SETTLEMENT & AMENDMENT CONCERNING A MANUFACTURING AGREEMENT DATED DECEMBER 2, DATED JUNE 20, 2011
Exhibit 10.25
|
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Double asterisks denote omissions.
|
SETTLEMENT & AMENDMENT CONCERNING A
MANUFACTURING AGREEMENT DATED DECEMBER 2,
2005 AND A COMMERCIAL SUPPLY AGREEMENT
DATED JUNE 20, 2011
| (1) | CMC ICOS BIOLOGICS, INC., |
| (2) | INSPIRATION BIOPHARMACEUTICALS, INC., |
THIS AGREEMENT is made on 20 November 2012
BETWEEN:
| (1) | CMC ICOS BIOLOGICS, INC., a corporation duly incorporated under the laws of the State of Washington and having its principal place of business at 00000 00xx Xxxxxx XX, Xxxxxxxx, XX, 00000, Xxxxxx Xxxxxx of America (hereinafter referred to as “CMC”); and |
| (2) | INSPIRATION BIOPHARMACEUTICALS, INC., a corporation duly incorporated under the laws of the State of Delaware and having its principal place of business at One Xxxxxxx Square, Building 1400E, Xxxxxxxxx, XX 00000, Xxxxxx Xxxxxx of America (hereinafter referred to as “IBI”), |
each referred to as a “Party” or collectively as “Parties”
WHEREAS:
| (A) | IBI is engaged in the development of products for the treatment of hemophilia including its proprietary recombinant Factor IX product described in BB-IND 13551 (known as “IB1001”) and its proprietary Factor VIIa product (known as “IB1007”); |
| (B) | CMC and IBI are parties to a Manufacturing Agreement dated December 2, 2005 (as amended) under which CMC agreed to provide biological manufacturing and development services on behalf of IBI (the “MSA”); |
| (C) | CMC and IBI are also parties to a Commercial Supply Agreement dated June 20, 2011 (as amended) under which CMC has agreed to reserve certain slots and capacity in its facilities and to provide certain services each relating to the manufacture for commercial supply of IB1001 (the “CSA”); |
| (D) | On October 30, 2012 (the “Petition Date”), IBI filed a voluntary petition for relief under chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the District of Massachusetts (the “Bankruptcy Court”) commencing Case Number 12-18687-WCH (the “Bankruptcy Case”) and IBI remains in possession of its property and continues to manage its business as a debtor in possession pursuant to sections 1107 and 1108 of the Bankruptcy Code; and |
| (E) | the Parties now wish, subject to Bankruptcy Court approval, to amend the CSA and agree to a settlement in respect of the current outstanding sums due under the MSA and CSA. |
IN CONSIDERATION of the mutual promises contained in this Agreement, the Parties hereby AGREE AS FOLLOWS:
| 1. | DEFINITIONS |
| 1.1 | Capitalised terms used in this Agreement which are not defined in this Agreement shall have the same meaning defined for such term in the CSA (as amended by this Agreement). |
2
| Notwithstanding the foregoing, the following terms defined in this clause 1.1 shall have the respective meanings set forth below: |
| “Acquisition Payment” | any and all consideration (whether cash or non-cash), value, monies, rights, benefits or waivers (including any future or conditional payments) payable or received, directly or indirectly, in exchange for the bona fide sale, transfer, issuance, grant or license of any assets, rights, benefits, stock, shares or control of IBI existing from time to time, and where such consideration is other than for cash, such non-cash consideration shall be valued on an arms-length, good faith, market-value basis; | |||
| “Additional Services” | (i) the HCP Development Services and those additional development services with respect to the latest Process (appended to this Agreement at Schedule 1-A), (ii) additional Services agreed upon by the Parties and set forth in a work order appended to this Agreement as Schedule 1-B, and (iii) the manufacture of Conformance Batches of Product; | |||
| “Conformance Batch(es)” | three (3) consecutive manufacturing Batches of IB1001 Product under GMP for the purpose of validating the changes in the modified Process; | |||
| “CSA Breach” | means IBI’s default under the CSA, specifically, the non-payment of any and all outstanding invoices under the CSA for Services provided by CMC prior to October 30, 2012; | |||
| “Factor VIIa Cell Banks” | all cell banks and cell lines (including master cell banks and research cell banks) owned, held or controlled by or on behalf of IBI which have been developed or constructed for the manufacture of Factor VIIa Product, including all intermediates and progeny of the same; | |||
| “Factor VIIa Product” | means IBI’s proprietary, recombinant Factor VIIa product known as IB-1007; | |||
| “Factor VIIa Product Assets” | means (i) Factor VIIa Cell Banks, and (ii) all biological and raw materials, process data and methodology (including manufacturing processes), clinical and pre-clinical data, media formulations, methodologies, IPRs, documents, records, know-how, regulatory submissions and approvals, correspondence and reports (including regulatory authority interactions), and all other materials concerning or relating to the Factor VIIa Product; | |||
| “HCP Development Services” | means those development services stipulated and identified in the Statement of Work appended to this Agreement at Schedule 1-A; | |||
3
| “IPRs” | all intellectual property rights, including (without limitation) patents and patent rights, supplementary protection certificates, xxxxx patents, utility models, trademarks, database rights, rights in designs, copyrights (whether or not any of these are registered or capable of being registered) and including all applications and the right to apply for registered protection of the foregoing and all inventions, trade secrets, know-how, techniques and confidential information and other proprietary knowledge and information, and all rights and forms of protection of a similar nature or having equivalent or similar effect to any of these which may subsist anywhere in the world, in each case for their full term and together with any renewals or extensions; | |||
| “MSA Breach” | means IBI’s default under the MSA, specifically, the non-payment of those outstanding invoices under the MSA for work performed by CMC prior to October 30, 2012; | |||
| “Person” | any individual, partnership, firm, corporation, company, association, trust, unincorporated organisation or other entity in each case in any jurisdiction; | |||
| “UNC License” | the Amended and Restated License Agreement dated November 28, 2008, including any amendments thereto, entered into between The University of North Carolina and IBI; | |||
| “Upfront Acquisition Payment” | Acquisition Payments made, received or payable that are triggered by the completion of a sale, transfer, issuance, grant or license of any assets, rights, benefits, stock, shares or control of IBI. | |||
| 1.2 | In this Agreement (except where the context otherwise requires): |
| 1.2.1 | any reference to a recital, clause (unless identified as being in another document) or appendix is to the relevant recital, clause or appendix of or to this Agreement and any reference to a sub-clause or paragraph is to the relevant sub-clause or paragraph of the clause or appendix in which it appears; |
| 1.2.2 | the table of contents and clause headings are included for convenience only and shall not affect the interpretation of this Agreement; |
| 1.2.3 | use of the singular includes the plural and vice versa and use of any gender includes the other genders; |
| 1.2.4 | any reference to “persons” includes natural persons, firms, partnerships, companies, corporations, associations, organizations, governments, states, governmental or state agencies, foundations and trusts (in each case whether or not having separate legal personality and irrespective of the jurisdiction in or under the law of which it was incorporated or exists); |
4
| 1.2.5 | a reference to a “Party” is a reference to a party to this Agreement and a reference to a “Party” includes a reference to that Party’s successors in title, permitted assignees and transferees (if any) and in the case of an individual, to his or her estate and personal representatives; |
| 1.2.6 | a reference to “writing” does not include email; |
| 1.2.7 | any phrase introduced by the terms “including”, “include”, “in particular” or a similar expression shall be construed as illustrative and shall not limit the sense of the words preceding those terms. |
| 1.3 | The Appendices form an integral part of this Agreement and shall have effect as if set out in full in the body of this Agreement and any reference to this Agreement includes the Appendices. |
| 1.4 | Where there is any inconsistency between the Appendices and the main body of this Agreement, the conflicting terms of the main body of this Agreement shall, unless expressly specified to the contrary, prevail. |
| 2. | SETTLEMENT & RELEASE OF CERTAIN OBLIGATIONS |
| 2.1 | In consideration of and subject to IBI’s compliance with all other terms and conditions of this Agreement, including without limitation the provisions of Clauses 3, 4 and 8, CMC hereby waives, releases and discharges IBI from: |
| 2.1.1 | its obligation to cure the CSA Breach as well as any interest charges on the sums due under the CSA Breach; |
| 2.1.2 | its obligation to cure the MSA Breach as well as any interest charges on the sums due under the MSA Breach; and |
| 2.1.3 | its obligation to order and pay for those Batches of Product which are the subject of Firm Orders and Semi-Binding Orders included in the most recent Forecast under the CSA provided by IBI to CMC. |
| 2.2 | In consideration of the waiver granted by CMC hereunder, IBI hereby releases CMC from its obligation to manufacture Batches of Product under the CSA in accordance with those Forecasts existing as of the Effective Date, provided, however, that the foregoing shall not affect CMC’s obligation to perform the Additional Services pursuant to the terms of this Agreement. |
| 2.3 | RELEASES |
| 2.3.1 | IBI warrants and represents that it has no claim, whether known or unknown, against CMC or its Affiliates in law or equity which IBI or its Affiliates, assigns and/or transferees have as of the Effective Date arising out of or in connection with the MSA or the CSA. Accordingly, IBI hereby waives, releases and discharges CMC from any claim, action, recovery or other liability existing as |
5
| of the Effective Date, whether known or unknown, against CMC or its Affiliates in law or equity which IBI or its Affiliates, assigns and/or transferees, have arising out of or in connection with the MSA and the CSA. |
| 2.3.2 | CMC warrants and represents that it has no claim, whether known or unknown, against IBI or its Affiliates in law or equity which CMC or its Affiliates, assigns and/or transferees have as of the Effective Date arising out of or in connection with the MSA or, the CSA. Accordingly, CMC hereby waives, releases and discharges IBI from any claim, action, recovery or other liability existing as of the Effective Date, whether known or unknown, against IBI or its Affiliates in law or equity which CMC or its Affiliates, assigns and/or transferees, have arising out of or in connection with the MSA and the CSA. |
