RIGHT OF REFERENCE AND LICENSE AGREEMENT
Certain identified information has been excluded from the exhibit because it is both (i) not material and (ii) would likely cause competitive harm to the Company, if publicly disclosed. Double asterisks denote omissions.
RIGHT OF REFERENCE AND LICENSE AGREEMENT
THIS RIGHT OF REFERENCE AND LICENSE AGREEMENT (this “Agreement”), dated as of February 8, 2019 (the “Effective Date”), is made and entered into by and between GlaxoSmithKline Intellectual Property (No. 2) Limited, a company organized under the laws of England and Wales and having a place of business at 000 Xxxxx Xxxx Xxxx, Xxxxxxxxx, Xxxxxxxxx XX0 0XX England (“GIP2”), GlaxoSmithKline LLC, a Delaware limited liability company having a place of business at 0000 X. Xxxxxxxxxxxx Xxxx, Xxxxxxxxxxxx, XX 00000-0000 (“GSK LLC”) and Glaxo Group Limited, a company organized under the laws of England and Wales and having a place of business at 000 Xxxxx Xxxx Xxxx, Xxxxxxxxx, Xxxxxxxxx XX0 0XX England (“GGL”) (XXX0, XXX LLC and GGL are collectively referred to herein as “GSK”), and Fulcrum Therapeutics, Inc., a Delaware corporation having a place of business at 00 Xxxxxxxxxx Xxxxxx, Xxxxxxxxx, XX 00000 (“Fulcrum”). GSK and Fulcrum may be referred to herein individually as a “Party” and collectively as the “Parties”.
RECITALS
WHEREAS, GSK (or its Affiliate) is the owner of the Losmapimod IND and the GSK Intellectual Property (each as defined below);
WHEREAS, Fulcrum desires to obtain a right of reference to the Losmapimod IND and an exclusive license under the GSK Intellectual Property; and
WHEREAS, GSK desires to grant to Fulcrum such rights in accordance with the terms and conditions of this Agreement and the Equity Documents (defined below), which will be executed simultaneously with this Agreement.
NOW THEREFORE, in consideration of the premises and mutual covenants herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
ARTICLE I
DEFINITIONS
Section 1.1. Definitions.
The following terms, as used herein, have the following meanings:
“Affiliate” means any Person that directly or indirectly, whether de jure or de facto, is controlled by, controlling or under common control with, a Party, but only for so long as such control shall continue. For purposes of this definition, “control” (including, with correlative meanings, “controlled by”, “controlling” and “under common control with”) means, with respect
to a Person, possession, direct or indirect, of (a) the power to direct or cause direction of the management and policies of such Person (whether through ownership of securities or partnership or other ownership interests, by contract or otherwise), or (b) at least 50% of the voting securities (whether directly or pursuant to any vested and exercisable option, warrant or other similar arrangement) or other comparable equity interests of such Person. A Person shall cease to be an “Affiliate” under this Agreement upon the date that such Person no longer satisfies the requirements set forth in this definition.
“Business Day” means any day except Saturday, Sunday or any day on which banks are generally not open for business in New York, NY United States or London, England, but in any event excluding the nine (9) consecutive calendar days beginning on December 24th and continuing through and including January 1st of each Calendar Year during the Term.
“Calendar Quarter” means each of the consecutive three (3) month periods ending on March 31, June 30, September 30 and December 31 of any Calendar Year.
“Calendar Year” means each twelve (12) month period commencing on January 1 and ending on December 31.
“Commercially Reasonable Efforts” means, with respect to Fulcrum’s obligations under this Agreement, the carrying out of such obligations or tasks with a level of efforts and resources consistent with the commercially reasonable practices of a company of similar size and resources in the biopharmaceutical industry for the research, development or commercialization of a pharmaceutical product of commercial potential comparable to that of the applicable Licensed Product at a similar stage of development or commercialization, taking into account efficacy, safety, patent and regulatory exclusivity, anticipated or approved labeling, present and future market potential, competitive market conditions, costs and risks of development and commercialization, relevant scientific and technical considerations and the profitability of the applicable Licensed Product in light of pricing and reimbursement issues; provided, that Fulcrum shall not take into consideration any amounts owed to GSK under this Agreement. Commercially Reasonable Efforts shall be determined on a market-by-market and Licensed Product-by-Licensed Product basis, and it is anticipated that the level of efforts required shall be different for different markets and Licensed Products and shall change over time, reflecting changes in the status of the Licensed Product and markets involved.
“Compound” means the small molecule p38 inhibitor known as Losmapimod, which molecule is identified on Schedule 1.1 of this Agreement, attached hereto and incorporated herein.
“Control” means, with respect to any item of Data, Patents or other proprietary right, the possession of the right, whether directly or indirectly, and whether by ownership, license, or other right (other than pursuant to this Agreement), to grant an exclusive license under such Data, Patents or other proprietary right.
“Data” means, with respect to the Compound, (i) the documents and data stored on an encrypted hard drive transferred to Fulcrum by GSK prior to the Effective Date and identified
within GSK as “FT04Feb2019” and (ii) the documents, data and information required to be delivered pursuant to Section 2.5(b).
“Debarred” or “Debarment” means, with respect to a Person, that such Person (a) is debarred by the FDA pursuant to Section 306 of the United States Federal Food, Drug and Cosmetic Act, or is the subject of conviction described in such section (or is subject to a similar sanction of any other applicable Regulatory Authority), (b) is the subject of an FDA debarment investigation or proceeding (or similar proceeding of any other applicable Regulatory Authority), or (c) has been charged with or convicted under U.S. Law for conduct relating to the development or approval, or otherwise relating to the regulation, of any product under the Generic Drug Enforcement Act of 1992.
“EMA” means the European Medicines Agency and any successor entity.
“Equity Documents” has the meaning set forth in Section 3.1 of this Agreement.
“Exploit” means to research, develop, design, make, use, sell, offer for sale, license, lease, supply, distribute, provide, perform, practice, import, export or otherwise make available, commercialize or exploit any technology, or to allow any Person to do any of the foregoing.
“EU” means the economic, scientific and political organization of member states known as the European Union, as its membership may be altered from time to time, and any successor thereto. Notwithstanding the foregoing, the EU shall be deemed to include the United Kingdom for purposes of this definition regardless of whether or not the United Kingdom is a member state of the European Union.
“FDA” means the United States Food and Drug Administration and any successor agency thereto.
“Field” means all therapeutic uses in humans.
“First Commercial Sale” means, with respect to a Licensed Product in a particular country, the first sale of such Licensed Product by a Selling Party to a Third Party for end use or consumption in such country.
“FSHD” means facioscapulohumeral muscular dystrophy.
“Generic Version” means a product that is (i) approved for the same indication as a Licensed Product and contains the Compound; and (ii) sold by a Third Party that is not a Sublicensee of Fulcrum, under a Regulatory Approval granted to a Third Party.
“GSK Intellectual Property” means the GSK Patents and the Data.
“GSK Patents” means the Patents listed on Schedule 1.2 attached hereto and incorporated herein, and all counterpart Patents thereof, and all corresponding Patents throughout the world.
“IND” means an investigational new drug application filed with the FDA.
“Indemnified Party” has the meaning set forth in Section 8.3(a).
“Law” means any federal, state, local, national or supra-national law (both common and statutory law and civil and criminal law), treaty, convention, rule, directive, legislation, ordinance, regulatory code (including, without limitation, statutory instruments, guidance notes, circulars, directives, decisions, rules and regulations) or similar provision having the force of law or an order of any Regulatory Authority or any self-regulatory organization.
“Licensed Product” means any pharmaceutical preparation that contains the Compound as an active pharmaceutical ingredient, in any and all forms, presentations, delivery systems, dosages and formulations.
“Losmapimod IND” means IND No. [**], previously filed with FDA by GSK or its Affiliates for the Compound, and all amendments of any of the foregoing.
“Marketing Authorization Application” means: (a) in the United States, a New Drug Application (as defined in Title 21, Section 314.50 et seq. of the U.S. Code of Federal Regulations or any successor regulations), including any amendment or supplement thereto, and (b) in any other country or regulatory jurisdiction, an application for Regulatory Approval required for marketing or sale of a Licensed Product in such country or regulatory jurisdiction, including any amendment or supplement thereto.
