COLLABORATION AGREEMENT
| 1. | THE PARTIES |
| This Agreement is made effective November 15, 2007 (the “Effective Date”) by and between Ceres, Inc., a Delaware corporation with principal offices at 0000 Xxxxxx Xxxxxx Xxxx., Xxxxxxxx Xxxx, XX 00000, hereinafter “Ceres,” and the Institute of Crop Sciences of the Chinese Academy of Agricultural Sciences, 00 Xxxxx Xxxxxxxxxxxxx Xxxxxx, Xxxxxxx Xxxxxxxx, Xxxxxxx 000000, People’s Republic of China, hereinafter “ICS.” |
| THE PARTIES HERETO COVENANT AND AGREE AS FOLLOWS: |
| 2. | PROGRAM |
| 2.1 | Ceres will deliver to ICS material and information as described in ANNEX I (jointly referred to as the “Ceres Material”). ICS will inform Ceres of and obtain any permits or authorizations and proceed to any notifications which are required to import the Ceres Material in China and transfer it to the premises of ICS. Ceres will only deliver the Ceres Material after receipt of written notice from ICS that all required permits, authorizations and notifications have been obtained, which notice will include a copy of any such permits, authorizations and/or notifications. Upon receipt of a delivery of Ceres Material, ICS will sign and return the Receipt Form enclosed with the Ceres Material to Ceres, attn: Mr. Chuan-xxx Xx. | ||
| 2.2 | ICS agrees to perform the activities defined in ANNEX I which shall be referred to hereinafter as the “Program.” For all activities, ICS shall strictly comply with the standard operating protocols referred to hereinafter as “SOPs”, which will be provided by Ceres or jointly agreed upon by the Parties. Further, ICS shall strictly comply with any Stewardship Guidelines which may be provided by Ceres. The Stewardship Guidelines applicable as of the Effective Date are in ANNEX IV. Ceres may provide written updated from time to time. The Program includes provision of defined deliverables to Ceres. | ||
| 2.3 | In performing the Program activities ICS undertakes to comply with the work plan and time schedule set forth in ANNEX I and with all applicable laws, rules and regulations. Program activities will be performed by employees to be hired by ICS or under ICS’ responsibility, by its Authorized Subcontractors in accordance with Article 5. ICS will make available all laboratory, greenhouse, and field space and all relevant equipment and supplies required to perform the Program, except that certain chemicals and supplies not available in China will be provided by Ceres (delivery conditions of Article 2.1 to apply), all as defined in ANNEX I. | ||
| 2.4 | ICS undertakes not to make any use of the Ceres Material which is not strictly necessary for performing the Program activities in accordance with ANNEX I. |
Page 1 of 47
| 2.5 | The Ceres Material will be used only at premises under the control of ICS or its Authorized Subcontractors, located at Beijing, Hainan or Nanjing. | ||
| 2.6 | Upon termination of the Program, and except to the extent a different agreement is reached pursuant to Article 7.4, ICS will destroy any remaining Ceres Material and any material and information obtained under the Program, and will send Ceres forthwith an attestation of such destruction. | ||
| 2.7 | ICS shall have the right, subject to compliance with all the conditions stated hereinafter, to subcontract certain parts of the Program activities under its own responsibility to the third parties identified hereinafter or otherwise agreed to in writing by Ceres (“Authorized Subcontractors”). | ||
| South China University of Tropical Agriculture Chinese Academy of Tropical Agriculture Sciences Xxxxxxx, Xxxxxx 000000 Xxxxx Xxxxxx Xxxxx, PhD, Professor, Xxxx |
|||
| National Key Laboratory for Crop Genetics and Germplasm Enhancement Xxxxxxx Xxxxxxxxxxxx Xxxxxxxxxx Xxxxxxx 000000 Xxxxx Xxxxxxx Xxx, PhD, Professor |
|||
| The following terms and conditions shall apply to ICS’ Authorized Subcontractors: (a) the Authorized Subcontractors shall perform defined activities on behalf and for the benefit of ICS in exchange for a fee or other tangible consideration; (b) the Authorized Subcontractors shall deliver all the results of the Authorized Subcontractors’ activities under the subcontract to ICS and Ceres only, and assign ownership of any inventions made during the performance of the subcontracting activities to Ceres or to ICS for further assignment to Ceres, without any further remuneration; (c) the Authorized Subcontractors shall not have the right to use any results, whether information or material, for any purpose whatsoever other than the performance of the subcontract; (d) the Authorized Subcontractors shall be bound by confidentiality obligations consistent with the terms and obligations of this Agreement and shall be bound to comply with all SOP’s, Stewardship Guidelines, laws, rules and regulations that govern their activities; and (e) ICS will provide to Ceres a draft copy of any subcontract ICS is planning to conclude at least fifteen (15) days before the scheduled signature date and will provide a true copy of any subcontract to Ceres within thirty (30) days of its execution. |
| 3. | REPORTS |
| 3.1 | During the entire Program, ICS and Ceres will participate in monthly conference calls and provide detailed quarterly reports regarding Program progress, discoveries made and results obtained during the implementation of the Program, |
Page 2 of 47
| management, tracking and data transfer. The type of information to be included in the reports and formatting is set forth in ANNEX I and ANNEX II. Authorized Subcontractors may participate in conference calls as appropriate. The reports provided by ICS will include the same information regarding Authorized Subcontractors’ activities as for ICS’ own activities. | |||
| 3.2 | During the entire Program, ICS will promptly communicate to Ceres any information on the Program activities or the results obtained or observed that Ceres may ask. Between reports, ICS will also spontaneously communicate to Ceres any unexpected observations or results. |
| 4. | REMUNERATION. PAYMENT |
| 4.1 | Ceres will remunerate ICS as set forth in ANNEX I for the implementation of the Program, in accordance with the payment schedule set forth in such annex and the detailed Program budget in ANNEX III. | ||
| 4.2 | The payments will be made on the dates set forth in the payment schedule in ANNEX I provided that Ceres has received a corresponding invoice from ICS (detailing the actual resources used by ICS during the applicable period) at least thirty (30) days in advance, by bank transfer to ICS’s account mentioned in its invoice. |
| 5. | PROGRAM STAFFING |
| 5.1 | ICS will hire employees with the qualifications set forth in ANNEX I, to perform the Program activities on a full-time basis. Upon mutual agreement of the Parties, one or more of said employees may be hired by Authorized Subcontractors instead of ICS. | ||
| 5.2 | More detailed qualifications and criteria for hiring and evaluating the performance of the employees referred to in Article 5.1 will be determined by mutual agreement. Ceres will have the right to participate in the interview and performance review process and a joint decision will be made on each person to be hired. | ||
| 5.3 | Subject to the prior written agreement of both Parties on a case-by-case basis, ICS graduate students may be involved in certain Program activities. | ||
| 5.4 | ICS will provide quarterly updates to the Collaboration Committee on anticipated personnel changes at ICS and its Authorized Subcontractors to the extent relevant for the Program. |
| 6. | EXCHANGE OF SCIENTISTS |
| 6.1 | From time to time, ICS employees who are scientists working on Program activities may perform such activities temporarily at Ceres’ premises in Thousand Oaks, California, U.S.A. (“Visiting Scientists”). |
Page 3 of 47
| 6.2 | The Parties will mutually agree on each Visiting Scientist and the dates of his/her stay at Ceres. Not more than one (1) Visiting Scientist will be at Ceres at any given time, and the duration of each stay will not exceed three (3) months. | ||
| 6.3 | ICS shall bear the entire cost related to its Visiting Scientists, including but not limited to salary, other allowances and benefits, social security, housing, meals and travel, and shall be responsible for obtaining all necessary permits or documents required for the stay and work abroad. However, for practical purposes certain of such costs, to be mutually agreed, may be paid by Ceres and deducted from payments due to ICS pursuant to this Agreement. Such costs to be deducted shall be agreed to in writing by the Parties and properly documented. | ||
| 6.4 | ICS shall take out appropriate insurance for its Visiting Scientists, including but not limited to health insurance, travel insurance and insurance for damages to third parties and Ceres. | ||
| 6.5 | Visiting Scientists shall be subject to all internal rules applicable at Ceres when working there and they will sign appropriate agreements prepared by Ceres to that effect, including an amendment to their Agreement on Confidentiality and Assignment of Inventions. Their access to Ceres’ premises shall be restricted to the normal working hours applicable there. Their attendance of internal company seminars and meeting shall be subject to prior approval of Ceres’ Chief Scientific Officer. | ||
| 6.6 | Visiting Scientists shall have no access to any Ceres technology apart from the technology which is used in the Program. |
| 7. | COLLABORATION COMMITTEE |
| 7.1 | The Parties shall establish a Collaboration Committee to oversee the implementation of the Program. The Collaboration Committee will consist of four (4) members, each Party to appoint two (2) members. The Collaboration Committee will meet monthly by teleconference and quarterly at ICS. | ||
| 7.2 | The responsibilities of the Collaboration Committee will be as follows: |
| 7.2.1 | Reviewing progress of work relative to timelines and deliverables. | ||
| 7.2.2 | Implementation and maintenance of process flows, SOPs, Stewardship Guidelines and key performance indicators referred to hereinafter as “KPIs”. | ||
| 7.2.3 | Recommending to the Parties actions as necessary to ensure successful implementation of the Program. | ||
| 7.2.4 | Managing resources within the Program. | ||
| 7.2.5 | Discussing field trials and advancement of selected genes. |
Page 4 of 47
| 7.2.6 | Recommending to the Parties corrective measures as appropriate with respect to compliance shortfalls or failures. | ||
| 7.2.7 | Referring issues to designated senior technical and/or business officers of Ceres and ICS where resolution is not obtainable. |
| 7.3 | Any decision or recommendation of the Collaboration Committee will require the consent of at least one (1) of the representatives appointed by either Party. |
| 8. | OWNERSHIP. INTELLECTUAL PROPERTY RIGHTS. EXPLOITATION |
| 8.1 | This Agreement does not bring any change to the ownership and intellectual property rights relating to the Ceres Material. ICS acknowledges that the Ceres Material may be covered by patents or patent applications of Ceres. | ||
| 8.2 | Ceres will exclusively own any material, information, data, technology, or other findings or inventions resulting from the Program (hereinafter “Results”). Subject to Article 8.6, Ceres will have the exclusive rights to protect any of the Results through patents or similar protection, and any intellectual property rights arising therefrom will belong exclusively to Ceres. If ICS is prevented by law or otherwise from assigning ownership or protecting all rights in the Results as contemplated by this Article 8.2, ICS shall take all steps necessary to give effect to this clause, including but without limitation procuring that its employees, consultants, Authorized Subcontractors, agents or otherwise shall take all steps necessary to give effect to this clause. | ||
| 8.3 | In case ICS or an Authorized Subcontractor has made or contributed to any invention forming part of the Results, ICS shall promptly inform Ceres thereof, giving detailed information in a confidential written report. ICS shall, to the extent that rights do not automatically vest in Ceres by virtue of the forgoing, assign its rights in any such invention to Ceres. ICS shall render such assistance as may be required for assigning any rights ICS inventors may have in such invention to Ceres or Ceres’ designee and for protecting such invention, including but not limited to the signing of documents. Any inventor of ICS will be recognized in patent applications on an invention which is part of the Results in accordance with U.S. patent law. If ICS is prevented by law or otherwise from assigning its rights in inventions to Ceres as contemplated by Article 8.2, ICS hereby grants Ceres all licenses, to the fullest extent possible, to effectuate the intent of this Agreement that Ceres be assigned such inventions, and to otherwise effectuate the purposes of this Agreement. ICS will cause its Authorized Subcontractors to comply with the provisions of Articles 8.1 through 8.3 as if they were ICS. | ||
| 8.4 | Subject to Article 8.5, Ceres will have the exclusive right to commercialize any of the Results. | ||
| 8.5 | At any time during the term of this Agreement, if ICS is interested in commercializing a selected Result or Results in rice in China, ICS will notify Ceres thereof in writing. Subject to any obligations of Ceres vis-à-vis third |
Page 5 of 47
| parties at the time of such notification, Ceres will negotiate with ICS in good faith about the grant by Ceres to ICS of a royalty-bearing license to commercialize such selected Result(s) in rice in China and/or potential collaborative business approaches for the commercialization of such selected Result(s) in rice in China. The terms and conditions of such agreement may include the filing by ICS of patent applications or other intellectual property rights on selected Results in China. If no such agreement is signed six (6) months after the aforementioned notification by ICS, neither Party shall have any obligation to enter into such an agreement. |
| 9. | CONFIDENTIALITY |
| 9.1 | ICS will treat any and all information and material communicated or transferred to it by Ceres pursuant to this Agreement (including but not limited to the Ceres Material) and any Results as strictly confidential and will not use the same for any purpose other than as expressly allowed by this Agreement nor disclose or transfer the same to any third party other than its employees or other collaborators or Authorized Subcontractors (or graduate students, if decided by the Parties jointly) necessary to carry out the Program and bound by appropriate secrecy and non-use undertakings. | ||
| 9.2 | The obligations of ICS in Article 9.1 shall not apply to any information, material or Results which ICS can demonstrate by competent evidence: |
| - | were lawfully in ICS’s possession without restriction prior to disclosure by Ceres, as evidenced by written records of ICS; | ||
| - | were in the public domain prior to disclosure by Ceres, or subsequently comes in the public domain through no fault, action or omission of ICS; or | ||
| - | are made available to ICS, without any restriction, by a third party who had the right to do so. |
| 9.3 | If ICS wishes to disclose certain information relating to the Program to Chinese government authorities with a view to obtaining a grant, ICS will inform Ceres thereof, and the Parties will jointly define the information to be submitted to such authorities. To the extent feasible under applicable law, ICS will make a reasonable effort to obtain a protective order or other confidential treatment requiring that the information so disclosed be used only for the purposes for which disclosure was made. | ||
| 9.4 | ICS will require all individuals performing Program activities to sign a confidentiality agreement with Ceres, as per the model agreement in ANNEX V. |
| 10. | PUBLICATIONS |
| 10.1 | “Publication” and “Publish” shall include any discussion with or presentation to a third party, other than parties referred to in Article 9.1. Examples of Publications include, without limitation: presentation at a conference, submission for |
Page 6 of 47
| publication to a journal, submission of joint proposals, posting information on a website, posters, abstracts, Ph.D. dissertations, and informal oral discussions. | |||
