EX-10.10 11 d600976dex1010.htm EX-10.10
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Exhibit 10.10
Execution Version
This License Agreement (this “Agreement”) is made effective as of October 22, 2013 (the “Effective Date”).
|
BETWEEN: | FB HEALTH S.p.A., a company existing under the laws of Italy | |
| (hereinafter referred to as the “Licensor”) | ||
| AND: | ALZHEON, INC., a company existing under the laws of the state of Delaware | |
| (hereinafter referred to as “Alzheon”) | ||
| (the Licensor and Alzheon are each hereafter referred to individually as a “Party” and together as the “Parties”). |
WHEREAS, the Licensor is the owner of, or otherwise Controls (including pursuant to that certain License Agreement by and between the Licensor and BHI Limited Partnership (“Xxxxxx”), of even date herewith (the “Xxxxxx License Agreement”)), certain patents, patent applications, technology, know-how and scientific and technical information relating to the Licensed Products (as such term is defined below);
WHEREAS, Alzheon desires to obtain the exclusive right from the Licensor to Develop, make, modify, enhance, improve and use such patents, patent applications, technology, know-how and scientific and technical information for the Field, and manufacture, market, sell, offer for sale, import, distribute and use the Licensed Products in the Territory in the Field, and to obtain certain other rights of the Licensor under the Xxxxxx License Agreement;
WHEREAS, the Licensor and Alzheon are parties to that certain Letter Agreement with Xxxxxx, of even date herewith, (the “Letter Agreement”), which, among other things, clarifies the rights and obligations of the Licensor, Xxxxxx and Alzheon with respect to this Agreement and the Xxxxxx License Agreement; and
WHEREAS, the Licensor desires to grant such rights to Alzheon on the terms and conditions of this Agreement.
NOW, THEREFORE, in consideration of the mutual covenants contained herein, and for other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the Parties hereby agree as follows:
1.
| 1. | DEFINITIONS |
Whenever used in this Agreement with an initial capital letter, the terms defined in this Article 1 shall have the meanings specified.
“Action” shall have the meaning for such term set forth in Section 7.6.
“Affiliate” shall mean, with respect to a Party or a Third Party, any corporation, firm, limited liability company, partnership or other entity which directly controls or is controlled by or is under common control with such Party or Third Party. “Control”, for purposes of this definition only, means ownership, directly or indirectly through one or more Affiliates, of fifty percent (50%) or more of the shares entitled to vote for the election of directors, in the case of a corporation, or fifty percent (50%) or more of the equity interests in the case of any other type of legal entity, status as a general partner in any partnership, or any other arrangement whereby such Party or Third Party controls or has the right to control the Board of Directors or equivalent governing body of a corporation or other entity.
“Alzheon” shall have the meaning for such term set forth in the Recitals.
“Alzheon Indemnitees” shall have the meaning for such term set forth in Section 10.2.
“Auditing Party” shall have the meaning for such term set forth in Section 4.5.2.
“Bankruptcy Code” shall mean Title 11 of the United States Code, as amended.
“Xxxxxx” shall have the meaning for such term set forth in the Recitals.
“Xxxxxx License Agreement” shall have the meaning for such term set forth in the Recitals.
“BLU8499 Patent Rights” shall have the meaning for such term set forth in Section 7.1.1.
“Celtic Agreement” shall have the meaning for such term set forth in Section 8.1.18.
“Change of Control” means, with respect to a Party, (a) a bona fide merger, consolidation, business combination, recapitalization, liquidation, dissolution or similar transaction or series of related transactions involving the Party and a Third Party pursuant to which the stockholders of the Party immediately preceding such transaction or transactions hold less than a majority of the equity interests in the surviving or resulting entity of such transaction or transactions; (b) any Third Party “person” (as such term is used in Sections 13(d) and 14(d)(2) of the Securities Exchange Act of 1934, as amended) becomes a “beneficial owner” (as such term is defined in Rule 13d-3 promulgated under such Act) (other than the applicable Party), directly or indirectly, of securities of such Party (or the surviving company of such merger, consolidation or other such transaction or transactions, as applicable) representing fifty percent (50%) or more of the combined voting power of such Party’s (or such surviving company’s) then outstanding securities; or (c) a sale or other disposition in one or a series of related transactions by a Party of all or substantially all of such Party’s assets.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
2.
“Commercially Reasonable Efforts” shall mean, with respect to the Development and commercialization of Licensed Products by a Party, that level of efforts and resources which [ * ]. Commercially Reasonable Efforts shall be determined on a market-by-market basis for each Licensed Product.
“Confidential Information” shall mean, with respect to a Party (the “Receiving Party”), all information, which is disclosed by the other Party (the “Disclosing Party”) to the Receiving Party hereunder or to any of its employees, consultants, legal or financial advisors, Affiliates, licensees or sublicensees (“Representatives”), except to the extent that such information, (a) as of the date of disclosure is demonstrably known to the Receiving Party or its Representatives, as shown by written documentation, other than by virtue of a prior confidential disclosure to such Party or its Representatives by the Disclosing Party or its predecessors; (b) as of the date of disclosure is in, or subsequently enters, the public domain, through no fault or omission of the Receiving Party or its predecessors; (c) is obtained by the Receiving Party or its Representatives from a Third Party having a lawful right to make such disclosure free from any obligation of confidentiality or fiduciary duty to the Disclosing Party or its predecessors; or (d) is independently developed by or for the Receiving Party or its Representatives without reference to or reliance upon any Confidential Information of the Disclosing Party or its predecessors as demonstrated by competent written records.
“Control” or “Controlled” shall mean, with respect to any Patent Rights or Know-How, the possession by a Party of the ability to grant a license or sublicense of such Patent Rights or Know-How as provided for herein without violating the terms of any arrangement or agreements between such Party and any Third Party, unless otherwise provided for in this Agreement.
“Development” and “Develop” shall mean, with respect to any Licensed Product, all activities relating to research and development including without limitation, all pre-clinical and non-clinical research and development activities (including formulation and reformulation activities), all human and veterinary clinical studies, all activities relating to developing the ability to manufacture any Licensed Product or any component thereof (including, without limitation, process development work), and all other activities relating to seeking, obtaining and/or maintaining any Regulatory Approvals from any Regulatory Authority, including the publishing of certain data and other results as may be necessary in connection with the foregoing.
“Dispute” shall have the meaning for such term set forth in Section 12.1.
“Effective Date” shall have the meaning for such term set forth in the Recitals.
“EMA” shall mean the European Medicines Agency or any successor agency thereto.
“Expert” shall have the meaning for such term set forth in Section 12.5.1.
“Field” shall mean [ * ]. For avoidance of doubt, the Field specifically excludes [ * ].
“First Commercial Sale” shall mean, on a country-by-country and Licensed Product-by-Licensed Product basis, the first Sale of a Licensed Product by Alzheon or any of its Affiliates or permitted sublicensees to a Third Party in a country for use in the Field after such Licensed
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
3.
Product has been granted Regulatory Approval in such country. For the sake of clarity, transfers or dispositions of Licensed Product for charitable, promotional (including samples), pre-clinical, clinical or regulatory purposes shall not constitute “Sales” for purposes of this definition.
“GAAP” shall mean the International Financial Reporting Standards and the official pronouncements issued by the International Accounting Standards Board.
“ICC Rules” shall have the meaning for such term set forth in Section 12.4.2.
“Improvements” shall have the meaning for such term set forth in Section 3.1.3.
“Indemnitee” shall mean the Alzheon Indemnitee or the Licensor Indemnitee.
“Indemnitor” shall have the meaning for such term set forth in Section 10.3.
“Infringement” shall have the meaning for such term set forth in Section 7.5.
“Insolvency Event” shall have the meaning for such term set forth in Section 11.4.
“Inventory Sale Period” shall have the meaning for such term set forth in Section 11.6.3.
“Italy Commercialization Agreement” shall have the meaning for such term set forth in Section 3.2.2(a).
“Italy Net Sales” shall have the meaning for such term set forth in Section 4.2.
“Know-How” shall mean and include any and all unpatented (whether or not patentable) proprietary ideas, inventions, discoveries, Confidential Information, biologic materials, data, results, formulae, designs, specifications, scientific methods, business plans and methods, processes, formulations, techniques, know-how, technical information (including, without limitation, structural and functional information), process information, pre-clinical information, clinical information, and any and all proprietary biological, chemical, pharmacological, toxicological, pre-clinical, clinical, assay, control and manufacturing data and materials.
“Laws” shall mean all provisions of all (a) constitutions, treaties, laws, statutes, codes, ordinances, orders, decrees, rules, regulations and municipal by-laws, whether domestic, foreign or international, (b) judgments, orders, writs, injunctions, decisions, rulings, decrees and words of any governmental or regulatory body or authority, and (c) all policies, voluntary restraints, practices and guidelines of any Regulatory Authority, in each case binding on or affecting the Party referred to in the context in which such word is used.
“Letter Agreement” shall have the meaning for such term set forth in the Recitals.
“Licensed Data” shall mean any and all in vitro, in vivo, pre-clinical and clinical data related to the Licensed Products that is Controlled by the Licensor on the Effective Date or during the Term, including all such data that is Controlled by the Licensor pursuant to the Xxxxxx License Agreement.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
4.
“Licensed Know-How” shall mean all Know-How Controlled by the Licensor on the Effective Date or during the Term that is necessary or useful for the Development, manufacture or commercialization of the Licensed Products, including the Licensed Data, any Know-How that is solely and exclusively related to the Licensed Patent Rights and all Know-How that is Controlled by the Licensor pursuant to the Xxxxxx License Agreement.
“Licensed Patent Rights” shall mean (i) all Patents Rights Controlled by the Licensor on the Effective Date or during the Term that are necessary for the Development, manufacture or commercialization of the Licensed Products, including all Patent Rights with respect to the patents and patent applications listed in Schedule A and Schedule B hereto and all Patent Rights Controlled by the Licensor pursuant to the Xxxxxx License Agreement and (ii) all Patent Rights in the Improvements Controlled by the Licensor during the Term.
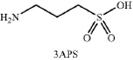
“Licensed Product” shall mean any product that contains 3-amino-1-propanesulfonic acid (“3-APS”) or any prodrug, analog, salt, free acid, free base, clathrate, solvate, hydrate, hemihydrate, anhydride, ester, chelate, conformer, congener, crystal form, crystal habit, polymorph, amorphous solid, homolog, isomer, stereoisomer, enantiomer, racemate, isotopic or radiolabeled equivalent, metabolite or conjugates of 3-APS.
“Licensed Technology” shall mean collectively the Licensed Patent Rights and the Licensed Know-How.
“Licensor” shall have the meaning for such term set forth in the Recitals.
“Licensing Revenue” shall mean any consideration (including all upfront and milestone payments) paid by a Third Party to Alzheon, one of its Affiliates or one of its or their sublicensees, in exchange for a grant to such Third Party of a sublicense or other right, license, privilege or immunity under or with respect to the Licensed Technology (including in relation to commercial sales), provided that Licensing Revenue shall exclude (i) Royalties and (ii) any and all fees paid to Alzheon, one of its Affiliates or one of its or their sublicensees for [ * ]. For greater certainty, consideration paid in transactions between or among Alzheon and its Affiliates shall be excluded from the computation of the Licensing Revenue, but Licensing Revenue shall include consideration paid in transactions between or among Alzheon’s, its Affiliates or its or their sublicensees and Third Parties, but only to the extent that such consideration qualifies as “Licensing Revenue” as defined in the immediately preceding sentence. Where (a) [ * ], (b) [ * ]; or (c) [ * ], the consideration payable as Licensing Revenue shall be [ * ].
“Licensor Indemnitees” shall have the meaning for such term set forth in Section 10.1.
“Net Sales” shall mean, with respect to a Licensed Product, the gross amount invoiced for sales of any Licensed Product in the Territory directly or in arm’s length sales by Alzheon (and its authorized Affiliates) or permitted sublicensees to Third Parties, less the following deductions from such gross amounts which are actually incurred, allowed, accrued or specifically allocated to such Licensed Product: (a) [ * ]; (b) [ * ]; (c) [ * ]; (d) [ * ]; (e) [ * ]; (f) [ * ]; (g) [ * ]; and (h) [ * ].
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
5.
In the case of any sale or other disposal for value, such as barter or counter-trade, of a Licensed Product, or part thereof, other than in an arm’s length transaction exclusively for cash, Net Sales shall be calculated as above [ * ] of such Licensed Product in the country of sale or disposal, as determined in accordance with GAAP.
Notwithstanding the foregoing, Net Sales shall not be imputed to transfers of Licensed Product for use in any clinical trial of a Licensed Product, for bona fide charitable purposes or for compassionate use, or for samples of Licensed Product, provided that [ * ].
Notwithstanding the foregoing, in the event a Licensed Product is sold as a Combination Product, Net Sales shall be calculated by multiplying the Net Sales of the Combination Product by the fraction A/(A+B), where A is the gross invoice price of the Licensed Product when sold separately in a country and B is the gross invoice price of the other active ingredient(s) included in the Combination Product when sold separately in such country. In the event no such separate sales are made by Alzheon (or its authorized Affiliates) or its permitted sublicensees, Net Sales of the Combination Product shall be calculated in a manner to be negotiated and agreed upon by the Parties, reasonably and in good faith, prior to [ * ], which shall be based upon [ * ]. For purposes of this definition, “Combination Product” means any product that comprises a Licensed Product sold in conjunction with another active component so as to be a combination product (whether packaged together or in the same therapeutic formulation).
