EX-10.33 41 d39288dex1033.htm EX-10.33 *** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406...
Exhibit 10.33
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
Dated December 19, 2014
by and between
SYNDAX PHARMACEUTICALS, INC.
and
KYOWA HAKKO KIRIN CO., LTD.
CONFIDENTIAL
EXECUTION VERSION
LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
THIS LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT (as modified or amended in accordance with the terms hereof, the “Agreement”) is entered into as of December 19, 2014 (the “Effective Date”) by and between Syndax Pharmaceuticals, Inc., a Delaware corporation having a place of business at 000 Xxxxxx Xxxx Xxxx, Xxxxx 000, Xxxxxxx, XX 00000 XXX (“Syndax”), and Kyowa Hakko Kirin Co., Ltd., a Japanese corporation having a place of business at 0-0-0 Xxxxxxxxx, Xxxxxxx-xx, Xxxxx 000-0000, Xxxxx (“KHK”).
RECITALS
WHEREAS, Syndax possesses and/or has licensed from Bayer certain Patent and other IP rights to ENTINOSTAT and related backup compounds and possesses certain additional intellectual property rights and proprietary interests related to ENTINOSTAT and related backup compounds;
WHEREAS, KHK wishes to obtain license and sublicenses from Syndax under the Syndax Patents and the Syndax Know-How with respect to the Compound and the Product in the Territory and Syndax is willing to grant such licenses to KHK and permitted and authorized to grant such sublicenses in accordance with the terms of the Bayer License Agreement and in accordance to the terms and conditions set forth herein;
WHEREAS, the Parties wish to set forth additional terms and conditions applicable to the Development and Commercialization of the Product in the Territory; and
WHEREAS, Syndax has obtained Bayer’s consent to the license granted by Syndax to KHK under this Agreement (as attached hereto as Exhibit C) which is acceptable in form and content to KHK.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants contained herein, and other valuable consideration received by the Parties, the Parties hereto agree as follows:
ARTICLE 1
DEFINITIONS
For the purposes of this Agreement, the following definitions shall apply, and the terms defined herein in plural shall include the singular and vice-versa.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
1
“Action Date” means, with respect to a legal action in connection with a Product Infringement, the date that is the earlier of (a) *** following receipt or delivery of notice and evidence of Product Infringement pursuant to Section 10.3.1, and (b) *** before the date after which a legal action would be substantively limited or compromised with respect to the remedies available against the alleged Third Party infringer.
“Additional Syndax Patent” means all Patents in the Territory that are not Controlled by Syndax on the Effective Date but are Controlled by Syndax at any time during the Term after the Effective Date and Cover the Development, Manufacturing, or Commercialization of, the Compound or Products in the Territory; provided, however, that the Additional Syndax Patents shall not include any Patents licensed from a Third Party after the Effective Date unless it is licensed pursuant to a Third Party License; further provided, however, that after KHK’s election to exclude a Patent from Additional Syndax Patents pursuant to Section 2.10, such excluded Patent thereafter shall no longer constitute an Additional Syndax Patent. Without limiting the foregoing, Additional Syndax Patents shall include those Patents listed in Exhibit A under the heading “Additional Syndax Patents” as updated from time to time by Syndax pursuant to Section 2.10. Additional Syndax Patents shall include any Patents in the Territory that become Bayer Patents after the Effective Date.
“Affiliate” means, with respect to a Party hereto, any corporation, partnership, joint venture or other business entity which, at the time of determination, is controlled by, controlling or under common control with such Party. For purposes of the definition of “Affiliate”, “control” means direct or indirect beneficial ownership of more than fifty percent (50%) of the voting stock, equity or participating profit interest of such corporation or other business entity (provided that, if Applicable Law requires a minimum percentage of local ownership, control shall be established by direct or indirect beneficial ownership of one hundred percent (100%) of the maximum ownership percentage that may, under such Applicable Law, be owned by foreign interests) or the power to direct or cause the direction of the management or policies of a Party, whether through the ownership of voting securities, by contract or otherwise. When used in reference to an entity that is not Party (such as the reference to an Affiliate of Bayer in Section 12.2), “Affiliate” shall have the same meaning, applied mutatis mutandis.
“Agreement” shall have the meaning set forth in the first paragraph of this License Agreement.
“API” means active pharmaceutical ingredients of a Product.
“Applicable Law” means all applicable laws, statutes, rules, regulations, directives, decisions, ordinances, guidelines and other pronouncements of any Governmental Authority.
“Auditor” shall have the meaning set forth in Section 9.3.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
2
“Back-Up Compound” shall mean any compounds (and any metabolite, salt, polymorph, hydrate, semihydrate or degradant thereof) Controlled by Syndax at any time during the Term that (a) is an HDAC Class I Selective Inhibitor other than Entinostat (and other than any metabolite, salt, polymorph, hydrate, semihydrate or degradant of Entinostat) and (b) has been is being developed by Syndax, as of the Effective Date or at any time during the Term, to prevent, treat, and/or diagnose cancer.
“Bayer” means Bayer Pharma AG (formerly Bayer Schering Pharma AG).
“Bayer Intellectual Property” means the Bayer Know-How and the Bayer Patents.
“Bayer Know-How” means the Bayer Know-How as defined in the Bayer License Agreement.
“Bayer License Agreement” means that certain License, Development and Commercialization Agreement entered into between Bayer and Syndax, dated as of March 26, 2007, as amended.
“Bayer IP Enforcement Action” shall have the meaning set forth in Section 10.3.2.
“Bayer Patents” means the Bayer Patents as defined in the Bayer License Agreement.
“Business Day” means a day (other than a Saturday, Sunday or national holiday) on which banking institutions in each of Tokyo, Japan, Seoul, Korea and New York City, New York are open to the public for conducting business and neither is authorized or required by law to close.
“Claim” shall have the meaning set forth in Section 12.1.
“Clinical Quality Agreement” shall have the meaning set forth in Section 7.3.
“Clinical Supply Agreement” shall have the meaning set forth in Section 7.2.
“Clinical Trials” means human clinical trials with respect to the use of the Product in the Field.
“CMC” means, with respect to any NDA, the Chemistry, Manufacturing and Controls section of such NDA.
“Commercial Quality Agreement” shall have the meaning set forth in Section 7.3.
“Commercial Supply Agreement” shall have the meaning set forth in Section 7.2.
“Commercialize” or “Commercialization” means all of the activities usually and customarily undertaken by a pharmaceutical company to accomplish the packaging, storage,
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
3
distribution, sale, Marketing, import, export, post-marketing activities and compliance with applicable legal and regulatory requirements relating to the foregoing activities in respect of active pharmaceutical ingredients, compounds and products similar to Entinostat or the Products that are the subject of this Agreement. Commercialization expressly excludes Manufacturing activities.
“Commercially Reasonable Efforts” means the level of effort and standard practices consistent with the usual practices followed by similarly-situated pharmaceutical companies in pursuing research, development, commercialization and marketing of their pharmaceutical products with comparable potential, market, risk, and revenues, taking into account all relevant factors including product labeling or anticipated labeling, present and future market potential, past performance of such product and such Party’s other pharmaceutical products that are of similar market potential, financial return, medical and clinical considerations, present and future regulatory environment and competitive market conditions, all as measured by the facts and circumstances at the time such efforts are due.
“Competing Product” means any pharmaceutical product (other than a Product) to which both of the following applies: (1) it has the same indication as a Product sold in the Territory, and (2) it contains an active pharmaceutical ingredient that is an HDAC Class I Selective Inhibitor. Solely for purposes of this definition, all forms of cancer shall be considered to be the same indication.
“Compound” means Entinostat (and any metabolite, salt, polymorph, hydrate, semihydrate or degradant thereof that Syndax may develop during the Term) and all Back-Up Compounds.
“Confidential Information” means all confidential or proprietary information, materials and Know-How (whether or not patentable) of a Party, including the Syndax Know-How, other invention disclosures, technology, libraries, targets, compounds, economic information, business or research strategies and trade secrets, and any and all embodiments thereof, disclosed by a Party to the other Party, whether in oral, written or other form, which is either marked “confidential” or “proprietary” or, if disclosed orally or in other intangible form that cannot be so marked, in such case identified as confidential or proprietary at the time of disclosure and described in a writing which is marked “confidential” or “proprietary” and transmitted to the receiving Party within *** of such disclosure. Without limiting the foregoing, the terms and existence of this Agreement shall constitute the Confidential Information of each Party hereto.
“Control” means, with respect to any material, information, or intellectual property right, that a Party owns or has a license or right to such material, information, or intellectual property right and has the ability to grant to the other Party access, a license, or a sublicense (as applicable) to such material, information, or intellectual property right on the terms and conditions set forth herein without violating the terms of any then-existing agreement or other arrangement with any Third Party.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
4
“Cover(ed)” means, with respect to any Patent and the subject matter at issue, that, but for a license granted under a such Patent, the sale, offer for sale, exportation, importation or manufacture of the subject matter at issue would infringe such Patent, or in the case of a Patent that is a patent application, would infringe a claim in a Patent issued from such patent application if the currently pending claims of such patent application were to be issued as a Patent.
“Develop”, “Developing” or “Development” means all activities relating to non-clinical, preclinical studies and Clinical Trials, toxicology testing, statistical analysis and reporting, necessary or reasonably useful or otherwise requested or required by a Regulatory Authority as a condition or in support of obtaining or maintaining all Regulatory Approvals for the Compound or Product in the applicable indication and applicable territory, but expressly excludes basic research or any activities related to Commercialization or Manufacturing. For clarity, “Develop” shall include conducting in vitro, in vivo or in silico studies for the purpose of determining which indication to pursue or for the purpose of supporting Commercialization of the Product.
“Development Data” means any and all research data, pharmacology data, chemistry, manufacturing and control data, preclinical data, clinical data and all other documentation (including raw data) compiled, developed or generated with respect to the Compound or Product.
“Development Plan” shall have the meaning set forth in Section 4.2.1.
“Disclosing Party” shall have the meaning set forth in Section 14.1.
“DMFs” shall mean drug master files.
“Dollars” or “$” means United States dollars.
“ECOG License Agreement” shall have the meaning set forth in Section 2.3.4(i).
“Effective Date” shall have the meaning set forth in the Preamble.
“Enforcement Action” shall have the meaning set forth in Section 10.3.3.
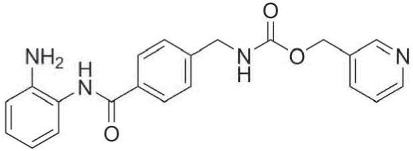
“Entinostat” means the compound 3-Pyridylmethyl N-{4-[(2-aminophenyl)carbonyl] benzyl}carbamate (also known as N-(2-aminophenyl)-4-[N-(pyridin-3-ylmethoxycarbonyl) aminomethyl]benzamide), with the molecular formula C21H20N4O3 and the structure set forth below, and all formulations and crystal forms thereof.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
5
“Existing Third Party License” means the Bayer License Agreement and any other agreement pursuant to which Syndax, as of the Effective Date, has licensed Third Party IP. Notwithstanding the foregoing, that certain Exclusive License Agreement between Syndax and the ***, dated as of *** is deemed not to be an Existing Third Party License.
“FDA” means the United States Food and Drug Administration, including any successor drug regulatory entity thereto.
“FDCA” means the United States Federal Food, Drug and Cosmetic Act of 1938, as amended from time to time, and the regulations promulgated thereunder.
“Field” means the prevention, treatment and diagnosis of any human disease; provided, however, that with respect to the grant of a sublicense herein to KHK under the rights licensed to Syndax pursuant to the Bayer License Agreement, “Field” is limited to “the treatment of any human disease” or any other uses or field the Syndax later acquires under the Bayer License Agreement during the Term.
“First Commercial Sale” means, with respect to a Product, the first sale of such Product by or on behalf of KHK or its Affiliates to a Third Party (including wholesalers or distributors), after receipt of Regulatory Approval for such Product in the Territory.
“First Indication” shall have the meaning set forth in Section 4.3.
“Generic Entry” shall have the meaning set forth in Section 8.5.1(iii).
“Generic Product” means, with respect to the Product in a given country, a product sold in such country by a Third Party (other than a sublicensee of KHK or any other Third Party authorized to sell such product by, or otherwise in the chain of distribution of, KHK or a KHK Affiliate or Sublicensee) that (a) contains the same active ingredient(s) as the Product, or any base form, salt form, prodrug form, ester, ether, isomer, crystalline polymorph, hydrate or solvate of such active ingredients (but no more pharmaceutically active ingredients than is contained in the Product), and (b) is approved or registered for use in such country pursuant to any drug approval process based solely on (A) (x) reference to a Regulatory Approval for such Product held by KHK, Syndax, or one of their respective Affiliates or licensees, whether in such country or in another country, and/or (y) reference to other publicly available clinical data with respect to such Product generated by KHK, Syndax, or one of their respective Affiliates or licensees, and (B) a demonstration of bioequivalence to such Product.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
6
“Good Clinical Practices” or “GCP” means, as applicable, (i) the then-current standards, practices and procedures promulgated or endorsed by the FDA for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials, including the requirements set forth in 21 C.F.R. Parts 11, 50, 54, 56, 312, and 314 and including any related regulatory requirements imposed by the FDA, and (ii) any comparable regulatory standards, practices and procedures in jurisdictions outside of the U.S., in each case as they may be updated from time to time, that provide assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of trial subjects are protected.
“Good Laboratory Practices” or “GLP” means all applicable Good Laboratory Practice standards, including, as applicable, (i) as set forth in the then-current good laboratory practice standards promulgated or endorsed by the FDA as defined in 21 C.F.R. Part 58, and (ii) the equivalent Applicable Law in any relevant country, each as may be amended and applicable from time to time.
“Good Manufacturing Practices” or “GMP” means all applicable Good Manufacturing Practices including, as applicable, (i) the principles detailed in the U.S. Current Good Manufacturing Practices, 21 C.F.R. Sections 210, 211, 601 and 610, (ii) the principles detailed in the ICH Q7A guidelines, and (iii) the equivalent Applicable Law in any relevant country, each as may be amended and applicable from time to time.
“Governmental Authority” means any multinational, federal, state, local, municipal or other governmental authority of any nature (including any governmental division, prefecture, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal), in each case, having jurisdiction over the applicable subject matter.
“HDAC Class I Selective Inhibitor” means any compound that (a) has greater than *** inhibition against HDAC class I at *** and (b) has less than *** inhibition against *** at ***.
“IND” means (a) an Investigational New Drug Application (as defined in 21 C.F.R. Part 312) that is required to be submitted to the FDA before beginning clinical testing of a Product in human subjects, or any successor application or procedure, or (b) any counterpart of an Investigational New Drug Application (such as an import drug license) that is required in the Territory before beginning clinical testing of a Product in human subjects in such country.
“Initial Delivery of Information and Materials” shall have the meaning set forth in Section 2.3.1.
“Initial Product” means the Product containing Entinostat that as of the Effective Date is being developed by Syndax and is the subject of an Investigational New Drug (as defined in the FDCA) application in the United States, as such Product may be modified in the ordinary course of Development.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
7
“Initial Syndax Patents” means all Patents in the Territory that are Controlled by Syndax at the time of the Effective Date that Cover the Development, Manufacturing, or Commercialization of, the Compound or Products in the Territory. Without limiting the foregoing, Initial Syndax Patents includes those Patents listed in Exhibit A under the heading “Initial Syndax Patents”. Initial Syndax Patents includes all Bayer Patents in the Territory that exist on the Effective Date.
“Inspection” shall have the meaning set forth in Section 9.3.
“Interest Rate” means the annual rate equal to the prime rate quoted in the Wall Street Journal (as available at its website) plus ***, calculated daily on the basis of a three hundred sixty (360) day year, or if lower, the maximum rate permitted by Applicable Law.
“Invention” means any invention, process, machine, formulation, manufacture, use or composition of matter related to the Compound or the Product, and any improvement thereof.
“Joint Invention” shall have the meaning set forth in Section 10.1.
“JSC” shall have the meaning set forth in Section 3.1.
“KHK” shall have the meaning set forth in the first paragraph of this Agreement.
“KHK Development Partner” shall have the meaning set forth in Section 2.3.4(ii).
“KHK Improvements” shall have the meaning set forth in Section 2.4.1.
“KHK Improvements License” shall have the meaning set forth in Section 2.4.1.
“KHK Parties” shall have the meaning set forth in Section 5.7.2.
“KHK Trademarks” shall have the meaning set forth in Section 10.8.1.
“Know-How” means any and all proprietary information, including all patentable and non-patentable inventions, discoveries, technologies, methods, knowledge, know-how, trade secrets, experience, skill, techniques, disclosure claims, formulas, processes, procedures, compounds, compositions of matter, assays, materials, specifications, descriptions, results and data (including physical, chemical, biological, toxicological, pharmacological, manufacturing, regulatory, analytical, commercial, pre-clinical and clinical data). Notwithstanding the foregoing, Know-How excludes Patents.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
8
“Knowledge” shall have the meaning set forth in Section 11.4.
“Losses” shall have the meaning set forth in Section 12.1.
“Manufacture” or “Manufacturing” means all activities related to the manufacture and/or supply of the Product, or any ingredient thereof, including manufacturing for clinical use or commercial sale, in-process and Product testing, handling, transport, and storage of Product and ongoing stability tests and regulatory activities related to any of the foregoing, excluding any activities related to PTP packaging or final packaging for the Product.
“Marketing” means all activities related to the marketing or sales promotion of the Product in the Territory.
“Marketing Authorization Holder” means a person who possesses all Regulatory Approvals for any particular indication in the Territory in such person’s name and who will manage all interactions with Regulatory Authorities regarding such Regulatory Approval.