“Net Sales” means the gross amounts billed or invoiced by Fulcrum, its Affiliates and Sublicensees (each, a “Selling Party”) to any Third Party that is not a Sublicensee with respect to sales of Licensed Products in the Territory, less the following:
(a) discounts, credits, refunds, wholesaler allowances, inventory management fees and rebates actually allowed by Fulcrum, its Affiliates or Sublicensees with respect to Licensed Products;
(b) sales, import, export, customs, and value added taxes, and duties directly imposed on the Licensed Products and actually paid by Fulcrum, its Affiliates or Sublicensees, excluding taxes on the net income of Fulcrum, its Affiliates and Sublicensees;
(c) outbound freight, postage, duties, transportation, handling and insurance costs actually paid by Fulcrum, its Affiliates or Sublicensees with respect to Licensed Products;
(d) billed or invoiced amounts written off as uncollectible by Fulcrum, its Affiliates or Sublicensees with respect to Licensed Products, provided that if any such written off amount is thereafter collected, such amount shall be included in Net Sales in the Calendar Quarter in which it is collected;
(e) annual fees due under Section 9008 of the United States Patient Protection and Affordable Care Act of 2010 (Pub. L. No. 111-48) allocable to sales of Licensed Product; and
(f) amounts actually allowed or credited on returns of sales of Licensed Products by Fulcrum, its Affiliates or their Sublicensees.
In no event shall any particular amount identified above be deducted more than once in calculating Net Sales (i.e., no “double counting” of deductions). All discounts, allowances, credits, rebates, and other deductions shall be fairly and equitably allocated to the Licensed Product and other product(s) of Fulcrum, and of its Affiliates and Sublicensees, such that the Licensed Products do not bear a disproportionate portion of such deductions.
Such amounts shall be determined in accordance with either (i) U.S. Generally Accepted Accounting Principles (“GAAP”) or (ii) International Financial Reporting Standards (“IFRS”) depending on the accounting standard used by the applicable Selling Party in such Selling Party’s financial reporting, case consistently applied.
“Patent” means all: (a) letters patent (including inventor’s certificates), including any substitution, extension, registration, confirmation, validation, reissue, re-examination, supplementary protection certificates, confirmation patents, patent of additions, renewal or any like filing thereof; (b) pending applications for letters patent (including applications for inventor’s certificates), including any continuation, division or continuation-in-part thereof and any provisional applications; and (c) any United States and international counterparts to any of (a) and (b) above.
“Person” means an individual, corporation, partnership, limited liability company, association, trust or other entity or organization, including a government or political subdivision or an agency or instrumentality thereof.
“Phase 1 Clinical Trial” means a study of a Licensed Product in healthy individuals or patients, the principal purpose of which is a preliminary determination of safety, tolerability, pharmacological activity or pharmacokinetics, as further defined in 21 C.F.R. 312.21(a), or the corresponding regulations in any jurisdiction or country other than the United States, or any amended or successor regulations, to permit the design of further clinical trials.
“Phase 2 Clinical Trial” means a study of a Licensed Product in the Field in human patients designed or intended to determine initial efficacy, pharmacological effect or dose range or regimen, as further defined in 21 C.F.R. 312.21(b), or the corresponding regulations in any jurisdiction or country other than the United States, or any amended or successor regulations, to permit the design of further clinical trials.
“Phase 3 Clinical Trial” means a pivotal study in the Field in human patients with a defined dose or a set of defined doses of a Licensed Product designed or intended to ascertain efficacy and safety of such Licensed Product for the purpose of enabling the preparation and submission of a Marketing Authorization Application to the competent Regulatory Authority in a country of the Territory, as further defined in 21 C.F.R. 312.21(c), or the corresponding regulations in any jurisdiction or country other than the United States, or any amended or successor regulations.
“Regulatory Approval” means any and all approvals (including supplements, amendments, and pre- and post-approvals), licenses, registrations or authorizations (or waivers) of any national, supra-national (e.g., the European Commission or the Council of the EU), regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, that are necessary for the manufacture, distribution, use, import, transport, promotion, marketing, offer for sale or sale of a product in a regulatory jurisdiction; but excluding any pricing and reimbursement approval.
“Regulatory Authority” means the applicable national (e.g., the FDA), supra-national (e.g., the European Commission or the Council of the EU), regional, state or local regulatory agency, department, bureau, commission, council or other governmental authority that, in each case, regulates or governs the Exploitation of the Compound or Licensed Product or the granting of Regulatory Approval of a Licensed Product in a regulatory jurisdiction.
“Regulatory Exclusivity” means any exclusive marketing rights or data exclusivity rights granted by a Regulatory Authority (other than Patents) with respect to a Licensed Product sold in a given country, including orphan drug exclusivity, new chemical entity exclusivity, data exclusivity or pediatric exclusivity, which confers an exclusive commercialization period during which Fulcrum or its Affiliates or Sublicensees have the exclusive right to market and sell a Licensed Product in such country.
“Senior Management Designees” has the meaning set forth in Section 10.4(b) of this Agreement.
“Service Provider” means a contract research organization, a contract manufacturing organization or other similar service provider that provides drug development services to Fulcrum.
“Spreadsheet” has the meaning set forth in Section 2.5(b) of this Agreement.
“Sublicensee” means a Third Party that has been granted a sublicense under the rights granted to Fulcrum pursuant to Section 2.3 of this Agreement.
“Supplemental Data” has the meaning set forth in Section 2.5(c) of this Agreement.
“Term” has the meaning set forth in Section 9.1 of this Agreement.
“Territory” means worldwide.
“Third Party” means any Person other than GSK or Fulcrum or any of their respective Affiliates.
“Third Party Claim” means any, action, suit, proceeding, liability or obligation brought by a Third Party.
ARTICLE II
LICENSE GRANT; DATA TRANSFER
Section 2.1. Right of Reference.
GSK hereby grants Fulcrum a right of reference as to the Losmapimod IND. Within [**] following the Effective Date GSK shall deliver, or shall cause to be delivered, to Fulcrum an executed Letter of Authorization (in the form of Exhibit A) to the FDA confirming that Fulcrum, its Affiliates and its Sublicensees have the right to reference the Losmapimod IND in connection with their Exploitation of the Compound and Licensed Products as provided under this Agreement.
Section 2.2. License Grant.
Subject to the terms and conditions of this Agreement, GSK, on behalf of itself and its Affiliates, shall and hereby does, effective as of the Effective Date, grant Fulcrum an exclusive (subject to Section 2.4), royalty-bearing, sublicensable (subject to Article IV) license under the GSK Intellectual Property to Exploit the Compound and Licensed Products in the Field in the Territory.
Section 2.3. Sublicenses.
(a) Subject to Sections 2.3(b) and 4.1, Fulcrum may sublicense the rights granted to it by GSK under Section 2.2. Each sublicense to a Sublicensee must be granted pursuant to a written sublicense agreement consistent with the requirements of this Agreement applicable thereto, and Fulcrum shall provide to GSK, within [**] after the execution of such sublicense agreement, a complete copy thereof, which copy Fulcrum may redact for financial terms and other information not required to evidence Fulcrum’s compliance with the sublicensing terms of this Agreement.
(b) Commencing on the Effective Date and continuing until the date on which Fulcrum first submits an IND to the FDA for a Licensed Product, Fulcrum shall not sublicense any of the rights as to Licensed Products in the United States granted to Fulcrum by GSK under Section 2.2 to any Third Party without GSK’s prior written consent; provided, however, that the terms of this Section 2.3(b) shall not apply to any sublicense by Fulcrum to any Service Provider.
(c) Fulcrum shall remain responsible for its obligations, including as set forth in Sections 3.2, 3.3 and 3.4, that have been delegated, subcontracted or sublicensed to any of its Affiliates or Sublicensees, and Fulcrum shall be liable for any failure by any Affiliate or Sublicensee of Fulcrum to comply with the terms of this Agreement to the extent that compliance with such terms has been delegated, subcontracted or sublicensed to such Affiliate or Sublicensee.
Section 2.4. Rights Retained by GSK.
Fulcrum shall receive only those rights of GSK expressly granted by GSK under the provisions of this Agreement, and any right of GSK not expressly granted to Fulcrum under the provisions of this Agreement shall be retained by GSK. For the avoidance of doubt, (a) GSK
and its Affiliates shall retain the right, without the right to grant sublicenses, to conduct nonclinical research under the GSK Intellectual Property; and (b) with Fulcrum’s consent (as described below), GSK’s [**] may, under the GSK Patents, [**] solely relating to the use of Licensed Product in connection with the [**]; provided, however, that, (i) prior to [**], GSK shall seek Fulcrum’s consent thereto and, in connection therewith, shall [**] to Fulcrum for its review, and (ii) Fulcrum shall determine whether to grant such consent based on such protocol and all other relevant factors, which consent Fulcrum shall not unreasonably withhold or delay. This Agreement creates no obligation for GSK or any of its Affiliates to remove or segregate the Data from its systems for any purpose.