| 3. | FUND PAYMENTS |
| 3.1 | The Parties acknowledge that pursuant to the provisions of clause 7 of the CSA, IBI has paid CMC the Fund comprising US$[**] US dollars) and that: |
| 3.1.1 | US$[**] US dollars) of the Fund shall be irrevocably paid to CMC for the settlement of the CSA Breach and MSA Breach (“IBI Payment”) and shall cease to be part of the Fund or subject to the CSA, and such IBI Payment shall be non-refundable and not repayable under clause 7.2.2 of the CSA or otherwise; |
| 3.1.2 | clause 7.2 of the CSA shall be amended such that Fund shall now mean a fund with an amount of US$[**] US dollars); |
| 3.1.3 | IBI shall not be obliged to replenish the Fund beyond a value of US$[**] US dollars); provided, however, that if IBI or any party who acquires all or substantially all of IBI’s rights to IB1001 (the “Acquiror”) decides to increase the Maximum Value above sixteen (16) Batches, IBI or the Acquiror must comply with Section 5.2 hereof; and, |
| 3.1.4 | IBI hereby waives and forever releases CMC from any rights IBI may have in respect of the IBI Payment. |
| 4. | FEES & PAYMENTS |
| 4.1 | Appendix 3 of the CSA shall be deleted and replaced in its entirety with Schedule 2 of this Agreement. Schedule 2 contains pricing for all Batches other than Conformance Batches, which shall be included in the payments set forth in Section 4.2 below. |
| 4.2 | The Parties acknowledge that IBI has already paid CMC the non-refundable sum of US$[**] toward the performance of the Additional Services. In addition to that payment, IBI shall pay the following amounts to CMC: |
| 4.2.1 | as consideration for CMC performing Additional Services from the Effective Date up to and including 31 January 2013 (the “Pre-pay Period”), IBI shall |
6
| make an initial payment of US$[**] US dollars) split into two equal installments as follows (i) the first payment of US$[**] US dollars) due on 30 November 2012 and (ii) a second payment of US$[**] US dollars) due on 10 December 2012; and |
| 4.2.2 | (i) US $[**] US dollars) for services provided in February 2013, to be paid prior to February 1, 2013, and (ii) US $[**] US dollars) to be paid prior to March 1, 2013, for services provided in March and April 2013 in accordance with the timeline set forth in Schedule 1-A and Schedule 1-B; provided, that the payments set forth in this Clause 4.2.2 shall only be made by IBI for each month(s) (or part thereof) beyond the Pre-pay Period during which IBI requests, in IBI’s sole discretion and in accordance with Clause 6.1.4, that CMC continue to perform the Additional Services. |
| 4.3 | In consideration of the waiver under Clause 2 and subject to the approval of the Bankruptcy Court, IBI (or any successor trustee or liquidating agent) shall pay consideration from the sale of the Combined Assets (as such term is defined in the “Motion for Order Approving Bidding Procedures in Connection with Marketing and Proposed Sale of Substantially All of Its Assets, Credit Bid Waiver Threshold, and Release of Certain Claims” dated November 5, 2012 filed in the Bankruptcy Case (“Bidding Procedures Motion”)) to CMC in accordance with the “Waterfall Distribution” attached as Exhibit C to the Bidding Procedures Motion. Specifically: |
| 4.3.1 | [**]%) percent of the aggregate of all Upfront Acquisition Payments in excess of US $[**], subject to a maximum payment of US $[**], shall be paid to CMC as set forth in the Waterfall Distribution; and, |
| 4.3.2 | [**]%) percent of the aggregate of all Acquisition Payments, subject to a maximum payment (including any payment due under Clause 4.3.1) of US $[**], shall be paid to CMC as set forth in the Waterfall Distribution (collectively the “Acquisition Consideration”). For the removal of doubt, the total Acquisition Consideration shall not exceed US$[**]. |
| 4.4 | The Acquisition Consideration as set forth in Section 4.3 above shall be paid to CMC after such sums are paid by the Successful Bidder, who will be approved by the Bankruptcy Court after the Sale Hearing (as such terms are defined in the Bidding Procedures Motion). Specifically, the amount set forth in Section 4.3.1 shall be paid to CMC immediately after payments on Priority One, Two, Three and Four Distributions are made under the Waterfall Distribution and the amount set forth in Section 4.3.2 shall be paid to CMC no later than payment on other Priority Six Distributions are made under the Waterfall Distribution. If the Waterfall Distribution is not approved by an order of the Bankruptcy Court or if no distribution is due to CMC pursuant to the Waterfall Distribution that is ultimately approved by the Bankruptcy Court, CMC shall have an allowed unsecured claim in the amount of US $10,000,000, with recourse only against the Fund. |
7
| 5. | VARIATION TO CAPACITY OBLIGATIONS |
| 5.1 | CMC’s obligations under the CSA with respect to its capacity for manufacture of Product shall be amended such that CMC shall now be required to maintain a maximum capacity of sixteen (16) Batches per full Year for the production of IB1001 and, prior to 1 January 2014, shall not be in breach of its obligations under the CSA to meet the timeline stipulated in the non-binding Forecast submitted by IBI provided that it has used Commercially Reasonable Endeavors to manufacture such Batches. Accordingly, the Parties further agree that: |
| 5.1.1 | any Forecasts in respect of Batches to commence manufacture prior to 1 January 2014 shall be non-binding and shall not, notwithstanding the provisions of the CSA, comprise Firm Orders or Semi-Binding Orders; provided, however, that if any Purchase Order issued by IBI is less than the Forecast or exceeds the Forecast by less than 20%, then the Forecast shall become binding once that Purchase Order is issued by IBI; |
| 5.1.2 | references to the maximum number of Batches in clause 5.1 of the CSA that may be requested in any Calendar Quarter shall be amended from eight (8) Batches to four (4) Batches, being two (2) per each of Line 1 Suite or Line 2 Expansion; |
| 5.1.3 | the Maximum Value definition defined in clause 5.5 of the CSA shall be amended from thirty-two (32) Batches per full Year to sixteen (16) Batches per full Year; |
| 5.1.4 | IBI shall provide a Forecast (i) for Q2 2013 by January 31, 2013, and (ii) for Q3 and Q4 2013 by March 31, 2013; and |
| 5.1.5 | The provisions of clauses 5.7.1 to 5.7.3 (inclusive) of the CSA shall cease to have effect on Forecasts for the calendar years of 2012 and 2013. |
| 5.2 | Upon request, CMC shall enter into good faith negotiations with IBI or the Acquiror to increase the Maximum Value of Batches of IB1001 that may be ordered per full Year provided that any increase in the Maximum Value shall require, in IBI or the Acquiror’s sole discretion, either (i) an additional payment of US$[**] US dollars) to the Fund or (ii) the renegotiation of the CSA, which renegotiated terms shall include a provision for an additional cash payment to CMC. |
| 6. | CMC SERVICES & MANUFACTURING OBLIGATIONS |
| 6.1 | CMC shall include the Additional Services as Services under the CSA subject to the following: |
| 6.1.1 | IBI continuing to comply with the terms of the CSA; |
| 6.1.2 | IBI continuing to comply with all of the terms of this Agreement; |
8
| 6.1.3 | the Conformance Batches shall count towards the Maximum Value; |
| 6.1.4 | in respect of Additional Services to be performed beyond the Pre-pay Period, IBI shall be required to provide CMC with at least ten (10) Business Days’ notice in advance of each additional month in which it wishes CMC to undertake the Additional Services; and |
| 6.1.5 | If IBI requests that CMC perform Additional Services beyond 30 April 2013, IBI shall pay for such Additional Services pursuant to the terms of the CSA. |
| 6.2 | Except for the Conformance Batches, which are to be paid for pursuant to Clause 4.2, prior to 1 January 2014 and notwithstanding the provisions of clause 7 of the CSA or any payment terms contained therein, IBI shall be obliged to pay CMC per Batch (as listed in Schedule 2 to this Agreement) in four (4) equal installments of twenty five percent (25%) of the price per Batch due as follows: (i) on the date on which an order for that Batch is placed; (ii) on the date the applicable vial(s) of Cell Line are proposed to be thawed by CMC to commence manufacture of the Batch, which date shall be notified to IBI by CMC; (iii) on the completion of manufacture of the Batch; and (iv) upon delivery of the Batch by CMC to IBI. After January 1, 2014, payments for Batches shall be in accordance with Clause 7 of the CSA. |
| 7. | FORECASTS & STEERING COMMITTEE |
| 7.1 | IBI shall continue to provide Forecasts during 2013 in accordance with Clause 5.1.4 above. Accordingly, the Parties hereby agree that: |
| 7.1.1 | IBI shall at all times provide Forecasts which are good faith estimates of its requirements for the numbers of Batches required in the applicable Calendar Quarter(s); and |
| 7.1.2 | all Forecasts issued in accordance with the CSA (as amended by this Agreement) that identify Batches for manufacture on or after 1 January 2014 shall be binding on the Parties pursuant to the terms of the CSA provided that any Forecasts for Q1 2014 must be provided to CMC by 30 June 2013. |
| 7.2 | The Parties hereby agree that Xxxxx Pharma S.A.S. shall be entitled to appoint a Committee Member to the Joint Steering Committee to participate in all meetings of the Joint Steering Committee (the “Ipsen Committee Member”). The Ipsen Committee Member shall only have the right to participate as an observer and shall have no voting right or right of veto. |
| 8. | FACTOR VIIA ASSETS |
| 8.1 | In consideration of the waivers under the CSA and MSA agreed to hereunder by CMC, IBI hereby: |
| 8.1.1 | licenses to CMC on an exclusive (to the exclusion of IBI, its successors, assigns and licensees), worldwide, royalty free basis, such license being freely assignable: (i) all IPR relating to the Factor VIIa Product licensed to IBI and (ii) |
9
| all IPR relating to the Factor VIIa Product owned by IBI including, but not limited to, the patents set out in Schedule 3 to this Agreement, to make, have made, use and sell the Factor VIIa Product; and |
| 8.1.2 | assigns to CMC, free of any encumbrances, all rights to (i) the Factor VIIa Cell Banks; and (ii) all other Factor VIIa Product Assets other than IPR. |
For the avoidance of doubt, IBI shall retain all rights under any and all IPR licensed to CMC pursuant to Clause 8.1.1 above to the extent such IPR is necessary or useful to make, have made, use or sell IB1001 or IBI’s other proprietary products.
