RXi-CSHL LICENSE AGREEMENT
Exhibit 10.2
RXi-CSHL LICENSE AGREEMENT
This License Agreement is made and entered into effective as of March 15th, 2007 (the “Effective
Date”), by and between Cold Spring Harbor Laboratory (hereafter “CSHL”), having an address of Xxx
Xxxxxxxx Xxxx, Xxxx Xxxxxx Xxxxxx, XX 00000 and RXi Pharmaceuticals Corp., (hereafter “Company”),
having an address of Xxx Xxxxxxxxxx Xxxxx, Xxxxxxxxx, XX, Xxxxxxxxxxxxx 00000.
R E C I T A L S
WHEREAS, CSHL is the owner by assignment or otherwise of the inventions claimed in the United
States patent applications listed in Exhibit A, attached hereto and incorporated herein by
reference, pertaining to CSHL’s inventions entitled “Methods and Composition for RNA Interference”
(in part, the “Patent Rights”, as further defined herein), the Know-How (as defined herein)
(collectively, the foregoing defined as the “CSHL Intellectual Property”); and
WHEREAS, Company intends, among other things, to develop ribonucleic acid interference-based
products (“RNAi”) products in the human therapeutic field; and
WHEREAS, Company desires to obtain a non-exclusive license to certain CSHL intellectual property,
including the Patent Rights identified in Exhibit A in the Therapeutic Field (as defined herein),
as well as the Research Field and for purposes of providing the Licensed Services as provided
herein; and
WHEREAS, CSHL is willing to grant Company a non-exclusive license on the terms set forth in this
Agreement.
THEREFORE, CSHL and Company agree to state such license as follows:
1. Definitions.
1.1. “Biological Materials” means tangible biological materials that, to the extent that
CSHL has a right to transfer such materials, are necessary for the effective exercise of the Patent
Rights and are actually provided by Xx. Xxxxxx or a member of Xx. Xxxxxxx Xxxxxx’x lab to the
Company, as well as additional tangible materials that are routinely produced through use of those
materials, including, for example, any progeny derived from a cell line, monoclonal antibodies
produced by hybridoma cells, DNA or RNA replicated from isolated DNA or RNA, recombinant proteins
produced through use of isolated DNA or RNA, and substances routinely purified from a source
material included in the original materials (such as, recombinant proteins isolated from a cell
extract or supernatant by non-proprietary affinity purification methods). Xx. Xxxxxx will secure
the approval of CSHL and/or, as needed, the Xxxxxx Xxxxxx Medical Institute (HHMI) prior to the
transfer of any such Biological Material to Company.
1
1.2. “Confidential Information” means any confidential or proprietary information furnished
by one party (the “Disclosing Party”) to the other party (the “Receiving Party”), as further
described and limited by Section 7 below, in connection with this Agreement.
1.3. “Therapeutic Field” means the use of short hairpin RNA (shRNA) for (i) drug discovery
or (ii) the development of therapeutic drugs and drug targets or (iii) use in a drug-screening
program.
1.4. “Research Field” means the use of shRNA, under the Patent Rights, by Company for its
internal scientific research and development.
1.5. “Licensed Product” means any “Covered Product” or “Developed Product.”
(a) “Covered Product” means any product that (i) the manufacture, use, sale, offer for
sale, or import of which, but for the license granted in this Agreement, would infringe one or more
Valid Claims under the Patent Rights, as if such Valid Claims were present in an issued or granted
patent; or (ii) incorporates some portion of one or more Biological Materials proprietary to CSHL;
or (iii) cannot be manufactured, used, or sold without using some portion of the Know-How.
(b) “Developed Product” means any product that is not a Covered Product and is discovered
or developed (i) using either any composition or method that would infringe one or more Valid
Claims under the Patent Rights, as if such Valid Claims were present in an issued or granted patent
or (ii) using the Know-How.
1.6. “Licensed Service” means any service that (a) cannot be developed or performed without
using at least one process that infringes one or more claims under the Patent Rights, (b) uses some
portion of one or more Biological Materials, or (c) uses some portion of the Know-How.
1.7. “Net Sales” means the gross amount billed or invoiced on sales by Company of Licensed
Products and Licensed Services, less the following: (a) customary trade, quantity, or cash
discounts to non-affiliated brokers or agents to the extent actually allowed and taken; (b) amounts
repaid or credited by reason of rejection or return; (c) to the extent separately stated on
purchase orders, invoices, or other documents of sale, any taxes or other governmental charges
levied on the production, sale, transportation, delivery, or use of a Licensed Product or Licensed
Service which is paid by or on behalf of Company; and (d) outbound transportation costs prepaid or
allowed and costs of insurance in transit; and (e) allowance for bad debt that is customary and
reasonable for the industry and in accordance with generally accepted accounting principles.
Notwithstanding anything to the contrary in this Section 1.7, and without otherwise acting as a
limitation, “Net Sales” does not include sales or transfers of Licensed Products where such sales
are solely for clinical testing purposes or are made to an Affiliate for its internal research
purposes or to a Co-Marketer for the purpose of commercial sale. No rights under the Patent Rights
are granted to such third parties under this Agreement.
2
If Company receives non-monetary consideration for any Licensed Products or Licensed Services, Net
Sales are calculated based on the fair market value of that consideration. If Company uses or
disposes of a Licensed Product in the provision of a commercial service other than a Licensed
Service, the Licensed Product is sold and the Net Sales are calculated based on the sales price of
the Licensed Product to an independent third party during the same Royalty Period or, in the
absence of sales, on the fair market value of the Licensed Product as determined by the parties in
good faith.
1.8. “Patent Rights” means the United States patent applications listed on Exhibit
A and any divisional, continuation, or continuation-in-part of those patent applications to the
extent the claims are directed to subject matter specifically described therein, as well as any
patents issued on these patent applications and any reissues or reexaminations of those patents,
and any foreign counterparts to those patents and patent applications. Exhibit A shall be
periodically amended to include any additional Patent Rights that may arise. Patent Rights include
improvements to the Patent Rights controlled by CSHL and made by Xxxxxxx Xxxxxx as a member of the
scientific staff of CSHL to the extent that such an improvement (a) is in the field of shRNA; (b)
is dominated by another patent or application within the Patent Rights; and (c) is conceived and
reduced to practice within two (2) years of the Effective Date.