Section 2.5. Data Transfer.
(a) To the extent not already provided prior to the Effective Date, within [**] following the Effective Date, GSK shall cooperate with Fulcrum’s reasonable instructions to effect the transfer of electronic copies of the Data to Fulcrum (the “Data Transfer”).
(b) Commencing on the Effective Date, GSK shall use commercially reasonable efforts to deliver to Fulcrum (i) those SAS datasets related to the Compound, which are set forth on Schedule 2.5(b), within [**] following the Effective Date, and (ii) as requested by Fulcrum in a spreadsheet named “GSK Information Requests_vMaster 05 Feb (version 2) sent to GSK on February 5, 2019 by [**] of Fulcrum to [**] of GSK (the “Spreadsheet”): (A) the drug substance and placebo batch records related to the physical material of the Compound being transferred to Fulcrum as described in Section 2.6 below, which are set forth in request number [**] and request numbers [**] (inclusive) of the Spreadsheet, within [**] following the Effective Date and (B) the Microsoft Word and PDF files for all nonclinical and clinical study reports related to the Compound, which are set forth in request numbers [**] (inclusive) of the Spreadsheet, within [**] following the Effective Date to the extent not previously delivered by GSK to Fulcrum. If, upon expiration of the applicable timeframes in this Section 2.5(b), GSK has not delivered all Data described in this Section 2.5(b) then GSK and Fulcrum shall use good faith efforts to implement an appropriate alternative means to provide Fulcrum with any such Data that GSK has not delivered within the applicable timeframes in this Section 2.5(b), to the extent such Data is available.
(c) For a period of [**] commencing on the Effective Date, Fulcrum may request, and GSK shall use commercially reasonable efforts to provide, any Data not provided in the initial Data Transfer. During such [**] period commencing on the Effective Date, Fulcrum may also request that GSK provide information that is not Data that is reasonably related to the Compound and may be useful to Fulcrum for further development of, or the submission of regulatory filings relating to, the Compound for the treatment of FSHD (such information, “Supplemental Data”), to the extent such Supplemental Data is in GSK’s (or its Affiliates’) possession and is Controlled by GSK (or its Affiliates), and GSK shall use commercially reasonable efforts to locate or provide any such requested Supplemental Data. Any Supplemental Data that may be provided by GSK to Fulcrum as provided herein shall be deemed to be Data.
Section 2.6. Material Transfer.
GSK shall provide to Fulcrum the physical material of the Compound, including clinical placebo tablets, which are set forth on Schedule 2.6 attached hereto and incorporated herein, and which physical transfer GSK shall initiate within [**] of the Effective Date with respect to all physical material of the Compound set forth on Schedule 2.6.
Section 2.7. Reports.
Within [**] after then end of each Calendar Year during the Term, Fulcrum shall provide a written report to GSK, which report shall summarize in reasonable detail all of the development, regulatory and commercialization activities conducted by or on behalf of Fulcrum or its Affiliates with respect to the Compound and Product in the Territory.
ARTICLE III
EQUITY ISSUANCE; PAYMENTS
Section 3.1. Equity Issuance.
(a) Issuance of Equity. In partial consideration for the licenses and other rights granted to Fulcrum hereunder, Fulcrum shall issue to GSK twelve million five hundred thousand (12,500,000) shares of Fulcrum’s Series B Preferred Stock pursuant to a subscription agreement and any other agreements by and between GSK and Fulcrum and dated as of the date hereof, relating to the issuance of such shares and any other rights related thereto to GSK (collectively, the “Equity Agreements”).
(b) Equity Agreements. As a condition precedent to the effectiveness of this Agreement, the Parties shall have duly authorized, executed and delivered the all of the Equity Agreements and performed their respective obligations that are required to be performed thereunder.
Section 3.2. Development and Regulatory Milestone Payments.
Fulcrum shall make each of the development and regulatory milestone payments set forth in the table below in this Section 3.2 to GSK within [**] after the first achievement (whether by or on behalf of Fulcrum or any of its Affiliates or, subject to Section 2.3, Sublicensees) of the corresponding milestone event set forth in the table below in this Section 3.2 by the first Licensed Product to achieve such a milestone event. Fulcrum shall notify GSK within [**] of the achievement of each development and regulatory milestone event. Each milestone payment set forth in the table below in this Section 3.2 shall be paid only once during the Term, for the first time any Licensed Product reaches such milestone event during the Term, and regardless of the number of Licensed Products that achieve such milestone event, the number of times such milestone event is achieved by any Licensed Product, or the number of additional Licensed Products that subsequently achieve any such milestone event. Notwithstanding the foregoing, in the event any regulatory milestone event in (c) through (e) is achieved, prior to the achievement of a development milestone in (a) or (b) (the “skipped milestone”), the applicable skipped
milestone will become due and payable together with the applicable regulatory milestone in (c), (d) or (e). For clarification, the total milestone payments that may become payable under this Section 3.2 if all milestone events in the table below in this Section 3.2 are achieved is thirty-seven million five hundred thousand dollars ($37,500,000).
|
Milestone Event |
|
Milestone Payment |
| |
|
(a) Initiation of the first Phase 2 Clinical Trial for a Licensed Product |
|
$ |
2,500,000 |
|
|
(b) [**] |
|
[** |
] | |
|
(c) [**] |
|
[** |
] | |
|
(d) [**] |
|
[** |
] | |
|
(e) [**] |
|
[** |
] | |
Section 3.3. Sales Milestone Payments.
Fulcrum shall make each of the following one-time sales milestone payments to GSK within [**] after the end of the Calendar Quarter in which aggregate annual Net Sales of the first Licensed Product in the Territory to reach the threshold(s) specified in the table below in this Section 3.3. Fulcrum shall notify GSK promptly of the achievement of each such sales threshold. If more than one sales threshold is reached in any given Calendar Year, then the applicable milestone payment for each such achievement shall be due and owing with respect to such Calendar Year. Each milestone payment set forth in the table below in this Section 3.3 shall be paid only once during the Term, for the first time any Licensed Product reaches such sales milestone threshold, and regardless of the number of Licensed Products that achieve such sales milestone threshold, the number of times such milestone event is achieved by any Licensed Product, or the number of additional Licensed Products for which such milestone event is subsequently achieved. For clarification, the total milestone payments that may become payable under this Section 3.3 if all sales milestone thresholds in the table below in this Section 3.3 are achieved is sixty million dollars ($60,000,000).
|
Threshold for Aggregate Annual |
|
Milestone Payment |
|
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
Section 3.4. Royalty Payments.
(a) Royalty Rates. Fulcrum shall pay to GSK tiered royalties on the aggregate annual Net Sales of Licensed Products sold in the Territory during each Calendar Year quarterly within [**] after the end of such Calendar Quarter during the Royalty Term at the applicable rate(s) set forth below with such royalties to be calculated by multiplying the applicable incremental amount of Net Sales of such Licensed Products in the Territory in such Calendar Quarter by the corresponding royalty rate set forth in the table below and by subsequently making the applicable adjustments in accordance with Section 3.4(c).
|
Annual Net Sales of Licensed Product |
|
Royalty Rate |
|
|
For that portion of annual Net Sales less than or equal to $[**] |
|
[** |
]% |
|
For that portion of annual Net Sales greater than $[**] and less than or equal to $[**] |
|
[** |
]% |
|
For that portion of annual Net Sales greater than $[**] |
|
[** |
]% |
(b) Royalty Term. Royalties under this Section 3.4 shall be payable on a country-by-country basis in the Territory during the period commencing on the First Commercial Sale of such Licensed Product in such country and continuing on until the earlier of (i) approval of a Generic Version of such Licensed Product by the applicable Regulatory Agency on a country-by-country basis; and (ii) the tenth (10th) anniversary of the First Commercial Sale of such Licensed Product in such country (the “Royalty Term”). Upon expiration of the Royalty Term for any Licensed Product in a given country, the licenses granted to Fulcrum under Section 2.2 with respect to such Licensed Product in such country shall automatically become fully paid-up, perpetual and royalty-free and shall survive any expiration or termination of this Agreement, and Net Sales of such Licensed Product in such country shall thereafter be excluded from aggregate annual Net Sales of such Licensed Product for purposes of calculating royalties pursuant to Section 3.4(a).
