IN WITNESS WHEREOF, the Parties, intending to be legally bound hereby, have caused this Amendment to be executed by their duly authorized representatives as of the Amendment Effective Date. For and on behalf of Nektar Therapeutics: Signature: /s/...

Exhibit 10.35 [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission. AMENDMENT NO. 1 TO STRATEGIC COLLABORATION AGREEMENT This AMENDMENT NO. 1 (this “Amendment”) to the Agreement (as defined below) is entered into as of January 9, 2020 (the “Amendment Effective Date”) by and between Nektar Therapeutics, a Delaware corporation, headquartered at 000 Xxxxxxx Xxx Xxxxxxxxx Xxxxx, Xxxxx 000, Xxx Xxxxxxxxx, XX 00000 (“Nektar”) and Xxxxxxx-Xxxxx Squibb Company, a Delaware corporation, with offices at 000 X. 00xx Xxxxxx, 00xx xxxxx, Xxx Xxxx, XX 00000 (“BMS”). Nektar and BMS may be referred to herein individually as a “Party,” or collectively as the “Parties.” RECITALS WHEREAS, the Parties have entered into a Strategic Collaboration Agreement dated as of February 13, 2018 and effective as of April 3, 2018 (the “Agreement”); WHEREAS, the Parties have agreed to a revised version of the Joint Development Plan that supersedes and replaces any prior versions thereof, including the initial Joint Development Plan attached to the Agreement as Schedule 3.1; WHEREAS, as a result of their agreement on a revised Joint Development Plan, the Parties wish to amend the list of Collaboration Therapies attached to the Agreement as Schedule 1.43; and WHEREAS, the Parties, pursuant to Section 17.9 of the Agreement, wish to formalize their agreement on the revised Joint Development Plan and agree on certain additional amendments pursuant to the terms and conditions hereof. NOW, THEREFORE, in consideration of the foregoing premises and the mutual promises and covenants contained herein, the receipt and sufficiency of which is hereby acknowledged, the Parties agree as follows: 1. DEFINITIONS The terms in this Amendment with initial letters capitalized that are not defined herein shall have the meaning set forth in the Agreement. 2. AMENDMENTS 2.1 Joint Development Plan (a) Pursuant to Sections 3.1(b), 3.6(b) and 3.6(c) of the Agreement, the Parties, on the basis of a meeting of the JDC held on [***], and through other discussions held in accordance with the Agreement have agreed to a revised Joint Development Plan. The revised Joint Development Plan, effective as of the Amendment Effective Date, is attached hereto as Appendix A. The Parties hereby waive the requirement to have the revised Joint Development Plan attached hereto as Appendix A approved at a meeting of the JDC. This revised Joint Development Plan replaces and supersedes, as of the Amendment Effective Date, the initial Joint Development Plan attached as Schedule 3.1 to the Agreement. (b) As a result of their agreement on this revised Joint Development Plan, the Parties are hereby released from any and all obligations or restrictions in respect of any of the Initial Trials that are ACTIVE/102462316.2

not listed in the revised Joint Development Plan attached hereto as Appendix A (including relating to the conduct thereof). (c) Subject to Section 2.1(g), for each of the Collaboration Studies contemplated in the revised Joint Development Plan, Appendix A sets forth, for each Collaboration Study, either its Diligence Date or a confirmation that the Diligence Date has been met, provided that such Diligence Dates shall remain subject to Allowable Delays. (d) For purposes of this Amendment and to the actual knowledge of BMS’ [***], BMS is not aware of any circumstance that would justify to delay, on the basis of the application of the Commercially Reasonable Efforts standard of the Agreement, the commencement (and solely the commencement), by the applicable Diligence Date, of any of the Collaboration Studies referred to in the Joint Development Plan attached as Appendix A that has not started as of the Amendment Effective Date. For purposes of this Amendment and to the actual knowledge of Nektar’s [***], Nektar is not aware of any circumstance that would justify to delay, on the basis of the application of the Commercially Reasonable Efforts standard of the Agreement, the commencement (and solely the commencement), by the applicable Diligence Date, of any of the Collaboration Studies referred to in the Joint Development Plan attached as Appendix A that has not started as of the Amendment Effective Date. (e) As a result of their agreement on the revised Joint Development Plan, the Parties agree to a revised Schedule 1.43 (Collaboration Therapies) to the Agreement. Such revised Schedule 1.43, attached hereto as Appendix C, replaces and supersedes, as of the Amendment Effective Date, the initial version of Schedule 1.43 attached to the Agreement. For clarity, as of the Amendment Effective Date, the restrictions set forth in Section 7.3(d) of the Agreement will no longer apply to any Collaboration Therapy that is not listed in the revised Schedule 1.43 attached hereto as Appendix C. For additional clarity, the restrictions set forth in Section 7.3(d) of the Agreement will apply to first line non-small-cell lung cancer Collaboration Therapy even if there is, as of the Amendment Effective Date, no Collaboration Study associated with that Collaboration Therapy. (f) BMS shall have the right, at its sole discretion, to terminate co-funding of its pro rata share of the Development Costs for the Adjuvant Melanoma Collaboration Study by notice in writing to Nektar in the event that the Metastatic Melanoma Collaboration Study fails to meet the primary endpoint of progression-free survival (the “Adjuvant Melanoma Co-Funding Termination Right”). In the event that any primary or co-primary endpoint is not reached in the Metastatic Melanoma Collaboration Study, the Parties, with the understanding that the health and welfare of patients is of foremost importance, agree to meet and confer to discuss whether there is a need to inform patients, physicians or study sites involved in the Adjuvant Melanoma Collaboration Study of such endpoints not having been reached or other relevant information in the Metastatic Melanoma Collaboration Study, and if so, the means and timeframe to do so. In the event BMS duly exercises its Adjuvant Melanoma Co-Funding Termination Right, Nektar shall have the right, in its sole discretion, to continue the Adjuvant Melanoma Study as a Combined Therapy Independent Study pursuant to the Agreement. (g) For the avoidance of doubt, notwithstanding anything herein or in the Agreement to the contrary, nothing in this Amendment or the Agreement should be read or construed as creating any obligation on either Party to agree to conduct any Phase III Study or registrational Clinical Trial of a [***]. The gating criteria included in Appendix A in this respect are for guidance purposes only. [***] [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission. Page 2

