving effect to any adjustments to the Series B-2 Conversion Price resulting from the Dilutive Issuance that is the subject of this calculation. For the purposes of any adjustment of the Series B-2 Conversion Price pursuant to this Section 7(e)(i), the following provisions shall be applicable.
a. In the case of the issuance of Common Stock in whole or in part for cash, the consideration shall be deemed to be the amount of cash paid therefor after deducting therefrom any discounts, commissions or other expenses allowed, paid or incurred by the Corporation for any underwriting or otherwise in connection with the issuance and sale thereof, plus the value of any property other than cash received by the Corporation, determined as provided in Section 7(e)(i)(b) hereof, plus the value of any other consideration received by the Corporation determined as set forth in Section 7(e)(i)(c) hereof.
b. In the case of the issuance of Common Stock for a consideration in whole or in part in property other than cash, the value of such property other than cash shall be deemed to be the fair market value of such property as determined in good faith by the Board of Directors, irrespective of any accounting treatment; provided, however, that such fair market value of such property as determined by the Board of Directors shall not exceed the aggregate Current Market Price (as defined in Section 7(e)(viii) hereof) of the shares of Common Stock or such other securities being issued, less any cash consideration paid for such shares, determined as provided in Section 7(e)(i)(a) hereof and less any other consideration received by the Corporation for such shares, determined as set forth in Section 7(e)(i)(c) hereof.
c. In the case of the issuance of Common Stock for consideration in whole or in part other than cash or property, the value of such other consideration shall be deemed to be the aggregate par value of such Common Stock (or the aggregate stated value if such Common Stock has no par value).
d. In the case of the issuance of options or other rights to purchase or subscribe for Common Stock or the issuance of securities by their terms convertible into or exchangeable or exercisable for Common Stock or options to purchase or other rights to subscribe for such convertible or exchangeable or exercisable securities:
i. the aggregate maximum number of shares of Common Stock deliverable upon exercise of such options to purchase or rights to subscribe for Common Stock shall be deemed to have been issued at the time such options or rights were issued and for a consideration equal to the consideration (determined in the manner provided in Sections 7(e)(i)(a), (b) and (c) hereof), if any, received by the Corporation upon the issuance of such options or rights plus the minimum purchase price provided in such options or rights for the Common Stock covered thereby (the consideration in each case to be determined in the manner provided in Sections 7(e)(i)(a), (b) and (c) hereof);
ii. the aggregate maximum number of shares of Common Stock deliverable upon conversion of, or in exchange for, any such convertible or exchangeable securities or upon the exercise of options to purchase or rights to subscribe for such convertible or exchangeable securities and subsequent conversion or exchange thereof shall be deemed to have been issued at the time such securities were issued or such options or rights were issued and for a consideration equal to the consideration received by the Corporation for any such securities and related options or rights (excluding any cash received on account of accrued interest or accrued dividends), plus the minimum additional consideration, if any, to be received by the Corporation upon the conversion or exchange of such securities or the exercise of any related options or rights (the consideration in each case to be determined in the manner provided in Sections 7(e)(i)(a), (b) and (c) hereof);
iii. if there is any change (whether automatic pursuant to the terms contained therein or as a result of the amendment of such terms) in the exercise price of, or number of shares deliverable upon exercise of, any such options or rights or upon the conversion or exchange of any such convertible or exchangeable securities (other than a change resulting from the original antidilution provisions thereof in place at the time of issuance of such security), then the Series B-2 Conversion Price shall automatically be readjusted in proportion to such change (notwithstanding the foregoing, no adjustment pursuant to this clause shall have the effect of increasing the Series B-2 Conversion Price to an amount which exceeds the lower of (i) the Series B-2 Conversion Price on the original adjustment date, or (ii) the Series B-2 Conversion Price that would have resulted from any Dilutive Issuances between the original adjustment date and such readjustment date);
iv. upon the expiration of any such options or rights or the termination of any such rights to convert or exchange such convertible or exchangeable securities (or in the event that the change that precipitated an adjustment pursuant to Section 7(e)(i)(d)(iii) hereof is reversed or terminated, or expires), then the Series B-2 Conversion Price shall be automatically readjusted to the Series B-2 Conversion Price that would have been obtained had such options, rights or convertible or exchangeable securities not been issued; and
v. if the terms of any option or convertible security (excluding options or convertible securities which, upon exercise, conversion or exchange thereof, would entitle the holder thereof to receive shares of Common Stock which are Excluded Stock), the issuance of which was not a Dilutive Issuance, are revised after the Series B-2 Original Issuance Date (either automatically pursuant the provisions contained therein or as a result of an amendment to such terms) to provide for either (1) any increase in the number of shares of Common Stock issuable upon the exercise, conversion or exchange of any such option or convertible security or (2) any decrease in the consideration payable to the Corporation upon such exercise, conversion or exchange, then such option or convertible security, as so amended, and the shares of Common Stock subject thereto shall be deemed to have been issued effective upon such increase or decrease becoming effective.
e. In the case of the issuance of shares of Common Stock on more than one date that are a part of one transaction or a series of related
transactions and that would result in an adjustment to the Series B-2 Conversion Price pursuant to the terms of this Section 7(e), and such issuance dates occur within a period of no more than 90 days from the first such issuance to the final such issuance, then, upon the final such issuance, the Series B-2 Conversion Price shall be readjusted to give effect to all such issuances as if they occurred on the date of the first such issuance (and without giving effect to any additional adjustments as a result of any such subsequent issuances within such period).
(ii) “Excluded Stock” shall mean:
a. Common Stock issued upon conversion of any shares of Preferred Stock, including any shares of Common Stock issuable upon conversion of any dividends accrued on such Preferred Stock;
b. Common Stock issued or issuable to officers, directors or employees of, or consultants, advisors or independent contractors to, the Corporation, pursuant to any written agreement, plan or arrangement to purchase, or rights to subscribe for, such Common Stock approved by the Board of Directors, including each of the Preferred Directors (as defined in the Series A Certificate);
c. Common Stock issued as a stock dividend or distribution payable in shares of Common Stock, or capital stock of any other class issuable upon any subdivision, recombination, split-up or reverse stock split of all the outstanding shares of such class of capital stock;
d. Common Stock or other securities issued or issuable to banks, lenders, equipment lessors or landlords, provided that each such issuance is approved by the Board of Directors, including, but not limited to, warrants to acquire Common Stock held by Silicon Valley Bank (or its affiliates, successors and assignees), warrants to purchase Preferred Stock issued to GE Capital Equity Investments, Inc. or any of its affiliates (“GECEI”) and Oxford Finance Corporation or any of its affiliates (“OFC”) pursuant to a debt financing approved by the Board of Directors (the “GE Financing”), shares of Preferred Stock issued or issuable to GECEI in connection with the GE Financing or upon exercise by GECEI or OFC of warrants issued in the GE Financing and shares of common stock issuable upon conversion of any such shares of Preferred Stock issued to GECEI or OFC pursuant to the GE Financing;
e. Common Stock or other securities issued or issuable to suppliers or third party service providers in connection with the provision of goods or services or to other third parties in connection with sponsored research agreements, collaboration agreements, development agreements, strategic partnerships or alliances, corporate partnerships, joint ventures or other licensing transactions, provided that each such transaction and related issuance is approved by the Board of Directors, including, but not limited to, (A) any shares of Preferred Stock or Common Stock issued or issuable to Ipsen Pharma SAS (“Ipsen”), pursuant to the terms of that certain License Agreement, as amended and may be amended with the approval of the Board of Directors of the Corporation and in effect from time to time, by and between the Corporation and Ipsen as payment for milestones in lieu of cash payments and (B) shares of
Series A-5 Stock issued pursuant to the Stock Issuance Agreement (as defined in Section 3(d) of the Series A Certificate) and the issuance of Series A-6 Stock issued or to be issued as dividends on such Series A-5 Stock, and shares of Common Stock issuable upon conversion of any such shares of Series A-5 Stock and Series A-6 Stock;
f. Common Stock or other securities issued or issuable pursuant to the acquisition by the Corporation of any other corporation, partnership, joint venture, trust or other entity by any merger, stock acquisition, reorganization, or purchase of substantially all assets or otherwise in which the Corporation or its stockholders of record immediately prior to the effective date of such transaction, directly or indirectly, own a majority of the voting power of the acquired entity or the resulting entity after such transaction, in each case so long as such transaction is approved by the Board of Directors;
g. Common Stock or other securities, the issuance of which is approved by the Required Investor Majority, with such approval expressly waiving the application of the anti-dilution provisions of this Section 7 as a result of such issuance;
h. Preferred Stock or Common Stock issued or issuable pursuant to any warrant outstanding as of the date hereof or any warrant and any shares of Preferred Stock or Common Stock, or Common Stock issued upon conversion of any Preferred Stock, issued in connection with the Qualified Financing, any shares of Preferred Stock or Common Stock upon exercise thereof and any Common Stock issuable upon conversion of such Preferred Stock issued upon exercise thereof;
i. Common Stock or Convertible Securities actually issued upon the exercise of Options or shares of Common Stock actually issued upon the conversion or exchange of Convertible Securities, in each case provided such issuance is pursuant to the terms of such Option or Convertible Security; and
j. All Series B-2 Stock and other securities issued pursuant to the Series B-2 Purchase Agreement, and all shares of Common Stock issued or issuable upon conversion of any such shares of Series B-2 Stock or exercise or conversion of any such other securities.
(iii) If the number of shares of Common Stock outstanding at any time after the Series B-2 Original Issuance Date (as defined in Section 8) is increased by a stock dividend payable in shares of Common Stock or by a subdivision or split-up of shares of Common Stock, then, following the record date fixed for the determination of holders of Common Stock entitled to receive such stock dividend, subdivision or split-up, the Series B-2 Conversion Price shall be appropriately decreased in the form of a Proportional Adjustment (as defined in Section 8) so that the number of shares of Common Stock issuable on conversion of each share of Series B-2 Stock shall be increased in proportion to such increase in outstanding shares.
(iv) If the number of shares of Common Stock outstanding at any time after the Series B-2 Original Issuance Date is decreased by a combination of the outstanding shares of
Common Stock, then, following the record date for such combination, the Series B-2 Conversion Price shall be appropriately increased in the form of a Proportional Adjustment so that the number of shares of Common Stock issuable on conversion of each share of Series B-2 Stock shall be decreased in proportion to such decrease in outstanding shares.
(v) Except as otherwise contemplated in the Series B-2 Purchase Agreement, if at any time after the Series B-2 Original Issuance Date, the Corporation shall make or issue, or fix a record date for the determination of holders of Common Stock entitled to receive, a dividend or other distribution payable in securities of the Corporation (other than shares of Common Stock) or in cash or other property, then and in each such event provision shall be made so that the holders of the Series B-2 Stock shall receive upon conversion thereof in addition to the number of shares of Common Stock receivable thereupon, the kind and amount of securities of the Corporation, cash or other property which they would have been entitled to receive had the Series B-2 Stock been converted into Common Stock on the date of such event and had they thereafter, during the period from the date of such event to and including the conversion date, retained such securities receivable by them as aforesaid during such period, giving application to all adjustments called for during such period under this paragraph with respect to the rights of the holders of the Series B-2 Stock; and provided further, however, that no such adjustment shall be made if the holders of Series B-2 Stock simultaneously receive a dividend or other distribution of such securities, cash, or other property in an amount equal to the amount of such securities, cash, or other property as they would have received if all outstanding shares of Series B-2 Stock had been converted into Common Stock on the date of such event.
(vi) Subject to the provisions of Section 4(e) above, in the event, at any time after the Series B-2 Original Issuance Date, of any capital reorganization, or any reclassification of the capital stock of the Corporation (other than a change in par value or from par value to no par value or from no par value to par value or as a result of a stock dividend or subdivision, split-up or combination of shares), or the consolidation or merger of the Corporation with or into another person (other than a consolidation or merger in which the Corporation is the continuing corporation and which does not result in any change in the powers, designations, preferences and rights, or the qualifications, limitations or restrictions, if any, of the capital stock of the Corporation) or of the sale or other disposition of all or substantially all the properties and assets of the Corporation in their entirety to any other person (any such transaction, an “Extraordinary Transaction”), then the Corporation shall provide appropriate adjustment in the form of a Proportional Adjustment to the Series B-2 Conversion Price with respect to each share of Series B-2 Stock outstanding after the effectiveness of such Extraordinary Transaction such that each share of Series B-2 Stock outstanding immediately prior to the effectiveness of the Extraordinary Transaction shall be convertible into the kind and number of shares of stock or other securities or property of the Corporation, or of the corporation resulting from or surviving such Extraordinary Transaction, that a holder of the number of shares of Common Stock deliverable (immediately prior to the effectiveness of the Extraordinary Transaction) upon conversion of such share of Series B-2 Stock would have been entitled to receive upon such Extraordinary Transaction. The provisions of this Section 7(e)(vi) shall similarly apply to successive Extraordinary Transactions.
(vii) All calculations under this Section 7(e) shall be made to the nearest one-tenth of a cent ($.001) or to the nearest one-tenth of a share, as the case may be.
(viii) For the purpose of any computation pursuant to Section 7(d), Section 3(a) hereof or this Section 7(e), the “Current Market Price” at any date of one share of Common Stock shall be defined as the average of the daily closing prices for the 20 consecutive Business Days ending on the fifth (5th) Business Day before the day in question (as adjusted for any stock dividend, split-up, combination or reclassification that took effect during such 20 Business Day period), determined as follows:
a. If the Common Stock is listed or admitted for trading on a national securities exchange, then the closing price for each day shall be the last reported sales price regular way or, in case no such reported sales took place on such day, the average of the last reported bid and asked prices regular way, in either case on the principal national securities exchange on which the Common Stock is listed or admitted to trading.
b. If the Common Stock is not at the time listed or admitted for trading on any such exchange, then such price shall be equal to the last reported bid and asked prices on such day as reported by the NASD OTCBB or the National Quotation Bureau, Inc., or any similar reputable quotation and reporting service if such quotation is not reported by the NASD OTCBB or the National Quotation Bureau, Inc.
c. If the Common Stock is not traded in such manner that the quotations referred to in this Section 7(e)(viii) are available for the period required hereunder, then the Current Market Price shall be the fair market value of such share, as determined in good faith by a majority of the entire Board of Directors.
(ix) In any case in which the provisions of this Section 7(e) shall require that an adjustment shall become effective immediately after a record date for an event, the Corporation may defer until the occurrence of such event (A) issuing to the holder of any shares of Series B-2 Stock converted after such record date and before the occurrence of such event the additional shares of capital stock issuable upon such conversion by reason of the adjustment required by such event over and above the shares of capital stock issuable upon such conversion before giving effect to such adjustment, and (B) paying to such holder any cash amounts in lieu of fractional shares pursuant to Section 7(d) hereof; provided, however, that the Corporation shall deliver to such holder a due xxxx or other appropriate instrument evidencing such holder’s right to receive such additional shares and such cash upon the occurrence of the event requiring such adjustment.
(x) If a state of facts shall occur that, without being specifically controlled by the provisions of this Section 7, would not fairly protect the conversion rights of the holders of the Series B-2 Stock in accordance with the essential intent and principles of such provisions, then the Board of Directors shall make an adjustment in the application of such provisions, in accordance with such essential intent and principles, so as to protect such conversion rights.
(f) Whenever the Series B-2 Conversion Price shall be adjusted as provided in Section 7(e) hereof, the Corporation shall forthwith file and keep on record at the office of the Secretary of the Corporation and at the office of its transfer agent or at such other place as may be designated by the Corporation, a statement, signed by both its President or Chief Executive
Officer and its Treasurer or Chief Financial Officer, showing in detail the facts requiring such adjustment and the Series B-2 Conversion Price that shall be in effect after such adjustment. The Corporation shall also cause a copy of such statement to be sent by first-class, certified mail, return receipt requested, postage prepaid, to each Series B-2 Stockholder at such holder’s address appearing on the Corporation’s records. Where appropriate, such copy shall be given in advance of any such adjustment and shall be included as part of a notice required to be mailed under the provisions of Section 7(g) hereof.
(g) In the event the Corporation shall propose to take any action of the types described in Section 7(e)(i), (iii), (iv) or (v) hereof, or any other Event of Sale, other than the transactions contemplated by the Series B-2 Purchase Agreement, the Corporation shall give notice to each Series B-2 Stockholder in the manner set forth in Section 7(f) hereof, which notice shall specify the record date, if any, with respect to any such action and the date on which such action is to take place. Such notice shall also set forth such facts with respect thereto as shall be reasonably necessary to indicate the effect of such action (to the extent such effect may be known at the date of such notice) on the Series B-2 Conversion Price with respect to the Series B-2 Stock, and the number, kind or class of shares or other securities or property which shall be deliverable or purchasable upon each conversion of Series B-2 Stock. In the case of any action (other than any action contemplated or required by the Series B-2 Purchase Agreement) that would require the fixing of a record date, such notice shall be given at least 20 days prior to the record date so fixed, and in the case of any other action, such notice shall be given not later than 30 days following the taking of such proposed action.
(h) The Corporation shall pay all documentary, stamp or other transactional taxes attributable to the issuance or delivery of shares of capital stock of the Corporation upon conversion of any shares of Series B-2 Stock; provided, however, that the Corporation shall not be required to pay any taxes which may be payable in respect of any transfer involved in the issuance or delivery of any certificate for such shares in a name other than that of the Series B-2 Stockholder in respect of which such shares of Series B-2 Stock are being issued.
(i) The Corporation shall reserve out of its authorized but unissued shares of Common Stock, solely for the purpose of effecting the conversion of the Series B-2 Stock, sufficient shares of Common Stock to provide for the conversion of all outstanding shares of Series B-2 Stock.
(j) All shares of Common Stock which may be issued in connection with the conversion provisions set forth herein will, upon issuance by the Corporation, be validly issued, fully paid and nonassessable, not subject to any preemptive or similar rights, and free from all taxes, liens or charges with respect thereto created or imposed by the Corporation.
8. Definitions. As used in this Certificate of Designations, the following terms shall have the corresponding meanings:
“Business Day” shall mean any day other than a Saturday, Sunday or day on which banks are closed in the city and state where the principal executive office of the Corporation is located.
“Convertible Securities” shall mean any evidences of indebtedness, shares or other securities directly or indirectly convertible into or exchangeable for Common Stock, but excluding Options.
“Series B-2 Original Issuance Date” shall mean the date of issuance by the Corporation of the first share of Series B-2 Stock to be issued by the Corporation.
“Series B-2 Original Purchase Price” shall mean, with respect to the Series B-2 Stock, $61.42 per share, subject, for all purposes other than Section 7 hereof (which provisions shall be applied in accordance with their own terms), to Proportional Adjustment.
“Option” shall mean rights, options or warrants to subscribe for, purchase or otherwise acquire Common Stock or Convertible Securities.
“Proportional Adjustment” shall mean an adjustment made to the price of the Series B-2 Stock upon the occurrence of a stock split, reverse stock split, stock dividend, stock combination reclassification or other similar change with respect to such security, such that the price of one share of the Series B-2 Stock before the occurrence of any such change shall equal the aggregate price of the share (or shares or fractional share) of such security (or any other security) received by the holder of the Series B-2 Stock with respect thereto upon the effectiveness of such change.
“Qualified Financing” shall mean the transaction involving the issuance of shares of Series B-2 Stock and warrants to purchase Common Stock pursuant to the terms of the Series B-2 Purchase Agreement.
“Series B-2 Purchase Agreement” shall mean that certain Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement, dated as of the date of the filing of this Certificate with the Secretary of State of the State of Delaware, by and among the Corporation and the “Investors” party thereto.
9. Special Mandatory Conversion.
(a) Trigger Events. In the event that (i) the Series B-2 Majority shall have elected to convert all shares of Series B-2 Stock, (ii) the closing of a firm commitment underwritten public offering of shares of Common Stock occurs on or prior to June 30, 2014, or (iii) the Common Stock of the Corporation becomes listed for trading on a national securities exchange after June 30, 2014, then each share of Series B-2 Stock shall be converted automatically into shares of Common Stock, at the then effective conversion rate (subject to the last sentence of this Section 9(a), as applicable). Each of the conversions set forth in this Section 9(a) is referred to as a “Special Mandatory Conversion.” Notwithstanding anything contained herein to the contrary, including without limitation Section 3 hereof, all accrued but unpaid dividends on shares of Series B-2 Stock, including without limitation the Series B-2 Accruing Dividend, shall be paid in cash or
shares of Common Stock, with the decision of whether to pay any such dividend in cash or in shares of Common Stock being made at the sole discretion of the Board of Directors (calculated based on the then effective Series B-2 Conversion Price), upon the conversion of such shares of Series B-2 Stock in connection with a Special Mandatory Conversion. In the event of the closing of a firm commitment underwritten public offering of shares of Common Stock that results in the automatic conversion of the Series B-2 Stock on or prior to June 30, 2014 pursuant to clause (ii) of this Section 9(a) in which the public offering price per share is less than the Series B-2 Conversion Price in effect immediately prior to the issuance of such Common Stock, then, in lieu of any adjustment to the Series B-2 Conversion Price pursuant to Section 7(e)(i) above in connection with such Dilutive Issuance, the Series B-2 Conversion Price in effect immediately prior to such issuance shall automatically be reduced to a price equal to such public offering price per share.
(b) Procedural Requirements. Upon a Special Mandatory Conversion, each holder of shares of Series B-2 Stock converted pursuant to Section 9(a) shall be sent written notice of such Special Mandatory Conversion and the place designated for mandatory conversion of all shares of Series B-2 Stock. Upon receipt of such notice, each holder of such shares of Series B-2 Stock shall surrender his, her or its certificate or certificates for all such shares (or, if such holder alleges that such certificate has been lost, stolen or destroyed, a lost certificate affidavit and agreement reasonably acceptable to the Corporation to indemnify the Corporation against any claim that may be made against the Corporation on account of the alleged loss, theft or destruction of such certificate) to the Corporation at the place designated in such notice. If so required by the Corporation, certificates surrendered for conversion shall be endorsed or accompanied by written instrument or instruments of transfer, in form satisfactory to the Corporation, duly executed by the registered holder or by his, her or its attorney duly authorized in writing. All rights with respect to the Series B-2 Stock so converted, including the rights, if any, to receive notices and vote (other than as a holder of Common Stock), will terminate at the time of the Special Mandatory Conversion (notwithstanding the failure of the holder or holders thereof to surrender the certificates for such shares at or prior to such time), except only the rights of the holders thereof, upon surrender of their certificate or certificates therefor (or lost certificate affidavit and agreement), to receive the items provided for in the next sentence of this Section 9(b). As soon as practicable after the Special Mandatory Conversion and the surrender of the certificate or certificates (or lost certificate affidavit and agreement) for Series B-2 Stock so converted, the Corporation shall issue and deliver to such holder, or to his, her or its nominees, a certificate or certificates for the number of full shares of Common Stock issuable on such conversion in accordance with the provisions hereof, together with cash as provided in Section 7(d) in lieu of any fraction of a share of Common Stock otherwise issuable upon such conversion and the payment of any declared but unpaid dividends on the shares of Series B-2 Stock converted, and a new certificate for the number of shares, if any, of Series B-2 Stock represented by such surrendered certificate and not converted pursuant to Section 9(a). Such converted Series B-2 Stock shall be retired and cancelled and may not be reissued as shares of such series, and the Corporation may thereafter take such appropriate action (without the need for stockholder action) as may be necessary to reduce the authorized number of shares of Series B-2 Stock accordingly.
(c) Duration of Section. This Section 9 and the rights and obligations of the parties hereunder shall automatically terminate on the consummation of a Liquidation or an Event of Sale.
[signature page follows]
IN WITNESS WHEREOF, RADIUS HEALTH, INC. has caused this Certificate to be executed by Xxxxxx X. Xxxx, its President and Chief Executive Officer, this 14th day of February, 2014.
|
|
RADIUS HEATH, INC. . | |
|
|
| |
|
|
|
|
|
|
By: |
|
|
|
Name: |
Xxxxxx X. Xxxx |
|
|
Title: |
President and Chief Executive Officer |
Exhibit B
Form of Warrant
THESE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR ANY APPLICABLE STATE SECURITIES LAWS AND HAVE BEEN ACQUIRED FOR INVESTMENT AND NOT WITH A VIEW TO, OR IN CONNECTION WITH, THE SALE OR DISTRIBUTION THEREOF. NO SUCH SALE OR DISTRIBUTION MAY BE EFFECTED WITHOUT AN EFFECTIVE REGISTRATION STATEMENT OR QUALIFICATION RELATED THERETO OR AN OPINION OF COUNSEL IN A FORM SATISFACTORY TO THE COMPANY THAT SUCH REGISTRATION OR QUALIFICATION IS NOT REQUIRED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR ANY APPLICABLE STATE SECURITIES LAWS.
Date of Issuance: , 2014
RADIUS HEALTH, INC.
WARRANT TO PURCHASE SHARES OF COMMON STOCK
THIS CERTIFIES THAT, for value received, (the “Holder”) is entitled to purchase from Radius Health, Inc., a Delaware corporation (the “Company”), subject to the terms and conditions of this Warrant, at any time prior to the Expiration Date (as defined below), shares of the Company’s Common Stock, par value $.0001 per share (“Common Stock”), at an exercise price per Warrant Share of $6.142 (the “Exercise Price”). The shares of Common Stock purchasable upon exercise of this Warrant are referred to herein as the “Warrant Shares”. The Warrant Shares and the Exercise Price are subject to further adjustment as set forth in Section 2.
1 Exercise of Warrant.
1.1 Term. This Warrant shall terminate and no longer be exercisable on , 2019. For purposes of this Warrant, (a) “Expiration Date” shall mean the date upon which this Warrant expires in accordance with the terms of this Section 1.1, and (b) “Purchase Agreement” shall mean that certain Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement, dated as of February 14, 2014, by and among the Company and the other parties thereto.
1.2 Method. This Warrant may be exercised by the Holder, in whole or in part, by:
(a) the surrender of this Warrant (with the Notice of Exercise form attached hereto as Attachment A and the Investment Representation Statement attached hereto as Attachment B duly executed) at the principal office of the Company; and
(b) the payment to the Company, by check, wire or cancellation of indebtedness, of an amount equal to the Exercise Price per share multiplied by the number of Warrant Shares then being purchased.
1.3 Net Exercise. In lieu of Section 1.2 hereof, the Holder may elect to convert this Warrant or any portion thereof (the “Conversion Right”), by surrender of this Warrant at the principal office of the Company together with notice of the Holder’s intention to
exercise the Conversion Right, into that number of Warrant Shares computed using the following formula:
X = Y(A-B)
A
Where:
X = The number of Warrant Shares to be issued to the Holder upon exercise of the Conversion Right.
Y = The number of Warrant Shares for which this Warrant is being exercised.
A = The Fair Market Value (as defined below) of one Warrant Share at the time the Conversion Right is exercised.
B = Exercise Price (as adjusted to the date of such calculation).
For purposes of Section 1.3, “Fair Market Value” shall mean:
(a) If the Warrant is exercised in connection with and contingent upon an initial public offering, and if the Company’s registration statement relating to such initial public offering has been declared effective by the Securities and Exchange Commission, then the initial “Price to Public” specified in the final prospectus with respect to such offering.
(b) If the Warrant is exercised in connection with and contingent upon an Event of Sale (as defined in the Company’s certificate of incorporation, as it may be amended and/or restated from time to time (the “Certificate”)), then the purchase price per share actually received by a holder of Common Stock.
(c) If the Warrant is exercised after the Common Stock is listed or admitted for trading on a national securities exchange, then the average of the daily closing prices of the Common Stock for the 20 consecutive trading days ending on the fifth (5th) trading day before the day in question (as adjusted for any stock dividend, split-up, combination or reclassification that took effect during such 20 trading day period) (or such shorter period of time during which such Common Stock was traded on such exchange), with the closing price for each day being the last reported sales price or, in case no such reported sales took place on such day, the average of the last reported bid and asked prices, in either case on the principal national securities exchange on which the Common Stock is listed or admitted to trading or, if the Common Stock is not at the time listed or admitted for trading on any such exchange, then such price shall be equal to the last reported bid and asked prices on such day as reported by the NASD OTCBB or the National Quotation Bureau, Inc., or any similar reputable quotation and reporting service if such quotation is not reported by the NASD OTCBB or the National Quotation Bureau, Inc.
(d) If none of the immediately preceding clauses (a), (b) or (c) is applicable, then the fair market value as determined in good faith by the majority of the entire Board of Directors of the Company.
In the event of 1.3(d), above, the Company’s Board of Directors shall prepare a certificate, to be signed by an authorized officer of Company, setting forth in reasonable detail the basis for and method of determination of the per share Fair Market Value of one Warrant Share.