| 8.2 | IBI hereby warrants and represents that: |
| 8.2.1 | IBI is free and able to grant the licence and assign the assets as set forth in clause 8.1; |
| 8.2.2 | IBI has not assigned or licensed any of the Factor VIIa Product Assets to any third party; |
| 8.2.3 | IBI shall keep confidential and not use for any purpose any of the Factor VIIa Product Assets other than to comply with its obligations under this Agreement; |
| 8.2.4 | IBI has disclosed the full terms of all licenses of IPR under which IBI receives a license in respect of the Factor VIIa Product and will disclose to CMC, within 30 days following the Effective Date and upon CMC’s request, any techniques or materials used for the manufacture of the Factor VIIa Product; |
| 8.2.5 | To IBI’s knowledge, all patent registration fees due or incurred prior to the Effective Date in respect of IPR in the Factor VIIa Product Assets have been paid; |
| 8.2.6 | IBI will use reasonable commercial efforts to obtain approval from the University of North Carolina (“UNC”), the owner of certain patents relating to the Factor VIIa Product which are licensed to IBI pursuant to the UNC License, to grant CMC the right to grant further sublicenses to the Factor VIIa Product; and |
| 8.2.7 | the UNC License will not terminate as a result of the Bankruptcy Case; provided, however, that IBI makes no representation or warranty as to whether an Acquiror would accept or reject the UNC License. |
| 8.3 | At the reasonable request of CMC and at CMC’s reasonable cost, IBI shall at all times hereafter do all such acts and execute all such documents as may reasonably be necessary or desirable to give effect to the transfer of the Factor VIIa Product Assets to CMC including, without limitation, disclosing all know-how and information required and liaising with any regulatory authorities. |
10
| 8.4 | IBI shall, upon CMC’s reasonable request and CMC’s expense (provided such costs are reasonably incurred): |
| 8.4.1 | transport and deliver to CMC or its designee the Factor VIIa Cell Banks, and transporting and storing the same in accordance with industry standards to preserve the viability and quality of the same; |
| 8.4.2 | deliver to CMC all documents, records, data, files and information in respect of the Factor VIIa Product Assets; |
| 8.4.3 | provide all documents containing know-how and information concerning the Factor VIIa Product in the possession or control of IBI (including without limitation all documents relating to any interactions with regulatory authorities); |
| 8.4.4 | provide CMC with reasonable access to IBI’s Head of Regulatory and procure his co-operation and assistance with CMC as may reasonably be required to help transfer knowledge and regulatory information concerning the Factor VIIa Product and the transfer of the Factor VIIa Product Assets; |
| 8.4.5 | notify CMC of all individuals, contractors, service providers and consultants who have worked on the development of the Factor VIIa Product with or on behalf of IBI and provide their professional contact details, to the extent IBI is in possession of such contact details. |
| 8.5 | CMC shall: |
| 8.5.1 | use diligent efforts to pursue development of and/or exploit the Factor VIIa Product, provided, however, that such diligence obligation shall be subject to CMC first receiving the right (as a sublicensee of IBI) to grant further sublicenses under the IPR associated with the Factor VIIa Product (such right to be procured for CMC by IBI pursuant to Clause 8.2.6 of this Agreement on terms consistent with those under the UNC License). CMC shall be free to develop or exploit any other factor VIIa product; |
| 8.5.2 | be responsible for all expenses associated with the IPR not already incurred by IBI at the Effective Date, and shall reimburse IBI for any such expenses associated with the IPR that continue to be incurred by IBI after the Effective Date. In addition, CMC has the option, at its sole discretion, to pay the outstanding expenses incurred by or on behalf of IBI in relation to such IPR prior to the Effective Date, subject to IBI first informing CMC of the value of such expenses; |
| 8.5.3 | be entitled to approach UNC to obtain a direct license from UNC in respect of the IPR associated with the Factor VIIa Product currently licensed to IBI by UNC (and sublicensed to CMC pursuant to Clause 8.1.1(i) hereof), which license shall be consistent with the sublicense grant set forth in Clause 8.1.1 hereof; provided, however, that CMC shall approach UNC in coordination with |
11
| IBI and IBI shall be present for all discussions (except for discussions of the economic terms of a direct license between UNC and CMC if and when UNC, CMC and IBI agree that a direct license between CMC and UNC is in the best interest of all three parties). |
| 8.6 | CMC acknowledges the disclaimer of warranty and limitation on UNC’s liability as set forth in Article 10 of the UNC License. |
| 8.7 | At any time after the Effective Date, if CMC intends to seek a partner to purchase and/or exclusively license the Factor VIIa Product Assets for the purposes of development and commercialisation of the Factor VIIa Product in Europe and/or the United States, then, subject to Clause 8.9, CMC shall so notify IBI and observe the following terms (“ROFR”): |
| 8.7.1 | CMC shall notify IBI in writing of its intent to sell and/or license the Factor VIIa Product Assets in the United States and/or Europe (“ROFR Notice”); |
| 8.7.2 | If IBI wishes to acquire the Factor VIIa Product Assets for the territory identified in the ROFR Notice, IBI shall serve written notice on CMC within fifteen (15) Business Days of receipt the ROFR Notice (“ROFR Acceptance”), receipt being deemed two (2) Business Days after sending where sent by pre-paid international courier; |
| 8.7.3 | If no ROFR Acceptance is served within the fifteen (15) Business Day period, then CMC shall be automatically released from the provisions of this Clause 8.7 and this Clause 8.7 shall cease thereafter to apply in respect of the territory that was the subject of the ROFR Notice unless the ROFR Notice was only with respect to either Europe or the United States; |
| 8.7.4 | If a ROFR Acceptance is served within the 15 Business Day period, then: |
| 8.7.4.1 | CMC and IBI shall, in good faith and at arm’s length, exclusively negotiate terms for the license or purchase of the Factor VIIa Product Assets for the development and commercialization of the Factor VIIa Product in the territory identified in the ROFR Notice, which terms shall be based on fair market value; |
| 8.7.4.2 | if a definitive agreement between the Parties cannot be consummated within ninety (90) days of the ROFR Acceptance (or such longer period as may be agreed between the Parties), CMC shall have no further obligation to negotiate with IBI under this Clause 8.7 and this Clause 8.7 shall cease thereafter to apply except as set forth in Clause 8.7.4.3 below; and |
| 8.7.4.3 | if CMC ultimately does not sell or license the Factor VIIa Product Assets within six (6) months from the end of the ninety (90) days following the ROFR Acceptance, the ROFR shall be reinstated. |
12
| 8.7.5 | Nothing in this Agreement shall prevent CMC from entering into development or subcontracting agreements with third parties concerning the Factor VIIa Product Assets nor from holding discussions with prospective partners for commercialisation rights to the Factor VIIa Product Assets for commercialisation of Factor VIIa Product in the United States and/or Europe, provided, however, that CMC observes the provisions of Clauses 8.7 and 8.8 hereof and that any such agreements are subject to Clauses 8.7 and 8.8 hereof. |
| 8.8 | At any time after the Effective Date, if IBI wishes to acquire from CMC the Factor VIIa Product Assets for the purposes of development and commercialisation of the Factor VIIa Product in Europe and the United States, then, subject to Clause 8.9, IBI shall so notify CMC in writing (“IBI Notice”) and the Parties shall observe the following terms (“IBI ROFR”): |
| 8.8.1 | CMC shall, in good faith, respond to IBI with a draft term sheet within forty-five (45) days of the date CMC receives the IBI Notice, setting out the terms on which CMC is prepared to assign or license those Factor VIIa Product Assets to IBI for development and commercialisation of the Factor VIIa Product for the United States and Europe; |
| 8.8.2 | Upon receipt of that term sheet: |
| 8.8.2.1 | CMC and IBI shall, in good faith and at arms length, exclusively negotiate terms for the license or purchase of the Factor VIIa Product Assets for the development and commercialization of the Factor VIIa Product in Europe and the United States, which terms shall be based on fair market value; |
| 8.8.2.2 | if a definitive binding agreement between the Parties cannot be consumated within ninety (90) days of the date the term sheet is provided by CMC (or such longer period as may be agreed between the Parties), CMC shall have no further obligation to negotiate with IBI under this Clause 8.8 and this Clause 8.8 shall cease thereafter to apply except as set forth in Clause 8.8.2.3 below; and |
| 8.8.2.3 | if CMC ultimately does not sell or license the Factor VIIa Product Assets within six (6) months from the end of the ninety (90) days following the date the term sheet is provided by CMC, the IBI ROFR shall be reinstated. |
| 8.9 | The ROFR and the IBI ROFR set forth in Clauses 8.7 and 8.8 hereof shall not apply (or otherwise restrict CMC) where CMC or any of its Affiliates elects to sell the Factor VIIa Product to third parties (or partner with them) for the purpose of developing a chemically modified version of the Factor VIIa Product, or any other factor VIIa product, which chemical modifications would affect the pharmacokinetic profile of the Factor VIIa Product. |
13
| 9. | OTHER AMENDMENTS TO THE CSA |
| 9.1 | The address for IBI under clause 19.10 of the CSA shall be amended to One Xxxxxxx Square, Building 1400E, Xxxxxxxxx, XX 00000, Xxxxxx Xxxxxx of America. |
| 10. | AGREEMENT |
| 10.1 | Save for the amendments to the CSA as expressly provided in this Agreement and the specific waivers under the CSA and MSA, no other amendments are made to the CSA or waivers given in respect of the CSA or MSA and all other terms and conditions of the CSA and MSA will remain in full force and effect and are not affected, varied or amended by this Agreement. |
| 10.2 | The CSA and MSA shall be subject to the provisions of this Agreement and where there is any conflict between the provisions of this Agreement and the CSA or MSA, the provisions of this Agreement shall prevail. |
| 11. | MISCELLANEOUS |
| 11.1 | Other than as expressly set out herein, no Party has relied upon any statement, representation, warranty, understanding, undertaking, promise or assurance in entering into this Agreement and no warranties, representations, covenants or guarantees express or implied are given, made or renewed by entering into this Agreement. |
| 11.2 | This Agreement may be executed in any number of counterparts, each of which will be an original, but all of which together will constitute one and the same instrument. The Agreement is not effective until each Party has executed at least one counterpart and the Bankruptcy Court has issued an order as set forth below in Section 11.8. |
| 11.3 | Any specific rights or remedies conferred on the Parties under this Agreement are in addition to and without prejudice to all other rights and remedies which any such Party may have available to it against the other or otherwise. |
| 11.4 | Nothing in this Agreement shall create or be deemed to create a partnership, joint venture or principal-agent relationship between the parties and no Party shall have authority to bind the other in any way. |
| 11.5 | No provision of this Agreement (or any document entered into in connection with this Agreement) shall be modified or varied without the written consent of the Parties. For the avoidance of doubt, no modification or variation of this Agreement shall be valid if made by email or fax, provided, however, that binding, executed amendments to this Agreement may be exchanged in PDF format. |
| 11.6 | Any notices to be served pursuant to this Agreement shall be valid only if served in accordance with the notice provisions of the CSA (as amended by this Agreement). |
| 11.7 | Section 19.6 of the CSA shall be amended to provide that IBI may, upon providing advance written notice to CMC, assign its rights (subject to the assignee also assuming and |
14
| accepting IBI’s obligations) under the MSA, the CSA, this Agreement and all amendments to the foregoing documents only to an Acquiror. Notwithstanding the foregoing, (i) no assignment shall relieve any Party of responsibility for the performance of any obligation that accrued prior to the effective date of such assignment and (ii) this Agreement and its terms shall be binding on its successors and assigns and IBI shall procure that this Agreement and its terms and effect shall be binding upon its successors and assigns. |
| 11.8 | This Agreement shall not be effective until approved by a final order of the Bankruptcy Court pursuant to Bankruptcy Code Sections 105, 363, and 365 and Rule 9019 of the Federal Rules of Bankruptcy Procedure, which order shall be satisfactory in form and substance to CMC. IBI shall seek approval of this Agreement by the Bankruptcy Court on an expedited basis. |
| 12. | GOVERNING LAW |
This Agreement shall be interpreted and governed, and all rights and obligations of the Parties shall be determined, in accordance with the laws of the State of Delaware (regardless of choice of law provisions). The Parties waive application of the provisions of the 1980 U.N. Convention on Contracts for the International Sale of Goods, as amended.