“Marketing Plan” shall have the meaning set forth in Section 6.2.
“MFDS” means the Ministry of Food and Drug Safety in Korea, or such other Regulatory Authority as may replace it or otherwise have the authority to grant Regulatory Approval for Product in Korea.
“MHLW” means the Ministry of Health, Labour and Welfare in Japan, or such other Regulatory Authority as may replace it or otherwise have the authority to grant Regulatory Approval for Product in Japan.
“NDA” means, in the United States, a New Drug Application (as defined in the FDCA), and/or any amendment or supplement thereto, and, in any other jurisdiction, the applicable local equivalent required in accordance with Applicable Law.
“Necessary Third Party License” shall have the meaning set forth in Section 2.9.
“Net Recovery” shall have the meaning set forth in Section 10.7.
“Net Sales” means, with respect to the Product, the gross amount invoiced by KHK or its Affiliates or Sublicensees for sales or other disposition of the Product in the Territory, less deductions for: (a) transportation charges, including insurance actually paid; (b) sales and excise taxes and duties and tariffs paid or allowed by a selling party and any other governmental charges imposed upon the production, inspection, use or sale of the Product; (c) any distributors fees, rebates or allowances, quantity or cash discounts, chargebacks, or fees actually granted in the ordinary course of business; (d) allowances or credits to customers, not in excess of the selling price of the Product, on account of governmental requirements, rejection, outdating or return of the Product. For the purpose of calculating Net Sales, the Parties recognize that KHK’s,
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
9
its Affiliates’ or Sublicensees’, customers may include parties in the chain of commerce who enter into agreements with KHK, its Affiliates or Sublicensees, as to price even though legal title to the Product does not pass directly from KHK, its Affiliates or Sublicensees, to such customers, and even though payment for such Product is not made by such customers to KHK, its Affiliates or Sublicensees, and that in such cases, chargebacks paid by KHK, its Affiliates and Sublicensees, to or through a Third Party (such as a wholesaler) can be deducted from gross revenues in order to calculate Net Sales. Sales between KHK and its Affiliates shall be excluded from the computation of Net Sales, except where such entities are end users, in which case Net Sales shall include sales between KHK and said Affiliates; provided however, that if said Affiliates are using such Product solely for research or clinical testing purposes, indigent or other public support programs, then such sales between KHK and said Affiliates shall be excluded from the computation of Net Sales. Upon the sale or other disposal of the Product (other than in a bona fide arms’ length transaction exclusively for money) or upon any use of the Product for purposes which do not result in a disposal of the Product in consideration of sales revenue customary in the country of use, such sale, other disposal or use shall be deemed to constitute a sale at the relevant open market price in the country in which the sale, other disposal or use occurs, or, if that price is not ascertainable, a reasonable price assessed on an arms’ length basis for the goods or services provided in exchange of the supply; provided, however, that the disposal (but not sale) by KHK, its Affiliates or Sublicensees of Product for promotional sampling (as is customary in the pharmaceutical industry in the applicable countries within the Territory) shall not be included in Net Sales. For Product that is sold in currencies other than Dollars, Net Sales shall be converted into Dollars at the exchange rate of the last Business Day of the applicable calendar quarter, where the applicable exchange rate shall be the New York Closing Snapshot rate published in the Wall Street Journal (as available at its website), or if no such buying rate is available for the relevant currency, at a rate mutually agreed by the Parties. KHK acknowledges and agrees that the calculation of Net Sales under this Agreement is intended to match exactly the calculation of Net Sales under the Bayer License Agreement.
“Net Selling Price” means the amount calculated in accordance with the following formula: Net Sales of a Product in a calendar year divided by the total sales volume of such Product in the same calendar year.
“*** Development Data” shall have the meaning set forth in Section 2.3.4(i).
“XXX Xxxxx” means the reimbursement price of the Product established from time to time by relevant Governmental Authority in the Territory.
“Out-of-Pocket Costs” means costs actually paid or accrued to Third Parties.
“Party” or “Parties” means KHK and/or Syndax.
“Patent” means (i) all issued and existing patents, any extensions, supplemental protection certificates, registrations, confirmations, substitutions, reissues, reexaminations or renewals thereof, and utility model filings; (ii) all pending provisional applications, non-provisional applications and converted provisional applications; and (iii) all continuation, divisional, or continuation-in-part patent applications.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
10
“Product” means any pharmaceutical formulation that contains the Compound.
“Product Complaint” means any written, verbal or electronic expression of dissatisfaction regarding any Product sold by or on behalf of KHK, its Affiliates or its permitted Sublicensees or distributors in the Territory, including reports of actual or suspected product tampering, contamination, mislabeling or inclusion of improper ingredients.
“Product Infringement” shall have the meaning set forth in Section 10.3.3.
“Promotional Materials” means all written, printed, video or graphic advertising, promotional, educational and communication materials (other than the Product labels and package inserts) for marketing, advertising and promoting of the Product in the Field in the Territory, for use (i) by a sales representative, a wholesaler or a distributor, or (ii) in advertisements, web sites or direct mail pieces.
“Prosecute” or “Prosecution” means the procedures and practices necessary or advisable to obtain and maintain Patents, including those procedures and practices necessary or advisable to prepare and file patent applications, respond to office actions and other requests of administrative agencies such as the U.S. Patent and Trademark Office and counterparts thereof outside the United States, and conduct interferences, reexaminations, reissues, oppositions, and the like before such administrative agencies and, if applicable, those procedures and practices necessary or advisable to obtain patent term restoration or supplemental protection certificates or their equivalents.
“Publications” shall have the meaning set forth in Section 14.6.
“PV Agreement” shall have the meaning set forth in Section 5.3.
“Quality Agreements” shall have the meaning set forth in Section7.3.
“Recall” shall have the meaning set forth in Section 5.7.2.
“Recall Costs” shall have the meaning set forth in Section 5.7.2.
“Receiving Party” shall have the meaning set forth in Section 14.1.
“Regulatory Approval” means the approval of a Governmental Authority necessary for the manufacturing, marketing and sale of the Product in a given country or regulatory jurisdiction, which may include the approval of an NDA or the provision of an import drug license.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
11
“Regulatory Authority” means the governmental entities or quasi-governmental entities or its agencies in each country of the Territory with the authority to any matters related to Regulatory Approvals, including the MHLW and the MFDS.
“Regulatory Materials” means regulatory applications, submissions, notifications, communications, correspondence, registrations, Regulatory Approvals and/or other filings made to, received from or otherwise conducted with a Regulatory Authority that are necessary in order to Develop, Manufacture, obtain marketing authorization, market, sell or otherwise Commercialize the Product in a particular country or regulatory jurisdiction (including any applicable DMFs, CMC data, or similar documentation) and all supporting documents in connection therewith, including any Development Data referenced or incorporated therein. Regulatory Materials include INDs, NDA’s, presentations, responses, and applications for other regulatory approvals.
“Reimbursement Cap” shall have the meaning set forth in Section 7.1.
“Royalty Term” shall have the meaning set forth in Section 8.6.
“Senior Officers” shall have the meaning set forth in Section 3.4.2.
“Sole Invention” shall have the meaning set forth in Section 10.1.
“Specifications” means the Manufacturing, performance, quality-control, and packaging and labeling specifications for the Initial Product in a given country in the Territory, which are initially as set forth in the applicable Regulatory Approval for the Initial Product and are set forth on Exhibit B, as such specifications may be amended from time to time pursuant to the terms of this Agreement and/or the Quality Agreements.
“Sublicensee” mean a permitted sublicensee of KHK in accordance with Section 2.2.
“Supply Agreement” shall have the meaning set forth in Section 7.2.
“Supply Failure” shall have the meaning set forth in Section 7.6.1.
“Syndax” shall have the meaning set forth in the first paragraph of this Agreement.
“Syndax Development Partner” shall have the meaning set forth in Section 2.3.4(i).
“Syndax Know-How” means any and all Know-How that is Controlled by Syndax (including the Bayer Know-How) at any time during the Term and that is necessary or useful for the Development, Manufacturing or Commercialization of the Compound or Products in the Territory; provided, however, that the Syndax Know-How shall not include any Know-How licensed from a Third Party after the Effective Date unless it is licensed pursuant to a Third Party License (it being understood that this proviso shall not prevent any Bayer Know-How from being included in Syndax Know-How).
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
12
“Syndax Licensee Improvements” shall have the meaning set forth in Section 2.11.
“Syndax Parties” shall have the meaning set forth in Section 5.7.2.
“Syndax Patents” means all Initial Syndax Patents and Additional Syndax Patents which shall be listed in Exhibit A, as updated from time to time.
“Syndax Trademarks” means any trademarks, and all registrations or applications for registration thereof, that are used or intended to be used in connection with the Commercialization of the Product, to the extent filed in the Territory and Controlled by Syndax or its Affiliates at any time during the Term.
“Technology Transfer” shall have the meaning set forth in Section 7.6.2
“Term” shall have the meaning set forth in Article 13.
“Territory” means Japan and Korea ***.
“Territory Trials” shall have the meaning set forth in Section 4.1.
“Third Party” means a person or entity other than a Party to this Agreement, its respective Affiliates and their employees and personnel.
“Third Party IP” means any Patent or Know-How that is licensed or sublicensed to Syndax by a Third Party and that either (a) is a Syndax Patent or Syndax Know-How or (b) would be a Syndax Patent or Syndax Know-How if Controlled by Syndax in the Territory.
“Third Party Licenses” shall mean (a) all Existing Third Party Licenses and (b) all other agreements between Syndax and Third Parties pursuant to which Syndax acquires rights under any Third Party IP that KHK elects to include in the Syndax Patent and/or Syndax Know-How pursuant to Section 2.9.
“Transfer Price” shall have the meaning set forth in Section 8.5
“Valid Claim” means a claim (including a process, use, or composition of matter claim) of an issued and unexpired Patent in the Syndax Patents that Covers the Development, Commercialization or Manufacture of the Compound or a Product and that has not been held invalid or unenforceable by a patent office, court or other governmental agency or an intergovernmental agency of competent jurisdiction, which holding is unappealable or unappealed within the time allowed for appeal, and that has not been admitted to be invalid by the owner through reissue, disclaimer or otherwise.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
13
The following Exhibits are attached hereto and expressly incorporated into this Agreement:
Exhibit A – Syndax Patents: (1) Initial Syndax Patents (2) Additional Syndax Patents
Exhibit B – Specifications
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
14
ARTICLE 2
LICENSE
2.1 Licenses to KHK.
2.1.1 Subject to the terms and conditions of this Agreement, Syndax hereby grants to KHK the exclusive (even as to Syndax) license in the Territory, with the right to sublicense (subject to Section 2.2), under the Syndax Patents and the Syndax Know-How, to (a) Develop Products for use in the Field in the Territory; and (b) Commercialize Products in the Field in the Territory. Upon request of KHK or its Affiliates or Sublicensees, and at their own cost and expense, Syndax shall fully cooperate, and shall use Commercially Reasonable Efforts to cause Bayer or other patent owner of Syndax Patents to fully cooperate, with KHK or its Affiliates or Sublicensees in registering with patent offices in the Territory the exclusive licenses (senyo jisshiken in Japan and the equivalent in Korea), including any sublicense, to Syndax Patents granted under this Agreement.
2.1.2 Subject to the terms and conditions of this Agreement, including the terms set forth in Section 10.8, Syndax hereby grants to KHK an exclusive (even as to Syndax) license, with the right to sublicense (subject to Section 2.2), to use the Syndax Trademarks solely in connection with the Commercialization of the Product in the Field in the Territory. The foregoing license shall be subject to KHK’s compliance with Syndax’s trademark guidelines and quality control provisions, as communicated to KHK from time to time. Upon request of KHK or its Affiliates or Sublicensees, and at their own cost and expense, Syndax shall fully cooperate with KHK or its Affiliates or Sublicensees in registering with trademark offices in the Territory the exclusive licenses (senyo shiyoken in Japan and the equivalent in Korea), including sublicenses, to Syndax Trademarks granted under this Agreement.
2.2 Sublicenses. KHK (or its Affiliates) shall be permitted to sublicense the rights granted to it hereunder, subject to the prior written consent of Syndax, which shall not be unreasonably withheld, conditioned or delayed. KHK, however, acknowledges that any of its sublicenses under the Syndax Patents licensed to Syndax from Bayer will require the prior written consent of Bayer, which shall not be unreasonably refused and which Syndax will use Commercially Reasonable Efforts to obtain, but Syndax shall not be liable to KHK if Bayer fails to provide such consent. KHK and the applicable Sublicensee shall document each such sublicense in writing, and the terms of the written sublicense shall be consistent with this Agreement. Without limiting the generality of the foregoing, each such written sublicense shall (a) require the applicable Sublicensee to comply with the terms of this Agreement; (b) require that, upon a termination of such sublicense, the Sublicensee must assign to KHK, and provide to KHK full copies of, all Regulatory Approvals and INDs, NDAs and other similar regulatory filings that relate to Products and/or Compounds and are owned or Controlled by such Sublicensee, (such that KHK will be able to, pursuant to Section 13.5.3, assign to Syndax, and provide Syndax with full copies of, all such Regulatory Approvals and regulatory filings upon termination of this Agreement); and (c) explicitly state that such sublicense will immediately terminate upon termination of this Agreement. KHK shall be responsible for its Sublicensee’s
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
15
actions and omissions with respect to this Agreement. Promptly after the execution of each written sublicense agreement, KHK shall provide to Syndax a true and complete copy of such agreement; provided, however, that any financial or other information may be redacted to the extent not applicable to the Sublicensee’s compliance with this Agreement. Syndax shall be permitted to provide such redacted copy to Bayer in confidence. Notwithstanding the foregoing, KHK shall be permitted to sublicense to its Affiliates the rights granted to it hereunder without obtaining the prior consent of Syndax.
2.3 Provision of Information, Materials and Assistance.
2.3.1 Initial Delivery of Information and Materials. In furtherance of the rights and licenses granted by Syndax to KHK under this Agreement, as soon as reasonably practicable, Syndax shall furnish to KHK a data package in electronic format that shall include the copies or embodiments of the Syndax Know-How (including any Development Data and Regulatory Materials included in such term) Controlled by Syndax as of the Effective Date (hereinafter the “Initial Delivery of Information and Materials”).
2.3.2 Ongoing Delivery of Information and Materials. During the Term, at no additional cost to KHK, Syndax shall promptly disclose and provide KHK with copies, reports and summaries of any additional Syndax Know-How (including any Development Data and Regulatory Materials included in such term) that comes to Syndax’s attention (or that are reasonably requested by KHK) and that have not previously been provided to KHK by Syndax. Syndax shall (a) *** Syndax’s licensees, research and clinical partners or other Third Parties with which Syndax conducts Development or any other activities with respect to the Product to *** and (b) ***. During the Term, at no additional cost to Syndax, KHK shall promptly disclose and provide Syndax with copies of Development Data and Regulatory Materials that comes to KHK’s attention (or that are reasonably requested by Syndax) and that have not previously been provided to Syndax by KHK provided, however, that KHK shall provide a brief summary description in English of each set of documents, but KHK shall not be required to prepare English translations of any documents. KHK shall (x) *** KHK’s Sublicensees, research and clinical partners or other Third Parties with which KHK conducts Development or any other activities with respect to the Product to *** and (y) ***.
2.3.3 Rights of Reference. At no additional cost to KHK, KHK, its Affiliates and its designees shall have the right to reference Regulatory Materials for applicable Products outside the Territory, to the extent such Regulatory Materials are Controlled by Syndax or its Affiliates or licensees (including any future licensees), in connection with any Regulatory Approvals that KHK or its Affiliates may seek for use of Product in the Field in the Territory. Syndax shall (a) *** Syndax’s licensees, research and clinical partners or other Third Parties with which Syndax conducts Development or any other activities with respect to the Product to *** and (b) ***. At no additional cost to Syndax, Syndax, its Affiliates and its designees shall have the right to reference Regulatory Materials for Products, to the extent such Regulatory Materials are Controlled by KHK or its Affiliates or Sublicensees, in connection with any Regulatory Approvals that Syndax or its Affiliates may seek for use of Product outside of the Territory. KHK shall (x) *** KHK’s Sublicensees, research and clinical partners or other Third Parties with which KHK conducts Development or any other activities with respect to the Product to *** and (y) ***.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
16
2.3.4 Use of Data.
(i) KHK shall have the right, without any additional payment, to access and use in support of Development, Regulatory Approval and/or Commercialization of Product by KHK, its Affiliates or Sublicensees in the Territory in the Field, any Development Data arising from the Development of Product by Syndax or its Affiliates, or their contractors, licensees or other Development partners, including institutions or investigators who conduct investigator-initiated clinical studies (a “Syndax Development Partner”) (including such Development Data arising from the clinical study that is the subject of the license agreement dated March 14, 2014 between Syndax and the Eastern Cooperative Oncology Group (the “ECOG License Agreement”) or clinical studies conducted for the Product outside the Territory by medical institutions, whether sponsored by Syndax or its Affiliates or licensees or initiated by a medical institution), provided that such access and use is consistent with the other terms of this Agreement. Syndax shall, at its own cost, provide KHK or its Affiliates or Sublicensees with such Development Data by way of electronic formats of (i) SEND and CDISC, and (ii) additionally, other formats, if any, in which Syndax files with FDA. Notwithstanding the foregoing, with respect to Development Data solely relating to *** (“*** Development Data”) that is generated by or on behalf of a Syndax Development Partner, KHK acknowledges that obtaining access to and/or the right to use any such *** Development Data may require providing payments or other consideration to licensee, and KHK (and not Syndax) shall be responsible for any such payments or other consideration in the Territory. To the extent that a Syndax Development Partner does not permit disclosure of its *** Development Data to KHK, Syndax shall not permit such Syndax Development Partner to access or use any *** Development Data generated by or on behalf of KHK except with KHK’s prior written consent. The foregoing shall be KHK’s sole remedy for any failure by Syndax to comply with the its obligations in this Section 2.3.4(i) with respect to Development Data from Syndax Development Partners.
