Confidential information redacted and filed separately with the Commission. Omitted portions indicated by [***] CONTRACT MANUFACTURING AND PACKAGING AGREEMENT
Exhibit 10.16
Confidential information redacted and filed separately with the Commission.
Omitted portions indicated by [***]
CONTRACT MANUFACTURING AND PACKAGING AGREEMENT
This Agreement (the “Agreement”), dated this 18 day of April, 2008, is between Annie’s Homegrown, Inc. (“Customer”), and Harmony Foods Corp (dba Santa Xxxx Nutritionals), a Delaware corporation (“Manufacturer”).
RECITALS
Customer desires Manufacturer to manufacture certain products identified on Exhibit A hereto and made a part hereof (the “Products”) to the specifications provided by Customer and under the terms and conditions described below, for sale by Customer in the Stores under the trademarks and trade names owned by Customer and identified on Exhibit B hereto and made a part hereof (the “Marks”).
AGREEMENT
In consideration of the foregoing and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1. Manufacture of Products.
(a) Manufacturer shall manufacture, label, package, store, and ship to Customer at the location Customer specifies the Products set forth in Exhibit A in accordance with good manufacturing practices prevailing in the industry and in strict compliance with the terms of this Agreement and the specifications, manufacturing process and quality control standards set forth in Exhibit C (as amended from time to time, the “Specifications”).
(b) Customer will provide Manufacturer with a forecast and access to Customer’s inventory information. Manufacturer will schedule production and order materials accordingly. Upon receipt of Manufacturer’s production schedule, Customer will issue a purchase order to Manufacturer. Manufacturer will ship the Products in the sizes and with the packaging specified, and shall label the Products with the Marks, using the labels specified by Customer, as provided in Section 4 below.
(c) The Parties agree that specific certified organic recipes and/or formulas for fruit snacks to be used by the Customer will remain exclusive to the Customer in the bunny shape and will not be offered by the Manufacturer to Whole Foods. Customer acknowledges that Manufacturer has and will continue to produce similar recipes and/or formulas for other customers. Manufacturer agrees to produce the unique die shapes as identified in Exhibit A solely and exclusively for the Customer.
2. Orders; Cancellations.
(a) The parties acknowledge that all Products will be ordered in such minimum quantities specified for each Product on Exhibit A by Customer or a Distributor in writing on a purchase order form either provided or approved by Manufacturer.
Confidential Treatment Requested
(b) Cancellations without charge may be made in writing by Customer or Distributor not less than 20 days prior to the date upon which the Products are labeled with the Marks. No cancellations may be made after Products have been labeled with the Marks.
(c) Customer acknowledges that it will accept actual conforming order quantities of +[***]%/-[***]% from accepted purchase order quantities. All Manufacturer invoices will reflect actual order quantities.
(d) Customer may reject and refuse to pay for Products within [***] days of delivery which (i) do not fully comply with the Specifications, (ii) have been damaged during storage or handling while the Products were in Manufacturer’s custody, or (iii) are not in compliance with the terms and conditions of this Agreement.
(e) Customer may reject and refuse to pay for Products which have been produced and packaged during a particular production run if quality assurance samples from that production run do not conform to the Specifications or are otherwise not in compliance with the terms and conditions of this Agreement.
(f) Risk of damage or loss to Products shall remain with Manufacturer until the same is received by Customer or a facility or consignee designated by Customer in accordance with the terms and conditions of this Agreement. All freight claims will be handled by the Manufacturer.
3. Compensation.
(a) The price per case for each Product is set forth on Exhibit A hereto. The price per case does includes the cost for GMA #1, soft wood pallets.
(b) Shipping costs to the designated destinations are not included in the listed price, but shall be handled on a prepaid basis by the [***]. Shipping shall be by rail to customer’s main distribution center in IL.
(c) All Products shall be shipped and invoiced to Customer or Distributor as specified in the applicable purchase order.
