THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. LICENSE AGREEMENT BY AND BETWEEN AMGEN INC. AND...
Exhibit 10.32
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
BY AND BETWEEN AMGEN INC.
AND
AMYLIN PHARMACEUTICALS, INC.
February 7, 2006
-1-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
This License Agreement (this “Agreement”) is made effective as of the 7th day of February, 2006 (the “Effective Date”) by and between Amgen Inc., a Delaware corporation with a principal place of business at Xxx Xxxxx Xxxxxx Xxxxx, Xxxxxxxx Xxxx, Xxxxxxxxxx 00000- 1799 (“Amgen”), and Amylin Pharmaceuticals, Inc., a Delaware corporation with a principal place of business at 0000 Xxxxx Xxxxxx Xxxxx, Xxx. 000, Xxx Xxxxx, Xxxxxxxxxx 00000 (“Amylin”). Amgen and Amylin are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, Amgen owns or controls certain patent rights and know-how relating to its proprietary compound referred to as leptin; and
WHEREAS, Amylin has capabilities in the development, manufacture and commercialization of pharmaceutical compounds; and
WHEREAS, Amylin desires to obtain from Amgen, and Amgen is willing to grant to Amylin, the exclusive license to develop, manufacture and commercialize leptin worldwide, on the terms and conditions set forth herein.
NOW, THEREFORE, based on the premises and the mutual covenants and obligations set forth below, and intending to be bound hereby, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
For purposes of this Agreement, the following terms shall have the meanings as set forth below:
1.1 “A-100 Leptin” means r-metHuLeptin, the polypeptide having the amino acid sequence which is set forth in Exhibit A-2.
1.2 “A-200 Leptin” means Fc-leptin fusion protein, including the Fc-fusion protein having the amino acid sequence which is set forth in Exhibit B.
1.3 “Active Component” means any active pharmaceutical ingredient other than Leptin which performs an identifiable therapeutic or prophylactic function when combined with Leptin.
1.4 “Affiliate” means an entity that, directly or indirectly, through one or more intermediaries, controls, is controlled by or is under common control with the party being referenced. For purposes of this definition, “control” means the possession, direct or indirect, of
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
the power to cause the direction of the management and policies of the applicable entity, whether through ownership of fifty percent (50%) or more of the voting securities of such Person, by contract or otherwise. An entity will be an Affiliate for purposes of this Agreement only so long as it satisfies the definition set forth herein.
1.5 “Agreement” means this License Agreement dated February 7, 2006 by and between Amgen and Amylin, as may be amended in accordance with its terms.
1.6 “Amgen” shall have the meaning ascribed to it in the opening paragraph of this Agreement.
1.7 “Amgen Indemnitees” shall have the meaning ascribed to it in Section 8.2.
1.8 “Amylin” shall have the meaning ascribed to it in the opening paragraph of this Agreement.
1.9 “Amylin Indemnitees” shall have the meaning ascribed to it in Section 8.1.
1.10 “Amylin Manufacturing Improvements” means any improvements to the Manufacturing Processes conceived, created or discovered by (a) Amylin or its Affiliates, (b) any CMO (as defined in Exhibit H) in performing work for Amylin, its Affiliates or Sublicensees, or (c) representatives, consultants or contractors of Amylin, its Affiliates or any CMO, in each case after the Effective Date but prior to the expiration of the Term or the earlier termination of the Agreement.
1.11 “Claim” shall have the meaning ascribed to it in Section 8.3.
1.12 “Combination Product” means any product sold by or on behalf of Amylin, its Affiliates or Sublicensee(s) which contains Leptin in combination with one or more Active Components.
1.13 “Commercialize” or “Commercialization” means those activities relating to the promotion, marketing, distribution and sale of Licensed Products, including Phase IV Trials or equivalent clinical trials conducted following Regulatory Approval to market a pharmaceutical product.
1.14 “Commercially Reasonable Efforts” means the level of efforts and resources commonly used in the pharmaceutical industry to develop, obtain regulatory approvals for, protect intellectual property relating to, and commercialize a product consistent with the efforts a similarly situated biopharmaceutical company would typically devote to a product at a similar stage in its product life and of similar market potential, profit potential and strategic value resulting from its own research efforts, based on information and conditions then-prevailing, including, without limitation, efficacy of the product, the competitiveness of alternative products in the marketplace, the patent and other proprietary position of the product, the likelihood of regulatory approval given the regulatory structure involved and the likelihood of adequate
-2-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
reimbursement. Commercially Reasonable Efforts shall be determined on a country-by-country (each country including its territories) basis for a particular Licensed Product, and it is anticipated that the level of effort will change over time reflecting changes in the status of the Licensed Product and the country (including its territories) involved.
1.15 “Confidential Information” means all confidential or proprietary information received or otherwise obtained by either Party from the other Party or its Affiliates pursuant to this Agreement, other than that portion of such information which:
(a) is now, or hereafter becomes, generally available to the public through no fault of the receiving Party, or its Affiliates, or any entity that obtained such information or materials from the receiving Party;
(b) the receiving Party or its Affiliates already possesses, as evidenced by its written records, predating receipt thereof from the other Party;
(c) is obtained from a Third Party without restriction who had the legal right to disclose the same to the receiving Party or its Affiliates; or
(d) has been independently developed by the receiving Party or its Affiliates without the aid, application or use of Confidential Information, as demonstrated by competent written proof.
Notwithstanding the preceding definition of Confidential Information, during the Term, the information identified in subparts (1), (2), (4) and (5) of the definition of Licensed Know-How and the information identified in subpart (6) of the definition of Licensed Know-How as it relates to Leptin shall be deemed the Confidential Information of each of Amgen and Amylin, subject to the exceptions in subparts (a), (c) and (d) of this Section 1.15.
1.16 “Control” means with respect to any (a) material, document, item of information, method, data or other know-how or (b) intellectual property right, the possession (whether by ownership or license, other than by a license granted pursuant to this Agreement) by a Party or its Affiliates of the ability to grant to the other Party access to use, ownership, a license and/or a sublicense as provided herein under such item or right without violating the terms of any agreement or other arrangement with any Third Party as of the time such Party would first be required hereunder to grant the other Party such access, ownership, license, or sublicense.
1.17 “Default” means with respect to either Party (i) that any representation or warranty of such Party set forth herein shall have been untrue in any material respect when made, (ii) such Party, or such Party’s Affiliate or Sublicensee, shall have materially breached this Agreement or (iii) such Party’s failure to pay to the other Party any payment on or before the last day when such payment is due.
1.18 “Dollar” means a United States dollar, and “$” shall be interpreted accordingly.
-3-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.19 “Drug Product” means the finished dosage form that contains a Drug Substance, generally, but not necessarily in association with other active or inactive ingredients, and not necessarily labeled or packaged.
1.20 “Drug Substance” means the substance or mixture of substances intended to be used in the manufacture of A-100 Leptin and that, when used in the production of A-100 Leptin, becomes an active ingredient of A-100 Leptin.
1.21 “FDA” means the United States Food and Drug Administration, or any successor thereto.
1.22 “FDCA” means the Federal Food, Drug & Cosmetic Act.
1.23 “Financial Default” shall have the meaning ascribed to it in Section 10.2(b).
1.24 “FTE” means the equivalent of the work of one (1) professional employee fulltime for one (1) year (consisting of at least a total of [*] or [*] per year (excluding vacations and holidays)).
1.25 “FTE Rate” means [*] per FTE.
1.26 “GAAP” means United States generally accepted accounting principles.
1.27 “Generic Date” means the date that a first Third Party (other than a Sublicensee) makes available for purchase, following receipt of regulatory approval therefor from any Regulatory Authority to market in a particular country, a product containing Leptin for the same indication as the applicable Licensed Product (other than a Combination Product) that is being Commercialized under this Agreement in such country; provided that, in the event Amgen provides to Amylin reasonable evidence that such Third Party has not made such product available for sale in such country during the first thirty (30) days after such Third Party receives approval from any Regulatory Authority to market such product in such country, then the Generic Date shall mean the date thereafter on which Amylin provides to Amgen reasonable evidence that such Third Party has made such product available for sale in such country.
1.28 “IND” means an Investigational New Drug application, as defined in 21 C.F.R. 312 or any successor regulation.
1.29 “Indemnified Party” shall have the meaning ascribed to it in Section 8.3.
1.30 “Indemnifying Party” shall have the meaning ascribed to it in Section 8.3.
1.31 “Launch Date” means the date on which a Licensed Product is first sold by Amylin, its Affiliates or Sublicensees to a Third Party in a country, after Regulatory Approval for the Licensed Product has been granted in such country.
-4-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.32 “Leptin” means (i) the OB Polypeptide and fragments and species variants thereof; (ii) A-100 Leptin; (iii) A-200 Leptin; and (iv) any other molecule or other moiety that agonizes the natural physiological receptor for A-100 Leptin (“Leptin Receptor”), including any glycosylated or PEGylated form of (i), (ii), (iii) or (iv), as well as any analog of (i), (ii), (iii) or (iv) disclosed in any patent or patent application within Licensed Patent Rights. Leptin shall not include any pharmaceutical component which antagonizes the Leptin Receptor.
1.33 “Leptin Patent Rights” means:
(a) the patents and patent applications identified in part 2 of Exhibit C;
(b) any and all patent applications that claim priority to any of the patents and patent applications identified in part 2 of Exhibit C hereto to the extent that the claims are directed to Leptin (including, without limitation, all divisional or continuation, in whole or in part, applications based on the patent applications described in (a) above);
(c) any and all foreign applications corresponding to the patent applications described in (a) and (b) above;
(d) any and all issued and unexpired patents resulting from any of the applications described in (a), (b) or (c) above; and
(e) any and all issued and unexpired reissues, reexaminations, renewals, or extensions of any of the patents described in (a) or (d) above.
1.34 “License Fee” shall have the meaning ascribed to it in Section 5.1.
1.35 “Licensed Know-How” means the following information that is Controlled by Amgen or its Affiliates as of the Effective Date or, to the extent applicable, that is Controlled by Amgen or its Affiliates and results from Amgen’s performance of the transfer of the Manufacturing Processes pursuant to Section 3.2(b) and Exhibit H: (1) the Regulatory Documents; (2) protocols, data and reports of clinical studies listed in Exhibit I; (3) the Manufacturing Information; (4) results of all investigator-sponsored clinical trials, including the Ongoing Clinical Trials listed in Exhibit E; (5) all research and preclinical data for Leptin and formulations of Leptin that are available, and (6) any such information which Amgen expressly designates in writing it intends to include as Licensed Know-How under this Agreement.
1.36 “Licensed Patent Rights” means the Leptin Patent Rights, the Other Licensed Patent Rights, the Rockefeller Patents and the UCSF Patent.
1.37 “Licensed Product” means a preparation containing Leptin. Licensed Product shall not include any product which antagonizes the Leptin Receptor.
1.38 “Licensed Technology” means the Licensed Patent Rights, Licensed Trademarks and Licensed Know-How.
-5-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.39 “Licensed Trademarks” means the registered Trademarks and applications for Trademarks listed in Exhibit D and all trademarks issuing from such applications, together with any renewals, modifications or extensions thereto.
1.40 “Losses” means costs and expenses (including, without limitation, reasonable legal expenses and attorneys’ fees), judgments, suits, actions, liabilities, fines, damages, assessments and/or other losses.
1.41 “Major Market Country” means the United States, the United Kingdom, France, Germany, Italy, Spain or Japan.
1.42 “Manufacturing Information” means all information Controlled by Amgen or its Affiliates as of the Effective Date or, to the extent applicable, that is Controlled by Amgen or its Affiliates and results from Amgen’s performance of the transfer of the Manufacturing Processes pursuant to Section 3.2(b) and Exhibit H, and within its possession that is necessary for the manufacture of A-100 Leptin or A-200 Leptin, including but not limited to information contained in the CMC section of any applicable Regulatory Documents and trade secrets.
1.43 “Manufacturing Processes” means the manufacturing processes (bulk drug substance and drug product) for (i) A-100 Leptin, as described in the Regulatory Documents as of the Effective Date, and, to the extent applicable, modifications made thereto that are Controlled by Amgen or its Affiliates and result from Amgen’s performance of the transfer of the Manufacturing Processes pursuant to Section 3.2(b) and Exhibit H, and (ii) A-200 Leptin, as described in the A-200 Leptin documentation set forth in Exhibit H as of the Effective Date, and, to the extent applicable, modifications made thereto that are Controlled by Amgen or its Affiliates and result from Amgen’s performance of the transfer of the Manufacturing Processes pursuant to Section 3.2(b) and Exhibit H.
1.44 “NDA” means a New Drug Application or Biologics License Application submitted to the FDA, or any successor application or procedure, or any equivalent application or filing outside the United States to seek Regulatory Approval in such jurisdiction.
1.45 “Net Sales” means, with respect to a certain time period, all revenues recognized in accordance with GAAP consistently applied, which are received from sales of Licensed Products sold by or for Amylin, its Affiliates and its Sublicensees in arm’s-length transactions to Third Parties (but not including sales relating to transactions between Amylin, its Affiliates and/or its respective Sublicensees and agents) during such time period, less the total of the following estimated and/or incurred charges or expenses: (a) trade, cash, prompt payment and/or quantity discounts; (b) returns, allowances and rebates, and chargebacks, other allowances or payments to government agencies; (c) retroactive price reductions applicable to sales of such Licensed Product; (d) reasonable fees paid to distributors, selling agents (excluding any sales representatives of Amylin or any of its Affiliates), group purchasing organizations and managed care entities; (e) bad debt; (f) freight, shipping, packing and insurance; and (g) nonrecoverable sales and other taxes based on sales prices (excluding income taxes) to the extent paid by Amylin, its Affiliates or Sublicensees (and not reimbursed by a Third Party); provided that the amounts in subsections (f) and (g) in the aggregate do not exceed [*]% of total revenues recognized for such time period for the sale of Licensed Products.
-6-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
All of the sales, and deductions taken above, shall be determined in accordance with GAAP.
Any disposal of Licensed Products for, or use of Licensed Products in, clinical or pre-clinical trials, given as free samples, including, without limitation, sample cards, or distributed for indigent programs shall not be included in Net Sales.
Upon any sale or other disposal of any Licensed Product that should be included within Net Sales for any consideration other than an exclusively monetary consideration on bona fide arm’s- length terms, then for purposes of calculating the Net Sales under this Agreement, such Licensed Product shall be deemed to be sold exclusively for money at the average sales price during the applicable reporting period generally achieved for such Licensed Product in the country in which such sale or other disposal occurred when such Licensed Product is sold alone and not with other products.
In the case of any Licensed Product that is a Combination Product, Net Sales for such Combination Product shall be calculated by multiplying actual Net Sales of such Combination Product by the fraction A/(A+B) where A is the invoice price of the Licensed Product (which does not contain any Active Component(s)), if sold separately, and B is the total invoice price of the Active Component(s) in the Combination Product, if sold separately. If, on a country-by-country basis, the Active Component(s) in the Combination Product is not sold separately in said country, Net Sales for the purpose of determining royalties of the Combination Product shall be calculated by multiplying actual Net Sales of such Combination Product by the fraction A/D, where A is the invoice price of the Licensed Product (which does not contain any Active Component(s)), if sold separately, and D is the invoice price of the Combination Product. If neither the Licensed Product (which does not contain any Active Component(s)) nor the Active Component(s) in the Combination Product is sold separately in a given country, the Parties shall determine Net Sales for such Combination Product by mutual agreement based on the relative contribution of the Licensed Product (which does not contain any Active Component(s)) and the Active Components in the Combination Product.
In the event a Licensed Product is sold with one or more other products or services for a single price (together, a “Multiple Product Offering”), Net Sales for such Multiple Product Offering shall be calculated by multiplying actual Net Sales of such Multiple Product Offering by the fraction A/(A+B) where A is the invoice price of the Licensed Product, if sold separately, and B is the total invoice price of the other products in the Multiple Product Offering, if sold separately. If, on a country-by-country basis, the other products in the Multiple Product Offering are not sold separately in said country, Net Sales for the purpose of determining royalties of the Multiple Product Offering shall be calculated by multiplying actual Net Sales of such Multiple Product Offering by the fraction A/D, where A is the invoice price of the Licensed Product, if sold separately, and D is the invoice price of the Multiple Product Offering. If neither the Licensed Product nor the other products are sold separately in a given country, the Parties shall determine Net Sales for such Multiple Product Offering by mutual agreement based on the relative contribution of the Licensed Product (excluding other products) and each other product in the Multiple Product Offering.
-7-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
For purposes of the two preceding paragraphs, the invoice price of a Licensed Product (which does not contain any Active Component(s)) sold separately for an indication designated as an “Orphan Product” under the U.S. Orphan Drug Act, as amended, shall not be used to calculate Net Sales for any Combination Product or Multiple Product Offering, except any such Combination Product or Multiple Product Offering that is used for an indication designated as an “Orphan Product” under the U.S. Orphan Drug Act, as amended.
1.46 “Neuro Field” means [*]
1.47 “NIH” means the National Institutes of Health of the U.S. Department of Health and Human Services, or any successor agency thereto.
1.48 “OB Polypeptide” means the polypeptide encoded by the ob (obese) gene. The human ob polypeptide has the amino acid sequence set forth in Exhibit A-1.
1.49 “Ongoing Clinical Trials” means those clinical trials listed on Exhibit E hereto, which are clinical trials of A-100 Leptin.
1.50 “Other Licensed Patent Rights” means:
(a) the patents and patent applications identified in part 3 of Exhibit C;
(b) any and all patent applications that claim priority to any of the patents and patent applications identified in part 3 of Exhibit C hereto to the extent that the claims are directed to Leptin (including, without limitation, all divisional or continuation, in whole or in part, applications based on the patent applications described in (a) above);
(c) any and all foreign applications corresponding to the patent applications described in (a) and (b) above;
(d) any and all issued and unexpired patents resulting from any of the applications described in (a), (b) or (c) above; and
(e) any and all issued and unexpired reissues, reexaminations, renewals, or extensions of any of the patents described in (a) or (d) above.
1.51 “Person” means an individual, corporation, limited liability company, partnership, association, trust, unincorporated organization, other entity or group.
1.52 “Phase IV Trial” means a clinical trial of a pharmaceutical product initiated in a country in an approved indication after receipt of Regulatory Approval for such product in such indication in such country, to delineate additional information about such product’s risks, benefits and optimal use, pursuant to 21 CFR 312.85.
-8-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.53 “Procedural Default” shall have the meaning ascribed to it in Section 10.2(e).
1.54 “Product Field” means all human and animal uses, including therapeutic, prophylactic, palliative and diagnostic uses, for the treatment of diseases and disorders, and the conduct of research and development in humans and animals.
1.55 “Regulatory Approval” means any approvals (including supplements, amendments, pre- and post-approvals and price approvals), licenses, registrations or authorizations of any national, supra-national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, necessary for the distribution, use or sale of a Licensed Product in a regulatory jurisdiction.
1.56 “Regulatory Authority” means the FDA or any counterpart of the FDA outside the United States.
1.57 “Regulatory Documents” means all regulatory documents and filings (including INDs), correspondence with Regulatory Authorities, annual reports and amendments thereto related to A-100 Leptin or A-200 Leptin.
1.58 “Rockefeller License” means that certain License Agreement between The Rockefeller University and Amgen dated April 14, 1995, as amended, a copy of which is attached to this Agreement as Exhibit F.
1.59 “Rockefeller Patents” means:
(a) the patents and patent applications identified in part 1 of Exhibit C:
(b) any and all patent applications that claim priority to any of the patents and patent applications identified in part 1 of Exhibit C hereto to the extent that the claims are directed to Leptin (including, without limitation, all divisional or continuation, in whole or in part, applications based on the patent applications described in (a) above);
(c) any and all foreign applications corresponding to the patent applications described in (a) and (b) above;
(d) any and all issued and unexpired patents resulting from any of the applications described in (a), (b) or (c) above; and
(e) any and all issued and unexpired reissues, reexaminations, renewals, or extensions of any of the patents described in (a) or (d) above.
Rockefeller Patents shall include the patent rights of subparts (b), (c), (d) and (e) above to the extent such patent rights are licensed to Amgen under the Rockefeller License.
1.60 “Royalty Term” means, on a Licensed Product-by-Licensed Product and country-by-country basis, the period from the Launch Date of such Licensed Product in such
-9-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
country until the later of: (i) expiration of the last-to-expire Valid Claim covering such Licensed Product in such country, (ii) expiration of any market exclusivity period granted by a Regulatory Authority with respect to such Licensed Product in such country (e.g., designation as an orphan product), or (iii) ten (10) years from the Launch Date of such Licensed Product in such country.
1.61 “Sublicensee” means a sublicensee, direct or indirect, of Amylin under Amylin’s rights pursuant to Sections 2.1, 2.2 and/or 2.3.
1.62 “Term” means the term of this Agreement as set forth in Section 10.1.
1.63 “Territory” means all countries of the world.
1.64 “Third Party” means any Person other than Amgen or Amylin or an Affiliate of either of them.
1.65 “Third Party Transaction” shall have the meaning ascribed to it in Section 2.6,
1.66 “Trademark” means any trade name, service xxxx, logo or trademark (whether or not registered), together with all goodwill associated therewith, and any renewals, extensions or modifications thereto.
1.67 “UCSF License” means that certain License Agreement between The Regents of the University of California and Amgen dated July 13, 2005, a copy of which is attached to this Agreement as Exhibit G.
1.68 “UCSF Patent” means;
(a) the patent identified in Exhibit G;
(b) any and all patent applications that claim priority to any of the patents and patent applications identified in Exhibit G hereto to the extent that the claims are directed to Leptin (including, without limitation, all divisional or continuation, in whole or in part, applications based on the patent applications described in (a) above);
(c) any and all foreign applications corresponding to the patent applications described in (a) and (b) above;
(d) any and all issued and unexpired patents resulting from any of the applications described in (a), (b) or (c) above; and
(e) any and all issued and unexpired reissues, reexaminations, renewals, or extensions of any of the patents described in (a) or (d) above.
UCSF Patent shall include the patent rights of subparts (b), (c), (d) and (e) above to the extent such patent rights are licensed to Amgen under the UCSF License.
-10-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.69 “Valid Claim” means an unexpired claim of an issued patent within the Licensed Patent Rights that has not been found to be unpatentable, invalid or unenforceable by a court or other authority in the country of the patent, from which decision no appeal is taken or can be taken.
ARTICLE 2
LICENSES
2.1 Patent License.
(a) Subject to the terms and conditions of this Agreement, Amgen hereby grants to Amylin an exclusive (even as to Amgen) license, with the right to grant sublicenses (subject to Amylin’s compliance with Section 2.4 of this Agreement), under the Leptin Patent Rights to make, have made, use, sell, have sold, offer for sale and import Licensed Products in the Territory within the Product Field.
(b) Subject to the terms and conditions of this Agreement, Amgen hereby grants to Amylin an exclusive (even as to Amgen) license, with the right to grant sublicenses (subject to Amylin’s compliance with Section 2.4 of this Agreement), under the Other Licensed Patent Rights to make, have made, use, sell, have sold, offer for sale and import Licensed Products in the Territory within the Product Field.
(c) Subject to the terms and conditions of this Agreement and the Rockefeller License, Amgen hereby grants to Amylin an exclusive (even as to Amgen) sublicense, with the right to grant further sublicenses (subject to Amylin’s compliance with Section 2.4 of this Agreement and the terms of the Rockefeller License), of Amgen’s exclusive rights under the Rockefeller Patents to make, use and sell Licensed Products in the Territory within the Product Field.
(d) Subject to the terms and conditions of this Agreement and the UCSF License, Amgen hereby grants to Amylin an exclusive (even as to Amgen) sublicense, with the right to grant further sublicenses (subject to Amylin’s compliance with Section 2.4 of this Agreement and the terms of the UCSF License), of Amgen’s non-exclusive rights under the UCSF Patent to make, have made, use, have used, sell, have sold, offer for sale, import, export or otherwise exploit or transfer physical possession of or title in Licensed Products in the Territory within the Product Field.
2.2 Trademark License. Amgen hereby grants to Amylin an exclusive (even as to Amgen) license, with the right to grant sublicenses (subject to Amylin’s compliance with Section 2.4 of this Agreement), under Amgen’s entire right, title and interest in and to the Licensed Trademarks, to use and display the Licensed Trademarks in connection with Licensed Products in the Territory within the Product Field. If Amylin decides to discontinue use of the Licensed Trademarks, it will notify Amgen in writing. Upon such written notification, the license granted under this Section 2.2 and the provisions in this Agreement granting Amylin rights with respect to the Licensed Trademarks shall terminate, and all such rights pertaining to the Licensed Trademarks under this Agreement shall revert to Amgen without further consideration.
-11-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
2.3 Know-How License. Subject to the terms and conditions of this Agreement, including Sections 2.5, 3.1 and 3.3, and the Rockefeller License (as applicable), Amgen hereby grants to Amylin an exclusive (even as to Amgen) license, with the right to grant sublicenses (subject to Amylin’s compliance with Section 2.4 of this Agreement), under the Licensed Know- How to make, have made, use, sell, have sold, offer for sale and import Licensed Products in the Territory within the Product Field.
2.4 Sublicenses. Amylin shall at all times be free to grant sublicenses to its Affiliates, provided that any further sublicense by any such Affiliate shall be subject to the terms and conditions of Amgen’s right of first offer under Section 2.6. Subject to Amgen’s right of first offer under Section 2.6, and only as expressly permitted under this Article 2, Amylin may grant sublicenses to Third Parties, which shall allow the grant of further sublicenses by such Third Parties; provided, however, that any such sublicense of rights to a Third Party, other than to a CMO (which is subject to Exhibit H), shall be subject to the prior written consent of Amgen, which shall not be unreasonably withheld or delayed; and provided further that any sublicense of Amylin’s rights under the Manufacturing Information shall only be granted to a Third Party contract manufacturer engaged by Amylin to manufacture Licensed Products. Notwithstanding the sublicensing of all or part of Amylin’s rights and obligations hereunder, Amylin shall remain responsible for the full and complete performance of all of Amylin’s obligations and duties under this Agreement. Amylin shall promptly notify Amgen of the granting of any sublicense hereunder and provide to Amgen a copy of the fully-executed agreement under which Amylin granted such sublicense (from which Amylin may redact any confidential information that is not necessary to disclose to Amgen for purposes of confirming compliance with this Agreement). Any such sublicense shall require the Sublicensee to comply with the obligations of Amylin as contained herein. Any such sublicense shall provide for the termination of the sublicense upon termination of this Agreement, except that such sublicense shall not terminate upon termination of this Agreement but instead shall remain in full force and effect if the Sublicensee is not then in material breach of its sublicense agreement and such Sublicensee provides to Amgen within thirty (30) days after termination of this Agreement a written agreement to be bound as licensee under the terms and conditions of this Agreement as to the field and territory in which such Sublicensee has been granted rights under its sublicense agreement. Amylin shall include in any sublicense agreement express language that the terms, conditions and obligations of any such sublicense are subject to the terms, conditions and obligations of this Agreement.
2.5 Retained Rights. Notwithstanding the licenses granted to Amylin pursuant to Sections 2.1 and 2.3, Amgen retains rights under the Licensed Technology (a) for the purpose of fulfilling its obligations under this Agreement; and (b) to conduct research, development, manufacturing and commercialization activities with respect to products other than Licensed Products (including Leptin).
2.6 Amgen Right of First Offer. Beginning on the Effective Date and expiring on the [*] anniversary of the Effective Date, Amylin hereby grants to Amgen a right of first offer for
-12-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
any out-license, partnership, co-development, commercialization, co-promotion or similar agreement related to Leptin or Licensed Products (“Third Party Transaction”). Prior to initiating discussions with a Third Party regarding a Third Party Transaction prior to the expiration of such [*] period, Amylin shall give Amgen written notice of its intention to seek a Third Party Transaction. Amgen shall notify Amylin in writing whether it elects to exercise its right of first offer, within [*] of Amylin’s written notice. If Amgen elects not to make an offer, Amylin shall be free to enter into discussions and an agreement with any Third Party regarding a Third Party Transaction. If Amgen elects to make an offer by providing written notice to Amylin within such [*] period or does not respond within such [*] period, the Parties shall negotiate in good faith for [*] from the date of Amylin’s notice with the goal of arriving at a mutually agreeable term sheet. If at the end of this time period the Parties have negotiated a mutually acceptable term sheet, then the Parties shall negotiate in good faith for up to [*] from the date of Amylin’s notice with the goal of arriving at a mutually agreeable definitive agreement. If at the end of the [*] time period the Parties have not negotiated a mutually agreeable term sheet or, if the Parties have negotiated a mutually agreeable term sheet within such period but have not entered into a definitive agreement by the end of the [*] time period, then Amylin shall be free to enter into discussions and an agreement with any Third Party regarding a Third Party Transaction. If Amylin has not consummated a Third Party Transaction within [*] after notifying Amgen of Amylin’s intention to seek a Third Party Transaction, then Amgen’s right of first offer under this Section 2.6 (including the effect of this sentence) shall thereafter apply anew with respect to a potential Third Party Transaction.
2.7 Amylin Right of First Negotiation. If Amgen Controls intellectual property rights in the Neuro Field related to Leptin that are not Licensed Patent Rights and it intends to partner such rights with a Third Party, Amgen will notify Amylin in writing of such partnership opportunity. Beginning on the Effective Date and expiring on the [*] anniversary of the Effective Date, Amgen hereby grants to Amylin a right of first negotiation for any license, partnership, co-development, commercialization, co-promotion or similar agreement related to Leptin, Licensed Products, or such intellectual property rights Controlled by Amgen to the extent they relate to Leptin or the use thereof, in the Neuro Field. Amylin shall notify Amgen in writing whether it elects to exercise its right of first negotiation within [*] of such notice. If Amylin elects not to make an offer or does not respond within such [*] period, Amgen shall be free to enter into discussions and an agreement with any Third Party regarding such partnership opportunity in the Neuro Field, If Amylin elects to make an offer by providing written notice to Amgen within such [*] period, the Parties shall negotiate in good faith for [*] from the date of Amgen’s notice with the goal of arriving at a mutually agreeable term sheet. If at the end of this time period the Parties have negotiated a mutually acceptable term sheet, the Parties shall negotiate in good faith for up to [*] from the date of Amgen’s notice with the goal of arriving at a mutually agreeable definitive agreement. If at the end of the [*] period the Parties have not negotiated a mutually agreeable term sheet or, if the Parties have negotiated a mutually agreeable term sheet within such period but have not entered into a definitive agreement by the end of the [*] time period, then Amgen shall be free to enter into discussions and an agreement with any Third Party regarding such partnership opportunity in the Neuro Field.
-13-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
2.8 License to Commercialize in Neuro Field. If Amgen desires to commercialize a product containing Leptin in the Neuro Field, Amgen shall provide notice to Amylin of its desire to obtain a license under Licensed Patent Rights. Amgen and Amylin agree to use commercially reasonable efforts to negotiate in good faith the terms of a license agreement necessary for Amgen to develop, manufacture and commercialize such product in the Neuro Field.
2.9 Covenant Not to Xxx. Amylin hereby covenants that it and its Affiliates, Sublicensees and assignees shall not xxx Amgen, its Affiliates and assignees for infringement of any Licensed Patent Rights and Licensed Know-How which are exclusively licensed to Amylin hereunder and under which Amylin has the right to xxx infringers, with respect to the noncommercial activities of Amgen, its Affiliates and its assignees using Leptin and Licensed Products in the Territory within the Neuro Field on and after the Effective Date.
2.10 Rockefeller License. Amylin agrees to comply directly with the obligations set forth in the Rockefeller License (including timely paying directly to The Rockefeller University or its successors or assigns all royalties, milestones and other payments payable to The Rockefeller University, or its successors or assigns thereunder, other than payments due under Section 6.9 of the Rockefeller License, which Amgen shall pay out of the amounts it receives under Section 5.1) that arise as a result of the activities of Amylin and its Affiliates and Sublicensees under this Agreement. Amgen agrees to comply directly with obligations of Amgen under the Rockefeller License, if any, other than the obligations which Amylin agrees to comply with directly pursuant to the preceding sentence. Each of Amgen and Amylin will promptly notify the other of any notice of default or breach that it receives from The Rockefeller University (or its successors or assigns) regarding the Rockefeller License. In the event The Rockefeller University notifies Amgen or Amylin of a default or breach under the Rockefeller License related to any failure by Amylin, its Affiliates and/or Sublicensees to perform any obligation or covenant under the Rockefeller License, Amgen shall have the right, but not the obligation, to take such actions and/or make such payments as reasonably necessary or appropriate to cure such default or breach, and Amylin shall promptly reimburse Amgen therefor. Solely to the extent necessary to avoid termination of Amylin’s sublicense under Sections 2.1(c) and 2.3 of this Agreement due to a termination of the Rockefeller License, Amylin shall have the right, but not the obligation, to take such actions and/or make such payments as reasonably necessary or appropriate to cure any default or breach of Amgen’s obligations under the Rockefeller License that Amgen has not cured. Neither party shall terminate, amend or modify or waive any rights under the Rockefeller License without the prior written consent of the other Party, which shall not be unreasonably withheld or delayed. Failure to perform Amylin’s obligations under this Section 2.10 shall constitute a Default under this Agreement. Amgen shall have no liability to Amylin for any termination or modification of the Rockefeller License arising out of or resulting from the failure of Amylin, its Affiliates and/or Sublicensees to abide by, comply with or perform under the terms, conditions or obligations of the Rockefeller License. Notwithstanding anything to the contrary in this Agreement, Amylin agrees that the licenses and rights granted under this Agreement with respect to the Rockefeller Patents, Rockefeller know-how and Rockefeller License are subject to all terms, conditions and obligations under the Rockefeller License. Amgen agrees to take all lawful steps reasonably necessary or requested by Amylin to permit Amylin to exercise and enforce Amgen’s rights under the Rockefeller License to the extent of Amylin’s rights under this Agreement.
-14-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
2.11 UCSF License. Amylin agrees to comply directly with the obligations set forth in the UCSF License (including timely paying directly to The Regents of the University of California or its successors or assigns all milestones, annual license maintenance fees and other payments payable to The Regents of the University of California, or its successors or assigns thereunder) that arise as a result of the activities of Amylin and its Affiliates and Sublicensees under this Agreement. Amgen agrees to comply directly with obligations of Amgen under the UCSF License, if any, other than the obligations which Amylin agrees to comply with directly pursuant to the preceding sentence. Each of Amgen and Amylin will promptly notify the other of any notice of default or breach that it receives from The Regents of the University of California (or its successors or assigns) regarding the UCSF License. In the event UCSF notifies Amgen or Amylin of a default or breach under the UCSF License related to any failure by Amylin, its Affiliates and/or Sublicensees to perform any obligation or covenant under the UCSF License, Amgen shall have the right, but not the obligation, to take such actions and/or make such payments as reasonably necessary or appropriate to cure such default or breach, and Amylin shall promptly reimburse Amgen therefor. Solely to the extent necessary to avoid termination of Amylin’s sublicense under Section 2.1(d) of this Agreement due to a termination of the UCSF License, Amylin shall have the right, but not the obligation, to take such actions and/or make such payments as reasonably necessary or appropriate to cure any default or breach of Amgen’s obligations under the UCSF License that Amgen has not cured. Neither party shall terminate, amend or modify or waive any rights under the UCSF License without the prior written consent of the other Party, which shall not be unreasonably withheld or delayed. Failure to perform Amylin’s obligations under this Section 2.11 shall constitute a Default under this Agreement. Amgen shall have no liability to Amylin for any termination or modification of the UCSF License arising out of or resulting from the failure of Amylin, its Affiliates and/or Sublicensees to abide by, comply with or perform under the terms, conditions or obligations of the UCSF License. Notwithstanding anything to the contrary in this Agreement, Amylin agrees that the licenses and rights granted under this Agreement with respect to the UCSF Patent and UCSF License are subject to all terms, conditions and obligations under the UCSF License. Amgen agrees to take all lawful steps reasonably necessary or requested by Amylin to permit Amylin to exercise and enforce Amgen’s rights under the UCSF License to the extent of Amylin’s rights under this Agreement.
2.12 Additional License. Amgen is party to a License Agreement with The Texas A&M University System dated as of November 29, 1993 (the “Additional License”). If Amgen obtains the right under the Additional License to sublicense to Amylin Amgen’s rights under the current terms of the Additional License, the Parties will enter into an amendment to this Agreement to include the Licensed Patent (as defined in the Additional License) within the Other Licensed Patent Rights on terms consistent with the terms applicable hereunder to sublicensed rights and Other Licensed Patent Rights. Amgen will contact The Texas A&M University System to request the right to grant such sublicense, but Amgen shall have no liability for the failure to obtain the right to grant such sublicense.
-15-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
ARTICLE 3
TRANSFER OF ASSETS; MANUFACTURING
3.1 Transfer of Assets.
(a) Licensed Know-How. As soon as is reasonably practicable after the Effective Date, Amgen will provide to Amylin the Licensed Know-How. The clinical data portion of the Licensed Know-How will be provided to Amylin in computer-readable, SAS transport format, where practicable and available (as specified in Exhibit I), and otherwise in printed format. All other portions of the Licensed Know-How will be provided to Amylin in written form, electronically if reasonably practicable and otherwise in hard copy documents. Data from all clinical trials conducted by or on behalf of Amgen will also be provided in signed clinical study reports. Data from all Ongoing Clinical Trials will also be provided in written reports, summaries or manuscripts where available. If, during the Term, information is identified that is Controlled by Amgen or its Affiliates and was as of the Effective Date, is reasonably necessary for the development, manufacture or commercialization of Leptin, and should have been included in the Licensed Know-How provided under this Section 3.1 but was not previously provided to Amylin pursuant to this Section 3.1, then Amgen will promptly provide such Licensed Know-How to Amylin. Upon Amylin’s reasonable request, Amgen shall provide reasonable consultation services (by teleconference or in-person during regular business hours) in support of Amylin’s regulatory and clinical efforts with respect to A-100 Leptin or A-200 Leptin, for a period of [*] after the Effective Date. Thereafter, for an additional period of [*], as reasonably requested by Amylin, until Regulatory Approval of a Licensed Product for severe lipodystrophy and/or congenital absence of leptin, Amgen will consider in good faith and, if Amgen has expertise and resources available, reasonably provide such assistance. Such consultation services may include, if requested by Amylin, Amgen’s participation in meetings or teleconferences with Amylin, preparation for such meetings or teleconferences, and time spent responding to other questions or requests of Amylin on clinical or regulatory matters. Amylin shall pay Amgen for such services at the FTE Rate in addition to all of Amgen’s reasonable external expenses, including travel, per diem and lodging for meetings and site visits. All such travel expenses will be charged in accordance with Amgen’s then-current travel policy (a true and complete copy of which shall be provided to Amylin upon request). Amgen shall invoice Amylin no more frequently than quarterly for FTE costs and reimbursable expenses incurred under this Section 3.1(a), and Amylin shall pay such invoiced amounts to Amgen within forty-five (45) days of receipt of each such invoice. In addition, Amgen shall also provide, without charge, assistance with the next annual report for X-000 Xxxxxx, X-000 Leptin, and two orphan indication reports to be filed with the FDA following the transfer of the Regulatory Documents to Amylin, including preparation by Amgen of that portion of the annual report describing activities conducted by Amgen. In addition, subject to Amgen’s rights of reference as described below in Section 3.1(b) and Amgen’s other rights pursuant to such section, promptly following the Effective Date, Amgen will submit to the FDA the information necessary to transfer
-16-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
ownership of the Regulatory Documents to Amylin. Immediately after Amgen’s submission of the necessary information, Amylin shall submit to the FDA the information necessary to transfer ownership of the Regulatory Documents to Amylin. As soon as is reasonably practical after the Effective Date, Amgen will assign and transfer (to the extent assignable and transferable) to Amylin, and Amylin will assume from Amgen or its Affiliates, the clinical trial contracts for the Ongoing Clinical Trials as set forth in Exhibit E hereto; provided, however, that Amgen shall remain responsible for all obligations of Amgen under such contracts which became due and payable or were required to be performed on or prior to the date of such assignment to Amylin. Amgen shall make reasonable efforts to complete such assignments within [*] after the Effective Date, and transfer to Amylin any data and results from the Ongoing Clinical Trials that Amgen possesses as of such date. Amylin shall reimburse Amgen for all Ongoing Clinical Trial expenses incurred by Amgen after the Effective Date.