2.2 Release and Waiver. EACH PARTY HEREBY FULLY AND IRREVOCABLY RELEASES AND WAIVES ANY CLAIM, WHETHER KNOWN OR UNKNOWN, IT HAS OR MAY HAVE FROM THE BEGINNING OF TIME TO THE AMENDMENT EFFECTIVE DATE AGAINST THE OTHER PARTY ARISING OUT OF, OR RELATING TO, (A) ANY FAILURE BY SUCH OTHER PARTY TO CONDUCT ANY OF THE INITIAL TRIALS OR (B) ANY OF THE INITIAL TRIALS NOT HAVING MET THEIR DILIGENCE DATE, INCLUDING AS SET FORTH IN SECTION 3.2(A) OF THE AGREEMENT. 2.3 Additional Milestone Payments (a) Additional, Non-creditable Milestone Payment. Within [***] following the achievement of the first patient, first visit in the first Phase III Study of a Combined Therapy consisting of a Product and the BMS Compound that is conducted as a Combined Therapy Collaboration Study (and not an Independent Study) in adjuvant melanoma (the “Additional Melanoma Milestone”), BMS shall pay, or cause to be paid, to Nektar an amount of twenty-five million U.S. Dollars ($25,000,000) (the “Additional Melanoma Milestone Payment”). The Additional Melanoma Milestone Payment payable pursuant to this Section 2.3(a) will be non-refundable and non-creditable and is in addition to all other payments that are, or may be due, to Nektar under the Agreement (as amended). The Additional Melanoma Milestone Payment shall be payable only one time regardless of the number of products that achieve such Additional Melanoma Milestone and regardless of the number of Indications for which such Additional Melanmoa Milestone is achieved. (b) Additional, Creditable Milestone Payments. (i) Additional MIBC Milestone Payment. Within [***] following the achievement of the first patient, first visit in the first Phase III Study of a Combined Therapy consisting of a Product and the BMS Compound that is conducted as a Combined Therapy Collaboration Study (and not an Independent Study) in Muscle Invasive Bladder Cancer (the “Additional MIBC Milestone”), BMS shall pay, or cause to be paid, to Nektar an amount of twenty-five million U.S. Dollars ($25,000,000) (the “Additional MIBC Milestone Payment”). The Additional MIBC Milestone Payment payable pursuant to this Section 2.3(b)(i) will be non-refundable, but will be fully creditable against any future Development Milestone Payment(s) payable by BMS to Nektar pursuant to Section 9.2(b) of the Agreement, until the full amount of such Additional MIBC Milestone Payment shall have been applied to Development Milestone Payments. The Additional MIBC Milestone Payment shall be payable only one time regardless of the number of Products that achieve such Additional MIBC Milestone and regardless of the number of Indications for which such Additional MIBC Milestone is achieved. (ii) Within [***] following the achievement of the first patient, first visit in the first Phase III Study of a Combined Therapy consisting of a Product and the BMS Compound that is conducted as a Combined Therapy Collaboration Study (and not an Independent Study) in First Line Non- Small-Cell Lung Cancer (the “Additional Lung Milestone”), BMS shall pay, or cause to be paid, to Nektar an amount of seventy-five million U.S. Dollars ($75,000,000) (the “Additional Lung Milestone Payment”). The Additional Lung Milestone Payment payable pursuant to this Section 2.3(b)(ii) will be non-refundable, but will be fully creditable against any future Development Milestone Payment(s) payable by BMS to Nektar pursuant to Section 9.2(b) of the Agreement, until the full amount of such Additional Lung Milestone Payment shall have been applied to Development Milestone Payments. The Additional Lung Milestone Payment shall be payable only one time regardless of the number of Products that achieve such Additional Lung Milestone and regardless of the number of Indications for which such Additional Lung Milestone is achieved. For the avoidance of doubt, notwithstanding anything herein or in the Agreement [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission. Page 3

to the contrary, nothing in this Amendment or the Agreement should be read or construed as creating any obligation on either Party to agree to conduct any Phase III Study or registration Clinical Trial of the Combined Therapy in [***] as a Combined Therapy Collaboration Study. 2.4 Combined Therapy Collaboration Studies Expenses. Schedule 6.3 of the Agreement is replaced in its entirety by the new version attached to this Amendment as Appendix D. 2.5 Right of Cross Reference. Section 4.2(b)(v) of the Agreement is replaced in its entirety by the following: “(v) to the extent necessary for the conduct of any Collaboration Study or Independent Study or BMS’s filing of a BLA or supplemental BLA as set forth in Section 10.1(b), providing BMS a Right of Cross-Reference to the relevant Regulatory Documentation, provided that, such Right of Cross-Reference shall terminate upon the expiration or termination of this Agreement for purposes of conducting any new Clinical Trials, except that in the case of termination for a Material Safety Issue pursuant to Section 16.4, such Right of Cross-Reference shall remain in effect solely (A) to the extent necessary to permit BMS to comply with any outstanding obligations required by a Regulatory Authority and/or Applicable Law or (B) as necessary to permit BMS to continue to dose subjects enrolled in each Collaboration Study or Independent Study through completion of the applicable Protocol if required by the applicable Regulatory Authority(ies) and/or Applicable Laws;” 2.6 Reimbursement for Opt-Out Development Costs. Section 7.4 of the Agreement is replaced in its entirety by the following: “7.4 Reimbursement for Opt-Out Development Costs. If a Monotherapy Independent Study or Combined Therapy Independent Study results in Regulatory Approval or a Label expansion of a BMS Asset or Nektar Asset (including the Nektar Compound), the non-funding Party shall reimburse the funding Party for the non-funding Party’s allocated share of Opt-Out Development Costs incurred by the funding Party for the applicable Monotherapy Independent Study or Combined Therapy Independent Study (using the principles set forth in Sections 5.3(c)(i), 6.2 and 6.3) for which the non-funding Party would have been responsible had such Independent Study been a Collaboration Study, plus an amount equal to either (i) [***] of such reimbursement in the event that the applicable Combined Therapy Independent Study studies the Combined Therapy of a Product and the BMS Compound (whether or not other compounds are included in such study), or (ii) [***] of such reimbursement in all other cases. Such reimbursed Opt-Out Development Costs (and the [***] or [***], as applicable, additional reimbursement for such Independent Study) shall be subject to the reconciliation procedures set forth in Section 9.7 but shall not be subject to the Development Cost Cap.” 2.7 Independent Studies. Appendix B hereto includes a list of Independent Studies currently being conducted or planned to be initiated by a Party. 2.8 [***] 2.9 [***] [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission. Page 4

2.10 Governance. The Parties agree that Allowable Delays shall include the time between the expiration of any contractually agreed timeframefor the provision by one Party to the other Party of information, comments or Study Data and the actual provision thereof. 3. PUBLICITY Upon execution of this Amendment, the Parties will issue a press release the contents of which is as attached hereto as Appendix E. The aforementioned press release will be issued within [***] before the opening of U.S. based stock market trading on [***]. 4. MISCELLANEOUS 4.1 This Amendment shall become effective on the Amendment Effective Date. 4.2 Unless and to the extent expressly amended by this Amendment, all the terms and conditions of the Agreement shall remain in full force and effect. 4.3 In the event of a conflict between the terms of this Amendment (or any attachments thereto) and the terms of the Agreement, the terms of this Amendment (including its attachments) shall prevail. 4.4 The headings used in this Amendment have been inserted for convenience of reference only and do not define or limit the provisions hereof. 4.5 This Amendment may be signed in any number of counterparts (facsimile and electronic transmission included), each of which shall be deemed an original, but all of which shall constitute one and the same instrument. 4.6 This Amendment and all claims relating to or arising out of this Amendment or the breach thereof shall be governed and construed in accordance with the internal laws of the State of New York, USA, excluding any choice of law rules that may direct the application of the laws of another jurisdiction. The United Nations Convention on Contracts for the International Sale of Goods shall not apply to this Agreement. Further, Disputes arising out of this Amendment, other than a JDC Dispute, JCC Dispute, JFC Dispute or JMC Dispute or a Publication Dispute or a dispute as to whether a Material Safety Issue exists, shall be resolved in accordance with Section 15.1 of the Agreement. No part of this Amendment changes the rights and obligations of the Parties under Article 15 of the Agreement. 4.7 This Amendment and the Agreement (as amended by the Amendment) constitute the entire understanding between the Parties with respect to the subject matter hereof, and supersede all prior agreements whether oral or written. No amendment, modification, waiver, release or discharge to this Amendment or the Agreement shall be binding upon the Parties unless in writing and duly executed by authorized representatives of both Parties. 4.8 This Amendment has been prepared jointly and shall not be strictly construed against either Party. No presumption as to construction of this Amendment shall apply against either Party with respect to any ambiguity in the wording of any provision(s) of this Amendment irrespective of which Party may be deemed to have authored the ambiguous provision(s). (signature page follows) [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission. Page 5

IN WITNESS WHEREOF, the Parties, intending to be legally bound hereby, have caused this Amendment to be executed by their duly authorized representatives as of the Amendment Effective Date. For and on behalf of Nektar Therapeutics: Signature: /s/ Xxxxxx X. Xxxxx Title: President and CEO Date: 01/09/20 For and on behalf of Xxxxxxx-Xxxxx Squibb Company: Signature: /s/ Xxxxxxx Xxxxxxxx Title: Executive VP, Strategy and Business Development Date: 01/09/20 Signature page to Amendment No. 1 to the Strategic Collaboration Agreement ACTIVE/102462316.2