(d) Payment for orders shall be net [***] days from the invoice date. Invoices shall specify the Products purchased, the quantities, the stock numbers, and the shipping charges for each order.
4. Labeling.
(a) Customer hereby grants Manufacturer a limited, non-exclusive, non-transferable license as to the Marks for the purpose of allowing Manufacturer to perform its obligations under this Agreement. Manufacturer agrees not to use, directly or indirectly, any other trademarks that are colorable imitations of or confusingly similar to the Marks. Manufacturer agrees that it shall not register the Marks listed on Exhibit B (including any colorable imitations, translations, or transliterations thereof) or participate directly or indirectly in such registration without Customer’s prior written consent. Manufacturer further acknowledges and agrees that if it
| Confidential Information Redacted |
Confidential Treatment Requested
has obtained or obtains in the future, in any country, a right, title, or interest in the Marks (including any colorable imitations, translations, or transliterations thereof), or in any other trademark or service xxxx owned by Customer, Manufacturer has acted or will act as an agent for the benefit of Customer for the limited purpose of obtaining such registrations and assigning them to Annie’s Homegrown, Inc. Furthermore, Manufacturer agrees that it shall immediately notify customer of any potential infringements of the Marks as it shall become aware. Manufacturer also agrees that it will not directly or indirectly (by causing others or otherwise) use or take any action challenging or opposing, or raise any questions concerning, the validity of the Marks. The design costs and all costs for prepress chares, including color separations and charges for preparing plates and all other costs related to developing the Marks shall be paid by Customer to the provider of such services. Customer shall, subject to the size specification provided by Manufacturer, provide Manufacturer with the design and color specification for the label for each Product covered by this Agreement and for each Xxxx to be used on the packaging for each Product
(b) Manufacturer shall be responsible for ensuring compliance with the labeling requirements of the Federal Food, Drug, and Cosmetic Act and other applicable federal and state food labeling laws and regulations.
(c) Manufacturer shall be responsible for ordering adequate supplies of labels and other packaging materials on behalf of Customer based on forecasts as provided by the Customer. Prior to placing any order for labels or packaging materials, Manufacturer shall deliver to Customer a recommendation as to the quantity of packaging to be ordered based on forecasts as provided by the Customer for Customer’s approval or modification. Manufacturer shall not place any order for labels or packaging materials without Customer’s prior written approval. Customer shall be responsible for any unused labels or packaging materials due to marketing formulation changes, not hitting sales forecast or item discontinuation. Manufacturer shall order [***] impressions of each film item and [***] of each carton.
(d) Customer shall apply for and obtain, at Customer’ cost, UPC codes required for labeling of the Products with the Marks. Customer shall supply the UPC codes to Manufacturer for printing on the labels used for the Products.
(e) Nothing herein is deemed to grant to Manufacturer any right or license to use the Marks for any purpose other than the labeling of Products for sale by Customer as provided in this Agreement.
5. Representations and Warranties.
(a) Manufacturer hereby represents to Customer that:
(i) Manufacturer has the full legal right, power and authority to enter into this Agreement.
(ii) This Agreement is the legal, valid, and binding obligation of Manufacturer, enforceable against Manufacturer in accordance with its terms, except as enforceability may be limited by bankruptcy, insolvency, or other similar laws of general application or by general principles of equity.
| Confidential Information Redacted |
Confidential Treatment Requested
(iii) All Products shall be manufactured and packaged in accordance with the Specifications and good manufacturing practices prevailing in the industry, the applicable provisions of the Hazard Analysis and Critical Control Point food safety program (“HAACP”), and as required by state or federal authorities. Manufacturer shall promptly notify Customer of any noncompliance with such practices or provisions. If Manufacturer learns of any condition that raises the possibility of finished Products being adulterated or misbranded within the meaning of any federal, state or local law, Manufacturer shall notify Customer within 24 hours of first notice. Manufacturer agrees to cooperate fully with Customer and to provide all information necessary for Customer to make a determination as to whether a product recall or market withdrawal is necessary. Manufacturer has provided to Customer a copy of its current Recall Policy and shall promptly provide to Customer all updates or amendments to such policy during the term of this Agreement.