(b) Reserved Rights. Amgen shall have a right to review, a right of access, a right of reference and a right to use and incorporate all Licensed Know-How to satisfy its obligations hereunder.
3.2 Manufacturing.
(a) Manufacturing Right. From and after the Effective Date, Amylin shall have the right and responsibility (subject to the terms of this Section 3.2 and Exhibit H) to manufacture and supply Licensed Products for development and Commercialization in the Territory within the Product Field and to make all decisions with respect thereto in its sole discretion, including without limitation, decisions related to process development work to support quality assurance, improving manufacturing cost/efficiency and commercial scale-up manufacturing.
(b) Transfer of Manufacturing Processes. Amgen shall transfer the Manufacturing Processes to the CMO(s) (as such term is defined in Exhibit H hereto). The rights and obligations of the Parties with respect to such transfer, together with a scope of Amgen’s technology transfer plan, are set forth in Exhibit H, which is incorporated by reference into this Agreement. Upon Amylin’s reasonable request, Amgen shall provide reasonable consultation services (by teleconference or in-person during regular business hours and upon reasonable advance notice) to support such transfer of the Manufacturing Processes, which may include performing activities specified in Exhibit H as requested by Amylin, Amgen’s participation in meetings or teleconferences with Amylin or the CMO, preparation for such meetings or teleconferences, and time spent responding to other questions or requests of Amylin or the CMO on manufacturing or quality-related matters (but shall not include the fill and finish services for the clinical supply of A-100 Leptin as set forth in Section 3.3 below). Amgen shall provide up to [*] [*] of such services to support the transfer under Section 3.1(a) and this Section 3.2(b) without additional payment under this Agreement. Time required beyond [*] shall be paid by Amylin at the FTE Rate. In addition, Amylin shall reimburse Amgen for all reasonable external expenses, including supplies and raw materials to be purchased for the demonstration of the production process, travel, per diem and lodging for meetings and site visits (including expenses incurred during the [*] of support). All such travel expenses will be charged in accordance with Amgen’s then-current travel policy. Amgen shall invoice Amylin no more frequently than quarterly for FTE costs and reimbursable expenses incurred under this Section 3.2(b), and
-17-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Amylin shall pay such invoiced amounts within [*] of receipt of each such invoice. Amgen’s obligation to provide manufacturing assistance or related technical support will expire on the earlier of (i) [*] after the completion of the Drug Substance pilot runs at the designated CMO (as set forth in Exhibit H), or (ii) [*].
3.3 Fill and Finish Services. After the Effective Date, at Amylin’s reasonable request, and subject to Section 3.8, Amgen shall provide fill and finish services for up to a maximum of [*] vials of lyophilized A-100 Leptin per calendar year for [*], beginning January 1, 2006, to support Amylin’s ongoing patient and clinical trial supply obligations. Subject to the prior sentence, for the period from the Effective Date through [*] after the transfer to Amylin of the IND for A-100 Leptin, Amgen shall ship labeled A-100 Leptin vials to the existing sites of Ongoing Clinical Trials and to existing extramural research sites with active contracts. For any new clinical or research sites initiated by Amylin, Amgen will ship unlabelled A-100 Leptin vials to a label facility designated by Amylin for Amylin’s subsequent label and distribution. Beginning [*] after the transfer to Amylin of the IND for A-100 Leptin through the remainder of the period that Amgen is providing fill and finish services, Amgen will be responsible for shipping unlabelled vials of GMP A-100 Leptin to a label facility designated by Amylin; and Amylin shall be responsible for labeling and shipping A-100 Leptin vials to their final destination. Amgen and Amylin will, promptly after the Effective Date, enter into a separate quality agreement regarding such clinical supply of A-100 Leptin to Amylin, which will include, without limitation, provision of a certificate confirming that such A-100 Leptin, both bulk and finished product, was manufactured under current Good Manufacturing Practices (“GMP”). Such A-100 Leptin vials will be filled from Amgen’s existing quantity of bulk A-100 Leptin manufactured and tested according to Good Manufacturing Practices under the FDCA. Amgen shall provide such A-100 Leptin vials throughout each calendar year as generally reflected in a mutually agreed upon Tentative Schedule for Delivery of A-100 Leptin Clinical Drug Product attached as Exhibit J. The schedule attached as Exhibit J shall be subject to manufacturing capacity and availability of GMP drug substance. Each shipment of A-100 Leptin under this Section 3.3 shall be accompanied or preceded by a certificate of analysis confirming that such A-100 Leptin conforms to the specifications set forth in the Regulatory Documents. In addition, Amgen will ship the A-100 Leptin vials to a location specified by Amylin (which will be within the contiguous U.S.). Amylin will compensate Amgen for these fill and finish services at a price of [*] per vial, FCA Amgen’s premises (Incoterms 2000) (it being understood that such price covers all activities related to such supply, and none of such activities shall be considered consultation services under this Agreement). All transportation costs, insurance and packaging materials are additional and the responsibility of Amylin. Amgen is under no obligation to manufacture additional A-100 Leptin, but may perform analytical testing services pursuant to Section 3.4. Amgen shall invoice Amylin no more frequently than quarterly for the cost of fill and finish services for vials supplied under this Section 3.3 plus transportation costs, insurance and packaging, and, except as set forth below in this Section 3.3, Amylin shall pay such invoiced amounts within forty-five (45) days of receipt of each such invoice. Amylin may reject any delivery of A-100 Leptin hereunder which does not comply with the specifications and GMP requirements set forth in the Regulatory Documents. Any such notice of rejection shall be in writing and shall indicate the reasons for such rejection. In order to reject delivery of a shipment
-18-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
of A-100 Leptin, Amylin must give written notice to Amgen of Amylin’s rejection of any delivery within [*] after receipt of such delivery. If no such notice of rejection is received, Amylin shall be deemed to have accepted such delivery of A-100 Leptin within [*] of delivery of the vials. After notice of rejection is given, Amylin shall cooperate with Amgen in determining whether rejection is necessary or justified. Amgen shall notify Amylin as promptly as reasonably possible whether it accepts Amylin’s basis for any rejection. If Amgen in good faith disagrees with Amylin’s determination that certain A-100 Leptin does not meet the specifications or GMP requirements set forth in the Regulatory Documents, such A-100 Leptin shall be submitted to a mutually acceptable Third Party laboratory. Such Third Party laboratory shall determine whether such A-100 Leptin meets the specifications aid GMP requirements set forth in the Regulatory Documents and the Parties agree that such laboratory’s determination shall be final and determinative. The Party whose position is shown to be incorrect as a result of the Third Party test shall bear all costs of the Third Party testing. Whether or not Amgen accepts Amylin’s basis for rejection, promptly on receipt of a notice of rejection of a batch of A-100 Leptin, and solely to the extent that GMP bulk product is available, Amgen shall use reasonable efforts at Amylin’s request to replace such rejected A-100 Leptin. Amylin’s sole remedy and Amgen’s sole liability in the event of a rejection of any A-100 Leptin delivered to Amylin hereunder shall be, at Amgen’s sole discretion and election, either a (i) replacement of such rejected A-100 Leptin; or (ii) refund of monies received from Amylin for such rejected A-100 Leptin. If the Third Party tester rules that the batch meets specifications and GMP requirements set forth in the Regulatory Documents, Amylin shall purchase that batch, irrespective of whether Amgen has already replaced it.
3.4 Analytical Testing Services. Upon Amylin’s reasonable request, until the earlier of (i) [*] after the Effective Date; or (ii) transfer of analytical methods as evidenced by approval of the method transfer reports, Amgen shall provide reasonable analytical testing services, including GMP stability and release testing; provided, however, that with respect to methods that are not being run by Amgen’s Quality Analytical Laboratories as of the Effective Date, such testing shall be solely limited to non-GMP testing. Amgen shall communicate to Amylin such analytical testing results by teleconference or in-person during regular business hours and upon reasonable advance notice and shall provide Amylin a certificate of analysis for GMP analytical testing results. Such analytical testing services, including communications/discussions with Amylin regarding such analytical testing and results, shall be provided at the FTE Rate. In addition, Amylin shall reimburse Amgen for all external expenses, including travel, per diem and lodging for any required off-site meetings. All such travel expenses will be charged in accordance with Amgen’s then-current travel policy. Amgen shall invoice Amylin no more frequently than quarterly for FTE costs and reimbursable expenses incurred under this Section 3.4, and Amylin shall pay such invoiced amounts within forty-five (45) days of receipt of each such invoice.
3.5 Materials. At Amylin’s reasonable request, Amgen shall provide to Amylin the following materials, to the extent such materials are within Amgen’s Control: existing quantities of A-100 Leptin and reference standards of A-100 Leptin, including cell banks, cell paste, intermediates, and purified bulk material, and A-200 Leptin cell banks. Amgen may retain
-19-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
research quantities of such materials for its own purposes consistent with the terms and conditions of this Agreement. Amgen has determined the value of these materials to be [*], which amount is included in the License Fee paid by Amylin. All transportation costs, insurance, and packaging materials are additional and the responsibility of Amylin.
3.6 Communications with Regulatory Authorities. From and after the Effective Date, except as set forth in this Agreement (including Section 3.1(a) for the transfer of the Regulatory Documents), Amylin shall be responsible for all contacts with Regulatory Authorities with respect to Licensed Products in the Territory within the Product Field. At Amylin’s request, Amgen may participate in such regulatory discussions, such participation to be paid for by Amylin at the FTE Rate. In addition, Amylin shall pay all of Amgen’s reasonable external expenses, including travel, per diem and lodging, with respect to such participation. All such travel expenses will be charged in accordance with Amgen’s then-current travel policy. Amgen shall invoice Amylin no more frequently than quarterly for FTE costs and reimbursable expenses incurred under this Section 3.6, and Amylin shall pay such invoiced amounts within forty-five (45) days of receipt of each such invoice.
3.7 U.S. Manufacturing. Amylin understands and acknowledges that, in accordance with the Rockefeller License and the rights sublicensed thereunder, Licensed Products for sale in the U.S. may be required to be manufactured substantially in the U.S., including Puerto Rico, unless a waiver is obtained from NIH with respect thereto and the Rockefeller License so permits. Amgen agrees to reasonably cooperate with Amylin to permit Amylin to seek to obtain the waiver from the NIH as promptly as practicable following the Effective Date.
3.8 Availability of GMP Supplies. The Parties acknowledge and agree that there is a limited supply of existing GMP bulk A-100 Leptin. Furthermore, the Parties acknowledge that the existing supply of GMP bulk A-100 Leptin is on a rolling stability program and may not meet specifications at any given time. To the extent that Amylin’s ongoing patient and clinical supply obligations exceed the available quantity of qualified GMP bulk A-100 Leptin, each Party shall be excused from performance of its obligations under this Agreement that require GMP bulk A-100 Leptin to the extent of such shortfall or degradation, and the Parties shall work together in good faith to allocate the remaining supply of GMP bulk A-100 Leptin based on patient needs, until supply of GMP bulk A-100 Leptin is available to Amylin from the CMO.
3.9 Services. For purposes of clarity, Amgen will provide up to [*], in the aggregate, of support services under Sections 3.1 (other than assistance with the next annual report for X-000 Xxxxxx, X-000 Leptin, and two orphan indication reports as contemplated in Section 3.1(a)), 3.2, 3.4, 3.6 and Exhibit H without payment therefor (other than reimbursable expenses as set forth in such provisions), and Amylin will pay for all time required for support services beyond [*] at the FTE Rate (in addition to the reimbursable expenses).
-20-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
ARTICLE 4
DILIGENCE
4.1 Diligence. Amylin shall use its Commercially Reasonable Efforts to develop, obtain Regulatory Approvals for and, following Regulatory Approval, Commercialize, and protect intellectual property (to the extent Amylin has control over protection of such intellectual property) with respect to, Licensed Products in the Territory within the Product Field during the Term. Additionally, without limiting the foregoing, Amylin shall use Commercially Reasonable Efforts to complete the filing of an NDA for a Licensed Product in a Major Market Country within forty-two (42) months of the Effective Date. If Amylin, its Affiliates and Sublicensees fail to satisfy the obligations under this Section 4.1 of this Agreement, such failure shall be deemed a Default.
4.2 Continuing Supply for Ongoing Clinical Trials. Amylin shall use commercially reasonable efforts to continue to supply to Xxxxxxxxx Xxxxx Xxxxx at Kyoto University and the NIH suitable clinical grade A-100 Leptin for human studies, at current planned supply levels, based upon a forecast provided by Amgen to Amylin, and subject to final regulatory approvals necessary for Amylin to provide such supplies, until December 31, 2006.
4.3 Negotiation of Japanese License. Amylin shall discuss in good faith the possibility of a license agreement with the potential licensee in Japan with whom Amgen has been in active discussions prior to the Effective Date for the development and commercialization of Licensed Products in Japan.
4.4 Reports. Within thirty (30) days after the end of each calendar year, Amylin shall provide to Amgen a written annual report concerning its efforts regarding development and Commercialization of the Licensed Products in the Territory as carried out during the prior twelve (12) months and as planned for the next twelve (12) months, which annual report shall include a summary of Amylin pre-clinical and development activities, the status of Amylin clinical trials and the then current schedule for clinical trials and for filing regulatory applications in each country, and the status of other approvals necessary to manufacture and market Licensed Products, including pricing and reimbursement approvals. Amylin shall also provide prompt written notice to Amgen of (i) any Regulatory Approval received for any Licensed Product in any country and (ii) the Launch Date for each Licensed Product in each country. The information contained in such reports and notices shall be deemed to be Amylin’s Confidential Information.
ARTICLE 5
CONSIDERATION; PAYMENTS; REPORTS
5.1 License Fee. Amylin shall pay to Amgen, within thirty (30) days after the Effective Date, [*], which includes the allocation of a portion of such payment to the materials transferred to Amylin pursuant to Section 3.5 (“License Fee”), The payment to Amgen shall be made in cash by wire transfer of immediately available funds into an account designated in writing by Amgen. The License Fee shall be nonrefundable and noncreditable against any milestones or other fees or payments due Amgen under this Agreement.
-21-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
5.2 Milestone Payment.
(a) Amylin shall pay to Amgen [*] upon successful completion of the [*] (as that term is defined in Exhibit H attached hereto), as demonstrated by either: [*]
(b) If the milestone payment under Section 5.2(a) has not already been paid to Amgen, [*], Amylin shall pay to Amgen [*] subject to a maximum cumulative payment of [*], which payments shall be payable within thirty (30) days after the end of each such month and shall be creditable against [*]. Notwithstanding the foregoing sentence, Amylin shall not be obligated to make any such [*] monthly payments for any month prior to the time that the NIH waiver described in Section 3.7 is granted.
(c) Amylin shall use good faith commercially reasonable efforts to achieve, or facilitate and cause the achievement of, the milestone event set forth in Section 5.2(a). This milestone payment shall be nonrefundable and noncreditable against any other fees or payments due Amgen under this Agreement. The Party first aware of the completion of the milestone event set forth in Section 5.2(a) shall promptly notify the other Party in writing thereof and Amylin shall pay to Amgen the milestone payment described in Section 5.2(a) within thirty (30) days following occurrence of the milestone event.
5.3 Royalties.
(a) Amylin shall pay royalties to Amgen on a country-by-country basis in the Territory on annual Net Sales of each Licensed Product that is claimed by a Valid Claim or has market exclusivity granted by a Regulatory Authority at the time of Regulatory Approval in the applicable country during the applicable Royalty Term at a rate of [*] percent ([*]%) (as may be reduced under Section 5.3(c)), to be paid within forty-five (45) days of the end of each calendar quarter for the preceding calendar quarter’s Net Sales.
(b) If, as of the time a Licensed Product receives Regulatory Approval in a country, such Licensed Product is not claimed by a Valid Claim in such country and does not have market exclusivity granted from a Regulatory Authority in such country with respect to such Licensed Product, then
(i) if a Valid Claim claiming such Licensed Product issues in such country or the Licensed Product is granted market exclusivity from a Regulatory Authority in such country within [*] after the date such Licensed Product received Regulatory Approval in such country, (A) Amylin shall pay to Amgen royalties on Net Sales of such Licensed Product in such country for the period from the Launch Date for such Licensed Product in such country through the date that such Valid Claim that claims such Licensed Product in such country issues or market exclusivity for such Licensed Product is granted by a Regulatory Authority in such country at a rate of [*] percent ([*]%) (as may be reduced under Section 5.3(c)), to be paid
-22-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
within forty-five (45) days of the date that such Valid Claim that claims such Licensed Product in such country issues or market exclusivity for such Licensed Product is granted by a Regulatory Authority in such country; and (B) Amylin shall pay royalties to Amgen on Net Sales of such Licensed Product in such country from the date such Valid Claim that claims such Licensed Product in such country issues or market exclusivity for such Licensed Product is granted by a Regulatory Authority in such country during the remaining Royalty Term for such Licensed Product at a rate of [*] percent ([*]%) [*], to be paid within forty-five (45) days of the end of each calendar quarter for the preceding calendar quarter’s Net Sales;
(ii) if clause (i) above does not apply and a Valid Claim claiming such Licensed Product issues in such country or the Licensed Product is granted market exclusivity from a Regulatory Authority in such country after the date such Licensed Product received Regulatory Approval in such country, (A) Amylin shall pay to Amgen royalties on Net Sales of such Licensed Product in such country for the period from the Launch Date for such Licensed Product in such country through the date that such Valid Claim that claims such Licensed Product in such country issues or market exclusivity for such Licensed Product is granted by a Regulatory Authority in such country at a rate of [*] percent ([*]%), to be paid within forty-five (45) days of the date that such Valid Claim that claims such Licensed Product in such country issues or market exclusivity for such Licensed Product is granted by a Regulatory Authority in such country; and (B) Amylin shall pay royalties to Amgen on Net Sales of such Licensed Product in such country from the date such Valid Claim that claims such Licensed Product in such country issues or market exclusivity for such Licensed Product is granted by a Regulatory Authority in such country during the remaining Royalty Term for such Licensed Product at a rate of [*] percent ([*]%) (as may be reduced under Section 5.3(c)), to be paid within forty-five (45) days of the end of each calendar quarter for the preceding calendar quarter’s Net Sales; and
(iii) if neither clause (i) nor clause (ii) above apply and a Valid Claim claiming such Licensed Product issues in such country or the Licensed Product is granted market exclusivity from a Regulatory Authority in such country after [*] after the date such Licensed Product received Regulatory Approval in such country, Amylin shall pay royalties to Amgen on Net Sales of such Licensed Product in such country from the date such Valid Claim that claims such Licensed Product in such country issues or market exclusivity for such Licensed Product is granted by a Regulatory Authority in such country during the remaining Royalty Term for such Licensed Product at a rate of [*] percent ([*]%) (as may be reduced under Section 5.3(c)), to be paid within forty-five (45) days of the end of each calendar quarter for the preceding calendar quarter’s Net Sales.
(c) Following both (i) the Generic Date in a country; and (ii) if the Licensed Product is claimed by a Valid Claim in such country, expiration of the last-to-expire Valid Claim covering the applicable Licensed Product in such country, the royalty rate for purposes of calculating royalties payable to Amgen on Net Sales of such Licensed Product in such country under this Section 5.3 during the remainder of the applicable Royalty Term shall be reduced to a rate of [*] percent ([*]%).
-23-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
5.4 Third Party Royalties. If Amylin is required to pay royalties to one or more Third Parties greater than [*]% cumulatively (on an annual basis) on annual Net Sales of Licensed Products in any country (other than royalties due under the Rockefeller License) for a patent license required, in Amylin’s reasonable judgment, in order to sell such Licensed Products, [*]% of such royalties (meaning the royalties in excess of [*]%) actually paid by Amylin to such Third Parties (net of any offsets against royalties payable to such Third Party(ies), including The Rockefeller University) is creditable against royalties due to Amgen for such period, provided that the royalties due to Amgen in any calendar quarter shall not be reduced by more than [*]%. Such royalties in excess of [*]% of Net Sales cumulatively due to such Third Parties for a patent license in a particular country or territory shall be creditable only against the royalties due to Amgen hereunder on Net Sales in such country or territory. For the avoidance of doubt, royalties paid to a Third Party for a patent license as set forth in this Section 5.4 would qualify for this deduction only to the extent required and paid for Commercializing Licensed Products, and royalties required for access to broad technology platforms would not qualify.
5.5 Payments; Reports. Payment of all sums due hereunder shall be made to Amgen by wire transfer, or electronic funds transfer (EFT), in accordance with payment transfer instructions to be provided by Amgen. Beginning with the calendar quarter in which the Launch Date of the first Licensed Product occurs until the expiration of Amylin’s obligation to pay royalties, royalty payments and reports of the sale of Licensed Products for each calendar quarter will be calculated and delivered to Amgen under this Agreement within forty five (45) days of the end of each such calendar quarter, unless otherwise specifically provided herein. Each payment of royalties shall be accompanied by a report of Net Sales of Licensed Products in sufficient detail to permit confirmation of the accuracy of the royalty payment made, including and on a country-by-country basis, the number of Licensed Products sold, the gross sales and Net Sales of Licensed Products and deductions taken from gross sales by category as set forth in the definition of Net Sales to arrive at the Net Sales calculation, the royalties payable (in Dollars), the method used to calculate the royalty and the exchange rates used. The total royalty due for the sale of Licensed Products during such calendar quarter shall be paid at the time such report is made. Amylin will keep complete and accurate records pertaining to the sale or other disposition of Licensed Products in sufficient detail to permit Amgen to confirm the accuracy of all payments due hereunder.
5.6 Exchange Rate. With respect to Net Sales invoiced or expenses incurred in Dollars, the Net Sales or expense due to Amgen hereunder shall be expressed in Dollars. With respect to Net Sales invoiced or expenses incurred in a currency other than Dollars, the Net Sales invoiced or expenses incurred shall be converted into the Dollar equivalent using a rate of exchange which corresponds to the rate used by the invoicing or incurring Party, for the respective reporting period, related to recording such Net Sales or expenses in its books and records that are maintained in accordance with GAAP. All payments shall be made in Dollars. If at any time legal restrictions in any country in the Territory prevent the prompt remittance of any payments with respect to sales in that country, Amylin shall have the right and option upon written notice to Amgen to make such payments by depositing the amount thereof in local currency to Amgen’s account (or such other designated nominee by Amgen) in a bank or depository in such country.
-24-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
5.7 Late Payments. Any undisputed amounts not paid by Amylin when due under this Agreement or disputed amounts that become subsequently due upon resolution of such dispute which are not paid by Amylin within fifteen (15) days of such resolution, shall be subject to interest from and including the date payment is due through and including the date upon which Amylin has made a wire transfer of immediately available funds into an account designated by Amgen, at a rate equal to LIBOR (London InterBank Offer Rate) plus [*] percent ([*]%).
5.8 Taxes. In the event that laws, rules or regulations require Amylin to withhold Taxes with respect to any payment to be made by Amylin pursuant to this Agreement, Amylin will notify Amgen of such withholding requirement prior to making the payment to Amgen and provide such assistance to Amgen, including the provision of such documentation as may be required by a tax authority, as may be reasonably necessary in Amgen’s efforts to claim an exemption from or reduction of such taxes. Amylin will, in accordance with such laws, rules or regulations, withhold taxes from the amount due, and remit such taxes to the appropriate tax authority. Amylin shall provide to Amgen original copies of all official receipts evidencing such tax obligation together with written evidence of payment within fifteen (15) days following such payment. If taxes are paid to a tax authority, Amylin shall provide reasonable assistance to Amgen to obtain a refund of taxes withheld, or obtain a credit with respect to taxes paid.
5.9 Audit. Amylin shall keep complete and accurate records of the underlying revenue and expense data relating to the calculations of Net Sales and payments required under this Agreement. Amgen shall have the right, at its own expense and no more than once per year, to have an independent, certified public accountant, selected by Amgen and reasonably acceptable to Amylin, review such records of Amylin upon reasonable notice (which shall be no less than thirty (30) days prior written notice) and during regular business hours and under obligations of strict confidence, for the sole purpose of verifying the basis and accuracy of payments required and made under this Agreement within the prior thirty-six (36) month period; provided that, if royalties are owed for retroactive periods pursuant to Section 5.3(b)(i) or (ii), then for a thirty-six (36) month period after the date such royalties first become due under Section 5.3(b)(i) or (ii), Amgen shall have the right to audit records related to the period of the Royalty Term prior to such date. No calendar year may be audited more than one time. Notwithstanding the foregoing, in the event that Amylin restates its earnings, and such restatement would impact the royalty due to Amgen for any period(s) previously audited, or Amylin revises a report or makes a further payment for a period for which a report or payment was previously provided or due to Amgen under Section 5.5, which report or payment reflects a material change in the amount of royalties due for the prior period and Amgen has previously audited such period, then Amgen shall have the right to re-audit the affected time period(s) solely with respect to verifying the effect, if any, such restatement or revision has on royalties due with respect to such period(s). Amylin shall receive a copy of each audit report promptly from Amgen. Should the inspection lead to the discovery of a discrepancy to Amgen’s detriment, Amylin shall pay the amount of the discrepancy within thirty (30) days after being notified
-25-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
thereof. Amgen shall pay the full cost of the inspection unless the discrepancy is greater than [*] percent ([*]%), in which case Amylin shall pay to Amgen the reasonable cost charged by such accountant for such inspection.
ARTICLE 6
INTELLECTUAL PROPERTY
6.1 Prosecution and Maintenance.
(a) Amylin shall be responsible (either in-house or using mutually acceptable outside counsel) for the filing, prosecution, defense and maintenance of the Leptin Patent Rights before all patent authorities in the Territory, including oppositions and interferences. If Amylin determines in its sole discretion not to file, prosecute, defend or maintain any claim or patent application or patent within Leptin Patent Rights in any country, then Amylin shall provide Amgen with thirty (30) days prior written notice of such determination to provide Amgen with the right and opportunity to file, prosecute, defend and maintain such claim or patent application or patent.
(b) Amylin shall have the right to direct the filing, prosecution, defense and maintenance of the Rockefeller Patents only to the extent Amgen has such rights under the Rockefeller License. Prior to abandoning any patent prosecution or maintenance with respect to any Rockefeller Patents, Amylin shall notify Amgen thereof and Amgen shall thereafter have the right to exercise all of Amgen’s rights under the Rockefeller License with respect to the filing, prosecution, defense and maintenance of such Rockefeller Patent(s), including the abandonment thereof.
(c) Amylin shall be responsible (either in-house or using mutually acceptable outside counsel) for the filing, prosecution, defense and maintenance of the Licensed Trademarks before all trademark authorities in the Territory, including oppositions. If Amylin determines in its sole discretion not to file, prosecute, defend or maintain any of the Licensed Trademarks in any country, then Amylin shall provide Amgen with thirty (30) days prior written notice of such determination to provide Amgen with the right and opportunity to file, prosecute, defend and maintain such Licensed Trademarks.
(d) Each Party shall designate a lead patent counsel, either in-house or at a mutually agreeable outside law firm, or, in the case of Amgen, a lead patent agent to be the patent contact person for all ongoing patent matters hereunder between Amgen or Amylin. A Party may change such patent contact person from time to time by written notice delivered to the other Party.
(e) All costs associated with the filing, prosecution, maintenance and defense of Leptin Patent Rights, Rockefeller Patents and Licensed Trademarks under this Section 6.1, including the costs and expenses of mutually acceptable outside counsel, shall be borne by Amylin. As between the Parties, Amgen will pay all costs associated with the filing, prosecution and maintenance of Leptin Patent Rights, Rockefeller Patents and Licensed Trademarks that have accrued and become due and payable prior to the Effective Date.
-26-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
6.2 Infringement by Third Parties.
(a) Amylin shall have the first right, but not the obligation, at its own expense, to enforce Leptin Patent Rights against Third Parties and to defend the Leptin Patent Rights against any challenges in the Territory within the Product Field. In the event Amylin shall so elect, Amylin shall control any such action; provided that, Amgen shall, at its own expense, be entitled to participate in, and to have counsel selected by Amgen participate in, such action. Recoveries in any actions under this Section 6.2(a) shall be used first to reimburse the Parties’ costs and expenses (including attorneys’ fees) for such action (on an equal basis) and any remainder shall belong to Amylin, except that Amgen shall receive out of any such remaining recovery received by Amylin an amount as follows: (i) as to ordinary damages, Amgen shall receive payment equivalent to payments that would have been due to Amgen under this Agreement had the infringing sales that Amylin lost to the infringer been made by Amylin and (ii) as to special or punitive damages, such amount shall be allocated between the Parties in the same proportion as ordinary damages under clause (i); and provided further that the amounts paid under (i) and (ii) shall not exceed 50% of the total recovery of Amylin from such action or proceeding.
(b) Amylin shall have the right to enforce and defend the Rockefeller Patents only to the extent of Amgen’s rights under the Rockefeller License with respect thereto.
(c) In the event Amylin does not commence an enforcement and/or defense action pursuant to Section 6.2(a) or (b) within sixty (60) days after Amylin first notifies Amgen or Amgen first notifies Amylin of potential infringement of the Leptin Patent Rights or the Rockefeller Patents in the Territory (or of the filing of a declaratory judgment action, in the case of defense actions), Amgen shall be entitled to bring and prosecute such an action at its own expense and to retain one hundred percent (100%) of any recoveries in such action, unless Amylin participates, Amylin shall, at its own expense, be entitled to participate in, and to have counsel selected by Amylin participate in, such action. Recoveries in any actions in which Amylin participates under this Section 6.2(c) shall be used first to reimburse the Parties’ costs and expenses (including attorneys’ fees) for such action (on an equal basis) and any remainder shall belong to Amgen.
(d) Each Party shall promptly notify the other Party upon becoming aware of any potential Third Party infringement of the Licensed Patent Rights.
(e) Neither Party shall enter into any settlement of any action under this Section 6.2 that affects the other Party’s rights or interests under this Agreement without such other Party’s written consent, which consent shall not be unreasonably withheld or delayed.
6.3 Defense. Each Party shall promptly notify the other Party upon receiving written notice of any potential infringement, or any Third Party claim or action against Amgen or
-27-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Amylin or any of their Affiliates or Sublicensees for possible infringement, of a Third Party patent right resulting from the development or Commercialization of Licensed Product. Subject to the indemnification and defense obligations of the Parties under Article 8, each Party shall be responsible for defending, and shall control the defense of, any such action brought against such Party. The Parties shall confer with each other and cooperate in the defense of any such action in which both Amgen and Amylin are named parties. Neither Party shall enter into any settlement of any action under this Section 6.3 that affects the other Party’s rights or interests under this Agreement without such other Party’s written consent, which consent shall not be unreasonably withheld or delayed. Notwithstanding the foregoing, nothing in this Section 6.3 shall obligate either Party to defend against any action referenced in this Section 6.3. In addition, in connection with any such defense by Amylin, it shall confer and cooperate with The Rockefeller University with respect thereto, and comply with such other obligations, as required under the Rockefeller License.
6.4 Other Licensed Patent Rights. Amgen shall be solely responsible for, and have complete discretion in controlling and making decisions with respect to, filing, prosecution, defense and maintenance of the Other Licensed Patent Rights before all patent authorities in the Territory, including but not limited to oppositions and interferences, and enforcement of the Other Licensed Patent Rights. Amgen shall provide to Amylin on an annual basis a summary of the status of the Other Licensed Patent Rights. Amylin may request enforcement of Other Licensed Patent Rights, and Amgen will consider and determine, in its sole discretion, whether to allow Amylin to enforce such patent rights.
6.5 Cooperation. Each Party agrees to reasonably cooperate with the other Party in the filing, prosecution, maintenance, defense and enforcement of Leptin Patent Rights, Rockefeller Patents and Other Licensed Patent Rights, as set forth in this Article 6, including joining an action or proceeding if reasonably requested, signing any necessary legal papers, and providing the other Party with data or other information reasonably requested in support thereof. Each Party shall keep the other Party reasonably informed of the substantive developments with respect to any enforcement or defensive actions under this Article 6 regarding Leptin Patent Rights.
6.6 Marking. All Licensed Products shall be marked with the patent numbers of issued patents within Licensed Patent Rights that cover such Licensed Products, to the extent permitted by law in countries in which such markings have notice value against infringers of patents.
ARTICLE 7
REPRESENTATIONS, WARRANTIES AND COVENANTS
7.1 Representations and Warranties of Amgen. As of the Effective Date, Amgen hereby represents and warrants to Amylin as follows:
-28-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
(a) Corporate Existence and Power. Amgen is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware, and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted.
(b) Authority and Binding Agreement. Amgen has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder. Amgen has taken all necessary corporate action on its part required to authorize the execution and delivery of the Agreement and the performance of its obligations hereunder. The Agreement has been duly executed and delivered by Amgen and constitutes a legal, valid and binding obligation of Amgen that is enforceable against it in accordance with its terms.
(c) No Conflict. The execution, delivery and performance of this Agreement by Amgen does not conflict with, and would not result in a breach of, any material agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, nor violate any material law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
(d) Patent Rights. Amgen has the right to grant the licenses under the Licensed Patent Rights granted hereunder and has not assigned, transferred, conveyed or licensed its right, title and interest in the Licensed Patent Rights in a manner inconsistent with the terms of this Agreement. The Licensed Patent Rights constitute all patents and patent applications Controlled by Amgen or its Affiliate as of the Effective Date that claim the composition of matter or a method of use of, or that specifically claim a method of manufacturing, A-100 Leptin or A-200 Leptin. There is no pending litigation or, to Amgen’s knowledge, written threat of litigation that has been received by Amgen (and has not been resolved by taking a license or otherwise), which alleges that Amgen’s activities with respect to the Leptin Patent Rights or A-100 Leptin or A-200 Leptin have infringed or misappropriated any of the intellectual property rights of any Third Party.
(e) Regulatory Matters. The Regulatory Documents constitute all regulatory filings Controlled by Amgen or its Affiliates with respect to A-100 Leptin and A-200 Leptin in any regulatory jurisdiction in the Territory. Amgen has not used any employee or consultant who is or has been debarred by any Regulatory Authority or, to the best of Amgen’s knowledge, is or has been the subject of debarment proceedings by any Regulatory Authority in the course of development of Leptin or any Licensed Product.
(f) Ongoing Clinical Trials. The Ongoing Clinical Trials constitute all ongoing clinical trials of A-100 Leptin in which Amgen is involved. Amgen will provide to Amylin true and complete copies of each such Ongoing Clinical Trial contract, including any and all amendments thereof.
(g) Licenses. The Rockefeller License and UCSF License attached hereto as Exhibit F and Exhibit G, respectively, are true and complete copies of those agreements between Amgen and The Rockefeller University and Amgen and The Regents of the University of
-29-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
California, respectively. Each of the Rockefeller License and the UCSF License is in full force and effect as of the Effective Date, and Amgen is not aware of any material breach of the Rockefeller License or UCSF License that would be expected to result in the termination of the Rockefeller License or UCSF License, respectively.
(h) Disclaimer. EXCEPT AS EXPRESSLY SET FORTH IN THIS SECTION 7.1, ALL MATERIALS AND INFORMATION PROVIDED HEREUNDER ARE BEING PROVIDED “AS IS” AND WITHOUT ANY REPRESENTATIONS OR WARRANTIES. EXCEPT AS EXPRESSLY SET FORTH IN THIS SECTION 7.1, AMGEN MAKES NO REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, OF ANY KIND, INCLUDING AS TO MERCHANTABILITY, FITNESS FOR A PARTICULAR USE OR NON-INFRINGEMENT. SPECIFICALLY, AMGEN DOES NOT WARRANT THE VALIDITY OR ENFORCEABILITY OF THE LICENSED PATENT RIGHTS, OR LICENSED TRADEMARKS, AND MAKES NO REPRESENTATIONS WHATSOEVER WITH REGARD TO THE SCOPE OF THE LICENSED PATENT RIGHTS OR LICENSED TRADEMARKS, OR THAT THE LICENSED PATENT RIGHTS, LICENSED KNOW-HOW OR LICENSED TRADEMARKS MAY BE EXPLOITED WITHOUT INFRINGING OTHER PATENTS OR OTHER INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES.
7.2 Representations and Warranties of Amylin. As of the Effective Date, Amylin hereby represents and warrants to Amgen as follows:
(a) Corporate Existence and Power. Amylin is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware, and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted.
(b) Authority and Binding Agreement. Amylin has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder. Amylin has taken all necessary corporate action on its part required to authorize the execution and delivery of the Agreement and the performance of its obligations hereunder. The Agreement has been duly executed and delivered by Amylin and constitutes a legal, valid and binding obligation of Amylin that is enforceable against it in accordance with its terms.
(c) No Conflict. The execution, delivery and performance of this Agreement by Amylin does not conflict with, and would not result in a breach of, any material agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, nor violate any material law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
(d) Disclaimer. EXCEPT AS EXPRESSLY SET FORTH IN THIS SECTION 7.2, AMYLIN MAKES NO REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, OF ANY KIND.
-30-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
7.3 Mutual Covenant. Each Party hereby covenants to the other Party that it will not enter into any agreement with any Third Party that is in conflict with this Agreement, and will not take any action that would in any way prevent it from performing its obligations under this Agreement, or that would otherwise materially conflict with or adversely affect the performance of its obligations under this Agreement.
7.4 Additional Covenants.
(a) No Misappropriation or Infringement. Amylin covenants to Amgen that Amylin shall not knowingly misappropriate or infringe any trade secret, patent or other intellectual property of another party in its activities to develop, manufacture or Commercialize Licensed Products.
(b) No Debarment. Amylin covenants to Amgen that, in the course of the development and Commercialization of Licensed Products during the Term, Amylin shall not knowingly use any employee or consultant who is or has been debarred by any Regulatory Authority or, to the best of Amylin’s knowledge, is or has been the subject of debarment proceedings by any Regulatory Authority. Amgen covenants to Amylin that, in the course of the supply and testing activities under Sections 3.3 and 3.4, Amgen shall not knowingly use any employee or consultant who is or has been debarred by any Regulatory Authority or, to the best of Amgen’s knowledge, is or has been the subject of debarment proceedings by any Regulatory Authority.
