EXCLUSIVE DISTRIBUTION AGREEMENT
Exhibit 10.1
EXECUTION VERSION
CONFIDENTIAL TREATMENT
REQUESTED
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT WHICH HAVE BEEN REDACTED ARE
MARKED WITH BRACKETS (“[***]”). THE OMITTED
MATERIAL HAS BEEN FILED SEPARATELY
WITH
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
This Exclusive Distribution Agreement (this “Agreement”)
is dated this 16th day of April, 2008, but is effective as of May 1, 2008 (the “Effective Date”), by and between CryoLife, Inc., 0000 Xxxxxxx Xxxx., XX, Xxxxxxxx, XX 00000 (“CryoLife”) and Medafor, Inc., 0000
Xxxxxxx Xxxx., Xxxxx 000, Xxxxxxxxxxx, XX 00000 (“Medafor”).
A. Medafor
is in the business of developing, manufacturing and selling proprietary hemostatic products, including the Product (as defined below).
B. CryoLife develops and commercializes biomaterials and preserves and distributes human tissue for vascular
and cardiac transplant applications.
C. Medafor desires to appoint CryoLife as the exclusive distributor of the Product
throughout the Territory (as defined below) for use in applications in the Field (as defined below), and CryoLife desires to accept such appointment, all in accordance with the terms and conditions of this Agreement.
In consideration of the mutual covenants contained in this Agreement, CryoLife and Medafor agree as follows:
ARTICLE I
DEFINITIONS AND RULES OF CONSTRUCTION
1.1. Definitions.
(a) Terms Defined in this Article. For purposes of this Agreement, the following terms shall have the following meanings:
“Affiliates” as it relates to a Party, shall mean any Person controlling, controlled by or under common
control with a Party.
“Annual Minimum” shall have the meaning ascribed to it in Section 2.2.
“Annual Minimum
Dispute Notice” shall have the meaning ascribed to it in Section 2.2.
“Applicable Laws” means all applicable common law, statutes, ordinances, rules, regulations or orders of any Governmental Authority, including Regulatory Laws.
“Bellows Applicator” means the bellows applicator manufactured by Medafor for use with MPH
Product.
“Business Day” means any day other than a Saturday, a Sunday or a day on which banks in New York are
authorized or obligated by law or executive order to remain closed.
“China”
means the People’s Republic of China
“Claim”
shall have the meaning ascribed to it in Section 7.2.
“CGMP” shall have the meaning ascribed to it in Section 4.2.
“Commercially Reasonable Efforts” means, with respect to a Party’s diligence in satisfying an
obligation under this Agreement, that the Party applies the level of efforts, expertise and resources that it would apply in the ordinary course of business to satisfy a comparable obligation and that such actions be taken in good
faith. In determining whether a Party is applying Commercially Reasonable Efforts, the potential benefit to such Party not taking such efforts (e.g. benefit in receipt of payments for products that are sold inappropriately through a
Crossover) shall not be considered. At the reasonable request of the non-offending Party, as that term is used in Section 2.1, Commercially Reasonable Efforts will include ceasing to
provide Products or MPH Product to the offending distributor.
“Confidential
Information” shall have the meaning ascribed to it in Section 6.1.
“Crossover” shall have the meaning ascribed to in Section 2.1.
“CryoLife Applicator” means any applicator developed by CryoLife for use with the MPH Product.
“CryoLife IP” shall have the meaning ascribed to it in Section 7.1.
“CryoLife
Marks” means the brand names and marks CryoLife designates pursuant to Section 2.4 for use on Product packaging.
“Department of Defense Hospitals” those hospitals that are run by the U.S. Department of Defense and located
on any United States military facility anywhere in the world. Notwithstanding the foregoing, hospitals run by the Veterans Administration shall not be deemed “Department of Defense Hospitals”, regardless of their location.
“Distribute” shall be the meaning ascribed to it in Section 2.1.
“Effective Date” means May 1, 2008.
“Failure to
Supply” shall have the meaning ascribed to it in Section 10.2.
“FDA” means the United States Food and Drug Administration or any successor agency having the administrative authority to grant Marketing Approval in the
United States.
“Field” means: (i) all applications in cardiac surgery and vascular surgery in the
United States; and (ii) all general surgery applications outside the United States including cardiac and vascular surgeries, but excluding orthopedic and ear, nose, and throat surgeries. For avoidance of doubt, the parties agree that neurosurgery
and topical applications are also excluded from the definition of “Field”.
- 2 -
“Field Action”
means any correction or removal action by CryoLife or Medafor due to safety, efficacy, quality or regulatory compliance concerns, including actions to recover title to or possession of, or to halt distribution of, Products that previously
have been shipped to customers.
“Forecast” shall be the meaning ascribed to it in Section 3.1.
“Governmental
Authority” means the United States and any other country in which the Product is manufactured, marketed, sold, tested, investigated or otherwise regulated, and all states or other political subdivisions thereof and supranational
bodies applicable thereto, including the European Union, and all agencies, commissions, officials, courts or other instrumentalities of the foregoing.
“HemArrest” means HemArrest, Inc., a Minnesota corporation.
“Insolvency
Event” means that the Party (a) has commenced a voluntary proceeding under any insolvency law, (b) had an involuntary proceeding commenced against it under any insolvency law which has continued undismissed or unstayed for sixty (60)
consecutive days, (c) had a receiver, trustee or similar official appointed for it or for any substantial part of its property, (d) made an assignment for the benefit of creditors or (e) had an order for relief entered with respect to it
by a court of competent jurisdiction under any insolvency law. For purposes hereof, the term “insolvency law” means any applicable bankruptcy, insolvency or other similar law now or hereafter in effect.
“Intellectual Property” means (a) discoveries, inventions, improvements, concepts and ideas, whether or
not patentable, (b) works of authorship fixed in a tangible medium of expression, (c) Trademarks, (d) trade secrets and know-how and (e) all proprietary rights relating thereto, including all applications, registrations and
renewals in connection therewith.
“Initial Term” shall have the meaning ascribed to it in Section 10.1.
“Inventory
Cost” shall mean the specific and direct manufacturing or procurement cost to Medafor associated with the manufacturing or procurement of such inventory (but shall not include overhead, markup or carrying costs).
“ISO” shall have the meaning ascribed to it in Section 4.2.
“Losses” shall have the meaning ascribed to it in Section 9.1.
“Marketing Approval” means, with respect to any country or jurisdiction, the act of the applicable
Regulatory Authority that is necessary under applicable Regulatory Laws for the manufacture, marketing, distribution and sale of the Product in that country or jurisdiction, and satisfaction of all applicable regulatory and notification requirements
and, to the extent applicable, the grant of Pricing Approval.
- 3 -
“Medafor IP”
shall have the meaning set forth in Section 7.2.
“MPH
Product” means Medafor’s proprietary product for effecting hemostasis at the site of a wound, cut or incision using a patented technology known as MPH® (Microporous Polysaccharide Hemospheres). The MPH Product for surgical use is currently referred to as Arista®. MPH Product includes all product
improvements, modifications, additions and refinements thereto made by Medafor during the Term.
“Other
Manufacturing Laws” shall have the meaning ascribed to it in Section 4.2.
“Party” means CryoLife or Medafor, as the context requires.
“Person”
means any individual, group or entity, including Governmental Authorities.
“Pricing
Approval” means, with respect to any country or jurisdiction in which Governmental Authorities determine the pricing at which products will be reimbursed, the approval, agreement, determination or decision by the applicable authorities
establishing that pricing.
“Product” means a Product Applicator containing MPH Product per the Specifications.
“Product Applicator” means a Bellows Applicator or a CryoLife Applicator. The term “Product
Applicator” includes all product applicator improvements, modifications, additions and refinements thereto made by Medafor or CryoLife, as applicable, during the Term.
“Product Complaint” means any expression by a Third Party of dissatisfaction relating to the identity,
durability, reliability, safety, efficacy or performance of any Product, including actual or suspected product tampering, contamination, mislabeling or misformulation.
“Product Information” shall have the meaning ascribed to it in Section 2.3.
“QSR”
shall have the meaning ascribed to it in Section 4.2.
“Regulatory
Authority” means, with respect to any country or jurisdiction, any Governmental Authority involved in granting Marketing Approval or Pricing Approval or in administering Regulatory Laws in that country or jurisdiction, including the FDA
in the United States.
“Regulatory Laws” means all Applicable Laws governing (i) the import, export,
testing, investigation, manufacture, marketing or sale of the Product, (ii) establishing recordkeeping or reporting obligations, (iii) any Field Action or (iv) similar regulatory matters.
- 4 -
“Right of Renewal” shall have the meaning ascribed to it in Section 10.1.
“Specifications” means, collectively, (i) Medafor’s design and functionality specifications relating to the Product set forth in Exhibit A and (ii) any specifications for manufacturing, testing, storing, packaging, shipping or labeling (including CryoLife’s labeling and packaging requirements and
instructions as set forth herein) the Product set forth in any approved application for Marketing Approval and any supplements and amendments thereto.
“Term” shall have the meaning ascribed to it in Section 10.1
“Territory” shall mean the United States, Canada, the United Kingdom and Germany commencing on the Effective
Date. Beginning January 1, 2009, the Territory shall expand to mean the entire world except for the countries of Japan and China. Notwithstanding the foregoing, in no event shall the Territory include any Department of Defense
Hospitals. Although the Territory shall initially include the United States, the parties agree that in the United States the Territory shall exclude those certain enumerated hospitals listed on Exhibit B in the United States until the times set forth therein.
“Third Party” means any Person other than a Party or its Affiliates.
“Trademarks” means all trademarks, service marks, trade dress, logos and trade names, together with all translations, adaptations, derivations and combinations thereof (including all
goodwill associated therewith), and all applications, registrations and renewals in connection therewith.
“United
Kingdom” means the United Kingdom of Great Britain and North Ireland.
“United States” means the United States of America, including its territories, commonwealths and possessions.
“Year” shall mean the period commencing the Effective Date and ending
June 30, 2009, or any subsequent twelve month period commencing July 1 and ending on the following June 30.
“Year 1” “Year 2” and “Year 3” shall have the meanings
ascribed to them in Section 2.2.
1.2. Rules of Construction.
(a) When a reference is made in this Agreement to a Recital, an Article, a Section, a Schedule or an Exhibit, such reference is to a Recital, Article or Section of, or a Schedule
or an Exhibit to, this Agreement, unless otherwise indicated. All Exhibits and Schedules attached hereto are incorporated in this Agreement by reference.
(b) Whenever the words
“include,” “includes” or “including” are used in this Agreement, they shall be understood to be followed by the words “without limitation.”
- 5 -
(c) Pronouns, including
“he,” “she” and “it,” when used in reference to any Person, shall be deemed applicable to entities or individuals, male or female, as appropriate in any given case.
(d) Article, Section and other
headings contained in this Agreement are for reference purposes only and are not intended to describe, interpret, define or limit the scope, extent or intent of any provision of this Agreement.