“Option” shall have the meaning for such term set forth in Section 3.2.2.
“Option Exercise Deadline” shall have the meaning for such term set forth in Section 3.2.2.
“Option Exercise Notice” shall have the meaning for such term set forth in Section 3.2.2.
“Option Trigger Notice” shall have the meaning for such term set forth in Section 3.2.2.
“Other Licensed Patent Rights” shall have the meaning for such term set forth in Section 7.1.2.
“Parteq AA” shall have the meaning for such term set forth in Section 8.1.14.
“Parteq Agreement” shall have the meaning for such term set forth in Section 8.1.14.
“Parteq LA” shall have the meaning for such term set forth in Section 8.1.14.
“Party” and “Parties” shall have the meaning for such term set forth in the Recitals.
“Patent Rights” shall mean the rights and interests in and to (a) issued patents; (b) pending patent applications and any related patent applications filed in the future claiming priority thereto, including all provisional applications, non-provisional applications, international (PCT) applications, substitutions, renewals, divisionals, continuations, continued prosecution applications or continuations-in-part, and all patents granted thereon or issuing therefrom; (c) patents of addition, reissues, re-examinations, extensions, confirmations, registrations, revalidations, revisions and restorations by existing or future extension or restoration mechanisms, including supplementary protection certificates or the equivalent thereof, granted
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
6.
with respect to any of the foregoing; (d) registration patents, inventor’s certificates, utility models or confirmation patents granted on or issuing from any of the foregoing; (e) any form of government-issued right substantially similar to any of the foregoing, in each case with respect to clauses (a) through (e), in any country or patent examining or granting jurisdiction in the Territory.
“Pending Claim” shall mean a bona fide pending claim in a patent application within the Licensed Patent Rights that (a) has not been disclaimed, revoked, withdrawn or abandoned; (b) has not been unappealably cancelled or rejected by any administrative agency or other body of competent jurisdiction; (c) has not been declared unpatentable by any administrative agency, under a decision of a court or other body of competent jurisdiction that is unappealable or unappealed within the time allowed for appeal; or (d) has not been lost through an interference proceeding. Notwithstanding the foregoing, any patent application within the Licensed Patent Rights listed in Schedule A that has been pending for more than [ * ] from [ * ] shall not be considered to contain any Pending Claims for purposes of this Agreement.
“Person” shall mean an individual, a limited liability company, a joint venture, a corporation, a partnership, an association, a trust, a division or an operating group of any of the foregoing or any other entity or organization.
“Regulatory Approval” shall mean any and all approvals (including pricing and reimbursement approvals), product and establishment licenses, registrations or authorizations of any kind of any Regulatory Authority necessary for the development, pre-clinical and/or human clinical testing, manufacture, quality testing, supply, use, storage, importation, export, transport, marketing and sale of a Licensed Product (or any component thereof) in any country or other jurisdiction in the Territory.
“Regulatory Authorities” shall mean any applicable supranational, national, federal, state, provincial or local regulatory agency, department, bureau or other governmental entity of any country or jurisdiction in the Territory, having responsibility in such country or jurisdiction for any regulatory approvals of any kind in such country or jurisdiction, and any successor agency or authority thereto.
“Right of Reference” shall mean the authority to rely upon, and otherwise use, an investigation for the purpose of obtaining a regulatory approval, including the ability to make available the underlying raw data from the investigation for audit by the applicable Regulatory Authority, if necessary.
“Royalties” shall have the meaning for such term set forth in Section 4.1.1.
“Royalty Term” shall mean, on a country-by-country and Licensed Product-by-Licensed Product basis, the period of time commencing on the date of the First Commercial Sale of a Licensed Product in a country and ending on the date on which there is no Pending Claim or Valid Claim covering the use, manufacture, sale, offer for sale or importation of such Licensed Product in such country.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
7.
“Sell”, “Sale”, “Sold” shall mean the sale, rent, lease, license or other form of distribution of a Licensed Product, to an end-user, distributor, or any other Person, either directly or through a chain of distribution, for financial consideration, but excludes any returns.
“Sued Party” shall have the meaning for such term set forth in Section 7.7.1.
“Term” shall have the meaning for such term set forth in Section 11.1.
“Territory” shall mean all countries and jurisdictions of the world.
“Third Party” shall mean any Person other than Alzheon, the Licensor and their respective Affiliates.
“Third Party Payments” shall have the meaning for such term set forth in Section 4.1.2.
“TPC Agreement” shall have the meaning for such term set forth in Section 8.1.16.
“Valid Claim” shall mean a claim in an issued, unexpired patent within the Licensed Patent Rights that (a) has not been finally cancelled, withdrawn, abandoned or rejected by any administrative agency or other body of competent jurisdiction, (b) has not been revoked, held invalid, or declared unpatentable or unenforceable in a decision of a court or other body of competent jurisdiction that is unappealable or unappealed within the time allowed for appeal, (c) has not been rendered unenforceable through disclaimer or otherwise, or (d) has not been lost through an interference proceeding.
“Vivimind Field” shall mean the use of a product as a nutraceutical or dietary supplement but specifically excluding any pharmaceutical classification.
“Vivimind Product” shall mean any “Licensed Product” as defined in the Vivimind License Agreement.
“Vivimind License Agreement” shall mean that certain License Agreement by and between the Licensor and BHI Limited Partnership, of even date herewith.
| 2. | GRANT OF RIGHTS |
| 2.1 | License to Alzheon. Subject to the terms and conditions of this Agreement, the Licensor hereby grants to Alzheon a royalty-bearing, irrevocable (except as set forth herein), exclusive (even as to the Licensor) license or sublicense (as applicable) in the Territory, including the right to grant sublicenses solely in accordance with Section 2.3, under the Licensed Technology to: (a) use, have used, Develop, have Developed, make, have made, modify, have modified, enhance, have enhanced, improve and have improved the Licensed Technology and the Licensed Products for the Field and (b) use, have used, manufacture, have manufactured, market, sell, offer for sale, have sold, import, have imported, distribute and have distributed the Licensed Products in the Field in the Territory. Alzheon acknowledges and agrees that, with respect to the Licensed Technology that is Controlled by the Licensor pursuant to the Xxxxxx License Agreement, |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
8.
| the sublicense to Alzheon granted herein, and Alzheon’s obligations with regard thereto, shall at all times be subject to the applicable terms and conditions of the Xxxxxx License Agreement. |
| 2.2 | Restriction to License. For avoidance of doubt, the Parties agree that (a) Alzheon is not granted any rights hereunder to use, have used, Develop, have Developed, make, have made, modify, have modified, enhance, have enhanced, improve, have improved the Licensed Technology or the Licensed Products outside the Field, or to manufacture, have manufactured, market, sell, offer for sale, have sold, import, have imported, distribute or have distributed the Licensed Products outside the Field and (b) the Licensor shall retain no rights to use, have used, Develop, have Developed, make, have made, modify, have modified, enhance, have enhanced, improve, have improved the Licensed Technology or the Licensed Products for the Field, or to manufacture, have manufactured, market, sell, offer for sale, have sold, import, have imported, distribute or have distributed the Licensed Products in the Field. |
| 2.3 | Right to Sublicense. Alzheon shall have the right to grant sublicenses to all or any portion of its rights under the license granted pursuant to Section 2.1. Any sublicense granted hereunder shall be in writing and contain terms not materially less protective of the Licensor’s rights than those contained herein. Alzheon shall remain liable to the Licensor for any breaches of this Agreement that result from the acts or omissions of any sublicensee. Alzheon shall provide the Licensor with a copy of each sublicense granted by Alzheon pursuant to this Section 2.3, provided that Alzheon may redact those portions of such sublicense that are not reasonably necessary for the Licensor to verify compliance with this Agreement. |
| 2.4 | Right of Reference. The Licensor hereby grants to Alzheon and its Affiliates and sublicensees an exclusive Right of Reference to, and a royalty-bearing, irrevocable (except as set forth herein), exclusive (even as to the Licensor) license or sublicense (as applicable) to access and use, the Licensed Data (and any regulatory submissions, clinical dossiers and regulatory approvals that include such Licensed Data) for any and all purposes, other than developing, commercializing and/or seeking regulatory approval for the Vivimind Product in the Vivimind Field. The Licensor shall, at Alzheon’s request, provide a signed statement in accordance with 21 C.F.R. 314.50(g)(3) (or any analogous Law outside the United States), or otherwise provide appropriate notification of such right to the applicable Regulatory Authority, to the extent necessary for Alzheon and its Affiliates and sublicensees to exercise the Right of Reference granted hereunder. |
| 2.5 | Rights in Bankruptcy. |
| 2.5.1 | All rights and licenses now or hereafter granted by the Licensor to Alzheon under or pursuant to this Agreement, including, for the avoidance of doubt, the licenses granted to Alzheon pursuant to Section 2. 1, are, for all purposes of Section 365(n) of the Bankruptcy Code, licenses of rights to “intellectual property” as defined in the Bankruptcy Code. Upon the occurrence of any Insolvency Event with respect to the Licensor, the Licensor agrees that Alzheon, as licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
9.
| and elections under the Bankruptcy Code. Further, each Party agrees and acknowledges that, notwithstanding anything to the contrary in this Agreement, all payments by Alzheon to the Licensor hereunder, other than the Royalties, do not constitute royalties within the meaning of Section 365(n) of the Bankruptcy Code or relate to licenses of intellectual property hereunder. The Licensor shall, during the Term, create and maintain current copies or, if not amenable to copying, detailed descriptions or other appropriate embodiments, to the extent feasible, of all Licensed Technology. The Licensor and Alzheon acknowledge and agree that “embodiments” of intellectual property within the meaning of Section 365(n) include, without limitation, all physical embodiments of the Licensed Know-How. If (a) a case under the Bankruptcy Code is commenced by or against the Licensor, (b) this Agreement is rejected as provided in the Bankruptcy Code and (c) Alzheon elects to retain its rights hereunder as provided in Section 365(n) of the Bankruptcy Code, the Licensor (in any capacity, including debtor-in-possession) and its successors and assigns (including a trustee) shall comply with the provisions of this Section 2.5.1. |
| (a) | The Licensor shall provide to Alzheon all Licensed Technology (including all embodiments thereof) held by the Licensor and such successors and assigns, or otherwise available to them, immediately upon Alzheon’s written request. Whenever the Licensor or any of its successors or assigns provides to Alzheon any of the Licensed Technology (or any embodiment thereof) pursuant to this Section 2.5.1(a), Alzheon shall have the right to perform the Licensor’s obligations hereunder with respect to such Licensed Technology, but neither such provision nor such performance by Alzheon shall release the Licensor from liability resulting from rejection of the license or the failure to perform such obligations. |
| (b) | The Licensor shall not interfere with Alzheon’s rights under this Agreement, or any agreement supplemental hereto, to the Licensed Technology (including any embodiments thereof), including any right to obtain such Licensed Technology (or such embodiments) from another entity, to the extent provided in Section 365(n) of the Bankruptcy Code. |
| 2.5.2 | All rights, powers and remedies of Alzheon provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the Bankruptcy Code) in the event of the commencement of a case under the Bankruptcy Code with respect to the Licensor. The Parties agree that they intend the following rights to apply to the maximum extent permitted by law, and to be enforceable under Bankruptcy Code Section 365(n): |
| (a) | the right of access to any intellectual property (including all embodiments thereof) of the Licensor, or any Third Party with whom the Licensor contracts to perform an obligation of the Licensor under this Agreement, and, in the case of the Third Party, which is necessary for the manufacture, use, sale, offering for sale, import or export of Licensed Products; and |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
10.
| (b) | the right to contract directly with any Third Party with whom the Licensor contracts to perform an obligation of the Licensor under this Agreement to complete such contracted work. |
| 2.6 | Initial Information Transfer. Within a reasonable period of time after the Effective Date (but in no event later than [ * ] after the Effective Date), the Licensor shall make available or cause to be made available to Alzheon, in a mutually-agreed upon format, (a) the Licensed Data and any other material data included in the Licensed Know-How and (b) other information regarding the Licensed Technology that is [ * ]. During the Term, the Licensor shall transfer or make available or shall cause to be transferred or made available to Alzheon, in a mutually agreed upon format, all new Licensed Know-How that comes into the Control of the Licensor within [ * ] after such Licensed Know-How comes into the Licensor’s control. |
| 3. | DEVELOPMENT AND COMMERCIALIZATION OF LICENSED PRODUCTS |
| 3.1 | Research and Development |
| 3.1.1 | Alzheon Rights. The Licensor hereby sublicenses to Alzheon on the Effective Date all of its rights under the Xxxxxx License Agreement with respect to the Development of the Licensed Products in the Territory for the Field, and Alzheon acknowledges and agrees that all such rights, and Alzheon’s obligations with regard thereto, shall at all times be subject to the applicable terms and conditions of the Xxxxxx License Agreement. On and after the Effective Date, Alzheon shall have sole and exclusive control, authority and responsibility over the Development of the Licensed Products in the Territory for the Field. |
| 3.1.2 | Alzheon Diligence Obligations. In accordance with Section 2.3 of the Xxxxxx License Agreement, Alzheon shall fully satisfy, on behalf of the Licensor, the following obligations: |
| (a) | during the Term, Alzheon shall use Commercially Reasonable Efforts to Develop and seek to commercialize at least one Licensed Product in the Field in the Territory; |
| (b) | Alzheon shall or shall cause one of its sublicensees to, dose the first healthy individual in any study of a Licensed Product in the Field no later than * ]; and |
| (c) | Alzheon shall or shall cause one of its sublicensees to, dose the first patient in a clinical trial of a Licensed Product in its respective Field no later * ]. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
11.