| 10.2 | Subject to Ceres prior written approval, which will not unreasonably be withheld, ICS may proceed to Publication of selected Results provided that: (i) no confidential information of Ceres is revealed thereby, (ii) ICS shall take into account any suggestions which may be formulated by Ceres, and (iii) at least thirty (30) days prior to the submission to a publisher or presentation to any third party, ICS delivers copies of the proposed Publication to Ceres for review. At Ceres’ request, ICS shall, for a reasonable period up to ninety (90) days from initial delivery to Ceres, delay revealing any patentable subject matter in the disclosure in order to permit the filing of patent applications. In any Publication, the Parties shall consider joint authorship and acknowledge the contributions and publications of the other as scientifically appropriate. |
| 11. | WARRANTIES. LIMITED LIABILITY |
| 11.1 | ICS acknowledges that the Ceres Material is of an experimental nature and will take all reasonable precautions to prevent any damage or injury by the Ceres Material. ICS will use its best efforts to prevent any theft or unauthorized use of the Ceres Material or any Results. | ||
| 11.2 | ICS warrants that the Ceres Material will exclusively and restrictedly be used under suitable containment conditions, and in accordance with all applicable regulations, SOP’s and Stewardship Guidelines, and it will not be used on human subjects. ICS will be responsible for obtaining any authorizations or permits or proceeding to any notifications which may be required for its activities with the Ceres Material. | ||
| 11.3 | ICS warrants that its Authorized Subcontractors will comply with the terms and conditions of this Agreement. | ||
| 11.4 | Ceres declines any liability for any damage which may be caused by the Ceres Material or the Program activities to ICS or any Authorized Subcontractor or any third party. If Ceres is held to indemnify a third party for any damage related to the conduct of the Program activities, ICS will indemnify and hold Ceres harmless for the amounts paid by Ceres to such third party. | ||
| 11.5 | Neither Party shall be liable for indirect, special, remote, incidental or consequential damages or loss of profit in connection with this Agreement or its implementation. |
| 12. | GENERAL CONDITIONS |
| 12.1 | Amendments: This Agreement, including its annexes, may only be amended by a written document signed by duly authorized representatives of the Parties. | ||
| 12.2 | Ambiguities: In case of ambiguity between this Agreement and its annexes, the contents of this Agreement shall prevail. |
Page 7 of 47
| 12.3 | Number of copies: This Agreement including its annexes is being made in two (2) copies, one for each Party. | ||
| 12.4 | Assignment / Subcontracts: Ceres has concluded this Agreement with ICS in view of ICS’s specific qualifications and ICS shall not have the right to assign any of its rights or obligations under this Agreement nor to sub-contract any part of the Program activities to any third party, except with the prior written approval of Ceres as provided in Article 2.7 on Authorized Subcontractors. Ceres has the right to assign its rights and obligations under this Agreement to any third party. Further, Ceres has the right to entrust the implementation of all or part of its obligations under this Agreement to any of its affiliates. | ||
| 12.5 | Invalid provisions: If any provision of this Agreement is or becomes invalid, is ruled illegal by a court of competent jurisdiction or is deemed unenforceable under the current applicable law from time to time in effect during the term of this Agreement, the remainder of this Agreement will not be affected or impaired thereby and will continue to be construed to the maximum extent permitted by law. In lieu of each provision which is invalid, illegal or unenforceable, there will be substituted or added as part of this Agreement by mutual written agreement of the Parties, a provision which will be as similar as possible in economic and business objectives as intended by the Parties to such invalid, illegal or unenforceable provision, but will be valid, legal and enforceable. | ||
| 12.6 | Equitable Remedies: It is understood and agreed that money damages would not be a sufficient remedy for any breach of this Agreement by ICS and that Ceres is entitled to equitable relief, including injunction and specific performance, as a remedy for any such breach. Such remedies shall not be deemed to be the exclusive remedies for a breach by ICS of this Agreement but shall be in addition to all other remedies available at law or equity to Ceres. | ||
| 12.7 | Governing Law / Jurisdiction: This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the State of California without regard to principles of conflicts of law. The Parties agree that any dispute regarding the interpretation or validity of, or otherwise arising out of this Agreement, shall be subject to the exclusive jurisdiction of the California State Courts of San Francisco County or, in the event of federal jurisdiction, the United States District Courts of the Northern District of California, and each Party agrees to submit to the personal and exclusive jurisdiction and venue of the courts and not to seek the transfer of any case or proceeding out of such courts. | ||
| 12.8 | Notices: Any notice, notification or report permitted or required by this Agreement shall be sent by (i) facsimile with written confirmation copy, (ii) registered mail or (iii) a recognized private mail carrier service, and such notice shall be effective on the date received as indicated by the facsimile imprint date in the case of (i), or the carrier receipt of delivery to the addressee in the case of (ii) and (iii), if sent and addressed as follows or to such other address as may be designated by a Party in writing: |
Page 8 of 47
| If to Ceres: | Ceres, Inc. | |||
| 0000 Xxxxxx Xxxxxx Xxxx. | ||||
| Xxxxxxxx Xxxx, Xxxxxxxxxx, 00000 | ||||
| XXX | ||||
| Attention: Vice President of Product Development | ||||
| cc: Legal Department | ||||
| Telephone Number: (000) 000-0000 | ||||
| Facsimile Number: (000) 000-0000 | ||||
| If to ICS: | Institute of Crop Sciences | |||
| Chinese Academy of Agricultural Sciences | ||||
| 00 Xxxxx Xxxxxxxxxxxxx Xxxxxx | ||||
| Xxxxxxx Xxxxxxxx | ||||
| Xxxxxxx 000000 | ||||
| People’s Republic of China | ||||
| Attention: Mr. Jian-Min Wan, Director | ||||
| Telephone number: | ||||
| Facsimile number: |
| 13. | DURATION |
| 13.1 | This Agreement will enter into force on the date first written hereinabove and will remain in full force and effect until the delivery by ICS of the last report provided for in this Agreement, or until the last payment is due by Ceres pursuant to this Agreement is made, whatever is later. Notwithstanding the foregoing, Ceres will have the right to terminate this Agreement unilaterally at any time by giving sixty (60) days’ prior written notice to ICS. | ||
| 13.2 | Either Party will have the rights to terminate this Agreement unilaterally by registered letter addressed to the other Party in case such other Party has committed a breach of any of its obligations under this Agreement and has failed to remedy such breach within thirty (30) days from the receipt of a registered letter specifying the breach. | ||
| 13.3 | Expiration or termination of this Agreement will not relieve either Party of any obligation accruing prior to such expiration or termination. The provisions of Articles 8, 9, 10, 11, 12.5, 12.6 and 12.7 will survive the expiration or termination of this Agreement. |
Page 9 of 47
| Institute of Crop Sciences Chinese Academy of Agricultural Sciences |
Ceres, Inc. | |||||||
By: Name: |
/s/ Jian-Min Wan
|
By: Name: |
/s/ Xxxxx Xxxxxx
|
|||||
Title:
|
Director | Title: | Vice President of Product Development | |||||
By: Name: |
/s/ Hu-Qu Zhai
|
By: Name: |
/s/ Xxxxxxx Xxxxxxx
|
|||||
Title:
|
President | Title: | Chief Scientific Officer | |||||
Page 10 of 47
| 1. | CERES MATERIAL |
| Within 30 days from the Effective Date Ceres will provide approximately [***] gene constructs in vectors designed for Agrobacterium-mediated rice transformation. At the beginning of each calendar quarter thereafter, for a total of 12 quarters or as otherwise agreed by Ceres and ICS, Ceres will provide approximately an additional [***] gene constructs. Each group of constructs delivered to ICS will also contain target trait information. In addition, within 30 days from the Effective Date Ceres will provide a list of up to [***] pairs of selected events for which it would like the corresponding transgenes combined into a single rice line using genetic crossing. At the beginning of each calendar year thereafter, for a total of four years or as otherwise agreed by Ceres and ICS, Ceres will provide an additional list of up to [***] pairs of selected events to be crossed. The total number of slots for constructs to be transformed into rice and multiple-transgene lines to be generated by genetic crossing is [***]. The rice lines generated will be phenotyped and assayed for selected traits. At its option, Ceres can increase the number of gene constructs for transformation to [***] per quarter and [***] crosses per year. In addition, ICS and Ceres will collaborate on the QTL analysis of biomass in rice. The Program will continue for 24 months after the final delivery of gene constructs. ICS will submit a final report within 30 days after the end of such 24-month period. |
| 2. | PROGRAM ACTIVITIES |
| The goal of the program is to generate and evaluate carefully selected plant gene constructs of various origins under field conditions and under selected assay conditions by using a high through-put rice transformation system. A second goal is to combine transgenes and stack selected transgene-induced traits using genetic crosses between transgenic rice plants. A third goal is to perform a QTL analysis of biomass in rice to determine the identity of sequences that are associated with target biomass traits. Information generated from this program is intended to be used for crop improvement. |
| 2.1 | CERES ACTIVITIES |
| 2.1.1 | High throughput cloning (HTPC): Selection and construction of genes, transformation of Agrobacterium cells, delivery of constructs to ICS in Agrobacterium (or transgenic seeds) and quality control of the HTPC pipeline on a quarterly basis. |
| 2.1.1.1 | Generate construct tracking numbers for all constructs, e.g. CW00001. | ||
| 2.1.1.2 | List of target traits or predicted phenotype for each construct. |
Page 11 of 47
| 2.1.2 | Prioritization: Prioritization of delivered constructs for transformation, prioritization of existing events for genetic crossing, and prioritization of existing events for plant assay. | ||
| 2.1.3 | Quality control: Deliver gene-specific primers and vector primer sequences to ICS for use in quality control experiments using gene-specific PCR. Verify quality control processes at ICS and at SCUTA as per SOP R1 and R2 in ANNEX IV. | ||
| 2.1.4 | Data formats: Prescribe data formats for recording phenotyping results, assay results and data transfer (ANNEX II). | ||
| 2.1.5 | Internet server: Establish and maintain an independent web-accessible server for use by ICS, SCUTA and Ceres. | ||
| 2.1.6 | IT support: Establish a Program database. Provide expertise and training for electronic data capture and for database management. |
| 2.2 | ICS ACTIVITIES |
| 2.2.1 | Rice transformation and rice genetic crosses: Perform transformation and genetic crosses in rice, approximately [***] per year, for a total of [***] for this Agreement. Written SOPs for all phenotyping and assays will be provided by Ceres or developed by ICS in consultation with Ceres Trait Managers by the end of year two of the Agreement. ICS will also track and deliver quarterly updates for selected Program KPIs (ANNEX II). |
| 2.2.1.1 | Transformation: Transform up to [***]constructs per quarter and produce and collect seeds from [***] independent events (T0 plants) for each construct (SOP R1). Maintain updated Program database that includes articles 2.2.1.1.1 to 2.2.1.1.5. Update Ceres monthly. |
| 2.2.1.1.1 | T0 production: See the Transformation Table and KPI 1.1 (ANNEX II). | ||
| 2.2.1.1.2 | T0 plant phenotyping: See SOP R1. Include descriptions, images and dates. | ||
| 2.2.1.1.3 | T1 seed production: See SOP R3, the Seed Production Table and KPI 1.3 (ANNEX IV). | ||
| 2.2.1.1.4 | Quality control: See SOP R1 and R2, the Transformation Table, the Quality Control Table and KPI 1.2 (ANNEX IV). | ||
| 2.2.1.1.5 | OPTION: Ceres can elect to exercise an option to increase the number of constructs from [***] to [***] per quarter. |
Page 12 of 47
| 2.2.1.2 | T1 phenotyping — plant growth and development: Analyze T1 progeny from all events from article 2.2.1.1 for growth and development phenotypes. Maintain updated Program database that includes articles 2.2.1.2.1 to 2.2.1.2.2. Update Ceres monthly. |
| 2.2.1.2.1 | T1 plant phenotyping: See SOP R2 (ANNEX IV). Include descriptions, coded comments, images and data entry dates. Maintain a phenotype tracking database. | ||
| 2.2.1.2.2 | T2 seed production: See SOP R3 and the Seed Production Table (ANNEX II, Table 3). |
| 2.2.1.3 | T2 phenotyping: Upon request selected lines will be chosen for additional phenotyping. Resources used for T2 plant phenotyping should not exceed prescribed Program resources without the consent of the Collaboration Committee. See SOP R2 (ANNEX IV). | ||
| 2.2.1.4 | Trait assays: Selected lines will be assayed for drought tolerance, low N tolerance or composition by NIR. SOPs are presented in ANNEX II. |
| 2.2.1.4.1 | Drought tolerance: See the drought tolerance assay SOP R4 (ANNEX IV). | ||
| 2.2.1.4.2 | Low N tolerance: See the low nitrogen assay SOP R5 (ANNEX IV). | ||
| 2.2.1.4.3 | NIR analysis: See SOP R6 (ANNEX IV). Analyze plant material selected by Ceres for composition using NIR. | ||
| 2.2.1.4.4 | Genotyping quality control: All events that are selected for assaying will be sequence validated using gene-specific PCR. In addition, Ceres may request plant material or plant DNAs for QC analysis. | ||
| 2.2.1.4.5 | Images: Photographs will be taken of all plants that display a distinct morphological phenotype or response to assay conditions affecting plant growth, development, biomass, fertility, stem morphology, tiller number or leaf morphology. Images with and without labels and plant descriptions will be stored in an image database and the Image Log Table (ANNEX II, Table 6). |
| 2.2.1.5 | OPTION: Ceres can elect to exercise an option to increase the repertoire of measurements and assays. |
Page 13 of 47
| 2.2.1.5.1 | New SOPs can be developed at ICS in consultation with Ceres as authorized by the Collaboration Committee. | ||
| 2.2.1.5.2 | Resources for trait assay development and utilization should not exceed prescribed resources without the consent of the Collaboration Committee. |
| 2.2.1.6 | Genetic crossing of transgenics: Perform up to [***] crosses per year to stack and combine selected transgenes into a single line. Crosses and events will be requested and prioritized by Ceres. Crosses will be reviewed and scheduled by the Collaboration Committee. The parent lines for crosses will be germinated two times each year — in April for crosses made by ICS and in October for crosses made by SCUTA. |
| 2.2.1.6.1 | Homozygous T2 lines for crossing will be identified using herbicide treatments at ICS or SCUTA. | ||
| 2.2.1.6.2 | Each cross requested will be given a unique CW identification number, e.g. CW50001, CW50002. Parents, F1 and F2 seeds will be tracked in the Genetic Crossing Table (ANNEX II). | ||
| 2.2.1.6.3 | Ceres Trait Managers will specify the number of F1 seeds that need to be produced for each trait assay. | ||
| 2.2.1.6.4 | OPTION: Ceres can elect to exercise an option to increase the number of crosses to [***] per year. |
| 2.2.1.7 | F1 phenotyping: Grow F1 progeny and parents in the field side-by-side, e.g. row 1, parent 1; row 2, F1; row 3, parent 2. Phenotype as per SOP R2. |
| 2.3 | QTL analysis of biomass: Genetically map quantitative trait loci responsible for target biomass traits in rice. |
| 2.3.1 | Target traits for QTL analysis. Plant height, growth rate, flowering time, productive tiller number, stem thickness, biomass, and stem composition (as determined by NIR). | ||
| 2.3.2 | Select parent lines and crosses for biomass QTL analysis. Parent lines, descriptions and available crosses are listed in ANNEX II. Screen parents for usable marker polymorphisms. | ||