(e) Standard variations on
defined terms (such as the plural form of a term defined in the singular form, and the past tense of a term defined in the present tense) shall be deemed to have meanings that correlate to the meanings of the defined terms.
ARTICLE II
DISTRIBUTION
2.1. Exclusive Distribution Rights.
(a) Medafor hereby grants to
CryoLife, and CryoLife hereby accepts, the exclusive right to promote, market, sell and distribute (collectively, “Distribute”) the Product throughout the Territory for all uses and applications in the Field. Medafor shall
not, directly or indirectly, Distribute, or permit Distribution of, Products or the MPH Product anywhere in the Territory for any uses or applications in the Field, either on its own behalf or through any Affiliate or Third Party. CryoLife
shall not, directly or indirectly, Distribute, or permit Distribution of, the Product anywhere in the Territory for any uses or applications outside of the Field, either on its own behalf or through any Affiliate or Third Party.
(b) Both Parties acknowledge
and agree that, notwithstanding their efforts to the contrary, given the nature of the industry, the distribution of products and that both Parties employ distributors, there may be inadvertent sales “Crossover” by either or both
Parties, i.e. inadvertent sales unknown to CryoLife of Product into applications outside of the Field and inadvertent sales unknown to Medafor of MPH Product or Products into applications in the Field. Upon learning of such inadvertent sales, the
non-offending Party shall give the other Party written notice of the facts and circumstances of the inadvertent sale, and the Party receiving such notice will use its Commercially Reasonable Efforts to prevent additional prohibited sales and report
what steps it has taken to prevent a reoccurrence. Such inadvertent sales shall not be considered a material breach of this Section, provided the other Party takes prompt and Commercially Reasonable Efforts to prevent additional prohibited sales and
timely reports what steps it has taken to prevent a reoccurrence to the non-offending Party.
(c) Medafor represents and warrants that attached hereto as Schedule 2.1 is a list of all agreements that permit
others to Distribute Products in the Field (or if such agreement is oral, a description of such oral arrangement). Medafor represents and warrants that:
(i) it has delivered to
CryoLife the most current and complete copies of each of the agreements listed on Schedule 2.1 (or if such agreement is oral, that is has summarized all terms of such oral
arrangement);
- 6 -
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT
WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
(ii)
by the Effective Date there shall be no agreement or arrangements to which it is a party or by which
it is bound that would permit others to Distribute Products in the Field in the United States, Canada, the United Kingdom or Germany; provided however that distribution rights that allow for the promotion, sale or distribution of the Product in
Department of Defense Hospitals shall not be deemed a violation of this Section; and provided, further and notwithstanding the foregoing, the distribution by the three distributors disclosed on Schedule 2.1 that have agreements that do not expire or terminate on the Effective Date shall not be deemed a violation of this Section to the limited extent that they permit promotion, sale
or distribution to those hospitals within the United States that are identified on Exhibit B;
(iii) by January 1, 2009 there
shall be no agreements or arrangements to which it is a party or by which it is bound that would permit others to Distribute Products in the Field outside of the United States, Canada, the United Kingdom or Germany, Japan or China; or
(iv)
it will not take any action with respect to any of its agreements with third parties that may expose
CryoLife to any liability.
(d) Medafor represents and
warrants that other than the existing distributors identified on Schedule 2.1, it does not currently have any other agents, representatives, or distributors entitled to Distribute
Products for use in the Field within the Territory, and that there is no restriction, covenant, or agreement to which it is a party or by which it is bound that would prevent or delay Medafor from providing to CryoLife the exclusive distribution
rights contained in this Agreement. Medafor agrees that it will not, directly or indirectly, undertake any action, omit to take any action, or enter into any agreement that will prevent or delay the enjoyment by CryoLife of the full
benefits of the exclusive distribution relationship provided in this Agreement. Medafor agrees to direct all sales inquiries respecting the Product within the Field in the Territory to CryoLife immediately during the Term of this
Agreement. Medafor represents and warrants that its termination of agreements that permit others to Distribute Products in the Field in the Territory shall not cause CryoLife any Losses. CryoLife agrees to direct all sales
inquires respecting the Product outside the Field immediately to Medafor during the Term of this Agreement.
2.2. Annual Minimums.
(a) During the Initial Term, CryoLife agrees to order the following minimum Product amounts (the “Annual Minimum”) during the periods set forth below:
| Year 1 - (May 1, 2008 –
June 30, 2009): |
$[***] | |
| Year 2 - (July 1, 2009 –
June 30, 2010): |
$[***] | |
| Year 3 - (July 1, 2010 –
June 30, 2011): |
$[***] | |
(b) After Year 3, CryoLife
agrees that the minimums for Years 4-6 shall be as set forth below:
| Year 4 - (July 1, 2011 –
June 30, 2012): |
$[***] | |
| Year 5 - (July 1, 2012 –
June 30, 2013): |
$[***] | |
| Year 6 - (July 1, 2013 –
June 30, 2014): |
$[***] |
- 7 -
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN
FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
The parties expressly acknowledge and agree that CryoLife’s obligation
to purchase the Annual Minimums set forth in Sections 2.2(a) and 2.2(b) is based on CryoLife’s unimpaired ability
to sell the Product under private label, beginning on the Effective Date throughout the United States, Canada, Germany and the United Kingdom (except for those specific hospitals set forth on Exhibit B); and beginning on January 1, 2009, throughout out the entire Territory. In the event that CryoLife is unable to sell such Products under private label as set forth
herein in the Territory, as a result of impairment (unless such impairment is caused directly by CryoLife) then such Annual Minimums shall be equitably adjusted downward as follows: CryoLife shall provide written notice to Medafor of the
impairment and its proposed reduction based on such impairment. Medafor shall have fifteen (15) days from receipt of such notice to notify CryoLife in writing that it disagrees with CryoLife’s reduction with such notice also
detailing the proposed number Medafor believes is the appropriate reduction, if any, in the Annual Minimum. In the event that Medafor fails to notify CryoLife, the Annual Minimum for the Year in question shall be reduced per
CryoLife’s notice set forth above. In the event that Medafor notifies CryoLife that it disagrees with CryoLife, the parties shall have fifteen (15) days to resolve the dispute, after which, after which, either Party may notify the
other Party that it requests that an arbitrator decide upon the appropriate reduction pursuant to Schedule 2.2 attached hereto pursuant to so called “Baseball” arbitration
(such notice, the “Annual Minimum Dispute Notice”). The parties agree that the equitable adjustment downward for purposes of the “Baseball” arbitration shall be based on the following factors: CryoLife’s
projection of sales in such country(ies), the number of medical procedures that the Product could be sold for use in such country(ies), sales of the Product in similarly situated countries, and other similar facts that the parties deem to be
important.
(c) The parties acknowledge and agree that the Annual Minimums are based on Medafor’s current sales of the
Product and therefore, Medafor represents and warrants that it has delivered to CryoLife, prior to the execution of this Agreement, Medafor’s current sales information for the first quarter of 2008 for the Product and that such information is
true, correct and complete.
(d) During Year 1, CryoLife
agrees to submit purchase orders for the Products as follows: (i) for the period between the Effective Date and July 31, 2008, CryoLife will submit to Medafor a purchase order for at least $500,000 of Products and such purchase order shall be
submitted upon the execution of this Agreement by the Parties; (ii) for the period between August 1, 2008 and October 31, 2008 and by no later than June 15, 2008, CryoLife shall submit to Medafor a purchase order for at least $750,000 of
Products; (iii) for the remainder of Year 1, CryoLife shall submit monthly purchase orders for $[***] worth of Products with the understanding that each monthly purchase order shall not be deemed effective
unless it is submitted in accordance with Section 3.2. CryoLife’s obligations under this subsection shall be equitably adjusted in the event of any reduction in the
Annual Minimum for Year 1.
(e) After the conclusion of
Year 1 and during the remainder of the Term, CryoLife will submit purchase orders for at least 15% of the applicable Annual Minimum for the calendar quarter commencing July 1, 25% for the calendar quarter commencing October 1, 25% for the calendar
quarter commencing January 1, and 35% for the calendar quarter commencing April 1. Such purchase orders may be submitted at any time prior to and including the first date of the applicable calendar quarter and should be consistent with
the three (3) month portion of the rolling forecast set forth in Section 3.1. CryoLife may submit purchase orders monthly provided that for a purchase order to be effective
it must be submitted in accordance with Section 3.2.
- 8 -
(f) The foregoing minimum purchases may be reduced by CryoLife in any given period to the extent of any prior purchase by CryoLife in excess of the minimum amounts specified above
for any and all preceding periods. The inability of CryoLife to meet any minimum purchase requirement by reason of Product returns pursuant to Section 2.6, any breach of this Agreement by Medafor resulting in an impairment to CryoLife’s
ability to sell into any portion of the Territory based on the timelines set forth herein, supply interruption by Medafor, force majeure, or any Product recall shall not cause CryoLife to be in default under this Section.
(g) During Year 1, all Product
purchases shall be for Products in 3 gram and 5 gram volumes. Thereafter, Medafor agrees to make additional volume Product and configurations available for purchase, as reasonably requested by CryoLife, at prices and volumes to be mutually
negotiated and agreed to by the Parties.
2.3. Marketing and Sales Activities.
(a) CryoLife shall have sole control and authority over its marketing activities for the Products. All business decisions concerning the sales and marketing of Products
within the Field and the Territory, including the price, product packaging and configuration, and other sale and promotional terms thereof, will be within the sole discretion of CryoLife, except that CryoLife shall provide Medafor with a reasonable
opportunity to review and approve all marketing materials relating to the Products for purposes of compliance with Regulatory Laws and to reasonably protect its rights in its trademarks and copyrights, which approval shall not be unreasonably
withheld or delayed. CryoLife shall comply with the appropriate quality control instructions of Medafor as to the form and manner in which such Medafor trademarks shall be used In its discretion, CryoLife may conduct clinical trials for
the Products in order to support CryoLife’s marketing and sales efforts. CryoLife agrees to provide Medafor with written results of such clinical trials for Medafor’s records and uses in areas outside of the Field.
(b) Medafor agrees to provide
to CryoLife all reasonable technical assistance, including necessary information related to the Product in the Field to CryoLife in order for CryoLife to market the Product and so CryoLife may develop its own technical, scientific and clinical
information files for purposes of obtaining new product committee approvals at target hospitals. Medafor agrees to provide reasonable training CryoLife’s sales force regarding the Products either at Medafor’s facilities or in
the Field; provided that CryoLife shall be responsible for the costs and expenses of CryoLife personnel incurred in connection with Medafor providing such train-the trainer technical assistance and training and CryoLife shall reimburse Medafor for
its incurred travel expenses. Medafor agrees to provide CryoLife with copies of its product handling manuals, sales literature, promotion materials, training materials, videos, demonstration kits and other applicable information for the
Products. To the extent possible Medafor shall provide CryoLife in electronic form and format all such marketing materials and information described in this Section (collectively all such information described in this Section is
“Product Information”).