| 3.1.3 | Arising Intellectual Property. During the Term, all right, title and interest in and to any enhancement, invention or discovery created, conceived, identified, or reduced to practice by Alzheon, any of its Affiliates or any sublicensee or by a Third Party on behalf of Alzheon that relies on or incorporates the Licensed Technology (an “Improvement”) shall be assigned by Alzheon, or Alzheon shall cause such right, title and interest to be assigned by such Third Party, to the Licensor or its designee. The Parties acknowledge and agree that, pursuant to Section 3.1.3 of the Xxxxxx License Agreement, the Licensor is required to assign to Xxxxxx all Improvements that rely on or incorporate any Licensed Technology that is Controlled by the Licensor pursuant to the Xxxxxx License Agreement, but such Improvements shall be Controlled by the Licensor pursuant to the Xxxxxx License Agreement and sublicensed to Alzheon pursuant to Section 2.1 of this Agreement. |
| 3.2 | Commercialization. |
| 3.2.1 | Alzheon Rights. The Licensor hereby sublicenses to Alzheon on the Effective Date all of its rights under the Xxxxxx License Agreement with respect to the commercialization of the Licensed Products in the Territory for the Field, and Alzheon acknowledges and agrees that all such rights, and Alzheon’s obligations with regard thereto, shall at all times be subject to the applicable terms and conditions of the Xxxxxx License Agreement. Without limiting any rights of Alzheon herein, but subject to Section 3.2.2 and Article 7, on and after the Effective Date, Alzheon shall have sole and exclusive control, authority and responsibility over all commercialization of Licensed Products in the Territory for the Field, including without limitation (a) all activities relating to manufacture and supply of all Licensed Products; (b) all marketing, promotion, sales, distribution, import and export activities relating to any Licensed Product; and (c) all activities relating to any regulatory filings, registrations, applications and Regulatory Approvals relating to any of the foregoing. |
| 3.2.2 | Licensor Option to Commercialize Licensed Products in Italy. Notwithstanding any sublicense hereunder to Alzheon by the Licensor, the Licensor shall have the exclusive right and option, in its sole discretion, to obtain exclusive rights to commercialize the Licensed Products in Italy on the terms and conditions set forth in this Section 3.2.2 (the “Option”). If Alzheon intends to submit a marketing authorization application for any Licensed Product in the Field in Italy (whether to the EMA under the centralized EMA filing procedure or to the applicable Regulatory Authority in Italy), Alzheon shall so notify the Licensor in writing and deliver a copy of such application to the Licensor (the “Option Trigger Notice”). The Licensor may exercise the Option with respect to the Licensed Products by delivering written notice thereof (the “Option Exercise Notice”) to Alzheon no later than [ * ] after receiving the Option Trigger Notice (the “Option Exercise Deadline”). |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
12.
| (a) | If the Licensor delivers an Option Exercise Notice to Alzheon prior to the Option Exercise Deadline, then Alzheon and the Licensor shall thereafter negotiate in good faith and attempt to agree upon a distribution and commercialization agreement in a mutually-agreed form, pursuant to which Alzheon shall grant the Licensor an exclusive, royalty-free, fully paid-up license under the Licensed Technology and Improvements to commercialize the Licensed Products in the Field in Italy (the “Italy Commercialization Agreement”). The Italy Commercialization Agreement shall contain commercially reasonable, arms-length terms and conditions customary to the distribution and commercialization of products similar to the Licensed Products and shall include the terms set forth on Exhibit A attached hereto. [ * ]. |
| (b) | If (i) the Licensor notifies Alzheon prior to the Option Exercise Deadline that it will not exercise the Option or (ii) the Licensor fails to deliver an Option Exercise Notice to Alzheon on or prior to the Option Exercise Deadline, then Alzheon shall thereafter be free to commercialize the Licensed Products in the Field in Italy or to engage any Third Party to undertake all or any portion of such commercialization activities and the Licensor shall thereafter have no further rights under this Section 3.2.2 or otherwise with respect to the commercialization of the Licensed Products in Italy. |
| 3.3 | Updates and Reports. During the Term, Alzheon shall keep the Licensor currently, and no less frequently than [ * ], advised of its (and its Affiliates’ and sublicensees’) Development and commercialization activities with respect to the Licensed Products in the Field in the Territory. Without limiting the generality of the foregoing, Alzheon shall provide to the Licensor: |
| 3.3.1 | on a [ * ] basis, written materials summarizing the activities undertaken by or on behalf of Alzheon with respect to the Development and commercialization of Licensed Products in the Field in the Territory, which materials shall include a reasonably detailed description of Alzheon’s efforts to achieve the diligence obligations set forth in Section 3.1.2; |
| 3.3.2 | prompt written notice of the obtaining of any Regulatory Approval in relation to any Licensed Product in the Territory; |
| 3.3.3 | prompt written notice of the occurrence of the First Commercial Sale of any Licensed Product in each country and jurisdiction of the Territory; and |
| 3.3.4 | prompt written notice upon the receipt of any Licensing Revenue. |
Alzheon shall be deemed to be in compliance with the requirements of this Section 3.3 so long as it provides the information and notices described above in Sections 3.3.1 through 3.3.4, as well as any additional information requested by the Licensor that is [ * ].
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
13.
| 3.4 | Ownership of Submissions and Regulatory Approvals. Subject to Section 11.6, Alzheon shall own all Regulatory Approvals for Licensed Products and any other submissions to any Regulatory Authority with respect to the Licensed Product in the Field in the Territory. |
| 3.5 | Complaints; Recalls. During the Term, Alzheon shall be responsible for all matters relating to recalls and medical inquiries relating to the Licensed Products in the Field in the Territory and shall do so in conformity with all applicable Laws. |
| 3.6 | Adverse Drug Events. During the Term, Alzheon shall be responsible for all matters relating to the reporting of adverse events concerning the Licensed Products in the Territory, including the creation and maintenance of global safety database(s) for adverse drug events, and shall do so in conformity with all applicable Laws. |
| 4. | PAYMENTS AND ROYALTIES |
| 4.1 | Royalty and Licensing Payments |
| 4.1.1 | Royalty and Licensing Rates. In consideration of the grant of the license and the covenants and representations hereunder, and subject to the other terms of this Agreement. Alzheon shall pay to the Licensor the aggregate of (i) during the Royalty Term for any Licensed Product in any country in the Territory, a royalty amount equal to [ * ] of Net Sales of such Licensed Product in such country (the “Royalties”) and (ii) during the Term, [ * ] of all Licensing Revenues. For the sake of clarity, if any Licensing Revenue is received by an Affiliate or a sublicensee of Alzheon and such Affiliate or sublicensee pays a portion of such Licensing Revenue to Alzheon, Alzheon shall be required to pay the Licensor an amount equal to [ * ] of the total amount of Licensing Revenue received by such Affiliate or sublicensee, regardless of the amount paid to Alzheon. For example, if an Affiliate or sublicensee of Alzheon receives total consideration of [ * ] in Licensing Revenue and pays Alzheon [ * ] of such Licensing Revenue pursuant to the terms of the agreement between Alzheon and such Affiliate or sublicensee, then Alzheon would only be required to pay the Licensor [ * ] of Licensing Revenue received by such Affiliate or sublicensee. |
| 4.1.2 | Payments under the Parteq Agreement and the TPC Agreement. In further consideration of the grant of the license and the covenants and representations hereunder, and subject to the other terms of this Agreement, for the duration of the Term, Alzheon shall pay, in accordance with Section 8.3, any amounts due or that may become due by the Licensor under the Parteq Agreement and the TPC Agreement as a result of the Development or commercialization of the Licensed Products by Alzheon or any of its sublicensees pursuant to this Agreement (the “Third Party Payments”). Any such Third Party Payments shall be calculated in accordance with the relevant provisions of the Parteq Agreement or the TPC Agreement, as applicable. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
14.
| 4.2 | Third Party Licenses. If (a) Alzheon reasonably determines that it is [ * ] to obtain a license from a Third Party under a Patent Right or other right to Develop, manufacture or commercialize a Licensed Product in the Field in the Territory and (b) the Licensor is commercializing the Licensed Products in Italy pursuant to the Italy Commercialization Agreement, then Alzheon shall be responsible for payment of any amounts owed to such Third Party pursuant to such license and the Licensor shall reimburse Alzheon [ * ], provided that the [ * ]. |
| 4.3 | Fully Paid-Up, Royalty Free Licenses. Following the expiration of the Royalty Term for any Licensed Product in any country or other jurisdiction in the Territory, no further Royalties shall be payable in respect of Sales of such Licensed Product in such country or jurisdiction and, thereafter, the licenses granted to Alzheon under this Agreement with respect to such Licensed Product in such country or jurisdiction shall automatically become fully paid-up, perpetual, irrevocable, royalty-free, non-exclusive licenses. |
| 4.4 | Payment Terms |
| 4.4.1 | Payment of Royalties and Licensing Revenues. Alzheon shall make any payments owed to the Licensor hereunder in arrears, within [ * ] from the end of each calendar quarter in which such payment accrues. Each payment shall be accompanied by a report for each country in the Territory in which sales of Licensed Products occurred and/or with respect to which Licensing Revenues were received in the calendar quarter covered by such statement, specifying: (a) the gross sales and Net Sales of each Licensed Product, if any, in such country’s currency; (b) the Royalties and Licensing Revenue payable to the Licensor in Canadian dollars; and (c) the calculation of any Third Party Payments due with respect to such country. |
| 4.4.2 | No Payment Due. For greater certainty, no Royalties shall be due to Licensor on Licensed Products which were not Sold; i.e. Licensed Products that were returned or that were provided to an end-user without financial consideration, such as a sample, for example. |
| 4.4.3 | Accounting. All payments hereunder shall be made to the Licensor in Italy in Canadian dollars. Conversion of foreign currency to Canadian dollars, if applicable, shall be made at the conversion rate of the Bank of Canada on the last business day of the calendar quarter preceding the applicable calendar quarter. |
| 4.4.4 | Interest. In the event that any payment due hereunder is not made when due, interest shall accrue at a rate per annum equal to the lesser of [ * ], calculated on the number of days such payments are paid after the date such payments are due and compounded monthly. |
| 4.4.5 | Taxes. All payments paid by Alzheon to the Licensor under this Agreement are exclusive of, and Alzheon shall pay any sales, use, rental, custom, value added, consumption or other taxes, duties, levies, fees or charges that may be assessed in any jurisdiction resulting from or arising under this Agreement, except for |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
15.
| withholding taxes (to the extent applicable) which Alzheon shall be permitted to deduct from the applicable payments prior to making such payments to the Licensor. The Licensor shall collect and remit such taxes, duties, levies, fees or charges as required under applicable Law. |
| 4.5 | Records Retention; Review |
| 4.5.1 | Payments. Alzheon and its Affiliates shall keep for at least [ * ] from the end of the calendar year to which they pertain, complete and accurate books and records sufficient to support and confirm the calculation of gross invoiced sales, Net Sales, exchange rates, Royalties, Licensing Revenues and Third Party Payments under this Article 4. |
| 4.5.2 | Review. At the request of the Licensor or Xxxxxx (the “Auditing Party”), upon at least [ * ] prior written notice, and at the expense of the Auditing Party (except as otherwise provided herein), Alzheon shall permit an independent certified accounting firm selected by the Auditing Party, and reasonably acceptable to Alzheon, to inspect (during regular business hours) the relevant records required to be maintained by Alzheon and its Affiliates under this Section 4.5. The independent certified accounting firm shall be entitled to review the then-preceding [ * ] of records required to be maintained by Alzheon and its Affiliates under this Section 4.5 solely for purposes of verifying the accuracy of the reports submitted by Alzheon pursuant to Section 4.4.1 and the Licensing Revenues, Royalties and Third Party Payments due hereunder. Such right may be exercised by the Auditing Parties only [ * ]. If any review reveals a deficiency in the calculation of payments resulting in an underpayment to the Licensor, Alzheon shall promptly pay the Licensor the amount remaining to be paid, and if such underpayment is by [ * ] or more for [ * ], Alzheon shall pay the reasonable documented out-of-pocket expenses of the review. Any independent certified accounting firm that performs an inspection pursuant to this Section 4.5.2 shall enter into a written confidentiality agreement with Alzheon on terms substantially similar to those set forth in Article 5 of this Agreement. |
| 4.5.3 | [ * ]. |
| 5. | TREATMENT OF CONFIDENTIAL INFORMATION |
| 5.1 | Confidentiality Obligations. The Licensor and Alzheon each recognize that the other Party’s Confidential Information constitutes highly valuable and proprietary confidential information. The Licensor and Alzheon each agree that it will keep confidential, and will cause its officers, employees, consultants, agents, Affiliates, licensees and sublicensees to keep confidential, all Confidential Information of the other Party. Neither the Licensor nor Alzheon nor any of their respective officers, employees, consultants, agents, Affiliates, licensees or sublicensees shall use Confidential Information of the other Party for any purpose whatsoever other than exercising any rights granted to it or reserved by it hereunder or performing its obligations hereunder. Without limiting the foregoing, each Party may disclose Confidential Information of the other Party to the extent such |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
16.