1.9. “Know-How” means know-how, technical information, research and development
information, test results, and data which are owned or Controlled by CSHL and that have been
actually provided by Xx. Xxxxxx or a member of Xx. Xxxxxxx Xxxxxx’x lab to the Company, and which
(a) have been developed by Xx. Xxxxxxx Xxxxxx and his associates in his laboratory at CSHL (either
as an employee of Xxxxxx Xxxxxx Medical Institute or CSHL) as of the Effective Date or (b) are
subsequently developed by Xx. Xxxxxxx Xxxxxx at CSHL as an employee of Xxxxxx Xxxxxx Medical
Institute. Xx. Xxxxxx will designate in writing or orally all Know-How disclosed by him to
Company.
1.10. “Royalty Period” means the partial calendar quarter commencing on the date on which
the first Licensed Product is sold or used or the first Licensed Service is performed and every
complete or partial calendar quarter thereafter during which either (a) this Agreement remains in
effect or (b) Company has the right to complete and sell work-in-progress and inventory of Licensed
Products or perform Licensed Services pursuant to Section 8.6.
1.11. “Control” or “Controlled” means the possession of the ability to grant access
to or a license as provided for herein without violating the terms of any agreement or other
arrangement with any third party.
1.12. “Bona Fide Collaborator” means a third party having a bona fide research
collaboration with Company, and such collaboration is undertaken by Company and such third party
pursuant to a written agreement, (a) having a specific plan of research; (b) having a finite term;
(c) granting Company intellectual proprietary rights in any invention resulting from such
collaboration; and (d) granting Company commercial rights in any Licensed Product or Licensed
Service resulting from such collaboration.
3
1.13. “Co-Marketer” means a third party that makes or sells a Licensed Product or a
Licensed Service developed by Company and does so for the benefit, in whole or in part, of the
Company but only where such third party qualifies for and has actually entered into, in advance, a
Co-Marketer License under Section 2.4 of this Agreement or is otherwise licensed in writing by CSHL
to do so.
1.14. “Xx. Xxxxxxx Xxxxxx’x lab” means the Xxxxxx Xxxxxx Medical Institute lab at CSHL
where Xx. Xxxxxxx Xxxxxx is the principal investigator and the scientists in that lab that are
under his direct supervision or control.
1.15. “Valid Claim” shall mean a claim in a pending patent application, or an unexpired and
issued patent that has not been revoked, held invalid, declared unpatentable or unenforceable in a
decision of a court or other body of competent jurisdiction, in a decision that is unappealable or
not appealed within the time allowed for appeal.
1.16. “Date of First Sale” shall mean the time at which Licensed Product, or Licensed
Services is/are transferred by Company to a third party for consideration.
1.17. “Affiliate” shall mean a corporation, company, or partnership, or other entity which
is controlled by Company. For the purposes of this Section 1.17 only, “control” shall mean (A) the
ownership or control of at least fifty percent (50%) or more of (i) the stock (or other securities
or voting rights) having the right to vote for directors or other governing authority thereof or
(ii) ownership interest or (B) in any country where the local law shall not permit foreign equity
participation of fifty percent (50%) or more, then the ownership or control of the maximum
percentage of such outstanding stock or voting rights permitted by local law.
2. Grant of Rights.
2.1. License Grants.
(a) Patent Rights and Biological Materials. CSHL grants to Company a non-exclusive,
worldwide, royalty-bearing license (without the right to sublicense) under its commercial rights in
the Patent Rights and Biological Materials to develop, make, have made, use, offer to sell and sell
Licensed Products and to develop and perform Licensed Services in Therapeutic Field and in Research
Field.
(b) Know-How. CSHL grants to Company a non-exclusive, worldwide royalty-bearing license
(without the right to sublicense) under its commercial rights in the Know-How necessary for the
effective exercise of the Patent Rights, and/or necessary to develop, make, have made, use, offer
to sell and sell Licensed Products and to develop and perform Licensed Services in Therapeutic
Field and in Research Field.
2.2. Rights of the U.S. Government.
To the extent that any invention claimed in the Patent Rights has been funded by the U.S. federal
government, this Agreement and the grant of any rights in Patent Rights are subject to and
4
governed by federal law as set forth in 35 U.S.C. §§201-211, and the regulations promulgated
thereunder, as amended, or any successor statutes or regulations. Company acknowledges that these
statutes and regulations reserve to the federal government a royalty-free, non-exclusive,
non-transferable license to practice any government-funded invention claimed in the Patent Rights.
If any term of this Agreement fails to conform to those laws and regulations, the relevant term is
an invalid provision and shall be modified by the parties pursuant to Section 10.11.
2.3. Additional Research Licensees.
CSHL agrees to grant a non-exclusive license, in the Research Field only (no sales), under
substantially similar terms as are in this Agreement, to up to three (3) companies that qualify as
Bona Fide Collaborators except that each such Additional Research Licensee shall pay CSHL, a
License Fee of $100,000 and a License Maintenance Fee of $100,000 per year.
2.4. Co-Marketer Licensees.
CSHL agrees to grant a non-exclusive license in the Therapeutic Field (to develop, make, have made,
use, market, and sell only), under substantially similar terms as are in this Agreement (including
but not limited to the royalty provisions in Section 4.5), to up to three (3) companies that
qualify as Co-Marketers, except that each such Co-Marketer Licensee shall also pay CSHL a License
Fee of $250,000 and a License Maintenance Fee of $75,000 per year.
The payments in Section 2.3 and this Section 2.4 shall begin on the Effective Date of each such
Additional Research License or Co-Marketer License.
3. Company Obligations Relating to Commercialization.
3.1 Within eighteen (18) months after the Effective Date, Company shall successfully undertake a
public or private offering or raising or otherwise committing at least ten million dollars
($10,000,000) to the business of Company using RNA interference technology (the
“Financing”).]
