MANUFACTURING SERVICES AGREEMENT FOR SUBASSEMBLY (does not apply to cap)
Exhibit 10.20
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
MANUFACTURING SERVICES AGREEMENT FOR SUBASSEMBLY
(does not apply to cap)
This Manufacturing Services Agreement (this “Agreement”), dated as of December 21, 2018 (the “Effective Date”), is by and among, on the one hand, OptiNose US, Inc., duly organized and existing under the laws of Delaware and having offices located at 0000 Xxxxx Xxxx Xxxx, Xxxxx 000, Xxxxxxx, XX 00000 (referred to herein as "OptiNose US"), OptiNose UK Ltd. duly organized and existing under the laws of England and having offices located at Hunts Rise, South Xxxxxxx Park, Wiltshire, SN3 4TG, England (referred to herein as "OptiNose UK"), and OptiNose AS, duly organized and existing under the laws of Norway and having offices located at Xxxxxxxxxxxxx 00, 0000 Xxxx, Xxxxxx (referred to herein as "OptiNose Norway", and collectively with OptiNose US and OptiNose UK, “OptiNose”), and, on the other hand, and Advance Mold & Manufacturing, Inc., d/b/a Vision Technical Molding, a Connecticut corporation having offices located at 00 Xxxxxx Xxxx, Xxxxxxxxxx, XX 00000 (“VTM”).
WHEREAS, VTM is a device development and manufacturing company skilled in the design, development, manufacture and assembly of medical devices and delivery systems and of their components;
WHEREAS, VTM and OptiNose previously entered into a Molded Parts Agreement dated October 24, 2017 (the “Molded Parts Agreement”) whereby VTM molded various product components and product cap for OptiNose;
WHEREAS, concurrent with the execution of this Agreement, the parties shall enter into an amendment to the Molded Parts Agreement whereby the parties will remove VTM’s future manufacture for OptiNose of various product components thereunder (other than the product cap noted therein); and
WHEREAS, OptiNose desires to retain VTM to assemble and supply DSAs (as defined herein) for use by OptiNose in the subsequent production of Finished Product (as defined herein) under FDA and other regulations for the benefit of patients.
In consideration of the mutual covenants and agreements hereinafter set forth, the parties agree as follows:
ARTICLE I
DEFINITIONS
[***].
“Action” shall have the meaning set forth in Section 12.03.
“Affiliate” means any Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, another Person. The term
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
“control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Agreement” shall have the meaning set forth in the preamble.
“Approved Vendor List” shall mean the list of vendors approved in writing by OptiNose and VTM to provide the Inventory or services specified in the Xxxx of Materials for a DSA.
“Xxxx of Materials” shall mean the Inventory and OptiNose Components that comprise the DSA, as set forth in the Specifications.
“Binding Period” shall have the meeting set forth in Section 2.07.
“Capacity” means the facility space, equipment, utilities, maintenance capabilities, infrastructure, human capital, and other capabilities in sufficient volume needed to manufacture DSAs, except for the OptiNose Equipment.
“Confidential Information” means any information that is treated as confidential by a Disclosing Party, whether tangible or intangible, including, without limitation, any and all specification information, techniques, discoveries, inventions, processes, know-how, patent applications and related information, inventor certificates, trade names, trade secrets, methods of production, technology, other proprietary information, other intellectual property, information pertaining to business operations and strategies, and information pertaining to pricing, and marketing of Finished Product or Inventory. Confidential Information shall not include information that: (a) is already known to the Receiving Party without restriction on use or disclosure prior to receipt of such information from the Disclosing Party; (b) is or becomes generally known by the public other than by breach of this Agreement by, or other wrongful act of, the Receiving Party; (c) is developed by the Receiving Party independently of, and without reference to, any Confidential Information of the Disclosing Party; or (d) is received by the Receiving Party from a third party who is not under any obligation to the Disclosing Party to maintain the confidentiality of such information.
“Defaulting Party” shall have the meaning set forth in Section 15.02.
“Defective Product” means any DSA that fails to conform to the Specifications, Quality Agreement or applicable Laws or that contains a Latent Defect or Patent Defect.
“Deliverables” means all documents, work product and other materials that are delivered to OptiNose hereunder or prepared by or on behalf of VTM in the course of performing the services under this Agreement.
“Disclosing Party” means a party that discloses Confidential Information under this Agreement.
“DSA” means a device subassembly manufactured under this Agreement.
2
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
“Effective Date” shall have the meaning set forth in the preamble of this Agreement.
[***].
“FDA” means the U.S. Food & Drug Administration.
“Excess Inventory” shall mean any DSA, partially completed DSA or Inventory (in each instance, to the extent procured by or on-order with VTM) that is not required for consumption to satisfy the next [***] of demand for DSAs under the then-current Forecast.
“FD&C Act” means the Federal Food, Drug, and Cosmetic Act, as amended, and includes the rules, regulations and guidances promulgated thereunder (including, without limitation, current Good Manufacturing Practices).
“Finished Product” means the full saleable product unit for XHANCE® (formerly known as OPN-375) including without limitation active ingredient, delivery system, container closure system, and market package.
“Forecast” shall have the meeting set forth in Section 2.07.
“Force Majeure Event” shall have the meaning set forth in Section 17.01.
“Intellectual Property Rights” means all (a) patents, patent disclosures and inventions (whether patentable or not), (b) trademarks, service marks, trade dress, trade names, logos, corporate names and domain names, together with all of the goodwill associated therewith, (c) copyrights and copyrightable works (including computer programs), mask works, and rights in data and databases, (d) trade secrets, know-how and other confidential information, and (e) all other intellectual property rights, in each case whether registered or unregistered and including all applications for, and renewals or extensions of, such rights, and all similar or equivalent rights or forms of protection in any part of the world.
“Inventory” shall mean any materials, parts, components and inputs needed for VTM to manufacture the DSAs in accordance with the Specifications, whether made by VTM or procured from a third-party. (other than OptiNose Components) that are procured by or on-order with VTM in accordance with the applicable Lead Time for use in the manufacture of DSAs pursuant to a Purchase Order or Forecast from OptiNose, which may include (unless the context requires otherwise) Special Inventory and Minimum Order Inventory.
“Inventory Procurement Lead Time” shall mean, with respect to any particular item of Inventory, the longer of (a) the lead time to obtain such Inventory as recorded on VTM’s system of record or (b) the actual lead time.
“Latent Defect” means a defect where any DSA fails to conform to the Specifications, Quality Agreement or applicable Laws, which could not reasonably have been discovered upon receipt and physical inspection of the DSA by OptiNose or its designee.
3
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
“Law” means any statute (including without limitation the FD&C Act), law, ordinance, regulation, rule, code, order, constitution, treaty, common law, judgment, decree, other requirement or guidance, or rule of law of any federal, state, local or foreign government or political subdivision thereof, or any arbitrator, court or tribunal of competent jurisdiction that is applicable: (a) to the obligations of VTM in supplying OptiNose with the DSAs, and performing any related activities under other terms of this Agreement, or (b) to the obligations of OptiNose.
“Lead Time” shall mean the Inventory Procurement Lead Time plus the manufacturing cycle time required from the delivery of the Inventory at VTM’s facility to the completion of the manufacture, assembly and test processes for the DSA.
“Long Lead Time Inventory” shall mean Inventory with Lead Times exceeding the period covered by the accepted Purchase Orders for the DSA.
“Losses” means all losses, damages, liabilities, deficiencies, actions, judgments, interest, awards, penalties, fines, costs or expenses of whatever kind, including reasonable attorneys’ fees and the cost of enforcing any right to indemnification hereunder and the cost of pursuing any insurance providers.
“Minimum Order Inventory” shall mean Inventory purchased in excess of requirements for Purchase Orders and Forecast because of minimum lot sizes required by the vendor.
“NDA” means a new drug application filed with the FDA.
“Obsolete Inventory” shall mean any partially completed DSA or Inventory that is any of the following: (a) removed from the Xxxx of Materials for a DSA by an engineering change; or (b) no longer on an active Xxxx of Materials for a DSA.
“OptiNose” shall have the meaning set forth in the preamble.
“OptiNose Components” shall mean the consigned inventory that has the meaning set forth in Section 2.02(a).
“OptiNose Equipment” means equipment (i) that can be used only for production of DSA’s under this Agreement or (ii) that OptiNose desires to have dedicated solely to the production of DSA’s under this Agreement.
“OptiNose Indemnitee” shall have the meeting set forth in Section 12.02.
“OptiNose Information” means any documents, data, know-how, trade secrets, methodologies, software and other information (Confidential Information or otherwise) provided to VTM by or on behalf of OptiNose or developed by VTM on behalf of OptiNose, including without limitation computer programs, reports and specifications.
“OptiNose Supply Relationship Manager” shall have the meaning set forth in Section 7.01(a)
4
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
“Patent Defect” means any instance where any DSA fails to conform to the Specifications, Quality Agreement or applicable Laws, where such failure is or was discoverable upon reasonable physical inspection upon receipt by OptiNose or its designee.
“Person” means an individual, corporation, partnership, joint venture, limited liability company, governmental authority, unincorporated organization, trust, association or other entity.
“Production Materials” shall mean materials that are consumed in the production processes to manufacture DSAs including without limitation, [***]; Production Materials do not include any such production materials that have been specified by OptiNose.
“Purchase Order” shall have the meaning set forth in Section 2.09(a).
“Quality Agreement” means that Quality Agreement between OptiNose and VTM to be entered between the parties related to production of the DSAs.
“Quantitative Defect” means any instance in which VTM has delivered a quantity that is [***] less than, or [***] greater than, the quantity stated in any invoice or xxxx of lading.
“Receiving Party” means a party that receives or acquires Confidential Information directly or indirectly under this Agreement.
“Special Inventory” shall mean any mutually agreed Inventory acquired by VTM to support flexibility, demand requirements, safety stock or pricing discounts.
“Specifications” shall mean the procedures, standards, quality control and other data and requirements for each DSA, which shall include, without limitation: Bills of Materials, designs, schematics, assembly drawings, process documentation, specifications according to the Device Master Record for the DSA, current revision number, and an Approved Vendor List.