1.4 Delivery; Certificate. Upon receipt by the stock transfer agent or warrant agent of the Company at its office, together with, if applicable, the aggregate Exercise Price, the Holder shall be deemed to be the holder of record of the applicable Warrant Shares, notwithstanding that the stock transfer books of the Company shall then be closed or that certificates representing such Warrant Shares shall not then be actually delivered to the Holder. The Company shall, as soon as practicable after the exercise of this Warrant in accordance with the terms hereof, direct its stock transfer agent to prepare a certificate for the Warrant Shares purchased in the name of the Holder. If this Warrant should be exercised in part only, the Company shall, as soon as practicable after the surrender of this Warrant, execute and deliver a new Warrant evidencing the rights of the Holder thereof to purchase the balance of the Warrant Shares purchasable hereunder.
1.5 Exercise Upon a Company Sale. Notwithstanding anything herein to the contrary, upon and effective as of the occurrence of an Event of Sale (as defined in the Certificate), to the extent not previously exercised, this Warrant shall automatically be exercised by the Holder pursuant to Section 1.3 herein without any further action necessary on the part of the Holder (a “Sale Exercise”) unless the Holder notifies the Company in writing to the contrary prior to such automatic exercise; provided, however, that such automatic exercise shall not occur and this Warrant shall instead be terminated upon and effective as of the occurrence of an Event of Sale (as defined in the Certificate) if the Exercise Price equals or exceeds the Fair Market Value calculated in accordance with Section 1.3(b) hereof in connection with such Sale Exercise.
1.6 Automatic Exercise. To the extent this Warrant is not previously exercised, it shall be deemed to have been automatically converted in accordance with Section 1.3 hereof (even if not surrendered) as of immediately before its expiration, involuntary termination or cancellation if the then-Fair Market Value of a Warrant Share exceeds the then-Exercise Price, unless Holder notifies Company in writing to the contrary prior to such automatic exercise.
2 Adjustment of Exercise Price and Number of Warrant Shares. The number and kind of Warrant Shares purchasable upon the exercise of this Warrant and the Exercise Price shall be subject to adjustment from time to time upon the occurrence of the following events:
2.1 Subdivision or Combination. If the Company at any time prior to the Expiration Date shall subdivide or combine its Common Stock, the Exercise Price shall be proportionately decreased (and the number of Warrant Shares issuable upon exercise of this Warrant proportionately increased to the nearest whole) in the case of a subdivision or the Exercise Price shall be proportionately increased (and the number of Warrant Shares issuable
upon exercise of this Warrant proportionately decreased to the nearest whole) in the case of a combination.
2.2 Reclassification, Reorganization and Consolidation. In case of any reclassification, capital reorganization or change in the type of securities of the Company issuable upon exercise of this Warrant (other than as a result of a subdivision, combination or stock dividend provided for in Section 2.1 above or Section 2.3 below) or consolidation or merger involving the Company in which the Common Stock is converted into or exchanged for securities, cash or other property, then, as a condition of such reclassification, reorganization, change, consolidation or merger, lawful provision shall be made, and duly executed documents evidencing the same from the Company or its successor shall be delivered to the Holder, so that the Holder shall have the right at any time prior to the expiration of this Warrant to purchase, at a total price equal to that payable upon the exercise of this Warrant, the kind and amount of shares of stock and other securities or property receivable in connection with such reclassification, reorganization, change, consolidation or merger by a holder of the same number and type of securities as were purchasable as Warrant Shares by the Holder immediately prior to such reclassification, reorganization, change, consolidation or merger. In any such case appropriate provisions shall be made with respect to the rights and interest of the Holder so that the provisions hereof shall thereafter be applicable with respect to any shares of stock or other securities or property deliverable upon exercise hereof, and appropriate adjustments shall be made to the Exercise Price per Warrant Share payable hereunder, provided the aggregate Exercise Price shall remain, as nearly as reasonably may be, the same.
2.3 Stock Dividends. If the Company at any time prior to the Expiration Date shall pay a dividend with respect to Common Stock payable in Common Stock (except any distribution accounted for in the foregoing Section 2.1), then the Exercise Price shall be adjusted (and the number of Warrant Shares issuable upon exercise of this Warrant proportionately increased), from and after the record date for shareholders entitled to receive such dividend or distribution, to that price determined by multiplying the Exercise Price in effect immediately prior to such record date by a fraction (a) the numerator of which shall be the total number of shares of Common Stock outstanding immediately prior to such dividend or distribution, and (b) the denominator of which shall be the total number of shares of Common Stock outstanding immediately after such dividend or distribution.
2.4 Adjustment Certificate. Whenever the Exercise Price shall be adjusted as provided in Section 2 hereof, the Company shall forthwith file and keep on record at the office of the Secretary of the Company and at the office of its transfer agent or at such other place as may be designated by the Company, a statement, signed by both its President or Chief Executive Officer and its Treasurer or Chief Financial Officer, showing in detail the facts requiring such adjustment and the Exercise Price that shall be in effect after such adjustment. The Company shall also cause a copy of such statement to be sent by first-class, certified mail, return receipt requested, postage prepaid, to the Holder at such Holder’s address appearing on the Company’s records. Where appropriate, such copy shall be given in advance of any such adjustment.
3 Fractional Warrant Shares. No fractional Warrant Shares will be issued in connection with any exercise hereunder, but in lieu of such fractional shares the Company shall make a cash payment therefor upon the basis of the Exercise Price then in effect.
4 Stock Fully Paid; Reservation of Warrant Shares. All Warrant Shares issuable upon the exercise of the rights represented by this Warrant will, upon issuance, be fully paid and nonassessable. During the period within which the rights represented by this Warrant may be exercised, the Company will at all times have authorized and reserved for the purpose of issuance upon exercise of the purchase rights evidenced by this Warrant, a sufficient number of shares of Common Stock to provide for the exercise of the rights represented by this Warrant. In the event that there is an insufficient number of shares of Common Stock reserved for issuance pursuant to the exercise of this Warrant, the Company will take appropriate action to authorize an increase in the capital stock to allow for such issuance or similar issuance acceptable to the Holder.
5 Securities Laws; Transfer.
5.1 Compliance with Securities Act. The Holder, by acceptance hereof, agrees that this Warrant and the Warrant Shares are being acquired for investment and that it will not offer, sell or otherwise dispose of this Warrant or any Warrant Shares except under circumstances which will not result in a violation of the Securities Act of 1933, as amended (the “Act”). Upon exercise of this Warrant, the Holder hereof shall confirm in writing, in the form attached hereto as Attachment B, that the Warrant Shares so purchased are being acquired for investment and not with a view toward distribution or resale. In addition, the Holder shall provide such additional information regarding such Holder’s financial and investment background as the Company may reasonably request. This Warrant and all Warrant Shares (unless registered under the Act) shall be stamped or imprinted with a legend in substantially the following form:
THESE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR ANY APPLICABLE STATE SECURITIES LAWS AND HAVE BEEN ACQUIRED FOR INVESTMENT AND NOT WITH A VIEW TO, OR IN CONNECTION WITH, THE SALE OR DISTRIBUTION THEREOF. NO SUCH SALE OR DISTRIBUTION MAY BE EFFECTED WITHOUT AN EFFECTIVE REGISTRATION STATEMENT OR QUALIFICATION RELATED THERETO OR AN OPINION OF COUNSEL IN A FORM SATISFACTORY TO THE COMPANY THAT SUCH REGISTRATION OR QUALIFICATION IS NOT REQUIRED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR ANY APPLICABLE STATE SECURITIES LAWS.
5.2 Transferability of Warrant. In connection with any transfer by Holder of this Warrant, the Company may require the transferee to provide the Company with written representations and warranties substantially similar to Holder’s representations and warranties set forth in Attachment B, and may require Holder to provide a legal opinion, in form and substance satisfactory to Company and its counsel, that such transfer is exempt from the registration and prospectus delivery requirements of the Act; provided, that the Company shall not require Holder to provide an opinion of counsel if the transfer is to an affiliate of Holder,
provided that such affiliate is an “accredited investor” as defined in Regulation D promulgated under the Act. Any transferee (including, without limitation, any affiliate of Holder) shall take this Warrant subject to all of the terms and conditions thereof and such transferee’s rights under this Warrant shall be subject to such transferee’s compliance with all of the terms and conditions of this Warrant that are applicable to Holder. Following any transfer of this Warrant, at the request of either the Company or the transferee, the transferee shall surrender this Warrant to the Company in exchange for a new warrant of like tenor and date, executed by Company. Subject to the foregoing, this Warrant is transferable on the books of the Company at its principal office by the registered Holder hereof upon surrender of this Warrant properly endorsed. Upon any partial transfer, Company will execute and deliver to Holder a new warrant of like tenor with respect to the portion of this Warrant not so transferred. Holder shall not have any right to transfer any portion of this Warrant to any direct competitor of Company.
5.3 Disposition of Warrant Shares. The Holder agrees not to make any disposition of all or any portion of the Warrant Shares unless and until (a) the Holder shall have notified the Company of the proposed disposition and shall have furnished the Company with a detailed statement of the circumstances surrounding the proposed disposition, (b) the transferee has agreed in writing for the benefit of the Company to be bound by this Section 5 and (c):
(i) there is then in effect a registration statement under the Act covering such proposed disposition and such disposition is made in accordance with such registration statement; or
(ii) the Holder shall have furnished the Company with an opinion of counsel, reasonably satisfactory to the Company, that such disposition will not require registration of the Warrant Shares under the Act; provided that the Company will not require opinions of counsel for transactions made pursuant to Rule 144 except in unusual circumstances.
5.4 Market Standoff. Each Holder agrees, in connection with the Company’s initial public offering (the “IPO”) of its equity securities (other than pursuant to a registration statement on Form S-8), and upon request of the Company or the underwriters managing such offering, (a) not to sell, make any short sale of, loan, grant any option for the purchase of or otherwise dispose of any of the Warrants or the Warrant Shares (other than those included in the registration, if any) without the prior written consent of the Company or such underwriters, as the case may be, for such period of time not to exceed one hundred eighty (180) days (or such longer period of time as may be required to accommodate regulatory restrictions on (i) the publication or other distribution of research reports and (ii) analyst recommendations and opinions, including, but not limited to, the restrictions contained in FINRA Rule 2711(f)(4) or NYSE Rule 472(f)(4), as applicable, (or any successor rules or amendments thereto))) from the effective date of such registration as may be requested by the Company or such underwriters and (b) to execute any agreement regarding (a) above as may be requested by the Company or underwriters at the time of the public offering; provided that such request is made of all officers, directors and 1% and greater Stockholders of the Company and each such person shall be similarly bound. The Company may impose stop transfer instructions to enforce this Section 5.4.
6 Rights of Stockholders. No Holder of this Warrant shall be entitled to vote or receive dividends or be deemed the holder of capital stock or any other equity securities of the Company, nor shall anything contained herein be construed to confer upon the Holder of this Warrant, as such, any of the rights of a stockholder of the Company or any right to vote for the election of directors or upon any matter submitted to stockholders at any meeting thereof, or to give or withhold consent to any corporate action (whether upon any recapitalization, issuance of stock, reclassification of stock, change of par value or change of stock to no par value, consolidation, merger, conveyance, or otherwise) or to receive notice of meetings, or to receive dividends or subscription rights or otherwise until this Warrant has been exercised and the Warrant Shares shall have become deliverable, as provided herein.
7 Registration Rights. The Company agrees that the Warrant Shares issued and issuable upon the exercise or conversion of this Warrant, shall have registration rights pursuant to and as set forth in the Company’s Fourth Amended and Restated Stockholders’ Agreement, as amended and in effect from time to time (the “Stockholders’ Agreement”). The foregoing referenced registration rights are subject to and conditioned upon the Holder, at the time of exercise of this Warrant, being a party to the Stockholders’ Agreement or becoming a party to the Stockholders’ Agreement by executing and delivering to the Company an Instrument of Adherence thereto and such registration rights will be governed by the terms of the Stockholders’ Agreement.
8 Miscellaneous.
8.1 Governing Law. The terms and conditions of this Warrant shall be governed in all respects by the internal laws of the Commonwealth of Massachusetts without regard to conflicts of laws principles that would result in the application of the laws of any other jurisdiction.
8.2 Successors and Assigns. This Warrant shall be binding upon any successors or assigns of the Company and inure to the benefit of the Holder and any successors or assigns.
8.3 Waivers and Amendments. This Warrant is one of a series of Warrants (collectively, the “Warrants”) that were originally issued by the Company pursuant to the Purchase Agreement. This Warrant and any provisions hereof may be changed, waived, discharged or terminated only by an instrument in writing signed by the Company and the Holders of Warrants representing a majority of the number of Warrant Shares then issuable upon the exercise of the Warrants, provided, however, that the consent of the holder of this Warrant shall be required if such amendment or waiver adversely affects such holder in a disproportionate manner than the holders of the other Warrants.
8.4 Loss of Warrant. Upon receipt of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Warrant and, in the case of any such loss, theft or destruction, upon delivery of an indemnity agreement reasonably satisfactory in form and amount to the Company, or, in the case of any such mutilation, upon surrender and cancellation of such Warrant, the Company will execute and deliver a new Warrant of like terms.
8.5 Headings. The headings in this Warrant are for purposes of convenience and reference only, and shall not be deemed to constitute a part hereof.
8.6 Notices. All notices and other communications given or made pursuant hereto shall be in writing and shall be deemed effectively given: (a) upon personal delivery to the party to be notified, (b) when sent by confirmed facsimile if sent during normal business hours of the recipient, and if not so confirmed, then on the next business day, (c) forty-eight (48) hours after having been sent by registered or certified mail, return receipt requested, postage prepaid, or after being deposited in the U.S. mail, postage prepaid, or (d) one (1) day after deposit with a nationally recognized overnight courier, specifying next day delivery, with written verification of receipt, in the case of the Holder, addressed to the Holder at the address set forth on the signature page hereto and, in the case of the Company, to Radius Health, Inc., 000 Xxxxxxxx, Xxxxx Xxxxx, Xxxxxxxxx, Xxxxxxxxxxxxx 00000, Attention: Chief Financial Officer, with a copy to Xxxxxx & Xxxxxxx LLP, Xxxx Xxxxxxx Tower, 20th Floor, 000 Xxxxxxxxx Xxxxxx, Xxxxxx, Xxxxxxxxxxxxx 00000, Attention: Xxxxx X. Xxxxxxxxx; or as subsequently modified by written notice to the other party.
8.7 Counterparts. This Warrant may be executed in two or more counterparts (including, but not limited to, by facsimile, PDF or other electronic copy), each of which shall be deemed an original and all of which together shall constitute one instrument.
(Signature page follows)
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by a duly authorized officer.
|
|
RADIUS HEALTH, INC. | |
|
|
| |
|
|
| |
|
|
By: |
|
|
|
Name: Xxxxxx X. Xxxx | |
|
|
Its: Chief Executive Officer and President | |
|
|
| |
|
|
| |
|
ACKNOWLEDGED: |
| |
|
|
| |
|
[HOLDER NAME] |
| |
|
|
| |
|
|
| |
|
By: |
|
|
|
Name: |
|
|
|
Its: |
|
|
WARRANT SIGNATURE PAGE
ATTACHMENT A
NOTICE OF EXERCISE
TO: RADIUS HEALTH, INC.
o The undersigned hereby elects to purchase shares of Common Stock of Radius Health, Inc. pursuant to the terms of the attached Warrant, and tenders herewith payment of the purchase price of such shares in full.
o The undersigned hereby elects to convert the attached Warrant into Warrant Shares in the manner specified in Section 1.3 of the Warrant. This conversion is exercised with respect to of the shares covered by the Warrant.
[Check the box next to the paragraph above that applies.]
2. Please issue a certificate or certificates representing said shares of Common Stock in the name of the undersigned or in such other name as is specified below:
|
|
Name: |
|
|
|
|
|
|
|
|
|
Address: |
|
|
|
|
|
|
|
3. The undersigned represents that the aforesaid shares of stock are being acquired for the account of the undersigned for investment and not with a view to, or for resale in connection with, the distribution thereof and that the undersigned has no present intention of distributing or reselling such shares. In support thereof, the undersigned has executed an Investment Representation Statement attached hereto as Attachment X.
|
|
XXXXXX | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
By: |
|
|
|
|
|
|
|
Title: |
|
|
|
|
|
|
|
Date: |
|
ATTACHMENT B
INVESTMENT REPRESENTATION STATEMENT
In connection with the exercise or conversion of a Warrant to purchase shares of Common Stock (the “Warrant Shares”) of Radius Health, Inc. (the “Company”), the undersigned (the “Holder”) hereby represents and warrants to the Company the following:
(a) Investment Experience. It is an “accredited investor” within the meaning of Rule 501(a) of the Securities Act of 1933, as amended (the “Act”), and has substantial experience in evaluating and investing in private placement transactions of securities in companies similar to the Company so that it is capable of evaluating the merits and risks of its investment in the Company and has the capacity to protect its own interests. It is aware of the Company’s business affairs and financial condition and has acquired sufficient information about the Company to reach an informed and knowledgeable decision to acquire the Warrant Shares.
(b) Purchase Entirely for Own Account. The Warrant Shares are being acquired for investment for the Holder’s own account, not as a nominee or agent, and not with a view to the resale or distribution of any part thereof. Holder has no present intention of selling, granting any participation in, or otherwise distributing the Warrant Shares. The Holder further represents that it does not have any contract, undertaking, agreement or arrangement with any person to sell, transfer or grant participations, to such person or to any third person, with respect to the Warrant Shares.
(c) Restricted Securities. The Holder understands that the Warrant Shares are characterized as “restricted securities” under the federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that under such laws and applicable regulations such Warrant Shares may be resold without registration under the Act only in certain limited circumstances. In this connection, the Holder represents that it is familiar with Rule 144, as presently in effect, and understands the resale limitations imposed thereby and by the Act. The Holder must bear the economic risk of this investment indefinitely unless the Warrant Shares are registered pursuant to the Act, or an exemption from registration is available. The Holder understands that the Company has no present intention of registering the Warrant Shares. The Holder also understands that there is no assurance that any exemption from registration under the Act will be available and that, even if available, such exemption may not allow the Holder to transfer all or any portion of the Warrant Shares under the circumstances, in the amounts or at the times the Holder might propose.
|
|
| |
|
|
HOLDER | |
|
|
| |
|
|
By: |
|
|
|
Name: |
|
|
|
Title: |
|
|
|
|
|
|
|
Date: |
|
Exhibit C
Form of Fourth Amended and Restated Stockholders’ Agreement
FOURTH AMENDED AND RESTATED STOCKHOLDERS’ AGREEMENT
THIS FOURTH AMENDED AND RESTATED STOCKHOLDERS’ AGREEMENT, dated this 14th day of February, 2014, is entered into by and among (i) Radius Health, Inc., a Delaware corporation (the “Corporation”), (ii) those holders of shares of the Corporation’s Common Stock, par value $.0001 per share (“Common Stock”), listed on Schedule 1 hereto (hereinafter referred to collectively as the “Common Stockholders”), (iii) those holders of shares of the Corporation’s Series B-2 Convertible Preferred Stock, par value $.0001 per share (“Series B-2 Preferred Stock”), listed on Schedule 2 hereto (hereinafter referred to collectively as the “Series B-2 Stockholders”), (iv) those holders of shares of the Corporation’s Series B Convertible Preferred Stock, par value $.0001 per share (“Series B Preferred Stock”), listed on Schedule 3 hereto (hereinafter referred to collectively as the “Series B Stockholders”), (v) those holders of shares of the Corporation’s Series A-1 Convertible Preferred Stock, par value $.0001 per share (“Series A-1 Preferred Stock”), listed on Schedule 4 hereto (hereinafter referred to collectively as the “Series A-1 Stockholders”), (vi) those holders of shares of the Corporation’s Series A-2 Convertible Preferred Stock, par value $.0001 per share (“Series A-2 Preferred Stock”), listed on Schedule 5 hereto (hereinafter referred to collectively as the “Series A-2 Stockholders”), (vii) those holders of the shares of the Corporation’s Series A-3 Convertible Preferred Stock, par value $.0001 per share (“Series A-3 Preferred Stock”), listed on Schedule 6 hereto (hereinafter referred to collectively as the “Series A-3 Stockholders”), (viii) those holders of shares of the Corporation’s Series A-4 Convertible Preferred Stock, par value $.0001 per share (“Series A-4 Preferred Stock”), listed on Schedule 7 hereto (hereinafter referred to collectively as the “Series A-4 Stockholders”), (ix) that certain holder of shares of the Corporation’s Series A-5 Convertible Preferred Stock, par value $.0001 per share (“Series A-5 Preferred Stock”), listed on Schedule 8 hereto (hereinafter referred to as the “Series A-5 Stockholder”) and (x) any person or entity that becomes a party hereto pursuant to Section 17 hereof or otherwise (the “Additional Stockholders”).
WITNESSETH:
WHEREAS, the Corporation and the Series B-2 Stockholders have entered into a Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement, dated as of the date hereof (the “Stock Purchase Agreement”), in connection with which the Corporation has agreed to sell shares Series B-2 Preferred Stock and warrants to purchase shares of Common Stock (the “Series B-2 Financing”), and the Corporation desires to grant to the Series B-2 Stockholders certain registration and other rights with respect to such shares;
WHEREAS, the Corporation and certain of the other parties hereto entered into a Third Amended and Restated Stockholders’ Agreement, dated April 23, 2013 (the “Prior Agreement”), which Prior Agreement the requisite persons desire to amend and restate in its entirety as set forth herein; and
WHEREAS, as a condition to Series B-2 Stockholders entering into the Stock Purchase Agreement, the Common Stockholders, Series B Stockholders, Series A-1 Stockholders, Series A-2 Stockholders, Series A-3 Stockholders, Series A-4 Stockholders, Series A-5 Stockholder and Series A-6 Stockholder (as hereinafter defined) have agreed to certain restrictions on their rights to dispose of their shares of Common Stock (as hereinafter defined) and Preferred Stock (as hereinafter defined) as contained in this Agreement.
NOW, THEREFORE, in consideration of the foregoing and of the respective covenants and undertakings of the Corporation and the Stockholders (as hereinafter defined) hereunder and under the Stock Purchase Agreement, the parties hereto do hereby agree as follows:
Definitions. As used herein, the following terms shall have the following respective meanings:
30-Day Period shall have the meaning set forth in Section 2.3(b) hereof.
Board means the Board of Directors of the Corporation.
BB Bio means BB Biotech Ventures II, L.P. including any successor thereto or any assignee of the interest, in whole or in part, of BB Bio under this Agreement
Brookside means Brookside Capital Partners Fund L.P., a Delaware limited partnership, including any successor thereto or any assignee of the interest, in whole or in part, of Brookside Capital Partners Fund L.P. under this Agreement.
Brookside Group means: (i) Brookside; (ii) any investment fund limited partnership now existing or hereafter formed which is affiliated with or under common control with one or more general partners of any general partner of Brookside (a “Brookside Fund”); (iii) any limited partners or affiliates of Brookside or any other Brookside Fund; and (iv) any successors or assigns of any of the foregoing.
Certificate means the certificate of incorporation of the Corporation, as amended and/or restated from time to time, including the Series A-1 Certificate, the Series B Certificate and the Series B-2 Certificate.
Commission means the U.S. Securities and Exchange Commission.
Common Stock shall have the meaning set forth in the first paragraph of this Agreement.
Convertible Securities means any evidences of indebtedness, shares or other securities directly or indirectly convertible into or exchangeable for Common Stock, but excluding Options.
Demand Notice shall have the meaning set forth in Section 3.4(a) hereof.
Effective Date means the date on which the automatic conversion of all of the Preferred Stock occurs upon the listing of the Common Stock on a national securities exchange.
Equity Percentage means, as to any Series B Stockholder, Series B-2 Stockholder or Other Preferred Stockholder, as applicable, that percentage figure which expresses the ratio that (a) the number of shares of issued and outstanding Common Stock then owned by such Series B Stockholder, Series B-2 Stockholder or Other Preferred Stockholder bears to (b) the aggregate number of shares of issued and outstanding Common Stock then owned by all Series B Stockholders, Series B-2 Stockholders and Other Preferred Stockholders. For purposes solely of the computation set forth in clauses (a) and (b) above and the right of oversubscription (as set forth in Section 2.3(d)), all issued and outstanding securities held by the Series B Stockholders, Series B-2 Stockholders and Other Preferred Stockholders that are convertible into or exercisable or exchangeable for shares of Common Stock (including any issued and issuable shares of Preferred Stock) or for any such convertible, exercisable or exchangeable securities, shall be treated as having been so converted, exercised or exchanged at the rate or price at which such securities are convertible, exercisable or exchangeable for shares of Common Stock in effect at the time in question (which, for purposes of Section 2.3 of this Agreement, shall be at the time of delivery by the Corporation of the notice of the Offer contemplated by Section 2.3(b)), whether or not such securities are at such time immediately convertible, exercisable or exchangeable.
Exchange Act means the Securities Exchange Act of 1934, as amended.
Excess Securities shall have the meaning set forth in Section 2.3(d) hereof.
Excess Securities Notice shall have the meaning set forth in Section 2.3(d) hereof.
Excess Securities Period shall have the meaning set forth in Section 2.3(d) hereof.
Excluded Securities means:
(i) Common Stock issued upon conversion of any Preferred Shares, including any shares of Common Stock issuable upon conversion of any dividends accrued on such Preferred Shares;
(ii) Common Stock issued or issuable to officers, directors or employees of, or consultants, advisors or independent contractors to, the Corporation, pursuant to any written agreement, plan or arrangement to purchase, or rights to subscribe for, such Common Stock approved by the Board, including each of the Investor Directors;
(iii) Common Stock issued as a stock dividend or distribution payable in shares of Common Stock, or capital stock of any other class issuable upon any subdivision, recombination, split-up or reverse stock split of all the outstanding shares of such class of capital stock;
(iv) Common Stock or other securities issued or issuable pursuant to the acquisition by the Corporation of any other corporation, partnership, joint venture, trust or other entity by any merger, stock acquisition, reorganization, or purchase of substantially all assets or otherwise in which the Corporation or its stockholders of record immediately prior to the effective date of such transaction, directly or indirectly, own a majority of the voting power of the acquired entity or the resulting entity after such transaction, in each case so long as such transaction is approved by the Board;
(v) Common Stock or other securities issued or issuable to banks, lenders, equipment lessors or landlords, provided that each such issuance is approved by the Board of Directors, including warrants to acquire Common Stock held by Silicon Valley Bank (or its affiliates, successors and assignees), warrants to purchase Preferred Stock issued to GE Capital Equity Investments, Inc. or any of its affiliates (“GECEI”) and Oxford Finance Corporation or any of its affiliates (“OFC”) pursuant to a debt financing approved by the Board of Directors (the “GE Financing”), shares of Preferred Stock issued or issuable to GECEI in connection with the GE Financing or upon exercise by GECEI or OFC of warrants issued in the GE Financing and shares of common stock issuable upon conversion of any such shares of Preferred Stock issued to GECEI or OFC pursuant to the GE Financing;
(vi) Common Stock or other securities issued or issuable to suppliers or third party service providers in connection with the provision of goods or services or to other third parties in connection with sponsored research agreements, collaboration agreements, development agreements, strategic partnerships or alliances, corporate partnerships, joint ventures or other licensing transactions, provided that each such transaction and related issuance is approved by the Board of Directors, including (A) any shares of Preferred Stock or Common Stock issued or issuable to Ipsen Pharma SAS (“Ipsen”), pursuant to the terms of that certain License Agreement, as amended and may be amended with the approval of the Board of Directors of the Corporation and in effect from time to time, by and between the Corporation and Ipsen as payment for milestones in lieu of cash payments and (B) shares of Series A-5 Preferred
Stock issued pursuant to the Stock Issuance Agreement (as defined in Section 3(d) of the Series A-1 Certificate) and the issuance of Series A-6 Preferred Stock issued or to be issued as dividends on such Series A-5 Preferred Stock, and shares of Common Stock issuable upon conversion of any such shares of Series A-5 Preferred Stock and Series A-6 Preferred Stock;
(vii) Common Stock or other securities, the issuance of which is approved by the Majority Investors, with such approval expressly waiving the application of the right of first refusal provisions of Section 2.3 of this Agreement as a result of such issuance;
(viii) Preferred Stock or Common Stock issued or issuable pursuant to any warrant outstanding as of the date hereof or any warrant and any shares of Preferred Stock or Common Stock, or Common Stock issued upon exercise of any Preferred Stock, issued in connection with the Series B-2 Financing or any equity financing of the Corporation that has occurred at any time prior to the date hereof, including a warrant for shares of Series A-1 Preferred Stock issued or issuable to Leerink Xxxxx and any warrants issued pursuant to the terms of the Stock Purchase Agreement, any shares of Preferred Stock or Common Stock upon exercise thereof and any Common Stock issuable upon conversion of such Preferred Stock issued upon exercise thereof;
(ix) Common Stock or Convertible Securities actually issued upon the exercise of Options or shares of Common Stock actually issued upon the conversion or exchange of Convertible Securities, in each case provided such issuance is pursuant to the terms of such Option or Convertible Security;
(x) All shares of Series B-2 Preferred Stock and other securities issued pursuant to the Stock Purchase Agreement, and all shares of Common Stock issued or issuable upon conversion of any such shares of Series B-2 Preferred Stock or exercise or conversion of any such other securities; and
(xi) Common Stock issued or issuable in the Company’s first underwritten public offering of its Common Stock under the Securities Act.