15
SCHEDULE 1-A
Amendment 1 to the CSA
AMENDMENT 1
TO COMMERCIAL SUPPLY (MANUFACTURING SERVICES) AGREEMENT
BETWEEN
CMC ICOS BIOLOGICS, INC. (“CMC ICOS”) AND INSPIRATION
BIOPHARMAEUTICALS, INC. (“Inspiration”)
dated June 17, 2011 (the “Effective Date”)
Date of Amendment: 29 Sept. 2012
WHEREAS, CMC ICOS and Inspiration entered into a Commercial Supply (Manufacturing Services) Agreement, effective June 17, 2011, as amended (the “Agreement”); and
WHEREAS, by executing this Amendment Inspiration makes a commitment to pay for Manufacturing Capacity and other Services, and CMC ICOS reserves such Manufacturing Capacity for Inspiration; and
WHEREAS, CMC ICOS and Inspiration have executed this Amendment for the purpose of setting forth such terms and conditions.
NOW, THEREFORE, the Parties agree as follows:
Any defined term used herein that is not defined herein has the definition ascribed to such term in the Agreement.
| I. | Product |
“Product” means Recombinant Human Blood Coagulation Factor IX
| II | Scope of Services |
Attached as Appendix A
| III. | Timing |
Attached as Appendix B
| IV. | Price and Payment Terms |
Attached as Appendix C
| Amendment 1 | - 1 - | Confidential |
IN WITNESS WHEREOF, this Amendment No. 1 has been executed by the Parties hereto through their duly authorized officers as of the Effective Date.
| INSPIRATION BIOPHARMACEUTICALS, INC. | CMC ICOS BIOLOGICS, INC. | |||||||
| By: | /s/ Xxxx Xxxxxxx |
By: | /s/ [Illegible] | |||||
| Xxxx Xxxxxxx | ||||||||
| Date: 29 Sept. 2012 | Date: Oct. 11, 2012 | |||||||
| Amendment 1 | - 2 - | Confidential |
FACTOR IX
APPENDIX A
SCOPE OF SERVICES
Objective
The objective of this amendment is to develop, implement and validate a process operation to reduce the levels of HCP present in the Factor IX API. This amendment includes three parts:
| Stage 1: | Bench scale development, optimization and characterization of a new process operation |
| Stage 2: | Method validation/re-validation to support new in-process testing |
| Stage 3: | Implementation and Validation of new process operation into GMP manufacturing |
Assumptions
| • | The activities shown in the attached timeline will be re-set (day-for-day) when work on HCP reduction is re-started upon the signature of this amendment |
| • | If in the course of development and implementation the scope of work deviates significantly from the scope outlined below, a new amendment will be prepared or the work will be charged as out-of-scope |
| • | Out-of-scope work associated with HCP reduction and not covered as part of this amendment or covered in a superseding amendment will be charged at $XXX/hr |
| • | The goal of the work is to develop a new downstream process that results in: |
| • | A process that results in API with HCP of less than approximately 50 ng/mg |
| • | No impact to product quality as measured by API release assays |
| • | Minimum impact to yield and validated state of process |
| • | The immunoblot will not be run at CMC and will not be used for product release |
| • | Silver Stain gels will be considered characterization assays; these methods will not be validated |
| Amendment 1 | - 3 - | Confidential |
Stage 1 – Bench scale development, optimization and characterization of a new process operation
Objective
The objective of this Stage is to develop a modified downstream process that results in:
| • | A process that results in API with HCP of less than approximately 50 ng/mg |
| • | No impact to product quality as measured by API release assays |
| • | Minimum impact to yield and validated state of process |
After development of the modified process, a bench scale model will be qualified and the modified process characterized
Prerequisites and Assumptions
| • | Work that has already been completed is summarized in the Work Scope below |
| • | It is assumed that the modified downstream process (called A03) that will be implemented and validated will include the implementation of a new HIC column that will be operated in bind-elute mode with the elution being a step elution |
| • | If the modified downstream process requires additional changes beyond the HIC column, development of these additional changes will be considered out-of-scope |
| • | If additional Characterization Studies not outlined below are required the studies will be considered out-of-scope |
| • | See attached timeline and associated assumptions for outline of timing of execution of this work (Appendix B) |
Scope of Work
| • | The Scope outlined below is a high level non-inclusive summary of work that has been completed to-date |
| • | Explore options for reducing HCP level through exploratory bench scale development work including: |
| • | Harvest Clarification (filtration): Reduction of HMW impurities with a modified clarification filter train. Specifically, replace CUNO filters with Millipore X0HC. |
| • | Hydrophobic Interaction Chromatography and Membrane technology: Flow through mode of operation for removal of HMW impurities, with high product yield (>90%) and ease of incorporation into the process |
| • | Tangential Flow Filtration: Size-based separation of F90 from HMW impurities using TFF with High product yield (>90%). |
| • | Process Optimization for the HIC membrane based on preliminary work. |
| • | Evaluate alternate salts and salt concentrations for promoting HIC binding |
| • | Determine appropriate membrane/filter capacity |
| • | Execute TFF experiments subsequent to HIC filtration to ensure salt solution does not negatively impact product during formulation step |
| • | Column Chromatography Alternate Collection/ Pooling |
| • | Evaluate alternate elution collection or pooling strategies on the three chromatography columns |
| Amendment 1 | - 4 - | Confidential |
| • | Combine alternate collection or pooling strategies with and without the HIC membrane |
| • | Evaluate revised wash steps for IEX1 and IEX3 |
| • | Evaluate additional wash steps for IEX1 and determine impact on HCP levels in IEX1 eluate |
| • | Develop new IEX3 wash strategy: |
| • | Load column in absence of calcium |
| • | Perform salt wash |
| • | Elute Factor IX with Calcium |
| • | Determine impact to HCP levels in eluate and confirm no product quality impact |
| • | Evaluation of Different Separation Technologies |
| • | Compare advantages of resins vs. membranes, and flowthrough vs. elution strategies |
| • | Screen three alternate HIC ligands (one phenyl and two butyl) |
| • | Further evaluate phenyl resin and one butyl resin in bind-elute mode |
| • | Evaluate impact of different salt types, and salt concentrations on column performance |
| • | Evaluate gradient vs. step elution options |
| • | Based on development work completed to-date, the modified process is assumed to consist of the implementation of a HIC column that will be operated in bind-elute mode with the elution being a step elution. Using this base new process, complete one full bench scale run (the “pre demo run”) where the modified process is executed from start-to-finish (IEX1 through TFF) |
| • | The Scope outlined below represents the remaining bench scale work required to complete development, characterization, implementation and validation of the modified process |
| • | Unit Operation optimization and Process Lock-in |
| • | Perform series of DOE studies on the new HIC column unit operation |
| • | Optimize operation to minimize yield loss and maximize HCP removal |
| • | Evaluate loading |
| • | Evaluate salt concentration used for step elution |
| • | Evaluate peak collection criteria |
| • | Confirm no impact to product quality |
| • | Bench Scale Demonstration Runs |
| • | Execute four bench scale demonstration runs under the optimized process conditions both prior to and during at-scale process validation runs. Latter demonstration runs are included to evaluate other feedstreams and/or to provide linkage between bench scale and at-scale operations |
| • | Demonstration runs will start at IEX1 or, if material is available, will start at the HIC step using material from IEX3 collection pool |
| • | The bench scale demonstration runs will consist of the new HIC column, followed by UFDF. The runs will be conducted at the setpoints or selected range limits which were determined by process development and which are transferred to manufacturing for scale up. |
| Amendment 1 | - 5 - | Confidential |
| • | Bench Scale Model Qualification |
| • | Develop and approve Bench Scale Model Qualification Protocol for the HIC step |
| • | Three runs will be performed in DPD with the same setpoints as used at-scale |
| • | Material for the Qualification will come from at-scale manufacturing (e.g., IEX3 collection pool) |
| • | Draft and approve Bench Scale Model Qualification Report |
| • | Bench Scale Process Characterization |
| • | Develop and approve Characterization Protocol |
