EXECUTION VERSION -2- 70472309_1 2. Sale of Product. 2.1. Generally. During the Term, Customer hereby commits to purchase from Novavax the Vaccine, which will be supplied in [***] (the “Product”), in an amount equal to the aggregate quantity of...

Exhibit 10.36 CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (i) NOT MATERIAL AND (ii) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. EXECUTION VERSION ADVANCED PURCHASE AGREEMENT This ADVANCED PURCHASE AGREEMENT (this “Agreement”) is made as of 31 December 2020 (the “Effective Date”), by and between NOVAVAX, INC., incorporated and registered in the State of Delaware, with a principal place of business at ▇▇ ▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇ ▇.▇.▇. (“Novavax”), and The Commonwealth of Australia as represented by the Department of Health, with offices at ▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇, ▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇ (“Customer”). Novavax and Customer may individually be referred to herein as a “Party” and, collectively, as the “Parties.” RECITALS WHEREAS, Novavax is currently developing a novel NVX-CoV2373 vaccine, consisting of a stable, prefusion protein made using its proprietary nanoparticle technology and coformulated with its proprietary Matrix-M™ adjuvant (the “Vaccine”), which is intended to prevent SARS-CoV-2 (“COVID-19”) in humans; WHEREAS, Novavax intends to seek government issued licenses, registrations, authorizations and approvals necessary to commercialize the Vaccine, including without limitation, emergency exemptions, Authorisations or provisional approvals (“Regulatory Approval”) to permit use of the Vaccine in Australia (the “Territory”); WHEREAS, in advance of Regulatory Approval in the Territory, Customer wishes to pre-order an aggregate number of doses of Vaccine from Novavax designed to vaccinate [***], to be supplied subject to the terms and conditions of this Agreement; WHEREAS, in reliance on such commitment by Customer, Novavax agrees to commence commercial manufacture of the Vaccine prior to Regulatory Approval for supply to, and distribution by, Customer to individuals in the Territory; NOW, THEREFORE, in consideration of the mutual covenants, terms and conditions set forth below, and for other good and valuable consideration the sufficiency and receipt of which is acknowledged by each Party, the Parties agree as follows: 1. Effects of COVID-19. Novavax and Customer hereby acknowledge and agree that to make Vaccine available [***] Novavax will commence manufacture of the Vaccine in advance of Regulatory Approval in the Territory and that the use, deployment and administration of the Vaccine by Customer to individuals in the Territory will occur under COVID-19 pandemic conditions. The terms and conditions of this Agreement, including with respect to Product pricing, refund terms, indemnification and limitations of liability, reflect this understanding.

EXECUTION VERSION -2- 70472309_1 2. Sale of Product. 2.1. Generally. During the Term, Customer hereby commits to purchase from Novavax the Vaccine, which will be supplied in [***] (the “Product”), in an amount equal to the aggregate quantity of Vaccine doses set forth in Exhibit A (the “Aggregate Amount”). If during the Term Customer desires to purchase an additional quantity of Vaccine doses, up to the Maximum Aggregate Additional Amount identified in Exhibit A, then Customer shall notify Novavax in writing, and the Parties will negotiate, in good faith, whether Novavax has supply sufficient to provide the requested additional quantity of Vaccine doses at the time of Customer’s request. If the Parties agree on the quantity of, and Delivery Schedule for, an additional amount of Vaccine doses (“Additional Amount”), Customer shall issue a Purchase Order for the Additional Amount in accordance with Section 2.3.1. 2.2. Use, Donation and Resale. 2.2.1. Use. Novavax grants Customer the right to use any Product supplied hereunder (a) solely to vaccinate individuals in the Territory against COVID-19, subject to Section 2.2.2 and Section 2.2.3, and (b) in accordance with the terms and conditions of this Agreement. 2.2.2. Donation and Resale. Notwithstanding the territory restriction set forth in Section 2.2.1 and subject to Customer’s obligation to indemnify Novavax as set forth in Section 9, Novavax grants Customer the right to donate or resell some or all of the Product to one or more countries outside of the Territory or an organisation (including an NGO) that Customer may determine in its absolute discretion (“Regional Partners”), provided that (i) the intended purpose of such donation or resale is to vaccinate individuals against COVID-19, (ii) [***], (iii) [***] and (iv) [***]. 2.2.3. Conditions for Donation and Resale. Customer expressly acknowledges and agrees that, in connection with any donation or resale of Product to a Regional Partner: (A) [***], (B) [***], (C) [***], and (D) [***]. Customer acknowledges and agrees Customer shall be responsible for [***]. 2.3. Purchase Order. 2.3.1. Purchase Order. Within [***] of the Effective Date, Customer will deliver a purchase order (“Purchase Order”) for the Aggregate Amount to Novavax together with the Customer’s order number and invoice address. If Customer elects to purchase an Additional Amount, it will deliver a Purchase Order for the Additional Amount to Novavax together with the Customer’s order number and invoice address. 2.3.2. Confirmation. Provided the Purchase Order contains accurate quantity and pricing information in accordance with Section 2.3.1, Novavax will confirm a Purchase Order within [***] of receipt; provided, however, that Novavax

EXECUTION VERSION -3- 70472309_1 will be deemed to have accepted a Purchase Order if no confirmation is received prior to the expiry of such [***] period. 2.4. Delivery. Novavax intends to deliver monthly shipments of Product to Customer until the Aggregate Amount is supplied. Based upon Novavax’s projections and expectations as of the Effective Date, the anticipated quarterly delivery schedule of the Product is set forth in Exhibit B (“Delivery Schedule”). Customer acknowledges that the Delivery Schedule may change due to the impact of several variables including, but not limited to, speed of clinical trial enrollment and accrual of events, manufacturing delays and/or timing of Regulatory Approval in the Territory. Novavax will use reasonable endeavours to deliver, or ensure that the Sponsor delivers, the Product to Customer in accordance with the Delivery Schedule and will, on at least a [***] basis, communicate any anticipated changes to the Delivery Schedule to Customer including any anticipated variances permitted by Section 2.5. At least [***] in advance of each anticipated shipment under the Delivery Schedule, Novavax will confirm to the Customer in writing the date of delivery of the Product. Delivery of the first shipment of Product under the Delivery Schedule is expected to be a date as soon as practicable after receipt of Regulatory Approval in the Territory when sufficient Product is available for the first shipment in the Delivery Schedule. Novavax will [***] notify Customer in writing by email when the Product is available for Delivery and for Customer’s inspection. 2.5. Variance. Without limiting Section 2.6, Customer hereby acknowledges and agrees that the Delivery Schedule is an estimate only and that notwithstanding anything herein to the contrary, (a) the quantity of Vaccine actually delivered each Calendar Quarter may vary by [***] of the Aggregate Amount and (b) the actual date of delivery may vary within [***] of the delivery date projected by Novavax pursuant to Section 2.4; provided that Novavax first notifies Customer of such variances [***] in advance of each anticipated shipment of Product under the Delivery Schedule. 2.6. Short Supply. If Novavax receives Regulatory Approval in the Territory, but reasonably believes that it will not be able to supply Customer with quantities of Vaccine within the variances permitted by Section 2.5, then Novavax shall [***] notify Customer in writing of such circumstances, including [***] (“Remedial Plan”). Where such inability to supply results from Novavax’s inability to manufacture or source sufficient quantities of Vaccine doses to supply all of its customers, Novavax shall deliver to Customer [***] for the period of short supply. Novavax shall consider in good faith any reasonable changes to the Remedial Plan proposed by Customer. 2.6.1. If the Remedial Plan does not resolve such inability or failure to supply to within [***] of the first missed or under delivery giving rise to the Remedial Plan, the Customer may, [***], cancel delivery of the Product that was scheduled for delivery [***].

EXECUTION VERSION -4- 70472309_1 2.6.2. If failure to supply is still ongoing after [***] of the initial missed or under delivery giving rise to the Remedial Plan, Customer may upon written notice to Novavax cancel future deliveries of Product and terminate the Agreement. 2.6.3. If Customer elects to cancel delivery of Product pursuant to this Section 2.6, [***]. Subject to Section 7.2.2 and Section 7.5, the remedies in this Section 2.6 shall be Customer’s sole recourse and Novavax’s entire liability with respect to any failure to supply. 2.7. Inconsistent Terms. All terms and conditions contained in any prior or subsequent oral or written communication, including terms and conditions contained in the Purchase Order, that are different from or in addition to this Agreement are hereby rejected by the Parties and will neither expand nor modify either Party’s obligations under this Agreement. 2.8. Non-exclusivity of arrangements. Novavax acknowledges and agrees that the arrangement under this Agreement is non-exclusive and Customer may acquire any product comparable to the Vaccine under any process or arrangement and from any vaccine supplier as it sees fit and by entering this Agreement Customer is not restricted from entering into any international or multilateral agreements for supply of product comparable to the Vaccine. 3. Delivery and Acceptance 3.1. Delivery, Title and Risk of Loss. Product will be delivered to a point of entry in Australia mutually agreed by the Parties, acting reasonably (the “Point of Entry”). Product will not be delivered until after all import and customs clearance requirements in respect of the Products have been completed, including the batch testing and release process required by the TGA, and Novavax (or Sponsor) has provided all Delivery Documents to Customer. Novavax will [***] notify, or ensure that the Sponsor notifies, Customer in writing when Product is ready for collection by Customer (or its nominee) at the Point of Entry. Delivery of the Product will be deemed to take place [***]. Title to Product shall pass to Customer [***]. For clarity, Novavax (and Sponsor, as applicable) will be [***] for importing Product into the Territory (excluding payment of any customs duties, which shall be the [***] of the Customer), obtaining a batch release letter from the TGA (including [***]) and unloading Product from the transportation carrier at Point of Entry. [***]; if Customer fails to handle the Product with due care, maintain cold chain requirements throughout the Acceptance Period and that failure results in damage to the Product or otherwise causes such Product to be unsuitable for use, then Customer acknowledges and agrees the resultant damage will not be cause for non-Acceptance under Section 3.4. After Acceptance, Customer will be solely responsible for distributing Product in the Territory.