Expiration Date means the earlier of (i) the third anniversary of the date on which the Common Stock is first listed for trading on a national securities exchange and (ii) February 14, 2019.
Extra Observer shall have the meaning set forth in Section 4.3(b) hereof.
Extra Purchaser shall have the meaning set forth in Section 4.3(b) hereof.
F2 Biosciences means F2 Biosciences III, L.P., a Cayman Islands limited partnership.
FINRA means the Financial Industry Regulatory Authority.
G3 Investor means each of HCV II, Saints Capital IV, L.P. and Wellcome.
G3 Observer shall have the meaning set forth in Section 4.3(a) hereof.
GE Financing shall have the meaning set forth in the definition of “Excluded Securities” above.
GEHFS shall have the meaning set forth in the definition of “Excluded Securities” above.
Group means: (i) as to any Stockholder that is a corporation or other entity, any and all of the venture capital limited partnerships or corporations now existing or hereafter formed that are affiliated with or under common control with one or more of the controlling stockholders of such Stockholder and any predecessor or successor thereto; (ii) in the case of any member of the HCV Group, any other member of the HCV Group; (iii) in the case of any member of the MPM Group, any other member of the MPM Group; (iv) in the case of any member of the Brookside Group, any other member of the Brookside Group; (v) in the case of any member of the Oxford/Saints Group, any other member of the Oxford/Saints Group; and (vi) in the case of Wellcome, any successor trustee of the Wellcome Trust or additional trustee or trustees of the Wellcome Trust from time to time, or any company whose shares are all held directly or indirectly by the Wellcome Trust, or any nominee or custodian of any such person.
HCV Group means: (i) HCV VII; (ii) any venture capital limited partnership now existing or hereafter formed which is affiliated with or under common control with one or more general partners of any general partner of HCV VII (an “HCV Fund”); (iii) any limited partners or affiliates of HCV VII or any other HCV Fund; and (iv) any successors or assigns of any of the foregoing.
HCV VII means HealthCare Ventures VII, L.P. a Delaware limited partnership, including any successor thereto or any assignee of the interest, in whole or in part, of HCV VII under this Agreement.
Holder or Holders means the holder or holders, as the case may be, from time to time of Registrable Securities.
Immediate Family Member means a child, stepchild, grandchild, parent, stepparent, grandparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, including adoptive relationships, of a natural person referred to herein.
Independent Directors shall have the meaning set forth in Section 4.2(b) hereof.
Industry Expert Director shall have the meaning set forth in Section 4.2(b) hereof.
Investor Directors shall have the meaning set forth in Section 4.2(b) hereof.
Initiating Holders means, collectively, Holders who properly initiate a registration request under this Agreement.
Investors means each of the persons listed on Schedule 2, Schedule 3 or Schedule 4 hereto, severally, but not jointly and severally.
Ipsen shall have the meaning set forth in the definition of “Excluded Securities” above.
Majority Investors means (i) the holders of not less than 70% of the outstanding shares of Series B Preferred Stock, (ii) the holders of not less than 70% of the outstanding shares of Series B-2 Preferred Stock and (ii) the Senior Majority (as defined in the Series A-1 Certificate), collectively.
Majority G3 Investors means the G3 Investor(s) holding a majority of the shares of capital stock of the Corporation (on an as-converted basis) held by all G3 Investors, including, with respect to each G3 Investor, all shares of capital stock of the Corporation (on an as-converted basis) held by members of such G3 Investor’s Group.
MPM means MPM Capital L.P.
MPM Group means (i) MPM BioVentures III, L.P., (ii) MPM BioVentures III QP. L.P., (iii) MPM BioVentures III GmbH & Co. Beteiligungs KG, (iv) MPM BioVentures III Parallel Fund, L.P., (v) MPM Asset Management Investors 2003 VIII LLC, (vi) MPM Bio IV NVS Strategic Fund, L.P., (vii) any other venture capital limited partnership now existing or hereafter formed which is affiliated with or under common control with the foregoing or one or more general partners of the foregoing, and (viii) any successors or assigns of the foregoing.
Notice of Acceptance shall have the meaning set forth in Section 2.3(c) hereof.
OFC shall have the meaning set forth in the definition of “Excluded Securities” above.
Offer shall have the meaning set forth in Section 2.3(b) hereof.
Offered Securities means, except for Excluded Securities, (i) any shares of Common Stock, Preferred Stock or any other equity security of the Corporation, (ii) any debt security, (iii) any capitalized lease with any equity feature with respect to the Corporation, or (iv) any option, warrant or other right to subscribe for, purchase or otherwise acquire any such equity security, debt security or capitalized lease.
Option means rights, options or warrants to subscribe for, purchase or otherwise acquire Common Stock or Convertible Securities.
Other Preferred Stockholder means any holder of shares of Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, Series A-5 Preferred Stock or Series A-6 Preferred Stock.
Other Shares shall have the meaning set forth in Section 3.6(b) hereof.
Oxford means OBP IV — Holdings LLC.
Oxford/Saints Group means (i) Oxford Bioscience Partners IV L.P., (ii) mRNA Fund II L.P., (iii) OBP IV — Holdings LLC, (iv) mRNA II — Holdings LLC, (v) Saints Capital VI, L.P., (vi) any other venture capital limited partnership now existing or hereafter formed which is affiliated with or under common control with the foregoing or one or more general partners of the foregoing, and (vii) any successors or assigns of the foregoing.
Person (whether or not capitalized) means an individual, corporation, partnership, limited partnership, limited liability company, syndicate, trust, association or other entity.
Preferred Shares means shares of Series B-2 Preferred Stock, Series B Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock and Series A-5 Preferred Stock and shares of the Corporation’s Series A-6 Convertible Preferred Stock, par value $.0001 per share (the “Series A-6 Preferred Stock”, with any holder of shares of Series A-6 Preferred Stock being referred to herein as a “Series A-6 Stockholder”).
Preferred Stock means the Preferred Stock, par value $.0001 per share, of the Corporation.
Preferred Stockholders means, collectively, the holders of shares of Preferred Stock of the Corporation.
Prior Agreement shall have the meaning set forth in the third paragraph of this Agreement.
Qualified Public Offering means any public offering of shares of the Corporation’s capital stock in connection with which the Common Stock becomes listed for trading on a national securities exchange.
Refused Securities shall have the meaning set forth in Section 2.3(f) hereof.
Registrable Securities means (i) the Common Stock issued or issuable upon the conversion of the Preferred Stock, (ii) the Common Stock issued or issuable upon exercise of the Warrants (as defined in the Stock Purchase Agreement) and the warrants issued pursuant to the Series B Stock Purchase Agreement, (iii) any Common Stock, or any Common Stock issued or issuable (directly or indirectly) upon conversion and/or exercise of any other securities of the Corporation, acquired by the Investors or any member of an Investor’s Group after the date hereof, (iv) any Common Stock issued as (or issuable upon conversion or exercise of any warrant, right or other security that is issued as) a dividend or other distribution with respect to, or in exchange for or in replacement of, the shares referenced in clause (i) above; excluding in all cases, however, any Registrable Securities sold by a Person in a transaction in which the applicable rights under this Agreement are not assigned pursuant to Section 7, and excluding any shares for which registration rights have terminated pursuant to Section 3.13.
Registrable Senior Securities means the Registrable Securities issued or issuable upon conversion of, or in respect of, the Senior Preferred Stock.
Restricted Stock means all shares of capital stock of the Corporation, including (i) all shares of Common Stock, (ii) all shares of Preferred Stock, (iii) all shares of capital stock of the Corporation into which such shares may be converted or for which they may be exchanged or exercised and (iv) all other shares of capital stock issued or issuable by way of stock splits, stock dividends, stock combinations, recapitalizations or like occurrences on such shares.
ROFR Stockholders shall have the meaning set forth in Section 2.3(a) hereof.
Rule 145 means Rule 145 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
Securities Act means the Securities Act of 1933, as amended.
Selling Expenses means all underwriting discounts, selling commissions, and stock transfer taxes applicable to the sale of Registrable Securities, and fees and disbursements of counsel for any Holder.
Senior Preferred Stock means shares of Series B-2 Preferred Stock, Series B Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock and Series A-3 Preferred Stock.
Series A-1 Certificate means the Certificate of Designations of the Series A-1 Convertible Preferred Stock, Series A-2 Convertible Preferred Stock, Series A-3 Convertible Preferred Stock, Series A-4 Convertible Preferred Stock, Series A-5 Convertible Preferred Stock and Series A-6 Convertible Preferred Stock of the Corporation filed by the Corporation with the Secretary of State of the State of Delaware on May 17, 2011, as amended from time to time.
Series A-1 Directors shall have the meaning set forth in Section 4.2(b) hereof.
Series A-1 Preferred Stock shall have the meaning set forth in the first paragraph of this Agreement.
Series A-1 Stock Purchase Agreement means that certain Series A-1 Stock Purchase Agreement, dated as of May 17, 2011, by and among the Corporation and the Investors referenced therein.
Series A-2 Preferred Stock shall have the meaning set forth in the first paragraph of this Agreement.
Series A-3 Preferred Stock shall have the meaning set forth in the first paragraph of this Agreement.
Series A-4 Preferred Stock shall have the meaning set forth in the first paragraph of this Agreement.
Series A-5 Preferred Stock shall have the meaning set forth in the first paragraph of this Agreement.
Series A-6 Preferred Stock shall have the meaning set forth in the definition of “Preferred Shares” above.
Series A-1 Stockholders shall have the meaning set forth in the first paragraph of this Agreement.
Series A-2 Stockholders shall have the meaning set forth in the first paragraph of this Agreement.
Series A-3 Stockholders shall have the meaning set forth in the first paragraph of this Agreement.
Series A-4 Stockholder shall have the meaning set forth in the first paragraph of this Agreement.
Series A-5 Stockholder shall have the meaning set forth in the first paragraph of this Agreement.
Series A-6 Stockholder shall have the meaning set forth in the definition of “Preferred Shares” above.
Series B Certificate means the Certificate of Designations of the Series B Convertible Preferred Stock filed by the Corporation with the Secretary of State of the State of Delaware on April 23, 2013, as amended from time to time.
Series B-2 Certificate means the Certificate of Designations of the Series B-2 Convertible Preferred Stock filed by the Corporation with the Secretary of State of the State of Delaware on the date hereof, as amended from time to time.
Series B-2 Financing shall have the meaning set forth in the second paragraph of this
Agreement.
Series B Preferred Stock shall have the meaning set forth in the first paragraph of this Agreement.
Series B-2 Preferred Stock shall have the meaning set forth in the first paragraph of this Agreement.
Series B Stock Purchase Agreement means the Series B Convertible Preferred Stock and Warrant Purchase Agreement, dated April 23, 2013, by and among the Corporation and the Series B Stockholders named therein.
Series B Stockholders shall have the meaning set forth in the first paragraph of this Agreement.
Series B-2 Stockholders shall have the meaning set forth in the first paragraph of this Agreement.
Stock Purchase Agreement shall have the meaning set forth in the second paragraph of this Agreement.
Stockholders means all holders of capital stock of the Corporation.
Tax and Taxes shall have the meanings set forth in Section 2.9 hereof.
Transfer shall include any disposition of any Restricted Stock or of any interest therein which would constitute a sale thereof within the meaning of the Securities Act.
Wellcome means The Wellcome Trust Limited, as trustee of the Wellcome Trust.
Certain Covenants of the Corporation.
Meetings of the Board of Directors. The Corporation shall call, and use its best efforts to have, regular meetings of the Board not less often than quarterly. The Corporation shall promptly pay all reasonable and appropriately documented travel expenses and other out-of-pocket expenses incurred by directors who are not employed by the Corporation in connection with attendance at meetings to transact the business of the Corporation or attendance at meetings of the Board or any committee thereof.
Reservation of Shares of Common Stock and Preferred Stock, Etc. The Corporation shall at all times have authorized and reserved out of its authorized but unissued shares of Common Stock a sufficient number of shares of Common Stock to provide for the conversion of the Preferred Shares. Neither the issuance of the Preferred Shares nor the shares of Common Stock issuable upon the conversion of the Preferred Shares shall be subject to a preemptive right of any other Stockholder.
Right of First Refusal.
(a) The Corporation shall not issue, sell or exchange, agree to issue, sell or exchange, or reserve or set aside for issuance, sale or exchange, any Offered Securities, unless in each case the Corporation shall have first offered to sell to the Series B-2 Stockholders, the Series B Stockholders, the Series A-1 Stockholders, the Series A-2 Stockholders and the Series A-3 Stockholders
(collectively, the “ROFR Stockholders”) all of such Offered Securities on the terms set forth herein. Each ROFR Stockholder shall be entitled to purchase up to its Equity Percentage of the Offered Securities. Each ROFR Stockholder may delegate its rights and obligations with respect to such Offer to one or more members of its Group, which members shall thereafter be deemed to be “ROFR Stockholders” for the purpose of applying this Section 2.3 to such Offer.
(b) The Corporation shall deliver to each ROFR Stockholder written notice of the offer to sell the Offered Securities, specifying the price and terms and conditions of the offer (the “Offer”). The Offer by its terms shall remain open and irrevocable for a period of 30 days from the date of its delivery to such ROFR Stockholders (the “30-Day Period”), subject to extension to include the Excess Securities Period (as such term is hereinafter defined).
(c) Each ROFR Stockholder shall evidence its intention to accept the Offer by delivering a written notice signed by such ROFR Stockholder, as applicable, setting forth the number of shares that such ROFR Stockholder elects to purchase (the “Notice of Acceptance”). The Notice of Acceptance must be delivered to the Corporation prior to the end of the 30-Day Period. The failure by a ROFR Stockholder to exercise its rights hereunder shall not constitute a waiver of any other rights or of the right to receive notice of and participate in any subsequent Offer.
(d) If any ROFR Stockholder fails to exercise its right hereunder to purchase its Equity Percentage of the Offered Securities, the Corporation shall so notify the other ROFR Stockholders in a written notice (the “Excess Securities Notice”). The Excess Securities Notice shall be given by the Corporation promptly after it learns of the intention of any ROFR Stockholder not to purchase all of its Equity Percentage of the Offered Securities, but in no event later than ten (10) business days after the expiration of the 30-Day Period. The ROFR who or which have agreed to purchase their Equity Percentage of the Offered Securities shall have the right to purchase the portion not purchased by such ROFR Stockholders (the “Excess Securities”), on a pro rata basis, by giving notice within ten (10) business days after receipt of the Excess Securities Notice from the Corporation. The twenty (20) business day period during which (i) the Corporation must give the Excess Securities Notice to the applicable ROFR Stockholders, and (ii) each of them must then give the Corporation notice of their intention to purchase all or any portion of their pro rata share of the its Excess Securities, is hereinafter referred to as the “Excess Securities Period.”
(e) If the ROFR Stockholders tender their Notice of Acceptance prior to the end of the 30-Day Period, indicating their intention to purchase all of the Offered Securities, or, if prior to the termination of the Excess Securities Period the ROFR Stockholders tender Excess Securities Notices to purchase all of the Excess Securities, the Corporation shall schedule a closing of the sale of all such Offered Securities. Upon the closing of the sale of the Offered Securities to be purchased by the ROFR Stockholders and the Excess Securities to be purchased by ROFR Stockholders, each ROFR Stockholder shall (i) purchase from the Corporation that portion of the Offered Securities and Excess Securities, as applicable, for which it tendered a Notice of Acceptance and an Excess Securities Notice, as applicable, upon the terms specified in the Offer, and (ii) execute and deliver an agreement further restricting transfer of such Offered Securities substantially as set forth in Section 3.1, 3.2 and 3.3 of this Agreement. In addition, with respect to the Offered Securities and Excess Securities being purchased by the ROFR Stockholders, the Corporation shall provide each such ROFR Stockholder with the rights and benefits set forth in this Agreement. The obligation of the ROFR Stockholders to purchase such Offered Securities and Excess Securities, as applicable, is further conditioned upon the preparation of a purchase agreement embodying the terms of the Offer, which shall be reasonably satisfactory in form and substance to such ROFR Stockholder and each of their respective counsels.
(f) The Corporation shall have ninety (90) days from the expiration of the 30-Day Period, or the Excess Securities Period, if applicable, to sell the Offered Securities (including the Excess Securities) refused by the ROFR Stockholders (the “Refused Securities”) to any other person or persons, but only upon terms and conditions which are in all material respects (including price and interest rate) no more favorable to such other person or persons, and no less favorable to the Corporation, than those set forth in the Offer. Upon and subject to the closing of the sale of all of the Refused Securities (which shall include full payment to the Corporation), each ROFR Stockholder shall (i) purchase from the Corporation those Offered Securities and Excess Securities, as applicable, for which it tendered a Notice of Acceptance and an Excess Securities Notice, if applicable, upon the terms specified in the Offer, and (ii) execute and deliver an agreement restricting transfer of such Offered Securities and Excess Securities, as applicable, substantially as set forth in Sections 3.1, 3.2 and 3.3 of this Agreement. In addition, with respect to the Offered Securities or Excess Securities being purchased by the ROFR Stockholders, the Corporation shall provide each such ROFR Stockholder with the rights and benefits set forth in this Agreement. The Corporation agrees, as a condition precedent to accepting payment for and making delivery of any Refused Securities to any executive officer, employee, consultant or independent contractor of or to the Corporation, or to any other person, to have each and every such person execute and deliver this Agreement, as may be modified or amended from time to time pursuant to Section 11 hereof, to the extent such purchaser has not already executed this Agreement. The obligation of the ROFR Stockholders to purchase such Offered Securities and Excess Securities, as applicable, is further conditioned upon the preparation of a purchase agreement embodying the terms of the Offer, which shall be reasonably satisfactory in form and substance to such ROFR Stockholder and each of their respective counsels.
(g) In each case, any Offered Securities not purchased either by the ROFR Stockholders or by any other person in accordance with this Section 2.3 may not be sold or otherwise disposed of until they are again offered to the ROFR Stockholders under the procedures specified in Paragraphs (a), (b), (c), (d), (e) and (f) hereof.
(h) Each ROFR Stockholder may, by prior written consent, waive its rights under this Section 2.3. Such a waiver shall be deemed a limited waiver and shall only apply to the extent specifically set forth in the written consent of such ROFR Stockholder.
(i) This Section 2.3 and the rights and obligations of the parties hereunder shall automatically terminate on the consummation of a Qualified Public Offering.
Filing of Reports Under the Exchange Act. For so long as the Common Stock is registered under the Exchange Act, the Corporation shall comply with all reporting requirements of the Exchange Act and shall comply with all other public information reporting requirements of the Commission as a condition to the availability of an exemption from the Securities Act for the sale of any of the Registrable Securities (including any such exemption pursuant to Rule 144 thereof, as amended from time to time, or any successor rule thereto or otherwise). The Corporation shall cooperate with each Holder in supplying such information as may be necessary for such holder to complete and file any information reporting forms presently or hereafter required by the Commission as a condition to the availability of an exemption from the Securities Act (under Rule 144 thereof or otherwise) for the sale of any Registrable Securities.
Directors’ & Officers’ Insurance. The Corporation shall continue to maintain a directors’ and officers’ liability insurance policy covering all directors, observers and executive officers of the Corporation.
Properties and Business Insurance. The Corporation shall continue to maintain from responsible and reputable insurance companies or associations valid policies of insurance against such casualties, contingencies and other risks and hazards and of such types and in such amounts as is customary for similarly situated businesses.
Preservation of Corporate Existence. The Corporation shall preserve and maintain its corporate existence, rights, franchises and privileges in the jurisdiction of its incorporation, and qualify and remain qualified as a foreign corporation in each jurisdiction in which (i) such qualification is necessary or desirable in view of its business and operations or the ownership or lease of its properties or (ii) the failure to so qualify would have a material adverse effect on the business, properties, assets or condition (financial or otherwise) of the Corporation.
Compliance with Laws. The Corporation shall comply with all applicable laws, rules, regulations, requirements and orders of the United States or any applicable foreign jurisdiction in the conduct of its business including all labor, employment, wage and hour, health and safety, environmental, health insurance, health information security, privacy, data protection and data transfer laws, and shall adopt and monitor policies and procedures designed to comply with all such applicable laws, rules, regulations and orders, except where noncompliance would not have a material adverse effect on the business, properties, assets or condition (financial or otherwise) of the Corporation.
Payment of Taxes. The Corporation will pay and discharge all lawful Taxes (as defined below) before such Taxes shall become in default and all lawful claims for labor, materials and supplies which, if not paid when due, might become a lien or charge upon its property or any part thereof; provided, however, that the Corporation shall not be required to pay and discharge any such Tax, assessment, charge, levy or claim so long as the validity thereof is being contested by or for the Corporation in good faith by appropriate proceedings and an adequate reserve therefore has been established on its books. The term “Tax” (and, with correlative meaning, “Taxes”) means all United States federal, state and local, and all foreign, income, profits, franchise, gross receipts, payroll, transfer, sales, employment, use, property, excise, value added, ad valorem, estimated, stamp, alternative or add-on minimum, recapture, environmental, withholding and any other taxes, charges, duties, impositions or assessments, together with all interest, penalties, and additions imposed on or with respect to such amounts, or levied, assessed or imposed against the Corporation.
Management Compensation. The Board (upon the recommendation of the Compensation Committee or otherwise) shall determine the compensation to be paid by the Corporation to its executive officers. Any grants of capital stock or options to employees, officers, directors or consultants of the Corporation and its Subsidiaries shall be made pursuant to a plan, agreement or arrangement approved by the Board.
No Further Pay-to-Play Provisions. The Corporation hereby covenants and agrees that at no time after the date of this Agreement, without the prior written consent of each of F2 Biosciences, Wellcome, BB Bio, one member of the HCV Group, one member of the MPM Group, one member of the Brookside Group and one member of the Oxford/Saints Group, shall it enter into any agreement or amend the Certificate to implement terms that would automatically convert Preferred Shares into shares of Common Stock, or impose any other penalty on the holder of Preferred Shares, solely because the holders of such Preferred Shares fail to participate at any level in a transaction pursuant to which the Corporation raises funds through the issuance of debt or equity securities.
Confidentiality, Assignment of Inventions and Non-Competition Agreements for Key Employees. The Corporation shall cause each person who becomes an employee of or a consultant to the Corporation subsequent to the date hereof, and who shall have or be proposed to have access to
confidential or proprietary information of the Corporation, to execute a confidentiality, assignment of inventions, and non-competition agreement in form and substance approved by the Board prior to the commencement of such person’s employment by the Corporation in such capacity.
Duration of Section. Sections 2.5 through 2.12 and the rights and obligations of the parties hereunder shall automatically terminate on the earlier of (i) the consummation of an Event of Sale (as defined in the Certificate) or (ii) the automatic conversion of all of the Preferred Stock of the Corporation pursuant to the terms and conditions of the Certificate upon the listing, or the admitting for trading, of the Common Stock on a national securities exchange.
Transfer of Securities.
Restriction on Transfer. The Restricted Stock shall not be transferable, except upon the conditions specified in this Section 3, which conditions are intended solely to ensure compliance with the provisions of the Securities Act in respect of the Transfer thereof. In addition, no Restricted Stock shall be transferred unless, as conditions precedent to such transfer, the transferee thereof agrees in writing to be bound by the obligations of the transferring Stockholder hereunder.
Restrictive Legend. Each certificate evidencing any Restricted Stock and each certificate evidencing any such securities issued to subsequent transferees of any Restricted Stock shall (unless otherwise permitted by the provisions of Section 3.3 or 3.10 hereof) be stamped or otherwise imprinted with a legend in substantially the following form:
THE SECURITIES REPRESENTED BY THIS CERTIFICATE HAVE BEEN ACQUIRED FOR INVESTMENT AND HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR ANY STATE SECURITIES LAW. THE SECURITIES MAY NOT BE PLEDGED, HYPOTHECATED, SOLD OR TRANSFERRED IN THE ABSENCE OF AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAW OR AN EXEMPTION THEREFROM UNDER SUCH ACT OR LAW.
Notice of Transfer. By acceptance of any Restricted Stock, the holder thereof agrees to give prior written notice to the Corporation of such holder’s intention to effect any Transfer and to comply in all other respects with the provisions of this Section 3.3. Each such notice shall describe the manner and circumstances of the proposed Transfer and shall be accompanied by: (a) the written opinion of counsel for the holder of such Restricted Stock or, at such holder’s option, a representation letter of such holder, addressed to the Corporation (which opinion and counsel, or representation letter, as the case may be, shall be reasonably acceptable to the Corporation), as to whether, in the case of a written opinion, in the opinion of such counsel such proposed Transfer involves a transaction requiring registration of such Restricted Stock under the Securities Act and applicable state securities laws or an exemption thereunder is available, or, in the case of a representation letter, such letter sets forth a factual basis for concluding that such proposed transfer involves a transaction requiring registration of such Restricted Stock under the Securities Act and applicable state securities laws or that an exemption thereunder is available, or (b) if such registration is required and if the provisions of Section 3.4 hereof are applicable, a written request addressed to the Corporation by the holder of such Restricted Stock describing in detail the proposed method of disposition and requesting the Corporation to effect the registration of such Registrable Securities pursuant to the terms and provisions of Section 3.4 hereof; provided, however, that (y) in the case of a Transfer by a holder to a member of such holder’s Group, no such opinion of counsel or representation letter of the holder shall be necessary, provided that the transferee agrees in writing to be subject to Sections 3.1, 3.2, 3.3, 3.10 hereof to the same extent as if
such transferee were originally a signatory to this Agreement, and (z) in the case of any holder of Restricted Stock that is a partnership, no such opinion of counsel or representation letter of the holder shall be necessary for a Transfer by such holder to a partner of such holder, or a retired partner of such holder who retires after the date hereof, or the estate of any such partner or retired partner if, with respect to such Transfer by a partnership, (i) such Transfer is made in accordance with the partnership agreement of such partnership, and (ii) the transferee agrees in writing to be subject to the terms of Sections 3.1, 3.2, 3.3, 3.10 hereof to the same extent as if such transferee were originally a signatory to this Agreement. If in an opinion of counsel or as reasonably concluded from the facts set forth in the representation letter of the holder (which opinion and counsel or representation letter, as the case may be, shall be reasonably acceptable to the Corporation), the proposed Transfer may be effected without registration under the Securities Act and any applicable state securities laws or “blue sky” laws, then the holder of Restricted Stock shall thereupon be entitled to effect such Transfer in accordance with the terms of the notice delivered by it to the Corporation. Each certificate or other instrument evidencing the securities issued upon such Transfer (and each certificate or other instrument evidencing any such securities not Transferred) shall bear the legend set forth in Section 3.2 hereof unless: (a) in such opinion of such counsel or as can be concluded from the representation letter of such holder (which opinion and counsel or representation letter shall be reasonably acceptable to the Corporation) the registration of future Transfers is not required by the applicable provisions of the Securities Act and state securities laws, or (b) the Corporation shall have waived the requirement of such legend; provided, however, that such legend shall not be required on any certificate or other instrument evidencing the securities issued upon such Transfer in the event such transfer shall be made in compliance with the requirements of Rule 144 (as amended from time to time or any similar or successor rule) promulgated under the Securities Act. The holder of Restricted Stock shall not effect any Transfer until such opinion of counsel or representation letter of such holder has been given to and accepted by the Corporation (unless waived by the Corporation) or, if applicable, until registration of the Registrable Securities involved in the above-mentioned request has become effective under the Securities Act. In the event that an opinion of counsel is required by the registrar or transfer agent of the Corporation to effect a transfer of Restricted Stock in the future, the Corporation shall seek and obtain such opinion from its counsel, and the holder of such Restricted Stock shall provide such reasonable assistance as is requested by the Corporation (other than the furnishing of an opinion of counsel) to satisfy the requirements of the registrar or transfer agent to effectuate such transfer. Notwithstanding anything to the contrary herein, the provisions of this Section 3.3 and of Sections 3.1 and 3.2 shall not apply, and shall be deemed of no force or effect, with respect to shares of capital stock of the Corporation that are subject to a re-sale registration statement under the Securities Act, provided that such registration statement has been declared, and continues to remain, effective by the Commission.
Registration Rights.
(j) Form S-1 Demand. If (i) at any time after 180 days after the Effective Date, the Corporation receives a request from Holders of a majority of the Registrable Securities then outstanding that the Corporation file a Form S-1 registration statement with respect to at least thirty percent (30%) of the Registrable Securities then outstanding or (ii) the Corporation receives a request from Holders of a majority of the Registrable Senior Securities that the Corporation file a Form S-1 registration statement with respect to the Registrable Senior Securities then outstanding, then the Corporation shall (1) within 20 days after the date such request is given, give notice thereof (the “Demand Notice”) to all Holders other than the Initiating Holders; and (2) as soon as practicable, and in any event within 60 days after the date such request is given by the Initiating Holders, file a Form S-1 registration statement under the Securities Act covering all Registrable Securities that the Initiating Holders requested to be registered and any additional Registrable Securities requested to be included in such registration by any other Holders, as specified by notice given by each such Holder to the Corporation within 20 days of
the date the Demand Notice is given, and in each case, subject to the limitations of Sections 3.4(c), 3.4(f) and 3.6.
