SCALE UP AGREEMENT
EXHIBIT 10.56
REDACTED VERSION
By and between
ImmunoGen, Inc., a corporation organized under the laws of the Commonwealth of Massachusetts, with its principal offices at 000 Xxxxxx Xxxxxx, Xxxxxxxxx, Xxxxxxxxxxxxx 00000-0000, X.X.X. (“ImmunoGen”)
and
SICOR—Società Italiana Corticosteroidi S.r.l., an Italian corporation with single shareholder, having its principal place of business at Xxx Xxxxxxxxxx 00, 00000 Xxx (XX), Xxxxx (“Sicor”)
with regards to the Maytansinoid Products DM1 and DM4 (these and other capitalized terms as defined hereinbelow).
WHEREAS, under a certain Technology Transfer and Development Agreement between ImmunoGen and Sicor, effective as of November 12, 2004 and amended on June 21, 2006 and December 15, 2006 (as so amended, the “Technology Transfer Agreement”), Sicor has provided and continues to provide ImmunoGen with expertise, technical assistance and advice in connection with ImmunoGen fermentation and chemical synthesis technologies in order to have such processes utilizable for the production of maytansinoid derivative compounds at the industrial scale;
WHEREAS, in view of ImmunoGen’s intention to prepare for [***] and with development of one or more Drug Products, Sicor has made certain investments and is carrying out certain preparatory activities, necessary as preliminary steps to scale-up the production of the Ansamitocins and DMx compounds to be used in such Drug Products;
WHEREAS, ImmunoGen now desires that Sicor scale-up the aforementioned processes, with the goal of producing one or both of the Products in cGMP conditions;
WHEREAS, during such scale-up activities, ImmunoGen is interested in having Sicor manufacture scale-up batches of such Products;
WHEREAS, ImmunoGen also desires Sicor to carry out certain preliminary regulatory work and other scale-up activities, including the GMP-related Activities, regarding Maytansinol, certain Ansamitocins and the Products, in connection with ImmunoGen plans to submit one or more Health Registrations for the Drug Product(s);
WHEREAS, ImmunoGen intends to enter into a Supply Agreement (the “[***] Supply Agreement”) with [***] (“[***]”) pursuant to which ImmunoGen will provide [***] with certain Products manufactured by Sicor under this Agreement;
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
WHEREAS, Sicor is willing to carry out such scale-up activities and GMP-related Activities, and to supply to ImmunoGen scale-up batches of the Products, under the terms and conditions set forth hereinbelow;
NOW, THEREFORE, the parties hereto agree to the following:
1. Definitions. Any term or concept defined only in the Technology Transfer Agreement, or defined in this Agreement by reference to a definition in the Technology Transfer Agreement, shall have the meaning, and be interpreted and integrated using the terms and conditions, set forth in the Technology Transfer Agreement. Any other terms defined in both the Technology Transfer Agreement and this Agreement shall be have the meaning set forth in this Agreement. Subject to the foregoing, whenever used in this Agreement the terms defined in this Article 1 shall have the following specified meanings.
1.1. “Batch” shall mean any Product that is produced by Sicor during the same cycle of Manufacture, as defined by the applicable Master Batch Records.
1.2. “Certificate of Analysis” shall mean a document prepared by Sicor in the same form as generally used by Sicor for such purpose and in a form and format substantially in the form attached hereto as Exhibit A that (i) lists all analytical tests and standards Sicor uses to evaluate a Batch and/or any intermediate of a Product used in the production of a Batch and (ii) certifying the results of each of the foregoing.
1.3. “cGMP” shall mean the then current good manufacturing practices applicable to the manufacture of bulk active pharmaceutical ingredients for drug products destined for marketing in the United States or the European Union, in accordance with the applicable parts of Title 21 of the United States Code of Federal Regulations, the Guide to Good Manufacturing Practices for Medical Products as promulgated under European Directive 2003-94-EC, and ICH7A, each as amended or updated from time to time, as implemented by the respective Regulatory Authorities thereof.
1.4. “Downstream Processing” shall mean the downstream processing activities to be conducted by Sicor using the DSP Technologies and any Project Inventions which the parties agree are applicable to the conduct of the Scale-up Project.
1.5. “Effective Date” shall mean 27 April 2007.
1.6. “Fermentation Process” shall mean the fermentation process activities to be conducted by Sicor using the ImmunoGen Fermentation Process Technologies and any Project Inventions which the parties agree are applicable to the conduct of the Scale-up Project.
1.7. “GMP-related Activities” shall have the meaning set forth in Article 3.
1.8. “Manufacture” shall mean the manufacture and quality control testing (including in-process, release, and stability testing) of Ansamitocin(s) or Products, as appropriate, conducted in accordance with this Agreement.
1.9. “Manufacturing Documentation” shall mean all Master Batch Records and any other executed batch records with all associated documentation, including but not limited to [***] and [***] associated with each Batch, as further defined in Appendix 4.
2
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
1.10. “Manufacturing Process” shall mean any and all processes (or step in any process) used or planned to be used by Sicor in the Manufacture of the Products for ImmunoGen or the Supply Partners.
1.11. “Manufacturing Registrations” shall mean any and all applicable technical, medical or scientific licenses, registrations, authorizations and/or approvals (including, without limitation, manufacturing approvals and authorizations) that are required by any Regulatory Authority for the Manufacture of Product, as amended or supplemented from time to time. For the sake of clarification, “Manufacturing Registrations” shall not mean any of the aforementioned that are applicable solely to drug master files or similar regulatory filings.
1.12. “Master Batch Records” (or “MBRs”) shall mean the written descriptions of the Manufacturing Process used hereunder for each Product, which shall include the technical requirements and specifications with regard to the manufacturing methods, packaging process, and storage methods and procedures, as applicable, and as further clarified, and subject to, Section 1 of Appendix 4.
1.13. “Mutual Confidentiality Agreement” shall mean that certain Mutual Confidentiality Agreement effective as of [***], by and among ImmunoGen, [***] and Sicor.
1.14. “Product” shall mean either DM1 or DM4; “Products” shall mean both DM1 and DM4.
1.15. “Regulatory Guidelines and Requirements” shall mean any federal, state, local, national and supra-national laws, statutes, rules and regulations, including the cGMP, and any other regulatory norms or guidelines, in effect at any particular time, issued or required by the FDA and the European Medicines Agency or other competent legislative bodies or Regulatory Authorities of the United States and the European Union, applicable to a particular activity hereunder, including the Manufacture of Product. For purposes of clarity, “Regulatory Guidelines and Requirements” shall not mean any regulatory norms or guidelines that are specifically applicable to drug master files for active pharmaceutical ingredient or advanced intermediate, or similar regulatory filings for such substances, including but not limited to norms or guidelines concerning their preparation, submission, maintenance and updating, even where such files or submissions may be required of the manufacturer of such substances in connection with the filing and/or approval of Health Registrations.