3.2. Indemnification.
(a) Indemnity by Company. Company shall indemnify, defend, and hold harmless CSHL and its
trustees, officers, faculty, students, employees, and agents and their respective successors, heirs
and assigns (the “CSHL Indemnitees”), against any liability, damage, loss, or expense
(including reasonable attorneys fees and expenses of litigation) incurred by or imposed upon any of
the CSHL Indemnitees in connection with any third party claims, suits, actions, demands or
judgments arising out of any theory of liability (including without limitation actions in the form
of tort, warranty, or strict liability and regardless of whether the action has any factual basis)
(collectively, a “Claim”) concerning any product, process, or service that is made, used,
or sold pursuant to any right or license granted under this Agreement. However, indemnification
does not apply to any liability, damage, loss, or expense to the extent directly attributable to
(i) the negligent activities or intentional misconduct of the CSHL Indemnitees; or (ii) the
settlement of a Claim by CSHL Indemnitees without the prior written approval of Company. Company
shall
5
have the sole right to settle such claim or suit; provided that Company shall not settle or
compromise any such claim or suit in a manner that is inconsistent with this Agreement, that
includes any statement of liability, fault of, or by CSHL (or any of the CSHL Indemnitees) or
involves any payment from any CSHL Indemnitee or include any statement, administration of
wrongdoing on the part of CSHL (or any of CSHL Indemnitees), or that could result in any adverse
affect on CSHL (or any of CSHL Indemnitees) or any of the Licensed Patents.
(b) Procedures. Subject to the other terms of this Agreement, CSHL Indemnitees agree to
provide the Company with prompt written notice of any claim, suit, action, demand, or judgment for
which indemnification is sought under this Agreement. Company agrees, at its own expense, to
provide attorneys reasonably acceptable to such Indemnitees to defend against any Claim. Each of
the CSHL Indemnitees shall cooperate fully with Company in the defense and will permit Company to
conduct and control the defense and the disposition of the claim, suit, or action (including all
decisions relative to litigation, appeal, and settlement). However, any such Indemnitee may retain
its own counsel, at the expense of the indemnifying party (such expenses to be reasonable and
pre-approved by the indemnifying party), if representation of the Indemnitee by the counsel
retained by the indemnifying party causes a conflict of interest as ultimately determined by said
counsel. Company agrees to keep CSHL Indemnitees informed of the progress in the defense and
disposition of the Claim and to consult with CSHL Indemnitees regarding any proposed settlement.
(c) Insurance. Subject to the other terms of this Agreement, Company shall maintain
insurance that is reasonably adequate to fulfill any potential obligation to the Indemnitees, but
not less than one million dollars ($1,000,000) for injuries to any one person arising out of a
single occurrence and five million dollars ($5,000,000) for injuries to all persons arising out of
a single occurrence. Company shall provide CSHL, upon request, with written evidence of insurance.
Company shall continue to maintain such insurance after the expiration or termination of this
Agreement during any period in which Company continues (a) to make, use, or sell a product that was
a Licensed Product under this Agreement or (b) to perform a service that was a Licensed Service
under this Agreement, and thereafter for a period of five (5) years.
(d) Product Liability Insurance. Commencing one month prior to the First Sale of Licensed
Products or Licensed Services, Company shall maintain comprehensive general liability insurance,
including products liability insurance, with reputable and financially secure liability carriers,
reasonably acceptable to Licensor to cover the activities of Company, in an amount customary in the
pharmaceutical industry and sufficient to protect the interests of CSHL. Such insurance shall
include CSHL, HHMI and their respective trustees, officers, employees, and agents as additional
insured. Company shall furnish a certificate of insurance evidencing such coverage one month prior
to the date of First Sale of any Licensed Product or Licensed Service with thirty (30) days written
notice to Licensor of cancellation or material change.
3.3. Use of CSHL Name. Subject to the other terms of this Agreement, in accordance with
Section 7.2, and except as otherwise required by Applicable Law, Company may not use the name “Cold
Spring Harbor Laboratory” or “CSHL” or any variation of that name in connection with the marketing
or sale of any Licensed Products or Licensed Services.
6
3.4. Marking of Licensed Products. To the extent commercially feasible and consistent with
prevailing business practices, Company shall xxxx all Licensed Products that are manufactured or
sold under this Agreement with the number of each issued patent under the Patent Rights that
applies to a Licensed Product.
3.5. Compliance with Law. Company shall comply with all local, state, federal, and
international laws and regulations relating to the development, manufacture, use, and sale of
Licensed Products and Licensed Services. Company expressly agrees to comply with the following:
(a) Company shall be responsible for obtaining all necessary approvals from the United States Food
& Drug Administration and any similar governmental authorities of any foreign jurisdiction in which
Company intends to make, use, or sell Licensed Products or to perform Licensed Services.
(b) Company shall comply with all United States laws and regulations controlling the export of
commodities and technical data, including without limitation all Export Administration Regulations
of the United States Department of Commerce. Among other things, these laws and regulations
prohibit or require a license for the export of certain types of commodities and technical data to
specified countries. Company hereby gives written assurance that it will comply with all United
States export control laws and regulations, that it bears sole responsibility for any violation of
those laws and regulations, and that it will indemnify, defend, and hold CSHL harmless (in
accordance with Section 3.2.) for the consequences of any violation.
(c) To the extent that any invention claimed in the Patent Rights has been partially funded by the
United States government, and only to the extent required by applicable laws and regulations,
Company agrees that any Licensed Products used or sold in the United States will be manufactured
substantially in the United States or its territories. Current law provides that if domestic
manufacture is not commercially feasible under the circumstances, CSHL may seek a waiver of this
requirement from the relevant federal agency on behalf of Company.