(iv) All manufacturing and packaging of the Products shall be conducted in a clean and sanitary environment.
(v) All Products shall be merchantable and fit for human consumption.
(vi) Consistent with the terms of Section 4, Manufacturer has in place appropriate procedures to assure that the Product labels are compliant with applicable federal and state requirements and that the correct label is applied to each Product.
(vii) All products identified as being “organic” shall be produced in accordance with the National Organic Program, the organic food regulations adopted pursuant to the Federal Organic Foods Production Act, and applicable certifying bodies.
(viii) Manufacturer is not subject to, nor is it aware of any pending or threatened order, injunction, enforcement action or other proceeding by any local, state or federal governmental agency regarding the manufacturing processes, storage conditions, or purity of any products produced by Manufacturer.
(ix) Manufacturer shall make available to Customer, at Customer’s request, the results of all federal, state and local inspection reports and sanitation audits conducted from [***] days before to [***] days after the Term of this Agreement and relating to or affecting (1) Manufacturer’s facilities, or (2) equipment, raw materials, ingredients, packaging materials, labeling, work in process or Products located therein. Manufacturer shall not disclose information in such audits relating to other Customer materials or ingredients. Manufacturer shall immediately notify Customer of any such inspections or audits or any other information that indicates the presence of any bacteriological agent or any substance considered by health authorities as being indicative of either unsanitary practices or of public health concern.
(x) Manufacturer shall submit to Customer such quality control records and reports as are reasonably requested by Customer. Manufacturer shall retain Products from each production run for the full shelf life of the Product plus six (6) months. Manufacturer shall send production samples to Customer upon request at any time and at Customer’s cost. Manufacturer shall permit Customer to visit Manufacturer’s facility at reasonable internals and upon reasonable notice during regular business hours to observe manufacturing and storage activities with respect to the Products.
| Confidential Information Redacted |
Confidential Treatment Requested
(xi) The signing and delivery of this Agreement by Manufacturer and the performance by Manufacturer of all of Manufacturer’s obligations under this Agreement will not breach any agreement to which Manufacturer is a party, or give any person the right to accelerate any obligation of Manufacturer; violate any law, judgment, or order to which Manufacturer is subject; or require the consent, authorization, or approval of any person, including but not limited to any governmental body.
(b) Customer hereby represents to Manufacturer that:
(i) Customer has the full legal right, power and authority to enter into this Agreement.
(ii) Customer (or its affiliates) is the exclusive owner of the Marks, that it has the right to grant the non-exclusive license described above, that it has not granted or agreed to grant any assignment, license, right or privilege which conflicts with the express provisions of this Agreement.
(iii) This Agreement is the legal, valid, and binding obligation of Customer, enforceable against Customer in accordance with its terms, except as enforceability may be limited by bankruptcy, insolvency, or other similar laws of general application or by general principles of equity.
(iv) The signing and delivery of this Agreement by Customer and the performance by Customer of all of Customer’s obligations under this Agreement will not breach any agreement to which Customer is a party, or give any person the right to accelerate any obligation of Customer; violate any law, judgment, or order to which Customer is subject; or require the consent, authorization, or approval of any person, including but not limited to any governmental body.