- 9 -
(c) Portions of the Product Information may be incorporated into CryoLife’s materials used for the promotion of the Products. Medafor represents and warrants that
the Product Information shall be accurate and complete in all material respects, and undertakes to update any such Product Information when any information included therein becomes outdated, inaccurate, or misleading. CryoLife shall have
the right to produce, at its expense, promotional material, Products handling manuals, instructions for use, and other written information relating to the Products that is based in whole or in part on the material supplied by Medafor subject to the
limitations set forth above.
(d) Medafor will cooperate
with CryoLife in the sponsorship and planning of seminars and marketing events for Products.
(e) Medafor shall furnish without charge to CryoLife, any market surveys and related information prepared by or for Medafor pertaining to the market for the Products in the
Territory. CryoLife will treat such information as Medafor IP in accordance with the applicable provisions of Section 7.1.
2.4. Branding. The Products shall be sold under CryoLife’s brand names as directed by CryoLife; provided however, that CryoLife’s branding will use the MPH® trademark owned by
Medafor on its external packaging in a manner reasonably acceptable to Medafor and in accordance with all Applicable Laws and in accordance with appropriate quality control instructions of Medafor as to form and manner of
use. Notwithstanding the foregoing, the parties have agreed that CryoLife may use the name Hemostase as the private label and to the extent necessary Medafor agrees to consent to the use and filing of the name Hemostase MPH® or Hemostase MPH® trademarks by CryoLife with appropriate Governmental Authorities, if
CryoLife so desires. Medafor shall adapt packaging and labeling for the Products as instructed by CryoLife to meet CryoLife’s usual, normal and reasonable branding standards, which packaging shall be developed by
CryoLife. CryoLife, subject to Medafor’s reasonable acceptance, shall have final approval over all packaging and labeling of the Products.
2.5. Sample Products. Medafor shall provide to CryoLife sterile samples of Product at no charge according to the following commitment:
(a) Year 1- 800 boxes of 5
gram
(b) Year 2- 400 boxes of 5
gram
Unless otherwise agreed, samples shall be delivered to CryoLife at the commencement of Year 1 and Year 2 in accordance with forecasts that in the case of Year 1 have already
been delivered to Medafor, and that will be delivered to Medafor in conjunction with the forecasts set forth in Section 3.1 for Year 2. CryoLife may purchase additional sterile samples of boxes of 3 gram or 5 gram product at a fixed price of $125 per box over the Initial Term. If requested by Medafor, CryoLife will
make all reasonable efforts to document the no charge use of these samples. CryoLife shall use sample units only for demonstrations and marketing purposes and may not resell any samples.
2.6. Acceptance of Products. CryoLife shall have twenty (20) Business Days to inspect and to verify that the Products conform to the applicable firm order and the Specifications from the date
of arrival of such Products at the point of delivery. Unless CryoLife rejects any Product within such twenty (20) Business Day period in writing to Medafor specifying the reason for such rejection, the entire shipment shall be deemed
accepted by CryoLife. Any Product rejected in accordance with the preceding sentence shall, if requested by Medafor be either returned to Medafor or CryoLife shall provide such other evidence of the deficiency of the Products to
Medafor. CryoLife will follow Medafor’s reasonable instructions to return such Products or to otherwise dispose of them, and will not take any action in relation to such Products until it receives such instructions from
Medafor. Medafor shall have fifteen (15) Business Days from CryoLife’s notice to check, verify, test and respond to any claims by CryoLife that Products are not in conformity with the Specifications, before agreeing to replace
the defective nonconforming product. Medafor shall bear all costs of return (including freight and insurance) for defective or non conforming Product and shall
either replace the defective or nonconforming Product without charge (including payment of freight and insurance for delivery of the replacement Product) or, at CryoLife’s request, refund to CryoLife the entire amount paid in connection with
the rejected Product (including transportation and shipping charges. All such replacement product shall be shipped to CryoLife within thirty five (35) calendar days. Nothing in
this Section, including the exercise of rights hereunder, shall be construed as a waiver of CryoLife’s indemnification rights, its warranty rights or any other common law or statutory remedies. CryoLife agrees to reimburse Medafor
for any freight and insurance costs incurred by Medafor with respect to Product originally rejected by CryoLife, that Medafor determines through verification and/or testing was not defective and conformed to Specifications.
- 10 -
ARTICLE III
PURCHASING
3.1. Forecasts. On a quarterly basis and twenty (20) days before the end of each quarter, CryoLife shall provide
to Medafor twelve (12) month rolling forecasts of the anticipated quarterly quantities and mix of the Products that CryoLife expects to order (each, a “Forecast”), which shall correspond to at least the minimum purchase quantities
in Section 2.2 of this Agreement provided that such forecasts shall not be binding unless and until CryoLife delivers a purchase order to Medafor.
3.2. Firm Orders. Medafor shall supply to CryoLife the minimum quantities as described in Section 2.2 of this agreement against purchase
orders placed by CryoLife. In regard to purchase orders placed above the minimum purchase requirements as per Section 2.2, Medafor shall fulfill all firm additional orders for the
Product submitted by CryoLife provided that such orders are not more than 50% above the minimums set forth in Section 2.2. CryoLife shall place any firm purchase orders, so that they
have been received by Medafor no less than thirty-five (35) calendar days prior to the requested ship date. Firm purchase orders may be submitted via regular mail or facsimile to the following:
Medafor, Inc.
0000 Xxxxxxx Xxxx., Xxxxx 000
Xxxxxxxxxxx, XX 00000
Attn: Xxxx Xxx
Fax No.: 000-000-0000
- 11 -
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT
WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
Each firm order shall be deemed accepted upon receipt by Medafor. Except as otherwise provided herein, Medafor may not reject any purchase order received from
CryoLife unless such purchase order: (a) has not been forecasted pursuant to Section 3.1 and (b) is not consistent with the Annual Minimums to the extent the order exceeds the
forecasted amount. If Medafor rejects a purchase order due to a force majeure event or as otherwise provided herein, Medafor must give notice within two (2) Business Days of receipt of such purchase order (and before receipt of payment)
and must advise CryoLife in writing of the reason. In the event of such rejection, CryoLife’s annual minimum requirements as set forth in Section 2.2 shall be adjusted
accordingly.
3.3. Transfer Pricing.
(a) Subject to Section 3.3(b) below, the transfer pricing for the Products ordered by CryoLife during the Initial
Term shall be as set forth in Exhibit C. Medafor represents and warrants that its current pricing in the Territory to its distributors and for direct sales by Medafor, if
any, has been delivered to CryoLife and that such information delivered to CryoLife is true, correct and complete. The parties acknowledge that in the event that CryoLife is unable to obtain appropriate margins, the parties will, in good
faith, negotiate an equitable adjustment to such pricing.
(b) The transfer pricing set
forth in Exhibit C is for finished Products (i.e. packaged, labeled and sterilized). CryoLife shall be responsible for all applicable (i) sales, use, value-added or
similar taxes imposed by any Governmental Authority, and (ii) excises, duties, import fees and export fees. CryoLife shall be also responsible for all reasonable freight and delivery charges and any other costs for shipment of the Products
from Medafor’s facilities in Minneapolis, Minnesota to CryoLife’s facilities as specified in the applicable firm order. Title to products and all risk of loss shall pass from CryoLife to Medafor upon Medafor’s delivery
of Products to CryoLife’s designated shipper at Medafor’s Minneapolis, Minnesota location. Each shipment of Products from Medafor to CryoLife shall contain such quality control certificates reasonably requested by CryoLife
certifying that the Products shipped conform to the specifications.
3.4. Payment Terms. Medafor shall deliver to CryoLife an invoice
for each firm order, which invoice shall contain customary information for CryoLife to verify the invoice, including the quantity of Products delivered. Payment terms for undisputed amounts (excluding for the first and second quarters of
2008 which shall be paid immediately upon delivery of the inventory due) shall be [***] ([***]) days from when the Products are shipped in accordance with Section 3.5 or, if later, ten (10) days after date that CryoLife receives the applicable invoice. All payments hereunder will be made in United States dollars.
3.5. Shipping.
(a) Medafor shall at all times use its Commercially Reasonable Efforts to ship Products to CryoLife no later than the ship date set forth in the applicable firm order.
(b) Medafor shall package, label, store and ship all Products in compliance with Applicable Laws and in accordance with good commercial and industry
practice. CryoLife shall select the shipper. The Products shall be delivered to CryoLife sterile and ready for end-user sale and use, including all packaging, labeling, and instructions for use. Medafor shall package
the Products suitably for export and appropriately to prevent damage during shipment. The packing slip shall have the part number, purchase order number and delivery quantity.
- 12 -
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN
FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
(c) The Products shall be shipped F.O.B. Medafor facilities in Minneapolis, Minnesota. At
CryoLife’s expense, Medafor shall ship the Products to CryoLife as directed by CryoLife in the applicable firm purchase order.
ARTICLE IV
MANUFACTURING
4.1. Inventory.
(a) Medafor shall at all times
maintain a [***] ([***]) [***] supply of Products in inventory to support CryoLife’s
sales efforts based on the higher of (A) the total amount of Products ordered by CryoLife during the preceding [***] on a rolling basis, or (B) the minimum amount of Products CryoLife is required to purchase
pursuant to subsections 2.2(b) and 2.2(c) for the following [***] on a rolling basis.
(b) Medafor will notify CryoLife immediately in writing upon becoming aware of any supply shortage, or other interruption or potential interruption in the supply of any material,
component, or sub-assembly, in each case as it relates to Products.
(c) Medafor shall at all times
ensure that sufficient manufacturing capacity (including appropriate manufacturing, storage and distribution facilities and qualified personnel) is maintained to meet CryoLife’s forecasted demand plus 50%. If at any time Medafor
does not have enough component materials to fulfill, or other supply or manufacturing problems prevent Medafor from fulfilling, on a timely basis, its supply obligations to CryoLife for CryoLife’s purchase orders for Products, Medafor shall
promptly notify CryoLife of the nature and extent of the impairment to Medafor’s ability to supply and shall allocate its remaining manufacturing resources to CryoLife’s purchase orders for the Products first. Only after such
action is taken and completed shall Medafor allocate remaining manufacturing resources to other competitive products Medafor produces, so that CryoLife will not be disadvantaged in its ability to obtain Products during such impairment.
(d) In the event Medafor is
unable to fulfill CryoLife’s purchase orders for Products, CryoLife’s Annual Minimum and calendar quarter purchase requirements for the Year and subsequent Years shall be suspended until Medafor is able to fulfill CryoLife’s
purchase orders for at least one calendar quarter. Thereafter, CryoLife’s Annual Minimum and calendar quarter purchase requirements shall be reduced by a reasonable amount to account for the shortage and any resulting interruption of
CryoLife’s ability to supply its customers.