4. Consideration for Grant of Rights.
4.1. License Fee. Company shall pay to CSHL (a) $50,000 within sixty (60) days of the
Effective Date of this Agreement; and (b) an additional $50,000 upon the earlier of (i) the
granting of U.S. Patent application No. 09/866,557 by the U.S. Patent and Trademark Office or (ii)
the one year anniversary of the Effective Date if the Agreement has not yet been terminated. These
license fee payments are nonrefundable and are not creditable against any other payments due to
CSHL under this Agreement
4.2. License Maintenance Fee. Beginning on the first anniversary of the Effective Date,
and on each anniversary of the Effective Date thereafter during the term of the Agreement, Company
shall pay to CSHL $75,000 (license to include both Research Field and Therapeutic Field). This
annual license maintenance fee is nonrefundable and is not creditable against any other payments
due to CSHL under this Agreement
7
4.3. Milestone Payments.
(a) Company shall pay CSHL for Covered Products the following milestone payments within thirty (30)
days after the occurrence of each event achieved by Company:
| Milestone | Payment | |
Filing of IND or equivalent for each Covered Product
|
{***} | |
Entry into Phase II clinical trial or equivalent for each Covered Product
|
{***} | |
Entry into Phase III clinical trial or equivalent for each Covered Product
|
{***} | |
Filing for market approval (NDA or equivalent) in any country besides the United States
|
{***} | |
Date of First Sale of Covered Product in the United States
|
{***} | |
Date of First Sale for first three countries outside US
|
{***} |
(b) Company shall pay CSHL a one-time bonus of {***} upon the filing of the first IND
on a Covered Product.
(c) Company shall pay CSHL a one-time bonus of {***} in the event that cumulative Net
Sales of Covered Products by Company exceed $150 Million in one calendar year in the United States.
(d) Company shall pay CSHL for Developed Products for each milestone event set forth in subsection
(a), except that each payment shall be reduced by two-thirds (2/3) of the amount specified for
Covered Products.
(e) These milestone payments are nonrefundable and are not creditable against any other payments
due to CSHL under this Agreement.
(f) For any one Licensed Product, if Company or any Co-Marketer makes an equivalent payment under
another agreement with CSHL or any affiliate of CSHL with respect to that Licensed Product for a
milestone event that is specified in this Section 4.3, then payment under this Section is
satisfied, so that the aggregate payments under all CSHL (or its affiliates’) agreements with
Company or any Bona Fide Collaborator or Co-Marketer for that milestone, including this Agreement,
do not exceed the amount specified in this Section 4.3 for any one (1) Licensed Product.
4.4. Royalties. In partial consideration of the rights granted Company under the
Agreement, Company shall pay to CSHL the following royalties:
(a) Covered Products. Company shall pay to CSHL a royalty of {***} of Net Sales
of Covered Products by Company.
(b) Developed Products. Company shall pay to CSHL a royalty of {***} of Net Sales
of Developed Products by Company.
(c) Licensed Services. Company shall pay to CSHL a royalty of {***} of Net Sales
of Licensed Services by Company.
(d) These royalty payments are nonrefundable and are not creditable against any other payments due
to CSHL under this Agreement.
8
(e) For any one Licensed Product, if Company or any Co-Marketer makes an equivalent payment under
another agreement with the CSHL with respect to that Licensed Product for a royalty that is
specified in this Section 4.4, then payment under this Section is satisfied, so that the aggregate
payments under all CSHL agreements with Company or any Co-Marketer for that royalty including this
Agreement do not exceed the amount specified in this Section 4.4 for any one (1) Licensed Product.
(f) Expired or Abandoned Patent Rights. If during the Royalty Period, (a) a patent under
the Patent Rights which only covers a Covered Product or a Licensed Service, but not a Developed
Product has expired or has been abandoned in a particular country; and (b) if, as a result of such
expiration or abandonment, Company reduces its prices for Covered Products or Licensed Services in
that country, then Company and CSHL shall negotiate in good faith a reduction in the royalty rate
for Covered Products or Licensed Services to reflect the reduction in the Company’s gross margins
caused by the price reduction.
5. Royalty Reports; Payments; Records and Auditing.
5.1. First Sale. Company shall report to CSHL the date of First Sale of each Licensed
Product and the date of first commercial performance of each Licensed Service within thirty (30)
days after occurrence in each country.
5.2. Reports and Payments. Within sixty (60) days after the conclusion of each Royalty
Period, Company shall deliver to CSHL a report containing the following information:
(a) the number of Licensed Products sold to independent third parties in each country and the
number of Licensed Products used by Company in the provision of Licensed Services and other
services in each country;
(b) the number of Licensed Services provided by Company in each country;
(c) the gross sales price for each Licensed Product and the gross charge for each Licensed Service
by Company during the applicable Royalty Period in each country;
(d) calculation of Net Sales for the applicable Royalty Period in each country, including a listing
of applicable deductions;
(e) total royalty payable on Net Sales in United States dollars, together with the exchange rates
used for conversion; and
9
If no royalties are due to CSHL for any Royalty Period, the report shall so state. Concurrent with
this report, Company shall remit to CSHL any payment due for the applicable Royalty Period. All
reports under this Section 5.2 are Company Confidential Information.
5.3. Payments in United States Dollars. All payments due under this Agreement are payable
in United States dollars. Conversion of foreign currency to United States dollars shall be
made at the conversion rate existing in the United States (as reported in the Wall Street Journal)
on the last working day of the calendar quarter preceding the applicable Royalty Period. Payments
shall be without deduction of exchange, collection, or other charges.
5.4. Payments in Other Currencies. If by law, regulation, or fiscal policy of a particular
country, conversion into United States dollars or transfer of funds of a convertible currency to
the United States is restricted or forbidden, Company shall give CSHL prompt written notice of the
restriction, within the sixty-day payment deadline described in Section 5.2. Company shall pay any
amounts due CSHL through whatever lawful methods CSHL reasonably designates. However, if CSHL
fails to designate a payment method within thirty (30) days after CSHL is notified of the
restriction, Company may deposit payment in local currency to the credit of CSHL in a recognized
banking institution selected by Company and identified by written notice to CSHL, and that deposit
fulfills all obligations of Company to CSHL with respect to that payment.