APPENDIX A JOINT DEVELOPMENT PLAN (Effective as of January 9, 2020) Number Lead Tumor Phase Patient Population Study Design Diligence Date Patients Party 3 1L metastatic melanoma Bempeg + Nivo vs. Nivo 764 Achieved BMS Melanoma 3 Adjuvant melanoma Bempeg + Nivo vs. Nivo 1100 [***] NKTR 3 1L metastatic RCC doublet Bempeg + Nivo vs. TKI 600 Achieved NKTR Phase 1. Dose escalation cohort: Nivo + 6-20 [***] BMS Bempeg + axitinib Phase 2, gated study. Expansion cohort: Nivo + Bempeg + Axitinib vs Nivo + Axitinib (gated upon RCC 80 [***] BMS 1/2/3 RCC triplet acceptable safety profile from dose escalation (1) prior to initiation) Xxxxx 0, gated study. [***] 960 [***] BMS 2 1L metastatic UC Bempeg + Nivo 190 Achieved NKTR Bladder Muscle-invasive bladder Bempeg + Nivo vs Nivo 540 [***] BMS 3 cancer 1/2A Pediatric study Bempeg + Nivo [***] [***] BMS Other 1 Safety study - Xxxxx Xxxxx 0 Bempeg + Nivo 20 Achieved BMS 1 Safety study - [***] Bempeg + Nivo [***] [***] BMS Bempeg = Bempegaldesleukin; Nivo = Nivolumab PIVOT-02 – Parties have agreed to stop additional enrollment, continue to provide patient follow up and conclude the study. [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission.

APPENDIX B INDEPENDENT STUDIES (as of January 9, 2020) Tumor Study Lead Trial Design End Point(s) FPFV Number of Patients Party Non-small cell lung PROPEL - Nektar Bempeg + Pembrolizumab Safety, XXX Achieved Approximately 135 cancer (NSCLC) NSCLC Phase 1/2 Non-small cell lung NSCLC Dose BMS Nivo + Bempeg 0.009 mg/kg vs. XXX [***] 180 cancer (NSCLC) Optimization Nivo + Bempeg 0.006 mg/kg vs. Nivo + Phase 1/21 Ipi Multiple Solid Tumor Reveal Phase 1/2 Nektar NKTR-262 + bempeg and in Safety, XXX Achieved Phase 1: Approximately 48 Indications combination with Bempeg + Nivo Phase 2: Approximately 400 Squamous Cell Head Phase 1/2 Pfizer Avelumab in combination with bempeg Safety, XXX, PSA Achieved 20-40 for each combination and Neck Cancer with or without talazoparib or response rate enzalutamide Metastatic Colorectal and Prostate Cancer Unresectable or Phase 2 Bioxcel BXCL701 in combination with avelumab Safety, XXX [***] Approximately 52 Metastatic Pancreatic and bempeg Adenocarcinoma Locally advanced or Phase 2 Vaccibody VB10.NEO or VB10.NEO plus bempeg Safety, XXX [***] Approximately 50 metastatic solid tumors including melanoma, NSCLC, clear RCC, urothelial cancer or SCCHN Sarcoma IST IST by Bempeg + Nivo XXX Achieved Approximately 85 MSKCC [***] Bempeg = Bempegaldesleukin Ipi = Ipilimumab Nivo = Nivolumab [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission.
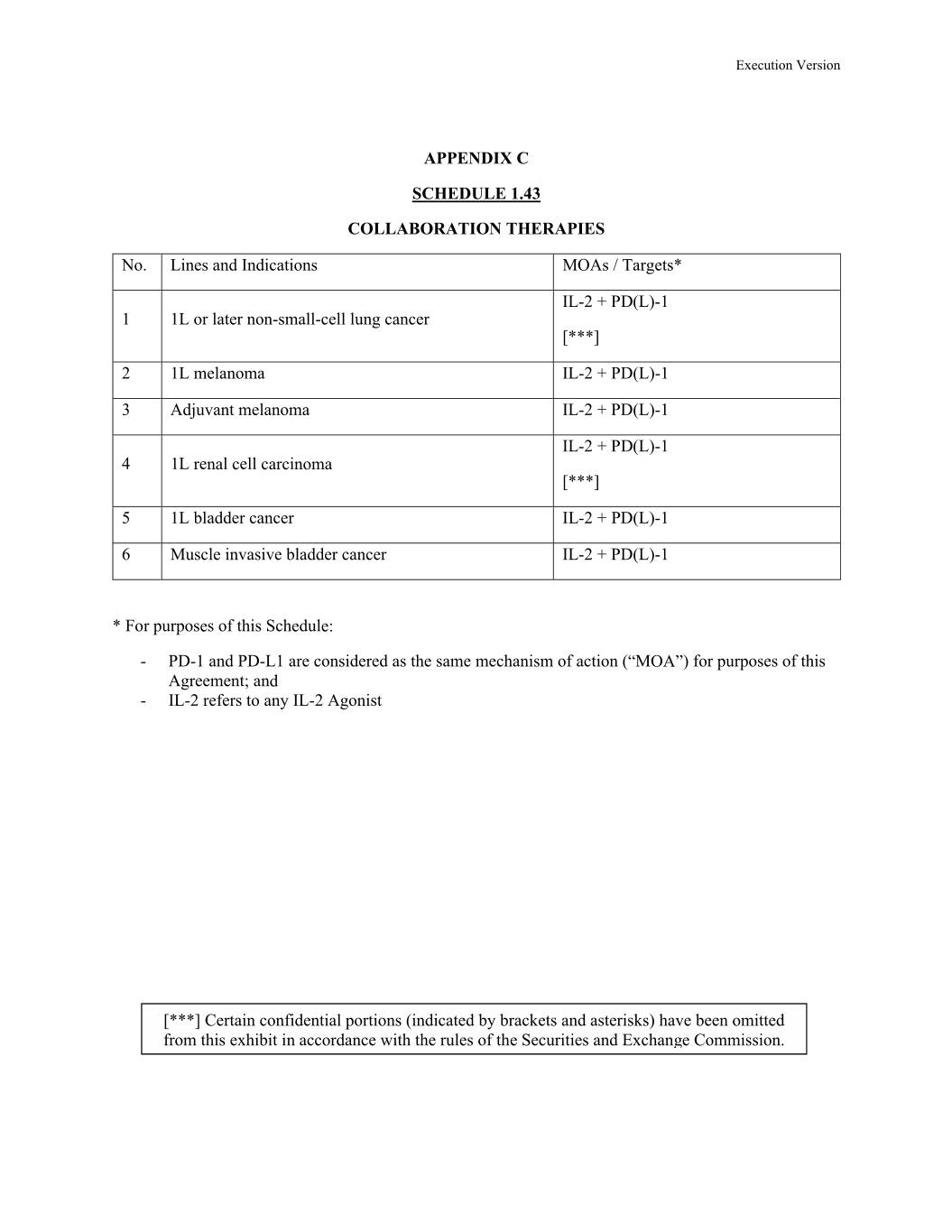
Execution Version APPENDIX C SCHEDULE 1.43 COLLABORATION THERAPIES Xx. Xxxxx xxx Xxxxxxxxxxx XXXx / Xxxxxxx* XX-0 + PD(L)-1 1 1L or later non-small-cell lung cancer [***] 2 1L melanoma IL-2 + XX(X)-0 0 Xxxxxxxx xxxxxxxx XX-0 + PD(L)-1 IL-2 + PD(L)-1 4 1L renal cell carcinoma [***] 5 1L bladder cancer IL-2 + PD(L)-1 6 Muscle invasive bladder cancer IL-2 + PD(L)-1 * For purposes of this Schedule: - PD-1 and PD-L1 are considered as the same mechanism of action (“MOA”) for purposes of this Agreement; and - IL-2 refers to any IL-2 Agonist [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission.

