TECHNOLOGY TRANSFER AND DEVELOPMENT SERVICES SUBCONTRACT THIS TECHNOLOGY TRANSFER AND DEVELOPMENT SERVICES SUBCONTRACT (the “Subcontract”) is made as of September 17, 2009 (the “Effective Date”) by and between Diosynth RTP Inc., a Delaware...
Exhibit 10.52
Confidential Materials Omitted and Filed Separately with the Securities and Exchange Commission
|
|
TECHNOLOGY TRANSFER AND DEVELOPMENT SERVICES SUBCONTRACT THIS TECHNOLOGY TRANSFER AND DEVELOPMENT SERVICES SUBCONTRACT (the “Subcontract”) is made as of September 17, 2009 (the “Effective Date”) by and between Diosynth RTP Inc., a Delaware corporation, located at 000 X. Xxxxxx Xxxxxxx Xxxx, Xxxxxxxxxxx, Xxxxx Xxxxxxxx 00000 (“Diosynth”), and PharmAthene, Inc., a Delaware corporation, located at Xxx Xxxx Xxxxx, Xxxxx 000, Xxxxxxxxx, XX 00000 (“PharmAthene”). Each of Diosynth and PharmAthene may be referred to herein separately as a “Party” and collectively as the “Parties.” WHEREAS, PharmAthene owns rights to a recombinant protective antigen for anthrax vaccine (rPA) product (the “Product”); WHEREAS, PharmAthene has been awarded a prime contract in respect of the development of an anthrax vaccine, numbered NO-A1-30052 with the National Institutes of Health (“NIH”), an agency of the U.S. Government, which contract was recently transferred from NIH to the Biomedical Advanced Research and Development Authority (“BARDA”) within the Department of Health and Human Services (as amended from time to time, and together with any successor or replacement contract (s), collectively the “Prime Contract”); WHEREAS, PharmAthene anticipates further funding of its development of recombinant protective antigen (rPA) for anthrax (rPA); WHEREAS, Diosynth is a contract manufacturing organization (“CMO”) providing a range of services related to the development and manufacture of biopharmaceutical products; WHEREAS, prior to the date hereof, another CMO provided certain development and manufacturing services to PharmAthene related to the supply of the bulk drug substance for the Product (“Drug Substance”); WHEREAS, PharmAthene desires to transfer the technology related to, among other things, the development, scale-up and manufacture of the Drug Substance from the prior CMO to Diosynth (the “Technology Transfer”); WHEREAS, Diosynth desires to assist PharmAthene with the Technology Transfer to its organization and facilities and to begin development work related to the manufacturing process for the Drug Substance in support of the Prime Contract (all of the foregoing, including the Technology Transfer and all other services included in the Scope, collectively, the “CMO Services”); WHEREAS, PharmAthene and Diosynth may in the future decide to amend this Subcontract to add additional development services and provisions relating to the Drug Substance, including without limitation validation, verification and conformance manufacturing; |
|
|
5 Scope, or associated Program timeline may be changed. As used herein, “Change Order” means a written document signed by both Parties describing in reasonable detail a change in the Program Price and Payment Schedule, the Scope, and/or associated Program timeline. (b) Change Orders must be agreed by both Parties in writing, and consent to a Change Order shall not be unreasonably withheld, conditioned or delayed by either Party. Either Party may propose in writing a change to the Program Price and Payment Schedule, the Scope and/or associated Program timeline, which shall contain the terms of such proposed change in reasonable detail. In such event, Diosynth shall prepare and deliver to PharmAthene within ten (10) days a proposed Change Order which shall include a statement of all effects on the Program Price and Payment Schedule, the Scope and/or associated Program timeline. The proposed Change Order shall include (i) cost information in reasonable detail for all Pass- Through Costs and (ii) a fixed price for all other services related to the proposed change(s). Diosynth agrees that such Pass-Through Costs and fixed price quote will be commercially reasonable. Within ten (10) days thereafter, PharmAthene shall respond to Diosynth indicating whether it approves or rejects the proposed Change Order, and if the Change Order is approved the Parties shall promptly execute same, whereupon the Change Order shall be effective to modify the Program Price and Payment Schedule, the Scope and/or associated Program timeline. In the event that the Parties cannot agree on the terms of any Change Order and the Parties do not resolve same promptly through good faith negotiations, then the dispute procedures of Article 26 hereof shall apply. Unless directed otherwise by PharmAthene, Diosynth shall continue to perform all work not affected by the proposed Change Order in accordance with this Subcontract during the pendency