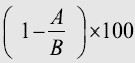LICENSE AGREEMENT
Exhibit 10.40
[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and the registrant customarily and actually treats as private and confidential.
This License Agreement (including any exhibits attached hereto, this “Agreement”) is made and is effective this 22nd day of September, 2023 (the “Effective Date”) between Century Therapeutics, Inc., a Delaware corporation (“Century”) having an address at 0000 Xxxxxx Xxxxxx, Xxxxxxxxxxxx, XX 00000 XXX and FUJIFILM Cellular Dynamics Inc., a Wisconsin corporation (“CDI”) having an address at 000 Xxxxxxx Xxxxx Xxxxxxx, XX 0000 0 XXX. Century and CDI are each referred to as a “Party” and collectively referred to as the “Parties.”
Recitals
WHEREAS, Century is a biotechnology company interested in development and commercialization of immunotherapy products based on T cells, NK cells, dendritic cells, macrophages and monocytes derived from human iPSC (including TiPSC);
WHEREAS, CDI possesses and controls certain patent rights and know-how related to (i) human iPSC (including TiPSC)-derived T cells, NK cells, dendritic cells, macrophages and monocytes and (ii) Licensed Products (as defined below);
WHEREAS, on September 18, 2018, Century and CDI entered into a License Agreement, as amended by a First Amendment to License Agreement effective as of March 23, 2021 and as amended by a certain letter agreement effective as of January 7, 2022 with respect to the BMS Collaboration Agreement (as defined in such letter agreement) and as amended by a Second Amendment to License Agreement effective as of September 22, 2023 (the “2018 License”) pursuant to which, CDI granted Century Inc. an exclusive license under the Licensed Technology to Exploit the Licensed Products within the Field (as defined in the 2018 License) in the Territory (as defined in the 2018 License);
WHEREAS, Century wishes to obtain, and CDI wishes to grant to Century, a nonexclusive license under the Licensed Technology (as defined below) for the research, development and commercialization of the Licensed Products (as defined below) in the Territory (as defined below) within the field of therapeutic use for immune-mediated inflammatory diseases, including autoimmune diseases, in humans, other than cancer immunotherapeutic use, with the right to grant sublicenses (through multiple tiers), in all cases subject to the terms and conditions of this Agreement;
WHEREAS, CDI wishes to obtain, and Century wishes to grant to CDI, a non-exclusive, worldwide license under the Century Licensed Technology (as defined below) and the Century Manufacturing Technology (as defined below) for the research, development and commercialization of the CDI CDMO Products (as defined below), with the right to grant sublicenses (through multiple tiers), in all cases subject to the terms and conditions of this Agreement;
NOW THEREFORE, CDI and Century, intending to be legally bound, agree as follows:
Article 1
Definitions
“Affiliate” means, with respect to a Party, any Person that controls, is controlled by, or is under common control with such Party. For purposes of this definition, “control” shall refer to (a) in the case of
a Person that is a corporate entity, direct or indirect ownership of more than fifty percent (50%) of the stock or shares having the right to vote for the election of directors of such Person and (b) in the case of a Person that is not a corporate entity, the possession, directly or indirectly, of the power to direct, or cause the direction of, the management or policies of such Person, whether through the ownership of voting securities, by contract or otherwise.
“Bankruptcy Code” has the meaning set forth in Section 2.5.
“BLA” means a Biologies License Application as defined in the United States Federal Food, Drug, and Cosmetic Act and the regulations promulgated thereunder (21 C.F.R. §§314 et seq).
“Business Days” means a day that is not a Saturday, Sunday or a day on which banking institutions in Boston, Massachusetts or Wisconsin are authorized by Law to remain closed.
“Calendar Quarter” means each three-month period beginning on January 1, April 1, July 1 and October 1 of each Calendar Year; provided, however, that as it relates to the First Commercial Sale of the Licensed Product, the first Calendar Quarter shall be comprised of the time period beginning on the date of First Commercial Sale and ending at the end of the Calendar Quarter during which such First Commercial Sale occurs. “Quarterly” means once during each Calendar Quarter.
“Calendar Year” means each successive period of twelve (12) months commencing on January 1 and ending on December 31; provided, however, that the first Calendar Year hereunder shall commence on the Effective Date and the final Calendar Year hereunder shall end on the effective date of termination or expiration of this Agreement.
“CDI CDMO Customer” means a Third Party who receives CDI CDMO Services and cells or other deliverables generated in the performance of CDI CDMO Services.
“CDI CDMO Services” means contract services provided by or on behalf of CDI to research, develop or manufacture CDI CDMO Products for any Third Party.
“CDI CDMO Product” means products for the treatment of diseases in humans, including products consisting of cells that are or are modifications of T cells, NK cells, dendritic cells, macrophages or monocytes. For the sake of clarity, such “modifications” exclude materials or substances extracted, isolated from, or secreted by, such modified or unmodified cells.
“Century Know-How” means (i) all Know-How Owned or Controlled by Century or its Affiliates, subject to Section 10.3, (other than the Licensed Know-How) as of the Effective Date, and (ii) all Know-How that is first made, conceived, reduced to practice or otherwise discovered by, or on behalf of, CDI after the Effective Date in the course of CDI’s performance of the Services (as defined in the Collaboration Agreement) pursuant to the Collaboration Agreement or Activities (as defined in the Supply Agreement) pursuant to the Supply Agreement but is Owned by Century in accordance with the Collaboration Agreement or the Supply Agreement, as applicable, and that is reasonably necessary to practice the Licensed Technology, to perform CDI CDMO Services or to Exploit a CDI CDMO Product.
“Century Licensed Technology” means Century Patent Rights and Century Know-How. For clarity, Century Licensed Technology includes Improvements of the Licensed Technology Owned or Controlled by Century or its Affiliates during the Term.
“Century Manufacturing Technology” means any Know-How and other inventions, improvements, discoveries or developments, whether or not patentable, Owned or Controlled by Century
or its Affiliates during the Term that are conceived of, reduced to practice, discovered, tested or developed by or on behalf of Century in connection with Century’s exercise of its rights under the 2018 License, the Collaboration Agreement or the Supply Agreement, in each case to the extent related to manufacturing, analytical development and/or process development. For purposes of clarity, “Century Manufacturing Technology” does not include technologies of gene editing, targets and binders and switches but does include, without limitation, any and all of the following: (i) strategies, criteria or methods for analytical test development and quality control test development for drug substances and/or drug products including, without limitation: determination of the respective tests that are required for intermediates, release and characterization; the development of the appropriate tests; and stability studies and shelf life determination; (ii) criteria and/or methods for cell expansion and scale up process; methods for, and results from, a suite of in vitro and in vivo assays that test cell performance and function; and evaluation analysis of such results; (iii) criteria, methods or processes relating to any of the following: closed unit operations (examples, include, without limitation: closed continuous or batch centrifugation); scale up of cell expansion and cell harvest steps; process choreography, i.e., the optimal order of combining streams, transferring streams, sampling and measuring intermediates, and defining hold times; identification of standard, or design of custom made, product contact containers for combining streams, transferring streams, and sampling; cell cryopreservation; drug product vial filling and visual inspection at large scale; and shipping and clinical handling and administration of cryopreserved products; and (iv) pre IND regulatory and Chemistry CMC strategies, interactions (meetings and briefing books), and composition of an IND-enabling technical package supporting development of an immunotherapy for cancer (where “IND” means an investigational new drug application with the FDA, or the equivalent application in any foreign jurisdiction filed with another Regulatory Authority).
“Century Patent Rights” means all Patent Rights Owned or Controlled by Century or its Affiliates, subject to Section 10.3, as of the Effective Date (other than the Licensed Patent Rights) that are reasonably necessary to practice the Licensed Technology, to perform CDI CDMO Services or to Exploit a CDI CDMO Product.
“Collaboration Agreement” means the Master Collaboration Agreement dated as of October 21, 2019 between Century and CDI, as amended by Amendment No. 1 to Master Collaboration Agreement dated as of July 17, 2020, by Amendment No. 2 to Master Collaboration Agreement dated as of March 23, 2021, by Amendment No. 3 to Collaboration Agreement dated as of March 29, 2021, by Amendment No. 4 to Master Collaboration Agreement dated as of July 29, 2022 and by a letter agreement dated as of March 7, 2022, by Amendment No. 5 to Master Collaboration Agreement effective as of September 22, 2023 and as may be further amended.
“Commercialize” means to engage in any and all activities directed to transporting, storing, marketing, detailing, promoting, distributing, importing, exporting, using, offering to sell or selling a product.
“Commercially Reasonable Efforts” means efforts consistent with the efforts and resources as commonly used by a pharmaceutical or biotechnology company, as applicable, of comparable size and resources of such Party for a product at a similar stage of research, development or commercialization having similar product characteristics at a similar stage in its development or product life, taking into account relevant factors including patent coverage, relative safety and efficacy, product profile, the competitiveness of the marketplace, the proprietary position of such product, the regulatory structure involved, the market potential of such product and other relevant factors, including comparative technical, legal, scientific, medical and/or economic factors, all as measured by the facts and circumstances in effect at the time when the relevant activities are conducted.
