Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Asterisks denote omissions. BISx INTERNATIONAL LICENSE AGREEMENT
Exhibit 10.3
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Asterisks denote omissions.
Securities and Exchange Commission. Asterisks denote omissions.
THIS Agreement is made and entered into as of April 14, 2009 (the “Effective Date”), by and between
ASPECT MEDICAL SYSTEMS, INC. (“Aspect”), a Delaware, U.S.A. corporation having offices at ▇▇▇
▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇.▇.▇. and Philips Medizin Systeme Böblingen GmbH with
offices at ▇▇▇▇▇▇▇-▇▇▇▇▇▇▇-▇▇▇▇▇▇▇ ▇, ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ (“Philips”).
WHEREAS, Aspect possesses certain intellectual and industrial property rights;
WHEREAS, Aspect is willing to grant, and Philips desires to acquire, non-exclusive worldwide
rights to use such rights in accordance with the terms and conditions hereinafter set forth; and
WHEREAS, Aspect and Philips wish to provide Philips customers with a more effective supply of
BIS Sensors (as defined below) at competitive prices.
NOW, THEREFORE, in consideration of the premises and mutual promises, terms and conditions
hereinafter set forth, and other good and valuable consideration, the receipt and sufficiency of
which are hereby acknowledged, Aspect and Philips (the “Parties”) do hereby agree as follows:
| 1. | DEFINITIONS |
As used herein, the following terms shall have the following definitions.
| 1.1 | Affiliates. “Affiliates” of a Party hereto shall mean companies which are controlled by, control or are under common control with such Party. A company shall be considered an “Affiliate” for only so long as such control exists. For the purposes of this definition, partnerships or similar entities where a majority-in-interest of its partners or owners are a Party hereto and/or Affiliates of such Party shall also be deemed to be Affiliates of such Party. | ||
| 1.2 | Agreement Term. “Agreement Term” shall mean the period beginning on the Effective Date and ending on the date of termination or expiration of this Agreement, as the case may be. | ||
| 1.3 | Aspect Products. “Aspect Products” shall mean those products available for purchase by Philips and its Affiliates as set forth in Exhibit A (Aspect Products and Purchase Prices). | ||
| 1.4 | BIS. “BIS” shall mean the Bispectral Index™ which is Aspect’s proprietary processed EEG parameter that directly measures the hypnotic effects of anesthetic and sedative agents on the brain. | ||
| 1.5 | BIS Engine. “BIS Engine” shall mean Aspect’s processing unit for deriving the BIS from the raw EEG signal. |
| 1.6 | BIS Product. “BIS Product” shall mean the BISx System and/or the BIS Engine, as the case may be. | ||
| 1.7 | BIS Ready. “BIS Ready” shall mean a Philips Patent Monitor with (a) a BIS port standard on the monitor ready to accept a BISx System, with BIS software installed on the host monitor, or (b) at least one available multi-connector port if such a dedicated BIS port is not utilized. | ||
| 1.8 | BIS Sensor. “BIS Sensor” shall mean a single use disposable or semi-reusable sensor manufactured by Aspect for use with the BISx System or the BIS Engine/DSC-XP in the OR, ICU and other sedation locations that is required to generate Aspect’s Bispectral Index. These sensors include the BIS Quatro Sensor, the BIS Extend Sensor and the BIS Pediatric Sensor. | ||
| 1.9 | BISx. “BISx” shall mean a device that acquires up to two channels of EEG and computes BIS and other EEG parameters, uniting the functionality of the existing Aspect’s BIS Engine and DSC-XP. BISx shall include enhancements and new features relating to consciousness monitoring that are made generally available by Aspect during the term of this Agreement. | ||
| 1.10 | BISx System. “BISx System” shall mean the system comprised of a BISx device with a Monitor Cable and a Patient Interface Cable (“PIC”) designed for use with the Philips Patient Monitor and is also known, including in Exhibit A, as a “BISx Kit”. | ||
| 1.11 | Business Day. “Business Day” shall mean a day on which Aspect’s offices are open for business in Norwood, Massachusetts, U.S.A., and on which Philips’ offices are open for business in Boeblingen, Germany. | ||
| 1.12 | Contract Year. “Contract Year” shall mean the 12-month period commencing on the Effective Date, and then each 12-month period thereafter. | ||
| 1.13 | Documentation. “Documentation” shall mean any and all user manuals, programmer’s guides, configuration guides, circuit diagrams, and help files related to the Software. | ||
| 1.14 | DSC-XP. “DSC-XP” shall mean Aspect’s digital signal converter using XP technology. The DSC-XP is a small box that is kept close to the patient that converts the analog EEG signals to digital signals for processing the BIS value. | ||
| 1.15 | Intellectual Property Rights. “Intellectual Property Rights” shall mean all forms of protection afforded by law to inventions, models, designs or technical information, and applications therefor or which otherwise arise or are enforceable under the laws of the United States, any other jurisdiction or any bi-lateral or multi-lateral treaty regime, including, but not limited to, any and all patents and patent applications (including additions, reissues, divisionals, continuations, continuations-in-part, substitutions, re-examinations, patent term extensions, registrations, supplemental protection term certificates, and renewals |
| of any of the foregoing), utility models, trademarks, trade secrets, registered and unregistered designs including mask works, copyrights, and moral rights. |
| 1.16 | IntelliVue Clinical Network. “IntelliVue Clinical Network” shall mean the software that enables wireless and wire line communications transmission of physiologic measurement data between Philips IntelliVue bedside monitors, central stations, applications servers, database servers and telemetry devices. | ||
| 1.17 | Licensed Technology. “Licensed Technology” shall mean the Aspect Products, the Software, Documentation and the Technical Information. | ||
| 1.18 | License Term. “License Term” shall mean the period beginning on the Effective Date and ending on the date of termination or expiration of this Agreement, as the case may be. | ||
| 1.19 | Material Product Change. “Material Product Change” shall mean any of the following changes to a BIS Product or any component thereof: |
| (a) | BIS Product or service design changes that affect regulatory compliance or safety or materially affect form, fit, function, reliability, serviceability, performance, functional interchangeability or interface capability of the BIS Product; | ||
| (b) | production process changes that materially affect design and/or production specifications; | ||
| (c) | change of manufacturing facility location or change of ownership of the manufacturing facility (to the extent such change in ownership is known to Aspect); | ||
| (d) | changes that modify Aspect’s quality system beyond editorial enhancements or continuous efficiency improvements in workflows; or | ||
| (e) | changes to regulatory status (including environmental compliance status) which affect any certification of Aspect or the manufacturing facility for the BIS Product. |
| 1.20 | Monitor Cable. “Monitor Cable” is a cable that connects the BISx to the Philips Patient Monitor and is also known, including in Exhibit A, as a “Host Cable”. The Monitor Cable connector will be supplied by Aspect or its manufacturer using designs supplied by Philips for final assembly with the BIS Products. | ||
| 1.21 | “Open Source Software” shall mean any software that is licensed under Open License Terms. | ||
| 1.22 | “Open License Terms” shall mean terms that require as a condition of use, modification and/or distribution of a work: |
1. the making available of source code or other materials preferred for
modification, or
2. the granting of permission for creating derivative works, or
3. the reproduction of certain notices or license terms in derivative works or
accompanying documentation, or
4. the granting of a royalty-free license to any party under Intellectual Property
Rights regarding the work and/or any work that contains, is combined with or
otherwise is based on the work.
| 1.23 | Philips Patient Monitor. “Philips Patient Monitor” shall mean all Philips multi-parameter patient monitors designed by Philips to accept a BIS Product. | ||
| 1.24 | Software. “Software” shall mean Aspect software programs that are integrated into the BIS Product. | ||
| 1.25 | Technical Information. “Technical Information” shall mean the trade secrets, know-how, computer programs (including copyrights in said software), knowledge, technology, means, methods, processes, practices, formulas, techniques, procedures, technical assistance, designs, drawings, apparatus, written and oral rectifications of data, specifications, assembly procedures, schematics and other valuable information of whatever nature, whether confidential or not, and whether proprietary or not, which is now (or hereafter, during the Agreement Term, becomes) licensable by Aspect and which is necessary for Philips’ integration, sale, distribution, use, installation, servicing or testing of an Aspect Product as permitted under this Agreement. | ||
| 1.26 | U.S. Dollars. “U.S. Dollars” shall mean lawful money of the United States of America, in immediately available funds. | ||
| 1.27 | Philips BIS Products Solution. “Philips BIS Products Solution” shall mean the BIS Products in combination with Philips Patient Monitors. |
| 2. | LICENSES TO INTELLECTUAL PROPERTY |
| 2.1 | Licenses. |
| (a) | For the term of this Agreement, subject to the terms and conditions of this Agreement, Aspect agrees to sell and Philips agrees, on behalf of itself and its Affiliates, to purchase the Aspect Products listed in Exhibit A (Aspect Products and Purchase Prices) and each Party agrees to negotiate an amendment to Exhibit A to include BIS-x4 when available (including the negotiation of the price thereof). | ||
| The Aspect Products purchased from Aspect under this Agreement shall only be used as part of, incorporated into, or integrated with, systems and products of Philips which Philips, its Affiliates, distributors or sub-distributors sell or lease to third-party users in the regular course of business. Philips, its Affiliates, distributors or sub-distributors shall only sell Aspect-approved accessories in connection with any BIS Product. Aspect agrees to provide Philips with all information in its possession that |
| is necessary for the interfacing and operation of the BIS Products with Philips Patient Monitors. Aspect agrees to provide Philips with information in its possession that will allow Philips to provide Aspect Products-specific service and support to the end-user. |
| (b) | Subject to the terms and conditions of this Agreement, during the term of this Agreement and, thereafter, solely as provided in Section 12.3(b)(i), Aspect hereby grants Philips a non-exclusive and non-transferable world-wide license (i) with the right to sublicense solely to Philips’ Affiliates, distributors and sub-distributors, to import, and (ii) with the right to sublicense solely to Philips’ Affiliates, distributors, sub-distributors and customers, to use, display and copy the Software provided by Aspect solely in connection with the sale and operation of the Aspect Products for use with a Philips Patient Monitor. | ||
| (c) | Subject to the terms and conditions of this Agreement, during the term of this Agreement and, thereafter, solely as provided in Section 12.3(b)(i), Aspect hereby grants Philips a non-exclusive and non-transferable world-wide license (i) with the right to sublicense solely to Philips’ Affiliates, distributors and sub-distributors, to copy, make alterations and derivative works, sell copies of, distribute, and import the Documentation provided by Aspect, and (ii) with the right to sublicense solely to Philips’ Affiliates, distributors, sub-distributors and customers, to use and display such Documentation and the derivative works thereof created in accordance with clause 2.1(c)(i), in each case solely in connection with the sale and operation of the Aspect Products for use with a Philips Patient Monitor. | ||
| (d) | All rights granted to customers to use the Software and Documentation with the Aspect Products shall survive any termination of the Agreement as long as the Software and Documentation is distributed to customers who are bound by terms at least as protective of the Software and Aspect’s Intellectual Property Rights therein as the terms set forth in Sections 2.1(e) and 2.1(f) of this Agreement; Philips shall be responsible for communicating these requirements to its customers and the customers of its Affiliates, distributors and sub-distributors. Any sublicense to a customer to use the Software and Documentation shall automatically terminate on the customer’s breach of any of the terms set forth in Section 2.1(e) or 2.1(f). | ||