1.16. “Run” shall mean a single fermentation run of the Manufacturing Process used hereunder to produce Ansamitocins at the [***] liter fermentation scale at the Sicor Facility, and the harvest, recovery, purification, quality testing and release of Ansamitocins. For purposes of this Agreement, “[***] liter fermentation scale” shall mean a fermentation carried out in a fermenter with a [***]-liter nominal volume.
1.17. “Scale-up Batches” shall mean each and every Batch of the Products Manufactured by Sicor pursuant to Section 2.3 hereof.
1.18. “Scale-Up Conforming Product” shall mean Product which, at the time of delivery to ImmunoGen, conforms to cGMP as applicable to non-validated processes, the Tentative Specifications and any other Specifications agreed to by the Parties in writing, and the applicable Master Batch Records.
3
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
1.19. ““Scale-up Project” shall mean the production of Scale-up Batches as set forth in Section 2.3, and the associated pre-requisite scale-up activities and the GMP-related Activities set forth in Section 2.2 and Appendix 1, and Article 3, respectively.
1.20. “Specifications” shall mean the specifications and the quality control testing procedures applicable to the Manufacture by Sicor of each of the Products, as set forth in Appendix 2 hereto, as amended in writing by the parties. “Tentative Specifications” shall have the meaning set forth in the same Appendix 2.
1.21. “Term” shall mean the period from the Effective Date to the date of termination or expiration of this Agreement, pursuant to Article 6 hereof.
4
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Additional Definitions. Each of the following definitions is set forth in the section of this Agreement or the Technology Transfer Agreement indicated below:
|
Definition |
|
|
|
Section |
|
Affiliate |
|
1.3 of Technology Transfer Agreement |
||
|
Ansamitocin P3 |
|
1.2 of Technology Transfer Agreement |
||
|
Ansamitocins |
|
1.2 of Technology Transfer Agreement |
||
|
Applicable Acceptance Tests |
|
2.4 |
||
|
Change of Control |
|
1.5 of Technology Transfer Agreement |
||
|
Claims |
|
8.3.1 |
||
|
DM1 |
|
1.7 of Technology Transfer Agreement |
||
|
DM4 |
|
1.7 of Technology Transfer Agreement |
||
|
Drug Product |
|
1.3 of Technology Transfer Agreement |
||
|
DSP Technologies |
|
1.8 of Technology Transfer Agreement |
||
|
FDA |
|
1.9 of Technology Transfer Agreement |
||
|
Full Process Development |
|
1.13 of Technology Transfer Agreement |
||
|
Health Registrations |
|
1.14 of Technology Transfer Agreement |
||
|
ICC |
|
2,4 |
||
|
Indemnitee |
|
8.3.3 |
||
|
ImmunoGen Fermentation Process Technologies |
|
1.18 of Technology Transfer Agreement |
||
|
ImmunoGen Indemnitees |
|
8.3.1 |
||
|
ImmunoGen Notice |
|
2.4 |
||
|
Intermediates |
|
2.3.2.2 |
||
|
Laboratory |
|
2.4 |
||
|
Losses |
|
8.3.1 |
||
|
Maytansinol |
|
1.24 of Technology Transfer Agreement |
||
|
Maytansinoid Products |
|
1.25 of Technology Transfer Agreement |
||
|
Minimum Yield |
|
2.3.1 |
||
|
[***] |
|
1.27 of Technology Transfer Agreement |
||
|
Preparatory Work for the Scale-up Project |
|
6.1(a)(i) of Technology Transfer Agreement |
||
|
Regulatory Authority |
|
1.32 of Technology Transfer Agreement |
||
|
Supply Partner |
|
1.36 of Technology Transfer Agreement |
||
|
Sicor Confidential Information |
|
1.33 of Technology Transfer Agreement as supplemented by 2.11.1 hereof |
||
|
Sicor Facility |
|
1.34 of Technology Transfer Agreement |
||
|
Sicor Indemnitees |
|
8.3.2 |
||
|
Strain |
|
1.35 of Technology Transfer Agreement |
||
|
Targeted Requirements |
|
1.37 of Technology Transfer Agreement |
||
Appendices
Appendix 1 Scale up Project
Appendix 2 Specifications
5
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Appendix 3 cGMP-Related Activities
Appendix 4 Manufacturing Documentation
Exhibits
Exhibit A Form of Certificate of Analysis
2.1. Preparatory Activities. The parties hereby acknowledge and agree that Sicor has begun and is continuing to carry out the Preparatory Work for the Scale-up Project as contemplated in the Technology Transfer Agreement.
2.2. Scale-up to [***] Liter Scale Fermentation. Sicor shall begin scale-up of the Fermentation Process and the Downstream Processing of Ansamitocins to the [***] liter fermentation scale in accordance with Appendix 1 attached hereto.
2.3. Scale-up Batches
2.3.1. Fermentation Scale-up. As part of the scale-up activities set forth in Section 2.2, Sicor shall Manufacture Ansamitocins in [***] Runs in accordance with Sub-section 2.3.3, to be used by Sicor in the Manufacture and supply of Product set forth in the following Sub-section. In the event that the Manufacture of DM1 and DM4 as set forth in Paragraph 2.3.2.1 does not result in at least [***] grams of such substances and of any other Maytansinoid Products consumed in performing analytical sampling and/or any other activities performed during such Runs agreed to in writing by ImmunoGen (the “Minimum Yield”), Sicor shall Manufacture Ansamitocins carrying out [***] Run. For purposes of determining the Minimum Yield, the quantity of said other Maytansinoid Products shall be considered on a proportional basis calculated from actual yields, as per the following example (said example not to be deemed indicative in any manner of the yields or sampling expected.
If [***] gram of Ansamitocins (from Fermentations Scale-up) is used to Manufacture [***] gm DM1, and [***] grams of Ansamitocins are consumed for sampling, then for purpose of determining the Minimum Yield, it will be considered that [***] gram-equivalents of DM1 were used for sampling purposes.
Furthermore, notwithstanding any of the foregoing, if prior to startup of the Chemical Synthesis Scale-up, laboratory scale production demonstrates a significantly lower yield (difference of [***]) of DM1 (from Ansamitocins) relative to that of DM4, then the overall quantities of DM4 required to reach the Minimum Yield (after subtracting DM1 and the aforementioned Maytansinoid Products) shall be adjusted proportionately.
6
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
2.3.2. Chemical Synthesis Scale-up
2.3.2.1. Upon completion of the Manufacture of Ansamitocins set forth in Sub-section 2.3.1 hereof, Sicor shall Manufacture and supply to ImmunoGen (a) [***] Scale-up Batches of DM1, and (b) [***] Scale-up Batches of DM4, or such other number of batches of each Product as are agreed to by the parties. To the extent Sicor is obligated under Sub-section 2.3.1 hereof to Manufacture Ansamitocins in a [***] Run in accordance with Section 2.3.3, ImmunoGen shall inform Sicor as to whether such Ansamitocins should be used to Manufacture DM1 or DM4, and Sicor shall Manufacture such Product from the [***] Run in accordance with Appendix 1.