of any dispute regarding such proposed Change Order, provided that PharmAthene shall continue to comply with its payment obligations hereunder. Notwithstanding the preceding sentence, Diosynth shall not be required to continue performance of the CMO Services during the pendency of any dispute to the extent that performance of such work would be impossible in the absence of such a Change Order. Except in the case of a stop work order as described in Section 2.3 below, each Party shall notify the other within fifteen (15) days after first becoming aware of the existence of circumstances giving rise to a Change Order. (c) Except as otherwise provided herein, all notices to be furnished by Diosynth shall be sent to PharmAthene’s Director of Contracts at the address set forth in Article 20 with a copy to PharmAthene’s General Counsel at the same address. 2.3 Stop-Work Orders. (a) PharmAthene may, at any time, by written order to Diosynth, require Diosynth to stop all, or any part, of the work called for by the Scope for a period of ninety (90) days after the order is delivered to Diosynth, and for any further period to which the Parties may agree. The order shall be specifically identified as a stop-work order issued under this Section 2.3. Upon receipt of the order, Diosynth shall immediately comply with its terms and take all reasonable steps to minimize the incurrence of costs allocable to the work covered by the order during the period of work stoppage. Within a period of 90 days after a stop-work |
|
|
3 (i) As soon as practicable after PharmAthene provides Diosynth with a notice of rejection, the Parties shall undertake an investigation of such Product Deliverables in accordance with Article 9 of the Quality Agreement. Upon commencement of the investigation, PharmAthene shall immediately deliver to Diosynth all potentially Deviating Product. The Parties shall cooperate in good faith during such investigation and use reasonable best efforts to complete the investigation. Diosynth shall notify PharmAthene within five (5) days of the conclusion of such investigation, whether Diosynth accepts or disputes PharmAthene’s assertion(s) that certain Product Deliverables are a Deviating Product. (ii) After the completion of such investigation (or earlier, if agreed to by the Parties), at PharmAthene’s request, whether or not Diosynth accepts PharmAthene’s assertion that certain Product Deliverables are Deviating Product, Diosynth shall process and deliver replacement Product Deliverables (“Replacement Product”) as soon as reasonably possible, provided that Diosynth shall not be required to cease, interrupt or otherwise disturb campaigns in progress. If Diosynth disputes in writing whether the Product Deliverables are Deviating Product or whether such Deviating Product was caused by the failure of Diosynth, PharmAthene shall pay Diosynth, within thirty (30) days of such Replacement Product request: (A) for such Replacement Product in accordance with the prices set forth in the Program Price and Payment Schedule and (B) for all Pass-Through Costs purchased for such Replacement Product, plus a fee equal to seven and one half percent (7.5%) of such actual costs. Following completion of an investigation and dispute resolution if any pursuant to this Section 3.4 (c), Diosynth shall reimburse PharmAthene such payment, in accordance with Section 3.4 (c)(iv), to the exent such Deviating Product is agreed or determined to be caused by the failure of Diosynth. (iii) If Diosynth disputes PharmAthene’s assertion that certain Product Deliverables are Deviating Product or Diosynth believes that the Deviating Product was not caused by any failure of Diosynth, then the Parties shall work together in good faith to resolve the dispute. If the Parties are unable to resolve such a dispute through good faith negotiation, then the dispute procedures of Article 26 hereof shall apply. (iv) If Diosynth accepts PharmAthene’s assertion or if it is determined through the dispute procedures of Article 26 hereof that such Deviating Product was caused by the failure of Diosynth and PharmAthene previously paid for the Replacement Product, then Diosynth shall, within thirty (30) days of such acceptance or determination, reimburse PharmAthene for the price of the Replacement Product to the extent that such Deviating Product was caused by the failure of Diosynth |
|
|