“Confidential Information” means any confidential or proprietary information furnished by one Party (or its Affiliate) to the other Party (or its Affiliate) in connection with this Agreement, whether during the Term or prior to the execution hereof as expressly specified below, provided that such information is (a) specifically designated as confidential at the time of disclosure or confirmed as confidential within [***] days of disclosure or (b) reasonably identifiable by an individual familiar with the industry as confidential or proprietary. Without limiting the generality of the foregoing, Confidential Information includes:
(a)non-public information disclosed by Century to CDI in reports submitted by Century to CDI pursuant to Section 3.3;
(b)non-public information disclosed by Century to CDI relating to patent application prosecution files for the Licensed Patent Rights; and
(c)the terms of this Agreement
“Controlled” means, with respect to Patent Rights or Know-How as such relates to a Party, that such Party owns or has a license or sublicense to such Patent Rights or Know- How and has the legal right to grant a license or sublicense, including having received any necessary Third Party consents, to such Patent Rights or Know-How to the other Party as provided for in this Agreement, or has the ability to assign its right, title and interest in and to such Patent Rights or Know-How to the other Party, without violating the terms of any agreement or other arrangement with any Third Party.
“Cover” means with respect to a Patent Right, that the Exploitation of a given product or item would infringe a Valid Patent Claim of such Patent Right (in the absence of ownership of, or a license under, such Patent Right). Cognates of the word “Cover” have correlative meanings.
“European Union” or “E.U.” means the economic, scientific, and political organization of member states known as the European Union, as its membership may be altered from time to time, and any successor thereto, and includes, for purposes of this Agreement, Great Britain (whether Great Britain is a member of the E.U. or not).
“Exploit” or “Exploitation” means, with respect to a particular Licensed Product or CDI CDMO Product, as the case may be, to make, have made, use, have used, manufacture, have manufactured, sell, have sold, offer for sale, have offered for sale, import, have imported, export and have exported, including to research, develop, commercialize or otherwise exploit such Licensed Product or CDI CDMO Product.
“FDA” means the United States Food and Drug Administration and any successor agency thereto.
“Field” means any immunotherapeutic use for immune-mediated inflammatory diseases, including autoimmune diseases in humans, other than cancer immunotherapeutic use.
“First Commercial Sale” means, with respect to a given Licensed Product on a country-by-country basis, the first invoiced commercial sale for monetary value for use or consumption by the end user of such Licensed Product in any country in the Territory after the Regulatory Approval for such Licensed Product has been obtained in such country.
“ICC” has the meaning set forth in Section 10.11(b).
“Improvements” means any Intellectual Property arising during the Term that is an improvement to the Licensed Technology from the 2018 License and was developed by either Party or Third Parties
acting on their behalf while performing activities under the 2018 License, the Collaboration Agreement or the Supply Agreement and all intellectual property rights (including Patent Rights) thereto.
“IND” means an investigational new drug application with the FDA, or the equivalent application in any foreign jurisdiction filed with another Regulatory Authority.
“Indemnified Party” shall have the meaning set forth in Section 8.3.
“Indemnifying Party” shall have the meaning set forth in Section 8.3.
“Indication” means a discrete clinically recognized form of a disease. For clarity, a discrete clinically recognized form of a disease that is treated in one or more different subpopulations within a population of patients having such disease shall not be treated as separate Indications for purposes of this Agreement (e.g., front-line treatment, relapsed refractory treatment and maintenance treatment of the same disease shall not be considered different Indications).
“Intellectual Property” means ideas, concepts, discoveries, inventions, developments, Know-How, trade secrets, techniques, methodologies, modifications, innovations, improvements, writings, documentation, electronic code, data and rights (whether or not protectable under state, federal or foreign patent, trademark, copyright or similar laws) or the like, whether or not written or otherwise fixed in any form or medium, regardless of the media on which contained and whether or not patentable or copyrightable.
“Know-How” means any and all commercial, technical, regulatory, scientific and other know-how and information, knowledge, technology, materials (including biological and chemical materials), methods, processes, practices, standard operating procedures, formulae, instructions, skills, techniques, procedures, assay protocols, experiences, ideas, technical assistance, designs, drawings, assembly procedures, specifications, regulatory filings, data and results (including biological, chemical, pharmacological, toxicological, pharmaceutical, physical and analytical, pre-clinical, clinical, safety, regulatory, manufacturing and quality control data and know-how, including study designs and protocols), whether or not confidential, proprietary or patentable, in written, electronic or any other form.
“Law” means all laws, statutes, rules, codes, regulations, orders, judgments or ordinances applicable to a Party, this Agreement or the activities contemplated hereunder.
“Licensed Know-How” means all Know-How that is Owned or Controlled by CDI as of the Effective Date that is reasonably necessary to Exploit a Licensed Product, including, without limitation, any Know-How set forth on Exhibit A. For clarity, Licensed Know-How shall not include any Know-How related to the reprogramming of human somatic cells to iPSCs and Century’s rights in such Know-How, if any, are as set forth in the Reprogramming License.
“Licensed Patent Rights” means the Patent Rights Owned or Controlled by CDI as of the Effective Date that are reasonably necessary to Exploit a Licensed Product, including, without limitation, any Patent Rights listed on Exhibit B. For clarity, Licensed Patent Rights shall not include any Patent Rights that are licensed to Century by CDI pursuant to the Reprogramming License.
“Licensed Product” means products for the treatment of disease in humans consisting of cells that are or are modifications of T cells, NK cells, dendritic cells, macrophages and monocytes derived from human iPSC (including TiPSC). For the sake of clarity, such “modifications” exclude materials or substances extracted, isolated from, or secreted by, such modified or unmodified cells. A separate Licensed Product is one for which, under applicable Laws, it is required that there be in effect a separate BLA with
respect to the composition of matter or active ingredient of such Licensed Product as compared to the composition(s) of matter or active ingredient(s) of any other Licensed Product.
“Licensed Technology” means Licensed Patent Rights and Licensed Know-How. For clarity, Licensed Technology includes Improvements of the Century Licensed Technology Owned or Controlled by CDI as of the Effective Date.
“Major Market Countries” means France, Germany, Italy, Spain, and the United Kingdom.
“Net Sales” means the gross amounts invoiced by Century or its Sublicensees for sales of Licensed Product in finished package form (ready for use by the ultimate consumer) in the Territory to a Third Party, including, but not limited to, sales to wholesalers or other customers in each country in bona fide, arm’s length transactions. In the event Century does not sell directly to such customers in one or more countries, electing instead to utilize another party as a distributor to those customers, it is understood that Net Sales shall include sales by the distributor rather than Century’s sales to the distributor. In determining Net Sales, certain deductions may be taken against the gross amount invoiced. These allowable deductions are the following, but only insofar as these items are commercially reasonable under the circumstances, documented in writing, pertain specifically to Century’s sale of the Licensed Products, were actually included and accounted for in the gross amount invoiced, and were not given in exchange for anything of value (such as data, in-kind consideration, or commitments to purchase other products or services):
(a)(i) discounts actually allowed and taken with respect to Licensed Products, including cash discounts, discounts to managed care or similar organizations or government organizations, administrative fees paid to pharmacy benefits managers; (ii) rebates actually paid or credited, including government rebates such as Medicaid chargebacks or rebates; (iii) retroactive price reductions or allowances actually allowed or granted from the billed amount; and (iv) promotional allowances actually granted to customers as reflected on the same invoice as for the sale of Licensed Product;
(b)credits or allowances actually granted upon claims, rejections or returns of such sales of Licensed Products, including government mandated recalls and recalls that Century reasonably believes are in the best interest of the consumer;
(c)taxes, duties or other governmental charges levied on or measured by the invoiced amount, as adjusted for rebates, charge-backs and refunds; and
(d)freight, postage, shipping and insurance charges to the extent included on the same invoice by Century or its Sublicensee for delivery of such Licensed Products.
(e)In the case of discounts on packages of products or services which include Licensed Product in those countries of the Territory in which such is legally permissible (“Packages”), the discount applied to Licensed Product within the Package shall be no greater than the discount determined by discounting the list price of the Licensed Product in the Package by the average percentage discount of list prices of all products of Century in the same Package, calculated as follows:
Average percentage Discount on a Particular Package | = |
|
where A equals the total discounted value of a particular Package of products, and B equals the sum of the undiscounted value of the same Package of products. Century shall provide CDI with reasonable documentation supporting the percentage discounts with respect to each product within such Package.
(f)A “sale” of a Licensed Product is deemed to occur upon the invoicing, or if no invoice is issued, upon the earlier of shipment or transfer of title in the Licensed Product to a Third Party.
(g)With respect to Combination Products, if Licensed Products are sold in the form of Combination Products containing one or more therapeutically active ingredients other than the Licensed Product, Net Sales for the Combination Product will be calculated by multiplying actual Net Sales of such Combination Product by the fraction A/(A+B) where A is the invoice price of a Licensed Product if sold separately, and B is the total invoice price of any other active component or components, or devices, in the combination, if sold separately. If, on a country-by-country basis, the other active component or components in the combination are not sold separately in said country, Net Sales for the purpose of determining royalties of the Combination Product shall be calculated by multiplying actual Net Sales of such Combination Product by the fraction A/C where A is the invoice price of either the Licensed Product, if sold separately, and C is the invoice price of the Combination Product. If, on a country-by-country basis, neither the Licensed Product nor the other active component or components of the Combination Product is sold separately in said country, Net Sales for the purposes of determining royalties of the Combination Product shall be determined by the Parties in good faith based on the relative value of the Licensed Product and the additional active ingredients that are included in the Combination Product.
