CO-DEVELOPMENT AND LICENSE AGREEMENT between SINO BIOPHARMACEUTICAL CO., LTD. and AMBRX, INC. Dated as of January 13, 2020
Exhibit 10.10
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
CO-DEVELOPMENT AND LICENSE AGREEMENT
between
SINO BIOPHARMACEUTICAL CO., LTD.
and
AMBRX, INC.
Dated as of January 13, 2020
1
This CO-DEVELOPMENT AND LICENSE AGREEMENT (this “Agreement”), effective as of January 13, 2020 (the “Effective Date”), is between Ambrx, Inc., a Delaware corporation having its principal business address at 00000 Xxxxx Xxxxxx Xxxxx Xxxx, Xx Xxxxx, Xxxxxxxxxx 00000, XXX, for and on behalf of itself and its Affiliates (together with its Affiliates, “Ambrx”), and Sino Biopharmaceutical Co., Ltd., a company registered under the laws of the Cayman Islands, with its registered address in Cricket Square, Xxxxxxxx Drive, X.X. Xxx 0000, Xxxxx Xxxxxx, XX0-0000, Xxxxxx Islands, for and on behalf of itself and its Affiliates (together with its Affiliates, “Sino”). Ambrx and Sino may each be referred to herein individually as a “Party” or, collectively, as the “Parties.”
RECITALS
WHEREAS, Ambrx owns and/or controls Ambrx Background Technology (as hereinafter defined) and has rights to Licensed Intellectual Property Rights (as hereinafter defined) with respect to the Research Program (as hereinafter defined);
WHEREAS, Sino is a pharmaceutical company engaged in research, Development, and Commercialization of pharmaceutical products, including the human therapeutic products in the Sino Territory (as hereinafter defined);
WHEREAS, Sino desires to obtain an exclusive license under the Licensed Intellectual Property Rights (as defined below) in the Sino Territory upon the terms and conditions set forth herein, and Ambrx desires to grant such a license, in order for Sino to Develop, make, use, sell and offer for sale the Licensed Products (as hereinafter defined) for the prevention or treatment of human diseases and human conditions in the Sino Territory; and
WHEREAS, Sino desires to obtain assistance from Ambrx and Ambrx desires to offer such assistance to Sino to Develop the Research Program and the Licensed Products in the Sino Territory under world-class standards.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants contained herein, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
ARTICLE 1
DEFINITIONS
As used in this Agreement, the following terms shall have those meanings set forth in this Article 1 unless the context dictates otherwise.
| 1.1 | “Affiliate” shall mean, with respect to any Person, any other Person that directly or indirectly controls, is controlled by, or is under common control with such Person. A Person shall be deemed to control another Person if such Person possesses the power to direct or cause the direction of the management, business and policies of such Person, |
2
| whether through the ownership of fifty percent (50%) or more of the voting securities of such Person by voting agreement, by contract or otherwise. |
| 1.2 | “Agreement” shall have the meaning set forth in the introductory paragraph. |
| 1.3 | “Ambrx” shall have the meaning set forth in the introductory paragraph. |
| 1.4 | “Ambrx Background Technology” shall mean Know-How and Patent Rights that are owned and/or Controlled by Ambrx or any of its Affiliates as of the Effective Date or during the Term of this Agreement that is not Ambrx Research Program IP, including Ambrx Core Technology IP and the Ambrx Core Technology Platform. |
| 1.5 | “Ambrx Cell Materials” shall mean (a) the CHO cell lines Controlled by Ambrx that [***], (b) the E. coli strains Controlled by Ambrx that express [***], and (c) the special and proprietary supporting materials for (a) and (b) Controlled and provided by Ambrx. For clarity, Ambrx Cell Materials shall not include proprietary mammalian and bacterial cell lines used to create the Ambrx Cell Materials. |
| 1.6 | “Ambrx Core Technology IP” shall mean Ambrx Core Technology Patents and Ambrx Core Technology Know-How. |
| 1.7 | “Ambrx Core Technology Know-How” shall mean all Know-How owned or Controlled by Ambrx that is related to Ambrx Core Technology Platform. |
| 1.8 | “Ambrx Core Technology Patents” shall mean all Patent Rights that are Controlled by Ambrx that claim the Ambrx Core Technology Platform. |
| 1.9 | “Ambrx Core Technology Platform” shall mean Ambrx’s proprietary platform technology, as described in more detail in Exhibit 1.9, which includes but is not limited to: (i) Ambrx’s proprietary mammalian and bacterial cell lines used to create the Ambrx Cell Materials; (ii) the ReCODE™ platform; (iii) the EuCODE™ platform; and (iv) other technologies, such as conjugation chemistry technology. |
| 1.10 | “Ambrx Indemnified Parties” shall have the meaning set forth in Section 9.1. |
| 1.11 | “Ambrx Research Program Invention” shall mean any Research Program Invention discovered, generated, made or reduced to practice solely by Ambrx’s employees, independent contractors or consultants. |
| 1.12 | “Ambrx Research Program IP” shall mean all Ambrx Research Program Patents and Ambrx Research Program Know-How. |
| 1.13 | “Ambrx Research Program Know-How” shall mean all Know-How that is related to and arises from an Ambrx Research Program Invention. |
| 1.14 | “Ambrx Research Program Patents” shall mean any Patent Right that claims an Ambrx Research Program Invention. |
3
| 1.15 | “Ambrx Territory” shall mean all parts of the world except the Sino Territory. |
| 1.16 | “Ambrx Third Party License” shall have the meaning set forth in Section 2.4.1. |
| 1.17 | “Applicable Laws” shall mean the applicable laws of any jurisdiction which are applicable to any of the Parties or their respective Affiliates in carrying out activities hereunder or to which any of the Parties or their respective Affiliates in carrying out the activities hereunder is subject by law or by agreement, and shall include all statutes, enactments, acts of legislature, laws, ordinances, rules, Regulations, notifications, guidelines, policies, directions, directives and orders of any statutory authority, tribunal, board, or court or any central or state government or local authority or other governmental entity in such jurisdictions. |
| 1.18 | “Biosimilar Product” means, with respect to a Licensed Product, a biologic product: (a) for which Regulatory Approval is obtained by referencing Regulatory Filings of such Licensed Product; (b) that is approved for use in such country (or region) pursuant to a Regulatory Approval process governing approval of interchangeable or biosimilar biologics as described in 42 U.S.C. § 262, or a similar process for Regulatory Approval in any country (or region) outside the United States, or any other similar provision that comes into force, or is the subject of a notice with respect to such Licensed Product under 42 U.S.C. § 262(l)(2) or any other similar provision that comes into force in such country (or region); and (c) is sold in the same country as such Licensed Product by any Third Party that is not a Sublicensee of Sino or its Affiliates with respect to the Ambrx Research Program IP and Joint Research Program IP. |
| 1.19 | “Bispecific Molecule” shall mean any molecule that only binds to two biological targets simultaneously, and shall exclude any molecule that binds more than two biological targets or less than two biological targets simultaneously. |
| 1.20 | “BLA” means a Biologics License Application filed with the FDA in the United States, as defined in Title 21 of the U.S. Code of Federal Regulations, Section 601.2 et seq., or any non-U.S. counterpart of the foregoing. |
| 1.21 | “Calendar Quarter” shall mean the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31. |
| 1.22 | “Calendar Year” shall mean each successive period of twelve (12) months commencing on January 1 and ending on December 31. |
| 1.23 | “cGMP” or “Current Good Manufacturing Practice” shall mean the applicable then-current good manufacturing practice standards for manufacturing of pharmaceuticals or biologicals, as set forth in the United States Federal Food, Drug and Cosmetic Act, 21 U.S.C. §§ 301, as amended from time to time, together with any similar standards of good manufacturing practice as required by the FDA and other relevant Regulatory Authority. |
4
| 1.24 | “China GAAP” shall mean Generally Accepted Accounting Principles for the People’s Republic of China. |
| 1.25 | “Claimant” shall have the meaning set forth in Section 6.4.1. |
| 1.26 | “Claimant License Fee” shall have the meaning set forth in Section 6.5. |
| 1.27 | “Clinical Trial” means any Phase I Clinical Trial, Phase II Clinical Trial, or Phase III Clinical Trial. |
| 1.28 | “CMC Data” means any data included in the “Chemistry, Manufacturing and Controls” portion of a Regulatory Filings or in any supporting Development reports thereto, in each case, with respect to any Licensed Product in any country in the world. |
| 1.29 | “Commercialize” or “Commercialization” means any and all activities directed to the marketing and commercialization of the Licensed Product after Regulatory Approval, including pre-launch and post-launch marketing, promoting, distribution, detailing or commercially selling the Licensed Product (as well as importing and exporting activities in connection therewith). |
| 1.30 | “Commercially Reasonable Efforts” shall mean, with respect to the efforts to be expended by a Party [***]. |
| 1.31 | “Compensation” shall have the meaning set forth in Section 6.5. |
| 1.32 | “Confidential Information & Materials” shall mean any and all proprietary and/or confidential information, materials and data, including all scientific, pre-clinical, clinical, regulatory, process, formulation, manufacturing, marketing, financial and commercial information or data, compounds, cells and cell lines, whether communicated in writing or orally or by any other method, which are provided by one Party to the other Party prior to or during the Term of this Agreement. The fact that an item is known to the public shall not be taken to exclude the possibility that a compilation including the item, and/or a Development relating to the item, is (and remains) not known to the public. |
| 1.33 | “Control,” “Controls” or “Controlled by” shall mean, with respect to any Patent Rights, Know-How, Confidential Information & Materials, or other intellectual property assets or other items or rights, as applicable, possession by the Party granting the applicable right, license, access or release to the other Party as provided herein of the power and authority, whether arising by ownership, license, or other authorization, to disclose and deliver such Know-How, Confidential Information & Materials, and to grant and authorize under such Patent Rights, Know-How, Confidential Information & Materials the right, license, access or release, as applicable of the scope granted to such other Party in this Agreement without giving rise to any violation of the term of any written agreement with any Third Party existing at the time such disclosure is first made or such right, license access or release first comes into effect hereunder. Notwithstanding anything to the contrary in this Agreement, in the event that a Third Party merges or consolidates with or acquires a Party or an Affiliate of a Party, or a Party or an Affiliate of a Party transfers to a Third Party all or substantially |
5
| all of its assets to which this Agreement relates (such Third Party and its Affiliates immediately prior to such merger, consolidation or transfer (the “Acquisition Transaction”, collectively, the “Acquiring Entities”), then (a) any intellectual property or materials owned or Controlled by any Acquiring Entity (and not Controlled by such Party or its Affiliates) immediately prior to the effective date of such Acquisition Transaction, and (b) any intellectual property or materials independently developed or acquired by or on behalf of any Acquiring Entity after an Acquisition Transaction without accessing or practicing any Patent Rights, Know-How, Confidential Information & Materials made available to such Party under this Agreement, shall not be deemed be Controlled by such Party or its Affiliates after the effective date of such Acquisition Transaction for purposes of this Agreement. |
| 1.34 | “Cover” shall mean, with respect to Patent Rights and materials, products and services, that the research, Development, making, using, offering to sell, selling, importing, or exporting of such materials, products and services, would, but for the a license under such Patent Rights, infringe a Valid Claim of such Patent Rights in the Sino Territory. |
| 1.35 | “Development” or “Develop” means any and all research and development activities necessary or useful to obtain Regulatory Approval for a Licensed Product, including but not limited to all non-clinical, Scientific Development, Pre-Clinical development and clinical activities, research and development of companion diagnostics for use in connection with Clinical Trials of Licensed Products, as well as approved Licensed Products, drug development activities, animal pharmacology, toxicology, statistical analysis and report writing, activities to generate chemistry-manufacturing-and-control information, the distribution of Licensed Products for use in Clinical Trials (including placebos and comparators), Licensed Product approval and registration, and regulatory affairs related to the foregoing. When used as a verb, “Develop” means to engage in development. |
| 1.36 | “Development Plan” shall mean the plan setting forth the Scientific Development, Pre-Clinical Development, clinical, manufacturing and regulatory activities and timelines relating to the Development of Licensed Products in the Field to the completion of the Phase I Clinical Trials to be performed by Sino in the Sino Territory and by Ambrx in the Ambrx Territory for each of the Licensed Products, which plan shall be updated by the Joint Steering Committee from time to time. |
| 1.37 | “Disclosing Party” shall have the meaning set forth in Section 7.1. |
| 1.38 | “Dispute” shall have the meaning set forth in Section 10.7. |
| 1.39 | “Due Diligence Period” shall have the meaning set forth in Section 11.1. |
| 1.40 | “Effective Date” shall mean the date first set forth in the introductory paragraph. |
| 1.41 | “Exclusive Existing Third Party Licenses” shall have the meaning set forth in Section 2.4.2. |
6
| 1.42 | “Existing Third Party License” shall have the meaning set forth in Section 2.4.2. |
| 1.43 | “FDA” shall mean the United States Food and Drug Administration, or any successor thereto. |
| 1.44 | “Field” shall mean all indications and uses, including all human disease indications and therapeutic uses. |
| 1.45 | “Filing Party” shall have the meaning set forth in Section 6.2.1(I). |
| 1.46 | “First Commercial Sale” shall mean, with respect to any Licensed Product, the first sale to the general public of such Licensed Product in the Sino Territory after all required marketing and pricing approvals have been granted, or otherwise permitted, by the governing health authority of Sino Territory such as NMPA. “First Commercial Sale” shall not include the provision of any Licensed Product for use in Clinical Trials or for compassionate use, in each case, if such use is not compensated, prior to the receipt of necessary Marketing Authorization. |
| 1.47 | “Future Improvements” shall have the meaning set forth in Section 4.3.3. |
| 1.48 | “Future Third Party License” shall have the meaning set forth in Section 2.4.1. |
| 1.49 | “Generic Competition” shall mean the sale of Biosimilar Products in the Sino Territory by a Third Party. |
| 1.50 | “GLP” means the applicable then-current good laboratory practice standards as are required by applicable Regulatory Authorities or Applicable Law in the relevant jurisdiction, including in the United States, those promulgated or endorsed by the FDA in U.S. 21 C.F.R. Part 58, or the equivalent thereof as promulgated or endorsed by the applicable Regulatory Authorities outside of the United States. |
| 1.51 | “HKIAC” shall have the meaning set forth in Section 10.8. |
| 1.52 | “IND” shall mean (a) an investigational new drug application filed with the FDA for authorization for the investigation of a product, and (b) any of its foreign equivalents as filed with the applicable Regulatory Authorities in other countries or regulatory jurisdictions in the Sino Territory, as applicable. |
| 1.53 | “Indemnified Party” shall have the meaning set forth in Section 9.3. |
| 1.54 | “Indemnifying Party” shall have the meaning set forth in Section 9.3. |
| 1.55 | “Infringement” shall have the meaning set forth in Section 6.3.1. |
| 1.56 | “Initial Compounds” shall mean (a) the [***], and (b) the [***]. For clarity, in the circumstances of a Reload Compound under Section 3.2, with respect to any Initial Compound selected as a PCC by the Parties for Pre-Clinical Development and subsequently agreed by the Parties to cease Development under Section 3.2, any |
7
| improvements, modifications or upgrades of such ceased Initial Compound confirmed and accepted by the Parties as a Reload Compound shall forthwith not be considered as an Initial Compound. |
| 1.57 | “Initial PCC Requirements” shall have the meaning set forth in Section 4.2.2. |
| 1.58 | “Invention” shall mean any process, method, composition of matter, article of manufacture, discovery or finding that is conceived or reduced to practice. |
| 1.59 | “Joint Research Program Invention” shall mean any Research Program Inventions discovered, generated, made or reduced to practice in the performance of the Development Plan jointly by the Parties or any of their employees, independent contractors or consultants. |
| 1.60 | “Joint Research Program IP” shall mean all Joint Research Program Patents and Joint Research Program Know-How. |
| 1.61 | “Joint Research Program Know-How” shall mean all Know-How that is related to or arises from a Joint Research Program Invention. |
| 1.62 | “Joint Research Program Patents” shall mean any Patent Right that claims a Joint Research Program Invention. |
| 1.63 | “Joint Steering Committee” shall mean the entity organized and acting pursuant to Article 3. |
| 1.64 | “Know-How” shall mean all existing and future unpatented technical and other information or materials which are not in the public domain including information comprising or relating to discoveries, Inventions, data, designs, formulae, methods, models, assays, Development Plans, procedures, designs for experiments and tests and results of experimentation and testing, information related to cells or cell lines, processes (including manufacturing processes, specifications and techniques), laboratory records, chemical, pharmacological, toxicological, clinical, analytical and quality control data, trial data, case report forms, data analyses, reports or summaries and information contained in submissions to and information from ethical committees and regulatory authorities. Know-How includes rights protecting Know-How. |
| 1.65 | “License” shall mean all of the rights granted by Ambrx to Sino by this Agreement under the Licensed Intellectual Property Rights pursuant to Section 2.2.1. |
| 1.66 | “Licensed Compounds” shall mean (a) the Initial Compounds and (b) any Reload Compound that is selected by the unanimous decision of the Joint Steering Committee as a PCC in accordance with Section 3.2, which unanimous decision shall be reached without invoking casting vote of its chairperson under Section 3.4.2. |
| 1.67 | “Licensed Intellectual Property Rights” shall mean all existing (as of the Effective Date) and future Patent Rights and Know-How that are Controlled by Ambrx that are necessary or useful for the manufacture, Development or Commercialization of the Licensed |
8
| Compounds and the Reload Compound (if any), including but not limited to the Ambrx Background Technology, Platform Improvements, and any Patent Rights and Know-How Controlled by Ambrx under the Existing Third Party Licenses, Ambrx Third Party Licenses and Future Third Party Licenses. |
| 1.68 | “Licensed Product” shall mean any pharmaceutical product containing a Licensed Compound as an active pharmaceutical ingredient. |
| 1.69 | “Losses” shall have the meaning set forth in Section 9.1. |
| 1.70 | “MAA” means a Marketing Authorization Application, BLA, NDA, or similar application, as applicable, and all amendments and supplements thereto, submitted to the FDA, European Medicines Agency, or any equivalent filing in a country or regulatory jurisdiction other than the United States or the European Union with the applicable Regulatory Authority (including the NMPA), to obtain marketing approval for a pharmaceutical, biological, or diagnostic product, in a country or in a group of countries. |
| 1.71 | “Marketing Authorization” shall mean all approvals from NMPA necessary to market and sell a Licensed Product in the Sino Territory or a Regulatory Authority in a corresponding jurisdiction outside the Sino Territory. |
| 1.72 | “Milestone Events” shall have the meaning set forth in Section 5.2. |
| 1.73 | “Milestone Payments” shall have the meaning set forth in Section 5.2. |
| 1.74 | “NDA” means a New Drug Application submitted to the FDA, or any successor application or procedure, as more fully defined in 21 C.F.R. § 314.50 et seq. |
| 1.75 | “Negotiation Period” shall have the meaning set forth in Section 11.1. |
| 1.76 | “Net Sales” shall mean with respect to a Licensed Product, the total gross amounts invoiced by or on behalf of Sino or its Affiliate or Sublicensee for sales of such Licensed product after deducting, if not previously deducted, from the amount invoiced or received: |
| (a) | [***]; |
| (b) | [***]; |
| (c) | [***]; |
| (d) | [***]; |
| (e) | [***]; |
| (f) | [***]; |
| (g) | [***]; |
9
| (h) | [***]; |
| (i) | [***]; and |
| (j) | [***]. |
Any individual items that are estimated and deducted in calculating Net Sales shall be periodically (but at least on a Calendar Quarter basis) trued up and adjusted by Sino consistent with its customary practices and in accordance with China GAAP. Any deductions subsequently reversed shall be included in Net Sales for the royalty period in which such deductions are reversed. In no event shall the total amount of deductions made in accordance with Items 1.76(f) through 1.76(j) above during any period exceed [***] ([***]) of the gross invoice price for such period. The calculation of Net Sales hereunder shall be in accordance with China GAAP and Sino’s and/or its Affiliates’ customary accounting policies, applied consistently across periods, and:
(1) Transfer or sale of a Licensed Product within Sino, between Sino and an Affiliate, or between Sino and a non-Affiliate Third Party in which Sino has equity interest shall not be considered a sale, commercial use or disposition for the purpose of the foregoing paragraphs;
(2) in the event that Sino has to transfer or sell any Licensed Product to a non-Affiliate Third Party in which Sino has equity interest, Sino and Ambrx shall jointly discuss and determine the value of Net Sales; and
(3) in the event that Sino receives consideration for any Licensed Products in the case of transactions not at arm’s length with a non-Affiliate of Sino, Net Sales will be calculated based on the fair market value of such consideration or transaction, assuming an arm’s length transaction made in the ordinary course of business.