(ii) Syndax shall have the right, without any additional payment, to access and use in support of Development, Regulatory Approval and/or Commercialization of Product by Syndax, its Affiliates or licensees outside the Territory in the Field, any Development Data arising from the Development of Product by KHK or its Affiliates, or their contractors, licensees or other Development partners, including institutions or investigators who conduct investigator-initiated clinical studies (a “KHK Development Partner”), provided that such use is consistent with the other terms of this Agreement. Notwithstanding the foregoing, with respect to *** Development Data that is generated by or on behalf of a KHK Development Partner, Syndax acknowledges that obtaining access to and/or the right to use any such *** Development Data may require providing payments or other consideration to licensee, and Syndax (and not KHK) shall be responsible for any such payments or other consideration outside the Territory. To the extent that a KHK Development Partner does not
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
17
permit disclosure of its *** Development Data to Syndax, KHK shall not permit such KHK Development Partner to access or use any *** Development Data generated by or on behalf of Syndax except with Syndax’s prior written consent. The foregoing shall be Syndax’s sole remedy for any failure by KHK to comply with the its obligations in this Section 2.3.4(ii) with respect to Development Data from KHK Development Partners.
(iii) This Section 2.3.4 shall not restrict or otherwise affect either Party’s access to data on adverse drug reactions, adverse events and other relevant drug safety matters with respect to Products during the Term, the exchanged of which will be governed exclusively by the PV Agreement.
2.3.5 Assistance. In addition to the foregoing, Syndax shall provide to KHK at no additional cost to KHK all reasonable assistance as KHK may request in order to assist KHK with obtaining Regulatory Approval for the Product in the Territory; provided, however, that KHK shall be responsible for ***. Notwithstanding the foregoing, until KHK or its Affiliate or Sublicensees obtain Regulatory Approval for the Initial Product in each country in the Territory, Syndax shall, at its own cost, *** or cause *** to *** the *** outside of the *** and *** in connection with any ***. If any raw data is missing, destroyed or cannot be traced and any additional studies are required to be conducted by KHK or its Affiliates or Sublicensees as a result thereof, the Parties shall ***.
2.4 Improvements by KHK.
2.4.1 License to Syndax; Grant Back. In the event KHK or a KHK Affiliate discovers *** (“KHK Improvements”), KHK or its Affiliate (as the case may be) shall own all rights in and to any such KHK Improvements, provided, however, that KHK hereby grants to Syndax, subject to the applicable provisions contained herein, a perpetual, royalty-free, fully paid, sublicenseable license, under such KHK Improvements Controlled by KHK or its Affiliates, to use such KHK Improvements outside of the Territory in connection with the research, Development, Manufacturing, or Commercialization of Products (the “KHK Improvements License”). To the extent that a Syndax licensee (including a sublicensee) does not permit a Syndax Licensee Improvement (defined below in Section 2.11) to be licensed to KHK, Syndax shall not permit such Syndax licensee (including any sublicensee) to use any KHK Improvements except with KHK’s prior written consent. Such KHK Improvements License shall be exclusive solely with respect to Products, and as a result KHK shall be free to use and exploit the KHK Improvements in connection with Products in the Territory or products other than Products anywhere in the world, without any obligations to Syndax. For avoidance of doubt, the KHK Improvements License shall automatically terminate if this Agreement is terminated by KHK in accordance with Section 13.3. The foregoing shall be KHK’s sole remedy for any failure by Syndax to comply with the obligation in the first sentence of Section 2.11.
2.4.2 Responsibility for Prosecution of Patents. KHK shall have the sole discretion, at its own cost, for the Prosecution of any Patents for the KHK Improvements in or
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
18
outside the Territory, including any decision whether to file a patent application for such KHK Improvements. In the event that KHK fails to further Prosecute any Patent of a KHK Improvement owned by KHK outside of the Territory, or to conduct any interference, reexamination, re-issue or opposition with respect thereto, KHK shall promptly notify Syndax (which in no event will be less than *** prior to the date on which such Patent will become abandoned, such payment is due or such proceeding is scheduled to occur). Thereafter, Syndax shall, at its sole discretion and cost, have the right to Prosecute such Patent in such countries as it deems appropriate, and conduct any interference, re-examination, re-issue or opposition with respect thereto and KHK shall assign, at no additional cost to Syndax, all right, title and interest in and to such Patents owned by KHK outside the Territory and shall cooperate, at no additional cost to Syndax, as reasonably requested by Syndax in connection with such actions by Syndax.
2.5 No Rights by Implication. KHK’s rights with regard to the Syndax Patents and Syndax Know-How shall be limited to those rights specified in Sections 2.1–2.5, and, as between the Parties, Syndax retains all other rights related thereto. For avoidance of doubt, no rights are granted to KHK under this Agreement to Develop or Commercialize the Product outside the Territory.
2.6 Territory and Ex-Territory Restrictions. KHK hereby covenants and agrees that it shall not (and shall cause its Affiliates, Sublicensees and subcontractors not to), either directly or indirectly, market, distribute or sell the Compound or Product outside the Territory. Without limiting the generality of the foregoing, with respect to such countries outside the Territory, KHK shall not (and shall cause its Affiliates, Sublicensees and subcontractors not to) (i) unless otherwise agreed by the Parties, engage in any advertising activities relating to the Product directed solely to customers located in such countries, or (ii) solicit orders from any prospective purchaser located in such countries.
2.7 Non-Competition.
2.7.1 During the Term, neither Party shall, directly or indirectly, Commercialize a Competing Product in the Territory.
2.7.2 If Syndax or any of its Affiliates, acquires, is acquired by, merges with, or otherwise enters into a combination with, an entity that owns or has a license or other right to, a Competing Product, then Syndax and/or its Affiliates (or the surviving or acquiring entity, as applicable) shall have the right to commercialize such Competing Product in the Territory provided that Syndax or its Affiliate (or the surviving or acquiring entity, as applicable) notifies KHK of such Competing Product in writing no later than the Required Notice Date (as defined below) and thereafter performs one of the following acts:
(i) divest such Competing Product on a country-by-country basis and notify KHK in writing of such divestiture; provided that an agreement with a Third Party for such divestiture shall be completed within *** after the Required Notice Date; or
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
19
(ii) establish *** to ensure that *** who are *** (A) are *** and (B) are not ***.
For the avoidance of doubt, in no event shall Syndax or its Affiliates (or the surviving or acquiring entity, as applicable) be entitled to use any Confidential Information of KHK for any other purpose (including the research, development or commercialization of such Competing Product) than the purposes permitted under Article 14.
2.7.3 If KHK or any of its Affiliates acquires, is acquired by, merges with, or otherwise enters into a combination with, an entity that owns or has a license or other right to a Competing Product, then KHK and/or its Affiliates (or the surviving or acquiring entity, as applicable) shall have the right to commercialize such Competing Product in the Territory provided that KHK or its Affiliate (or the surviving or acquiring entity, as applicable) notifies Syndax of such Competing Product in writing no later than the Required Notice Date (as defined below) and thereafter performs one of the following acts:
(i) divest such Competing Product on a country-by-country basis and notify Syndax in writing of such divestiture; provided that an agreement with a Third Party for such divestiture shall be completed within *** after the Required Notice Date; or
(ii) immediately terminate this Agreement (in which case the notice delivered above shall be deemed to be a notice of termination).
For the avoidance of doubt, in no event shall KHK or its Affiliates (or the surviving or acquiring entity, as applicable) be entitled to use any Confidential Information of Syndax for any other purpose (including the research, development or commercialization of such Competing Product) than the purposes permitted under Article 14.
2.7.4 As used herein, “Required Notice Date” means the date that is *** prior to the *** of ***; provided that the Required Notice Date shall in no event be *** the *** following the consummation of the transaction described in Section 2.7.2 or 2.7.3, as applicable.
2.8 Performance by Affiliates, Subcontractors or Sublicensees.
2.8.1 Affiliates. The Parties recognize that each may perform some or all of its obligations under this Agreement through Affiliates; provided, however, that each Party shall remain responsible for and shall be guarantor of the performance by its Affiliates and shall cause its Affiliates to comply with the provisions of this Agreement in connection with such performance. Each Party hereby expressly waives any requirement that the other Party exhausts any right, power or remedy, or proceeds against an Affiliate, for any obligation or performance hereunder prior to proceeding directly against such Party.
2.8.2 Subcontractors. The Parties recognize that each Party may perform some or all of its obligations under this Agreement through subcontractors; provided, however, that each
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
20
Party shall remain responsible for and shall be guarantor of the performance by its subcontractors and shall cause its subcontractors to comply with the provisions of this Agreement in connection with such performance. Each Party hereby expressly waives any requirement that the other Party exhaust any right, power or remedy, or proceeds against a subcontractor, for any obligation or performance hereunder prior to proceeding directly against such Party.
2.8.3 Sublicensees. To the extent that KHK sublicenses any of the rights or obligations granted to it hereunder in accordance with the terms hereof, as between KHK and Syndax, KHK shall remain responsible for performing its obligations hereunder and ensuring that any such Sublicensees comply with this Agreement.
2.9 Third Party Licenses. If, during the Term, Syndax acquires any Third Party IP under any agreement other than an Existing Third Party License and such agreement includes sublicensable rights in the Territory, Syndax shall promptly notify KHK of such agreement and disclose payments under such agreement that are relevant to, or otherwise allocable to, the Territory. KHK may, in its sole discretion, provide a written confirmation to Syndax that it wishes such Third Party IP to be included in the Syndax Patents and/or Syndax Know-How (as applicable). Effective solely upon such confirmation, such agreement shall be deemed to be a Third Party License, and the Syndax Patents and/or Syndax Know-How (as applicable) shall be deemed to include such Third Party IP. Syndax shall bear all royalties, costs or other compensation payable to any Third Party under any Third Party License to Third Party IP that *** by *** of the *** (as such *** as of the Effective Date) in the Territory (a “Necessary Third Party License”). For Third Party Licenses that are not Necessary Third Party Licenses, KHK shall bear all royalties, costs or other compensation payable to any Third Party under any such Third Party License for the Territory as a result of the grant of such Third Party License to KHK or the exercise or sublicensing of such Third Party License by KHK, its Affiliates, or its sublicensees.
2.10 Additional Syndax Patents. Prior to Syndax’s filing of any Patent application for an Additional Syndax Patent, Syndax shall provide KHK a sufficient period and opportunity to review and comment on any documents that will be filed as part of such Patent application. Whenever Syndax files a Patent application or otherwise obtains Control of a Patent after the Effective Date, Syndax shall promptly notify KHK and such Patent shall automatically constitute an Additional Syndax Patent unless KHK notifies Syndax within *** (or within such reasonable extension of time beyond *** as KHK may request) after receiving notice of such Patent that KHK wishes to exclude such Patent from Additional Syndax Patents. If KHK elects to exclude such Patent and provides notification of such election within the aforementioned time period, such Patent shall thereafter be deemed not to be an Additional Syndax Patent. Syndax shall provide KHK with an updated list of Additional Syndax Patents on at least an annual basis.
2.11 Syndax Licensee Improvements. Notwithstanding Section 2.9, in the event that Syndax’s licensees (including any sublicensees) discover *** (“Syndax Licensee
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
21
Improvements”), Syndax shall ensure that all licensees (and sublicensees) of Syndax shall be required to grant a perpetual, royalty-free, fully paid, freely sublicenseable, non-exclusive license under such Syndax Licensee Improvements Controlled by such licensee (or sublicensee) so that, subject to the applicable provisions contained herein, KHK may have access to and use such Syndax Licensee Improvements in the Territory free of charge under this Agreement. To the extent that a KHK Sublicensee does not permit a KHK Improvement to be licensed to Syndax, KHK shall not permit such KHK Sublicensee to use any Syndax Licensee Improvements except with Syndax’s prior written consent; provided, however, that the foregoing shall not in any way limit any KHK Sublicensee’s rights under any Third Party License.
ARTICLE 3
JOINT STEERING COMMITTEE
3.1 Formation of JSC. Within *** after the Effective Date, the Parties shall establish and designate the members of a joint committee to consist of an equal number of representatives from each Party (the “JSC”).
3.2 Composition. The JSC shall be composed of *** members selected by Syndax and *** members selected by KHK, all of whom shall have appropriate background and authority to consult, discuss and monitor matters assigned to the JSC hereunder. Each Party shall designate one of its members as a co-chair of the JSC. Each Party is free to select and change members of the JSC immediately upon notice to the other Party. Additional participants may be invited by any member to attend meetings where appropriate (e.g., personnel from regulatory affairs or outside consultants). Such additional participants shall not be deemed to have, or have any rights or responsibilities of, a member of such committee or working group.
3.3 JSC Meetings. The JSC shall meet at least *** during the first *** and thereafter at least ***. Members of the JSC may participate in meetings in person, by telephone or by video conference; provided, however, that the Parties Syndax and KHK shall each use reasonable efforts to at least one (1) such meeting during each year to be attended in person by their respective representatives, alternating between the United States and Japan. Additional representatives of either Party may be permitted to attend JSC meetings, subject to prior notice to and the approval of the other Party, not to be unreasonably withheld. The Parties shall cooperate in preparing written summaries of the substance of significant discussions covered at JSC meetings, with the host company preparing the initial draft of the summary.
3.4 Responsibility and Authority.
3.4.1 The JSC shall be the primary forum to:
(i) facilitate the exchange of data, information, materials and results between the Parties, and monitor the Development and Commercialization activities of the Parties.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
22
(ii) discuss and monitor the Development Plan and Commercialization activities and regulatory strategies of the Parties hereunder;
(iii) review and discuss the overall strategy for obtaining, maintaining and enforcing patent protection and market and data exclusivity for the Product in the Field in the Territory;
(iv) establish working groups pursuant to Section 3.4.5 on an as-needed basis, oversee the activities of all working groups so established, and address disputes or disagreements arising in all such working groups; and
(v) have such other responsibilities as may be assigned to the JSC pursuant to this Agreement or as may be mutually agreed upon by the Parties in writing from time to time.
3.4.2 The JSC shall be an instrument for discussion and consultation, and shall not be a decision making body. Votes will not be taken or recorded. If a disagreement among the parties is not resolved through good faith discussions of the JSC, or if the JSC is no longer functioning, the matter shall be referred for further discussion by, with respect to each Party, a senior representative of such Party responsible for the performance and management of this Agreement (collectively, the “Senior Officers”). The Senior Officers shall use reasonable efforts to discuss and resolve any matters referred to them promptly and in good faith. If the Senior Officers are unable to reach agreement with respect to a particular matter after good faith discussions, the matter shall be referred for review and resolution by each Party’s Chief Executive Officer or Chairman or his/her designee of each, who shall use reasonable efforts to negotiate and resolve matters referred to them promptly and in good faith.
3.4.3 KHK shall have the final say and may act in accordance with its discretionary judgment with respect to any decision concerning the Development or Commercialization of a Product in the Territory, subject to the terms of this Agreement. KHK shall consult with Syndax in good faith and shall use Commercially Reasonable Efforts to appropriately respond to any reasonable concerns of Syndax on any matter that relates to the Product. KHK shall use reasonable efforts to avoid any situation that: (i) materially impairs or is reasonably likely to impair any rights or assets of Syndax or any of its Affiliates; (ii) results in or is reasonably likely to result in Syndax being in breach of the Bayer Agreement; (iii) results in or is reasonably likely to result in an increased financial obligation for Syndax or any of its Affiliates or an increased obligation for Syndax or its Affiliates to utilize any resources; or (iv) creates any safety or regulatory issues that could reasonably be expected to have a material effect on the Development of the Product outside the Territory.
3.4.4 The JSC shall have only the powers or functions assigned expressly to it in this Article 3 and elsewhere in this Agreement, and shall not have any power to amend, modify or waive compliance with this Agreement or determine whether a breach of this Agreement has occurred. In furtherance thereof, each Party shall retain the rights, powers and discretion granted
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
23
to it under this Agreement and no such rights, powers or discretion shall be delegated or vested in the JSC unless such delegation or vesting of rights is expressly provided for in this Agreement or the Parties expressly so agree in writing.
3.4.5 In addition to the JSC, the Parties may establish JSC’s working groups to focus on discussions and information sharing concerning Development, Regulatory, supply of Product, Commercialization or other areas of concern. To the extent that KHK plans to conduct a Territory Trial that is part of a global Clinical Trial being conducted by Syndax or its Affiliates or a Syndax Development Partner, the Parties shall discuss whether additional processes and information sharing may be necessary and/or advisable to ensure coordination between such Territory Trial and the global Clinical Trial.
ARTICLE 4
DEVELOPMENT
4.1 Development Responsibilities. KHK shall, during the Term, have sole authority and responsibility for the Development of Products in the Field in the Territory, including the conduct of Clinical Trials of Product in the Territory in support of Regulatory Approval in the Territory (collectively, “Territory Trials”). In the event that Syndax or its designee conducts a global Clinical Trial in countries including the United States and Europe for ***, KHK, in its sole discretion, shall have the right to join such global Clinical Trial.
4.2 Development Plan.
4.2.1 Initial Preparation. Within *** after the Initial Delivery of Information and Material, KHK shall present to the JSC a development plan which shall outline the applicable general guidelines and governing plans, processes and procedures pursuant to which KHK will seek Regulatory Approval for the Product in *** (the “Development Plan”). The Development Plan shall be expanded by KHK to include Development activities for *** as soon as reasonably practicable, but in any event prior to the commencement of any Territory Trials in ***.