(e) If this Agreement is
terminated by CryoLife (other than for Medafor’s breach of this Agreement or pursuant to Section 10.2(c) or 10.2(e)) then CryoLife shall reimburse Medafor for the total Inventory Cost of up to [***] ([***]) [***] inventory maintained pursuant to Section 4.1(a) unless CryoLife directs that inventory be
shipped to CryoLife pursuant to Section 10.3 (in which case CryoLife shall pay the amounts for the inventory as set forth herein); provided that inventory already paid for by CryoLife
shall be deducted from this amount.
- 13 -
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN
FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
(f) If CryoLife requires any changes to any CryoLife-specific components during the Term, outside of a required change by Regulatory Authorities which renders such
CryoLife-specific components unusable for sale by CryoLife, then CryoLife shall reimburse Medafor for the Inventory Cost of the CryoLife-specific components that are rendered unusable due to such change; provided, however, that the total amount of
reimbursement under this Section 4.1(f) shall only be paid for inventory that has been kept as part of the [***] ([***]
) [***] inventory set forth in Section 4.1(a).
4.2. Manufacturing. Medafor will manufacture the Products in accordance with the then current (i) Specifications, (ii) applicable regulations relating to current Good Manufacturing
Practices (“CGMP”) and similar protocols required by the United States Food, Drug and Cosmetic Act and similar laws and regulations in foreign jurisdictions within the Territory that regulate the manufacture, distribution, and sale of
the Products for use in the Field, all as may be amended from time to time (“Other Manufacturing Laws”), quality system regulations (“QSR”) of the United States
Food and Drug Administration, including master device and lot history records, and ISO 13485 requirements (including appropriate certification) (“ISO”), MDD requirements, CMDCAS requirements, and (iii) other pertinent rules and
regulations of any Regulatory Authorities within the Territory that have approved the sale of the Products. Upon CryoLife’s request, Medafor shall provide CryoLife with written evidence of compliance with the criteria set forth
herein. During the Term, Medafor will maintain, or cause to be maintained, the Product manufacturing facility’s registration as a certified medical device manufacturing facility with all applicable Regulatory Authorities or cause
such facility to be maintained such that the facility would pass an audit for compliance with CGMP, QSR, Other Manufacturing Laws and ISO. Medafor shall maintain ongoing quality assurance and testing policies sufficient to satisfy its
obligations under this Agreement and Medafor’s standard quality assurance policies. Medafor shall maintain throughout the Term and for the specified shelf life of the Product (or for such longer period as may be required by
Applicable Laws) accurate and complete records relating to its manufacture and testing of the Products, including all records required under Applicable Laws.
4.3. Change Labeling. Medafor shall not make any changes to the Products (including materials, packaging, and directions for use), the Specifications, the raw materials, component suppliers, or
manufacturing process for the Products, which in any case could reasonably be expected to adversely affect the form, fit, function, regulatory status, or efficacy of the Products or patient safety, unless approved by CryoLife in writing in advance,
which approval may not be unreasonably denied. Without limiting the foregoing, all such changes (including changes required by law) shall be submitted to CryoLife no later than sixty (60) calendar days prior to Medafor’s proposed
date of implementation for such change. Unless CryoLife notifies Medafor in writing that it disapproves of such change during the thirty (30) calendar day period following the notification of such change or if such a proposed change is
otherwise required by law, regulation, or directive, Medafor shall be authorized to implement such change and shall be responsible for properly communicating and implementing such change, including with respect to any of Medafor’s
vendors. Without limiting the foregoing, the following changes shall be deemed governed by this Section: (i) use of any nonconforming material in the manufacture of any of the Products in variance with the Specifications; (ii)
implementation of any deviation that could affect the handling, sterility, safety, or efficacy of any of the Products and be at variance with the Specifications; or (iii) implementation of any corrective action that could affect the safety or
efficacy of the Products.
- 14 -
4.4. Product Warranty. Medafor warrants to CryoLife that the Product, when delivered in accordance with the applicable firm order, will (i) conform to the Specifications, (ii) have
been manufactured, tested, stored, packaged, labeled and shipped in compliance with Applicable Laws and in accordance with applicable Regulatory Authorities, including all Marketing Approvals and (iii) be free of defects in design, material,
engineering, fabrication and workmanship in accordance with the Specifications. The foregoing warranty shall be in effect with respect to each Product for the labeled shelf life of the Product. Medafor further warrants to CryoLife
that the Product, when delivered, shall be free and clear of any liens, security interests or encumbrances of any nature whatsoever. The above warranty is contingent upon the proper use of the Products and will not apply to Products that
have been removed from their original packaging or altered before resale by CryoLife. In addition, this warranty shall not apply to defects or failure due to: (a) the delivery of Product with items not provided by Medafor; (b) any party
other than Medafor or an authorized Medafor representative modifying the Product; or (c) storage of Products in an environment not intended or recommended by Medafor. EXCEPT AS OTHERWISE SET FORTH IN THIS AGREEMENT OR PROVIDED IN ANY
INDEMNIFICATION, EACH PARTY DISCLAIMS ALL WARRANTIES, EXPRESS OR IMPLIED INCLUDING ANY WARRANTIES AS TO MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
ARTICLE V
REGULATORY MATTERS
5.1. Compliance with Laws. Each Party shall comply in all material respects with all Applicable Laws that pertain to its activities under this Agreement and, except as otherwise provided for
herein, shall bear the entire cost and expense of such compliance.
5.2. Marketing Approvals. Medafor represents and warrants to CryoLife that it has applied for and received FDA approval and a CE marking for the Product under Medafor brand, and that such
approval and CE marking are in good standing and have never been revoked or suspended for any reason. Medafor has no reason to believe that such approval or CE marking will be revoked or suspended for any reason. In addition, Medafor represents and
warrants to CryoLife that as of March 18, 2008 it has applied for all approvals necessary in Canada, Germany, the United Kingdom and the United States to allow CryoLife to Distribute
Product under a private label and that it had received all such approvals in Germany, the United Kingdom and the United States. All costs and expenses for maintaining the FDA approval and the CE marking for the Product shall be at
Medafor’s expense. At CryoLife’s request, Medafor shall use its Commercially Reasonable Efforts to obtain and maintain additional Marketing Approvals for the Product (under the CryoLife brand discussed under Section 2.4) throughout the Territory (and Medafor understands that any delay in receiving such Marketing Approvals shall reduce the Annual Minimums in accordance with Section 2.2). Medafor shall have primary responsibility for all communications, submissions and interactions with the Regulatory Authorities for the purpose of obtaining and
maintaining Marketing Approvals. CryoLife shall provide reasonable personnel assistance to Medafor with respect to such Marketing Approvals. Medafor hereby grants to CryoLife the fully paid-up right to use any and all regulatory approvals
and clearances related to the Product owned by or licensed to Medafor and existing as of the date hereof or obtained during the Term.
- 15 -
5.3. Actions by Regulatory Authorities. Medafor shall
be responsible to Regulatory Authorities throughout the Territory as the manufacturer of the Products. If either Party receives notice of an actual or threatened inspection, investigation, inquiry, recall, import or export ban, product
seizure, enforcement proceeding or similar action by a Regulatory Authority with respect to the Product or a Party’s activities in connection with the Product, it will notify the other Party within forty-eight (48) hours after its receipt of
notice of the action and will promptly deliver to the other Party copies of all relevant documents received from the Regulatory Authority. Any notice respecting a recall or action that in any way restricts the ability of either Party to
Distribute Products shall be delivered to the other Party promptly upon receipt. The Parties shall cooperate in response to the action, including providing information and documentation as requested by the Regulatory Authority. If the
action primarily concerns CryoLife’s activities, then CryoLife shall have primary responsibility to respond to the Regulatory Authority; otherwise, Medafor shall have primary responsibility to respond. In either case, upon request
of the responding Party, the other Party shall provide consulting advice and assistance with the response. In addition, each Party shall promptly notify the other and provide to the other a copy or transcription, if available, of any
communication from any Regulatory Authority relating to the Products, the marketing thereof, or any related matter and shall keep the other Party reasonably apprised of regulatory interactions and similar activities with Regulatory Authorities in
connection with the Products. In the event of such action by a Regulatory Authority that impedes CryoLife’s ability to sell the Product, the Annual Minimums shall be adjusted equitably downward to reflect such impediment in
accordance with the impairment adjustment and “Baseball” arbitration mechanism set forth in Section 2.2.
5.4. Regulatory Audit. Medafor will permit authorized representatives of any Regulatory Authority to inspect Medafor’s plant and production facilities (and will secure the same rights
with respect to any third party plant and production facilities) relating to or used in connection with the manufacture of Products and will promptly notify CryoLife when Medafor receives notice of any such inspection. Medafor will advise
CryoLife of the findings of any regulatory inspection and will promptly take the necessary steps to correct any compliance deficiencies found by the Regulatory Authority relating to the production of Products. Medafor further agrees to
use its reasonable best efforts to provide to CryoLife such documentation or conduct such analyses as CryoLife may reasonably request in connection with any regulatory submission or audit concerning Products. CryoLife will permit
authorized representatives of any Regulatory Authority to inspect CryoLife’s facilities relating to distribution of Products and will promptly notify Medafor when CryoLife receives notice of any such inspection. CryoLife will advise
Medafor of the findings of any regulatory inspection and will promptly take the necessary steps to correct any compliance deficiencies found by the Regulatory Authority relating to CryoLife’s activities with Products.
- 16 -
5.5. Inspections.
(a) CryoLife shall have the
right to have its representatives present at the plant or plants at which the Products are manufactured during normal business hours to conduct an initial and periodic inspections of such facilities and manufacturing procedures for compliance with
CGMP, QSR, Other Manufacturing Laws and ISO, the Specifications and CryoLife’s quality assurance requirements and to inspect Medafor’s inventory of the Products, work-in-process, raw materials to be used for the Products, production
records, design history file, quality manuals, regulatory dossiers, and such other matters as may be pertinent to proper quality assurance of the Products to be delivered hereunder. CryoLife agrees to give Medafor a minimum of five
Business Days’ prior notice of any such inspection and each representative of CryoLife may be required by Medafor to sign an appropriate confidentiality agreement. Medafor shall promptly use its Commercially Reasonable Efforts to
take such action as is required to correct any deficiencies identified by CryoLife relating to the production of the Products. Medafor further agrees to use its best efforts to provide such documentation or conduct such analyses as
CryoLife may reasonably request in connection with any regulatory submission or audit.
(b) At CryoLife’s reasonable request, Medafor will perform a quality system assessment of the vendors who provide Medafor with raw components/materials, sub-assemblies or
contract services for any Product.
5.6. Product Complaints and Reports. The Parties shall each collect and record Product Complaints (and any other events required to be recorded under Applicable Laws) in accordance with
Applicable Laws and their standard procedures and policies in effect from time to time. Each Party shall provide to the other Party reports of such complaints or events within seventy-two (72) hours after receipt. Medafor shall
be responsible for investigating all Product Complaints, shall promptly respond to such complaints and shall copy CryoLife on any response made by Medafor. Medafor shall be responsible for submitting to the Regulatory Authorities all
required reports and other materials, including annual reports, distribution reports and safety reports. Each Party shall immediately notify the other Party of any information it learns concerning the safety or efficacy of the Product,
regardless of whether formal reporting to any Regulatory Authority is required.