| 1.77 | “NMPA” shall mean National Medical and Pharmaceutical Administration in the People’s Republic of China, or any successor thereto. |
| 1.78 | “Non-Exclusive Existing Third Party License” shall have the meaning set forth in Section 2.4.2. |
| 1.79 | “Originating Licensor” shall have the meaning set forth in Section 2.7.1. |
| 1.80 | “Party” or “Parties” shall have the meaning set forth in the introductory paragraph. |
| 1.81 | “Patent Rights” shall mean any and all rights under any of the following, whether existing now or in the future, and whether or not filed: (i) a United States, international or foreign patent, utility model, design registration, certificate of invention, patent of addition or substitution, or other governmental grant for the protection of Inventions or industrial designs anywhere in the world, including any reissue, renewal, re-examination or extension thereof; and (ii) any application for any of the foregoing, including any international, provisional, divisional, continuation, continuation-in-part or continued prosecution application. |
10
| 1.82 | “[***]” shall mean the Bispecific Molecules set forth in Exhibit 1.82 wherein (a) [***] and (b) [***]. |
| 1.83 | “PEGylated IL-2 Molecule” shall mean the pegylated version of biological molecule Interleukin-2 through the site-specific conjugation with the incorporated unnatural amino acid set forth in Exhibit 1.83 modified by Ambrx under this Agreement meant to have reduced or abolished binding to alpha receptor (CD25). |
| 1.84 | “Pending Product Specific Patent Rights” shall have the meaning set forth in Section 2.1.4. |
| 1.85 | “Person” shall mean any natural person, corporation, firm, business trust, joint venture, association, organization, company, partnership or other business entity, or any government or any agency or political subdivision thereof. |
| 1.86 | “Phase I Clinical Trial” shall mean a human clinical trial in any country that would satisfy the requirements of 21 C.F.R. § 312.21(a), as may be amended, or the foreign equivalent thereof. |
| 1.87 | “Phase II Clinical Trial” shall mean a human clinical trial in any country that would satisfy the requirements of 21 C.F.R. § 312.21(b), as may be amended, or the foreign equivalent thereof. |
| 1.88 | “Phase III Clinical Trial” shall mean a human clinical trial in any country that would satisfy the requirements of 21 C.F.R. § 312.21(c), as may be amended, or the foreign equivalent thereof. |
| 1.89 | “Platform Improvements” shall have the meaning set forth in Section 6.1.4. |
| 1.90 | “PRC” shall mean the People’s Republic of China. |
| 1.91 | “Pre-Clinical Candidate” or “PCC” shall mean (a) each Initial Compound that meets the Initial PCC Requirements that is selected by the Joint Steering Committee in accordance with Section 3.2 and developed under the Development Plan; and (b) the Reload Compound (if any) that meets the Initial PCC Requirements and the further criteria set by the unanimous decision of Joint Steering Committee without invoking casting vote of its chairperson under Section 3.4.2, in each case to start the Development activities under Section 3.2 to enable IND filing by the Parties in their respective territories, including for completion of the Scientific Development by Ambrx and the subsequent Pre-Clinical Development by Sino in scale-up production, process optimization and other Development activities in regulatory aspects for Successful Dual IND Filing with Regulatory Authority. |
| 1.92 | “Pre-Clinical Data” shall mean data and information generated in the performance of Pre-Clinical Development. |
| 1.93 | “Pre-Clinical Development” shall mean those Development activities occurring in the Research Term subsequent to Scientific Development that are essential in preparation for |
11
| a Successful Dual IND Filing, commencing from delivery of a PCC to a Successful Dual IND Filing in all cases as provided in the applicable Development Plan. |
| 1.94 | “Product Specific Patent Rights” shall have the meaning set forth in Section 2.1.1. |
| 1.95 | “Receiving Party” shall have the meaning set forth in Section 7.1. |
| 1.96 | “Regulations” means regulations, statutes, rules, guidelines and procedures promulgated by any Regulatory Authority pursuant to Applicable Laws, including current GLP and current cGMP. |
| 1.97 | “Regulatory Approval” means all approvals, licenses, and authorizations of the applicable Regulatory Authority necessary for the marketing and sale of a pharmaceutical, biological, or diagnostic product for a particular indication in a country or region, including XXXx (if any). |
| 1.98 | “Regulatory Authority” shall mean any applicable government regulatory authority involved in granting approvals for the manufacturing, marketing, reimbursement and/or pricing of the Licensed Product or Licensed Compound in the Sino Territory or outside the Sino Territory, including, in the Sino Territory, NMPA, and, in the United States, the FDA and any successor governmental authority having substantially the same function. |
| 1.99 | “Regulatory Data” means any and all research data, pharmacology data, CMC Data, Safety Data, Pre-Clinical Data, clinical data and all other documentation submitted, or required to be submitted, to Regulatory Authorities in association with Regulatory Filings and Regulatory Approvals for Licensed Products (including any applicable drug master files or similar documentation). |
| 1.100 | “Regulatory Filing” means, with respect to the Licensed Compounds/Licensed Products, any submission to a Regulatory Authority of any appropriate regulation applications specific to the Licensed Compounds/Licensed Products, and shall include any submission to a regulatory advisory board any supplement or amendment thereto, regulatory registrations, applications, authorizations, and approvals (including approvals of XXXx, supplements and amendments, pre- and post-approvals, pricing approvals, and labeling approvals), Regulatory Approvals, and other submissions made to or with any Regulatory Authority for research, Development, manufacture, or Commercialization of a pharmaceutical, biological, or diagnostic product in a regulatory jurisdiction, together with all related correspondence to or from any Regulatory Authority and all documents referenced in the complete regulatory chronology for each MAA, including all drug master files (if any), INDs, BLAs, and NDAs, and foreign equivalents of any of the foregoing. |
| 1.101 | “Reload Compound” shall have the meaning set forth in Section 3.2. |
| 1.102 | “Reload Patent Rights” shall have the meaning set forth in Section 3.2. |
12
| 1.103 | “Remaining Compensation” shall have the meaning set forth in Section 6.5. |
| 1.104 | “Research Program” shall mean the research and Development program for the [***] and Reload Compound (if any) conducted under this Agreement, including, for clarity, under the Development Plan. |
| 1.105 | “Research Program Invention” shall mean any Invention discovered, generated, made or reduced to practice in the performance of the Research Program by the Parties that are not improvements to the Ambrx Core Technology Platform. |
| 1.106 | “Research Term” shall mean the period commencing on the Effective Date, and, unless this Agreement is earlier terminated, ending on the later to occur of (a) the fourth (4th) anniversary of the Effective Date, or (b) the sixth (6th) anniversary of the Effective Date in case of Development of a Reload Compound; and (c) the first Successful Dual IND Filing for a Licensed Product. |
| 1.107 | “Review Period” shall have the meaning set forth in Section 11.1. |
| 1.108 | “Right of Reference” means the “right of reference or use” defined in 21 C.F.R. § 314.3(b), or its equivalents outside the United States, and shall include the right to allow the applicable Regulatory Authority in a country to have access to relevant information (by cross-reference, incorporation by reference or otherwise) contained in Regulatory Filings (and any data contained or referenced therein) filed with such Regulatory Authority. |
| 1.109 | “Royalty Term” shall have the meaning set forth in Section 5.5. |
| 1.110 | “Rules” shall have the meaning set forth in Section 10.8. |
| 1.111 | “Safety Data” means any adverse event (as such term is used in the meaning set forth in Title 21 of the United States Code of Federal Regulations § 312.32 or its equivalents in the Sino Territory) information from human trials and all results from non-clinical safety studies, including toxicology and safety pharmacology data, with respect to a Licensed Product required by one or more Regulatory Authorities to be collected or to be reported to such Regulatory Authorities under Applicable Laws, but excluding any information related to the efficacy of the Licensed Product. |
| 1.112 | “Scientific Development” means the activities to be undertaken by Ambrx under the Research Program to generate the Pre-Clinical Candidates for the Licensed Compounds. |
| 1.113 | “Selected Improvements” shall have the meaning set forth in Section 4.3.3. |
| 1.114 | “Sino” shall have the meaning set forth in the introductory paragraph. |
| 1.115 | “Sino Indemnified Parties” shall have the meaning set forth in Section 9.2. |
13
| 1.116 | “Sino Investee” shall have the meaning set forth in Section 11.3. |
| 1.117 | “Sino Paid-up Amount” shall have the meaning set forth in Section 6.5. |
| 1.118 | “Sino Research Program Invention” shall mean any Research Program Invention discovered, generated, made or reduced to practice solely by Sino’s employees, independent contractors or consultants. |
| 1.119 | “Sino Research Program IP” shall mean all Sino Research Program Patents and Sino Research Program Know-How. |
| 1.120 | “Sino Research Program Know-How” shall mean all Know-How that is related to or arises from a Sino Research Program Invention. |
| 1.121 | “Sino Research Program Patents” shall mean any Patent Right that claims a Sino Research Program Invention. |
| 1.122 | “Sino Territory” shall mean all cities, zones, provinces, territories and other divisions or regions in and throughout the People’s Republic of China, and for the purpose of this Agreement, shall include the Hong Kong Special Administrative Region, the Macau Special Administrative Region and Taiwan. |
| 1.123 | “Sublicense” shall have the meaning set forth in Section 2.7.1. |
| 1.124 | “Sublicensee” shall have the meaning set forth in Section 2.7.1. |
| 1.125 | “Sublicensor” shall have the meaning set forth in Section 2.7.1. |
| 1.126 | “Successful Dual IND Filing” means an IND that is suitable for filing with and complies with the Regulations of both the NMPA and the FDA and which within thirty (30) days after submission to the FDA is not placed on clinical hold due to any concerns regarding Pre-Clinical Data or the sufficiency thereof. |
| 1.127 | “Taxes” shall have the meaning set forth in Section 5.11. |
| 1.128 | “Term” shall have the meaning set forth in Section 10.1. |
| 1.129 | “Third Party” shall mean a Person or entity other than Ambrx, Sino or their Affiliates. |
| 1.130 | “Third Party Manufacturer” shall have the meaning set forth in Section 4.6.1. |
| 1.131 | “Valid Claim” shall mean: (a) a claim of an issued and unexpired patent within a Patent Right that has not been (i) held permanently revoked, unenforceable, unpatentable or invalid by a decision of a court or governmental body of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, (ii) rendered unenforceable through disclaimer or otherwise, (iii) abandoned or (iv) permanently lost through an interference or opposition proceeding without any right of appeal or review; or (b) a pending claim of a pending patent application within the Patent Rights that (i) has been |
14
| asserted and continues to be prosecuted in good faith and (ii) has not been abandoned or finally rejected without the possibility of appeal or refiling. |
| 1.132 | “Withdrawal Notice” shall have the meaning set forth in Section 3.6. |
ARTICLE 2
ASSIGNMENTS, LICENSE, DEVELOP, COMMERCIALIZATION
| 2.1 | ASSIGNMENTS OF PRODUCT SPECIFIC PATENT RIGHTS IN THE SINO TERRITORY. |
| 2.1.1 | In consideration of and subject to payment of the upfront payment under Section 5.1, and subject to the assignment provisions of Section 3.2, Ambrx hereby irrevocably undertakes to assign and transfer to Sino or its designated Affiliate, at no additional consideration, any existing and future Patent Rights Controlled by Ambrx (or its Affiliates) in the Sino Territory that Cover solely the Licensed Compounds and/or the Licensed Products, including but not limited to any Patent Rights that claim the modifications, or method of use of any of the Licensed Compounds and/or the Licensed Products in the Sino Territory and the right to claim priority in Patent Right applications in the Sino Territory, but in each case excluding Ambrx Background Technology and any Patent Rights Controlled by Ambrx that claim any product or composition of matter other than a Licensed Compound and/or a Licensed Product (the “Product Specific Patent Rights”), to the full end of the term for which such Product Specific Patent Rights may be granted by the relevant government department or administration in charge of patent registrations in the Sino Territory, to the effect that Sino or its designated Affiliate shall become the sole registered owner or applicant (as applicable) of the Product Specific Patent Rights in the Sino Territory. A list of the existing Product Specific Patent Rights is set forth on Exhibit 2.1.1. |
| 2.1.2 | Upon Sino’s presentation of proof of upfront payment under Section 5.1, Ambrx shall sign an assignment agreement and/or all other necessary documents required for assignment of Patent Rights applications or change of applicant of the existing Product Specific Patent Rights in the Sino Territory to Sino or it designated Affiliate. |
| 2.1.3 | With respect to the Product Specific Patent Rights that are pending or issued in the Sino Territory, Ambrx shall assign the Product Specific Patent Rights pending or issued in the Sino Territory to Sino or its designated Affiliate within 60 days from the Effective Date to the extent assignable under Applicable Law, and as promptly as possible thereafter if not permitted to be assigned by Applicable Law within such 60 days. |
| 2.1.4 | With respect to the Patent Rights that are pending (including Patent Cooperation Treaty patent applications and provisional patent applications) or provisional that are eligible to claim priority to Product Specific Patent Rights in the Sino Territory (“Pending Product Specific Patent Rights”), Ambrx shall, within twenty (20) days after receipt of the registration or application numbers of such Pending Product Specific Patent Rights, notify Sino in writing of such pending or provisional Pending Product Specific Patent Rights. Upon Sino’s written request, Ambrx shall execute all documents and instruments, provide copy of all documents relating to the Pending Product Specific Patent Rights and shall do |
15
| all lawful acts, as may be reasonably necessary for assignment of Patent Right applications or change of applicant of the Pending Product Specific Patent Rights in the Sino Territory to Sino, so as to enable Sino or its designated Affiliate to make Patent Right applications or priority claims of such Pending Product Specific Patent Rights in the Sino Territory. |
| 2.2 | GRANT OF EXCLUSIVE LICENSES TO USE THE LICENSED INTELLECTUAL PROPERTY RIGHTS. |