| (e) | Philips shall not, without the express written consent of Aspect, disassemble, decompile or otherwise reverse engineer the Aspect Products or any part thereof (including the Software). Philips agrees to obligate its distributors, sub-distributors, its Affiliates and purchasers of Aspect Products not to disassemble, decompile or otherwise reverse engineer the Aspect Products or any part thereof without the express written consent of Aspect. If applicable law requires that Philips or any of its distributors, sub-distributors, Affiliates or customers have access to the source code of the Software, Philips shall notify Aspect if any such party desires such access and Aspect shall have the option to (i) perform the work to derive any required information at Aspect’s usual consulting rate, payable by |
| such party, or (ii) allow such party to access the source code solely for the legally required purpose. | |||
| (f) | Title to and ownership of any and all proprietary rights in or related to the Software and Documentation thereof shall at all times remain with Aspect or its licensor(s). Nothing in this Agreement shall be construed as a sale of any rights in the Software or Documentation. Any references in this Agreement to sale, resale or purchase of the Aspect Products, or similar references, shall, with respect to the Software and the Documentation, mean licenses of the Software and Documentation pursuant to Sections 2.1(b) and (c). | ||
| (g) | Philips and its customers shall have the right to create a reasonable number of backup copies of the Software, Documentation and any Technical Information solely for disaster recovery and/or contingency purposes. |
| 2.2 | Use of Technical Information. | ||
| Subject to the terms and conditions of this Agreement, Aspect grants to Philips a paid-up, non-exclusive worldwide right and license, with the right to sublicense solely to Philips’ Affiliates, during the Agreement Term to use, display, copy and import the Technical Information in connection with the exercise by Philips and its Affiliates of its rights and licenses granted in Section 2.1, and for no other purpose and Philips may distribute such Technical Information to its Affiliates solely for such purpose. All Technical Information shall be considered to be the Proprietary Information of Aspect for the purposes of Section 8. | |||
| 2.3 | Philips Trademarks, Service Marks, and Trade Names. | ||
| Philips on behalf of its ultimate parent Koninklijke Philips Electronics NV (“KPENV”) hereby grants Aspect a non-exclusive license under those certain trademarks as listed on Exhibit F (the “Philips Trademarks”) for the sole purpose of promoting the Aspect Products in promotional material, advertisement and/or in written technical literature [**] (“Purpose”). Such license is granted provided Aspect fully satisfies all of the provisions relating to/imposed on Aspect as set forth below: |
| (a) | Aspect in its use of the Philips Trademarks shall adhere to the trademark guidelines of Philips and KPENV; | ||
| (b) | Aspect shall use the Philips Trademarks consistent and solely in accordance with the Purpose; | ||
| (c) | Aspect shall use the Philips Trademarks only at trade shows and for advertising within brochures and marketing materials (“Licensed Use”); | ||
| (d) | Aspect shall not use the Philips Trademarks without Philips’ prior written permission wherein permission must be agreed to by Philips for each type of Licensed Use; |
| (e) | Aspect agrees to use the Philips Trademarks only in the form and manner provided and approved by Philips; | ||
| (f) | Aspect shall make available to Philips a sample of each type of Licensed Use (final version) for purposes of verification of usage being in accordance with the terms and conditions of this Section 2.3. | ||
| (g) | Notwithstanding anything to the contrary in this Section 2.3, (i) Aspect may identify Philips as a customer of Aspect, including without limitation on Aspect’s web site during the Agreement Term, the form and content of which is subject to Philips’ review and approval as set forth in this Section 2.3, such approval not to be unreasonably withheld or delayed; and (ii) all promotional material, advertisements and/or written technical literature using Philips Trademarks that were previously developed and approved by Philips under the agreement dated the 6th day of August, 1999, by and between Aspect and Philips (formerly Hewlett-Packard GmbH), as amended through the Effective Date (“1999 Agreement”) during the period from January 1, 2007 until the Effective Date of this Agreement shall remain approved under this Agreement; | ||
| (h) | Philips hereby reserves all rights, title and interest in and to the Philips Trademarks not specifically granted herein; | ||
| (i) | Philips shall at all times, anywhere in the world, and whether or not in competition with Aspect, have the right to use and/or authorize the use of the Philips Trademarks, or any portion thereof, in any way Philips may desire; | ||
| (j) | Aspect shall indemnify, defend and hold Philips, and all other legal entities which are, directly or indirectly, owned or controlled by KPENV and all of each of their respective officers, directors and employees harmless from and against any and all losses, costs, claims, damages (including court costs and attorney’s fees), liability, demands or expenses, which may arise out of or derive in any way from Aspect’s use of the Philips Trademarks except in the event of a breach of the representations or warranties by Philips as set forth in this Section 2.3. Philips hereby agrees to defend, indemnify and hold Aspect harmless from any claims arising out of Philips breach of any representations, warranties or agreements as set forth in this Section 2.3; | ||
| (k) | Philips represents and warrants that the use of the licensing rights under the Philips Trademarks granted to Aspect under this Section 2.3 shall not infringe upon the common law or statutory trademark rights of any third parties; |
| (l) | Aspect agrees that it will do nothing inconsistent with the ownership of the Philips Trademarks by KPENV and that all uses of same shall inure to the benefit of and be on behalf of KPENV; | ||
| (m) | Aspect agrees that nothing in this Agreement shall give Aspect any right, title or interest in the Philips Trademarks other than the right to use the Philips Trademarks in accordance with this Section 2.3; | ||
| (n) | Philips shall have the sole right and discretion to bring infringement or unfair competition proceedings involving the Philips Trademarks. Under no circumstances shall Aspect take any action or has any right to enforce Philips’s rights in the Philips Trademarks; | ||
| (o) | Aspect’s license of the Philips Trademarks in accordance with this Section 2.3 is expressly conditioned on Aspect’s execution and delivery of this Agreement and continued compliance with the terms of this Section 2.3. Philips may terminate this Agreement immediately if Aspect breaches any term or condition contained in this Section 2.3 which has not been cured within thirty (30) days after written notice of breach is delivered to Aspect. Aspect agrees to immediately discontinue the use of the Philips Trademarks if such cure is not complete within this thirty (30) day time period. Aspect further agrees that upon termination of this Agreement, all rights in the Philips Trademarks and the goodwill connected therewith shall remain the property of KPENV; | ||
| (p) | In the event that Philips decides, at its sole discretion, as a result of a cease and desist demand from a third party (or an agreement resulting therefrom) or a judicial order to cease and desist from using any one or more of the Philips Trademarks, Aspect shall immediately cease using said any one or more of the Philips Trademarks; | ||
| (q) | Aspect shall not use any other trademark in combination with any of the Philips Trademarks without prior written approval of Philips which shall be in Philips’ sole discretion; and | ||
| (r) | In the event of expiration or termination of this Agreement, the licensing rights under the Philips Trademarks of this Section 2.3 immediately terminates. Upon termination of this Agreement any reference to Philips and its trademarks must be promptly removed from any literature or other display and any existing literature containing any reference to Philips and its trademarks must no longer be distributed. Aspect may not use the Philips Trademarks in connection with the delivery by Aspect of any services. |
| 2.4 | Aspect Trademarks, Service Marks and Trade Names. |
| (a) | Aspect hereby grants Philips the right to use the trademarks, service marks and trade names claimed by Aspect listed in Exhibit E hereto, as may be |
| amended from time to time by mutual agreement of the Parties’ respective relationship managers (the “Aspect Trademarks”) on a non-exclusive basis only for the License Term and solely for display or advertising purposes in connection with the Aspect Products manufactured and sold in accordance with this Agreement, the form and content of which is subject to Aspect’s review and approval, such approval not to be unreasonably withheld or delayed. All display and advertising material using the Aspect Trademarks that were developed and approved under the 1999 Agreement shall remain approved under this Agreement. During the License Term, Philips and its Affiliates may also use, without Aspect’s prior written consent, Philips or third-party trademarks, service marks and trade names other than the Aspect Trademarks in connection with the BIS Products; provided, however Philips shall, and shall ensure that its Affiliates, will make reasonable efforts to use the Aspect Trademarks in a manner which makes them at least as large and prominent as any other similar third party trademarks, service marks, or trade names appearing on any such label, display or advertisement. Philips may not use the Aspect Trademarks in connection with the delivery by Philips of any services. |
| (b) | Philips and its Affiliates shall be required to ▇▇▇▇ those Philips Patient Monitors that are BIS Ready with Aspect’s “BIS Ready” logo in form of a laser printed, printed, or sticky label. The marking will be of a mutually agreeable size and will be placed at a mutually agreeable location on such Philips Patient Monitor. Notwithstanding the foregoing, if no mutually agreeable place or size can be determined then Philips’ labeling obligations shall be considered fulfilled with providing one loose sticky label “BIS Ready” with each such monitor shipped. Aspect shall provide the required number of “BIS Ready” sticky labels to Philips free of charge. | ||
| (c) | Each of Philips and its Affiliates shall |
| (i) | whenever it uses the Aspect Trademarks in any technical literature, product literature or advertisement, include a statement at least once in each piece of literature that the Aspect Trademarks are trademarks claimed by Aspect; | ||
| (ii) | use the Aspect Trademarks in compliance with Aspect’s guidelines for using the Aspect Trademarks; | ||
| (iii) | not modify any of the Aspect Trademarks in any way and not use any of the Aspect Trademarks on or in connection with any goods or services other than the Aspect Products. |
| 2.5 | Right to Sublicense. | ||
| Except as agreed to subject to the terms of this Agreement, Philips shall not have the right to sublicense any of the rights or licenses granted hereunder without Aspect’s prior written consent in Aspect’s sole discretion; provided, however it is understood that Philips shall have the right to grant sublicenses consistent with the terms and conditions of this Agreement to Philips’ Affiliates, distributors, sub-distributors, and customers without Aspect’s prior written consent, |
| provided, however, that Philips shall not have the right to grant any sublicense to customers under the Aspect Trademarks. |
| 2.6 | No Rights by Implication. | ||
| No rights or licenses with respect to Licensed Technology are granted or deemed granted hereunder or in connection herewith, other than those rights or licenses expressly granted in this Agreement. | |||
| 2.7 | Software and Computer Programs. | ||
| Philips shall retain and shall not, without the express written consent of Aspect, alter or obscure any notices, markings or other insignia which are affixed to the Software, the Documentation or any part thereof at the time of delivery of such Software or such Documentation. The Software shall be considered to be the Proprietary Information of Aspect for the purposes of Section 8. | |||
| 2.8 | Limited Warranty. | ||
| Aspect represents and warrants to Philips that, as of the Effective Date, Aspect knows of no claim by any third-party of infringement by Aspect on such party’s patent, trademark, copyright, trade secret or other Intellectual Property Rights covering the Aspect Products, Software, Documentation, or Technical Information. Aspect further represents and warrants that it owns all right, title, and interest in and to the Aspect Products, Software, Documentation, and Technical Information, including all Intellectual Property Rights thereto, or has the full lawful right to grant the licenses to the Licensed Technology as set forth herein. | |||
| 2.9 | DISCLAIMER OF WARRANTY. | ||
| EXCEPT FOR THE EXPRESS WARRANTIES SET FORTH HEREIN, INCLUDING BUT NOT LIMITED TO EXHIBIT D, ASPECT MAKES NO OTHER EXPRESS OR IMPLIED WARRANTY, STATUTORY OR OTHERWISE, CONCERNING THE LICENSED TECHNOLOGY OR ANY OTHER INFORMATION COMMUNICATED TO PHILIPS, INCLUDING WITHOUT LIMITATION NO WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE, AND NO WARRANTIES AS TO QUALITY OR THE USEFULNESS OF THE LICENSED TECHNOLOGY FOR ITS INTENDED PURPOSE. |