EXECUTION VERSION -5- 70472309_1 3.2. Delivery Documents. [***], Novavax will provide, or ensure that Sponsor provides, to Customer the usual documentation provided for the Product, including [***] (“Delivery Documents”). 3.3. Cooperation. Novavax agrees to cooperate, and ensure that Sponsor cooperates, with the TGA including in relation to the testing of the Product including in relation to requisite batch testing requirements. Novavax agrees that the TGA may provide Customer with information relating to the Product, including information relating to the testing contemplated by this Section 3.3 and Novavax’s manufacturing process and supply chain in respect of the Product (including details of any Special Vendors). 3.4. Acceptance. Customer (or its nominee) will, [***] after Customer is notified under Section 3.1 that the Product is ready for collection and Novavax (or Sponsor) has provided all Delivery Documents to Customer (“Acceptance Period”), visually inspect such delivery to confirm that the Product has been supplied in the correct quantity and appears, from a visual inspection only, to constitute Conforming Product. Notwithstanding the foregoing, Customer may request to extend the Acceptance Period for an additional [***] period with reasonable advance notice and a detailing of the circumstances for such extension and Novavax will reasonably and in good faith consider such extension request and provide written notice of approval to Customer if granted. Without limiting any other rights Customer may have at Law or under this Agreement, if Customer determines that any shipment of Product contains any non-Conforming Product, then Customer shall have the right to reject the portion of the applicable delivery that constitutes non-Conforming Product by providing Novavax with written notice of such rejection prior to the expiry of the Acceptance Period. Customer will be deemed to have accepted a delivery of Product if not rejected prior to expiry of the Acceptance Period. 4. Product Warranty. 4.1. Limited Product Warranty. Novavax warrants to Customer that, upon delivery of Product to the Point of Entry, Product will (a) materially conform to the specifications for such Product as set forth on Exhibit C hereto (the “Specifications”) and be free from defects (including any latent defects), (b) comply with the applicable Regulatory Approval in the Territory for such Vaccine, including shelf-life requirements and any other conditions, requirements or directions of the TGA, and (c) have been manufactured, packaged, handled, stored, transported and cold-chain maintained in accordance with the Specifications, Novavax’s relevant standard operating procedures in relation to the manufacture and delivery of the Product, and cGMP. Product satisfying clauses (a)-(c) hereof, “Conforming Product”. Any claims by Customer that the Product fails to meet this warranty must be made by the Customer within [***] of Acceptance of the Product as set forth in Section 3.4.

EXECUTION VERSION -6- 70472309_1 4.2. Remedies For Non-Conforming Product. If Novavax accepts Customer’s rejection of Product as set forth in Section 3.4, accepts Customer’s warranty claim in Section 4.1 or if the Independent Expert determines that any Product is non-Conforming Product as set forth in Section 4.3, then Novavax shall, at Novavax’s option and at no additional charge to Customer, either (a) replace the non-Conforming Product or (b) credit or refund the Price of such non-Conforming Products. If Novavax so requests, Customer shall, [***], return any non-Conforming Products to Novavax; otherwise, Customer shall dispose of Product in compliance with applicable Laws [***]. 4.3. Disputes. If Novavax disputes Customer’s rejection of Product pursuant to Section 3.4 or Customer’s warranty claim pursuant to Section 4.1, then Novavax will provide Customer written notice of such dispute (“Warranty Dispute Notice”) no later than [***] after the date of the notice from Customer that it does not Accept the Product or that Product is non-Conforming Product. Such dispute shall be resolved by having an independent, mutually acceptable, qualified third party expert (the “Independent Expert”) promptly examine the Product subject to the dispute. Failing agreement in writing of an Independent Expert within [***] of receipt of the Warranty Dispute Notice, the Independent Expert will be (a) [***]. If a person is nominated by [***] as the Independent Expert under this Section 4.3, the Parties agree to do all things reasonably necessary to effect that nomination (including signing the person’s engagement letter, agreeing to indemnify the person and agreeing to pay the fees and expenses of the person) [***]. The non-prevailing Party shall bear all out-of-pocket costs and expenses associated with the Independent Expert’s determination, including any reasonable out-of-pocket costs incurred by the prevailing Party in connection therewith. 4.4. Disclaimer. THE REMEDIES SET FORTH IN SECTION 4.2 AND SECTION 4.3 SHALL BE CUSTOMER’S SOLE AND EXCLUSIVE REMEDY AND NOVAVAX’S ENTIRE LIABILITY FOR NON-ACCEPTANCE OF PRODUCT UNDER SECTION 3.4 OR ANY BREACH OF THE LIMITED WARRANTY SET FORTH IN SECTION 4.1. 5. Payment Terms. 5.1. Advance Payment. Customer shall pay to Novavax an upfront payment of [***] of the Total Price as set forth on Exhibit A (the “Advance Payment”) in accordance with this Section 5. [***]. [***] of the Advance Payment is non-refundable. The remaining [***] of Advance Payment (“Refundable Portion”) is refundable only as provided in Section 2.6.3, Section 7.2.2 and Section 7.5. 5.2. Price. The Total Price, Per-Unit Price and Per-Unit Delivery Price (each excluding GST) for Product are as set forth on Exhibit A (collectively, the “Price”). The Price includes [***]. Any additional shipping charges, including charges for expedited shipping, more frequent deliveries or multiple locations, will be agreed between the Parties and charged to the Commonwealth. The Price is exclusive of any and all governmental taxes, including, without limitation, GST, value added tax, customs,

EXECUTION VERSION -7- 70472309_1 charges or levies of every kind that may apply upon sale, transfer, importation or shipment of Product to the Point of Entry under any applicable Laws but excluding any GST, valued added tax or similar tax payable in respect of importation of the Product into Australia to the extent that the importer is entitled to a full credit for such tax and excluding taxes on income. Subject to those exceptions, Customer will be solely responsible for all such taxes, charges and levies to the extent they relate to supply of Product to Customer. 5.3. [***]. [***]. 5.4. Invoices. Novavax shall submit invoices to Customer for (a) the Advance Payment upon [***] which invoices shall be directed to the following person and address (or to such other person or address if Customer notifies Novavax in writing pursuant to Section 13.2 that invoices should be sent to such other person or address): [***] Each invoice must include the following information: (a) Customer’s order number, as set out in the Purchase Order and (b) details of the contract point of contact (including the name of the current point of contact). Novavax must provide all invoices in a soft copy format that is a Word, Excel, JPEG, PDF, PNG, TIFF, EML, CSV or CFDI file. Invoices shall be deemed to be received when the sender receives a confirmation from [***] confirming receipt. If an invoice is found to have been rendered incorrectly after it has been paid, any underpayment or overpayment will be recoverable by or from Novavax, as the case may be, and, without limiting recourse to other available remedies, may be offset against any amount subsequently due to Novavax. Each invoice for a delivery of Product shall reflect the actual quantities of Vaccine shipped to the Point of Entry, together with the Per-Unit Delivery Price, the total Delivery Price and the total amount of GST (if any) to be paid under such invoice. All amounts set forth in each invoice (a) for the Advance Payment shall be payable within [***], and made in United States Dollars (“USD”). For clarity, Customer will not be responsible for paying any bank charges. In the event Customer disputes all or any portion of an invoice submitted to it in accordance with this Section 5, then such dispute shall be resolved in accordance with Section 13.6. Customer will not be required to pay any amount disputed in good faith, unless such amount is finally determined to be owed to Novavax in accordance with the dispute resolution procedure set forth in Section 13.6, in which case, such amount will bear interest at the rate of [***]. Except as expressly set out in this Agreement or required by Law, no offset or deduction from any invoice is permitted. 5.5. GST. 5.5.1. Unless this Agreement expressly states otherwise, all consideration to be provided under this Agreement is exclusive of GST.