(c) Royalty Adjustments.
(i) During any applicable Royalty Term, following the expiration of Regulatory Exclusivity for any Licensed Product on a country-by-country basis, the royalty rates in Table 3.4(a) applicable to Net Sales of such Licensed Products in such country will be reduced by [**] percent ([**]%).
(ii) If Fulcrum or any of its Affiliates or Sublicensees, as applicable, determines, in its reasonable judgment, that it is necessary to obtain a license from any Third Party (each a “Third Party License”) under any Patents in order to Exploit a Licensed Product in a country, then Fulcrum may deduct [**] percent ([**]%) of any license fees under such Third Party License payable by Fulcrum or any Affiliate or
Sublicensee in any Calendar Quarter to such Third Party with respect to sales of such Licensed Product in such country under such Third Party License from the royalty payment that would otherwise be due with respect to Net Sales of such Licensed Product in such country in such Calendar Quarter pursuant to Section 3.4(a) (as adjusted by Section 3.4(c)(i), as applicable); provided, however, that in no event shall any royalty payment to GSK on Net Sales of any Licensed Product in any country in any Calendar Quarter be reduced to less than [**] percent ([**]%) of the royalties that would otherwise be owed to GSK with respect to Net Sales of such Licensed Product under Section 3.4(a) (as adjusted by Section 3.4(c)(i), if applicable). Any amount of royalties paid to such Third Party which is entitled to be deducted under this Section 3.4(c)(ii) but is not deducted as a result of the foregoing limitation shall be carried over and applied against royalties payable to GSK in respect of such Licensed Product in such country in subsequent Calendar Quarters until the full deduction is taken.
Section 3.5. Reports; Audits.
(a) Until the expiration of the Term as to all Licensed Products in all countries, Fulcrum agrees to make written reports to GSK within [**] after the end of each Calendar Quarter covering sales by Fulcrum, its Affiliates and Sublicensees of any Licensed Product that is subject to Fulcrum’s royalty obligations hereunder on a country-by-country basis during such Calendar Quarter. Each such written report shall provide (a) the Net Sales during such Calendar Quarter, in US dollars, (b) the royalties payable, in US dollars, which accrued hereunder with respect to such Net Sales and (c) the exchange rate used in calculating any of the foregoing. The information contained in each report under this Section 3.5 shall be considered Confidential Information of Fulcrum and its Affiliates.
(b) Fulcrum agrees, upon not less than [**] prior written notice, to permit, and to require its Affiliates and Sublicensees to permit, an independent accounting firm selected by GSK and reasonably acceptable to Fulcrum to examine the books and records relating to any Licensed Product for the purpose of verifying reports provided by Fulcrum under Section 3.5(a). Such audit shall not (i) be performed more frequently than [**] period (unless a previous audit during such [**] period revealed a material discrepancy with respect to such period), or (ii) be repeated for any Calendar Quarter, and shall be conducted for the sole purpose of verifying the accuracy and completeness of all financial, accounting and numerical information and calculations provided under this Agreement. The independent accounting firm shall have the right to make copies of relevant portions of Fulcrum’s books and records; provided that any such copies shall be the Confidential Information of Fulcrum, shall be protected by appropriate confidentiality obligations and shall not be shared with GSK or any other Person. The independent accounting firm will prepare and provide to the Parties a written report stating only whether the reports submitted, and amounts paid hereunder were correct or incorrect, and the amounts of any discrepancies. GSK shall bear the cost of any audit conducted under this Section 3.5(b) unless a discrepancy greater than [**] percent ([**]%) over any Calendar Year is found, in which case Fulcrum shall bear the cost of such audit.
Section 3.6. Payment Method.
All Royalty Payments shall be made in US dollars in immediately available funds via either a bank wire transfer, an ACH (automated clearing house) mechanism, or any other means of electronic funds transfer, at Fulcrum’s election, to a bank account specified by GSK in a notice at least [**] before the payment is due.
Section 3.7. Late Payments.
If Fulcrum shall fail to make a timely Royalty Payment pursuant to the terms of this Agreement, GSK shall provide written notice of such failure to Fulcrum (a “Late Payment Notice”), and interest shall accrue on the past due amount starting on the date of the Late Payment Notice at the prime or equivalent rate per annum quoted by the Wall Street Journal, Eastern Edition on the first Business Day after such payment is due, plus [**] percent ([**]%), computed for the actual number of days after the date of the Late Payment Notice that the payment was past due.
ARTICLE IV
RIGHT OF FIRST NEGOTIATION
Section 4.1. Negotiation Right.
Commencing on the Effective Date and until the completion of a Phase 2 Clinical Trial for a Licensed Product, if Fulcrum wishes to sublicense to any Third Party any of the rights granted to Fulcrum by GSK under Section 2.2 in any country(ies) in the Territory other than the United States, Fulcrum shall, prior to entering into a sublicense agreement with any Third Party, notify GSK in writing of Fulcrum’s desire to sublicense its rights to a Third Party and the terms on which Fulcrum proposes to grant such sublicense; provided, however, that the terms of this Section 4.1 shall not apply to any sublicense to any Service Provider. GSK shall have [**] from receipt of such notice to decide whether to enter into further negotiations with Fulcrum for GSK or its Affiliate to Exploit Licensed Product in the applicable country(ies) in the Territory other than the United States. If GSK does not elect for it or its Affiliate to Exploit Licensed Product in the applicable country(ies) in the Territory other than the United States or does not respond to Fulcrum’s notice within such [**] period, subject to Section 2.3, Fulcrum may sublicense the rights granted to Fulcrum by GSK under Section 2.2 in the applicable country(ies) in the Territory other than the United States to any Third Party, subject to Section 2.3. If GSK elects within such [**] period to negotiate for it or its Affiliate to Exploit Licensed Product in the applicable country(ies) in the Territory other than the United States, then the Parties shall negotiate in good faith for a period of not more than [**] after such election by GSK. If the Parties have not reached an agreement within such [**] period, then Fulcrum shall have the right to (i) enter into negotiations with any Third Party for the sublicense of its rights granted to Fulcrum by GSK under Section 2.2 in the applicable country(ies) in the Territory other than the United States and (ii) grant sublicenses in the applicable country(ies) in the Territory without further obligations to negotiate with GSK, provided that any such sublicense (x) is subject to Section 2.3 and (y) does not include terms that are in the aggregate less favorable than those offered by GSK. For the avoidance of doubt, GSK’s right of first negotiation under this Section 4.1 shall expire upon the first completion of a Phase 2 Clinical Trial for a Licensed Product.
ARTICLE V
INTELLECTUAL PROPERTY
Section 5.1. Patent Maintenance.
(a) GSK Patents. All decisions and actions with respect to the maintenance of the GSK Patents shall be the responsibility of Fulcrum, at Fulcrum’s sole cost and expense. Fulcrum may abandon or discontinue the maintenance of any GSK Patent in a country; provided that Fulcrum first notifies GSK in writing at least [**] in advance of the due date of any payment or other action that is required to maintain such GSK Patent, and, upon such notice, GSK shall have the option, but not the obligation, to maintain such GSK Patent in the Territory at its sole cost and expense.
(b) Patent Term Extensions. As between the Parties, Fulcrum shall have the sole right, but not the obligation, at its sole cost and expense, to file for and seek to obtain patent term extensions (including any pediatric exclusivity extensions as may be available) or supplemental protection certificates or their equivalents in any country with respect to the GSK Patents based on Regulatory Approvals for the GSK Patents.
(c) Data Exclusivity. With respect to data exclusivity periods, Fulcrum shall have the sole right, but not the obligation, at its sole cost and expense, to seek, maintain and enforce all such data exclusivity periods available for Licensed Products.
Section 5.2. Patent Enforcement.