| 2.2.1 | In consideration of and subject to the terms and conditions of this Agreement (including payment of the upfront payment under Section 4.1 5.1), Ambrx hereby grants to Sino exclusive right and license, with the right to grant Sublicenses subject to Section 2.7, under all existing and future the Licensed Intellectual Property Rights to Develop, make, have made and manufacture solely the Licensed Compounds and Licensed Products solely for use in the Field in the Sino Territory during the Term of this Agreement, provided that (a) for the Licensed Intellectual Property Rights self-owned by Ambrx, such grant shall be for a perpetual right with royalties payable in accordance with Article 5; (b) for Licensed Intellectual Property Rights constituted under the Existing Third Party Licenses, [***] (i) to the full end of the term or extension or renewed term under the relevant Existing Third Party Licenses; and (ii) in the event of a new agreement with the same Third Party licensor the subject matter of which include license for Patent Rights covered under the Existing Third Party Licenses, for the term or any extension or renewed term under such new agreement; and (c) for Licensed Intellectual Property Rights constituted under the Ambrx Third Party Licenses and the Future Third Party Licenses, such grant shall be (i) to the full end of the term or any extension or renewal under such agreements; and (ii) in the event of a new agreement with the same Third Party licensor the subject matter of which include license for Patent Rights covered under the Ambrx Third Party Licenses or the Future Third Party Licenses, for the term or extension or renewed term under the new agreement, in each case with royalty fees only payable upon First Commercial Sale of any Licensed Products and subject to royalty set-off in accordance with Section 5.6 (Third Party Royalty Set-off), in each case for so long as Ambrx Controls such Patent Rights. A list of the Licensed Intellectual Property Rights in existence as of the Effective Date is set forth in Exhibit 2.2.1 (Licensed Intellectual Property Rights self-owned by Ambrx) and Exhibit 2.4.2 (Existing Third Party Licenses). |
| 2.2.2 | Subject to the terms and conditions of this Agreement and as partial consideration for the rights granted by Ambrx under the License, Sino hereby grants to Ambrx an exclusive, perpetual, sub-licensable, royalty-free right and license in the Ambrx Territory, under Sino Research Program IP and the Product Specific Patent Rights, to make, use, sell, offer for sale, import and export products in the Ambrx Territory during the Term of this Agreement. |
| 2.2.3 | Ambrx agrees that during the Term of this Agreement, it will not grant any exclusive right and license under the Licensed Intellectual Property Rights to any Third Party to Develop, have Developed, use, manufacture, have manufactured, sell, offer for sale and have sold any product containing any Licensed Products for use in the Field and in the Sino Territory. |
| 2.3 | AMBRX RETAINED RIGHTS. The License granted by Ambrx to Sino under Section 2.2.1 is exclusive for use in the Field in the Sino Territory. Nothing in this |
16
| Agreement shall preclude Ambrx from granting license to use the Ambrx Background Technology and Ambrx Core Technology Platform for use not falling within scope of the Field in the Sino Territory. |
| 2.4 | THIRD PARTY LICENSE. |
| 2.4.1 | In the event that, during the Term of this Agreement and after the Effective Date, Ambrx (as licensee) enters into agreement(s) with any Third Party (as licensor) in respect of Licenses for rights in the Field to any Valid Claim of any issued Patent Right or Patent Right application issued to such Third Party that may be necessary for Sino’s exercise of its rights pursuant to Section 2.2 herein in the Sino Territory (an “Ambrx Third Party License”), for future Third Parties, Ambrx shall secure the right (a) to grant the Sublicense under this Section in any Ambrx Third Party License, (b) for licenses to Patent Rights that, but for such license, would be infringed by the making, using, selling, offering for sale or importation of a Licensed Product at the time and in the country in the Sino Territory in which such activity occurs (“Future Third Party License”). If Sino is required to pay certain royalty payments to such a Third Party under any such Ambrx Third Party License or Future Third Party License, either directly to the Third Party or indirectly to such Third Party through Ambrx, [***]. |
| 2.4.2 | A list of Ambrx’s existing Third Party licenses (“Existing Third Party License”) that are exclusive (“Exclusive Existing Third Party Licenses”) and non-exclusive (“Non-Exclusive Existing Third Party Licenses”) in each case that are essential for Development of each of the Licensed Compounds in the Territory is set forth in Exhibit 2.4.2. |
| 2.5 | NO ASSERTION BY AMBRX. So long as Sino is in compliance with the terms and conditions of this Agreement, Ambrx shall not assert against any claims for infringement of any Licensed Intellectual Property Rights by Sino’s permitted exercise of its rights hereunder solely for the purpose of Developing, making, having made, using, selling, offering for sale or having sold any Licensed Product in the Field in the Sino Territory during the Term of this Agreement. |
| 2.6 | RIGHTS OF REFERENCE; CLINICAL DATA RIGHTS. |
| 2.6.1 | Subject to the terms of this Agreement, Ambrx hereby grants Sino, its Affiliates and Sublicensees (solely to the extent permitted to make or own any Regulatory Filings or Regulatory Approvals) access to, and a Right of Reference with respect to (a) Regulatory Filings, Regulatory Approvals and all corresponding documentation, and (b) all Regulatory Data (including Safety Data and CMC Data contained or referenced in any Regulatory Filings), and all corresponding documentation, in each case ((a) and (b)) (i) to the extent Controlled by Ambrx or its Affiliates at any time during the Term, (ii) associated with the Pre-Clinical Development and/or the Phase I Clinical Trial conducted hereunder for a Licensed Product in the Field and (iii) for the sole purpose of Sino exercising its rights under the License in the Sino Territory during the Term. Upon written request from Sino, Ambrx shall provide to Sino or its Affiliates or Sublicensees (if permitted) a cross- |
17
| reference letter or similar communication to the applicable Regulatory Authority to effectuate such Right of Reference. |
| 2.6.2 | Subject to the terms of this Agreement and effective on the Effective Date, Sino hereby grants Ambrx, its Affiliates and their Sublicensees access to, and a Right of Reference with respect to: (a) Sino’s and its Affiliates’ and their Sublicensees’ Regulatory Filings and Regulatory Approvals and all corresponding documentation Controlled by Sino or its Affiliates or their Sublicensees at any time during the Term; and (b) all Regulatory Data (including Safety Data and CMC Data contained or referenced in any Regulatory Filings), and all corresponding documentation, in each case ((a) and (b)) (i) to the extent Controlled by Sino or its Affiliates at any time during the Term, (ii) associated with Pre-Clinical Development and/or the Phase I Clinical Trial conducted hereunder for a Licensed Product, and (iii) for the sole purpose of researching, Developing, making, having made, manufacturing, seeking and securing Regulatory Approval for and importing, exporting, selling and commercializing the Licensed Products in the Ambrx Territory. Upon written request from Ambrx, Sino shall provide to Ambrx, its Affiliates and Sublicensees (as applicable) a cross-reference letter or similar communication to the applicable Regulatory Authority to effectuate such Right of Reference. Notwithstanding anything in this Agreement to the contrary, the foregoing right of access and Right of Reference in this Section 2.6.2 shall survive any expiration or early termination of this Agreement for any reason. |
| 2.7 | SUBLICENSES. |
| 2.7.1 | Any Sublicense by either Party as sublicensor (the “Sublicensor”) of the rights granted to such Party under this Agreement to a Third Party or an Affiliate as sublicensee (“Sublicensee”) shall (a) require prior written approval from the party granting the originating license (“Originating Licensor”), such approval not to be unreasonably conditioned, withheld or delayed, (b) be consistent with the terms of this Agreement, and (c) shall include an obligation for the Sublicensee to comply with the applicable obligations of the sublicensing Party set forth in this Agreement. The Sublicensor shall be responsible and liable for the conduct and activities of each Sublicensee as if performed by the Sublicensor hereunder. The Sublicensor shall not grant any Sublicense hereunder that would impose obligations on the Sublicensee greater than those obligations of the Originating Licensor. The Sublicensor shall provide to the Originating Licensor a copy of each sublicense hereunder (“Sublicense”) promptly after entering into such Sublicense, which shall permit verification by the Originating Licensor of compliance with the provisions of this Agreement. |
| 2.8 | NO OTHER GRANT OF RIGHTS. Except as expressly provided herein, nothing in this Agreement will be construed to confer any ownership interest, license or other rights upon Sino by implication, estoppel or otherwise as to any technology, intellectual property rights, products or biological materials of Ambrx, or any other entity, regardless of whether such technology, intellectual property rights, products or biological materials are dominant, subordinate or otherwise related to any Ambrx Background Technology or Licensed Intellectual Property Rights. |
18
ARTICLE 3
JOINT STEERING COMMITTEE
| 3.1 | MEMBERS. The Parties shall establish a Joint Steering Committee (the “Joint Steering Committee”), which shall comprise six (6) members, three (3) designated by Sino and three (3) by Ambrx (or such other number as the Parties may agree in writing). The initial members of the Joint Steering Committee are set forth on Exhibit 3.1. Any member of the Joint Steering Committee may be represented at any meeting by a designee who is appointed by the Party designating such member for such meeting and who has authority to act on behalf of such member, as evidenced by written notice from the Party designating such member to the chairperson of the Joint Steering Committee. The chairperson of the Joint Steering Committee shall be designated by Ambrx. The initial chairperson is designated on Exhibit 3.1. Each Party shall be free to replace its representative members with new appointees who have authority to act on behalf of such Party on the Joint Steering Committee, on prior written notice to the other Party. |
| 3.2 | RESPONSIBILITIES. The Joint Steering Committee shall be responsible for providing oversight and coordinating the Scientific Development, Pre-Clinical Development, clinical Development and cGMP activities related to the Research Program and Development Plan, including: (a) maximizing the global opportunity and profitability of the Licensed Product while aligning the strategic, logistical and financial considerations of each Party; (b) reviewing and discussing the implementation of the Development Plan; (c) reviewing, discussing and approving amendments and updates to the Development Plan; (d) directing Development activities for the Licensed Product in accordance with the Development Plan; (e) aligning cGMP manufacturing activities and investments to provide the Licensed Compounds and Licensed Products for Clinical Trials and commercial supply in accordance with the Development Plan; and (f) selecting [***] and [***]. If the Parties mutually agreed to cease Development of a PCC after the relevant IND-enabling activities (including manufacture of such PCC for use in such IND-enabling activities) have been completed under the applicable Development Plan, then the Parties shall replace such PCC, no more than [***] during the Term, each time with a molecule that is Controlled by Ambrx upon mutual agreement, (“Reload Compound”) for Development under this Agreement, including with respect to a Successful Dual IND Filing for such Reload Compound, for no additional consideration under Section 5.1 (Upfront Payment). For clarity, (a) such replacement may only occur up to [***] during the Term, which may be (i) [***]; or (ii) [***]; and (b) any modifications, reworkings, improvements or upgrades of the Initial Compound for which the Parties mutually agreed to cease Development after the relevant IND-enabling activities can be considered as a Reload Compound. After completion of the due diligence of the Patent Rights that Cover such Reload Compound (“Reload Patent Rights”) to the satisfaction of Sino, Sino shall reassign the Product Specific Patents Rights for the replaced Licensed Compound to Ambrx, and concurrently Ambrx shall assign Reload Patent Rights to Sino. |
| 3.3 | MEETINGS. The Joint Steering Committee shall meet as frequently as the Parties deem appropriate during the first four (4)-year period following the Effective Date but no less frequently than once a Calendar Quarter (or more frequently, as agreed upon by the Parties) |
19
| thereafter, on such dates and at such times as the Parties shall agree, on ten (10) days’ written notice to the other Party unless such notice is waived by the other Party. The Joint Steering Committee may convene or be polled or consulted from time to time by means of telecommunications, video conferences or correspondence, as deemed necessary or appropriate by the Parties. To the extent that meetings are held in person, they shall alternate between the offices of the Parties unless the Parties otherwise agree. The chairperson shall be responsible for sending notices of meetings to all members. |
| 3.4 | DECISIONS. |
| 3.4.1 | A quorum for a meeting of the Joint Steering Committee shall require the presence of at least two (2) Ambrx members (or designees) and at least two (2) Sino members (or designees) in person or by telephone. All decisions made or actions taken by the Joint Steering Committee shall be made by consensus by its members, with the Ambrx members present at a meeting cumulatively having one (1) vote and the Sino members present at a meeting cumulatively having one (1) vote. |
| 3.4.2 | In the event that a consensus cannot be reached by the Joint Steering Committee with respect to a matter that is a subject of its decision-making authority within thirty (30) days after the matter is first brought before the Joint Steering Committee, then the matter shall be decided unanimously by the CEO of Ambrx and the CEO of Sino or by their designated representative. If the CEO of Ambrx and the CEO of Sino or their designated representative cannot reach a unanimous decision, then the chairperson of the Joint Steering Committee shall have the final decision-making authority; provided that (a) [***]; and (b) [***]. Notwithstanding the foregoing, in no event shall final decision-making authority be used (i) to require the other Party to violate any Applicable Law or any agreement it may have with any Third Party, (ii) to amend the terms and conditions of the Agreement, or (iii) to require the other Party to conduct any activities outside the scope of the Development Plan. |
| 3.5 | MINUTES. Within fifteen (15) days after each Joint Steering Committee meeting, the chairperson of the Joint Steering Committee shall prepare and distribute minutes of the meeting, which shall provide a description in reasonable detail of the discussions had at the meeting and a list of any actions, decisions or determinations approved by the Joint Steering Committee at such meeting. The chairperson of the Joint Steering Committee shall be responsible for circulation of all draft and final minutes. Draft minutes shall be circulated to all members of the Joint Steering Committee sufficiently in advance of the next meeting to allow review and comment prior to the meeting. Minutes shall be approved or disapproved, and revised as necessary, at the next meeting. Final minutes shall be distributed to the members of the Joint Steering Committee. |
| 3.6 | WITHDRAWAL. At any time during the Term and for any reason, either Party shall have the right to withdraw from participation in the Joint Steering Committee upon written notice to the other Party (a “Withdrawal Notice”), which shall be effective immediately upon receipt. Following the issuance of a Withdrawal Notice and subject to this Section 3.6, the withdrawing Party’s representatives on the Joint Steering Committee shall not participate in any meetings of the Joint Steering Committee. If, at any time, following |
20
| the issuance of a Withdrawal Notice, the withdrawing Party wishes to resume participation in the Joint Steering Committee, the withdrawing Party shall notify the other Party in writing and, thereafter, the withdrawing Party’s representatives on the Joint Steering Committee shall be entitled to attend any subsequent meeting of, and to participate in the activities of, the Joint Steering Committee as if a Withdrawal Notice had not been issued by the withdrawing Party. Following the withdrawing Party’s issuance of a Withdrawal Notice, unless and until the withdrawing Party resumes participation in the Joint Steering Committee in accordance with this Section 3.6: (i) all meetings of the Joint Steering Committee shall be held at the other Party’s facilities; and (ii) all decisions of the Joint Steering Committee shall be made by the remaining Party; (iii) the withdrawing Party shall have the right to continue to receive the minutes of the meetings of the Joint Steering Committee, but shall not have the right to approve the minutes for any such meeting held after the withdrawing Party’s issuance of the Withdrawal Notice. In any event, withdrawal from the Joint Steering Committee shall not impair Sino and Ambrx’s rights to receive reports or disclosures under Section 3.5. |