| 2.3.3 | F2 analysis. [***] ([***]) F2 populations, [***] individuals in size, will be used for QTL analysis. Identify the [***] top biomass producing plants from each cross. Extract DNAs and map major QTLs. The estimated number of |
Page 14 of 47
| project samples to be analyzed is [***]. Save seeds from all top biomass plants. | |||
| 2.3.4 | Analyze composition of parent lines, F1 and F2 plants using NIR. Save stem samples from all parents, F1 and selected F2 plants. Perform NIR analysis on samples at ICS or at Ceres using SOP R6 (ANNEX IV). |
| 2.4 | Quarterly delivery of transgenic rice seeds and biological materials to Ceres. |
| 2.4.1 | ICS will work in good faith to obtain phytosanitary certification and ship seeds requested by Ceres to the USDA in Maryland for inspection and release to Ceres. This should take no more than three (3) months. |
| 3. | Reports |
| 3.1 | Deliver Program Reports to the Collaboration Committee on a quarterly basis at the end of each calendar quarter. Reports shall include, but not be limited to: |
| 3.1.1 | KPI summary. Gather KPI data according KPIs listed in Section 1 ANNEX II and improve underlying processes. | ||
| 3.1.2 | Transformation update: Updated Transformation Table including the identification and number of constructs transferred to rice and summary of T0 QC analysis (ANNEX II, Table 1 and 2). | ||
| 3.1.3 | T1 and T2 seed production update: Updated Seed Production Table (ANNEX II, Table 3). | ||
| 3.1.4 | T1 plant phenotyping update: Summary of T1 plant phenotyping including descriptions, measurements and images. |
| 3.1.4.1 | Phenotyping descriptions to be based on articles 2.2.1.2 and 2.2.1.3 of this ANNEX and in accordance with a coded comments system (SOP R2). |
| 3.1.5 | Trait assay update: Summary of trait assay results, measurements and images. | ||
| 3.1.6 | Genetic crossing update: Updated Genetic Crossing Table (ANNEX II,Table 4) including crosses made and F1 seed collected. | ||
| 3.1.7 | Summary of QTL Mapping: markers used, measurements, mapping results, major loci identified. |
| 4. | Resources to be made available by the Parties |
| 4.1 | ICS will make available the following resources for the implementation of the Program (in addition to employees): |
Page 15 of 47
| 4.1.1 | Laboratory space, fully equipped, installed and operational: At least [***] square meters ([***] square feet) for media preparation and molecular studies | ||
| 4.1.2 | Tissue culture space: At least [***] square meters ([***] square feet) for tissue culture. | ||
| 4.1.3 | Screenhouse and droughthouse space for growing primary transgenics and T1 plants: At least [***] square meters ([***] square feet). | ||
| 4.1.4 | Greenhouse space for germination, hydroponic assays, sample drying and crosses: At least [***] square meters ([***] square feet). | ||
| 4.1.5 | Field space for evaluation of T1 and T2 plants: at least [***] square meters ([***] square feet). | ||
| 4.1.6 | Supplies, chemicals, utilities as required. |
| 4.2 | SCUTA will make available the following resources for the implementation of the Program (in addition to employees): |
| 4.2.1 | Laboratory space, fully equipped, installed and operational: at least [***] square meters ([***] square feet) for seed storage and molecular studies | ||
| 4.2.2 | Screenhouse space for growing primary transgenic plants and T1 plants: At least [***] square meters ([***] square feet). | ||
| 4.2.3 | Supplies, chemicals, utilities as required. |
| 4.3 | At the request of ICS and SCUTA, Ceres will provide chemicals, supplies and IT training required for the Program activities which are not available in China. Delivery conditions of Article 2.1 of the Agreement to apply. |
| 5. | Remuneration and payment schedule |
| 5.1 | Ceres will pay for employees for the Program referred to in Article 5.1 of the Agreement at the rates indicated below (including salary, benefits and partial support for apartment rental). |
| 5.1.1 | IT specialist, $[***] | ||
| 5.1.2 | Post-doctoral fellow, $[***] | ||
| 5.1.3 | PhD student, $[***] | ||
| 5.1.4 | BS/MS level, $[***] | ||
| 5.1.5 | Technical/field staff, $[***] |
Page 16 of 47
| 5.2 | Ceres will pay for use of [***] square feet of laboratory space at ICS at a rate of $[***] per 10 square feet per year (approximately $[***] per year), including water, electricity and other utilities. | ||
| 5.3 | Ceres will pay for use of [***] square feet of laboratory space at SCUTA at a rate of $[***] per 10 square feet per year (approximately $[***] per year), including water, electricity and other utilities. | ||
| 5.4 | Ceres will pay for use of [***] square feet of tissue culture room at a rate of $[***] per 10 square feet per year (approximately $[***] per year), including water, electricity, utilities and maintenance. | ||
| 5.5 | Ceres will pay for use of [***] square feet of ICS greenhouse space at a rate of $[***] per 10 square feet per year (approximately $[***] per year), including water, electricity, heating and others. | ||
| 5.6 | Ceres will pay for the installation of greenhouse lights at ICS at a one time cost of $[***]. | ||
| 5.7 | Ceres will pay for the use of [***] square feet of ICS screenhouse and droughthouse space at a rate of $[***] per 10 square feet per year (approximately $[***] per year). Includes screening film, soil preparation, water, plant care and pest control. | ||
| 5.8 | Ceres will pay for the use of up to [***] square feet of specially segregated and controlled access field space at Langfang Field Station for transgenic rice plants at a rate of $[***] per 10 square feet per year plus room, board and transportation for ICS breeder and field staff (approximately $[***] per year). Space has already been approved by the Ministry of Agriculture for the growth of transgenic rice. Includes soil preparation, water and pest management. | ||
| 5.9 | Ceres will pay for the use of [***] square feet of SCUTA screenhouse at a rate of $[***] per 10 square feet (approximately $[***] per year). Includes soil preparation, water and pest management. | ||
| 5.10 | Ceres will pay $[***] per year for growth media, supplies for QC, supplies for Southerns, northerns, PCR, RT-PCR, assays, QTL mapping, chemicals and utilities for ICS (including direct purchases by Ceres outside China). | ||
| 5.11 | Ceres will pay $[***] per year for supplies, chemicals and utilities for SCUTA (including direct purchases by Ceres outside China). | ||
| 5.12 | Ceres will pay $[***] per year for 5 round trips travel to Hainan for ICS @ [***] CNY airfare, [***] CNY for 3 days hotel and meals and [***] CNY for ground transportation. | ||
| 5.13 | Ceres will pay $[***] per year for 5 round trips travel to Beijing for SCUTA @ [***] CNY airfare [***] CNY for 3 days hotel and meals, [***] CNY for ground transportation. |
Page 17 of 47
| 5.14 | Ceres will pay $[***] per year to support ICS research activities. | ||
| 5.15 | Ceres will pay $[***] per year to support SCUTA research activities. | ||
| 5.16 | Payments to ICS will be made quarterly, within 30 days of Ceres’ receipt of an invoice from ICS. An initial quarterly payment of $[***] will be made after the Effective Date within 30 days of Ceres’ receipt of an invoice from ICS. Disbursement of Program funds to SCUTA is the responsibility of ICS. | ||
| 5.17 | Budget summary |
| 5.17.1 | Table 1: Budget summary for Program. | ||
| 5.17.2 | Table 2: Budget summery for Program Option. | ||
| 5.17.3 | Detailed Program budget: see ANNEX III, Table 1. | ||
| 5.17.4 | In the case that the exchange rate for one USD to RMB (RENMINBI), as quoted by the Financial Management Service (FMS) of the U.S. Department of the Treasury (DOT), and monitored by ICS on each anniversary of the effective date, declines by 10 percent from 7.544 to less than 6.790 or increases by 10 percent to greater than 8.300, then ICS or Ceres can propose that the payment schedule in Table 1 be recalculated by Ceres and that future payments in USD be adjusted based on the then current USD to RMB exchange rate. |
| 5 YEAR | ||||||||||||||||||||||||
| TOTAL IN | ||||||||||||||||||||||||
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | USD | |||||||||||||||||||
Lab space, plant growth, expendables, administration |
[***] | [***] | [***] | [***] | [***] | [***] | ||||||||||||||||||
Project FTEs |
[***] | [***] | [***] | [***] | [***] | [***] | ||||||||||||||||||
TOTAL CONTRACT |
[***] | [***] | [***] | [***] | [***] | [***] | ||||||||||||||||||
Page 18 of 47
| DATE | SHIPME | NUM_RE | REGENE | PLANTLE | SOIL_TR | T0_PHEN | T1_SEED | T1_EVEN | ||||||||||||||||||||||||||||||||||||||||||
| XXXX | RECEI | NT_NU | AGRO_G | INFECTIO | SISTANT | RATION | TS_GENE | ANSPLAN | T0_TO_S | T0_LOCA | OTYPING | _HARVE | TS_HARV | |||||||||||||||||||||||||||||||||||||
| S_ID | CW_LINE | VED | MBER | STATUS | REASON | ROWTH | N_DATE | _CALLI | DATE | RATED | T_DATE | OIL | TION | _NOTES | ST_DATE | ESTED | ||||||||||||||||||||||||||||||||||
1 |
CW00001 | 12/1/04 | 01 | FINISHED | INSUFFICIENT T1 EVENTS | Yes | 12/18/04 | 23 | 2/26/05 | 18 | 5/15/05 | ICS | Broad leaves | 9/28/05 | 8 | |||||||||||||||||||||||||||||||||||
2 |
CW00002 | 12/1/04 | 01 | FINISHED | INSUFFICIENT T1 EVENTS | Yes | 12/18/04 | 20 | 2/26/05 | 15 | 5/15/05 | ICS | 9/28/05 | 7 | ||||||||||||||||||||||||||||||||||||
3 |
CW00003 | 12/1/04 | 01 | FINISHED | INSUFFICIENT T1 EVENTS | Yes | 12/18/04 | 21 | 2/26/05 | 10 | 5/15/05 | ICS | 9/28/05 | 6 | ||||||||||||||||||||||||||||||||||||
4 |
CW00004 | 12/1/04 | 01 | FINISHED | INSUFFICIENT T1 EVENTS | Yes | 12/18/04 | 21 | 2/26/05 | 9 | 5/15/05 | ICS | 9/28/05 | 6 | ||||||||||||||||||||||||||||||||||||
| Column | Description | |
TRANS_ID
|
Unique barcode for a particular transformation event | |
CW_LINE
|
CW identifier supplied by Ceres | |
DATE_RECEIVED
|
Date constructs were received by ICS | |
SHIPMENT_NUMBER
|
Batch number of shipment | |
STATUS
|
Current status of construct: Received, In Process, Finished, Failed | |
REASON
|
Only for Failed constructs, explain why they failed | |
AGRO_GROWTH
|
Yes/No — did Agro grow | |
INFECTION_DATE
|
Date calli infection was performed | |
NUM_RESISTANT_CALLI
|
Number of resistant calli resulting from infection | |
REGENERATION_DATE
|
Date plantlet regeneration started | |
PLANTLETS_GENERATED
|
Number of plantlets resulting from regeneration | |
SOIL_TRANSPLANT_DATE
|
Date plantlets were transplanted to soil | |
T0_TO_SOIL
|
Number of T0 plants transplanted to soil | |
T0_LOCATION
|
Location of T0 plants (ICS, SCUTA) | |
T0_PHENOTYPING_NOTES
|
Any observations made while T0 plants are in soil | |
T1_SEED_HARVEST_DATE
|
Date of T1 seed harvest | |
T1_EVENTS_HARVESTED
|
Number of T1 seed lots harvested |
| Date of | Generation of | Container | Amount | |||||||||||||||||||
| CW_ID | Harvest | Harvested By | Seed | Location | Building | Room | (Barcode) | (g) | Notes | |||||||||||||
CW00006
|
1/1/2006 | LZ | T1 | SCUTA | AAA | XXX | 123456 | 2 | Notes on germination, amount used (2.1.2006) | |||||||||||||
CW00003
|
1/1/2006 | LZ | T1 | SCUTA | BBB | XXX | 123457 | 3 | Poor germination (1.1.2007) | |||||||||||||
CW00006-01
|
6/1/2006 | T2 | ICS | YYY | 123458 | 4 | ||||||||||||||||
CW00010
|
7/1/2007 | CYW | F1 | ICS | YYY | 123459 | 0.5 | |||||||||||||||
CW00010-01
|
10/1/2007 | XZ | F2 | ICS | YYY | 123460 | 2 | |||||||||||||||
Page 19 of 47
| Pollination | P1 | P2 | Deliver | Harvest | ||||||||||||||||||
| CW_ID | Date | Xxxxxxx | Parent 1  |
Trait | Parent 2  |
Trait | Requester | Reason | By | Date | Project | |||||||||||
CW50010
|
4/1/2007 | CYW | CW00006-01-01 | Drought | CW00003-01-01 | Drought | JKO | Stacking test | 6/1/2007 | 4/1/2007 | Product Development | |||||||||||
CW50011
|
4/1/2007 | CYW | CW00006-01-01 | Drought | CW00002-01-01 | Drought | JKO | Stacking test | 6/1/2007 | 4/1/2007 | Product Development | |||||||||||
CW50012 |
||||||||||||||||||||||
CW50013 |
| Parent | Plant Height (m) | Tillers | Flowering time | |||||||||
P1
|
KN | 2 | Less | Late | ||||||||
P2
|
DWR-4 | N/A | Fast growing, stem elongation | Average | ||||||||
P3
|
Nanjing 6 | >1.7 | High, strong stems | Late | ||||||||
P4
|
Kuidao | 2 | N/A | N/A | ||||||||
P5
|
601 | 0.8 | N/A | Average | ||||||||
| Image | ||||||||||||||
| CW_ID | Image _ID | Description | Trait | Phenotype | Image Type | Location | ||||||||
CW00196-04
|
87654321 | Whole plant and controls | Biomass | Increased biomass | Phenotype | SCUTA | ||||||||
CW00006-01
|
87654322 | Plot image | Drought | Drought tolerance | Assay Result | ICS | ||||||||
CW00006-01-01
|
87654323 | Field shot | Drought | Drought tolerance | Field Trial | CHANGPING | ||||||||
| Measurement | Plant Position | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | Row | CW_Event | Measurement | _Code | Name | Date | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | Plant_Status | 1 | 5/31/2007 | bugs | blight | sick | yellow | |||||||||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | Is this KI? | 2 | 5/21/2007 | Y | ||||||||||||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | Height | 3 | 6/1/2007 | 72 | 68 | 59 | 68 | 66 | 60 | |||||||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | Flowering_Time | 4 | 6/1/2007 | y | y | |||||||||||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | Flowering_Time | 4 | 6/2/2007 | y | y | y | y | |||||||||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | Flowering_Time | 4 | 6/3/2007 | y | y | y | ||||||||||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | QC_result | 7 | 6/7/2007 | + | — | + | ||||||||||||||||||||||||||||||||||||||||||||||||||
10 |
1 | CW00222-01 | Biomass | 5 | 6/28/2007 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Page 20 of 47
| 1. | Key Performance Indicators (KPIs) |
| 1.1. | Number of constructs yielding >10 T0 plants delivered each quarter vs. all constructs delivered each quarter. | ||
| 1.2. | Number of constructs sequence validated in T0 plants each quarter vs. all constructs assayed in T0 each quarter. | ||
| 1.3. | Number of constructs yielding T1 seeds from >10 events each quarter vs. all T1 seeds delivered each quarter. | ||
| 1.4. | Number of constructs sequence validated in T1 events each quarter vs. all events assayed in T1. | ||
| 1.5. | Time from infection to T1 seed harvest each quarter. | ||
| 1.6. | Number of constructs evaluated for predicted phenotype or trait. | ||
| 1.7. | Number of constructs yielding predicted phenotype or trait vs. all constructs phenotyped or assayed. | ||
| 1.8. | Number of constructs yielding novel phenotypes vs. all constructs phenotyped and assayed. | ||
| 1.9. | Number of constructs showing yield drag or negative traits vs. all constructs analyzed. |
| 2. | Screenhouse Maps |
| 2.1. | Plot map for Screenhouse 1 and Screenhouse 2 at SCUTA |
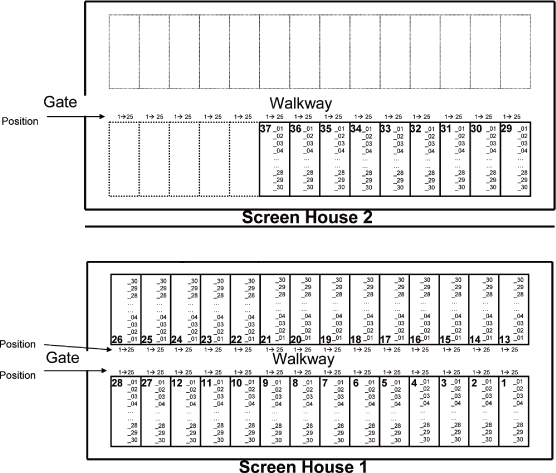
Page 21 of 47
Page 22 of 47
Page 23 of 47
Page 24 of 47
| 1. | Plantlet transport. Transgenic rice plantlets will be transported by ICS personnel from the lab to the field in a clearly labeled, secured and sealed container. Unused plants will be destroyed by autoclaving according to SOP R7. |
| 2. | Plant growth. Transgenic rice will be grown in a secure and contained growth room, greenhouse, screenhouse or in a field approved for the growth of transgenic rice. No commercial rice plants will be grown in the same greenhouse, screenhouse or approved field with transgenic rice. |
| 3. | Seed storage. Each seed line will be contained within a sealed clearly labeled envelope or a sealed paper bag. Each seed line is inventoried and stored inside a locked cabinet inside a secure room. Access to the seed storage cabinets is limited to those involved in the experiments. Seed utilization and disposal will be tracked using a log system. Unused seeds will be destroyed by autoclaving according to SOP R7. |
| 4. | Seed transport. Each seed line will be transported in a sealed, clearly labeled envelope. Envelopes will be shipped in a clearly labeled, secured and sealed container within a shipping box to prevent spillage if the outside packaging gets damaged. |
| 5. | Seed destruction. The maintenance of a transgenic rice seed log is the responsibility of ICS. The inventory of transgenic seeds will be reviewed annually. Seed stocks designated by Ceres as no longer needed will be destroyed by autoclaving as described in SOP R7. Their destruction will be recorded in the seed log. |
Page 25 of 47