2.3.2.2. Sicor shall carry out the assessments on the Scale-up Batches as set forth in Appendix 1. Without limiting the foregoing, Sicor shall (a) test a representative sample from each Scale-up Batch in accordance with the schedule set forth on Appendix 1, (b) provide and submit to ImmunoGen (i) a Certificate of Analysis for the Ansamitocins and Maytansinol (the “Intermediates”) used to Manufacture each Scale-up Batch within [***] days after completion of testing and (ii) a Certificate of Analysis for each Scale-up Batch approved by Sicor for delivery to ImmunoGen [***] of each such Scale-Up Batch and (c) provide ImmunoGen with a copy of the original set of Manufacturing Documentation associated with each Scale-Up Batch as described in Appendix 4 attached hereto, provided that, until and unless the parties have agreed to [***] governing the provision of the Manufacturing Documentation set forth in Sections 9-11 of said Appendix 4, Sicor may provide such Manufacturing Documentation on a voluntary basis, but shall not be contractually obliged to do so.
2.3.3. All Product supplied under this Section 2.3 shall conform to cGMP applicable for non-validated processes and to the Tentative Specifications and shall be delivered to ImmunoGen in accordance with Section 5.3 of the Technology Transfer Agreement (excluding the pro-forma nature of the relative invoices, which shall not be applicable to Product supplied under this Section).
7
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
2.4. Inspection, Acceptance and Rejection. ImmunoGen shall have [***] days from receipt of each Scale-up Batch at the ImmunoGen designated delivery site in accordance with Sub-sections 2.3.1 and/or 2.3.2 to conduct acceptance testing to determine whether the Scale-up Batch constitutes Scale-Up Conforming Product. In the event that any Scale-up Batch fails to constitute Scale-Up Conforming Product, ImmunoGen shall notify Sicor in writing within such [***] day period (the “ImmunoGen Notice”). Except as otherwise provided below, (a) Sicor shall, at Sicor’s own cost (including freight and insurance) deliver additional or replacement quantities of such Scale-up Batch failing to constitute Scale-Up Conforming Product to ImmunoGen and (b) in the event that [***] Scale-up Batch fails to constitute Scale-Up Conforming Product, ImmunoGen and [***] shall have the rights set forth in Section 2.11.2. Sicor may analyze any Scale-Up Conforming Products rejected for nonconformity. In case of any disagreement between the parties as to whether a Scale-up Batch constitutes Scale-Up Conforming Product, the claim shall be submitted for resolution to an independent testing organization mutually agreed upon by the parties which meets current Good Laboratory Practices as defined by the FDA from time to time (the “Laboratory”), the appointment of which shall not be unreasonably withheld or delayed by either party. Should the parties fail to agree upon a Laboratory within [***] from the day either party first proposes an independent testing organization, either disputing party shall have the right to have recourse instead to the International Centre for Technical Expertise of the International Chamber of Commerce (“ICC”), in accordance with the ICC’s Rules for Technical Expertise, with the determination of said Centre to be deemed that of a Laboratory for purposes of this Section. The determination of such entity with respect to all or part of any shipment of Scale-up Batch shall be final and binding upon the parties. The fees and expenses of the Laboratory making such determination shall be paid by the party against which the determination is made. For purposes of this Agreement, any Scale-up Batch shall be deemed to have been accepted by ImmunoGen upon the first to occur of (i) the issuance by ImmunoGen to Sicor of a written notice of acceptance; (ii) the failure of ImmunoGen to provide Sicor with the ImmunoGen Notice on or before expiration of the [***] period described above; and (iii) the determination by the Laboratory that the Scale-up Batch constitutes Scale-Up Conforming Product as described above.
2.5. Subsequent to completion of the scale-up activities set forth and described in Sections 2.2 and 2.3 and Appendix 1 hereof, the parties hereto shall discuss ImmunoGen’s need for other pre-commercial cGMP production of DM1 and/or DM4 or other Maytansinoid Product(s), prior to and additional to any [***] supplies as contemplated in Section 2.3 hereof, and to the extent ImmunoGen determines such production is required, negotiate in good faith the necessary terms and conditions relative thereto, including any necessary extension of the Term.
2.6. Sicor undertakes to use reasonable commercial and technical efforts to meet the time periods set forth in Appendix 1. The parties agree that to the extent that the failure to meet any of such time periods is due to scientific or technical reasons inherent to the experimental nature of the scale-up activities and beyond Sicor’s reasonable control, Sicor will not be held liable for such failure. In the event that Sicor, at any time during the term of this Agreement, reasonably believes that it will be unable to supply ImmunoGen in a timely manner with the full quantities of Product as detailed in Appendix 1, Sicor shall provide prompt written notice of same to ImmunoGen
2.7. Sample Retention. Sicor shall take and retain, for such period as may be required by Regulatory Guidelines and Requirements, samples of Ansamitocins, Maytansinol and Product
8
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Manufactured under this Agreement sufficient to perform at least full duplicate quality control testing. When required for complying with specific requirements of Regulatory Authorities or other Regulatory Guidelines and Requirements, Sicor shall submit to ImmunoGen amounts from such samples, as required under the particular circumstances, as well as the relative quality control and other records (including, but not limited to, raw data) as ImmunoGen may reasonably request in writing in order for ImmunoGen to meet such requirements. Throughout the term of this Agreement, ImmunoGen also agrees to take and retain samples of Product Manufactured and supplied hereunder, sufficient to perform at least full duplicate quality control testing, for use to meet such specific requirements as described above.
2.8. Record Retention. All batch records relating to the Manufacture of Product by Sicor hereunder shall be retained and archived by Sicor for a period of [***] years following completion of the applicable Batch, or such longer period as may be required by Regulatory Guidelines and Requirements. Sicor shall, upon the expiration of each retention period and in any case prior to destroying any such records, provide written notice to ImmunoGen identifying the records that are no longer subject to this Section 2.8 and shall, if requested in writing by ImmunoGen within [***] days of its receipt of Sicor’s notice, provide copies of such records to ImmunoGen.
2.9. No Use of Third Party Subcontractors. Sicor acknowledges and agrees that, with the exception of analytical testing of the Products or other Maytansinoid Products, Sicor may not perform any of its obligations regarding the Manufacture and supply of the Products, through any Third Party subcontractors without the prior written consent of ImmunoGen, such consent not to unreasonably withheld or delayed. The parties acknowledge that Sicor has requested and received ImmunoGen’s consent for use of the following third party contractors for carrying out applicable parts of the Preparatory Work for the Scale-up Project and/or of this Agreement:
· [***] for the execution of the [***]; and
· [***] for [***].
2.10. Sicor Facility; Equipment. Except as otherwise provided for in Section 2.9 hereof, or consented to by ImmunoGen pursuant to such Section, Sicor shall conduct all Manufacturing of Product at the Sicor Facility and shall maintain at the Sicor Facility all equipment and other items used to Manufacture Products. Sicor shall maintain the Sicor Facility, to the extent used for the Manufacture of Product hereunder, and all such equipment and other items in a state of repair and operating efficiency consistent with the requirements of the Specifications, cGMP and all other applicable Regulatory Guidelines and Requirements that affect the Manufacture of Product.