“Standard Cost” shall mean, as applicable, (a) the quoted cost of Inventory represented on the Xxxx of Materials current at the time such Inventory is acquired; or (b) the value of any services required to be performed hereunder on work-in-progress at the time such services are performed.
“Term” shall have the meaning set forth in Section 14.01.
“VTM” shall have the meaning set forth in the preamble.
“VTM Supply Relationship Manager” shall have the meaning set forth in Section 6.01(a).
“VTM Equipment” means any and all equipment, systems, or facilities owned or leased, by or on behalf of VTM and made available for either direct or indirect performance by VTM under this Agreement.
“VTM Personnel” means all employees, contractors, and consultants, engaged by VTM to perform under this Agreement.
5
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
“VTM Tools” means all documents, data, know-how, methodologies, software and other information provided by or used by VTM in connection with performance by VTM under this Agreement, in any case developed or acquired by VTM independently of this Agreement and of other services performed by VTM for OptiNose prior to or after the date hereof.
ARTICLE II
MANUFACTURE AND SUPPLY OF DSAS
Section 2.01 Manufacture and Supply. During the Term and subject to the terms and conditions set forth herein, VTM shall procure Inventory and manufacture and assemble DSAs in compliance with the Specifications, Quality Agreement, applicable Laws and the other terms of this Agreement and deliver them to OptiNose or its designee. Delivery of the DSAs shall be EXW (“Ex-Works”) VTM’s manufacturing facility 00 Xxxxxx Xxxx, Xxxxxxxxxx, XX 00000 (INCOTERMS 2000); [***] shall arrange for the DSAs to be picked up by a carrier identified and paid by [***] or its designee. During the Term, VTM shall use [***] to ensure that it has the Capacity to meet all of OptiNose’s requirements for DSAs in a timely manner based on the applicable Forecast under this Agreement; provided that if new or additional OptiNose Equipment is required, VTM will inform OptiNose with sufficient lead time for such OptiNose Equipment to be acquired by OptiNose [***] and qualified for use under this Agreement taking into account normal equipment malfunctions and breakdowns not preventable through normal maintenance; Capacity metrics will be agreed upon and equipment will be initiated if projected utilization for the following year exceeds [***], or if OptiNose deems to initiate a project for business continuity.
Section 2.02 Inputs for Supply of DSA’s.
(a) OptiNose is responsible for negotiation of agreements and payment to third parties for the items listed on Exhibit B (the “Third Party Components”) and was responsible for the agreements and payment for the Pre-Existing Inventory (as defined in Exhibit A) listed on Exhibit B (collectively with the Third Party Components, the “OptiNose Components”), in each instance for use in the manufacture of DSAs.
(b) OptiNose will manage the relationship with suppliers of the OptiNose Components identified in Exhibit B, placing orders for Third Party Components, arranging delivery to VTM of the OptiNose Components at OptiNose’s cost, and ensuring that such suppliers perform in accordance with requirements of OptiNose. VTM shall manage suppliers of the OptiNose Components within VTM’s quality systems as appropriate to ensure compliance with the quality agreements referenced in this Section 2.02(b). OptiNose may initiate the addition or replacement of suppliers for each OptiNose Component upon [***] written notice to VTM and will work with VTM to execute such addition or replacement according to relevant OptiNose and VTM procedures at OptiNose’s expense.
6
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(c) If the suppliers of the OptiNose Components cause the assembly line at VTM to halt production due to lack of OptiNose Components, and if VTM has provided OptiNose at least [***] advance written notice of VTM’s projected or actual shortage of such OptiNose Components, then subject to the following sentence, VTM may invoice OptiNose for the reasonable costs incurred by VTM for such idle VTM operator time, which shall not exceed $[***]per week for [***] shift, or $[***] per week if running [***] shifts, in the aggregate, for which VTM shall provide OptiNose with a detailed breakdown of such costs and which shall solely include the compensation of the operators (and not any other overhead, facility costs, or profit of VTM), such amount to be the sole amount payable by OptiNose with respect to such idle time. If OptiNose provides VTM with at [***] prior written notice of any such lack of OptiNose Components then VTM shall use its [***] efforts to reassign its staff and otherwise mitigate its costs, which shall be deducted from any amounts due by OptiNose to VTM for such lack of OptiNose Components.
(d) If any time after [***] VTM’s production of DSA requires less operators than anticipated or no operators at all, and then an increase in demand requires operators to be retrained to manufacture and assemble the DSAs, (1) OptiNose shall pay for such retraining at a rate [***], and (2) OptiNose must provide at least [***] prior written notice before VTM shall be required to effectuate such an increase.
(e) At OptiNose’s request, VTM shall warehouse up to [***] pallets of the OptiNose Components listed on Exhibit B. The parties will mutually agree to any additional warehousing and associated cost. Notwithstanding the foregoing, VTM shall at OptiNose’s request warehouse [***] any and all OptiNose Components that VTM previously manufactured for OptiNose pursuant to the Molded Parts Agreement.
(f) [***] is responsible for the negotiation, payment, purchase, and delivery to VTM of all Inventory, but not the OptiNose Components. Appropriate agreements and documentation of quality related requirements will also be the responsibility of [***].
Section 2.03 Specifications. The Specifications may be amended in accordance with the terms of this Agreement, the Quality Agreement, or as required by the FDA or other similar regulatory authority, and by the written agreement of the parties. VTM shall not make changes to the Specifications without OptiNose’s prior written approval as provided for in the Quality Agreement.
Section 2.04 Validation and Other Services. Before manufacturing DSAs under this Agreement, VTM will have completed validation necessary and appropriate for such manufacture in accordance with the Specifications, applicable protocols- and the other requirements of this Agreement and the Quality Agreement.
7
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Section 2.05 Engineering Changes. Either party may request that VTM incorporate engineering changes into the DSAs or Specifications by providing a written description of the proposed engineering change sufficient to permit the parties to evaluate the feasibility and cost of the proposed change. VTM shall proceed with engineering changes when the parties have agreed in writing upon the changes to the Specifications, delivery schedule and adjustments to the Pricing, [***]. In all instances VTM shall use [***] to incorporate engineering changes requested by OptiNose.
Section 2.06 Pricing.
(a) [***], the initial pricing for all DSAs and any associated services for such calendar year shall be the respective column as set forth on Exhibit A based on the [***] Forecast in such calendar year. At the end of each calendar year, if the actual volume of DSA production would have resulted in a lower applicable price per unit had the pricing been based on such respective column (i.e. if the pricing was initially based at the beginning of such calendar year on the [***] column but [***] DSA were actually assembled during such calendar year, then the pricing will be based solely on the [***] column in Exhibit A), then VTM shall apply the appropriate credit due OptiNose against future production until the full credit has been issued (if the amount of the credit is over [***], the credit will be applied [***]), while in the event the actual volume of DSA production would have resulted in a higher applicable price per unit had the pricing been based on such respective column (i.e. if the pricing was initially based at the beginning of such calendar year on the [***] column but [***] DSA were actually assembled during such calendar year, then the pricing will be based solely on the [***] column in Exhibit A), then VTM shall invoice OptiNose for the appropriate amount. Notwithstanding the foregoing, for the calendar year 2019 the charge shall be based on the column [***] set forth in Exhibit A less any credit due to OptiNose per Exhibit A, and [***]. [***].
(b) VTM will use [***] to engage in continuous improvement processes with respect to the manufacture of the DSAs and any such resulting cost reductions shall be applied to lower the pricing set forth in Exhibit A hereto. [***]. Additionally, the price may be adjusted prospectively for any costs directly related to any changes in the Specifications and/or levels of service requested by OptiNose and agreed to by VTM, in writing. VTM shall provide sufficient documentation to support any price adjustment in accordance with this Section [***].
(c) Commencing on [***] and [***] thereafter, during the Term of this Agreement, the unit price of each DSA shall be increased or decreased, which adjustment shall become effective on January 1 and July 1, respectively, of the applicable calendar year, by the [***] provided, that, with respect to price increases of [***], (A) VTM shall have used [***] to negate, defer or reduce proposed [***] cost increases, and (B) the parties shall have discussed in good faith such increases and VTM shall have provided OptiNose with reasonable documentation to explain and support such increase or
8
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
decrease. [***]. This pricing review may be triggered immediately if the price of [***] increases or decreases by more [***] at any time between the scheduled review periods, in which case such change will not be considered for any price change to be implemented under the preceding sentence in this Section 2.06(c).
Section 2.07 Forecasts. Commencing on January 1, 2019, OptiNose shall provide VTM with a non-binding, rolling [***] forecast of its DSA requirements by month to VTM (“Forecast”). The portion of the Forecast for the first [***] period shall be binding (a “Binding Period”) and the remaining [***] shall be for planning purposes and not binding (a “Non-Binding Period”). OptiNose shall place Purchase Orders for the Binding Period of the Forecast in accordance with Section 2.09 of this Agreement. The Forecast shall be updated [***] by OptiNose no later than the [***] day of each calendar month with the Binding Period updated with each Forecast to include the new [***] of the going forward [***] rolling forecast. VTM shall participate in periodic sales and operations planning meetings with OptiNose and other suppliers as OptiNose [***] deems appropriate. VTM shall provide OptiNose monthly inventory reports of DSAs, OptiNose Components and all other Inventory inventoried by VTM solely for the manufacture of DSAs.
Section 2.08 Use of Forecasts. VTM will reference the latest available Forecasts when ordering Inventory (excluding the OptiNose Components which shall be ordered by OptiNose) necessary or appropriate to fulfill the forecasted DSA requirements, taking into account necessary lead times, volume-based pricing, the applicable Inventory’ expiration periods, the Binding Period, and any Purchase Orders for DSAs outside the Binding Period. VTM may purchase such Inventory (including minimum quantities required by suppliers) on the basis of OptiNose’s most recent Forecasts for the applicable Binding Period and, further, the Forecast for the longer period in the case of such Inventory having a longer lead time than [***]. Notwithstanding anything contained in this Agreement to the contrary and other than as set forth in Section 3.09(d), in no case shall VTM maintain more than a [***] supply of Inventory (other than OptiNose Components) based on the then [***] rolling forecast without OptiNose’s prior written consent. Beginning on July 1, 2020 and on an annual basis thereafter, if less than [***] DSAs are assembled over the prior [***] period (and such shortage is due to OptiNose ordering less than this amount and not due to any production delays attributable to VTM or otherwise), OptiNose will pay a fee equal to the number of DSAs below [***] not assembled over the relevant [***] period multiplied by [***] of the assembly fee set forth in Exhibit A (for example, if only [***] DSAs were assembled during the relevant [***] period then the amount due is $[***] ([***] multiplied by $[***] ([***] of $[***]))].