EXECUTION VERSION -8- 70472309_1 5.5.2. If a Party (the “Supplying Party”) makes a supply under or in connection with this Agreement in respect of which GST is payable (“Taxable Supply”), the recipient of the Taxable Supply (“Recipient”) must, subject to the prior receipt of a tax invoice, pay to the Supplying Party, an additional amount equal to the GST payable on the Taxable Supply. This Section 5.5 does not apply to GST payable in respect of importations. 5.5.3. If a Party must reimburse or indemnify another party for a Loss, the amount to be reimbursed or indemnified is first reduced by any input tax credit the other Party (or the representative member of a GST group of which is other Party is a member) is entitled to for the Loss, and then increased in accordance with Section 5.5.2. 5.5.4. If an adjustment event occurs in relation to a Taxable Supply, the Supplying Party must issue an adjustment note to the Recipient in relation to that Taxable Supply within [***] after becoming aware of the adjustment. 5.5.5. A term which has a defined meaning in the GST Law has the same meaning when used in this Section 5.5, unless the contrary intention appears. 5.5.6. This Section 5.5 will survive the termination of this Agreement. 5.6. Withholdings. If a Law requires Customer to withhold or deduct an amount in respect of Taxes from a payment made to Novavax pursuant to this Agreement, then Customer agrees to pay an additional amount required to be withheld or deducted to the relevant Agency in accordance with the applicable Law and to provide evidence of payment thereof to Novavax. 6. Intellectual Property. As between Customer and Novavax, Customer hereby acknowledges and agrees that all rights, title and interests in, to and under any Intellectual Property Rights that relate to the Vaccine are and shall remain the sole and exclusive property of Novavax. Except as otherwise set out in this Agreement, Novavax does not grant Customer and right, title or interest in, to or under any such Intellectual Property Rights or any other intellectual property owned or controlled by Novavax. However, Novavax grants Customer a royalty-free, worldwide, non-exclusive license under Novavax’s Intellectual Property Rights to use the Vaccine solely for the purposes of distributing Vaccine in the Territory [***] in accordance with this Agreement. Novavax grants Customer a royalty-free, world-wide, non-exclusive license (including the right to sub-license to third parties acting on Customer’s behalf) to use, reproduce, publish, electronically transmit and distribute any Agreement Material solely for the purposes of fulfilling Customer’s purposes under this Agreement and for the Customer’s reporting and accountability requirements. For the avoidance of doubt, this does not include Intellectual Property Rights relating to the development, construction or manufacture of the Vaccine itself. To the extent Customer, directly or indirectly, creates, discovers, reduces to practice or otherwise generates Intellectual Property Rights in the Vaccine, such Intellectual Property Rights will be solely owned by, and immediately vest on creation in, Novavax. Customer shall assign, and hereby does assign, to Novavax all such Intellectual Property Rights, and will take

EXECUTION VERSION -9- 70472309_1 reasonable actions requested by Novavax, [***], to record and confirm Novavax’s ownership thereof, including if requested by Novavax executing formal documentation evidencing Novavax’s ownership thereof. 7. Term; Termination; Effects of Termination. 7.1. Term. This Agreement shall become effective upon the Effective Date and, unless sooner terminated as set forth in Section 7.2, shall continue in force and effect until the later of (a) the end of the Pandemic Period, (b) the date that is three (3) years from the Effective Date; (c) Novavax has delivered to Customer an amount of Product equal to the Aggregate Amount (the “Term”). 7.2. Termination. 7.2.1. Material Breach. 7.2.1.1.Customer Termination. Customer may terminate this Agreement at any time prior to expiration of the Term upon written notice to Novavax if Novavax materially breaches this Agreement and (a) such breach is not cured within [***] of written notice to Novavax describing such breach and requiring Novavax to cure the breach or (b) the breach is not capable of being cured (for the avoidance of doubt, failure to meet a deadline shall not be considered a breach not capable of being cured). 7.2.1.2.Novavax Termination. Novavax may terminate this Agreement at any time prior to expiration of the Term upon written notice to Customer if Customer (a) materially breaches Section 2.2, Section 6 or Section 12 and such breach is (i) not cured within [***] of Novavax providing written notice to the Customer requiring the Customer to cure the breach or (ii) not capable of being cured (for the avoidance of doubt, failure to meet a deadline shall not be considered a breach not capable of being cured); or (b) fails to pay an undisputed amount when due and such breach is not cured within [***] of Novavax providing written notice to the Customer requiring the Customer to cure the breach. The Parties agree that Novavax may only terminate this Agreement pursuant to this Section 7.2.1.2. 7.2.2. Regulatory Approval. If Novavax fails to receive Regulatory Approval of the Vaccine in the Territory on or before [***] then Customer may terminate this Agreement [***] upon written notice to Novavax. Customer’s sole and exclusive remedy for Novavax’s failure to receive Regulatory Approval of the Vaccine in the Territory are those set out in this Section 7.2.2 and Section 7.5. 7.3. Customer Termination. Without prejudice to its rights under this Agreement and at Law, Customer may terminate this Agreement, by notice in writing to Novavax,

EXECUTION VERSION -10- 70472309_1 [***] if Novavax (a) suffers an Insolvency Event and fails to emerge within [***] of initiation of such Insolvency Event; (b) ceases to carry on its business; (c) suffers a change in Control or assigns or novates its rights otherwise than in accordance with the requirements of this Agreement; or (d) disposes of the whole or any material part of its assets or business (whether in a single transaction or a series of related or unrelated transactions), other than to carry out a reconstruction or amalgamation of the corporate group constituting Novavax and its Related Bodies Corporate and which has a material adverse effect on Novavax’s ability to supply the Vaccine as contemplated under this Agreement. 7.4. ARTG registration. Without prejudice to its rights at Law, Customer may terminate this Agreement by notice in writing to Novavax, [***] if, [***]. 7.5. Effects of Expiration or Termination. Upon expiry of the Term or any early termination of this Agreement, (a) neither Party shall be relieved of any obligation that accrued prior to such effective date of termination, (b) except as otherwise expressly provided herein, all rights and obligations of each Party hereunder will cease and (c) each Party shall return or destroy all Confidential Information of the other Party that is in its possession pursuant to the requirements of Section 12.5. If this Agreement is terminated under Section 7.2.1.1 (provided such termination is related to Novavax’s failure to deliver Product), 7.2.2 or 7.4, Novavax must refund to Customer the Refundable Portion of the Advance Payment attributable to the undelivered Product as at the date of termination within [***] of receipt of a termination notice under that Section. In the event Novavax terminates this Agreement pursuant to Section 7.2.1.2, Customer must pay all amounts due and payable to Novavax pursuant to Agreement as at the date of termination. 8. Development and Regulatory Matters. 8.1. Development of Vaccine. The Parties acknowledge and agree that Novavax will use reasonable endeavours to develop the Vaccine, but the successful development of the Vaccine is not guaranteed. 8.2. Labelling. Novavax must ensure that all Product is labelled and packaged in accordance with the applicable Regulatory Approval, Specifications and GMP standards, and otherwise in compliance with TGA labelling requirements unless an Exemption applies. 8.3. Approach to Regulatory Approval. Novavax shall use commercially reasonable efforts to obtain, and ensure that Sponsor obtains, Regulatory Approval of the Vaccine in the Territory from the TGA, including (a) Authorisation, (b) GMP clearance from the TGA or equivalent regulator to manufacture the Vaccine overseas, in order to meet the requirements of the TG Act with regard to manufacture of the Vaccine (“GMP Clearance”) and (c) to the extent the Vaccine is required to be registered on the ARTG under the TG Act and is not Exempt, registration of the Vaccine on the ARTG, [***]. Novavax will notify Customer in writing (email being sufficient) when the Vaccine is granted any Regulatory

EXECUTION VERSION -11- 70472309_1 Approval in the Territory and provide to Customer evidence of any Authorisations, GMP Clearance and registration of the Vaccine on the ARTG for the purposes of this Section 8.3. Customer and Novavax both acknowledge that regulatory harmonization and streamlined logistics requirements, including packaging, labelling, and post-marketing requirements across all participating high income countries (HICs), is of the utmost importance to the timely approval and delivery of a COVID-19 vaccine during the pandemic. Customer acknowledges that any country-specific requirements in the Territory regarding packaging, labelling, or release logistics may delay delivery of Product. 8.4. Documentation. Novavax shall prepare and maintain, and ensure that the Sponsor prepares and maintains, all documentation required by the TGA in relation to the safety, manufacture, delivery and storage of Product, including batch records and file samples, properly stored, from each lot or batch of Product manufactured and shipped under this Agreement as well as shipping records for each batch shipped (including records of cold-chain compliance). Upon request, such documentation will [***] be made available to Customer (in English) for review. In addition, Novavax will maintain appropriate records and supporting documentation to confirm that each invoice is limited to the actual quantities of Product shipped to the Point of Entry, which records and documentation will be maintained by Novavax for [***], following Acceptance of the relevant batch of Product by Customer pursuant to Section 3.4. Customer will have the right, [***], to inspect any such records. Customer may appoint an independent person to assist in inspections during normal business hours at the place such records are normally kept. In the event any such inspection reveals an overpayment by Customer, Novavax will [***] credit Customer the amount of such overpayment against future deliveries of Product, unless there are no such future deliveries, in which case, Novavax will [***] refund Customer for the amount of such overpayment. 8.5. Regulatory Assistance. Customer will, within the framework of its competencies, reasonably support Novavax in obtaining Regulatory Approval for the Vaccine in the Territory, including accelerated Regulatory Approval processes. The Parties acknowledge, and Customer represents to Novavax, that TGA is part of Customer but operates independently in accordance with the TG Act and that, in entering into this Agreement, Customer is not acting as, or influencing the TGA or in any regulatory capacity. 8.6. Recalls. Novavax must [***] notify Customer of any issues that could potentially lead to a recall of the Product and provide all relevant information to Customer [***]. As distributor of the Product in the Territory, Customer (or its nominee) shall use reasonable endeavours to assist Novavax in implementing any Product recalls (a) required by controlling regulatory agencies and (b) voluntary withdrawals requested by Novavax, with respect to each, in the Territory. Novavax shall initiate and handle, or ensure that Sponsor initiates and handles, any recalls in a timely, prudent and skillful manner, in compliance with all applicable Laws. Novavax will, at the [***] of Customer, [***] (a) replace any recalled and unexpired Product if it has supply of Product that can be made available or (b)