“Owned” means, with respect to Patent Rights or Know-How that a Person owns, solely or jointly, in each case with the ability to grant a right, license, or access to such material, information or Intellectual Property right to another Person on the terms and conditions set forth herein, without violating the terms of any agreement or other arrangement with any of such Person’s Affiliates and/or Third Party(ies). “Ownership” has the correlative meaning.
“Patent Rights” means with respect to any patents or patent applications, (a) such patents or patent applications; (b) patents issuing from such patent applications; (c) substitutions, divisionals, renewals, continuations or continuations-in-part (only to the extent of claims that are entitled to the priority date of the parent application); (d) patents of addition, restorations, extensions, supplementary protection certificates, registration or confirmation patents, patents resulting from post-grant proceedings, re-issues and re-examinations; (e) other patents or patent applications claiming and entitled to claim priority to (i) such patents and patent applications specified in (a), (b), (c) or (d) or (ii) any patent or patent application from which such patents and patent applications specified in (a), (b), (c) or (d) claims and is entitled to claim priority (f) all rights of priority attendant to such listed in (a) through (e); and (g) in each case of such patents and patent applications described in (a) through (e), including all counterparts and foreign equivalents thereof tiled in any country, territory or jurisdiction in the world.
“Person” means any natural person or any corporation, company, partnership, joint venture, firm or other entity, including a Party, or any government or agency or political subdivision thereof.
“Phase 1 Clinical Trial” means, as to a specific Licensed Product, a human clinical trial of a product in any country designed to satisfy the requirements of 21 C.F.R. §312.21(a) as amended from time to time, and intended to determine metabolism and pharmacologic actions of a drug in humans, the side effects associated with increasing doses and, if possible, to gain early evidence of efficacy, or any comparable trial under applicable Laws in the United States or the corresponding regulation in jurisdictions other than the United States.
“Phase 3 Clinical Trial” means a human clinical trial of a Licensed Product in any country that satisfies the requirements of 21 C.F.R. § 312.21(c). For clarity, a trial called a Phase 2/3 trial shall be considered a Phase 3 trial if it satisfies the requirements of 21 C.F.R. § 312.21(c).
“Prosecution and Maintenance” or “Prosecute and Maintain” means, with respect to the applicable Patent Rights, the preparation, filing, prosecution and maintenance of such Patent Rights, as well as re-examinations, reissues, appeals, and requests for patent term adjustments and patent term extensions with respect to such Patent Rights, together with the initiation or defense of interferences, the initiation or defense of oppositions, post grant review, and other similar proceedings with respect to the particular Patent Rights, and any appeals therefrom. For clarification, “Prosecution and Maintenance” or “Prosecute and Maintain” shall not include any other enforcement actions taken with respect to Patent Rights.
“Regulatory Approval” means, with respect to a country or territory, the approvals (including any applicable governmental price and reimbursement approvals), licenses, registrations or authorizations of Regulatory Authorities necessary for the commercialization of a pharmaceutical product in such country or territory, including, as applicable, approval of an BLA or comparable filing in the United States or approval of a comparable filing in any other country or jurisdiction.
“Regulatory Authority” means a federal, national, multinational, state, provincial or local regulatory agency, department, bureau or other governmental entity with authority over the testing, manufacture, use, storage, import, promotion, marketing or sale of a product in the applicable country.
“Regulatory Documentation” means all applications, registrations, licenses, authorizations and approvals (including all Regulatory Approvals), all correspondence submitted to or received from Regulatory Authorities (including minutes and official contact reports relating to any communications with any Regulatory Authority) and all supporting documents and all clinical studies and tests, relating to the Licensed Products and all data contained in any of the foregoing, including all INDs, Regulatory Approvals, regulatory drug lists, advertising and promotion documents, manufacturing data, drug master files, clinical data, adverse event files and complaint files.
“Reprogramming License” means that certain agreement entered into between the Parties under which CDI grants a non-exclusive license to Century under patent rights in relation to reprogramming technology under the terms and conditions therein, as amended by a First Amendment to License Agreement effective as of March 23, 2021, as amended by a certain letter agreement effective as of January 7, 2022 with respect to the BMS Collaboration Agreement (as defined in such letter agreement), as amended by a Second Amendment to License Agreement effective as of September 22, 2023, and as may be further amended.
“SEC Filing” has the meaning set forth in Section 6.4(d).
“Sublicensee” has the meaning set forth in Section 2.3.
“Supply Agreement” means the Manufacturing and Supply Agreement dated as of March 23, 2021 between Century and CDI, and as may be amended.
“Term” means the term of this Agreement as provided in Section 9.1.
“Territory” means worldwide, but excluding any country(ies) eliminated from the Territory pursuant to Section 9.4.
“Third Party” means any Person other than a Party. For avoidance of doubt, Third Party will include each Party’s Affiliates.
“United States” or “U.S.” means the United States of America and its territories and possessions (including the District of Columbia and Puerto Rico).
“Valid Patent Claim” means a claim in any Licensed Patent Rights, which claim has not expired or held invalid by a non-appealed or unappealable decision by a court or other appropriate body of competent jurisdiction. In addition, and when referring to the European Union or to any of its member states, a claim shall not be a Valid Patent Claim, unless it is issued or pending in its regional or national phase, in each and all members of the European Union or the European Patent Convention, whichever has the largest number of member states, as of the Effective Date of this Agreement. For the purpose of royalty determination and payment, any claim being prosecuted in a pending patent application shall be deemed to be a “Valid Patent Claim” provided such claim is not pending for more than [***] years from the earliest filing date of the patent application in which case it shall cease to be considered a Valid Patent Claim until the patent issues.
Article 2
License Grants; Technology Transfer
2.1License Grants to Century. Subject to the terms and conditions of this Agreement, CDI hereby grants to Century:
(a)a non-exclusive, non-transferable (except in accordance with Section 10.3), royalty-bearing, sublicensable (with the ability to sublicense through multiple tiers) license under the Licensed Technology to Exploit the Licensed Products within the Field in the Territory. Notwithstanding any term or implication herein to the contrary and for the sake of clarity, the foregoing license excludes any license or right under the Licensed Patent Rights (i) to manufacture, have manufactured, sell, have sold, offer for sale or have offered for sale any product or service to any third party in furtherance of any research or development of a Licensed Product other than a Licensed Product that is being researched, developed or commercialized by or on behalf of Century or Sublicensees, or (ii) to grant any sublicense to any Third Party to manufacture, have manufactured, sell, have sold, offer for sale or have offered for sale any product or service to any other Third Party in furtherance of any research or development of a product other than a Licensed Product that is being researched, developed or commercialized by or on behalf of Century or Sublicensees; and
(b)a non-exclusive, non-transferable (except in accordance with Section 10.3), license and right of reference, with the right to grant sublicenses through multiple tiers and further rights of reference, under any Regulatory Documentation Owned and Controlled by CDI and any master file included in the Regulatory Documentation as of the Effective Date so long as CDI Owns or Controls such Regulatory Documentation, to Exploit the Licensed Products within the Field in the Territory.
2.2License Grants to CDI. Notwithstanding anything to the contrary in the Collaboration Agreement and subject to the terms and conditions of this Agreement, Century hereby grants to CDI a fully paid-up, perpetual, irrevocable, non-exclusive, non-transferable (except in accordance with Section 10.3), sublicensable (with the ability to sublicense through multiple tiers) worldwide license under the Century Licensed Technology and Century Manufacturing Technology for (i) CDI CDMO Services and (ii) the Exploitation of CDI CDMO Products by CDI CDMO Customers.
2.3Sublicensing
(a)Each Party shall have the right to grant sublicenses under the licenses granted to it, with respect to Century under Section 2.1, and with respect to CDI under Section 2.2, in each case, to Third Parties (each, a “Sublicense”) through multiple tiers; provided that any such sublicense granted to a Third Party shall be pursuant to a written agreement and each sublicense shall be subject to all relevant restrictions and limitations set forth in this Agreement. Each Party shall be responsible for each of its Sublicensees complying with all obligations of such Party under this Agreement that are applicable to sublicenses and any breach by a Sublicensee under any such sublicense shall be deemed to be a breach of such Party under this Agreement. Each Party shall notify the other Party in writing of the identity of Sublicensees without delay.
(b)If this Agreement is terminated for any reason other than by Century pursuant to Section 9.4, then, at the option of any Sublicensee of a terminating Party not in default of the applicable sublicense (or any provision of this Agreement applicable to such Sublicensee), a terminating Party shall use Commercially Reasonable Efforts to execute a possible direct license arrangement with such Sublicensee under, and subject to the terms and conditions of, this Agreement.
2.4No Implied License. Except as explicitly set forth in this Agreement, neither Party shall acquire any license, intellectual property interest or other rights, by implication or otherwise, in any Know-How or under any Patent Rights Owned or Controlled by the other Party or its Affiliates.