5.7. Traceability. Medafor shall maintain manufacturing and traceability records with respect to the Products,
including records by lot number. CryoLife shall maintain distribution records for the Product per 21 C.F.R. 820.160 for the purpose of maintaining traceability to the customer to facilitate recalls or field actions as
necessary. Medafor may audit CryoLife’s distribution records for the Product upon a minimum of five (5) Business Days prior notice of such audit and each representative of Medafor may be required by CryoLife to sign an appropriate
confidentiality agreement.
5.8. Field Actions. If either Party in good faith determines that a removal, correction, recall or other Field Action involving the Product or its labeling is warranted (whether or not required
by a Regulatory Authority), such Party shall immediately notify the other Party in writing and shall advise such other Party of the reasons underlying its determination that a removal, correction, recall or other Field Action is
warranted. The Parties shall consult with each other as to any action to be taken in regard to such removal, correction, recall or other Field Action. If, after consultations, either Party in good faith believes that such a
removal, correction, recall or Field Action should be undertaken with respect the Product or its labeling, the Parties shall cooperate in carrying out the same. Medafor shall be responsible for all of CryoLife’s reasonable
out-of-pocket costs and expenses, including the cost of the Products and the replacement cost of the Products, quality control testing and notification in the event of removals, corrections, recalls or other Field Actions with respect to any Product
unless such removal, correction, recall or other Field Action was due to an act or omission by CryoLife. Medafor shall be responsible for any required reporting to Regulatory Authorities with respect to any removal, correction, recall or
other Field Action involving the Product or its labeling, provided it copies CryoLife on the same. In the event of a recall of any Products, Medafor shall promptly correct noted deficiencies relating to its manufacturing, packaging,
labeling, testing and Medafor’s storage or handling of Products, if applicable, or cause the vendor of any material, component, or sub-assembly incorporated into such Products to do likewise with respect to such material, component, or
sub-assembly and CryoLife shall correct noted deficiencies related to matters for which it is responsible.
- 17 -
5.9. Post-Market Clinical Studies. Each Party shall
inform the other Party in the event that such Party becomes aware of post-market clinical studies being conducted with the product. Each Party shall inform the other Party in the event that they become aware of published literature or
unpublished reports of data from any clinical or non-clinical laboratory studies involving the product.
ARTICLE VI
CONFIDENTIALITY
6.1. Confidentiality. In the course of their activities pursuant to this Agreement, the Parties anticipate that they may disclose Confidential Information to one another and either Party may,
from time to time, be either the disclosing Party or the recipient of Confidential Information. The Parties wish to protect such Confidential Information in accordance with this Section 6.1. The provisions of this Section shall apply to disclosures furnished to or received by a Party and its employees, agents and representatives (which may include
employees, agents and representatives of its Affiliates). Each Party shall advise its employees, agents and representatives of the requirements of this Section and shall be responsible to ensure their compliance with such
provisions.
(a) For purposes hereof,
“Confidential Information” with respect to a disclosing Party means all information, in any form or media, concerning the disclosing Party that the disclosing Party furnishes to the recipient, whether furnished before or after the
Effective Date, and all notes, analyses, compilations, studies and other materials, whether prepared by the recipient or others, that contain or reflect such information; provided, however, that Confidential Information does not include information
that (i) is or hereafter becomes generally available to the public other than as a result of a disclosure by the recipient, (ii) was already known to the recipient prior to receipt from the disclosing Party, as evidenced by prior written
documents in its possession not subject to an existing confidentiality obligation to the disclosing Party, (iii) is disclosed to the recipient on a non-confidential basis by a person who is not in default of any confidentiality obligation to
the disclosing Party or (iv) is developed by or on behalf of the recipient without reliance on confidential information received hereunder. The contents of this Agreement shall be deemed to be Confidential Information of each Party.
- 18 -
(b) The recipient of
Confidential Information shall (i) maintain its confidentiality using efforts and precautions at least as great as those it uses and takes to protect its own confidential information and trade secrets; (ii) use such Confidential
Information solely in connection with the discharge of its obligations under this Agreement and (iii) not disclose such Confidential Information to any person other than those of its agents and representatives who need to know such Confidential
Information in order to accomplish the objectives for which it was disclosed. Notwithstanding the foregoing, the recipient of Confidential Information may disclose it to the extent necessary to comply with Applicable Laws, stock exchange
rules, or with an order issued by a court or Regulatory Authority with competent jurisdiction; provided that, in connection with such disclosure, the recipient uses commercially reasonable efforts to obtain confidential treatment or an appropriate
protective order, to the extent available, with respect to such Confidential Information.
(c) Upon request of the disclosing Party, the recipient of Confidential Information shall promptly redeliver to the disclosing Party all Confidential Information provided to the
recipient in tangible form, and the recipient shall not retain any copies, extracts or other reproductions, in whole or in part, of such Confidential Information. All notes or other work product prepared by the recipient based upon or
incorporating Confidential Information of the disclosing Party shall be destroyed, and such destruction shall be certified in writing to the disclosing Party by an authorized representative of the recipient who supervised such
destruction. Notwithstanding the foregoing, in-house legal counsel to the recipient shall be permitted to retain in its files one copy of all Confidential Information to evidence the scope of the Party’s obligation of
confidentiality.
(d) The obligations under this
Section shall remain in effect from the Effective Date through the third anniversary of the expiration or termination of this Agreement.
(e) In addition to any other remedies available in law or equity, the disclosing Party shall be entitled to temporary and permanent injunctive relief in the event of a breach (or
threatened breach) under this Section.
(f) The provisions of this
Section shall supersede and replace any prior agreements between the Parties relating to Confidential Information covered hereby; provided that notwithstanding the foregoing the Parties acknowledge and agree that upon execution of this Agreement by
the Parties that certain Non-Disclosure Agreement, dated December 13, 2007 between the Parties shall be deemed terminated as of the date of execution but those terms set forth therein shall survive in accordance with their terms.
(g) Notwithstanding the
provisions of this Article VI, the parties acknowledge they may desire (or be required) to make a public announcement, issue a press release or provide similar publicity with respect to this Agreement or the transactions contemplated herein, and
each Party shall notify the other Party of its intent to make such publicity and deliver a draft of such publicity to the other Party. Neither Party shall make any public announcement, press release or similar public pronouncement with
respect to this Agreement or the transactions contemplated herein without the consent of the other Party regarding the content, time and manner of such publicity; provided that neither Party shall unreasonably withhold its consent under this section
and nothing in this Article VI shall prevent either Party from timely making any disclosure required by law or by the New York Stock Exchange.
- 19 -
ARTICLE VII
INTELLECTUAL PROPERTY RIGHTS
7.1. Intellectual Property/Information and Ideas. CryoLife acknowledges Medafor's exclusive right, title and interest in Medafor's patents and know-how related to the manufacture, regulatory
approval, or distribution of the Bellows Applicator and the MPH Product and to the trademarks, trade names, trade dress and methods of presentation relating to the Bellows Applicator and the MPH Product (including the Medafor
Xxxx). Medafor acknowledges CryoLife’s exclusive right, title and interest in the CryoLife Marks and all Intellectual Property rights therein or related thereto, including the CryoLife Applicator (the “CryoLife
IP”). Except as otherwise expressly provided herein, nothing contained herein shall be construed to convey any right, interest or title in or to the Medafor IP to CryoLife, or any right, interest or title in or to the CryoLife IP to
Medafor.
7.2. IP Representations. Medafor hereby represents and warrants to, and covenants with, CryoLife as follows:
(a) Medafor, and only Medafor,
owns or holds valid and enforceable rights to exclusively manufacture, Distribute, use or license (to the extent a license is required) any and all Intellectual Property that is necessary (i) to manufacture and sell the MPH Product, the Bellows
Applicator, and the Products or to permit others to manufacture or sell the MPH Product, the Bellows Applicator, and the Products (except for the CryoLife Applicator, if any, for which CryoLife grants Medafor a royalty free license during the Term
of this Agreement solely for the purposes of Medafor performing its obligations hereunder), (ii) CryoLife to Distribute the Product as contemplated by this Agreement and (iii) for Medafor to grant to CryoLife the Distribution rights under
this Agreement (such Intellectual Property rights collectively, the “Medafor IP”).
(b) Medafor owns or licenses all right, title and interest in and to the Medafor IP, subject only to those rights retained by HemArrest pursuant to that certain Second Amended and
Restated Exclusive License and Royalty Agreement, dated November 1, 2004, by and between HemArrest and Medafor, as amended by that certain Addendum, dated February 15, 2005, by and between HemArrest and Medafor, a copy of which has been provided to
CryoLife, as amended by that certain Agreement, dated May 10, 2006, by and among HemArrest, Medafor, Xxx X. Xxxxx, Xxxxxx X. Xxxxxxxxxx and Xxxxx X. Xxxxx.
(c) Medafor has not granted
any license, covenant not to xxx or other right that would be inconsistent with or conflict with the grant of the exclusive Distribution rights under this Agreement. Medafor also agrees that if in the event HemArrest has or granted or
does grant any license, covenant not to xxx or other right that would be inconsistent with or conflict with the grant of the exclusive Distribution rights under this Agreement then it would be considered a material breach of this Agreement by
Medafor and that CryoLife would be entitled to indemnification pursuant to Sections 7.4 and 9.1.
- 20 -
(d) No Person has asserted any
claim, suit, proceeding, action or demand (a “Claim”) with respect to any of the Medafor IP, which Claim (i) challenges the validity of Medafor’s interest in the Medafor IP, (ii) alleges that Medafor’s use or
practice of the Medafor IP infringes, misappropriates or violates the rights of any Person or (iii) seeks to enjoin or restrain Medafor’s use or practice of the Medafor IP in any manner that would interfere with the transactions
contemplated by this Agreement. Medafor has no knowledge that any Person intends to assert such a Claim. Medafor also agrees that in the event that if HemArrest has knowledge that a Person intends to assert such a Claim then it
would be considered a material breach of this Agreement by Medafor and that CryoLife would be entitled to indemnification pursuant to Sections 7.4 and 9.1.
(e) To Medafor’s
knowledge no Intellectual Property or contract rights of others will be infringed by (i) the development, manufacture, distribution or sale of the Products by Medafor or CryoLife as contemplated by this Agreement, (ii) the entering into of this
Agreement, or (iii) the performance of this Agreement by either Party. Medafor agrees however that if in the event that any Intellectual Property or contract rights of others are infringed by (i) the development, manufacture, distribution
or sale of the Products by Medafor or CryoLife as contemplated by this Agreement, (ii) the entering into of this Agreement, or (iii) the performance of this Agreement by either Party then it would be considered a material breach of this Agreement by
Medafor and that CryoLife would be entitled to indemnification pursuant to Sections 7.4 and 9.1.