EXECUTION VERSION -12- 70472309_1 refund or credit the Price attributable to the recalled and unexpired Product (and in any case within [***] unless otherwise agreed by Novavax and Customer). All costs incurred in responding to recalls and market withdrawals shall be borne by [***]. 8.7. Summary reports. Until delivery of the Aggregate Amount, Novavax will provide Customer with a written report, on a minimum [***] basis or other period agreed between the Parties (“Summary Report”) that details: [***]. The Parties acknowledge that the Summary Report is intended to be a high-level update regarding the progress of the Vaccine development and supply, and may include Confidential Information. Novavax will, at the request of Customer, provide a brief verbal discussion of each Summary Report. Novavax or third party confidential information contained in a Summary Report will be treated as Novavax’s Confidential Information for the purposes of Section 12. 8.8. Notifications. Novavax must [***] notify Customer in writing if (a) [***]. Novavax must [***] and fully inform the TGA in respect of any information required to be supplied to the TGA under the TG Act in respect of the Vaccine, and, if requested by Customer, it will supply such information upon request to Customer. 8.9. Manufacturing Facilities. Novavax may not supply Product under this Agreement from facilities other than the Manufacturing Facilities without first obtaining approval from the TGA for an alternative manufacturing site and obtaining Customer’s prior written consent. If Novavax seeks consent to supply Product from facilities other than the Manufacturing Facilities, and has obtained approval from the TGA to use an alternative manufacturing site, Customer’s consent may not be unreasonably withheld and will be deemed to have been provided if (a) the request is made in writing and complies with the requirements set out in Section 13.2 and (b) Customer fails to provide consent or notify Novavax that it does not provide consent within [***] after receipt of the request. 8.10. Specifications. Novavax will provide to Customer on a confidential basis (a) the draft Specifications provided to the TGA with the application for Regulatory Approval, within [***] of submitting that application to the TGA; and (b) the final Specifications which are the specifications for the Product determined by Novavax and approved by the TGA, reflecting data arising from the trials (including safety and efficacy requirements) and the Regulatory Approval, within [***] of the granting of the Regulatory Approval by the TGA. 9. Indemnification. 9.1. By Customer. Notwithstanding any contrary provision of this Agreement and to the fullest extent not prohibited by applicable Laws Customer (“Indemnifying Party” for the purposes of this Section 9.1) will release and indemnify Novavax and its affiliates, Sponsor and its or their respective officers, directors, employees, agents and contractors (each an “Indemnified Party” for the purposes of this Section 9.1) from and against any and all claims, demands, causes of action, damages,

EXECUTION VERSION -13- 70472309_1 losses, liabilities, costs, expenses (including legal fees and litigation expenses), penalties, fines, settlements and judgments (collectively, “Losses”) resulting from a Claim to the extent that Claim is arising out of or in connection with any one or more of [***]. Notwithstanding any provision of this Agreement to the contrary, the provisions of this indemnity shall apply and be binding on Indemnifying Party regardless of whether any defect in Product causing any Losses originates from the testing, development, manufacture, delivery, export, import, distribution, sale, offer for sale, administration, use or deployment of Product. [***]. Indemnifying Party’s liability for any Losses under this Section shall be reduced proportionally to the extent the relevant Losses would have been avoided or mitigated but for the Indemnified Party’s failure to take reasonable steps to mitigate those Losses. 9.2. Deed Poll. The rights under Section 9.1 are for the benefit of, and may be exercised by, the Sponsor. This document takes effect as a deed poll in favour of the Sponsor and is enforceable by the Sponsor. 9.3. Procedure. Any Indemnified Party shall [***] notify Indemnifying Party in writing of any Claim made against an Indemnified Party, specifying the basis given for such Claim; provided that [***]. Indemnifying Party shall, within [***] after receiving notice of a Claim from the Indemnified Party, notify the Indemnified Party whether Indemnifying Party elects to undertake the defense of or, [***]. The election by Indemnifying Party to undertake the defense of a Claim shall not preclude the Indemnified Party from participating or continuing to participate in such defense, so long as the Indemnified Party [***]. If Indemnifying Party elects not to defend or settle the Claim itself, the Indemnified Party may assume the defense of the Claim, in which case Indemnifying Party shall, [***]. Novavax acknowledges that the Commonwealth must comply with government policy in the conduct of litigation (including the Legal Services Directions 2017 made under section 55ZF of the Judiciary ▇▇▇ ▇▇▇▇ (Cth)). 9.4. Third Party Intellectual Property Infringement. In the event that Product is found to infringe any third party Intellectual Property Rights, the Parties will discuss in good faith potential solutions to permit the ongoing supply of Product by Novavax and use by Customer. 10. Representations and Covenants. 10.1. Mutual Representations. Each Party hereby represents and warrants to the other Party, as of the Effective Date, that: 10.1.1. it has all requisite power and authority, corporate or otherwise, to execute, deliver and perform this Agreement; 10.1.2. this Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms; 10.1.3. the execution, delivery and performance of this Agreement by such Party does not conflict with any agreement, instrument or understanding, oral or

EXECUTION VERSION -14- 70472309_1 written, to which such Party is bound, nor violate any applicable Law or any order, writ, judgment, injunction, decree, determination or award of any court or governmental body or administrative or other agency presently in effect and applicable to such Party; and 10.1.4. in the performance of this Agreement, it shall comply with all applicable Laws. 10.2. Novavax’s Covenant. Novavax hereby covenants to Customer that, at the time of delivery to the Point of Entry, Customer will have good title to the delivered Products, free and clear of all liens, encumbrances and security interests. 10.3. Disclaimer. EXCEPT FOR THOSE REPRESENTATIONS, WARRANTIES AND COVENANTS EXPRESSLY SET FORTH IN SECTION 4.1 OR THIS SECTION 10, TO THE FULLEST EXTENT NOT PROHIBITED BY APPLICABLE LAW, NOVAVAX EXPRESSLY DISCLAIMS ALL OTHER REPRESENTATIONS, WARRANTIES AND COVENANTS OF ANY KIND, WHETHER EXPRESS OR IMPLIED, WRITTEN OR ORAL, BY FACT OR LAW, INCLUDING ANY IMPLIED REPRESENTATIONS, WARRANTIES AND COVENANTS OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, SATISFACTORY QUALITY, NON-INFRINGEMENT AND ANY REPRESENTATIONS OR WARRANTIES OR CONDITIONS OR GUARANTEES ARISING FROM STATUTE, COURSE OF DEALING OR USAGE OF TRADE. EACH PARTY ACKNOWLEDGES THAT IT HAS NOT ENTERED INTO THIS AGREEMENT IN RELIANCE UPON ANY WARRANTY OR REPRESENTATION. FURTHER, THE PARTIES HEREBY ACKNOWLEDGE AND AGREE THAT NOTHING CONTAINED IN THIS AGREEMENT SHALL BE CONSTRUED AS A WARRANTY, EITHER EXPRESS OR IMPLIED, THAT NOVAVAX WILL OBTAIN A POSITIVE CLINICAL OUTCOME OR THAT THE PRODUCT WILL RECEIVE REGULATORY APPROVAL IN THE TERRITORY. 11. Limitation of Liability. 11.1. [***]. [***]. 12. Confidential Information. 12.1. Definition. “Confidential Information” means any and all non-public or proprietary information provided by or on behalf of a Party to the other Party in connection with this Agreement or to which a Party obtains access as a consequence of entering into or performing this Agreement (in each case whether before, on or after the Effective Date), whether or not marked as “CONFIDENTIAL” or “PROPRIETARY,” and whether provided prior to, on or after the Effective Date, including all technical, scientific, business, commercial and other know-how, information, trade secrets, methods, processes, practices, formulae, instructions, techniques, designs, drawings, data or results, but expressly excluding any