4.2.2 Contents. The Development Plan shall contain sufficient operational and technical detail, including timelines to provide clear deliverables and milestones for the management of Development, including a description of any Territory Trials that KHK anticipates will be conducted by or for KHK, its Affiliates, or its Sublicensees in the subsequent twenty-four (24) months.
4.2.3 Amendments. On an annual basis (no later than October of each calendar year), or more often as the Parties deem appropriate by mutual agreement, KHK shall prepare amendments to the then-current Development Plan for consultation in and monitoring by the JSC. Each such amended Development Plan shall specify the items described in Sections 4.2.1 and 4.2.2. Such amended Development Plan shall cover the next calendar year (as well as any later periods as reasonably determined by KHK).
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
24
4.2.4 Performance. KHK shall use Commercially Reasonable Efforts to perform the tasks and obligations in the Development Plan, substantially in accordance with the time schedules set forth therein. The Parties shall communicate in good faith and in a timely manner so that each Party is appropriately informed of the other Party’s efforts and activities aimed toward Regulatory Approval in their respective territories.
4.3 Diligent Efforts. KHK shall use Commercially Reasonable Efforts to Develop *** Product in the Territory and to obtain Regulatory Approval therefor in ***. Without limiting the generality of foregoing, KHK agrees that, at minimum, it will pursue Development of, and seek Regulatory Approval for, the Initial Product in the Territory with respect to *** for *** with the *** including, *** (the “First Indication”), for which Syndax intends to pursue Regulatory Approval of in ***. The activities of KHK’s Affiliates and Sublicensees shall be attributed to KHK for the purposes of evaluating KHK’s fulfillment of the obligations set forth in this Section 4.3.
4.4 Costs. KHK shall bear all costs in connection with the Development of Product in the Territory and seeking Regulatory Approval of the Product in the Territory. Notwithstanding the foregoing, in the case of any Territory Trial that is part of a global Clinical Trial being conducted by Syndax or its designee, KHK shall only bear those costs to be paid to any medical institution or investigator for clinical studies of Product in the Territory and those costs in connection with seeking Regulatory Approval of the Product in the Territory.
ARTICLE 5
REGULATORY
5.1 KHK Obligations.
5.1.1 INDs; NDAs. KHK shall be responsible for obtaining Regulatory Approval for the Product in the Territory, including drafting, preparation, filing, and maintenance of INDs in the Territory, NDAs in the Territory, and all other applications in connection with seeking Regulatory Approvals for Product in the Territory. KHK shall timely provide Syndax with, and shall thereafter update, copies of all material correspondence, submissions and exchanges between KHK and any Regulatory Authorities in the Territory with respect to Product. KHK shall provide a brief summary description in English of each set of documents, but KHK shall not be required to prepare English translations of any documents.
5.1.2 Regulatory Materials. The Parties intend that the Development Plan will set forth the agreed regulatory strategy for seeking Regulatory Approval of Products in the Territory. KHK shall draft, prepare, file, and maintain Regulatory Materials in the Territory, as well as seek Regulatory Approval for Product in the Territory, in a manner consistent with such strategy. Syndax shall fully cooperate with and assist KHK in complying with any of KHK’s regulatory obligations with respect to Product within the Territory, including those described in Sections 2.3.4 and 4.4. Syndax shall use Commercially Reasonable Efforts to provide any information or documentation requested by KHK to meet a deadline set by the relevant Regulatory Authority or by Applicable Law.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
25
5.1.3 Regulatory Authority Inspection
(i) If a Regulatory Authority in or outside the Territory desires to conduct an inspection or audit of Syndax’s facility or a facility under contract with Syndax with regard to the Product, Syndax shall cooperate and, if applicable, cause the contract facility to cooperate with such Regulatory Authority during such inspection or audit. If Syndax, rather than KHK, receives the inspection or audit observations of such Regulatory Authority in the Territory, Syndax shall promptly provide a copy of such observations to KHK. Syndax (and/or its contractor) shall prepare the response to any such observations, but the submission of the response to the applicable Regulatory Authority shall be subject to KHK’s review or comments, which Syndax shall consider in good faith. Syndax agrees to conform its activities under this Agreement to any commitments made in such a response, except to the extent it believes in good faith that such commitments violate Applicable Law.
(ii) If a Regulatory Authority in or outside the Territory desires to conduct an inspection or audit of KHK’s facility or a facility under contract with KHK with regard to the Product, KHK shall cooperate and, if applicable, cause the contract facility to cooperate with such Regulatory Authority during such inspection or audit. If KHK rather than Syndax, receives the inspection or audit observations of such Regulatory Authority outside the Territory, KHK shall promptly provide a copy of such observations to Syndax. KHK shall prepare the response to any such observations, but the submission of the response to the applicable Regulatory Authority shall be subject to Syndax’s review or comments, which KHK shall consider in good faith. KHK agrees to conform its activities under this Agreement to any commitments made in such a response, except to the extent it believes in good faith that such commitments violate Applicable Law.
5.2 Manufacturing-Related Filings. Notwithstanding the provisions of Sections 4.4 and 5.1, Syndax shall be solely responsible, at its sole cost, for preparing those portions of any Regulatory Materials related to the Manufacture of the Product for sale in the Field in the Territory, including any DMFs and CMC (or equivalent) sections of any Regulatory Materials, and will promptly provide to KHK (or permit KHK to reference, as applicable) such Regulatory Materials for use in compiling, supporting and maintaining regulatory filings in the Territory. In the event that Syndax or its designee elects to file a DMF (or equivalent) for the Product in the Territory, Syndax shall, at its own cost, file and maintain, or cause such designee to file and maintain, such DMF (or equivalent) for the Product in the Territory. The foregoing shall only apply to the extent that Syndax is Manufacturing the Compound or the Product under Article 7.
5.3 Pharmacovigilance. Syndax shall be the global safety database holder and shall be responsible for all aspects of pharmacovigilance of the Product. Syndax (or its designee) shall be responsible for the collection, review, assessment, tracking and filing of information related to adverse events associated with the Product in the countries outside the Territory as well as, to the
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
26
extent required under Applicable Law, compiling and communicating to Regulatory Authorities outside the Territory. KHK shall reasonably cooperate with and assist Syndax upon request as required by Syndax in order to comply with Syndax’s regulatory obligations with respect to the Product outside of the Territory. KHK shall be responsible for the collection, review, assessment, tracking and filing of information related to adverse events associated with the Product in the Field in the Territory. Not later than the date of the first IND submission in the Territory or such earlier date required by Applicable Law with respect to the Parties’ activities hereunder, the Parties shall enter into a separate, written pharmacovigilance agreement (the “PV Agreement”) containing the specific terms, conditions and obligations of the Parties with respect to the collection, reporting and monitoring of all adverse events, risk management activities and other relevant drug safety matters with respect to Products during the Term. The PV Agreement shall ensure that adverse event and other safety information is exchanged according to a schedule that will permit each Party (and its designees or licensees) to comply with Applicable Law and regulatory requirements in their respective markets.
5.4 Information Sharing. The Parties will comply with all information sharing obligations contained in this Agreement or any related agreement, including the Clinical Supply Agreement, the Commercial Supply Agreement, the Quality Agreements and the PV Agreement.
5.5 Communications with Regulatory Authorities. KHK shall be solely responsible for all communications and other dealings with Regulatory Authorities in the Territory with respect to Product, subject to the information sharing required by Section 5.1.1 and Section 5.4, subject to Syndax’s obligations with respect to DMFs pursuant to Section 5.2 and subject to Applicable Law. Syndax shall not communicate with any Regulatory Authority in the Territory regarding any Product unless explicitly requested or permitted in writing to do so by KHK, or unless so ordered by such Regulatory Authority or otherwise required by Applicable Law, in which case Syndax shall immediately provide notice of such order or legal requirement to KHK. Syndax (or its designee) shall be solely responsible for any communications with Regulatory Authorities outside of the Territory with respect to Product. KHK shall not communicate with any Regulatory Authority outside the Territory regarding any Product unless explicitly requested or permitted in writing to do so by Syndax, or unless so ordered by such Regulatory Authority or otherwise required by Applicable Law, in which case KHK shall immediately provide notice of such order or legal requirement to Syndax.
5.6 Ownership. To the extent permitted by Applicable Law, KHK shall be the legal and beneficial owner of all Regulatory Approvals for Product in the Territory. To the extent permitted by Applicable Law, all Regulatory Materials for Products in the Territory shall be filed in the name of KHK or its Affiliate or Sublicensee.
5.7 Recalls.
5.7.1 Procedures. In the event that any Governmental Authority threatens or initiates any action to remove the Product from the market in the Field whether inside the
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
27
Territory or outside the Territory (in whole or in part), the Party receiving notice thereof shall notify the other Party of such communication immediately, but in no event later than *** after receipt of such notification, and in any case in accordance with Applicable Law. Notwithstanding the foregoing, in all cases KHK shall determine whether to initiate any recall or withdrawal of the Product in the Field in the Territory, and Syndax shall determine whether to initiate any recall or withdrawal of the Product outside the Territory, provided, however, that each Party shall perform any recalls or withdrawals to the extent required or mandatory under Applicable Law. With respect to voluntary recalls or withdrawals other than to the extent required or mandatory under Applicable Law, before KHK initiates a recall or withdrawal, the Parties shall use Commercially Reasonable Efforts to promptly meet telephonically or in person to discuss in good faith the reasons therefor, provided, however, that such discussions shall not delay any action that KHK reasonably believes has to be taken in relation to any recall or withdrawal. In the event of any such recall or withdrawal in the Territory, KHK, as the distributor of the Product, shall determine the necessary actions to be taken, and, shall implement such action, considering in good faith any reasonable input provided by Syndax. Each Party shall maintain complete and accurate records of any recalls or withdrawals in its territory for such periods as may be required by Applicable Law, but in no event for less than ***.
5.7.2 Costs. Without prejudice to other rights and remedies set forth in this Agreement, or any other agreement executed pursuant to this Agreement, including the Clinical Supply Agreement, the Commercial Supply Agreement, the PV Agreement and the Quality Agreement, all internal and external costs (excluding any costs or damages arising from a Claim by a Third Party to which a Party is entitled to indemnification under Article 12) incurred by the Parties in connection with implementing a recall or withdrawal (a “Recall”) with respect to the Product in the Field in the Territory (“Recall Costs”) shall be allocated between Syndax and KHK as follows:
(i) in the event, and to the extent, that the Recall arises as a result of: (a) defective Product supplied by Syndax under the Commercial Supply Agreement; (b) negligence or willful misconduct of Syndax or Bayer or their respective Affiliates, directors, officers, employees representative, contractors and agents (each a “Syndax Party” and, collectively, the “Syndax Parties”); (c) a breach of this Agreement, or any other agreement executed pursuant to this Agreement, including the Clinical Supply Agreement, the Commercial Supply Agreement, the PV Agreement or the Quality Agreements, by any Syndax Party, (d) the failure by any Syndax Party to comply with Applicable Law; or (e) a determination by an applicable Regulatory Authority in the Territory that incidents, activities, non-compliance or other material issues outside of the Territory necessitate such Recall, Syndax shall bear all Recall Costs; provided, however that this Section 5.7.2(i) shall not apply to the extent any such Recall Costs arise out of (x) the negligence or willful misconduct of KHK, its Affiliates or Sublicensees, and their respective directors, officers, employees, representatives, contractors and agents (each a “KHK Party” and, collectively, the “KHK Parties”), (y) the breach of this Agreement or any other agreement executed pursuant to this Agreement by any KHK Party, or (z) the failure by any KHK Party to comply with Applicable Law;
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
28
(ii) in the event, and to the extent, that the Recall arises as a result of (a) negligence or willful misconduct of any KHK Party in its Development, Commercialization, or Manufacturing activities (including such negligence or willful misconduct of ay KHK Party in those activities in connection with the Initial Product), or (b) any breach of this Agreement or any other agreement executed pursuant to this Agreement by any KHK Party, KHK shall bear all Recall Costs; provided, however that this Section 5.7.2(ii) shall not apply to the extent any such Recall Costs arise out of (x) the negligence or willful misconduct of any Syndax Party, or (y) the breach of this Agreement or any other agreement executed pursuant to this Agreement (including the Clinical Supply Agreement, the Commercial Supply Agreement, the PV Agreement or the Quality Agreements), by any Syndax Party (including any Product Manufacturing breach by any Syndax Party or any failure by any Syndax Party to supply a Product in compliance with the representations and warranties herein applicable thereto), or (z) the failure by any Syndax Party to comply with Applicable Law; or
(iii) in all other cases, Recall Costs shall be borne by the Parties in the following ratio: ***.
ARTICLE 6
COMMERCIALIZATION
6.1 Commercialization Responsibilities. KHK shall, during the Term, have sole authority and responsibility for the Commercialization of Products in the Field in the Territory. KHK will be the sole Marketing Authorization Holder in the Territory.
6.2 Marketing Plan. After the first Regulatory Approval of the Product in the Territory, KHK shall provide Syndax with its summary of the annual Marketing plan (the “Marketing Plan”), which shall include a summary of the Marketing strategy. KHK shall use Commercially Reasonable Efforts to perform the tasks and obligations in the Marketing Plan.
6.3 Medical Activities. KHK shall annually inform Syndax of any outsourced research, publication strategy, or supply of Product to physician initiated clinical trials.
6.4 Diligent Efforts. For each Product that receives Regulatory Approval in a country in the Territory, KHK shall use Commercially Reasonable Efforts to Commercialize such Product in the Field in such country. The activities of KHK’s Affiliates and Sublicensees shall be attributed to KHK for the purposes of evaluating KHK’s fulfillment of the obligations set forth in this Section 6.4.
6.5 Promotional Materials. KHK shall be solely responsible for the design and supply of Promotional Materials for the Product in the Territory. KHK shall own all right, title and interest in and to any Promotional Materials created by KHK hereunder relating to the Product in the Field in the Territory, but excluding in any event the Syndax name and corporate logo and the Syndax Trademarks.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
29
6.6 Medical Inquiries for the Product. With regard to any Product sold by or on behalf of KHK (or any of its Affiliates or Sublicensees), KHK shall be responsible for receiving all medical questions or inquiries in each country in the Territory (including, if applicable, setting up a call center in connection therewith), in each case in accordance with Applicable Law and this Agreement. Syndax shall promptly forward any and all medical questions or inquiries which it receives with respect to any Product sold by or on behalf of KHK (or any of its Affiliates or Sublicensees) in the Territory to KHK in accordance with Applicable Law, and KHK shall immediately forward to Syndax any and all medical questions or inquiries that it receives with respect to a Product sold outside of the Territory, in each case in accordance with Applicable Law.
ARTICLE 7
MANUFACTURE AND SUPPLY
7.1 Supply Responsibilities. Subject to the terms and conditions of this Article 7, the Quality Agreements and the other provisions of this Agreement, Syndax shall supply KHK’s total requirements of Initial Product (for *** and both for use in Clinical Trials in the Territory and for commercial sale in the Territory) in accordance with the Specifications, the Clinical Supply Agreement, the Commercial Supply Agreement and the Quality Agreements, which shall include provisions to address the quality of the Product and related regulatory issues, forecasting, order, delivery, transfer price, product specifications and other customary provisions applicable to the clinical or commercial supply of pharmaceuticals. The Parties shall agree on contact procedures among Syndax, the relevant Syndax contract manufacturers and suppliers and KHK, its Affiliates and Sublicensees, including a process by which KHK can directly communicate with Syndax’s contract manufacturers and suppliers. Further, the Parties shall agree on *** taking into account the ***. For the avoidance of doubt, subject to Section 15.5, nothing herein shall require Syndax to supply to KHK Initial Product having Specifications that differ materially from the specifications being used by Syndax in other Territories, unless and to the extent the Parties so agree in one or both of the Supply Agreements. Unless otherwise specified in the Supply Agreements, all Product will be supplied as bulk tablets. As between the Parties, such supply by Syndax shall be at Syndax’s cost, subject to the payment of the transfer price described in Section 7.5 below. KHK shall conduct PTP packaging and final packaging in the Territory, and Syndax shall reimburse KHK for the cost of PTP packaging and final packaging as a credit against the Transfer Price, up to a maximum of *** per package (the “Reimbursement Cap”).
7.2 Supply Agreements. Within *** after the Effective Date, the Parties shall enter into a manufacturing and supply agreement (the “Clinical Supply Agreement”), which shall set forth the terms and conditions pursuant to which Syndax shall supply Initial Product to KHK for use in Clinical Trials in the Territory. At least *** in advance of the ***, the Parties shall enter into a commercial manufacturing and supply agreement (the “Commercial Supply Agreement” and with the Clinical Supply Agreement, the “Supply Agreements”), which shall set forth the terms and conditions pursuant to which Syndax shall supply Initial Product to KHK for commercial sale in the Territory. The Clinical Supply Agreement and the Commercial Supply Agreement shall be negotiated by the Parties in good faith, shall be consistent with the supply terms described in this Article 7.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
30
7.3 Quality Agreements. At the same time that the Parties enter into the Clinical Supply Agreement, the Parties shall enter into a quality agreement addressing the quality and safety of the Initial Product for use in Clinical Trials in the Territory and related regulatory issues relating thereto (the “Clinical Quality Agreement”), on terms to be negotiated by the Parties in good faith. At the same time that the Parties enter into the Commercial Supply Agreement, the Parties shall enter into a quality agreement addressing the quality and safety of the Initial Product for Commercialization in the Territory and related regulatory issues relating thereto (the “Commercial Quality Agreement”), on terms to be negotiated by the Parties in good faith. (the Clinical Quality Agreement and the Commercial Quality Agreement hereinafter collectively the “Quality Agreements”.)