| 3. | CHANGES TO BIS PRODUCTS AND PATIENT MONITORS |
| 3.1 | Notifications of Changes to BIS Products. | ||
| Aspect shall have the right, at any time and from time to time, to make substitutions and modifications to BIS Products that, in Aspect’s reasonable judgment, do not constitute Material Product Changes, and Aspect shall not be required to provide Philips with notification of any such substitutions or modifications. | |||
| 3.2 | Material Product Changes. | ||
| In the event that any proposed substitution or modification is expected by Aspect to be a Material Product Change, Aspect shall give Philips written notice of such |
| proposed substitution or modification as early as possible in the development process, but in any case no later than the earlier of (i) at least [**] days prior to the planned release of such change if no significant changes are likely to be required to be implemented by Philips in Philips Patient Monitors, or (iii) at least [**] prior to the planned release of such modifications if significant changes are also likely to be required to be implemented by Philips in Philips Patient Monitors. If Philips does not provide written approval of any such proposed changes (which approval shall not be unreasonably withheld) Aspect shall continue to deliver the unchanged version of the BIS Product for at least [**] according to the regular forecast mechanism and, [**] prior to the termination of supply, offer Philips a final opportunity to purchase up to [**]% of the volume ordered in the previous [**]. Aspect shall provide the appropriate verification and validation information for evaluating the effect of the change on the BIS Products. |
| 3.3 | Required Changes. | ||
| Notwithstanding anything contained in this Agreement to the contrary, Aspect reserves the right from time to time during the License Term to require Philips, after consulting with Philips, to modify or improve any Aspect Product (including without limitation the software programs used in connection with the BIS Product) if the modification or improvement reasonably relates to efficacy or patient safety. Provided that such modifications are feasible and commercially reasonable, Philips shall implement those modifications to the BIS Products and associated Philips Patient Monitors being manufactured or to be manufactured and to modify and improve BIS Products previously manufactured and shipped to customers in order to incorporate such changes on a schedule at Philips’ reasonable discretion. If such modifications, improvements, or changes are implemented by Philips, it will be with Aspect’s reasonable assistance and, to the extent that the required change is caused by the BIS Product (other than the Monitor Cable), at Aspect’s expense. | |||
| 3.4 | Notification of Discontinuance. | ||
| Aspect agrees to notify Philips in writing not less than [**] in advance of the discontinuance of any BIS Product. Philips shall be able to place orders for [**] after receipt of the written notice in any case. In addition, Philips shall be entitled to determine its lifetime-buy quantities and place a corresponding last purchase order no later than [**] prior to the planned obsolescence date. |
| 4. | INCIDENT REPORTING AND CORRECTIVE ACTIONS |
| 4.1 | Aspect shall immediately, but no later than within 48 hours, provide Philips with a written notice upon Aspect becoming aware of the occurrence of any of the following events: (i) Aspect recalls any BIS Product, or ceases or suspends the sale of any BIS Product due to any problem which relates to such BIS Product’s efficacy or patient safety in any country; (ii) any defect of any Licensed Technology, which relates to such BIS Product’s efficacy or patient safety, is published, publicly reported or made known to the public by any third party, or found by or reported to Aspect; or (iii) any Licensed Technology is determined to have contributed to or caused a death or serious injury. |
| 4.2 | Philips shall immediately, but no later than within 48 hours, provide Aspect with a written notice upon Philips becoming aware of the occurrence of any of the following events: (i) Philips identifies the need to recall its BIS Products, or ceases or suspends the sale of its BIS Products due to any problem which Philips believes relates to such BIS Product’s efficacy or patient safety in any country, it being understood that any decision to proceed with a recall of BIS Products sold hereunder shall not be made by Philips before consulting with Aspect; (ii) any defect of any BIS Product or the Licensed Technology, which relates to such BIS Product’s efficacy or patient safety, is published, publicly reported or made known to the public by any third party, or found by or reported to Philips; or (iii) any BIS Product or the Licensed Technology is determined to have contributed to or caused a death or serious injury, or any BIS Product or the Licensed Technology malfunctioned and if that malfunction occurred again, it would be likely to contribute to or cause a death or serious injury. | ||
| 4.3 | Aspect, itself or through its Affiliates, is responsible for determining complaints and Medical Device Reporting (“MDRs”) and related submissions to the FDA and other regulatory authorities for the BIS Products. Philips, itself or through its Affiliates, is responsible for determining complaints and MDRs and related submissions to the FDA and other regulatory authorities for the Philips BIS Products Solution (other than the BIS Products). | ||
| 4.4 | Subject to the provisions of Sections 4.1, 4.2 and 4.3 above, Philips is responsible for the overall Philips BIS Products Solution and is therefore responsible to perform recalls and corrective actions with respect thereto. | ||
| 4.5 | Any corrective action will be at Philips’ expense if clearly caused by the Philips Patient Monitor. Philips will reimburse Aspect for Aspect’s documented cost directly resulting from such corrective actions within 60 days of completion of the corrective action. | ||
| 4.6 | Aspect will reimburse Philips for Philips’ documented cost directly resulting from such corrective actions within 60 days of completion of the corrective action if clearly caused by any BIS Product, as described in Exhibit B. | ||
| 4.7 | Philips and Aspect will share the cost if both Parties’ products contribute to the cause, proportionate to the extent of each Party’s respective degree of cause as mutually agreed. Aspect’s Vice President of Regulatory and Quality Affairs and a senior business director, together with the most senior executive with regulatory responsibility and a senior business director from Philips Healthcare Informatics/Patient Monitoring business unit, will negotiate in good faith a reasonable settlement of the issue. If after twenty (20) Business Days, the Parties in good faith fail to agree the issue will be escalated for resolution in accordance with Section 13.4(b). |
| 5. | PRODUCT DEVELOPMENT (BIS PRODUCTS AND MONITOR CABLE) | |
| The existing implementation of BIS in Philips Patient Monitors utilizing the BIS Products has been developed under a previous development contract. Both Parties anticipate that there will be advances in the BIS technology over time and agree that reasonable efforts should be made, if mutually agreed, to keep the Philips BIS implementation up-to-date according to the latest Aspect technology and performance. Therefore, an ongoing project is established between the companies to cover these ongoing improvement efforts. |
| 5.1 | Project Management. |
| 5.1.1 | Each Party shall appoint a “Project Manager” who shall oversee and manage the joint R&D project on a day-to-day basis. | ||
| 5.1.2 | The Project Managers shall meet regularly based on the project needs to assess the project status and discuss and resolve any issues or problems. These meetings may be held face-to-face or as telephone or videoconferences. | ||
| 5.1.3 | Each Party shall bear its own development, communication and travel costs. |
| 5.2 | Philips Development Responsibilities. | ||
| Philips will be responsible for any changes (design or material) to the Philips Patient Monitor to integrate the BIS Products with the Philips Patient Monitor and the visualization of BIS data. This includes the Philips Patient Monitor connector and the appropriate communication software to interface the BIS Products with Philips Patient Monitors. |
| 5.2.1 | Philips will be responsible for the necessary Philips Patient Monitors hardware and software (changes, upgrades, maintenance) to allow the user to configure BIS parameters, to display the BIS-related information on the Philips Patient Monitor display, and to pass on BIS-related data through the IntelliVue Clinical Network and other Philips Patient Monitor outputs. | ||
| 5.2.2 | Philips shall make reasonable commercial efforts to follow Aspect’s guidelines and recommendations concerning processing and visualization of BIS data with respect to the operation and visualization logic, philosophy and visual, audible and haptic experience (“look-and-feel”) of Philips Patient Monitors and other Philips products that store, process, display or provide patient data. | ||
| 5.2.3 | Philips shall be responsible for testing and validation of the BIS Products integration with the Philips Patient Monitors. | ||
| 5.2.4 | Depending on the complexity of the connector assembly, Philips acknowledges, subject to good faith negotiation and mutual agreement of the Parties, that it may be involved in the manufacturing and assembly process of any new Monitor Cable required to interface Philips Patient Monitors with the BISx System. | ||
| 5.2.5 | Philips shall assume regulatory responsibility for the combination of the BIS Products with Philips Patient Monitors as provided in Section 9 of this Agreement. |
| 5.3 | Aspect Development Responsibilities. |
| For changes to the current BIS Products, Aspect shall provide Philips with the necessary Technical Information to support the integration of the new or modified BIS Products with Philips Patient Monitors. This includes information to facilitate the development and/or refinement of a Philips Patient Monitor connector, of an appropriate communications software to interface the BIS Products to the Philips Patient Monitors, and of an appropriate user interface for BIS in the Philips Patient Monitor. Aspect shall inform Philips and provide said Technical Information as early in the development process as possible, in any case no later than (i) any other Aspect partner (who has an agreement with similar terms and conditions as this Agreement) gets informed about the plans and specifications, (ii) six (6) months prior to commercial availability for any change that may affect the specifications and documentation of the Philips BIS Products Solution, or (iii) twelve (12) months prior to commercial availability for any change that requires changes in the Philips Patient Monitors as well. | |||
| 5.3.1 | Aspect shall design, develop and test the BIS Products according to the mutually agreed upon specifications. | ||
| 5.3.2 | Aspect shall make commercially reasonable efforts to maintain backward compatibility with installed base Philips Patient Monitors for any new or changed BIS Products and/or 2-channel BIS sensors. | ||
| 5.3.3 | Upon Philips completing the integration of the BIS Products into a Philips Patient Monitor (including when any new model of a BIS Ready Philips Patient Monitor is developed/introduced), Aspect will “certify” this integration. This entails Aspect verifying the accurate display on the Philips Patient Monitor. Once successfully verified, Aspect will provide Philips with a letter so certifying. To facilitate the certification process, Philips will lend to Aspect a Philips Patient Monitor for testing purposes. Said Philips Patient Monitor will be provided to Aspect, at Philips’ cost and expense, within thirty (30) days after execution of this Agreement, and will be kept updated by Philips to the latest available state as far as the BIS functionality is concerned. Aspect will return such equipment to Philips, at Aspect’s cost and expense, within thirty (30) days after termination of this Agreement. |
| 6. | SALES BY ASPECT TO PHILIPS |
| 6.1 | Offer and Acceptance; Pricing. Philips’ and its Affiliates’ purchases of Aspect Products during the first calendar year shall be at the prices set forth in Exhibit A (Aspect Products and Purchase Prices), except as noted in Sections 6.2 and 6.4. The Parties have agreed, subject to applicable law, to initiate at least once a year discussions on market conditions and trends as well as transfer pricing of Aspect Products. | ||
| 6.2 | Additional costs which have been agreed upon in writing (both NRE and incremental unit cost) for customization of the BIS Products (mold color, labeling) will be borne by Philips. Philips shall be responsible for any charges associated with scrap of inventory due to any subsequent changes made to any BIS Product that were initiated by Philips. |
| 6.3 | For each purchase by Philips or its Affiliates from Aspect, Philips or such Affiliate shall present a purchase order to Aspect (a “Purchase Order”). Each Purchase Order shall be deemed an offer to purchase and, unless Philips or such Affiliate is notified in writing to the contrary within five (5) Business Days after Aspect receives it, such Purchase Order shall be deemed accepted by Aspect. | ||