|
Protocol Number: | R1 | ||
| Property of Ceres, Inc. |
Protocol Name: | Agrobacterium-mediated Rice Transformation |
||
| Authors: Chuan-xxx Xx | Date Issued: | |||
| Approved By: | Version Number: | 1.0 |
Purpose:
|
This protocol is established to produce a large number of transgenic rice plants using Ceres’ promoter and selectable marker gene. | |
References:
|
“Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA.” Hiei, Y., Ohta, S., Komari, T. and Kumashiro, T. Plant J. 6, 271-282 (1994). | |
Safety:
|
Follow universal lab safety technique. Properly dispose of transgenic waste in designated receptacles. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. |
| Disposable Supplies: | Reagents and Stock Solutions: | |
100 x 20 xx Xxxxx dishes (25373-143; VWR)
|
2,4-Dichlorophenoxyacetic acid (Sigma) 2mg/mL |
|
Disposable pipettes 10mL, 25mL (53106-431;
VWR)
|
Kinetin (Sigma) 1mg/ml | |
Autoclave bags (11215-796; VWR)
|
Naphthalene Acetic Acid (Sigma) | |
50 xx Xxxxxxx conical tube (Xxxxxx Scientific)
|
Bialaphos 5mg/mL (Bialaphos; Shinyo Sangyo) | |
Gloves (32916-532; Microflex Corp.)
Sundae Cup (Xxxxx Xxxxx Paper Co.)
|
Carbenicillin (1000; Agribio) 250mg/mL | |
Whatman filter paper (VWR)
|
Sucrose (S-0389; Sigma) | |
Eppendorf tubes
|
N6 basal medium (Phytotechnology) | |
Pipette tips
|
MS basal medium (Phytotechnology) | |
12 ml culture tubes (FALCON)
|
Gelrite (Sigma) | |
| Agarose (EMD Chemicals Inc.) | ||
Capital Equipment: |
||
Laminar Flow Hood (Nuaire)
|
Non-disposable Supplies: | |
66 Series Incubator (Percival Scientific)
|
Magenta Boxes (GA7; Magenta Corp) | |
Micro-centrifuge (Heraeus Instrument) |
||
22°C growth chamber (Percival Scientific) |
||
28°C growth chamber (Percival Scientific) |
Page 26 of 47
Start Product(s):
|
Wild-type rice seeds | |
End Product(s):
|
Plants that have been genetically transformed with appropriate constructs |
| A. | Induce calli of good quality from mature rice seeds |
| 1. | Dehusk mature seeds and discard any spotted ones. Do not use seeds which have been stored for more than 2 years at room temperature. Do not use seeds harvested from plants grown in high humidity and high temperature conditions. | ||
| 2. | Transfer dehusked seeds to a 50 ml conical tube (such as CORNING 430290). | ||
| 3. | Rinse seeds in 70% ethanol for 1 min. | ||
| 4. | Pour out ethanol and rinse seeds with autoclaved distilled water. | ||
| 5. | Add 24 ml autoclaved distilled water and then 6 ml Clorox Bleach (Chlorox contains 5.25 % sodium hypochlorite, so the final concentration is 1.05% ). | ||
| 6. | Shake the tube occasionally for 30 min. | ||
| 7. | Pour out the Clorox solution and rinse seeds with sterile water 4 times. | ||
| 8. | Dry the seeds on autoclaved Kimwipes for a few minutes. | ||
| 9. | Transfer seeds to semi-solid N6-P medium, 10 seeds in each Petri dish (100 x 20 mm, FALCON). Each Petri dish contains 30 ml N6-P medium (see Appendix A for N6-P). Dishes are sealed with surgical tapes to allow air exchange. | ||
| 10. | Induce calli at 28°C under cold fluorescent light. Many granular calli should be formed within 4 weeks on most of the seeds (Fig. 1). Calli of good quality consist of small spherical cells with dense cytoplasm, which are competent for transformation (Fig. 2). The calli can be used directly for Agrobacterium infection, or subcultured for later use. Do not use calli older than 2 months (from the seed sterilization date). |
| B. | Infection and co-cultivation of calli with Agrobacterium cells |
| 1. | Pick up a single Agrobacterium clone from plate and culture it in 2 xx XXX liquid medium in a sterile tube on a shaker (140 rpm) overnight. Include appropriate antibiotics depending on marker genes in a vector at 50 ug/ml. | ||
| 2. | Transfer the liquid culture to 1.5 ml Eppendorf tube and centrifuge it at 7,000 rpm for 5 min. | ||
| 3. | Discard the supernatant and resuspend cells in N6-AS liquid medium (see Appendix B for N6-AS). | ||
| 4. | Adjust cell density with the liquid N6-AS medium. The density can be measured by a spectrometer. The optimal OD600 value varies significantly depending on Agrobacterium strains and vectors. The optimal value means there is no over-growth of Agrobacterium cells in 3-day co-cultivation. | ||
| 5. | Mix xxxx xxxxx with bacterium cells. This can be done in a 50 ml sterile conical tube. Shake the tube by hand gently for 3 min. The ratio of calli to bacterium solution is not a critical factor affecting transformation efficiency. Several grams of calli can be in 15 ml bacterium suspension. |
Page 27 of 47
| 6. | Pour out bacterium solution. Transfer calli onto autoclaved Kimwipes paper to remove extra solution. | ||
| 7. | Culture calli on autoclaved filter paper placed on the N6-AS semi-solid medium. Each 100 x 20 mm dish contains 20 ml N6-AS. Seal the dish with surgical tape. | ||
| 8. | Co-culture calli and bacterium cells at 22 °C in dark for 4-6 days. | ||
| 9. | Note: Calli should remain healthy during co-cultivation (Fig. 3). | ||
| 10. | Less than 10% of the calli should be over-infected at the end of co-cultivation (Fig. 4). | ||
| 11. | Efficiency of Agrobacterium infection can be examined by using the GFP gene as a reporter (Fig. 5). |
| C. | Selection of transformed calli |
| 1. | Transfer the infected calli to a sterile 50 ml tube containing 35 ml sterile distilled water. | ||
| 2. | Shake the tube for a few seconds and pour out water. Repeat washing 6 times. | ||
| 3. | For the final wash, add carbenicillin or cefotaxime at the concentration of 500 mg/ml. | ||
| 4. | Transfer calli onto autoclaved Kimwipes paper to remove extra water. | ||
| 5. | Culture calli on the semi-solid N6-P medium supplemented with 250 mg/ml carbenicillin or cefotaxime at 28 °C under cold fluorescent light for 7 days. Each 100 x 20 mm dish contains 30 ml N6-P medium, 30 calli on each dish. Dishes are sealed with surgical tape. | ||
| 6. | Subculture calli on the N6-P medium supplemented with 250 mg/ml carbenicillin or cefotaxime and 100 mg/ml paramomycin for 16 days. The Gelrite is replaced by Agarose (7-8 g/l) in the N6-P medium. | ||
| 7. | Subculture calli on the N6-P medium supplemented with 250 mg/ml carbenicillin or cefotaxime and 100 mg /ml paramomycin for another 16 days. Use agarose to replace Gelrite in N6-P medium. Paramomycin-resistant calli should start to be visible at the end of the 2nd selection. | ||
| 8. | Note: fast-growing transformed cells must be visible during second selection (Fig. 6). Non-transformed wild-type calli must be completely killed by selection (Fig. 7). |
| D. | Regeneration of transgenic plants |
| 1. | Transfer independent resistant calli to the N6-R plant regeneration medium (see Appendix C for N6-R). Add 50 mg/l paramomycin to N6-R medium. Each 100 x 20 mm dish contains 30 ml N6-R medium, 10 calli on each dish. Dishes are sealed with surgical tape. Culture calli at 28 °C under cold fluorescent light for 2 weeks. | ||
| 2. | Transfer calli to fresh N6-R medium. Do not add paramomycin to N6-R medium for this subculture. Culture calli at 28 °C under cold fluorescent light until shoots and roots are formed. | ||
| 3. | Transfer plantlets to Magenta boxes containing 30 ml MS-F medium (see Appendix D for MS-F). Grow plantlets at 28 °C under cold fluorescent light for 10-14 days. |
Page 28 of 47
| 4. | Acclimate in vitro regenerated plants. Plant containers will be opened 4 days before transplanting in order to xxxxxx plantlets. | ||
| 5. | Transfer plants to pots or to soil. Label T0 plants systematically with CW ID and event number, e.g. CW00001-01. Generate and maintain greenhouse and field maps for all T0 plants. Note: transformed plants must be resistant to paramomycin, and non-transgenic seedlings are albinos when treated with kanamycin (Fig. 8). |
| E. | Quality assurance |
| 1. | Validate transgene identity in transgenic calli or T0 regenerants for each construct using gene-specific primers and PCR. | ||
| 2. | Process three independent samples for each construct along with appropriate positive and negative controls. |
| F. | T0 plant phenotyping |
| 1. | Grow and maintain T0 plants normally throughout phenotyping. | ||
| 2. | T0 observations and measurements include: |
| a. | Fertility: No entry (normal), 1 (reduced). Include date of observation. | ||
| b. | Morphology: Morphological traits will be described using prescribed coded comments (TBD). | ||
| c. | Images: Photographs will be taken of all T0 plants with distinctive morphological phenotypes affecting plant growth, development, biomass and seed production, including fertility, stem morphology, tiller number and leaf morphology. Each photograph should include a Ceres white board labeled with construct name, event number and date and be written with a black broad tip erasable marker. | ||
| d. | Labeled images with plant descriptions will be stored in the project database and image log with the date of entry. |
| G. | T1 seed harvest and tracking |
| 1. | T1 seed numbering. Assign a unique event number to each plant for T1 seed harvesting, e.g. CW00001-01. | ||
| 2. | Harvest T1 seeds on the panicle and placed into an envelope. Label the envelope with the construct and event number, date of harvest and store individually. | ||
| 3. | Log each envelope of seed collected in the Seed Production Table. Include the room and storage container numbers. |
| H. | Pest management |
| 1. | Spray low noxious pesticides to control the plant hoppers and borers 1-3 times during the vegetative phase. |
Page 29 of 47
N6 basal salt mixture (Sigma) |
1 bag (1 liter size) | |
KI |
0.8 | |
CoCI2×6H2O |
0.025 | |
XxXX0x0X0X |
0.000 | |
XxXxX0x0X0X |
0.25 | |
Glycine |
2 | |
Myo-inositol |
000 | |
Xxxxxxxx XXX |
0 | |
Xxxxxxxxxx XXX |
1 | |
Nicotinic acid |
1 | |
Proline |
2,800 | |
Casamino acid |
300 | |
Sucrose |
30,000 | |
2.4-D |
2 | |
pH |
5.6 | |
Gelrite |
4,000 | |
(For Bar marker and Biolaphos selection)
Or
Agarose |
7,000-8,000 | |
(For NPT II marker and Paramomycin selection)
Autoclave at 121 °C for 15 min. |
N6 basal salt mixture (Sigma) |
1 bag (1 liter size) | |
KI |
0.8 | |
CoCI2×6H2O |
0.025 | |
XxXX0x0X0X |
0.000 | |
XxXxX0x0X0X |
0.25 | |
Glycine |
2 | |
Myo-inositol |
000 | |
Xxxxxxxx XXX |
0 | |
Xxxxxxxxxx XXX |
1 | |
Nicotinic acid |
1 | |
Casamino acid |
1,000 | |
Sucrose |
30,000 | |
Glucose |
10,000 | |
2.4-D |
2 | |
pH |
5.6 | |
Gelrite (semi-solid medium) |
4,000 | |
Autoclave at 121 °C for 15 min. |
||
Add in acetosyringone at the final concentration of 200 mM just before use. |
N6 basal salt mixture (Sigma) |
1 bag (1 liter size) | |
KI |
0.8 |
Page 30 of 47
CoCI2×6H2O |
0.025 | |
XxXX0x0X0X |
0.000 | |
XxXxX0x0X0X |
0.25 | |
Glycine |
2 | |
Myo-inositol |
000 | |
Xxxxxxxx XXX |
0 | |
Xxxxxxxxxx XXX |
1 | |
Nicotinic acid |
1 | |
Casamino acid |
1,000 | |
Sucrose |
25,000 | |
Sorbitol |
25,000 | |
Kinetin |
2 | |
NAA |
0.05 | |
pH |
5.6 | |
Agarose (Sigma Type I-A) |
8,000 | |
Autoclave at 121 °C for 15 min. |
||
Appendix D — MS-F medium (mg per liter)
MS basal salt mixture (Sigma) |
1/2 bag (1 liter size) | |
Glycine |
2 | |
Myo-inositol |
100 | |
Thiamine HCI |
5 | |
Pyridoxine HCI |
1 | |
Nicotinic acid |
1 | |
Sucrose |
15,000 | |
pH |
5.6 | |
Gelrite |
2,500 | |
Autoclave at 121 °C for 15 min. |
Page 31 of 47
Page 32 of 47
 Property of Ceres, Inc. |
||||||||
| Protocol Number: | R2 | |||||||
| Protocol Name: | Plant Growth and Development Phenotyping |
|||||||
Authors:
|
Chuan-xxx Xx | Date Issued: | July 16, 2007 | |||||
Approved By:
|
Version Number: | 1.0 | ||||||
Purpose:
|
This protocol is for the phenotyping of T1 and T2 transgenic rice plants. | |
Safety:
|
Follow universal lab safety techniques. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. |
Stock Solution: herbicide |
0.4% Finale |
| 1. | Assign an experiment number to seedlings started together and record events included in the experiment. | ||
| 2. | Soak seeds in 2% limewater for 2-3 days at room temperature, and then wash them completely using clean water. | ||
| 3. | Sow seeds in flats. | ||
| 4. | Herbicide selection — eight days (summer) or twelve days (winter) after germination, spray seedlings with the herbicide Finale® to remove non-transgenic segregants. Respray 5 days after the first spraying to minimize escapes. |
| 1. | Herbicide resistant seedlings at 4-5 leaf stage will be transplanted to field, ten transgenic lines for each construct, fifteen plants for each line, one row wild type plants every eleven rows as shown in Figure 1. | ||
| 2. | Save and score at least 30 seedlings for herbicide tolerance after herbicide sensitive plants have clearly died. Store numbers of herbicide sensitive and resistant seedlings in the Data Collection Table (ANNEX II). | ||
| 3. | Field layout — the distance between rows is 26.7 cm and the distance between plants within a row is 13.4 cm. Two guard rows will be grown for each plot. Walk lane will be 50 cm wide. |
Page 33 of 47
| 4. | A plot map or a screenhouse map (ANNEX II) will be used to track each plant in each experiment. |
| 5. | Measurements to be taken for all T1 plants |
| a. | Flowering time: Number of days from transplanting to heading of the earliest panicle. | ||
| b. | Plant height: From the ground to the top of the tallest panicle. | ||
| c. | Effective tiller number: Number of tillers bearing a fertile panicle. | ||
| d. | Seed yield: Weight of seeds while still on panicles, including the or envelope. |
| 6. | General observations To be noted in cases where the frequency is higher than expected based on control plants. Use coded comments whenever possible to describe plants (see ANNEX IV, Data Collection Table). A phenotyping update should be presented monthly to the Collaboration Committee. |
| a. | Growth vigor: Normal (no entry), improved (1), poor (2) | ||
| b. | Leaf color: Normal (no entry), darker green (1), yellow-green (2) | ||
| c. | Fertility: Normal (no entry), reduced (1). | ||
| d. | Lodging tolerance: Normal (no entry), increased lodging (1). | ||
| e. | Resistance to pests and diseases: Normal (no entry), increased resistance (1), hypersensitive (2). | ||
| f. | Stress tolerance (drought, cold, heat): Normal (no entry), increased tolerance (1), hypersensitive (2). | ||
| g. | Flag leaf color at maturity: Normal (no entry), more green than controls (1), more yellow than controls (2). |
Page 34 of 47
| h. | Flag leaf angle from vertical: Normal (no entry), decreased leaf angle (1), increased leaf angle (2). |
| 7. | Optional measurements for selected events (upon request from Ceres) |
| a. | Fertility2: Number of filled seeds divided by number of spikelets on a panicle (average of 3 panicles). | ||
| b. | Panicle size: Number of seeds per panicle, averaged from three panicles. | ||
| c. | Seed yield2: Weight of total seeds less panicles and bag for lines showing 10 percent increase. | ||
| d. | Seed fill rate: Number of days from flowering to when 50% of seeds are yellow. |
| 1. | Events with highly penetrant phenotypes will be genotype confirmed using gene specific PCR. New phenotypes will be reported monthly to the Collaboration Committee. | ||
| 2. | Images. Photographs will be taken of T1 or T2 plants with distinctive morphological phenotypes affecting plant growth, development, biomass and seed production including fertility, stem morphology, tiller number and leaf morphology. Each photograph should include a Ceres white board labeled with the construct name, the event number and date and be written with a black broad tip erasable marker. | ||
| 3. | Labeled images with plant descriptions will be stored in the project database and image log with the date of entry. |
Page 35 of 47
 |
Protocol Number: | R3 | ||||
| Property of Ceres, Inc. | Protocol Name: | Rice Seed Harvesting | ||||
Authors:
|
Chuan-xxx Xx | Date Issued: | July 16, 2007 | |||
Approved By:
|
Version Number: | 1.0 | ||||
Purpose:
|
Harvest and track seeds from transgenic T0, and T1 rice plants. | |
Safety:
|
Follow universal lab safety techniques. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. |
| A. | T1 seed harvest |
| 1. | Harvest T1 seeds from individual T0 plants. | ||
| 2. | Harvest seeds by removing whole panicles. | ||
| 3. | Store in labeled envelopes with CW ID, event number and date, e.g. CW00001-01, MM/DD/YY. | ||
| 4. | Log harvested seed in the Seed Production Table (ANNEX II). |
| B. | T2 seed harvest |
| 1. | Harvest T2 seeds from individual T1 plants. | ||
| 2. | Harvest seeds by removing whole panicles. | ||
| 3. | Store in appropriately labeled paper envelopes with CW ID, event number, progeny number and date, e.g. CW00001-01-10, MM/DD/YY. | ||
| 4. | Log harvested seed in the Seed Production Table. |
| CW_ID | Date of Harvest | Harvested By | Generation of Seed | Location | Building | Room | Container
(Barcode) |
Amount (g) | Notes | |||||||||||||||||||||||||||
CW00006 |
1/1/2006 | LZ | T1 | SCUTA | AAA | XXX | 123456 | 2 | Notes on germination, amount used (2.1.2006) | |||||||||||||||||||||||||||
CW00003 |
1/1/2006 | LZ | T1 | SCUTA | BBB | XXX | 123457 | 3 | Poor germination (1.1.2007) | |||||||||||||||||||||||||||
CW00006-01 |
6/1/2006 | T2 | ICS | YYY | 123458 | 4 | ||||||||||||||||||||||||||||||
CW00010 |
7/1/2007 | CYW | F1 | ICS | YYY | 123459 | 0.5 | |||||||||||||||||||||||||||||
CW00010-01 |
10/1/2007 | XZ | F2 | ICS | YYY | 123460 | 2 | |||||||||||||||||||||||||||||
Page 36 of 47
 |
Protocol Number: | R4 | ||||||
| Property of Ceres, Inc. | Protocol Name: | Rice Drought Assay | ||||||
Authors:
|
Ning Su, Xxxxxx Xxxx, Chuan-xxx Xx |
Date Issued: | July 16, 2007 | |||||
Approved By:
|
Version Number: | 1.0 | ||||||
Purpose:
|
Test selected transgenic rice lines for enhanced drought tolerance. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. | |
Safety:
|
Follow universal lab safety techniques. Properly dispose of transgenic waste. |
Stock Solutions:
|
||
0.4% Finale |
| A. | Seedling Germination |
| 1. | Choose 50 seeds of good quality for each event to be assayed. The seeds of wild type should be prepared at the same time as a control. | ||
| 2. | Soak the seeds at room temperature for 3 days. | ||
| 3. | Sow the seeds in flats and cover the flats with a net or plastic to protect from animals. | ||
| 4. | Check for seed germination every day. Make sure there is enough water and the temperature is not too high. | ||
| 5. | Spray the herbicide Finale at day 8 after seed sowing, or at the time >95% seeds have germinated. (Add 4 ml Finale in one liter of water. Shake the solution thoroughly before spraying). Note: Do not spray the wild type control. | ||
| 6. | Spray the seedlings again with the Finale solution of the same concentration 5 days after the first spraying. | ||
| 7. | Count herbicide resistant and herbicide sensitive seedlings 8 days after the second spraying. Then allow the seedlings to grow another week before transplanting to soil. |
| B. | Transplant seedlings to pots |
| 1. | Transfer 2 transgenic (good resistance to herbicide) and 2 wild type seedlings to a pot. Use four pots for each event tested. Water the plants daily until the second tiller is visible. (2 xx xxxx). Note: Soil must be mixed uniformly and each pot must contain the same amount of soil. |
Page 37 of 47
| 2. | Stop watering plants. Record for each plant the date when leaves start to xxxx (the leaves start to roll up). Special attention should be paid to any difference between the transgenic and control plants within a pot. | ||
| 3. | Re-water the pot if all plants in a pot have wilted. Harvest grains and biomass (above ground only) when the plants mature. Dry up and weigh grain yield and biomass. | ||
| 4. | Take pictures for any observed phenotypes. |
Page 38 of 47