6. Covenant
Manufacturer covenants and agrees as follows:
(a) Manufacturer shall provide Customer with Products of consistent quality composed of safe and wholesome ingredients, manufactured, labeled, packaged, stored, and shipped under conditions compliant with all applicable federal, state, and local requirements including but not limited to applicable laws, regulations, and guidelines adopted by (i) the Food and Drug Administration (“FDA”) pursuant to the Federal Food, Drug, and Cosmetic Act, as amended (the “Act”) and the Public Health Security and Bioterrorism Preparedness and Response Act (the “Bioterrorism Act”), including but not limited to the food safety, composition, labeling, registration, and manufacturing provisions and current industry good manufacturing practices; (ii) the United States Department of Agriculture (“USDA”) and the Food Safety Inspection Service (“FSIS”), including but not limited to the National Organic Program and the organic food regulations adopted pursuant to the Federal Organic Foods Production Act; and (iii) applicable state and local authorities responsible for regulating the manufacture, storage, and shipment of food products and establishments, including without limitation, the California Organic Foods Act, as amended, and all applicable organic food certification programs.
Confidential Treatment Requested
(b) Manufacturer shall follow the Specifications provided in Exhibit C for Products identified in Exhibit A and shall follow applicable laws, rules, and regulations, in its purchasing, manufacturing, labeling, packaging, storing, and shipping of the Products. (i) All finished Products and all raw materials, ingredients, processing aids, and packaging material (collectively, “Product Supplies”) (a) shall be stored and shipped under sanitary conditions, in strict compliance with all federal, state, and local laws, rules, regulations, and guidelines including applicable current Good Manufacturing Practice and any other applicable FDA, USDA, or FSIS guidelines and regulations; (b) shall be manufactured, labeled, and packaged in strict compliance with all federal, state, and local laws, rules, regulations, and guidelines, including current Good Manufacturing Practice, current industry practices, and FDA and USDA standards of identity; (c) shall be wholesome, merchantable, fit for their intended purpose, and fit for human consumption consistent with the Specifications, current Good Manufacturing Practice, industry practices, and applicable laws, regulations, and requirements. (ii) All finished Products shall be packaged in accordance with Specifications and Manufacturer warrants that it has procedures in place to assure the finished Products will be labeled in a manner consistent with the Specifications and federal, state, and local requirements.
(c) All Products that are manufactured, labeled, packaged, stored, and shipped pursuant to this Agreement for Customer, and all packaging and other materials that come into contact with such Products, will not at the time of shipment to Customer, or any designated consignee, be adulterated, contaminated, or misbranded within the meaning of the Act or any other federal, state, or local law, rule, or regulation, and that such Products, packaging and other materials shall not constitute articles prohibited from introduction into interstate commerce under the provisions of Sections 301, 402, 403, 404, 405, 409, or 505 of the Act, and Manufacturer also specifically warrants that it will register and fully comply with all applicable requirements under the Bioterrorism Act and the FDA’s implementing regulations.
(d) Manufacturer shall provide at least [***] days prior written notice of its intent to modify the recipe, ingredients or specifications of any Product prior to implementing any changes. Customer may object to any such proposed modification by delivering written notice of such objection within [***] days of receipt of notice of the proposed modification. If Customer does not object to such proposed modification as provided herein, Customer will be deemed to have consented to such modification. Whether Customer has consented to such modification or shall be deemed to have consented to such modification, Customer shall own all right, title and interest in such modification.
(e) If no agreement is reached allowing the requested modification to the Product within [***] days of Manufacturer’s receipt of Customer’s objection thereto, then Customer shall have the right to terminate this Agreement solely with respect to the particular product or products that are the subject of the proposed modification.
(f) All of the above covenants of quality and conformance with applicable federal, state, and local requirements and industry manufacturing standards shall apply equally to Products enumerated in Exhibit A and any modifications thereto anticipated by Section 6(d).
| Confidential Information Redacted |
Confidential Treatment Requested
(g) Manufacturer shall provide written notice of shortages of ingredients the absence of which would decrease Product availability immediately upon learning of such shortages.
(h) All books and records maintained or retained pursuant to this Agreement shall be retained by Manufacturer for a period of at least [***] years, or longer if so required by federal, state, or local laws, rules, or regulations.