Execution Version APPENDIX D SCHEDULE 6.3 COMBINED THERAPY COLLABORATION STUDY DEVELOPMENT COST ALLOCATION Combined Therapy Collaboration Study Development Cost Allocation: Combinations with Products Nektar BMS Third Party Doublet with the BMS Compound or any other single BMS 32.5% 67.5% - Asset or Third Party Asset sourced by BMS Doublet with any other Nektar Asset or Third Party Asset 82.5% 17.5% - sourced by Nektar Triplet with 2 BMS Assets (which may include the BMS 22% 78% - Compound) [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] In the event that a Collaboration Study includes multi-arm comparative studies that draw on more than one combination described in the above table (e.g., a doublet with a BMS Compound plus a triplet with 1 BMS Asset plus 1 Nektar Asset) the Development Cost allocations between Nektar and BMS shall be a blended rate based [***]. Using the example from the prior sentence, [***] [***] Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit in accordance with the rules of the Securities and Exchange Commission.

Execution Version APPENDIX E Press Release (See attached)

Execution Version Jan 10, 2020 Nektar Therapeutics and Xxxxxxx-Xxxxx Squibb Amend Strategic Collaboration Agreement for bempegaldesleukin Plus Opdivo (nivolumab) SAN FRANCISCO & NEW YORK--(BUSINESS WIRE)--Jan. 10, 2020-- Nektar Therapeutics (Nasdaq:NKTR) and Xxxxxxx-Xxxxx Squibb Company (NYSE:BMY) announced today the companies have agreed to a new joint development plan to advance bempegaldesleukin (bempeg) plus Opdivo (nivolumab) into multiple new registrational trials. The revision to the strategic collaboration agreement includes a new joint development plan under which Nektar and Xxxxxxx-Xxxxx Squibb will expand the active clinical development program for bempeg plus nivolumab from three ongoing registrational trials in first-line metastatic melanoma, first-line cisplatin-ineligible metastatic urothelial cancer and first-line metastatic renal cell carcinoma (RCC) to include two additional registrational trials in adjuvant melanoma and in muscle-invasive bladder cancer. In addition, a Phase 1/2 dose escalation and expansion study will be initiated to evaluate bempeg plus nivolumab in combination with axitinib in first-line RCC in order to support a future registrational trial. The costs for these studies will be shared based upon the cost-sharing outlined in the terms of the original collaboration agreement. Also as part of the new strategic collaboration agreement, Xxxxxxx-Xxxxx Squibb will independently conduct and fund a Phase 1/2 dose optimization and expansion study in first-line non-small-cell lung cancer with bempeg and nivolumab. “Xxxxxxx-Xxxxx Squibb and Nektar view bempeg as an important asset and IL-2 as an important target,” said Xxxxx Xxxxxxx, M.D., head of oncology development, Xxxxxxx-Xxxxx Squibb. “We look forward to expanding the registrational program currently underway for bempeg and are committed to the development of potential new combination therapies to address the unmet needs of patients living with cancer.” “We are pleased to move forward with this new set of registrational trials for bempeg, including the addition of an important Phase 3 study in adjuvant melanoma which builds on the existing metastatic melanoma study and our Breakthrough Therapy Designation,” said Nektar President & CEO Xxxxxx X. Xxxxx. “We now have a comprehensive plan to target multiple indications and have the opportunity to continue to collaborate on development with other companies in indications outside of those in the BMS and Nektar joint development program.” About Opdivo Opdivo is a programmed death-1 (PD-1) immune checkpoint inhibitor that is designed to uniquely harness the body’s own immune system to help restore anti-tumor immune response. By harnessing the body’s own immune system to fight cancer, Opdivo has become an important treatment option across multiple cancers. Opdivo’s leading global development program is based on Xxxxxxx-Xxxxx Squibb’s scientific expertise in the field of Immuno-Oncology, and includes a broad range of clinical trials across all phases, including Phase 3, in a variety of tumor types. To date, the Opdivo clinical

Execution Version development program has treated more than 35,000 patients. The Opdivo trials have contributed to gaining a deeper understanding of the potential role of biomarkers in patient care, particularly regarding how patients may benefit from Opdivo across the continuum of PD- L1 expression. In July 2014, Opdivo was the first PD-1 immune checkpoint inhibitor to receive regulatory approval anywhere in the world. Opdivo is currently approved in more than 65 countries, including the United States, the European Union, Japan and China. In October 2015, the Company’s Opdivo and Yervoy combination regimen was the first Immuno-Oncology combination to receive regulatory approval for the treatment of metastatic melanoma and is currently approved in more than 50 countries, including the United States and the European Union. About Yervoy Yervoy is a recombinant, human monoclonal antibody that binds to the cytotoxic T-lymphocyte- associated antigen-4 (CTLA-4). CTLA-4 is a negative regulator of T-cell activity. Yervoy binds to CTLA-4 and blocks the interaction of CTLA-4 with its ligands, CD80/CD86. Blockade of CTLA-4 has been shown to augment T-cell activation and proliferation, including the activation and proliferation of tumor infiltrating T-effector cells. Inhibition of CTLA-4 signaling can also reduce T-regulatory cell function, which may contribute to a general increase in T-cell responsiveness, including the anti-tumor immune response. On March 25, 2011, the U.S. Food and Drug Administration (FDA) approved Yervoy 3 mg/kg monotherapy for patients with unresectable or metastatic melanoma. Yervoy is approved for unresectable or metastatic melanoma in more than 50 countries. There is a broad, ongoing development program in place for Yervoy spanning multiple tumor types. U.S. FDA-APPROVED INDICATIONS FOR OPDIVO® OPDIVO® (nivolumab) as a single agent is indicated for the treatment of patients with unresectable or metastatic melanoma. OPDIVO® (nivolumab), in combination with YERVOY® (ipilimumab), is indicated for the treatment of patients with unresectable or metastatic melanoma. OPDIVO® (nivolumab) is indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving OPDIVO. OPDIVO® (nivolumab) is indicated for the treatment of patients with metastatic small cell lung cancer (SCLC) with progression after platinum-based chemotherapy and at least one other line of therapy. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. OPDIVO® (nivolumab) is indicated for the treatment of patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. OPDIVO® (nivolumab), in combination with YERVOY® (ipilimumab), is indicated for the treatment of patients with intermediate or poor risk, previously untreated advanced renal cell carcinoma (RCC). OPDIVO® (nivolumab) is indicated for the treatment of adult patients with classical Hodgkin lymphoma (cHL) that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and brentuximab vedotin or after 3 or more lines of systemic therapy that includes autologous HSCT. This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.

Execution Version OPDIVO® (nivolumab) is indicated for the treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) with disease progression on or after platinum-based therapy. OPDIVO® (nivolumab) is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum- containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. OPDIVO® (nivolumab), as a single agent, is indicated for the treatment of adult and pediatric (12 years and older) patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer (CRC) that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. OPDIVO® (nivolumab), in combination with YERVOY® (ipilimumab), is indicated for the treatment of adults and pediatric patients 12 years and older with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer (CRC) that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. OPDIVO® (nivolumab) is indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials. OPDIVO® (nivolumab) is indicated for the adjuvant treatment of patients with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection. U.S. FDA-APPROVED INDICATIONS FOR YERVOY® (ipilimumab) YERVOY® (ipilimumab) is indicated for the treatment of unresectable or metastatic melanoma in adults and pediatric patients (12 years and older). YERVOY® (ipilimumab) is indicated for the adjuvant treatment of patients with cutaneous melanoma with pathologic involvement of regional lymph nodes of more than 1 mm who have undergone complete resection, including total lymphadenectomy. IMPORTANT SAFETY INFORMATION WARNING: IMMUNE-MEDIATED ADVERSE REACTIONS YERVOY can result in severe and fatal immune-mediated adverse reactions. These immune-mediated reactions may involve any organ system; however, the most common severe immune-mediated adverse reactions are enterocolitis, hepatitis, dermatitis (including toxic epidermal necrolysis), neuropathy, and endocrinopathy. The majority of these immune-mediated reactions initially manifested during treatment; however, a minority occurred weeks to months after discontinuation of YERVOY.