5 (i) If Diosynth is in default of its material obligations under this Subcontract, then PharmAthene may notify Diosynth in writing of any such default. (1) Diosynth shall have a period of twenty (20) days from the date of receipt of such notice within which to cure such default, and shall diligently pursue efforts to cure such default at all reasonable times. If Diosynth fails to cure within such twenty (20) day period, then PharmAthene shall have the right to immediately terminate all or any part of this Subcontract by providing written notice to Diosynth. In the event of such termination, PharmAthene shall not be liable to pay for any work not Accepted; provided however that PharmAthene’s Acceptance shall not be unreasonably withheld, conditioned or delayed. Notwithstanding the foregoing, Diosynth shall upon request deliver to PharmAthene any supplies and materials, manufacturing materials, and manufacturing information that Diosynth has specifically produced or acquired for this Subcontract and PharmAthene shall compensate Diosynth for the reasonable cost of such supplies, materials and/or information and any costs associated with these deliverables. (2) With respect to production runs or other activities that are not capable of being cured in twenty (20) days from the date of receipt of such notice, Diosynth shall initiate actions during such twenty (20) day period that are reasonably anticipated to cure the default within a reasonable period (not to exceed one hundred and fifty (150) days), and thereafter cure such default within a reasonable period (not to exceed one hundred and fifty (150) days). If Diosynth fails to cure within such period, then PharmAthene shall have the right to immediately terminate all or any part of this Subcontract by providing written notice to Diosynth in accordance with Section 4.2(a)(i)(1). (ii) If PharmAthene is in default of its material obligations under this Subcontract, Diosynth shall promptly notify PharmAthene in writing of any such default. PharmAthene shall have a period of twenty (20) days from the date of receipt of such notice within which to cure such default provided that it diligently continues efforts to cure such default at all reasonable times. With respect to non-monetary performances that are not capable of being cured in twenty (20) days from the date of receipt of such notice, PharmAthene shall initiate actions during such twenty (20) day period that are reasonably anticipated to cure the default within a reasonable period (not to exceed sixty (60) days), and thereafter cure such default within a reasonable period (not to exceed sixty (60) days). If PharmAthene fails to so cure, then Diosynth shall have the right to immediately terminate this Subcontract by providing written notice to Diosynth. |
|
|
8 personnel, PharmAthene may request that Diosynth provide to PharmAthene reasonable consulting services related to the manufacturing, quality control, quality assurance, and the CMC (chemistry, manufacturing and control) part of the Drug Substance registration process; provided however that Diosynth shall not be required to provide consulting services under this Section 4.4 for so long as PharmAthene has not paid Diosynth amounts due to Diosynth hereunder. PharmAthene shall pay Diosynth for all reasonable expenses related to the consulting services provided under this Section 4.4, and shall compensate Diosynth at its then current rates for such consulting services. All technical assistance to be provided by Diosynth pursuant to this Section 4.4 shall be in accordance with a plan provided to Diosynth by PharmAthene and agreed upon by the Parties. To the extent transferable, Diosynth shall also transfer any license(s), permit(s), or approval(s) obtained specifically for the manufacturing of the Drug Substance. The Parties agree to work in good faith for the transfer of any information and materials related to the Drug Substance, PharmAthene’s Intellectual Property Rights and any Diosynth Intellectual Property Rights required under this Section 4.4(a). (b) Subject to the limitations set forth in Section 4.4(a), the technology transfer contemplated by Section 4.4(a) shall include without limitation at least the following activities: (i) Diosynth shall provide PharmAthene access to any and all Diosynth Intellectual Property Rights (as defined in Article 8) that are used in the manufacture of the Drug Substance in accordance with the Subcontract and to support regulatory filings for the Drug Substance (including, without limitation, analytical testing methods, protocols, process descriptions, batch records, specifications and other process and manufacturing data and documentation); (ii) Diosynth shall provide PharmAthene with reasonable access to Diosynth employees with expertise in development and manufacturing the Drug Substance to answer questions related to such technology transfer; and (iii) Diosynth shall allow PharmAthene reasonable access to the Facility at reasonable times on business days for any reasonable purpose connected with such technology transfer; provided, however, that such access must be scheduled in advance with Diosynth and may not interfere with Diosynth’s business operations (such training sessions, assistance, cooperation, access