Section 2.09 Purchase Orders.
(a) The terms and conditions contained in this Agreement shall prevail over any terms and conditions of any Purchase Order, acknowledgment form or other form instrument exchanged by the
9
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
parties. OptiNose shall submit purchase orders specifying: (a) the number of units of DSAs to be manufactured, (b) the price (determined in accordance with Exhibit A hereto) and (c) the expected delivery date (“Purchase Orders”). Unless otherwise agreed, a Purchase Order shall not request a shipment date sooner than [***] days from the date of the Purchase Order unless agreed to separately by both parties. VTM shall confirm acceptance of Purchase Orders and projected dates of shipment within [***] days of receiving a Purchase Order. Failure of VTM to confirm any Purchase Order within the [***] day period shall be deemed to be acceptance of such Purchase Order, price and delivery.
(b) For any Binding Period, OptiNose shall submit Purchase Orders that aggregately meet at least [***] of the Forecast for such Binding Period, and VTM shall fulfill such Purchase Orders. If the Purchase Orders for a month in the Binding Period in aggregate exceed the Forecast for such month by an amount between [***], VTM shall supply such excess under this Agreement, provided, however, that, in any consecutive [***] in a Binding Period, VTM shall not be required to supply DSAs in aggregate in excess of [***]. If such Purchase Orders in aggregate exceed the Forecast for such month in the Binding Period by more than [***], VTM shall use [***] to fill such orders, but shall not be in breach of this Agreement if VTM does not accept such portion of the order in excess of such [***], as applicable. VTM shall promptly advise OptiNose to what extent VTM can fulfill such excess amount above [***], as applicable, which amount shall be considered part of the accepted Purchase Order hereunder.
Section 2.10 Release of DSAs. Upon completion of manufacture and associated testing documentation [***] and before shipping each batch, VTM shall send batch documentation, prepared pursuant to the Quality Agreement, to OptiNose’s designee for review and approval. If OptiNose or its designee advises VTM of any issues or concerns regarding such batch documentation in accordance with this Agreement, VTM shall promptly rectify any such issue or concern and reissue updated batch documentation for review and approval within the time periods set forth in the Quality Agreement. Upon OptiNose’s (or OptiNose’s designee) receipt of complete batch documentation for such shipment, OptiNose’s designee shall, within the time period set forth in the Quality Agreement, review and respond as to whether the batch is approved for shipment. [***].
Section 2.11 Non- or late Deliveries. In the event that VTM is unable to make delivery by a ready to ship date specified in the applicable Purchase Order, VTM shall immediately notify OptiNose (and any designee of OptiNose) of such delay and provide the date of availability for the shipment. If VTM fails to deliver the DSAs in the quantities ordered in any Purchase Order within [***] of the date specified in such Purchase Order, then in addition to, and without waiver or limitation of any of its other rights hereunder, at law or in equity, in all instances VTM shall use [***] to timely deliver DSAs even if there were prior delays in the OptiNose Components due to [***]. If shipment of Product is more than [***] late from the most recent agreed upon shipping date pursuant to the
10
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
accepted Purchase Order, then promptly following OPN’s written request for expedited shipping, VTM shall be responsible for the cost of such expedited shipping over and above standard shipping costs for that shipment, but only if the reason for such expedited shipping is attributable to a cause within VTM’s reasonable control. In the event that there is an actual or anticipated delay in shipping beyond [***] or other project delay due to events within VTM’s control, OPN may request an immediate escalation of the issue to VTM’s senior management at the subsidiary level (e.g., Vice-President, Precision Plastics), and failing resolution of the issue in [***], to Flex’s senior management at the corporate level (e.g., President, Medical Segment). Failing resolution at such point, the matter shall be further escalated pursuant to Exhibit D attached hereto after each [***] period.
Section 2.12 Manager Meetings. The parties shall meet periodically [***] at meetings to be organized by the OptiNose Supply Relationship Manager and the VTM Supply Relationship Manager to discuss, agree upon, and oversee implementation of initiatives to plan Capacity and OptiNose Equipment requirements, improve the DSA manufacturing process to improve quality and to reduce cost and price for the benefit of both parties. Participants in such meetings will be agreed to by the parties. For each such agreed initiative, the parties shall agree on the capital and expense to implement the initiative, the party to provide funds for such capital and expense, the expected cost savings to result, and an equitable sharing of the cost and other benefits from the initiative after recoupment of the funds provided for the initiative (which sharing shall take into account a reasonable return on investment for the party providing the funds to implement such initiative). Additionally, promptly following the execution of this Agreement, the parties shall use [***] to agree upon reporting metrics, which shall include but are not limited to [***].
ARTICLE III
EQUIPMENT, INVENTORY AND WORK IN PROGRESS
Section 3.01 OptiNose Equipment
(a) VTM acknowledges that the OptiNose Equipment [***], is owned by OptiNose and that OptiNose may place identifying tags on the OptiNose Equipment confirming and providing notice of OptiNose’s ownership. VTM shall not permit [***] and shall only use the OptiNose Equipment for the manufacture of the DSAs hereunder or other activities for OptiNose. VTM hereby disclaims any interest, to the extent it has any, in the OptiNose Equipment and agrees to execute and deliver any agreements or other documents evidencing OptiNose’s ownership of such OptiNose Equipment. In the event that any OptiNose Equipment is to be shipped from VTM to OptiNose or any third party, [***] shall be responsible for all reasonable packing and shipping costs related thereto.
(b) VTM shall, [***], maintain the OptiNose Equipment in good working order (including maintenance and repair in the ordinary course and calibration, if needed) such that the OptiNose
11
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Equipment enables VTM to produce DSAs according to the Specifications and otherwise in accordance with this Agreement. OptiNose shall be responsible for [***].
(c) At OptiNose’s written request, VTM shall make available at VTM’s facility such OptiNose Equipment as OptiNose may designate, [***], and VTM shall provide [***] at [***] in transitioning such OptiNose Equipment to OptiNose [***].
Section 3.02 Inventory and Work In Progress. The parties acknowledge that the OptiNose Components are significant inputs to the DSAs by value and that the OptiNose Components will be the property of OptiNose at all times that such OptiNose Components are in VTM’s possession. [***], VTM shall not [***].
Section 3.03 Bailment Agreements. VTM agrees to enter a bailment agreement with OptiNose in the form reasonably acceptable to both parties for the OptiNose Equipment, and the OptiNose Components.
Section 3.04 Inventory. Authorization. OptiNose’s accepted Purchase Orders and each Forecast shall constitute authorization for VTM to procure, without OptiNose’s prior approval, but subject to the [***] limitation set forth in Section 2.08: (a) Inventory to manufacture the DSAs covered by such Purchase Orders and Forecast based on the applicable Lead Times, including Long Lead Time Inventory; (b) Minimum Order Inventory reasonably required to support OptiNose’s Purchase Orders and Forecast; and (c) any Special Inventory which is separately authorized in writing by OptiNose.
Section 3.05 Supply Chain Management. Purchases from Approved Vendor List. VTM shall maintain an Approved Vendor List. VTM shall purchase Inventory required to manufacture the DSA only from vendors listed on such Approved Vendor List.
Section 3.06 Vendor Warranties for Inventory. To the extent VTM actually receives from a vendor of Inventory or services the benefit arising from said vendor’s warranty obligations related to its Inventory or services, including OptiNose Components, VTM shall transfer such benefit to OptiNose (without any actual liability for such vendor’s warranty obligations) related to the following warranties with regard to the Inventory or services: (i) conformance of the Inventory or services with the vendor’s specifications; (ii) that the Inventory or services shall be free from defects in design, materials, or workmanship; (iii) that the Inventory or services shall comply with environmental regulations and all other applicable Laws; and (iv) that the Inventory or services shall not infringe the intellectual property rights of third parties. Nothing contained in this Section 3.06 is intended to limit the warranties provided by VTM under Section 4.02 of this Agreement or any other obligation of VTM under this Agreement.
12
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Section 3.07 OptiNose Responsibility for Inventory. Subject to the other terms of this Section 3, OptiNose is financially responsible under the conditions provided in this Agreement for all Inventory purchased by VTM under this Section 3 that can only be used for manufacturing the DSAs. VTM agrees to maintain sufficient level of Inventory to manufacture the DSAs in accordance with the Forecast and Purchase Orders and the terms of this Agreement; provided, that in the event such Inventory is not used in connection with the manufacture of DSA then VTM shall use [***] to return or utilize such Inventory and if they are unable to do so OptiNose shall reimburse VTM for such Inventory, as the case may be.
Section 3.08 Quantity Increases and Shipment Schedule Changes.
(a)For any accepted Purchase Order, OptiNose may request an increase in the quantity of DSAs ordered. All DSA quantity increases in excess of the amount set forth in such Purchase Order (“Excess Request”) shall require VTM’s approval, which is subject to Inventory, OptiNose Component and capacity availability. VTM shall use [***] to meet any DSA quantity increases in an Excess Request.
(b)For any accepted Purchase Order, OptiNose may request a reschedule of the expected delivery date set forth in the applicable Purchase Order not to exceed [***] days. All DSA reschedules in excess of [***] days require VTM’s approval, which, in its reasonable discretion, may or may not be granted. If VTM agrees to accept a reschedule of any length of time, and if there are extra costs to meet such reschedule, then OptiNose shall be liable for such extra costs, provided, that VTM provides OptiNose advance notice of such costs and OptiNose elects to proceed with such reschedule.
(c)Any delays in the normal production or interruption in the workflow process caused by OptiNose’s changes to the Specifications shall be considered a reschedule of any affected Purchase Orders for purposes of this Section 3.08 for the period of such delay.