| disclosure is reasonably necessary in (a) filing and prosecuting patent applications and maintaining patents which are filed in accordance with the provisions of this Agreement, or (b) filing, prosecuting or defending litigation in accordance with the provisions of this Agreement, or (c) complying with applicable Laws, regulations, court orders or the requirements of any nationally recognized security exchange, quotation system or over-the-counter market on which such Party has its securities listed or traded; provided, however, that if a Party is required to make any such disclosure of the other Party’s Confidential Information, it will give reasonable advance written notice to the other Party of such disclosure requirement and will use reasonable efforts to assist such other Party in efforts to secure confidential treatment of such information required to be disclosed. |
| 5.2 | Limited Disclosure and Use. The Licensor and Alzheon each agree that it may disclose the other Party’s Confidential Information to its Affiliates, licensees or sublicensees or any of its or their officers, employees, consultants or agents, provided that such disclosure shall (a) be made only if and to the extent reasonably necessary to carry out its rights and obligations under this Agreement; (b) be limited to the maximum extent possible consistent with such rights and obligations; and (c) only be made to Persons who are bound by written confidentiality obligations no less stringent than those set forth in this Article 5. The Licensor and Alzheon each further agree not to disclose or transfer the other Party’s Confidential Information to any Third Parties under any circumstance without the prior written approval of the other Party (such approval not to be unreasonably withheld or delayed), except (i) as otherwise required by Law, (ii) to any prospective or actual investors, lenders or other financing sources, prospective or actual acquirers, strategic partners, licensees or sublicensees who are bound by written confidentiality obligations no less stringent than those set forth in this Article 5 and (iii) as otherwise expressly permitted by this Agreement. Each Party shall take such action, and shall cause its Affiliates, licensees or sublicensees to take such action, to preserve the confidentiality of each other’s Confidential Information as it would customarily take to preserve the confidentiality of its own Confidential Information, and in no event, shall treat the other Party’s Confidential Information with less than reasonable care. Each Party, upon the request of the other Party, will return all the Confidential Information disclosed or transferred to it by the other Party pursuant to this Agreement, including all copies and extracts of documents and all manifestations in whatever form, within [ * ] of the termination or expiration of this Agreement; provided, however, that a Party may retain Confidential Information of the other Party relating to any license or right to use Licensed Technology which survives such termination, and one copy of all other Confidential Information may be retained in inactive archives or by legal counsel solely for the purpose of establishing the contents thereof. |
| 5.3 | Bankruptcy Procedures. All Confidential Information disclosed by one Party to the other, including all intellectual property rights therein, shall remain the property of the Disclosing Party. In the event that a court or other legal or administrative tribunal, directly or through an appointed master, trustee or receiver, assumes partial or complete control over the assets of either Party in connection with an Insolvency Event with respect to such Party, the bankrupt or insolvent Party shall promptly notify the court or other tribunal (a) that Confidential Information received from the other Party under this |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
17.
| Agreement remains the property of the other Party and (b) of the confidentiality obligations under this Agreement. In addition, the bankrupt or insolvent Party shall, to the extent permitted by Law, take all steps necessary or desirable to maintain the confidentiality of the other Party’s Confidential Information and to ensure that the court, other tribunal or appointee maintains such information in confidence in accordance with the terms of this Agreement. |
| 5.4 | Publicity. Subject to the terms hereof including, without limitation, Section 5.1, neither Party may publicly disclose the existence or terms or any other matter of fact regarding this Agreement or the Xxxxxx License Agreement without the prior written consent of the other Party; provided, however, that either Party may make such a disclosure (a) to the extent required by Law or any regulation or requirements of any nationally recognized securities exchange, quotation system or over-the-counter market on which such Party has its securities listed or traded; (b) to any prospective or actual investors, lenders or other financing sources, prospective or actual acquirers, strategic partners, licensees or sublicensees who are bound by written confidentiality obligations no less stringent than those set forth in this Article 5; or (c) to persons or entities permitted disclosure of Confidential Information pursuant to Section 5.2, but only to the extent and on the same terms and conditions as set forth in Section 5.2. In the event that a Party is required to disclose the existence or terms or any other matter of fact regarding this Agreement in accordance with clause (a) of this Section 5.4, it will, to the extent permitted, provide reasonable advance written notice to the other Party of such disclosure requirement and will use reasonable efforts to assist such other Party in efforts to secure confidential treatment of such information required to be disclosed. |
| 5.5 | Use of Name. Neither Party shall employ or use the name of the other Party in any promotional materials or advertising without the prior express written permission of the other Party as to each such use, except as otherwise previously set forth in a permitted disclosure pursuant to Section 5.4. |
| 6. | GOVERNANCE |
| 6.1 | Alliance Managers. Promptly after the Effective Date, each Party shall designate a single representative to manage all of the activities contemplated under this Agreement. Such designated representatives will be responsible for the day-to-day coordination of the activities of the Parties with respect to the Licensed Products and will serve to facilitate communication between the Parties. Such designated representatives shall have experience and knowledge appropriate for managers with such project management responsibilities. Each Party may change its designated representative from time to time upon notice to the other Party. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
18.
| 7. | PROVISIONS CONCERNING THE FILING, PROSECUTION AND MAINTENANCE OF PATENT RIGHTS |
| 7.1 | Patent Filing, Prosecution and Maintenance. |
| 7.1.1 | Alzheon Responsibilities. Subject to the terms of Sections 7.2, 7.3 and 7.4, the Parties agree that Alzheon shall prepare, file, prosecute, obtain and maintain, [ * ] and acting through patent attorneys or agents of its choice, all Licensed Patent Rights with respect to the patents and patent applications listed in Schedule B attached hereto and all Patent Rights with respect to Improvements (the “BLU8499 Patent Rights”) throughout the Territory. The Licensor hereby assigns to Alzheon on the Effective Date all of its rights under the Xxxxxx License Agreement with respect to the preparation, filing, prosecuting, obtaining and maintenance of the BLU8499 Patent Rights (other than such rights that are sublicensed to Alzheon pursuant to Section 2.1), and Alzheon acknowledges and agrees that all such rights, and Alzheon’s obligations with regard thereto, shall at all times be subject to the applicable terms and conditions of the Xxxxxx License Agreement. In the event that Alzheon at any time or from time to time determines not to maintain any BLU8499 Patent Right in any country or other jurisdiction in the Territory and such determination is not made for legitimate strategic reasons, it shall provide the Licensor with prior written notice of the same (given not less than [ * ] prior to any applicable deadline with respect to maintaining any such right in the applicable Territory), in which case the Licensor may, by notice in writing, thereafter prepare, file, prosecute, obtain and maintain such BLU8499 Patent Right on its own behalf and [ * ]. |
| 7.1.2 | Licensor Responsibilities. Subject to the terms of Sections 7.2, 7.3 and 7.4, the Parties agree that, as between the Parties, the Licensor shall prepare, file, prosecute, obtain and maintain, [ * ] and acting through patent attorneys or agents of its choice, all Licensed Patent Rights other than the BLU8499 Patent Rights, including the Patent Rights with respect to the patents and patent applications listed on Schedule A (the “Other Licensed Patent Rights”), throughout the Territory; provided, however, that the Parties acknowledge and agree that Xxxxxx shall prepare, file, prosecute, obtain and maintain, [ * ] and acting through patent attorneys or agents of its choice, the Other Licensed Patent Rights that are Controlled by the Licensor pursuant to the Xxxxxx License Agreement. In the event that the Licensor or Xxxxxx at any time or from time to time determines not to maintain any Other Licensed Patent Rights in any country or other jurisdiction in the Territory [ * ], the Licensor shall provide Alzheon with prior written notice of the same (given not less than [ * ] prior to any applicable deadline with respect to maintaining any such right in the applicable Territory), in which case Alzheon may, by notice in writing to the Licensor, thereafter prepare, file, prosecute, obtain and maintain such Other Licensed Patent Rights on its own behalf and [ * ]. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
19.
| 7.2 | Expense Reimbursement. Alzheon shall reimburse to the Licensor [ * ] per year in connection with all preparation, filing, prosecution and maintenance costs incurred by the Licensor and/or Xxxxxx with respect to the Other Licensed Patent Rights resulting from Section 7.1.2. The aforementioned [ * ] shall be reduced to [ * ] for the calendar years [ * ] (inclusively) and further reduced to [ * ] for calendar years [ * ] and following (until [ * ]). |
| 7.3 | Cooperation. The Parties agree to cooperate in the preparation, filing, prosecution and maintenance of all patent applications within the Licensed Patent Rights, including obtaining and executing necessary powers of attorney and assignments by the named inventors, providing relevant technical reports to the filing Party concerning the invention disclosed in such patent application, obtaining execution of such other documents which shall be needed in the filing and prosecution of such patent applications, and, as requested, updating each other regarding the status of such patent applications. Without limiting the generality of the foregoing, each Party shall keep the other Party reasonably informed of the status of the filing, prosecution and maintenance of each patent and patent application within the Licensed Patent Rights, including [ * ]. |
| 7.4 | Divisional and Continuation. The Parties agree that, upon Alzheon’s request, the Licensor shall pursue additional patent claims under the Other Licensed Patent Rights through the filing of one or more divisionals or continuations in order to provide protection in respect of a product being developed by Alzheon. Alzheon shall promptly (and in any event within [ * ] of presentation of an invoice therefor by the Licensor) pay [ * ] incurred by the Licensor in connection with such request (including, without limitation, [ * ]). Alzheon may direct the drafting of such divisionals and continuations and review and comment on such filings, provided that, subject to Section 7.1.2, Alzheon shall have final decision making authority in all matters relating to the drafting, reviewing and approving of filings made in relation to such divisionals and continuations. [ * ]. For avoidance of doubt, any preparation, filing, prosecution, obtaining and maintaining obligations with respect to any divisional and continuation related to the BLU8499 Patent Rights shall be subject to the provisions of Section 7.1.1. |
| 7.5 | Notice of Infringement. If, during the Term, either Party learns of any actual, alleged or threatened infringement by a Third Party of any Licensed Patent Rights in the Territory (an “Infringement”), such Party shall promptly notify the other Party and shall provide such other Party with available evidence of such Infringement. |
| 7.6 | Infringement of Patent Rights. As between the Parties, Alzheon shall have the first right (but not the obligation), at Alzheon’s expense and with legal counsel of its own choice, to bring suit or take other appropriate legal action (an “Action”) against any Infringement. The Licensor hereby assigns to Alzheon on the Effective Date all of its rights under the Xxxxxx License Agreement to take Action against any Infringement, and Alzheon acknowledges and agrees that all such rights, and Alzheon’s obligations with regard thereto, shall at all times be subject to the applicable terms and conditions of the Xxxxxx License Agreement. Licensor shall have the right, at its own expense, to participate in any such Action by Alzheon using counsel of Licensor’s own choice; provided, however, |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
20.
| that under no circumstances shall the foregoing affect the right of Alzheon to bring an Action against any Infringement. [ * ]. Under no circumstances shall Alzheon be entitled to settle any Action in the Territory in respect of Licensed Patent Rights in any way that would [ * ] without the prior written consent of the Licensor, which consent shall not be unreasonably withheld or delayed. Any damages, monetary awards or other amounts recovered, whether by judgment or settlement, pursuant to any Action taken under this Section 7.6, in respect of the Territory, shall be applied as follows: |
| (a) | [ * ]; and |
| (b) | [ * ]. |
If a Party brings any Action under this Section 7.6, the other Party agrees to be joined as party plaintiff if necessary to prosecute such Action, and to give the Party bringing such Action reasonable assistance and authority to file and prosecute the suit; provided, however, that neither Party shall be required to [ * ].
| 7.7 | Third Party Claims. |
| 7.7.1 | If the Development or commercialization of a Licensed Product is alleged by a Third Party to infringe a Third Party Patent Right or misappropriate Third Party Know-How, the Party that becomes aware of such allegation shall promptly notify the other Party and shall provide such other Party with a reasonably detailed description of such claim. If a Third Party sues a Party (the “Sued Party”) alleging that the Development or commercialization of a Licensed Product by or on behalf of the Sued Party infringes such Third Party’s Patent Right(s) or misappropriates such Third Party’s Know-How, then the Sued Party shall have the sole right (but not the obligation) to defend such suit. At the Sued Party’s request and cost, the other Party shall reasonably assist the Sued Party in the defense of such Third Party suit and shall join such suit if deemed a necessary party. If the other Party does not join such Third Party suit, then the Sued Party shall report the status of such Third Party suit to the other Party on a quarterly basis prior to and during the pendency of such Third Party suit. If Alzheon is the Sued Party, then Alzheon shall not settle such Third Party suit in any way that would [ * ] without the prior written consent of the Licensor, as applicable, which consent shall not be unreasonably withheld or delayed. If the Licensor is the Sued Party, then the Licensor shall not settle such Third Party suit in any way that would [ * ] without the prior written consent of Alzheon, which consent shall not be unreasonably withheld or delayed. Subject to the Parties’ respective indemnification obligations under Article 10, all litigation expenses associated with a Third Party suit (including settlement costs, royalties paid in settlement of such suit and the payment of damages to the Third Party) shall be [ * ]. In the event that a Sued Party desires to settle a Third Party suit but such settlement would [ * ], then such other Party shall be, notwithstanding the foregoing, [ * ]. For avoidance of doubt, such other Party shall be required to [ * ]. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
21.