EXECUTION VERSION -15- 70472309_1 information that (a) at the time of disclosure, is in the public domain, (b) after disclosure, becomes part of the public domain by publication or otherwise, through no fault of the receiving Party or its affiliates, (c) at the time of disclosure, is already in the receiving Party’s or its affiliates’ possession, except through prior disclosure by the disclosing Party, without any obligation of confidentiality or any restriction on its use, and such possession can be properly documented by the receiving Party or its affiliates in its written records, and was not made available to the receiving Party or its affiliates by any person or party owing an obligation of confidentiality to the disclosing Party, (d) is rightfully made available to the receiving Party or its affiliates from sources independent of the disclosing Party and (e) is independently discovered or developed by or on behalf of the receiving Party or its affiliates without the aid, use of, access to or application of any Confidential Information of the disclosing Party. For clarity, specific aspects or details of Confidential Information will not be deemed to be within the public domain or in the possession of the receiving Party merely because the Confidential Information is embraced by more general information in the public domain or in the possession of the receiving Party. 12.2. Obligation to Maintain in Confidence; Permitted Disclosure. Each Party agrees to (a) protect and maintain in confidence the disclosing Party’s Confidential Information using the same degree of care that it employs to protect the confidentiality of its own confidential information (but never less than a reasonable standard of care), (b) not disclose to any person or entity any of the disclosing Party’s Confidential Information without prior written approval from the disclosing Party; provided that the receiving Party may disclose such Confidential Information to its affiliates, and to its and their officers, directors, employees, contractors, agents, Australian, State and Territory government entities as necessary, Ministers, Ministerial advisers, professional advisors or consultants who are bound by confidentiality obligations at least as restrictive as those set forth in this Section 12 and who reasonably need to know such Confidential Information in the performance of the receiving Party’s obligations under this Agreement, or for the purpose of governmental reporting including requests for information by the responsible Minister, a House of the Parliament of the Commonwealth of Australia or Parliamentary Committee and provided such information is designated as ‘Confidential’ (c) ensure the full compliance of each of its affiliates and its and their officers, directors, employees, contractors or agents who have access to the disclosing Party’s Confidential Information with the confidentiality and non-use obligations in this Section 12 and (d) not use such Confidential Information for any purpose other than performing its obligations under this Agreement. The Customer may disclose all or any part of the Confidential Information to the extent necessary to enable Customer’s performance under this Agreement and its distribution, administration, and use of Product, provided, however, that it ensures that any receiving party are bound by confidentiality obligations at least as restrictive as those set forth in this Section 12. Each Party acknowledges and agrees that its failure to comply with the provisions of this Section 12 may cause irreparable harm to the other Party that cannot be adequately compensated for in damages and, accordingly, that each Party will be entitled to claim, in addition to any other

EXECUTION VERSION -16- 70472309_1 remedies available to it, interlocutory and permanent injunctive relief to restrain any anticipated, present or continuing breach of this Section 12 without the need to post bond or other security. The terms of this Agreement will be the Confidential Information of both Parties. 12.3. Disclosures Required by Law. Subject to the remainder of this Section 12.3, each Party may disclose the Confidential Information of the other Party to the extent that such disclosure is, in the reasonable opinion of the receiving Party’s legal counsel, required to be disclosed pursuant to applicable Law (including the rules of any stock exchange) or a valid order of a court of competent jurisdiction or a supra-national, national, regional, state, provincial or local governmental body of competent jurisdiction. Prior to making any such disclosure, the receiving Party shall promptly advise the disclosing Party of the requirement to disclose as soon as the receiving Party becomes aware that such a requirement might become effective in order that, where possible, the disclosing Party may seek a protective order or such other remedy as the disclosing Party may consider appropriate in the circumstances. The receiving Party shall reasonably cooperate with the disclosing Party (at the disclosing Party’s cost) in seeking a protective order or other relief. The receiving Party shall disclose only that portion of the disclosing Party’s Confidential Information that it is required to disclose. 12.4. Survival. The provisions of this Section 12 shall survive for a period of [***] from the date of any expiration or termination of this Agreement, but shall survive [***] with respect to any Confidential Information that is a trade secret for as long as such information remains a trade secret. 12.5. Return or Destruction. A Party may request that the other Party return or destroy any of its Confidential Information that is in the other Party’s possession at any time upon written notice to the other Party. Upon expiration or termination of this Agreement, each Party shall return or destroy, at the other Party’s written election, all Confidential Information of the other Party that is in its possession as of the date of expiration or termination. Notwithstanding the foregoing, each Party may retain [***] of the other Party’s Confidential Information including to ensure its compliance with this Agreement, and no Party will be required to destroy copies of the other Party’s Confidential Information that are included on disaster recover/backup tapes that are maintained by a Party pursuant to a bona fide disaster recovery plan. If requested by a Party, the returning or destroying Party will certify in writing to the other Party that such return or destruction has occurred. 13. Miscellaneous. 13.1. Force Majeure. Each Party’s obligations of performance under this Agreement will be temporarily suspended and excused for the period of interruption to the extent any failure of performance is caused by (a) fire, earthquake, storm (including hurricanes, snow storms, blizzards or ice storms), hail, flood, act of war or terrorism, riot, civil commotion, pandemic, epidemic or embargo, (b) any other event or omission beyond the reasonable control of such Party (collectively, a

EXECUTION VERSION -17- 70472309_1 “Force Majeure”); provided that the non-performing Party is without fault in causing the failure or delay, and the failure or delay could not have been prevented by reasonable precautions and cannot reasonably be circumvented by the non-performing Party at its reasonable expense through the use of alternate sources, work around plans or other means. The onus of establishing a Force Majeure event rests on the Party asserting that a Force Majeure event exists. The non-performing Party will [***] notify the other Party of the anticipated period of interruption due to a Force Majeure, and describe at a reasonable level of detail the circumstances causing the delay, and will take all reasonable measures to forthwith remedy the interruption and recommence performance whenever and to whatever extent possible without delay. Each Party acknowledges and agrees that the effects of COVID-19 may be considered a Force Majeure to the extent it satisfies the requirements of this Section 13.1. If a delay or failure of Novavax to perform its obligations due to an event of Force Majeure exceeds [***]. 13.2. Notices. Any notice given under this Agreement must be in writing and delivered either to the addresses set forth below in person or via overnight courier (or to such other addresses of which the Parties may from time to time be notified in writing), with a PDF copy sent by email: If to Novavax: Novavax, Inc. ▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ ▇.▇.▇. Attn: [***] Email: [***] If to Customer: ▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ Attn: [***] Email: [***] Copy: [***] Such notice will be deemed to have been given: (a) if delivered by post, on the second (2nd) business day (at the place of delivery) after posting with an internationally recognized overnight delivery service or (b) if sent by email, upon written acknowledgement of the receiving Party. 13.3. Entire Agreement. This Agreement, including any schedules or exhibits hereto, contains the entire and exclusive agreement between the Parties in connection with

EXECUTION VERSION -18- 70472309_1 the subject matter thereof and supersede all prior and collateral agreements, understandings, communications, representations and warranties between the Parties in relation thereto, including without limitation, the Heads of Terms between the Parties dated 4 November 2020. 13.4. Amendment. No amendment or modification or supplement of this Agreement, including this provision, will be valid unless made in a writing signed by an authorized representative of each Party specifically referring to this Agreement. 13.5. Public Announcements. Novavax agrees not to issue any press release or other public statement relating to this Agreement or otherwise disclosing the discussions in relation to this Agreement, entry into, existence and/or contents of this Agreement or the transactions contemplated hereby without the prior written consent of the Customer, except to the extent required by applicable Laws or stock exchange rules, in which case the Customer shall be given advance written notice of such disclosure. Novavax will consult the Customer prior to issuing any press release or other public announcement with respect to this Agreement. Subject to compliance with Section 12, Customer is not restricted from making a public announcement and other public statements as to the entry into, existence and/or general contents of this Agreement or the transactions contemplated hereby and, where reasonably practicable, agrees to provide Novavax advance written notice of any such public announcement. 13.6. Dispute Resolution; Equitable Relief. 13.6.1. With respect to any, dispute or controversy (“Dispute”) between the Parties and arising in whole or in part in connection with this Agreement, including whether a breach has occurred or been appropriate cured, the Parties shall first use good faith efforts to resolve such Dispute and, if such Dispute is not resolved within [***] from the date such Dispute arose, then either Party may submit the Dispute to binding arbitration in [***]. All matters so submitted to arbitration will be settled by three (3) arbitrators in accordance with the [***]. In the event of a conflict between [***] and this Agreement, this Agreement shall govern. Each Party will designate an arbitrator and the Parties will cause the designated arbitrators to mutually agree upon and to designate a third arbitrator who will serve as chairperson. The Parties shall arrange for a hearing to occur and be completed within [***] after the appointment of the third (3rd) arbitrator, which hearing shall last no longer than [***], unless the arbitral panel believes a longer period is required, in which case the hearing may last [***]. The Parties will cause the arbitrators to decide the matter to be arbitrated within [***] after the close of evidence unless the chairperson arbitrator determines, at the request of any Party or on his or her own initiative, that such time period should be extended, in which case such time period may not be extended beyond an additional [***]. The final decision of the majority of the arbitrators shall be in writing, in all events follow governing law and will be furnished to all the