| 3.7 | TERM. The Joint Steering Committee shall exist until completion of Phase I Clinical Trial for a Licensed Product. Afterwards, each Party shall nominate an alliance manager to communicate and mutually coordinate activities. |
ARTICLE 4
DEVELOPMENT, REGULATORY, MANUFACTURING AND COMMERCIALIZATION
| 4.1 | DEVELOPMENT OVERVIEW. The Parties shall collaborate in the Development of Licensed Products under this Agreement in order to achieve a Successful Dual IND Filing for each of the [***], [***], and Reload Compound (if any), and thereafter, up until the completion of the Phase I Clinical Trial in the Field set forth in and in accordance with the Development Plan and Section 4.3 below. In the course of performance of its obligations under this Agreement, each Party shall co-operate and provide assistance to the other Party to achieve the common intent of successful Commercialization of the Licensed Products for sale in the respective territories of the Parties. Thereafter, the Parties may continue the Research Program or conduct Development activities in their respective territories separately in accordance with Section 4.4 below. |
| 4.2 | DEVELOPMENT RESPONSIBILITIES AND DEVELOPMENT PLAN. |
| 4.2.1 | As of the Effective Date, the Parties have agreed to undertake Scientific Development, Pre-Clinical Development and clinical Development under the Development Plan up until the completion of the Phase I Clinical Trials in the Field described therein in a collaborative manner, including with respect to (i) the indication(s) to be Developed; (ii) the proposed Development activities and allocation thereof between the Parties, (iii) the timeline for initiation and completion of activities, including target milestones, (iv) manufacturing process development, (v) clinical supply arrangements, and (vi) the Phase I Clinical Trial activities. Either Party may propose to the Joint Steering Committee and the other Party revisions to the Development Plan, with supporting evidence, such as technical, clinical or regulatory reasons that underline the proposed revisions. The Joint Steering Committee |
21
| shall approve the Development Plan within sixty (60) days after the Effective Date, and may amend the Development Plan from time to time after the Effective Date, pursuant to the decision-making mechanism as set forth in Section 3.4.2. The Parties shall use Commercially Reasonable Efforts to achieve the Successful Dual IND Filing. |
| 4.2.2 | Subject to further allocation of responsibilities under the Development Plan in Section 4.2.1 above, the Parties have agreed that Ambrx shall be responsible for Scientific Development of the Licensed Compounds and shall use Commercially Reasonable Efforts to provide PCCs of the Licensed Compounds that meet the requirements set forth in Exhibit 4.2.2 (“Initial PCC Requirements”), provided that, the Reload Compounds (if any) shall meet the additional and further success criteria set by the unanimous decision of the Joint Steering Committee without invoking casting vote by its chairperson under Section 3.4.2. |
| 4.2.3 | Except as expressly stated elsewhere in this Agreement (including Section 4.3.1), each Party shall bear the internal costs incurred by such Party in undertaking its activities under this Agreement, including under the Development Plan and with respect to the creation and submission of any Regulatory Filings for use by such Party. For clarity, Ambrx shall bear all the costs incurred by Ambrx for the Scientific Development of the PCCs of the Licensed Compounds and Sino shall bear all the costs for preparing the regulatory docket for dual IND filing of the Licensed Compounds in the PRC and the United States, as well as the costs for Pre-Clinical Development of the Initial Compounds, or as otherwise agreed to by the Parties. |
| 4.2.4 | The Parties anticipate that the Pre-Clinical Development for Licensed Compounds will be undertaken in stages by a contract research organization selected by Sino, which shall be suitably qualified and experienced in handling dual IND filing in the PRC and the United States. If, during such Pre-Clinical Development for a Licensed Compound, such Licensed Compound generates negative safety or efficacy signals or fails to meet the Initial PCC Requirements, a Reload Compound may be selected as a replacement in accordance with Section 3.2, and such Reload Compound may undergo Pre-Clinical Development with Ambrx and Sino sharing the costs equally until the end of Pre-Clinical Development. |
| 4.3 | RESEARCH PROGRAMS UNDER THE DEVELOPMENT PLAN; FUNDING. |
| 4.3.1 | Up until completion of the Phase I Clinical Trial for the Licensed Product described in the Development Plan, the Parties shall collaborate in the Development of the Licensed Product in accordance with the Development Plan, including budgets (if any) and timelines set forth in the Development Plan. Each Party shall use Commercially Reasonable Efforts to carry out the activities assigned to it under the Development Plan. |
| 4.3.2 | AMBRX CELL MATERIALS. Ambrx shall provide the Ambrx Cell Materials to Sino in accordance with the Development Plan. Sino shall use the Ambrx Cell Materials solely to further develop a manufacturing process for the Licensed Compounds and Licensed Products (including for cGMP scale-up) in accordance with the Development Plan. Sino shall not reverse engineer, chemically analyze, disassemble, or modify the Ambrx Cell Materials. Sino shall only have the right to provide the Ambrx Cell Materials to Third |
22
| Party contract manufacturers that are manufacturing the Licensed Products in connection with Sino exercising its rights under the License or to fulfill Sino’s supply obligations to Ambrx under this Agreement. Other than such Third Party contract manufacturers, Sino shall not provide the Ambrx Cell Materials to any Third Party without Ambrx’s prior written consent. |
| 4.3.3 | OPT OUT AND OPT IN SELECTED IMPROVEMENTS. Subject to the Licensed Intellectual Property Rights granted to Sino under Section 2.1.2 2.2.1, for any future improvements in (i) the Licensed Intellectual Property Rights; (ii) the Patent Rights constituted under Ambrx Third Party License; (iii) the Patent Rights constituted under Future Third Party Licenses; and (iv) the Ambrx Cell Material cell lines used by Ambrx to Manufacture Licensed Products for use in Clinical Trials or Commercialization by Ambrx, ((i), (ii), (iii) and (iv) collectively referred to as “Future Improvements”), Ambrx shall notify Sino in writing of such Future Improvements and Sino shall have an option, exercisable in its sole discretion from time to time during the Term, to opt out of or into such Future Improvements for Development of any of the Licensed Compounds or the Licensed Products during the Term in the Sino Territory. For clarity, (i) Future Improvements for which Sino has exercised its option to opt out shall not be included in Licensed Intellectual Property Rights; (ii) Future Improvements for which Sino has exercised its option to opt-in (“Selected Improvements”) shall be included in the Licensed Intellectual Property Rights in accordance with Section 2.2.1, effective as from the date of the Sino opt-in notice. Ambrx shall provide personnel and assistance to support Sino’s implementation of the Selected Improvements, and Sino shall only be liable to pay the full time equivalent cost of such support to Ambrx, calculated based on the number of days spent on the support and by reference to the basic salary of such supporting personnel without regard to other benefits. Except for the full time equivalent costs of the Ambrx personnel support and subject to royalties that may be payable under Section 5.3 and offsettable under Section 5.6, Sino shall not be liable to pay any additional fees and costs to Ambrx or to any Third Party for the Selected Improvements. |
| 4.4 | OUTSIDE THE DEVELOPMENT PLAN. Other than rights and obligations set forth in the Development Plan, Sino shall be solely responsible for conducting and paying for all Development and Commercialization of the Licensed Products in or for the Sino Territory, and Ambrx shall be solely responsible for conducting and paying for the Development and Commercialization of the Licensed Products outside the Sino Territory. Subject to Section 3.4.2, Sino shall have decision-making authority for (and an obligation to fund) additional activities not included in the Development Plan that Sino deems necessary or desirable for the Sino Territory; provided that such additional activities would not be reasonably likely to affect the Development or Commercialization of the Licensed Product outside the Sino Territory or outside the Field. |
| 4.5 | REGULATORY. Sino shall file and own all INDs and other Regulatory Filings required in connection with Sino’s Development of the Licensed Compounds and Licensed Products in the Sino Territory. Ambrx shall have the right to conduct Development and file and own INDs and other Regulatory Filings for the Development of the Licensed Compounds and |
23
| Licensed Products and to make its own filings with Regulatory Authorities outside the Sino Territory with respect thereto. |
| 4.5.1 | SAFETY DATA EXCHANGE. No later than initiation by Sino of a Clinical Trial in the Sino Territory, Sino shall enter into a Safety Data exchange agreement with Ambrx and/or its other licensee regarding the Licensed Product, which shall set forth standard operating procedures governing the collection, investigation, reporting and exchange of information concerning adverse drug reactions/experiences sufficient to permit each Party to comply with its regulatory and other legal obligations within the applicable timeframes. Such Safety Data exchange agreement shall identify which Party shall be responsible for the timely reporting of all relevant adverse drug reactions/experiences, product quality, product complaints and Safety Data relating to the Licensed Product to the appropriate Regulatory Authorities in and outside the Sino Territory in accordance with all Applicable Law. Such agreement shall allow each Party to comply with all regulatory and legal requirements regarding the management of Safety Data by providing for the exchange of relevant information in the appropriate format within applicable timeframes. Unless otherwise mutually agreed by the Parties, Ambrx or its other licensee shall maintain a global safety database for the Licensed Product, and Sino shall maintain one or more safety database(s) for the Licensed Product in the Field and the Sino Territory. |
| 4.6 | MANUFACTURING. |
| 4.6.1 | With respect to each Licensed Compound, prior to the initiation of Phase I Clinical Trial to be conducted under the Development Plan, Ambrx shall provide Pre-Clinical Candidate for each such Licensed Compound, having completed the Scientific Development of the PCC as agreed by the unanimous decision of the Joint Steering Committee without invoking casting vote of its chairperson under Section 3.4.2. Sino shall be responsible for and shall perform all cGMP manufacturing activities and Pre-Clinical Development (including cGMP scale-up) related to manufacturing the Licensed Compounds and Licensed Products in a manner in order to achieve a Successful Dual IND Filing, and the Parties shall co-operate and provide assistance to each other in support of seeking Successful Dual IND Filing. Sino (by itself or through its Affiliate or a Third Party contract manufacturer appointed by Sino) (“Third Party Manufacturer”) shall manufacture all Licensed Compounds and Licensed Products in accordance with cGMP applicable to the United States and the equivalent standard or Current Good Manufacturing Practice in the PRC in order to support a Successful Dual IND Filing. Sino’s Affiliate or the Third Party Manufacturer shall perform the technology transfer of the manufacturing process for each of the Licensed Products in accordance with the Development Plan [***], provided that any activities in addition to the activities described in the Development Plan would be [***]. Ambrx shall provide technical support (including upon mutual agreement on-site assistance) relating to the Ambrx Cell Materials, and Licensed Compounds with respect to manufacturing Licensed Compound and Licensed Products to Sino or its Affiliate or the Third Party Manufacturer [***]. |
| 4.6.2 | Upon request by Sino during the Research Term and for the period prior to the First Commercial Sale of a Licensed Product, for purposes of establishing manufacturing capability for each Licensed Compound and/or Licensed Product, Ambrx shall enable a |
24
| technology transfer to Sino or its Affiliate or the Third Party Manufacturer the manufacturing process required to manufacture the Licensed Compounds and/or the Licensed Products in the Sino Territory. Such enablement shall include a written description of the technology, together a copy of all relevant documents (including, without limitation, production manuals and experiment protocols), as well as on-site technical assistance at the facility designated by Sino, provided that [***]. |
| 4.6.3 | Sino shall be responsible for manufacturing the Licensed Compound and Licensed Products for the Phase I Clinical Trials conducted by Sino under this Agreement in support of Regulatory Approval in the Sino Territory through a Third Party Manufacturer. The Third Party Manufacturer costs, all fees and costs arising from engagement of the Third Party Manufacturer or manufacturing activities in the Sino Territory shall be borne by the Parties in accordance with Sections 4.2.3 and 4.2.4. Sino shall provide Ambrx with sufficient clinical supply to [***]. For the purpose of advancing Development and Commercialization of the Licensed Compounds and/or the Licensed Products in their respective territories, Sino and Ambrx agree to share all Regulatory Data and Regulatory Filings within sixty (60) days after the relevant submission to or obtaining of the grant of Regulatory Approval from the Regulatory Authority in their respective territories. Upon expiry of the Research Term, the Parties shall negotiate in good faith terms for a supply agreement under which Sino would fulfill Ambrx’s clinical and commercial needs for each Licensed Product and further Clinical Trials. |
| 4.7 | COMMERCIALIZATION. |
| 4.7.1 | Sino (itself and through its Affiliates and Sublicensees, as applicable) shall be solely responsible, at its own expense, for marketing, selling, offering for sale, distributing, promoting and otherwise commercializing the Licensed Product in the Field in the Sino Territory. |
| 4.7.2 | Sino shall use Commercially Reasonable Efforts to obtain Regulatory Approval for and Commercialize each Licensed Compound and Licensed Product in each country in the Sino Territory. |
| 4.8 | COMPLIANCE WITH APPLICABLE LAWS. Sino shall conduct, and shall cause its Affiliates and Sublicensees to conduct, all Development, regulatory, manufacturing and Commercialization activities with respect to Licensed Compounds and Licensed Products in the Field in the Sino Territory in compliance with all Applicable Laws, including good scientific and clinical practices under the Applicable Laws of the country in which such activities are conducted. |
| 4.9 | EXCLUSIVITY. Subject to the terms and conditions of this Agreement, during the Term (a) Ambrx and its Affiliates shall not in the Sino Territory, and (b) Sino itself, any Sino Affiliate or the Sino Investee, each that has been assigned any rights or obligations under this Agreement, and each anywhere in the world shall not, in each case (a) and (b) research, Develop, manufacture or Commercialize, directly or indirectly, by itself or with a Third Party, any of the following: (i) [***]; (ii) any project related to a Bispecific Molecule that [***]; (iii) [***]; and (iv) a biological molecule that is [***]. For the avoidance of doubt, |
25