| 6.4 | Aspect’s sale prices shall be FCA (Incoterms 2000) Norwood, Massachusetts, U.S.A. The initial prices set forth in Exhibit A (Aspect Products and Purchase Prices) are based on current material and labor costs for the Aspect Products. Starting with the second (2nd) Contract Year, Aspect may increase the then current sale prices if Aspect’s actual manufacturing cost of the Aspect Products increases from the current cost by more than [**]%; provided, however that: (i) such change may be made only [**] effective as of the first day of April with the prior written notice to be given by Aspect no later than the last day of December of the preceding year, after consulting with Philips; and (ii) the annual increase shall be [**], based on evidence provided to Philips by Aspect. Aspect may [**] at any time during the year with immediate effect by giving Philips written notice. No price change shall affect Purchase Orders offered by Philips and accepted by Aspect prior to the date such price change becomes effective | ||
| 6.5 | Delivery. | ||
| Unless Philips requests otherwise, all Aspect Products ordered by Philips shall be packed for shipment and storage in accordance with Aspect’s standard commercial practices. It is Philips’ obligation to notify Aspect of any special packaging requirements (which shall be at Philips’ expense if such requirement is in excess of the scope of normal and necessary packaging for export). Aspect shall deliver BIS Products into the possession of a common carrier designated by Philips or its Affiliate, in Norwood, Massachusetts, U.S.A. no later than the date specified for such delivery on the relevant Purchase Order. Risk of loss and damage to a BIS Products shall pass to Philips or its Affiliate upon the delivery thereof to the common carrier designated by Philips or such Affiliate. If Philips or its Affiliate does not designate a common carrier by the specified delivery date, then Aspect may do so on Philips’ or its Affiliate’s behalf. All claims for non-conforming shipments must be made in writing to Aspect within thirty (30) days of the passing of risk of loss and damage; any claims not made within this time period shall be deemed waived. | |||
| 6.6 | Forecast. | ||
| Philips shall furnish to Aspect a non-binding quarterly forecast during the term of this Agreement with the number and type of BIS Products for which Philips expects to submit orders for the following [**]. | |||
| 6.7 | Sales Reporting. |
| 6.7.1 | Philips will report the number of BIS Products sold by Philips, or its Affiliates by hospital (where possible) on a quarterly basis . Should hospital-level data not be available to Philips, Philips will then report BIS Products sold at the next most accurate level, e.g. distributor name, location). |
| 6.7.2 | K-Options. Philips will provide detail of K-Options (as defined in Exhibit A) sold by Philips or its Affiliates by hospital (where possible).Should hospital-level data not be available to Philips, Philips will then report K-Options sold at the next most accurate level, e.g. distributor name, location. |
| 6.8 | Method of Payment. | ||
| All amounts due and payable with respect to Aspect Products delivered by Aspect in accordance with this Section 6 shall be paid in full within [**] days from the end of the month of the date of Aspect’s invoice therefor. All such amounts shall be paid in U.S. Dollars by wire transfer, to such banks or accounts as Aspect may from time to time designate in writing. All costs incurred in connection with such wire transfer shall be the responsibility of Philips. Whenever any amount hereunder is due on a day which is not a day on which banks in Norwood, Massachusetts, U.S.A. or Boeblingen, Germany, are open for business, such amount shall be paid on the next Business Day. Amounts hereunder shall be considered to be paid as of the day on which funds are received by Aspect’s banks. No part of any amount payable to Aspect hereunder may be reduced due to any counterclaim, set-off, adjustment or other right which Philips might have or assert against Aspect, any other party or otherwise. | |||
| 6.9 | Limited Warranty. |
| (a) | With respect to the BIS Products, Aspect makes the warranties set forth in Exhibit D attached hereto and made a part hereof. Under no circumstances shall the warranties set forth in Exhibit D hereto apply to a BIS Product which has been customized, modified, damaged or misused by Philips or any third party in any manner without Aspect’s authorization. Except as provided elsewhere in this Agreement, Philips’ sole remedy for a non-conforming BIS Product is, at Aspect’s election, the repair or replacement thereof. THE PROVISIONS OF THE FOREGOING WARRANTIES ARE IN LIEU OF ANY OTHER WARRANTY, WHETHER EXPRESS OR IMPLIED, WRITTEN OR ORAL (INCLUDING ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NONINFRINGEMENT). | ||
| (b) | After expiration of the Warranty Period (as defined in Exhibit D), Aspect shall undertake repairs of BIS Products or shall provide parts for repairs by Philips, at reasonable cost to Philips. Both Parties hereby agree on the charge for such repairs and parts as provided in Exhibits A and G. |
| 6.10 | Priority of Agreement. | ||
| In the event of any discrepancy between any Purchase Order and this Agreement, the terms of this Agreement shall govern. | |||
| 6.11 | Service and Support. | ||
| Except as otherwise provided in this Agreement, Service and Support provisions from the 1999 Agreement provided herein as Exhibit G shall continue under this Agreement. Notwithstanding the foregoing, the Parties shall negotiate in good faith a new service agreement with a target execution date of March 31, 2009. |
| 7. | DISTRIBUTION OF BIS SENSORS |
| 7.1 | Philips has provided to Aspect a list, attached hereto as Exhibit A, Part E — “Pre-existing Supply Obligation List”, identifying each and every customer with which Philips has entered into a long-term BIS Sensor purchasing contract that extends beyond January 1st, 2009 (“Long-Term Sensor Customers”), along with anticipated BIS Sensor purchase quantities for the remainder of the contract term with respect to each such Long-Term Sensor Customer. | ||
| 7.2 | Philips shall not, and shall ensure that its Affiliates, distributors and sub-distributors shall not, market or distribute BIS Sensors to customers that are not Long-Term Sensor Customers and shall not market or distribute BIS Sensors to any Long-Term Sensor Customer in excess of the applicable quantities set forth in Exhibit A Part E. For clarity, Philips may sell [**] specified in Exhibit A solely in connection with the new sale of the Philips BIS Products Solution. | ||
| 7.3 | Subject to Section 7.4, Aspect shall sell BIS Sensors to Philips’ customers that are not Long Term Sensor Customers, either directly by Aspect, through its Affiliates or through distributors in each country or region. | ||
| 7.4 | Aspect will ensure that Philips customers that are not Long-Term Sensor Customers have an alternative outlet (which may include Aspect or its Affiliates or a third party distributor) from which to purchase BIS Sensors so that Philips customers are not forced to purchase BIS Sensors directly from [**]. In the event Aspect does not provide such an alternative BIS Sensor outlet in a country or region, Aspect (or its local Affiliate) will grant [**] distribution rights for BIS Sensors in such country or region with terms, conditions and pricing consistent with those of similar distributors in other countries or regions offering the same level of marketing, customer, and technical support as the local Philips Affiliate is prepared to offer. Aspect shall have the right to terminate any such agreement upon six (6) months notice once Aspect has established an alternative BIS Sensor outlet for such country or region. Aspect will provide regular updates to Philips regarding the progress toward the establishment of alternative BIS Sensor outlets. | ||
| 7.5 | Philips hereby agrees not to enter into, and to ensure that none of its Affiliates, distributors or sub-distributors enter into, any new BIS Sensor purchase contracts and not to renew or extend the term of any BIS Sensor supply contracts with any Long-Term Sensor Customer. | ||
| 7.6 | Aspect agrees to honor Philips BIS Sensor purchase commitments for Long-Term Sensor Customers by selling directly to the Philips’ central procurement site currently located at Boeblingen, Germany. Aspect will honor current BIS Sensor pricing for pre-existing supply contracts to Long-Term Sensor Customers using the price set forth in Exhibit A Part D in order for Philips to supply BIS Sensors to Long-Term Sensor Customers until the earlier of the date on which (i) each such contract expires or (ii) the end-user chooses to buy directly from Aspect. | ||
| 7.7 | If any dispute arises between the Parties regarding the status of any contracts for supply of BIS Sensors to Long-Term Sensor Customers, Aspect will have the right at its own expense to have an independent auditor review such contracts. |
| 7.8 | If any sales by Philips or its Affiliates, distributors or sub-distributors of BIS Sensors are made to any customer in contravention of Sections 7.2 and/or 7.5, Philips will cease such sales and Philips will retroactively pay Aspect an additional $[**] previously sold to customers in contravention of Sections 7.2 and/or 7.5. In addition, Philips shall reimburse Aspect for the cost of any audit that confirmed the existence of any such sales. | ||
| 7.9 | Compensation for US-Based BIS Product Sales. |
| 7.9.1 | Installed Base Payments. Subject to Section 7.11.1, for each Aspect BIS Sensor sold by Aspect to Philips US customers for use with Philips BIS Products that were sold prior to the Effective Date under the 1999 Agreement, Aspect shall pay Philips [**] billed by Aspect for such Aspect BIS Sensors (“Installed Base Payments”). | ||
| 7.9.2 | US Discount Program. Aspect will provide a discount to Philips of $[**] for each BIS Product purchased by Philips or its Affiliates, distributors or sub-distributors for sale to end user customers in the US after the Effective Date. Said discount shall be applied at time of invoicing of each BIS Product by Aspect. | ||
| 7.9.3 | The purpose of the payments under sections 7.9.1 and 7.9.2 is to compensate Philips at a fair market value amount for the promotion, sales and servicing by Philips of the BIS Products and to ensure equitable access for health care providers to all technologies. If any court opinion, federal agency ruling, federal or state enforcement action or interpretation (including any settlement) or DHHS Office of Inspector General advisory opinion alters the interpretation or application of the federal anti-kickback laws such that, in the written opinion of legal counsel for either Party, the compensation payments set forth in this Section 7.9 may no longer be compliant with such laws, the Parties agree to engage in good faith negotiations to revise the compensation provisions to the extent necessary to remain compliant with the federal anti-kickback laws, including potentially restructuring or eliminating payments hereunder. If the Parties are unable to reach agreement on the terms of the revision, the dispute shall be resolved in accordance with Section 13.4 herein. |
| 7.10 | Compensation for Sales of BIS Products Outside the United States. |
| 7.10.1 | For the year 2008, Aspect shall pay to Philips a fixed commission of $[**]. | ||
| 7.10.2 | Should BIS Sensor volumes sold to Philips exceed [**] units from July 1st, 2008 to December 31st 2008, the $[**] compensation paid by Aspect to Philips pursuant to Section 7.10.1 will be reduced by $[**] for each BIS Sensor exceeding [**] units. | ||
| 7.10.3 | Outside US Discount Program. Aspect will provide a discount to Philips of $[**] for each BIS Product purchased by Philips or its Affiliates, distributors or sub- |
| distributors for sale to end user customers outside the US after the Effective Date. Said discount shall be applied at time of invoicing of each BIS Product by Aspect. |
| 7.10.4 | Philips will have the right to hire (at its own expense) an independent auditor to verify both the commission and the discount calculations. |
| 7.11 | Additional Compensation Details. |
| 7.11.1 | With respect to Installed Base Payments within the United States, BIS Sensor commissions will be paid for a period of [**] full years for each Philips-sold BIS Product from the date of installation at the customer site, which may have been prior to the Effective Date. Aspect shall pay commissions due Philips on a [**] basis. With such [**] payments, Aspect shall provide to Philips a list of Philips United States customers to which such sales were made, the number of BIS Sensors sold in that [**], and the net revenue billed for the sale of the BIS Sensors by Aspect. | ||