(k) Form S-3 Demand. If at any time when it is eligible to use a Form S-3 registration statement, the Corporation receives a request from Holders of at least ten percent (10%) of the Registrable Securities then outstanding that the Corporation file a Form S-3 registration statement with respect to outstanding Registrable Securities of such Holders having an anticipated aggregate offering price, net of Selling Expenses, of at least $10.0 million, then the Corporation shall (i) within 10 days after the date such request is given, give a Demand Notice to all Holders other than the Initiating Holders; and (ii) as soon as practicable, and in any event within 45 days after the date such request is given by the Initiating Holders, file a Form S-3 registration statement under the Securities Act covering all Registrable Securities requested to be included in such registration by any other Holders, as specified by notice given by each such Holder to the Corporation within 20 days of the date the Demand Notice is given, and in each case, subject to the limitations of Sections 3.4(c), 3.4(f) and 3.6.
(l) Notwithstanding the foregoing obligations, if the Corporation furnishes to Holders requesting a registration pursuant to this Section 3.4 a certificate signed by the Corporation’s chief executive officer stating that in the good faith judgment of the Board it would be materially detrimental to the Corporation and its stockholders for such registration statement to be filed and it is therefore necessary to defer the filing of such registration statement, then the Corporation shall have the right to defer taking action with respect to such filing, and any time periods with respect to filing or effectiveness thereof shall be tolled correspondingly, for a period of not more than 90 days after the request of the Initiating Holders is given; provided, however, that the Corporation may not invoke this right more than twice in any 12-month period.
(m) The Corporation shall not be obligated to effect, or to take any action to effect, any registration pursuant to Section 3.4(a) (i) after the Corporation has effected two registrations pursuant to Section 3.4(a); or (ii) if the Initiating Holders propose to dispose of shares of Registrable Securities that may be immediately registered on Form S-3 pursuant to a request made pursuant to Section 3.4(b). The Corporation shall not be obligated to effect, or to take any action to effect, any registration pursuant to Section 3.4(b) (i) during the period that is 30 days before the Corporation’s good faith estimate of the date of filing of, and ending on a date that is 90 days after the effective date of, a Corporation-initiated registration, provided, that the Corporation is actively employing in good faith commercially reasonable efforts to cause such registration statement to become effective; or (ii) if the Corporation has effected two registrations pursuant to Section 3.4(b) during the 12-month period immediately preceding the date of such request. A registration shall not be counted as “effected” for purposes of this Section 3.4(d) until such time as the applicable registration statement has been declared effective by the Commission, unless the Initiating Holders withdraw their request for such registration, elect not to pay the registration expenses therefor, and forfeit their right to one demand registration statement pursuant to Section 3.4(a), in which case such withdrawn registration statement shall be counted as “effected” for purposes of this Section 3.4(d).
(n) Existing Registration Statement. The Corporation shall prepare and file with the Commission such amendments and supplements to the Corporation’s Registration Statement on Form S-1 (Reg. No. 333-175091) and the prospectus used in connection therewith as may be requested by the Holders of a majority of the Registrable Senior Securities and necessary to keep such registration statement effective until the earlier of (i) the sale of all Registrable Securities covered thereby or (ii) such time as the Registrable Securities registered on such registration statement are included in another registration statement covering the sale of such Registrable Securities, and to comply with the provisions of the Securities Act with respect to the sale or other disposition of all Registrable Securities covered by
such registration statement.
(o) The Corporation shall not be required to include in any registration statement an amount of securities that would exceed the maximum number of shares that can be included therein in accordance with the Securities Act and the rules and regulations promulgated thereunder. In the event that not all Registrable Securities that Holders desire to include in a registration statement can be included in any one registration statement, then the Registrable Securities to be included shall be allocated among Holders on a pro rata basis based on the total number of Registrable Securities held by all Holders that have not been included in a registration statement.
Piggyback Registration.
(p) Each time that the Corporation proposes for any reason to register any of its securities under the Securities Act, other than (i) a registration relating to the sale of securities to employees of the Corporation or a subsidiary pursuant to a stock option, stock purchase or similar plan; (ii) a registration relating to a Rule 145 transaction; (iii) a registration on any form that does not include substantially the same information as would be required to be included in a registration statement covering the sale of the Registrable Securities; or (iv) a registration in which the only Common Stock being registered is Common Stock issuable upon conversion of debt securities that are also being registered, the Corporation shall promptly give written notice of such proposed registration to all Holders, which notice shall also constitute an offer to such Holders to request inclusion of any Registrable Securities in the proposed registration.
(q) Each Holder shall have 30 days from the receipt of such notice to deliver to the Corporation a written request specifying the number of Registrable Securities such Holder intends to sell and the Holder’s intended method of disposition.
(r) In the event that the proposed registration by the Corporation is, in whole or in part, an underwritten public offering of securities of the Corporation, any request under Section 3.5(b) may specify that the Registrable Securities be included in the underwriting (i) on the same terms and conditions as the shares of Common Stock, if any, otherwise being sold through underwriters under such registration, or (ii) on terms and conditions comparable to those normally applicable to offerings of common stock in reasonably similar circumstances in the event that no Common Stock other than Registrable Securities are being sold through underwriters under such registration.
(s) Upon receipt of a written request pursuant to Section 3.5(b), the Corporation shall promptly use its best efforts to cause all such Registrable Securities to be registered under the Securities Act, to the extent required to permit sale or disposition as set forth in the written request.
Underwriting Requirements.
(t) If, pursuant to Section 3.4, the Initiating Holders intend to distribute the Registrable Securities covered by their request by means of an underwriting, they shall so advise the Corporation as a part of their request made pursuant to Section 3.4, and the Corporation shall include such information in the Demand Notice. The underwriter(s) will be selected by the Corporation and shall be reasonably acceptable to the Initiating Holders holding a majority of the shares of the Corporation’s capital stock then held by all Initiating Holders (calculated on an as-converted basis). In such event, the right of any Holder to include such Holder’s Registrable Securities in such registration shall be conditioned upon such Holder’s participation in such underwriting and the inclusion of such Holder’s
Registrable Securities in the underwriting to the extent provided herein. All Holders proposing to distribute their securities through such underwriting shall (together with the Corporation) enter into an underwriting agreement in customary form with the underwriter(s) selected for such underwriting. Notwithstanding any other provision of this Section 3.6, if the managing underwriters advise the Initiating Holders in writing that marketing factors require a limitation on the number of shares to be underwritten, then the Initiating Holders shall so advise all Holders of Registrable Securities that otherwise would be underwritten pursuant hereto, and the number of Registrable Securities that may be included in the underwriting shall be allocated among such Holders of Registrable Securities, including the Initiating Holders, in proportion (as nearly as practicable) to the number of Registrable Securities owned by each Holder or in such other proportion as shall mutually be agreed to by all such selling Holders; provided, however, that the number of Registrable Securities held by the Holders to be included in such underwriting shall not be reduced unless all other securities are first entirely excluded from the underwriting.
(u) In connection with any offering involving an underwriting of shares of the Corporation’s capital stock pursuant to Section 3.5, the Corporation shall not be required to include any of the Holders’ Registrable Securities in such underwriting unless the Holders accept the terms of the underwriting as agreed upon between the Corporation and its underwriters, and then only in such quantity as the underwriters in their sole discretion determine will not jeopardize the success of the offering by the Corporation. If the managing underwriter(s) of any proposed registration under Section 3.5 determines and advises in writing that the inclusion of all Registrable Securities proposed to be included in the underwritten public offering, together with any other Common Stock proposed to be included therein by holders other than the Holders (such other shares hereinafter collectively referred to as the “Other Shares”) would interfere with the successful marketing of the Corporation’s securities, then the total number of such securities proposed to be included in such underwritten public offering shall be reduced, (i) first by the shares requested to be included in such registration by the holders of Other Shares, (ii) second, if necessary, by all Registrable Securities which are not Registrable Senior Securities and (iii) third, if necessary, so that (A) one-half (1/2) of the securities to be included consist of the securities proposed to be issued by the Corporation, and (B) one-half (1/2) of the securities to be included consist of the Registrable Senior Securities proposed to be included in such registration by the holders thereof, allocated among such Holders on a pro rata basis calculated based upon the number of Registrable Senior Securities sought to be registered by each such holder; provided, that the aggregate number of securities proposed to be included in such registration by the holders of Registrable Senior Securities shall only be reduced hereunder if and to the extent that such securities exceed twenty-five percent (25%) of the aggregate number of securities included in such registration. The shares of Common Stock that are excluded from the underwritten public offering pursuant to the preceding sentence shall be withheld from the market by the holders thereof for a period, not to exceed 90 days from the closing of such underwritten public offering, that the managing underwriter reasonably determines as necessary in order to effect such underwritten public offering. For purposes of the provision in this Section 3.6(b) concerning apportionment, for any selling Holder that is a partnership, limited liability company, or corporation, the partners, members, retired partners, retired members, stockholders, and affiliates of such Holder, or the estates and Immediate Family Members of any such partners, retired partners, members, and retired members and any trusts for the benefit of any of the foregoing Persons, shall be deemed to be a single “selling Holder,” and any pro rata reduction with respect to such “selling Holder” shall be based upon the aggregate number of Registrable Securities owned by all Persons included in such “selling Holder,” as defined in this sentence.
(v) For purposes of Section 3.4, a registration shall not be counted as “effected” if, as a result of an exercise of the underwriter’s cutback provisions in Section 3.6(a) or (b), fewer than fifty percent (50%) of the total number of Registrable Senior Securities that Holders have
requested to be included in such registration statement are actually included.
Preparation and Filing. If and whenever the Corporation is under an obligation pursuant to the provisions of Sections 3.4 and/or 3.5 to use its best efforts to effect the registration of any Registrable Securities, the Corporation shall, as expeditiously as practicable:
(w) prepare and file with the Commission a registration statement with respect to such securities and use its best efforts to cause such registration statement to become and remain effective in accordance with Section 3.7(b) hereof;
(x) prepare and file with the Commission such amendments and supplements to such registration statement and the prospectus used in connection therewith as may be necessary to keep such registration statement effective until the earlier of (i) the sale of all Registrable Securities covered thereby or (ii) nine months from the date such registration statement first becomes effective, and to comply with the provisions of the Securities Act with respect to the sale or other disposition of all Registrable Securities covered by such registration statement;
(y) furnish to each holder whose Registrable Securities are being registered pursuant to this Section 3 such number of copies of any summary prospectus or other prospectus, including a preliminary prospectus, in conformity with the requirements of the Securities Act, and such other documents as such holder may reasonably request in order to facilitate the public sale or other disposition of such Registrable Securities;
(z) use its best efforts to register or qualify the Registrable Securities covered by such registration statement under the securities or blue sky laws of such jurisdictions as each holder whose Registrable Securities are being registered shall reasonably request, and do any and all other acts or things which may be necessary or advisable to enable such holder to consummate the public sale or other disposition in such jurisdictions of such Registrable Securities; provided, however, that the Corporation shall not be required to consent to general service of process for all purposes in any jurisdiction where it is not then subject to process, qualify to do business as a foreign corporation where it would not be otherwise required to qualify or submit to liability for state or local taxes where it is not otherwise liable for such taxes;
(aa) at any time when a prospectus covered by such registration statement and relating thereto is required to be delivered under the Securities Act within the appropriate period mentioned in Section 3.7(b) hereof, notify each holder whose Registrable Securities are being registered of the happening of any event as a result of which the prospectus included in such registration, as then in effect, includes an untrue statement of a material fact or omits to state a material fact required to be stated therein or necessary to make the statements therein not misleading in the light of the circumstances then existing and, at the request of such holder, prepare, file and furnish to such holder a reasonable number of copies of a supplement to or an amendment of such prospectus as may be necessary so that, as thereafter delivered to the purchasers of such shares, such prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein not misleading in the light of the circumstances then existing;
(bb) if the Corporation has delivered preliminary or final prospectuses to the holders of Registrable Securities that are being registered and after having done so the prospectus is amended to comply with the requirements of the Securities Act, the Corporation shall promptly notify such holders and, if requested, such holders shall immediately cease making offers of Registrable Securities and return all prospectuses to the Corporation. The Corporation shall promptly provide such
holders with revised prospectuses and, following receipt of the revised prospectuses, such holders shall be free to resume making offers of the Registrable Securities; and
(cc) furnish, at the request of any holder whose Registrable Securities are being registered, on the date that such Registrable Securities are delivered to the underwriters for sale in connection with a registration pursuant to this Agreement if such securities are being sold through underwriters, or on the date that the registration statement with respect to such securities becomes effective if such securities are not being sold through underwriters, (i) an opinion, dated such date, of the counsel representing the Corporation for the purposes of such registration, in form and substance as is customarily given to underwriters in an underwritten public offering, addressed to the underwriters, if any, and to the holder or holders making such request, and (ii) a letter dated such date, from the independent certified public accountants of the Corporation, in form and substance as is customarily given by independent certified public accountants to underwriters in an underwritten public offering, addressed to the underwriters, if any, and to the holder or holders making such request.
Expenses. The Corporation shall pay all expenses incurred by the Corporation in complying with this Section 3, including all registration and filing fees (including all expenses incident to filing with the FINRA), fees and expenses of complying with the securities and blue sky laws of all such jurisdictions in which the Registrable Securities are proposed to be offered and sold, printing expenses and fees and disbursements of counsel (including with respect to each registration effected pursuant to Sections 3.4 and 3.5, the reasonable fees and disbursements of a counsel for the holders of Registrable Securities that are being registered pursuant to this Section 3, such counsel for the holders of Registrable Securities shall be designated by a vote of the holders of a majority of the Registrable Securities to be included in such registration); provided, however, that all underwriting discounts and selling commissions applicable to the Registrable Securities covered by registrations effected pursuant to Section 3.4 or 3.5 hereof shall be borne by the seller or sellers thereof, in proportion to the number of Registrable Securities sold by each such seller or sellers.
Indemnification.
(dd) In the event of any registration of any Registrable Securities under the Securities Act pursuant to this Section 3 or registration or qualification of any Registrable Securities pursuant to Section 3.7(d) hereof, the Corporation shall indemnify and hold harmless the seller of such shares, each underwriter of such shares, if any, each broker or any other person acting on behalf of such seller and each other person, if any, who controls any of the foregoing persons, within the meaning of the Securities Act, against any losses, claims, damages or liabilities, joint or several, to which any of the foregoing persons may become subject under the Securities Act or otherwise, insofar as such losses, claims, damages or liabilities (or actions in respect thereof) arise out of or are based upon an untrue statement or alleged untrue statement of a material fact contained in any registration statement under which such Registrable Securities were registered under the Securities Act, any preliminary prospectus or final prospectus contained therein, or any amendment or supplement thereto, or any document incident to registration or qualification of any Registrable Securities pursuant to Section 3.7(d) hereof or arise out of or are based upon the omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein not misleading or, with respect to any prospectus, necessary to make the statements therein, in light of the circumstances under which they were made, not misleading, or any violation by the Corporation of the Securities Act or any state securities or blue sky laws applicable to the Corporation and relating to action or inaction required of the Corporation in connection with such registration or qualification under the Securities Act or such state securities or blue sky laws. The Corporation shall reimburse on demand such seller, underwriter, broker or other person acting on behalf of such seller and each such controlling person for any legal or any other expenses
reasonably incurred by any of them in connection with investigating or defending any such loss, claim, damage, liability or action; provided, however, that the Corporation shall not be liable in any such case to the extent that any such loss, claim, damage or liability arises out of or is based upon an untrue statement or alleged untrue statement or omission or alleged omission made in said registration statement, preliminary or final prospectus or amendment or supplement thereto or any document incident to registration or qualification of any Registrable Securities pursuant to Section 3.7(d) hereof, in reliance upon and in conformity with written information furnished to the Corporation by such seller, underwriter, broker, other person or controlling person specifically for use in the preparation hereof.
(ee) Before Registrable Securities held by any prospective seller shall be included in any registration pursuant to this Section 3, such prospective seller and any underwriter acting on its behalf shall have agreed to indemnify and hold harmless (in the same manner and to the same extent as set forth in paragraph (a)) the Corporation, each director of the Corporation, each officer of the Corporation who signs such registration statement and any person who controls the Corporation within the meaning of the Securities Act, with respect to any untrue statement or omission from such registration statement, any preliminary prospectus or final prospectus contained therein, or any amendment or supplement thereto, if such untrue statement or omission was made in reliance upon and in conformity with written information furnished to the Corporation through an instrument duly executed by such seller or such underwriter specifically for use in the preparation of such registration statement, preliminary prospectus, final prospectus or amendment or supplement; provided, however, that the maximum amount of liability in respect of such indemnification shall be limited, in the case of each prospective seller, to an amount equal to the net proceeds actually received by such prospective seller from the sale of Registrable Securities effected pursuant to such registration.
(ff) Promptly after receipt by an indemnified party of notice of the commencement of any action involving a claim referred to in Section 3.9(a) or (b) hereof, such indemnified party will, if a claim in respect thereof is to be made against the indemnifying party under this Section 3.9, give written notice to the latter of the commencement of such action. In case any such action is brought against an indemnified party, the indemnifying party will be entitled to participate in and to assume the defense thereof jointly with any other indemnifying party similarly notified to the extent that it may wish, with counsel reasonably satisfactory to such indemnified party, and, after notice to such indemnified party from the indemnifying party of its election to assume the defense thereof, the indemnifying party shall be responsible for any legal or other expenses subsequently incurred by such indemnifying party in connection with the defense thereof; provided, however, that, if any indemnified party shall have reasonably concluded that there may be one or more legal defenses available to such indemnified party which are different from or additional to those available to the indemnifying party, or that such claim or litigation involves or could have an effect upon matters beyond the scope of the indemnity agreement provided in this Section 3.9, the indemnifying party shall not have the right to assume the defense of such action on behalf of such indemnified party, and such indemnifying party shall reimburse such indemnified party and any person controlling such indemnified party for the fees and expenses of counsel retained by the indemnified party which are reasonably related to the matters covered by the indemnity agreement provided in this Section 3.9. The indemnifying party shall not make any settlement of any claims in respect of which it is obligated to indemnify an indemnified party or parties hereunder, without the written consent of the indemnified party or parties, which consent shall not be unreasonably withheld.
(gg) In order to provide for just and equitable contribution to joint liability under the Securities Act, in any case in which either (i) any Holder exercising rights under this Agreement, or any controlling person of any such holder, makes a claim for indemnification pursuant to this Section 3.9, but it is judicially determined (by the entry of a final judgment or decree by a court of
competent jurisdiction and the expiration of time to appeal or the denial of the last right of appeal) that such indemnification may not be enforced in such case notwithstanding the fact that this Section 3.9 provides for indemnification in such case, or (ii) contribution under the Securities Act may be required on the part of any such holder or any such controlling person in circumstances for which indemnification is provided under this Section 3.9; then, in each such case, the Corporation and such holder will contribute to the aggregate losses, claims, damages or liabilities to which they may be subject as is appropriate to reflect the relative fault of the Corporation and such holder in connection with the statements or omissions which resulted in such losses, claims, damages or liabilities, it being understood that the parties acknowledge that the overriding equitable consideration to be given effect in connection with this provision is the ability of one party or the other to correct the statement or omission which resulted in such losses, claims, damages or liabilities, and that it would not be just and equitable if contribution pursuant hereto were to be determined by pro rata allocation or by any other method of allocation which does not take into consideration the foregoing equitable considerations. Notwithstanding the foregoing, (i) no such holder will be required to contribute any amount in excess of the proceeds to it of all Registrable Securities sold by it pursuant to such registration statement, and (ii) no person or entity guilty of fraudulent misrepresentation, within the meaning of Section 11(f) of the Securities Act, shall be entitled to contribution from any person or entity who is not guilty of such fraudulent misrepresentation.
(hh) Notwithstanding any of the foregoing, if, in connection with an underwritten public offering of any Registrable Securities, the Corporation, the holders of such Registrable Securities and the underwriters enter into an underwriting or purchase agreement relating to such offering which contains provisions covering indemnification among the parties, then the indemnification provision of this Section 3.9 shall be deemed inoperative for purposes of such offering.
Removal of Legends, Etc. Notwithstanding the foregoing provisions of this Section 3, the restrictions imposed by this Section 3 upon the transferability of any Restricted Stock shall cease and terminate when (a) any such Restricted Stock are sold or otherwise disposed of in accordance with the intended method of disposition by the seller or sellers thereof set forth in a registration statement or such other method contemplated by Section 3 hereof that does not require that the securities transferred bear the legend set forth in Section 3.2 hereof, including a Transfer pursuant to Rule 144 or a successor rule thereof (as amended from time to lime), or (b) the holder of Restricted Stock has met the requirements for transfer of such Restricted Stock pursuant to subparagraph (b)(1) of Rule 144 or a successor rule thereof (as amended from time to time) promulgated by the Commission under the Securities Act. Whenever the restrictions imposed by this Section 3 have terminated, a holder of a certificate for Restricted Stock as to which such restrictions have terminated shall be entitled to receive from the Corporation, without expense, a new certificate not bearing the restrictive legend set forth in Section 3.2 hereof and not containing any other reference to the restrictions imposed by this Section 3.
Lock-up Agreement.
(ii) In no event shall any Stockholder be permitted, during the period commencing on the date hereof and ending on the date of the listing of the Common Stock on a national securities exchange, to sell, assign, transfer, make a short sale of, loan, or grant any option for the purchase of, any shares of Common Stock for a price that is less than $6.142 per share (subject to proportionate and equitable adjustment upon any stock split, stock dividend, reverse stock split or similar event affecting the Common Stock that becomes effective after the date of this Agreement) or any other shares of capital stock of the Company for an effective price that is less than $6.142 per share on an as-converted to Common Stock basis (subject to proportionate and equitable adjustment upon any stock split, stock dividend, reverse stock split or similar event affecting the Common Stock that becomes effective after the date of this Agreement), except (x) with the prior written consent of the Company or
(y) to a member of such Stockholder’s Group.
(b) Each Stockholder agrees further that, if the Company or a managing underwriter so requests of such Stockholder in connection with a registered public offering of securities of the Company, such Stockholder will not, without the prior written consent of the Company or such underwriters, sell, assign, transfer, make a short sale of, loan, grant any option for the purchase of, or exercise registration rights with respect to any shares of Common Stock or shares of capital stock or other securities of the Corporation convertible into or exercisable for, whether directly or indirectly, shares of Common Stock, other than to a member of such Stockholder’s Group, during the period of (i) 180 days following the closing of the first public offering of securities offered and sold for the account of the Corporation that is registered under the Securities Act, or (ii) 90 days following the closing of any other public offering of securities offered and sold for the account of the Corporation that is registered under the Securities Act; provided that such request is made of all officers, directors and 1% and greater Stockholders and each such person shall be similarly bound; and, provided, further, that nothing in this Section 3.11(b) shall prevent any Stockholder from participating in any registered public offering of the Corporation as a selling stockholder or security holder.
(jj) In the event that the Corporation releases or causes to be released any Stockholder from any restrictions on transfer set forth in the foregoing provisions of this Section 3.11, the Corporation shall release or cause to be released all other Stockholders in similar fashion and any such release of all Stockholders shall be implemented on a pro rata basis.
Furnish Information. It shall be a condition precedent to the obligations of the Corporation to take any action pursuant to this Section 3 with respect to the Registrable Securities of any selling Holder that such Holder shall furnish to the Corporation such information regarding itself, the Registrable Securities held by it, and the intended method of disposition of such securities as is reasonably required to effect the registration of such Holder’s Registrable Securities.
Termination of Registration Rights. The right of any Holder to request registration or inclusion of Registrable Securities in any registration pursuant to this Section 3 shall terminate upon the earlier to occur of: (a) such time as Rule 144 or another similar exemption under the Securities Act is available for the sale of all of such Holder’s shares without volume, manner of sale or other limitation during a three-month period without registration; and (b) the Expiration Date.
Election of Directors.
Intentionally Omitted.
Voting for Directors. At the first annual meeting of the Stockholders of the Corporation after the Initial Closing (as defined in the Stock Purchase Agreement), and thereafter at each annual meeting and each special meeting of the Stockholders of the Corporation called for the purposes of electing directors of the Corporation, and at any time at which Stockholders of the Corporation shall have the right to, or shall, vote or consent to the election of directors, then, in each such event, each Stockholder shall vote all shares of Preferred Stock, Common Stock and any other shares of voting stock of the Corporation then owned (or controlled as to voting rights) by it, him or her, whether by purchase, exercise of rights, warrants or options, stock dividends or otherwise:
(kk) to fix and maintain the number of directors on the Board at eight (8);
(ll) to the extent entitled under the Certificate as in effect as of the date of
this Agreement, to elect as Directors of the Corporation on the date hereof and in any subsequent election of Directors the following individuals:
i. in the case of the two (2) directors to be elected by the holders of Series A-1 Preferred Stock under the Certificate, two (2) individuals to be designated by the affirmative vote or written consent of the holders of a majority of the outstanding shares of Series A-1 Preferred Stock (the “Series A-1 Directors”), who shall initially be Xxxxxxx Xxxxxxx and Xxxxxx Xxxxxxxxx.
ii. in the case of the one (1) director to be elected by the G3 Holders (as defined in the Series A-1 Certificate), one (1) individual to be designated by the affirmative vote or written consent of the holders of at least 70% of the shares of Common Stock issuable upon conversion of the outstanding shares of Series B Preferred Stock and Series B-2 Preferred Stock, voting together as a single class on an as-converted to Common Stock basis (the “Series B Director”), who shall initially be Xxxxxx Xxxxx-Embiricos.
iii. in the case of the one (1) director to be elected by MPM, one (1) director to be designated by the affirmative vote or written consent of MPM, provided that such director be an individual with particular expertise in the development of pharmaceutical products, as reasonably determined by MPM, if any (the “Industry Expert Director” and together with the Series B Director and the Series A-1 Directors, the “Investor Directors”), who shall initially be Xxxxxxxxx Xxxxxx, provided, further, however, that in order to be eligible to vote or consent with respect to the designation of an individual as a nominee for election as the Industry Expert Preferred Director, MPM together with members of the MPM Group must hold greater than twenty percent (20%) of the Preferred Stock purchased under the Series A-1 Stock Purchase Agreement by MPM and members of the MPM Group.
iv. in the case of the remaining directors to be elected by the holders of Preferred Stock and Common Stock, voting together as a single class, under the Certificate, four (4) individuals as follows:
1.1.ll.iv.1. three industry or market experts, each of whom shall be designated by a majority of the other members of the Board, including a majority of the Investor Directors (the “Independent Directors”), and who shall initially be Xxxx Xxxxxxxx, Xxxx Xxxxxx and Xxxx Xxxxxx; and
1.1.ll.iv.2. the Chief Executive Officer of the Corporation, who shall initially be Xxxxxx X. Xxxx.
Observer Rights.
(mm) The Majority G3 Investors shall have the right to appoint one observer to the Board (the “G3 Observer”), provided, however, that in order for any G3 Investor to be eligible to participate in the appointment of an individual as the G3 Observer, such G3 Investor, together with members of such G3 Investor’s Group, must continue to hold greater than seventy five percent (75%) of the Series A-1 Preferred Stock originally purchased by such G3 Investor and members of such G3 Investor’s Group pursuant to the Series A-1 Stock Purchase Agreement and greater than seventy five percent (75%) of the Series B Preferred Stock originally purchased by such G3 Investor and members of such G3 Investor’s Group pursuant to the Series B Stock Purchase Agreement. The G3 Observer shall have the right to attend all meetings of the Board in a non-voting observer capacity, and the Corporation shall provide to the G3 Observer all materials provided to the members of the Board and notice of such meetings, all in the manner and at the time provided to the members of the Board; provided, however, that
the Corporation reserves the right to exclude such representatives from access to any material or meeting or portion thereof if the Corporation believes upon advice of counsel that such exclusion is necessary to preserve the attorney-client privilege or to protect highly confidential information, the disclosure of which should not be made to any person who does not have a fiduciary or other similar duty to the Corporation. The decision of the Board with respect to the privileged or confidential nature of such information shall be final and binding. The rights of each G3 Investor under this Section 4.3(a) may only be assigned in connection with the transfer of all of the Preferred Stock held by such G3 Investor to the assignee. In addition and without limiting the foregoing, in the event that the Majority G3 Investors appoint any person to be the G3 Observer under this Section 4.3(a) who, in the good faith determination of the Board, has conflicting interests with the Corporation, then the Corporation shall have the right, at any time and from time to time, to exclude the G3 Observer from access to any meeting, or any portion thereof, and/or deny the G3 Observer access to any information and documents, or any portions thereof.