(d)Cancellations. OptiNose may not cancel all or any portion of DSA quantity of an accepted Purchase Order without VTM’s prior written approval, which, in its [***], may or may not be granted; provided, however, that any reduction of the DSAs ordered in an accepted Purchase Order which reduction is equal to or less than [***] shall not require VTM’s approval and shall not be subject to the other terms and conditions of this Section 3.08; and, provided, further, that VTM will not withhold its approval of any cancellation in the event VTM is able to utilize the production capability that would have been utilized to produce product or otherwise provide services for another customer.
Section 3.09 Excess and Obsolete Inventory.
OptiNose shall be responsible for the following:
a.Excess Inventory. When applicable, VTM shall report the Excess Inventory to OptiNose on a monthly basis. VTM shall advise whether such Excess Inventory was due to a decrease within the first [***] period of a Forecast in DSA production hereunder or due to VTM’s purchase of Inventory
13
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
beyond the amount reasonably necessary for the next [***] of demand for DSAs under the then-current Forecast. Such Excess Inventory reports shall be deemed agreed to by OptiNose, unless OptiNose provides a written objection within [***] days of the end of the calendar month in which such report is received by OptiNose. Upon notice from VTM to OptiNose of any Excess Inventory pursuant to a report, VTM shall attempt to cancel or amend any pending orders for such inventory to the extent such inventory is not reasonably likely to be necessary for any Forecasts hereunder or otherwise for VTM to comply with its obligations hereunder.
b.Purchase of Excess Inventory. OptiNose shall pay VTM for Excess Inventory, as identified by VTM in each monthly report, and not objected to by OptiNose or returned/cancelled by VTM, in each instance pursuant to the process set forth above in Section 3.09(a), at a price equal to (as applicable) the price from the price list set forth in Exhibit A for any finished DSAs, the proportionate amount of such price for any partially completed DSAs, or the listed price for individual components, in each instance such price to be the price in existence at the time such Excess Inventory was acquired, used or completed; provided that if any such completed DSAs, partially completed DSAs or other Excess Inventory are subsequently utilized by VTM for the manufacture and supply of DSAs under this Agreement, VTM shall provide OptiNose a credit for the amount previously paid by OptiNose for such completed DSA, partially completed DSA or other Excess Inventory pursuant to this Section 3.09(b); [***]. In connection with any payments made by OptiNose to VTM pursuant to this Section 3.09(b), at VTM’s discretion and OptiNose’s request (1) VTM shall safely store such Excess Inventory at the rate to be mutually agreed to by the parties, and/or (2) OptiNose can, [***], have some or all of such Excess Inventory stored by a third party for VTM’s potential future use.
c.Obsolete Inventory. When applicable, VTM shall report the Obsolete Inventory to OptiNose on a monthly basis. OptiNose’s failure to object to VTM’s Obsolete Inventory report (or failure to deny its responsibility for such inventory) within [***] days of the end of the calendar month in which such report is received by OptiNose shall constitute its acceptance of VTM’s Obsolete Inventory report. After a validation period, which shall not exceed [***] from the date of such report, OptiNose shall purchase the Obsolete Inventory (and pay in accordance with Section 2 of this Agreement) at a price equal to (as applicable) the price from the price list set forth in Exhibit A for any finished DSAs, the proportionate amount of such price for any partially completed DSAs, or the listed price for individual components.
d.Notwithstanding the foregoing in Sections 3.09(a), (b) and (c) above, for those OptiNose Components set forth on Exhibit C, the [***] period noted in Sections 3.09(a) and (b) shall instead be [***] with respect to the items set forth in Exhibit C for which VTM shall also be required to maintain at least [***] but not more than [***] of available inventory at all times.
14
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
e.Prior to invoicing OptiNose for the amounts due pursuant to Section this 3.09, VTM shall use [***] for a period not to exceed [***] to return for refund unused Inventory from Excess Inventory and Obsolete Inventory and to otherwise mitigate the amounts payable by OptiNose. OptiNose shall submit payment for the amounts identified and invoiced pursuant to this Section 3.09 in accordance with the terms for payment set forth above in Section 2. At OptiNose’s discretion, VTM shall ship the Excess Inventory and Obsolete Inventory to OptiNose promptly following said payment by OptiNose, or destroy such Excess Inventory and Obsolete Inventory, at [***]. [***].
f.For changes (including cancellation and reschedules) that are not consistent with Sections 3.08 or 3.09, [***] shall be responsible for any vendor cancellation charges incurred.
ARTICLE IV
DEFECTIVE PRODUCT
Section 4.01 Notification of Defective Product. OptiNose or its designee shall notify VTM within:
◦ | [***] days after receiving a shipment of DSAs if it determines that such shipment contains a Quantitative Defect, |
◦ | [***] days after receiving a shipment of DSAs if it determines that such shipment contains a Patent Defect, and |
◦ | [***] days after OptiNose becomes aware of a Latent Defect. |
OptiNose or its designee shall provide VTM a sample of what it alleges contains a Latent or Patent Defect, subject to compliance with the foregoing notice requirements and the provisions of Section 4.02, below.
Section 4.02 Resolution of Defective Product.
(a) Patent Defect and Latent Defect. VTM warrants and covenants that: (i) for a period of [***] from invoice date, such DSAs shall be free from defects in workmanship; (ii) be manufactured and delivered in accordance with the terms of this Agreement, the Specifications, and the Quality Agreement, (iii) it shall perform its obligations under this Agreement using personnel of required skill, experience and qualifications and in a professional and workmanlike manner in accordance with generally recognized industry standards, (iv) it is in compliance with, and shall perform under this Agreement in compliance with, all applicable Laws, and (v) OptiNose will receive good and valid title to all DSAs, free and clear of all encumbrances and liens of any kind (collectively, the “Warranty”).
(b) Notwithstanding anything else in this Agreement, this Warranty does not apply to, and VTM makes no representations or warranties whatsoever with respect to any of: (i) defects resulting from adherence to the Specifications, or any written instructions provided by or on behalf of OptiNose; (ii) the design of the DSAs; (iii) DSAs that have been abused, damaged, altered or misused or
15
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
mishandled by any person or entity after title passes to OptiNose; or (iv) defects resulting from tooling, designs or written instructions produced or supplied by OptiNose.
(c) Upon any failure of a DSA to comply with this Warranty, VTM shall, [***]; provided that OptiNose’s inspection or approval shall not excuse Supplier’s responsibility with respect to Latent Defects. [***] shall bear all of the risk, and shipping costs, associated with DSAs that have been returned to VTM for which there is no defect found under the Warranty.
(d) OptiNose or its designee must obtain a Returned Material Authorization (RMA) Number from VTM prior to returning any DSA. The RMA must be indicated on the return package as notice to VTM’s Shipping Department to accept shipment. Any package not so marked will be returned at [***] expense. [***] is responsible for risk of loss and all costs associated with the return to VTM of the DSAs; provided that [***] shall reimburse [***] for such costs if it is determined that the DSA is non-conforming. For purposes of this Agreement, a DSA shall be deemed to be “non-conforming” if it does not meet the Specifications or the other requirements of this Warranty. If OptiNose and VTM are unable to agree as to whether a DSA is conforming, then after [***] to resolve the disagreement, and subject to, and without waiver or limitation of OptiNose’s and/or VTM’s rights and remedies hereunder, at law and/or in equity, either party may submit a sample of such DSA to a mutually agreed upon independent third party testing laboratory which is an expert in the industry and which will expertly apply the agreed upon testing protocol in order to determine whether the DSA is conforming or non-conforming. The independent laboratory’s results shall be final and binding for purposes of determining whether payment is owed (but not for purposes of any pending or potential product liability litigation which shall be governed by Article XII). If the parties or the independent laboratory determine that the DSA was non-conforming, then in addition to, and without waiver or limitation of OptiNose’s rights and remedies hereunder, at law and/or in equity, OptiNose shall [***]. If the parties or the independent laboratory determine the DSA was conforming, OptiNose shall [***]. Unless otherwise agreed to by the parties in writing, the costs associated with testing and review of a DSA pursuant to this Section shall be borne by [***].
(e) The above warranties are given in lieu of any other representation or warranty, express or implied, and including but not limited to the implied warranty of merchantability or fitness for a particular purpose.
(f) Quantitative Defect. If OptiNose believes that a shipment of DSAs hereunder has a Quantitative Defect, OptiNose shall notify VTM within the applicable period. If VTM agrees with such Quantitative Defect, VTM will promptly, and in no event more than [***] days, ship sufficient DSAs at OptiNose’s direction to remedy such Quantitative Defect. If VTM does not agree with OptiNose’s determination that such shipment has a Quantitative Defect, then after [***] to resolve the disagreement, and subject to, and without waiver or limitation of OptiNose’s and/or VTM’s rights and
16
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
remedies hereunder, at law and/or in equity, VTM may require a mutually agreed upon independent third party to determine whether the shipment had a Quantitative Defect. The independent party’s results shall be final and binding for purposes of determining whether VTM is obligated to ship additional DSAs, and the costs of such independent third party shall be borne by [***]. If such results indicate that the shipment had a Quantitative Defect, OptiNose shall be entitled to [***].
ARTICLE V
RECORDS AND REGULATORY MATTERS
Section 5.01 Recordkeeping. VTM shall maintain true and accurate books, records, inventory of Inventory and finished DSAs, test and laboratory data, reports and all other information relating to Manufacturing under this Agreement, including all information required to be maintained by all Law. Such information shall be maintained for the period specified in the Quality Agreement or longer if required under Law. VTM shall provide or make such information available to OptiNose upon request and shall notify and provide OptiNose with advance notice and opportunity to obtain such information at the end of the retention period.
Section 5.02 Regulatory Compliance. VTM will be responsible to maintain all permits and licenses required by any Law with respect to the facility and its equipment for the manufacture and delivery of DSAs and will manufacture and deliver the DSAs in accordance with the requirements of this Agreement, including the Quality Agreement, the Specifications and applicable Laws. In addition, during the Term, at OptiNose’s request VTM will provide [***] assistance [***] with all regulatory matters relating to the manufacturing of the DSAs and services under this Agreement. Each Party intends and commits to cooperate to satisfy all Law within the scope of its respective responsibilities under this Agreement.