(c) Compliance with Applicable Law. Amylin covenants to comply with all statutes and regulations (including statutes, regulations and guidance of Regulatory Authorities) applicable to its activities under this Agreement.
(d) Compliance with Trademark Specifications. Amylin covenants to maintain such quality standards as are consistent with the manner in which Amylin maintains its own trademarks in any use of the Licensed Trademarks, but in no event less than reasonable quality standards, and as are necessary or appropriate to maintain the value of the Licensed Trademarks, including not using or displaying the Licensed Trademarks in any manner that might dilute, tarnish, disparage or reflect adversely on Amgen, its Affiliates or the Licensed Trademarks. During the Term, Amylin shall provide, at the request of Amgen, representative samples of items bearing the Licensed Trademarks (including Licensed Product brochures, advertising and other promotional literature).
ARTICLE 8
INDEMNIFICATION
8.1 Indemnification by Amgen. Amgen hereby agrees to defend, hold harmless and indemnify Amylin and its Affiliates, and each of their respective officers, directors and employees (collectively, the “Amylin Indemnitees”), from and against any and all Losses arising out of (i) any of Amgen’s representations and warranties set forth in Section 7.1 of this
-31-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Agreement being untrue in any material respect when made; (ii) Amgen’s failure to perform, in any material respect, any covenant or agreement of Amgen set forth in this Agreement; and (iii) Amgen’s gross negligence or willful misconduct; except, in each case, to the extent any such Losses result from the gross negligence or willful misconduct of Amylin Indemnitees or from the breach of any representation or warranty hereunder or of this Agreement by Amylin.
8.2 Indemnification by Amylin. Amylin hereby agrees to defend, hold harmless and indemnify Amgen and its Affiliates, and each of their respective officers, directors and employees (collectively, the “Amgen Indemnitees”), from and against any and all Losses arising out of (i) any of Amylin’s representations and warranties set forth in Section 7.2 of this Agreement being untrue in any material respect when made; (ii) Amylin’s failure to perform, in any material respect, any covenant or agreement of Amylin set forth in this Agreement; (iii) the practice by Amylin, its Affiliates or Sublicensees of the licenses granted to Amylin under Sections 2.1, 2.2 and 2.3; and (iv) the development, manufacture or Commercialization of Leptin and/or any Licensed Product by or for Amylin, its Affiliates or Sublicensees; except, in each case, to the extent any such Losses result from the gross negligence or willful misconduct of Amgen Indemnitees or from the breach of any representation or warranty hereunder or of this Agreement by Amgen.
8.3 Indemnification Procedures. Each Party (Amgen on behalf of Amgen Indemnitees, or Amylin on behalf of Amylin Indemnitees) will promptly notify the other Party when it becomes aware of a claim for which indemnification may be sought hereunder (a “Claim”). To be eligible to be indemnified for a Claim, a Person seeking indemnification (the “Indemnified Party”) shall (i) provide the Party required to indemnify such Person (the “Indemnifying Party”) with prompt written notice of the Claim giving rise to the indemnification obligation under this Article 8, provided that, the failure to provide such prompt notice shall not relieve the Indemnifying Party of any of its obligations under this Article 8 except to the extent the Indemnifying Party is actually prejudiced thereby; (ii) provide the Indemnifying Party with the exclusive ability to defend (with the reasonable cooperation of the Indemnified Party) against the Claim; and (iii) not settle, admit or materially prejudice the Claim, without the Indemnifying Party’s prior written consent. The Indemnified Party shall reasonably cooperate with the Indemnifying Party, at the Indemnifying Party’s expense, in the defense of any Claim. Notwithstanding the foregoing, the Indemnified Party shall have the right to participate in and have its own counsel participate in any action or proceeding for which the Indemnified Party seeks to be indemnified by the Indemnifying Party. Such participation shall be at the Indemnified Party’s expense, unless (i) the Indemnifying Party and the Indemnified Party shall have mutually agreed to the retention of such counsel or (ii) the named parties to any such proceeding (including any impleaded parties) include both the Indemnifying Party and the Indemnified Party and representation of both parties by the same counsel would be inappropriate due to actual or potential differing interests between them. The Indemnifying Party’s obligations under Section 8.1 or 8.2, as the case may be, shall not apply to the extent of the Indemnified Party’s failure to take reasonable action to mitigate any Losses. The Indemnifying Party shall not settle or compromise, or consent to the entry of any judgment with respect to, any Claim, without the prior written consent of the Indemnified Party, which will not be unreasonably withheld or delayed.
-32-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
8.4 Insurance. Amylin shall, at its own expense, procure and maintain during the Term and for a period of five (5) years thereafter, insurance policy/policies, including product liability insurance, adequate to cover its obligations hereunder and which are consistent with normal business practices of prudent companies similarly situated. Such insurance shall not be construed to create a limit of Amylin’s liability with respect to its indemnification obligations under this Article 8. Amylin shall provide Amgen with written evidence of such insurance upon request. Amylin shall provide Amgen with prompt written notice of cancellation, non-renewal or material change in such insurance or self-insurance which could materially adversely affect the rights of Amgen hereunder and shall use commercially reasonable efforts to provide such notice at least thirty (30) days prior to any such cancellation, non-renewal or material change. Amylin’s insurance hereunder shall be primary with respect to the obligations for which Amylin is liable hereunder and Amgen’s insurance shall be non-contributing with respect to the obligations for which Amgen is to be indemnified by Amylin hereunder. Amgen’s insurance hereunder shall be primary with respect to the obligations for which Amgen is liable hereunder and Amylin’s insurance shall be non-contributing with respect to the obligations for which Amylin is to be indemnified by Amgen hereunder.
8.5 Limitation of Liability. EXCEPT FOR LIABILITY FOR BREACH OF OBLIGATIONS UNDER ARTICLE 9 OR FRAUD OR COMPARABLE INTENTIONAL MISCONDUCT, NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE OTHER PARTY ANY INDIRECT, SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES IN CONNECTION WITH THIS AGREEMENT OR ANY LICENSE GRANTED HEREUNDER. The limitations set forth in this Section 8.5 shall not apply with respect to either Party’s indemnification obligations under Sections 8.1 or 8.2 for Third Party Claims.
ARTICLE 9
CONFIDENTIALITY
9.1 Treatment of Confidential Information. The Parties agree that during the Term, and for a period of five (5) years after this Agreement expires or terminates, a Party receiving Confidential Information of the other Party shall (i) maintain in confidence such Confidential Information; (ii) not disclose such Confidential Information to any Third Party without prior written consent of the disclosing Party, except for disclosures to its Sublicensees who agree to be bound by obligations of non-disclosure and non-use at least as stringent as those contained in this Article 9 and disclosures permitted under Section 9.3; and (iii) not use such Confidential Information for any purpose other than the performance of this Agreement.
9.2 Treatment of Manufacturing Information. In addition to the other provisions herein, Amylin recognizes that maintaining the confidentiality and trade secret nature of the Manufacturing Information requires a higher level of vigilance than other Confidential
-33-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Information, and agrees to (i) maintain in confidence Manufacturing Information with the same degree of care that Amylin uses to protect its own like information, (ii) strictly limit access to and use of Manufacturing Information to employees, representatives, consultants and contractors of Amylin and the CMO(s) with a need to know such information, and (iii) use Manufacturing Information and trade secrets only for producing Licensed Product(s) in the Product Field, Amylin shall ensure that any Person having access to the Manufacturing Information will be made aware of its highly confidential nature and will agree to be bound by confidentiality terms no less stringent than those in this Agreement. Amgen agrees to maintain in confidence Manufacturing Information as it relates to A-100 Leptin and A-200 Leptin with the same degree of care that Amgen uses to protect its own like information. The obligations under this Section 9.2 shall survive and continue in effect for a period of twenty (20) years following any expiration or termination of this Agreement. Each of Amgen and Amylin acknowledge and agree that Confidential Information disclosed to the CMO selected hereunder, and any Confidential Information of the other Party received from such CMO, shall not cause such Confidential Information to fall within any exceptions within the definition of Confidential Information herein or otherwise cease to be Confidential Information of the applicable Party.
9.3 Authorized Disclosure. If, based upon the advice of legal counsel skilled in the subject matter, a Party is required to disclose Confidential Information of the other Party to comply with an applicable law, regulation, legal process, or order of a government authority or court of competent jurisdiction, the Party may disclose such Confidential Information only to the Person required to receive such disclosure; provided, however, that the Party required to disclose such Confidential Information shall (a) to the extent permitted by such law, regulation, process, order or rules, first have given prompt (but in no event less than five (5) business days) advance notice to such other Party to enable it to seek any available exemptions from or limitations on such disclosure requirement and shall reasonably cooperate in such efforts by the other Party, (b) furnish only the portion of the Confidential Information which is legally required; (c) use all reasonable efforts to secure confidential protection of such Confidential Information, and (d) continue to perform its obligations of confidentiality set out herein. Each Party may disclose Confidential Information of the other Party to Regulatory Authorities to the extent such disclosure is reasonably necessary in regulatory filings required for the development and/or commercialization of Licensed Products. In addition, each Party may disclose Confidential Information of the other Party (other than Manufacturing Information) to the extent such disclosure is reasonably necessary in the following instances: filing or prosecuting patents as permitted by this Agreement; disclosure to The Rockefeller University and The Regents of the University of California to the extent necessary to fulfill obligations under the Rockefeller License and UCSF License, respectively, in accordance with this Agreement and the Rockefeller License or the UCSF License, as applicable; and disclosure to Sublicensees and potential Sublicensees, contractors, employees and consultants who need to know such information for the development, manufacture and commercialization of Licensed Products, to bankers, lawyers, accountants, agents or other Third Parties in connection with due diligence or similar investigations, and to potential Third Party investors in confidential financing documents; provided that any such Sublicensee, contractor, employee, consultant, banker, lawyer, accountant, agent or Third Party is bound by obligations of confidentiality and non-use at least as
-34-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
restrictive as those set forth herein. In the case of each disclosure, the Party making such disclosure shall use reasonable efforts to obtain confidential treatment of any such disclosure, and shall not disclose Confidential Information of the other Party other than is reasonably necessary.
9.4 Publicity; Terms of Agreement. The Parties shall treat the existence and material terms of this Agreement as confidential and shall not disclose such information to Third Parties without the prior written consent of the other Party or except as provided in Section 9.3 (treating such information as Confidential Information for purposes of Section 9.3). The Parties agree that upon execution of this Agreement or shortly thereafter, either Party may issue a press release, which shall be subject to prior review and approval by the other Party, not to be unreasonably withheld or delayed. Except for such press release or as otherwise required by applicable law or applicable stock exchange requirements, neither Amgen nor Amylin shall issue or cause the publication of any other press release or public announcement with respect to the transactions contemplated by this Agreement without the express prior approval of the other Party, which approval shall not be unreasonably withheld or delayed; provided that, each of Amgen and Amylin may make any public statement in response to questions by the press, analysts, investors or those attending industry conferences or financial analyst calls, or issue press releases, so long as any such public statement or press release is not inconsistent with prior public disclosures or public statements approved by the other Party pursuant to this Section 9.4 and which do not reveal non-public information about the other Party. If, in the reasonable opinion of a Party’s legal counsel, a public announcement of the transactions contemplated by the Agreement is required by applicable laws or applicable stock exchange requirements, then, to the extent permissible by law, such Party will provide the other with notice reasonable under the circumstances (but in no event less than ten (10) days prior to disclosure) of such intended announcement and will consult with the other Party with respect to the nature and scope of the required announcement (which shall be limited to the information reasonably required to be disclosed). In addition to the foregoing, with respect to complying with the disclosure requirements of the Securities and Exchange Commission or other regulatory agencies, in connection with any required filing of this Agreement with such agency, the Parties shall consult with one another concerning which terms of this Agreement shall be requested to be redacted in any public disclosure of the Agreement by the agency, and each Party shall seek confidential treatment by the agency in public disclosure of the Agreement by the agency for the definitions of Leptin, Licensed Products and Neuro Field, the exhibits, and any dollar amounts set forth herein.
9.5 Injunctive Relief. Given the nature of the Confidential Information and the competitive damage that would result to a Party upon unauthorized disclosure, use or transfer of its Confidential Information to any Third Party, the Parties agree that monetary damages may not be a sufficient remedy for any breach of this Article 9. In addition to all other remedies, a Party shall be entitled to seek specific performance and injunctive and other equitable relief as a remedy for any breach or threatened breach of this Article 9.
-35-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
ARTICLE 10
TERM AND TERMINATION
10.1 Term. This Agreement shall expire upon the expiration of the last Royalty Term for any Licensed Product with respect to which Amylin has a license under this Agreement, unless earlier terminated pursuant to this Article 10. Upon expiration of the Royalty Term with respect to a Licensed Product in any country and payment in full of all amounts owed to Amgen hereunder with respect to such Licensed Product in such country, the licenses granted in Sections 2.1, 2.2 and 2.3 for such Licensed Product in such country shall become non-exclusive, fully paid up and irrevocable, and shall survive any expiration (but not early termination) of this Agreement.
10.2 Termination.
(a) Termination for Convenience. Following payment of the License Fee under Section 5.1, Amylin shall have the right to terminate this Agreement for convenience by giving [*] prior written notice to Amgen; provided, however, that if Amylin elects to terminate this Agreement prior to completion of the transfer of the Manufacturing Processes to Amylin’s CMO and payment of the milestone payment under Section 5.2(a), Amylin shall pay a termination fee of [*] to Amgen (subject to the credit that may be available under Section 5.2(b)). Such termination fee shall be paid to Amgen by wire transfer or electronic funds transfer (EFT) in accordance with payment transfer instructions to be provided by Amgen, with payment to be made within [*] of such written notice by Amylin to Amgen.
(b) Termination for Bankruptcy/Insolvency. A Party may terminate this Agreement in the event any of the following occurs with respect to the other Party (each, a “Financial Default”): (a) such Party files a petition in bankruptcy or makes a general assignment for the benefit of creditors or otherwise acknowledges in writing insolvency, or is adjudged bankrupt, and such Party (i) fails to assume this Agreement in any such bankruptcy proceeding within [*] after filing or (ii) assumes and assigns this Agreement to a Third Party; (b) such Party goes into or is placed in a process of complete liquidation; (c) a trustee or receiver is appointed for any substantial portion of such Party’s business and such trustee or receiver is not discharged within [*] after appointment; (d) any case or proceeding shall have been commenced or other action taken against such Party in bankruptcy or seeking liquidation, reorganization, dissolution, a winding-up arrangement, composition or readjustment of its debts or any other relief under any bankruptcy, insolvency, reorganization or similar act or law of any jurisdiction now or hereafter in effect and is not dismissed or converted into a voluntary proceeding governed by clause (a) above within [*] after filing; or (e) there shall have been issued a warrant of attachment, execution, distraint or similar process against any substantial part of the property of such Party and such event shall have continued for a period of [*] and none of the following has occurred: (i) it is dismissed, (ii) it is bonded in a manner reasonably satisfactory to the other Party, or (iii) it is discharged.
-36-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
(c) Termination for Amylin Default. Upon any Default by Amylin under this Agreement, Amgen may notify Amylin of such Default and, in the event such Default shall be a payment Default, require that Amylin cure such Default within [*] of Amgen’s notice, or in the event such Default shall be a Default other than a payment Default, require that Amylin cure such Default within [*] of Amgen’s notice; provided that, if such Default cannot be cured within the [*]cure period, this Agreement shall not terminate if Amylin has made diligent efforts to cure such breach within the [*] period and this Agreement shall remain in effect for up to [*] after notice of breach as long as Amylin continues to use diligent efforts to pursue the cure and there is a reasonable expectation that cure will be effected within such [*] period. In the event Amylin shall not have cured the Default by the end of the applicable grace period, Amgen may terminate this Agreement immediately upon written notice to Amylin. The Parties agree that there may be circumstances in which monetary damages may not be a sufficient remedy for improper termination of this Agreement under this Section 10.2(c). In such cases, in addition to all other remedies, Amylin shall be entitled to seek preliminary injunctive relief as a remedy for any improper termination of this Agreement under this Section 10.2(c).
(d) Termination for Amgen Default. Upon any Default by Amgen under this Agreement, Amylin may notify Amgen in writing of such Default and require that Amgen cure such Default within [*] of Amylin’s notice; provided that, if such Default cannot be cured within the [*] cure period, this Agreement shall not terminate if Amgen has made diligent efforts to cure such breach within the [*] period and this Agreement shall remain in effect for up to [*] after notice of breach as long as Amgen continues to use diligent efforts to pursue the cure and there is a reasonable expectation that cure will be effected within such [*] period. In the event Amgen shall not have cured the Default by the end of such grace period, Amylin may terminate this Agreement immediately upon written notice to Amgen. The Parties agree that there may be circumstances in which monetary damages may not be a sufficient remedy for improper termination of this Agreement under this Section 10.2(d). In such cases, in addition to all other remedies, Amgen shall be entitled to seek preliminary injunctive relief as a remedy for any improper termination of this Agreement under this Section 10.2(d).
(e) Other Termination. The following shall be immediate material breaches of this Agreement that Amylin shall have no right to cure: (a) a Financial Default by Amylin; or (b) a lawsuit or reexamination or protest proceeding or the equivalent filed by Amylin, its Affiliate or Sublicensee against Amgen or its Affiliates seeking a declaratory judgment or determination that any claim(s) of the Licensed Patent Rights is invalid, unenforceable, of narrower scope or otherwise not patentable (a “Procedural Default”). Upon the occurrence of a Financial Default or Procedural Default, Amgen shall have the right to terminate this Agreement at any time upon written notice to Amylin.
10.3 Effects of Termination.
(a) Upon termination of this Agreement pursuant to Section 10.2 (excluding any termination of this Agreement by Amylin pursuant to Section 10.2(b) or 10.2(d)), all licenses and assignments granted hereunder to Amylin shall revert to Amgen, all sublicenses granted by Amylin under the rights or licenses granted to Amylin under this Agreement shall terminate,
-37-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
except that such sublicenses shall not terminate upon termination of this Agreement but instead shall remain in full force and effect to the extent provided in Section 2.4. Amylin shall immediately cease all development and Commercialization of Licensed Products and return to Amgen all physical manifestations of the Licensed Technology and Amgen Confidential Information (including Manufacturing Information).
(b) Upon such termination, Amylin agrees to promptly transfer to Amgen (including making such filings as may be required with Regulatory Authorities and other governmental authorities of the Territory to effect such transfer), at Amgen’s request, all Amylin Manufacturing Improvements and any Amylin equipment used in the Manufacturing Processes that can be transferred to Amgen, and, effective upon such termination, Amylin will be deemed to have hereby granted to Amgen and its Affiliates an irrevocable, royalty-free, fully paid-up, non-exclusive, fully transferable, worldwide, perpetual license, with the right to grant sublicenses, under the Amylin Manufacturing Improvements solely to make, have made and use Licensed Products in the Product Field in the Territory. In addition, upon such termination, Amylin agrees to promptly transfer to Amgen (including making such filings as may be required with Regulatory Authorities and other governmental authorities of the Territory to effect such transfer), at Amgen’s request, the following with respect only to the Licensed Products and subject to Section 10.3(e) below: (i) ownership of all regulatory documents and Regulatory Approvals applicable to Licensed Products Controlled by Amylin (including the Regulatory Documents); (ii) all pre-clinical (including toxicology) and clinical study protocols, data and reports applicable to Licensed Products Controlled by Amylin; and (iii) all Amgen-sponsored or investigator-sponsored clinical trial results, and the results of Ongoing Clinical Trials, and (iv) such other information, data, and documents applicable to Licensed Products Controlled by Amylin that are reasonably requested by Amgen to permit Amgen and its Affiliates to develop, manufacture and commercialize Licensed Products in the Territory after the termination date, including but not limited to any documents related to the prosecution, maintenance, defense and enforcement of the Licensed Patent Rights.
(c) Amgen shall reimburse Amylin for reasonable costs incurred by Amylin to effect the transfer of information related to the Manufacturing Processes, Amylin Manufacturing Improvements, analytical testing methods, and other information and costs related to the transfer of Leptin manufacturing under this Section 10.3, except that if this Agreement is terminated pursuant to Section 10.2(a) or (c), then Amylin shall bear all such costs incurred. Amgen shall not reimburse costs incurred by Amylin to effect the transfer of research, clinical, or regulatory information except in the case of consulting and advisory services provided by Amylin and reasonably requested by Amgen. Amgen shall pay to Amylin reasonable value (as determined by the parties in good faith) for any transferred equipment under this Section 10.3.
(d) Effective upon such termination, Amylin will be deemed to have hereby granted to Amgen and its Affiliates an irrevocable, royalty-free, fully paid-up, non-exclusive, fully transferable, worldwide, perpetual license, with the right to grant sublicenses, under any intellectual property Controlled by Amylin as of the effective date of such termination (including patents, copyrights, trademarks, trade secrets and know-how) that incorporates, uses or is derived from Licensed Technology solely to make, have made, use, sell, offer to sell or import Licensed Products in the Product Field in the Territory.
-38-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
(e) Notwithstanding anything to the contrary herein, Sections 10.3(b)(i)-(iv) and 10.3(d) above shall in no event apply with regard to (i) any Combination Product, (ii) any proprietary delivery technology used with or as part of any Licensed Product and (iii) any regulatory documents, Regulatory Approvals, protocols, data, reports, results or intellectual property to the extent related to either (i) or (ii). Upon request of Amgen, Amylin agrees to negotiate with Amgen in good faith a license, on mutually agreeable terms, for Amgen to research, develop, manufacture, and commercialize any Combination Product and/or any proprietary delivery technology used with or as part of any Licensed Product solely to the extent that such license would be required by Amgen to manufacture or commercialize Licensed Products.
(f) Upon such termination, at the request of Amgen, Amylin agrees to negotiate with Amgen in good faith to sell and transfer to Amgen any inventory of Licensed Products. Amylin further agrees, upon such termination, at the request of Amgen, to negotiate in good faith with Amgen to assign to Amgen any contracts with Third Party manufacturers, suppliers, clinical trial organizations or clinical trial sites related to Licensed Products,
(g) Upon such termination, the Parties will cooperate in good faith to establish a transition plan to effectuate the transfers contemplated under this Section 10.3 in a manner that preserves continuity of clinical and commercial supply with respect to any Licensed Products that are being developed and/or commercialized as of the effective date of the termination.
10.4 Survival. The following provisions shall survive any expiration or termination of this Agreement: Articles 1, 8, 9 (other than Amgen’s confidentiality obligations under Section 9.2), 10 and 11, and Sections 2.9, 2.10, 2.11, 5.9, 7.1(h) and 7.2(d). Termination of this Agreement shall not relieve the Parties of any liability which accrued hereunder prior to the effective date of such termination nor preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach or Default of this Agreement nor prejudice either Party’s right to obtain performance of any obligation. The remedies provided in this Article 10 are not exclusive of any other remedies a Party may have in law or equity.
-39-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
ARTICLE 11
MISCELLANEOUS
11.1 Entire Agreement; Amendment. This Agreement, including the exhibits, constitutes the entire agreement between the Parties (or their Affiliates) related to the subject matter hereof. All prior and contemporaneous negotiations, representations, warranties, agreements, statements, promises and understandings related to the subject matter hereof are superseded by and merged into and extinguished and completely expressed by this Agreement, including the exhibits. No Party shall be bound by or charged with any written or oral agreements, representations, warranties, statements, promises or understandings not specifically set forth in this Agreement, including the exhibits. As of the Effective Date, the Confidential Disclosure Agreement dated April 18, 2005 (Amgen Reference #200501894) between Amgen and Amylin (the “Confidentiality Agreement”), is hereby superseded by this Agreement, provided that all Confidential Information (as defined in the Confidentiality Agreement) disclosed thereunder shall be treated as Confidential Information disclosed under, and subject to the terms of, this Agreement. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party.
11.2 Notices. Any notice required or permitted to be given under this Agreement shall be in writing and shall be deemed to have been sufficiently given for all purposes (i) when delivered, if sent by recognized overnight courier or personally delivered, or (ii) upon confirmation of receipt, if sent by facsimile transmission (provided a duplicate hard copy is promptly delivered by one of the other foregoing means), in each case using the mailing addresses of the Parties as set forth below (or such other mailing address of which a Party is notified pursuant to this Section 11.2):
| For Amylin: | Amylin Pharmaceuticals, Inc. 0000 Xxxxx Xxxxxx Xxxxx, Xxxxx 000 Xxx Xxxxx, Xxxxxxxxxx 00000 Facsimile: (000) 000-0000 Attn: Vice President, Business Development | |
| With a copy to: | Amylin Pharmaceuticals, Inc. 0000 Xxxxx Xxxxxx Xxxxx, Xxxxx 000 Xxx Xxxxx, Xxxxxxxxxx 00000 Facsimile; (000) 000-0000 Attn: General Counsel | |
-40-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| For Amgen: | Amgen Inc. Xxx Xxxxx Xxxxxx Xxxxx Xxxxxxxx Xxxx, XX 00000-0000 Facsimile: [*] Attn: Corporate Secretary | |
| With a copy to: | Amgen Inc. Xxx Xxxxx Xxxxxx Xxxxx Xxxxxxxx Xxxx, XX 00000-0000 Facsimile: [*] Attn: Vice President, Licensing | |
11.3 Governing Law; Judicial Resolution. This Agreement shall be governed and construed in accordance with the laws of the State of California, as applied to agreements executed and performed entirely within the State of California, without regard to any applicable principles of conflicts of law. Each of the Parties hereto hereby irrevocably and unconditionally consents to submit to the exclusive jurisdiction of the courts of the State of California and of the United States of America located in the State of California for any matter arising out of or relating to this Agreement and the transactions contemplated hereby, and agrees not to commence any litigation relating thereto except in such courts. Each of the Parties hereto hereby irrevocably and unconditionally waives any objection to the laying of venue of any matter arising out of this Agreement or the transactions contemplated hereby in the courts of the State of California or of the United States of America located in the State of California and hereby further irrevocably and unconditionally waives and agrees not to plead or claim in any such court that any such matter brought in any such court has been brought in an inconvenient forum. The Parties agree that a final judgment in any such matter shall be conclusive and may be enforced in other jurisdictions by suits on the judgment or in any other manner provided by law. Notwithstanding the foregoing, any dispute, controversy or claim relating to the scope, validity, enforceability or infringement of any patent right shall be submitted exclusively to the competent court having jurisdiction over the disputed patent.
11.4 Interpretation. Amgen and Amylin have each participated in negotiations and due diligence and consulted their respective counsel regarding this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any provisions of this Agreement.
11.5 Assignment. Neither Party may assign or transfer this Agreement or any rights or obligations hereunder, by operation of law or otherwise, without the prior written consent of the other Party, except that a Party may make such an assignment or transfer, by operation of law or otherwise, without the other Party’s consent to Affiliates or to an entity that acquires all or substantially all of the business of Amgen and its Affiliates or Amylin and its Affiliates, respectively, whether in a merger, consolidation, reorganization, acquisition, sale or otherwise. To the extent any rights and/or obligations of a Party are held by an Affiliate of such Party then
-41-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
any business transaction, change in control of a majority of the voting power or other event that, in each case, causes such Affiliate to cease to be an Affiliate of the Party, shall be deemed an assignment of the rights and/or obligations held by such former Affiliate and require prior written consent of the other Party. Notwithstanding the foregoing, Amgen shall have the right to assign its rights to payment pursuant to Article 5 without Amylin’s consent. This Agreement shall be binding on the successors and permitted assigns of the assigning Party, and the name of a Party appearing herein shall be deemed to include the name(s) of such Party’s successors and permitted assigns to the extent necessary to carry out the intent of this Agreement. Any assignment or attempted assignment by either Party in violation of the terms of this Section 11.5 shall be null and void and of no legal effect.
11.6 Performance by Affiliates. Each of Amgen and Amylin acknowledge that obligations under this Agreement may be performed by Affiliates of Amgen and Amylin. Each of Amgen and Amylin guarantee performance of this Agreement by its Affiliates, notwithstanding any assignment to Affiliates in accordance with Section 11.5 of this Agreement.
11.7 Severability. In the event that any one or more of the provisions contained herein, or the application thereof in any circumstances, is held invalid, illegal or unenforceable in any respect for any reason, the Parties shall negotiate in good faith with a view to the substitution therefor of a suitable and equitable provision in order to carry out, so far as may be valid and enforceable, the intent and purpose of such invalid provision; provided, however, that the validity, legality and enforceability of any such provision in every other respect and of the remaining provisions contained herein shall not be in any way impaired thereby, it being intended that all of the rights and privileges of the Parties hereto shall be enforceable to the fullest extent permitted by law.
11.8 Headings. The heading for each article and section in this Agreement has been inserted for convenience of reference only and is not intended to limit or expand on the meaning of the language contained in the particular article or section.
11.9 Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of the Agreement.
11.10 Independent Contractors. The relationship between Amylin and Amgen created by this Agreement is solely that of independent contractors. This Agreement does not create any agency, distributorship, employee-employer, partnership, joint venture or similar business relationship between the Parties. Neither Party is a legal representative of the other Party, and neither Party can assume or create any obligation, representation, warranty or guarantee, express or implied, on behalf of the other Party for any purpose whatsoever. Each Party shall use its own discretion and shall have complete and authoritative control over its employees and the details of performing its obligations under this Agreement.
11.11 Use of Name. Except as expressly permitted in Section 2.2 (Trademark License), no right, express or implied, is granted to Amylin by this Agreement to use in any manner any
-42-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Trademark of Amgen or its Affiliates. Except as expressly permitted in Section 2.2 (Trademark License), Amylin shall not use or allow its representatives to use, any name or Trademark of Amgen or its Affiliates, or the name of any of their employees, or any derivatives thereof, for purposes of any promotion, publicity or advertising without Amgen’s prior written consent, which may be withheld at Amgen’s sole discretion. Amylin may use Amgen’s name to the extent it is included on labels attached to the vials Amgen fills and labels in accordance with its performance of the fill and finish services set forth in Section 3 .3.
11.12 No Waiver. A Party’s consent to or waiver, express or implied, of the other Party’s breach of its obligations hereunder shall not be deemed to be or construed as a consent to or waiver of any other breach of the same or any other obligations of the other Party. A Party’s failure to complain of any act, or failure to act, by the other Party, to declare the other Party in default, to insist upon the strict performance of any obligation or condition of this Agreement or to exercise any right or remedy consequent upon a breach thereof, no matter how long such failure continues, shall not constitute a waiver by such Party of its rights hereunder, of any such breach, or of any other obligation or condition. A Party’s consent in any one instance shall not limit or waive the necessity to obtain such Party’s consent in any future instance and in any event no consent or waiver shall be effective for any purpose hereunder unless such consent or waiver is in writing and signed by the Party granting such consent or waiver.
11.13 Fees and Expenses. Regardless of whether or not the transactions contemplated by this Agreement are consummated, each Party shall bear its own fees and expenses incurred in connection with the negotiation and execution of this Agreement.
11.14 No Set-Off. Neither Party shall have any right to set-off any amount owed to such first Party by the other Party or an Affiliate thereof under this Agreement, another agreement or otherwise from any amount owed by such first Party to the other Party hereunder, without the prior written consent of the other Party.
11.15 No Other Rights. The Parties acknowledge and agree that, except as expressly set forth in this Agreement, neither Party grants any rights or licenses to the other Party under this Agreement nor shall either Party have any rights or obligations under this Agreement.
11.16 Parties in Interest. This Agreement shall be binding upon and inure solely to the benefit of each Party hereto and its respective successors and permitted assigns, and nothing in this Agreement, express or implied, is intended to or shall confer upon any other person any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement (with the exception of Amylin Indemnitees and Amgen Indemnitees under Sections 8.1 and 8.2, respectively).
11.17 Rules of Construction. The use in this Agreement of the term “including” (or any cognates thereof, such as “include” or “includes”) means “including (or the applicable cognate thereof), without limitation.” The words “herein,” “hereof,” “hereunder,” and other words of similar import refer to this Agreement as a whole, including the exhibits, and not to any particular section, subsection, paragraph, subparagraph or clause contained in this Agreement. All references to sections and exhibits mean those sections of this Agreement and the exhibits attached to this Agreement, except where otherwise stated.
-43-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
11.18 Counterparts. This Agreement may be executed in two (2) or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
11.19 Precedence. In the event of any conflict between this Agreement and any of the exhibits attached hereto, this Agreement shall control; provided, however, that notwithstanding the foregoing, in the event of any conflict between this Agreement and Exhibit H (Transfer of Manufacturing Processes), the terms and conditions of Exhibit H shall control.
-44-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
IN WITNESS WHEREOF, the Parties have executed this Agreement by their duly authorized representatives as of the Effective Date.
| AMGEN INC. | AMLYN PHARMACEUTICALS, INC. | |||||||
| By: | /s/ Xxxxx X. Xxxxxxx |
By: | /s/ Xxxxxx X. Xxxxxxxx | |||||
| Print Name: | Xxxxx X. Xxxxxxx | Print Name: | Xxxxxx X. Xxxxxxxx | |||||
| Title: | Vice President, Licensing | Title: | Chief Operating Officer | |||||
-45-
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Exhibit A-1
PROTEIN SEQUENCE OF OB POLYPEPTIDE
Full-length sequence
[*]
Mature sequence
[*]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT A-2
PROTEIN SEQUENCE OF A-100 LEPTIN
[*]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT B
PROTEIN SEQUENCE OF A-200 LEPTIN
[*]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT C
LICENSED PATENT RIGHTS
Part 1
[See attached list of Rockefeller Patents]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Argentina |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| ARIPO |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Armenia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Barbados |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Belarus |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Benin |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Brazil |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Bulgaria |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Burkina Faso |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Cameroon |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Central African Republic |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Chad |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Chile |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| China |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Columbia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Congo |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Czech Republic |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Democratic Peoples Republic of Korea |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Estonia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Finland |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Gabon |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Georgia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Germany |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Germany |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Great Britain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Guinea |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Hong Kong |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Hungary |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Iceland |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Israel |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Israel |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Ivory Coast |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Japan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Kazakstan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Kenya |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Kyrgyzstan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Latvia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Lesotho |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Liberia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Lithuania |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Madagascar |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Malawi |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Mali |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mauritania |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mongolia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| New Zealand |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Niger |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Norway |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| OAPI |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Peru |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Philippines |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Poland |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Republic of Korea |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Republic of Moldova |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Romania |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Russia |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Saudi Arabia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Senegal |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Yugoslavia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Singapore |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Slovakia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Slovenia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Sri Lanka |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Sudan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Swaziland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Tajikistan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| The former Yugoslav Republic of Macedonia |
ALL | [*] | [*] | [*] | [*] | |||||||||||||
| Togo |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Trinidad and Tobago |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Turkey |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Turkmenistan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Uganda |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Ukraine |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Uzbekistan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Vatican City |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Venezuela |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Venezuela |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Vietnam |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Exhibit C
LICENSED PATENT RIGHTS
Part 2
[See attached list of Leptin Patent Rights]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Australia |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Canada |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Australia |
ALL | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Austria |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belgium |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Denmark |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Europe |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Finland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| France |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Germany |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Great Britain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Greece |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Ireland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Italy |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Luxembourg |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Monaco |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Netherlands |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Portugal |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Spain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Sweden |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Switzerland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Albania |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Australia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Austria |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belgium |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Denmark |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Europe |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Finland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| France |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Germany |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Great Britain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Greece |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Hong Kong |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Ireland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Israel |
ALL | [*] | [*] | [*] | [*] | |||||||||||||
| Israel |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Italy |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Latvia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Lithuania |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Luxembourg |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Netherlands |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| New Zealand |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| New Zealand |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| New Zealand |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Portugal |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Romania |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Slovenia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Spain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Sweden |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Switzerland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
PEN | [*] | [*] | [*] | [*] |
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Argentina |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Armenia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Armenia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Australia |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Azerbaijan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Azerbaijan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belarus |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belarus |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Brazil |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Bulgaria |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| China |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Czech Republic |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Eurasia |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| Eurasia |
NAT | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Hong Kong |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Hungary |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Israel |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Kazakstan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Kazakstan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Korea, Republic of (South) |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Kyrgyzstan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Kyrgyzstan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Moldova, Republic of |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Moldova, Republic of |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| New Zealand |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| New Zealand |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Norway |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| PCT |
CH2 | [*] | [*] | [*] | [*] | |||||||||||||
| Poland |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Russia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Russia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Slovakia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Taiwan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Tajikistan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Tajikistan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Turkmenistan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Turkmenistan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Yugoslavia |
PEN | [*] | [*] | [*] | [*] |
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Austria |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belgium |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| China |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Europe |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| France |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Germany |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Great Britain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Hong Kong |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Hungary |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Ireland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Israel |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Italy |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Korea, Republic of (South) |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Luxembourg |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Monaco |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Netherlands |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Spain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Switzerland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Taiwan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Australia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Austria |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belgium |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Cyprus |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Denmark |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Europe |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Finland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| France |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Germany |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Great Britain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Greece |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Ireland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Italy |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Luxembourg |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
GRÀ | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Netherlands |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Portugal |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Spain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Sweden |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Switzerland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Europe |
ALL | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title | Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| Australia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Honq Kong |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Poland |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| WO |
N2 (ABD) | [*] | [*] | [*] | [*] | |||||||||||||
| WO |
N2 (ABD) | [*] | [*] | [*] | [*] | |||||||||||||
| WO |
ABD | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT C
LICENSED PATENT RIGHTS
Part 3
[See attached list of Other Licensed Patent Rights]
Notwithstanding anything in the Agreement or this Exhibit C to the contrary, Other Licensed Patent Rights shall not include [*]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| Austria |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belgium |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Switzerland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| China |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Germany |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Denmark |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Europe |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Spain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Finland |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| France |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Great Britain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Greece |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Ireland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Israel |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Italy |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Japan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Luxembourg |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Monaco |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Netherlands |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Norway |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Portugal |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Sweden |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||||
| [*] |
[*] | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| AL |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| AT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| AU |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| BE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| CA |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| CH |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| DE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| DK |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| EP |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||||
| ES |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| FI |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| FR |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| GB |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| GR |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| IE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| IL |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| IT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| JP |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| LT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| LU |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| LV |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MC |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MX |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| NL |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| NZ |
ALL | [*] | [*] | [*] | [*] | |||||||||||||
| PT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| RO |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| SE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| SI |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| South Africa |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Taiwan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| AL |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| AT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| BE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| CA |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| CH |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| CY |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| DE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| DK |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| EP |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| ES |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| FI |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| FR |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| GB |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| GR |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| HK |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| IE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| IT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| JP |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| LT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| LU |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| LV |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MC |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MK |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MX |
PEN | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| NL |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| PT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| RO |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| SE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| SI |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| TW |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| WO |
N2 | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| Australia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Bulgaria |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Czech Republic |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Hungary |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Poland |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Slovakia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| Canada |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Hong Kong |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Taiwan |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| Bulgaria |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Canada |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Czech Republic |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Hungary |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Mexico |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Poland |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Slovakia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Yugoslavia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Australia |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
PEN | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| China |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Europe |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Hungary |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| Israel |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Korea |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| Austria |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Australia |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Belgium |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Canada |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Switzerland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Cyprus |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Germany |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Denmark |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Europe |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| Spain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Finland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| France |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Great Britain |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Greece |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Ireland |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Italy |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Japan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| Luxembourg |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Monaco |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Mexico |
ALL | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Netherlands |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Portugal |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Sweden |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| Taiwan |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
PEN | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| AL |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| AT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| AU |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| BE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| CA |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| CH |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| CN |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| CY |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| DE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| DK |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| EP |
NAT | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| ES |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| FI |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| FR |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| GB |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| GR |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| HU |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| IE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| IL |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| IT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| JP |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| KR |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| LT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| LU |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| LV |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MC |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MK |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| MX |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| NL |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| PT |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| RO |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| SE |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| SI |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| TW |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | |||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| ZA |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST.