(a) Notification. If either Party becomes aware of any existing or threatened infringement of any GSK Patent in the Field in the Territory, including (i) any such existing or threatened infringement on account of a Third Party’s Exploitation of the Compound or any Generic Version of a Licensed Product in the Field in any country in the Territory, or (ii) any certification filed by a Third Party in the United States pursuant to the Drug Price Competition and Patent Term Restoration Act of 1984 (or any successor legislation) or similar provisions in other jurisdictions, in connection with an abbreviated new drug application or a paper new drug application (or equivalent) with respect to the Compound or any Licensed Product in the Field in any country in the Territory, or any other similar Third Party communication, including notices pursuant to §§ 101 and 103 of such act from any person or entity who has filed an abbreviated new drug application or a paper new drug application (or equivalent) with respect to the Compound or any Generic Version of a Licensed Product in the Field ((i) and (ii), collectively, “Competitive Infringement”), it shall promptly notify the other Party in writing to that effect, and the Parties will consult with each other regarding any actions to be taken with respect to such Competitive Infringement.
(b) Right to Enforce. Fulcrum shall have the first right, but shall not be obligated, to bring and control an infringement action with respect to any Competitive Infringement of any GSK Patent, at Fulcrum’s sole cost and expense. If Fulcrum does not bring such an action with respect to a GSK Patent (or settle or otherwise secure the abatement of such infringement) prior to the earlier of: (i) [**] following GSK’s receipt or delivery of the notice under Section 5.2(a), or (ii) [**] before the deadline, if any, set forth in the applicable Laws for the filing of such
actions, GSK shall have the right, but not the obligation, to bring and control any such action, at its own expense and by counsel of its own choice.
(c) Cooperation. Each Party shall cooperate fully with the enforcing Party in such enforcement, at such enforcing Party’s request and expense, including joining such action as a party plaintiff if required by applicable Laws to pursue such action. The enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts and shall reasonably consider the other Party’s comments on any such efforts. The non-enforcing Party shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall at all times cooperate fully with the enforcing Party. Neither Party shall have the right to settle any patent infringement litigation under this Section 5.2 in a manner that diminishes the rights or interests of the other Party without the prior written consent of such other Party, such consent not to be unreasonably withheld, conditioned or delayed.
(d) Expenses and Recoveries. The enforcing Party bringing a claim, suit or action under this Section 5.2 shall be solely responsible for any expenses incurred by such Party as a result of such claim, suit or action. If such Party recovers monetary damages in such claim, suit or action, except as otherwise agreed by the Parties in connection with a cost-sharing arrangement, such recovery shall be allocated first to the reimbursement of any expenses incurred by the Parties in such litigation, and any remaining amounts shall be shared as follows: the remaining amount of any such recovery shall be deemed Net Sales for the Calendar Quarter in which such recovery payment is received by the Parties, and GSK shall receive royalty payments on such amounts as set forth in Section 3.4.
ARTICLE VI
REPRESENTATIONS AND WARRANTIES; GSK COVENANTS
Section 6.1. Representations and Warranties of Both Parties.
Each Party hereby represents and warrants to the other Party, as of the Effective Date, that:
(a) Such Party is duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation and has full corporate power and authority to enter into this Agreement and to carry out the provisions hereof;
(b) Such Party has taken all necessary action on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder;
(c) This Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, binding obligation, enforceable against it in accordance with the terms hereof;
(d) The execution, delivery and performance of this Agreement by such Party does not and will not conflict with any agreement or any provision thereof, or any instrument or understanding, oral or written, to which it is or becomes a party or by which it is or becomes
bound, nor violate any Law or regulation of any court, governmental body or administrative or other agency having jurisdiction over such Party; and
(e) No government authorization, consent, approval, license, exemption of, or filing or registration with any court or governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, under any Laws currently in effect, is necessary for its execution and delivery of this Agreement.
Section 6.2. Additional Representations and Warranties of GSK.
GSK hereby represents and warrants to Fulcrum, as of the Effective Date, that:
(a) GSK or its Affiliates Control the Losmapimod IND and the GSK Intellectual Property and is entitled to grant the rights and licenses under the Losmapimod IND and the GSK Intellectual Property specified herein; and
(b) GSK has not granted a right of reference to the Losmapimod IND or licensed the GSK Intellectual Property to any Third Party.
Section 6.3. GSK Covenants.
(a) GSK shall not grant to any Third Party any rights that would be inconsistent with Fulcrum’s rights hereunder.
(b) GSK shall not assign, transfer, convey or otherwise encumber its right, title and interest in and to the Losmapimod IND or the GSK Intellectual Property in a manner that conflicts with any rights granted to Fulcrum hereunder.
Section 6.4. Fulcrum Covenants. During the Term:
(a) Fulcrum hereby covenants that it and its Affiliates shall use Commercially Reasonable Efforts to develop and commercialize a Licensed Product for FSHD in the Territory.
(b) Fulcrum and its Affiliates shall conduct, and shall use Commercially Reasonable Efforts to cause its Sublicensees and Service Providers to conduct, all activities contemplated under this Agreement in accordance with applicable Law.
(c) Fulcrum and its Affiliates will conduct its business and all activities with respect to the Licensed Product in compliance with the Foreign Corrupt Practices Act of 1977, the UK Bribery Act of 2010 and any other applicable anti-corruption laws.
(d) Neither Fulcrum nor any of its Affiliates will knowingly use in any capacity in connection with the Exploitation of any Licensed Product, any Person who has been Debarred. Fulcrum shall notify GSK immediately upon becoming aware that any Person who is performing services with respect to any Licensed Product has been Debarred or if any claim is pending or, to the best of Fulcrum’s knowledge, threatened, relating to the Debarment of Fulcrum, its Affiliates or any Person in connection with the Exploitation of any Licensed Product. For the avoidance of doubt, neither Fulcrum nor any of its Affiliates shall have any liability to GSK or any of its
Affiliates as a result of any Debarred Person’s involvement in the Exploitation of any Licensed Product to the extent Fulcrum, having instituted the policies and procedures set forth in Section 6.4(e), had no knowledge of such Person’s Debarment or any claim (whether pending or threatened) relating to the Debarment of such Person, provided that Fulcrum takes prompt action to terminate such Debarred Person’s involvement in the Exploitation of any Licensed Product upon learning of such Debarment or claim relating to the Debarment of such Person.
(e) Fulcrum and its Affiliates will maintain internal policies and procedures designed to reasonably ensure that neither Fulcrum nor any of its Affiliates will knowingly use in any capacity in connection with the Exploitation of any Licensed Product, any Person who has been Debarred.
Section 6.5. No Other Warranties.
EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY OF ANY KIND, EITHER EXPRESS, IMPLIED, STATUTORY OR OTHERWISE, WITH RESPECT TO THE SUBJECT MATTER OF THIS AGREEMENT (INCLUDING, WITHOUT LIMITATION, THE DATA, LOSMAPIMOD IND, COMPOUND, ANY LICENSED PRODUCT OR GSK INTELLECTUAL PROPERTY), INCLUDING ANY WARRANTY OF MERCHANTABILITY, NONINFRINGEMENT, OR FITNESS FOR A PARTICULAR PURPOSE. EACH PARTY DISCLAIMS ANY REPRESENTATION OR WARRANTY THAT THE EXPLOITATION OF LICENSED PRODUCT PURSUANT TO THIS AGREEMENT WILL BE SUCCESSFUL.
ARTICLE VII
CONFIDENTIALITY
Section 7.1. Confidentiality.
(a) During the Term and for [**] thereafter, each Party shall maintain Confidential Information (as defined in Section 7.1(b)) of the other Party in confidence, and shall not disclose, divulge or otherwise communicate such Confidential Information to others (except for agents, directors, officers, employees, consultants, subcontractors, Affiliates, advisors, licensees, sublicensees, partners and potential licensees, sublicensees and partners (collectively, “Agents”) under obligations of confidentiality at least as restrictive as set forth in this Article VII) or use it for any purpose other than in connection with the Exploitation of the Compound or any Licensed Product pursuant to this Agreement, and each Party shall exercise commercially reasonable efforts to prevent the unauthorized disclosure of such Confidential Information by any of its Agents, which efforts shall be at least as diligent as those generally used by such Party in protecting its own confidential and proprietary information. Each Party will be responsible for a breach of this Section 7.1 by its Agents. For clarity, Fulcrum may disclose Confidential Information of GSK (a) to governmental authorities (i) to the extent desirable to obtain or maintain INDs or regulatory approvals for the Compound or any Licensed Product within the Territory and (ii) in order to respond to inquiries, requests or investigations by governmental authorities; (b) to outside consultants, scientific advisory boards, managed care organizations, and non-clinical and clinical investigators to the extent necessary to Exploit the Compound or
any Licensed Product; (c) to the extent useful to develop, manufacture and commercialize the Compound or any Licensed Product; and (d) to the extent necessary or useful in order to enjoy its rights under this Agreement; provided that Fulcrum shall obtain the same confidentiality obligations from any Third Parties to which it discloses the Confidential Information of GSK as it obtains with respect to its own similar types of confidential information.