Section 5.03 Regulatory Correspondence. VTM shall notify OptiNose in accordance with the Quality Agreement of any notice, correspondence, and the result of any inspection(s) by or with the FDA or any Regulatory Authority (including without limitation any 483, warning letter, or similar correspondence) concerning an actual or potential regulatory deficiency, noncompliance or problem that directly or indirectly relates to the manufacturing of the DSAs or any of the services provided by VTM under this Agreement. VTM shall notify OptiNose in accordance with the Quality Agreement of any other notice or correspondence, and the result of any inspection(s), with the FDA or any Regulatory Authority that is reasonably likely to impact or directly relates to the manufacture of DSAs or other performance under this Agreement. In all of the foregoing notifications, VTM shall provide OptiNose with copies of any such notices, correspondences, or results of inspection in accordance with the Quality Agreement subject to any other customer confidentiality requirements of VTM; provided VTM shall attempt to reasonably redact any such other customer confidential information in order to provide OptiNose with such information. Furthermore, VTM shall send a draft
17
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
to OptiNose of all correspondence VTM intends to send to any Regulatory Authority with any substantial relation to DSAs. For all correspondence with a Regulatory Authority related directly to DSAs that is in response to any 483, warning letter, regulatory deficiency or other problem relating to the manufacture of DSAs, VTM shall consult with, and reasonably consider the input of, OptiNose on the draft correspondence before such correspondence is sent to the Regulatory Authorities. Regarding all interactions with Regulatory Authorities, both parties shall make [***] to act expeditiously in cooperating with each other and responding to Regulatory Authorities.
Section 5.04 Governmental Inspections and Requests. VTM shall as soon as [***] in accordance with the Quality Agreement inform OptiNose in writing of any inspection, notice or request for inspection, and other regulatory action, by any regulatory agency relating to the manufacture of DSAs and/or, in the case of a facility to the extent related to VTM’s manufacturing, packaging, testing and storage of DSAs at such facility, so that OptiNose has as much advance notice as possible to enable it to, as applicable and relevant, participate in preparation and/or strategy regarding and/or attend the inspection. VTM shall permit the OptiNose’s representatives to be present during any such inspection related to DSAs ([***]), including being present at any inspection of the facility to the extent such inspection is related to VTM’s manufacturing, packaging, testing or storage of the DSAs. As provided in Section 5.03, VTM will provide OptiNose with the results of all regulatory inspection or audits related to the DSAs after VTM’s receipt of such results in accordance with the Quality Agreement.
Section 5.05 Recall. In the event VTM believes a recall, field alert, product withdrawal or field correction may be necessary with respect to any DSA provided under this Agreement, VTM shall as soon as practicable notify OptiNose in writing. VTM will not act to initiate a recall, field alert, product withdrawal or field correction with respect to the DSAs. In the event OptiNose believes a recall, field alert, product withdrawal or field correction may be necessary with respect to any DSA provided under this Agreement, OptiNose shall immediately notify VTM in writing and VTM shall provide [***] cooperation and assistance to OptiNose. The cost of any recall, field alert, product withdrawal or field correction, and any assistance in connection therewith, shall be borne by [***]. For avoidance of doubt and subject to applicable Laws, OptiNose shall have the ultimate and final authority to initiate a field alert or recall of the Finished Product.
Section 5.06 Inspections and Audits by OptiNose. [***], representatives of OptiNose shall have access upon [***] prior notice to VTM’s facility where it manufactures DSAs for the purpose of: (a) conducting inspections of such facility and VTM’s maintenance and usage of the equipment utilized in the manufacture of the DSAs, (b) performing quality control and quality assurance (including without limitation cGMP) audits (c) witnessing the manufacture, storage or transportation of the DSAs or the Inventory, (d) verifying the storage of the OptiNose Components, and (d) requiring cycle counts by VTM (and adjustments to inventory as necessary). OptiNose shall have access to the
18
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
results of any tests performed by VTM relating to DSAs and Inventory and the processes or the Inventory that VTM’s purchases directly from a third party used in their manufacture. Such inspections shall not relieve VTM of any of its obligations under this Agreement or create new obligations on the part of OptiNose. This right of inspection can be exercised [***] (and as often as necessary for cause), subject to a written notice to VTM given in accordance with the time periods specified in the Quality Agreement, or at any time for cause. VTM shall permit such inspection during normal business hours at reasonable and mutually acceptable times [***]. At all times, OptiNose’s representatives shall be accompanied by VTM personnel and follow all site reasonable health and safety policies of VTM. Each inspection, audit and witnessing shall be subject, at all times, to VTM’s confidentiality and non-disclosure obligations to its other third party customers.
ARTICLE VI
ADDITIONAL VTM OBLIGATIONS
Section 6.01 VTM shall:
(a) appoint a VTM employee to serve as a primary contact with respect to this Agreement and who will have the expertise and authority to act on behalf of VTM in connection with matters pertaining to this Agreement (the “VTM Supply Relationship Manager”);
(b) [***] maintain the same VTM Supply Relationship Manager throughout the Term, however VTM has the right to replace the VTM Supply Relationship Manager [***];
(c) before the date on which the services under this Agreement are to start, obtain, and at all times during the Term maintain, all necessary licenses and consents and comply with all Laws;
(d) prior to any VTM Personnel performing any services hereunder: (i) ensure that such VTM Personnel are suitably trained, skilled, experienced and qualified to perform such services; and (ii) ensure that such VTM Personnel have the legal right to work in the United States; and
(e) maintain complete and accurate records relating to the provision of services under this Agreement, including records of the time spent and Inventory and OptiNose Components used by VTM in providing such services in such form as [***] shall [***]. During the Term and for a period of [***] thereafter, upon OptiNose’s written request, VTM shall allow OptiNose or OptiNose’s representative to inspect and make copies of such manufacturing and quality records in connection with the provision of the services under this Agreement; provided that any such inspection shall take place during regular business hours no more than [***] (which limit shall not include any inspections for cause) and OptiNose provides VTM with [***] advance written notice.
Section 6.02 VTM is responsible for all VTM Personnel and for the payment of their compensation, including, if applicable, withholding of income taxes, and the payment and
19
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
withholding of social security and other payroll taxes, unemployment insurance, workers’ compensation insurance payments and disability benefits.
Section 6.03 VTM acknowledges that time is of the essence with respect to VTM’s obligations hereunder and that prompt and timely performance of all such obligations is strictly required.
Section 6.04 The obligations of VTM under this Agreement shall be performed fully within the facility located at 71 and 00 Xxxxxx Xxxx, Xxxxxxxxxx, XX 00000 Xxxxxx Xxxxxx, unless approved in writing in advance by OptiNose.
Section 6.05 Upon VTM’s receipt of OptiNose Components, VTM shall promptly inspect such OptiNose Components in accordance with the applicable inspection criteria provided by OptiNose and ensure that such OptiNose Components provided by each such supplier meets the applicable specifications for such OptiNose Components. VTM shall do such inspection within [***] days of its receipt of such OptiNose Components. Should the OptiNose Components fail any such inspection or should VTM identify any issues with the OptiNose Components, VTM shall provide OptiNose and the applicable supplier of such OptiNose Components notice of such failure and/or issue within [***] day.
ARTICLE VII
ADDITIONAL OPTINOSE’S OBLIGATIONS
Section 7.01 OptiNose shall:
(a) appoint an OptiNose employee to serve as the primary contact with respect to this Agreement and who will have the expertise and authority to act on behalf of OptiNose with respect to matters pertaining to this Agreement (the “OptiNose Supply Relationship Manager”);
(b) respond [***] to any VTM request to provide direction, information, approvals, authorizations or decisions that are reasonably necessary for VTM to perform in accordance with this Agreement;
(c) provide such information as VTM may reasonably request and OptiNose considers [***] necessary in order to perform under this Agreement;
(d) obtain and maintain all necessary licenses and consents and comply with all Law to the extent necessary for OptiNose’s performance under the Agreement; and
(e) obtain and maintain throughout the term of this Agreement insurance on the OptiNose Equipment in [***] amounts and coverage.
20
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
ARTICLE VIII
FEES AND EXPENSES; PAYMENT TERMS
FEES AND EXPENSES; PAYMENT TERMS
Section 8.01 VTM shall issue invoices to OptiNose upon delivery to OptiNose in accordance with Section 2.01 of DSAs with pricing pursuant to Exhibit A for such DSAs produced, and OptiNose shall pay all properly invoiced amounts due to VTM within [***] days after the date of the invoice except for any amounts disputed by OptiNose in good faith (subject to OptiNose’s match process for Purchase Order, invoice and receipt). VTM shall provide, for OptiNose’s review and prior written approval, statements of work with budgetary allowances for any services not required to be provided by VTM at its costs by the quality agreement or this Agreement. VTM will provide invoices to those services if incurred. All payments hereunder shall be in US dollars and made by check or wire transfer. The provisions of this Agreement shall govern over any terms and conditions listed on any invoice or Purchase Order. A service fee of [***]% per month will be added to all accounts more than [***] days past due, and [***] is responsible for all collection and attorneys’ fees and costs required to collect unpaid amounts.
Section 8.02 [***] shall be responsible for all sales, use and excise taxes, and any other similar taxes, duties and charges of any kind imposed by any federal, state or local governmental entity on any amounts payable to VTM. [***]. In no event shall [***] pay or be responsible for any taxes imposed on, or with respect to, [***] income, revenues, gross receipts, personnel or real or personal property or other assets.