| 7.11.2 | This [**] payment shall be provided to Philips no later than [**] days following the end of each [**]. In the event that Aspect’s standalone BIS monitors, the BIS modules and/or the BIS or BISx systems of or sold by other original equipment manufacturers have also been installed at such sites/regions in addition to Philips BIS Products, Philips will be entitled to a commission only on BIS Sensor sales intended for use with Philips BIS Products. A pro rata determination will be based on the total number of BIS units of different types installed at such locations during the period according to Aspect’s installed base records. Such installed base records shall be subject to the same [**] year period as that provided for Philips-sold BIS Products for the purposes of calculating commissions according to this Section 7. To facilitate such calculations, Philips will be responsible for providing Aspect with the documentation required in Section 6.7, and Aspect shall be responsible for providing Philips with accurate information regarding the total number of non-Philips units which have been installed, and are in use, at such sites/regions. The information on total installed BIS systems at Philips accounts is auditable by an independent auditor at Philips’ expense, provided that such independent auditor (i) is bound by obligations of confidentiality and non-use at least as protective of Aspect’s records as the provisions of Section 8 are with respect to Proprietary Information, and (ii) shall disclose to Philips only the percentage of total installed BIS systems that are installed at Philips accounts. In the event such audit identifies an underpayment by Aspect of at least five percent (5%), Aspect shall reimburse Philips for the cost of such audit. The information underlying the reports provided by Philips pursuant to Section 6.7 is auditable by an independent auditor at Aspect’s expense, provided that such independent auditor (a) is bound by obligations of confidentiality and non-use at least as protective of Philips’ records as the provisions of Section 8 are with respect to Proprietary Information, and (b) shall disclose to Aspect only the information necessary to document any discrepancies |
| in the reports. In the event such audit identifies an overpayment by Aspect of at least five percent (5%), Philips shall reimburse Aspect for the cost of such audit. |
| 8. | CONFIDENTIAL INFORMATION |
| 8.1 | Agreement. | ||
| This Agreement and all documents, drawings, manuals and other materials related thereto and transmitted between the Parties shall be treated as Proprietary Information (as defined in Section 8.2) by the Parties and their employees and such information shall not be disclosed to any third party except as provided by this Agreement. | |||
| 8.2 | Confidentiality Obligations. | ||
| Each Party (the “disclosing Party”) has a proprietary interest in information which it discloses to the other Party (the “receiving Party”), whether in connection with this Agreement or otherwise, which is (a) a trade secret, confidential or proprietary information, (b) not publicly known, and (c) annotated by a legend, stamp or other written identification as confidential or proprietary information, or if disclosed orally, is identified as confidential or proprietary by a written instrument within 30 days of such disclosure (referred to herein as “Proprietary Information”). The receiving Party shall disclose the Proprietary Information of the disclosing Party only to those of its agents and employees to whom it is necessary in order properly to carry out their duties as limited by the terms and conditions hereof. Both during and after the Agreement Term, all disclosures by the receiving Party to its agents and employees shall be held in strict confidence by such agents and employees. During and after the Agreement Term, the receiving Party, its agents and employees shall not use the Proprietary Information for any purpose other than in connection with discharging its duties pursuant to this Agreement. The receiving Party shall, at its expense, return to the disclosing Party the Proprietary Information of the disclosing Party or certify its destruction as soon as practicable after the termination or expiration of this Agreement but in no event later than thirty (30) days after such expiration or termination. During the Agreement Term and thereafter, all such Proprietary Information shall remain the exclusive property of the disclosing Party. Each Party shall ensure that any consultants or subcontractors that it may engage in connection with its obligations under this Agreement (including Affiliates of the receiving Party) are bound by obligations of confidentiality and nonuse with respect to the Proprietary Information of the other Party at least as protective as those set forth in this Section 8. | |||
| 8.3 | Exceptions. | ||
| Notwithstanding anything contained in this Agreement to the contrary, the receiving Party shall not be liable for a disclosure of the Proprietary Information of the disclosing Party if the information so disclosed: (a) was in the public domain at the time of disclosure without breach of this Agreement; or (b) was known to or contained in the records of the receiving Party from a source other than the disclosing Party at the time of disclosure by the disclosing Party to the receiving Party and can be so demonstrated; or (c) becomes known to the receiving Party from a source other than the disclosing Party without breach of |
| this Agreement by the receiving Party and can be so demonstrated; or (d) was disclosed pursuant to court order or as otherwise compelled by law, provided that the receiving Party gives the disclosing Party prior written notice of such compelled disclosure, if permitted, and assists the disclosing Party in its reasonable efforts to prevent or limit such disclosure. | |||
| 9. | QUALITY ASSURANCE / REGULATORY MATTERS |
| 9.1 | Certification Status. | ||
| Both Parties agree to maintain, as applicable, ISO13485, CAN/CSA-ISO 13485:03 certified by a Health Canada recognized Registrar, European directive 93/42/EEC Annex II (“MDD AX-II”) certification status and compliance with the U.S. Food and Drug Administration’s (“FDA”) Quality System Regulation (“QSR”), the European Medical Device Directive (“MDD”), and other appropriate regulations pertinent to the development, manufacturing and marketing of medical products similar to the BIS Products. | |||
| 9.2 | Regulatory Responsibility — Aspect. | ||
| In particular, Aspect shall be responsible, with respect to BIS Products, for achieving and maintaining FDA 510(k) clearance, Canadian Medical Device Licence, and compliance with the EU Council Directive 93/42/EEC (DoC). | |||
| 9.3 | Regulatory Requirements — Philips. | ||
| Philips shall assume the regulatory responsibility to achieve regulatory approvals/registrations for the combination of the Aspect Products and Philips Patient Monitors. Aspect shall provide commercially reasonable support to Philips as required in the process of obtaining regulatory approvals by making available to Philips any required information, data, certificates, or technical files in the requested formats as requested by the regulatory authorities. | |||
| 9.4 | Product Complaints and Incident Reporting. | ||
| Philips and Aspect shall inform each other (in writing) quarterly of any and all customer complaints that have come to their attention during the prior quarter regarding the Aspect Products or BIS Sensors that were used in conjunction with a Philips Patient Monitor and relating to Aspect technology. Both Parties will cooperate and use reasonable efforts to resolve such customer complaints. Closure of any such customer complaints relating to the Philips BIS Patient Monitor Solution will occur when Philips notifies Aspect that the problem is resolved, except that closure of any such customer complaint relating solely to BIS Products or BIS Sensors will occur when Aspect notifies Philips that the problem is resolved. | |||
| Philips and Aspect shall inform each other in writing of all incidents relating to Aspect Products within 48 hours of making a determination that such event requires reporting under any applicable regulatory or other governmental reporting requirements, including without limitation incidents involving death or serious injury, malfunctions that, if recurrent, may cause or contribute to death or serious injury or other material quality problems or concerns; provided, however, for the purposes of clarity, if such reportable event involves a third party product |
| into which the Aspect Product has been incorporated, but the cause of reportable incident was some other aspect or attribute of such third party product, then the notification requirement in this paragraph shall not apply. Aspect will be responsible for reporting such incidents to the appropriate regulatory authority for the Aspect Products. Philips will be responsible for reporting such incidents to the appropriate regulatory authority for the Philips BIS Products Solution (other than the BIS Products) or Philips Patient Monitor. Both Parties shall fully cooperate with each other as may be necessary to comply with any reporting obligations regarding such incidents or quality concerns. If FDA or other authorities contact either Party to inquire about or investigate the Aspect Products sold to Philips under this Agreement, the contacted Party, unless required to maintain confidentiality by such authorities, shall inform the other Party immediately thereof. The Parties shall cooperate closely to clear any regulatory issues or potential regulatory issues promptly. | |||
| 9.5 | Traceability. | ||
| Philips agrees to maintain traceability through Aspect serial number and/or lot code for all Aspect Products shipped to Philips. |
| 10. | COMMUNICATION | |
| All communication in conjunction with this Agreement shall be directed to the appropriate person and address listed in Exhibit C (Contact Persons / Addresses). |
| 11. | INDEMNIFICATIONS |
| 11.1 | In Favor of Aspect. |
(A) Philips hereby agrees to indemnify and hold harmless Aspect, its Affiliates and all
officers, directors, employees and agents thereof from all liabilities, claims, damages,
losses, costs, expenses, demands, suits and actions (including without limitation attorneys’
fees, expenses and settlement costs) (collectively, “Damages”), arising out of personal
injuries and/or tangible property damages, to the extent (a) caused by a defect in the Philips
Patient Monitor or in the design of the Monitor Cable, (b) relating to the failure of Philips
to incorporate the Aspect Products with Philips Patient Monitors in accordance with the
Technical Information provided by Aspect hereunder, or (c) relating to any delay or failure by
Philips to implement any modification or improvement set forth in Section 3.3 (Required
Changes).
(B) Philips hereby agrees to indemnify and hold harmless Aspect, its Affiliates and all
officers, directors, employees and agents thereof from all Damages arising out of any
third-party infringement claim to the extent the infringement is caused solely by the Philips
Patient Monitor or compliance with Philips’ specifications for design of the Monitor Cable
provided such specifications cannot be implemented in a commercially reasonable non-infringing
manner.
| 11.2 | In Favor of Philips. |
(A) Aspect hereby agrees to indemnify and hold harmless Philips, its Affiliates and
all
officers, directors, employees and agents thereof from all Damages, arising out of personal
injuries and/or tangible property damages, to the extent (a) caused by a defect in the
manufacture of the Monitor Cable or a defect in any other Aspect Product or (b) relating to any
inaccuracy in the Technical Information provided by Aspect hereunder with respect to the Aspect
Products (excluding the Monitor Cable).
(B) Aspect hereby agrees to indemnify and hold harmless Philips, its Affiliates and all
officers, directors, employees and agents thereof from all Damages arising out of any claim
that: (a) the Licensed Technology infringes on the intellectual property rights of third
parties, provided that neither Philips nor its Affiliates own such intellectual property; or
(b) use of the Aspect Trademarks in accordance with Section 2.4 above infringes on the
trademark, service ▇▇▇▇ or trade name rights of third parties, provided that neither Philips
nor its Affiliates own such trademark, service ▇▇▇▇ or trade name; further provided, however
that:
Aspect shall have no obligation for any claim of infringement arising from:
| (i) | any combination by Philips of the Aspect Products with any other product not supplied or approved in writing by Aspect where such infringement would not have occurred but for such combination; | ||
| (ii) | the adaptation or modification of the Aspect Products not performed or not authorized by Aspect, where such infringement would not have occurred but for such adaptation or modification; | ||
| (iii) | the misuse of the Aspect Products or the use of any Aspect Product in an application for which it was not designed by Aspect, where such infringement would not have occurred but for such use or misuse; | ||
| (iv) | compliance with Philips’ specifications for design of the Monitor Cable provided such specifications cannot be implemented in a commercially reasonable non-infringing manner; or | ||
| (v) | infringement to the extent solely caused by a Philips Patient Monitor. |
Notwithstanding anything to the contrary, nothing in this Agreement restricts Philips from
asserting whatever intellectual property rights it may have in the Licensed Technology against
Aspect.