| • | Using the Qualified Bench Scale Model, perform Process Characterization studies on the bench scale using a DOE strategy for new HIC column unit operation |
| • | Characterization parameters are to-be-determined but may include: |
| • | Column loading |
| • | Salt concentration |
| • | pH |
| • | Full and fractional factorial designs will be considered |
| • | Characterization studies will include analysis of HCP levels as well as product quality analysis via SE-HPLC, RP-HPLC and N-linked glycan analysis |
| • | Draft and approve Characterization Summary Report summarizing results of process characterization. The approved Executive Summary of this report is a required prerequisite for at-scale Process Validation |
| • | Stability Validation of New High Salt Feedstreams (HIC Load & Eluate) |
| • | HIC load and elution samples from representative small-scale or large scale runs will be evaluated for stability against aggregation and/degradation |
| • | Data will be used to support intermediate hold times in Manufacturing |
| • | Resin Cleaning and Lifetime Validation (15 cycles) |
| • | An HIC cycling study, plus blank runs, will be performed to validate resin cleaning and reuse |
| • | Data will be used to validate maximum number of cycles for Manufacturing |
| • | Viral Clearance Validation |
| • | The HIC step will not be validated as a virus reduction step |
| • | Viral clearance of the nano-filtration step will be re-validated for the new high salt feedstream |
Deliverables
| • | Bench Scale Model Qualification Protocol (client approval not required) |
| • | Bench Scale Model Qualification Report |
| • | Bench Scale Characterization Protocol (client approval not required) |
| • | Bench Scale Characterization Report |
| Amendment 1 | - 6 - | Confidential |
Stage 2 – Method validation/re-validation to support new in-process testing
Objective
The objective of this Stage is to validate and/or re-validate all analytical methods required to support the modified downstream process
Prerequisites and Assumptions
| • | It is assumed that the modified downstream process (called A03) that will be implemented and validated will include the implementation of a new HIC column that will be operated in bind-elute mode with the elution being a step elution |
| • | If additional methods not outlined below need development, qualification and/or validation the work will be considered out-of-scope of this Amendment |
Scope of Work
| • | Re-validate HCP test method TME-0488 (F90 specific HCP ELISA) with additional background matrices to enable testing of HCP levels for HIC eluates |
| • | Develop a sample preparation procedure for samples in a high salt matrix so that they are suitable for assay in the HCP assay |
| • | Validate the HCP assay for use at the HIC step |
| • | Re-validate the assay for the BDS (API) step; this is required to validate new dilutions that are necessary in order to assay for the lower HCP levels that are achieved in the new downstream process |
| • | Re-validate bioburden test method with additional background matrices to enable testing of bioburden levels for HIC eluates |
| • | Re-validate endotoxin test method with additional background matrices to enable testing of endotoxin levels for HIC eluates |
| Amendment 1 | - 7 - | Confidential |
Stage 3 – Implementation and Validation of new process operation into GMP manufacturing
Objective
The objective of this Stage is to implement the modified downstream process into at-scale manufacturing, initiate GMP manufacturing and complete Process Validation
Prerequisites and Assumptions
| • | The initial at-scale implementation of the modified downstream process included the implementation of a HIC flow-through membrane filter (Engineering run 12-20) and the combination of the HIC membrane filter with a reduced pooling of IEX3 fractions (Engineering run 12-30). These runs demonstrated that the HCP levels in the API would not meet the targeted level of 50 ng/mg. Thus, it is assumed the HIC membrane filter will not be part of the modified process |
| • | It is assumed that the modified downstream process (called A03) that will be implemented and validated will include the implementation of a new HIC column that will be operated in bind-elute mode with the elution being a step elution |
| • | Prerequisites for the initiation (thaw) of a GMP batch are outlined in the Scope of Work below |
| • | Prerequisites for the initiation (thaw) of the Process Validation campaign are outlined in the Scope of Work below |
| • | It is assumed that data only from Line A will be used to validate the modified process. The validation of Line B is not within the scope of this amendment |
| • | See attached timeline and associated assumptions for outline of timing of execution of this work (Appendix B) |
Scope of Work
| • | Implement HIC membrane flow-through filter into at-scale Engineering run (note: this work was completed as part of Engineering Run 12-20 and Engineering Run 12-30) |
| • | Develop draft batch records & operational implementation plan |
| • | Executed Engineering Run 12-20: Implement HIC filter into downstream process |
| • | Executed Engineer Run 12-30: Combine reduced fraction pooling from IEX3 with the implementation of the HIC filter |
| • | Performed parallel scale-down studies on the bench scale |
| • | Performed analytics for load, flow through and strip (ELISA and SDS-PAGE) and shipped material for immunoblot |
| • | Implement modified downstream process developed on the Bench Scale into at-scale Engineering run |
| • | The batch price for the Engineering batches are not within the scope of this amendment |
| • | Develop draft batch records and operational implementation plan for the implementation of a modified wash step for IEX3. Batch records have been developed but the process change will not be implemented |
| Amendment 1 | - 8 - | Confidential |
| • | Develop draft batch records and operational implementation plan for the implementation of a new HIC column that will be operated in bind-elute mode with the elution being a step elution |
| • | Execute Engineering Batch |
| • | Perform the standard set of analytics on the Engineering Batch that is normally performed during GMP manufacturing |
| • | Execute additional Engineering Batches until the initiation of GMP manufacturing |
| • | If the modified downstream process requires changes prior to initiation of GMP manufacturing, the implementation of the additional changes will be considered out-of-scope |
| • | Implement modified downstream process for GMP manufacturing |
| • | The following are prerequisites prior to thaw for a GMP manufacturing batch: |
| • | QST revised and approved |
| • | A minimum of one bench-scale demonstration run completed where the modified process is executed in entirety on the bench scale and the results are back from the analytical analysis (minimally, testing of the API for HCP, SDS-PAGE, SE-HPLC, RP-HPLC, and N-linked glycans) |
| • | Master Batch Records (MBRs) may be approved in a rolling fashion |
| • | Materials are on-site and released |
| • | Test Methods approved and qualified for API and IPC to support specifications |
| • | In absence of a complete set of data back from a bench scale run, and at the sole discretion of the CMC Quality Assurance Department, a thaw to initiate a GMP run may occur under the following scenario: |
| • | At least one bench-scale demonstration run has been completed with the analytical results pending |
| • | Prior to thaw of the GMP batch, a Change Request (CR) will be initiated for the new downstream process. The CR will include key acceptance criteria that must be achieved by the time of the inoculation of the 750 L bioreactor. If the criteria are not met, the batch will be terminated. The CR content will be at CMC discretion and the acceptance criteria for moving forward will be the sole discretion of the CMC quality department |
| • | Approve the MBRs for GMP production |
| • | Approve the QST |
| • | Execute GMP batch (note: the batch price is not included as part of this amendment) |
| • | Perform standard GMP testing on the GMP batch |
| • | Perform at-scale Validation |
| • | The following are prerequisites prior to thaw for Process Validation: |
| • | All GMP Lot prerequisites listed above |
| • | A minimum of one GMP at-scale successful run with analytical results back |
| • | Characterization executive summary report approved with key content including: |
| • | Summarize process changes for modified process |
| Amendment 1 | - 9 - | Confidential |
| • | Captures previous risk assessments for reduction of HCP |
| • | Summarizes bench and at-scale development |
| • | Process risk assessment completed/approved g assess impact of changes for modified process to the validated state and supporting studies |
| • | PVMP approved |
| • | CPPs/CQAs redefined g update upstream and downstream PERs (or equivalent summary document) |
| • | PV protocols approved |