7.4 Product Complaints. With regard to any Product sold by or on behalf of KHK (or any of its Affiliates or Sublicensees), KHK shall be responsible for receiving all product complaints, in each case in accordance with Applicable Law and this Agreement. KHK shall inform Syndax of such product complaints which KHK believes to have been likely caused by the Manufacture by Syndax or its designee, and Syndax shall be primarily responsible for finding the cause of such product complaints related to the Manufacture of the Products and preparing an explanation thereof (but only to the extent that Syndax or its designee Manufactured the Product in question), which procedure shall be set forth in the Quality Agreement.
7.5 Transfer Price. With respect to Initial Product or API supplied under the Clinical Supply Agreement, Syndax shall supply reasonable quantities of such Initial Product or API to KHK, free of charge to KHK, using inventory existing on the Effective Date or produced in manufacturing runs conducted after the Effective Date by or on behalf of Syndax in the ordinary course of business from time to time, subject to maximum quantities to be specified in the Clinical Supply Agreement based on KHK’s then-current development plans in the Territory (together with commercially reasonable margin of error). With respect to Initial Product supplied under the Commercial Supply Agreement, Syndax shall supply such Initial Product to KHK at the Transfer Price, as described in Section 8.5. Invoicing and payment terms for the Transfer Price shall be set forth in the Commercial Supply Agreement.
7.6 Manufacture by KHK. KHK shall not be permitted to Manufacture Product or Compound except as described in Section 7.6.1 below.
7.6.1 Manufacturing Option. Syndax grants to KHK the option to elect to receive from Syndax a non-exclusive license to Manufacture the Product or the Compound inside or outside the Territory for Commercialization in the Territory, which option may be exercised by KHK solely in the event that Syndax and its designee is unable or unwilling to supply such Product to KHK in a timely manner and in compliance with quality standards (a “Supply Failure”). The Supply Agreements shall include objective criteria for determining whether a Supply Failure has occurred. In the event that KHK exercises such option, KHK shall have the
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
31
right to Manufacture, or have Manufactured, such Product or Compound in whole or part in or outside the Territory (excluding ***, which are the subject of an exclusive manufacturing license to a Third Party), subject to KHK’s compliance with reasonable manufacturing and clinical safety standards and applicable regulatory requirements. In the event that KHK elects to have one or more of Syndax’s existing contract manufacturers Manufacture the Product or the Compound, Syndax shall reasonably cooperate to allow KHK to enter into a direct contract manufacturing agreement with such contract manufacturers. Any Product Manufactured by KHK pursuant to the foregoing rights shall be exclusively sold in the Territory. Any regulatory activities that are necessary for KHK to perform the foregoing Manufacture of the Product or the Compound shall be performed by the Parties in accordance with Section 4.3. The Commercial Supply Agreement shall include mutually agreed royalty payments to Syndax that, upon transfer of Manufacturing responsibility to KHK, will replace the Transfer Price payments in Section 8.5 so as to provide equitable compensation to Syndax for the licenses granted by Syndax to KHK.
7.6.2 Technology Transfer. In the event that KHK exercises the option set forth in Section 7.6.1, Syndax shall transfer to KHK any Syndax Know-How that is necessary in order to allow and enable KHK, its Affiliates or Sublicensees or their contract manufacturers to practice such rights, and shall use Commercially Reasonable Efforts to facilitate the practice of such Manufacturing rights by KHK, its Affiliate or its Sublicensee, at KHK’s cost, including performing such actions and making such filings, necessary and sufficient in order to enable KHK to practice the foregoing Manufacturing rights in accordance with Applicable Law (the “Technology Transfer”). If KHK, its Affiliates or Sublicensees or their contract manufacturers begin Manufacturing a Product to be supplied by Syndax to KHK, its Affiliates or Sublicensees or their contract manufacturers for the Territory, Syndax’s Manufacturing and supply obligations for such Product shall cease.
7.7 Supply After Expiration/Termination of this Agreement. In the event of the expiration of this Agreement or the termination of this Agreement by KHK in accordance with Section 13.3.1, so long as KHK or an Affiliate or Sublicensee continues sales of a Product then being supplied by Syndax to KHK or an Affiliate or Sublicensee in the Territory, Syndax shall continue to supply the Product to KHK or an Affiliate or Sublicensee in accordance with the provisions of this Agreement and the Commercial Supply Agreement. The Parties shall negotiate the supply price of the Product to be applicable after the expiration of this Agreement or termination of this Agreement, provided however that in no event the supply price shall exceed ***. Notwithstanding the foregoing, if (a) either Party gives notice to the other Party at least *** prior to the expected expiration of this Agreement (provided that Syndax has not already transferred to KHK the responsibility for Manufacturing the Product), and (b) Syndax undertakes the Technology Transfer and such Technology Transfer is completed within the *** notice period, then Syndax’s Manufacturing and supply obligations for any Products included in such Technology Transfer shall cease.
7.8 Formulation of Product. Both during the Term and thereafter, Syndax shall not change the formulation or the manufacturing process of any Product to be supplied for the Territory without providing KHK with at least *** prior notice to allow KHK to ***. In no
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
32
event shall Syndax implement any such change that would reasonably be expected to require KHK to amend or otherwise modify its Regulatory Materials in a manner that requires approval of a Regulatory Authority, unless it obtains the prior written consent of KHK. The foregoing obligations shall not apply if KHK or its Affiliate or Sublicensee begins Manufacturing such Product for the Territory.
7.9 New Dosage Form. Either Party may request that consideration be made of the manufacture and sale of a tablet other than ***. In such case, the Parties will investigate the costs of Development, reduction of manufacturing costs, and other practical commercial considerations. If the Parties agree to manufacture and sell such tablet, *** and *** shall receive *** of *** (in comparison with the ***) through ***. Alternatively, if the Parties agree to manufacture and sell such tablet and one Party agrees to ***, such Party may ***.
ARTICLE 8
PAYMENTS
8.1 Upfront Payments. In consideration of the licenses and rights granted hereunder, KHK shall pay $17,500,000 to Syndax within thirty (30) days after the Effective Date. Such fee shall be non-refundable and shall not be creditable against any other amount due to Syndax pursuant to this Agreement.
8.2 Equity. Provided that Syndax and KHK have obtained all necessary authorizations, approvals and signatures of their respective boards of directors and shareholders, on the Effective Date, KHK and Syndax will enter into a “Series B-1 Preferred Stock Purchase Agreement,” attached hereto as Exhibit D, (for which the terms, including those of agreements and other arrangements between the shareholders, are identical those of B-1 preferred shares) pursuant to which, subject to the terms and conditions thereof, KHK shall purchase 670,062 Series B-1 Preferred Shares of Syndax at a price equal to $11.193 (Eleven Point One Nine Three Dollars) per share for an aggregate purchase price of $7,500,003.97 (Seven Million Five Hundred Thousand and Three Dollars and Ninety-Seven Cents).
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
33
8.3 Development Milestone Payments. KHK shall pay to Syndax each of the following milestone payments upon the first achievement of each such milestone event, within *** following the occurrence of such event (it being understood that such events may not occur in the order set forth below). Each of the following milestones will be payable only once:
| Number | Event | Payment | ||||
| 1 | *** | $ | *** | |||
| 2 | *** | $ | *** | |||
| 3 | *** | $ | *** | |||
| 4 | *** | $ | *** | |||
| 5 | *** | $ | *** | |||
| 6 | *** | $ | *** | |||
|
|
| |||||
| Total Possible Milestone Payments | $ | 50 million | ||||
|
|
|
*** KHK shall promptly notify Syndax in writing of the occurrence of each milestone event under this Section 8.3. Each such payment shall be non-refundable and shall not be creditable against any other amount due to Syndax pursuant to this Agreement. One payment shall be made with respect to the achievement of each of the six (6) milestone events above regardless of the number of the Products, or indications or preparation forms within a Product for which such milestone event is achieved. *** If milestone 2 above is achieved prior to milestone 1 being achieved, then milestone 1 shall be deemed to have be achieved as of the achievement of milestone 2, and the payments for both milestones 1 and 2 shall be due. For milestones 5 and 6 above, the payment will not be triggered by ***.
8.4 Commercialization Milestone Payments. KHK shall pay to Syndax each of the milestone payments set forth below within *** after Net Sales of all Licensed Products in the Territory first exceed the indicated value in a calendar year. KHK shall promptly notify Syndax of the occurrence of the first achievement of each such sales level. Each of the following milestones will be payable only once:
| Number |
Calendar Year Net Sales of Products in Territory | Payment | ||||
| 1 | $*** | $ | *** | |||
| 2 | $*** | $ | *** | |||
| 3 | $*** | $ |
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
34
8.5 Transfer Price. For Product supplied to KHK under the Commercial Supply Agreement, KHK shall pay to Syndax a transfer price (the “Transfer Price”), calculated as follows:
8.5.1 Japan.
(i) If the initial XXX Xxxxx for the first Product that receives Regulatory Approval in Japan is ***, then the Transfer Price for Product in Japan shall be equal to *** for the ***, and *** for the ***; provided, however, that immediately following the earlier of (A) the *** and (B) the ***, the Transfer Price for Product in Japan shall be ***.
(ii) If the initial XXX Xxxxx for the first Product that receives Regulatory Approval in Japan is ***, then the Transfer Price for Product in Japan shall be equal to ***.
(iii) Notwithstanding the foregoing, upon the listing of an XXX Xxxxx for the first Generic Product in Japan (“Generic Entry”) the Transfer Price for such Product shall be ***.
(iv) If at any time prior to Generic Entry, the XXX Xxxxx in effect ***, the Parties shall *** a *** to *** and shall amend the Commercial Supply Agreement accordingly.
(v) In *** of each year, an estimated Transfer Price shall be proposed by KHK’s (and agreed by Syndax) based upon KHK’s estimate of the likely Net Selling Price for the following calendar year. The estimated Transfer Price shall be used for the purchase and sale of Product supplied to KHK during the entire calendar year. As soon as is practicable after the end of each calendar quarter, the actual Transfer Price shall be determined based upon the actual Net Selling Price calculations for such calendar quarter. The Parties shall promptly settle the overpayment or underpayment of the estimated Transfer Price versus the actual Transfer Price on a quarterly basis, it being understood that any overpayment to Syndax will be settled through a credit against future Transfer Price payments. The details of the calculation method and deductions to be made shall be set forth in the Commercial Supply Agreement.
(vi) Notwithstanding the foregoing, *** in the *** is ***, the Parties shall *** should be *** the *** of the above (i) or the *** of the above (iv) for the ***.
8.5.2 Korea. The Parties shall separately discuss in good faith and determine within *** of the Effective Date the Transfer Price applicable to Korea.
8.6 Royalties. If Net Sales for all Products in the Territory exceeds $*** in any calendar year, KHK shall pay to Syndax a royalty equal to *** of the portion of the Net Sales exceeding $*** for the remainder of such calendar year. Royalty payment obligations resulting from Product sales during a particular calendar quarter shall be paid within *** after the end of such calendar quarter. The term of the royalties payable under this Agreement (the “Royalty Term”) shall commence on the Effective Date and, unless earlier terminated in accordance with the terms of this Agreement, shall continue on a country-by-country basis, until the later of (i) the date all Valid Claims of the last effective Patent among the Syndax Patents expires or is abandoned, withheld, or is otherwise invalidated in such country, and (ii) fifteen (15) years from the date of the First Commercial Sale of a Product in the Territory. Upon the expiration of the
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
35
Royalty Term, with respect to a country in the Territory, the license granted to KHK hereunder shall be an exclusive, fully paid-up, royalty-free, irrevocable and perpetual license. The applicable exchange rate between the Dollar and any other currency shall be the New York Closing Snapshot rate published in the Wall Street Journal (as available at its website) on the last business day of such calendar quarter, or if no such rate is available for the relevant currency, at a rate mutually agreed by the Parties.
8.7 Manner of Payment. Except for the Transfer Price and as otherwise agreed between the Parties, all payments under this Agreement shall be in Dollars in immediately available funds and shall be made by wire transfer to such bank account as may be designated from time to time by either Party.
8.8 Late Payment. All payments by one Party to the other Party under this Agreement shall earn interest from the date due until paid at the Interest Rate.
8.9 Underpayment; Overpayment. If an Inspection reveals an underpayment, then KHK shall promptly make up such underpayment with interest at the Interest Rate from the date payment was owed. If an Inspection (as defined below in Section 9.3) revealed an overpayment, then Syndax shall promptly refund such overpayment with interest at the Interest Rate from the date such overpayment was made.
8.10 Withholding Taxes. Each Party shall pay any and all taxes in their respective countries of residence levied in respect of any net profit achieved by any such Party. If Applicable Law requires that either Party withhold any taxes from the amounts paid to the other Party hereunder, the paying Party shall deduct such taxes from the amounts paid to the other Party hereunder, make timely payment of such taxes to the proper taxing authority for the account of the other Party and send proof of such payment to the other Party within *** following such payment. Further, the paying Party shall provide the other Party with copies of any tax receipts for any such taxes paid, together with copies of all pertinent communications from or with governmental authorities with respect thereto. The Parties shall reasonably cooperate in completing and filing documents required under the provisions of any Applicable Law in connection with the making of any required Tax payment or withholding payment, or in connection with any claim to an exemption from, reduction of, or a refund of or credit for any such payment to the extent available under Applicable Law. Notwithstanding the deadlines for making payments set forth herein, the Parties recognize that some delay may be inevitable if Syndax requests that steps be taken to obtain exemption from any withholding tax that may be applicable.
8.11 Limitation on Duration. Any claim by one Party to the other Party for any payment obligation arising under this Agreement must be made within *** of the *** of such payment obligation. No Party shall be liable to the other Party for any claim for the payment of a payment obligation arising under this Agreement that is ***.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
36
ARTICLE 9
STATEMENTS, RECORDS AND INSPECTION
9.1 Statements. All payments made to Syndax under Article 8 shall be accompanied by a written statement and supporting documentation setting forth in reasonable detail the calculation thereof, including a calculation of the relevant Net Sales, in reasonable detail. Without limiting the foregoing, no later than *** after each calendar quarter (commencing with the first calendar quarter in which there are any Net Sales), KHK shall provide to Syndax a written statement listing Net Sales by each country of the Territory in local currency and in US Dollars using the currency conversion described in the definition of “Net Sales” in Article 1, regardless of whether royalties are due under Section 8.6.
9.2 Record Keeping. KHK shall keep and maintain, and shall cause its Affiliates and Sublicensees to keep and maintain, complete and accurate books of account and adequate records of all sales and other dispositions of the Product in sufficient detail to permit Syndax to confirm the accuracy of reported milestones and other payments due hereunder, including general accounting ledgers, invoice/sale registers, original invoices and shipping documents, tax returns, inventory and manufacturing records, license, sublicense and distributor agreements and price lists, and shall retain such books and records for a period of *** from the last day of the calendar quarter in which such sales or dispositions were made, or longer, if required.
9.3 Inspection. Subject to the terms set forth below, Syndax may from time to time, not exceeding once every calendar year, audit (each such audit, an “Inspection”) the books and records of KHK, its Affiliates and/or Sublicensees, as the case may be, to the extent necessary in order to verify the accuracy of any payment made under this Agreement or any Net Sales reported hereunder, or, in the event of KHK’s failure to make payment or other amounts hereunder, to obtain information as to the payment payable for any such period by KHK to Syndax. Any such Inspection shall be conducted only by an internationally-recognized firm of certified public accountants selected by Syndax (the “Auditor”), and reasonably acceptable to KHK or such Affiliate or Sublicensee, and shall be on reasonable notice and during normal business hours. The Auditor shall disclose to Syndax and KHK only whether the information as to the payments is correct or incorrect and the specific details concerning any discrepancies, or in the case of KHK’s failure to make payment or other amounts hereunder, such information as would have been included in a report submitted pursuant to Section 9.1. No other information shall be provided to Syndax. Such Inspection shall be at Syndax’ cost, unless an underpayment discrepancy is determined and such discrepancy is *** or greater, in which case, KHK shall reimburse Syndax for costs incurred by Syndax, including the examination costs charged by the Auditor. If the Auditor concludes that an overpayment was made, such overpayment shall be fully creditable against amounts payable in subsequent payment periods. In any event, KHK shall pay past due amounts identified in the Inspection within *** after the date KHK receives the report of the Auditor.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
37
ARTICLE 10
INTELLECTUAL PROPERTY
10.1 Ownership of Inventions. Each Party shall own any Inventions made solely by its employees, agents or independent contractors in conducting its activities hereunder (each a “Sole Invention”). Each Party shall be responsible, at its cost, for the Prosecution of any Patents claiming its Sole Inventions. The Parties acknowledge that during the Term one or more employees, agents or contractors of one Party jointly with one or more employees, agents or contractors of the other Party may have conceived and/or reduced to practice or may conceive and/or reduce to practice one or more Inventions that are necessary or useful for the Development or Commercialization of any Compound or Product (such Inventions being “Joint Inventions”). For purposes of this Agreement, the determination of the identities of the inventors of any Sole Inventions or Joint Inventions shall be made in accordance with the laws of inventorship under United States patent law. The Parties agree that Joint Inventions shall be jointly owned by the Parties. If any Joint Inventions shall arise, the Parties shall negotiate in good faith to determine their respective rights and obligations with respect thereto.