| the above restrictions on the Sino Affiliate and/or the Sino Investee shall only cover the period during which the rights and obligations under this Agreement are effectively assigned and assumed by the relevant Sino Affiliate and/or the Sino Investee. In the event that there is any re-assignment of rights and obligations from the relevant Sino Affiliate and/or Sino Investee (in each case as an assignor) to a new permitted assignee designated by Sino, the above restrictions shall no longer apply to the assigning Sino Affiliate and/or the Sino Investee as from the date on which such re-assignment of the rights or obligations are made effective. For clarity, the foregoing does not restrict either Party from researching, Developing, manufacturing or Commercializing any of the following: (1) [***], (2) [***], (3) [***], (4) [***], (5) [***], and (6) [***]. |
ARTICLE 5
MONETARY OBLIGATIONS, REPORTS AND AUDITS
| 5.1 | UPFRONT PAYMENT. Sino shall pay to Ambrx, within [***] ([***]) business days of the Effective Date, a one-time payment in cash of Ten Million Dollars ($10,000,000 USD), which payment shall be non-refundable (other than as set forth in Section 6.5 and Section 10.3A) and non-creditable and not subject to set off. |
| 5.2 | MILESTONE PAYMENTS. As set forth in the following table, Sino shall make the following payments in cash (the “Milestone Payments”) to Ambrx upon achievement of each of the milestone events set forth in the tables below (the “Milestone Events”) for any Licensed Products by Sino or its Affiliates or Sublicensees. Each Milestone Payment shall be payable by Sino to Ambrx within thirty (30) days after the achievement of the corresponding Milestone Event and receipt of invoice from Ambrx with respect to each of the Licensed Products. Such payments shall be non-refundable and non-creditable and not subject to set-off (other than as set forth in Section 6.5). |
The following Milestone Payments shall apply for each Licensed Product:
| Milestone Event |
Milestone Payment | |
| [***] |
[***] | |
| [***] |
[***] | |
| [***] |
[***] | |
| [***] |
[***] | |
| [***] |
[***] |
| 5.3 | ROYALTIES PAYABLE BY SINO. Subject to the terms and conditions of this Agreement, Sino shall pay Ambrx royalties in an amount equal to the following percentage of Net Sales of each Licensed Product sold by Sino, its Affiliates or Sublicensees: |
| 5.3.1 | [***] ([***]) of such Net Sales of each Licensed Product in the Sino Territory in each Calendar Year up to and including Net Sales of [***] ([***]); |
26
| 5.3.2 | [***] ([***]) of such Net Sales of each Licensed Product in the Sino Territory in each Calendar Year for the portion of such Net Sales exceeding [***] ([***]) up to and including [***] ([***]); and |
| 5.3.3 | [***] ([***]) of such Net Sales of each Licensed Product in the Sino Territory in each Calendar Year for the portion of such Net Sales exceeding [***] ([***]). |
| 5.4 | KNOW-HOW ROYALTY. Notwithstanding the provisions of Section 5.3 above, in the event that (a) the manufacture, use or sale of Licensed Products by Sino or its Affiliates in a country in the Sino Territory is not Covered by a Valid Claim of the Patent Right within the Licensed Intellectual Property Rights or Sino Research Program Patents or Ambrx Research Program Patents in such country, or (b) Generic Competition occurs in a country in the Sino Territory, then the royalty payments due to Ambrx [***]. For clarity, upon the first occurrence of (a) or (b) above, the royalty payments due to Ambrx shall be [***]. |
| 5.5 | ROYALTY TERM. Royalties on the Licensed Product at the rates set forth above in Section 5.3 shall be calculated, reported and paid by Sino on a quarterly basis during the period commencing with the First Commercial Sale of such Licensed Product in a country in the Sino Territory and continue until the expiration of [***] ([***]) years after such First Commercial Sale of the Licensed Product in such country (the “Royalty Term”). |
| 5.6 | THIRD PARTY ROYALTY SET-OFF. If Sino, under an agreement with a Third Party for licenses to Patent Rights that, but for such license, would be infringed by the making, using, selling, offering for sale or importation of a Licensed Product at the time and in the country in which such activity occurs, is required to pay any royalty that is as a result of Sino’s exercise of its rights under such Third Party license, Sino may offset [***] ([***]) of any royalty payments actually paid by Sino to all such Third Parties due under such licenses in the aggregate with respect to sales of Licensed Products against the royalty payments that are due to Ambrx; provided that in no event shall the royalty payments to Ambrx with respect to such Licensed Products be [***] ([***]) of the amount otherwise due (“Set-Off Limit”). |
| 5.7 | THIRD PARTY PAYMENTS. Subject to the terms of this Agreement and Sections 4.2.3 and 4.2.4, each Party shall be responsible for and at its sole expense shall pay all amounts owing by such Party to any Third Party for Development activities under the Development Plan performed by Third Parties. |
| 5.8 | REPORTS. During the Term following the First Commercial Sale of the Licensed Product, Sino shall furnish to Ambrx a quarterly written report for the Calendar Quarter showing the gross and Net Sales of all Licensed Products subject to royalty payments sold by Sino and its Affiliates in the Sino Territory during the reporting period and the royalties payable under this Agreement. Reports shall be due on the forty-fifth (45th) day following the close of each Calendar Quarter. Royalties shown to have accrued by each royalty report shall be due and payable on the date such royalty report is due. Sino and its Affiliates shall keep complete and accurate records in sufficient detail to enable the royalties payable hereunder to be determined. |
27
| 5.9 | AUDITS. |
| 5.9.1 | ACCOUNTING FIRM. Upon the written request of Ambrx and not more than once in each Calendar Year, Sino shall permit a qualified and reputable independent certified public accounting firm selected by Ambrx and approved by Sino, such approval not to be unreasonably withheld, at Ambrx’s expense, to have access during normal business hours to such of the records of Sino’s designated Affiliate that is Developing and/or Commercializing Licensed Products that are related to the production and sales of Licensed Products as may be reasonably necessary to verify the accuracy of the royalty reports pursuant to Section 5.8 for any Calendar Year ending not more than thirty-six (36) months prior to the date of such request. The accounting firm shall disclose to Ambrx and Sino whether the royalty reports are correct or incorrect and the amount of any discrepancy. |
| 5.9.2 | ACCESS. In order to fulfill the auditing, the accounting firm so selected shall have the right to access, examine, review and copy, the books or accounts of any Affiliate designated by Sino for sales of the Licensed Products, such access shall be restricted to the extent relevant to determine the accuracy of the royalty reports, and shall include the relevant procurement/distribution agreements and other purchase/sales contracts, purchase/sales orders, operation records, tax paid to local government, and itemized tax for the Licensed Products, and to make enquiries with respect to such Sino Affiliate, to the extent reasonably necessary for determining the accuracy of the royalty reports. The relevant Sino Affiliate shall not unreasonably restrict the accounting firm’s access to its premises during normal business hours. |
| 5.9.3 | PAYMENT AND FEES. If such accounting firm identifies a discrepancy made during such period, the appropriate Party shall pay the other Party the amount of the discrepancy within thirty (30) days of the date Ambrx delivers to Sino such accounting firm’s written report so concluding, or as otherwise agreed upon by the Parties. The fees charged by such accounting firm shall be paid by Ambrx, provided, however, that if such audit uncovers an underpayment of royalties by Sino that exceeds [***] ([***]) of the total royalties owed for the period in question, the fees of such accounting firm shall be equally shared by Ambrx and Sino. |
| 5.9.4 | SUBLICENSES. Sino shall include in each Sublicense granted by it pursuant to this Agreement a provision requiring the Sublicensee to make reports to Sino, to keep and maintain records of sales made pursuant to such Sublicense and to grant access to such records by Ambrx’s independent accountant to the same extent required of Sino under this Agreement. |
| 5.9.5 | CONFIDENTIALITY. Ambrx shall treat all financial information subject to review under this Section 5.9.5 or under any Sublicense agreement in accordance with the terms of Article 7 of this Agreement, and shall cause its accounting firm to enter into an acceptable confidentiality agreement with Ambrx, or with Sino and/or its Affiliates or Sublicensee, obligating it to retain all such information in confidence pursuant to such confidentiality agreement. |
28
| 5.10 | PAYMENT EXCHANGE RATE; LATE PAYMENTS. All royalty payments due hereunder shall be paid in United States dollars by wire transfer to a bank account designated by Ambrx. Any payments or portions thereof due hereunder which are not paid on the date such payments are due under this Agreement shall bear interest [***], calculated on the number of days such payment is delinquent. If the royalty payments are paid in any currency other than United States Dollars, the Parties shall apply the [***]. |
| 5.11 | TAX WITHHOLDING. All payments under this Agreement shall be net of any taxes or duties, other than income taxes of Ambrx, owed thereon, which taxes (other than Ambrx income taxes) shall be the responsibility of Sino (“Taxes”). Ambrx shall be liable for all income and/or other taxes (including interest) imposed upon any royalty payments made by Sino to Ambrx under this Agreement. In the event Applicable Laws require withholding of Taxes, Sino shall notify Ambrx in writing of the amount of tax payable in advance, before it makes such withholding payments and subtracts the amount thereof from the payments. Sino shall submit appropriate proof of payment of the withheld Taxes to Ambrx and shall provide Ambrx with the official receipts within a reasonable period of time. Notwithstanding the foregoing, to the extent permitted by Applicable Laws and upon the request of Ambrx, Sino shall use Commercially Reasonable Efforts to apply for approvals from competent PRC tax authorities, on behalf of Ambrx, for reduction or exemption of applicable PRC taxes on the payments made by Sino to Ambrx, before it makes the withholding payments and subtracts the amount thereof from the payments due to Ambrx. |
| 5.12 | PAYMENT PROCEDURES. Sino shall be responsible for obtaining any and all governmental approval/registration procedures (if legally required) and foreign exchange-related procedures/formalities in connection with repatriation of any payments made by Sino to Ambrx under the Agreement to the bank accounts designated by Ambrx outside China, and Ambrx will provide reasonable assistance if necessary. |
ARTICLE 6
INTELLECTUAL PROPERTY
| 6.1 | OWNERSHIP OF INTELLECTUAL PROPERTY. |
| 6.1.1 | OWNERSHIP OF SELF-CONTROLLED IP OUTSIDE THIS AGREEMENT. As between the Parties, each Party will retain ownership of all Patent Rights, Know-How and other intellectual property rights that are Controlled by such Party prior to the Effective Date or are otherwise developed, made, conceived of or reduced to practice by such Party. |
| 6.1.2 | OWNERSHIP OF LICENSED COMPOUNDS, LICENSED PRODUCTS AND PRODUCT SPECIFIC PATENTS. As between Sino and Ambrx, Sino is the sole owner of all existing and future rights, titles and interests in and to the Licensed Compounds, the Licensed Products and the Product Specific Patent Rights in each case in the Sino Territory, and Ambrx is the sole owner of all existing and future rights, titles and interests in the Licensed Compounds and the Licensed Products in each case in the Ambrx Territory. |
29
| 6.1.3 | OWNERSHIP OF RESEARCH PROGRAM IP INDEPENDENTLY DEVELOPED BY EACH PARTY. |
| (I) | Sino is the sole owner: |
| (a) | any Sino Research Program Patents in the Sino Territory; and |
| (b) | any Ambrx Research Program Patents in the Sino Territory. |
| (II) | Ambrx is the sole owner of any Ambrx Background Technology and: |
| (a) | any Sino Research Program Patents in the Ambrx Territory; and |
| (b) | any Ambrx Research Program Patents in the Ambrx Territory. |
| 6.1.4 | OWNERSHIP OF PLATFORM IMPROVEMENTS. Ambrx is the sole owner of any improvements to the Ambrx Core Technology Platform made, conceived of, reduced to practice, or generated by either or both of the Parties (such improvements “Platform Improvements”). So long as the Ambrx Core Technology Patents relevant to the Platform Improvements remains valid, Sino hereby assigns to Ambrx all of Sino’s right, title and interest in, to and under all Platform Improvements and all intellectual property rights therein. |
| 6.1.5 | OWNERSHIP OF JOINT RESEARCH PROGRAM PATENTS. |
| (a) | With respect to interests in Joint Research Program Patents in the Sino Territory, Sino is the sole owner; and |
| (b) | With respect to interests in Joint Research Program Patents in the Ambrx Territory, Ambrx is the sole owner. |
| 6.1.6 | OWNERSHIP OF INVENTION OUTSIDE THE FIELD. Ownership of Invention by each Party outside the Field shall be owned by the Parties in the following manner: |
| (a) | With respect to any interests arising from any Invention outside the Field, which is discovered, generated, made or reduced to practice solely by Sino’s employees, independent contractors or employees in the course of performance of the Research Program, such interests in the Sino Territory shall be solely owned by Sino and such interests in the Ambrx Territory shall be solely owned by Ambrx. |
| (b) | With respect to any interests arising from any Invention outside the Field, which is discovered, generated, made or reduced to practice solely by Ambrx’ s employees, independent contractors or employees in the course of performance of the Research Program, such interests in the Sino Territory shall be solely owned by Sino and such interests in the Ambrx Territory shall be solely owned by Ambrx. |
30
| 6.1.7 | ASSIGNMENT. Each Party shall assign and hereby assigns to the other Party such Party’s right title and interest in, to and under the intellectual property rights (including Patent Rights) that are assigned to such other Party under this Section 6.1. |
| 6.2 | FILING, PROSECUTION AND MAINTENANCE OF PATENTS. |
| 6.2.1 |
(I) JOINT RESEARCH PROGRAM IP. Each Party shall have the right to file Patent Right applications for Joint Research Program Patents, with respect to Sino in the Sino Territory, and with respect to Ambrx in the Ambrx Territory and thereafter prosecute and maintain Patent Rights for such Joint Research Program Patents in its territory. In the event that any Party files such Patent Right applications and thereafter prosecutes and maintains Patent Rights for such Joint Research Program Patents in its territory (such Party, the “Filing Party”), upon the request of the Filing Party, the other Party shall co-operate and shall execute such documents and perform such ministerial acts, at the costs and expenses of the Filing Party, as may be reasonably necessary for the Filing Party to continue such prosecution or maintenance of Patent Rights claiming such Joint Research Program Patents. With respect to a given Joint Research Program Patent, the Filing Party may elect to file or may elect not to file in a particular country and, if so, the Filing Party shall notify the other Party.
(II) PATENT RIGHTS FOR RESEARCH PROGRAM IP. Sino shall have the right, at its sole cost and expense, to file Patent Right applications in the Sino Territory for (i) Patent Rights for Sino Research Program IP; (ii) Patent Rights for Ambrx Research Program IP; and (iii) Product Specific Patent Rights; and Ambrx shall have the right, at its sole cost and expense, to file Patent Right applications in the Ambrx Territory for (iv) Patent Rights for Ambrx Research Program IP; and (v) Patent Rights for Sino Research Program IP. In the event that any Filing Party files such Patent Right applications and thereafter prosecutes and maintains Patent Rights for such Patent Rights under this (i) to (v) of this paragraph, upon the request of the Filing Party, the other Party shall co-operate and shall execute such documents and perform such ministerial acts, at the costs and expenses of the Filing Party, as may be reasonably necessary to continue such prosecution or maintenance of such Sino Research Program Patents and Ambrx Research Program.