| • | All MBRs approved prior to thaw (no rolling approval) |
| • | The documentation and Validation strategy is shown in the figure below |
| • | Items shown in grey are not part of the scope of this amendment |
| • | The numbers list the order of prioritization for the documents |
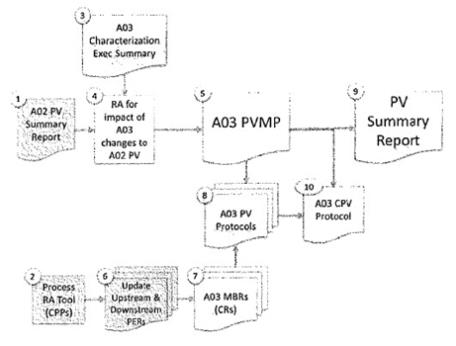
| • | Complete documentation and Validation strategy as outlined in the Figure above |
| • | A03 Characterization Executive Summary (#3 above) |
| • | High-level summary document that outlines |
| • | Process changes for modified process |
| • | Captures previous risk assessments for reduction of HCP |
| • | Bench and at-scale development |
| • | Risk Assessment for Impact of A03 Changes to A02 Process Validation (#4 above) |
| • | Draft and approve Risk Assessment on Unit Operation by Unit Operation basis |
| • | Risk Assessment will guide PVMP and scope of Validation |
| • | A03 Process Validation Master Plan (PVMP, #5 above) |
| • | Draft and approve PVMP for the A03 process |
| Amendment 1 | - 10 - | Confidential |
| • | PVMP will require a minimum of three at-scale batches to be completed |
| • | A03 MBRs (#7 above) |
| • | Revise and approve MBRs |
| • | A03 Process Validation Protocols (#8 above) |
| • | Draft and approve Validation Protocols on a Unit Operation basis required to validate the A03 process |
| • | PV Summary Report (#9 above) |
| • | Will be completed after successful execution of at-scale validation batches |
| • | Draft and approve PV summary report. Completion of this report will document the completion of the at-scale Process Validation of the A03 process |
| • | A03 CPV Protocol (#10 above) |
| • | Update and approve the new Continued Process Validation Protocol to support the A03 Process |
| • | Execute three at-scale validation batches per the strategy outlined in the PVMP |
| • | Price of the validation batches is not included in the scope of this amendment |
| • | Complete Process Validation protocols for all required Unit Operations |
| • | Perform analytical testing as outlined in the PV protocols and MBRs |
Deliverables
| • | Documentation as outlined above |
Estimated Timeline
| • | At-scale validation is anticipated to start in December 2012 |
| Amendment 1 | - 11 - | Confidential |
FACTOR IX
APPENDIX B
TIMING
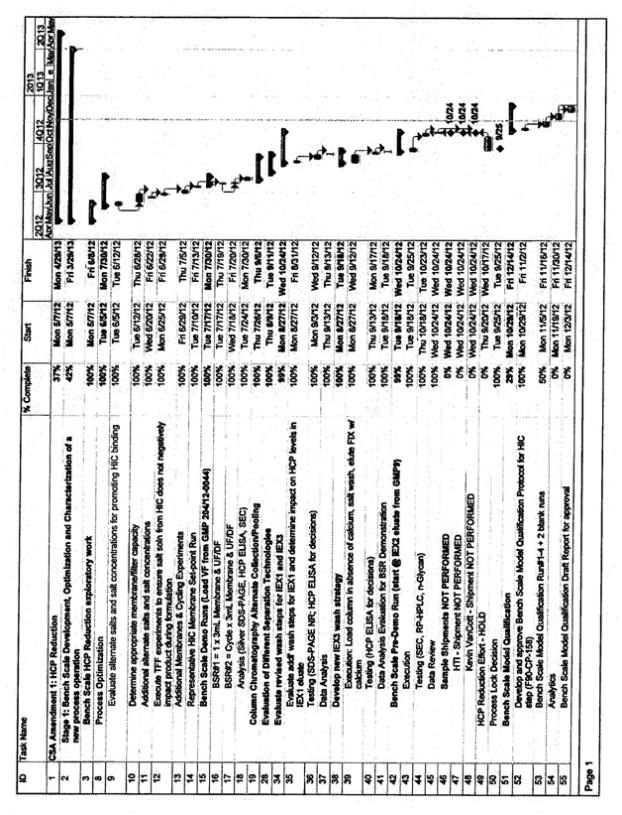
See attached timeline
Assumptions to Attached Timeline
| • | Adherence to timeline and deliverables will be managed per the terms outlined in the Commercial Supply Agreement (CSA) |
| • | CMC will provide a weekly timeline update to Inspiration. This will include an update for the tasks for percent completion, actual start dates and actual/target dates for completion |
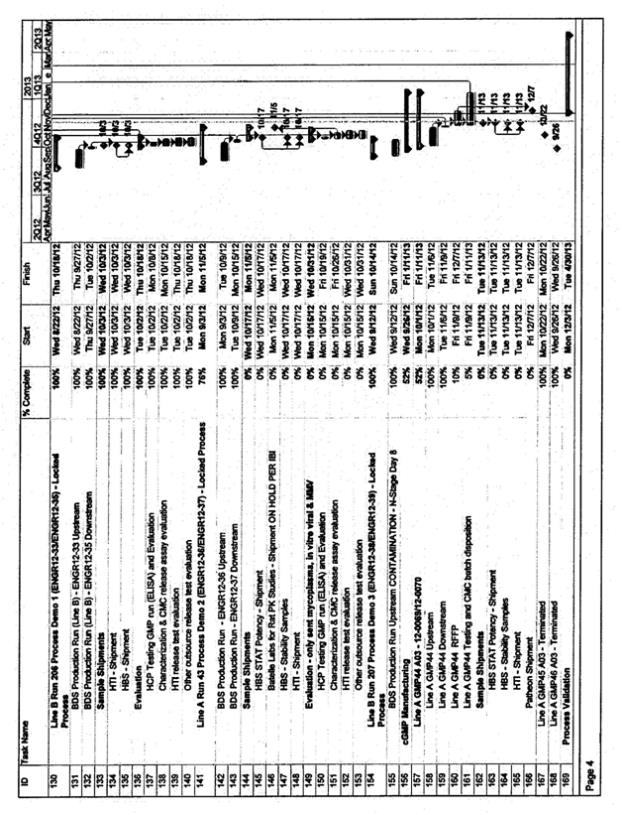
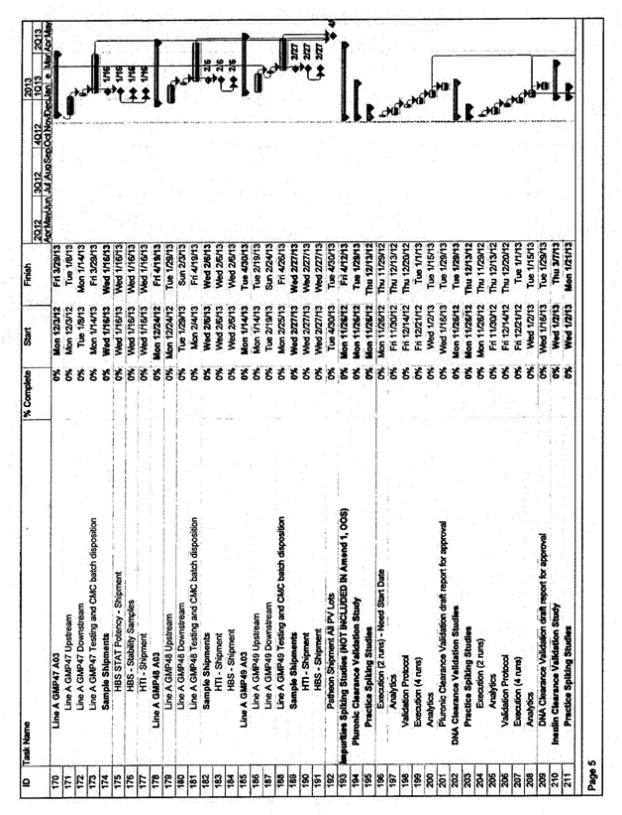
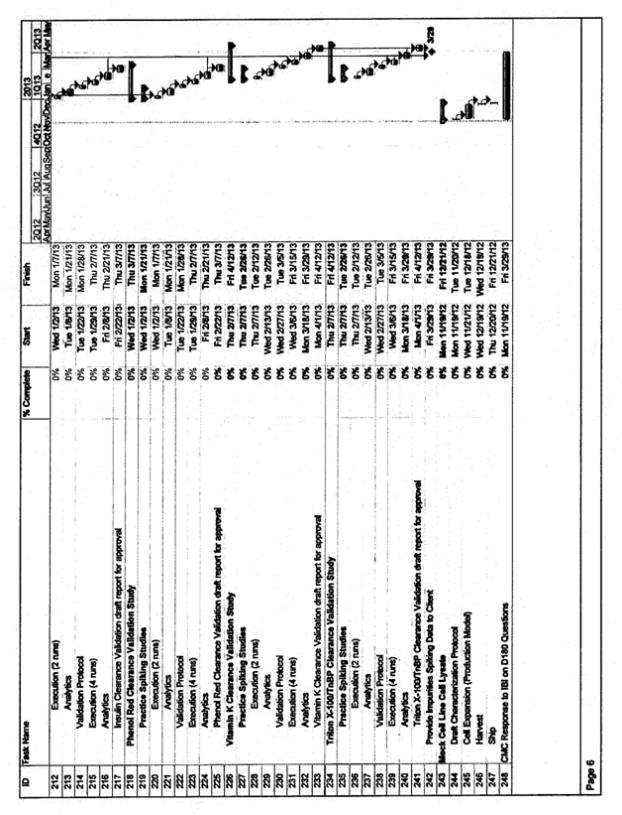
| • | The timeline for activities supporting at-scale validation, including bench scale model qualification, bench scale characterization, viral clearance, resin lifetime validation, and stability validation will need finalization. This work will need to be prioritized against other deliverables associated with the MAA and BLA filings. CMC and Inspiration will prioritize and finalize the timing for these activities after signature of this amendment. The target date for completing the prioritization is 14 days after signature of the amendment |
| • | The attached timeline assumes that the Validation campaign will occur in Line A and occur in manufacturing slots already reserved by Inspiration. Inspiration has requested evaluation of an option for two Inspiration manufacturing batches scheduled in Line B in the December 2012 to January 2013 timeframe be eliminated so as to execute batches only in Line A. This would provide the ability to compress the timing for the Process Validation/manufacturing batches in Line A and thus enables a more timely completion of the Process Validation batches and subsequent reports. Such a change would have financial considerations as well as implications for the Line B validation and approval plans. These implications and considerations are not subject of this amendment and will need to be resolved per the terms of the relevant amendments and the CSA. The timeline compression options will be evaluated and agreed to by both parties after signature of this amendment. The target date for completing this agreement is 14 days after signature of the amendment |
| • | The attached timeline for Validation is based on the process knowledge known to-date. Delays (due to contract negotiations, delay in the re-start of the HCP reduction work, technical challenges, or change in plans for the process to be implemented) will impact the proposed timeline |
| Amendment 1 | - 12 - | Confidential |
FACTOR IX
APPENDIX C
PRICE AND PAYMENT TERMS
| Stage |
Services |
Price and Payment Terms for Stage | ||||
| [**] | ||||||
| Stage 1 | Bench scale development, optimization and characterization of a new process operation |
• |
[**]% payable upon initiation of Stage | |||
|
• |
[**]% payable upon completion of Stage | |||||
|
[**] | ||||||
| Stage 2 | Method validation/re-validation to support new in-process testing | •
|
[**]% payable upon initiation of Stage
| |||
| • | [**]% payable upon completion of Stage | |||||
|
[**] | ||||||
| Stage 3 | Implementation and Validation of new process operation into GMP manufacturing | •
|
[**]% payable upon initiation of Stage
| |||
| • | [**]% payable upon completion of Stage | |||||
Note
| • | Final payment for each stage (the [**]% due upon completion) is contingent upon IBI approving stage deliverables |
Additional Costs
In addition to the costs outlined above:
| • | Materials. |
| • | Development General Materials’ costs shall be covered by a flat [**] percent ([**]%) fee applied to the price of the applicable stage. For clarity, Development General Materials include all the elementary chemicals and laboratory raw materials that are typically required in the process of biological development and used outside of the cGMP area (including, but not limited to, kits, reagents, tubing, single-use bags, pipettes, salts, etc). Development General Material’ costs may be invoiced to Inspiration up to sixty (60) days in advance of the commencement of the applicable Stage. |
| • | Development Specific Materials include specific raw materials that are unique to the Inspiration’s project and will be necessary for work performed outside of CMC ICOS’ cGMP areas. Development Specific Materials shall be invoiced at the vendor’s list price plus a handling charge of [**]%. An initial estimated invoice may be sent to Inspiration sixty (60) calendar days prior to start of the relevant Stage. A complete detailed invoice setting out any additional payment required by Inspiration or that a credit i Inspiration shall be sent on completion of the Stage. |
| Amendment 1 | - 13 - | Confidential |
| • | A General Manufacturing Consumables Fee of $[**] will be invoiced in advance of all Manufacturing Stages. |
| • | Manufacturing Materials listed on the Xxxx of Materials shall be invoiced at the vendor’s list price plus a handling charge of [**]%. An initial estimated invoice will be sent to Inspiration sixty (60) calendar days prior to start of the relevant Stage. A complete detailed invoice setting out any additional payment required by Inspiration or that a credit is due to Inspiration shall be sent on completion of the Stage. |
| • | External testing and other external costs will be invoiced as specified in the Agreement, i.e. at vendor’s list price plus a handling charge of [**] percent ([**]%). |
| • | CMC ICOS will invoice Inspiration on a monthly basis for any packing, shipping and handling charges (handling charges are $500/domestic and $1000/international shipment). |
| • | Necessary travel and related costs will be passed through to Inspiration and will be consistent with CMC ICOS’ internal travel policy. |
| • | Out of scope work under this Amendment No. 1 to the Agreement, if requested by Inspiration and agreed to by Inspiration and CMC ICOS Biologics in writing via a Change Order, will be invoiced at $[**] per FTE hour. |
| Amendment 1 | - 14 - | Confidential |
SCHEDULE 1-B
Amendment 2 to the CSA
AMENDMENT 2
TO COMMERCIAL SUPPLY (MANUFACTURING SERVICES) AGREEMENT
BETWEEN
CMC ICOS BIOLOGICS, INC. (“CMC ICOS”) AND INSPIRATION
BIOPHARMACEUTICALS, INC. (“Inspiration”)
dated June 17, 2011 (the “Effective Date”)
Date of Amendment: , 2012
WHEREAS, CMC ICOS and Inspiration entered into a Commercial Supply (Manufacturing Services) Agreement, effective June 17, 2011, as amended (the “Agreement”); and
WHEREAS, by executing this Amendment Inspiration makes a commitment to pay for Manufacturing Capacity and other Services, and CMC ICOS reserves such Manufacturing Capacity for Inspiration; and
WHEREAS, CMC ICOS and Inspiration have executed this Amendment for the purpose of setting forth such terms and conditions.