Execution Version Assess patients for signs and symptoms of enterocolitis, dermatitis, neuropathy, and endocrinopathy, and evaluate clinical chemistries including liver function tests (LFTs), adrenocorticotropic hormone (ACTH) level, and thyroid function tests, at baseline and before each dose. Permanently discontinue YERVOY and initiate systemic high-dose corticosteroid therapy for severe immune-mediated reactions. Immune-Mediated Pneumonitis OPDIVO can cause immune-mediated pneumonitis. Fatal cases have been reported. Monitor patients for signs with radiographic imaging and for symptoms of pneumonitis. Administer corticosteroids for Grade 2 or more severe pneumonitis. Permanently discontinue for Grade 3 or 4 and withhold until resolution for Grade 2. In patients receiving OPDIVO monotherapy, fatal cases of immune-mediated pneumonitis have occurred. Immune-mediated pneumonitis occurred in 3.1% (61/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, immune-mediated pneumonitis occurred in 6% (25/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated pneumonitis occurred in 4.4% (24/547) of patients. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated pneumonitis occurred in 1.7% (2/119) of patients. In Checkmate 205 and 039, pneumonitis, including interstitial lung disease, occurred in 6.0% (16/266) of patients receiving OPDIVO. Immune-mediated pneumonitis occurred in 4.9% (13/266) of patients receiving OPDIVO: Grade 3 (n=1) and Grade 2 (n=12). Immune-Mediated Colitis OPDIVO can cause immune-mediated colitis. Monitor patients for signs and symptoms of colitis. Administer corticosteroids for Grade 2 (of more than 5 days duration), 3, or 4 colitis. Withhold OPDIVO monotherapy for Grade 2 or 3 and permanently discontinue for Grade 4 or recurrent colitis upon re-initiation of OPDIVO. When administered with YERVOY, withhold OPDIVO and YERVOY for Grade 2 and permanently discontinue for Grade 3 or 4 or recurrent colitis. In patients receiving OPDIVO monotherapy, immune-mediated colitis occurred in 2.9% (58/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, immune- mediated colitis occurred in 26% (107/407) of patients including three fatal cases. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated colitis occurred in 10% (52/547) of patients. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated colitis occurred in 7% (8/119) of patients. In a separate Phase 3 study of YERVOY 3 mg/kg, severe, life-threatening, or fatal (diarrhea of ≥7 stools above baseline, fever, ileus, peritoneal signs; Grade 3-5) immune-mediated enterocolitis occurred in 34 (7%) patients. Across all YERVOY-treated patients in that study (n=511), 5 (1%) developed intestinal perforation, 4 (0.8%) died as a result of complications, and 26 (5%) were hospitalized for severe enterocolitis. Immune-Mediated Hepatitis OPDIVO can cause immune-mediated hepatitis. Monitor patients for abnormal liver tests prior to and periodically during treatment. Administer corticosteroids for Grade 2 or greater transaminase elevations. For patients without HCC, withhold OPDIVO for Grade 2 and permanently discontinue OPDIVO for Grade 3 or 4. For patients with HCC, withhold OPDIVO and administer corticosteroids if AST/ALT is within normal limits at baseline and increases to >3 and up to 5 times the upper limit of normal (ULN), if AST/ALT is >1 and up to 3 times ULN at baseline and increases to >5 and up to 10 times the ULN, and if AST/ALT is >3 and up to 5 times ULN at baseline and increases to >8 and up to 10 times the ULN. Permanently discontinue OPDIVO and administer corticosteroids if AST or ALT increases to >10 times the ULN or total bilirubin increases >3 times the ULN. In patients receiving OPDIVO monotherapy,

Execution Version immune-mediated hepatitis occurred in 1.8% (35/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, immune-mediated hepatitis occurred in 13% (51/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune- mediated hepatitis occurred in 7% (38/547) of patients. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated hepatitis occurred in 8% (10/119) of patients. In Checkmate 040, immune-mediated hepatitis requiring systemic corticosteroids occurred in 5% (8/154) of patients receiving OPDIVO. In a separate Phase 3 study of YERVOY 3 mg/kg, severe, life-threatening, or fatal hepatotoxicity (AST or ALT elevations >5x the ULN or total bilirubin elevations >3x the ULN; Grade 3-5) occurred in 8 (2%) patients, with fatal hepatic failure in 0.2% and hospitalization in 0.4%. Immune-Mediated Neuropathies In a separate Phase 3 study of YERVOY 3 mg/kg, 1 case of fatal Guillain-Barré syndrome and 1 case of severe (Grade 3) peripheral motor neuropathy were reported. Immune-Mediated Endocrinopathies OPDIVO can cause immune-mediated hypophysitis, immune-mediated adrenal insufficiency, autoimmune thyroid disorders, and Type 1 diabetes mellitus. Monitor patients for signs and symptoms of hypophysitis, signs and symptoms of adrenal insufficiency, thyroid function prior to and periodically during treatment, and hyperglycemia. Administer hormone replacement as clinically indicated and corticosteroids for Grade 2 or greater hypophysitis. Withhold for Grade 2 or 3 and permanently discontinue for Grade 4 hypophysitis. Administer corticosteroids for Grade 3 or 4 adrenal insufficiency. Withhold for Grade 2 and permanently discontinue for Grade 3 or 4 adrenal insufficiency. Administer hormone-replacement therapy for hypothyroidism. Initiate medical management for control of hyperthyroidism. Withhold OPDIVO for Grade 3 and permanently discontinue for Grade 4 hyperglycemia. In patients receiving OPDIVO monotherapy, hypophysitis occurred in 0.6% (12/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, hypophysitis occurred in 9% (36/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, hypophysitis occurred in 4.6% (25/547) of patients. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated hypophysitis occurred in 3.4% (4/119) of patients. In patients receiving OPDIVO monotherapy, adrenal insufficiency occurred in 1% (20/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, adrenal insufficiency occurred in 5% (21/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, adrenal insufficiency occurred in 7% (41/547) of patients. In MSI- H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, adrenal insufficiency occurred in 5.9% (7/119) of patients. In patients receiving OPDIVO monotherapy, hypothyroidism or thyroiditis resulting in hypothyroidism occurred in 9% (171/1994) of patients. Hyperthyroidism occurred in 2.7% (54/1994) of patients receiving OPDIVO monotherapy. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, hypothyroidism or thyroiditis resulting in hypothyroidism occurred in 22% (89/407) of patients. Hyperthyroidism occurred in 8% (34/407) of patients receiving this dose of OPDIVO with YERVOY. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, hypothyroidism or thyroiditis resulting in hypothyroidism occurred in 22% (119/547) of patients. Hyperthyroidism occurred in 12% (66/547) of patients receiving this dose of OPDIVO with YERVOY. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, hypothyroidism or thyroiditis resulting in hypothyroidism occurred in 15% (18/119) of patients. Hyperthyroidism occurred in 12% (14/119) of patients. In patients receiving OPDIVO monotherapy, diabetes occurred in 0.9% (17/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg,