to Diosynth employees and access to the Facility shall be limited to (i) PharmAthene employees and (ii) any consultants hired by PharmAthene and approved by Diosynth, with such approval not to be unreasonably denied, provided each consultant signs a confidentiality agreement that consistent with the confidentiality obligations established in this Subcontract. PharmAthene shall provide Diosynth with a list of proposed visitors to the Facility at least one (1) business day in advance of such visit. PharmAthene shall pay Diosynth for all reasonable expenses related to Diosynth’s provision of Facility access under this Section 4.4(b). (c) The technology transfer contemplated in Section 4.4(a) and (b) above shall be provided promptly and in accordance with the terms of this Subcontract for the provision of CMO Services. In the event that this Subcontract is terminated (i) by Diosynth due to a default of PharmAthene under Section 4.2(a) or due to an Insolvency Event with respect to PharmAthene described in Section 4.2(c), or (ii) by PharmAthene under Section 4.2(b), PharmAthene shall pay for the technology transfer contemplated in Section 4.4(a) and (b) above at Diosynth’s then-current hourly rates. If this Subcontract is terminated by |
|
|
17 or both to which the provisions of the SAFETY Act apply to limit the liability of PharmAthene and/or Diosynth, each party retains responsibility for Losses (as defined below), including business interruption Losses, that it sustains, and for Losses sustained by its own employees resulting from an Act of Terrorism when Drug Substance has been deployed in defense against, response to, or recovery from such act, but only to the extent that such Party’s liability is actually limited by the application of the SAFETY Act. 10.2 PharmAthene Indemnification. PharmAthene agrees to defend, indemnify and hold Diosynth, its Affiliates, officers, directors, employees and agents harmless against any and all losses, damages, fines, costs, claims, demands, judgments and liability (including reasonable legal fees and court costs) (collectively, “Losses”) to which the provisions of the SAFETY Act do not apply resulting from, or relating to: (i) the negligence, gross negligence or intentional misconduct of PharmAthene; (ii) the infringement or alleged infringement of the Product on the intellectual property rights of a third party; (iii) PharmAthene’s breach of its agreements, representations or warranties under this Subcontract or (iv) the handling, distribution, storage, sales, use or testing of the Drug Substance and/or Product that is manufactured by Diosynth in accordance with this Agreement, including without limitation Section 2.1(a) and the Scope; except in any such case to the extent that any such Losses are due to the negligence or intentional misconduct of Diosynth or its Affiliates or their respective officers, employees, contractors or agents;. 10.3 Diosynth Indemnification. Diosynth agrees to defend, indemnify and hold PharmAthene, its Affiliates, officers, directors, employees and agents harmless against any and all Losses to which the provisions of the SAFETY Act do not apply, resulting from, or relating to (i) the negligence, gross negligence or intentional misconduct of Diosynth or its subcontractors; (ii) the infringement or alleged infringement of any Diosynth Intellectual Property, including without limitation any Diosynth Background IP, on the intellectual property rights of a third party; (iii) Diosynth’s breach of its agreements, representations or warranties under this Subcontract or (iv) failure to take safety precautions to prevent known risks of the Sponsor Deliverables, the Drug Substance or the Product as communicated in writing to Diosynth by PharmAthene; except in any such case to the extent that any such Losses are due to the negligence or intentional misconduct of PharmAthene or its Affiliates or their respective officers, employees, contractors or agents. 10.4 Indemnification Procedures. (a) All indemnification claims in respect of a Party, its Affiliates or their respective directors, officers, employees and agents (each, an “Indemnitee”) will be made solely by the applicable Party (the “Indemnified Party”). The Indemnified Party will give the indemnifying Party (the “Indemnifying Party”) prompt written notice (an “Indemnification Claim Notice”) of any Losses for which Indemnified Party intends to request indemnification under any Section of this Article 10. Each Indemnification Claim Notice must contain a description of the claim and the nature and amount of such Loss, to the extent that the nature and amount of |
|
|