| 7.7.2 | The Licensor hereby assigns to Alzheon on the Effective Date all of the Licensor’s rights under Section 7.7 of the Xxxxxx License Agreement with respect to any Third Party suits brought against Xxxxxx alleging that the Development or commercialization of a Licensed Product by or on behalf of Xxxxxx infringes a Third Party Patent Right or misappropriates Third Party Know-How and such Licensed Product is being Developed and/or commercialized by Alzheon pursuant to this Agreement. Alzheon acknowledges and agrees that all such rights, and Alzheon’s obligations with regard thereto, shall at all times be subject to the applicable terms and conditions of the Xxxxxx License Agreement. |
| 7.8 | Covenant Not to Take Action Adverse to Licensed Technology. Alzheon hereby covenants and agrees (and, with respect to its Affiliates, licensees and sublicensees, represents and warrants) that neither it nor any of its Affiliates, licensees or sublicensees shall institute or participate in, or assist any Third Party to institute or participate in, formal proceedings against, or otherwise take any action adverse to, the Licensor’s or any of its Affiliates’, licensees’ or sublicensees’ rights or asserted rights in respect of the Licensed Technology, including the Licensed Patent Rights, including without limitation through any interference or similar proceeding, or in connection with any challenge to the ownership, validity or enforceability of the Licensed Technology, including the Licensed Patent Rights or any claims thereof. |
| 8. | REPRESENTATIONS, WARRANTIES AND COVENANTS |
| 8.1 | Licensor Representations. The Licensor represents and warrants to Alzheon that, as of the Effective Date: |
| 8.1.1 | it is a corporation or entity duly organized and validly existing under the Laws of the state or other jurisdiction of its incorporation or formation; |
| 8.1.2 | the execution and delivery of this Agreement and the performance of the transactions contemplated hereby have been duly authorized by all appropriate Licensor corporate actions; |
| 8.1.3 | this Agreement is a legal and valid obligation binding upon the Licensor and enforceable in accordance with its terms, and the execution, delivery and performance of this Agreement by the Parties does not conflict with any agreement, instrument or understanding to which the Licensor is a party of or by which it is bound; |
| 8.1.4 | it has the power and authority to perform its obligations hereunder; |
| 8.1.5 | it has the full right, power and authority to grant all of the rights, title and interests in the licenses granted to Alzheon hereunder; |
| 8.1.6 | the Licensor is the sole owner of the Licensed Technology or Controls the Licensed Technology (i.e., has sufficient rights to grant to Alzheon the licenses and sublicenses contemplated by this Agreement); |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
22.
| 8.1.7 | the Licensor has not received any written notice or threat of any material suit, legal claim, action, proceeding (other than patent oppositions) or investigation against the Licensor or any of its Affiliates that relates to any Licensed Product; |
| 8.1.8 | the Licensor has not received any written notice threatening any orders, injunctions or decrees of any Regulatory Authority or other governmental body applicable to any of the Licensed Products; |
| 8.1.9 | the Licensor is not actually aware of, nor has Licensor received any written notice of, any allegations or claims that the Licensed Technology infringes or misappropriates any intellectual property right of any Third Party; |
| 8.1.10 | none of the Licensed Patent Rights have been adjudged invalid or unenforceable in whole or in part, other than abandoned patents; |
| 8.1.11 | the Licensor has not received any written notice of any suit, claim, action or proceeding challenging or seeking to deny or restrict, directly or indirectly, the rights of the Licensor or any of its Affiliates in any of the Licensed Technology; |
| 8.1.12 | to the Licensor’s knowledge, no Third Party is infringing or misappropriating the Licensed Technology; |
| 8.1.13 | except as disclosed by the Licensor to Alzheon prior to the Effective Date, none of the following events have occurred with respect to any Licensed Product: (a) any material failure to comply with any regulatory requirement applicable to the conduct of a clinical trial involving the Licensed Product, (b) any material failure to comply with the protocol or applicable ethical standards for any clinical trial involving the Licensed Product; (c) any unexpected adverse drug reaction occurring during any clinical trial involving the Licensed Product that the Licensor suspected was drug-related; or (d) any serious adverse drug event concerning the Licensed Product; |
| 8.1.14 | the Licensor has provided Alzheon with a complete copy of the License Agreement dated January 1, 1999 between Parteq Research and Development Innovations and Neurochem Inc., including all amendments and supplements thereto (the “Parteq LA”), and the Assignment Agreement dated February 1, 2006 between Parteq Research and Development Innovations and Neurochem Inc., including all amendments and supplements thereto (the “Parteq AA” and collectively with the Parteq LA, the “Parteq Agreement”); |
| 8.1.15 | the Parteq LA was terminated, replaced and superseded in all respects by the Parteq AA; |
| 8.1.16 | the Licensor has provided Alzheon with a complete copy of the Contribution Agreement dated November 17, 1999 between Her Majesty the Queen in Right of Canada and Neurochem Inc., related to TPC Project No: 720-460609, including all amendments and supplements thereto (the “TPC Agreement”); |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
23.
| 8.1.17 | each of the Parteq AA and TPC Agreement are in full force and effect, and has not been modified or amended except as set forth in the amendments and supplements provided to Alzheon prior to the Effective Date, and no party to the Parteq AA or the TPC Agreement is in default with respect to any material obligation thereunder; |
| 8.1.18 | [ * ]; |
| 8.1.19 | the Licensor has provided Alzheon with a complete copy of the Vivimind License Agreement; and |
| 8.1.20 | the Licensor has provided Alzheon with a complete copy of the Xxxxxx License Agreement. |
| 8.2 | Alzheon Representations. Alzheon represents and warrants to the Licensor that, as of the Effective Date: |
| 8.2.1 | it is a corporation or entity duly organized and validly existing under the Laws of the state or other jurisdiction of its incorporation or formation; |
| 8.2.2 | the execution and delivery of this Agreement and the performance of the transactions contemplated hereby have been duly authorized by all appropriate corporate actions of Alzheon; |
| 8.2.3 | this Agreement is a legal and valid obligation binding upon Alzheon and enforceable in accordance with its terms, and the execution, delivery and performance of this Agreement by the Parties does not conflict with any agreement, instrument or understanding to which Alzheon is a party of or by which it is bound; |
| 8.2.4 | it has the power and authority to perform its obligations hereunder; and |
| 8.2.5 | to Alzheon’s knowledge, no Third Party is infringing or misappropriating the Licensed Technology. |
| 8.3 | Licensor Covenants. |
| 8.3.1 | The Licensor shall not execute or otherwise permit any amendment or modification of, or waiver of any provision of, the Xxxxxx License Agreement without the prior written consent of Alzheon. The Licensor shall take all actions reasonably necessary to enforce and maintain its rights under the Xxxxxx License Agreement. The Licensor shall not make any election or exercise any right or option (or omit to take any action) which would terminate or relinquish in whole or in part any right under the Xxxxxx License Agreement. The Licensor shall comply with all of its obligations under the Xxxxxx License Agreement, including by paying all amounts due to Xxxxxx thereunder. The Licensor shall take such actions as shall be necessary to keep in full force and effect the Xxxxxx License |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
24.
| Agreement. The Licensor shall give prompt notice to Alzheon, together with a detailed summary of outstanding issues if Alzheon so requests, of any notice received from or given to Xxxxxx of any actual or alleged defaults, breaches, violations, proposed amendments or proposed modifications of, or any proposed waivers under, the Xxxxxx License Agreement. In the event that the Licensor receives any such notice from Xxxxxx regarding an actual or alleged default, breach or violation by the Licensor, the Licensor shall promptly notify Alzheon, and [ * ] cure such default, breach or violation or otherwise proceed. |
| 8.3.2 | The Licensor shall not license, sell, assign or otherwise transfer to any Person (including any Affiliate of the Licensor) any Licensed Technology, or assign or otherwise transfer the Xxxxxx License Agreement or any of its rights or obligations thereunder to any Person (including any Affiliate of the Licensor) (or agree to do any of the foregoing) except to the extent permitted by, and in compliance with, Section 13.7. The Licensor shall not incur or permit to exist (and shall cause each of its Affiliates not to incur or permit to exist), with respect to any Licensed Technology, any lien, encumbrance, charge, security interest, mortgage, liability, grant of license to Third Parties or other restriction (including in connection with any indebtedness). |
| 8.3.3 | The Licensor shall not, without the prior written consent of Alzheon (which consent shall not be unreasonably withheld or delayed), amend the Vivimind License Agreement in a manner [ * ]. The Licensor shall notify Alzheon promptly following any termination of the Vivimind License Agreement. |
| 8.3.4 | In the event that the Licensor receives notice from Xxxxxx that it intends to amend the Parteq Agreement or the TPC Agreement, the Licensor shall promptly notify Alzheon, and [ * ]. |
| 8.3.5 | Neither the Licensor nor any of its Affiliates shall conduct, or assist, authorize or enable any Third Parties to conduct, any development of any Vivimind Product in the Vivimind Field other than development of products containing a Vivimind Product or a salt thereof in combination with another nutraceutical or dietary supplement for the Vivimind Field. |
| 8.3.6 | Neither the Licensor nor any of its Affiliates shall seek or obtain, or enable any Third Parties to seek or obtain, regulatory approval of any Vivimind Product outside of the Vivimind Field. The Licensor shall, and shall cause its Affiliates and sublicensees, to provide Alzheon with an opportunity to review and comment on any application for regulatory approval of any Vivimind Product for the Vivimind Field at least [ * ] prior to submitting such application to the relevant Regulatory Authority and to [ * ]. |
| 8.3.7 | The Licensor shall perform, and shall cause its Affiliates and sublicensees to perform, all activities with respect to the commercialization of the Vivimind Product in accordance with applicable Laws. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
25.
| 8.3.8 | The Licensor shall keep Alzheon currently, and no less frequently than [ * ], advised of its (and its Affiliates’ and sublicensees’) development and commercialization activities with respect to the Vivimind Products in the Vivimind Field in the Territory. Without limiting the generality of the foregoing, the Licensor shall provide to Alzheon: |
| (a) | on a [ * ] basis, written materials summarizing the activities undertaken by or on behalf of the Licensor with respect to the development and commercialization of Vivimind Products in the Vivimind Field in the Territory; |
| (b) | prompt written notice of the obtaining of any regulatory approval in relation to any Vivimind Product in the Territory; and |
| (c) | prompt written notice of the occurrence of the sale of any Vivimind Product in the Vivimind Field in each country and jurisdiction of the Territory. |
| 8.3.9 | The Licensor shall promptly report to Alzheon any negative health effects associated with the use of any Vivimind Product in the Vivimind Field of which the Licensor becomes aware. Upon Alzheon’s request, the Parties shall meet and discuss the appropriate actions to be taken in response to such negative health effects, including whether the Vivimind Product should be recalled or withdrawn from the market, and the Licensor shall reasonably consider any suggestions or recommendations made by Alzheon. |
| 8.3.10 | In the event that [ * ] and [ * ], then the Option granted to the Licensor under Section 3.2.2 or, if the Option has been exercised and the Parties have executed the Italy Commercialization Agreement, the Italy Commercialization Agreement shall terminate automatically upon the expiration of such [ * ] period without any further action by Alzheon. |
| 8.4 | Alzheon Covenants. In accordance with Section 4.1.2, Alzheon shall assume any and all of the payment and notice obligations set forth in the Parteq Agreement and the TPC Agreement with respect to the Third Party Payments. Licensor shall have the right from time to time (i) to cause Alzheon to direct any Third Party Payment required pursuant to the Parteq Agreement or the TPC Agreement to be paid to the Licensor, which the Licensor shall then pay to the beneficiary thereof on its own behalf under the terms of said agreements or (ii) to cause Alzheon to direct any Third Party Payment required pursuant to the Parteq Agreement or the TPC Agreement directly to the beneficiary thereof under the terms of said agreements. |
| 8.5 | No Other Warranties. |
| 8.5.1 | Except as expressly set forth in Section 8.1 above, nothing in this Agreement is or shall be construed as a warranty or representation by the Licensor: (a) as to the ownership, validity or scope of the Licensed Technology, including the Licensed |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
26.
| Patent Rights and Licensed Know-How hereunder; or (b) that anything used, Developed, modified, enhanced, improved, manufactured, sold, offered for sale, distributed or otherwise disposed of under any license granted pursuant to this Agreement is or will be free from infringement or misappropriation of patents, trademarks, copyrights and other rights of Third Parties. |
| 8.5.2 | EXCEPT AS EXPRESSLY SET FORTH IN THIS ARTICLE 8, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED. THE LICENSOR EXPRESSLY DISCLAIMS, TO THE FULLEST EXTENT PERMITTED BY LAW, ALL OTHER EXPRESS OR IMPLIED WARRANTIES, INCLUDING THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, SATISFACTORY QUALITY AND NON-INFRINGEMENT. IN PARTICULAR, EACH PARTY ACKNOWLEDGES THAT THE RIGHTS GRANTED TO SUCH PARTY HEREIN ARE PROVIDED “AS IS, WHERE IS”. |
| 9. | INSURANCE |
| 9.1 | During the Term, each Party shall carry and maintain at its expense, general comprehensive all risk insurance and product liability insurance, in an amount not less than [ * ] per occurrence. All such insurance shall (a) be issued by insurers of recognized responsibility and who are reasonably acceptable by the other Party, (b) [ * ], and (c) contain a specific clause whereby the other Party shall be notified by the insurer at least [ * ] before any cancellation or termination of any such insurance and a certificate of insurance evidencing same shall be delivered to the other Party prior to any payment being made by the other Party hereunder. Notwithstanding the foregoing, Alzheon shall not be obliged to carry product liability insurance until it commences testing of any Licensed Product in humans. |
| 9.2 | Neither the failure of a Party to comply with any or all of the insurance provisions of this Agreement nor the failure to secure endorsements on the policies as may be necessary to carry out the terms and provisions of this Agreement shall be construed to limit or relieve such Party of any of its obligations under this Agreement including, without limitation, those set out in this Article 9. |
| 10. | INDEMNIFICATION AND LIMITATION OF LIABILITY |
| 10.1 | Alzheon Indemnity. Alzheon shall indemnify, defend and hold harmless the Licensor, its Affiliates and their respective successors, heirs and assigns (the “Licensor Indemnitees”) from and against any liability, damage, loss or expense (including reasonable attorneys’ fees and expenses of litigation) incurred by or imposed upon such the Licensor Indemnitees, or any of them, in connection with any Third Party claims, suits, actions, demands or judgments, including, without limitation, personal injury and product liability matters, to the extent arising out of (a) any actions or omissions, or alleged actions or omissions, of Alzheon and its Affiliates in connection with the use, |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
27.