2.5Section 365(n) of the Bankruptcy Code. All rights and licenses granted under or pursuant to any section of this Agreement are and will otherwise be deemed to be for purposes of Section 365(n) of the United States Bankruptcy Code (Title 11, U.S. Code) as amended or any comparable Law outside the United States (the “Bankruptcy Code”), licenses of rights to “intellectual property” as defined in Section 101 (35 A) of the Bankruptcy Code. Each licensing Party agrees that a licensed Party, as licensee of such rights under this Agreement, will retain and may fully exercise all of its rights and elections under the Bankruptcy Code or any other provisions of Law outside the United States that provide similar protection for “intellectual property.” Any agreement supplemental hereto will be deemed to be “agreements supplementary to” this Agreement for purposes of Section 365(n) of the Bankruptcy Code.
Article 3
Development, Commercialization and Manufacture
3.1General. As between the Parties, each Party shall be solely responsible for developing and preparing any and all regulatory filings for, obtaining Regulatory Approval of, and commercializing its own Licensed Products or CDI CDMO Products, as the case may be, in the Field and in the Territory, in its sole discretion.
3.2Diligence. Century shall use Commercially Reasonable Efforts, or shall cause one or more of its Affiliates and Sublicensees to use Commercially Reasonable Efforts, to develop, seek Regulatory Approval of and commercialize at least one (1) Licensed Product in the United States in the Field.
3.3Progress Reports. Century shall provide, within [***] days after the end of each Calendar Year, a status progress report to CDI that summarizes the status of Century’s research, development and Commercialization efforts and activities with respect to Licensed Products during such Calendar Year.
3.4Compliance. Each Party shall, and shall ensure that its Affiliates and Sublicensees, and its and their subcontractors, conduct all development, manufacture and Commercialization of Licensed Products and CDI CDMO Products, as the case may be, in compliance with all applicable Laws.
Article 4
FINANCIALS AND REPORTING
4.1License Fee. Century shall pay to CDI a nonrefundable license fee of [***] within [***] days of the Effective Date. Such payment is not subject to any future performance by either Party under this Agreement.
4.2Milestone Payments. Century shall pay to CDI on a Licensed Product-by-Licensed Product basis, each of the following milestone payments (each of which shall be non-refundable and non-creditable), which shall become due and payable when each Licensed Product hereunder first achieves each of the following Development milestones, regardless of whether such Development milestone is achieved by Licensee or its Sublicensee:
(a)[***]: [***]:
(b)[***]: [***];
(c)[***]: [***];
(d)[***]: [***]. For example, and without limitation:
[***] | [***] |
[***] | [***] |
[***] | [***] |
[***] | [***] |
(e)[***]: [***];
(f)[***]: [***];
(g)[***]: [***];
(h)[***]: [***].
4.3Sales Milestones. Century shall pay to CDI the following one-time sales-based milestone payments on a Licensed Product-by-Licensed Product basis, based on the total Net Sales of such Licensed Product in a given Calendar Year in the Territory by Century and Sublicensees. For clarity, the milestone payments listed below shall be made only once for each Licensed Product upon the first achievement of each relevant milestone by such Licensed Product regardless of the number of Calendar Years in which such Licensed Product achieves any particular milestone.
4.4Notice of Event Milestone Achievement. Century shall notify CDI in writing within [***] Business Days following the achievement of each milestone event set forth in Section 4.2 and within
[***] Business Days of the end of the Calendar Year in which any milestone event set forth in Section 4.3 is achieved. For clarity, in such notice, Century shall identify each particular milestone event that was achieved by reference to its description in this Agreement and, with respect to a milestone event set forth in Section 4.2, Century shall state whether Licensee or its Sublicensee achieved the event and, if the milestone event was achieved by a Sublicensee, the name of such Sublicensee.
4.5Royalty Rate for Licensed Products Developed and Commercialized by Century. Century shall pay CDI a non-refundable and non-creditable royalty based on Net Sales of Licensed Products sold by Century or its Sublicensees, on a Licensed Product-by-Licensed Product and country-by-country basis for each Calendar Year according to the following schedule:
(a)[***] on Annual Net Sales for a Licensed Product in countries where a Valid Patent Claim of a Licensed Patent Right Covering the manufacture, use or sale of a Licensed Product exists or in countries where the Licensed Product being sold is imported from a country where a Valid Patent Claim of a Licensed Patent Right Covering the manufacture of a Licensed Product exists; and
(b)in countries where no Valid Patent Claim of a Licensed Patent Right Covering the manufacture, use or sale of a Licensed Product exists and where the Licensed Product being sold is imported from a country where no Valid Patent Claim of a Licensed Patent Right Covering the manufacture of a Licensed Product exists, [***] on Annual Net Sales for all Licensed Products the manufacture of which utilizes Licensed Know-How.
(c)For the purpose of this section, “Annual Net Sales” will be determined on a Calendar Year basis.
4.6Third Party Patents. If a Patent of a Third Party should exist in any country during the term of this Agreement Covering the manufacture, use or sale of any Licensed Product, and if it should prove in Century’s reasonable judgment (as supported by an opinion from outside patent counsel which counsel is acceptable to both Parties) necessary to avoid infringement of such Patent for Century to make, have made, use, have used, sell, offer for sale, import, and otherwise Exploit Licensed Products in said country, then Century shall be entitled to a credit against the royalty payments due hereunder of an amount equal to [***] of the royalty paid to such Third Party, not to exceed [***] of the royalty due under clause (a) of Section 4.5 of this Agreement, arising from the manufacture, use or sale of the Licensed Product in said country.
4.7Compulsory License. If at any time and from time to time a Third Party in any country shall, under the right of a compulsory license granted or ordered to be granted by a competent governmental authority, manufacture, use or sell any Licensed Product, with respect to which royalties would be payable pursuant to Article 4 hereof, then Century may reduce the royalty on sales in such country of such Licensed Product to the rate of [***].
4.8Currency Restrictions. Except as expressly provided otherwise in this Section 4.8, all royalties shall be paid in U.S. Dollars. If, at any time, legal restrictions prevent the prompt remittance of part of or all of the royalties with respect to any country where Licensed Products are sold, Century shall have the right and option to make such payments by depositing the amount thereof in local currency to GDI’s accounts in a bank or depository in such country.
4.9Royalty Period. The royalty payments set forth above shall be payable for each Licensed Product on a Licensed Product-by-Licensed Product and country-by-country basis from the date of First Commercial Sale of such Licensed Product in such country until the later of: (a) [***] years from the date of First Commercial Sale after first Regulatory Approval of a Licensed Product in such country; (b) until
the last to expire of any Valid Patent Claim of a Licensed Patent Right encompassing the making, using, selling, offering for sale or importing of the Licensed Product; or (c) the date on which any applicable regulatory, pediatric, orphan drug or data exclusivity expires in such country (such period, the “Royalty Term”). Once Century has paid CDI for the full term of the periods of time set forth in (a), (b) and (c) in the immediately preceding sentence for a Licensed Product on a country-by-country basis, it shall have a perpetual, paid up, no fee, royalty-free license pursuant to Section 2.1 under Licensed Know-How for such Licensed Product in such country notwithstanding any changes, improvements, or modifications thereafter made to such Licensed Product.
4.10Notice of First Commercial Sale. Century shall notify CDI in writing of the First Commercial Sale in each country within the Territory within [***] Business Days following the occurrence thereof.
4.11Quarterly Royalty Reports. Within [***] days after the Calendar Quarter in which any First Commercial Sale occurs, and within [***] days after each Calendar Quarter thereafter, Century shall deliver to CDI a written report setting forth, on a Licensed Product-by-Licensed Product and country-by-country basis, the Net Sales of each Licensed Product during such Calendar Quarter, the applicable gross amounts invoiced, and the allowable deductions taken against such gross amounts in accordance with the definition of “Net Sales” to arrive at Net Sales, the relevant royalty rate, the amount of any adjustment to the royalty amount otherwise payable hereunder is made pursuant to Section 4.6 and the name of the Third Party to which royalty was paid as the basis for such adjustment, the country of manufacture of each Licensed Product, and the royalty payment due and payable hereunder with respect to such Net Sales or stating that no royalty payment is due with respect to such Calendar Quarter.
4.12Timing and Nature of Payments. Century shall pay to CDI the appropriate milestone payment within [***] calendar days after achievement of each milestone event set forth in Section 4.2 and within [***] calendar days after the end of the Calendar Year in which each milestone event set forth in Section 4.3 was achieved. Century shall pay to CDI royalty payments by the date on which the report setting forth such royalty payment is due to be provided by Century to CDI in accordance with Section 4.11. Century shall be responsible for all payments that are due to CDI but have not been paid by Century’s Sublicensees or Commercialization partners.
4.13Mode of Payment. All payments to CDI hereunder shall be made by wire transfer of immediately available funds in the requisite amount in the appropriate currency in accordance with Section 4.8, without deduction of exchange, collection, wiring fees, bank fees, or any other charges, to the account designated by CDI; provided, however, that any notice by CDI of a change in such account shall not be effective until [***] days after receipt thereof by Century.
4.14Financial Records. Century shall keep accurate and complete books and records, including, without limitation, of gross invoiced sales, Net Sales, and royalty payments, and license agreements with third parties for which adjustments to the royalty paid hereunder are made, in sufficient detail to enable CDI to determine and verify the accuracy and completeness of the reports delivered to CDI under this Article 4 and the amounts due hereunder to CDI (“Financial Records”).