18 such Loss are known at such time. The Indemnified Party will furnish promptly to the Indemnifying Party copies of all papers and official documents received in respect of any Losses. (b) At its option, the Indemnifying Party may assume the defense of any third party claims, suits, actions and demands (each a “Third Party Claim”) by giving written notice to the Indemnified Party within thirty (30) days after the Indemnifying Party’s receipt of an Indemnification Claim. (c) Upon assuming the defense of a Third Party Claim, the Indemnifying Party may appoint as lead counsel in the defense of the Third Party Claim any legal counsel selected by the Indemnifying Party and acceptable to the Indemnified Party in its reasonable judgment. In the event the Indemnifying Party assumes the defense of a Third Party Claim, the Indemnified Party will immediately deliver to the Indemnifying Party all original notices and documents (including court papers) received by any lndemnitee in connection with the Third Party Claim. Should the Indemnifying Party assume the defense of a Third Party Claim, the Indemnifying Party will not be liable to the Indemnified Party or any other lndemnitee for any legal expenses subsequently incurred by such Indemnified Party or other lndemnitee in connection with the analysis defense or settlement of the Third Party Claim. (d) Without limiting the preceding Section 10.4(c), any Indemnitee will be entitled to participate in, but not control, the defense of such Third Party Claim and to employ counsel of its choice for such purpose; provided, however, that such employment will be at the Indemnitee’s own expense unless (i) the employment thereof has been specifically authorized by the Indemnifying Party in writing, or (ii) the Indemnifying Party has failed to assume the defense and employ counsel in accordance with the preceding Section 10.4(b), in which case the Indemnified Party will control the defense. (e) With respect to any Losses relating solely to the payment of money damages in connection with a Third Party Claim and that will not result in the Indemnitee becoming subject to injunctive or other relief or otherwise adversely affect the business of the Indemnitee in any manner, and as to which the Indemnifying Party will have acknowledged in writing the obligation to indemnify the lndemnitee hereunder, the Indemnifying Party will have the sole right to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Loss, on such terms as the Indemnifying Party, in its sole discretion, will deem appropriate, and will transfer to the Indemnified Party all amounts which said Indemnified Party will be liable to pay prior to the entry of judgment. With respect to all other Losses in connection with a Third Party Claim, where the Indemnifying Party has assumed the defense of the Third Party Claim in accordance with Section 10.4(b), the Indemnifying Party will have authority to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Losses provided it obtains the prior written consent of the Indemnified Party (which consent will be at the Indemnified Party’s sole and absolute discretion). The Indemnifying Party will not be liable for any settlement or other disposition of Losses by an lndemnitee that is reached without the written consent of the Indemnifying Party. Regardless of whether the Indemnifying Party chooses to defend or prosecute any Third Party Claim, no |
|
|
23 shall be void and of no effect. Notwithstanding the foregoing, either Party shall be entitled, without the prior written consent of the other Party, to assign all or part of its rights under this Subcontract to a purchaser of all or substantially all of its assets, or an entity with which it may merge where it is not the surviving company, provided that the assignee agrees in writing to assume all obligations undertaken by its assignor in this Subcontract and, with respect to an assignment by Diosynth, the assignee possesses adequate technical capabilities to perform the CMO Services. The terms of this Subcontract shall inure to the benefit of all successors and permitted assigns. 19. Governing Law. This Subcontract and any Scope will be governed by and construed and interpreted in accordance with the law of the State of Maryland without reference to conflicts of law principles that may dictate application of the laws of another jurisdiction, except that any applicable flow-down clauses as set forth in Exhibit C will be construed and interpreted according to the federal common law of government contracts as enunciated and applied by federal judicial bodies, Boards of Contract Appeal, and quasi-judicial agencies of the U.S. Government. 20. Notices. Any notice, approval, instruction or other written communication required or permitted hereunder shall be sufficient if made or given to the other Party by personal delivery, courier service or Certified First Class U.S. Mail to the mailing address set forth below: If to PharmAthene: PharmAthene, Inc. Xxx Xxxx Xxxxx, Xxxxx 000 Xxxxxxxxx, XX 00000 Attn: Director of Contracts with separate correspondence to PharmAthene, Inc. Xxx Xxxx Xxxxx, Xxxxx 000 Xxxxxxxxx, XX 00000 Attn: General Counsel If to Diosynth: Diosynth RTP Inc. 000 X Xxxxxx Xxxxxxx Xxxx Xxxxxxxxxxx, Xxxxx Xxxxxxxx 00000 Attn: Xxxxxx Xxxxxxx or Xxxxxxx Xxxxx with separate correspondence to: 23 |