2.11. Audit of Sicor Facility. Sicor agrees that ImmunoGen, [***] and their respective agents shall have the right, upon reasonable prior notice to Sicor, to inspect the Sicor Facility and audit Sicor’s quality systems during normal business hours at times reasonably agreed to with Sicor in order to ascertain compliance by Sicor with the terms of this Agreement, including but not limited to, inspection of (a) the materials used in the Manufacture of the Ansamitocins and Product, (b) the holding facilities for such materials, (c) equipment, (d) the quality control procedures and systems and (e) all records relating to such manufacturing, quality control and quality assurance. The parties agree that, except under extraordinary circumstances and needs, such inspections shall be limited to [***] per calendar year. Any information disclosed in writing, orally or by inspection of tangible objects, including but not limited to general Sicor SOPs reviewed by
9
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
ImmunoGen during such audits, shall be considered Sicor Confidential Information and protected as such by ImmunoGen pursuant to the terms of the Technology Transfer Agreement and by [***] pursuant to the Mutual Confidentiality Agreement.
2.12. Batch and Run Failures. Without limiting the rights of ImmunoGen and [***] under Section 2.11, in the event that (i) [***] Scale-up Batch [***] to [***], or (ii) [***] Run [***] to [***] [***], based on the [***][***] during the Technology Transfer Agreement, or (iii) any [***][***] to [***][***][***] or other [***], ImmunoGen, [***] and their respective employees and agents shall have the right to meet with Sicor at the Sicor Facility or such other location as may be mutually agreeable to the Parties to discuss and otherwise have input on the steps to be taken into consideration in order to [***] such [***] and the possible presence of at most [***] employee from each of ImmunoGen and [***] during certain of such [***][***].
2.13. Government Inspections. Sicor, in accordance with Regulatory Guidelines and Requirements, shall permit representatives of any Regulatory Authority having jurisdiction over the Manufacture of Product and/or marketing of Drug Products in countries where ImmunoGen or Supply Partners are submitting Health Registrations, including the FDA, to inspect the Sicor Facility in conjunction with the Manufacture, storage, handling and shipping of the Product. ImmunoGen will inform Sicor of such countries to the extent necessary for Sicor to meet and comply with the foregoing obligation and any other obligations under this Agreement. Sicor shall promptly advise ImmunoGen if Sicor receives a notice of an impending inspection or if an authorized agent of the FDA or other Regulatory Authority visits the Sicor Facility with respect to Product Manufactured for ImmunoGen and shall, if requested by ImmunoGen, permit ImmunoGen to be present at the Sicor Facility during any such inspection. Sicor shall furnish to ImmunoGen non-confidential copies of any report regarding Product Manufactured for ImmunoGen, including any FDA Form 483 notices (or comparable notices of other governmental agencies), regulatory letters or similar documents received from such Regulatory Authority and the application of such report to such Product, if any, within [***] days of Sicor’s receipt of such report.
2.14. Communication and Meetings. To the extent requested by ImmunoGen and subject to the final sentence of this Section 2.14, (a) Sicor shall meet with ImmunoGen and [***] not more than [***] per [***] at the Sicor Facility or such other meeting place as the parties may agree to discuss the Scale-up Project; (b) Sicor shall participate in any meetings of the joint project committee, established by ImmunoGen and [***] under the [***] Supply Agreement; and (c) Sicor shall permit [***] to attend any meeting of Sicor and ImmunoGen that involves the Sicor Scale-up Project in a non-decision-making, observer capacity. For purposes of clarity, all meetings of the joint project committee shall be conducted in accordance with the following guidelines:
The joint project committee shall establish a schedule of times for regular [***] (or as often as otherwise agreed to by ImmunoGen, [***] and (to the extent involving Sicor) Sicor) meetings, taking into account the planning needs and timetable of the Project and the responsibilities of the joint project committee. The meetings of the joint project committee shall be held by teleconference.
2.15. Information and Assistance. To the extent requested by ImmunoGen and solely as applicable for Products Manufactured hereunder with non-validated processes, Sicor shall provide ImmunoGen and [***] with such reasonable information and assistance relating to Sicor’s
10
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Manufacture of Products hereunder as either party may reasonably require for purposes of applying for and maintaining any regulatory submissions which are Health Registrations for any Drug Products of ImmunoGen or [***] containing Product Manufactured by Sicor hereunder, including, without limitation, providing ImmunoGen and/or [***] with all reports, authorizations, certificates, methodologies, and other documentation in the possession or under the control of Sicor relating to the Manufacture of Product (or any component thereof). Such reports, authorizations, certificates, methodologies and other documentation, shall not be deemed to be included or be required to be included in drug master files or other similar regulatory filings mentioned in Section 1.15 hereof. The parties shall use reasonable efforts to have Sicor provide all such assistance by telephonic conference calls; provided, that, to the extent any in-person meeting is required for such assistance, it shall be on such terms as may be reasonably requested by Sicor.
3. GMP-related and Quality Control Activities.
3.1. During scale-up and any other operations contemplated under Article 2 hereof, Sicor will conduct in a diligent manner the regulatory activities, quality assurance and analytical activities set forth in Appendix 3 appropriate for cGMP production in accordance with all other Regulatory Guidelines and Requirements.
4. Payments.
4.1. Scale-up and GMP-related Activities. In consideration of the scale-up activities and production carried out by Sicor, including the associated pre-requisite activities set forth in Sections 2.2 and Article 3 hereof, ImmunoGen shall pay to Sicor a non-refundable amount of Euro Five Million (€5,000,000.00[***]percent of such amount will be due from ImmunoGen [***]and [***]of such amount will be due from ImmunoGen [***][***]and [***] of such amount will be due from ImmunoGen [***]and the [***]and [***]and[***]respectively and the [***]as provided [***] Sicor shall provide ImmunoGen with a written invoice with respect to the occurrence of each event for which a payment is due under this Section 4.1 (in the case of the [***], with [***][***], as appropriate, with the [***][***][***], provided with [***][***][***]) and ImmunoGen shall pay Sicor the amount set forth in each such invoice within [***] days of receipt thereof(except for the [***][***] which shall be due within [***] days of the aforementioned ImmunoGen [***] of [***][***]).
4.2. All payments set forth in Section 4.1 hereof shall be exclusive of any Value Added Tax or any other taxes, duties of government charges due in respect of the products, services or other taxable or chargeable items of which such payments are being made. Any applicable Value Added Tax, as invoiced by Sicor, shall be paid by ImmunoGen as part of payment for Sicor performance under this Agreement. In the event Sicor is required to prepay any other such tax or fee, ImmunoGen will reimburse Sicor promptly upon receipt by ImmunoGen of appropriate documentation supporting Sicor’s prepayment. When applicable, any such charges shall be stated as separate line items on Sicor’s invoice(s).