10.2 Prosecution of Patents.
10.2.1 Responsibility for Prosecution of Patents. Syndax shall be responsible, at its sole discretion and cost, for the Prosecution of the Syndax Patents in the Territory (including, for the avoidance of doubt, any Bayer Patents in the Territory) and shall consult and cooperate with KHK in such Prosecution in accordance with this Article 10.2. Upon *** prior written notice to KHK, Syndax may elect to abandon or discontinue the Prosecution of any Syndax Patent in the Territory and/or not to file, pay the maintenance fees, or conduct any further activities with respect to the Syndax Patents. In the event Syndax declines to file or, having filed, fails to further prosecute or maintain any Syndax Patents in the Territory or to conduct any interferences, re-examinations, re-issues, or oppositions with respect thereto, Syndax shall promptly notify KHK (such notification to be given as early as possible, which is no event will be less than *** prior to the date on which said Syndax Patents in the Territory will become abandoned, such payment is due or such proceeding is schedules to occur). Thereafter, KHK shall, at its sole discretion and cost, have the right to Prosecute such Syndax Patents in such countries in the Territory as it deem appropriate, and conduct any interferences, reexaminations, re-issues, or oppositions with respect thereto. Syndax shall assign, and shall cause Bayer or its Affiliates to assign, without additional charge to KHK, all right, title and interest in and to such Syndax Patents to KHK, and shall cooperate, and shall cause Bayer to cooperate, at KHK’s cost, in any manner reasonably requested by KHK in connection with such actions by KHK; except that Bayer shall not be required to communicate directly with any inventors of Syndax Patents owned by Bayer who are not employees of Bayer or its Affiliates. For the avoidance of doubt, any Syndax Patent (including, for the avoidance of doubt, any Bayer Patents in the Territory) assigned to KHK as set forth in the preceding sentence in this Section 10.2 shall cease being a Syndax Patent for all purposes under this Agreement.
10.2.2 Cooperation. Syndax shall at all times during the Term keep KHK advised of the status of Syndax Patents and shall, upon the request of KHK, promptly provide KHK with copies of the requested documents relating to the Prosecution of the Syndax Patents in the Territory conducted on or before the Effective Date, and further shall promptly provide KHK
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
38
with copies of all documents relating to the Prosecution of the Syndax Patents in the Territory to be conducted during the Term, including promptly providing copies of any documents that Syndax or Bayer receives from such patent office, including any notice of interference, reissues, re-examinations, oppositions or requests for patent term extension. Syndax shall provide KHK sufficient period and opportunity to review and comment on any documents relating the Syndax Patents or the Additional Syndax Patents which will be filed in any patent office in the Territory in advance of the filing dates. During the Term, the Parties will use Commercially Reasonable Efforts to provide any information necessary to assist with such Prosecution.
10.2.3 Bayer Patents. For clarity, nothing herein is intended to modify Bayer’s rights and obligations with respect to the Prosecution of Bayer Patents, as set forth in the Bayer License Agreement.
10.3 Syndax Patent Enforcement and Defense.
10.3.1 Notification. If either Party becomes aware of any circumstance, claim or proceeding that relate to the Syndax Patents that may adversely affect the validity, title or enforceability of the Syndax Patents in the Territory, or any actual, alleged or threatened infringement of the Syndax Patents in the Territory, such Party shall promptly notify the other Party thereof in writing. In addition, if KHK becomes aware of any other circumstance, claim or proceeding relating to the Syndax Patents or any actual, alleged or threatened infringement thereof outside of the Territory, it shall promptly notify Syndax thereof, in writing.
10.3.2 Bayer Intellectual Property. The Parties acknowledge that, with respect to any Bayer Intellectual Property: (a) Bayer, by itself, or through an Affiliate, has the right (but not the obligation) to initiate and conduct, at its sole cost, legal proceedings to enforce the Bayer Intellectual Property in the Territory against any infringement or misappropriation by Third Parties or defend any declaratory judgment action involving the Bayer Patents in the Territory (an “Bayer IP Enforcement Action”); and (b) if, within *** following receipt of a written notice of an infringement or misappropriation of any Bayer Intellectual Property or written notice of a declaratory judgment action alleging invalidity or unenforceability of Bayer Intellectual Property, Bayer fails to initiate a Bayer IP Enforcement Action, then Syndax will have the right (but not the obligation) to initiate and conduct the Bayer IP Enforcement Action in its own name and at its sole cost. Once Syndax has the right to initiate and conduct a Bayer IP Enforcement Action in accordance with the (b) above, at KHK’s option, KHK shall be permitted, but not obligated, to initiate and conduct the Bayer IP Enforcement Action in the Territory. After the execution of this Agreement, Syndax shall take all reasonable efforts to ***. Syndax agrees to be joined as a party plaintiff, at KHK’s cost, in any Bayer IP Enforcement Action initiated and conducted by KHK, if requested by KHK, and Syndax agrees to exercise its right under the Bayer License Agreement to have Bayer joined as a party plaintiff, at KHK’s cost, in any Bayer IP Enforcement Action initiated and conducted by KHK, if requested by KHK.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
39
10.3.3 Other Syndax Patents. KHK shall have the right but not the obligation to institute, at its sole cost, an action (each an “Enforcement Action”) against a Third Party for infringement of any Syndax Patent in the Territory (other than Bayer Patents in the Territory) on account of such Third Party’s manufacture, use, offer for sale, or sale of a Product in the Field (a “Product Infringement”). If KHK institutes any such Enforcement Action against Product Infringement in the Territory, then Syndax may, at its option, elect to participate in such action, in which case Syndax shall bear its costs (including the fees and costs of any separate counsel) arising from such election to participate. In the event KHK fails to institute an Enforcement Action against any Product Infringement (or settle or otherwise secure the abatement of such Product Infringement) by the Action Date, Syndax may, at its option and own cost, institute an Enforcement Action against such Product Infringement.
10.3.4 Cooperation. Each Party shall reasonably cooperate and execute all necessary documents and take appropriate actions, at the cost of the prosecuting Party, to allow the other Party to institute and prosecute any Bayer IP Enforcement Actions or Enforcement Actions as provided in this Section 10.3. If either Party, although willing, is unable to institute or prosecute any Bayer IP Enforcement Action or Enforcement Action solely in its own name in any jurisdiction in the Territory in accordance with this Section 10.3, the other Party shall, if lawful to do so, join such action and shall execute all documents necessary to institute and prosecute such Bayer IP Enforcement Action or Enforcement Action. In connection with any such Bayer IP Enforcement Action or Enforcement Action, the Parties shall cooperate fully and shall provide each other with any information or assistance that either reasonably requests. Each Party shall keep the other informed of developments in any such Bayer IP Enforcement Action or Enforcement Action. Nothing in this Section 10.3.4 shall require Syndax to perform any actions in violation of the Bayer License Agreement.
10.3.5 Settlement. Neither Party shall enter into any settlement or compromise of any Bayer IP Enforcement Action or Enforcement Action that admits or concedes that any aspect of the Syndax Patents or Syndax Know-How is invalid or unenforceable, without the prior written consent of the other Party.
10.4 Defense Actions. If any Party receives notice by counterclaim, declaratory judgment action or otherwise, alleging the invalidity or unenforceability of any Syndax Patent, it shall bring such fact to the attention of the other Party, including all relevant information related to such claim. Where such allegation is made in an opposition, reexamination, interference or other patent office proceeding, the provisions of Section 10.1 shall apply. Where such allegation is made in a counterclaim to a suit or other action brought under Section 10.3, the applicable provisions of Section 10.3 shall apply. Where such allegation is made in a declaratory judgment or other court action, the Party who prosecuted such Syndax Patent shall have the first right to defend such action at its own cost, provided that if a Party pursuant to Section 10.3 elects to bring an infringement counterclaim, the provisions of Section 10.3 shall thereafter apply. If the Party with the first right to defend a Syndax Patent elects not to defend such action, it shall so notify the other Party in writing, and the other Party shall have the right to defend such action, at the other Party’s cost. For any such action involving a Syndax Patent, the non-defending Party
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
40
shall provide to the defending Party all reasonable assistance in such defense, at the defending Party’s request and cost; and the defending Party shall keep the other Party regularly informed of the status and progress of such efforts, and shall reasonably consider the other Party’s comments on any such efforts.
10.5 Infringement of Third Party Rights. Each Party shall promptly notify the other Party in writing of any allegation, claim or suit that the manufacture, use or sale of any Product infringes or misappropriates a Third Party’s Patent or other Intellectual Property. Subject to Article 12, each Party shall have the sole right to control any defense of any such claim involving alleged infringement of such Third Party rights by such Party, at its own cost and by counsel of its own choice,. In the event that settlement of such an allegation, claim or suit by Syndax (whether as a result of a suit against Syndax or as a result of Syndax’s indemnification of KHK under Section 12.1(v)) results in Syndax receiving a license to Third Party IP, Section 2.9 shall apply.
10.6 Settlement. Neither Party shall settle any Bayer IP Enforcement Action, Enforcement Action, or any action under Section 10.4 or 10.5 or consent to the entry of any judgment or settlement or otherwise compromise any such action or suit in any way that admits or concedes that any of the Syndax Patents is invalid or unenforceable or that may otherwise adversely affect the other Party’s rights or interests without the other Party’s prior written consent, which shall not be unreasonably withheld or delayed.
10.7 Awards. Any award paid to either Party by a Third Party found to have infringed or misappropriated Bayer Intellectual Property in the Territory as a result of a Bayer IP Enforcement Action (whether by way of settlement or otherwise) shall be applied first to reimburse the Party that initiated and conducted the Bayer IP Enforcement Action (or Bayer, if Bayer initiated and conducted such action) for all costs and, if after such reimbursement, any funds shall remain from such an award, said funds shall be allocated as set forth in the Bayer License Agreement, except that KHK shall be entitled to (a) *** of any and all *** in connection with a Bayer IP Enforcement Action in the Territory and (b) *** of any and all *** in connection with a Bayer IP Enforcement Action in the Territory, which *** shall be *** in the ***. Any award paid by a Third Party to either Party as a result of any Enforcement Action or Defense Action contemplated by Section 10.3 or Section 10.5, as applicable, whether by way of settlement as contemplated by Section 10.5 or otherwise, shall be allocated first pro rata to the reimbursement of any costs incurred by the Parties in such Enforcement Action or Defense Action, and any remaining amounts (the “Net Recovery”) shall be allocated to the enforcing Party. Any Net Recovery received by KHK shall be *** in the ***.
10.8 Trademarks.
10.8.1 General. Within *** of the Effective Date, Syndax shall provide KHK a complete list of the Syndax Trademarks, including the application or registration number, class, product/services and status of each Syndax Trademark. Syndax shall promptly update such list
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
41
upon the registration of any additional Syndax Trademark. Syndax shall, apply for the registration of *** in ***, as candidates for the latin name and local transliterations of the global product name for the Product no later than the ***. Syndax shall discuss in good faith with KHK prior to the applications for the registration of trademarks for such local transliterations in each country in the Territory. The Parties shall discuss the trademarks to be used for the Product in the Territory following Syndax’s final decision of the name of the Product to be used worldwide. KHK shall, at its discretion on a country-by-county basis, have the right to either use the Syndax Trademarks or trademarks selected and owned by KHK (the “KHK Trademarks”) with respect to the Development, Commercialization (including packaging of the Product) or Manufacture (if KHK exercises its option to Manufacture Products in accordance with Section 7.6.1) of the Product in the Territory.
10.8.2 Syndax Trademarks. In the event KHK elects to use the Syndax Trademarks for the Product in one or more countries in the Territory pursuant to Section 10.8.1, the following shall apply:
(i) All rights arising from the use of the Syndax Trademarks in the Territory during the Term shall inure to Syndax’s benefit.
(ii) All Products with which the Syndax Trademarks are used shall conform to all requirements of the applicable Regulatory Authority in the Territory.
(iii) Syndax shall use Commercially Reasonable Efforts to have the Syndax Trademarks registered in the applicable country(-ies) of the Territory and shall keep KHK regularly informed of the completion of such registration process and provide KHK with an updated list of registration numbers for such Syndax Trademarks. Syndax shall use Commercially Reasonable Efforts to maintain the registrations of the Syndax Trademarks in the applicable country(-ies) of the Territory. All such efforts by Syndax shall be at *** cost.
10.8.3 Infringement of Syndax Trademarks. KHK shall, as soon as practicable after receiving notice of any potential infringement of a Syndax Trademark in the Territory, inform Syndax of any such potential infringement. Syndax shall have the first right and discretion to bring infringement or unfair competition proceedings involving the Syndax Trademark in the Territory and Syndax shall bear all costs in connection with any such proceedings. KHK shall cooperate with Syndax in any such proceedings, including by giving testimony and producing documents and materials supporting the Syndax Trademark, and shall endeavour to cause the employees of KHK, as appropriate, to cooperate with Syndax, all at Syndax’s cost. Any recoveries obtained as a result of any infringement litigation undertaken by Syndax alone or in settlement of such infringement shall be ***. KHK may elect to participate with Syndax in any infringement or unfair competition action undertaken by Syndax hereunder in the Territory, at KHK’s cost, and any recovery obtained shall be ***. Should Syndax fail to institute infringement proceedings in the a country in the Territory where KHK has elected to use the Syndax Trademark, KHK shall have the right but shall not be obligated, to bring suit for such infringement under its name and at its own cost. Syndax
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
42
shall cooperate with KHK in any such proceedings, including giving testimony and producing document and material supporting the Syndax Trademark and shall endeavour to cause the employees of Syndax, as appropriate, to cooperate with KHK, all at KHK’s cost. Any recoveries obtained in suit for trademark infringement litigation or in settlement of such infringement undertaken without Syndax’s involvement shall be ***.
10.8.4 KHK Trademarks. To the extent KHK elects to use a KHK Trademark in addition or as an alternative to the Syndax Trademarks in connection with the Commercialization of the Products in the Territory, KHK shall be responsible for the selection, adoption, registration, maintenance and defense of such KHK Trademarks, as well as all costs and costs associated therewith. KHK shall own all KHK Trademarks and all rights arising from the use of the KHK Trademarks in the Territory shall inure to KHK’s benefit.
ARTICLE 11
REPRESENTATIONS AND WARRANTIES; DISCLAIMER
11.1 Mutual Representation and Warranties. Each Party represents and warrants to the other that (a) it has all requisite corporate power and authority to enter into this Agreement and to perform its obligations under this Agreement, (b) the execution, delivery and performance of this Agreement have been duly authorized and shall not result in any violation of, be in conflict with or constitute a default under any contract, obligation or commitment to which it is a Party or by which it is bound or under any Applicable Law, and (c) this Agreement is the legally valid and binding obligation of such Party, enforceable against such Party in accordance with its terms, subject to the effects of bankruptcy, insolvency or other laws of general application affecting the enforcement of creditor rights and judicial principles affecting the availability of specific performance and general principles of equity, whether enforceability is considered a proceeding at law or equity.
11.2 KHK Representations and Warranties. KHK warrants, represents and covenants that all of its activities related to the Products pursuant to this Agreement shall comply with all Applicable Law in the Territory.
11.3 Syndax Representation and Warranties. Except as set forth in a written disclosure schedule delivered by Syndax to KHK concurrently with the execution and delivery of this Agreement, if any, Syndax hereby represents and warrants to KHK that, as of the Effective Date:
11.3.1 Syndax is the owner or licensee of and Controls the Syndax Patents and the Syndax Know-How.
11.3.2 Syndax has the authority to grant the licenses and rights as set forth in this Agreement.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
43
11.3.3 The Syndax Patents owned by Syndax are, and to the Knowledge of Syndax, the Syndax Patents licensed to Syndax are, free and clear of all liens, security interests or other encumbrances.
11.3.4 To the Knowledge of Syndax or any of its Affiliates, no Third Party is infringing any Syndax Patents in the Territory or has misappropriated any Syndax Know-How in the Territory.
11.3.5 Exhibit A contains a complete and correct list of the Syndax Patents as of the Effective Date.
11.3.6 There are no pending reissue, reexamination, interference, opposition or similar proceedings, in each case with respect to the Syndax Patents in the Territory that are owned by Syndax, and to the Knowledge of Syndax, there are no pending reissue, reexamination, interference, opposition or similar proceedings, in each case with respect to the Syndax Patents in the Territory that are licensed to Syndax. Syndax has not received written notice as of the Effective Date of any threatened claims or litigation or any reissue, reexamination, interference, opposition or similar proceedings seeking to invalidate or otherwise challenge the Syndax Patents in the Territory.
11.3.7 To the Knowledge of Syndax, the Commercialization, Development, Manufacture, use, sale, offer for sale, or importation by Syndax or KHK (or their respective Affiliates or permitted sublicensee), as applicable, of the Product in the Territory for use in the Field does not infringe any issued Patent of any Third Party in the Territory.
11.3.8 All Product Manufactured and supplied hereunder by, or under authority of, Syndax shall be Manufactured and supplied such that the Product furnished by Syndax to KHK under this Agreement:
(i) will be manufactured, handled, stored and shipped by Syndax in compliance with all Applicable Law, including GMPs; and
(ii) will, at the time delivered by Syndax, not contain any material that would cause the Product to be adulterated or misbranded within the meaning of Applicable Law; and
11.3.9 Syndax has complied with all Applicable Law in all material respects, including any disclosure requirements, in connection with the filing, prosecution and maintenance of the Syndax Patents owned by Syndax in the Territory.
11.3.10 Syndax has previously delivered or made available to KHK a true copy of the Bayer License Agreement, redacted only to obscure financial and other terms that have not previously been publicly disclosed, and the Bayer License Agreement has not been modified, supplemented or amended relative to the version delivered or made available to KHK. Syndax has performed all necessary obligations under the Bayer Agreement in order to enter into this Agreement.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
44
11.3.11 The Bayer License Agreement is in full force and effect. Syndax is not in breach of the Bayer License Agreement, and, to the Knowledge of Syndax, Bayer is not in breach of the Bayer License Agreement.