| 11.2.1 | In the event that any of the Licensed Technology is held in a suit or proceeding to infringe any intellectual property rights of a third party, and the use of any of the Licensed Technology is enjoined or Aspect reasonably believes that it is likely to be found to infringe or likely to be enjoined, Aspect shall, at its sole cost and expense, either (i) procure for Philips the right to continue manufacturing, using and selling the Licensed Technology subject to the terms of this Agreement, or (ii) replace the Licensed Technology with a non-infringing alternative of equivalent functionality. If neither (i) nor (ii) are practicable, either Party may terminate this Agreement, effective immediately, upon giving the other Party written notice. |
| 11.2.2 | This Section 11.2 constitutes Philips’ exclusive remedy in the event that any of the Licensed Technology and/or the Aspect Trademarks infringe on the intellectual property rights of third parties. |
| 11.3 | Indemnification Procedures. | ||
| In the event that any person intends to claim indemnification pursuant to this Agreement, (an “Indemnitee”), it shall promptly notify the indemnifying Party (the “Indemnitor”) in writing of such alleged liability, provided that the failure to promptly notify the Indemnitor shall not relieve the Indemnitor of any obligation under this Agreement except to the extent such failure to provide prompt notice adversely impairs the Indemnitor’s ability to defend against the claim, suit or proceeding. |
| 11.3.1 | The Indemnitor shall have the sole right to control the defense and settlement thereof, provided, that (i) the Indemnitor may not consent to imposition of any obligation or restriction on, or the admission of any wrongdoing on the part of, the Indemnitee in any settlement unless mutually agreed among Aspect and Philips, (ii) Indemnitor shall keep Indemnitee fully informed and permit the Indemnitee to participate (at Indemnitee’s expense) as the Indemnitee may reasonably request, and (iii) Indemnitee may, without affecting its right to indemnity hereunder, defend and settle any such claim, suit or proceeding if Indemnitor declines to defend against such claim, suit or proceeding or files for bankruptcy. The Indemnitee shall reasonably cooperate with the Indemnitor and its legal representatives in the investigation of any action, claim or liability covered by this Agreement, at the expense of the Indemnitor. | ||
| 11.3.2 | The Indemnitee shall not, except at its own cost, voluntarily make any payment or incur any expense with respect to any claim or suit without the prior written consent of Indemnitor, which Indemnitor shall not be required to give, provided that the Indemnitee may, without affecting its right to indemnity hereunder, defend and settle any such claim, suit or proceeding if the Indemnitor declines to take responsibility or files for bankruptcy. |
| 11.4 | Partial Indemnification. | ||
| In the event a claim is based partially on an indemnified claim described in Sections 11.1 and/or 11.2 above and partially on a non-indemnified claim, or is based partially on a claim described in Section 11.1 above and partially on a claim described in Section 11.2 above, any Damages incurred in connection with such claims are to be apportioned between the Parties in accordance with the degree of cause attributable to each Party as determined under Section 11.5 below. | |||
| 11.5 | Determination of Indemnification Obligations. The extent of any indemnification obligations set forth in this Section 11 shall be determined according to the degree of cause attributable to each Party as determined in one of the following manners: |
| (a) | by the holding of a court of competent jurisdiction in a proceeding in which both Parties had the opportunity to be a party; or | ||
| (b) | by the terms of any settlement agreement mutually agreed by the Parties; or | ||
| (c) | in accordance with the procedures set forth in Section 13.4. |
| 12. | TERMINATION OR EXPIRATION |
| 12.1 | Term and Renewal. | ||
| Unless it is terminated earlier pursuant to this Section 12, the initial term of this Agreement shall commence on the Effective Date and shall continue for a period of three (3) years (“Initial Term”). After the Initial Term this Agreement shall be automatically extended for a maximum of two successive 1 (one) year periods unless either Party provides written notice to the other Party of its desire not to renew this Agreement at least 3 months prior to the applicable anniversary date of this Agreement. The term of this Agreement may be renewed by mutual written agreement of the Parties prior to the expiration of the Initial Term. | |||
| 12.2 | Termination for Cause. | ||
| Upon the occurrence of a material breach or default as to any obligation hereunder by either Party and the failure of the breaching Party to cure within thirty (30) days after receiving written notice thereof from the non-breaching Party of such material breach or default, this Agreement may be terminated by the non-breaching Party by giving written notice of termination to the breaching Party after such thirty (30) day period, such termination being immediately effective upon the giving of such notice of termination. | |||
| 12.3 | After Termination or Expiration. | ||
| The Parties agree that, once this Agreement is terminated or expires, Philips shall immediately cease: |
(a) any use or practice of the Licensed Technology; and
(b) any use or sale of the Aspect Products; provided, however
that:
| (i) | Philips shall have the right to sell Aspect Products which are in Philips’ possession at the time of such termination or expiration for a period of six (6) months after such termination or expiration; | ||
| (ii) | Aspect or any third party designated by Aspect shall sell to Philips the parts necessary to repair the BIS Products and shall grant to Philips the right to repair BIS Products, for a period reasonably deemed that BIS Products are used by the customers, but no more than for a period of 7 years; and | ||
| (iii) | Aspect or any third party designated by Aspect shall continue to supply end-users for no more than 7 years with BIS Sensors to use with BIS Products. |
| 12.4 | Payment Obligations Continue. |
| Upon termination or expiration of this Agreement, nothing shall be construed to |
| release Aspect from its BIS Sensor commission payment obligations provided in Section 7 herein with respect to BIS Products sold by Philips prior to the date of such termination or expiration or in accordance with Section 12.3(b), or Philips from its obligations to pay Aspect any and all amounts accrued but unpaid pursuant to Section 6 above prior to the date of such termination or expiration. |
| 12.5 | No Damages for Termination. | ||
| The Parties agree that if either Party terminates this Agreement pursuant to Section 12.2, then the terminating Party shall not be liable for damages or injuries suffered by the other Party solely as a result of that termination, unless otherwise expressly provided herein. |
| 13. | MISCELLANEOUS |
| 13.1 | LIMITATION ON LIABILITY. |
| (a) | EACH PARTY’S LIABILITY TO THE OTHER PARTY ARISING OUT OF THE MANUFACTURE, SALE OR SUPPLYING OF PRODUCTS OR THEIR USE, OR DISPOSITION OF THEIR OBLIGATIONS OR RESPONSIBILITIES UNDER THIS AGREEMENT, WHETHER BASED UPON WARRANTY, CONTRACT, TORT OR OTHERWISE, SHALL NOT EXCEED THE GREATER OF (A) FIVE MILLION DOLLARS ($5,000,000.00) OR (B) THE SUM OF (i) THE TOTAL ACTUAL PURCHASE PRICE PAID OR PAYABLE BY PHILIPS FOR ALL ASPECT PRODUCTS PURCHASED HEREUNDER AND (ii) ALL AMOUNTS PAID OR PAYABLE BY PHILIPS TO ASPECT FOR SERVICE AND SUPPORT PROVIDED UNDER THIS AGREEMENT. | ||
| (b) | IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER PARTY FOR SPECIAL, INCIDENTAL, CONSEQUENTIAL, OR OTHER INDIRECT DAMAGES (INCLUDING, BUT NOT LIMITED TO, LOSS OF PROFITS, LOSS OF DATA OR LOSS OF USE DAMAGES) ARISING OUT OF THE MANUFACTURE, SALE OR SUPPLYING OF PRODUCTS OR THE OBLIGATIONS OR RESPONSIBILITIES UNDER THIS AGREEMENT. | ||
| (c) | THE LIMITATIONS IN THIS SECTION 13.1 WILL NOT APPLY TO CLAIMS FOR DAMAGES FOR BODILY INJURY (INCLUDING DEATH) AND DAMAGE TO TANGIBLE PERSONAL PROPERTY FOR WHICH A PARTY IS LEGALLY LIABLE AND/OR A PARTY’S INDEMNIFICATION OBLIGATIONS CONTAINED IN THIS AGREEMENT. |
| 13.2 | Assignment. | ||
| This Agreement and the rights and obligations hereunder may not be assigned, delegated or transferred by either Party without the prior written consent of the other Party; provided, however that the other Party’s consent shall not be required with respect to any assignment, delegation or transfer by a Party to (i) an Affiliate of such Party; (ii) the purchaser of all or substantially all of the (A) assets related to this Agreement or (B) stock of such Party, through merger, |
| consolidation or otherwise; or (iii) Aspect’s assignment to any third party(ies) of its right to collect payment hereunder. To the extent permitted by this Agreement, this Agreement shall be binding upon and inure to the benefit of the permitted successors and assigns of both Parties. |
| 13.3 | Governing Law. | ||
| This Agreement shall be construed and governed according to, and any judicial proceedings shall be conducted in accordance with the laws of the Commonwealth of Massachusetts, U.S.A., excluding its conflicts of laws principles. | |||
| 13.4 | Dispute Resolution. |
| (a) | Any dispute, controversy or claim arising out of or relating to this Agreement or to a breach hereof, including its interpretation, performance or termination, shall be resolved by good faith negotiations between the Parties, who in all cases shall endeavor to resolve all disputes promptly on mutually acceptable, fair and reasonable terms. | ||
| (b) | If after twenty (20) Business Days the Parties in good faith fail to resolve any such dispute, the dispute will be escalated for resolution by the Vice President of Emerging Technologies (or designee) for Aspect and the Vice President and General Manager of its Measurements and Monitoring business unit (or designee) for Philips. If such negotiations do not reach a resolution within thirty (30) days after such escalation, either Party shall thereafter have the right to institute judicial proceedings to resolve such dispute, controversy, or claim. |
| 13.5 | Entire Agreement. | ||
| Except as expressly set forth herein, this Agreement supersedes and cancels any previous agreements or understandings, whether oral, written or implied, heretofore in effect, including the 1999 Agreement, and sets forth the entire agreement between Aspect and Philips and its Affiliates with respect to the Aspect Products and the BIS Products. As of the Effective Date, and notwithstanding any requirements of notice prior to termination, the Parties agree that any such previous agreements or understandings shall be terminated, and effective only for determining the Parties’ respective rights and obligations with respect to any products or services ordered (except for any compensation payments from Aspect to Philips, which shall be governed by Sections 7.9 and 7.10 of this Agreement and not by any provision of the 1999 Agreement), or information exchanged or obtained, under such agreements or understandings, provided, however, that any information subject to any duties of nondisclosure or nonuse under any agreement superseded by this Agreement pursuant to this Section 13.5 shall be deemed “Proprietary Information” subject to the obligations set forth in Section 8 hereof. No modification or change may be made in this Agreement except by written instrument duly signed by a duly authorized representative of each Party. | |||
| 13.6 | Notices. | ||
| All notices given under this Agreement shall be in writing and shall be addressed to the Parties at their respective addresses and email addresses, and to the attention of the individuals set forth in Exhibit C. Either Party may change |
| its address, telecopy number and contact person for purposes of this Agreement by giving the other Party written notice of its new address, email address or contact person. Any such notice if given or made by registered or recorded delivery international air mail letter shall be deemed to have been received on the date actually if made by email transmission shall be deemed to have been received at the time of dispatch, unless such date of deemed receipt is not a day on which banks in the receiving Party’s home city are open for business, in which case the date of deemed receipt shall be the next day on which banks in the receiving Party’s home city are open for business. |
| 13.7 | Waivers. | ||
| None of the conditions or provisions of this Agreement shall be held to have been waived by any act or knowledge on the part of either Party, except by an instrument in writing signed by a duly authorized officer or representative of such Party. Further, the waiver by either Party of any right hereunder or the failure to enforce at any time any of the provisions of this Agreement, or any rights with respect thereto, shall not be deemed to be a waiver of any other rights hereunder or any breach or failure of performance of the other Party. | |||
| 13.8 | Responsibility for Taxes. | ||
| Taxes now or hereafter imposed with respect to the transactions contemplated hereunder shall be the responsibility of Philips, and if paid or required to be paid by Aspect, the amount thereof shall be added to and become a part of the amounts payable by Philips hereunder. | |||
| 13.9 | Severability. | ||
| If, under applicable law or regulation, any provision of this Agreement is invalid or unenforceable or directly or indirectly affects the validity of any other material provision(s) of this Agreement (“Severed Clause”), it is mutually agreed that this Agreement shall endure except for the Severed Clause. The Parties shall consult and use their best efforts to agree upon a valid and enforceable provision which shall be a reasonable substitute for such Severed Clause in light of the intent of this Agreement. | |||
| 13.10 | Counterparts. | ||
| This Agreement may be executed in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. | |||
| 13.11 | Relationship of the Parties. |
| (a) | The relationship between Aspect and Philips shall not be construed to be that of employer and employee, or to constitute a partnership, joint venture or agency of any kind. Neither Party shall have any right to enter into any contracts or commitments in the name of, or on behalf of, the other Party, or to bind the other Party in any respect whatsoever. | ||
| (b) | Philips shall not obligate or purport to obligate Aspect by issuing or making any affirmations, representations, warranties or guarantees with respect to BIS Products to any third party, other than the warranties described in Exhibit D hereto. |
| (c) | Each Party may perform its obligations and duties hereunder through one or more of its Affiliates, and Affiliates of the Parties are expressly granted certain rights herein; provided, that each such Affiliate shall be bound by the corresponding obligations of such Party and the relevant Party shall remain liable hereunder for the performance of all their respective obligations hereunder. |
| 13.12 | Survival of Contents. | ||
| Notwithstanding anything else in this Agreement to the contrary, the Parties agree that Sections 2.1(d)-(g), 2.3, 2.4, 2.6, 2.7, 2.9, 3.3, 4, 6.9, 6.10, 7.7, 7.8, 8 (for seven (7) years, except perpetually for Software and Technical Information), 9.4 (second paragraph only), 9.5, 10, 11, 12.3, 12.4, 12.5 and 13 shall survive the termination or expiration of this Agreement, as the case may be. | |||
| 13.13 | Compliance With Laws. | ||
| Each of Philips and Aspect covenants that all of its activities under or pursuant to this Agreement shall comply with all applicable laws, rules and regulations. Philips, with Aspects’ support and all necessary assistance, shall be responsible for obtaining all licenses, permits and approvals which are necessary or advisable for sales of BIS Products in all jurisdictions and for the performance of its duties hereunder. | |||
| 13.14 | Headings. | ||
| Any headings contained herein are for directory purposes only, do not constitute a part of this Agreement, and shall not be employed in interpreting this Agreement. | |||
| 13.15 | Language. | ||
| All written material, correspondence, Technical Information, notices and oral assistance supplied by either Party hereunder shall be in the English language. | |||
| 13.16 | Exhibits. | ||
| The following Exhibits shall be part of this Agreement: |
| EXHIBIT A | Aspect Products and Purchase Prices | |||
| EXHIBIT B | Specifications: BIS Products | |||
| EXHIBIT C | Contact Persons / Addresses | |||
| EXHIBIT D | Warranty | |||
| EXHIBIT E | Aspect Trademarks | |||
| EXHIBIT F | Philips Trademarks | |||
| EXHIBIT G | Service, Support and Repair |
IN WITNESS WHEREOF, the Parties hereto have signed this Agreement under seal.