(b) Confidential Information. “Confidential Information” means all trade secrets or other proprietary information, including any proprietary data and materials (whether or not patentable or protectable as a trade secret), regarding a Party’s or its Affiliate’s or licensor’s technology, products, business, financial status or prospects or objectives regarding the Licensed Products that is disclosed by a Party to the other Party. All information disclosed prior to the Effective Date by a Party to the other Party pursuant to the Mutual Non-Disclosure Agreement by and between the Parties, dated as of June 4, 2018 through the Effective Date (the “Confidentiality Agreement”), shall be deemed “Confidential Information” of such disclosing Party. Notwithstanding the foregoing, there shall be excluded from the foregoing definition of Confidential Information any of the foregoing that:
(i) either before or after the date of the disclosure to the receiving Party is lawfully disclosed to the receiving Party by a Third Party without any violation of any obligation to the other Party; or
(ii) either before or after the date of the disclosure to the receiving Party, becomes published or generally known to the public through no fault or omission on the part of the receiving Party or its Agents; or
(iii) is independently developed by or for the receiving Party without reference to or reliance upon the disclosing Party’s Confidential Information as demonstrated by contemporaneous written records of the receiving Party.
Notwithstanding the foregoing, the receiving Party may disclose the disclosing Party’s Confidential Information if it is required to be disclosed to comply with applicable Laws, to defend or prosecute litigation or to comply with governmental regulations or the regulations or requirements of any stock exchange, provided that the receiving Party promptly provides prior notice of such disclosure to the other Party and uses commercially reasonable efforts to avoid or minimize the degree of such disclosure.
(c) Terms of this Agreement. Neither Party may disclose the terms of this Agreement except to the extent required to comply with applicable Laws or legal process, including the rules and regulations of the U.S. Securities and Exchange Commission, or similar agency in any country other than the United States, or of any stock exchange, including Nasdaq. Notwithstanding the foregoing, before disclosing the terms of this Agreement with respect to any filings with the U.S. Securities and Exchange Commission or similar agency in any country other than the United States or of any stock exchange, including Nasdaq, on which securities issued by a Party or a Party’s Affiliate are traded, the Parties will, prior to any such disclosure, agree upon the redaction of certain provisions of this Agreement, and each Party will use commercially reasonable efforts to seek confidential treatment for such terms as may be reasonably requested by the other Party.
(d) Return of Confidential Information. Upon the termination of this Agreement (other than on expiration of this Agreement following which Fulcrum’s license rights hereunder survive in accordance with Section 9.1), each Party shall return the other Party’s Confidential Information or destroy the other Party’s Confidential Information at the other Party’s request; provided, that each Party may retain one (1) copy of the other Party’s Confidential Information for archival purposes only and neither Party shall be required to return or destroy Confidential Information of the other Party stored on automatically created back-up computer systems. Notwithstanding the foregoing, each Party shall continue to be bound by its obligations of confidentiality under this Article VII.
(e) Publicity. The Parties agree that following the Effective Date Fulcrum may issue a mutually agreed press release announcing the execution of this Agreement. Any press release or other public announcement relating to the terms of this Agreement will first be reviewed and approved in writing by both Parties, such approval not to be unreasonably withheld; provided, however, that any disclosure the minimum information of which is required by applicable Law (including the rules of a securities exchange), as reasonably advised by the disclosing Party’s counsel, may be made without the prior consent of the other Party, although the other Party will be given prompt notice of any such legally required disclosure and to the extent practicable will be provided an opportunity to comment on the proposed disclosure and the disclosing Party will consider in good faith any comments provided by the other Party on such proposed disclosure.
ARTICLE VIII
INDEMNIFICATION
Section 8.1. Indemnification by GSK.
GSK will defend, indemnify and hold harmless Fulcrum, its Affiliates and its and their respective directors, officers, employees and agents (each a “Fulcrum Indemnitee”) from and against any and all Third Party Claims (including without limitation reasonable attorneys’ fees and court costs) that constitute, or arise out of or in connection with:
(a) any misrepresentation or breach of warranty made by GSK under Sections 6.1 and 6.2;
(b) any default by GSK or its Affiliates in the performance or observance of any of its covenants or agreements hereunder; or
(c) the negligence or willful misconduct of any of GSK or its Affiliates in connection with GSK’s performance of this Agreement;
except, in each case ((a)-(c)) for those Third Party Claims for which Fulcrum, in whole or in part, has an obligation to indemnify GSK pursuant to Section 8.2 hereof, as to which Third Party Claims each Party shall indemnify the other to the extent of their respective Liability for the Third Party Claim.
Section 8.2. Indemnification by Fulcrum.
Fulcrum will defend, indemnify and hold harmless GSK, its Affiliates and its and their respective directors, officers, employees and agents (each a “GSK Indemnitee”) from and against any and all Third Party Claims (including without limitation reasonable attorneys’ fees and court costs) that constitute, or arise out of or in connection with:
(a) any misrepresentation or breach of warranty made by Fulcrum under Section 6.1;
(b) any default by Fulcrum, its Affiliates or any Sublicensees in the performance or observance of any of its covenants or agreements hereunder;
(c) the Exploitation of the Compound or Licensed Products by Fulcrum, its Affiliates or Sublicensees, including all Third Party Claims involving death or bodily injury caused or allegedly caused as a result of the Exploitation of the Compound or Licensed Products by Fulcrum or its Affiliates or its Sublicensees inside the Territory;
(d) any actual or alleged infringement of any trademark, patent right or other intellectual property right, or misappropriation of any trade secret, of any Third Party as a result of the Exploitation of the Compound or Licensed Products by Fulcrum or its Affiliates or its Sublicensees inside the Territory; or
(e) the negligence or willful misconduct of any of Fulcrum, its Affiliates or Sublicensees in connection with Fulcrum’s performance of this Agreement;
except, in each case ((a)-(e)) for those Third Party Claims for which GSK, in whole or in part, has an obligation to indemnify Fulcrum pursuant to Section 8.1 hereof, as to which Third Party Claims each Party shall indemnify the other to the extent of their respective Liability for the Third Party Claim.
Section 8.3. Procedure.
(a) A Fulcrum Indemnitee or a GSK Indemnitee seeking indemnification pursuant to this Article VIII (an “Indemnified Party”) shall give prompt written notice to the Party from whom indemnification is sought (the “Indemnifying Party”) of the commencement or assertion of any Third Party Claim for which indemnification may be sought (it being understood and agreed, however, that the failure by an Indemnified Party to give notice of a Third Party Claim as provided in this Section 8.3 shall not relieve the Indemnifying Party of its indemnification obligation under this Agreement except and only to the extent that such Indemnifying Party is actually prejudiced as a result of such failure to give notice).
(b) Within [**] after delivery of such notification, the Indemnifying Party may, upon written notice thereof to the Indemnified Party, assume control of the defense of such Third Party Claim with counsel reasonably satisfactory to the Indemnified Party. If the Indemnifying Party does not assume control of such defense, the Indemnified Party shall control such defense and, without limiting the Indemnifying Party’s indemnification obligations, the Indemnifying Party shall reimburse the Indemnified Party for all costs and expenses, including reasonable attorneys’ fees and disbursements, incurred by the Indemnified Party in defending itself within [**] after receipt of any invoice therefor from the Indemnified Party. The Party not controlling such
defense may participate therein at its own expense; provided, however, that, if the Indemnifying Party assumes control of such defense and the Indemnified Party in good faith concludes, based on written advice from outside counsel, that the Indemnifying Party and the Indemnified Party have conflicting interests with respect to such Third Party Claim sufficiently adverse to make unadvisable the representation by the same counsel of both Parties under Law, ethical rules or equitable principles, the Indemnifying Party shall be responsible for the reasonable fees and expenses of a single counsel to the Indemnified Party in connection therewith. The Party controlling such defense shall keep the other Party advised of the status of such Third Party Claim and the defense thereof and shall consider recommendations made by the other Party with respect thereto.