NOW, THEREFORE, the Parties agree as follows:
Any defined term used herein that is not defined herein has the definition ascribed to such term in the Agreement.
| I. | Product |
“Product” means Recombinant Human Blood Coagulation Factor IX
| II. | Scope of Services |
Attached as Appendix A
| III. | Timing |
Attached as Appendix B
| IV. | Price and Payment Terms |
Attached as Appendix C
IN WITNESS WHEREOF, this Amendment No. 1 has been executed by the Parties hereto through their duly authorized officers as of the Effective Date.
| INSPIRATION BIOPHARMACEUTICALS, INC. | CMC ICOS BIOLOGICS, INC. | |||||||
| By: | /s/ [Illegible] |
By: |
| |||||
| Date: | 11/20/12 | Date: |
| |||||
FACTOR IX
APPENDIX A
SCOPE OF SERVICES
Objective
The objective of this amendment is to define the additional activities that will be executed at CMC in support of the process validation and regulatory filings for Factor IX. This work is outlined below.
Assumptions
| • | The attached timeline summarizes activities outlined in this work statement. The timeline shows some activities extending into April 2013 and beyond (e.g., lot release, completion and approval of final reports, etc.). However, the timeline is built around the assumption that CMC and IBI will work to deliver all necessary information (e.g., process validation data, data from bench scale work, draft reports, etc.) in time to support a complete response to the EMA due at the end of April 2013. The target date for the completion/generation of this work is the end of March 2013 in order to enable sufficient time to generate the response for the EMA. |
| • | Many of the activities outlined below will require material (e.g., harvest fluid, column eluates, etc) taken from an at-scale manufacturing run. Thus, material taken from the conformance run(s) will be a pre-requisite for these activities |
| • | If in the course of execution of this Amendment the scope of work deviates significantly from the scope outlined below, a new amendment will be prepared |
| • | CMC will support sample shipment to domestic locations only |
Scope of Work
Resin Cleaning and Lifetime Validation
| • | Execute bench scale resin and cleaning and lifetime validation for the new HIC chromatography step |
| • | Develop validation protocol (target approximately 15 cycles) |
| • | Execute HIC cycling study plus blank runs, on the bench scale; data will be used to validate maximum number of cycles for Manufacturing |
| • | Summarize work in Validation Report |
Viral Clearance Validation
| • | The HIC step will not be validated as a virus reduction step |
| • | Viral clearance of the nano-filtration step will be re-validated for the new high salt feedstream |
| • | Develop validation protocol |
| • | Execute virus reduction studies at CMC and third party vendor |
| • | Summarize results in Validation report |
Impurities Spiking Studies
| • | Scope of work and estimates are based on draft version of VAL-30180-01, F90 Process Related Impurity Clearance Validation Plan. Changes to the scope may affect effort and duration estimates. |
| • | The scope of impurity clearance is limited to the seven process-related impurities that were part of the F90 process validation protocols: Triton X-100, tri-n-butyl phosphate (TnBP), phenol red, insulin, vitamin K, Pluronic F-68, and CHO host cell DNA |
| • | Protocols will be written for process-related impurity clearance. Clearance for each impurity will be a combination of results from at-scale GMP runs and small-scale spiking runs. The use of at-scale GMP runs vs. small scale runs is determined by whether spiking studies are necessary to demonstrate impurity clearance. |
| • | At least three runs will be performed or assayed for each impurity. Spiked runs involving a chromatography column will be followed by an unspiked run to check for any column carryover. The exception to the above description is DNA spiking, for which small-scale spiking studies will be performed on all three column chromatography steps to ascertain total process clearance capacity. Selected impurities may be spiked simultaneously if preliminary experiments indicate there is no interference with either elution profiles or with assay results. |
| • | Impurity clearance report(s) will summarize the total clearance capacity (i.e. validated impurity clearance capacity of all tested steps in the F90 purification process) for each process-related impurity. |
Day 120 Commitments
| • | Redefine process control strategy |
| • | Develop project plan for change to definitions of CPPs, CQAs |
| • | Perform modified risk assessments for each unit operation and all parameters under current process control strategy |
| • | Revise PERs (PJRs and PRAs will not be revised) |
| • | Revise CPV protocol to set new baseline for parameters |
| • | Revise CPV summary report template |
| • | Additional activities required for each F90 batch |
| • | Data entry and verification for CPV |
| • | Additional assays from revised QST |
Generation of Material for HCP ELISA Method Development
| • | Execute bench scale bioreactor/spinner process using mock cell line under approved protocol |
| • | Collect cells from bioreactor/spinner and generate cell lysate per protocol |
| • | Ship cell lysate material to support development of next generation ELISA |
FACTOR IX
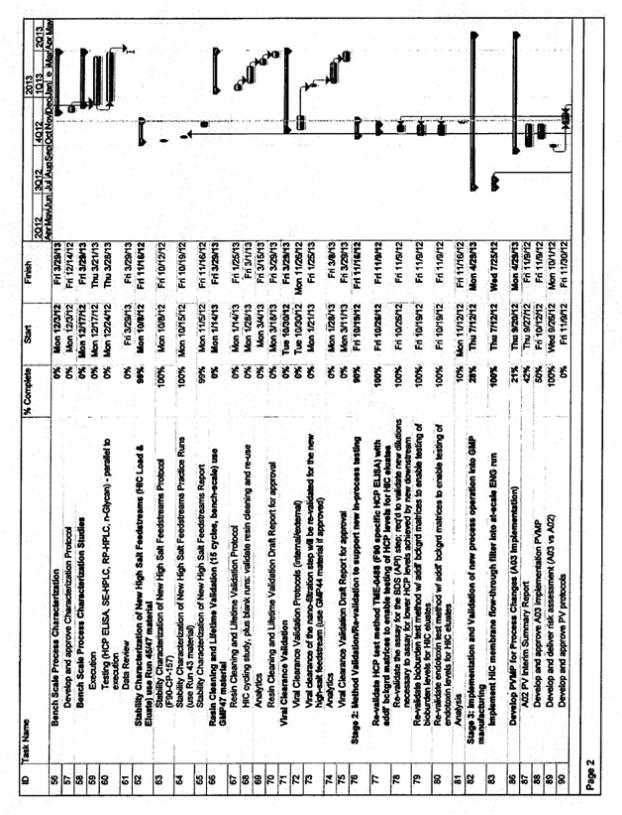
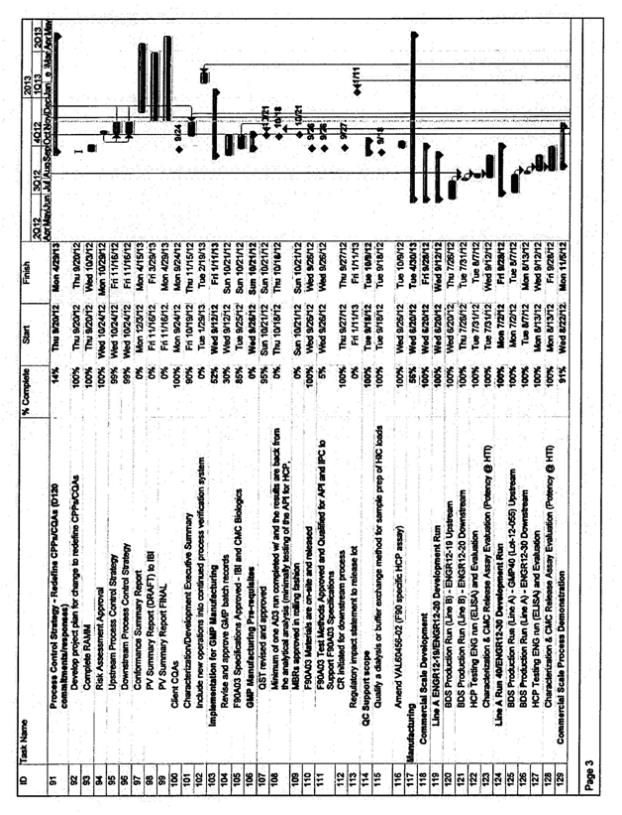
APPENDIX B
TIMING
See attached timeline
Assumptions to Attached Timeline
| • | CMC will provide a regular timeline updates. This will include an update for the tasks for percent completion, actual start dates and actual/target dates for completion |
| • | The attached timeline assumes that the Validation campaign will occur in Line A and occur in manufacturing slots already reserved by Inspiration. |
| • | The attached timeline for Validation is based on the process knowledge known to-date. |
FACTOR IX
APPENDIX C
PRICE AND PAYMENT TERMS
| • | IBI will pay CMC per the following terms: |
| • | $[**] due February 1, 2013 |
| • | $[**] due March 1, 2013 |
SCHEDULE 2
Price per Batch
APPENDIX THREE
Price
Note: all prices are subject to an annual price index increase
Price per Batch up until 31 December 2015
Shall be US $[**] per Batch delivered up to and including 31 December 2015. This amount is exclusive of the cost of raw materials.
Price per Batch from 1 January 2016 until 31 December 2016
Shall be US $[**] per Batch. This amount is exclusive of the cost of raw materials.
Should capacity for manufacture of Product be increased beyond sixteen (16) Batches per Year in accordance with this Agreement, CMC and IBI may, in good faith, consider pricing for Batches in 2016 to be calculated according to the following formula rather than US $[**] but in the absence of agreement the price per Batch shall remain at US $[**] per Batch.