4.15Currency Exchange. In the case of Net Sales of any Licensed Product invoiced in a currency other than U.S. Dollars, such Net Sales shall be calculated and reported in, and royalty payments by Century to CDI with respect thereto shall be converted to, U.S. Dollars in accordance with Century’s procedures that are generally applied by Century for calculating and making such currency conversions; provided that such procedures are in compliance with generally accepting accounting principles then in effect in the United States and are the same procedures that Century uses in preparing its audited financial statements, consistently applied.
4.16Audit and Financial Records. Financial Records under this Agreement shall be open for examination during reasonable business hours for a period of [***] years from creation of individual records. Upon the written request of CDI but not more often than once each year, at CDI’s expense, Century shall permit an independent public accounting firm of national prominence selected by CDI and reasonably acceptable to Century to have access during normal business hours to those records of Century as may be reasonably necessary for the sole purpose of verifying the accuracy and completeness of the reports delivered to CDI under this Article 4 and the amounts due hereunder to CDI. Century shall include in each Sublicense or Commercialization agreement entered into by it pursuant to this Agreement, a provision requiring, among others, the Sublicensee or Commercialization partner to keep and maintain adequate Financial Records pursuant to such Sublicense or Commercialization agreement and to grant access to such records by the aforementioned independent public accountant for the reasons specified in this Agreement. The report prepared by such independent public accounting firm, a copy of which shall be sent or otherwise provided to Century by such independent public accountant at the same time as it is sent or otherwise provided to CDI, shall contain the conclusions of such independent public accountant regarding the audit and will specify that the amounts paid to CDI pursuant thereto were correct or, if incorrect, the amount of any underpayment or overpayment. If such independent public accounting firm’s report shows any underpayment, Century shall remit or shall cause its Sublicensees or Commercialization partners to remit to CDI within [***] days after Century’s receipt of such report, (i) the amount of such underpayment and (ii) if such underpayment exceeds [***] of the total amount owed for the Calendar Year then being audited, the reasonable and necessary fees and expenses of such independent public accountant performing the audit, subject to reasonable substantiation thereof. If such independent public accounting firm’s report shows any overpayment, Century shall receive a credit equal to such overpayment against the royalty otherwise payable to CDI.
4.17Interest Due. In case of any delay in payment by Century to CDI, interest on the overdue payment shall accrue from the date when payment first became due until the date on which the late payment is made to CDI at an annual interest rate, compounded monthly, equal to the prime rate as reported in The Wall Street Journal, as determined for each month on the last business day of that month, assessed from the day payment was initially due. The foregoing interest shall be due from Century without any special notice.
4.18Tax Withholding.
(a)Century will make all payments to CDI under this Agreement without deduction or withholding for taxes except to the extent that any such deduction or withholding is required by law in effect at the time of payment.
(b)Any tax required to be withheld on amounts payable under this Agreement will promptly be paid by Century on behalf of CDI to the appropriate governmental authority, and Century will furnish CDI with proof of payment of such tax. Any such tax required to be withheld will be an expense of and borne by CDI.
(c)Century and CDI will cooperate with respect to all documentation required by any taxing authority or reasonably requested by Century to secure a reduction in the rate of applicable withholding taxes.
(d)If Century had a duty to withhold taxes in connection with any payment it made to CDI under this Agreement but Century failed to withhold, and such taxes were assessed against and paid by Century, then CDI will indemnify and hold harmless Century from and against such taxes (including interest). If Century makes a claim under this Section 4.18(d), it will comply with the obligations imposed by this Section 4.18(b) as if Century had withheld taxes from a payment to CDI.
Article 5
Intellectual Property Protection and Related Matters
5.1Ownership. As between the Parties, each Party or its Affiliates shall solely own all Intellectual Property, including Patent Rights related thereto, made, conceived, reduced to practice, or otherwise discovered, whether prior to, on or after the Effective Date, solely by employees, agents and consultants of such Party or its Affiliates. For purposes of determining ownership under this Section 5.1, inventorship shall be determined in accordance with the rules of inventorship under U.S. patent laws.
5.2Prosecution and Maintenance of Licensed Patent Rights. As between the Parties, CDI shall have the sole and exclusive right, but not the obligation, to Prosecute and Maintain, in its absolute and sole discretion, all Licensed Patent Rights worldwide within the Field. As between the Parties, Century shall have the sole and exclusive right, but not the obligation, to Prosecute and Maintain, in its absolute and sole discretion, all Century Patent Rights worldwide.
5.3Third Party Infringement.
(a)Each Party shall notify the other Party promptly of any knowledge it acquires of any actual or potential infringements of the Licensed Patent Rights with respect to any activities of a Third Party in the Field in any country in the world and of any actual or potential infringements of the Century Patent Rights with respect to any activities of a Third Party in any country in the world and shall provide the other Party with all available evidence regarding such known or suspected infringement or unauthorized use. Notwithstanding any provision to the contrary, CDI is not obligated to enforce the Licensed Patent Rights against such Third Party.
(b)As between the Parties, CDI shall have the sole and exclusive right, but not the obligation, to enforce the Licensed Patent Rights against any such Third Party. As between the Parties, Century shall have the sole and exclusive right, but not the obligation, to enforce the Century Patent Rights against any such Third Party.
5.4Patent Invalidity Claim. During the Term, each Party shall promptly notify the other Party in the event of any legal or administrative action by any Third Party against a Licensed Patent Right of which such Party becomes aware, including any nullity, revocation, reexamination or compulsory license proceeding or similar proceeding.
5.5Patent Term Extensions. As between the Parties, CDI shall have the sole right to obtain patent term extensions or supplemental protection certificates or their equivalents with respect to any Licensed Patent Right in the Field in any country worldwide, and Century shall reasonably cooperate with CDI in connection therewith.
5.6Patent Marking. Century shall comply with the patent marking statutes in each country in which Licensed Products are sold by or on behalf of Century and/or its Affiliates or Sublicensees.
Article 6
Confidentiality
6.1Confidential Obligations. Each Party shall (a) maintain in strict confidence the Confidential Information of the other Party to the same extent such Party maintains its own confidential information, but in no event less than a reasonable degree of care, (b) not disclose such Confidential Information to any Third Party without the prior written consent of the other Party (except as permitted pursuant to Section 6.3 below), and (c) not use such Confidential Information for any purpose except those
expressly permitted by this Agreement. The obligations of confidentiality, non-disclosure and non-use under this Section 6.1 shall be in full force during the Term and for a period for [***] years thereafter. Each Party, upon the request of the other Party, will return all copies of or destroy (and certify such destruction in writing) the Confidential Information disclosed or transferred to it by the other Party pursuant to this Agreement, within [***] days of such request or, if earlier, the termination or expiration of this Agreement; provided, however that a Party may retain (i) Confidential Information of the other Party which expressly survives such termination pursuant to this Agreement, and (ii) one (1) copy of all other Confidential Information in archives solely for the purpose of establishing the contents thereof; provided, further, that a Party is not required to return or destroy Confidential Information contained in electronic back-ups unless and until such Confidential Information is accessed.
6.2Exceptions to Confidentiality. Notwithstanding the foregoing, the obligations of confidentiality set forth in Section 6.1 shall not apply to information that, in each case as demonstrated by competent written documentation:
(a)is publicly disclosed or made generally available to the public by the disclosing Party, either before or after it becomes known to the receiving Party;
(b)was known to the receiving Party, without any obligation to keep it confidential, prior to the date of first disclosure by the disclosing Party to the receiving Party, as shown by the receiving Party’s files and records;
(c)is subsequently disclosed to the receiving Party by a Third Party lawfully in possession thereof without obligation to keep it confidential and without a breach of such Third Party’s obligations of confidentiality;
(d)has been publicly disclosed or made generally available to the public other than through any act or omission of the receiving Party or its Affiliates or their subcontractors or Sublicensees in breach of this Agreement; or
(e)has been independently developed by the receiving Party without the aid, application or use of the disclosing Party’s Confidential Information (the competent written proof of which must be contemporaneous with such independent development).
6.3Authorized Disclosure. Notwithstanding Section 6.1, a Party may disclose Confidential Information of the other Party to the extent such disclosure is reasonably necessary in the following instances:
(a)Prosecuting and Maintaining Patent Rights in accordance with this Agreement; provided that the non-filing Party whose Confidential Information is being disclosed is given a reasonable opportunity to review the proposed disclosure of such Confidential Information and the filing Party considers in good faith any comments provided by the non-filing Party;
(b)Communicating and making filings with Regulatory Authorities or otherwise complying with applicable Laws or submitting information to tax or other governmental authorities; provided that if a Party is required by Law to make any public disclosure of Confidential Information of the other Party, to the extent it may legally do so, it will give reasonable advance written notice to the other Party of such disclosure and will use its reasonable efforts to secure confidential treatment of such Confidential Information prior to its disclosure (whether through protective orders or otherwise), and shall disclose only that portion of the Confidential Information which is legally required to be disclosed; for Regulatory Approval of Licensed Products or to research, develop, make, have made, use, have used, offer
to sell, sell, import, export, Commercialize or otherwise exploit Licensed Products in accordance with this Agreement;
(c)Subject to Section 6.4(b) or Section 6.4(c), as applicable, to its Affiliates, and to prospective and actual acquirers, lenders, licensees, and sublicensees (including Sublicensees and CDI CDMO Customers), and to each of their employees, consultants, contractors, agents, accountants, lawyers, advisors, investors and underwriters, in each case only on a need to know basis, each of whom, in the case of Third Parties, prior to disclosure must be bound by written or professional ethical obligations of confidentiality and non-use equivalent in scope to those set forth in this Article 6 and a disclosing Party shall be responsible for any breach of confidentiality by a receiving Party to which Confidential Information is disclosed under this Section 6.3(c); or
(d)To the extent mutually agreed to in writing by the Parties.