Execution Version diabetes occurred in 1.5% (6/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, diabetes occurred in 2.7% (15/547) of patients. In a separate Phase 3 study of YERVOY 3 mg/kg, severe to life-threatening immune-mediated endocrinopathies (requiring hospitalization, urgent medical intervention, or interfering with activities of daily living; Grade 3-4) occurred in 9 (1.8%) patients. All 9 patients had hypopituitarism, and some had additional concomitant endocrinopathies such as adrenal insufficiency, hypogonadism, and hypothyroidism. Six of the 9 patients were hospitalized for severe endocrinopathies. Immune-Mediated Nephritis and Renal Dysfunction OPDIVO can cause immune-mediated nephritis. Monitor patients for elevated serum creatinine prior to and periodically during treatment. Administer corticosteroids for Grades 2-4 increased serum creatinine. Withhold OPDIVO for Grade 2 or 3 and permanently discontinue for Grade 4 increased serum creatinine. In patients receiving OPDIVO monotherapy, immune-mediated nephritis and renal dysfunction occurred in 1.2% (23/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, immune-mediated nephritis and renal dysfunction occurred in 2.2% (9/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated nephritis and renal dysfunction occurred in 4.6% (25/547) of patients. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated nephritis and renal dysfunction occurred in 1.7% (2/119) of patients. Immune-Mediated Skin Adverse Reactions and Dermatitis OPDIVO can cause immune-mediated rash, including Xxxxxxx-Xxxxxxx syndrome (SJS) and toxic epidermal necrolysis (TEN), some cases with fatal outcome. Administer corticosteroids for Grade 3 or 4 rash. Withhold for Grade 3 and permanently discontinue for Grade 4 rash. For symptoms or signs of SJS or TEN, withhold OPDIVO and refer the patient for specialized care for assessment and treatment; if confirmed, permanently discontinue. In patients receiving OPDIVO monotherapy, immune-mediated rash occurred in 9% (171/1994) of patients. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg, immune-mediated rash occurred in 22.6% (92/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated rash occurred in 16% (90/547) of patients. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, immune-mediated rash occurred in 14% (17/119) of patients. In a separate Phase 3 study of YERVOY 3 mg/kg, severe, life-threatening, or fatal immune- mediated dermatitis (eg, Xxxxxxx-Xxxxxxx syndrome, toxic epidermal necrolysis, or rash complicated by full thickness dermal ulceration, or necrotic, bullous, or hemorrhagic manifestations; Grade 3-5) occurred in 13 (2.5%) patients. 1 (0.2%) patient died as a result of toxic epidermal necrolysis. 1 additional patient required hospitalization for severe dermatitis. Immune-Mediated Encephalitis OPDIVO can cause immune-mediated encephalitis. Evaluation of patients with neurologic symptoms may include, but not be limited to, consultation with a neurologist, brain MRI, and lumbar puncture. Withhold OPDIVO in patients with new-onset moderate to severe neurologic signs or symptoms and evaluate to rule out other causes. If other etiologies are ruled out, administer corticosteroids and permanently discontinue OPDIVO for immune-mediated encephalitis. In patients receiving OPDIVO monotherapy, encephalitis occurred in 0.2% (3/1994) of patients. Fatal limbic encephalitis occurred in one patient after 7.2 months of exposure despite discontinuation of OPDIVO and administration of corticosteroids. Encephalitis occurred in one patient receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg (0.2%) after 1.7 months of exposure. Encephalitis occurred in one RCC patient receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg (0.2%) after approximately 4 months of exposure. Encephalitis occurred in

Execution Version one MSI-H/dMMR mCRC patient (0.8%) receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg after 15 days of exposure. Other Immune-Mediated Adverse Reactions Based on the severity of the adverse reaction, permanently discontinue or withhold OPDIVO, administer high-dose corticosteroids, and, if appropriate, initiate hormone-replacement therapy. Across clinical trials of OPDIVO monotherapy or in combination with YERVOY, the following clinically significant immune-mediated adverse reactions, some with fatal outcome, occurred in <1.0% of patients receiving OPDIVO: myocarditis, rhabdomyolysis, myositis, uveitis, iritis, pancreatitis, facial and abducens nerve paresis, demyelination, polymyalgia rheumatica, autoimmune neuropathy, Guillain-Barré syndrome, hypopituitarism, systemic inflammatory response syndrome, gastritis, duodenitis, sarcoidosis, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), motor dysfunction, vasculitis, aplastic anemia, pericarditis, and myasthenic syndrome. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Xxxx-Xxxxxxxx-Xxxxxx-like syndrome, which has been observed in patients receiving OPDIVO and may require treatment with systemic steroids to reduce the risk of permanent vision loss. Infusion Reactions OPDIVO can cause severe infusion reactions, which have been reported in <1.0% of patients in clinical trials. Discontinue OPDIVO in patients with Grade 3 or 4 infusion reactions. Interrupt or slow the rate of infusion in patients with Grade 1 or 2. In patients receiving OPDIVO monotherapy as a 60-minute infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients. In a separate study in which patients received OPDIVO monotherapy as a 60-minute infusion or a 30-minute infusion, infusion-related reactions occurred in 2.2% (8/368) and 2.7% (10/369) of patients, respectively. Additionally, 0.5% (2/368) and 1.4% (5/369) of patients, respectively, experienced adverse reactions within 48 hours of infusion that led to dose delay, permanent discontinuation or withholding of OPDIVO. In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, infusion-related reactions occurred in 2.5% (10/407) of patients. In RCC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, infusion-related reactions occurred in 5.1% (28/547) of patients. In MSI-H/dMMR mCRC patients receiving OPDIVO 3 mg/kg with YERVOY 1 mg/kg, infusion-related reactions occurred in 4.2% (5/119) of patients. Complications of Allogeneic Hematopoietic Stem Cell Transplantation Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1 receptor blocking antibody. Transplant-related complications include hyperacute graft-versus- host-disease (GVHD), acute GVHD, chronic GVHD, hepatic xxxx-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1 receptor blocking antibody prior to or after an allogeneic HSCT. Embryo-Fetal Toxicity Based on their mechanisms of action, OPDIVO and YERVOY can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with an

Execution Version OPDIVO- or YERVOY- containing regimen and for at least 5 months after the last dose of OPDIVO. Increased Mortality in Patients with Multiple Myeloma when OPDIVO is Added to a Thalidomide Analogue and Dexamethasone In clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials. Lactation It is not known whether OPDIVO or YERVOY is present in human milk. Because many drugs, including antibodies, are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from an OPDIVO-containing regimen, advise women to discontinue breastfeeding during treatment. Advise women to discontinue breastfeeding during treatment with YERVOY and for 3 months following the final dose. Serious Adverse Reactions In Checkmate 037, serious adverse reactions occurred in 41% of patients receiving OPDIVO (n=268). Grade 3 and 4 adverse reactions occurred in 42% of patients receiving OPDIVO. The most frequent Grade 3 and 4 adverse drug reactions reported in 2% to <5% of patients receiving OPDIVO were abdominal pain, hyponatremia, increased aspartate aminotransferase, and increased lipase. In Checkmate 066, serious adverse reactions occurred in 36% of patients receiving OPDIVO (n=206). Grade 3 and 4 adverse reactions occurred in 41% of patients receiving OPDIVO. The most frequent Grade 3 and 4 adverse reactions reported in ≥2% of patients receiving OPDIVO were gamma-glutamyltransferase increase (3.9%) and diarrhea (3.4%). In Checkmate 067, serious adverse reactions (74% and 44%), adverse reactions leading to permanent discontinuation (47% and 18%) or to dosing delays (58% and 36%), and Grade 3 or 4 adverse reactions (72% and 51%) all occurred more frequently in the OPDIVO plus YERVOY arm (n=313) relative to the OPDIVO arm (n=313). The most frequent (≥10%) serious adverse reactions in the OPDIVO plus YERVOY arm and the OPDIVO arm, respectively, were diarrhea (13% and 2.2%), colitis (10% and 1.9%), and pyrexia (10% and 1.0%). In Checkmate 017 and 057, serious adverse reactions occurred in 46% of patients receiving OPDIVO (n=418). The most frequent serious adverse reactions reported in ≥2% of patients receiving OPDIVO were pneumonia, pulmonary embolism, dyspnea, pyrexia, pleural effusion, pneumonitis, and respiratory failure. In Checkmate 032, serious adverse reactions occurred in 45% of patients receiving OPDIVO (n=245). The most frequent serious adverse reactions reported in at least 2% of patients receiving OPDIVO were pneumonia, dyspnea, pneumonitis, pleural effusions, and dehydration. In Checkmate 025, serious adverse reactions occurred in 47% of patients receiving OPDIVO (n=406). The most frequent serious adverse reactions reported in ≥2% of patients were acute kidney injury, pleural effusion, pneumonia, diarrhea, and hypercalcemia. In Checkmate 214, serious adverse reactions occurred in 59% of patients receiving OPDIVO plus YERVOY and in 43% of patients receiving sunitinib. The most frequent serious adverse reactions reported in ≥2% of patients were diarrhea, pyrexia, pneumonia, pneumonitis, hypophysitis, acute kidney injury, dyspnea, adrenal insufficiency, and colitis; in patients treated with sunitinib, they were pneumonia, pleural effusion, and dyspnea. In Checkmate 205 and 039, adverse reactions leading to discontinuation occurred in 7% and dose delays due to adverse reactions occurred in 34% of patients (n=266). Serious adverse reactions occurred in 26% of patients. The most frequent serious adverse reactions reported in ≥1% of patients were pneumonia, infusion-related reaction, pyrexia, colitis or diarrhea, pleural effusion, pneumonitis, and rash. Eleven patients died from causes other than disease progression: 3 from adverse reactions within 30 days of the last OPDIVO dose, 2 from infection