|
|
|
|
|
1 Exhibit A Scope of Work for Technology Transfer, Scale-Up and cGMP Manufacturing of rPA Drug Substance Diosynth Biotechnology CONFIDENTIAL |
|
|
35 EXHIBIT B Program Price and Payment Schedule |
|
|
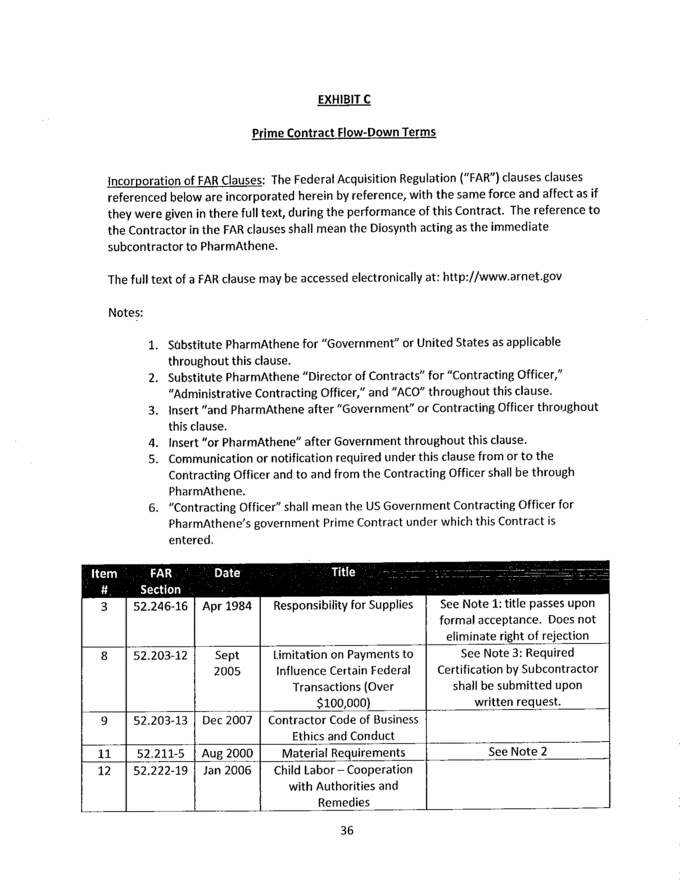
36 EXHIBIT C Prime Contract Flow-Down Terms Incorporation of FAR Clauses: The Federal Acquisition Regulation (“FAR”) clauses clauses referenced below are incorporated herein by reference, with the same force and affect as if they were given in there full text, during the performance of this Contract. The reference to the Contractor in the FAR clauses shall mean the Diosynth acting as the immediate subcontractor to PharmAthene. The full text of a FAR clause may be accessed electronically at: xxxx://xxx.xxxxx.xxx Notes: 1. Substitute PharmAthene for “Government” or United States as applicable throughout this clause. 2. Substitute PharmAthene “Director of Contracts” for “Contracting Officer,” “Administrative Contracting Officer,” and “ACO” throughout this clause. 3. Insert “and PharmAthene after “Government” or Contracting Officer throughout this clause. 4. Insert “or PharmAthene” after Government throughout this clause. 5. Communication or notification required under this clause from or to the Contracting Officer and to and from the Contracting Officer shall be through PharmAthene. 6. “Contracting Officer” shall mean the US Government Contracting Officer for PharmAthene’s government Prime Contract under which this Contract is entered. ItemFAR- #.SectionDateTitle 352.246-16Apr 1984Responsibility for SuppliesSee Note 1: title passes upon formal acceptance. Does not eliminate right of rejection 852.203-12Sept 2005Limitation on Payments to Influence Certain Federal Transactions (Over $100,000)See Note 3: Required Certification by Subcontractor shall be submitted upon written request. 952.203-13Dec 2007Contractor Code of Business Ethics and Conduct 1152.211-5Aug 2000Material RequirementsSee Note 2 1252.222-19Jan 2006Child Labor – Cooperation with Authorities and Remedies |
|
|
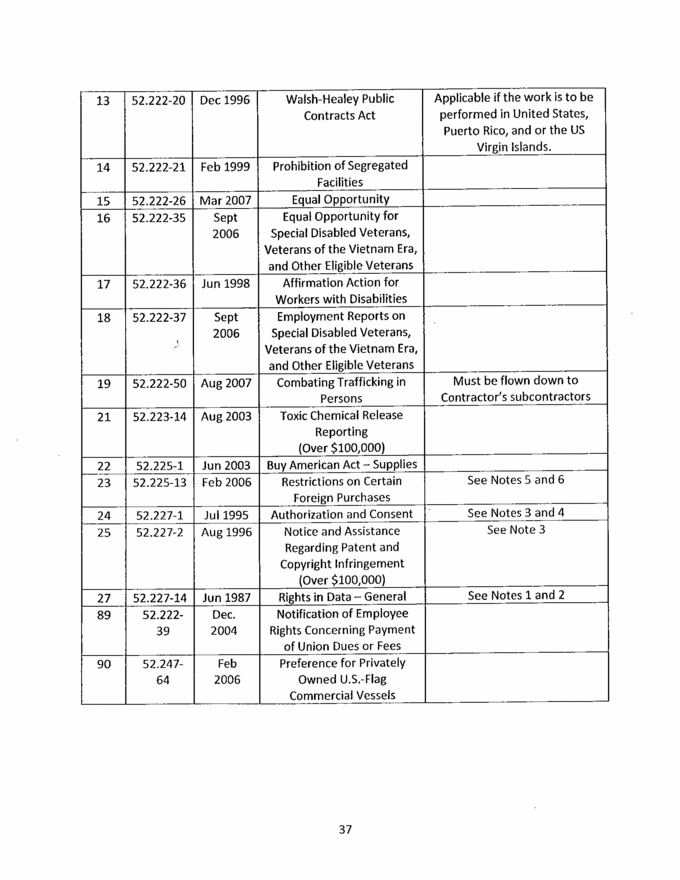
37 13 52.222-20 Dec 1996 Xxxxx-Xxxxxx Public Contracts Act Applicable if the work is to be performed in United States, Puerto Rico, and or the US Virgin Islands. 14 52.222-21 Feb 1999 Prohibition of Segregated Facilities 15 52.222-26 Mar 2007 Equal Opportunity 16 52.222-35 Sept 2006 Equal Opportunity for Special Disabled Veterans, Veterans of the Vietnam Era, and Other Eligible Veterans 17 52.222-36 Jun 1998 Affirmation Action for Workers with Disabilities 18 52.222-37 Sept 2006 Employment Reports on Special Disabled Veterans, Veterans of the Vietnam Era, and Other Eligible Veterans 19 52.222-50 Aug 2007 Combating Trafficking in Persons Must be flown down to Contractor’s subcontractors 21 52.223-14 Aug 2003 Toxic Chemical Release Reporting (Over $100,000) 22 52.225-1 Jun 2003 Buy American Act – Supplies 23 52.225-13 Feb 2006 Restrictions on Certain Foreign Purchases See Notes 5 and 6 24 52.227-1 Jul 1995 Authorization and Consent See Notes 3 and 4 25 52.227-2 Aug 1996 Notice and Assistance Regarding Patent and Copyright Infringement (Over $100,000) See Note 3 27 52.227-14 Jun 1987 Rights in Data – General See Notes 1 and 2 89 52.222-39 Dec. 2004 Notification of Employee Rights Concerning Payment of Union Dues or Fees 90 52.247-64 Feb 2006 Preference for Privately Owned U.S.-Flag Commercial Vessels |
|
|
38 EXHIBIT D Quality Agreement |
|
|
16 Definitions and Abbreviations Analytical Test Methods Methods used for analytical testing, including Standard Test Methods and Compendial Methods. Anomalous A result that does not follow the expected trend, either in comparison with other batches or with respect to previous results collected during a stability study. API Active Pharmaceutical Ingredient, may be used interchangeably with Bulk Drug Substance. Approval The term “Approval” is defined as concurrence between PharmAthene and Diosynth, such as agreement on a proposed Change, as evidenced in writing and signed by both companies’ Authorized Quality Representatives. Approved Supplier A supplier who has met minimum approval standards and who has been approved to provide required items or services that may impact product quality. Authorized Quality Representatives An individual named within the Quality Agreement with the authority to resolve any disputes or conflicts relating to this Quality Agreement in a timely and equitable manner and in compliance with all applicable quality and regulatory requirements. Batch Batches are defined as the material represented at the end of the processing step for Bulk Drug Substance. Batch Packet Relevant documentation to be transferred to PharmAthene to facilitate the release of a Batch. This packet consists of copies of QA reviewed; • executed processing batch records •all Deviations and NOEs, which references CAPA’s initiated •investigations •in-process and release assay results including raw data •Certificate of Analysis (CoA) or analytical results •QA disposition of product statement •batch genealogy (when applicable) •restriction summary •facility summary including associated laminar flow environmental data |