(nn) In the event that the Board approves the grant of the rights contained herein to a purchaser of shares of Series B-2 Preferred Stock under the Stock Purchase Agreement after the date hereof (any such purchaser with respect to whom the Board has approved the granting of such rights, the “Extra Purchaser”), upon such Extra Purchaser becoming a party hereto and a Stockholder hereunder, such Extra Purchaser shall have the right to appoint an observer to the Board (the “Extra Observer”) as long as such Extra Purchaser holds greater than seventy five percent (75%) of the Series B-2 Preferred Stock originally purchased by such purchaser pursuant to the Stock Purchase Agreement. The Extra Observer shall have the right to attend all meetings of the Board in a non-voting observer capacity, and the Corporation shall provide to the Extra Observer all materials provided to the members of the Board and notice of such meetings, all in the manner and at the time provided to the members of the Board; provided, however, that the Corporation reserves the right to exclude such representatives from access to any material or meeting or portion thereof if the Corporation believes upon advice of counsel that such exclusion is necessary to preserve the attorney-client privilege or to protect highly confidential information, the disclosure of which should not be made to any person who does not have a fiduciary or other similar duty to the Corporation. The decision of the Board with respect to the privileged or confidential nature of such information shall be final and binding. The Extra Purchaser’s rights under this Section 4.3(b) may only be assigned in connection with the transfer of all of the Preferred Stock held by the Extra Purchaser to the assignee. In addition and without limiting the foregoing, in the event that the Extra Purchaser appoints any person to be the Extra Observer under this Section 4.3(b) who, in the good faith determination of the Board, has conflicting interests with the Corporation, then the Corporation shall have the right, at any time and from time to time, to exclude the Extra Observer from access to any meeting, or any portion thereof, and/or deny the Extra Observer access to any information and documents, or any portions thereof.
Cooperation of the Corporation. The Corporation shall use its best efforts to effectuate the purposes of this Section 4, including (a) taking such actions as are necessary to convene annual and/or special meetings of the Stockholders for the election of directors and (b) promoting the adoption of any necessary amendment of the by-laws of the Corporation and the Certificate.
Notices. The Corporation shall provide the Series B Stockholders, the Series B-2 Stockholders, the Series A-1 Stockholders and MPM with at least twenty (20) days’ prior notice in writing of any intended mailing of notice to the Stockholders of a meeting at which directors are to be elected, and such notice shall include the names of the persons designated by the Corporation pursuant to this Section 4. The Series B Stockholders, the Series B-2 Stockholders, the Series A-1 Stockholders and MPM shall notify the Corporation in writing at least three (3) days prior to such mailing of the persons designated by them respectively pursuant to Section 4.2 above as nominees for election to the Board. In the absence of any notice from the Series B Stockholders, the Series B-2 Stockholders, the
Series A-1 Stockholders and MPM, the director(s) then serving and previously designated by the Series B Stockholders, the Series B-2 Stockholders, the Series A-1 Stockholders and MPM, as applicable, shall be renominated.
Removal. Except as otherwise provided in this Section 4, no Stockholder shall vote to remove any member of the Board designated in accordance with the foregoing provisions of this Section 4 unless the party or group of stockholders, as applicable, who designated such director (the “Designating Party”) shall so vote or otherwise consent, and, if the Designating Party shall so vote or otherwise consent, then the non-designating Stockholders shall likewise so vote. Any vacancy on the Board created by the resignation, removal, incapacity or death of any person designated under the foregoing provisions of this Section 4 may be filled by another person designated by the original Designating Party. Each Stockholder shall vote all shares of voting stock of the Corporation owned or controlled by such Stockholder in accordance with each such new designation.
Quorum. A quorum for any meeting of the Board of Directors shall consist of a majority of all directors; provided, that a majority of the Investor Directors then serving as directors of the Corporation is in attendance at such meeting. If, at any meeting, a quorum is not present for any reason, then another Board meeting may be convened within no less than two (2) and no more than ten (10) business days and, at such meeting, a majority of all directors shall constitute a quorum for all purposes.
Committees. Each of the Investor Directors serving as a director of the Corporation shall have the right to sit on any committee of the Board.
Duration of Section. This Section 4 and the rights and obligations of the parties hereunder shall automatically terminate on the earlier of (i) the consummation of an Event of Sale (as defined in the Certificate) or (ii) the automatic conversion of all of the Preferred Stock of the Corporation pursuant to the Certificate as a result of the listing, or the admitting for trading, of the Common Stock on a national securities exchange. Prior to such termination, the rights and obligations of any Preferred Stockholder under this Section 4 shall terminate upon the date on which such Preferred Stockholder or its Group no longer owns any Preferred Stock, whereupon the obligations of the remaining Stockholders to vote in favor of the designee of such Preferred Stockholder shall also terminate.
Indemnification.
Indemnification of Investors. In the event that any Preferred Stockholder or any director, officer, employee, affiliate or agent thereof (the “Indemnitees”), become involved in any capacity in any action, proceeding, investigation or inquiry in connection with or arising out of any matter related to the Corporation or any Indemnitee’s role or position with the Corporation, the Corporation shall reimburse each Indemnitee for its legal and other expenses (including the cost of any investigation and preparation) as they are incurred by such Indemnitee in connection therewith. The Corporation also agrees to indemnify each Indemnitee, pay on demand and protect, defend, save and hold harmless from and against any and all liabilities, damages, losses, settlements, claims, actions, suits, penalties, fines, costs or expenses (including attorneys’ fees) (any of the foregoing, a “Claim”) incurred by or asserted against any Indemnitee of whatever kind or nature, arising from, in connection with or occurring as a result of this Agreement or the matters contemplated by this Agreement; provided, however, that the Corporation shall not be required to indemnify any Indemnitee hereunder in connection with any matter as to which a court of competent jurisdiction has made a final non-appealable determination that such Indemnitee has acted with gross negligence or willful or intentional misconduct in connection therewith. The foregoing agreement shall be in addition to any rights that any
Indemnitee may have at common law or otherwise.
Advancement of Expenses. The Corporation shall advance all expenses reasonably incurred by or on behalf of the Indemnitees in connection with any Claim or potential Claim within twenty (20) days after the receipt by the Corporation of a statement or statements from the Indemnitee requesting such advance payment or payments from time to time.
Remedies. In case any one or more of the covenants and/or agreements set forth in this Agreement shall have been breached by any party hereto, the party or parties entitled to the benefit of such covenants or agreements may proceed to protect and enforce its or their rights, either by suit in equity and/or action at law, including, but not limited to, an action for damages as a result of any such breach and/or an action for specific performance of any such covenant or agreement contained in this Agreement. The rights, powers and remedies of the parties under this Agreement are cumulative and not exclusive of any other right, power or remedy which such parties may have under any other agreement or law. No single or partial assertion or exercise of any right, power or remedy of a party hereunder shall preclude any other or further assertion or exercise thereof.
Successors and Assigns. Except as otherwise expressly provided herein, this Agreement shall bind and inure to the benefit of the Corporation and each of the Stockholder parties hereto and the respective successors and permitted assigns of the Corporation and each of the Stockholder parties hereto (including any member of a Stockholder’s Group). Subject to the requirements of Section 3 hereof, this Agreement and the rights and duties of the any Stockholder set forth herein may be freely assigned, in whole or in part, by such Stockholder to any member of their respective Group, provided such transferee is an “affiliate” of such Stockholder, as the case may be, as such term is defined under Rule 501 of the Securities Act (it being recognized and agreed that each member of the Oxford/Saints Group shall be deemed to be “affiliates” of each other for this purpose). Subject to the requirements of Section 3 hereof, the rights under this Agreement may be assigned (but only with related obligations) by a Holder to a transferee of Registrable Securities that, after such transfer, holds at least 100,000 shares of Registrable Securities (subject to appropriate adjustment for stock splits, stock dividends, combinations and other recapitalizations). For the purposes of determining the number of shares of Registrable Securities held by a transferee, the holdings of a transferee (a) that is an affiliate or stockholder of a Holder; (b) who is a Holder’s Immediate Family Member; or (c) that is a trust for the benefit of an individual Holder or such Holder’s Immediate Family Member shall be aggregated together and with those of the transferring Holder; provided further that all transferees who would not qualify individually for assignment of rights shall have a single attorney-in-fact for the purpose of exercising any rights, receiving notices, or taking any action under this Agreement. Any transferee from a Stockholder to whom rights under Section 3 are transferred shall, as a condition to such transfer, deliver to the Corporation a written instrument by which such transferee identifies itself (together with its address), gives the Corporation notice of the transfer of such rights, identifies the securities of the Corporation owned or acquired by it and agrees to be bound by the obligations imposed hereunder to the same extent as if such transferee were a Stockholder hereunder. A transferee to whom rights are transferred pursuant to this Section 7 will be thereafter deemed to be a Stockholder for the purpose of the execution of such transferred rights and may not again transfer such rights to any other person or entity, other than as provided in this Section 7. Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective successors and permitted assignees any rights, remedies, obligations or liabilities under or by reason of this Agreement, except as expressly provided herein.
Duration of Agreement. The rights and obligations of the Corporation and each Stockholder set forth herein shall survive indefinitely, unless and until, by the respective terms of this Agreement, they are no longer applicable.
Entire Agreement. This Agreement, together with the other writings referred to herein or delivered pursuant hereto which form a part hereof, contains the entire agreement among the parties with respect to the subject matter hereof and amends, restates and supersedes all prior and contemporaneous arrangements or understandings with respect thereto, including the Prior Agreement.
Notices. All notices, requests, consents and other communications hereunder to any party shall be deemed to be sufficient if contained in a written instrument delivered in person or duly sent by first class registered, certified or overnight mail, postage prepaid, or telecopied with a confirmation copy by regular mail, addressed or telecopied, as the case may be, to such party at the address or telecopier number, as the case may be, set forth below or such other address or telecopier number, as the case may be, as may hereafter be designated in writing by the addressee to the addressor listing all parties:
i. if to the Corporation, to:
Radius Health, Inc.
000 Xxxxxxxx
Xxxxx Xxxxx
Xxxxxxxxx, XX 00000
Attention: Chief Executive Officer Telecopier: (000) 000-0000
with a copy to:
Xxxxxx & Xxxxxxx LLP
Xxxx Xxxxxxx Tower, 20th Floor
000 Xxxxxxxxx Xxxxxx
Xxxxxx, XX 00000
Attention: Xxxxx X. Xxxxxxxxx
Telecopier: (000) 000-0000
ii. if to the Investors, as set forth on Schedule 2, Schedule 3 or Schedule 4; to the Common Stockholders, as set forth on Schedule 1; to the holders of Series A-2 Preferred Stock, as set forth on Schedule 5; to the holders of Series A-3 Preferred Stock, as set forth on Schedule 6; to the holders of Series A-4 Preferred Stock, as set forth on Schedule 7; to the holder of Series A-5 Preferred Stock and/or Series A-6 Preferred Stock, as set forth on Schedule 8.
All such notices, requests, consents and communications shall be deemed to have been received (a) in the case of personal delivery, on the date of such delivery, (b) in the case of mailing, on the third business day following the date of such mailing, (c) in the case of overnight mail, on the first business day following the date of such mailing, and (d) in the case of facsimile transmission, when confirmed by facsimile machine report.
Changes. The terms and provisions of this Agreement may not be modified or amended, or any of the provisions hereof waived, temporarily or permanently, except pursuant to the written consent of the Corporation and the Majority Investors, and to the extent that there is a material adverse effect of any such modification or amendment on the rights and obligations of the holders of shares of Series A-4 Preferred Stock, Series A-5 Preferred Stock or Series A-6 Preferred Stock, respectively, in a manner more adverse than such effect on the holders of Series A-1 Preferred Stock, Series A-2 Preferred Stock or Series A-3 Preferred Stock, respectively, a majority in combined voting power of the such more affected series then outstanding, determined in accordance with Section 6(a) of the Series A-1 Certificate. Additional parties who become parties to this Agreement pursuant to an instrument of adherence will not
constitute a change under this Section 11. Notwithstanding the foregoing, (a) any modification or amendment to this Agreement that would adversely affect one Series B Stockholder, Series B-2 Stockholder, Series A-1 Stockholder, Series A-2 Stockholder or Series A-3 Stockholder in a manner that is directed specifically to such Series B Stockholder, Series B-2 Stockholder, Series A-1 Stockholder, Series A-2 Stockholder or Series A-3 Stockholder, rather than to all Series B Stockholders, Series A-1 Stockholders, Series A-2 Stockholders and Series A-3 Stockholders, shall be subject to the approval of each such Series B Stockholder, Series B-2 Stockholder, Series A-1 Stockholder, Series A-2 Stockholder or Series A-3 Stockholder, as applicable, (b) any modification or amendment to Section 2.11 hereof shall be subject to the further approval of F2 Biosciences, Wellcome, BB Bio, at least one member of HCV Group, one member of the MPM Group, one member of the Brookside Group and one member of the Oxford/Saints Group, (c) any modification to Section 4.2(b)(i) shall be subject to the further approval of Stockholders holding a majority of the outstanding shares of Series A-1 Preferred Stock, (d) any modification to Section 4.2(b)(ii) shall be subject to the further approval of Stockholders holding at least 70% of the shares of Common Stock issuable upon conversion of the outstanding shares of Series B Preferred Stock and Series B-2 Preferred Stock, voting together as a single class on an as-converted to Common Stock basis, (e) any modification to Section 4.2(b)(iii) shall be subject to the further approval of at least one member of the MPM Group, (f) any modification to Section 4.3(a) shall be subject to the further approval of the Majority G3 Investors and (g) any modification to Section 4.3(b) shall be subject to the further approval of the Extra Purchaser, if any. It is understood that any separate consent required pursuant to the foregoing clauses (a) - (g) would not be required if any such adverse effect results from the application of criteria uniformly to all Stockholders even if such application may affect Stockholders differently.
Counterparts. This Agreement may be executed in any number of counterparts, each such counterpart shall be deemed to be an original instrument and all such counterparts together shall constitute but one agreement.
Headings; Interpretation. The headings of the various sections of this Agreement have been inserted for convenience of reference only, and shall not be deemed to be a part of this Agreement. All references in this Agreement to “including” shall be deemed to mean “including without limitation.”
Nouns and Pronouns. Whenever the context may require, any pronouns used herein shall include the corresponding masculine, feminine or neuter forms, and the singular form of names and pronouns shall include the plural and vice-versa.
Severability. Any provision of this Agreement that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction.
Governing Law. This Agreement shall be governed by and construed in accordance with the laws of the Commonwealth of Massachusetts, excluding choice of law rules thereof that would result in the application of the laws of any other jurisdiction; provided that Sections 2, 3 and 4 of this Agreement shall be governed by and construed in accordance with the General Corporation Law of the State of Delaware, as to matters within the scope thereof.
Additional Parties. Notwithstanding anything to the contrary contained herein, any Stockholder may become a party to this Agreement following the delivery to, and written acceptance by, the Corporation of an executed Instrument of Adherence to this Agreement in the form attached hereto as Annex A. No action or consent by Stockholder parties hereto shall be required for such joinder to this
Agreement by such additional Stockholder, so long as such additional Stockholder has agreed in writing to be bound by all of the obligations as Stockholder party hereunder as indicated in the Instrument of Adherence and the Instrument of Adherence has been accepted in writing by the Corporation.
Waiver; Amendment; Termination. The parties to this Agreement hereby agree and acknowledge that the Corporation has, as of, and at all times prior to, the date of this Agreement, complied with all of its obligations under the Prior Agreement and that the Corporation shall have no liability or obligation to pay any damages under the Prior Agreement with respect to any right, covenant or obligation arising under the Prior Agreement at any time prior to the date of this Agreement. All provisions of, rights granted and covenants made in, the Prior Agreement are hereby waived, released and superseded, with full retrospective and prospective effect, in their entirety and shall have no further force or effect.
IN WITNESS WHEREOF the parties hereto have executed this Agreement on the date first above written.
|
|
CORPORATION: | |
|
|
| |
|
|
RADIUS HEALTH, INC. | |
|
|
| |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxx X. Xxxx | |
|
|
Title: President & Chief Executive Officer | |
|
|
| |
|
|
INVESTORS: | |
|
|
| |
|
|
F2 BIOSCIENCE III, L.P. | |
|
|
| |
|
|
By: F2 Bioscience GP, Ltd., General Partner | |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxx Xxxxx-Embiricos | |
|
|
Title: Director of Investment Adviser | |
|
|
To F2 Bioscience III, L.P. | |
|
|
| |
|
|
BB BIOTECH VENTURES II, L.P. | |
|
|
| |
|
|
On behalf of BB Biotech Ventures GP (Guernsey) Limited as General Partners to BB Biotech Ventures II, L.P. | |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxx Xxxxxxx | |
|
|
Title: Director | |
|
|
| |
|
|
BIOTECH GROWTH N.V. | |
|
|
| |
|
|
By: |
|
|
|
Name: H.J. van Neutegem | |
|
|
Title: Managing Director | |
|
|
| |
|
|
MPM BIOVENTURES III, L.P. | |
|
|
|
|
|
|
By: |
MPM BioVentures III GP, L.P., |
|
|
|
its General Partner |
|
|
By: |
MPM BioVentures III LLC, |
|
|
|
its General Partner |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxxx Xxxxxxx | |
|
|
Title: Series A Member |
|
|
MPM BIOVENTURES III-QP, L.P. | ||
|
|
|
| |
|
|
By: |
MPM BioVentures III GP, L.P., | |
|
|
|
its General Partner | |
|
|
By: |
MPM BioVentures III LLC, | |
|
|
|
its General Partner | |
|
|
|
| |
|
|
By: |
| |
|
|
Name: Xxxxxxx Xxxxxxx | ||
|
|
Title: Series A Member | ||
|
|
| ||
|
|
MPM BIOVENTURES III GMBH & CO. BETEILIGUNGS KG | ||
|
|
|
| |
|
|
By: |
MPM BioVentures III GP, L.P., | |
|
|
|
in its capacity as the Managing Limited Partner | |
|
|
By: |
MPM BioVentures III LLC, | |
|
|
|
its General Partner | |
|
|
|
| |
|
|
By: |
| |
|
|
Name: Xxxxxxx Xxxxxxx | ||
|
|
Title: Series A Member | ||
|
|
| ||
|
|
MPM BIOVENTURES III PARALLEL FUND, L.P. | ||
|
|
|
| |
|
|
By: |
MPM BioVentures III GP, L.P., | |
|
|
|
its General Partner | |
|
|
By: |
MPM BioVentures III LLC, | |
|
|
|
its General Partner | |
|
|
|
| |
|
|
By: |
| |
|
|
Name: Xxxxxxx Xxxxxxx | ||
|
|
Title: Series A Member | ||
|
|
| ||
|
|
MPM ASSET MANAGEMENT INVESTORS 2003 BVIII LLC | ||
|
|
|
| |
|
|
By: |
| |
|
|
Name: Xxxxxxx Xxxxxxx | ||
|
|
Title: Manager | ||
|
|
| ||
|
|
MPM BIO IV NVS STRATEGIC FUND, L.P. | ||
|
|
|
| |
|
|
By: |
MPM BioVentures IV GP LLC, its General Partner | |
|
|
By: |
MPM BioVentures IV LLC, | |
|
|
|
its Managing Member | |
|
|
By: |
|
|
|
Name: Xxxxxxx Xxxxxxx | |
|
|
Title: Member | |
|
|
| |
|
|
HEALTHCARE PRIVATE EQUITY LIMITED PARTNERSHIP | |
|
|
|
|
|
|
By: |
Waverley Healthcare Private Equity Limited, |
|
|
its general partner | |
|
|
|
|
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
OBP IV — HOLDINGS LLC | |
|
|
| |
|
|
By: OXFORD BIOSCIENCE PARTNERS IV., L.P. | |
|
|
By: OBP Management iv, L.P. | |
|
|
| |
|
|
By: |
|
|
|
Name: Xxxxxxxx Xxxxxxx | |
|
|
Title: General Partner | |
|
|
| |
|
|
By: SAINTS CAPITAL GRANITE, L.P. | |
|
|
By: SAINTS CAPITAL GRANITE, LLC | |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxx Xxxxxxx | |
|
|
Title: Managing Director | |
|
|
| |
|
|
MRNA II - HOLDINGS LLC | |
|
|
| |
|
|
By: MRNA FUND II, L.P. | |
|
|
By: OBP MANAGEMENt II , L.P. | |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxxxx Xxxxxxx | |
|
|
Title: General Partner | |
|
|
| |
|
|
By: SAINTS CAPITAL GRANITE, L.P. | |
|
|
By: SAINTS CAPITAL GRANITE, LLC | |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxx Xxxxxxx | |
|
|
Title: Managing Director |
|
|
THE WELLCOME TRUST LIMITED, | |
|
|
AS TRUSTEE OF THE WELLCOME TRUST | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
BROOKSIDE CAPITAL PARTNERS FUND, L.P. | |
|
|
| |
|
|
By: |
|
|
|
Name: Xxxxxx Xxxxxxxxxx | |
|
|
Title: General Counsel | |
|
|
| |
|
|
NORDIC BIOSCIENCE CLINICAL | |
|
|
DEVELOPMENT VII A/A | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
IPSEN PHARMA SAS | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
The Xxxxxxxx Family Trust dated August 15, 2003 | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
| |
|
|
Xx. Xxxxxxx X. Xxxxxxxx | |
|
|
| |
|
|
| |
|
|
Xx. Xxxxxx X. Xxxxxx | |
|
|
| |
|
|
The Xxxxx X. Xxxxxxxx Revocable Trust | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
Xxxxxx X. Xxxxxxxxx Revocable Trust dated March 17, 2000 |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
HEALTHCARE VENTURES VII, L.P. | |
|
|
|
|
|
|
By: |
HealthCare Partners VII, L.P. |
|
|
|
Its General Partner |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxxx Xxxxxxxxx | |
|
|
Title: Administrative Partner | |
|
|
| |
|
|
The Xxxx X. Xxxxxxxxx Revocable Trust, | |
|
|
Dated March 17, 2000 | |
|
|
| |
|
|
By: |
|
|
|
Name: Xxxx Xxxxxxxxx, M.D. | |
|
|
Title: Trustee | |
|
|
| |
|
|
| |
|
|
H. Xxxx Xxxxxxx III | |
|
|
| |
|
|
H2 ENTERPRISES, LLC | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
Xxxxxxxxx Family Trust UTD 3/18/92 | |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxx X. Xxxxxxxxx | |
|
|
Title: Co-Trustee | |
|
|
| |
|
|
By: |
|
|
|
Name: Margarethe. Xxxxxxxxx | |
|
|
Title: Co-Trustee | |
|
|
| |
|
|
The Xxxxxxx Trust dated 2/6/83 | |
|
|
| |
|
|
By: |
|
|
|
Name: Xxxxxxx X. Xxxxxxx | |
|
|
Title: Co-Trustee | |
|
|
| |
|
|
By: |
|
|
|
Name: Xxx X. Xxxxxxx, | |
|
|
Title: Co-Trustee |
|
|
Xxxx Trust dated l-1-02 | |
|
|
| |
|
|
By: |
|
|
|
Name: F. Xxxxxxx Xxx Xxxx | |
|
|
Title: Trustee | |
|
|
| |
|
|
| |
|
|
Xxxxxxx X. Xxxxxxxxx | |
|
|
| |
|
|
| |
|
|
| |
|
|
Xxxxxxx Xxxxxxxxxx, M.D. | |
|
|
| |
|
|
| |
|
|
Xxxxxxxx X. Xxxxxxxxxx | |
|
|
| |
|
|
Dr. Xxxx Xxxxx, Jr and Xxxxxxx X. Xxxxx | |
|
|
Irrevocable Trust for Xxxxxxx X. Xxxxx | |
|
|
dated 6-15-05 | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
| |
|
|
Xxxx Xxxxxx Xxxxx, M.D. | |
|
|
| |
|
|
| |
|
|
| |
|
|
Xxxx X. Katzenellenbogen, PhD. | |
|
|
| |
|
|
Xxxx X. Katzenellenbogen Trust | |
|
|
Under Agreement Dated August 2, 1999 | |
|
|
|
|
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
| |
|
|
Xxxxxx X. Xxxxxxxxxxxxxxxx, PhD | |
|
|
| |
|
|
| |
|
|
Xxxx Xxxxxxxxx |
|
|
BOARD OF TRUSTEES OF THE UNIVERSITY OF ARKANSAS | |
|
|
| |
|
|
By: |
|
|
|
Name: | |
|
|
Title: | |
|
|
| |
|
|
| |
|
|
| |
|
|
Xxxxxxxx X. Xxxx | |
|
|
| |
|
|
Xxxx Trust dated l-1-02 | |
|
|
|
|
|
|
By: |
|
|
|
Name: F. Xxxxxxx Xxx Xxxx | |
|
|
Title: Trustee | |
|
|
| |
|
|
| |
|
|
Xxxxxxxxx Xxxxxxxx, PhD. | |
|
|
| |
|
|
| |
|
|
Xxxxxx X. Xxxxx, PhD. | |
|
|
| |
|
|
| |
|
|
Xxxxxx X. Xxxxxxxxx, M.D. | |
|
|
| |
|
|
| |
|
|
Xxxxxxxx X. Xxxxxxx, PhD. | |
|
|
| |
|
|
| |
|
|
Xxxxx XxXxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxxx X. Xxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxxx Xx | |
|
|
| |
|
|
| |
|
|
Xxxxxxx X’Xxxxx, PhD. | |
|
|
| |
|
|
| |
|
|
Alwyn Xxxxxxx Xxxxxxx, M.D. | |
|
|
| |
|
|
| |
|
|
Xxxxxxxx Xxxxxxx | |
|
|
| |
|
|
| |
|
|
| |
|
|
Xxxxx Xxxxxxxx |
|
|
| |
|
|
Xxxxx Xxxxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxxxxx X. Xxxxxxxxxx, M.D. | |
|
|
| |
|
|
| |
|
|
Xxxxx X. Xxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxxxx Xxxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxxxxx Xxxxxxxxx | |
|
|
| |
|
|
| |
|
|
E. Xxxxx Xxxxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxx Xxxxxxx Xxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxx X’Xxx | |
|
|
| |
|
|
| |
|
|
Xxxxx Xxxxxxxx Xxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxxxxxxxx Xxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxx Xxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxx Xxxxxxxx | |
|
|
| |
|
|
| |
|
|
Xxxxx Xxxxx | |
|
|
| |
|
|
F2 BIOSCIENCE IV, L.P. | |
|
|
| |
|
|
By: F2 Bioscience XX XX Ltd., General Partner | |
|
|
| |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxx Xxxxx-Embiricos | |
|
|
Title: | |
|
|
| |
|
|
F2 BIO VENTURES V, L.P. | |
|
|
| |
|
|
By: F2 Bio Ventures GP Ltd., General Partner. | |
|
|
| |
|
|
|
|
|
|
By: |
|
|
|
Name: Xxxxxx Xxxxx-Embiricos | |
|
|
Title: |
Schedule 1
List of Common Stockholders
|
Name of Common Stockholder |
|
Address of Record |
|
Xxxxxxxx X. Xxxxxxx, Ph.D |
|
0000 Xxxxxxxx Xxx Xxxxxxxxxx, XX 00000 |
|
Xxxxx Xxxxxxxx, Ph.D |
|
00 Xxxxxxx Xxxxxx Xxxxxxx Xxxxx, XX 00000 |
|
H2 Enterprises, LLC |
|
c/o H. Xxxx Xxxxxxx, III. Esq. Xxxxx Xxxx, LLP 000 Xxxx Xxxxxxx Xxxxxx, Xxxxx 0000 Xxxxxx Xxxx, XX 00000 |
|
Xxxxxxxxx Family Trust, UTD 3/18/92 |
|
00000 Xxx Xx. Xxxxxxx Xxx Xxx, XX 00000 |
|
Xxxxxx L, Xxxxx, Ph.D |
|
00000 Xxxxxxxxx Xxxxx Xxxxxx Xxxx, XX 00000 |
|
Xxxxxx X. Xxxxxxxxxxxxxxxx, Ph.D |
|
000 Xxxx Xxxxxxxxxxxx Xxx Xxxxxx, XX 00000 |
|
Xxxx X. Katzenellenbogen, Ph. D |
|
000 Xxxx Xxxxxxxxxxxx Xxx Xxxxxx, XX 00000 |
|
Xxxx X. Katzenellenbogen Trust Under Agreement dated August 2, 1999 |
|
000 Xxxx Xxxxxxxxxxxx Xxx Xxxxxx, XX 00000 |
|
Xxxxxxxxx Xxxxxxxx, Ph.D |
|
00 Xxxxxxxx Xxxxxx Xxxx Xxxxx, XX 00000 |
|
Xxxx Xxxxxxxxx |
|
00 Xxxxxxxx Xxxx Xxxxxxx, XX 00000 |
|
Xx. Xxxxxxx X. Xxxxxxxxx |
|
00 Xxxxx Xxxxx Xxxxxx Xxxxxx Xxxx XX 00000 |
|
Xxxxxxx X’Xxxxx, Ph. D |
|
0000 Xxxxx Xxxxx Xx Xxxxxx Xxxx, XX 00000 |
|
Xxxxxxxx X. Xxxxxxxxxx, M.D. |
|
Xxxxxxxxxx 00 0000 XX the Hague The Netherlands |
|
Alwyn Xxxxxxx Xxxxxxx, M.D. |
|
00 Xxxxx Xxx Xxx Xxxxxxx Xxxxxxx, XX 00000 |
|
Xxxx Xxxxxx Xxxxx, Xx., M.D. |
|
00 Xxxxxxxxx Xxxxxx Xxxxxxxxx, XX 00000 |
|
Dr. Xxxx Xxxxx Jr and Xxxxxxx X. Xxxxx Irrevocable Trust for Xxxxxxx X. Xxxxx, dated 6/15/05 |
|
00 Xxxxxxxxx Xxxxxx Xxxxxxxxx, XX 00000 |
|
Xxxxxxx Xxxxxxxxxx, M.D. |
|
000 Xxxx Xxx Xxxxxx Xxxxxx, XX 00000 |
|
Xxxxxxxx X. Xxxxxxxxxx |
|
000 Xxxxxx Xxxxxx, Xxx 0 Xxxxxx, XX 00000 |
|
Xxxx Trust, F. Xxxxxxx Xxx Xxxx, Trustee |
|
0000 Xxxxxxx 000 Xxxx Xxxxxxxx, XX 00000 |
|
Xxxxx X. Xxxxx |
|
0000 Xxxxxxx Xx. Xxxx Xxxxx, XX 00000 |
|
Name of Common Stockholder |
|
Address of Record |
|
Xxxxxx X. Xxxxxx, Xx. |
|
XX Xxx 000000 Xxx Xxxxxxxxx, XX 00000 |
|
Board of Trustees of the University of Arkansas |
|
University of Arkansas c/o UAMS Bioventures Technology Licensing & Life Science Incubator 0000 Xxxx Xxxxxxx Xx #000 Xxxxxx Xxxx, XX 00000-0000 |
|
Xxxxxx X. Xxxxxxxxx, M.D. |
|
00 Xxxxxxxxx Xxxxxx Xxxx, XX 00000 |
|
The Kind X. Xxxxxxxxx Revocable Trust, dated March 17, 2000 |
|
00 Xxxxx Xxxxx Xxxx Xxxxxx Xxxx, XX 00000 |
|
Xxxx Xxxxxx |
|
Radius Health, Inc. 000 Xxxxxxxx, Xxxxx Xxxxx Xxxxxxxxx, XX 00000 |
|
Xxxx Xxxxxx |
|
Radius Health, Inc. 000 Xxxxxxxx, Xxxxx Xxxxx Xxxxxxxxx, XX 00000 |
|
Xxxxx X’Xxx |
|
000 Xxxx Xxxxxx, Xxxxxxx, XX 00000 |
|
Xxxxx XxXxxxxx |
|
000 Xxxxxxxxx Xxxxxx Xxxxxxxxx, XX 00000 |
|
Xxxxxx Xx |
|
00 Xxxxxxxx Xxx. Xxxxxxxx, XX 00000 |
|
Xxxxxxx Pitzelle |
|
0000 X Xxxxx Xxx Xxxxxx, XX 00000 |
|
Xxxxx Xxxxxxxx |
|
000 Xxxxxx Xxxxxx #0 Xxxxxxxxx, XX 00000 |
|
Xxxxxxx Xxxxxxx |
|
000 Xxxxxx Xxxxxx Xxxxxxxxx, XX 00000 |
|
Xxxxxxxx Xxxxxxxxx |
|
00 Xxxxx Xxxx Xxxxxx, XX 00000 |
|
E. Xxxxx Xxxxxxxx |
|
00 Xxxxxxx Xx Xxxxxxxxx, XX 00000 |
|
Xxxxxxxxxxx Xxxxxx |
|
0000 Xxxxxxxxx Xx. Xxxx Xxxxxx, XX 00000 |
|
Xxxxxxxx X. Xxxx |
|
0000 Xxxx Xxxxx Xxxx Xxxxxxxxxxxx, XX 00000 |
|
Xxxxx Xxxxx |
|
000 Xxx Xxxxxxxx Xx. Xxx Xxxxx, XX 00000-0000 |
|
Name of Common Stockholder |
|
Address of Record |
|
Xxxxx Xxxxxxxx |
|
00 Xxxx Xx. Xxxxxx, XX 00000-0000 |
|
Xxxxx Xxxxx |
|
00 Xxxxxxx Xx. Xxxxx Xxxxxxx, XX 00000-0000 |
Schedule 2
List of Series B-2 Stockholders
|
Name |
|
Address of Record |
|
F2 Bioscience IV X.X.