7. Indemnification.
(a) Manufacturer shall indemnify and hold harmless Customer, its officers, directors, officers, agents and employees from and against any and all damages, losses, liabilities, claims, suits, costs and expenses (including attorney fees) (collectively, “Claims”) resulting from or relating to any breach by Manufacturer of any provision, warranty or covenant, or any non-fulfillment of any obligation by Manufacturer, under this Agreement. Manufacturer further agrees to indemnify and hold harmless Customer and its officers, directors and agents, from and against any and all damages, loss, cost, liability or expense (including attorney fees and costs) incurred by any such party in connection with any complaints, demands, claims, or legal actions alleging illness, injury, death, or damage as a result of the consumption or use of any Product; provided that the Manufacturer shall not be required to indemnify Customer against a defect or defects in the Product that independent investigation shows originated after the Product left the custody of the Manufacturer.
(b) Customer agrees to defend, indemnify and hold harmless Manufacturer, its directors, officers, agents and employees from and against any and all damages, losses, liabilities, claims, suits, costs and expenses (including attorney fees) (collectively, “Claims”) resulting from or relating to any breach by Customer of any provision, warranty or covenant, or any nonfulfillment of any obligation by Customer, under this Agreement. Customer further agrees to indemnify and hold harmless Manufacturer and its officers, directors and agents, from and against any and all damages, loss, cost, liability or expense (including attorney fees and costs) incurred by any such party in connection with any complaints, demands, claims, or legal actions alleging illness, injury, death, or damage as a result of the consumption or use of any Product that independent investigation shows was caused by a defect in the Product that originated after the Product left the custody of Manufacturer.
(c) If either party becomes aware of any incident involving potential contamination of any Product sold hereunder, the party shall provide immediate telephone notice to the other party, and the parties shall cooperate with each other to identify and remove from sale any Products suspected of contamination; provided, however, that Customer reserves the right to direct any such investigation and to determine the actions to be taken in response to the investigation.
8. Term.
(a) This Agreement shall run for a period of one (1) year from the date hereof. This Agreement shall renew automatically each year for a period of one (1) year unless either party provides written notice of non-renewal to the other party at least thirty (30) days prior to the expiration of the then-current term.
| Confidential Information Redacted |
Confidential Treatment Requested
(b) Manufacturer may terminate this Agreement immediately:
(i) If Customer fails to make any payment due under this Agreement and such nonpayment continues 10 days after written notice from Manufacturer that any payment due hereunder is more than 30 days late;
(ii) If Customer fails to perform any other obligation under this Agreement within 15 days of notice from Manufacturer specifying such failure, or if such failure cannot be cured within such 15-day period, then Customer shall not be in default hereunder so long as Customer commences cure within such 15-day period; or
(iii) If Customer becomes insolvent, a receiver is appointed to the possession of all or substantially all of Customer’s property, Customer makes a general assignment for the benefit of creditors or files a voluntary petition in bankruptcy, or Customer is the subject of an involuntary petition in bankruptcy and such involuntary petition is not dismissed within one hundred twenty (120) days of filing.
(c) Customer may terminate this Agreement immediately:
(i) If Manufacturer fails to perform or meet any material term or condition hereof and has failed to correct same within 30 days after written notice of such failure by Customer;
(ii) If Manufacturer or its agents or representatives, without Customer’s consent, has adulterated any Products or has substituted or added, with respect to any instruction, specification, formula, manufacturing process or quality control standard or any procedure set forth in this Agreement or any exhibit hereto, an ingredient, component, process or procedure not called for thereby, or has altered or omitted an ingredient, component, process or procedure called for thereby (provided that, for avoidance of doubt, no cure period shall be required prior to termination under this provision);
(iii) If Manufacturer ceases to do business as a going concern or ceases to conduct its operations in the normal course of business, becomes insolvent, a receiver is appointed to the possession of all or substantially all of Manufacturer’s property, Manufacturer makes a general assignment for the benefit of creditors or files a voluntary petition in bankruptcy, or Manufacturer is the subject of an involuntary petition in bankruptcy and such involuntary petition is not dismissed within one hundred twenty (120) days of filing; or
(iv) At any time after the initial one-year term of this Agreement upon 60 days’ written notice to Manufacturer of Customer’s election to terminate this Agreement.