|
Protocol Number: | R5 | ||||
Property of Ceres,
Inc. |
||||||
| Protocol Name: | Low N Assay | |||||
Authors: Chuan-xxx Xx
|
Date Issued: | July 16, 2007 | ||||
Approved By:
|
Version Number: | 1.0 | ||||
Purpose:
|
Test selected transgenic rice lines for low N tolerance. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. | |
Safety:
|
Follow universal lab safety techniques. Properly dispose of transgenic waste. |
Stock Solution: herbicide |
0.4% Finale |
Equipment: |
SPAD 502 chlorophyll meter, Spectrum Technologies, Inc. (xxxx://xxx.xxxxxxxxxx.xxx/Xxxxxxxxxxx_Xxxxxx/xxxxx.xxxx) |
| A. | Seed germination |
| 1. | Choose 50 seeds of good quality for each event to be assayed. The seeds of wild type should be prepared at the same time as a control. | ||
| 2. | Soak the seeds at room temperature for 3 days. | ||
| 3. | Sow the seeds in flats and cover the flats with net or plastic to protect from animals. | ||
| 4. | Check for seed germination every day. Make sure there is enough water and that the temperature is not too high. | ||
| 5. | Spray seedlings with the herbicide Finale at day 8 after seed sowing, or at the time >95% seeds have germinated. Note: Do not spray the wild type control. (Add 4 ml Finale in one liter of water. Shake the solution thoroughly before spraying). | ||
| 6. | Spray the seedlings again with the Finale solution of the same concentration 5 days after the first spraying. | ||
| 7. | Count herbicide resistant and herbicide sensitive seedlings 8 days after the second spraying. Then allow the seedlings to grow another week. | ||
| 8. | Supply fertilizer in the field one day before transplanting. |
| B. | Transplant seedlings to the field |
| 1. | Transplant 15 seedlings per row, 2 rows per event. Transplant one row of wild type controls every 3 rows so that each row of transgenics has an immediate control neighbor. |
Page 39 of 47
| 2. | Supply fertilizer at day 5 and 10, respectively, after transplanting. Stop fertilizer supply until plants mature. | ||
| 3. | Observe plant growth daily and compare between transgenic and control plants to see if there is any difference, especially on leaf color. Take pictures for any observed phenotypes. | ||
| 4. | Record chlorophyll levels regularly (Minolta SPAD-502 chlorophyll meter, Spectrum technologies, Inc or a comparable instrument) until the termination of the experiment. | ||
| 5. | When plants reach maturity measure shoot length, tiller number and yield. |
Page 40 of 47