Execution Version 8 to 9 months after completing OPDIVO, and 6 from complications of allogeneic HSCT. In Checkmate 141, serious adverse reactions occurred in 49% of patients receiving OPDIVO (n=236). The most frequent serious adverse reactions reported in ≥2% of patients receiving OPDIVO were pneumonia, dyspnea, respiratory failure, respiratory tract infection, and sepsis. In Checkmate 275, serious adverse reactions occurred in 54% of patients receiving OPDIVO (n=270). The most frequent serious adverse reactions reported in ≥2% of patients receiving OPDIVO were urinary tract infection, sepsis, diarrhea, small intestine obstruction, and general physical health deterioration. In Checkmate 142 in MSI-H/dMMR mCRC patients receiving OPDIVO with YERVOY, serious adverse reactions occurred in 47% of patients. The most frequent serious adverse reactions reported in ≥2% of patients were colitis/diarrhea, hepatic events, abdominal pain, acute kidney injury, pyrexia, and dehydration. In Checkmate 040, serious adverse reactions occurred in 49% of patients (n=154). The most frequent serious adverse reactions reported in ≥2% of patients were pyrexia, ascites, back pain, general physical health deterioration, abdominal pain, and pneumonia. In Checkmate 238, Grade 3 or 4 adverse reactions occurred in 25% of OPDIVO-treated patients (n=452). The most frequent Grade 3 and 4 adverse reactions reported in ≥2% of OPDIVO-treated patients were diarrhea and increased lipase and amylase. Serious adverse reactions occurred in 18% of OPDIVO- treated patients. Common Adverse Reactions In Checkmate 037, the most common adverse reaction (≥20%) reported with OPDIVO (n=268) was rash (21%). In Checkmate 066, the most common adverse reactions (≥20%) reported with OPDIVO (n=206) vs dacarbazine (n=205) were fatigue (49% vs 39%), musculoskeletal pain (32% vs 25%), rash (28% vs 12%), and pruritus (23% vs 12%). In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO plus YERVOY arm (n=313) were fatigue (62%), diarrhea (54%), rash (53%), nausea (44%), pyrexia (40%), pruritus (39%), musculoskeletal pain (32%), vomiting (31%), decreased appetite (29%), cough (27%), headache (26%), dyspnea (24%), upper respiratory tract infection (23%), arthralgia (21%), and increased transaminases (25%). In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO arm (n=313) were fatigue (59%), rash (40%), musculoskeletal pain (42%), diarrhea (36%), nausea (30%), cough (28%), pruritus (27%), upper respiratory tract infection (22%), decreased appetite (22%), headache (22%), constipation (21%), arthralgia (21%), and vomiting (20%). In Checkmate 017 and 057, the most common adverse reactions (≥20%) in patients receiving OPDIVO (n=418) were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite. In Checkmate 032, the most common adverse reactions (≥20%) in patients receiving OPDIVO (n=245) were fatigue (45%), decreased appetite (27%), musculoskeletal pain (25%), dyspnea (22%), nausea (22%), diarrhea (21%), constipation (20%), and cough (20%). In Checkmate 025, the most common adverse reactions (≥20%) reported in patients receiving OPDIVO (n=406) vs everolimus (n=397) were fatigue (56% vs 57%), cough (34% vs 38%), nausea (28% vs 29%), rash (28% vs 36%), dyspnea (27% vs 31%), diarrhea (25% vs 32%), constipation (23% vs 18%), decreased appetite (23% vs 30%), back pain (21% vs 16%), and arthralgia (20% vs 14%). In Checkmate 214, the most common adverse reactions (≥20%) reported in patients treated with OPDIVO plus YERVOY (n=547) vs sunitinib (n=535) were fatigue (58% vs 69%), rash (39% vs 25%), diarrhea (38% vs 58%), musculoskeletal pain (37% vs 40%), pruritus (33% vs 11%), nausea (30% vs 43%), cough (28% vs 25%), pyrexia (25% vs 17%), arthralgia (23% vs 16%), decreased appetite (21% vs 29%), dyspnea (20% vs 21%), and vomiting (20% vs 28%). In Checkmate 205 and 039, the most common adverse reactions (≥20%) reported in patients receiving OPDIVO (n=266) were upper respiratory tract infection (44%), fatigue (39%), cough (36%), diarrhea (33%), pyrexia (29%), musculoskeletal pain (26%), rash (24%), nausea (20%) and pruritus (20%). In Checkmate 141, the most common adverse reactions (≥10%) in patients receiving OPDIVO (n=236) were cough and dyspnea at a higher incidence than investigator’s choice. In Checkmate 275, the most common adverse reactions (≥20%) reported in patients receiving OPDIVO (n=270) were fatigue