REDACTED MATERIAL IS MARKED WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES
AND EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Publication No. |
Grant No. |
Grant Date |
EXP Date |
Inventor | |||||||||
| EP |
PEN | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| JP |
PEN | [*] | [*] | [*] | [*] | |||||||||||||
| US |
GRA | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
| US |
PEN | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status | Short Title |
Application No. | Application Date |
Publication No. | Grant No. | Grant Date | EXP Date | Inventor | |||||||||
| WO |
N2 | [*] | [*] | [*] | [*] | [*] | ||||||||||||
| US |
PEN | [*] | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| City |
Status |
Short Title |
Application No. |
Application Date |
Inventor | |||||
| WO |
ABD | [*] | [*] | [*] | [*] | |||||
| WO |
ABD | [*] | [*] | [*] | [*] | |||||
| WO |
N2 (ABD) | [*] | [*] | [*] | [*] | |||||
| WO |
N2 (ABD) | [*] | [*] | [*] | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT D
LICENSED TRADEMARKS
The Licensed Trademarks are:
[*]
[*]
[See attached list of registrations and/or applications of such Trademarks]
D-1
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| Monday, February 06, 2006 |
Trademark List |
|||||||||||||||
| Trademark |
Client Matter/Subcase Country Name |
Status Class(es) |
Application Number/Date |
Registration Number/Date |
||||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | [ | *] | ||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| Monday, February 06, 2006 |
Trademark List |
|||||||||||||||
| Trademark |
Client Matter/Subcase Country Name |
Status Class(es) |
Application Number/Date |
Registration Number/Date |
||||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| Monday, February 06, 2006 |
Trademark List |
|||||||||||||||
| Trademark |
Client Matter/Subcase Country Name |
Status Class(es) |
Application Number/Date |
Registration Number/Date |
||||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| Monday, February 06, 2006 |
Trademark List |
|||||||||||||||
| Trademark |
Client Matter/Subcase Country Name |
Status Class(es) |
Application Number/Date |
Registration Number/Date |
||||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | ALLOWED 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Opposed 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| Monday, February 06, 2006 |
Trademark List |
|||||||||||||||
| Trademark |
Client Matter/Subcase Country Name |
Status Class(es) |
Application Number/Date |
Registration Number/Date |
||||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Pending 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| Monday, February 06, 2006 |
Trademark List |
|||||||||||||||
| Trademark |
Client Matter/Subcase Country Name |
Status Class(es) |
Application Number/Date |
Registration Number/Date |
||||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | ALLOWED 05 Int. | [ | *] | ||||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
| [*] |
Owner Name: Product Name: |
[ | *] | Registered 05 Int. | [ | *] | [ | *] | ||||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT E
ONGOING CLINICAL TRIALS
| Protocol Number |
Investigator |
Short Desc | ||
| 990755 |
Mantzoros | Fasting | ||
| 20000141 |
Xxxxxxxxx | Obese | ||
| 20000709 |
Xxxxxxxx | VLCD | ||
| 20000745 |
Licinio | Leptin Deficiency (Turkish) | ||
| 20000773 |
Mantzoros | Amen-Athlets | ||
| 20010222 |
Xxxx-Xxxxxxx | French peds | ||
| 20010728 |
Schambelan | UCSF-HIV | ||
| 20010769 |
Xxxxxx | 1265 Rollover open lipo | ||
| 20020129 |
Nakoa | Japan | ||
| 00000000 |
Garg | Lipo-Blind | ||
| 20020382 |
Mantzoros | Signaling | ||
| 20020385 |
Xxxxxxxxxxxxxxx | HIV-metab | ||
| 20020700 |
Garg | HIV | ||
| 20020701 |
Garg | 1265 Rollover open lipo | ||
| 20030181 |
Xxxxxx | XX Rollover | ||
| 20030208 |
Brook | Vascular | ||
| 20040155 |
Xxxxxxx | Leptin Sensitivity | ||
| 20040161 |
Mantzoros | HA | ||
| 20050119 |
Oral | HA | ||
| 20040193 |
Xxxxx | LTTDM |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT F
ROCKEFELLER LICENSE
[See attached]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
LICENSE AGREEMENT
LICENSE AGREEMENT (the “Agreement”) made as of the fourteenth day of April, 1995 by and between The Rockefeller University, an education corporation organized and existing under the laws of the State of New York, having an office at 1200 Xxxx Xxxxxx, Xxx Xxxx, Xxx Xxxx 00000-0000 (“Rockefeller”), and Amgen Inc., a corporation organized and existing under the laws of the State of Delaware, having an office at Amgen Center, 1800 XxXxxxxxxxx Xxxxx, Xxxxxxxx Xxxx, Xxxxxxxxxx 00000-0000 (“Amgen”).
WITNESSETH:
WHEREAS, scientists employed by Rockefeller and the Xxxxxx Xxxxxx Medical Institute have developed valuable technology and know-how related to the identification of an obesity gene and its significance having been described in the U.S. patent applications listed on Exhibit “A” attached hereto;
WHEREAS, Rockefeller owns the entire right, title and interest in (a) the U.S. patent applications listed on Exhibit “A,” by assignment from the inventors and the Xxxxxx Xxxxxx Medical Institute, and (b) the related know-how;
WHEREAS, it is the policy of Rockefeller to encourage the use of inventions for the greatest possible public benefit;
WHEREAS, Amgen has advised Rockefeller that it shares that view and in furtherance thereof desires to obtain the exclusive right and license, with the right to sublicense, to make, use, and sell such technology, together with relevant know-how for commercial development and application, all in the manner hereinafter provided.
NOW, THEREFORE, in consideration of the undertakings and of the covenants and conditions contained herein, and intending to be bound hereby, the parties agree as follows:
1. DEFINITIONS
The following terms shall have the meaning assigned to them below when used in this Agreement:
1.1 Amgen shall mean Amgen and Amgen Affiliates.
common control with Amgen. For the purposes of this definition, control shall mean the direct or indirect ownership of at least fifty percent (50%) or, if less than fifty percent (50%), the maximum percentage as allowed by applicable law of (i) the stock shares entitled to vote for the election of directors or (ii) ownership Interest.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.3 “Commercially Reasonable Efforts” shall mean efforts and resources commonly used In the research-based pharmaceutical industry for a product at a similar stage in its product life of similar market potential taking into account efficacy, the competitiveness of alternative products in the marketplace, the patent and other proprietary position of the product, the likelihood of regulatory approval given the regulatory structure involved, the profitability of the product and alternative products and other relevant factors. Commercially Reasonable Efforts shall be determined on a market by market basis for a particular Product, and it is anticipated that the level of effort will change over time reflecting changes in the status of the Product and the market involved.
1.4 “Confidential Information” shall mean information, which, if written or embodied in a tangible item or product, is marked “confidential” by the disclosing party on first being provided to the receiving party or, if oral, is reduced to writing, which is marked “confidential” by the disclosing party and provided to the receiving party within thirty (30) days of the oral disclosure.
1.5 “Effective Date” shall mean the day and year first written above.
1.6 “Field” shall mean technology related to ob genes, ob gene products, and molecules that modulate or mediate their action and/or regulation.
1.7 “Improvements” shall mean any inventions, discoveries, materials, and/or information in the Field, which are derived from data or biological materials described or claimed in Licensed Patent Rights or Technical Information.
1.8 “IND” shall mean an Investigational New Drug Application filed with the United States FDA, which is allowed to go into effect (e.g., for which no clinical hold is issued), and which is required for the clinical testing in the United States of a human therapeutic product.
1.9 “Licensed Patent Rights” shall mean:
| (a) | the patent applications and patents set forth in Exhibit “A”; |
| (b) | any Improvements pursuant to the terms of Subparagraph 2.6; |
| (c) | any patent rights pursuant to the scientific collaboration under Section 12; and |
| (d) | all patent applications which are divisions, continuations, continuations-in-part, reissues, renewals, foreign counterparts of the applications described in Subparagraphs (a), (b), and (c) hereof, and all patents which may issue thereon. |
1.10 “NDA” shall mean a New Drug Application accepted for filing with the United States FDA, to obtain marketing approval of a human drug therapeutic product.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.11 “Net Sales” shall mean all revenues, recognized in accordance with generally accepted accounting principles consistently applied, received from sales or other dispositions by Amgen, or a sublicensee of Amgen, to a third party, less returns and allowances (actually paid or allowed, including, but not limited to, prompt payment and volume discounts, chargebacks from wholesalers and other allowances granted to customers or wholesalers, whether in cash or trade), bad debt, freight, shipping, packing, insurance, rebates, and sales and other taxes based on sales prices when included in gross sales, but not including taxes when assessed on income derived from such sales.
1.12 “Party” shall mean either Amgen or Rockefeller; “Parties” shall mean both Amgen and Rockefeller.
1.13 “Phase III Licensing Trial” shall mean a phase III human clinical trial, the design of which will, if the endpoint(s) are met, provide data that Amgen and FDA agree will support licensure of the product. Amgen will make reasonable efforts to reach such agreement with FDA prior to initiation of the trial.
1.14 “PLA” shall mean a Product License Application filed with and accepted by the United States FDA, to obtain marketing approval of a human biological therapeutic product.
1.15 “Product(s)” shall mean any and all products the making, using, or selling of which employ (a) Licensed Patent Rights; or (b) know-how in the Field that is hereafter developed at Rockefeller, which is given to Amgen on an exclusive, confidential basis as part of Technical Information, and which is not available from any other source.
1.16 “Technical Information” shall mean all technical data, information, materials and know-how in which Rockefeller has or acquires rights, whether before or after the Effective Date, which are needed or useful to practice Licensed patent Rights.
1.17 “Territory” shall mean the world.
2. LICENSE GRANT
2.1 Rockefeller hereby grants to Amgen the sole and exclusive right and license, with the right to sublicense, under Licensed Patent Rights and Technical Information to make, use and sell Products in the Territory, excepting only to the extent that Rockefeller’s right to do so may be limited under the provisions of 35 Xxxxxx Xxxxxx Xode, Section 201, et seq., and regulations and rules promulgated thereunder.
2.2 Rockefeller hereby grants to Amgen the non-exclusive right and license, with the right to sublicense, under Technical Information to make, use and sell products other than Products in the Territory, excepting only to the extent that Rockefeller’s right to do so may be limited under the provisions of 35 Xxxxxx Xxxxxx Xode, Section 201, et seq., and regulations and rules promulgated thereunder.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
2.3 Any right granted in this Agreement greater than that permitted under Public Law 96-517 or Public Law 98-620 shall be subject to modification as may be required to conform to the provisions of that statute.
2.4 Rockefeller shall retain the right to make and to use, solely for its internal research purposes and not for any commercial purpose, any and all biological materials which are claimed or described In Licensed Patent Rights (“Biological Materials”).
2.5 The Parties acknowledge that Rockefeller has granted to the Xxxxxx Xxxxxx Medical Institute a paid-up, non-exclusive, nan-transferable, irrevocable license to use the Licensed Patent Rights, Biological Materials and Technical information for non-commercial purposes, but with no right to sub-license.
2.6 For a period of three (3) years from the Effective Date, any Improvements in the Field made at Rockefeller or any Xxxxxx Xxxxxx Medical Institute laboratory at Rockefeller, in which Rockefeller holds rights, shall become part of Licensed Patent Rights or Technical Information hereunder, as the case may be.
2.7 Amgen represents that It currently manufactures only in the United States and has no current plans to manufacture outside of the United Stales, but retains the right to do so. Amgen agrees that Products sold in the United Stales shall be manufactured substantially in the United States including Puerto Rico.
3. TRANSFER OF BIOLOGICAL MATERIALS
3.1 During the term of this Agreement, Rockefeller may provide samples of Biological Material to academic investigators at academic institutions that are not affiliated with a commercial entity (a “Material Transfer”); provided, however, that any Material Transfer shall be subject to prior written approval by Amgen, which approval will not be unreasonably withheld, and shall be made under the terms and conditions of the Material Transfer Agreement attached hereto as Exhibit ‘B”. Upon deciding to be a party to a Material Transfer, Rockefeller shall provide at least thirty (30) days prior written notice to Amgen, such written notice indicating the name and address of the proposed recipient and the intended use of the Biological Material. Amgen agrees to provide a written response to each such notice within thirty (30) days of receipt by Amgen. It is the purpose of this procedure to enable Amgen to determine whether each Material Transfer is consistent with Amgen’s ongoing development program. Without limiting the foregoing, any Material Transfer to any employee of the Xxxxxx Xxxxxx Medical Institute shall be accomplished by full execution of a Material Transfer Agreement in the form of Exhibit “B” and further shall be subject to representations by Xxxxxx Xxxxxx Medical Institute (i) that the transferee of Biological Material has not and shall not enter into a collaboration agreement with any commercial entity in the Field and (ii) that no consulting agreement executed by the transferee of the Biological Material shall provide for transfer of any information or rights relating to the Biological Material to a commercial entity; but, notwithstanding anything in this Subparagraph 3.1 to the contrary, such Material Transfer shall not be subject to the prior approval of either Party. Any rights that Rockefeller may obtain
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
through a Material Transfer shall be subject to the terms and conditions of this Agreement. Copies of any executed Material Transfer Agreement, any written reports or proposed publications submitted by Institution to Rockefeller thereunder, and any notification of an invention or discovery thereunder, shall be promptly provided to Amgen by Rockefeller.
3.2 In the event that Rockefeller receives any request for Biological Material from a commercial entity, Amgen shall decide, in its sole discretion after considering any recommendations by Rockefeller, whether to provide samples of Biological Materials to such commercial parties. Any and all Biological Materials provided to commercial entities shall be done by Amgen.
4. UNDERTAKINGS BY AMGEN AND ROCKEFELLER
4.1 Amgen undertakes to use Commercially Reasonable Efforts to identify and develop Product(s) and, following obtaining regulatory marketing approval, to commence appropriate marketing efforts for one or more Products.
4.2 Rockefeller undertakes to promptly provide to Amgen all Technical Information and Licensed Patent Rights that exist as of the Effective Date of this Agreement. On a quarterly basis, during the term of the collaboration agreement under Section 12 or, in any case, for a period of three (3) years from the Effective Date, Rockefeller shall provide to Amgen a written description of all additional Technical Information and Licensed Patent Rights since the last disclosure to Amgen.
5. PATENTS
5.1 Within thirty (30) days of the Effective Date, Amgen agrees to reimburse Rockefeller for alt of its unreimbursed patent costs expended up to the Effective Date for the preparation, filing, prosecution and maintenance of Licensed Patent Rights, said amount being $50,963.73 as of March 21, 1995.
5.2 Except in connection with the exercise of its rights to abandon patent prosecution or maintenance pursuant to Subparagraph 5.4 hereof, Amgen shall continue to reimburse Rockefeller promptly for such additional preparation, filing, prosecution, and maintenance costs as shall be incurred with respect to each such patent application or patent licensed hereunder during the term of such license.
5.3 Rockefeller shall select qualified independent patent counsel reasonably acceptable to Amgen to prepare, file, prosecute and maintain all patent applications and patents Included in Licensed Patent Rights. Such counsel shall meet and consult at least quarterly with Amgen and/or its designated patent officers and counsel in order to keep them properly advised of the status of patent matters in the normal course, and shall include providing Amgen with copies of all correspondence sent to and received from any patent office, and providing Amgen with the opportunity to review, comment and consult on such papers relating to the Licensed Patent Rights before filing with any patent office through designated patent counsel. Amgen
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
shall be entitled to determine the countries in which it wishes to obtain and maintain patent protection under this Agreement, and patent applications shall be filed in such countries by Rockefeller.
5.4 Amgen shall be free, at any time and at its sole option, to abandon patent prosecution or maintenance with respect to any patent application or patent within Licensed Patent Rights in any country of the Territory. Amgen shall promptly advise Rockefeller of its election not to proceed with and/or to abandon the preparation, filing, prosecution, or maintenance of any patent application or patent licensed hereunder, at least thirty (30) days before a due date to allow Rockefeller, at its own cost, to effectuate such preparation, filing, prosecution, or maintenance if it so desires; and Amgen shall, at the request of Rockefeller, take whatever steps may be necessary or appropriate, reasonable and proper, to return to Rockefeller the rights licensed from Rockefeller to the specific patent application or patent within Licensed Patent Rights which it proposes to abandon.
5.5 Nothing herein is intended or shall be construed as obligating Rockefeller to apply for any U.S. or foreign patents at its own expense, or to defend any patent or patent application which may be included in the Licensed Patent Rights hereunder.
5.6 Nothing herein is intended or shall be construed as obligating Amgen to maintain its license with respect to any patent or application which may be included in the Licensed Patent Rights hereunder or to continue to finance the preparation, filing, prosecution or maintenance of any patent application or patent in any country or jurisdiction.
5.7 During the term of this Agreement, Rockefeller agrees to use its best efforts to ensure that Dr. Xxxxxxx Xxxxxxxx and any co-inventors of Licensed Patent Rights (and/or other Rockefeller or Xxxxxx Xxxxxx Medical Institute employees as might reasonably be requested for assistance by Amgen) will be available to cooperate with Amgen at Amgen’s request and expense for prosecution of Licensed Patent Rights.
6. FINANCIAL CONSIDERATION
6.1 In consideration of the rights and license granted hereunder, Amgen shall pay or cause to be paid to Rockefeller the amounts set forth below at the times indicated below:
| (a) | Initial License Fee: A one-time non-refundable payment of [*] shall be due and payable upon the satisfaction of all conditions to effectiveness set forth in Section 26 herein; |
| (b) | Milestone payments: One-time milestone payments shall be due and payable in the following amounts within thirty (30) days of the following occurrences: |
| (i) | [*] |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| (ii) | [*] |
| (iii) | [*] |
| (iv) | [*] |
Only one milestone shall be payable under each of 6.1 (b) (i), (ii), (iii) and (iv) no matter how many Products are developed under this Agreement
| (c) | Amgen shall pay to Rockefeller royalties, based upon the following incremental rates, on annual Net Sales of any product, the selling of which would, in the absence of this Agreement, infringe a valid claim of an issued, unexpired patent within the Licensed Patent Rights in the country in which the product is sold by Amgen or a sublicensee thereof (a “Patented Product”): |
| (i) | for that portion of annual Net Sales of a Patented Product in the Territory less than [*], a royalty of [*] percent ([*]%) of Net Sales; and |
| (ii) | for that portion of annual Net Sales of a Patented Product in the Territory equal to or greater than [*] and less than or equal to [*], a royalty of [*] percent ([*]%) of Net Sales; and |
(iii) for that portion of annual Net Sales of a Patented Product in the Territory greater than [*], a royalty of [*] percent ([*]%) of Net Sales.
Royalties shall be payable to Rockefeller by Amgen pursuant to this Subparagraph (c) on a Patented Product by Patented Product and country by country basis for the lives of the pertinent patent(s) within Licensed Patent Rights. Thereafter, Amgen shall have a fully paid up license to make, use, and sell that Patented Product in that country.
| (d) | Amgen shall pay to Rockefeller, for Net Sales of a product the selling of which would in the absence of this Agreement, and assuming such claim were issued, infringe a claim in a pending patent application included within Licensed Patent Rights in the country in which the product is sold by Amgen or a sublicensee thereof (a “Patent Pending Product”), a royalty on Net Sales equal to [*] the royalty rates set forth in Subparagraph 6.1 (c) (i), (ii) and (iii) applied to annual Net Sales of such Patent Pending Product in the Territory. Royalties shall be payable to Rockefeller by |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| Amgen pursuant to this Subparagraph (d) on a Patent Pending Product by Patent Pending Product and country by country basis for a period of up to [*] beginning on the date of the first commercial sale of a Patent Pending Product in a given country. Thereafter, Amgen shall have a fully paid up license for that Patent Pending Product in that country. In the event a patent subsequently issues on such Patent Pending Product during the term of this Agreement, then the payment of royalties by Amgen shall be determined in accordance with Subparagraph 6.1 (c). |
| (e) | For all Products other than Patented Products or Patent Pending Products (“Know-How Product”), Amgen shall pay to Rockefeller, for Net Sales of such Know-How Product a royalty of [*] percent ([*]%) applied to annual Net Sales of such Know-How Product in the Territory. Royalties shall be payable to Rockefeller by Amgen pursuant to this Subparagraph (e) on a Know-How Product by Know-How Product and country by country basis for a period of [*] beginning on the date of the first commercial sale of such Know-How Product in a given country. Thereafter, Amgen shall have a fully paid up license for that Know-How Product in that country. |
6.2 The obligation to pay royalties hereunder is imposed only once with respect to the sale of a Product regardless of the number of claims which cover such Product and only under one of Subparagraph 6.1 (c), (d), or (e).
6.3 There shall be no obligation to pay Rockefeller royalties on the sale of Products by Amgen to sublicensees for resale to unrelated third parties, but in such instances, the obligation to pay royalties shall only arise upon the sale by Amgen or sublicensees to such unrelated third parties.
6.4 Amgen shall have the right to sublicense third parties under Licensed Patent Rights and Technical Information to make, use and sell Products in the Territory, provided only that Amgen shall disclose the identity of the sublicensee(s) to Rockefeller. Products sold, leased, or otherwise disposed of by Amgen’s sublicensee shall be considered to be sales, leases or disposals of products by Amgen for purposes of determining Net Sales and the calculation of royalty payments and the preparation of royalty reports under this Agreement.
6.5 Rockefeller agrees to use reasonable efforts, short of litigation, to resist any claim by a third party which might affect the exclusive nature of any license granted hereunder. In the event that during the term of this Agreement the license granted herein is rendered non-exclusive in any country of the Territory as a result of any governmental, judicial or other action, then the royalty provided in Subparagraph 6.1 (c), (d), or (e) hereof due to Rockefeller from Amgen on the affected Products) in that country shall be reduced by [*]. Amgen’s obligation to pay to Rockefeller a royalty on the affected Product pursuant to Subparagraph 6.1 (c), (d), or (e) in that country shall expire/terminate no later than [*] (in the case of 6.1 (c) or (d)) or [*] (in the case of 6.1 (e)) [*]. Thereafter, Amgen shall have a fully paid up license to make, use, and sell the affected Produces) in that country.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
6.6 Upon commencement of commercial sales of Products which generate a royalty to Rockefeller pursuant to this Agreement, Amgen shall, within ninety (90) days after the end of each calendar quarter of each year, make quarterly reports to Rockefeller showing the aggregate Net Sales of Products sold, leased or otherwise disposed of in the Territory during such period and the calculation of royalties thereon. Any royalty then due and payable shall be included with such report.
6.7 Amgen’s records that are necessary to determine the correctness of any payment of royalties hereunder shall be open to inspection for five (5) years following the end of the period to which they pertain by an independent Certified Public Accountant designated by Rockefeller and reasonably acceptable to Amgen, at reasonable times and from time to time, during normal business hours, and upon reasonable notice, for the sole purpose of verifying the accuracy of the reports and amount of royalty payments due. Any information obtained by Rockefeller from Amgen pursuant to this Subparagraph 6.7 shall be deemed Confidential Information under this Agreement. Should such Inspection lead to the discovery of a greater than ten percent (10%) discrepancy in reporting to Rockefeller’s detriment, Amgen agrees to pay the full cost of such inspection.
6.8 All payments due hereunder shall be paid with deduction of taxes or other fees which may be imposed by any foreign government on royalties payable to Rockefeller and which shall be paid by Amgen, provided, however, if Amgen shall be reimbursed or receive credit for such taxes Amgen shall pay to Rockefeller the amount deducted for taxes.
7. INFRINGEMENT
7.1 Rockefeller agrees to notify Amgen promptly of any evidence of third party infringement of the Licensed Patent Rights and all details of such infringement of Licensed Patent Rights of which it becomes aware.
7.2 Amgen, or its sublicensees, shall have the right but not the obligation, in its own name, to institute patent infringement proceedings against third parties based on any Licensed Patent Rights licensed hereunder. The expense of any such proceedings, including lawyers’ fees and costs, shall be borne by Amgen. Each Party shall execute all necessary and proper documents and take all other appropriate action required to institute and prosecute such proceedings. If Amgen or its sublicensee elects to commence an action for infringement and Rockefeller is a legally indispensable party to such action, Rockefeller shall have the right to assign to Amgen its right, title and Interest in the subject patent(s) or application(s) (subject to its obligations to the U.S. government) in lieu of joining as an indispensable party, should that be sufficient for purposes of commencing and maintaining the action. Regardless of such assignment or not, however, Rockefeller shall cooperate fully with Amgen in such action upon request by Amgen. During the term of this Agreement, Rockefeller agrees to use its best efforts to ensure that Dr. Xxxxxxx Xxxxxxxx and any co-inventors of Licensed Patent Rights (and/or other Rockefeller or Xxxxxx Xxxxxx Medical Institute employees as might reasonably be requested for assistance by Amgen) will be available to cooperate with Amgen at Amgen’s request and expense In connection with such action.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
7.3 Any award paid by third parties as a result of such patent infringement proceedings (whether by way of settlement or otherwise) shall first be applied toward reimbursement for the legal fees and expenses incurred, and the excess, if any, shall be treated as Net Sales, prorated over the period of the alleged infringement, of Products, on which Amgen shall pay the applicable royalty. So long as such infringement proceedings continue, Amgen shall be permitted to reserve any royalty due to Rockefeller on sales of the affected Product in the country in question until such time as the proceedings have been concluded. If the patent is finally held to be valid and infringed by any such third party, the reserved royalty shall thereupon be promptly paid to Rockefeller; if such patent is finally held to be unenforceable or invalid, then Rockefeller shall not be entitled to the reserved royalty and no further royally shall be due by Amgen or its sublicensees under that patent; royalties theretofore paid may be retained.
7.4 Should Amgen In any calendar year be required to pay royalties in any country under third party patents in order to make, use, or sell a Product hereunder, Amgen shall have the right to deduct such royalties from any royalties due Rockefeller in that country, up to a maximum of [*], provided, however, that the royalty owed to Rockefeller shall never be less than [*] of the amount otherwise payable by Amgen to Rockefeller on Net Sales of such Product pursuant to Section 6 in the affected country. In the event unlicensed competition should render it impossible for Amgen to make an acceptable profit in any country of the Territory, the Parties shall meet to discuss an appropriate further reduction in the royalty due Rockefeller for that country.
7.5 Amgen shall have the first right, but not the obligation, to defend any suit against Amgen or its sublicensees alleging infringement of any third party patent right arising out of the manufacture, use, or sale of a Product by Amgen or its sublicensees. Rockefeller and Amgen shall confer with each other and cooperate during the defense of any such action. If Amgen finds it necessary or desirable for Rockefeller to join Amgen as a party, Rockefeller shall execute all papers or perform such other acts as may reasonably be required by Amgen. Rockefeller agrees to use its best efforts to ensure that Dr. Xxxxxxx Xxxxxxxx and any co-inventors of Licensed Patent Rights (and/or other Rockefeller or Xxxxxx Xxxxxx Medical Institute employees as might reasonably be requested for assistance by Amgen) will be available to cooperate with Amgen at Amgen’s request and expense for pursuing such action. Rockefeller shall be entitled to participate in and have counsel selected by it participate in any such action. Amgen shall bear the costs and expenses associated with any such suit or action. So long as such Infringement proceedings by a third party continue, Amgen shall be permitted to reserve the royalty payable to Rockefeller on the sales of any affected Product in the country in question until such time as the proceedings have been concluded. If the third party patent right is finally held to be uninfringed, unenforceable or invalid, then any reserved amount shall be promptly paid to Rockefeller. If the third party patent right is finally held to be valid and infringed by Amgen or its sublicensees or Amgen or the sublicensees enter into a settlement of such proceedings, Amgen or its sublicensees shall pay the full amount of such royalties, damages and/or settlement amounts due to such third party.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
7.6 Unless abandoned or terminated, the licenses herein granted shall continue for the lives of any patents licensed hereunder as the same or the effectiveness thereof may be extended by an governmental authority, rule or regulation applicable thereto. Thereafter, upon expiration of all Licensed Patent Rights in a country, Amgen shall have a fully paid-up license to make, use and sell the Products(s) in that country.
7.7 In any infringement suit that Amgen may institute to enforce or defend the Licensed Patent Rights pursuant to this Agreement, Rockefeller, at the request and expense of Amgen, shall cooperate in all respects and, to the extent possible, have its employees testify when requested and make available relevant records, papers, information, samples, specimens, and the like.
8. ABANDONMENT
Should Amgen decide at any time during the term hereof that it will no longer pursue commercial development of all Products licensed hereunder, Amgen shall promptly notify Rockefeller of its decision and upon request from Rockefeller, shall take whatever steps are necessary to assure reversion to Rockefeller of the rights licensed hereunder to such Products.
9. TIMES AND CURRENCIES OF PAYMENT
9.1 Royalties due hereunder shall be payable in United States Dollars. Where the Net Sales has been collected in a foreign currency, it shall be converted into the United States Dollar equivalent, at the rate of exchange for buying funds (as reported in The Wall Street Journal) for the last business day of the royalty period (or, in the event The Wall Street Journal does not report such conversion rate, then another reliable and generally accepted source agreeable to the Parties shall be used).
9.2 If Amgen or its sublicensees under this Agreement are unable to convert a foreign currency into United States Dollars for reasons beyond its control, or is restricted by law or regulation from remitting royalty payments from any country of sale, Amgen shall cause such payment to be made to Rockefeller by deposit to the credit and account of Rockefeller or its designated nominee in any commercial bank designated by Rockefeller in the applicable country. Amgen shall deliver to Rockefeller proper evidence of such deposit
10. PUBLICITY
10.1 Except as may be required by law or as may be required to be disclosed in Amgen’s filings with the United States Securities and Exchange Commission, Amgen will not use directly or by implication the name of Rockefeller or the name of the Xxxxxx Xxxxxx Medical institute, or the name of any member of the faculty or staff of Rockefeller or the Xxxxxx Xxxxxx Medical institute and/or their faculty or staff without the prior written approval of Rockefeller and/or the Xxxxxx Xxxxxx Medical Institute and their faculty or staff member involved.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
10.2 Except as may be required by law, Rockefeller will not use directly or by implication the name of Amgen or the name of any member of the staff of Amgen without the prior written approval of Amgen and its staff member involved.
11. INDEMNIFICATION AND PRODUCT LIABILITY
Amgen agrees to indemnify, defend and hold harmless Rockefeller and its trustees, officers, agents, faculty, medical and professional staff, employees, and students and the Xxxxxx Xxxxxx Medical Institute, and its trustees, officers, agents, faculty, medical and professional staff, employees and students from any and all liability, damage, loss or expense including reasonable attorneys’ fees and expenses of litigation) in connection with any claims, suits, action or judgments for personal injury or property damage arising out of Amgen’s (or any sublicensee of Amgen) acquisition, use, design, manufacture, promotion or sale of any Product licensed hereunder or otherwise arising out of acts or omissions of Amgen (or any sublicensee of Amgen) under this Agreement, except to the extent that such claim arises from Rockefeller’s or the Xxxxxx Xxxxxx Medical Institute’s willful misconduct or gross negligence. Amgen agrees, at its own expense, to provide attorneys reasonably acceptable to Rockefeller and the Xxxxxx Xxxxxx Medical Institute to defend against any actions brought or filed against any party indemnified hereunder with respect to this indemnity regardless of whether such action was properly or rightfully brought. Amgen further agrees to obtain and maintain in force product liability insurance coverage or a program of self insurance reasonably acceptable to Rockefeller (with coverage tor claims of bodily injury and property damage) before proceeding with marketing any Products in amounts at normal, customary and commercially acceptable levels, as appropriate to the risk as determined by reference to reliable standards in the industry, such Insurance to specifically name Rockefeller and the Xxxxxx Xxxxxx Medical Institute as additional insureds, and to provide written evidence of insurance to Rockefeller upon request. Amgen shall maintain this product liability insurance beyond expiration or termination of the Agreement during
| (a) | the period that any Product is commercially distributed or sold by Amgen or a sublicensee, and |
| (b) | a reasonable period thereafter which in no event shall be less than 5 years. |
12. COLLABORATION
It is the intention of the Parties and the Xxxxxx Xxxxxx Medical Institute to endeavor to form a scientific collaboration in order to facilitate future research and to enhance or support Licensed Patent Rights. Such a collaboration will be governed by a Research Collaboration Agreement, such agreement to be subject to the approval of the Xxxxxx Xxxxxx Medical Institute.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
13. CONFIDENTIALITY
Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, the Parties agree that, for the term of this Agreement and for five (5) years thereafter, the receiving Party shall keep confidential and shall not publish or otherwise disclose and shall not use for any purpose other than as provided for in this Agreement any Confidential Information furnished to it by the other Party pursuant to this Agreement, except to the extent that it can be established by the receiving Party by competent proof that such Confidential Information:
| (a) | was already known to the receiving Party at the time of disclosure by the other Party; |
| (b) | was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party; |
| (c) | became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement; |
| (d) | was disclosed to the receiving Party, by a third party who had no obligation to the disclosing Party not to disclose such information to others; |
| (e) | was independently discovered or developed by the receiving Party without the use of Confidential Information belonging to the disclosing Party; or |
| (f) | was information that the receiving Party believes in good faith is required to be disclosed to comply with any applicable law, regulation or order of a government authority or court of competent jurisdiction (including any securities laws applicable to a Party), in which event the receiving Party shall use all reasonable efforts to advise the other Party in advance of the need for such disclosure. |
14. NO WARRANTY
14.1 Nothing in this Agreement shall be construed as:
| (a) | a warranty or representation by Rockefeller as to the validity of Licensed Patent Rights; |
| (b) | a warranty or representation by Rockefeller that anything made, used, sold or otherwise disposed of under the license granted in this Agreement is or will be free from infringement of patents, copyrights, trademarks, registered design or other intellectual property or contractual rights; |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| (c) | an obligation by Rockefeller to bring or prosecute actions or suits against third parties for infringement of patents, copyrights, trademarks, registered design or other intellectual properly or contractual rights, or |
| (d) | the conferring by Rockefeller or any of its faculty or staff of rights to use in advertising or sales publicity or promotion or otherwise the name, symbol, xxxx or designation of Rockefeller and/or the Xxxxxx Xxxxxx Medical Institute except in accordance with the express terms of this Agreement. |
14.2 SUBJECT TO THE EXPRESS TERMS OF THIS AGREEMENT, ROCKEFELLER EXPRESSLY DISCLAIMS ANY AND ALL IMPLIED OR EXPRESS WARRANTIES AND MAKES NO EXPRESS OR IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE OF THE LICENSED PATENT RIGHTS OR ANY BIOLOGICAL MATERIAL OR TECHNICAL OR OTHER INFORMATION SUPPLIED BY ROCKEFELLER.
15. LEGAL COMPUANCE
Amgen agrees to comply with all laws and regulations applicable to its activities contemplated hereunder.
16. NOTICES
Any Notice required to be given pursuant to this Agreement shall be made by personal delivery, by courier service or, if by mail, by registered or certified mail, return receipt requested, by one Party to the other Party at the addresses noted below.
In the case of Amgen, notice should be sent to:
Amgen Inc.
Amgen Center
0000 XxXxxxxxxxx Xxxxx
Xxxxxxxx Xxxx, XX 00000-0000
Attention: Secretary
cc: Senior Vice President, Research
Vice President, Product Licensing
In the case of Rockefeller, notice should be sent to:
The Rockefeller University
0000 Xxxx Xxxxxx
Xxx Xxxx, Xxx Xxxx 00000-0000
Attention: Office of the General Counsel
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
17. LAW TO GOVERN
This Agreement shall be interpreted and governed in accordance with the laws of the Stale of New York, except that questions affecting the construction and effect of any patent shall be determined by the law of the country in which the patent was granted.
18. ASSIGNMENT18
This Agreement may not be assigned by either Party without the prior written consent of the other; provided, however, shall, Amgen may assign this Agreement to any successor by merger of Amgen or purchase or sale of substantially all of its property and assets in a manner such that the assignor shall remain liable and responsible for the performance and observance of all its duties and obligations hereunder. This Agreement shall be binding upon the successors and permitted assigns of Amgen and the name of Amgen appearing herein shall be deemed to include the names of such Party’s successors and permitted assigns to the extent necessary to carry out the intent of this Agreement. Any assignment not in accordance with this Section shall be void.
19. TERMINATION
19.1 This Agreement shall remain in full force and effect until terminated as provided herein. Amgen shall have the right to terminate this Agreement at any time from and after the first anniversary of the Effective Date upon ninety (90) days’ prior written notice to Rockefeller. Such termination shall automatically terminate the license provided in Section 2 hereof, but shall not affect the right of Rockefeller to retain any and all payments theretofore made (or due and payable) hereunder up to the effective date of termination, including, without limitation, any payments provided for in Section 6 hereof. In the event of such termination, Amgen agrees to discuss in good faith the transfer to Rockefeller of clinical data developed by Amgen on Products), subject to payment of appropriate compensation to Amgen. Under no circumstances shall Amgen be required to divulge any Amgen proprietary or confidential information concerning process development, manufacturing, sales, or marketing.
19.2 Either Party may terminals this Agreement in the event of a material breach by the other Party, provided only that the offending Party is given notice of the breach and a reasonable time, not to exceed ninety (90) days, in which to cure such breach.
19.3 Any termination of this Agreement and/or license granted hereunder shall also terminate any sublicense therefor.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
20. REPRESENTATIONS
Rockefeller represents:
20.1 Rockefeller is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder. The person executing this Agreement on Rockefeller’s behalf has been duly authorized to do so by all requisite legal action.
20.2 This Agreement is a legal and valid obligation binding upon Rockefeller and enforceable in accordance with its terms. The execution, delivery and performance of this Agreement by Rockefeller does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, nor violate any material law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
20.3 Rockefeller has not, and will not during the term of this Agreement, grant any right to any third party in the Territory which would conflict with the rights granted to Amgen hereunder.
20.4 Rockefeller hereby represents that it has no knowledge of any claim asserting that the Licensed Patent Rights or Technical Information fall within the scope of any rights of any third parties.
20.5 Rockefeller hereby represents that it has no knowledge of any infringement of any of the Licensed Patent Rights.
Amgen represents:
20.6 Amgen is duly organized and validly existing under the laws of Delaware and has full corporate power and authority to enter into this Agreement and carry out the provisions hereof.
20.7 Amgen is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder. The person executing this Agreement on Amgen’s behalf has been duly authorized to do so by all requisite corporate action.
20.8 This Agreement is a legal and valid obligation binding upon Amgen, and enforceable in accordance with its terms. The execution, delivery and performance of this Agreement by Amgen does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, nor violate any material law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
20.9 Subject to the provisions of Section 26, Amgen is aware of no action, suit or inquiry or investigation instituted by any U.S. federal or state governmental agency which questions or threatens the validity of this Agreement.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
21. RESOLUTION OF DISPUTES
The Parties agree that in the event of a dispute between them arising from, concerning, or in any way relating to this Agreement, the Parties shall undertake good faith efforts to resolve the same amicably between themselves.