|
|
Xxxxxx Xxxxx Xxxxx Xxxxxx Xxxxxx PO Box 309 Xxxxxx Town KY1-1104 Grand Cayman Cayman Islands |
|
F2 Bio Ventures V X.X.
|
|
Xxxxxx Xxxxx Xxxxx Xxxxxx Xxxxxx PO Box 309 Xxxxxx Town KY1-1104 Grand Cayman Cayman Islands |
|
BB Biotech Ventures II, L.P. |
|
Trafalgar Court Les Banques St. Xxxxx Port Guernsey Channel Islands GY1 3QL
With copies to Xxxxxx Xxxxxxxxx Bellevue Asset Management Xxxxxxxxxx 00 0000 Xxxxxxxx Xxxxxxxxxxx |
|
Biotech Growth N.V. |
|
Xxxxxxx 00 Xxxxxxx |
Schedule 3
List of Series B Stockholders
|
Name |
|
Address of Record |
|
F2 Bioscience III, X.X. |
|
Xxxxxx Xxxxx Xxxxx Xxxxxx Xxxxxx PO Box 309 Xxxxxx Town KY1-1104 Grand Cayman Cayman Islands |
|
MPM BioVentures III, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM BioVentures III - QP, L.P |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM BioVentures III GmbH & Co. Beteiligungs KG |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM BioVentures III Parallel Fund, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Asset Management Investors 2003 BVIII LLC |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bio IV NVS Strategic Fund, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
Brookside Capital Partners Fund, L.P. |
|
Attn: Brookside Legal Department Xxxx Capital, LLC Xxxx Xxxxxxx Tower 000 Xxxxxxxxx Xx. Xxxxxx XX 00000 |
|
BB Biotech Ventures II, L.P. |
|
Trafalgar Court Les Banques Xx. Xxxxx Xxxx Xxxxxxxx Xxxxxxx Xxxxxxx XX0 0XX |
|
Name |
|
Address of Record |
|
|
|
With copies to Xxxxxx Xxxxxxxxx Bellevue Asset Management Xxxxxxxxxx 00 0000 Xxxxxxxx Xxxxxxxxxxx |
|
Biotech Growth N.V. |
|
Xxxxxxx 00 Xxxxxxx |
Schedule 4
List of Series A-1 Stockholders
|
Name |
|
Address of Record |
|
BB Biotech Ventures II, L.P. |
|
Trafalgar Court Les Banques St. Xxxxx Port Guernsey Channel Islands GY1 3QL
With copies to Xxxxxx Xxxxxxxxx Bellevue Asset Management Xxxxxxxxxx 00 0000 Xxxxxxxx Xxxxxxxxxxx |
|
BB Biotech Growth N.V. |
|
Xxxxxxx 00 Xxxxxxx |
|
HealthCare Ventures VII, L.P. |
|
00 Xxxxxx Xxxxxx Xxxxxxxxx, XX 00000 |
|
MPM BioVentures III, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM BioVentures III - QP, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bio IV NVS Strategic Fund, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM BioVentures III GmbH & Co. Beteiligungs KG |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM BioVentures III Parallel Fund, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Asset Management Investors 2003 BVIII LLC |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
Healthcare Private Equity Limited Partnership (Registered Number SL004769) |
|
Xxxxxxxxx Xxx, Xxxxxxxx Xxxxxx Xxxxxxxxx, XX0 0XX |
|
Name |
|
Address of Record |
|
|
|
United Kingdom |
|
Xx. Xxxxxxx X. Xxxxxxxx |
|
Xxxxx University School of Medicine Veterans Affairs Medical Center 0000 Xxxxxxxxx Xxxx Xxxxxxx, XX 00000 |
|
The Wellcome Trust Limited as trustee of the Wellcome Trust
|
|
000 Xxxxxx Xxxx Xxxxxx XX0 0XX Xxxxxxx |
|
SECTION 4.SAINTS CAPITAL VI, L.P.,
|
|
000 Xxxxxxx Xxxxxx, Xxxxx 0000 Xxx Xxxxxxxxx, XX 00000 Attention: Xxxxx Xxxxxxx |
|
H. Xxxx Xxxxxxx, III |
|
Suite 2000 000 Xxxx Xxxxxxx Xxxxxx Xxxxxx Xxxx, Xxxxxxxx 00000 |
|
The Xxxxxxxx Family Trust 2/15/03 |
|
XX Xxx 0000 Xxxxxx Xxxxx Xx, XX 00000 |
|
The Xxxxxxx Trust dated 2/6/83 |
|
0000 Xx Xxxxx Xxxxx Xxxx Xx Xxxxx, XX 00000 |
|
Brookside Capital Partners Fund, L.P.
|
|
Attn: Brookside Legal Department Xxxx Capital, LLC Xxxx Xxxxxxx Tower 000 Xxxxxxxxx Xx. Xxxxxx XX 00000 |
|
Xxxxx X. Xxxxxxxx Revocable Trust |
|
0000 Xxxxx Xxxxxx, # 000 Xxx Xxxxxxxxx, XX 00000 |
Schedule 5
List of Series A-2 Stockholders
|
Name of Stockholder |
|
Address of Record |
|
MPM Bioventures III Funds |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bioventures III-QP, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bioventures III GMBH & Co. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bioventures III Parallel Fund, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Asset Management Investors 2003 |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bio IV NVS Strategic Fund |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
Wellcome Trust |
|
000 Xxxxxx Xxxx Xxxxxx XX0 0XX Xxxxxxx |
|
HealthCare Ventures VII |
|
00 Xxxxxx Xxxxxx Xxxxxxxxx, XX 00000 |
|
OBP IV Holdings, LLC |
|
c/o Oxford Bioscience Partners 000 Xxxxxxxx Xxxxxx Xxxxx 0000 Xxxxxx, XX 00000 |
|
mRNA Fund II Holdings, LLC |
|
c/o Oxford Bioscience Partners 000 Xxxxxxxx Xxxxxx Xxxxx 0000 Xxxxxx, XX 00000 |
|
BB Biotech Ventures II, L.P. |
|
Trafalgar Court Les Banques St. Xxxxx Port Guernsey Channel Islands GY1 3QL
With copies to Xxxxxx Xxxxxxxxx Bellevue Asset Management Xxxxxxxxxx 00 0000 Xxxxxxxx Xxxxxxxxxxx |
|
Healthcare Private Equity Limited Partnership (Registered Number SL004769) |
|
Edinburgh One, Xxxxxxxx Xxxxxx Xxxxxxxxx, XX0 0XX Xxxxxx Xxxxxxx |
|
Xx. Xxxxxxx X. Xxxxxxxx |
|
Xxxxx University School of Medicine Veterans Affairs Medical Center 0000 Xxxxxxxxx Xxxx Xxxxxxx, XX 00000 |
Schedule 6
List of Series A-3 Stockholders
|
Name of Stockholder |
|
Address of Record |
|
MPM Bioventures III Funds |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bioventures III-QP, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bioventures III GMBH & Co. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Bioventures III Parallel Fund, L.P. |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
MPM Asset Management Investors 2003 |
|
c/o MPM Capital 000 Xxxxxxxxx Xxxxxx 00xx Xxxxx Xxxxxx, XX 00000 |
|
HealthCare Ventures VII |
|
00 Xxxxxx Xxxxxx Xxxxxxxxx, XX 00000 |
|
OBP IV Holdings, LLC |
|
c/o Oxford Bioscience Partners 000 Xxxxxxxx Xxxxxx Xxxxx 0000 Xxxxxx, XX 00000 |
|
mRNA Fund II Holdings, LLC |
|
c/o Oxford Bioscience Partners 000 Xxxxxxxx Xxxxxx Xxxxx 0000 Xxxxxx, XX 00000 |
Schedule 7
List of Series A-4 Stockholders
|
Name of Stockholder |
|
Address of Record |
|
Xx. Xxxxxxx X. Xxxxxxxx |
|
Xxxxx University School of Medicine Veterans Affairs Medical Center 0000 Xxxxxxxxx Xxxx Xxxxxxx, XX 00000 |
|
H. Xxxx Xxxxxxx, III |
|
Suite 2000 000 Xxxx Xxxxxxx Xxxxxx Xxxxxx Xxxx, Xxxxxxxx 00000 |
|
The Xxxxxxxx Family Trust 2/15/03 |
|
XX Xxx 0000 Xxxxxx Xxxxx Xx, XX 00000 |
|
The Xxxxxxx Trust dated 2/6/83 |
|
0000 Xx Xxxxx Xxxxx Xxxx Xx Xxxxx, XX 00000 |
Schedule 8
List of Series A-5 and A-6 Stockholder
|
Name of Stockholder |
|
Address of Record |
|
Nordic Bioscience Clinical Development VII A/S |
|
Herlev Xxxxxxxxx 000 0000 Xxxxxx Xxxxxxx Attn: Clinical Trial Leader & Medical Advisor / Clinical Studies Phone: 00.0000.0000 Fax: 00.0000.0000 |
Annex A
Instrument of Adherence to Fourth Amended and Restated
Stockholders’ Agreement dated February 14, 2014
Reference is hereby made to that certain FOURTH AMENDED AND RESTATED STOCKHOLDERS’ AGREEMENT (the “Agreement”), dated the 14th day of February, 2014, entered into by and among (i) Radius Health, Inc., a Delaware corporation (the “Corporation”) and the Stockholder parties thereto. Capitalized terms used herein without definition shall have the respective meanings ascribed thereto in the Agreement.
The undersigned (the “New Stockholder Party”), in order to become the owner or holder of shares of and all other shares of the Corporation’s capital stock hereinafter acquired, of the Corporation (the “Acquired Shares”), hereby agrees that, from and after the date hereof, the undersigned has become a party to the Agreement in the capacity of a party to the Agreement, and is entitled to all of the benefits under, and is subject to all of the obligations, restrictions and limitations set forth in, the Agreement that are applicable to such Stockholder parties and shall be deemed to have made all of the representations and warranties made by such Stockholder parties thereunder. This Instrument of Adherence shall take effect and shall become a part of the Agreement on the latest date of execution by both the New Stockholder Party and the Corporation.
Executed under seal as of the date set forth below under the laws of the Commonwealth of Massachusetts.
|
|
Print Name: |
|
|
|
|
|
|
|
|
|
|
|
Signature: |
|
|
|
|
Name: |
|
|
|
Title: |
Accepted:
|
RADIUS HEALTH, INC. | |||
|
| |||
|
| |||
|
By: |
|
| |
|
Name: |
|
| |
|
Title: |
|
| |
|
|
|
| |
|
Date: |
|
|
|
NOTICE OF EXERCISE
RADIUS HEALTH, INC.
Disclosure Schedule (“Schedule”) to the Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement, dated February 14, 2014 (the “Purchase Agreement”)
The following Schedule is delivered by Radius Health, Inc., a Delaware corporation (the “Corporation”), in connection with the Initial Closing under the Purchase Agreement. This Schedule sets forth exceptions to the representations and warranties of the Corporation to be given at the Initial Closing (which exceptions shall be deemed to be representations and warranties as if made under the Purchase Agreement). The information in this Schedule is provided as of the Initial Closing Date.
The disclosure of any item or information in this Schedule shall not be construed as an admission that such item or information is material to the Corporation, and any inclusion in the Schedule shall expressly not be deemed to constitute an admission, or otherwise imply, that any such item or information is material or creates measures of materiality for the purposes of the Purchase Agreement. Nothing in this Schedule constitutes an admission of any liability or obligation of the Corporation to any third party, nor an admission to any third party against the Corporation’s interests.
With respect to any matter that is clearly disclosed in any Section of this Schedule in such a way as to make its relevance to the information called for by another Section of this Schedule readily apparent, such matter shall be deemed to have been included in the Schedule in response to such other Section, notwithstanding the omission of any appropriate cross-reference thereto. The Section numbers referred to in this Schedule correspond to the Section numbers in the Purchase Agreement. Capitalized terms not otherwise defined in this Schedule shall have the meanings set forth in the Purchase Agreement.
Schedule 5.2
Capitalization
1. Capitalization of the Corporation following the Initial Closing *:
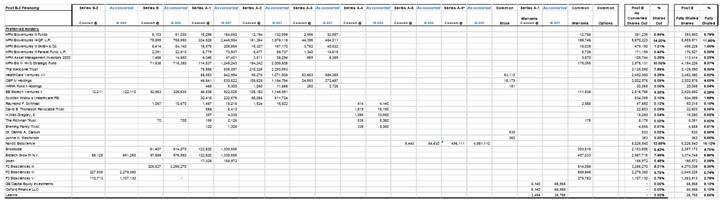
* Fully diluted shares include options to be granted to the Company’s Chief Executive Officer, Chief Medical Officer and Chief Development Officer in February 2014 in accordance with their respective employment agreements. The number of shares subject to the Sign-On Option (as defined in the employment agreement entered into by the Company with its current Chief Executive Officer) is an estimate. Such share number may change once the calculation of the number of shares subject to such option is finally completed pursuant to the terms of such agreement.
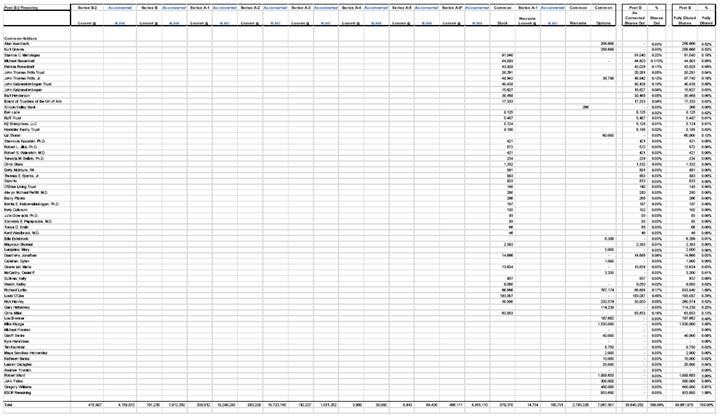
2. In connection with that certain Loan and Security Agreement, dated as of August 6, 2004, by and between the Corporation and Silicon Valley Bank (“SVB”), as amended, the Corporation issued to SVB a warrant currently exercisable for the purchase of an aggregate of up to 266 shares of Common Stock at $15.00 per share (as amended, the “SVB Warrant”). The SVB Warrant expires in August 2014 and was 100% vested as of the grant date.
3. Series A Convertible Redeemable Preferred Stock Purchase Agreement, dated November 14, 2003, by and among the Corporation and the stockholders listed on the schedules thereto.
4. Series B Convertible Redeemable Preferred Stock Purchase Agreement, dated November 14, 2003, by and among the Corporation and the stockholders listed on the schedules thereto.
5. Series C Convertible Redeemable Preferred Stock Purchase Agreement, dated December 15, 2006, by and among the Corporation and the Investors referenced therein, as amended by Amendment No. 1 thereto, dated February 12, 2007, Amendment No. 2 thereto, dated February 22, 2007, Amendment No. 3 thereto, dated August 17, 2007, and Amendment No. 4 thereto, dated November 14, 2008.
6. Series A-1 Convertible Preferred Stock Purchase Agreement, dated April 25, 2011, by and among the Corporation and the Investors referenced therein, as amended by Amendment No. 1 thereto, dated May 11, 2011.
7. Series B Convertible Preferred Stock and Warrant Purchase Agreement, dated April 23, 2013, by and among the Company and the Investors listed therein.
8. Certificate of Incorporation of the Corporation as filed with the Secretary of State of the State of Delaware on February 4, 2008, as amended by (i) a Certificate of Designations of the Series A-1 Convertible Preferred Stock, Series A-2 Convertible Preferred Stock, Series A-3 Convertible Preferred Stock, Series A-4 Convertible Preferred Stock, Series A-5 Convertible Preferred Stock and Series A-6 Convertible Preferred Stock of the Corporation and a Certificate of Ownership and Merger, each as filed with the Secretary of State of the State of Delaware on May 17, 2011, (ii) a Certificate of Amendment to the Series A-1 Certificate, as filed with the Secretary of State of the State of Delaware on January 19, 2012, (iii) a Certificate of Amendment to the Series A-1 Certificate, as filed with the Secretary of State of the State of Delaware on March 8, 2012, (iv) a Certificate of Amendment to the Series A-1 Certificate, as filed with the Secretary of State of the State of Delaware on October 8, 2012 (the Certificate of Designations referred to in clause (i) above, as amended by the Certificates of Amendment referred to in clauses (ii) and (iii) above and in this clause (iv), being referred to herein as the “Series A-1 Certificate”), (v) a Certificate of Designations of the Series B Convertible Preferred Stock of the Corporation, as filed with the Secretary of State of the State of Delaware on April 23, 2013 and (vi) a Certificate of Designations of the Series B-2 Convertible Preferred Stock of the Corporation, as filed with the Secretary of State of the State of Delaware on February 14, 2014.
9. Loan and Security Agreement, dated May 23, 2011, by and among the Corporation, General Electric Capital Corporation as agent and a lender, and Oxford Finance LLC as a lender.
10. First Amendment to Loan and Security Agreement, dated February 27, 2012, by and among the Company, General Electric Capital Corporation and Oxford Finance LLC.
11. Second Amendment to Loan and Security Agreement, date March 16, 2012, by and among the Company, General Electric Capital Corporation and Oxford Finance LLC.
12. Third Amendment to Loan and Security Agreement, dated May 29, 2012, by and among the Company, General Electric Capital Corporation and Oxford Finance LLC.
13. Promissory Note, dated May 23, 2011, issued by the Corporation to General Electric Capital Corporation in the principal amount of $12,500,000.
14. Promissory Note, dated May 23, 2011, issued by the Corporation to Oxford Finance LLC in the principal amount of $3,125,000.
15. Promissory Note, dated May 23, 2011, issued by the Corporation to Oxford Finance LLC in the principal amount of $9,375,000.
16. Promissory Note, dated May 23, 2011, issued by the Corporation to Oxford Finance LLC in the principal amount of $6,250,000.
17. Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock, dated May 23, 2011, issued by the Corporation to GE Capital Equity Investments.
18. Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock, dated May 23, 2011, issued by the Corporation to Oxford Finance LLC.
19. Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock, dated November 21, 2011, issued by the Corporation to GE Capital Equity Investments.
20. Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock, dated November 21, 2011, issued by the Corporation to Oxford Finance LLC.
21. Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock, dated May 29, 2012, issued by the Company and GE Capital Equity Investments.
22. Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock, dated May 29, 2012, the Company and Oxford Finance LLC.
23. In connection with that certain Letter Agreement by and between the Corporation and Leerink Xxxxx LLC (“Leerink”), dated September 24, 2010 (the “Leerink Agreement”), the Corporation issued three warrants to purchase Series A-1 Convertible Preferred Stock of the Corporation in connection with each of the Stage I Closing, the Stage II Closing and the Stage III Closing(the “Leerink Warrants”).
24. Amended and Restated Stock Issuance Agreement by and between the Corporation and Nordic Bioscience Clinical Development VII A/S (“Nordic”), dated May 16, 2011.
25. Amendment No. 1, dated February 21, 2013, to Amended and Restated Stock Issuance Agreement, dated as of May 16, 2011, by and between the Corporation and Nordic.
26. Series A-1 Convertible Preferred Stock Issuance Agreement, dated May 11, 2011, by and between the Corporation and Ipsen Pharma SAS, on behalf of itself and its Affiliates (collectively, “Ipsen”).
Schedule 5.6(a)
Business of the Corporation
None.
Schedule 5.6(b)
Business of the Corporation
A. Research, Development and/or Service Agreements
1. Work Order by and between the Corporation and Veristat, Inc., dated May 17, 2011, as amended May 22, 2012, April 1, 2013 and July 1, 2013.
2. Amended Letter of Payment Authorization by and between the Corporation and Xxxxxxx River Laboratories, dated November 12, 2012.
3. Letter Agreement by and between the Corporation and Xxxxxxx Xxxxxxxxx, dated December 20, 2012.
4. Letter of Intent by and between the Corporation and Xxxxxx Pharma International GMBH, dated December 26, 2013.
5. Letter Agreement by and between the Corporation and FTI Consulting, dated January 23, 2013.
B. ERISA
1. Radius Health, Inc. 401(k) Plan
2. Radius Health, Inc. Flexible Spending Account Plan
C. Indemnification Agreements
1. Please see general commercial agreement indemnification provisions included in the material license agreements listed above or included as exhibits to the Public Filings.
D. Other Agreements
1. The Corporation has granted each of Xxxx Xxxxxxxx and Xxxx Xxxxxx registration rights with respect to shares issuable upon exercise of granted options.
2. The Corporation has granted registration rights to SVB pursuant to the terms of the SVB Warrant.
E. Stock Option Agreements
The Corporation has entered into Stock Option Agreements with the following individuals**:
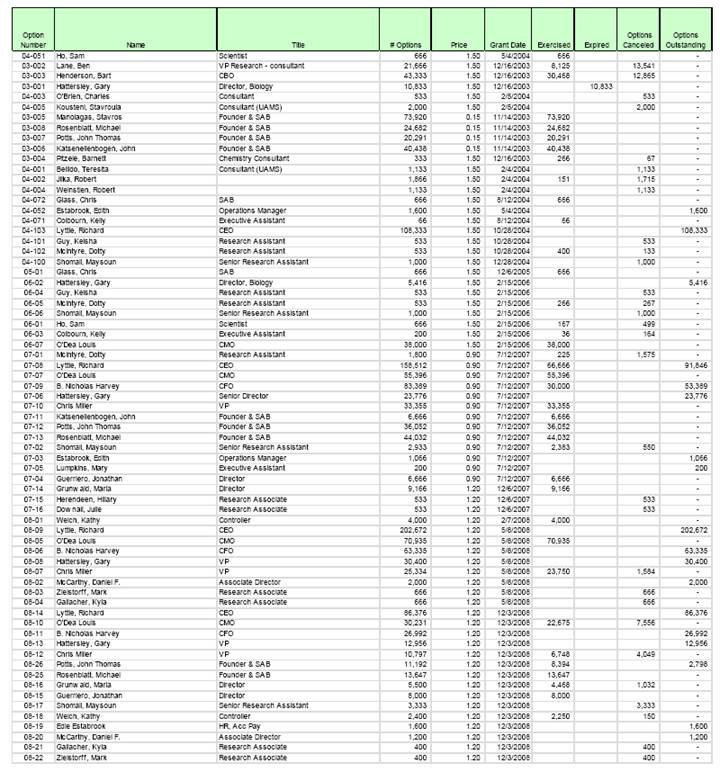
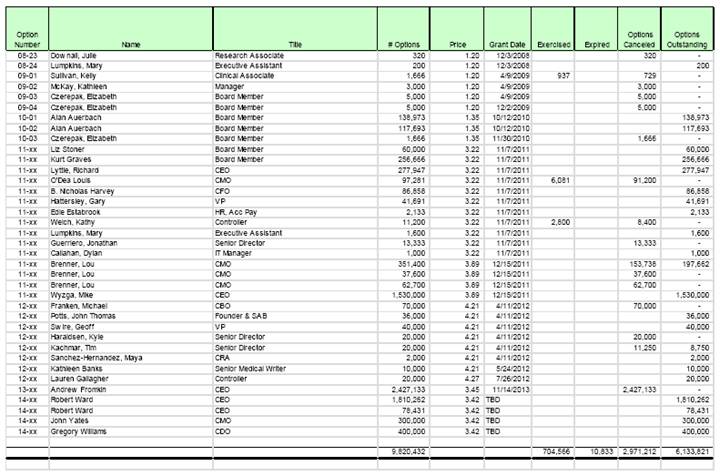
** Options outstanding include options to be granted to the Company’s Chief Executive Officer, Chief Medical Officer and Chief Development Officer in February 2014 in accordance with their respective employment agreements. As reflected in the table above, the number of shares subject to the Sign-On Option (as defined in the employment agreement entered into by the Company with its current Chief Executive Officer) is estimated today to be 78,431. Such share number may change once the calculation of the number of shares subject to such option is finally completed pursuant to the terms of such agreement.