6.4Press Releases and Other Permitted Disclosures.
(a)CDI and Century each agree not to disclose any of the terms and conditions of this Agreement to any Third Party, except as described below in this Section 6.4 or as otherwise agreed in writing by the Parties. The Parties have agreed on a press release to be issued by each Party announcing this Agreement after the Effective Date in substantially the form set forth in Exhibit C; provided that the timing of the publication and release of such press release is in the sole discretion of an announcing Party. Each Party shall have the right to disclose the existence of this Agreement, including that the fact that such Party has received a license to the other Party’s Licensed Technology or Century Technology, as applicable without disclosing the Licensed Know-How or Century Know-How. Subject to the other provisions of this Agreement, no other press release, public statement or public disclosure concerning the existence or terms of this Agreement shall be made, either directly or indirectly, by either Party without the prior written approval of the other Party. If disclosure of the terms and conditions of this Agreement or its filing publicly is required by applicable Law or applicable stock exchange regulation, or by order or other ruling of a competent court or governmental authority, as set forth in Section 6.4(d), then CDI or Century or its respective Affiliates, as the case may be, may also disclose such terms or this Agreement in a public statement or disclosure subject to Section 6.4(b). Once any public statement or public disclosure has been approved in accordance with this Section 6.4, then either Party may appropriately communicate information contained in such permitted statement or disclosure.
(b)Century may disclose the terms and conditions of this Agreement to (i) its Affiliates, employees, consultants, agents or professional advisors (including attorneys, accountants and actual and prospective investment bankers), (ii) actual or potential investors, lenders, licensees, sublicensees, licensors or collaborators, or (iii) acquirers or merger partners that have entered into a letter of intent or are actively negotiating a definitive acquisition or merger agreement with Century; in each case under the foregoing clause (i), (ii) or (iii), under obligations of confidentiality at least as restrictive as those set forth herein, and solely in connection with Century performing its obligations or exercising its rights under this Agreement or for the purpose of assisting the recipient with evaluating and entering into a transaction with Century; provided, however, that Century shall redact financial terms from any such disclosure made to any actual or potential licensee, sublicensee or collaborator.
(c)CDI may disclose the terms and conditions of this Agreement to (i) its Affiliates, employees, consultants, agents or professional advisors (including attorneys, accountants and actual and prospective investment bankers), (ii) actual or potential investors, lenders, licensees, sublicensees, or CDI CDMO Customers, or (iii) acquirers or merger partners that have entered into a letter of intent or are actively negotiating a definitive acquisition or merger agreement with CDI; in each case under the foregoing clause (i), (ii) or (iii), under obligations of confidentiality at least as restrictive as those set forth herein, and solely
in connection with CDI performing its obligations or exercising its rights under this Agreement or for the purpose of assisting the recipient with evaluating and entering into a transaction with CDI; provided, however, that CDI shall redact financial terms from any such disclosure made to any actual or potential licensee, sublicensee or CDI CDMO Customer.
(d)Notwithstanding the foregoing provisions of this Article 6, a Party or its Affiliates may disclose the existence and terms of this Agreement or the Confidential Information of the other Party where required, as reasonably determined by the legal counsel of the disclosing party, by applicable Law, by applicable stock exchange regulation or by order or other ruling of a competent court or other governmental authority, although, to the extent practicable, the other Party shall be given at least [***] Business Days advance written notice of any such legally required disclosure to comment and the disclosing party shall reasonably consider such comments provided by such other Party on the proposed disclosure. In case either Party or its Affiliates is obliged to publicly disclose or file this Agreement as a “material agreement” in accordance with applicable Law or applicable stock exchange regulations (“SEC Filing”), this Agreement shall be redacted by the filing party to the extent permissible upon the reasonable advice of legal counsel, and the filing party shall provide the other Party a copy of such redacted agreement in advance of such SEC Filing to give reasonable opportunity the other Party to review and comment on the scope of such redaction in consultation with their own counsel; provided that the filing arty shall consider in good faith any comments provided by such other Party.
Article 7
Representations, Warranties, and Covenants
7.1Representations of Authority. Each Party represents and warrants to the other that as of the Effective Date it has full right, power and authority to enter into this Agreement and to perform its respective obligations under this Agreement.
7.2Consents. Each Party represents and warrants that as of the Effective Date all necessary consents, approvals and authorizations of all government authorities and other Persons required to be obtained by such Party in connection with execution, delivery and performance of this Agreement have been obtained.
7.3No Conflict. Each Party represents and warrants that, as of the Effective Date, the execution and delivery of this Agreement and the performance of such Party’s obligations hereunder (a) do not conflict with or violate any requirement of applicable Laws and (b) do not conflict with, violate or breach or constitute a default of, or require any consent under, any contractual obligations of such Party, except such consents as have been obtained as of the Effective Date.
7.4Employee, Consultant and Advisor Obligations. Each Party represents and warrants that, as of the Effective Date, each of its and its Affiliates’ employees, consultants and advisors has executed an agreement or has an existing obligation under law obligating such employee, consultant or advisor to maintain the confidentiality of Confidential Information to the extent required under Article 6.
7.5Intellectual Property.
(a)CDI represents and warrants to Century with respect to the Licensed Patent Rights, and Century represents and warrants to CDI with respect to the Century Patent Rights, as follows that (i) it owns or Controls the entire right, title and interest in and to the Licensed Patent Rights or Century Patent Rights, as the case may be, free and clear of all liens, charges and encumbrances, (ii) it has the right to grant to the other Party the rights and licenses under the Licensed Patent Rights and Century Patent Rights granted in this Agreement and has not previously assigned, transferred, conveyed or otherwise encumbered its right,
title and interest in such Patent Rights in any manner inconsistent with the terms hereof, and will not take any of the foregoing actions in any manner inconsistent with the terms hereof, (iii) none of the Licensed Patent Rights or Century Patent Rights was fraudulently procured from the relevant governmental patent granting authority, (iv) as of the Effective Date, there is no written claim or demand of any Person pertaining to, or any proceeding which is pending or threatened, that asserts the invalidity, misuse or unenforceability of the Licensed Patent Rights or Century Patent Rights or challenges its ownership of such Patent Rights or makes any adverse claim with respect thereto, including any claim that such Patent Rights infringe or misappropriate Intellectual Property of any Third Party, and, to the knowledge of such Party (without any duty to investigate), there is no basis for any such claim, demand or proceeding, (v) to the knowledge of such Party (without any duty to investigate), as of the Effective Date, the Licensed Patent Rights or Century Patent Rights are not being infringed by any Third Party, and (vi) to the knowledge of such Party (without any duty to investigate) as of the Effective Date, none of its Affiliates own or control, as of the Effective Date, any Patent Rights pertaining to differentiation of human iPSC that are reasonably necessary to Exploit a Licensed Product.
(b)During the Term, each Party shall use Commercially Reasonable Efforts to retain and maintain sufficient legal or beneficial title and ownership of, or sufficient license rights under, any Licensed Patent Rights or Century Patent Rights to enable such Party to grant the licenses and rights to such Patent Rights that would be granted to the other Party under, and as reasonably necessary to practice, such Patent Rights in accordance with the license to be granted under this Agreement.
7.6No Warranties. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH HEREIN, THE PARTIES MAKE NO REPRESENTATIONS AND EXTEND NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT, OR NON-MISAPPROPRIATION OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS ARE MADE OR GIVEN BY OR ON BEHALF OF A PARTY. EXCEPT AS EXPRESSLY STATED IN THIS AGREEMENT, ALL REPRESENTATIONS AND WARRANTIES, WHETHER ARISING BY OPERATION OF LAW OR OTHERWISE, ARE HEREBY EXPRESSLY EXCLUDED.
Article 8
Indemnification; Insurance; and Limitation on Damages
8.1By Century. Century agrees to defend CDI, its Affiliates and their respective directors, officers, employees, consultants and agents at Century’s cost and expense, and shall indemnify and hold harmless CDI and its Affiliates and their respective directors, officers, employees, consultants and agents from and against any liabilities, losses, costs, damages, fees or expenses arising out of any Third Party (excluding any Affiliate)-claim, suit, action or demand relating to (a) any breach by Century of any of its representations, warranties or obligations pursuant to this Agreement, (b) personal injury, property damage or other damage resulting from the Exploitation of Licensed Products in the Field within the Territory by or on behalf of Century or its Affiliates or Sublicensees or (c) the willful misconduct or grossly negligent acts of Century, its Affiliates, subcontractors, Century or Sublicensees (excluding CDI and its Affiliates as Licensees or Sublicensees of Century hereunder); excluding, in each case (a), (b) and (c), any damages or other amounts for which CDI has an obligation to indemnify Century pursuant to Section 8.2, as to which damages or amounts each Party shall indemnify the other to the extent of their respective liability for such damages or amounts.