|
Protocol Number: | R6 | ||||
Property of Ceres,
Inc.
|
Protocol Name: | NIR Composition Assay | ||||
Authors: Xxxxxx Xxxxx, Xxxxx
Xxxxxx
|
Date Issued: | July 16, 2007 | ||||
Approved By:
|
Version Number: | 1.0 | ||||
Purpose:
|
The following instructions describe a sample collection and preparation protocol for collection of NIR spectra using the Bruker Optics MPA FT-NIR. | |
Safety:
|
Follow universal lab safety techniques. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. |
Capital Equipment:
|
Disposable Supplies: | |
Bruker Optics MPA
(xxx.xxxxxxxxxxxx.xxx).
|
Bruker part number: 301061 (package of 10 glass petri dishes, 94x20 mm, Xxxxxx DUROPLAN). | |
Part number: IN312/C rotating sample
cup (xxx.xxxxxxxxxxxx.xxx).
|
Standard NIST RM8491 sugarcane bagasse (see xxxxx://xxxxxx.xxxx.xxx/xxxx_xxxxxx.xxx?xxxx0000; and xxxxx://xxxxxx.xxxx.xxx/xxxxxxxxxxxxxx/xxxx_xxxxxxxxxxxxxx.xxx?xxxx0000) | |
Part number: IN312-B adaptor for glass petri
dishes (xxx.xxxxxxxxxxxx.xxx). |
| A. | Sample collection |
| 1. | Harvest fully senesced plants according to your region’s harvest time. | ||
| 2. | Harvest 5 randomly selected individual plants from each variety at each test site (if multiple reps, more samples are fine). Combine these plants into one sample. The target amount for each sample is minimum 20 g dry weight. | ||
| 3. | For very large plants, a sub-sample can be collected if care is taken to obtain a representative sub sample of the entire plant. | ||
| 4. | Freshly cut biomass samples should be placed in some sort of breathable bag or sack. e.g. Xxxxx paper, mesh, muslin or burlap. | ||
| 5. | Each bag should be labeled with a Sample ID — a unique sample identifier, written ON THE BAG (labels/tag, etc can fall off). For perennial species, record any information about the sample collection process that will be needed if we wish to return to the same plants in subsequent years. |
Page 41of 47
| 6. | Sample bags with cut biomass should bee sealed to prevent spilling — e.g. stapled , sewn, tied. |
| B. | Drying |
| 1. | Samples should be dried to < 10% moisture content prior to milling. | ||
| 2. | Ideally, samples should be dried for several days in a forced air oven set 45°C. **Keep oven at or below 45°C to maintain sample integrity. | ||
| 3. | Air drying is OK, provided the ambient relative humidity allows the samples to reach moisture content <10%. |
| C. | Milling |
| 1. | Mill dried biomass samples in a standard laboratory knife mill to pass a 2mm screen. Collect the entire milled sample to minimize separation of plant tissues. | ||
| 2. | Check blade sharpness periodically; dull blades cause temperatures to rise, compromising sample integrity and yield large amount of fines, skewing chemistry & conversion results); Ceres’ preferred xxxxx are Wiley Model 4 mill or Cyclone mill (for smaller sample amounts). | ||
| 3. | Clean mill thoroughly between samples to avoid cross contamination of samples. | ||
| 4. | Milled samples should be stored in sealed quart or gallon Ziploc freezer bag at room temperature. | ||
| 5. | After milling place sample ID and information inside each sample bag, include a 3” x 5” index card with these six pieces of information. For handwritten cards — please use a Sharpie or other permanent marker — preferably black. For printed labels, please use Ceres’ label template. |
| • | Sample ID | ||
| • | Plant Number (1-5) | ||
| • | Variety | ||
| • | Location | ||
| • | Harvest Date (MM/YYYY) | ||
| • | Weight of milled sample |
| D. | Spectroscopy |
| 1. | Turn on the instrument and lamp at least one hour prior to running samples | ||
| 2. | Check instrument dessicant and replace if necessary. | ||
| 3. | Run instrument diagnostics. Do not proceed until instrument passes all tests. | ||
| 4. | Run the QC sample each day at the beginning and end of each batch of samples. | ||
| 5. | Using a metal spatula, thoroughly mix the sample prior to removing a sample for spectroscopy. Avoid shaking the sample or any actin that cause the sample to separate by particle size. | ||
| 6. | Using a small scoop spatula, take four aliquots of material from different parts of the bag. Transfer each aliquot to a clean, dry Petri dish. |
Page 42 of 47
| 7. | **Close the Petri dish. Hold it by using your thumb on the cover and your middle finger at the base it (such as when holding a CD disk)and mix the material by shaking from left to right a couple of times. Avoid tapping or any action that causes the sample to separate by particle size. | ||
| 8. | Be careful at all times to avoid touching the Petri dish in areas within the spectroscopic window. | ||
| 9. | Make sure that your sample holder in the machine is free of any debris. Remove the top of the Petri dish and place only the base of your dish with the sample in it in the sample holder | ||
| 10. | Scan the sample using the instrument method provided. Repeat from ** until three spectra have been collected. | ||
| 11. | Average the three spectra and save the averaged spectrum. Only this spectrum will be forwarded to Ceres for analysis |
Page 43 of 47
 Property of Ceres, Inc. |
Protocol Number: | R7 | ||||
| Protocol Name: | Destruction of Biomaterials | |||||
Authors:
|
Xxxx Xxxxxxx | Date Issued: | July 16, 2007 | |||
Approved By:
|
Version Number: | 1.0 | ||||
Purpose:
|
Insure that all CW seed are eventually destroyed. | |
Safety:
|
Practice universal safety techniques in the lab. |
Start Product(s):
|
CW line seed stocks. | |
End Product(s):
|
All CW seeds should be destroyed by the end of the contract. |
| 1. | Must maintain a log of all CW seed stocks generated (see Seed Production Table, ANNEX IV). | |
| 2. | Store individual stocks in a sealed clearly labeled envelope or a sealed paper bag. Each seed line is logged and stored inside a locked metal cabinet inside a locked room. Access to the seed storage cabinets is limited to those involved in the experiments. | |
| 3. | Track seed utilization in the seed log. Review list of CW seed stocks annually with Ceres. Destroy unneeded seed stocks by autoclaving at 121 °C for 15 min. |
Page 44 of 47
[NAME] ________________________, an individual, of [ADDRESS]
| 1. | Purpose. | ||
| Ceres has entered into a Collaboration Agreement with ICS effective November 15, 2007 (the “Collaboration Agreement”). In the framework of the Collaboration Agreement, (i) the Receiving Party may have access to certain information of a confidential or proprietary nature of Ceres and (ii) the Receiving Party may make or contribute to inventions that will be assigned to Ceres pursuant to such agreement. | |||
| 2. | Confidential Information. | ||
| Confidential Information is any information disclosed by Ceres in the framework of the Collaboration Agreement and any material, information, data, technology or other findings or inventions resulting from the work performed under the Collaboration Agreement. Confidential Information may be disclosed or provided in oral, written, graphic, photographic or any other form or may be acquired by the Receiving Party through inspection, observation or overhearing, whether advertent or inadvertent. | |||
| 3. | Protection of Confidential Information. |
| 3.1 | The Receiving Party agrees (i) to hold Ceres’ Confidential Information in strict confidence and to take all reasonable precautions to protect such Confidential Information (ii) not to divulge any of the Confidential Information or any information derived therefrom to any third person except ICS employees, collaborators or graduate students performing work under the Collaboration Agreement, and (iii) not to make any use whatsoever at any |
Page 45 of 47
| time of the Confidential Information except for the purpose of the implementation of the Collaboration Agreement. | |||
| 3.2 | The obligations in Section 3.1 shall not apply with respect to any information that the Receiving Party can demonstrate by competent evidence: |
| - | was lawfully in his/her possession without restriction prior to disclosure by Ceres, as evidenced by written records of the Receiving Party; | ||
| - | was in the public domain prior to disclosure by Ceres, or subsequently comes in the public domain through no fault, action or omission of the Receiving Party; or | ||
| - | is made available to the Receiving Party, without any restriction, by a third party who had the right to do so. |
| 4. | Ownership. | ||
| The Confidential Information including any material support containing Confidential Information will remain the exclusive property of Ceres and the Receiving Party will not acquire any right, title, license or interest on or to the Confidential Information, the supports containing Confidential Information, or any patent covering Confidential Information. | |||
| 5. | Inventions. | ||
| Pursuant to the Collaboration Agreement, ICS shall promptly inform Ceres of, and assign its rights in, any invention made or contributed to during the implementation of such agreement. In order to enable ICS to comply with such obligation, the Receiving Party shall promptly disclose to the Research Director at ICS and, to the extent that rights do not automatically vest in ICS, assign to ICS any invention made or contributed to by her/him in the framework of the implementation of the Collaboration Agreement. The Receiving Party shall render such assistance to ICS and Ceres as may be required for assigning the aforementioned rights to Ceres or Ceres’ designee and for protecting such invention, including but not limited to the signing of documents. The Receiving Party will be recognized in patent applications on the inventions referred to in this Article 5 in accordance with U.S. patent law. | |||
| 6. | Term. | ||
| This Agreement will be valid until all Confidential Information is in the public domain. | |||
| 7. | Notices. | ||
| Any notice or request delivered pursuant to this Agreement shall be deemed given when actually received or three days after mailed first class postage prepaid or dispatched by courier service to the Receiving Party’s address set forth hereinafter (whichever is earliest): |
Page 46 of 47
If to Ceres, Inc.:
|
Ceres, Inc. | ||
| 0000 Xxxxxx Xxxxxx Xxxx | |||
| Xxxxxxxx Xxxx, Xxxxxxxxxx 00000 | |||
| Telephone Number: (000) 000-0000 | |||
| Facsimile Number: (000) 000-0000 | |||
| Attention: Vice President of Product Development | |||
| cc: Legal Department | |||
If to the Receiving Party:
|
[NAME] | ||
| [ADDRESS] | |||
| or at such address as shall be designated in writing by such Party to the other Party upon at least ten (10) days prior notice thereof. |
| 8. | Governing Law/Jurisdiction. | ||
| This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the State of California without regard to principles of conflicts of law. The Parties agree that any dispute regarding the interpretation or validity of, or otherwise arising out of this Agreement, shall be subject to the exclusive jurisdiction of the California State Courts of San Francisco County or, in the event of federal jurisdiction, the United States District Courts of the Northern District of California, and each Party agrees to submit to the personal and exclusive jurisdiction and venue of the courts and not to seek the transfer of any case or proceeding out of such courts. |
| CERES, INC. | RECEIVING PARTY | |||||
Signature:
|
Signature: | |||||
Name:
|
Chuan-xxx Xx | Name: | ||||
Title:
|
Manager, Crop Transformation and Gene Evaluation | Title: | ||||
Signature: |
||||||
Name:
|
Xxxxx Xxxxxx | |||||
Title:
|
Vice President of Product Development | |||||
Page 47 of 47
| 1. | The Parties agree that the activities defined in the [***] Program Plan in ANNEX I-A attached hereto will be included in the Program. Activities under the Program Plan in ANNEX I relating to rice will be decreased as directed by Ceres. Whenever reference is made in the Agreement to ANNEX I, except in Article 5.1, this reference shall forthwith read “ANNEX I and ANNEX I-A”. | ||
| 2. | The Program Budget in ANNEX III remains unchanged and covers both the activities defined in ANNEX I (as decreased pursuant to Ceres’ directors) and the activities defined in ANNEX I-A. It is anticipated that the decrease of the activities under ANNEX I for Years 3 through 5 will amount to approximately fifteen percent (15%) of the Program Budget. | ||
| 3. | The type of information with respect to [***] Program Plan activities to be included in the reports and formatting is set forth in ANNEX I-A and ANNEX II-A attached hereto. | ||
| 4. | ICS agrees to comply with the Additional Stewardship Guidelines included in ANNEX IV-A attached hereto. The Stewardship Guidelines in ANNEX IV and the Additional Stewardship Guidelines in ANNEX IV-A apply to all activities under the Agreement, whether defined in ANNEX I or in ANNEX I-A. | ||
| 5. | The Parties agree that this Amendment I is effective as of March 5, 2009. | ||
| 6. | For the remainder, the Agreement remains unchanged and this Amendment I shall form an integral part thereof. |
Page 1 of 28
| Institute of Crop Sciences | Ceres, Inc. | |||||
| Chinese Academy of Agricultural Sciences | ||||||
By:
|
/s/ Jian-Min Wan | By: | /s/ Xxxxx Xxxxxxx | |||
Name:
|
Jian-Min Wan | Name: | Xxxxx Xxxxxxx | |||
Title:
|
Director | Title: | Director of Trait Development | |||
By:
|
/s/ Hu-Qu Zhai | By: | /s/ Xxxxxxx Xxxxxxx | |||
Name:
|
Hu-Qu Zhai | Name: | Xxxxxxx Xxxxxxx, CBE, FRS | |||
Title:
|
President | Title: | Chief Scientific Officer | |||
Page 2 of 28
| to the Collaboration Agreement between Ceres and ICS dated November 15, 2007 |
| 1.1 [***] PROGRAM ACTIVITIES | ||
| The goal of the [***] PROGRAM ACTIVITIES project is to generate and evaluate selected [***] gene constructs [***] . | ||
| CERES ACTIVITIES | ||
| 1.1.1 | Design of constructs: Constructs will be made according to the specifications below. Ceres will provide: |
| 1.1.1.1 | The six [***] vectors (plasmid and map), and sequences for [***] | ||
| 1.1.1.2 | Protocol for establishing [***] for transformation | ||
| 1.1.1.3 | Protocol for T-DNA integration analysis | ||
| 1.1.1.4 | [***] protocol and [***] for [***] quantification | ||
| 1.1.1.5 | A list of [***] for [***] testing |
| 1.1.2 | [***] Transformation: Ceres will [***] provide a [***] transformation protocol. | ||
| 1.1.3 | Prioritization: Prioritization of constructs for transformation, prioritization of existing events for genetic crossing, and prioritization of existing events for plant assay. | ||
| 1.1.4 | Quality control: Suggest gene-specific primer sequences and vector primer sequences for use in quality control experiments using gene-specific PCR. | ||
| 1.1.5 | Data formats: Prescribe data formats for recording results (ANNEX II). |
| 1.2 | ICS ACTIVITIES |
| 1.2.1 | Gene Construction: Develop up to 10 constructs based on DNA sequence information and maps provided by Ceres. Ceres will provide the following vectors: [***]. |
Page 3 of 28
| 1.2.2 | Perform transformation according to the [***] Transformation Protocol. ICS will also track and deliver quarterly updates for selected Program KPIs (ANNEX II). |
| 1.2.2.1 | Transformation: Transform up to 5 constructs into [***] lines provided by Ceres. Produce and collect seeds from up to 100 independent events (T0 plants) for each construct (SOP R1). Maintain updated Program database that includes articles 2.2.1.1.1 to 2.2.1.1.5. Update Ceres monthly. |
| 1.2.2.1.1 | T0 production: See the Transformation Table and KPI 1.1 (ANNEX II). | ||
| 1.2.2.1.2 | T0 plant phenotyping: See SOP R1. Include descriptions, images and dates. | ||
| 1.2.2.1.3 | T1 seed production: See SOP R3, the Seed Production Table and KPI 1.3 (ANNEX IV). | ||
| 1.2.2.1.4 | Quality control: See SOP R1 and R2, the Transformation Table, the Quality Control Table and KPI 1.2 (ANNEX IV). |
| 1.2.2.2 | T1 phenotyping — plant growth and development: Analyze T1 progeny from all events from article 2.2.1.1 for growth and development phenotypes. Maintain updated Program database that includes articles 2.2.1.2.1 to 2.2.1.2.2. Update Ceres monthly. |
| 1.2.2.2.1 | T1 plant phenotyping: See SOP R2 (ANNEX IV). Include descriptions, coded comments, images and data entry dates. Maintain a phenotype tracking database. | ||
| 1.2.2.2.2 | T2 seed production: See SOP R3 and the Seed Production Table (ANNEX II, Table 3). |
| 1.2.2.3 | T2 phenotyping: Upon request selected lines will be chosen for additional phenotyping. Resources used for T2 plant phenotyping should not exceed prescribed Program resources without the consent of the Collaboration Committee. See SOP R2 (ANNEX IV). |
| 1.2.2.4 | Trait assays: Selected lines will be assayed for [***]. SOPs to be discussed. |
| 1.2.2.4.1 | [***]: Selected events will be assayed for [***]. |
Page 4 of 28
| 1.2.2.4.2 | Genotyping quality control: All events that are selected for assaying will be sequence validated using gene-specific PCR. |
| 1.2.2.4.3 | Images: Photographs will be taken of all plants that display a distinct morphological phenotype. Images with and without labels and plant descriptions will be stored in an image database and the Image Log Table (ANNEX II, Table 6). |
| 2. | REPORTS |
| 2.1 | Deliver Program Reports to the Collaboration Committee on a quarterly basis at the end of each calendar quarter. Reports shall include, but not be limited to: |
| 2.1.1 | KPI summary. Gather KPI data according KPIs listed in Section 1 ANNEX II and improve underlying processes. | ||
| 2.1.2 | Transformation update: Updated Transformation Table including the identification and number of constructs transferred to [***] and summary of T0 QC analysis (ANNEX II, Table 1 and 2). | ||
| 2.1.3 | T1 and T2 seed production update: Updated Seed Production Table (ANNEX II, Table 3). | ||
| 2.1.4 | T1 plant phenotyping update: |
| 2.1.4.1 | Phenotyping descriptions |
| 2.1.4.1.1 | Seedling emergence stand count 30 days post planting | ||
| 2.1.4.1.2 | Seeding vigor rating | ||
| 2.1.4.1.3 | Plant height in cm at flowering (if notably different from parent) | ||
| 2.1.4.1.4 | Tillering (if notably different from parent) | ||
| 2.1.4.1.5 | Plant health rating (1-9) at flowering | ||
| 2.1.4.1.6 | Lodging | ||
| 2.1.4.1.7 | [***] | ||
| 2.1.4.1.8 | [***] | ||
| 2.1.4.1.9 | [***] |
Page 5 of 28
| 2.1.5 | Trait assay update: Summary of trait assay results, measurements and images. |
| 3. RESOURCES AND WORK PLAN | ||
| Resources as specified in the Agreement will be allocated to this project as follows: | ||
| Ceres will deliver a vector backbone for [***] to China. The work will be divided into 2 phases. The first phase will include vector construction to preliminary event selection. The second phase will be the testing and development phase. | ||
| Duration | ||||||||
| Task | FTE | (months) | ||||||
Phase I — Vector construction to preliminary event
selection |
[***] | [***] | ||||||
[***] synthesis and cloning |
[***] | [***] | ||||||
[***] transformation protocol development and
validation in China |
[***] | [***] | ||||||
[***] transformation and plant regeneration (200 each) |
[***] | [***] | ||||||
Grow and observe primary transformants in ICS
greenhouse |
[***] | [***] | ||||||
Transgenic plant (seedlings) analysis and [***]
expression level measuring (RT-PCR, [***]) |
[***] | [***] | ||||||
Test [***] efficacy with transgenic plants |
[***] | [***] | ||||||
Transgenic plant seed collection |
[***] | [***] | ||||||
Segregation analysis |
[***] | [***] | ||||||
Insert quality evaluation |
[***] | [***] | ||||||
Phase II — [***] development |
||||||||
[***] |
[***] | [***] | ||||||
[***] |
[***] | [***] | ||||||
[***] |
[***] | [***] | ||||||
[***] |
[***] | [***] | ||||||
Selection of preferred events |
[***] | |||||||
Additional [***] testing |
[***] | [***] | ||||||
[***] event selection and [***] |
||||||||
| 4. REMUNERATION AND PAYMENT SCHEDULE |
| 4.1 | As specified in the Agreement. |
Page 6 of 28
| Number of | Regeneration | Plantlets | Soil Transplant | T0 | T1 Harvest | T1 Events | ||||||||||||||||||||
| Trans ID | Construct ID | Construct Date | Made by | Agro Growth | Infection Date | Resistant [***] | date | Generated | date | location | Notes | Date | Harvested | |||||||||||||
| Notes on | ||||||||||||||||||||||||||
| germination, amount | ||||||||||||||||||||||||||
| 1 | 1/1/2009 | LZ | Yes | 2/1/2009 | 50 | 6/1/09 | 40 | 9/1/09 | ICS | used (10/1/09) | 12/1/09 | 30 | ||||||||||||||
| Poor | ||||||||||||||||||||||||||
| 2 | 1/1/2009 | LZ | Yes | 2/1/2009 | 20 | 6/1/09 | 10 | 9/1/09 | ICS | germination (10/1/09) | 12/1/09 | 9 | ||||||||||||||
| 3 | 6/1/2009 | No | 7/1/2009 | 21 | 12/1/09 | 10 | 3/1/09 | ICS | 8 | |||||||||||||||||
| 4 | 7/1/2009 | [***] | No | 8/1/2009 | 21 | 1/1/10 | 9 | 4/1/10 | ICS | 7 | ||||||||||||||||
| 5 | 10/1/2009 | XZ | Yes | 11/1/2009 | 60 | 3/1/10 | 50 | 6/1/10 | ICS | 45 |
| Column N | Description | |
Trans ID
|
Unique number for a particular transformation event | |
Construct ID
|
Number assigned to the construct | |
Construct date
|
Date the construct was completed | |
Made by
|
Who made the construct | |
Agro growth
|
Yes/No did the agro grow | |
Infection date
|
Date [***] infection was performed | |
Number of Resistant [***]
|
Number of resistant [***] resulting from the infection | |
Regeneration date
|
Date plantlet regeneration started | |
Plantlets regenerated
|
Number of resistant [***] which give rise to plants | |
Soil transplant date
|
Date plantlets were transplanted to soil | |
T0 location
|
Location of T0 plants | |
Notes
|
Any observations made while T0 plants are in soil | |
T1 harvest date
|
Date of T1 seed harvest | |
T1 events harvested
|
Number of T1 seed lots harvested |
| Date of | Generation | Container | ||||||||||||||||
| [***]_ID | Harvest | Harvested By | of Seed | Location | Building | Room | (Barcode) | Amount (g) | Notes | |||||||||
| Notes on | ||||||||||||||||||
| germination, amount | ||||||||||||||||||
| [***] | 1/1/2009 | LZ | T1 | SCUTA | AAA | XXX | 123456 | [***] | used (2.1.2006) | |||||||||
| Poor germination | ||||||||||||||||||
| [***] | 1/1/2009 | LZ | T1 | SCUTA | BBB | XXX | 123457 | [***] | (1.1.2007) | |||||||||
| [***] | 6/1/2009 | T2 | ICS | YYY | 123458 | [***] | ||||||||||||
| [***] | 7/1/2009 | CYW | F1 | ICS | YYY | 123459 | [***] | |||||||||||
| [***] | 10/1/2009 | XZ | F2 | ICS | YYY | 123460 | [***] |
| Image | ||||||||||||
| [***]_ID | Image _ID | Description | Trait | Phenotype | Image Type | Location | ||||||
| [***] | 87654321 | Whole plant and controls | [***] | None observed | Phenotype | SCUTA | ||||||
| [***] | 87654322 | Plot image | [***] | [***] | Assay Result | ICS | ||||||
| [***] | 87654323 | Field shot | [***] | None [***] | Field Trial | CHANGPING |
| Plant Position | ||||||||||||||||||||||||||||||||||||||||||||||||
| Measurement | ||||||||||||||||||||||||||||||||||||||||||||||||
| Block | Row | CW Event | Measurement | Code | Name | Date | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | Plant Status | 1 | 5/31/2007 | [***] | [***] | sick | yellow | |||||||||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | Is this KI? | 2 | 5121/2007 | Y | ||||||||||||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | Height | 3 | 6/1/2007 | 72 | 68 | 59 | 68 | 66 | 60 | |||||||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | Flowering Time | 4 | 6/1/2007 | Y | Y | |||||||||||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | Flowering Time | 4 | 6/2/2007 | Y | Y | Y | Y | |||||||||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | Flowering Time | 4 | 6/3/2007 | Y | Y | Y | ||||||||||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | QC result | 7 | 6/7/2007 | + | - | + | ||||||||||||||||||||||||||||||||||||||||
10
|
1 | GW00222-01 | Biomass | 5 | 6/28/2007 | |||||||||||||||||||||||||||||||||||||||||||
| 1. | Key Performance Indicators (KPIs) |
| 1.1. | Number of constructs yielding >10 T0 plants delivered each quarter vs. all constructs delivered each quarter. | ||
| 1.2. | Number of constructs sequence validated in T0 plants each quarter vs. all constructs assayed in T0 each quarter. | ||
| 1.3. | Number of constructs yielding T1 seeds from >10 events each quarter vs. all T1 seeds delivered each quarter. | ||
| 1.4. | Number of constructs sequence validated in T1 events each quarter vs. all events assayed in T1. | ||
| 1.5. | Time from infection to T1 seed harvest each quarter. | ||
| 1.6. | Number of constructs evaluated for predicted phenotype or trait. | ||
| 1.7. | Number of constructs yielding predicted phenotype or trait vs. all constructs phenotyped or assayed. | ||
| 1.8. | Number of constructs yielding novel phenotypes vs. all constructs phenotyped and assayed. | ||
| 1.9. | Number of constructs showing yield drag or negative traits vs. all constructs analyzed. |
| 2. | Screenhouse Maps |
| 2.1. | Plot map for Screenhouse 1 and Screenhouse 2 at SCUTA |
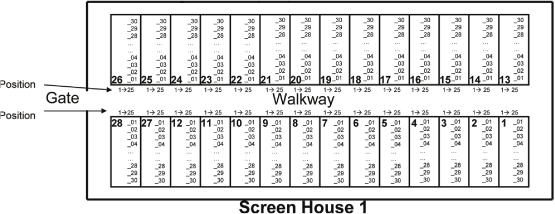
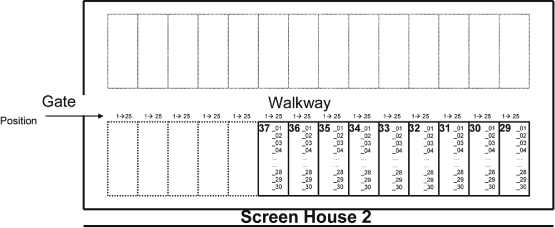
| to the Collaboration Agreement between Ceres and ICS dated November 15, 2007 |
| 1. | the Excellence Through Stewardship Quality Management Program Guide to Maintaining Plant Product Integrity of Biotechnology-derived Plants (provided herewith). |
| 2. | Ceres has licensed in the bar gene from Bayer BioScience, N.V. (“Bayer”). Pursuant to the License Agreement with Bayer Ceres has to comply with and charge its subcontractors with the Stewardship Guidelines set forth hereinafter or any reasonable stewardship guidelines as Bayer may provide. | |
| BAYER and LICENSEE agree they have a common interest to ensure that any material containing the bar Gene alone or in combination with the 35S Promoter is handled in such a way that no safety or regulatory issues arise from its use. Therefore, the Parties agree that LICENSEE will maintain an internal stewardship program ensuring the appropriate handling of the aforesaid material in order to assure particularly, but not exclusively containment, confinement and traceability. | ||
| The LICENSEE confirms that all employees and/or contractors working at any stage with the Material will be trained to ensure compliance with the stewardship program and appropriate documentation will be maintained as evidence of training and compliance. | ||
| The LICENSEE shall follow all necessary biosafety requirements during the handling and any transportation of the Material. | ||
| The Parties agree that BAYER may request a stewardship audit to be conducted by an independent auditor(s) agreed upon by both Parties, a maximum of one (1) time per calendar year per location where research is taking place (except in case of default; see below). The auditor(s) will, upon BAYER’s request, notify LICENSEE of desire to conduct an audit, giving minimum of five (5) working days notice of such audit. It is expressly agreed upon by the Parties that non-inspection or non-action on the part of BAYER shall not be construed in any way as an approval of procedures and precautions practiced by LICENSEE and does not remove, reduce or affect in any way the responsibility and liability of LICENSEE as set forth in the Agreement. | ||
| BAYER will specify to the auditor(s) which aspects of LICENSEE’s use of the bar Gene alone or in combination with the 35S Promoter are to be audited, and specifically what activities in relation to such use must be taken into account. This can include specific handling, containment and monitoring procedures (laboratory, greenhouse or field use, etc.); locations (e.g. audit of all activities involving the bar Gene alone or in combination with the 35S Promoter at a particular LICENSEE location); or combinations thereof, and will be communicated to LICENSEE upon notification of an audit. | ||
| LICENSEE will provide to auditor access to any and all stewardship documentation (laboratory notebooks, records of shipment, copies of legal permits for field trials, records of monitoring, etc.) to allow auditor(s) to make a full assessment of LICENSEE’s compliance |
Page 10 of 28
| with local requirements and use of the bar Gene alone or in combination with the 35S Promoter. LICENSEE agrees to share other documentation (stewardship guidelines, best practices, SOPs, etc.) with auditor(s), as requested. LICENSEE agrees to allow auditor(s) to question LICENSEE’s staff concerning specific practices and procedures of relevance. LICENSEE will also provide to auditor(s) access of reports of all external audits conducted by regulatory authorities as part of their compliance procedures, the conclusions of which will be transferred to BAYER subject to BAYER’s confidentiality obligations in Article 10. | ||
| Auditor(s) will prepare a report, to be provided simultaneously to both Parties, indicating the following: Compliance with applicable legal requirements (Yes/No); if Yes, any specific or general recommendations for improvements; if No, nature and location of defaults and recommended actions to correct such. It is expressly understood that the auditor(s) will not provide BAYER with any confidential information concerning LICENSEE’s use of the bar Gene alone or in combination with the 35S Promoter. | ||
| LICENSEE will have sixty (60) days to remedy such default to BAYER’s satisfaction. BAYER has the right to request a follow-up audit, at LICENSEE’s expense, to verify corrective action has been taken and the default remedied. | ||
| Any deviation from these Stewardship Guidelines should be reported to Xxxxxx Xxxxxx, Telephone: x00(0)0000 000000, E-mail: Xxxxxx.Xxxxxx@XXXXXxxxxxxxxxxx.xxx |
| 3. | the following guidelines are provided to prevent the pilferage, vandalism, or escape of transgenic [***] in China. |
| 3.1 | Plantlet transport. Transgenic [***] plantlets will be transported by ICS personnel from the lab to the field in a clearly labeled, secured and sealed container. Unused plants will be destroyed by autoclaving according to SOP R7. | ||
| 3.2 | Plant growth. Transgenic [***] will be grown in a secure and contained growth room, greenhouse, screenhouse or in a field approved for the growth of transgenic rice. No [***] rice plants will be grown in the same greenhouse, screenhouse or approved field with transgenic [***] . | ||
| 3.3 | Seed storage. Each seed line will be contained within a sealed clearly labeled envelope or a sealed paper bag. Each seed line is inventoried and stored inside a locked cabinet inside a secure room. Access to the seed storage cabinets is limited to those involved in the experiments. Seed utilization and disposal will be tracked using a log system. Unused seeds will be destroyed by autoclaving according to SOP R7. | ||
| 3.4 | Seed transport. Each seed line will be transported in a sealed, clearly labeled envelope. Envelopes will be shipped in a clearly labeled, secured and sealed container within a shipping box to prevent spillage if the outside packaging gets damaged. |
| 3.5 | Seed destruction. The maintenance of a transgenic [***] seed log is the responsibility of ICS. The inventory of transgenic seeds will be reviewed annually. Seed stocks designated by Ceres as no longer needed will be destroyed by autoclaving as described in SOP R7. Their destruction will be recorded in the seed log. |