G. Consulting Agreements
The Corporation has entered into consulting agreements with the following parties:
|
Party |
|
Effective Date |
|
Dr. John Bilezidian |
|
9/14/2005 |
|
Dr. Xxxxx Xxxxxx |
|
10/24/2005 |
|
Xxxxxxxx Associates, Inc |
|
6/18/2004 |
|
Xxxxxxxx Xxxxxxx |
|
6/24/2004 |
|
Dr. M. Xxxxxxxxx Xxxxxx |
|
1/3/2006 |
|
IntaPro LLC |
|
3/22/2005 |
|
Access BIO, LC |
|
7/8/2005 |
|
Xx. Xxxx X. Xxxxxxx |
|
5/3/2004 |
|
SVC Associates, Inc |
|
10/25/2005 |
|
Xxxxxx X Xxxxxxxxxxx |
|
7/23/2004 |
|
Xx. Xxxxxxxx Creinin |
|
10/17/2005 |
|
Frame and Xxxxxx Consulting LLP |
|
7/21/2005 |
|
Xx. Xxxxxxxxxxx Xxxxx |
|
9/1/2004 |
|
Xx. Xxxxxxx X. Xxxxx |
|
4/21/2005 |
|
TLG Consulting Inc |
|
7/1/2005 |
|
Xxxxxx X. Xxxxxxxx |
|
4/18/2006 |
|
Xxxxxx Xxxxx |
|
6/24/2004 |
|
Xx. Xxxx Xxxxxxxxxxxxxxxx |
|
11/14/2003 |
|
Xxxxx Xxxxxxx |
|
11/18/2005 |
|
Xxxxxxxxx Xxxxxxxx |
|
6/24/2004 |
|
Xxxxxxx Xxxxxxxxxxxx |
|
1/1/2004 |
|
Xxxxxx Xxxxxxx |
|
07/26/2004 |
|
Xxxxxx Xxxxxxx |
|
07/23/2004 |
|
Xx. Xxxxxxx X. Xxxxxxxxx |
|
00/00/0000 |
|
Xx. Xxxxxxx X. Xxxxxxxxx |
|
12/14/2005 |
|
Musso and Associates LLC |
|
06/24/2005 |
|
Musso and Associates LLC |
|
03/08/2006 |
|
Xxxxxx X. Xxxx |
|
11/22/2005 |
|
Xxxxxxx Xxxxxx |
|
6/4/2004 |
|
Xxxxxxx X’Xxxxx |
|
06/24/2004 |
|
Skokie Valley Consulting |
|
12/01/2003 |
|
XX Xxxxxx & Associates LLC |
|
07/08/2005 |
|
Xx. Xxxx Xxxxxx Xxxxx, Xx |
|
11/14/2003 |
|
Xx. Xxxxx Xxxxx |
|
07/22/2005 |
|
Dr. Xxxxxxx Xxxxxxxxxx |
|
11/14/2003 |
|
Xxxxxx X. Xxxxx |
|
07/08/2005 |
|
Xxx Xxxxx |
|
07/13/2004 |
|
Xxxxxxx Xxxxx |
|
07/13/2004 |
|
KellySci Consulting Inc. |
|
11/16/2007 |
|
Xxxxxx X. Xxxx |
|
06/22/2005 |
|
Xxxxxx Xxxxxxxxx |
|
06/24/2004 |
|
ChanTest Inc |
|
11/08/2005 |
|
Diamond BioPharm Ltd |
|
10/26/2007 |
|
Diamond BioPharm Ltd |
|
8/15/2007 |
|
Diamond BioPharm Ltd |
|
8/15/2007 |
|
Team Consulting Ltd |
|
8/18/2007 |
|
Xxxx Xxxxxxxxxx |
|
10/23/2007 |
|
Xxxx Xxxxxxxxxx |
|
10/10/2006 |
|
INTAPRO LLC |
|
3/22/2005 |
|
Xxx Xxxxxxxxx |
|
3/6/2008 |
|
Skokie Valley Consulting Corp. |
|
12/1/2003 |
|
Target Health Inc. |
|
4/14/2006 |
|
LGL Consulting LLC |
|
4/28/2008 |
|
Xxxxx Xxxxxx |
|
3/26/2008 |
|
Xxxxxxxx Consulting Services LLC |
|
8/15/2008 |
|
Xxxxx X. Xxxxxxxx a.k.a. d/b/a Organized Minds |
|
6/1/2008 |
|
XX Xxxxxx & Associates LLC |
|
9/15/2008 |
|
Matrix BioAnalytical Laboratories Inc |
|
4/11/2008 |
|
Xxxx Xxxxx Xxxxxxxxxx |
|
2/9/2009 |
|
Xxxxx Xxxxxxxx |
|
3/4/2009 |
|
Professor Xxxxxx Xxxxx |
|
4/14/2009 |
|
Xx. Xxxxx Xxxxxxx |
|
7/20/2009 |
|
Frame and Xxxxxx Consulting LLP |
|
5/5/2009 |
|
Access Bio LC |
|
8/20/2009 |
|
Xxxxx Xxxxxx, MD |
|
3/27/2009 |
|
Xxxx-Xxxxxxxx Sibi |
|
11/2/2009 |
|
Diamond Biopharm Ltd |
|
11/9/2009 |
|
Xxxxx Xxxxxx, MD |
|
12/12/2009 |
|
Xxxxx Xxxxxxx |
|
1/19/2010 |
|
Safety Partners, Inc. |
|
06/03/2004 |
|
Xxxx Xxxxxxxxxx |
|
10/10/2006 |
|
Xxxx Xxxxxxxxxx |
|
10/23/2007 |
|
Xxxxxx Xxxxxxxx |
|
07/24/2004 |
|
Xxxxxxxx Xxxxx |
|
11/13/2009 |
|
Goldmann Consulting LLC |
|
7/5/2010 |
|
Duck Flats Pharma LLC |
|
8/2/2010 |
|
Xxxxxx Xxxxx |
|
11/29/2010 |
|
Welsh Consulting |
|
1/20/2011 |
|
Xxxxxxx Xxxxx |
|
5/3/2011 |
|
Xxxxx Xxxxxx |
|
5/30/2011 |
|
Diamond Compliance Ltd. |
|
10/13/2011 |
|
Diamond Biopharm Ltd. |
|
10/13/2011 |
|
Xxxxx Xxxxxxxxx |
|
10/13/2011 |
|
Xxxx Xxxxx |
|
10/14/2011 |
|
Bone Research |
|
08/12/2011 |
|
Xxxxxxx Xxxxxxx |
|
06/07/2011 |
|
Xxxx Xxxxxxxx |
|
06/13/2011 |
|
Biologics Consulting |
|
05/03/2011 |
|
Premier Research International |
|
08/12/2011 |
|
Xxxxx Xxxxxx (amendment) |
|
11/14/2011 |
|
Xxxx Xxxxxxxxxx |
|
11/28/2011 |
|
Frame and Xxxxxx |
|
11/03/2011 |
|
Xxxxx Xxxxxx |
|
11/14/2011 |
|
Xxxxxxx X. Xxxxx |
|
11/18/2011 |
|
XX Xxxxxx & Associates LLC |
|
12/20/2011 |
|
Chimera Consulting |
|
01/23/2012 |
|
Xxxxxx Xxxxxxx, RN |
|
02/15/2012 |
|
Xxxxxx X. Xxxx |
|
03/02/2012 |
|
Drug Development and Regulatory Consultants LLC |
|
03/13/2012 |
|
Xxxxxxx Xxxxxxxx |
|
04/18/2012 |
|
TPQ Consultancy Ltd |
|
04/25/2012 |
|
Xxxxxx X. Xxxxxxxxx |
|
04/27/2012 |
|
SafeBridge Consultants Inc. |
|
05/15/2012 |
|
L.E.K. Consulting LLC |
|
05/22/2012 |
|
Xxxxxxx X. Xxxx, MD |
|
06/28/2012 |
|
Xxxxxx Xxxxx, MD |
|
07/03/2012 |
|
Xxxxxx X. XxXxxxxxx |
|
08/03/2012 |
|
Xxxxxxx Xxxxx-Xxxx |
|
08/09/2012 |
|
Xxxxxxx Xxxxxxxxx |
|
12/22/2012 |
|
Xxxxxxx X. Xxxxx |
|
01/24/2013 |
|
Xxxxx Xxxxxx |
|
03/14/2013 |
|
Gemini Staffing Consultants, Inc. |
|
05/31/2013 |
|
Xxxx Xxxxxx |
|
07/02/2013 |
|
The Xxxxxx Group LLC |
|
07/03/2013 |
|
Xxxxxx Xxxxx |
|
07/09/2013 |
|
Xx. Xxxx Xxxxxxxxxx |
|
07/11/2013 |
|
FaegreBD Consulting |
|
07/22/2013 |
|
MarketSense Ltd |
|
08/02/2013 |
|
Xxxxxxx Xxxx |
|
11/20/2013 |
|
Xxxx Xxxxxxxx |
|
11/25/2013 |
|
Xxxx Xxxxx, M.D. |
|
11/25/2013 |
|
Xxxxxx Xxxxxx |
|
12/02/2013 |
|
Xxxxxxx Xxxxxxx |
|
12/03/2013 |
|
Pharmagellan |
|
12/31/2013 |
|
LEK Consulting, LLC |
|
01/02/2014 |
|
Cabot Law PC |
|
01/03/2014 |
|
Avalere |
|
01/16/2014 |
|
FTI Consulting |
|
01/23/2014 |
|
Orbit Advisors Limited |
|
01/23/2014 |
|
XX Xxxxxx Oncology Consulting LLC |
|
01/25/2014 |
|
Xxxxx X. Xxxxxx, LLC |
|
01/28/2014 |
|
Xxx Xxxxxxxxx |
|
01/28/2014 |
|
Xxxx Xxxxxx Xxxxx, Xx., M.D. |
|
01/30/2014 |
|
Xxxxxxx Xxxxxxxxxx, M.D. |
|
01/30/2014 |
|
Xxxxxx Xxxxxxx, RN LLC |
|
02/01/2014 |
H. Employment Agreements
See Attachment A to this Schedule 5.6 for the Form of Letter Agreement entered into by and between the Corporation and its at-will employees.
Attachment A to
Schedule 5.6
Form of Employee Letter Agreement
,20
Dear :
It is my pleasure to offer you the position of at Radius Health, Inc. (the “Company”). As you know, I am excited about the contributions that I expect you will make to the success of the Company. Accordingly, if you accept this offer, I would like us to agree that you could start at Radius on or before (the “Start Date”). This offer may be accepted by you by countersigning where indicated at the end of this letter.
DUTIES AND EXTENT OF SERVICE
As , you will report to and you will have responsibility for performing those duties as are customary for, and are consistent with, such position, as well as those duties that may be designated to you from time to time. As you know, your employment will be contingent upon your agreeing to abide by the rules, regulations, instructions, personnel practices, and policies of the Company and any changes therein that the Company may adopt from time to time, and your execution of the Company’s standard Nondisclosure, Developments, and Non-Competition Agreement.
COMPENSATION AND BENEFITS
Your salary will be $ per semi-monthly pay period, minus applicable taxes and withholdings. Such salary may be adjusted from time to time in accordance with normal business practice and in the sole discretion of the Company. You will also be eligible for a performance-based annual bonus opportunity of up to % of your annualized salary.
You will be entitled to three weeks paid vacation annually. You will also be entitled to participate in such employee benefit plans and fringe benefits as may be offered or made available by the Company to its employees.
STOCK OPTIONS
At the first meeting of the Company’s Board of Directors following your Start Date, it is my intention that the Company will recommend to the Board of Directors that you receive a common stock option grant of shares. This offer is contingent upon approval by the Board of Directors of your option grant specifically. Promptly after the Grant Date, the Company and you will execute and deliver to each other the Company’s then standard form of stock option agreement, evidencing the Option and the terms thereof. As you know, the Option shall be subject to, and governed by, the terms and provisions of the Plan and your stock option agreement.
NONDISCLOSURE, DEVELOPMENTS AND NON-COMPETITION
As you know, prior to commencing, and as a condition to your employment with the Company, all employees are required to agree to sign a copy of the Company’s standard Nondisclosure, Developments, and Non-Competition Agreement. The Company will ask you to sign this agreement after you have signed and returned this letter and prior to or on your Start Date.
At-Will Employment
This letter shall not be construed as an agreement, either expressed or implied, to employ you for any stated term, and shall in no way alter the Company’s policy of employment at-will, under which both you and the Company remain free to terminate the employment relationship, with or without cause, at any time, with or without notice. Similarly, nothing in this letter shall be construed as an agreement, either express or implied, to pay you any compensation or grant you any benefit beyond the end of your employment with the Company.
Full Services to Company
The Company requires that, as a full-time employee, you devote your full business time, attention, skill, and efforts to the tasks and duties of your position as assigned by the Company.
NO CONFLICTING OBLIGATION AND OBLIGATIONS
You represent and warrant that the performance by you of any or all of the terms of this letter agreement and the performance by you of your duties as an employee of the Company do not and will not breach or contravene (i) any agreement or contract (including, without limitation, any employment or consulting agreement, any agreement not to compete or any confidentiality or nondisclosure agreement) to which you are or may become a party on or at an time after the Start Date or (ii) any obligation you may otherwise have under applicable law to any former employer or to any person to whom you have provided, provide or will provide consulting services.
I am pleased on behalf of Radius to extend this offer to have you join us. This is an exciting time for Radius and we would be delighted to have you as part of our organization.
Please acknowledge your acceptance of this offer and the terms of this letter agreement by signing below and returning a copy to me.
Sincerely,
I hereby acknowledge that I have had a full and adequate opportunity to read, understand and discuss the terms and conditions contained in this letter agreement prior to signing hereunder.
Dated this day of , 2013
Date this day of , 20
|
|
|
Schedule 5.6(e)
Business of the Corporation
On July 31, 2012, the Corporation received a letter from legal advisors to Pharmarama International Limited (“PIL”) claiming that the Corporation had breached a contract for PIL to supply dosage units of Forsteo for the Phase 3 clinical study of the Corporation’s BA058 Injection product and claiming €258,000 in foregone profits, plus any other additional losses incurred by PIL, in relation to a specified quantity of unordered Forsteo units. PIL threatened to commence proceedings after August 14, 2012. The Corporation has considered the claim and has provided a full response rejecting it.
Schedule 5.6(f)
Business of the Corporation
None.
Schedule 5.6(g)
Business of the Corporation
Indemnification Agreement, dated November 14, 2003, by and between the Corporation and Xxxxxxx Xxxxxxxxxx, M.D.
Indemnification Agreement, dated November 14, 2003, by and between the Corporation and Xxxxxxxxxxx Xxxxxxxxx.
Indemnification Agreement, dated November 14, 2003, by and between the Corporation and Xxxxxxxxx Xxxxxx.
Indemnification Agreement, dated November 14, 2003, by and between the Corporation and Xxxxxx Xxxxxxxx, M.D.
Indemnification Agreement, dated October 12, 2010, by and between the Corporation and Xxxx Xxxxxxxx.
Indemnification Agreement, dated May 17, 2011, by and between the Corporation and Ansbert X. Xxxxxxx.
Indemnification Agreement, dated May 17, 2011, by and between the Corporation and C. Xxxxxxx Xxxxxx Xxxxxx.
Indemnification Agreement, dated May 17, 2011, by and between the Corporation and Xxxxxx Muenchbach.
Indemnification Agreement, dated May 17, 2011, by and between the Corporation and Xxxxxxxx Xxxxxxx.
Indemnification Agreement, dated May 17, 2011, by and between the Corporation and Xxxx Xxxxxx.
Indemnification Agreement, dated May 17, 2011, by and between the Corporation and Xxxxxxxxx Xxxxxx.
Indemnification Agreement, dated December 5, 2011, by and between the Corporation and Xxxxxxx X. Xxxxx.
Indemnification Agreement, dated April 23, 2013, by and between the Company and Xxxx Xxxxxx.
Indemnification Agreement, dated April 23, 2013, by and between the Company and Xxxxxx Xxxxx-Embiricos.
Schedule 5.12(a)
Intellectual Property
The Corporation has registered its trademark rights in the following marks:
RADIUS, Registration No. 3687535, Registration Date 22 Sep 2009
Domain name: xxx.xxxxxxxxxxx.xxx
See also Attachment A to this Schedule 5.12(a).
Attachment A to
Schedule 5.12(a)
RADIUS PATENT AND PATENT APPLICATION SUMMARY
(as of January 22, 2014)
RAD-1901
A. Owned by Radius
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Title |
|
3803.1016-000 (US) |
|
61/127,025 (5/09/08) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
Expired |
|
Pharmaceutical Combinations and Methods of Using Same |
|
3803.1016-001 (PCT) |
|
PCT/US2009/002885 (5/07/09) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
Expired
Published as WO2009/137104 11/12/09 |
|
Pharmaceutical Combinations and Methods of Using Same |
|
3803.1022-000 (US) |
|
61/334,095 (5/12/10) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
Expired |
|
Therapeutic Regimens |
|
3803.1022-001 (PCT) |
|
PCT/US2011/036311 (5/12/11) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
Expired
Published as WO2011/143469 11/17/11 |
|
Therapeutic Regimens |
|
3803.1022-002 (US) |
|
13/697,230 (5/12/11) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
Pending
Published as US20130053448 2/28/13 |
|
Therapeutic Regimens |
|
3803.1022-003 (CA) |
|
2799183 (5/12/11) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
Pending |
|
Therapeutic Regimens |
|
3803.1022-004
|
|
11781299 |
|
Xxxxxxx X. Xxxxxx, |
|
Pending
|
|
Therapeutic Regimens |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Title |
|
(EP) |
|
(5/12/11) |
|
Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
2568806 3/20/13 |
|
|
|
3803.1022-005 (MX) |
|
MX/a/2012/013014 (5/12/11) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxxx, Xxxxx X’Xxx |
|
Pending |
|
Therapeutic Regimens |
B. Jointly Owned by Radius and Eisai
|
HBSR Docket No. |
|
Application Number (Pub. Number-Date) and Filing Date |
|
Inventors |
|
Status |
|
Title |
|
3803.1001-000 (US) |
|
60/816,191 (6/23/06) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxx, Xxxx Xxxxxxxxxx |
|
U.S. Provisional; (Expired 06/23/07) |
|
Treatment of Vasomotor Symptoms With Selective Estrogen Receptor Modulators |
|
3803.1001-002 (PCT) |
|
PCT/US2007/014598 (WO2008/002490-1/3/08) 6/22/07 |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxx, Xxxx Xxxxxxxxxx |
|
Expired |
|
Treatment of Vasomotor Symptoms With Selective Estrogen Receptor Modulators |
C. Solely Owned by Radius
|
HBSR Docket No. |
|
Application Number (Pub. Number-Date) and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1001-003 (US) |
|
12/308,640 (6/22/07)
(US 2010/0105733 A1-4/29/2010) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxx, Xxxx Xxxxxxxxxx |
|
U.S. National Stage of PCT Pending |
|
Treatment of Vasomotor Symptoms With Selective Estrogen Receptor Modulators |
|
3803.1001-004 (Europe) |
|
EP Xxx. No 2 037 905 07796378.3 (6/22/07)
(EP2037905-3/25/09) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxx, Xxxx Xxxxxxxxxx |
|
EP Regional Phase Granted |
|
Treatment of Vasomotor Symptoms With Selective Estrogen Receptor Modulators |
|
3803.1001-005 (Canada) |
|
2656067 (6/22/07) |
|
Xxxxxxx X. Xxxxxx, Xxxx Xxxxxxxxx, Xxxx Xxxxxxxxxx |
|
Canadian National Stage of PCT Pending |
|
Treatment of Vasomotor Symptoms With Selective Estrogen Receptor Modulators |
D. Licensed to Radius by Eisai
|
HBSR Reference No. |
|
Country |
|
App.and/or Patent Number (Pub. Number-Date) and Filing Date |
|
Status |
|
Short Description |
|
3803.0020-000 |
|
PCT |
|
PCT/JP2003/016808 (12/25/03) Publication No. WO 2004/058682 (7/15/04) |
|
Expired |
|
Selective Estrogen Receptor Modulators |
|
3803.0020-001 |
|
USA |
|
US. Patent No. 7,612,114 11/158,245 (2006/0116364-6/1/06) 6/22/05 |
|
Granted Continuation-in-Part of U.S. Designation of PCT |
|
Selective Estrogen Receptor Modulators |
|
3803.0020-002 |
|
Europe |
|
EP 2003782904 (1577288 A1-9/21/05) 12/25/03 |
|
Pending European Regional Stage of PCT |
|
Selective Estrogen Receptor Modulators |
|
3803.0020-003 |
|
Australia |
|
2003292625 B2 (2003292625 A1-7/22/2004) 12/25/03 |
|
Granted 11/6/2008 Australian National Stage of PCT |
|
Selective Estrogen Receptor Modulators |
|
3803.0020-004 |
|
Canada |
|
2512000 12/25/03 |
|
Granted 8/9/2011 Canadian National Stage of PCT |
|
Selective Estrogen Receptor Modulators |
|
0000.0000-000 |
|
Xxxxx |
|
2829/DELNP/2005 (12/25/2003) |
|
Pending Indian National Stage of PCT |
|
Selective Estrogen Receptor Modulators |
|
HBSR Reference No. |
|
Country |
|
App.and/or Patent Number (Pub. Number-Date) and Filing Date |
|
Status |
|
Short Description |
|
3803.0020-007 |
|
United States |
|
U.S. Patent No.: 7,960,412 Application No. 12/544,965 8/20/2009 Publication No. US2009/0325930 12/31/2009
Granted 6/14/11 |
|
Granted |
|
Selective Estrogen Receptor Modulators |
|
3803.0020-008 |
|
United States |
|
US Patent No. 8,399,520 3/19/13 Application No. 13/048,391 3/15/2011 |
|
Granted |
|
Selective Estrogen Receptor Modulators |
SARMS
A. Owned by Radius
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1015-000 (US) |
|
61/066,697 (02/22/08) |
|
Xxxxx X. Xxxxxx
|
|
Expired
|
|
Selective Androgen Receptor Modulators |
|
3803.1015-001 (US) |
|
61/132,353 (6/18/08) |
|
Xxxxx X. Xxxxxx |
|
Expired |
|
Selective Androgen Receptor Modulators |
|
3803.1015-002 (US) |
|
61/205,727 (1/21/09) |
|
Xxxxx X. Xxxxxx |
|
Expired |
|
Selective Androgen Receptor Modulators |
|
3803.1015-003 (PCT) |
|
PCT/US2009/001035 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Expired |
|
Selective Androgen Receptor Modulators |
|
3803.1015-004 (US) |
|
12/378,812 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Granted US Patent No. 8,067,448 11/29/11 |
|
Selective Androgen Receptor Modulators |
|
3803.1015-005 (US) |
|
12/541,489 (8/14/09) |
|
Xxxxx X. Xxxxxx |
|
Granted US Patent No. 8,268,872 9/18/12
Published as US2010/041721 2/18/10 |
|
Selective Androgen Receptor Modulators |
|
3803.1015-006 (US) |
|
12/806,636 (8/17/10) |
|
Xxxxx X. Xxxxxx |
|
Granted US Xxx. No. 8,455,525
Published as US2011/0224267 9/15/11 |
|
Selective Androgen Receptor Modulators |
|
3803.1015-007 (Australia) |
|
2009215843 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Granted Xxx. No. 2009215843 12/22/11 |
|
Selective Androgen Receptor Modulators |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1015-008 (Brazil) |
|
PI09078444 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1015-009 (Canada) |
|
2716320 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1015-010 (Europe) |
|
09712082.8 (2/19/09)
|
|
Xxxxx X. Xxxxxx |
|
Pending
Published as EP2260028 12/15/10 |
|
Selective Androgen Receptor Modulators |
|
3803.1015-011 (India) |
|
6324DELNP2010 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1015-012 (Japan) |
|
2010547633 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Granted Xxx. No. 5308458 (7/5/13) |
|
Selective Androgen Receptor Modulators |
|
3803.1015-013 (Mexico) |
|
MXA2010009162 (2/19/09) |
|
Xxxxx X. Xxxxxx |
|
Granted Patent No. 301658 7/25/12 |
|
Selective Androgen Receptor Modulators |
|
3803.1015-014 (United States) |
|
13/570,417 (8/9/12) |
|
Xxxxx X. Xxxxxx |
|
Granted Xxx. No. 8,629,167 (1/14/14) |
|
Selective Androgen Receptor Modulators |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1021-000 (US) |
|
61/301,492 (2/4/10) |
|
Xxxxx X. Xxxxxx
|
|
Expired |
|
Selective Androgen Receptor Modulators |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1021-001 (PCT) |
|
PCT/US2011/023768 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
Expired
Published as WO2011/097496 8/11/11 |
|
Selective Androgen Receptor Modulators |
|
3803.1021-002 (US) |
|
13/576,754 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
US National Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1021-003 (AU) |
|
2011212813 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
AU National Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1021-004 (BR) |
|
BR1120120195514 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
BR National Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1021-005 (CA) |
|
2788907 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
CA National Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1021-006 (EP) |
|
11740437.6 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
EP Regional Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1021-007 (IN) |
|
7188/DELNP/2012 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
IN National Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1021-008 (JP) |
|
Pending (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
JP National Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1021-009 (MX) |
|
MXA2012008995 (2/4/11) |
|
Xxxxx X. Xxxxxx |
|
MX National Stage of PCT Pending |
|
Selective Androgen Receptor Modulators |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1023-000 (US) |
|
61/361,168 (7/2/10) |
|
Xxxxx X. Xxxxxx
|
|
Expired |
|
Selective Androgen Receptor Modulators |
|
3803.1023-001 (US) |
|
13/175,307 (7/1/11) |
|
Xxxxx X. Xxxxxx
|
|
Pending
Published as US2012/004270 1/5/12 |
|
Selective Androgen Receptor Modulators |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1024-000 (US) |
|
61/387,440 (9/28/10) |
|
Xxxxx X. Xxxxxx
|
|
Expired |
|
Selective Androgen Receptor Modulators |
|
3803.1024-001 (PCT) |
|
PCT/US2011/053375 (9/27/11) |
|
Xxxxx X. Xxxxxx
|
|
Expired
Published as WO2012/047617 4/12/12 |
|
Selective Androgen Receptor Modulators |
|
3803.1024-002 (US) |
|
13/876,609 (3/28/13) |
|
Xxxxx X. Xxxxxx
|
|
Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1024-003 (AU) |
|
2011312490 (9/27/11) |
|
Xxxxx X. Xxxxxx
|
|
Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1024-004 (BR) |
|
1120130076852 (9/27/11) |
|
Xxxxx X. Xxxxxx
|
|
Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1024-005 (EP) |
|
11767355.8 (9/27/11) |
|
Xxxxx X. Xxxxxx
|
|
Pending |
|
Selective Androgen Receptor Modulators |
|
3803.1024-006 (JP) |
|
2013530412 (9/27/11) |
|
Xxxxx X. Xxxxxx
|
|
Pending |
|
Selective Androgen Receptor Modulators |
BA058
A. Jointly Owned by Radius and 3M
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1025-000 (US) |
|
61/478,466 (4/22/11) |
|
Hattersley, Hansen, Xxxxxxxx |
|
Expired |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-001 (US) |
|
61/578,120 (12/20/11) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Expired |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-002 (US) |
|
13/452,412 (4/20/12) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-003 (PCT) |
|
PCT/US12/34510 (4/20/12) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Expired DOR Met |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-004 (US) |
|
13/791,170 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-005 (US) |
|
13/791,360 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-006 (Australia) |
|
2012245301 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-007 (Brazil) |
|
BR1120130271531 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-008 (Canada) |
|
2833571 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-009 (China) |
|
TBD (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-010 (Europe) |
|
TBD (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-011 (Hong Kong) |
|
TBD (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
DOR to be met |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-012 (India) |
|
9811DELNP2013 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-013 (Israel) |
|
228942 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1025-014 (Japan) |
|
TBD (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-015 (Mexico) |
|
MXa2013012349 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-016 (New Zealand) |
|
618003 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-017 (Russia) |
|
2013151314 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-018 (Singapore) |
|
2013078324 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
0000.0000-000 (Xxxxx Xxxxx) |
|
1020137031111 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
|
3803.1025-020 (Ukraine) |
|
201313547 (3/8/2013) |
|
Hattersley, Hansen, Xxxxxxxx and Zhang |
|
Pending |
|
Method of Drug Delivery for PTH, PTHrp and Related Peptides |
B. Jointly Owned by Radius and Ipsen
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1004-000 (US) |
|
60/848,960 (10/3/06) |
|
Xxxxxxx X. Xxx, Nathalie Mondoly, Xxxxxxxx Xxxxxx, Xxxx Xxxxxxxxx and Xxxxxxx X. Xxxxxx |
|
Expired |
|
PTHrP Formulation |
|
3803.1004-002 (PCT) |
|
PCT/US2007/021216 (10/3/07) |
|
Xxxxxxx X. Xxx, Nathalie Mondoly, Xxxxxxxx Xxxxxx, Xxxx Xxxxxxxxx and Xxxxxxx X. Xxxxxx |
|
Expired |
|
PTHrP Formulation |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1004-003 (US) |
|
12/151,975 (5/9/08) |
|
Xxxxxxx X. Xxx, Nathalie Mondoly, Xxxxxxxx Xxxxxx, Xxxx Xxxxxxxxx and Xxxxxxx X. Xxxxxx |
|
Granted US Patent No. 7,803,770 (9/28/10) |
|
PTHrP Formulation |
|
3803.1004-004 (Australia) |
|
0000000000 (10/3/07) |
|
Xxxxxxx X. Xxx, Nathalie Mondoly, Xxxxxxxx Xxxxxx, Xxxx Xxxxxxxxx and Xxxxxxx X. Xxxxxx |
|
Granted Patent No. 0000000000 4/5/12 |
|
PTHrP Formulation |
|
3803.1004-005 (Brazil) |
|
PU07198213 (10/3/07) |
|
Xxxxxxx X. Xxx, Nathalie Mondoly, Xxxxxxxx Xxxxxx, Xxxx Xxxxxxxxx and Xxxxxxx X. Xxxxxx |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-006 (Canada) |
|
2664734 (10/3/07) |
|
Xxxxxxx X. Xxx, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-007 (China) |
|
200780037021.9 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted Patent No. 838064 9/14/11 |
|
PTHrP Formulation |
|
3803.1004-008 (Europe) |
|
07870768.4 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending
Published as 2073789 7/1/09 |
|
PTHrP Formulation |
|
3803.1004-009 (India) |
|
2340DELNP2009 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-010 (Israel) |
|
197926 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1004-011 (Japan) |
|
2009531434 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted Pat. No. 5375611 10/4/13 |
|
PTHrP Formulation |
|
3803.1004-012 (Korea) |
|
1020097008736 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-013 (Mexico) |
|
MXA2009003569 (10/3/07)
|
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted
Patent No 303348 9/12/12 |
|
PTHrP Formulation |
|
3803.1004-014 (New Zealand) |
|
576682 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted
Patent No. 576,682 12/11/12 |
|
PTHrP Formulation |
|
3803.1004-015 (Norway) |
|
20091545 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-016 (Russian Federation) |
|
2009116531 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-017 (Singapore) |
|
2009922401 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted Patent No. 151061 7/13/12 |
|
PTHrP Formulation |
|
3803.1004-018 (Ukraine) |
|
200904264 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted Patent No. 98776 6/25/12 |
|
PTHrP Formulation |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1004-019 (US) |
|
12/311,418 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted US Pat No. 8,148,333 4/3/12 |
|
PTHrP Formulation |
|
3803.1004-020 (PCT) |
|
PCT/US2009/002868 (5/8/09) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
DORs not met |
|
PTHrP Formulation |
|
3803.1004-021 (Hong Kong) |
|
09109160.6 (10/2/09) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-023 (CN) |
|
201110220104.X (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending
Published 102274492 12/14/11 |
|
PTHrP Formulation |
|
3803.1004-024 (BR) |
|
|
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-025 (New Zealand) |
|
Application No. 596095 (10/3/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Abandoned |
|
PTHrP Formulation |
|
3803.1004-027 (Singapore) |
|
2011071784 (10/03/07) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-028 (Australia) |
|
2012201490 (3/13/12) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Granted Pat. No. 2012201490 B2 8/22/13 |
|
PTHrP Formulation |
|
HBSR Docket No. |
|
Application Number and Filing Date |
|
Inventors |
|
Status |
|
Short Description |
|
3803.1004-029 (US) |
|
13/438,086 (4/3/12) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Pending |
|
PTHrP Formulation |
|
3803.1004-030 (US) |
|
13/790,186 (3/8/13) |
|
Michael J. Dey, Nathalie Mondoly, Benedice Rigaud, Bart Henderson and Richard C. Lyttle |
|
Abandoned |
|
PTHrP Formulation |
C. Ipsen Patents Licensed to Radius Covering Ba058
|
Ipsen Reference No. |
|
Country |
|
Application Number Filing Date and Priority |
|
Status |
|
Short Description |
|
038/US2 |
|
USA |
|
08/779,768 (01/07/97) |
|
U.S. Patent 5,969,095 (10/19/99) Expires 3/29/16 |
|
Claims BA058 |
|
038/US/PCT2/EP |
|
Europe |
|
96924355.9 (01/30/98)
Regional Phase entry of PCT/US96/11292 |
|
European Patent 0 847 278 (09/24/03) Expires 07/03/16 Activation in AT, BE, CH, DE, DK, ES, FI, FR, Gb, GR, IE, IT, LU, MC, NL, PT, SE; Ext: AL, LT, LV, SI Now Abandoned in AL, LT, LU, MC, SI, LV |
|
Analogs of PTH |
|
Ipsen Reference No. |
|
Country |
|
Application Number Filing Date and Priority |
|
Status |
|
Short Description |
|
038/US/PCT2/AU |
|
Australia |
|
64834/96 (07/03/96)
Regional Phase entry of PCT/US96/11292 |
|
Patent No. 707094 (07/01/99) Expires 07/03/16 |
|
Analogs of PTH |
|
038/US/PCT2/CA |
|
Canada |
|
2,226,177 (07/03/96)
Regional Phase entry of PCT/US96/11292 |
|
Patent No.:2,226,177 Expires 7/3/2016 |
|
Analogs of PTH |
|
038/US/PCT2/CN-C |
|
China |
|
2006 10100113.4 (7/21/06)
Divisional of 2004 10005427.7 |
|
Patent No.: ZL200610100113 Expires 7/3/2016 |
|
Analogs of PTH |
|
038/US/PCT2/HU |
|
Hungary |
|
P9901718 (07/03/96)
Regional Phase entry of PCT/US96/11292 |
|
Patent No.: 226935 Expires 7/3/2016 |
|
Analogs of PTH |
|
038/US/PCT2/KR-D |
|
Korea |
|
2004-706341 (04/28/04) Divisional of 1998-0700249 |
|
Patent No. 0563112 (3/15/06) Expires 07/03/16 |
|
Analogs of PTH |
|
038/US/PCT2/NZ |
|
New Zealand |
|
312899 (01/20/98)
Regional Phase entry of PCT/US96/11292 |
|
Patent No. 312899 (02/08/00) Expires 07/03/16 |
|
Analogs of PTH |
|
038/US/PCT2/MX |
|
Mexico |
|
PA/a/1998/000418 (07/03/96) |
|
Patent No. 222317 (08/26/04) Expires 07/03/16 |
|
Analogs of PTH |
|
Ipsen Reference No. |
|
Country |
|
Application Number Filing Date and Priority |
|
Status |
|
Short Description |
|
|
|
|
|
Regional Phase entry of PCT/US96/11292 |
|
|
|
|
|
038/US/PCT2/PL |
|
Poland |
|
P.325905 (01/12/98)
Regional Phase entry of PCT/US96/11292 |
|
Patent No. 186710 (08/07/03) Expires 07/03/16 |
|
Analogs of PTH |
|
038/US/PCT2/RU |
|
Russia |
|
98/102406 (7/3/96) |
|
Patent No. 2,157,699 (10/20/00) |
|
Analogs of PTH |
|
038/US/PCT2/SG |
|
Singapore |
|
9706046.1 (7/3/96) |
|
Patent No. P-51260 (10/16/01) |
|
Analogs of PTH |
|
038/US/TW |
|
Taiwan |
|
85108390 (07/11/96)
|
|
Patent No. 153897 (08/07/02)
|
|
Analogs of PTH |
|
038/US3/PCT2/US2 |
|
USA |
|
09/399,499 (09/20/99) |
|
Patent No. 6,544,949 (04/08/03) Expires 03/29/16
|
|
Claims a method of treating osteoporosis with BA058 and a pharmaceutical composition including BA058 |
|
038/US/PCT2/CN-C/HK |
|
HK |
|
Filing 07103960.3 4/16/2007 |
|
Patent No. HK1096976 Expires 7/3/2016 |
|
Analogs of PTH |
Schedule 5.12(b)
Intellectual Property
See items listed as “Jointly Owned” or “Licensed to Radius” in Item 3 of Attachment A to Schedule 5.12(a).