(c) The Indemnified Party shall not agree to any settlement of such Third Party Claim without the prior written consent of the Indemnifying Party, which consent shall not be unreasonably withheld, delayed or conditioned; provided, that, if the Indemnifying Party does not assume the defense of such Third Party Claim in accordance with Section 8.3(b), the Indemnified Party may thereafter agree to a settlement of such Third Party Claim that does not impose obligations on the Indemnifying Party that are more burdensome than the Indemnifying Party’s indemnification obligations under this Agreement. The Indemnifying Party shall not agree to any settlement of such Third Party Claim or consent to any judgment in respect thereof that does not include a complete and unconditional release of the Indemnified Party from all liability with respect thereto, that imposes any liability or obligation on the Indemnified Party or that acknowledges fault by the Indemnified Party, without the prior written consent of the Indemnified Party.
(d) Any indemnification hereunder for a Third Party Claim shall be made net of any insurance proceeds actually recovered by the Indemnified Party from unaffiliated Third Parties for such Third Party Claim to the extent that such insurance proceeds actually reduce the amount of such Third Party Claim; provided, however, that if, following the indemnification payment to the Indemnified Party of any amount under this Article VIII, such Indemnified Party recovers any such insurance proceeds in respect of the Third Party Claim for which such indemnification payment was made, the Indemnified Party shall promptly pay the Indemnifying Party an amount equal to the excess of the indemnification payment received over the amount of the indemnification payment that would have been due if the insurance proceeds had been received, realized or recovered before the indemnification payment was made.
(e) The Parties agree and acknowledge that the provisions of this Article VIII represent any Indemnified Party’s exclusive recourse with respect to any Losses for which indemnification is provided to such Indemnified Party under this Article VIII.
Section 8.4. Limitation of Liability.
No Consequential Damages. NEITHER PARTY NOR ANY OF THEIR RESPECTIVE AFFILIATES SHALL BE LIABLE TO THE OTHER PARTY OR ITS AFFILIATES FOR SPECIAL, INDIRECT, INCIDENTAL, EXEMPLARY, CONSEQUENTIAL DAMAGES OR LOSS OF PROFITS OR OPPORTUNITIES OR DIMINUTION OF GOODWILL ARISING OUT OF THIS AGREEMENT BASED ON CONTRACT, TORT OR ANY OTHER LEGAL THEORY; PROVIDED, HOWEVER, THAT NOTHING IN THIS SECTION 8.4 IS
INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF EITHER PARTY UNDER THIS AGREEMENT WITH RESPECT TO THIRD PARTY CLAIMS OR THE WILLFUL MISCONDUCT, INTENTIONAL BREACH OR FRAUD OF THE OTHER PARTY.
ARTICLE IX
TERM
Section 9.1. Term.
The term of this Agreement (the “Term”) shall commence on the Effective Date and, unless earlier terminated as provided in this Article IX, shall continue in full force and effect, on a county-by-country and Licensed Product-by-Licensed Product basis until the expiration of the Royalty Term in each country in the Territory, at which time (unless earlier terminated) this Agreement shall expire with respect to such Licensed Product in such country and Fulcrum shall have a fully paid-up, royalty-free and perpetual license under the GSK Intellectual Property with respect to such Licensed Product in such country. This Agreement shall terminate in its entirety on the date this Agreement has expired with respect to all Licensed Products in all countries in the Territory.
Section 9.2. Termination for Cause.
Either Party (the “Non-Breaching Party”) may, without prejudice to any other remedies available to it under Law or in equity, terminate this Agreement if the other Party (the “Breaching Party”) shall have materially breached in the performance of its obligations hereunder, and such breach shall have continued for (i) [**] in the case of a breach of any obligation other than a payment obligation or (ii) [**] in the case of a breach of any payment obligation hereunder (as applicable, the “Cure Period”) after written notice thereof was provided to the Breaching Party by the Non-Breaching Party, such notice describing the alleged breach. Any such termination of this Agreement under this Section 9.2 shall become effective [**] after written termination notice from the Non-Breaching Party following expiration of the Cure Period, unless the Breaching Party has cured such breach prior to the expiration of the applicable Cure Period.
Section 9.3. Effect of Termination; Accrued Rights and Obligations.
Termination of this Agreement for any reason shall not release either Party from any liability that, at the time of such termination, has already accrued or that is attributable to a period prior to such termination (including payment obligations accrued pursuant to Article III prior to the effective date of termination) nor preclude either Party from pursuing any right or remedy it may have hereunder or at Law or in equity with respect to any breach of this Agreement. It is understood and agreed that monetary damages may not be a sufficient remedy for any breach of this Agreement and that the non-breaching Party may be entitled to seek injunctive relief as a remedy for any such breach.
Section 9.4. Survival.
The rights and obligations set forth in this Agreement shall extend beyond the Term or termination of this Agreement only to the extent expressly provided for in this Agreement or to the extent required to give effect to a termination of this Agreement or the consequences of a termination of this Agreement as expressly provided for in this Agreement. Without limiting the generality of the foregoing, it is agreed that the provisions of Article I, Section 2.1, Article VI, Article VII, Article VIII, Section 9.1 (with respect to the licenses granted therein), Section 9.4 and Article X shall survive expiration or termination of this Agreement for any reason.
ARTICLE X
MISCELLANEOUS PROVISIONS
Section 10.1. Notices.
All notices, communications and deliveries under this Agreement will be made in writing signed by or on behalf of the Party making the same, shall specify the Section under this Agreement pursuant to which it is given or being made and shall be deemed given (a) when delivered personally, (b) when transmitted by facsimile or other electronic transmission (receipt confirmed), (c) on the close of business, local time of the recipient, on the third (3rd) Business Day after the day it is so placed in the mail or, if earlier, the time of actual receipt following mailing by registered or certified mail (return receipt requested) or (d) on the first Business Day following deposit with an overnight delivery service of national reputation, in each case to the applicable addressee at the addresses or facsimile number set forth below. Any notice that is addressed and mailed in the manner herein provided shall be conclusively presumed to have been duly given to the Party to which it is addressed.
If to GSK:
GlaxoSmithKline LLC
0000 X. Xxxxxxxxxxxx Xxxx
Xxxxxxxxxxxx, XX 00000-0000, Xxxxxx Xxxxxx
Attention: Worldwide Business Development
with a copy to:
Vice President & Associate General Counsel
Legal- Business Development & Corporate
GlaxoSmithKline
0000 X. Xxxxxxxxxxxx Xxxx/ XX0000
Xxxxxxxxxxxx, XX 00000-0000, Xxxxxx Xxxxxx
If to Fulcrum:
Fulcrum Therapeutics
00 Xxxxxxxxxx Xxxxxx
Xxxxxxxxx, XX 00000
Attn: Xxxxx Xxxxxx, Chief Operating Officer
With a copy to:
WilmerHale LLP
00 Xxxxx Xxxxxx
Xxxxxx, XX 00000
Attn: Xxxxxx X. Xxxxxxx
Telephone: (000) 000-0000
Facsimile: (000) 000-0000
Section 10.2. Schedules and Exhibits; Entire Agreement; Amendments.
The Schedules and Exhibits to this Agreement are hereby incorporated into this Agreement and are hereby made a part of this Agreement as if set out in full in this Agreement. This Agreement, together with the Schedules and Exhibits hereto, set forth all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties hereto and supersede and terminate all prior agreements and understanding between the Parties. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties other than as set forth herein and therein. This Agreement may not be amended, modified or supplemented except by written agreement of the Parties expressly referencing this Agreement.
Section 10.3. Assignment.
Neither this Agreement nor any right, interest or obligation hereunder may be assigned, pledged or otherwise transferred by either Party, whether by operation of law or otherwise, without the prior consent of the other Party; provided, that either Party may assign its rights hereunder to (a) any of its respective Affiliates or (b) an acquirer of all or substantially all of the business or assets of such Party relating to the subject matter of this Agreement, whether by merger, sale of assets or otherwise, provided, further that in each case of (a) and (b) with respect to either Party, such Person to which such Party assigns this Agreement expressly agrees in writing to assume and be bound by all obligations of such Party under this Agreement and a copy of such written agreement by such assignee shall be provided to the other Party within [**] after such assignment.
Section 10.4. Controlling Law; Dispute Resolution.
(a) Controlling Law. This Agreement shall be governed by and construed and enforced in accordance with the internal laws of the State of Delaware without reference to its choice of law rules; provided, that those matters pertaining to the validity or enforceability of patent rights shall be interpreted and enforced in accordance with the laws of the territory in which such patent rights exist.