5. Supply of Products [***].
5.1. [***], the parties hereto shall negotiate in good faith [***] pursuant to Section 6.1(c), Appendix 6 and Appendix 7 of the Technology Transfer Agreement. Such negotiations shall also
11
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
consider, to the extent feasible, the practical experience and results obtained during the scale-up activities contemplated herein.
6. Term.
6.1. This Agreement shall come into force on the Effective Date and, unless terminated earlier as provided herein, shall terminate upon the date of payment of the last sum due hereunder for the scale-up activities and Scale-up Batches, or upon the date when the last activities required to be performed hereunder are performed, whichever date shall last occur unless specifically extended by further written agreement.
6.2. Termination for Force Majeure. If it becomes apparent to either party hereto at any time during the performance of the scale-up activities, the production of the Scale-up Batches and/or the GMP-related Activities that it will not be possible to carry out such activities for scientific reasons or as a result of Force Majeure (as described in Article 11 below), the parties shall permit [***] business days for discussion to resolve, if possible, the scientific issue or Force Majeure issue giving rise to the problem. If the parties fail to resolve the problem within this [***] day period, either party shall have the right to terminate this Agreement, effective upon written notice to the other. In the event of such termination for the production activities carried out under Articles 2 and 3 and pursuant to Appendix 1 and Appendix 3 hereof, Sicor shall be paid an amount calculated as a proportion of the amounts due under such Section 4.1, based on the actual time spent on activities performed by Sicor relative to the overall estimated time established for all such activities.
6.3. Early Termination for other Reasons. Either party hereto may terminate this Agreement effective upon written notice if either of the following events occurs:
(a) the other party commits a breach of this Agreement and the breach is not remedied within [***] days after the receipt of notice identifying the breach, requiring its remedy and stating the intent of the party to terminate in the absence of remedy; or
(b) the other party (i) becomes unable to pay its debts as they become due, (ii) suspends payment of its debts, (iii) enters into or becomes subject to corporate rehabilitation or bankruptcy proceedings or liquidation or dissolution, (iv) makes an assignment for the benefit of its creditors or (v) seeks relief under any similar laws for debtor’s relief.
12
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
6.4. Effect of Termination. Upon the expiration or termination of this Agreement, the provisions of article 11.4 paragraphs a) through d) of the Technology Transfer Agreement shall be applied mutatis mutandis; provided, that (a) all references in such Section 11.4 paragraphs a) through d to the Project shall be deemed to refer to the Scale-up Project as defined hereunder; and (b) the rights and obligations under Section 4.1 of this Agreement, respectively, shall not survive early termination of this Agreement by ImmunoGen for material breach by Sicor.
7. Intellectual Property Rights; Manufacturing Documentation.
7.1. Intellectual Property Rights. The parties recognize and agree that it is not specifically intended that the activities contemplated hereunder would give rise to new technology or intellectual property. All provisions of Article 7 of the Technology Transfer Agreement shall, in any event, be applied mutatis mutandis to this Agreement; provided, that all references to the Project in the Technology Transfer Agreement shall be deemed to refer to the Scale-up Project.
7.2. Manufacturing Documentation. Notwithstanding anything to the contrary in this Agreement or in the Technology Transfer Agreement all Manufacturing Documentation shall be jointly-owned by the parties and the use and disclosure by the parties of such Manufacturing Documentation shall be subject to the following limitations: (a) ImmunoGen may (i) use all Manufacturing Documentation for any and all purposes without restriction, (ii) disclose to [***], under appropriate obligations of confidentiality and restricted use, all Manufacturing Documentation, and (iii) disclose to any Third Party other than [***] the Manufacturing Documentation listed in Sections 4, 6, 7 and 12 of Appendix 4 for any and all purposes without restriction, provided that ImmunoGen shall consult with Sicor as to which Manufacturing Documentation from Section 4 is so disclosed and (b) Sicor may (i) use Manufacturing Documentation solely for [***] and (ii) disclose Manufacturing Documentation to (A) Regulatory Authorities, but solely to the extent required to meet general regulatory requirements of such Regulatory Authorities and in order to implement or carry out this Agreement and the aforementioned activities and (B) any Marketing Partner if and solely to the extent (1) such disclosure is reasonably approved by ImmunoGen and (2) the relative documents and information regarding any Batches, or parts thereof, for which the Products so Manufactured, or intermediates thereof, are also supplied to such Marketing Partner. For purposes of clarity, under no circumstances shall Sicor use or disclose Manufacturing Documentation for any purpose not described in this Section 7.2.
8. Representations. Warranties and Indemnification.
8.1. All provisions, representations, warranties and exclusions of Sections 8.1, 8.3 and 8.4 of the Technology Transfer Agreement shall be applied mutatis mutandis to this Agreement; provided, that all references to the Project in the Technology Transfer Agreement shall be deemed to refer to the Scale-up Project.
8.2. Additional Representations of Sicor. Sicor further represents and warrants to ImmunoGen that:
13
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
8.2.1. At the time of transfer to ImmunoGen of title and risk to each Product pursuant to the applicable delivery terms, such Product: (a) will have been Manufactured and shipped in accordance with the applicable Specifications, cGMP, the Manufacturing Process used hereunder and Regulatory Guidelines and Requirements, and (b) will not be adulterated or misbranded under the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 321 et seq., as amended, or under any other Regulatory Guidelines and Requirements.
8.2.2. The Manufacture of Product hereunder shall be performed with requisite care, skill and diligence, in accordance with industry standards by individuals who are appropriately trained, experienced and qualified.
8.2.3. Sicor has not been debarred, nor is subject to a pending debarment and that Sicor will not use in any capacity, in connection with the services to be performed under this Agreement, any person who has been debarred pursuant to Section 306 of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 335a, or who is the subject of a conviction described in such section. Sicor agrees to inform ImmunoGen in writing immediately if Sicor or any person who is performing services under this Agreement is debarred or is the subject of a conviction described in Section 306, or if any action, suit, claim, investigation, or legal or administrative proceeding is pending or, to the best of Sicor’s knowledge, is threatened, relating to the debarment or conviction of Sicor or any person performing services hereunder.
8.2.4. Sicor has and shall maintain during the Term all Manufacturing Registrations required to perform its obligations under this Agreement.