Certain rights licensed to 3M Company (“3M”) pursuant to that certain Development and Clinical Supplies Agreement with 3M dated June 19, 2009, as amended.
Schedule 5.12(c)
Intellectual Property
See items listed as “Jointly Owned” or “Licensed to Radius” in Item 3 of Attachment A to Schedule 5.12(a).
Certain rights licensed to 3M pursuant to that certain Development and Clinical Supplies Agreement with 3M dated June 19, 2009, as amended.
Schedule 5.12(e)
Intellectual Property
None.
Schedule 5.12(f)
Intellectual Property
See items listed as “Jointly Owned” or “Licensed to Radius” in Item 3 of Attachment A to Schedule 5.12(a).
Certain rights licensed to 3M pursuant to that certain Development and Clinical Supplies Agreement with 3M dated June 19, 2009, as amended.
Schedule 5.12(g)
Intellectual Property
See items listed as “Jointly Owned” or “Licensed to Radius” in Item 3 of Attachment A to Schedule 5.12(a).
Certain rights licensed to the Corporation pursuant to that certain Development and Clinical Supplies Agreement with 3M dated June 19, 2009, as amended.
Schedule 5.12(h)
Intellectual Property
None.
Schedule 5.12(i)
Intellectual Property
None.
Schedule 5.14(a)
Title to Properties
None.
Schedule 5.14(b)
Title to Properties
Sublease by and between the Corporation and Sonos, Inc., dated January 14, 2011, for the property located at 201 Broadway, Cambridge, Massachusetts.
Schedule 5.16
ERISA
Radius Health, Inc. 401(k) Plan
Radius Health, Inc. Flexible Spending Account Plan
Schedule 5.17
Use of Proceeds
The Corporation anticipates using the net proceeds from the sale of the Series B-2 Preferred Stock to further the conduct and completion of the ongoing clinical trials of its product candidates, to bolster the Corporation’s balance sheet to improve negotiation leverage with potential partners, to continue to make regular payments to its lenders pursuant to the terms of its agreements with such lenders, and to pay its employees, consultants and vendors pursuant to its obligations as an employer or the terms of its agreements with such employees consultants and vendors, as applicable. The amounts actually expended by the Corporation for the above purposes may vary significantly depending on numerous factors, including future revenue growth, if any, the progress of the Corporation’s research and development efforts and technological advances and hence, the Corporation’s management will retain broad discretion in the allocation of the net proceeds from the sale of the Series B-2 Preferred Stock.
Schedule 5.18
Permits and Other Rights; Compliance with Laws
None.
Schedule 5.19
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
DOMESTIC PACKAGE |
|
Travelers Property Casualty Co. of America |
|
ZLP14P32338 |
|
01/30/2014 - 01/30/2015 |
|
$7,639 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
PROPERTY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Replacement Cost Valuation on Personal Property |
|
|
|
|
|
|
|
|
| |
|
Special Form Causes of Loss including Equipment Breakdown |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Scheduled Location |
|
|
|
|
|
|
|
|
| |
|
201 Broadway |
|
|
|
|
|
|
|
|
| |
|
Cambridge , MA 02139 |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Limits |
|
|
|
|
|
|
|
|
|
|
|
Business Personal Property |
$490,000 |
|
|
|
|
|
|
|
|
|
|
Business Income/Extra Expense |
$250,000 |
|
|
|
|
|
|
|
|
|
|
Property at Unscheduled Location |
$50,000 |
|
|
|
|
|
|
|
|
|
|
Property in Transit |
$50,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deductibles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property |
$2,500 |
|
|
|
|
|
|
|
|
|
|
Business Income Waiting Period |
24 Hours |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
DOMESTIC PACKAGE |
|
Travelers Property Casualty Co. of America |
|
ZLP14P32338 |
|
01/30/2014 - 01/30/2015 |
|
$7,639 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
GENERAL LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
General Aggregate Limit |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
DOMESTIC PACKAGE |
|
Travelers Property Casualty Co. of America |
|
ZLP14P32338 |
|
01/30/2014 - 01/30/2015 |
|
$7,639 |
| |
|
Products/Completed Operations Aggregate |
Excluded |
|
|
|
|
|
|
|
|
|
|
Each Occurrence Limit |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Advertising Injury and Personal Injury Limit |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Premises Damage |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Medical Expense |
$10,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EMPLOYEE BENEFITS LIABILITY (CLAIMS-MADE) |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Each Claim Limit |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Aggregate Limit |
$3,000,000 |
|
|
|
|
|
|
|
|
|
|
Deductible |
$0 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
FOREIGN PACKAGE |
|
Travelers Property Casualty Co. of America |
|
ZPP14P32363 |
|
01/30/2014 - 01/30/2015 |
|
$2,500.00 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
INTERNATIONAL GENERAL LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
General Aggregate Limit |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
Products/Completed Operations Aggregate |
Excluded |
|
|
|
|
|
|
|
|
|
|
Each Occurrence Limit |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Advertising Injury and Personal Injury Limit |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Premises Damage |
$300,000 |
|
|
|
|
|
|
|
|
|
|
Medical Expense |
$10,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INTERNATIONAL AUTOMOBILE |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
HIRED & NON-OWNED AUTO LIABILITY — EXCESS/DIC |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Combined Single Limit for Bodily Injury and/or Property Damage |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
FOREIGN PACKAGE |
|
Travelers Property Casualty Co. of America |
|
ZPP14P32363 |
|
01/30/2014 - 01/30/2015 |
|
$2,500.00 |
| |
|
Medical Payments |
$10,000 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
FOREIGN PACKAGE |
|
Travelers Property Casualty Co. of America |
|
ZPP14P32363 |
|
01/30/2014 - 01/30/2015 |
|
$2,500.00 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
FOREIGN VOLUNTARY WORKERS’ COMPENSATION |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Description of Covered Employees |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
International Executive Employees - State of Hire Benefits |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Other International Employees - Country of Origin Benefits |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
EMPLOYER’S LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Limits of Liability |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bodily Injury by Accident (Each Accident) |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Bodily Injury by Disease (Aggregate) |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Bodily Injury by Disease (Each Employee) |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Coverage Extensions |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Repatriation/Transportation - Each Person |
$25,000 |
|
|
|
|
|
|
|
|
|
|
Repatriation/Transportation - Aggregate |
$50,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ACCIDENTAL DEATH & DISMEMBERMENT |
100,0000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
KIDNAP & RANSOM |
$100,000 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
COMMERCIAL AUTOMOBILE |
|
Charter Oak Fire Ins. Co. |
|
BA5881P528 |
|
01/30/2014 - 01/30/2015 |
|
$250.00 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
HIRED & NON-OWNED AUTO LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Combined Single Limit for Bodily Injury and/or Property Damage |
$1,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HIRED CAR PHYSICAL DAMAGE |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Limit |
Actual Cash Value |
|
|
|
|
|
|
|
|
|
|
Comprehensive Deductible |
$0 |
|
|
|
|
|
|
|
|
|
|
Collision Deductible |
$500 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
WORKERS’ COMPENSATION |
|
Phoenix Insurance Co. |
|
UB9485C327 |
|
01/30/2014 - 01/30/2015 |
|
$7,098 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
COVERAGE A — WORKERS’ COMPENSATION Statutory in MA |
|
|
|
|
|
|
|
Subject to Audit |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
COVERAGE B — EMPLOYER’S LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Bodily Injury by Accident (Each Accident) |
$500,000 |
|
|
|
|
|
|
|
|
|
|
Bodily Injury by Disease (Aggregate) |
$500,000 |
|
|
|
|
|
|
|
|
|
|
Bodily Injury by Disease (Each Employee) |
$500,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVERAGE C — OTHER STATES except |
|
|
|
|
|
|
|
|
| |
|
ND, OH, WA, WY & those listed in Coverage A |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Note: Coverage C has limitations. Please contact WGA if you begin operations in any state not listed under Coverage A. |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| ||
|
WORKERS’ COMPENSATION |
|
Phoenix Insurance Co. |
|
UB9485C327 |
|
01/30/2014 - 01/30/2015 |
|
$7,098 |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Based on estimated payroll as follows: |
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
MA |
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Class Code |
Class Description |
Payroll |
|
|
|
|
|
|
|
|
|
|
8810 |
Clerical Office Employees NOC |
$2,600,000 |
|
|
|
|
|
|
|
|
|
|
4512 |
Biomedical Research Laboratories |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
COMMERCIAL UMBRELLA |
|
Travelers Property Casualty Co. of America |
|
ZUP14P32351 |
|
01/30/2014 - 01/30/2015 |
|
$3,495.00 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
LIMITS OF LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Per Occurrence |
$5,000,000 |
|
|
|
|
|
|
|
|
|
|
General Aggregate |
$5,000,000 |
|
|
|
|
|
|
|
|
|
|
Self-Insured Retention |
$10,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Underlying Coverages Domestic General Liability Domestic Employee Benefits Liability Domestic Employer’s Liability Domestic Automobile Liability Foreign General Liability Foreign Employer’s Liability Foreign Automobile Liability |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
PRODUCTS LIABILITY |
|
Federal Insurance Company |
|
35854927 |
|
01/30/2014 - 01/30/2015 |
|
$51,988.00 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
LIMITS OF LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Aggregate Limit |
$10,000,000 |
|
|
|
|
|
|
|
|
|
|
Each Occurrence Limit |
$10,000,000 |
|
|
|
|
|
|
|
|
|
|
Deductible — Each Event |
$25,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retroactive Dates: |
|
|
|
|
|
|
|
|
|
|
|
$5,000,000 |
1/20/2006 |
|
|
|
|
|
|
|
|
|
|
$5,000,000 excess of $5,000,000 |
7/17/2008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rated on Number of Participants |
138 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Claims-Made Coverage |
|
|
|
|
|
|
|
|
|
|
|
Defense Costs included in Limit of Liability |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
WORLD WIDE TRANSIT |
|
Allianz Global Corporate Specialty-AGCS |
|
OC91225900 |
|
03/25/2013 - 03/25/2014 |
|
$31,750.00 |
| |
|
|
|
|
|
|
|
|
|
|
| |
|
Transit Limits |
|
|
|
|
|
|
|
|
| |
|
Any One Vessel/Connecting Conveyance |
$1,200,000 |
|
|
|
|
|
|
|
|
|
|
Any One Aircraft/Connecting Conveyance |
$1,200,000 |
|
|
|
|
|
|
|
|
|
|
Any One Truck or Railroad Car |
$1,200,000 |
|
|
|
|
|
|
|
|
|
|
Any One Package Shipped by Government or Private Mail or Parcel Post |
$7,500 |
|
|
|
|
|
|
|
|
|
|
Deductible |
$2,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Storage Limits |
|
|
|
|
|
|
|
|
|
|
|
Catalent CTS Limited |
$8,500,000 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
WORLD WIDE TRANSIT |
|
Allianz Global Corporate Specialty-AGCS |
|
OC91225900 |
|
03/25/2013 - 03/25/2014 |
|
$31,750.00 |
| |
|
CLA Centro Logistico Anhanguera |
$3,000,000 |
|
|
|
|
|
|
|
|
|
|
World Courier Argentina |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Vetter Pharma Fertigung GmbH |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
Beaufour Ipsen Industrie |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
Almac Clinical Services |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
Almac Clinical Services (UK) |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
Lonza braine SA |
$1,500,000 |
|
|
|
|
|
|
|
|
|
|
3M Drug Delivery Systems |
$2,000,000 |
|
|
|
|
|
|
|
|
|
|
Any One Unnamed Location |
$250,000 |
|
|
|
|
|
|
|
|
|
|
30 Clinical Sites |
$3,200,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deductible |
$5,000 |
|
|
|
|
|
|
|
|
|
|
Flood/Wind/Earthquake Deductible |
$25,000 |
|
|
|
|
|
|
|
|
|
* No Single Location to exceed $1,100,000
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
MANAGEMENT LIABILITY |
|
Federal Insurance Company |
|
82108432 |
|
07/23/2013 - 07/23/2014 |
|
$35,411.00 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
DIRECTORS’ & OFFICERS’ LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum Aggregate Limit |
$5,000,000 |
|
|
|
|
|
|
|
|
|
|
Dedicated Limit for Executives |
$500,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deductibles: |
|
|
|
|
|
|
|
|
|
|
|
Non-Indemnified Loss |
$0 |
|
|
|
|
|
|
|
|
|
|
Indemnified Loss |
$25,000 |
|
|
|
|
|
|
|
|
|
|
Company Loss |
$25,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prior & Pending Litigation Date |
4/29/2005 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Claims-Made Coverage |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
|
|
WORLD WIDE TRANSIT |
|
Allianz Global Corporate Specialty-AGCS |
|
OC91225900 |
|
03/25/2013 - 03/25/2014 |
|
$31,750.00 |
|
|
Defense Costs included in Limit of Liability |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
MANAGEMENT LIABILITY |
|
Federal Insurance Company |
|
82108432 |
|
07/23/2013 - 07/23/2014 |
|
$35,411.00 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
EMPLOYMENT PRACTICES LIABILITY |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum Aggregate Limit |
$5,000,000 |
|
|
|
|
|
|
|
|
|
|
Third Party Liability Limit |
$5,000,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deductibles: |
|
|
|
|
|
|
|
|
|
|
|
EPL |
$5,000 |
|
|
|
|
|
|
|
|
|
|
Third Party |
$5,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prior & Pending Litigation Date |
4/29/2005 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Claims-Made Coverage |
|
|
|
|
|
|
|
|
|
|
|
Defense Costs included in Limit of Liability |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
FIDUCIARY LIABILITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum Aggregate Limit |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Deductible |
$0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prior & Pending Litigation Date |
4/29/2005 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Claims-Made Coverage |
|
|
|
|
|
|
|
|
|
|
|
Defense Costs included in Limit of Liability |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
MANAGEMENT LIABILITY |
|
Federal Insurance Company |
|
82108432 |
|
07/23/2013 - 07/23/2014 |
|
$35,411.00 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
CRIME COVERAGE |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee Theft |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Premises |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
In Transit |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Forgery |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Computer Fraud |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Funds Transfer Fraud |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Money Orders & Counterfeit Currency |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Credit Card Fraud |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Client |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Expense |
$100,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deductible (all but Expense) |
$5,000 |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
MANAGEMENT LIABILITY |
|
Federal Insurance Company |
|
82108432 |
|
07/23/2013 - 07/23/2014 |
|
$35,411.00 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
SPECIAL COVERAGE |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Special Coverage |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Custody |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Expense |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accidental Loss |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
(i) Loss of Life |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
(ii) Mutilation |
100% |
|
|
|
|
|
|
|
|
|
|
COVERAGE AND LIMITS |
|
INSURANCE COMPANY |
|
POLICY NUMBER |
|
TERM |
|
PREMIUM |
| |
|
MANAGEMENT LIABILITY |
|
Federal Insurance Company |
|
82108432 |
|
07/23/2013 - 07/23/2014 |
|
$35,411.00 |
| |
|
(iii) All other |
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Legal Liability Costs |
$1,000,000 |
|
|
|
|
|
|
|
|
|
|
Emergency Political Repatriation |
$500,000 |
|
|
|
|
|
|
|
|
|
|
Disappearance Investigation Expense |
$250,000 |
|
|
|
|
|
|
|
|
|
|
Express Kidnap Costs |
$250,000 |
|
|
|
|
|
|
|
|
|
|
Hostage Crisis Costs |
$250,000 |
|
|
|
|
|
|
|
|
|
Schedule 5.20
Board of Directors
1. The Corporation has agreed, in Section 6(b) of the Series A-1 Certificate to allocate four of the eight seats on the Board of Directors to certain of its existing stockholders.
2. Pursuant to Section 4.3 of the Stockholders’ Agreement, the Corporation has granted to one or more investors the right to appoint one or more observers to the Board of Directors.
Schedule 5.22(a)
Environmental Matters
None.
Schedule 5.22(d)
Environmental Matters
1. The following chemicals, radioactive materials, or other potentially harmful materials or substances were present or used on the premises previously subleased and occupied by the Corporation at 300 Technology Square, Cambridge, Massachusetts 02139:
|
Less than 10 liters |
|
Less than 1 liter |
|
Less than 1 kg |
|
Less than 10g |
|
Acetone |
|
acetic acid |
|
4-C2-Hydroxyethyl-1- piperazineethanesulfonic acid |
|
Adenoslne 5’ Triposphate Disodium |
|
Ethanol |
|
acetonitrile |
|
acrylamide |
|
All Gel Dydrazide |
|
Isopentyl alcohol |
|
Benzyl alcohol |
|
agarose |
|
abendonate |
|
Methanol |
|
butanol |
|
albumin |
|
Bromopherol blue |
|
Wescodyne |
|
chloroform |
|
aromoblum acetate |
|
Cholecalciferol |
|
Xylene |
|
Citric Acid |
|
beta-cyclodextrin |
|
Dihydroxytestosterone |
|
bleach |
|
Coomassie Blue Stain |
|
Calcium Chloride |
|
estradiol |
|
|
|
DEPC water |
|
Carbamychofine Chloride |
|
Ethldium Bromide |
|
|
|
diethyl pyrocarbonate |
|
Chloramidopropyl (Dimethylammonio)-2 Hydroxy-1-Propoanesultonate |
|
Forskolin |
|
|
|
dimethylacetamide |
|
Chloramphenicol |
|
Geneticin |
|
|
|
dimethylformamide |
|
dextran sulfate |
|
Heparin Sodium |
|
|
|
dimethylsuloxide |
|
dithothrepl |
|
ketamine |
|
|
|
Ethyl Acetate |
|
ettylepadiamine tetraacetic acid |
|
Lipopolysaccharide |
|
|
|
Ethyl Alcohol |
|
Gelatin |
|
nandrolone |
|
|
|
Ethylene Glycol |
|
Glucose |
|
Phorbal — 12-myristate 13 acetate |
|
|
|
formic acid |
|
glycine |
|
raloxfene |
|
|
|
glycerol |
|
Guanldine thcyanate |
|
streptavicin |
|
|
|
heptane |
|
hemaloxylin |
|
tamoxlene |
|
|
|
Hexane |
|
Hepes Free Acid |
|
testosterone |
|
|
|
hydrochloric acid |
|
Hexamine cobalt trichloride |
|
tetracycline hydrochloride |
|
|
|
hydrogen peroxide |
|
Histopaque 1077 |
|
toluidine blue |
|
|
|
isoflurane |
|
magnesium chloride |
|
xyazine |
|
|
|
isopropyl alcohol |
|
Magnesium Sulfate |
|
xylene canole |
|
|
|
mercapioethanol |
|
melthycellulose |
|
|
|
|
|
octane |
|
methylmethacrylate |
|
|
|
|
|
pH calibration buffers 4, 7, 10 |
|
paraffin wax |
|
|
|
|
|
phenol |
|
Paraformaldehyde |
|
|
|
|
|
phenopthalein |
|
Phosphate buffered saline |
|
|
|
|
|
phosphate buffered saline |
|
polyvinlypyrolidone |
|
|
|
|
|
phosphoric acid |
|
Potassium Acetate |
|
|
|
|
|
polyoxyethylene |
|
potassium chloride |
|
|
|
|
|
potassium hydroxide |
|
Potassium Hydroxide |
|
|
|
|
|
Propanol |
|
sodium Acetate |
|
|
|
|
|
propylene glycol |
|
sodium Azide |
|
|
|
|
|
sulphuric acid |
|
sodium bicarbonate |
|
|
|
|
|
Tetramethylbenzidine |
|
Sodium Carbonate |
|
|
|
|
|
trichioroacatic acid |
|
Sodium chloride |
|
|
|
|
|
triethanplamine |
|
Sodium hydroxide |
|
|
|
|
|
Tritton X-100 |
|
Sodium phosphate |
|
|
|
|
|
|
|
Sucrose |
|
|
|
|
|
|
|
Tetraethylammonium chloride |
|
|
|
|
|
|
|
Thioglycolic acid |
|
|
|
|
|
|
|
trishydorchloride |
|
|
|
Human Cell Line |
|
Murine Cell Line |
|
Other Cell Lines |
|
|
|
|
|
|
|
HeLa |
|
C2C12 |
|
HEK293 |
|
HOB |
|
ST2 |
|
COS |
|
SaOs2 |
|
MC3T3E1 |
|
|
|
MG63 |
|
U32 |
|
|
|
U2OS |
|
OB6 |
|
|
|
|
|
UMR106 |
|
|
|
|
|
MLOY4 |
|
|
|
Radioactive Material |
|
Chemical/Physical Form |
|
Maximum Possession Limit |
|
|
|
|
|
|
|
A. Hydrogen-3 |
|
A. Any |
|
A 20 millicuries |
|
B. Carbon-14 |
|
B. Any |
|
B. 5 millicuries |
|
C. Phosphorus-32 |
|
C. Any |
|
C. 20 millicuries |
|
D. Phosphorus-33 |
|
D. Any |
|
D. 20 millicuries |
|
E. Sulfur-35 |
|
E. Any |
|
E. 20 millicuries |
|
F. Iodine-125 |
|
G. Bound |
|
G. 5 millicuries |
Schedule 5.22(e)
Environmental Matters
1. The premises previously subleased by the Corporation and located at 300 Technology Square, Cambridge, Massachusetts 02139.
Schedule 5.22(f)
Environmental Matters
None.