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version | |||
| Number: |
| 3.2.1. | Dispose the unused Agrobacterium culture in the biological waste container | ||
| 3.2.2. | Dispose all the excess [***] and plant materials in the biological waste container |
| • | To avoid misidentification, request HTPC group to use printed labels on tubes when handing over Agrobacterium to transformation group. The label should contain Gemini ID, date of inoculation and the initials of the person who inoculated the culture. |
| • | Agrobacterium culture tubes during re-inoculation, keep the tubes that belong to the same Gemini ID together. Check the label on the tubes before each inoculation. | ||
| • | Always check the label on the lid of the Petri dish before subculturing. | ||
| • | Sub-culture just one construct at a time to avoid mix up. Once you finish subculturing a construct, label the plates and then go on to the next construct. | ||
| • | Print labels to avoid mislabeling the Petri dish. |
| • | Print labels to avoid mislabeling. | ||
| • | Count the number of plates used for sub-culturing each construct. In the next stage, make sure that the count matches. For example, if you used 15 plates to subculture one construct from co-cultivation to selection, make sure that there are 15 plates when you transfer the [***] from selection to regeneration. | ||
| • | Enter all the data in the work sheet, so it is easy to refer. |
Page 13 of 28

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version | |||
| Number: |
| • | Check the database to verify if you are handing over the transformants for the right person who requested the transformation of that particular construct. | ||
| • | Ask the requestor, to sign off those transformants to indicate that they have received the plants. | ||
| • | Enter the hand off information in the oracle database and worksheets. The hand off information should include the date of hand off, the person to whom the transformants were delivered and the number of transformants that were delivered. |
| • | Request the HTPC group to send the list of constructs that have to be used for transformation. The list should contain the following information |
| • | Gemini ID | ||
| • | Name of the requestor | ||
| • | Project |
| • | Target organism | ||
| • | Number of transformants needed | ||
| • | Selection marker |
| • | Refer to 5.1. |
| • | Refer to 4.4. |
| • | Refer to 5.4. |
| • | Refer to [***] Integrity Concerns (No. 4) |
| • | Create worksheets for each transformation experiment. | ||
| • | Enter data in the worksheets immediately after a specific task has been completed. | ||
| • | Enter the data in oracle database. |
Page 14 of 28