| 6.2.2 | REVIEW AND CONSULTATION. In each case in connection with the foregoing with respect to Patent Rights, as applicable, the Filing Party (a) shall keep the other Party advised of the status of the actual and prospective patent filings; (b) upon the other Party’s written request, shall provide advance copies of any papers related to the filing, prosecution and maintenance of such patent filings; (c) shall give the other Party an opportunity to review the text of the application before filing and shall consult with the other Party with respect thereto; (d) shall give the other Party an opportunity to review and comment on any documents relating to such patent filings that will be filed in any patent office at least twenty (20) days before such filing and give due consideration to such substantive, non-cumulative comments; (e) shall supply the other Party with a copy of the application as-filed, together with notice of its filing date and serial number; and (f) shall promptly give notice to the other Party of the grant, lapse, revocation, surrender, invalidation or |
31
| abandonment of any Patent Rights for which it is responsible for the filing, prosecution or maintenance hereunder (provided that the filing Party shall give at least thirty (30) days’ prior written notice to the other Party of any desire to cease prosecution and/or maintenance of such Patent Rights). With respect to a given Patent Right, the Filing Party may elect not to prosecute and maintain such Patent Right, and, if so, the Filing Party shall notify the other Party and the other Party shall have the right (but not the obligation) to prosecute and maintain such Patent Right. |
| 6.2.3 | COSTS. The Filing Party shall be responsible for the costs of filing Patent Right applications and procuring and maintaining Patent Rights for such Joint Research Program Patents, Ambrx Research Program Patents and Sino Research Program Patents that it has a right to file, prosecute and maintain under this Article 6. |
| 6.3 | ENFORCEMENT OF PATENT RIGHTS. |
| 6.3.1 | NOTICE. Each Party shall promptly notify the other Party of any infringement or possible infringement by a Third Party of any Patent Rights licensed or assigned to Sino under this Agreement. Further, Ambrx shall give Sino, and Sino shall give Ambrx, notice of any infringement of any Patent Rights that a Filing Party has the right to prosecute and maintain in the Sino Territory, or any misappropriation or misuse of Joint Research Program Know-How, Ambrx Research Program Know-How or Sino Research Program Know-How, that may come to Ambrx’s or Sino’s attention (“Infringement”). Sino and Ambrx shall thereafter consult and cooperate fully to determine a course of action, including, but not limited to, the commencement of legal action by Sino and/or Ambrx, to terminate any Infringement of such Patent Rights or any misappropriation or misuse of such Know-How, as applicable. |
| 6.3.2 | SUIT BY SINO. Sino shall have the first right, but not the obligation, to initiate and prosecute such legal action at its own expense and in the name of Sino to terminate any Infringement in the Sino Territory, as applicable. Should Sino elect to bring suit against an infringer, Sino shall keep Ambrx reasonably informed of the progress of the action and shall give Ambrx a reasonable opportunity in advance to consult with Sino and offer its views about major decisions affecting the litigation. Sino shall give careful consideration to Ambrx’s views, but shall have the right to Control the action; provided, however, that if the validity and/or enforceability of the Ambrx Background Technology (including the Ambrx Core Technology IP) is raised by the infringer in the action, or if Sino’s License to a Valid Claim in the suit terminates, Ambrx may elect to take control of the action pursuant to Section 6.3.3. Should Sino elect to bring suit against an infringer and Ambrx is joined as party plaintiff in any such suit, Sino shall have the right to approve the counsel selected by Sino to represent Sino and Ambrx, such approval not to be unreasonably withheld. The expenses of such suit or suits that Sino elects to bring, including any expenses of Ambrx incurred in conjunction with the prosecution of such suits or the settlement thereof, shall be paid for entirely by Sino and Sino shall hold Ambrx free, clear and harmless from and against any and all costs of such litigation, including reasonable attorneys’ fees. Sino shall not compromise or settle such litigation without the prior written consent of Ambrx, which consent shall not be unreasonably withheld or delayed. In the event Sino exercises its right to xxx pursuant to this Section 6.3.2, it shall first reimburse itself out of any sums recovered |
32
| in such suit or in settlement thereof for all costs and expenses of every kind and character, including reasonable attorneys’ fees, necessarily incurred in the prosecution of any such suit. If, after such reimbursement, any funds shall remain from said recovery, then Ambrx shall receive an amount equal to [***] ([***]) of such funds and the remaining [***] ([***]) of such funds shall be retained by Sino. |
| 6.3.3 | SUIT BY AMBRX. If Sino does not take action in the prosecution, prevention or termination of any infringement pursuant to Section 6.3.2 above, and has not commenced negotiations with the infringer for the discontinuance of said infringement, within ninety (90) days after receipt of notice to Sino by Ambrx of the existence of an Infringement, Ambrx may elect to do so. Should Ambrx elect to bring suit against an infringer and Sino is joined as party plaintiff in any such suit, Ambrx shall have the right to select the counsel to represent Ambrx and Sino unless otherwise conflicted out. The expenses of such suit or suits that Ambrx elects to bring, including any expenses of Sino incurred in conjunction with the prosecution of such suits or the settlement thereof, [***]. In the event Ambrx exercises its right to xxx pursuant to this Section 6.3.3 it shall first reimburse itself out of any sums recovered in such suit or in settlement thereof for all costs and expenses of every kind and character, including reasonable attorneys’ fees, necessarily incurred in the prosecution of any such suit. If, after such reimbursement, any funds shall remain from said recovery, then Sino shall receive an amount equal to [***] ([***]) of such funds and the remaining [***] ([***]) of such funds shall be retained by Ambrx. Notwithstanding the foregoing, Ambrx shall have the right to initiate and prosecute any legal action(s) relating to Licensed Intellectual Property Rights or Ambrx Background Technology outside the Sino Territory at its own expense. |
| 6.3.4 | COOPERATION. Each Party agrees to cooperate fully in any action under this Article 6 that is controlled by the other Party, provided that the controlling Party reimburses the cooperating Party promptly for any costs and expenses incurred by the cooperating Party in connection with providing such assistance. |
| 6.3.5 | DECLARATORY JUDGMENT & INVALIDITY CHALLENGE. If a declaratory judgment action is brought naming Sino and/or any of its Affiliates as a defendant, or a claim alleging invalidity or unenforceability of any Valid Claims within the in Ambrx Research Program Patents or Sino Research Program Patents or Joint Research Program Patents in the Sino Territory, it shall be handled in the same manner as set forth in Section 6.4. |
| 6.4 | INFRINGEMENT ACTIONS BY THIRD PARTIES. |
| 6.4.1 | NOTICE. Each Party shall notify the other Party promptly in writing of any claim of, or action for, infringement of any Patent Rights owned or licensed by Third Parties (“Claimant”) which is threatened, made or brought against either Party by reason of either Party’s performance of its obligations under this Agreement or Development, manufacture, use or sale of any Licensed Products in the Sino Territory. |
| 6.4.2 | DEFENSE. In the event that such an action for infringement is commenced by a Claimant solely against a Party or both Parties jointly and/or any of their respective Affiliates, as the |
33
| case may be, with respect to a Licensed Product Developed and Commercialized by Sino and/or its Affiliate in the Sino Territory, Sino shall use best efforts to defend such action at its own expense, and Ambrx hereby agrees to use best efforts to assist and cooperate with Sino in the defense of such suit, including but not limited to providing the necessary information and materials Controlled by Ambrx for preparing the defense and counter-claim. |
| 6.4.3 | PAYMENT OBLIGATION. During the pendency of any such action, Sino shall continue to pay all royalties and other payments due hereunder, provided that no injunctive relief has been declared by the relevant court which prohibit use of the relevant Product Specific Patent Rights or the relevant Licensed Intellectual Property Rights in the Sino Territory. |
| 6.5 | UNSUCCESSFUL DEFENSE AND SETTLEMENT. In the event that Sino loses an action for infringement of Patent Rights brought by a Claimant, Ambrx shall, within 30 days of the Court’s award of damages to the Claimant, [***]. Alternatively, if a settlement agreement is reached between Sino and the Claimant to pay the Claimant (i) [***]; (ii) [***]: (a) [***]; (b) [***] (i) [***]; and (ii) [***]. For clarity, the [***] and the [***] aggregating with the [***] shall not exceed [***] of the total royalties payable to Ambrx during the Term. |
ARTICLE 7
CONFIDENTIALITY & PUBLICATIONS
| 7.1 | NONDISCLOSURE OBLIGATION. Except to the extent expressly authorized by this Agreement, the Parties agree that, during the Term of this Agreement and for seven (7) years thereafter, each Party and its Affiliates, if any (collectively, a “Receiving Party”), shall use their best efforts to keep Confidential Information & Materials completely confidential, shall not publish or otherwise disclose to any Third Party and shall not use for any purpose other than the performance of this Agreement both the financial terms of this Agreement and any information furnished to it by the other Party or its Affiliates, if any (collectively, a “Disclosing Party”) (and shall ensure that its and its Affiliates’ respective directors, officers, employees or agents do likewise), except to the extent that it can be established by the Receiving Party by competent proof that such information: (i) is, or hereafter becomes, generally available to the public other than by reason of any default by the Receiving Party with respect to its confidentiality obligations hereunder; (ii) was already known to the Receiving Party at the time of disclosure by the Disclosing Party; (iii) was lawfully disclosed to the Receiving Party by a Third Party not in default of any confidentiality obligation to the Disclosing Party; or (iv) is independently Developed by or for the Receiving Party without reference to or reliance upon the information furnished by the Disclosing Party. |
| 7.2 | EXCLUSIONS TO CONFIDENTIALITY. The restrictions contained in Section 7.1 shall not apply to any Confidential Information & Materials in the hands of a Receiving Party that (i) are submitted by the Receiving Party to governmental authorities to facilitate the issuance of Marketing Authorization for Licensed Products in the Sino Territory, provided that reasonable measures shall be taken to assure confidential treatment of such information, if practicable, or (ii) are otherwise required to be disclosed in compliance with |
34
| Applicable Laws (including, without limitation, to comply with any governmental or stock exchange disclosure requirements) or an order by a court or other regulatory body having competent jurisdiction; provided, however, that if a Receiving Party is required to make any such disclosure of the Disclosing Party’s Confidential Information & Materials, such Receiving Party shall, except where impracticable for necessary disclosures (for example to physicians conducting studies or to health authorities), give reasonable advance notice to the other Party of such disclosure requirement and, except to the extent inappropriate in the case of Patent Right applications or otherwise, will use its best efforts to secure confidential treatment of such Confidential Information & Materials required to be disclosed. In addition, any press release or other public announcement permitted by the terms of Section 7.4 hereof shall be excluded from the provisions of Section 7.1. |
| 7.3 | PUBLICATION. Sino and Ambrx each acknowledge the other Party’s interest in publishing the results of its research in order to obtain recognition within the scientific community and to advance the state of scientific knowledge. Each Party also recognizes the mutual interest in obtaining valid patent protection and in protecting business interests and trade secret information. Consequently, except for disclosures permitted pursuant to Section 7.2, either Party, its employees or its consultants wishing to make a publication with respect to the Development or clinical results regarding the Licensed Products hereunder shall deliver to the other Party a copy of the proposed written publication or an outline of an oral disclosure at least sixty (60) days prior to submission for publication or presentation. The reviewing Party shall have the right (a) to propose modifications to the publication or presentation for patent reasons, trade secret reasons or business reasons or (b) to request a reasonable delay in publication or presentation in order to protect patentable information. If the reviewing Party requests a delay, the publishing Party shall delay submission or presentation for a period of one hundred and twenty (120) days to enable Patent Right applications protecting each Party’s rights in such information to be filed in accordance with Article 67 above. Upon expiration of such one hundred and twenty (120) days, the publishing Party shall be free to proceed with the publication or presentation. If the reviewing Party requests modifications to the publication or presentation, the publishing Party shall edit such publication to prevent disclosure of trade secret or proprietary business information prior to submission of the publication or presentation. |
| 7.4 | PUBLICITY/USE OF NAMES. No disclosure of the existence, or the terms, of this Agreement may be made by either Party. Notwithstanding the foregoing, disclosure by either Party to its business partners or other institutions in the ordinary and usual course of business or with the bona fide intent to pursue the objective of this Agreement shall be permitted, provided that such disclosure shall be to the minimum extent possible and a confidentiality agreement shall be entered into with the recipient on terms no less exacting than the confidentiality provisions set forth in this Agreement. Neither Party shall use the name, trademark, trade name or logo of the other Party, its Affiliates or their respective employees in any publicity, promotion, news release or disclosure relating to this Agreement or its subject matter, without the prior express written permission of the other Party, except as may be required by Applicable Law or as permitted pursuant to Section 7.2; provided that in the event disclosure is required by Applicable Law, the |
35
| Disclosing Party shall use good-faith efforts to give the non-Disclosing Party an opportunity, with reasonable advance notice, to review and comment on any proposed disclosure. Notwithstanding Section 7.4 herein, Sino and Ambrx shall make reasonable effort to issue a mutually agreed joint press release as shown in Exhibit 7.4 regarding the execution of this Agreement and the cooperation between both Parties, provided, further, that any further press release to be issued by one Party mentioning the execution of this Agreement or naming the other Party shall be approved in advance by the other Party. |
| 7.5 | INJUNCTIVE RELIEF. The Parties acknowledge that monetary damages alone may not adequately compensate the Disclosing Party in the event of a material breach by the Receiving Party of this Article 7, and that, in addition to all other remedies available to the Disclosing Party under this Agreement, at law or in equity, to the extent permitted by Applicable Laws, it shall be entitled to seek injunctive relief for the enforcement of its rights under this Article 7. |
ARTICLE 8
REPRESENTATIONS AND WARRANTIES
| 8.1 | MUTUAL REPRESENTATIONS AND WARRANTIES. Each Party represents and warrants to the other Party the following as of the Effective Date and covenants during the Term that: |
| 8.1.1 | CORPORATE POWER. Such Party is duly organized and validly existing under the laws of the country/state of its organization, and has full legal and corporate power and authority to enter into this Agreement and to perform its obligations hereunder. |
| 8.1.2 | DUE AUTHORIZATION AND EXECUTION. The execution and delivery of this Agreement and the consummation of the transactions contemplated hereby have been duly authorized by the necessary corporate actions of such Party. This Agreement has been duly executed by such Party. This Agreement and any other documents contemplated hereby constitute valid and legally binding obligations of such Party enforceable against it in accordance with their respective terms, except to the extent that enforcement of the rights and remedies created thereby is subject to bankruptcy, insolvency, reorganization, moratorium and other similar laws of general application affecting the rights and remedies of creditors. |
| 8.1.3 | NON-CONTRAVENTION. The execution, delivery and performance by such Party of this Agreement and any other agreements and instruments contemplated hereunder will not (i) in any material respect violate any statute, Regulation, judgment, order, decree or other restriction of any governmental authority to which such Party is subject, (ii) violate any provision of the corporate charter, by-laws or other organizational documents of such Party, or (iii) constitute a material violation or breach by such Party of any provision of any material contract, agreement or instrument to which such Party is a party or to which such Party may be subject although not a party. |
36
| 8.2 | REPRESENTATIONS BY AMBRX. Ambrx represents and warrants to Sino the following as of the Effective Date: |
| 8.2.1 | to Ambrx’s knowledge, the Licensed Intellectual Property Rights exist and are not invalid or unenforceable, in whole or in part; |
| 8.2.2 | it has not previously (i) assigned, transferred, conveyed or otherwise encumbered its right, title and/or interest in Licensed Intellectual Property Rights related to the [***] in the Field in the Sino Territory, or (ii) granted any rights to any Third Parties, in either case that would conflict with the rights granted to Sino hereunder; |
| 8.2.3 | to Ambrx’s knowledge, it is (a) the sole and exclusive owner of the Licensed Intellectual Property Rights, (b) the sole and exclusive licensee of the Exclusive Existing Third Party Licenses, and (c) the non-exclusive licensee of the Non-Exclusive Existing Third Party Licenses, in each case related to the [***] in the Sino Territory and the Ambrx Territory; |
| 8.2.4 | the Exclusive Existing Third Party Licenses are in force and effect, and Ambrx has not received any notice of breach or termination with respect to any Existing Third Party License; |
| 8.2.5 | to Ambrx’s knowledge, there are no claims, judgments or settlements against or owed by Ambrx and no pending or threatened claims or litigation relating to Licensed Intellectual Property Rights in the Sino Territory and the Ambrx Territory; and Ambrx is not aware of any infringement or misappropriation of the intellectual property rights of any Third Party in the Sino Territory and the Ambrx Territory arising from any research and development of the Licensed Compounds by Ambrx prior to the Effective Date; |
| 8.2.6 | to Ambrx’s knowledge, there are no claims, judgments or settlements against or owed by the licensor of the Existing Third Party License relating to the Third Party License in the Sino Territory and the Ambrx Territory; and |
| 8.2.7 | save and except the Existing Third Party Licenses set forth in Exhibit 2.4.2, there are no other Existing Third Party Licenses to which Ambrx is a licensee for use of any Patent Rights or Know-How that are necessary or useful for the manufacture, Development or Commercialization of the Licensed Compounds and the Licensed Products. Ambrx shall update the Existing Third Party License exhibit for any changes and addition of Ambrx Third Party Licenses and Future Third Party Licenses on a regular basis. |
| 8.3 | REPRESENTATIONS BY SINO. Sino represents and warrants to Ambrx as of the Effective Date and covenants thereafter, to Ambrx that: |
| 8.3.1 | Sino and its Affiliates will use the Licensed Intellectual Property Rights and Ambrx Core Technology IP solely for the purpose of the Development, use, manufacture or sale of the Licensed Products in the Sino Territory strictly in accordance with the terms of this Agreement and not for any other purpose. |
37
| 8.3.2 | Sino and its Affiliates shall invest sufficient resources and funds and use Commercially Reasonable Efforts to achieve Milestone Events so as to Develop and Commercialize Licensed Products in the Sino Territory. |
ARTICLE 9
INDEMNIFICATION & INSURANCE
| 9.1 | INDEMNIFICATION BY SINO. Sino hereby agrees to indemnify, hold harmless and defend Ambrx, its Affiliates and their respective officers, directors, agents, employees, successors and assigns (collectively, the “Ambrx Indemnified Parties”) against any and all direct losses, costs, expenses, fees or damages arising out of or relating to claims, allegations, suits, actions or proceedings (collectively, “Losses”) asserted by any Third Party, whether governmental or private, arising out of or relating to (i) the Development, manufacture, use, sale or other disposition of Licensed Products by Sino or its Affiliates or Sublicensees under this Agreement, (ii) failure to perform its obligations under this Agreement by Sino, its Affiliates or their respective officers, directors, agents or employees, (iii) the breach of any of Sino’s covenants, representations or warranties under this Agreement, or (iv) the negligence or willful misconduct by Sino, its Affiliates or their respective officers, directors, agents or employees, in performing any obligations under this Agreement, except in each case ((i) to (iv)) for those Losses as to which Ambrx has an obligation to indemnify Sino pursuant to Section 9.2, as to which Losses each Party shall indemnify the other to the extent of their respective liability; provided, however, that Sino shall not be obligated to indemnify Ambrx for any Losses to the extent that such Losses arises as a result of gross negligence or wilful misconduct on the part of Ambrx or any of its Affiliates. |