8.2By CDI. CDI agrees to defend Century, its Affiliates and their respective directors, officers, employees, consultants and agents at CDI’s cost and expense, and shall indemnify and hold harmless Century and its Affiliates and their respective directors, officers, employees, consultants and agents from and against any liabilities, losses, costs, damages, fees or expenses arising out of any Third
Party (excluding any Affiliate) claim, suit, action or demand relating to (a) any breach by CDI of any of its representations, warranties or obligations pursuant to this Agreement, (b) personal injury, property, damage or other damage resulting from the Exploitation of CDI CDMO Products in the Field in the Territory by or on behalf of CDI, its Affiliates or Sublicensees or (c) the willful misconduct or grossly negligent acts of CDI, its Affiliates, subcontractors, licensees or Sublicensees (excluding Century and its Affiliates as licensees or Sublicensees to CDI hereunder); excluding, in each case (a), (b) and (c), any damages or other amounts for which Century has an obligations to indemnify CDI pursuant to Section 8.1, as to which damages or amounts each Party shall indemnify the other to the extent of their respective liability for such damages or amounts.
8.3Procedures. A Person entitled to indemnification under this Article 8 (an “Indemnified Party”) shall give prompt written notification to the Party from whom indemnification is sought (the “Indemnifying Party”) of any claim, suit, action or demand for which indemnification is sought under this Agreement; provided, however, that no delay or failure on the part of an Indemnified Party in so notifying the Indemnifying Party shall relieve the Indemnifying Party of any liability or obligation hereunder except to the extent of any damage or liability caused by or arising out of such delay or failure. Within [***] days after delivery of such notification, the Indemnifying Party may, upon written notice thereof to the Indemnified Party, assume control of the defense of such claim, suit, action or demand with counsel reasonably satisfactory to the Indemnified Party. If the Indemnifying Party does not assume control of such defense the Indemnified Party shall control such defense. The Party not controlling such defense may participate therein with counsel of its own choosing at its own expense; provided that, the Indemnified Party shall have the right to retain its own counsel, at the expense of the Indemnifying Party, if representation of such Indemnified Party by the counsel retained by the Indemnifying Party would be inappropriate because of actual or potential differences in the interests of such Indemnified Party and any other party represented by such counsel. The Indemnified Party shall not agree to any settlement of such action, suit, proceeding or claim without the prior written consent of the Indemnifying Party, which shall not be unreasonably withheld, delayed or conditioned.
8.4Insurance. Century shall procure and maintain insurance or self-insurance, including general liability insurance and, starting at the time at which a Licensed Product first enters clinical testing in human subjects by or on behalf of Century or Sublicensees, product liability insurance, in each case adequate to cover its obligations hereunder and consistent with normal business practices of prudent companies similarly situated, which insurance shall identify CDI as an additional insured starting at the time at which a Licensed Product first enters clinical testing in human subjects by or on behalf of Century or Sublicensees. It is understood that any such insurance shall not be construed to create a limit of each Party’s liability with respect to its indemnification obligations under this Article. Century shall provide CDI with written evidence of such insurance upon request. Century shall provide CDI with written notice at least [***] days prior to the cancellation, non-renewal or material change in such insurance or self-insurance which could adversely affect rights hereunder.
8.5No Consequential or Punitive Damages. EXCEPT WITH RESPECT TO (A) THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF EITHER PARTY UNDER THIS AGREEMENT WITH RESPECT TO THIRD PARTY CLAIMS, SUITS; ACTIONS OR DEMANDS, OR (B) A BREACH OF THE CONFIDENTIALITY AND NON-USE OBLIGATIONS OF ARTICLE 6. NEITHER PARTY HERETO WILL BE LIABLE FOR INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, EXEMPLARY OR PUNITIVE DAMAGES, INCLUDING LOST PROFITS, ARISING FROM OR RELATING TO THIS AGREEMENT, REGARDLESS OF ANY NOTICE OF SUCH DAMAGES.
Article 9
Term and Termination
9.1Term. This Agreement shall become effective as of the Effective Date and shall otherwise remain in effect, unless terminated as set forth in this Article 9 (the “Term”).
9.2Termination for Material Breach.
(a)Any failure by a Party (the “Breaching Party”) to comply with any of its material obligations contained in this Agreement (such failure, a “Material Breach”) shall entitle the other Party (the “Non-Breaching Party”) to give to the Breaching Party written notice specifying the nature of the Material Breach, requiring the Breaching Party to cure such Material Breach.
(b)If such Material Breach is not cured within [***] days after the receipt of notice pursuant to Section 9.2(a) above, the Non-Breaching Party shall be entitled to terminate this Agreement on written notice to the Breaching Party and without prejudice to any of its other rights conferred on it by this Agreement; provided that if a Material Breach cannot reasonably be cured within such [***]-day period and the Breaching Party promptly delivers a plan to cure such Material Breach (reasonably acceptable to the Non-Breaching Party) and cures such Material Breach up to a maximum cure period of [***] days from the date of initial notice; further provided, however, that if the Breaching Party disputes the existence of a Material Breach, the matter shall be submitted for resolution in accordance with Section 10.11, and the Breaching Party shall not have the right to terminate this Agreement unless and until a final decision of Material Breach is rendered under Section 10.11 and the Breaching Party fails to cure such Material Breach within [***] days thereafter.
9.3Termination for Bankruptcy. To the extent allowed under applicable Law, either Party shall have the right to terminate this Agreement in the event that the other Party admits its inability to pay its debts generally as they become due or in the event of the commencement of any proceeding in or for bankruptcy, insolvency, dissolution or winding up by or against the other Party (other than pursuant to a corporate restructuring), or the appointment of a receiver or similar agent by a court of competent jurisdiction, that is not dismissed or otherwise disposed of within [***] days thereafter and/or the administrator of the bankruptcy estate or the Party under in-court restructuring has not, within [***] days after the receipt of an inquiry from the other Party, confirmed that the bankruptcy estate or the Party under in-court restructuring will adopt this Agreement.
9.4Termination for Convenience. Century may terminate this Agreement, at any time and for any reason or no reason, in its entirety or on an indication-by-indication basis, a Licensed Product-by-Licensed Product basis (to exclude such product from the Field) or country-by-country basis (to eliminate such country from the Territory), by providing [***] days’ prior written notice to CDI, provided that, for purposes of clarity, in such event, GDI’s license under Section 2.2 shall survive any such termination by Century for Convenience. The termination shall become effective at the end of the [***]-day period.
9.5Effects of Termination.
(a)Generally. Expiration or termination of this Agreement shall not relieve the Parties of any obligation accruing prior to such expiration or termination. Any expiration or termination of this Agreement shall be without prejudice to the rights of either Party against the other accrued or accruing under this Agreement prior to expiration or termination. Termination of this Agreement shall be in addition to, and shall not prejudice, the Parties’ remedies at law or in equity, including the Parties’ ability to receive legal damages or equitable relief with respect to any breach of this Agreement, regardless of whether or not such breach was the reason for the termination. In the event of any early termination of this Agreement, the Parties will work together in good faith to determine and implement reasonable wind-down procedures with respect to relevant Licensed Product-related activities ongoing at the time of such termination, which shall include continuation of the licenses granted to Century hereunder (and subject to the continuing terms
and conditions of this Agreement) to permit Century and Sublicensees to continue and complete any ongoing clinical trials of Licensed Products and to make or have made such Licensed Products as necessary to continue and complete such clinical trials; provided, however, in that the event such termination is by CDI pursuant to Section 9.2, such Licensed Product-related activities shall be limited to those necessary for Century to comply with regulatory obligations, or medical or ethical obligations to patients consistent with industry standards. For purposes of clarity, CDI’s license under Section 2.2 shall survive any expiration or termination of this Agreement.
(b)Return or Destruction of Confidential Information. Upon the expiration or termination of this Agreement, each Party shall promptly return or destroy all of such other Party’s Confidential Information that relates to a Licensed Product or that was provided by or on behalf of such other Party hereunder that is in the possession or control of such Party (or any of its Affiliates), except that (i) such Party will have the right maintain one (1) copy of intangible Confidential Information of such other Party for legal and archival purposes; and (ii) CDI will have the right to retain Century Confidential Information that is embodied in the Century Licensed Technology or the Century Manufacturing Technology and as is reasonably necessary to exercise its rights under Section 2.2 and CDI’s retention of such Confidential Information will remain subject to the terms of Article 6.
9.6Survival. The following provisions shall survive the expiration or termination of this Agreement: Article 1 (Definitions) (to the extent necessary to give effect to other surviving provisions), Article 5 (Intellectual Property), Article 6 (Confidentiality), Article 8 (Indemnification; Insurance; and Limitation on Damages) (other than Section 8.4 (Insurance)) and Article 10 (Miscellaneous Provisions), and Sections 2.2 (License Grants to CDI), 2.3(b) (Sublicensing), 9.5 (Effects of Termination) and this Section 9.6 (Survival).