8.3. Indemnification.
8.3.1. Sicor. Subject to Sub-section 8.3.2 and Section 9.1, [***] shall [***] and [***][***], its [***], their [***],[***] and [***], and their [***],[***] and [***] (the “[***]”),[***] and [***] ([***] and [***] of [***]) (collectively, “[***]”)[***] or [***] any one or more of the [***] as a [***] of [***] or [***] of any [***],[***] and [***] and [***] of [***] and the [***] (collectively, “[***]”)[***] of (a) the [***] or [***], or (b) [***] or [***] in the [***]; provided, however, that [***] be [***] to [***] or [***] a [***] for [***] to the [***] that [***] from (a) the [***] or [***] of an [***], or (b) the [***] of [***] or [***] by [***] under this Agreement. In the [***] of [***],[***], or other [***][***] an [***] by a [***] shall [***] of the [***] or [***], and [***] and [***], at its [***], the [***] of [***],[***] or other [***]and its [***], keeping [***] of the [***] of the [***] and/or [***]. The [***] shall [***] with [***] and [***], at [***] and [***], be [***] in [***],[***] or other [***] be [***] for [***] or [***] by the [***]
14
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
8.3.2. ImmunoGen. [***] to [***] and [***],[***] and [***],[***] and [***] and [***] (the “[***]”)[***] and [***] by or [***] or [***] of the [***], as a [***] or [***] of (a) the [***] of [***] or [***] by [***] this [***], or (b) the [***] for [***] or [***] by [***] or [***] or [***] of [***], of [***], or (c) [***] or [***] or [***] in the [***] of [***]; provided, however, that [***] be [***] to [***] or [***] a [***] for [***] to the [***] that [***] from (i) the [***] or [***] of an [***] or (ii) the [***] of any [***] by [***] to [***] the [***] of [***]. In the [***] of [***], or other [***] a [***] by a [***],[***] in [***] of the [***] or other [***], and [***] shall [***] and [***], at its [***], the [***] of the [***] or other [***] and its [***] of the [***] of the [***] and/or [***]. The [***] shall [***] and [***], at [***] and [***], be [***] in any [***],[***] or other [***]. [***] be [***] for [***] or [***] by the [***].
8.3.3. Identification of [***]. An "[***]" means [***] with [***] to [***], and [***] with[***] to [***]. An "[***]" means [***] of the [***] with respect to [***], and [***] of the [***] with [***] to [***].
8.3.4. Indemnification Procedures. An [***] which intends to [***] under [***] or [***] shall [***] in writing of any [***] or other [***] in [***] of [***] the [***], its [***], or any of [***] and [***] to [***]; provided, however, that [***] or [***] in [***] from [***] to the [***] that the [***] is [***]. [***] the [***], at its [***], to [***] and/or [***] or other [***], at the [***], and [***] to the [***] of such [***] or [***] by the [***]; provided, however, [***] the [***] or [***] any [***] the [***] in [***] to [***]. [***] or [***] be [***] the [***] of the [***], not to be [***], and the [***] not be [***] for any [***] or [***] as [***]. [***], its [***] and their [***] and [***] with the [***] and its [***] in the [***] and [***] of [***] or [***] by this [***], all at the [***] of the [***].[***] the [***], but not the[***], to be [***] by [***] of its own [***] and at its [***].
8.3.5. Exclusion of VAT. Where a Party to this Agreement is required to [***] or [***] an [***], such obligation to [***] or [***] [***] extend to any value added tax payable by the [***] in respect of the cost, expense or other matter [***] or [***], [***] any such value added tax which cannot normally be offset from payables of value added tax to the competent tax authorities.
8.3.6. [***]
[***]
[***]
[***]
8.3.7. [***]
[***]
15
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
[***]
[***]
9. Limitation of Liability. All provisions of Article 9 the Technology Transfer Agreement shall be applied mutatis mutandis to this Agreement; provided, that, all references to Appendices 4 and 5 in the Technology Transfer Agreement shall be deemed to refer to Appendices 1, 2 and 3 of this Agreement, all references to the Project shall be deemed to refer to the Scale-up Project, and all references to the Technology Transfer Agreement shall be deemed to refer to this Agreement.
10. Confidentiality. All provisions of Article 10 of the Technology Transfer Agreement shall be applied mutatis mutandis to this Agreement; provided, that, all references to the Project in the Technology Transfer Agreement shall be deemed to refer to the Scale-up Project.
11. Force Majeure. Any delay in the performance of any of the duties or obligations of either party hereto (except the payment of money) caused by an event outside the affected party’s reasonable control, including, without limitation, fire, flood, storm, explosions, earthquakes, electrical outages, damage or failure of key equipment, Internet service providers, commotion, riots, insurrection, acts of God or the public enemy, strikes or other labor disturbances, accidents or shipping difficulties, embargoes, restrictions on the use of any material, any inadequate supply of material, or any lawful government, intergovernmental or supranational authority or body, shall not be considered a breach of this Agreement and the time required for performance shall be extended for a period equal to the period of such delay. The party so affected shall give prompt notice to the other party of such cause, and shall take whatever reasonable steps are appropriate in that party’s discretion to relieve the effect of such cause as rapidly as possible.
12. Severability. In the event that any provision in this Agreement becomes wholly or partially invalid, the effectiveness of the remaining provisions shall not be affected. The parties hereto undertake to replace any such invalid provision by a valid one which most closely corresponds with the economic intention of the invalid provision.
13. Assignment. All provisions of Sections 12.11 and 12.12 of the Technology Transfer Agreement shall be applied mutatis mutandis to this Agreement.
14. Successor in Interest. This Agreement shall be binding upon and inure to the benefit of the parties hereto, their Affiliates, successors and permitted assigns.
15. Entire Agreement. This Agreement shall constitute the entire agreement between the parties hereto with regards to the Scale-up Project and shall supersede any other agreements, whether oral or written, express or implied, as they pertain to the Scale-up Project. This Agreement shall not be changed or modified except by written instrument signed by both parties.
16. Notice. Any notice required hereunder may be served by either party on the other by personal delivery, or by sending the same, first class registered mail or by international courier service or by facsimile (in the latter case, promptly confirmed by personal delivery, first class registered mail or international courier service) addressed to the other party at its address first indicated hereinabove, or to
16
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
such other address as shall have been notified in writing to the sending party by the receiving party from time to time, and shall take effect upon receipt by the addressee. In the case of Sicor, said notice shall be served upon or addressed to the attention of the Managing Director with a copy to the Contracts Administrator.
17. Waiver. The waiver by either party hereto of any right hereunder, or of the failure to perform, or of a breach by the other party of any of the terms hereof shall not be deemed a waiver of any other right hereunder or of any other breach or failure by said other party, whether of a similar nature or otherwise.
18. Governing Law and Arbitration. All provisions of Section 12.4 of the Technology Transfer Agreement shall be applied mutatis mutandis to this Agreement; provided, that, all references to the Project in the Technology Transfer Agreement shall be deemed to refer to the Scale-up Project.
19. Property Rights. Except as provided for under Article 7 hereof, nothing otherwise contained in this Agreement shall be construed as granting or conferring any rights by license or otherwise, expressly, implicitly, or otherwise for any invention, discovery or improvement made, conceived or acquired prior to or after the date of this Agreement with regard to the subject matter hereof.