5.5. Late Payments. Any undisputed payments by Company that are not paid on or before the
date payments are due under this Agreement bear interest to the extent permitted by law at one
percentage point above the Prime Rate of interest as reported in the Wall Street Journal on the
date payment is due, with interest calculated based on the number of days that payment is
delinquent.
5.6. Method of Payment. All payments under this Agreement should be made from U.S. bank
and to “COLD SPRING HARBOR LABORATORY” and sent to the address identified below. Each payment
should reference this Agreement and identify the obligation under this Agreement that the payment
satisfies.
Payee Information:
COLD SPRING HARBOR LABORATORY Bungtown Road X.X. Xxx 000 Xxxx Xxxxxx Xxxxxx, Xxx Xxxx 00000 Attn: Xx. Xxxx Xxxxxxx Director, Office of technology Transfer |
For wire transfer by Company:
CSHL Bank:
|
The Chase Manhattan Bank ABA No. 021 000 021 000 Xxxx Xx. Xxxxxxxxxx, XX 00000 |
10
Payee:
|
Cold Spring Harbor Laboratory Account no. 777521385 |
5.7. Withholding and Similar Taxes. Company shall be responsible for all the taxes and
wire transfer fees or the like related with Royalty payments and all other payments due to CSHL
under this Agreement.
5.8. Auditing, Records and Reporting. Company shall keep and maintain records of sales and
the discovery and development of Licensed Products and Licensed Services pursuant to this
Agreement. Such records shall be open to inspection at any reasonable time during normal business
hours not more often than once each calendar year within five (5) years after the royalty period to
which such records relate by Certified Public Accountant selected by CSHL, to whom Company has no
reasonable objection, who October 29, 2007 shall have the right to examine and make abstracts of
the records kept pursuant to this Agreement and report findings of said examination of records to
CSHL insofar as it is necessary to evidence any mistake or impropriety on the part of Company.
6. Patents and Infringement.
6.1. Responsibility for CSHL Patent Rights. CSHL has the sole rights for and controls the
preparation, filing, prosecution, and maintenance of all CSHL Patent Rights, using patent counsel
of CSHL’s choice.
6.2. Notification of Infringement. Company agrees to reasonably provide written notice to
CSHL promptly after becoming aware of any infringement of the Patent Rights. CSHL is not obligated
to prosecute any such infringers and CSHL’s failure to do so does not in any way release Company of
its obligations under this Agreement.
7. Confidential Information; Publications; Publicity.
7.1. Confidential Information.
(a) Designation. Confidential Information that is disclosed in writing shall be marked
with a legend indicating its confidential status (such as, “Confidential” or “Proprietary”).
Confidential Information that is disclosed orally or visually shall be documented in a written
notice prepared by the Disclosing Party and delivered to the Receiving Party within thirty (30)
days of the date of disclosure. The notice shall summarize the Confidential Information disclosed
to the Receiving Party and reference the time and place of disclosure.
(b) Obligations. For a period of five (5) years after disclosure of any portion of
Confidential Information, the Receiving Party shall (i) maintain Confidential Information in
confidence, except that the Receiving Party may disclose or permit the disclosure of any
Confidential Information to its trustees or directors, officers, employees, consultants, and
11
advisors who are obligated to maintain the confidential nature of Confidential Information and who
need to know Confidential Information for the purposes of this Agreement; (ii) use Confidential
Information solely for the purposes of this Agreement; and (iii) allow its trustees or directors,
officers, employees, consultants, and advisors to reproduce the Confidential
Information only to the extent necessary for the purposes of this Agreement, with all reproductions
being Confidential Information.
(c) Exceptions. The obligations of the Receiving Party under Subsection 7.1.(b) above do
not apply to the extent that the Receiving Party can demonstrate that Confidential Information (i)
was in the public domain prior to the time of its disclosure under this Agreement; (ii) entered the
public domain after the time of its disclosure under this Agreement through means other than an
unauthorized disclosure resulting from an act or omission by the Receiving Party; (iii) was
independently developed or discovered by the Receiving Party without use of the Confidential
Information as demonstrated by contemporaneous documentation; (iv) is or was disclosed to the
Receiving Party at any time, whether prior to or after the time of its disclosure under this
Agreement, by a third party having no fiduciary relationship with the Disclosing Party and
having no obligation of confidentiality with respect to the Confidential Information; or (v) is
required to be disclosed to comply with applicable laws or regulations or with a court or
administrative order, provided that the Disclosing Party receives reasonable prior written notice
of the intent to disclose and is afforded an opportunity to oppose, limit or secure confidential
treatment for such required disclosure.
(d) Ownership and Return. The Receiving Party acknowledges that the Disclosing Party (or a
third party entrusting its own information to the Disclosing Party) owns the Confidential
Information in the possession of the Receiving Party. Upon expiration or termination of this
Agreement, or at the request of the Disclosing Party, the Receiving Party shall return to the
Disclosing Party all originals, copies, and summaries of documents, materials, and other tangible
manifestations of Confidential Information in the possession or control of the Receiving Party,
except that the Receiving Party may retain one copy of the Confidential Information in the
possession of its legal counsel solely for the purpose of monitoring its obligations under this
Agreement.
7.2. Publicity.
7.2.1 Press Release Announcing this Agreement. CSHL shall allow Company to use the names
Cold Spring Harbor Laboratory and Xx. Xxxxxxx Xxxxxx in conjunction with a press release announcing
the signing of this Agreement. Company shall submit a draft press release to CSHL and CSHL shall
review the same within twenty-four (24) hours of submission. CSHL shall work diligently with
Company to approve a press release acceptable to both parties so that such a release can be
published as soon as possible after execution of this Agreement.
7.2.2 Restrictions. Other than the press release referred to in Section 7.2.1, above,
Company may not use the name Cold Spring Harbor Laboratory or the names of any of its trustees,
officers, faculty, students, employees, or agents, or any adaptation of their names, or any terms
of this Agreement in any promotional material or other public announcement or disclosure without
the prior written consent of CSHL. The foregoing notwithstanding, Company
12
may disclose that information without the consent of CSHL in any prospectus, offering memorandum, or other document
or filing but only to the extent legally required by applicable securities laws or other applicable
law or regulation, provided that Company provides CSHL at
least ten (10) days prior written notice of the proposed text for the purpose of giving CSHL the
opportunity to comment on the text.