ARTICLE IX
INTELLECTUAL PROPERTY RIGHTS; OWNERSHIP
INTELLECTUAL PROPERTY RIGHTS; OWNERSHIP
Section 9.01 Except as set forth in Section 9.03, OptiNose is, and shall be, the sole and exclusive owner of all right, title and interest in and to any Intellectual Property Rights generated in connection with VTM’s performance under this Agreement, and to any of the Deliverables, including all Intellectual Property Rights therein. VTM agrees, and will cause its VTM Personnel to agree, that with respect to any Intellectual Property Rights or Deliverables that may qualify as “work made for hire” as defined in 17 U.S.C. §101, such Intellectual Property Rights and Deliverables are hereby deemed a “work made for hire” for OptiNose. To the extent that any of the Intellectual Property Rights or Deliverables hereunder do not immediately vest in OptiNose or do not constitute a “work made for hire”, VTM hereby irrevocably assigns on behalf of itself and all VTM Personnel, and, [***], shall cause the VTM Personnel to irrevocably assign to OptiNose, in each case without additional consideration, all right, title and interest throughout the world in and to such Intellectual Property Rights and Deliverables, including all Intellectual Property Rights therein. VTM hereby waives on behalf of itself and all VTM Personnel, and, [***], shall cause the VTM Personnel to irrevocably waive, to the extent permitted by applicable Law, any and all claims VTM and/or such VTM Personnel
21
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
may now or hereafter have in any jurisdiction to so-called “moral rights” or rights of droit moral with respect to such Intellectual Property Rights and Deliverables.
Section 9.02 Upon the request of OptiNose, VTM shall, and shall cause the VTM Personnel to, promptly take such further actions, including execution and delivery of all appropriate instruments of conveyance, as may be necessary to assist OptiNose to prosecute, register, perfect or record its rights in or to any Deliverables.
Section 9.03 VTM and its licensors are, and shall remain, the sole and exclusive owners of all right, title and interest in and to the VTM Tools, including all Intellectual Property Rights therein. VTM represents and warrants that the DSAs delivered hereunder shall not incorporate any VTM Tools, and that OptiNose can use and/or sell the DSAs without the requirement of any approval and/or license from VTM
Section 9.04 OptiNose and its licensors are, and shall remain, the sole and exclusive owner of all right, title and interest in and to the OptiNose Information, including all Intellectual Property Rights therein. VTM shall have no right or license to use any OptiNose Information, except solely during the Term of the Agreement to the extent necessary to perform under this Agreement. All other rights in and to the OptiNose Information are expressly reserved by OptiNose.
ARTICLE X
CONFIDENTIAL INFORMATION
CONFIDENTIAL INFORMATION
Section 10.01 The Receiving Party agrees:
(a) not to disclose or otherwise make available Confidential Information of the Disclosing Party to any third party without the prior written consent of the Disclosing Party; provided, however, that the Receiving Party may disclose the Confidential Information of the Disclosing Party to its Affiliates, and their officers, employees, consultants and legal advisors who have a “need to know”, who have been apprised of this restriction and who are themselves bound by nondisclosure obligations at least as restrictive as those set forth in this Article X, provided, that, the Receiving Party shall be responsible for any disclosure or use of Confidential Information by such persons or entities that is contrary to the terms of this Agreement;
(b) to use the Confidential Information of the Disclosing Party only for the purposes of performing its obligations under the Agreement or, in the case of OptiNose, to make use of the services under this Agreement and Deliverables; and
(c) to promptly notify the Disclosing Party in the event it becomes aware of any loss or disclosure of any of the Confidential Information of the Disclosing Party.
Section 10.02 If the Receiving Party becomes legally compelled to disclose any Confidential Information, the Receiving Party shall provide: (i) prompt written notice of such
22
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
requirement so that the Disclosing Party may seek, at its sole cost and expense, a protective order or other remedy; and (ii) [***] assistance, [***], in opposing such disclosure or seeking a protective order or other limitations on disclosure. If, after providing such notice and assistance as required herein, the Receiving Party remains required by Law to disclose any Confidential Information, the Receiving Party shall disclose no more than that portion of the Confidential Information which, on the advice of the Receiving Party’s legal counsel, the Receiving Party is legally required to disclose and, upon the Disclosing Party’s request, shall use commercially reasonable efforts to obtain assurances from the applicable court or agency that such Confidential Information will be afforded confidential treatment.
ARTICLE XI
REPRESENTATIONS AND WARRANTIES
Section 11.01 Each party further represents and warrants to the other party that:
(a) it is duly organized, validly existing and in good standing as a corporation or other entity as represented herein under the laws and regulations of its jurisdiction of incorporation, organization or chartering;
(b) it has the full right, power and authority to enter into this Agreement, to grant the rights and licenses granted hereunder and to perform its obligations hereunder;
(c) the execution of this Agreement by its representative whose signature is set forth at the end hereof has been duly authorized by all necessary corporate action of the party;
(d) when executed and delivered by such party, this Agreement will constitute the legal, valid and binding obligation of such party, enforceable against such party in accordance with its terms; and
(e) it will comply at all times with the provisions of applicable Laws of the United States (and, as applicable, analogous such laws in any other territories where regulatory approval is sought) regarding debarment and will upon request certify in writing to the other parties that none of its employees nor any person providing services in connection with this Agreement have been debarred under the provisions of such laws.
Section 11.02 VTM further represents and warrants to OptiNose that:
(a) it shall perform the services under this Agreement using personnel of required skill, experience and qualifications and in a professional and workmanlike manner in accordance with generally recognized industry standards for similar services and shall devote adequate resources to meet its obligations under this Agreement;
(b) it is in compliance with, and shall perform under this Agreement in compliance with, all applicable Laws;
23
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(c) Upon delivery of DSAs to OptiNose in accordance with Section 2.1, OptiNose will receive good and valid title to all Deliverables, free and clear of all encumbrances and liens of any kind;
(d) the DSAs provided under this Agreement shall be manufactured and delivered in strict compliance with the terms of this Agreement, including (i) the Specifications; (ii) all Laws relating to the manufacture of the DSAs, including without limitation the FD&C Act and cGMPs; and (iii) the Quality Agreement; and
(e) as of the date hereof, there are no pending or, to VTM’s knowledge, threatened claims, litigation or other proceedings pending against VTM by any third party, in each case, excluding any infringement or claim, litigation or other proceedings to the extent arising out of (x) any OptiNose Information or any instruction, information, designs, specifications or other materials provided by OptiNose to VTM, (y) use of the Deliverables in combination with any materials or equipment not supplied or specified by VTM, if the infringement would have been avoided by the use of the Deliverables not so combined, and (z) any modifications or changes made to the Deliverables by or on behalf of any Person other than VTM.
Section 11.03 EXCEPT FOR THE EXPRESS WARRANTIES IN THIS AGREEMENT, EACH PARTY HEREBY DISCLAIMS ALL WARRANTIES, EITHER EXPRESS, IMPLIED, STATUTORY, OR OTHERWISE UNDER THIS AGREEMENT, INCLUDING ALL IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE.
ARTICLE XII
INDEMNIFICATION
INDEMNIFICATION
Section 12.01 OptiNose acknowledges that VTM has no control over, and is not responsible for, the manner in which the DSAs will be used or otherwise dealt with by OptiNose. OptiNose shall defend, indemnify and hold VTM and VTM’s Affiliates and their officers, directors, employees, agents, successors and permitted assigns (each, a “VTM Indemnitee”) harmless from and against any and all third party suits, claims, losses, demands, liabilities, damages, costs and expenses (including reasonable attorneys’ fees) (“Losses”) and agree to assume all responsibility for any and all actions, claims, or demands arising out of or in any way connected with, and any and all amounts which VTM and/or OptiNose becomes obligated to pay, caused by or resulting directly or indirectly from the use or operation of the DSAs, including any intellectual property claims, except to the extent VTM is required to indemnity OptiNose for such Loss pursuant to Section 12.02.
Section 12.02 VTM agrees to defend, indemnify and hold harmless OptiNose and OptiNose’s Affiliates and their officers, directors, employees, agents, successors and permitted assigns (each, an “OptiNose Indemnitee”) from and against, any and all Losses as follows: (a) any
24
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
actual or alleged injury or damage to any person (including death) or property caused, or alleged to be caused, by a DSA sold by VTM to OptiNose hereunder, but solely to the extent such injury or damage has been caused by the breach by VTM of its Warranty set forth in Article IV, including but not limited to the failure of the DSA to conform to the Specifications; and (b) any actual or alleged infringement or misappropriation of the intellectual property rights (including any industrial design rights, database rights or any other form of intangible or business property rights) of any third party, but solely to the extent that such infringement or misappropriation is caused by a process, VTM Tools, or Production Materials that VTM elects to use to manufacture, assemble or test the DSAs; however, VTM shall not have any obligation to indemnify OptiNose if such claim would not have arisen but for VTM’s manufacture, assembly or test of the DSA in accordance with the Specifications.
Section 12.03 The party seeking indemnification hereunder shall promptly notify the indemnifying party in writing of any third party claim, suit, action or proceeding (“Action”) and cooperate with the indemnifying party [***]. The indemnifying party shall immediately take control of the defense and investigation of such Action and shall employ counsel of its choice to handle and defend the same, [***]. The indemnifying party shall not settle any Action in a manner that adversely affects the rights of the indemnified party without the indemnified party’s prior written consent. The indemnified party’s failure to perform any obligations under this Section 12.3 shall not relieve the indemnifying party of its obligations under this Section 12.3 except to the extent that the indemnifying party can demonstrate that it has been materially prejudiced as a result of such failure. The indemnified party may participate in and observe the proceedings [***].
ARTICLE XIII
LIMITATION OF LIABILITY
LIMITATION OF LIABILITY
Section 13.01 EXCEPT AS OTHERWISE PROVIDED IN SECTION 13.02, IN NO EVENT WILL EITHER PARTY BE LIABLE TO THE OTHER OR TO ANY THIRD PARTY FOR ANY LOSS OF USE, REVENUE OR PROFIT OR FOR ANY CONSEQUENTIAL, INCIDENTAL, INDIRECT, EXEMPLARY, SPECIAL OR PUNITIVE DAMAGES WHETHER ARISING OUT OF BREACH OF CONTRACT, TORT (INCLUDING NEGLIGENCE) OR OTHERWISE, WHETHER OR NOT SUCH PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. FURTHERMORE, EXCEPT WITH RESPECT TO THE EQUITABLE EXCEPTIONS, IN NO EVENT WILL VTM BE LIABLE FOR THE VALUE OF THE INTERNAL TIME OF OPTINOSE’S EMPLOYEES TO REMEDY A BREACH.