Execution Version (46%), musculoskeletal pain (30%), nausea (22%), and decreased appetite (22%). In Checkmate 142 in MSI-H/dMMR mCRC patients receiving OPDIVO as a single agent, the most common adverse reactions (≥20%) were fatigue (54%), diarrhea (43%), abdominal pain (34%), nausea (34%), vomiting (28%), musculoskeletal pain (28%), cough (26%), pyrexia (24%), rash (23%), constipation (20%), and upper respiratory tract infection (20%). In Checkmate 142 in MSI-H/dMMR mCRC patients receiving OPDIVO with YERVOY, the most common adverse reactions (≥20%) were fatigue (49%), diarrhea (45%), pyrexia (36%), musculoskeletal pain (36%), abdominal pain (30%), pruritus (28%), nausea (26%), rash (25%), decreased appetite (20%), and vomiting (20%). In Checkmate 040, the most common adverse reactions (≥20%) in patients receiving OPDIVO (n=154) were fatigue (38%), musculoskeletal pain (36%), abdominal pain (34%), pruritus (27%), diarrhea (27%), rash (26%), cough (23%), and decreased appetite (22%). In Checkmate 238, the most common adverse reactions (≥20%) reported in OPDIVO- treated patients (n=452) vs ipilimumab-treated patients (n=453) were fatigue (57% vs 55%), diarrhea (37% vs 55%), rash (35% vs 47%), musculoskeletal pain (32% vs 27%), pruritus (28% vs 37%), headache (23% vs 31%), nausea (23% vs 28%), upper respiratory infection (22% vs 15%), and abdominal pain (21% vs 23%). The most common immune-mediated adverse reactions were rash (16%), diarrhea/colitis (6%), and hepatitis (3%). In a separate Phase 3 study of YERVOY 3 mg/kg, the most common adverse reactions (≥5%) in patients who received YERVOY at 3 mg/kg were fatigue (41%), diarrhea (32%), pruritus (31%), rash (29%), and colitis (8%). Please see U.S. Full Prescribing Information for OPDIVO and YERVOY, including Boxed WARNING regarding immune-mediated adverse reactions for YERVOY. Checkmate Trials and Patient Populations Checkmate 037–previously treated metastatic melanoma; Checkmate 066–previously untreated metastatic melanoma; Checkmate 067–previously untreated metastatic melanoma, as a single agent or in combination with YERVOY; Checkmate 017–second-line treatment of metastatic squamous non-small cell lung cancer; Checkmate 057–second-line treatment of metastatic non-squamous non-small cell lung cancer; Checkmate 032–small cell lung cancer; Checkmate 025–previously treated renal cell carcinoma; Checkmate 214–previously untreated renal cell carcinoma, in combination with YERVOY; Checkmate 205/039–classical Hodgkin lymphoma; Checkmate 141–recurrent or metastatic squamous cell carcinoma of the head and neck; Checkmate 275–urothelial carcinoma; Checkmate 142–MSI-H or dMMR metastatic colorectal cancer, as a single agent or in combination with YERVOY; Checkmate 040–hepatocellular carcinoma; Checkmate 238–adjuvant treatment of melanoma. About Xxxxxxx-Xxxxx Squibb Xxxxxxx-Xxxxx Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Xxxxxxx-Xxxxx Squibb, visit us at XXX.xxx or follow us on LinkedIn, Twitter, YouTube, Facebook, and Instagram. Celgene and Juno Therapeutics are wholly owned subsidiaries of Xxxxxxx-Xxxxx Squibb Company. In certain countries outside the U.S., due to local laws, Celgene and Juno Therapeutics are referred to as, Celgene, a Xxxxxxx-Xxxxx Squibb company and Juno Therapeutics, a Xxxxxxx-Xxxxx Squibb company. About the Xxxxxxx-Xxxxx Squibb and Ono Pharmaceutical Collaboration In 2011, through a collaboration agreement with Ono Pharmaceutical Co., Xxxxxxx-Xxxxx Squibb expanded its territorial rights to develop and commercialize Opdivo globally, except in Japan, South Korea and Taiwan, where Ono had retained all rights to the compound at the time. On July 23, 2014, Ono and Xxxxxxx-Xxxxx Squibb further expanded the companies’

Execution Version strategic collaboration agreement to jointly develop and commercialize multiple immunotherapies – as single agents and combination regimens – for patients with cancer in Japan, South Korea and Taiwan. About Nektar Therapeutics Nektar Therapeutics is a biopharmaceutical company with a robust, wholly-owned R&D pipeline of investigational medicines in oncology, immunology and pain as well as a portfolio of approved partnered medicines. Nektar is headquartered in San Francisco, California, with additional operations in Huntsville, Alabama and Hyderabad, India. Further information about the company and its drug development programs and capabilities may be found online at xxxx://xxx.xxxxxx.xxx. About Bempegaldesleukin (BEMPEG, NKTR-214) Bempeg is an investigational, first-in-class, CD122-preferential IL-2 pathway agonist designed to provide rapid activation and proliferation of cancer-killing immune cells, known as CD8+ effector T cells and natural killer (NK) cells, without over activating the immune system. The agent is designed to stimulate these cancer-killing immune cells in the body by targeting CD122-specific receptors found on the surface of these immune cells. CD122, which is also known as the Interleukin-2 receptor beta subunit, is a key signaling receptor that is known to increase proliferation of these effector T cells.1 In clinical and preclinical studies, treatment with bempegaldesleukin resulted in expansion of these cells and mobilization into the tumor micro- environment.2,3 Bempegaldesleukin has an antibody-like dosing regimen similar to the existing checkpoint inhibitor class of approved medicines. Xxxxxxx-Xxxxx Squibb Forward Looking Statements This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 regarding, among other things, the research, development and commercialization of pharmaceutical products and the collaboration with Nektar Therapeutics. All statements that are not statements of historical facts are, or may be deemed to be, forward-looking statements. Such forward-looking statements are based on historical performance and current expectations and projections about our future financial results, goals, plans and objectives and involve inherent risks, assumptions and uncertainties, including internal or external factors that could delay, divert or change any of them in the next several years, that are difficult to predict, may be beyond our control and could cause our future financial results, goals, plans and objectives to differ materially from those expressed in, or implied by, the statements. These risks, assumptions, uncertainties and other factors include, among others, that the expected benefits of, and opportunities related to, the collaboration with Nektar Therapeutics may not be realized by Xxxxxxx-Xxxxx Squibb or may take longer to realize than anticipated and that Opdivo, in combination with bempegaldesleukin, may not achieve their primary study endpoints or receive regulatory approval for the indications described in this release in the currently anticipated timeline or at all and, if approved, whether such combination treatment for such indications described in this release will be commercially successful. No forward-looking statement can be guaranteed. Forward-looking statements in this press release should be evaluated together with the many risks and uncertainties that affect Xxxxxxx-Xxxxx Squibb’s business and market, particularly those identified in the cautionary statement and risk factors discussion in Xxxxxxx-Xxxxx Squibb’s Annual Report on Form 10-K for the year ended December 31, 2018, as updated by our subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the Securities and Exchange Commission. The forward-looking statements included in this document are made only as of the date of this document and except as otherwise required by applicable law, Xxxxxxx-Xxxxx Squibb undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, changed circumstances or otherwise.

Execution Version Nektar Therapeutics Cautionary Note Regarding Forward-Looking Statements This press release contains forward-looking statements which can be identified by words such as: "will," "move forward," "plan" and similar references to future periods. Examples of forward- looking statements include, among others, statements we make regarding the therapeutic potential of bempegaldesleukin ("bempeg") in combination with nivolumab, and the availability of results and outcomes from studies of the therapies based on bempeg combinations. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, anticipated events and trends, and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward- looking statements. Important factors that could cause our actual results to differ materially from those indicated in the forward-looking statements include, among others: (i) our statements regarding the therapeutic potential of bempeg are based on preclinical and clinical findings and observations; (ii) bempeg is currently in clinical development and the risk of failure remains high and failure can unexpectedly occur at any stage for one or more of the cancer indications being studied prior to regulatory approval due to lack of sufficient efficacy, safety considerations or other factors that impact drug development; (iii) data reported from ongoing preclinical and clinical trials are necessarily interim data only and the final results will change based on continuing observations; (iv) scientific discovery of new medical breakthroughs is an inherently uncertain process and the future success of potential of bempeg is therefore very uncertain and unpredictable; (v) the timing of the commencement or end of clinical studies and the availability of clinical data may be delayed or unsuccessful due to regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, changing standards of care, evolving regulatory requirements, clinical trial design, clinical outcomes, delays caused by our collaboration partners, and enrollment competition; (vi) patents may not issue from our patent applications for our drug candidates, patents that have issued may not be enforceable, or additional intellectual property licenses from third parties may be required; and (vii) certain other important risks and uncertainties set forth in Nektar's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 7, 2019. Any forward-looking statement made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. References: 1. Boyman, J., et al., Nature Reviews Immunology, 2012, 12, 180-190. 2. Charych, D., et al., Clin Can Res; 22(3) February 1, 2016 3. Diab, A., et al., Journal for ImmunoTherapy of Cancer 2016, 4(Suppl 1): P369 For Xxxxxxx-Xxxxx Squibb Media: xxxxx@xxx.xxx 000-000-0000

Execution Version Investors: Xxx Xxxxx, 000-000-0000, xxxxxxx.xxxxx@xxx.xxx Xxxx Xxxxxxx, 000-000-0000, xxxxxxxx@xxxxxxx.xxx For Nektar Investors: Xxxxxx Xx of Nektar Therapeutics 000-000-0000 Media: Xxx Xxxxxxx of 1AB 000-000-0000 xxx@0xxxxxxx.xxx