8. Term and Termination.
8.1. Term. This Agreement commences on the Effective Date and remains in effect until (a)
the expiration of all issued patents within the Patent Rights or (b) for a period of ten (10) years
after the Effective Date if no patents have issued within the Patent Rights within that ten-year
period, unless earlier terminated in accordance with the provisions of this Agreement.
8.2. Voluntary Termination by Company. Company may terminate this Agreement for any reason
upon ninety (90) days prior written notice to CSHL, but the obligation to pay the milestones and
royalties under Sections 4.3 and 4.4, respectively shall survive any such Termination to the extent
that the sale of Licensed Products and Licensed Services are in accordance with Section 8.6.
8.3. Termination for Default. If either party commits a material breach of its obligations
under this Agreement and fails to cure that breach within sixty (60) days after receiving written
notice of the breach, the other party may terminate this Agreement immediately upon written notice
to the party in breach. If the alleged breach involves nonpayment of any undisputed amounts due
CSHL under this Agreement, Company has only one opportunity to cure a material breach for which it
receives notice as described above. Any subsequent material breach by Company will entitle CSHL to
terminate this Agreement immediately upon written notice to Company, without the sixty-day cure
period.
8.4. Force Majeure. Neither party is responsible for delays resulting from causes beyond
its reasonable control, including without limitation fire, explosion, flood, war, strike, or riot,
provided that the nonperforming party uses commercially reasonable efforts to avoid or remove those
causes of nonperformance and continues performance under this Agreement with reasonable dispatch
whenever the causes are removed.
8.5. Termination by CSHL. This Agreement may also be terminated by CSHL immediately upon
written notice if Company or any of its Affiliates (a) provokes an interference with any of the
Patent Rights, (b) commences any action challenging the scope, validity or enforceability of any of
the Patent Rights or (c) to the extent Company has such control, encourages, assists or, permits
any third party to do any of the foregoing.
8.6. Effect of Termination. In addition to the survival provisions in Section 8.2, the
following provisions survive the expiration or termination of this Agreement Articles 1, 5 and 9;
Sections 3.2, 3.5, 4.3, 4.4, 6.3, 7.1, 7.2, 8.6, and 10.9 Upon the early termination of this
Agreement, Company may complete and sell any work-in-progress and inventory of Covered Products and
Licensed Services that exist as of the effective date of termination, provided that (a) Company is
current in payment of all amounts due CSHL under this Agreement, (b) Company
13
pays CSHL the applicable royalty on sales of Licensed Products and Licensed Services in accordance with the terms
of this Agreement, and (c) Company completes and sells all work-in-progress and inventory of
Licensed Products and Licensed Services within twelve (12) months
after the effective date of termination. Notwithstanding the foregoing, Company may continue to
provide Licensed Services as defined in Section 1.6(b) or 1.6(c) following termination. The
obligation to pay milestones and royalties on Developed Products under Sections 4.3(a), 4.3(d) and
4.4(b), and on Licensed Services under Section 4.4(c) shall survive termination.
9. Dispute Resolution.
9.1. Procedures Mandatory. The parties agree that any dispute arising out of or relating
to this Agreement will be resolved solely by means of the procedures set forth in this Article, and
that these procedures constitute legally binding obligations that are an essential provision of
this Agreement. However, all procedures and deadlines specified in this Article may be modified
only by written agreement of the parties. If either party fails to observe the procedures of this
Article, as modified by their written agreement, the other party may bring an action for specific
performance in any court of competent jurisdiction.
9.2. Dispute Resolution Procedures.
(a) Negotiation. In the event of any dispute arising out of or relating to this Agreement,
the affected party shall notify the other party, and the parties shall attempt in good faith to
resolve the matter within ten (10) days after the date of notice (the “Notice Date”). Any
disputes not resolved by good faith discussions shall be referred to senior executives of each
party, who shall meet at a mutually acceptable time and location within thirty (30) days after the
Notice Date and attempt to negotiate a settlement or resolution.
(b) Mediation. If the matter remains unresolved within sixty (60) days after the Notice
Date, or if the senior executives fail to meet within thirty (30) days after the Notice Date,
either party may initiate mediation upon written notice to the other party, whereupon both parties
shall engage in a mediation proceeding under the then current CPR Institute for Dispute Resolution
(“CPR”) Model Procedure for Mediation of Business Disputes, except that specific provisions of this
Section override inconsistent provisions of the CPR Model Procedure. The mediator will be selected
from the CPR Panels of Neutrals. If the parties cannot agree upon the selection of a mediator
within ninety (90) days after the Notice Date, then upon the request of either party, the CPR shall
appoint the mediator. The parties shall attempt to resolve the dispute through mediation until one
of the following occurs: (i) the parties reach a written settlement or resolution; (ii) the
mediator notifies the parties in writing that they have reached an impasse; (iii) the parties agree
in writing that they have reached an impasse; or (iv) the parties have not reached a settlement or
resolution within one hundred twenty (120) days after the Notice Date.
(c) Trial Without Jury. If the parties fail to resolve the dispute through mediation, or
if neither party elects to initiate mediation, each party may pursue any other remedies legally
available to resolve the dispute.
9.3. Preservation of Rights Pending Resolution.
14
(a) Performance to Continue. Each party shall continue to perform its obligations under
this Agreement pending final resolution of any dispute arising out of or relating to this
Agreement. However, a party may suspend performance of its obligations during any period in which
the other party fails or refuses to perform its obligations.
(b) Provisional Remedies. Although the procedures specified in this Article are the
exclusive procedures for resolution of disputes arising out of or relating to this Agreement,
either party may seek a preliminary injunction or other provisional equitable relief if, in its
reasonable judgment, that action is necessary to avoid irreparable harm to itself or to preserve
its rights under this Agreement.