(b) Negotiation. The Parties shall endeavor to resolve in good faith any disputes or conflict arising from or relating to the subject matter of this Agreement, failing which either Party may submit such dispute for resolution to the members of their senior management as each Party may designate to handle such disputes from time to time (“Senior Management Designees”). If such Senior Management Designees are unable to resolve such dispute within [**] after such conflict is submitted to them for resolution, either Party may refer the dispute for mediation as set forth in Section 10.4(c).
(c) Mediation. If the Parties are unable to resolve a dispute arising out of or relating to this Agreement through the negotiation procedures set forth in Section 10.4(b), then at the end of such [**] period, the Parties agree to submit such dispute for confidential mediation under the CPR Mediation Procedure then in effect at the start of mediation with the International Institute for Conflict Prevention & Resolution (xxx.xxxxxx.xxx). Unless otherwise agreed, the Parties shall select a mediator from the CPR Panels of Distinguished Neutrals. If the Parties cannot agree, they shall defer to the CPR to select a mediator. The cost of the mediator shall be borne equally by the Parties. Any dispute not resolved within [**] (or within such other time period as may be agreed to by the Parties in writing) after appointment of a mediator shall be finally resolved by arbitration pursuant to Section 10.4(d).
(d) Arbitration. If the Parties are unable to resolve a dispute arising out of or relating to this Agreement through the negotiation procedures set forth in Section 10.4(b) and the mediation procedures set forth in Section 10.4(c) within the timeframes set forth in such Sections, the Parties agree that they shall submit such dispute for final settlement via binding arbitration. The arbitration shall be conducted under the Commercial Arbitration Rules of the American Arbitration Association in effect at the time of the arbitration, except as they may be modified herein or by mutual agreement of the Parties, but need not be under the auspices of the American Arbitration Association, and heard before a single arbitrator as selected in accordance with the Commercial Arbitration Rules. Such arbitration shall be held in Wilmington, Delaware and shall be conducted in English. Except as otherwise determined by the arbitrator, each Party shall be responsible for its own expenses in connection therewith. The arbitration award shall be final and binding upon the Parties, and judgement on the award may be entered in any court having jurisdiction thereof.
(e) For the avoidance of doubt, from the date any such dispute is referred to the Senior Management Designees in accordance with Section 10.4(b) until such time as the dispute has become finally settled or resolved, any cure periods, if applicable, shall be suspended and no termination of this Agreement by either Party as provided in this Agreement shall become effective.
(f) As between the Parties, notwithstanding anything herein to the contrary, any dispute, controversy or claim relating to the scope, validity, enforceability or infringement of any GSK Patent Rights shall not be subject to arbitration, but shall be submitted to a court of competent jurisdiction in the jurisdiction in which such Patent rights were granted or arose.
(g) Notwithstanding the Parties agreement to arbitrate, the Parties hereby agree that a Party may apply to any court of law or equity of competent jurisdiction for specific performance
or injunctive relief to enforce or prevent any violation of the provisions of Article VII of this Agreement.
Section 10.5. Severability.
Any provision of this Agreement which is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions of this Agreement, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. To the extent permitted by Law, the parties waive any provision of Law which renders any such provision prohibited or unenforceable in any respect.
Section 10.6. Counterparts.
This Agreement may be executed in two or more counterparts (delivery of which may be by facsimile, or via email as a portable document format (.pdf)), each of which will be deemed an original, and it will not be necessary in making proof of this Agreement or the terms of this Agreement to produce or account for more than one of such counterparts.
Section 10.7. No Third Party Beneficiaries.
Except for indemnification obligations hereunder to any Indemnified Party, nothing expressed or implied in this Agreement is intended, or shall be construed, to confer upon or give any Person other than the Parties, and their successors or permitted assigns, any rights, remedies, obligations or liabilities under or by reason of this Agreement, or result in such Person being deemed a third party beneficiary of this Agreement.
Section 10.8. Further Assurances.
Following the Effective Date, each of the Parties shall deliver to the other such further information and documents and shall execute and deliver to the others such further instruments and agreements as the other Party shall reasonably request to consummate or confirm the transactions provided for in this Agreement, to accomplish the purpose of this Agreement or to assure to such other Party the benefits of this Agreement.
Section 10.9. Transaction Costs.
Each Party shall bear its own expenses incident to the preparation, negotiation, execution and delivery of this Agreement and the performance of its obligations hereunder.
[Signature page follows]
IN WITNESS WHEREOF, the Parties have caused this Agreement to be duly executed, as of the date first above written.
|
|
|
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED | |
|
|
|
| |
|
|
|
| |
|
|
|
By: |
/s/ Xxxxxx Xxxxxxx |
|
|
|
Name: |
Xxxxxx Xxxxxxx |
|
|
|
Title: |
Director |
|
|
|
|
|
|
|
|
|
|
|
|
|
GLAXOSMITHKLINE LLC | |
|
|
|
| |
|
|
|
| |
|
|
|
By: |
/s/ Xxxxxxx Xxxxx |
|
|
|
Name: |
Xxxxxxx Xxxxx |
|
|
|
Title: |
Assistant Secretary |
|
|
|
|
|
|
|
|
|
|
|
|
|
GLAXO GROUP LIMITED | |
|
|
|
| |
|
|
|
| |
|
|
|
By: |
/s/ Xxxx Xxxxxx |
|
|
|
Name: |
Xxxx Xxxxxx |
|
|
|
Title: |
Authorised Signatory For and on behalf Edinburgh |
|
|
|
Pharmaceutical Industries Limited Corporate Director | |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
| |
|
|
|
| |
|
|
|
By: |
/s/ Xxxxxx X. Xxxxx |
|
|
|
Name: |
Xxxxxx X. Xxxxx |
|
|
|
Title: |
President & CEO |
(Signature Page to Right of Reference and License Agreement)
SCHEDULE 1.1
LOSMAPIMOD COMPOUND
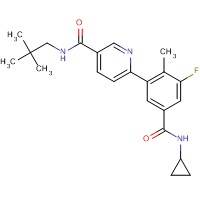
Formula: C22H26FN3O2
Molecular Weight: 383.459
GSK Compound Number: GW856553
SCHEDULE 1.2
GSK PATENTS
(Follows)
|
Case No. |
|
Country |
|
Application Number |
|
Application |
|
Grant Date |
|
Patent Number |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**]
SCHEDULE 2.5(b)
SAS DATASETS
All SAS datasets related to the studies listed below (to be provided in current format):
[**]
SCHEDULE 2.6
MATERIAL TRANSFER
Losmapimod Material Transfer: 7.5 mg tablets, placebo tablets and API
Tablets (located in [**] and [**])
|
Lot Number |
|
Material |
|
Quantity |
|
Expiry date |
|
Status |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
Placebo tablets (located in [**])
|
Lot Number |
|
Material |
|
Quantity |
|
Expiry date |
|
Status |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
API, available for clinical development (located in [**])
|
Lot Number |
|
Material |
|
Quantity |
|
Expiry date |
|
Status |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
API, “development batches”, not to be used for clinical material (located in [**])
|
Lot Number |
|
Material |
|
Quantity |
|
Expiry date |
|
Status |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
EXHIBIT A
LETTER OF AUTHORIZATION
[GSK Letterhead]
Dear Division Director at FDA Division of Neurology Products,
Pursuant to a request from Fulcrum Therapeutics (“Fulcrum”), GSK hereby authorizes the United States Food and Drug Administration (“FDA”) to refer to the information contained in the withdrawn GSK-sponsored losmapimod INDs (IND No. [**]) in their entirety and all information therein in connection with any filing or application made by Fulcrum Therapeutics, including without limitation Fulcrum Therapeutics’ PIND [**] filing for its FTX-1821 product for treatment of Facioscapulohumeral muscular dystrophy (FSHD).
As indicated by my signature below, I am authorized to provide this authorization on behalf of GSK and my full name, title, address, email address, telephone number, are listed below.
If you have any questions, please contact me at [**].
Sincerely,
Xxx Xxxx
Vice President, Global Regulatory Affairs, Therapeutic Group
RD Chief Regulatory Office
GSK
0000 X. Xxxxxxxxxxxx Xxxx, Xxxxxxxxxxxx, Xxxxxxxxxxxx, 00000-0000, Xxxxxx Xxxxxx
Office Phone [**]
Mobile Phone [**]