| 9.2 | INDEMNIFICATION BY AMBRX. Ambrx hereby agrees to indemnify, hold harmless and defend Sino, its Affiliates and their respective officers, directors, agents, employees, successors and assigns (collectively, the “Sino Indemnified Parties”) against any and all losses, costs, expenses, fees or damages arising out of or relating to claims, allegations, suits, actions or proceedings asserted by any Third Party, whether governmental or private, arising out of or relating to (i) failure to perform its obligations under this Agreement by Ambrx, its Affiliates or their respective officers, directors, agents or employees, (ii) the breach of any of Ambrx’s covenants, representations or warranties under this Agreement, or (iii) the negligence or willful misconduct by Ambrx, its Affiliates or their respective officers, directors, agents or employees, in performing any obligations under this Agreement, except in each case ((i) to (iii)) for those Losses as to which Sino has an obligation to indemnify Ambrx pursuant to Section 9.1, as to which Losses each Party shall indemnify the other to the extent of their respective liability; provided, however, that Ambrx shall not be obligated to indemnify Sino for any Losses to the extent that such Losses arises as a result of gross negligence or wilful misconduct on the part of Sino or any of its Affiliates. |
| 9.3 | PROCEDURE. If a Party is seeking indemnification under this Article 9 (the “Indemnified Party”), it shall inform the other Party (the “Indemnifying Party”) of the claim giving rise to the obligation to indemnify pursuant to this Article 9 as soon as reasonably practicable after receiving notice of the claim (provided, however, any delay or |
38
| failure to provide such notice shall not constitute a waiver or release of, or otherwise limit, the Indemnified Party’s rights to indemnification under, as applicable, this Article 9 except to the extent that such delay or failure materially prejudices the Indemnifying Party’s ability to defend against the relevant claims). The Indemnifying Party shall have the right to assume the defense of any such claim for which it is obligated to indemnify the Indemnified Party. The Indemnified Party shall cooperate with the Indemnifying Party and the Indemnifying Party’s insurer as the Indemnifying Party may reasonably request, and at the Indemnifying Party’s cost and expense. The Indemnified Party shall have the right to participate, at its own expense and with counsel of its choice, in the defense of any claim or suit that has been assumed by the Indemnifying Party. The Indemnifying Party shall not settle any claim without the prior written consent of the Indemnified Party, which the Indemnifying Party may provide in its sole discretion. The Indemnified Party shall not settle or compromise any such claim without the prior written consent of the Indemnifying Party, not to be unreasonably withheld. |
| 9.4 | INSURANCE. Each Party shall obtain and acquire customary and reasonable insurance for activities conducted under Clinical Trials, in accordance with applicable laws and regulations in the territory of each Party. |
ARTICLE 10
TERM & TERMINATION
| 10.1 | TERM. The term of this Agreement shall commence on the Effective Date and, unless earlier terminated as provided in this Article 10, shall continue in full force and effect until the expiration of the Royalty Term with respect to Licensed Products in the Sino Territory (the “Term”). |
| 10.2 | EFFECT OF EXPIRATION. Following the expiration of this Agreement with respect to a Licensed Product in the Sino Territory pursuant to Section 10.1, (a) the License granted to Sino under Section 2.2.1, comprising (i) the Licensed Intellectual Property Rights self-owned by Ambrx, (ii) the Licensed Intellectual Property Rights constituted under the Existing Third Party Licenses, and (iii) the Licensed Intellectual Property Rights constituted under the Ambrx Third Party Licenses and Future Third Party Licenses shall continue to be in full force and effect for Sino to make, have made, use, sell, offer for sale, have sold and export such Licensed Product in the Sino Territory, in accordance with the terms under Section 2.2.1; (b) the License granted to Ambrx under Section 2.2.2 shall continue to be in full force and effect for Ambrx to make, have made, use, sell, offer for sale, have sold and export such Licensed Product in the Ambrx Territory; and (c) Sino shall continue to retain all of Sino’s right, title and interest in, to and under the Product Specific Patent Rights and Reload Patent Rights (if any) acquired during the Term of this Agreement. |
| 10.3 | TERMINATION BY SINO WITHOUT CAUSE. Sino may terminate this Agreement upon six (6) months’ prior written notice to Ambrx. |
| 10.3A | REQUEST FOR REFUND AND TERMINATION BY SINO DUE TO AMBRX’S FAILURE TO DELIVER PCC ON TIME. |
39
| 10.3A(1) | In the event that Ambrx fails to provide any one PCCs of the Licensed Compounds that meet the Initial PCC Requirements on or before June 30, 2020 or such later date as further agreed by the Parties, Sino shall have the right to request a refund of [***] of the upfront payment under Section 5.1 (being [***] ([***])) by serving a refund notice to Ambrx. If Sino exercises such a discretion, Ambrx shall return [***] of the upfront payment to Sino within [***] business days from the date of the refund notice; and Sino shall reassign all the Product Specific Patent Rights that is/are specific only to the delayed PCC to Ambrx within [***] days upon receipt of [***] of the upfront payment. All fees and costs related to the reassignment of such Product Specific Patent Rights shall be borne by Ambrx. For clarity, Product Specific Patent Rights that are useful for Development and Commercialization of PCC delivered on-time shall not be reassigned. |
| 10.3A(2) | In the event that Ambrx fails to provide the PCCs of the Licensed Compounds that meet the Initial PCC Requirements on or before June 30, 2020 or such later date as further agreed by the Parties, Sino shall have the right to terminate this Agreement unilaterally by serving a termination notice to Ambrx. If Sino exercises such a discretion, Ambrx shall return the upfront payment under Section 5.1 to Sino within 10 business days from the date of the termination notice; and Sino shall reassign all the Product Specific Patents Rights to Ambrx within 60 days upon receipt of the upfront payment. All fees and costs related to the reassignment of the Product Specific Patent Rights shall be borne by Ambrx. |
| 10.4 | TERMINATION FOR DEFAULT. Each Party shall have the right to terminate this Agreement, upon notice to the other Party, in the event that: |
| 10.4.1 | Such other Party materially defaults with respect to any of its material obligations under this Agreement and does not cure such default within sixty (60) days after the receipt of a notice from the non-breaching Party specifying the nature of, and requiring the remedy of, such default (or, if such default cannot be cured within such sixty (60)-day period, if the breaching Party does not commence and diligently continue actions to cure same during such sixty (60)-day period); |
| 10.4.2 | Such other Party fails to use Commercially Reasonable Efforts to perform its respective obligations under the Agreement in accordance with the timelines set forth therein; |
| 10.4.3 | The other Party shall have: (i) voluntarily commenced any proceeding or filed any petition seeking relief under the bankruptcy, insolvency or other similar laws of any jurisdiction, (ii) applied for, or consented to, the appointment of a receiver, trustee, custodian, sequestrator, conciliator, administrator or similar official for it or for all or substantially all of its property, (iii) filed an answer admitting the material allegations of a petition filed against or in respect of it in any such proceeding, (iv) made a general assignment for the benefit of creditors of all or substantially all of its assets, (v) admitted in writing its inability to pay all or substantially all of its debts as they become due, or (vi) taken corporate action for the purpose of effecting any of the foregoing; or |
| 10.4.4 | An involuntary proceeding shall have been commenced, or any involuntary petition shall have been filed, in a court of competent jurisdiction seeking: (i) relief in respect of the other Party, or of its property, under the bankruptcy, insolvency or similar laws of any |
40
| jurisdiction, (ii) the appointment of a receiver, trustee, custodian, sequestrator, conciliator, administrator or similar official for such other Party or for all or substantially all of its property, or (iii) the winding-up or liquidation of such other Party; and, in each case, such proceeding or petition shall have continued undismissed for sixty (60) days, or an order or decree approving or ordering any of the foregoing shall have continued unstayed, unappealed and in effect for thirty (30) days. |
| 10.5 | EFFECT OF TERMINATION. |
| 10.5.1 | TERMINATION OF RIGHTS. Effect of Termination of this Agreement shall be in accordance with Section 10.5 and Section 10.6. |
(I) Upon termination of this Agreement by Ambrx pursuant to Section 10.4 or by Sino pursuant to Section 10.3, (a) all rights and Licenses granted to Sino under Article 2 shall continue to be in full force and effect (excluding Ambrx Territory), (b) the License granted to Ambrx under Section 2.2.2 shall continue to be in full force and effect (excluding the Sino Territory), and (c) Sino shall continue to retain all of Sino’s right, title and interest in, to and under the Product Specific Patent Rights and the Reload Patent Rights (if any).
(II) Upon termination of this Agreement by Sino pursuant to Section 10.4, the rights and Licenses granted to Ambrx under Section 2.2.2 shall be retained by Ambrx.
(III) Upon termination of this Agreement by Sino pursuant to Section 10.3A(2) and subject to the refund of the upfront payment by Ambrx to Sino, the Product Specific Patent Rights assigned to Sino shall be reassigned and reverted to Ambrx in accordance with Section 10.3A(2) and the Licenses granted to Sino under Article 2 shall be null and void.
| 10.5.2 | NO RELEASE. Termination of this Agreement for any reason shall not release any Party hereto from any liability which, at the time of such termination, has already accrued to the other Party or which is attributable to a period prior to such termination, nor shall such termination preclude either Party from pursuing any rights and remedies it may have hereunder or at law or in equity which accrued or are based upon any event occurring prior to such termination. |
| 10.5.3 | SINO’S RETAINED RIGHTS AFTER TERMINATION. After the date of termination by either party pursuant to Section 10.4 or by Sino pursuant to Section 10.3, Sino and its Affiliates shall have the right to (a) [***]; and (b) [***] and (c) [***]; provided that, [***]. For clarity, this Section 10.5.3 does not apply to termination pursuant to Section 10.3A(2). |
| 10.5.4 | TRANSFER OF INFORMATION, MATERIALS AND REGULATORY FILINGS. Notwithstanding the foregoing, upon termination of this Agreement by either party pursuant to Section 10.4 or by Sino pursuant to Section 10.3, each Party shall provide the other Party with the right to reference, cross-reference, review, have access to, incorporate and use all documents and other materials filed by or on behalf of the other Party and its Affiliates with any Regulatory Authority in furtherance of applications for Marketing Authorization of the Parties in their respective territories with respect to the Licensed Product. Each Party shall be entitled to freely and exclusively use and to grant others the |
41
| right to use all such materials and documents delivered pursuant to this Section 10.5.4 for use outside their respective territories. For clarity, this Section 10.5.4 does not apply to termination pursuant to Section 10.3A(2). |
| 10.5.5 | RETURN OF CONFIDENTIAL INFORMATION & MATERIALS. Subject to Section 10.5.1, upon any termination of this Agreement, each Party shall promptly return to the other Party all Confidential Information & Materials or Know-How received from the other Party, except as reasonably required to exercise any surviving rights or licenses hereunder. |
| 10.6 | ACCRUED RIGHTS; SURVIVAL. |
| 10.6.1 | Termination or expiration of this Agreement for any reason shall be without prejudice to any rights which shall have accrued to the benefit of either Party prior to such termination or expiration. Such termination or expiration shall not relieve either Party from obligations which are expressly indicated to survive termination or expiration of this Agreement. The rights of the Parties upon termination described in this Agreement shall not be exclusive of any other rights or claims at law or in equity that either Party may have against the other arising out of this Agreement. |
| 10.6.2 | Termination, relinquishment or expiration of this Agreement pursuant to Section 10.3 (Termination by Sino Without Cause) and Section 10.4 (Termination for Default) shall not terminate each Party’s obligation to pay all royalties, Milestone Payments and other monetary obligations that may have accrued hereunder prior to such termination. All of the Parties’ respective rights and obligations under Section 2.1.1 (Assignments of Product Specific Patent Rights in the Sino Territory); Section 2.2 (Grant of Exclusive Licenses to Use the Licensed Intellectual Property Rights); Section 2.6 (Rights of Reference; Clinical Data Rights), Section 6.1 (Ownership of Intellectual Property), Section 10.5 (Effect of Termination) and Section 11.5 (Notices), Article 1 (Definitions), Article 7 (Confidentiality & Publications), Article 9 (Indemnification & Insurance), Section 10.2 (Effect of Expiration), Section 10.6 (Accrued Rights; Survival), Section 10.7 (Dispute Resolution), and Section 11.6 (Applicable Law) shall survive termination, relinquishment or expiration hereof. |
| 10.6.3 | Termination, relinquishment or expiration of this Agreement pursuant to Section 10.3A(2) shall not terminate Article 1 (Definitions), Article 7 (Confidentiality & Publications), Article 9 (Indemnification & Insurance), Section 10.6 (Accrued Rights; Survival), Section 10.7 (Dispute Resolution), and Section 11.6 (Applicable Law), which Sections shall survive termination and relinquishment hereof. |
DISPUTE RESOLUTION
| 10.7 | DISPUTES. Subject to Section 10.8, upon the written request of either Party to the other Party, any claim, dispute or controversy as to the breach, enforcement, interpretation or validity of this Agreement (a “Dispute”) will be referred to the executive officers (or such executive officer’s designee with decision-making authority) for attempted resolution. In the event such executives are unable to resolve such Dispute within thirty (30) days after |
42
| the initial written request, then, upon the written demand of either Party, the Dispute shall be subject to arbitration, as provided in Section 10.8, except as expressly set forth in Section 10.8. |
| 10.8 | ARBITRATION. Any Dispute that cannot be resolved pursuant to Section 10.7 will be referred to and finally resolved by arbitration in accordance with the International Chamber of Commerce (the “Rules”) by the Hong Kong International Arbitration Centre (“HKIAC”), by an arbitral tribunal composed of three (3) arbitrators, with each Party appointing one (1) arbitrator and the third arbitrator to be selected by mutual agreement of the two (2) arbitrators appointed by the Parties. The foregoing arbitration proceedings may be commenced by either Party by notice to the other Party. All arbitration proceedings will be conducted in the English language. The allocation of expenses of the arbitration, including reasonable attorney’s fees, will be determined by the arbitrators, or, in the absence of such determination, each Party will pay its own expenses. All rulings by the arbitrators will be final. |
| 10.9 | EXCEPTIONS. Nothing contained in this Agreement shall deny either Party the right to seek, upon good cause, injunctive or other equitable relief from a court of competent jurisdiction in the context of an emergency or prospective irreparable harm, and such an action may be filed and maintained notwithstanding any ongoing Dispute resolution discussions or arbitration proceedings. In addition, either Party may bring an action in any court of competent jurisdiction to resolve Disputes pertaining to the validity, construction, scope, enforceability, infringement or other violations of Patent Rights or other intellectual property rights, and no such claim shall be subject to arbitration pursuant to Section 10.8. |
ARTICLE 11
MISCELLANEOUS
| 11.1 | RIGHT OF FIRST OFFER FOR CHINA OPPORTUNITIES. In the event that Ambrx (for itself and on behalf of its Affiliates) consider to develop [***] in China, Ambrx shall first notify Sino in writing, and Sino shall have [***] ([***]) days (“Review Period”) to notify Ambrx in writing that Sino wishes to negotiate for rights to [***] in China. If Sino delivers such written notice prior to the expiration of the Review Period, followed by Sino’s performance of due-diligence investigation on [***] in no more than [***] days (“Due Diligence Period”), then Ambrx and Sino shall [***] (either inside or outside the Field) in China for a period of [***] ([***]) days (“Negotiation Period”). Ambrx (for itself and on behalf of its Affiliates) shall not enter into any [***] for any such [***] in China unless and until the Review Period and, if applicable, the Due Diligence Period and the Negotiation Period have expired without the Parties coming to terms under this Section 11.1. |
| 11.2 | FORCE MAJEURE. Neither Party shall be held liable to the other Party or be deemed to have defaulted under or breached this Agreement for failure or delay in performing any obligation under this Agreement to the extent that such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party, potentially including embargoes, war, acts of war (whether war shall be declared or not), acts of terrorism, insurrections, riots, civil commotions, strikes, lockouts or other labor |
43
| disturbances, fire, floods, or other acts of God, or acts, omissions or delays in acting by any governmental authority or the other Party. The affected Party shall notify the other Party of such force majeure circumstances as soon as reasonably practical, and shall promptly undertake all reasonable efforts necessary to cure such force majeure circumstances. |
| 11.3 | ASSIGNMENT. Except as provided in this Section 11.3, this Agreement may not be assigned or otherwise transferred, nor may any right or obligation hereunder be assigned or transferred, by either Party without the consent of the other Party. Notwithstanding the foregoing, (i) Sino may assign this Agreement and its obligations hereunder in whole or in part to an entity in which Sino owns not less than [***] equity interest (“Sino Investee”), provided that Sino shall be jointly and severally liable under this Agreement upon such assignment taking effect, (ii) either Party may, without the other Party’s consent, assign this Agreement and its rights and obligations hereunder in whole or in part to an Affiliate; provided, however, that (a) the assigning Party must notify the other Party at least twenty (20) days prior to completion of any such assignment, and (b) in the event of an assignment to an Affiliate, the assigning Party shall be jointly and severally liable for all activities of such Affiliate pursuant to this Agreement. Further, each Party may assign this Agreement to any assignee of all or substantially all of such Party’s business to a successor in interest in connection with the transfer or sale of all or substantially all of its business or assets to which this Agreement relates, or in the event of such Party’s merger, consolidation or similar transaction. Any permitted assignee shall assume all obligations of its assignor under this Agreement. This Agreement is binding upon the permitted successors and assigns of the Parties. Any attempted assignment not in accordance with this Section 11.3 shall be void. |
| 11.4 | SEVERABILITY. If any one or more of the provisions contained in this Agreement is held invalid, illegal or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions contained herein shall not in any way be affected or impaired thereby, unless the absence of the invalidated provision(s) adversely affects the substantive rights of the Parties. The Parties shall in such an instance use their good faith efforts to replace the invalid, illegal or unenforceable provision(s) with valid, legal and enforceable provision(s) which, insofar as practical, implement the purposes of this Agreement. |
| 11.5 | NOTICES. All notices which are required or permitted hereunder shall be in writing and sufficient if delivered personally, sent by facsimile (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), sent by internationally recognized overnight courier or sent by registered or certified mail, postage prepaid, return receipt requested, addressed as follows: |
| If to Ambrx, to: | Ambrx, Inc. | |
| 00000 Xxxxx Xxxxxx Xxxxx Xxxx | ||
| Xx Xxxxx, XX 00000 | ||
| Attn: Office of General Counsel | ||
| Facsimile No.: (000) 000-0000 |
With a copy to:
00
| Xxxxxx Xxxxxx LLP 000 Xxxxxxxxxx Xxxxxx | ||
| Xxxxx 0000 | ||
| Xxx Xxxxxxxxx, Xxxxxxxxxx 00000 | ||
| XXX | ||
| Attn: Xxx Xxxxx, Partner | ||
| Facsimile No.: x0 000-000-0000 |
If to Sino, to:
Xxxx 0000, Xxxxxx Xxxxx, Xxxxxxxxxx Xxxxx,
| Xxxx Biopharmaceutical Limited 0 Xxxxxxx Xxxx, Xxxxxxx, | ||
| Xxxx Xxxx | ||
| Attention: Xxxxx Xxxxx | ||
| Facsimile No.: x000 0000 0000 |
or to such other address(es) as the Party to whom notice is to be given may have furnished to the other Party in writing in accordance herewith. Any such notice shall be deemed to have been given: (a) when delivered, if personally delivered or sent by facsimile on a business day (or if delivered or sent on a non-business day, then on the next business day); (b) on the business day after dispatch, if sent by internationally recognized overnight courier; or (c) on the fifth (5th) business day following the date of mailing, if sent by mail.