(c) Statute of Limitations. The parties agree that all applicable statutes of limitation
and time-based defenses (such as, estoppel and laches) are tolled while the procedures set forth in
Subsections 9.2.(a) and 9.2(b) are pending. The parties shall take any actions necessary to
effectuate this result.
10. Miscellaneous.
10.1. Representations and Warranties. CSHL represents that its employees and members of
its staff have assigned to CSHL their entire right, title, and interest in the Patent Rights and
Biological Material and Know How and the other CSHL Intellectual Property and that it has authority
to grant the rights and licenses set forth in this Agreement. NEITHER CSHL, NOR COMPANY, MAKES ANY
OTHER WARRANTIES CONCERNING THE PATENT RIGHTS, KNOW-HOW, AND BIOLOGICAL MATERIALS, INCLUDING
WITHOUT LIMITATION ANY EXPRESS OR IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE, AND IN THE CASE OF COMPANY ITS OBLIGATIONS, AND THE PRODUCTS AND SERVICES IT WILL BE
DEVELOPING AND COMMERCIALIZING HEREUNDER. SPECIFICALLY, CSHL MAKES NO WARRANTY OR REPRESENTATION
(A) REGARDING THE VALIDITY OR SCOPE OF THE PATENT RIGHTS, (B) THAT THE EXPLOITATION OF THE PATENT
RIGHTS, BIOLOGICAL MATERIALS, KNOW-HOW OR ANY LICENSED PRODUCT OR LICENSED SERVICE WILL NOT
INFRINGE ANY PATENTS OR OTHER INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY, AND (C) THAT ANY THIRD
PARTY IS NOT CURRENTLY INFRINGING OR WILL NOT INFRINGE THE PATENT RIGHTS.
10.2 Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR ANY SPECIAL,
CONSEQUENTIAL, LOST PROFIT, EXPECTATION, PUNITIVE OR OTHER INDIRECT DAMAGES IN CONNECTION WITH ANY
CLAIM ARISING OUT OF OR RELATED TO THIS AGREEMENT, WHETHER GROUNDED IN TORT (INCLUDING NEGLIGENCE),
STRICT LIABILITY, CONTRACT, OR OTHERWISE.
10.3. Compliance with Law and Policies. Company agrees to comply with applicable law and
the conflict of interest policies of CSHL in the area of technology transfer and shall promptly
notify CSHL of any violation that Company knows or has reason to believe has occurred or is likely
to occur.
15
10.4. Counterparts. This Agreement may be executed in one or more counterparts, each of
which is an original, and all of which together are one instrument.
10.5. Headings. All headings are for convenience only and do not affect the meaning of any
provision of this Agreement.
10.6. Binding Effect. This Agreement is binding upon and inures to the benefit of the
parties and their respective permitted successors and assigns.
10.7. Assignment. Neither party may assign this Agreement without the prior written
consent of the other party, which consent may not be unreasonably withheld, except that Company may
assign this Agreement to an or to a successor in connection with the merger, consolidation, or sale
of all or substantially all of its assets or that portion of its business to which this Agreement
relates.
10.8. Amendment and Waiver. This Agreement may be amended, supplemented, or otherwise
modified only by means of a written instrument signed by both parties. Any waiver of any rights or
failure to act in a specific instance relates only to that instance and is not an agreement to
waive any rights or fail to act in any other instance.
10.9. Governing Law. This Agreement shall be governed by and construed in accordance with
the laws of the State of New York irrespective of any conflicts of law principles.
10.10. Notice. Any notices required or permitted under this Agreement shall be in writing,
shall specifically refer to this Agreement, and shall be sent by recognized national overnight
courier, or registered or certified mail, postage prepaid, return receipt requested, to the
following addresses:
If to CSHL:
COLD
SPRING HARBOR LABORATORY Bungtown Road X.X. Xxx 000 Xxxx Xxxxxx Xxxxxx, Xxx Xxxx 00000 |
Attn: Xx.
Xxxx Xxxxxxx Director, Office of technology Transfer |
If to Company: |
RXi
Corporation Suite 650 00000 Xxx Xxxxxxx Xxxx. Xxx Xxxxxxx, XX 00000 |
16
Attn: |
Chief Executive Officer |
All notices under this Agreement are effective upon receipt. A party may change its contact
information immediately upon written notice to the other party in the manner provided in this
Section.
10.11 Severability. If any provision of this Agreement is held invalid or unenforceable
for any reason, the invalidity or unenforceability does not affect any other provision of this
Agreement, and the parties shall negotiate in good faith to modify the Agreement to preserve (to
the extent possible) their original intent. If the parties fail to reach a modified agreement
within sixty (60) days after the relevant provision is held invalid or unenforceable, then the
dispute shall be resolved in accordance with the procedures set forth in Article 9. While the
dispute is pending resolution, this Agreement shall be construed as if the provision were deleted
by agreement of the parties.
10.12. Entire Agreement. This Agreement constitutes the entire agreement between the
parties with respect to its subject matter and supersedes all prior agreements or understandings
between the parties relating to its subject matter.
The parties have caused this Agreement to be executed by their duly authorized representatives as
of the date hereof.