11.3.12 Other than the Bayer License Agreement, there are no Existing Third Party Licenses.
11.3.13 Syndax has disclosed or made available to KHK all material scientific and technical information which is known to, and in the possession of, Syndax that Syndax reasonably believes relates to the safety and efficacy of the Product for use in the Field in the Territory.
11.3.14 Syndax has disclosed or otherwise made available to KHK all material written correspondence between Syndax and any Governmental Authorities regarding the Product for use in the Field in the Territory.
11.3.15 Neither Syndax nor any of its Affiliates has any Knowledge of any safety, efficacy, or regulatory issues that would reasonably be expected to preclude Syndax or KHK from carrying out Development, Manufacturing or Commercialization of the Product in the Field in the Territory in compliance with the Applicable Law and as otherwise expressly contemplated to be done hereunder.
11.3.16 To the Knowledge of Syndax, Syndax and its Affiliates have conducted all Development and Manufacturing of Compounds and Products in accordance and compliance with all Applicable Law, including, to the extent applicable, GLPs, GCPs and GMPs.
11.3.17 In the course of the Development of the Product, Syndax has not used any employee that is debarred by the FDA under the Generic Drug Enforcement Act of 1992 (or by any analogous agency or under any analogous Applicable Law in the Territory).
11.4 Knowledge Standard. As used in this Article 11, “Knowledge” and its derivatives, means that the relevant Party has actual knowledge of such fact or matter.
11.5 DISCLAIMER. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATIONS NOR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING ANY EXPRESS OR IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE WITH RESPECT TO THE LICENSES GRANTED HEREUNDER, THE COMPOUND, ANY PRODUCTS THAT MAY BE DEVELOPED OR COMMERCIALIZED HEREUNDER, SYNDAX PATENTS, SYNDAX KNOW-HOW, WITH
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
45
RESPECT TO ANY PRODUCTS OR SERVICES PROVIDED BY SYNDAX OR WITH RESPECT TO THE ABILITY OF KHK TO CAUSE THE DEVELOPMENT OR COMMERCIALIZATION ACTIVITIES HEREUNDER TO BE EFFECTIVE OR PROFITABLE. IN ADDITION, NOTHING IN THIS AGREEMENT IS TO BE CONSTRUED AS A REPRESENTATION OR WARRANTY THAT ANY PRODUCT SHALL BE OR IS CAPABLE OF BEING SUCCESSFULLY DEVELOPED OR COMMERCIALIZED OR GRANTED REGULATORY APPROVAL BY ANY REGULATORY AUTHORITY.
ARTICLE 12
INDEMNITY AND LIMITED LIABILITY
12.1 Syndax Indemnity. Syndax shall indemnify, hold harmless and defend the KHK Parties from and against any and all losses, damages, liabilities, judgments, fines, and amounts paid in settlement, in each case paid or payable to a Third Party claimant, as well as any other out-of-pocket costs and costs of defense (including reasonable attorneys’ fees and costs) (the “Losses”), on an as-incurred basis, resulting directly or indirectly from any claim, demand, suit, action or proceeding brought or initiated by a Third Party (the “Claim”) against them, to the extent that such Claim arises out of (i) a defective Product supplied by Syndax under the Commercial Supply Agreement; (ii) negligence or a willful misconduct of any Syndax Party; (iii) a breach of this Agreement, or any other agreement executed pursuant to this Agreement (including the Clinical Supply Agreement, the Commercial Supply Agreement, the PV Agreement or the Quality Agreements), by any Syndax Party, (including any Product Manufacturing breach by any Syndax Party or any failure by any Syndax Party to supply Product that complies with the representations and warranties herein applicable thereto); (iv) the failure by any Syndax Party to comply with Applicable Law; or (v) any allegation that the Development, Commercialization or Manufacture of Product in the Territory infringes any Third Party IP where such Third Party IP would necessarily be infringed by the Development, Commercialization or Manufacture of the Initial Product (as such Initial Product exists as of the Effective Date) in the Territory; provided, however, that the indemnities contained in this Section 12.1 shall not apply to the extent any such Loss or Claim arises out of (i) the negligence or willful misconduct of any KHK Party, (ii) the breach or alleged breach of this Agreement by any KHK Party, or (iii) failure by any KHK Party to comply with Applicable Law. In the case of a Claim falling with the scope of Section 12.1(v), Syndax reserves the right to resolve such Claim by obtaining a license to the applicable Third Party IP that is sublicenseable to KHK hereunder, subject to any consents of KHK required by Section 12.3. Syndax shall have no further obligations under Section 12.1(v) if Syndax obtains such a license at no cost to KHK, and KHK refuses to include such license as a Third Party License pursuant to Section 2.9.
12.2 KHK Indemnity. KHK shall indemnify, hold harmless and defend the Syndax Parties from and against any and all Losses, on an as-incurred basis, resulting directly or indirectly from any Claim against them to the extent that such Claim arises out of (i) the negligence or willful misconduct of any KHK Party in its Development, Commercialization or Manufacturing activities (including such negligence or willful misconduct of any KHK Party in those activities in connection with the Initial Product); or (ii) any breach of this Agreement or
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
46
any other agreement executed pursuant to this Agreement by any KHK Party, (iii) the failure by any KHK Party to comply with Applicable Law; provided, however, that the indemnities contained in this Section 12.2 shall not apply to the extent any such Loss or Claim arises out of (i) the negligence or willful misconduct of any Syndax Party; or (ii) the breach of this Agreement or any other agreement executed pursuant to this Agreement (including the Clinical Supply Agreement, the Commercial Supply Agreement, the PV Agreement or the Quality Agreements) by any Syndax Party or any failure by any Syndax Party to supply Product that complies with the representations and warranties herein applicable thereto; or (iii) the failure by any Syndax Party to comply with Applicable Law.
12.3 Indemnification Procedures. The indemnified Party shall notify the indemnifying Party in writing promptly upon becoming aware of any Claim to which such indemnification may apply. The indemnifying Party shall be relieved of its obligation of indemnification to the extent, and only to the extent, the indemnifying Party is prejudiced by any failure of the indemnified Party to provide the indemnifying Party with the foregoing notice of any such Claim within a reasonable period of time. The indemnifying Party shall have the right to assume and control the defense of the Claim at its own cost. If the right to assume and have sole control of the defense is exercised by the indemnifying Party, the indemnified Party shall have the right to participate in, but not control, such defense at its own cost and the indemnifying Party’s indemnity obligations shall be deemed not to include attorneys’ fees and litigation costs incurred by the indemnified Party after the assumption of the defense by the indemnified Party. If the indemnifying Party does not assume the defense of the Claim, the indemnified Party may defend the Claim at the indemnifying Party’s cost, but shall have no obligation to do so. The indemnified Party shall not settle or compromise the Claim without the prior written consent of the indemnifying Party, and the indemnifying Party shall not settle or compromise the Claim in any manner which would have an adverse effect on the indemnified Party without the consent of the indemnified Party, which consent, in each case, shall not be unreasonably withheld or delayed. The indemnified Party shall reasonably cooperate with the indemnifying Party and shall make available to the indemnifying Party all pertinent information under the control of the indemnified Party.
12.4 Limitation of Liability. NOTWITHSTANDING ANYTHING IN THIS AGREEMENT OR OTHERWISE, EXCEPT FOR EITHER PARTY’S INDEMNIFICATION OBLIGATIONS SET FORTH IN THIS ARTICLE 12 OR A PARTY’S BREACH OF ITS OBLIGATIONS UNDER ARTICLE 14, NEITHER PARTY SHALL BE LIABLE TO THE OTHER OR A THIRD PARTY UNDER ANY CONTRACT, NEGLIGENCE, STRICT LIABILITY, OR OTHER LEGAL OR EQUITABLE THEORY FOR ANY SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, EXEMPLARY, OR CONSEQUENTIAL DAMAGES.
12.5 Insurance. Each Party shall maintain, and shall require its Affiliates and licensees hereunder to maintain, a commercial general liability and product liability insurance program on terms customary in the pharmaceutical industry covering all activities and obligations of it, and, as the case may be, its Affiliates, hereunder, or other programs with comparable coverage, up to and beyond the expiration or termination of this Agreement during (i) the period that any
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
47
Product is being commercially distributed or sold by a Party, its Affiliates or licensees, and (ii) a commercially reasonable period thereafter. In lieu of the insurance coverage described above, each Party shall have the right to undertake a program of self-insurance to cover its indemnity obligations hereunder, with financial protection comparable to that arranged by it for its own protection with regard to other products in its product line. Each Party shall provide the other with proof of such insurance program at the other Party’s written request.
ARTICLE 13
TERM AND TERMINATION
13.1 Term. The term of this Agreement (the “Term”) shall commence on the Effective Date and, unless earlier terminated in accordance with the terms of this Agreement, shall continue on a country-by-country and Product-by-Product basis, until the expiration of the Royalty Term with respect to such Product and such country.
13.2 Termination for Convenience. KHK shall have the right to terminate this Agreement, in its entirety, upon *** written notice thereof to Syndax.
13.3 Termination for Breach or Insolvency.
13.3.1 Either Party shall have the right to terminate this Agreement upon written notice to the other Party if such other Party, after receiving written notice from the terminating Party identifying a material breach by such other Party of its obligations under this Agreement, fails to cure such material breach within *** from the date of such notice. In the event of a dispute as to whether an uncured material breach exists, this Agreement shall remain in full force and effect and no Party shall be released from its obligations hereunder unless and until a final arbitral or court determination is made with respect to the matter under the dispute resolution procedures set forth in Article 16 (or under the arbitration process described Section 13.7.3, to the extent applicable).
13.3.2 Either Party shall have the right to terminate this Agreement in its entirety: (i) upon the filing by the other Party in any court or agency pursuant to any statute or regulation of any state or country a petition in bankruptcy or insolvency or for reorganization or for an arrangement or for the appointment of a receiver or trustee of Party or of its assets, (ii) if the other Party proposes a written agreement of composition or extension of its debts, (iii) if the other Party is served with an involuntary petition against it, filed in any insolvency proceeding, and such petition is not dismissed within *** after the filing thereof, (iv) if the other Party proposes or is a Party to any dissolution or liquidation, or (v) if the other Party makes an assignment for the benefit of creditors.
13.4 Termination for Patent Challenge. Syndax may terminate this Agreement in its entirety upon written notice to KHK if KHK or any Affiliate, directly or indirectly, individually or in association with any other person or entity, commences any action or proceeding that challenges the validity, enforceability or scope of any Syndax Patent in the Territory. KHK shall (a) *** KHK’s Sublicensees, *** and (b) ***.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
48
13.5 Effects of Termination. Without limiting any other legal or equitable remedies that a Party may have, if this Agreement is terminated prior to its natural expiration, then the following provisions shall apply:
13.5.1 Termination of Licenses; Withdrawal. The licenses granted to KHK hereunder shall expire or terminate with respect to such Product in such country, and KHK shall cease all use of and activities with respect to the Compound, Product, Syndax Patents and Syndax Know-How, including any Development or Commercialization of the Product. KHK shall discontinue making any representation and withdraw registrations regarding its status as a licensee of Syndax for such Product in such country. All sublicenses granted by KHK to Affiliates or Third Parties under the Syndax Patents and/or Syndax Know-How shall immediately terminate.
13.5.2 Inventory. Except in the case of termination by Syndax pursuant to Section 13.3.1, KHK and its Affiliates will be entitled, during the period ending on the last day of the *** following the effective date of such termination, to sell any inventory of Product affected by such termination that remains on hand as of the effective date of the termination, so long as KHK pays to Syndax any royalty payments and other amounts payable hereunder, in accordance with the terms and conditions set forth in this Agreement and otherwise complies with the terms set forth in this Agreement (in each case as though this Agreement had not been terminated). Notwithstanding the foregoing, Syndax shall have the right, at any time following termination, to purchase from KHK any or all of such Product and work-in-progress at a price to be mutually agreed in good faith.
13.5.3 Regulatory Materials. Except in the case of termination by KHK pursuant to Section 13.3.1, to the extent permitted by Applicable Law, KHK shall transfer and assign to Syndax all Regulatory Materials and Regulatory Approvals with respect to Product in the Territory that are Controlled by KHK or its Affiliates, if any, at no cost to Syndax.
13.5.4 Licenses to Syndax. Except in the case of termination by KHK pursuant to Section 13.3.1, KHK shall grant to Syndax (i) an exclusive, royalty-free, fully paid license under any Patents or Know-How of KHK Improvements Controlled by KHK or its Affiliates relevant to the Development, Manufacturing, or Commercialization of the Product existing at the time of such termination in the Territory, only to the extent necessary to continue the Development, Manufacture and Commercialization of the then-existing Product in the Territory and (ii) an exclusive license under the KHK Trademarks, to use such KHK Trademarks in connection with the Product for the product name in any country of the Territory where such Trademark is then used; provided that Syndax shall pay to KHK *** of Net Sales of Products using such then used KHK Trademarks for the product name on a country-by-country basis as consideration for the license granted in this Section 13.5. For the avoidance of doubt, in no event shall Syndax be entitled to use any KHK Trademarks which is or consists of, or contains
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
49
in part, KHK’s corporate name (e.g., “Kyowa Hakko Kirin”), corporate logo or design (e.g., “KYOWA KIRIN”) or any part thereof (e.g., “Kyowa,” “Kirin,” “Kyowa Hakko”) or similar language or marks or any derivative (including any translations or transliterations thereof).
13.6 Expiration of this Agreement. Upon expiration of this Agreement, KHK shall have an exclusive, fully paid-up, royalty-free, irrevocable and perpetual license to Develop, Commercialize and Manufacture the Product in the Territory. In addition, Syndax covenants in favor of KHK not to enforce against KHK with respect to such Product any Patent Controlled by Syndax which Patent has issued after the expiration or termination of this Agreement from a patent application filed by Syndax during the term of this Agreement. Notwithstanding the foregoing, in the event that KHK uses a Syndax Trademark after the expiration of the Term, KHK shall pay to Syndax *** of Net Sales of Products using such Syndax Trademark on a country-by-country basis as consideration for the license granted in Section 2.1.2, for so long as KHK continues to use such trademark in connection with sales of Product. Such payments shall be governed by Sections 8.7-8.10 and Article 9, all of which shall survive expiration of this Agreement, as necessary. The Parties shall negotiate in good faith the supply price of the Product to be applicable after the expiration of the Term, provided that the supply price shall not exceed the supply price effective at the time of expiration of the Agreement.
13.7 Effects of Material Breach by Syndax.
13.7.1 If KHK has the right to terminate this Agreement pursuant to Section 13.3.1 due to an Significant Syndax Breach (as defined below) that has not been cured by the end of the *** period specified therein, then KHK may elect, by written notice to Syndax, either (a) to terminate this Agreement pursuant to Section 13.3.1 or, in the alternative, (b) continue this Agreement. If KHK elects to continue this Agreement, then, for any Product ordered on or after the date that Syndax receives notice of such election from KHK, the *** shall be *** (excluding the *** following ***, which shall remain unchanged), and, for the sake of clarity, all other provisions of this Agreement shall remain in full force and effect without change.
13.7.2 As used herein, “Significant Syndax Breach” means a material breach by Syndax of (a) its obligation to ***, or (b) ***
13.7.3 In the event that Syndax disputes whether an uncured Significant Syndax Breach has occurred, Syndax shall have the right to submit the dispute to binding arbitration administered by the American Arbitration Association (“AAA”). The arbitration shall be conducted in English in New York City, New York, USA pursuant to the AAA Commercial Arbitration Rules then in effect, by a single arbitrator with not less than fifteen (15) years of relevant experience in the subject matter of the dispute. The arbitrator shall only decide whether an uncured Significant Syndax Breach has occurred and shall have no authority to award damages of any kind or require any other type of remedy other than *** described in Section 13.7.1 above. The decision of the arbitrator shall be made in writing and shall be binding on the Parties, subject to Section 13.7.5. Each Party shall bear its own costs and expenses and attorneys’ fees and an equal share of the arbitrator’s fees and any administrative fees of arbitration. The existence, content, or results of any such arbitration shall be deemed to be the Confidential Information of each of the Parties hereunder.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
50
13.7.4 In the event that Syndax submits to arbitration a dispute as to the existence of an uncured Significant Syndax Breach, this Agreement shall remain in full force and effect, the *** set forth above shall not occur and no Party shall be released from its obligations hereunder unless and until a final determination is made with respect to the matter by the arbitrator.
13.7.5 Regardless of whether Syndax elects to arbitrate a dispute as to the existence of an uncured Significant Syndax Breach, both Parties reserve the right to seek resolution of any dispute hereunder (including any dispute as to the existence of a Significant Syndax Breach, whether such Significant Syndax Breach was cured in a timely manner or the proper damages owed to KHK as a result of a Significant Syndax Breach) pursuant to Section 16.7. To the extent any resolution pursuant to Section 16.7 is contrary to the results of an arbitration under Section 13.7.3, the former shall control. To the extent that, following a *** pursuant to Section 13.7.1 KHK receives an award of damages for a Significant Syndax Breach that does not expressly take into account the past and future economic effect of such ***, such *** shall be reasonably adjusted by good faith discussion and agreement of the Parties with the aim to prevent double benefit to either Party.
13.8 Accrued Rights. Termination or expiration of this Agreement for any reason shall be without prejudice to any rights that shall have accrued to the benefit of a Party prior to such termination or expiration.
13.9 Remedies Not Exclusive. Except as expressly provided herein, the rights and remedies provided in this Agreement shall be cumulative and not exclusive of any rights or remedies provided under Applicable Law.