Article 10
Miscellaneous Provisions
10.1Governing Law: Language. This Agreement and all disputes arising out of or related to this Agreement shall be construed and the respective rights of the Parties determined in accordance with the laws of the State of Wisconsin, U.S.A., excluding application of any conflict of laws. This Agreement and all communications related to it, or to any dispute or controversy arising out of it, shall be conducted in English.
10.2Notice. Any notices required or permitted by this Agreement shall be in writing, shall specifically refer to this Agreement, and shall be sent by hand, recognized national overnight courier, confirmed electronic mail or registered or certified mail, postage prepaid, return receipt requested, to the following address of the Parties:
If to CDI:
FUJIFILM Cellular Dynamics, Inc.
000 Xxxxxxx Xxxxx
Xxxxxxx, XX 00000
Attention: Director, Intellectual Property
Email: xxxx-xxxxxxxxx@xxxxxxxxxxxxxxxx.xxx
With a copy to:
FUJIFILM Cellular Dynamics, Inc.
000 Xxxxxxx Xxxxx
Madison, WI 53711
Attention: General Counsel
and
Email: xxxxxxxxxxxxxxx@xxxxxxxx.xxx
If to Century:
Century Therapeutics, Inc.
00 X. 00xx Xxxxxx, 00xx Xxxxx
Xxxxxxxxxxxx, XX 00000
Attention: General Counsel
Email: xxxxx@xxxxxxxxx.xxx
All notices under this Agreement shall be deemed effective upon receipt. A Party may change its contact information immediately upon written notice to the other Party in the manner provided in this Section 10.2.
10.3Assignment. Neither Party may, without the consent of the other Party, assign or transfer any of its rights and obligations hereunder; provided that each Party may assign this Agreement, in its entirety, without the consent of the other Party, (a) in connection with a sale or transfer of all or substantially all of the business and assets of each Party to which this Agreement relates, including by way of merger, consolidation, transfer, or sale of assets related to this Agreement or (b) to an Affiliate or in connection with a reorganization, provided, further that in each case each Party shall ensure that upon any assignment or transfer under this Section 10.3 the assignee or transferee or an Affiliate, as the case may be, agrees in writing, in form and substance reasonably satisfactory to the other Party, to perform and comply with all obligations of such Party under, and to be bound by the terms and conditions of, this Agreement as if such assignee or transferee or an Affiliate were a direct party to this Agreement. Any assignment in circumvention of the foregoing shall be void. Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and permitted assigns. For clarity, if Century is involved in a change of control with a Third Party, then: (a) the Patent Rights and Know-How controlled by such Third Party (or any Affiliate thereof, excluding Century as a result of such transaction) existing as of the date of closing of such change of control (if such Third Party becomes the assignee of this Agreement); or (b) the Patent Rights and Know-How controlled by such Third Party (if such Third Party remains an Affiliate of Century), in each case, will be excluded from the definition of Century Know-How and Century Patent Rights.
10.4Entire Agreement. This Agreement constitutes the entire agreement between the Parties with respect to its subject matter and supersedes all prior agreements or understandings between the Parties relating to its subject matter.
10.5Interpretation. The captions and headings to this Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions of this Agreement. Unless specified to the contrary, references to Articles, Sections or Exhibits mean the particular Articles, Sections or Exhibits to this Agreement and references to this Agreement include all Exhibits hereto. Unless context otherwise clearly requires, whenever used in this Agreement: (a) the words “include” or “including” shall be construed as incorporating, also, “but not limited to” or “without limitation;” (b) the word “day” or “year” means a calendar day or year unless otherwise specified; (c) the word “notice”, “consent”, or “approval” and references to other written communications contemplated under this Agreement shall mean notice, consent, approval or communication in writing (whether or not specifically stated); (d) the words “hereof,” “herein,” “hereby” and derivative or similar words refer to this Agreement (including any
Exhibits); (e) the word “or” shall be construed as the inclusive meaning identified with the phrase “and/or;” (t) provisions that require that a Party or the Parties hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter or otherwise; (g) words of any gender include the other gender; (h) words using the singular or plural number also include the plural or singular number, respectively; and (i) the word “law” (or “laws”) when used herein means any applicable, legally binding statute, ordinance, resolution, regulation, code, guideline, rule, order, decree, judgment, injunction, mandate or other legally binding requirement of a government entity, together with any then- current modification, amendment and re-enactment thereof, and any legislative provision substituted therefor. The Parties and their respective counsel have had an opportunity to fully negotiate this Agreement. If any ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties, and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any provision of this Agreement. No prior draft of this Agreement shall be used in the interpretation or construction of this Agreement.
10.6Amendment and Waiver. This Agreement may be amended, supplemented, or otherwise modified only by means of a written instrument signed by an authorized representative of both Parties. Any waiver of any right or failure to act in a specific instance shall relate only to such instance and shall not be construed as an agreement to waive any right or fail to act in any other instance, whether or not similar.
10.7Severability. In the event that any provision of this Agreement shall be held invalid or unenforceable for any reason, such invalidity or unenforceability shall not affect any other provision of this Agreement The Parties shall consult one another and use reasonable efforts to agree upon a valid and enforceable provision that is a reasonable substitute for the invalid or unenforceable provision and this Agreement shall be automatically amended to reflect such mutually agreed provision.
10.8Use of Name. Neither Party shall use the other Party’s name (except in connection with disclosures permitted under Article 6) or logo without the other Party’s express prior written consent, which consent may be granted in the context of the Parties mutually approving a press release or other public disclosure related to this Agreement.
10.9Counterparts. This Agreement may be executed in counterparts, each of which counterparts, when so executed and delivered, shall be deemed to be an original, and all of which counterparts, taken together, shall constitute one and the same instrument.
10.10Force Majeure. Neither Party will be responsible for delays resulting from causes beyond the reasonable control of such Party, including fire, explosion, flood, war, strike, or riot, provided that the nonperforming Party uses Commercially Reasonable Efforts for a company of its size and resources to avoid or remove such causes of nonperformance and continues performance under this Agreement with reasonable dispatch whenever such causes are removed.
10.11Dispute Resolution.
(a)Escalation. If any dispute arises out of or relates to this Agreement, the Parties agree to first seek to resolve such dispute by referring such dispute to the respective Chief Executive Officers of each Party for resolution. Such referral shall take place within [***] days after a written request by either Party to the other Party that resolution by the Chief Executive Officers be attempted. If, within [***] days following the dispute being referred to the Chief Executive Officers of the Parties, the Chief Executive Officers of the Parties have not resolved the dispute, and a Party wishes to pursue the matter, such Party may initiate binding arbitration in accordance with Section 10.11(b).
(b)Alternative Dispute Resolution. Any dispute arising out of or relating to this Agreement that has not been resolved pursuant to Section 10.11(a) shall be finally settled, in Tokyo in the event Century is a claimant and in New York in the event CDI is a claimant, under the Rules of Arbitration of the International Chamber of Commerce (“ICC”). The arbitral tribunal shall consist of three arbitrators, one nominated by the claimant in the request for arbitration, the second nominated by the respondent within [***] days of receipt of the request for arbitration, and the third, who shall act as presiding arbitrator, nominated by the two party appointed arbitrators within [***] days of the nomination of the second arbitrator. If any arbitrators are not nominated within these time periods, the ICC the International Court of Arbitration shall, upon the request of any Party, make the appointment(s).
(c)No Limitation. Nothing in this Section 10.11 shall be construed as limiting in any way the right of a Party to seek a preliminary injunction or other provisional relief with respect to any actual or threatened breach of this Agreement or to bring an action in aid of arbitration. Should any Party seek an injunction or other equitable relief, or bring an action in aid of arbitration, then for purposes of determining whether to grant such injunction or other equitable relief, or whether to issue any order in aid of arbitration, the dispute underlying the request for such injunction or other equitable relief, or action in aid of arbitration, may be heard by the court in which such action or proceeding is brought.
10.12No Third-Party Beneficiaries. No Person other than Century, CDI and their respective Affiliates, successors and permitted assignees hereunder, shall be deemed an intended beneficiary hereunder or have any right to enforce any obligation of this Agreement.
10.13Independent Contractors. It is expressly agreed that Century and CDI shall be independent contractors and that the relationship between Century and CDI shall not constitute a partnership, joint venture or agency. Neither Century nor CDI shall have the authority to make any statements, representations, or commitments of any kind, or to take any action, which shall be binding on the other Party, without the prior written consent of such other Party.
[remainder of page intentionally left blank]
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the date first set forth above.
FUJIFILM CELLULAR DYNAMICS, INC. | |
By: /s/Xxxxxxxx Xxxxxxxx Name: Xxxxxxxx Xxxxxxxx Xxxxx: President and Chief Executive Officer | By: /s/Xxxx Xxxxxxxx Name: Xxxx Xxxxxxxx, Ph.D. Title: Interim Chief Executive Officer |
Exhibit A
[***]
[***]
[***]
[***]
Exhibit B
[***]
| ||
[***] | [***] | [***] |
[***] | [***] | [***] |
[***] | [***] | [***] |
[***] | [***] | [***] |
[***] | [***] | [***] |
[***] | [***] | [***] |
[***] | [***] | [***] |
Exhibit C
[***]
[***]