| Philips Medizin Systeme Böblingen GmbH | ||
By: /s/ J. ▇▇▇▇ ▇▇▇▇▇▇▇▇▇
|
By: /s/ ▇. ▇▇▇▇ | |
Typed Name: J. ▇▇▇▇ ▇▇▇▇▇▇▇▇▇
|
Typed Name: ▇. ▇▇▇▇ | |
Title: CFO
|
Title: VP, GM | |
Date: 4/24/09
|
Date: 14-Apr-2009 | |
| Philips Medizin Systeme Böblingen GmbH | ||
| By: /s/ ▇▇▇▇▇▇ | ||
| Typed Name: ▇▇▇▇▇▇ | ||
| Title: Managing Director | ||
| Date: 14-Apr-2009 |
EXHIBIT A
ASPECT PRODUCTS AND PURCHASE PRICES
ASPECT PRODUCTS AND PURCHASE PRICES
A) BIS Products:
List price for the BIS Products: $[**]. Volume discounts are available as follows:
| Part # | Description | Aspect List Price | Philips Price | |||
186-0195-PH
|
BISx Kit | $[**] | See Below | |||
| As of Effective Date | ||
| Volume | Pricing (1) | |
| 0 — 1500 | $[**] | |
| 1501 — 2500 | $[**] | |
| 2501 — 3500 | $[**] | |
| >3500 | $[**] | |
For the purpose of calculating the volume discount for a given calendar year, all BIS Products
sold as reported by Philips according to Section 6.7 above during that calendar year (excluding
BIS Products that are provided free of charge) (“Reported Sales”) will be included in the total
volume discount calculation.
The initial pricing for a given calendar year is based on the total of BIS Products Reported
Sales in the prior calendar year. Philips will maintain the previous year pricing as long as
they maintain the Reported Sales volume levels required for that pricing. For example, if there
were Reported Sales of [**].
If a higher volume level of BIS Products is achieved during a given calendar year, the price on
purchases made after achieving the higher volume level will reflect the price associated with
the appropriate volume level achieved. All price adjustments are proactive and no credit will
apply retroactively to units purchased prior to achieving the volume break point. For example,
if midway through year 1, [**] are reported, the price on [**] will reflect the next volume
break. Achieving this higher volume level will also reduce the initial pricing for the
following year.
The initial volume discount will be calculated at the Effective Date based on the volume of BIS
Products previously purchased by Philips during the previous 12 month period under the 1999
Agreement. The discounts according to sections 7.9.2 and 7.10.3 shall be applied by Aspect in
addition to the volume discounts described above.
(1) Volume pricing before discount program provided in 7.9.2 and 7.10.3
B) BIS SENSORS
| • | For the purpose of this Agreement, Aspect will include [**] with each BIS Product shipped to Philips. |
| o | Price for the [**] will be $[**]. |
| • | Philips may also order [**] solely for resale to a customer together with the sale of any new BIS Product (the “K-Options”): |
| #K20: 50x BIS Quatro sensors — price is $[**] | |||
| #K21: 100x BIS Semi-reusable sensors — price is $[**] |
C) SERVICE PARTS:
| Part # | Description | Philips Price | ||
186-0199-PH
|
Philips BISx Kit (Refurbished) / Exchange | $[**] | ||
186-0201-PH
|
Philips BISx Host Cable Kit (Replacement) | $[**] | ||
186-0202-PH
|
Philips BISx BulkHead Kit (Replacement) | [**] | ||
186-0133
|
Philips BIS Sensor Simulator | $[**] | ||
186-0131
|
Philips PIC+ Cable (replacement) | $[**] |
D) PRE-EXISTING SENSOR PRICING AND QUANTITIES
As described in Section 7, “Distribution of BIS Sensors”, Philips will be allowed to order the
following quantities of BIS Sensors to meet pre-existing customer commitments:
Prices for the BIS Sensor quantities purchased pursuant to Section 7.6 will be $[**].
E) Pre-existing Supply Obligation List
[**]
EXHIBIT B
SPECIFICATIONS: BIS Products
SPECIFICATIONS: BIS Products
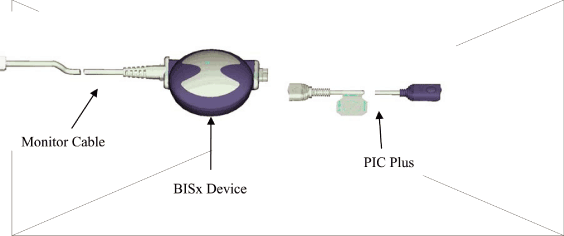
BISx is a device that acquires up to two channels of EEG and computes BIS and other EEG parameters,
uniting the functionality of the existing Aspect BIS Engine and DSC-XP. BISx is designed to mate
with Aspect’s XP platform 1 or 2 channel sensors. BISx has no display or user interface. It plugs
into a host monitor system for display of EEG and processed parameters. BISx is designed for use
wherever sedative drugs are administered, including but not limited to the following environments:
Operating rooms, Intensive Care Units, Procedural Sedation, and Clinical Research areas.
The standard BISx acquires EEG via single channel or two channel referential XP platform sensors
and XP compatible sensors. The inputs are protected against damage from electro-static discharge
(ESD), a direct hit from an electrocautery device, and defibrillation of the patient to which it is
attached. The BISx recovers from large signal saturation quickly. The BISx is resistant to
electro-surgical (ESU) interference.
The BISx is designed to be placed near the patient’s head. It is unobtrusive and conveniently
handled. It is sealed so as not to allow liquids to enter when splashed. The enclosure is not
painted, but rather is of materials that are a solid color throughout. The BISx includes a
convenient method for attaching and de-attaching the enclosure to surgical draperies, sheets, or an
IV pole.
The BISx is attached to the host monitoring system via a 9-foot monitor cable. The wire is narrow
and highly flexible. The monitor connection is integral to the enclosure (no pigtail), and may
require the use of tools for detachment from the device for service or replacement. The enclosure
is sealed against liquid ingress only when the monitor cable is attached. The connector on the host
monitor end is chosen in collaboration with the OEM host partner company. There are no adjustable
parts inside the BISx. The cables may be replaced without opening the enclosure.
The BISx connects to a sensor via the Aspect PIC Plus (Aspect part number 186-0107). The PIC Plus
is approximately 48 inches long. The PIC Plus connection is integral to the enclosure (no
pigtail), and can be detached from the device for service or replacement without the use of tools.
The enclosure is sealed against liquid ingress even when the PIC is detached.
Each BISx is given a unique serial identifier, allowing for electronic identification and tracking
of every device.
BISx can be connected and disconnected to an already powered up host monitor. The host monitor
should automatically detect its presence and configure it accordingly.
The BISx is a High Power, USB Powered Device which adheres to the power requirements of the USB 2.0
specification. The BISx requires 5 Vdc +/- 5% to operate and may draw up to 500 mA.