(d) Following any termination, (i) Customer shall take delivery of all finished Products previously identified to this Agreement by Manufacturer, and shall pay for same in accordance with this Agreement, (ii) Customer shall have the right to sell all remaining stock of Products in Customer’s possession, (iii) Customer shall reimburse Manufacturer for
Confidential Treatment Requested
Manufacturer’s actual cost for any unused labels and other packaging materials purchased in accordance with Section 4(c), and (iv) Customer shall reimburse Manufacturer for Manufacturer’s actual cost for any unused raw materials or ingredients ordered specifically for Products manufactured solely for Customer, to the extent that such raw materials or ingredients are not otherwise used by Manufacturer in its operations.
9. Confidentiality.
(a) “Confidential Information” means this Agreement and all confidential or otherwise proprietary business and technical information relating to the Parties and their respective businesses, including, without limitation, ideas, know-how, trade secrets, production, manufacturing and sales techniques, financial statements and data, recipes and formulas, sources of supply, advertising, actual and prospective customers, pricing, costing, and accounting procedures. Confidential Information does not include information that is in the public domain at the time of disclosure by the disclosing Party; that enters the public domain after disclosure by the disclosing Party through no fault of the receiving Party; that was or is separately disclosed to the receiving Party by a third party not itself subject to an obligation of confidentiality to the disclosing Party with respect to such information; or that was in the receiving Party’s possession at the time of disclosure by the disclosing Party.
(b) Each Party agrees to maintain the Confidential Information in strict confidence and, except to the extent expressly permitted in this Agreement or otherwise consented to in writing by the other Party, that the Confidential Information will not be disclosed by it or its “Representatives” (defined to include affiliates, directors, shareholders, officers, employees, agents, subcontractors, consultants, members, managers, advisors, or other representatives including legal counsel, accountants and, in the case of Customer, its Distributors) to any “Person” (defined to include individuals, partnerships, companies, limited liability companies, entities, corporations, or agents thereof) except with the specific prior written consent of the other.
(c) Both Parties agree that during the term of this Agreement and for a period of one (1) year after the termination hereof, unless otherwise agreed by the Parties, that each party and its employees and agents shall not contact, solicit, seek or in any way enter into an employment relationship with any employee of the other party as of the date of termination.
10. Insurance.
Manufacturer shall maintain commercial general liability insurance (including products liability and contractual liability), with limits of not less than $[***] combined single limit, for bodily injury or death to any person or persons and loss or damage to any property. Such insurance shall be written by an insurance carrier reasonably acceptable to both parties, and shall name the Customer as an additional insured. Its terms and conditions shall not be materially changed, altered (unless to increase coverage) or canceled until ten days after termination or cancellation of this Agreement; provided, however, that in the event of Manufacturer’s cancellation of insurance, Manufacturer shall be obligated to obtain tail coverage suitable to Customer. A certificate of such insurance coverage shall be furnished to the Customer upon execution of this Agreement and thereafter upon request.
| Confidential Information Redacted |
Confidential Treatment Requested
11. Miscellaneous.
(a) Relationship of Parties. Manufacturer and Customer are independent contractors for the purpose of this Agreement. Neither the execution, delivery nor performance of this Agreement will be construed to constitute either party as an agent or representative of the other for any purpose. Neither the execution, delivery nor performance of this Agreement will be deemed to establish a joint venture or partnership between the Parties. Except as otherwise provided herein, neither Party has the authority to (i) bind the other Party by or to any contract, representation, understanding, act or deed, (ii) represent that either Party is an agent of the other Party, or (iii) represent that either Party is responsible for the acts or omissions of the other Party.