Potential alternative pricing terms (not including materials and supplies and resins):
First five (5) million IU per Batch supplied at $[**]/IU
Next five (5) million IU per Batch supplied at $[**]/IU
Next five (5) million IU per Batch supplied at $[**]/IU
All IU per Batch in excess of fifteen (15) million IU supplied at $[**]/IU
Price per Additional Batch
Shall be determined according to CMC’s then current list price per batch, which as of the Effective Date is US$[**]
Payment Terms (for all other monies due under this Agreement for which payment terms are not expressly identified in the Agreement)
| • | 30 days net of invoice date. |
SCHEDULE 3
IPR Associated with Factor VIIa Product
Inspiration Factor VII Patent Portfolio 11-15-2012
| EWP Ref No. |
Ref No. |
Reference source |
Application No. / Patent No. |
ATTY REF NO. |
Country |
Filing Date |
Note | |||||||
| METHOD OF PRODUCING RECOMBINANT VITAMIN K DEPENDENT PROTEINS (Inspiration-UNC Co-Owned) | ||||||||||||||
| 90160P(311835) | INSPL024PR | Inspiration | 61/256,802 | US PROV | 30/10/2009 | EXPIRED | ||||||||
| 90160WO(311835) | INSPI.024WO | Inspiration | PCT/US2010/054581 | PCT | 28/10/2010 | PUBLISHED Nationalized | ||||||||
| 90160US(311835) | INSPI.024NP | Inspiration | 13/459,743 | US | 30/04/2012 | Published | ||||||||
| 90160EP(311835) | INSPI.024WO | Inspiration | 10827499.4 | EP | 10/28/2010 (PCT date) | Published | ||||||||
| 90160JP(311835) | TBD | Inspiration | awaiting | JP | 10/28/2010 (PCT date) | Pending | ||||||||
| RECOMBINANT VITAMIN K DEPENDENT PROTEINS WITH HIGH SIALIC ACID CONTENT AND METHODS OF PREPARING SAME (Inspiration UNC co-Owned) | ||||||||||||||
| 90207(311835) | INSPI.008NP | Inspiration | 12/597,456 | US | 23/10/2009 | PUBLISHED | ||||||||
| 90207P(311835) | INSPI.008PR | Inspiration | 607914,281 | US PROV | 26/04/2007 | CLOSED | ||||||||
| 90207WO(311835) | INSPI.008VPC | Inspiration | PCT/US2008/061822 | PCT | 28/04/2008 | EXPIRED | ||||||||
| 90207AU(311835) | INSPI.008VAU | Inspiration | 2008245524 | AU | 28/04/2008 | Pending | ||||||||
| 90207CA(311835) | INSPI.008VCA | Inspiration | 2683423 | CA | 28/04/2008 | Pending | ||||||||
| 90207EP(311835) | INSPI.008VEP | Inspiration | 8747060.5 | EP | 28/04/2008 | Pending | ||||||||
| 90207JP(311835) | INSPI.008VJP | Inspiration | 2010-506563 | JP | 28/04/2008 | PUBLISHED- pending | ||||||||
| 90207DIVEP (311835) | Inspiration | 12170422.5 | EP | 28/04/2012 | Pending- published | |||||||||
| CORRELATION OF SNPS IN THE VITAMIN K EPOXIDE REDUCTASE GENE AND WARFARIN DOSAGE (METHODS AND COMPOSITIONS FOR VITAMIN K EPOXIDE REDUCTASE) (UNC) | ||||||||||||||
| 0024(311835) | 04-0007 | UNC | 60/505,000 | 0000-000 PR | US PROV | 23/09/2003 | EXPIRED | |||||||
1
| EWP Ref No. |
Ref No. |
Reference source |
Application No. / Patent No. |
ATTY REF NO. |
Country |
Filing Date |
Note | |||||||
| 0024(311835) | 04-0007 | UNC | PCT/US04/031481 | 5470-401WO | PCTY | 23/09/2004 | EXPIRED | |||||||
| 0024(311835) | 04-0007 | UNC | 10/573,131 7,687,233 |
5470-401 | US | 18/04/2006 | ALLOWED | |||||||
| 0024(311835) | 04-0007 | UNC | 11/516,229 7,524,665 |
5470-401CT | US CON | 06/09/2006 | EXPIRES 09/23/2024 | |||||||
| 0024(311835) | 04-0007 | UNC | 11/699,930 7,482,141 |
5470-401CT2 | US CON | 30/01/2007 | EXPIRES 09/23/2024 | |||||||
| 0024(311835) | 04-0007 | UNC | 11/787,072 7,645,602 |
5470-401P | US CIP | 13/04/2007 | EXPIRES 09/23/2024 | |||||||
| 0024(311835) | 04-0007 | UNC | 12/612,154 | 5470-401PCT2 | US CON | 04/11/2009 | PENDING | |||||||
| 0024(311835) | 04-0007 | UNC | 2004275828 | 5470-401AU | AU | 23/09/2004 | PENDING | |||||||
| 0024(311835) | 04-0007 | UNC | 2539434 | 5470-401CA | CA | 23/09/2004 | PENDING | |||||||
| 0024(311835) | 04-0007 | UNC | 4789039.7 | 5470-401EP | EP | 23/09/2004 | PENDING | |||||||
| 0024(311835) | 04-0007 | UNC | 7109353.8 | 5470-401EP2 | EP DIV | 23/09/2004 | PENDING OPPOSITION | |||||||
| 0024(311835) | 04-0007 | UNC | 2006528251 | 5470-401JP | JP | 23/09/2004 | PENDING | |||||||
| 0024(311835) | 04-0007 | UNC | 61140030 | 5470-401HK | HK | 23/09/2004 | PENDING | |||||||
| 0024(311835) | 04-0007 | UNC | 81036847 | 5470-401HK2 | HK DIV | 23/09/2004 | PENDING | |||||||
| 0024(311835) | 04-0007 | UNC | 11156979.4 | 5470-401EP4 | EP DIV | 23/09/2004 | ||||||||
| VITAMIN K-DEPENDENT CARBOXYLASE (UNC) | ||||||||||||||
| 0026(311835) | 90-62 | UNC | 07/697,427 | 5470-24 | US | 08/05/1991 | ABANDONED | |||||||
| 0026(311835) | 90-62 | UNC | 07/756,250 5,268,275 |
5470-34 | US | 09/09/1991 | EXPIRED 12/7/2010 | |||||||
2
| EWP Ref No. |
Ref No. |
Reference source |
Application No. / Patent No. |
ATTY REF NO. |
Country |
Filing Date |
Note | |||||||
| 0026(311835) | 90-62 | UNC | PCT/US92/003853 | 5470-34.1WO | PCT | 08/05/1992 | EXPIRED | |||||||
| 0026(311835) | 90-62 | UNC | 2102702 | 5470.34.4CA | CA | 08/05/1992 | EXPIRES 05/08/2012 | |||||||
| 0026(311835) | 90-62 | UNC | 92913019.3 0587742 |
5470-34.2EP | EP | 18/05/1992 | EXPIRES 05/08/2012 | |||||||
| 0026(311835) | 90-62 | UNC | 92913019.3 692325441 |
5470-34.2DE | DE | 08/05/1992 | EXPIRES 05/08/2012 | |||||||
| 0026(311835) | 90-62 | UNC | 92913019.3 587742 |
5470-34.2DK | DK | 08/05/1992 | EXPIRES 05/08/2012 | |||||||
| 0026(311835) | 90-62 | UNC | 92913019.3 587742 |
5470-34.2FR | FR | 08/05/1992 | EXPIRES 05/08/2012 | |||||||
| 0026(311835) | 90-62 | UNC | 92913019.3 587742 |
5470-34.2GB | GB | 08/05/1992 | EXPIRES 05/08/2012 | |||||||
| 0026(311835) | 90-62 | UNC | 92913019.3 587742 |
5470-34.2ES | SE | 08/05/1992 | EXPIRES 05/08/2012 | |||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2AT | AT | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2BE | BE | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2CH | CH | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2ES | ES | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2GR | GR | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2IT | IT | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2XX | XX | 08/05/1992 | |||||||||
3
| EWP Ref No. |
Ref No. |
Reference source |
Application No. / Patent No. |
ATTY REF NO. |
Country |
Filing Date |
Note | |||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2XX | XX | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2MC | MC | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2NL | NL | 08/05/1992 | |||||||||
| 0026(311835) | 90-62 | UNC | 5470-34.2AU | AU | 08/05/1992 | |||||||||
| CHIMERIC FACTOR VII MOLECULES (UNC) | ||||||||||||||
| 0031(311835) | 08-0047 | UNC | 61/220,278 | 5400-000 | XX PROV | 25/06/2009 | EXPIRED | |||||||
| 0031(311835) | 08-0047 | UNC | PCT/US10/039934 WO 10/151736 |
5470-494WO | PCT | 25/06/2010 | EXPIRED | |||||||
| 0031(311835) | 08-0047 | UNC | 5470-494AU | AU | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 08-0047 | UNC | 5470-494BR | BR | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 08-0047 | UNC | 5470-494CA | CA | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 08-0047 | UNC | 5470-494CN | CN | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 08-0047 | UNC | 5470-494EP | EP | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 08-0047 | UNC | 5470-494IN | IN | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 04-0047 | UNC | 5470-494JP | JP | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 04-0047 | UNC | 5470-494RU | RU | 25/06/2010 | N/A | ||||||||
| 0031(311835) | 04-0047 | UNC | 12/823,382 | US | N/A | |||||||||
| 0031(311835) | 04-0047 | UNC | 13/097,609 (co-owned with Inspiration) | 5470-494IP | US | 29/04/2011 | N/A | |||||||
4
THIS AGREEMENT has been executed and delivered by or on behalf of the Parties on the date at the top of page 1.
| EXECUTED by CMC ICOS BIOLOGICS, Inc. acting by two directors or a director and the secretary |
) ) ) ) |
/s/ Xxxxx Xxxxxxxx XXXXX XXXXXXXX Director
/s/ Xxxxxxx Xxxxxxx Xxxxxxx Xxxxxxx Director/Secretary | ||
| EXECUTED by INSPIRATION BIOPHARMACEUTICALS, Inc. |
) ) |
/s/ [Illegible] Chief Executive Officer | ||
| Amendment to the CSA agreed to by CMC Luxembourg S.a.r.l | ||||
| EXECUTED by CMC LUXEMBOURG S.a.r.l. acting by two directors or a director and the secretary |
) ) ) ) |
/s/ Xxxxx Xxxxxxxx XXXXX XXXXXXXX Director
/s/ Glads Laostsev GLADS LAOSTSEV Director/Secretary | ||