22. FORCE MAJEURE
The Parties shall not be liable in any manner for failure or delay in fulfillment of all or part of this Agreement, directly or indirectly caused by acts of God, governmental orders or restrictions, war, war-like condition, revolution, riot, looting, strike, lockout, fire, flood or other similar or dissimilar causes or circumstances beyond the non-performing Party’s control. The non-performing Party shall promptly notify the other Party of the cause or circumstance and shall recommence its performance of its obligations as soon as practicable after the cause or circumstance ceases.
23. SEVERABILITY
In the event that any one or more of the provisions of this Agreement should for any reason be held by any court or authority havingjurisdiction over this Agreement to be invalid, illegal or unenforceable, such provision or provisions shall be reformed to approximate as nearly as possible the intent of the parties, and if unenforceable, shall be divisible and deleted in such jurisdiction and the remainder of the Agreement shall remain in full force and effect; elsewhere, the Agreement shall not be affected.
24. INDEPENDENT CONTRACTORS
The relationship between Rockefeller and Amgen is that of independent contractors. Rockefeller and Amgen are not joint venturers, partners, principal and agent, master and servant, employer or employee, and have no other relationship other than independent contracting parties. Rockefeller shall have no power to bind or obligate Amgen In any manner, other than as is expressly set forth in this Agreement. Likewise, Amgen shall have no power to bind Rockefeller in any manner, other than as is expressly set forth in this Agreement
25. ENTIRE UNDERSTANDING
This Agreement represents the entire understanding between the Parties concerning the subject matter hereof.
26. CONDITIONS TO EFFECTIVENESS
This Agreement shall become effective upon the full and complete satisfaction of the following conditions:
| (a) | termination of any waiting period(s) under the provisions of 15 U.S.C. 18A (the Xxxx-Xxxxx-Xxxxxx Antitrust Improvements Act of 1976); and |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| (b) | no judgment, injunction, order or decree shall prohibit, or threaten to prohibit, this Agreement as of the date of termination of all such waiting period(s). |
27. GOOD FAITH DEALINGS
The Parties agree to deal with each other in good faith.
28. PUBLICATIONS
Rockefeller agrees that each proposed publication that describes or discloses Licensed Patent Rights or Technical information, before submission to a publisher, will be submitted to Amgen for review in connection with preservation of exclusive patent rights. Amgen shall have thirty (30) days in which to review each publication, which may be extended for an additional thirty (30) days when Amgen provides substantial and reasonable need for such extension. By mutual agreement, this period may be further extended for not more than an additional three (3) months. When requested by Rockefeller in advance, Amgen, at its discretion, may allow for simultaneous submission of the publication to the publisher. Scientists at both Rockefeller and Amgen will be expected to treat matters of authorship in a proper collaborative spirit, giving credit where it is due and proceeding in a manner which fosters cooperation and communication, but will not do anything in this regard which will jeopardize the issuance of a valid patent.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
IN WITNESS WHEREOF, the Parties have caused this Agreement to be duly executed as of the day and year first above written.
| AMGEN INC. | ||
| By: | /s/ Xxxxxx Xxxxxx | |
| Title: | Senior Vice President | |
| Date: | 4/14/95 | |
| THE ROCKEFELLER UNIVERSITY | ||
| By: | /s/ Xxxxxxx Xxxxxx | |
| Title: | President | |
| Date: | 4/14/95 | |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT “A”
U.S.S.N. 08/292,345 filed August 17, 1994 (Parent)
U.S.S.N. 08/347,563 filed November 30, 1994 (CIP)
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT “B”
MATERIAL TRANSFER AGREEMENT
THIS MATERIAL TRANSFER AGREEMENT (“Agreement”) is entered into by and between Rockefeller University, 0000 Xxxx Xxxxxx, Xxx Xxxx, Xxx Xxxx 00000-0000 (“Rockefeller”) and , (“Institution”).
Whereas, Rockefeller and Amgen Inc. (“Amgen”) have entered into a License Agreement dated April 14,1995 under which Rockefeller has exclusively licensed to Amgen rights in Materials (defined below) and technology based on Materials;
Whereas, Rockefeller is willing to provide to you Rockefeller’s proprietary and mutually agreed additional materials (collectively “Material”) in accordance with the following terms:
Research Program. Institution shall undertake a research program (“Research Program”) as described in attached Appendix A. Institution agrees that the Research Program shall be conducted by or under the direct supervision of (“Investigator”). The Research Program may be modified, upon mutual written agreement of Rockefeller and Institution.
Reports. Institution shall furnish Rockefeller with a final written report summarizing the results of the Research Program within thirty (30) days of completion of the Research Program or termination of this Agreement. The final report will be sent to the person at Rockefeller who sent the Material or a designee thereof at Rockefeller. Rockefeller and Amgen shall have the unrestricted right to utilize all data and information developed under the Research Program in internal research.
Research Materials. In consideration of the services provided by Institution and the rights obtained by Rockefeller and Amgen under this Agreement, Rockefeller shall provide Investigator with a mutually agreed upon quantity of the Material. The Materials and all other materials arising out of the conduct of the Research Program (“Program Materials”) shall be used for the sole purpose of conducting the Research Program. Any Material or Program Materials remaining upon conclusion of this Agreement shall be returned to Rockefeller or, at Rockefeller’s option, destroyed, within thirty (30) days following completion of the Research Program or termination of this Agreement, or upon request of Rockefeller prior to completion of the Research Program. Institution shall not provide either Materials or Program Materials to any third party without the prior written approval of Rockefeller.
Confidentiality. For the term of this Agreement, and any subsequent extension, and for a period of five (5) years thereafter, Institution will not use, except as necessary for purposes of the Research Program, or disclose or provide to any third party without the prior written consent of Rockefeller any Confidential Information. For purposes of this Agreement, “Confidential Information” means all information, reagents, procedures, results, conclusions, and the like which are disclosed or provided to Institution by Rockefeller in connection with the Research Program. Institution shall have no obligation with respect to any portion of such Confidential Information which:
| (a) | is or later becomes generally available to the public by use, publication or the tike, through no fault of Institution; or |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| (b) | is obtained from a third party who had the legal right to disclose the same to Institution; or |
| (c) | Institution already possesses, as evidenced by its written records, predating receipt thereof from Rockefeller; or |
| (d) | was information that Institution believes in good faith is required to be disclosed to comply with any applicable taw, regulation or order of a government authority or court of competent jurisdiction, in which event Institution shall use all reasonable efforts to advise Rockefeller in advance of the need for such disclosure. |
Publications. Institution shall submit all scientific articles, manuscripts, abstracts, and posters or summaries of any oral presentations relating to the Research Program to Rockefeller thirty (30) days prior to submission for publication or presentation. Rockefeller shall have thirty (30) days to review and comment on each proposed presentation or publication. Institution shall delete any Confidential Information and postpone publication or presentation for up to forty-five (45) days upon request by Rockefeller in order to allow appropriate patent applications to be filed. These periods can be extended by mutual agreement of the parties.
Intellectual Property. Any inventions or discoveries made in performance of the Research Program solely by investigator and/or other personnel affiliated with Institution (“Sole Inventions”), will belong to Institution. Institution shall have the right to obtain patent protection for Sole inventions, at its expense. Any inventions or discoveries made in performance of the Research Program jointly by Investigator and/or other personnel affiliated with Institution and by Rockefeller’s employees or affiliated personnel (collectively, “Joint Inventions”), shall belong jointly to Institution and Rockefeller. Rockefeller shall have the right to obtain patent protection for any Joint inventions involving Rockefeller personnel, at its expense, unless otherwise agreed upon by Rockefeller. Institution shall promptly notify Rockefeller of all inventions and discoveries developed as a result of the Research Program.
Rockefeller and Amgen shall have an exclusive option for the term of this Agreement and [*] thereafter to obtain an exclusive worldwide license, with right to sublicense, for any such patent application, or patent issued thereon, filed for Sole Inventions and/or for Institution’s interest in Joint Inventions. The license shall be negotiated in good faith by the parties and provide for appropriate compensation to Institution, in the event the parties fail to reach a mutually acceptable licensing arrangement within [*] after commencing negotiations, Institution shall grant to Amgen a royalty-free non-exclusive license under any Sole Invention and thereafter Institution shall be entitled to negotiate in good faith with a third party for a license to any patent application or patent on Sole Inventions or Institution rights in Joint Inventions.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Warranty. Institution warrants that it is permitted to enter into this Agreement and that this Agreement is not inconsistent with other contractual arrangements of Institution.
Term. The term of this Agreement shall be for a period of one (1) year from the date of execution of this Agreement, unless extended by written agreement between the parties. Any rights or obligations set forth herein which of their nature are intended to extend beyond the termination of this Agreement including but not limited to the confidentiality and option provisions, shall survive any such termination.
Indemnification. Institution agrees to indemnify, defend, and hold Rockefeller harmless from any liability (including attorney’s fees) resulting from any claim or demand arising from use of the Material by Institution.
IN WITNESS WHEREOF, the parties hereto have caused this instrument to be executed by their respective duly authorized officers or representatives on the respective dates indicated below.
| Institution | Rockefeller University | |||||||
| By: |
|
By: |
| |||||
| Title: |
|
Title: |
| |||||
| Date: |
|
Date: |
| |||||
| AGREED:
Investigator | ||||||||
| By: |
| |||||||
| Title: |
| |||||||
| Date: |
| |||||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
APPENDIX A
Research Program
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Letterhead of AMGEN
Xxxxxx Xxxxxx, Ph.D
Senior Vice President
Research
May 10, 1995
Xxxxxxx X. Xxxxxxx, Esq.
The Rockefeller University
0000 Xxxx Xxxxxx
Xxx Xxxx, XX 00000-0000
| Re: | Letter Agreement to amend License Agreement between Rockefeller University (“Rockefeller”) and Amgen Inc. (“Amgen”) dated April 14, 1995 (the “Agreement”); Amgen Reference Number 942985 |
Dear Xx. Xxxxxxx:
Amgen and Rockefeller hereby agree to modify the above referenced Agreement as indicated below.
After Section 1.1, at the bottom, of page 1 of the Agreement, insert the following:
— 1.2 Amgen Affiliated(s) shall mean a person or entity that, directly or indirectly, through one or more intermediates, controls, is controlled by, or is under—.
In all other respects, the Agreement shall remain the same.
If this modification is satisfactory to Rockefeller, please have the appropriate representative sign both originals of this letter and return one fully executed original to Xxx Xxxx at Amgen.
| Sincerely, |
| /s/ Xxxxxx Xxxxxx |
| Xxxxxx Xxxxxx, Ph.D. Senior Vice President, Research |
Agreed: THE ROCKEFELLER UNIVERSITY
| By: | /s/ Xxxxxxx Xxxxxxx |
Date: 5/15/95 | ||||
| Name: | Xxxxxxx X. Xxxxxxx |
| cc: | Xxxxx Xxxxxxx |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
AMENDMENT TO LICENSE AGREEMENT
This AMENDMENT TO LICENSE AGREEMENT (this “Amendment”) dated January 1 , 2006 (“Amendment Date”) is altered into by and between The Rockefeller University, an education corporation organized and existing under the laws of the State of New York, with principal offices located at 0000 Xxxx Xxxxxx, Xxx Xxxx, Xxx Xxxx 00000-0000 (“Rockefeller”), and Amgen Inc., a Delaware corporation with principal offices located at Xxx Xxxxx Xxxxxx Xxxxx, Xxxxxxxx Xxxx, Xxxxxxxxxx 00000-0000 (“Amgen”).
WITNESSETH:
WHEREAS, Rockefeller and Amgen have entered into a License Agreement dated April 14, 1995, as amended and supplemented by a letter agreement dated May 10, 1995 (the “License Agreement”).
WHEREAS, Rockefeller and Amgen now wish to further amend certain terms and conditions set forth in the License Agreement.
NOW THEREFORE, in consideration of the foregoing and the covenants and promises contained in this Amendment, the Parties agree as follows:
1. Capitalized terms used but not defined herein shall have the meanings assigned to such terms in the License Agreement.
2. Section 1 of the License Agreement shall be amended to add (or in the case of the definition for “NDA”, such definition shall be deleted in its entirety and shall be superseded and replaced by the definition for “NDA” set forth hereinbelow) the following definitions:
“BLA” shall mean a Biological License Application and all amendments and supplements thereto submitted to the FDA.
“NDA” shall mean a New Drug Application, and all amendments and supplements thereto submitted to the FDA.
“Orphan Product” shall mean any designation of an indication for a Product as an “Orphan Product” under the U.S. Orphan Drug Act, as amended.
“SLD” shall mean severe lipodystrophy and/or congenital absence of leptin.
3. Subparagraph 6.1(b) of the License Agreement shall be amended in its entirety as follows:
(b) Milestone payments. One-time milestone payments shall be due and payable in the following amounts within thirty (30) days following first achievement by Amgen or its sublicensee of tile corresponding milestones as set forth below;
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
(i) If an NDA or BLA is submitted to the FDA for a Product for any indication within eighteen (18) months from the Amendment Date, the following milestones shall apply to such Product and all subsequent Products (and the milestones in Subparagraphs 6.1(b)(ii) and (iii) shall not apply):
| Milestone Event |
Milestone Payment | |
| [*] |
||
(ii) If an NDA or BLA is submitted to the FDA for a Product for any indication following the eighteenth (18th) month alter the Amendment Date, but before or during the forty second (42nd) month after the Amendment Date (and Subparagraph 6.l(b)(i) does not apply), the following milestones shall apply to such Product and all subsequent Products (and the milestones in Subparagraphs 6.l(b)(i) and (iii) shall not apply):
| Milestone Event |
Milestone Payment | |
| [*] |
||
(iii) If an NDA or BLA is submitted to the FDA for a Product for any indication after the forty second (42nd) month after the Amendment Date but before or during the sixtieth (60th) month after the Amendment Date (and neither Subparagraph 6.1(b)(i) nor (ii) apply), the following milestones shall apply (and the milestones in Subparagraphs 6.i(b)(i) and (ii) shall not apply):
| Milestone Event |
Milestone Payment | |
| [*] |
||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
For the avoidance of doubt, payment of any of the foregoing milestones set forth in Subparagraphs 6.1(b)(i), (ii) or (ill) shall be made only once for the first achievement of such milestone, and not with respect to each additional occasion that such milestone is achieved. Also, for purposes of the milestones set forth in Subparagraphs 6.l(b)(ii) and (iii), a Phase III Licensing Trial shall be deemed initiated upon enrollment of the first patient therefor. For purposes of Subparagraph 6.1, an NDA or BLA shall not require inclusion, of the CMC (Chemistry, Manufacturing and Controls) section to be deemed submitted to the FDA.
Notwithstanding anything in this Agreement to the contrary, to the extent the initiation of the first Phase III Licensing Trial for the first Orphan Product indication of a Product other than SLD, or the first non-Orphan Product indication of a Product other than SLD, occurs prior to the end of the forty-second (42nd) month after the Amendment Date and prior to Amgen or its sublicensee first submitting an NDA or BLA to the FDA for a Product for any indication, the applicable milestone payment under Subparagraph 6.1(b)(ii)(A) or (B) shall be paid to Rockefeller within thirty (30) days following first achievement by Amgen or its sublicensee of such Phase III Licensing Trial milestone event; provided, however, that if the first Phase III Licensing Trial for the first Orphan Product indication of a Product other than SLD, or the first non-Orphan Product indication of a Product other than SLD, occurs (i) within eighteen (18) months of the Amendment Date; and (ii) neither Amgen nor its sublicensee has first submitted an NDA or BLA to the FDA for a Product for any indication within eighteen (18) months after the Amendment Date, the applicable milestone payment under Subparagraph 6.1(b)(ii)(A) or (B) shall be paid to Rockefeller within thirty (30) days of the first day of the nineteenth (19th) month after the Amendment Date. Upon Amgen or its sublicensee first submitting an NDA or BLA to the FDA for a Product for any indication, to the extent that the timing of the filing results in a higher milestone payment (as set forth in Subparagraph 6.1(b)(iii)(A) or (B)) being due Rockefeller for the achievement of a prior milestone (e.g., a milestone payment has been made under Subparagraph 6.1(b)(ii)(A), but the timing of Amgen’s first submission of an NDA or
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
BLA to the FDA for a Product occurs after the forty-second (42nd) month after the Amendment Xxxx such that the milestones of Subparagraph 6.1(b)(iii) apply), Amgen shall pay to Rockefeller such difference within thirty (30) days of Amgen or its sublicensee first submitting such NDA or BLA to the FDA for a Product for any indication. By way of example, and not as a limitation, if Amgen initiates the first Phase III Licensing Trial for the first Orphan Product indication of a Product other than for SLD prior to first submitting an NDA or BLA to the FDA for a Product for any indication (and Subparagraph 6.1(b)(i) does not apply), Amgen shall pay to Rockefeller [*] in accordance with Subparagraph 6.1(b)(ii)(A); and then if Amgen first submits an NDA or BLA to the FDA for a Product for any indication after the forty second (42nd) month after the Amendment Date, Amgen shall pay Rockefeller an additional [*], which is the difference between the [*] initially paid to Rockefeller upon initiation of the first Phase III Licensing Trial for the first Orphan Product indication of a Product other than for SLD under Subparagraph 6.1(b)(ii)(A), and the [*] milestone payment due Rockefeller under Subparagraph 6.1(b)(iii)(A).
4. Subparagraph 6.1 of the License Agreement shall be amended to add the following:
(f) an NDA or BLA is submitted to the FDA by Amgen or its sublicensee within eighteen (18) months of the Amendment Date for a Product for SLD, then for each year for the term of this Agreement, the royalties shall be calculated pursuant to this Subparagraph 6.1(f) rather than Subparagraph 6.1(c). Amgen shall pay royalties on Patented Products at the rate of [*] percent ([*]%) for the first [*] in annual Net Sales. For any additional annual Net Sales, the royalty rates in Subparagraph 6.1(c) of this Agreement shall apply (and for the avoidance of doubt, the first [*] in annual Net Sales set forth in the preceding sentence shall not be subject to such royalty rates; thus the first Dollar of annual Net Sales after the first [*] in annual Net Sales shall be considered the first Dollar of annual Net Sales for purposes of determining the applicable annual Net Sales threshold in Subparagraph 6.l(c)(i), (ii) and (iii)). By way of example and not as a limitation, if Amgen achieved [*] in annual Net Sales for a Patented Product, the royalty rates applicable to such annual Net Sales would be a [*] percent ([*]%) royalty on the first [*], a [*] percent ([*]%) royalty on the next [*] (which is that portion of total annual Net Sales between [*] and [*], and a [*] percent ([*]%) royalty on [*], which is the balance of the annual Net Sales.
(g) If an NDA or BLA is submitted to the FDA by Amgen or its sublicensee after the forty second (42nd) month following the Amendment Date but before or during the sixtieth (60th) month after the Amendment Date for a Product for any indication (and an NDA or BLA has not been submitted to the FDA by Amgen or its sublicensee prior to or during the forty second (42nd) month following the Amendment Date for a Product for any indication), then for each year for the term of this Agreement, the royalties shall be calculated pursuant to this Subparagraph 6.1(g) rather than Subparagraph 6.1(c). Amgen shall pay royalties on Patented Products at the rate of [*] percent ([*]%) for the first [*] in cumulative Net Sales. Thereafter, for all additional Net Sales in the year in which [*] cumulative Net Sales is achieved and for all Net Sales in all subsequent years, the royalty rates in Subparagraph 6.1(c) of this Agreement shall apply (and for the avoidance of doubt, the first [*] in cumulative Net Sales set forth in the preceding sentence, shall not be subject to such royalty rates; thus all annual Net Sales in excess of the first [*] in cumulative Net Sales would be subject to the royalty rates set forth in Subparagraph 6.1(c) from
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
the first Dollar after the first [*] in cumulative Net Sales). By way of example and not as a limitation, if this Subparagraph 6.1(g) applies and Amgen achieves [*] in cumulative Net Sales for a Patented Product on March 31, 2009 (to which the foregoing [*] percent ([*]%) royalty would apply), and from April 1, 2009 to December 31, 2009, Amgen achieves Net Sales in the amount of [*], the royalty rates applicable to the Net Sales occurring from April 1, 2009 to December 31, 2009 would be a [*] percent ([*]%) royalty on [*] of such Net Sales, and a [*] percent ([*]%) royalty on the remaining [*] of such Net Sale.
(h) If no NDA or BLA has been submitted to the FDA by Amgen or its sublicensee by the fifth (5th) anniversary of the Amendment Date, Amgen or its sublicensee shall pay to Rockefeller [*] annually as a maintenance fee until an NDA or BLA has been submitted to the FDA by Amgen or its sublicensee for a Product for any indication, and Amgen or its sublicensee shall be subject to (on a prospective basis only) all unpaid original milestones described in Paragraph 6.1(b) of the License Agreement as in effect prior to the Amendment Date.
5. Subparagraph 6.5 of the License Agreement shall be amended in its entirety as follows:
6.5 Rockefeller agrees to use reasonable efforts, short of litigation, to resist any claim by a third party which might affect the exclusive nature of any license granted hereunder. In the event that during the term of this Agreement the license granted herein is rendered non-exclusive in any country of the Territory as a result of any governmental, judicial or other action, then the royalty provided in Subparagraph 6.1(c), (d), (e), (f) or (g) hereof due to Rockefeller from Amgen (and its sublicensees) on the affected Products) in that country shall be reduced by [*]. Amgen’s obligation to pay to Rockefeller a royalty on the affected Product pursuant to Subparagraph 6.1(c), (d), (e), (f) or (g) in that country shall expire/terminate no later than six (6) years (in the case of Subparagraph 6.1(c), (d), (f) or (g)) or five (5) years (in the case of Subparagraph 6.1(e)) from the date of first commercial sale of the affected Products) in that country. Thereafter, Amgen shall have a fully paid-up and perpetual license to make, use, and sell the affected Product(s) in that country.
6. Subpargraphs 6.9 and 6.10 are hereby added to the License Agreement as follows:
6.9 Sublicense Revenues. Within thirty (30) days of the date upon which any upfront license fees and other lump sum or one-time fees in consideration of a sublicense to the Licensed Patent Rights and Technical Information are paid to Amgen (including without limitation any other fee or payment for a sublicense to the Licensed Patent Rights and Technical Information which is paid to Amgen solely on the basis of the passage of time), Amgen shall pay to Rockefeller [*] percent ([*]%) of such amounts in accordance with Paragraph 9. To the extent that upfront license fees and other lump sum or one-time fees in consideration of a sublicense to the licensed Patent Rights and Technical Information are paid to Amgen in the form of equity securities, Amgen shall transfer, or cause to be issued to Rockefeller, [*] percent ([*]%) of the equity securities received by (or that would otherwise be issued to) Amgen, subject to applicable securities laws and other laws. As a condition to receiving such equity securities, Rockefeller acknowledges that it
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
may be required to execute certain typical and customary agreements relating to the purchase and sale of such securities, which agreements may require certain representations by Rockefeller and impose certain obligations on Rockefeller related to, among other things, registration, lock-ups, co-sale, first refusal, first offer and voting rights, if any, relating to such equity securities. The parties acknowledge and agree that Amgen, its affiliates, subsidiaries and agents shall have no liability whatsoever with respect to the equity securities transferred or issued to Rockefeller, including without limitation, any liability related to the value, liquidity, volatility, rights, preferences or any other aspect of such securities. For the avoidance of doubt, any milestone or other payments tied to future clinical, regulatory, operational or commercial outcomes or sales shall not be considered sublicense revenues for purpose of this Subparagraph 6.9, and shall be excluded from such amounts.
6.10 No Other Compensation. Other than as explicitly set forth (and as applicable) in this Section 6, and Subparagraph 5.2, Section 7 and Section 11, Amgen shall not be obligated to pay any additional fees, milestone payments, royalties or any other payments to Rockefeller regarding the Licensed Patent Rights and Technical Information.
7. Section 16 of the License Agreement is hereby amended to change Amgen’s address to the following:
Amgen Inc.
Xxx Xxxxx Xxxxxx Xxxxx
Xxxxxxxx Xxxx, Xxxxxxxxxx 00000-0000
Attention: Corporate Secretary
cc: Vice President, Licensing
8. Subparagraph 19.3 of the License Agreement shall be amended in its entirety as follows:
19.3 Upon any termination of this Agreement, any sublicense(s) granted by Amgen prior to termination of this Agreement shall remain in full force and effect, provided that (i) the sublicensee is not then in material breach of its sublicense agreement, and (ii) upon Rockefeller’s written request to the sublicensee, the sublicensee agrees in writing within thirty (30) days of receipt of such request, to be bound as licensee under the terms and conditions of this Agreement.
9. Section 24 of the License Agreement is hereby amended to include the following sentence:
HHMI is not a party to this Agreement and has no liability to any licensee, sublicensee, or user of anything covered by this Agreement, but HHMI is an intended third-party beneficiary under Subparagraphs 2.5, 3.1, 10.1, 14.1(d), and Section 11 of this Agreement and such Subparagraphs and Sections are for the benefit of HHMI and are enforceable by HHMI in its own name.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
10. Except as amended and supplemented hereby, all of the terms of the License Agreement, as amended and supplemented to date, shall remain and continue in full force and effect.
11. This Amendment may be executed in any number of counterparts, each of which need not contain the signature of more than one Party, but all such counterparts taken together shall constitute one and the same document.
12. Whenever possible, each provision of this Amendment will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Amendment is held to be prohibited by or invalid under applicable law, such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Amendment.
13. No amendment, modification or supplement of any provision of this Amendment shall be valid or effective unless made in writing and signed by a duly authorized officer of each Party.
14. This Amendment and the License Agreement constitute and contain the complete, final and exclusive understanding and agreement of the Parties with respect to the subject matter hereof and thereof, and cancel and supersede any and all prior negotiations, correspondence, understandings and agreements, whether oral or written, between the Parties, respecting the subject matter hereof and thereof.
15. Notwithstanding anything in the License Agreement to the contrary, Rockefeller acknowledges and agrees that Amgen may disclose the terms of the License Agreement and this Amendment, and Confidential Information of Rockefeller disclosed to Amgen under the License Agreement and this Amendment, to potential sublicensees of the Licensed Patent Rights and Technical Information under confidentiality obligations no less restrictive than those Amgen requires of parties to whom it may disclose its own confidential information.
16. This Amendment shall be binding upon and inure solely to the benefit of each Party and its respective successors and assigns, and nothing in this Amendment, express or implied, is intended to or shall confer upon any other person any right, benefit or remedy of any nature whatsoever under or by reason of this Amendment.
17. Each of Amgen and Rockefeller hereby represent and warrant that it has the right to enter into this Amendment and that the terms of this Amendment are not inconsistent with other contractual obligations, express or implied, which it may have.
18. Amgen and Rockefeller agree that if Amgen has not sublicensed its rights under the Licensed Patent Rights and Technical Information to a third party by March 31, 2006, this Amendment shall terminate without effect, in which case the terms and conditions of the License Agreement shall apply.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
IN WITNESS WHEREOF, the Parties hereto have executed this Amendment as of the date first above written.
AMGEN INC.
| By: | /s/ Xxxxx Xxxxxxx | |
| Xxxxx X. Xxxxxxx, Esq. | ||
| Title: | Vice President, Licensing |
THE ROCKEFELLER UNIVERSITY
| By: | /s/ Xxxxxxx X. Xxxx | |
| Title: | VP & General Counsel |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
CONFIDENTIAL
SECOND AMENDMENT TO LICENSE AGREEMENT
This SECOND AMENDMENT TO LICENSE AGREEMENT (this “Amendment”) dated as of January 15, 2008 (“Amendment Date”) is entered into by and between The Rockefeller University, an education corporation organized and existing under the laws of the State of New York, with principal offices located at 0000 Xxxx Xxxxxx, Xxx Xxxx, Xxx Xxxx 00000-0000 (“Rockefeller”), and Amgen Inc., a Delaware corporation ‘with principal offices located at Xxx Xxxxx Xxxxxx Xxxxx, Xxxxxxxx Xxxx, Xxxxxxxxxx 00000-0000 (“Amgen”).
WITNESSETH:
WHEREAS, Rockefeller and Amgen have entered into a License Agreement dated April 13, 1995, as amended and supplemented by a letter agreement dated May 10,1995 and an Amendment dated January 1, 2006 (the “License Agreement”);
WHEREAS, Rockefeller and Amgen now wish to further amend certain terms and conditions set forth in the License Agreement;
NOW, THEREFORE, in consideration of the foregoing and the covenants and promises contained in this Amendment, the Parties agree as follows:
1. Capitalized terms used but not defined herein shall have the meanings assigned to such terms in the License Agreement.
2. Section 1 of the License Agreement shall be amended to add the following definitions:
“Active Component” shall mean any active pharmaceutical ingredient other than the Product which performs an identifiable therapeutic or prophylactic function when combined with the Product, and which is not disclosed or enabled under the Licensed Patent Rights.
“Combination Product” means any product sold by or on behalf of Amgen, its Affiliates or Sublicensee(s) which contains the Product in combination with one or more Active Components. For avoidance of doubt, Combination Products are a subset of Products.
3. Section 1.11 of the License Agreement shall be deleted and replaced in its entirety with the following:
“1.11 “Net Sales” shall mean all revenues, recognized in accordance with generally accepted accounting principles consistently applied, received from sales or other dispositions by Amgen, or a sublicensee of Amgen, to a third party, less returns and allowances (actually paid or allowed, including, but not limited to, prompt payment and volume discounts, chargebacks from
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
wholesalers and other allowances granted to customers or wholesalers, whether in cash or trade), bad debt, freight, shipping, packing, insurance, rebates, and sales and other taxes based on sales prices when included in gross sales, but not including taxes when assessed on income derived from such sales.
In the case of a Product that is a Combination Product, Net Sales for such Combination Product shall be calculated by multiplying actual Net Sales of such Combination Product by the fraction A/(A+B) where A is the invoice price of the Product which does not contain any Active Component(s), if sold separately, and B is the total invoice price of the Active Component(s) in the Combination Product, if sold separately. If, on a country-by-country basis, the Active Component(s) in the Combination Product is not sold separately in said country, Net Sales for the purpose of determining royalties of the Combination Product shall be calculated by multiplying actual Net Sales of such Combination Product by the fraction A/D, where A is the invoice price of the Product which does not contain any Active Component(s), if sold separately, and D is the invoice price of the Combination Product. If neither the Product which does not contain any Active Component(s), nor the Active Component(s) in the Combination Product is sold separately in a given country, the Parties shall determine Net Sales for such Combination Product by mutual agreement based on the relative contribution of the Product which does not contain any Active Component(s) and the Active Components in the Combination Product; provided, however that if the parties are not able to mutually agree on the relative contribution of a Product which does not contain any Active Component(s) and the Active Component(s) in the Combination Product, then Net Sales for such Combination Product containing only one Active Component shall be fifty percent (50%) of the amount calculated in accordance with the first paragraph of this Section 1.11.
In the event a Product is sold with one or more other products or services for a single price (together, a “Multiple Product Offering”), Net Sales for such Multiple Product Offering shall be calculated by multiplying actual Net Sales of such Multiple Product Offering by the fraction A/(A+B) where A is the invoice price of the Product, if sold separately, and B is the total invoice price of the other products or services in the Multiple Product Offering, if sold separately. If, on a country-by-country basis, the other products or services in the Multiple Product Offering are not sold separately in said country, Net Sales for the purpose of determining royalties of the Multiple Product Offering shall be calculated by multiplying actual Net Sales of such Multiple Product Offering by the fraction A/D, where A is the invoice price of the Product, if sold separately, and D is the invoice price of the Multiple Product Offering. If neither the Product nor the other products or services are sold separately in a given country, the Parties shall determine Net Sales for such Multiple Product Offering by mutual agreement based on the relative contribution of the Product (excluding
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
other products) and each other product or service in the Multiple Product Offering; provided, however that if the parties are not able to mutually agree on the relative contribution of the Product (excluding other products) and each other product or service in the Multiple Product Offering, then Net Sales for such Multiple Product Offering containing only one other product or service shall be fifty percent (50%) of the amount calculated in accordance with the first paragraph of this Section 1.11.
For purposes of the two preceding paragraphs, the invoice price of the Product (which does not contain any Active Component(s)) sold separately for an indication designated as an “Orphan Product” under the U.S. Orphan Drug Act, as amended, shall not be used to calculate Net Sales for any Combination Product or Multiple Product Offering, except any such Combination Product or Multiple Product Offering that is used for an indication designated as an “Orphan Product” under the U.S. Orphan Drug Act, as amended.”
4. For the purposes of Section 6.1(e) of the License Agreement, a Know-How Product shall include any product, the selling of which would, in the absence of this Agreement, infringe a claim in an issued but expired patent included within the Licensed Patent Rights in the country in which the product is sold by Amgen or a sublicensee thereof, provided, however, that notwithstanding anything to the contrary set forth in the License Agreement, Amgen shall not be required to pay royalties for such Know-How Product for more than five (5) years including years of royalties paid for the same product pursuant to Section 6.1(c) or (d) of the License Agreement. However, if that certain License Agreement by and between Amgen and Amylin Pharmaceuticals, Inc. effective as of the 7th day of February 2006 expires or terminates during the term of the License Agreement, then any Know-How Product royalty payable to Rockefeller shall be governed by the original language in Section 6.1(e) of the License Agreement as in effect prior to the Amendment Date (rather than the amended language set forth in this Section 4 of this Amendment).
5. Section 6.9 of the License Agreement shall be deleted and replaced in its entirety with the following:
“6.9 Sublicense Revenues. Within thirty (30) days of the date upon which any upfront license fees and other lump sum or one-time fees in consideration of a sublicense to the Licensed Patent Rights and Technical Information are paid to Amgen or its sublicensee (including without limitation any other fee or payment for a sublicense to the Licensed Patent Rights and Technical Information which is paid to Amgen or its sublicensee solely on the basis of the passage of time), Amgen or its sublicensee shall pay to Rockefeller [*] percent ([*]%) of such amounts in accordance with Paragraph 9. To the extent that upfront license fees and other lump sum or one-time fees in consideration of a sublicense to the Licensed Patent Rights and Technical Information are paid to Amgen or its sublicensee in the form of equity securities, Amgen or its sublicensee shall transfer, or cause to be issued to Rockefeller, [*] percent ([*]%) of the equity
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
securities received by (or that would otherwise be issued to) Amgen or its sublicensee, subject to applicable securities laws and other laws. As a condition to receiving such equity securities, Rockefeller acknowledges that it may be required to execute certain typical and customary agreements relating to the purchase and sale of such securities, which agreements may require certain representations by Rockefeller and impose certain obligations on Rockefeller related to, among other things, registration, lock-ups, co-sale, first refusal, first offer and voting rights, if any, relating to such equity securities. The parties acknowledge and agree that Amgen, its sublicensees and their respective affiliates, subsidiaries and agents shall have no liability whatsoever with respect to the equity securities transferred or issued to Rockefeller, including without limitation, any liability related to the value, liquidity, volatility, rights, preferences or any other aspect of such securities. For the avoidance of doubt, any milestone or other payments tied to future clinical, regulatory, operational or commercial outcomes or sales shall not be considered sublicense revenues for purpose of this Subparagraph 6.9, and shall be excluded from such amounts. If any upfront license fees and other lump sum or one-time fees are paid to Amgen or its sublicensee in consideration of a sublicense to the Licensed Patent Rights and Technical Information as well as other intellectual property not subject to the License Agreement, the payment to Rockefeller pursuant to this Section 6.9 shall be reduced based on the relative contribution of the Licensed Patent Rights and Technical Information, on the one hand, and the other intellectual property not subject to the License Agreement, on the other hand; provided, however that if the parties are not able to mutually agree on the relative contribution of the Licensed Patent Rights and Technical Information, on the one hand, and the other intellectual property not subject to the License Agreement, on the other hand, then the payment to Rockefeller pursuant to this Section 6.9 shall be determined based on an equal contribution of the Licensed Patent Rights and Technical Information, on the one hand, and each other category of intellectual property not subject to the License Agreement, on the other hand.”
6. Except as amended and supplemented hereby, all of the terms of the License Agreement, as amended and supplemented to date, shall remain and continue in full force and effect.
7. This Amendment may be executed in any number of counterparts, each of which need not contain the signature of more than one Party, but all such counterparts taken together shall constitute one and the same document.
8. Whenever possible, each provision of this Amendment will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Amendment is held to be prohibited by or invalid under applicable law, such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Amendment.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
9. No amendment, modification or supplement of any provision of this Amendment shall be valid or effective unless made in writing and signed by a duly authorized officer of each Party.
10. This Amendment and the License Agreement constitute and contain the complete, final and exclusive understanding and agreement of the Parties with respect to the subject matter hereof and thereof, and cancel and supersede any and all prior negotiations, correspondence, understandings and agreements, whether oral or written, between the Parties, respecting the subject matter hereof and thereof.
11. Notwithstanding anything in the License Agreement to the contrary, Rockefeller acknowledges and agrees that Amgen may disclose the terms of the License Agreement and this Amendment and Confidential Information of Rockefeller disclosed to Amgen under the License Agreement and this Amendment, to potential and existing sublicensees of the Licensed Patent Rights and Technical Information under confidentiality obligations no less restrictive than those Amgen requires of parties to whom it may disclose its own confidential information.
12. This Amendment shall be binding upon and inure solely to the benefit of each Party and its respective successors and assigns, and nothing in this Amendment, express or implied, is intended to or shall confer upon any other person any rights, benefit or remedy of any nature whatsoever under or by reason of this Amendment.
13. Each of Amgen and Rockefeller hereby represent and warrant that it has the right to enter into this Amendment and that the terms of this Amendment are not inconsistent with other contractual obligations, express or implied, which it may have.
IN WITNESS WHEREOF, the Parties hereto have executed this Amendment as of the date first above written.
| AMGEN INC. | THE ROCKEFELLER UNIVERSITY | |||||||
| By: | /s/ Xxxxxx Xxxxxx |
By: | /s/ Xxxx Xxxxx | |||||
| Name: | Xxxxxx X. Xxxxxx | Name: | Xxxx Xxxxx | |||||
| Title: | Vice President, Strategy and Corporate Development | Title: | VP Scientific & Facility Operations | |||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT G
UCSF LICENSE; UCSF PATENT
[See attached]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
NON-EXCLUSIVE LICENSE AGREEMENT
between
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA
and
AMGEN, INC.