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version | |||
| Number: |
| • | Refer to 7.2. |
| • | Request the HTPC group to send a transformation request email with the list of constructs that have to be used for transformation. The list should contain the following information |
| • | Gemini ID | ||
| • | Name of the requestor | ||
| • | Project | ||
| • | Target organism | ||
| • | Number of transformants needed | ||
| • | Selection marker |
| • | Print the transformation request email and attach it to the worksheet. |
| • | Refer to 4.4. |
| • | Check the [***] of the [***] that was used for transformation. | ||
| • | Check if the right selection was used. | ||
| • | Check if a new vector was used. | ||
| • | Sequence the selection marker to confirm that it is intact | ||
| • | Analyze the promoter driving the selection marker gene | ||
| • | Analyze the effects of the trait gene |
| • | Refer to 5.1. |
| • | Refer to 4.4. |
Page 15 of 28

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version | |||
| Number: |
| • | Refer to 7.2. |
| 11.2. | Document Process Improvement plans and results |
| 11.3. | Document handoff to PI |
| • | Refer to 4.4. |
| • | Construct ID | ||
| • | Person to whom you will deliver the transgenic plants | ||
| • | How many events were requested | ||
| • | How many events were delivered | ||
| • | Material destroyed | ||
| • | Attach spreadsheet template to this SOP— [***] transformation database |
| Revision | Date | Description of Change | Approval and Date | |||||||||||
Page 16 of 28

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version | |||
| Number: |
MATERIALS |
||
Disposable Supplies:
|
Capital Equipment: | |
100 x 20 xx Xxxxx dishes (25373-143; VWR)
|
Laminar Flow Hood (Nuaire) |
Page 17 of 28

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version | |||
| Number: |
Gloves (32916-532; Microflex Corp.)
|
66 Series Incubator (Percival Scientific) | |
Whatman 113 filter papers (VWR)
|
Micro-centrifuge (Heraeus Instrument) | |
50 xx Xxxxxxx conical tube (Xxxxxx Scientific)
|
Stock Solutions: | |
3M first-aid tape (3M)
|
[***] | |
Sands
|
[***] | |
[***] |
[***] | |
[***] |
[***] | |
[***] |
[***] | |
[***] |
[***] | |
[***] |
[***] | |
[***] |
[***] | |
[***]
|
Magenta Boxes (GA7; Magenta Corp), | |
[***]
|
Forceps | |
[***] |
||
[***] |
||
[***] |
||
[***] |
||
[***] |
||
[***] |
||
[***] |
||
[***] |
Page 18 of 28

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version Number: |
[***] |
||||
[***] |
||||
[***] |
||||
[***] |
||||
[***] |
||||
[***] |
||||
[***] |
||||
[***]
|
[***] | |||
[***]
|
[***] |
| o [***] | ||
| o [***] |

|
Protocol Number: |
Leave this blank. | ||
Property of Ceres, Inc.
|
Protocol Name: | [***] Transformation | ||
Authors: Xxxxxxx Xxxxx
|
Date Issued: | 12/30/[***] | ||
Approved By: Chuan-Xxx Xx
|
Version Number: |
| o [***] | ||
| o [***] | ||
| [***] |
[***]
| o [***] | ||
| o [***] | ||
| o [***] |
| o [***] | ||
| o [***] | ||
| o [***] | ||
| o [***] |

|
Protocol | Leave this blank. | ||||
| Number: |
||||||
Property of Ceres, Inc.
|
Protocol Name: |
[***] Transformation | ||||
Authors:
|
Xxxxxxx Xxxxx | Date Issued: | 12/30/[***] | |||
Approved By:
|
Chuan-Xxx Xx | Version | ||||
| Number: |
Page 21 of 28

|
Protocol | Leave this blank. | ||||
| Number: |
||||||
Property of Ceres, Inc.
|
Protocol Name: |
[***] Transformation | ||||
Authors:
|
Xxxxxxx Xxxxx | Date Issued: | 12/30/[***] | |||
Approved By:
|
Chuan-Xxx Xx | Version | ||||
| Number: |
Page 22 of 28

|
Protocol | Leave this blank. | ||||
| Number: |
||||||
Property of Ceres, Inc.
|
Protocol Name: |
[***] Transformation | ||||
Authors:
|
Xxxxxxx Xxxxx | Date Issued: | 12/30/[***] | |||
Approved By:
|
Chuan-Xxx Xx | Version | ||||
| Number: |
Page 23 of 28

|
Protocol Number: R2 | |
| Property of Ceres, Inc. | Protocol Name: Plant Growth and Development Phenotyping | |
| Authors: Chuan-xxx Xx | Date Issued: December 30, 2008 | |
| Approved By: | Version Number: 1.0 |
Purpose:
|
This protocol is for the phenotyping of T1 and T2 transgenic [***] plants. | |
Safety:
|
Follow universal lab safety techniques. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. |
| 1. | Measurements to be taken for all T1 plants |
| a. | Flowering time: Number of days from transplanting to heading of the panicle. | ||
| b. | Plant height: From the ground to the top of the panicle. | ||
| c. | Seed yield: Weight of seeds while still on panicles, including the envelope. |
| 2. | General observations To be noted in cases where the frequency is higher than expected based on control plants. Use coded comments whenever possible to describe plants (see ANNEX IV, Data |
Page 24 of 28

|
Protocol Number: R2 | |
| Property of Ceres, Inc. | Protocol Name: Plant Growth and Development Phenotyping | |
| Authors: Chuan-xxx Xx | Date Issued: December 30, 2008 | |
| Approved By: | Version Number: 1.0 |
| Collection Table). A phenotyping update should be presented monthly to the Collaboration Committee. |
| a. | Growth vigor: Normal (no entry), improved (1), poor (2) |
| b. | Leaf color: Normal (no entry), darker green (1), yellow-green (2) | ||
| c. | Fertility: Normal (no entry), reduced (1). | ||
| d. | Lodging tolerance: Normal (no entry), increased lodging (1). | ||
| e. | [***] to [***] and [***]: Normal (no entry), increased [***] | ||
| f. | Stress tolerance (drought, cold, heat): Normal (no entry), increased tolerance (1), hypersensitive (2). | ||
| g. | Flag leaf color at maturity: Normal (no entry), more green than controls (1), more yellow than controls (2). | ||
| h. | Flag leaf angle from vertical: Normal (no entry), decreased leaf angle (1), increased leaf angle (2). |
| 7. | Optional measurements for selected events (upon request from Ceres) |
| a. | Fertility: Number of filled seeds divided by number of spikelets on a panicle (average of 3 panicles). | ||
| b. | Panicle size: Number of seeds per panicle, averaged from three panicles. | ||
| c. | Seed yield2: Weight of total seeds less panicles and bag for lines showing 10 percent increase. | ||
| d. | Seed fill rate: Number of days from flowering [***]. |
| 1. | Events with acceptable phenotypes will be genotype confirmed using gene specific PCR. | |
| 2. | Images. Photographs will be taken of T1 or T2 plants with distinctive morphological phenotypes affecting plant growth, development, biomass and seed production including |
Page 25 of 28
| Protocol Number: | R2 | |||||
 |
||||||
| Property of Ceres, Inc. |
Protocol Name: | Plant Growth and Development Phenotyping | ||||
Authors:
|
Chuan-xxx Xx | Date Issued: | December 30, 2008 | |||
Approved By:
|
Version Number: | 1.0 | ||||
| fertility, stem morphology, tiller number and leaf morphology. Each photograph should include a Ceres white board labeled with the construct name, the event number and date and be written with a black broad tip erasable marker. | ||
| 11. | Labeled images with plant descriptions will be stored in the project database and image log with the date of entry. |
Purpose:
|
Harvest and track seeds from transgenic T0, and T1 [***] plants. | |
Safety:
|
Follow universal lab safety techniques. | |
Stewardship:
|
Properly manage and dispose of transgenic plant material. |
| 1. | Harvest T1 seeds from individual T0 plants. | ||
| 2. | Harvest seeds by removing whole panicles. | ||
| 3. | Store in labeled envelopes with [***] ID (‘[***]’), event number and date, e.g. [***]00001-01, MM/DD/YY. | ||
| 4. | Log harvested seed in the Seed Production Table (ANNEX II). |
| 1. | Harvest T2 seeds from individual T1 plants. | ||
| 2. | Harvest seeds by removing whole panicles. | ||
| 3. | Store in appropriately labeled paper envelopes with CW ID, event number, progeny number and date, e.g. [***]00000-00-00, MM/DD/YY. | ||
| 4. | Log harvested seed in the Seed Production Table. |
| CW_ID | Date of Harvest | Harvested By | Generation of Seed | Location | Building | Room | Container (Barcode) | Amount (g) | Notes |
Page 26 of 28
| Protocol Number: | R2 | |||||
 |
||||||
| Property of Ceres, Inc. |
Protocol Name: | Plant Growth and Development Phenotyping | ||||
Authors:
|
Chuan-xxx Xx | Date Issued: | December 30, 2008 | |||
Approved By:
|
Version Number: | 1.0 | ||||
| CW_ID | Date of Harvest | Harvested By | Generation of Seed | Location | Building | Room | Container (Barcode) | Amount (g) | Notes | |||||||||
| [***] | 1/1/2006 | LZ | T1 | SCUTA | AAA | XXX | 123456 | [***] | Notes on germination, amount used (2.1.2006) | |||||||||
| [***] | 1/1/2006 | LZ | T1 | SCUTA | BBB | XXX | 123457 | [***] | Poor germination (1.1.2007) | |||||||||
| [***] | 6/1/2006 | T2 | ICS | YYY | 123458 | [***] | ||||||||||||
| [***] | 7/1/2007 | CYW | F1 | ICS | YYY | 123459 | [***] | |||||||||||
| [***] | 10/1/2007 | XZ | F2 | ICS | YYY | 123460 | [***] |
Page 27 of 28
| Protocol Number: | R7 | |||||
 |
||||||
| Property of Ceres, Inc. |
Protocol Name: | Destruction of Biomaterials | ||||
Authors:
|
Chuan-Xxx Xx | Date Issued: | July 16, 2007 | |||
Approved By:
|
Xxxxx Xxxxxxx | Version Number: | 1.0 | |||
Purpose:
|
Insure that all [***] seed are eventually destroyed. | |
Safety:
|
Practice universal safety techniques in the lab. | |
Start Product(s):
|
[***] line seed stocks. | |
End Product(s):
|
All [***] seeds should be destroyed by the end of the contract. |
| 1. | Must maintain a log of all [***] seed stocks generated (see Seed Production Table, ANNEX IV). | |
| 2. | Store individual stocks in a sealed clearly labeled envelope or a sealed paper bag. Each seed line is logged and stored inside a locked metal cabinet inside a locked room. Access to the seed storage cabinets is limited to those involved in the experiments. |
Page 28 of 28