| Development, making, modification, enhancement or improvement of the Licensed Technology or the Licensed Products, or the use, manufacture, marketing, Sale, offering for Sale, importation or distribution of the Licensed Products, including any claims that any such actions or omissions, or alleged actions or omissions, infringe or misappropriate any Third Party intellectual property rights; (b) any breach or alleged breach of a representation or warranty of Alzheon set forth herein; (c) any breach or alleged breach of this Agreement by Alzheon or, with respect to any Affiliate of Alzheon, any act or omission that would constitute a breach of this Agreement were such act or omission Alzheon’s own; or (d) the gross negligence or willful misconduct on the part of any Alzheon Indemnitee, except in each case to the extent caused by the gross negligence or willful misconduct of any Licensor Indemnitee (including the Licensor) or by breach of this Agreement by the Licensor. |
| 10.2 | Licensor Indemnity. The Licensor shall indemnify, defend and hold harmless Alzheon, its Affiliates and their respective successors, heirs and assigns (the “Alzheon Indemnitees”) from and against any liability, damage, loss or expense (including reasonable attorneys’ fees and expenses of litigation) incurred by or imposed upon such Alzheon Indemnitees, or any of them, in connection with any Third Party claims, suits, actions, demands or judgments, including, without limitation, personal injury and product liability matters, to the extent arising out of (a) any actions or omissions, or alleged actions or omissions, of the Licensor, or its Affiliates, licensees or sublicensees, in connection with the use, development, making, modification, enhancement or improvement of the Licensed Technology or the Licensed Products outside the Field or the Vivimind Products for the Vivimind Field, or the use, manufacture, marketing, Sale, offering for Sale, importation or distribution of the Licensed Products outside the Field or the Vivimind Products in the Vivimind Field, including any claims that any such actions or omissions, or alleged actions or omissions, infringe or misappropriate any Third Party intellectual property rights; (b) any breach or alleged breach of a representation or warranty of the Licensor set forth herein; (c) any breach or alleged breach of this Agreement by the Licensor or, with respect to any Affiliate of the Licensor, any act or omission that would constitute a breach of this Agreement were such act or omission the Licensor’s own; or (d) the gross negligence or willful misconduct on the part of any Licensor Indemnitee, except in each case to the extent caused by the gross negligence or willful misconduct of any Alzheon Indemnitee (including Alzheon) or by breach of this Agreement by Alzheon. |
| 10.3 | Indemnification Procedures. In the event that any Indemnitee is seeking indemnification under Section 10.1 or 10.2 above from a Party (the “Indemnitor”), the Indemnitee shall notify the Indemnitor of such claim with respect to such Indemnitee as soon as reasonably practicable after the Indemnitee receives notice of the claim. The Indemnitor (on behalf of itself and such Indemnitee) shall, at its sole cost and expense, have full control of and authority over the defense of the claim (including the right to settle the claim) and the other Party and the Indemnitee shall, at their own expense, cooperate as requested by the Indemnitor in the defense of the claim; provided, however, that the Indemnitee shall have the right to participate in (but not control) and be represented in any suit or action by independent counsel at its own expense and provided, further, that the Indemnitor may not settle such claim without the Indemnitee’s prior written consent, which shall not be unreasonably withheld or delayed, to the extent that such settlement materially affects the Indemnitee’s rights or obligations hereunder. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
28.
| 10.4 | Consequential Damages. EXCEPT WITH RESPECT TO ANY BREACH OF ARTICLE 5 OR INDEMNIFICATION OBLIGATIONS UNDER ARTICLE 10, IN NO EVENT SHALL EITHER PARTY BE LIABLE FOR ANY CONSEQUENTIAL, INCIDENTAL, INDIRECT, SPECIAL OR PUNITIVE DAMAGES (INCLUDING, BUT NOT LIMITED TO, DAMAGES FOR LOSS OF PROFITS, FOR BUSINESS INTERRUPTION, FOR FAILURE TO MEET ANY DUTY OF GOOD FAITH OR OF REASONABLE CARE OR FOR NEGLIGENCE) ARISING OUT OF OR IN ANY WAY RELATED TO THE USE OF OR INABILITY TO USE THE LICENSED TECHNOLOGY OR OTHERWISE UNDER OR IN CONNECTION WITH ANY PROVISION OF THIS AGREEMENT EVEN IF ANY REPRESENTATIVE OF THE OTHER PARTY HAS BEEN ADVISED OF THE POSSIBILITY THEREOF. |
| 10.5 | Maximum Extent. THE TERMS OF THIS ARTICLE 10 SHALL APPLY TO THE MAXIMUM EXTENT PERMITTED BY APPLICABLE LAW, EVEN IF THIS AGREEMENT OR ANY REMEDY FAILS ITS ESSENTIAL PURPOSE. |
| 11. | TERM AND TERMINATION |
| 11.1 | Term; Expiration. The term of this Agreement shall commence on the Effective Date and, unless earlier terminated under this Article 11, shall continue in full force and effect until the expiration of the last-to-expire Royalty Term (the “Term”). |
| 11.2 | Termination for Breach of Payment. This Agreement and the rights granted herein may be terminated by the Licensor upon any breach of any payment obligation hereunder by Alzheon, effective [ * ] after giving written notice to Alzheon of such termination. If such default or breach is cured within the foregoing [ * ], the notice shall be deemed automatically withdrawn and of no effect. |
| 11.3 | Remedies for Other Breach. |
| 11.3.1 | Subject to Sections 11.2 and 11.4, this Agreement and the rights granted herein may be terminated by either Party upon any material breach of this Agreement by the other Party, effective [ * ] after giving written notice to the breaching Party of such termination. If such default or breach is cured within the foregoing [ * ] period, the notice shall be deemed automatically withdrawn and of no effect. Notwithstanding the foregoing, if a Party is alleged to be in material breach and disputes such allegation through the dispute resolution procedures set forth in this Agreement, then the other Party’s right to terminate this Agreement shall be tolled for so long as such dispute resolution procedures are being pursued in good faith and, if it is finally and conclusively determined that the Party is in material breach, then prior to any termination becoming effective or any remedies being enforced, the breaching Party shall have the right to cure such material breach after such determination within a [ * ] cure period. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
29.
| 11.3.2 | If the Licensor materially breaches this Agreement, then Alzheon may, in lieu of exercising its right to terminate pursuant to Section 11.3.1, elect to continue this Agreement and terminate the Option granted to the Licensor under Section 3.2.2 or, if the Option has been exercised and the Parties have executed the Italy Commercialization Agreement, the Italy Commercialization Agreement. Alzheon shall notify the Licensor of such election in writing prior to the expiration of the [ * ] cure period under Section 11.3.1 and the termination of the Option or Italy Commercialization Agreement, as applicable, shall take effect automatically if the Licensor’s breach or default is not cured within such [ * ] cure period. |
| 11.4 | Termination for Bankruptcy. This Agreement may be terminated by written notice by either Party at any time during the Term if the other Party shall file in any court or agency, pursuant to any bankruptcy and/or insolvency statute or regulation of any state, province or country, a petition in bankruptcy or insolvency or for reorganization or for an arrangement or for the appointment of a receiver or trustee of that Party or of its assets, or if the other Party proposes a written agreement of composition or extension of its debts, or if the other Party shall be served with an involuntary petition against it, filed in any insolvency proceeding, and such petition shall not be dismissed within [ * ] after the filing thereof, or if the other Party shall propose or be a Party to any dissolution or liquidation, or if the other Party shall make an assignment for the benefit of its creditors (each, an “Insolvency Event”). |
| 11.5 | Alzheon Right of Termination. This Agreement may be terminated in its entirety at any time by Alzheon for any reason, effective upon at least [ * ] prior written notice to the Licensor. |
| 11.6 | Effects of Termination. |
| 11.6.1 | Termination by the Licensor for any Reason or by Alzheon without Cause. |
| (a) | Upon any termination of this Agreement by the Licensor hereunder or any termination of this Agreement by Alzheon pursuant to Section 11.5, as of the effective date of such termination, all licenses and sublicenses granted by the Licensor to Alzheon hereunder (other than the licenses and sublicenses granted pursuant to Section 4.3) and each sublicense granted by Alzheon and its Affiliates hereunder shall terminate immediately and automatically unless [ * ]. Alzheon shall immediately assign and transfer to the Licensor, [ * ], all of Alzheon’s right, title and interest in and to all Regulatory Approvals and other submissions to Regulatory Authorities with respect to the Licensed Products in the Territory. Alzheon shall immediately transfer to the Licensor all the Confidential Information disclosed, transferred to it, or generated by or on behalf of the Licensor in the context of this Agreement, including all copies and extracts of documents and all manifestations in whatever form, and all other data, if any, regarding clinical data, materials and information (including, without limitation, all sales and marketing materials) in the possession of Alzheon or one of its Affiliates relating to the Licensed Products or the Licensed Technology. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
30.
| (b) | The rights and obligations of the Parties under Section 7.8 shall survive the termination of this Agreement by the Licensor pursuant to Section 11.2 or 11.4. |
| 11.6.2 | Termination by Alzheon for Cause. |
| (a) | Upon any termination of this Agreement by Alzheon pursuant to Section 11.3 or 11.4, all licenses and sublicenses granted by the Licensor to Alzheon hereunder (other than the licenses and sublicenses granted pursuant to Section 4.3) and each sublicense granted by Alzheon and its Affiliates hereunder shall terminate immediately and automatically unless [ * ]. Alzheon shall immediately assign and transfer to the Licensor all of Alzheon’s right, title and interest in and to all Regulatory Approvals and other submissions to Regulatory Authorities with respect to the Licensed Product in the Territory. Alzheon shall immediately transfer to the Licensor all the Confidential Information disclosed, transferred to it, or generated by or on behalf of the Licensor in the context of this Agreement, including all copies and extracts of documents and all manifestations in whatever form, and all other data, if any, regarding clinical data, materials and information (including, without limitation, all sales and marketing materials) in the possession of Alzheon or one of its Affiliates relating to the Licensed Products or the Licensed Technology. |
| (b) | Notwithstanding any termination by Alzheon, Alzheon shall remain liable for, and shall promptly satisfy, any and all payment obligations which accrued prior to the effective date of termination. |
| 11.6.3 | Right to Sell Inventoried Licensed Products. On the date of termination, Alzheon will cease to use the Licensed Technology or to manufacture, assemble, market, Sell, import or distribute the Licensed Products except and to the extent permitted by this Section 11.6.3: |
| (a) | Except for a termination arising under Section 11.4, for a period not to exceed [ * ] following the date of termination, Alzheon may Sell any Licensed Products remaining in its inventory or stock (“Inventory Sale Period”). In such case, Alzheon will deliver to Licensor an accounting within [ * ] from expiry of the Inventory Sale Period. The accounting will specify, in or on such terms as Licensor may in its sole discretion require, the inventory or stock of Licensed Products manufactured and remaining unSold on expiry of the Inventory Sale Period. All manufactured and remaining unsold inventory or stock of Licensed Products shall be returned to Licensor at the expiry of the Inventory Sale Period, at Licensor’s cost and sole discretion. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
31.
| (b) | Subject to Section 11.6.3(a), Licensor shall provide instructions regarding any unSold Licensed Products, including having such Licensed Products stored, destroyed or Sold under its direction. |
| (c) | Alzheon shall continue to make payments to Licensor in the same manner specified in Article 4 on all Licensed Products that are Sold in accordance with this Section 11.6.3. |
| 11.7 | Remedies. Except as otherwise expressly set forth in this Agreement, the termination provisions of this Article 11 are in addition to any other relief and remedies available to either Party at law. |
| 11.8 | Surviving Provisions. Notwithstanding any provision herein to the contrary, (i) the provisions of Article 1 - Definitions, (ii) the rights and obligations of the Parties set forth in Articles 4 – Payments and Royalties, 5 - Treatment of Confidential Information, 8 – Representations and Warranties, 10 – Indemnification and Limitation of Liability, 12 – Disputes, 13 – Miscellaneous, Section 11.6 – Effects of Termination and this Section 11.8, and (iii) any rights or obligations otherwise accrued hereunder (including any accrued payment obligations) prior to the expiration or the effective date of termination of this Agreement, shall survive the expiration or termination of this Agreement for any reason. |
| 12. | DISPUTES |
| 12.1 | Dispute Resolution Procedures. Any dispute, controversy or claim between the Parties relating to the construction and/or interpretation of this Agreement (a “Dispute”) shall be resolved in accordance with the provisions of this Article 12. |
| 12.2 | First Level. All Disputes (other than disputes subject to expert resolution pursuant to Section 12.5) shall be referred to a senior representative of each Party for review, consideration and resolution. If such individuals are unable to resolve the Dispute within [ * ] after referral of the matter to them, then any of the Parties may by notice in writing submit the Dispute for resolution pursuant to Section 12.4. |
| 12.3 | Mediation. If the Dispute is not resolved within the period referred to in Section 12.2, the Parties shall attempt to resolve the Dispute with the aid of an impartial mediator who will attempt to facilitate resolution. The mediator shall be selected by agreement of the Parties. The mediation shall be treated as a settlement discussion and therefore shall be confidential. If the Dispute has not been resolved within [ * ] after the written notice beginning the mediation process, the mediation shall terminate and the Dispute may be submitted to arbitration in accordance with Section 12.4 within [ * ] following the termination of the mediation process by any Party by means of written notice of submission. |
| 12.4 | Arbitration. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
32.