Section 13.02 The exclusions and limitations in Section 13.01 shall not apply to the following (the “Equitable Exceptions”):
(a) damages or other liabilities arising out of or relating to a party’s failure to comply with its obligations under Article IX (Intellectual Property Rights; Ownership);
25
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(b) damages or other liabilities arising out of or relating to a party’s failure to comply with its obligations under Article X (Confidentiality);
(c) a party’s indemnification obligations under Article XII (Indemnification); and
(d) damages or other liabilities arising out of or relating to a party’s gross negligence, willful misconduct or intentional acts.
EXCEPT WITH RESPECT TO THE EQUITABLE EXCEPTIONS ABOVE AND NOTWITHSTANDING ANYTHING TO THE CONTRARY IN THIS AGREEMENT, VTM’S TOTAL LIABILITY TO OPTINOSE HEREUNDER SHALL BE SUBJECT TO AN AGGREGATE CAP IN ACCORDANCE WITH THE FOLLOWING: [***].
Section 13.03 No Double Recovery. Notwithstanding OptiNose’s rights under the Molded Parts Agreement, in the event of a dispute of a claim under either this Agreement or the Molded Parts Agreement, OptiNose is only entitled to recover under this Agreement with respect to the DSAs, and the Molded Parts Agreement with respect to the cap covered thereunder. Further, notwithstanding the preceding sentence, if there are any disputes or claims regarding Pre-Existing Inventory that OptiNose purchased from VTM prior to the date of this Agreement and such Pre-Existing Inventory would not be covered under this Agreement for any claims or disputes related thereto, then the parties agree and acknowledge that OptiNose maintains any and all rights set forth in the Molded Parts Agreements regarding such Pre-Existing Inventory.
ARTICLE XIV
TERM
Section 14.01 This Agreement shall commence as of the Effective Date and shall expire at 11:59PM on October 24, 2021, unless sooner terminated pursuant to Article XV. After the expiration of the initial term hereunder, this Agreement shall be automatically renewed for separate but successive one-year terms unless either party provides written notice to the other party that it does not intend to renew this Agreement ninety (90) days or more prior to the end of any term.
ARTICLE XV
TERMINATION; EFFECT OF TERMINATION
TERMINATION; EFFECT OF TERMINATION
Section 15.01 OptiNose, in its sole discretion, may terminate this Agreement:
(a) [***];
(b) by providing VTM written notice if VTM fails an inspection or suffers a hold, 483, warning letter, or other disciplinary action by the FDA or any other government authority and VTM fails to cure such inspection shortcoming, or remove or resolve such hold or disciplinary action in such a manner that the VTM facility passes re-inspection by the FDA or government authority and/or is free of the hold or disciplinary action, in good standing with FDA or such other government authority,
26
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
and is lawfully able to and does resume timely and conforming manufacture and delivery of OptiNose’s DSAs requirements in accordance with this Agreement within thirty days of such original inspection, or imposition of the hold or disciplinary action; or
(c) by providing VTM written notice if VTM fails to gain recommendation for approval by FDA to manufacture DSAs in accordance with this Agreement (with such recommendation being either unqualified or with any qualifications resolved to FDA’s acknowledged satisfaction) in a manner that does not delay such approval by the FDA.
Section 15.02 Either party may terminate this Agreement, effective upon written notice to the other party (the “Defaulting Party”), if the Defaulting Party:
(a) materially breaches this Agreement, and such breach is incapable of cure, or with respect to a material breach capable of cure, the Defaulting Party does not cure such breach within [***] days after receipt of written notice of such breach.
(b) (i) becomes insolvent or admits its inability to pay its debts generally as they become due; (ii) becomes subject, voluntarily or involuntarily, to any proceeding under any domestic or foreign bankruptcy or insolvency law, which is not fully stayed within seven business days or is not dismissed or vacated within forty-five days after filing; (iii) is dissolved or liquidated or takes any corporate action for such purpose; (iv) makes a general assignment for the benefit of creditors; or (v) has a receiver, trustee, custodian or similar agent appointed by order of any court of competent jurisdiction to take charge of or sell any material portion of its property or business.
Section 15.03 Upon expiration or termination of this Agreement for any reason:
(a) OptiNose shall have the right at any time after a notice of termination has been given or an event has occurred which, with the passage of time, will cause this Agreement to terminate to require VTM, as soon as reasonably practicable and in no more than [***] days from the effective date of termination, to make available for removal by OptiNose or its designee [***]: (i) all DSAs, all Inventory, all partially completed DSAs, all Deliverables and all OptiNose Information ([***]), and (ii) all OptiNose Equipment. Upon payment, as applicable, all of the foregoing items for removal shall be made available at the facility, [***], claims and encumbrances, and VTM shall provide [***] cooperation and assistance to OptiNose upon OptiNose’s written request [***] in transitioning the manufacture of DSAs and related services under this Agreement to an alternate supplier. [***].
(b) Each party shall (i) return to the other party all documents and tangible materials (and any copies) containing, reflecting, incorporating or based on the other party’s Confidential Information, (ii) if the other party requests, use [***] efforts to permanently erase all of the other party’s Confidential Information from its computer systems, and (iii) certify in writing to the other party that it has complied with the requirements of this clause; provided, however, that OptiNose may retain copies of any Confidential Information of VTM incorporated in the Deliverables or to the extent necessary to allow
27
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
it to make full use of the DSAs and any Deliverables; and provided further, however, that VTM shall retain such documents and tangible materials as are required to be maintained by VTM under Law.
(c) In no event shall OptiNose be liable for [***].
Section 15.04 The rights and obligations of the parties set forth in this Section 15.04 and Article I, Sections 5.01, 5.03, 5.04, 5.05, 5.06 and 6.01(e), Article IX, Article X, Article XI, Article XII, Article XIII, Section 15.03, Article XVI and Article XVIII, and any right or obligation of the parties in this Agreement which, by its nature, should survive termination or expiration of this Agreement, will survive any such termination or expiration of this Agreement. For purposes of clarity, in no event shall any termination or expiration of this Agreement excuse either party from any breach or violation of this Agreement or other obligation that occurred prior to such termination or expiration and, in each such case, full legal and equitable remedies shall remain available to address such issues.
ARTICLE XVI
INSURANCE
INSURANCE
Section 16.01 At all times during the Term and for a period of at least [***] thereafter, VTM shall procure and maintain, at its sole cost and expense, at least the following types and amounts of insurance coverage:
(a) Commercial General Liability/ Completed Operation Liability with limits no less than $[***] per occurrence, including bodily injury and property damage and products, which policy will include contractual liability coverage insuring the activities of VTM under this Agreement;
(b) Worker’s Compensation with employer’s liability limits no less [***];
(c) Errors and Omissions and/or Professional Liability with limits no less than $[***] per occurrence; and
(d) Umbrella Liability with limits no less than $[***] per occurrence and $[***] in the aggregate providing excess coverage of the primary Commercial General Liability, including Products/Completed Operations Liability.
Section 16.02 All insurance policies required pursuant to this Article XVI shall:
(a) be issued by an insurance company or insurance companies having an A.M. Best Rating of [***] or better;
(b) provide that such insurance be primary insurance and any similar insurance in the name of and/or for the benefit of OptiNose shall be excess and non-contributory; provided, however, this provision shall not apply to the insurance required by Section 16.01(b); and
28
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(c) name OptiNose and OptiNose’s Affiliates, including, in each case, all successors and permitted assigns, as Additional Insureds; provided, however, this provision shall not apply to the insurance required by Section 16.01(b).
Section 16.03 Upon the written request of OptiNose, VTM shall provide OptiNose with copies of the certificates of insurance for all insurance coverage required by this Article XVI, and shall not do anything to invalidate such insurance. This Article XVI shall not be construed in any manner as waiving, restricting or limiting the liability of either party for any obligations imposed under this Agreement (including but not limited to, any provisions requiring a party hereto to indemnify, defend and hold the other harmless under this Agreement).
ARTICLE XVII
FORCE MAJEURE
Section 17.01 No party shall be liable or responsible to the other party, nor be deemed to have defaulted under or breached this Agreement, for any failure or delay in fulfilling or performing any term of this Agreement, when and to the extent such failure or delay is caused by or results from acts beyond the affected party’s reasonable control, including, without limitation:
(a) acts of God;
(b) flood, fire or explosion;
(c) war, invasion, riot or other civil unrest;
(d) actions, embargoes or blockades in effect on or after the date of this Agreement;
(e) national or regional emergency;
(f) strikes, labor stoppages or slowdowns or other industrial disturbances; or
(g) compliance with any law or governmental order, rule, regulation or direction, or any action taken by a governmental or public authority, including but not limited to imposing an embargo, export or import restriction, quota or other restriction or prohibition, or failing to grant a necessary license or consent;
(each of the foregoing, a “Force Majeure Event”). A party whose performance is affected by a Force Majeure Event shall give notice to the other party, stating the period of time the occurrence is expected to continue and shall use [***] to end the failure or delay and minimize the effects of such Force Majeure Event.
29
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Section 17.02 During the Force Majeure Event, the non-affected party may similarly suspend its performance obligations until such time as the affected party resumes performance.
Section 17.03 The non-affected party may terminate this Agreement if such failure or delay continues for a period of [***] days or more and, [***].
ARTICLE XVIII
MISCELLANEOUS
MISCELLANEOUS
Section 18.01 Each party shall, upon the reasonable request, promptly execute such documents and perform such acts as may be necessary to give full effect to the terms of this Agreement.
Section 18.02 The relationship between the parties is that of independent contractors. Nothing contained in this Agreement shall be construed as creating any agency, partnership, joint venture or other form of joint enterprise, employment or fiduciary relationship between the parties, and neither party shall have authority to contract for or bind the other party in any manner whatsoever.
Section 18.03 Neither party shall issue or release any announcement, statement, press release or other publicity or marketing materials relating to this Agreement, or otherwise use the other party’s trademarks, service marks, trade names, logos, symbols or brand names, in each case, without the prior written consent of the other party, except to the extent necessary pursuant to any applicable securities exchange rule.