20. Manufacturing Authorizations and Health Registrations. Sicor shall be responsible for obtaining, at its expense, any authorizations or other licenses or permits, and any regulatory and government approvals, including all Manufacturing Registrations, necessary for the Manufacture and supply of the Product to ImmunoGen in accordance with the terms and conditions of this Agreement. Sicor shall provide to ImmunoGen all information in its possession reasonably relevant to specific methods of the Manufacture of the Products and any other information specific to the Products and relevant to FDA and analogous non-U.S. Health Registrations, including, but not limited to, IND, NDA and other Health Registrations, in a timely manner as required to enable punctual submission by ImmunoGen of necessary regulatory documentation. The aforementioned authorizations, licenses, permits and approvals, and the information contemplated under this Section, shall not be deemed to include or be required to be included in drug master files or other similar regulatory filings mentioned in Section 1.15 hereof.
21. Third Party Beneficiaries. Except as set forth in Sections 8.3.1 and 8.3.2, no third party (including, without limitation, any employees of either party) shall have or acquire any rights by reason of this Agreement. Notwithstanding anything to the contrary in this Agreement, (a) the parties hereby acknowledge and agree that ImmunoGen shall enter into the [***]pursuant to which ImmunoGen will supply [***]Sicor hereby acknowledges and agrees, in connection therewith, [***]
[Remainder of page intentionally left blank.]
17
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
IN WITNESS WHEREOF, each of the parties hereto has caused this Agreement in duplicate to be executed and to be retained by its duly authorized officers or representatives this 30th day of April 2007.
|
SIGNED BY |
|
/s/ Xx. XXXXXXXX FINE |
|
|
|
|
Xx. Xxxxxxxx Fine |
|
|
|
|
Managing Director |
|
|
|
|
duly authorized for and on behalf of |
|
|
|
|
SICOR—Società Italiana Corticosteroidi S.r.l. |
|
|
SIGNED BY. |
|
/s/ ING. XXXXXXX XXXXXXXX |
|
|
|
|
Ing. Xxxxxxx Xxxxxxxx |
|
|
|
|
General Manager |
|
|
|
|
duly authorized for and on behalf of |
|
|
|
|
SICOR—Società Italiana Corticosteroidi S.r.l. |
|
|
SIGNED BY |
|
/s/ XXXXXXX XXX XXXX |
|
|
|
|
Xxxxxxx Xxx Xxxx |
|
|
|
|
Senior Vice President, |
|
|
|
|
duly authorized for and on behalf of |
|
|
|
|
IMMUNOGEN, Inc. |
|
18
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
The production schedule will be organized in a way that the [***] ([***] and [***]) will be [***] and [***] by the [***][***][***] of [***][***][***] in the [***][***] according to the following scheme:
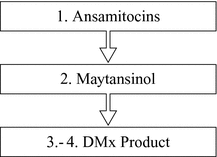
1. Ansamitocins: The production of Ansamitocins will be performed by means of fermentation, in the [***][***][***][***] Sicor, [***][***]. There are [***]and[***]other than by [***]the [***]of the this [***].
The process will [***]in [***]to the [***]
· [***]will be [***]in [***]and will [***]for [***]
· [***]will be [***]in a [***]of [***]of [***]and will [***]for [***]The [***]can be used [***]or [***]to [***]or [***]of [***].
[***]will be [***]in [***]of [***]of [***]and will [***]The [***]can be [***]as a [***]or in [***]
Appendix 1-1
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
[***]will be [***]in the [***]and will [***]The [***]of the [***]will [***]the [***]from [***]or [***]of [***]
The [***]will be [***]in a [***]
[***]and [***]are [***]to [***]in [***]the [***]of such [***]will be [***]on the [***]of the [***]
[***]will be [***]to [***]
The [***]in the [***]of [***]of [***]will be [***]and [***]by the [***]The [***]is [***]to [***]
If [***]in [***]with a [***]is [***]In [***]the [***]of [***]will [***]to [***]at [***]the [***]in the [***]of the [***]this [***]will be [***]to [***].
[***]is [***]to [***]on [***]
2. Maytansinol: The production of Maytansinol will be performed by mean of chemical transformation of Ansamitocins, there are no [***]and [***]other than by [***]during the [***]of the this [***].
The production of Maytansinol will be carried out in the [***][***][***] in the Sicor, [***][***].
[***]are [***]each [***]about [***]
Each [***]will be [***]and [***]by the [***]The [***]is [***]to [***]
The [***]will be [***]as [***]in a [***]from a [***]
The [***]is [***]to [***]from a [***].
[***]The [***]may [***]on the [***]and on the [***]of the [***]
The [***]will be used [***]by the [***]
The [***]is [***]to [***]the [***]of the [***]
The [***]of [***]will be [***]to [***]the [***]is [***]to [***]the [***]of the [***]of the
[***]see [***]
[***]is [***]to [***]
3. DM1: The production of DM1 will be performed by mean of chemical transformation of Maytansinol, there are no [***]and [***]other than by [***]the [***]of the this [***].
The production of DM1 will be carried out in the [***][***][***] in the Sicor, [***][***].
[***]are [***]each [***]about [***]
Each [***]will be [***]and [***]by the [***]The [***]is [***]to [***].
The [***]will be [***]as [***]a [***]of [***]to that [***]from [***]of [***]
[***]The [***]may [***]on the [***]of [***]and on the [***]of the [***]
The [***]will be [***]by the [***]
The [***]is [***]to [***]the [***]
The [***]of [***]will be [***]to [***]
· [***]is [***]to [***]
4. DM4: The production of DM4 will be performed by mean of chemical transformation of Maytansinol, there are [***]and [***]than [***]the [***]of the this [***].
Appendix 1-2
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
The production of DM4 will be carried out in the [***][***][***] in the Sicor, [***][***].
[***]
Each [***]will be [***]and [***]by the [***]The [***]is [***]to [***].
The [***]will be [***]as [***]a [***]of [***]to that [***]from [***]of [***]
· The [***]may [***]on the
[***]of [***]
[***]of the [***]
[***]of the [***]to be [***]
· [***]of ImmunoGen on [***]of [***]
The [***]will be [***]by the [***]
The [***]is [***]to [***]the [***]
The [***]of [***]will be [***]to [***]
[***]is [***]to [***]
All the above activities will be performed in accordance with Master Batch Records [***] of the present Agreement.
The [***]and [***]the [***]in [***]on the [***]may be [***]by such [***].
Appendix 1-3
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Appendix 2
DM1 shall be Manufactured and supplied according to the following Targeted Requirements as previously set forth in Appendix 2 of the Technology Transfer Agreement
|
TEST |
|
|
|
VARIABLE |
|
TARGET |
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
|
|
[***] |
|
[***] |
||
|
|
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
and as further developed by common agreement of the parties, prior to the start of other scale-up activities (the “Tentative Specifications”).
Specifications shall mean the Tentative Specifications, and after the scale-up activities, as further developed by common agreement of the parties..
Appendix 2-1
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Appendix 2
DM4 shall be Manufactured and supplied according to the following Targeted Requirements as previously set forth in Appendix 2 of the Technology Transfer Agreement
|
TEST |
|
|
|
VARIABLE |
|
TARGET |
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
|
|
[***] |
|
[***] |
||
|
|
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
|
[***] |
|
[***] |
|
[***] |
||
and as further developed by common agreement of the parties, prior to the start of other scale-up activities (the “Tentative Specifications”).