13.10 Survival. Except as expressly provided herein, the rights and obligations of the Parties under the following provisions shall survive the expiration or termination of this Agreement: Articles 1, 12, 13, 14, and 16; Sections 5.3, 5.4, 5.7, 8.6, 9.2, 9.3, 10.1, 10.5, and 11.5; and any other provisions which by their nature are intended to survive expiration or termination.
ARTICLE 14
CONFIDENTIAL INFORMATION
14.1 Confidentiality. Except for the purposes of this Agreement or otherwise agreed in writing, the Parties agree that, until the end of the Term of this Agreement and for *** thereafter, each Party receiving any Confidential Information (the “Receiving Party”) of the other Party (the “Disclosing Party”) shall keep such Confidential Information confidential and shall not publish or otherwise disclose or use such Confidential Information for any purpose
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
51
other than as provided for by this Agreement, except for any portion of the Confidential Information that the Receiving Party can establish by competent written proof:
(i) was already known to the Receiving Party (other than under an obligation of confidentiality) at the time of disclosure by the Disclosing Party;
(ii) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party;
(iii) became generally available or known, or otherwise became part of the public domain, after its disclosure to such Receiving Party through no fault of the Receiving Party or its Affiliates;
(iv) was disclosed to the Receiving Party on a non-confidential basis by a Third Party who had no confidentiality obligation to the Disclosing Party with respect to such information; or
(v) was independently discovered or developed by or on behalf of the Receiving Party without the use of the Confidential Information belonging to the Disclosing Party; or
This Section 14.1 shall not preclude a Party from making any disclosure that is required by Applicable Law or order of any Governmental Authority or tribunal of competent jurisdiction, provided that the Receiving Party gives the Disclosing Party timely, prior notification in order to give the Disclosing Party opportunity to take appropriate action to limit or prevent disclosure of such Confidential Information, takes reasonable steps as necessary to assist the Disclosing Party in protecting the confidentiality of the Confidential Information, and discloses only that portion of the Confidential Information that the Receiving Party is legally required to disclose.
Additionally, Syndax may disclose to Bayer any Confidential Information of KHK that Syndax receives hereunder solely to the extent required to comply with the terms and conditions of the Bayer License Agreement, provided that Bayer is obligated to maintain the confidentiality of KHK’s Confidential Information as set forth herein for the benefit of KHK.
14.2 Disclosures. The Receiving Party agrees that it shall limit dissemination of and access to the Disclosing Party’s Confidential Information to those employees and agents of the Receiving Party who have a direct need to know such Confidential Information for the purpose of this Agreement and who are under at least as stringent conditions of confidentiality and limitations on use as set forth in this Agreement.
14.3 Return. Upon expiration or termination of this Agreement for any reason, the Receiving Party shall return, or at the option of the Disclosing Party, certify destruction of, all Confidential Information and copies thereof; provided, however, that the Receiving Party may retain one (1) archival copy of the Confidential Information at a secure location solely for purposes of determining its continuing obligations or preserving its continuing rights under this Agreement.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
52
14.4 Confidentiality of Agreement Terms. The Parties acknowledge that the terms of this Agreement shall be treated confidentially as Confidential Information of both Parties. Notwithstanding the foregoing, such terms may be disclosed by a Party (but with financial terms redacted unless required in connection with a merger, acquisition or public or private offering) to investment bankers, investors, and potential licensees, collaborators, investors or acquirers and their respective advisors, in the context of a potential transaction, each of whom prior to disclosure must be bound by similar obligations of confidentiality and non-use at least equivalent in scope to those set forth in this Article 14 (except that the duration of such obligations may be for a shorter period, so long as such duration is commercially reasonable). In addition, a copy of this Agreement may be filed by a Party with the U.S. Securities and Exchange Commission, or similar regulatory agency in a country other than the United States or of any stock exchange or other securities trading institution, as required by Applicable Law. In connection with any such filing, such Party shall endeavor to obtain confidential treatment of economic and trade secret information.
14.5 Publicity.
14.5.1 During the Term, subject to the remainder of this Section 14.5, neither Party shall issue a press release or public announcement relating to Licensed Products and/or this Agreement without the prior written approval of the other Party, which approval shall not be unreasonably withheld or delayed.
14.5.2 The Parties hereby agree to coordinate the timing and content of their respective press releases announcing the execution of this Agreement.
14.5.3 KHK shall be permitted to issue press releases in connection with Development and Commercialization of the Product in the Territory and make accurate public statements with respect to KHK’s Development or Commercialization activities in the normal course of business; provided, however, that KHK shall provide Syndax with at least *** prior notice of such press release or public statement if it would reasonably be expected that such press release or public statement will have regulatory implications outside the Territory.
14.5.4 Notwithstanding the above, a Party may issue a press release or public announcement if and to the extent required by Applicable Law, including by the rules or regulations of the U.S. Securities and Exchange Commission or similar regulatory agency in a country other than the U.S. or the rules of any stock exchange (including the Tokyo Stock Exchange) or Nasdaq, in each case after first notifying the other Party of such planned press release or public announcement at least *** in advance of issuing such press release or making such public announcement (or with as much advanced notice as practicable under the circumstances if it is not practicable to provide notice at least *** in advance) for the sole purpose of allowing the other Party to review the proposed press release or public announcement for the inclusion of Confidential Information or the use of its name.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
53
14.5.5 KHK agrees that Syndax will have the right to issue a press release with respect to the occurrence of the following events under this Agreement, subject to KHK’s right to prior review of and opportunity to comment on the content of such press release: (i) initiation, completion and results of a clinical trial, (ii) filing and/or approvals of any regulatory applications; and (iii) commercial launch of a Product in a country or region.
14.6 Publications by KHK. KHK may prepare and submit, in its sole discretion, alone or in cooperation with any organization, contractor, investigator or other agent under contract with, or at the direction of, KHK as KHK may select, documents or materials for publication or peer review, or otherwise present any data or results of its, its Affiliates, or its licensee’s clinical studies or the Development or Commercialization of the Product (“Publications”), provided, however, that KHK shall submit such Publications to Syndax for review and comment at least *** prior to submission. Syndax shall review any proposed abstracts, manuscripts or summaries of presentations or other materials for publication and shall respond in writing to KHK within *** after receipt of the proposed disclosure with comments, if any. Subject to the foregoing, KHK shall not submit, as part of any Publications, any confidential data which would reasonably be expected to have a detrimental effect on Syndax’s ability to file, obtain or otherwise Prosecute any patent applications or other filings related thereto.
14.7 Publications by Syndax. Syndax may prepare and submit, in its sole discretion, alone or in cooperation with any organization, contractor, investigator or other agent under contract with, or at the direction of, Syndax as Syndax may select, documents or materials for publication or peer review, or otherwise present any data or results of its or its Affiliates’ clinical studies or the Development or Commercialization of the Product in the Territory or outside of the Territory; provided, however, that Syndax shall submit such Publications to KHK for review and comment at least *** prior to submission. KHK shall review any proposed abstracts, manuscripts or summaries of presentations or other materials for publication and shall respond in writing to Syndax within *** after receipt of the proposed disclosure with comments, if any. With respect to any Publications by investigators, licensees or other Third Parties, such Publications shall be subject to review under this Section 14.7 to the extent that Syndax has the right and ability to do so; provided, however, that Syndax shall use Commercially Reasonable Efforts to obtain such right and ability.
ARTICLE 15
ADDITIONAL COVENANTS
15.1 Reimbursement Price. Syndax covenants in favor of KHK that Syndax shall use best efforts to procure, or cause its licensees to procure, a reimbursement price (or its closest equivalent) for the Product *** in *** and to seek to have such reimbursement price published (to the extent such publication is available under Applicable Law).
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
54
15.2 ECOG Data. Syndax covenants in favor of KHK that, pursuant to the ECOG License Agreement, Syndax has the right to provide KHK with the Development Data arising from the clinical study that is the subject of the ECOG License Agreement.
15.3 Restriction on Amendment of Bayer License Agreement. Syndax shall not execute any amendment to the Bayer License Agreement that would have a material adverse effect on KHK’s rights under this Agreement.
15.4 Improvements to *** Aspects of Products. Taking into consideration the fact that *** are known to have *** with respect to *** or other *** in the ***, Syndax shall use Commercially Reasonable Efforts to proactively respond to any *** and shall use Commercially Reasonable Efforts to *** to ***.
ARTICLE 16
GENERAL PROVISIONS
16.1 Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by Syndax are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, if applicable, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that KHK, as licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code.
16.2 Notices. All notices or other communications required or permitted hereunder shall be in writing and delivered personally or by facsimile transmission (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), mailed by registered or certified mail (return receipt requested), postage prepaid, or sent by internationally-recognized overnight courier service, addressed as follows:
If to Syndax:
Address: 000 Xxxxxx Xxxx Xxxx
Xxxxx 000
Xxxxxxx, XX
00000 XXX
Fax: x0-000-000-0000
Attention: ***
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
55
If to KHK:
Address: 0-0-0 Xxxxxxxxx
Xxxxxxx-xx, Xxxxx 000-0000 Xxxxx
Fax: x00-0-0000-0000
Attention: Director, Business Development Department
or to such other address or facsimile number as the Party to whom notice is to be given may have furnished to the other Party in writing in accordance herewith. Any such communication shall be deemed to have been given (a) when delivered, if personally delivered or sent by facsimile transmission on a Business Day; (b) on the Business Day after dispatch, if sent by internationally-recognized overnight courier; and (c) on the *** following the date of mailing, if sent by mail.
16.3 Force Majeure. Neither Party shall be liable for delay or failure in the performance of any of its obligations hereunder if such delay or failure is due to causes beyond its reasonable control after taking reasonable precaution, including acts of God, fires, earthquakes, strikes and labor disputes, acts of war or terrorism, civil unrest or acts or omissions of any Governmental Authority; provided, however, that (a) all payment obligations of KHK hereunder shall not be affected by any events of force majeure, (b) the affected Party promptly notifies the other Party, and (c) the affected Party uses its Commercially Reasonable Efforts to avoid or remove such causes of non-performance and continues performance with the utmost dispatch whenever such causes are removed.
16.4 Assignment. Neither Party shall assign its rights or obligations under this Agreement without the prior written consent of the Party, which shall not be unreasonably withheld or delayed, except that (a) Syndax may assign this Agreement in whole without the consent of KHK to (i) Affiliates (provided, however, that Syndax will remain jointly and severally liable with, and will guarantee the performance of, the relevant Affiliate under this Agreement, and the relevant Affiliate assignee, will assume in writing all of Syndax’s obligations under this Agreement) or (ii) a successor to the business of Syndax relating to the Product, whether in a merger, sale of stock, sale of assets or other transaction, (b) KHK may make such an assignment without Syndax’ consent to (i) Affiliates (provided, however, that KHK will remain jointly and severally liable with, and will guarantee the performance of, the relevant Affiliate under this Agreement, and the relevant Affiliate assignee, will assume in writing all of KHK’s obligations under this Agreement) and (ii) a successor to the business of KHK, whether in a merger, sale of stock, sale of assets or other transaction. Any permitted assignment shall be binding on the successors of the assigning Party. Any assignment or attempted assignment by either Party in violation of the terms of this Section 16.4 shall be null, void and of no legal effect. Upon any assignment of this Agreement by Syndax to a permitted assignee (including any assignment by operation of law), the Syndax Patents and the Syndax Know-How shall exclude any Patents or Know-How owned or Controlled by such assignee or any Affiliate of such assignee prior to such assignment, or thereafter developed or made by such assignee or its Affiliates independently of the research, Development, Manufacturing, or Commercialization of Products.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
56
16.5 Waivers and Modifications. The failure of any Party to insist on the performance of any obligation, or the failure or delay to exercise any remedy, right, power or privilege, hereunder shall not be deemed to be a waiver of such obligation, remedy, right, power or privilege. Waiver of any breach of any provision hereof shall not be deemed to be a waiver of any other breach of such provision or any other provision. No waiver, modification, release or amendment of any obligation under or provision of this Agreement shall be valid or effective unless in writing and signed by the Parties hereto.
16.6 Choice of Law. This Agreement shall be governed by and shall be construed in accordance with the laws of the State of New York, U.S.A, without regard to any choice of law principles that would provide for the application of the laws of another jurisdiction. The Parties hereby exclude from this Agreement the application of the United Nations Convention on Contracts for the International Sale of Goods.
16.7 Dispute Resolution. Any controversy, dispute or claim arising out of or in connection with this Agreement, including the validity, inducement, or breach thereof, shall be resolved by the Parties in the following manner:
16.7.1 Informal Settlement. Either Party may initiate resolution of such dispute by providing to the other Party a concise statement of the initiating Party’s claims, together with relevant facts supporting them, and referring to this Section 16.7.1. For a period of *** from the date of such statement, or such longer period as the Parties may agree in writing, the Parties shall cause their respective Senior Officers to conduct good-faith negotiations in order to settle the dispute amicably.
16.7.2 Litigation. Any dispute, controversy or claim arising out of or relating to the validity, construction, interpretation, enforceability, breach, performance, application or termination of this Agreement that is not resolved pursuant to Section 16.7.1 may be submitted to a court of competent jurisdiction.
16.7.3 Waiver of Jury Trial. Each Party waives, to the fullest extent permitted by Applicable Law, any right it may have to a trial by jury with respect to litigation arising out of or relating to this Agreement. Each Party (i) certified that no representative, agent or attorney of the other Party has represented, expressly or otherwise, that such other Party would not, in the event of litigation, seek to enforce the foregoing waiver, and (ii) acknowledges that it has been induced to enter into this Agreement by, among other things, the mutual waivers and certifications set forth above in this Section 16.7.3.
16.8 Entire Agreement. This Agreement (including the Exhibits attached hereto) constitutes the entire agreement between the Parties as to the subject matter hereof, and supersedes all prior and contemporaneous negotiations, representations, agreements and understandings regarding the same.
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
57
16.9 Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments and take all such other actions as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
16.10 Relationship of the Parties. Each party is an independent contractor under this Agreement. Nothing contained herein is intended or is to be construed so as to constitute Syndax and KHK as partners, agents or joint venturers. Neither Party shall have any express or implied right or authority to assume or create any obligations on behalf of or in the name of the other Party or to bind the other Party to any contract, agreement or undertaking with any Third Party. The members of the JSC, and any working group thereof, shall be, and shall remain, employees or other representatives of Syndax or KHK, as the case may be.
16.11 Severability. If any provision hereof is held invalid, illegal or unenforceable in any respect, then, to the fullest extent permitted by Applicable Law, (a) all other provisions hereof shall remain in full force and effect and shall be liberally construed in order to carry out the intent of the Parties as nearly as may be possible, and (b) the Parties agree to use their best efforts to negotiate a provision, in replacement of the provision held invalid, illegal or unenforceable, that is consistent with Applicable Law and accomplishes, as nearly as possible, the original intention of the Parties with respect thereto. To the extent that the Parties are, in any event, unable to agree to the modifications made in accordance with (b), the applicable provision shall be deemed modified to the most limited extent possible in order to most fully carry out the intent of the original provision while complying with Applicable Law. To the fullest extent permitted by Applicable Law, each Party hereby waives any provision of law that would render any provision hereof prohibited or unenforceable in any respect.
16.12 Construction. References to Articles and Sections are references to Articles and Sections of this Agreement. Except where the context otherwise requires, wherever used, the singular shall include the plural, the plural the singular, the use of any gender shall be applicable to all genders and the word “or” is used in the inclusive sense. The captions of this Agreement are for convenience of reference only and in no way define, describe, extend or limit the scope or intent of this Agreement or the intent of any provision contained in this Agreement. The term “including” as used herein shall mean “including, but not limited to,” without limiting the generality of any description preceding such term. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties, and no rule of strict construction shall be applied against either Party.
16.13 Third Party Beneficiaries. No person or entity other than Syndax and KHK shall be deemed to be an intended beneficiary of this Agreement or have any right to enforce any obligation under this Agreement. Notwithstanding the foregoing, the Parties agree that Bayer shall be an intended third party beneficiary hereunder solely with respect to Bayer’s rights under the following provisions: Section 10.3.2, Article 14 (but only as to Confidential Information that is Bayer’s Confidential Information), and Article 16 (solely to the extent required to exercise the rights in the preceding sections).
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
58
16.14 Costs. Except as expressly set forth herein, KHK and Syndax shall each be solely responsible for their respective costs incurred in connection with the matters contemplated by this Agreement.
16.15 Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed to be an original and all of which together shall constitute one and the same agreement. Delivery of a counterpart by facsimile or by email in PDF format shall be deemed to be an original for purposes of this Section 16.15.
[signature page follows]
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
59
IN WITNESS WHEREOF, the Parties have duly executed this Agreement as of the date first written above.
| KYOWA HAKKO KIRIN CO., LTD | SYNDAX PHARMACEUTICALS, INC. | |||||||
| By: | /s/ Nobu Hanai | By: | /s/ Xxxxxx X. Xxxxxx | |||||
| Name: | Nobuo Hanai | Title: | Chief Financial Officer | |||||
| Title: | President & Chief Executive Officer |
[Signature Page to License, Development and Commercialization Agreement]
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Exhibit A
Syndax Patents
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
***
*** INDICATES 3 PAGES OF MATERIAL THAT WERE OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Exhibit B
Specifications
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
***
*** INDICATES 1 PAGE OF MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Exhibit C
Bayer’s Consent Letter
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
***
*** INDICATES 1 PAGE OF MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Exhibit D
Series B-1 Preferred Stock Purchase Agreement
*** INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
***
*** INDICATES 31 PAGES OF MATERIAL THAT WERE OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED.