EXECUTION VERSION -19- 70472309_1 Parties in such dispute. Judgment on such decision may be entered in any court having jurisdiction. 13.6.2. Notwithstanding any other terms of this Agreement, either Party may seek a preliminary injunction or other provisional equitable relief in any court of competent jurisdiction if, in its reasonable judgment, such action is necessary to avoid irreparable harm as permitted by applicable Law. 13.7. Governing Law. This Agreement is made subject to the laws of [***]. The United Nations Convention on Contracts for the International Sale of Goods and the United Nations Convention on the Limitation Period in the International Sale of Goods, if otherwise applicable, each as the same may be amended or superseded, are hereby expressly excluded and will not be applicable to this Agreement. 13.8. Assignment. Neither Party will assign (including by change of Control) all or any portion of this Agreement or any right or obligation under this Agreement without the other Party’s prior written consent, which consent will not be unreasonably withheld or delayed. Any unauthorized assignment by a Party will be null and void of no force or effect. This Agreement will bind and inure to the benefit of the successors and permitted assigns of the respective Parties. 13.9. Survival. In order that the Parties may fully exercise their rights and perform their obligations in connection with this Agreement, any provisions of this Agreement that are required to ensure such exercise or performance (including any obligations accrued as of the termination date) or which are intended by their terms or by necessary implication to survive will survive the expiration or termination of this Agreement, including Sections 5 (with respect to accrued but unpaid amounts and Section 5.5), 6, 7.5, 8, 9, 11, 12, 13.2, 13.3, 13.5, 13.6, 13.7, 13.9, 13.11, 13.12, 13.13, 13.17 and 16. 13.10. Waiver. Failure of either Party to exercise any right it has under this Agreement on one or more occasions will not operate or be construed as a waiver by such Party of its right to exercise the same right on another occasion. Any waiver must be in a writing signed by the waiving Party. 13.11. Severability. If any provision of this Agreement will be adjudicated to be invalid or unenforceable by a court of competent jurisdiction, it is the Parties’ intent that the remaining provisions of this Agreement will remain in full force and effect and the affected provision or portion thereof will be deemed modified so that it is enforceable to the maximum extent permissible to reflect as closely as possible the intentions of the Parties as evidenced from the provisions of this Agreement. 13.12. Independent Relationship of Parties; No Third-Party Beneficiary. The relationship of Novavax and Customer is that of independent contractors and under no circumstances will a Party, its agents or employees be partners, agents or representatives of another Party. Except as otherwise expressly provided in this Agreement, including Section 9.2 and any indemnification or limitation of liability

EXECUTION VERSION -20- 70472309_1 provision, nothing in this Agreement will be construed as creating any direct or beneficial right in or on behalf of any third party. 13.13. Interpretation; Section Headings. For purposes of this Agreement, (a) the plural will include the singular and the singular the plural, (b) any gender will include any other gender, (c) the terms “included” or “including” or any variation are not words of exclusion and will be read to include “without limitation,” (d) the terms “hereof” or “herein” or any variation are intended to apply to this Agreement as a whole, (e) the word “or” is not exclusive and will be interpreted to have the meaning commonly associated with the phrase “and/or,” (f) references to any applicable law, rule or regulation, or article, section or other division thereof, will be deemed to include the then-current amendments thereto or any replacement or successor applicable law, rule or regulation thereof and (g) the word the word “will” shall be construed to have the same meaning and effect as the word “shall.” The section headings used herein are intended for convenience of reference only and will not be considered in interpreting this Agreement. This Agreement shall be deemed to be the joint work product of the Parties and any rule of construction that a document shall be interpreted or construed against a drafter of such document shall not be applicable. 13.14. English Language. This Agreement shall be written and executed in, and all other communications under or in connection with this Agreement shall be in, the English language. Any translation into any other language shall not be an official version thereof, and in the event of any conflict in interpretation between the English version and such translation, the English version shall control. 13.15. Counterparts. This Agreement and all exhibits and schedules hereto may be executed and delivered by the Parties in one or more counterparts, each of which will be an original, and each of which may be delivered by facsimile, e-mail or other functionally equivalent electronic means of transmission and those counterparts will together constitute one and the same instrument. 13.16. Manufacturing in the Territory. In the event that onshore manufacturing capacity becomes available in the Territory, Customer will notify Novavax, and the Parties will discuss, in good faith, the terms on which Novavax could manufacture Product in the Territory, for the purposes of supplying Product to Customer under this Agreement. 13.17. Liability for Sponsor. The acts or omissions of the Sponsor will be deemed to be the acts or omissions of Novavax, and Novavax will be responsible and liable for those acts and omissions in all circumstances, irrespective of whether (a) Novavax authorised the acts or omissions; (b) the acts or omission were willful, deliberate, illegal or fraudulent; or (c) the acts or omissions were in contravention of instructions. 14. Insurance.

EXECUTION VERSION -21- 70472309_1 14.1. Insurance Requirements. On or before [***], Novavax must take out and maintain such types and amounts of liability insurance to cover liabilities relating to its activities under this Agreement as is normal and customary in the pharmaceutical industry generally, including the following insurance cover: (a) general products liability insurance, covering legal liability to pay for personal injury arising out of or in any way connected with the Vaccine, having a limit of indemnity of not less than USD[***] per occurrence and in annual aggregate; and (b) to the extent required by Law, workers’ compensation insurance in respect of Novavax’s liability for any Loss or Claim by a person employed or otherwise engaged, or deemed to be employed or otherwise engaged, by Novavax in connection with the performance of the terms of this Agreement. 14.2. Insurance Terms. The policy or policies of insurance arranged in accordance with this Section 14 must be maintained for a period of [***] after the expiration of the Term. 14.3. Evidence of Insurance. Novavax will upon request by Customer [***] provide certificates of insurance evidencing the insurances required to be effected or maintained by Novavax pursuant to this Agreement and such certificates must record the name of the insurer or the insurers, the insured, the type of policy, the policy number, the policy expiry date and the amount of cover and deductible. 15. Subcontracting. Novavax may subcontract the whole, or any part, of its obligations under this Agreement without the prior written consent of Customer, provided that (a) Novavax notifies Customer of any subcontract of the whole or any part of its obligations under this Agreement [***]; (b) Novavax shall remain responsible and liable for the acts or omissions of its subcontractors, as if they were acts or omissions of Novavax. 16. Privacy. 16.1. Privacy Obligations. Novavax does not intend to collect Personal Information in performance of its obligations under this Agreement. To the extent Novavax collects, uses or discloses Personal Information in performance of this Agreement, Novavax agrees (a) to use or disclose Personal Information only in accordance with applicable Laws, (b) not to do any act or engage in any practice which, if done or engaged in by Customer, would be in breach of the requirements of Division 2 of Part III of the Privacy Act, (c) not to use or disclose Personal Information or engage in an act or practice that would breach Australian Privacy Principle 7 (direct marketing) of Schedule 1 of the Privacy Act unless the use or disclosure is authorised by this Agreement or is necessary, directly or indirectly, to discharge an obligation under this Agreement, (d) to [***] notify Customer if Novavax becomes aware of a breach of any of the obligations contained in, or referred to in, this Section 16, whether by Novavax or any subcontractor, (e) to comply with

EXECUTION VERSION -22- 70472309_1 applicable Law, or (f) any directions, guidelines determinations or recommendations referred to in, or relating to the matters set out in applicable government policy as provided within this Agreement, to the extent that they are not inconsistent with the requirements of this Section 16, and (g) to ensure that any employee of Novavax who is required to deal with Personal Information for the purposes of this Agreement is made aware of the obligations of Novavax set out in this Section 16. 16.2. Subcontracts. Novavax agrees to ensure that any subcontract entered into for the purpose of processing Personal Information under this Agreement contains provisions to ensure that the subcontractor has the same awareness and obligations as Novavax has under this Section 16, including the requirement in relation to subcontracts. 16.3. Data Breaches. Novavax must notify Customer within [***] after it becomes aware, and keep Customer informed (a) of an obligation under the Privacy Act to report a breach (including an eligible data breach) in respect of its handling of Personal Information to the Information Commissioner or any third party; (b) that the Information Commissioner has started any action under the Privacy Act relevant to Novavax and the Agreement including (i) that the Information Commissioner is investigating a complaint against Novavax, (ii) an audit by the Information Commissioner, (iii) a request by the Information Commissioner to be given access to Novavax’s premises, or (iv) an injunction or other order against Novavax being sought by the Information Commissioner, in each case, to the extent relevant to the supply of the Vaccines under this Agreement. Novavax shall (A) act in accordance with any with Customer’s reasonable instructions in any related correspondence with the Information Commissioner or the relevant third party and keep Customer informed of the conduct, progress and outcome of any above outlined action including any determination made by the Information Commissioner, (B) ensure that Customer is kept informed of any assessment (or requirement to make an assessment) of a suspected eligible data breach, (C) take all reasonable measures, promptly and in accordance with the timeframes in the Privacy Act, including assisting Customer in any investigations to determine whether an eligible data breach has occurred, or if there are reasonable grounds to suspect and eligible data breach has occurred, and the requirements to notify the Information Commissioner and any third parties in respect of such eligible data breach, and (D) work with Customer to agree, who will issue notification of the eligible data breach to the Information Commissioner and relevant third parties if the eligible data breach is relevant to both Novavax and Customer. 16.4. Survival. The provisions of this Section 16 shall survive expiration or termination of this Agreement. 17. Commonwealth requirements 17.1. Freedom of Information. Novavax must comply with, and must do such things as may be reasonably necessary to assist Customer to comply with, the Freedom of