| 12.4.1 | If any Dispute is not resolved pursuant to Section 12.3, any Party may, within [ * ] after the initiation of the procedures set forth in Section 12.3, refer such Dispute to arbitration by serving written notice of its intention to arbitrate the Dispute to the other Party. |
| 12.4.2 | Arbitration of any Dispute shall be conducted in accordance with the rules of the International Chamber of Commerce (the “ICC Rules”). Such arbitration shall be conducted by one arbitrator appointed in accordance with the ICC Rules. |
| 12.4.3 | All arbitrations shall take place in the City of New York, in the State of New York, in the English language. |
| 12.4.4 | Any decision of the arbitrator shall be final and binding on the Parties. The costs and expenses of the arbitration shall be paid as the arbitrator determines. The Party to whom any amount is owed as a result of an award of the arbitrator shall be entitled to payment within [ * ] of the date of award. |
| 12.4.5 | Each of the Parties agrees to cooperate promptly and fully with the other Party with respect to all aspects of arbitration, including the appointment of the arbitrator and compliance with any requests or orders of the arbitrator. |
| 12.4.6 | During the arbitration proceeding, the Parties shall continue the performance of their respective obligations hereunder in the ordinary course. |
| 12.4.7 | Each Party may apply to any court of competent jurisdiction for appropriate temporary injunctive relief to avoid irreparable harm, maintain the status quo, or preserve the subject matter of the arbitration, in each case pending resolution of any arbitration proceeding. |
| 12.4.8 | EACH PARTY HERETO WAIVES ITS RIGHT TO TRIAL BY JURY OF ANY ISSUE WITHIN THE SCOPE OF THE AGREEMENT TO ARBITRATE AS SET FORTH IN SECTION 12.4.1. |
| 12.4.9 | EACH PARTY HERETO WAIVES ANY CLAIMS FOR ATTORNEYS’ FEES AND COSTS FROM THE OTHER. |
| 12.5 | Expert Disputes. The provisions of this Section 12.5 shall apply if [ * ] the Italy Commercialization Agreement determined by an Expert pursuant to Section 3.2.2(a). |
| 12.5.1 | [ * ]. |
| 12.5.2 | [ * ]. |
| 13. | MISCELLANEOUS |
| 13.1 | Notification. All notices, requests and other communications hereunder shall be in writing, shall be addressed to the receiving Party’s address set forth below or to such other address as a Party may designate by notice hereunder, and shall be either (a) |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
33.
| delivered by hand, (b) made by facsimile transmission (to be followed with written fax confirmation), (c) sent by private courier service providing evidence of receipt, or (d) sent by registered or certified mail, return receipt requested, postage prepaid. The addresses and other contact information for the Parties are as follows: |
| If to the Licensor: | FB Health S.p.A. | |||
| Xxx xxx Xxxxxx, 00 | ||||
| 00000 Xxxxxx Xxxxxx | ||||
| Italy | ||||
| Attention: | Xxxxx Xxxxxxxxx, President and | |||
| Chief Executive Officer | ||||
| Telecopier: | [ * ] | |||
| If to Alzheon: | Alzheon, Inc. | |||
| 000 Xxxxxx Xxxxxx, Xxxxx 0 | ||||
| Xxxxxxxxx, XX 00000 | ||||
| Attention: | Xxxxxx Xxxxx, MD, PhD, Founder, President | |||
| and Chief Executive Officer | ||||
| Telecopier: | [ * ] |
All notices, requests and other communications hereunder shall be deemed to have been given either (i) if by hand, at the time of the delivery thereof to the receiving Party at the address of such Party set forth above, (ii) if made by telecopy or facsimile transmission, at the time that receipt thereof has been acknowledged by electronic confirmation or otherwise, (iii) if sent by private courier, on the third (3rd) business day following the day such notice is delivered to the courier service, or (iv) if sent by registered or certified mail, on the fifth (5th) business day following the day such mailing is made.
| 13.2 | Governing Law. This Agreement will be construed, interpreted and applied in accordance with the Laws of the State of New York and the federal Laws of the United States of America applicable therein, excluding its body of law controlling conflicts of law. The provisions of the U.N. Convention on Contracts for the International Sale of Goods shall not apply to this Agreement. |
| 13.3 | Limitations. Except as expressly set forth elsewhere in this Agreement, neither Party grants to the other Party any right or license to any of its intellectual property. All rights not expressly granted are reserved to their owners. |
| 13.4 | Entire Agreement. This Agreement, including the Schedules and Exhibits attached hereto, and the Letter Agreement collectively constitute the entire agreement between the Parties with respect to the subject matter hereof and supersedes all prior representations, understandings and agreements between the Parties with respect to the subject matter hereof. No modification or amendment to this Agreement shall be effective unless in writing with specific reference to this Agreement and signed by authorized representatives of the Parties. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
34.
| 13.5 | Waiver. The terms or conditions of this Agreement may be waived only by a written instrument executed by the Party waiving compliance. The failure of either Party at any time or times to require performance of any provision hereof shall in no manner affect its rights at a later time to enforce the same. No waiver by either Party of any condition or term shall be deemed as a continuing waiver of such condition or term or of another condition or term. |
| 13.6 | Headings. Section and subsection headings are inserted for convenience of reference only and do not form part of this Agreement. |
| 13.7 | Assignment. |
| 13.7.1 | Except as otherwise provided herein, neither this Agreement nor any right or obligation hereunder may be assigned, delegated or otherwise transferred, in whole or part, by either Party without the prior, express written consent of the other Party, which consent shall not be unreasonably withheld or delayed, except that each Party shall always have the right, without such consent, (a) to perform any or all of its obligations and exercise any or all of its rights under this Agreement through any of its Affiliates; and (b) upon written notice to the other Party, to assign any or all of its rights and delegate or subcontract any or all of its obligations under this Agreement to (i) any of its Affiliates or (ii) a successor of all or substantially all of assets or business to which this Agreement relates, whether by way of merger, amalgamation, sale of stock, sale of assets or other transaction (or series of transactions). Each Party shall remain responsible for any failure to perform by any of its Affiliates to which it assigns, delegates or otherwise transfers any rights or obligations under this Agreement in accordance with this Section 13.7.1. Any permitted assignee shall assume all obligations of its assignor under this Agreement. Any purported assignment, delegation or other transfer in violation of this Section 13.7.1 shall be void. The terms and conditions of this Agreement shall be binding upon and inure to the benefit of the permitted successors and assigns of the Parties. |
| 13.7.2 | The Licensor shall not sell, assign or otherwise transfer the Xxxxxx License Agreement or any Licensed Technology to any Person (including any Affiliate of the Licensor) except to (x) a wholly-owned direct or indirect subsidiary of the Licensor or (y) a successor corporation or entity in accordance with Section 13.7.1(b)(ii), and solely in each case if, prior to any such sale, assignment or transfer, such transferee has acknowledged and confirmed in writing to Alzheon that, effective as of such sale, assignment or other transfer, such transferee shall be bound by this Agreement as if it were a party to it as and to the identical extent applicable to the Licensor with respect to the Xxxxxx License Agreement and Licensed Technology. |
| 13.8 | Non-Solicitation. During the Term and [ * ], neither Party shall, directly or indirectly, through any Affiliate, officer, director, agent or otherwise, solicit for employment any employee or contractor of the other Party, provided that the hiring of such employees through the use of general advertisements in publications shall be deemed not to violate this Section 13.8. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
35.
| 13.9 | Force Majeure. Neither Party shall be liable for failure of or delay in performing obligations set forth in this Agreement, and neither shall be deemed in breach of its obligations, if such failure or delay is due to natural disasters or any causes beyond the reasonable control of such Party. In event of such force majeure, the Party affected thereby shall use reasonable efforts to cure or overcome the same and resume performance of its obligations hereunder. |
| 13.10 | No Third-Party Beneficiary. The terms and provisions of this Agreement are intended solely for the benefit of each Party hereto, and it is not the intention of the Parties hereto to confer third-party beneficiary rights upon any other Person, other than the rights granted to Xxxxxx under Section 4.5 and the Alzheon Indemnitees and the Licensor Indemnitees in Article 10. |
| 13.11 | Construction. The Parties hereto acknowledge and agree that: (a) each Party and its counsel have reviewed and negotiated the terms and provisions of this Agreement and have contributed to its revision; (b) the rule of construction to the effect that any ambiguities are resolved against the drafting Party shall not be employed in the interpretation of this Agreement; and (c) the terms and provisions of this Agreement shall be construed fairly as to all Parties hereto and not in favor of or against any Party, regardless of which Party was generally responsible for the preparation of this Agreement. |
| 13.12 | Severability. If any provision(s) of this Agreement are or become invalid, are ruled illegal by any court of competent jurisdiction or are deemed unenforceable under then current applicable Law from time to time in effect during the Term, it is the intention of the Parties that the remainder of this Agreement shall not be affected thereby provided that a Party’s rights under this Agreement are not materially affected. The Parties hereto covenant and agree to renegotiate any such term, covenant or application thereof in good faith in order to provide a reasonably acceptable alternative to the term, covenant or condition of this Agreement or the application thereof that is invalid, illegal or unenforceable, it being the intent of the Parties that the basic purposes of this Agreement are to be effectuated. |
| 13.13 | Status The status of each Party under this Agreement shall be that of an independent contractor. Nothing in this Agreement is intended or shall be deemed to constitute a partner, agency, employer-employee, or joint venture relationship between the Parties or, except as expressly provided in this Agreement, to grant either Party the authority to bind or contract any obligation in the name of or on the account of the other Party or to make any statements, representations, warranties or commitments on behalf of the other Party. |
| 13.14 | Further Assurances. Each Party agrees to execute, acknowledge and deliver such further instructions, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
36.
| 13.15 | Counterparts. This Agreement may be executed simultaneously in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. |
| 13.16 | Interpretation. Unless a context otherwise requires, wherever used, (a) the singular shall include the plural, the plural the singular; (b) the use of any gender shall be applicable to all genders; (c) the word “or” is used in the inclusive sense (and/or); (d) the word “including” is used without limitation and shall mean “including without limitation”; and (e) all amounts set forth in this Agreement are expressed in Canadian dollars. |
| 13.17 | Headings. Section and subsection headings are inserted for convenience of reference only and do not form a part of this Agreement. |
[signature page follows]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
37.
Execution Version
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their duly authorized representative in originals.
| ALZHEON, INC. | FB HEALTH S.p.A. | |||||||
| Per: | /s/ Xxxxxx Xxxxx | Per: | /s/ Xxxxx Xxxxxxxxx | |||||
| Name: | Xxxxxx Xxxxx, MD, PhD | Name: | Xxxxx Xxxxxxxxx | |||||
| Title: | Founder, President and Chief Executive Officer | Title: | President and Chief Executive Officer |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE A
Licensed Patents
[ * ]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE B
BLU8499 Patents
[ * ]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE C
Excluded Disorders, Conditions and Diseases
[ * ]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
EXHIBIT A
Terms of Italy Commercialization Agreement
[ * ]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
AMENDMENT NO. 1 TO
LICENSE AGREEMENT
This Amendment No. 1 to License Agreement (this “Amendment”), effective as of February 27, 2015 (the “Amendment Effective Date”), amends the License Agreement effective as of October 22, 2013 by and between FB Health S.p.A., a company organized and existing under the laws of Italy (“Licensor”), and Alzheon, Inc., a Delaware (USA) corporation (“Alzheon”) (the “Agreement”). Capitalized terms used herein and not otherwise defined shall have the meanings ascribed to them in the Agreement.
WHEREAS Licensor and Alzheon desire to amend, in accordance with Section 13.4 of the Agreement, various aspects of the Agreement to, among other things: (i) expand the defined field of Alzheon’s licenses for certain Licensed Products; and (ii) modify certain specific diligence requirements.
NOW, THEREFORE, in consideration of the mutual covenants contained herein and for other good and valuable consideration, Licensor and Alzheon, intending to be legally bound, hereby agree that, as of the Amendment Effective Date:
1. Section 1 of the Agreement is hereby amended by:
a. inserting the following definitions in the appropriate alphabetical locations:
““ALZ-801 Compound” means the compound identified as such on Schedule D attached hereto and referred to by Alzheon as of the Amendment Effective Date as ALZ-801. For clarity, the ALZ-801 Compound is not Tramiprosate.”
““ALZ-801 Licensed Product” means each Licensed Product that contains (i) the ALZ-801 Compound and/or (ii) any prodrug, analog, salt, free acid, free base, clathrate, solvate, hydrate, hemihydrate, anhydride, ester, chelate, conformer, congener, crystal form, crystal habit, polymorph, amorphous solid, homolog, isomer, stereoisomer, enantiomer, racemate, isotopic or radiolabeled equivalent, metabolite or conjugate of the ALZ-801 Compound; provided that the compounds described in subsection (ii) shall exclude Tramiprosate. Notwithstanding anything to the contrary in this Agreement, if a Licensed Product falls within the definition of both an ALZ-801 Licensed Product and a Tramiprosate Licensed Product, it will be an ALZ-801 Licensed Product.