Specifications shall mean the Tentative Specifications, and after the scale-up activities, as further developed by common agreement of the parties.
Appendix 2-2
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Appendix 3
In the following paragraphs all the described activities, and the documents generated thereof, will be performed in accordance with the relevant SOPs enforced in Sicor at the moment of the performance of such activities.
Written documents, resulting from the below activities, will be prepared and provided to ImmunoGen in accordance with the provisions of Appendix 4 (for the documents cited therein).
1. [***]:
[***]in which the [***]of [***]and [***]will [***]will be [***]
[***]where not [***]will be [***]out [***]the [***]of the [***]
A [***]will be [***]and [***]
[***]will be [***]
At the [***]of the [***]a [***]will be [***]and [***]
1.1. [***]
[***]of [***]and [***]the [***]of the [***]will be [***]
A [***]will be [***]and [***]
[***]will be [***]
At the end of the [***]a [***]will be [***]and [***]
2. [***]:
2.1. [***]
For [***]in the [***]for [***]of [***]and [***]that are not [***]in use in [***]
The [***]will be [***]and [***]
Every [***]in the [***]of [***]and [***]will be [***]and [***]by the [***]in the [***].
2.2. [***]
[***]by [***]in [***]of the [***]of the [***]and [***]will be [***]
The [***]of such [***]will be [***]only if [***]will [***]it as [***]
In case an [***]will be [***]this will be made in [***]to [***]
[***]will be [***]in the form of [***]that will be [***]in the [***]
2.3. [***]and [***]
For each of the [***]and [***]to be [***]a [***]of [***]to be [***]for the [***]of such [***]will be [***]
Upon [***]of [***]by ImmunoGen[***]a [***]will be [***]and [***]
For the [***]for which it will be [***]to [***]a [***]of [***]will be [***]
[***]
A [***]will be [***]and [***]
[***]will be [***]
At the end of the [***]a [***]will be [***]and [***]
The [***]will be [***]the [***]of any such [***]and [***].
Appendix 3-1
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Upon [***]of the [***]and [***]each [***]will be [***]and [***]by the [***]The [***]will be [***]the [***]upon [***]and the [***]will be [***]if [***]all [***]A [***]will be then [***]this [***]will [***]all the [***]see [***]
3. [***]s
3.1 [***]
[***]and [***]will be [***]and [***]they are[***]along the [***]
3.2 [***]
3.2.1 [***]
For each of [***]and [***]that will [***]and [***]by the [***]a [***]will be [***]This [***]will be [***]by [***]to [***] to this [***]will be [***]in the [***]according to [***]
3.2.2 [***]
At the end of the [***]of each [***]the [***]will be [***]and [***]by [***]
[***]will be [***]and those [***]in [***]will be eventually [***]for the [***]of a new [***]
[***]will be [***]to the [***].
3.2.3 [***]
[***]will be issued and approved before their use in the process.
4. [***]
[***]will be [***]between [***]and [***]at the end of the [***]
[***]for detection of calculated allowed [***]will be [***]and[***].
4.1. [***]
A [***]will be issued
At the end of the [***]of [***]the [***]will be [***]and [***]will be [***]to the [***]
Due to the fact that a [***]can be [***]only [***]an [***]will be [***]
The [***]will be [***]when [***].
4.2. [***][***]
An [***]will be [***]
The [***]will be [***]
The [***]will not be [***]due to the fact that [***]of [***]and [***]outside the scope of this Agreement, are planned to be [***]in [***]
5. [***]
Even if these activities are not strictly related to[***]the [***]of [***]will be [***]of the [***]
If [***]a [***]for [***]from the one [***]for [***]will be [***]
6. [***]
As the last step of the [***]a [***]for each of the [***]and [***]will be [***]using [***]and [***]
The [***]of this [***]based on such information, will be agreed upon by the [***]
A [***]for [***]and [***]
Appendix 3-2
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Appendix
4
Manufacturing Documentation
|
# |
|
|
|
Document |
|
[***] |
|
Details |
|
1 |
|
[***] |
|
[***] |
|
[***]will be [***]at the [***]by ImmunoGen[***]will be [***]to [***]for [***]A [***]will be [***]to ImmunoGen for [***]which [***]shall not be [***]or [***]to [***]at [***]of the [***]will be [***]to ImmunoGen[***] [***]which [***]in a [***]or a [***]to the [***]of the [***]shall be made to [***]and [***]and [***]by [***]as appropriate with [***]of the [***]being provided to ImmunoGen. Such [***]shall be deemed the [***]for [***]of [***]of the [***] [***]from [***]which [***]in a [***]or a [***]to the [***]of the [***]will be reviewed and approved by ImmunoGen [***]the [***]used for the [***] |
||
|
2 |
|
[***] |
|
[***] |
|
[***]are reviewed at ImmunoGen’s discretion based upon [***][***]provided to ImmunoGen by [***]can be [***]to [***]in [***]. |
||
|
3 |
|
[***] |
|
[***] |
|
[***]of [***]will be provided to ImmunoGen [***]and [***]at [***] |
||
|
4 |
|
[***] |
|
[***] |
|
A [***]will be prepared by [***]to [***]the [***]and [***]for all the [***]in [***]A [***]the [***]and [***]with ImmunoGen[***]The [***]will be completed [***]of [***]of [***]for the [***]in the [***] |
||
|
5 |
|
[***] |
|
[***] |
|
[***]will provide [***]for [***]and [***]to ImmunoGen for [***]and [***]For [***]and [***]upon such [***]these will be considered [***]for [***]of the [***]The [***]the [***]and [***]will also [***]for [***]. |
||
|
6 |
|
[***] |
|
[***] |
|
A [***]will be provided to ImmunoGen [***][***]for each [***]or [***]The [***]and a [***]will be [***]with the [***]of the [***]for the [***]and the [***]will be provided with the [***] |
||
|
7 |
|
[***] |
|
[***] |
|
A [***]will be provided to ImmunoGen [***]for each [***]and [***]of the [***]for the [***]and the [***]will be provided [***] |
||
|
8 |
|
[***] |
|
[***] |
|
[***]will be [***]as [***]to a [***]of the [***]will [***]of such events from [***]within [***]of [***] [***]to [***]in the [***]will be [***]on the [***]to [***]will also be annotated[***]These [***]will be [***]into [***]as [***]see [***] |
||
|
9 |
|
[***] |
|
[***] |
|
[***]will provide copies of the [***]for [***]to ImmunoGen for review and [***]to [***] |
||
|
10 |
|
[***] |
|
[***] |
|
[***]will [***]of the [***]to ImmunoGen upon [***]of the [***] |
||
|
11 |
|
[***] |
|
[***] |
|
[***]will provide [***]of the [***]for [***]and [***]for [***] |
||
|
12 |
|
[***] |
|
[***] |
|
[***]will provide [***]for [***]and [***] |
||
Appendix 4-1
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
[***]
Portions of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