|
|
17 Bulk Drug Substance (BDS) Any substance or mixture of substances intended to be used in the manufacture of a drug (medicinal) product and that, when used in the production of a drug, becomes an active ingredient of the Drug Product. Such substances are intended to furnish pharmacological activity or other direct effect on the diagnosis, cure mitigation, treatment, or prevention of disease or to affect the structure and function of the body. Certificate of Analysis (CoA) An authentic document that states that a specific batch of material has been evaluated in accordance with the Item Specification for that material. Certificate of Conformance (CoC) A PharmAthene supplied document that states a specific lot of material has been evaluated by PharmAthene and conforms to all product and regulatory requirements for further manufacturing or release. cGMP Current Good Manufacturing Practices pursuant to (a) the U.S. Federal Food, Drug and Cosmetics Act as amended (21 USC 301 et seq.), (b) U.S. regulations in Title 21 of the U.S. Code of Federal Regulations Parts 210, 211, 600 and 610, (c) the EU Guide to Good Manufacturing Practice for Medicinal Products, Volume 4, Part lI including relevant sections of DIR 2003/94/EC, and (d) International Conference on Harmonization (ICH) Guidance for Industry Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients. Change Management Any change that: (a) has the potential to impact the quality of PharmAthene’s Product; (b) impacts the regulatory commitments and/or reporting requirements of the bulk drug substance; (c) requires re-qualification or re-validation of PharmAthene’s methods, process or reference standards; and/or (d) results in changing or modifying PharmAthene’s approved Item Specifications, test methods or any document approved by PharmAthene. Critical Raw Materials Critical raw materials are raw materials that have the potential to impact process performance attributes and/or product quality, compromise the final formulation components or combine structurally or chemically with the active pharmaceutical ingredient or drug product. Critical Consumable A consumable that comes into direct contact with the product post the CSIB stage. |
|
|
18 Date of Manufacturing At Diosynth determined from the first date of fill into final container or packaging for intermediate or Bulk Drug Substance. Deviation An unplanned event requiring investigation with 1) may affect the quality or compliance status of the product, process, materials, equipment or facility involved or 2) may not be in alignment with regulatory submissions. Disposition A recommendation given by Diosynth Quality on the suitability of the Intermediate or BDS for further processing. Drug Substance (DS) Synonymous with Bulk Drug Substance (BDS). Drug Product The dosage form in the final immediate packaging intended for clinical use. Exception An exception to a validation protocol is either a deviation from or modification to pre-established acceptance criteria; or an issue encountered after approval of the protocol that requires retesting or additional test plans. For Cause Visit The term “for cause visit” is used to describe site visits, other than internal audits or business discussions, for the purpose of reviewing documentation, facilities or processes related to a specific deviation affecting Product disposition. Item Specification A set of criteria to which a material must conform to be considered acceptable for its intended use. Master Batch Record A detailed description of PharmAthene specific production process outlining the different actions an operator has to perform to complete the BDS production process. A scaled copy of the master batch record is the batch production record. Notice of Events (XXX) Events which are not considered deviations. These events have no impact to products or materials because the incident is a departure from Standard Operating Procedures (SOPs), manufacturing/testing instructions, maintenance/calibration out of tolerances, environmental monitoring excursions, deemed minor in nature and can be verified by existing documentation available at the time of observation. Out-of-Specification (OOS) A test that is valid but the sample result does not comply with the established specification. In this case, “result” is defined as the final reportable value as determined according to the test |