For
“Methods for Restoring or Enhancing Reproductive Function in Reproductively Impaired Hosts”
[*]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
TABLE OF CONTENTS
| Article No. | Title | Page | ||||
| 1. | DEFINITIONS | 2 | ||||
| 2. | GRANT | 3 | ||||
| 3. | SUBLICENSES | 3 | ||||
| 4. | PAYMENT TERMS | 4 | ||||
| 5. | LICENSE ISSUE FEE | 4 | ||||
| 6. | LICENSE MAINTENANCE FEE | 5 | ||||
| 7. | MILESTONE PAYMENT | 5 | ||||
| 8. | DUE DILIGENCE | 5 | ||||
| 9. | PROGRESS REPORTS | 5 | ||||
| 10. | TERM OF THE AGREEMENT; TERMINATION | 6 | ||||
| 11. | USE OF NAMES AND TRADEMARKS | 7 | ||||
| 12. | LIMITED WARRANTY | 7 | ||||
| 13. | LIMITATION OF LIABILITY | 8 | ||||
| 14. | PATENT PROSECUTION AND MAINTENANCE | 8 | ||||
| 15. | PATENT INFRINGEMENT | 8 | ||||
| 16. | INDEMNIFICATION | 9 | ||||
| 17. | NOTICES | 10 | ||||
| 18. | ASSIGNABILITY | 10 | ||||
| 19. | WAIVER | 10 | ||||
| 20. | FORCE MAJEURE | 11 | ||||
| 21. | GOVERNING LAWS; ATTORNEYS FEES | 11 | ||||
| 22. | MISCELLANEOUS | 11 | ||||
| CONSENT TO SUBSTITUTION OF PARTY | 14 | |||||
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
NON-EXCLUSIVE LICENSE AGREEMENT
for
“Methods for Restoring or Enhancing Reproductive Function in Reproductively Impaired Hosts”
This Non-Exclusive License Agreement (this “Agreement”) is made effective as of this 13th day of July, 2005 (“Effective Date”), by and between The Regents of the University of California, a California corporation, having its statewide administrative offices at 0000 Xxxxxxxx Xxxxxx, 00xx Xxxxx, Xxxxxxx, Xxxxxxxxxx 00000-0000 and acting through its Office of Technology Management, University of California San Francisco, 000 Xxxxx Xxxxxx, Xxxxx 0000, Xxx Xxxxxxxxx, Xxxxxxxxxx 00000 (“The Regents”) and Amgen Inc., a Delaware corporation, having a principal place of business at Xxx Xxxxx Xxxxxx Xxxxx, Xxxxxxxx Xxxx, Xxxxxxxxxx 00000-0000 (together with its Affiliates, “Licensee”). The Regents and Licensee are sometimes referred to herein individually as a “Party” and collectively, as the “Parties”.
BACKGROUND
A. Certain inventions, generally characterized as methods for restoring or enhancing reproductive function in reproductively impaired hosts (collectively “Invention”), were made in the course of research at the University of California San Francisco, by Xx. Xxxxx X. Xxxxxx and are claimed in Patent Rights (as defined below).
B. Licensee wishes to obtain a non-exclusive license to Patent Rights (as defined below) from The Regents for the commercial development of the Invention, in accordance with the terms and conditions set forth herein and The Regents is willing to grant such non-exclusive license to Licensee as that the Invention may be developed and the benefits enjoyed by the general public.
C. The scope of such rights granted by The Regents is intended to extend to the scope of the patents and patent applications in Patent Rights, but only to the extent that The Regents controls rights in and to the Valid Claims of such Patent Rights,
D. Licensee is not a “small business firm” as defined in 15 U.S.C. §632.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein, the Parties agree as follows:
| 1. | DEFINITIONS |
As used in this Agreement, the following terms, whether used in the singular or plural, shall have the following meanings:
1.1 “Affiliate” means a person or entity that, directly or indirectly, through one or more intermediates, controls, is controlled by, or is under common control with Licensee. For the purposes of this definition, control shall mean the direct or indirect ownership of more than fifty percent (50%) or, if less than fifty percent (50%), the maximum percentage as allowed by applicable law or (i) the stock shares entitled to vote for the election of directors or (ii) ownership interest.
1.2 “Field of Use” means therapeutic, prophylactic, and diagnostic uses.
1.3 “First Commercial Sale” means, with respect to a particular Licensed Product, the first sale for end-use or consumption of such Licensed Product in a country after the governing health regulatory authority of such country has granted Regulatory Approval.
1.4 Licensed Product” means any product the manufacture, use, sale, offer for sale or import of which, but for the license granted in this Agreement, would infringe, or contribute to, or induce the infringement of, a Valid Claim under Patent Rights.
1.5 “Patent Rights” means, to the extent assigned to or otherwise controlled by The Regents, the following United States patents and patent applications:
| UC Case-Number |
United States Application Number Or United States Patent Number |
Filing or Issue Date | ||
| SF200S-049 |
U.S. PATENT NO. 5,773,416 | Issued June 30,1998 |
Patent Rights shall farther include, to the extent assigned to or otherwise controlled by The Regents, the corresponding foreign patents and patent applications and any reissues, extensions, substitutions, continuations, divisions and continuation-in-part applications (but only to the extent, however, that claims in the continuation-in-part applications are entirely supported in the specification and entitled to the priority date of the parent application) related thereto.
1.6 “Person” shall mean an individual, partnership, limited liability company, joint venture, corporation, trust, estate, unincorporated organization, or any other entity or any government or any department or agency thereof,
1.7 “Regulatory Approval” shall mean any and all approvals (excluding any applicable governmental price and reimbursement approvals), licenses, registrations, or authorizations of any federal, national, multinational, state, provincial or local regulatory agency, department, bureau or other governmental entity that are necessary and sufficient for the marketing and sale of a Licensed Product in a country or group of countries.
1.8 “Territory” shall mean worldwide.
2
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1.9 “Third Party” shall mean a Person other than Licensee or The Regents,
1.10 “Valid Claim” means a claim of an issued and unexpired patent within Patent Rights in any country that has not been found to be unpatentable, unenforceable or invalid by a court or other governmental agency of competent jurisdiction in the Territory, in an unappealed or unappealable decision, and which has not been disclaimed or admitted to be unpatentable, invalid or unenforceable through reissue, disclaimer or otherwise.
| 2. | GRANT |
2.1 Subject to the limitations and other terms and conditions set forth in this Agreement, The Regents grants to Licensee a non-exclusive license under its rights in and to Patent Rights to make, have made, use, have used, sell, have sold, offer for sale, import, export or otherwise exploit or transfer physical possession of or title in Licensed Products in the Territory in the Field of Use, where such license may be lawfully granted.
2.2 The Regents reserves and retains the non-exclusive right to make, use and practice the Invention and Patent Rights and any technology relating to any of the foregoing and to make and use any products and to practice any process that is the subject of the Patent Rights (and to grant any of the foregoing rights to other educational and non-profit institutions) for educational and research purposes, including without limitation, any sponsored research performed for or on behalf of commercial entities and including publication and other communication of any research results. Moreover, The Regents is free to issue additional licenses under the Patent Rights for commercial or non-commercial purposes. Notwithstanding anything in this Agreement to the contrary, nothing in this Agreement is intended, and nothing in this Agreement shall be deemed, to grant to the Regents any right or license, express or implied, under any Licensee intellectual property rights.
| 3. | SUBLICENSES |
3.1 The Regents also grants to Licensee the right to sublicense the rights granted to Licensee hereunder. Each sublicensee shall enter into a written sublicense agreement. Such sublicenses shall not exceed the scope of the license granted to Licensee hereunder, and the sublicensee shall agree to comply with the terms and conditions applicable to Licensee hereunder.
3.2 Licensee will notify The Regents at least thirty (30) days in advance of each sublicense to be granted. Licensee shall consider The Regents’ comments with respect to such sublicense(s); however, notwithstanding anything in this Section 3.2 or in this Agreement to the contrary, Licensee shall have sole control, discretion and election as lo any sublicenses to be granted.
3.3 Upon any expiration or termination of this Agreement for any reason, all sublicenses granted by Licensee prior lo the termination or expiration of this Agreement shall remain in full force and effect, provided that (i) the sublicensee is not then in material breach of
3
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
its sublicense agreement and (ii) upon The Regents’ written request to the sublicensee, the sublicensee agrees in writing within thirty (30) days of receipt of such request, to be bound to The Regents as licensor under the terms and conditions of this Agreement and to make any payment due The Regents hereunder directly to The Regents as if it were Licensee.
| 4. | PAYMENT TERMS |
4.1 The Regents shall submit invoices to Licensee for all amounts due from Licensee to The Regents, at least thirty (30) days prior to the due date of such payment. All invoices shall be submitted to Licensee at the following address:
[*]
Invoices not submitted to this address may be subject to delay or return.
4.2 If requested by Licensee, the Regents shall provide to Licensee a completed IRS Form W-9 within fifteen (15) days of such request from Licensee.
4.3 All consideration due The Regents hereunder will be payable and will be made m United States dollars by check payable to “The Regents of the University of California” or by wire transfer to the following account designated by The Regents. Licensee is responsible for all bank or other transfer charges.
| For Checks: | [*] | |
| For Wire Transfer: | [*] | |
| Note: In order to ensure that funds are properly credited to Licensee’s account, please reference [*] on all wire transfers. | ||
4.4 In the event that fees or other monies owed to The Regents are not received by The Regents within forty-five (45) days after the date such payments are due under this Agreement, such payments shall, after prior notice from UCSF and a thirty (30) business day cure period, bear interest at an annual rate of interest equal to [*] percent ([*]%) above the London Interbank Offered Rate (“LIBOR”), calculated on the number of days such payment is delinquent. In no event shall the annual interest rate exceed [*] percent ([*]%).
| 5. | LICENSE ISSUE FEE |
Licensee shall pay to The Regents a license issue fee of thirty-five thousand dollars ($35,000) within fifteen (15) days of the Effective Date. This fee is non-refundable, non- cancelable and is not an advance or otherwise creditable against any payments required to be paid under the terms of this Agreement.
4
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| 6. | LICENSE MAINTENANCE FEE |
Licensee shall pay to The Regents a license maintenance fee of [*] beginning on the first anniversary of the Effective Date and continuing annually on each anniversary of the Effective Date. The license maintenance fee is not due on any anniversary of the Effective Date if on that date Licensee is selling or otherwise exploiting Licensed Products. The license maintenance fee is non-refundable and if not an advance or otherwise creditable against any payments required to be paid under the terms of this Agreement.
| 7. | MILESTONE PAYMENT |
7.1 Licensee will pay to The Regents the following one-time non-refundable, non- creditable amount within thirty (30) days of first achievement by Licensee or its sublicensee:
7.1.1 [*]
7.2 For the avoidance of doubt, payment of the foregoing milestone shall be made only once for the first achievement of such milestone event, and not with respect to each additional occasion that such milestone event is achieved.
7.3 Other than as explicitly set forth (and as applicable) in Articles 5, 6 and 7 hereunder, Licensee shall not be obligated to pay any additional fees, milestone payments or any additional payments to The Regents under this Agreement.
| 8. | DUE DILIGENCE |
8.1 Licensee, upon execution of this Agreement, will use commercially reasonable efforts to develop Licensed Products. Upon First Commercial Sale of a Licensed Product in the United States, Licensee shall be deemed to have satisfied its obligations under this Section 8.1.
8.2 As between the Parties, Licensee will be responsible for obtaining all necessary Regulatory Approvals in each country where Licensee or its sublicensees manufactures, sells, uses, offers for sale or imports Licensed Products.
| 9. | PROGRESS REPORTS |
9.1 At The Regent’s written request to Licensee, Licensee shall provide to The Regents, no more than once per calendar year, a written summary report regarding the development of Licensed Products, including a summary description of the activities conducted within the prior year, and the identification of significant activities anticipated for the following year. Once Licensee achieves the milestone event set forth in Section 7.1.1. Licensee shall no longer be required to provide such summary reports to the Regents.
5
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| 10. | TERM OF THE AGREEMENT; TERMINATION |
10.1 Unless earlier terminated in accordance with this Section 10, this Agreement will remain in effect from the Effective Date until the expiration or abandonment of the last of the Patent Rights licensed hereunder.
10.2 The Regents may terminate this Agreement immediately upon the bankruptcy, liquidation, dissolution or cessation of operations of Amgen; or the filing of any voluntary petition for bankruptcy, dissolution, liquidation or winding-up of the affairs of Amgen; or any assignment by Amgen for the benefit of creditors; or the filing of any involuntary petition for bankruptcy, dissolution, liquidation or winding-up of the affairs of Amgen which is not dismissed within [*] of the date on which it is filed or commenced.
10.3 Any termination or expiration of this Agreement will not affect the rights and obligations set forth in the following Articles:
| Article 1 | Definitions | |
| Section 3.3 | Sublicenses | |
| Section 4.4 | Late Payments | |
| Article 10 | Term of the Agreement; Termination | |
| Article 11 | Use of Names And Trademarks | |
| Article 12 | Limited Warranty | |
| Article 13 | Limitation of Liability | |
| Article 14 | Patent Prosecution and Maintenance | |
| Article 15 | Infringement (but only as to infringement during the term of this agreement) | |
| Article 16 | Indemnification | |
| Article 17 | Notices | |
| Article 21 | Governing Laws; Attorneys Fees | |
| Article 22 | Miscellaneous |
10.4 The termination or expiration of this Agreement will not relieve Licensee of its obligation to pay any fees or other payments owed to The Regents at the time of such termination or expiration and will not impair any accrued right of The Regents.
10.5 If Licensee is in material breach of any material term or covenant of this Agreement, then The Regents may give written notice of such material default (“Notice of Default”) to Licensee. If Licensee fails to cure such material default within [*] after the effective date of such notice, then The Regents will have the right to immediately terminate this Agreement and its licenses by providing a written notice of termination (“Notice of Termination”) to Licensee.
6
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
10.6 Licensee has the right at anytime to terminate this Agreement by providing a Notice of Termination to The Regents. Termination of this Agreement will be effective [*] from the effective date of such notice.
| 11. | USE OF NAMES AND TRADEMARKS |
Nothing contained in this Agreement will be construed as conferring any right to either party to use in advertising, publicity or other promotional activities any name, trade name, trademark or other designation of the other party (including a contraction, abbreviation or simulation of any of the foregoing). Unless required by law or consented to in writing by an authorized officer of The Regents, the use by Licensee of the name “The Regents of the University of California” or the name of any campus of the University of California in advertising, publicity or other promotional activities is expressly prohibited.
| 12. | LIMITED WARRANTY |
12.1 The Regents represents and warrants to Licensee that it is the owner of U.S. Patent No, 5,773,416 and has the lawful right to grant this license to Licensee under this Agreement.
12.2 Except as expressly set forth in this Agreement, this license and the associated Patent Rights are provided by The Regents WITHOUT WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER WARRANTY OF ANY KIND, EXPRESS OR IMPLIED. THE REGENTS MAKES NO EXPRESS OR IMPLIED REPRESENTATION OR WARRANTY THAT THE INVENTION, PATENT RIGHTS OR LICENSED PRODUCTS, WILL NOT INFRINGE ANY PATENT, COPYRIGHT, TRADEMARK OR OTHER RIGHTS.
| 12.3 | This Agreement does not: |
| 12.3.1 | express or imply a warranty or representation as to the validity, enforceability, or scope of any Patent Rights; or |
| 12.3.2 | express or imply a warranty or representation that anything made, used, sold, offered for sale or imported or otherwise exploited under any license granted in this Agreement is or will be free from infringement of patents, copyrights, or other rights of third parties; or |
| 12.3.3 | obligate The Regents to bring or prosecute actions or suits against third parties for patent infringement; or |
| 12.3.4 | confer by implication, estoppel or otherwise any license or rights under any patents or other rights of The Regents other than Patent Rights, regardless of whether such patents are dominant or subordinate to Patent Rights; or |
7
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| 12.3.5 | obligate The Regents to furnish any patents, know-how, technology or information not provided in Patent Rights. |
| 13. | LIMITATION OF LIABILITY |
EXCEPT FOR LICENSEE’S INDEMNIFICATION OBLIGATIONS UNDER ARTICLE 16 FOR THIRD PARTY CLAIMS, NEITHER PARTY WILL BE LIABLE FOR ANY LOST PROFITS, COSTS OF PROCURING SUBSTITUTE GOODS OR SERVICES, LOST BUSINESS, ENHANCED DAMAGES FOR INTELLECTUAL PROPERTY INFRINGEMENT OR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, PUNITIVE OR OTHER SPECIAL DAMAGES SUFFERED BY THE OTHER PARTY OR ITS SUBLICENSEES (IF APPLICABLE) ARISING OUT OF OR RELATED TO THIS AGREEMENT FOR ALL CAUSES OF ACTION OF ANY KIND (INCLUDING TORT, CONTRACT, NEGLIGENCE, STRICT LIABILITY AND BREACH OF WARRANTY) EVEN IF A PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
| 14. | PATENT PROSECUTION AND MAINTENANCE |
The Regents, at its sole expense, will diligently prosecute and maintain the United States and foreign patents comprising the Patent Rights using counsel of its choice. The Regents’ counsel will take instructions only from The Regents.
| 15. | PATENT INFRINGEMENT |
15.1 Enforcement. In the event that Licensee learns of infringement of potential commercial significance of any patent licensed under this Agreement, Licensee will provide The Regents with (i) written notice of such infringement and (ii) with any evidence of such infringement available to it (the “Infringement Notice”). During the period in which, and in the jurisdiction where, Licensee has rights under this Agreement, Licensee will not notify a possible infringer of infringement or put such infringer on notice of the existence of the Patent Rights without first obtaining the written consent of The Regents.
15.2 Licensee will cooperate with The Regents, at The Regents’ expense, in litigation proceedings instituted hereunder. Any litigation proceedings will be controlled by The Regents at its sole expense.
15.3 Infringement Defense. Licensee shall have the sole right, at its own expense, to defend any suit brought against it or its sublicensee alleging infringement of a third party patent because of the manufacture, use or sale of a Licensed Product by Licensee or its sublicensee in the Territory. Upon request, The Regents shall cooperate with Licensee, at Licensee’s expense, in any such litigation.
8
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| 16. | INDEMNIFICATION |
16.1 Licensee will, and will require its sublicensees to, indemnify, hold harmless and defend The Regents and its officers, employees and agents, the sponsors of the research that led to the Invention, and the inventors of any invention claimed in the Patent Rights (including the Licensed Products contemplated hereunder)) and their employers (“Indemnitees”), against any and all claims, suits, losses, damage, costs, fees and expenses (“Losses”) resulting from, or arising out of, the exercise of this license granted to Licensee hereunder. This indemnification will include, but not be limited to, any product liability resulting from, or arising out of, exercise of the license granted to Licensee by The Regents in this Agreement. The indemnification obligations of this Section 16.1 shall not apply to the extent any Losses occur in connection with or arise out of any representation or warranty by The Regents set forth herein being untrue in any material respect when made or any material breach or material default by The Regents of any of its obligations hereunder,
16.2 During the term of this Agreement and for [*] following its termination or expiration, Licensee, at its sole cost and expense, will obtain and maintain the following insurance:
| 16.2.1 (a) | Comprehensive or Commercial Form General Liability Insurance (contractual liability Included) with limits as follows: |
| Each Occurrence |
[ | *] | ||
| Personal and Advertising Injury |
[ | *] | ||
| General Aggregate (commercial form only) |
[ | *] | ||
| (b) | Products liability, on a claims made basis, with a limit of [*] |
Notwithstanding the foregoing, to the extent that Licensee self-insures in a manner consistent with the standards and practices generally accepted in the biotechnology and pharmaceutical businesses, Licensee shall be deemed to be in compliance with all of the obligations set forth in Section 16.2 of this Agreement.
| 16.2.2 | The coverage and limits referred to in Paragraph 16.2.1 above will not in any way limit the liability of Licensee and Licensee will : |
| – | Provide for [*] advance written notice to The Regents of any cancellation; |
| – | extend status to The Regents as an additional insured under the coverage described above only with respect to Licensee’s obligations under Section 16.1 above; and |
9
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| – | include a provision that the coverage will be primary and will not participate with, nor will be excess over, any valid and collectable insurance or program of self-insurance maintained by The Regents |
16.3 The Regents will promptly notify Licensee in writing of any claim or suit brought against The Regents for which The Regents intends to invoke the provisions of this Article 16 (INDEMNIFICATION).
| 17. | NOTICES |
Any notice or other communication pursuant to this Agreement shall be sufficiently made or given, by hand on the date of delivery, by facsimile upon receipt of confirmed transmission, or on the date of mailing, if sent to such Party by certified first class mail, postage prepaid, or by overnight courier, addressed to it at its address below or as it shall designate by written notice given to the other Party:
| In the case of Licensee: |
Amgen Inc. | |||
| Xxx Xxxxx Xxxxxx Xxxxx | ||||
| Xxxxxxxx Xxxx, Xxxxxxxxxx 00000-0000 | ||||
| Telephone: | 000-000-0000 | |||
| Facsimile: | 000-000-0000 | |||
| Attention: | Vice President, Licensing | |||
| In the case of The Regents: |
[*] | |||
| 18. | ASSIGNABILITY |
This Agreement is personal to Licensee. Licensee may not assign or transfer this Agreement, including by merger, operation of law, or otherwise, without The Regents’ prior written consent; notwithstanding the foregoing, such consent will not be required in the case of assignment or transfer to a party that succeeds to all or substantially all of Licensee’s business or assets relating to this Agreement, whether by sale, merger, operation of law or otherwise, provided that such assignee or transferee promptly agrees to be bound by the terms and conditions of this Agreement and signs The Regents’ standard substitution of party letter (the form of which is attached hereto as Appendix A). Any attempted assignment by Licensee not in accordance with this Section 18 will be null and void. This Agreement is binding upon and will inure to the benefit of Parties, its successors and assigns.
| 19. | WAIVER |
No waiver by either Party of any breach or default of any of the covenants or agreements contained herein will be deemed a waiver as to any subsequent and/or similar breach or default. No waiver will be valid or binding upon the Parties unless made in writing and signed by a duly authorized officer of each Party.
10
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| 20. | FORCE MAJEURE |
20.1 The Parties shall not be responsible for any failure to perform due to the occurrence of any events beyond their reasonable control which render their performance impossible or onerous, including, but not limited to: accidents (environmental, toxic spill, etc.); acts of God; biological or nuclear incidents; casualties; earthquakes; fires; floods; inability to obtain suitable and sufficient labor, transportation, fuel and materials; local, national or state emergency; power failure and power outages; acts of terrorism; strike; and war; provided that any such force majeure event shall not relieve Licensee of its obligation to pay any fees or other payments accrued and payable to The Regents prior to the commencement of such force majeure event.
| 21. | GOVERNING LAWS; ATTORNEYS FEES |
21.1 THIS AGREEMENT WILL BE INTERPRETED AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF CALIFORNIA, excluding any choice of law rules that would direct the application of the laws of another jurisdiction and without regard to which Party drafted particular provisions of this Agreement, but the scope and validity of any patent or patent application will be governed by the applicable laws of the country of such patent or patent application.
21.2 The prevailing Party in any suit related to this Agreement will be entitled to recover its reasonable attorneys’ fees in addition to its costs and disbursements.
| 22. | MISCELLANEOUS |
22.1 The headings of the several sections are inserted for convenience of reference only and are not intended to be a part of or to affect the meaning or interpretation of this Agreement.
22.2 This Agreement is not binding on the Parties until it has been signed below on behalf of each Party. It is then effective as of the Effective Date.
22.3 No amendment or modification of this Agreement is valid or binding on the Parties unless made in writing and signed on behalf of each Party.
22.4 This Agreement embodies the entire understanding of the Parties with respect to the Patent Rights and supersedes all previous communications, representations or understandings, either oral or written, between the Parties relating to the Patent Rights.
22.5 In case any of the provisions contained in this Agreement is held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability will not affect any other provisions of this Agreement and this Agreement will be construed as if such invalid, illegal or unenforceable provisions had never been contained in it.
11
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
22.6 No provisions of this Agreement are intended or shall be construed to confer upon or give to any person or entity other than The Regents and Licensee any rights, remedies or other benefits under, or by reason of, this Agreement.
22.7 Each Party agrees to perform its obligations under this Agreement in accordance with all applicable federal, state and local laws and regulations.
22.8 The Regents and Licensee have each consulted counsel of their choice regarding this Agreement, and each acknowledges and agrees that this Agreement shall not be deemed to have been drafted by one Party or another and will be construed accordingly.
22.9 Each Party agrees to execute, acknowledge and deliver such further instruments and to perform all such other acts as may be necessary or appropriate to carry out the purposes and intent of this Agreement.
22.10 It is agreed that no waiver by either Party of any breach or default of any of the covenants or agreements herein set forth shall be deemed a waiver as to any subsequent and/or similar breach or default.
22.11 The captions to the several sections hereof are included merely for convenience of reference only and shall not affect the meaning or interpretation of any of the provisions of this Agreement
22.12 In performing their respective duties under this Agreement, each of the Parties will be operating as an independent contractor. Nothing contained herein will in any way constitute any association, partnership, or joint venture between the Parties hereto, or be construed to evidence the intention of the Parties to establish any such relationship. Neither Party will have the power to bind the other Party or incur obligations on the other Party’s behalf without the other Party’s prior written consent.
22.13 The Parties shall keep confidential and shall not disclose to a Third Party the terms of this Agreement, without the prior written consent of the other Party; provided that (i) Licensee may disclose the terms of this Agreement to potential sublicensees of the Patent Rights under confidentiality obligations, without the prior written consent of the Regents; (ii) the Parties may disclose the terms of this Agreement in response to a valid order of a court of other governmental body or as required by law, regulation or stock exchange rule, including as required by the California Public Records Act; provided however, that the obligated Party shall advise the other Party in advance of such disclosure to the extent practicable and permissible by such order, law, regulation or stock exchange rule and other applicable law; and (iii) The Regents may disclose to a Third Party the existence of a sublicensable non-exclusive license under the Patent Rights, but shall not disclose the name of Licensee or any sublicensee. The Regents shall keep confidential, and shall not disclose to any Third Party, any information provided by Licensee in accordance with Section 3.2 and Section 9 of this Agreement, including but not limited to the identity of potential sublicensees and any progress reports provided by Licensee to The Regents, without the prior written consent of Licensee. Nothing contained
12
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
herein will in any way restrict or impair the right of either Party to disclose any terms of this Agreement that a Party can demonstrate by written records is now, or becomes in the future, public knowledge other than through acts or omissions of such Party.
IN WITNESS WHEREOF, both The Regents and Licensee have executed this Agreement, in duplicate originals, by their respective and duly authorized officers on the day and year written.
| AMGEN INC. | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | |||||
| By: | /s/ Xxxxx Xxxxxxx | By: | /s/ Xxxx X. Xxxxxxxxxx | |||
| Name: | Xxxxx X. Xxxxxxx, Esq. | Name: | Xxxx X. Xxxxxxxxxx | |||
| Title: | Vice President, Licensing | Title: | Director, UCSF Office of Technology Management | |||
| Date: | 7/18/05 | Date: | 7/21/05 | |||
13
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Appendix A
[*]
CONSENT TO SUBSTITUTION OF PARTY
This CONSENT TO SUBSTITUTION OF PARTY (“Agreement”) is effective this day of 20 , among The Regents of the University of California (“The Regents”), a California corporation, having its statewide administrative offices at 0000 Xxxxxxxx Xxxxxx, 00xx Xxxxx, Xxxxxxx, Xxxxxxxxxx 00000-0000 and acting through its Office of Technology Management, University of California San Francisco, 000 Xxxxx Xxxxxx, Xxxxx 0000, Xxx Xxxxxxxxx, Xxxxxxxxxx 00000; Amgen Inc. (“Amgen”), a Delaware corporation, having a principal place of business at Xxx Xxxxx Xxxxxx Xxxxx, Xxxxxxxx Xxxx, Xxxxxxxxxx 00000-0000; and [new licensee name] [(“XXX”)] a corporation, having a principal place of business at .
BACKGROUND
A. The Regents and Amgen entered into a License Agreement effective July 13, 2005 (UC Control No. , entitled “Methods for Restoring or Enhancing Reproductive Function in Reproductively Impaired Hosts” (“License Agreement”), wherein Amgen was granted certain rights.
B. Amgen desires that [XXX] be substituted as Licensee (defined in the License Agreement) in place of Amgen, and The Regents is agreeable to such substitution.
C. [XXX] has read the License Agreement and agrees to abide by its terms and conditions.
The parties agree as follows:
1. [XXX] assumes all liability and obligations under the License Agreement and is bound by all its terms in all respects as if it were the original Licensee of the License Agreement in place of Amgen.
2. [XXX] is substituted for Amgen, provided that [XXX] assumes all liability and obligations under the License Agreement as if [XXX] were the original party named as licensee as of the effective date of the License Agreement.
3. The Regents releases Amgen from all liability and obligations under the License Agreement arising before or after the effective date of this Agreement.
The parties have executed this Agreement in triplicate originals by their respective authorized officers on the following day and year.
14
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| AMGEN INC. | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | |||||||
| By: |
|
By: |
| |||||
| (Signature) | ||||||||
| Name: |
|
By: Xxxx X. Xxxxxxxxxx | ||||||
| (Please print) | ||||||||
| Title: |
|
Title: Director, UCSF Office of Technology Management | ||||||
| Date: |
|
Date: |
| |||||
[XXX] COMPANY
| By: |
| |
| (Signature) | ||
| Name: |
| |
| (Please print) | ||
| Title: |
| |
| Date: |
| |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
Linking Adiposity and Central Neural Networks,” Science 269:546-549 (1995).
Xxxxxx, F. et a1., “Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin,” Nature Genetics 12:318-320 (1996).
Xxxxxxx, X.X., et al., “Effects of parabiosis of normal with genetically diabetic mice,” Am.
J. Physiology 217(5): 1298-1304 (1969).
Xxxxxxx, X.X., “Effects of Parabiosis of Obese with Diabetes and Normal Mice,” Diabetologia 9:294-298 (1973),
Xxxxxxx, M,L. et al., “Physiological Differences in Uteri of Obese Stock Mice,” J. Heredity 46(6):209-212 (1955).
Xxxxxxxxx, X.X. et al., “Leptin levels reflect body lipid content in mice: Evidence for diet induced resistance to leptin action,” Nature Medicine 1(12): 1311-1314 (1995).
Xxxxxx, R.E. et al., “Height and Weight at Menarche and a Hypothesis of Critical Body Weights and Adolescent Events,” Science 169:397-399 (1970).
Xxxxxx, R.E. et al., “Menstrual Cycles: Fatness as a Determinant of minimum Weight for Height Necessary for Their Maintenance or Onset,” Science 185:949-951 (1974).
Xxxxxx, R.E. et al., “Delayed Menarche and Amenorrhea in Ballet Dancers,” N. Eng. J. Med. 303(1):17-19 (1980).
Xxxxxx, R.E. et al., “Delayed Menarche and Amenorrhea of College Athletes in Relation to Age of Onset of Training,” J. Am. Med. Assoc. 246(14): 1559-1563 (1981).
Progress in Reproductive Biology and Medicine: Adipose Tissue and Reproduction Xxxxxx, R.E. ed., Karger, Basel, Switzerland, vol. 14 (1990).
Xxxxxx, X.X. et al., “Weight-Reducing Effects of the Plasma Protein Encoded by the obese Gene” Science 269:543-546 (1995),
Xxxxxxx B. et al., “Endocrine Activity of the Testis in Obese-Hyperglycaemic Mice,” Acta Endocrinologica 44:20-26 (1963).
Xxxxxxx, B., “Studies in Obese-Hyperglycemic Mice,” Xxx. New York Acad. Sci. 131 (l):541-558 (1965).
Xxxxxx, X.X., “Transplantation of Ovaries of the Obese Mouse,” Anat. Rec, 128(3):569 (1957).
Xxxxxxx, A.M. et al., “Obese, A new Mutation in the House Mouse,” J. Heredity 41:317-318 (1950).
Xxxxxxx, X.X, et al., “Body Weight and Food Intake as Inititiating Factors for Puberty in the Rat,” J. Physiol. 166:408-418 (1963).
Xxxx, X.X. et al., “Fertile, Obese Male Mice,” J. Heredity 45(2):56-58 (1954).
Xxxxxx, M. et al., “Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects,” Nature Medicine 1(11):1155-1161 (1995). Pelleymounter, M.A, et al., “Effects of the obese Gene Produce on Body Weight Regulation in ob/ob Mice,” Science 269:540-543 (1995).
Runner, M.N. et al., “Inherited hypofunction of the female pituitary in the sterile-obese syndrome in the mouse,” Genetics 39(6):990-991 (1954).
Runner, M.N. et al., “Inherited hypofunction of the female pituitary in the sterile-obese syndrome in the mouse,” Records Genetics Soc. of America 23:63-64 (1954).
Runner, M.N. et al., “Sterile, Obese Mothers,” J. Heredity 45(2);51-55 (1954).
Xxxxxxxxx, M. et al., “The Induction and Maintenance of Pregnancy in Prepuberal Mice,” J. Exper. Zoology 133(3):441-457 (1956).
Xxxxxxxxx, M. et al., “Pregnancy Induced in Genetically Sterile Mice,” J. Heredity 48(3):97- 100(1957).
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
Stiff, M.E. et al., “Plasma Gonadotropins in Prenatal and prepubertal Female Mice: Disorganization of Pubertal Cycles in the Absence of a Male,” Endocrinology 94(2):492-496 (1974).
Xxxxxxxxx, X.X. et al., “Reproductive Hormonal Function in the Genetically Obese (ob/ob)
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
( 10 of 10 )
| United States Patent |
5,773,416 | |||
| Xxxxxx |
Xxxx 30, 1998 | |||
Methods for restoring or enhancing reproductive function in reproductively impaired hosts
Abstract
A method for restoring reproductive function in a reproductively impaired male or female host is disclosed, the method comprising administering a leptin compound to the host for a time and in an amount sufficient to restore or enhance reproductive function. A method of accelerating the onset of puberty in a male or female host is also disclosed, the method comprising administering a leptin compound to the host for a time and in an amount sufficient to cause the onset of puberty.
| Inventors: | Xxxxxx; Xxxxx X. (San Francisco, CA) | |
| Assignee: | The Regents of the University of California (Oakland, CA) | |
| Appl. No.: | 735038 | |
| Filed: | October 22, 0000 | |
| Xxxxxxx X.X. Class: | 514/21 | |
| Intern’l Class: | A61K 038/00 | |
| Field of Search: | 514/21 | |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
References Cited [Referenced By]
Foreign Patent Documents
| WO 96/29405 | Sep., 1996 | WO. |
Other References
Xxxxxx, F. et al., “A broader role for leptin,” Nature Medicine, 2(7): 723-724 (1996). Xxxxxx, I.A. et al., “Leptin is a Metabolic Signal to the Reproductive System,” Endocrinology 137(7):3144-3147 (1996).
Xxxxxxxxx, L.A. et al., “Recombinant Mouse OB Protein: Evidence for a Peripheral Signal Linking Adiposity and Central Neural Networks,” Science 269:546-549 (1995).
Xxxxxx, F. et al., “Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin,” Nature Genetics 12:318-320 (1996).
Xxxxxxx, X.X., et al., “Effects of parabiosis of normal with genetically diabetic mice,” Am. J. Physiology 217(5):1298-134 (1969).
Xxxxxxx, X.X., “Effects of Parabiosis of Obese with Diabetes and Normal Mice,” Diabetologia 9:294-298 (1973).
Xxxxxxx, X.X. et al., “Physiological Differences in Uteri of Obese Stock Mice,” J. Heredity 46(6):209-212 (1955).
Xxxxxxxxx, X.X. et al., “Leptin levels reflect body lipid content in mice: Evidence for diet induced resistance to leptin action,” Nature Medicine 1(12): 1311-1314 (1995).
Xxxxxx, R.E. et al., “Height and Weight at Menarche and a Hypothesis of Critical Body Weights and Adolescent Events,” Science 169:397-399 (1970).
Xxxxxx, R.E. et al., “Menstrual Cycles: Fatness as a Determinant of Minimum Weight for Height Necessary for Their Maintenance or Onset,” Science 185:949-951 (1974).
Xxxxxx, R.E. et al., “Delayed Menarche and Amenorrhea in Ballet Dancers,” N. Eng. J. Med. 303(1):17-19 (1980).
Xxxxxx, R.E. et al., “Delayed Menarche and Amenorrhea of College Athletes in Relation to Age of Onset of Training,” J. Am. Med. Assoc. 246(14):1559-1563 (1981).
Progress in Reproductive Biology and Medicine: Adipose Tissue and Reproduction Xxxxxx, R.E. ed., Karger, Basel, Switzerland, vol. 14 (1990).
Xxxxxx, X.X. et al., “Weight-Reducing Effects of the Plasma Protein Encoded by the obese Gene” Science 269:543-546 (1995).
Xxxxxxx B., et al., “Endocrine Activity of the Testis in Obese-Hyperglycaemic Mice,” Acta Endocrinologica 44:20-26 (1963).
Xxxxxxx, B., “Studies in Obese-Hyperglycemic Mice,” Xxx. New York Acad. Sci. 131 (1):541-558 (1965).
Xxxxxx, X.X., “Transplantation of Ovaries of the Obese Mouse,” Anat. Rec, 128(3):569 (1957).
Xxxxxxx, A.M. et al., “Obese, A new Mutation in the House Mouse,” J. Heredity 41:317-318 (1950).
Xxxxxxx, X.X, et al., “Body Weight and Food Intake as Initiating Factors for Puberty in the Rat,” J. Physiol. 166:408-418 (1963).
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
Xxxx, X.X. et al., “Fertile, Obese Male Mice,” J. Heredity 45(2):56-58 (1954).
Xxxxxx, M. et al., “Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects,” Nature Medicine 1(11):1155-1161 (1995). Pelleymounter, M.A, et al., “Effects of the obese Gene Produce on Body Weight Regulation in ob/ob Mice,” Science 269:540-543 (1995).
Runner, M.N. et al., “Inherited hypofunction of the female pituitary in the sterile-obese syndrome in the mouse,” Genetics 39(6):990-991 (1954).
Runner, M.N. et al., “Inherited hypofunction of the female pituitary in the sterile-obese syndrome in the mouse,” Records Genetics Soc. of America 23:63-64 (1954).
Runner, M.N. et al., “Sterile, Obese Mothers,” J. Heredity 45(2):51-55 (1954).
Xxxxxxxxx, M. et al., “The Induction and Maintenance of Pregnancy in Prepuberal Mice,” J. Exper. Zoology 133(3):441-457 (1956).
Xxxxxxxxx, M. et al., “Pregnancy Induced in Genetically Sterile Mice,” J. Heredity 48(3):97- 100 (1957).
Stiff, M.E. et al., “Plasma Gonadotropins in Prenatal and prepubertal Female Mice: Disorganization of Pubertal Cycles in the Absence of a Male,” Endocrinology 94(2):492-496 (1974).
Xxxxxxxxx, X.X. et al., “Reproductive Hormonal Function in the Genetically Obese (ob/ob) Mouse,” Endocrinology 98(6):1359-1364 (1976).
Xxxxxxxx, X.X. et al., “Hypothalamic Dysfunction in Secondary Amenorrhea Associated with Simple Weight Loss,” N. Engl. J. Med. 297(21):1141-1145 (1977).
Xxxxxx, X.X. et al., “Recombinant ob Protein Reduces Feeding and Body Weight in the ob/ob Mouse,” J. Clin. Invest. 96:2065-2070 (1995).
Xxxxxxxxx, L. et al., “Sexual maturation in contemporary American girls,” Amer. J. Obstet. Gynec. 108(5):833-846 (1970).
Zhang, et al., “Positional cloning of the mouse obese gene and its human homologue,” Nature 372:425-432 (1994).