““Tramiprosate” means the compound identified as such on Schedule D attached hereto and referred to by Alzheon as of the Amendment Effective Date as tramiprosate.”
““Tramiprosate Licensed Product” means each Licensed Product that contains (i) Tramiprosate and/or (ii) any analog, salt, free acid, free base, clathrate, solvate, hydrate, hemihydrate, anhydride, ester, chelate, conformer, congener, crystal form, crystal
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
habit, polymorph, amorphous solid, homolog, isomer, stereoisomer, enantiomer, racemate, isotopic or radiolabeled equivalent, metabolite or conjugate of Tramiprosate. Notwithstanding anything to the contrary in this Agreement, if a Licensed Product falls within the definition of both an ALZ-801 Licensed Product and a Tramiprosate Licensed Product, it will be an ALZ-801 Licensed Product.”
b. deleting the definition of “Field” in its entirety and replacing it with the following definition:
““Field” shall mean:
(a) as applied to the ALZ-801 Compound and ALZ-801 Licensed Products, any and all uses, excluding any use in the mitigation, prevention, treatment, control or diagnosis of AA Amyloidosis;
(b) as applied to Tramiprosate and Tramiprosate Licensed Products, any and all uses excluding (i) any use within the Vivimind Field, and (ii) any use in the mitigation, prevention, treatment, control or diagnosis of AA Amyloidosis; and
(c) as applied to any Licensed Products other than the ALZ-801 Licensed Products and the Tramiprosate Licensed Products, the mitigation, prevention, treatment, control or diagnosis of [ * ]. For avoidance of doubt, the Field specifically excludes [ * ].
c. adding the following sentence to the end of the existing definition of “Licensed Product”
““For clarity, Licensed Products include ALZ-801 Licensed Products and Tramiprosate Licensed Products.”
2. Section 2.2 of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“Restriction to License. For avoidance of doubt, the Parties agree that:
a. Alzheon is not granted any rights hereunder to use, have used, Develop, have Developed, make, have made, modify, have modified, enhance, have enhanced, improve, have improved the Licensed Technology or the Licensed Products outside the Field, or to manufacture, have manufactured, market, sell, offer for sale, have sold, import, have imported, distribute or have distributed the Licensed Products outside the Field;
b. Licensor shall retain no rights to use, have used, Develop, have Developed, make, have made, modify, have modified, enhance, have enhanced, improve, have improved the ALZ-801 Compound, or Tramiprosate, the Licensed Technology or the Licensed Products for the Field, or to manufacture, have manufactured, market, sell, offer for sale, have sold, import, have imported, distribute or have distributed, the ALZ-801 Compound, Tramiprosate or the Licensed Products in the Field; and
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
c. Licensor shall not, during the Term, Develop, have Developed, manufacture, have manufactured, market, sell, offer for sale, have sold, import, have imported, distribute or have distributed any compound or product for the mitigation, prevention, treatment, control or diagnosis of AA Amyloidosis, or cause or assist, directly or indirectly, any Third Party to do any of the foregoing acts.”
3. Section 3.1.2 of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“Alzheon Diligence Obligations. In accordance with Section 2.3 of the Xxxxxx License Agreement, Alzheon shall fully satisfy, on behalf of the Licensor, the following obligations:
(a) during the Term, Alzheon shall use Commercially Reasonable Efforts to Develop at least one Licensed Product in the Field in the Territory, and after obtaining Regulatory Approval therefor, to commercialize such Licensed Product in the Field;
(b) Alzheon shall, or shall cause one of its sublicensees to, dose the first healthy individual in any study of a Licensed Product in the Field no later than [ * ]; and
(c) Alzheon shall, or shall cause one of its sublicensees to, dose the first patient in a clinical trial of a Licensed Product in the Field no later than [ * ].”
4. Section 3.4 of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“Ownership of Submissions and Regulatory Approvals. Subject to Section 11.6, Alzheon shall own all Regulatory Approvals for Licensed Products and any other submissions to any Regulatory Authority with respect to Licensed Products in the Field in the Territory.”
5. Section 13.1 of the Agreement is hereby amended by deleting the address for notices to Alzheon and replacing it with the following:
| If to Alzheon: | Alzheon, Inc. | |
| 000 Xxxxx Xxxxxx, Xxxxx 000 | ||
| Xxxxxxxxxx, XX 00000 | ||
| Attention: Xxxxxx Xxxxx, M.D., Ph.D. | ||
| President and CEO | ||
| Telecopier: [ * ] |
6. The Agreement is hereby amended by attaching Schedule D attached hereto as new Schedule D to the Agreement.
7. As consideration for the entering into of this Amendment, Alzheon hereby agrees to issue 72,992 of its common shares (on the same terms and conditions as its most recent financing completed on February 12, 2015 (as adjusted to reflect any consolidation, share split or other share reorganization completed since that date by Alzheon)) to a designee of Licensor, which shall be BHI Limited Partnership. For avoidance of doubt, such shares shall represent a payment amount equal to [ * ].
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
8. Except as expressly amended by this Amendment, all of the terms and conditions of the Agreement shall remain in full force and effect.
9. This Amendment will be construed, interpreted and applied in accordance with the Laws of the State of New York and the federal Laws of the United States of America applicable therein, excluding its body of law controlling conflicts of law. The provisions of the U.N. Convention on Contracts for the International Sale of Goods shall not apply to this Amendment.
10. This Amendment may be executed simultaneously in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
[Signature Page follows]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
IN WITNESS WHEREOF Licensor and Alzheon have executed this Amendment as of the respective dates set forth below.
| ALZHEON, INC. | FB HEALTH S.p.A. | |
| By: /s/ Xxxxxx Xxxxx | By: /s/ Xxxxx Xxxxxxxxx | |
| Name: Xxxxxx Xxxxx | Name: Xxxxx Xxxxxxxxx | |
| Title: President and CEO | Title: Chief Executive Officer | |
| Date: February 27, 2015 | Date: February 26, 2015 |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Schedule D
DESCRIPTION OF THE ALZ-801 COMPOUND and TRAMIPROSATE
| Tramiprosate
Chemical IUPAC Name: 3-amino-1-propanesulfonate
Structural Formula:
| ALZ-801 Compound
Chemical IUPAC Name: 3-(2-amino-3-methylbutanamido)propane-1-sulfonic acid)
Structural Formula:
|
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
| CONFIDENTIAL COPY | EXECUTION |
AMENDMENT NO. 2 TO
LICENSE AGREEMENT
This Amendment No. 2 to License Agreement (this “Second Amendment”), effective as of June 30, 2016 (the “Second Amendment Effective Date”), amends the License Agreement effective as of October 22, 2013 by and between FB Health S.p.A., a company organized and existing under the laws of Italy (“Licensor”), and Alzheon, Inc., a Delaware (USA) corporation (“Alzheon”), as previously amended by Amendment No. 1 effective as of February 27, 2015 (collectively, the “Agreement”). Capitalized terms used herein and not otherwise defined shall have the meanings ascribed to them in the Agreement.
WHEREAS Licensor and Alzheon desire to further amend the Agreement in accordance with Section 13.4 of the Agreement.
NOW, THEREFORE, in consideration of the mutual covenants contained herein and for other good and valuable consideration, Licensor and Alzheon, intending to be legally bound, hereby agree that, as of the Second Amendment Effective Date:
1. Section 1 of the Agreement is hereby amended by:
a. Inserting the following definition in the appropriate alphabetical location:
““Improvement Licensed Product” means a product, the composition of matter, manufacture or use of which is claimed in a Pending Claim or Valid Claim of a Patent Right included in part (ii) of the definition of “Licensed Patent Rights”.”
b. deleting the definition of “Field” in its entirety and replacing it with the following definition:
““Field” shall mean:
(a) as applied to the ALZ-801 Compound, ALZ-801 Licensed Products and, subject to the proviso below, Improvement Licensed Products, any and all uses, excluding [ * ];
(b) as applied to Tramiprosate and Tramiprosate Licensed Products, any and all uses excluding (i) [ * ], and (ii) [ * ]; and
(c) as applied to any Licensed Products other than the ALZ-801 Licensed Products, Tramiprosate Licensed Products and, to the extent set forth in the proviso below, Improvement Licensed Products, the mitigation, prevention, treatment, control or diagnosis of [ * ]. For avoidance of doubt, the Field specifically excludes [ * ];
[ * ].”
c. deleting the last sentence at the end of the existing definition of “Licensed Product” and replacing it with the following:
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
“For clarity, Licensed Products include ALZ-801 Licensed Products, Tramiprosate Licensed Products and Improvement Licensed Products.”
2. Section 2.2(c) of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“Licensor and its Affiliates shall not, during the Term, Develop, have Developed, manufacture, have manufactured, market, sell, offer for sale, have sold, import, have imported, distribute or have distributed any Licensed Product for the mitigation, prevention, treatment, control or diagnosis of [ * ], or cause or assist, directly or indirectly, any Third Party to do any of the foregoing acts.”
3. Section 3.1.2 of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“Alzheon Diligence Obligations. In accordance with Section 2.3 of the Xxxxxx License Agreement, Alzheon shall fully satisfy, on behalf of the Licensor, the following obligations:
(a) during the Term, Alzheon shall use Commercially Reasonable Efforts to [ * ];
(b) Alzheon shall, or shall cause one of its sublicensees to, [ * ]; and
(c) Alzheon shall, or shall cause one of its sublicensees to, [ * ].”
4. Section 3.1.3 of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“Arising Intellectual Property. During the Term, all right, title and interest in and to any enhancement, invention or discovery created, conceived, identified, or reduced to practice by Alzheon, by any of its Affiliates, by a Third Party on behalf of Alzheon, or by any sublicensee, that relies on or incorporates the Licensed Technology (an “Improvement”) [ * ].”
5. Section 11.6.1(a) of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“(a) Upon any termination of this Agreement by the Licensor hereunder or any termination of this Agreement by Alzheon pursuant to Section 11.5, as of the effective date of such termination, all licenses and sublicenses granted by the Licensor to Alzheon hereunder (other than the licenses and sublicenses granted pursuant to Section 4.3) shall terminate immediately and automatically. Each sublicense granted directly to a Third Party by Alzheon or its Affiliate that is then in effect shall survive termination as if granted directly by Licensor to the applicable sublicensee, provided that (i) such sublicense was granted in compliance with Section 2.3, (ii) [ * ]. Alzheon shall immediately assign and transfer to the Licensor, [ * ], all of Alzheon’s and its Affiliates’ right, title and interest in and to all Regulatory Approvals and other submissions to Regulatory Authorities with respect to the Licensed Products in the Territory. Alzheon shall immediately transfer to the Licensor all the Confidential Information disclosed,
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
transferred to it, or generated by or on behalf of the Licensor in the context of this Agreement, including all copies and extracts of documents and all manifestations in whatever form, and all other data, if any, regarding clinical data, materials and information (including, without limitation, all sales and marketing materials) in the possession of Alzheon or one of its Affiliates relating to the Licensed Products or the Licensed Technology.”
6. Section 11.6.2(a) of the Agreement is hereby amended by deleting the text thereof in its entirety and replacing it with the following:
“(a) Upon any termination of this Agreement by Alzheon pursuant to Section 11.3 or 11.4, all licenses and sublicenses granted by the Licensor to Alzheon hereunder (other than the licenses and sublicenses granted pursuant to Section 4.3) hereunder shall terminate immediately and automatically. Each sublicense granted directly to a Third Party by Alzheon or its Affiliate that is then in effect shall survive termination as if granted directly by Licensor to the applicable sublicensee, provided that (i) such sublicense was granted in compliance with Section 2.3 (ii) [ * ]. Alzheon shall immediately assign and transfer to the Licensor all of Alzheon’s and its Affiliates’ right, title and interest in and to all Regulatory Approvals and other submissions to Regulatory Authorities with respect to the Licensed Product in the Territory. Alzheon shall immediately transfer to the Licensor all the Confidential Information disclosed, transferred to it, or generated by or on behalf of the Licensor in the context of this Agreement, including all copies and extracts of documents and all manifestations in whatever form, and all other data, if any, regarding clinical data, materials and information (including, without limitation, all sales and marketing materials) in the possession of Alzheon or one of its Affiliates relating to the Licensed Products or the Licensed Technology.”
7. Except as expressly amended by this Second Amendment, all of the terms and conditions of the Agreement shall remain in full force and effect. From and after the Second Amendment Effective Date, all references to the Agreement shall mean the Agreement as amended by this Second Amendment.
8. This Second Amendment will be construed, interpreted and applied in accordance with the Laws of the State of New York and the federal Laws of the United States of America applicable therein, excluding its body of law controlling conflicts of law. The provisions of the U.N. Convention on Contracts for the International Sale of Goods shall not apply to this Second Amendment.
9. This Second Amendment may be executed simultaneously in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
[Signature Page Follows]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
IN WITNESS WHEREOF Licensor and Alzheon have executed this Second Amendment as of the respective dates set forth below.
| ALZHEON, INC. | FB HEALTH S.p.A. | |||||||
| Per: | /s/ Xxxxxx Xxxxx | Per: | /s/ Xxxxx Xxxxxxxxx | |||||
| Name: | Xxxxxx Xxxxx, MD, PhD | Name: | Xxxxx Xxxxxxxxx | |||||
| Title: | Founder, President and Chief Executive Officer | Title: | President and Chief Executive Officer |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.