|
|
19 method. Such a reportable value may be comprised of multiple individual determinations (i.e., replicates) as per the test method. Only reportable values are compared to specifications; therefore only a reportable value may constitute an OOS. Process Consumable Disposable equipment or equipment parts that may/may not come in contact with an intermediate or bulk drug substance. A consumable may be single or for multiple use, and may/may not be sterile. Process Consumables Process Consumables include any Process Consumable or Raw Material used in the manufacture of an intermediate or BDS that do not themselves participate in a chemical or biological reaction. Such other materials include: media, resins, filters, membranes, product-contact materials or surfaces, disposable analytical test kits, analytical columns dedicated to the Program, disposable containers, and subcontracted analytical testing are considered to be process consumables. Process Profiling A campaign of three (or more) batches performed at scale, in advance of Process Validation, to demonstrate the final process can perform effectively and reproducibly and meet the predefined acceptance criteria. Product Any (a) API/Bulk Drug Substance, or (b) Drug Product comprised of API/Bulk Drug Substance, or (c) intermediate(s) of (a) or (b), in each case as specified in the applicable Scope. Production Batch Record An accurate reproduction of a Master Batch Record used as instruction for and documentation of production activities. QC Raw Data Analytical worksheets or notebook pages used to record analyses, including details of preparation and expiry dates of reagents, sample and standard solutions and details of instruments used and associated printouts, sequences and methods. For HPLC, single injection reports for all injections, this includes integration events and the sequence table. For SDS-PAGE and IEF, original gel scans, processed gels and details of processing parameters. Any analytical report or CoA from a sub-contractor. Raw Material Any ingredient intended for use in the manufacture of an intermediate or API, including those that may not appear in the final formulation. These include chemicals used directly and/or indirectly in the manufacturing process. Release Dispositioned material is approved by PharmAthene for further processing, as evidence by Diosynth’s receipt of PharmAthene’s CoC. |
|
|
20 Significant Deviation An unplanned event requiring investigation which has a known serious negative impact on the quality of the product, process, materials, equipment or facility, represents a quality system failure with serious negative impact, or is not in alignment with regulatory submissions. Standard Test Methods An approved document describing a method of testing which establishes a particular course of action or way of performing an activity as established by Diosynth. Statement of Compliance A Diosynth QA Disposition of Product Statement stating that a specific Batch of BDS complies with all Product, GMP and regulatory requirements and is signed by an authorized representative of Diosynth. |
|
|
39 EXHIBIT E Earned Value Management System 1. Reporting a. Diosynth shall provide a detailed schedule in line with the Work Breakdown Structure (WBS), provided by PharmAthene. b. Diosynth shall submit monthly reports to PharmAthene compared against the agreed baseline plan. The monthly reports will be submitted electronically by the end of the 2nd business day following the end of each calendar month. c. Diosynths’ reporting will be submitted in accordance with the WBS structure. Each report must include: i. Monthly Technical Progress Report by work package; ii. Any schedule changes or slippages by work package; and iii. An updated cash flow analysis spreadsheet. 2. Schedule a. Setting the Baseline schedule i. PharmAthene will provide Diosynth with a WBS for its portion of the work. ii. Diosynth will provide a detailed schedule (Microsoft project xxxxx) that breaks down the tasks into activities that are four (4) weeks or less in duration. Some management activities may be longer, but tasks that are discretely measurable and/or have defined products should follow this guideline wherever possible. b. Monthly Schedule Status Reports i. Diosynth shall provide schedule updates to PharmAthene on a monthly basis (in accordance with the project schedule outlined above). Actual start and end dates must be reported and percentage (%) completion against all active activities. Any proposed changes to the forward planned activities should be listed, for discussion/agreement by PharmAthene. |