Primary Examiner: Jordan; Xxxxxxxx
Attorney, Agent or Firm: Xxxxxxxx and Xxxxxxxx and Crew LLP
Government Interests
This work was partly supported by NIH grant HLS 53762. The U.S. Government may have certain rights in this invention.
Parent Case Text
This application claims priority under 35 U.S.C. l19(C) to provisional application 60/006,106, filed Oct. 25, 1995.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
Claims
What is claimed is:
1. A method for restoring reproductive function in a reproductively impaired male or female host, wherein the host is reproductively impaired as a result of a hormonal deficiency, said method comprising administering a leptin compound to the host for a time and in an amount sufficient to restore or enhance reproductive function.
2. A method as in claim 1 wherein the leptin compound is administered to a female host from a time prior to impregnation through delivery of offspring.
3. A method as in claim 2, further comprising the administration of the leptin compound to a female host through lactation.
4. A method as in claim 1, wherein the leptin compound is a recombinant protein comprising the full length secreted form of leptin.
5. A method as in claim 1, wherein the leptin compound comprises at least a biologically active fragment of leptin.
6. A method as in claim 1, wherein the leptin compound comprises at least a biologically active fragment of leptin expressed as a fusion product in an expression vector.
7. A method as in claim 1, wherein the leptin compound is administered in a dosage from 0.1 ng/kg body weight to 100 mg/kg body weight.
8. A method as in claim 1, wherein the leptin compound is administered subcutaneously, intradermally, intravenously, intramuscularly, intraperitoneally, transdermally, orally, via pulmonary delivery, via intranasal delivery, via controlled release delivery, or via pump.
9. A method as in claim 1, wherein the leptin compound is administered continuously.
10. A method as in claim 1, wherein the leptin compound is administered in discrete doses.
11. A method as in claim 1, wherein the host suffers from a physiological defect of one or more hypothalamic, pituitary, or gonadal hormones.
12. A method as in claim 1, wherein the host is obese.
13. A method as in claim 1, wherein the host is non-obese.
14. A method as in claim 13, wherein the host has a body mass index of less than about 20.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
15. A method of accelerating the onset of puberty in a male or female host, the method comprising administering a leptin compound to the host for a time and in an amount sufficient to cause the onset of puberty.
16. A method as in claim 15, wherein the leptin compound is a recombinant protein comprising the full length secreted form of leptin.
17. A method as in claim 15, wherein the leptin compound comprises at least a biologically active fragment of leptin.
18. A method as in claim 15, wherein the leptin compound comprises at least a biologically active fragment of leptin expressed as a fusion product in an expression vector.
19. A method as in claim 15, wherein the leptin compound is administered in a dosage from 0.1 ng/kg body weight to 100 mg/kg body weight.
20. A method as in claim 15, wherein the leptin compound is administered subcutaneously, intradermally, intravenously, intramuscularly, intraperitoneally, transdermally, orally, via pulmonary delivery, via intranasal delivery, via controlled release delivery, or via pump.
21. A method as in claim 15, wherein the leptin compound is administered continuously.
22. A method as in claim 15, wherein the leptin compound is administered in discrete doses.
23. A method as in claim 15, wherein the host suffers from a physiological defect of one or more hypothalamic, pituitary, or gonadal hormones.
24. A method as in claim 15, wherein the host is obese.
25. A method as in claim 15, wherein the host is non-obese.
26. A method as in claim 25, wherein the host has a body mass index of less than about 20.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
Description
This application claims priority under 35 U.S.C. l19(C) to provisional application 60/006,106, filed Oct. 25, 1995.
BACKGROUND OF THE INVENTION
The sterility of male and female homozygous ob/ob mice was recognized since the original report of the ob mutation (Xxxxxxx et al. J. Hered. 41:317-318 (1950)). ob/ob females are always sterile whereas ob/ob males can occasionally become fertile if maintained on a restricted diet (Lane et al. J. Heredity 45:56-58 (1954)). The ovaries of ob/ob females are capable of producing viable eggs when transplanted into lean female recipients (Xxxxxx et al. Anat. Rec. 128:569 (1957)). Although early sexual development is normal, ovulation never follows and the mice remain prepuberal indefinitely. FSH, LH and testosterone levels are reduced in ob/ob females (Xxxxxxxxx et al. Endocrinology 98:1359-1364 (1976)), demonstrating the absence of a functional feedback from the hypothalamic-pituitary axis. Hypofunction of the pituitary gland in the female ob/ob mouse was demonstrated indirectly by showing that their in vivo uterine weights did not significantly change after bilateral ovariectomy (Runner et al. Genetics 39:990-991 (1954); Xxxxxxx et al. J. Heredity 46:209-212 (1955)) but did, however, respond to exogenous estrogen. Pituitary extracts administered to ob/ob females induced ovulation and conception, but not implantation (Runner, M. N. Rec. Genet. Soc. Am. 23:63-64 (1954)) which was achieved following treatment with gonadotropic hormones (Runner et al. J. Heredity 45:51-55 (1954)). Furthermore, the administration of high doses of progesterone maintained pregnancy for 19 days p.c., but did not enable the mothers to deliver the fetuses except after administration of relaxin which stimulated parturition and lactation (Xxxxxxxxx et al. J. Exp. Zool. 133:441-458 (1956); Xxxxxxxxx et al. J. Heredity 48:97-100 (1957)). The above findings demonstrated that the sterility of the ob/ob female is caused by an insufficiency of hormones at the hypothalamic-pituitary level rather than physical hindrance of copulatory activity by excess adipose tissue.
Xxxxxxx and Mitra (J. Physiol. (London) 166:408 (1963)) proposed that puberty is linked to body weight and more specifically to fat storage which is as they conclude, one of the signals responsible for the initiation of hypothalamic control of ovarian function. Xxxxxx and XxXxxxxx (Science 185:949 (1974)) related the loss or restoration of menstrual cycles in young girls to a minimum weight for height and reported that normal girls become relatively fatter from menarche to reproductive maturity. Therefore, these early and important findings established a relationship between initiation of reproduction and adiposity. In support of this relationship were the observations that very lean young female ballet dancers and college rowers (Xxxxxx et al. NEJM 303:17 (1980); Xxxxxx et al. JAMA 246:1559 (1981)) have delayed puberty, whereas obese girls have an acceleration of puberty (Xxxxxxxxx et al. Am. J. Obs. Gyn. 108:833 (1970)). Furthermore, the amenorrhea of extremely lean women was attributed to loss of fat and hypothalamic dysfunction (Vigersky et al. NEJM 297:1141 (1977)). Based on these findings, a “critical weight” hypothesis was suggested (Xxxxxx et al. Science 109:397 (1970)) extending the
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
assumption that a metabolic signal may be responsible for the initiation of reproduction. Moreover, adipose tissue has been viewed not only as an energy source but also as a direct regulator of female reproduction (R. E. Xxxxxx Adipose Tissue and Reproduction Progress in Reproductive Biology and Medicine vol. 14 (1990)) since it converts androgens to estrogens via aromatization (P. K. Sifteri J. Endocrinology 89:119 (1981)).
The cloning, expression, and biological activities of leptin, the ob gene product, were described in a number of references, such as Pelleymounter et al. Science 269:540-543 (1995); Halaas et al. Science 269:543-546 (1995), and Xxxxxxxxx et al. Science 269:546-549 (1995).
While a variety of hormonal and other treatments have been proposed for lack of fertility in males or females, none have been entirely successful, and there remains a need for identifying improved and/or alternative therapies for enhancing fertility. In particular, improved methods and compositions should be effective, have minimum side effects, optionally be compatible with other hormonal treatments, and contribute to conception, pregnancy maintenance, and/or delivery of viable fetuses. The instant invention addresses this need and more.
SUMMARY OF THE INVENTION
One aspect of the invention is a method for restoring reproductive function in a reproductively impaired male or female host, the method comprising administering a leptin compound to the host for a time and in an amount sufficient to restore or enhance reproductive function.
A further aspect of the invention is a method of accelerating the onset of puberty in a male or female host, the method comprising administering a leptin compound to the host for a time and in an amount sufficient to cause the onset of puberty.
The leptin compound is administered to a female host from a time prior to impregnation through delivery of offspring, and preferably through lactation. The leptin compound may be a recombinant protein comprising the full secreted form of leptin. In other embodiments the leptin compound may be a recombinant protein comprising at least a biologically active fragment of leptin. The leptin compound is administered in a dosage from about 0.1 ng/kg body weight to 100 mg/kg body weight, typically subcutaneously, intradermally, intravenously, intramuscularly, intraperitoneally, orally, transdermally, via pulmonary delivery, via intranasal delivery, via controlled release, or via pump. The leptin compound is typically administered continuously or in discrete doses. The male or female host may suffer from a physiological defect of one or more hypothalamic, pituitary, or gonadal hormones. The host may be obese or non-obese. In some embodiments the host has a body mass index of less than about 20.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 depicts a 12% SDS-polyacrylamide gel showing 1 .mu.l aliquots of the recombinant human leptin before and after dialysis and 2 .mu.l and 3 .mu.l after dialysis. A 1 .mu.l aliquot from the metal chelate affinity column flow through shows bacterial proteins not bound to the column.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
FIG. 2 depicts the body weights (a, b) and food intake (c, d) of ob/ob and db/db female mice during the 3 phases of leptin treatment for group 1 (a, c) and the single phase treatment for group 2 (b, d). In the first group, the 3 phases of the treatment are shown on top of each graph and outlined by vertical lines on each graph.
FIG. 3 depicts the body weight profiles of the two leptin treated ob/ob females after stabilization of their body weights and mating to a lean wild-type C57BL/6J male. The two mice showed copulatory plugs at days 46 and 54 of the treatment and gave birth at respectively, 64 and 73 days.
FIG. 4 depicts the PCR allele-typing of the R105X obesity mutation. The 8% polyacrylamide gel shows PCR products cleaved with Dde I from the homozygous C57BL/6J lean father, a leptin-treated homozygous ob/ob mother and their 5 newborn mice. The 472 bp PCR product generates upon cleavage with Dde I a constant 325 bp fragment and a 147 bp fragment diagnostic of the wild-type allele and 105 bp and 42 bp fragments diagnostic of the mutant ob allele. The newborn mice are all heterozygous at the ob locus as shown by the presence of both 147 bp and 105 bp fragments. The 42 bp fragment is off the gel. The DNA marker is .PHI.X cleaved with Hae III.
FIG. 5 depicts the time course of leptin clearance from the circulation of lean C57BL/6J females. Each point represents the mean .+-.SEM concentration of immunoreactive (IR) leptin in 3 adult females sacrificed at the indicated time. None of the 6 controls injected with PBS for the 0 time point had any detectable human leptin in their circulation, thus denoting the high specificity of the assay for human leptin on a mouse background. Note that leptin concentrations drop by almost 50% every hour, suggesting that the half-life of leptin is approximately 1 hour.
FIG. 6 depicts the effect of leptin treatment on body weight (A) and food intake (B) of prepuberal female mice. Either vehicle (solid circles) or leptin (open circles) treatment was initiated at 21 days of age when the animals were weaned. The addition of breeder males to both groups on day 27 resulted in a slight stabilization of body weight. Total food intake (B) was reduced by approximately 18% during the 6 days of treatment prior to the addition of the breeder males.
FIG. 7 depicts the acceleration of reproduction in leptin (white bar) versus vehicle-treated (black bar) mice. The number inside the bars represent the number of mice in each group. (A) Body weights of females at the time the copulatory plug was detected. PBS-treated mice had a mean body weight of 18.3. +-.0.3 g versus 15.9.+-.0.2 g for the leptin-treated group (P<0.001 by student’s t-test is denoted by the asterisk). (B) Percentage and age of mice with a copulatory plug in the PBS and leptin groups. Mice were compared at 3 age ranges of 30-39, 40-49 and 50-62 days of age. The asterisk denotes the statistical value of P=0.003 as determined by student’s t-test at 30-39 days of age. The age distribution of the leptin-treated mice is shifted to the left reflecting an earlier age of mating behavior. (C) Percentage of plugged mice from each group that delivered pups. 42% and 46% of plugged PBS and leptin-treated mice resulted in deliveries of newborn pups.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
FIG. 8 depicts the effects of exogenous leptin on body weight (A), vaginal opening (B), onset of estrous cycle (C), uteri, oviducts and ovaries (D), LH (E) and 17-0-estradiol (F) in vehicle (O or black bar) and leptin (O or open bar) treated-group. A, B and C reflect continuous measurements from 21 to 29 days of age whereas D, E and F show determinations performed at the time of sacrifice. Asterisks denote statistical significance by student’s t-test. Uteri, P<0.004; ovaries, P<0.0001; oviducts P=0.001; LH, P=0.007. D2, P, E and D1 denote respectively dipstrus, proestrus, estrus and metestrus.
FIG. 9 depicts the effect of human recombinant leptin treatment on body weight and endogenous mouse leptin levels. (A) Body weights of prepuberal mice treated either with vehicle (0, n=12) or leptin (0, n=12). (B) 4 mice from each group were sacrificed at 30, 35 and 39 days of age to collect blood for endogenous leptin level measurements. Each time point represents the mean and SEM of 4 mice. The asterisk denotes statistical significance of leptin levels between 30 and 39 days of age (P=0.015 by student’s t-test).
FIG. 10 depicts the effect of leptin treatment or food restriction on the body weights of ob/ob males. Body weights are expressed as means and s. e. m. of 5 animals per group. Standard errors for most points were too small to be shown on the plotted scale.
FIG. 11 depicts the hematoxylin and eosin stained sections of testis from lean, untreated ob/ob, food restricted ob/ob and leptin treated ob/ob mice. (A) Overall appearance of seminiferous tubules (100 .times,magnification). The tubules are hollow and contain little sperm in the untreated and food-restricted ob/ob males. Lean and leptin-treated ob/ob males show normal tubules with abundant sperm. (B) (400.times.) Seminiferous tubules and Leydig cells. This view shows the sperm deficient lumen of the seminiferous tubules and shrunk interstitial Leydig cells in untreated and food-restricted ob/ob males. Leptin treated ob/ob males regained a testicular histology comparable to the 9 lean wild-type males. (C) (l000.times.) Magnification showing interstitial Leydig cells. The untreated and food-restricted ob/ob males show cytoplasm shrinkage of the Leydig cells, whereas the leptin-treated ob/ob males have normal Leydig cells identical to lean males.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Exemplary methods for cloning and purifying leptin compounds are described in scientific literature. See, for example, Pelleymounter et al. Science 269:540-543 (1995); Halaas et al. Science 269:543-546 (1995), Xxxxxxxxx et al. Science 269:546-549 (1995), and Xxxxxx et al. Nat. Gen. 12:318-320 (1996). Leptin compounds suitable for use in the present invention, also termed “leptin” herein, may comprise a full-length leptin polypeptide obtained from a mammalian source, preferably human for the treatment of humans. Typically, the leptin compound will be a recombinant polypeptide. The recombinant polypeptide can be produced in mammalian, bacterial, or yeast expression systems. Suitable leptin compounds may also
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
comprise fragments, analogs, and derivatives, including truncated forms, of the full-length leptin polypeptide. Fusions of such compounds to a non-leptin polypeptide are included in the scope of the invention. In particular, a fusion protein incorporating the human leptin polypeptide used in the Examples section hereinafter, is at least one exemplary leptin compound which is suitable for use in the methods of the present invention.
Reproductively impaired female hosts suitable for treatment by the methods of the present invention will usually suffer from a deficiency in the hormonal system which supports ovulation, conception, maintenance of pregnancy, and/or delivery of offspring, typically being one or more hypothalamic, pituitary, or gonadal hormones. Administration of the leptin compound is believed to correct or help correct such deficiencies in the hypothalamic-pituitary-gonadal hormone system of the reproductively impaired female. “Reproductively impaired” is used interchangeably with infertile herein, and is intended to include amenorrheic females. Reproductively impaired females include those incapable of ovulation, conception, pregnancy maintenance, lactation, and/or delivery of full-term offspring, as well as those having difficulty in these areas. Administration of the leptin compound will restore and/or enhance the ability of such females to conceive and bear children. Similarly, infertile males include those incapable of impregnating females. “Sterile” is used interchangeably with “infertile” and “reproductively impaired” herein.
The male or female hosts treated by the method of the present invention may be obese or non-obese. It is believed that administration of the leptin compounds will restore hormonal function associated with obesity or gross alteration in body weight as well as other conditions which are not associated with obesity.
Thus, the present invention provides methods for restoring reproductive function in a reproductively impaired male or female host, the method comprising administering a leptin compound to the host for a time and in an amount sufficient to restore or enhance reproductive function. In some embodiments of this invention a leptin compound will be administered to a male or female host to accelerate the onset of puberty. “Puberty” as used herein includes the initiation and completion of the first menstrual cycle. Typically, the male or female host will have experienced a delayed onset of puberty or be at risk for a delayed onset of puberty, typically as a result of gross deviation in body weight, before treatment with a leptin compound. The leptin compound will usually be administered in a dosage from 0.1 ng/kg body weight to 100 mg/kg body weight. Amounts effective for this use will depend on, e.g., the leptin composition, the manner of administration, the weight and general state of health of the patient, and the judgment of the prescribing physician.
The leptin compound will typically be administered to a female host during at least a portion of the time from prior to impregnation through delivery of the offspring, usually during the entire period from prior to impregnation through delivery of the offspring, and preferably through lactation.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
The leptin compound will typically be administered to a male host during at least a portion of the time from prior to impregnation of a female to when impregnation has been confirmed.
The leptin compound will typically be administered to a host experiencing a delayed onset of puberty for at least a period prior to the onset of puberty through puberty, and possibly through a period of desired impregnation and delivery of offspring, typically in an amount sufficient to cause the onset of puberty.
The treated male or female host is typically human, but the method is effective with other mammalian hosts, such as mice. The host will usually suffer from an insufficiency of one or more hypothalamic, pituitary, or gonadal hormones of the type necessary for or otherwise involved in reproductive fertility. In some cases the hosts will be obese; in other cases the hosts may be non-obese. Obesity is defined as including having a body mass index (BMI; weight in kg/(height in meters).sup.2) over about 30. In some instances, the non-obese host will have a BMI of less than about 20.
The leptin compound may be administered subcutaneously, intradermally, intravenously, intramuscularly, intraperitoneally, via pulmonary delivery, via intranasal delivery, transdermally, orally, via controlled release, via pump, or by any other conventional route of administration for polypeptide drugs. Typically, the leptin compound will be administered continuously during the period of administration, i.e., being delivered at least once per day or via controlled release techniques, such as via transdermal patches.
In some embodiments, the invention provides compositions for administration which comprise a solution of leptin dissolved or suspended in an acceptable carrier, preferably an aqueous carrier. A variety of aqueous carriers may be used, e.g., water, buffered water, 0.8% saline, 0.3% glycine, hyaluronic acid and the like. These compositions may be sterilized by conventional, well known sterilization techniques, or may be sterile filtered. The resulting aqueous solutions may be packaged for use as is, or lyophilized, the lyophilized preparation being combined with a sterile solution prior to administration. The compositions may contain pharmaceutically acceptable auxiliary substances as required to approximate physiological conditions, such as pH adjusting and buffering agents, tonicity adjusting agents, wetting agents and the like, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, sorbitan monolaurate, triethanolamine oleate, etc.
The concentration of leptin in the pharmaceutical formulations can vary widely, i.e., from less than about 0.1%, usually at or at least about 2% to as much as 20% to 50% or more by weight, and will be selected primarily by fluid volumes, viscosities, etc., in accordance with the particular mode of administration selected.
For solid compositions, conventional nontoxic solid carriers may be used which include, for example, pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, talcum, cellulose, glucose, sucrose, magnesium carbonate, and the like. For oral administration, a pharmaceutically acceptable nontoxic composition is formed by incorporating
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
any of the normally employed excipients, such as those carriers previously listed, and generally 10%-95% of active ingredient, that is, one or more leptin compounds of the invention, and more preferably at a concentration of 25%-75%.
For aerosol administration, leptin is preferably supplied in finely divided form along with a surfactant and propellant. Typical percentages of leptin are 0.01%-20% by weight, preferably 1 %-10%. The surfactant must, of course, be nontoxic, and preferably soluble in the propellant. Representative of such agents are the esters or partial esters of fatty acids containing from 6 to 22 carbon atoms, such as caproic, octanoic, lauric, palmitic, stearic, linoleic, linolenic, olesteric and oleic acids with an aliphatic polyhydric alcohol or its cyclic anhydride. Mixed esters, such as mixed or natural glycerides may be employed. The surfactant may constitute 0.1%-20% by weight of the composition, preferably 0.25-5%. The balance of the composition is ordinarily propellant. A carrier can also be included, as desired, as with, e.g., lecithin for intranasal delivery.
The leptin compositions of the invention can additionally be delivered in a controlled release system such as a depot-type system, an encapsulated form, or an implant by techniques well-known in the art. The compositions of the invention can also be delivered via a pump, such as a minipump, to the male or female host.
The following examples are offered by way of illustration, not by way of limitation.
EXAMPLES
I. Rescuing Fertility in Females
| A. | Results and Discussion |
This study investigated the ability of leptin to correct the reproductive defect of the sterile female ob/ob mouse.
The human ob cDNA sequences spanning amino acids 22 to 167 (Zhang et al. Nature 372:425-32 (1994)) and representing the secreted ob protein were cloned into the expression vector pQE30. The 16 Kd ob protein was overexpressed in E.coli, purified and renatured by dialysis (FIG. 1).
The refolded leptin protein was injected intraperitoneally into 2 groups of experimental ob/ob female mice at a dose of 10 .mu.g/g of initial body weight. Control ob/ob mice were injected with identical volumes of phosphate buffered saline (PBS). The first group consisted of 4 ob/ob animals (2 controls and 2 experimentals) and the second group of 10 ob/ob animals (5 controls and 5 experimentals).
In the first group, the two experimental ob/ob female mice (ob-5 and ob-6), weighed respectively, 48.5 g and 51.7 g whereas the two control mice weighed 53.8 g and 50.0 g. In addition, 2 female db/db mice also received leptin injections to test for non-specific weight loss
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
as these mice are resistant to leptin effects, presumably due to a defect in the leptin receptor (Xxxxxxx et al. Am. J. Phys. 217:1298-1304 (1969); Xxxxxxx, X. X. Diabetoloqia 9:294-298 (1973)). Food intake and body weights were continuously measured to determine the efficacy of the treatment.
The leptin treatment for the first group consisted of 3 phases. In the first phase which lasted 8 days, the mice received two injections per day of PBS or leptin. At the end of the first phase, weight loss in the treated animals was 24% and 27% of their original body weight (FIG. 2A). In contrast, the body weights of both ob/ob PBS-controls and one leptin treated db/db mouse increased by 6% whereas the second db/db treated mouse lost 1% of body weight (FIG. 2A). Food intake of leptin treated ob/ob mice was markedly reduced to 13% of the food intake of control ob/ob mice (FIG. 2B). Withdrawal of the leptin injections in the second phase showed in the ob/ob mice, a rapid increase in food consumption and body weight, demonstrating the need for repeated leptin injections to maintain a biological effect (FIG. 2A and 2B). The third phase consisted of single daily leptin injections which produced a biological response similar to the first phase, albeit at a lower rate as evidenced by the slopes of the two body weight curves during phases 1 and 3 (FIG. 2A). Injections were continued for a total of 42 days in the two leptin treated ob/ob mice until their body weights stabilized to approximately 28 g and 25 g corresponding to 46% and 48% reductions in body weight. The body weights of the db/db mice changed minimally (FIG. 2A) whereas their food intake increased in parallel with that of PBS injected ob/ob controls (FIG. 2B).
In the second group, the experimental ob/ob mice were injected intraperitoneally once daily with a 10 .mu.g/g initial body weight of leptin for 30 days. The mean body weights of the 5 ob/ob controls after 30 days was 55.9.+-.2.0 as compared to 27.8.+-.0.9 for the 5 leptin treated ob/ob mice. The treated mice had lost 40% of their original body weight whereas the control mice increased their body weight by 13% (FIG. 2C and 2D).
After 6 weeks of leptin treatment, at a time where body weight had stabilized, the 2 PBS-controls and 2 leptin-treated mice (ob-5 and ob-6) from the first group were mated with a normal C57BL/6J male mouse. A copulatory plug was detected only in ob-5 and ob-6 shortly thereafter, but not in the controls. The formation of copulatory plugs demonstrates the occurrence of estrus and ovulation in the treated ob/ob females. Leptin injections were then continued at half the original dose. The treated females started to show a significant increase in body weight at day 12 p.c, and delivered newborn pups at 20.5 and 19.5 days p.c. (FIG. 3). Parturition occurred in both treated mice over at least 36 hours resulting in the delivery of 6 (ob-5) and 2 (ob-6) pups.
Although the mice were alive and fully developed at birth, none of the newborns survived. Five intact or partially eaten newborn mice were recovered from ob-5 and 2 mice from ob-6. DNA was extracted from the newborns to determine their genotypes at the ob locus. PCR primers bracketing codon 105 which is the site of the mutation in the ob/ob mouse, were designed and used for DNA amplification. The 472 bp amplified PCR product contains in the homozygous normal mouse a single Dde I site which produces 325 bp and 147 bp fragments upon cleavage with Dde I. However, the R105X obesity mutation generates an additional Dde I site which
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
results in fragments of 325 bp, 105 bp and 42 bp in the homozygous ob/ob mouse. PCR testing showed that the lean male mouse who fertilized both treated females was homozygous for the wild-type allele, whereas both mothers were homozygous for the mutation and all newborn mice were heterozygous for R105X (FIG. 4).
In the second group, 4 ob/ob controls and 4 ob/ob leptin-treated mice whose body weight stabilized as a result of the leptin treatment were also mated to C57 BL/6J wild-type lean males. All 4 leptin-treated, but not any of the control ob/ob mice, became pregnant. Furthermore, ob-5, who was under continuous leptin treatment even after delivery, became pregnant a second time, demonstrating that the entire process can be repeated.
Although the levels of reproductive hormones in the pregnant females were not measured, the entire process of ovulation, pregnancy and parturition could not have occurred in the absence of these hormones. The previous findings that the ob/ob females can respond to the effects of these hormones either exogenously or in vivo after ovarian transplants demonstrates that leptin can stimulate the production of hormones required for ovulation, pregnancy and parturition. Furthermore, these studies show that the sterility defect in adult ob/ob female mice is a reversible process that can be corrected with leptin treatment. Therefore, leptin is not only a satiety factor that can suppress appetite and promote lipolysis (Pelleymounter et al. Science 269:540-543 (1995); Halaas et al. Science 269:543-546 (1995); Xxxxxxxxx et al. Science 269:546-549 (1995); Xxxxxx et al. J. Clin. Inv. 96: 2065-2070 (1995)) but also plays an important role in reproductive physiology.
It is not clear why the newborn mice were eaten by the mothers. It is possible that daily handling and injection of the mothers throughout gestation and especially before and after delivery is a contributing factor to this effect. It is also possible that the mothers failed to nurse the newborns, from possibly a lack of prolactin secretion.
Rescue of the sterility phenotype in ob/ob females has important implications for the husbandry of this strain. Whereas ovarian transplants have been a successful approach to the maintenance and propagation of the ob/ob strain, an alternative solution, leptin treatment, is available as a result of the studies described herein.
| B. | Methods |
1. Expression and purification of the recombinant human ob protein.
Human ob cDNA sequences representing the mature secreted human protein (from amino acids 22 to 167) were amplified by RT-PCR from normal human fat mRNA using human specific cDNA primers based on the Genbank sequence U18915. The cDNA was subcloned by blunt-end ligation into pSK+ srf (Stratagene), recovered as a Bam HI restriction fragment and inserted into the expression vector pQE30 (Quiagen). The recombinant ob protein was thus under the control of a T5 bacteriophage promoter, had a hexahistidine tag at the amino terminus and a stop codon immediately following amino acid 167. The DNA construct was transformed and overexpressed
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
in E. coli following induction by IPTG. A crude protein lysate was prepared under denaturing conditions and the ob protein purified by metal chelate affinity chromatography on a nitrilo-tri-acetic acid (NTA) resin. The bound protein was finally eluted and recovered in 20 ml o 4.6M urea, 0.077 M Na2HPO4, 0.077M Tris pH 8.0 and 250 mM imidazole. The eluted protein was allowed to refold by slow dialysis of the denaturant successively in 4 M, 3 M, 2 M, 1 M, 0.5 M urea in phosphate buffered saline (PBS). Final dialysis was in 3 changes of PBS. Each dialysis step was performed for at 12-24 hours in 6000-8000 molecular weight cutoff (MWCO) tubing at 4,degree. C. against 50 volumes of solution. Aliquots of the refolded protein were used to determine its concentration by the Bradford protein assay (Biorad Laboratories). In addition, purity and recovery of the protein after dialysis were estimated by fractionation on a 12% stacking SDS-polyacrylamide gel. Electrophoresis was performed at 100V, 25 mA for 2 hours in 25 mM Tris, 250 mM glycine and 0.1% SDS. The gel was then stained with Coomassie Blue and extensively destained in 45% methanol, 10% acetic acid.
2. Leptin treatment of the ob/ob mice.
Homozygous female C57BL/6J-ob/ob and C57BL-KsJ-db/db mice were purchased from the Xxxxxxx Laboratories and housed at the UCSF Animal Care Facility under alternating 12-hours light and dark periods.
For the first group of mice, the initial phase of the treatment (days 1 -8) consisted twice daily (between 8- 9 a.m. and 5-6 p.m.) of either PBS or recombinant human leptin injections at a dose of 10 .mu.g/g of initial body weight. In the second phase (days 9-13), injections were completely withdrawn and in the third phase, which began at day 14, the mice received a single injection (between 5-6 p.m.) per day of either PBS or leptin. The second group received once daily a 10 .mu.g/g of initial body weight dose throughout the single phase treatment. The dose of leptin was reduced to half upon observation of the copulatory plug and continued at a single injection daily throughout gestation until delivery. The mice were weighed periodically and food consumption deduced by weighing the food left in the cage at periodic intervals. The dead newborn mice were frozen at -20.degree. C. until DNA extraction.
3. Genotyping of the newborn mice.
5 mm sections from the frozen embryos or tail of the ob/ob mother and lean father were obtained by excision. The tissue was first homogenized in 10 mM Tris-1 mM EDTA (TE) to generate a cell suspension. SDS was then added to 0.5% to lyse the cells and the mixture extracted with phenol- chloroform followed by ethanol precipitation. The DNAs were dissolved each in 50 .mu.l of TE and 5 .mu.l aliquots subjected to DNA amplification by the polymerase chain reaction (PCR). The PCR primers consisted of 5’ CTGGTTCTTCACGGATATCATTG 3’ and 5’ AGGGAGCAGCTCTTGGAGAA 3’. The amplification reaction was carried out in 50 .mu.l for 35 cycles at 00.xxxxxx, X.00.xxxxxx. C., and 00.xxxxxx. C. for 30 sec. after which a discrete 472 bp product was obtained. A 7 .mu.l aliquot was then digested with Dde I (New England Biolabs) and the digested products fractionated on a native 8% polyacrylamide gel. The gel was stained with ethidium bromide and the products visualized under ultraviolet light.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
| II. | Triggering of reproductive function in prepuberal normal female mice |
This study demonstrated that leptin is a critical signal for the initiation of reproduction. Without being limited to any one theory, leptin may stimulate GnRH release so as to initiate puberty.
| A. | Measurement of Leptin Half-life |
An advantage of using human leptin for these experiments lies in the ability to measure independently mouse endogenous and human exogenous leptin with specific radioimmunoassays that have virtually no cross reactivity with each other (<0.2%). Measurement of leptin levels with the human specific radioimmunoassay revealed undetectable levels in control mice, thus demonstrating the specificity of the assay.
The clearance time of exogenous human recombinant leptin was determined to calculate the frequency of injections in subsequent experiments as follows. Twenty eight C57 BL/6J adult females weighing 24.1+-.0.7 g, fed ad lib., were divided between a control and a leptin group. Six mice were injected with PBS vehicle solution and 21 others with a 4 .mu.g/g body weight dose of immunoreactive leptin. Control mice were sacrificed immediately after saline injection whereas 3 leptin-injected mice were sacrificed at each 1, 2, 3, 4, 5, and 7 hours. Blood was collected by cardiac puncture and the plasma separated by centrifugation and frozen at -20.degree. C. until use.
In the injected animals, immunoreactive leptin (IR-L) peaked at 1 hour and started to decline approximately by 50% each hour until it was undetectable at 7 hours post injection (See FIG. 5). Therefore, leptin has a half-life of approximately 1 hour. Consequently, leptin was administered daily in subsequent experiments between 5-7 p.m. just prior or soon after the onset of the dark period.
Because previous studies have shown that large amount of exogenous leptin are required to exert a biological effect in normal C57BL/6J mice (Pelleymounter et al. Science 269:540 (1995); Halaas et al. Science 269:543 (1995)), exogenous leptin was administered in these experiments at 2 .mu.g/g body weight (half the dose used in the clearance study).
| B. | Fertility of leptin-treated lean female mice |
To determine whether leptin-treated mice will reproduce earlier than control animals, fertility was selected as an endpoint of the experiment. The rationale of this study was derived from the hypothesis that if fat is a determinant for puberty, then leptin, which is secreted from fat may be a critical factor in initiating reproductive function. Therefore, treatment of prepuberal mice with exogenous leptin would “trick” neuroendocrine pathways involved in reproduction into acting as if the animal accumulated a certain amount of fat as reflected by the elevated levels of leptin in its circulation.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
Prepuberal C57BL/6J mice (n=25) were weaned at 21 days of age and divided into two groups. One group (n=13) weighing 10.2.+-.0.4 g received one daily injection of the human recombinant leptin whereas the other group (n=12) weighing 10.3 .+-.0.3 g received identical volumes of the phosphate buffered-saline (PBS) vehicle solution. Breeder males were then placed at day 28 in each cage at a ratio of one male per 3 females until a copulatory plug was detected in each female whose weight and age was recorded. Plugged females were kept under either treatment until 20 days after detection of the plug and were housed each in a separate cage to assess whether the copulatory plug was associated with a successful pregnancy.
All procedures were approved by the UCSF Committee on Animal Research. Two-week-old lactating prepuberal C57 BL/6J female pups were obtained from the Xxxxxxx Laboratories and allowed to recover for one week prior to initiation of the experiment. All male pups were removed from the litters at 2 weeks of age. Mice were housed at the UCSF Animal Care Facility and maintained at 00.xxxxxx. C. with a 12 hrs. light, 12 hrs. dark cycle (lights on at 6 a.m., off at 6 p.m.). Mice were treated with human recombinant leptin as described above. The leptin preparation was quantitated by radioimmunoassay (Linco Research, St. Louis Mo.) and injected intraperitoneally at a dose of 2 .mu.g/g body weight while control animals received phosphate buffered saline (PBS) injections. Prepuberal C57BL/6J mice (n=25) born on the same day were weaned at 21 days of age and housed in 7 cages consisting each of 3 mice and 1 cage of 4 mice. Animals from the same litter were placed into different cages such that no cage contained two mice from the same litter and divided into two groups. One group (n=13) weighing 10.2.+-.0.4 g received one daily injection of 2 .mu.g/g body weight IR-L whereas the other group (n=12) weighing 10.3.+-.0.3 g received identical volumes of the phosphate-buffered-saline (PBS) vehicle solution. The mice were continuously monitored for body weight, food intake and vaginal opening which occurred in both groups at 26-27 days of age. Young female mice were housed away from males and had no contact with males or their urine until day 28, when a sexually competent adult C57 BL/6J male was added to each of the 8 cages at a ratio of one male per 3 females to initiate mating. Plugged females were kept under either treatment until 20 days after detection of the plug and were housed each in a separate cage to assess whether the copulatory plug was associated with a successful pregnancy. All statistical P values in this study were calculated by two-sample t-test using a Macintosh computer equipped with statistical package.
Leptin treatment of the young mice resulted in a slowdown of growth as compared to the control group (FIG. 6A) and was statistically significant from the third day (P=0.003) and throughout the treatment. This effect was primarily due to a decrease in food intake, as shown in FIG. 6B.
If leptin is involved in signaling puberty and the onset of reproduction, then leptin-treated mice, despite having a lower body weight (but high leptin levels) as a result of the thinning effects of leptin, will attain reproductive maturity earlier than the vehicle treated group. Indeed, these results showed that at the time the copulatory plug was first detected, leptin-treated mice had reached a body weight of 15.9.+-.0.2 g as opposed to 18.3.+-.0.3 g for the vehicle group (FIG. 7A), accounting for a 13% difference in body weight (P<0.0001). Furthermore, leptin-treated mice were plugged at an earlier age than the vehicle treated group. Thus, between the ages of
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
30-39 days, plugs were detected in 85% of leptin-treated mice (11 of 13 mice, P=0.003) and 17% (2 of 12 mice) of vehicle treated mice. The remaining 2 leptin-treated mice (15%) were plugged at 44 and 46 days of age as opposed to 40-49 and 50-62 days of age for respectively 6 (50%) and 4 (33%) of vehicle-treated mice (FIG. 7B).
Therefore, leptin treatment resulted in a significant acceleration of behavioral estrus and mating capability when the copulatory plug was used as a reproductive index. Thus, the attainment of the appropriate age and weight that allows initiation of reproduction in the control group, is advanced in the leptin-treated group owing to the elevated levels of leptin that have signaled neuroendocrine pathways that the animal has accumulated enough fat since leptin was shown to be a brain marker for adiposity (Frederich et al. Nat. Med. 12:1311 (1995); Xxxxxx et al. Nat. Med. 11:1155 (1995)).
The proportion of plugged mice from both groups that carried out successful pregnancies at their first estrus was determined by delivery of newborn pups. Thus successful pregnancies and deliveries were comparable in both groups and consisted of 42% and 46% in vehicle and leptin groups, respectively (FIG. 7C). Altogether, leptin treatment resulted not only in an acceleration of behavioral estrus and mating but was also accompanied with normal pregnancies and deliveries, demonstrating successful ovulation among the leptin-treated mice.
To assess whether leptin treatment affected maturation of the reproductive tract in prepuberal mice, we determined in vehicle and leptin groups the timing of vaginal opening, initiation and progress of the first estrous cycle, the weights of uteri, ovaries and oviducts (which are excellent indices and bioassays of hormones action as well as LH and estradiol levels). Mice were weaned at 21 days of age and separated into PBS (n=12) and leptin (n=12) groups. Twenty four C57 BL/6J females were weaned at 21 days as above from different litters and divided equally with respect to litter of origin into vehicle (n=12) and leptin groups (n=12). Three animals were housed per cage with no litter mates placed in the same cage. The mice were treated with either PBS or leptin as above for 8 days and sacrificed on day 29 to collect blood, ovaries, oviducts and uteri. Dissection of each organ was carried out under a binocular microscope to ensure removal of contaminating tissues. Organs were weighed on a Metier AE160 high precision analytical balance.
Leptin treated mice grew at a slower rate than vehicle treated animals, demonstrating again the effect of leptin on body weight (FIG. 8A). At the reproductive level, vaginal opening occurred in respectively 2, 1, 3, and 6 leptin-treated mice at respectively 22, 23, 24 and 25 days of age. However vaginal opening occurred in 3, 6, 2 and 1 vehicle treated mice at respectively 25, 26, 27 and 28 days of age (FIG. 8B). Thus vaginal opening is advanced up to 4 days as a result of leptin treatment.