| 11.6 | APPLICABLE LAW. This Agreement shall be governed by and construed in accordance with the laws of the Hong Kong Special Administrative Region, without reference to any rules of conflict of laws or renvoi. The United Nations Convention on the Sale of Goods shall not apply to this Agreement. All Disputes arising from or in connection with this Agreement shall be submitted for arbitration in accordance with the United Nations Commission on International Trade Law Arbitration Rules in accordance with the HKIAC Procedures for the Administration of International Arbitration in force at the date of this Agreement. The place of arbitration shall be in Hong Kong at HKIAC. |
| 11.7 | ENTIRE AGREEMENT; AMENDMENTS. This Agreement together with the Schedules hereto contains the entire understanding of the Parties with respect to the subject matter hereof, including the Research Program and the Licenses granted hereunder. Any other express or implied agreements and understandings, negotiations, writings and commitments, either oral or written, with regard to the subject matter hereof, including the Research Program and/or the Licenses granted hereunder, are superseded by the terms of this Agreement. The Schedules to this Agreement are incorporated herein by reference and shall be deemed a part of this Agreement. This Agreement may be amended, or any term hereof modified, only by a written instrument duly executed by authorized representatives of both Parties hereto. |
45
| 11.8 | HEADINGS AND INTERPRETATION. The captions to the several Articles and Sections and subsections hereof are not a part of this Agreement, but are merely for convenience to assist in locating and reading the several Articles and Sections hereof. Any reference in this Agreement to an Article, Section, subsection, paragraph, clause, or Schedule or Exhibit shall be deemed to be a reference to an Article, Section, subsection, paragraph, clause, or Schedule or Exhibit of or to, as the case may be, this Agreement, unless otherwise indicated. Unless the context of this Agreement otherwise requires, (a) words of any gender include each other gender, (b) words such as “herein,” “hereof” and “hereunder” refer to this Agreement as a whole and not merely to the particular provision in which such words appear, (c) words using the singular shall include the plural, and vice versa, (d) whenever any provision of this Agreement uses the term “including” (or “includes” or words of similar import), such term shall not be limiting and such term shall be deemed to mean “including without limitation” (or “includes without limitation”), (e) the word “or” shall not be construed as exclusive, and (f) references to any Articles or Sections include Sections and subsections that are part of the reference Article or section (e.g., a section numbered “Section 2.2.1” would be part of “Section 2.2,” and references to “Article 2” or “Section 2.2” would refer to material contained in the subsection described as “Section 2”). |
| 11.9 | INDEPENDENT CONTRACTORS. It is expressly agreed that Ambrx and Sino shall be independent contractors and that the relationship between the Parties shall not constitute a partnership, joint venture or agency. Neither Ambrx nor Sino shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other Party, without the prior written consent of the other Party. |
| 11.10 | WAIVER. The waiver by either Party hereto of any right hereunder, or of any failure of the other Party to perform, or of any breach by the other Party, shall not be deemed a waiver of any other right hereunder or of any other breach by or failure of such other Party, whether of a similar nature or otherwise. |
| 11.11 | CUMULATIVE REMEDIES. No remedy referred to in this Agreement is intended to be exclusive, but each shall be cumulative and in addition to any other remedy referred to in this Agreement or otherwise available under law. |
| 11.12 | WAIVER OF RULE OF CONSTRUCTION. Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, the rule of construction that any ambiguity in this Agreement shall be construed against the drafting Party shall not apply. |
| 11.13 | BUSINESS DAY REQUIREMENTS. In the event that any notice or other action or omission is required to be taken by a Party under this Agreement on a day that is not a business day, then such notice or other action or omission shall be deemed to be required to be taken on the next occurring business day. |
| 11.14 | COUNTERPARTS. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the |
46
| same instrument. For purposes hereof, a scanned copy of this Agreement, including the signature pages hereto, will be deemed to be an original. |
| 11.15 | PRC REGULATORY MATTERS. If required under applicable laws and regulations, Sino shall be responsible for any and all PRC-related Regulatory Approvals, registrations and/or filings in connection with performance of this Agreement, including without limitation registering this Agreement with competent commission of commerce and providing registration certificate to Ambrx within sixty (60) days after execution of this Agreement; after Regulatory Approvals, registrations and/or filings are completed, Sino shall provide a copy of relevant certificates to Ambrx within thirty (30) days after obtaining such relevant certificates. |
| 11.16 | EXPORT LAWS. Notwithstanding anything to the contrary contained herein, all obligations of Ambrx and Sino are subject to prior compliance with the export Regulations of the United States and any other relevant country and such other laws and Regulations in effect in the United States and/or any other relevant country as may be applicable, and to obtaining all necessary approvals required by the applicable agencies of the governments of the United States and any other relevant countries. Ambrx and Sino shall cooperate with each other and shall provide assistance to the other as reasonably necessary to obtain any required approvals. |
| 11.17 | FURTHER ACTIONS. Each Party will execute, acknowledge and deliver such further instruments, and do all such other ministerial, administrative or similar acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement. |
| 11.18 | NO THIRD PARTY RIGHTS. The provisions of this Agreement are for the exclusive benefit of the Parties, and no other Person or entity shall have any right or claim against any Party by reason of these provisions or be entitled to enforce any of these provisions against any Party. |
| 11.19 | EXPENSES. Except as otherwise specifically provided in this Agreement, each Party (and its Affiliates) shall bear its own costs and expenses in connection with entering into this Agreement and the consummation of the transactions and performance of its obligations contemplated hereby. |
| 11.20 | EXTENSION TO AFFILIATES. Sino shall have the right to extend the rights, licenses, immunities and obligations granted in this Agreement to one or more of its Affiliates. All applicable terms and provisions of this Agreement shall apply to any such Affiliate to which this Agreement has been extended to the same extent as such terms and provisions apply to Sino. Sino shall remain fully liable for any acts or omissions of such Affiliates. |
| 11.21 | LANGUAGE. The official text of this Agreement is in the English language as written and spoken in the United States of America. Any text or version of this Agreement in another language, even if such text or version is made by translation or prepared by or executed by one or both of the Parties for a Party’s convenience, shall not be binding and shall have no force or effect. Without limiting the foregoing, in the event of any conflict |
47
| or inconsistency between the English text of this Agreement and any text or version of this Agreement in another language, the English text of this Agreement will prevail. |
[Remainder of this page is left intentionally blank]
48
IN WITNESS WHEREOF, the Parties have executed this Co-Development and License Agreement as of the Effective Date.
| SINO BIOPHARMACEUTICAL CO., LTD. | ||
| By: | /s/ Xxxxxxx Xxx | |
| Name: Xxx, Xxxxxxx Y Y | ||
| Title: Chairwoman | ||
| AMBRX, INC. | ||
| By: | /s/ Xxxx Xxxx | |
| Name: Xxxx, Xxxx | ||
| Title: Chief Executive Officer | ||
49
List of Exhibits
Exhibit 1.9: Ambrx Core Technology Platform
Exhibit 1.36: Development Plan
Exhibit 1.82: [***]
Exhibit 1.83: [***]
Exhibit 2.1.1: Product Specific Patent Rights
Exhibit 2.2.1: Licensed Intellectual Property Rights
Exhibit 2.4.2: Existing Third Party Licenses
Exhibit 3.1: Joint Steering Committee
Exhibit 4.2.2 : Initial PCC Requirements
Exhibit 7.4: Joint Press Release
Exhibit 1.9: Ambrx Core Technology Platform
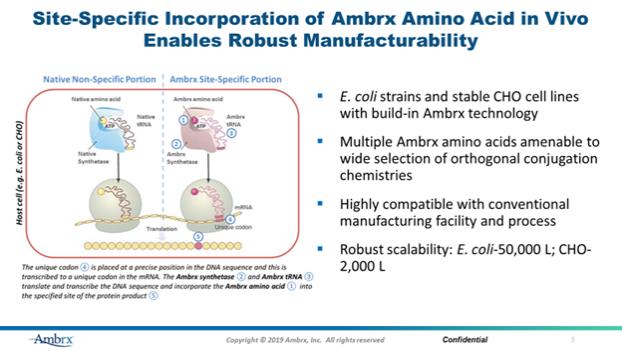
Exhibit 1.9 - 1
Exhibit 1.36: Development Plan
Clinical Plan will be updated (with Rodent Non-GLP toxicology plan)
Exhibit 1.36 - 1
Exhibit 1.82: [***]
The diagram and description below set forth the structure of the [***] as of the Effective Date, which may be modified by mutual agreement to the satisfaction of both Parties.
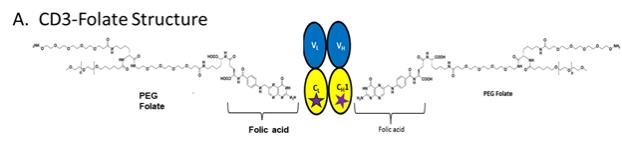
B. Amino Acid Sequence of Humanized Anti-CD3 Fab Lead Molecule
[***]
[***]
[***]
[***]
[***]
Exhibit 1.82 - 1
Exhibit 1.83: [***]
The diagram and description below set forth the structure of the [***] as of the Effective Date, which may be modified by mutual agreement to the satisfaction of both Parties.
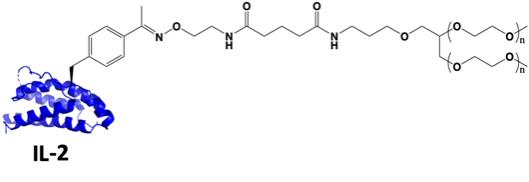
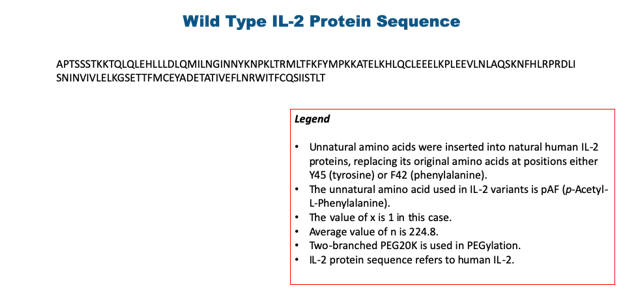
Exhibit 1.83 - 1
Exhibit 2.1.1: Product Specific Patent Rights
| Ambrx Docket Number |
Title |
Country |
Serial No. (Filing Date) |
Publication No. (Date) |
Patent No. (Issue Date) |
Priority |
Status Estimated | |||||||
| [***] | [***] | [***] | [***] [***] |
[***] [***] | ||||||||||
| [***] | [***] | [***] | [***] [***] |
[***] [***] | ||||||||||
| [***] | [***] | [***] | [***] [***] |
[***] | ||||||||||
| [***] | [***] | [***] | [***] [***] |
[***] [***] | ||||||||||
| [***] | [***] | [***] | [***] [***] |
[***] |
Exhibit 2.1.1 - 1
Exhibit 2.2.1: Licensed Intellectual Property Rights
Section A: List of Ambrx Core Technology Patents (Attached list)
Section B: List of Ambrx Non-Core Patents (Attached list)
Section C: List of Know-How
| • | Know-How is reflected/ covered in Sections A and B |
Exhibit 2.1.2 - 1
Exhibit 2.4.2: Existing Third Party Licenses
The agreements set forth herein have been provided by Ambrx with certain content redacted or omitted intentionally for confidentiality reasons. Sino is not aware of the full content of the agreements set forth herein.
| Reference |
Agreement |
Description |
Signed |
Exclusivity |
Term |
Expiry Date |
Royalty to be | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] [***] |
[***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] [***] |
[***] |
Exhibit 2.4.2 - 1
Exhibit 3.1: Joint Steering Committee
Ambrx
Xxxxx Xxxxx, VP Research
Xxxx Xxxxxxxx, VP Development
Xxxxxxx Xxxxxxxx, VP and Head of Chemistry
SINO
Names of 3 members from Sino’s side to be provided.
Exhibit 3.1 - 1
Exhibit 4.2.2: Initial PCC Requirements
[***]
PD study: [***].
PK study: [***].
Safety: [***].
2. [***]
In vitro studies: [***]
[***]
PK studies: [***].
PD studies: [***].
Safety: [***].
Exhibit 4.2.2 - 1
Ambrx Patent Rights – Core IP (December 2019)
Exhibit 7.4: Draft Joint Press Release
To be mutually agreed prior to release
Exhibit 7.4 - 1
Ambrx Patent Rights – Core IP (December 2019)
| Ambrx |
Title | Country | Serial No. (Filing Date) |
Publication No. (Date) |
Patent No. (Issue Date) |
Priority | Status Estimated Expiry | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] |
Exhibit 7.4 - 1