PHYSICAL SPECIFICATIONS:
BISx
Size: 95.3 mm (3.75”) diameter x 63.5 mm (2.5”) height
Weight: 227g (8.0 oz) without cables
456g (1.0 lb) with cables (Estimated)
Weight: 227g (8.0 oz) without cables
456g (1.0 lb) with cables (Estimated)
BISx Integral Cables
Monitor Cable: 2.74m (9ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
SAFETY SPECIFICATIONS:
| • | The BISx complies with the essential requirements of the Medical Device Directive 93/42/EEC, as well as IEC 60601-1 and IEC ▇▇▇▇▇-▇-▇▇. | |
| • | It is a Type BF applied part. It has internal optical coupling and an isolation transformer for patient isolation. | |
| • | It is protected against damage from defibrillation as long as the sensor is not located between the defibrillator pads and is resistant to artifact from electrosurgery. | |
| • | United States federal law restricts this device to sale by or on the order of a physician. | |
| • | BISx and cables are latex free. |
ENVIRONMENTAL SPECIFICATIONS:
Liquid Ingress Rating
IEC 529 IPX4
Temperature Range
Operating: 0 to 40°C (32 to 104°F)
Storage: -40 to 70°C (-40 to 158°F)
Storage: -40 to 70°C (-40 to 158°F)
Humidity
Operating: 10 to 95% RH at 40°C (104°F), non-condensing
Storage: 10 to 95% RH at 60°C (140°F), non-condensing
Storage: 10 to 95% RH at 60°C (140°F), non-condensing
Altitude Range
Operating: Up to 6,130 m (20,000 ft)
Storage: Up to 15,300 m (50,000 ft)
Storage: Up to 15,300 m (50,000 ft)
PERFORMANCE SPECIFICATIONS:
Noise (EEG Waveform)
|
< 0.3 µVRMS (2.0µV peak-to-peak) | |
BIS Numeric Update Frequency
|
Once per second | |
Bandwidth
|
0.25 to 100 Hz (- 3dB) | |
Impedance Measurement Range
|
0 to 999 kOhm | |
Input Impedance
|
>50 Mohm | |
Patient Leakage
|
<10µA | |
Measurement Ranges |
| Bispectral Index (BIS): | 0 to 100 | |||
| Electromyographic Strength (EMG): | 25 to 100 dB, where 1µV2=40dB | |||
| Signal Quality Index (SQI): | 0 to 100% | |||
| Suppression Ratio (SR): | 0 to 100% | |||
| Spectral Edge Frequency (SEF): | 0.5 to 30.0 Hz | |||
| Total Power: | 40 to 100 dB, where 1µV RMS=40dB | |||
| Burst Count: | 0 to 30 |
INTERFACE SPECIFICATIONS:
| Communication | ||||
| USB Interface: | Conforms to USB Rev. 1.1 Full-Speed protocol | |||
| Protocol: | Aspect proprietary | |||
| Power | 12 Vdc +/-25%, 400 mA maximum current draw | |||
| Connector | 3M Inc. 10120-6000E Connector, 20 position, .050” Mini D Ribbon (or equivalent) | |||
EXHIBIT C
CONTACT PERSONS/ADDRESSES
CONTACT PERSONS/ADDRESSES
Contact Persons and responsibilities at Aspect:
| Person | Title | Responsibility | ||||
▇▇▇▇▇▇▇ ▇▇▇▇▇▇
|
Director, Global OEM Partnerships | Relationship Manager | ▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇▇ ▇▇▇▇▇
|
▇▇. Director, Business Dvlmpt and Strategic Planning | Contract and Marketing | ▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇
|
Director, OEM Engineering | Project Manager | ▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇
|
▇▇. Director, RA/QA | Quality and Regulatory Matters | ▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇ ▇▇▇▇▇▇▇▇▇▇
|
▇▇. Support Representative | Technical Service | ▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇ |
Mailing Address:
Contact Persons and responsibilities at Philips:
| Person | Title | Responsibility | ||||
▇▇▇▇▇ ▇▇▇▇
|
Global Director, Product Marketing | Relationship Manager | ▇▇▇▇▇.▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇
|
Product Manager | Marketing | ▇▇▇▇▇▇.▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇▇▇ ▇▇▇▇▇
|
Q&R Manager | Quality and Regulatory | ▇▇▇▇▇.▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇▇▇ ▇▇▇▇▇▇
|
Product Support Engineer | Service and Support | ▇▇▇▇▇.▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇ | |||
▇▇▇▇▇ ▇▇▇▇▇▇▇
|
Contracts Analyst | Contracts | ▇▇▇▇▇.▇▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇ |
Mailing Address:
Philips Medizinsysteme Boblingen GmbH
▇▇▇▇▇▇▇-▇▇▇▇▇▇▇-▇▇▇▇▇▇▇ ▇
▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇
▇▇▇▇▇▇▇
▇▇▇▇▇▇▇-▇▇▇▇▇▇▇-▇▇▇▇▇▇▇ ▇
▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇
▇▇▇▇▇▇▇
EXHIBIT D
WARRANTY
WARRANTY
Aspect warrants to Philips that the BIS Products (“Warranted Product”) will be free from defects in
workmanship or materials, when given normal, proper, and intended usage for a period of 18 months
from the date of its initial shipment to Philips, or 12 months from the date of resale by Philips,
whichever period first expires (“Warranty Period”). This warranty shall not apply to expendable
components and supply items, such as, but not limited to, cables (except for failures occurring
within 180 days of receipt of shipment to Philips) or disposable items such as a BIS Sensor after
the expiration date marked on the BIS Sensor packaging. This warranty shall also not apply to any
component or workmanship of the Monitor Cable supplied by Philips, or any failure resulting from,
or relating to, the design of a Philips -specific connector (e.g. Multi-connector). Aspect’s
obligations under this warranty are to repair or replace any Warranted Product or part thereof that
Aspect reasonably determines to be covered by this warranty and to be defective in workmanship or
materials, provided that the Warranted Product is returned to the factory with freight prepaid.
Repair or replacement of BIS Products under this warranty does not extend beyond the Warranty
Period.
To request repair or replacement under this warranty, Philips should contact the Aspect Technical
Services contact as defined in Exhibit C. Aspect will authorize Philips to return the Warranted
Product (or part thereof) to Aspect. Aspect shall determine whether to repair or replace Warranted
Products and parts covered by this warranty and all Warranted Products or parts replaced shall
become Aspect’s property. In the course of warranty service, Aspect may, but shall not be
required, to make engineering improvements to the Warranted Product or part thereof. If Aspect
reasonably determines that a repair or replacement is covered by the warranty, Aspect shall bear
the costs of shipping the repaired or replacement Warranted Product to the Philips. All other
shipping costs shall be paid by Philips. Risk of loss or damage during shipments under this
warranty shall be borne by the Party shipping the Warranted Product. Warranted Products shipped by
Philips under this warranty shall be packaged in the original shipping container or equivalent
packaging to protect the Warranted Product. If Philips ships a Warranted Product to Aspect in
unsuitable packaging, any physical damage present in the Warranted Product upon receipt by Aspect
(and not previously reported) will be presumed to have occurred in transit and will be the
responsibility of Philips.
Unless authorized or instructed by Aspect in advance, this warranty does not extend to any
Warranted Products or part thereof: that have been subject to misuse, neglect or accident; that
have been damaged by causes external to the Warranted Product, including but not limited to failure
of or faulty electrical power; that have been used in violation of Aspect’s instructions; that have
been affixed to any nonstandard accessory attachment; on which the serial number has been removed
or made illegible; that have been modified by anyone other than Aspect; or that have been
disassembled, serviced or reassembled by anyone other than Aspect, unless authorized by Aspect.
Aspect shall have no obligation to make repairs, replacements, or corrections which result, in
whole or in part, from normal wear and tear. Aspect makes no warranty (a) with respect to any
products that are not Warranted Products, (b) with respect to any products purchased from a person
other than Aspect or an Aspect authorized distributor or (c) with respect to any product sold under
a brand name other than Aspect.
Aspect represents and warrants that, to the best of its knowledge after proper due diligence and
inquiry, that the Warranted Product to be provided to Philips for use or distribution by Philips
(including in Philips’ product packages or through a download from Philips’s website, or otherwise)
does not include any items that are subject to Open License Terms, including without limitation,
any Open Source Software. Aspect agrees that it will defend, indemnify and hold harmless Philips
and its customers against any and all losses, damages, costs and expenses arising from a breach by
Aspect of any of its obligations or representations under this paragraph, including, without
limitation, any third party claims in connection with any such breach.
EXHIBIT E
ASPECT TRADEMARKS
ASPECT TRADEMARKS
| Aspect Trademarks | Reference | |
Aspect ®
|
Aspect is a registered trademark of Aspect Medical Systems, Inc | |
A-2000 TM
|
A-2000 is a trademark of Aspect Medical Systems, Inc. | |
Bispectral Index ®
|
Bispectral Index is a registered trademark of Aspect Medical Systems, Inc. | |
BIS ®
|
BIS is a registered trademark of Aspect Medical Systems, Inc. | |

|
BIS logo is a registered trademark of Aspect Medical Systems, Inc. | |
BISx ™
|
BISx is a trademark of Aspect Medical Systems, Inc. | |
BIS Ready™
|
BIS Ready is a trademark of Aspect Medical Systems, Inc. | |
BIS VISTA ™
|
BIS VISTA is a trademark of Aspect Medical Systems, Inc. | |

|
BISx logo is a trademark of Aspect Medical Systems, Inc. | |

|
BIS Ready logo is a registered trademark of Aspect Medical Systems, Inc. |
EXHIBIT F
Philips Trademarks
Philips Trademarks
| Philips Trademarks | Reference | |
Philips [TM /®]
|
Philips is a [registered] trademark of [Koninklijke Philips Electronics N.V. Corporation (Netherlands)] | |
IntelliVue ®
|
IntelliVue is a registered trademark of Koninklijke Philips Electronics N.V. Corporation (Netherlands) | |

|
Philips logo is a registered trademark of [Koninklijke Philips Electronics N.V. Corporation (Netherlands)] |
EXHIBIT G
SERVICE, SUPPORT AND REPAIR
SERVICE, SUPPORT AND REPAIR
Service and Support.
| a) | Service and Support. Philips shall be responsible for providing installation, customer training, service and support (including repair) to its end customers for the Aspect Products sold hereunder and Philips shall bear all related costs incurred for labor, parts, or travel to perform such service. | ||
| b) | Central Repair Service. For the term of this Agreement, Aspect agrees to provide central repair service to Philips for Aspect Products sold hereunder at a charge and as further detailed below (Repair). | ||
| c) | Excessive Failure Rate. If the annual failure rate of Aspect’s BISx Kit excluding out-of-box failures and cables exceeds 9.5% (or such higher rate as may be applicable based on age of equipment) by more than 50% then Aspect shall reimburse Philips for any additional cost (including material and labor) incurred by Philips for repairing the units in excess of the above limit. | ||
| d) | Service Period. For a period of ten (10) years following the last delivery to Philips of the applicable Aspect Product ordered by Philips hereunder, Aspect shall make available repair service (or at Aspect’s sole discretion, exchange units for the Aspect Products) for purchase by Philips and third party users of the Aspect Products at Aspect’s then-current prices for such repair services and exchange units. After expiry of this ten (10) year period, Aspect may, in its sole discretion, continue to supply repair services (and/or exchange units for the Aspect Products) subject to the mutual written agreement of the Parties. | ||
| e) | Service Reporting. Aspect shall maintain a complete record of all repair activities performed on any Aspect Products received for repair, and will provide Philips with a monthly report on all service actions including failure and repair statistics at a sub-assembly levels set forth below (Repair). Service reports for each Aspect Product shall be sent via email to responsible procurement and technical marketing engineer (contact persons shown in Exhibit C). Root cause analysis is to be performed and reported by Aspect in case of abnormal failures, incidents and malfunctions. |
Repair
1. Repair Strategy:
Repair of defective units will be carried out by Aspect. The service strategy will be most likely
Full Unit Repair:
- Defective units will be shipped from Philips to Aspect for repair.
- Aspect repairs and ships the units within [**] calendar days to Philips after receipt.
Each repair unit will be labeled with an internal Philips order number and tracking number by Aspect. Tracking number and maybe other data must be labeled as barcode on the shipment carton by Aspect.
Each repair unit will be labeled with an internal Philips order number and tracking number by Aspect. Tracking number and maybe other data must be labeled as barcode on the shipment carton by Aspect.
2. Repair Price:
Repair consists of troubleshooting, repair, full testing (function, performance, safety) + admin
| To define for each Aspect Product: | ||||
Material cost:
|
Subassemblies/Repair Items | |||
Labor cost:
|
Troubleshooting | |||
| Testing | ||||
| Admin | ||||
Goal for full unit exchange: ARP (Average Repair Price)/ exchange unit
Right for Aspect to revise ARP on an annual basis.
Right for Aspect to revise ARP on an annual basis.
To define: Prices for NEW units/cables/sensors
3. Handling of Repair Costs:
Philips will pay the then-current ARP and freight costs for any defective unit that is returned to
Aspect. On a regular basis Philips shall determine the number of units under warranty that have
been replaced/repaired and Aspect shall reimburse Philips the ARP + freight costs.
With respect to repaired units with “No trouble found” classification, Aspect shall credit Philips
for the difference between ARP and the following amounts only: Troubleshooting, Testing, Admin +
Freight costs.