COLD SPRING HARBOR LABORATORY
|
||
By: Xxxx Xxxxxxx |
||
Date: |
||
By: |
Chief Executive Officer
Date: 3/15/07
17
EXHIBIT A
CSHL-P-010/HANNON01
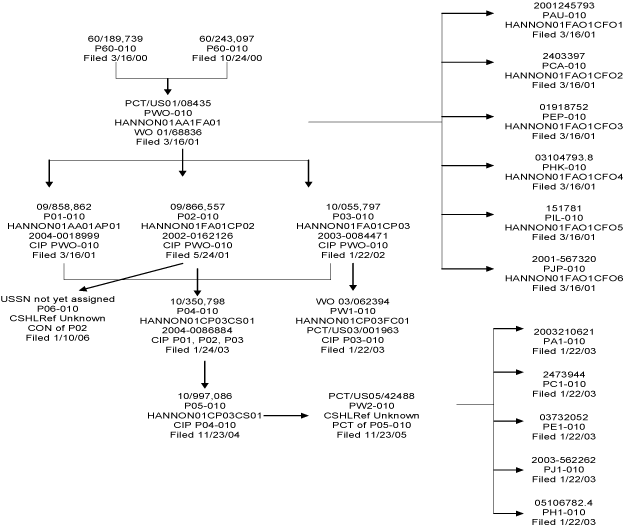
| Client-Matter/Subcase | Case | Application | Publication | Patent | Status | |||||||||
| Country Name | Type | Number/Date | Number/Date | Number/Date | Expiration Date | |||||||||
CSHL-P-010 A1
|
PCT | 2003210621 | Pending | |||||||||||
Australia
|
22-Jan-2003 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXXXXX, Xxxxx; XXXXX, Xxx; XXXXXX, Xxxxxxx X.; XXXXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx | |||||||||||||
CSHL-P-010 AU
|
ORD | 2001245793 | Pending | |||||||||||
Australia
|
16-Mar-2001 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
XXXX-X-000 XX
|
XXX | 0000000 | Xxxxxxx | |||||||||||
Xxxxxx
|
22-Jan-2003 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXXXXX, Xxxxx; XXXXX, Xxx; XXXXXX, Xxxxxxx X.; XXXXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx | |||||||||||||
CSHL-P-010 CA
|
ORD | 2403397 | Pending | |||||||||||
Canada
|
16-Mar-2001 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
CSHL-P-010 E1
|
PCT | 03732052.0 | 1546174 | Published | ||||||||||
European Patent Convention
|
22-Jan-2003 | 29-Jun-2005 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXXXXX, Xxxxx; XXXXX, Xxx; XXXXXX, Xxxxxxx X.; XXXXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx | |||||||||||||
CSHL-P-010 EP
|
ORD | 01918752.5 | 1272630 | Published | ||||||||||
European Patent Convention
|
16-Mar-2001 | 08-Jan-2003 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
CSHL-P-010 H1
|
ORD | 05106782.4 | 1073660 | Published | ||||||||||
Hong Kong
|
22-Jan-2003 | 14-Oct-2005 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXXXXX, Xxxxx; XXXXXX, Xxxxxxx X.; XXXXXXXX, Xxxxxxx X.; XXXXX, Xxx; XXXXXXX, Xxxxxxx | |||||||||||||
CSHL-P-010 HK
|
ORD | 03104793.8 | Published | |||||||||||
Hong Kong
|
07-Jul-2003 | 13-Oct-2003 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
CSHL-P-010 IL
|
ORD | 151781 | Pending | |||||||||||
Israel
|
17-Sep-2002 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
| Client-Matter/Subcase | Case | Application | Publication | Patent | Status | |||||||||
| Country Name | Type | Number/Date | Number/Date | Number/Date | Expiration Date | |||||||||
CSHL-P-010 JI
|
PCT | 2003-562262 | 533484 | Published | ||||||||||
Japan
|
22-Jan-2003 | 10-Nov-2005 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXXXXX, Xxxxx; XXXXX, Xxx; XXXXXX, Xxxxxxx X.; XXXXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx | |||||||||||||
CSHL-P-010 JP
|
ORD | 2001-567320 | 2003-52636 | Published | ||||||||||
Japan
|
16-Mar-2001 | 09-Sep-2003 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
CSHL-0-010 WI
|
ORD | US03/001963 | 03/062394 | National | ||||||||||
Patent Cooperation Treaty
|
22-Jan-2003 | 31-Jul-2003 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXXXXX, Xxxxx; XXXXX, Xxx; XXXXXX, Xxxxxxx X.; XXXXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx | |||||||||||||
CSHL-P-010 W2
|
ORD | US05/42488 | Pending | |||||||||||
Patent Cooperation Treaty
|
23-Nov-2005 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx X. | |||||||||||||
CSHL-P-010 WO
|
ORD | US01-08435 | WO01/68836 | National | ||||||||||
Patent Cooperation Treaty
|
16-Mar-2001 | 20-Sep-2001 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
CSHL-P-010 01
|
CIP | 09/858862 | 04/0018999 | Published | ||||||||||
| 16-May-2001 | 29-Jan-2004 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXX, Xxx; XXXXXXXXX, Xxxxx | |||||||||||||
XXXX-X-000 00 |
CIP | 090-866557 | 0162126-A1 | Allowed | ||||||||||
United States of America
|
24-May-2001 | 31-Oct-2002 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X. | |||||||||||||
CSHL-P-010 03 |
CIP | 10/055797 | 0084471 | Published | ||||||||||
United States of America |
22-Jan-2002 | 01-May-2003 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X. | |||||||||||||
CSHL-P-010 04 |
CIP | 10/350798 | 0086884 | Published | ||||||||||
United States of America |
24-Jan-2003 | 06-May-2004 | ||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx; XXXXXXXX, Xxxxxxx X.; XXXXXXX, Xxxxxxx | |||||||||||||
| Client-Matter/Subcase | Case | Application | Publication | Patent | Status | |||||||||
| Country Name | Type | Number/Date | Number/Date | Number/Date | Expiration Date | |||||||||
CSHL-P-010 05
|
CIP | 10/997086 | Pending | |||||||||||
United States of America
|
23-Nov-2004 | |||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | XXXXXX, Xxxxxxx X.; XXXXXXXX, Xxxxxxx X.; XXXXXX, Xxxxxxx X. | |||||||||||||
CSHL-P-010 -6
|
CON | 10-Jan-2006 | Pending | |||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||
CSHL-P-010 60
|
PRO | 60/189739 | Expired | |||||||||||
| 16-Mar-2000 | ||||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; BEACH, Xxxxx X. | |||||||||||||
CSHL-P-010 61
|
PRO | 60/243097 | Expired | |||||||||||
| 24-Oct-2000 | ||||||||||||||
| Title: | METHODS AND COMPOSITIONS FOR RNA INTERFERENCE | |||||||||||||
| Inventor(s): | HAMMOND, Scott, XXXXXX, Xxxxxxx X.; XXXXXXXXX, Xxxxx; XXXXX, Xxx | |||||||||||||