EXECUTION VERSION -23- 70472309_1 Information ▇▇▇ ▇▇▇▇ (Cth) and Freedom of Information Amendment (Reform) ▇▇▇ ▇▇▇▇ (Cth) (together the “FOI Act”). If required under the FOI Act, Customer will provide Novavax with a reasonable opportunity to avail itself of any applicable protections that are available to Novavax under the FOI Act, including the an opportunity to consult and make submissions in relation to Novavax’s trade secrets and other commercially valuable information contained in any Customer response to a request under the FOI Act. For the avoidance of doubt, Customer Confidential Information, Health Data and Customer documentation or other material will, to the extent applicable, be deemed to be documents of, and in the possession of, Customer for the purposes of the application of the FOI Act. 17.2. Anti-terrorism 17.2.1. Novavax must comply with its obligations (if any) under Part 4 of the Charter of United Nations ▇▇▇ ▇▇▇▇ (Cth) and the Charter of United Nations (Dealing with Assets) Regulations 2008 (Cth). 17.2.2. Novavax must comply with all applicable Laws dealing with the supply of goods, services and information to foreign nationals or institutions, including under the Customs ▇▇▇ ▇▇▇▇ (Cth) and the Weapons of Mass Destruction (Prevention of Proliferation) ▇▇▇ ▇▇▇▇ (Cth). 17.2.3. Novavax acknowledges that it is an offence to knowingly make any funds or assets available to a person or organisation on the list of all persons and entities who are subject to targeted financial sanctions under Australian sanctions law, available at ▇▇▇▇▇://▇▇▇.▇▇▇▇.▇▇▇.▇▇/▇▇▇▇▇▇▇▇▇▇▇▇▇-▇▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇/▇▇▇▇▇/▇ onsolidated-list. 17.2.4. If Novavax holds assets or funds belonging to a person or organisation on the list of persons and entities designated as terrorists, Novavax must immediately freeze those assets in accordance with all applicable Law. 17.3. Anti-money laundering. Novavax must comply with its obligations (if any) under the Anti-Money Laundering and Counter-Terrorism Financing ▇▇▇ ▇▇▇▇ (Cth) or similar applicable Laws. 17.4. Commonwealth policies: In so far as is reasonably practicable, Novavax must use its [***] to comply with any other Commonwealth policies or codes of conduct as notified to Novavax in writing by Customer from time to time to the extent such policies are applicable to Novavax’s obligations under this Agreement. 17.5. Work health and safety. Novavax must ensure that Novavax personnel carry out work under this Agreement in a manner which: 17.5.1. does not pose any avoidable health or physical safety risk to Customer personnel, Novavax personnel, or any other person;

EXECUTION VERSION -24- 70472309_1 17.5.2. complies at all times with all applicable work health and safety and workplace diversity legislation and any other applicable Laws where such personnel are located; and 17.5.3. when using or accessing facilities of Customer complies with Australian Government policies on work health and safety. For more information see: ▇▇▇▇▇://▇▇▇▇.▇▇▇▇▇▇▇▇▇.▇▇▇.▇▇/▇▇▇▇▇▇▇▇▇▇▇-▇▇▇-▇▇▇▇▇▇▇▇/▇▇▇▇-▇▇▇-▇▇▇▇▇▇▇▇▇/ whs-workplace-health-and-safety. 18. Definitions 18.1. Acceptance has the meaning given in Section 3.4 and Accept has the same meaning. 18.2. Acceptance Period has the meaning given in Section 3.4. 18.3. Additional Amount has the meaning given in Section 2.1. 18.4. Advance Payment has the meaning given in Section 5.1. 18.5. Agency means (a) government or government department or other body; (b) a governmental, semi-governmental or judicial person; or (c) a person (whether autonomous or not) who is charged with the administration of a Law, and unless stated otherwise, includes Commonwealth, State and Territory Agencies. 18.6. Aggregate Amount has the meaning given in Section 2.1. 18.7. Agreement Material means non-confidential documents, information, data or other material owned by Novavax or a third party (a) which are delivered to Customer in accordance with this Agreement, including any reports; or (b) copied or derived by Customer from documents referred to in clause (a) of this definition, but excludes all Intellectual Property Rights in the Vaccine. 18.8. ARTG means Australian Register of Therapeutic Goods. 18.9. Australian Disputes Centre has the meaning given in Section 4.3. 18.10. Authorisation means the approvals from the TGA for the Vaccine, which may initially be granted under an expedited marketing authorisation process but which may include additional requirements or directions of the TGA including requirements under the TG Act or other conditions on which the TGA grants the Authorisation (unless an Exemption applies) and Authorised has the corresponding meaning. 18.11. Calendar Quarter means the calendar quarters specified in the Delivery Schedule set forth in Exhibit B.

EXECUTION VERSION -25- 70472309_1 18.12. Certificate of Analysis means the certificate of analysis to accompany the Product delivered to the Customer, which certifies that the Product has been manufactured and tested in compliance with its Specification. 18.13. Claim means any claim, suit, proceeding, cause of action or demand of any kind, including those which are prospective or contingent and those the amount of which is not ascertained or ascertainable. 18.14. Commonwealth means the Commonwealth of Australia. 18.15. Commonwealth Record means a Commonwealth record as defined in the Archives ▇▇▇ ▇▇▇▇ (Cth). 18.16. Confidential Information has the meaning given in Section 12.1. 18.17. Conforming Product has the meaning given in Section 4.1. 18.18. Control means (a) to possess, directly or indirectly, the power to direct the management or policies of a person, whether through ownership of voting securities or by contract relating to voting rights or corporate governance, or (b) to own, directly or indirectly, fifty percent (50%) or more of the outstanding voting securities or other ownership interest of such person, or (c) in the case of a partnership, control of the general partner, and “Controls” and “Controlled” shall be construed accordingly. 18.19. Controller has the meaning it has in the Corporations Act. 18.20. Corporations Act means the Corporations ▇▇▇ ▇▇▇▇ (Cth). 18.21. COVID-19 as the meaning given in the Recitals. 18.22. Delivery Documents has the meaning given in Section 3.2. 18.23. Delivery Price means the Per Unit Delivery Price multiplied by the number of Vaccine doses delivered. 18.24. Delivery Schedule has the meaning given in Section 2.4. 18.25. Effective Date means the date of this Agreement. 18.26. Exemption means an exemption under the TG Act. Exempt and Exempting have a corresponding meaning. 18.27. GMP Clearance has the meaning given in clause 8.3. 18.28. Good Manufacturing Practice or GMP or cGMP means the current good manufacturing practice determined under section 36 of the TG Act.

EXECUTION VERSION -26- 70472309_1 18.29. Gross Negligence means an act or omission done with reckless disregard for a legal duty, whether consciously or not, for the consequences of the act or omission. 18.30. GST has the same meaning as in the GST Law. 18.31. GST Act means the A New Tax System (Goods and Services Tax) ▇▇▇ ▇▇▇▇ (Cth). 18.32. GST Law has the same meaning as in the GST Act and any regulations made pursuant to that Act. 18.33. Health Data means all data and information relating to Customer and its respective operations, facilities, customers, personnel, assets or programs, in connection with the Agreement in whatever form that data or information may exist and whether or not it was generated by or processed by or on behalf of Customer, or is stored in any Commonwealth Record. 18.34. Indemnified Party has the meaning given in Section 9. 18.35. Indemnifying Party has the meaning given in Section 9. 18.36. Independent Expert has the meaning given in Section 4.3. 18.37. Information Commissioner has the meaning given in section 3A of the Australian Information Commissioner ▇▇▇ ▇▇▇▇ (Cth). 18.38. Insolvency Event means, in respect of a person, any of the following events (a) it is (or states that it is) an insolvent under administration or insolvent (each as defined in the Corporations Act); or (b) it is in liquidation, in provisional liquidation, under administration or wound up or has had a Controller appointed to its property; or (c) it is subject to any arrangement (including a deed of company arrangement or scheme of arrangement), assignment, moratorium, compromise or composition, protected from creditors under any statute, or dissolved (in each case, other than to carry out a reconstruction or amalgamation while solvent on terms approved by the financier); or (d) an application or order has been made, resolution passed, proposal put forward or any other action taken, in each case in connection with that person, in respect of any of the above clauses; or (e) it is taken (under section 459F(1) of the Corporations Act) to have failed to comply with a statutory demand; or (f) it is the subject of an event described in section 459C(2)(b) or section 585 of the Corporations Act (or it makes a statement from which the Financier reasonably deduces it is so subject); or (g) it is otherwise unable to pay its debts when they fall due; or (h) something having a substantially similar effect to any of the things described in the above clauses happens in connection with that person under the law of any jurisdiction. 18.39. Intellectual Property Rights means copyrights, and all rights in relation to inventions, patents, registered and unregistered trade marks (including service marks), registered designs, circuit layouts, and all other rights resulting from