As a consequence of early vaginal opening, normal cyclicity was initiated in leptin treated mice prior to the control group (FIG. 8C) such that by day 29, 5 out of 12 leptin-treated mice had passed through estrus and progressed to metestrus (D1) stage thereby completing their first estrous cycle whereas none of the mice in the control group had reached that point. Mice, not exposed to males, have normally their first fully cornified smear 5-8 days after vaginal opening (Stiff et al. Endocrinology 84:492 (1974)).
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
A corollary of this experiment and the fertility experiment described above demonstrated that the biological actions of estrogens that are first evidenced by vaginal opening are not sufficient to elicit reproduction in control mice. However, in the experimental group, the presence of elevated levels of leptin with endogenous estrogens induce a rapid onset of reproduction. Thus, vaginal opening in mice lacking leptin is a sensitive index of estrogens action but not of fertility, whereas in leptin-treated mice, vaginal opening is associated with fertility.
Evidence of gonadal steroids action was determined by assessing the weights of reproductive organs which are targets for hormones that are released transiently, but that produce long term effects on target tissues. Thus, the action of estradiol can be best evidenced by its striking and stimulatory effects on the hypertrophy and hyperplasia of uterine tissues thereby promoting uterine growth. In leptin-treated prepuberal mice, uterine weights consisted of 20.8.+-.2.1 mg as opposed to 13.6.+-.0.8 mg in controls (P=0.004) representing a 53% increase. Similar effects were found on ovarian (4.8.+-.0.2 mg for vehicle vs. 6.6.+-.0.3 mg for leptin. P<0.0001) and oviducts weights (3.2.+-.0.2 for control vs. 4.6 .+-.0.3 for leptin, P=0.001) which increased respectively by 37.5% and 43.8% as a result of leptin treatment (FIG. 8D). Therefore, leptin treatment of the mouse at an early age resulted in a premature release of the hormones necessary for maturation of the reproductive tract.
Hormone levels for the gonadotropin LH and the gonadal steroid 17-.beta.-estradiol were determined at day 29, when the mice were sacrificed. LH was assayed on 50 .mu.l plasma using a rat specific immunoassay purchased from Peninsula Laboratories (Belmont, Calif.). 17-.beta.-estradiol levels were determined on 200 .mu.l plasma by an ultrasensitive radioimmunoassay purchased from Diagnostic System Laboratories (Webster, Tex.). Unknown samples from both assays fell into the linear portion of each standard curve. LH levels consisted in control and leptin-treated mice of respectively, 6.9.+-.0.4 ng/ml and 5.2.+-.0.4 ng/ml, a statistically significant decrease (P=0.007) of 24.6% (FIG. 8E). However, I7-.beta.-estradiol levels were comparable in both groups and consisted respectively of 10.3.+-.1.1 pg/ml and 9.5.+-.1.1 pg/ml for control and leptin-treated groups (FIG. 8F). Altogether, the results displayed in FIG. 8 show that leptin treatment of prepuberal mice causes at the reproductive level a concomitant stimulation of vaginal opening and ensuing first estrous cycle. These effects were mediated by the target actions of gonadal steroids which caused the reproductive tissues to mature earlier in leptin-treated mice. Because at the time of sacrifice, most control animals had not already ovulated, the findings that LH levels are elevated in control animals are consistent with the rising levels of LH prior to ovulation.
Conversely, the depressed levels of LH in leptin-treated animals reflect the completion or near completion of their first estrous cycle. Taken in this context, 17-.beta.-estradiol levels thus reflect antiparallel effects whereby estrogen levels are on the rise in control animals that are progressing towards completion of their first cycle but decreasing in most leptin-treated mice that have already proceeded either close or beyond their first cycle.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
In order to determine the levels of endogenous leptin at the time of reproductive maturation, C57 BL/6J females were weaned at 24 days of age and treated with either PBS (n=l 2) or human recombinant leptin (n=12). Four animals from each group were sacrificed at 30, 35 and 39 days of age and blood collected for leptin measurements. The rationale of this experimental was two-fold: to assess endogenous leptin levels in vehicle treated mice at the time of reproductive maturation and to find out whether production of endogenous leptin is affected by the administration of exogenous leptin. As in the previous experiment, leptin treatment resulted consistently in a slowdown of body weight growth as shown in FIG. 9A. Plasma leptin levels in the vehicle group at 30, 35 and 39 days of age (FIG. 9B) consisted of 2.3+-.0.1 ng/ml, 2.9.+-.0.4 ng/ml and 3.7.+-.0.4 ng/ml. A 61% statistically significant increase (P=0.015) in leptin was thus found between 30 and 39 days of age. Interestingly, in the treated group, leptin levels did not rise but stabilized at 2.9-3.3 ng/ml showing that exogenous leptin treatment suppressed endogenous leptin production thereby suggesting the presence of a leptin producing feedback loop which usually increases endogenous leptin as the mouse progresses to puberty.
Overall, these findings demonstrated that leptin acts as a signal for puberty essentially as evidenced by its effects on the acceleration of reproduction in prepuberal mice, on vaginal opening, on the onset of the first estrous cycle and on maturation of reproductive tissues which are accompanied by changes in LH and 17-.beta.-estradiol levels. The sites of actions of leptin could either be on the brain, on the ovaries or both since leptin receptors have also been identified on mouse ovaries (Chehab et al. Nat. Gen. 12:318- 320 (1996)). However, the brain appears to be the prime target since transplantation of either the ob/ob or the db/db defective ovary into a normal mouse restores its function independent of whether the original host has a leptin (ob/ob) or leptin-receptor (db/db) defect.
| III. | Rescue of Male Sterility |
| A. | Leptin treatment and food restriction of ob/ob males |
Experiments were approved by the UCSF Committee on Animal Research. The animals were purchased from the Jackson Laboratories (Bar Harbor, Mee) and maintained each in individual cages at the UCSF Animal Care facility under a 12 hr. dark light regimen (lights on at 6 a.m., off at 6 p.m.). Human recombinant leptin was prepared as described above. Ten morbidly obese adult homozygous ob/ob males housed in individual cages were equally divided into a food-restricted group (ob/ob 1-5) and a leptin-treated group (ob/ob 6-10). In order to accelerate weight loss, the recombinant protein was injected daily intraperitoneally at a single dose of 20 .mu.g/g initial body weight. The treatment was for 60 days to allow enough time for the food restricted ob/ob males to stabilize their body weight loss. Leptin-treated ob/ob males had continuous access to food whereas food-restricted ob/ob males were pair-fed to the leptin-treated group and allowed to consume daily 1 g of food (Purina-Mills, FormuLab Diet 5008) for 46 days and 3 g for the remaining 14 days. Water was provided without any restriction to both groups.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
| B. | Matings of ob/ob mice |
After 12 days of leptin treatment, 2 normal females were placed with each ob/ob male from both groups. Females with food-restricted ob/ob males were placed in the cage between 5 p.m. and 6 a.m. for mating after the ob/ob male had consumed the 1 g of food. These females were removed the next morning and placed collectively in cages where they access to food which they lacked of during the dark period. This cycle was repeated throughout the treatment.
| C. | Organ weights and histology |
In order to determine some of the parameters that contributed to their fertility, control and leptin-treated ob/ob mice were sacrificed by an overdose of 2.5% Avertin at day 61 along with 5 lean male C57/BL mice and 3 untreated ob/ob males which were fed ad lib. To gain further insights into the effects of leptin treatment on the anatomy of the testis, histological examination of testicular sections from lean, untreated ob/ob, food restricted ob/ob and leptin treated ob/ob mice were carried out. The seminal vesicles and testis of all animals were weighed and histology of the testis examined. The seminal vesicles and testis were dissected and weighed immediately. The testis were then fixed in fresh 4% paraformaldehyde in PBS for 48 hours and processed for paraffin embedding, sectioning and hematoxylin and eosin staining. Statistics were determined by two-sample Student’s t-test,
| D. | Food consumption and body weight of leptin-treated ob/ob males |
Mean daily food intake of each leptin treated ob/ob male during the first 12 days of treatment consisted of 0,8+0.1 g (Table 1). The food-restricted and leptin-treated groups entered the treatment, respectively, with 68.7+1.2 g and 65.6+2.2 g and lost respectively after 12 days of treatment 24.2+0.5% and 32.1+1.2% of their initial body weight (FIG. 10). Long term leptin treatment resulted in an increase of food consumption to approximately 2.9+0.3 g at day 45 (Table 1) which was used as a reference to increase the allotment of food to the food-restricted males for the last two weeks of treatment. The increase in food consumption of the leptin-treated ob/ob males could be as a result of partial resistance to the administered exogenous leptin. The body weights of 5 ob/ob mice treated for 12 days with a PBS vehicle solution did not significantly change (data not shown) and were thus excluded from subsequent matings since no weight loss was observed.
| E. | Fertility of ob/ob males |
Each ob/ob mouse was housed at day 12 with 2 lean females to determine if weight loss, whether induced by food-restriction or leptin treatment, was accompanied with fertility. Demonstration of fertility in the leptin-treated ob/ob males was determined by the delivery of newborn pups 19-20 days after detection of the copulatory plug in lean females.
Food restriction of the ob/ob males resulted in a significant weight loss of 40.6.+-.0.5% at 30 days and 48.1.+-.1.8% at 60 days. However, none of the lean females that were mated with the
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
food-restricted males had a copulatory plug nor any pregnancy resulted from diet-induced weight loss in the ob/ob males. Monitoring of body weight and food intake of the lean females revealed that they maintained a constant weight and consumed their food during the day since food was not available when they were placed with the food-restricted ob/ob males (data not shown). Therefore, induction of weight loss by food restriction in the ob/ob males did not result in correction of their sterility suggesting that excess adipose tissue is not the cause of sterility.
In contrast, mating of the 5 leptin-treated males with the 10 lean females resulted in copulatory plugs, pregnancies and deliveries in all the breeder females (Table II) demonstrating unequivocally successful functional reconstitution of the mate ob/ob reproductive system in the presence of leptin. Thus, correction of the sterility in ob/ob mice occurred despite an initial body weight of 65.6+2.2 g, thus indicating that accumulation of excessive fat does not permanently block the immaturity of the hypothalamic-pituitarygonadal axis which becomes functional as a result of leptin treatment.
F. Organ weights and testicular histology
Seminal vesicles weights were not statistically significant among the 4 groups. However, testis weight which is mostly indicative of overall spermatogenic activity and testosterone content was significantly different between lean and untreated ob/ob mice (P<0.001) and food-restricted versus leptin-treated ob/ob mice (P<0.001) as shown in Table III, Interestingly, comparison of the untreated ob/ob and food restricted ob/ob mice resulted in a 60% increase in testicular weight of the latter group (P<0.009) without, however, correction of their sterility. Thus, one effect of the leptin-induced fertility treatment is a normalization of testicular weight and function. Previous studies have shown that the overall structure of the ob/ob testis is abnormal and characterized most aberrantly by multinucleated spermatids, few spermatozoa and a small amount of interstitial Leydig tissue (STET) reduced by more than 50% (Heilman, B. Ann. N.Y. Acad, Sci. 131:541-558 (1965)). Consistent with these observations, two prominent histological features shown in FIG. 11 are noticeable. First, the lumen of the seminiferous tubules in the untreated and food restricted ob/ob males appear hollow and contain strikingly less sperm than the lean male mouse. Second, the interstitial Leydig cells of the obese mice are atrophied due visibly to a shrinkage in their cytoplasm. Food restriction of ob/ob males does not alter their abnormal histology and infertility as previously shown (Heilman et al. Acta Endocrinol. 44:20-26 (1963); Barash et al. Endocrinology 137:3144-3147 (1996)) and by the present experiments (FIG. 11).
In contrast, the seminiferous tubules of the leptin-treated ob/ob males become more abundant with mature sperms and the Leydig cells regain their usual morphology and clustering characteristic. Therefore, leptin treatment of the ob/ob males stimulates spermatogenesis and allows regeneration of the Leydig cells which should then be capable of producing adequate amounts of testosterone.
Therefore, the present study demonstrated that leptin treatment corrects the sterility of ob/ob males as effectively as in ob/ob females.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
All references cited herein are incorporated by reference in their entirety for all purposes.
As will be understood by those of skill in the art, the present invention may be embodied in other specific forms without departing from the spirit and essential characteristics thereof. Accordingly, reference should be made to the appended claims for a description of the scope of the invention.

THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
United States Patent: 5,773,416
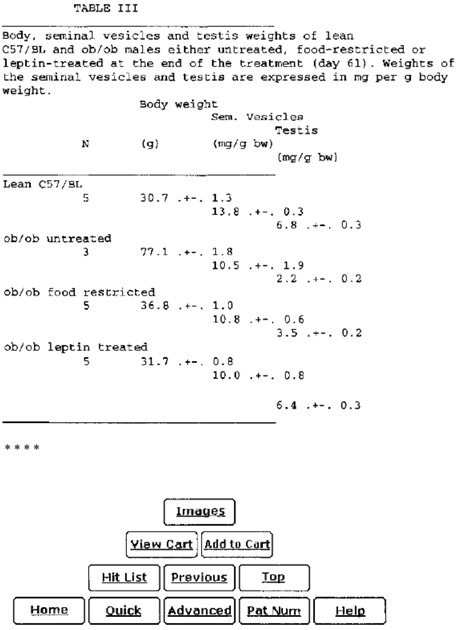
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT H
TRANSFER OF MANUFACTURING PROCESSES
1. Amgen will provide to Amylin, or at Amylin’s request, to its Third Party manufacturer(s) approved by Amgen as set forth below, access to the Manufacturing Information and other information within the Licensed Know-How for the manufacture of A-100 Leptin. Amylin will be the license holder for the Regulatory Documents and any supplements thereto. As between Amgen and Amylin, Amylin will be solely responsible for interacting with Regulatory Authorities regarding and for obtaining all necessary Regulatory Approvals for, the clinical and commercial manufacturing of Licensed Products for use or sale in the Territory within the Product Field.
2. Amylin shall select a Third Party manufacturer(s) for the manufacture of Licensed Products (such Third Party manufacturer(s) shall be referred to herein as “CMO”), The list of Amgen-approved Third Party manufacturers is attached hereto as Exhibit 1. Amylin may select a Third Party manufacturer that is not on Exhibit 1 subject to approval by Amgen, which approval shall not be unreasonably withheld or delayed. Amylin shall provide Amgen with the name of a proposed Third Party manufacturer, within [*] of the Effective Date. Amylin shall be responsible for promptly negotiating, in consultation with Amgen, the terms of the agreement pursuant to which such Third Party manufacturer shall manufacture and supply Licensed Products.
3. The Parties will effect the transfer of the Manufacturing Processes in accordance with this Exhibit H pursuant to a master transfer plan to be mutually agreed to by the Parties as promptly as possible following the Effective Date.
4. Amgen’s support pursuant to Section 3.2 shall be solely limited to the following activities, as more particularly set forth in the Leptin Technology Transfer, Technical Support and Documentation attached hereto as Exhibit 2:
• [*]
5. Each Party will designate in writing to the other Party a project leader, which individual will be a person of appropriate experience, skill and knowledge to oversee such Party’s activities contemplated under this Exhibit H. If any dispute between the Parties arises relating to Amgen’s provision of support under Section 4 of this Exhibit H. then either Party may give written notice thereof to the other Party. Within [*] of receipt of such notice, the project leaders will meet or confer, and attempt in good faith to resolve such disputed issue. If such project leaders are unable to resolve any such issue within [*] after receipt of such notice, then the matter shall be referred to a senior executive (or designee) of each Party. If such senior executives (or designees) are unable to resolve any such issue within [*] after such referral, either Party may pursue any remedy available to it at law or in equity.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT 1
LIST OF THIRD PARTY MANUFACTURERS
Options for Drug Substance CMO: [*]
Options for Drug Product CMO: [*]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT 2
LEPTIN TECHNOLOGY TRANSFER, TECHNICAL SUPPORT AND DOCUMENTATION
Introduction
The purpose of this document is to define the scope of the transfer of the Manufacturing Processes from Amgen to Amylin and/or Amylin’s designated CMO(s) approved by Amgen, as set forth herein, as well as define respective roles and responsibilities of the Parties. The Manufacturing Process for A-100 Leptin was developed by Amgen in the late 1990’s and was last run at commercial scale in the LakeCentre, CO facility. A joint technology transfer team will be formed including experts from Amgen as well as Amylin and the designated CMO(s) to facilitate and coordinate the transfer of Manufacturing Processes.
Analytical Methods for Drug Substance and Drug Product
| • | Amgen is transferring the methods “as is” and will not do any additional development work on the methods prior to the transfer (with the exception of the bioassay, Host Cell Protein assay, and DNA assay, which will require some additional development work prior to the transfer). |
| • | Amgen will not qualify/characterize, or validate the analytical methods. |
| • | Upon reasonable request and sufficient advance notice, and subject to Amgen resource availability, Amgen will provide lab training for the non-compendial methods for the A-100 process if deemed necessary by Amylin/CMO. Such training would be provided at Amgen Thousand Oaks (“ATO”) and each Party will bear its own costs and expenses incurred in connection with such training. |
| • | Amgen will provide master cell bank (MCB) and working cell bank (WCB). Amgen will perform any necessary retest/stability testing on the cell banks if they are to expire within the first six months of this Agreement. Amgen will also provide any reasonable cell banks and critical reagents necessary to perform any of the non-compendial analytical methods such as the bioassay and host cell protein assays. Amgen will also provide vials of bioassay master cell bank. |
| • | The CMO will re-qualify the MCB and WCB. |
| • | The following methods are considered to be compendial: |
| • | Protein concentration (AO 120) |
| • | Spectrophotometry (protein/DNA) (A0101) |
| • | pH (A0105) |
| • | Endotoxin (A0S20) |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| • | Appearance (A0221) |
| • | Moisture/Karl Fischer (A0406) |
| • | Pyrogen (USP) |
| • | Sterility (USP/EP) |
| • | Bioburden (A0736/A0743) |
| • | Amgen will provide consulting for the transferred A-100 Leptin analytical methods during their implementation by Amylin/CMOs/Amylin contractors. |
| • | Amgen will review the method qualification/characterization protocols and reports, but will not provide the experimental design for the qualification/characterization. |
| • | Method transfers may be required for different CMO sites for Drug Substance and Drug Product. |
| • | Amgen will transfer methods to one (1) designated CMO site for each of Drug Substance and Drug Product. |
| • | Amylin or its designated CMO will be responsible for additional method transfers. |
| • | Amgen will write the method transfer protocols and will transfer these protocols to the CMO. The CMO will execute the method transfer protocols and author the method transfer reports. Amylin and Amgen will approve the method transfer reports authored by the CMO. |
Drug Substance Technical Knowledge and Documentation
| • | Amgen will transfer the A-100 Leptin Manufacturing Process that was last run in LakeCentre, including: |
| • | Process descriptions (process transfer documents, including electronic and hard copy of all of the current versions of the manufacturing batch records). |
| • | Development reports where available. |
| • | Copies of lab notebooks where no report exists. |
| • | Copies of campaign summaries for the last LakeCentre campaign (including all the manufacturing batch records including process chromatograms and in-process data) and, where available, campaign summaries for any other campaign. |
| • | Product quality summaries (test results, including release and stability results, chromatograms, pictures of gels, etc., system suitability data for each run, Certificates of Analyses (C of As), etc.). |
| • | Analytical method descriptions (including electronic and hard copy of the controlled document for each method), |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| • | Amgen and Amylin will evaluate any process improvements and determine if any should be incorporated. |
| • | Amgen will perform at least three successful bench/pilot runs at Amgen to show process reproducibility. Amylin and/or the bulk CMO will be allowed to observe the runs. Success will be defined as meeting all product specifications (analytical testing performed by Amgen) consistent with current (as of Effective Date) clinical material of A-100 Leptin, yield > 5g/L for fermentation titer, and an overall purification yield > 11 % (within three (3) sigma of last LakeCentre campaign). |
| • | Amgen will review the characterization protocols and reports, but will not provide the experimental design for the characterization. |
| • | Amgen will also transfer the A-200 Leptin Manufacturing Process documentation, but such transfer will be solely limited to supplying Amylin with Amgen’s existing technical documentation (which will, if possible, include the master and working cell banks and will at least include a research cell bank), supporting written documentation and data, including the following: |
| • | Process descriptions (process transfer document). |
| • | Development reports where available. |
| • | Copies of campaign summaries where available. |
| • | Product quality summaries (test results, C of As, etc). |
| • | Analytical method descriptions. |
| • | Batch records. |
| • | For Drug Substance, Amgen will be responsible for the transfer through the successful completion of runs at the CMO, which will be defined as a maximum of three pilot runs at an appropriate scale as recommended by Amgen and that meet product specifications consistent with current (as of Effective Date) clinical material of A-100 Leptin, yield > 5g/L for fermentation titer, and an overall purification yield > 11% (within three (3) sigma of last LakeCentre campaign). |
| • | Amgen will be responsible for product specification testing for material manufactured during the CMO pilot runs. |
| • | Failure to meet acceptance criteria due to environmental factors at the CMO facility (i.e. bacterial contamination, high endotoxin, yield loss due to an assignable cause) will not be considered a failure if there is an assignable root cause. |
Drug Product Technical Knowledge and Documentation
| • | Amgen will transfer the A-100 Leptin fill-and-finish lyophilization process currently run at ATO-B20 (Amgen Clinical Manufacturing), including: |
| • | Process descriptions (including electronic and hard copy of the latest revision of the manufacturing batch record and the last batch record that was run at Parke-Dale, LakeCentre or Amgen’s pilot plant and any available campaign summary reports (or data if no such report is available) from such work). |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| • | Development reports where available. |
| • | Copies of lab notebooks where no report exists. |
| • | Manufacturing Procedures and Master Packaging Configurations. |
| • | Amgen is transferring the process and formulation “as is” and will not do any additional development work on the process prior to the transfer (except as expressly provided herein). |
| • | CMO for Drug Substance manufacture may be a different site and/or company than that used for manufacture of Drug Product. |
| • | Amgen will review the characterization protocols and reports written by Amylin/CMO but will not provide the experimental design for the characterization. |
| • | Drug Product transfer will not require additional pilot fills beyond the current ATO-B20 scheduling. Amylin/CMO will be invited to come to ATO, at Amylin/CMO’s own costs and expenses, to observe no more than four fill runs in B20 during 2006 at a time mutually convenient for both Parties. Any such observation will not occur prior to May, 2006 and will require at least three weeks prior notice to Amgen. Each observation will be limited to two persons and viewing of aseptic activities will occur behind glass. Amgen will provide certain analytical testing as provided in Section 3.4 of this Agreement. |
| • | For Drug Product, Amgen will be responsible for the transfer through the successful completion of runs at the CMO, which will be defined as a maximum of three runs at an appropriate pilot scale as recommended by Amgen and that meet product specifications consistent with current (as of Effective Date) clinical material of A-100 Leptin. |
| • | Amgen will be responsible for product specification testing for material manufactured during the CMO pilot runs. |
| • | Failure to meet acceptance criteria due to environmental factors at the CMO facility (i.e. bacterial contamination, high endotoxin) will not be considered a failure if there is an assignable root cause. |
| • | Amgen will transfer the fill-and-finish lyophilization process for the leptin placebo drug product. |
Stability and Reference Standard
| • | Amgen will transfer active stability studies (e.g. protocols, data, samples), and protocols and data from completed or suspended studies. |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
| • | Amgen will transfer reference standard material, including qualification data and characterization data. Amgen will supply all existing inventory of reference standard in addition to production and testing/release of a new lot of reference standard. |
| • | It will be the responsibility of Amylin to make additional reference standard material as and when needed. |
Technical Support
| • | Amgen is not responsible for the success of commercialization or runs after pilot runs. |
| • | Amgen’s role in the transfer process with respect to a CMO will be limited to a consulting role after the pilot runs at such CMO. |
| • | Consulting will be limited to reasonable requests for review of process data, batch documentation, process characterization plans, process validation protocols and reports and one onsite visit during the engineering and conformance runs to observe critical operations. |
| • | Consulting does not include any lab work in either Party’s analytical or process labs. |
| • | Amgen will perform such consulting activities, upon Amylin’s reasonable request, until the earlier of (i) twenty-four (24) months after the completion of the Drug Substance pilot runs at the designated CMO, or (ii) December 31, 2008. |
| • | Amylin will provide to Amgen a timeline through regulatory approval and an estimate of requirements for Amgen consulting services upon completion of each of the three transfer activities set forth in Section 5.2(a)(i). |
| • | Amgen will need to approve all access of the CMOs or Amylin staff to Amgen’s facilities and technology during the technology transfer. Approval is at Amgen’s sole discretion (subject to compliance with the technology transfer obligations of this Agreement). |
| • | Amgen will supply a list of all critical equipment to perform both processes to aid in selection of the appropriate CMOs. |
| • | Amylin will be responsible for negotiating and entering into, and performing under, the contract with the selected CMOs. |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT I
LIST OF AMGEN-SPONSORED CLINICAL TRIALS
| Study No |
Molecule(S) |
Short Study Title |
Type of Report | |||
| 950272 |
A-100 | Ascending dose, first-in-humans in uncomplicated obesity | Summary | |||
| 960176 |
A-100 | Follow-up, open-label in uncomplicated obesity | Summary | |||
| 960240 |
A-100 | Continuous SC infusion, multiple ascending dose | Summary | |||
| 970121 |
A-100 | IV multiple ascending dose | Summary | |||
| 970161 |
A-100 | Obese, leptin deficient - UK & Canada | Full | |||
| 970164 |
A-100 | SC 10 mg qd am; 10 mg qd pm, 10 mg bid in obese subjects | Summary | |||
| 970171 |
A-100 | Glycemic control in NIDDM treated with oral sulfonylurea | Abbreviated | |||
| 970188 |
A-100 | Glycemic control in obese, diet-treated NIDDM | Abbreviated | |||
| 970211 |
A-100 | IT ascending dose in severely obese | Summary | |||
| 970213 |
A-100 | Weight Loss After Induction | Summary | |||
| 970216 |
A-200 | SC PK | Summary | |||
| 970217 |
A-200 | Efficacy and safety | Summary | |||
| 980145 |
A-200, A-100 | Glucose & lipid Metabolism in Type 2 DM | Summary | |||
| 980219 |
A-100 | Glycemic control in obese type 2 DM treated w/metformin or metformin + sulfonylurea | Summary | |||
| 980225 |
A-100 | Glycemic control in obese, type 2 DM treated with insulin or insulin + sulfonylurea | Summary | |||
| 980236 |
A-100 | Efficacy in obese with low endogenous leptin | Summary | |||
| 980245 |
A-200 | SC bioavailability/PK obese | Summary | |||
| 990766 |
A-200 | Multiple dose SC PK in obese | Summary | |||
| 990768 |
A-200 | Efficacy and safety in obese | Summary | |||
| 200000107 |
A-200 | Efficacy for weight maintenance after VLCD-induced weight loss | Summary |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
EXHIBIT J
LIST OF AMGEN-SPONSORED CLINICAL TRIALS
TENTATIVE SCHEDULE FOR DELIVERY OF A-100 LEPTIN
CLINICAL DRUG PRODUCT
| Fill# |
Target vials release date | Target Quality | ||
| [*] |
||||
The foregoing clinical vial supply schedule is subject to availability of GMP drug substance. Actual vial supply timelines may vary due to fluctuations in manufacturing capacity and scheduling, as well as unforeseeable events. Amgen reserves the right to change this schedule at any time. Amgen will notify Amylin of any such changes.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.

|
Amylin Pharmaceuticals, Inc. 9360 Towne Centre Drive San Diego, CA 92121 USA | Tel (858)552 2200 Fax (858) 552 2212 xxx.xxxxxx.xxx |
July 23, 2012
Kathleen Denis
Associate Vice President/Office of Technology Transfer
The Rockefeller University
1230 York Avenue, 502 Founders Hall
New York, New York 10021
Re: License Agreement between Rockefeller University (“Rockefeller”) and Amgen Inc. (“Amgen”) dated April 14, 1995, as amended on May 10, 1995, January 1, 2006 and January 15, 2008 (the “Rockefeller/Amgen License Agreement”)
Dear Dr. Denis:
As you know. Rockefeller has exclusively licensed its rights in certain patent rights and technical information to Amgen pursuant to the Rockefeller/Amgen License Agreement. Amgen has exclusively sublicensed its rights under the Rockefeller/Amgen License Agreement to Amylin Pharmaceuticals, Inc. (“Amylin”) pursuant to the License Agreement between Amgen and Amylin, dated February 7, 2006, as amended (the “Amgen/Amylin License Agreement”). Pursuant to Section 2.10 of the Amgen/Amylin License Agreement, Amylin has agreed to comply directly with certain obligations of Amgen set forth in the Rockefeller/Am gen License Agreement, including payment of royalties, milestones and other payments to Rockefeller, arising as a result of the activities of Amylin and its affiliates and sublicensees pursuant to the Amgen/Amylin License Agreement.
By this letter agreement (the “Letter Agreement”), Amylin and Rockefeller desire to modify: (i) Amylin’s future payment obligations to Rockefeller under the Rockefeller/Amgen License Agreement that arise as a result of the activities of Amylin and its affiliates and sublicensees pursuant to the Amgen/Amylin License Agreement, and (ii) certain related provisions of the Rockefeller/Amgen License Agreement. Capitalized terms used but not otherwise defined in this Letter Agreement shall have the meanings provided in the Rockefeller/Amgen License Agreement.
Rockefeller and Amylin, intending to be legally bound, agree as follows:
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
1. In lieu of the payment obligations set forth in Subparagraphs 6.1(b), 6.1(c), 6.1(d). 6.1(e), 6.1(f), 6.1(g) and 6.1(h) of the Rockefeller/Amgen License Agreement, Amylin shall owe payments to Rockefeller as set forth below, and as between Amylin and Rockefeller, such Subparagraphs and Paragraphs 6.2, 6.5, 7.6 and 1.3 are explicitly superseded as provided herein.
2. Subparagraph 6.1(b) of the Rockefeller/Amgen License Agreement is replaced in its entirety with the following new Subparagraph 6.1(b):
“(b) Milestone Payments: Except as provided in subparagraph (iii) below, Amylin shall owe to Rockefeller one-time, non-re fundable milestone payments as set forth in subparagraphs (i) and (ii) below which shall be due and payable within thirty (30) calendar days following first achievement by Amylin or its affiliate or sublicensee of the corresponding milestones:
| (i) | [*] |
| (ii) | [*] |
| (iii) | [*] In the event that the aforementioned conditions have been satisfied, then such payment shall be made within sixty (60) calendar days of the effective date of the [*] and, once made, shall be non-refundable.[*] |
3. Subparagraphs 6.1(c), 6.1(d), 6.1(e), 6.1(f), 6.1(g) and 6.1(h) of the Rockefeller/Amgen License Agreement are replaced in their entirety with the following new Subparagraph 6.1(c):
“(c) Royalties. Amylin shall pay to Rockefeller royalties of [*] percent ([*]%) of Net Sales of any Patented Product for any Orphan Product indications of such Patented Product and [*] percent ([*]%) of Net Sales of any Patented Product for Non-Orphan Product indications of such Patented Product. For clarity, “Orphan Product” shall mean any Patented Product for which orphan designation is approved by the United States FDA and “Non-Orphan Product” shall mean a Patented Product which is not an Orphan Product. “Patented Product” means any Product (i) the selling of which would, in the absence of this Agreement, infringe a valid claim of an issued, unexpired patent within the Licensed Patent Rights in the country in which such Product is sold by Amgen or its affiliate or sublicensee, (ii) with respect to which a market exclusivity period has been granted by a governmental body or regulatory authority and is in effect in the country in which such Product is sold by Amgen or its affiliate or sublicensee, or (iii) with respect to which data exclusivity has been granted by a governmental body or regulatory authority and is in effect in the country in which such Product is sold by Amgen or its affiliate or sublicensee. Such royalties shall be payable on a Patented Product-by-Patented Product and country-by-country basis in the Territory from the date of first commercial sale of a given Patented Product in a given country until the later to occur of (a) expiration of the last to expire of a valid claim of an issued, unexpired patent within the Licensed Patent Rights in the country in which such Patented Product is sold by Amgen or its affiliate or sublicensee, (b) expiration of any market exclusivity period granted by a governmental
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
body or regulatory authority with respect to such Patented Product in such country, or (c) expiration of any data exclusivity period granted by a governmental body or regulatory authority with respect to such Patented Product in such country, and in the case of clause (c) above, only for so long as the total number of units of a Patented Product sold in a calendar quarter exceeds the total number of units of generic products (corresponding to the Patented Product) sold in the same calendar quarter (hereinafter the “Royalty Term”); provided that, with respect to Patented Products which are designated as Orphan Products in the United States, such data exclusivity period shall be deemed to be twelve (12) years from the date of U.S. FDA marketing approval thereof, and that, with respect to Patented Products which are not designated as Orphan Products in the United States, such data exclusivity period shall be deemed to be the data exclusivity period then in effect in the United States at the time of U.S. FDA approval of the applicable Patented Product. For purposes of this paragraph, “data exclusivity” means that neither the generic drug or biologic applicant nor the applicable governmental body or regulatory authority with respect to a particular country may refer or actually refers to, or may rely or actually relies on, the information of the original marketing authorization holder for the corresponding branded drug or biologic product (in this case, the applicable Patented Product).
4. Paragraph 6.2 of the Rockefeller/Amgen License Agreement is replaced in its entirety with the following new Paragraph 6.2:
| “6.2 | The obligation to pay royalties hereunder is imposed only once with respect to the sale of a Product regardless of the number of patent claims within the Licensed Patent Rights which cover such Product.” |
5. Paragraph 6.5 of the Rockefeller/Amgen License Agreement is replaced in its entirety with the following new Paragraph 6.5:
| “6.5 | Rockefeller agrees to use reasonable efforts, short of litigation, to resist any claim by a third party which might affect the exclusive nature of any license granted hereunder. In the event that during the term of this Agreement the license granted herein is rendered non-exclusive in any country of the Territory as a result of any governmental, judicial, or other action, then the royalty provided in Subparagraph 6.1(c) hereof due to Rockefeller on the affected Product(s) in that country shall be reduced by [*]. In such case, the obligation to pay Rockefeller a royalty on the affected Product(s) pursuant to Subparagraph 6.1(c) in that country shall expire/terminate no later than [*] from the date of first commercial sale of the affected Product(s) in that country. Thereafter, the license granted under this Agreement with respect to such affected Product(s) in such country shall be fully paid up.” |
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
6. Paragraph 7.6 of the Rockefeller/Amgen License Agreement is replaced in its entirety with the following new Paragraph 7.6:
| “7.6 | This Agreement shall expire with respect to Patented Product for which Amylin has a license under the Amgen/Amylin License Agreement upon (i) the expiration of the last Royalty Term for such Patented Product, or (ii) if the Amgen/Amylin License Agreement is terminated earlier in accordance with the termination provisions therein, such earlier termination date. Thereafter, except in the case of termination by Rockefeller based on Amylin’s uncured material breach, the license granted under this Agreement, and the sublicense granted to Amylin under the Amgen/Amylin License Agreement, with respect to such Patented Product in such country shall be fully paid up and irrevocable.” |
7. Paragraph 1.3 of the Rockefeller/Amgen License Agreement is replaced in its entirety with the following new Paragraph 1.3:
| “1.3 | “Commercially Reasonable Efforts” means the level of efforts and resources commonly used in the pharmaceutical industry to develop, obtain regulatory approvals for, protect intellectual property relating to, and commercialize a product consistent with the efforts a similarly situated biopharmaceutical company would typically devote to a product at a similar stage in its product life and of similar market potential, profit potential and strategic value resulting from its own research efforts, based on information and conditions then-prevailing, including, without limitation, efficacy of the product, the competitiveness of alternative products in the marketplace, the patent and other proprietary position of the product, the likelihood of regulatory approval given the regulatory structure involved and the likelihood of adequate reimbursement. Commercially Reasonable Efforts shall be determined on a country-by-country (each country including its territories) basis for a particular Licensed Product, and it is anticipated that the level of effort will change over time reflecting changes in the status of the Licensed Product and the country (including its territories) involved.” |
8. Rockefeller hereby generally, irrevocably, unconditionally and completely releases, acquits and forever discharges Amylin and Amgen, and all agents, representatives, employees, officers, directors, attorneys, successors and assigns thereof, from any and all claims, causes of action, damages, losses, attorneys’ fees, costs, whether known or unknown, suspected or unsuspected, matured or unmatured, of every kind and nature, at law or in equity, arising from or relating to claims or potential claims regarding any obligation to undertake to use Commercially Reasonable Efforts to identify, develop and market Product(s) under the Amgen/Rockefeller License Agreement prior to the date of this Letter Agreement. Rockefeller acknowledges and agrees that, notwithstanding any law, regulation, rule or order to the contrary, the foregoing release shall be effective whether or not such claims are currently known, unknown, foreseen, unforeseen, patent or latent, and whether or not Rockefeller becomes aware of facts in addition to or different from what is known to Rockefeller as of the date of this Letter Agreement.
9. Except as specifically amended by this Letter Agreement, the Amgen/Rockefeller License Agreement shall remain in full force and effect in accordance with its terms.
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
10. Should the Amgen/Amylin License Agreement terminate, the provisions of this Letter Agreement (excluding the paragraph 8 release provisions above) shall terminate, and the provisions of the Amgen/Rockefeller License Agreement in existence prior to the date of this Letter Agreement shall be reinstated, but, with respect to any remaining milestones, solely on a prospective basis. Notwithstanding the termination of this Letter Agreement, and for clarity, Amylin acknowledges that any non-refundable payments that have been made by it under this Letter Agreement shall not be refunded.
11. This Letter Agreement may be executed in counterparts, each of which shall be deemed an original document, and all of which, together with this writing, shall be deemed one and the same instrument.
[remainder of page left blank]
THIS EXHIBIT HAS BEEN REDACTED AND IS THE SUBJECT OF A
CONFIDENTIAL TREATMENT REQUEST. REDACTED MATERIAL IS MARKED
WITH [*] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND
EXCHANGE COMMISSION.
If the foregoing is acceptable to you, please sign and date this Letter Agreement in the space provided below and return it to me. Thank you for your prompt assistance with this matter.
Yours sincerely,
Amylin Pharmaceuticals, Inc.
| By: | /s/ Daniel M. Bradbury | |
| Name: | Daniel M. Bradbury | |
| Title: | President and Chief Executive Officer |
Agreed to and accepted as of the date first set forth above:
The Rockefeller University
| By: | /s/ Marc Tessier-Lavigne | |
| Name: | M.P.W | |
| Title: | President | |
Amgen Inc. acknowledges and agrees to this Letter Agreement for purposes of amending the Rockefeller/Amgen License Agreement as provided herein and amending Exhibit F (Rockefeller License) of the Amgen/Amylin License Agreement to reflect the amendment of the Rockefeller/Amgen License Agreement pursuant to this Letter Agreement:
| Amgen Inc. | ||
| By: | /s/ David Pacquad | |
| Name: | David Piacquad | |
| Title: | VP, Strategy & Corp. Dv. | |