(f) As of the Effective Date,
it has not granted and during the Term hereof, it will not grant any Person the right of first refusal to purchase all or substantially all of its assets, the assets constituting the Product or the MPH product.
7.3. Trademarks. CryoLife shall have the right and license to use Medafor’s Trademarks associated with the Product for Product marketing purposes and as may be necessary in order to
comply with applicable Regulatory Laws. CryoLife shall comply with the reasonable quality control instructions of Medafor as to the form and manner in which such Medafor Trademarks shall be used. Trademarks developed by
CryoLife for the Product shall be owned exclusively by CryoLife. Other than as expressly provided herein, no Party shall acquire or have any right or license to use the name or Trademarks of the other Party without its prior written
consent.
7.4. Infringement Notification. Each Party shall promptly notify the other Party of any and all infringements of the Medafor IP or of the CryoLife IP of which such Party becomes aware within
the Territory. Medafor shall, at its own cost, take any and all actions, legal or equitable, necessary to defend the Medafor IP against such infringements and to eliminate or minimize the consequences of any infringement of the Medafor IP
in the Field in the Territory. At Medafor’s request and expense, CryoLife will assist Medafor in taking action against any such infringements. In addition, and in addition to any responsibility of Medafor pursuant to Section 9.1, if any Product is held to constitute an infringement or misappropriation of any third party’s Intellectual Property right or if Medafor and CryoLife concur that any
Products constitutes an infringement or misappropriation, Medafor will at its expense either: (i) procure the right for CryoLife to continue distributing the Products in accordance with this Agreement at no additional cost to CryoLife, (ii) replace
the Product with a non-infringing and non-misappropriating equivalent product conforming to the Specifications at no additional cost to CryoLife, or (iii) modify the Product to make it non-infringing and non-misappropriating while conforming to the
Specifications at no additional cost to CryoLife. If Medafor is unable to secure sufficient rights to permit Cryolife to Distribute the Products in the manner contemplated by this Agreement, Medafor shall promptly repurchase all of
CryoLife’s Product inventory as provided in Section 10.4, and CryoLife shall be released of its obligation to Distribute the Products.
- 21 -
7.5. Patent Prosecution. At its own cost, Medafor
shall (or shall require HemArrest to) apply for, prosecute, and maintain all patents or rights to license or use the patents included in the Medafor IP in the Territory. Medafor
shall keep CryoLife reasonably informed as to the status of the prosecution and maintenance of such patents in the Territory and with respect to any actions regarding such patents in the Territory.
ARTICLE VIII
REPRESENTATIONS AND WARRANTIES
Each Party hereby represents and warrants to the other Party
that:
(a) It is a corporation duly
organized, validly existing and, if relevant in its jurisdiction of organization, in good standing under the laws of its jurisdiction of organization and that it is legally qualified to do business in each jurisdiction in which this Agreement may be
performed and where its activities hereunder require such qualification and has the power and authority to own, lease and operate its assets and to conduct the business now being conducted by it. It has all requisite power and authority
to enter into this Agreement and to perform its obligations hereunder.
(b) The execution, delivery and performance by it of this Agreement and the consummation by it of the transactions contemplated hereby have been duly authorized and approved by all
necessary corporate or equivalent action on its part. This Agreement has been duly executed and delivered by it and constitutes its legal, valid and binding obligation, enforceable against it in accordance with its terms, except as the
same may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws relating to or affecting creditors’ rights generally.
(c) The execution, delivery
and performance by it of this Agreement and the consummation by it of the transactions contemplated hereby do not and will not: (i) violate any Applicable Laws; (ii) conflict with, or result in the breach of any provision of,
its certificate of incorporation, bylaws or equivalent organizational documents; (iii) result in the creation of any lien or encumbrance of any nature upon any property being transferred or licensed by it pursuant to this Agreement; or
(iv) violate, conflict with, result in the breach or termination of, or constitute a default under (or event which, with notice, lapse of time or both, would constitute a default under), any permit, contract or agreement to which it is a party
or by which any of its properties or businesses are bound.
- 22 -
(d) No authorization, consent or approval of, or notice to or filing with, any Governmental Authority is required for the execution, delivery and performance by it of this
Agreement, other than Marketing Approvals that have not been obtained prior to the Effective Date.
(e) It is not a party to any litigation relating to, or that could reasonably be expected to affect its ability to perform its obligations under this Agreement.
ARTICLE IX
INDEMNIFICATION AND INSURANCE
9.1. Indemnification by Medafor.
(a) Medafor shall indemnify and hold harmless CryoLife and its respective shareholders, directors, officers, employees and agents from and against any and all liabilities, damages,
losses, penalties, fines, costs and expenses, including reasonable attorneys’ fees (“Losses”), paid or incurred by them in connection with any Claim based upon or arising from: (i) any use of the Products, including
Claims for personal injury, death or property damage; (ii) any allegations of infringement or violation of any Intellectual Property rights of a third party, as a result of the use, manufacture, sale or distribution of the Product (provided
that the provisions of this Section 9.1 shall be read consistent with the provisions of Section 7.4); (iii) any
breach by Medafor of any of its representations, warranties or obligations under this Agreement; (iv) any violation by Medafor of Applicable Laws; or (v) any negligent acts or omissions or wilful misconduct of Medafor or its Affiliates or
subcontractors or any of their respective employees or agents relating to the activities subject to this Agreement.
(b) CryoLife shall give Medafor prompt written notice of any Claim with respect to which Medafor’s indemnification obligations may apply, but any delay or failure of such
notice shall not excuse Medafor’s indemnification obligations except to the extent that Medafor’s legal position is prejudiced thereby. Medafor shall have the right to assume and control the defense and settlement of any such
Claim; except that CryoLife shall have the right to assume and control, at Medafor’s expense, the defense and settlement of any such Claim if: (i) CryoLife reasonably determines that there is a conflict of interest between
CryoLife and Medafor with respect to such Claim; (ii) Medafor fails to employ counsel reasonably satisfactory to CryoLife to represent CryoLife within a reasonable time after Medafor’s receipt of notice of the Claim or (iii) in the
reasonable opinion of counsel to CryoLife, the Claim could result in CryoLife becoming subject to injunctive or other non-monetary relief that could have a material adverse effect on CryoLife’s ongoing business. The Party not
controlling the defense shall have the right to participate in the Claim at its own expense, but in any event shall cooperate with the controlling Party in the investigation and defense of the Claim.
(c) If Medafor is entitled to,
and does, assume and control the defense and settlement of any Claim with respect to which its indemnification obligations apply, then Medafor shall not settle such Claim without CryoLife’s prior written consent (which consent shall not be
unreasonably withheld or delayed), unless (i) the sole relief provided in such settlement is monetary in nature and shall be paid in full by Medafor and (ii) such settlement does not include any finding or admission of a violation by
CryoLife of any Applicable Laws or Third Party’s rights. Whenever CryoLife assumes and controls the defense and settlement of a Claim with respect to which Medafor’s indemnification obligations applies, Medafor shall not
be liable for any settlement thereof effected by CryoLife unless CryoLife shall have obtained Medafor’s prior written consent to the proposed settlement (which consent shall not be unreasonably withheld or delayed).
- 23 -
9.2. Indemnification by CryoLife.
(a) CryoLife shall indemnify and hold harmless Medafor and its Affiliates and their respective shareholders, directors, officers, employees and agents from and against any and all
Losses, paid or incurred by them in connection with any Claim based upon or arising from: (i) any breach by CryoLife of any of its representations, warranties or obligations under this Agreement; (ii) any violation by CryoLife
of Applicable Laws or (iii) any negligent acts or omissions or wilful misconduct by CryoLife or its Affiliates or any of their respective employees or agents relating to the activities subject to this Agreement.
(b) Medafor shall give
CryoLife prompt written notice of any Claim with respect to which CryoLife’s indemnification obligations may apply, but any delay or failure of such notice shall not excuse CryoLife’s indemnification obligations except to the extent that
CryoLife’s legal position is prejudiced thereby. CryoLife shall have the right to assume and control the defense and settlement of any such Claim; except that Medafor shall have the right to assume and control, at CryoLife’s
expense, the defense and settlement of any such Claim if: (i) Medafor reasonably determines that there is a conflict of interest between CryoLife and Medafor with respect to such Claim; (ii) CryoLife fails to employ counsel
reasonably satisfactory to Medafor to represent Medafor within a reasonable time after CryoLife’s receipt of notice of the Claim or (iii) in the reasonable opinion of counsel to Medafor, the Claim could result in Medafor becoming subject
to injunctive or other non-monetary relief that could have a material adverse effect on Medafor’s ongoing business. The Party not controlling the defense shall have the right to participate in the Claim at its own expense, but in
any event shall cooperate with the controlling Party in the investigation and defense of the Claim.
(c) If CryoLife is entitled to, and does, assume and control the defense and settlement of any Claim with respect to which its indemnification obligations apply, then CryoLife
shall not settle such Claim without Medafor’s prior written consent (which consent shall not be unreasonably withheld or delayed), unless (i) the sole relief provided in such settlement is monetary in nature and shall be paid in full by
CryoLife and (ii) such settlement does not include any finding or admission of a violation by Medafor of any Applicable Laws or Third Party’s rights. Whenever Medafor assumes and controls the defense and settlement of a
Claim with respect to which CryoLife’s indemnification obligations applies, CryoLife shall not be liable for any settlement thereof effected by Medafor unless Medafor shall have obtained CryoLife’s prior written consent to the proposed
settlement (which consent shall not be unreasonably withheld or delayed).
9.3. Combined Obligations. To the extent that CryoLife and Medafor have indemnification obligations to one another
in connection with a single Claim, CryoLife and Medafor shall contribute to the aggregate damages arising from such Claim in such proportion as is appropriate to reflect their relative responsibilities for such damages, as well as any other relevant
equitable considerations. The amount paid or payable by CryoLife or Medafor for purposes of apportioning the aggregate damages shall be deemed to include all reasonable legal fees and expenses incurred by such Party in connection with
investigating, preparing for or defending against such Claim. Such finding of contribution shall be as agreed to in writing by the parties, or as determined by a judicial determination, in final, non-appealable form.
- 24 -
9.4. Limitation of Liability. Except as otherwise specifically stated herein, Medafor shall not be liable for any loss or damages claimed to have resulted from the use, operation, or
performance of the Products, regardless of the form of action; and the sole and exclusive remedies for breach of any or all warranties or representations and for Medafor’s liability of any kind are limited to repair or replacement of the
Product or actual damages for personal injury or to tangible personal property caused by the sole negligence of Medafor. EXCEPT TO THE EXTENT PROVIDED IN THIS ARTICLE IX, NEITHER PARTY
SHALL BE LIABLE TO THE OTHER PARTY FOR, AND EACH PARTY HEREBY WAIVES, SPECIAL, EXEMPLARY, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES OR ANY DAMAGE RESULTING FROM LOSS OF USE OR PROFITS.