(b) Impossibility. The Parties shall not be responsible for any failure to perform due to unforeseen circumstances or causes beyond their reasonable control, including but not limited to acts of God, war, riot, embargoes, acts of civil or military authorities, fires, floods, accidents, strikes, or shortages of transportation, facilities, fuel, energy, labor, or materials. In the event of any such delay, the Parties may defer performance hereunder for a period equal to the time of such delay.
(c) Severability. If any provision of this Agreement shall be prohibited or unenforceable by any applicable law, the provision shall be ineffective only to the extent and for the duration of the prohibition or unenforceability, without invalidating any of the remaining provisions.
(d) Waiver. The temporary, limited, or specific waiver of any term, provision, or condition of this Agreement or a breach thereof will not be considered a waiver of any other term, provision, or condition, or of any subsequent breach of the same term, provision, or condition.
(e) Entire Agreement. This Agreement embodies the entire understanding of the Parties and shall supersede all previous communications, representations or understandings either oral or written between the Parties relating to the subject matter hereof.
(f) Assignability. This Agreement shall be binding upon and be for the benefit of the Parties and their legal representatives, successors, and assigns. Neither party may assign this Agreement without the prior written consent of the other.
(g) Choice of Laws. This Agreement shall be interpreted and construed in accordance with the laws of the state of Oregon, without giving effect to choice of law rules. The Parties consent to jurisdiction and venue in the state and federal courts located in Multnomah County, Oregon.
(h) Notice. All notices, bills and payments shall be made in writing and may be given by personal delivery, via overnight courier requiring a signature for delivery, or by certified or registered mail, return receipt requested. Notices, bills and payments sent by mail should be addressed as follows:
| Customer: | Annie’s Homegrown, Inc. |
| 000 Xxxxxxx Xxxxx |
| Xxxx, XX 00000 |
| Attn: Xxxx Xxxxxxx, President |
| Manufacturer: |
Confidential Treatment Requested
and when so addressed shall be deemed given 5 days after deposited in the U.S. mail, first class, postage prepaid, and postmarked. In all other instances notices, bills, and payments shall be deemed given at the time of actual delivery. Changes may be made in the names and addresses of the person to whom notices, bills, and payments are to be given by giving notice pursuant to this section.
(i) Construction. Section headings are included for convenience, but shall not form a part of the Agreement or affect the interpretation of any part hereof. The word “including” is used in this Agreement in a non-exclusive sense and, unless otherwise expressly set forth, shall be interpreted as being illustrative and not limiting.
(j) Expenses. Each party shall bear its own expenses.
(k) Signatures. This Agreement may be signed in counterparts. A fax transmission of a signature page will be considered an original signature page. At the request of a party, the other party will confirm a fax-transmitted signature page by delivering an original signature page to the requesting party.
(l) Amendment. This Agreement may be amended only by a written document signed by the party against whom enforcement is sought.
| MANUFACTURER: | CUSTOMER: | |||||||
| SANTA XXXX NUTRITIONALS | ANNIE’S HOMEGROWN, INC. | |||||||
| By: | /s/ Xxxxxxx Xxxxxxxxxx | By: | /s/ Xxxxxx Xxxxxxx | |||||
| Name: | Xxxxxxx Xxxxxxxxxx | Name: | Xxxxxx Xxxxxxx | |||||
| Its: | CEO | Its: | COO | |||||
Confidential Treatment Requested
EXHIBIT A
Product Description
General Description: [***].
Ingredient Description: [***].