Section 18.04 All notices, requests, consents, claims, demands, waivers and other communications hereunder shall be in writing and shall be deemed to have been given (a) when delivered by hand (with written confirmation of receipt); (b) when received by the addressee if sent by a nationally recognized overnight courier (receipt requested); or (c) on the third day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective parties at the addresses indicated below (or at such other address for a party as shall be specified in a notice given in accordance with this Section 18.04.)
30
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
If to VTM: | VTM, LLC Attn: Chief Executive Officer 71 Utopia Xxxx Xxxxxxxxxx, XX 00000 Flex Health Xxxxxxxxx 0000 Xxxxxxx Xxxxxx Xxxxx Xxx Xxxx, XX Attn: Medical, General Counsel |
If to OptiNose: | OptiNose US, Inc. Attn: Chief Executive Officer 0000 Xxxxx Xxxx Xxxx, Xxxxx 000 Xxxxxxx, XX 00000 Xx OptiNose UK: OptiNose UK, Ltd. Xxxxx Xxxx Xxxxx Xxxxxxx Xxxx, Xxxxxxxxx XX0 0XX, Xxxxxxx Attention: Chief Executive Officer To OptiNose Norway: OptiNose AS Xxxxxxxxxxxxx 000000 Xxxx, Xxxxxx Attention: Chief Executive Officer |
In each instance, with cc to:
OptiNose US, Inc.
OptiNose US, Inc.
Attn: Chief Legal Officer
0000 Xxxxx Xxxx Xxxx, Xxxxx 000
Xxxxxxx, XX 00000
Section 18.05 For purposes of this Agreement, (a) the words “include,” “includes” and “including” shall be deemed to be followed by the words “without limitation”; (b) the word “or” is not exclusive; and (c) the words “herein,” “hereof,” “hereby,” “hereto” and “hereunder” refer to this Agreement as a whole. Unless the context otherwise requires, references herein: (x) to Sections and Exhibits refer to the Sections of, and Exhibits attached to, this Agreement; and (y) to a statute means such statute as amended from time to time and includes any successor legislation thereto and any regulations promulgated thereunder. This Agreement shall be construed without regard to any
31
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
presumption or rule requiring construction or interpretation against the party drafting an instrument or causing any instrument to be drafted. The Schedules, Exhibits and Statements of Work referred to herein shall be construed with, and as an integral part of, this Agreement to the same extent as if they were set forth verbatim herein.
Section 18.06 This Agreement, together with all Exhibits and any other documents incorporated herein by reference, constitutes the sole and entire agreement of the parties to this Agreement with respect to the subject matter contained herein, and supersedes all prior and contemporaneous understandings and agreements, both written and oral, with respect to such subject matter. For purposes of clarity, this Agreement shall not supersede the development agreements and other related project work between the parties not otherwise covered by this Agreement.
Section 18.07 Neither party may assign, transfer or delegate any or all of its rights or obligations under this Agreement (including without limitation to any subcontractors), without the prior written consent of the other party, which consent shall not be unreasonably withheld or delayed; provided, that, upon prior written notice to the other party, either party may assign the Agreement to an Affiliate of such party or to a successor of all or substantially all of the assets of such party through merger, reorganization, consolidation or acquisition; provided further, that, notwithstanding the foregoing, VTM may not make such an assignment without OptiNose’s prior written consent, not to be unreasonably withheld. No assignment shall relieve the assigning party of any of its obligations hereunder. Any attempted assignment, transfer or other conveyance in violation of the foregoing shall be null and void. This Agreement shall be binding upon and shall inure to the benefit of the parties hereto and their respective successors and permitted assigns.
Section 18.08 This Agreement is for the sole benefit of the parties hereto and their respective successors and permitted assigns and nothing herein, express or implied, is intended to or shall confer upon any other Person any legal or equitable right, benefit or remedy of any nature whatsoever, under or by reason of this Agreement.
Section 18.09 The headings in this Agreement are for reference only and shall not affect the interpretation of this Agreement.
Section 18.10 This Agreement may only be amended, modified or supplemented by an agreement in writing signed by each party hereto. No waiver by any party of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. Except as otherwise set forth in this Agreement, no failure to exercise, or delay in exercising, any rights, remedy, power or privilege arising from this Agreement shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
32
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Section 18.11 If any term or provision of this Agreement is invalid, illegal or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other term or provision of this Agreement or invalidate or render unenforceable such term or provision in any other jurisdiction. Upon such determination that any term or other provision is invalid, illegal or unenforceable, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the transactions contemplated hereby be consummated as originally contemplated to the greatest extent possible.
Section 18.12 This Agreement shall be governed by and interpreted in accordance with the laws of the State of New York, exclusive of conflict or choice-of-law rules, except to the extent there may be any conflict between the laws of the State of New York and the Incoterms of the International Chamber of Commerce, 2010 edition, in which case the Incoterms shall be controlling. The parties specifically agree that the 1980 United Nations Convention on Contracts for the International Sale of Goods, as may be amended from time to time, shall not apply to this Agreement. The parties hereby consent to the personal and exclusive jurisdiction and venue of the New York state courts and the federal courts located in New York, New York.
Section 18.13 Any controversy, claim, or dispute arising out of or relating to this order and not resolved by agreement of the parties shall be resolved in Arbitration in New York, New York. The decision and award of the arbitrator(s) shall be final and binding and may be entered in any court having jurisdiction thereof. The parties will pay their respective attorneys’ fees and equally share all other costs and expenses of the arbitration proceedings.
Section 18.14 Notwithstanding the foregoing, except with respect to enforcing claims for injunctive or equitable relief, any dispute, claim or controversy arising out of or relating in any way to this Agreement, any other aspect of the relationship between VTM and OptiNose or their respective affiliates and subsidiaries, the interpretation, application, enforcement, breach, termination or validity thereof (including, without limitation, any claim of inducement of this Agreement by fraud and a determination of the scope or applicability of this agreement to arbitrate), or its subject matter (collectively, “Disputes”) shall be determined by binding arbitration before one arbitrator. The arbitration shall be administered by JAMS conducted in accordance with the expedited procedures set forth in the JAMS Comprehensive Arbitration Rules and Procedures as those Rules exist on the Effective Date of this Agreement, including Rules 16.1 and 16.2 of those Rules. Notwithstanding anything to the contrary in this Agreement, the Federal Arbitration Act shall govern the arbitrability of all Disputes. The arbitration shall be held in New York, New York and it shall be conducted in the English language. The parties shall maintain the confidential nature of the arbitration proceeding and any award, including the hearing, except as may be necessary to prepare for or conduct the arbitration hearing on the merits, or except as may be necessary in connection with a court application for a
33
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
preliminary remedy, a judicial challenge to an award or its enforcement, or unless otherwise required by law or judicial decision. The arbitrator shall have authority to award compensatory damages only and shall not award any punitive, exemplary, or multiple damages and the parties waive any right to recover any such damages. Judgment on any award in arbitration may be entered in any court of competent jurisdiction. Notwithstanding the above, each party shall have recourse to any court of competent jurisdiction to enforce claims for injunctive and other equitable relief.
Section 18.15 IN THE EVENT OF ANY DISPUTE BETWEEN THE PARTIES, WHETHER IT RESULTS IN PROCEEDINGS IN ANY COURT IN ANY JURISDICTION OR IN ARBITRATION, THE PARTIES HEREBY KNOWINGLY AND VOLUNTARILY, AND HAVING HAD AN OPPORTUNITY TO CONSULT WITH COUNSEL, WAIVE ALL RIGHTS TO TRIAL BY JURY, AND AGREE THAT ANY AND ALL MATTERS SHALL BE DECIDED BY A JUDGE OR ARBITRATOR WITHOUT A JURY TO THE FULLEST EXTENT PERMISSIBLE UNDER APPLICABLE LAW. To the extent applicable, in the event of any lawsuit between the parties arising out of or related to this Agreement, the parties agree to prepare and to timely file in the applicable court a mutual consent to waive any statutory or other requirements for a trial by jury.
Section 18.16 Each party acknowledges that a breach by a party of Article IX (Intellectual Property Rights; Ownership) or Article X (Confidentiality) may cause the non-breaching party irreparable damages, for which an award of damages would not be adequate compensation and agrees that, in the event of such breach or threatened breach, the non-breaching party will be entitled to seek equitable relief, including a restraining order, injunctive relief, specific performance and any other relief that may be available from any court, in addition to any other remedy to which the non-breaching party may be entitled at law or in equity. Such remedies shall not be deemed to be exclusive but shall be in addition to all other remedies available at law or in equity, subject to any express exclusions or limitations in this Agreement to the contrary.
Section 18.17 In the event that any action, suit, or other legal or administrative proceeding is instituted or commenced by either party hereto against the other party arising out of or related to this Agreement, the prevailing party shall be entitled to recover its reasonable attorneys’ fees and court costs from the non-prevailing party.
Section 18.18 This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall be deemed to be one and the same agreement. A signed copy of this Agreement delivered by e-mail or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Agreement.
Section 18.19 [***].
[Signature Page Follows]
34
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
ADVANCE MOLD & MANUFACTURING, INC. By: /s/ Xxxxx Xxxxxxxxxxx Name: Xxxxx Xxxxxxxxxxx Its: General Manager | OPTINOSE US, INC. By: /s/ Xxxxx Xxxxxx Name: Xxxxx Xxxxxx Its: Chief Executive Officer |
OPTINOSE UK LTD. By: /s/ Xxxxx Xxxxxxx Name: Ricci Whitlow Its: VP, Tech Ops | |
OPTINOSE AS By: /s/ Xxxxx Xxxxxx Name: Xxxxx Xxxxxx Its: Chief Executive Officer | |
35
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT A
PRICING
[***]
***************
36
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT B
37
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
OPTINOSE COMPONENTS
[***]
38
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT C
OPTINOSE COMPONENTS WITH ADDITIONAL STORAGE TIME
[***]
39
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT D
ESCALATION MATRIX
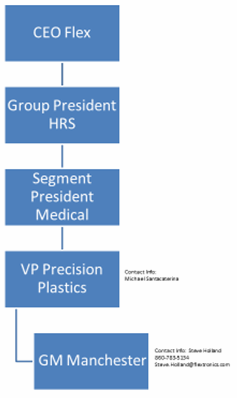
40
[***] Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
41