9.5. Insurance Provisions.
(a) During the Term of
this Agreement and for a period of seven years thereafter, Medafor shall procure and maintain, from a reputable insurer reasonably satisfactory to CryoLife, insurance in the amount of two million U.S. Dollars ($2,000,000) per occurrence or five
million U.S. Dollars ($5,000,000) in the aggregate, including product liability insurance, which is consistent with normal business practices of prudent companies similarly situated. Such insurance policy shall at all times name CryoLife
as an additional insured thereunder and Medafor shall provide a certificate of insurance to CryoLife evidencing such coverage. It is understood that such insurance shall not be construed to create a limit of Medafor’s liability with
respect to its indemnification obligations under this Agreement. Medafor shall provide CryoLife with written evidence of such insurance upon request. Medafor shall provide CryoLife with written notice at least 15 calendar days
prior to the cancellation, non-renewal, or material change in such insurance.
(b) During the Term of this Agreement and for a period of seven years thereafter, CryoLife shall procure and maintain, from a reputable insurer reasonably satisfactory to Medafor,
insurance in the amount of two million U.S. Dollars ($2,000,000) per occurrence or five million U.S. Dollars ($5,000,000) in the aggregate, including product liability insurance, which is consistent with normal business practices of prudent
companies similarly situated. Such insurance policy shall at all times name Medafor as an additional insured thereunder and CryoLife shall provide a certificate of insurance to Medafor evidencing such coverage. It is understood
that such insurance shall not be construed to create a limit of CryoLife’s liability with respect to its indemnification obligations under this Agreement. CryoLife shall provide Medafor with written evidence of such insurance upon
request. CryoLife shall provide Medafor with written notice at least 15 calendar days prior to the cancellation, non-renewal, or material change in such insurance.
- 25 -
ARTICLE X
TERM AND TERMINATION
10.1. Term.
(a) This Agreement shall take effect as of the Effective Date and shall remain in effect until June 30, 2011 (the “Initial Term”). At CryoLife’s option
(“Right of Renewal”), this Agreement may be renewed for an additional thirty-six (36) months period if CryoLife meets the Annual Minimums during the Initial Term. The Initial Term plus any renewal terms, if any, is referred to
herein as the “Term.”
(b) CryoLife may exercise its
Right of Renewal by giving Medafor written notice of its intent to renew at least sixty (60) days prior to the expiration of the Initial Term. If CryoLife does not exercise the Right of Renewal or does not qualify for the Right of
Renewal, then, within the last three (3) months of the Initial Term, the Parties shall discuss options for concluding a new agreement to take effect upon the expiration of this Agreement. Nothing in the preceding sentence shall require
either Party to enter into or be bound by a new agreement or shall be construed to require a minimum length of time for such discussions.
(c) If this Agreement is not renewed as provided above or after the expiration of the Term herein, CryoLife shall be free to continue to sell Products it has purchased from Medafor
unless and until Medafor repurchases such Products from CryoLife.
10.2. Termination. This Agreement may be terminated prior to the expiration of the Term as follows:
(a) By either Party for reason
of the other Party’s material breach of a duty or obligation under this Agreement by giving the other Party not less than thirty (30) days prior written notice of termination to the other Party which specifies such default and such other Party
fails to cure the material default during such thirty (30) day period; provided that CryoLife’s failure to make payments due for invoices set forth hereunder shall only be subject to a ten (10 day cure period; or
(b) By either Party forthwith
on written notice of termination to the other Party for the other Party's voluntary or involuntary petition of bankruptcy, or insolvency, or winding up of its operations; or in the event of nationalization, in whole or part, of the other Party; or
(c) By CryoLife upon thirty
(30) days written notice of termination to Medafor after Medafor fails on any two occasions within any twelve (12) month period to timely deliver Product, or material quantities of Products, that conform to the Specifications and for which Medafor
fails to timely deliver replacement Products that meet Specifications, ordered by CryoLife in accordance with the provisions of this Agreement (a “Failure to Supply”); or
(d) By
Medafor upon thirty (30) days written notice of termination to CryoLife if CryoLife fails to meet at least 80% of the Annual Minimum of any Year (except for Year 1), provided that in determining whether or not CryoLife has met the purchase
requirement for a particular period, any Products ordered by CryoLife in excess of the amount required for that period may be carried backward or forward to supplement the purchases of the preceding or following period, and any Products ordered by
CryoLife in the previous Year in excess of the Annual Minimum for such previous Year may be carried forward to supplement the inadequate inventory purchases of any period of the following Year; and provided further, that within thirty (30) days of
CryoLife’s receipt of Medafor’s written notice of termination, CryoLife does not submit purchase orders to make up for the shortfall during the thirty (30) day period;
- 26 -
(e) By CryoLife upon notice in
the event of any Product supply interruption pursuant to Sections 4.1(c) or (d) or any recall of Products provided
CryoLife delivers thirty (30) days prior written notice of termination to Medafor of such Product supply interruption or recall and Medafor fails to cure the default during such thirty (30) day period; or
(f) In the event CryoLife
fails to meet the minimum purchase requirements set forth in Section 2.2 (as modified by Section 10.2(d)),
Medafor’s sole and exclusive remedy is shall be to terminate the Agreement as provided subsubsection (d) above. CryoLife shall not be liable for any monetary damages that Medafor may incur as a result of any such
failure.
10.3. Effect of Termination. Notwithstanding anything to the contrary contained herein, upon termination or expiration of this Agreement other than due to an uncured breach of this Agreement by
CryoLife (outside of a failure to meet the minimum purchase requirements set forth in Section 2.2 as modified by Section
10.2(d)), (i) Medafor shall continue to fill all CryoLife purchase orders made in accordance with the provisions of this Agreement prior to the date of the initial notice of such termination or expiration; (ii) CryoLife shall continue to have
all rights necessary or appropriate to sell Products (including Products delivered pursuant to post-termination orders and any Products ordered by CryoLife prior to termination or expiration) for six (6) months following the date of termination or
expiration, and Medafor shall continue to comply with all of its duties and obligations hereunder necessary or appropriate to facilitate such sales by CryoLife; (iii) Medafor shall continue to comply with all of its duties and obligations hereunder
necessary or appropriate to permit CryoLife to fulfill its obligations to deliver Products pursuant to tenders or sales contracts outstanding at the time of such termination or expiration until such tenders or sales contracts have expired, including
Medafor’s obligation to fill any related CryoLife purchase orders, (iv) CryoLife shall continue to comply with its obligations under this Agreement; and (v) CryoLife shall make payments of all undisputed portions of invoices within ten (10)
days of receipt of such invoice (provided that if CryoLife has not paid all other undisputed invoices, payment shall be made COD for such instruments). Termination of this Agreement shall not affect rights and obligations of either Party
that may have accrued prior to the effective date of termination or any obligation that by its nature or express terms survives termination. Sections 1.1, 1.2, 2.1(c), 2.1(d), 2.1(e), 2.3, 2.4, 2.6, 3.2, 3.3, 3.4, 3.5, 4.1(e), 4.2, 4.3, 4.4 and Articles V-Article XI shall survive the
termination or expiration of this Agreement.
10.4. Inventory Repurchases. Upon termination or expiration of this Agreement for any reason other than for CryoLife’s material breach of its obligations hereunder, Medafor shall, at
CryoLife’s option, repurchase from CryoLife all Products that are commercially usable at the Transfer Price paid by CryoLife for such Products, and CryoLife shall return to Medafor any advertising or sales materials previously provided by
Medafor, if any. Within thirty (30) calendar days after any such termination of this Agreement, CryoLife shall provide Medafor an inventory of Products in its possession, including samples of Products, and information relating to the transfer prices
CryoLife paid for such Products. If Medafor disputes any information provided by CryoLife, it shall deliver a written notice thereof to CryoLife within fifteen (15) calendar days after receiving such information, in which case, the
Parties shall negotiate in good faith to resolve such dispute; provided that in the event that they fail to resolve the dispute within thirty (30) days after Medafor’s delivery of such notice of dispute, either party may take any judicial
action it deems necessary or appropriate. CryoLife shall deliver to Medafor all remaining Products, including samples, promptly after the expiration of the fifteen (15) calendar day period if no dispute is raised by Medafor, or after the
resolution of such dispute.
- 27 -
ARTICLE XI
MISCELLANEOUS
11.1. Agency. Neither Party is, nor shall be deemed to be, an employee, agent, partner or legal representative of the other Party for any purpose. Neither Party shall have the right,
power or authority to enter into any contracts in the name of, or on behalf of, the other Party, nor shall either Party have the right, power or authority to pledge the credit of the other Party in any way or hold itself out as having the authority
to do so.
11.2. Force Majeure. If the performance of any obligation under this Agreement is prevented, restricted or interfered with by reason of war, revolution, civil commotion, acts of terrorism,
blockade, embargo, material changes in Applicable Laws, strikes or similar event which is beyond the reasonable control of the Party affected and could not have been avoided with the exercise of due care, then the Party so affected shall, upon
giving prior written notice to the other Party, be excused from such performance to the extent of such prevention, restriction, or interference, provided that the Party so affected shall notify the other Party within ten (10) days of such
occurrence, shall use its Commercially Reasonable Efforts to avoid or remove such causes of nonperformance, and shall continue performance hereunder with reasonable dispatch whenever such causes are removed. If such conditions inhibiting
complete performance shall continue in excess of sixty (60) days, then the Party that is not affected by the force majeure event shall have the option, by delivery of written notice of termination to the affected Party, to terminate this
Agreement.
11.3. Binding Assignment. This Agreement shall be binding on CryoLife, Medafor, and their respective successors and assigns. Neither Party may assign its obligations under this Agreement or in
any way transfer its rights or obligations under this Agreement, directly or indirectly, without the prior written consent of the other Party, which consent shall not be unreasonably withheld.
11.4. Set Off. The existence of any Claim by a Party against the other Party or any of its affiliates, whether predicated upon this Agreement or otherwise, shall not entitle the claiming Party
to withhold or set-off any payments due to the other Party under this Agreement. If any governmental agency prevents performance of any duty of either Party under this Agreement, either Party may immediately suspend this Agreement and
each Party will be excused from further performance of its obligations under this Agreement unless and until governmental approval is obtained
- 28 -
11.5. Entire Agreement; Amendments. Except for that
certain Non-Disclosure Agreement referenced herein, this Agreement constitutes the entire agreement between the Parties hereto concerning its subject matter and supersedes all previous negotiations, agreements and commitments with respect
thereto. This Agreement shall not be released, discharged, amended or modified in any manner except by a written instrument signed by duly authorized officers of each of the Parties hereto.