EXECUTION VERSION -27- 70472309_1 intellectual activity in the industrial, scientific, literary or artistic fields and includes the right to apply for registration of any such rights. 18.40. Law means any applicable statute, regulation, by-law, ordinance or subordinate legislation in force from time to time anywhere including in Australia and overseas, whether made by a State, Territory, the Commonwealth, or a local government, and includes the common law and equity as applicable from time to time. 18.41. Loss has the meaning given in Section 9.1. 18.42. Manufacturing Facilities means the drug substance, adjuvant and fill finish manufacturing facilities used to manufacture the Vaccine supplied to Customer under this Agreement as listed in Exhibit D and approved by the TGA or which have otherwise received GMP Clearance acceptable to the TGA, and updated in accordance with Section 8.9. 18.43. Maximum Aggregate Additional Amount means the maximum amount of additional Vaccine doses that Customer is permitted to order under this Agreement in addition to the Aggregate Amount. 18.44. Pandemic Period means the COVID-19 pandemic period as declared by the World Health Organization. 18.45. Personal Information means information or an opinion (including information or an opinion forming part of a database), whether true or not, and whether recorded in a material form or not, about a natural person whose identity is apparent, or can reasonably be ascertained from the information or opinion. 18.46. Per Unit Delivery Price means, after the Advance Payment is made, the remainder of the Per Unit Price that is payable in respect of each Vaccine dose delivered as set forth in Exhibit A. 18.47. Per Unit Price means the purchase price per Vaccine dose as set forth in Exhibit A. 18.48. Point of Entry has the meaning given in Section 3.1. 18.49. Price has the meaning given in Section 5.2. 18.50. Privacy Act means the Privacy ▇▇▇ ▇▇▇▇ (Cth). 18.51. Product has the meaning given in Section 2.1 and includes any Additional Amount. 18.52. Purchase Order means an order submitted by Customer in accordance Section 2.3 to purchase Product. 18.53. Refundable Portion has the meaning given in Section 5.1.

EXECUTION VERSION -28- 70472309_1 18.54. Regional Partner has the meaning given in Section 2.2.2. 18.55. Regulatory Approval has the meaning given in the Recitals. 18.56. Related Body Corporate has the meaning it has in the Corporations Act. 18.57. Remedial Plan has the meaning given in Section 2.7. 18.58. Special Vendor means a supplier of raw material that is key to the manufacture of Vaccine (including vial manufacturers), as listed in Exhibit D and updated in accordance with Section 13.4. 18.59. Specifications has the meaning given in Section 4.1. 18.60. Sponsor means the person or company appointed by Novavax to import the Vaccine in Australia and perform the duties of a Sponsor under the TG Act. 18.61. Sponsor Agreement means a legally binding term sheet or agreement between Novavax and Sponsor relation to the importation of the Vaccine in the Territory. 18.62. Taxes means taxes, levies, imposts, charges and duties (including stamp and transaction duties) imposed by any Agency together with any related interest, penalties, fines and expenses in connection with them, except if imposed on, or calculated having regard to, the net income of Customer. 18.63. Term has the meaning given in Section 7.1. 18.64. Territory has the meaning given in the Recitals. 18.65. TGA means the Therapeutic Goods Administration. 18.66. TG Act means the Therapeutic Goods ▇▇▇ ▇▇▇▇ (Cth). 18.67. Total Price means the total amount payable by Customer to Novavax for the Product purchased as set forth in Exhibit A. 18.68. USD has the meaning given in Section 5.4. 18.69. Vaccine has the meaning given in the Recitals. 18.70. Warranty Dispute Notice has the meaning given in Section 4.3. 18.71. Willful Misconduct has the meaning given in Section 9.1. EXECUTED as an agreement and, in respect of Section 9.1 only, as a deed poll in favour of the Sponsor. [Remainder of page intentionally blank.]

EXECUTION VERSION -29- 70472309_1

[Signature Page to Advanced Purchase Agreement] IN WITNESS WHEREOF, the Parties hereto by their duly authorized officers have executed this Agreement as of the Effective Date. NOVAVAX, INC. By: /s/ ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ III ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ III Printed Name EVP, CLO Title: SIGNED by an authorised representative for The Commonwealth of Australia acting through and represented by the Department of Health (ABN 83 605 426 759) in the presence of: /s/ ▇▇▇▇ ▇▇▇▇▇▇ /s/ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Signature of witness Signature of authorised signatory ▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Name of Witness (block letters) Name of authorised signatory (block letters) Date: 31/12/2020 Acting Secretary Position of authorised signatory
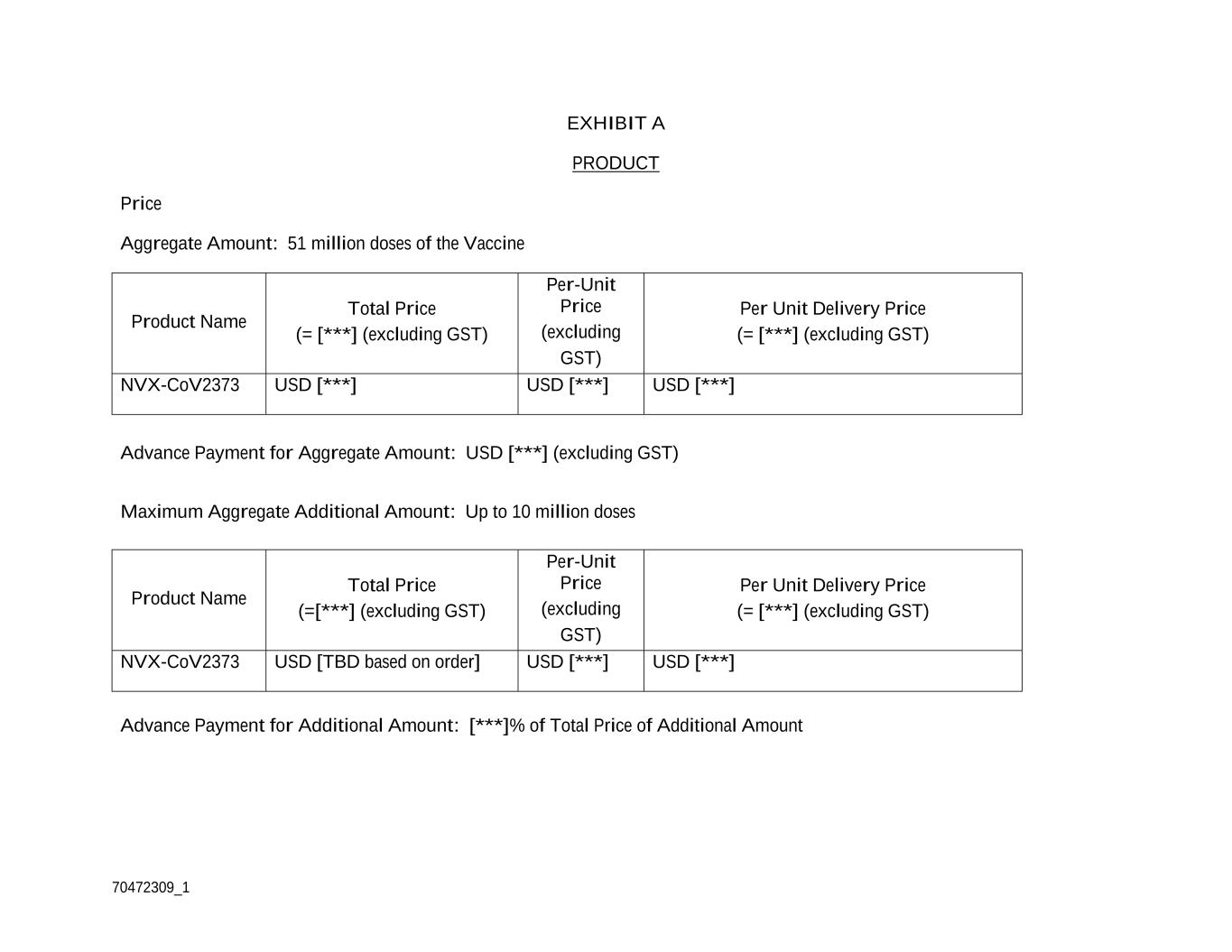
70472309_1 EXHIBIT A PRODUCT Price Aggregate Amount: 51 million doses of the Vaccine Product Name Total Price (= [***] (excluding GST) Per-Unit Price (excluding GST) Per Unit Delivery Price (= [***] (excluding GST) NVX-CoV2373 USD [***] USD [***] USD [***] Advance Payment for Aggregate Amount: USD [***] (excluding GST) Maximum Aggregate Additional Amount: Up to 10 million doses Product Name Total Price (=[***] (excluding GST) Per-Unit Price (excluding GST) Per Unit Delivery Price (= [***] (excluding GST) NVX-CoV2373 USD [TBD based on order] USD [***] USD [***] Advance Payment for Additional Amount: [***]% of Total Price of Additional Amount

70472309_1 EXHIBIT B DELIVERY SCHEDULE [Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]

70472309_1 EXHIBIT C SPECIFICATIONS [Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the specifications has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]

70472309_1 EXHIBIT D NOVAVAX [Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the manufacturing facilities and special vendors has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]