Two Items: [***]
Packaging Configuration:
[***]
[***]
Lead Time:
[***]
Pricing:
[***]
[***]
Minimum Order Quantity: [***].
| Confidential Information Redacted |
Confidential Treatment Requested
EXHIBIT B
TRADEMARKS AND TRADENAMES
| No. |
Country | Trademark: |
Application Number |
Goods |
Status | |||||
| 1. | US | NO WEIRD STUFF | 78/937,047 | International Class 30: Cereal, namely breakfast and processed cereals | Pending | |||||
| 2. | US | NO ARTIFICIAL ANYTHING | 77/008,539 | International Class 30: Cereal, namely breakfast and processed cereals | Pending | |||||
| 3. | US | ANNIE’S HOMEGROWN | 77/068,363 | International Class 30: Breakfast cereal | Pending | |||||
| 4. | US | BUNNY DESIGN | 77/068,358 | International Class 30: Breakfast cereal | Pending | |||||
| 5. | US | BUNNY LOVE | 77/112,092 | International Class 30: Breakfast cereal | Pending | |||||
| 6. | US | HEART DESIGN | 77/112,097 | International Class 30: Breakfast cereal | Pending | |||||
| 7. | US | BUNNY LOVE (and heart design) | 77/112,094 | International Class 30: Breakfast cereal | Pending | |||||
| 8. | US | HONEY BUNNIES | 77/112,103 | International Class 30: Breakfast cereal | Pending | |||||
| 9. | US | FLOWER DESIGN | 77/112,100 | International Class 30: Breakfast cereal | Pending | |||||
| 10. | US | CINNABUNNIES | 77/112,102 | International Class 30: Breakfast cereal | Pending | |||||
| 11. | US | BUNNY DESIGN | 77/112,101 | International Class 30: Breakfast cereal | Pending | |||||
| 12. | US | ANNIE’S | 77/184,917 | International Class 30: Breakfast cereal | Pending | |||||
| 13. | US | BREAKFAST WITH XXXXXX | 77/184,912 | International Class 30: Breakfast cereal | Pending | |||||
| 14. | US | NO ICKY ADDITIVES OR PESKY PRESERVATIVES | 77/184,906 | International Class 30: Breakfast cereal | Pending |
Confidential Treatment Requested
EXHIBIT C
SPECIFICATIONS
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested
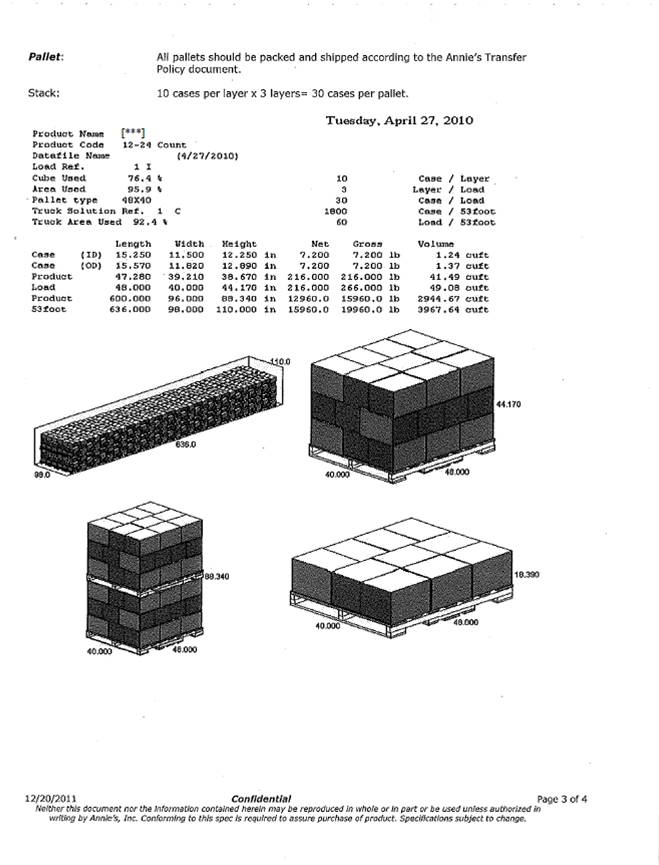
| Confidential Information Redacted |
Confidential Treatment Requested

| Confidential Information Redacted |
Confidential Treatment Requested