11.6. Partial Illegality. If any provision of this Agreement, or the application thereof to any Party or circumstances, shall be declared void, illegal or unenforceable, the remainder of this
Agreement shall be valid and enforceable to the extent permitted by Applicable Laws. In such event, the Parties shall use their best efforts to replace the invalid or unenforceable provision by a provision that, to the extent permitted by
Applicable Laws, achieves the purposes intended under the invalid or unenforceable provision. Any deviation by either Party from the terms and provisions of this Agreement in order to comply with Applicable Laws shall not be considered a
breach of this Agreement.
11.7. Headings and Captions. Headings and captions used herein are for convenience only and shall not be deemed part of the Agreement.
11.8. Waiver of Compliance. No provision of this Agreement shall be waived by any act, omission or knowledge of a Party or its agents or employees, except by an instrument in writing expressly
waiving such provision and signed by a duly authorized officer of the waiving Party, which waiver shall be effective only with respect to the specific obligation and instance described therein. A failure to exercise or a delay in exercising a right
or remedy provided by this Agreement or by Applicable Law shall not constitute a wavier of that right or remedy.
11.9. Notices. All notices and other communications in connection with this Agreement, other than firm purchase
orders which are governed by Section 3.2, shall be in writing and shall be sent to the respective Parties at the following addresses, or to such other addresses as may be
designated by the Parties in writing from time to time in accordance with this Section, by registered or certified mail, postage prepaid, or by express courier service or service fee prepaid:
To Medafor:
Medafor, Inc.
0000 Xxxxxxx Xxxx., Xxxxx 000
Xxxxxxxxxxx, XX 00000
Attn: Xxxx X. Xxxxx, CEO
To CryoLife:
CryoLife , Inc.
0000 Xxxxxxx Xxxx XX
Xxxxxxxx, XX 00000
Attn: General Counsel
- 29 -
All notices shall be deemed given and received (i) if delivered by hand, immediately, (ii) if sent by mail, three (3) Business Days after
posting, or (iii) if delivered by express courier service, the next Business Day in the jurisdiction of the recipient.
11.10. Counterparts. This Agreement may be executed in multiple counterparts, each of which shall be deemed to be an
original and all of which together shall be deemed to be one and the same instrument.
11.11. Further Actions. Each Party agrees, subsequent to the execution and delivery of this Agreement and without
any additional consideration, to execute, acknowledge and deliver such further documents and instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
11.12. Jointly Prepared. This Agreement has been prepared jointly and shall not be strictly construed against either
Party.
11.13. Third Party Rights. Except as otherwise expressly provided herein, this Agreement is not intended to confer
any benefits upon, or create any rights in favor of any Person other than the Parties.
11.14. Expenses. Except as otherwise expressly provided in this Agreement, each Party shall be responsible for its
own expenses incurred in connection with this Agreement and the transactions contemplated hereby.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK;
SIGNATURES APPEAR ON FOLLOWING PAGE]
- 30 -
| MEDAFOR, INC. |
|||
| |
By:
|
/s/ Xxxx X. Xxxxx | |
| Name: | Xxxx X. Xxxxx | ||
| Title: | Chief Executive Officer | ||
| |
By:
|
/s/ Xxxxx Xxxxxx Xxx | |
| Name: | Xxxxx Xxxxxx Xxx | ||
| Title: | Executive Vice President, Chief Operating Officer and Chief Financial Officer | ||
- 31 -
EXECUTION VERSION
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT
WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
EXHIBIT A
Specifications
Product Packaging/Labeling:
Ø The Products will be individually contained within moisture impermeable foil pouches that are compatible with gamma irradiation sterilization. Foil product pouches
along with product IFU(s) will be inserted into pre-printed dispensing boxes. Some product dispensing box may be individually shrink-wrapped prior to shipment for added environmental and shipping protection. All Products shall be boxed in
containers as agreed to as of the date hereof by the Parties.
Ø Each Product foil pouch label, whether pre-printed onto or adhered to the pouch, will include and contain any and
all applicable elements agreed to by the Parties as of the date hereof (including and required by Regulatory Authorities).
Ø All materials,
including foil pouches, ink, labels, and dispensing box, will be smudge resistant and compatible with manufacturing, sterilization and shipping activities.
Ø The Products and associated packaging/labeling materials will be subjected to the Method VDmax of radiation sterilization as described in AAMI TIR27, but will meet at least a minimum sterility assurance level of 10-6. The Products and all materials used in the Products shall be shipped and stored at room temperature
In addition to the
foregoing:
1) All Products shall meet the attached “[***]
Specifications” attached hereto; and
2) The Bellows Applicator specifications shall be composed of two components as set forth on the attached
documents labeled “LAMEPLAST CAP/TIP” and “Lamepast 9gm Bellows”.
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT
WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
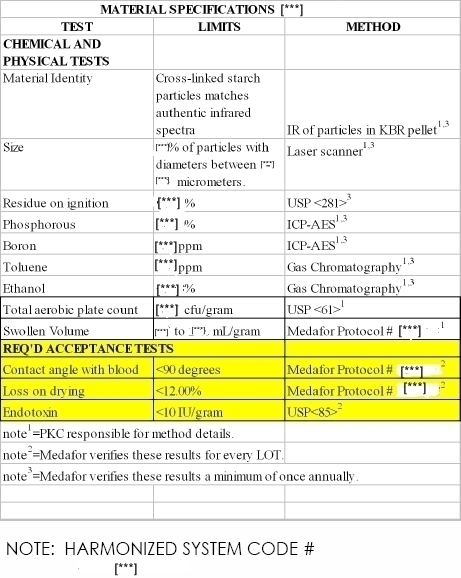 |
 [DIAGRAM OF THE HEMOSTASE MPH MATERIAL]  |
 |
 |
[***] – CONFIDENTIAL PORTIONS
OF THIS AGREEMENT WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
 | |
|
OD MAX
[***]
ID MIN [***]
ID MAX
[***]
OD MIN [***] [***]
TOTAL SLOPE
[***] [DIAGRAM OF THE LAMEPLAST CAP] [***] |
[DIAGRAM OF THE LAMEPLAST CAP] |
 |
 |
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN
FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
 | |
| [***] [***] [***]
[***] [DIAGRAM OF THE LAMEPLAST 9 XX XXXXXXX] [***] |
[DIAGRAM OF THE LAMEPLAST 9 XX XXXXXXX]
|
 |
 |
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT
WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
EXHIBIT B
Initial Exclusions from the Territory
Excluded United States Hospitals
The following United States hospitals shall not be deemed part of the Territory until the earlier of the date set forth across from such hospital, or the
termination of the distributor by Medafor that is distributing to such hospital:
| Distributor |
Name of Hospital |
CryoLife Start Date |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
October 1, 2008 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
- 2 -
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
| [***] |
[***] |
January 1, 2009 |
- 3 -
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT
WHICH HAVE BEEN REDACTED ARE MARKED WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
Exhibit C
United States
Transfer Prices:
| Year |
Transfer Price 3-gram |
Transfer Price for 5-gram |
| Year 1 (May 1, 2008 – June 30, 2009) |
$[***] |
$[***] |
| Year 2 (July 1, 2009 – June 30, 2010) |
$[***] |
$[***] |
| Year 3 (July 1, 2010 – June 30, 2011) |
$[***] |
$[***] |
| Year 4 (July 1, 2011 – June 30, 2012) |
$[***] |
$[***] |
Direct Sales Outside of United States (U.K. and Germany)
Transfer Prices:
| Year |
Transfer Price 3-gram |
Transfer Price for 5-gram |
| Year 1 (May 1, 2008 – June 30, 2009) |
$[***] |
$[***] |
| Year 2 (July 1, 2009 – June 30, 2010) |
$[***] |
$[***] |
| Year 3 (July 1, 2010 – June 30, 2011) |
$[***] |
$[***] |
| Year 4 (July 1, 2011 – June 30, 2012) |
$[***] |
$[***] |
Third Party Sales Outside of United States (Canada, EU
(excluding U.K. and Germany) and ROW)
Transfer Prices:
| Year |
Transfer Price 3-gram |
Transfer Price for 5-gram |
| Year 1 (May 1, 2008 – June 30, 2009) |
$[***] |
$[***] |
| Year 2 (July 1, 2009 – June 30, 2010) |
$[***] |
$[***] |
| Year 3 (July 1, 2010 – June 30, 2011) |
$[***] |
$[***] |
| Year 4 (July 1, 2011 – June 30, 2012) |
$[***] |
$[***] |
All transfer prices in Years 5 and 6 shall be adjusted as
follows:
The price shall increase, if at all based on the lesser of the following, (a) [***]% or (b) the increase in Federal
Bureau of Labor Statistics, Consumer Price Index (CPI) for all Urban Consumers (or its successor if one is substituted in the future) from the month of April of the year prior to the current year in which the adjustment must be made until the month
of March of the current year
- 4 -
SCHEDULE 2.1
[***] – CONFIDENTIAL PORTIONS OF THIS AGREEMENT
WHICH HAVE BEEN REDACTED ARE MARKED
WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED
SEPARATELY WITH UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
WITH BRACKETS (“[***]”). THE OMITTED MATERIAL HAS BEEN FILED
SEPARATELY WITH UNITED STATES SECURITIES AND EXCHANGE COMMISSION.
Current Distribution Agreements
(those United States distributors noted with a * will continue after the Effective Date in accordance with Section 2.1)
*[[***] – Pennsylvania]
*[[***] – Florida]
*[[***] – Ohio]
- 5 -
Schedule 2.2
“Baseball” Arbitration
In the event that
one Party delivers the other Party an Annual Dispute Notice in accordance with Section 2.2 the parties hereby agree that the dispute over the amount of the reduction for the Annual
Minimum reduction shall be settled by “Baseball” arbitration as follows:
| (a) |
There shall be a single arbitrator selected in accordance with the then current
rules of the American Arbitration Association (such arbitrator, the “Arbitrator”) |
| (b) |
Arbitration shall be held in Chicago, Illinois |
| (c) |
Once the Arbitrator is selected, the Arbitrator shall direct the Parties to attend
a hearing, and this hearing shall take place within twenty (20) calendar days of the appointment of the Arbitrator. |
| (d) |
Each Party shall have a maximum of one (1) hour in which to make oral submissions
to the Arbitrator at the hearing and a maximum of three (3) 8 inch by 11 inch pages, single sided in which to make written submissions to the Arbitrator. |
| (e) |
Each Party shall submit to the arbitrator and exchange with each other in advance
of the hearing, their last, best offer stating the name of the Party submitting such offer and the sum that constitutes their reduced Annual Minimum number. Such offer shall be delivered a minimum of forty-eight (48) hours before such
hearing. |
| (f) |
The Arbitrator shall have five (5) Business Days from the date of the hearing to
make a final and binding award, and the Arbitrator shall, in the making of such award be limited to awarding only one or the other of the two figures submitted in accordance with (e) above. |
| (g) |
Each Party shall bear its own costs and expenses for this “Baseball”
arbitration and the costs of this “Baseball” arbitration shall be allocated between the parties. No attorneys’ fees may be awarded. |
.
- 6 -
